Abstract
A global pandemic has resulted from the emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19). To control the spread of SARS-CoV-2 infection, several SARS-CoV-2 vaccines have been developed and administered in a wide range of age groups. Messenger ribonucleic acid (mRNA)-based COVID-19 vaccines are the most widely used. We present the case of an 88-year-old woman who was diagnosed with acute disseminated encephalomyelitis (ADEM) following her second mRNA COVID-19 vaccination. She was admitted to hospital with disturbed consciousness (Glasgow Coma Scale E1V1M4) and gaze-evoked nystagmus. Brain magnetic resonance imaging revealed bilateral presence of middle cerebellar peduncle sign. Following steroid pulse therapy, clinical symptoms improved. The occurrence of ADEM following COVID-19 vaccination does not question the importance of vaccination programs during the COVID-19 pandemic. COVID-19 vaccines have been administered to individuals of a wide range of ages, from children to older adults. Thus, ADEM could occur following COVID-19 vaccination at any age, although ADEM is rare in older adults.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Acute disseminated encephalomyelitis, mRNA, Complications, Adverse effects
Highlights
-
•
An 88-year-old woman was diagnosed with ADEM following mRNA COVID-19 vaccination.
-
•
ADEM may be a rare neurological complication of COVID-19 vaccination.
-
•
ADEM is rare in older adults but may occur following COVID-19 vaccination.
Dear Editor,
List of abbreviations
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| mRNA | messenger ribonucleic acid |
| ADEM | acute disseminated encephalomyelitis |
| CSF | cerebrospinal fluid |
| MCP | middle cerebellar peduncle |
| MRI | magnetic resonance imaging |
| T-cell | T lymphocyte |
| COVID-19 | coronavirus disease 2019 |
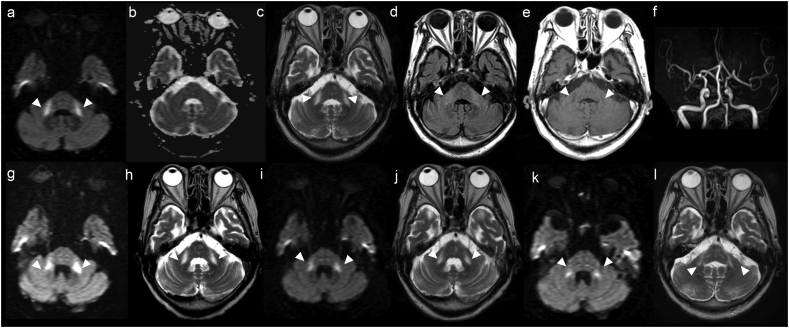
An 88-year-old Japanese woman was admitted to hospital with impaired consciousness (Glasgow Coma Scale: E1V1M4). She had a history of diabetes and Alzheimer's disease, and she had not had any recent infections. Twenty-nine days before admission, she had received her second dose of messenger ribonucleic acid (mRNA)-based BNT162 coronavirus disease 2019 (COVID-19) vaccine (Pfizer, New York City, NY). A clinical examination revealed impaired consciousness and gaze-evoked nystagmus; there were no signs of dysarthria, paralysis, or ataxia. Blood tests showed that the patient did not have hypoglycemia, electrolyte abnormalities, a vitamin deficiency, or abnormal thyroid function. Antibody tests for severe acute respiratory virus 2 (SARS-CoV-2) revealed antibodies against the spike protein, but not the nucleocapsid protein. Tests were also negative for antinuclear-, autoimmune vasculitis-, onconeural-, and anti-ganglioside antibodies. Cerebrospinal fluid (CSF) bacterial and fungal cultures, a CSF oligoclonal band screen, and a test for autoantibodies against myelin basic protein were all negative. A magnetic resonance imaging (MRI) brain scan at admission revealed signal abnormalities in the bilateral middle cerebellar peduncles (MCP; Fig. 1). The patient was treated with steroid pulse therapy (intravenous methylprednisolone, 1000 mg/day for three consecutive days), and the impaired consciousness and gaze-evoked nystagmus were found to improve. Further MRI brain scans revealed the signal abnormalities had decreased by day 31 and day 66 (Fig. 1).
Fig. 1.
MRI findings.
Day 1 in hospital: (a) diffusion-weighted image (DWI), (c) T2-weighted image, and (d) fluid-attenuated inversion recovery image all show hyperintensity of the middle cerebellar peduncles (MCP), although the apparent diffusion coefficient map (b) shows no obvious abnormality. (e) Contrast-enhanced MRI shows low signal intensity in the MCP. (f) MR angiography shows no abnormality.
Day 17: (g) DWI and (h) T2-weighted image both show no lesion improvement.
Day 31: (i) DWI and (j) T2-weighted image both show lesion improvement.
Day 66: (k) DWI and (l) T2-weighted image both show lesion improvement.
DWI, diffusion-weighted image; MCP, middle cerebellar peduncles; MRI, magnetic resonance imaging.
The patient met the diagnostic criteria for acute disseminated encephalomyelitis (ADEM) according to the International Pediatric Multiple Sclerosis Study Group [1]. An MRI scan revealed bilateral presence of the MCP sign, which is known to be observed in ADEM [2]. Previous studies reported a low incidence of ADEM among older adults, but this may be due to the fact that they are less likely to be vaccinated than younger people [3]. As the new COVID-19 vaccines have been administered to a wide range of age groups, it is possible that ADEM could occur following COVID-19 vaccination at any age. To the best of our knowledge, there have been two published reports of ADEM following mRNA COVID-19 vaccination and two reports following other types of COVID-19 vaccination (Table 1) [[4], [5], [6], [7]].
Table 1.
ADEM after COVID-19 vaccination.
| Cases | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Author | Vogrig et al. [4] | Kania et al. [5] | Cao et al. [6] | Rinaldi [7] | This study |
| Age | 56 | 19 | 24 | 45 | 88 |
| Sex | F | F | F | M | F |
| Country | Italy | Poland | China | Italy | Japan |
| Time from vaccination to onset | 14 days | 14 days | 14 days | 12 days | 29 days |
| Vaccine | BNT162 | mRNA-1273 | BBIBP-CorV | ChAdOx1nCoV-19 | BNT162 |
| Vaccine type | mRNA | mRNA | Inactivated | Adenoviral vector | mRNA |
| Developer | Pfizer | Moderna | Sinopharm | AstraZeneca | Pfizer |
| History | Recurrent herpes zoster, postinfectious rhombencephalitis |
Atopic dermatitis, depression | – | – | Diabetes, Alzheimer's disease |
| Symptoms | Gaze-evoked nystagmus, mild weakness on left upper limb, left ataxia | Severe headache, nuchal rigidity, urinary retention, bilateral Babinski signs | Somnolence, memory decline, headache, low-grade fever, muscle stiffness, extremity weakness, reduced appetite, seizure | Decreased visual acuity, gaze-evoked nystagmus dysarthria, dysphagia, weakness on right upper limb, right ataxia, numbness in upper extremities, trunk, and lower extremities, urinary incontinence | Impaired consciousness, gaze-evoked nystagmus |
| Lesion | Left MCP, bilateral cerebral white matter | Brain hemispheres, pons, the medulla oblongata, cerebellum, spinal cord | Bilateral cerebral white matter | Pons, right MCP, right thalamus, spinal cord | Bilateral MCP |
| Treatment | Prednisone | Steroid pulse, plasma exchange, ceftriaxone, acyclovir | IVIg, ceftriaxone, acyclovir, diazepam, levetiracetam | Steroid pulse, followed by oral prednisone | Steroid pulse |
| Prognosis | Mild ataxia remains | Almost all symptoms were improved except for a mild headache | Complete clinical recovery | Complete clinical recovery | Complete clinical recovery |
IVIg, intravenous immunoglobulin, MRI, magnetic resonance imaging; mRNA, messenger ribonucleic acid; MCP, middle cerebellar peduncles.
The rare occurrence of ADEM, a treatable complication, following COVID-19 vaccination does not detract from the public health imperative to vaccinate against COVID-19. Even the rare cases that have been reported were found to have favorable outcomes [[4], [5], [6], [7]]. However, clinicians should be aware that ADEM can potentially occur following mRNA-based COVID-19 vaccination, even among older adults.
Disclosures
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number: 19K17047). The authors declare that there are no conflicts of interest relevant to this work. Ethical compliance statement: this study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We obtained written informed consent for publication from the patient. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Declaration of Competing Interest
None.
Acknowledgment
We thank Bronwen Gardner, PhD, and Jessica Foxton, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Contributor Information
Mai Shimizu, Email: mai-shimizu@juntendo.ac.jp.
Kotaro Ogaki, Email: koogaki@juntendo.ac.jp.
Ryota Nakamura, Email: ryonaka@juntendo.ac.jp.
Eriko Kado, Email: e.kado.pq@juntendo.ac.jp.
Sho Nakajima, Email: synakaji@juntendo.ac.jp.
Naohide Kurita, Email: nkurita@juntendo.ac.jp.
Masao Watanabe, Email: masao-w@juntendo.ac.jp.
Kazuo Yamashiro, Email: kazuo-y@juntendo.ac.jp.
Nobutaka Hattori, Email: nhattori@juntendo.ac.jp.
Takao Urabe, Email: t_urabe@juntendo.ac.jp.
References
- 1.Krupp L.B., Banwell B., Tenembaum S., International Pediatric MS Study Group Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68:S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto K., Tokiguchi S., Furusawa T., Ishikawa K., Quardery A.F., Shinbo S., Sasai K. MR features of diseases involving bilateral middle cerebellar peduncles. Am. J. Neuroradiol. 2003;24:1946–1954. [PMC free article] [PubMed] [Google Scholar]
- 3.Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun. Rev. 2014;13:215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Vogrig A., Janes F., Gigli G.L., Curcio F., Del Negro I., D’Agostini S., Fabris M., Valente M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin. Neurol. Neurosurg. 2021;208 doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kania K., Ambrosius W., Tokarz Kupczyk E., Kozubski W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann. Clin. Transl. Neurol. 2021;8:2000–2003. doi: 10.1002/acn3.51447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao L., Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol. Belg. 2021 doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinaldi V., Bellucci G., Romano A., Bozzao A., Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: a case report. Mult. Scler. 2021 doi: 10.1177/13524585211040222. [DOI] [PubMed] [Google Scholar]