Abstract
Objective
The primary aim of this investigation was to analyze the microbiome in patients with combined periodontal-endodontic lesions.
Method
Patients with loose and/or painful teeth referred for treatment from March 2020 to December 2020 in the First People's Hospital of Jinzhong were recruited. Samples were collected from teeth diagnosed as chronic periodontics (PE), ulcerative pulpitis (PU), and retrograde pulpitis (RE). Genomic DNA was extracted. The quantitative polymerase chain reaction, targeting the 16S ribosomal RNA (rRNA), was adopted for the quantification of bacteria. Then, the V3-V4 hypervariable regions of the 16S rRNA gene were amplified and subjected to next-generation sequencing. The statistical analysis was performed by R software (V3.5.1).
Results
A total of 57 qualified samples were collected from 48 patients and analyzed (7 PE, 21 PU, and 19 RE). By linear discriminant analysis effect size, Kingella and Barnesiella were significantly increased in the periodontal pocket of retrograde pulpitis (RE-PE), compared with PE. The relative abundance of Clostridiales Incertae Sedis XI, Fusobacteriaceae, Fusobacterium, Parvimonas, Micrococcaceae, and Rothia was significantly increased in the pulp of retrograde pulpitis (RE-PU) than PU and RE-PE. Prevotella, Leptotrichia, Porphyromonas, Streptococcus, and Fusobacterium are consistently at a high abundance, across PU, RE-PE, and RE-PU.
Conclusion
The current study highlighted the evidence that a specific microbial community is associated with the occurrence of retrograde pulpitis. The microenvironment of the root canal and pulp chamber will select microbiota. This study offered insights into the pathogenesis of retrograde pulpitis.
1. Introduction
Firstly described by Simring and Goldberg in 1964 [1], combined periodontal-endodontic lesions have been classified into three categories based on the primary site [2]: (a) endodontic lesions with secondary periodontic involvement (retrograde pulpitis), (b) periodontic lesions with secondary endodontic involvement (retrograde periodontics), and (c) “TRUE” combined lesions. The periodontium and endodontium are tightly bonded embryonically, anatomically, and functionally, making combined periodontal-endodontic lesions a dilemma to diagnose and treat thoroughly [3, 4]. It has been widely accepted that the anatomical interconnection of periodontium and endodontium with pathogenic microorganism transmission is accused of inducing the former two types of diseases as above [5]. Several researchers have found the common microbial composition between infected root canals and advanced periodontitis through the traditional bacterial culture method [6–9]. However, restricted to the conventional research technique, only cultivable microbes could be detected, and the panoramic difference between infected root and derived periodontitis is still not known.
Being different from caries-caused pulpitis [10, 11], the infections of retrograde pulpitis are thought to be derived from those microorganisms that exist deep in the periodontal pocket [4, 12]. However, not all teeth with chronic periodontitis will progress into retrograde pulpitis eventually, inspiring us that apart from anatomical factors of root canals, discrepant microbiome profiles of periodontal pockets may also lead to the onset of pulpitis or not.
Several approaches have been applied in identifying pathogens. Culture and biochemical testing, with the advantage of low cost, is considered the golden standard of pathogen identification [13]. The main limitation of the culture and biochemical testing is that not all pathogen is cultivable. Next-generation sequencing (NGS), developed in 2005, can sequence billions of DNA fragments independently and simultaneously [14]. The characteristic of NGS is the ability to identify unculturable bacteria, shorter turnaround time, and more accurate results [15]. Thus, NGS has been widely adopted in the investigation of the association between microorganisms and oral diseases [16–19].
The principal aim of the present study is to compare the microbiome composition of chronic periodontitis (PE), ulcerative pulpitis (PU), and retrograde pulpitis (RE). The 16S rRNA gene sequencing technique was adopted to analyze the composition of the microbial community, aiming to elucidate the possible driving force for the development of periodontitis into retrograde pulpitis and to provide a theoretical basis for the early intervention of the disease.
2. Materials and Methods
2.1. Collection of Clinical Samples and Sampling Procedures
Patients with loose and/or painful teeth referred for treatment from March 2020 to December 2020 in the First People's Hospital of Jinzhong were screened and eligible patients were included. Teeth with probing depth (PD) ≥ 6 mm, attachment level (AL) ≥ 5 mm, looseness ≥ II°, and obvious alveolar bone resorption were diagnosed as PE. A tooth that had extensive caries lesions that led to pulp exposure without root canal treatment history and sensitivity to cold and or heat tests was diagnosed as PU. The diagnosis of RE included classical periodontics symptoms and the following criteria: (1) caries-free and intact crown; (2) spontaneous, cold, and/or heat-evoked localized or diffused pain; (3) no history of trauma; and (4) lack of periapical lesion radiographically with no sinus tracts. The summary of grouping abbreviations is presented in Table 1.
Table 1.
The summary of grouping abbreviations.
| Abbreviation | Grouping |
|---|---|
| PE | Chronic periodontics |
| PU | Ulcerative pulpitis |
| RE | Retrograde pulpitis |
| RE-PE | Periodontal pocket of retrograde pulpitis |
| RE-PU | Pulp of retrograde pulpitis |
Exclusion criteria include (1) long-term medication history or antibiotics are taken within the past 3 months; (2) women during pregnancy, lactation, or menstruation; (3) have periodontal and endodontic treatment during the past 3 months; (4) history of orthodontic treatment; and (5) smoking history.
The study was approved by the Ethics Committee of the First People's Hospital of Jinzhong (2020-006-01). Written informed consent was obtained from each patient.
2.2. Microbial DNA Extraction and Sequencing
Genomic DNA was extracted from samples by using the DNeasy Blood & Tissue Kit (Qiagen, Germany) according to the manufacturer's instructions. DNA quality was evaluated by absorbance ratios of A260 to A280 using spectrophotometry (NanoDrop8000, Thermo Scientific). Only DNA samples with a ratio of A260 to A280 higher than 1.8 were recognized as qualified samples and used for subsequent analysis. The hypervariable V3-V4 region of the bacteria 16S ribosomal RNA genes was amplified using the primer pair 5′-CCTACGGGRSGCAGCAG-3′ (forward primer) and 5′-GGACTACVVGGGTATCTAATC-3′ (reverse primer), with the following PCR conditions: 95°C for 3 min, followed by 30 cycles at 98°C for 20 s, 58°C for 15 s, and 72°C for 20s and a final extension at 72°C for 5 min. PCR reactions were performed in 30 μL mixture containing 15 μL of 2× KAPA Library Amplification ReadyMix, 1 μL of each primer (10 μM), 50 ng of template DNA, and ddH2O.
Sequencing was performed according to a previously described protocol [20, 21]. In brief, amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer's protocols and quantified using Qubit®2.0 (Invitrogen, USA). All quantified amplicons were pooled to equalize sequencing concentrations using Illumina MiSeq/PE250 (Illumina, Inc., CA, USA). The paired-end reads of 425 bp were overlapped on their 3′ ends for concatenation into original longer tags by using PANDAseq (https://github.com/neufeld/pandaseq, V2.9). DNA extraction, library construction, and sequencing were performed at Realbio Genomics Institute (Shanghai, China). Operational taxonomic units (OTUs) were clustered with 97% similarity by using USEARCH (V7.0.1090). Each representative tag was assigned to taxa by RDP Classifier (http://rdp.cme.msu.edu/). QIIME (V1.9.1) and R (V3.5.1) were also used to analyze and profile differences of results.
2.3. Statistical Analysis
The statistical analysis of alpha diversity, beta diversity, and statistically significant differences analysis was performed by R (V3.5.1) and QIIME (V1.9.1). Only average relative abundance > 0.1% would be counted in the statistical analysis. Values of P < 0.05 were considered to be statistically significant.
3. Results
3.1. Subject Characteristics
A total of 48 patients fulfilling the criteria were recruited. Among them, 23 (47.9%) were males and 25 (52.1%) were females. The average age of included patients was 45.2 years old (range 12-76 years old). Most of the teeth were molar (71.7%). The characteristics of recruited patients are shown in Table 2.
Table 2.
Study population characteristics.
| Characteristics | PU | PE | RE |
|---|---|---|---|
| Gender | |||
| Male | 8 | 9 | 6 |
| Female | 15 | 5 | 5 |
| Age (mean, range) | 36.7 (12-66) | 52.2 (38-76) | 53.9 (45-62) |
| Location | |||
| Anterior tooth | 4 | 2 | — |
| Premolar | 5 | 4 | — |
| Molar | 15 | 12 | 11 |
| Systematic conditions | |||
| Hashimoto's disease | 1 | — | — |
| Hypertension | 1 | 3 | 2 |
| Diabetes | — | 3 | 2 |
3.2. 16S rRNA Gene Sequencing
We sequenced 16S rRNA gene amplicons from 57 qualified samples (PE 17, PU 21, and RE 19). For RE, 10 samples were collected from the periodontal pocket (RE-PE) and 9 samples were collected from the pulp (RE-PU). All samples passed quality control. The number of total valid reads from 16S rRNA was 2.04 × 106, ranging from 29,733-38,987 reads (mean 35,763 reads). Using a 97% similarity level, a total of 1163 OTUs were detected. The detailed distribution of OTUs is shown in Figure S1.
3.3. The Differences of Microbial Community Structure among the PE, RE-PE, and RE-PU Groups
The alpha diversity analysis included community richness (Chao1 diversity index) and community evenness (Shannon diversity index). In general, the RE-PU yielded the lowest community richness, followed by RE-PE and PE (P < 0.05) (Figure 1(a)). For the community evenness, RE-PU was significantly lower than PE (Figure 1(b)). The beta-diversity was analyzed to depict divergence among PE, RE-PE, and RE-PU groups. The principal coordinates analysis (PCoA) diagram showed that three groups were relatively independent indicating significantly different microbial communities (Adonis analysis, P = 0.001) (Figure 1(c)). The distribution of the top 20 genera is depicted in Figure 1(d). Compared with RE-PE and PE, Porphyromonas, Leptotrichia, Saccharibacteria, and Selenomonas were significantly reduced in RE-PU, while Streptococcus, Parvimonas, Rothia, and Murdochiella were significantly rich in RE-PU. The linear discriminant analysis (LDA) effect size (LEfSe) analysis was adopted to find the species with an abundance that is significantly different among multiple groups. As shown in Figure 2, a total of 32 genera (represented by Clostridiales Incertae Sedis XI, Actinobacteria, and Parvimonas) were significantly abundant in RE-PU, 17 genera (represented by Porphyromonadaceae, Porphyromonas, Treponema, and Spirochaetes) were abundant in RE-PE, and 20 genera (represented by Synergistetes, Synergistales, Synergistia, and Synergistaceae) were abundant in PE.
Figure 1.
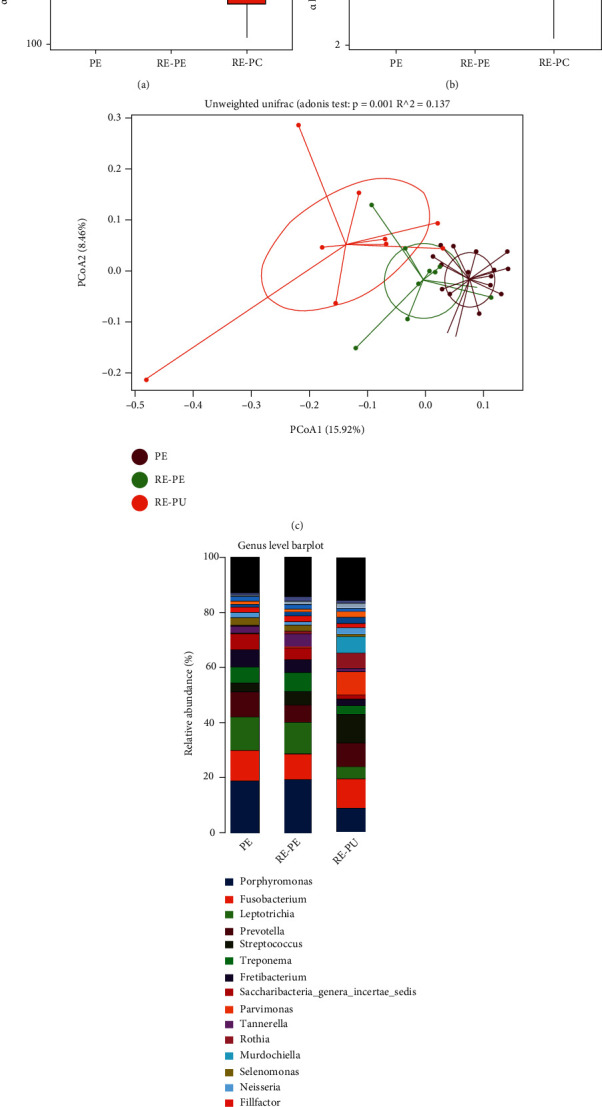
The analysis of microbial community structure of PE, RE-PE, and RE-PU groups. (a, b) The boxplot diagrams show alpha diversity index among PE, RE-PE, and RE-PU. (c) The plot of principal coordinates analysis (PCoA) shows intergroup distances by 2 principal coordinates. (d) The relative abundance of bacterial taxa at the genus level of PE, RE-PE, and RE-PU.
Figure 2.
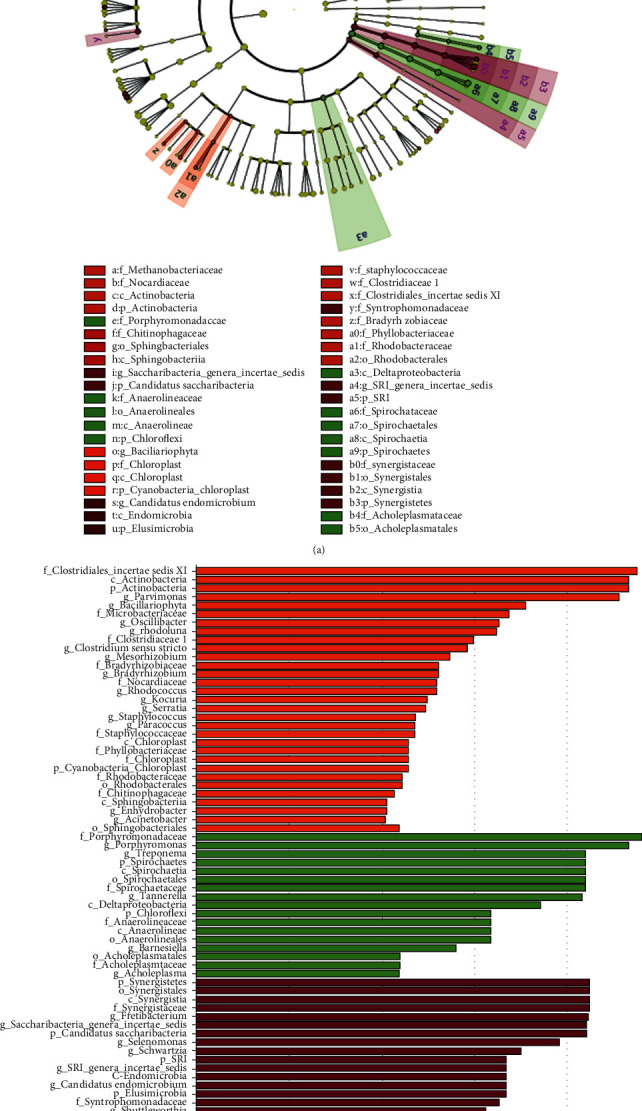
The linear discriminant analysis (LDA) effect size (LEfSe) profiles of PE, RE-PE, and RE-PU. (a) Cladograms indicating the phylogenetic distribution of bacterial lineages among 3 groups. The phylum, class, order, family, and genus levels are listed in order from inside to outside of the cladogram, and the labels for levels of order, family, and genus are abbreviated by a single letter. (b) LDA along with effect size measurements was applied to present the enriched bacterial genera of each group.
3.4. The Differences in Microbial Community Structure among the PU, RE-PU, and RE-PE Groups
Alpha diversity showed higher richness and evenness in RE-PE, while there are no statistically significant differences between PU and RE-PU (Figures 3(a) and 3(b)). PCoA diagram indicated three relatively independent groups (Figure 3(c)), testified by Adonis (P = 0.001), Anosim (P = 0.003), and MRPP (P = 0.002) analyses. Genera of the top 20 relative abundances were marked in Figure 3(d). In detail, the abundance of Leptotrichia, Selenomonas, and Capnocytophaga were significantly lower in RE-PU than PU and RE-PE. Meanwhile, the abundance of Streptococcus, Parvimonas, and Murdochiella was significantly higher in RE-PU, compared with PU and RE-PE. LEfSe analysis filtered genera with a significant difference in relative abundance are shown in Figure 4. In specific, RE-PE was rich in Porphyromonadaceae, Bacteroidia, Bacteroidales, Porphyromonas, and Bacteroidetes. For RE-PU, the relative abundance of Clostridiales Incertae Sedis XI, Fusobacteriaceae, Fusobacterium, Parvimonas, Micrococcaceae, and Rothia was significantly increased, while the most selectively enriched abundant genera for PU were Actinobacteria, Actinomycetales, Lactobacillales, and Bacilli.
Figure 3.
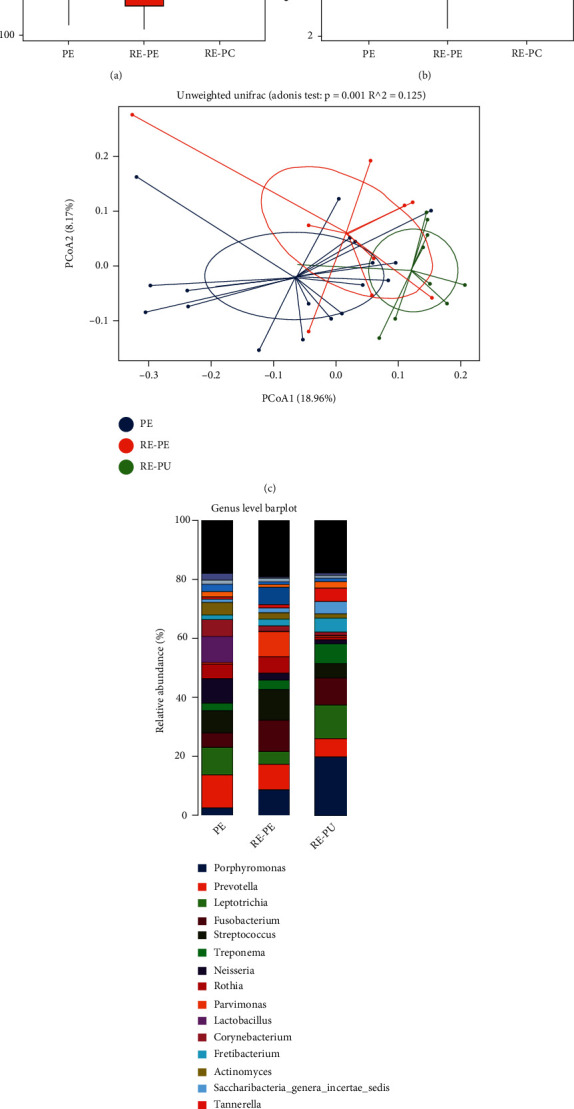
The analysis of microbial community structure of PU, RE-PU, and RE-PE groups. (a, b) The boxplot diagrams showed alpha diversity index among PU, RE-PU, and RE-PE. (c) The plot of principal coordinates analysis (PCoA) showed intergroup distances by 2 principal coordinates. (d) The relative abundance of top 20 bacterial taxa at the genus level for PU, RE-PE, and RE-PU.
Figure 4.
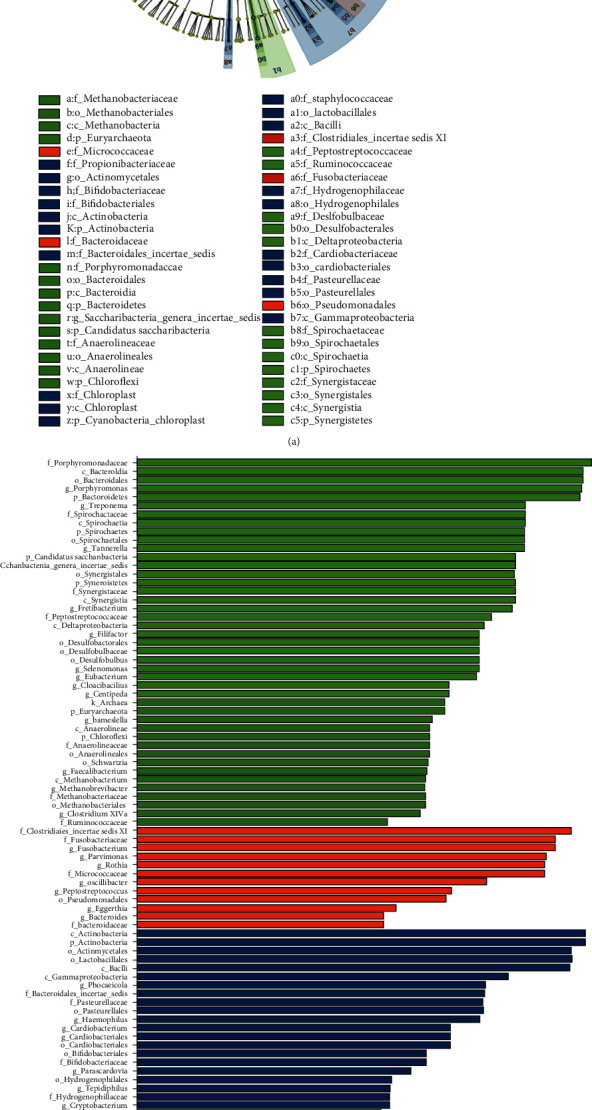
The linear discriminant analysis (LDA) effect size (LEfSe) profiles of PU, RE-PU, and RE-PE. (a) Cladograms indicating the phylogenetic distribution of bacterial lineages among 3 groups. The phylum, class, order, family, and genus levels are listed in order from inside to outside of the cladogram, and the labels for levels of order, family, and genus are abbreviated by a single letter. (b) LDA along with effect size measurements was applied to present the enriched bacterial genera of each group.
3.5. The Function Prediction of Microbial Community
The function of a differently detected microbial community was predicted using KEGG (http://www.kegg.jp/). The top 30 genes enriched by a differently detected microbial community are presented in Figure 5 (P < 0.05). The function of a differently detected microbial community was close for PE and RE-PE, compared with RE-PU. In specific, RNA polymerase sigma, methyl-accepting chemotaxis protein, glutathione S-transferase, acetyl-CoA C-acetyltransferase, and acyl-CoA thioester hydrolase were rich in PE and RE-PE (Figure 5(a)). Gene families involved in fatty acid biosynthesis, glycolysis/gluconeogenesis, fatty acid degradation, glutathione metabolism, pyrimidine metabolism, fatty acid metabolism, iron complex outer-membrane receptor protein, RNA polymerase sigma-70 factor, ECF subfamily, and chromosome partitioning protein were significantly enriched in RE-PU and RE-PE, while those responsible for galactose metabolism, proteasome, starch, and sucrose metabolism were significantly associated with PE (Figure 5(b)).
Figure 5.
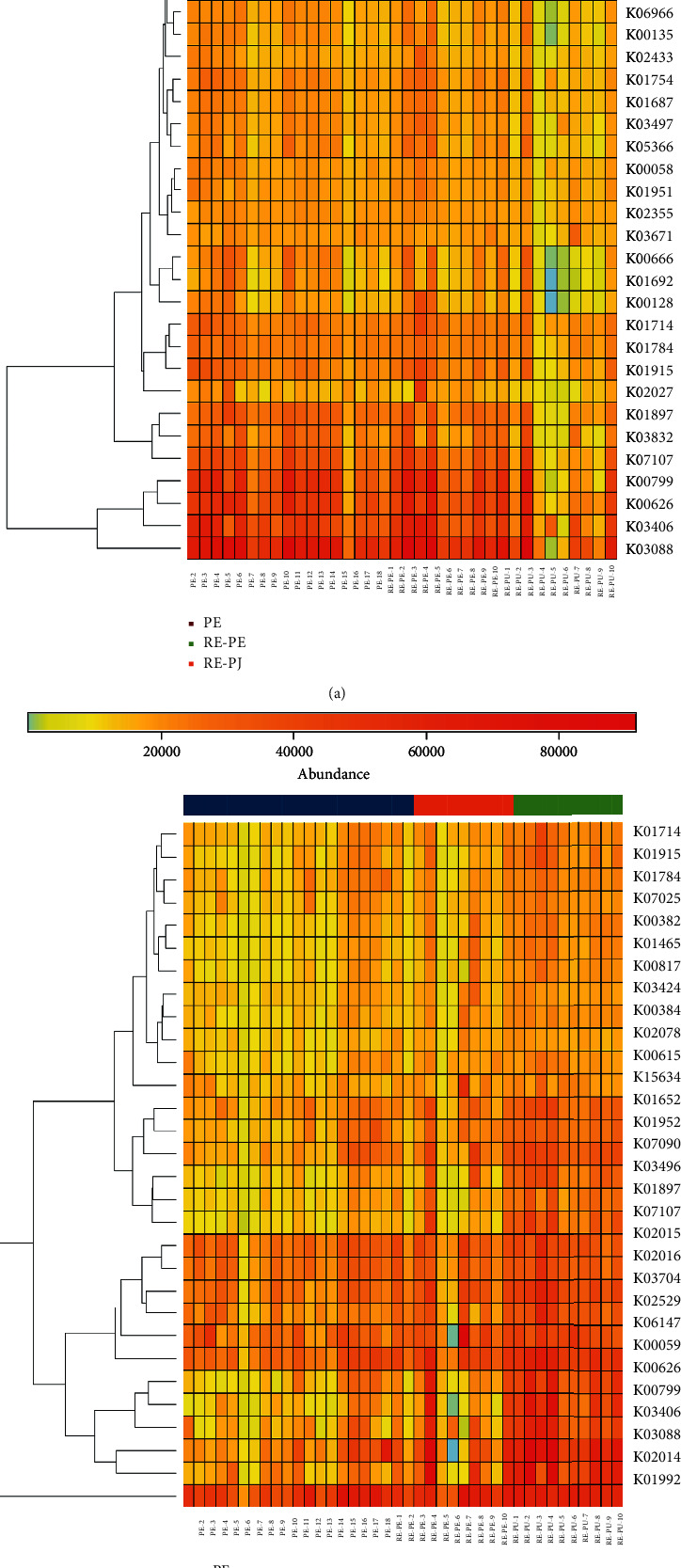
The heat map of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway categories based on differently detected genera of (a) PE, RE-PE, and RE-PU and (b) PU, RE-PE, and RE-PU.
4. Discussion
Combined periodontal-endodontic lesions, which can establish independently with or without communication between endodontic and periodontal components, are a kind of disease characterized by the interrelationship between the periodontal pocket and tooth pulp [22]. The prognosis of combined periodontal-endodontic lesions treated with traditional management is poor [23, 24]. Targeted therapy, aiming at the trigger event of combined periodontal-endodontic lesions, may have better performance and is eagerly needed. It is very important for clinicians to understand the pathogenesis process of combined periodontal-endodontic lesions.
The important role of microorganisms in oral diseases has been well recognized [20, 21, 25–28]. The specific mechanism of periodontal-endodontic lesions occurrence is still under debate; however, the important role of bacterial infection has been identified [6–8, 29, 30]. The transmission of microbial community and toxins between the periodontal pocket and the root canal was speculated to be the irritation [7]. Kipioti et al. found the similarity of flora between root canals and adjacent periodontal pockets of teeth with advanced periodontitis [6]. Kobayashi et al. also detected common microflora from root canals and periodontal pockets of caries-free teeth with advanced periodontitis [8]. Both of the studies above were conducted using the culture method only. Xia and Qi compared bacterial profiles using denaturing gradient gel electrophoresis (DGGE) between dental plaque and pulp of 13 teeth with combined periodontal-endodontic lesions [31]. However, the similarity of bacteria from dental plaque and pulp of the same tooth ranged from 13.1% to 62.5%. The conclusion might not be valid. Li et al. identified 43 genera/species from 20 patients' teeth with combined periodontal-endodontic lesions via DGGE [32]. The predominant genera were Porphyromonas sp. (13.9%), Filifactor sp. (12.5%), and Parvimonas sp. (11.1%). The most prevalent bacteria in the root canal and periodontal pocket were Filifactor alocis, Parvimonas micra, Porphyromonas gingivalis, and Tannerella forsythia. In general, previous studies revealed the possibility that the periodontal pocket was a source of root canal infection; however, due to the limited technique, the profile of differently distributed bacterial was not comprehensive. There was also a lack of follow-up research to elucidate the changing process after the root canal infection derived from the periodontal pocket.
It is interesting to note that not all teeth, which suffered from chronic periodontitis, will develop retrograde pulpitis and result in combined periodontal-endodontic lesions eventually. It is unclear which factor will decide the fate of the tooth. The present study showed the composition of the microbiota of RE-PE is differed from PE, indicating that different genera in periodontal pockets are the key to the occurrence of retrograde pulpitis. The fact that the microbiome of RE-PU was closer to RE-PE rather than PE further supported this viewpoint (Figure 1(c)). Highly rich genera in RE-PE may exert a pivotal role in the pulp invasion, leading to retrograde pulpitis. By LEfSe analysis, Kingella and Barnesiella are found to be highly rich in RE-PE than PE. Kingella is a genus of Gram-negative, aerobic, and facultatively anaerobic bacilli. Kingella is a normal flora in the oral cavity, being detected in both healthy and periodontitis subjects [33, 34]. To note, a study showed that Kingella was rich in biofilm collected from children with severe caries, indicating that Kingella might associate with invasion and erosion of enamel and dentin [35]. We speculate that predominate Kingella can degrade mineral substances and form the channel through which microbiota and secreted toxins can affect dental pulp. Barnesiella is reported to play an important role in several diseases as a major component of the gut microbiome [36–38]. A recent published study demonstrated that the abundance of Barnesiella would increase after Porphyromonas gingivalis infection, which was the etiological agent of periodontal disease [39]. It has been recognized that the gut microbiome is significantly associated with host immunity [40]. Thus, we speculated that Barnesiella may be able to affect the immune microenvironment of the periodontal pocket, damage the immune barrier, and accelerate the invasion of pathogenic microorganisms. The function of Barnesiella in periodontitis should be further investigated.
Previous studies showed that as the periodontal inflammation progresses, alteration of local flora might happen gradually [41–43]. To our knowledge, there is no study investigating the microbiota shift in retrograde pulpitis. The PCA analysis revealed that the composition of the microbiota of RE-PU is closer to PU rather than RE-PE. To note, all teeth diagnosed as combined periodontal-endodontic lesions are caries-free. Thus, the infection resource of retrograde pulpitis is only periodontal pockets. The significantly different microbiota composition between RE-PE and RE-PU indicates that the microenvironment has selected the microorganism by local pressures. The results of the alpha diversity analysis also support the assumption. The score of RE-PU is significantly lower than the PE and RE-PE groups, indicating that some predominant microorganisms are existing in the infected dental pulp.
The composition of microorganisms is significantly different among PU, RE-PE, and RE-PU. In detail, Clostridiales Incertae Sedis XI, Parvimonas, Clostridium, Peptostreptococcaceae, and Filifactor are more frequently detected in RE-PU, compared with PU and RE-PE. Clostridiales Incertae Sedis XI was created in 2001, and family members of Clostridiales Incertae Sedis XI were pathogen of septic arthritis, necrotizing pneumonia, chronic rhinosinusitis, and bacterial vaginosis [44–50]. This is the first time to illustrate the significant role of Clostridiales Incertae Sedis XI in oral disease. The function of Clostridiales Incertae Sedis XI is merit to be further explored. Delima et al. found that smoke cessation would lead to a decrease in the prevalence of Parvimonas [51]. The abundance of Parvimonas was proved to be rich in smokers with periodontitis than nonsmokers [52, 53]. Clostridium is a genus of gram-positive obligate anaerobes, including several significant human pathogens. Vigil et al. isolated Clostridium from 22 refractory periapical cases via growth culture [54]. Medina-Palacios et al. identified that Clostridium was one of the most common genera in refractory apical periodontitis [55]. One possible mechanism that Clostridium contributed to destruction is degrading Type IV collagen, which is the major component of basement membranes, via proteinases [56]. Several researchers have identified the abnormal accumulation of Peptostreptococcaceae in both chronic periodontitis and aggressive periodontitis [57–61]. In this study, we firstly reported Peptostreptococcaceae is rich in RE-PU. This adds a growing body of evidence that the periodontal pocket is the resource of infection for retrograde pulpitis. The role of Peptostreptococcaceae in pathogenesis of retrograde pulpitis needs to be further investigated. Filifactor is a genus of Gram-positive and anaerobic bacterium, containing Filifactor alocis, which is a diagnostic indicator of periodontal disease [62]. The most important characteristic of Filifactor alocis is the ability to survive in the oxidative stress-rich environment, especially periodontal pocket. Compared with P. gingivalis, Filifactor alocis is more resistant to H2O2-induced oxidative stress [63]. Moreover, it is interesting to note that the survival of P. gingivalis is significantly increased when cocultured with Filifactor alocis, indicating that Filifactor alocis may have the ability to detoxify the microenvironment and thereby improve the survival of pathogenic microorganism. Further study revealed the core role of “superoxide reductase” of Filifactor alocis in resistance to superoxide radicals [64]. Profound synergistic interactions among Filifactor alocis and oral bacteria have been confirmed by in vitro studies, suggesting the central role of Filifactor alocis in the microbial community of periodontal pocket [62, 63, 65, 66] and may majorly contribute retrograde pulpitis development.
Overall, this study is the largest cohort so far to investigate the microbiota for combined periodontal-endodontic lesions. The present study suggests that specific microbiota of periodontal pocket may be associated with the occurrence of retrograde pulpitis. The shift of microbial communities from periodontitis to retrograde pulpitis is investigated and revealed that the microenvironment of the root canal and pulp chamber can select predominate microflora. Due to the poor prognosis of retrograde pulpitis, more profound research and a better understanding of microorganisms might give us a hint on targeted medication to prevent the occurrence of retrograde pulpitis in the future.
Acknowledgments
This work was supported by the Scientific Research Project of Shanxi Provincial Health Commission (No. 2021161).
Contributor Information
Jiannan Liu, Email: laurence_ljn@163.com.
Xinhua Liu, Email: xinhua765@163.com.
Data Availability
The data that support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Ping Sun and Zhiyong Guo contributed data collection, analysis, and interpretation equally to this work and share first authorship. Daiping Guo, Jian Wang, Tingting Wu, and Tingjun Li collected and processed samples. Jiannan Liu and Xinhua Liu contributed conception, design, and data acquisition and drafted the manuscript equally to this work and are co-corresponding authors. Ping Sun and Zhiyong Guo contributed equally to this work.
Supplementary Materials
Figure S1: the Venn diagram contouring the distribution of operational taxonomic units (OTUs) for (a) PE, RE-PE, and RE-PU and (b) PU, RE-PE, and RE-PU.
References
- 1.Simring M., Goldberg M. The pulpal pocket approach: retrograde periodontitis. The Journal of Periodontology . 1964;35(1):22–48. doi: 10.1902/jop.1964.35.1.22. [DOI] [Google Scholar]
- 2.Simon J. H., Glick D. H., Frank A. L. The relationship of endodontic-periodontic lesions. Journal of Periodontology . 1972;43(4):202–208. doi: 10.1902/jop.1972.43.4.202. [DOI] [PubMed] [Google Scholar]
- 3.Harrington G. W., Steiner D. R., Ammons W. F., Jr. The periodontal-endodontic controversy. Periodontology 2000 . 2002;30(1):123–130. doi: 10.1034/j.1600-0757.2002.03012.x. [DOI] [PubMed] [Google Scholar]
- 4.Raja V. S., Emmadi P., Namasivayam A., Thyegarajan R., Rajaraman V. The periodontal-endodontic continuum: a review. Journal of Conservative Dentistry . 2008;11(2):54–62. doi: 10.4103/0972-0707.44046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotstein I., Simon J. H. S. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontology 2000 . 2004;34(1):165–203. doi: 10.1046/j.0906-6713.2003.003431.x. [DOI] [PubMed] [Google Scholar]
- 6.Kipioti A., Nakou M., Legakis N., Mitsis F. Microbiological findings of infected root canals and adjacent periodontal pockets in teeth with advanced periodontitis. Oral Surgery, Oral Medicine, and Oral Pathology . 1984;58(2):213–220. doi: 10.1016/0030-4220(84)90139-7. [DOI] [PubMed] [Google Scholar]
- 7.Kerekes K., Olsen I. Similarities in the microfloras of root canals and deep periodontal pockets. Endodontics & Dental Traumatology . 1990;6(1):1–5. doi: 10.1111/j.1600-9657.1990.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T., Hayashi A., Yoshikawa R., Ookuda K., Hara K. The microbial flora from root canals and periodontal pockets of non-vital teeth associated with advanced periodontitis. International Endodontic Journal . 1990;23(2):100–106. doi: 10.1111/j.1365-2591.1990.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 9.Balaei-Gajan E., Shirmohammadi A., Abashov R., Agazadeh M., Faramarzie M. Detection of enterococcus faecalis in subgingival biofilm of patients with chronic refractory periodontitis. Medicina Oral, Patología Oral y Cirugía Bucal . 2010;15(4):e667–e670. doi: 10.4317/medoral.15.e667. [DOI] [PubMed] [Google Scholar]
- 10.Rôças I. N., Lima K. C., Assunção I. V., Gomes P. N., Bracks I. V., Siqueira J. F., Jr. Advanced caries microbiota in teeth with irreversible pulpitis. Journal of Endodontia . 2015;41(9):1450–1455. doi: 10.1016/j.joen.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J., Wu Z., Niu K., et al. Microbiome of deep dentinal caries from reversible pulpitis to irreversible pulpitis. Journal of Endodontics . 2019;45(3):302–309.e1. doi: 10.1016/j.joen.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Ricucci D., Siqueira J. F., Jr. Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. Journal of Endodontia . 2010;36(1):1–15. doi: 10.1016/j.joen.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Sayed S., Cherniak W., Lawler M., et al. Improving pathology and laboratory medicine in low-income and middle-income countries: roadmap to solutions. Lancet . 2018;391(10133):1939–1952. doi: 10.1016/S0140-6736(18)30459-8. [DOI] [PubMed] [Google Scholar]
- 14.Kwong J. C., Mccallum N., Sintchenko V., Howden B. P. Whole genome sequencing in clinical and public health microbiology. Pathology . 2015;47(3):199–210. doi: 10.1097/PAT.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhamad Rizal N. S., Neoh H. M., Ramli R., et al. Advantages and limitations of 16S rRNA next-generation sequencing for pathogen identification in the diagnostic microbiology laboratory: perspectives from a middle-income country. Diagnostics (Basel) . 2020;10(10):p. 816. doi: 10.3390/diagnostics10100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulhaq A., Halboub E., Homeida H. E., et al. Tongue microbiome in children with autism spectrum disorder. Journal of Oral Microbiology . 2021;13(1):p. 1936434. doi: 10.1080/20002297.2021.1936434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu Fanas S., Brigi C., Varma S. R., Desai V., Senok A., D'souza J. The prevalence of novel periodontal pathogens and bacterial complexes in stage II generalized periodontitis based on 16S rRNA next generation sequencing. Journal of Applied Oral Science . 2021;29, article e20200787 doi: 10.1590/1678-7757-2020-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böttger S., Zechel-Gran S., Schmermund D., et al. Microbiome of odontogenic abscesses. Microorganisms . 2021;9(6):p. 1307. doi: 10.3390/microorganisms9061307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zorba M., Melidou A., Patsatsi A., Ioannou E., Kolokotronis A. The possible role of oral microbiome in autoimmunity. International Journal of Women's Dermatology . 2020;6(5):357–364. doi: 10.1016/j.ijwd.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen L. H., Xiao E., Wang E. B., Zheng H., Zhang Y. High-throughput sequencing analysis of microbial profiles in the dry socket. Journal of Oral and Maxillofacial Surgery . 2019;77(8):1548–1556. doi: 10.1016/j.joms.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Huang X., Zheng H., An J., Chen S., Xiao E., Zhang Y. Microbial profile during pericoronitis and microbiota shift after treatment. Frontiers in Microbiology . 2020;11:p. 1888. doi: 10.3389/fmicb.2020.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Fouzan K. S. A new classification of endodontic-periodontal lesions. International Journal of Dentistry . 2014;2014:5. doi: 10.1155/2014/919173.919173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho Y. D., Lee J. E., Chung Y., et al. Collaborative management of combined periodontal-endodontic lesions with a palatogingival groove: a case series. Journal of Endodontia . 2017;43(2):332–337. doi: 10.1016/j.joen.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S., Tewari S., Tewari S., Mittal S. Effect of time lapse between endodontic and periodontal therapies on the healing of concurrent endodontic-periodontal lesions without communication: a prospective randomized clinical trial. Journal of Endodontia . 2015;41(6):785–790. doi: 10.1016/j.joen.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Siqueira J. F., Jr., Rôças I. N. Diversity of endodontic microbiota revisited. Journal of Dental Research . 2009;88(11):969–981. doi: 10.1177/0022034509346549. [DOI] [PubMed] [Google Scholar]
- 26.Xiao E., He L., Wu Q., et al. Microbiota regulates bone marrow mesenchymal stem cell lineage differentiation and immunomodulation. Stem Cell Research & Therapy . 2017;8(1):p. 213. doi: 10.1186/s13287-017-0670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao E., Mattos M., Vieira G. H. A., et al. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host & Microbe . 2017;22(1):120–128.e4. doi: 10.1016/j.chom.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren Y., Su S., Liu X., Zhang Y., Zhao Y., Xiao E. Microbiota-derived short-chain fatty acids promote BMP signaling by inhibiting histone deacetylation and contribute to dentinogenic differentiation in murine incisor regeneration. Stem Cells and Development . 2020;29(18):1201–1214. doi: 10.1089/scd.2020.0057. [DOI] [PubMed] [Google Scholar]
- 29.Simon J. H., Glick D. H., Frank A. L. The relationship of endodontic-periodontic lesions. Journal of Endodontia . 2013;39(5):e41–e46. doi: 10.1016/j.joen.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Fujii R., Muramatsu T., Yamaguchi Y., et al. An endodontic-periodontal lesion with primary periodontal disease: a case report on its bacterial profile. The Bulletin of Tokyo Dental College . 2014;55(1):33–37. doi: 10.2209/tdcpublication.55.33. [DOI] [PubMed] [Google Scholar]
- 31.Xia M., Qi Q. Bacterial analysis of combined periodontal-endodontic lesions by polymerase chain reaction-denaturing gradient gel electrophoresis. Journal of Oral Science . 2013;55(4):287–291. doi: 10.2334/josnusd.55.287. [DOI] [PubMed] [Google Scholar]
- 32.Li H., Guan R., Sun J., Hou B. Bacteria community study of combined periodontal-endodontic lesions using denaturing gradient gel electrophoresis and sequencing analysis. Journal of Periodontology . 2014;85(10):1442–1449. doi: 10.1902/jop.2014.130572. [DOI] [PubMed] [Google Scholar]
- 33.Chen C. Distribution of a newly described species, Kingella oralis, in the human oral cavity. Oral Microbiology and Immunology . 1996;11(6):425–427. doi: 10.1111/j.1399-302X.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 34.Noguera-Julian M., Guillén Y., Peterson J., et al. Oral microbiome in HIV-associated periodontitis. Medicine (Baltimore) . 2017;96(12, article e5821) doi: 10.1097/MD.0000000000005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai N., Homma H., Sakurai A., Takahashi N., Shintani S. Microbiomes of colored dental biofilms in children with or without severe caries experience. Clinical and Experimental Dental Research . 2020;6(6):659–668. doi: 10.1002/cre2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S. C., Xiao Y., Wu R. T., et al. Comparative analysis of type 2 diabetes-associated gut microbiota between Han and Mongolian people. Journal of Microbiology . 2021;59(7):693–701. doi: 10.1007/s12275-021-0454-8. [DOI] [PubMed] [Google Scholar]
- 37.Valdez-Palomares F., Nambo-Venegas R., Uribe-García J., et al. Intestinal microbiota fingerprint in subjects with irritable bowel syndrome responders to a low FODMAP diet. Food & Function . 2021;12(7):3206–3218. doi: 10.1039/D0FO03162C. [DOI] [PubMed] [Google Scholar]
- 38.Zheng S., Zhu Y., Wu W., et al. A correlation study of intestinal microflora and first-episode depression in Chinese patients and healthy volunteers. Brain and Behavior . 2021;11(8, article e02036) doi: 10.1002/brb3.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simas A. M., Kramer C. D., Weinberg E. O., Genco C. A. Oral infection with a periodontal pathogen alters oral and gut microbiomes. Anaerobe . 2021;71, article 102399 doi: 10.1016/j.anaerobe.2021.102399. [DOI] [PubMed] [Google Scholar]
- 40.Schluter J., Peled J. U., Taylor B. P., et al. The gut microbiota is associated with immune cell dynamics in humans. Nature . 2020;588(7837):303–307. doi: 10.1038/s41586-020-2971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen B., Li L. L., Zhang Q., Liu J., Cheng Q., Yan F. H. Comparison of subgingival microbial profile of aggressive periodontitis, chronic periodontitis and periodontally healthy individuals. Zhonghua Kou Qiang Yi Xue Za Zhi . 2020;55(7):466–474. doi: 10.3760/cma.j.cn112144-20200413-00207. [DOI] [PubMed] [Google Scholar]
- 42.Moore W. E. C., Moore L. V. H. The bacteria of periodontal diseases. Periodontology 2000 . 1994;5(1):66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 43.Nibali L., Sousa V., Davrandi M., et al. Differences in the periodontal microbiome of successfully treated and persistent aggressive periodontitis. Journal of Clinical Periodontology . 2020;47(8):980–990. doi: 10.1111/jcpe.13330. [DOI] [PubMed] [Google Scholar]
- 44.Hugon P., Ramasamy D., Robert C., Couderc C., Raoult D., Fournier P. E. Non-contiguous finished genome sequence and description of Kallipyga massiliensis gen. nov., sp. nov., a new member of the family Clostridiales Incertae Sedis XI. Standards in Genomic Sciences . 2013;8(3):500–515. doi: 10.4056/sigs.4047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain S., Bui V., Spencer C., Yee L. Septic arthritis in a native joint due to Anaerococcus prevotii. Journal of Clinical Pathology . 2008;61(6):775–776. doi: 10.1136/jcp.2007.053421. [DOI] [PubMed] [Google Scholar]
- 46.LaButti K., Pukall R., Steenblock K., et al. Complete genome sequence of Anaerococcus prevotii type strain (PC1) Standards in Genomic Sciences . 2009;1(2):159–165. doi: 10.4056/sigs.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulger-Toprak N., Liu C., Summanen P. H., Finegold S. M. Murdochiella asaccharolytica gen. nov., sp. nov., a Gram-stain-positive, anaerobic coccus isolated from human wound specimens. International Journal of Systematic and Evolutionary Microbiology . 2010;60(5) Part 5:1013–1016. doi: 10.1099/ijs.0.015909-0. [DOI] [PubMed] [Google Scholar]
- 48.Pépin J., Deslandes S., Giroux G., et al. The complex vaginal flora of West African women with bacterial vaginosis. PLoS One . 2011;6(9, article e25082) doi: 10.1371/journal.pone.0025082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veloo A. C. M., Erhard M., Welker M., Welling G. W., Degener J. E. Identification of Gram-positive anaerobic cocci by MALDI-TOF mass spectrometry. Systematic and Applied Microbiology . 2011;34(1):58–62. doi: 10.1016/j.syapm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Jung M. Y., Cho J. H., Shin Y., et al. Peptoniphilus rhinitidis sp. nov., isolated from specimens of chronic rhinosinusitis. Anaerobe . 2014;30:30–34. doi: 10.1016/j.anaerobe.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Delima S. L., McBride R. K., Preshaw P. M., Heasman P. A., Kumar P. S. Response of subgingival bacteria to smoking cessation. Journal of Clinical Microbiology . 2010;48(7):2344–2349. doi: 10.1128/JCM.01821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zambon J. J., Grossi S. G., Machtei E. E., Ho A. W., Dunford R., Genco R. J. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. Journal of Periodontology . 1996;67(Supplement 10S):1050–1054. doi: 10.1902/jop.1996.67.10s.1050. [DOI] [PubMed] [Google Scholar]
- 53.van Winkelhoff A. J., Bosch-Tijhof C. J., Winkel E. G., Reijden W. A. Smoking affects the subgingival microflora in periodontitis. Journal of Periodontology . 2001;72(5):666–671. doi: 10.1902/jop.2001.72.5.666. [DOI] [PubMed] [Google Scholar]
- 54.Vigil G. V., Wayman B. E., Dazey S. E., Fowler C. B., Bradley D. V., Jr. Identification and antibiotic sensitivity of bacteria isolated from periapical lesions. Journal of Endodontia . 1997;23(2):110–114. doi: 10.1016/S0099-2399(97)80256-7. [DOI] [PubMed] [Google Scholar]
- 55.Medina-Palacios S. E., Vitales-Noyola M., López-González E., González-Amaro A. M., Méndez-González V., Pozos-Guillén A. Root canal microorganisms and their antibiotic susceptibility in patients with persistent endodontic infections, with and without clinical symptoms. Odontology . 2021;109(3):596–604. doi: 10.1007/s10266-020-00580-2. [DOI] [PubMed] [Google Scholar]
- 56.Uitto V. J. Degradation of basement membrane collagen by proteinases from human gingiva, leukocytes and bacterial plaque. Journal of Periodontology . 1983;54(12):740–745. doi: 10.1902/jop.1983.54.12.740. [DOI] [PubMed] [Google Scholar]
- 57.Wei Y., Shi M., Zhen M., et al. Comparison of subgingival and buccal mucosa microbiome in chronic and aggressive periodontitis: a pilot study. Frontiers in Cellular and Infection Microbiology . 2019;9:p. 53. doi: 10.3389/fcimb.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lourenço T. G., Heller D., Silva-Boghossian C. M., Cotton S. L., Paster B. J., Colombo A. P. V. Microbial signature profiles of periodontally healthy and diseased patients. Journal of Clinical Periodontology . 2014;41(11):1027–1036. doi: 10.1111/jcpe.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zakaria M. N., Takeshita T., Shibata Y., et al. Microbial community in persistent apical periodontitis: a 16S rRNA gene clone library analysis. International Endodontic Journal . 2015;48(8):717–728. doi: 10.1111/iej.12361. [DOI] [PubMed] [Google Scholar]
- 60.Shi M., Wei Y., Nie Y., et al. Alterations and correlations in microbial community and metabolome characteristics in generalized aggressive periodontitis. Frontiers in Microbiology . 2020;11:p. 573196. doi: 10.3389/fmicb.2020.573196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui X., Liu J., Xiao W., Chu Y., Ouyang X. Subgingival microbiome in Chinese patients with generalized aggressive periodontitis compared to healthy controls. Archives of Oral Biology . 2019;101:92–99. doi: 10.1016/j.archoralbio.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Chen H., Liu Y., Zhang M., et al. A Filifactor alocis-centered co-occurrence group associates with periodontitis across different oral habitats. Scientific Reports . 2015;5(1):p. 9053. doi: 10.1038/srep09053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aruni A. W., Roy F., Fletcher H. M. Filifactor alocis has virulence attributes that can enhance its persistence under oxidative stress conditions and mediate invasion of epithelial cells by porphyromonas gingivalis. Infection and Immunity . 2011;79(10):3872–3886. doi: 10.1128/IAI.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishra A., Aja E., Fletcher H. M. Role of superoxide reductase FA796 in oxidative stress resistance in Filifactor alocis. Scientific Reports . 2020;10(1):p. 9178. doi: 10.1038/s41598-020-65806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlafer S., Riep B., Griffen A. L., et al. Filifactor alocis--involvement in periodontal biofilms. BMC Microbiology . 2010;10(1):p. 66. doi: 10.1186/1471-2180-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q., Wright C. J., Dingming H., Uriarte S. M., Lamont R. J. Oral community interactions of Filifactor alocis in vitro. PLoS One . 2013;8(10, article e76271) doi: 10.1371/journal.pone.0076271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: the Venn diagram contouring the distribution of operational taxonomic units (OTUs) for (a) PE, RE-PE, and RE-PU and (b) PU, RE-PE, and RE-PU.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.


