Abstract
Background and Purpose:
Despite zinc’s role as an anti-oxidant and anti-inflammatory agent, prospective studies relating zinc levels to ischemic stroke risk are lacking. To examine the association between serum zinc levels and incidence of ischemic stroke in a US population.
Methods:
Using a case–cohort study nested within the REGARDS (REasons for Geographic and Racial Differences in Stroke) cohort, participants were randomly selected from the REGARDS cohort to generate a sub-cohort (n=2,346). All incident ischemic stroke cases as of September 2012 (n=660) were included, with 62 incident cases overlapping in the sub-cohort. Serum zinc levels were measured at baseline. Barlow-weighted Cox’s proportional hazards regression models were used to calculate multivariable-adjusted hazard ratios (HR) and the corresponding 95% confidence intervals (CI) of ischemic stroke by serum zinc levels.
Results:
The median zinc level for the sub-cohort was 121.19 μg/dL (interquartile range 104.86 to 140.39 μg/dL). Serum zinc levels were inversely associated with incidence of ischemic stroke after adjustment for potential confounders (quartile 4 vs. quartile 1: HR=0.78, 95% CI: 0.61-0.98, p=0.03 for trend). When stratified by pre-specified factors (sex, race, region), only sex showed a significant modification (p=0.03 for interaction). The inverse association was more pronounced among females (quartile 4 vs. quartile 1: HR=0.58, 95% CI: 0.41-0.84, p<0.01 for trend) than males (quartile 4 vs. quartile 1: HR=1.08, 95% CI: 0.78-1.51, p=0.92 for trend).
Conclusions:
Serum zinc concentration was inversely associated with incidence of ischemic stroke, especially among women, indicating that low zinc levels may be a risk factor for ischemic stroke.
Keywords: strokes, zinc, prospective study, Stroke, Epidemiology, Lifestyle, Prevention
Introduction
Stroke is a leading cause of death in the US.1 Stroke rates vary by sex, ethnic background, and geographic location, with higher rates observed among African Americans, or residents of the “Stroke Belt” (states where stroke mortality exceeds the rest of the US).2,3 Thus, determining modifiable risk factors for stroke and potential reasons for the variations in stroke rates is a priority for public health intervention.
A component of 300+ enzymes and 1000+ transcription factors from all six enzyme classes,4–6 zinc is involved in a diverse array of processes, from DNA synthesis, RNA transcription, growth, development, and reproduction (e.g. fetal development, testosterone secretion),7 as well as maintaining homeostasis, proper immune function, and reducing oxidative stress. Given zinc’s critical role in immune function, specifically as an anti-oxidant and anti-inflammatory agent,8–12 the potential of zinc deficiency as a risk factor for atherosclerosis and subsequent stroke has previously been posited.13
However, data directly relating zinc levels to either ischemic or hemorrhagic stroke risk are sparse. Previous preclinical studies of zinc and ischemic stroke have mainly focused on potential effects of zinc following a stroke event.14–16 Some cross-sectional studies found serum zinc levels were lower among acute ischemic or hemorrhagic stroke patients17,18 and low serum zinc was associated with increased ischemic stroke severity.19 Some studies investigating other cardiovascular outcomes found that zinc levels from diet and serum were associated with lower rates of subclinical atherosclerosis,20 hypertension,21 total cardiovascular diseases,22–24 and coronary artery disease.21,25,26
Given zinc’s role in the regulation of inflammation and oxidative stress and the impact of inflammation on atherosclerosis (and by extension stroke), the potential relation between zinc and stroke risk warrants further investigation. Therefore, this study aimed to examine the prospective association of serum zinc levels with the incidence of ischemic stroke in the US general population.
Methods
REGARDS
The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study is a national, population-based, prospective longitudinal study examining geographic and racial variation in stroke incidence and mortality in the US, as well as potential stroke risk factors. Detailed methodological and study design of REGARDS have been described in Howard et al.2 and in the Supplemental Methods. In brief, the REGARDS study cohort included 30,239 African American and Caucasian participants aged ⩾45 years. Participants were enrolled between January 2003 and October 2007. After an initial physical assessment, participants were contacted every six months to assess occurrence of stroke events. Oral and written informed consent was obtained from all participants. The REGARDS study received approval from the institutional review boards of all participating institutions.
The present study – case-cohort study design
Using a case-cohort study design, participants were randomly selected from the entire REGARDS cohort (n=30,239) to generate a sub-cohort (n=2,666). Participants included in the sub-cohort had at least 1 follow-up with a fixed sampling probability of 9% in each stratum jointly classified by age (<55, 55–64, 65–74, 75–84, and ⩾85 years), sex (male and female), race (African American and Caucasian), and stroke region residency (non-Stroke Belt, Stroke Belt, and Stroke Buckle).27,28 The sub-cohort was supplemented by 728 incident cases of ischemic stroke that were identified in the entire REGARDS study through September 2012. Thus, due to random selection, the sub-cohort also contained 78 incident cases of ischemic stroke. Participants who had stroke at baseline (n=169) or without serum zinc measurements (n=203) were excluded. Therefore, the final data set was comprised of 2,944 participants, including 2,346 participants in the random sub-cohort (62 incident cases) and 660 incident ischemic stroke cases (see Figure 1). The data that support the findings of this study are available from the corresponding author and REGARDS coordinating center upon reasonable request.
Figure 1.
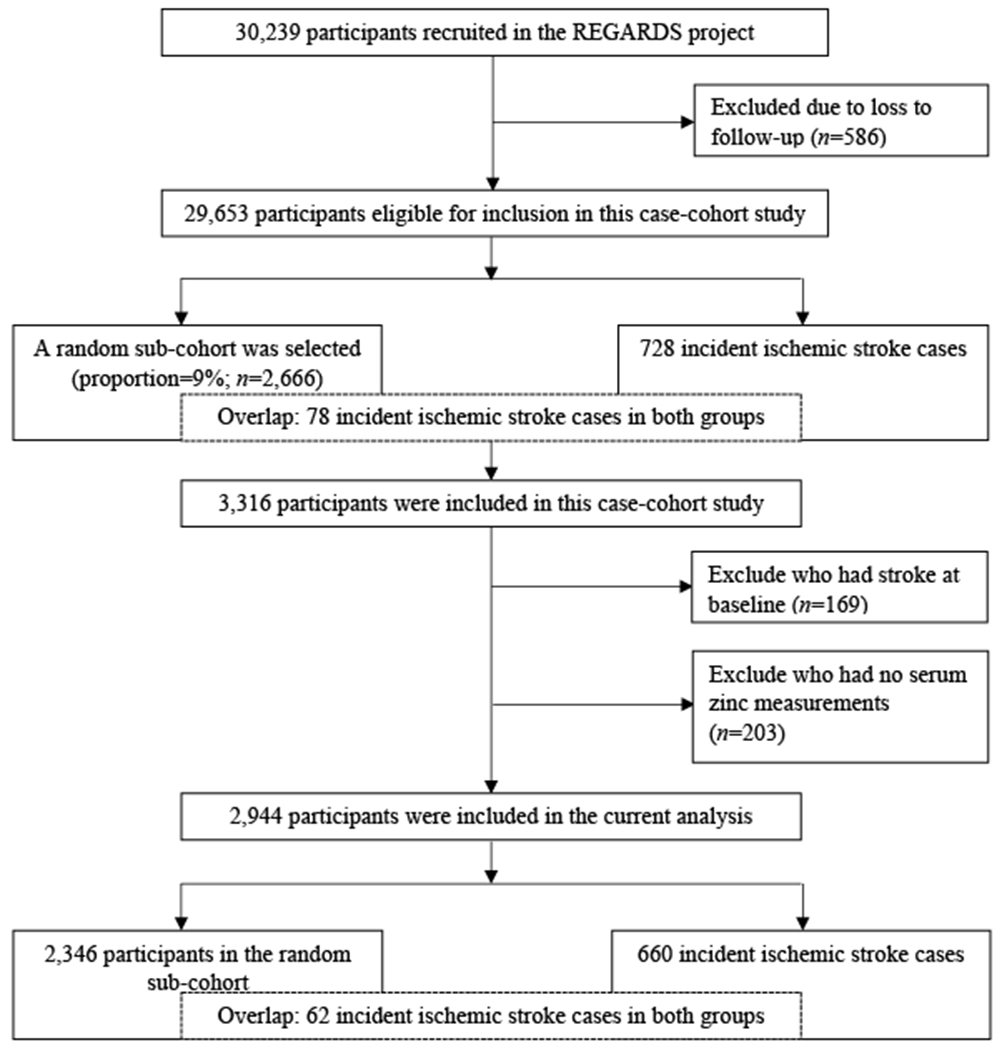
Flow chart of sub-cohort and cases sampling.
Laboratory Analyses
Fasting blood and urine samples were collected during the home visit at baseline and were centrifuged and shipped overnight with ice packs to a central laboratory at University of Vermont where they were stored at −80°C.29 Serum zinc levels were measured using a NEXION 300X quadrupole inductively coupled plasma mass spectrometer with kinetic energy discrimination to remove polyatomic interferences (ICP-MS; Perkin Elmer, Waltham, MA).30 Urinary cadmium was measured by NEXION 300X quadrupole ICP-MS (Perkin Elmer, MA, USA) operated with a dynamic reaction cell to eliminate interference from urinary molybdenum.30
Outcome, Exposure, and Covariates
Every 6 months, follow-up phone interviews were conducted to assess time to incident ischemic stroke (outcome measured). Incident ischemic stroke was adjudicated from hospital records reviewed by neurologists. Medical records were pursued for all self-reported suspected stroke and transient ischemic attack and a sub-sample of stroke symptoms. The primary exposure was serum zinc concentration. Demographic information (e.g. age, sex, race, and region) was collected through self-report during a computer assisted telephone interview. Socioeconomic and lifestyle factors as well as medical histories were also collected via computer assisted telephone interview, and included education, smoking status, alcohol consumption, physical activity, hypertension, history of myocardial infarction, history of atrial fibrillation, diabetes mellitus, and dyslipidemia. Details on these covariate measures are provided in the Supplementary Methods.
Statistical Analysis
Baseline characteristics of the incident ischemic stroke cases and of the sub-cohort were summarized. Serum zinc concentration was classified in quartiles or as a continuous variable. Quartiles of serum zinc were calculated based on the random sub-cohort. Analysis of variance, Kruskal-Wallis test, or χ2 test, as appropriate, was used to compare participants’ characteristics across quartiles of serum zinc levels in the sub-cohort.
The Barlow31 weighting method of the Cox proportional hazards regression model was used to examine the association between serum zinc levels and the incidence of ischemic stroke. Linear association was examined by using the continuous variable of zinc concentrations with values over 95th percentile (calculated based on the random sub-cohort) excluded. Multivariable-adjusted hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) using the lowest quartile of serum zinc concentration as the referent were estimated in 3 sequential models. Model 1 was adjusted for geo-demographics, including age, sex (female or male), race (African American or Caucasian), age-sex and age-race interactions, and US region (Stroke Buckle, the rest of Stroke Belt, or non-Stroke-Belt region). Model 2 was additionally adjusted for socioeconomic status and lifestyle factors that were likely associated with both zinc levels and stroke risk, including education levels (less than high school, high school, some college, or college graduates), smoking status (never, former, or current smokers), alcohol consumption (never, former, or current drinkers), physical activity (none, 1 – 3 times/week, or ⩾4 times/week), and BMI (<25.0, 25.0-29.9, or ⩾30.0 kg/m2).32,33 Model 3 was further adjusted for medical histories (hypertension, myocardial infarction, atrial fibrillation, diabetes, and dyslipidemia; yes or no) and urinary cadmium, which was found to interact with zinc on ischemic stroke risk in this cohort.30,34
Stratified analyses by pre-specified factors were performed to explore potential effect modifiers that included sex, race, and region. Interactions were examined to determine if there were significant effect modifications. Values of p ≤ 0.05 were considered statistically significant. SAS version 9.4 was used for all analysis (SAS Institute, Cary, NC).
Results
Table 1 shows baseline characteristics of the sub-cohort by quartiles of serum zinc levels. Within the random sub-cohort (n=2,346), just over half (54.7%) of participants were female and 39.9% were African American, with an average age of 64.6±9.3 years. The median zinc level for the random sub-cohort was 121.19μg/dL (interquartile range 104.86 μg/dL to 140.39 μg/dL). Participants with higher serum zinc levels were more likely to be younger, Caucasian, more physically active, and have a normal BMI. Additionally, they were less likely to have diabetes mellitus. Among ischemic stroke cases (n=660), 48.1% were female and 40.9% were African American, with an average age of 70.2±8.5 years.
Table 1.
Baseline characteristics of the study population by quartiles of serum zinc levels, the REGARDS Trace Element Study.a
| Cases | Random sub-cohort | Quartiles of serum zinc levels (μg/dL) |
P value | ||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (<105.98) | Quartile 2 (105.98-122.78) | Quartile 3 (122.78-144.59) | Quartile 4 (≥144.59) | ||||
| n (cases/sub-cohort) | 660 | 2,346 | 186/583 | 166/588 | 163/587 | 145/588 | -- |
| Median zinc level (IQR) | 118.22 (102.50-135.88) | 121.19 (104.86-140.39) | 95.85 (89.70-101.38) | 113.82 (109.82-118.17) | 131.99 (126.87-137.63) | 160.92 (151.35-176.28) | -- |
| Age (year) | 70.19±8.52 | 64.60±9.30 | 66.30±9.47 | 64.03±9.09 | 64.09±9.25 | 64.01±9.22 | <0.01 |
| Female (%) | 48.11 | 54.69 | 24.55 | 25.88 | 24.40 | 25.18 | 0.73 |
| African American (%) | 40.87 | 39.86 | 28.66 | 25.13 | 21.28 | 24.92 | <0.01 |
| Region (%) | 0.97 | ||||||
| Stroke Buckle | 21.72 | 21.27 | 25.65 | 25.25 | 24.05 | 25.05 | |
| The rest of Stroke Belt | 34.99 | 34.23 | 25.65 | 25.16 | 24.78 | 24.41 | |
| Non-Stroke-Belt region | 43.29 | 44.50 | 23.85 | 24.90 | 25.67 | 25.57 | |
| Education (%) | 0.18 | ||||||
| Less than high school | 15.38 | 11.81 | 31.41 | 25.27 | 22.74 | 20.58 | |
| High school graduate | 29.26 | 23.67 | 23.78 | 27.21 | 23.06 | 25.95 | |
| Some college | 26.24 | 27.55 | 24.61 | 23.68 | 26.32 | 25.39 | |
| College graduate | 29.11 | 36.97 | 23.64 | 24.68 | 26.07 | 25.61 | |
| Smoking status (%) | 0.26 | ||||||
| Never | 41.33 | 46.21 | 25.37 | 24.08 | 25.92 | 24.63 | |
| Former | 41.63 | 39.17 | 25.35 | 26.66 | 24.27 | 23.72 | |
| Current | 17.04 | 14.62 | 21.87 | 23.91 | 24.20 | 30.03 | |
| Alcohol consumption (%) | 0.25 | ||||||
| Never | 31.67 | 30.05 | 27.09 | 24.26 | 21.99 | 26.67 | |
| Former | 22.02 | 17.77 | 24.94 | 24.70 | 25.18 | 25.18 | |
| Current | 46.30 | 52.17 | 23.53 | 25.65 | 26.72 | 24.10 | |
| Physical activity (%) | <0.01 | ||||||
| None | 34.00 | 32.80 | 28.78 | 24.31 | 25.23 | 21.68 | |
| 1-3 times/week | 36.17 | 36.08 | 22.70 | 26.40 | 25.45 | 25.45 | |
| ≥4 times/week | 29.83 | 31.12 | 22.30 | 24.38 | 24.93 | 28.39 | |
| BMI (kg/m2, %) | 0.03 | ||||||
| <25.0 | 26.29 | 24.14 | 22.14 | 24.11 | 24.82 | 28.93 | |
| 25.0-29.9 | 39.67 | 38.06 | 24.92 | 24.24 | 26.16 | 24.69 | |
| ≥30.0 | 34.04 | 37.80 | 26.57 | 26.57 | 24.17 | 22.69 | |
| Hypertension (%) | 73.68 | 57.32 | 26.29 | 25.09 | 24.65 | 23.97 | 0.24 |
| Myocardial infarction (%) | 21.14 | 10.96 | 25.20 | 24.41 | 23.23 | 27.17 | 0.81 |
| Atrial fibrillation (%) | 14.99 | 8.57 | 27.41 | 22.34 | 25.89 | 24.37 | 0.71 |
| Diabetes (%) | 28.33 | 20.54 | 30.88 | 23.53 | 23.74 | 21.85 | <0.01 |
| Dyslipidemia (%) | 64.33 | 56.86 | 25.21 | 23.69 | 26.05 | 25.06 | 0.29 |
| Urinary cadmium (μg/g creatinine) | 0.61±0.70 | 0.53±0.45 | 0.51±0.39 | 0.56±0.56 | 0.52±0.40 | 0.54±0.40 | 0.37 |
Abbreviations: BMI, body mass index; REGARDS, REasons for Geographic and Racial Differences in Stroke.
Results are presented by means ± standard deviations, medians (inter-quartile ranges) or proportions. Quartiles of serum zinc were calculated based on the random sub-cohort (n=2,492). P values are for any difference across the quartiles of serum zinc levels in the random sub-cohort by using analysis of variance, Kruskal-Wallis test, or chi-squared test as appropriate.
Table 2 reports multivariable-adjusted hazard ratios of incident ischemic stroke by quartiles of serum zinc levels. Serum zinc levels were inversely associated with incidence of ischemic stroke after adjustment for potential confounders (quartile 4 vs. quartile 1: HR=0.78, 95% CI: 0.61-0.98, p=0.03 for trend).
Table 2.
Multivariable-adjusted HRs (95% CIs) of incident ischemic stroke by quartiles of serum zinc levels, the REGARDS Trace Element Study.a–e
| Quartiles of serum zinc levels (μg/dL) |
P for trend | ||||
|---|---|---|---|---|---|
| Quartile 1 (<105.98) | Quartile 2 (105.98-122.78) | Quartile 3 (122.78-144.59) | Quartile 4 (≥144.59) | ||
| Sub-cohort | 583 | 588 | 587 | 588 | -- |
| No. of cases | 186 | 166 | 163 | 145 | -- |
| Model 1c | 1.00 (Ref.) | 1.03 (0.83-1.27) | 0.99 (0.80-1.23) | 0.88 (0.71-1.10) | 0.12 |
| Model 2d | 1.00 (Ref.) | 0.97 (0.78-1.21) | 0.93 (0.75-1.16) | 0.85 (0.68-1.07) | 0.051 |
| Model 3e | 1.00 (Ref.) | 0.98 (0.78-1.22) | 0.93 (0.74-1.17) | 0.78 (0.61-0.98) | 0.03 |
Abbreviations: CI, confidence interval; HR, hazard ratio; No., number; Ref., referent; REGARDS, REasons for Geographic and Racial Differences in Stroke.
All models were constructed using Barlow-weighted Cox proportional hazards regression analysis. P for trend was examined by using the continuous variable of zinc concentrations with values over 95th percentile excluded.
Quartiles of serum zinc were calculated based on the random sub-cohort (n=2,346).
Model 1 was adjusted for age, sex, race, age-sex and age-race interactions, and US region.
Model 2 was additionally adjusted for education levels, smoking status, alcohol consumption, physical activity, and BMI.
Model 3 was additionally adjusted for medical histories of hypertension, myocardial infarction, atrial fibrillation, diabetes, and dyslipidemia, and urinary cadmium concentrations.
Table 3 presents associations between serum zinc levels and incident ischemic stroke stratified by pre-specified factors (sex, race, and region). Only sex showed significant effect modification (p=0.03 for interaction); the inverse association persisted among females but not males. Among females, the multivariable-adjusted HR was 0.58 comparing the highest quartile of serum zinc levels to the lowest (95% CI: 0.41-0.84, p<0.01 for trend). Among males, the multivariable-adjusted HR was 1.08 comparing the highest quartile of serum zinc levels to the lowest (95% CI: 0.78-1.51, p=0.92 for trend). Neither race nor region significantly modified the association between serum zinc levels and incidence of ischemic stroke.
Table 3.
Associations [HRs (95% CIs)] between serum zinc levels and incident ischemic stroke stratified by pre-specified factors, the REGARDS Trace Element Study.a,b
| Serum zinc levels [mean (SD)] | No. of cases | No. of participants in sub-cohort | Quartiles of serum zinc levels (μg/dL) |
P for trend | ||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 (<105.98) | Quartile 2 (105.98-122.78) | Quartile 3 (122.78-144.59) | Quartile 4 (≥144.59) | |||||
| Sex | ||||||||
| Female | 123.57 (26.37) | 318 | 1,283 | 1 (Ref.) | 0.81 (0.59-1.11) | 0.75 (0.54-1.03) | 0.58 (0.41-0.84) | <0.01 |
| Male | 124.32 (26.72) | 342 | 1,063 | 1 (Ref.) | 1.36 (0.97-1.90) | 1.26 (0.90-1.75) | 1.08 (0.78-1.51) | 0.92 |
| P for interaction | -- | -- | -- | 0.03 | ||||
| Race | ||||||||
| African American | 121.81 (26.90) | 270 | 935 | 1 (Ref.) | 1.08 (0.75-1.55) | 1.13 (0.79-1.61) | 0.74 (0.50-1.09) | 0.04 |
| Caucasian | 125.31 (26.20) | 390 | 1,411 | 1 (Ref.) | 0.91 (0.68-1.22) | 0.83 (0.62-1.12) | 0.78 (0.57-1.06) | 0.33 |
| P for interaction | -- | -- | -- | 0.35 | ||||
| Region | ||||||||
| Stroke Buckle | 124.08 (27.02) | 143 | 499 | 1 (Ref.) | 0.85 (0.50-1.46) | 0.85 (0.48-1.48) | 0.74 (0.43-1.28) | 0.41 |
| The rest of Stroke Belt | 122.98 (26.65) | 231 | 803 | 1 (Ref.) | 0.80 (0.55-1.17) | 0.83 (0.57-1.21) | 0.60 (0.39-0.91) | 0.015 |
| Non-Stroke-Belt region | 124.56 (26.19) | 286 | 1,044 | 1 (Ref.) | 1.19 (0.84-1.70) | 1.01 (0.71-1.43) | 1.00 (0.69-1.44) | 0.97 |
| P for interaction | -- | -- | -- | 0.16 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio; No., number; Ref., referent; REGARDS, REasons for Geographic and Racial Differences in Stroke; SD, standard deviation.
All models were constructed using Barlow-weighted Cox proportional hazards regression analysis with adjustment for covariates in model 3 Table 2. P for trend and P for interaction were examined by using the continuous variable of zinc concentrations with values over 95th percentile excluded.
Quartiles of serum zinc levels were calculated based on the random sub-cohort (n=2,346).
Discussion
Our findings from this case-cohort study support a significant inverse association between serum zinc levels and incidence of ischemic stroke in the US general population. This inverse association was attenuated among men, but persisted among women.
The wide distribution of zinc in the soil may contribute to the serum zinc variation observed in the US population.34 However, insufficient zinc intake is a common issue among the elderly. Previous studies found approximately half of the older adults had adequate dietary zinc intake.35,36 Belbraouet et al.37 defined risk of zinc deficiency as serum zinc levels <0.70 mg/L (=70 μg/dL), while Miyata38 used 84-159 μg/dL as the normal adult range of serum zinc. The median zinc level for this random sub-cohort was 121.19 μg/dL, falling within the normal/adequate range for adult serum zinc levels. Still, findings in this study suggest high zinc levels may protect against the risk of ischemic stroke.
To the best of our knowledge, the present study is the first prospective study to assess the association between serum zinc levels and ischemic stroke incidence. The limited previous studies have mainly focused on exploring potential effects of zinc after a stroke event.14–16 Two cross-sectional studies found serum zinc levels were significantly lower among acute ischemic stroke patients compared with controls17 and low serum zinc was associated with increased stroke severity among acute ischemic stroke patients.19 Evidence from studies that examined the association between serum zinc and other cardiovascular outcomes is in concordance with our findings. Dietary zinc levels were inversely associated with hypertension21 and subclinical atherosclerosis.20 In addition, zinc levels from both serum and diet were consistently associated with lower cardiovascular disease/coronary artery disease risk or mortality in most of the previous studies.21–24
Infection and inflammation are important contributing factors for stroke development among other traditional stroke risk factors and genetic susceptibility.39 Notably, zinc plays an essential role in immune function, 40,41 acting as an anti-oxidant and anti-inflammatory agent.8–12 Thus, zinc may have a protective effect in reducing inflammation and oxidative stress.42 For some chronic diseases (e.g. atherosclerosis and subsequent stroke), contemporaneous zinc deficiency may increase the production of inflammatory cytokines7 and increase the susceptibility of endothelial cells to the damages of oxidative stress.13 Therefore, zinc may be involved in the pathophysiology of stroke, with increased zinc levels being protective against stroke.
In this study, we found a significant sex-zinc interaction; the inverse association between serum zinc levels and ischemic stroke risk is more pronounced in women than men, which may be one explanation for disparities in stroke rates by sex. Similar to other studies,43 the serum zinc levels were not substantially different in women and men in this population. In a previous report of REGARDS, Howard et al.44 examined ischemic stroke risk by sex. Among participants younger than 75 years of age, women generally had a lower stroke risk compared with men. However, this pattern was not observed for oldest age group (75+ years). These findings suggest the possibility that the differential effects of zinc might be explained by its critical function in the metabolism of sex hormones and reproductive cycle, which are involved in the pathways protecting against cerebrovascular disease.45 Through its structural and function roles in certain proteins, zinc finger proteins are commonly involved in genetic regulation and expression, including nuclear hormone receptors.46,47 Some previous studies found Zinc Finger Protein 202 gene was associated with ischemic heart disease and myocardial infarction in women, but not in men.48 Therefore, zinc may better protect against stroke in women, but the mechanism underlying the possible interaction between zinc and sex is still unclear and warrants further investigation. Given the previous literature is limited and this study is the first one on this topic, the findings observed here provide data for future investigation.
Our study was limited by using serum zinc measurement at baseline only, allowing for possible within-person variation to affect our findings. However, zinc concentrations in blood are generally stable in humans despite small changes in zinc intake.49 There is also no evidence that the within-person variation in serum zinc is differential in this population. Thus, our findings based on the baseline zinc exposure should not be substantially biased. In addition, several compounds and elements may enhance or inhibit zinc absorption. Phytate from food sources (e.g. cereals), iron given in supplement form, and cadmium can inhibit zinc absorption, while some proteins from animal meat enhance zinc absorption.50–52 Although we did not have data on all of these nutrients or contaminants, we adjusted for urinary level of cadmium, which has been found to interact with zinc on ischemic stroke in this cohort. Moreover, similar to other observational studies, the residual confounding from dietary, environmental, and other social factors cannot be completely ruled out. The adjusted lifestyle factors and medical histories were self-reported, which may introduce reporting bias in these covariates. Nevertheless, our final model was adjusted for a comprehensive list of important factors shown in literature, and the results were generally consistent. Finally, we were not able to stratify the analyses by subtypes of ischemic stroke. Among the ischemic stroke cases in this study, about half of them were unknown or other subtypes that were not defined. The rest of the study sample was largely reduced, thereby not allowing for the examination of the association of interest for each subtype.
Of note, ischemic and hemorrhagic strokes have different pathophysiology and should be investigated separately, but most previous studies are not able to separate them due to lack of information on stroke types. Thus, the present study only focused on ischemic stroke as the outcome. Additionally, studies have suggested that serum zinc is an objective measurement of zinc levels that reflects long-term exposure and total body burden compared to dietary zinc that is prone to measurement errors. The use of serum zinc as well as adjudication of stroke substantially reduce the possibility of measurement errors and misclassification, which is crucial for us to understand the health impact of zinc exposure in the general population. Another strength of the present study is that it is based on a large sample of African American and Caucasian men and women from across the continental US, which facilitates the exploration of potential effect modifiers and improves generalizability.
In conclusion, this national, population-based, prospective longitudinal study shows an inverse association between serum zinc concentration and incidence of ischemic stroke, particularly, in women. Maintaining appropriately high zinc levels is of great clinical and public health significance. Further studies are needed to establish causal inference and better understand the mechanisms of action.
Supplementary Material
Acknowledgments
The authors thank the other investigators, staff, and participants of the REGARDS project for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Sources of Funding
This work was supported by the National Institutes of Health [grant number R01ES021735]. The REGARDS research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and Department of Health and Human Service. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Non-standard Abbreviations and Acronyms
- REGARDS
REasons for Geographic and Racial Differences in Stroke
Footnotes
Publisher's Disclaimer: Disclaimer
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, the National Institutes of Health, or the National Institute for Occupational Safety and Health.
Disclosures
No conflict of interest to report.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Howard VJ, Katholi C, Oli MK, Huston S. Decline in US stroke mortality: an analysis of temporal patterns by sex, race, and geographic region. Stroke. 2001;32(10):2213–2220. doi: 10.1161/hs1001.096047 [DOI] [PubMed] [Google Scholar]
- 4.McCall KA, Huang C, Fierke CA. Function and mechanism of zinc metalloenzymes. J Nutr. 2000;130(5):1437S–1446S. doi: 10.1093/jn/130.5.1437S [DOI] [PubMed] [Google Scholar]
- 5.Prasad AS. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr. 2009;28(3):257–265. doi: 10.1080/07315724.2009.10719780 [DOI] [PubMed] [Google Scholar]
- 6.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73(1):79–118. [DOI] [PubMed] [Google Scholar]
- 7.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86(4):521–534. doi: 10.1007/s00204-011-0775-1 [DOI] [PubMed] [Google Scholar]
- 8.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8(3):281–291. doi: 10.1016/0891-5849(90)90076-U [DOI] [PubMed] [Google Scholar]
- 9.Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr. 1999;18(2):152–158. doi: 10.1080/07315724.1999.10718843 [DOI] [PubMed] [Google Scholar]
- 10.Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130(5):1447S–1454S. doi: 10.1093/jn/130.5.1447S [DOI] [PubMed] [Google Scholar]
- 11.Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43(5):370–377. doi: 10.1016/j.exger.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 12.Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol. 2012;26(2-3):66–69. doi: 10.1016/j.jtemb.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 13.Beattie JH, Kwun I-S. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr. 2004;91(2):177–181. doi: 10.1079/BJN20031072 [DOI] [PubMed] [Google Scholar]
- 14.Galasso SL, Dyck RH. The role of zinc in cerebral ischemia. Mol Med. 2007;13(7-8):380. doi: 10.2119/2007-00044.Galasso [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shabanzadeh A, Shuaib A, Yang T, Salam A, Wang CX. Effect of zinc in ischemic brain injury in an embolic model of stroke in rats. Neurosci Lett. 2004;356(1):69–71. doi: 10.1016/j.neulet.2003.10.073 [DOI] [PubMed] [Google Scholar]
- 16.Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852(2):268–273. doi: 10.1016/S0006-8993(99)02095-8 [DOI] [PubMed] [Google Scholar]
- 17.Munshi A, Babu S, Kaul S, Shafi G, Rajeshwar K, Alladi S, Jyothy A. Depletion of serum zinc in ischemic stroke patients. Methods Find Exp Clin Pharmacol. 2010;32(6):433. doi: 10.1358/mf.2010.32.6.1487084 [DOI] [PubMed] [Google Scholar]
- 18.Karadas S, Sayın R, Aslan M, Gonullu H, Kati C, Dursun R, Duran L, Gonullu E, Demir H. Serum levels of trace elements and heavy metals in patients with acute hemorrhagic stroke. J Membr Biol. 2014;247(2):175–180. doi: 10.1007/s00232-013-9621-0 [DOI] [PubMed] [Google Scholar]
- 19.Bhatt A, Farooq MU, Enduri S, Pillainayagam C, Naravetla B, Razak A, Safdar A, Hussain S, Kassab M, Majid A. Clinical significance of serum zinc levels in cerebral ischemia. Stroke Res Treat. 2010;2010. doi: 10.4061/2010/245715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YJ, Choi BY, Chun B-Y, Kweon S-S, Lee Y-H, Park PS, Kim MK. Dietary zinc intake is inversely related to subclinical atherosclerosis measured by carotid intima-media thickness. Br J Nutr. 2010;104(8):1202–1211. doi: 10.1017/S0007114510001893 [DOI] [PubMed] [Google Scholar]
- 21.Singh RB, Niaz MA, Rastogi SS, Bajaj S, Gaoli Z, Shoumin Z. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr. 1998;17(6):564–570. doi: 10.1080/07315724.1998.10718804 [DOI] [PubMed] [Google Scholar]
- 22.Chu A, Foster M, Samman S. Zinc Status and risk of cardiovascular diseases and type 2 diabetes mellitus—a systematic review of prospective cohort studies. Nutrients. 2016;8(11):707. doi: 10.3390/nu8110707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D-H, Folsom AR, Jacobs DR Jr. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women’s Health Study. Am J Clin Nutr. 2005;81(4):787–791. doi: 10.1093/ajcn/81.4.787 [DOI] [PubMed] [Google Scholar]
- 24.Reunanen A, Knekt P, Marniemi J, Mäki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996;50(7):431–437. [PubMed] [Google Scholar]
- 25.Eshak ES, Iso H, Yamagishi K, Maruyama K, Umesawa M, Tamakoshi A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J Nutr Biochem. 2018;56:126–132. doi: 10.1016/j.jnutbio.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 26.Soinio M, Marniemi J, Laakso M, Pyörälä K, Lehto S, Rönnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care. 2007;30(3):523–528. doi: 10.2337/dc06-1682 [DOI] [PubMed] [Google Scholar]
- 27.Cai J, Zeng D. Sample size/power calculation for case–cohort studies. Biometrics. 2004;60(4):1015–1024. doi: 10.1111/j.0006-341X.2004.00257.x [DOI] [PubMed] [Google Scholar]
- 28.Cushman M, Judd SE, Howard VJ, Kissela B, Gutiérrez OM, Jenny NS, Ahmed A, Thacker EL, Zakai NA. N-Terminal Pro–B-type Natriuretic Peptide and Stroke Risk: The Reasons for Geographic and Racial Differences in Stroke Cohort. Stroke. 2014;45(6):1646–1650. doi: 10.1161/STROKEAHA.114.004712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabagambe EK, Judd SE, Howard VJ, Zakai NA, Jenny NS, Hsieh M, Warnock DG, Cushman M. Inflammation biomarkers and risk of all-cause mortality in the reasons for geographic and racial differences in stroke cohort. Am J Epidemiol. 2011;174(3):284–292. doi: 10.1093/aje/kwr085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, Xun P, Tsinovoi C, McClure LA, Brockman J, MacDonald L, Cushman M, Cai Jianwen, Kamendulis L, Mackey J, et al. Urinary cadmium concentration and the risk of ischemic stroke. Neurology. Published online 2018:10–1212. doi: 10.1212/WNL.0000000000005856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. Published online 1994:1064–1072. doi: 10.2307/2533444 [DOI] [PubMed] [Google Scholar]
- 32.Mares-Perlman JA, Subar AF, Block G, Greger JL, Luby MH. Zinc intake and sources in the US adult population: 1976-1980. J Am Coll Nutr. 1995;14(4):349–357. [DOI] [PubMed] [Google Scholar]
- 33.Unkiewicz-Winiarczyk A, Bagniuk A, Gromysz-Kałkowska K, Szubartowska E. Calcium, magnesium, iron, zinc and copper concentration in the hair of tobacco smokers. Biol Trace Elem Res. 2009;128(2):152–160. [DOI] [PubMed] [Google Scholar]
- 34.White J, Welch R, Norvell W. Soil zinc map of the USA using geostatistics and geographic information systems. Soil Sci Soc Am J. 1997;61(1):185–194. [Google Scholar]
- 35.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the US population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. J Nutr. 2000;130(5):1367S–1373S. doi: 10.1093/jn/130.5.1367S [DOI] [PubMed] [Google Scholar]
- 36.Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr. 2002;132(11):3422–3427. doi: 10.1093/jn/132.11.3422 [DOI] [PubMed] [Google Scholar]
- 37.Belbraouet S, Biaudet H, Tébi A, Chau N, Gray-Donald K, Debry G. Serum zinc and copper status in hospitalized vs. healthy elderly subjects. J Am Coll Nutr. 2007;26(6):650–654. doi: 10.1080/07315724.2007.10719643 [DOI] [PubMed] [Google Scholar]
- 38.Miyata S [Zinc deficiency in the elderly]. Nihon Ronen Igakkai Zasshi Jpn J Geriatr. 2007;44(6):677–689. [PubMed] [Google Scholar]
- 39.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34(10):2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC [DOI] [PubMed] [Google Scholar]
- 40.Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14(4):277–285. doi: 10.1016/j.autrev.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 41.Maares M, Haase H. Zinc and immunity: An essential interrelation. Arch Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 42.Bao B, Prasad AS, Beck FW, Fitzgerald JT, Snell D, Bao GW, Singh T, Cardozo LJ. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010;91(6):1634–1641. doi: 10.3945/ajcn.2009.28836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buxaderas S, Farré-Rovira R. Whole blood and serum zinc levels in relation to sex and age. Rev Esp Fisiol. 1985;41(4):463–470. [PubMed] [Google Scholar]
- 44.Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA. Sex and Race Differences in the Association of Incident Ischemic Stroke With Risk Factors. JAMA Neurol. 2019;76(2):179–186. doi: 10.1001/jamaneurol.2018.3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurn PD, Brass LM. Estrogen and stroke: a balanced analysis. Stroke. 2003;34(2):338–341. doi: 10.1161/01.STR.0000054051.88378.25 [DOI] [PubMed] [Google Scholar]
- 46.Prasad AS. Zinc: an overview. Nutrition. 1995;11(1 Suppl):93–99. [PubMed] [Google Scholar]
- 47.Favier AE. The role of zinc in reproduction. Biol Trace Elem Res. 1992;32(1-3):363–382. [DOI] [PubMed] [Google Scholar]
- 48.Stene MC, Frikke-Schmidt R, Nordestgaard BG, Steffensen R, Schnohr P, Tybjærg-Hansen A. Zinc Finger Protein 202: a new candidate gene for ischemic heart disease: The Copenhagen City Heart Study. Atherosclerosis. 2006;188(1):43–50. doi: 10.1016/j.atherosclerosis.2005.10.014 [DOI] [PubMed] [Google Scholar]
- 49.Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US); 2001. https://www.ncbi.nlm.nih.gov/books/NBK222310/ [PubMed] [Google Scholar]
- 50.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130(5):1374S–1377S. doi: 10.1093/jn/130.5.1374S [DOI] [PubMed] [Google Scholar]
- 51.Lonnerdal B Dietary factors influencing zinc absorption. J Nutr. 2000;130(5):1378S–1383S. doi: 10.1093/jn/130.5.1378S [DOI] [PubMed] [Google Scholar]
- 52.Sandstrom B Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr. 2001;85(S2):S181–S185. doi: 10.1049/BJN2000312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


