Supplemental Digital Content is available in the text.
Keywords: COVID-19, hospital mortality, incidence, intensive care units, patient discharge, pulmonary embolism
Background and Purpose:
The frequency of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) varies in the current literature, and risk factors are unknown. We assessed the incidence, risk factors, and outcomes of acute ischemic stroke in hospitalized patients with COVID-19.
Methods:
We included patients with a laboratory-confirmed SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) infection admitted in 16 Dutch hospitals participating in the international CAPACITY-COVID registry between March 1 and August 1, 2020. Patients were screened for the occurrence of acute ischemic stroke. We calculated the cumulative incidence of ischemic stroke and compared risk factors, cardiovascular complications, and in-hospital mortality in patients with and without ischemic stroke.
Results:
We included 2147 patients with COVID-19, of whom 586 (27.3%) needed treatment at an intensive care unit. Thirty-eight patients (1.8%) had an ischemic stroke. Patients with stroke were older but did not differ in sex or cardiovascular risk factors. Median time between the onset of COVID-19 symptoms and diagnosis of stroke was 2 weeks. The incidence of ischemic stroke was higher among patients who were treated at an intensive care unit (16/586; 2.7% versus nonintensive care unit, 22/1561; 1.4%; P=0.039). Pulmonary embolism was more common in patients with (8/38; 21.1%) than in those without stroke (160/2109; 7.6%; adjusted risk ratio, 2.08 [95% CI, 1.52–2.84]). Twenty-seven patients with ischemic stroke (71.1%) died during admission or were functionally dependent at discharge. Patients with ischemic stroke were at a higher risk of in-hospital mortality (adjusted risk ratio, 1.56 [95% CI, 1.13–2.15]) than patients without stroke.
Conclusions:
In this multicenter cohort study, the cumulative incidence of acute ischemic stroke in hospitalized patients with COVID-19 was ≈2%, with a higher risk in patients treated at an intensive care unit. The majority of stroke patients had a poor outcome. The association between ischemic stroke and pulmonary embolism warrants further investigation.
See related article, p 3987
Coronavirus disease 2019 (COVID-19) has affected millions of people worldwide. The clinical course of COVID-19 may be complicated by venous and arterial thromboembolic events.1,2 Pulmonary embolism (PE) accounts for the majority of these events, but other cardiovascular complications, including ischemic stroke, have also been reported. In contrast to early reports suggesting an increased risk of ischemic stroke among patients hospitalized with COVID-19, results from later reports are less consistent.3–17 The occurrence of ischemic stroke varied, ranging from 0.01% to 6.9%. This may be explained, in part, by differences in study design, sample size, case-findings methods, and settings. Studies that reported clinical details have suggested an increased severity of stroke symptoms, more cryptogenic strokes, and a worse outcome,3,4,18 including higher in-hospital mortality rates,3–5 in patients with COVID-19 than in those without. Nevertheless, large cohort studies reporting data on stroke details are limited, as ischemic strokes were often not assessed by neurologists. In addition, little data are available on the relationship between ischemic stroke and other cardiovascular complications in patients with COVID-19. To improve our understanding of the relationship between COVID-19 and ischemic stroke, we assessed risk factors, time course, hospital setting, the relationship with other cardiovascular complications, stroke severity, and outcomes of ischemic stroke in patients hospitalized with COVID-19 during the first wave of the pandemic across 16 centers in the Netherlands.
Methods
Study Design
This study was conducted within the CAPACITY-COVID international patient registry (www.capacity-covid.eu; https://www.clinicaltrials.gov; unique identifier: NCT04325412). Details regarding CAPACITY-COVID have been outlined elsewhere.19 In short, the case report form of the International Severe Acute Respiratory and Emerging Infection Consortium was extended within CAPACITY-COVID to collect in-depth information on cardiovascular history, medication, and cardiac and thromboembolic events in patients hospitalized with COVID-19. STROCORONA (Stroke in Corona Patients) was incorporated as a substudy within CAPACITY-COVID, to obtain additional information on neurovascular history and the occurrence of ischemic stroke during hospitalization, including data on vascular risk factors, etiology, severity, and outcome. Sixteen Dutch hospitals participated in STROCORONA. Ethical approval was obtained in all participating hospitals, and the necessity of a consent procedure was determined to conform local regulations. The majority of participating sites had an opt-out approach.20 The data of this study can be made available upon reasonable request to the data access committee of CAPACITY-COVID.
Study Population and Data Collection
We included adult patients with a laboratory-confirmed SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) infection (determined by a positive polymerase chain reaction test result from a nasopharyngeal swab) who were admitted to a hospital during the first wave of the pandemic in the Netherlands (March 1 to August 1, 2020). Patients who were strongly suspected of COVID-19 were retested. If their tests remained negative, they were excluded from the current study. We retrieved data on demographics, comorbidities, prehospital medication, the need of mechanical ventilation, treatment at a high-dependency or intensive care unit (ICU) during admission, in-hospital mortality, and the occurrence of cardiac or thromboembolic complications: deep vein thrombosis, PE, acute coronary syndrome, endocarditis, and new-onset atrial fibrillation. Outcome definitions of cardiac and thromboembolic complications have been reported previously.20 For STROCORONA, patient files of all cases were systematically screened and scored by neurologists or other physicians with experience in stroke research per hospital to identify ischemic stroke during hospitalization. In addition, data on prior transient ischemic attack, ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, or vascular dementia were collected. Ischemic stroke was defined as a sudden onset of focal neurological signs originating from the brain or retina that persisted for >24 hours or until death, confirmed with neuroimaging demonstrating either infarction in the corresponding vascular territory or absence of another apparent cause.21 We recorded whether patients had been examined by or under supervision of a neurologist. We graded stroke severity at the time of diagnosis with the National Institutes of Health Stroke Scale and collected data on acute stroke treatment (intravenous thrombolysis, endovascular treatment, and antithrombotic treatment), timing (median time between onset of COVID-19 symptoms and stroke diagnosis), and imaging findings (vascular territory, intracranial large vessel occlusion). We classified stroke etiology with the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria and scored stroke outcome at discharge with the modified Rankin Scale.22
Statistical Analysis
Baseline characteristics are summarized with descriptive statistics as median (interquartile range [IQR]), mean (SD), or frequencies (proportions) where appropriate. We performed a quality check of the data set and recoded entry errors as missing data. We did not impute missing values (Table I in the Data Supplement). We calculated the cumulative incidence of ischemic stroke with corresponding 95% CIs and stratified results according to age and sex. Since 3 participating hospitals only included patients with cardiovascular risk factors or patients for whom a cardiologist was consulted during admission, a sensitivity analysis excluding these three centers was performed. We compared the occurrence of other cardiovascular complications and in-hospital mortality between patients with and without ischemic stroke with χ2 or Student t tests as appropriate and calculated risk ratios with Poisson regression.23 We adjusted risk ratios for age, sex, and treatment on an ICU. For stroke outcome, we calculated the proportion of ischemic stroke patients with an unfavorable outcome (death or dependency [modified Rankin Scale score of ≥3]) at discharge. We report our findings in accordance with the RECORD guidelines (Reporting of Studies Conducted Using Observational Routinely-Collected Data; Table II in the Data Supplement).
Results
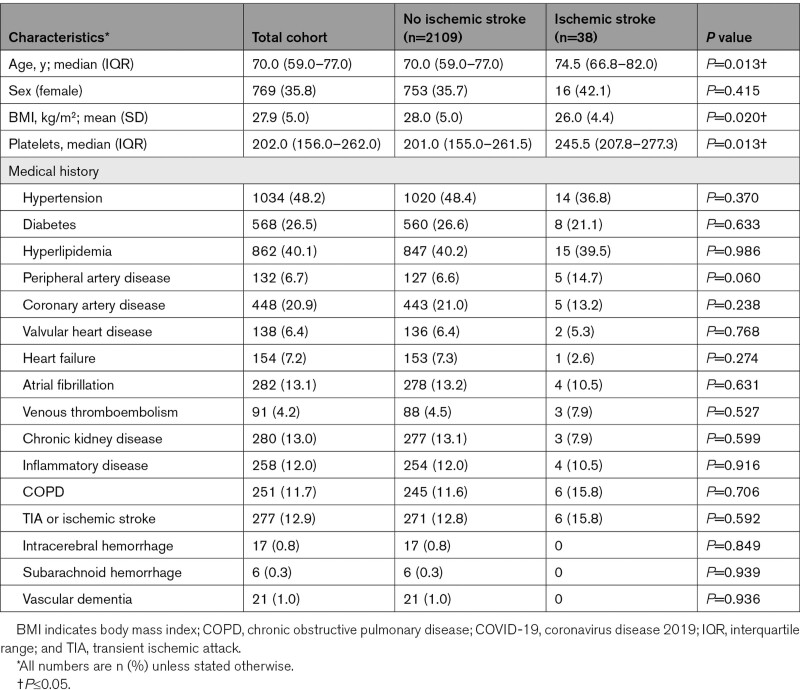
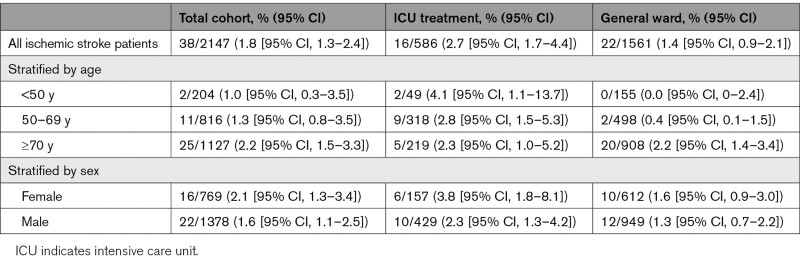
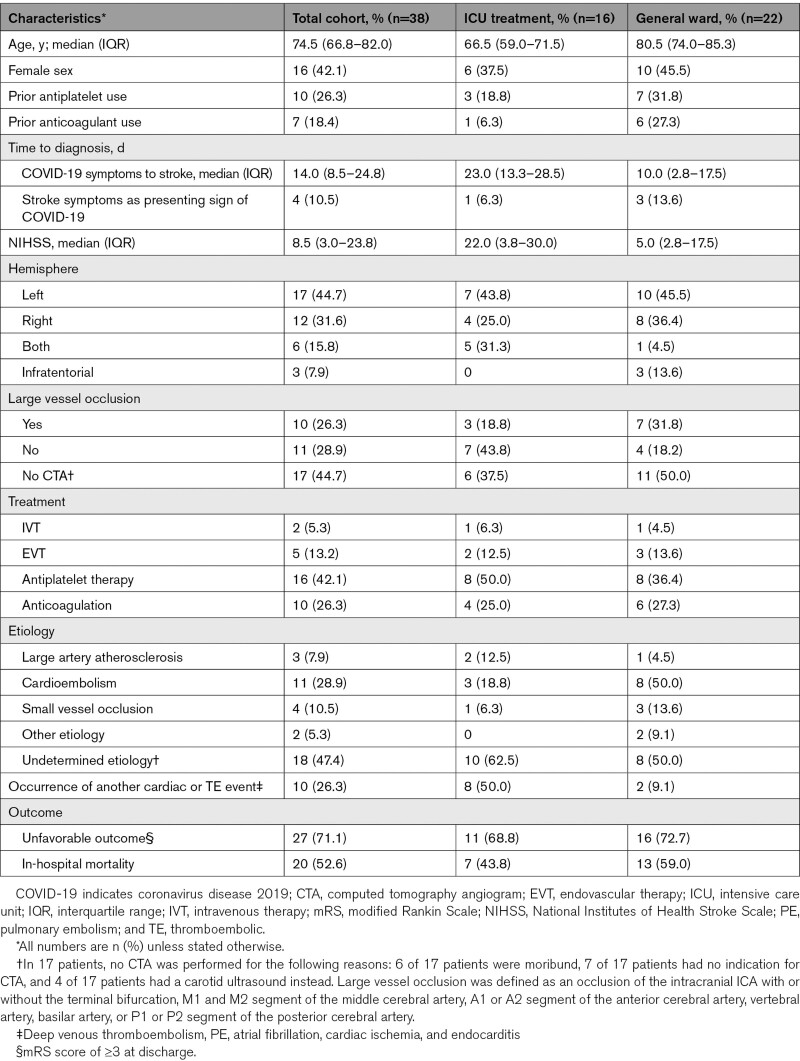
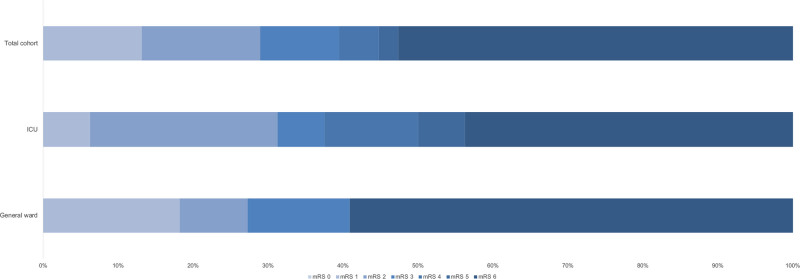
We included a total of 2147 patients in STROCORONA (Figure I in the Data Supplement). Table 1 shows the baseline characteristics. The median age was 70.0 years (IQR, 59.0–77.0); about one-third of the patients were female (769; 35.8%), and cardiovascular comorbidities were common. Of all patients, 586 (27.3%) received treatment at an ICU. In general, patients treated at an ICU were younger and had fewer comorbidities than patients treated on a general ward only (Table III in the Data Supplement). Ischemic stroke occurred in 38 of 2147 (1.8% [95% CI, 1.3%–2.4%]) patients (Table 2). All ischemic strokes were diagnosed by a neurologist. These patients were older than patients without ischemic stroke, had a lower BMI, and had higher platelet counts at baseline but did not differ in terms of sex, cardiovascular comorbidities, and prehospital medication (Table 1; Table IV in the Data Supplement). After stratification by age, no differences in cardiovascular risk factors between patients with and without ischemic stroke were found (Table V in the Data Supplement). In a sensitivity analysis excluding 3 hospitals that excluded patients without cardiovascular risk factors or cardiologist consultation, baseline characteristics and cumulative stroke incidence were similar (Table VI in the Data Supplement). The median time between the onset of COVID-19 symptoms and stroke diagnosis was 14 days (IQR, 9–25 days) for all patients, 23 days (IQR, 13–29) for patients who received ICU treatment, and 10 days (IQR, 3–18) for patients treated on a general ward only (P=0.031; Figure 1; Table 3). The cumulative incidence of ischemic stroke was 2.7% in patients who were treated at an ICU (16/586 [95% CI, 1.7%–4.4%]) and 1.4% in patients who only received treatment on a general ward (22/1561 [95% CI, 0.9%–2.1%]; P=0.039). Age- and sex-stratified cumulative incidence is given in Table 2 and details about stroke severity, subtype, imaging, treatment, and outcome in Table 3. Stroke patients treated at an ICU were younger than those treated at a general ward only (ICU, 63.4 years [SD, 15.2]; general ward, 79.2 [SD, 8.1]; P<0.001), frequently had other thromboembolic events (ICU, 8/16 [50%]; general ward, 2/22 [9.1%]; P=0.020), and had more severe strokes (ICU: median National Institutes of Health Stroke Scale score, 22.0; IQR, 3.8–30.0; general ward, 5.0; IQR, 2.8–17.5; P=0.050; Table 3). Eighteen patients (47.4%) had a stroke of undetermined etiology; however, in 6 (33.3%), the diagnostic workup was incomplete because they were moribund. Differences between patients with and without cryptogenic stroke are summarized in Table VII in the Data Supplement and an overview of the available laboratory, imaging, telemetry, and other investigations in each patient is provided in Table VIII in the Data Supplement. The occurrence of other cardiovascular complications in patients with and without ischemic stroke is given in Table IX in the Data Supplement. PE was more common in patients with ischemic stroke (8/38 [21.1%] versus 160/2109 [7.6%]; P=0.002), also after adjustment for age, sex, and treatment on an ICU (adjusted risk ratio, 2.08 [95% CI, 1.52–2.84]). Patients with PE and ischemic stroke had higher median platelet counts at baseline (285×109/L; IQR, 223–556) than patients with PE without ischemic stroke (230×109/L; IQR, 180–306; P=0.026). The median time between onset of COVID-19 symptoms and PE diagnosis was 18 days (IQR, 12–25 days) for all patients, 19 days (IQR, 12–26) for patients who received ICU treatment, and 14 days (IQR, 8–21) for patients treated on a general ward only (P=0.04). In 5 of 8 (62.5%) patients with PE and ischemic stroke, PE was diagnosed before ischemic stroke. Three-quarters of the patients with ischemic stroke (27/38 [71.1%]) had a modified Rankin Scale score of ≥3 at discharge (Figure 2). Patients with ischemic stroke were at a higher risk of in-hospital mortality (adjusted risk ratio, 1.56 [95% CI, 1.13–2.15]) than patients without ischemic stroke. Age- and sex-stratified cumulative in-hospital mortality is shown in Table X in the Data Supplement. A timeline of admissions and in-hospital mortality during the first wave is given for the participating centers in the Netherlands in Figure II in the Data Supplement.
Table 1.
Baseline Characteristics of Hospitalized Patients With COVID-19, Stratified by Diagnosis of Ischemic Stroke
Table 2.
Cumulative Incidence of Ischemic Stroke in Patients With and Without Treatment at an ICU, Stratified by Age and Sex
Figure 1.
Median time between the onset of coronavirus disease 2019 (COVID-19) symptoms and diagnosis of ischemic stroke in patients treated at an intensive care unit (ICU) or on a general ward.
Table 3.
Characteristics of Ischemic Stroke in Patients With COVID-19 Treated at an ICU or on a General Ward
Figure 2.
Outcome of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) assessed with the modified Rankin Scale (mRS) at discharge in patients with and without treatment at an intensive care unit (ICU).
Discussion
In this Dutch multicenter study, the overall cumulative incidence of ischemic stroke was 1.8% in patients hospitalized with COVID-19, with a higher rate of ischemic stroke in patients who needed treatment at an ICU (2.7%). Patients with ischemic stroke were older but did not have more cardiovascular risk factors when compared with patients without ischemic stroke. In addition, patients with ischemic stroke were twice as likely to have PE and were at higher risk of in-hospital mortality.
The overall incidence of ischemic stroke of 1.8% in hospitalized patients with COVID-19 is in line with previous hospital-based COVID-19 cohorts, which reported cumulative incidences ranging between 1.0% and 2.4%.3–5 Lower stroke rates have been found in studies that reported on a combination of hospitalized and nonhospitalized patients.6,24 Higher rates of up to 6.9% have been reported in ICU populations or other selective populations.5,25,26 In addition, the variation in ischemic stroke incidence may also be explained by other factors. First, most studies were performed in Asia and North America, with only a few European cohorts.3–5,7 Geographic variation may explain some of the heterogeneity, with a higher incidence reported in Asia.3 Second, regional differences in COVID-19 surges may have resulted in a higher threshold for seeking medical attention in pandemic areas, especially for patients with mild stroke symptoms. Third, in most studies, ischemic stroke was recorded as one of the various cardiovascular events, with case ascertainment often not performed by neurologists or stroke physicians.6–8,27 This may have resulted in a systematic bias in the estimation of the cumulative stroke incidence among hospitalized patients with COVID-19 in these studies.
In contrast to some of the previous cohorts,25,28 our findings suggest that patients with COVID-19 and ischemic stroke did not have more cardiovascular risk factors than patients without a stroke. One explanation for this discrepancy may be that older patients with more vascular risk factors may not have been hospitalized or admitted to an ICU, because of treatment restrictions or patient preferences, which may have led to reduced survival rates in this group.29 In addition, the greater severity of COVID-19 illness among hospitalized patients, especially those treated at an ICU, as well as the increased risk of medical complications during hospitalization, may, at least partially, have contributed to stroke risk in hospitalized COVID-19 patients without vascular risk factors.30 To our knowledge, this is the first study to report on an association between PE and ischemic stroke in hospitalized patients with COVID-19.
Acute respiratory infections in general can act as a trigger for the short-term risk of ischemic stroke and myocardial infarction and are associated with a high risk of cardiovascular-related death.31 Two recent studies have compared the occurrence of ischemic stroke in hospitalized patients with COVID-19 versus those with influenza. One study found that patients with COVID-19 appeared to have an increased stroke risk (COVID-19, 1.6%; influenza, 0.2%), whereas the other study found the risk of ischemic stroke to be similar in patients with COVID-19 (1.2%) and influenza (1.2%).9,28 In SARS-CoV and Middle-East Respiratory Syndrome, the occurrence of ischemic stroke has only been reported sporadically.32 Pathophysiological mechanisms that could link COVID-19 to thromboembolic events include direct viral-induced endotheliitis, postinfectious immune-mediated responses, prothrombotic coagulopathy, and the occurrence of a hyperinflammatory state, with elevated d-dimer levels and antiphospholipid antibodies frequently found in patients with COVID-19 and thromboembolic complications.2 Platelet counts varied across studies, but severe COVID-19 was often associated with thrombocytopenia.2,25 Several studies have found that patients with COVID-19 who had ischemic stroke were more likely to die.3–5 It remains unclear whether this association with an increased mortality is driven by disease severity and the prothrombotic state triggered by COVID-19. Other confounding factors, such as impeded functional recovery due to fever and infection and withdrawal of care in patients with COVID-19 and ischemic stroke, may also play a role.33,34
Our study has limitations. First, different forms of bias should be considered in observational research. Hospitalized patients with COVID-19, and in particular, those requiring treatment at an ICU, represent a selected group. Numerous factors may have influenced whether patients sought emergency care, were admitted to a hospital, and received intensive treatment. Some patients with COVID-19 and ischemic stroke may have died before reaching the hospital, and milder affected patients or those with treatment restrictions may have stayed at home.35 This may have underestimated the overall rate of ischemic stroke in patients hospitalized with COVID-19. In addition, we used data from a registry primarily set up to detect cardiac and thromboembolic complications in patients with COVID-19. To assure complete and systematic case ascertainment for ischemic stroke, medical records of all eligible patients were revisited by neurologists or other physicians with experience in stroke research. The high caseload of COVID-19 patients in some hospitals, in combination with contagion containment and sedation on an ICU, may have impeded imaging investigations to diagnose ischemic strokes, especially among moribund patients. This may have resulted in an overestimation of the percentage of strokes with undetermined etiology. Among patients with PE and ischemic stroke, the diagnostic workup to rule out a patent foramen ovale was often not performed. In contrast, the relatively large proportion of patients with a cardioembolic etiology may reflect the accessibility of telemetry. In addition, laboratory findings should be interpreted with caution, as these were recorded in different stages of the disease and d-dimers were only selectively tested. Furthermore, as ischemic stroke was the primary outcome of this study, we did not report data on other neurological complications, such as intracerebral hemorrhage and cerebral venous thrombosis.2 Finally, we only included patients with COVID-19 admitted during the first wave of the pandemic and were unable to adjust for changes in management and treatment strategies that occurred over time. This may hamper the generalizability of our results to later phases of the pandemic. A recent comparison between the second and first waves in the Netherlands has shown a decline in in-hospital mortality rates of patients with COVID-19.7 Due to the novelty of this pandemic, comparisons with hospital populations from previous years and across different waves should, however, be interpreted with caution.7,36 The main strength of the CAPACITY-COVID consortium is that it is a multidisciplinary collaborative effort to systematically record thromboembolic complications in patients with COVID-19 in a longitudinal fashion. By incorporating STROCORONA, we were able to extend this large registry with cerebrovascular expertise and detailed ischemic stroke data and to link various cardiovascular complications in hospitalized patients with COVID-19.
Conclusions
In conclusion, the overall cumulative incidence of ischemic stroke in hospitalized patients with COVID-19 was ≈2%, with a higher risk in patients treated at an ICU. The finding that patients with COVID-19 and ischemic stroke were twice as likely to have PE than patients without stroke warrants further investigation. Our findings underscore the importance of appropriate antithrombotic strategies and increased awareness of stroke symptoms in hospitalized patients with COVID-19.
Article Information
Acknowledgments
We want to express our gratitude to all sites and researchers involved in the CAPACITY-COVID collaborative consortium. A list of all participating organizations is given in Table XI in the Data Supplement.
Sources of Funding
This work was supported by the Dutch Heart Foundation (2020B006 CAPACITY), Novartis Global, Novo Nordisk Nederland, Servier Nederland, and Daiichi Sankyo Nederland.
Disclosures
Dr Sluis is supported by the European Union’s Horizon 2020 Research and Innovation Programme (grant No. 634809). Dr Linschoten is supported by the Alexandre Suerman Stipend of the University Medical Center Utrecht. Dr Asselbergs is supported by the University College London Hospitals National Institute for Health Research Biomedical Research and CardioVasculair Onderzoek Nederland 2015-12 eDETECT. Dr Wermer is supported by a grant from the Dutch Heart Foundation (Dr Dekker grant 2016T086), a VIDI grant from ZonMw/NWO (91717337), and a grant for CORONIS from ZonMW and the Dutch Heart Foundation. She served as a consultant for Biogen without payment. Dr van der Worp reports a grant from ZonMW for CORONIS during the conduct of the study, grants from Stryker outside the submitted work, and served as a consultant to Bayer and LivaNova, with fees paid to his institution. He participates in a different observational study assessing the impact of coronavirus disease 2019 (COVID-19) on cerebral ischemic lesions. Dr Algra is supported by a grant from the Dutch Heart Foundation (Dr Dekker grant 2016T023). The other authors report no conflicts.
Supplemental Materials
Online Figures I and II
Online Tables I–XI
Supplementary Material
Appendix
Collaborators of the CAPACITY-COVID Consortium: Richard C.J.M. Donders, MD, PhD (Department of Neurology, Diakonessenhuis Hospital, Utrecht, the Netherlands); D. Martijn O. Pruissen, MD, PhD (Department of Neurology, Diakonessenhuis Hospital, Utrecht, the Netherlands); Aaf F.M. Kuijper, MD, PhD (Department of Cardiology, Spaarne Gasthuis, Haarlem, the Netherlands); Clara E.E. van Ofwegen- Hanekamp, MD, PhD (Department of Cardiology, Diakonessenhuis Utrecht, Utrecht, the Netherlands); Rik S. Hermanides, MD, PhD (Department of Cardiology, Isala Hospital, Zwolle, the Netherlands); Hortence E. Haerkens-Arends, MD (Department of Cardiology, Jeroen Bosch Hospital, ‘s-Hertogenbosch, the Netherlands); Rutger L. Anthonio, MD (Department of Cardiology, Treant Zorggroep, Emmen, the Netherlands); Mireille E. Emans, MD, PhD (Department of Cardiology, Ikazia Hospital, Rotterdam, the Netherlands); René A. Tio, MD, PhD (Department of Cardiology, Catharina Hospital, Eindhoven, the Netherlands; Department of Educational Development and Research in the Faculty of Health, Medicine and Life Sciences, Catharina Hospital, Eindhoven, the Netherlands); Jur M. ten Berg, MD, PhD (Department of Cardiology, St. Antonius Hospital, Nieuwegein, the Netherlands); Björn E. Groenemeijer, MD, PhD (Department of Cardiology, Gelre Hospital Apeldoorn, Apeldoorn, the Netherlands); Ron Pisters, MD, PhD (Department of Cardiology, Rijnstate Hospital, Arnhem, the Netherlands); P. Marc van der Zee, MD, PhD (Department of Cardiology, St. Jansdal Hospital, Harderwijk, the Netherlands); Hans-Marc J. Siebelink, MD, PhD (Department of Cardiology, Heart Lung Center, Leiden University Medical Center, Leiden, the Netherlands); Derk O. Verschure, MD, PhD (Department of Cardiology, Zaans Medical Center, Zaandam, the Netherlands); Matthijs F.L. Meijs, MD, PhD (Department of Cardiology, Medisch Spectrum Twente, Enschede, the Netherlands); Astrid Schut, MSc (The Dutch Network for Cardiovascular Research, Utrecht, the Netherlands); Robert G. Tieleman, MD, PhD (Department of Cardiology, Martini Hospital, Groningen, the Netherlands); Wanda Hermans-van Ast, PhD (Durrer Center, Netherlands Heart Institute, Utrecht, the Netherlands); Jeroen Schaap, MD, PhD (Department of Cardiology, Amphia Hospital, the Netherlands; The Dutch Network for Cardiovascular Research, Utrecht, the Netherlands); Lucia S. Jewbali, MD (Department of Cardiology, Erasmus MC University Medical Center, Rotterdam, the Netherlands; Department of Intensive Care, Erasmus MC University Medical Center, Rotterdam, the Netherlands); Peter C. Smits, MD, PhD (Department of Cardiology, Maasstad Hospital, Rotterdam, the Netherlands); Pim van der Harst, MD, PhD (Department of Cardiology, Division of Heart and Lungs, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands); Maarten van Smeden, PhD (Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands); Wiek H. van Gilst, MD, PhD (Department of Cardiology, University Medical Center Groningen, Groningen, the Netherlands).
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- ICU
- intensive care unit
- IQR
- interquartile range
- PE
- pulmonary embolism
- TOAST
- Trial of ORG 10172 in Acute Stroke Treatment
A list of the CAPACITY-COVID Collaborative Consortium is given in the Appendix.
This manuscript was sent to Stephen M. Davis, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.034787.
For Sources of Funding and Disclosures, see page 3984.
Contributor Information
Marijke Linschoten, Email: m.p.m.linschoten@umcutrecht.nl.
Julie E. Buijs, Email: jbuijs@spaarnegasthuis.nl.
J. Matthijs Biesbroek, Email: j.m.biesbroek@umcutrecht.nl.
Heleen M. den Hertog, Email: m.h.den.hertog@isala.nl.
Tessa Ribbers, Email: tessa.ribbers@radboudumc.nl.
Dennis J. Nieuwkamp, Email: d.nieuwkamp@jbz.nl.
Reinier C. van Houwelingen, Email: r.vanhouwelingen@treant.nl.
Andreas Dias, Email: a.dias@ikazia.nl.
Ingeborg W.M. van Uden, Email: inge.v.uden@catharinaziekenhuis.nl.
Joost P. Kerklaan, Email: j.kerklaan@antoniusziekenhuis.nl.
H. Paul Bienfait, Email: p.bienfait@gelre.nl.
Sarah E. Vermeer, Email: svermeer@rijnstate.nl.
Sonja W. de Jong, Email: S.W.de.Jong@stjansdal.nl.
Mariam Ali, Email: mariama9908@gmail.com.
Marieke J.H. Wermer, Email: m.j.h.wermer@lumc.nl.
Marieke T. de Graaf, Email: graaf.m@zaansmc.nl.
Paul J.A.M. Brouwers, Email: pjam.brouwers@wxs.nl.
Folkert W. Asselbergs, Email: f.asselbergs@ucl.ac.uk.
L. Jaap Kappelle, Email: l.kappelle@umcutrecht.nl.
H. Bart van der Worp, Email: h.b.vanderworp@umcutrecht.nl.
References
- 1.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Requena M, Olivé-Gadea M, Muchada M, García-Tornel Á, Deck M, Juega J, Boned S, Rodríguez-Villatoro N, Piñana C, Pagola J, et al. COVID-19 and stroke: incidence and etiological description in a high-volume center. J Stroke Cerebrovasc Dis. 2020;29:105225. doi: 10.1016/j.jstrokecerebrovasdis.2020.105225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siepmann T, Sedghi A, Simon E, Winzer S, Barlinn J, de With K, Mirow L, Wolz M, Gruenewald T, Schroettner P, et al. Increased risk of acute stroke among patients with severe COVID-19: a multicenter study and meta-analysis. Eur J Neurol. 2021;28:238–247. doi: 10.1111/ene.14535 [DOI] [PubMed] [Google Scholar]
- 6.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76:2060–2072. doi: 10.1016/j.jacc.2020.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaptein FHJ, Stals MAM, Grootenboers M, Braken SJE, Burggraaf JLI, van Bussel BCT, Cannegieter SC, Ten Cate H, Endeman H, Gommers DAMPJ, et al. Dutch COVID & Thrombosis Coalition. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID-19 in the second and first wave. Thromb Res. 2021;199:143–148. doi: 10.1016/j.thromres.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etkin Y, Conway AM, Silpe J, Qato K, Carroccio A, Manvar-Singh P, Giangola G, Deitch JS, Davila-Santini L, Schor JA, et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, Raju M, Lobanova I, Suri MFK, Naqvi SH, French BR, et al. Acute Ischemic Stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52:905–912. doi: 10.1161/STROKEAHA.120.031786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makda A, Kumar S, Kumar A, Kumar V, Rizwan A. The frequency of neurological symptoms in COVID-19 patients at a tertiary care hospital in Pakistan. Cureus. 2020;12:e10360. doi: 10.7759/cureus.10360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nersesjan V, Amiri M, Christensen HK, Benros ME, Kondziella D. Thirty-day mortality and morbidity in COVID-19 positive vs. COVID-19 negative individuals and vs. individuals tested for influenza A/B: a population-based study. Front Med (Lausanne). 2020;7:598272. doi: 10.3389/fmed.2020.598272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esenwa C, Cheng NT, Lipsitz E, Hsu K, Zampolin R, Gersten A, Antoniello D, Soetanto A, Kirchoff K, Liberman A, et al. COVID-19-associated carotid atherothrombosis and stroke. AJNR Am J Neuroradiol. 2020;41:1993–1995. doi: 10.3174/ajnr.A6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, Snyder T, Berger S, Yang D, Granger A, et al. A Prospective Study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96:e575–e586. doi: 10.1212/WNL.0000000000010979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York Healthcare System. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegler JE, Cardona P, Arenillas JF, Talavera B, Guillen AN, Chavarría-Miranda A, de Lera M, Khandelwal P, Bach I, Patel P, et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 multinational registry. Int J Stroke. 2021;16:437–447. doi: 10.1177/1747493020959216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51:e219–e222. doi: 10.1161/STROKEAHA.120.030995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fifi JT, Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol. 2020;19:713–715. doi: 10.1016/S1474-4422(20)30272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linschoten M, Uijl A, Schut A, Jakob CEM, Romao LR, Bell RM, McFarlane E, Stecher M, Zondag AGM, van Iperen EPA, et al. Clinical presentation, disease course and outcome of COVID-19 in hospitalized patients with and without pre-existing cardiac disease – a cohort study across sixteen countries. medRxiv. Preprint posted online March 12, 2021. doi: 10.1101/2021.03.11.21253106 [DOI] [PubMed] [Google Scholar]
- 20.Linschoten M, Peters S, van Smeden M, Jewbali LS, Schaap J, Siebelink HM, Smits PC, Tieleman RG, van der Harst P, van Gilst WH, et al. ; CAPACITY-COVID Collaborative Consortium. Cardiac complications in patients hospitalised with COVID-19. Eur Heart J Acute Cardiovasc Care. 2020;9:817–823. doi: 10.1177/2048872620974605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lansky AJ, Messé SR, Brickman AM, Dwyer M, van der Worp HB, Lazar RM, Pietras CG, Abrams KJ, McFadden E, Petersen NH, et al. Proposed standardized neurological endpoints for cardiovascular clinical trials: an academic research consortium initiative. J Am Coll Cardiol. 2017;69:679–691. doi: 10.1016/j.jacc.2016.11.045 [DOI] [PubMed] [Google Scholar]
- 22.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 23.Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ. 2012;184:895–899. doi: 10.1503/cmaj.101715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annie F, Bates MC, Nanjundappa A, Bhatt DL, Alkhouli M. Prevalence and outcomes of acute ischemic stroke among patients ≤50 years of age with laboratory confirmed COVID-19 infection. Am J Cardiol. 2020;130:169–170. doi: 10.1016/j.amjcard.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan S, Xiao M, Han F, Xia P, Bai X, Chen H, Zhang H, Ding X, Zhao H, Zhao J, et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol. 2020;11:806. doi: 10.3389/fneur.2020.00806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantador E, Núñez A, Sobrino P, Espejo V, Fabia L, Vela L, de Benito L, Botas J. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J Thromb Thrombolysis. 2020;50:543–547. doi: 10.1007/s11239-020-02176-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linschoten M, Nab L, van der Horst ICC, Tieleman R, Asselbergs FW. Response to “early hydroxychloroquine but not chloroquine use reduces ICU admission in COVID-19 patients”. Int J Infect Dis. 2021;103:560–561. doi: 10.1016/j.ijid.2020.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5:456–463. doi: 10.1002/acn3.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umapathi T, Kor AC, Venketasubramanian N, Lim CC, Pang BC, Yeo TT, Lee CC, Lim PL, Ponnudurai K, Chuah KL, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39:3029–3035. doi: 10.1161/STROKEAHA.108.521583 [DOI] [PubMed] [Google Scholar]
- 35.Rinkel LA, Prick JCM, Slot RER, Sombroek NMA, Burggraaff J, Groot AE, Emmer BJ, Roos YBWEM, Brouwer MC, van den Berg-Vos RM, et al. Impact of the COVID-19 outbreak on acute stroke care. J Neurol. 2021;268:403–408. doi: 10.1007/s00415-020-10069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz JM, Libman RB, Wang JJ, Sanelli P, Filippi CG, Gribko M, Pacia SV, Kuzniecky RI, Najjar S, Azhar S. Cerebrovascular complications of COVID-19. Stroke. 2020;51:e227–e231. doi: 10.1161/STROKEAHA.120.031265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huisman MV, Coppens M, Eikenboom J, Kamphuisen PW, Klok E, Middeldorp S, Kruip M, Meijer K, van den Toorn L, Wester J, et al. Leidraad COVID-19 coagulopathie. 2020. https://www.demedischspecialist.nl/sites/default/files/Leidraad%20COVID-19%20coagulopathie.pdf.
- 38.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. RECOVERY. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.