Abstract
Obesity contributes to reduced life expectancy because of its link with type 2 diabetes and cardiovascular disease. Yet targeting this poorly diagnosed, ill-defined and under-addressed modifiable risk factor remains a challenge. In this review, we emphasize that the tendency among healthcare professionals to amalgam all forms of obesity altogether as a single entity may contribute to such difficulties and discrepancies. Obesity is a heterogeneous condition both in terms of causes and health consequences. Attention should be given to two prevalent subgroups of individuals: 1- patients who are overweight or moderately obese with excess visceral adipose tissue; and 2- patients with severe obesity, the latter group having distinct additional health issues related to their large body fat mass. The challenge of tackling high cardiovascular risk forms of obesity through a combination of personalized clinical approaches and population-based solutions is compounded by the current obesogenic environment and economy.
Keywords: visceral obesity, massive obesity, ectopic fat deposition, cardiovascular disease, type 2 diabetes
CONDENSED ABSTRACT
The tendency among healthcare professionals to amalgam all forms of obesity altogether as a single entity contributes to the challenges in addressing this modifiable risk factor. Obesity is a heterogeneous condition pointing to the use of the term “obesities”. Attention should be given to two prevalent subgroups of individuals: 1- patients who are overweight or moderately obese with excess visceral adipose tissue; and 2- patients with severe obesity, who often have health issues related to excess total body fat accumulation. The challenge of tackling high cardiovascular risk forms of obesity is compounded by the current obesogenic environment and economy.
Introduction - Clinical Vignettes
CASE #1 (Figure 1):
Figure 1: Sedentary 49-year-old Man with Visceral Obesity.
Despite being nonobese, this individual developed coronary heart disease. BMI: body mass index
A 49-year-old sedentary man with acute chest pain is sent to the coronary catheterization lab for progressive debilitating angina. The angiogram reveals the culprit lesion responsible for his symptoms: significant stenosis of the mid-portion of the left anterior descending artery. A drug-eluting stent is placed and the patient recovers completely. The patient’s medical record does not reveal major alterations in traditional cardiovascular disease (CVD) risk factors. Apart from being sedentary and stressed out by a demanding executive job, the patient weighs 185 pounds (83.9 kg) at 5’8”(1.73 m) is not obese (body mass index [BMI] of 28 kg/m2) with a waist circumference of 125 cm. He does not smoke (tobacco, cannabis), has a blood pressure of 135/85 mmHg, has a low-density lipoprotein cholesterol (LDL-C) of 125 mg/dl (3.2 mmol/l) and does not have diabetes. Because radial access was used and the procedure was performed without complications, the patient is discharged from the hospital within 24 hours with statin, beta-blockers, ACE inhibitors and antithrombotic treatments in line with clinical guidelines. The patient leaves with recommendations on how to improve his diet and to exercise, but no specific counselling or follow-up of his lifestyle habits is provided.
CASE #2 (Figure 2):
Figure 2: Sedentary 41-year-old Woman with Severe Obesity.
This patient has symptoms of heart failure. BMI: body mass index
A 41-year-old female computer programmer presents to her primary care physician with symptoms of exertional dyspnea, nighttime cough and pedal edema for the last 4 months. Being 5’2” (1.57 m) tall and weighing 285 pounds (129.3 kg), she has a BMI of 52 kg/m2. She also has shortness of breath when she bends over to tie her shoes. Blood pressure is 147/91 mmHg. Physical exam is significant for severe obesity, faint crackles in both lung fields, presence of S4 and mild pitting edema of her lower extremities. The echocardiogram shows concentric severe left ventricular hypertrophy and left ventricular ejection fraction of 56%. Diastolic parameters are suggestive of grade 2 diastolic dysfunction with enlarged left atrium, elevated filling pressure and mild pulmonary hypertension. There is no significant valvular disease. She shows dysglycemia as well as low high-density lipoprotein cholesterol (HDL-C) (39 mg/dl; 1.00 mmol/l) and high triglyceride (204 mg/dl; 2.3 mmol/l) levels with LDL-C at 148 mg/dl (3.8 mmol/l). She is placed on antihypertensive therapy and a low dose loop diuretic, with recommendations on how to improve her diet and to exercise, but without follow-up of her lifestyle habits, evaluation for bariatric surgery or pharmacological treatment.
2. Why cardiologists should pay attention to obesity
The present paper is based on the observation that the number of cases such as those in the vignettes is increasing in tertiary cardiology centers. This is an alarming situation because these patients are often young and may not always have major abnormalities in their traditional CVD risk factors. Regarding Case #1, the apparently harmless variation in traditional CVD risk factors observed hides a poorly recognized and undiagnosed condition that puts him at higher risk for premature coronary artery disease (CAD): excess visceral adipose tissue (VAT) and ectopic fat. As discussed later, due to his apparently harmless and common BMI, he was not diagnosed with the presence of excessive “inner” body fat. This issue has been well documented more than 10 years ago (1). Because this largely unrecognized form of overweight/obesity is associated with metabolic abnormalities increasing the risk of premature CAD and of other adverse cardiovascular outcomes, we will review the evidence supporting the notion that cardiologists should pay more attention to this high-risk form of overweight/obesity.
For Case #2, severe obesity (WHO class III, BMI ≥40 kg/m2) and a sedentary lifestyle contribute to early onset of stage B and subsequently stage C heart failure (2), often with preserved rather than reduced left ventricular ejection fraction but with diastolic dysfunction along with evidence for end-organ damage and marked hemodynamic stress. We address issues relevant to this rapidly expanding group of patients who are at high risk for heart failure and other obesity-related comorbidities that should now be a major focus area for cardiologists.
3. From obesity to obesities: heterogeneous causes and consequences
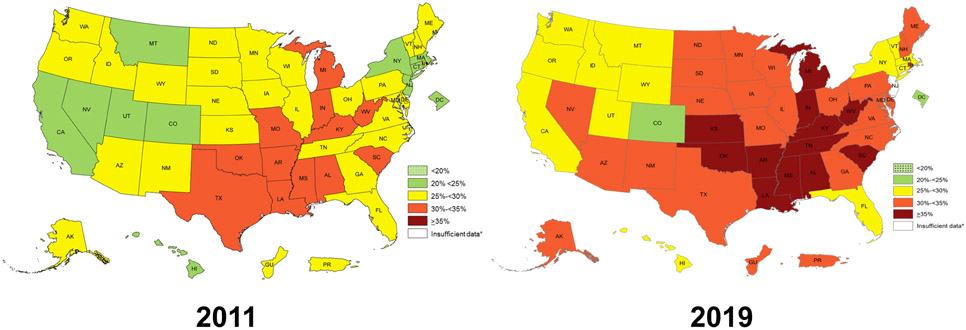
The prevalence of obesity, defined by excess body fat causing prejudice to health (3) has increased globally over the last few decades (4). Data from the Behavioral Risk Factor Surveillance System (Figure 3) have confirmed over the last decade the continuing growth in the prevalence of obesity (defined by a BMI of ≥30 kg/m2) in the U.S., with 12 states showing a prevalence ≥35% (5). Using direct measurements of height and weight, the National Health and Nutrition Examination Survey documented that the age-adjusted prevalence of obesity was even higher reaching 42.4% of the adult U.S. population in 2017-18 (6). Despite these staggering figures, obesity remains a puzzling and challenging CVD modifiable risk factor for clinicians regarding its causes, health consequences and management (3). There is a long list of environmental and biological factors leading to excess body fat accumulation causing prejudice to health, although a discussion on this complex topic is beyond the scope of this article.
Figure 3: Prevalence of Obesity in the U.S. in 2011 and 2019.
Data are derived from the Behavioral Risk Factor Surveillance System which used reported weight and height. Obesity is defined by a body mass index of ≥30 kg/m2. From reference 5.
Despite the well-documented J-shaped relationship between BMI and mortality (3), patients with obesity are quite heterogeneous in terms of CVD risk factors and abnormal cardiac features (7). This phenomenon has even led some investigators to propose the term “metabolically healthy” obesity (8) to describe a group of patients at much lower health risk than expected from their body fat excess. Whether there is a subgroup of truly “metabolically healthy obese” individuals remains a debated issue, and they are likely less prevalent than initially proposed (9, 10). Most importantly, it may be simply a matter of time for these presumably low-risk individuals to eventually develop cardiovascular comorbidities (11-13). Nevertheless, these findings highlight the remarkable individual variation in the CVD risk profile observed even within the same BMI category or at the same amount of total body fat. Such heterogeneity makes it difficult to position obesity as a whole among the modifiable CVD risk factors.
4. Visceral obesity: a high-risk form of overweight/obesity
The use of imaging techniques to study body composition and assess adiposity has been a remarkable advance in obesity research (14-16). First with computed tomography (CT) and then with magnetic resonance imaging (MRI), it has become possible to visualize and assess with great accuracy the size of various body fat depots. Reviewing the extensive body of work conducted using these imaging techniques is beyond the scope of this paper and many comprehensive review articles have already addressed this topic (10, 14, 17-23). These studies have consistently shown that: 1- at any BMI value, there is substantial individual variation in abdominal adiposity; and 2- such variation in body fat topography is predictive of marked differences in the cardiometabolic risk profile. Irrespective of BMI, excess VAT has been associated with insulin resistance and type 2 diabetes (T2D). The overall dysmetabolic state observed among individuals with visceral obesity is not limited to insulin resistance, T2D and atherogenic dyslipidemia. It also includes alterations in the profile of inflammatory cytokines, including those secreted by adipose tissue itself (adipokines) (24, 25), generating a chronic state of low-grade inflammation (26).
Although visceral obesity is clearly associated with many metabolic abnormalities (17), causality of the relationship between expanded VAT and clinical outcomes remains unclear (27). Three nonexclusive scenarios have been proposed to explain the increased cardiometabolic risk of visceral obesity (17-19, 22):
1- The enlarged VAT mass exposes the liver, through the portal circulation, to high levels of free fatty acids which impair hepatic lipid and carbohydrate metabolism particularly in the postprandial state (28-30). This increased fatty acid flux may contribute to tissue insulin resistance, increased hepatic glucose production and beta cell dysfunction (31, 32). Such high plasma fatty acid flux in the face of hyperinsulinemia is associated with liver steatosis (33), higher risk of developing T2D (34) and increased cardiovascular risk score (35). However, VAT does not contribute as much to the hepatic or systemic fatty acid fluxes as do subcutaneous adipose tissues (27).
2- The expanded visceral fat depot becomes infiltrated by pro-inflammatory macrophages and this process is accompanied by altered secretion of adipocytokines (such as increased interleukin-6 and tumor necrosis factor-alpha and reduced adiponectin), leading to chronic, low-grade inflammation, with adverse local and systemic metabolic consequences, as reviewed (36, 37).
3- Excess visceral adiposity is a marker of dysfunctional subcutaneous adipose tissue, which could be either absent or, more often, unable to fully play its role as a protective lipid storage organ. Excess visceral fat accumulation may, therefore, be a consequence of the saturation of the capacity of subcutaneous adipose tissue to act as a protective storage site when facing caloric surplus (38). Such capacity of subcutaneous adipose tissue to handle and store an energy excess varies widely and may explain the low cardiometabolic risk of some individuals (39, 40). Genetic and acquired lipodystrophies lead to excessive ectopic lipid deposition in lean tissues, with a high risk of developing T2D and CVD (41, 42). Similarly, large-scale genomic studies have demonstrated that a genetic score linked to high circulating insulin, triglyceride and low HDL-C levels is associated with T2D and CVD events, underpinned by reduced peripheral adipose tissue mass (43).
Tracer and imaging studies have shown reduced storage of dietary fat per volume of abdominal fat tissue in individuals with obesity (44-46). This is associated with increased partitioning of fat in the myocardium and reduced left ventricular ejection fraction in patients with prediabetes (47). Both reduced intra-abdominal uptake and increased myocardial partitioning of dietary fat are corrected after modest weight loss from lifestyle changes in prediabetes (48) or within 12 days after bariatric surgery in patients with T2D and severe obesity (49). In contrast, 7-day overfeeding in healthy individuals leads to rapid increases in subcutaneous adipose tissue storage of dietary fat, reducing the exposure of the heart and skeletal muscles to potentially toxic dietary fats during weight gain and the development of insulin resistance (50). These studies suggest that metabolic flexibility of adipose tissues helps dealing with periods of caloric excesses. Dynamic metabolic adaptation to store dietary fats in different adipose tissue depots may vary depending on the dysmetabolic state: it is preferentially retained in subcutaneous adipose tissues in the healthy state, while intra-abdominal adipose tissues play a greater role in the dysmetabolic state. Although harmful over the long term, visceral fat expansion may represent a defense response against ectopic fat deposition.
An increasing number of studies support the notion that accumulation of lipids in ectopic sites is a consequence of dysfunctional adipose tissues (17-20, 22) and that such dysfunction clearly relates to adverse clinical outcomes. In other words, body fat quality and location matter. Ectopic fat depots have been postulated to contribute to cardiovascular complications, both indirectly through altered downstream CVD risk factors, as well as directly through lipotoxicity of circulating fatty acids, inflammatory, biologically active adipocytokines and a host of other molecular mechanisms (7, 10, 17, 19, 20, 51, 52). Because these notions are derived from observational and epidemiological studies, it is important to emphasize the relevance of performing randomized CVD outcome trials testing the impact of interventions targeting visceral fat loss. In the meantime, the heterogeneity of obesity represents a challenge for clinicians who obviously need more than weight and height to identify adiposity phenotypes contributing to increase CVD risk. One practical approach may be to utilize the incidental fat distribution information that cardiologists obtain through imaging, for example, as a motivational tool to educate and counsel patients. Much like coronary artery calcium scoring leads to downstream preventive therapies and improved health behaviors, simply by virtue of providing concrete “visual” evidence of atherosclerosis, body fat imaging demonstrating a visceral/ectopic fat phenotype may be a powerful motivational tool for behavioral change and more aggressive preventive practices.
5. Severe obesity: a newly emerging condition in cardiology
In addition to individuals affected by visceral obesity, those with severe obesity (BMI values ≥40 kg/m2 or BMI ≥35 kg/m2 with at least one comorbidity) (53, 54) now represent 9.2% of the U.S. population and this category has been growing rapidly (6). Estimates show that by 2030, 25% of the U.S. population will live with severe obesity (55). The NCD Risk Factor Collaboration has provided the most extensive data on obesity prevalence worldwide in the past 40 years (56). These figures are a source of major concern both for clinical practice and public health.
Bariatric surgery is indicated in adult patients with a BMI ≥35 kg/m2 and at least one major obesity-related complication such as diabetes, hypertension, hyperlipidemia, CAD, severe reflux or obstructive sleep apnea among others; or in patients with BMI ≥40 kg/m2 without obesity-related diseases (54). To the cardiologist, this simple recommendation means that all patients in secondary prevention — patients with heart failure or with CVD risk factors and a BMI ≥35 kg/m2 — are potential candidates for bariatric surgery. Because this condition is termed “massive”, “morbid”, or “severe” obesity, cardiologists may think that most of their patients do not meet this diagnostic criterion. Patients with a BMI ≥35 kg/m2 are common in daily cardiology practice. Using BMI ≥40 kg/m2 as a threshold, severe obesity may represent as much as 19.7% of heart failure patients (57); using BMI ≥35 kg/m2 as the cut-off, it may represent up to 7.5% of patients who underwent coronary artery bypass grafting surgery (58). Patients with severe obesity are among those with the highest risk for morbidity and cardiovascular mortality. This group also tends to be younger with a high proportion of females and individuals from minorities (59, 60). Severe obesity is associated with additional major health issues that require specific attention compared to less severe and more common forms of obesity (21, 55, 59, 61, 62).
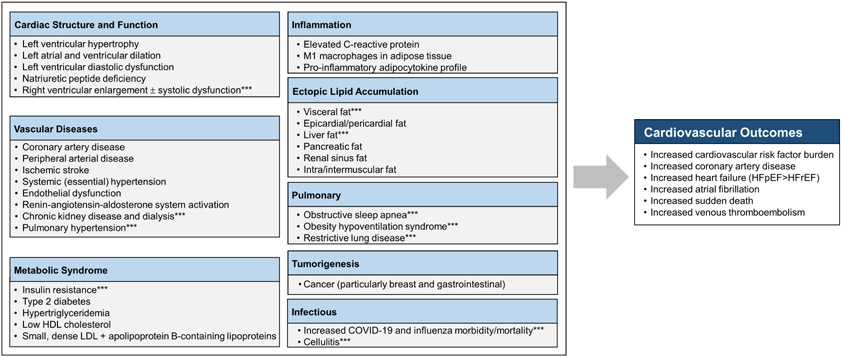
Thus, on the basis of the remarkable differences in phenotypic expression between the visceral vs. severe forms of obesity, cardiologists must be aware that the health risk of overweight/obesity cannot be solely and properly diagnosed on the basis of excess weight or elevated BMI alone. Among overweight and moderately obese patients, excess VAT accumulation and deposition of fat in undesired sites are key drivers of unfavorable health outcomes, irrespective of the patient’s body weight (7, 10, 17-20) (Figure 4). Severe obesity must also be recognized as it is associated with important health issues, and dealing with this high-risk obesity phenotype is required in terms of health risk and clinical management (21) (Figure 4).
Figure 4: Cardiovascular and Systemic Effects of Obesities.
In addition to cardiovascular outcomes, high-risk forms of obesities are associated with various combinations of abnormalities causing prejudice to patients’ health and quality of life. ***Commonly seen with severe obesity COVID-19: coronavirus disease 2019, HDL: high-density lipoprotein, HFpEF: heart failure with preserved ejection fraction, HFrEF: heart failure with reduced ejection fraction, LDL: low-density lipoprotein
6. Obesities: a challenge for clinical assessment and CVD risk management
Although the foundation of CVD risk assessment for primary prevention remains the estimation of 10-year absolute risk using a risk calculator, the BMI has not emerged as a component of the Framingham or Pooled Cohort Equations (PCE) risk estimator functions (63), in contrast to the NICE risk calculator in the UK (https://qrisk.org/three/). While obesity is associated with worsening cardiovascular risk factors and increased risk for cardiovascular events, some of the events may not be fully explained by CVD risk factors incorporated in the PCE (7). Furthermore, the rate of observed events among some subsets of individuals who are obese may be lower than among those with a normal weight, a finding frequently referred to as the “obesity paradox” (64). A recent investigation including >37,000 participants found that the PCE overestimates the risk of CVD across the spectrum of BMI with less optimal prediction in the highest risk groups (e.g. those with BMI >40 kg/m2). Moreover, approaches that have considered clinical measurements of obesity (e.g. BMI, waist circumference, and high-sensitivity C-reactive protein) as biomarkers into the PCE have failed to improve risk estimation compared with the standard PCE (65). It is important to keep in mind that: 1- PCE risk estimates could possibly be improved with measurements of visceral adiposity and ectopic fat; 2- high-risk adiposity phenotypes are key upstream drivers of altered CVD risk factors considered in the PCE.
Clinical tools other than BMI to estimate body fat and quantify the associated health risks include waist circumference (23), waist-to-hip ratio, waist-to-height ratio, bio-impedance, and dual-energy x-ray absorptiometry as well as many other indices (66). All of these methods do not directly measure visceral adiposity, so caution should be used when these anthropometric tools suggest only a mild increase in abdominal obesity and when laboratory and other markers (such as hypertriglyceridemia and excess liver fat by imaging) are indicative of a major expansion of the VAT depot. In the absence of direct, imaging-based tools such as CT and MRI for clinical use, a combination of anthropometry and laboratory markers may aid in the differentiation of high-risk adiposity phenotypes. Visceral obesity is often accompanied by more liver fat content driving an increased production of triglyceride-rich very-low-density lipoproteins. The combined presence of an elevated waist circumference and increased triglyceride levels has been associated with a high probability (~80%) for large VAT mass (67). Several studies have since confirmed the notion that the “hypertriglyceridemic-waist” phenotype is a simple and quick clinical tool to screen for the presence of excess VAT and ectopic fat. Within every BMI category, the presence of increased waist circumference (≥90 cm in men and ≥85 cm in women) accompanied by elevated triglyceride concentrations (≥177 mg/dl [2 mmol/l] in men and ≥133 mg/dl [1.5 mmol/l] in women) is predictive of visceral obesity. The joint ESC/EAS guidelines categorized high BMI, high waist circumference, or non-alcoholic steatohepatitis (NASH) as CVD risk modifiers (68). Other tools to estimate visceral adiposity have been proposed and also include waist girth and triglyceride levels such as the lipid accumulation product (69) and the Visceral Adiposity Index (70).
7. Clinical management of high-risk obesity phenotypes
Lifestyle and prevention/remission of T2D in visceral obesity
It is well-documented that a daily regimen of moderate intensity endurance exercise (e.g. walking 30 min) acutely increases insulin sensitivity (71). Regular moderate intensity exercise leads to improvements in glucose tolerance and insulin levels even in the absence of weight loss (72, 73). A daily walk of 30-45 min is the simplest recommendation for patients with visceral obesity. Even with stable weight, data from the EPIC-Norfolk Study have shown that physically active abdominally obese individuals with features of the metabolic syndrome were at 50% lower risk for CAD compared to sedentary abdominally obese individuals, this was true in men and women (74). Beyond weight loss, physical activity should be considered as an important therapeutic objective in the management of the high-risk visceral obesity. Imaging studies have suggested that the improvements in glucose tolerance and insulin levels associated with regular endurance exercise were more closely related to the concomitant loss of VAT than to weight loss per se (75, 76). Discordant changes in body weight vs. visceral adiposity with regular exercise are expected, some high responders for VAT loss showing no or trivial weight loss. This phenomenon may be explained by skeletal muscle mass accretion with exercise in some sedentary patients (76, 77).
Lifestyle modification should be the cornerstone of any prevention strategy for CVD in all obesities and stages of chronic disease (Figure 5). The most recent ACC/AHA and ESC/EAS guidelines on the primary prevention of CVD specifically recognize that plant-based and Mediterranean diets, along with increased fruit, nut, vegetable, legumes, and lean vegetable or animal protein (preferably fish) consumption, with the inherent soluble and insoluble vegetable fibers, have consistently been associated with lower risk of all-cause mortality than standard diets (68, 78). These diets, along with potential benefits on atherosclerosis and endothelial function (79), may also improve visceral adiposity, especially when combined with caloric restriction (80). Recent guidelines also recommend minimizing sugar-sweetened beverage intake as their increased consumption is correlated with higher risk for insulin resistance, VAT gain and mortality (81, 82).
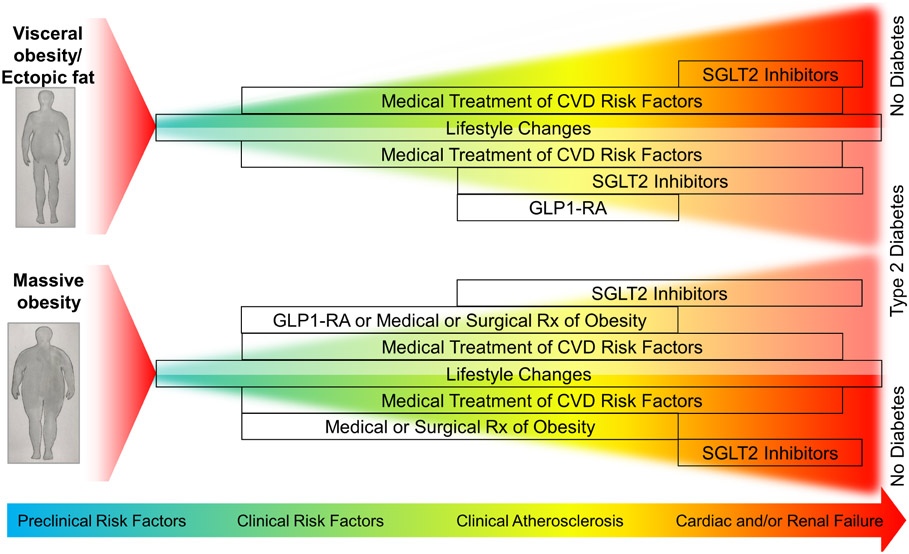
Figure 5: Management of Obesities throughout the Spectrum of Disease Progression.
Lifestyle changes are recommended in all obesities. Medical treatment of cardiovascular disease (CVD) risk factors is also indicated, except perhaps in very advanced renal and/or heart failure where statin therapy is not recommended. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are indicated in patients with type 2 diabetes (T2D) in the presence of CVD in all obesities. SGLT2 inhibitors are also indicated in patients with heart and/or kidney failure. Glucagon-like protein-1 receptor agonists (GLP1-RA) are indicated in patients with T2D with CVD. Liraglutide is indicated for treatment of obesity. Weight loss drugs/bariatric surgery should be considered in high-risk patients with severe obesity.
Physical activity is an essential component of a lifestyle strategy to prevent adverse cardiovascular events (83). Considerable evidence supports recommendations for aerobic physical activity to lower CVD risk (84-86). One useful marker of sedentary behavior is a low level of cardiorespiratory fitness, which has been shown to be a powerful predictor of CVD risk (86). Meeting physical activity guidelines allows poorly fit individuals to increase cardiorespiratory fitness so that they are no longer in a high-risk category (86). This is why cardiorespiratory fitness, which does not have to be assessed by a maximal exercise test, should be considered as another important vital sign (86). VAT may be reduced by exercise without significant weight loss (20, 87). A recent meta-analysis of 3602 participants from 17 randomized controlled trials demonstrated that exercise interventions resulted in greater reduction in VAT relative to weight loss than did pharmacological interventions, suggesting that tracking weight loss alone underestimates its cardiovascular benefits (87). High-risk patients with visceral obesity should be advised to participate in comprehensive lifestyle programs supporting participants in adhering to quantitative and qualitative re-calibration of their diet and physical activity.
Clinical approaches for severe obesity
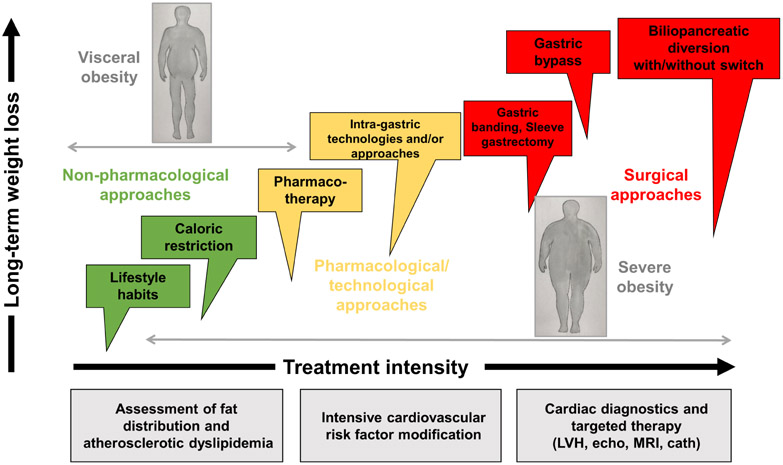
Guidelines suggest that clinical teams should provide counseling and promote lifestyle modifications before considering weight-loss surgery (53, 54, 88, 89). However, for most patients with severe obesity, such modifications alone are frequently ineffective for long-term weight loss maintenance and durable metabolic recovery. In the Look AHEAD trial, 74% of individuals living with severe obesity undergoing intensive behavioral intervention did not maintain a weight loss ≥10% of initial body weight after 4 years. Accordingly, few benefits were observed in this subgroup for CVD risk factors (90).
A few different options are available including restrictive or restrictive/malabsorptive surgeries (61). Bariatric surgery in combination with lifestyle modification including exercise (91) can result in significant long-term weight loss (20 to 40% of initial body weight) and improvement or, in some cases, remission of obesity-related diseases, including T2D, sleep apnea, fatty liver disease and hypertension (92). .Advanced patient age alone is not a contraindication to bariatric surgery. The outcomes and complication rates for patients >60 years of age appear to be comparable to those of a younger population regardless of the surgical procedure performed (93). Contraindications for bariatric surgery include recent substance abuse, non-stable psychiatric conditions, diagnosis of cancer or life expectancy <5 years (54).
8. Pharmacological management of diabetes and CVD: the adiposity connection
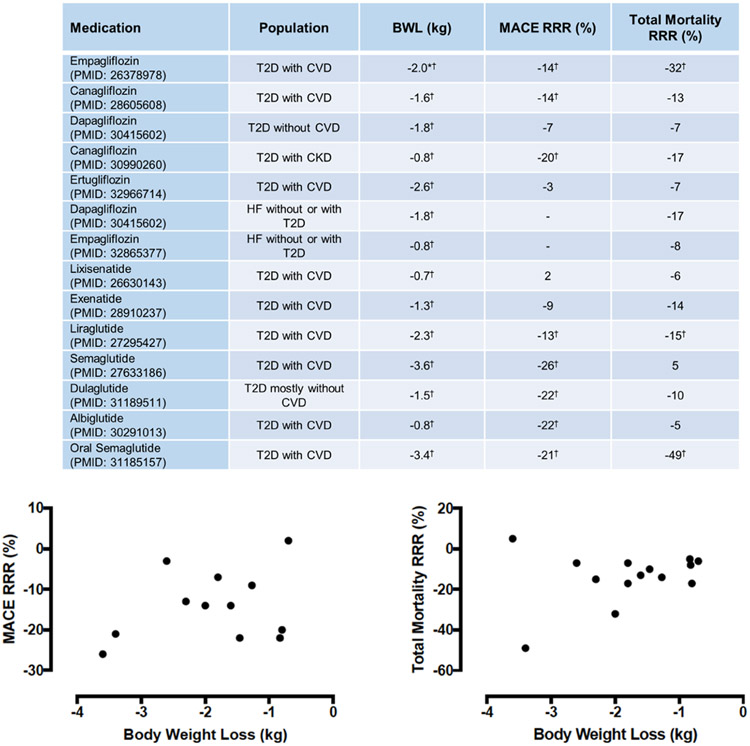
Despite its neutral effect on body weight and composition, metformin is still the first line of pharmacological therapy in most patients with T2D given its possible cardiovascular benefits, low cost and safety (94). Metformin exerts its glucose lowering effect through reduction in hepatic gluconeogenesis, but the underlying cellular mechanisms are still much debated (95). Metformin, however, does not alter the caloric balance of the body and its added cardiovascular value, if it exists, is limited. In contrast, glucagon-like peptide 1 receptor agonists (GLP1-RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitors have proven cardiovascular benefits at least in some populations and are now recommended as first-line therapy in patients with T2D and overt CVD (94) (Figure 5). GLP1-RA exert their glucose lowering effect by a combination of mechanisms (96). They increase the glucose-dependent stimulation of insulin secretion (i.e. incretin effect), that, in contrast to insulin treatment, does not cause hypoglycemia. They also slow gastric emptying and inhibit glucagon secretion. Finally, GLP1-RA increase satiety and reduce food intake, which leads to weight loss and improved insulin sensitivity over time. The GLP1-RA liraglutide (97), semaglutide (98), dulaglutide (99) and albiglutide (100), but not lixisenatide (101) and exenatide (102), have shown significant reduction of MACE vs. placebo in randomized, controlled clinical trials (Figure 6). SLGT2 inhibitors induce blood glucose-dependent urinary glucose and sodium losses, leading to rapid reduction in blood glucose and blood pressure, with a caloric loss leading to modest weight loss (103). These drugs also increase ketone body and glucagon levels and stimulate hepatic glucose production, but these mechanisms are still of unclear significance regarding their cardiovascular benefits. The SGLT2 inhibitors empagliflozin (104), canagliflozin (105), but not ertugliflozin (106), significantly reduced MACE vs. placebo in T2D populations mostly in the setting of secondary cardiovascular prevention (Figure 6). Dapagliflozin did not show significant reduction in MACE in patients with T2D who were mostly in a primary prevention setting (107) (Figure 6). All SGLT2 inhibitors tested thus far in large randomized clinical trials have reported reduced hospitalisation for heart failure (104-108). Dapagliflozin and empagliflozin have proven efficacy for the reduction of heart failure-related clinical events in patients with or without diabetes and left ventricular systolic dysfunction, with a 17% reduction of all-cause mortality with dapagliflozin, whereas empagliflozin did not show a significant decrease in mortality (109, 110). SGLT2 inhibitors have been shown to be effective to delay the progression of kidney failure in patients with chronic kidney disease (108, 111). Thus, they are the treatment of choice in T2D with cardiac and/or renal failure (Figure 5).
Figure 6: Weight Loss Induced by SGLT2 Inhibitors and GLP1-RA and MACE/Mortality.
Relationship between reported difference in average body weight loss (BWL, in kg) between the active treatment vs. placebo and relative risk reduction (RRR) of 3-point major adverse cardiovascular events (MACE) (left panel) or total mortality (right panel) from major adverse cardiovascular outcome trials with sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide-1 receptor agonists (GLP1-RA) in patients with type 2 diabetes (T2D). CKD: chronic kidney disease; CVD: cardiovascular disease; HF: heart failure. * Difference in average weight loss estimated from data reported in a graph. † P <0.05 between the active vs. placebo treatment groups.
Multiple mechanisms have been proposed to explain the benefits of GLP1-RA and SGLT2 inhibitors on cardiometabolic health (96, 103, 112, 113). A common denominator between these two classes is their capacity to induce significant weight loss (Figure 6), notably VAT loss. Several studies have documented a reduction in VAT and ectopic fat with SGLT2 inhibitors. Dapagliflozin treatment leads to reduction in VAT in patients with T2D (114) and intra-hepatic fat loss in patients with T2D and NASH (115-117). Dapagliflozin and canagliflozin were also shown to reduce epicardial fat mass in patients with T2D (118, 119). Empagliflozin was shown to reduce VAT-derived glycerol flux from intracellular lipolysis contributing to hepatic gluconeogenesis (120), suggesting a direct mechanistic link between reduced VAT and improvement of glucose control. VAT and ectopic fat losses have also been documented with GLP1-RA treatment. Liraglutide treatment led to more fat loss than lifestyle treatment in patients with prediabetes or T2D, despite similar total body weight loss (121). Reduction of VAT with liraglutide was associated with improvement in intra-hepatic fat, albuminuria and glucose control (122, 123). Liraglutide was also seen to reduce VAT and ectopic fat in participants without prediabetes or diabetes who were nevertheless at high cardiovascular risk due to obesity and metabolic syndrome. In this study, VAT and liver fat losses were correlated with improvements in inflammation and fasting blood glucose, even among those with normal baseline glucose tolerance. Treatment with liraglutide, semaglutide, dulaglutide or exenatide was also found to induce a rapid reduction in epicardial or intra-hepatic fat (124-128). Other studies failed to detect significant reduction of VAT and other ectopic fat depots with liraglutide vs. placebo despite weight and subcutaneous adipose tissue loss (129). Semaglutide and canagliflozin led to similar weight and VAT loss in a rare controlled trial comparing a GLP1-RA and an SGLT2 inhibitor (130).
All cardiovascular outcome trials performed with SGLT2 inhibitors or GLP1-RA in patients with T2D have shown a significant reduction in body weight vs. placebo (Figure 6). However, direct evidence that their cardiovascular benefits stem from VAT loss is still lacking. Any weight loss is associated with reduction in major cardiovascular risk factors (131), but large randomized trials investigating the effect of weight loss achieved by lifestyle changes [Look AHEAD (132)], sibutramine [SCOUT (133)], or lorcaserin [CAMELLIA TIMI 61 (134)] failed to demonstrate superiority in cardiovascular event reduction in their primary intention-to-treat analysis. Yet, post-hoc analyses of the Look AHEAD and SCOUT trials showed significant reduction in cardiovascular events in patients who lost significant amounts of weight (135, 136). The results of ongoing and future cardiovascular outcome trials using GLP1-RA and/or SGLT2 inhibitors for the treatment of non-diabetic subjects with high-risk visceral obesity may provide such evidence.
9. Revisiting the clinical vignettes
CASE #1:
This patient eventually enrolled in a cardiometabolic imaging study. Investigation of his regional adiposity by MRI and magnetic resonance spectroscopy revealed that despite being overweight and not obese, he had a massive accumulation of VAT (visceral adiposity in the 95th percentile for his age and BMI), which was accompanied by a high liver fat content (25% fat/water fraction) and by a large accumulation of epicardial/pericardial adipose tissue (90th percentile for his age and BMI). His waist circumference of 115 cm and elevated fasting triglyceride concentration (248 mg/dl; 2.8 mmol/l) confirmed that he had the “hypertriglyceridemic-waist” phenotype predictive of visceral obesity, dyslipidemia and insulin resistance (67, 137). Apolipoprotein B and high-sensitivity C-reactive protein levels were high suggesting increased concentrations of atherogenic lipoproteins and a state of chronic, subclinical inflammation. Overall nutritional quality was investigated by a simple food-based nutritional questionnaire (138) and was found to be poor. The patient was managed with a simple lifestyle intervention program (Figure 7), in which he was followed initially on a bi-monthly basis by a certified nutritionist and a kinesiologist for 6 months, then by monthly interactions. Simple food-based recommendations were provided (cutting by 50% the frequency of poor-quality fast food intake with a corresponding increase in fruits and vegetables and less processed foods). The patient was instructed to gradually increase his daily walking time to attain moderate-intensity activity for at least 150 min/week and at least 10,000 steps per day. After proper adaptation, the patient became able to walk 30-60 minutes per day at a pace of 100 steps per minute, 5 days per week, and his walking sessions were confirmed by recording on a portable device. A year later, he had lost 11 pounds (5 kg) of body weight and his waist circumference was reduced by 8 cm. Imaging of his abdomen and liver revealed that visceral adiposity had been reduced by 30%, with a 60% reduction in liver fat. Features of the atherogenic dyslipidemic profile had all improved (reduction in triglyceride concentrations and increase in HDL-C levels) in addition to the expected reduction in LDL-C achieved by his concomitant statin therapy. The substantial improvements in the patient’s cardiometabolic risk profile were well beyond those expected from the rather small weight loss.
Figure 7: Steps in the Clinical Management of Obesities.
Initial assessment of the form of obesity (visceral vs. severe) is key in the determination of the therapeutic approach. Whereas lifestyle changes inducing limited weight loss could nevertheless positively impact ectopic fat and related cardiometabolic risk in patients with visceral obesity, more severe forms of obesity may require additional diagnostic tools and management approaches as a function of disease progression. LVH: left ventricular hypertrophy; MRI: magnetic resonance imaging.
CASE #2:
Unfortunately, despite her young age, this patient had already developed stage C, NYHA class III heart failure with preserved left ventricular ejection fraction (HFpEF), likely related to her severe obesity. Her echocardiogram suggested significant diastolic dysfunction with evidence for secondary WHO group 2 pulmonary hypertension (due to left sided heart disease). She also had severe left ventricular hypertrophy despite only mild, stage 2 hypertension. All these cardiac abnormalities are expected complications from her severe obesity, resulting from the associated significant hemodynamic and metabolic stresses. A NT-proBNP level should be obtained, but interpreted with caution given the known inverse relationship between BMI and natriuretic peptide levels: a relatively low value does not indicate absence of heart failure. Nevertheless, NT-proBNP when elevated is a helpful prognostic indicator in the presence of severe obesity (139) but will increase after weight loss due to bariatric surgery (140). Before initiating obesity-specific heart failure treatment, additional evaluations should be conducted to rule out secondary causes of obesity (severe hypothyroidism, Cushing’s syndrome, etc.) and other complications that may play a role in the development of cardiac dysfunction (obstructive sleep apnea, obesity hypoventilation syndrome, pulmonary hypertension, T2D, chronic kidney disease). Although loop diuretics and antihypertensive therapies are the mainstay of treatment for HFpEF, other treatments could be used to target the underlying defect of obesity and its consequences. Spironolactone should be considered since it was associated with a significant improvement in the risk for cardiovascular death, heart failure hospitalization, or aborted cardiac arrest among patients with obesity-related HFpEF (141). A targeted weight-loss program including caloric restriction, increased symptom-limited physical activity, pharmacological therapy (142), and evaluation for bariatric surgery should be initiated (Figure 7). Bariatric surgery is associated with significantly lower risk of incident MACE among patients with T2D and obesity (143) and is linked with improvement in multiple parameters related to heart failure in patients with HFpEF (62, 144). SGLT2 inhibitors should be used in patients with T2D and heart failure. As mentioned, dapagliflozin reduces total mortality (110) whereas empagliflozin reduces hospitalizations (109) in individuals with left ventricular systolic dysfunction. SGLT2 inhibitors are now recommended in these populations (145). In addition to ongoing trials with these drugs in patients with diabetes and heart failure, other studies currently test their effect in those with HFpEF (146, 147).
10. The obesogenic environment as a barrier to clinical management
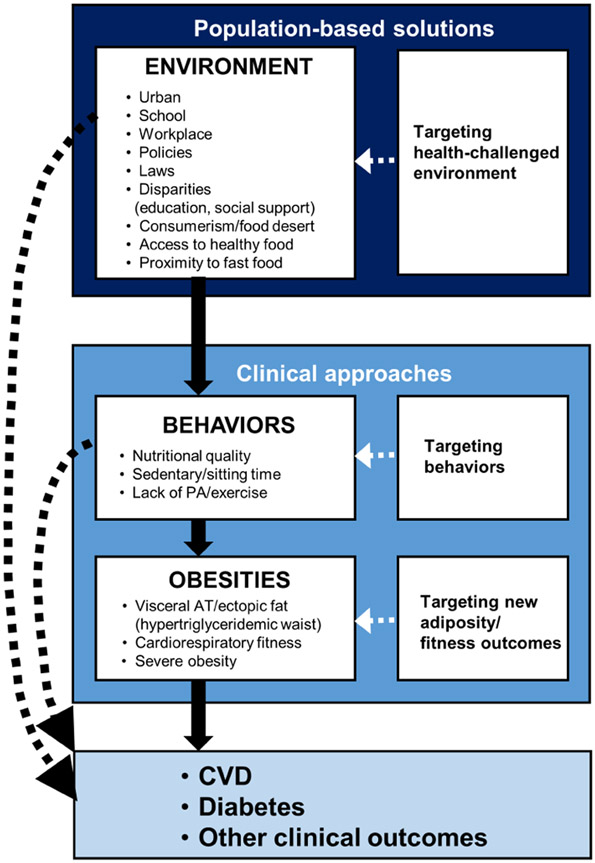
In spite of the considerable progress made over the past 50 years in our understanding of human body composition, regulation of energy balance, lipid and carbohydrate metabolism and the overall biology of human adiposity, the prevalence of obesity is still increasing worldwide (56). Clinical approaches targeting weight loss as a primary outcome have had little impact at the population level. Because of the absence of mechanism-targeted treatment, the “statin-like” approach to manage high CVD risk forms of obesity does not exist yet, despite some promising results obtained with some classes of anti-diabetic drugs. As emphasized in the recently published Canadian obesity guidelines (54), managing obesity is much more than a simple recommendation to eat less and move more (Figure 8). Proper global evaluation of the patient’s history is necessary to identify potential psycho-social causes (e.g.: low socio-economic status, food insecurity, built environment/neighborhood, crime rates, local food establishments, access to healthy nutrition and walkable neighborhood, depressive episodes, personal crisis, social support, etc.) that represent some of the barriers in adopting and maintaining a healthy lifestyle over the long term. It is also obvious that our current living and work environments are not always promoting health: proliferation of sedentary jobs, access to highly processed and energy-dense foods that are heavily marketed and affordable, lack of environments promoting physical activity and healthy eating, urban environments lacking infrastructures such as sidewalks, proximity stores that can be reached by safe walking, parks, bicycle paths, proliferation of suburbs and long commutes, etc. (148, 149). The European guidelines (ESC/EAS) recommend that social deprivation or psychosocial stress should be taken into consideration in assessing CVD risk (68) whereas the 2019 ACC/AHA guideline on primary prevention of CVD also recommends that social determinants of health be considered (150). Not addressing the basic, unmet social needs considerably reduces the probability of being successful in the attempt of the multidisciplinary team in recalibrating lifestyle in high-risk obesities for the optimal management of cardiometabolic risk as well as physical and mental well-being. Because obtaining such information requires time, resources and additional expertise, cardiologists must play an important leadership role in recommending the development of multidisciplinary clinical teams (nutritionists, kinesiologists, psychologists, etc.) as well as the implementation of the public health policies required to have a long-term impact on the socio-economic causes of obesity.
Figure 8: Clinical Approaches and Public Health Solutions to tackle Obesities.
As high-risk forms of obesity result from the complex interactions of biological, behavioral, psycho-social and environmental factors, the current obesity epidemic will not be curbed until an integrated set of population-based solutions and clinical approaches are put in place, going beyond body weight/weight loss as the single assessment/management outcome. AT: adipose tissue, CVD: cardiovascular disease; PA: physical activity.
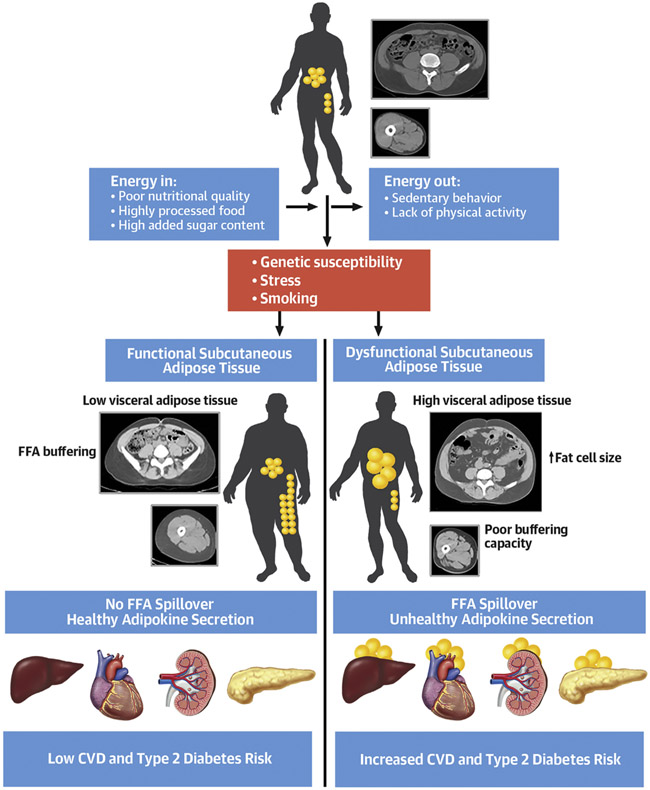
Central Illustration: Key Factors Involved in Visceral Obesity and Related Cardiometabolic Risk.
Subcutaneous adipose tissue plays an important role in the storage of excess calories resulting from a positive energy imbalance. When facing caloric surplus, functional subcutaneous adipose tissue expands to allow the storage of excess energy, a process that also limits accumulation of fat in intra-abdominal adipose depots (visceral adipose tissue). In the presence of dysfunctional subcutaneous adipose tissue, the resulting lipid spillover must then be stored in visceral adipose tissue as well as in normally lean tissues (heart, liver, skeletal muscle, kidney, pancreas), a process referred to as ectopic fat deposition.
Table 1:
Simple Clinical Tools to Assess/Manage Abdominal Obesity
|
HIGHLIGHTS.
Individual differences in regional body fat distribution are a key factor in determining associated health risks.
Excess visceral fat storage is often accompanied by accumulation of fat in normally lean tissues such as the heart, liver, kidneys, pancreas, and skeletal muscle.
Elevated waist circumference is associated with hazard to health at any level of body mass index and should be monitored routinely in clinical practice.
Patients at cardiovascular risk benefit from lifestyle interventions that reduce waist circumference even without weight loss.
Funding:
JPD is the Scientific Director of the International Chair on Cardiometabolic Risk supported by the Fondation de l’Université Laval. Research from JPD discussed in this paper has been and is currently supported by the Canadian Institutes of Health Research (Foundation grant: FDN-167278) as well as by the Fondation of the Québec Heart and Lung Institute. ACC holds the Canada Research Chair in Molecular Imaging of Diabetes. IJN has received a grant from NIH/NIDDK (K23 DK106520).
LIST OF ABBREVIATIONS
- BMI
body mass index
- GLP1-RA
glucagon-like peptide 1 receptor agonists
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- MACE
major adverse cardiovascular events
- NASH
non-alcoholic steatohepatitis
- NDPP
National Diabetes Prevention Program
- PCE
Pooled Cohort Equations
- T2D
type 2 diabetes
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JPD has no relationships relevant to the paper to declare. ACC received funding by Eli Lilly, HLS Therapeutics, Janssen, Novartis Pharmaceuticals Canada, and Novo Nordisk Canada as a consultant. AT received research funding from Johnson & Johnson Medical Companies, Medtronic and Bodynov for studies unrelated to this manuscript, in addition to consulting fees from Bausch Health and Novo Nordisk. IJN has received speaking and consultancy fees from Boehringer Ingelheim, Merck, AMRA Medical and a grant from Novo Nordisk. PP has received honoraria for continuing medical education/consultant/expert events from Abbott, Amgen, AstraZeneca, Bayer, Bausch Health, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Servier.
References
- 1.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J 2007;28:2087–93. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, et al. Obesity. Nat Rev Dis Primers 2017;3:17034. [DOI] [PubMed] [Google Scholar]
- 4.G.B.D. Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Adult obesity prevalence maps. https://www.cdc.gov/obesity/data/prevalence-maps.html#overall. Accessed September 29, 2020.
- 6.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief No. 360, February 2020. Available at: https://www.cdc.gov/nchs/products/databriefs/db360.htm. Accessed September 29, 2020. [PubMed] [Google Scholar]
- 7.Powell-Wiley TM, Poirier P, Burke L, Després J-P, Gordon-Larsen P, Lavie CJ Jr, Lear SA, Ndumele C, Neeland I, Sanders P, St-Onge M-P; on behalf of the American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e•••–e•••. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008;168:1617–24. [DOI] [PubMed] [Google Scholar]
- 9.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest 2019;129:3978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: Clinical challenges and implications for management. Circulation 2018;137:1391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camhi SM, Must A, Gona PN, et al. Duration and stability of metabolically healthy obesity over 30 years. Int J Obes (Lond) 2019;43:1803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinosa De Ycaza AE, Donegan D, Jensen MD. Long-term metabolic risk for the metabolically healthy overweight/obese phenotype. Int J Obes (Lond) 2018;42:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen L, Netterstrom MK, Johansen NB, et al. Metabolically healthy obesity and ischemic heart disease: a 10-year follow-up of the Inter99 study. J Clin Endocrinol Metab 2017;102:1934–42. [DOI] [PubMed] [Google Scholar]
- 14.Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990;10:497–511. [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Léger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol 1992;72:787–95. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes 1983;7:437–45. [PubMed] [Google Scholar]
- 17.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. [DOI] [PubMed] [Google Scholar]
- 18.Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008;28:1039–49. [DOI] [PubMed] [Google Scholar]
- 19.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301–13. [DOI] [PubMed] [Google Scholar]
- 20.Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715–25. [DOI] [PubMed] [Google Scholar]
- 21.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020; 126:1477–500. [DOI] [PubMed] [Google Scholar]
- 22.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 23.Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020;16:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008;29:2959–71. [DOI] [PubMed] [Google Scholar]
- 25.Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21:E439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartier A, Côté M, Lemieux I, et al. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism 2009;58:1452–8. [DOI] [PubMed] [Google Scholar]
- 27.Jensen MD. Visceral fat: Culprit or canary? Endocrinol Metab Clin North Am 2020;49:229–37. [DOI] [PubMed] [Google Scholar]
- 28.Goossens GH, Moors CC, Jocken JW, et al. Altered skeletal muscle fatty acid handling in subjects with impaired glucose tolerance as compared to impaired fasting glucose. Nutrients 2016;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Normand-Lauzière F, Frisch F, Labbé SM, et al. Increased postprandial nonesterified fatty acid appearance and oxidation in type 2 diabetes is not fully established in offspring of diabetic subjects. PLoS One 2010;5:e10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab 2011;300:E255–62. [DOI] [PubMed] [Google Scholar]
- 32.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 2018;98:2133–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bril F, Barb D, Portillo-Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017;65:1132–44. [DOI] [PubMed] [Google Scholar]
- 34.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: Results from the San Antonio Metabolism study. Diabetes 2017;66:815–22. [DOI] [PubMed] [Google Scholar]
- 35.Ryden M, Arner P. Cardiovascular risk score is linked to subcutaneous adipocyte size and lipid metabolism. J Intern Med 2017;282:220–8. [DOI] [PubMed] [Google Scholar]
- 36.Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015;23:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol 2021;320:C375–C91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23:201–29. [DOI] [PubMed] [Google Scholar]
- 39.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol 2015;11:90–100. [DOI] [PubMed] [Google Scholar]
- 40.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–59. [DOI] [PubMed] [Google Scholar]
- 41.Hussain I, Patni N, Garg A. Lipodystrophies, dyslipidaemias and atherosclerotic cardiovascular disease. Pathology 2019;51:202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann JP, Savage DB. What lipodystrophies teach us about the metabolic syndrome. J Clin Invest 2019;130:4009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lotta LA, Gulati P, Day FR, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet 2017;49:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 2003;285:E1282–8. [DOI] [PubMed] [Google Scholar]
- 45.McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitrou P, Boutati E, Lambadiari V, et al. Rates of lipid fluxes in adipose tissue in vivo after a mixed meal in morbid obesity. Int J Obes (Lond) 2010;34:770–4. [DOI] [PubMed] [Google Scholar]
- 47.Labbé SM, Grenier-Larouche T, Noll C, et al. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes 2012;61:2701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labbé SM, Noll C, Grenier-Larouche T, et al. Improved cardiac function and dietary fatty acid metabolism after modest weight loss in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab 2014;306:E1388–96. [DOI] [PubMed] [Google Scholar]
- 49.Carreau AM, Noll C, Blondin DP, et al. Bariatric surgery rapidly decreases cardiac dietary fatty acid partitioning and hepatic insulin resistance through increased intra-abdominal adipose tissue storage and reduced spillover in type 2 diabetes. Diabetes 2020;69:567–77. [DOI] [PubMed] [Google Scholar]
- 50.Noll C, Montastier E, Amrani M, et al. Seven-day overfeeding enhances adipose tissue dietary fatty acid storage and decreases myocardial and skeletal muscle dietary fatty acid partitioning in healthy subjects. Am J Physiol Endocrinol Metab 2020;318:E286–E96. [DOI] [PubMed] [Google Scholar]
- 51.Sletten AC, Peterson LR, Schaffer JE. Manifestations and mechanisms of myocardial lipotoxicity in obesity. J Intern Med 2018;284:478–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carpentier AC. Abnormal myocardial dietary fatty acid metabolism and diabetic cardiomyopathy. Can J Cardiol 2018;34:605–14. [DOI] [PubMed] [Google Scholar]
- 53.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 54.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ 2020;192:E875–E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–50. [DOI] [PubMed] [Google Scholar]
- 56.N.C.D. Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joyce E, Lala A, Stevens SR, et al. Prevalence, profile, and prognosis of severe obesity in contemporary hospitalized heart failure trial populations. JACC Heart Fail 2016;4:923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chassé M, Mathieu P, Voisine P, et al. The underestimated belly factor: Waist circumference is linked to significant morbidity following isolated coronary artery bypass grafting. Can J Cardiol 2016;32:327–35. [DOI] [PubMed] [Google Scholar]
- 59.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogden CL, Fryar CD, Martin CB, et al. Trends in obesity prevalence by race and Hispanic origin-1999-2000 to 2017-2018. JAMA 2020;24:1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piché ME, Auclair A, Harvey J, Marceau S, Poirier P. How to choose and use bariatric surgery in 2015. Can J Cardiol 2015;31:153–66. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez Flores M, Aguilar Salinas C, Piché ME, Auclair A, Poirier P. Effect of bariatric surgery on heart failure. Expert Rev Cardiovasc Ther 2017;15:567–79. [DOI] [PubMed] [Google Scholar]
- 63.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]; J Am Coll Cardiol. 2014. July 1;63(25 Pt B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009;53:1925–32. [DOI] [PubMed] [Google Scholar]
- 65.Khera R, Pandey A, Ayers CR, et al. Performance of the Pooled Cohort Equations to estimate atherosclerotic cardiovascular disease risk by body mass index. JAMA Netw Open 2020;3:e2023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 2020;370:m3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist. A marker of the atherogenic metabolic triad (hyperinsulinemia, hyperapolipoprotein B, small, dense LDL) in men? Circulation 2000;102:179–84. [DOI] [PubMed] [Google Scholar]
- 68.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. [DOI] [PubMed] [Google Scholar]
- 69.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care 2006;29:151–3. [DOI] [PubMed] [Google Scholar]
- 70.Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010;33:920–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 1996;335:1357–62. [DOI] [PubMed] [Google Scholar]
- 72.Ross R Does exercise without weight loss improve insulin sensitivity? Diabetes Care 2003;26:944–5. [DOI] [PubMed] [Google Scholar]
- 73.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 1998;49:235–61. [DOI] [PubMed] [Google Scholar]
- 74.Broekhuizen LN, Boekholdt SM, Arsenault BJ, et al. Physical activity, metabolic syndrome, and coronary risk: the EPIC-Norfolk prospective population study. Eur J Cardiovasc Prev Rehabil 2011;18:209–17. [DOI] [PubMed] [Google Scholar]
- 75.Borel AL, Nazare JA, Smith J, et al. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity (Silver Spring) 2012;20:1223–33. [DOI] [PubMed] [Google Scholar]
- 76.Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol 2009;5:319–25. [DOI] [PubMed] [Google Scholar]
- 77.Després JP. Obesity and cardiovascular disease: weight loss is not the only target. Can J Cardiol 2015;31:216–22. [DOI] [PubMed] [Google Scholar]
- 78.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 79.Torres-Pena JD, Rangel-Zuniga OA, Alcala-Diaz JF, Lopez-Miranda J, Delgado-Lista J. Mediterranean diet and endothelial function: A review of its effects at different vascular bed levels. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Panizza CE, Lim U, Yonemori KM, et al. Effects of intermittent energy restriction combined with a Mediterranean diet on reducing visceral adiposity: A randomized active comparator pilot study. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenkins DJA, Dehghan M, Mente A, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med 2021;384:1312–22. [DOI] [PubMed] [Google Scholar]
- 82.Ma J, McKeown NM, Hwang SJ, Hoffmann U, Jacques PF, Fox CS. Sugar-sweetened beverage consumption is associated with change of visceral adipose tissue over 6 years of follow-up. Circulation 2016;133:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet 2017;390:2643–54. [DOI] [PubMed] [Google Scholar]
- 84.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC Health Promotion Series. J Am Coll Cardiol 2018;72:1622–39. [DOI] [PubMed] [Google Scholar]
- 85.Myers J, McAuley P, Lavie CJ, Després JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis 2015;57:306–14. [DOI] [PubMed] [Google Scholar]
- 86.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 2016;134:e653–e99. [DOI] [PubMed] [Google Scholar]
- 87.Rao S, Pandey A, Garg S, et al. Effect of exercise and pharmacological interventions on visceral adiposity: A systematic review and meta-analysis of long-term randomized controlled trials. Mayo Clin Proc 2019;94:211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract 2016;22 Suppl 3:1–203. [DOI] [PubMed] [Google Scholar]
- 89.Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. Diabetes Care 2016;39:861–77. [DOI] [PubMed] [Google Scholar]
- 90.Unick JL, Beavers D, Bond DS, et al. The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med 2013;126:236–42, 42 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Egberts K, Brown WA, Brennan L, O'Brien PE. Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg 2012;22:335–41. [DOI] [PubMed] [Google Scholar]
- 92.Auclair A, Martin J, Bastien M, et al. Is there a role for visceral adiposity in inducing type 2 diabetes remission in severely obese patients following biliopancreatic diversion with duodenal switch surgery? Obes Surg 2016;26:1717–27. [DOI] [PubMed] [Google Scholar]
- 93.Giordano S, Victorzon M. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging 2015;10:1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2020. Diabetes Care 2020;43:S98–S110. [DOI] [PubMed] [Google Scholar]
- 95.LaMoia TE, Shulman GI. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev 2021;42:77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab 2021;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–51. [DOI] [PubMed] [Google Scholar]
- 99.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–30. [DOI] [PubMed] [Google Scholar]
- 100.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–29. [DOI] [PubMed] [Google Scholar]
- 101.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57. [DOI] [PubMed] [Google Scholar]
- 102.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perry RJ, Shulman GI. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem 2020;295:14379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 105.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 106.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–35. [DOI] [PubMed] [Google Scholar]
- 107.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 108.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 109.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 110.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 111.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- 112.Drucker DJ. The ascending GLP-1 road from clinical safety to reduction of cardiovascular complications. Diabetes 2018;67:1710–9. [DOI] [PubMed] [Google Scholar]
- 113.Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020;17:761–72. [DOI] [PubMed] [Google Scholar]
- 114.Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–31. [DOI] [PubMed] [Google Scholar]
- 115.Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab 2019;21:285–92. [DOI] [PubMed] [Google Scholar]
- 116.Arase Y, Shiraishi K, Anzai K, et al. Effect of sodium glucose co-transporter 2 inhibitors on liver fat mass and body composition in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus. Clin Drug Investig 2019;39:631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Latva-Rasku A, Honka MJ, Kullberg J, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: A randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care 2019;42:931–7. [DOI] [PubMed] [Google Scholar]
- 118.Iacobellis G, Gra-Menendez S. Effects of dapagliflozin on epicardial fat thickness in patients with type 2 diabetes and obesity. Obesity (Silver Spring) 2020;28:1068–74. [DOI] [PubMed] [Google Scholar]
- 119.Yagi S, Hirata Y, Ise T, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr 2017;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neeland IJ, de Albuquerque Rocha N, Hughes C, Ayers CR, Malloy CR, Jin ES. Effects of empagliflozin treatment on glycerol-derived hepatic gluconeogenesis in adults with obesity: A randomized clinical trial. Obesity (Silver Spring) 2020;28:1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santilli F, Simeone PG, Guagnano MT, et al. Effects of liraglutide on weight loss, fat distribution, and beta-cell function in obese subjects with prediabetes or early type 2 diabetes. Diabetes Care 2017;40:1556–64. [DOI] [PubMed] [Google Scholar]
- 122.Bouchi R, Nakano Y, Fukuda T, et al. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J 2017;64:269–81. [DOI] [PubMed] [Google Scholar]
- 123.van Eyk HJ, Paiman EHM, Bizino MB, et al. A double-blind, placebo-controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovasc Diabetol 2019;18:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) 2017;25:311–6. [DOI] [PubMed] [Google Scholar]
- 125.Iacobellis G, Villasante Fricke AC. Effects of semaglutide versus dulaglutide on epicardial fat thickness in subjects with type 2 diabetes and obesity. J Endocr Soc 2020;4:bvz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dutour A, Abdesselam I, Ancel P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 2016;18:882–91. [DOI] [PubMed] [Google Scholar]
- 127.Yan J, Yao B, Kuang H, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: The effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology 2019;69:2414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Neeland IJ, Marso SP, Ayers CR, et al. Effects of liraglutide on visceral and ectopic fat in adults with overweight/obesity at high cardiovascular risk: a randomized clinical trial. Presented at: American Diabetes Association Scientific Sessions; June 25-29, 2021. [DOI] [PubMed] [Google Scholar]
- 129.Bizino MB, Jazet IM, de Heer P, et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia 2020;63:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McCrimmon RJ, Catarig AM, Frias JP, et al. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia 2020;63:473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zomer E, Gurusamy K, Leach R, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev 2016;17:1001–11. [DOI] [PubMed] [Google Scholar]
- 132.Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010;363:905–17. [DOI] [PubMed] [Google Scholar]
- 134.Bohula EA, Wiviott SD, McGuire DK, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med 2018;379:1107–17. [DOI] [PubMed] [Google Scholar]
- 135.Caterson ID, Finer N, Coutinho W, et al. Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes Metab 2012;14:523–30. [DOI] [PubMed] [Google Scholar]
- 136.Look AHEAD Research Group, Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lemieux I, Poirier P, Bergeron J, et al. Hypertriglyceridemic waist: A useful screening phenotype in preventive cardiology? Can J Cardiol 2007;23:23B–31B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bailey RL, Miller PE, Mitchell DC, et al. Dietary screening tool identifies nutritional risk in older adults. Am J Clin Nutr 2009;90:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh S, Pandey A, Neeland IJ. Diagnostic and prognostic considerations for use of natriuretic peptides in obese patients with heart failure. Prog Cardiovasc Dis 2020;63:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin J, Bergeron S, Pibarot P, et al. Impact of bariatric surgery on N-terminal fragment of the prohormone brain natriuretic peptide and left ventricular diastolic function. Can J Cardiol 2013;29:969–75. [DOI] [PubMed] [Google Scholar]
- 141.Cohen JB, Schrauben SJ, Zhao L, et al. Clinical phenogroups in heart failure with preserved ejection fraction: Detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail 2020;8:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pirlet C, Poirier P, Cieza T, et al. Clinical impact of weight-loss pharmacotherapy in patients with atherosclerotic cardiovascular disease. Am J Cardiovasc Drugs 2021;21:271–81. [DOI] [PubMed] [Google Scholar]
- 143.Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019;322:1271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McDowell K, Petrie MC, Raihan NA, Logue J. Effects of intentional weight loss in patients with obesity and heart failure: a systematic review. Obes Rev 2018;19:1189–204. [DOI] [PubMed] [Google Scholar]
- 145.O'Meara E, McDonald M, Chan M, et al. CCS/CHFS Heart Failure Guidelines: Clinical Trial Update on Functional Mitral Regurgitation, SGLT2 Inhibitors, ARNI in HFpEF, and Tafamidis in Amyloidosis. Can J Cardiol 2020;36:159–69. [DOI] [PubMed] [Google Scholar]
- 146.Anker SD, Butler J, Filippatos GS, et al. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail 2019;21:1279–87. [DOI] [PubMed] [Google Scholar]
- 147.Williams DM, Evans M. Dapagliflozin for heart failure with preserved ejection fraction: Will the DELIVER study deliver? Diabetes Ther 2020;11:2207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bluher M Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288–98. [DOI] [PubMed] [Google Scholar]
- 149.Rajan S, McKee M, Rangarajan S, et al. Association of symptoms of depression with cardiovascular disease and mortality in low-, middle-, and high-income countries. JAMA Psychiatry 2020;77:1052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]