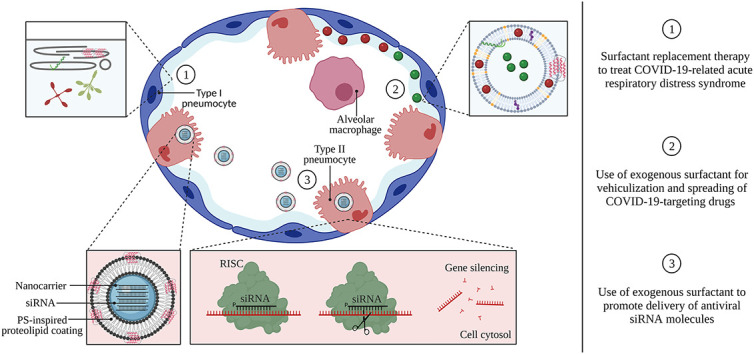
Abstract
The COVID-19 pandemic has wielded an enormous pressure on global health care systems, economics and politics. Ongoing vaccination campaigns effectively attenuate viral spreading, leading to a reduction of infected individuals, hospitalizations and mortality. Nevertheless, the development of safe and effective vaccines as well as their global deployment is time-consuming and challenging. In addition, such preventive measures have no effect on already infected individuals and can show reduced efficacy against SARS-CoV-2 variants that escape vaccine-induced host immune responses. Therefore, it is crucial to continue the development of specific COVID-19 targeting therapeutics, including small molecular drugs, antibodies and nucleic acids. However, despite clear advantages of local drug delivery to the lung, inhalation therapy of such antivirals remains difficult. This review aims to highlight the potential of pulmonary surfactant (PS) in the treatment of COVID-19. Since SARS-CoV-2 infection can progress to COVID-19-related acute respiratory distress syndrome (CARDS), which is associated with PS deficiency and inflammation, replacement therapy with exogenous surfactant can be considered to counter lung dysfunction. In addition, due to its surface-active properties and membrane-interacting potential, PS can be repurposed to enhance drug spreading along the respiratory epithelium and to promote intracellular drug delivery. By merging these beneficial features, PS can be regarded as a versatile biomaterial to combat respiratory infections, in particular COVID-19.
Keywords: Coronavirus disease-19, Inhalation therapy, Lung delivery, Pulmonary surfactant, Nanomedicine, Severe acute respiratory syndrome coronavirus-2, Small-interfering RNA, Antiviral drugs
Graphical abstract
1. Introduction
In December 2019, the Chinese authorities identified severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) as a new emerging β-coronavirus, capable of infecting humans and causing respiratory pathology. Infection with SARS-CoV-2 can result in the development of coronavirus disease-19 (COVID-19), which poses a serious threat to global public health, echoed by an estimated global mortality rate of more than four million people, as reported by the World Health Organization (WHO) [1]. Since its emergence, SARS-CoV-2 has rapidly spread across countries by which, as of March 2020, the WHO officially declared the outbreak as a pandemic. This health crisis led to worldwide efforts to combat COVID-19 by directly targeting SARS-CoV-2 infection (e.g. with antivirals), symptomatic treatment (e.g. via steroids), as well as through the optimization and standardization of supportive care management such as prone positioning and oxygenation [[2], [3], [4]]. All these efforts have the goal to prevent the development of respiratory failure, the most common cause of COVID-19 mortality [5]. Importantly, global vaccination campaigns are currently ongoing and will further progress in the coming months, leading to a reduction of infections, hospitalizations and mortality [6]. However, the availability of both preventive measures as well as (combination) therapies for already infected patients might be most effective. Therefore, in response to the urgency to fight the escalating COVID-19 pandemic, many existing drugs have been screened with the aim to identify compounds that could be successfully repurposed, since such an approach is more time- and cost-efficient and is correlated to higher success rates compared to the development of novel anti-SARS-CoV-2 drugs [[7], [8], [9], [10]]. A variety of compounds from diverse drug classes including antivirals (e.g. remdesivir, favipiravir and lopinavir/ritonavir), antiparasitics (e.g. chloroquine and hydroxychloroquine), monoclonal antibodies (e.g. tocilizumab) and steroids (e.g. dexamethasone) have been investigated in (pre)-clinical trials [11]. In this regard, the broad-spectrum nucleotide mimic remdesivir is approved for emergency use as a post-infection treatment for COVID-19 in around 50 countries [12,13]. Next to this, the WHO strongly recommends the use of dexamethasone in severe COVID-19 patients that require oxygenation or mechanical ventilation [14]. In addition, many groups have investigated the potential of combining different (repurposed) drugs to achieve improved therapeutic outcomes. In this aspect, remdesivir is often combined with anti-inflammatory drugs or immunomodulators to both target SARS-CoV-2 as well as inflammatory pathways. This led to the approval of remdesivir and the immunomodulator baricitinib for emergency use by the Food and Drug Administration (FDA). Similarly, also combination therapies of casirivimab and imdevimab as well as bamlanivimab and estesevimab (i.e. monoclonal antibodies (mAbs) that target the spike protein of SARS-CoV-2) were granted emergency use approval by the FDA [15].
However, apart from this, advancements in the development of SARS-CoV-2-specific therapeutics are limited [16]. This can in part be explained by antiviral drug candidates often having limited influence on critical parameters such as the need for oxygenation or mortality [17]. However, the development of such specific antivirals as an add-on to vaccination is crucial, especially for already infected patients, individuals who are not yet vaccinated or in cases where vaccination does not provide sufficient protection (e.g. upon the emergence of SARS-CoV-2 variants) or causes adverse events (e.g. anaphylaxis and cerebral thrombosis) [18]. In this context, Seley-Radtke, Roche and Altea are developing a nucleotide analog (AT527) to directly target SARS-CoV-2 infection after oral administration, which is currently evaluated in a phase III clinical trial (i.e. the MORNINGSKY trial). The aim is to administer this antiviral drug candidate to both non-hospitalized and hospitalized patients, as well as to prevent infection in people who experienced a high-risk contact [19]. Next to this, two oral antiviral drugs by Merck and Pfizer (i.e. molnupiravir and paxlovid, respectively) are currently under clinical development. Here, molnupiravir was initially developed to treat influenza, while paxlovid is a SARS-CoV-2-specific drug. Both candidates already showed to significantly reduce the number of hospitalizations and deaths of COVID-19 infected patients [20].
Infection with SARS-CoV-2 occurs through binding of the spike protein on the outer shell of the virus to the angiotensin-converting enzyme-2 (ACE-2) receptor, expressed on a variety of host cell types throughout the respiratory system. In particular, there is a significant expression of the ACE-2 receptor on type II pneumocytes, the alveolar epithelial cell type responsible for the production and secretion of PS [[21], [22], [23]]. In severe cases, infection with SARS-CoV-2 leads to the development of CARDS, associated with excessive inflammation, respiratory failure and potentially death [[24], [25], [26], [27]]. The administration of exogenous surfactants to patients suffering from both neonatal (NRDS) and acute respiratory distress syndrome (ARDS) has been widely investigated. This so-called ‘surfactant replacement therapy’ (SRT) is currently the standard-of-care to reduce mortality in premature infants with surfactant-deficient lungs [28]. Although clinical trials have failed to demonstrate improved survival in ARDS [24,29,30], the current COVID-19 pandemic has invigorated the use of exogenous surfactants to treat CARDS, resulting in several ongoing clinical trials [[31], [32], [33], [34], [35]]. In addition to this well-documented application, this review additionally aims to highlight two distinct modes of action of PS, which could prove highly beneficial in the fight against COVID-19. First, due to its surface-spreading properties, PS can be used as a drug delivery vehicle to achieve improved spreading of COVID-19-targeting drugs along the respiratory surface, as well as to support drug delivery into more distal regions such as the alveolar spaces [2,8,36,37]. In addition, formulating drugs inside PS liposomal carriers can hitchhike down the endogenous surfactant recycling pathway to target specific cell types such as type II pneumocytes or alveolar macrophages (AMs) [[38], [39], [40]]. Secondly, besides improving the extracellular availability of various therapeutics in the lung, PS has the newfound potential to promote the intracellular delivery of membrane-impermeable drugs. In particular, inhalation therapy with small interfering RNA (siRNA) is a promising approach to address acute respiratory viral infections, both by targeting viral proteins responsible for infectivity or replication, as well as host-related proteins that play a crucial role in viral infection or disease severity [41,42]. To be functional, siRNA molecules require cytosolic delivery in the target cell of interest, for which they are typically encapsulated into nanoparticles (i.e. nanomedicines). Unfortunately, current state-of-the-art siRNA nanomedicines have difficulties in overcoming the many extra- and intracellular barriers upon topical administration to the lung, leading to inefficient cytosolic delivery [43,44]. In this regard, it was recently disclosed by Raemdonck and co-workers that exogenous PS has the unexpected property of promoting cytosolic siRNA delivery by polymeric nanomedicines [[45], [46], [47], [48], [49], [50]]. As such, exogenous PS can be regarded as a multifaceted biomaterial, enabling both direct treatment of COVID-19-related lung dysfunction as well as improved pulmonary delivery of small molecular- and macromolecular drugs.
2. Severe acute respiratory syndrome coronavirus-2
Coronaviruses belong to the family of Coronaviridae, which is further subdivided in four genera (α, β, γ, δ) [51,52]. They are enveloped, single-stranded (ss) RNA viruses with a genome ranging from 26 to 32 kilobases (kb) [53,54]. The ~30 kb long genome of SARS-CoV-2 encodes four structural proteins including the nucleocapsid (N), spike (S), membrane (M) and envelope (E) proteins [41] and a variety of non-structural proteins such as RNA-dependent RNA polymerase and helicase [55]. The spike protein is responsible for the remarkable morphology shared by Coronaviridae, typified by the projection of crown-like structures on their surface [54,55].
Life-threatening lower respiratory tract infections were reported for severe acute respiratory syndrome coronavirus-1 (SARS-CoV-1) in 2003 and Middle Eastern respiratory syndrome coronavirus (MERS-CoV) in 2012 [56,57]. Since December 2019, SARS-CoV-2, the third virus of the β-coronavirus group, is responsible for the current pandemic [55].
As briefly mentioned above, infection with SARS-CoV-2 occurs through binding of the viral spike protein to the ACE-2 receptor, mainly expressed in the pulmonary system on endothelial cells and type II pneumocytes. Of note, the ACE-2 receptor is also expressed in other organs such as the intestine, heart and kidney, explaining the extrapulmonary symptoms such as diarrhea, nausea, cardiac injury and acute kidney injury [51,58,59]. As shown in Fig. 1 , viral entry occurs via direct fusion as well as via endocytic entry routes. The former mechanism of entry requires initial spike protein priming by a host serine protease, transmembrane protease serine 2 (TMPRSS2), which cleaves the protein into two subunits, S1 and S2. In the case of endocytic entry, the spike protein is activated by cathepsin B and L, two cysteine proteases present in the endosomal compartment, followed by fusion between the viral envelope and the endosomal membrane. In this way, SARS-CoV-2 can also infect host cells that lack TMPRSS2 [[21], [22],]. Once the SARS-CoV-2 genome enters the cytosol, it is transcribed by the viral RNA-dependent RNA polymerase and translated into viral proteins by host cell ribosomes. Finally, mature virions are released via exocytosis, where they can further infect surrounding cells [23].
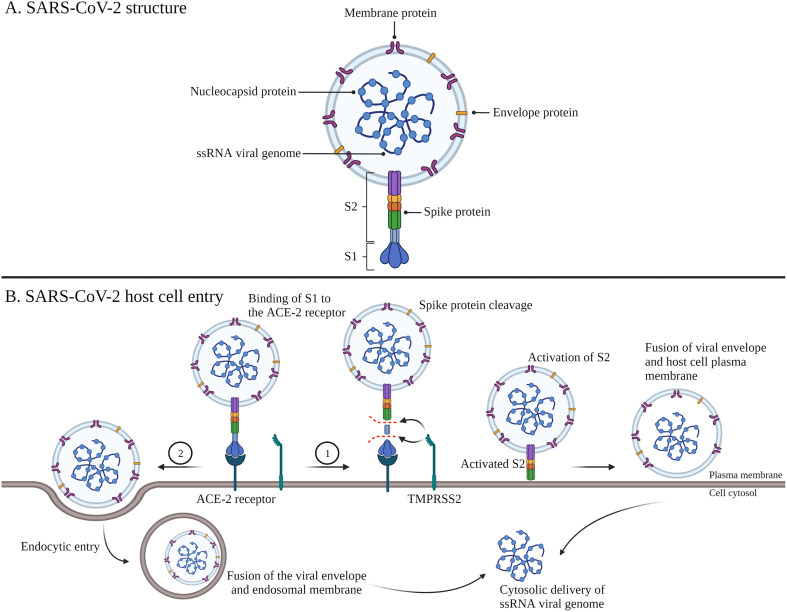
Fig. 1.
SARS-CoV-2 structure and host cell entry mechanisms. The single stranded (ss) RNA viral genome encodes four structural proteins including the nucleocapsid, membrane, envelope and spike protein (A). Binding of S1 of the viral spike protein to the ACE-2 receptor on host cells induces 1) spike protein cleavage by TMPRSS2, followed by activation of S2 and viral internalization via direct fusion of the viral envelope and the host cell plasma membrane or 2) viral internalization via endocytic entry, followed by fusion between the viral envelope and the endosomal membrane (B). Abbreviations: SARS-CoV-2; severe acute respiratory syndrome coronavirus-2, ACE-2; angiotensin-converting enzyme-2, TMPRSS2; transmembrane protease serine 2, S1; subunit 1, S2; subunit 2. Adapted from ‘Mechanisms of SARS-CoV-2 Viral Entry’, by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates
Despite the complete pathophysiological pattern of COVID-19 remaining elusive, infection results in a broad spectrum of clinical manifestations, ranging from asymptomatic to life-threatening. Most common features of moderate COVID-19 include fever, cough, headache, fatigue, myalgia and shortness of breath [53]. Especially older patients and patients with co-morbidities such as obesity, diabetes and cardiovascular disease are at risk to develop respiratory failure with differing levels of hypoxia and diffuse lung infiltrates, associated with multi-organ failure (which necessitates hospitalization in the intensive care unit (ICU) and mechanical ventilation) and a high mortality rate [2,51,54,58,60]. Of note, an increasing number of reports describe persistent complications post-infection that can last more than 4 weeks since the onset of symptoms [[61], [62], [63]].
3. Pulmonary surfactant
3.1. Endogenous pulmonary surfactant: Composition and function
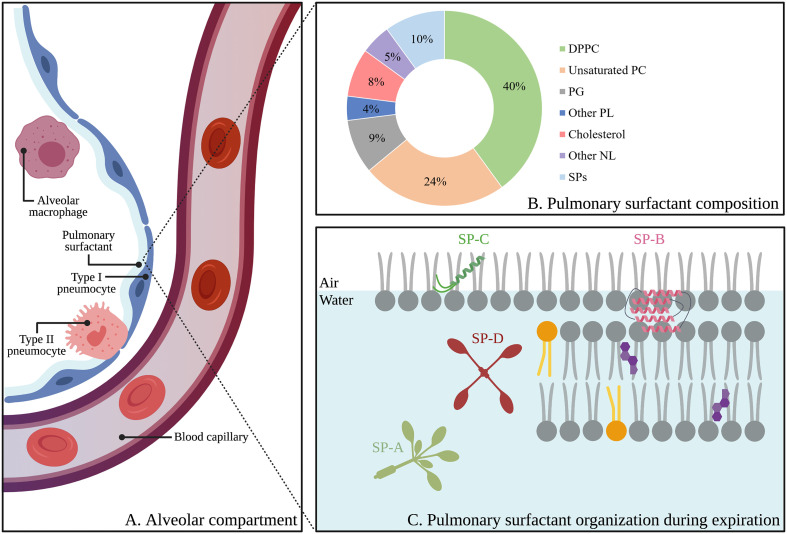
PS is a surface-active biomaterial present at the thin layer of fluid that lines the alveolar epithelial surface, where it lowers the surface tension upon the formation of surfactant films at the air-liquid interface (Fig. 2A) [64]. These interfacial films prevent alveolar collapse during expiration and thereby secure proper gas exchange [2,[65], [66], [67]]. The presence of PS is crucial for pulmonary homeostasis, as its absence, deficiency or inactivation is correlated with severe pulmonary diseases [66,68]. As shown in Fig. 2B, PS is mainly composed of lipids (~90 wt%) with dipalmitoylphosphatidylcholine (DPPC), a saturated phospholipid, being most abundant (~40 wt%). In addition, anionic lipids (~15 wt%) such as phosphatidylglycerol (PG), phosphatidylinositol (PI) and phosphatidylserine (PS) and neutral lipids such as cholesterol (~8 wt%) contribute to this lipid fraction [65,67]. The remaining 10 wt% consists of four highly specialized surfactant proteins (SPs), which can be divided in two groups based on their structural, physicochemical and functional properties, i.e. the large and hydrophilic proteins SP-A (~6 wt%) and SP-D (~1.5 wt%) and the smaller, hydrophobic proteins SP-B (~1 wt%) and SP-C (~1.5 wt%).
Fig. 2.
Schematic representation of the alveolar compartment, including the alveolar epithelium (i.e. type I- and type II pneumocytes), alveolar macrophages and pulmonary surfactant (A). Proteolipid composition of pulmonary surfactant (wt%) (B). Organization of pulmonary surfactant and lipid-protein interactions during expiration, according to the squeeze-out model. Grey, orange and purple lipids represent saturated lipids, unsaturated lipids and cholesterol, respectively (C). Abbreviations: DPPC; dipalmitoylphosphatidylcholine, PC; phosphatidylcholine, PG; phosphatidylglycerol, PL; phospholipid, NL; neutral lipid, SPs; surfactant proteins, SP-A; surfactant protein-A, SP-B; surfactant protein-B, SP-C; surfactant protein-C, SP-D; surfactant protein-D. Created with BioRender.com (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
PS is synthetized by type II pneumocytes and stored in highly packed intracellular organelles called lamellar bodies (LBs), which have a spherical shape and comprise concentrically organized membranes. Subsequently, upon fusion of the LBs with the plasma membrane, PS is secreted into the alveolar lumen where it partly reorganizes into tubular myelin. Tubular myelin consists of multi-layered lipid-protein structures in the liquid phase, stabilized by SP-A. The presence of SP-B and SP-C subsequently induces the adsorption of these structures onto the interface (Fig. 3 ) [69]. Upon expiration, unsaturated lipids and cholesterol are excluded from these interfacial films (i.e. the squeeze-out model), resulting in the formation of a rigid DPPC monolayer that reduces the surface tension to near 0 N/m ( Fig. 2C) [2].
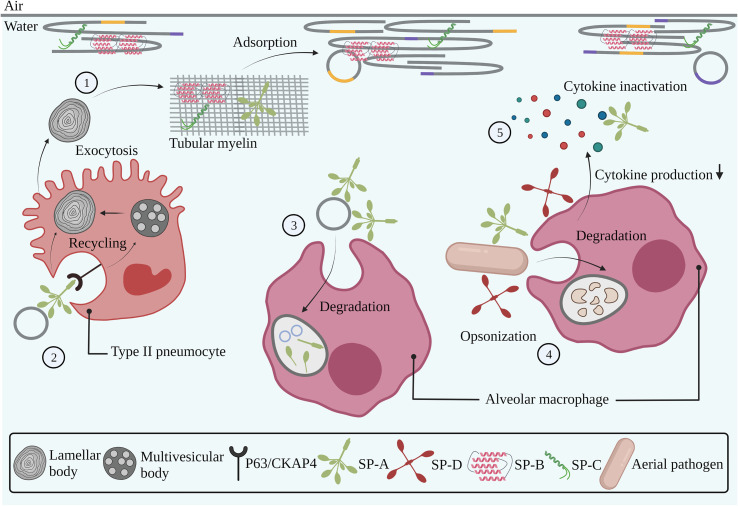
Fig. 3.
Interaction of endogenous pulmonary surfactant with pulmonary cells, cellular membranes and the immune system. Pulmonary surfactant is produced by type II pneumocytes in concentrically organized lamellar bodies, which are partly converted into tubular myelin upon secretion into the alveolar lumen. The presence of SP-B and SP-C in tubular myelin drives the adsorption of surfactant membranes towards the air-liquid interface (1). Binding of SP-A to the P63/CKAP4 receptor expressed by type II pneumocytes induces the uptake and recycling of used surfactant components (2). Degradation of used surfactant components occurs via uptake and phagocytosis by alveolar macrophages (3). SP-A and SP-D are involved in the pulmonary innate immune system via opsonization of aerial pathogens, followed by phagocytosis by alveolar macrophages (4). SP-A and SP-D modulate inflammatory responses via interactions with immune cells, thereby reducing cytokine production, as well as via direct binding and inactivation of soluble cytokines (5). Grey, orange and purple lines represent saturated, unsaturated and cholesterol-rich domains, respectively. Abbreviations: SP-A; surfactant protein-A, SP-B; surfactant protein-B, SP-C; surfactant protein-C, SP-D; surfactant protein-D. Created with BioRender.com (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
About 65% of PS components (i.e. ‘used’ surfactant) is reutilized via recycling mechanisms by type II pneumocytes [70]. As depicted on Fig. 3, after binding of SP-A to the P63/CKAP4 receptor expressed on these cells [71,72], surfactant lipid- and protein complexes (mainly phospholipids and SP-A) are taken up via receptor-mediated endocytosis. While SP-A is secreted into the extracellular space via recycling vesicles, phospholipids are stored in multivesicular bodies (MVBs) and are eventually directed towards LBs, by which the above-mentioned secretion, reorganization- and adsorption processes resume [70].
3.1.1. Hydrophilic surfactant proteins
Next to the well-known biophysical function essential for mammalian breathing, PS also contributes to the pulmonary innate immune system, protecting the alveoli from invading pathogens and other foreign particulate matter [29,65]. These properties are mainly assigned to SP-A and SP-D, two oligomeric proteins that belong to the family of collectins of which the role in the innate immune system can be explained by a dual mode-of-action [65,73]. First, it is reported that SP-A and SP-D have immunomodulatory properties. More specifically, as shown in Fig. 3, these proteins can bind to a variety of receptors on innate immune cells (e.g. AMs and monocytes) via their carbohydrate recognition domain (CRD) or N-terminal region, thereby modulating cytokine production [[74], [75], [76]]. In addition, SP-A and SP-D can directly bind to soluble pro-inflammatory mediators such as tumor necrosis factor (TNF)α and lipopolysaccharide (LPS), which results in the inhibition of their downstream inflammatory actions (Fig. 3) [65,77,78]. Secondly, SP-A and SP-D bind to carbohydrates, phospholipids and glycolipids present on bacteria, viruses, yeasts and fungi, as well as to neutrophils and DNA via their CRD. This so-called ‘opsonization’ of pathogens and particles results in phagocytosis by immune cells such as AMs (Fig. 3) [[79], [80], [81]]. Next to its crucial role in the protection of alveoli against potentially harmful matter, it is reported that SP-D contributes to surfactant homeostasis, as its absence results in the accumulation of PS in the airways due to an aberrant recycling process, although the exact underlying mechanism is still unknown [82,83].
3.1.2. Hydrophobic surfactant proteins
SP-B and SP-C are cationic and amphiphilic proteins, found in and between PS-associated lipid bilayers, and are essential for surfactant adsorption and stabilization at the alveolar air-liquid interface. Both proteins modulate membranes through combined electrostatic and hydrophobic protein-lipid interactions via cationic and aromatic residues, respectively [2,69,84,85]. SP-B belongs to the saposin-like protein (SAPLIP) family. All members of this family are characterized by a so-called ‘saposin fold’, consisting of α-helical domains, six conserved cysteines that form three intramolecular disulfide bridges as well as conserved hydrophobic regions [86]. As depicted in Fig. 2B, the SP-B monomer (79aa) has a complex three-dimensional structure consisting of five cationic amphiphilic α helices that mediate its peripheral orientation in surfactant membranes. Moreover, via an intermolecular disulfide bridge, SP-B assembles into a covalent homodimer, which is believed to be important for its surface activity and the promotion of membrane-membrane interactions, of which the latter may induce membrane fusion [87,88].
In addition, SP-B homodimers can assemble into larger oligomers with a ring-shaped channel configuration. These oligomers are believed to mediate translocation of lipids between membranes and towards the air-liquid interface, a key initial step to obtain proper surfactant structure and alveolar dynamics. Moreover, next to its role in dense packing of surfactant components in LBs, it is described that extracellular SP-B also induces the secretion of PS from these intracellular organelles via interaction with and activation of receptors on the plasma membrane of type II pneumocytes. The pleiotropic effects of SP-B at the alveolar air-liquid interface appear indispensable for mammalian breathing, as SP-B deficiency, for example due to mutations in the SP-B gene SFTPB, leads to fatal respiratory failure [89,90].
SP-C is a 35aa lipoprotein that, in contrast to SP-B, displays a transmembrane orientation due to its α-helical conformation and the presence of two palmitoylated cysteine residues (Fig. 2B) [91]. Although the absence of SP-C in PS is correlated to chronic respiratory diseases, it does not fully impair breathing as is the case with SP-B deficiency [92,93]. In this regard, although the specific role of SP-C in surfactant layers is not yet fully understood, it is clear that its presence is crucial for proper surfactant structure, recycling and dynamics during repetitive breathing cycles [94,95].
Next to the importance of SP-B and SP-C in surfactant dynamics and stability as described above, recent studies have elucidated the contribution of these proteins, together with phospholipids, to immunomodulatory processes, based on their anti-inflammatory and antibacterial properties [65,[96], [97], [98], [99], [100], [101], [102]]. More in-depth information on the structure and function of the SPs can be found in comprehensive reviews on the topic [83,88,[103], [104], [105]].
3.2. Exogenous pulmonary surfactant: Surfactant replacement therapy
PS has become the standard-of-care in the prevention and treatment of NRDS after Avery and Mead discovered the correlation between NRDS and surfactant deficiency [28,106]. Since the lungs of preterm infants are not yet fully developed, there is no production of endogenous surfactant, requiring the supplementation of exogenous clinical surfactants to avoid alveolar collapse and respiratory failure [24,68].
3.2.1. First-generation synthetic surfactant preparations
The clinical use of exogenous surfactant is not a recent finding. The potential of SRT was already discovered in 1953, where the nebulization of the non-ionic detergent Triton WR-1339 (Alevaire®) was probed as a treatment for neonatal asphyxia. Since its administration was associated with fatal outcomes, additional efforts were done to improve safety and efficacy of SRT. Further research led to the development of first-generation protein-free synthetic surfactants such as Colfosceril Palmitate (Exosurf®), which contains a mixture of DPPC and two spreading agents (i.e. hexadecanol and tyloxapol) [107] and Pumactant (ALEC®), composed of DPPC and PG (Table 1 ) [108].
Table 1.
Protein- and phospholipid composition of surfactant preparations of different origin.
| Preparation | Origin | SP-B | SP-C | Phospholipids (PL) | Others | Ref. |
|---|---|---|---|---|---|---|
| First-generation synthetic surfactant preparations | ||||||
| Colfosceril (Exosurf®) |
Synthetic | – | – | 13.5 mg/mL (DPPC) |
Hexadecanol Tyloxapol |
[109] |
| Pumactant (ALEC®) |
Synthetic | – | – | 40 mg/mL (DPPC, PG) |
– | [109] |
| Animal-derived surfactant preparations | ||||||
| Poractant Alfa (Curosurf®) |
Minced porcine lung extract | 2–3.7 mg/mM PL |
5–11.6 mg/mM PL | 80 mg/mL |
Tripalmitoylglycerol Palmitic acid |
[109,110] |
| Beractant (Survanta®) |
Minced bovine lung extract | 0–1.3 mg/mM PL |
1–20 mg/mM PL |
25–30 mg/mL |
– | [109,110] |
| Calfactant (Infasurf®) |
Calf lung lavage | 5.4 mg/mM PL |
8.1 mg/mM PL |
35 mg/mL |
– | [109,110] |
| SF-RI I (Alveofact®) |
Bovine lung lavage | 2–5.6 mg/mM PL |
1–12 mg/mM PL |
40 mg/mL |
– | [109] |
| Second-generation synthetic surfactant preparations | ||||||
| Lucinactant (Surfaxin®) |
Synthetic | 19.8 mg/mM PL (KL4) |
– | 30 mg/mL (DPPC, POPG) |
Palmitic acid | [109] |
| CHF5633 (Under clinical investigation, phase II) |
Synthetic | 0.2% (Mini-Bleu) |
1.5% (SP-C33Leu) |
80 mg/mL (DPPC, POPG) |
– | [111,112] |
| Minisurf (Under preclinical investigation) |
Synthetic | 3% (SMB) |
– | DPPC, POPC, POPG | – | [111] |
3.2.2. Animal-derived surfactant preparations
The discovery of the importance of SP-B and SP-C for rapid adsorption and stability of surfactant membranes paved the way towards the use of animal-derived surfactants [[113], [114], [115], [116]]. Indeed, studies that compared animal-derived surfactants and protein-free synthetic surfactants showed that the latter failed to reduce the surface tension, due to the lack of SP-B and SP-C. In addition, it is reported that the administration of animal-derived surfactants results in lower needs for ventilation, reduced risk of pneumothorax and a lower mortality rate. Consequently, protein-free synthetic preparations were withdrawn from the market [117,118].
A variety of animal-derived surfactants, which differ in origin and proteolipid content, are widely used in NRDS management (Table 1). In 1983, Curstedt and Robertson were the first to investigate the porcine-derived Poractant Alfa (Curosurf®) [2,119]. Following studies led to the development of other animal-derived surfactants such as Beractant (Survanta®), Calfactant (Infasurf®) and SF-RI I (Alveofact®), all from bovine origin [103,108,120].
Various studies have compared the efficacy among different animal-derived preparations, however with conflicting results. In general, there are no significant differences between preparations from bovine origin. On the contrary, different studies reported on the superiority of Curosurf®, compared to both Survanta® and Infasurf®, albeit the administered dose of Curosurf® was systematically higher. Therefore, in order to draw clear conclusions, randomized and controlled trials that compare the efficacy of animal-derived surfactants at the same dose are needed [121,122].
3.2.3. Second-generation synthetic surfactant preparations
Animal-derived preparations are associated with batch-to-batch variabilities, limited supply and a theoretical risk of pathogen transmission. Together with the fact that extraction and purification from animal lungs are time-consuming and expensive, the focus of SRT has shifted to the development of synthetic alternatives [103]. Due to the complex three-dimensional structure and the high hydrophobicity of SP-B and SP-C, production of their complete native form via chemical synthesis or recombinant technologies without loss of function is not yet possible [103,123]. Therefore, there is a need to develop simpler SP-B and SP-C analogues or mimics that can, at least partially, replicate their structure and, most importantly, their biophysical function.
KL4 (21aa, sinapultide) is a cationic amphiphilic peptide with four lysine (K)-tetraleucine (L4) repeats that was clinically approved by the FDA in 2012 as the first synthetic peptide for substitution of SP-B in clinical surfactants. The sequence of KL4 is based on the hydrophobic/hydrophilic ratio in the C-terminal part of SP-B. Upon combination with DPPC, palmitoyloleoylphosphatidylglycerol (POPG) and palmitic acid, this synthetic formulation (Surfaxin®) is as effective as its animal-derived counterparts in the treatment of NRDS [123,124].
Given the importance of both the N- and C-terminal segment for the surface activity of SP-B, Walther and Waring studied the in vitro and in vivo surface activity of Mini-B (MB) and Super Mini-B (SMB), peptides that comprise two amphiphilic α helices of native SP-B, located at the N- and C termini, which are linked by two disulfide bridges [125]. The SMB peptide additionally contains the seven first N-terminal amino acids (FPIPLPY) [126]. The high hydrophobicity of this sequence fosters interaction with surfactant membranes, explaining the higher surface activity of SMB compared to MB [127,128]. Additional efforts to develop a peptide with increased hydrophobicity and α-helical structure as well as reduced susceptibility to oxidation led to the design of Mini-BLeu, a MB analogue in which two methionine residues are replaced by leucine [129].
In contrast to SP-B, the specific amino acid composition of SP-C seems to be less important for its biophysical function, since SP-C analogues that solely mimic the α-helical structure of the transmembrane part of native SP-C can fully replicate its surface-active properties [130,131]. This observation led to the development of SP-C33Leu, which deviates from native SP-C with respect to the removal or substitution of specific amino acids [103,130,132].
Currently, CHF5633 is the most advanced synthetic surfactant preparation and is under clinical investigation in a phase II trial for NRDS after proof of equal efficacy compared to Curosurf® and Survanta® in in vitro and in vivo models [[133], [134], [135], [136]]. CHF5633 contains peptide analogues of both SP-B and SP-C (i.e. Mini-BLeu and recombinantly synthesized SP-C33Leu), together with DPPC and POPG [2,103]. In addition, a surfactant preparation that contains a mixture of DPPC, palmitoyloleoylphosphatidylcholine (POPC), POPG and SMB (i.e. Minisurf), is currently under preclinical investigation [137].
4. Pulmonary surfactant in the fight against COVID-19
4.1. Surfactant replacement therapy to treat COVID-19-related acute respiratory distress syndrome
The clinical success of SRT in NRDS has rationalized attempts to broaden the therapeutic application of clinical surfactants to other lung diseases like ARDS. ARDS is a severe form of acute lung injury [25] and is incited by direct lung insults (e.g. due to pneumonia or gastric content aspiration) as well as indirect systemic causes (e.g. extrapulmonary sepsis, nonthoracic trauma or burn injury) and is associated with high morbidity and mortality [[25], [138], [139]]. Clinical manifestations include surfactant deficiency and inactivation [2,66], pulmonary edema, hypoxemia and suppressed respiration [64]. These symptoms are the result of acute outbursts of pro-inflammatory cytokines and an affected endothelial barrier, which lead to a continuous cycle of pulmonary inflammation and tissue damage [25,[39], [140], [141], [142]]. Due to conflicting clinical outcomes, with several clinical trials on adult patients showing no improvement in survival despite enhanced oxygenation, currently no comparable SRT is available to treat patients suffering from ARDS [24,29,30]. The main reason for these suboptimal outcomes is that next to the underlying surfactant deficiency, also inactivation of endogenous and exogenous surfactant occurs due to the presence of surface-active serum proteins and inflammatory molecules in the alveolar lumen, as will be explained later [139].
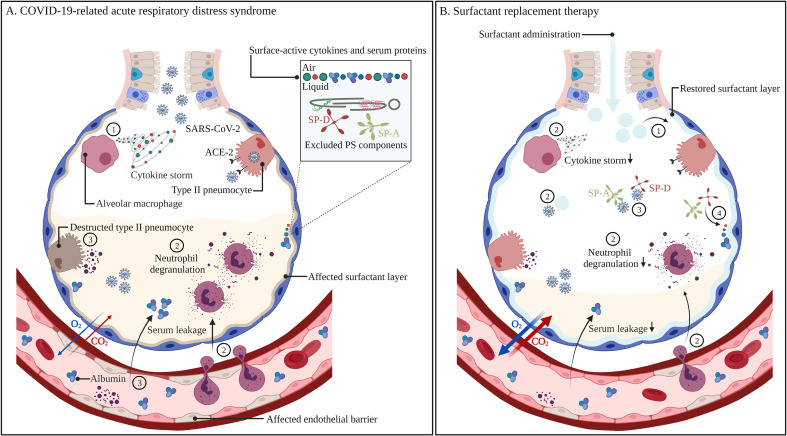
The high expression level of the ACE-2 receptor on the cellular surface of type II pneumocytes makes this cell type particularly vulnerable to infection with SARS-CoV-2. As a result, SARS-CoV-2 infection is reported to result in decreased endogenous PS levels, as well as altered PS composition and mutations [143]. Of importance, COVID-19-related mortality is mostly caused by a continuous, uncontrolled and exaggerated immune response. This is explained by the recruitment of inflammatory cells such as monocytes and macrophages, which subsequently secrete cytokines and chemokines, resulting in a so-called ‘cytokine storm’. Subsequent neutrophil influx and degranulation leads to the destruction of type II pneumocytes and endothelial cells, resulting in the impairment of surfactant production and secretion, a disrupted endothelial barrier, serum leakage into the alveolar spaces and the development of CARDS (Fig. 4 ) [[24], [25], [26], [27],144]. Moreover, the ACE-2 receptor is involved in anti-inflammatory and antifibrotic mechanisms, which protect the lung from injuries. Extensive binding of SARS-CoV-2 to this receptor thus reduces its lung-protective capacities [27].
Fig. 4.
Non-treated (A)versus pulmonary surfactant-treated COVID-19-related acute respiratory distress syndrome (B). Infection with SARS-CoV-2 results in the recruitment of alveolar macrophages, which produce high levels of cytokines, also referred to as a cytokine storm (1). Subsequent neutrophil recruitment and degranulation (2) leads to the destruction of type II pneumocytes and endothelial cells, resulting in reduced surfactant production and secretion, serum leakage in the alveolar spaces and surfactant inactivation by surface-active cytokines and serum proteins that adsorb to the air-liquid interface, thereby excluding endogenous PS components (3). Improper surfactant function leads to collapsed alveoli, aberrant gas exchange and respiratory failure. The administration of exogenous surfactant can supplement the affected endogenous PS pool (1), as well as dampen the inflammatory response via interactions with immune cells, cytokines and SARS-CoV-2 (2). Exogenous SP-A and SP-D can prevent viral infection via binding and neutralization of the spike protein, thereby preventing its interaction with the ACE-2 receptor on type II pneumocytes (3). Exogenous SP-A and SP-D grants more resistance towards surfactant inactivation (4). Recovery of the surfactant layer as well as reduced inflammation leads to less cellular damage, reduced serum leakage in the alveolar spaces, enhanced gas exchange and thus the prevention of respiratory failure. Abbreviations: COVID-19; coronavirus disease-19, SARS-CoV-2; severe acute respiratory syndrome coronavirus-2, ACE-2; angiotensin-converting enzyme-2, SP-A; surfactant protein-A, SP-D; surfactant protein-D. Created with BioRender.com
Surprisingly, CARDS does not follow the common clinical pattern in terms of lung mechanics, alveolar damage and radiological presentations typically seen with ARDS [145], but is rather classified as an atypical pneumonia that resembles the pathophysiology of NRDS [24].
Based on the distinctive clinical manifestations of CARDS and the correlation to surfactant deficiency and inactivation, it is clear that results from past ARDS trials cannot simply serve as a reference to its potential in treating patients with CARDS [145]. Therefore, surfactant treatment to anticipate on the progression of severe lung injury in COVID-19 patients has been proposed as a potential treatment for COVID-19 [2,24,30]. Indeed, a study by Gerosa et al. showed that infection with SARS-CoV-2 results in the overexpression of SP-A inside the alveolar spaces. Here, it is important to note that SP-A is present in rather condensed masses, which implies its inactivation or dysregulation [146]. Another study by Islam et al. subsequently showed that, next to affected levels and structures of SP-A, there is a dysregulation of a variety of genes involved in pulmonary surfactant production and activation, as well as its turnover and metabolism [147]. Next to this, Kerget et al. reported a significant increase of SP-D levels in blood samples of infected vs. non-infected individuals. Importantly, SP-D levels were higher in patients who developed CARDS and who did not survive SARS-CoV-2 infection. These results highlight the importance of serum SP-D as a biomarker for disease progression, as well as underscore the potential of SRT in severely ill COVID-19 patients due to the leakage of SP-D from the alveolar spaces into the systemic circulation, induced by an affected alveolar integrity [148]. Likewise, a study by Tong et al. revealed that serum SP-D levels were significantly higher in patients with severe COVID-19 [149].
As shown in Fig. 4, the administration of exogenous surfactants is reported to have several beneficial features, i.e. (1) restoration of the endogenous surfactant pool, thereby reducing the surface tension and work of breathing, and (2) mitigating the activation of the innate immune system and preventing inflammatory damage, which is mainly assigned to the presence of anionic lipids in the surfactant preparation. POPG and PI are reported to have immunomodulatory properties via interaction with toll-like receptors (TLRs) and viruses. More specifically, in vitro experiments revealed that these anionic lipids are capable of suppressing the activation of both TLR2 and TLR4 in AMs [[99], [150]]. In addition, in vitro experiments in primary human bronchial epithelial cells (HBECs) showed that both POPG and PI could bind to respiratory syncytial virus (RSV), thereby preventing infection and production of interleukin (IL)-6 and IL-8. These findings were confirmed in vivo in RSV-infected mice, where intranasal administration of POPG and PI resulted in a decreased neutrophil- and lymphocyte count in lung lavage, as well as a significant reduction in viral load [[98], [151], [152]]. Likewise, in vitro studies in HBECs and Madin-Darby Canine Kidney (MDCK) cells showed that POPG and PI prevent infection with H3N2 influenza A virus (IAV). Subsequent in vivo studies also demonstrated protection of mice from H1N1-IAV infection [153].
It is worth mentioning that the optimal composition and origin of surfactant preparations for the treatment of CARDS and NRDS might differ. First, all clinical surfactants currently on the market to prevent NRDS are devoid of the hydrophilic proteins SP-A and SP-D, albeit that their presence can add to a more effective treatment of CARDS. SP-A and SP-D could promote the clearance of SARS-CoV-2 through binding of their CRD to the glycosylated spike protein [154], as well as modulate the inflammatory response correlated to infection and associated with disease severity (Fig. 4) [154,155]. Earlier in vitro studies already demonstrated that SP-A and SP-D have antiviral properties against CoVs, where SP-A and SP-D were able to bind and neutralize HCoV-229E [156,157]. Furthermore, the antiviral properties of SP-A and SP-D have been confirmed for a variety of other viruses [[158], [159], [160], [161], [162]].
As shown in Fig. 4, the addition of SP-A and SP-D can also grant more resistance to surfactant inactivation [2,163]. A damaged endothelial barrier results in the leakage of serum into the alveolar lumen, as extensively described in severely ill COVID-19 patients suffering from CARDS. In the same manner as surface-active inflammatory molecules (e.g. IL-1 and TNF), surface-active serum proteins (e.g. albumin) can adsorb to the air-liquid interface, creating a steric and electrostatic barrier that prevents effective surfactant adsorption [[164], [165], [166]]. Interestingly, recent studies have shown that the addition of non-adsorbing hydrophilic macromolecules such as polyethyleneglycol (PEG) and hyaluronic acid also prevents surfactant inactivation in a similar way [139].
Since animal-derived SP-A and SP-D could also induce immunogenic reactions in adults, addition of these proteins to the formulation would require synthesis of human homologues [2]. Efforts to develop recombinant fragments of SP-A (rfhSP-A) and SP-D (rfhSP-D) have been undertaken. Most importantly, these fragments are able to mimic the antipathogenic and immunomodulatory properties of the native proteins [105]. In this regard, a recent study by Madan et al. reported that rfhSP-D successfully neutralized SARS-CoV-2 and reduced infection in clinical samples, where it is more potent in inhibiting viral replication and infectivity compared to remdesivir [167]. In addition, it has been shown that rfhSP-D binds to the S1 subunit of the viral spike protein, thereby preventing its interaction with the ACE-2 receptor [168].
Secondly, focusing on the development of synthetic surfactants might become even more important in the treatment of CARDS. Indeed, synthetic surfactants seem more resistant to inactivation [169]. In addition, it has been reported that CHF5633 reduces LPS-induced pro-inflammatory cytokine release from human monocytes, a valuable property given the marked inflammatory nature of COVID-19 [[170], [171], [172]].
In addition to higher doses and/or more frequent administrations [173], also an alternative pulmonary delivery mode might be required. In neonates suffering from NRDS, surfactants are typically administered via an intratracheal bolus instillation, yet this delivery mode is invasive and associated with a non-homogenous distribution throughout the lung tissue. Taking into account the larger surface area of the conducting airways in adults, there is a risk for more significant loss of surfactant along its way to the alveoli. Therefore, non-invasive delivery modes such as surfactant aerosolization using ultrasonic or jet nebulizers are currently under development [[174], [175], [176], [177]], and have already proven to be safe in animal models without functional alterations of the surfactant preparation [[178], [179], [180]].
Based on the above-mentioned beneficial features of surfactant administration to COVID-19 patients, several clinical trials have been initiated to investigate the safety, efficacy and practical feasibility of this treatment option and are summarized in Table 2 [2,181].
Table 2.
Overview of ongoing clinical trials investigating the potential of surfactant replacement therapy in COVID-19 management. Abbreviations: NA; not announced, BLES; bovine lipid extraction surfactant.
| Preparation | Dose | Dose frequency | Initiation | Delivery mode | Sample size | Phase | Ref. |
|---|---|---|---|---|---|---|---|
| Bovactant (Alveofact®) | 1080–3240 mg/kg (45 mg/mL) |
3/day | Within 24 h of ventilation | Nebulization | 24 | NA | [31] |
| BLES® | 50 mg/kg (27 mg/mL) |
≤ 3/day | As soon as possible/within 48 h of ventilation | Intratracheal instillation | 20 | I/II | [32] |
| Poractant Alfa (Curosurf®) | 48 mg/kg (16 mg/mL) |
NA | Within 72 h of ventilation | Endobronchial administration | 20 | II | [33] |
| Lucinactant (Surfaxin®) | 80 mg/kg | NA | At the time of ventilation | Intratracheal instillation | 30 | II | [34] |
| Poractant Alfa (Curosurf®) | 30 mg/kg (80 mg/mL) |
3/day | Within 48 h of ventilation | Intratracheal instillation | 85 | II | [35] |
In addition, the off-label use of surfactants to treat CARDS has already been reported in the literature.
Busani et al. reported on the administration of Curosurf® to five critically ill patients suffering from COVID-19-related pneumonia and low lung compliance. Patients received surfactant during one month at a dosage of 30 mg/kg via intratracheal intubation, initiated shortly after the onset of invasive mechanical ventilation. The results showed improvement of oxygenation after one hour in four patients and after six hours in all patients. Additionally, over the course of 30 days, a survival rate of 80% was observed despite the critical condition of the included patients [181].
A single-center retrospective case-control pilot study was reported by Piva et al., where seven COVID-19 patients suffering from CARDS received Curosurf® through bronchoscopy for two months at a dose of 720 mg in 150 mL normal saline for a total of ten instillations. These patients were matched to 14 COVID-19 patients with similar disease severity, receiving only supportive care. Despite the infection risk for healthcare providers related to the bronchoscopy procedure, the personnel did not develop COVID-19, indicating the feasibility of surfactant therapy via this delivery mode. In addition, a favorable safety profile was observed, where none of the patients receiving surfactant developed acute decompensation. Despite the limited and preliminary data regarding treatment efficacy, surfactant delivery and retention in the lungs following bronchoscopy was confirmed. However, future clinical trials using this mode of delivery are required to confirm a possible reduction in total time on mechanical ventilation and long-term mortality [182].
Heching et al. reported on the administration of Infasurf® at a dosage of 20 mg/kg to a critically ill COVID-19 patient. Surfactant was directly dispersed into the lungs via a tracheobronchial suction catheter passed through the endotracheal tube. An improvement of oxygenation was seen after 18 h, which further improved after 48 h and resulted in the removal of the patient from extracorporeal membrane oxygenation (ECMO) and extubation [29].
Although validation on the efficacy, safety and feasibility by sufficiently powered clinical trials is required, the outcomes as mentioned above already give an optimistic indication on the potential of exogenous surfactant administration to COVID-19 patients.
4.2. Use of exogenous pulmonary surfactant for vehiculization and spreading of COVID-19-targeting drugs
As the lungs are the major target tissue of SARS-CoV-2 infection, pulmonary delivery of COVID-19-targeting drugs is an attractive alternative for intravenous administration. Local application to the lung has a variety of advantages as it is non-invasive, grants direct access to target cells, allows a rapid onset of action and prevents systemic side effects [183,184]. A variety of new and existing drug molecules and approaches are envisioned as a potential treatment for COVID-19, including antiviral therapies as well as therapeutics that target pathophysiological pathways associated to infection [2]. In this regard, this section will discuss the potential to exploit exogenous PS as a drug delivery vehicle or carrier to enhance drug spreading and adsorption or drug targeting to specific cell types after local pulmonary delivery, leading to improved therapeutic outcomes.
4.2.1. Antivirals
Antiviral drugs might be most suitable for treating COVID-19, as they specifically target the viral life cycle and viral spreading. As the pandemic unfolded, the number of clinical trials that evaluated the repurposing of existing drugs as antivirals concurrently increased, since this repurposing strategy has several advantages compared to the development of new drugs [[7], [185]].
However, the randomized evaluation of COVID-19 therapy (RECOVERY) trial showed no clinical benefit from the use of hydroxychloroquine or lopinavir/ritonavir [186,187]. As a result, Gilead's remdesivir, a repurposed antiviral drug that acts as a nucleoside analogue to inhibit the RNA-dependent RNA polymerase of SARS-CoV-2 [188], is the only antiviral drug approved for the treatment of COVID-19 thus far. The approval of remdesivir was grounded on three randomized controlled trials, where the administration of remdesivir resulted in a shorter recovery time and a reduction in respiratory tract infection compared to the placebo group.
4.2.2. Antibodies
Another approach to prevent viral entry is the development of mAbs or antibody-based products that for example bind the viral spike protein, thereby blocking its interaction with the ACE-2 receptor. In this regard, Wrapp et al. reported successful neutralization of the SARS-CoV-2 spike protein by heavy chain-only antibodies (HCAbs). Moreover, by the construction of a bivalent Fc-fusion (i.e. a tail-to-head combination of two HCAbs fused to the Fc domain of human IgG1), it also neutralizes SARS-CoV-2 pseudoviruses [52]. Likewise, Koenig et al. screened an HCAb library, where four HCAbs seemed to efficiently neutralize SARS-CoV-2 via binding to two distinct epitopes on the RBD, which allows combination of HCAbs to obtain improved neutralization efficiencies. In this regard, a bivalent HCAb (i.e. VHH VE) was able to neutralize SARS-CoV-2 by a rather unusual mode-of-action. Here, the binding of VHH VE to the RBD induced fusion, leading to irreversible changes in spike protein structure, thereby preventing its subsequent interaction with the ACE-2 receptor on host cell membranes. Importantly, VHH VE was also able to neutralize SARS-CoV-2 variants [189]. Focusing on inhalation therapy, HCAbs have several advantages compared to conventional mAbs. First, they have a high robustness as they are able to refold after denaturation. In addition, they are small and have a high thermal- and chemical stability and solubility. Next to targeting the spike protein, also neutralization of inflammatory molecules such as interleukins can be of interest. In this regard, the RECOVERY trial probed the potential of tocilizumab, a recombinant humanized anti-IL-6 mAb. Results showed improved survival in hospitalized COVID-19 patients with confirmed hypoxemia and systemic inflammation [190].
4.2.3. Corticosteroids
Since COVID-19 disease severity is correlated to inflammatory lung injury, the administration of corticosteroids is an arguable treatment option to temper inflammation pathways and prevent the development of respiratory failure. In this regard, the RECOVERY trial investigated the impact of oral and intravenous administered dexamethasone (once daily, 10 days) on the 28-day mortality. Dexamethasone treatment resulted in a lower mortality rate in patients that required invasive mechanical ventilation or oxygenation, compared to patients receiving usual care. As a result, next to remdesivir, also dexamethasone was approved for hospitalized COVID-19 patients requiring oxygenation with or without mechanical ventilation. Given the many advantages of local pulmonary delivery as described above and its wide application in other respiratory diseases including asthma and COPD, also inhalation corticosteroids (ICS) have been considered for local COVID-19 treatment. Ramakrishnan et al. performed a randomized open-label controlled phase II trial (i.e. steroids in COVID-19, STOIC) of inhaled budesonide within the first week of mild COVID-19 symptom onset. Results showed that early administration of inhaled budesonide reduced the hazard of urgent medical care requirements and shortened the recovery time, compared to patients receiving conventional care [191]. A recent study by Yamaya et al. showed that pre-treatment of primary human nasal- and tracheal epithelial cells with a combination of budesonide, glycopyrronium and formoterol lowered viral titers of HCoV-229E and modulated infection-induced inflammation by decreasing the release of IL-6, IL-8 and IFNβ [192]. In addition, budesonide and formoterol lowered TNFα release induced by other respiratory viruses such as rhinoviruses and RSV in bronchial epithelial cells in vitro [193]. Notably, recent studies reported on the clinical potential of inhaled ciclesonide. Upon local administration, this corticosteroid has an anti-inflammatory effect as well as antiviral activities, as it lowers SARS-CoV-2 replication and cytotoxic potential in Vero cells in vitro [194]. Following this data, Covis Pharma group recently announced favorable safety and efficacy results from a double-blind randomized controlled phase III trial investigating ciclesonide administration via a metered-dose inhaler (MDI) in non-hospitalized, symptomatic COVID-19 patients. Results showed faster relief of symptoms and a reduced risk for hospitalization by day 30, compared to placebo. Importantly, no adverse events were reported up to 60 days post-treatment [195]. To improve therapeutic results upon inhalation, Lammers et al. described the promise of dexamethasone nanomedicines. As the encapsulation of drugs into nanoparticles results in enhanced phagocytosis by AMs, dexamethasone could be more specifically delivered to the cells that play a key role in lung inflammation [196].
4.2.4. Pulmonary surfactant-assisted drug delivery and spreading
In general, despite the availability of many interesting drugs to treat COVID-19, deep penetration of drugs into the lungs and drug deposition in the alveolar spaces is challenging. Impaction of aerosolized drugs on bifurcations will lead to mucociliary clearance and hence subtherapeutic outcomes. Most important, as described above, critically ill COVID-19 patients additionally suffer from acute respiratory distress, which is correlated with the development of hypoxic, collapsed lung regions. These regions are even more difficult to reach following inhalation therapy due to a reduced surface area that is accessible for drug deposition and the inability of the patients to properly use inhalation devices [36].
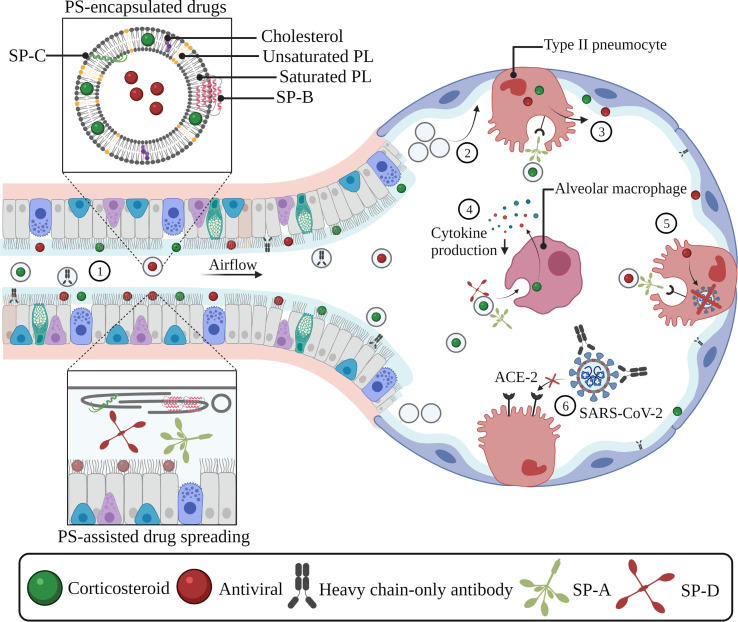
Therefore, recent studies have evaluated the use of exogenous PS as a vehicle or liposomal carrier for inhalation therapy of COVID-19-targeting drugs. Upon so-called ‘surfactant vehiculization’ (i.e. co-delivery of drugs and an exogenous surfactant preparation, Fig. 5 ), one can expect improved drug spreading and adsorption along the entire respiratory epithelial surface due to the excellent and rapid surface-spreading of PS, followed by drug release during consecutive compression/expansion cycles upon breathing [2,37]. Owing to the ability of exogenous surfactant to improve lung dynamics as mentioned above, this treatment strategy can be seen as a double-edged sword to attain improved therapeutic outcomes and provides new opportunities for local pulmonary drug delivery, e.g. in CARDS.
Fig. 5.
Exogenous pulmonary surfactant as a vehicle or liposomal carrier for COVID-19-targeting drugs. Encapsulation of drugs inside exogenous PS can improve drug spreading and adsorption along the entire pulmonary epithelium (1). The administration of exogenous surfactant can supplement the reduced or inactivated endogenous surfactant pool, which prevents alveolar collapse and facilitates the delivery and deposition of inhaled drugs in deeper lung regions (2). SP-A-mediated uptake in type II pneumocytes induces drug- and surfactant recycling, which allows further drug spreading along the alveolar interface (3). SP-A- and SP-D-mediated drug internalization by alveolar macrophages allows anti-inflammatory drugs (e.g. corticosteroids) to interfere with the production of pro-inflammatory cytokines (4). SP-A-mediated uptake of antivirals in type II pneumocytes results in the reduction of viral replication processes and/or viral infectivity via various mechanisms of action (5). PS-assisted delivery of monoclonal-antibody based products (e.g. heavy chain-only antibodies) allows them to bind to viral components (e.g. the spike protein), thereby preventing interactions with the ACE-2 receptor thus reducing their infectivity (6). Abbreviations: SP-A; surfactant protein-A, SP-B; surfactant protein-B, SP-C; surfactant protein-C, SP-D; surfactant protein-D, ACE-2; angiotensin-converting enzyme-2, SARS-CoV-2. severe acute respiratory syndrome coronavirus-2, PL; phospholipid, PS; pulmonary surfactant. Created with BioRender.com
In addition, liposomal surfactant carriers (i.e. drugs formulated inside surfactant liposomes, Fig. 5) can mediate active targeting of drugs. As mentioned above, during consecutive breathing cycles, used PS components are released from surfactant films and recycled through uptake into type II pneumocytes and repackaging in LBs. Next to this, used PS components can also be processed and degraded by AMs. In case the PS-encapsulated drugs are carried along with this depleted pool of PS components, they can also benefit from this recycling mechanism to promote further spreading along the lung surface or enable internalization by these specific cell types [39,40,197].
Given the amphiphilic nature of PS, a variety of therapeutics with different physicochemical properties can be incorporated, even enabling combination therapy by loading several drugs into an exogenous lung surfactant formulation, as shown in Fig. 5 [198]. Hidalgo et al. confirmed that hydrophobic drugs such as corticosteroids experience enhanced transport and spreading along the respiratory surface upon formulation in exogenous PS [199]. In addition, Ya-Min et al. compared the therapeutic effect of aerosolized dexamethasone and co-aerosolization of dexamethasone and PS in an acute lung injury (ALI) mouse model. While dexamethasone alone did not improve lung function in terms of PaO2, co-delivery with PS resulted in a reduction of lung index, lung injury scores and levels of the pro-inflammatory cytokines TNFα, IL-1β and IL-6 in bronchoalveolar lavage (BAL) [198].
PS-assisted pulmonary drug delivery of other small molecules has also been investigated. Here, the immunomodulating agent tacrolimus retained its immunosuppressive efficacy after lung transplantation and in respiratory infections upon encapsulation in lung surfactant-like carriers. In addition, the combination of the aminoglycoside antibiotic tobramycin and clinical surfactant leads to improved survival rates in mice suffering from Klebsiella pneumonia infection [200,201]. Next to small molecules, also the delivery of biological drugs including antimicrobial peptides, antibodies, antioxidant enzymes and viral particles have shown to benefit from this particular encapsulation approach [[202], [203], [204], [205]].
A recent publication reported the potential of co-aerosolizing the cationic amphiphilic compound ambroxol and surfactant in the form of ambroxol-loaded- and coated DPPC nanoparticles as a treatment for CARDS. Ambroxol is a mucolytic drug that is widely used in a variety of pulmonary diseases [206]. Moreover, it is reported to have anti-inflammatory, anti-oxidant, antiviral and antibacterial properties, as well as to stimulate the production- and secretion of PS [207,208]. Upon co-delivery, the surfactant fraction can supplement the deficient or inactivated endogenous surfactant pool, while ambroxol can further fight the disease by the array of therapeutic properties as described above. This approach was established by an earlier meta-analysis on the administration of high-dose ambroxol to patients suffering from ARDS, of which results showed an improvement in oxygenation, as well as a reduction of superoxide dismutase (SOD), TNFα and IL-6 levels in serum [209].
4.3. Use of exogenous pulmonary surfactant to promote delivery of antiviral siRNA molecules
4.3.1. siRNA therapeutics as an effective antiviral therapy
As briefly mentioned above, there is a clear unmet medical need for more specific anti-SARS-CoV-2 therapies targeting key steps in the viral life cycle. A promising strategy is the use of the RNA interference (RNAi) mechanism, where siRNA molecules can be designed to specifically and selectively target viral and/or host-related proteins, without affecting host cell homeostasis [210].
Upon inhalation and delivery into the target cell cytosol, these double-stranded siRNA molecules will bind to the RNA-induced silencing complex (RISC). After digestion of the lagging strand, the activated RISC employs the retained guide strand to bind to a complementary region on the target mRNA, followed by its enzymatic degradation. Notably, this approach ensures a very effective treatment due to the catalytic nature of this gene silencing mechanism, requiring relatively few siRNA molecules per cell to induce potent gene knockdown [[211], [212], [213], [214], [215]]. In addition, the screening of siRNA libraries to identify lead molecules with promising antiviral effects is not as time-consuming as the de novo development of small molecules or antibody(−based) therapeutics, an attractive feature given the rapid spread of viral infections such as SARS-CoV-2 [17].
Efforts to exploit the RNAi mechanism to treat respiratory infections have already been made, with a first report by Bitko and Barik in 2001 showing successful in vitro antiviral activity against RSV via siRNA-mediated knockdown of the RSV phosphoprotein (i.e. the smaller subunit of RNA-dependent RNA polymerase) [216]. In addition, to anticipate on the outbreaks of SARS-CoV-1 and MERS-CoV, a variety of siRNA molecules have been designed that effectively target viral RNA-dependent RNA polymerase, helicase, proteolytic enzymes and structural proteins [217].
As briefly mentioned above, both a variety of SARS-CoV-2 proteins responsible for infectivity or viral replication as well as host-related proteins that play a crucial role in viral infection or disease severity are interesting siRNA targets for an effective antiviral therapy. In severe cases, COVID-19-related morbidity and mortality is caused by an exaggerated pulmonary immune response. More specifically, an upregulation of INFγ, TNFα, IL-6, IL-10 and IL-12 is reported, where especially IL-6 seems to promote the inflammatory cascade [58,[218], [219], [220], [221]]. In addition, the upregulation of high-mobility group box 1 (HMGB1) and sphingosine-1-phosphate lyase (S1PLyase) is observed, where the latter has a more upstream mode-of-action, as its downregulation results in the reduction of TNFα- and IL-6 levels [141,142]. Other studies additionally report on increased levels of IL-2, IL-7, granulocyte-colony stimulating factor (G-CSF), inducible protein-10 (IP-10), monocyte chemo-attractant protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α), where IP-10 is correlated to disease deterioration and death [51,222,223].
Although downregulation of the inflammatory cascade is a warranted approach to reduce disease severity, translational suppression of viral proteins might be a more direct and specific strategy. The genomic sequence of SARS-CoV-2 was released early after its discovery, which facilitated the development of siRNA libraries to target SARS-CoV-2 viral ssRNA coding regions. Currently, several companies are focusing on the development of siRNA molecules to target SARS-CoV-2. Alnylam Pharmaceuticals has synthetized more than 350 siRNA hits that target highly conserved regions in the SARS-CoV-2 genome [224]. Likewise, OilX Pharmaceuticals holds a patent on 30 siRNA molecules targeting conserved regions among coronaviral transcripts. Lastly, Sirnaomics focuses on the development of siRNA molecules targeting proteins involved in SARS-CoV-2 infection and replication [225].
RNAi-based suppression of all four structural proteins (S, E, M and N) can avoid cellular entry and viral assembly, where targeting the spike- and envelope protein have attracted most attention. Since the spike protein is responsible for viral entry into host cells, downregulating this protein will result in its absence in newly assembled viral particles, leading to exocytosis of viruses with reduced infectivity [225]. In this regard, Gallicano et al. showed successful in vitro spike protein suppression in HEK293 cells and human airway tracheal cells, without compromising cell growth and viability [226]. Next to this, the envelope protein is involved in viral assembly and release into the extracellular environment. The sequence of the envelope protein seems to be highly conserved [225], which allows the development of siRNA molecules that will not lose their efficiency upon mutation of the virus, an important consideration taking into account the emergence of SARS-CoV-2 variants [227].
Next to structural proteins, also proteins involved in viral replication are envisioned. In this regard, the sequences coding for protease 3CL, RNA-dependent RNA polymerase and helicase are known to be highly conserved among β-coronaviruses [225].
It is worth noting that the RNAi mechanism allows the combination of different siRNA molecules into one synergistic treatment. In this way, multiple viral- and host-related genes can be targeted simultaneously, leading to significantly enhanced therapeutic efficiencies [[228], [229], [230], [231]].
4.3.2. Nanoparticle-assisted cytosolic delivery of siRNA molecules following inhalation therapy
After inhalation, siRNA molecules need to be safely guided to the target cell cytosol, for which they are generally formulated into nanoparticles. However, besides the many advantages of local pulmonary delivery of nanomedicines, the lung also poses many extra- and intracellular barriers that can hamper siRNA delivery [232,233]. In general, predicting the fate of inhaled nanoparticles is complex since it is determined by a variety of parameters. Next to particle-related properties (e.g. mean mass aerodynamic diameter (MMAD), hydrophobicity and charge), also patient-related properties (e.g. breathing patterns) as well as the anatomy, physiology and pathological state of the lung are involved. An important anatomical barrier towards pulmonary siRNA delivery is the highly branched structure of the lungs, where the fate of aerosolized particles following inhalation therapy will strongly depend on their MMAD. Nanomedicines formulated in aerosolized microparticles (e.g. dry powders or nebulized droplets) that sediment in the bronchial or alveolar region will subsequently need to cross the respiratory mucus or pulmonary surfactant layer to reach the underlying bronchial or alveolar epithelial cells, respectively [[234], [235], [236]]. In addition, nanoparticles are prone to clearance by respiratory immune cells such as macrophages, which can be present at both interfaces [237]. In this regard, investigating nanoparticle/PS interactions are crucial since such interactions can both alter the nanoparticles' distribution profile, targeting potential and drug delivery efficiency, as well as affect the endogenous PS layer [83]. However, the design of such studies is challenging since it requires the use of materials or models that closely resemble the composition of endogenous PS. Indeed, clinically used surfactants can be obtained easily but have several limitations; they are devoid of the hydrophilic proteins SP-A and SP-D and both the phospholipid- and SP-B/SP-C fractions are often lower compared to physiological conditions. Therefore, the use of patient-derived PS (e.g. via BAL) is more desired, however requires more invasive procedures. Several studies report on the greater affinity of surfactant proteins to smaller particles, leading to enhanced toxicity of these particles towards endogenous PS [[238], [239], [240]]. In this regard, SP-A and SP-D will more easily adsorb to hydrophilic nanoparticles, leading to particle opsonization and enhanced uptake by AMs and lung dendritic cells [241,242]. In addition, Wohlleben et al. showed that SP-A adsorption may induce interactions with PS lipids, thereby facilitating particle diffusion through the liquid phase and enhancing particle delivery to desired cell types [243]. On the other hand, SP-B and SP-C have a higher affinity for hydrophobic particles, which is more detrimental for the biophysical function of PS given the importance of SP-B and SP-C in surfactant adsorption and stability [197]. In this context, cationic and hydrophilic particles seem more biocompatible since their intrinsic properties mitigate interactions with SP-B and SP-C [[244], [245], [246]]. Importantly, Beck-Broichsitter et al. showed that pre-coating of particles with PS components such as SP-B and SP-C can avoid their interaction with endogenous PS, thus reducing the risk for PS toxicity or a change in the particles' characteristics [246]. In contrast to the adsorption of surfactant proteins, Raesch et al. elucidated that the composition of the lipid corona was independent from the particles' properties [234].
However, one of the most difficult barriers to overcome for siRNA therapeutics is situated at the intracellular level. When nanoparticles interact with the cellular plasma membrane, they are typically taken up via endocytosis, confining them inside early endosomes. These organelles further mature towards late endosomes and finally fuse with lysosomes, which are characterized by an acidic pH (~4.5–5) and the presence of degrading enzymes, including nucleases [247]. As the RNAi machinery resides in the cytosol, endosomal escape of nanoparticles and/or their siRNA cargo into the cell cytosol before lysosomal fusion occurs is required to induce target gene silencing. Despite the many efforts towards the design of effective nanoformulations in the last decades, even for state-of-the-art nanocarriers the endosomal escape efficiency remains very low (< 1–2%) [44]. Importantly, since the lungs of COVID-19 patients are already highly sensitized due to the activation of inflammatory pathways, not only an effective, but also a biocompatible nanoparticle for inhalation therapy of siRNA therapeutics is highly sought after. In this regard, exploiting bio-inspired materials is a research area gaining attention.
As mentioned above, PS can be regarded as an extracellular barrier upon inhalation of nanomedicines. More specifically, surfactant components can adsorb to the surface of the nanoparticle, creating a biomolecular corona. In addition, due to the presence of negatively charged surfactant components (e.g. PG, PI and PS), there is a risk for nanocarrier aggregation or siRNA decomplexation prior to endocytic uptake [37,83,197,248]. In this regard, De Backer et al. investigated the colloidal stability and transfection efficiency of siRNA-loaded cationic dextran nanogels (siNGs) in the presence of two clinically available animal-derived surfactants (i.e. Curosurf® and Infasurf®) [45]. These polysaccharide nanoparticles are biodegradable due to the presence of carbonate ester crosslinks that interconnect the dextran backbone and were previously shown to have a high loading capacity for siRNA molecules [249]. Moreover, siNGs showed efficient delivery of siRNA in vitro in lung epithelial and alveolar macrophage cell lines (i.e. H1299_eGFP and MH-S) [250].
Despite a significant reduction of siNG cellular uptake upon surfactant exposure, gene silencing was maintained in both cell lines, indicating that the presence of surfactant can further promote the cytosolic delivery of siNGs. These initial results led to the development of a bio-inspired hybrid nanoparticle composed of a siNG core layered with a surfactant shell (Curosurf®). In addition to the above-mentioned beneficial effects of PS (i.e. surfactant supplementation, improved biodistribution and anti-inflammatory effects) as well as the promoted siRNA delivery, this anionic surfactant coating is expected to improve in vivo stability (e.g. mitigating the interaction with and decomplexation of siRNA by mucins and endogenous PS components), as well as nanocarrier biocompatibility [46].
Following in vivo studies that probed the administration of uncoated- and Curosurf®-coated siNGs via pharyngeal aspiration in mice showed that only the latter could induce gene suppression in resident AMs, reaching 70% knockdown of the pan-leukocyte marker CD45 at a dosage of 1 mg/kg [47].
Clinical translation of the above-mentioned core-shell nanocomposites requires testing the long-term stability as well as the delivery efficiency following aerosolization. In this regard, previous studies already showed the feasibility to lyophilize PS preparations of different origins without compromising their surface-activities [[251], [252], [253]]. Considering this, Merckx et al. assessed lyophilization of Curosurf®-coated siNGs, followed by reconstitution and nebulization using a state-of-the-art vibrating mesh nebulizer. Importantly, neither the lyophilization and reconstitution process, nor the subsequent nebulization negatively impacted the physicochemical properties and the biological performance of the particles. Of note, no stabilizing agents such as cryo- and lyoprotectants were required to achieve this feat [48].
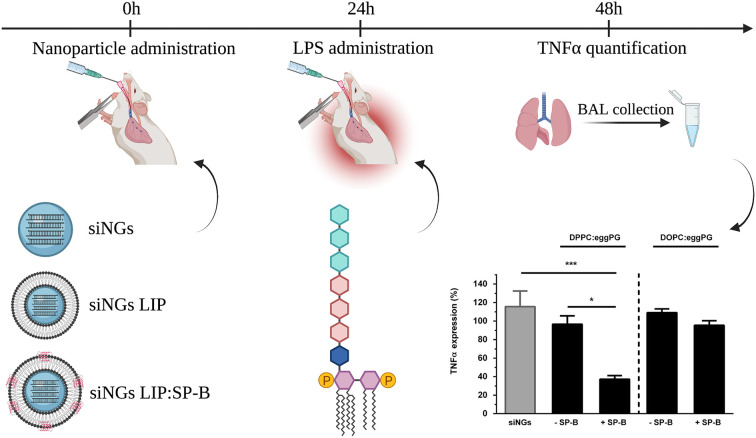
Further research by Merckx et al. revealed that the cationic amphiphilic SP-B is the key component in lung surfactant responsible for the improved siRNA delivery by siNGs, which was demonstrated both in vitro and in vivo in an LPS-induced acute lung injury model, targeting the pro-inflammatory cytokine TNFα (Fig. 6 ) [49]. This discovery enabled to reduce the complexity of the surfactant coat by replacing Curosurf® with a simplified SP-B proteolipid mixture. In addition, the latter enabled to investigate the impact of the lipid components on the delivery-promoting activity of SP-B in more detail. In vitro studies demonstrated the importance of a fluid lipid bilayer to support SP-B, with cholesterol not exceeding physiological levels, while the type of anionic lipid was less critical [50].
Fig. 6.
Schematic overview of relative TNFα silencing in a murine, LPS-induced acute lung injury (ALI) model. Intratracheal administration of anti-TNFα siRNA was performed using uncoated nanogels (siNGs) or nanogels coated with a surfactant-inspired proteolipid composition (DPPC or DOPC:PG 85:15, LIP), with or without SP-B (siNGs LIP, siNGs LIP:SP-B), followed by LPS administration after 24 h. TNFα levels were quantified in BAL fluid, obtained 24 h after LPS stimulation. TNFα expression levels of mice treated with anti-TNFα siRNA are normalized to mice treated with control siRNA (siCTRL). Only siRNA delivery using siNGs coated with DPPC:PG and supplemented with SP-B leads to substantial gene silencing. All values are a mean ± standard deviation (SD) from four independent repeats (n = 4). Statistical analysis was performed via One-Way ANOVA followed by a Tukey's multiple comparison test. Abbreviations: TNFα; tumor necrosis factor α, LPS; lipopolysaccharide, siNGs; siRNA-loaded nanogels, SP-B; surfactant protein-B, BAL; bronchoalveolar lavage. Data adopted from [49], with permission. Created with BioRender.com
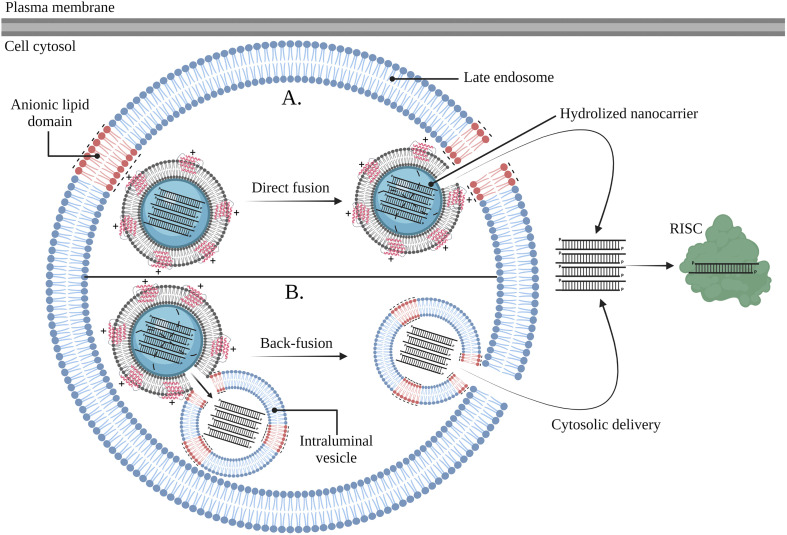
Guagliardo et al. further revealed that SP-B fosters endosomal escape of siRNA via membrane fusion events. This mode-of-action in part explains the enhanced in vitro gene silencing efficiency when SP-B is present in a more fluid lipid microenvironment, as the latter allows lateral diffusion of SP-B molecules and the formation of SP-B homodimers or larger oligomers with improved membrane-interaction potential [[254], [255]]. More specifically, in vitro results showed that SP-B-mediated fusion was pH independent and required the presence of anionic lipids in the opposing membrane. As shown in Fig. 7 , these anionic lipids are believed to electrostatically interact with the cationic SP-B, guaranteeing close membrane contact to provoke membrane fusion followed by cytosolic delivery of encapsulated siRNA molecules. Interestingly, a similar mechanism of action is described for arginine-rich cell penetrating peptides (CPPs), as these residues allow electrostatic interactions with anionic lipids in the endosomal compartment. For example, a disulfide-bonded dimer of HIV-TAT (i.e. dHIV-TAT) fosters endosomal escape by binding to bis(monoacylglycerol)phosphate (BMP) lipids in the late endosomal compartment. Upon binding, fusion occurs between bilayers containing BMP, followed by content leakage and endosomal escape of the cargo [256].
Fig. 7.
SP-B-mediated cytosolic delivery of siRNA molecules occurs via the promotion of fusion events. The surfactant-coated siNGs are internalized by endocytosis and reside in the late endosomal compartment upon endosomal maturation. The cationic SP-B can interact with anionic lipids in the membrane of late endosomes, followed by direct fusion of the surfactant-coated siNGs with the endosomal membrane, hydrolysis of the NGs and cytosolic delivery of the siRNA molecules (A). The cationic SP-B can interact with anionic lipids in intraluminal vesicles (ILVs), followed by fusion between surfactant-coated siNGs and ILVs, hydrolysis of the NGs, translocation of the siRNA molecules and cytosolic delivery upon back-fusion between the ILVs and the endosomal membrane (B). Abbreviations: RISC; RNA-induced silencing complex. Created with BioRender.com
Similar to SRT, also this treatment approach might benefit from the development of SP-B analogues that can mimic the siRNA delivery properties of native SP-B. In this regard, Qiu et al. showed succesfull siRNA delivery into alveolar- and bronchial epithelial cells (i.e. A549 and BEAS-2B) in vitro upon complexation with the cationic KL4 peptide [257]. Following studies by the same group showed in vitro mRNA delivery in lung epithelial cells via complexation with a (12-mer) PEGylated KL4 peptide. To obtain a suitable microformulation for local pulmonary delivery, these complexes were formulated into a dry powder without compromising the delivery efficiency of the particles. Intratracheal administration of the mRNA/PEG12KL4 complexes via a liquid or powder aerosol in mice led to succesful mRNA delivery in deeper lung regions without inducing inflammation [258].
5. Conclusions
Although the COVID-19 pandemic is gradually losing strength due to global vaccination initiatives, the slow-moving global vaccine distribution, residual infections of vaccinated, non-vaccinated and immunosuppressed individuals as well as the possible emergence of resistant viral variants advocate the continued development of effective COVID-19 treatment strategies. In this regard, pulmonary administration of exogenous pulmonary surfactant (i.e. SRT) could greatly contribute to COVID-19 management as these preparations could supplement the deficient or inactivated endogenous surfactant pool as well as directly target SARS-CoV-2 and alleviate inflammation-related symptoms upon combination with small molecular- and macromolecular drugs.
First, although typical ARDS did not show benefit from SRT in early clinical trials, the resemblance of the clinical manifestations of CARDS and NRDS rationalizes its re-investigation. Indeed, NRDS is treatable with SRT, reflected by strongly reduced mortality rates and need for oxygenation. However, besides the importance to identify COVID-19 patients that might benefit from this treatment strategy, simply re-claiming the surfactant preparations or doses applied for NRDS will likely not suffice. For example, additional research is needed regarding the optimal composition of surfactant preparations to assure resistance to inactivating agents, where further advances in the development of synthetic surfactants and the addition of non-adsorbing proteins or polymers could prove beneficial. Another important aspect is finding a substitute for the intratracheal delivery route, e.g. non-invasive surfactant delivery via aerosolization, where a balance must be found between patient comfort and protecting the personnel from infection.
Secondly, as the lungs are the main target for SARS-CoV-2 infection, inhalation of COVID-19-targeting drugs (e.g. (repurposed) antivirals, steroids and antibody(−based) therapeutics) is an attractive administration route. However, especially in severely ill COVID-19 patients it is challenging to target the more distal airways upon inhalation since these patients experience hypoxic and collapsed lung regions. Here, due to its excellent surface-spreading properties, pulmonary surfactant can serve as a vehicle or carrier for COVID-19-targeting drugs. This approach can be regarded as a combination therapy where the administration of surfactant can both regenerate the affected endogenous surfactant pool as well as improve drug spreading and adsorption along the respiratory surface and aid active drug targeting to specific cell types.
Lastly, the deployment of siRNA-based antivirals can alleviate the challenges associated with small molecular antivirals as this therapy is highly specific, allows to target different genes of interest simultaneously and is able to inhibit otherwise undruggable targets. To guarantee target cell entry and protection of the siRNA payload from degradation, these molecules are generally formulated into nanosized carriers. Unfortunately, to date no nanoformulation is available that allows safe and efficient siRNA delivery following inhalation. In response to this unmet need, Raemdonck and co-workers disclosed the ability of the pulmonary surfactant-associated SP-B to enhance the cytosolic delivery of siRNA. Given its endogenous nature, pulmonary surfactant-based nanoparticles could pave the way to the development of biocompatible surfactant-inspired formulations that enable efficient delivery of antiviral siRNAs to the lungs of COVID-19 patients. Moreover, it is anticipated that such formulations could be more widely used for treatment of other respiratory pathologies as well.
Declaration of Competing Interest
There are no conflicts of interest to disclose.
Acknowledgements
LH is a doctoral fellow of the Research Foundation-Flanders, Belgium (FWO, grant 1S56121N). SDS and KR additionally acknowledge the Ghent University Special Research Fund (BOF12-GOA-014 and BOF19-GOA-008). KR acknowledges funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 101002571, RESPIRNA).
References
- 1.World Health Organization . WHO COVID-19 Dashboard [Internet] WHO; 2021. https://covid19.who.int/ [Google Scholar]
- 2.Veldhuizen R.A.W., Zuo Y.Y., Petersen N.O., Lewis J.F., Possmayer F. The COVID-19 pandemic: a target for surfactant therapy? Expert Rev. Respir. Med. 2021;15:597–608. doi: 10.1080/17476348.2021.1865809. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C., Gao C., Xie Y., Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect. Dis. 2020;20:510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haleeqa M.A., Alshamsi I., Al Habib A., Noshi M., Abdullah S., Kamour A., Ibrahim H. Optimizing supportive care in COVID-19 patients: A multidisciplinary approach. J. Multidiscip. Healthc. 2020;13:877–880. doi: 10.2147/JMDH.S264168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirastschijski U., Dembinski R., Maedler K. Lung surfactant for pulmonary barrier restoration in patients with COVID-19 pneumonia. Front. Med. 2020;7:254. doi: 10.3389/fmed.2020.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar Dey S., Mahbubur Rahman M., Raihan Siddiqi U., Howlader A., Tushar A. Analyzing the global impact of COVID-19 vaccination Progress: A result-oriented storytelling approach. MedRxiv. 2021 Submitted for publication. [Google Scholar]
- 7.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo A., Garcia-Mouton C., Autilio C., Carravilla P., Orellana G., Islam M.N., Bhattacharya J., Bhattacharya S., Cruz A., Pérez-Gil J. Pulmonary surfactant and drug delivery: Vehiculization, release and targeting of surfactant/tacrolimus formulations. J. Control. Release. 2021;329:205–222. doi: 10.1016/j.jconrel.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzzi P.H., Mercatelli D., Ceraolo C., Giorgi F.M. Master regulator analysis of the SARS-CoV-2/human interactome. J. Clin. Med. 2020;9:982. doi: 10.3390/jcm9040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jirjees F., Saad A.K., Al Hano Z., Hatahet T., Al Obaidi H., Bashi Y.H.D. COVID-19 treatment guidelines: do they really reflect best medical practices to manage the pandemic? Infect. Dis. Rep. 2021;13:259–284. doi: 10.3390/idr13020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kupferschmidt K., Cohen J. WHO Launches Global Megatrial of the Four Most Promising Coronavirus Treatments [Internet] 2020. https://www.science.org/content/article/who-launches-global-megatrial-four-most-promising-coronavirus-treatments Available from.
- 13.Gilead. U.S. Food and Drug Administration Approves Gilead's Antiviral Veklury® (remdesivir) for Treatment of COVID-19 [internet]. 2021. Available from: https://www.gilead.com/news-and-press/press-room/press-releases/2020/10/us-food-and-drug-administration-approves-gileads-antiviral-veklury-remdesivir-for-treatment-of-covid19.
- 14.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinbolade S., Coughlan D., Fairbairn R., et al. Combination therapies for COVID-19: an overview of the clinical trials landscape. Br. J. Clin. Pharmacol. 2021;47:777–780. doi: 10.1111/bcp.15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez M.A. Lack of effectiveness of repurposed drugs for COVID-19 treatment. Front. Immunol. 2021;12:635371. doi: 10.3389/fimmu.2021.635371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Solidarity Trial Consortium Repurposed antiviral drugs for COVID-19-interim WHO solidarity trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brüssow H. Clinical trials with antiviral drugs against COVID-19: some progress and many shattered hopes. Environ. Microbiol. 2021;23:6364–6376. doi: 10.1111/1462-2920.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atea Pharmaceuticals . Atea Pharmaceuticals announces first patient dosed in global phase 3 MORNINGSKY trial of AT-527 for treatment of COVID-19. Atea Pharmaceuticals; 2021. [Google Scholar]
- 20.Ledford H. COVID antiviral pills: what scientists still want to know. Nature. 2021;599:358–359. doi: 10.1038/d41586-021-03074-5. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein S.H. The chemistry, biology and pharmacology of the cyclopentenone prostaglandins. Prostaglandins Other Lipid Mediat. 2020;148 doi: 10.1016/j.prostaglandins.2020.106408. 106408. [DOI] [PubMed] [Google Scholar]
- 25.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J. Clin. Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurka D.P., Balk R.A. Critical Care Medicine. 3rd. Elsevier; 2008. Acute respiratory failure; pp. 773–794. [Google Scholar]
- 28.Ramanathan R. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J. Perinatol. 2009;29:38–43. doi: 10.1038/jp.2009.31. [DOI] [PubMed] [Google Scholar]
- 29.Heching M., Lev S., Shitenberg D., Dicker D., Kramer M.R. Surfactant for the treatment of ARDS in a patient with COVID-19. Chest. 2021;160:9–12. doi: 10.1016/j.chest.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattel F., Giordano S., Bertiond C., Lupia T., Corcione S., Scaldaferri M., Angelone L., De Rosa F.G. Use of exogenous pulmonary surfactant in acute respiratory distress syndrome (ARDS): role in SARS-CoV-2-related lung injury. Respir. Physiol. Neurobiol. 2021;288:103645. doi: 10.1016/j.resp.2021.103645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ClinicalTrials.gov. ClinicalTrials.Gov; 2020. A Clinical Trial of Nebulized Surfactant for the Treatment of Moderate to Severe COVID-19 (COVSurf) [Internet]https://clinicaltrials.gov/ct2/show/NCT04362059 [Google Scholar]
- 32.Lawson Health Research Institute ClinicalTrials.gov. London's Exogenous Surfactant Study for COVID19 (LESSCOVID) [Internet] 2021. https://clinicaltrials.gov/ct2/show/NCT04375735 Available from.
- 33.ClinicalTrials.gov . ClinicalTrials.Gov; 2020. Curosurf® in Adult Acute Respiratory Distress Syndrome Due to COVID-19 (Caards-1) [Internet]https://clinicaltrials.gov/ct2/show/NCT04384731 [Google Scholar]
- 34.ClinicalTrials.gov . The Safety and Preliminary Tolerability of Lyophilized Lucinactant in Adults with Coronavirus Disease 2019 (COVID-19) [Internet] ClinicalTrials.gov; 2020. https://clinicaltrials.gov/ct2/show/NCT04389671 [Google Scholar]
- 35.ClinicalTrials.gov . Poractant Alfa - Curosurf and SARS-COV-19 ARDS (Covid- 19) [Internet] ClinicalTrials.gov; 2020. https://clinicaltrials.gov/ct2/show/NCT04502433 [Google Scholar]
- 36.Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidalgo A., Cruz A., Pérez-Gil J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015;95:117–127. doi: 10.1016/j.ejpb.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 38.García-Mouton C., Hidalgo A., Arroyo R., Echaide M., Cruz A., Pérez-Gil J. Pulmonary surfactant and drug delivery: an interface-assisted carrier to deliver surfactant protein SP-D into the airways. Front. Bioeng. Biotechnol. 2021;8:1556. doi: 10.3389/fbioe.2020.613276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz G., Muller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- 40.Andreeva A.V., Kutuzov M.A., Voyno-Yasenetskaya T.A. Regulation of surfactant secretion in alveolar type II cells. Am. J. Phys. Lung Cell. Mol. Phys. 2007;293:259–271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 41.Uludağ H., Parent K., Aliabadi H.M., Haddadi A. Prospects for RNAi therapy of COVID-19. Front. Bioeng. Biotechnol. 2020;8:916. doi: 10.3389/fbioe.2020.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta A., Michler T., Merkel O.M. siRNA therapeutics against respiratory viral infections—what have we learned for potential COVID-19 therapies? Adv. Healthc. Mater. 2021;10:2001650. doi: 10.1002/adhm.202001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Y., Merkel O.M. Pulmonary delivery of siRNA via polymeric vectors as therapies of asthma. Arch. Pharm. (Weinheim) 2015;348:681–688. doi: 10.1002/ardp.201500120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilleron J., Querbes W., Zeigerer A., Borodovsky A., Marsico G., Schubert U., Manygoats K., Seifert S., Andree C., Stöter M., Epstein-Barash H., Zhang L., Koteliansky V., Fitzgerald K., Fava E., Bickle M., Kalaidzidis Y., Akinc A., Maier M., Zerial M. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 45.De Backer L., Braeckmans K., Demeester J., De Smedt S.C., Raemdonck K. The influence of natural pulmonary surfactant on the efficacy of siRNA-loaded dextran nanogels. Nanomedicine. 2013;8:1625–1638. doi: 10.2217/nnm.12.203. [DOI] [PubMed] [Google Scholar]
- 46.De Backer L., Braeckmans K., Stuart M.C.A., Demeester J., De Smedt S.C., Raemdonck K. Bio-inspired pulmonary surfactant-modified nanogels: A promising siRNA delivery system. J. Control. Release. 2015;206:177–186. doi: 10.1016/j.jconrel.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 47.De Backer L., Naessens T., De Koker S., Zagato E., Demeester J., Grooten J., De Smedt S.C., Raemdonck K. Hybrid pulmonary surfactant-coated nanogels mediate efficient in vivo delivery of siRNA to murine alveolar macrophages. J. Control. Release. 2015;217:53–63. doi: 10.1016/j.jconrel.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 48.Merckx P., Lammens J., Nuytten G., Bogaert B., Guagliardo R., Maes T., Vervaet C., De Beer T., De Smedt S.C., Raemdonck K. Lyophilization and nebulization of pulmonary surfactant-coated nanogels for siRNA inhalation therapy. Eur. J. Pharm. Biopharm. 2020;157:191–199. doi: 10.1016/j.ejpb.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Merckx P., De Backer L., Van Hoecke L., Guagliardo R., Echaide M., Baatsen P., Olmeda B., Saelens X., Pérez-Gil J., De Smedt S.C., Raemdonck K. Surfactant protein B (SP-B) enhances the cellular siRNA delivery of proteolipid coated nanogels for inhalation therapy. Acta Biomater. 2018;78:236–246. doi: 10.1016/j.actbio.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Guagliardo R., Herman L., Penders J., Zamborlin A., De Keersmaecker H., Van de Vyver T., Verstraeten S., Merckx P., Mingeot-Leclercq M.-P., Echaide M., Pérez-Gil J., Stevens M.M., De Smedt S.C., Raemdonck K. Surfactant protein B promotes cytosolic siRNA delivery by adopting a virus-like mechanism of action. ACS Nano. 2021;15:8095–8109. doi: 10.1021/acsnano.0c04489. [DOI] [PubMed] [Google Scholar]
- 51.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W., Roose K., van Schie L., Hoffmann M., Pöhlmann S., Graham B.S., Callewaert N., Schepens B., Saelens X., McLellan J.S. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181:1004–1015. doi: 10.1016/j.cell.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavalcante-Silva L.H.A., Carvalho D.C.M., de Almeida Lima É., Galvão J.G.F.M., de França da Silva J.S., de Sales-Neto J.M., Rodrigues-Mascarenhas S. Neutrophils and COVID-19: the road so far. Int. Immunopharmacol. 2021;90:107233. doi: 10.1016/j.intimp.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burrell C.J., Howard C.R., Murphy F.A. Fenner and White’s Medical Virology. Elsevier; 2017. Coronaviruses; pp. 437–446. [Google Scholar]
- 56.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 57.Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J. Virol. 2010;84:3134–3146. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menezes M.C.S., Pestana D.V.S., Gameiro G.R., da Silva L.F.F., Baron Ė., Rouby J.J., Auler J.O.C. SARS-CoV-2 pneumonia-receptor binding and lung immunopathology: a narrative review. Crit. Care. 2021;25:53. doi: 10.1186/s13054-020-03399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nadim M.K., Forni L.G., Mehta R.L., Connor M.J., Liu K.D., Ostermann M., Rimmelé T., Zarbock A., Bell S., Bihorac A., Cantaluppi V., Hoste E., Husain-Syed F., Germain M.J., Goldstein S.L., Gupta S., Joannidis M., Kashani K., Koyner J.L., Legrand M., Lumlertgul N., Mohan S., Pannu N., Peng Z., Perez-Fernandez X.L., Pickkers P., Prowle J., Reis T., Srisawat N., Tolwani A., Vijayan A., Villa G., Yang L., Ronco C., Kellum J.A. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat. Rev. Nephrol. 2020;16:747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salah H.M., Arthur J.M., Mehta J.L. Implications of renal ACE2 expression in the age of COVID-19. Eur. Heart J. 2020;41:4589–4591. doi: 10.1093/eurheartj/ehaa872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tenforde M.W., Kim S.S., Lindsell C.J., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network. MMWR Morb. Mortal. Wkly. Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nieman G.F., Al-Khalisy H., Kollisch-Singule M., Satalin J., Blair S., Trikha G., Andrews P., Madden M., Gatto L.A., Habashi N.M. A physiologically informed strategy to effectively open, stabilize, and protect the acutely injured lung. Front. Physiol. 2020;11:227. doi: 10.3389/fphys.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulz A., Pagerols Raluy L., Kolman J.P., Königs I., Trochimiuk M., Appl B., Reinshagen K., Boettcher M., Trah J. The inhibitory effect of Curosurf® and Alveofact® on the formation of neutrophil extracellular traps. Front. Immunol. 2021;11:582895. doi: 10.3389/fimmu.2020.582895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blanco O., Pérez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat respiratory distress syndrome: role of the different components in an efficient pulmonary surfactant. Eur. J. Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 67.Parra E., Pérez-Gil J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem. Phys. Lipids. 2015;185:153–175. doi: 10.1016/j.chemphyslip.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Whitsett J.A., Wert S.E., Weaver T.E. Diseases of pulmonary surfactant homeostasis. Annu. Rev. Pathol. Mech. Dis. 2015;10:371–393. doi: 10.1146/annurev-pathol-012513-104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pérez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim. Biophys. Acta Biomembr. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Wright J.R. Clearance and recycling of pulmonary surfactant. Am. J. Phys. Lung Cell. Mol. Phys. 1990;259:259. doi: 10.1152/ajplung.1990.259.2.L1. [DOI] [PubMed] [Google Scholar]
- 71.Bates S.R., Dodia C., Tao J.Q., Fisher A.B. Surfactant protein-A plays an important role in lung surfactant clearance: evidence using the surfactant protein-A gene-targeted mouse. Am. J. Physiol. Cell. Mol. Physiol. 2008;294:325–333. doi: 10.1152/ajplung.00341.2007. [DOI] [PubMed] [Google Scholar]
- 72.Bates S. P63 (CKAP4) as an SP-A receptor: implications for surfactant turnover. Cell. Physiol. Biochem. 2010;25:41–54. doi: 10.1159/000272062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ariki S., Nishitani C., Kuroki Y. Diverse functions of pulmonary collectins in host defense of the lung. J. Biomed. Biotechnol. 2012;2012:532071. doi: 10.1155/2012/532071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sano H., Chiba H., Iwaki D., Sohma H., Voelker D.R., Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J. Biol. Chem. 2000;275:22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]
- 75.Barrow A.D., Palarasah Y., Bugatti M., Holehouse A.S., Byers D.E., Holtzman M.J., Vermi W., Skjødt K., Crouch E., Colonna M. OSCAR is a receptor for surfactant protein D that activates TNF-α release from human CCR2 + inflammatory monocytes. J. Immunol. 2015;194:3317–3326. doi: 10.4049/jimmunol.1402289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olde Nordkamp M.J.M., van Eijk M., Urbanus R.T., Bont L., Haagsman H.P., Meyaard L. Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J. Leukoc. Biol. 2014;96:105–111. doi: 10.1189/jlb.3AB0213-092RR. [DOI] [PubMed] [Google Scholar]
- 77.Minutti C.M., García-Fojeda B., Sáenz A., de las Casas-Engel M., Guillamat-Prats R., de Lorenzo A., Serrano-Mollar A., Corbí Á.L., Casals C. Surfactant protein A prevents IFN-γ/IFN-γ receptor interaction and attenuates classical activation of human alveolar macrophages. J. Immunol. 2016;197:590–598. doi: 10.4049/jimmunol.1501032. [DOI] [PubMed] [Google Scholar]
- 78.Arroyo R., Khan M.A., Echaide M., et al. SP-D attenuates LPS-induced formation of human neutrophil extracellular traps (NETs), protecting pulmonary surfactant inactivation by NETs. Commun. Biol. 2019;2:470. doi: 10.1038/s42003-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veith N.T., Tschernig T., Gutbier B., Witzenrath M., Meier C., Menger M., Bischoff M. Surfactant protein A mediates pulmonary clearance of Staphylococcus aureus. Respir. Res. 2014;15:85. doi: 10.1186/s12931-014-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benne C.A., Benaissa-Trouw B., van Strijp J.A.G., Kraaijeveld C.A., van Iwaarden J.F. Surfactant protein A, but not surfactant protein D, is an opsonin for influenza A virus phagocytosis by rat alveolar macrophages. Eur. J. Immunol. 1997;27:886–890. doi: 10.1002/eji.1830270413. [DOI] [PubMed] [Google Scholar]
- 81.Gil M., McCormack F.X., LeVine A.M. Surfactant protein A modulates cell surface expression of CR3 on alveolar macrophages and enhances CR3-mediated phagocytosis. J. Biol. Chem. 2009;284:7495–7504. doi: 10.1074/jbc.M808643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Korfhagen T.R., Sheftelyevich V., Burhans M.S., Bruno M.D., Ross G.F., Wert S.E., Stahlman M.T., Jobe A.H., Ikegami M., Whitsett J.A., Fisher J.H. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J. Biol. Chem. 1998;273:28438–28443. doi: 10.1074/jbc.273.43.28438. [DOI] [PubMed] [Google Scholar]
- 83.Guagliardo R., Pérez-Gil J., De Smedt S.C., Raemdonck K. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J. Control. Release. 2018;291:116–126. doi: 10.1016/j.jconrel.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Lopez-Rodriguez E., Pérez-Gil J. Structure-function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim. Biophys. Acta Biomembr. 2014;1838:1568–1585. doi: 10.1016/j.bbamem.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 85.Pérez-Gil J., Weaver T.E. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda) 2010;25:132–141. doi: 10.1152/physiol.00006.2010. [DOI] [PubMed] [Google Scholar]
- 86.Olmeda B., García-Álvarez B., Pérez-Gil J. Structure-function correlations of pulmonary surfactant protein SP-B and the saposin-like family of proteins. Eur. Biophys. J. 2013;42:209–222. doi: 10.1007/s00249-012-0858-9. [DOI] [PubMed] [Google Scholar]
- 87.Beck D.C., Ikegami M., Na C.L., Zaltash S., Johansson J., Whitsett J.A., Weaver T.E. The role of homodimers in surfactant protein B function in vivo. J. Biol. Chem. 2000;275:3365–3370. doi: 10.1074/jbc.275.5.3365. [DOI] [PubMed] [Google Scholar]
- 88.Wüstneck N., Wüstneck R., Pérez-Gil J., Pison U. Effects of oligomerization and secondary structure on the surface behavior of pulmonary surfactant proteins SP-B and SP-C. Biophys. J. 2003;84:1940–1949. doi: 10.1016/S0006-3495(03)75002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clark J.C., Wert S.E., Bachurski C.J., Stahlman M.T., Stripp B.R., Weaver T.E., Whitsett J.A. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc. Natl. Acad. Sci. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nogee L.M., DeMello D.E., Dehner L.P., Colten H.R. Deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N. Engl. J. Med. 1993;328:406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- 91.Johansson J., Szyperski T., Wüthrich K., Curstedt T. The NMR structure of the pulmonary surfactant-associated polypeptide SP-C in an apolar solvent contains a valyl-rich α-helix. Biochemistry. 1994;33:6015–6023. doi: 10.1021/bi00185a042. [DOI] [PubMed] [Google Scholar]
- 92.Glasser S.W., Burhans M.S., Korfhagen T.R., Na C.L., Sly P.D., Ross G.F., Ikegami M., Whitsett J.A. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc. Natl. Acad. Sci. 2001;98:6366–6371. doi: 10.1073/pnas.101500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomas A.Q., Lane K., Phillips J., Prince M., Markin C., Speer M., Schwartz D.A., Gaddipati R., Marney A., Johnson J., Roberts R., Haines J., Stahlman M., Loyd J.E. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am. J. Respir. Crit. Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 94.Roldan N., Goormaghtigh E., Pérez-Gil J., Garcia-Alvarez B. Palmitoylation as a key factor to modulate SP-C-lipid interactions in lung surfactant membrane multilayers. Biochim. Biophys. Acta Biomembr. 2015;1848:184–191. doi: 10.1016/j.bbamem.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 95.Gómez-Gil L., Pérez-Gil J., Goormaghtigh E. Cholesterol modulates the exposure and orientation of pulmonary surfactant protein SP-C in model surfactant membranes. Biochim. Biophys. Acta Biomembr. 2009;1788:1907–1915. doi: 10.1016/j.bbamem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 96.Mulugeta S., Beers M.F. Surfactant protein C: its unique properties and emerging immunomodulatory role in the lung. Microbes Infect. 2006;8:2317–2323. doi: 10.1016/j.micinf.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 97.Ikegami M., Whitsett J.A., Martis P.C., Weaver T.E. Reversibility of lung inflammation caused by SP-B deficiency. Am. J. Physiol. Cell. Mol. Physiol. 2005;289:962–970. doi: 10.1152/ajplung.00214.2005. [DOI] [PubMed] [Google Scholar]
- 98.Numata M., Chu H.W., Dakhama A., Voelker D.R. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proc. Natl. Acad. Sci. U.S.A. 2009;107:320–325. doi: 10.1073/pnas.0909361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuronuma K., Mitsuzawa H., Takeda K., Nishitani C., Chan E.D., Kuroki Y., Nakamura M., Voelker D.R. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J. Biol. Chem. 2009;284:25488–25500. doi: 10.1074/jbc.M109.040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L., Johansson J., Ridsdale R., Willander H., Fitzen M., Akinbi H.T., Weaver T.E. Surfactant protein B propeptide contains a saposin-like protein domain with antimicrobial activity at low pH. J. Immunol. 2010;184:975–983. doi: 10.4049/jimmunol.0900650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Augusto L.A., Li J., Synguelakis M., Johansson J., Chaby R. Structural basis for interactions between lung surfactant protein C and bacterial lipopolysaccharide. J. Biol. Chem. 2002;277:23484–23492. doi: 10.1074/jbc.M111925200. [DOI] [PubMed] [Google Scholar]
- 102.Garcia-Verdugo I., Garcia de Paco E., Espinassous Q., Gonzalez-Horta A., Synguelakis M., Kanellopoulos J., Rivas L., Chaby R., Pérez-Gil J. Synthetic peptides representing the N-terminal segment of surfactant protein C modulate LPS-stimulated TNF-α production by macrophages. Innate Immun. 2009;15:53–62. doi: 10.1177/1753425908100500. [DOI] [PubMed] [Google Scholar]
- 103.Johansson J., Curstedt T. Synthetic surfactants with SP-B and SP-C analogues to enable worldwide treatment of neonatal respiratory distress syndrome and other lung diseases. J. Intern. Med. 2019;285:165–186. doi: 10.1111/joim.12845. [DOI] [PubMed] [Google Scholar]
- 104.Martínez-Calle M., Parra-Ortiz E., Cruz A., Olmeda B., Pérez-Gil J. Towards the molecular mechanism of pulmonary surfactant protein SP-B: at the crossroad of membrane permeability and interfacial lipid transfer. J. Mol. Biol. 2021;433:166749. doi: 10.1016/j.jmb.2020.166749. [DOI] [PubMed] [Google Scholar]
- 105.Watson A., Madsen J., Clark H.W. SP-A and SP-D: dual functioning immune molecules with antiviral and immunomodulatory properties. Front. Immunol. 2021;11:3692. doi: 10.3389/fimmu.2020.622598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Avery M.E., Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA. J. Dis. Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 107.Dechant K.L., Faulds D. Colfosceril palmitate: a review of the therapeutic efficacy and clinical tolerability of a synthetic surfactant preparation (exosurf neonatal) in neonatal respiratory distress syndrome. Drugs. 1991;42:877–894. doi: 10.2165/00003495-199142050-00009. [DOI] [PubMed] [Google Scholar]
- 108.Moya F., Maturana A. Animal-derived surfactants versus past and current synthetic surfactants: current status. Clin. Perinatol. 2007;34:145–177. doi: 10.1016/j.clp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Logan J.W., Moya F.R. Animal-derived surfactants for the treatment and prevention of neonatal respiratory distress syndrome: summary of clinical trials. Ther. Clin. Risk Manag. 2009;5:251. doi: 10.2147/tcrm.s4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jeon G.W. Surfactant preparations for preterm infants with respiratory distress syndrome: past, present, and future. Korean J. Pediatr. 2019;62:155. doi: 10.3345/kjp.2018.07185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walther F.J., Gordon L.M., Waring A.J. Waring, Advances in synthetic lung surfactant protein technology. Exp. Rev. Resp. Med. 2019;13:499–501. doi: 10.1080/17476348.2019.1589372. [DOI] [PubMed] [Google Scholar]
- 112.Mikolka P., Curstedt T., Feinstein R., Larsson A., Grendar M., Rising A., Johansson J. Impact of synthetic surfactant CHF5633 with SP-B and SP-C analogues on lung function and inflammation in rabbit model of acute respiratory distress syndrome. Phys. Rep. 2021;9:e14700. doi: 10.14814/phy2.14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Obladen M. History of surfactant up to 1980. Biol. Neonate. 2005;87:308–316. doi: 10.1159/000084878. [DOI] [PubMed] [Google Scholar]
- 114.Silverman W.A., Andersen D.H. Controlled clinical trial of effects of alevaire mist on premature infants. J. Am. Med. Assoc. 1955;157:1093–1096. doi: 10.1001/jama.1955.02950300021004. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi A., Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem. Biophys. Res. Commun. 1986;135:527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- 116.Fujiwara T., Chida S., Watabe Y., Maeta H., Morita T., Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;315:55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 117.Soll R.F., Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst. Rev. 2001;2:CD000144. doi: 10.1002/14651858.CD000144. [DOI] [PubMed] [Google Scholar]
- 118.Ardell S., Pfister R.H., Soll R. Animal derived surfactant extract versus protein free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database Syst. Rev. 2015;8:CD000144. doi: 10.1002/14651858.CD000144.pub2. [DOI] [PubMed] [Google Scholar]
- 119.Curstedt T., Halliday H.L., Speer C.P. A unique story in neonatal research: the development of a porcine surfactant. Neonatology. 2015;107:321–329. doi: 10.1159/000381117. [DOI] [PubMed] [Google Scholar]
- 120.Halliday H.L. Surfactants: past, present and future. J. Perinatol. 2008;28:47–56. doi: 10.1038/jp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh N., Halliday H.L., Stevens T.P., Suresh G., Soll R., Rojas-Reyes M.X. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2015;12:CD010249. doi: 10.1002/14651858.CD010249.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramanathan R. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J. Perinatol. 2009;292:38–43. doi: 10.1038/jp.2009.31. [DOI] [PubMed] [Google Scholar]
- 123.Braide-Moncoeur O., Tran N.T., Long J.R. Peptide-based synthetic pulmonary surfactant for the treatment of respiratory distress disorders. Curr. Opin. Chem. Biol. 2016;32:22–28. doi: 10.1016/j.cbpa.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Zhang L., Cao H.Y., Zhao S., Yuan L.J., Han D., Jiang H., Wu S., Wu H.M. Effect of exogenous pulmonary surfactants on mortality rate in neonatal respiratory distress syndrome: A network meta-analysis of randomized controlled trials. Pulm. Pharmacol. Ther. 2015;34:46–54. doi: 10.1016/j.pupt.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Walther F.J., Gordon L.M., Waring A.J. Design of surfactant protein B peptide mimics based on the saposin fold for synthetic lung surfactants. Biomed. Hub. 2016;1:3. doi: 10.1159/000451076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharifahmadian M., Sarker M., Palleboina D., Waring A.J., Walther F.J., Morrow M.R., Booth V. Role of the N-terminal seven residues of surfactant protein B (SP-B) PLoS One. 2013;8:72821. doi: 10.1371/journal.pone.0072821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walther F.J., Waring A.J., Hernandez-Juviel J.M., Gordon L.M., Wang Z., Jung C.L.L., Ruchala P., Clark A.P., Smith W.M., Sharma S., Notter R.H., Morty R.E. Critical structural and functional roles for the N-terminal insertion sequence in surfactant protein B analogs. PLoS One. 2010;5:8672. doi: 10.1371/journal.pone.0008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Notter R.H., Wang Z., Walther F.J. Activity and biophysical inhibition resistance of a novel synthetic lung surfactant containing super-mini-B DATK peptide. PeerJ. 2016;4:1528. doi: 10.7717/peerj.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calkovska A., Linderholm B., Haegerstrand-Björkman M., Pioselli B., Pelizzi N., Johansson J., Curstedt T. Phospholipid composition in synthetic surfactants is important for tidal volumes and alveolar stability in surfactant-treated preterm newborn rabbits. Neonatology. 2016;109:177–185. doi: 10.1159/000442874. [DOI] [PubMed] [Google Scholar]
- 130.Johansson J., Nilsson G., Stromberg R., Robertson B., Jornvall H., Curstedt T. Secondary structure and biophysical activity of synthetic analogues of the pulmonary surfactant polypeptide SP-C. Biochem. J. 1995;307:535–541. doi: 10.1042/bj3070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nilsson G., Gustafsson M., Vandenbussche G., Veldhuizen E., Griffiths W.J., Sjovall J., Haagsman H.P., Ruysschaert J.M., Robertson B., Curstedt T., Johansson J. Synthetic peptide-containing surfactants. Evaluation of transmembrane versus amphipathic helices and surfactant protein C poly-valyl to poly-leucyl substitution. Eur. J. Biochem. 1998;255:116–124. doi: 10.1046/j.1432-1327.1998.2550116.x. [DOI] [PubMed] [Google Scholar]
- 132.Clercx A., Vandenbussche G., Curstedt T., Johansson J., Jornvall H., Ruysschaert J.M. Structural and functional importance of the C-terminal part of the pulmonary surfactant polypeptide SP-C. Eur. J. Biochem. 1995;229:465–472. [PubMed] [Google Scholar]
- 133.Johansson J., Some M., Linderholm B.M., Almlén A., Curstedt T., Robertson B. A synthetic surfactant based on a poly-Leu SP-C analog and phospholipids: effects on tidal volumes and lung gas volumes in ventilated immature newborn rabbits. J. Appl. Physiol. 2003;95:2055–2063. doi: 10.1152/japplphysiol.00153.2003. [DOI] [PubMed] [Google Scholar]
- 134.Almlén A., Vandenbussche G., Linderholm B., Haegerstrand-Björkman M., Johansson J., Curstedt T. Alterations of the C-terminal end do not affect in vitro or in vivo activity of surfactant protein C analogs. Biochim. Biophys. Acta Biomembr. 2012;1818:27–32. doi: 10.1016/j.bbamem.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 135.Seehase M., Collins J.J.P., Kuypers E., Jellema R.K., Ophelders D.R.M.G., Ospina O.L., Pérez-Gil J., Bianco F., Garzia R., Razzetti R., Kramer B.W. New surfactant with SP-B and C analogs gives survival benefit after inactivation in preterm lambs. PLoS One. 2012;7:e47631. doi: 10.1371/journal.pone.0047631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sato A., Ikegami M. SP-B and SP-C containing new synthetic surfactant for treatment of extremely immature lamb lung. PLoS One. 2012;7:e39392. doi: 10.1371/journal.pone.0039392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walther F.J., Gordon L.M., Waring A.J. Advances in synthetic lung surfactant protein technology. Expert Rev. Respir. Med. 2019;13:499–501. doi: 10.1080/17476348.2019.1589372. [DOI] [PubMed] [Google Scholar]
- 138.Perl M., Lomas-Neira J., Venet F., Chung C.S., Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev. Respir. Med. 2011;5:115–126. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taeusch H.W., De La Serna J.B., Pérez-Gil J., Alonso C., Zasadzinski J.A. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys. J. 2005;89:1769–1779. doi: 10.1529/biophysj.105.062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin definition. J. Am. Med. Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 141.Bohr A., Tsapis N., Andreana I., Chamarat A., Foged C., Delomenie C., Noiray M., El Brahmi N., Majoral J.P., Mignani S., Fattal E. Anti-inflammatory effect of anti-TNF-α siRNA cationic phosphorus dendrimer nanocomplexes administered intranasally in a murine acute lung injury model. Biomacromolecules. 2017;18:2379–2388. doi: 10.1021/acs.biomac.7b00572. [DOI] [PubMed] [Google Scholar]
- 142.Oh B., Lee M. Combined delivery of HMGB-1 box A peptide and S1PLyase siRNA in animal models of acute lung injury. J. Control. Release. 2014;175:25–35. doi: 10.1016/j.jconrel.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 143.Wang S., Li Z., Wang X., Zhang S., Gao P., Shi Z. The role of pulmonary surfactants in the treatment of acute respiratory distress syndrome in COVID-19. Front. Pharmacol. 2021;12:1640. doi: 10.3389/fphar.2021.698905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soiza R.L., Donaldson A.I.C., Myint P.K. Vaccine against arteriosclerosis: an update. Ther. Adv. Vacc. 2018;9:259–261. [Google Scholar]
- 146.Gerosa C., Fanni D., Cau F., Ravarino A., Senes G., Demontis R., Coni P., Piras M., Orru G., Coghe F., Congiu T., La Nasa G., D’Aloja E., Saba L., Faa G. Immunohistochemical findings in the lungs of COVID-19 subjects: evidence of surfactant dysregulation. Eur. Rev. Med. Pharmacol. Sci. 2021;25:4639–4643. doi: 10.26355/eurrev_202107_26257. [DOI] [PubMed] [Google Scholar]
- 147.Islam A.B.M.M.K., Khan M.A.A.K. Lung transcriptome of a COVID-19 patient and systems biology predictions suggest impaired surfactant production which may be druggable by surfactant therapy. Sci. Rep. 2020;10:1–17. doi: 10.1038/s41598-020-76404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kerget B., Kerget F., Koçak A.O., Kızıltunç A., Araz Ö., Uçar E.Y., Akgün M. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID-19? Lung. 2020;198:777–784. doi: 10.1007/s00408-020-00393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tong M., Xiong Y., Zhu C., et al. Serum surfactant protein D in COVID-19 is elevated and correlated with disease severity. BMC Infect. Dis. 2021;21:737. doi: 10.1186/s12879-021-06447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kandasamy P., Numata M., Berry K.Z., Fickes R., Leslie C.C., Murphy R.C., Voelker D.R. Structural analogs of pulmonary surfactant phosphatidylglycerol inhibit toll-like receptor 2 and 4 signaling. J. Lipid Res. 2016;57:993–1005. doi: 10.1194/jlr.M065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Numata M., Grinkova Y.V., Mitchell J.R., Chu H.W., Sligar S.G., Voelker D.R. Nanodiscs as a therapeutic delivery agent: inhibition of respiratory syncytial virus infection in the lung. Int. J. Nanomedicine. 2013;8:1417–1427. doi: 10.2147/IJN.S39888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Numata M., Kandasamy P., Nagashima Y., Fickes R., Murphy R.C., Voelker D.R. Phosphatidylinositol inhibits respiratory syncytial virus infection. J. Lipid Res. 2015;56:578–587. doi: 10.1194/jlr.M055723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Numata M., Kandasamy P., Nagashima Y., Posey J., Hartshorn K., Woodland D., Voelker D.R. Phosphatidylglycerol suppresses influenza A virus infection. Am. J. Respir. Cell Mol. Biol. 2012;46:479–487. doi: 10.1165/rcmb.2011-0194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Joel Funk C., Wang J., Ito Y., Travanty E.A., Voelker D.R., Holmes K.V., Mason R.J. Infection of human alveolar macrophages by human coronavirus strain 229E. J. Gen. Virol. 2012;93:494–503. doi: 10.1099/vir.0.038414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leth-Larsen R., Zhong F., Chow V.T.K., Holmskov U., Lu J. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology. 2007;212:201–211. doi: 10.1016/j.imbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van Iwaarden J.F., van Strijp J.A., Ebskamp M.J., Welmers A.C., Verhoef J., van Golde L.M. Surfactant protein A is opsonin in phagocytosis of herpes simplex virus type 1 by rat alveolar macrophages. Am. J. Physiol. Cell. Mol. Physiol. 1991;261:204–209. doi: 10.1152/ajplung.1991.261.2.L204. [DOI] [PubMed] [Google Scholar]
- 159.Li L., Zheng Q., Zhang Y., Li P., Fu Y., Hou J., Xiao X. Antiviral activity of recombinant porcine surfactant protein A against porcine reproductive and respiratory syndrome virus in vitro. Arch. Virol. 2016;161:1883–1890. doi: 10.1007/s00705-016-2838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McKenzie Z., Kendall M., Mackay R.M., Tetley T.D., Morgan C., Griffiths M., Clark H.W., Madsen J. Nanoparticles modulate surfactant protein A and D mediated protection against influenza A infection in vitro. Philos. Trans. R. Soc. B Biol. Sci. 2014;370:20140049. doi: 10.1098/rstb.2014.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Perino J., Thielens N.M., Crouch E., Spehner D., Crance J.M., Favier A.L. Protective effect of surfactant protein D in pulmonary vaccinia virus infection: implication of A27 viral protein. Viruses. 2013;5:928–953. doi: 10.3390/v5030928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hillaire M.L.B., Van Eijk M., Vogelzang-van Trierum S.E., Fouchier R.A.M., Osterhaus A.D.M.E., Haagsman H.P., Rimmelzwaan G.F. Recombinant porcine surfactant protein D inhibits influenza A virus replication ex vivo. Virus Res. 2014;181:22–26. doi: 10.1016/j.virusres.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 163.Laffey J.G., Kavanagh B.P. Insight into acute respiratory distress syndrome: from models to patients. Am. J. Respir. Crit. Care Med. 2017;196:18–28. doi: 10.1164/rccm.201612-2415CI. [DOI] [PubMed] [Google Scholar]
- 164.Krafft M.P. Overcoming inactivation of the lung surfactant by serum proteins: A potential role for fluorocarbons? Soft Matter. 2015;11:5982–5994. doi: 10.1039/c5sm00926j. [DOI] [PubMed] [Google Scholar]
- 165.Alig T.F., Warriner H.E., Lee L., Zasadzinski J.A. Electrostatic barrier to recovery of dipalmitoylphosphatidylglycerol monolayers after collapse. Biophys. J. 2004;86:897–904. doi: 10.1016/S0006-3495(04)74165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schürch S., Green F.H.Y., Bachofen H. Formation and structure of surface films: captive bubble surfactometry. Biochim. Biophys. Acta Mol. Basis Dis. 1998;1408:180–202. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 167.Madan T., Biswas B., Varghese P.M., Subedi R., Pandit H., Idicula-Thomas S., Kundu I., Rooge S., Agarwal R., Tripathi D.M., Kaur S., Gupta E., Gupta S.K., Kishore U. A recombinant fragment of human surfactant protein D binds spike protein and inhibits infectivity and replication of SARS-CoV-2 in clinical samples. Am. J. Respir. Cell. Mol. Biol. 2021;65:41–53. doi: 10.1165/rcmb.2021-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hsieh M.H., Beirag N., Murugaiah V., Chou Y.C., Kuo W.S., Kao H.F., Madan T., Kishore U., Wang J.Y. Human surfactant protein D binds S1 and receptor binding domain of spike protein and acts as an entry inhibitor of SARS-CoV-2 pseudotyped viral particles in vitro. Front Immunol. 2021;12:1613. doi: 10.3389/fimmu.2021.641360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Herting E., Rauprich P., Stichtenoth G., Walter G., Johansson J., Robertson B. Resistance of different surfactant preparations to inactivation by meconium. Pediatr. Res. 2001;50:44–49. doi: 10.1203/00006450-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 170.Fehrholz M., Glaser K., Seidenspinner S., Ottensmeier B., Curstedt T., Speer C.P., Kunzmann S. Impact of the new generation reconstituted surfactant CHF5633 on human CD4+ lymphocytes. PLoS One. 2016;11:e0153578. doi: 10.1371/journal.pone.0153578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Glaser K., Fehrholz M., Curstedt T., Kunzmann S., Speer C.P. Effects of the new generation synthetic reconstituted surfactant CHF5633 on proand anti-inflammatory cytokine expression in native and LPS-stimulated adult CD14+ monocytes. PLoS One. 2016;11:e0146898. doi: 10.1371/journal.pone.0146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Glaser K., Fehrholz M., Henrich B., Claus H., Papsdorf M., Speer C.P. Anti-inflammatory effects of the new generation synthetic surfactant CHF5633 on ureaplasma-induced cytokine responses in human monocytes. Expert Rev. Anti-Infect. Ther. 2017;15:181–189. doi: 10.1080/14787210.2017.1259067. [DOI] [PubMed] [Google Scholar]
- 173.Walmrath D., Grimminger F., Pappert D., Knothe C., Obertacke U., Benzing A., Günther A., Schmehl T., Leuchte H., Seeger W. Bronchoscopic administration of bovine natural surfactant in ARDS and septic shock: impact on gas exchange and haemodynamics. Eur. Respir. J. 2002;19:805–810. doi: 10.1183/09031936.02.00243402. [DOI] [PubMed] [Google Scholar]
- 174.Shah S. Exogenous surfactant: intubated present, nebulized future? J. Pediatr. 2011;7:11–15. doi: 10.1007/s12519-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 175.Rzepka-Wrona P., Skoczynski S., Wrona D., Barczyk A. Inhalation techniques used in patients with respiratory failure treated with noninvasive mechanical ventilation. Can. Respir. J. 2018;2018:8959370. doi: 10.1155/2018/8959370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dhand R. How should aerosols be delivered during invasive mechanical ventilation? Respir. Care. 2017;62:1343–1367. doi: 10.4187/respcare.05803. [DOI] [PubMed] [Google Scholar]
- 177.Willson D.F. Aerosolized surfactants, anti-inflammatory drugs, and analgesics. Respir. Care. 2015;60:774–790. doi: 10.4187/respcare.03579. [DOI] [PubMed] [Google Scholar]
- 178.Marks L.B., Notter R.H., Oberdorster G., McBride J.T. Ultrasonic and jet aerosolization of phospholipids and the effects on surface activity. Pediatr. Res. 1983;17:742–747. doi: 10.1203/00006450-198309000-00012. [DOI] [PubMed] [Google Scholar]
- 179.Bahlmann H., Sun B., Nilsson G., Curstedt T., Robertson B. Aerosolized surfactant in lung-lavaged adult rats: factors influencing the therapeutic response. Acta Anaesthesiol. Scand. 2000;44:612–622. doi: 10.1034/j.1399-6576.2000.00521.x. [DOI] [PubMed] [Google Scholar]
- 180.Schermuly R.T., Günther A., Weissmann N., Ghofrani H.A., Seeger W., Grimminger F., Walmrath D. Differential impact of ultrasonically nebulized versus tracheal-instilled surfactant on ventilation-perfusion (VA/Q) mismatch in a model of acute lung injury. Am. J. Respir. Crit. Care Med. 2000;161:152–159. doi: 10.1164/ajrccm.161.1.9812017. [DOI] [PubMed] [Google Scholar]
- 181.Busani S., Dall’Ara L., Tonelli R., Clini E., Munari E., Venturelli S., Meschiari M., Guaraldi G., Cossarizza A., Ranieri V.M., Girardis M. Surfactant replacement might help recovery of low-compliance lung in severe COVID-19 pneumonia. Ther. Adv. Respir. Dis. 2020;14:1–6. doi: 10.1177/1753466620951043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Piva S., DiBlasi R.M., Slee A.E., Jobe A.H., Roccaro A.M., Filippini M., Latronico N., Bertoni M., Marshall J.C., Portman M.A. Surfactant therapy for COVID-19 related ARDS: a retrospective case–control pilot study. Respir. Res. 2021;22:1–8. doi: 10.1186/s12931-020-01603-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ali M. Handbook of Non-invasive Drug Delivery Systems. Elsevier; 2010. Pulmonary drug delivery; pp. 209–246. [Google Scholar]
- 184.Joshi N., Shirsath N., Singh A., et al. Endogenous lung surfactant inspired pH responsive nanovesicle aerosols: pulmonary compatible and site-specific drug delivery in lung metastases. Sci.Rep. 2014;4:7085. doi: 10.1038/srep07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 186.Horby P.W., Mafham M., Bell J.L., Linsell L., Staplin N., Emberson J., Palfreeman A., Raw J., Elmahi E., Prudon B., Green C., Carley S., Chadwick D., Davies M., Wise M.P., Baillie J.K., Chappell L.C., Faust S.N., Jaki T., Jefferey K., Lim W.S., Montgomery A., Rowan K., Juszczak E., Haynes R., Landray M.J. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.The RECOVERY collaborative group Effect of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Farnung L., Siewert A., Höbartner C., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12:1–7. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koenig P.A., Das H., Liu H., Kümmerer B.M., Gohr F.N., Jenster L.M., Schiffelers L.D.J., Tesfamariam Y.M., Uchima M., Wuerth J.D., Gatterdam K., Ruetalo N., Christensen M.H., Fandrey C.I., Normann S., Tödtmann J.M.P., Pritzl S., Hanke L., Boos J., Yuan M., Zhu X., Schmid-Burgk J.L., Kato H., Schindler M., Wilson I.A., Geyer M., Ludwig K.U., Hällberg B.M., Wu N.C., Schmidt F.I. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021;371:6530. doi: 10.1126/science.abe6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Horby P.W. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ramakrishnan S., Nicolau D.V., Langford B., Mahdi M., Jeffers H., Mwasuku C., Krassowska K., Fox R., Binnian I., Glover V., Bright S., Butler C., Cane J.L., Halner A., Matthews P.C., Donnelly L.E., Simpson J.L., Baker J.R., Fadai N.T., Peterson S., Bengtsson T., Barnes P.J., Russell R.E.K., Bafadhel M. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021;9:763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamaya M., Nishimura H., Deng X., Sugawara M., Watanabe O., Nomura K., Shimotai Y., Momma H., Ichinose M., Kawase T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir. Investig. 2020;58:155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bochkov Y.A., Busse W.W., Brockman-Schneider R.A., Evans M.D., Jarjour N.N., McCrae C., Miller-Larsson A., Gern J.E. Budesonide and formoterol effects on rhinovirus replication and epithelial cell cytokine responses. Respir. Res. 2013;14:98. doi: 10.1186/1465-9921-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., Shimojima M., Fukushi S. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J. Virol. 2020;95:e01648–20. doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Covis Pharma. Covis Pharma Group announces top-line safety and efficacy data from a phase 3 placebo controlled COVID-19 study using inhaled corticosteroid (ciclesonide) [Internet]. 2021. Available from: https://covispharma.com/index.php/press-release-3/.
- 196.Lammers T., Sofias A.M., van der Meel R., Schiffelers R., Storm G., Tacke F., Koschmieder S., Brümmendorf T.H., Kiessling F., Metselaar J.M. Dexamethasone nanomedicines for COVID-19. Nat. Nanotechnol. 2020;15:622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hidalgo A., Cruz A., Pérez-Gil J. Pulmonary surfactant and nanocarriers: toxicity versus combined nanomedical applications. Biochim. Biophys. Acta Biomembr. 2017;1859:1740–1748. doi: 10.1016/j.bbamem.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 198.Yan Y.M., Li Y.D., Song X.L., Liu M., Diao F., Wang Y., Sun Y., Wang Z.H., Lu J. Therapeutic effects of inhaling aerosolized surfactant alone or with dexamethasone generated by a novel noninvasive apparatus on acute lung injury in rats. J. Trauma Acute Care Surg. 2012;73:1114–1120. doi: 10.1097/TA.0b013e318265cbe9. [DOI] [PubMed] [Google Scholar]
- 199.Hidalgo A., Salomone F., Fresno N., Orellana G., Cruz A., Pérez-Gil J. Efficient interfacially driven vehiculization of corticosteroids by pulmonary surfactant. Langmuir. 2017;33:7929–7939. doi: 10.1021/acs.langmuir.7b01177. [DOI] [PubMed] [Google Scholar]
- 200.Van’t Veen A., Mouton J.W., Gommers D., Lachmann B. Pulmonary surfactant as vehicle for intratracheally instilled tobramycin in mice infected with Klebsiella pneumoniae. Br. J. Pharmacol. 1996;119:1145–1148. doi: 10.1111/j.1476-5381.1996.tb16016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Canadas O., Guerrero R., Garcıa-Canero R., Orellana G., Menendez M., Casals C. Characterization of liposomal tacrolimus in lung surfactant-like phospholipids and evaluation of its immunosuppressive activity. Biochemistry. 2004;43:9926–9938. doi: 10.1021/bi036227z. [DOI] [PubMed] [Google Scholar]
- 202.Banaschewski B.J.H., Veldhuizen E.J.A., Keating E., Haagsman H.P., Zuo Y.Y., Yamashita C.M., Veldhuizen R.A.W. Antimicrobial and biophysical properties of surfactant supplemented with an antimicrobial peptide for treatment of bacterial pneumonia. Antimicrob. Agents Chemother. 2015;59:3075–3083. doi: 10.1128/AAC.04937-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Herting E., Gan X., Rauprich P., Jarstrand C., Robertson B. Combined treatment with surfactant and specific immunoglobulin reduces bacterial proliferation in experimental neonatal group B streptococcal pneumonia. Am. J. Respir. Crit. Care Med. 1999;159:1862–1867. doi: 10.1164/ajrccm.159.6.9810047. [DOI] [PubMed] [Google Scholar]
- 204.Walther F.J., David-Cu R., Lopez S.L. Antioxidant-surfactant liposomes mitigate hyperoxic lung injury in premature rabbits. Am. J. Phys. 1995;269:613–617. doi: 10.1152/ajplung.1995.269.5.L613. [DOI] [PubMed] [Google Scholar]
- 205.Katkin J.P., Husser R.C., Langston C., Welty S.E. Exogenous surfactant enhances the delivery of recombinant adenoviral vectors to the lung. Hum. Gene Ther. 1997;8:171–185. doi: 10.1089/hum.1997.8.2-171. [DOI] [PubMed] [Google Scholar]
- 206.Kantar A., Klimek L., Cazan D., Sperl A., Sent U., Mesquita M. Ambroxol for the treatment of children with acute and chronic respiratory diseases: an overview of efficacy and safety. Multidiscip. Respir. Med. 2020;15:511. doi: 10.4081/mrm.2020.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Malerba M., Ragnoli B. Ambroxol in the 21st century: pharmacological and clinical update. Expert Opin. Drug Metab. Toxicol. 2008;4:1119–1129. doi: 10.1517/17425255.4.8.1119. [DOI] [PubMed] [Google Scholar]
- 208.Paleari D., Rossi G.A., Nicolini G., Olivieri D. Ambroxol: a multifaceted molecule with additional therapeutic potentials in respiratory disorders of childhood. Expert Opin. Drug Discovery. 2011;6:1203–1214. doi: 10.1517/17460441.2011.629646. [DOI] [PubMed] [Google Scholar]
- 209.Kumar P. Co-aerosolized pulmonary surfactant and ambroxol for COVID-19 ARDS intervention: what are we waiting for? Front. Bioeng. Biotechnol. 2020;8:577172. doi: 10.3389/fbioe.2020.577172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sridharan K., Gogtay N.J. Therapeutic nucleic acids: current clinical status. Br. J. Clin. Pharmacol. 2016;82:659–672. doi: 10.1111/bcp.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wilson R.C., Doudna J.A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Raemdonck K., Vandenbroucke R.E., Demeester J., Sanders N.N., De Smedt S.C. Maintaining the silence: reflections on long-term RNAi. Drug Discov. Today. 2008;13:917–931. doi: 10.1016/j.drudis.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dorsett Y., Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat. Rev. Drug Discov. 2004;3:318–329. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- 214.Kim D.H., Rossi J.J. RNAi mechanisms and applications. Biotechniques. 2008;44:613–616. doi: 10.2144/000112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aagaard L. RNAi therapeutics: principles, prospects and challenges. Adv. Drug Deliv. Rev. 2020;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bitko V., Barik S. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 2001;1:1–11. doi: 10.1186/1471-2180-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 220.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., Caricchio R., Mahmud S., Hazen M.M., Halyabar O., Hoyt K.J., Han J., Grom A.A., Gattorno M., Ravelli A., De Benedetti F., Behrens E.M., Cron R.Q., Nigrovic P.A. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Carnero Contentti E., Correa J. Immunosuppression during the COVID-19 pandemic in neuromyelitis optica spectrum disorders patients: a new challenge. Mult. Scler. Relat. Disord. 2020;41:102097. doi: 10.1016/j.msard.2020.102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang Y., Shen C., Li J., et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020;146:119–127. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ghosh S., Firdous S.M., Nath A. siRNA could be a potential therapy for COVID-19. EXCLI J. 2020;19:528–531. doi: 10.17179/excli2020-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Uludağ H., Parent K., Aliabadi H.M., Haddadi A. Prospects for RNAi therapy of COVID-19. Front. Bioeng. Biotechnol. 2020;8:916. doi: 10.3389/fbioe.2020.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ian Gallicano G., Casey J.L., Fu J., Mahapatra S. Molecular targeting of vulnerable RNA sequences in SARS CoV-2: identifying clinical feasibility. Gene Ther. 2020:1–8. doi: 10.1038/s41434-020-00210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cyranoski D. Alarming COVID variants show vital role of genomic surveillance. Nature. 2021;589:337–338. doi: 10.1038/d41586-021-00065-4. [DOI] [PubMed] [Google Scholar]
- 228.Garbuzenko O.B., Ivanova V., Kholodovych V., Reimer D.C., Reuhl K.R., Yurkow E., Adler D., Minko T. Combinatorial treatment of idiopathic pulmonary fibrosis using nanoparticles with prostaglandin E and siRNA(s) Nanomedicine. 2017;13:1983–1992. doi: 10.1016/j.nano.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zheng M., Liu Y., Wang Y., Zhang D., Zou Y., Ruan W., Yin J., Tao W., Park J.B., Shi B. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv. Mater. 2019;31:1903277. doi: 10.1002/adma.201903277. [DOI] [PubMed] [Google Scholar]
- 230.Aliabadi H.M., Mahdipoor P., Bisoffi M., Hugh J.C., Uludağ H. Single and combinational siRNA therapy of cancer cells: probing changes in targeted and nontargeted mediators after siRNA treatment. Mol. Pharm. 2016;13:4116–4128. doi: 10.1021/acs.molpharmaceut.6b00711. [DOI] [PubMed] [Google Scholar]
- 231.Parmar M.B., Meenakshi Sundaram D.N., Remant R.B., Maranchuk R., Montazeri Aliabadi H., Hugh J.C., Löbenberg R., Uludağ H. Combinational siRNA delivery using hyaluronic acid modified amphiphilic polyplexes against cell cycle and phosphatase proteins to inhibit growth and migration of triple-negative breast cancer cells. Acta Biomater. 2018;66:294–309. doi: 10.1016/j.actbio.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 232.Merkel O.M., Zheng M., Debus H., Kissel T. Pulmonary gene delivery using polymeric nonviral vectors. Bioconjug. Chem. 2012;23:3–20. doi: 10.1021/bc200296q. [DOI] [PubMed] [Google Scholar]
- 233.Merkel O.M., Rubinstein I., Kissel T. siRNA delivery to the lung: what’s new? Adv. Drug Deliv. Rev. 2014;75:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Raesch S.S., Tenzer S., Storck W., Rurainski A., Selzer D., Ruge C.A., Pérez-Gil J., Schaefer U.F., Lehr C.M. Proteomic and lipidomic analysis of nanoparticle corona upon contact with lung surfactant reveals differences in protein, but not lipid composition. ACS Nano. 2015;9:11872–11885. doi: 10.1021/acsnano.5b04215. [DOI] [PubMed] [Google Scholar]
- 235.Hu G., Jiao B., Shi X., Valle R.P., Fan Q., Zuo Y.Y. Physicochemical properties of nanoparticles regulate translocation across pulmonary surfactant monolayer and formation of lipoprotein corona. ACS Nano. 2013;7:10525–10533. doi: 10.1021/nn4054683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Theodorou I.G., Ruenraroengsak P., Gow A., Zhang J.J., Chung K.F., Tetley T.D., Ryan M.P., Porter A.E. Effect of pulmonary surfactant on the dissolution, stability and uptake of zinc oxide nanowires by human respiratory epithelial cells. Nanotoxicology. 2017;10:1351–1362. doi: 10.1080/17435390.2016.1214762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Geiser M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol. Med. Pulm. Drug Deliv. 2010;23:207–217. doi: 10.1089/jamp.2009.0797. [DOI] [PubMed] [Google Scholar]
- 238.Fan Q., Wang Y.E., Zhao X., Loo J.S., Zuo Y.Y. Adverse biophysical effects of hydroxyapatite nanoparticles on natural pulmonary surfactant. ACS Nano. 2011;5:6410–6416. doi: 10.1021/nn2015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kodama A.T., Kuo C.C., Boatwright T., Dennin M. Investigating the effect of particle size on pulmonary surfactant phase behavior. Biophys. J. 2014;107:1573–1581. doi: 10.1016/j.bpj.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bakshi M.S., Zhao L., Smith R., Possmayer F., Petersen N.O. Metal nanoparticle pollutants interfere with pulmonary surfactant function in vitro. Biophys. J. 2008;94:855–868. doi: 10.1529/biophysj.107.106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ruge C.A., Kirch J., Cañadas O., Schneider M., Pérez-Gil J., Schaefer U.F., Casals C., Lehr C.M. Uptake of nanoparticles by alveolar macrophages is triggered by surfactant protein A. Nanomedicine. 2011;7:690–693. doi: 10.1016/j.nano.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 242.Ruge C.A., Schäfer U.F., Kirch J., Schneider M., Cañadas O., Pérez-Gil J., Casals C., Lehr C.M. Role of surfactant proteins in the interaction of nanoparticles with the air-blood-barrier. Pneumologie. 2011;65 [Google Scholar]
- 243.Wohlleben W., Driessen M.D., Raesch S., et al. Influence of agglomeration and specific lung lining lipid/protein interaction on short-term inhalation toxicity. Nanotoxicology. 2016;10:970–980. doi: 10.3109/17435390.2016.1155671. [DOI] [PubMed] [Google Scholar]
- 244.Norde W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interf. Sci. 1986;25:267–340. doi: 10.1016/0001-8686(86)80012-4. [DOI] [PubMed] [Google Scholar]
- 245.Beck-Broichsitter M., Ruppert C., Schmehl T., Guenther A., Betz T., Bakowsky U., Seeger W., Kissel T., Gessler T. Biophysical investigation of pulmonary surfactant surface properties upon contact with polymeric nanoparticles in vitro. Nanomedicine. 2011;7:341–350. doi: 10.1016/j.nano.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 246.Beck-Broichsitter M., Ruppert C., Schmehl T., Günther A., Seeger W. Biophysical inhibition of synthetic vs. naturally-derived pulmonary surfactant preparations by polymeric nanoparticles. Biochim. Biophys. Acta Biomembr. 2014;1838:474–481. doi: 10.1016/j.bbamem.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 247.Scott C.C., Vacca F., Gruenberg J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014;31:2–10. doi: 10.1016/j.semcdb.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 248.De Backer L., Cerrada A., Pérez-Gil J., De Smedt S.C., Raemdonck K. Bio-inspired materials in drug delivery: exploring the role of pulmonary surfactant in siRNA inhalation therapy. J. Control. Release. 2015;220:642–650. doi: 10.1016/j.jconrel.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 249.Raemdonck K., Naeye B., Buyens K., Vandenbroucke R.E., Høgset A., Demeester J., De Smedt S.C. Biodegradable dextran nanogels for RNA interference: focusing on endosomal escape and intracellular siRNA delivery. Adv. Funct. Mater. 2009;19:1406–1415. [Google Scholar]
- 250.Jensen L.B., Griger J., Naeye B., Varkouhi A.K., Raemdonck K., Schiffelers R., Lammers T., Storm G., De Smedt S.C., Sproat B.S., Nielsen H.M., Foged C. Comparison of polymeric siRNA nanocarriers in a murine LPS-activated macrophage cell line: gene silencing, toxicity and off-target gene expression. Pharm. Res. 2012;29:669–682. doi: 10.1007/s11095-011-0589-0. [DOI] [PubMed] [Google Scholar]
- 251.Echaide M., Vazquez-Sanchez S., Cruz A., Pérez-Gil J. Functional and structural characterization of a lyophilized pulmonary surfactant directly applied onto the air-liquid interface. Biophys. J. 2020;118:388. [Google Scholar]
- 252.Gregory T.J., Irshad H., Chand R., Kuehl P.J. Deposition of aerosolized lucinactant in nonhuman primates. J. Aerosol Med. Pulm. Drug Deliv. 2020;33:21–33. doi: 10.1089/jamp.2018.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mazela J., Merritt T.A., Terry M.H., Gregory T.J., Blood A.B. Comparison of poractant alfa and lyophilized lucinactant in a preterm lamb model of acute respiratory distress. Pediatr. Res. 2012;721:32–37. doi: 10.1038/pr.2012.46. [DOI] [PubMed] [Google Scholar]
- 254.Olmeda B., García-Álvarez B., Gómez M.J., Martínez-Calle M., Cruz A., Pérez-Gil J. A model for the structure and mechanism of action of pulmonary surfactant protein B. FASEB J. 2015;29:4236–4247. doi: 10.1096/fj.15-273458. [DOI] [PubMed] [Google Scholar]
- 255.Guagliardo R., Merckx P., Zamborlin A., De Backer L., Echaide M, Pérez-Gil J., et al. Nanocarrier lipid composition modulates the impact of pulmonary surfactant protein B (SP-B) on cellular delivery of siRNA. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Erazo-Oliveras A., Najjar K., Truong D., Wang T.Y., Brock D.J., Prater A.R., Pellois J.P. The late endosome and its lipid BMP act as gateways for efficient cytosolic access of the delivery agent dfTAT and its macromolecular cargos. Cell Chem. Biol. 2016;23:598–607. doi: 10.1016/j.chembiol.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Qiu Y., Chow M.Y.T., Liang W., Chung W.W.Y., Mak J.C.W., Lam J.K.W. From pulmonary surfactant, synthetic KL4 peptide as effective siRNA delivery vector for pulmonary delivery. Mol. Pharm. 2017;14:4606–4617. doi: 10.1021/acs.molpharmaceut.7b00725. [DOI] [PubMed] [Google Scholar]
- 258.Qiu Y., Man R.C.H., Liao Q., Kung K.L.K., Chow M.Y.T., Lam J.K.W. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J. Control. Release. 2019;314:102–115. doi: 10.1016/j.jconrel.2019.10.026. [DOI] [PubMed] [Google Scholar]