Abstract
Background
Safe, effective vaccines against coronavirus disease 2019 (Covid-19) are urgently needed in children younger than 12 years of age.
Methods
A phase 1, dose-finding study and an ongoing phase 2–3 randomized trial are being conducted to investigate the safety, immunogenicity, and efficacy of two doses of the BNT162b2 vaccine administered 21 days apart in children 6 months to 11 years of age. We present results for 5-to-11-year-old children. In the phase 2–3 trial, participants were randomly assigned in a 2:1 ratio to receive two doses of either the BNT162b2 vaccine at the dose level identified during the open-label phase 1 study or placebo. Immune responses 1 month after the second dose of BNT162b2 were immunologically bridged to those in 16-to-25-year-olds from the pivotal trial of two 30-μg doses of BNT162b2. Vaccine efficacy against Covid-19 at 7 days or more after the second dose was assessed.
Results
During the phase 1 study, a total of 48 children 5 to 11 years of age received 10 μg, 20 μg, or 30 μg of the BNT162b2 vaccine (16 children at each dose level). On the basis of reactogenicity and immunogenicity, a dose level of 10 μg was selected for further study. In the phase 2–3 trial, a total of 2268 children were randomly assigned to receive the BNT162b2 vaccine (1517 children) or placebo (751 children). At data cutoff, the median follow-up was 2.3 months. In the 5-to-11-year-olds, as in other age groups, the BNT162b2 vaccine had a favorable safety profile. No vaccine-related serious adverse events were noted. One month after the second dose, the geometric mean ratio of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing titers in 5-to-11-year-olds to those in 16-to-25-year-olds was 1.04 (95% confidence interval [CI], 0.93 to 1.18), a ratio meeting the prespecified immunogenicity success criterion (lower bound of two-sided 95% CI, >0.67; geometric mean ratio point estimate, ≥0.8). Covid-19 with onset 7 days or more after the second dose was reported in three recipients of the BNT162b2 vaccine and in 16 placebo recipients (vaccine efficacy, 90.7%; 95% CI, 67.7 to 98.3).
Conclusions
A Covid-19 vaccination regimen consisting of two 10-μg doses of BNT162b2 administered 21 days apart was found to be safe, immunogenic, and efficacious in children 5 to 11 years of age. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04816643.)
The BNT162b2 vaccine (Pfizer–BioNTech) is a lipid nanoparticle formulation containing nucleoside-modified mRNA encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral spike glycoprotein.1 The BNT162b2 vaccine received emergency use authorization from the Food and Drug Administration (FDA) in December 2020 for the prevention of coronavirus disease 2019 (Covid-19) in persons 16 years of age or older, with subsequent expansion to 12-to-15-year-olds in May 2021, and to 5-to-11-year-olds on October 29, 2021, on the basis of data from this trial.2 BNT162b2 recently received U.S. licensure for immunization in persons 16 years of age or older.2
Results of an ongoing phase 1–2–3 clinical study in healthy persons 12 years of age or older support the safety, immunogenicity, and efficacy of a two-dose series of 30-μg BNT162b2 injections administered 21 days apart.3-5 BNT162b2 has been shown to have an acceptable safety profile characterized by transient mild-to-moderate injection-site pain, fatigue, and headache, was more immunogenic among 12-to-15-year-olds than among young adults, and was 95 to 100% efficacious in preventing Covid-19 illness from 7 days to approximately 2 months after the second dose.3,4 Vaccine efficacy against Covid-19 from 7 days to 6 months after the second dose was 91%,6 with a similar estimated real-world effectiveness from 8 to 28 days after the second dose.7
Although Covid-19 is generally milder in children than in adults, severe illness and long-term complications, including multisystem inflammatory syndrome in children (MIS-C), can occur after the primary infection.8,9 School-age children represent a high proportion of Covid-19 cases,10 and they may play an important role in the transmission of SARS-CoV-2,11,12 including spread of the highly transmissible B.1.617.2 (or delta) variant.13,14 At the end of September 2021, persons younger than 18 years of age represented more than a quarter of weekly U.S. cases and 1.6 to 4.2% of cumulative hospitalizations.10 Covid-19–associated hospitalizations among children have increased steadily since early July 2021 in the United States; prevalence among 5-to-11-year-old children reached an all-time high of 1.1 per 100,000 population in late September.15 The pandemic has also interrupted education and has adversely affected children’s social and emotional development and mental health.16-19 Therefore, the availability of safe and efficacious vaccines for school-age children is critical.
Methods
Participants and Oversight
This phase 1 dose-level identification study and ongoing phase 2–3 safety, immunogenicity, and efficacy trial investigate the administration of the BNT162b2 vaccine to healthy participants 6 months to 11 years of age. Herein we present the results for children 5 to 11 years of age through the cutoff date (September 6, 2021); results for children 2 to 4 years and 6 months to less than 2 years of age are not yet available. Participants will be followed for 2 years after receipt of the first dose, including monitoring for potential cases of Covid-19 and MIS-C.
Participants were recruited by study site personnel. Children with no or stable preexisting conditions were eligible to participate, except those with an immunocompromising or immunodeficiency disorder, those with a history of MIS-C, or those receiving immunosuppressive therapy (including cytotoxic agents and systemic glucocorticoids). In addition, in the phase 1 study, children with a previous clinical or virologic Covid-19 diagnosis were excluded. Further inclusion and exclusion criteria and information on study responsibilities and ethical study conduct, including informed consent, are summarized in the Supplementary Appendix, available with the full text of this article at NEJM.org. The protocol contains additional details and is available at NEJM.org. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Procedures
Phase 1, Open-Label, Dose-Finding Study
In 5-to-11-year-olds, a vaccination regimen involving intramuscular (deltoid) injection of two doses of BNT162b2 administered 21 days apart was initiated at a 10-μg dose level on the basis of acceptable safety of the 30-μg dose level in 12-to-15-year-olds, described in the efficacy study.4 At each planned dose level (10 μg, 20 μg, and 30 μg), four sentinel participants received injections of BNT162b2, with vaccination followed by a planned 2-day pause. If no safety events of concern were noted, the remaining 12 participants in the group were vaccinated. After confirmation of an acceptable safety assessment at 10 μg by the internal review committee (see the Supplementary Appendix), vaccination began at the 20-μg dose level. The same process was followed for the 30-μg dose level.
Phase 2–3 Randomized Trial among Children 5 to 11 Years of Age
Through the use of an interactive Web-based system, participants were randomly assigned in a 2:1 ratio to receive two doses, 21 days apart, of either BNT162b2 at the dose level selected during phase 1 (10 μg) or saline placebo. In phase 2–3, all participants and study personnel, with the exception of those preparing or administering the injections, were unaware of group assignments. After 6 months, or after participants become eligible for Covid-19 vaccination according to local or national recommendations, placebo recipients will be offered the BNT162b2 vaccine.
Safety
Safety evaluations included assessment of reactogenicity events reported by a parent or guardian through the use of an electronic diary for 7 days after each dose. Data on unsolicited adverse events, including confirmed diagnoses of myocarditis or pericarditis, were collected from the first dose through 1 month after the second dose. Data on serious adverse events will be collected from the first dose through 6 months after the second dose.
Immunogenicity
For all participants in phase 1 and for a subset of participants in phase 2–3, blood samples were collected for immunogenicity assessments, which included determination of SARS-CoV-2 neutralization titers as described previously.20 Serum samples collected from 5-to-11-year-olds and 16-to-25-year-olds in this study were assayed in parallel to ensure comparability of titers. Analyses were conducted in participants who were without serologic or virologic evidence of past SARS-CoV-2 infection. In phase 1, SARS-CoV-2 neutralizing geometric mean titers (GMTs) were measured in serum samples obtained at 7 days after the second dose from participants who could be evaluated (Table S1). In phase 2–3, we calculated the ratio of GMTs (geometric mean ratio) and the difference between the percentage of participants with seroresponse among 5-to-11-year-olds and that among 16-to-25-year-olds at 1 month after the second dose, GMTs at baseline and 1 month after the second dose, and geometric mean fold rises from baseline to 1 month after the second dose. Seroresponse was defined by an increase in titers by a factor of at least 4 from baseline or by titers that were at least 4 times the lower limit of quantitation if the baseline measurement was less than the lower limit of quantitation.
Efficacy
Vaccine efficacy against confirmed Covid-19 with onset at least 7 days after the second dose was described both in participants without evidence of previous SARS-CoV-2 infection and in all participants. Methods for identifying SARS-CoV-2 infection and Covid-19 are summarized in the Supplementary Appendix.
Statistical Analysis
The sample size for phase 1 and the total sample size for phase 2–3 were not based on statistical hypothesis testing. Safety end points for the safety population (Table S1) are presented descriptively as counts, percentages, and associated Clopper–Pearson two-sided 95% confidence intervals. Adverse events and serious adverse events are categorized according to terms in the Medical Dictionary for Regulatory Activities (MedDRA), version 24.0, for each group.
Effectiveness was inferred by an “immunobridging” approach, in which neutralizing titers elicited by BNT162b2 in 5-to-11-year-olds were compared with titers elicited by BNT162b2 in 16-to-25-year-olds (in whom efficacy had been demonstrated4), with formal noninferiority hypothesis testing. The immunobridging subset of 5-to-11-year-olds (those whose neutralizing titers were compared) consisted of 485 participants enrolled from U.S. and European sites (322 in the BNT162b2 group and 163 in the placebo group). The immunobridging comparison used data that could be evaluated from the immunogenicity population of the immunobridging subset. A random sample of 350 participants 16 to 25 years of age from an earlier study4 (300 in the 30-μg BNT162b2 group and 50 in the placebo group [included for blinding purposes]) was selected with the use of a simple random-sample selection procedure as the immunobridging control age group. Immunobridging success as specified in the protocol was based on the geometric mean ratio of neutralizing titers of serum samples drawn 1 month after the second dose and was declared if the lower limit of the 95% confidence interval for the geometric mean ratio (5-to-11-year-olds to 16-to-25-year-olds) was greater than 0.67 and the geometric mean ratio point estimate was 0.8 or greater. FDA communication received after the database lock for the reported analyses proposed increasing the geometric mean ratio point estimate for immunobridging success to 1.0 or greater.
Immunobridging success based on the difference in seroresponse was declared if the lower limit of the 95% confidence interval for the difference in percentages of participants (5–11 years minus 16–25 years) with seroresponse was greater than –10%. A sample size of 225 BNT162b2 recipients with data that could be evaluated in both age groups provided 90.4% and 92.6% power to demonstrate immunobridging on the basis of the geometric mean ratio and the difference in seroresponse, respectively.
Vaccine efficacy was defined as 100×(1–IRR), where IRR is the rate ratio of confirmed Covid-19 illness in the BNT162b2 group to that in the placebo group. The associated two-sided 95% confidence interval for vaccine efficacy was derived with the Clopper–Pearson method, adjusted for surveillance time (defined as the total time in 1000 person-years for the given end point across all participants within each group at risk for the end point).
Results
Participants
Phase 1
From March 24 through April 14, 2021, a total of 50 children 5 to 11 years of age were screened for inclusion at four U.S. sites, and 48 received escalating doses of the BNT162b2 vaccine (Figure 1). Half the children were male, 79% were White, 6% were Black, 10% were Asian, and 8% were Hispanic or Latinx. The mean age was 7.9 years (Table S2).
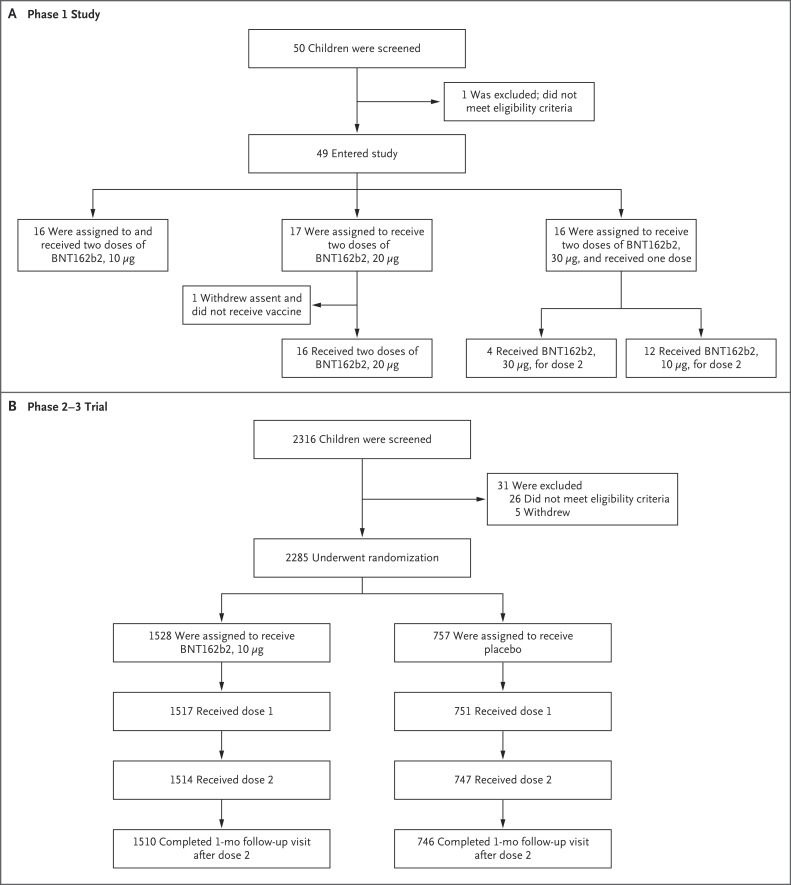
Figure 1. Screening, Randomization, and Vaccine and Placebo Administration among 5-to-11-Year-Old Children in the Phase 1 Study and the Phase 2–3 Trial.
Participants who discontinued the vaccination regimen could remain in the study. In the phase 2–3 trial, reasons for not receiving the first dose included withdrawal (14 children), no longer meeting eligibility criteria (2 children), and protocol deviation (1 child). Discontinuations or withdrawals after the first dose were due to a decision by the parent or guardian or by the participant, except one, for which the reason was classified as “other.” In the phase 2–3 trial, one participant who was randomly assigned to receive placebo was administered BNT162b2 in error for both doses. Therefore, 1518 participants received dose 1 of BNT162b2 and 750 participants received dose 1 of placebo.
Phase 2–3
From June 7 through June 19, 2021, a total of 2316 children 5 to 11 years of age were screened for inclusion and 2285 underwent randomization across 81 sites in the United States, Spain, Finland, and Poland; 2268 participants received injections, with 1517 randomly assigned to receive BNT162b2 and 751 assigned to receive placebo (Figure 1). One participant who was randomly assigned to receive placebo was administered BNT162b2 in error for both doses; therefore, 1518 participants received dose 1 of BNT162b2 and 750 participants received dose 1 of placebo. More than 99% of participants received a second dose. At the data cutoff date, the median follow-up time was 2.3 months (range, 0 to 2.5); 95% of participants had at least 2 months of available follow-up safety data after the second dose. Overall, 52% were male, 79% were White, 6% were Black, 6% were Asian, and 21% were Hispanic or Latinx (Table 1). The mean age was 8.2 years; 20% of children had coexisting conditions (including 12% with obesity and approximately 8% with asthma), and 9% were SARS-CoV-2–positive at baseline. Apart from younger age and a lower percentage of Black and Hispanic or Latinx 5-to-11-year-olds (6% and 18%, respectively) than 16-to-25-year-olds (12% and 36%, respectively), demographic characteristics were similar among the 5-to-11-year-old and 16-to-25-year-old BNT162b2 recipients who were included in the immunobridging subset (Table S3).
Table 1. Demographic and Clinical Characteristics of Children in the Phase 2–3 Trial.*.
| Characteristic | BNT162b2 (N=1518)† |
Placebo (N=750)† |
Total (N=2268) |
|---|---|---|---|
| Male sex — no. (%) | 799 (52.6) | 383 (51.1) | 1182 (52.1) |
| Race — no. (%)‡ | |||
| White | 1204 (79.3) | 586 (78.1) | 1790 (78.9) |
| Black | 89 (5.9) | 58 (7.7) | 147 (6.5) |
| Asian | 90 (5.9) | 47 (6.3) | 137 (6.0) |
| Multiracial | 109 (7.2) | 49 (6.5) | 158 (7.0) |
| Other or not reported | 26 (1.7) | 10 (1.3) | 36 (1.6) |
| Hispanic or Latinx ethnicity — no. (%)‡ | 319 (21.0) | 159 (21.2) | 478 (21.1) |
| Country — no. (%) | |||
| United States | 1073 (70.7) | 531 (70.8) | 1604 (70.7) |
| Finland | 158 (10.4) | 81 (10.8) | 239 (10.5) |
| Spain | 162 (10.7) | 78 (10.4) | 240 (10.6) |
| Poland | 125 (8.2) | 60 (8.0) | 185 (8.2) |
| Age at vaccination — yr | |||
| Mean ±SD | 8.2±1.93 | 8.1±1.97 | 8.2±1.94 |
| Median (range) | 8.0 (5–11) | 8.0 (5–11) | 8.0 (5–11) |
| Obese — no. (%)§ | 174 (11.5) | 92 (12.3) | 266 (11.7) |
| Coexisting conditions¶ | 312 (20.6) | 152 (20.3) | 464 (20.5) |
| Baseline SARS-CoV-2 infection status — no. (%)‖ | |||
| Positive | 133 (8.8) | 65 (8.7) | 198 (8.7) |
| Negative | 1385 (91.2) | 685 (91.3) | 2070 (91.3) |
Results shown are for the safety population (see Table S1). Percentages may not total 100 because of rounding.
One participant who was randomly assigned to receive placebo was administered BNT162b2 in error; this participant received two doses of BNT162b2 and is included in the BNT162b2 column.
Race and ethnicity were reported by the participants or by their parents or guardians.
Obesity was defined as a body-mass index (the weight in kilograms divided by the square of the height in meters) at or above the 95th percentile according to the U.S. Centers for Disease Control and Prevention growth charts.
Coexisting conditions are those that increase the risk of severe Covid-19 disease (i.e., one or more prespecified underlying conditions as defined in Kim et al.,21 obesity, or both).
A positive SARS-CoV-2 status required a positive N-binding antibody result at the first vaccination visit, a positive nucleic acid amplification test result at the first vaccination visit, or a medical history of Covid-19.
Phase 1 Safety and Immunogenicity
Most local reactions were mild to moderate, and all were transient (Fig. S1A and Table S4). Fever was more common in the 30-μg dose-level group than in the 10-μg and 20-μg dose-level groups after the first and second doses (Fig. S1B). All four sentinel participants in the 30-μg dose-level group who received the second 30-μg dose had mild-to-moderate fever within 7 days; the remaining 12 participants in the 30-μg dose-level group received a 10-μg second dose approximately 1 month after the first dose, as recommended by the internal review committee after selection of the phase 2–3 dose. Adverse events from the first dose through 1 month after the second dose were reported by 43.8% of participants who received two 10-μg doses of BNT162b2, 31.3% of those who received two 20-μg doses, and 50.0% of those who received two 30-μg doses (Table S6). One severe adverse event (grade 3 pyrexia) in a 10-year-old participant began the day of the second 20-μg dose of BNT162b2, with temperature reaching 39.7°C (103.5°F) the day after vaccination and resolving the following day. Antipyretic medications were used, and the investigator considered the event to be related to receipt of the BNT162b2 vaccine.
Serum neutralizing GMTs 7 days after the second dose were 4163 with the 10-μg dose of BNT162b2 and 4583 with the 20-μg dose (Fig. S2). On the basis of these safety and immunogenicity findings, the 10-μg dose level was selected for further assessment in 5-to-11-year-olds in phase 2–3.
Phase 2–3 Safety
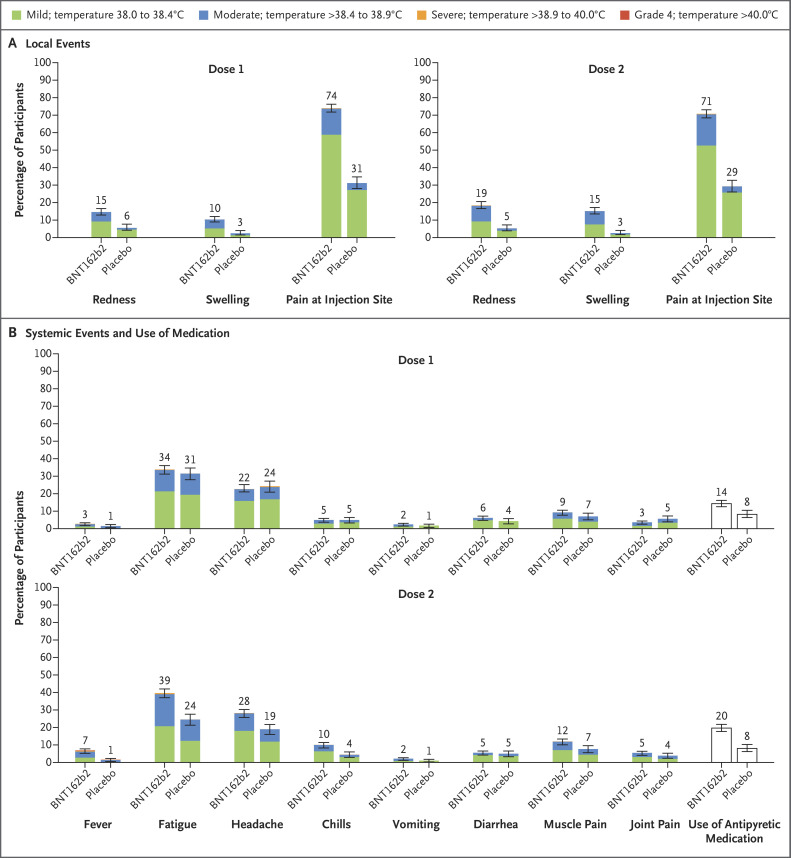
BNT162b2 recipients reported more local reactions and systemic events than placebo recipients (Figure 2). The reactions and events reported were generally mild to moderate, lasting 1 to 2 days (Table S4). Injection-site pain was the most common local reaction, occurring in 71 to 74% of BNT162b2 recipients. Severe injection-site pain after the first or second dose was reported in 0.6% of BNT162b2 recipients and in no placebo recipients. Fatigue and headache were the most frequently reported systemic events. Severe fatigue (0.9%), headache (0.3%), chills (0.1%), and muscle pain (0.1%) were also reported after the first or second dose of BNT162b2. Frequencies of fatigue, headache, and chills were similar among BNT162b2 and placebo recipients after the first dose and were more frequent among BNT162b2 recipients than among placebo recipients after the second dose. In general, systemic events were reported more often after the second dose of BNT162b2 than after the first dose. Fever occurred in 8.3% of BNT162b2 recipients after the first or second dose. Use of an antipyretic among BNT162b2 recipients was more frequent after the second dose than after the first dose. One BNT162b2 recipient had a temperature of 40.0°C (104°F) 2 days after the second dose; antipyretics were used, and the fever resolved the next day.
Figure 2. Local Reactions and Systemic Events Reported in the Phase 2–3 Trial within 7 Days after Injection of BNT162b2 or Placebo.
Panel A shows local reactions and Panel B shows systemic events after the first and second doses in recipients of the BNT162b2 vaccine (dose 1, 1511 children; dose 2, 1501 children) and placebo (dose 1, 748 or 749 children; dose 2, 740 or 741 children). The numbers refer to the numbers of children reporting at least one “yes” or “no” response for the specified event after each dose; responses may not have been reported for every type of event. Severity scales are summarized in Table S5; fever categories are designated in the key. The numbers above the bars are the percentage of participants in each group with the specified local reaction or systemic event. 𝙸 bars represent 95% confidence intervals. One participant in the BNT162b2 group had a fever of 40.0°C after the second dose.
From the first dose through 1 month after the second dose, adverse events were reported by 10.9% of BNT162b2 recipients and 9.2% of placebo recipients (Table S7). Slightly more BNT162b2 recipients (3.0%) than placebo recipients (2.1%) reported adverse events that were considered by the investigators to be related to the vaccine or placebo. Severe adverse events were reported in 0.1% of BNT162b2 recipients and 0.1% of placebo recipients. Three serious adverse events in two participants were reported by the cutoff date; all three (postinjury abdominal pain and pancreatitis in a placebo recipient and arm fracture in a BNT162b2 recipient) were considered to be unrelated to the vaccine or placebo. No deaths or adverse events leading to withdrawal were reported.
Lymphadenopathy was reported in 10 BNT162b2 recipients (0.9%) and 1 placebo recipient (0.1%). No myocarditis, pericarditis, hypersensitivity, or anaphylaxis in BNT162b2 recipients was reported. Four rashes in BNT162b2 recipients (observed on the arm, torso, face, or body, with no consistent pattern) were considered to be related to vaccination; the rashes were mild and self-limiting, and onset was typically 7 days or more after vaccination. No safety differences were apparent when the data were analyzed according to baseline SARS-CoV-2 infection status.
Phase 2–3 Immunogenicity
The geometric mean ratio of neutralizing GMTs for 10 μg of BNT162b2 in 5-to-11-year-olds to that for 30 μg of BNT162b2 in 16-to-25-year-olds 1 month after the second dose was 1.04 (95% confidence interval [CI], 0.93 to 1.18) (Table 2), a ratio meeting the immunobridging criterion of a lower boundary of the two-sided 95% confidence interval greater than 0.67, the predefined point estimate of a geometric mean ratio of 0.8 or greater, and the FDA-requested point estimate criterion of a geometric mean ratio of 1.0 or greater. In both age groups, 99.2% of participants achieved seroresponse 1 month after the second dose. The difference between the percentage of 5-to-11-year-olds who achieved seroresponse and the percentage in 16-to-25-year-olds was 0.0 percentage points (95% CI, –2.0 to 2.2), which also met an immunobridging criterion.
Table 2. Results of Serum SARS-CoV-2 Neutralization Assay 1 Month after the Second Dose of BNT162b2 among Participants 5 to 11 and 16 to 25 Yr of Age.*.
| Age Group | BNT162b2 Dose Level |
No. of Participants |
GMT (95% CI)† | Geometric Mean Ratio, 5-to-11-yr-olds vs. 16-to-25-yr-olds (95% CI)‡ |
|---|---|---|---|---|
| 5–11 yr | 10 μg | 264 | 1197.6 (1106.1–1296.6) | 1.04 (0.93–1.18) |
| 16–25 yr | 30 μg | 253 | 1146.5 (1045.5–1257.2) | — |
Results are those that could be evaluated for participants in the immunogenicity population of the immunobridging subset (Table S1) who had no serologic or virologic evidence of past or current SARS-CoV-2 infection up to the visit 1 month after the second dose and who had no history of Covid-19. Twenty-eight of 322 participants 5 to 11 years of age and 27 of 300 participants 16 to 25 years of age were excluded from the immunogenicity population; the most common reasons were that the participant did not have at least one valid and determinate immunogenicity result within 28 to 42 days after the second dose (13 and 21 participants, respectively), which included those who either did not have blood drawn at 1 month or did not have blood drawn within the specified time window, and protocol deviation (10 and 4 participants, respectively). Participants could be excluded for more than one reason. Among those in the population with data that could be evaluated, 30 participants who were 5 to 11 years of age and 20 participants who were 16 to 25 years of age were further excluded because they did not meet the requirement of “without evidence of infection” for the primary comparison.
Geometric mean titers (GMTs) and two-sided 95% confidence intervals were calculated by exponentiation of the mean logarithm of the titers and the corresponding confidence intervals (based on Student’s t distribution). Assay results below the lower limit of quantitation were set to 0.5 times the lower limit of quantitation.
The geometric mean ratio and two-sided 95% confidence intervals were calculated by exponentiation of the mean difference of the logarithms of the titers (the 5-to-11-year-old cohort minus the 16-to-25-year-old cohort) and the corresponding confidence intervals (based on Student’s t distribution). The immunobridging criterion was met because the lower boundary of the two-sided confidence interval for the geometric mean ratio was greater than 0.67 and the point estimate of the geometric mean ratio was 0.8 or more.
Serum-neutralizing GMTs 1 month after the second dose of BNT162b2 were 1198 in 5-to-11-year-olds and 1147 in 16-to-25-year-olds (Fig. S3); corresponding GMTs among placebo recipients were 11 and 10. Geometric mean fold rises from baseline to 1 month after the second dose were 118.2 in 5-to-11-year-olds and 111.4 in 16-to-25-year-olds; corresponding geometric mean fold rises among placebo recipients were 1.1 and 1.0. Of note, the neutralizing GMTs reported in phase 1 are from serum samples obtained 7 days after the second dose (during immune response expansion) and the GMTs in phase 2–3 are from serum samples obtained 1 month after the second dose.
Phase 2–3 Efficacy
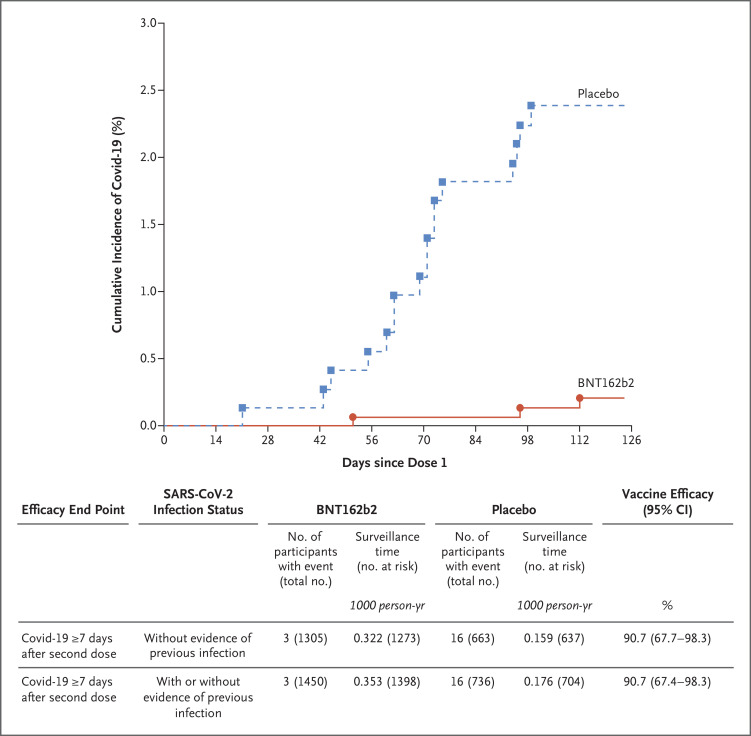
Among participants without evidence of previous SARS-CoV-2 infection, there were three cases of Covid-19 (with onset 7 days or more after the second dose) among BNT162b2 recipients and 16 among placebo recipients; the observed vaccine efficacy was 90.7% (95% CI, 67.7 to 98.3). Among all participants with data that could be evaluated, regardless of evidence of previous SARS-CoV-2 infection, no additional cases were reported; the observed vaccine efficacy was 90.7% (95% CI, 67.4 to 98.3) (Figure 3). No cases of severe Covid-19 or MIS-C were reported.
Figure 3. Vaccine Efficacy in Children 5 to 11 Years of Age.
The graph represents the cumulative incidence of the first occurrence of Covid-19 after the first dose of vaccine or placebo. Each symbol represents cases of Covid-19 starting on a given day. Results shown in the graph are all available data for the efficacy population, and results shown in the table are those for the efficacy population that could be evaluated (defined in Table S1). Participants without evidence of previous infection were those who had no medical history of Covid-19 and no serologic or virologic evidence of past SARS-CoV-2 infection before 7 days after the second dose (i.e., N-binding serum antibody was negative at the first vaccination visit, SARS-CoV-2 was not detected in nasal swabs by nucleic acid amplification test at the vaccination visits, and nucleic acid amplification tests were negative at any unscheduled visit before 7 days after the second dose). The cutoff date for the efficacy evaluation was October 8, 2021. Surveillance time is the total time in 1000 person-years for the given end point across all participants within each group at risk for the end point. The time period for Covid-19 case accrual was from 7 days after the second dose to the end of the surveillance period. The 95% confidence intervals for vaccine efficacy were derived by the Clopper–Pearson method, adjusted for surveillance time.
Discussion
Two 10-μg doses of the BNT162b2 vaccine administered 21 days apart were safe, immunogenic, and 90.7% effective against Covid-19 in 5-to-11-year-old children. Evaluation of BNT162b2 in children was undertaken for several reasons. With the prioritization of older age groups for vaccination, school-age children now account for an increasing proportion of cases and hospitalizations as compared with their proportion early in the pandemic,10,22,23,24 and school outbreaks are occurring.14,25 Direct benefits of preventing SARS-CoV-2 infection in children include protection against severe disease, hospitalizations, and severe or long-term complications, such as MIS-C. Indirect benefits include the likelihood of reduced transmission in the home and in school settings, including transmission affecting vulnerable persons, and safer in-person learning.26-29 Without effective Covid-19 vaccines for this age group, children could potentially become ongoing reservoirs of infection and sources of newly emerging variants.26,30 Covid-19–associated school closures and quarantines also have social and economic costs for families and caregivers.16-19 Widespread vaccination across age groups is therefore essential in ongoing efforts to curtail the pandemic.
On the basis of phase 1 safety and immunogenicity results, 10 μg of BNT162b2 was selected as the dose level to be studied in 5-to-11-year-olds in the phase 2–3 trial. This dose level was associated with mainly low-grade local and systemic adverse events lasting 1 to 2 days; the frequency and severity of fever were low. As compared with adults and adolescents in the pivotal trial, 5-to-11-year-olds reported a higher incidence of injection-site redness (15 to 19%, vs. 5 to 7%) and swelling (10 to 15%, vs. 5 to 8%), but a generally lower incidence of systemic events, including fever (3 to 7%, vs. 1 to 20%) and chills (5 to 10%, vs. 6 to 42%).3,4 Lymphadenopathy was reported in 0.9% of 5-to-11-year-old BNT162b2 recipients, an incidence similar to that in 12-to-15-year-olds (0.8%) but higher than that observed in adults (0.3%).3,4 Four potentially vaccine-related rashes were reported, too few to determine whether the pattern was similar to that observed in adult BNT162b2 recipients.31 No MIS-C cases were reported, although surveillance continues. Neither myocarditis nor pericarditis was observed, a finding consistent with the low frequency of these adverse events with real-world use of BNT162b2 in other age groups.32
The robust virus-neutralizing response observed in 5-to-11-year-olds was similar to that seen in 16-to-25-year-olds from the pivotal trial, which demonstrated 95% vaccine efficacy among persons at least 16 years of age from 7 days to approximately 2 months after the second dose.3 Immunobridging to data from 16-to-25-year-olds, alongside efficacy assessments, was used to support emergency use authorization in 12-to-15-year-olds.4 The observed high efficacy of BNT162b2 in 5-to-11-year-olds is consistent with the immunobridging results and the high efficacy demonstrated in the pivotal trial.3,4,6
This study describes immunization against SARS-CoV-2 infection with an mRNA vaccine in children younger than 12 years of age and documents the safety, immunogenicity, and efficacy of a Covid-19 vaccine in this population; trials of other vaccines are under way. Limitations of the study include the lack of longer-term follow-up to assess the duration of immune responses, efficacy, and safety. However, longer-term follow-up from this study, which will continue for 2 years, should provide clarification. This study was also not powered to detect potential rare side effects of BNT162b2 in 5-to-11-year-olds. However, the safety of BNT162b2 observed in the study combined with widespread use of BNT162b2 in older populations should provide reassurance. Moreover, an expanded cohort of 5-to-11-year-olds is being assessed in the present study, and additional safety assessments are in progress. Further limitations are that concomitant administration of BNT162b2 with other vaccines was not assessed, and cell-mediated responses to immunization are not yet available.
The data reported herein support vaccination of 5-to-11-year-old children with two 10-μg doses of the BNT162b2 vaccine. Evaluation of BNT162b2 in younger children is ongoing.
Acknowledgments
We thank Sheena Hunt, Ph.D., and Tricia Newell, Ph.D., of ICON (North Wales, PA), who wrote the first draft of the manuscript under direction from the authors, with funding from Pfizer. We are grateful to all the participants who volunteered for this study and to the caregivers who allowed them to participate. We also thank all the study site personnel for their contributions to this study. We especially acknowledge members of the data monitoring committee, who have been reviewing the trial safety data: Jonathan Zenilman, Kathryn Edwards, Lawrence Stanberry, Robert Belshe, Steven G. Self, Heather Lipkind, and Robert Phillips Heine. We also acknowledge the following persons for their contributions to this work: Pfizer colleagues Greg Adams, Neda Aghajani Memar, Priscilla Alba, Ayman Ayoub, Gabriela Bassani, Molly Bennett, Mark Boaz, Mary Ellen Boers, Christopher Bowen, Donna Boyce, Michelle Bryson, Patrick Caubel, Andrea Cawein, Sherri Charlton, Darren Cowen, Yvette Crawley, Kimberly Ann Cristall, Carmel Devlin, Julie Donato, Saumita Dubey, Camilla Farrell, Beth Fetzer, Emily Graham, Caitlin Hansen, Elisa Harkins Tull, Mark Harre, Marie-Pierre Hellio Le Graverand-Gastineau, Cindy Ingerick-Holt, Martha Iwamoto, Luis Jodar, Hui Kim, Esther Ladipo, Rod MacKenzie, Jason McKinley, Patience Makinde, Robert Maroko, Ruchi Mathur, Shawn Musselman, Sagaya Mythili, Brendan O’Neill, Jason Painter, Marina Palombini, Elisabeth Pantazis-Butera, Pinky Patel, Vishal Patel, Elizabeth Paulukonis, Mark Pepin, Allison Pfeffer, Kellie Lynn Richardson, Elizabeth Rogers, Melinda Rottas, Carol Schaffer, Ian Schochet, Judy Sewards, Rupal Shah, Noushad Shahulhameed, Monish Shetty, Marianne Simone, Helen Smith, Sima Toussi, Dina Tresnan, Sarah Tweedy, Erica Weaver, Hayley Wyper, Gabriel Zegrean, Liping Zhang, the Vaccines Clinical Assay Team, the Vaccines Assay Development Team, and all the Pfizer colleagues not named here who contributed to the success of this study; and BioNTech colleagues Elizabeth Adams, Rene Bartz, Meghan Bushway, Alexandra Kemmer Brück, Zakaria Khondker, Kimberly Krüger, Christian Miculka, Orkun Orzhelvaci, Ruben Rizzi, Svetlana Shpyro, and Anna Sokolowska.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on November 9, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by BioNTech and Pfizer.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021;595:572-577. [DOI] [PubMed] [Google Scholar]
- 2.Comirnaty and Pfizer–BioNTech COVID-19 Vaccine. Food and Drug Administration, Silver Spring, MD, October 29, 2021. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine).
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comirnaty (Covid-19 vaccine, mRNA) prescribing information. Mainz, Germany: BioNTech Manufacturing, 2021. (https://www.fda.gov/media/151707/download).
- 6.Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021;385:1761-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L, Bromberg M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 delta variant infection, Israel, 2021. Emerg Infect Dis 2021;27:2919-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsabouri S, Makis A, Kosmeri C, Siomou E. Risk factors for severity in children with coronavirus disease 2019: a comprehensive literature review. Pediatr Clin North Am 2021;68:321-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). 2021. (https://www.cdc.gov/mis/hcp/index.html).
- 10.American Academy of Pediatrics. Children and COVID-19: state-level data report. 2021. (https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/).
- 11.Rumain B, Schneiderman M, Geliebter A. Prevalence of COVID-19 in adolescents and youth compared with older adults in states experiencing surges. PLoS One 2021;16(3):e0242587-e0242587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szablewski CM, Chang KT, Brown MM, et al. SARS-CoV-2 transmission and infection among attendees of an overnight camp — Georgia, June 2020. MMWR Morb Mortal Wkly Rep 2020;69:1023-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. COVID data tracker. 2021. (https://covid.cdc.gov/covid-data-tracker/#datatracker-home).
- 14.Lam-Hine T, McCurdy SA, Santora L, et al. Outbreak associated with SARS-CoV-2 B.1.617.2 (delta) variant in an elementary school — Marin County, California, May–June 2021. MMWR Morb Mortal Wkly Rep 2021;70:1214-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVID-NET. Laboratory-confirmed COVID-19-associated hospitalizations. 2021. (https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html).
- 16.McKune SL, Acosta D, Diaz N, et al. Psychosocial health of school-aged children during the initial COVID-19 safer-at-home school mandates in Florida: a cross-sectional study. BMC Public Health 2021;21:603-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engzell P, Frey A, Verhagen MD. Learning loss due to school closures during the COVID-19 pandemic. Proc Natl Acad Sci U S A 2021;118(17):e2022376118-e2022376118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Bueno R, López-Sánchez GF, Casajús JA, Calatayud J, Tully MA, Smith L. Potential health-related behaviors for pre-school and school-aged children during COVID-19 lockdown: a narrative review. Prev Med 2021;143:106349-106349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UNICEF. COVID-19 and children. 2020. (https://data.unicef.org/covid-19-and-children/).
- 20.Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delahoy MJ, Ujamaa D, Whitaker M, et al. Hospitalizations associated with COVID-19 among children and adolescents — COVID-NET, 14 states, March 1, 2020–August 14, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1255-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston LE, Chevinsky JR, Kompaniyets L, et al. Characteristics and disease severity of US children and adolescents diagnosed with COVID-19. JAMA Netw Open 2021;4(4):e215298-e215298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel DA, Reses HE, Cool AJ, et al. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0–17 years — United States, August 2020–August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1249-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jehn M, McCullough JM, Dale AP, et al. Association between K–12 school mask policies and school-associated COVID-19 outbreaks — Maricopa and Pima Counties, Arizona, July–August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1372-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson EJ, Campbell JD, Creech CB, et al. Warp speed for coronavirus disease 2019 (COVID-19) vaccines: why are children stuck in neutral? Clin Infect Dis 2021;73:336-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klass P, Ratner AJ. Vaccinating children against Covid-19 — the lessons of measles. N Engl J Med 2021;384:589-591. [DOI] [PubMed] [Google Scholar]
- 28.Parks SE, Zviedrite N, Budzyn SE, et al. COVID-19-related school closures and learning modality changes — United States, August 1–September 17, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1374-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul LA, Daneman N, Schwartz KL, et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr 2021:175;1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman E. How the unvaccinated threaten the vaccinated for COVID-19: a Darwinian perspective. Proc Natl Acad Sci U S A 2021;118(39):e2114279118-e2114279118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grieco T, Maddalena P, Sernicola A, et al. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: an observational study. Dermatol Ther 2021. October 7 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai C, Peng Y, Shen E, et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther 2021;29:2794-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.