ABSTRACT
Exosomes are nano-sized extracellular vesicles (30–160 nm diameter) with lipid bilayer membrane secrete by various cells that mediate the communication between cells and tissue, which contain a variety of non-coding RNAs, mRNAs, proteins, lipids and other functional substances. Adipose tissue is important energy storage and endocrine organ in the organism. Recent studies have revealed that adipose tissue-derived exosomes (AT-Exosomes) play a critical role in many physiologically and pathologically functions. Physiologically, AT-Exosomes could regulate the metabolic homoeostasis of various organs or cells including liver and skeletal muscle. Pathologically, they could be used in the treatment of disease and or that they may be involved in the progression of the disease. In this review, we describe the basic principles and methods of exosomes isolation and identification, as well as further summary the specific methods. Moreover, we categorize the relevant studies of AT-Exosomes and summarize the different components and biological functions of mammalian exosomes. Most importantly, we elaborate AT-Exosomes crosstalk within adipose tissue and their functions on other tissues or organs from the physiological and pathological perspective. Based on the above analysis, we discuss what remains to be discovered problems in AT-Exosomes studies and prospect their directions needed to be further explored in the future.
KEYWORDS: Adipose tissue-derived exosomes, composition, isolation, identification, cancer, cardiovascular diseases
1. Introduction
Adipose tissue (AT) is a main energy storage organ, which is a loose connective tissue with stromal vascular fraction (SVF), adipose-derived stem cells (ADSCs), adipose tissue macrophages (ATMs), T cells, preadipocytes and mature adipocytes. Literatures have shown that AT, as an endocrine organ, secretes a variety of adipokines that affect the body’s homoeostasis, including leptin, adiponectin, resistin and adipsin [1–3]. In terms of biogenesis, exosomes are a kind of extracellular vesicles (EVs). EVs are heterogeneous membrane vesicles that have been proven to be secreted by all types of cells [4]. They contain various types of particles with a wide range of physical properties and biological origins, being mainly divided into two categories based on different sizes [5]. One is 50–1000 nm vesicles formed by the sprouting of the plasma membrane, mainly including ectosomes (ie, shedding vesicles, microparticles and microvesicles) and apoptotic bodies, the other is exosomes with a size of 40–160 nm from the endosomal membrane. In physiological and pathological conditions, different types of cells secrete exosomes into the extracellular environment and body fluids [6], including blood [7], urine [8], amniotic fluid [9], spermatozoa [10]. Exosomes have a wide range of functions related to biological processes, including transfer of functional proteins and nucleic acids [11], immune response [12], elimination of unwanted substances, nutrition [13], surface receptor-mediated cell signalling [14] and cancer metastasis [15–17]. We use the keyword ‘exosome’ to search for relevant literature in the NCBI PubMed Database (https://pubmed.ncbi.nlm.nih.gov/), observing that exosomes-related literatures have risen sharply in recent years (Figure 1(a)). Studies on the biological origin, transport and function of exosomes will broaden the understanding of unknown intercellular communication and tissue homoeostasis in a mammal. Due to the lipid bilayer membrane structure, exosomes protect their coated substances and target specific cells or organs. Furthermore, exosomes have been used as disease biomarkers [18] and therapeutic agents [19] for disease diagnosis and drug carrier studies.
Figure 1.
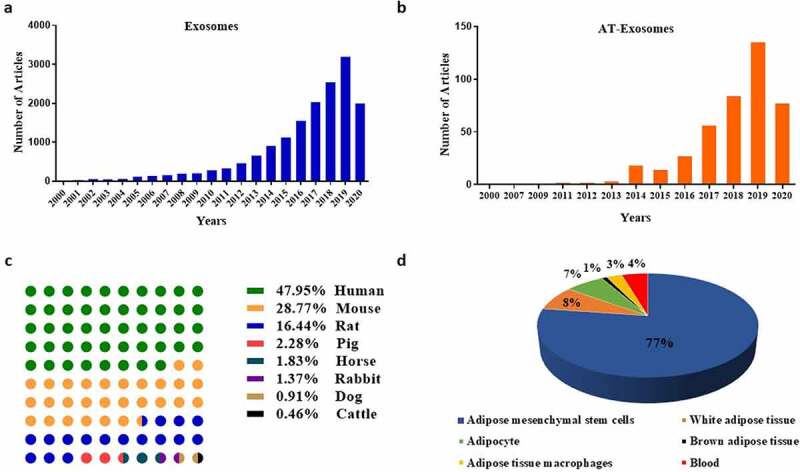
Classification and statistics of articles related to exosomes and AT-Exosomes. (a). Number of exosomes literatures along with year. (b). Number of AT-Exosomes articles along with year. (c). The literatures of AT-Exosomes in different species including human, mouse, rat, pig, horse, rabbit, dog and cattle. (d). Source of AT-Exosomes. AT-Exosomes, adipose tissue-derived exosomes
In recent years, AT has been proven to play an important physiological function by releasing AT-derived exosomes (AT-exosomes) to other specific tissues and organs. Its hypertrophy changes the miRNA profile of exosomes in plasma and affects glucose absorption and lipid metabolism in mice [20]. The exosomes derived from adipocytes (Adipocytes-Exosomes) affected the mTOR signal of the hypothalamus to regulate the appetite and body weight of mice [21]. Moreover, exosomes secreted by human adipose tissue mesenchymal stem cells (ADSCs-Exosomes) had a positive effect on the treatment of Alzheimer’s disease [22]. They were confirmed to affect the growth and migration of liver cancer, ovarian cancer, breast cancer [23–25]. We use the keywords ‘adipose’ and ‘exosome’ to search and classify relevant kinds of literature in the NCBI PubMed database. The studies of AT-Exosomes show an upward trend year by year similar to exosomes (Figure 1(b)). Additionally, by the analysis of the functions of different species and cell sources, we find that ADSCs-Exosomes are the most concerned by researchers (Figure 1(c.d)), because ADSCs are pluripotent cells that are easy to obtain and are cultured in large quantities with clinical therapeutic potential. In the above studies, the extraction and identification methods of AT-Exosomes are the research basis. Furthermore, studies about the internal components of AT-Exosomes help us make better use of this biological endogenous delivery to play a variety of regulatory functions. In this review, we summarize the components of AT-Exosomes, the methods of isolation and identification, as well as their physiological and pathological functions.
2. AT-Exosome composition
Given the universality and particularity of exosomes cargoes in adipose tissue, it was divided into two parts. The composition of universal substances is mainly caused by the biogenesis of exosomes, including some membrane proteins and key proteins of vesicle formation, which are contained in all of the exosomes. Specific substances are mainly molecules related to the metabolites secreted by adipose tissue and lipid storage, without in other tissue. In this section, we summarize the universal and specific components of AT-Exosomes.
2.1. Universality composition
The universality of exosomal composition is largely determined by its biogenesis. The biological process mainly includes the reverse budding of cytoplasmic membrane to form multivesicular endosome, the fusion of mature endosome and cell membrane, and the secretion of exosomes to the extracellular environment [26]. According to the origin and biogenesis of exosomes, all exosomes contain proteins involved in membrane transport and fusion (GTPases, annexins, flotillin) [27–30], tetraspanin proteins (CD9, CD63, CD81, CD82) [30–33], Heat shock proteins (Hsc70, Hsp90) [33,34], vesicle-forming proteins (Alix, TSG101) [30,33], lipid-associated proteins and phospholipases [35]. This has also become an important marker to identify exosomes [21,36,37]. In addition to proteins, exosomes contain lipids such as cholesterol, ceramides, sphingolipids, and long-chain glycerophospholipid, as well as a variety of RNAs including mRNA, miRNA, other non-coding RNAs (snRNA, snoRNA, scaRNA, piRNA, lncRNA and circRNA), tRNA and rRNA. Interestingly, most of the RNAs in exosomes are 20–200 nt in length.
2.2. Specificity composition
The specificity of the internal components of AT-Exosomes is reflected in their different sources of species and cells, determining their different biological functions (Table 1). The gluteal fat releases exosomal Hotair (HOX transcript antisense RNA) that promoted the proliferation of intestinal stem cells and progenitor cells [38]. In addition, AT-Exosomes are rich in lipids, lipid-related mRNAs and proteins [39–43]. Cargoes of AT-Exosomes have functions similar to AT and affect lipid synthesis and homoeostasis of target organs and cells. ADSCs-Exosomes contained miR-125a which acted on endothelial cells and promoted angiogenesis [44]. ADSCs-Exosomes were shown to secrete exosomes containing miR-4792, miR-320b, and miR-320a to inhibit the viability of ovarian cancer cells [11]. Moreover, ADSCs-Exosomes were detected to be rich in lncRNA metastasis associated lung adenocarcinoma-transcript 1 (MALAT1), lncGm37494 and other lncRNAs to perform a variety of important physiological functions [45–51], mainly including enhancing the regeneration of neurons in the injured area, repairing spinal cord injury, stimulating wound healing, angiogenesis, and improving hypoxia-induced cardiac injury. Furthermore, the exosomal microRNA-34a secreted by adipocytes inhibited the polarization of M2 macrophages and promoted fat inflammation caused by obesity [52]. Besides, adipocytes-Exosomes circRNA circ-DB to target ubiquitin-specific protease 7 (USP7) to promote the growth of hepatocellular carcinoma [53]. Our laboratory has devoted itself to the function of the ingredients in AT-Exosomes. The previous study indicated that resistin-containing exosomes secreted from adipocytes caused fatty degeneration of the liver in mice [54], suggesting that AT-Exosomes are new potential targets for the treatment of obesity and related hepatorenal syndrome.
Table 1.
Cargo in AT-exosomes in different species
| Species Cargo |
Human | Mouse | Rat | Pig | Cattle |
|---|---|---|---|---|---|
| microRNA | miR-125a [44], miR-4792, miR-320b, miR-320a [11], miR-145 [158] | miR-27a [55], miR-155 [56,145], miR-92a [57], microRNA-34a [52] | miR-214 [58], miR-191 [59], miR-450a-5p [60], miR-126, miR-130a, miR-132, miR-let7c [61] | miR-148a, miR-532-5p, miR-378, let-7 f [39] | miR-142-5p [40] |
| lncRNA | lncRNA MALAT1 [45–50] anti-NOS2a, DLG2A5, GAS5, HOTAIRM1, lincRNAp21, lincRNA-VLDLR, NEAT1 [50] |
LexGm37494 [51], Hotair [38] | |||
| circRNA | circ-DB [53], circ-0001359 [62], circ-0000250 [63] | ||||
| tRNA | tRNA CTC [79] | ||||
| mRNA | ANXA4, CLTC, CCT2 [64], MDFIC, HGF, CEBPA, ARRB1 [39] | CPT1A, HSL, PLIN, ATGL, FABP4 [40] | |||
| protein | IL6 [65], NEP [66], PTRF [67], Alpha-1-Antitrypsin [68] | FASN, G6PD, ACC [41], aP2 [42], eNAMPT [69], resistin [54] | caveolin 1, AQP7, adenylate kinase 2, catalase, liver carboxylesterase [70] |
We conduct a statistical analysis of the AT-Exosomes in the retrieval articles mentioned above (Figure 2), finding that the studies on microRNA components accounted for 74.12%. This is mainly due to the short length of microRNA and the complete microRNA sequence in exosomes, which makes it easy to perform biological functions. Furthermore, the current study methods including RNA-sequencing analysis, proteomics and lipidomics related to exosomal composition are limited. From a long-term perspective, the components of AT-Exosomes need to be further supplemented. It will also help us better understand the principles of exosomes and the communication between cells and tissue, this endogenous biological medium to perform physiological and pathological functions.
Figure 2.
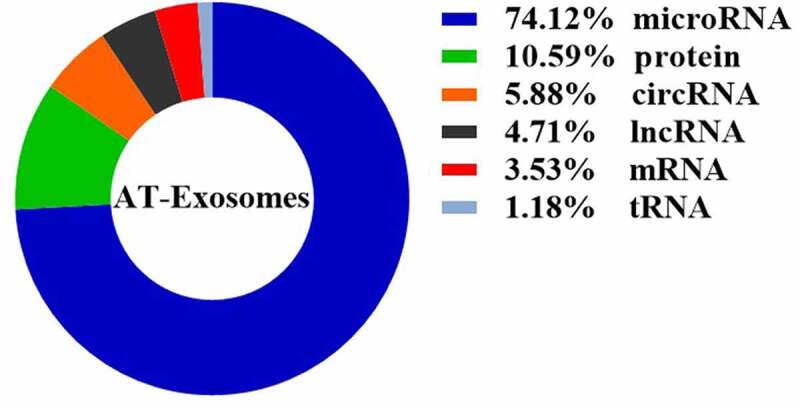
The proportion of components in AT-Exosomes
3. AT-Exosome isolation
In recent years, studies on the function of AT-exosomes have become a hot spot. The basic method is successfully isolates and detect them without disturbing natural form. In this part, we briefly summarize the principles and methods of several commonly used AT-Exosomes separation, and further analyse their respective advantages and disadvantages (Table 2).
Table 2.
The features of AT-Exosomes isolation methods
| Characteristics based on |
Method | Instrument requirement | Separation time | Shape & structure | Concentration | Purity | References |
|---|---|---|---|---|---|---|---|
| Size | Differential ultracentrifugation | high | long | destroy | normal | normal | [71–73] |
| Gradient ultracentrifugation | high | long | protect | low | high | [84,85] | |
| Ultrafiltration | normal | short | destroy | normal | normal | [43,62,74] | |
| Size-exclusion chromatography | high | short | protect | high | normal | [75,95] | |
| Water soluble | Polymer precipitation | low | short | destroy | high | low | [46,76,147] |
| Marker protein | Immunoaffinity capture | low | short | destroy | high | low | [108] |
3.1. Ultracentrifugation
Ultracentrifugation is called the ‘gold standard’ for exosome extraction according to the different sedimentation coefficients of exosomes and other substances [77,78]. It is mainly divided into differential ultracentrifugation and density gradient ultracentrifugation.
3.1.1. Differential ultracentrifugation
The differential ultracentrifugation is based on the size of exosomes. It is suitable for the extraction of exosomes in various body fluids [79–82]. The differential centrifugation method has the advantages of simple operation and low cost. However, the exosome samples prepared by this method have high levels of protein aggregation and lipoprotein contamination [83], and the purity is low. This method requires high sample volume and equipment requirements. In addition, ultra-high-speed centrifugation may cause exosome fragmentation, which is not conducive to subsequent quantitative and functional studies.
3.1.2. Gradient ultracentrifugation
The gradient ultracentrifugation is based on the size and density of exosomes. Density gradient centrifugation is mainly a combination of differential centrifugation and density centrifugation [84,85]. The principle is that exosomes will be suspended in liquids of similar density or composition after centrifugation. Exosomes have been reported to have a density between 1.13–1.19 g/ml [86]. The most commonly used solvents are mainly sucrose and iodixanol. The method of sample primary centrifugation is consistent with gradient centrifugation. The difference is that, 30% sucrose or iodixanol should be added to the bottom of the centrifuge tube before ultracentrifugation, and ultrahigh-speed centrifugation of 100,000–120,000 × g should be used for at least 75 min. In the next step, the suspension is resuspended by adding pre-chilled PBS, centrifuged at 100,000–120,000 × g and washed again, and the resulting precipitate (exosomes) is resuspended in a solvent according to different subsequent needs.
The density of the exosomes extracted by density gradient centrifugation is high in purity, which avoids protein contamination to a certain extent and protects the morphology of exosomes [87]. However, this method isolates exosomes with EVs similar to exosomes size, reducing the production of exosomes and separation efficiency.
3.1.3. Ultrafiltration
The separation of exosomes by ultrafiltration is similar to the traditional filtration principle, mainly using ultrafine nano-membranes with different MWCO (molecular weight cut-off) to separate extracellular vesicles of different sizes [88–90]. The main process of extracting exosomes by ultrafiltration is relatively simple. Various body fluids and cell culture supernatants are sequentially passed through filters to remove small particles such as cell debris, free proteins, and large vesicles such as apoptotic bodies, and collect exosomes smaller than 200 nm.
Compared with the ultracentrifugation method, the ultrafiltration method greatly shortens the experimental time, and has lower requirements for experimental equipment. However, this method easily causes vesicle congestion on the nanofiltration membrane to damage the membrane. Pressurization during filtration also destroys the natural form and structure of the exosomes, causing exosomes rupture and reducing the recovery rate [88,91]. In addition, exosomes separated based on size alone are likely to be mixed with nanoparticles of comparable size, resulting in exosome contamination and affecting exosomes purity [92].
3.2. Size-exclusion chromatography
The highlighted advantage of this separation method is that it does not destroy the structure and integrity of exosomes [93]. Under this separation method, the exosomes are observed to be normal in structure and the size of vesicles is about 80–200 nm. After treatment of the cells, there is no significant reduction in cell viability [94]. Furthermore, the characteristic of no interaction with another recently reported method for separating exosomes based on size is size-exclusion chromatography (SEC), also known as gel chromatography, molecular exclusion chromatography, etc., which is a type of liquid chromatography [95–97]. The separation mechanism of exclusion chromatography is three-dimensional exclusion, and there is no interaction between the sample components and the stationary phase. The packing material of the chromatographic column is a gel, which is an inert surface and contains many pores or three-dimensional network materials of different sizes. When the sample to be separated passes through the chromatographic column, the components of different sizes can penetrate into the gel pores at different depths. The large component molecules cannot enter the small pores or even be completely repelled and are quickly eluted, then the separated exosomes are obtained.
The stationary phase ensures a high separation efficiency [92,98]. Generally, we recommend that the separation method of size exclusion chromatography is more suitable for the separation, identification and subsequent functional study of exosomes. However, this method has higher requirements on instruments, showing wide size distribution of exosomes after isolating. Meanwhile, there are contaminants similar in size to exosomes, including protein aggregates and lipoproteins. Therefore, some researchers have used it in conjunction with ultrafiltration to remove possible contaminants [98–100].
3.3. Polymer precipitation
The polymer precipitation method mainly uses highly hydrophilic polymers to interact with water molecules around the exosomes to form a hydrophobic microenvironment, thereby allowing the exosomes to settle down [101,102]. The currently used hydrophilic polymers are mainly polydiethanol [103]. First, centrifuge at 3000 × g for 15 min to remove cell debris and apoptotic bodies, and aspirate the supernatant. Aspirate the supernatant and filter with a 0.22 μm filter. The suspension and the hydrophilic polymer solution were incubated overnight at 4°C. Then, centrifuge at a speed of 1500 × g for about 15 min, and discard the supernatant. The resulting precipitate (exosomes) will be resuspended in a solvent according to different subsequent needs.
The advantages of the polymer precipitation method are mainly simple operation, high yield and no need for complicated equipment. Therefore, it is often used in the production of commercial kits [47,104,105]. However, the polymer also precipitates various water-soluble substances in the exudate, including nucleic acids, lipoproteins, proteins, and even viruses. Thereby, it may lead to a high probability of exosome contamination by this method [106], causing great difficulties for the subsequent morphological observation and functional exploration of exosomes.
3.4. Immunoaffinity capture
The immunoaffinity capture method mainly uses specific proteins on the surface of exosomes for separation. As mentioned above, all exosomes contain some specific proteins and cell membrane components, so they can be used for the capture of exosomes based on immunoaffinity [107]. This includes proteins involved in membrane transport and fusion (GTPases, annexins, flotillin), four types of transmembrane proteins (CD9, CD63, CD81, CD82), heat shock proteins (Hsc70, Hsp90), vesicle-forming proteins (Alix, TSG101) and lipid-associated proteins and phospholipase. Existing institutions have used this feature to develop kits for isolating exosomes based on different markers.
Similar to the polymer precipitation method, the immunoaffinity capture method is also simple to operate and does not require expensive experimental machinery [108]. Simultaneous separation and extraction take a short time. Non-physiological pH-worthy eluents are used during the operation of this method, so it inevitably destroys the physiological structure of exosomes and affects the subsequent functional studies. Additionally, immunocapture of specific proteins is limited to exosomes with known antigens and may be affected by the heterogeneity of exosomes [109].
We have analysed and summarized the methods of AT-Exosomes separation in a number of studies which is special for adipose tissue, the extraction process are as follows: (1) Centrifuge at a low speed (300 × g) for about 10 min to remove impurities and live cells in the sample (this part should be noted that when separating the body fluids of high concentration exosomes such as plasma, use pre-chilled PBS first dilution). (2) Collect the supernatant, centrifuge at 2000 × g for 10–20 min, and centrifuge at 10,000 × g for 30–40 min. This process is to remove dead cells and cell debris. (3) Aspirate the supernatant, filter at 0.22 μm and enter into ultracentrifugation. (4) The filtered liquid is centrifuged for at least 70 min by ultra-high speed of 100,000–120,000 × g, discard the supernatant, and resuspend the pellet in PBS. (5) 100,000–120,000 × g centrifugation to wash the precipitate, and then resuspend the obtained precipitate (exosomes) using solvents according to different needs.
4. AT-Exosome identification
According to the structure, size and formation process of exosomes, the identification of exosomes currently mainly includes marker protein expression detection mentioned above, electron microscope observation and nanoparticle tracking analysis (NTA). Of course, these methods were also used to identify AT-Exosomes.
4.1. Marker protein detection
Based on the formation process and principle of exosomes mentioned above, exosomes have a series of specifically expressed proteins. In these years of research, a large number of experimenters have used this feature to carry out preliminary auxiliary identification of the isolated and extracted exosomes [110–114]. Among them, the specific proteins commonly used for detection mainly include CD63, CD9, TSG101 and Alix.
4.2. Electron microscope observation
Electron microscope observation is mainly divided into transmission electron microscope and cryo-electron microscope. Under the transmission electron microscope (TEM), the exosomes show a saucer-like structure, and its size is roughly judged to be between 40–160 nm [115]. Exosome samples are first fixed with 2% paraformaldehyde. Suspended droplets are taken on the carbon membrane copper mesh and dried to be negatively stained with dye [115–118]. Do not need to undergo complicated TEM sample preparation operations such as fixation, dehydration, embedding, and ultrathin sectioning [119], but instead directly stain the sample homogenate suspension. The negative staining technique is not only simple and fast but also the amount of dyeing solution is very small and the resolution is high.
However, some researchers point out that the saucer-like structure under the transmission electron microscope of exosomes is most likely caused by collapse after drying [120,121] Meanwhile, the fixed staining in TEM sample preparation and vacuum observation during imaging may also affect the size and morphology of exosomes [122]. In contrast, the exosomes observed by cryo-electron microscopy are round, which seems to be more consistent with the shape of the organism [123,124]. The preparation of the low-temperature electron microscope sample is that the exosome suspension is directly adsorbed on the porous carbon grid, sucked dry at 95%–99% humidity and quickly immersed in liquid ethane. This process does not require fixation and staining and is observed at −180°C, which maximizes the size and shape of the isolated exosomes. Therefore, more and more researchers choose to use this method to identify and semi-quantitate exosomes [125–127]. To ensure the staining effect and exosomal morphology, it is necessary to carefully select dyes and grasp the staining time.
4.3. Nanoparticle tracking analysis
NTA is a technology developed based on the principle of light scattering and Brownian motion of particles in suspension [128,129]. It detects the concentration and size distribution of vesicles with a diameter of 10 nm–1 μm and has recently been used for quantitative detection of exosomes [130–132]. The minimum size of vesicles using traditional flow detectors is about 500 nm, and a few improved detectors can only detect particles of 200 nm [133], which is not in line with the small size of ‘40–160 nm’ unique to exosomes. In the NTA experiment, a laser beam is used to pass through the sample chamber, and the exosomes suspend in the beam path scattered the light, making it easy to see them through a 20× magnification microscope equipped with a camera. The camera runs at 30 frames per second (fps) and captures video files of particles [134]. From the collected video records, the displacement of each particle is tracked and plotted as a function of time, and its hydrodynamic diameter is calculated using the Stokes-Einstein equation. After calibration with microspheres of known concentration, the absolute size distribution of the vesicles in the suspension can be obtained [135].
The detection cycle of NTA technology is very short, and it can measure more than 1000 particles in only 60 s [136]. In addition, NTA has 405 nm, 488 nm, 532 nm and 635 nm lasers with four different wavelengths to choose from, with corresponding filters, so that it can analyse fluorescent samples. There are specific markers on the surface of exosomes, including CD63, HSP70 and TSG-101 [137,138]. Under complex background conditions, using fluorescent antibodies to label exosomes, researchers can use the fluorescence measurement function of NTA to measure exosomes, and the results are more reliable than flow cytometry [139,140].
However, NTA testing also has certain limitations. When there are a large number of large-sized vesicles in the sample, the overlapping may affect the identification and tracking of small vesicles by the instrument. Consistent with this, the same problem occurs when the exosomes concentration in the resuspension solution is too large [130]. Therefore, this technique requires the concentration of exosome suspension to be controlled between 108–109 /mL [141,142]. Thereby it needs a good grasp of the density of the exosomal suspension, and sample pretreatments such as dilution or concentration if necessary.
Above all, we introduce three methods to obtain AT-exosome better, but none of them is perfect. Most of the studies use the combination of differential ultracentrifugation, marker protein detection, electron microscope observation, and NTA to isolate and identify exosomes. So far there are no methods to identify specific AT-exosomes, but we can make full use of the multi-omics to exploit the specific marker protein, thereby identifying the AT-exosomes in the future. Furthermore, with the development of the technology, it is possible to identify the specific exosomes. SP-IRIS, with higher sensitivity and accuracy, can characterize the particle size difference exosomes from different sources. Daniel et al use the SP-IRIS to explore the size difference of the exosomes from serum, finding that the exosomes from CD9 were ~10 nm larger than those from other sources [143]. Further SP-IRIS research still needed to be performed to uncover the methods which can identify AT-exosomes specifically.
5. Function of AT-Exosomes
As the largest energy storage and secretory organ, adipose tissue has attracted more and more attention to its secretion and function of exosomes. By classifying statistics of research papers retrieved from the database, we summarize the important roles of AT-Exosomes in both physiological and pathological aspects (Figures 3 and 4).
Figure 3.
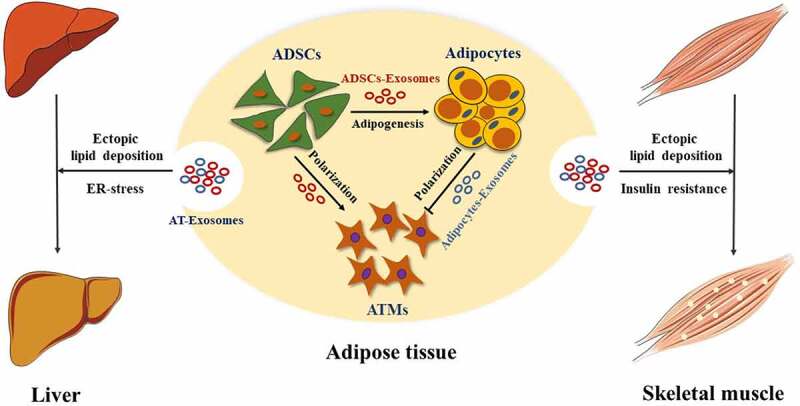
AT-Exosomes modulate ATMs polarization and adipogenesis in adipose tissue, liver and skeletal muscle. ADSCs, adipose tissue mesenchymal stem cells. ATMs, adipose tissue macrophages. ER-stress, endoplasmic reticulum stress
Figure 4.
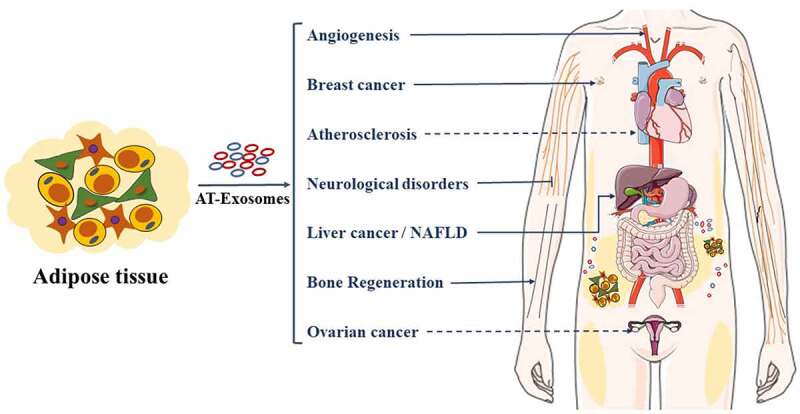
AT-Exosomes control human cancers and other diseases. AT-Exosomes may arrive target organs mainly through blood circulation. NAFLD, Non-alcoholic fatty liver
5.1. AT-Exosomes physiological function
5.1.1. AT-Exosomes function within adipose tissue
The heterogeneity of cells in adipose tissue determines that there will be crosstalk between cell exosomes in adipose tissue. The hypertrophy of adipose tissue causes the infiltration and activation of ATMs, which further affects body weight and produces insulin resistance. Deng et al first found AT-exosomes and showed that AT-exosomes of obese mice activated ATMs, leading to increased production of proinflammatory cytokines IL-6 and TNF-α in 2009. This process enhanced the migration of ATMs to adipose tissue and liver and promotes the development of insulin resistance [84]. Adipocytes-Exosomal miRNA-34a secreted by adipocytes inhibited M2 macrophage polarization and promoted obesity-induced fat inflammation [52]. Melatonin acted on ATMs by promoting the secretion of exosomes by adipocytes, reducing fat inflammation [116]. Further, Adipocytes-Exosomes contained lipid droplets and were absorbed by ATMs [43]. Additionally, in the white adipose tissue of diet-induced obese mice, ADSCs-Exosomes promoted the polarization of M2 macrophages and reduced the inflammatory response in mice [144]. Meanwhile, ATMs also released miRNA-rich exosomes to act on fat cells, liver cells and skeletal muscle cells, affecting the body’s metabolic homoeostasis [145]. In summary, ADSCs, adipocytes and ATMs in adipose tissue use exosomes as a biological medium to interact with each other to jointly maintain metabolic homoeostasis (Figure 3). However, AT-Exosome research is still needed to further reveal the underlying mechanisms and specific signalling molecules and pathways in adipose tissue.
5.1.2. AT-Exosome function in liver
The liver is an organ with complex functions in the organism, playing a vital physiological role in metabolism, detoxification, digestion, lipid synthesis and storage. Adipose and liver tissue interact with hormones and other biologically active factors to jointly maintain the body’s homoeostasis [146]. A study has shown that exosomal miRNA derived from brown adipose tissue (BAT) affected liver gene expression. Under cold stress conditions, miR-132-3p in exosomes secreted by BAT promoted liver fat production [147]. Melatonin reduced the transport volume of Adipocytes-Exosomal resistin from adipocytes to liver cells, thereby further alleviating liver fatty degeneration caused by endoplasmic reticulum stress [54]. Interestingly, ADSCs promoted the release of liver exosomes [148], and the miR-130a-3p derived from liver exosomes reduced glucose tolerance by targeting PH domain leucine-rich repeat protein phosphatase 2 (PHLPP2) in adipocytes [36]. Taken together, these findings indicate that there is indispensable exosome-mediated crosstalk between adipose tissue and the liver, revealing the potential of exosome-based therapies to control liver fat production and further reduce liver steatosis (Figure 3).
5.1.3. AT-Exosome function in skeletal muscle
As the largest organ of mammals, skeletal muscle is responsible for basic functions including exercise, breathing and metabolism. Studies have shown that metabolic disorders of adipose tissue affected the metabolism of fatty acids and the release of adipokines, further causing ectopic lipid deposition in skeletal muscle [149]. Adipocytes-Exosomes were regarded as a new type of adipokine, which affects the lipid metabolism in skeletal muscle [150]. 3T3-L1 Adipocytes-Exosomal miR-27a was shown to inhibit insulin signalling in C2C12 cells through PPARγ. Interestingly, ATMs-exosomal miR-155 also promoted skeletal muscle insulin resistance through PPARγ [145]. PPARγ knockout in adipocytes attenuated the thickening of the myocardium caused by miR-200a in exosomes [151]. It further suggests that adipose tissue and skeletal muscle are in close communication by their exosomes (Figure 3).
5.2. AT-Exosomes pathological function
5.2.1. AT-Exosomes and tumours
AT-Exosomes play an important role in the growth and migration of liver cancer, ovarian cancer, breast cancer and other cancers (Figure 4). ADSCs-Exosomes promoted the growth and migration of hepatocellular carcinoma [152]. Mechanistically, miR-23a/b and circ-DB were transported into liver cancer cells via ADSCs-Exosomes, thereby promoting the growth and migration of liver cancer cells [23,53]. Moreover, some researchers have proposed that ADSCs-Exosomes mediated exogenous transport of miR-199a-3p and miR-122 to increase the chemical sensitivity of hepatocellular carcinoma [153,154]. ADSCs-Exosomal miR-21 transferred to ovarian cancer cells, inhibiting the apoptosis of ovarian cancer cells by binding to the target apoptotic protease activating factor-1 (APAF1) and giving them chemoresistance [155]. However, ADSCs-Exosomal microRNAs inhibited the proliferation of ovarian cancer cells and promoted their apoptosis [16]. ADSCs-Exosomes also promoted the migration of breast cancer cells [156]. Global gene expression profile analysis showed that breast cancer cells treated with ADSCs-Exosomes upregulated cancer-related signalling genes and activated Wnt signalling pathways. In addition, ADSCs-Exosomes targeted inducible costimulatory molecule to promote anti-tumour immunity of lung adenocarcinoma [157], increased collagen beta (1-O) galactosyltransferase 2 (COLGALT2) expression to promote osteosarcoma proliferation and diffusion [17], inhibited prostate cancer cells proliferation, and induced prostate cancer cell apoptosis through miR-145 [158]. As mentioned above, AT-Exosomes have different effects on the proliferation, apoptosis and differentiation of different cancer cells. Furthermore, for the same type of cancer cell, the effects of different components in AT-Exosomes on the recipient cells are also inconsistent. In summary, ADSCs-Exosomes have been shown to effectively promote the proliferation or migrate of certain cell types and significantly reduce the proportion of apoptotic cells. Additional work is needed to enhance the use of AT-Exosomes in tumour therapy and confirm the optimal concentration for human use, as it will increase the feasibility and safety of AT-Exosome therapy in clinical applications.
5.2.2. AT-Exosomes and obesity-related diseases
AT-Exosomes have been shown to affect diabetes, non-alcoholic fatty liver disease (NAFLD) and cardiovascular diseases induced by insulin resistance (Figure 4). AT-Exosomes induced insulin resistance by activating ATMs polarization [84]. In this process, ATM-exosomal miR-210 regulated glucose uptake and mitochondrial activity, thereby promoting the onset of obesity and diabetes in mice [159]. Evidence showed that BAT-Exo significantly mitigated the metabolic syndrome in HFD mice by being involved in catalytic processes to promoted oxygen consumption in recipient cells [160]. Obesity changed the miRNA profile of plasma exosomes in mice, including the up-regulation of miR-122, miR-192, miR-27a-3p and miR-27b-3p, causing glucose intolerance and insulin resistance [161]. Additionally, cystatin C levels in AT-Exosomes were positively related, and monocyte marker CD14 levels were negatively related to metabolic complications of obesity, being used as a potential marker for cardiovascular-related diseases [162]. As a common obesity-related metabolic disease, the effect of ss-Exosomes on atherosclerosis was controversial. But more details about the molecular mechanism must be elucidated. ADSCs-Exosomes played a role in promoting atherosclerosis by regulating the formation and polarization of macrophage foam cells [163], whereas ADSCs-Exosomes protected endothelial cells from atherosclerosis [164]. Exosomes from visceral adipose tissue integrated into liver cells and induced dysregulation of TGF-β pathway members in vitro and offered an intriguing possibility for the pathogenesis of NAFLD [165]. Although AT-Exosomes can be used as diagnostic markers and therapeutic targets for obesity-related metabolic diseases. Currently, there are more and more studies on ADSCs-Exos related to obesity, and more studies are needed to investigate the understanding of exosome functions in obesity.
5.2.3. AT-Exosomes and angiogenesis
Angiogenesis is a key biological process that affects development, skeletal muscle hypertrophy, menstruation, pregnancy and wound healing (Figure 4). It mainly relies on the extensive signal transduction network formed by endothelial cells, parietal cells, vascular smooth muscle cells, pericytes and immune cells. ADSCs-Exosomes have been reported in numerous articles related to angiogenesis. Studies showed that ADSCs-exosomes stimulated the proliferation and migration of microvascular endothelial cells to promote angiogenesis by platelet-derived growth factor [166]. Mechanically, ADSCs-Exosomes was proved to be rich in small RNAs including miR-125a, miR-199-3p, miR-181b-5p and miR-423-5p, affecting the proliferation and migration of endothelial cells by binding their target genes [44,167–169]. In addition, hypoxia-induced ADSCs-Exosomes improved angiogenesis through activating the PKA signalling pathway and upregulating the expression of vascular endothelial growth factor (VEGF) [170]. In conclusion, although there are few effective clinical treatments for angiogenesis at present, cell-free therapies such as wound healing may be a valuable tool in promoting recovery after injury.
5.2.4. AT-Exosomes and bone regeneration
AT-Exosomes play an important role in the repair and regeneration of bone tissue and affect the proliferation, apoptosis, differentiation, ageing of osteoblasts, inducing bone damage caused by osteoarthritis and age-related bone loss (Figure 4). ADSCs-Exosomes and 3T3-L1 Adipocytes-Exosomes promoted the proliferation and osteogenic differentiation of human primary osteoblasts and 3T3-L1 precursor adipocytes [171,172]. And the exosomes derived from human adipose stem cells (hADSCs-Exo) The results indicate that hADSCs-Exo could be absorbed by hADSCs and induce osteogenic differentiation at 15 μg/ml [173]. Above all, they ensure optimal concentration which could promote the proliferation and migration of hADSCs. After ADSCs were pretreated by TNF-α, their therapeutic effect was much better. Moreover, ADSCs-Exosomes enriched in miR-375 improved the osteogenic differentiation of human bone marrow mesenchymal stem cells [174]. Meanwhile, ADSCs-exosomes inhibited the inflammation and oxidative stress of osteoblasts [175] and upregulated the expression of miR-145 and miR-221 [176] to promote cartilage production, improve the anti-ageing effect of osteoblasts and repress osteoarthritis. ADSCs-Exosomes alleviated osteocyte apoptosis and osteocyte-mediated osteoclastogenesis induced by hypoxia/serum deprivation [177]. Low-dose laser irradiation promoted ADSCs-Exosomes therapeutic effect [178]. ADSCs-Exosomes contained specific substances that induced osteogenic differentiation of cancer stem cells, being used to reprogram cancer stem cells into non-tumorigenic cells [179]. Recent studies indicated that hydrogel loaded with ADSCs-Exosomes promoted the bone regeneration in rat skull defect models, providing a basis for clinical applications [174]. Currently, AT-Exosomes-related studies on bone regeneration are rare and more investigations are still needed to expand our understanding of exosome functions in bone tissue.
5.2.5. AT-Exosomes and neurological disorders
AT-Exosomes have the potential to treat neurological diseases (Figure 4). ADSCs-Exosomes improved the survival and proliferation of neurons after injury [46]. Mechanistically, ADSCs-Exosomal MALAT1 mediates protein kinase C δ II (PKCδII) splicing, thereby improving the survival rate of neurons. ADSCs-Exosomes significantly enhanced neurite outgrowth in vitro, and ultraviolet radiation reduced the effect of ADSCs-Exosomes on neurite outgrowth [180]. After being internalized by Schwann cells (SCs), ADSCs-Exosomes significantly promoted SCs proliferation, migration, apoptosis, myelination and secretion of neurotrophic factors [181,182]. Additionally, ADSCs-Exosomes inhibited the activation of microglia by the NF-kB and MAPK pathways, reducing the cytotoxicity of activated microglia and preventing neuroinflammation [183]. Some researchers proposed that ADSCs-Exosomes may be used as an effective treatment tool for tissue engineering nerves, increasing the growth of neurites and enhancing regeneration of the sciatic nerve in vivo [184]. However, more details about the molecular mechanism must be elucidated, and clinical trials of the regeneration of the nerve must be conducted. Moreover, miR-133b exogenously modified ADSCs-Exosomes promoted the recovery of nerve function in animals with spinal cord injury by affecting signal pathways related to axon regeneration [104].
5.2.6. AT-Exosomes and other disease
AT-Exosomes have the potential to treat other diseases. Evidence shows that exosomes derived from MuSCs control important immunomodulatory effects to protect acute colitis induced by DSS [185]. Although the potential for treating acute colitis is significant, additional work is needed to confirm the optimal concentration. Furthermore, ADSCs-Exo also regulates the recovery after injury. Liu et al isolated the exosomes from mesenchymal stromal cell and added to the TSCs, the result showed that ADSCs-Exo could be absorbed by TSCs and activated the SMAD2/3 and SMAD1/5/9 pathways to promote the proliferation, migration [186]. ADSCs-exosomes also could promote hair follicle regeneration in vivo [187]. Nevertheless, even more details about the underlying molecular mechanism should be explored. Evidence suggests that ADSCs-Exo regulates the regeneration of the myelin sheath by reducing autophagy of injured SCs via miRNA-26b which could downregulate the expression of Kpna2 [188]. Above all, the function of adipose-derived exosomes is very powerful. At present, the research on the function of exosomes is further deepened, and more studies will focus on the study of exosomes in the future.
6. Future outlooks in AT-Exosomes
AT-Exosomes are media produced internally by organisms, which have good protection for their contents and target specific organs and cells to exert biological functions. This review introduces the research progress and universal and specific cargoes of AT-Exosomes. To explore the biological function of AT-Exosomes, we summarize the principles and procedures of main methods for the isolation and identification of AT-Exosomes. Importantly, we focus on the effects of AT-exosomes on organism tissues or organs in physiology and pathology.
As mentioned above, the methods of separating AT-Exosomes have their advantages and disadvantages. To date, ultracentrifugation is still regarded as the ‘gold standard’. Moreover, through the analysis of the separation time, cost and purity of various methods, we recommend size exclusion chromatography in addition to ultracentrifugation and commercial kits based on water-soluble extraction. It has the advantages of low cost and short separation time but also has problems of low purity and serious pollution. Therefore, new separation methods also should be developed to maximize the purity of AT-Exosomes and maintain their shape and activity. The physiological functions of AT-Exosomes are currently limited to tracking in vivo and phenotypic investigations, and the mechanism of crosstalk between AT-Exosomes and other tissues or organs is unclear. Therefore, exosome research still needs to be performed to further uncover the underlying mechanisms and specific signalling molecules and pathways. Pathologically, ADSCs-Exosomes have been currently used as therapeutic materials. However, target-specific organs, biological safety and inter-species rejection of ADSCs-Exosomes need to be further explored. Meanwhile, to date, the number of organ diseases that can be effectively treated by AT-Exosomes are limited. More studies are needed to explore this promising area. Future studies on the function of AT-Exosomes will not only help us better understand the crosstalk between mammalian different tissues and organs, but also are expected to fully use their biological functions for related cancer diagnosis and diseases treatment.
Funding Statement
This work is supported by the National Natural Science Foundation of China (31872979 and 31572366 to WJ).
Author contributions
Weijun Pang and Tiantian zhao: The conception of the review, investigation and selection of references, paper revision and editing and approval of the final version; Rui Zhao: Writing —original draft preparation, review and editing, and search of references; Zhaozhao He, Rui Cai: Supervision of the paper, complementation of references and writing —review and editing. All authors have read and agreed to the published version of the manuscript.
Date availability statement
Included as appendix 1and 2.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Żelechowska P, Brzezińska-Błaszczyk E, Kusowska A, et al. The role of adipokines in the modulation of lymphoid lineage cell development and activity: an overview. Obes Rev. 202021:e13055. [DOI] [PubMed] [Google Scholar]
- [2].Fischer J, Völzke H, Kassubek J, et al. Associations of a panel of Adipokines with fat deposits and metabolic phenotypes in a general population. Obesity (Silver Spring). 2020;28(8):1550–1559. [DOI] [PubMed] [Google Scholar]
- [3].Li M, Li C, Liu Y, et al. Decreased secretion of adiponectin through its intracellular accumulation in adipose tissue during tobacco smoke exposure. Nutr Metab (Lond). 2020;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hu W, Liu C, Bi ZY, et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression. metastasis and cancer immunology. Mol Cancer. 2020;19(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kalluri R, LeBleu VS.. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].He C, Zheng S, Luo Y, et al. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liang M, Yu S, Tang S, et al. A panel of plasma exosomal miRNAs as potential biomarkers for differential diagnosis of thyroid nodules. Front Genet. 2020;11:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zheng H, Guan S, Wang X, et al. Deconstruction of heterogeneity of size-dependent exosome subpopulations from human urine by profiling N-Glycoproteomics and phosphoproteomics simultaneously. Anal Chem. 2020;92(13):9239–9246. [DOI] [PubMed] [Google Scholar]
- [9].Liu R, Zhang W, Luo M, et al. ITRAQ-based proteomics and in vitro experiments reveals essential roles of ACE and AP-N in the renin-angiotensin system-mediated congenital ureteropelvic junction obstruction. Exp Cell Res. 2020;393(1):112086. [DOI] [PubMed] [Google Scholar]
- [10].Chen X, Zheng Y, Lei A, et al. Early cleavage of preimplantation embryos is regulated by tRNAGln-TTG derived small RNAs present in mature spermatozoa. J Biol Chem. 2020;295(32):10885–10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ding C, Qian C, Hou S, et al. Exosomal miRNA-320a is released from hAMSCs and regulates SIRT4 to prevent reactive oxygen species generation in POI. Mol Ther Nucleic Acids. 2020;21:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Orinska Z, Hagemann PM, Halova I, et al. Tetraspanins in the regulation of mast cell function. Med Microbiol Immunol. 2020;209(4):531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sanwlani R, Fonseka P, Chitti SV, et al. Milk-derived extracellular vesicles in inter-organism, cross species communication and drug delivery. Proteomes. 2020;8(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Camussi G, Deregibus MC, Bruno S, et al. Exosomes/microvesicles as amechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. [DOI] [PubMed] [Google Scholar]
- [15].Zhang N, Nan A, Chen L, et al. Circular RNA circ-SATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. 2020;19(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reza A, Choi YJ, Yasuda H, et al. Human adipose mesenchymal stem cell-derived exosomal-miRN as are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 2016;6:38498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Y, Chu Y, Li K, et al. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol. 2020;8:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang M, Chen D, Zhang F, et al. Serum exosomal hasmiR-135b-5p serves as a potential diagnostic biomarker in steroid-induced osteonecrosis of femoral head. Am J Transl Res. 2020;12(5):2136–2154. [PMC free article] [PubMed] [Google Scholar]
- [19].Norouzi-Barough L, Asgari Khosro Shahi A, Mohebzadeh F, et al. Early diagnosis of breast and ovarian cancers by body fluids circulating tumor-derived exosomes. Cancer Cell Int. 2020;20:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Castaño C, Kalko S, Novials A, et al. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115(48):12158–12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gao J, Li X, Wang Y, et al. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol (Oxf). 2018;228:e13339. [DOI] [PubMed] [Google Scholar]
- [22].Katsuda T, Oki K, Ochiya T. Potential application of extracellular vesicles of human adipose tissue- derived mesenchymal stem cells in Alzheimer’s disease therapeutics. Methods Mol Biol. 2015;1212:171–181. [DOI] [PubMed] [Google Scholar]
- [23].Liu Y, Tan J, Ou S, et al. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J Physiol Biochem. 2019;75(3):391–401. [DOI] [PubMed] [Google Scholar]
- [24].García-Contreras M, Vera-Donoso CD, Hernández-Andreu JM, et al. Therapeutic potential of human adipose-derived stem cells (ADSCs) from cancer patients: a pilot study. PLoS One. 2014;9:e113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kuhbier JW, Bucan V, Reimers K, et al. Observed changes in the morphology and phenotype of breast cancer cells in direct co-culture with adipose-derived stem cells. Plast Reconstr Surg. 2014;134(3):414–423. [DOI] [PubMed] [Google Scholar]
- [26].Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. [DOI] [PubMed] [Google Scholar]
- [27].Li Z, Fang R, Fang J, et al. Functional implications of Rab27 GTPases in cancer. Cell Commun Signal. 2018;16(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pick H, Alves AC, Vogel H. Single-vesicle assays using liposomes and cell-derived vesicles: from modeling complex membrane processes to synthetic biology and biomedical applications. Chem Rev. 2018;118(18):8598–8654. [DOI] [PubMed] [Google Scholar]
- [29].Chaudhary P, Gibbs LD, Maji S, et al. Serum exosomal-annexin A2 is associated with African-American triple-negative breast cancer and promotes angiogenesis. Breast Cancer Res. 2020;22(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu H, Zhou J, Tang J, et al. Identification of serum exosomal lncRNA MIAT as a novel diagnostic and prognostic biomarker for gastric cancer. J Clin Lab Anal. 2020;34:e23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Escola JM, Kleijmeer MJ, Stoorvogel W, et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–20127. [DOI] [PubMed] [Google Scholar]
- [32].Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and d- efine a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. [DOI] [PubMed] [Google Scholar]
- [33].Marzano M, Bejoy J, Cheerathodi MR, et al. Differential effects of extracellular vesicles of lineage-specific human pluripotent stem cells on the cellular behaviors of isogenic cortical spheroids. Cells. 2019;8(9):993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Taha EA, Ono K, Eguchi T. Roles of extracellular HSPs as biomarkers in immune surveillance and I- mmune evasion. Int J Mol Sci. 2019;20(18):4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23(2):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu J, Dong T, Chen T, et al. Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism. 2020;103:154006. [DOI] [PubMed] [Google Scholar]
- [37].Murdica V, Giacomini E, Makieva S, et al. In vitro cultured human endometrial cells release extracellular vesicles that can be uptaken by spermatozoa. Sci Rep. 2020;10(1):8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu X, Bai D, Liu X, et al. Sedentary lifestyle related exosomal release of Hotair from gluteal-femoral fat promotes intestinal cell proliferation. Sci Rep. 2017;7:45648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Eirin A, Riester SM, Zhu XY, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Muroya S, Ogasawara H, Nohara K, et al. Coordinated alteration of mRNA-microRNA transcriptomes associated with exosomes and fatty acid metabolism in adipose tissue and skeletal muscle in grazing cattle. Asian-Australas J Anim Sci. 2020;33(11):1824–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sano S, Izumi Y, Yamaguchi T, et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445(2):327–333. [DOI] [PubMed] [Google Scholar]
- [42].Ertunc ME, Sikkeland J, Fenaroli F, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res. 2015;56(2):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Flaherty SE 3rd, Grijalva A, Xu X, et al. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363(6430):989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liang X, Zhang L, Wang S, et al. secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129(11):2182–2189. [DOI] [PubMed] [Google Scholar]
- [45].Cooper DR, Wang C, Patel R, et al. Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv Wound Care (New Rochelle). 2018;7(9):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].El Bassit G, Patel RS, Carter G, et al. MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCδII in HT22 cells. Endocrinology. 2017;158(1):183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].He L, Zhu C, Jia J, et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci Rep. 2020;40:BSR20192549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Patel NA, Moss LD, Lee JY, et al. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflammation. 2018;15(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xia W, Chen H, Xie C, et al. Long-noncoding RNA MALAT1 sponges microRNA-92a-3p to inhibit doxorubicin-induced cardiac senescence by targeting ATG4a. Aging (Albany NY). 2020;12(9):8241–8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Patel RS, Carter G, El Bassit G, et al. Adipose-derived stem cells from lean and obese humans show depot specific differences in their stem cell markers, exosome contents and senescence: role of protein kinase C delta (PKCδ) in adipose stem cell niche. Stem Cell Investig. 2016;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shao M, Jin M, Xu S, et al. Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization. Inflammation. 2020;43(4):1536–1547. [DOI] [PubMed] [Google Scholar]
- [52].Pan Y, Hui X, Hoo RLC, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129(2):834–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang H, Deng T, Ge S, et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2019;38(15):2844–2859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [54].Rong B, Feng R, Liu C, et al. Reduced delivery of epididymal adipocyte-derived exosomal resistin is essential for melatonin ameliorating hepatic steatosis in mice. J Pineal Res. 2019;66(4):e12561. [DOI] [PubMed] [Google Scholar]
- [55].Yu Y, Du H, Wei S, et al. Adipocyte-DERIVED EXOSOMal MiR-27a induces insulin resistance in skeletal muscle through repression of PPARγ. Theranostics. 2020;8(8):2171–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wei M, Gao X, Liu L, et al. Visceral adipose tissue derived exosomes exacerbate colitis severity via pro-inflammatory MiRNAs in high fat diet fed mice. ACS Nano. 2020;14(4):5099–5110. [DOI] [PubMed] [Google Scholar]
- [57].Chen Y, Buyel JJ, Hanssen MJ, et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun. 2016;7:11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li KC, Chang YH, Hsu MN, et al. Baculovirus-mediated miR-214 knockdown shifts osteoporotic ASCs differentiation and improves osteoporotic bone defects repair. Sci Rep. 2017;7(1):16225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang C, Wang P, Mohammed A, et al. Function of adipose-derived mesenchymal stem cells in monocrotaline-induced pulmonary arterial hypertension through miR-191 via regulation of BMPR2. Biomed Res Int. 2019;16:2858750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang Y, Yu M, Dai M, et al. MiR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci. 2017;130(6):11. [DOI] [PubMed] [Google Scholar]
- [61].Zhu LL, Huang X, and Yu W, et al. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50(2):115. [DOI] [PubMed] [Google Scholar]
- [62].Shang Y, Sun Y, Xu J, et al. Exosomes from mmu circ 0001359-modified ADSCs attenuate airway remodeling by enhancing FoxO1 signaling-mediated M2-like macrophage activation. Mol Ther Nucleic Acids. 2020;19:951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shi R, Jin Y, Hu W, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318(5):c848–C856. [DOI] [PubMed] [Google Scholar]
- [64].Conley SM, Shook JE, Zhu XY, et al. Metabolic syndrome induces release of smaller extracellular vesicles from porcine mesenchymal stem cells. Cell Transplant. 2019;28(9–10):1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pu CM, Liu CW, Liang CJ, et al. Adipose-dipose-derived stem cells protect skin flaps against ischemia/reperfusion injury via IL-6 expression. J Invest Dermatol. 2017;137(6):1353–1362. [DOI] [PubMed] [Google Scholar]
- [66].Katsuda T, Tsuchiya R, Kosaka N, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3(6):1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Perez-Diaz S, Garcia-Sobreviela MP, Gonzalez-Irazabal Y, et al. PTRF acts as an adipokine contributing to adipocyte dysfunctionality and ectopic lipid deposition. J Physiol Biochem. 2018;74(4):613–622. [DOI] [PubMed] [Google Scholar]
- [68].Bari E, Ferrarotti I, Di Silvestre D, et al. Adipose mesenchymal extracellular vesicles as Alpha-1-Antitrypsin physiological delivery systems for lung regeneration. Cells. 2019;8(9):965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yoshida M, Satoh A, Lin JB, et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019;30(2):329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lee JE, Moon PG, Lee IK, et al. Proteomic analysis of extracellular vesicles released by adipoc- ytes of Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Protein J. 2015;34(3):220–235. [DOI] [PubMed] [Google Scholar]
- [71].Bai Y, Han YD, Yan XL, et al. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem Biophys Res Commun. 2018;500(2):310–317. [DOI] [PubMed] [Google Scholar]
- [72].Chai HT, Sheu JJ, Chiang JY, et al. Early administration of cold water and adipose derived mesenchymal stem cell derived exosome effectively protects the heart from ischemia-reperfusion injury. Am J Transl Res. 2019;11(9):5375–5389. [PMC free article] [PubMed] [Google Scholar]
- [73].Cunnane EM, Lorentz KL, Ramaswamy AK, et al. Extracellular vesicles enhance the remodeling of cell-free silk vascular scaffolds in rat aortae. ACS Appl Mater Interfaces. 2020;12(24):26955–26965. [DOI] [PubMed] [Google Scholar]
- [74].Ni J, Li H, Zhou Y, et al. Therapeutic potential of human adipose-derived stem cell exosomes in stress urinary incontinence - an in vitro and in vivo study. Cell Physiol Biochem. 2018;48(4):1710–1722. [DOI] [PubMed] [Google Scholar]
- [75].Jayabalan N, Lai A, Ormazabal V, et al. Adipose tissue exosomal proteomic profile reveals a role on placenta glucose metabolism in gestational diabetes mellitus. J Clin Endocrinol Metab. 2019;104(5):1735–1752. [DOI] [PubMed] [Google Scholar]
- [76].De Silva N, Samblas M, Martínez JA, et al. Effects of exosomes from LPS-activated macrophag- es on adipocyte gene expression, differentiation, and insulin-dependent glucose uptake. J Physiol Biochem. 2018;74(4):59–568. [DOI] [PubMed] [Google Scholar]
- [77].Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin rec- eptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844–1851. [PubMed] [Google Scholar]
- [78].Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- [79].Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen KH, Chen CH, Wallace CG, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7(46):74537–74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cui X, He Z, Liang Z, et al. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-Catenin signaling pathway. J Cardiovasc Pharmacol. 2017;70(4):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shen T, Zheng Q, Luo H, et al. Exosomal miR-19a from adipose-derived stem cells suppresses differentiation of corneal keratocytes into myofibroblasts. Aging (Albany NY). 2020;12(5):4093–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014;3.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58(11):2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kranendonk ME, Visseren FL, Van Herwaarden JA, et al. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring). 2014;22(10):2216–2223. [DOI] [PubMed] [Google Scholar]
- [86].van der Pol E, Böing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. [DOI] [PubMed] [Google Scholar]
- [87].Paolini L, Zendrini A, Di Noto G, et al. Residual matrix from different separation techniques impacts exosome biological activity. Sci Rep. 2016;6:23550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Soda N, Rehm BHA, Sonar P, et al. Advanced liquid biopsy technologies for circulating biomarker detection. J Mater Chem B. 2019;7(43):6670–6704. [DOI] [PubMed] [Google Scholar]
- [89].Peterson MF, Otoc N, Sethi JK, et al. Integrated systems for exosome investigation. Methods. 2015;87:31–45. [DOI] [PubMed] [Google Scholar]
- [90].Zhang X, Chen L, Xiao B, et al. Circ_0075932 in adipocyte-derived exosomes induces inflammation and apoptosis in human dermal keratinocytes by directly binding with PUM2 and promoting PUM2-mediated activation of AuroraA/NF-κB pathway. Biochem Biophys Res Commun. 2019;511(3):551–558. [DOI] [PubMed] [Google Scholar]
- [91].Alvarez ML, Khosroheidari M, Kanchi Ravi R, et al. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82(9):1024–1032. [DOI] [PubMed] [Google Scholar]
- [92].Vergauwen G, Dhondt B, Van Deun JD, et al. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci Rep. 2017;7(1):2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].An M, Wu J, Zhu J, et al. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J Proteome Res. 2018;17(10):3599–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, et al. Size Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6:33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhu D, Johnson TK, Wang Y, et al. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther. 2020;11(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ax E, Jevnikar Z, Cvjetkovic A, et al. T2 and T17 cytokines alter the cargo and function of airway epithelium-derived extracellular vesicles. Respir Res. 2020;21(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ludwig N, Gillespie DG, Reichert TE, et al. Purine metabolites in tumor-deriveed exosomes may facilitate immune escape of head and neck squamous cell carcinoma. Cancers (Basel). 2020;2(6):1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shu S, Yang Y, Allen CL, et al. Ernstoff MS, Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles. 2020;9(1):1692401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;27:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rood IM, Deegens JK, Merchant ML, et al. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 2010;78(8):810–816. [DOI] [PubMed] [Google Scholar]
- [101].Tao Y, Tang Y, Yang Z, et al. Exploration of serum exosomal LncRNA TBILA and AGAP2-AS1 as promising biomarkers for diagnosis of non-small cell lung cancer. Int J Biol Sci. 2020;16(3):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ryu KJ, Lee JY, Park C, et al. Isolation of small extracellular vesicles from human serum using a combination of ultracentrifugation with polymer-based precipitation. Ann Lab Med. 2020;40(3):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Holman NS, Church RJ, Nautiyal M, et al. Hepatocyte-derived exosomes promote liver immune tolerance: possible implications for idiosyncratic drug-induced liver injury. Toxicol Sci. 2019;170(2):499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ren ZW, Zhou JG, Xiong ZK, et al. Effect of exosomes derived from MiR-133b-modified ADSCs on the recovery of neurological function after SCI. Eur Rev Med Pharmacol Sci. 2019;23(1):52–60. [DOI] [PubMed] [Google Scholar]
- [105].Jin J, Wang Y, Zhao L, et al. Exosomal miRNA-215-5p derived from adipose-derived stem cells attenuates epithelial-mesenchymal transition of podocytes by inhibiting ZEB2. Biomed Res Int. 2020;2685305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cao F, Gao Y, Chu Q, et al. Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis. 2019;40(23–24):3092–3098. [DOI] [PubMed] [Google Scholar]
- [107].Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].An Y, Zhao J, Nie F, et al. Exosomes from Adipose-Derived Stem Cells(ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. 2019;9(1):12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Willms E, Johansson HJ, Mäger I, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Géminard C, Nault F, Johnstone RM, et al. Characteristics of the interaction between Hsc70 and the transferrin receptor in exosomes released during reticulocyte maturation. J Biol Chem. 2001;276(13):9910–9916. [DOI] [PubMed] [Google Scholar]
- [111].Yang X, Meng S, Jiang H, et al. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J Surg Res. 2011;171(2):826–832. [DOI] [PubMed] [Google Scholar]
- [112].Chairoungdua A, Smith DL, Pochard P, et al. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhao L, Jiang X, Shi J, et al. Exosomes derived from bone marrow mesenchymal stem cells overexpressing microRNA-25 protect spinal cords against transient ischemia. J Thorac Cardiovasc Surg. 2019;157(2):508–517. [DOI] [PubMed] [Google Scholar]
- [114].Zieren RC, Dong L, Pierorazio PM, et al. Extracellular vesicle isolation from human renal cancer tissue. Med Oncol. 2020;37(4):28. [DOI] [PubMed] [Google Scholar]
- [115].Crescitelli R, Lässer C, Szabó TG, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Liu Z, Gan L, Zhang T, et al. Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2018;64(1). [DOI] [PubMed] [Google Scholar]
- [117].Jin J, Shi Y, Gong J, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sun L, Zhu M, Feng W, et al. Exosomal miRNA Let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxid Med Cell Longev. 2019;16:4506303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Du X, Zhou L, and Aw YC, et al. ORP5 localizes to ER-lipid droplet contacts and regulates the level of PI(4)P on lipid droplets. J Cell Biol. 2020;219(1):e201905162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cizmar P, Yuana Y. Detection and characterization of extracellular vesicles by transmission and Cryo-Transmission electron microscopy. Methods Mol Biol. 2017;1660:221–232. [DOI] [PubMed] [Google Scholar]
- [122].Noble JM, Roberts LM, Vidavsky N, et al. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J Struct Biol. 2020;210(1):107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7(12):5157–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Whitham M, Parker BL, Friedrichsen M, et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27(1):237–251. [DOI] [PubMed] [Google Scholar]
- [125].Monsivais LA, Sheller-Miller S, and Russell W, et al. Fetal membrane extracellular vesicle profiling reveals distinct pathways induced by infection and inflammation in vitro. Am J Reprod Immunol.2020; 84(3):e13282. [DOI] [PubMed] [Google Scholar]
- [126].Emelyanov A, Shtam T, and Kamyshinsky R, et al. Cryo-electron microscopy of extracellular vesicles from cerebrospinal fluid. 2020;PLoS One. 15(1):e0227949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gámez-Valero A, Campdelacreu J, Vilas D, et al. Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer’s disease and dementia with Lewy bodies. Transl Neurodegener. 2019;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Dasgupta D, Nakao Y, Mauer AS, et al. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology. 2020;159(4):1487–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wang LQ, Liu TL, Liang PH, et al. Characterization of exosome-like vesicles derived from Taenia pisiformis cysticercus and their immunoregulatory role on macrophages. Parasit Vectors. 2020;13(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gardiner C, Ferreira YJ, Dragovic RA, et al. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles. 2013;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Han P, Bartold PM, Salomon C, et al. Salivary small extracellular vesicles associated miRNAs in periodontal Status-A pilot study. Int J Mol Sci. 2020;21(8):2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Zou J, Guo Y, Wei L, et al. Long noncoding RNA POU3F3 and α-Synuclein in plasma L1-CAM exosomes combined with β-Glucocerebrosidase activity: potential predictors of Parkinson’s disease. Neurotherapeutics. 2020;17(3):1104–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chandler WL, Yeung W, Tait JF. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J Thromb Haemost. 2011;9(6):1216–1224. [DOI] [PubMed] [Google Scholar]
- [134].Szatanek R, Baj-Krzyworzeka M, Zimoch J, et al. The methods of choice for Extracellular Vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gu X, Li Y, Chen K, et al. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J Cell Mol Med. 2020;24(13):7515–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Varga Z, Yuana Y, Grootemaat AE, et al. Towards traceable size determination of extracellular vesicles. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Curley N, Levy D, Do MA, et al. Sequential deletion of CD63 identifies topologically distinct scaffolds for surface engineering of exosomes in living human cells. Nanoscale. 2020;12(22):12014–12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chanteloup G, Cordonnier M, Isambert N, et al. Membrane-bound exosomal HSP70 as a biomarker for detection and monitoring of malignant solid tumours: a pilot study. Pilot Feasibility Stud. 2020;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Carnell-Morris P, Tannetta D, Siupa A, et al. Analysis of extracellular vesicles using fluorescence nanoparticle tracking analysis. Methods Mol Biol. 2017;1660:153–173. [DOI] [PubMed] [Google Scholar]
- [140].Wang J, Guo R, Yang Y, et al. The novel methods for analysis of exosomes released from endothelial cells and endothelial progenitor cells. Stem Cells Int. 2016;2639728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Soo CY, Song Y, Zheng Y, et al. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136(2):192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Parsons MEM, McParland D, Szklanna PB, et al. A protocol for improved precision and increased confidence in nanoparticle tracking analysis concentration measurements between 50 and 120 nm in biological fluids. Front Cardiovasc Med. 2017;68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bachurski D, Schuldner M, Nguyen PH, et al. Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles. 2019;8(1):1596016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Zhao H, Shang Q, Pan Z, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235–247. [DOI] [PubMed] [Google Scholar]
- [145].Ying W, Riopel M, Bandyopadhyay G, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–384. [DOI] [PubMed] [Google Scholar]
- [146].Xu A, Wang Y, Keshaw H, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2001;112(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kariba Y, Yoshizawa T, Sato Y, et al. Brown adipocyte-derived exosomal miR-132-3p suppress hepatic Srebf1 expression and thereby attenuate expression of lipogenic genes. Biochem Biophys Res Commun. 2020;530(3):500–507. [DOI] [PubMed] [Google Scholar]
- [148].Chaker D, Mouawad C, Azar A, et al. Inhibition of the RhoGTPase Cdc42 by ML141 enhances hepatocyte differentiation from human adipose-derived mesenchymal stem cells via the Wnt5a/PI3K/miR-122 pathway: impact of the age of the donor. Stem Cell Res Ther. 2018;9(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(1):881–887. [DOI] [PubMed] [Google Scholar]
- [150].Samuelson I, Vidal-Puig AJ. Fed-EXosome: extracellular vesicles and cell-cell communication in metabolic regulation. Essays Biochem. 2018;62(2):0165–175. [DOI] [PubMed] [Google Scholar]
- [151].Fang X, Stroud MJ, and Ouyang K, et al. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight. 2016;1(16):e89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ko SF, Yip HK, Zhen YY, et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient natural killer T-Cell responses, and histopathological features. Stem Cells Int. 2015;853506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Lou G, Chen L, Xia C, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Au Yeung CL, Co NN, Tsuruga T, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13–20. [DOI] [PubMed] [Google Scholar]
- [157].Fan X, Wang J, Qin TZ, et al. Exosome miR-27a-3p secreted from adipoyteytes targets ICOS to promote antitumor immunity in lung adenocarcinoma. Thorac Cancer. 2020;11(6):1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Takahara K, Ii M, Inamoto T, et al. MicroRNA-145 mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem Cells Dev. 2016;25(17):1290–1298. [DOI] [PubMed] [Google Scholar]
- [159].Tian F, Tang P, Sun Z, et al. MiR-210 in exosomes derived from macrophages under high glucose promotes mouse diabetic obesity pathogenesis by suppressing NDUFA4 expression. J Diabetes Res. 2020;6894684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Zhou X, Li Z, Qi M, et al. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10(18):8197–8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Hubal MJ, Nadler EP, Ferrante SC, et al. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25(1):102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kranendonk ME, de Kleijn DP, Kalkhoven E, et al. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc Diabetol. 2014;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Xie Z, Wang X, Liu X, et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc. 2018;7(5):e007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Xing X, Li Z, Yang X, et al. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging (Albany NY). 2020;12(4):3880–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Koeck ES, Iordanskaia T, Sevilla S, et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res. 2014;192(2):268–275. [DOI] [PubMed] [Google Scholar]
- [166].Lopatina T, Bruno S, Tetta C, et al. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Du L, Li G, Yang Y, et al. Exosomes from microRNA-199-3p-modified adipose-derived stem cells promote proliferation and migration of endothelial tip cells by downregulation of semaphorin 3A. Int J Clin Exp Pathol. 2018;11(10):4879–4888. [PMC free article] [PubMed] [Google Scholar]
- [168].Yang Y, Cai Y, Zhang Y, et al. Exosomes secreted by adipose-derived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen-glucose deprivation in vitro through MicroRNA-181b/TRPM7 axis. J Mol Neurosci. 2018;65(1):74–83. [DOI] [PubMed] [Google Scholar]
- [169].Xu F, Xiang Q, Huang J, et al. Exosomal miR-423-5p mediates the proangiogenic activity of human adipose-derived stem cells by targeting Sufu. Stem Cell Res Ther. 2019;10(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Xue C, Shen Y, Li X, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27(7):456–465. [DOI] [PubMed] [Google Scholar]
- [171].Lu Z, Chen Y, Dunstan C. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A. 2017;23(21–22):1212–1220. [DOI] [PubMed] [Google Scholar]
- [172].Du W, Su L, Zhang N, et al. Exosomes derived from preadipocytes improve osteogenic differe-ntiation, potentially via reduced miR‑223 expression. Mol Med Rep. 2019;19(2):951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhu M, Liu Y, Qin H, et al. Osteogenically-induced exosomes stimulate osteogenesis of human adipose-derived stem cells. Cell Tissue Bank. 2021;22(1):77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Chen S, Tang Y, Liu Y, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52(5):e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, et al. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47(1):11–25. [DOI] [PubMed] [Google Scholar]
- [176].Zhao C, Chen JY, Peng WM, et al. Exosomes from adipose‑derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR‑145 and miR‑221. Mol Med Rep. 2020;21(4):1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Ren L, Song ZJ, Cai QW, et al. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem Biophys Res Commun. 2019;508(1):138–144. [DOI] [PubMed] [Google Scholar]
- [178].Zhu CT, Li T, Hu YH, et al. Exosomes secreted by mice adipose-derived stem cells after low-level laser irradiation treatment reduce apoptosis of osteocyte induced by hypoxia. Eur Rev Med Pharmacol Sci. 2017;21(24):5562–5570. [DOI] [PubMed] [Google Scholar]
- [179].Lee KS, Choi JS, Cho YW. Reprogramming of cancer stem cells into non-tumorigenic cells using ste- m cell exosomes for cancer therapy. Biochem Biophys Res Commun. 2019;512(3):511–516. [DOI] [PubMed] [Google Scholar]
- [180].Ching RC, Wiberg M, Kingham PJ. Chwann cell-like differentiated adipose stem cells promote ne- urite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther. 2018;9(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Chen J, Ren S, Duscher D, et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J Cell Physiol. 2019;234(12):23097–23110. [DOI] [PubMed] [Google Scholar]
- [182].Liu CY, Yin G, Sun YD, et al. Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci Ther. 2020;26(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Feng N, Jia Y, Huang X. Exosomes from adipose-derived stem cells alleviate neural injury caused by microglia activation via suppressing NF-kB and MAPK pathway. J Neuroimmunol. 2018;334:576996. [DOI] [PubMed] [Google Scholar]
- [184].Bucan V, Vaslaitis D, Peck CT, et al. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol Neurobiol. 2019;56(3):812–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Heidari N, Abbasi-Kenarsari H, Namaki S, et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236(8):5906–5920. [DOI] [PubMed] [Google Scholar]
- [186].Liu H, Zhang M, Shi M, et al. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res Ther. 2021;12(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Wu J, Yang Q, Wu S, et al. Adipose-derived stem cell exosomes promoted hair regeneration. Tissue Eng Regen Med. 2021;18(4):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Yin G, Yu B, Liu C, et al. Exosomes produced by adipose-derived stem cells inhibit schwann cells autophagy and promote the regeneration of the myelin sheath. Int J Biochem Cell Biol. 2021;132:105921. [DOI] [PubMed] [Google Scholar]


