Abstract
Mobile applications and paired devices allow individuals to self-monitor physical activity, dietary intake, and weight fluctuation concurrently. However, little is known regarding patterns of use of these self-monitoring technologies over time and their implications for weight loss. The objectives of this study were to identify distinct patterns of self-monitoring technology use and to investigate the associations between these patterns and weight change. We analyzed data from a 6-month weight loss intervention for school district employees with overweight or obesity (N = 225). We performed repeated measures latent profile analysis (RMLPA) to identify common patterns of self-monitoring technology use and used multiple linear regression to evaluate the relationship between self-monitoring technology use and weight change. RMLPA revealed four distinct profiles: minimal users (n = 65, 29% of sample), activity trackers (n = 124, 55%), dedicated all-around users (n = 25, 11%), and dedicated all-around users with exceptional food logging (n = 11, 5%). The dedicated all-around users with exceptional food logging lost the most weight (X2[1,225] = 5.27, p = .0217). Multiple linear regression revealed that, adjusting for covariates, only percentage of days of wireless weight scale use (B = −0.05, t(212) = −3.79, p < .001) was independently associated with weight loss. We identified distinct patterns in mHealth self-monitoring technology use for tracking weight loss behaviors. Self-monitoring of weight was most consistently linked to weight loss, while exceptional food logging characterized the group with the greatest weight loss. Weight loss interventions should promote self-monitoring of weight and consider encouraging food logging to individuals who have demonstrated consistent use of self-monitoring technologies.
Keywords: mHealth, Physical activity, Diet, Weight loss, Feedback
Lay Summary
Mobile applications and paired devices now enable users to track their physical activity levels, dietary intake, and weight fluctuations all in one user interface. We know that tracking each of these behaviors generally facilitates weight loss, but it is not clear how people with overweight or obesity may tend to use these multiple functions together when trying to lose weight. In a sample of 225 school district employees with overweight or obesity, we investigated whether there were common patterns in tracking these behaviors over time, and whether patterns were associated with weight loss. We identified groups reflecting four common patterns, which we termed the minimal users (n = 65, 29%), activity trackers (n = 124, 55%), dedicated all-around users (n = 25, 11%), and dedicated all-around users with exceptional food logging (n = 11, 5% of sample). The dedicated all-around users with exceptional food logging was the only group that reliably lost weight and was characterized by high tracking of activity, diet, and weight. Overall, regular use of the weight scale was most strongly associated with weight loss. It may be useful to broadly encourage self-monitoring of weight, and selectively encourage food logging to individuals amenable to this self-monitoring technology.
Implications.
Practice: Distinct technology usage patterns for self-monitoring physical activity, dietary intake, and weight scale use were observed in groups of individuals with overweight or obesity participating in a worksite weight loss program, and these patterns have implications for weight loss.
Policy: Weight loss interventions may be more effective if they take into account common self-monitoring technology usage patterns.
Research: Research is needed to investigate how to best capitalize on the multifeatured functionality of self-monitoring technologies for weight loss.
Introduction
Consistent self-monitoring is a behavior change technique emphasized by numerous behavior change theories [1–3]. Self-monitoring of physical activity levels, dietary intake, and weight is widely prescribed for weight loss and weight loss maintenance [4], and evidence generally supports the efficacy of self-monitoring of these behaviors for achieving weight loss outcomes [5]. Emerging mobile health (mHealth) technologies may facilitate self-monitoring of physical activity levels, dietary intake, and weight [6]. The near-ubiquity of mobile devices and their ability to reduce the burden of regular self-monitoring make them attractive intervention options for weight-related behavioral intervention. These technologies are increasingly featured as components of weight loss interventions [7], and are particularly promising because electronically delivered weight loss interventions have the potential to be delivered with high fidelity to diverse populations at relatively low cost [5]. Indeed, mHealth intervention options for self-monitoring energy balance-related behaviors have been shown to yield relatively high adherence rates (e.g., greater than 85% over 3 months) [6] that can compare favorably to more traditional methods of self-monitoring [8]. Further, mHealth weight loss interventions can be effective [9, 10], and evidence suggests that some such interventions can be comparable in efficacy to more resource-intensive, in-person approaches [11].
Historically, intervention options for self-monitoring physical activity, dietary intake, and weight were necessarily offered piecewise (e.g., a behavioral intervention might have provided a pedometer, a food log, and a weight scale; participants might have been instructed to each use separately). However, mHealth intervention options are increasingly multifeatured and able to facilitate self-monitoring these behaviors concurrently while providing integrated feedback (e.g., Fitbit offers physical activity trackers, the ability to log food electronically, and a wireless weight scale; data from these sources can be combined to inform energy balance-related messaging). This approach to weight loss intervention is increasingly being used with some success [12–14]. While evidence suggests that higher use of self-monitoring technologies for tracking physical activity [15], dietary intake [15, 16], and weight fluctuation [15, 17, 18] may each individually yield better weight loss outcomes, research is limited with respect to how these mHealth self-monitoring technologies tend to be used together in practice and how combinatorial usage patterns influence weight loss outcomes.
As mHealth technologies for self-monitoring physical activity, dietary intake, and weight become increasingly utilized as components of behavioral intervention, it is critical to understand how these intervention components are used, and the extent to which they, independently and in concert, facilitate weight loss. The first aim of this study was to investigate the associations between mHealth self-monitoring technology use for tracking physical activity, dietary intake, and weight with device-measured weight loss in individuals with overweight or obesity participating in a worksite weight loss program. The second aim was to identify distinct usage patterns of these self-monitoring technologies and investigate how these patterns related to weight loss. Based on the extant literature, we hypothesized that use of each self-monitoring behavior would be independently associated with increased weight loss [6]. Further, we hypothesized that we would observe three profiles corresponding to high, moderate, and minimal use of all mHealth self-monitoring technologies and that those characterized by high use of self-monitoring technologies would tend to lose the most weight [15, 19].
METHODS
We examined data from two waves (2017–2018 and 2018–2019 school years) of the Vibrant Lives Plus Program. Vibrant Lives Plus was a 6-month worksite weight loss program school district employees with overweight or obesity near Houston, Texas (spanning from November to May). The program was a part of the Pasadena Vibrant Community, an initiative to unite individuals, schools, workplaces, and other key stakeholders to make long lasting changes in people’s lives. The program included 16 didactic lessons based on the Diabetes Prevention Program (DPP) that participants received by email or mail over the course of 26 weeks [20]. Program content was reinforced by 10 text messages sent each week. Text messages were framed using behavior change theories and targeted various constructs to supplement DPP-derived content (e.g., knowledge, self-regulation, outcome expectations, social support, etc.).
Participants received a Fitbit Flex 2 wireless physical tracker and a Fitbit Aria weight scale and were encouraged to use these devices and to log their daily food intake in the Fitbit mobile app regularly over the course of the program (Fitbit Inc., San Francisco, CA). Text message reminders were sent automatically to remind participants to sync their Fitbit devices. Another aspect of the program was the inclusion of various (approximately monthly) “challenges” featured throughout the program. For example, there was a “No Gain Challenge” to lose or maintain weight throughout the holiday season, and a “Spring in to Action Challenge” to achieve 150 active minutes during the week of spring break. These featured challenges generally encouraged the use of self-monitoring technologies. In half of the schools, participants who gained weight or did not lose at least three percent of their body weight after 12 weeks (n = 38) were offered three motivational telephone coaching sessions from a dietician and/or exercise physiologist.
We administered questionnaires at baseline and postprogram regarding participants’ sociodemographic characteristics and health status. We used Fitabase (Small Steps Labs, San Diego, CA) to gather participants’ physical activity tracker, food logging, and wireless weight scale data over the course of the program. We excluded individuals who did not use any of the three technologies from the analytic sample.
Weight
We calculated the dependent variable, weight change, by subtracting baseline weight from follow-up weight. We determined baseline weight with Fitbit Aria wireless scale data by taking the average of participants’ weight measurements during week 1. If no data from week 1 were available, we used the average for week 2, and so on, going up to week 4 if necessary. We performed a similar procedure to obtain participants’ follow-up weight (i.e., we prioritized wireless weight scale data from week 26 if available, then from weeks 25, 24, and 23, respectively).
Physical activity
To determine Fitbit physical activity tracker use, we calculated the percentage of days of valid device wear over the course of the program. We defined a valid wear day to be one in which participants obtained at least 1,500 steps on their device [21, 22].
Dietary intake
To determine food logging technology use, we calculated the percentage of days over the course of the program that participants logged at least 800 calories of food [23].
Weight scale use
To determine wireless weight scale use, we calculated the percentage of days over the course of the program that participants weighed themselves at least once with the Aria device.
Statistical methods
We calculated Pearson correlations between physical activity tracker use, food logging technology use, and wireless weight scale use, and weight change. We evaluated separate multiple linear regression models of the associations between device-measured weight change and the percentage of days over the 6-month study period that participants (a) wore the physical activity tracker and logged at least 1,500 steps, (b) used the dietary self-monitoring feature and logged at least 800 calories of food, and (c) used the wireless weight scale. All models adjusted for age, race/ethnicity (non-Hispanic white or other), sex, educational attainment (bachelor’s degree and higher or not), marital status (married or other), study wave, whether or not the participant received additional phone calls as per the program protocol, height, and baseline weight. We further evaluated added variable plots for each self-monitoring technology use behavior. To determine the association of each self-monitoring variable with weight change while adjusting for the others, we evaluated a single multiple linear regression model with terms for all three technologies and potentially confounding covariates.
We conducted repeated measures latent profile analysis (RMLPA) to identify clusters of individuals reflective of patterns of self-monitoring technology use over time. Latent profile analysis is a model-based technique for identifying clusters defined by commonalities in continuous measurement scale variables, and RMLPA extends this by allowing for the identification of discreet profiles while accounting for interdependent data due to nested time points. We chose this model because it is particularly useful for identifying and characterizing patterns of behavior that can emerge over time, and it lends itself to distal outcome analyses whereby we could evaluate the link between identified profiles and eventual weight change. Similar procedures have been used to identify patterns of adherence to health-related behaviors over the course of weight loss interventions [24]. First, we repeated the above calculations to determine participants’ percentage of days of physical activity tracker use, food logging technology use, and wireless weight scale use separately for the first and second 3 months of the program. We selected this time period because previous literature has identified a novelty period of approximately 3 months for mHealth device usage, after which users may be more likely to discontinue use [25]. To determine the number of profiles, we evaluated and compared models with two to eight profiles. Due to the use of repeated measures and otherwise highly correlated variables, we specified the model to estimate all covariances between indicator variables (thus conducting conventional multivariate mixture models with varying means, equal variances, and equal covariances). As recommended by Nylund et al., we chose among candidate models by comparing Bayesian Information Criterion values, conducting bootstrap likelihood ratio tests, and considering the interpretability of solutions [26, 27]. We created a heat map using each participant’s most probable latent profile classifications to visualize differences between profiles.
We regressed weight change on the latent profile construct, adjusting for the covariates enumerated above using the BCH stepwise regression procedure recommended by Bakk and Vermunt [28]. To do so, we used Mplus’ manual BCH functionality with robust maximum likelihood estimation [28, 29]. To minimize the chance of converging on local maxima, we ensured that the best log likelihood value was repeated across multiple starting values [30]. We assumed that participant-level missing data were missing at random and used a random forest-based, nonparametric imputation procedure to handle missingness. The nominal alpha for all analyses was set to p < .05. The RMLPA and the BCH stepwise regression procedure were conducted using Mplus version 8.3 (Muthén & Muthén, Los Angeles, CA). All other data analyses and procedures were conducted in R version 3.6.3.
RESULTS
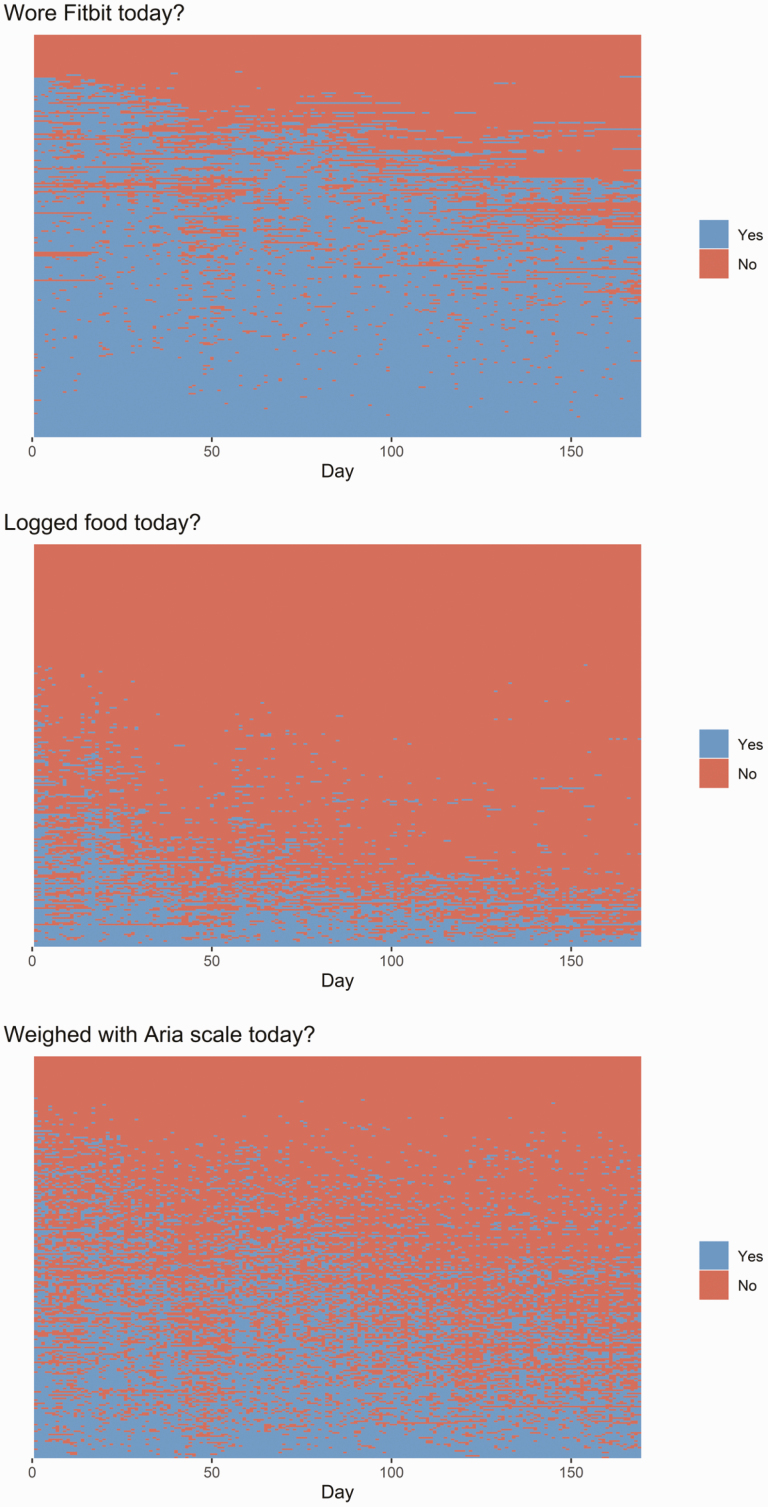
Two hundred and forty-five individuals participated in Vibrant Lives Plus over the 2017–2018 and 2018–2019 waves. Of those, 225 used at least one of the three mHealth technologies and thus comprised this study’s analytic sample. Participants were mostly female (Table 1). The average age of the sample was 42.8 years (SD = 10), and 164 participants (72.9%) had obesity. In total, 61 participants had missing outcome data before imputation (27%). On average, over the course of the 6-month program participants wore the Fitbit device 71.9% of days (SD = 28.9, Mdn = 83.4), logged food 18.8% of days (SD = 22.6, Mdn = 10.2), and used the wireless weight scale 39.1% of days (SD = 26.7, Mdn = 37.0). Adherence to self-monitoring behaviors gradually tapered off over the course of the 26-week behavioral intervention, with notable dips in use occurring over the winter and spring break holidays (about day 50 and 120, respectively, see Fig. 1).
Table 1.
| Participant characteristics
| Characteristic | Category | Number (%) |
|---|---|---|
| Gender | Male | 19 (8.4) |
| Female | 206 (91.6) | |
| Education level | HS diploma/GED | 23 (10.2) |
| Technical school or some college | 43 (19.1) | |
| Bachelor’s degree | 78 (34.7) | |
| Graduate school | 81 (36.0) | |
| Marital status | Single | 38 (16.9) |
| Married or living with significant other | 155 (68.9) | |
| Divorced or separated | 32 (14.2) | |
| Race/ethnicity | Black or African American | 19 (8.4) |
| Hispanic | 85 (37.8) | |
| Non-Hispanic White | 110 (48.9) | |
| Other | 11 (4.9) |
Fig 1.
| Self-monitoring technology use over the course of the 6-month program. Each row corresponds to one participant; participants arrayed by frequency of use for each technology.
Self-monitoring technology use and weight loss
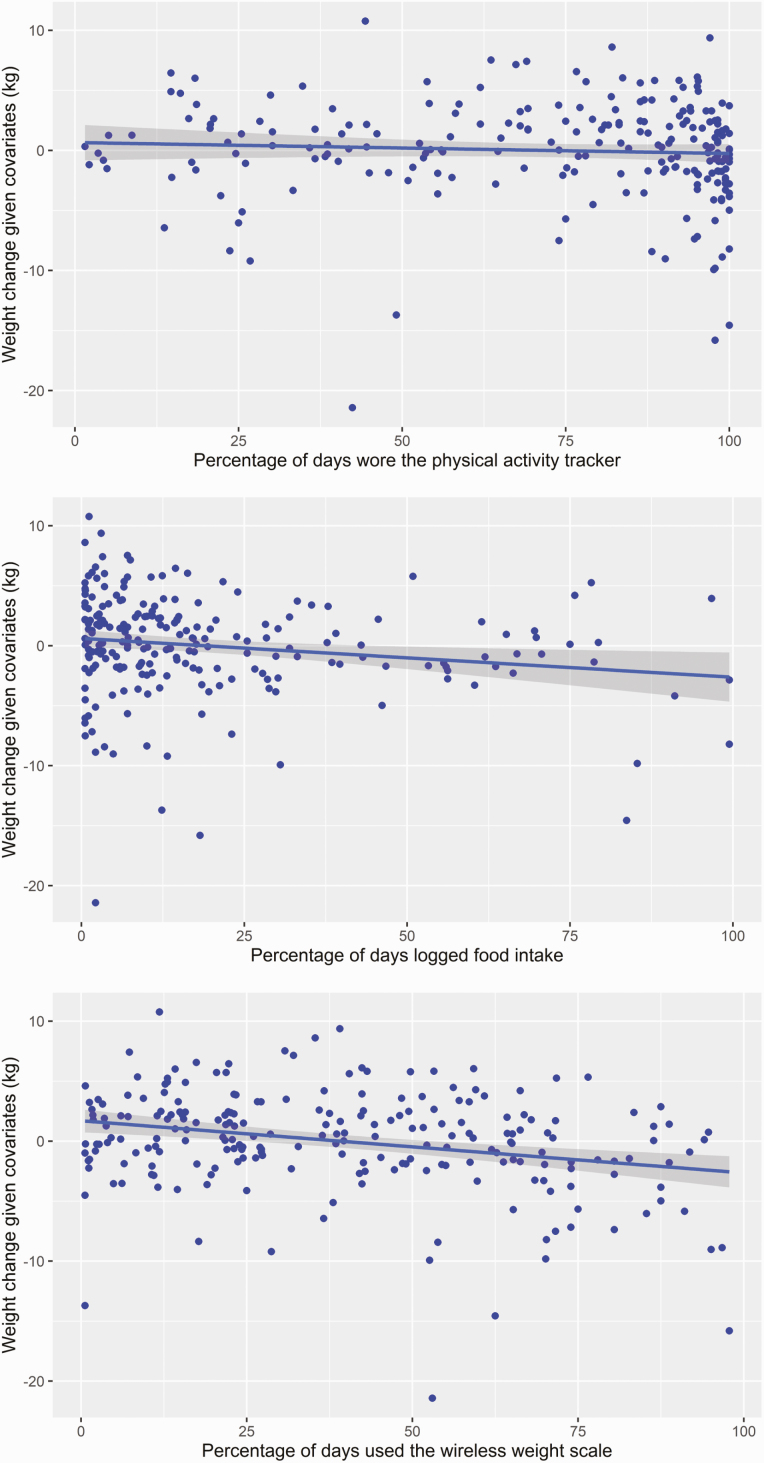
The average weight lost over the program was 2.03 kg (SD = 4.49). Pearson correlations indicated that physical activity tracker use, food logging, and wireless weight scale use had medium to strong, positive correlations with one another, and that each of these behaviors was independently associated with weight loss (Table 2). Results partially supported our first hypothesis (self-monitoring behaviors would be associated with increased weight loss). Separate multiple linear regression models, adjusting for covariates, suggested that a higher percentage of days of food logging (B = −0.04, t(214) = −2.76, p = .006) and a higher percentage of days of weight scale use were each associated with greater weight loss (B = −0.05, t(214) = −4.36, p < .001; see Fig. 2). Percentage of days of physical activity tracker use was not associated with weight change in its model. In the model which included terms for all three self-monitoring technology use behaviors, the significant association between food logging and weight loss was no longer significant (B = −0.02, p = .092), while percentage of days of weight scale use remained significant (B = −0.05, p < .001; Table 3).
Table 2.
| Pearson correlations between weight change and self-monitoring behaviorsa
| Weight change (%) | Fitbit use | Food logging | Scale use | |
|---|---|---|---|---|
| Weight change (%) | 1 | |||
| Fitbit use | −0.174 | 1 | ||
| Food logging | −0.251 | 0.411 | 1 | |
| Scale use | −0.328 | 0.488 | 0.401 | 1 |
aAll correlations statistically significant at the alpha < .01 level.
Fig 2.
| Added variable plots for use of self-monitoring technologies and weight change.
Table 3.
| Regression results for self-monitoring technology use predicting weight change
| Predictor | b | b | sr 2 | sr 2 | Fit |
|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||
| (Intercept) | −3.25 | [−17.69, 11.19] | |||
| Fitbit wear (%) | 0.01 | [−0.01, 0.04] | .00 | [−.01, .02] | |
| Dietary intake (%) | −0.02 | [−0.05, 0.00] | .01 | [−.01, .04] | |
| Weight scale use (%) | −0.05** | [−0.07, −0.02] | .06 | [.00, .11] | |
| Age (years) | −0.04 | [−0.11, 0.02] | .01 | [−.01, .03] | |
| Race/ethnicity (ref. is non-Hispanic white) | −0.01 | [−1.24, 1.22] | .00 | [−.00, .00] | |
| Sex (ref. is female) | −0.25 | [−2.63, 2.12] | .00 | [−.00, .00] | |
| Education (ref. is less than bachelor’s) | 0.54 | [−0.75, 1.82] | .00 | [−.01, .01] | |
| Marital status (ref. is single) | 0.41 | [−1.12, 1.95] | .00 | [−.01, .01] | |
| Study wave (ref. is year 1) | 1.17* | [0.03, 2.32] | .02 | [−.01, .05] | |
| Intervention (ref. is no support) | 0.37 | [−0.76, 1.51] | .00 | [−.01, .01] | |
| Baseline height (inches) | 0.15 | [−0.07, 0.37] | .01 | [−.01, .03] | |
| Baseline weight (kg) | −0.07** | [−0.10, −0.04] | .07 | [.01, .13] | |
| R 2 = .178** |
Note. A significant b-weight indicates the semi-partial correlation is also significant. b represents unstandardized regression weights. sr2 represents the semi-partial correlation squared. LL and UL indicate the lower and upper limits of a confidence interval, respectively.
*p < .05.
**p < .01.
Latent profile analysis
Results from an RMLPA suggested more than the hypothesized number of profiles. After comparing candidate model fit indices (Table 4) and considering the interpretability of the solutions, we selected the model with four profiles. The entropy of this model was 0.969 and the average probabilities of most likely latent profile membership were ≥0.975 for all profiles, suggesting that individual participants tended to be accurately classified.
Table 4.
| Model comparison fit indices
| Number of profiles | BIC | LMR (p) | Entropy | Range of profile sizes (proportions) |
|---|---|---|---|---|
| 8 | −868.092 | 0.128 | 0.965 | 0.02–0.48 |
| 7 | −860.753 | 0.707 | 0.934 | 0.04–0.47 |
| 6 | −875.804 | 0.03 | 0.977 | 0.03–0.52 |
| 5 | −841.727 | 0.054 | 0.974 | 0.03–0.55 |
| 4 | −793.67 | 1e−04 | 0.969 | 0.05–0.54 |
| 3 | −683.375 | 0.023 | 0.997 | 0.05–0.84 |
| 2 | −535.709 | 0.008 | 0.989 | 0.12–0.88 |
BIC Bayesian information criteria; LMR Lo–Mendell–Rubin test.
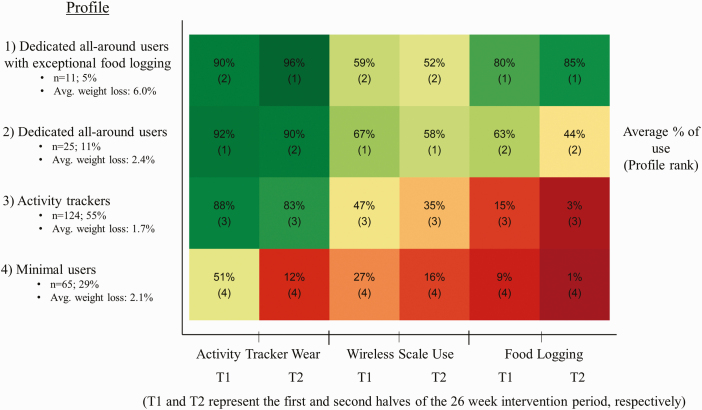
Figure 3 shows that nearly a third of participants (n = 65, 29%) comprised the minimal users. This group evidenced limited use of all self-monitoring technologies, and their use of all technologies reduced substantially from the first half of the program to the second. This group was the relative youngest and had the highest body mass index at baseline (Table 5). Members of this group lost an average of 2.1% of body weight (2.10 kg; SD = 4.45) over the course of the program.
Fig 3.
| Heat map of the typical technology use for each profile over the course of the 6-month program. Each square corresponds to its profile’s average percentage of days of using the mHealth technology over the corresponding time period.
Table 5.
| Participant characteristics by profile
| Characteristic | Category | Dedicated all-around users+ (N = 11) | Dedicated all-around users (N = 25) | Activity trackers (N = 124) | Minimal users (N = 65) |
|---|---|---|---|---|---|
| Mean (SD) | |||||
| Age (years) | 49.4 (8.7) | 45.4 (10.9) | 43.9 (9.5) | 38.4 (9.4) | |
| Baseline BMI (kg/m2) | 33.9 (7.3) | 31.6 (5.4) | 35.5 (7.0) | 36.3 (7.4) | |
| Average weight change (kg) | −5.7 (4.5) | −2.2 (4.4) | −1.6 (4.4) | −2.1 (4.4) | |
| Count (%) | |||||
| Gender | Male | 0 (0.0) | 2 (8.0) | 14 (11.3) | 3 (4.6) |
| Female | 11 (100.0) | 23 (92.0) | 110 (88.7) | 62 (95.4) | |
| Education level | HS diploma/GED | 1 (9.1) | 5 (20.0) | 9 (7.3) | 8 (12.3) |
| Technical school or some college | 1 (9.1) | 2 (8.0) | 27 (21.8) | 13 (20.0) | |
| Bachelor’s degree | 3 (27.3) | 7 (28.0) | 46 (37.1) | 22 (33.8) | |
| Graduate school | 6 (54.5) | 11 (44.0) | 42 (33.9) | 22 (33.8) | |
| Marital status | Single | 2 (18.2) | 4 (16.0) | 19 (15.3) | 13 (20.0) |
| Married or living with significant other | 9 (81.8) | 19 (76.0) | 88 (71.0) | 39 (60.0) | |
| Divorced or separated | 0 (0.0) | 2 (8.0) | 17 (13.7) | 13 (20.0) | |
| Race/ethnicity | Black or African American | 1 (9.1) | 2 (8.0) | 12 (9.7) | 4 (6.2) |
| Hispanic | 1 (9.1) | 8 (32.0) | 46 (37.1) | 30 (46.2) | |
| Non-Hispanic White | 8 (72.7) | 14 (56.0) | 59 (47.6) | 29 (44.6) | |
| Other | 1 (9.1) | 1 (4.0) | 7 (5.6) | 2 (3.1) |
BMI body mass index.
The majority of participants in the weight loss program were classified as activity trackers (n = 124, 55%). This group was characterized by consistent use of the physical activity tracking device over both halves of the study program period, low wireless weight scale use, and minimal food logging. Members of this group lost an average of 1.7% of body weight (1.64 kg; SD = 4.39) over the course of the program.
The dedicated all-around users profile (n = 25, 11%) was characterized by high use of all three self-monitoring technologies. This group had, on average, the highest use of the wireless weight scale. In this group, the average use of all self-monitoring technologies decreased from the first half of the program to the second half. Members of this group lost an average of 2.4% of body weight, or 2.15 kg (SD = 4.40), over the course of the program.
The smallest profile was that of the dedicated all-around users with exceptional food logging (n = 11, 5%; Fig. 3). This profile was characterized by relatively high use of all three self-monitoring technologies for both halves of the program and especially consistent food logging. Interestingly, for this group the average percentage of days using the physical activity tracker and logging food was even higher for the second half of the program than it was for the first half. This group was the relative oldest (Table 5). Members of this group lost an average of 6.0% of body weight (5.65 kg; SD = 4.50), over the course of the program.
BCH stepwise procedure regression indicated that the dedicated all-around users with exceptional food logging tended to lose significantly more weight than the activity trackers (the majority and referent group; X2[1,225] = 5.27, p = .0217). There was not a statistically significant difference in weight loss between the referent group and either the dedicated all-around users or the minimal users.
Discussion
As multifeatured mHealth technologies for self-monitoring physical activity, food intake, and weight become increasingly utilized as central components of weight loss interventions, it is important to understand how these technologies are used and how usage patterns are linked to weight loss. To the best of our knowledge, this study is the first to identify and characterize distinct latent profiles reflecting patterns of mHealth self-monitoring technology use over time for physical activity, food logging, and self-weighing behaviors in individuals with overweight or obesity participating in a weight loss program. We hypothesized that there would be three latent profiles, corresponding to high, moderate, and minimal use of all self-monitoring technologies. We identified four discrete profiles, corresponding to minimal users, activity trackers, dedicated all-around users, and dedicated all-around users with exceptional food logging. The majority of participants (55%) comprised the activity trackers group. Weight loss was limited in this group, and only membership in the smallest group (5%), the dedicated all-around users with exceptional food logging, was associated with significantly more weight loss.
In this study, we also investigated the associations between self-monitoring technology use for tracking physical activity, dietary intake, and weight with device-measured weight change. We hypothesized that use of each technology would be associated with weight loss. Findings indicated that a higher percentage of days of weight scale use was associated with greater weight loss; we did not observe evidence to indicate that percentage of days of physical activity tracker use or percentage of days of logging dietary intake was independently associated with weight loss. Thus, our findings suggest that regularly using weight self-monitoring technologies may be particularly important for facilitating weight loss. This finding is in accord with previous literature emphasizing the importance of high consistency of self-monitoring of weight for weight loss in individuals with overweight or obesity [15, 31]. Other research has found that daily self-monitoring of weight can provide weight loss benefits beyond just self-monitoring most days of the week [32], and that breaks in self-monitoring for as little as 1 week may predict weight gain [33]. Research suggests that use of self-monitoring technology for tracking weight may tend to decrease over time [6], and this was observed in all latent profiles in the present study. Given the apparent effectiveness and accessibility of self-monitoring of weight, it may be that encouraging consistent and sustained use of this self-monitoring technology should be a priority in weight loss interventions; indeed, this messaging may be relevant for individuals in all latent profiles identified in the present study.
A study conducted by Zheng et al. investigated patterns of self-weighing with a wireless weight scale in individuals participating in a weight loss intervention and identified three groups: high/consistent, moderate/declined, and minimal/declined [19]. They found that the high/consistent group (which constituted 75% of the sample and regularly self-weighed 7 days per week) tended to be more adherent to their physical activity and dietary intake goals and lost the most weight. Their findings generally accord with the clustering patterns observed in the present study, except that the frequency and consistency of self-weighing was notably higher in their study than in ours. This is perhaps because the current worksite weight loss program was relatively low in intensity. The behavioral intervention featured by Zheng et al. featured regular, in-person treatment sessions, and it has been postulated that such human support can increase adherence to digital interventions by fostering a sense of personal accountability to a coach who is trustworthy, benevolent, and has expertise [34]. More research is needed to elucidate the apparent trade-off between preserving the ready scalability of digital lifestyle interventions and maximizing effective engagement via more resource-intensive intervention options, such as the provision of human support.
Food logging was the least utilized of the three self-monitoring technologies, and the dedicated all-around users with exceptional food logging comprised the smallest profile. Previous literature has indicated that use of self-monitoring technology for food logging may tend decrease over time [6], and this was the case for all latent profiles except the dedicated all-around users with exceptional food logging. Self-monitoring of food intake may be perceived as burdensome for individuals; although mHealth technology may reduce participant burden (e.g., individuals can use their phone to scan bar codes to automatically log packaged food items, and easily repeat previously logged meals), user burden may yet impede sustained use for most people. Indeed, consistent food logging itself may be reflective of a priori, high-quality motivation for weight loss. Previous research has indicated that individuals participating in an electronic weight loss intervention who realized clinically significant weight loss outcomes at 16 weeks tended to exhibit sustained increases in autonomous motivation for weight loss, and that this relationship was mediated by self-monitoring of physical activity, dietary intake, and weight [35]. The dynamic between motivation, self-monitoring, and weight loss outcomes may further be influenced by individual characteristics. A negative affective response to reviewing the data associated with the self-monitoring of these behaviors, e.g., may make some individuals more likely to skip subsequent weigh-ins. Indeed, weight-related information avoidance has been shown to be negatively associated with weight self-monitoring and outcomes [36]. Conversely, people who had higher intrinsic motivation for losing weight may have been more engaged in self-monitoring but also with other weight loss behaviors (e.g., calorie restriction and exercise), thus losing weight independent of the self-monitoring. Future research should clarify the relationship between self-monitoring technology use and latent motivational constructs and investigate how to best target these potentially modifiable constructs. Furthermore, the development of less burdensome methods for self-monitoring of food intake is needed.
Given the relatively low uptake of self-monitoring technology use for dietary intake, it may be that taking a one-size-fits-all approach to categorically promote food logging could be inefficient. Instead, targeted efforts to incrementally increase or maintain this behavior in groups of individuals who have demonstrated some interest in food logging may be a more sensible strategy. The latent profile identified in the present study of dedicated, all-around users may represent an appropriate group to target in this way. Individuals in this group generally demonstrated a moderate degree of food logging, and it may be that strategically allocating intervention resources to encourage these individuals to engage in more frequent food logging may make this group more likely to achieve the weight loss outcomes observed in the dedicated all-around users with exceptional food logging. Emerging just-in-time, adaptive intervention techniques may be useful to this end [37]. For example, intervention options that may serve to increase food logging consistency, such as phone calls with study staff [13], may be employed contingent upon an individual demonstrating the technology use patterns characteristic of the dedicated, all-around users.
Consistent with previous studies, physical activity tracker use in this study was high [6, 13, 38, 39]. Results from RMLPA indicated that wear was high for all profiles except the minimal users. The largest latent profile identified was that of the activity trackers, which constituted a majority of study participants. Interestingly, the novelty effect associated with physical activity tracker use that has been identified in previous literature was pronounced only in the minimal users [40]. The dedicated all-around users with exceptional food logging increased their use of the physical activity tracker and food logging (perhaps due to winter holidays suppressing engagement in the first half of the program). It may be that the apparent interest in this technology can be leveraged to increase self-monitoring of weight. For example, activity trackers may be able to provide reminders to use the weight scale (as they sometimes do to break up sedentary time) or provide cues to action at opportune times (such as when the device detects that the user first wakes up in the morning). Alternatively, it may be possible to incorporate some of the features that make activity trackers enjoyable into weight logging, such as the incorporation of gamification elements or celebrating successes in weigh-in adherence.
While the relationship between self-monitoring of physical activity and weight loss is not entirely clear, there is some previous literature to suggest that electronic physical activity tracking can support weight loss [6, 41]. Despite being the most consistently used self-monitoring technology investigated, regression analyses indicated physical activity tracker wear was not associated with weight loss in this study. This is perhaps not surprising, as physical activity alone, without concomitant changes in dietary intake, may be relatively inefficient for achieving weight loss [42]. Further, physical activity trackers are tools that may facilitate self-monitoring, but it is not clear to what degree wearing these devices necessarily corresponds to self-monitoring of physical activity. Self-monitoring is believed to facilitate behavior change by increasing awareness of progress towards desired goals, allowing individuals to appreciate progress or take corrective action as necessary [2]. It is possible that simply wearing wearable devices may be apt to become an automatic process of habit and so diverge from the process of effortful self-monitoring. Supplementing the provision of wearable devices with the promotion of complementary behavior change techniques, such as goal-setting, action planning, feedback on performance, and review of previously set goals, may help increase intervention efficacy [2, 43]. As technologies continue to develop, behavioral scientists’ expertise will be valuable for leveraging passively collected, individual-level data for promoting sustained behavior change and positive health outcomes [44, 45].
This study must be considered in the context of its limitations. First, the observational nature of the evaluation precludes causal inference. It may have been that participants who experienced weight loss were more likely to subsequently self-monitor their weight, rather than the self-monitoring behavior preceding weight loss. In this sense, self-monitoring may have served to confirm or validate weight loss, and perhaps increase self-efficacy for weight loss. While the design of the current study has limitations, the practical nature of this investigation may provide added insight for how self-monitoring technologies are used in more generalizable circumstances than had these data come from a highly controlled efficacy trial. While the study sample was relatively ethnically diverse, the external validity of this study is limited by a small sample size that was mostly female and relatively well educated. Further, participants elected to take part in a weight loss program; thus, their motivation for weight loss may not be broadly generalizable. The study duration was 6 months, and thus we were unable to pursue our research aims in the context of long-term maintenance of weight loss. Long-term use of self-monitoring technologies and maintenance of weight loss is particularly challenging, and future research should investigate how our results may or may not hold over longer time intervals. Furthermore, based on our review of the extant literature, we opted to parse time into two 3-month periods. It is possible that different patterns may have emerged if a different number of time periods was used (e.g., using four time periods instead of two). While the approach used in the present study supported model parsimony, it is important to note that a more granular parsing of time may have yielded different results. Another limitation of the current study is that the weight assessment was not necessarily the gold standard. However, the use of device-measured weight assessment is generally preferable to self-report [46, 47]. Finally, this study is limited by its exclusion of nonusers from the analysis. This group could not be included due to the nature of the analyses, but they may represent a district subgroup with different weight-related motivations, behaviors, and outcomes.
Conclusions
We identified distinct usage patterns of technologies for self-monitoring physical activity, dietary intake, and weight fluctuation in individuals with overweight and obesity participating in a worksite weight loss program. Findings emphasize the importance of self-monitoring of weight for achieving weight loss. Individuals who were characterized by highly consistent food logging tended to lose the most weight after 6 months. It may be beneficial to generally encourage self-monitoring of weight, and selectively encourage food logging to individuals who have demonstrated dedicated use of self-monitoring technologies.
Acknowledgments
This research was made possible by the Center for Energy Balance in Cancer Prevention and Survivorship at The University of Texas MD Anderson Cancer Center. The Pasadena Vibrant Community is an initiative of The University of Texas MD Anderson Cancer Center made possible by an investment from and collaboration with Shell Oil Company.
Funding
Michael C. Robertson was supported by the National Cancer Institute of the National Institutes of Health under Award Number F31 CA236433. This study used MD Anderson’s Assessment, Intervention, and Measurement (AIM) Core, a shared resource supported by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (P30 CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflict of Interest: None declared.
Authors’ Contributions
: M.C.R. conceived and designed the analysis, performed the analysis, and wrote the paper; M.R. revised the paper; Y.L. conceived and designed the analysis and revised the paper; I.W. conceived and designed the analysis and revised the paper; N.P. revised the paper; L.G. collected the data and revised the paper; T.L. collected the data and revised the paper; C.P.D. conceived and designed the analysis and revised the paper; K.M.B.-E. provided the data, conceived and designed the analysis, and wrote the paper.
Ethical Approval: The University of Texas MD Anderson Cancer Center institutional review board approved the analysis of these data.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Transparency Statements: The analytic code used to conduct the analyses presented in this study may be made available by emailing the corresponding author. Deidentified data from this study are not available in a public archive; they may be made available by emailing the corresponding author as allowable by institutional IRB standards. This study was a secondary data analysis and thus was not formally registered. The analysis plan was not formally preregistered. Materials used to conduct the study are not publicly available.
References
- 1. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]
- 2. Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality—social, clinical, and health psychology. Psychol Bull. 1982;92(1):111–135. [PubMed] [Google Scholar]
- 3. Bandura A. The evolution of social cognitive theory. In: Smith KG, Hitt MA, eds. Great Minds in Management: The Process of Theory Development. Oxford, England: Oxford University Press on Demand.. 2005:9–35. [Google Scholar]
- 4. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34(4):841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butryn ML, Godfrey KM, Martinelli MK, Roberts SR, Forman EM, Zhang F. Digital self-monitoring: does adherence or association with outcomes differ by self-monitoring target? Obes Sci Pract. 2020;6(2):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teasdale N, Elhussein A, Butcher F, et al. Systematic review and meta-analysis of remotely delivered interventions using self-monitoring or tailored feedback to change dietary behavior. Am J Clin Nutr. 2018;107(2):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turner-McGrievy GM, Beets MW, Moore JB, Kaczynski AT, Barr-Anderson DJ, Tate DF. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Inform Assoc. 2013;20(3):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu F, Kong X, Cao J, et al. Mobile phone intervention and weight loss among overweight and obese adults: a meta-analysis of randomized controlled trials. Am J Epidemiol. 2015;181(5):337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang J, Sereika SM, Chasens ER, Ewing LJ, Matthews JT, Burke LE. Effect of adherence to self-monitoring of diet and physical activity on weight loss in a technology-supported behavioral intervention. Patient Prefer Adherence. 2012;6:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas JG, Bond DS, Raynor HA, Papandonatos GD, Wing RR. Comparison of smartphone-based behavioral obesity treatment with gold standard group treatment and control: a randomized trial. Obesity (Silver Spring). 2019;27(4):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rogers RJ, Lang W, Barone Gibbs B, et al. Applying a technology-based system for weight loss in adults with obesity. Obes Sci Pract. 2016;2(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ross KM, Wing RR. Impact of newer self-monitoring technology and brief phone-based intervention on weight loss: a randomized pilot study. Obesity (Silver Spring). 2016;24(8):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spring B, Pellegrini CA, Pfammatter A, et al. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obesity (Silver Spring). 2017;25(7):1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pourzanjani A, Quisel T, Foschini L. Adherent use of digital health trackers is associated with weight loss. PLoS One. 2016;11(4):e0152504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harvey J, Krukowski R, Priest J, West D. Log often, lose more: electronic dietary self-monitoring for weight loss. Obesity (Silver Spring). 2019;27(3):380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity (Silver Spring). 2013;21(9):1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ingels JS, Misra R, Stewart J, Lucke-Wold B, Shawley-Brzoska S. The effect of adherence to dietary tracking on weight loss: using HLM to model weight loss over time. J Diabetes Res. 2017;2017:6951495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Y, Burke LE, Danford CA, Ewing LJ, Terry MA, Sereika SM. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40(9):1392–1396. [DOI] [PubMed] [Google Scholar]
- 20. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tudor-Locke C, Barreira TV, Schuna JM Jr. Comparison of step outputs for waist and wrist accelerometer attachment sites. Med Sci Sports Exerc. 2015;47(4):839–842. [DOI] [PubMed] [Google Scholar]
- 22. Chu AH, Ng SH, Paknezhad M, et al. Comparison of wrist-worn Fitbit Flex and waist-worn ActiGraph for measuring steps in free-living adults. PLoS One. 2017;12(2):e0172535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Payne JE, Turk MT, Kalarchian MA, Pellegrini CA. Defining adherence to dietary self-monitoring using a mobile app: a narrative review. J Acad Nutr Diet. 2018;118(11):2094–2119. [DOI] [PubMed] [Google Scholar]
- 24. Fitzpatrick SL, Coughlin JW, Appel LJ, et al. Application of latent class analysis to identify behavioral patterns of response to behavioral lifestyle interventions in overweight and obese adults. Int J Behav Med. 2015;22(4):471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin G, Feng Y, Jarrahi MH, Gafinowitz N. Beyond novelty effect: a mixed-methods exploration into the motivation for long-term activity tracker use. JAMIA Open. 2019;2(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nasserinejad K, van Rosmalen J, de Kort W, Lesaffre E. Comparison of criteria for choosing the number of classes in Bayesian finite mixture models. PLoS One. 2017;12(1):e0168838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model. 2007;14(4):535–569. [Google Scholar]
- 28. Bakk Z, Vermunt JK. Robustness of stepwise latent class modeling with continuous distal outcomes. Struct Equ Model. 2016;23(1):20–31. [Google Scholar]
- 29. Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: using the BCH method in Mplus to estimate a distal outcome model and an arbitrary secondary model. Mplus Web Notes. 2014;21(2):1–22. [Google Scholar]
- 30. Geiser C. Data Analysis With Mplus. New York, NY: Guilford Press; 2012. [Google Scholar]
- 31. Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med. 2005;30(3):210–216. [DOI] [PubMed] [Google Scholar]
- 32. Steinberg DM, Bennett GG, Askew S, Tate DF. Weighing every day matters: daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Diet. 2015;115(4):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Helander EE, Vuorinen AL, Wansink B, Korhonen IK. Are breaks in daily self-weighing associated with weight gain? PLoS One. 2014;9(11):e113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Webber KH, Tate DF, Ward DS, Bowling JM. Motivation and its relationship to adherence to self-monitoring and weight loss in a 16-week Internet behavioral weight loss intervention. J Nutr Educ Behav. 2010;42(3):161–167. [DOI] [PubMed] [Google Scholar]
- 36. Schumacher LM, Martinelli MK, Convertino AD, Forman EM, Butryn ML. Weight-related information avoidance prospectively predicts poorer self-monitoring and engagement in a behavioral weight loss intervention. Ann Behav Med. 2020;55(2):kaaa034. doi: 10.1093/abm/kaaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med. 2018;52(6):446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL. Randomized trial of a Fitbit-based physical activity intervention for women. Am J Prev Med. 2015;49(3):414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hartman SJ, Nelson SH, Cadmus-Bertram LA, Patterson RE, Parker BA, Pierce JP. Technology- and phone-based weight loss intervention: pilot RCT in women at elevated breast cancer risk. Am J Prev Med. 2016;51(5):714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neve MJ, Collins CE, Morgan PJ. Dropout, nonusage attrition, and pretreatment predictors of nonusage attrition in a commercial Web-based weight loss program. J Med Internet Res. 2010;12(4):e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewis ZH, Lyons EJ, Jarvis JM, Baillargeon J. Using an electronic activity monitor system as an intervention modality: a systematic review. BMC Public Health. 2015;15(1):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bensimhon DR, Kraus WE, Donahue MP. Obesity and physical activity: a review. Am Heart J. 2006;151(3):598–603. [DOI] [PubMed] [Google Scholar]
- 43. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009;28(6):690–701. [DOI] [PubMed] [Google Scholar]
- 44. Hekler E, Tiro JA, Hunter CM, Nebeker C. Precision health: the role of the social and behavioral sciences in advancing the vision. Ann Behav Med. 2020;54(11):805–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schembre SM, Liao Y, Robertson MC, et al. Just-in-time feedback in diet and physical activity interventions: systematic review and practical design framework. J Med Internet Res. 2018;20(3):e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pebley K, Klesges RC, Talcott GW, Kocak M, Krukowski RA. Measurement equivalence of e-scale and in-person clinic weights. Obesity (Silver Spring). 2019;27(7):1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ross KM, Wing RR. Concordance of in-home “smart” scale measurement with body weight measured in-person. Obes Sci Pract. 2016;2(2):224–248. [DOI] [PMC free article] [PubMed] [Google Scholar]