Abstract
Throughout the body, lymphatic fluid movement supports critical functions including clearance of excess fluid and metabolic waste. The glymphatic system is the analog of the lymphatic system in the CNS. As such, the glymphatic system plays a key role in regulating directional interstitial fluid movement, waste clearance, and, potentially, brain immunity. The glymphatic system enables bulk movement of CSF from the subarachnoid space along periarterial spaces, where it mixes with interstitial fluid within the parenchyma before ultimately exiting from the parenchyma via perivenous spaces. This review focuses on important questions about the structure of this system, why the brain needs a fluid transport system, and unexplored aspects of brain fluid transport. We provide evidence that astrocytes and blood vessels determine the shape of the perivascular space, ultimately controlling the movement of perivascular fluid. Glymphatic fluid movement has the potential to alter local as well as global transport of signaling molecules and metabolites. We also highlight the evidence for cross talk among the glymphatic system, cardiovascular system, gastrointestinal tract, and lymphatic system. Much remains to be studied, but we propose that the glymphatic/lymphatic system acts as a cornerstone in signaling between the brain and body.
Keywords: astrocyte, cerebrospinal fluid, choroid plexus, glymphatic, peptides, perivascular space
Introduction
The awake, active brain builds up metabolic waste such as amyloid-β, which negatively affects neural functions if not removed. The glymphatic hypothesis postulates that the restorative function of sleep is a consequence of basic housekeeping whereby the glymphatic system “sweeps” the brain clear of waste by providing a continuous flow of fluid across the brain and out to the periphery, thereby counteracting protein accumulation and the development of neurodegenerative diseases such as Alzheimer's disease (Nedergaard and Goldman, 2020).
The glymphatic system enables bulk movement of CSF from the subarachnoid space along periarterial spaces, where it mixes with interstitial fluid (ISF) within the parenchyma before ultimately exiting from the parenchyma via perivenous spaces (Fig. 1) and drains into the peripheral lymphatic system (Iliff et al., 2012). This fluid movement occurs through a mixture of advection and diffusion, and enables rapid exchange of fluid between the tissue and the perivascular space with a net directionality toward the venous system (Thomas, 2019). Physiologic drivers such as arterial pulsatility, vasomotion, and respiration establish this directionality (Rennels et al., 1985; Iliff et al., 2013b; Kiviniemi et al., 2016; Mestre et al., 2018a; Fultz et al., 2019; van Veluw et al., 2020).
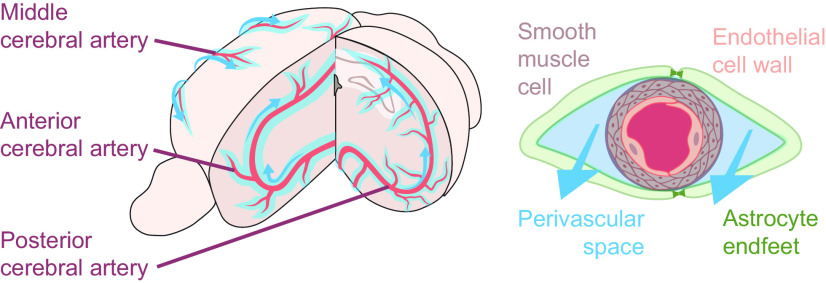
Figure 1.
The vascular network is a scaffold for glymphatic fluid transport along the perivascular spaces. Glymphatic fluid (light blue) enters the brain via the perivascular space of the major arteries (red; left). Arteries and veins are lined by perivascular spaces, where astrocyte end feet (green) cover smooth muscle cells (gray) and the endothelial wall of the vasculature (pink; right). This perivascular unit is a critical component of the glymphatic system, and its geometry is biologically optimized to promote fluid movement (blue arrows).
Evidence of CSF entering the periarterial space is not novel (Brierley, 1950; Rennels et al., 1985; Hadaczek et al., 2006); nor is the concept that CSF mixes with interstitial fluid (Levin et al., 1970; Kimelberg et al., 1978; Vladić et al., 2009) or even the hypothesis that this pathway may be a waste removal system (Lewis, 1877; Obersteiner, 1890), although the concept was insufficiently tested and thus prematurely discounted (Woollam and Millen, 1954). The features unique to discovery of the glymphatic system were as follows: (1) the direction of fluid transport, starting with entry of CSF at the periarterial space followed by exit of “dirty” interstitial fluid along the perivenous spaces; (2) a metabolic waste product, amyloid-β, was shown to be exported via the glymphatic system; (3) the fluid transport depends on polarized expression of the water channel aquaporin 4 (AQP4) in the vascular end feet of astrocytes; and (4) fluid transport and CSF entry into the neuropil exhibit a striking upregulation during sleep, paralleled by an increase in the clearance of metabolic waste (Iliff et al., 2012; Xie et al., 2013; Kress et al., 2014; Lundgaard et al., 2017, 2018; Mestre et al., 2018b).
Since its elucidation in 2012, the glymphatic system has provoked controversy, primarily because of a lack of data and adequate tools to characterize noninvasively a low-pressure fluid transport system residing in an electrically active organ encased within the rigid walls of the skull (Mestre et al., 2020a). Procedures associated with acute injection of tracers, such as opening the skull and insertion of a cannula, will effectively inactivate the glymphatic system (Mestre et al., 2018b; Plog et al., 2019). An equally troublesome issue is presented by the postmortem disappearance of fluid-filled CSF perivascular spaces and the reallocation of CSF tracers to other compartments, which invalidates the use of histology for characterization of brain fluid transport (Mestre et al., 2018a; Ma et al., 2019). Similar phenomena are quite commonly reported in the broader literature pertaining to interstitial fluid movement. For example, lymphatic capillaries were thought to be absent from skeletal muscle be cause the capillaries fully collapse in tissue preparations (Schmid-Schönbein, 1990). The more recent discovery of prelymphatic chambers in peripheral human interstitium demonstrates that large interstitial fluid compartments exist in all organs, but disappear during histologic procedures, highlighting the necessity of microscopy in vivo for the study of basic fluid transport (Benias et al., 2018). Finally, the new knowledge that bulk fluid movement through the brain is actively regulated by sleep (Xie et al., 2013; Lundgaard et al., 2017), anesthesia (Benveniste et al., 2017; Gakuba et al., 2018; Hablitz et al., 2019; Lilius et al., 2019), and time of day (Taoka et al., 2018; Cai et al., 2020; Hablitz et al., 2020) makes it inappropriate to compare previous studies in anesthetized animals with current work that includes the state of brain activity and time of day as important variables.
Caveats and controversies of the glymphatic hypothesis, along with their implications for neuropathology, have been reviewed at length in the recent literature (Kent and Mistlberger, 2017; Abbott et al., 2018; Rasmussen et al., 2018; Sun et al., 2018; Thomas, 2019; Nedergaard and Goldman, 2020; Troili et al., 2020; Wardlaw et al., 2020; Mestre et al., 2020a). For this reason, we place more focus in this review on important questions that remain to be addressed, fundamental questions about why the brain needs a fluid transport system, and the most exciting unexplored aspects of brain fluid transport.
In this review, we first compare and contrast the glymphatic system in the brain with the more traditional lymphatic system of the periphery, as both systems are essential for tissue homeostasis, interstitial fluid movement, immune function, and waste clearance. From there, we focus our efforts onto the perivascular space: what is known and unknown about the anatomy, and how differential regulation of the astrocytes and vasculature might alter global and local waste clearance. Then, we expand our questions beyond the perivascular space to how neuronal activity, intrinsically unique to the CNS, may alter interstitial fluid flow. In the final sections of this review, we ask the following question: does the glymphatic system have a role beyond simply “cleaning the brain”? We provide potential mechanisms of glymphatic bulk fluid signaling, whereby CSF may carry neuromodulators and/or vasoactive compounds to the perivascular space, changing interstitial fluid dynamics and brain activity. Finally, it has become increasingly clear that the brain does not work in isolation. We discuss known models of cross talk among the CNS, circulatory system, gastrointestinal (GI) track, and immune system, and how the glymphatic system may be a cornerstone in the communication between the brain and the body.
Comparison of glymphatic and lymphatic functions
Throughout the body, lymphatic fluid movement supports critical functions including clearance of excess fluid and metabolic waste and regulating tissue immunity (Miteva et al., 2010; Petrova and Koh, 2020). Tissues lacking traditional lymphatic capillaries develop alternative means of waste clearance and immune surveillance. In the eye, for example, Schlemm's canal, an endothelial-lined compartment that exhibits lymphatic markers (Grüntzig and Hollmann, 2019), drains fluid from the cornea (Petrova and Koh, 2018). Additionally, there is a glymphatic clearance system that exports intraocular fluid along the optic nerve (Wang et al., 2020). Both pathways ultimately convey fluid to the traditional lymphatic system. Based on the physiological requirements for local distribution of blood-derived nutrients and removal of metabolic waste, it is not surprising that the brain has compensated for its lack of lymphatic capillaries by developing an analogous perivascular perfusion system to maintain tissue homeostasis.
In peripheral tissue, blood capillaries provide a constant influx of an ultrafiltrate of plasma. The inflowing fluid percolates around the cells, dispersing glucose and other nutrients, while removing waste products via drainage to lymphatic vessels, which ultimately return the fluid to the venous circulation (Scallan et al., 2016; Breslin et al., 2018). In the brain, the blood–brain barrier (BBB) restricts ultrafiltration of plasma in most regions. Likely as a compensation, the CNS produces its own fluid, CSF, in the choroid plexus (Redzic et al., 2005). However, a portion of CSF production may still occur via the influx of plasma across the vast surface area of the microvasculature (Rasmussen et al., 2021). After production, CSF is, in part, circulated into the brain parenchyma along the periarterial spaces (Fig. 1; Mestre, 2018a). Thus, both in peripheral tissues and CNS, fluid entry occurs at the arterial segment of the microvascular bed, likely driven by arterial pulsatility that propels fluid influx into the tissue. However, the efflux routes of interstitial fluid differ. While lymphatic capillaries are the primary drainage path in peripheral tissues, interstitial fluid exits the CNS along the perivenous spaces and cranial/spinal nerves (Iliff et al., 2012, 2013b; Rangroo Thrane et al., 2013). CSF and interstitial fluid eventually drain from the CNS via a traditional lymphatic network located in the meninges (Aspelund et al., 2015; Louveau et al., 2015, 2018; Da Mesquita et al., 2018a; Ahn et al., 2019), as well as along nerve sheaths in the cribriform plate, which lead to cervical lymphatic vessels (Kida et al., 1993), with both pathways leading to the superficial and deep cervical lymphatic nodes (Raper et al., 2016; Cao et al., 2018; Zou et al., 2019). Ultimately, all CSF drains into the venous circulatory system, either indirectly through the lymphatic system or directly via the arachnoid villi. Thus, interstitial fluid transport is directional, with distinct influx and efflux routes both in peripheral tissues and the CNS. Lymphatic vessels are endowed with valves to prevent fluid backflow (Breslin, 2014). Whether the glymphatic system exhibits analogous one-way gating mechanisms within the neuropil to support directional fluid movement remains to be explored.
The glymphatic system can clear potassium, waste metabolites such as lactate, and peptides/proteins including amyloid-β and tau, along with a variety of contrast agents and tracers (Iliff et al., 2012, 2013a; Xie et al., 2013; Kress et al., 2014; Lundgaard et al., 2017; Eide and Ringstad, 2019; Monai et al., 2019). Clearance kinetics of tracer injected into the neuropil depends on the injection site (Cserr et al., 1981; Szentistványi et al., 1984), suggesting the existence of regional differences in waste clearance. Also, in support of regional differences in glymphatic function, Alzheimer's disease model mice exhibited lower CSF/ISF exchange in the rostral cortex compared with caudal cortex, which favored tau protein deposition in posterior regions (Harrison et al., 2020). These findings in brain parallel results in the gastrointestinal tract, where lymph composition and immune function differ between anatomic regions of the organ (Esterházy et al., 2019). Glymphatic clearance kinetics also vary across sleep state (Xie et al., 2013) as well as time of day (Hablitz et al., 2020), with increased clearance during both sleep and the inactive phase, demonstrating that the mechanism and routes of fluid efflux from brain are more complex than a simple plumbing system.
We still do not know precisely where in the brain interstitial fluid moves, and whether there are areas of pooling or slow flow within the brain. Additionally, details of where and how the glymphatic system connects to the lymphatic system remain unknown. Indeed, our current knowledge of the anatomic pathways of interstitial fluid clearance within the brain is rudimentary, relying on experimental procedures with invasive intracranial injections, sampling at discrete time points, and bulk transfer of tracers.
Open questions about the perivascular space, astrocytes, and flow
The perivascular space (PVS) is distinct from the highly complex and convoluted interstitial space of the brain parenchyma, and is a critical feature of the glymphatic system. The PVS surrounds the cerebral vasculature and is lined by astrocyte end-feet plastered alongside the pericytes and endothelial cells that form the BBB (Figs. 1, 2; Simard et al., 2003; Troili et al., 2020). Estimates of the extent of astrocytic coverage around capillaries range from 64% to 100% (Sasaki and Mannen, 1981; Bertossi et al., 1993; Simard et al., 2003; Oberheim et al., 2009; Korogod et al., 2015; Munk et al., 2019), likely because of the variable effects of postmortem histologic analysis and low-resolution microscopy. For example, three-dimensional electron microscopy reconstruction of a single hippocampal capillary showed tiled and interlocking end feet covering blood vessels and pericytes (Mathiisen et al., 2010), whereas another study based on cryofixation reported a coverage of only 64% in transversely sectioned capillaries (Korogod et al., 2015). Recent work has demonstrated a better correlation between astrocyte end foot size and vessel diameter in arteries than for veins, though these correlations were evaluated only in penetrating and ascending vessels (Wang et al., 2021). However, the thickness of the astrocyte ensheathment of the vasculature is relatively consistent in arteries, capillaries, and veins (McCaslin et al., 2011). Beyond these few studies limited to cerebral cortex, there is very little detailed information on end foot coverage of the arterial or venous walls, or how this may relate to the perivascular space. This lack of quantitative information about the PVS directly limits the applicability of models of glymphatic fluid flow, in which the geometry of the perivascular space dictates the calculated rate of CSF influx into the neuropil (Schain et al., 2017; Mestre et al., 2018a; Tithof et al., 2019).
Figure 2.
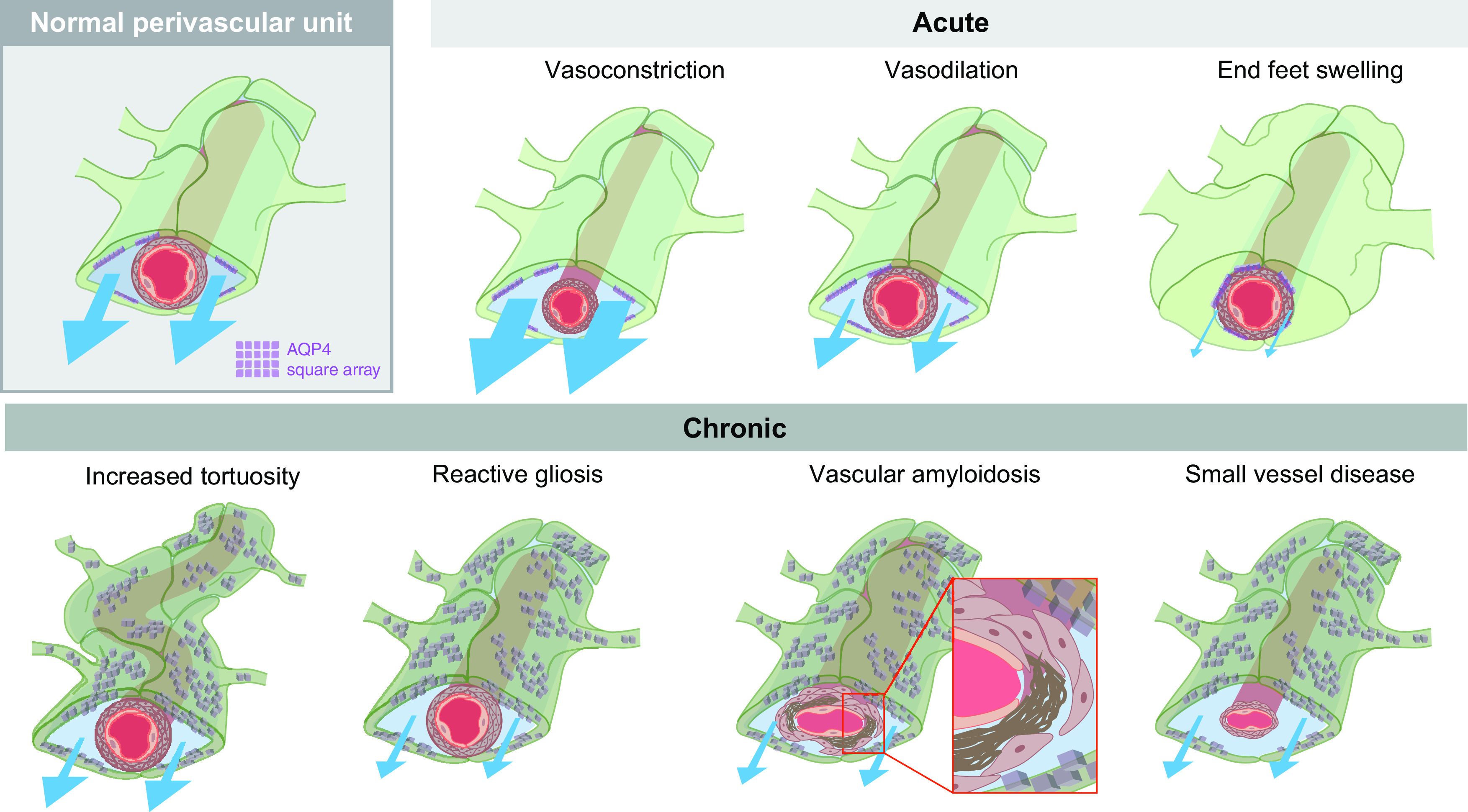
The perivascular space (PVS) can be modulated by changes to both astrocytes and the vasculature. The normal perivascular unit is composed of astrocyte end feet (green) covering smooth muscle cells (tan) and endothelial cell walls (pink) of the vascular network, promoting CSF (blue) movement along these channels. AQP4 (purple) is located in square arrays on the vascular-adjacent end feet of astrocytes. Acute changes to either the vasculature or astrocyte end feet can alter glymphatic fluid movement. Vasoconstriction increases the PVS, increasing flow (indicated by blue arrows; Mestre et al., 2020b). This is in contrast to vasodilation that is expected to decrease flow. Swelling of astrocytic end feet can alter the size of the PVS space in the setting of pathology (e.g., spreading depression; Schain et al., 2017), but it is possible that changes in the vascular end feet of astrocytes are a physiological mechanism by which glymphatic function is controlled. Chronic pathologic changes may also impair CSF influx (bottom). We hypothesize that vasculature changes such as increased tortuosity with aging alter fluid flow. Reactive gliosis (shown as a dark green color and mislocalized AQP4), is a common hallmark of neuropathology (Ikeshima-Kataoka, 2016; Verkhratsky et al., 2016; Wang and Parpura, 2016; Kovacs et al., 2018), which will, most likely decrease flow. Vascular amyloidosis, characterized by amyloid-β plaques (brown) accumulating between the smooth muscle cells and the endothelial cell wall, and small-vessel disease, characterized by altered vascular shape and enlarged perivascular spaces, both decrease glymphatic flow.
Acute changes in the geometry of the PVS on vasoconstriction, vasodilation, or astrocyte swelling have the potential to affect the movement of glymphatic fluid (Fig. 2). Evidence for this hypothesis comes from mouse disease models. For example, in a model of acute stroke, ischemic spreading depolarization triggers the constriction of blood vessels, thus widening the PVS (Fig. 2) and enabling a rapid influx of CSF to the parenchyma (Mestre et al., 2020b). In cortical spreading depression, a rapid neuronal depolarization event that is frequently associated with migraine aura, the PVS closes, resulting in reduced interstitial clearance of tracer (Schain et al., 2017). Spreading depression causes rapid vasodilation followed by vasoconstriction. It has been proposed that the vasoconstriction phase correlates with astrocytic end foot swelling, and underlies a reduction of flow in the PVS (Rosic et al., 2019). However, the magnitude of vasoreactivity or end foot swelling necessary to perturb glymphatic flow is unknown.
The PVS and vascular compartments are dynamic volume spaces that provide the brain with a mechanism to couple hyperemia and waste clearance. In humans, neuronal slow waves occurring during sleep are coupled to hemodynamic oscillations, which in turn are coupled to CSF flow. Specifically, a nocturnal peak in 0.2–4 Hz neuronal activity triggers increased cerebral blood flow, which reduces the amount of CSF movement in the ventricle and the brain parenchyma, directly supporting the concept of blood/CSF volume switching (Fultz et al., 2019). Acute hypertension increases the stiffness of the arterial wall, resulting in decreased pulsatility and PVS fluid flow by as much as 50% (Mestre et al., 2018a), highlighting the interplay between the vasculature and the PVS in glymphatic function. There is also evidence that posture can effect glymphatic flow (Lee et al., 2015), but whether this is related to known changes in cerebral blood flow (Foley et al., 2005; Kose and Hatipoglu, 2012), changes in intracranial pressure (Andresen et al., 2015), and/or changes in sympathetic noradrenergic tone (Stewart, 2012) remain unknown. Given that astrocytes are a critical component of the neurovascular unit and participate directly in the regulation of cerebral blood flow (Iadecola and Nedergaard, 2007), it seems likely that astrocytes regulate volume dynamics between the vasculature and perivascular spaces.
The primary evidence for astrocytic regulation of glymphatic fluid movement, beyond the spatial organization of the PVS, is that AQP4 facilitates glymphatic fluid transport (Iliff et al., 2012; Mestre et al., 2018b). The expression of AQP4 is normally highly polarized toward the plasma membrane of the astrocytic end feet facing the PVS (Fig. 2) and is anchored by the dystrophin-associated complex (Waite et al., 2012; Zhang et al., 2014). Mislocalization of AQP4 from astrocytic end feet has been linked to glymphatic malfunction in multiple lines of work (Kress et al., 2014; Ren et al., 2017; Lundgaard et al., 2018; Mestre et al., 2018b; Ohene et al., 2019; Wei et al., 2019; Harrison et al., 2020; Xue et al., 2020; Liu et al., 2020b). Though AQP4 polarization toward the vascular end feet constitutes a key regulatory mechanism, it is likely that astrocytes can alter glymphatic function by additional mechanisms.
It is clear that glymphatic function is highly dependent on optimized perivascular spaces with low resistance to fluid flow, yet very few studies have tested whether long-term remodeling of the shape, permeability, and patency of the PVS is linked to glymphatic dysfunction. With increased age, glymphatic flow decreases, alongside increased reactive gliosis and a reduction in the polarized expression of AQP4 toward the vascular end feet of astrocytes (Fig. 2; Kress et al., 2014). Reactive gliosis, particularly manifested by increased GFAP and mislocalized AQP4, is a hallmark of neuropathology (Ikeshima-Kataoka, 2016; Verkhratsky et al., 2016; Wang and Parpura, 2016; Kovacs et al., 2018) and could potentially indicate alterations to astrocytic end foot morphology, although this conjecture has not been tested. Additionally, the tortuosity of the brain vasculature increases with aging (Fig. 2; Thore et al., 2007), which likely presents an impediment to fluid flow.
The functionality and shape of the cerebrovasculature also changes in disease. In cerebral amyloid angiopathy, Aβ1-40 accumulates in vessel walls, causing vessel weakening and collapse of the perivascular space (Smith, 2018; Gatti et al., 2020). In small-vessel disease, which is a frequent complication of hypertension and diabetes, arterial stiffening and remodeling of the cerebral arteries cause enlargement of the PVS (Fig. 2; Mestre et al., 2017; Lerman et al., 2019). The functional importance of this chronic PVS remodeling has yet to be established, leading to several unresolved questions, including the following. If the curvature and surface area of the vasculature increases, can the astrocytic end feet compensate? Would changes in astrocyte volume or morphology, such as occurring during reactive gliosis, directly impact local glymphatic flow? And, finally, do the borders of the PVS ever break down, and how would this effect polarized fluid flow?
Astrocytes are morphologically complex cells. In addition to the vascular end feet, astrocytes extend innumerable fine, irregular processes that exhibit local Ca2+ signaling independent of the soma and other processes (Tong et al., 2013; Shigetomi et al., 2016; Verkhratsky and Nedergaard, 2018). The astrocyte and its processes can change rapidly in volume, within a matter of seconds (Takano et al., 2005; Risher et al., 2009; Sherpa et al., 2016). This process appears to be independent of AQP4 and occurs under conditions of high K+ such as during increased neuronal activity (Walch et al., 2020). This is consistent with reduced interstitial space measurements during wakefulness (Xie et al., 2013), and most likely is a prime mechanism for rapid alterations in glymphatic flow. More chronic changes to astrocyte morphology have been found in the hypothalamus. Astrocytes can extend and retract fine processes across sleep states (Bellesi et al., 2015), hydration state (Hawrylak et al., 1998), during pregnancy and lactation (Theodosis, 2002; Theodosis et al., 2008), and in response to the light/dark cycle (Lavialle and Servière, 1993; Lavialle et al., 2001, 2011). Such morphologic changes can last from several hours to days and are generally reversible. In the case of lactation, glial processes retract from the supraoptic nucleus (SON) of the hypothalamus (Fig. 3) and remain retracted until pup weaning, when the glial processes extend back into the region (Hatton, 1997). It is unknown whether astrocytic morphologic changes alter glymphatic function.
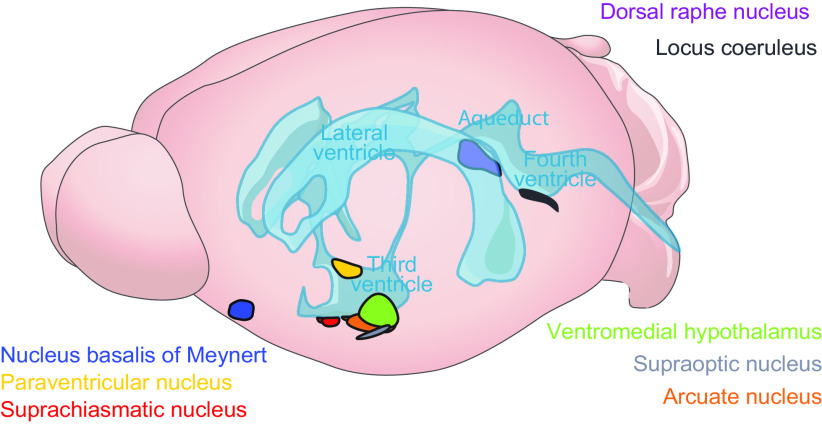
Figure 3.
Anatomical localization of key brain regions is strategically placed around CSF reservoirs. The hypothalamus is located along the third ventricle and base of the brain above the basal cisterns, a prime position for CSF signaling. It contains the suprachiasmatic nucleus (red), arcuate nucleus (orange), paraventricular nucleus (yellow), ventromedial hypothalamus (green), and the supraoptic nucleus (navy), which is a hub of peptidergic signaling that controls basic biological functions such as circadian timing, reproduction, feeding, hydration, and more. The nucleus basalis of Meynert (blue), dorsal raphe nucleus (purple), and locus coeruleus (gray) are also primed for brain-wide CSF-mediated cholinergic, serotonergic, and noradrenaline signaling.
Glymphatic–neuronal interactions
The brain is a unique organ because of the complexity of its neuronal networks, which signal to execute complex behaviors, tasks, and thought. Neural signaling (i.e., synaptic transmission and propagation of action potentials) requires tight regulation over interstitial ion concentrations (Rasmussen et al., 2020). For example, simply changing the interstitial ion concentration can induce arousal state changes, with lower interstitial Ca2+, Mg2+, and H+, and higher K+ inducing awake brain activity (Ding et al., 2016). Astrocytes are highly dynamic regulators of interstitial K+ concentrations and pH, and likely influence the concentrations of several other ions, as well as the interstitial space volume (Verkhratsky and Nedergaard, 2018). It is presently unknown whether the glymphatic system independently contributes to state-dependent changes in interstitial ion concentrations, but there are grounds to speculate that fluid transport and the ion composition of the interstitial fluid are intimately linked.
The strongest evidence linking brain-wide neuronal activity and glymphatic activity is derived from sleep studies. Non-rapid eye movement (NREM) sleep consists of four stages with different patterns of brain electrical activity including the phase dominated by large amplitude, synchronous, slow oscillations of 1–4 Hz. There is strong evidence that glymphatic flow increases to promote the delivery of larger CSF volumes to the brain during NREM sleep (Xie et al., 2013), and under anesthetic regimens characterized by a high prevalence of 1–4 Hz activity (Hablitz et al., 2019). In humans, the volume of CSF movement within the fourth ventricle is also increased during NREM sleep (Fultz et al., 2019), and AQP4 haplotype is associated with changes in NREM sleep architecture (Ulv Larsen et al., 2020), suggesting a link between fluid movement within the brain and neuronal activity. During REM sleep, the cortex exhibits low-voltage, rapid desynchronized neuronal activity that superficially resembles the pattern during wakefulness. It remains to be established whether glymphatic flow declines during REM sleep, as during waking. Mechanisms mediating the functional relationship between neuronal activity and bulk fluid movement have not been tested; nor have the above observations been applied to investigations of sleep deprivation or insomnia.
Synchronizing distinct populations of neurons may influence local hemodynamics and fluid flow to ultimately change local solute transport. This phenomenon has been shown for CSF dynamics across the brain as a whole (Fultz et al., 2019), but has yet to be demonstrated for regional brain or perivascular-localized waste clearance. Sensory manipulations targeted to network activity that were reduced in different pathologic states including aging and neurodegeneration, such as gamma oscillations (∼40 Hz) seen during response to external stimuli (Jafari et al., 2020), may improve glymphatic clearance. Support for this hypothesis is found in mouse models of Alzheimer's disease, where the stimulation of sensory systems can alter local waste buildup. Inducing synchronous gamma oscillations via 40 Hz light flickers is reported to decrease amyloid burden in the visual cortex (Iaccarino et al., 2016). Entraining the auditory system via 40 Hz tone evokes decreased amyloid protein buildup across the entire cortex via a glia- and vascular-dependent mechanism (Martorell et al., 2019).
Glymphatic bulk fluid signaling
To this point, we have focused on CSF/ISF exchange and waste clearance, which is likely the primary function of the glymphatic system. Albeit less studied, glymphatic transport may fulfill additional roles, including the widespread distribution of signaling molecules within brain. CSF may also play a largely unacknowledged role in transporting CNS signaling molecules to peripheral tissues, which we shall discuss here.
CSF is primarily produced by the choroid plexus located in the ventricles and then undergoes directional transport from the lateral ventricle to the third ventricle, through the aqueduct of Sylvius, and finally to the fourth ventricle, where it exits the brain via the foramina of Magendie and Luschka. From the foramina, CSF enters the cisterna magna and is either shunted out of CNS via the meningeal lymphatic system or redirected back into the brain by the glymphatic system. The entry path for the glymphatic system is via the pontine cistern, where CSF moves along the perivascular space surrounding the basilar artery to the circle of Willis. CSF then ascends along the perivascular spaces of the anterior, middle, and posterior cerebral arteries. Rodent studies have demonstrated that during sleep, CSF transport along the glymphatic system is fast (minutes to hours; Xie et al., 2013) compared with during the active phase, where CSF redistributes to the mandibular lymph nodes in an equally fast manner (Hablitz et al., 2020).
The choroid plexus secretes or transfers from blood a host of solutes, metabolites, vitamins, and carrier proteins to CSF (please see next section for details). Remarkably, most of the brain regions that produce signaling molecules with the potential for long-range volume transmission are located along the ventricular path of CSF transport (Fig. 3). The classical neuromodulators such as acetylcholine, noradrenaline, and serotonin, which are thought to participate in volume transmission, are produced by cell groups generally lying just below the ependymal lining of the ventricles or the pial lining of the ventral brain surface. The nucleus basalis of Meynert, which contains most of the cholinergic neurons in the CNS, is located in the basal forebrain region just on top of the pial membrane facing the optic nerve, while its medial boundary meets the wall of the lateral ventricle (Liu et al., 2015). The locus coeruleus, which is the main source of norepinephrine in the CNS, is located along the lateral wall of the fourth ventricle. The serotonergic dorsal raphé nucleus is positioned centrally in the midline of the brainstem, but sends extensive ascending projections to the wall of the lateral, third, and fourth ventricles (Simpson et al., 1998). Although it remains to be demonstrated, the spatial distribution of acetylcholine-, norepinephrine-, and serotonin-secreting neurons or their projections suggests that these neuromodulators may be distributed by CSF transport following their release from the axonal terminals (Agnati et al., 1986; Vizi et al., 2010; Taber and Hurley, 2014; Veening and Barendregt, 2015). In contrast, the dopamine neurons of the substantia nigra are located in the ventral midbrain posterior to the cerebral peduncle and are thus not in direct contact with the brain surfaces. Neither do projections from these dopaminergic neurons terminate at the ventricular surface. This may reflect a necessity for local, tightly regulated dopaminergic signaling and/or a mechanism to protect the rest of the brain from CSF-driven global dopaminergic neuromodulation.
Perhaps the most important example of brain–CSF communication is presented by the hypothalamus. The hypothalamus controls fundamental physiological and behavioral functions such as eating, drinking, regulating body temperature, neuroendocrine release, and internal circadian timing. Anatomically, the hypothalamus is in prime position to access the CSF, as it surrounds the third ventricle, is ventrally in contact with the CSF pool of the basal cisterns (Fig. 3), and has specialized cells, tanycytes, that can directly interact with the CSF pool in the ventricles (Rodríguez et al., 2010). The hypothalamus is a hub of peptidergic signaling, including neuropeptide Y and pro-opiomelanocortin in the arcuate nucleus, arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) in the suprachiasmatic nucleus, and oxytocin and AVP in the supraoptic and paraventricular nuclei. Biomarkers of the melanocortin system are implicated in appetite suppression, and melanin-concentrating hormone, which is important for initiation of feeding, is present in the CSF, enabling brain- and body-wide signaling (Kim et al., 2014; Page-Wilson et al., 2017; Noble et al., 2018). Hypothalamic peptides such as VIP and AVP are present in CSF (Burbach, 1982) and can directly alter vascular tone (Henning and Sawmiller, 2001; Pelletier et al., 2014), thus potentially regulating glymphatic flow through the neuropil. The plethora of peptidergic neuromodulators that participate in CSF/ISF-mediated volume transmission, and the vasoreactive action of many of these peptides, support a model of hypothalamic bulk fluid signaling.
Nuclei of hypothalamus densely innervate the pituitary gland, an endocrine organ at the base of the brain (Spencer and Deak, 2017; Qin et al., 2018). A distinct feature of the pituitary is its localization outside the CNS barriers of the brain, spinal cord, and CSF. The pituitary gland is positioned in an indentation of the skull known as the sella turcica and is separated from the subarachnoid space by the diaphragma sellae, a dural membrane that prevents CSF from accessing the gland (Fig. 4). As a consequence of this arrangement, pituitary hormones are secreted directly into the blood, avoiding CSF transport within CNS (Patel et al., 2021; Rawindraraj et al., 2021).
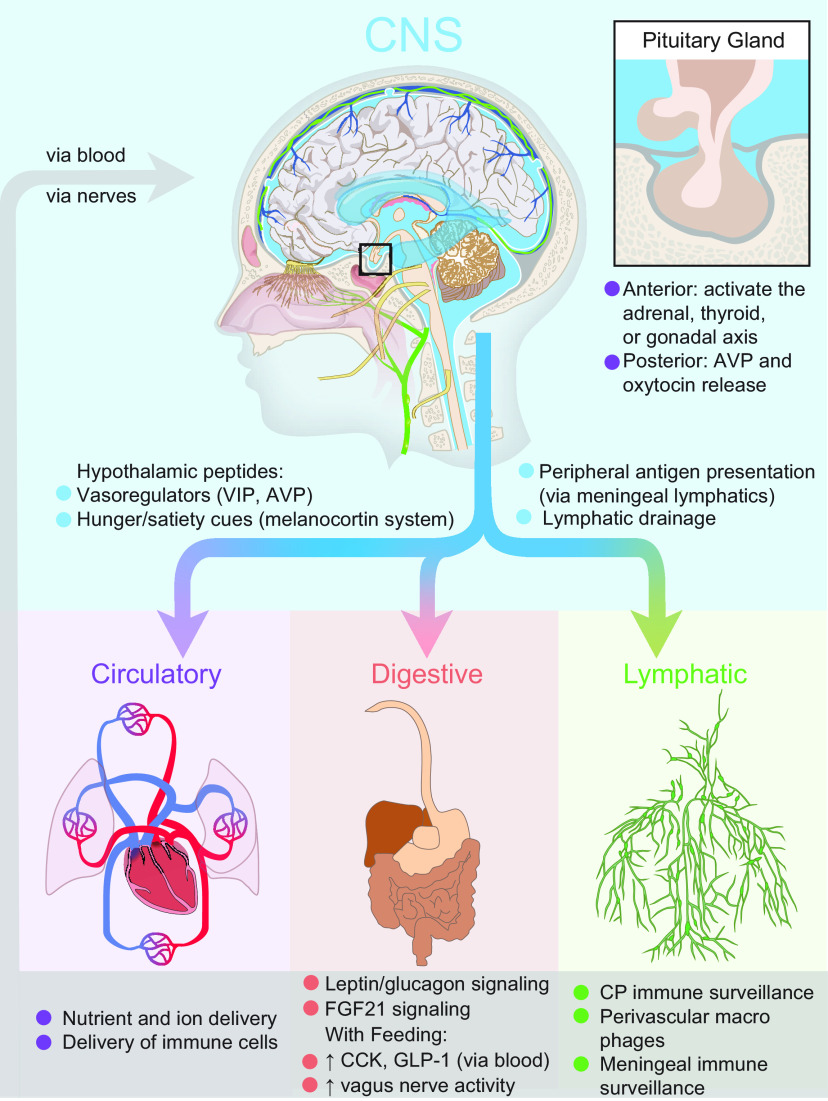
Figure 4.
The glymphatic system as an interface between the brain and body. The brain can communicate to the circulatory, digestive, and lymphatic systems by secreting signaling molecules to CSF, driving fluid to the meningeal lymphatics for antigen presentation, and ultimately draining to the lymph nodes of the lymphatic system. A unique feature of the brain is the hypothalamic signaling to the pituitary gland, where neurons can either induce neuroendocrine signaling in the anterior pituitary, or directly release peptides such as AVP and oxytocin into the blood vessels of the posterior pituitary. Feedback between these systems is potentially bidirectional when considering blood composition, feeding and fasting metabolites, and immune surveillance. The pituitary gland is uniquely shielded from direct interaction with the CSF pool and direct CNS signaling by its location in an indentation of the skull covered by the diaphragma sellae, a dural membrane.
The pituitary is divided into two lobes that are functionally and developmentally distinct (Kiecker, 2018). The posterior pituitary gland contains axonal projections from magnocellular neurosecretory neurons located in the SON and paraventricular nucleus (PVN) of hypothalamus. These neurons secrete oxytocin and AVP, which are stored in the posterior pituitary and then are released into the blood. In addition, the magnocellular neurosecretory cells can release oxytocin and AVP from their dendrites within the parenchyma of the hypothalamus. Dendrites from the SON form a plexus lying just below the pial surface of the SON, whereas the dendrites from PVN form a bundle at the subependymal region of the third ventricle, suggesting that oxytocin and vasopressin are distributed via CSF transport within the CNS (Heimer-McGinn et al., 2013), in addition to their peripheral actions. Cortical signaling by oxytocin and AVP alter social and cognitive function without having direct synaptic pathways (Ludwig and Leng, 2006; Meyer-Lindenberg et al., 2011; Abramova et al., 2020). The organization of the hypothalamus and posterior pituitary suggests that oxytocin and AVP could exert their effects on social and cognitive function through a combination of central and peripheral regulation of glymphatic function, where blood-borne peptides change vascular tone and interstitial fluid-mediated peptidergic signaling changes synaptic tone.
Parvocellular hypothalamic neurons secrete neurohormones that are exported to the anterior pituitary via hypophyseal portal blood vessels, thus avoiding CSF transport and distribution within CNS. These neurohormones control the release of growth hormone, prolactin, adrenocorticotropin, luteinizing hormone, follicle-stimulating hormone, and thyroid-stimulating hormone, ultimately inducing body-wide stress, sex, and metabolic responses. Exploration of neuroendocrine regulation of glymphatic flow is in its infancy. Activation of the adrenal gland is a classic example of a hypothalamic–pituitary cascade, which initiates a concerted, body-wide stress response. Chronic stress can cause glymphatic malfunction (Wei et al., 2019; Liu et al., 2020b), but it remains unknown how acute stress changes brain fluid movement.
Text box 1:
Bringing it all together with the circadian system
Sleep and circadian rhythmicity are evolutionarily conserved behavioral and physiological states, serving to restore bodily functions and, in the case of circadian rhythms, predicting changes in food availability, temperature, predation, and other factors essential to ensure survival. Sleep and circadian timing both regulate glymphatic function. In this section, we summarize the relevant literature and generate new hypotheses about the mechanisms and function of circadian rhythms and sleep in glymphatic function.
In humans, most brain regions exhibit endogenous rhythms in neuronal excitability, with peaks in excitability occurring within a 4 h window across the brain (Muto et al., 2016). This synchronized daily activity enables complex behaviors to have phase-appropriate performance peaks, such that learning and memory capacities peak during the active phase (Iyer et al., 2014; Smarr et al., 2014). Recent work has shown that, in addition to synchronized neuronal excitability, the distribution of CSF in the brain and lymph nodes is under circadian control, with increased glymphatic influx and clearance from the brain during the rest phase, and increased lymphatic drainage during the active phase (Hablitz et al., 2020). If we consider rhythmic, synchronized neuronal activity, the circadian cycle of glymphatic flow, and the earlier hypothesis that synchronized neuronal activity can drive fluid flow, one might suppose that fluid flow in the brain may act as a circadian entrainment mediator, whereby individual brain oscillators drive rhythmic fluid flow to the next oscillator in line, thus synchronizing brain activity across the 24 h cycle.
We have already introduced the hypothesis that the hypothalamus is a key signaling area for bulk flow, based on its anatomic location and highly enriched concentrations of neuropeptides. The mammalian suprachiasmatic nucleus (SCN) of the hypothalamus is considered the circadian pacemaker; synchronizing and driving daily rhythms in gene expression, metabolism, physiology, and behavior (Mohawk and Takahashi, 2011). Classic work demonstrated that grafting SCNs of rhythmic animals into the third ventricle of SCN-lesioned animals restores behavioral rhythmicity (Silver and LeSauter, 1993) and showed that this effect was dependent on a diffusible factor (Silver et al., 1996), suggesting that the SCN may directly communicate with the CSF pool to synchronize circadian rhythms. The SCN has a very distinct localization of neuropeptides (Morin et al., 2006). VIP neurons, which are responsible for maintaining intrinsic rhythmicity (Aton et al., 2005; Todd et al., 2020), are located in the ventral portion above the optic chiasm. AVP neurons, which regulate plasticity of rhythms (Mieda, 2019; Rohr et al., 2020), are found in the dorsal portion of the SCN along the third ventricle. Although both VIP and AVP are present within CSF (Burbach, 1982), how these enter the CSF pool remains unknown. Based on the localization of these vasoactive peptides (Henning and Sawmiller, 2001; Pelletier et al., 2014), it is tempting to hypothesize that the mechanism of action whereby VIP released near the basal cisterns impacts vascular tone of the glymphatic system, whereas AVP release into the ventricles—upstream in the CSF pathway—is able to tune fluid flow in “downstream” areas. Whether AVP is released by the SCN into the ventricles, perhaps via direct innervation (Taub et al., 2021), a small portion of tanycytes within the SCN (Wen et al., 2020), or a separate transependymal method, remains unknown.
One such downstream area may be the pineal gland, which sits near the center of the parenchyma, is highly vascularized, and is bathed in CSF. CSF tracers accumulate in the pineal gland of mice and rats (Iliff et al., 2013a; Benveniste et al., 2017). In rodents, the SCN triggers a multisynaptic pathway that activates sympathetic preganglionic neurons in the spinal cord to release norepinephrine from terminals innervating the pineal gland, thus inducing nighttime melatonin production on demand for immediate release (Ganguly et al., 2002; Saper et al., 2005). The primary function of melatonin is to entrain circadian rhythms and improve sleep timing, along with a host of other beneficial pleiotropic effects (Aulinas, 2000; Pandi-Perumal et al., 2006). Nightly melatonin secretion is the primary biomarker for the circadian phase in humans and declines with aging, leading to alterations in sleep timing (Pace-Schott and Spencer, 2011). Though it is widely accepted that melatonin is secreted directly to the blood, this is primarily because of early literature reports correlating melatonin production in the pineal gland to blood plasma levels (Hedlund et al., 1977; Illnerova et al., 1978). Anatomical studies of the pineal gland give conflicting results, suggesting an intact BBB in fetal tissue (Moller, 1974) and, perhaps, subregion differences in pineal BBB permeability in adults (Duvernoy and Risold, 2007). Although melatonin is found in the CSF (Bruce et al., 1991; Skinner and Malpaux, 1999), it is unclear whether this melatonin pool derives from rapid diffusion from blood across lipid membranes, or whether the pineal gland directly secretes this powerful chronobiotic molecule directly to the CSF to entrain the brain.
The circadian system is just one example of several concerted biological responses that might be controlled by glymphatic—and CSF—movement. We highlight the circadian system as an example of how neuronal activity, CSF movement, and bulk fluid signaling might interact to impact neuronal function, physiology, and, ultimately, behavior.
CSF goes to glymphatics and beyond
Above, we discussed how the glymphatic system can be regulated by the cardiovascular system, neuronal function, and, perhaps, by CSF/ISF bulk flow of neurotransmitters and peptides. We have discussed the pathway of fluid movement from the ventricles to the glymphatic and lymphatic systems of the body. Finally, we hypothesized that the while the glymphatic system clears waste, it is also a mechanism of brain-wide signaling that could dramatically impact the survival of an organism adapting to a new environment. Here, we expand on known CSF-localized signaling cues originating from the blood, gut, or immune system. We discuss how they impact glymphatic function and highlight new questions in brain–body interactions.
Cross talk between the blood and the brain
The vascular system carries oxygen and glucose along with other nutrients and immune cells throughout the body and is critical to maintain tissue homeostasis. As discussed above, the BBB limits the transfer of solutes between blood and brain. The brain obtains an ultrafiltrate of blood via the choroid plexus, a highly vascularized ependymal cell layer lining the ventricles, which is responsible for the bulk of CSF production (Redzic et al., 2005). As such, the choroid plexus may be considered the “starting point” of the glymphatic system. In addition to transferring signaling molecules like growth factors from the blood into the brain, the choroid plexus can also contribute to brain homeostasis by producing and secreting into the CSF a host of enzymes and growth factors such as transthyretin, insulin-like growth factor II, and interleukin-1β (Benarroch, 2016). Interestingly, the choroid plexus also expresses receptors for norepinephrine, melatonin, and AVP, among other hypothalamic-derived neuropeptides, which, on activation, can modulate the rate of CSF production (Nilsson et al., 1992). How CSF production rates impact glymphatic flow in rodent brain remains unexplored because of a lack of proper techniques (Orešković et al., 2003; Orešković and Klarica, 2014). Recent work has shown that physiological processes that reduce glymphatic function like age and wakefulness also suppress CSF production, yet other manipulations showed that there is no correlation between CSF production and glymphatic fluid transport (Liu et al., 2020a). It thus remains unknown whether CSF production and glymphatic function are interdependent, but it is clear that the choroid plexus is a key player in the feedback loop among the brain, glymphatic system, and CSF production from the blood (Fig. 4).
It is important to note that BBB restriction to influx of an ultrafiltrate of plasma is not consistent across brain regions, specifically in the circumventricular organs in the hypothalamus where the interstitial fluid of these regions are barricaded by defined glial borders from the rest of the brain (Rodríguez et al., 2010). Also, the BBB does not necessarily preclude the regulation of transport between the blood and parenchyma of ions, oxygen, molecules, and even cells under pathologic conditions (Daneman and Prat, 2015). Although the BBB is clearly necessary for brain health, how the BBB alters or interacts with glymphatic fluid flow is at this point completely unknown.
There is bidirectional signaling between the vascular compartment and the brain. As discussed above, the vascular compartment uses the choroid plexus as a gateway to provide access for signaling molecules to the CNS. The brain communicates directly with the vascular compartment via the posterior pituitary gland (Fig. 4). Peptidergic neuronal projections in the posterior pituitary from oxytocin and AVPergic neurons arising from the paraventricular nucleus (Fig. 3) release neuropeptides directly into the vascular system, with downstream effects on cardiovascular tone, kidney function, and other functions (Palkovits, 1984). Feedback from this peripheral pathway can alter cerebral blood flow at a more global level compared with local/bulk hypothalamic signaling, perhaps altering PVS volume to increase or decrease glymphatic flow depending on brain state.
The gut–brain axis
Above, we have discussed how the glymphatic system clears metabolic waste from the brain. One might anticipate that this fluid pathway would be receptive to satiety and hunger cues. We now discuss modes of cross talk between the gastrointestinal tract itself and the brain (Fig. 4). Intravenous administration of triglycerides alters the composition of CSF lipids (Hanson et al., 2019), and glymphatic transport can distribute lipids across the brain (Rangroo Thrane et al., 2013). Alterations in the blood/brain/CSF distribution of triglycerides also cause a dysregulation of central leptin signaling (Banks et al., 2018), which controls appetitive behavior. These pathways support the hypothesis that after absorption in the GI tract and passage through the liver, food-derived lipids and other hydrophobic molecules can enter the CSF from the blood via the choroid plexus and across the BBB. In the brain, the major carrier of cholesterol is apolipoprotein E (APOE), which is abundantly produced by astrocytes as well as by the choroid plexus (Xu et al., 2006), suggesting that APOE might be distributed in brain by fluid movement in the perivascular space. APOE isoforms, when delivered by the CSF, enter periarterial spaces with different efficacy (Achariyar et al., 2016). Interestingly, APOE genotype is the strongest genetic risk factor known for late-onset sporadic Alzheimer's disease (Belloy et al., 2019), and glymphatic function is reduced in mouse models of AD (Peng et al., 2016; Da Mesquita et al., 2018b; Harrison et al., 2020). Thus, it is possible that differential distribution of lipids derived from the gut along the perivascular spaces directly contributes to brain pathology.
The vagus nerve provides a direct neural connection from the gut to the brain. Upon feeding, the visceral branches of the vagus nerve are activated by a variety of neuroendocrine cues, including cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1), that are produced by enteroendocrine cells of the gut (Dockray, 2009; Krieger et al., 2015). Upon food deprivation, grehlin, orexin-a, and reduced CCK levels can reduce firing and retrograde signaling by the vagus nerve (Dockray, 2009). Vagus nerve stimulation leads to increased glymphatic influx (Cheng et al., 2020). Feeding may inherently increase glymphatic function to promote the intracerebral circulation of hypothalamic satiety cues. The gut peptide GLP-1 has also gained significant attention in neurodegeneration research (Grieco et al., 2019), and it is a matter of interest that treatment with a GLP-1 receptor agonist reduced CSF production (Botfield et al., 2017). Similarly, fibroblast growth factor 21 (FGF21) is produced by the liver (von Holstein-Rathlou and Gillum, 2019) and, when injected into the CSF, can inhibit sugar and alcohol appetite (von Holstein-Rathlou et al., 2016) via activation of the paraventricular nucleus and regulation of the hypothalamic–pituitary–thyroid axis (Yilmaz et al., 2018).
Vagal nerve stimulation, GLP-1 signaling, and CSF-derived FGF21 regulation of the hypothalamic–pituitary–thyroid access provide additional putative links among gut, brain, and glymphatic function extending beyond traditional cues such as insulin, leptin, and glucagon signaling.
When discussing the gut–brain axis, it would be impossible not to mention the microbiome, which is the host of bacteria present within the digestive tract that is essential not only for digestion, but can also impact neuroendocrine and inflammatory responses in the gut. The microbiome has been implicated in almost every disease of the nervous system, although the mechanisms of action are relatively unclear (Martin et al., 2018). There is some evidence that APOE isoform influences microbiota composition (Parikh et al., 2020). The microbiota can regulate factors such as GLP-1, thus ultimately changing vagus nerve activity (Everard and Cani, 2014; Martin et al., 2018). Finally, microbiota composition can somehow alter tight junctions at the BBB (Braniste et al., 2014), potentially dysregulating the composition of CSF/ISF that the choroid plexus normally maintains. Presently available evidence makes it tempting to suggest a link between the microbiome and glymphatic function, though we cannot yet propose a pathway other than via vagal retrograde signaling or BBB–glymphatic interactions.
Immune surveillance between the brain and the CNS
A primary feature of the traditional lymphatic system is to monitor the state of tissue inflammation. It is thus no surprise that immune surveillance is at play at every point of the pathway for CSF production and movement (Fig. 4). The choroid plexus is host to numerous leukocyte immune cells residing in the space between the leaky vessels and the ependymal cells, which provide continuous immune surveillance and enable macrophage invasion to the CSF after brain tissue damage (Shechter et al., 2013; Schwartz and Baruch, 2014). Perivascular macrophages associated with large brain vessels clear cellular debris and monitor CSF (Faraco et al., 2017). In the interstitial space, microglia and astrocytes clear debris locally, and can secrete chemokines and cytokines to the ISF/CSF (Pranzatelli, 2018; Afridi et al., 2020). Ultimately, CSF drains to the cervical lymphatic vessels and nodes (Kida et al., 1993; Louveau et al., 2018; Da Mesquita et al., 2018a; Ahn et al., 2019), which modulate the recruitment of immune cells from the blood (Breslin et al., 2018). In addition to blood-derived immune cells, the meninges contain a pool of myeloid immune cells from the bone marrow in the skull and vertebra (Cugurra et al., 2021), and this new discovery may alter our understanding of immune cell recruitment to perivascular spaces during CNS repair.
Though immune cross talk between the brain and periphery is anatomically possible, it remains completely unexplored whether/how glymphatic function may change systemic immune responses. Immune challenges, such as acute lipopolysaccharide injections, impairs glymphatic function (Manouchehrian et al., 2021). There is evidence that CSF distribution between the lymph nodes and brain is dependent on AQP4 and is controlled by circadian timing (Hablitz et al., 2020). Perhaps such a pathway for increasing CSF delivery directly to the lymph nodes is the key to mobilization of immune responses during the day (Haspel et al., 2020). Additionally, we note that AQP4 knock-out mice have reduced neuroimmune pathology in models of meningitis and experimental autoimmune encephalomyelitis (Papadopoulos and Verkman, 2005; Li et al., 2009). Glymphatic–lymphatic cross talk may also occur in the dural sinuses, where brain-derived antigens accumulate in the dural sinuses, are captured by antigen precursor cells, and trigger a local immune response (Rustenhoven et al., 2021). Recent work in meningeal lymphatic biology has demonstrated that γδ17 T cells can regulate anxiety-like behavior, while CD4+ T-cell signaling alters learning (Radjavi et al., 2014; Alves de Lima et al., 2020), suggesting that cross talk among the lymphatic system, the glymphatic system, and the brain as a whole may do more than simply protect against disease, but may also regulate fundamental animal behaviors.
Closing remarks
We have shown how the glymphatic system is the lymphatic analog of the brain, with a central role in regulating directional interstitial fluid movement, waste clearance, and potentially brain immunity. Astrocytes and blood vessels determine the shape of the PVS, ultimately controlling the movement of perivascular fluid. Glymphatic fluid movement has the potential to alter local (within a brain region) as well as global (brain-wide) transport of signaling molecules as well as metabolites known to be implicated in homeostasis and specific behaviors. There is a potential for cross talk among the glymphatic system, cardiovascular system, gastrointestinal tract, and the lymphatic system. Much remains to be studied, but we are of the opinion that the glymphatic/lymphatic system acts as a cornerstone in the architecture of the brain and body signaling.
To harness therapeutically the glymphatic system to reduce disease burden, we must first better understand the full range of biological functions of CSF flow. Specifically, future work should focus on how to manipulate the availability of CSF to supply the glymphatic system, and to elucidate the underlying physiological mechanisms that redistribute CSF between the brain and body.
Footnotes
This research was supported by Department of Health and Human Services/National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants R01-NS-100366 (to M.N.) and RF1-AG-057575 (to M.N.); US Department of Defense, US Army, US Army Research, Development and Engineering Command, Army Research Office Grant MURI W911NF1910280 (to M.N.); Fondation Leducq Transatlantic Networks of Excellence Program; Novo Nordisk Foundation; Lundbeck Foundation; and the EU Horizon 2020 Research and Innovation Program Grant 666881 (to M.N.). We thank Dan Xue for graphic illustrations.
The authors declare no competing financial interests.
References
- Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG (2018) The role of brain barriers in fluid movement in the CNS: is there a “glymphatic” system? Acta Neuropathol 135:387–407. 10.1007/s00401-018-1812-4 [DOI] [PubMed] [Google Scholar]
- Abramova O, Zorkina Y, Ushakova V, Zubkov E, Morozova A, Chekhonin V (2020) The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 83:102079. 10.1016/j.npep.2020.102079 [DOI] [PubMed] [Google Scholar]
- Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, Holtzman DM, Nedergaard M, Deane R (2016) Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener 11:74. 10.1186/s13024-016-0138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afridi R, Lee WH, Suk K (2020) Microglia gone awry: linking immunometabolism to neurodegeneration. Front Cell Neurosci 14:246. 10.3389/fncel.2020.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F (1986) A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand 128:201–207. 10.1111/j.1748-1716.1986.tb07967.x [DOI] [PubMed] [Google Scholar]
- Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, Jeong Y, Park SH, Koh GY (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572:62–66. 10.1038/s41586-019-1419-5 [DOI] [PubMed] [Google Scholar]
- Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, Kipnis J (2020) Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol 21:1421–1429. 10.1038/s41590-020-0776-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen M, Hadi A, Petersen LG, Juhler M (2015) Effect of postural changes on ICP in healthy and ill subjects. Acta Neurochir (Wien) 157:109–113. 10.1007/s00701-014-2250-2 [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212:991–999. 10.1084/jem.20142290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED (2005) Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8:476–483. 10.1038/nn1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulinas A (2000) Physiology of the pineal gland and melatonin. South Dartmouth, MA: MDText.com. [Google Scholar]
- Banks WA, Farr SA, Salameh TS, Niehoff ML, Rhea EM, Morley JE, Hanson AJ, Hansen KM, Craft S (2018) Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int J Obes (Lond) 42:391–397. 10.1038/ijo.2017.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, de Vivo L, Tononi G, Cirelli C (2015) Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol 13:66. 10.1186/s12915-015-0176-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloy ME, Napolioni V, Greicius MD (2019) A quarter century of APOE and Alzheimer's disease: progress to date and the path forward. Neuron 101:820–838. 10.1016/j.neuron.2019.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2016) Choroid plexus–CSF system: recent developments and clinical correlations. Neurology 86:286–296. 10.1212/WNL.0000000000002298 [DOI] [PubMed] [Google Scholar]
- Benias PC, Wells RG, Sackey-Aboagye B, Klavan H, Reidy J, Buonocore D, Miranda M, Kornacki S, Wayne M, Carr-Locke DL, Theise ND (2018) Structure and distribution of an unrecognized interstitium in human tissues. Sci Rep 8:4947. 10.1038/s41598-018-23062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, Lu H (2017) Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology 127:976–988. 10.1097/ALN.0000000000001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi M, Roncali L, Nico B, Ribatti D, Mancini L, Virgintino D, Fabiani G, Guidazzoli A (1993) Perivascular astrocytes and endothelium in the development of the blood-brain barrier in the optic tectum of the chick embryo. Anat Embryol (Berl) 188:21–29. 10.1007/BF00191448 [DOI] [PubMed] [Google Scholar]
- Botfield HF, Uldall MS, Westgate CSJ, Mitchell JL, Hagen SM, Gonzalez AM, Hodson DJ, Jensen RH, Sinclair AJ (2017) A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci Transl Med 9:eaan0972. 10.1126/scitranslmed.aan0972 [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Guan NL, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158. 10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW (2014) Mechanical forces and lymphatic transport. Microvasc Res 96:46–54. 10.1016/j.mvr.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL (2018) Lymphatic vessel network structure and physiology. Compr Physiol 9:207–299. 10.1002/cphy.c180015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley JB (1950) The penetration of particulate matter from the cerebrospinal fluid into the spinal ganglia, peripheral nerves, and perivascular spaces of the central nervous system. J Neurol Neurosurg Psychiatry 13:203–215. 10.1136/jnnp.13.3.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Tamarkin L, Riedel C, Markey S, Oldfield E (1991) Sequential cerebrospinal fluid and plasma sampling in humans: 24-hour melatonin measurements in normal subjects and after peripheral sympathectomy. J Clin Endocrinol Metab 72:819–823. 10.1210/jcem-72-4-819 [DOI] [PubMed] [Google Scholar]
- Burbach JP (1982) Neuropeptides and cerebrospinal fluid. Ann Clin Biochem 19:269–277. 10.1177/000456328201900416 [DOI] [PubMed] [Google Scholar]
- Cai X, Qiao J, Kulkarni P, Harding IC, Ebong E, Ferris CF (2020) Imaging the effect of the circadian light-dark cycle on the glymphatic system in awake rats. Proc Natl Acad Sci U S A 117:668–676. 10.1073/pnas.1914017117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Xu H, Feng W, Su D, Xiao M (2018) Deletion of aquaporin-4 aggravates brain pathology after blocking of the meningeal lymphatic drainage. Brain Res Bull 143:83–96. 10.1016/j.brainresbull.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Cheng KP, Brodnick SK, Blanz SL, Zeng W, Kegel J, Pisaniello JA, Ness JP, Ross E, Nicolai EN, Settell ML, Trevathan JK, Poore SO, Suminski AJ, Williams JC, Ludwig KA (2020) Clinically-derived vagus nerve stimulation enhances cerebrospinal fluid penetrance. Brain Stimul 13:1024–1030. 10.1016/j.brs.2020.03.012 [DOI] [PubMed] [Google Scholar]
- Cserr HF, Cooper DN, Suri PK, Patlak CS (1981) Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol 240:F319–F328. 10.1152/ajprenal.1981.240.4.F319 [DOI] [PubMed] [Google Scholar]
- Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, Baker W, Papadopoulos Z, Drieu A, Blackburn S, Kanamori M, Brioschi S, Herz J, Schuettpelz LG, Colonna M, Smirnov I, Kipnis J (2021) Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 373:eabf7844. 10.1126/science.abf7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Fu Z, Kipnis J (2018a) The meningeal lymphatic system: a new player in neurophysiology. Neuron 100:375–388. 10.1016/j.neuron.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, et al. (2018b) Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature 560:185–191. 10.1038/s41586-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7:a020412. 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, O'Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M (2016) Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352:550–555. 10.1126/science.aad4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ (2009) The versatility of the vagus. Physiol Behav 97:531–536. 10.1016/j.physbeh.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Risold PY (2007) The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev 56:119–147. 10.1016/j.brainresrev.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Eide PK, Ringstad G (2019) In vivo imaging of molecular clearance from human entorhinal cortex: a possible method for preclinical testing of dementia. Gerontol Geriatr Med 5:2333721419889739. 10.1177/2333721419889739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterházy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, Mucida D (2019) Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569:126–130. 10.1038/s41586-019-1125-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, Cani PD (2014) Gut microbiota and GLP-1. Rev Endocr Metab Disord 15:189–196. 10.1007/s11154-014-9288-6 [DOI] [PubMed] [Google Scholar]
- Faraco G, Park L, Anrather J, Iadecola C (2017) Brain perivascular macrophages: characterization and functional roles in health and disease. J Mol Med (Berl) 95:1143–1152. 10.1007/s00109-017-1573-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley LM, Hitchens TK, Kochanek PM, Melick JA, Jackson EK, Ho C (2005) Murine orthostatic response during prolonged vertical studies: effect on cerebral blood flow measured by arterial spin-labeled MRI. Magn Reson Med 54:798–806. 10.1002/mrm.20621 [DOI] [PubMed] [Google Scholar]
- Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD (2019) Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366:628–631. 10.1126/science.aax5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakuba C, Gaberel T, Goursaud S, Bourges J, Di Palma C, Quenault A, de Lizarrondo SM, Vivien D, Gauberti M (2018) General anesthesia inhibits the activity of the “glymphatic system". Theranostics 8:710–722. 10.7150/thno.19154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Coon SL, Klein DC (2002) Control of melatonin synthesis in the mammalian pineal gland: the critical role of serotonin acetylation. Cell Tissue Res 309:127–137. 10.1007/s00441-002-0579-y [DOI] [PubMed] [Google Scholar]
- Gatti L, Tinelli F, Scelzo E, Arioli F, Di Fede G, Obici L, Pantoni L, Giaccone G, Caroppo P, Parati EA, Bersano A (2020) Understanding the pathophysiology of cerebral amyloid angiopathy. Int J Mol Sci 21:3435. 10.3390/ijms21103435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M, Giorgi A, Gentile MC, d'Erme M, Morano S, Maras B, Filardi T (2019) Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front Neurosci 13:1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüntzig J, Hollmann F (2019) Lymphatic vessels of the eye - old questions - new insights. Ann Anat 221:1–16. 10.1016/j.aanat.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M (2019) Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 5:eaav5447. 10.1126/sciadv.aav5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M (2020) Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun 11:4411. 10.1038/s41467-020-18115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, Park JW, Bankiewicz K (2006) The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther 14:69–78. 10.1016/j.ymthe.2006.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AJ, Banks WA, Bettcher LF, Pepin R, Raftery D, Craft S (2019) Cerebrospinal fluid lipidomics: effects of an intravenous triglyceride infusion and apoE status. Metabolomics 16:6. 10.1007/s11306-019-1627-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, Ahmed Z, Fisher A, Meftah S, Murray TK, Ottersen OP, Nagelhus EA, O'Neill MJ, Wells JA, Lythgoe MF (2020) Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain 143:2576–2593. 10.1093/brain/awaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel JA, Anafi R, Brown MK, Cermakian N, Depner C, Desplats P, Gelman AE, Haack M, Jelic S, Kim BS, Laposky AD, Lee YC, Mongodin E, Prather AA, Prendergast BJ, Reardon C, Shaw AC, Sengupta S, Szentirmai É, Thakkar M (2020) Perfect timing: circadian rhythms, sleep, and immunity—an NIH workshop summary. JCI Insight 5:e131487. 10.1172/jci.insight.131487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI (1997) Function-related plasticity in hypothalamus. Annu Rev Neurosci 20:375–397. 10.1146/annurev.neuro.20.1.375 [DOI] [PubMed] [Google Scholar]
- Hawrylak N, Fleming JC, Salm AK (1998) Dehydration and rehydration selectively and reversibly alter glial fibrillary acidic protein immunoreactivity in the rat supraoptic nucleus and subjacent glial limitans. Glia 22:260–271. [DOI] [PubMed] [Google Scholar]
- Hedlund L, Lischko MM, Rollag MD, Niswender GD (1977) Melatonin: daily cycle in plasma and cerebrospinal fluid of calves. Science 195:686–687. 10.1126/science.841305 [DOI] [PubMed] [Google Scholar]
- Heimer-McGinn V, Murphy AC, Kim JC, Dymecki SM, Young PW (2013) Decreased dendritic spine density as a consequence of tetanus toxin light chain expression in single neurons in vivo. Neurosci Lett 555:36–41. 10.1016/j.neulet.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning RJ, Sawmiller DR (2001) Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res 49:27–37. 10.1016/s0008-6363(00)00229-7 [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai LH (2016) Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540:230–235. 10.1038/nature20587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376. 10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Ikeshima-Kataoka H (2016) Neuroimmunological implications of AQP4 in astrocytes. Int J Mol Sci 17:1306. 10.3390/ijms17081306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H (2013a) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123:1299–1309. 10.1172/JCI67677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M (2013b) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33:18190–18199. 10.1523/JNEUROSCI.1592-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illnerova H, Backström M, Sääf J, Wetterberg L, Vangbo B (1978) Melatonin in rat pineal gland and serum; rapid parallel decline after light exposure at night. Neurosci Lett 9:189–193. 10.1016/0304-3940(78)90070-8 [DOI] [PubMed] [Google Scholar]
- Iyer R, Wang TA, Gillette MU (2014) Circadian gating of neuronal functionality: a basis for iterative metaplasticity. Front Syst Neurosci 8:164. 10.3389/fnsys.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari Z, Kolb BE, Mohajerani MH (2020) Neural oscillations and brain stimulation in Alzheimer's disease. Prog Neurobiol 194:101878. 10.1016/j.pneurobio.2020.101878 [DOI] [PubMed] [Google Scholar]
- Kent BA, Mistlberger RE (2017) Sleep and hippocampal neurogenesis: implications for Alzheimer's disease. Front Neuroendocrinol 45:35–52. 10.1016/j.yfrne.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Kida S, Pantazis A, Weller RO (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19:480–488. 10.1111/j.1365-2990.1993.tb00476.x [DOI] [PubMed] [Google Scholar]
- Kiecker C (2018) The origins of the circumventricular organs. J Anat 232:540–553. 10.1111/joa.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JD, Leyva S, Diano S (2014) Hormonal regulation of the hypothalamic melanocortin system. Front Physiol 5:480. 10.3389/fphys.2014.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Kung D, Watson RE, Reiss FL, Biddlecome SM, Bourke RS (1978) Direct administration of methotrexate into the central nervous system of primates. Part 1: distribution and degradation of methotrexate in nervous and systemic tissue after intraventricular injection. J Neurosurg 48:883–894. 10.3171/jns.1978.48.6.0883 [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, Nedergaard M (2016) Ultra-fast magnetic resonance encephalography of physiological brain activity - glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36:1033–1045. 10.1177/0271678X15622047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korogod N, Petersen CC, Knott GW (2015) Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife 4:e05793. 10.7554/eLife.05793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose G, Hatipoglu S (2012) Effect of head and body positioning on cerebral blood flow velocity in patients who underwent cranial surgery. J Clin Nurs 21:1859–1867. 10.1111/j.1365-2702.2012.04134.x [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Yousef A, Kaindl S, Lee VM, Trojanowski JQ (2018) Connexin-43 and aquaporin-4 are markers of ageing-related tau astrogliopathy (ARTAG)-related astroglial response. Neuropathol Appl Neurobiol 44:491–505. 10.1111/nan.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861. 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JP, Langhans W, Lee SJ (2015) Vagal mediation of GLP-1's effects on food intake and glycemia. Physiol Behav 152:372–380. 10.1016/j.physbeh.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Lavialle M, Servière J (1993) Circadian fluctuations in GFAP distribution in the Syrian hamster suprachiasmatic nucleus. Neuroreport 4:1243–1246. 10.1097/00001756-199309000-00008 [DOI] [PubMed] [Google Scholar]
- Lavialle M, Begue A, Papillon C, Vilaplana J (2001) Modifications of retinal afferent activity induce changes in astroglial plasticity in the hamster circadian clock. Glia 34:88–100. 10.1002/glia.1044 [DOI] [PubMed] [Google Scholar]
- Lavialle M, Aumann G, Anlauf E, Pröls F, Arpin M, Derouiche A (2011) Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A 108:12915–12919. 10.1073/pnas.1100957108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H (2015) The effect of body posture on brain glymphatic transport. J Neurosci 35:11034–11044. 10.1523/JNEUROSCI.1625-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, Reckelhoff JF, Iadecola C, Coffman TM (2019) Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 73:e87–e120. 10.1161/HYP.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin VA, Fenstermacher JD, Patlak CS (1970) Sucrose and inulin space measurements of cerebral cortex in four mammalian species. Am J Physiol 219:1528–1533. 10.1152/ajplegacy.1970.219.5.1528 [DOI] [PubMed] [Google Scholar]
- Lewis B (1877) The relationships of the nerve-cells of the cortex to the lymphatic system of the brain. Proc R Soc 16:355–356. [Google Scholar]
- Li L, Zhang H, Verkman AS (2009) Greatly attenuated experimental autoimmune encephalomyelitis in aquaporin-4 knockout mice. BMC Neurosci 10:94. 10.1186/1471-2202-10-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius TO, Blomqvist K, Hauglund NL, Liu G, Stæger FF, Bærentzen S, Du T, Ahlström F, Backman JT, Kalso EA, Rauhala PV, Nedergaard M (2019) Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Control Release 304:29–38. 10.1016/j.jconrel.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Liu AK, Chang RC, Pearce RK, Gentleman SM (2015) Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer's and Parkinson's disease. Acta Neuropathol 129:527–540. 10.1007/s00401-015-1392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Mestre H, Sweeney AM, Sun Q, Weikop P, Du T, Nedergaard M (2020a) Direct measurement of cerebrospinal fluid production in mice. Cell Rep 33:108524. 10.1016/j.celrep.2020.108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hao J, Yao E, Cao J, Zheng X, Yao D, Zhang C, Li J, Pan D, Luo X, Wang M, Wang W (2020b) Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav Immun 89:357–370. 10.1016/j.bbi.2020.07.022 [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, et al. (2018) CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21:1380–1391. 10.1038/s41593-018-0227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7:126–136. 10.1038/nrn1845 [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, Nedergaard M (2017) Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 37:2112–2124. 10.1177/0271678X16661202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Wang W, Eberhardt A, Vinitsky HS, Reeves BC, Peng S, Lou N, Hussain R, Nedergaard M (2018) Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep 8:2246. 10.1038/s41598-018-20424-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Ries M, Decker Y, Müller A, Riner C, Bücker A, Fassbender K, Detmar M, Proulx ST (2019) Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol 137:151–165. 10.1007/s00401-018-1916-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manouchehrian O, Ramos M, Bachiller S, Lundgaard I, Deierborg T (2021) Acute systemic LPS-exposure impairs perivascular CSF distribution in mice. J Neuroinflammation 18:34. 10.1186/s12974-021-02082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CR, Osadchiy V, Kalani A, Mayer EA (2018) The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 6:133–148. 10.1016/j.jcmgh.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell AJ, Paulson AL, Suk HJ, Abdurrob F, Drummond GT, Guan W, Young JZ, Kim DN, Kritskiy O, Barker SJ, Mangena V, Prince SM, Brown EN, Chung K, Boyden ES, Singer AC, Tsai LH (2019) Multi-sensory gamma stimulation ameliorates Alzheimer's-associated pathology and improves cognition. Cell 177:256–271.e22. 10.1016/j.cell.2019.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103. 10.1002/glia.20990 [DOI] [PubMed] [Google Scholar]
- McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM (2011) In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab 31:795–806. 10.1038/jcbfm.2010.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Kostrikov S, Mehta RI, Nedergaard M (2017) Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 131:2257–2274. 10.1042/CS20160381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH (2018a) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9:4878. 10.1038/s41467-018-07318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Plá V, Du T, Kress BT, Wang X, Plog BA, Thrane AS, Lundgaard I, Abe Y, Yasui M, et al. (2018b) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7:e40070. 10.7554/eLife.40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Mori Y, Nedergaard M (2020a) The brain's glymphatic system: current controversies. Trends Neurosci 43:458–466. 10.1016/j.tins.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, Mortensen KN, Stæger FF, Bork PAR, Bashford L, Toro ER, Tithof J, Kelley DH, Thomas JH, Hjorth PG, Martens EA, Mehta RI, Solis O, Blinder P, Kleinfeld D, et al. (2020b) Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367:eaax7171. 10.1126/science.aax7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011) Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12:524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Mieda M (2019) The network mechanism of the central circadian pacemaker of the SCN: do AVP neurons play a more critical role than expected? Front Neurosci 13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA (2010) Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res 106:920–931. 10.1161/CIRCRESAHA.109.207274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Takahashi JS (2011) Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci 34:349–358. 10.1016/j.tins.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller M (1974) The ultrastructure of the human fetal pineal gland. I. Cell types and blood vessels. Cell Tissue Res 152:13–30. 10.1007/BF00224208 [DOI] [PubMed] [Google Scholar]
- Monai H, Wang X, Yahagi K, Lou N, Mestre H, Xu Q, Abe Y, Yasui M, Iwai Y, Nedergaard M, Hirase H (2019) Adrenergic receptor antagonism induces neuroprotection and facilitates recovery from acute ischemic stroke. Proc Natl Acad Sci U S A 116:11010–11019. 10.1073/pnas.1817347116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Shivers KY, Blanchard JH, Muscat L (2006) Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience 137:1285–1297. 10.1016/j.neuroscience.2005.10.030 [DOI] [PubMed] [Google Scholar]
- Munk AS, Wang W, Bèchet NB, Eltanahy AM, Cheng AX, Sigurdsson B, Benraiss A, Mäe MA, Kress BT, Kelley DH, Betsholtz C, Møllgård K, Meissner A, Nedergaard M, Lundgaard I (2019) PDGF-B is required for development of the glymphatic system. Cell Rep 26:2955–2969.e3. 10.1016/j.celrep.2019.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto V, Jaspar M, Meyer C, Kussé C, Chellappa SL, Degueldre C, Balteau E, Shaffii-Le Bourdiec A, Luxen A, Middleton B, Archer SN, Phillips C, Collette F, Vandewalle G, Dijk DJ, Maquet P (2016) Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science 353:687–690. 10.1126/science.aad2993 [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA (2020) Glymphatic failure as a final common pathway to dementia. Science 370:50–56. 10.1126/science.abb8739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Lindvall-Axelsson M, Owman C (1992) Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Res Brain Res Rev 17:109–138. 10.1016/0165-0173(92)90011-a [DOI] [PubMed] [Google Scholar]
- Noble EE, Hahn JD, Konanur VR, Hsu TM, Page SJ, Cortella AM, Liu CM, Song MY, Suarez AN, Szujewski CC, Rider D, Clarke JE, Darvas M, Appleyard SM, Kanoski SE (2018) Control of feeding behavior by cerebral ventricular volume transmission of melanin-concentrating hormone. Cell Metab 28:55–68.e7. 10.1016/j.cmet.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287. 10.1523/JNEUROSCI.4707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obersteiner H (1890) The anatomy of the central nervous organs in health and in disease. London: Charles Griffin. [Google Scholar]
- Ohene Y, Harrison IF, Nahavandi P, Ismail O, Bird EV, Ottersen OP, Nagelhus EA, Thomas DL, Lythgoe MF, Wells JA (2019) Non-invasive MRI of brain clearance pathways using multiple echo time arterial spin labelling: an aquaporin-4 study. Neuroimage 188:515–523. 10.1016/j.neuroimage.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orešković D, Klarica M (2014) Measurement of cerebrospinal fluid formation and absorption by ventriculo-cisternal perfusion: what is really measured? Croat Med J 55:317–327. 10.3325/cmj.2014.55.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orešković D, Klarica M, Vukic M, Marakovic J (2003) Evaluation of ventriculo-cisternal perfusion model as a method to study cerebrospinal fluid formation. Croat Med J 44:161–164. [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM (2011) Age-related changes in the cognitive function of sleep. Prog Brain Res 191:75–89. 10.1016/B978-0-444-53752-2.00012-6 [DOI] [PubMed] [Google Scholar]
- Page-Wilson G, Nguyen KT, Atalayer D, Meece K, Bainbridge HA, Korner J, Gordon RJ, Panigrahi SK, White A, Smiley R, Wardlaw SL (2017) Evaluation of CSF and plasma biomarkers of brain melanocortin activity in response to caloric restriction in humans. Am J Physiol Endocrinol Metab 312:E19–e26. 10.1152/ajpendo.00330.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M (1984) Neuropeptides in the hypothalamo-hypophyseal system: lateral retrochiasmatic area as a common gate for neuronal fibers towards the median eminence. Peptides 5 [Suppl 1]:35–39. 10.1016/0196-9781(84)90262-6 [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: nature's most versatile biological signal? FEBS J 273:2813–2838. 10.1111/j.1742-4658.2006.05322.x [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS (2005) Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem 280:13906–13912. 10.1074/jbc.M413627200 [DOI] [PubMed] [Google Scholar]
- Parikh IJ, Estus JL, Zajac DJ, Malik M, Maldonado Weng J, Tai LM, Chlipala GE, LaDu MJ, Green SJ, Estus S (2020) Murine gut microbiome association with APOE alleles. Front Immunol 11:200. 10.3389/fimmu.2020.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, Jessu R, Tiwari V (2021) Physiology, posterior pituitary. In: StatPearls. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Pelletier JS, Dicken B, Bigam D, Cheung PY (2014) Cardiac effects of vasopressin. J Cardiovasc Pharmacol 64:100–107. 10.1097/FJC.0000000000000092 [DOI] [PubMed] [Google Scholar]
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R (2016) Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol Dis 93:215–225. 10.1016/j.nbd.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Koh GY (2018) Organ-specific lymphatic vasculature: from development to pathophysiology. J Exp Med 215:35–49. 10.1084/jem.20171868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Koh GY (2020) Biological functions of lymphatic vessels. Science 369: 10.1126/science.aax4063 [DOI] [PubMed] [Google Scholar]
- Plog BA, Lou N, Pierre CA, Cove A, Kenney HM, Hitomi E, Kang H, Iliff JJ, Zeppenfeld DM, Nedergaard M, Vates GE (2019) When the air hits your brain: decreased arterial pulsatility after craniectomy leading to impaired glymphatic flow. J Neurosurg. Advance online publication. Retrieved August 5, 2021. 10.3171/2019.2.JNS182675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli MR (2018) Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: targeting chemokines/cytokines. Front Immunol 9:557. 10.3389/fimmu.2018.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Li J, Tang K (2018) The paraventricular nucleus of the hypothalamus: development, function, and human diseases. Endocrinology 159:3458–3472. 10.1210/en.2018-00453 [DOI] [PubMed] [Google Scholar]
- Radjavi A, Smirnov I, Kipnis J (2014) Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav Immun 35:58–63. 10.1016/j.bbi.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangroo Thrane V, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, Deane R, Nagelhus EA, Nedergaard M (2013) Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 3:2582. 10.1038/srep02582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper D, Louveau A, Kipnis J (2016) How do meningeal lymphatic vessels drain the CNS? Trends Neurosci 39:581–586. 10.1016/j.tins.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17:1016–1024. 10.1016/S1474-4422(18)30318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen R, O'Donnell J, Ding F, Nedergaard M (2020) Interstitial ions: a key regulator of state-dependent neural activity? Prog Neurobiol 193:101802. 10.1016/j.pneurobio.2020.101802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MK, Mestre H, Nedergaard M (2021) Fluid transport in the brain. Physiol Rev 10.1152/physrev.00031.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawindraraj AD, Basit H, Jialal I (2021) Physiology, anterior pituitary. In: StatPearls. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J (2005) The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol 71:1–52. 10.1016/S0070-2153(05)71001-2 [DOI] [PubMed] [Google Scholar]
- Ren H, Luo C, Feng Y, Yao X, Shi Z, Liang F, Kang JX, Wan JB, Pei Z, Su H (2017) Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J 31:282–293. 10.1096/fj.201600896 [DOI] [PubMed] [Google Scholar]
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA (1985) Evidence for a “paravascular” fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326:47–63. 10.1016/0006-8993(85)91383-6 [DOI] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA (2009) Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia 57:207–221. 10.1002/glia.20747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez EM, Blázquez JL, Guerra M (2010) The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31:757–776. 10.1016/j.peptides.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Rohr KE, Telega A, Savaglio A, Evans JA (2020) Vasopressin regulates daily rhythms and circadian clock circuits in a manner influenced by sex. Horm Behav 127:104888. 10.1016/j.yhbeh.2020.104888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosic B, Dukefoss DB, Åbjørsbråten KS, Tang W, Jensen V, Ottersen OP, Enger R, Nagelhus EA (2019) Aquaporin-4-independent volume dynamics of astroglial endfeet during cortical spreading depression. Glia 67:1113–1121. 10.1002/glia.23604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, Papadopoulos Z, Kanamori M, Salvador AF, Baker W, Lemieux M, Da Mesquita S, Cugurra A, Fitzpatrick J, Sviben S, Kossina R, Bayguinov P, Townsend RR, Zhang Q, Erdmann-Gilmore P, et al. (2021) Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184:1000–1016.e27. 10.1016/j.cell.2020.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Lu J, Chou TC, Gooley J (2005) The hypothalamic integrator for circadian rhythms. Trends Neurosci 28:152–157. 10.1016/j.tins.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Sasaki H, Mannen H (1981) Morphological analysis of astrocytes in the bullfrog (Rana catesbeiana) spinal cord with special reference to the site of attachment of their processes. J Comp Neurol 198:13–35. 10.1002/cne.901980104 [DOI] [PubMed] [Google Scholar]
- Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ (2016) Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 594:5749–5768. 10.1113/JP272088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R (2017) Cortical spreading depression closes paravascular space and impairs glymphatic flow: implications for migraine headache. J Neurosci 37:2904–2915. 10.1523/JNEUROSCI.3390-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein GW (1990) Microlymphatics and lymph flow. Physiol Rev 70:987–1028. 10.1152/physrev.1990.70.4.987 [DOI] [PubMed] [Google Scholar]
- Schwartz M, Baruch K (2014) The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J 33:7–22. 10.1002/embj.201386609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M (2013) Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38:555–569. 10.1016/j.immuni.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa AD, Xiao F, Joseph N, Aoki C, Hrabetova S (2016) Activation of β-adrenergic receptors in rat visual cortex expands astrocytic processes and reduces extracellular space volume. Synapse 70:307–316. 10.1002/syn.21908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Patel S, Khakh BS (2016) Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol 26:300–312. 10.1016/j.tcb.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, LeSauter J (1993) Efferent signals of the suprachiasmatic nucleus. J Biol Rhythms 8 [Suppl]:S89–S92. [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN (1996) A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382:810–813. 10.1038/382810a0 [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M (2003) Signaling at the gliovascular interface. J Neurosci 23:9254–9262. 10.1523/JNEUROSCI.23-27-09254.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Fisher TM, Waterhouse BD, Lin RC (1998) Projection patterns from the raphe nuclear complex to the ependymal wall of the ventricular system in the rat. J Comp Neurol 399:61–72. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Malpaux B (1999) High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology 140:4399–4405. 10.1210/endo.140.10.7074 [DOI] [PubMed] [Google Scholar]
- Smarr BL, Jennings KJ, Driscoll JR, Kriegsfeld LJ (2014) A time to remember: the role of circadian clocks in learning and memory. Behav Neurosci 128:283–303. 10.1037/a0035963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE (2018) Cerebral amyloid angiopathy as a cause of neurodegeneration. J Neurochem 144:651–658. 10.1111/jnc.14157 [DOI] [PubMed] [Google Scholar]
- Spencer RL, Deak T (2017) A users guide to HPA axis research. Physiol Behav 178:43–65. 10.1016/j.physbeh.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM (2012) Mechanisms of sympathetic regulation in orthostatic intolerance. J Appl Physiol (1985) 113:1659–1668. 10.1152/japplphysiol.00266.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, Yuan H, Colvin RA, Yang XY (2018) Lymphatic drainage system of the brain: a novel target for intervention of neurological diseases. Prog Neurobiol 163-164:118–143. 10.1016/j.pneurobio.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Szentistványi I, Patlak CS, Ellis RA, Cserr HF (1984) Drainage of interstitial fluid from different regions of rat brain. Am J Physiol 246:F835–844. 10.1152/ajprenal.1984.246.6.F835 [DOI] [PubMed] [Google Scholar]
- Taber KH, Hurley RA (2014) Volume transmission in the brain: beyond the synapse. J Neuropsychiatry Clin Neurosci 26:iv, 1–4. 10.1176/appi.neuropsych.13110351 [DOI] [PubMed] [Google Scholar]
- Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR, Nedergaard M (2005) Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A 102:16466–16471. 10.1073/pnas.0506382102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka T, Jost G, Frenzel T, Naganawa S, Pietsch H (2018) Impact of the glymphatic system on the kinetic and distribution of gadodiamide in the rat brain: observations by dynamic MRI and effect of circadian rhythm on tissue gadolinium concentrations. Invest Radiol 53:529–534. 10.1097/RLI.0000000000000473 [DOI] [PubMed] [Google Scholar]
- Taub A, Carbajal Y, Rimu K, Holt R, Yao Y, Hernandez AL, LeSauter J, Silver R (2021) Arginine Vasopressin-Containing Neurons of the Suprachiasmatic Nucleus Project to CSF. eNeuro 8:ENEURO.0363-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT (2002) Oxytocin-secreting neurons: a physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol 23:101–135. 10.1006/frne.2001.0226 [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH (2008) Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88:983–1008. 10.1152/physrev.00036.2007 [DOI] [PubMed] [Google Scholar]
- Thomas JH (2019) Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J R Soc Interface 16:20190572. 10.1098/rsif.2019.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR (2007) Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol 66:337–345. [DOI] [PubMed] [Google Scholar]
- Tithof J, Kelley DH, Mestre H, Nedergaard M, Thomas JH (2019) Hydraulic resistance of periarterial spaces in the brain. Fluids Barriers CNS 16:19. 10.1186/s12987-019-0140-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd WD, Venner A, Anaclet C, Broadhurst RY, De Luca R, Bandaru SS, Issokson L, Hablitz LM, Cravetchi O, Arrigoni E, Campbell JN, Allen CN, Olson DP, Fuller PM (2020) Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat Commun 11:4410. 10.1038/s41467-020-17197-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Shigetomi E, Looger LL, Khakh BS (2013) Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist 19:274–291. 10.1177/1073858412468794 [DOI] [PubMed] [Google Scholar]
- Troili F, Cipollini V, Moci M, Morena E, Palotai M, Rinaldi V, Romano C, Ristori G, Giubilei F, Salvetti M, Orzi F, Guttmann CRG, Cavallari M (2020) Perivascular unit: this must be the place. The anatomical crossroad between the immune, vascular and nervous system. Front Neuroanat 14:17. 10.3389/fnana.2020.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulv Larsen SM, Landolt HP, Berger W, Nedergaard M, Knudsen GM, Holst SC (2020) Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol 18:e3000623. 10.1371/journal.pbio.3000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP, Greenberg SM, Bacskai BJ (2020) Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105:549–561.e5. 10.1016/j.neuron.2019.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Barendregt HP (2015) The effects of beta-endorphin: state change modification. Fluids Barriers CNS 12:3. 10.1186/2045-8118-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98:239–389. 10.1152/physrev.00042.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Steardo L, Parpura V, Montana V (2016) Translational potential of astrocytes in brain disorders. Prog Neurobiol 144:188–205. 10.1016/j.pneurobio.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES, Fekete A, Karoly R, Mike A (2010) Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br J Pharmacol 160:785–809. 10.1111/j.1476-5381.2009.00624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladić A, Klarica M, Bulat M (2009) Dynamics of distribution of 3H-inulin between the cerebrospinal fluid compartments. Brain Res 1248:127–135. 10.1016/j.brainres.2008.10.044 [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, Gillum MP (2019) Fibroblast growth factor 21: an endocrine inhibitor of sugar and alcohol appetite. J Physiol 597:3539–3548. 10.1113/JP277117 [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, et al. (2016) FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 23:335–343. 10.1016/j.cmet.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite A, Brown SC, Blake DJ (2012) The dystrophin-glycoprotein complex in brain development and disease. Trends Neurosci 35:487–496. 10.1016/j.tins.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Walch E, Murphy TR, Cuvelier N, Aldoghmi M, Morozova C, Donohue J, Young G, Samant A, Garcia S, Alvarez C, Bilas A, Davila D, Binder DK, Fiacco TA (2020) Astrocyte-selective volume increase in elevated extracellular potassium conditions is mediated by the Na(+)/K(+) ATPase and occurs independently of aquaporin 4. ASN Neuro 12:1759091420967152. 10.1177/1759091420967152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MX, Ray L, Tanaka KF, Iliff JJ, Heys J (2021) Varying perivascular astroglial endfoot dimensions along the vascular tree maintain perivascular-interstitial flux through the cortical mantle. Glia 69:715–728. 10.1002/glia.23923 [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Eberhardt A, Yang Y, Kusk P, Xu Q, Förstera B, Peng S, Shi M, Ladrón-de-Guevara A, Delle C, Sigurdsson B, Xavier ALR, Ertürk A, Libby RT, Chen L, Thrane AS, Nedergaard M (2020) An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med 12:eaaw3210. 10.1126/scitranslmed.aaw3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Parpura V (2016) Central role of maladapted astrocytic plasticity in ischemic brain edema formation. Front Cell Neurosci 10:129. 10.3389/fncel.2016.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, Tannenbaum A, Ballerini L, Rungta RL, Boido D, Sweeney M, Montagne A, Charpak S, Joutel A, Smith KJ, Black SE (2020) Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 16:137–153. 10.1038/s41582-020-0312-z [DOI] [PubMed] [Google Scholar]
- Wei F, Song J, Zhang C, Lin J, Xue R, Shan LD, Gong S, Zhang GX, Qin ZH, Xu GY, Wang LH (2019) Chronic stress impairs the aquaporin-4-mediated glymphatic transport through glucocorticoid signaling. Psychopharmacology (Berl) 236:1367–1384. 10.1007/s00213-018-5147-6 [DOI] [PubMed] [Google Scholar]
- Wen S, Ma D, Zhao M, Xie L, Wu Q, Gou L, Zhu C, Fan Y, Wang H, Yan J (2020) Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat Neurosci 23:456–467. 10.1038/s41593-020-0586-x [DOI] [PubMed] [Google Scholar]
- Woollam DHM, Millen JW (1954) Perivascular spaces of the mammalian central nervous system. Biological Reviews 29:251–283. 10.1111/j.1469-185X.1954.tb00597.x [DOI] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 342:373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y (2006) Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 26:4985–4994. 10.1523/JNEUROSCI.5476-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Liu N, Zhang M, Ren X, Tang J, Fu J (2020) Concomitant enlargement of perivascular spaces and decrease in glymphatic transport in an animal model of cerebral small vessel disease. Brain Res Bull 161:78–83. 10.1016/j.brainresbull.2020.04.008 [DOI] [PubMed] [Google Scholar]
- Yilmaz U, Tekin S, Demir M, Cigremis Y, Sandal S (2018) Effects of central FGF21 infusion on the hypothalamus-pituitary-thyroid axis and energy metabolism in rats. J Physiol Sci 68:781–788. 10.1007/s12576-018-0595-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Pu T, Feng W, Lu M, Zheng Y, Du R, Xiao M, Hu G (2019) Blocking meningeal lymphatic drainage aggravates Parkinson's disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener 8:7. 10.1186/s40035-019-0147-y [DOI] [PMC free article] [PubMed] [Google Scholar]