Abstract
Background
BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine that has been deployed in India. The results of the phase 3 trial have shown clinical efficacy of BBV152. We aimed to evaluate the effectiveness of BBV152 against symptomatic RT-PCR-confirmed SARS-CoV-2 infection.
Methods
We conducted a test-negative, case-control study among employees of the All India Institute of Medical Sciences (a tertiary care hospital in New Delhi, India), who had symptoms suggestive of COVID-19 and had an RT-PCR test for SARS-CoV-2 during the peak of the second wave of the COVID-19 pandemic in India between April 15 and May 15, 2021. Cases (test-positives) and controls (test-negatives) were matched (1:1) on the basis of age and gender. The odds of vaccination with BBV152 were compared between cases and controls and adjusted for level of occupational exposure (to COVID-19), previous SARS-CoV-2 infection, and calendar time, using conditional logistic regression. The primary outcome was effectiveness of two doses of BBV152 (with the second dose received at least 14 days before testing) in reducing the odds of symptomatic RT-PCR-confirmed SARS-CoV-2 infection, expressed as (1 – odds ratio) × 100%.
Findings
Between April 15 and May 15, 2021, 3732 individuals had an RT-PCR test. Of these, 2714 symptomatic employees had data on vaccination status, and 1068 matched case-control pairs were available for analysis. The adjusted effectiveness of BBV152 against symptomatic COVID-19 after two doses administered at least 14 days before testing was 50% (95% CI 33–62; p<0·0001). The adjusted effectiveness of two doses administered at least 28 days before testing was 46% (95% CI 22–62) and administered at least 42 days before testing was 57% (21–76). After excluding participants with previous SARS-CoV-2 infections, the adjusted effectiveness of two doses administered at least 14 days before testing was 47% (95% CI 29–61).
Interpretation
This study shows the effectiveness of two doses of BBV152 against symptomatic COVID-19 in the context of a huge surge in cases, presumably dominated by the potentially immune-evasive delta (B.1.617.2) variant of SARS-CoV-2. Our findings support the ongoing roll-out of this vaccine to help control the spread of SARS-CoV-2, while continuing the emphasis on adherence to non-pharmacological measures.
Funding
None.
Translation
For the Hindi translation of the abstract see Supplementary Materials section.
Introduction
On Jan 16, 2021, India began to roll out its COVID-19 vaccination drive with two vaccines, Oxford–AstraZeneca ChAdOx1 nCoV-19 (a chimpanzee adenoviral vector vaccine manufactured by Serum Institute of India; Covishield) and BBV152 (a whole-virion inactivated vaccine manufactured by Bharat Biotech; Covaxin). Initially, only health-care workers and front-line workers (eg, police, paramilitary forces, sanitation workers, and disaster management volunteers) were eligible for vaccination. Eligibility was extended to include Indian residents older than 60 years or aged 45–60 years with comorbidities on March 1, 2021, and then to all residents older than 45 years on April 1, 2021. Currently, vaccination is being offered to all adults aged 18 years or older in India in the national COVID-19 vaccination drive.
Approval for BBV152 was granted on the basis of safety and immunogenicity data from phase 1 and phase 2 trials.1, 2 Subsequently, the phase 3 trial (NCT04641481) reported a vaccine efficacy of 77·8% (95% CI 65·2–86·4) against symptomatic laboratory-confirmed SARS-CoV-2 infection.3 The reported efficacy against severe disease was 93·4% (95% CI 57·1–99·8), against asymptomatic infection was 63·6% (29·0–82·4), and against the delta (B.1.617.2) variant of concern was 65·2% (33·1–83·0).3
Assessment of vaccine effectiveness after licensure is an essential component of any vaccine roll-out, because performance in a real-world setting often differs from the measured efficacy under controlled trial conditions.4 As a mass vaccination strategy is the most promising path to an end to the pandemic, effectiveness data for BBV152 are required to guide future policy decisions.
Research in context.
Evidence before this study
We searched PubMed and medRxiv for research articles from March 11, 2020, to Oct 10, 2021, with no language restrictions, using the search terms “SARS-CoV-2”, “effectiveness”, “B.1.617”, and “BBV152”. Previous phase 1 and phase 2 studies of BBV152 suggested the vaccine was safe and immunogenic. The phase 3 trial found a vaccine efficacy of 77·8% (95% CI 65·2–86·4) against symptomatic laboratory-confirmed SARS-CoV-2 infection (with a median of 146 days of follow-up, after one dose). The reported efficacy against severe disease was 93·4% (95% CI 57·1 to 99·8), against asymptomatic infection was 63·6% (29·0–82·4), and against the delta (B.1.617.2) variant of concern was 65·2% (33·1–83·0).
Added value of this study
This study is, to our knowledge, the first real-world observational study assessing vaccine effectiveness of BBV152. We found vaccine effectiveness of 50% (95% CI 33–62) for complete vaccination at least 14 days before testing, against symptomatic laboratory-confirmed COVID-19. Considering the timing and context of the study, the results reflect vaccine effectiveness in surge conditions attributable to the delta variant of SARS-CoV-2, which was the predominant variant of concern during the second wave of the COVID-19 pandemic in India.
Implications of all the available evidence
Our findings, together with other emerging data, point to the possible immune evasive potential of the delta variant of SARS-CoV-2, and underscore the need to ensure complete vaccination is done in a timely manner, while continuing to implement and adhere to the non-pharmacological interventions to prevent the spread of the virus, such as physical distancing and face masks. Further real-world studies should assess the effectiveness of BBV152 against severe outcomes of SARS-CoV-2 infection, such as hospitalisation, severe disease, and death.
We aimed to evaluate the effectiveness of BBV152 against symptomatic RT-PCR-confirmed SARS-CoV-2 infection.
Methods
Study design and participants
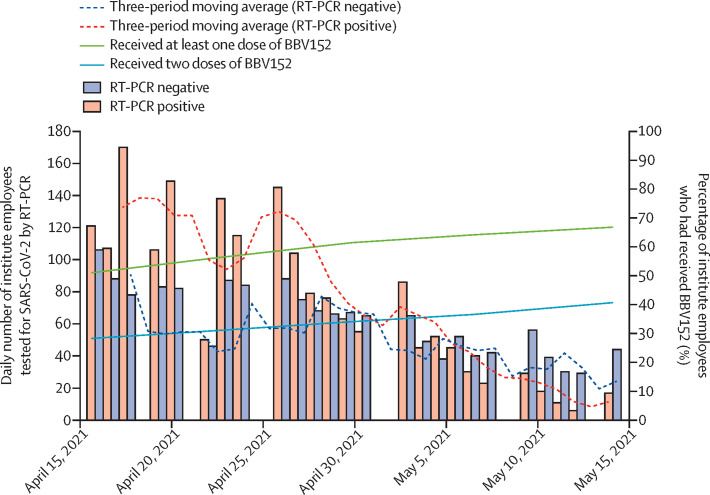
We conducted a test-negative, case-control study at the All India Institute of Medical Sciences, New Delhi, India, which is a tertiary care hospital and COVID-19 treatment centre. The hospital's COVID-19 vaccination centre exclusively offered the BBV152 vaccine from Jan 16, 2021, onwards. All institute employees had equal opportunity to access this vaccine regardless of age, comorbidity, socioeconomic status, place of residence, nature of work, previous SARS-CoV-2 infection, or COVID-19 serological status. Therefore, the majority of the approximately 23 000 employees of the institute who are vaccinated have received BBV152 (figure 1 ). The institute's COVID-19 sample collection facility offers RT-PCR testing for SARS-CoV-2 to employees and their family members who have self-reported symptoms suggestive of COVID-19, or have a history of high-risk exposure to a laboratory-confirmed case of COVID-19.
Figure 1.
Daily number of employees tested for SARS-CoV-2 by RT-PCR and cumulative coverage of BBV152 vaccination among institute employees, April 15–May 15, 2021
We included all employees of the hospital who had symptoms suggestive of COVID-19 and had been tested for SARS-CoV-2 by RT-PCR at the institute's COVID-19 sample collection facility between April 15 and May 15, 2021, the peak of the second wave of the COVID-19 pandemic in India. We excluded people who had an invalid test result that precluded the assignment of an outcome (RT-PCR positive or negative), were asymptomatic at the time of testing, had received the ChAdOx1 nCoV-19 vaccine, or had missing data regarding dates of vaccination. Data were retrieved from records of the institute sample collection facility and vaccination centre and analysis was done on a deidentified dataset. Consent was not sought from the study participants for use of the collected data, with due approval from the ethics committee.
The study protocol was approved by the institute ethics committee before the study commenced (IECPG-344/28.05.2021).
Procedures
Information was retrieved from the institute's COVID-19 sample collection facility, which routinely records data pertaining to demographics, symptoms at the time of testing, occupation and location of workplace, previous SARS-CoV-2 testing, and vaccination status by oral recall for all employees who are tested.
Vaccination status and exact dates of receipt of the first and second doses as reported by the participants were verified using the records of the institute's vaccination centre. The same method was used to collect missing data for participants whose vaccination status and dates of receipt were absent from the sample collection facility records. Participants who had missing vaccination data despite these approaches were excluded. Data regarding presence and dates of previous COVID-19 testing (RT-PCR, cartridge-based nucleic acid amplification test, or rapid antigen test) from the sampling facility records were re-ascertained by searching the participants' electronic health record using their unique hospital identification number. Definitions of symptoms suggestive of COVID-19, high-risk exposure, previous SARS-CoV-2 infection, and level of occupational exposure are provided in the appendix 2 (p 2).
Participants were divided into two groups on the basis of their definitive RT-PCR result: symptomatic test-positive (cases) and symptomatic test-negative (controls). Cases and controls were then matched (1:1) on the basis of age and gender. Vaccination status was compared between cases and controls.
Statistical analysis
At the time of the conduct of the study, there was an absence of published data on the efficacy of BBV152 (except for a few press releases). As such, a minimum sample size of 1133 cases and 1133 controls was calculated, assuming an effectiveness of 50% (due to absence of definitive efficacy information, and emerging in-vitro data about immune evasiveness of the delta variant), vaccination coverage in the population of at least 30% with at least one dose, a precision of plus or minus 10%, and a type I error rate of 0·05. In practice, all participants who met the eligibility criteria during the enrolment period were included.
We matched one test-negative control to each case according to age (exact matching by completed years) and gender. The matching ratio of 1:1 was agreed upon due to the relative paucity of the number of controls (ie, those who were RT-PCR-negative), as the study was conducted in surge conditions with a high test-positivity rate. Due to the small sample size, only the most relevant matching factors were chosen to balance the ability to reduce bias and to enrol a sufficient number of case-control pairs to achieve the desired power. A conditional logistic regression model was used to estimate the odds ratio (OR) comparing the odds of vaccination between symptomatic test-positive and symptomatic test-negative participants in the matched set. We estimated both unadjusted and adjusted ORs, accounting for covariates that were selected a priori on the basis of their known associations with SARS-CoV-2 infection and COVID-19 vaccine receipt, including previous SARS-CoV-2 infection, level of occupational exposure, and calendar time. To account for the influence of changing test-positivity rates on vaccine effectiveness, we adjusted for the calendar time by characterising the participants as having been tested in either the first, second, or third 10-day period within the 30 days of the study. However, we did not choose calendar time as a matching factor as it would have led to a substantial loss of sample size and thus, statistical power, owing to the differential depletion of test-positive and test-negative participants at different timepoints in the study period (the former at the start of the study period and the latter at the end due to declining test-positivity rate towards the end of the study period). Participants with previous SARS-CoV-2 infection were included in the primary analysis and adjusted for in accordance with emerging literature on this matter.5, 6 Vaccine effectiveness was calculated using the formula: effectiveness=(1 – OR) × 100%. The reference group for vaccination status was individuals who had not received any vaccine dose by the date of RT-PCR testing.
Effectiveness was estimated separately for those who received only one dose of BBV152 and those who received two doses, and was also calculated for different time intervals after the administered doses.
The primary outcome was effectiveness of two doses of BBV152 (with the second dose received at least 14 days before testing) in reducing the odds of symptomatic RT-PCR-confirmed SARS-CoV-2 infection. Other outcomes were the effectiveness of two doses administered at least 28 days and at least 42 days before testing. Effectiveness was also estimated separately for each 10-day period within the 30 days of the study, by gender, and after excluding those with previous SARS-CoV-2 infection.
We also performed a sensitivity analysis on the unmatched population of test-positive and test-negative participants using a multivariable logistic regression model adjusted for age, gender, previous SARS-CoV-2 infection, level of occupational exposure, and 10-day period of testing.
A p value of less than 0·05 was considered significant and all p values are two-sided. All analyses were conducted using Stata version 16.
Role of the funding source
There was no funding source for this study.
Results
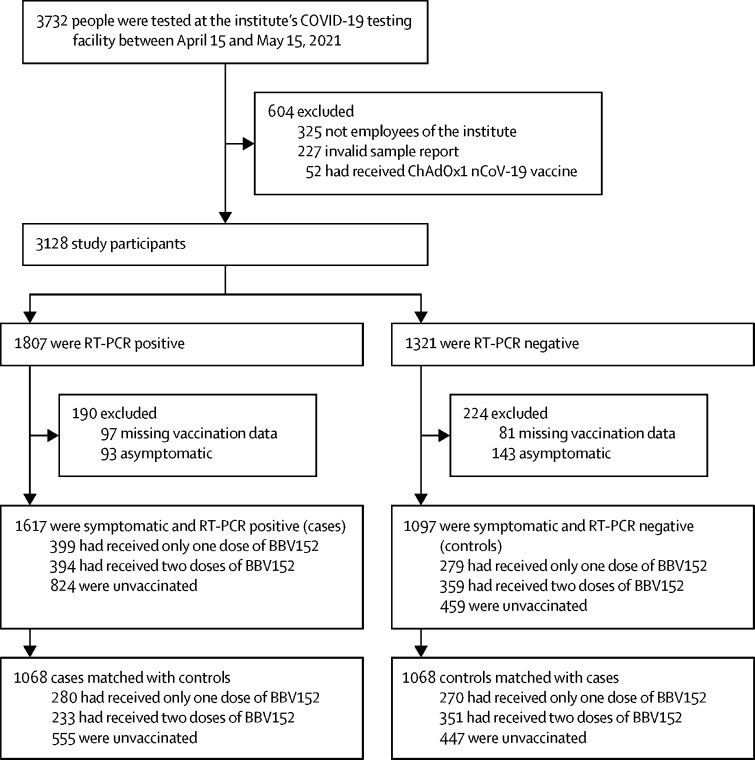
Between April 15 and May 15, 2021, 3732 people were tested for SARS-CoV-2 at the institute's COVID-19 sample collection facility. After excluding people who were not institute employees (n=325), had invalid test reports (n=227), had received the ChAdOx1 nCoV-19 vaccine (n=52), had missing data on vaccination status and dates (n=178), and were asymptomatic at the time of testing (n=236), 2714 symptomatic tested participants remained, of whom 1617 tested positive for SARS-CoV-2 (cases) and 1097 tested negative (controls; figure 2 ). The characteristics of participants who were excluded due to missing vaccination data were broadly similar between cases and controls, except for a higher proportion of people working in COVID-19 areas among cases than among controls (23 [23%] of 97 cases vs eight [10%] of 81 controls).
Figure 2.
Study profile
After matching by age and gender, a final set of 1068 matched case-control pairs were available for analysis (table 1 ). Among participants in the control group, 119 (11·1%) of 1068 had a documented previous SARS-CoV-2 infection, which was significantly higher than in the test-positive group (38 [3·6%] of 1068). The majority of eligible participants were tested in the first and second 10-day periods, when the test-positivity rate was at its peak (there was a gradual decline in test-positivity towards the end of the study period, from May 6 to May 15, 2021). The median interval between receipt of the last vaccine dose and the end of the study period (May 15, 2021) for those who had received one dose was 37 days (range 7–119) and two doses was 50 days (5–103). Cases had a higher odds of working in a COVID-19 area than did controls after adjusting for age, gender, previous SARS-CoV-2 infection, calendar time, and number of doses of BBV152 received, although this was not significant (adjusted OR 1·13 [95% CI 0·87–1·46]; p=0·34).
Table 1.
Participant characteristics
| Symptomatic SARS-CoV-2 RT-PCR-positive participants (n=1068) | Symptomatic SARS-CoV-2 RT-PCR-negative participants (n=1068) | Unvaccinated participants (n=1002) | Participants vaccinated with only one dose of BBV152 (n=550) | Participants vaccinated with two doses of BBV152 (n=584) | ||
|---|---|---|---|---|---|---|
| Age, years | ||||||
| <30 | 473 (44·3%) | 473 (44·3%) | 465 (46·4%) | 268 (48·7%) | 213 (36·5%) | |
| 31–40 | 361 (33·8%) | 361 (33·8%) | 342 (34·1%) | 177 (32·2%) | 203 (34·8%) | |
| 41–50 | 161 (15·1%) | 161 (15·1%) | 136 (13·6%) | 74 (13·5%) | 112 (19·2%) | |
| 51–60 | 68 (6·4%) | 68 (6·4%) | 54 (5·4%) | 29 (5·3%) | 53 (9·1%) | |
| >60 | 5 (0·5%) | 5 (0·5%) | 5 (0·5%) | 2 (0·4%) | 3 (0·5%) | |
| Gender | ||||||
| Female | 486 (45·5%) | 486 (45·5%) | 472 (47·1%) | 279 (50·7%) | 221 (37·8%) | |
| Male | 582 (54·5%) | 582 (54·5%) | 530 (52·9%) | 271 (49·3%) | 363 (62·2%) | |
| Previous SARS-CoV-2 infection | 38 (3·6%) | 119 (11·1%) | 68 (6·8%) | 46 (8·4%) | 43 (7·4%) | |
| Occupational exposure | ||||||
| Works in COVID-19 area | 175 (16·4%) | 172 (16·1%) | 181 (18·1%) | 81 (14·7%) | 85 (14·6%) | |
| Works in non-COVID-19 area | 893 (83·6%) | 896 (83·9%) | 821 (81·9%) | 469 (85·3%) | 499 (85·5%) | |
| 10-day period of testing | ||||||
| April 15–25, 2021 (first 10 days) | 565 (52·9%) | 450 (42·1%) | 527 (52·6%) | 241 (43·8%) | 247 (42·3%) | |
| April 26–May 5, 2021 (second 10 days) | 405 (37·9%) | 401 (37·5%) | 345 (34·4%) | 234 (42·6%) | 227 (38·9%) | |
| May 6–15, 2021 (third 10 days) | 98 (9·2%) | 217 (20·3%) | 130 (13·0%) | 75 (13·6%) | 110 (18·8%) | |
| Vaccination status | ||||||
| Vaccinated with only one dose of BBV152 | 280 (26·2%) | 270 (25·3%) | .. | .. | .. | |
| Vaccinated with two doses of BBV152 | 233 (21·8%) | 351 (32·9%) | .. | .. | .. | |
| Unvaccinated | 555 (52·0%) | 447 (41·8%) | .. | .. | .. | |
Data are n (%).
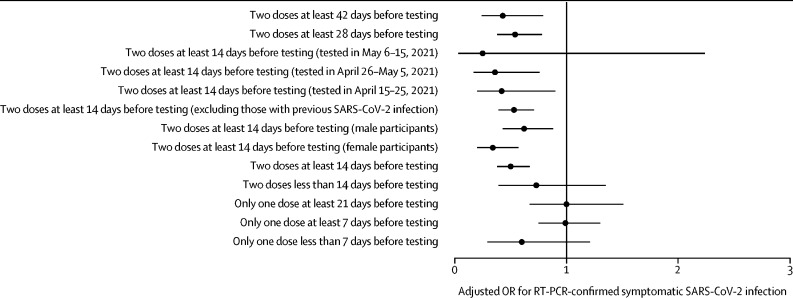
The unadjusted effectiveness of two doses of BBV152 against symptomatic RT-PCR-confirmed SARS-CoV-2, with an interval of at least 14 days between administration of the second dose and day of testing, was 53% (95% CI 38–64; table 2 ). After adjustment, the effectiveness was estimated to be 50% (33–62; figure 3 ). The adjusted effectiveness of two doses administered at least 28 days before testing was 46% (95% CI 22–62) and administered at least 42 days before testing was 57% (21–76). After excluding participants with previous SARS-CoV-2 infections, the adjusted effectiveness of two doses administered at least 14 days before testing was 47% (29–61). There was a trend towards better adjusted effectiveness for two doses administered at least 14 days before testing in women (66% [95% CI 43–80]) than in men (38% [12–57]), although this was not significant.
Table 2.
Estimated vaccine effectiveness against laboratory-confirmed symptomatic SARS-CoV-2 infection
| Symptomatic SARS-CoV-2 RT-PCR-positive | Symptomatic SARS-CoV-2 RT-PCR-negative | Unadjusted OR (95% CI) | Unadjusted p value | Adjusted OR (95% CI)* | Adjusted p value* | Vaccine effectiveness (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Unvaccinated | 555 | 447 | 1 (ref) | .. | 1 (ref) | .. | .. | |
| Only one dose of BBV152 | ||||||||
| Tested at least 7 days after first dose | 246 | 235 | 0·91 (0·70 to 1·18) | 0·46 | 0·99 (0·75 to 1·30) | 0·95 | 1% (−30 to 25) | |
| Tested less than 7 days after first dose | 34 | 35 | 0·75 (0·38 to 1·46) | 0·40 | 0·60 (0·29 to 1·21) | 0·15 | 40% (−21 to 71) | |
| Tested at least 21 days after first dose | 124 | 110 | 0·88 (0·60 to 1·28) | 0·49 | 1·00 (0·67 to 1·51) | 0·97 | −1% (−51 to 33) | |
| Two doses of BBV152 | ||||||||
| Tested at least 14 days after second dose | 189 | 293 | 0·47 (0·36 to 0·62) | <0·0001 | 0·50 (0·38 to 0·67) | <0·0001 | 50% (33 to 62) | |
| Tested less than 14 days after second dose | 44 | 58 | 0·74 (0·42 to 1·32) | 0·31 | 0·73 (0·39 to 1·35) | 0·32 | 27% (−35 to 61) | |
| Tested at least 28 days after second dose | 130 | 192 | 0·49 (0·35 to 0·68) | <0·0001 | 0·54 (0·38 to 0·78) | 0·0009 | 46% (22 to 62) | |
| Tested at least 42 days after second dose | 46 | 93 | 0·37 (0·21 to 0·64) | 0·0005 | 0·43 (0·24 to 0·79) | 0·0065 | 57% (21 to 76) | |
| Two doses of BBV152 with second dose at least 14 days before testing, by 10-day period of testing | ||||||||
| Tested in April 15–25, 2021 | 90 | 100 | 0·44 (0·23 to 0·89) | 0·023 | 0·42 (0·20 to 0·90) | 0·026 | 58% (10 to 80) | |
| Tested in April 26–May 5, 2021 | 78 | 117 | 0·39 (0·20 to 0·79) | 0·0086 | 0·36 (0·17 to 0·76) | 0·0071 | 64% (24 to 83) | |
| Tested in May 6–15, 2021 | 21 | 76 | 0·50 (0·09 to 2·72) | 0·42 | 0·25 (0·03 to 2·24) | 0·22 | 75% (−124 to 97) | |
| Unvaccinated, by 10-day period of testing | ||||||||
| Tested in April 15–25, 2021 | 305 | 222 | .. | .. | .. | .. | .. | |
| Tested in April 26–May 5, 2021 | 204 | 141 | .. | .. | .. | .. | .. | |
| Tested in May 6–15, 2021 | 46 | 84 | .. | .. | .. | .. | .. | |
| Female participants | ||||||||
| Two doses of BBV152 with second dose at least 14 days before testing | 66 | 118 | 0·36 (0·22 to 0·58) | <0·0001 | 0·34 (0·20 to 0·57) | <0·0001 | 66% (43 to 80) | |
| Unvaccinated | 271 | 201 | .. | .. | .. | .. | .. | |
| Male participants | ||||||||
| Two doses of BBV152 with second dose at least 14 days before testing | 123 | 175 | 0·54 (0·39 to 0·76) | 0·0004 | 0·62 (0·43 to 0·88) | 0·0075 | 38% (12 to 57) | |
| Unvaccinated | 284 | 246 | .. | .. | .. | .. | .. | |
| Excluding those with previous SARS-CoV-2 infection | ||||||||
| Two doses of BBV152 with second dose at least 14 days before testing | 186 | 263 | 0·49 (0·37 to 0·66) | <0·0001 | 0·53 (0·39 to 0·71) | <0·0001 | 47% (29 to 61) | |
| Unvaccinated | 535 | 399 | .. | .. | .. | .. | .. | |
Data are n unless otherwise stated. OR=odds ratio.
Adjusted for for previous SARS-CoV-2 infection, occupational exposure, and calendar time (10-day period of testing).
Figure 3.
Adjusted BBV152 vaccine effectiveness by subgroup
Error bars are 95% CIs. OR=odds ratio.
The estimated effectiveness of two doses of BBV152 administered at least 14 days before testing was numerically higher among participants who were tested in the third 10 days (75% [95% CI −124 to 97]) and second 10 days (64% [24 to 83]) of the 30-day study period than in those who were tested in the first 10 days (58% [10 to 80]). The estimated adjusted effectiveness of the one dose of BBV152 administered at least 7 days before testing was 1% (95% CI −30 to 25) and administered at least 21 days before testing was −1% (−51 to 33).
The results were broadly consistent in the sensitivity analysis in the unmatched population (1617 cases and 1097 controls; appendix 2 pp 3–4). In this analysis, the adjusted effectiveness of two doses of BBV152 administered at least 14 days before testing was 44% (95% CI 32–55).
Discussion
This test-negative, case-control study showed two doses of BBV152 (with the second dose administered at least 14 days before RT-PCR testing) had an effectiveness of 50% (95% CI 33–62) against symptomatic RT-PCR-confirmed SARS-CoV-2, in a high-risk population (hospital employees), under surge conditions which were presumably dominated by the potentially immune-evasive delta variant of concern. The vaccine effectiveness did not appear to vary with further increase in the duration beyond 14 days after receiving the second dose. Although there appears to be a trend towards better effectiveness in women than in men, this might be attributable to differences in the roles that men and women have in the hospital rather than any biological differences, and needs cautious interpretation considering the wide 95% CIs.
The vaccine effectiveness estimated in our study is lower than the efficacy announced after completion of the phase 3 trial, despite a similar testing strategy being used (SARS-CoV-2 RT-PCR testing for participants with symptoms suggestive of COVID-19 determined through weekly telephone follow-up).3 Several factors might be responsible for the observation of a lower effectiveness in this study. First, the population included in our study comprised only hospital employees, who might have been exposed to a higher risk of SARS-CoV-2 infection than the general population. The study was conducted during the peak of the second wave of COVID-19 in India, with high test-positivity rates for both hospital employees and residents of Delhi. On April 26, 2021, the test-positivity rate for Delhi was around 35%,7 which was the highest it had been since the beginning of the pandemic. Thus, our results might only reflect the performance of BBV152 under such surge conditions. Second, the prevalence of circulating variants of concern, especially the delta variant, might have contributed to lower effectiveness of BBV152. Although in-vitro studies have shown neutralisation of these variants by both convalescent and post-vaccine sera, the neutralisation titres are several times lesser against variants, particularly the delta variant.8, 9 The phase 3 trial of BBV152 was conducted during a period when the overall test-positivity rate was low, and the prevalence of the delta variant among positive cases was largely unknown. By contrast, at the end of April, 2021, a period during which this study was conducted, the delta variant was the dominant strain, making up more than 80% of all sequenced genomes as per the reports from the Indian SARS-CoV-2 Genome Sequencing Consortia.10
Our results support the evidence showing that vaccine effectiveness takes a few weeks (at least 14 days) to develop and requires the full two doses to achieve maximum effectiveness. The modest effectiveness of a single dose of BBV152, even after intervals of 7 and 21 days, is consistent with data from other studies on different vaccine platforms and underscores the need for rapid vaccine roll-out in the population, while continuing to implement and adhere to non-pharmacological measures, such as physical distancing and the use of face masks.11, 12
Another inactivated whole-virus vaccine, CoronaVac, showed mixed results for vaccine effectiveness in the field. In the setting of high SARS-CoV-2 transmission dominated by the gamma (B.1.1.28.1) variant in Manaus, Brazil, the adjusted effectiveness of two doses of CoronaVac (14 days or longer after administration of the second dose) against symptomatic COVID-19 among health workers was 37%.13 Similarly, in another study, which was also conducted in a surge setting (predominantly due to the gamma variant), among the older population of São Paulo, Brazil, the adjusted effectiveness of CoronaVac against symptomatic COVID-19 was 42%.11 In our setting, the impact of a surge or high-transmission reflected by test-positivity rate and daily incidence was further magnified by the circulating delta variant, which was the primary variant of concern dominating the second wave of COVID-19 in India. The notion of a reduction in vaccine effectiveness due to the surge is further highlighted by the higher effectiveness in the second and third 10-day periods of testing compared with the first 10-day period, when the test-positivity was climbing before reaching a peak on April 26, 2021 (although wide CIs necessitate cautious interpretation). Despite these factors, the estimated effectiveness for BBV152 from our study is modestly higher than reported for CoronaVac.
When compared with emerging data from the UK on effectiveness of the BNT162b2 and ChAdOx1 nCoV-19 vaccines against symptomatic disease due to the delta variant, our estimates are lower at 50% (compared with 88% and 67%, respectively).12 A number of factors might explain this disparity in results, including different vaccine platforms, different dosing intervals, and a greater surge of cases in India than in the UK.
This study was conducted in a population that was primarily offered the BBV152 vaccine, thus presenting a unique opportunity to evaluate its real-world effectiveness. Although institute employees had the opportunity to access the ChAdOx1 nCoV-19 vaccine at other centres, considerable efforts would have been required to access it compared with the ease of access to BBV152 at the institute itself. This is reflected in that only 52 employees were excluded for having received the ChAdOx1 nCoV-19 vaccine. Also, we used a test-negative design, which helped to control for biases which are often difficult to account for in conventional observational studies, including those related to health-seeking behaviour, access to testing, and case ascertainment. First, there was good comparability between cases and controls as both came from the same population, thus reducing variations normally seen in case-control studies where participants might be from different backgrounds or communities (selection bias). All employees had equal opportunities and access to vaccination, as well as to testing for SARS-CoV-2. Both cases and controls were tested for the same reasons (symptoms or exposure history), which minimised bias due to differences in health-seeking behaviours between groups. Additionally, vaccination history was recorded at the testing facility before the specimen was collected for RT-PCR, thus preventing differences in the recording of vaccination history between cases and controls (recall bias).
Our study has several limitations. The exact duration of symptoms at the time of testing was not recorded. Thus, employees who presented late into the course of their illness might have had false-negative results, due to low clinical sensitivity of RT-PCR later in the course of the illness resulting in a biased estimate of vaccine effectiveness (outcome misclassification bias). Even during a surge, it is expected that several symptomatic people who test negative for SARS-CoV-2 might have had infections due to other circulating respiratory viruses. The opposite would have also been possible, with employees falsely having been classified as cases due to recent infection with persistent shedding of non-viable virus, thereby resulting in possible outcome misclassification. Nonetheless, the impact of both of these factors should be minimal, considering the performance of the RT-PCR test and need for presence of symptoms at the time of testing. The direction of bias introduced by the presence of false-negatives would depend on the proportion vaccinated versus unvaccinated among the participants with a false-negative test. Although our sample size allowed for a relatively precise estimate of vaccine effectiveness of two doses of BBV152 with an interval of at least 2 weeks between the administration of second dose and day of testing (the primary outcome), our study was underpowered to estimate effectiveness for different time intervals after vaccination or to determine if effectiveness changed over time. Another limitation was the absence of data on comorbidities. The presence of comorbid conditions might affect health-seeking behaviour as well as vaccine effectiveness. However, the hospital policy mandated that all employees receive vaccination regardless of the presence or absence of underlying comorbid conditions (except pregnancy and lactation, or known severe allergy).
Another concern might be the completeness of data regarding previous COVID-19 infection, as there was no baseline serological testing. Given that our study was conducted in a resource-limited setting, baseline serological testing was not feasible or not available in all participants. The institute had a policy of providing SARS-CoV-2 testing to all employees who either had an influenza-like illness or a high-risk exposure to a known COVID-19 case. This recommendation ensured that only a small proportion of truly infected people were missed (ie, those who were infected but were asymptomatic and did not have a high-risk exposure). Although there remains the possibility of employees undergoing COVID-19 testing at other centres that might have been missed in our dataset, the effect of this should be negligible as the majority of previous COVID-19 tests performed by institute employees would have been done at the institute testing facility and thus captured in our electronic health records. Testing at the institute was available to employees at no cost, without delay, and with a short turnaround time, whereas testing at other centres would have incurred costs and was often difficult to access, with long waiting periods, especially during surges.
We did not have data pertaining to the prevalence of SARS-CoV-2 variants among the RT-PCR-positive patients. Therefore, we could not definitively estimate the vaccine effectiveness against symptomatic COVID-19 due to specific variants of concern. Regardless, our results were presumably influenced by the delta variant as it was the dominant circulating strain at the time of the conduct of the study. Future studies should examine the vaccine escape potential of the delta variant using pseudovirus neutralisation assays (incorporating the spike mutations of the delta variant into the pseudovirus) with post-vaccination sera collected from patients vaccinated with BBV152. Finally, our study did not estimate the vaccine effectiveness against severe disease and mortality, which are clinically meaningful endpoints that require assessment in future studies.
In conclusion, this study showed the effectiveness of two doses of BBV152 against symptomatic RT-PCR-confirmed SARS-CoV-2 in the setting of a surge in cases presumably dominated by the delta variant. The effectiveness should be analysed in the context of the surge conditions and the possible immune evasive potential of the delta variant. Our findings underscore the need for rapid roll-out to ensure complete vaccination with two doses is done in a timely manner while continuing to implement and adhere to non-pharmacological interventions, especially during surges in cases.
Data sharing
Deidentified participant data, including statistical analysis plan and code, will be made freely available after publication upon request to the corresponding author (manishsoneja@gmail.com).
Declaration of interests
We declare no competing interests.
Contributors
DD, ARK, MS, RL, and AMi conceptualised the study, determined the methodology, and wrote the initial draft of the manuscript. DD, ARK, MS, RL, AMi, SN, PKo, RK, AA, AMa, and GTM performed data collection and curation, and cross-checked dates of vaccination. AR, NN, PJ, LD, AC, MB, SS, NW, and RG performed project management roles as team leaders. Sample collection facility data was collated and provided by AK, VH, and PKu, who attest its reliability. Vaccination centre data were collated and provided by NRG, VH, and KM, who attest their reliability. RMP and SP did the final statistical analysis. DD and ARK accessed and verified the data. All authors reviewed and edited the manuscript and approve the final version. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ella R, Reddy S, Blackwelder W, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)02000-6. published online Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanquet G, Valenciano M, Simondon F, Moren A. Vaccine effects and impact of vaccination programmes in post-licensure studies. Vaccine. 2013;31:5634–5642. doi: 10.1016/j.vaccine.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 5.WHO Evaluation of COVID-19 vaccine effectiveness. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1
- 6.Kahn R, Schrag SJ, Verani JR, Lipsitch M. Identifying and alleviating bias due to differential depletion of susceptible people in post-marketing evaluations of COVID-19 vaccines. medRxiv. 2021 doi: 10.1101/2021.07.15.21260595. published online July 19. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Government of Delhi: Department of Information and Publicity Delhi Fights Corona: COVID-19 response updates from the Delhi Government. https://delhifightscorona.in/
- 8.Sapkal GN, Yadav PD, Ella R, et al. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J Travel Med. 2021;28 doi: 10.1093/jtm/taab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav PD, Sapkal GN, Abraham P, et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab411. published online May 7. [DOI] [PubMed] [Google Scholar]
- 10.Dhar MS, Marwal R, Radhakrishnan VS, et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. medRxiv. 2021 doi: 10.1101/2021.06.02.21258076. published online June 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: test negative case-control study. BMJ. 2021;374 doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hitchings MDT, Ranzani OT, Torres MSS, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am. 2021;1 doi: 10.1016/j.lana.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data, including statistical analysis plan and code, will be made freely available after publication upon request to the corresponding author (manishsoneja@gmail.com).