Abstract
Objective
This study investigated, in a large pediatric population, whether magnetic resonance imaging (MRI) evidence of mediobasal hypothalamic (MBH) gliosis is associated with baseline or change over 1 year in body adiposity.
Methods
Cross-sectional and prospective cohort analyses were conducted within the Adolescent Brain Cognitive Development (ABCD) Study. Study 1 included 169 children with usable baseline T2-weighted MRI images and anthropometrics from baseline and 1-year follow-up study visits. Signal ratios compared T2 signal intensity in MBH and 2 reference regions (amygdala [AMY] and putamen) as a measure of MBH gliosis. Study 2 included a distinct group of 238 children with overweight or obesity to confirm initial findings in an independent sample.
Results
In Study 1, MBH/AMY signal ratio was positively associated with BMI z-score (β=4.27 P<0.001). A significant interaction for the association of MBH/AMY signal ratio with change in BMI z-score suggested relationships differed by baseline weight status. Study 2 found that higher MBH/AMY signal ratios associated with an increase in BMI z-score for children with overweight (β=0.58 P=0.01), but not those with obesity (β=0.02 P=0.91).
Conclusions
Greater evidence of hypothalamic gliosis by MRI is associated with baseline BMI z-score and predicts adiposity gain in young children at risk of obesity.
Keywords: obesity, MRI, gliosis, hypothalamus, pediatric obesity
Introduction
Understanding factors that drive weight gain in young children and promote development of childhood obesity are critical goals for improving child and adult health. Genetic (1), environmental, and behavioral factors are well recognized contributors (2). In addition, strong evidence from preclinical models has implicated a critical role for the central nervous system (CNS) in obesity pathogenesis (3, 4), but the majority of these mechanistic findings await translational studies to evaluate their relevance to weight gain and pediatric obesity.
Rodent models of diet-induced obesity demonstrate that inflammation and reactive gliosis – activation and proliferation of glial cell populations (5) – occur in the key brain region for regulation of energy homeostasis, the arcuate nucleus of the hypothalamus (6). In humans, T2-weighted magnetic resonance imaging (MRI) can be used to non-invasively and reliably detect evidence of gliosis in the mediobasal hypothalamus (MBH), which encompasses the arcuate nucleus (7). As seen in adults (6–8), studies of children and adolescents (9, 10) have demonstrated that stronger evidence of MBH gliosis by MRI is associated with higher adiposity as assessed by BMI z-scores, total body fat mass, and visceral fat mass (9). However, previous pediatric studies were cross-sectional, enrolled small samples, and compared only children with obesity to those of normal weight (NW), excluding the overweight category (9). Consequently, prior work has not addressed whether hypothalamic gliosis precedes weight gain in humans (as it does in rodents (6)) and, therefore, it provides limited insight into the potential for hypothalamic gliosis to play a mechanistic role in obesity development in humans.
In a sample of children from a large, population-based, pediatric observational cohort study, the current study tested whether radiologic evidence of hypothalamic gliosis was associated with greater body adiposity and/or gain in adiposity over 1 year and whether these relationships varied based on a child’s baseline weight status.
Methods
Study 1 and Study 2 include data from two independent subsamples of participants from the Adolescent Brain Cognitive DevelopmentSM Study (11) and are available at DOI 10.15154/1520153.
Participants
Study 1
The initial sample included the first available 558 female and male participants who underwent T2-weighted image acquisition on a 3-T GE (GE Healthcare, Chicago, Illinois) MR scanner. Exclusion criteria were siblings, MRI artifacts (e.g. movement, blood vessel) in our regions of interest (ROIs), and missing or problematic anthropometric data (baseline or 1-yr follow-up). The final sample consisted of N=169 participants (Supporting Information Figure S1A).
Study 2
An independent cohort focused on participants with overweight (OV) and obesity (OB) was selected to further address the main findings from Study 1. From an initial sample of 1,763 participants, which did not include children from Study 1, with an available baseline T2-weighted image acquired on a GE MRI scanner, children with BMI ≥ 85th percentile (for age and sex) were selected. Exclusion criteria were the same as those that were applied for Study 1, resulting in a final sample comprised of 238 OV or OB participants (Supporting Information Figure S1B).
Data Collection for Studies 1 and 2
Demographics and Anthropometrics
Participants were from 6 study sites. Parents reported child race and ethnicity and family income (12). Pubertal status was based on child report through the Pubertal Development Scale (13). Weight, height, and waist circumference were measured at baseline and 1-yr follow-up visits (12). We used BMI z-score to ensure that age-and sex-specific changes could be interpreted relative to other children of the same age and sex (14). Baseline BMI-percentile was used to classify weight groups (normal weight (NW) BMI<85th percentile, OV between the 85th and 94.9th percentiles, with OB: ≥ 95th percentile) (14). BMI z-scores (14), waist/height ratio (15), and changes in BMI z-score and waist/height ratio (1-yr follow-up minus baseline) were calculated.
MRI acquisition and analysis
Scans were acquired following the ABCD Study imaging protocol and the sequence used in the current study was the 3D T2-weighted fast spin echo (16). For analysis, coronal slices through the hypothalamus were evaluated on OsiriX Imaging Software version 11 (Pixmeo SARL, Bernex, CH). Reviewers were blinded to weight classification. The slice immediately posterior to the optic chiasm was selected as previously described (6, 7, 9, 17). Within the same slice, ROIs in the left and right MBH were identified, as well as control regions in the left and right amygdala (AMY) and putamen (PUT; Figure 1A). Mean ROI T2 signal intensity was obtained for each side and a bilateral mean calculated for each ROI. In order to allow comparison across participants and sites, MBH signal intensity was normalized via a ratio (6) comparing MBH with amygdalar signal intensity, providing our primary measure (MBH/AMY signal ratio). Two control ratios were calculated: a negative control ratio, comparing putamen with amygdala signal intensities (PUT/AMY signal ratio), which does not test the MBH region; and a positive control ratio, MBH with putamen signal intensities (MBH/PUT signal ratio) were compared, which tests the MBH region using an alternate reference region. Study 1 had a single rater for all scans (LES). Two raters (LES and SK) performed scan analyses for Study 2 with a 142/96 proportion. For Study 2, all scans were pre-assigned to a primary reviewer for data capture, then were cross assigned to verify interrater reliability in 30 randomly selected scans. Intraclass correlation (ICC) for T2 signal ratios demonstrated excellent agreement among raters (MBH/AMY ICC: 77.0%; PUT/AMY ICC: 89.5%; MBH/PUT ICC: 81.9%, all P<0.001).
Figure 1. MRI evidence of hypothalamic gliosis in association with body adiposity in children.
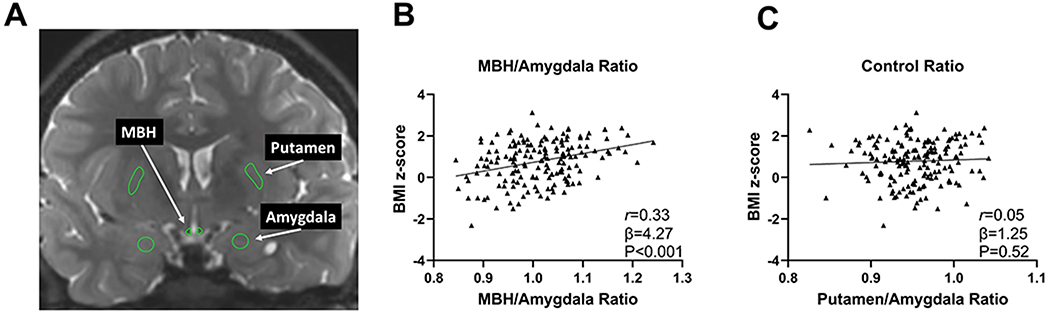
(A) Representative coronal slice of T2-weighted magnetic resonance imaging to assess T2 signal intensity from regions of interest in the MBH, amygdala, and putamen. Association of (B) MBH/amygdala and (C) putamen/amygdala T2 signal ratios with BMI z scores in children (n = 169). P values were calculated by linear regression and adjusted for age, sex, and study site. Pearson correlation coefficient was calculated for descriptive purposes. MBH, mediobasal hypothalamus
Behavioral variables
For Study 2, available data from the ABCD Study on sleep, screen time, physical activity, and diet quality (12) were assessed as potential confounders (or mediators) of relationships between T2 signal ratios and changes in BMI z-score. At the baseline visit, data included parent-report of child’s sleep function (12, 18) and daily screen time and child report of the number of days in the past week that they were physically active for at least 60 min (12). At the 1-yr follow-up visit, a parent-reported modified food frequency questionnaire was collected; greater total points suggest better diet quality (12).
Statistics
Means and (SDs) are reported unless otherwise noted. For descriptive purposes, comparisons were by chi-square test (categorical) or linear regression (continuous, normally distributed). Multiple linear regression tested the association between outcome variables (adiposity measures at baseline or changes in adiposity over 1-yr) and predictors (T2 signal ratios). In order to test for effect modification by baseline weight status, models included an interaction term (T2 signal ratio*group). The significance of the interaction was tested by Wald test and simple slopes were evaluated for each weight group. Models were repeated using the negative and positive control ratios as predictors. All models were adjusted a priori for sex, age, and study site. Additional covariates were added for secondary analyses. Statistical analyses were conducted in Stata version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) and graphing completed with GraphPad Prism version 8.0 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
Study 1
General characteristics
Participant characteristics (N=169) are in Table 1. A total of 56% of children were classified as NW, 20% as OV, and 24% as OB, but this distribution varied by study site (Χ2(10)=21.03, P=0.02). Follow-up visits occurred, on average, 11 (1) months after baseline visits. On average, children’s total and central adiposity remained stable over that time (Table 1). Change in BMI z-score over 1 year was 0.07 (0.39) for NW, −0.07 (0.38) for children with OV, and −0.04 (0.22) for children with OB. For our primary measure, a trend was present for the relationship of age to MBH/AMY signal ratio. No sex, study site or puberty status differences were found (Supporting Information Table S1). Variable results were found when assessing the relationships with and potential confounding by age, sex, study site, and puberty with control ratios (Supporting Information Table S1).
Table 1.
General characteristics of Study 1 sample (total n=169)
| Age at baseline, y | 9.9 | 0.6 | |
| Age at 1-yr follow-up a , y | 10.8 | 0.6 | |
| Sex, female participants | 85 | 50% | |
| Race b | White | 105 | 63% |
| Black | 20 | 12% | |
| Asian | 5 | 3% | |
| Native American | 2 | 1% | |
| Mixed (≥ 2 races) | 25 | 15% | |
| Other races | 10 | 6% | |
| Ethnicity b | Hispanic | 43 | 26% |
| Socioeconomic status c | up to $49,999 | 55 | 36% |
| $50,000 through $99,999 | 40 | 26% | |
| $100,000 or more | 57 | 38% | |
| Pubertal development scale d | pre-puberty | 44 | 29% |
| early puberty | 49 | 32% | |
| mid puberty | 54 | 36% | |
| late & post-puberty | 4 | 3% | |
|
| |||
| BMI z-score | 0.77 | 1.02 | |
| Waist/Height ratio | 0.49 | 0.07 | |
| BMI p95 at baseline, % | 17.2 | 22.8 | |
| Body weight classification e | Normal weight | 95 | 56% |
| Overweight | 34 | 20% | |
| With obesity | 40 | 24% | |
| Change in BMI z-score | 0.02 | 0.36 | |
| Change in Waist/Height ratio | 0.0 | 0.0 | |
| BMI p95 at 1-yr follow-up f , % | 16.4 | 15.1 | |
|
| |||
| T2 signal ratio | MBH/Amygdala | 1.01 | 0.08 |
| Putamen/Amygdala | 0.95 | 0.04 | |
| MBH/Putamen | 1.06 | 0.09 | |
Data are reported as mean and SD, or n and %. Abbreviations: BMI p95, percentage of the 95th percentile; MBH, mediobasal hypothalamus.
On average 11 ± 1 months after baseline visit.
n=167;
n=152;
n=151;
Change in BMI z-score and Change in waist/height ratio calculated by 1-yr follow-up minus baseline.
Defined by BMI-for age and sex-percentile by CDC (14);
Participants with obesity baseline (n=40) and 1-yr follow up (n=42).
Hypothalamic gliosis and baseline adiposity
Bilateral MBH/AMY signal ratio was positively associated with baseline BMI z-score (Figure 1B) and waist/height ratio (β=0.24, P=0.001), but the negative control ratio (PUT/AMY) was unrelated to BMI z-score (Figure 1C) or waist/height ratio (β=0.04, P=0.77). For the positive control ratio (MBH/PUT), significant associations were again present for both BMI z-score (β=3.48, P<0.001) and waist/height ratio (β=0.20, P=0.003). Both left (β=3.37, P<0.001) and right MBH/AMY signal ratios (β=2.23, P=0.001) were significantly associated with BMI z-score.
Hypothalamic gliosis and change in body adiposity over 1-yr
Among a representative sample of children from the ABCD Study, MBH/AMY T2 signal ratio was not associated with change in BMI z-score from baseline to 1-yr follow-up (β=−0.13, P=0.73), nor were the negative and positive control ratios (PUT/AMY: β=0.66, P=0.34; MBH/PUT: β=−0.27, P=0.41). We then tested for effect modification by baseline weight status and found a significant interaction of MBH/AMY signal ratio by weight group (Figure 2A). In a subgroup analysis, higher baseline MBH/AMY signal ratio was associated with 1-year increase in BMI z score among children with OV (Figure 2B). This association was not found for children with OB (Figure 2C) or those of NW (Figure 2D). No interaction was found for the negative control ratio [PUT/AMY: F(5,158):2.09, P=0.07], but it was present for the positive control ratio [MBH/PUT: F(5,158):2.31, P=0.047]. Given unplanned subgroup analyses and sensitivity analyses in the OV group that revealed two statistically influential data points, we therefore conducted a fully powered cohort study selecting for children with OV and OB in an independent sample (Study 2).
Figure 2. Evidence of hypothalamic gliosis and change in BMI z-score over 1 year.
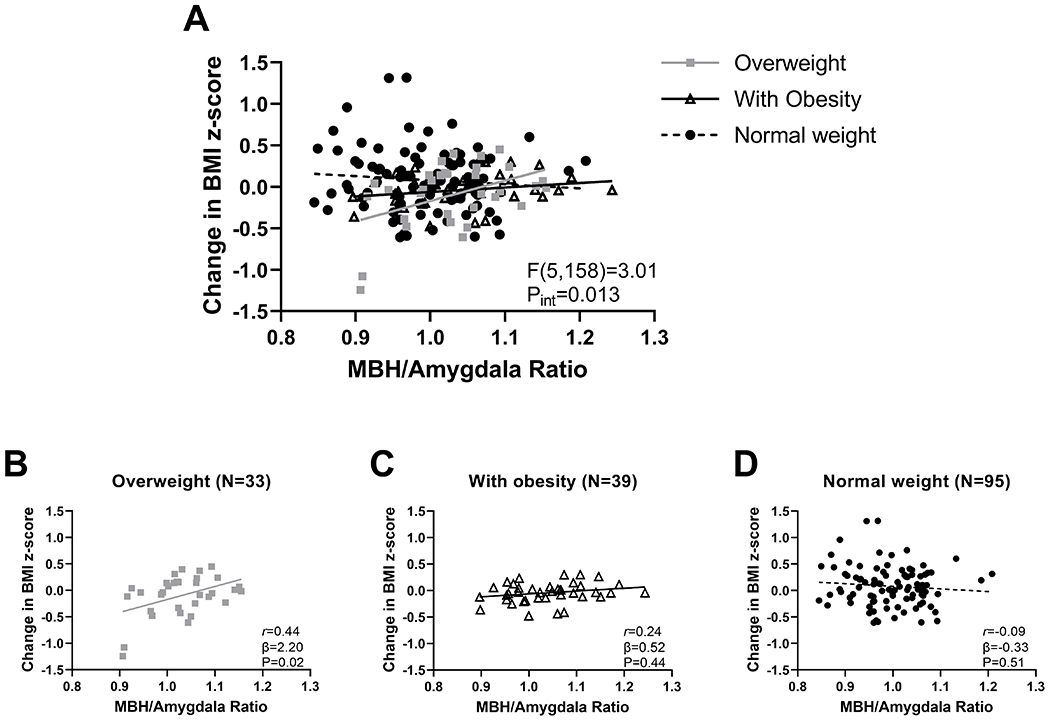
(A) Association of MBH/amygdala signal ratio and change in BMI z score differs by weight group (n = 167). Stratified analyses by weight group for children with (B) overweight, (C) obesity, and (D) normal weight for association of MBH/amygdala T2 signal ratio with BMI z score. P values determined by linear regression with an interaction term (panel A) followed by assessing simple effects at each weight group (panels B-D) and adjusted for age, sex, and study site. Pearson correlation coefficient was calculated for descriptive purposes. MBH, mediobasal hypothalamus; pint, p value for the Wald test that examined the T2 signal ratio-by-weight group interaction
Study 2.
General characteristics
Study 2 enrolled children with OV (N=114) and OB (N=124). The groups did not differ in age (β=0.003, P=0.97) or change in BMI z-score (β=0.01, P=0.64) and had similar distributions among study sites, sex, race, ethnicity, and socioeconomic status (Table 2).
Table 2.
Characteristics by group of children in Study 2 sample
| OV (n=114) | OB (n=124) | ||||
|---|---|---|---|---|---|
| Age at baseline, y | 9.9 | 0.6 | 9.9 | 0.6 | |
| Age at 1-yr follow-up, y | 10.9 | 0.6 | 10.9 | 0.6 | |
| Sex, female participants | 53 | 47% | 58 | 47% | |
| Race a | White | 61 | 55% | 58 | 50% |
| Black | 12 | 11% | 18 | 15% | |
| Asian | 5 | 4% | 2 | 2% | |
| Native American | 3 | 3% | |||
| Mixed (≥ 2 races) | 18 | 16% | 24 | 21% | |
| Other races | 12 | 11% | 14 | 12% | |
| Ethnicity a | Hispanic | 41 | 36% | 52 | 42% |
| Socioeconomic status b | up to $49,999 | 44 | 40% | 53 | 50% |
| $50,000 through $99,999 | 22 | 20% | 39 | 37% | |
| $100,000 or more | 44 | 40% | 14 | 13% | |
| Pubertal development scale c | pre-puberty | 15 | 15% | 29 | 26% |
| early puberty | 42 | 41% | 32 | 29% | |
| mid puberty | 40 | 40% | 44 | 40% | |
| late & post-puberty | 4 | 4% | 5 | 5% | |
|
| |||||
| BMI z-score | 1.37 | 0.17 | 2.04 | 0.26 | |
| Waist/Height ratio | 0.51 | 0.04 | 0.58 | 0.05 | |
| BMI p95 at baseline, % | 14.9 | 12.6 | |||
| Change in BMI z-score | 0.01 | 0.27 | 0.03 | 0.15 | |
| Change in Waist/Height ratio | 0 | 0 | 0.01 | 0 | |
| Body weight classification at 1-yr follow-up d | Normal weight | 11 | 10% | ||
| Overweight | 83 | 73% | 6 | 5% | |
| With obesity | 20 | 17% | 118 | 95% | |
| BMI p95 at 1-yr follow-up e , % | 15.7 | 13.0 | |||
|
| |||||
| T2 signal ratio | MBH/Amygdala | 1.13 | 0.09 | 1.13 | 0.10 |
| Putamen/Amygdala | 0.97 | 0.05 | 0.97 | 0.05 | |
| MBH/Putamen | 1.16 | 0.09 | 1.17 | 0.10 | |
Data are reported as mean and SD or n and %. Change in BMI z-score and Change in waist/height ratio calculated by 1-yr follow-up minus baseline. Abbreviations: BMI p95, percentage of the 95th percentile; MBH, mediobasal hypothalamus; OV, overweight; OB, with obesity.
Missing data: OV (race n=3, ethnicity n=1); OB (race n=8, ethnicity n=1).
Missing data: OV (n=13); OB (n=14).
Missing data: OV (n=4); OB (n=18).
Defined by BMI-for age and sex-percentile by CDC (14);
Participants with obesity at 1-yr follow up (n=138).
Hypothalamic gliosis and change in body adiposity in children with OV or OB
Similar to Study 1, the relationship of MBH/AMY signal ratio with change in BMI z-score tended to differ by weight group (Figure 3A). Higher baseline MBH/AMY signal ratio was associated with 1-yr increases in BMI z-score for children with OV (Figure 3B) but not for children with OB (Figure 3C). The PUT/AMY signal ratio was uncorrelated (interaction F(3,231):0.41, P=0.75; OV: β=‒0.16, P=0.67; OB: β=‒0.42, P=0.33). The positive control ratio (MBH/PUT) substantiated that greater evidence of MBH gliosis was associated with increases in BMI z-score solely for children with OV (interaction F(3,231):3.45, P=0.02; OV: β=0.67, P=0.002; OB: β=0.13, P=0.48). In order to consider the impact of known behavioral factors that can contribute to weight gain in children, among the subsample of children with OV with complete data for behavioral measures (N=101), a secondary analysis that included additional covariates for sleep, diet quality, screen time, and physical activity was performed and showed a persistent trend relating MBH/AMY signal ratio to change in BMI z-score (β=0.61, P=0.052).
Figure 3. Evidence of hypothalamic gliosis and change in BMI z-score in Study 2’s independent sample of children with overweight and with obesity.
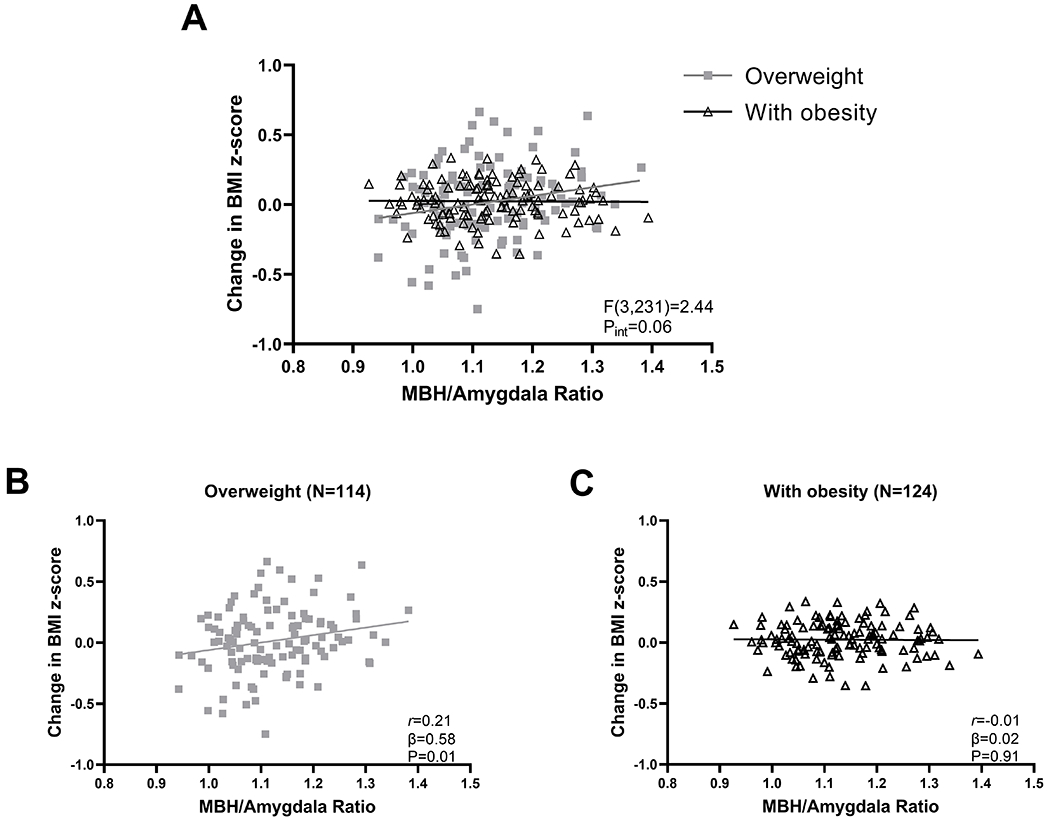
(A) Effect of weight group on the association of MBH/Amygdala signal ratio with change in BMI z-score. Stratified analyses by weight group for children with overweight (B) or obesity (C) for association of MBH/Amygdala T2 signal ratio with BMI z-score. MBH, mediobasal hypothalamus. N, number of participants. Pint, P-value for the Wald test used to determine significant T2 signal ratio-by-weight group interaction. P-values determined by linear regression with an interaction term (A) followed by assessing simple effects at each weight group (B and C), adjusted for age, sex, and study site. Pearson’s correlation coefficient calculated for descriptive purposes.
Combined analysis
For the purpose of a group comparison, we combined children from Study 1 and Study 2 to assess if mean T2 signal ratios would differ by weight group. In unadjusted analyses, NW children had lower MBH/AMY signal ratio when compared to both children with OV (β=0.11, P<0.001) and with OB (β=0.11, P<0.001). Weight groups did not differ for the negative control ratio (Supporting Information Table S2).
Discussion
Since the discovery of MRI evidence of hypothalamic gliosis in humans (6), questions have persisted about whether gliosis precedes weight gain in humans and could thereby contribute to obesity pathogenesis. Our current findings build on previous studies conducted in humans by showing, in the largest pediatric population assessed to date, a positive cross-sectional association between T2 signal in the MBH and BMI z-score in children, independent of sex, age, and study site. Control ratios confirmed that the findings were absent in a control region in the putamen and were irrespective of the reference region selected as the comparator. Furthermore, we found significantly elevated MBH T2 signal ratios when comparing NW with OV and OB groups. Although there was no overall association between signal ratios and one-year changes in adiposity in the prospective analysis of Study 1, a significant interaction demonstrated that the relationship of MBH T2 signal ratios with change in BMI z-score varied based on a child’s baseline weight status. Findings, confirmed in the independent Study 2 sample targeting children in OV and OB groups, show that children whose BMI z-score placed them in the overweight category were the only group for whom a higher baseline MBH T2 signal ratio was prospectively related to greater gains in BMI z-score over one year. In sum, in a large, population-based sample of children, we provide further evidence that hypothalamic gliosis is present in young children with overweight and obesity, as well as the first longitudinal evidence that a greater degree of MBH gliosis predicts a subsequent increase in body adiposity among susceptible individuals.
Prior cross-sectional neuroimaging studies have detected evidence of gliosis in the MBH in association with obesity, first in adults (6, 7, 17) and then in small samples of children (9, 10). Other MRI-derived metrics, such as proton diffusivity and tissue water content, provide further evidence that hypothalamic microstructure varies in adults with obesity (19, 20). In addition to total adiposity, a link has also been demonstrated between MBH gliosis and visceral adiposity in both children (9) and adult men (8), a relationship replicated in the current sample using the waist/height ratio as a marker. In several of these studies, lateralized effects were described for the association between T2 signal in the MBH and body adiposity in adults (6, 7, 17) and children (10). We investigated data from left and right MBH/AMY ratios separately and found consistent and robust associations on both sides. Our bilateral associations differ from earlier reports where unilateral associations were observed, perhaps due to our larger sample size and/or the younger age of our participants. In addition to in vivo approaches using imaging, one postmortem histopathological study of humans with obesity found increased microglial number and dystrophy in the MBH but not cortex (21). Importantly, the histopathological findings of postmortem samples from human adults have validated the use of T2-weighted MRI to assess MBH tissue characteristics and detect evidence of gliosis, by demonstrating that increased T2 signal (brightness) in the MBH is associated with the presence of astrocytosis (7). Thus, convergent evidence across age groups and methodologies establishes obesity-associated alterations in hypothalamic tissue composition that are consistent with gliosis.
These data highlight the potential translational significance of rodent studies demonstrating that hypothalamic inflammation and gliosis not only precede weight gain (6) but are both necessary and sufficient for excessive weight gain and diet-induced obesity (22, 23). Activation and reactive conformational changes of astrocytes and microglia in the arcuate nucleus of the MBH start early in the face of a dietary stimulus (6). Moreover, when animal models of diet-induced obesity are chronically exposed to high-fat diet, loss of proopiomelanocortin (POMC) neurons in the arcuate nucleus has been noted (6), which may incur negative functional consequences or impair energy homeostasis (6). Furthermore, studies in rodents have elucidated how synaptic plasticity in hypothalamic neuronal circuits is fundamental for body weight and food intake regulation (24). Advances in the study of glial cells have demonstrated that non-neuronal cells, such as astrocytes and microglia, are active participants in synaptic plasticity (25) and go beyond the initial conception of glia as solely neuron structural support cells. The role of hypothalamic neuron-glia interactions for the regulation of neuroendocrine processes such as energy balance are under investigation (26, 27). The current findings provide translational insight into the relevance of such preclinical data to human obesity pathogenesis.
Our findings show that children in the overweight group with higher T2 signal within the MBH demonstrate greater increases in adiposity over the subsequent 1-year follow-up period. These are the first data suggesting that hypothalamic gliosis may contribute to excessive weight gain. Prior studies demonstrate that children of normal weight and with obesity tend to maintain their weight status from a young age until adulthood (28). On the contrary, children with overweight, who outnumber children with obesity in several countries (29), are at an increased risk for the development of obesity, and its co-morbidities, later in adolescence and adult life. In addition, this group of children can also regress to a healthy weight category (30–32). Importantly, the extent to which the presence of MBH gliosis is causally related to adiposity gain or loss in children with overweight cannot be determined from this observational cohort study. However, in face of the accumulating preclinical evidence that remodeling may alter hypothalamic regulation of energy balance, persistent or permanent hypothalamic gliosis must be investigated as a possible contributing factor toward elevated long-term risk for childhood and adult obesity (31, 32) among susceptible individuals.
On the contrary, MRI evidence of MBH gliosis was not predictive of adiposity gain in the OB group. We may theorize that once obesity is in place, even for this young pediatric population, the cellular inflammatory reaction of gliosis in the MBH becomes less influential. Alternatively, there may be a “ceiling effect” to BMI z-score gain, the time frame of only 11 months may have been too limited to detect further increases in adiposity amongst the children with obesity, or BMI z-score may have been too insensitive a measure to detect changes in total adiposity in this cohort. As the ABCD Study continues to release data, we can further examine the predictive role of hypothalamic gliosis on longitudinal changes in adiposity among children with obesity, including central adiposity.
Through a multiple regression analysis, we found that behavioral factors known to be related to childhood obesity, such as diet and sleep quality, physical activity, and screen time (2, 33), exerted minimal influence on the association between hypothalamic gliosis and adiposity gain. When measures of these variables were added into the model, the magnitude of the association (beta values) was essentially unchanged, although the p-value was reduced to borderline significance. This may have been due to reduced power given the smaller sample size of children with complete data and the increased number of covariates in the model. Given the preliminary nature of this analysis, further investigation is warranted. Although demonstrated in rodents, it is unknown if dietary excess is a causal mechanism of hypothalamic gliosis in humans. Moreover, other behavioral, genetic, and perinatal environmental factors, such as consumption of ultra-processed foods, variants in the fat mass and obesity-associated (FTO) gene, and gestational diabetes, play important roles in both obesity and central regulation of energy homeostasis (34–36) and could participate as potential determinants of MBH gliosis in children and adults. Studies of diet with rodent models have demonstrated that the reversal of a high-fat to chow diet promotes reduction of astrocytosis and microgliosis in the arcuate nucleus (37). In adult women with obesity and type 2 diabetes, weight loss by bariatric surgery partially reduced MRI evidence of MBH gliosis (38), providing further evidence that substantial changes in diet and/or body weight can alter MBH gliosis. Future studies with comprehensive dietary assessments and lifestyle interventions would help to elucidate the specific role of diet in human hypothalamic gliosis.
Our current study is limited to findings in children aged between 9 and 12 years and data collected by the ABCD Study. Additionally, the MRI approach applied in the current study and previous adult studies (6, 17) is a “snapshot” of the tissue characteristics at that moment and we did not examine changes over time. Importantly, T2 signal hyperintensity cannot provide definitive evidence of tissue or cellular level hypothalamic structure as it can also identify other brain tissue changes, such as edema, sclerosis, and infection (39, 40). However, these pathologies are unlikely because the ABCD Study enrolled healthy participants (41), and MR images underwent evaluation by a neuroradiologist (42). Furthermore, waist/height ratio and BMI z-score are indirect measures of central and total adiposity, respectively, and the behavioral factors included in this study were based on parent- or self-report measures of diet, sleep, physical activity, and screen time. Future studies could benefit from more accurate measurements of body composition and in-depth behavioral assessments.
In conclusion, by applying MRI in a large, population-based study of children, we found evidence that hypothalamic gliosis precedes adiposity gain in children at risk of obesity by virtue of their overweight weight status. These longitudinal data further imply that hypothalamic gliosis may contribute to this group’s vulnerability to develop childhood obesity and its consequent elevated risk for cardiometabolic disorders later in life. Future studies are needed to better understand whether interventions or preventive strategies could alleviate hypothalamic gliosis in childhood, reduce weight gain, and improve health over the lifespan.
Supplementary Material
Study Importance.
What is already known?
Structural abnormalities (gliosis) in the hypothalamus may participate in obesity pathophysiology.
What does this study add?
We show MRI evidence that hypothalamic gliosis is associated with body adiposity in children.
Evidence of hypothalamic gliosis was shown to predict gain in adiposity specifically for children at risk of obesity.
How might these results change the direction of research?
Our findings contribute to understanding the role of the hypothalamus in the increased vulnerability of children with overweight transitioning to obesity.
Modifiable factors should be sought that reduce hypothalamic gliosis and potentially forestall adiposity gain in young children.
Acknowledgements
The authors would like to thank Allison Shapiro and Catherine Pihoker for their review and valuable comments.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI 10.15154/1520153 and the fast-track data release. DOIs can be found at https://nda.nih.gov/study.html?id=1019. The raw data are available at https://nda.nih.gov/edit_collection.html?id=2573. Instructions on how to create an NDA study are available at https://nda.nih.gov/training/modules/study.html.
Funding:
This work was supported by funding provided by the National Institutes of Health (DK117623) and the University of Washington Nutrition and Obesity Research Center (P30 DK035816).
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Pérusse L, Bouchard C. Role of genetic factors in childhood obesity and in susceptibility to dietary variations. Ann Med 1999;31:19–25. [DOI] [PubMed] [Google Scholar]
- 2.Leech RM, McNaughton SA, Timperio A. The clustering of diet, physical activity and sedentary behavior in children and adolescents: A review. Int J Behav Nutr Phys Act 2014;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz MW, Porte D. Diabetes, obesity, and the brain. Science 2005;307:375–9. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr Rev 2017;38:267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014;81:229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaler JP, Yi C, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schur EA, Melhorn SJ, Oh S-K, et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 2015;23:2142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkseth KE, Rubinow KB, Melhorn SJ, et al. Hypothalamic Gliosis by MRI and Visceral Fat Mass Negatively Correlate with Plasma Testosterone Concentrations in Healthy Men. Obesity (Silver Spring) 2018;26:1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sewaybricker LE, Schur EA, Melhorn SJ, et al. Initial evidence for hypothalamic gliosis in children with obesity by quantitative T2 MRI and implications for blood oxygen-level dependent response to glucose ingestion. Pediatr Obes 2019;14:e12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sewaybricker LE, Melhorn SJ, Papantoni A, et al. Pilot multi-site and reproducibility study of hypothalamic gliosis in children. Pediatr Obes 2021;16:e12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jernigan TL. 2019, Adolescent Brain Cognitive Development Study (ABCD), National Institute of Mental Health Data Archive, Version #2573, DOI 10.15154/1503209. [DOI] [Google Scholar]
- 12.Barch DM, Albaugh MD, Avenevoli S, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci 2018;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health 1993;14:190–5. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002:1–190. [PubMed] [Google Scholar]
- 15.Martin-Calvo N, Moreno-Galarraga L, Martinez-Gonzalez MA. Association between body mass index, waist-to-height ratio and adiposity in children: A systematic review and meta-analysis. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey BJ, Cannonier T, Conley MI, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci 2018;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreutzer C, Peters S, Schulte DM, et al. Hypothalamic Inflammation in Human Obesity Is Mediated by Environmental and Genetic Factors. Diabetes 2017;66:2407–2415. [DOI] [PubMed] [Google Scholar]
- 18.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC) construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res 1996;5:251–261. [DOI] [PubMed] [Google Scholar]
- 19.Thomas K, Beyer F, Lewe G, et al. Higher body mass index is linked to altered hypothalamic microstructure. Sci Rep 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kullmann S, Abbas Z, Machann J, et al. Investigating obesity-associated brain inflammation using quantitative water content mapping. J Neuroendocrinol 2020;32:1–13. [DOI] [PubMed] [Google Scholar]
- 21.Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol 2016;132:361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdearcos M, Douglass JD, Robblee MM, et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab 2017;26:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 2017;6:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Cáceres C, Balland E, Prevot V, et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci 2019;22:7–14. [DOI] [PubMed] [Google Scholar]
- 25.Douglass JD, Dorfman MD, Thaler JP. Glia: silent partners in energy homeostasis and obesity pathogenesis. Diabetologia 2017;60:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clasadonte J, Prevot V. The special relationship: Glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol 2018;14:25–44. [DOI] [PubMed] [Google Scholar]
- 27.Nuzzaci D, Cansell C, Liénard F, et al. Postprandial Hyperglycemia Stimulates Neuroglial Plasticity in Hypothalamic POMC Neurons after a Balanced Meal. Cell Rep 2020;30:3067–3078.e5. [DOI] [PubMed] [Google Scholar]
- 28.Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med 2018;379:1303–1312. [DOI] [PubMed] [Google Scholar]
- 29.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham SA, Kramer MR, Venkat Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 2014;370:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twig G, Yaniv G, Levine H, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med 2016;374:2430–2440. [DOI] [PubMed] [Google Scholar]
- 32.Ryder JR, Jacobs DR, Sinaiko AR, Kornblum AP, Steinberger J. Longitudinal Changes in Weight Status from Childhood and Adolescence to Adulthood. J Pediatr 2019;214:187–192.e2. [DOI] [PubMed] [Google Scholar]
- 33.Börnhorst C, Wijnhoven TMA, Kunešová M, et al. WHO European Childhood Obesity Surveillance Initiative: Associations between sleep duration, screen time and food consumption frequencies. BMC Public Health 2015;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouret S, Levin BE, Ozanne SE. Gene-Environment Interactions Controlling Energy and Glucose Homeostasis and the Developmental Origins of Obesity. Physiol Rev 2015;95:47–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalle Molle R, Fatemi H, Dagher A, Levitan RD, Silveira PP, Dubé L. Gene and environment interaction: Is the differential susceptibility hypothesis relevant for obesity? Neurosci Biobehav Rev 2017;73:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkseth KE, Guyenet SJ, Melhorn SJ, et al. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology 2014;155:2858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Sande-Lee S, Melhorn SJ, Rachid B, et al. Radiologic evidence that hypothalamic gliosis is improved after bariatric surgery in obese women with type 2 diabetes. Int J Obes 2020;44:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briellmann RS, Kalnins RM, Berkovic SF, Jackson GD. Hippocampal pathology in refractory temporal lobe epilepsy: T2-weighted signal change reflects dentate gliosis. Neurology 2002;58:265–271. [DOI] [PubMed] [Google Scholar]
- 40.Jackson GD, Williams SR, Weller RO, et al. Vigabatrin-induced lesions in the rat brain demonstrated by quantitative magnetic resonance imaging. Epilepsy Res 1994;18:57–66. [DOI] [PubMed] [Google Scholar]
- 41.Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci 2018;32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark DB, Fisher CB, Bookheimer S, et al. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: The ABCD experience. Dev Cogn Neurosci 2018;32:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


