Abstract
Objective:
To evaluate the proliferation and morphology of human osteoblasts cultured on two brands of mini-implants after 24, 48, and 72 hours, in addition to the chemical composition found on their surface.
Materials and Methods:
Two brands of mini-implant (Morelli and Neodent) were evaluated; polystyrene was used as a control group (n = 3). Osteoblasts were cultured on the surface of sterilized mini-implants in a CO2 incubator at different time periods (24, 48, and 72 hours). Osteoblast proliferation was quantified by scanning electron microscopy using up to 5000× magnification, and cell morphology was analyzed by a single observer. For the chemical analysis, spectroscopy X-ray fluorescence was used to identify and quantify chemical components on the surface of the mini-implants.
Results:
Two-way ANOVA showed no significant interaction between the factors studied (P = 0.686). A Tukey test revealed no significant difference in osteoblast proliferation between the mini-implants at all studied periods; however, a difference in cell proliferation was detected between the Neodent and the control group (P = .025). For all groups, time had a direct and positive effect on osteoblast proliferation (P < .001). The significant elements present in both brands of mini-implants were titanium, aluminum, vanadium, and iron.
Conclusions:
Osteoblast proliferation was present on the mini-implants studied, which increased over time; however, no significant difference between brands was observed. No difference was seen between the mini-implants evaluated in terms of chemical composition. Cell adhesion after 72 hours suggests that areas of bone remodeling can be achieved, thus initiating the process of mini-implant anchorage.
Keywords: Osteoblast, Mini-implants
INTRODUCTION
The use of a stable point of anchorage has broadened the possibilities and predictability of orthodontic treatments that avoid tooth extraction and orthognathic surgery in patients with unfavorable bone support. This type of anchorage has also been indicated in adult patients who would normally decline extraoral appliances, as well as in those having a complete dentition lacking edentulous gaps.1–4 Mini-implants are relatively easy to insert and remove at a relatively low cost, with no need to wait lengthy periods between installation and force application.5,6
Many types of anchorage, including conventional osseointegrated implants, nonosseointegrated appliances, miniplates, and mini-implants have been proposed.7,8 Due to their preestablished treatment time, mini-implants depend on mechanical retention and hence may not osseointegrate.9–11
The material used to manufacture mini-implants must be nontoxic, biocompatible, and mechanically resistant to support tension and corrosion.7 Therefore, commercially pure titanium (cp Ti) is the material of choice, as it is biocompatible and presents satisfactory mechanical properties.12,13 Nevertheless, mini-implants are fine components that must bear orthodontic loads and be resistant to fracture. In order to improve material composition, an alloy was obtained by incorporating aluminum (Al) and vanadium (V) into the cp Ti (Ti-6Al-4V). This titanium alloy, known as grade 5 Ti, confers higher fatigue resistance than does cp Ti, yet maintains properties such as resistance to corrosion and low toxicity.11,14,15
The chemical and topographic features of mini-implant surfaces are regarded as important in terms of their interface with bone cells because of their high quality of osseointegration,13 since osteoblast morphology can be influenced by composition and type of surface treatment.16 If corrosion sets in because of the chemical composition of the alloy, encapsulated tissue may be formed secondarily to the release of metallic ions and other byproducts of corrosion, as well as ionic contamination of organic tissues, thus compromising cell adhesion.17
At the completion of orthodontic treatment, the ideal scenario would be to remove the mini-implant by simply unscrewing it with minimal trauma, followed by bone regeneration.18 However, some studies have demonstrated that mini-implants can osseointegrate. Certain difficulties in their removal, including fractures—mainly in the neck of the mini-implant—have been observed, creating the need to remove them surgically or bury them subgingivally.19,20
Therefore, the aim of this study was to analyze the chemical constitution of mini-implant metal alloys, detailing the chemical component profile and rate at which each component occurs, and to associate those findings with in situ proliferation and cell morphology of human osteoblasts in vitro during the initial stages of cell adhesion.
MATERIALS AND METHODS
In order to evaluate cell adhesion, we used cultured human osteoblasts as the experimental units, seeding them on the surface of the mini-implants. The study factors were mini-implants and polystyrene, on two experimental levels of mini-implant, according to commercial brand (Morelli [Dental Morelli, Sorocaba, SP, Brazil] and Neodent [Neodent, Curitiba, PR, Brazil]), and a control, which consisted of a polystyrene disk (TPP-Biosystems, Curitiba, PR, Brazil). Three levels of time were chosen to assess osteoblast proliferation (24, 48, and 72 hours). The sample size consisted of three experimental units of each brand of mini-implant or polystyrene at each level of time (n = 3); the total sample size was 9 in an experimental design of 3 × 3. The response variable was cell adhesion, which was evaluated both quantitatively and qualitatively according to proliferation and cell morphology, respectively. Two experimental units (n = 2) for each commercial brand were used to analyze response in terms of chemical composition of the mini-implants. The response variable was the presence of each chemical as assessed quantitatively in percentage according to the other chemicals via X-ray fluorescence spectroscopy. The specifications of the mini-implants and polystyrene are described in Table 1 and illustrated in Figure 1.
Table 1.
Specifications of the Mini-Implants and the Polystyrene Used as Control
Figure 1.
Materials investigated. (A) Morelli mini-implant. (B) Neodent mini-implant. (C) Polystyrene, used as control.
This study was approved by the Ethics Committee in Research (registration 2012/0383).
Osteoblast Proliferation and Cell Morphology
Human osteoblast cell lines were obtained from the cell bank of the dental school, which were purchased from American Type Culture Collection, Manassas, Va (No. CRL2594). The osteoblasts were cultured in Alfa Minimum Essential Medium (Nutricell, Campinas, SP, Brazil) and supplemented with 10% fetal bovine serum (Sigma, St Louis, Mo). All procedures involving live cell culture were performed in a laminar flow cabinet (Veco, Bio Seg 09, Campinas, SP, Brazil) to prevent contamination.
The mini-implants and polystyrene were placed horizontally on a petri dish and stabilized using a metallic matrix to prevent movement during transportation. The mini-implant-and-matrix set was sterilized in ethylene oxide prior to seeding with osteoblasts.
The cells were kept in a CO2 incubator (Thermo Fisher Scientific 3110, Marietta, Ohio) at 37°C in a humid atmosphere containing 95% oxygen and 5% carbon dioxide. The culture medium was changed every 2 days.
Osteoblast adhesion to the mini-implants was evaluated using the trypan blue vital dye exclusion method at 24, 48, and 72 hours of culture on the mini-implants. Ten microliters of the cell suspension was diluted in 10µL of trypan blue dye (Sigma, Steimheim, Germany) from which 10µL was placed in a hemocytometer (Neubauer Chamber; Fisher Scientific, Pittsburgh, Pa) and counted under an inverted phase contrast microscope (Nikon, Eclipse TS100, Tokyo, Japan). The total number of cells present on each plate at different times was obtained as follows:21
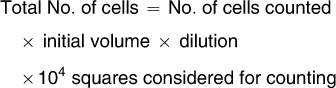 |
Cell morphology analysis was performed on a scanning electron microscope (SEM) (Philips, XL30, Amsterdam, The Netherlands) at 24, 48, and 72 hours. The cells were cultured as described above and fixed using Karnovsky solution with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4, for 1 hour at room temperature. They were then washed in the same buffer before successive dehydration to critical point, drying in a Bal-tec drying unit (Balzers Union, Bal-Tec CPD 030, Brunswick, Ohio). The specimens were positioned on metal stubs and analyzed under the SEM using 30×, 50×, 500×, and 2500×, up to 5000 times. The images were analyzed by a single operator and then processed using Adobe Photoshop (Adobe Systems, San Jose, Calif).
Analysis of Chemical Composition
Identification of the composition of the alloys was performed using an X-ray fluorescence spectrophotometer (Niton, XLI 818, São Bernardo do Campo, SP, Brazil) following verification against the international standard ARM-P/NIARM 35B, certificate No. 35B-101592 ARM-F. Type and quantity of metals present, as per the elements Ti, V, Al, and Fe, were evaluated via their atomic numbers, which were reverted into tables.
The mini-implants were irradiated at the collar region from a distance of 5 cm so that the X-ray beam was at a right angle to the long axis of the mini-implants, which remained in their sterile wrapping to prevent contamination by residues from other metals. The diameter of the collimator used was 20 mm, the value used to qualify and quantify the metals found.
Statistical Analysis
Data from the chemical analyses were obtained in terms of percentages of the constituents evaluated. Cell morphology was described in terms of cell type and shape. The proliferation data were assessed for normality and homogeneity of variance and analyzed using two-way analysis of variance. Multiple comparisons were analyzed using the Tukey test. All statistics were performed on SPSS 20 (SPSS Inc, Chicago, Ill) at a significance level of 5%.
RESULTS
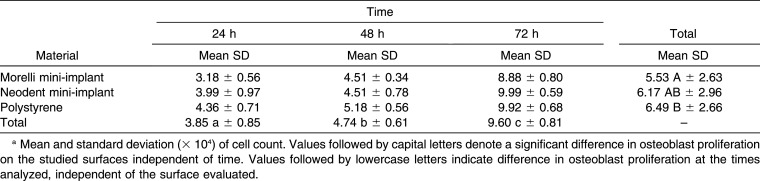
Analysis of cell proliferation demonstrated no significant interaction between the factors mini-implants/polystyrene and time (P = .686). The Tukey test revealed no significant difference in osteoblast proliferation between the Neodent and Morelli mini-implants at any time period, and only for the latter was a significant difference observed when compared with polystyrene, which was used as a control (P = .025). Time influenced osteoblast proliferation on all surfaces studied (mini-implants and polystyrene; P < .001), with the lowest values at 24 hours, intermediate at 48 hours, and highest at 72 hours (Table 2).
Table 2.
Osteoblast Proliferation on the Surface of the Mini-Implants or Polystyrene Over Timea
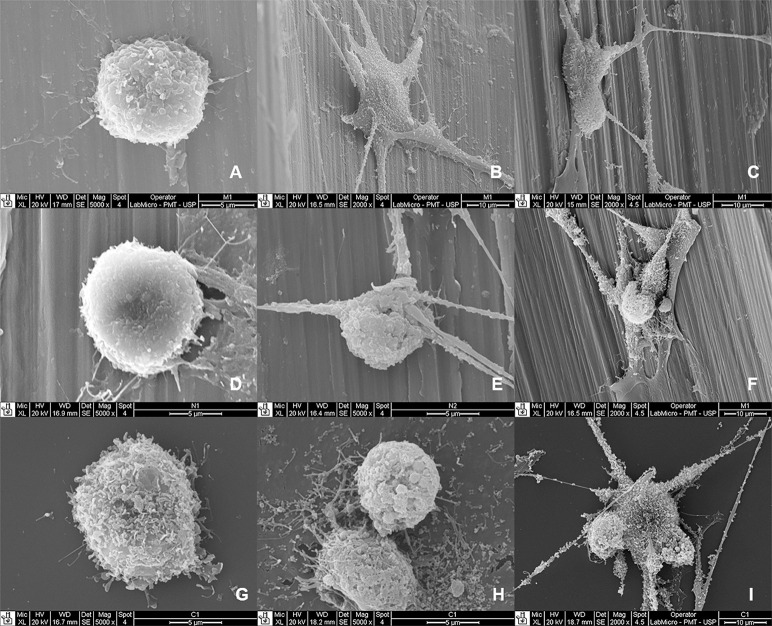
Cell variations were observed on the surface of the mini-implants and polystyrene for the different time periods. Figures 2, 3, and 4 show the results observed on the surface of the mini-implants and polystyrene at 24, 48, and 72 hours. At 24 hours, the cells demonstrated typical morphological features, with a rounded shape and a few elongations (lamellipodia and phyllopodia). At 48 hours, the osteoblasts proliferating onto the mini-implants presented morphological features that varied from round to stellate, with more frequent elongations and an increased number of cells. At 72 hours, an even greater increase in cell numbers was observed for both mini-implants. The osteoblasts presented a predominantly stellate shape with numerous elongations.
Figure 2.
Osteoblasts at 24, 48, and 72 hours on the mini-implants and polystyrene. (A to C) Morelli; (D to F) Neodent; (G to I) polystyrene.
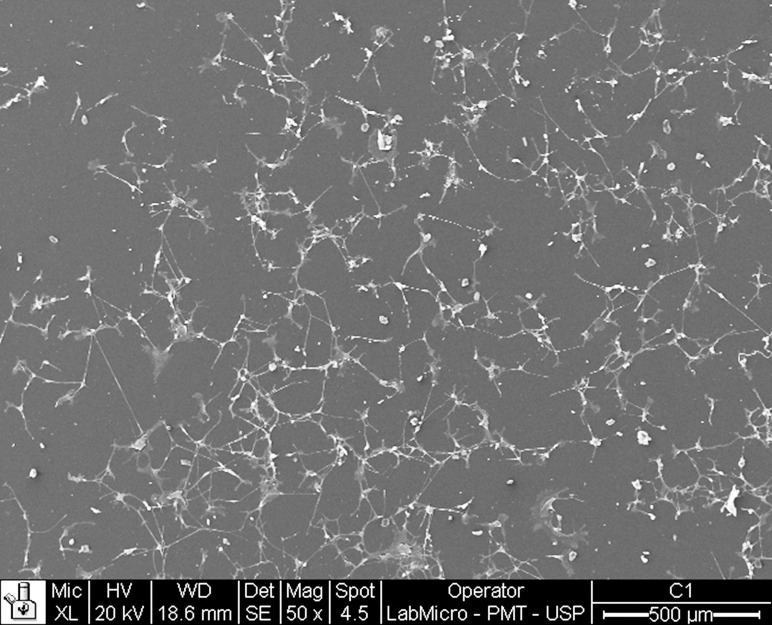
Figure 3.
Osteoblast proliferation at 72 hours for polysterene. Cell spread can be seen with numerous elongations (50× magnification).
Figure 4.
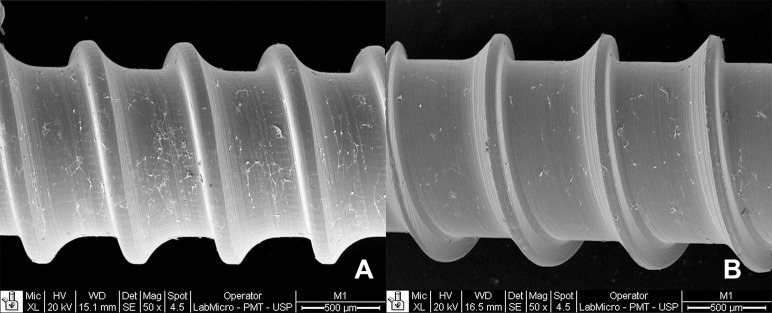
Osteoblast proliferation on the surface of the mini-implant at 72 hours. Although the cell proliferation and composition were similar for both mini-implants, a higher spread can be seen over the Morelli mini-implant (A) than over the Neodent mini-implant (50× magnification).
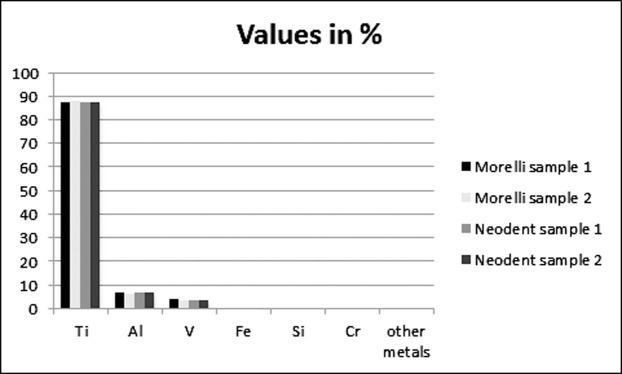
Data relating to the chemical analysis are presented in Figure 5. The most abundant elements in all the alloys analyzed were Ti, Al, V, and Fe. The mini-implants with the highest amounts of Ti were the Morelli from sample 2 (88.37%), followed by Neodent sample 2 (87.93%), Neodent sample 1 (87.92%), and Morelli sample 1 (87.73%), but these small differences were not significant.
Figure 5.
Results of spectrophotometric analysis of the chemical composition (in percentage) of the Morelli and Neodent mini-implants.
DISCUSSION
Materials used in the manufacturing of mini-implants must be nontoxic and biocompatible, as well as mechanically resist tension and corrosion.7 Therefore, cp Ti is the material of choice, as it possesses both characteristics.12,13,22 Incorporating Al and V into the alloy (Ti-6Al-4V) can enhance the composition of mini-implants to withstand orthodontic loads and resist fracture. Such Ti alloys, known as grade 5 and grade 23, provide higher fatigue resistance than does cp Ti without losing resistance to corrosion and maintaining low toxicity.14,23
According to the manufacturers, Neodent mini-implants are rated at grade 5 Ti6Al4V in their chemical composition (ASTM-136), while the Morelli mini-implants rate grade 23 Ti6Al4V (ASTM-136). In the present study, chemical analysis showed that heavy metals were present in both brands of mini-implants, with the most abundant elements in the alloys being Ti, Al, V, and Fe. The mini-implants of both commercial brands studied exhibited values and types of chemical components within the standards specified by their manufacturers and as shown by Malkoç et al.,24 which made them clinically biocompatible and without adverse effects on osteoblasts24 despite the mild differences in chemical elements.
Previous studies have shown that osteoblasts proliferate on the alloys studied11,15,24 and that the mini-implants used for temporary anchorage in orthodontics may partially osseointegrate.19,20 Also, both implant and thread design may influence insertion and removal torques, highlighting the importance of selecting an adequate design according to cortical bone thickness.25 Furthermore, mini-implant diameter and, most important, thread pitch have a significant effect on stability, which is not the case for length.26 Surface modification by sandblasting and acid etching has a beneficial effect on implant biocompatibility.12,27–29 In the present study, even with the surface visibly smooth both to the naked eye and at 50× magnification (Figure 4), osteoblast proliferation occurred within the irregularities observed by SEM, favoring cell proliferation and spread into the dipped areas (Figure 2), thus corroborating the literature regarding treated surfaces.12,13,18 No significant difference was observed in terms of osteoblast proliferation onto the Neodent and Morelli mini-implants; however, only for the latter was the proliferation different from that on the control, polystyrene. This result may be related to the surface similarity between the Neodent mini-implant and polystyrene (used as the control in cell-culture studies) regarding surface energy. According to Poulsson et al.,30 surface energy is a factor that can influence cell adhesion.
A direct relationship has been reported between surface roughness and implant anchorage, as evaluated by measuring reverse torque wherein anchorage seems to become more stable as the bone tissue matures during healing.12,13,18 In this respect, Conserva et al.16 observed that the microscopic aspect of the implant surface and osteoblast morphology seemed to be influenced by the type of surface treatment at 6, 24, and 72 hours and that machined surfaces retard cell proliferation as observed under SEM.
In the present study, as the time of contact between the cells and surface increased, so did cell proliferation, suggesting a favorable biological relationship between the cells and surfaces, including machined smooth surfaces. From 24 hours to 72 hours, cell morphology underwent some changes, including variation in shape from round to stellate, with more elongations. The number of adhered cells was also greater, which supports the previous findings of cell proliferation.16,24
Based on the similar patterns of cell proliferation and cell morphology, both brands might be suited for clinical use. The cell adhesion observed suggests that areas of bone remodeling can be achieved, thus initiating the process of mini-implant anchorage. Nevertheless, further studies are needed to investigate the speed and time of osseointegration in mini-implants.
CONCLUSIONS
Osteoblast proliferation was successful on the mini-implant surface, which increased over time without a significant difference between commercial brands.
The most frequently observed elements present in the alloys were Ti, Al, V, and Fe, a characteristic that did not differ significantly between brands.
REFERENCES
- 1.Block MS, Hoffman DR. A new device for absolute anchorage for orthodontics. Am J Orthod Dentofacial Orthop. 1995;107:251–258. doi: 10.1016/s0889-5406(95)70140-0. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara J, Daimaruya T, Umemori M, et al. Distal movement of mandibular molars in adult patients with the skeletal anchorage system. Am J Orthod Dentofacial Orthop. 2004;125:130–138. doi: 10.1016/j.ajodo.2003.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Palagi LM, Sabrosa CE, Gava EC, Baccetti T, Miguel JA. Long-term follow-up of dental single implants under immediate orthodontic load. Angle Orthod. 2010;80:807–811. doi: 10.2319/021010-86.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura M, Sannohe M, Nagasaka H, Igarashi K, Sugawara J. Nonextraction treatment with temporary skeletal anchorage devices to correct a Class II Division 2 malocclusion with excessive gingival display. Am J Orthod Dentofacial Orthop. 2014;145:85–94. doi: 10.1016/j.ajodo.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Carano A, Velo S, Leone P, Siciliani G. Clinical application of the miniscrew anchorage system. J Clin Orthod. 2005;39:9–24. [PubMed] [Google Scholar]
- 6.Zhang L, Zhao Z, Li Y, Wu J, Zheng L, Tang T. Osseointegration of orthodontic micro-screws after immediate and early loading. Angle Orthod. 2010;80:354–360. doi: 10.2319/021909-106.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morais LS, Serra GG, Muller CA, et al. Titanium alloy mini-implants for orthodontic anchorage: immediate loading and metal ion release. Acta Biomater. 2007;3:331–339. doi: 10.1016/j.actbio.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Samuels RH, Brezniak N. Orthodontic facebows: safety issues and current management. J Orthod. 2002;29:101–107. doi: 10.1093/ortho/29.2.101. [DOI] [PubMed] [Google Scholar]
- 9.Chaddad K, Ferreira AF, Geurs N, Reddy MS. Influence of surface characteristics on survival rates of mini-implants. Angle Orthod. 2008;78:107–113. doi: 10.2319/100206-401.1. [DOI] [PubMed] [Google Scholar]
- 10.Salmória KK, Tanaka OM, Guariza-Filho O, Camargo ES, de Souza LT, Maruo H. Insertional torque and axial pull-out strength of mini-implants in mandibles of dogs. Am J Orthod Dentofacial Orthop. 2008;133:790.e15–e22. doi: 10.1016/j.ajodo.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Rivadeneira J, Barrio DA, Arrambide G, Gambino D, Bruzzone L, Etcheverry SB. Biological effects of a complex of vanadium (V) with salicylaldehyde semicarbazone in osteoblasts in culture: mechanism of action. J Inorg Biochem. 2009;103:633–642. doi: 10.1016/j.jinorgbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Elias CN, Oshida Y, Lima JH, Muller CA. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J Mech Behav Biomed Mater. 2008;1:234–242. doi: 10.1016/j.jmbbm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz Z, Raz P, Zhao G, et al. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90:2485–2498. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bucci-Sabattini V, Cassinelli C, Coelho PG, Minnici A, Trani A, Dohan Ehrenfest DM. Effect of titanium implant surface nanoroughness and calcium phosphate low impregnation on bone cell activity in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:217–224. doi: 10.1016/j.tripleo.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Cotrim-Ferreira FA, Quaglio CL, Peralta RPV, Carvalho PEG, Siqueira DF. Metallographic analysis of the internal microstructure of orthodontic mini-implants. Braz Oral Res. 2010;24:438–442. doi: 10.1590/s1806-83242010000400011. [DOI] [PubMed] [Google Scholar]
- 16.Conserva E, Menini M, Ravera G, Pera P. The role of surface implant treatments on the biological behavior of SaOS-2 osteoblast-like cells: an in vitro comparative study. Clin Oral Implants Res. 2013;24:880–889. doi: 10.1111/j.1600-0501.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 17.Arcuri C, Muzzi F, Santini F, Barlattani A, Giancotti A. Five years of experience using palatal mini-implants for orthodontic anchorage. J Oral Maxillofac Surg. 2007;65:2492–2497. doi: 10.1016/j.joms.2007.06.651. [DOI] [PubMed] [Google Scholar]
- 18.Gotfredsen K, Berglundh T, Lindhe J. Anchorage of titanium implants with different surface characteristics: an experimental study in rabbits. Clin Implant Dent Relat Res. 2000;2:120–128. doi: 10.1111/j.1708-8208.2000.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 19.Fávero LG, Pisoni A, Paganelli C. Removal torque of osseointegrated mini-implants: an in vivo evaluation. Eur J Orthod. 2007;29:443–448. doi: 10.1093/ejo/cjm062. [DOI] [PubMed] [Google Scholar]
- 20.Vande Vannet B, Sabzevar MM, Wehrbein H, Asscherickx K. Osseointegration of miniscrews: a histomorphometric evaluation. Eur J Orthod. 2007;29:437–442. doi: 10.1093/ejo/cjm078. [DOI] [PubMed] [Google Scholar]
- 21.Freshney I. Culture of Animal Cells A Manual of Basic Technique and Specialized Applications 6th ed. New York: Wiley-Blackwell; 2010. [Google Scholar]
- 22.Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw: experience from a 10-year period. Scand J Plast Reconstr Surg. 1977;11:1–132. [PubMed] [Google Scholar]
- 23.Casaglia A, Dominici F, Pachì F, Turlà R, Cerroni L. Morphological observations and fractological considerations on orthodontics miniscrews. Minerva Stomatol. 2010;59:465–476. [PubMed] [Google Scholar]
- 24.Malkoç S, Öztürk F, Çörekçi B, Bozkurt BS, Hakki SS. Real-time cell analysis of the cytotoxicity of orthodontic mini-implants on human gingival fibroblasts and mouse osteoblasts. Am J Orthod Dentofacial Orthop. 2012;141:419–426. doi: 10.1016/j.ajodo.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Song YY, Cha JY, Hwang CJ. Mechanical characteristics of various orthodontic miniscrews in relation to artificial cortical bone thickness. Angle Orthod. 2007;77:979–985. doi: 10.2319/090606-363.1. [DOI] [PubMed] [Google Scholar]
- 26.Topcuoglu T, Bicakci AA, Avunduk MC, Sahin Inan ZD. Evaluation of the effects of different surface configurations on stability of miniscrews. Scientific World Journal. doi: 10.1155/2013/396091. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CL, Yu JH, Liu HL, Lin CH, Lin YS. Evaluation of contributions of orthodontic mini-screw design factors based on FE analysis and the Taguchi method. J Biomech. 2010;43:2174–2181. doi: 10.1016/j.jbiomech.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 28.Cha JY, Takano-Yamamoto T, Hwang CJ. The effect of miniscrew taper morphology on insertion and removal torque in dogs. Int J Oral Maxillofac Implants. 2010;25:777–783. [PubMed] [Google Scholar]
- 29.Chang CS, Lee TM, Chang CH, Liu JK. The effect of microrough surface treatment on miniscrews used as orthodontic anchors. Clin Oral Implants Res. 2010;20:1178–1184. doi: 10.1111/j.1600-0501.2009.01728.x. [DOI] [PubMed] [Google Scholar]
- 30.Poulsson AH, Mitchell SA, Davidson MR, Johnstone AJ, Emmison N, Bradley RH. Attachment of human primary osteoblast cells to modified polyethylene surfaces. Langmuir. 2009;25:3718–3727. doi: 10.1021/la801820s. [DOI] [PubMed] [Google Scholar]