SUMMARY
In mammals, changes in weight elicit responses that favor a return to one’s previous weight and promote weight stability. It has been hypothesized that palatable sweet and high-fat foods disturb the defense of body weight, leading to weight gain. We find that increasing sweetness or percent calories from fat increases diet palatability but that only increases in nutritive fat content increase caloric intake and body weight. In a mouse model of overfeeding that activates weight defense, high-fat diets, but not sweetened diets, attenuate the defense of body weight, leading to weight gain. The ability of a palatable, high-fat diet to increase food intake does not require tasting or smelling the food. Instead, the direct infusion of a high-fat diet into the stomach increases the ad libitum intake of less palatable, low-fat food. Post-oral sensing of percent calories from fat modulates feeding behavior to alter weight stability.
In brief
Gallop et al. find that increasing fat concentration, but not sweetness, attenuates defense against weight gain. Orexigenic effects of high-fat diet (HFD) occur when mice neither taste nor smell the diet. Infusing HFD into the stomach increases caloric intake, suggesting that post-oral detection of high-fat content drives increased consumption.
Graphical Abstract
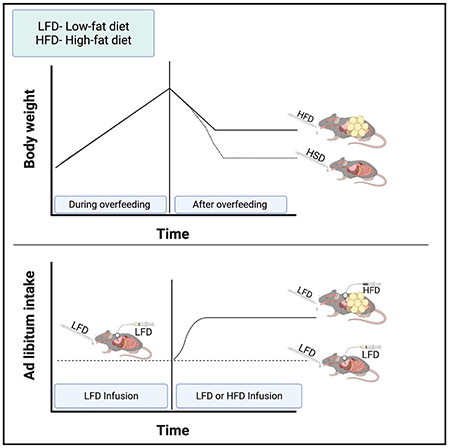
INTRODUCTION
Over the course of a typical year, human body weight remains largely stable despite day-to-day variation in caloric intake and activity (Jéquier and Tappy, 1999; Passmore, 1971, 1982). This stability occurs even in the absence of efforts to monitor or maintain a specific body mass and depends upon neuroendocrine responses that promote a return to one’s initial weight when weight is reduced or increased (Finkelstein et al., 2012; Leibel et al., 1995; Levin and Dunn-Meynell, 2000; Ravussin et al., 2014, 2018; Schwartz et al., 2017). Following successful weight loss, an increase in hunger and a reduction in energy expenditure favor weight gain and contribute to low rates of long-term success in maintaining weight loss (Fothergill et al., 2016; Keys et al., 1945; Leibel et al., 1995; Heymsfield and Wadden, 2017; Penney and Kirk, 2015; Saeidi et al., 2012). With the identification of leptin a quarter century ago, a key component of the neuroregulatory system that defends against weight loss and promotes long-term weight stability was discovered (Ahima et al., 1996; Myers et al., 2010; Zhang et al., 1994). When individuals intentionally lose weight, a reduction in fat mass lowers circulating leptin concentration that is sensed by several populations of neurons, including ones in the hypothalamus and nucleus of the solitary tract (Fothergill et al., 2016; Ravussin et al., 2014; Schwartz et al., 2000). These leptin-responsive neurons regulate the activity of circuits that increase hunger and reduce energy expenditure, thus favoring weight gain and restoration (Rosenbaum et al., 2010; Schwartz et al., 2017).
An opposite response occurs when an individual intentionally gains weight. Overfeeding and forced weight gain in mammals reduce hunger and increase energy expenditure, leading to a rapid return to one’s previous body mass (Ernersson et al., 2010; Jen and Hansen, 1984; Ravussin et al., 2018; Rosenbaum et al., 2003; White et al., 2010). The precision to which individuals return to their previous weight after overfeeding is comparable to and mirrors the weight gain response seen after weight loss. However, this response is much less well studied, with key signals and neuronal populations largely unknown (Flier and Maratos-Flier, 2017; Ravussin et al., 2014, 2018) Although leptin is clearly the signal that defends against weight loss, and its absence leads to a syndrome of severe obesity; studies in rodents reveal that an increased concentration of leptin is not the signal that mediates the response to weight gain (Ravussin et al., 2018; White et al., 2010). Mice defend against weight gain even if the circulating leptin concentration is artificially fixed as fat mass expands (Ravussin et al., 2018).
The long-term signals that indicate the size of fat stores are not the only cues that regulate energy balance and feeding behavior. Gastrointestinal (GI)-derived hormones and autonomic signals that sense distension and caloric content activate populations of neurons that rapidly modulate feeding behavior. Direct infusion of nutrients into the GI tract activates these sensing systems in the stomach and small intestine that, within minutes, relay the presence of nutrients to centers to reduce food intake (Ferreira et al., 2012; Glendinning et al., 2012; Goldstein et al., 2021; Su et al., 2017). These rapid anorectic responses are initiated by distinct regions of the small intestine that sense specific macronutrients, e.g., triglycerides in the jejunum (Goldstein et al., 2021; Kaelberer et al., 2018). They are thought to regulate the rate of food intake and have been harnessed in the development of anti-obesity therapies. For example, glucagon-like peptide 1 (GLP-1) produced by L-cells in the small intestine is released when calories are sensed, and its actions rapidly reduce food intake (Baggio and Drucker, 2007; Barrera et al., 2011). Chronic activation of GLP-1 receptors through pharmacologic agonists can counter the weight stability mechanisms and has proven to be a potent weight-loss strategy, leading to the approval of several agonists as weight-loss medications (Grill, 2020; Williams et al., 2020).
Despite systems that limit weight gain, there has been a steady increase in mean body mass index and the prevalence of obesity throughout much of the world during the past three decades. In the United States, most adults experience a slow (~0.5 kg) increase in weight per year during adulthood (Ball et al., 2002; Matsushita et al., 2009). Although at odds with a simple model of body weight stability, the slow yearly increase has been attributed to “drift” (Speakman, 2007) that is caused, in part, by the availability of highly palatable and calorically dense foods (Alkerwi et al., 2015; Hall et al., 2019; Johnson and Wardle, 2014; Mendonça et al., 2016; Zheng et al., 2009). By analogy to the anorectic effect of the GLP-1 receptor agonist attenuating the response to reduced weight, many have hypothesized that palatable foods provide a chronic reward stimulus that attenuates the defense against weight gain. Consistent with this model, in most strains of mice and rats, ad libitum access to palatable diets increases caloric intake, leading to weight gain (Berthoud and Zheng, 2012; Corbit and Stellar, 1964; Denis et al., 2015; Levin and Dunn-Meynell, 2002).
But, in addition to the oral sensing of components that contributes to palatability, mice can sense carbohydrate or fat content by post-oral mechanisms. An infusion of sucrose or fat directly into the stomach, thereby bypassing orophyrangeal sensing of taste and smell, can condition a preference in response to macronutrients (Ackroff and Sclafani, 2014; Cassie et al., 2019; Sclafani, 2001; Su et al., 2017; Tan et al., 2020; Zukerman et al., 2011, 2013). Although the ability to taste fat has been debated, the oropharyngeal sensing of fat, i.e., smell and texture, has been implicated in the ability of palatable high-fat foods to increase long-term food intake and body weight in rodents and humans (Ackroff and Sclafani, 2014; Corbit and Stellar, 1964; Massiera et al., 2010). However, these studies have largely considered short-term responses, i.e., conditioned licking rather than long-term feeding and weight effects. In addition, a confounding factor in these studies of post-oral signaling is the lack of a control for calories; much of the previous work on post-ingestive signaling uses water, rather than a substance of equal caloric density, as the control infusion. More recent studies in mice have raised the possibility that specific lipids are sensed in the small intestine and their presence relayed by vagal afferents to the NTS to increase food intake (Ferreira et al., 2012; Goldstein et al., 2021; Kleberg et al., 2015; Tan et al., 2020).
To understand whether access to palatable foods attenuates the system that defends against weight gain, we used an overfeeding mouse model of weight defense (Ravussin et al., 2018). All diets had the same caloric density (1 kcal/mL), but we varied the percentage of calories derived from sucrose or fat to modulate palatability (food preference). We tested whether more palatable diets increased food intake and body weight and whether palatable diets would attenuate various components of the system that defend against weight gain. For diets whose availability did attenuate the weight defense, we tested whether bypassing oropharyngeal sensing would eliminate this response.
RESULTS
Sweetening diets low in fat content increases preference but not caloric intake
Palatability is the relative pleasure provided by a food, and in rodent studies, it is typically measured as the preference of one food over another. In clinical studies, foods with a high density of simple, sweet carbohydrates are typically more palatable and preferred to foods with low carbohydrate content (Bellisle, 2015; Drewnowski et al., 2012; Han et al., 2020). Similarly, mice of most strains prefer sweetened foods to non-sweetened diets (Bachmanov et al., 2001; Glendinning et al., 2012; Pinhas et al., 2012; Sclafani, 2006). We assessed the ability of non-caloric sweeteners and sucrose to increase the preference of a variety of diets, including bovine-milk-based diets and commercially available enteral and oral diets. In this study and all studies in the experiment, diets that were directly compared had the same caloric density, which in the majority of studies was 1 kcal/mL. This design permitted us to assess the effect of fat, carbohydrate, and protein density on preference and intake without confounders related to total caloric density or volume. We also found that liquid diets permitted greater precision for measuring consumption. Nutritional breakdown and caloric density are shown in Table S1. In a preliminary set of feeding studies, we determined the concentrations of the non-nutritive sweeteners sucralose and saccharin that were most palatable (Figure S1, and not shown). In preference tests, sucrose (30% kcals) increased the palatability of every diet tested, while non-nutritive sweeteners only increased the palatability of some diets and never as much as 30% kcals of sucrose (Figure 1; Figure S1).
Figure 1. Sweetened diets are more palatable than unsweetened diets but do not increase caloric intake or body weight.
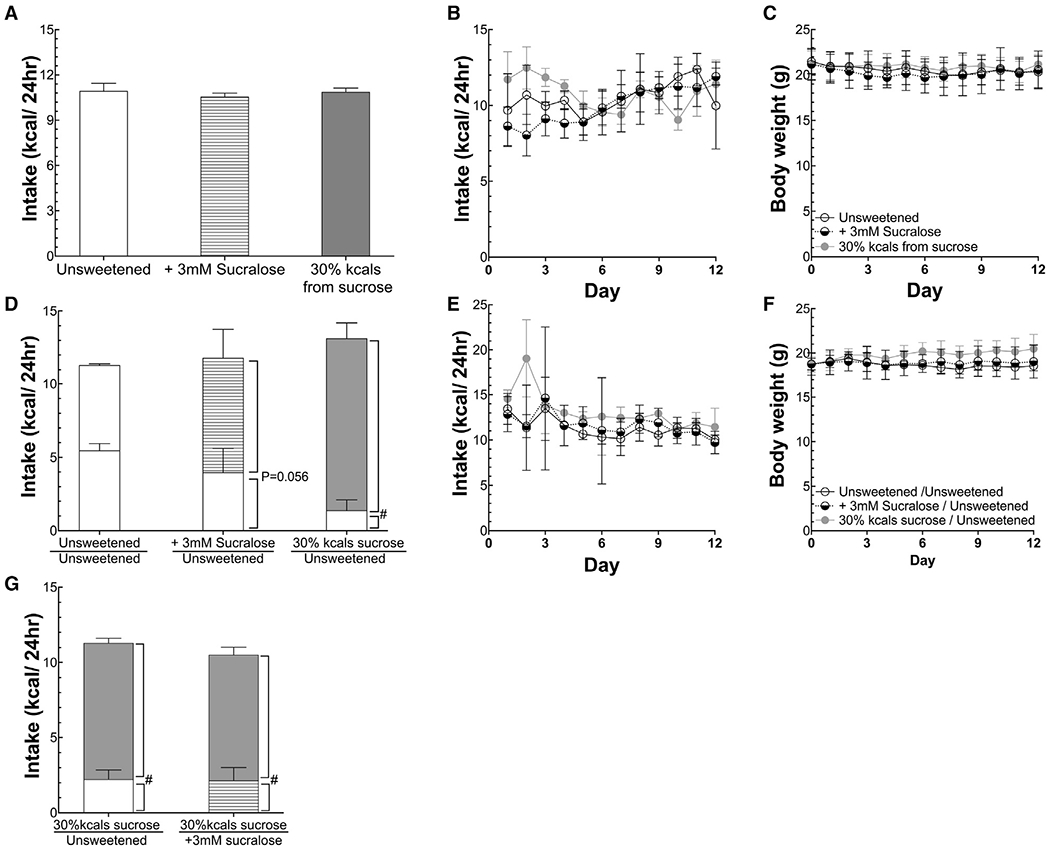
(A and B) Mean and daily caloric intake of mice provided sweetened and unsweetened diets ad libitum.
(C) Body weight of mice in (A) and (B).
(D) Mean caloric intake broken down by diet of mice simultaneously provided ad libitum both a sweetened and unsweetened diet. Mice offered only an unsweetened diet by two feeders served as controls.
(E and F) Daily total caloric intake and body weight of mice in (D).
(G) Mean caloric intake of mice simultaneously provided an unsweetened and sucrose-sweetened diet, and mean caloric intake of mice simultaneously provided dietssweetened with sucrose or the non-nutritive sweetener sucralose. Data are means ± standard deviation. #p < 0.05, comparing intake during the two-choice test. A paired t test was used for within-group analyses, and ANOVA was used for comparisons among groups. n = 5/group. The unshaded bars represent unsweetened intake; striped, gray bars represent sucralose-sweetened diet; and dark-gray bars represents 30% kcal sucrose diet.
In all the cases tested, when diets were made more palatable by sweetening, either with sucrose or a non-nutritive sweetener, mice did not increase caloric intake over 12 days of study (Figure 1; Figure S1); this finding is in contrast to the ability of sucrose or non-nutritive sweeteners to increase water intake several-fold (Figure S2). Consistent with no increase in caloric intake, there was no difference in the body mass of mice offered base diets or sweetened diets (Figure 1).
Percent calories from fat rather than taste preference regulates caloric intake
Previous studies have found that fat content alters the palatability of food and in rodents modulates food intake and body weight (Bray et al., 2004; Corbit and Stellar, 1964; Sclafani and Ackroff, 2018a, 2018b). However, most rodent studies have not controlled for overall caloric density. To test whether increasing the percentage of calories derived from fat while maintaining caloric density would alter diet preference and calorie intake, we compared the preference and intake of diets in which calories derived from fat varied from 10% to 60% while caloric density was constant (1 kcal/mL). Using a dairy-based diet, we compared the preference of mice for high-fat diet (HFD) and low-fat diet (LFD), which derived 60% or 10%, respectively, of their calories from milk fat (Table S1). During a two-choice test when mice were offered both a HFD and LFD, mice preferred the HFD (Figure 2A; 12.5 ± 0.8 kcals versus 1.6 ± 0.9 kcals, p < 0.0001). When the total caloric density is fixed, increasing the percent calories from fat, by necessity, lowers the density of the remaining macronutrients. To determine whether the preference for HFD versus LFD was driven by lower protein density, we provided mice with access to HFD and LFD with the same protein density. The protein difference was not driving the preference for a HFD over a LFD; mice still preferred a HFD to the LFD when protein content was held constant (Figure 2G, HFD versus LFD, 13.7 ± 3.5 kcals versus 4.4 ± 2.9 kcals; p = 0.012). Additionally, when the protein content was greater in the HFD than that in the LFD, the HFD was still preferred (Figure 2H; 13.3. ± 2.9 versus 3.4 ± 1.6 kcals, p < 0.001).
Figure 2. Increasing percent calories from fat increases palatability and intake independent of protein or carbohydrate density.
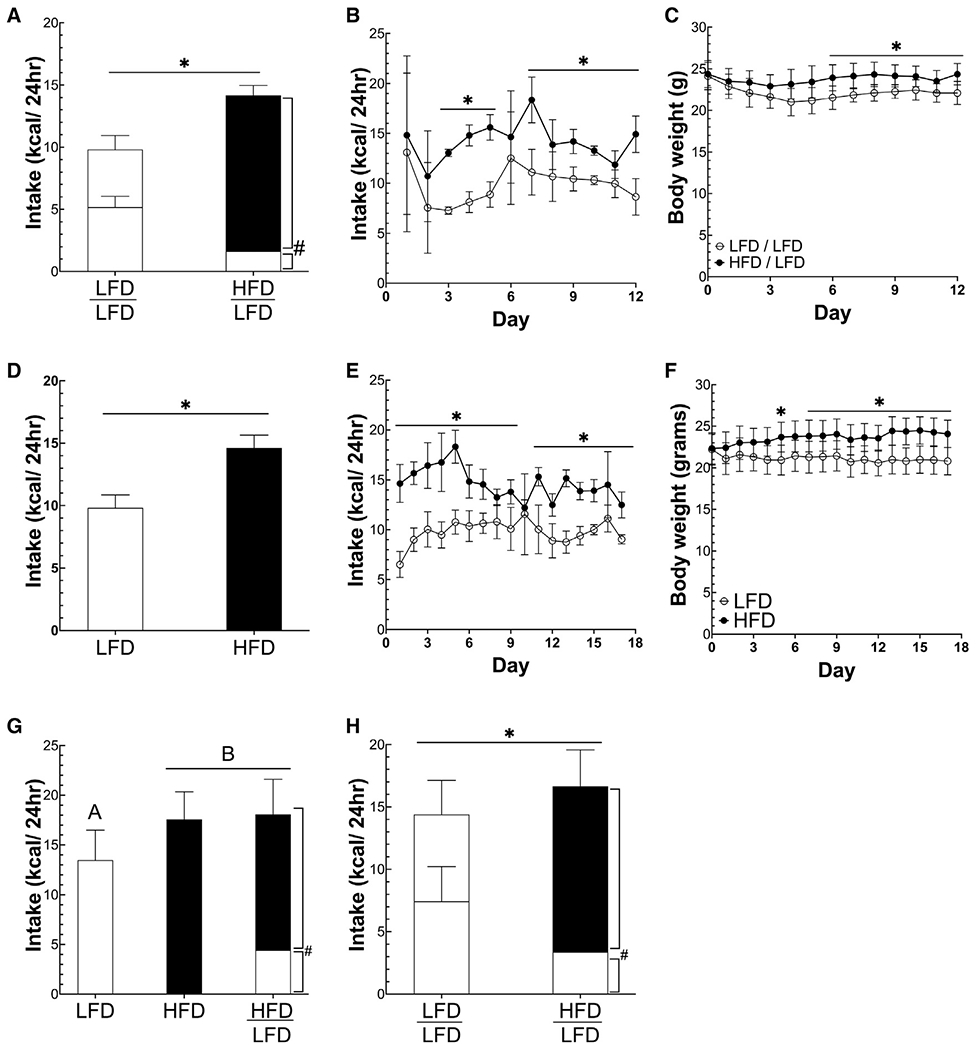
(A and B) Mean and daily caloric intake of mice offered either a low-fat diet (LFD; 10% of calories from fat) or a high-fat diet (HFD; 60% of calories from fat).
(C) Daily body weight of mice offered either a LFD (10% of calories from fat) or a HFD (60% of calories from fat).
(D and E) Mean and daily caloric intake of mice offered both a LFD and HFD or LFD.
(F) Daily body weight of mice offered both a LFD and HFD or only LFD.
(G) Caloric intake of mice offered either a LFD, HFD, or both HFD and LFD in which carbohydrate content was equal.
(H) Caloric intake of mice offered both a HFD and LFD or only a LFD in which protein content was equal. *p < 0.05 between groups using Student’s t test, #p < 0.05 for diet within-group analyses using a paired t test. Different letters represent significant differences calculated using ANOVA to compare three groups. All data are presented as mean ± standard deviation. n = 5/group. Unshaded bars show LFD intake, and black bars represent HFD intake.
Although we found that increasing the palatability of diets by increasing sweetness did not affect intake or weight over a 2-week period, increasing percent calories from fat did. When mice were offered either a HFD or LFD, mice offered the HFD consumed 49% more calories than mice offered the LFD (14.6 ± 1.0 kcals versus 9.8 ± 1.0 kcals, p < 0.0001) and weighed 16% more (24.1 ± 1.6 g versus 20.8 ± 1.6 g, p < 0.05) (Figures 2D–2F). Although both groups appear to initially lose weight, we have observed that weight loss can occur when mice are switched from a solid diet to a liquid, such as at the beginning of a study. Presumably, this weight loss is due to a decrease in intestinal contents, and the HFD seems to protect against this initial loss by increasing intake, causing HFD-fed mice to weigh more than LFD-fed mice. When mice were offered both a LFD and a HFD, they divided their food intake between the LFD and HFD, but the total caloric intake matched the intake of mice only offered the HFD. This result occurred in diets containing both dairy-derived (Figures 2A–2F) and plant-derived fats (Figures 2G and 2H).
Absorbable percent calories from fat rather than dietary preference regulates caloric intake
Although increasing the sweetness of a LFD did not increase caloric intake, we tested whether sweetening a HFD would further increase its palatability and lead to higher ad libitum caloric intake. We found that a sucralose-sweetened HFD was preferred to an unsweetened HFD (8.8 ± 0.44 versus 4.1 ± 0.62 kcal/day, p = 0.022), but total caloric intake did not increase (Figure 3A). All groups offered at least one choice of a HFD consumed significantly more than mice offered only LFD choices (Figure 3A), and all mice with access to a HFD consumed a similar number of calories (Figures 3A, 3B, and S3). Consistent with increased caloric intake, all groups offered at least one choice of a HFD weighed more than mice that only had access to LFDs.
Figure 3. Density of digestible fat rather than taste preference regulates caloric intake.
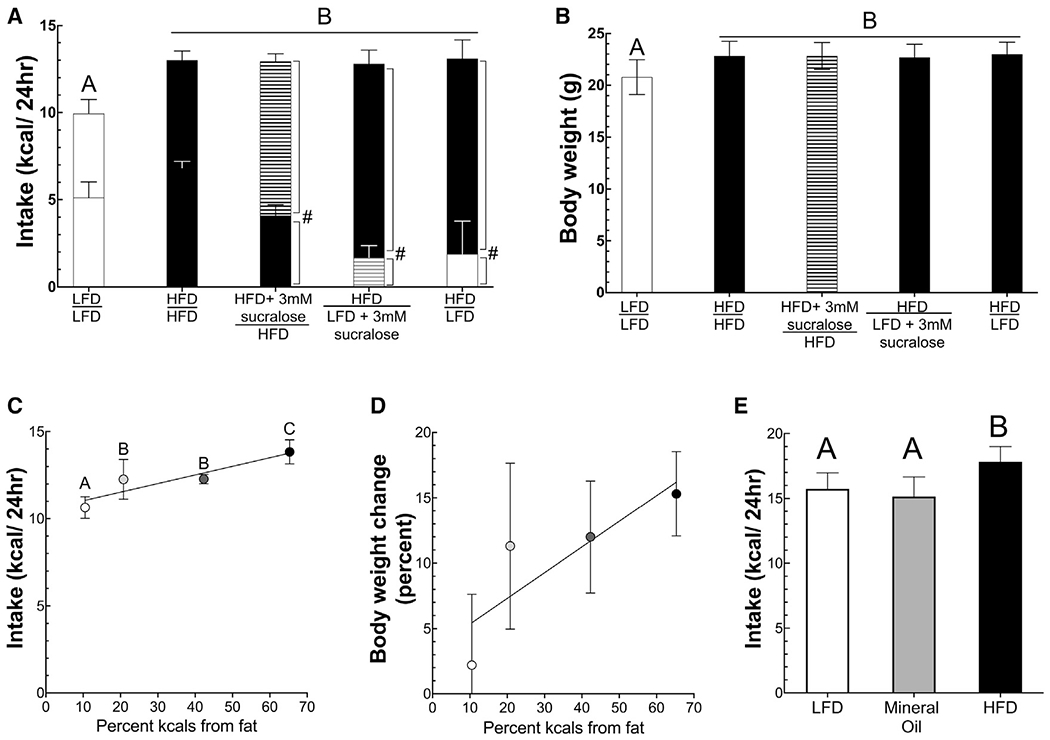
(A and B) Caloric intake and body weight among mice offered ad libitum access to several pairs of diets, including LFD and HFD supplemented with the sweetener sucralose. (A) Unshaded bars show unsweetened LFD intake, black bars show unsweetened HFD intake, striped black is sucralose-sweetened HFD, and striped gray is sucralose-sweetened LFD. (B) The bar shading corresponds to the more preferred diet as described above.
(C and D) Caloric intake and percent change in body weight over 4 weeks on diets of differing fat content.
(E) Caloric intake of mice fed either a LFD (10% calories from digestible fat), HFD (60% calories from digestible fat), or mineral oil diet (10% of calories from digestible fat but supplemented with mineral oil as described in the STAR Methods). Average intake from 6 days is shown. Bar shading in (E): unshaded is LFD, black is HFD, and gray is mineral oil diet. Data are presented as mean ± standard deviation. Different letters represent significant differences between groups. ANOVA with post hoc t tests using Benjamini-Hochberg correction was used for between-group statistical testing. #p < 0.05 for within-group diet preference by paired t test. .
To assess whether higher fat content increases caloric intake in a graded or threshold fashion, mice were offered ad libitum access to diets with the same caloric density (1 kcal/ml) but differing percent calories from fat (Table S1). Mice were offered these diets for 4 weeks. The average caloric intake was positively and linearly associated with percent calories from fat of the diets (intake = 0.049 × percent kcals from fat + 10.5kcals, p < 0.001, R = 0.78) (Figure 3C). As expected, body weight also increased as percent calories from fat increased, with the percent increase in body weight being positively associated with percent calories from fat (percent change in body weight = 2.89% + 0.169% × %kcals from fat, R = 0.519, p < 0.05) (Figure 3D).
To assess the latency of the response to fat content, we performed short-term feeding studies. On consecutive days, mice were given 30-min access to diets (all 1 kcal/ml) with 5.4%, 10.5%, or 65.3% kcals derived from fat. Mice were split into three groups, with each group receiving the three diets in different orders, and the three-diet sequence was repeated two times, making the total length of the experiment 3 days. Offering two rounds with each diet allowed us to assess both acute and learned intake. In a mixed model, both fat content and first round versus second round were significant predictors of intake (p < 0.0001) (Figure S4). On the first introduction, mice consumed different amounds of a HFD (65.3% calories from fat) than the two LFDs (LFD1: 10.5% calories from fat; LFD2: 5.3 calories from fat). During the initial introduction, they consumed more of the HFD than either LFD (HFD: 1.42 ± 0.52 kcals; LFD1: 0.84 ± 0.31 kcals [p < 0.0005]; LFD2: 0.64 ± 0.33 kcals [p < 0.0001], using Tukey HSD test). However, when mice were given the diets a second time, caloric intake of each of the diets was increased from first to second introduction, suggesting a learned aspect to feeding behavior that did not depend on percent calories from fat (LFD2: 0.64 ± 0.33 versus 0.99 ± 0.43 kcals, p < 0.05; LFD1: 0.84 ± 0.31 kcals versus 1.33 ± 0.26 kcals, p = 0.001; HFD1: 1.42 ± 0.52 kcals versus 2.20 ± 0.33, p < 0.000001) (Figure S4).
Although it is clear from the short access tests and the literature that mice prefer HFDs, we next tested whether higher fat content in the absence of a hydrolysable, digestible lipid was sufficient to increase caloric intake. To do this test, we offered mice a LFD, a HFD, or a diet matched to the HFD in lipid content but in which the lipid above that in the LFD was provided by non-digestible mineral oil, i.e., the mineral oil diet was made by replacing some of the water in the LFD with enough mineral to match the lipid density of the HFD. Thus, the diet contained the same caloric density and digestible macronutrient profile as the LFD but was matched to the HFD in terms of lipid content. At the end of 6 days, only the HFD containing the digestible lipid increased caloric intake, and mice consumed equal, albeit lower, amounts of the mineral oil diet and LFD as compared to the HFD (HFD: 17.8 ± 1.17, LFD: 15.7 ± 1.23, mineral oil: 15.1 ± 1.52; ANOVA, p < 0.01; Figure 3E). It is worth noting that mice used in this study were 12 weeks of age rather than the 6-week-old mice used in other experiments, causing them to be much larger and consume more calories.
Post-oral sensing of percent calories from fat modulates caloric intake
It has been postulated that the increased palatability of HFDs is derived from some pleasurable aspect of oropharyngeal sensing of lipids (Ackroff and Sclafani, 2014; Ferreira et al., 2012). Most investigators have hypothesized that the palatable taste, texture, or smell of fat drives increased feeding when mice are offered a HFD. To test whether post-orally sensing percent calories from fat is sufficient for a HFD to increase caloric intake, we used a gastric infusion system to bypass naso-oral sensing, delivering a HFD directly to the stomach. In the next studies, “i” or “o” in front of the diet name indicates whether the diet was infused or was taken orally, respectively, ad libitum by the mice. In each of several different experimental designs, mice were only provided ad libitum access to oLFD (lipid source: dairy) and infused with either HFD or LFD (Figure 4A; Table S1). We predicted that intragastric (IG) infusion of palatable iHFD would increase the ad libitum, oral intake of an LFD.
Figure 4. Ad libitum caloric intake is regulated by percent calories from fat of food in the gastrointestinal tract.
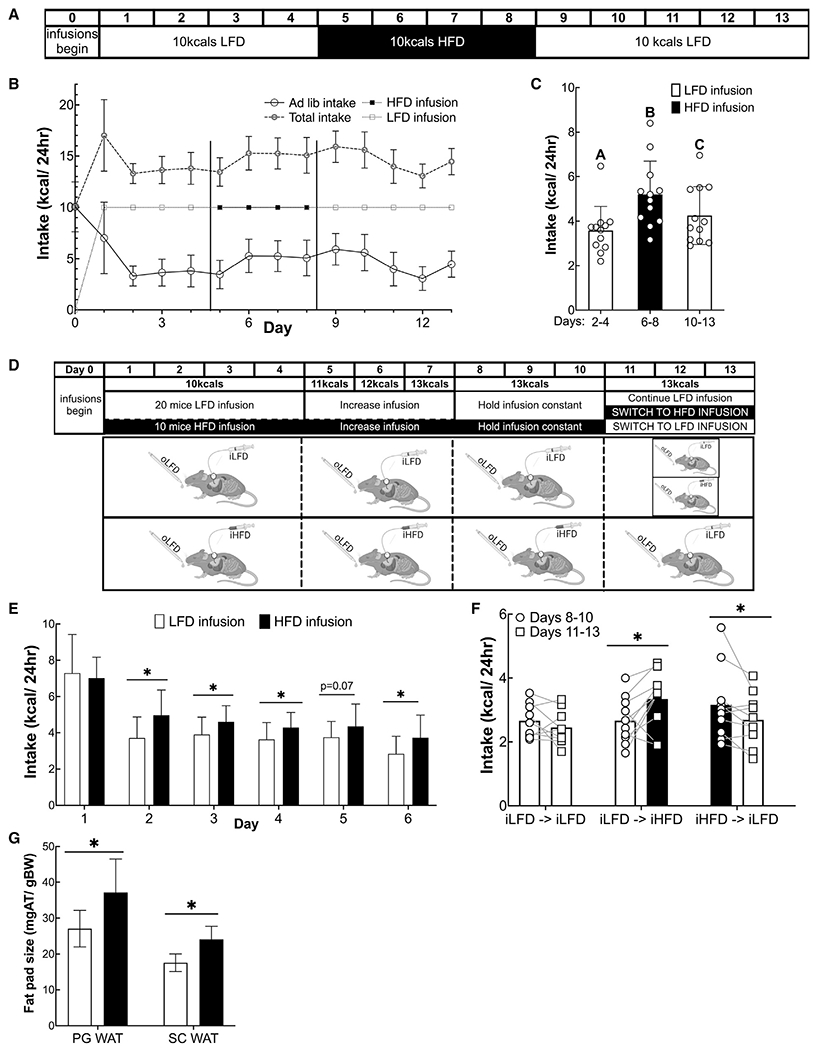
(A) Experimental design for data shown in (B) and (C) (n = 14).
(B) Daily ad libitum and infused caloric intake, and total caloric intake. Vertical lines indicate when the infusion diets were switched.
(C) Mean caloric intake during each infusion period excluding the first day of each period to allow for acclimation. Columns represent group averages, and open shapes are individual data points. Data are displayed as mean ± standard deviation. Different letters represent significantly different means as calculated with ANOVA and post hoc t test with a Benjamini-Hochberg correction.
(D) Experimental design for data in (E)–(G) (n = 10–19/group in E and G; and in F, n = 9–10/group). Animals were infused with 10 kcals on days 1–4, and this amount was gradually increased to 13 kcals/day at which it remained for the rest of the experiment. On day 11, infused diets were switched from infused LFD (iLFD) to infused HFD (iHFD) or iHFD to iLFD, and the control group remained on iLFD.
(E) Daily ad libitum intake over days 1–6.
(F) Mean ad libitum intake days 8–10 compared to intake on days 11–13.
(G) Epididymal fat mass as a fraction of total body weight. *p < 0.05 as calculated by Student’s t test in (E) and (G) or paired t test in (F).
In a first set of experiments, we determined whether the percent calories from fat of infused diets would modulate the ad libitum consumption oLFD by mice. Animals were IG infused with a fixed amount of calories (10 kcal/day) that was below their daily requirements and that varied in their percent calories from fat. Overall, the diets had the same caloric density (1 kcal/ml) and protein content (10% calories from protein), but carbohydrate density was inversely modulated compared to percent calories from fat. Mice were initially infused with iLFD (10% of calories from fat). Once ad libitum and total caloric intake stabilized for several days, the infused diet was switched to iHFD (60% calories from fat) and then back to iLFD. Consistent with post-oral sensing of fat, ad libitum oLFD intake increased when mice received iHFD IG (Figures 4B and 4C).
We next tested whether iHFD would induce a greater intake of a less palatable oLFD across multiple groups of mice (Figure 4D) and remove any confounders of a crossover-study design. Consistent with post-oral sensing of percent calories from fat, mice with iHFD ate 21% ± 6.1% more calories than a group of iLFD animals (Figure 4E). Even by infusing a higher number of calories (13 kcal/day), we observed the same dependency in which the delivery of a diet higher in the percentage of fat increased ad libitum oLFD. When mice were switched from iLFD to iHFD, their oral ad libitum caloric intake increased (2.67 ± 0.73 to 3.35 ± 0.89 kcals/day; p < 0.05), whereas switching from iHFD to iLFD IG infusion reduced the oral ad libitum caloric intake (3.16 ± 1.15 to 2.70 ± 0.81 kcals; p < 0.05) (Figure 4F). Although we did not observe a difference in body weight between the groups during this short experiment, perigonadal fat-pad weights (PG WAT) and subcutaneous fat-pad weights (s.c. WAT) were greater in mice that received the HFD (Figure 4G; PG WAT: 27.1 ± 5.1 mg/g of body weight versus 37.1 ± 9.3 mg/g of body weight, p < 0.05; s.c. WAT: 17.5 ± 2.4 mg/g of body weight versus 24.1 ± 3.6 mg/g of body weight, p < 0.005). We also measured blood glucose concentration on days 10 and 13 to test whether low blood glucose concentration resulting from fewer carbohydrates in iHFD than iLFD could explain the increased ad libitum intake. However, we found that mice receiving iHFD had higher rather than lower blood glucose concentration than those receiving iLFD.
To assess the effect of percent calories from fat on feeding behavior when animals received enough calories IG to meet nearly all of their daily caloric needs, we infused mice with increasing amounts of iLFD until their ad libitum caloric intake of oLFD had decreased to ~15% of their initial caloric intake (Figures 5A and 5B). One-half of the mice then continued to receive the iLFD IG at this rate, while the remaining mice received an equal number of calories and volume IG, but in the form of an iHFD. Consistent with a post-oral sensing system driving increased food intake, mice receiving a iHFD increased their ad libitum intake of oLFD, and consequently, the total caloric intake from what they were consuming during the iLFD (iLFD: 1.7 ± 1.0 kcals/day versus iHFD: 2.3 ± 1.3 kcals/day, p < 0.05), whereas there was no change in ad libitum intake for mice that had only received iLFD (Figure 5C). This increase in net calories was sufficient to increase the weight of iHFD mice compared with that of iLFD mice (iLFD: 21.8 ± 1.2 g versus iHFD: 24.0 ± 0.9 g, p < 0.05) (Figure 5D). We next determined whether iHFD would induce eating of a less palatable oLFD in mice in which their caloric needs were met by IG infusion. IG infusion of an iLFD was titrated so that the ad libitum intake of the oLFD was less than ~0.5 kcal/day. When this value was achieved, the infusion was switched to iHFD at the same caloric infusion rate. Consistent with post-oral sensing of percent calories from fat driving feeding behavior, mice ate more oLFD during the iHFD period (iLFD: 0.6 ± 0.3 kcals versus iHFD: 1.5 ± 0.7 kcals, p < 0.01) (Figures 5E–5G).
Figure 5. Post-oral sensing of a HFD increases ad libitum caloric intake and body weight in the absence of oral taste sensing.
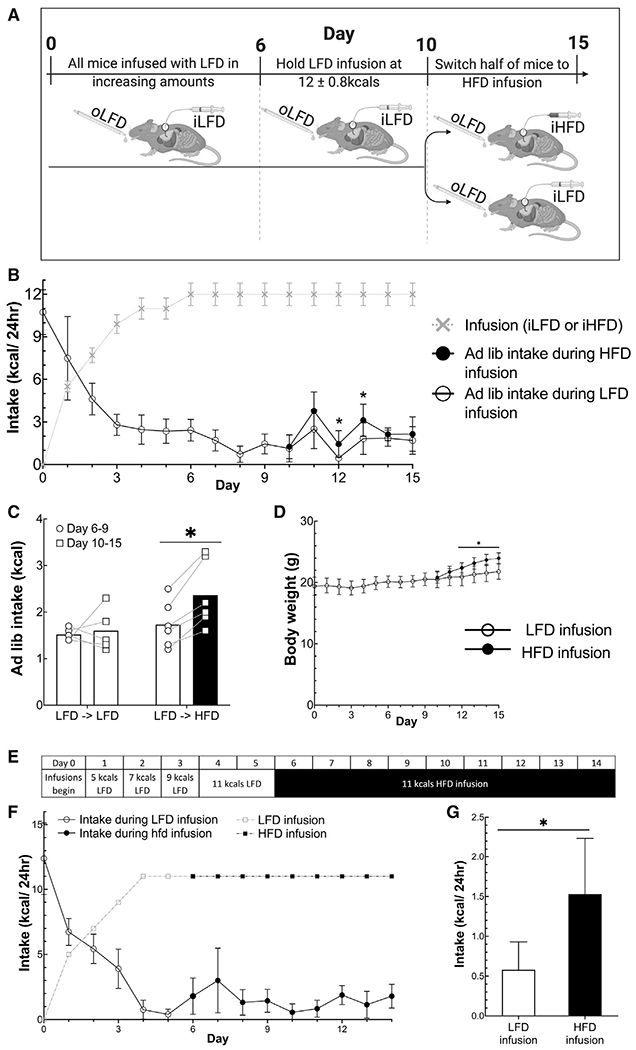
(A) Design of the experiment for data in (B)–(E) in which all animals are given ad libitum access to oLFD. n = 5–6/group.
(B) Infused kcals and ad libitum kcals consumed.
(C) Average ad libitum intake during 11-kcals infusions with LFD or HFD.
(D) Body weight during infusions.
(E) Experimental design for (F) and (G) (n = 6).
(F) Infused and ad libitum caloric intake.
(G) Average intake of days 4–5 during iLFD compared with that of days 6–14 during iHFD. Data are displayed as mean ± standard deviation or as individual data points in (C). *p < 0.05, comparing iLFD diet group to iHFD using a Student’s t test except in (C) and (G) for which paired t tests were used for within-group comparisons. Unshaded bars show intake during iLFD while black bars show intake during iHFD.
We confirmed that increased caloric intake during iHFD gastric infusion was not due to fewer calories being infused in HFD than LFD by measuring the calories in each diet by bomb calorimetry. To further confirm that the iHFD was not hypocaloric to the iLFD, we infused mice with either the HFD or LFD while removing ad libitum access to food to test the effect of the two diets on body weight. We found that iHFD mice actually gain slightly more weight than iLFD mice (Figure S5), implying an addition effect of percent calories from fat on energy expenditure/absorption. Nonetheless, increased ad libitum intake by mice receiving iHFD was not due to a reduced caloric density of infusions. Because protein density was greater in the iHFD than that in the iLFD, we tested whether protein content was driving the increased feeding behavior. We found that even when the iHFD contained less protein than the iLFD, IG iHFD still increased the ad libitum intake of oLFD (Figure 5F).
Higher percent calories from fat, rather than dietary preference, attenuates defense of body weight “set point”
Our demonstration that IG infusion of HFDs increase ad libitum caloric intake and fat mass and the long-standing observation that diets high in fat increase body weight and fat mass in many strains of mice suggest that fat content attenuates signals that limit food intake and defend against weight gain. We previously developed a model to study systems that defend against weight gain. In this paradigm, mice are overfed for a period of 1 to several weeks to enforce weight gain. Once overfeeding is stopped, mice remain anorectic for 1 to 2 days before slowly increasing food intake at a rate inversely related to excess fat and until they return to their previous body mass. This response is not mediated by an elevation in leptin (Ravussin et al., 2018). To test whether the hypophagic/anorectic responses to overfeeding are attenuated by access to a palatable diet, mice were overfed IG and offered ad libitum access to a either an oLFD (dairy-based, 10% of calories derived from fat) or oHFD (dairy-based, 60% of calories derived from fat) (Table S1). Control mice were infused with the same volume of saline and offered ad libitum access to oLFD (Figure 6A).
Figure 6. Access to a HFD attenuates defense of body weight.
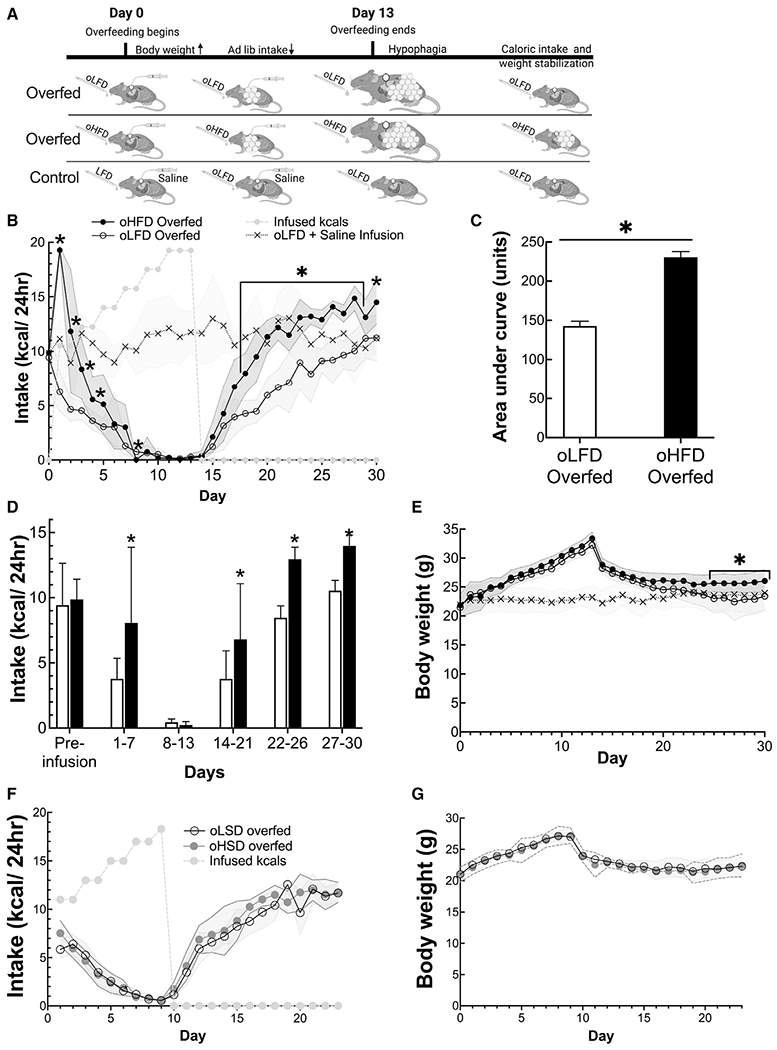
(A) Experimental design showing the three groups and their ad libitum and infused diets.
(B) Ad libitum caloric intake of overfed and control mice.
(C) Total ad libitum caloric intake is greater in mice offered oHFD than mice offered oLFD as measured as area under the curves.
(D) Ad libitum caloric intake during periods of overfeeding protocol.
(E) Daily body weight of control, oLFD, and oHFD during overfeeding experiment.
(F and G) Ad libitum caloric intake and body weight of mice that were overfed and had ad libitum oral access to low-sweetness diet (oLSD) or ad libitum access to an oral high-sweetness diet (oHSD). Data are presented as mean ± standard deviation shown by either error bars of the shaded regions in the line graphs. *p < 0.05, comparing the LFD and HFD overfed groups using a Student’s t test. Unshaded bars show oLFD intake, and black bars show intake during oHFD.
During the initial period as gastric infusion of calories was increased, mice with ad libitum access to oHFD consumed more calories than those who had access to oLFD (Figures 6B and 6E). All overfed mice, whether they had access to oHFD or oLFD, became anorectic by day 9. However, once overfeeding was ceased, the mice with access to oHFD consumed significantly more kcals during all time periods (days 14–21, 22–26, and 27–30) than mice with access to oLFD (Figure 6C). Following the infusion period, mice that were offered ad libitum access to an oLFD returned back to their original body weight and matched the body weight of control mice (control: 24.0 ± 2.9 g versus 23.4 ± 2.4 g) (Figure 6G). In contrast, mice that had access to an oHFD did not return to their original body weight trajectory but instead maintained a new higher body weight (26.1 ± 2.0 g) than the control and LFD-fed mice (overfed oLFD: 23.4 ± 2.5 g; p < 0.05) (Figure 6G).
The ability of a HFD to attenuate the weight defense response was not reproduced by a palatable high sucrose/sweetened diet (oHSD). When mice were overfed and had ad libitum access to either a low sucrose/sweetened diet (oLSD) or the more palatable oHSD that derived 30% of its calories from sucrose (Figure 1D), they did not eat more than mice offered an unsweetened LFD (Figure 6F), and body weights were the same in the two groups (Figure 6G).
DISCUSSION
Mammals maintain body weight stability by defending what has been described as a set point. Although the response to perturbations in weight are robust, the factors that determine that set point remain very poorly defined. The palatability of food has been proposed as being a factor that modulates this set point, and the observation that increases in the percent calories from fat of food increase caloric intake and the defense of a higher body weight support this notion. A comparison of fat, carbohydrate, and protein content across several mouse strains found that fat, but not carbohydrate content, of diets modulates long-term food intake and body weight (Hu et al., 2018). A similar finding in humans was observed when a HFD, but not a high-carbohydrate diet, increased caloric intake during an inpatient study (Hall et al., 2021). Our findings here are consistent with these observations and suggest that taste and smell do not drive this effect, but rather, post-oral sensing of the percentage of digestible fat in the GI track is sufficient to increase ad libitum caloric intake. Furthermore, a recent study reported that olfactory sensing of a HFD was not sufficient to increase ad libitum intake and postulated that post-oral sensing may be responsible for this feeding behavior (Boone et al., 2021).
In contrast to the findings here, Licholai and colleagues found that providing a fixed amount of a HFD diet orally while maintaining ad libitum access to a LFD did not increase caloric intake above what mice ate when only given access to a LFD. The reason for this difference is not clear, although the authors noted that mice ate as much as 50% of the fixed high-fat food in 30 min during the light cycle (Licholai et al., 2018). This consumption may have created a “binge”-like phenotype rather than the typical meal pattern seen in mice placed on an ad libitum HFD (Davis et al., 2021; Hatori et al., 2012). The relatively large binge eating of a HFD may have had a satiating effect (Cassie et al., 2019; Maljaars et al., 2008), i.e., a preload of HFD given in one dose may suppress food intake. Our results and this study both agree that intake and body weight increase with fat content on an ad libitum diet and are driven by hedonic signals (Licholai et al., 2018). Although hedonic signals from a palatable diet are thought to be derived from the taste, taste and post-oral signaling are not traditionally disentangled when examining the hedonic drive to consume a palatable diet.
Post-oral sensing of macronutrients is known to participate in several responses. Nutrients delivered directly to the GI tract can condition preferences. In classic experiments, Scalfani and colleagues demonstrated that infusing sucrose or triglycerides into the stomachs of rodents can condition a flavor preference (Ackroff and Sclafani, 2014; Sclafani, 2001, 2010; Zukerman et al., 2011). Recently, a circuit that mediates the conditioned response to sucrose has been mapped. Sucrose, although not non-nutritive sweeteners, through vagal afferents activate neurons in the caudal nucleus of the solitary tract that underlie sucrose preference (Tan et al., 2020). However, unlike the post-oral flavor preference response, the post-oral response that increases feeding behavior and alters defense of body weight is induced as a graded response to percent calories from fat, is not responsive to sucrose, and does not require conditioning. Diets high in fat have also been implicated in altering dopaminergic reward systems in a manner that leads rodents to prefer diets high in fat content (Ferreira et al., 2012; Kleberg et al., 2015). Strikingly, the GI infusion of a HFD was able to increase the intake of oLFD, suggesting a broad effect on feeding behavior.
The infusion of macronutrients in the GI tract, including protein, carbohydrates, and fat, all have been shown to induce satiety or reduce hunger signals (Maggio et al., 1983; Maljaars et al., 2008; Oesch et al., 2005; Su et al., 2017). Physiologically, this response is important for regulating meal size and transit time. Vagal sensory pathways that attenuate the action of AGRP-expressing neurons in the hypothalamus have been implicated in this response (Goldstein et al., 2021). Distinct from this short-term anorectic response to IG nutrient infusion, we found that increasing percent calories from fat of infused food has an orexigenic effect on ad libitum feeding that becomes apparent after approximately 24 h and leads to increases in body weight. The pathways that mediate this orexigenic response are likely distinct from the anorectic and taste preference responses. Identification of neuronal populations activated in a graded response to percent calories from fat in the GI tract and on a timescale of hours will provide a step in constructing temporal-spatial circuit maps that integrate nutrient sensing and feeding behavior.
The design of the studies here, in which all diets were maintained at the same caloric density, permitted us to demonstrate that mice are able to sense the fraction of calories attributable to micronutrients, independent of effects on overall caloric density. This result raises an intriguing question of how this is accomplished. Are there systems that assess the caloric contribution of each macronutrient (carbohydrate, protein, and fat) and then discern when digestible fat comprises a larger fraction of the total calories? Although the molecular mechanisms are difficult to imagine, such a system might provide an evolutionary advantage of being able to identify the most calorically dense food during times when the availability of calories is limiting (Warwick and Weingarten, 1995).
Conclusions
Although the increased consumption of fat has been attributed to its pleasant taste and texture, we have found that oropharyngeal sensing is not required for increased intake and that digestible fat in the gastrointestinal tract will increase feeding behavior and weight, even when only low-fat food is available. The apparent palatability of food that drives increased calorie intake is driven by post-oral signals that can increase consumption independent of taste.
Limitations of the study
The experiments of this study rely on interpreting qualitative data, namely, the palatability or pleasure of mice, in a quantitative way. Although preference tests were used as a proxy to correlate dietary preference with increased palatability, there is no way to assess the mouse’s perception of palatability. Preference tests have long been used to assess taste preference and conditioned preference in mice; however, there is no standard definition of palatability beyond the implied meaning of pleasurable taste.
Another limitation of the study is the timescale of the experiments. It is unknown whether the increased intake on a HFD would persist for weeks or months if allowed to continue. In this regard, we were bound by the limitations of our infusion system, as a longer infusion period resulted in clogged catheters necessitating removal of mice from the study. This system posed a number of challenges, such as a maximal tolerated infusion rate, which varied by diet as well as the 10+ h infusions being incompatible with certain diets that would separate or clog the system.
Our studies were carried out only in one strain of mice, namely, C57BL/6J. Mouse strains vary in their feeding behavior and dietary preferences. These studies provide a foundation on which others can be performed to assess that degree to which a similar physiology exists in other mice.
Finally, by keeping all diets to the same caloric density, changing the amount of any macronutrient would invariably change the concentrations of the others. Thus, although the diets were matched by caloric density and protein content when feasible, carbohydrate content varied inversely with fat content.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Anthony Ferrante (awf7@cumc.columbia.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All experiments used wild-type were C57bl6 male mice from Jax (Bar Harbor, ME). All studies were conducted in accordance with protocols approved by the Columbia University Institutional Animal Care and Use Committee. Upon arrival, mice were placed on a chow diet (PicoLab Rodent Diet 20) and were housed in a pathogen-free barrier facility maintained at 22–24C with a 12:12-h dark-light cycle (lights on at 0700 h). Mice were 5 weeks old at the time of purchase and were acclimated to facilities for one week so that they were six to eight weeks old at the beginning of experiments or at the time of surgery. For all studies, mice were individually housed with 24-hour ad-libitum access to food and water. All experimental diets were liquid diets at a density of 1kcal/mL and were delivered in 25mL serological pipettes (Fisher scientific, 13-676-10M). Water was provided in BioServ 50mL liquid feeder bottles (VWR international, 89067-822).
METHOD DETAILS
Caging and diet delivery
Mice were singly housed in plastic shoebox cages with wood chip bedding. For preference tests, cages had a metal grid and filter top with space for a water bottle. During preference tests, a small incision was made in the filter top, and two serological pipettes were places through the incision side by side with their tips sticking through spaces in the metal grid top. During infusion, no metal grid was used, and filter tops were fitted with a rubber stopper in the middle to hold the infusion swivel and tether which connected to the button on the mouse allowing the mouse a complete range of motion. As no metal grid was available to hold the feeding pipettes, pipettes were placed through the back of the cage. In the back of the cage, the screw and bolt which allow connection to a watering system were removed creating a hole in the back of the cage for the serological pipette to fit into. Sanding blocks (KingOrigin Sanding Sponge 4” × 2-5/8” × 1”) were cut to size and affixed to the back of the cage to support and hold the pipette in place. A silicon ear plug was used at the top of the pipette to create a seal and hold to the liquid in the pipette. New diets and pipettes were prepared daily, and diets were stored at 4 degrees until time of use.
Preference Testing
Mice were given concurrent, ad libitum access to two liquid diets of the same caloric density. Diets were positioned next to each other with the tip of the pipette sticking through the metal wire cage top. Positions of diets were switched every other day to control for place preference. Preference tests lasted eight to 12 days as stated in each experiment.
Diets
All diets were liquid and provided ad libitum to animals with concurrent ad libitum access to water. Diet compositions and components are shown in Table S1.
Intragastric Feeding
Catheter
To make the catheters, a 10cm length of Micro-Renathane® 0.037” x 0.023” per ft. Braintree Scientific MRE 037 100FT is pushed through a hole in the center of a square of Bard Mesh Flat Sheet 10”x14” (112660 eSutures) and held in place with Krazy glue.
Surgery
Mice are anesthetized with isoflurane (using a SomnoSuite, Kent Scientific, Torrington, CT) and surgical area is shaved and cleaned using alternating betadine and alcohol. Mice are treated with the analgesics Buprenorphine (.05mg/kg) and carprofen (5mg/kg) and lubricant is applied to the eyes.
A small 0.5cm incision is made on the back of the mouse below the skull. Using curved forceps, tunnel is made running from the incision on the back to the midline on the front. Next a 3cm incision is made on the front beginning from below the xyphoid process and extending down the midline. The peritoneum is opened, and the stomach is gently withdrawn of the cavity and stabilized with sterile gauze moistened with sterile 0.9% saline. An 18 g needle (BD 305185) is used to make a small puncture hole in the stomach. The tip of the catheter is pushed inside the hole while the mesh of the catheter remains on the outside. One suture (5-0 Surgipro blue 36” CV-23 taper, double armed, eSutures VP556X-SD) is placed in each corner of the mesh to affix the mesh to the walls of the stomach to hold the catheter in place. The stomach is then returned to the abdominal cavity, and the end of the catheter is pushed through the tunnel that was made at the beginning of surgery so that it exits the body via the incision on the back. The peritoneum is then sutured closed (5-0 Polysorb undyed 18” P-12 cutting, eSutures SL5636) and the abdominal skin is closed with wound clips. The mouse is then flipped over to allow access to the intrascapular region and the end of the catheter. The catheter is cut to size to remove excess length and is then attached to 22 g Button (Mouse VAB button Instech VAB62BS/22 Mouse) and small amount of Krazy Glue is applied to reinforce the attachment between the catheter and button. The button is sutured into the intrascapular muscle and the skin is closed around the button (5-0 Monosof black 18” P-12 cutting, eSutures SN5666). Mice are returned to their home cages and are kept on a hot water circulator until normal ambulation is observed.
Overfeeding infusions
lasted 14 hours and began at 20:00 one hour after onset of the dark cycle. Infusions started at 12mL of Ensure and increased in volume as described previously (Ravussin et al., 2018). The mice are individually housed in their home cages and the buttons on the mouse are attached to a swivel and tether system that allows free movement throughout the cage. Mice are allowed to recover for one week before being attached to the tethers. Harvard Apparatus Infusion pump and BD sterile syringes are used to infuse a liquid diet into the stomach of the mice.
Post-ingestive effects infusions
Mice were weighed and fed at the onset of the light cycle. Diet infusions began immediately after. Infusions terminated before the dark cycle and were done at a rate of 1ml/hr. When Intralipid was used as the fat source infusions lasted 20 hours with a maximum infusion rate of 0.65ml/hour as mice developed GI issues when a faster infusion rate was used. When dairy or formula diets were used, infusions were given over 8-10 hours during the light cycle at 1-1.5mL/hr. In all cases, infusions began in the light cycle immediately after body weight and food intake measurements were taken.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data are displayed as mean ± SD. t tests were performed in Excel and ANOVAs and regression analysis were performed using R. Post hoc analyses were performed as a t test with a Benjamini-Hochberg correction. Mixed model analyses were performed in Graphpad Prism 8. Statistical significance was prospectively defined as α < 0.05.
Graphpad Prism 8 was used for making all graphs, and experimental schematics and diagrams were created using BioRender.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Half and Half | Land O’ Lakes | N/A |
| Maltodextrin | Sigma-Aldrich | Cat # - 419699 |
| Intralipid | Sigma-Aldrich | Cat# - I141-100ML |
| Whey Protein | GNA Pro Performance | Cat # - 369951 |
| Dry non-fat Milk Powder | Village Farm | N/A |
| Similac Alimentum | Abbott Nutrition | Cat # - 64715 |
| Ensure | Abbott Nutrition | Cat # - 63071 |
| Sucrose | Sigma-Aldrich | Cat # 573113-5KG |
| Sucralose | Sigma-Aldrich | Cat # - 69293-100G |
| AIN 93 Vitamin Mix | Dyets | Cat # - 310025 |
| AIN 93 Mineral Mix | Dyets | Cat# - D210025 |
| Saccharin | Sigma-Aldrich | Cat # - 240931 |
| PicoLab rodent diet 20 | LabDiets | Cat # - 5053 |
| Mineral Oil | Walgreen’s | NDC - 0363-0831-43 |
| Natrel 2% milk | Amazon | ASIN # - B085Z6KFZ2 |
| Experimental models: Organisms/strains | ||
| WT mice C57bl6J | Jax | Cat # - 000664 |
| Software and algorithms | ||
| R | CRAN R Project | https://cran.r-project.org/ |
| BioRender | BioRender | BioRender.com |
| GraphPad prism 8 | GraphPad Prism | https://www.graphpad.com/scientific-software/prism/ |
| Excel | Microsoft | https://www.microsoft.com/en-us/microsoft-365/excel |
| Other | ||
| SomnoSuite Anesthesia | Kent Scientific | Cat # - SSXWTY |
| Water circulating heat-pump HTP- 1500 | Adroit Medical Systems | Cat # - 6021615 |
| Buprenorphine hydrochloride (0.3mg/mL) | Covetrus | Cat # - 59122 |
| Carprofen (Rimadyl) | Covetrus | Cat# - 10000319 |
| Micro-renathane | Braintree Scientific | Cat # - MRE 037 100FT |
| Bard Mesh Monofilament Polypropylene | e-Sutures | Cat # - 112660 |
| Krazy Glue | Elmer’s Products Inc. | Cat# - |
| Mouse VAB button | Instech Laboratories Inc. | Cat # - VAB62BS/22 |
| Puralube Vet Ointment | Dechra Veterinary Products | NDC - 17033-211-38 |
| 5-0 Polysorb undyed 18” P-12 cutting | e-Sutures | Cat # - SL5636 |
| 5-0 Surgipro blue 36” CV-23 taper, double armed | e-Sutures | Cat # - VP556X-SD |
| 5-0 Monosof black 18” P-12 cutting | e-Sutures | Cat # - SN5666 |
| 18 Gauge Needle | BD | Cat # - 305185 |
| Triple Antibiotic Ointment | Globe | NDC- 69396-002-20 |
| Mouse VAH infusion tether | Instech Laboratories Inc. | Cat # - KVAH62T |
| Harvard Apparatus Infusion Pumps | Harvard Apparatus | Cat # - 703007 |
| BD Slip tip syringes 10ml or 20ml | Fisher Scientific | Cat # - NC1134733 |
| Liquid Diet Feeding tubes | VWR International | Cat # - 89067-822 |
| Rubber Stoppers | Fisher Scientific | Cat # - 14-130P |
| Sanding Sponge 4” x 2-5/8” x 1” | SackOrange | UPC #-751474692429 |
| Mack’s Pillow Soft Silicone Earplugs | Amazon | ASIN # - B003LZQGN6 |
| Serological Pipettes for diet delivery | Fisher Scientific | Cat # - 13-676-10M |
Highlights.
In mice, increasing percent calories from fat and sweetness increase diet palatability
Increasing fat content but not sweetness increases food intake and weight gain
Intragastric infusion of food dense in fat increases intake of low-palatable food
High-fat dense food but not sweetened food attenuates the defense against weight gain
ACKNOWLEDGMENTS
These studies were supported by research grants to A.W.F. from NIH (DK066525) and the Russell Berrie Foundation, technical support was provided from the New York Nutrition Obesity Research Center (DK026687) and the Columbia Diabetes Research Center (DK063608), and fellowship support was provided to M.R.G. (DK122711). We thank John Glendinning, Lori Zeltser, and Rudy Leibel for advice and suggestions and Chloé Berland for help with care of mice.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.109845.
DECLARATION OF INTERESTS
A.W.F. is supported by a research grant from Boehringer-Ingelheim Pharmaceuticals. The authors declare no other competing interests.
REFERENCES
- Ackroff K, and Sclafani A (2014). Post-oral fat stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol. Behav 129, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, and Flier JS (1996). Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252. [DOI] [PubMed] [Google Scholar]
- Alkerwi A, Crichton GE, and Hébert JR (2015). Consumption of readymade meals and increased risk of obesity: findings from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study. Br. J. Nutr 113, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, and Beauchamp GK (2001). Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem. Senses 26, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, and Drucker DJ (2007). Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157. [DOI] [PubMed] [Google Scholar]
- Ball K, Brown W, and Crawford D (2002). Who does not gain weight? Prevalence and predictors of weight maintenance in young women. Int. J. Obes. Relat. Metab. Disord 26, 1570–1578. [DOI] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D’Alessio DA, and Seeley RJ (2011). GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat. Rev. Endocrinol 7, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellisle F (2015). Intense Sweeteners, Appetite for the Sweet Taste, and Relationship to Weight Management. Curr. Obes. Rep 4, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, and Zheng H (2012). Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav 107, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone MH, Liang-Guallpa J, and Krashes MJ (2021). Examining the role of olfaction in dietary choice. Cell Rep. 34, 108755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Paeratakul S, and Popkin BM (2004). Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol. Behav 83, 549–555. [DOI] [PubMed] [Google Scholar]
- Cassie N, Anderson R, Wilson D, Mercer JG, and Barrett P (2019). Fat, carbohydrate and protein by oral gavage in the rat can be equally effective for satiation. Physiol. Behav 207, 41–47. [DOI] [PubMed] [Google Scholar]
- Corbit JD, and Stellar E (1964). Palatability, food intake, and obesity in normal and hyperphagic rats. J. Comp. Physiol. Psychol 58, 63–67. [DOI] [PubMed] [Google Scholar]
- Davis JA, Paul JR, Yates SD, Cutts EJ, McMahon LL, Pollock JS, Pollock DM, Bailey SM, and Gamble KL (2021). Time-restricted feeding rescues high-fat-diet-induced hippocampal impairment. iScience 24, 102532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis RG, Joly-Amado A, Webber E, Langlet F, Schaeffer M, Padilla SL, Cansell C, Dehouck B, Castel J, Delbès AS, et al. (2015). Palatability Can Drive Feeding Independent of AgRP Neurons. Cell Metab. 22, 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Mennella JA, Johnson SL, and Bellisle F (2012). Sweetness and food preference. J. Nutr 142, 1142S–1148S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernersson A, Nystrom FH, and Lindström T (2010). Long-term increase of fat mass after a four week intervention with fast food based hyper-alimentation and limitation of physical activity. Nutr. Metab. (Lond.) 7, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JG, Tellez LA, Ren X, Yeckel CW, and de Araujo IE (2012). Regulation of fat intake in the absence of flavour signalling. J. Physiol 590, 953–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, and Dietz W (2012). Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med 42, 563–570. [DOI] [PubMed] [Google Scholar]
- Flier JS, and Maratos-Flier E (2017). Leptin’s Physiologic Role: Does the Emperor of Energy Balance Have No Clothes? Cell Metab. 26, 24–26. [DOI] [PubMed] [Google Scholar]
- Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, and Hall KD (2016). Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 24, 1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Gillman J, Zamer H, Margolskee RF, and Sclafani A (2012). The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice. Physiol. Behav 107, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein N, McKnight AD, Carty JRE, Arnold M, Betley JN, and Alhadeff AL (2021). Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 33, 676–687.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ (2020). A Role for GLP-1 in Treating Hyperphagia and Obesity. Endocrinology 161, bqaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V, et al. (2019). Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 30, 67–77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD, Guo J, Courville AB, Boring J, Brychta R, Chen KY, Darcey V, Forde CG, Gharib AM, Gallagher I, et al. (2021). Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat. Med 27, 344–353. [DOI] [PubMed] [Google Scholar]
- Han P, Mohebbi M, Seo H-S, and Hummel T (2020). Sensitivity to sweetness correlates to elevated reward brain responses to sweet and high-fat food odors in young healthy volunteers. Neuroimage 208, 116413. [DOI] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. (2012). Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, and Wadden TA (2017). Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med 376, 254–266. [DOI] [PubMed] [Google Scholar]
- Hu S, Wang L, Yang D, Li L, Togo J, Wu Y, Liu Q, Li B, Li M, Wang G, et al. (2018). Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab. 28, 415–431.e4. [DOI] [PubMed] [Google Scholar]
- Jen K-LC, and Hansen BC (1984). Feeding behavior during experimentally induced obesity in monkeys. Physiol. Behav 33, 863–869. [DOI] [PubMed] [Google Scholar]
- Jéquier E, and Tappy L (1999). Regulation of body weight in humans. Physiol. Rev 79, 451–480. [DOI] [PubMed] [Google Scholar]
- Johnson F, and Wardle J (2014). Variety, palatability, and obesity. Adv. Nutr 5, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, and Bohórquez DV (2018). A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A, Brozek J, Henschel A, Mickelsen O, and Taylor HL (1945). Experimental Starvation in Man. Air Force Office Of Scientific Research (Arlington, VA). https://apps.dtic.mil/sti/pdfs/ADA473351.pdf.
- Kleberg K, Jacobsen AK, Ferreira JG, Windeløv JA, Rehfeld JF, Holst JJ, de Araujo IE, and Hansen HS (2015). Sensing of triacylglycerol in the gut: different mechanisms for fatty acids and 2-monoacylglycerol. J. Physiol 593, 2097–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, and Hirsch J (1995). Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med 332, 621–628. [DOI] [PubMed] [Google Scholar]
- Levin BE, and Dunn-Meynell AA (2000). Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 278, R231–R237. [DOI] [PubMed] [Google Scholar]
- Levin BE, and Dunn-Meynell AA (2002). Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol 282, R46–R54. [DOI] [PubMed] [Google Scholar]
- Licholai JA, Nguyen KP, Fobbs WC, Schuster CJ, Ali MA, and Kravitz AV (2018). Why Do Mice Overeat High-Fat Diets? How High-Fat Diet Alters the Regulation of Daily Caloric Intake in Mice. Obesity (Silver Spring) 26, 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio CA, Greenwood MR, and Vasselli JR (1983). The satiety effects of intragastric macronutrient infusions in fatty and lean Zucker rats. Physiol. Behav 31, 367–372. [DOI] [PubMed] [Google Scholar]
- Maljaars PWJ, Symersky T, Kee BC, Haddeman E, Peters HPF, and Masclee AAM (2008). Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int. J. Obes 32, 1633–1639. [DOI] [PubMed] [Google Scholar]
- Massiera F, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, Mohsen-Kanson T, Amri EZ, and Ailhaud G (2010).A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J. Lipid Res 51, 2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Mizoue T, Takahashi Y, Isogawa A, Kato M, Inoue M, Noda M, and Tsugane S; JPHC Study Group (2009). Taste preferences and body weight change in Japanese adults: the JPHC Study. Int. J. Obes 33, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Mendonça RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, and Bes-Rastrollo M (2016). Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr 104, 1433–1440. [DOI] [PubMed] [Google Scholar]
- Myers MG Jr., Leibel RL, Seeley RJ, and Schwartz MW (2010). Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol. Metab 21, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch S, Degen L, and Beglinger C (2005). Effect of a protein preload on food intake and satiety feelings in response to duodenal fat perfusions in healthy male subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol 289, R1042–R1047. [DOI] [PubMed] [Google Scholar]
- Passmore R (1971). The regulation of body-weight in man. Proc. Nutr. Soc 30, 122–127. [DOI] [PubMed] [Google Scholar]
- Passmore R (1982). Reflexions on energy balance. Proc. Nutr. Soc 41, 161–165. [DOI] [PubMed] [Google Scholar]
- Penney TL, and Kirk SFL (2015). The Health at Every Size paradigm and obesity: missing empirical evidence may help push the reframing obesity debate forward. Am. J. Public Health 105, e38–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, Kakuriev L, Guskova E, Fuzailov I, Touzani K, et al. (2012). Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol. Behav 105, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Leibel RL, and Ferrante AW Jr. (2014). A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 20, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Edwin E, Gallop M, Xu L, Bartolomé A, Kraakman MJ, Le-Duc CA, and Ferrante AW Jr. (2018). Evidence for a Non-leptin System that Defends against Weight Gain in Overfeeding. Cell Metab. 28, 289–299.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, and Leibel RL (2003). Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R183–R192. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Kissileff HR, Mayer LES, Hirsch J, and Leibel RL (2010). Energy intake in weight-reduced humans. Brain Res. 1350, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi N, Nestoridi E, Kucharczyk J, Uygun MK, Yarmush ML, and Stylopoulos N (2012). Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. Int. J. Obes. (Lond.) 36, 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D Jr., Seeley RJ, and Baskin DG (2000). Central nervous system control of food intake. Nature 404, 661–671. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, and Leibel RL (2017). Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev 38, 267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A (2001). Post-ingestive positive controls of ingestive behavior. Appetite 36, 79–83. [DOI] [PubMed] [Google Scholar]
- Sclafani A (2006). Sucrose motivation in sweet “sensitive” (C57BL6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol. Behav 87, 734–744. [DOI] [PubMed] [Google Scholar]
- Sclafani A, and Ackroff K (2018a). Greater reductions in fat preferences in CALHM1 than CD36 knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R576–R585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, and Ackroff K (2018b). Role of lipolysis in postoral and oral fat preferences in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R434–R441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Glass DS, Margolskee RF, and Glendinning JI (2010). Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 299, R1643–R1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR (2007). A nonadaptive scenario explaining the genetic predisposition to obesity: the “predation release” hypothesis. Cell Metab. 6, 5–12. [DOI] [PubMed] [Google Scholar]
- Su Z, Alhadeff AL, and Betley JN (2017). Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep. 21, 2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HE, Sisti AC, Jin H, Vignovich M, Villavicencio M, Tsang KS, Goffer Y, and Zuker CS (2020). The gut-brain axis mediates sugar preference. Nature 580, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick ZS, and Weingarten HP (1995). Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am. J. Physiol 269, R30–R37. [DOI] [PubMed] [Google Scholar]
- White CL, Purpera MN, Ballard K, and Morrison CD (2010). Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol. Behav 100, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Nawaz A, and Evans M (2020). Drug Therapy in Obesity: A Review of Current and Emerging Treatments. Diabetes Ther. 11, 1199–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, and Friedman JM (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
- Zheng H, Lenard NR, Shin AC, and Berthoud HR (2009). Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int. J. Obes. (Lond.) 33, S8–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, and Sclafani A (2011). Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol 301, R1635–R1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, and Sclafani A (2013). Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. Am. J. Physiol. Regul. Integr. Comp. Physiol 305, R840–R853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


