Graphical abstract
Keywords: SNM1A, Squaramide, Thiosquaramide, Interstrand crosslink repair, Zinc-binding group, Nucleoside, Nuclease inhibitor
Abstract
SNM1A is a zinc-dependent nuclease involved in the removal of interstrand crosslink lesions from DNA. Inhibition of interstrand crosslink repair enzymes such as SNM1A is a promising strategy for improving the efficacy of crosslinking chemotherapy drugs. Initial studies have demonstrated the feasibility of developing SNM1A inhibitors, but the full potential of this enzyme as a drug target has yet to be explored. Herein, the synthesis of a family of squaramide- and thiosquaramide-bearing nucleoside derivatives and their evaluation as SNM1A inhibitors is reported. A gel electrophoresis assay was used to identify nucleoside derivatives bearing an N-hydroxysquaramide or squaric acid moiety at the 3′-position, and a thymidine derivative bearing a 5′-thiosquaramide, as candidate SNM1A inhibitors. Quantitative IC50 determination showed that a thymidine derivative bearing a 5′-thiosquaramide was the most potent inhibitor, followed by a thymidine derivative bearing a 3′-squaric acid. UV–Vis titrations were carried out to evaluate the binding of the (thio)squaramides to zinc ions, allowing the order of inhibitory potency to be rationalised. The membrane permeability of the active inhibitors was investigated, with several compounds showing promise for future in vivo applications.
1. Introduction
Interstrand crosslinks (ICLs) are a highly cytotoxic form of DNA damage, as they prevent unwinding of the double helix and thereby inhibit DNA replication and transcription. Crosslinking agents are therefore widely used as chemotherapy drugs. However, certain cancers can develop resistance to crosslinking agents through overexpression of the enzymes involved in ICL repair pathways.1, 2, 3, 4 Developing inhibitors for these enzymes is a promising strategy for improving the efficacy of chemotherapy for drug-resistant cancers. Inhibitors for DNA repair enzymes such as O6-methylguanine methyltransferase and poly (ADP-ribose) polymerase, are approved for use in cancer treatment.5, 6, 7
The main ICL repair pathway in eukaryotic cells occurs after stalling of a replication fork at the site of an ICL. A key enzyme in this process is SNM1A, a 5′-3′ exonuclease which hydrolyses the phosphodiester backbone of the DNA past the site of the ICL.8, 9 Cells deficient in SNM1A show increased sensitivity to crosslinking agents such as SJG-136 and the anticancer drug mitomycin C.10 SNM1A is therefore a promising drug target, as inhibitors of this enzyme could be used to resensitise chemotherapy-resistant tumours.
A crystal structure of a truncated version of SNM1A has been reported, and shows a single zinc ion in the active site.11 However the crystal structure of the closely related enzyme SNM1B shows two zinc ions in the active site.11 It has been suggested that the active form of SNM1A contains a second zinc ion, or perhaps another metal cation, which is more loosely bound and therefore not observed in the crystal structure.8 These two zinc ions are predicted to activate a water molecule for nucleophilic attack on a phosphodiester in the DNA backbone, and stabilise a build-up of negative charge on the phosphodiester as it undergoes hydrolysis.
Previously reported inhibitors of SNM1A include the β-lactam antibiotics cephalosporins, which show strong inhibition in vitro but have low membrane permeability,12 and several hits identified from a high throughput screening of a library of bioactive compounds, which have an unclear mechanism of action.13 The development of substrate-mimic inhibitors for SNM1A has shown promising initial results. These compounds are based on a nucleoside scaffold, appended with a zinc-binding group (ZBG) to enhance binding to the active site. Inhibitors of this type which have demonstrated efficacy in in vitro testing include a thymidine derivative bearing a hydroxamic acid ZBG at the 5′-position14 and several malonate-bearing thymidine derivatives.15 There remains a need however for further development of substrate-mimic SNM1A inhibitors to fully investigate the potential of this under-studied enzyme as a drug target.
In this work, we report the synthesis and testing of a series of substrate-mimic SNM1A inhibitors bearing squaramide or thiosquaramide ZBGs. Squaramides can chelate cations through their two carbonyl oxygen atoms,16 and N-hydroxysquaramides have shown promise as ZBGs in inhibitors for metalloproteases.17, 18 An oligonucleotide bearing a squaramide at the 5′-terminus has been shown to bind to SNM1A.19 However, the use of squaramides as metal chelators in biological applications has not been fully explored. In particular, we hypothesised that squaramides bearing substituents containing an additional oxygen atom could show improved binding to SNM1A due to chelation of the proposed second zinc ion in the SNM1A active site (Fig. 1A). Squaramides can also function as phosphate bioisosteres,20, 21 which is desirable for mimicking the natural DNA substrate of SNM1A. Thiosquaramides have more acidic NH protons than their oxo analogues,22 and up to now have principally been investigated for their anion binding properties22 and applications in organocatalysis.23, 24 However due to the affinity of sulfur for zinc, and the greater polarisation of thiosquaramides, we hypothesised that they could prove to be more potent ZBGs than their oxo analogues. For some squaramide-based metalloprotease inhibitors, replacement of one of the squaramide carbonyl groups with a thiocarbonyl moiety improved the inhibitory effect, however analogues containing two thiocarbonyls have not been explored to date.17
Fig. 1.
A) Design of squaramide-bearing substrate-mimic inhibitors of SNM1A based on a schematic view of substrate binding to the enzyme active site. B) Structures of squaramide and thiosquaramide motifs used as zinc-binding groups in SNM1A inhibitors.
A range of nucleoside derivatives bearing differently substituted squaramides or thiosquaramides at the 3′- or 5′-position, as well as a dinucleoside linked through a thiosquaramide in place of a phosphodiester, have been synthesised (Fig. 1B). Several modified ribonucleosides have been prepared for comparison with deoxyribonucleoside inhibitors, as SNM1A can hydrolyse RNA as well as DNA in vitro.8, 9 These compounds have been tested as inhibitors of SNM1A in competition with oligonucleotide substrates, with several showing biological activity. The interaction of these compounds with zinc ions has been studied in UV–Vis titrations, and these results allow the relative potency observed in SNM1A inhibition assays to be rationalised. The membrane permeability of the candidate SNM1A inhibitors has been quantified using a parallel artificial membrane permeability assay (PAMPA).
2. Results and discussion
A series of thymidine derivatives 2–4 bearing a squaramide group at the 5′-position were synthesised (Scheme 1). Squaramides 2 and 3 bear substituents containing an additional hydroxyl group, while compound 4 contains a squaric acid moiety, aimed at improving binding to active site zinc ions in SNM1A. Intermediate 1 was prepared as previously described,14, 19 and used for the generation of further functionalised squaramides 2–4. Intermediate 1 was reacted with ethanolamine to prepare squaramide 2 in 93% yield. Separately, reaction of squaryl monoamide 1 with 2-aminophenol produced squaramide 3, which is analogous to squaramide 2 in the positioning of the side-chain hydroxyl group, but has less conformational flexibility, which we reasoned could improve binding to zinc due to a lower entropic penalty. Intermediate 1 was also hydrolysed under basic conditions to provide squaric acid 4 in 33% yield.25
Scheme 1.
Synthesis of thymidine derivatives 2–4 bearing squaramides at the 5′-position.
Thymidine derivatives bearing squaramides at the 3′-position (6–9) were synthesised for comparison in biological assays with compounds 1–4 bearing the squaramide moiety at the 5′-position (Scheme 2). 3′-Amino-3′-deoxythymidine (5), prepared as previously described,15 was reacted with diethyl squarate to prepare squaryl monoamide 6, a key intermediate for preparation of further functionalised squaramides 7–9. Intermediate 6 was reacted with ethanolamine, and separately with N-methylhydroxylamine hydrochloride, to furnish squaramide 7 and N-hydroxysquaramide 8 respectively. Hydrolysis of squaryl monoamide 6 under basic conditions yielded squaric acid derivative 9 in a modest 22% yield.
Scheme 2.
Synthesis of thymidine derivatives 6–9 bearing squaramides at the 3′-position.
In vitro studies have shown that SNM1A can hydrolyse RNA as well as DNA,8, 9 indicating that modified ribonucleosides could potentially function as SNM1A inhibitors. Furthermore, use of a ribonucleoside scaffold in place of a deoxyribonucleoside provides the potential for further functionalisation at the 2′-position for future optimisation of lead compounds. We therefore synthesised several uridine derivatives bearing squaramides at the 3′-position.
To enable addition of a squaramide moiety at the 3′-position, uridine derivative 21 containing a 3′-amino group was prepared (Scheme 3). Reaction of uridine (10) with tert-butyldimethylsilyl chloride gave the 2′,5′-protected isomer 11 as the major product in 89% yield.26 3′-Amino uridine derivative 21 was prepared from 11 through modification of a previously reported synthetic scheme.27, 28 Oxidation of protected uridine derivative 11 to ketone 12, followed by condensation with hydroxylamine produced an oxime-bearing uridine derivative isolated as a mixture of E/Z isomers 15 and 16. Formation of previously unidentified side products 13 and 14, was also observed. These were presumably formed via silyl migration from the 2′- to the 3′-position in the enolate form of ketone 12. Reducing the concentration of the reaction by approximately one third (from 0.88 M of hydroxylamine and 0.17 M of compound 12 to 0.56 M of hydroxylamine and 0.11 M of 12) reduced the formation of side products 13 and 14 from 13% yield to a trace amount, and increased the yield of the desired products 15 and 16 from 67% to 86%. Attempts to reduce oxime isomers 15/16 were unproductive. However, after selective deprotection of the 5′-O-tert-butyldimethylsilyl group, E/Z oxime isomers 17 and 18 were successfully reduced using sodium triacetoxyborohydride formed in situ from sodium borohydride and acetic acid. Formation of trace amounts of alkylated side product 19 was observed, likely due to acetylation of hydroxylamine product 20 by boron triacetate followed by reduction of the resulting amide. Hydroxylamine product 20 was formed stereoselectively, which can be attributed to coordination of the 5′-hydroxyl group of oximes 17/18 to boron.29 Hydrogenation of hydroxylamine 20 afforded amine 21, the key intermediate allowing further functionalisation with squaramide moieties at the 3′-position of uridine derivatives.
Scheme 3.
Synthesis of 3′-aminouridine derivative 21.
Uridine derivative 22 containing a squaramide at the 3′-position was prepared in 98% yield by reaction of amine 21 with diethyl squarate (Scheme 4). The silyl protecting group of squaramide 22 was removed with TBAF to provide the product 23 in 99% yield. Squaryl monoamide 23 acted as a key intermediate in the preparation of squaramides 24–27. Squaryl monoamide 23 was treated with ethanolamine, and separately with 2-aminophenol to produce squaramides 24 and 25 respectively. N-Hydroxysquaramide 26 was prepared in 51% yield by reaction of squaryl monoamide 23 with N-methylhydroxylamine hydrochloride. Hydrolysis of squaryl monoamide 23 under basic conditions provided squaric acid derivative 27 in 57% yield.
Scheme 4.
Synthesis of uridine derivatives 23–27 bearing squaramides at the 3′-position.
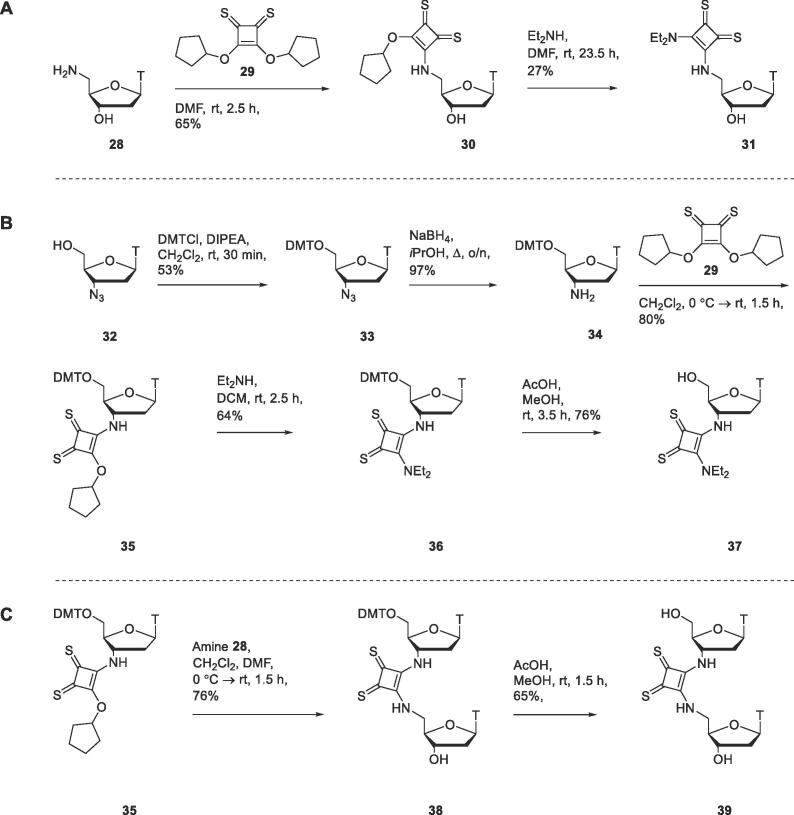
A series of thiosquaramide-bearing nucleosides 31, 37 and 39 were prepared to test whether these sulfur-containing functional groups would prove to be more effective inhibitors of the zinc metalloenzyme SNM1A than their oxo analogues. 5′-Aminothymidine 28, prepared as previously described,19 was reacted with dicyclopentyl dithiosquarate30 29 to prepare thiosquaryl monoamide 30 in 65% yield (Scheme 5A). Thiosquaryl monoamide 30 was reacted with diethylamine to provide thiosquaramide 31 in 27% yield.
Scheme 5.
A) Synthesis of a thymidine derivative 31 bearing a 5′-thiosquaramide. B) Synthesis of a thymidine derivative 37 bearing a 3′-thiosquaramide. C) Synthesis of a dinucleoside 39 containing a bridging thiosquaramide in place of a phosphodiester linkage.
To prepare a thymidine derivative bearing a thiosquaramide at the 3′-position, azidothymidine (32) was reacted with 4,4′-dimethoxytrityl chloride to generate protected thymidine derivative 33 in 53% yield (Scheme 5B).31 Azide 33 was reduced to amine 34,32 which was further reacted with dicyclopentyl dithiosquarate 29 to provide thiosquaryl monoamide 35. Initially a low yield of 35 was obtained, however when the reaction was carried out in the dark, compound 35 was prepared in 80% yield. It was observed that a number of the other thiosquaramides appeared to decompose after exposure to light for several hours. This is consistent with the reported photoactivity of thiocarbonyls, which can be excited by visible light.33, 34 Following this observation care was taken to shield thiosquaramides from light during subsequent reactions. Further reaction of thiosquaryl monoamide 35 with diethylamine provided thiosquaryl diamide 36 in 64% yield. Deprotection of thiosquaramide 36 furnished the target compound 37 in 76% yield.
We hypothesised that binding to SNM1A could be improved by using a dinucleoside inhibitor rather than a mononucleoside, more closely mimicking the natural DNA substrate and increasing the number of possible stabilising non-covalent interactions with the enzyme. This strategy was previously applied in the development of SNM1A inhibitors bearing malonate/malonamide ZBGs; dinucleosides linked through a malonamide were slightly more effective inhibitors than their mononucleoside analogues bearing a malonamide at the 3′-position.15 A dinucleoside 39 containing a thiosquaramide linkage was therefore prepared (Scheme 5C). Thiosquaryl monoamide 35 was reacted with 5′-amine 28 to produce thiosquaramide 38. The DMT protecting group was removed under acidic conditions to provide the target dinucleoside 39 in 65% yield.
The ability of the squaramide- and thiosquaramide-bearing nucleoside derivatives to inhibit SNM1A was first evaluated using a gel electrophoresis-based assay, as previously described.12, 19 The nucleoside derivatives were incubated at a concentration of 1 mM in solution with SNM1A at 37 °C for 5 min, before addition of a 21mer oligonucleotide substrate, labelled with a fluorophore at the 3′-terminus, and further incubation for 1 h. The extent of digestion of the oligonucleotide substrate was examined by polyacrylamide gel electrophoresis (PAGE). SNM1A does not show significant nuclease activity on DNA strands of 8 nucleotides or less,9 and digestion of the oligonucleotide substrate to this length therefore indicates full activity of the enzyme. The presence of longer oligonucleotides in the PAGE readout is indicative of effective inhibition of SNM1A. Thymidine was included in this assay as a control to verify that inhibitory effects result from the squaramide or thiosquaramide modifications.
The results of the inhibitor screen show that thymidine derivatives 1–4 bearing squaramide groups at the 5′-positon do not inhibit SNM1A to any significant extent (Fig. 2A). Compound 40, a squaramide without a substituent that could contribute to chelation of zinc, was prepared as previously described14 and tested as an additional control in this experiment, exhibiting no inhibition of SNM1A. This is consistent with previous results that showed compounds 1 and 40 do not inhibit SNM1A.14 Squaramides 2–4 did not show improved binding to SNM1A despite their additional functionalisation. Testing of thymidine derivatives 6–9 bearing squaramides at the 3′-position however revealed that compounds 8 and 9 inhibit SNM1A (Fig. 2B), as does uridine derivative 26 (Fig. 2C), an analogue of thymidine derivative 8. Squaramide-bearing nucleoside derivatives thus appear to inhibit SNM1A more effectively when the squaramide moiety is placed at the 3′-position rather than the 5′-position of the inhibitor. Compound 9 bearing a 3′-squaric acid inhibits SNM1A, while compound 4 bearing a 5′-squaric acid does not. The squaric acid and N-hydroxysquaramide moieties appeared to be more effective than the other squaramides tested.
Fig. 2.
Biological evaluation of SNM1A inhibitors through visualisation of the extent of digestion of a 21mer fluorescent oligonucleotide substrate by denaturing PAGE. SNM1A (2.5 nM) was pre-incubated with the modified nucleosides (1 mM) for 5 min before the oligonucleotide substrate (80 nM) was added, and then incubated for a further 1 h. nt = nucleotides. A) Evaluation of thymidine derivatives 2–4 bearing a squaramide at the 5′-position. B) Evaluation of thymidine derivatives 6–9 bearing a squaramide at the 3′-position. C) Evaluation of uridine derivatives 23–27 bearing a squaramide at the 3′-position. D) Evaluation of thiosquaramides 31, 37 and 39.
Thiosquaramides 31, 37 and 39 were also screened in the gel-electrophoresis based assay. Contrary to the trend observed for squaramides, thymidine derivative 31 bearing a thiosquaramide at the 5′-position showed greater inhibition of SNM1A than thymidine derivative 37 bearing a thiosquaramide at the 3′-position (Fig. 2D). This may indicate a different binding mode to SNM1A due to the larger steric bulk of thiosquaramides relative to their oxo analogues. Thiosquaramide-linked dinucleoside 39 inhibited SNM1A less effectively than either of the thiosquaramide-bearing mononucleosides 31 and 37. This was likely due to the conformational rigidity of the thiosquaramide linkage of dinucleoside 39, restricting the molecule to a less favourable binding conformation, different to that of the natural DNA substrate. Inclusion of a squaramide internucleotide linkage in oligonucleotides has previously been found to cause distortion of normal duplex structure.21
Following their identification as candidate SNM1A inhibitors, compounds 8, 9, 26, and 31 were tested individually at concentrations ranging from 1 mM to 3 µM (Fig. 3). This showed that thymidine N-hydroxysquaramide 8 is a slightly stronger inhibitor, showing inhibition at 333 µM, (Fig. 3A) than its uridine analogue 26, which only shows significant inhibition at 1 mM (Fig. 3C). When tested simultaneously in the same assay (Fig. 2B), thymidine derivative 9 bearing a squaric acid moiety appears to show slightly more inhibition of SNM1A at 1 mM than thymidine derivative 8 bearing an N-hydroxysquaramide, although it is difficult to confirm this difference in inhibitory effect based on individual testing of compounds 8 and 9 (Fig. 3A and 3B) as both show a degree of inhibition at 333 µM. N-Hydroxysquaramides such as 8 and 26 can chelate to zinc through formation of a 6-membered ring, while chelation of squaric acid 9 to zinc through formation of a 5-membered ring is considered less favourable due to the larger bite angle.35 However, these results indicate that increased electrostatic interaction with zinc due to the negative charge of compound 9 in its deprotonated form may be a more important factor in determining inhibitory potency. Thymidine derivative 31 bearing a thiosquaramide at the 5′-position appears to be the most potent of all the SNM1A inhibitors tested, showing inhibition at a concentration of 100 µM (Fig. 3D).
Fig. 3.
Evaluation of SNM1A inhibitors at a range of concentrations from 1 mM to 3 µM through visualisation of the extent of digestion of a fluorescent oligonucleotide substrate by denaturing PAGE. SNM1A (2.5 nM) was pre-incubated with the modified nucleosides for 5 min before the 21-mer oligonucleotide substrate (125 nM) was added and a further 60 min incubation. nt = nucleotides. A) Evaluation of thymidine 3′-N-hydroxysquaramide 8. B) Evaluation of thymidine 3′-squaric acid 9. C) Evaluation of uridine 3′-N-hydroxysquaramide 26. D) Evaluation of thymidine 5′-thiosquaramide 31.
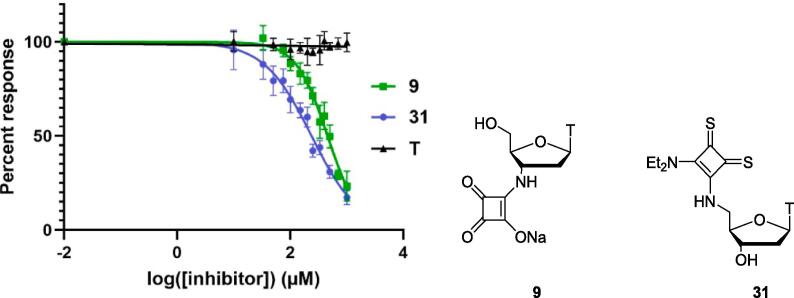
Following identification of the most promising compounds 9 and 31, a real-time fluorescence assay was carried out,12, 14 to determine the IC50 values for inhibition of SNM1A (Fig. 4). Unmodified thymidine was also tested in this assay as a control, showing no inhibition of SNM1A. Thymidine derivative 31 bearing a 5′-thiosquaramide again proved to be the most potent inhibitor, with an IC50 of 238 µM. The IC50 of thymidine derivative 9 with a squaric acid moiety at the 3′-position was found to be 456 µM. Thymidine derivative 8 bearing a 3′-N-hydroxysquaramide and thymidine derivative 37 bearing a 3′-thiosquaramide were also tested in this assay for comparison with squaric acid 9 and 5′-thiosquaramide 31, to confirm the trends in inhibitory potency seen in the gel electrophoresis assays. Some inhibition of SNM1A by N-hydroxysquaramide 8, and to a lesser extent by thiosquaramide 37 was observed (Fig. S1), however the IC50 values for these compounds could not be accurately determined as they appeared to be above the concentration range tested. These results confirm the trend observed in the gel electrophoresis assays; 5′-thiosquaramide 31 is a more potent inhibitor than 3′-thiosquaramide 37, and thymidine derivative 9 with a squaric acid moiety at the 3′-position is a more potent inhibitor than thymidine derivative 8 bearing an N-hydroxysquaramide at the 3′-position. 5′-Thiosquaramide 31 is the most effective of all the inhibitors tested.
Fig. 4.
IC50 determination of modified nucleosides 9 and 31 in a real-time fluorescence assay. Thymidine (T) is included as a control. Error bars were generated from 6 independent repeats.
To investigate whether SNM1A inhibition occurs through coordination of active site zinc ions by the squaramide moieties, and to rationalise the relative inhibitory potency of compounds 8, 9 and 31, solutions of these compounds in MeCN were used in UV–Vis titrations with Zn(ClO4)2 (Fig. S2). The titration data were analysed by global non-linear regression using the ReactLab Equilibria software to elucidate binding modes. The experimental binding constants obtained are summarised in Table 1. For thymidine derivative 8 bearing a 3′-N-hydroxysquaramide, the best fit to the data was obtained using a two-species model in which 8 binds to a metal ion in both a 1:1 and 1:2 ligand-metal ratio. The equilibrium constant calculated for formation of the 1:1 species was 3.0 × 104 M−1, and the equilibrium constant for formation of the 1:2 species was significantly lower at 6.6 × 101 M−1. Thymidine derivative 9 bearing a squaric acid at the 3′-position exhibited stronger binding to zinc, with an equilibrium constant of 4.0 × 104 M−1 for 1:1 ligand-metal binding. The data indicated squaric acid 9 could participate 2:1 ligand-metal binding in MeCN solution with an equilibrium constant of 3.2 × 106 M−1, although a 2:1 ligand-metal binding mode is unlikely to be accommodated within the SNM1A active site. The strongest zinc binding was observed when Zn(ClO4)2 was titrated against thymidine derivative 31 bearing a thiosquaramide at the 5′-position. The data from this titration were best fit by a three-species model in which ligand 31 binds to metal ions in a 1:1, 2:1, and 1:2 ratio. The equilibrium constant calculated for 1:1 ligand-metal binding was 6.8 × 105 M−1. For 2:1 ligand-metal binding the calculated equilibrium constant was 1.2 × 106 M−1, and 1:2 ligand-metal binding was less significant with an equilibrium constant of 1.7 × 102 M−1. Although these titrations should be considered as qualitative, given that MeCN solution is not a close representation of the SNM1A active site environment, the order of 1:1 binding strength to zinc ions observed, thiosquaramide 31 > squaric acid 9 > N-hydroxysquaramide 8, is the same as the trend in the potency of SNM1A inhibition observed in both the gel electrophoresis and real-time fluorescence assays. This supports the hypothesis that the compounds inhibit SNM1A through binding to the active site zinc ion(s).
Table 1.
Equilibrium constants for binding of squaramides to Zn2+ calculated from UV–Vis titration data.
| Ligand-metal binding constants |
|||
|---|---|---|---|
| Ligand | K1:1 | K1:2 | K2:1 |
| 8 | 3.0 × 104 M−1 | 6.6 × 101 M−1 | – |
| 9 | 4.0 × 104 M−1 | – | 3.2 × 106 M−1 |
| 31 | 6.8 × 105 M−1 | 1.7 × 102 M−1 | 1.2 × 106 M−1 |
To evaluate the potential of the SNM1A inhibitors for future use in in vivo applications, a parallel artificial membrane permeability assay (PAMPA)36 was carried out to examine the membrane permeability of the biologically active compounds 8, 9, 26, 31, 37 and 39 (Fig. S3). Thymidine derivative 8 bearing 3′-N-hydroxysquaramide, and thymidine derivative 9 bearing a squaric acid at the 3′-position were found to be membrane permeable, with logPe values of −4.6 ± 0.4 and −4.1 ± 0.6 respectively. Unlike its thymidine analogue 8, uridine derivative 26 bearing a 3′-N-hydroxysquaramide was found to be impermeable, with a logPe of −7.0 ± 0.1. Of the thiosquaramides, 5′-thiosquaramide 31 was found to have a low degree of membrane permeability, with a logPe value of −6.5 ± 0.6. 3′-Thiosquaramide 37 and bridging thiosquaramide 39 showed no membrane permeability, with logPe values of −7.01 ± 0.04 and −7.084 ± 0.005 respectively. The three most potent inhibitors, 8, 9, and 31, all show some degree of membrane permeability, demonstrating their potential for future biological applications.
3. Conclusion
In summary, we have synthesised a range of nucleoside derivatives bearing squaramide and thiosquaramide modifications. These compounds were tested as inhibitors of SNM1A in gel electrophoresis and real-time fluorescence assays. Their physical properties were also examined in a PAMPA assay to determine membrane permeability, and in UV–Vis titrations to investigate their interaction with zinc ions. Although compounds bearing a squaramide group at the 5′-position proved ineffective, nucleoside derivatives 8, 9, and 26 bearing a squaramide moiety at the 3′-position demonstrated inhibition of SNM1A. Gel electrophoresis assays showed that thymidine derivative 8 bearing an N-hydroxysquaramide at the 3′-position is a more effective inhibitor of SNM1A than its uridine analogue 26. This is consistent with the enzyme’s preference for DNA substrates over RNA. Compound 9 bearing a squaric acid moiety at the 3′-position was a more effective inhibitor than either of the N-hydroxysquaramides 8 and 26. UV–Vis titrations showed that this could be attributed to a stronger interaction of squaric acid 9 with zinc ions compared to N-hydroxysquaramide 8. Interestingly, thymidine derivative 31 bearing a thiosquaramide at the 5′-position proved to be a more effective inhibitor of SNM1A than compound 37 in which the thiosquaramide is placed at the 3′-position, contrary to the trend observed for the oxo squaramides. We hypothesise that this is because when the more sterically bulky thiosquaramide moiety is placed at the 3′-position, it causes the nucleobase and the deoxyribose ring to be displaced from their ideal binding position in the SNM1A active site, resulting in steric clashes with amino acid residues. Although the testing of the oxo squaramides suggests that the ideal placement of a less bulky ZBG appears to be at the 3′-position, 5′-thiosquaramide 31 is nonetheless the most potent of any of the inhibitors tested, with an IC50 value of 238 µM. UV–Vis titrations with Zn(ClO4)2 indicate that this is due to a much stronger interaction of the thiosquaramide with zinc ions compared to that observed for oxo squaramides. Finally, an assay was carried out to determine the membrane permeability of the SNM1A inhibitors to evaluate their potential for future in vivo applications. Squaramides 8 and 9 showed a high degree of membrane permeability, while the most potent inhibitor thiosquaramide 31 was permeable to a lesser degree. Taken together, the results of this study demonstrate the potential of squaramide and thiosquaramide moieties for targeting zinc-dependent enzymes in biological applications and provide insights useful for the further development of substrate-mimic inhibitors of SNM1A.
Funding
This work was supported by the Irish Research Council (GOIPG/2017/1453) and the Wellcome Trust Trinity College Dublin (TCD) institutional strategic support fund.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmc.2021.116369.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chu G. Cellular responses to cisplatin: the roles of DNA-binding proteins and DNA repair. J Biol Chem. 1994;269:787–790. [PubMed] [Google Scholar]
- 2.Bowden N.A. Nucleotide excision repair: why is it not used to predict response to platinum-based chemotherapy? Cancer Lett. 2014;346:163–171. doi: 10.1016/j.canlet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Helleday T., Petermann E., Lundin C., Hodgson B., Sharma R.A. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 4.Spanswick V.J., et al. Repair of DNA interstrand crosslinks as a mechanism of clinical resistance to melphalan in multiple myeloma. Blood. 2002;100:224–229. doi: 10.1182/blood.v100.1.224. [DOI] [PubMed] [Google Scholar]
- 5.Helleday T. Putting poly (ADP-ribose) polymerase and other DNA repair inhibitors into clinical practice. Curr Opin Oncol. 2013;25:609–614. doi: 10.1097/CCO.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 6.Sabharwal A., Middleton M.R. Exploiting the role of O6-methylguanine-DNA-methyltransferase (MGMT) in cancer therapy. Curr Opin Pharmacol. 2006;6:355–363. doi: 10.1016/j.coph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Madhusudan S., Hickson I.D. DNA repair inhibition: a selective tumour targeting strategy. Trends Mol Med. 2005;11:503–511. doi: 10.1016/j.molmed.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Sengerová B., et al. Characterization of the human SNM1A and SNM1B/Apollo DNA repair exonucleases. J Biol Chem. 2012;287:26254–26267. doi: 10.1074/jbc.M112.367243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hejna J., Philip S., Ott J., Faulkner C., Moses R. The hSNM1 protein is a DNA 5’-exonuclease. Nucleic Acids Res. 2007;35:6115–6123. doi: 10.1093/nar/gkm530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang A.T., et al. Human SNM1A and XPF-ERCC1 collaborate to initiate DNA interstrand cross-link repair. Genes Dev. 2011;25:1859–1870. doi: 10.1101/gad.15699211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allerston C.K., et al. The structures of the SNM1A and SNM1B/Apollo nuclease domains reveal a potential basis for their distinct DNA processing activities. Nucleic Acids Res. 2015;43:11047–11060. doi: 10.1093/nar/gkv1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.Y., et al. Cephalosporins inhibit human metallo β-lactamase fold DNA repair nucleases SNM1A and SNM1B/apollo. Chem Commun. 2016;52:6727–6730. doi: 10.1039/c6cc00529b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzon B., et al. Identification of Bioactive SNM1A Inhibitors. ACS Omega. 2021;6:9352–9361. doi: 10.1021/acsomega.0c03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty W., et al. A hydroxamic-acid-containing nucleoside inhibits DNA repair nuclease SNM1A. Org Biomol Chem. 2019;17:8094–8105. doi: 10.1039/c9ob01133a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dürr E.-M., McGouran J.F. Probing the binding requirements of modified nucleosides with the DNA nuclease SNM1A. Molecules. 2021;26:320. doi: 10.3390/molecules26020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quiñonero D., et al. Squaramide as a binding unit in molecular recognition. Chem Phys Lett. 2000;326:247–254. [Google Scholar]
- 17.Onaran M.B., Comeau A.B., Seto C.T. Squaric acid-based peptidic inhibitors of matrix metalloprotease-1. J Org Chem. 2005;70:10792–10802. doi: 10.1021/jo0517848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charton J., Déprez B.P., Déprez-Poulain R.F. Synthesis of a 200-member library of squaric acid N-hydroxylamide amides. Bioorganic Med Chem Lett. 2008;18:4968–4971. doi: 10.1016/j.bmcl.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Dürr E.-M., et al. Squaramide-based 5’-phosphate replacements bind to the DNA repair exonuclease SNM1A. ChemistrySelect. 2018;3:12824–12829. doi: 10.1002/slct.201803375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha A., Panda S., Paul S., Manna D. Phosphate bioisostere containing amphiphiles: a novel class of squaramide-based lipids. Chem Commun. 2016;52:9438–9441. doi: 10.1039/c6cc04089f. [DOI] [PubMed] [Google Scholar]
- 21.Sato K., Seio K., Sekine M. Squaryl group as a new mimic of phosphate group in modified oligodeoxynucleotides: synthesis and properties of new oligodeoxynucleotide analogues containing an internucleotidic squaryldiamide linkage. J Am Chem Soc. 2002;124:12715–12724. doi: 10.1021/ja027131f. [DOI] [PubMed] [Google Scholar]
- 22.Busschaert N., et al. Thiosquaramides: pH switchable anion transporters. Chem Sci. 2014;5:3617–3626. doi: 10.1039/C4SC01629G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy S., et al. New enantiopure binaphthyl-cinchona thiosquaramides: synthesis and application for enantioselective organocatalysis. New J Chem. 2019;43:5948–5959. [Google Scholar]
- 24.Rombola M., Sumaria C.S., Montgomery T.D., Rawal V.H. Development of chiral, bifunctional thiosquaramides: enantioselective Michael additions of barbituric acids to nitroalkenes. J Am Chem Soc. 2017;139:5297–5300. doi: 10.1021/jacs.7b01115. [DOI] [PubMed] [Google Scholar]
- 25.Seio K., Miyashita T., Sato K., Sekine M. Synthesis and properties of new nucleotide analogues possessing squaramide moieties as new phosphate isosters. European J Org Chem. 2005;2005:5163–5170. [Google Scholar]
- 26.Bege M., et al. A low-temperature, photoinduced thiol–ene click reaction: a mild and efficient method for the synthesis of sugar-modified nucleosides. Org Biomol Chem. 2017;15:9226–9233. doi: 10.1039/c7ob02184d. [DOI] [PubMed] [Google Scholar]
- 27.Kojima N., Szabo I.E., Bruice T.C. Synthesis of ribonucleic guanidine: replacement of the negative phosphodiester linkages of RNA with positive guanidinium linkages. Tetrahedron. 2002;58:867–879. [Google Scholar]
- 28.Ogawa A., Tanaka M., Sasaki T., Matsuda A. Nucleosides and Nucleotides. 180. Synthesis and antitumor activity of nucleosides that have a hydroxylamino group instead of a hydroxyl group at the 2’- or 3’-position of the sugar moiety. J Med Chem. 1998;41:5094–5107. doi: 10.1021/jm980466g. [DOI] [PubMed] [Google Scholar]
- 29.Robins M.J., Samano V., Johnson M.D. Periodinane oxidation, selective primary deprotection, and remarkably stereoselective reduction of tert-butyldimethylsilyl-protected ribonucleosides. Synthesis of 9-(β-D-xylofuranosyl)adenine or 3’-deuterioadenosine from adenosine. J Org Chem. 1990;55:410–412. [Google Scholar]
- 30.Rombola M., Rawal V.H. Dicyclopentyl dithiosquarate as an intermediate for the synthesis of thiosquaramides. Org Lett. 2018;20:514–517. doi: 10.1021/acs.orglett.7b03549. [DOI] [PubMed] [Google Scholar]
- 31.Gogoi K., Gunjal A.D., Phalgune U.D., Kumar V.A. Synthesis and RNA binding selectivity of oligonucleotides modified with five-atom thioacetamido nucleic acid backbone structures. Org Lett. 2007;9:2697–2700. doi: 10.1021/ol070990u. [DOI] [PubMed] [Google Scholar]
- 32.Chen J.-K., Schultz R.G., Lioyd D.H., Gryaznov S.M. Synthesis of oligodeoxyribonucleotide N3′→P5′ phosphoramidates. Nucleic Acids Res. 1995;23:2661–2668. doi: 10.1093/nar/23.14.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coyle J.D. The photochemistry of thiocarbonyl compounds. Tetrahedron. 1985;41:5393–5425. [Google Scholar]
- 34.Maciejewski A., Steer R.P. The photophysics, physical photochemistry, and related spectroscopy of thiocarbonyls. Chem Rev. 1993;93:67–98. [Google Scholar]
- 35.Solans X., et al. Coordination modes of the squarate ligand: syntheses and crystal structures of six copper(II) squarate complexes. Inorg Chem. 1990;29:775–784. [Google Scholar]
- 36.Schmidt, D. & Lynch, J. Millipore Corporation Application Note. Lit. No. AN1728EN00 (2003).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.