Abstract
Background:
Peanut allergy is common, life-threatening, and without therapeutic options. We evaluated peanut epicutaneous immunotherapy (EPIT) by using Viaskin Peanut for peanut allergy treatment.
Objective:
We sought to evaluate the clinical, safety, and immunologic effects of EPIT for the treatment of peanut allergy. Methods: In this multicenter, double-blind, randomized, placebo-controlled study, 74 participants with peanut allergy (ages 4–25 years) were treated with placebo (n = 25), Viaskin Peanut 100 μg (VP100; n = 24) or Viaskin Peanut 250 μg (VP250; n = 25; DBV Technologies, Montrouge, France). The primary outcome was treatment success after 52 weeks, which was defined as passing a 5044-mg protein oral food challenge or achieving a 10-fold or greater increase in successfully consumed dose from baseline to week 52. Adverse reactions and mechanistic changes were assessed.
Results:
At week 52, treatment success was achieved in 3 (12%) placebo-treated participants, 11 (46%) VP100 participants, and 12 (48%) VP250 participants (P = .005 and P = .003, respectively, compared with placebo; VP100 vs VP250, P = .48). Median change in successfully consumed doses were 0, 43, and 130 mg of protein in the placebo, VP100, and VP250 groups, respectively (placebo vs VP100, P = .014; placebo vs VP250, P =.003). Treatment success was higher among younger children (P = .03; age, 4–11 vs >11 years). Overall, 14.4% of placebo doses and 79.8% of VP100 and VP250 doses resulted in reactions, predominantly local patch-site and mild reactions (P = .003). Increases in peanut-specific IgG4 levels and IgG4/IgE ratios were observed in peanut EPIT-treated participants, along with trends toward reduced basophil activation and peanut-specific TH2 cytokines.
Conclusions:
Peanut EPIT administration was safe and associated with a modest treatment response after 52 weeks, with the highest responses among younger children. This, when coupled with a high adherence and retention rate and significant changes in immune pathways, supports further investigation of this novel therapy.
Keywords: Peanut hypersensitivity, food allergy, immunotherapy, IgE, desensitization, epicutaneous
Peanut allergy is the most common life-threatening food allergy, with an overall prevalence of 0.5% to 1%1,2 and a 3-fold increase noted from 1997–2008.2 In addition to being a key culprit in food-induced mortality, peanut allergy is associated with reduced quality of life and health economic effect.3–5 Currently, there is no US Food and Drug Administration–approved treatment for peanut allergy, with management consisting of a peanut-free diet and access to self-injectable epinephrine.6 Despite active avoidance, the risk of an adverse reaction from exposure is ongoing.7,8 For all these reasons, an effective treatment for peanut allergy would be highly desirable.
Recent efforts have focused on development of allergen-specific immunotherapeutic approaches to treat peanut allergy.9–15 These approaches are designed to alter immunologic responses to induce short-term desensitization (elimination of reactivity while receiving therapy) and longer-term sustained unresponsiveness (elimination of reactivity while off therapy). Subcutaneous immunotherapy has proved to be unsafe for the treatment of peanut allergy.16,17 Sublingual immunotherapy has been demonstrated to induce modest clinical benefits while being well tolerated.10,12,18,19 Oral immunotherapy (OIT) has been shown to induce desensitization in most participants and sustained unresponsiveness in a minority, although adverse reactions are common.9,11,14,20–22
Epicutaneous immunotherapy (EPIT) is an emerging modality for the treatment of food allergy. Epicutaneous delivery of antigen has shown benefits when used to treat grass pollen allergy in adults.23,24 Murine studies indicate that epicutaneously applied antigen modulates TH2 immune responses25 through antigen-driven activation of dendritic cells with subsequent immune modulation through trafficking to lymph nodes.26,27 A pilot study of milk EPIT in 19 infants with milk allergy and children showed trends toward clinical efficacy with acceptable safety in participants treated for 3 months.28 A phase I study of peanut EPIT demonstrated safety and tolerability by using Viaskin Peanut (DBV Technologies, Montrouge, France) during a 2-week treatment period.15 The purpose of the current study was to further evaluate peanut EPIT delivered by means of Viaskin Peanut, specifically evaluating clinical desensitization, safety, and immunomodulation after 52 weeks of blinded treatment.
METHODS
Study design and participant selection
This multicenter, randomized, double-blind, placebo-controlled, phase II study compared 2 doses of Viaskin Peanut versus placebo in children and young adults with peanut allergy. The primary end point was the proportion of participants with a successful outcome after 52 weeks of blinded treatment, with treatment success defined as either passing a double-blind, placebo-controlled oral food challenge (OFC) with 5044 mg of peanut protein at week 52 or by a 10-fold or greater increase in the successfully consumed dose (SCD) of peanut protein compared with the baseline OFC. Secondary end points included comparison of the 100- and 250-μg Viaskin Peanut doses, safety, and modulation of immune parameters.
Inclusion criteria included the following: (1) 4 to 25 years of age, (2) physician-diagnosed peanut allergy or a convincing clinical history of peanut allergy, (3) positive skin prick test (SPT) response to peanut (wheal size ≥3 mm greater than that elicited by the saline control) or peanut-specific IgE level of greater than 0.35 kilounits of antibody (kUA)/L, and (4) positive baseline OFC result to a cumulative dose of 1044 mg or less peanut protein. Subjects with a history of severe anaphylaxis (previous hypotension, neurologic compromise, or mechanical ventilation) to peanut were excluded. See Table E1 in this article’s Online Repository at www.jacionline.org for detailed inclusion/exclusion criteria.
Enrollment and randomization
Participants were randomly assigned to double-blind peanut EPIT by using Viaskin Peanut 100 μg (VP100), Viaskin Peanut 250 μg (VP250), or placebo (1:1:1) at each of 5 clinical Consortium of Food Allergy Research (CoFAR) sites (75 total participants). The study was blinded through 52 weeks (Fig 1). Enrollment and randomization of younger participants (ages 4-<6 years) was paused after the first 10 participants were enrolled for a predetermined interim Data Safety Monitoring Board (DSMB) safety review after 35 days of dosing.
FIG 1.
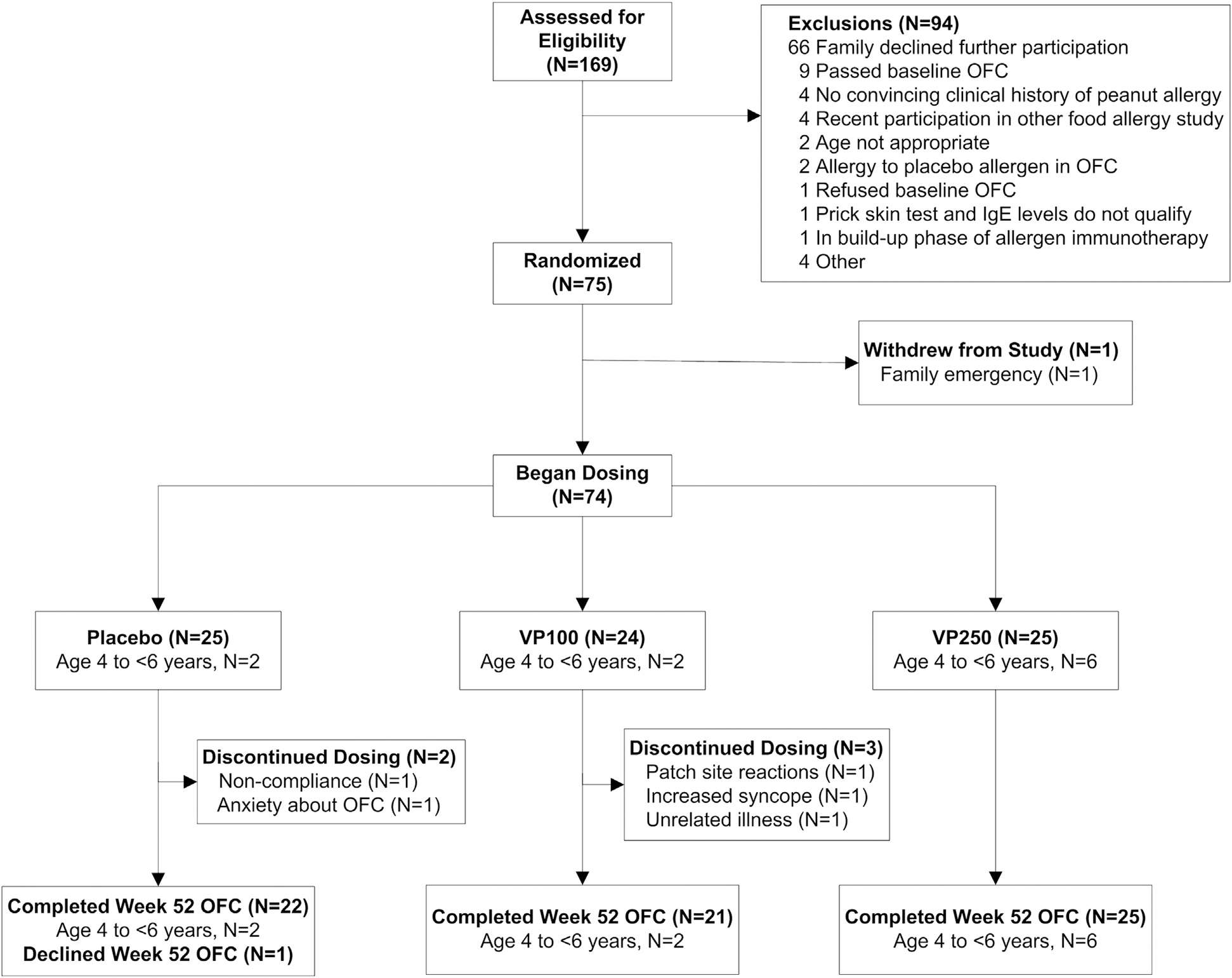
CONSORT diagram. Enrollment and randomization of younger participants aged 4 to less than 6 years was conducted as in the full study population, as indicated. Enrollment was paused after the first 10 participants were enrolled for a predetermined interim DSMB safety review after 35 days (21 days of escalation and 14 days of maintenance) of dosing to ensure tolerability of the study product. Because of completed study enrollment, no further participants in the 4- to less than 6-year-old age range were enrolled after the DSMB review.
Study product
The Viaskin Peanut patch used for this study is comprised of an epicutaneous delivery system containing a dry deposit of a formulation of peanut protein extract manufactured by DBV Technologies SA. The peanut extract is an unmodified lyophilized product derived from the extraction and freeze-drying of defatted peanut flour made from raw peanuts. A liquid formulation of the extract is then deposited on the backing of an occlusive chamber by using electrospraying. The Viaskin patch has a diameter of 26 mm, with an inner diameter of 18 mm containing the peanut protein. The matching Viaskin placebo is the same device devoid of any peanut protein but containing excipients included in the active patch.
EPIT dosing protocol
The Viaskin patch, plus optional Tegaderm covering, was placed on the upper arm (age >11 years) or the interscapular space (4–11 years) in a clockwise rotation by using 1 of 6 application sites at 24-hour intervals. Graduated dosing was performed with the same strength patch by increasing the time worn as follows: week 1, 3 h/d; week 2, 6 h/d; and week 3, 12 h/d. This was followed by patch application for 24 h/d beginning on day 22.
Participants were monitored in the research unit on days 1 and 2 for adverse reactions. If significant local reactions (ie, grade 3 or grade 4 skin reactions; see Table E2 in this article’s Online Repository at www.jacionline.org for grading criteria) occurred, participants were instructed to remove the patch immediately and contact the study team for further instructions regarding subsequent patch application. For persistent patch-site reactions, the patch was removed, and the participant was instructed to apply the patch for the length of time that it was tolerated for the following 3 days, followed by an increase in duration of patch application every 3 to 4 days until tolerated for a 24-hour period.
Usual medications, including topical corticosteroids and calcineurin inhibitors, were continued but not within 1 inch of the patch site. Oral and topical antihistamines and topical 1% hydrocortisone were approved for the treatment of patch-site reactions, with more potent topical steroids reserved for limited use with more bothersome reactions.
Adherence and safety assessments
Participants were contacted by telephone monthly and returned to the research unit at the start of weeks 2 to 4 and at completion of weeks 12, 24, 36, and 52 to review tolerability of the study drug, adherence, and any adverse events.
Adherence to daily dosing was assessed by using 2 methods. Participants maintained daily diary logs, recording the date and time of patch application and removal during the first 6 months of therapy. Thereafter, dosing logs were only used to record missed doses, doses removed prematurely, or doses associated with adverse symptoms. Dosing logs were reviewed by study personnel at each visit. Participants were also instructed to return all used and unused patches at each visit.
Participants were also monitored for patch-site reactions during scheduled visits and as needed if symptoms were reported. Skin changes at the patch site were scored as grade 0 to 4 by using a standardized scoring system (see Table E2). Symptoms extending outside of the patch site or involving systemic reactions were recorded, and the severity of allergic reactions was reported by using a customized grading system (see Table E3 in this article’s Online Repository at www.jacionline.org).
Predetermined rules for potential discontinuation of dosing included occurrence of systemic reactions during any stage of dosing, occurrence of any grade 4 patch-site reaction, more than 3 episodes of grade 3 patch-site reactions, or 2 or more consecutive grade 3 patch-site reactions. Adverse events, serious adverse events, and accidental exposures to peanut were reported throughout the study.
OFCs
At study entry, an OFC was conducted to a cumulative amount of 1044 mg of peanut protein administered in doses every 15 minutes by using a modified PRACTALL Protocol.29 The OFC was repeated at week 52 to a cumulative dose of 5044 mg of peanut protein (see the Methods section in this article’s Online Repository at www.jacionline.org).
SPTs
SPTs using the GREERPick device with peanut extract (Greer Laboratories, Lenoir, NC) and saline and histamine controls were performed at enrollment and 24 and 52 weeks after study entry, as previously described.10
In vitro assays
Mechanistic studies were conducted to assess the immunomodulatory effect of peanut EPIT by using serial testing of a variety of immune parameters. Serum peanut-specific IgE and IgG4 levels were measured by using the ImmunoCAP 250 (Thermo Fisher Scientific, Waltham, Mass). Basophil activation was measured based on CD63 upregulation by using flow cytometry in response to peanut extract stimulation of whole blood.10 Peanut-specific T-cell activation and phenotype were assessed by using flow cytometry with CD154 as an activation marker and intracellular staining for IL-4, IL-13, IFN-γ, and IL-10 (see the Methods section in this article’s Online Repository).
Ethics
Institutional review boards at each clinical site approved the protocol and consent forms. The study was conducted under a US Food and Drug Administration investigational new drug application and monitored by the National Institute of Allergy and Infectious Diseases DSMB. Written informed consent was obtained from parents/guardians, with assent of those more than 7 years of age.
Statistical analysis
The target sample size of 75 participants (randomized 1:1:1 and stratified by site) was selected to provide 95% power, assuming a 5% success rate for the primary end point in the placebo arm compared with 50% in each of the active arms. Power was determined by using a 1-sided exact unconditional binomial test (Barnard) with an α value of .0125 for each comparison of active to placebo treatment. Alternate success definitions were also compared between the active and placebo arms by using the Barnard test. Continuous variables were contrasted between treatment groups by using the Kruskal-Wallis test, followed by Wilcoxon rank sum tests for pairwise group comparisons. Safety data were contrasted between treatment groups by using the percentage of doses per participant and performed by using the Kruskal-Wallis test, followed by Wilcoxon rank sum tests for pairwise group comparisons.
Immunologic, activated basophil, and T-cell studies were contrasted between treatment groups over time by using repeated-measures models, accounting for within-participant correlation by using a Toeplitz covariance structure. Log10 transformations were applied as needed.
Prespecified exploratory analyses were performed to assess the effect of age on treatment effect by using logistic regression models for binary outcomes and Spearman correlations and linear regression models for continuous outcomes. The primary end point (VP250 vs placebo and VP100 vs placebo) was assessed at the .0125 significance level, mechanistic analyses were assessed at the .01 significance level to control for the multiplicity of analyses, and all other exploratory analyses were assessed at the .05 level. Primary end point P values were computed with StatXact (version 10; Cytel, Cambridge, Mass). All other analyses were performed with SAS (version 9.3 or higher; SAS Institute, Cary, NC).
RESULTS
Study population
The CONSORT diagram is represented in Fig 1: 169 participants were screened, 84 had a baseline OFC, 75 were randomized, and 74 received study treatment, with 1 participant withdrawing after randomization but before treatment initiation. The analysis population consists of 74 treated participants (25 in the placebo group, 24 in the VP100 group, and 25 in the VP250 group). As shown in Table I, the majority of participants were male (62.2%), and the median age was 8.2 years (range, 4–20 years). There were no significant differences in baseline demographic characteristics, comorbid atopic diseases, or immunologic measurements across treatment groups. The median baseline peanut SPT response was 12.8 mm, the median peanut IgE level was 78.2 kUA/L, and the median SCD was 44 mg of peanut protein.
TABLE I.
Baseline characteristics by treatment group*
| Placebo | VP100 | VP250 | Total | |
|---|---|---|---|---|
|
| ||||
| Sex, no. (%) | ||||
| Male | 16 (64.0) | 14 (58.3) | 16 (64.0) | 46 (62.2) |
| Female | 9 (36.0) | 10 (41.7) | 9 (36.0) | 28 (37.8) |
| Age (y), median (range) | 8.5 (4.8–20.3) | 8.4 (4.1–16.6) | 7.7 (4.2–14.4) | 8.2 (4.1–20.3) |
| Other allergic disease, no. (%) | ||||
| Asthma | 12 (48.0) | 16 (66.7) | 13 (52.0) | 41 (55.4) |
| Atopic dermatitis | 12 (48.0) | 11 (45.8) | 15 (60.0) | 38 (51.4) |
| Other food allergy | 20 (80.0) | 21 (87.5) | 20 (80.0) | 61 (82.4) |
| Atopic dermatitis total score, median (range) | 0.0 (0.0–7.0) | 0.0 (0.0–7.0) | 0.0 (0.0–7.0) | 0.0 (0.0–7.0) |
| Peanut SPT (mm), median (range) | 13.5 (3–39.5) | 11.8 (4.5–32.0) | 12.5 (6.0–25.5) | 12.8 (3–39.5) |
| Peanut IgE (kUA/L), median (range) | 58.0 (0.8–213.0) | 84.6 (0.4–213.0) | 92.1 (0.52–202.0) | 78.2 (0.4–213.0) |
| Peanut IgG4 (mg/L), median (range) | 1.1 (0.02–7.0) | 0.6 (0.03–2.4) | 0.5 (0.03–3.0) | 0.7 (0.02–7.0) |
| Peanut IgG4/IgE ratio,† median (range) | 3.8 (0.5–3571.4) | 2.5 (0.4–101.6) | 3.5 (0.6–74.5) | 3.6 (0.4–3571.4) |
| Total IgE (kU/L), median (range) | 360 (21.3–3334) | 452 (61.1–5169) | 472 (83.5–2143) | 452 (21.3–5169) |
| Peanut IgE/total IgE ratio (%), median (range) | 16.9 (0.4–43.7) | 12.1 (0.6–59.4) | 13.7 (0.4–54.8) | 13.9 (0.4–59.4) |
| Baseline OFC SCD (mg protein), median (range) | 44 (0–444) | 44 (0–444) | 14 (0–444) | 44 (0–444) |
There were no statistically significant differences between treatment groups in any baseline characteristics.
Peanut IgG4/IgE ratio was calculated by converting IgG4 levels from milligrams of antibody per liter to nanograms per milliliter and converting IgE levels from kUA per liter to nanograms per milliliter with the following formula: (IgG4 × 1000) ÷ (IgE × 2.4).
Three placebo-treated participants withdrew/discontinued dosing (2 because of anxiety before the week 52 OFC and 1 because of noncompliance), as did 3 participants from the VP100 group (1 because of grade 3/4 patch reactions, 1 because of non–study-related syncopal episodes, and 1 because of non–study-related illness). All of these participants were considered failures for the primary end point.
Efficacy of peanut EPIT
Table II presents results for the primary end point. For the placebo group, 3 (12.0%) participants met the primary end point compared with 11 (45.8%) for the VP100 group and 12 (48.0%) for the VP250 group. Only 1 participant (placebo) passed the week 52 OFC. Comparison of the treatment groups revealed significant differences between the placebo-treated participants and both active treatment arms (P =.005 and P =.003, respectively), with no difference between the VP100 and VP250 groups (P =.48).
TABLE II.
Week 52 OFC results by treatment group
| Placebo |
VP100 |
VP250 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | Percent | No. | Percent | No. | Percent | No. | Percent | |
|
| ||||||||
| Treatment success (primary end point)* | ||||||||
| Failure | 22 | 88.0 | 13 | 54.2 | 13 | 52.0 | 48 | 64.9 |
| Success | 3 | 12.0 | 11 | 45.8 | 12 | 48.0 | 26 | 35.1 |
| SCD ≥1044 mg of protein† | ||||||||
| Failure | 22 | 88.0 | 21 | 87.5 | 18 | 72.0 | 61 | 82.4 |
| Success | 3 | 12.0 | 3 | 12.5 | 7 | 28.0 | 13 | 17.6 |
| SCD ≥1044 mg of protein and 10-fold increase from baseline‡ | ||||||||
| Failure | 23 | 92.0 | 22 | 91.8 | 21 | 84.0 | 66 | 89.2 |
| Success | 2 | 8.0 | 2 | 8.3 | 4 | 16.0 | 8 | 10.8 |
Primary end point: The success criterion met was a 10-fold increase in SCD over baseline in all but 1 placebo-treated subject, who had no reaction and passed the week 52 OFC.
P = .005, placebo versus VP100; P = .003, placebo versus VP250; P = .48, VP100 versus VP250.
Post hoc analysis: P = .54, placebo versus VP100; P = .12, placebo versus VP250; P = .12, VP100 versus VP250.
Post hoc analysis: P = .55, placebo versus VP100; P = .26, placebo versus VP250; P = .27, VP100 versus VP250.
Post hoc analyses were undertaken to assess 2 additional efficacy end points (Table II). First, we compared the proportion of participants in each group who had an SCD of at least 1044 mg of protein at the week 52 OFC, which was achieved in 3 (12.0%) placebo-treated participants, 3 (12.5%) VP100-treated participants, and 7 (28.0%) VP250-treated participants (P = not significant for all comparisons). Second, we compared the number of participants who had an SCD of at least 1044 mg of protein plus at least a 10-fold increase in SCD at the week 52 OFC, revealing that only 2 (8.0%) placebo-treated participants, 2 (8.3%) VP100-treated participants, and 4 (16.0%) VP250-treated participants met this stricter definition of success (P = not significant for all comparisons).
Table III shows the SCD for the week 52 OFC, as well as the change in SCD from baseline (Fig 2, A). The placebo group had a median change in SCD of 0 mg of protein (interquartile range [IQR], −40.0 to 1.0) compared with median changes of 43 mg of protein (IQR, 0.0 to 140) in the VP100 group and 130 mg of protein (IQR, 30 to 600) in the VP250 group. Median change in SCD was significantly different among the 3 treatment groups (P = 0.003, Kruskal-Wallis test), as well as between the placebo and VP100 and VP250 groups (placebo vs VP100, P =.014; placebo vs VP250, P =.003; VP100 vs VP250, P =.41).
TABLE III.
1 Week 52 SCD and change from baseline
| Placebo | VP100 | VP250 | Total | |
|---|---|---|---|---|
|
| ||||
| Week 52 SCD (mg of protein) | ||||
| No. | 22 | 21 | 25 | 68 |
| Median | 14 | 144 | 144 | 144 |
| Minimum | 1 | 44 | 0 | 0 |
| Maximum | 5044 | 2044 | 2044 | 5044 |
| Change in SCD (mg of protein)* | ||||
| No. | 22 | 21 | 25 | 68 |
| Median | 0 | 43 | 130 | 40 |
| Minimum | −440 | −300 | −300 | −440 |
| Maximum | 4600 | 2040 | 2040 | 4600 |
P = .003 comparing all 3 groups, P = .014 for placebo versus VP100, P = .003 for placebo versus VP250, and P = .41 for VP100 versus VP250.
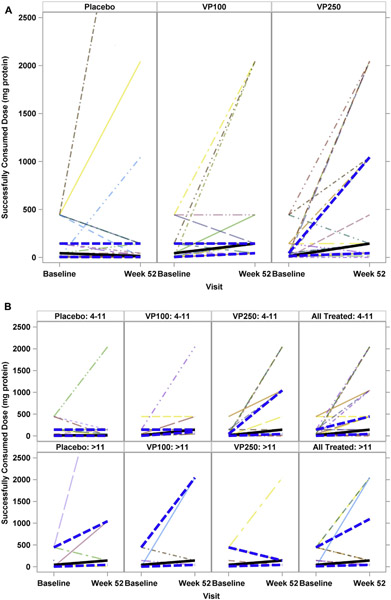
FIG 2.
SCD from baseline to the week 52 OFC. A, Analysis by treatment group. B, Analysis by age and treatment group. Top panels represent the 4- to 11-year-old age group. Bottom panels represent the greater than 11-year-old age group. Solid lines represent median values, and hatched lines represent the upper and lower IQR.
As a preplanned exploratory analysis, we assessed the potential effects of age on outcomes (Fig 2, B, and Table IV and see Table E4 in this article’s Online Repository at www.jacionline.org). We fit a model with the primary end point as the outcome with age as a continuous variable and with age as a dichotomous variable when comparing participants 11 years or younger with those older than 11 years. Both approaches revealed a statistically significant age-by-treatment interaction, with a successful outcome being more common in younger participants (P = .03, dichotomous analysis; P = .006, continuous model). In the subgroup of participants 11 years or younger, treatment success was achieved in 1 (6%) placebo-treated child, 10 (59%) VP100-treated children, and 11 (61%) VP250-treated children (P = .0006 and P = .0003, respectively, compared with placebo; VP100 vs VP250, P = .98).
TABLE IV.
Week 52 OFC results by treatment group and age group
| Treatment group |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo |
VP100 |
VP250 |
All |
|||||||||||||||||||
| 4–11 y |
>11 y |
All |
4–11 y |
>11 y |
All |
4–11 y |
>11 y |
All |
4–11 y |
>11 y |
||||||||||||
| No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | |
|
| ||||||||||||||||||||||
| Treatment success | ||||||||||||||||||||||
| Failure | 17 | 94.4 | 5 | 71.4 | 22 | 88.0 | 7 | 41.2 | 6 | 85.7 | 13 | 54.2 | 7 | 38.9 | 6 | 85.7 | 13 | 52.0 | 31 | 58.5 | 17 | 81.0 |
| Success | 1 | 5.6 | 2 | 28.6 | 3 | 12.0 | 10 | 58.8 | 1 | 14.3 | 11 | 45.8 | 11 | 61.1 | 1 | 14.3 | 12 | 48.0 | 22 | 41.5 | 4 | 19.0 |
| SCD ≥1044 mg of protein | ||||||||||||||||||||||
| Failure | 17 | 94.4 | 5 | 71.4 | 22 | 88.0 | 16 | 94.1 | 5 | 71.4 | 21 | 87.5 | 12 | 66.7 | 6 | 85.7 | 18 | 72.0 | 45 | 84.9 | 16 | 76.2 |
| Success | 1 | 5.6 | 2 | 28.6 | 3 | 12.0 | 1 | 5.9 | 2 | 28.6 | 3 | 12.5 | 6 | 33.3 | 1 | 14.3 | 7 | 28.0 | 8 | 15.1 | 5 | 23.8 |
| SCD ≥1044 mg of protein and 10-fold increase from BL | ||||||||||||||||||||||
| Failure | 18 | 100.0 | 5 | 71.4 | 23 | 92.0 | 16 | 94.1 | 6 | 85.7 | 22 | 91.7 | 14 | 77.8 | 7 | 100.0 | 21 | 84.0 | 48 | 90.6 | 18 | 85.7 |
| Success | 0 | 0.0 | 2 | 28.6 | 2 | 8.0 | 1 | 5.9 | 1 | 14.3 | 2 | 8.3 | 4 | 22.2 | 0 | 0.0 | 4 | 16.0 | 5 | 9.4 | 3 | 14.3 |
Logistic regression analysis was performed to determine whether any additional baseline factors other than age predicted treatment success (see Table E5 in this article’s Online Repository at www.jacionline.org). Only an SCD of less than 44 mg at baseline was statistically associated with a successful outcome (P = .0001). This association might only reflect that a lower baseline SCD results in easier attainment of the primary end point; baseline SCD was not significantly correlated with change in SCD from baseline to week 52. Notably, the presence or severity of atopic dermatitis at baseline was not predictive of treatment response.
Safety and adherence
Table V presents dosing symptoms by dose, participant, and percentage of doses per participant for each treatment. Overall, 14.4% of placebo doses resulted in a reaction compared with 79.8% of VP100 and VP250 doses. The majority of reactions were mild and limited to the patch site. Grade 2 or greater patch-site reactions occurred with 1.6% of placebo doses (no grade 3 or 4 reactions) compared with 18.7% of VP100 doses and 23.4% of VP250 doses. One grade 4 patch-site reaction occurred with the VP100 dose in a 12-year-old participant 34 days after enrollment. Reactions extending past the patch site occurred with 1.5% of placebo doses, 8.9% of VP100 doses, and 16.2% of VP250 doses.
TABLE V.
Dosing symptoms by treatment group
|
Dosing symptoms by dose
| |||||||||||||||||||||||||
| Treatment group | No. of doses |
Any reaction
|
Patch-site reactions
|
Reaction extended past patch site
|
Non–patch-site reaction
|
Non–patch-site symptoms
|
Symptoms >8 h
|
Treated
|
|||||||||||||||||
|
Any patch-site reaction
|
Grade 2 patch-site reaction
|
Grade 3 patch-site reaction
|
Grade 4 patch-site reaction
|
Mild symptoms
|
Moderate symptoms
|
Severe symptoms
|
|||||||||||||||||||
| No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | ||
|
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 8418 | 1216 | 14.4 | 1200 | 14.3 | 128 | 1.5 | 0 | 0.00 | 0 | 0.0 | 133 | 1.58 | 17 | 0.2 | 17 | 0.2 | 0 | 0.00 | 0 | 0.00 | 982 | 11.7 | 106 | 1.3 |
| VP100 | 8121 | 6482 | 79.8 | 6479 | 79.8 | 1513 | 18.6 | 6 | 0.01 | 1 | 0.0 | 720 | 8.87 | 20 | 0.2 | 18 | 0.2 | 1 | 0.01 | 0 | 0.00 | 4989 | 61.4 | 2218 | 27.3 |
| VP250 | 9033 | 7205 | 79.8 | 7203 | 79.7 | 2110 | 23.4 | 7 | 0.00 | 0 | 0.0 | 1466 | 16.23 | 6 | 0.1 | 6 | 0.1 | 0 | 0.00 | 0 | 0.00 | 6397 | 70.8 | 2142 | 23.7 |
|
| |||||||||||||||||||||||||
| Dosing symptoms by subject | |||||||||||||||||||||||||
|
| |||||||||||||||||||||||||
| No. of subjects | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | No. | Percent | |
|
| |||||||||||||||||||||||||
| Placebo | 25 | 22 | 88.0 | 22 | 88.0 | 6 | 24.0 | 0 | 0.0 | 0 | 0.0 | 8 | 32.00 | 3 | 12.0 | 3 | 12.0 | 0 | 0.00 | 0 | 0.00 | 15 | 60.0 | 9 | 36.0 |
| VP100 | 24 | 24 | 100.0 | 24 | 100.0 | 22 | 91.7 | 4 | 16.7 | 1 | 4.2 | 22 | 91.67 | 8 | 33.3 | 7 | 29.2 | 1 | 4.17 | 0 | 0.00 | 22 | 91.7 | 23 | 95.8 |
| VP250 | 25 | 25 | 100.0 | 25 | 100.0 | 25 | 100.0 | 5 | 20.0 | 0 | 0.0 | 25 | 100.00 | 3 | 12.0 | 3 | 12.0 | 0 | 0.00 | 0 | 0.00 | 25 | 100.0 | 25 | 100.0 |
|
| |||||||||||||||||||||||||
| Dosing symptoms by percentage of doses per subject | |||||||||||||||||||||||||
|
| |||||||||||||||||||||||||
| No. of doses, median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||||||||||
|
| |||||||||||||||||||||||||
| Placebo | 357 (350–364) | 1.6 (0.5–11.9) | 1.6 (0.5–7.9) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0.3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.5 (0–2.8) | 0 (0–0.3) | ||||||||||||
| VP100 | 357 (345–367) | 93.9 (73.9–98.3) | 92.8 (73.9–98.3) | 6.8 (1.6–28.4) | 0 (0–0) | 0 (0–0) | 2.8 (1.2–6.9) | 0 (0–0.3) | 0 (0–0.3) | 0 (0–0) | 0 (0–0) | 76.7 (22.5–94.5) | 8.9 (0.7–53) | ||||||||||||
| VP250 | 361 (352–370) | 96.1 (75.5–98.3) | 96.1 (75.5–98.3) | 7.4 (1.6–36.8) | 0 (0–0) | 0 (0–0) | 7.6 (1.7–22.6) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 93.6 (27–96.1) | 16.2 (4.9–37.7) | ||||||||||||
Non–patch-site reactions were uncommon, reported in 0.2% of placebo and VP100 doses and 0.1% of VP250 doses. One participant in the VP100 dose group experienced systemic hives that lasted 2 to 4 hours and responded to oral antihistamines. The most commonly reported treatment was topical corticosteroids, followed by oral antihistamines. No epinephrine was used for the treatment of dosing symptoms.
The median percentage of doses per participant with a patch-site reaction was 1.6% for placebo-treated participants compared with 92.8% for VP100-treated participants and 96.1% for VP250-treated participants, whereas for non–patch-site reactions, the median was 0% doses per participant for all groups. The median percentage of doses per participant with a treated reaction was 0% for the placebo group compared with 8.9% for the VP100 group and 16.2% for the VP250 group.
Significant differences were observed for any dosing reaction, patch-site reactions, duration of reaction, doses requiring treatment, and severity of the patch-site reaction. Pairwise group comparisons identified all of the above as being lower in the placebo group compared with the VP100 and VP250 treatment groups. No statistically significant differences were observed between the VP100 and VP250 groups (see Table E6 in this article’s Online Repository at www.jacionline.org). Three unrelated severe adverse events were observed during the study: syncopal episodes, abdominal pain, and migraine headache.
Reported compliance with treatment was overall excellent. A total of 26,372 doses were expected, with 25,611 (97.1%) administered: 97.0% in the 4- to 11-year-olds and 97.4% in those older than 11 years.
Immunologic outcomes
Fig 3 shows immunoglobulin results by treatment at baseline and weeks 12, 24, and 52. When assessing global treatment effects over time, significant differences were observed between treatment groups for log10 peanut IgG4 levels (P < .0001) and log10 peanut IgG4/peanut IgE ratios (P < .0001). In particular, participants receiving active treatment had increases in both peanut IgG4 levels (Fig 3, B) and IgG4/IgE ratios(Fig 3, C) when compared with those receiving placebo. No differences over time between treatments were seen for log10 peanut IgE levels (P = .37), total IgE levels (P = .54), or percentage of peanut IgE (P = .23).
FIG 3.
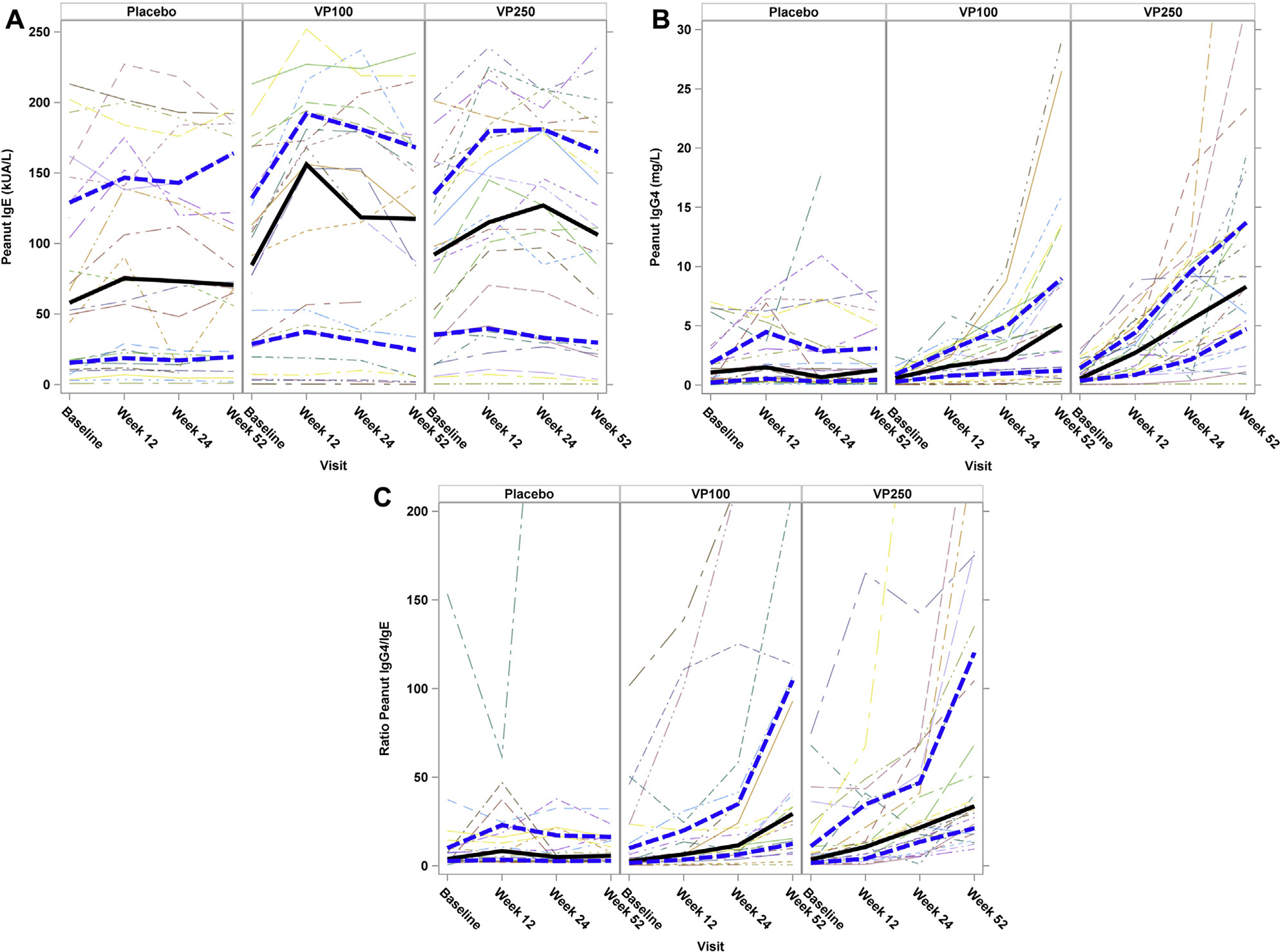
Immune mechanistic assessments over time by treatment group. A, Change in peanut-specific IgE levels over time. No significant differences over time were seen between treatment groups (P = .37). B, Change in peanut-specific IgG4 levels over time. A significant difference over time was seen between treatment groups (P < .0001), with a larger increase noted among the active Viaskin Peanut groups compared with the placebo group. C, Change in the peanut IgG4/IgE ratio over time. A significant difference over time was seen between treatment groups (P < .0001), with a larger increase noted among the active Viaskin Peanut groups compared with the placebo group. Solid lines represent median values, and hatched lines represent the upper and lower IQR.
Fig 4 shows peanut SPT results by treatment at baseline and weeks 24 and 52. A significant difference was not observed between treatment groups (P = .17). However, when the change in SPT response was examined from baseline to week 52, an apparent dose effect was noted, with a reduction in SPT size in the VP250 group (median, −2.5 [IQR, −7.5 to 0.5]; P = .02) but not within the VP100 (median, −3.25 [IQR, −7.0 to 3.0]; P = .07) or placebo (median, −2.0 [IQR, −5.0 to 1.5]; P =.27) groups.
FIG 4.
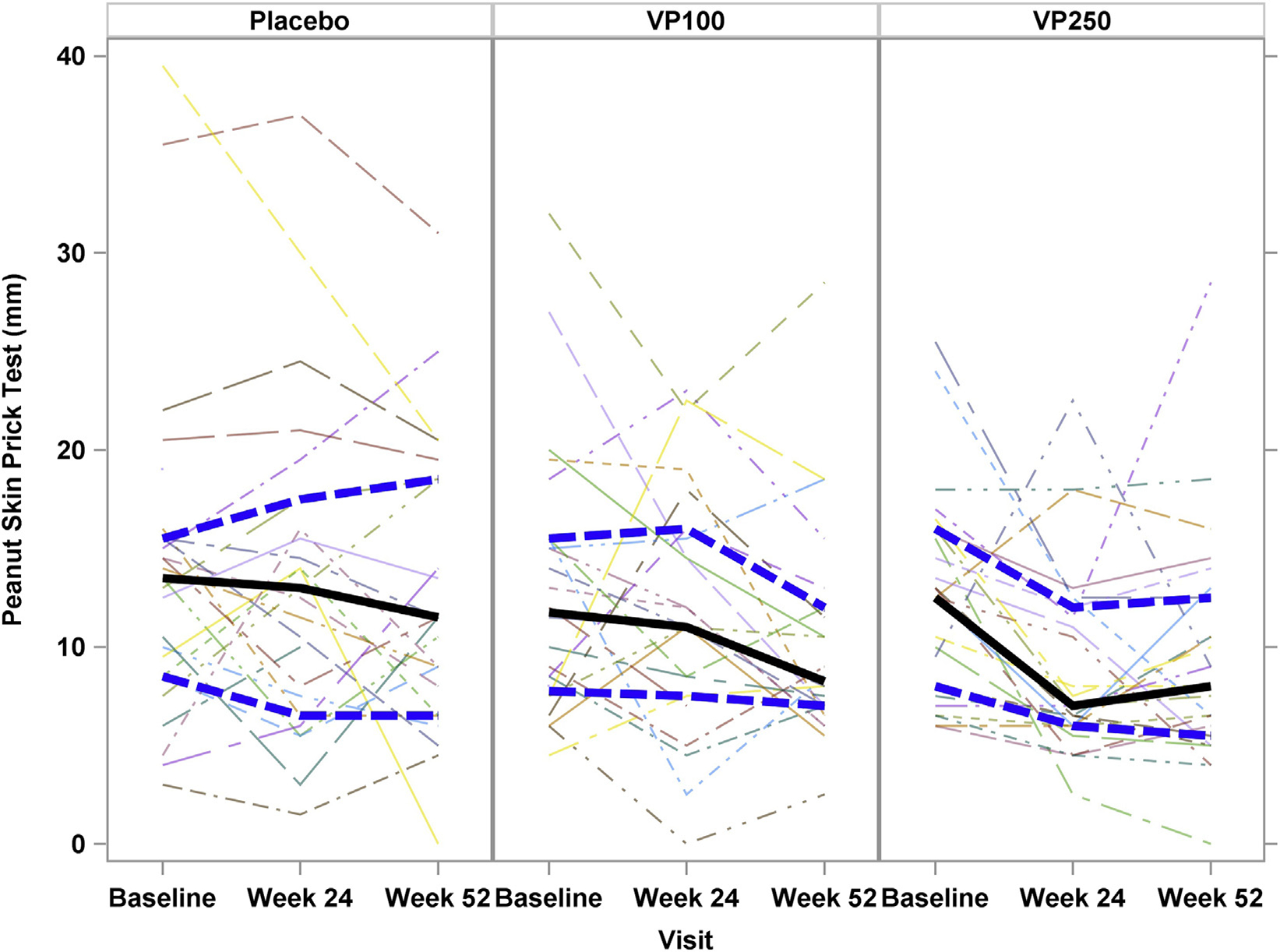
SPT results over time by treatment group. No significant difference was noted among treatment groups over time; however, when examined within a treatment group, a decrease in SPT size was noted in the VP250 group (P = .02). Solid lines represent median values, and hatched lines represent the upper and lower IQR.
When assessing global treatment effects on peanut-induced basophil activation, significant differences were observed at a stimulant dose of 0.01 μg (P < .0001) but not at higher doses. These data are consistent with a shift in threshold of reactivity to peanut rather than a loss of reactivity to peanut. This effect at a dose of 0.01 μg was evident beginning at 12 weeks for both the VP100 and VP250 treatment groups (see Fig E1 in this article’s Online Repository at www.jacionline.org).
T-cell studies are summarized in Table E7 in this article’s Online Repository at www.jacionline.org. At baseline, 50% and 42% of peanut-responsive CD154+CD4+ T cells were positive for IL-4 and IL-13, respectively, compared with 3% positive for IFN-γ and 4% positive for IL-10. Statistical analysis for these studies applied a more stringent P value of .01 because of the number of tests performed. No T-cell results reached this level of significance, but a global treatment effect over time on IL-4– and IL-13–producing cells trended toward significance (P = .059 and P = .040 for IL-4 and IL-13, respectively). Median frequencies of IL-4– and IL-13–producing T cells were lower compared with those in placebo-treated subjects at the VP250 dose but not at the VP100 dose.
Finally, data were analyzed to assess for relationships between baseline age and mechanistic outcomes at week 52. Independent of treatment group, lower age at baseline was correlated with an increasing peanut IgG4/IgE ratio (rho = −0.31, P = .010), as well as with larger decreases from baseline in percentages of CD63+ cells for stimulant levels of 0.1 μg and 0.01 μg (rho = 0.33 and 0.31, respectively; P ≤ .01). Within groups, for VP100 participants, lower age at baseline correlated with higher week 52 peanut IgG4 levels (rho = −0.57, P = .005) and greater change from baseline to week 52 in peanut IgG4/IgE ratios (rho = −0.56, P = .007). Correlations between baseline age and other mechanistic factors at week 52 were not significant for the other treatment groups.
DISCUSSION
Exploration for effective treatment options for peanut and other common food allergies remains on the forefront of priorities for clinicians and researchers. EPIT has shown promise in murine studies and early clinical trials as a potential therapeutic option. This multicenter, randomized, controlled trial is the first to comprehensively evaluate the clinical, safety, and immunologic effects of EPIT for the treatment of peanut allergy.
Our findings indicate that peanut EPIT delivered through the Viaskin Peanut patch is safe in our study population of children with peanut allergy, which excluded only children who have experienced severe anaphylaxis. Our findings also indicate that peanut EPIT is potentially effective, with evidence of immune modulation consistent with other forms of immunotherapy. Our findings demonstrate a modest but statistically significant treatment effect, which manifested as a 10-fold or greater increase in OFC SCD from baseline to week 52 among active treatment groups compared with placebo. The effect of treatment was more evident in the younger age group (66% of the VP250 group and 59% of the VP100 group compared with 6% of the placebo group), with little or no effect demonstrated in participants older than 11 years. In addition, we did not demonstrate significant treatment effects when considering other potentially meaningful outcomes in a post hoc analysis, such as the proportion of participants achieving an SCD of 1044 mg or greater or those with both a 10-fold increase and an SCD of 1044 mg or greater, and in fact, only 1 subject passed the full 52-week OFC, and that subject was receiving placebo.
The VIPES trial (a phase IIb study with Viaskin Peanut) had similar findings with regard to age, also finding that younger participants achieved more benefit from EPIT when compared with older participants.30 This suggests that responses to immunotherapy might be more robust in younger patients, as also seen in other studies of both food allergens and aeroallergens.31,32 Food immunotherapy studies are currently ongoing in younger children, which might help shed further light on this topic, and future studies of EPIT might help to determine whether the poorer responses in older participants are more related to inadequate doses or immunologic differences between younger and older participants.
Adherence to treatment was high in this study, with 97% of expected doses administered through week 52 and only 1 withdrawal caused by local cutaneous grade 3/4 reactions. This finding is similar to the greater than 96% adherence rate reported in the phase I peanut EPIT trial of 100 participants (ages 6–50 years), in which only 3 participants discontinued the trial because of treatment-related reactions.15
The safety of peanut EPIT with Viaskin Peanut was extensively evaluated in this trial. Although patch-site reactions were very common and occurred more frequently in the active treatment groups compared with the placebo group, most were mild (≤grade 2). A small proportion of participants (18.9% overall) had non–patch-site reactions that were also mostly mild and responsive to oral antihistamines or topical corticosteroids. No reactions required epinephrine.
It is important to consider these results in the context of other therapies under study for the treatment of peanut allergy. EPIT with Viaskin Peanut was generally well tolerated after 1 year of treatment and induced a modest but statistically significant increase in OFC SCD, with a median increase of 130 mg of protein (approximately 1/2 peanut) in the VP250 group and 43 mg of protein in the VP100 group. In comparison, OIT is associated with more adverse reactions, including anaphylaxis, but has been shown to induce robust changes in challenge thresholds of 5,000 to 10,000 mg.9,11,14,21,33,34 Sublingual immunotherapy is associated with fewer adverse reactions than OIT, but changes in challenge SCD are also more modest, with our CoFAR study of a similar design demonstrating a change in SCD of approximately 500 mg.10,12,18 The current EPIT study will extend treatment through 130 weeks, thus providing an important opportunity to assess adherence and clinical efficacy with more extended treatment. This essential balance between safety and efficacy will be of key importance in evaluating these therapies because they move toward clinical use in the coming years.
This is the first study of peanut EPIT to comprehensively evaluate immunologic mechanisms associated with treatment. The immunomodulation noted with active treatment, including increases in peanut-specific IgG4 levels and IgG4/IgE ratios, is consistent with changes seen with other forms of food immunotherapy.11,14,33–36 The trends seen in both basophil and T-cell responses suggest that exposure to peanut through intact skin might modulate TH2 responses and basophil reactivity. Future analyses at week 130 will determine whether prolonged treatment leads to further downregulation of these responses.
This study is limited by several factors. It is possible that the primary end point, allowing for just a 10-fold change in challenge threshold, was not sufficiently stringent. Exclusion of participants with a prior history of severe anaphylaxis, as in all other food immunotherapy trials that include double-blind, placebo-controlled food challenges in children to date, might influence the results of the study, especially those related to safety and tolerability end points. Although age effects appear to be important, the study was not designed to detect an age effect independent of a treatment effect. The mechanistic studies while using novel T-cell assays were limited in scope based on blood volume. We also acknowledge that blinding of the intervention might have been compromised by the differential rate of patch-site reactions noted between the placebo and active treatment groups. However, because patch-site reactions were seen in all groups, it is unlikely that the patch-site reactions influenced the intervention during the conduct of the blinded portion of the study.
In summary, peanut EPIT with Viaskin Peanut is generally well tolerated and associated with modest but statistically significant clinical and immunologic responses after 52 weeks of active treatment, with the greatest effect noted among the younger participants. Adherence and study retention were high, and although local reactions are common, EPIT appears safe in this study of children with peanut allergy. Additional time on therapy is needed to determine whether the modest clinical changes noted will be enhanced after a longer duration of therapy and will provide clinically meaningful protection from anaphylaxis. These results will be forthcoming, with open-label dosing of participants through 130 weeks in the continuation phase of this study.
Supplementary Material
Key messages.
Peanut EPIT is associated with modest treatment response in children with peanut allergy after 52 weeks of blinded therapy, with a higher response noted among younger children.
The vast majority of children treated with peanut EPIT had mild patch-site reactions; none had serious reactions, and none required epinephrine with dosing.
Immunologic changes were associated with peanut EPIT and were similar to changes noted with other forms of immunotherapy for food allergy.
Acknowledgments
The following persons provided study coordination and support: C. Bronchick, K. Brown-Engelhardt, S. Carlisle, L. Christie, J. Gau, M. Groetch, A. Hiegel, S. House, J. Kamilaris, S. Leung, K. Morgan, K. Mudd, S. Noone, M. Paterakis, J. Payne, D. Pearson, K. Peyton, R. Reames, J. Sikes, G. Spears, P. Steele, J. Stone, and M. Taylor. We thank C. Agashe, A. Grishin, M. Masilamani, M. Kamalakannan, M. Mishoe, and J. Grabowska and the Human Immune Monitoring Core at the Icahn School of Medicine at Mount Sinai for their contributions to mechanistic studies. We thank the staff of the clinical research unit at each institution and the Statistical and Clinical Coordinating Center; without their participation, the study could not have been done. Greer (Lenoir, NC) and Phadia (Uppsala, Sweden) generously provided reagents. We thank DBV Technologies for providing study drug. We thank J. Poyser, Project Manager for the CoFAR Program (National Institute of Allergy and Infectious Diseases). Finally, we thank the families who kindly participated. The study was designed by the investigators of the CoFAR, with Dr Jones as study chair. The data were gathered by the investigators, and analyzed by biostatisticians at EMMES Corporation. The manuscript was written collaboratively by Dr. Jones and reviewed and edited by the authors. The decision and approval to publish was made by the authors, as investigators in CoFAR, the EMMES Corporation and the National Institute of Allergy and Infectious Diseases leadership.
This study is registered with ClinicalTrials.gov with ID NCT01904604. The study is supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grants U19AI066738 and U01AI066560. The project was also supported by NIH/National Center for Advancing Translational Sciences (NCATS) grant nos. UL1 TR0001082 (Colorado), UL1 TR000067 (Mount Sinai), UL1 TR000039 (Arkansas), UL1 TR000083 (North Carolina), and UL1 TR000424 (Johns Hopkins) from the National Center for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Support for this trial was also provided by DBV Technologies (Montrouge, France) through funds provided to the Consortium of Food Allergy Research (CoFAR). Protocol development, study conduct, data analysis, and manuscript development were conducted independently of DBV.
Abbreviations used
- CoFAR
Consortium of Food Allergy Research
- CPE
Crude peanut extract
- DSMB
Data Safety Monitoring Board
- EPIT
Epicutaneous immunotherapy
- IQR
Interquartile range
- kUA
Kilounits of antibody
- OFC
Double-blind, placebo-controlled oral food challenge
- OIT
Oral immunotherapy
- SCD
Successfully consumed dose
- SPT
Skin prick test
- VP100
Viaskin Peanut 100 μg
- VP250
Viaskin Peanut 250 μg
Footnotes
Disclosure of potential conflict of interest: S. M. Jones receives grant support form FARE, Aimmune Technologies, DBV Technologies, the National Peanut Board, and the National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID); serves as a consultant for Stallergenes; and receives payment for lectures from the Kansas City Allergy Society, Mercy Children’s Hospital, Riley Children’s Hospital, Southwestern Medical School–Children’s Medical Center, the European Academy of Allergy and Clinical Immunology, the New York Allergy & Asthma Society, the University of Iowa-Seebohm Lectureship in Allergy, and the Iowa Society of Allergy, Asthma & Immunology. S. H. Sicherer receives grant support from the NIAID and FARE and royalties from UpToDate and serves as an Associate Editor of the Journal of Allergy and Clinical Immunology: In Practice. A. W. Burks receives grant funding from the NIH, Wallace Foundation and Food Allergy & Anaphylaxis; serves on the board for FARE, the NIH AITC Review Panel, the NIH HAI Study Section, Stallergenes, the World Allergy Organization, and the American Academy of Allergy, Asthma & Immunology; serves as a consultant for ActoGeniX, Adept Field Solutions, Dow AgroSciences, ExploraMed Development, Genentech, GLG Research, Insys Therapeutics, Merck, Regeneron Pharmaceuticals, Sanofi-Aventis US, SRA International, Valeant Pharmaceuticals North America, and Epiva Biosciences; manuscript preparation from the American Society for Microbiology; and holds stock in Allertein and Mastcell Pharmaceuticals. D. Y. M. Leung receives grant funding from the NIH. R. W. Lindblad receives grant funding from the NIH. P. Dawson receives grant funding from the NIH. A. K. Henning receives grant funding from the NIH. D. Chiang receives grant funding from the NIH. B. P. Vickery receives grant support from the NIAID, is an employee of Aimmune Therapeutics, and holds stock options in Aimmune Therapeutics. R. D. Pesek receives grant funding from the NIH, Kedrion, AstraZeneca, Parrigo Nutritionals, and Danone Research. H. A. Sampson receives grant support from the NIAID and DBV Technologies; serves as a consultant for Allertein therapeutics, Genentech/Roche, Sanofi, Stallergenes, Sanone, and Merck; is a part time employee of DBV Technologies; receives royalties from UpToDate and Elsevier; and received stock from DBV Technologies. R. A. Wood receives grant support from the NIAID, DBVAimmune, Astellas, and HAL-Allergy; serves as a consultant for Stallergenes and Sanofi; and received royalties from UpToDate. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9–17. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol 2010;125:1322–6. [DOI] [PubMed] [Google Scholar]
- 3.Bollinger ME, Dahlquist LM, Mudd K, Sonntag C, Dillinger L, McKenna K. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol 2006;96:415–21. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr 2013;167: 1026–31. [DOI] [PubMed] [Google Scholar]
- 5.Walkner M, Warren C, Gupta RS. Quality of life in food allergy patients and their families. Pediatr Clin North Am 2015;62:1453–61. [DOI] [PubMed] [Google Scholar]
- 6.NIAID-Sponsored Expert Panel, Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010;126(suppl):S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW, Jones SM, et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics 2012;130:e25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JW, Kagan R, Verreault N, Nicolas N, Joseph L, St Pierre Y, et al. Accidental ingestions in children with peanut allergy. J Allergy Clin Immunol 2006;118: 466–72. [DOI] [PubMed] [Google Scholar]
- 9.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, Carmargo Lopes de Oliveira L, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol 2010;126:83–91.e1. [DOI] [PubMed] [Google Scholar]
- 10.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 2013;131:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol 2009;124:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 2011;127:640–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol 2013;132:1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol 2011;127:654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SM, Agbotounou WK, Fleischer DM, Burks AW, Pesek RD, Harris MW, et al. Safety of epicutaneous immunotherapy for the treatment of peanut allergy: a phase 1 study using the Viaskin Patch. J Allergy Clin Immunol 2016;137:1258–61. [DOI] [PubMed] [Google Scholar]
- 16.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol 1997;99:744–51. [DOI] [PubMed] [Google Scholar]
- 17.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol 1992;90:256–62. [DOI] [PubMed] [Google Scholar]
- 18.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol 2015;135:1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burks AW, Wood RA, Jones SM, Sicherer SH, Fleischer DM, Scurlock AM, et al. Sublingual immunotherapy for peanut allergy: long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol 2015;135:1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin SJ, Vickery BP, Kulis MD, Kim EH, Varshney P, Steele P, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol 2013;132:476–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol 2009;124:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 2014;133:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senti G, Graf N, Haug S, Ruedi N, von Moos S, Sonderegger T, et al. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J Allergy Clin Immunol 2009;124:997–1002. [DOI] [PubMed] [Google Scholar]
- 24.Senti G, von Moos S, Tay F, Graf N, Sonderegger T, Johansen T, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol 2012;129:128–35. [DOI] [PubMed] [Google Scholar]
- 25.Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Dupont C, et al. The regulatory T cells induction by epicutaneous immunotherapy is sustained and mediates long-term protection from eosinophilic disorders in peanut-sensitized mice. Clin Exp Allergy 2014;44:867–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Benhamou P-H, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol 2011;186:5629–37. [DOI] [PubMed] [Google Scholar]
- 27.Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Dupont C, et al. Epicutaneous immunotherapy-induced regulatory T cells could migrate to more various sites of allergen exposure compared to sublingual or subcutaneous immunotherapy in mice sensitized to peanut [abstract] . J Allergy Clin Immunol 2014;133(suppl):AB48. [Google Scholar]
- 28.Dupont C, Kalach N, Soulaines P, Legoue-Morillon S, Piloquet H, Benhamou PH. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol 2010;125:1165–7. [DOI] [PubMed] [Google Scholar]
- 29.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol 2012;130:1260–74. [DOI] [PubMed] [Google Scholar]
- 30.Sampson HA, Agbotounou W, Thebault C, Ruban C, Martin L, Yang WH, et al. Epicutaneous immunotherapy (EPIT) is effective and safe to treat peanut allergy: a multi-national double-blind placebo-controlled randomized phase IIb trial. J Allergy Clin Immunol 2015;135:AB390. [Google Scholar]
- 31.Adkinson NF Jr, Eggleston PA, Eney D, Goldstein EO, Schuberth KC, Bacon JR, et al. A controlled trial of immunotherapy for asthma in allergic children. N Engl J Med 1997;336:324–31. [DOI] [PubMed] [Google Scholar]
- 32.Vickery BP, Berglund J, French JP, Hamilton DK, Herlihy L, Kim EH, et al. High rate of sustained unresponsiveness with early-intervention peanut oral immunotherapy [abstract]. J Allergy Clin Immunol 2015;135:AB155. [Google Scholar]
- 33.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 2012; 367:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 2008;122:1154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohil A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 2014;133:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, et al. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy 2012;42:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.