Abstract
Introduction
There are robust associations between use of anticholinergic medicines and adverse effects in older people. However, the nature of these associations for older people living with frailty is yet to be established.
Objectives
The aims were to identify and investigate associations between anticholinergics and adverse outcomes in older people living with frailty and to investigate whether exposure is associated with greater risks according to frailty status.
Methods
MEDLINE, CINAHL, EMBASE, Cochrane Database of Systematic Reviews, Web of Science and PsycINFO were searched to 1 August 2019. Observational studies reporting associations between anticholinergics and outcomes in older adults (average age ≥ 65 years) that reported frailty using validated measures were included. Primary outcomes were physical impairment, cognitive dysfunction, and change in frailty status. Risk of bias was evaluated using the Cochrane Risk of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool. Meta-analysis was undertaken where appropriate.
Results
Thirteen studies (21,516 participants) were included (ten community, one residential aged-care facility and two hospital studies). Observed associations included reduced ability for chair standing, slower gait speeds, poorer physical performance, increased risk of falls and mortality. Conflicting results were reported for grip strength, timed up and go test, cognition and activities of daily living. No associations were observed for transitions between frailty states, psychological wellbeing or benzodiazepine-related adverse reactions. There was no clear evidence of differences in risks according to frailty status.
Conclusions
Anticholinergics are associated with adverse outcomes in older people living with frailty; however, the literature has significant methodological limitations. There is insufficient evidence to suggest greater risks based on frailty, and there is an urgent need to evaluate this further in well-designed studies stratifying by frailty.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-021-00256-5.
Key Points
| Risks associated with anticholinergic burden among older people are well established; however, the nature of associations for those specifically living with frailty are not. |
| This review highlights that in older people with frailty, limited observational evidence indicates associations with reduced ability for chair standing, slower gait speeds, poorer physical performance, increased risk of falls and mortality. |
| Conflicting associations were reported for outcomes such as grip strength and cognition. No associations were observed for outcomes such as transitions between frailty states or change in psychological wellbeing. |
| Few studies within this review stratified by frailty grade, with no clear evidence of differential effects of anticholinergics by frailty. |
| There is a deficiency of studies investigating anticholinergic exposure with a frailty focus. Further research is needed to better inform the use of anticholinergics among older people living with frailty. |
Introduction
Frailty is a condition characterised by loss of biological reserves, failure of physiological mechanisms and vulnerability to adverse outcomes after relatively minor stressor events, such as prescription of a new medication [1]. Older people with frailty are especially vulnerable to experiencing adverse drug reactions (ADRs), with available evidence suggesting this is due to age-related physiological changes impacting on pharmacokinetics and pharmacodynamics [2]. The identification and stratification of older people living with frailty is therefore becoming increasingly important for medicines optimisation, with prescribing requiring constant vigilance, particularly when considering the safety of drug therapies [3]. The potential for medicines to become stressors to older people with frailty can result in a sudden and disproportionate deterioration in physical, mental and social wellbeing [4]. Balancing safe and effective prescribing with minimising risks of medication-related harm in older people with frailty is a priority for the UK National Health Service (NHS), which has recently adopted a population-based frailty stratification approach to medication reviews in primary care [5].
Among the medications that may result in a deterioration of older adults’ wellbeing, anticholinergic medications, specifically those which block acetylcholine by competitively binding to central and peripheral muscarinic receptors [6], have a particularly high rate of ADRs among older adults with advancing frailty [1, 7]. These include delirium, blurred vision, dry mouth, dry eyes, dizziness, heart rhythm disturbance, constipation, urinary retention and orthostatic hypotension, which can be harmful and are commonly observed among older persons [8–10]. There is also robust evidence of cumulative adverse effects of multiple anticholinergic medicines [11–14], referred to as anticholinergic burden [15], found to be associated with adverse outcomes including physical impairment, falls, cognitive dysfunction and all-cause mortality [11–14]. Older people are more likely to be exposed to potent anticholinergic medicines, with the prevalence of such prescribing increasing in this population [16]. This is despite the availability of criterion-based resources, such as the Beers and STOPP/START criteria, which focus on the list of anticholinergic medicines that should be avoided, or used with caution in certain clinical scenarios [17, 18]. There are concerns for older people living with frailty, who are particularly vulnerable to the adverse effects associated with anticholinergic burden [3].
To date, studies investigating anticholinergic burden have typically only focused on the older population as a whole, reporting varying degrees of association with adverse outcomes [11–13]. However, the associations between anticholinergic medication exposure and adverse outcomes specifically in older people living with frailty remains unclear, and whether frailty severity has a risk-modifying role is yet to be established. Additionally, there is an absence of randomised controlled trial (RCT) evidence and long-term safety data from drug trials that could support further understanding of the safety of these medicines in frail patients, typically due to underrepresentation of older populations [19].
This systematic review aims to:
Identify and investigate the current evidence for the associations between anticholinergic medication exposure and adverse health outcomes in older people living with frailty.
Investigate whether anticholinergic medication exposure is associated with greater risk of adverse outcomes for older people according to frailty status.
Methods
A protocol for this review was prospectively registered with PROSPERO https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=140046; reference CRD42019140046), and is reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (Appendix 1 of the supplementary information file; see the Electronic Supplementary Material [ESM]) [20].
Inclusion Criteria
Prospective and retrospective cohort studies and case–control studies were eligible. The study population were older adults in primary, secondary or tertiary care. Studies were included if samples had an average age of 65 years and over, were exposed to anticholinergic medicines, and were living with frailty. An average age of 65 years and over was selected for inclusion rather than a strict cut-off of 65 years, to expand the search more widely, acknowledging that this approach may also yield studies with participants younger than 65. Studies were not excluded entirely based on including younger participants, providing the average age across the sample was 65 and over, representing an older sample as a whole.
The age of 65 was used as this typically defines older people in general [21], and it is the age to typically define older people in clinical practice guidelines for pharmacotherapy [22]. Also, the age of 65 was selected with a view to focusing on older people with frailty, rather than just older people. A systematic review reporting the prevalence of frailty found that it increased steadily with age, but only around 4% of 65- to 69-year-olds were considered to be living with frailty [23]. The landmark study by Fried and colleagues which developed the frailty phenotype model also limited data analysis to those aged 65 and over [24]. Therefore, it was decided that an average age of 65 and over was the most suitable lower threshold for inclusion, with the perspective that the prevalence of frailty in younger ages would be low.
Frailty
Studies were included if any validated frailty assessment measure was used and if the study population, on average, was frail according to at least one frailty measure. In studies stratifying frailty status using the phenotype model, pre-frailty was defined as the presence of one or two components from the following five components: weakness, slow walking speed, exhaustion, low physical activity and unintentional weight loss. The presence of three or more of these components characterised frailty [24]. Where frailty was not explicitly identified, studies were included if they reported measures of the clinical features of frailty, defined by the phenotype model only [24]. Established thresholds for frailty measures were used; for example, > 0.8 m/s for walking speed (gait) [25], > 12 s to complete the timed up and go test (TUGT) [26] and a grip strength of < 20 kg (females) or < 30 kg (males) [24]. A broad definition of frailty was adopted for this review, which may contribute to the heterogeneity of the outcome measures; however, we understand this cannot be avoided, due to the various classifications of frailty.
Anticholinergic Exposure
Anticholinergic exposure was defined as either (1) any medication with antimuscarinic activity according to the reference list by Salahudeen et al. [12] or (2) anticholinergic burden, where any method of characterising anticholinergic burden was specified by name, e.g. Drug Burden Index (DBI) [27]. Where there was uncertainty in confirming anticholinergic status, the review team came to a consensus opinion based on pharmacological/medical expertise. If anticholinergic exposure was a dichotomised composite variable including multiple drug types, this composite measure was included where at least 60% of the constituent drugs had anticholinergic properties.
Outcomes
The primary outcomes were physical impairment, cognitive dysfunction and a change in frailty status. Outcomes representing physical impairment included, but were not limited to, reduced grip strength, slower TUGT and chair stand tests, falls and any other outcome which could characterise such impairment. Outcomes representing cognitive dysfunction included, but were not limited to, lower Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) scores, for example. Any specific instrument was considered as an outcome measure of physical impairment, activities of daily living (ADL) and cognitive dysfunction.
Secondary outcomes were any other adverse outcome that could be associated with anticholinergic exposure. These included, but were not limited to, unplanned hospitalisation, institutionalisation in to care homes or nursing homes, mortality, change in quality of life and ADRs.
Information Sources
The following databases were searched from inception to 1st August 2019, using an inclusive search strategy developed with the support of experienced librarians at the University of Bradford: MEDLINE (EBSCO), CINAHL (EBSCO), EMBASE, the Cochrane Database of Systematic Reviews, Web of Science and PsycINFO (EBSCO). Search strategies for each database are available (Appendix 2 of the Supplementary Information file; see the ESM). Search terms represented three main domains: elderly/frailty, anticholinergics and epidemiological filters domain.
Study Selection
Two reviewers (DM and MH, HZ, OT or IM) screened titles and abstracts of identified studies. Full texts were then retrieved to determine eligibility for inclusion (DM and MH, HZ, OT or IM). One reviewer (DM) screened 100% of studies at each stage, and the other reviewers screened 25% each. A screening flowchart was developed to assist the reviewers at both stages (Appendix 3 of the Supplementary Information file; see the ESM). Disagreements were settled by consensus discussion with a third reviewer (AC or DP). The search strategy and study selection process had no language restrictions, and where eligible studies were in a language other than English, the lead author was contacted to request an English version. If unavailable, it was translated using Google Translate [28] and the translation accuracy was assessed by a pharmacologist literate in both English and the language of the manuscript. Forward and backward citation analyses of the selected studies were performed using Publish or Perish™ software [29] and Web of Science. Studies identified by citation analysis were subject to the same independent study selection process.
Data Extraction
A standardised proforma was developed and piloted by two reviewers (DM and MH), and subsequent changes were made to improve the data extraction tool. Two reviewers (DM and MH, HZ, OT or IM) independently extracted data using the final standardised proforma for the outcome measures. One reviewer (DM) extracted data for 100% of the studies, with the independent extraction process being shared as equal as possible amongst the other reviewers. There was no principal summary measure prioritised for extraction, and measures included, but were not limited to, odds ratios (ORs), hazard ratios (HRs), incident rate ratios (IRRs), unstandardised and standardised correlation coefficients and measures of average and spread. Disagreements were settled by consensus discussion with a third reviewer (AC or DP). Covariate-adjusted and unadjusted data were extracted; however, only covariate-adjusted data were prioritised for the meta-analysis and narrative synthesis, as they provide more reliable estimates of outcome associations. Adjustment variables and models were recorded using the proforma, and where authors presented multiple adjusted results, the full model was prioritised for extraction. Two independent reviewers (DM and OT or DP) extracted data for the meta-analysis. Lead authors of studies were contacted where additional data were required.
Assessment of Risk of Bias
Two independent reviewers (DM and MH, HZ, OT or IM) assessed risk of bias using the Cochrane Risk of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool [30]. One reviewer (DM) assessed risk of bias for 100% of the studies, and the independent review was shared as equal as possible amongst the reviewers. Disagreement in the quality assessment was resolved by consensus discussion with a third reviewer (AC or DP). A table summarising how an overall risk of bias judgement can be reached is available (Appendix 4 of the Supplementary Information file; see the ESM).
Synthesis of Results
Meta-analysis
We identified studies where adjusted data could be appropriately synthesised for meta-analysis, and sample sizes and coefficients with 95% confidence intervals (CIs) were extracted, specifically for the outcome of interest. We synthesised data for meta-analysis using the Hedges-Olkin method, with a Fisher Z transformation of coefficients [31], and generated summary forest plots using MedCalc, version 19.4.0 [32]. Statistical heterogeneity was assessed using the I2 statistic, to determine whether the summary coefficient from the fixed effects (I2 < 50%) or random effects (I2 ≥ 50%) modelling should be adopted [33]. Estimates were included in the pooled analysis, where they were adjusted for a minimum of age and sex.
Narrative Synthesis
Data unsuitable for meta-analysis were summarised using a narrative synthesis, following guidance developed by Popay et al. [34].
Results
Literature Search
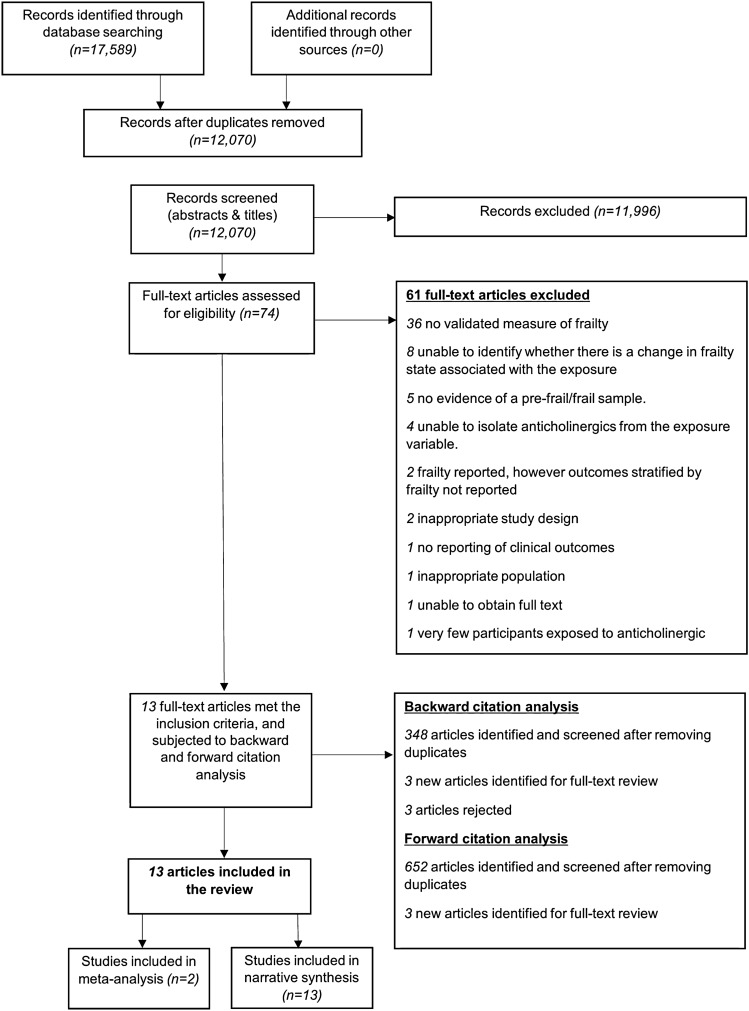
Details of the study selection are summarised in the PRISMA flowchart (Fig. 1). The search identified 17,589 studies, of which seventy four were retrieved for full-text review. Of these, thirteen met the eligibility criteria and were included within this systematic review [35–47]. Reasons for excluding the sixty one studies are available (Appendix 5 of the Supplementary Information file; see the ESM). Backward and forward citation analysis on 28th November 2019 revealed 1000 studies (after removing duplicates), of which six were identified for full-text review; none of which met eligibility criteria.
Fig. 1.
A PRISMA diagram reporting the identification of include studies. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Two studies reported multiple outcomes, in which one outcome (grip strength) was possible to synthesise within a meta-analysis [39, 45]. We attempted to contact authors of two studies for further data to ascertain eligibility, but this was without success; therefore, both were excluded [48, 49].
Study Characteristics
Four prospective cohort studies [35, 40, 41, 43], three retrospective cohort studies [37, 44, 46], four cross-sectional studies [36, 38, 39, 42], one case–control study [47] and one study including both a cross-sectional and retrospective cohort design were included [45], with a total of 21,516 participants (Table 1). The prospective cohort studies had a mean follow-up of 2.4 years (range 9 months to 5 years) and a median sample size of 1257 (range 204–12,405) [35, 40, 41, 43]. The retrospective cohort studies had a mean follow-up of 3.75 years (range 1–8 years) and a mean sample size of 1183 (range 602–1793) [37, 44–46], and the case–control study had a sample size of 428, with a follow-up of 1 year [47]. Ten studies were based in the community [36–39, 41–45, 47], one of which was in self-care retirement villages [39]. One study was based in a residential aged care facility [46], one study included older adults recruited based on attending a hospital outpatient department [40], and one was based in a tertiary care inpatient hospital setting [35]. Four studies were conducted in Europe [38, 42–44], three in North America [36, 37, 40], four in Australia [35, 39, 41, 46], one in Malaysia [47] and one in Japan [45]. All studies were in the English language, except for one, which has an abstract published in English and the full-text in Japanese, which was translated [45].
Table 1.
Characteristics of included studies
| Study | Study design | Year | Country | Healthcare setting | Sample size Study duration |
Mean [median] age | % male (%) | Anticholinergic exposure | Frailty measure (threshold used by study authors) | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Bennett et al. [35] | Prospective cohort | 2014 | Australia | Tertiary hospital |
204 9 months |
80.5 | 34.8 | One or more FRIDs | Edmonton Frail Scale (> 8 deficit points) |
Falls Functional decline Hospitalisation Institutionalisation |
| Cao et al. [36] | Cross-sectional | 2007 | USA | Community | 932 | 78 | 0 | DBI | (Difficulty in 2 or more functional domains out of a possible 8, e.g. gait speed, chair stands, grip strength, mobility, balance) |
Cognitive function ADL Chair stands Balance Gait speed Mobility Upper extremity function Hand grip strength |
| Cossette et al. [37] | Retrospective cohort | 2017 | Canada | Community |
1793 3 years |
74.4 | 48 | ACB score | Fried (fit = no components, pre-frail = 1 or 2 components, frail = 3+ components) |
PCS MCS |
| Gnjidic et al. [38] | Cross-sectional | 2012 | Finland | Community | 700 | 81.3 | 30.6 | DBI |
Gait speed (not defined) Chair stands test (not defined) TUGT (not defined) Grip strength (not defined) |
Gait speed Chair stands test Hand grip strength IADL score Barthel Index TUG test |
| Gnjidic et al. [39] | Cross-sectional | 2012 | Australia | Self-care retirement villages | 115 | 82.3 | 27 | DBI | Grip strength (f < 20 kg, m < 30 kg) |
SPPB score Grip strength |
| Hanlon et al. [40] | Prospective cohort | 2006 | USA | Outpatients |
808 1 year |
Not given (46.4% aged > 75 years) | 98 | Exposed to at least one anticholinergic drug/drug group (e.g. ‘anticholinergics’, ‘opioids’, ‘tricyclic antidepressants’) | (Met 2 or more of 10 criteria for frailty: dependence in at least one ADL, stroke within 3 months, previous falls, difficulty ambulating, malnutrition, dementia, depression, unplanned admission in last 3 months, prolonged bed rest, or incontinence) |
ADRs Preventable ADRs |
| Jamsen et al. [41] | Prospective cohort | 2016 | Australia | Community |
1705 5 years |
76 | 100 | DBI | Fried (fit = no components, pre-frail = 1–2 components, frail = 3+ components) |
Transitions between frailty states, and death Pre-frail—fit Pre-frail—frail Pre-frail—death Frail—pre-frail Frail—death |
| Landi et al. [42] | Cross-sectional | 2007 | Italy | Community | 364 | 85.8 | 33 | Drugs that demonstrate serum anticholinergic activity in literature |
Gait speed (not defined) Grip strength (not defined) |
Gait speed SPPB Grip strength ADL score IADL score |
| Martinot et al. [43] | Prospective cohort | 2018 | France | Community |
12,405 3 years |
Not given (67.7% aged > 65 years) |
74 |
Exposed to at least 1 PIM using Laroche list (anticholinergics reported as, e.g., ‘tricyclic antidepressants’, ‘antipsychotics’, ‘long-acting benzodiazepines’) |
Strawbridge questionnaire (difficulty in 2 or more domains) | Changes in frailty status (frailty to fit state) |
| Porter et al. [44] | Retrospective cohort | 2019 | UK | Community |
1154 Up to 8 years lookback |
78.8 | 37.9 |
At least 1 PIM (anticholinergics identified using ACB scale and reported as, e.g., ‘tricyclic antidepressants’, ‘antipsychotics’, ‘benzodiazepines’) |
Fried (fit = no components, pre-frail = 1–2 component, frail = 3+ components) | Mortality |
| Sato et al. [45] | Cross-sectional and retrospective cohort | 2017 | Japan | Community | 306 (cross-sectional) | [87] | 44.4 | DBI |
Grip strength (not defined) TUGT (not defined) |
ADL score IADL score MMSE PGC Grip strength One-leg balance Repetition standing TUGT |
|
176 (cohort) 3 years |
[90] | 46.6 | ||||||||
| Wilson et al. [46] | Retrospective cohort | 2011 | Australia | RACFs |
602 1 year |
85.7 | 29.1 | DBI |
Gait speed** (not defined) Grip strength** (not defined) |
Falls |
| Zia et al. [47] | Case–control | 2016 | Malaysia | Community |
428 1 year |
75.3 (fallers) | 31.9% | ACB |
TUGT (≥ 13.5 s) Grip strength (f < 20 kg, m < 30 kg) |
Falls |
| 72.1 (non-fallers) | 33.3% |
ACB Anticholinergic Cognitive Burden scale, ADL Activities of Daily Living, ADR adverse drug reaction, DBI Drug Burden Index, f female, FRID fall-risk–increasing drug, IADL Instrumental Activities of Daily Living, m male, MCS Mental Component Summary, MMSE Mini-Mental State Examination, PCS Physical Component Summary, PGC Philadelphia Geriatric Center Morale Scale, PIM potentially inappropriate medicine, RACF residential aged care facility, SPPB Short Physical Performance Battery, TUGT timed up and go test
**Gait speed and grip strength were reported in a different study conducted by the first author, however, using the exact same cohort
Participant Characteristics
The mean participant age was 80.3 years (SD 3.73), reported in nine studies [35–39, 41, 42, 44, 46]. One study reported a median age of 87 years (range 86–88 years) in the cross-sectional analyses [45]. Two studies did not report average age, and instead one study reported 46.4% of participants were aged over 75 years [40] and another study reported 67.7% were aged over 65 years (range 58–73) [43]. Across the included studies, 55.4% of participants were female (range 0–100%) [35–47].
Assessment of Frailty
Participants with pre-frailty/frailty were identified by study authors in six studies [35, 37, 40, 41, 43, 44], according to the phenotype model [24], Edmonton Frail Scale [50], the Strawbridge questionnaire [51] or 10-point frailty criteria [52]. Among these, the mean prevalence of frailty was 40.9% (range 3.7–100%). Pre-frailty was also reported in two studies, with 43.9% [37] and 45.9% [44] of their respective study samples deemed pre-frail. The remaining studies reported at least one frailty measure, but did not explicitly define frailty as per the previously mentioned frailty measures [36, 38, 39, 42, 45–47]. These included gait speed (m/s), TUGT (s), chair stands tests (s) and grip strength (kg) (Table 1).
Identifying Anticholinergic Exposures
The DBI [27] and the Anticholinergic Cognitive Burden (ACB) scale [53] were used to characterise the exposure of anticholinergic drugs in nine studies [36–39, 41, 44–47]. Others included fall-risk–increasing drugs (FRIDs) [35], prescription of at least one anticholinergic drug [40], prescription of at least one drug with demonstrated serum anticholinergic activity [42] or prescription with at least one potentially inappropriate medicine (PIM) that had anticholinergic properties [43] (Table 1).
Primary Outcomes: Physical Impairment
Grip Strength
Two cross-sectional studies investigated the association between a one-unit increase in DBI score and grip strength, and data were synthesised within a meta-analysis with a pooled sample size of 421. One study was conducted by Gnjidic et al., among older people living in self-care retirement villages in Australia (n = 115) [39], and the other by Sato et al., among community-dwelling older people in Japan (n = 306) [45]. Sato et al. also reported 3-year follow-up data; however, only cross-sectional data were extracted for meta-analysis. With an I2 value of 98.2%, a random effects model was selected to conduct the analysis. Results showed that a one-unit increase in DBI was not associated with weaker grip strength (kg) among older participants with frailty living in the community (pooled adjusted (adj.) coefficient 0.48 [95% CI − 0.286 to 0.869]) (Appendix 6 of the Supplementary File; see the ESM) [39, 45].
There were mixed results among four community-based cross-sectional studies, which could not be included within the meta-analysis. Two demonstrated that anticholinergic exposure was associated with weaker grip strength. In a US study by Cao et al., which included female participants if they had difficulty in two or more functional domains (n = 932), exposure to anticholinergic medication was associated with increased odds for weaker grip strength (defined as < 18 kg strength in the dominant hand) (adj. OR 2.4 [95% CI 1.1–5.3]) [36], and in a study in Italy by Landi et al. (n = 364), exposure to anticholinergics was associated with a weaker grip strength compared to non-exposure (adj. mean 28.88 (SE ± 1.05) vs 31.33 (SE ± 0.81), p = 0.05) [42]. In contrast, two studies observed no association between anticholinergic medication and weaker grip strength among older people deemed to be living with frailty. In a study in Finland by Gnjidic et al. (n = 700), anticholinergic exposure (DBI > 0) was not associated with weaker grip strength (change in adj. coefficient − 0.98 [95% CI − 2.05 to 0.08]) [38], and in the 3-year follow-up data presented by Sato et al. (n = 176), a one-unit increase in DBI was also not associated with a weaker grip strength (change in adj. coefficient − 0.78 [95% CI − 2.44 to 0.88]) (Table 2) [45].
Table 2.
Primary outcomes: summary of reported results
| Primary outcomes | Study | Frailty measure (mean/median) [SD] | Total number (%) of pre-frail/frail participants | AC exposure (description) | Outcome | Adjusted results* | Method of adjustment/controlling for confounding |
|---|---|---|---|---|---|---|---|
|
Physical impairment |
Bennett et al. [35] | Edmonton Frail Scale | Frail: 103 (50.4%) | One or more FRIDs (participants exposed to AC FRIDs on admission n = 131 [64.2%]; participants exposed to AC FRIDs on discharge n = 161 [78.9%]) | Falls | Total: OR 1.7 (95% CI 1.3–2.1)* | Age, sex, living status, comorbidity, ADL and IADL, fall risk factors and alcohol use |
| Fit: OR 2.4 (95% CI 1.3–6.1)* | |||||||
| Frail: OR 1.5 (95% CI 1.1–1.9)* | |||||||
| Functional decline | Total: OR 1.3 (95% CI 1.1–1.6)* | ||||||
| Fit: OR 1.4 (95% CI 0.9–2.0) | |||||||
| Frail: OR 1.2 (95% CI 1.0–1.5)* | |||||||
| Cao et al. [36] | Difficulty in 2 or more functional domains out of a possible 8 (e.g. gait speed, chair stands, grip strength, mobility, balance) |
932 (100%) Assumed based on the inclusion criteria |
DBI (dichotomised ‘anticholinergic’ burden variable used to identify participants exposed to anticholinergic burden) | Difficulty in chair stands | Full model: OR 4.2 (95% CI 2.0–8.7)* | Age, race, education, depression, arthritis, visual impairment, hearing impairment, hypertension, ischemic heart disease, congestive heart failure, pulmonary disease, osteoporosis, diabetes mellitus, cancer, spinal disc disease, hip fracture, spinal stenosis, Parkinson's disease and peripheral arterial disease | |
| Poor balance | Full model: OR 4.9 (95% CI 2.0–12.0)* | ||||||
| Slow gait speed | Full model: OR 3.6 (95% 1.6–8.0)* | ||||||
| Poor mobility | Full model: OR 3.2 (95% CI 1.5–6.9)* | ||||||
| Poor upper extremity function | Full model: OR 2.7 (95% CI 1.3–5.4)* | ||||||
| Weak grip strength | Full model: OR 2.4 (95% CI 1.1–5.3)* | ||||||
| Cossette et al. [37] | Fried |
Pre-frail: 787 (43.9%) Frail: 67 (3.7%) |
ACB score (change in beta coefficient for every 1-unit change in ACB score) | PCS | Non-frail: ß −0.30 (95% CI − 0.54 to − 0.06)* | Parsimonious model: time, sex, age, number of co-morbidities, Geriatric Depression Scale | |
| Frail/pre-frail: ß − 0.61 (95% CI − 0.88 to − 0.33)* | |||||||
| Gnjidic et al. [38] |
Gait speed (mean 1.3 m/s) [± 0.4] Chair stands test (mean 16.7 s) [± 7.8] TUGT (mean 13.9 s) [± 9.3] Grip strength (mean 20.0 kg) [± 10.2] |
Not reported | DBI (change in coefficient where DBI score > 0) (dichotomised) | Gait speed | − 0.13 (95% CI − 0.19 to − 0.08)* | Age, sex, education, comorbidities, self-reported status and cognitive impairment | |
| Chair stands test | 1.11 (95% CI 1.05–1.16)* | ||||||
| Grip strength (kg) | − 0.98 (95% CI − 2.05 to 0.08) | ||||||
| TUGT | 1.13 (95% CI 1.07–1.19)* | ||||||
| Gnjidic et al. [39] | Grip strength (mean 18.0 kg) [± 7.5] | Not reported | DBI (change in coefficient for every 1-unit change in DBI score) | SPPB | − 1.28 (95% CI − 2.53 to − 0.04)* | Age, sex, education, comorbidities, cognitive functioning, depression and sleep disturbance | |
| Grip strength (kg) | 0.10 (95% CI − 2.54 to 2.74) | ||||||
| Landi et al. [42] | Gait speed (m/s) | Not reported | Drugs that have demonstrated serum anticholinergic activity in literature (users of anticholinergics—dichotomised) | Gait speed (m/s) | Non-users AC drugs (mean): 0.49 (SE ± 0.01) | Age, gender, smoking, physical activity level, living alone, BMI, dementia, congestive heart failure, lung diseases, diabetes, delirium, history of falls | |
| Users of AC drugs (mean): 0.47 (SE ± 0.02) | |||||||
| SPPB | Non-users AC drugs (mean): 6.90 (SE ± 0.19)* | ||||||
| Users of AC drugs (mean): 6.19 (SE ± 0.25)* | |||||||
| Grip strength (kg) | Non-users AC drugs (mean): 31.33 (SE ± 0.81)* | ||||||
| Users of AC drugs (mean): 28.88 (SE ± 1.05)* | |||||||
| Sato et al. [45] |
Grip strength (median 19.3 kg) IQR 15.3, 23.5 TUGT (median 12.6 s) IQR 10.5, 15.4 |
Not reported | DBI (change in coefficient for every 1-unit change in DBI score) | Grip strength (kg) | Cross-sectional: 0.73 (95% CI − 2.02 to 0.57) | Age, sex, high blood pressure, diabetes, heart disease, cancer, stroke | |
| Cohort at 3 years: − 0.78 (95% CI − 2.44 to 0.88) | |||||||
| One-leg balance (duration in seconds) | Cross-sectional: − 0.32 (95% CI − 4.57 to 3.93) | ||||||
| Cohort at 3 years: 1.89 (95% CI − 1.49 to 5.28) | |||||||
| Repetition standing (no. of times over 30 s) | Cross-sectional: − 1.30 (95% CI − 2.79 to 0.20) | ||||||
| Cohort at 3 years: 0.08 (95% CI − 1.77 to 1.93) | |||||||
| TUGT | Cross-sectional: 0.53 (95% CI − 2.46 to 3.52) | ||||||
| Cohort at 3 years: 0.38 (95% CI − 2.00 to 2.75) | |||||||
| Wilson et al. [46] |
Gait speed** (mean 0.56 m/s) [± 0.21] Grip strength** (mean 19.9 kg) [± 8.1] |
Not reported | DBI (stratified in to DBI < 1, DBI ≥ 1) | Falls | DBI < 1: IRR 1.61 (95% CI 1.17–2.23)* | Age, sex, history of falls, cognitive impairment, depressive symptoms, comorbidities, use of a walking aid, polypharmacy and incontinence | |
| DBI ≥ 1: IRR 1.90 (95% CI 1.30–2.78)* | |||||||
| Zia et al. [47] |
TUGT ≥ 13.5 s n = 168, 39.3% Reduced right grip strength (kg) n = 290, 67.8% Reduced left grip strength (kg) n = 313, 73.1% (≤ 20 kg women, ≤ 30 kg men) |
Not reported | ACB (characterised as ACB score ≥ 1) | Falls | Model 9: OR 1.4 (95% CI 0.8–2.4) | Reduced right grip strength + age, gender, number of comorbidities | |
| Model 10: OR 1.4 (95% CI 0.85–2.4) | Reduced left grip strength + age, gender, number of comorbidities | ||||||
| Model 11: OR 1.3 (95% CI 0.76–2.1) | TUGT ≥ 13.5 (s) + age, gender, number of comorbidities | ||||||
| Model 12: OR 1.2 (95% CI 0.7–2.1) | Functional reach ≤ 18 cm + age, gender, number of comorbidities | ||||||
| Cognitive dysfunction | Cao et al. [36] | Difficulty in 2 or more functional domains out of a possible 8 (e.g. gait speed, chair stands, grip strength, mobility, balance) |
932 (100%) Assumed based on the inclusion criteria |
DBI (dichotomised ‘anticholinergic’ burden variable used to identify participants exposed to anticholinergic burden) | Poor cognitive function (MMSE score ≤ 26) | Full model: OR 2.4 (95% CI 1.1–5.1)* | Age, race, education, depression, arthritis, visual impairment, hearing impairment, hypertension, ischemic heart disease, congestive heart failure, pulmonary disease, osteoporosis, diabetes mellitus, cancer, spinal disc disease, hip fracture, spinal stenosis, Parkinson's disease, and peripheral arterial disease |
| Cossette et al. [37] | Fried |
Pre-frail: 787 (43.9%) Frail: 67 (3.7%) |
ACB score (change in beta coefficient for every 1-unit change in ACB score) | MCS | Non-frail: ß 0.04 (95% CI − 0.16 to 0.24) | Parsimonious model: time, sex, age, number of comorbidities, Geriatric Depression Scale | |
| Frail/pre-frail: ß 0.30 (95% CI 0.04–0.57)* | |||||||
| Sato et al. [45] |
Grip strength (median 19.3 kg) IQR 15.3, 23.5 TUGT (median 12.6 s) IQR 10.5, 15.4 |
Not reported | DBI (change in coefficient for every 1-unit change in DBI score) | MMSE | Cross-sectional: − 1.50 (95% CI − 2.96 to − 0.03)* | Age, sex, high blood pressure, diabetes, heart disease, cancer, stroke | |
| Cohort at 3 years: − 0.21 (95% CI − 1.78 to 1.35) | |||||||
| Change in frailty status | Jamsen et al. [41] | Fried |
Not reported; however, number of transitions reported: From pre-frail state: 603 stationary 172 to fit 114 to frail 200 to death From frail state: 73 stationary 35 to pre-frail 108 to death 3 to fit |
DBI (association with 1-unit increase in DBI) | Transitions between frailty states, and death (excluding transitions from fit state) | Pre-frail to fit: HR 0.90 (95% CI 0.59–1.36) | Age, diagnosis of dementia or mild cognitive impairment at baseline, comorbidity, education level, and living status |
| Pre-frail to frail: HR 1.03 (95% CI 0.76–1.40) | |||||||
| Pre-frail to death: HR 1.18 (95% CI 0.89–1.56) | |||||||
| Frail to pre-frail: HR 0.65 (95% CI 0.33–1.27) | |||||||
| Frail to death: HR 0.92 (95% CI 0.73–1.16) | |||||||
| Martinot et al. [43] | Strawbridge questionnaire (difficulty in 2 or more domains) |
Frailty (2012) = 1664 (14.0%) Frailty (2013) = 1766 (14.2%) Frailty (2014) = 1945 (16.6%) |
Exposed to at least 1 PIM using Laroche list (anticholinergics reported as, e.g., ‘tricyclic antidepressants’, ‘long-acting benzodiazepines’) | Changes in frailty state (frailty to fit state) | Anticholinergics: HR 0.84 (95% CI 0.64–1.09) | Age, gender, self-perceived social position, marital status, BMI, tobacco consumption, number of chronic diseases, and polypharmacy | |
| Other anticholinergics with questionable efficacy: HR 0.86 (95% CI 0.67–1.11) | |||||||
| Long-acting benzodiazepines: HR 0.89 (95% CI 0.70–1.14) | |||||||
| Concomitant use of ≥ 2 benzodiazepines: HR 0.93 (95% CI 0.61–1.41) | |||||||
| Concomitant use of ≥ 2 antidepressants: HR 0.57 (95% CI 0.25–1.32) | |||||||
| Prolonged use of benzodiazepines: HR 1.01 (95% CI 0.74–1.37) |
AC anticholinergic, ACB Anticholinergic Cognitive Burden scale, ADL Activities of Daily Living, BMI body mass index, CI confidence interval, DBI Drug Burden Index, FRID fall-risk–increasing drug, HR hazard ratio, IADL Instrumental Activities of Daily Living, IQR interquartile range, IRR incident rate ratio, MCS Mental Component Summary, MMSE Mini-Mental State Examination, OR odds ratio, PCS Physical Component Summary, PIM potentially inappropriate medicine, SPPB Short Physical Performance Battery, TUGT timed up and go test
*Represents significant associations determined as p ≤ 0.05
**Gait speed and grip strength were reported in a different study conducted by the first author, however, using the exact same cohort
Gait Speed, TUGT and Chair Stands Tests
In the US cross-sectional study by Cao et al. (n = 932) [36], a sub-group of 158 participants exposed to anticholinergic medicines as per the DBI were at increased odds of having a slower gait speed (< 0.64 m/s) (adj. OR 3.6 [95% 1.6–8.0]). In the study of community-dwelling older people in Finland (n = 700), Gnjidic et al. found that a DBI score > 0 was associated with reduced gait speed over a 10-m distance, with a change in adjusted coefficient of − 0.13 (95% CI − 0.19 to − 0.08) [38]. However, Landi et al.’s study of community-dwelling participants in Italy (n = 364) reported no difference in adjusted mean gait speed between those exposed and not-exposed to anticholinergics (0.47 [SE ± 0.02] vs 0.49 [SE ± 0.01] [p = 0.70]) (Table 2) [42].
Gnjidic et al. [38] reported an association between anticholinergic exposure (DBI score > 0) and poorer performance of the TUGT (change in adj. coefficient 1.13 [95% CI 1.07–1.19]). However, Sato et al. found no association between a one-unit increase in DBI score and poorer performance in the TUGT, reporting a change in adjusted coefficient of 0.53 (95% CI − 2.46 to 3.52) in the cross-sectional data and 0.38 (95% CI − 2.00 to 2.75) at the 3-year follow-up (Table 2) [45].
In the US study by Cao et al., participants exposed to anticholinergic medicines were at significantly increased odds of difficulty in chair stands (inability to stand up five times from a chair without using the arms) (adj. OR 4.2 [95% CI 2.0–8.7]) [36]. Poorer performance in chair stands was also observed by Gnjidic et al. in those exposed to a DBI score > 0, with a longer time needed to complete five chair stands in those exposed (change in adj. coefficient 1.11 [95% CI 1.05–1.16]) (Table 2) [38].
Falls
In a retrospective cohort study of older participants living with frailty in Australian residential aged care facilities (n = 602), Wilson et al. reported that anticholinergic medication exposure (DBI ≥ 1) was associated with a higher risk of falls (adj. IRR 1.90 [95% CI 1.30–2.78]), compared to no exposure (adj. IRR 1.61 [95% CI 1.17–2.23]) (Table 2) [46].
In a case–control study of community-dwelling older participants living with frailty in Malaysia (n = 428), no association between exposure to anticholinergics and falls was observed after adjustments for age, gender and the number of comorbidities, in addition to the following variables: reduced right grip strength (adj. OR 1.4 [95% CI 0.8–2.4]), reduced left grip strength (adj. OR 1.4 [95% CI 0.85–2.4]), TUGT ≥ 13.5(s) (adj. OR 1.3 [95% CI 0.76–2.1]) and functional reach ≤ 18 cm (adj. OR 1.2 [95% CI 0.7–2.1]) (Table 2) [47].
Other Outcomes Related to Physical Impairment
In the US cross-sectional study by Cao et al. (n = 932) [36], exposure to anticholinergic medicines was associated with increased odds of poor balance (inability to hold a full tandem stand for 10 s) (adj. OR 4.9 [95% CI 2.0–12]), poor mobility (great difficulty in walking half a mile, walking across a small room, climbing ten steps, stooping, crouching or kneeling) (adj. OR 3.2 [95% CI 1.5–6.9]) and poor extremity function (great difficulty in using fingers to grasp or to handle, raising arms up over the head or lifting and carrying 10 lb) (adj. OR 2.7 [95% CI 1.3–5.4]) (Table 2).
Two cross-sectional studies reported associations between anticholinergic medication exposure and the Short Physical Performance Battery (SPPB) test [39, 42]. In a different study conducted by Gnjidic et al. involving community-dwelling older adults in Australia living with frailty (n = 115), a one-unit increase in the DBI score was associated with a reduced SPPB score, with a change in adjusted coefficient of − 1.28 (95% CI − 2.53 to − 0.04) [39]. In the study conducted by Landi et al. with community-dwelling older participants in Italy (n = 364), non-users of anticholinergic drugs had an adjusted mean SPPB score of 6.90 (SE ± 0.19), compared to an adjusted mean score of 6.19 in users (SE ± 0.25) (p = 0.05), suggesting a reduction in physical performance in those exposed (Table 2) [42].
In the study by Sato et al. involving community-dwelling older participants in Japan, a one-unit increase in DBI was found not to be associated with impaired performance in a one-leg balance test (duration in seconds) and the number of repetition stands over 30 s test in cross-sectional data (change in adj. coefficient − 0.32 [95% CI − 4.57 to 3.93] and − 1.30 [95% CI − 2.79 to 0.20], respectively) and at 3-year follow-up (change in adj. coefficient 1.89 [95% CI − 1.49 to 5.28] and 0.08 [95% CI − 1.77 to 1.93], respectively) (Table 2) [45].
Primary Outcomes: Cognitive Dysfunction
Two cross-sectional studies reported an association between anticholinergic burden and cognitive dysfunction [36, 45]. Cao et al. observed that older women living in the community in the USA and exposed to anticholinergics (n = 932) were at increased odds of impaired cognitive function (characterised by an MMSE score ≤ 26), with an adjusted OR of 2.4 (95% CI 1.1–5.1) [36]. Sato et al. observed that in older community-dwelling Japanese participants (n = 306), a one-unit increase in the DBI score was associated with a lower MMSE score cross-sectionally (change in adj. coefficient − 1.50 [95% CI − 2.96 to − 0.03]) [45]. However, this association was not evident at 3-year follow-up (change in adj. coefficient − 0.21 [95% CI − 1.78 to 1.35]) (Table 2).
Primary Outcomes: Change in Frailty Status
In a prospective cohort study in Australia by Jamsen et al., 1705 community-dwelling male participants were studied over 5 years, using the DBI as the measure of anticholinergic burden and the Fried criteria to measure frailty [41]. A one-unit increase in DBI score was not associated with transitions in frailty status: from pre-frailty to frailty (adj. HR 1.03 [95% CI 0.76–1.40], n = 114); from pre-frailty to death (adj. HR 1.18 [95% CI 0.89–1.56], n = 200); from frailty to death (adj. HR 0.92 [95% CI 0.73–1.16], n = 108) [41]. There was also no evidence that increasing anticholinergic burden was associated with reverse transitions of frailty state: from pre-frailty to a fit state (adj. HR 0.90 [95% CI 0.59–1.36], n = 172); from frailty to pre-frailty (adj. HR 0.65 [95% CI 0.33–1.27], n = 35) (Table 2).
In a prospective cohort study by Martinot et al. in Australia, 12,405 community-dwelling participants were studied over a 3-year period, with associations between exposure to at least one PIM and frailty transitions investigated, using the Strawbridge questionnaire to characterise frailty [43]. In participants exposed to PIMs of an anticholinergic nature, there was no association with transitions from frailty to a fit state (adj. HR 0.84 [95% CI 0.64–1.09]). Specifically, there was also no association between the following anticholinergic medicine groups and transitions from a frail to fit state: prescription of long-acting benzodiazepines (adj. HR 0.89 [95% CI 0.70–1.14]); concomitant use of two or more benzodiazepines (adj. HR 0.93 [95% CI 0.61–1.41]); prolonged use of benzodiazepines (adj. HR 1.01 [95% CI 0.74–1.37]); the concomitant use of two or more antidepressants (adj. HR 0.57 [95% CI 0.25–1.32]) (Table 2).
Secondary Outcomes
Activities of Daily Living
Four cross-sectional studies reported associations between anticholinergic exposure and outcomes relating to ADL, including Instrumental Activities of Daily Living (IADL) and Barthel Index scores [36, 38, 42, 45].
In the study by Cao et al. among community-dwelling participants in the USA (n = 932), exposure to anticholinergic burden was associated with disability in ADL (adj. OR 3.4 [95% CI 1.7–6.9]) [36]. Disability in ADL was categorised in to three groups: mild (no more than some difficulty with at least one ADL), moderate (unable to do at least one ADL or a great difficulty in two or more ADL) and severe (unable to do at least two ADL or a great difficulty with three or more) (Table 3).
Table 3.
Secondary outcomes: summary of reported results
| Secondary outcomes | Study | Frailty measure(s) (mean/median) [SD] | Total number (%) of pre-frail/frail participants | AC exposure (description) | Outcome | Adjusted results* | Method of adjustment/controlling for confounding |
|---|---|---|---|---|---|---|---|
| ADL | Cao et al. [36] | Difficulty in 2 or more functional domains out of a possible 8 (e.g. gait speed, chair stands, grip strength, mobility, balance) |
932 (100%) Assumed based on the inclusion criteria |
DBI (dichotomised ‘anticholinergic burden’ variable used to identify participants exposed to anticholinergic burden) | Disability in ADL | Full model: OR 3.4 (95% CI 1.7–6.9)* | Age, race, education, depression, arthritis, visual impairment, hearing impairment, hypertension, ischemic heart disease, congestive heart failure, pulmonary disease, osteoporosis, diabetes mellitus, cancer, spinal disc disease, hip fracture, spinal stenosis, Parkinson's disease, and peripheral arterial disease |
| Gnjidic et al. [38] |
Gait speed (mean 1.3 m/s) [± 0.4] Chair stands test (mean 16.7 s) [± 7.8] TUGT (mean 13.9 s) [± 9.3] Grip strength (mean 20.0 kg) [± 10.2] |
Not reported | DBI (change in coefficient where DBI score > 0) (dichotomised) | IADL score | − 0.61 (95% CI − 0.84 to − 0.39)* | Age, sex, education, comorbidities, self-reported status and cognitive impairment | |
| Barthel Index | − 3.21 (95% CI − 4.68 to − 1.75)* | ||||||
| Landi et al. [42] | Gait speed | Not reported | Drugs that have demonstrated serum anticholinergic activity in literature (users of anticholinergics—dichotomised) | ADL score (MDS-HC instrument) | Non-users AC drugs (mean): 1.23 (SE ± 0.12)* | Age, gender, smoking, physical activity level, living alone, BMI, dementia, congestive heart failure, lung diseases, diabetes, delirium, history of falls | |
| Users of AC drugs (mean): 1.68 (SE ± 0.15)* | |||||||
| IADL score (MDS-HC instrument) | Non-users AC drugs (mean): 2.71 (SE ± 0.11)* | ||||||
| Users of AC drugs (mean): 3.47 (SE a 0.14)* | |||||||
| Sato et al. [45] |
Grip strength (median 19.3 kg) IQR 15.3, 23.5 TUGT (median 12.6 s) IQR 10.5, 15.4 |
Not reported | DBI (change in coefficient for every 1-unit change in DBI score) | ADL score | Cross-sectional: − 0.95 (95% CI − 4.91 to 3.01) | Age, sex, high blood pressure, diabetes, heart disease, cancer, stroke | |
| Cohort at 3 years:− −6.31 (95% CI − 11.61 to − 1.01)* | |||||||
| IADL score | Cross-sectional: − 0.63 (95% CI − 0.99 to − 0.27)* | ||||||
| Cohort at 3 years: − 0.34 (95% CI − 0.79 to 0.10) | |||||||
| ADRs | Hanlon et al. [40] | Met 2 or more of 10 criteria for frailty (dependence in at least one ADL, stroke within 3 months, previous falls, difficulty ambulating, malnutrition, dementia, depression, unplanned admission in the last 3 months, prolonged bed rest, or incontinence) | 808 (100%) | Exposed to at least one anticholinergic drug/drug group (e.g. ‘anticholinergics’, ‘opioids’, ‘tricyclic antidepressants’) | Any ADRs | Benzodiazepines: HR 1.23 (95% CI 0.95–1.58) | Multivariate model derived using stepwise procedures using all candidate variables listed in Tables 1 and 2 of the article; however, final candidate variables chosen not reported |
| Warfarin: HR 1.51 (95% CI 1.22–1.87)* | |||||||
| Preventable ADRs | Warfarin (adjusted): HR 1.50 (95% CI 1.08–2.11)* | ||||||
| Hospitalisation/institutionalisation | Bennett et al. [35] | Edmonton Frail Scale | Frail: 103 (50.4%) | One or more FRIDs (participants exposed to AC FRIDs on admission = 131 [64.2%], participants exposed to AC FRIDs on discharge = 161 [78.9%]) | Hospitalisation | Total: OR 1.1 (95% CI 0.9–1.4) | Age, sex, living status, comorbidity, ADL and IADL, fall risk factors and alcohol use |
| Fit: OR 1.3 (95% CI 0.8–4.6) | |||||||
| Frail: OR 1.0 (95% CI 0.8–1.4) | |||||||
| Institutionalisation | Total: OR 1.3 (95% CI 1.1–1.6)* | ||||||
| Fit: OR 1.3 (95% CI 0.8–2.1) | |||||||
| Frail: OR 1.3 (95% CI 1.0–1.6)* | |||||||
| Death | Porter et al. [44] | Fried |
Pre-frail: 530 (45.9%) Frail: 420 (36.4%) |
Exposed to at least 1 PIM (anticholinergics identified using ACB scale and reported as, e.g., ‘tricyclic antidepressants’, ‘antipsychotics’, ‘benzodiazepines’) | Mortality |
Antipsychotics: Fit: HR 3.60 (95% CI 0.40–31.99) Pre-frail: HR 2.89 (95% CI 1.26–6.66)* Frail: HR 3.34 (95% CI 1.37–8.12) * |
Age, gender, MMSE score, care home residence and comorbidities. |
|
Anticholinergics: Fit: HR 1.29 (95% CI 0.16–10.61) Pre-frail: HR 1.05 (95% CI 0.61–1.79) Frail: HR 1.23 (95% CI 0.76–2.01) | |||||||
|
Tricyclic antidepressants: Fit: not reported Pre-frail: HR 1.84 (95% CI 0.98–3.44) Frail: HR 0.90 (95% CI 0.55–1.48) | |||||||
|
Benzodiazepines: Fit: HR 0.92 (95% CI 0.11–7.78) Pre-frail: HR 1.40 (95% CI 0.66–2.97) Frail: HR 0.43 (95% CI 0.21–0.86)* | |||||||
|
Other antidepressants: Fit: HR 0.86 (95% CI 0.23–3.20) Pre-frail: HR 1.12 (95% CI 0.67–1.89) Frail: HR 0.74 (95% CI 0.49–1.12) | |||||||
| Psychological functioning | Sato et al. [45] |
Grip strength (median 19.3 kg) IQR 15.3, 23.5 TUGT (median 12.6 s) IQR 10.5, 15.4 |
Not reported. Frailty not explicitly identified | DBI (change in coefficient for every 1-unit change in DBI score) | PGC | Cross-sectional: − 0.72 (95% CI − 1.79 to 0.35) | Age, sex, high blood pressure, diabetes, heart disease, cancer, stroke |
| Cohort at 3 years: − 1.00 (95% CI − 2.47 to 0.48) |
AC anticholinergic, ACB Anticholinergic Cognitive Burden scale, ADL Activities of Daily Living, ADR adverse drug reaction, BMI body mass index, DBI Drug Burden Index, FRID fall-risk–increasing drug, MDS-HC Minimum Data Set for Home Care Assessment instrument, HR hazard ratio, IADL Instrumental Activities of Daily Living, IQR interquartile range, MMSE Mini-Mental State Examination, OR odds ratio, PGC Philadelphia Geriatric Center Morale Scale, PIM potentially inappropriate medicine, TUGT timed up and go test
*Represents significant associations determined as p ≤ 0.05
In the study by Gnjidic et al. of community-dwelling older people in Finland (n = 700), anticholinergic exposure was associated with impaired functional status, with a change in adjusted IADL coefficient of − 0.61 (95% CI − 0.84 to − 0.39) and a change in Barthel Index adjusted coefficient of − 3.21 (95% CI − 4.68 to − 1.75) where, in both, a lower score indicated greater impairment of function (Table 3) [38].
In the study by Landi et al. among community-dwelling participants in Italy (n = 364), anticholinergic exposure was associated with greater impairment of functional status [42]. Function was measured using the Minimum Data Set for Home Care Assessment instrument (MDS-HC), where impairment was indicated by higher scores on scales for ADL and IADL that ranged from 0 to 7 [42]. Anticholinergic exposure was associated with a higher adjusted mean score for ADL of 1.68 (SE ± 0.15) compared to an adjusted mean score of 1.23 (SE ± 0.12) for non-exposure (p = 0.03), and for IADL, an adjusted mean score of 3.47 (SE ± 0.14) compared to an adjusted mean score of 2.71 (SE ± 0.11) with non-exposure (p < 0.001) (Table 3).
In the study by Sato et al. involving community-dwelling older participants in Japan, a one-unit increase in DBI score was not associated with a change in ADL adjusted coefficient at baseline (− 0.95 [95% CI − 4.91 to 3.01]), but a change in ADL adjusted coefficient at 3 years of − 6.31 (95% CI − 11.61 to − 1.01) [45]. For IADL, a one-unit increase in DBI was associated with a change in adjusted coefficient of − 0.63 at baseline (95% CI − 0.99 to − 0.27), but this was not maintained at 3 years, with no statistically significant change in IADL adjusted coefficient observed (− 0.34 [95% CI − 0.79 to 0.10]). The number of participants at follow-up was significantly fewer than at baseline, with a difference of 130 participants (Table 3) [45].
Adverse Drug Reactions
In a US prospective cohort study by Hanlon et al., community-dwelling older participants living with frailty were followed up in an outpatient setting after a hospital stay (n = 808), and the associations of commonly prescribed medications with ‘any ADRs’ and specifically ‘preventable ADRs’ were investigated [40]. Assessment of prescribing, monitoring, dispensing and adherence errors were undertaken to determine ADR preventability, through clinical consensus. Among anticholinergic medications in the adjusted analyses, warfarin use was associated with ‘any ADRs’ (adj. HR 1.51 [95% CI 1.22–1.87]). Warfarin use was also associated with ‘preventable ADRs’ (adj. HR 1.50 [95% CI 1.08–2.11]). There was no association between benzodiazepines and ‘any ADRs’ (adj. HR 1.23 [95% CI 0.95–1.58]). Adjusted data for associations between benzodiazepine use and ‘preventable ADRs’ were unavailable (Table 3).
Psychological Functioning
In the study by Sato et al. involving community-dwelling older participants in Japan [45], psychological functioning was investigated in association with anticholinergic medication exposure, where psychological functioning was measured by the Philadelphia Geriatric Center Morale Scale [54]. Anticholinergic exposure was not associated with a change in psychological functioning at baseline (change in adj. coefficient − 0.72 [95% CI − 1.79 to 0.35]) or at 3-year follow-up (change in adj. coefficient − 1.00 [95% CI − 2.47 to 0.48]) (Table 3).
Risk of Outcomes Stratified by Frailty Status
Three cohort studies reported the association between anticholinergic medication exposure and adverse health outcomes stratified by frailty status, separating participants into groups of non-frail/fit, pre-frail and frail [35, 37, 44].
In a prospective cohort study in Australia by Bennett et al., 204 frail older participants within a tertiary hospital setting were studied over a 9-month period, investigating the association between FRID exposure and physical impairment, falls, hospitalisation (after a fall) and institutionalisation (nursing home admission or rehabilitation hospital) (Tables 2 and 3) [35]. Frailty was measured by the Edmonton Frail Scale. Overall, exposure to at least one FRID was associated with falls (adj. OR 1.7 [95% CI 1.3–2.1]). Among those with frailty, FRIDs exposure was associated with an adjusted OR of 1.5 [95% CI 1.1–1.9]) for falls, whereas among fit older people, the falls risk associated with FRID exposure was greater (adj. OR 2.4 [95% CI 1.3–6.1]). Overall, exposure to one or more FRIDs was associated with 1.3 times the odds of functional decline (adj. OR 1.3 [95% CI 1.1–1.6]), defined as an increase in Katz ADL score by 2 points from admission after 2 months [55]. Among older people with frailty, FRID exposure was associated with 1.2 times the odds of functional decline (adj. OR 1.2 [95% CI 1.0–1.5]), but in fit older people, no association was observed (adj. OR 1.4 [95% CI 0.9–2.0]) (Table 2). There was no association between FRID exposure and hospitalisation overall (adj. OR 1.1 [95% CI 0.9–1.4]) or in sub-populations defined by frailty status: in those who were fit (adj. OR 1.3 [95% CI 0.8–4.6]) and those with frailty (adj. OR 1.0 [95% CI 0.8–1.4]). FRID exposure was associated with increased odds of institutionalisation overall (adj. OR 1.3 [95% CI 1.1–1.6]) and in those with frailty (adj. OR 1.3 [95% CI 1.0–1.6]), but not in fit older people (adj. OR 1.3 [95% CI 0.8–2.1]) (Table 3).
In a retrospective cohort study by Cossette et al., 1793 older community-dwelling participants in Canada were studied over a 3-year period, investigating the change in coefficients for the Physical Component Summary (PCS) and Mental Component Summary (MCS) of the 36-item Short Form Survey (SF-36) questionnaire, for every one-unit change in ACB score (Table 2) [37]. Thirty-three per cent of the study population were taking at least one anticholinergic drug as per the ACB scale, with 22% scoring a total ACB score of 1–2. Frailty was measured using the phenotype model, characterising participants in to two categories: fit or pre-frail/frail. Among fit older people, a one-unit increase in the ACB score was associated with a change in PCS adjusted coefficient of −0.30 (95% CI − 0.54 to − 0.06). In the pre-frailty/frailty group, a one-unit increase in ACB was associated with a change in PCS adjusted coefficient of − 0.61 (95% CI − 0.88 to − 0.33). For the MCS outcome there was no statistically significant association with a one-unit change in ACB score in the non-frail group, reporting a change in adjusted coefficient of 0.04 (95% CI − 0.16 to 0.24). In the frail/pre-frail group, this was associated with a change in adjusted coefficient of 0.30 (95% CI 0.04–0.57).
In a retrospective cohort study by Porter et al. of community-dwelling older adults with cognitive impairment in the UK (n = 1154), the effect of PIMs on all-cause mortality was investigated, stratified by frailty status [44]. 17.7% (n = 204) were identified as fit, 45.9% (n = 530) were pre-frail and 36.4% (n = 420) were frail, as per the phenotype model. Exposure to one or more anticholinergic PIMs was not associated with a difference in mortality overall (adj. HR 3.60 [95% CI 0.40–31.99]). However, specifically in those with pre-frailty and frailty, exposure to anticholinergic PIMs was also not associated with increased mortality risk (adj. HR 1.05 [95% CI 0.61 to 1.79] and adj. HR 1.23 [95% CI 0.76 to 2.01], respectively). In those exposed to antipsychotics, there was no association with increased risk of mortality in fit older people (adj. HR 3.60 [95% CI 0.40–31.99]). In those with pre-frailty, antipsychotic prescription was associated with increased risk of mortality (adj. HR 2.89 [95% CI 1.26–6.66]), and this risk was greater in those with frailty (adj. HR 3.34 [95% CI 1.37–8.12]). There was no association between benzodiazepine prescription and mortality in the sub-population defined as fit (adj. HR 0.92 [95% CI 0.11–7.78]) or pre-frail (adj. HR 1.40 [95% CI 0.66–2.97]). Benzodiazepine prescription was associated with a decreased risk of mortality in the sub-population defined as frail (adj. HR 0.43 [95% CI 0.21–0.86]). The use of tricyclic antidepressants was not associated with greater subsequent mortality in any of the frailty sub-groups: fit older people (data unavailable), pre-frail participants (adj. HR 1.84 [95% CI 0.98–3.44]) and frail participants (adj. HR 0.90 [95% CI 0.55–1.48]). This was also the case for the ‘other antidepressants’ group: fit older people (adj. HR 0.86 [95% CI 0.23–3.20]), pre-frail (adj. HR 1.12 [95% CI 0.67–1.89] and frail (adj. HR 0.74 [95% CI 0.49–1.12]) (Table 3).
Risk of Bias Within Studies
All studies were identified as being at serious risk of bias from selection of participants. One study was additionally identified as being at further serious risk of bias due to missing data [45] (Table 4). The main concerns regarding selection bias included self-selection of participants [38, 39, 47], loss to follow-up or gaps in follow-up [35, 41, 43–46] and participants not followed from first exposure of anticholinergics [35–47]. Additionally, the characterisation of participants as frail if they could not complete some clinical assessments potentially introduced case ascertainment bias [44]. Finally, bias due to residual confounding was identified as a potential issue for all thirteen included studies.
Table 4.
Risk of bias assessment using the Risk of Bias in Non-randomized Studies—of Interventions (ROBINS-I) tool
| Study | Bias due to confounding | Selection bias | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Bennett [35] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Cao [36] | Moderate | Serious | Low | Low | Low | Low | Low | Serious |
| Cossette [37] | Moderate | Serious | Low | Low | Unclear | Low | Low | Serious |
| Gnjidic [38] | Moderate | Serious | Low | Low | Low | Low | Low | Serious |
| Gnjidic [39] | Moderate | Serious | Moderate | Low | Low | Low | Low | Serious |
| Hanlon [40] | Moderate | Serious | Low | Low | Low | Low | Low | Serious |
| Jamsen [41] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Landi [42] | Moderate | Serious | Low | Low | Unclear | Low | Moderate | Serious |
| Martinot [43] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Porter [44] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Sato [45] | Moderate | Serious | Moderate | Low | Serious | Moderate | Moderate | Serious |
| Wilson [46] | Moderate | Serious | Low | Low | Moderate | Low | Low | Serious |
| Zia [47] | Moderate | Serious | Moderate | Low | Low | Low | Moderate | Serious |
Discussion
Key Findings
This review is the first to the authors’ knowledge to summarise associations between anticholinergic medications and key outcomes among frail older populations. This review identified that anticholinergic medications are associated with reduced ability for chair standing, slower gait speeds, increased risk of falls, increased risk of mortality and poorer physical performance, among older people living with frailty. Conflicting results were reported for the effect of anticholinergics on grip strength, TUGT, cognition and ability to perform ADL. Anticholinergics were not shown to be associated with transitions between frailty states, psychological wellbeing or ADRs with benzodiazepine use.
This review identifies that older people living with frailty are at greater risk of adverse outcomes when exposed to anticholinergic medicines; however, there is insufficient evidence to suggest that frailty grade can modify these risks. The three studies that stratified by frailty grade did not clearly demonstrate evidence of a differential effect of anticholinergic medications because of frailty status [35, 37, 44], and therefore it remains unclear whether exposure amongst those with advancing frailty presents greater risks than for fit older people.
The difference between fit older adults and those with frailty in the association between anticholinergics and adverse outcomes did appear to vary among different outcomes, however. For example, PCS scores appeared to differ across the frailty spectrum in those exposed to anticholinergic medicines. Every one-unit increase in the ACB score was associated with a doubling in the reduction of the PCS score amongst those deemed pre-frail/frail, compared to fit older people, representing a greater decrease in physical function. However, from a clinical perspective, the effects were small and likely not relevant [37]. There is much evidence that frailty is graded; however, in this study the pre-frail and frail were considered one group in the reporting of results, limiting the inferences that can be made across the frailty spectrum. On the other hand, falls as an adverse event appeared to be uniform from anticholinergic medicines across the frailty spectrum. Although both the frail and the fit admitted to a tertiary hospital setting were at increased risk of falls associated with FRID exposure, there was no clinically significant difference in risk between people with frailty and fit older people [35]. This may suggest that the risk is apparent for older people as a whole, regardless of frailty grade. Although the majority of medicines used to characterise the FRID exposure variable were of an anticholinergic nature, FRIDs were not exclusively anticholinergic, and therefore challenges inferences made specifically for anticholinergics in this study.
Overall, this review has identified limited evidence with a specific focus on associations between anticholinergic medications and adverse events among older adults with frailty. Such associations among older people in general have been studied extensively internationally [11–13, 56]; however, few observational studies have focused on older people living with frailty, and the risks associated with anticholinergic burden in this population are therefore not fully understood. This highlights a significant gap in the literature in an area becoming increasingly important within medicines optimisation.
Findings in Context of Wider Research Literature
This review is consistent with other systematic reviews in finding that anticholinergic medications are associated with increased risks among older people [11–13, 56]; however, unlike this review, those reviews did not have a focus on frailty. Additionally, the lack of associations observed within some analyses within this review is also consistent with other literature, complementing Welsh et al.’s perspectives that associations are not as clear as others have suggested, and the risks associated with anticholinergic burden are not fully understood [11].
Nishtala et al. reported that exposure to DBI drugs in older people was associated with falls, subsequently leading to hospitalisations [57]. In another study, increasing DBI exposure was found to be associated with slower gait speeds in 1705 individuals over 70 years old [58], with similar findings to studies in this review [36, 38, 42]. Also consistent with this review, there are mixed results for cognition, with a 2-year longitudinal study observing associations between drugs with anticholinergic activity and cognitive dysfunction [59]; however, a lack of association with cognitive dysfunction has also been reported within the literature [11]. Frailty was not identified within these study populations, and therefore the results represent older populations in general; however, this systematic review can complement this existing literature, highlighting how similar findings can also be identified among older adults deemed to be living with frailty.
Interestingly, this review identified a study which did not observe an association between benzodiazepine use and ‘any ADRs’ among older people with frailty [40]. This finding is inconsistent with the wider literature as there is general acceptance that such medicines can cause adverse effects, such as sedation and cognitive decline, particularly among older people [60, 61], and that long-acting benzodiazepines are often involved in patients with ADRs [62]. The multivariate model used for the benzodiazepine analyses accounted for seventeen potential risk-factors, and it is possible that over-adjustment may have occurred, whereby variables on the causal pathway from exposure to outcome may have been controlled for. This could potentially reduce the precision of the results. The use of warfarin was found to be associated with ‘any ADRs’ and ‘preventable ADRs’, however. It is thought that warfarin may have mild anticholinergic activity [53]; however, its association with increased risk of ADRs is more likely to be related to its bleeding risk than antimuscarinic effects [63]; therefore, this has not been considered a significant finding in the context of anticholinergic burden.
Since completion of this review, a new cross-sectional study has been published (June 2020) [64], studying 520 community-dwelling older adults selected from a geriatric outpatient clinic in Turkey. Frailty was identified using the phenotype model. The use of any drugs with possible or definite anticholinergic activity (defined by the ACB scale) was associated with an increased risk of falls in frail participants (adj. OR 3.84 [95% CI 1.48–9.93], p = 0.006) and pre-frail participants (adj. OR 2.71 [95% CI 1.25–5.89], p = 0.012), but not in fit older adults. This supports the hypothesis that frailty grade may potentially modify the risks of adverse outcomes when exposed, as in this case the risk of falls among the frail was significantly greater than in the pre-frail. However, the study was limited by its cross-sectional design, meaning that the total duration of exposure to ACB medicines could not be assessed. Furthermore, there were concerns over the reliance on self-reported data from participants. In addition, the lack of observed association in fit participants challenges direct comparisons across the whole frailty spectrum, and it remains unclear whether the risks amongst those with advancing frailty truly are greater.
Strengths and Limitations of the Review
This systematic review focuses on older adults with frailty, which is an area much understudied. Robustly capturing a wide array of anticholinergic exposures, and frailty measures, with the use of clinical assessments and validated frailty screening tools enabled greater capture of relevant studies, particularly where anticholinergic burden and frailty were not the main focus of the included studies. Methods were pre-specified in a published protocol, and following the comprehensive search strategy, a fully independent review process was maintained throughout, from the initial screening of titles and abstracts to full-text review, to data extraction, to the risk of bias assessment, and finally data synthesis. The overall large number of studies included together with the robust methodology utilised for selection and data analysis can be considered as a strength of this study.
One limitation is that this review is only representative of literature up to the search date of 1st August 2019. Although forward and backward citation analysis was conducted, it is possible that more recently published studies that met the inclusion criteria were omitted. A re-run of the comprehensive search was conducted in April 2021 yielding 588 studies; however, screening of titles, abstracts and full texts was not conducted, and it is unclear whether any studies would have met the inclusion criteria. The search was limited to studies containing phrases relating to ‘frailty’ in the title or abstract, and yielded only nine studies, of which two would have been eligible for screening of full-texts as per the methods [64, 65]. However, it is possible more newly published studies reported the characteristics of a frail sample, without explicitly using phrases such as ‘frailty’ in the texts. Therefore, a full systematic screening approach is required, as was undertaken for the 13 included studies within this review, to identify any further studies specifically focusing on frail older people.
Only six out of the 13 included studies explicitly reported frailty [35, 37, 40, 41, 43, 44], and therefore the full samples of the remaining seven included studies were assumed to be living with frailty by the review team based on assessment of average clinical parameters of frailty measures [36, 38, 39, 42, 45–47]. This may not have been a true reflection of the entire sample, and consequently there may have been older participants who were fit. Measurement of frailty was generally categorised within studies in to fit, pre-frail or frail, and thresholds varied; however, it is accepted that frailty is graded and exists on a spectrum [24]. There is much debate about the thresholds used to characterise frailty, and these have not been standardised [66]. Only three studies stratified samples by frailty [35, 37, 44], and as the other included studies did not stratify, it was not possible to compare associations across the frailty spectrum in these studies.
With the inclusion criteria allowing for the selection of studies with an average sample age of 65 or more, one included study had an age range of 58–73 [43], and one study selected participants aged 60 and over [41]. Arguably, these could represent younger populations, and the outcomes reported for younger participants may have impacted on the overall outcomes reported for full samples. However, as these two studies had overall samples with an average age of 65 and over, they were more representative of an older population. Therefore, it is unlikely that the inclusion of younger participants would have impacted overall results of the individual studies, and indeed the overall conclusions of this systematic review. It is also possible that a requirement of an average age of 65 and over may also have prevented the inclusion of studies undertaken in developing countries, where older age is considered to commence earlier, such as 60 or over. However, this did not prevent the inclusion of one study from a developing country (Malaysia) [47], in which the sample had an average age of 75.3 among those who had fallen and 72.1 among the non-fallers.
Anticholinergic measures were heterogeneous, which made synthesis of the data challenging, in addition to the wide variety of outcomes reported and the diversity of metrics used. This highlights the importance of standardising these metrics. The DBI, considered as an anticholinergic exposure in this review, encompasses an anticholinergic component; however, it also includes a sedative component. Therefore, this does not exclusively represent anticholinergic medicines; a factor which should be considered when interpreting findings in the context of anticholinergics only.
Each study was deemed to be at serious risk of bias, due to concerns around selection biases. None of the thirteen included studies followed participants from the initiation of the exposure, and inevitably a period of follow-up was lost. Selection biases also arise if participants volunteer themselves for participation, which was a recruitment method used by two studies in this review after advertising through leaflets, media and word of mouth [39, 47]. In another study, control subjects volunteered themselves, whereas the cases were enrolled after participating in a separate study [47]. Ascertainment bias could also be an issue in another study, where participants were categorised as frail if data were missing due to the inability to complete clinical assessments [44]. Also, five of the thirteen studies included cross-sectional analyses, and therefore as the exposure and outcome are simultaneously assessed, the temporal relationship between exposure and outcome cannot be ascertained.
It is also possible that inappropriate adjustments may have occurred and may have contributed to bias. In the study investigating the association between DBI exposure and transitions between frailty states and death [41], it could be argued that there was a risk of confounding within those exposed to DBI medicines, whereby undetermined underlying factors could possibly be the cause of both the DBI exposure and the frailty progression. The covariates identified within this study were adjusted for as confounders, which allowed for the study of associations, but did not apply suitable methods to ameliorate bias for causal inference. It is possible that factors influencing anticholinergic exposure are also likely to influence participants’ frailty state and may have contributed to bias. Residual confounding that remains after adjusting for confounders is another significant issue for the included studies.
Finally, where associations between anticholinergic exposure and adverse outcomes have been identified in frail populations, causal assumptions must also be considered. Frailty in its own right can also be associated with increasing anticholinergic exposure and polypharmacy [67]. One must question whether increased anticholinergic exposure can contribute to the cause of adverse outcomes, such as physical impairment, or whether frailty or multimorbidity in their own right have greater contributions, which in turn can increase anticholinergic prescribing [3]. None of the 13 studies in this review applied rigorous causal inference methodology, which therefore limits the causal inferences that can be made from the associations presented.
Implications for Clinical Practice and Future Research
This review reinforces that frailty status should be an important consideration for clinicians when prescribing anticholinergic medicines to older people. As evidenced by various associations with adverse outcomes, anticholinergic burden is a concern in older people living with frailty, and clinicians should continue to practice vigilance, including the use of deprescribing interventions to ensure prescribing is appropriate. Prescribing patterns can differ between frail and non-frail older people, particularly as multimorbidity in advancing frailty is thought to further drive polypharmacy [68, 69]. Additionally, frail older people are more likely to be prescribed symptomatic therapies, increasing the potential for additional anticholinergic medicines to be prescribed [68], and therefore anticholinergic burden levels may differ between the frail and non-frail. Where possible, frailty should be identified, and stratified among older patients, to assist clinicians with anticholinergic prescribing decisions, with a view to limiting associated risks.
This review has also identified mixed results for outcomes such as grip strength, TUGT and cognitive dysfunction among older people with frailty; therefore, there is an urgent need for further high-quality research to investigate the impact of anticholinergic prescribing on older people living with this syndrome.
Importantly, this review also highlights how it is still unclear whether anticholinergic medication exposure is associated with greater risk of adverse outcomes for older people according to frailty status, and therefore whether those with advancing frailty are at greater risk than fit older people. Improving knowledge in this area is of importance, particularly as older patients are being identified, prioritised and proactively targeted for medication reviews, based on frailty grade [5]. Those exposed to anticholinergic medicines should continue to be identified and prioritised for review. However, there remains a knowledge gap surrounding the risk modifying potential of frailty grade, with regards to adverse outcomes associated with anticholinergic burden. Few studies in this area have stratified by frailty grade; therefore, future observational research should identify and stratify frailty within study populations using robust methods, as recommended by the European Medicines Agency in clinical investigations involving medicines [70].
Conclusions
This systematic review of observational studies has identified that prescribing anticholinergic medications is associated with poorer physical function, increased risk of falls and increased mortality among older adults living with frailty. Mixed results were seen for grip strength, TUGT, cognitive dysfunction and ability to perform ADL, and no associations were observed for transitions in frailty states, lower psychological wellbeing or ADRs with benzodiazepine use. It remains uncertain whether those with advancing frailty are at a greater risk from anticholinergic medicines compared with older people deemed to be fit or whether older people are generally at greater risk, regardless of their frailty status. There is an urgent need for this issue to be further evaluated within studies that robustly minimise bias and stratify by frailty.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the following: all members of the Safe Use of Medicines theme at the National Institute for Health Research (NIHR) Yorkshire and Humber Patient Safety Translational Research Centre (YHPSTRC) for their support with conceptualisation of this review; Anne Costigan, Maureen Pinder and Jennifer Rowland (University of Bradford) for help with library searching; and Hiroyuki Takita (Centre for Applied Pharmacokinetic Research [CAPKR], University of Manchester) for support in translating and interpreting Japanese literature.
Declarations
Funding
DM is funded by a doctoral training fund from the NIHR YHPSTRC. DM is also part-funded by Health Data Research UK (HDR UK North, Award Ref. CFC0124). MH is funded by an Academic Clinical Fellowship from the NIHR. OT is funded by a doctoral fellowship from the Dunhill Medical Trust, UK [RTF107/0117]. AC is part-funded by the NIHR Applied Research Collaboration, Yorkshire & Humber, and part-funded by Health Data Research UK (HDR UK North, Award Ref. CFC0124). This research was funded by the NIHR YHPSTRC. The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. This work was supported by Health Data Research UK, an initiative funded by UK Research and Innovation Councils, NIHR and the UK devolved administrations, and leading medical research charities.
Conflicts of interest/Competing interests
The authors have no conflicts of interest to declare.
Availability of data and material
The search strategy utilised is published in Appendix 2 of the supplementary information file (see the ESM).
Code availability
Not applicable.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DM, MH, OT, HZ and IM. The first draft of the manuscript was written by DM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have consented to the publication of the manuscript in its present form.
References
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilmer SN, Gnjidic D, Le Couteur DG. Thinking through the medication list: appropriate prescribing and deprescribing in robust and frail older patients. Aust Fam Physician. 2012;41(12):924. [PubMed] [Google Scholar]
- 4.Clegg A, Young J. The frailty syndrome. Clin Med (Lond). 2011;11(1):72–75. doi: 10.7861/clinmedicine.11-1-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody D, Lyndon H, Stevens G. Toolkit for general practice in supporting older people living with frailty. NHS Engl. 2017;1(2):8–14. [Google Scholar]
- 6.Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium—theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516–524. doi: 10.1111/bcp.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landi F, Dell'Aquila G, Collamati A, Martone AM, Zuliani G, Gasperini B, et al. Anticholinergic drug use and negative outcomes among the frail elderly population living in a nursing home. J Am Med Dir Assoc. 2014;15(11):825–829. doi: 10.1016/j.jamda.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Fox C, Smith T, Maidment I, Chan W-Y, Bua N, Myint PK, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing. 2014;43(5):604–615. doi: 10.1093/ageing/afu096. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman JA., 3rd Managing anticholinergic side effects. Prim Care Companion J Clin Psychiatry. 2004;6(Suppl 2):20–23. [PMC free article] [PubMed] [Google Scholar]
- 10.Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462. doi: 10.1177/014107680009300903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh TJ, van der Wardt V, Ojo G, Gordon AL, Gladman JRF. Anticholinergic drug burden tools/scales and adverse outcomes in different clinical settings: a systematic review of reviews. Drugs Aging. 2018;35(6):523–538. doi: 10.1007/s40266-018-0549-z. [DOI] [PubMed] [Google Scholar]
- 12.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15(1):31. doi: 10.1186/s12877-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardwell K, Hughes CM, Ryan C. The association between anticholinergic medication burden and health related outcomes in the ‘Oldest Old’: a systematic review of the literature. Drugs Aging. 2015;32(10):835–848. doi: 10.1007/s40266-015-0310-9. [DOI] [PubMed] [Google Scholar]
- 14.Richardson K, Fox C, Maidment I, Steel N, Loke YK, Arthur A, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:k1315. doi: 10.1136/bmj.k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishtala PS, Salahudeen MS, Hilmer SN. Anticholinergics: theoretical and clinical overview. Expert Opin Drug Saf. 2016;15(6):753–768. doi: 10.1517/14740338.2016.1165664. [DOI] [PubMed] [Google Scholar]
- 16.Grossi CM, Richardson K, Savva GM, Fox C, Arthur A, Loke YK, et al. Increasing prevalence of anticholinergic medication use in older people in England over 20 years: cognitive function and ageing study I and II. BMC Geriatr. 2020;20(1):267. doi: 10.1186/s12877-020-01657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panel BtAGSBCUE American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 18.O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bressler R, Bahl JJ. Principles of drug therapy for the elderly patient. Mayo Clin Proc. 2003;78(12):1564–1577. doi: 10.4065/78.12.1564. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . Definition of an older or elderly person. WHO; 2010. [Google Scholar]
- 22.Singh S, Bajorek B. Defining 'elderly' in clinical practice guidelines for pharmacotherapy. Pharm Pract (Granada). 2014;12(4):489. doi: 10.4321/s1886-36552014000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collard RM, Boter H, Schoevers RA, Voshaar RCO. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 25.Castell MV, Sánchez M, Julián R, Queipo R, Martín S, Otero Á. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Fam Pract. 2013;14:86. doi: 10.1186/1471-2296-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bischoff HA, Stähelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32(3):315–320. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- 27.Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781–787. doi: 10.1001/archinte.167.8.781. [DOI] [PubMed] [Google Scholar]
- 28.Google. Google Translate. California, USA. 2021. https://translate.google.com/about/. Accessed 02 Jan 2021.
- 29.Harzing A-W. Publish or Perish. 2007. https://harzing.com/resources/publish-or-perish. Accessed 2020.
- 30.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; 2014. [Google Scholar]
- 32.MedCalc . MedCalc statistical software. 19.4.0 ed. Ostend: Belgium MedCalc Software Ltd; 2020. [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme version 1. Lancaster University; 2006. p. b92. [Google Scholar]
- 35.Bennett A, Gnjidic D, Gillett M, Carroll P, Matthews S, Johnell K, et al. Prevalence and impact of fall-risk-increasing drugs, polypharmacy, and drug-drug interactions in robust versus frail hospitalised falls patients: a prospective cohort study. Drugs Aging. 2014;31(3):225–232. doi: 10.1007/s40266-013-0151-3. [DOI] [PubMed] [Google Scholar]
- 36.Cao YJ, Mager DE, Simonsick EM, Hilmer SN, Ling SM, Windham BG, et al. Physical and cognitive performance and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clin Pharmacol Ther. 2008;83(3):422–429. doi: 10.1038/sj.clpt.6100303. [DOI] [PubMed] [Google Scholar]
- 37.Cossette B, Bagna M, Sene M, Sirois C, Lefebvre GP, Germain O, et al. Association between anticholinergic drug use and health-related quality of life in community-dwelling older adults. Drugs Aging. 2017;34(10):785–792. doi: 10.1007/s40266-017-0486-2. [DOI] [PubMed] [Google Scholar]
- 38.Gnjidic D, Bell JS, Hilmer SN, Lönnroos E, Sulkava R, Hartikainen S. Drug Burden Index associated with function in community-dwelling older people in Finland: a cross-sectional study. Ann Med. 2012;44(5):458–467. doi: 10.3109/07853890.2011.573499. [DOI] [PubMed] [Google Scholar]
- 39.Gnjidic D, Le Couteur DG, Abernethy DR, Hilmer SN. Drug Burden Index and beers criteria: impact on functional outcomes in older people living in self-care retirement villages. J Clin Pharmacol. 2012;52(2):258–265. doi: 10.1177/0091270010395591. [DOI] [PubMed] [Google Scholar]
- 40.Hanlon JT, Pieper CF, Hajjar ER, Sloane RJ, Lindblad CI, Ruby CM, et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol Ser A Biol Sci Med Sci. 2006;61(5):511–515. doi: 10.1093/gerona/61.5.511. [DOI] [PubMed] [Google Scholar]
- 41.Jamsen KM, Bell JS, Hilmer SN, Kirkpatrick CMJ, Ilomäki J, Le Couteur D, et al. Effects of changes in number of medications and Drug Burden Index exposure on transitions between frailty states and death: the concord health and ageing in men project cohort study. J Am Geriatr Soc. 2016;64(1):89–95. doi: 10.1111/jgs.13877. [DOI] [PubMed] [Google Scholar]
- 42.Landi F, Russo A, Liperoti R, Cesari M, Barillaro C, Pahor M, et al. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81(2):235–241. doi: 10.1038/sj.clpt.6100035. [DOI] [PubMed] [Google Scholar]
- 43.Martinot P, Ré B, Zins M, Goldberg M, Ankri J, Herr M. Association between potentially inappropriate medications and frailty in the early old age: a longitudinal study in the GAZEL cohort. J Am Med Dir Assoc. 2018;19(11):967–73.e3. doi: 10.1016/j.jamda.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Porter B, Arthur A, Savva GM. How do potentially inappropriate medications and polypharmacy affect mortality in frail and non-frail cognitively impaired older adults? A cohort study. BMJ Open. 2019;9(5):e026171. doi: 10.1136/bmjopen-2018-026171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato R, Arai Y, Abe Y, Takayama M, Urushihara H. The drug burden of anticholinergics and sedatives and influence on outcomes in the community-living oldest old: The Tokyo Oldest Old survey on Total Health (TOOTH) survey. Nihon Ronen Igakkai Zasshi Jpn J Geriatr. 2017;54(3):403–416. doi: 10.3143/geriatrics.54.403. [DOI] [PubMed] [Google Scholar]
- 46.Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al. Associations between Drug Burden Index and falls in older people in residential aged care. J Am Geriatr Soc. 2011;59(5):875–880. doi: 10.1111/j.1532-5415.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- 47.Zia A, Kamaruzzaman S, Myint PK, Tan MP. Anticholinergic burden is associated with recurrent and injurious falls in older individuals. Maturitas. 2016;84:32–37. doi: 10.1016/j.maturitas.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Kersten H, Hvidsten LT, Gløersen G, Wyller TB, Wang-Hansen MS. Clinical impact of potentially inappropriate medications during hospitalization of acutely ill older patients with multimorbidity. Scand J Prim Health Care. 2015;33(4):243–251. doi: 10.3109/02813432.2015.1084766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kröger E, Simard M, Sirois M-J, Giroux M, Sirois C, Kouladjian-O'Donnell L, et al. Is the Drug Burden Index related to declining functional status at follow-up in community-dwelling seniors consulting for minor injuries? Results from the Canadian Emergency Team Initiative Cohort Study. Drugs Aging. 2019;36(1):73–83. doi: 10.1007/s40266-018-0604-9. [DOI] [PubMed] [Google Scholar]
- 50.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53(1):S9–S16. doi: 10.1093/geronb/53B.1.S9. [DOI] [PubMed] [Google Scholar]
- 52.Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 53.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–320. doi: 10.2217/1745509X.4.3.311. [DOI] [Google Scholar]
- 54.Lawton MP. The Philadelphia geriatric center morale scale: a revision. J Gerontol. 1975;30(1):85–89. doi: 10.1093/geronj/30.1.85. [DOI] [PubMed] [Google Scholar]
- 55.Fuentes-García A. Katz activities of Daily Living Scale. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht: Springer; 2014. pp. 3465–3468. [Google Scholar]
- 56.Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209–220. doi: 10.1111/bcp.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23(7):753–758. doi: 10.1002/pds.3624. [DOI] [PubMed] [Google Scholar]
- 58.Gnjidic D, Cumming RG, Le Couteur DG, Handelsman DJ, Naganathan V, Abernethy DR, et al. Drug Burden Index and physical function in older Australian men. Br J Clin Pharmacol. 2009;68(1):97–105. doi: 10.1111/j.1365-2125.2009.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 60.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use. CNS Drugs. 2004;18(1):37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- 61.Gudex C. Adverse effects of benzodiazepines. Soc Sci Med. 1991;33(5):587–596. doi: 10.1016/0277-9536(91)90216-Y. [DOI] [PubMed] [Google Scholar]
- 62.Laroche M-L, Charmes J-P, Nouaille Y, Picard N, Merle L. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol. 2007;63(2):177–186. doi: 10.1111/j.1365-2125.2006.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167(13):1414–1419. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 64.Naharci MI, Tasci I. Frailty status and increased risk for falls: the role of anticholinergic burden. Arch Gerontol Geriatr. 2020;90:104136. doi: 10.1016/j.archger.2020.104136. [DOI] [PubMed] [Google Scholar]
- 65.Lim R, Kalisch Ellett LM, Widagdo IS, Pratt NL, Roughead EE. Analysis of anticholinergic and sedative medicine effects on physical function, cognitive function, appetite and frailty: a cross-sectional study in Australia. BMJ Open. 2019;9(9):e029221. doi: 10.1136/bmjopen-2019-029221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Moulis F, Moulis G, Balardy L, Gérard S, Montastruc F, Sourdet S, et al. Exposure to atropinic drugs and frailty status. J Am Med Dir Assoc. 2015;16(3):253–257. doi: 10.1016/j.jamda.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Villani ER, Vetrano DL, Liperoti R, Palmer K, Denkinger M, van der Roest HG, et al. Relationship between frailty and drug use among nursing homes residents: results from the SHELTER study. Aging Clin Exp Res. 2021 doi: 10.1007/s40520-021-01797-z. [DOI] [PubMed] [Google Scholar]
- 69.Gutiérrez-Valencia M, Izquierdo M, Cesari M, Casas-Herrero Á, Inzitari M, Martínez-Velilla N. The relationship between frailty and polypharmacy in older people: a systematic review. Br J Clin Pharmacol. 2018;84(7):1432–1444. doi: 10.1111/bcp.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Committee for Medicinal Products for Human Use (CHMP) (2018) Reflection paper on physical frailty: instruments for baseline characterization of older populations in clinical trials. EMA/CHMP/778709/2015
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.