Abstract
We performed a pairwise and network meta-analysis to compare pathological complete response (pCR) among neoadjuvant chemotherapy in patients with triple-negative breast cancer. We searched PubMed for randomized clinical trials between January 1, 2000 and December 1, 2020. Abstracts from meetings were also searched. A frequentist random-effect model was applied to compare pCR and toxicities. The P-score was used to rank treatment effects. Nineteen trials with 16 treatments and 7794 patients were included. On the basis of SoC, the addition of carboplatin (OR = 1.82, 95% CI, 1.24 to 2.68, P < .01) and the addition of checkpoint inhibitors (OR = 1.69, 95% CI, 1.23 to 2.32, P < .01) increased pCR in pairwise meta-analysis; compared with paclitaxel, nab-paclitaxel did not improve pCR rates (OR = 1.81, 95% CI, .80 to 4.12, P = .16). The anthracycline-sparing regimen led to similar pCR compared with the anthracycline-containing regimen (OR = 1.50, 95% CI, .82 to 2.76, P = .19). In network meta-analysis, the addition of carboplatin plus a PD-1 inhibitor (pembrolizumab), carboplatin plus bevacizumab, and carboplatin plus veliparib ranked as the top three treatments for achieving pCR, with corresponding P-scores of .91, .84, and .72, respectively. Among patients with homologous recombination deficiency, the addition of carboplatin (OR = 1.31, 95% CI, .69 to 2.50, P = .41) or carboplatin plus PARP inhibitors (OR = 1.19, 95% CI, .58 to 2.47, P = .63) did not increase pCR. For triple-negative breast cancer, combining carboplatin with taxane-anthracycline-containing neoadjuvant chemotherapy could be the standard of care, and the combination containing checkpoint inhibitor is promising. However, their role in long-term oncologic outcome remains to be determined.
Keywords: neoadjuvant chemotherapy, triple-negative breast cancer, pathological response rate, checkpoint inhibitor, toxicity
What do we already know about this topic?
Neoadjuvant chemotherapy with taxane-anthracycline-based regimens is a preferred approach for high-risk early triple-negative breast cancer.
How does your research contribute to the field?
Carboplatin-containing regimens as neoadjuvant chemotherapy for triple-negative breast cancer could be the standard of care and adding a checkpoint inhibitor to such regimens is promising.
What are your research’s implications toward theory, practice, or policy?
A new standard of neoadjuvant treatment in triple-negative breast cancer should be supported by long-term oncologic outcome.
Introduction
Triple-negative breast cancer is characterized by a lack of estrogen receptor, progesterone receptor, and human epidermal growth factor 2 expression, but it represents a highly heterogeneous subtype in breast cancer. 1 Neoadjuvant chemotherapy is a preferred approach for high-risk early triple-negative breast cancer. 2 Regimens with taxanes and anthracyclines are standard of care, resulting in a pathological complete response (pCR) rate in approximately 40% of patients. 3 pCR has been validated as a surrogate for disease-free and overall survival for patients with early triple-negative breast cancer who received neoadjuvant chemotherapy. 4
Platinum and poly (ADP-ribose) polymerase (PARP) inhibitors both exhibit antitumor activities against triple-negative breast cancer in the metastatic setting,5,6 and they have been shown to increase pCR rates in triple-negative breast cancer when added to standard neoadjuvant chemotherapy.7,8 In addition, breast cancers with homologous recombination deficiency, including BRCA1/2-proficient tumors, are relatively sensitive to platinum and PARP inhibitors. 9
Immunotherapy with checkpoint inhibitors has also shown antitumor activities when combined with chemotherapy in triple-negative breast cancer. 9 A phase 1b trial of neoadjuvant pembrolizumab plus chemotherapy reported remarkable pCR rates in triple-negative breast cancer. 10 Despite all efforts to improve pCR, the role of neoadjuvant chemotherapy on long-term survival remains undefined in triple-negative breast cancer, and pCR improvement is usually accompanied by increased toxicity.
By searching relevant trials investigating neoadjuvant chemotherapy in triple-negative breast cancer, we conducted a pairwise and network meta-analysis to compare pCR rates and toxicities among different regimens. We also performed subgroup analysis in patients with homologous recombination-deficient tumors.
Methods
Study Eligibility and Identification
We searched PubMed and Cochrane Central Register of Controlled Trials for prospective randomized controlled trials that investigated neoadjuvant chemotherapy in patients with early triple-negative breast cancer. For unpublished studies, we searched abstracts from meetings of the American Society of Clinical Oncology, the San Antonio Breast Cancer Symposium, and the St. Gallen Conference. We limited our search between January 1, 2000 and December 1, 2020. This study was conducted under the recommendations of the PRISMA protocol.
The following terms were used for the search: breast neoplasm, cancer, or carcinoma; triple-negative; neoadjuvant chemotherapy; platinum; checkpoint inhibitor; PARP inhibitor; systemic review; meta-analysis; and randomized controlled trials.
Inclusion criteria of trials for this analysis were those with the following characteristics: (1) randomized controlled trials included triple-negative breast cancer; (2) early breast cancer; and (3) published with available data of pCR and toxicities.
Exclusion criteria of trials for this analysis were those with the following characteristics: (1) non-randomized trials; (2) phase-I trials; (3) without sufficient results for pCR; and (4) with metastatic breast cancer.
Data Extraction and Assessment for Risk of Bias
We extracted the following information from the included trials: study name, year of publication or meeting presentation, trial phase, sample size, pCR rates, and regimens. The numbers of patients who achieved pCR were directly extracted for analysis. A pCR (ypT0/isypN0) was defined as the absence of any invasive tumor cells in the breast and lymph nodes. Data extraction was conducted by 2 authors (X. L and J. L) independently, and disagreement was resolved by discussion with the third author (S.W). Cochrane Collaboration Recommendations for Assessment was used to evaluate the risk of bias (RoB2) by 2 authors, and discrepancy was resolved by discussion. The resulting plot was produced by the R software using the robvis package. Publication bias was assessed by the funnel plot and the Egger’s test.
Statistical Analysis
We conducted a traditional pairwise meta-analysis of pCR rates among 5 subgroups: taxane-anthracycline-containing regimens with carboplatin vs without; regimens with PARP inhibitors plus carboplatin vs without; regimens with checkpoint inhibitors vs without; regimens with nab-paclitaxel vs that with paclitaxel; and regimens with anthracycline vs without. The pooled OR was estimated using a general variance-based method.
Next, we performed network meta-analysis to rank the effects of treatments regarding pCR and toxicity (including neutropenia, anemia, thrombopenia, peripheral neuropathy, and vomiting). The pooled OR was estimated by the frequentist weighted least-squares algorithm, which is more user-friendly due to easier computation and programming but produces similar results and rankings for network analysis.11,12
Using the netrank function of the netmeta package in R, the P-score was obtained for each treatment and was used to rank treatment effects and toxicities, with higher scores indicating a greater probability for an event to occur.
Heterogeneity and inconsistency in the network were assessed by the Q total statistic, which can be decomposed into within-design heterogeneity and between-design inconsistency. The built-in net heat function enables the visualization of inconsistency between direct and indirect evidence in a network estimate, which was applied in this study. The red color in the corresponding graph indicates high inconsistency; blue indicates low inconsistency. When significant heterogeneity and inconsistency were found, a random-effect model was used; otherwise, a fixed-effect model was employed.
All analyses were performed with R (version i386.3.3.2). A statistical test with P<.05 was considered significant. Hypothesis tests were two-sided.
Results
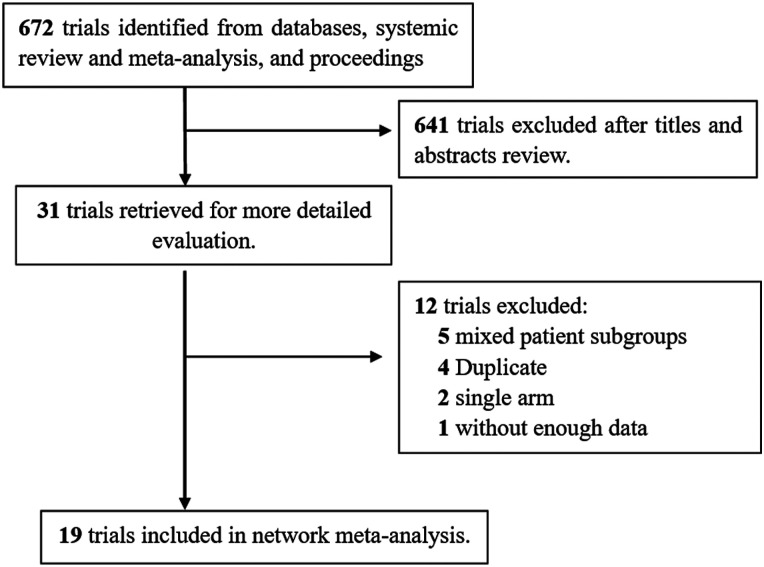
For network meta-analysis, we identified 19 trials including 14 full publications13,15-22,24-26,29,31 and 5 meeting abstracts14,23,27,28,30 by the search strategy, as illustrated in the flow chart (Figure 1). Of the 19 trials (with 7794 patients), 15 were limited to patients with triple-negative breast cancer; for the 4 remaining trials (the GeparSepto, 13 GeparOLA, 14 ISPY2, 15 and the ETNA 16 trial), only patients with triple-negative breast cancer were included in the analysis. Three trials were designed with multi-arms (the CALGB 40603 17 and BrightNess 18 trials). The risk of bias for all included trials was low (Figure S1). The funnel plot and linear regression test (P = .052 for Egger’s test) both indicated little publication bias. (Figure S2)
Figure 1.
Flow chart of the literature search and article selection process.
For analysis of patients whose tumors showed homologous recombination deficiency, 2 studies limited to patients with confirmed status of homologous recombination deficiency (the TBCRC 030 32 and TBCRC 031 29 trials) and subgroups in 4 trials that reported pCR rates (the GeparOLA, 14 BrightNess, 18 GeparSixto, 19 and the GeparOcto 20 trial) were included, with a total of 551 patients.
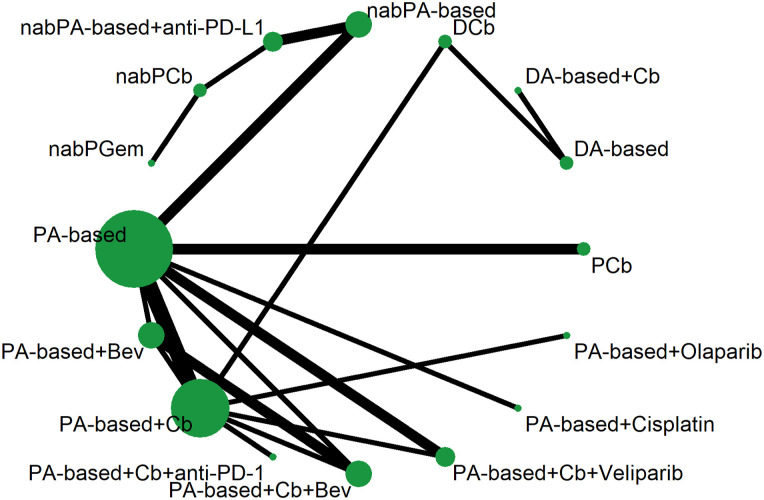
The main characteristics of the included trials are shown in Table 1. Sixteen treatments formed the network, as depicted in Figure 2. Regimens using paclitaxel and an anthracycline (epirubicin or doxorubicin), designated the PA-based regimen, were considered the reference arm in the network. The investigation arms involved agents such as platinum agents (carboplatin or cisplatin), PARP inhibitors (veliparib or olaparib), checkpoint inhibitors (pembrolizumab, durvalumab, or atezolizumab), and nab-paclitaxel (Table S1).
Table 1.
Main characteristics of included studies.
| Study | Year | Phase | TNBC patients, n | Treatments | pCR rate, % | Ref |
|---|---|---|---|---|---|---|
| GEICAM | 2012 | II | 46 | EC-D | 30 | 21 |
| 48 | EC→DCb | 30 | ||||
| Ando M et al | 2014 | II | 91 | P→CEF | 17.6 | 22 |
| 88 | PCb→CEF | 31.8 | ||||
| GeparSixto | 2014 | II | 157 | P+A+Beva | 36.9 | 19 |
| 158 | P+A+Beva+Cb | 53.2 | ||||
| Mexico | 2017 | II | 31 | P→AC | 39.2 | 23 |
| 30 | PCis→AC | 58.6 | ||||
| CALGB 40603 | 2015 | II | 107 | P→AC | 39 | 17 |
| 105 | P+Bev→AC | 43 | ||||
| 111 | P+Cb→AC | 49 | ||||
| 110 | P+Cb+Bev→AC | 60 | ||||
| GeparSepto | 2016 | III | 137 | P→AC | 26 | 13 |
| 139 | nabP→AC | 48 | ||||
| I-SPY2 | 2016 | II | 44 | P-AC | 26 | 15 |
| 72 | P+Cb+Veli→AC | 51 | ||||
| Zhang et al | 2016 | II | 47 | PCb | 38.6 | 24 |
| 44 | PE | 14 | ||||
| WSG-ADAPT-TN | 2017 | II | 146 | nabP+Cb | 45.9 | 25 |
| 178 | nabP+Gem | 28.7 | ||||
| BrightNess | 2018 | III | 158 | P→AC | 31 | 18 |
| 160 | P+Cb→AC | 58 | ||||
| 316 | P+Cb+Veli→AC | 53 | ||||
| ETNA | 2018 | II | 110 | P→AC | 37.3 | 16 |
| 109 | nabP→AC | 41.3 | ||||
| GeparNeuvo | 2019 | II | 86 | nabP→AC | 53.4 | 37 |
| 88 | nabP+Durv→AC+Durv | 44.2 | ||||
| GeparOLA | 2019 | II | 50 | P+Olap→AC | 56 | 14 |
| 27 | P+Cb→AC | 59.3 | ||||
| NeoTRIP | 2019 | III | 138 | nabP+Cb+Ate | 43.5 | 27 |
| 142 | nabP+Cb | 40.8 | ||||
| GeparOcto | 2019 | II | 470 | E→P→C | 48 | 20 |
| 475 | PMCb | 48.3 | ||||
| NeoStop | 2019 | II | 48 | PCb→AC | 55 | 28 |
| 52 | DCb | 52 | ||||
| KEYNOTE 522 | 2020 | III | 401 | P+Cb+Pem→AC+Pem | 64.8 | 26 |
| 201 | P+Cb→AC | 51.2 | ||||
| NeoCart | 2020 | II | 44 | EC→D | 38.6 | 30 |
| 44 | DCb | 61.4 | ||||
| TBCRC 030* | 2019 | II | 71 | Cis | 38 | 32 |
| 67 | P | 43.3 | ||||
| TBCRC 031* | 2020 | II | 60 | Cis | 18 | 29 |
| 58 | AC | 26 | ||||
| IMpassion031 | 2020 | III | 168 | nabP→AC | 41 | 31 |
| 165 | nabP+Ate→AC+Ate | 58 |
A, doxorubicin; Ate, atezolizumab; Bev, bevacizumab; C, cyclophosphamide; Cb, carboplatin; Cis, cisplatin; D, docetaxel; Durv, durvalumab; E, epirubicin; F, fluorouracil; Gem, gemcitabine; M, non-pegylated liposomal doxorubicin; NabP, nab-paclitaxel; Ola, olaparib; P, pacalitaxel; pCR, pathological complete response; PD-1, program death-1; PD-L1, program death ligand-1; Pem, pembrolizumab; TNBC, triple-negative breast cancer; Veli, veliparib. *These 2 trials were not included in the network meta-analysis.
Figure 2.
Network of comparisons of sixteen treatments. Each node denoted each treatment. The width of the lines was proportional to the number of trials comparing 2 treatments, and the size of the node was proportional to the sample size of each treatment.
The Q total statistic indicated heterogeneity and inconsistency in the network (Q = 30.56, P < .01). The subtotal Q statistic for heterogeneity indicated that the within-study design was the main source for heterogeneity (Q = 22.70, P < .001), whereas the subtotal Q statistic for inconsistency showed little between-design inconsistency (Q = 7.86, P = .09). Therefore, we used a random-effect model in this study for all network analyses, and the net heat plot indicated low inconsistency when a random-effect model was used (Figure S3).
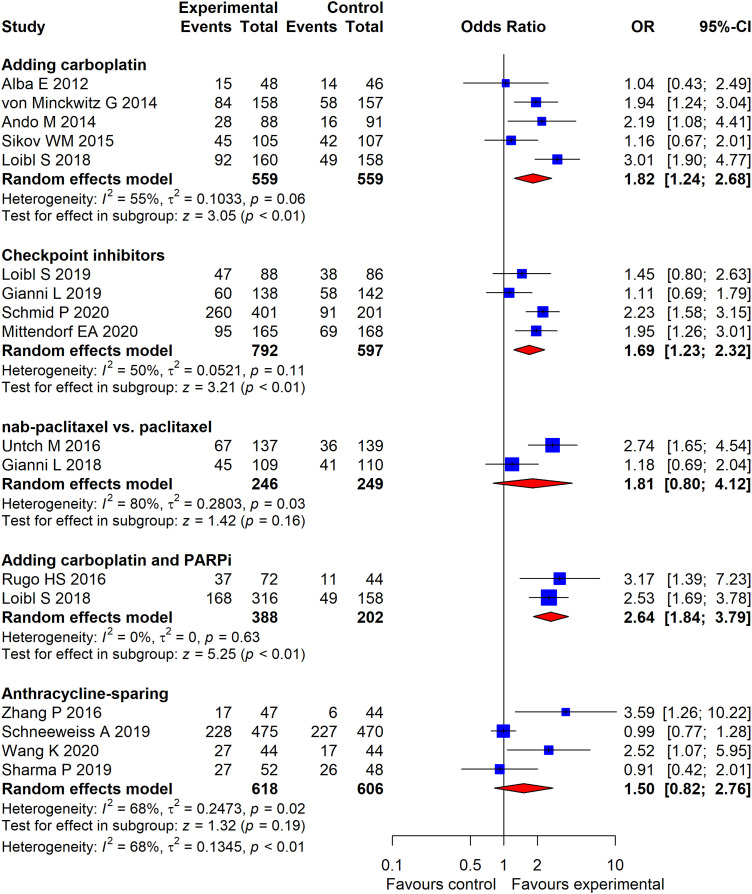
In pairwise meta-analysis of pCR (Figure 3), adding carboplatin to the PA-based regimen increased pCR (OR = 1.82, 95% CI, 1.24 to 2.68, P < .01); adding carboplatin and PARP inhibitors to the PA-based regimen also increased pCR rates (OR = 2.64, 95% CI, 1.84 to 3.79, P < .01). The addition of checkpoint inhibitors to chemotherapy led to a significant improvement in pCR (OR = 1.69, 95% CI, 1.23 to 2.32, P < .01). Compared with paclitaxel, nab-paclitaxel did not improve pCR rates (OR = 1.81, 95% CI, .80 to 4.12, P = .16). The anthracycline-sparing regimen led to similar pCR compared with the anthracycline-containing regimen (OR = 1.50, 95% CI, .82 to 2.76, P = .19).
Figure 3.
Forest plot of odds ratios for pathological complete response of 5 subgroup analyses by pairwise meta-analysis. The squares in the figure represent odds ratios (OR) for each trial, and the horizontal lines indicate the 95% confidential interval (CI) for the OR. The diamond represents the pooled OR, as based on a random-effect method. All statistical tests were 2-sided.
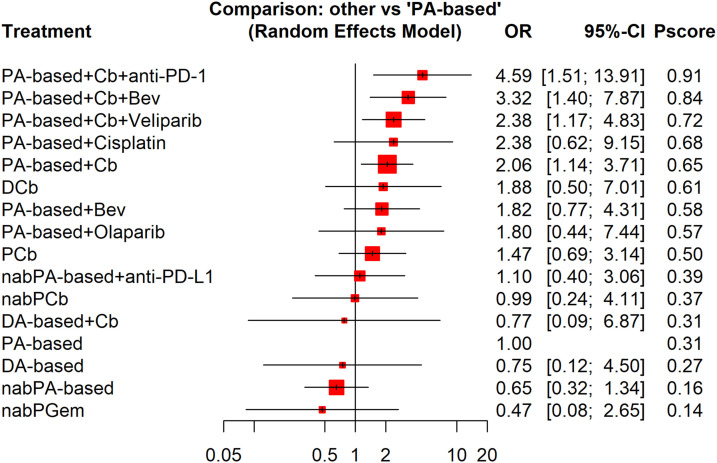
In network meta-analysis of pCR (Figure 4), on the basis of a PA-based regimen, the addition of carboplatin plus a PD-1 inhibitor (pembrolizumab), carboplatin plus bevacizumab, and carboplatin plus PARP inhibitors ranked as the top three treatments to achieve pCR, with corresponding P-scores of .91, .84, and .72, respectively. Network meta-analysis also showed that three anthracycline-sparing treatments (paclitaxel plus carboplatin, docetaxel plus carboplatin, and nab-paclitaxel plus carboplatin) induced comparable pCR when compared with the standard PA-based treatment. Table 2 presents the pairwise comparisons of the 16 treatments.
Figure 4.
Forest plot of odds ratios for pathological complete response of neoadjuvant therapies compared with the reference arm (paclitaxel-anthracycline-based therapy) by network meta-analysis. The squares in the figure represent hazard ratios (OR) for each trial, and the horizontal lines indicate the 95% confidential interval (CI) for the OR. The diamond represents the pooled OR, as based on a random-effect method. All statistical tests were 2-sided.
Table 2.
Pairwise comparisons of pathological complete response among the 16 treatments.
| PA-based +Cb+anti-PD-1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.38 .34-5.53 |
PA-based +Cb+Bev | ||||||||||||||
| 1.91 .51-7.25 |
1.39 .44-4.36 |
PA-based +Cb+Veliparib | |||||||||||||
| 1.93 .30-12.40 |
1.40 .26-7.59 |
1.01 .20-5.03 |
PA-based +Cisplatin | ||||||||||||
| 2.23 .80-6.24 |
1.61 .64-4.09 |
1.16 .50-2.71 |
1.15 .25-5.44 |
PA-based +Cb | |||||||||||
| 2.44 .48-12.30 |
1.77 .37-8.38 |
1.27 .28-5.76 |
1.26 .17-9.24 |
1.09 .31-3.81 |
DCb | ||||||||||
| 2.52 .63-10.07 |
1.82 .84-3.93 |
1.31 .42-4.13 |
1.30 .24-7.07 |
1.13 .45-2.86 |
1.03 .22-4.89 |
PA-based +Bev | |||||||||
| 2.55 .46-13.99 |
1.84 .36-9.56 |
1.33 .27-6.58 |
1.32 .17-10.35 |
1.14 .29-4.44 |
1.04 .17-6.60 |
1.01 .20-5.24 |
PA-based +Olaparib | ||||||||
| 3.03 .70-13.06 |
2.19 .63-7.59 |
1.58 .52-4.86 |
1.57 .31-8.04 |
1.36 .48-3.84 |
1.24 .24-6.30 |
1.20 .35-4.16 |
1.19 .22-6.56 |
PCb | |||||||
| 4.86 .78-30.46 |
3.52 .67-18.60 |
2.54 .52-12.30 |
2.52 .35-18.15 |
2.18 .48-9.96 |
1.99 .28-14.24 |
1.93 .37-10.20 |
1.91 .25-14.63 |
1.61 .32-7.99 |
nabPA-based
+anti-PD-L1 |
||||||
| 5.42 .64-45.53 |
3.92 .54-28.52 |
2.83 .42-19.14 |
2.81 .30-26.64 |
2.43 .38-15.67 |
2.22 .24-20.92 |
2.15 .30-15.64 |
2.13 .21-21.32 |
1.79 .26-12.38 |
1.11 .38-3.28 |
nabPCb | |||||
| 5.92 .51-68.61 |
4.29 .39-47.75 |
3.09 .29-33.38 |
3.07 .20-46.09 |
2.66 .29-24.54 |
2.43 .39-15.28 |
2.35 .21-26.19 |
2.32 .17-31.44 |
1.96 .17-22.73 |
1.22 .08-17.99 |
1.09 .06-19.89 |
DA-based +Cb | ||||
|
4.58
1.37-15.37 |
3.32
1.31-8.42 |
2.39
1.11-5.14 |
2.37 .58-9.75 |
2.06 1.09-3.88 |
1.88 .46-7.63 |
1.82 .72-4.61 |
1.80 .40-8.05 |
1.51 .67-3.44 |
.94 .24-3.75 |
.85 .15-4.88 |
.77 .08-7.82 |
PA-based | |||
| 6.15 .77-48.87 |
4.45 .59-33.76 |
3.21 .44-23.45 |
3.19 .30-34.22 |
2.76 .46-16.68 |
2.52 .69-9.21 |
2.45 .32-18.52 |
2.42 .25-22.99 |
2.03 .25-16.20 |
1.27 .12-13.33 |
1.14 .09-15.14 |
1.04 .28-3.84 |
1.34 .20-9.04 |
DA-based | ||
|
7.04
1.67-29.68 |
5.10
1.51-17.17 |
3.68
1.23-10.96 |
3.65 .73-18.31 |
3.16
1.16-8.63 |
2.89 .58-14.34 |
2.80 .83-9.41 |
2.76 .51-14.96 |
2.32 .75-7.21 |
1.45 .46-4.52 |
1.30 .27-6.24 |
1.19 .10-13.64 |
1.54 .70-3.35 |
1.14 .15-8.98 |
nabPA-based | |
|
11.44
1.05-124.1 |
8.28 .87-79.03 |
5.98 .67-53.51 |
5.93 .49-71.72 |
5.13 .60-44.08 |
4.69 .39-56.37 |
4.55 .48-43.35 |
4.49 .35-57.10 |
3.78 .41-34.50 |
2.35 .51-10.78 |
2.11 .72-6.18 |
1.93 .09-42.58 |
2.50 .32-19.47 |
1.86 .11-30.68 |
1.62 .24-10.87 |
nabPGem |
Each cell represents the pairwise comparison of treatment in the column versus treatment in the row, which contains the odds ratio and its 95% confidence interval underneath.
Significant hazard ratios are in bold.
PA, pacalitaxel-anthracycle; PD-1, program death 1; Cb, carboplatin; Bev, bevacizumab; D, docetaxel; NabP, nab-paclitaxel; Gem, gemcitabine.
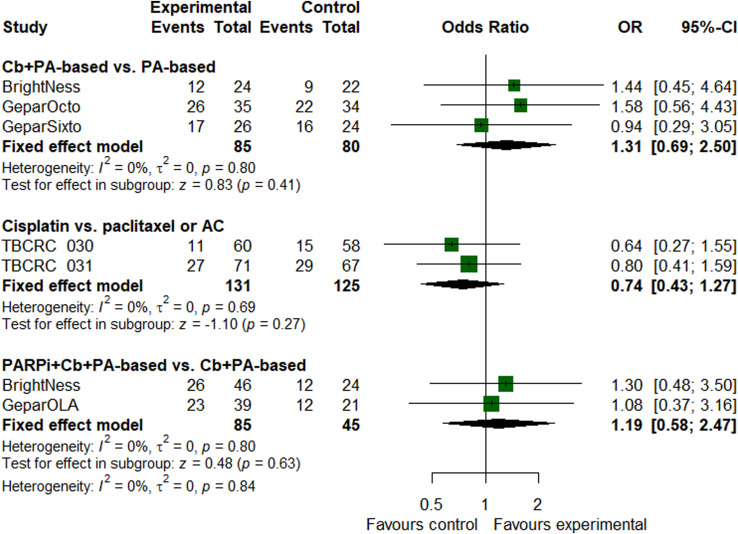
On the basis of paclitaxel and anthracycline, the addition of carboplatin (OR = 1.31, 95% CI, .69 to 2.50, P = .41) or carboplatin plus PARP inhibitors (OR = 1.19, 95% CI, .58 to 2.47, P = .63) did not increase pCR for patients with homologous recombination deficiency (Figure 5). Cisplatin yielded similar pCR rates as paclitaxel or doxorubicin-cyclophosphamide did (OR = .74, 95% CI, .43 to 1.27, P = .27).
Figure 5.
Forest plot of odds ratios for pathological complete response in patients with homologous recombination deficiency tumors.
Regarding nonhematologic toxicities, the nab-paclitaxel-containing regimen ranked highest in neuropathy development (Figure S4), although no differences in vomiting were observed among all regimens (Figure S5). In terms of hematologic adverse events, carboplatin plus PARP inhibitors caused the most neutropenia (Figure S6) and thrombocytopenia (Figure S7), and carboplatin plus bevacizumab caused more anemia than any other treatments (Figure S8).
Discussion
Early triple-negative breast cancer is associated with a high risk of recurrence. 34 Neoadjuvant chemotherapy induces relatively high pCR rates, and Oxford meta-analysis has confirmed the role of pCR in prognosis in early triple-negative breast cancer. 4 Indeed, neoadjuvant chemotherapy in triple-negative breast cancer is an active area of research involving novel agents in the pursuit of higher pCR rates. This pairwise and network study compared pCR rates among 16 neoadjuvant regimens, with recent advances in early triple-negative breast cancer.
Nab-paclitaxel has been compared with paclitaxel in 2 large randomized trials.13,18 In the GeparSepto study, 13 nab-paclitaxel significantly enhanced the pCR rate by 22% (48% vs 26%), although the ETNA study was unable to repeat these results. 18 Based on pairwise and network analyses in our study, nab-paclitaxel did not increase pCR rates compared with paclitaxel. Regarding invasive disease-free survival, the updated GeparSepto study showed a significant benefit for nab-paclitaxel in the overall study population but not for patients with triple-negative breast cancer. 35 Taking toxicity and cost into account, nab-paclitaxel was not recommended as a substitute for paclitaxel, but it remained a reasonable treatment choice for patients who had contradictions to paclitaxel.
Platinum salts have been extensively evaluated in triple-negative breast cancer in both metastatic and neoadjuvant settings. The results of our study support incorporating carboplatin into taxane- and anthracycline-based regimens to induce higher pCR rates. A recent meta-analysis had the same recommendation. 36 However, platinum-based neoadjuvant chemotherapy is associated with worse hematological toxicities. Although the GeparSixto study found a significant improvement in disease-free survival, 37 the CALGB 40603 study did not. 38 Neither of these 2 studies demonstrated a benefit in overall survival.
When carboplatin is used as a component in the neoadjuvant setting, an anthracycline-free regimen is of substantial clinical interest.25,28,30 The advantages of anthracyclines include shortened treatment duration, avoidance of potential cardiac toxicity, and possible pCR improvement. The combination of a taxane (docetaxel, paclitaxel, or nab-paclitaxel) with carboplatin in our study resulted in comparable pCR rates when compared with taxane and anthracycline-based regimens.
PARP inhibitors exhibit synergistic antitumor effects when combined with carboplatin in triple-negative breast cancer. Two studies included in the present meta-analysis added veliparib and carboplatin to standard neoadjuvant treatment. The phase 2 I-SPY study reported pCR rates that nearly doubled in patients who received veliparib plus carboplatin compared to those who received a standard neoadjuvant AT-based regimen. 15 However, the phase III BrightNess trial showed that the addition of veliparib did not increase pCR rates in patients who received paclitaxel and carboplatin. 18 Similarly, our network meta-analysis detected no improvement in pCR rates when adding veliparib to a regimen containing carboplatin, taxane, and anthracycline. Overall, not all patients with triple-negative breast cancer respond to PARP inhibitors. Theoretically, only tumors with homologous recombination deficiency, such as alterations in BRCA 1/2 and ATM genes, are sensitive to PARP inhibitors. The GeparOLA trial enrolled patients with homologous recombination deficiency and showed that the addition of olaparib to paclitaxel resulted in pCR rates similar to those obtained with the addition of carboplatin to paclitaxel, though formal comparison between the 2 arms was not planned. 14
Nonetheless, our subgroup pairwise meta-analysis in patients with homologous recombination deficiency did not find an increase in the pCR rate with the addition of carboplatin or carboplatin plus PARP inhibitors to standard neoadjuvant. As anthracyclines inhibit DNA replication and transcription, they might be potent drugs against tumors with homologous recombination deficiency. 39 In this context, regimens containing anthracyclines were of sufficient efficacy for these tumors. However, these findings should be interpreted cautiously because they are based on patients from unplanned subgroup analysis from each included trial.
The TBCRC 031 and TBCRC 030 trials both only enrolled patients with homologous recombination deficiency and used investigational cisplatin in a comparison with doxorubicin-cyclophosphamide or paclitaxel,32,33 respectively, showing similar pCR rates between the cisplatin and control arms. The results from the meta-analysis of these 2 trials in our study were in line with these reports. However, neither trial used established regimens that usually contain a taxane-anthracycline backbone. Available evidence did not support platinum alone as neoadjuvant therapy for TNBC.
The addition of checkpoint inhibitors to chemotherapy can improve efficacy in patients with metastatic TNBC,40,41 prompting trials to investigate such agents in the neoadjuvant setting. In the phase 1b trial KEYNOTE-173, neoadjuvant pembrolizumab and chemotherapy induced a pCR of 60%. 10 The subsequent phase III KEYNOTE-522 trial validated data from the KEYNOTE-173 trial. Our meta-analysis included 3 studies investigating three checkpoint inhibitors, with 1 study using the anti-PD-1 antibody pembrolizumab 29 and 2 using the anti-PD-L1 antibodies atezolizumab 27 and durvalumab. 26 The conventional pairwise meta-analysis showed increased pCR with the combination of checkpoint inhibitors and chemotherapy, but the network meta-analysis showed that only the combination of an anti-PD-1 antibody (pembrolizumab in this study) and chemotherapy led to higher pCR, whereas the combination of an anti-PD-L1 antibody (atezolizumab and durvalumab) and chemotherapy did not. It should be noted that the chemotherapy backbones in these trials were different, which may impact the efficacy of checkpoint inhibitors. Moreover, the duration of immunotherapy and the patients’ baseline characteristics varied among trials. Finally, the effect of immunotherapy might be better measured by survival outcomes, not only among patients with pCR but also among those with residual disease. Currently, no study in this area has reported conclusive evidence of improved long-term survival outcomes in patients with TNBC who had received neoadjuvant checkpoint inhibitors.
Several limitations existed in the present study. First, significant heterogeneity was detected. Some obvious factors contributed to such heterogeneity: different study designs, multiple treatment arms, and various patient characteristics. Five included trials were presented as abstracts without detailed information. In addition, some trials enrolled small proportions of patients other than those with TNBC who were excluded from the analysis. Accordingly, a random-effect model was used. Second, our study compared pCR among different neoadjuvant regimens in TNBC but was unable to evaluate survival outcomes, which is a substantial limitation for most meta-analyses. Long-term oncologic outcomes were the goal of neoadjuvant therapy for this patient population, which has a high risk of relapse. However, in most trials, follow-up was insufficient to assess survival. We believe that a meta-analysis based on individual patient data would yield more informative results.
Conclusion
In conclusion, the results from our pairwise and network meta-analysis did not support nab-paclitaxel as a routine substitute for paclitaxel but do support the addition of carboplatin to standard neoadjuvant regimens. The role of PARP inhibitors remains to be investigated. For tumors with homologous recombination deficiency, taxane-anthracycline-containing regimens should still be the standard of care. Finally, the combination of anti-PD-1 antibody pembrolizumab and chemotherapy was associated with the highest pCR rates, but its impact on long-term survival remains to be determined.
Supplemental Material
Supplemental Material, sj-pdf-1-inq-10.1177_00469580211056213 for Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Pairwise and Network Meta-Analysis of Pathological Complete Response by Zeng-Jie Weng, Sheng-Xi Wu, He-San Luo, Ze-Sen Du, Xu-Yuan Li and Jia-Zhou Lin in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Supplemental Material, sj-pdf-2-inq-10.1177_00469580211056213 for Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Pairwise and Network Meta-Analysis of Pathological Complete Response by Zeng-Jie Weng, Sheng-Xi Wu, He-San Luo, Ze-Sen Du, Xu-Yuan Li and Jia-Zhou Lin in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Jia-Zhou Lin https://orcid.org/0000-0001-9307-4794
References
- 1.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134-1150. [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N, Gluz O. Neoadjuvant therapy for triple negative and HER2-positive early breast cancer. Breast. 2017;34(suppl):S99-S103. [DOI] [PubMed] [Google Scholar]
- 4.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. [DOI] [PubMed] [Google Scholar]
- 5.Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat Med. 2018;24(5):628-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.JJJ Geenen, Linn SC, Beijnen JH, Schellens JHM. PARP inhibitors in the treatment of triple-negative breast cancer. Clin Pharmacokinet. 2018;57(4):427-437. [DOI] [PubMed] [Google Scholar]
- 7.La Belle A, Khatib J, Schiemann WP, Vinayak S. Role of platinum in early-stage triple-negative breast cancer. Curr Treat Options Oncol. 2017;18(11):68. [DOI] [PubMed] [Google Scholar]
- 8.Papadimitriou M, Mountzios G, Papadimitriou CA. The role of PARP inhibition in triple-negative breast cancer: Unraveling the wide spectrum of synthetic lethality. Cancer Treat Rev. 2018;67:34-44. [DOI] [PubMed] [Google Scholar]
- 9.Garufi G, Palazzo A, Paris I, et al. Neoadjuvant therapy for triple-negative breast cancer: potential predictive biomarkers of activity and efficacy of platinum chemotherapy, PARP- and immune-checkpoint-inhibitors. Expet Opin Pharmacother. 2020;21(6):687-699. [DOI] [PubMed] [Google Scholar]
- 10.Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569-581. [DOI] [PubMed] [Google Scholar]
- 11.Ribassin-Majed L, Marguet S, Lee AWM, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017;35(5):498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: A review of currently available automated packages. PLoS One. 2014;9(12):e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): A randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345-356. [DOI] [PubMed] [Google Scholar]
- 14.Fasching PA, Jackisch C, Rhiem K, et al. GeparOLA: A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients (pts) with HER2-negative early breast cancer (BC) and homologous recombination deficiency (HRD). J Clin Oncol. 2019;37(15 suppl):506. [Google Scholar]
- 15.Rugo HS, Olopade OI, DeMichele A, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianni L, Mansutti M, Anton A, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women With ERBB2/HER2-negative breast cancer-the evaluating treatment with neoadjuvant abraxane (ETNA) Trial: A randomized phase 3 clinical trial. JAMA Oncol. 2018;4(3):302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loibl S, O'Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497-509. [DOI] [PubMed] [Google Scholar]
- 19.Minckwitz Gvon, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014;15(7):747-756. [DOI] [PubMed] [Google Scholar]
- 20.Schneeweiss A, Möbus V, Tesch H, et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): A randomised phase III trial. Eur J Cancer. 2019;106:181-192. [DOI] [PubMed] [Google Scholar]
- 21.Alba E, Chacon JI, Lluch A, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat. 2012;136(2):487-493. [DOI] [PubMed] [Google Scholar]
- 22.Ando M, Yamauchi H, Aogi K, et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res Treat. 2014;145(2):401-409. [DOI] [PubMed] [Google Scholar]
- 23.Martinez MCA, Arce-Salinas C, Alvarado-Miranda A, et al. Randomized phase II trial to evaluate the safety and efficacy of neoadjuvant cisplatin in combination with taxanes-anthracyclines vs taxanes-anthracyclines alone in locally advanced triple negative breast cancer. J Clin Oncol. 2017;33(15 suppl):e12024. [Google Scholar]
- 24.Zhang P, Yin Y, Mo H, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget. 2016;7(37):60647-60656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluz O, Nitz U, Liedtke C, et al. Comparison of neoadjuvant nab-paclitaxel+carboplatin vs nab-paclitaxel+gemcitabine in triple-negative breast cancer: Randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst. 2018;110(6):628-637. [DOI] [PubMed] [Google Scholar]
- 26.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 27.Gianni L, Huang CS, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Cancer Res. 2020;80(4 suppl). [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Kimler BF, O'Dea A, et al. Results of randomized phase II trial of neoadjuvant carboplatin plus docetaxel or carboplatin plus paclitaxel followed by AC in stage I-III triple-negative breast cancer (NCT02413320). J Clin Oncol. 2019;37(15 suppl):516. [Google Scholar]
- 29.Tung N, Arun B, Hacker MR, et al. TBCRC 031: Randomized phase II study of neoadjuvant cisplatin versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial). J Clin Oncol. 2020;38(14):1539-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Wu Z, Lin Y, et al. Neoadjuvant docetaxel + carboplatin versus epirubicin+cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): Results from a multicenter, randomized controlled, open-label, phase II trial. J Clin Oncol. 2020;38(15suppl):586. [DOI] [PubMed] [Google Scholar]
- 31.Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090-1100. [DOI] [PubMed] [Google Scholar]
- 32.Mayer EL, Abramson VG, Jankowitz RC, et al. TBCRC 030: A randomized phase II study of preoperative cisplatin versus paclitaxel in TNBC—Evaluating the homologous recombination deficiency (HRD) biomarker. J Clin Oncol. 2019;37(15suppl):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saw S, Lim J, Lim SH, Wong M, Lim C, Yap YS. Patterns of relapse after neoadjuvant chemotherapy in breast cancer: implications for surveillance in clinical practice. Breast Cancer Res Treat. 2019;177(1):197-206. [DOI] [PubMed] [Google Scholar]
- 34.Untch M, Jackisch C, Schneeweiss A, et al. NAB-paclitaxel improves disease-free survival in early breast cancer: GBG 69-GeparSepto. J Clin Oncol. 2019;37(25):2226-2234. [DOI] [PubMed] [Google Scholar]
- 35.Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann Oncol. 2018;29(7):1497-1508. [DOI] [PubMed] [Google Scholar]
- 36.Loibl S, Weber KE, Timms KM, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018;29(12):2341-2347. [DOI] [PubMed] [Google Scholar]
- 37.Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279-1288. [DOI] [PubMed] [Google Scholar]
- 38.Sikov WM, Polley MY, Twohy E, et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/- carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol. 2019;37(15 suppl):591. [Google Scholar]
- 39.Treszezamsky AD, Kachnic LA, Feng Z, Zhang J, Tokadjian C, Powell SN. BRCA1- and BRCA2-deficient cells are sensitive to etoposide-induced DNA double-strand breaks via topoisomerase II. Cancer Res. 2007;67(15):7078-7081. [DOI] [PubMed] [Google Scholar]
- 40.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44-59. [DOI] [PubMed] [Google Scholar]
- 41.Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38(15 suppl):1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-inq-10.1177_00469580211056213 for Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Pairwise and Network Meta-Analysis of Pathological Complete Response by Zeng-Jie Weng, Sheng-Xi Wu, He-San Luo, Ze-Sen Du, Xu-Yuan Li and Jia-Zhou Lin in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Supplemental Material, sj-pdf-2-inq-10.1177_00469580211056213 for Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Pairwise and Network Meta-Analysis of Pathological Complete Response by Zeng-Jie Weng, Sheng-Xi Wu, He-San Luo, Ze-Sen Du, Xu-Yuan Li and Jia-Zhou Lin in INQUIRY: The Journal of Health Care Organization, Provision, and Financing