Abstract
Activation of the mouse mammary tumor virus (MMTV) promoter by the glucocorticoid receptor (GR) is associated with a chromatin structural transition in the B nucleosome region of the viral long terminal repeat (LTR). Recent evidence indicates that this transition extends upstream of the B nucleosome, encompassing a region larger than a single nucleosome (G. Fragoso, W. D. Pennie, S. John, and G. L. Hager, Mol. Cell. Biol. 18:3633–3644). We have reconstituted MMTV LTR DNA into a polynucleosome array using Drosophila embryo extracts. We show binding of purified GR to specific GR elements within a large, multinucleosome array and describe a GR-induced nucleoprotein transition that is dependent on ATP and a HeLa nuclear extract. Previously uncharacterized GR binding sites in the upstream C nucleosome region are involved in the extended region of chromatin remodeling. We also show that GR-dependent chromatin remodeling is a multistep process; in the absence of ATP, GR binds to multiple sites on the chromatin array and prevents restriction enzyme access to recognition sites. Upon addition of ATP, GR induces remodeling and a large increase in access to enzymes sites within the transition region. These findings suggest a dynamic model in which GR first binds to chromatin after ligand activation, recruits a remodeling activity, and is then lost from the template. This model is consistent with the recent description of a “hit-and-run” mechanism for GR action in living cells (J. G. McNally, W. G. Müller, D. Walker, and G. L. Hager, Science 287:1262–1264, 2000).
The mouse mammary tumor virus (MMTV) long terminal repeat (LTR) has been a useful model for studies on the relationship between chromatin structure and transcriptional activation. When integrated in cellular chromosomes, the MMTV LTR promoter adopts a specific chromatin organization consisting of six positioned nucleosome families, nucleosome region A (Nuc-A) to Nuc-F (14, 38). Activation of the MMTV promoter by steroid hormones is associated with a region-specific chromatin structural transition detected as an increase in sensitivity to nucleases (38, 39), chemical probes (38), or restriction enzymes (1). This nucleoprotein remodeling event is implicated, in turn, in the secondary binding of transcription factors that are excluded by nonremodeled chromatin (2, 4, 8, 22, 37, 45–47).
Glucocorticoid receptor (GR)-induced remodeling was originally associated with one nucleosome family (Nuc-B) in the LTR-phased array (1, 4, 22, 37, 38, 46, 47); four receptor binding sites are associated with this nucleosome family (−70 to −190). Recently, however, the region of GR-induced hypersensitivity has been found to extend upstream of the Nuc-B region to −295 (15). This transition region not only encompasses an area larger than that attributed to core histones, but is asymmetrically positioned with respect to the Nuc-B family. It is therefore difficult to model this transition as a simple nucleosomal event.
One possible explanation for this observation is that hormone activation produces a change in higher order chromatin structure and some feature of the chromatin fiber causes the transition to be asymmetric with respect to nucleosome family location. Alternatively, regulatory elements may exist upstream of the Nuc-B region that are involved in the chromatin transition in that region. Although most studies have focused on the hormone response elements (HREs) located in the Nuc-B region, the original GR footprinting experiments detected weak HREs upstream of Nuc-B (33). Several investigations have also identified elements in the region upstream of the distal HRE that are important for regulation of MMTV transcription in various cell types (6, 17, 41).
To further investigate the nature of the GR-induced chromatin transition, we reconstituted the MMTV promoter into chromatin in vitro utilizing the Drosophila chromatin assembly system (3). Using a polynucleosome template reconstituted with a 1.8-kb fragment of the promoter region, we show site-specific binding of purified, activated, rat GR to glucocorticoid response elements (GREs) in the Nuc-B region (−70 to −190; GRE1, -2, -3, and -4). Several different analyses show that, in the context of chromatin, GR binds to two additional upstream GREs (GRE5 and -6; positioned between −299 and −274) in the 3′ half of the Nuc-C family.
In the presence of a HeLa cell nuclear extract and ATP, we also find that purified GR will induce a DNase I-hypersensitive transition in the reconstituted MMTV promoter that maps to a region similar to that observed in vivo. When examined at higher resolution by restriction enzyme access, we find that boundaries of the receptor-induced transition are identical to those observed in vivo. That is, the remodeled region includes all sites within the Nuc-B family, but also extends upstream into the Nuc-C family. GR-dependent, in vitro chromatin remodeling in this region requires the presence of GRE5 and -6. Furthermore, in transfection analysis, removal of these sites reduces hormone activation by 50% in mouse mammary epithelial 34i and NIH 3T3 cells. These findings indicate that the asymmetric position of the chromatin transition is based, at least in part, on the position of the GR binding sites and deemphasize the role for a unique nucleosomal configuration.
We also report evidence that GR does not remain statically bound to remodeled chromatin. In the absence of ATP, GR binds to recognition elements in the LTR and prevents access of restriction enzymes whose sites are sterically obstructed by the presence of bound receptor. Upon addition of ATP and remodeling factors, access to these sites is dramatically increased, indicating that the receptor is no longer resident at these binding sites. These results suggest that the ligand-activated receptor undergoes a binding followed by a disengagement step that requires ATP hydrolysis. Together with recent evidence obtained by direct observation of GR interaction with target sites in chromatin in living cells (27), these findings argue that receptor undergoes constant and rapid exchange with HREs in the continued presence of ligand.
MATERIALS AND METHODS
Materials.
GR was purified from CHO cells containing amplified copies of the murine GR cDNA (21) and was purified by a previously described procedure (51). HeLa nuclear extract was prepared by the method of Shapiro et al. (44).
Plasmid constructions and site-directed mutagenesis.
Plasmid pGEM3zf(−)-LTRCAT, containing the MMTV LTR upstream of CAT, was constructed by cloning a 2.9-kb PstI-BamHI fragment from pUC-LTRCAT into the 3.2-kb PstI-BamHI fragment from pGEM3zf(−) (Promega). Deletion mutants of the LTR driving luciferase were constructed by inserting PCR fragments at the KpnI and SacI sites of plasmid pMLuc, which contains the C3H strain LTR sequence from −109 (SacI) to +110 (31). The PCRs were conducted with a common 5′ end primer containing a GC clamp, a KpnI site, 22-bp of the LTR sequence, from −1184 to −1163 (oligo 1038, 5′-GCGCT CGGTA CCCTG CAGCA GAAAT GGTTG AACT-3′), and various 3′ primers containing a clamp, a SacI site, and the appropriate LTR sequence. These reactions yielded fragments with 3′ ends at −110 (oligo 1039, 5′-GAGCG CGAGC TCAGA TCAGA ACCTT TGATA CCAA-3′), −230 (oligo 1040, 5′-GAGCG CGAGC TCAAG GCTAT TCATA ATAAC TCAT-3′), and −310 (oligo 1041, 5′-GAGCG CGAGC TCTGG AAAAT CTTTC CCCAA AA GT-3′). After restriction cleavage, the fragments were cloned into pMLuc to produce pFL-Luc, pFLΔB-Luc (deletion from −229 to −110), and pFLΔBC-Luc (deletion from −309 to −110). The plasmid pFLΔC-Luc was constructed by inserting a PCR fragment from −230 to −110, made with oligos 1039 and 1461 (5′-GCGCT CGAGC TCTTA TTGGC CCAAC CTTGC GGTT-3′), into the SacI site of pFLΔBC-Luc.
Site-directed mutagenesis of GRE5 and -6 (GRE5/6m) was performed with the QuickChange procedure (Stratagene). Oligonucleotides 1621 (TACCA AGGAG ACTCC AGTGG CTGGA CTAAT GAATT CTTAT TCTG) and 1624 (CAGAA TAAGA ATTCA TTAGT CCAGC CACTG GAGTC TCCTT GGTA) were used for the mutagenesis. GRE2 and -3 mutants (GRE2/3m) and the GRE4 mutant (GRE4m) were generated with similar strategies.
Reconstitution of nucleosomal arrays.
Reconstitutions were performed on a 1.8-kb NcoI-SphI or 2.1-kb NcoI-BstUI fragment containing the MMTV promoter from the pGEM3zf(−)-LTRCAT plasmid. The DNA was biotinylated by filling in the NcoI 3′ end by using Klenow polymerase (NEB) and the nucleotides α-S-dTTP, α-S-dGTP, α-S-dCTP (Sigma), and biotin-dATP (BRL) (42). Biotin-labeled DNA (20 μg) was immobilized to 2.5 mg of streptavidin beads (Dynabeads M-280; Dynal) by using the KiloBase binder kit (Dynal). After washing according to manufacturer's specifications, the bead-DNA mixture was resuspended in embryo extract buffer (10 mM HEPES, pH 7.6, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM EGTA, 10% glycerol, 10 mM β-glycerophosphate, 1 mM dithiothreitol, and 1 mM AEBSF) containing 0.05% NP-40 (EX-N), 0.001% thimersol, 0.3 mg of bovine serum albumin (BSA) per ml, and 1 mM AEBSF to a DNA concentration of 0.1 mg/ml. The amount of DNA immobilized to the beads was analyzed by digesting a small amount of reconstitution reaction with EcoRI to liberate a fragment and then performing ethidium-stained agarose gel electrophoresis.
Late or early Drosophila embryo extracts were obtained by using the procedure of Becker and Wu (3). Generally, chromatin was reconstituted by using 2 μg of immobilized DNA and 1.5 mg of early Drosophila extracts or 1 mg of late Drosophila extracts with 2 μg of histone octamers from mouse mammary epithelial (1471.1) cells in a total of 200 μl according to the method of Sandaltzopoulus et al. (42). After rotation for 4 h at room temperature, the reconstitution extract was removed from the chromatin by using a magnet. The chromatin was incubated at room temperature for 5 min with 0.05% Sarkosyl and 0.3 mg of BSA per ml in EX-N buffer to remove Drosophila remodeling and assembly complexes. The chromatin was washed once each with cold 200 mM NaCl–EX-N buffer and EX-N buffer containing 0.3 mg of BSA per ml and was stored in a solution containing EX-N buffer, 0.3 mg of BSA per ml, and protease inhibitors. Reconstitutions were analyzed by digesting 25 μl at each time point with 3 U of micrococcal nuclease per μl and 0.3 mM CaCl2 for 0, 1, and 5 min at room temperature. Reactions were stopped with 6.3 μl of 2.5% Sarkosyl-0.1 M EDTA and then incubated for 1 h with 1 μl of 0.5-mg/ml RNase A (Boehringer Mannheim). Proteins were digested overnight at 37°C with 4 μl of 10-mg/ml proteinase K and 0.2% sodium dodecyl sulfate (SDS), DNA was ethanol precipitated, and digested products were analyzed on a 1% agarose gel.
GR.
The GR used in these experiments was expressed in a WCL2 CHO cell line (21) and purified as described (51). The fraction isolated after Mono-Q chromatography was utilized; this GR contains only a small amount of associated proteins as judged by silver staining after SDS gel electrophoresis, has a high affinity for a GRE-containing oligonucleotide, and is able to activate transcription from the MMTV LTR promoter in vitro (51).
Restriction enzyme accessibility.
Restriction enzyme accessibility assays were performed in individual wells of a Costar 96-well polypropylene plate. Reconstituted chromatin fragments attached to magnetic beads were washed in the 96-well plate by using a Dynal MPC-96 magnet. A 40-μl reaction mixture containing 0.04 μg of chromatin was incubated at room temperature for 20 min with indicated amounts of GR or GR buffer, 0 or 3 μg of HeLa nuclear extract, and 1 mM ATP in EX-N plus 0.1 mg of BSA buffer per ml. Reactions were initiated by adding 4 μl of restriction enzyme diluted in EX buffer and incubating at 37°C for 15 min. Amounts of enzymes in the reactions were 10 U of SacI, 1 U of AlwNI, 10 U of StuI, and 2 U of PstI. Reactions were stopped by the addition of 10 μl of 2.5% Sarkosyl-0.1 M EDTA. The reaction supernatants were removed by using the magnet, were replaced with 100 μl of deproteination buffer (10 mM Tris plus 2 M NaCl plus 0.1% SDS plus 0.1% NP-40), and were incubated at 37°C for 15 to 30 min. Beads were washed with 50 μl of deproteination buffer, then twice with 50 μl of Tris-EDTA (TE) plus 0.1% NP-40, resuspended in 30 μl of labeling solution (0.5 μl of [α-32P]3′-dATP [NEN], 0.5 μl of terminal transferase [NEB], 3 μl of 10× buffer [NEB 4], 3 μl of 10 CoCl2, 23 μl of H2O), and incubated at 37°C for 2 h. Beads were washed twice with TE plus 0.1% NP-40. The DNA fragments were removed from beads by digesting with EcoRI and were run on a 1% agarose gel which was dried then exposed to a Molecular Dynamics PhosphorImager screen.
Digested and undigested chromatin was quantitated by using ImageQuant software. Accessibility of a site to a specific enzyme was determined by the fractional cleavage, F(x), calculated by dividing the amount of digested chromatin by the total amount of chromatin. The change in fractional cleavage, ΔF(x), for a particular sample was given by the formula F(x)sample-F(x)control (15). Since ATP caused a slight decrease in F(x) compared to control without ATP, all ΔF(x)'s in ATP were obtained by subtracting from F(x) of control with ATP.
Electrophoretic mobility shift assays on agarose gels.
Chromatin for agarose gel analysis was obtained by reconstitution as described above without streptavidin beads. The 1.8-kb NcoI-SphI fragment DNA was labeled by using Klenow polymerase (NEB) and the nucleotides [α-S]-dTTP, [α-S]-dGTP, [α-S]-dATP, and [32P]dCTP. After reconstitution, chromatin (100 μl) was treated with Sarkosyl (final concentration, 0.05%) and was purified by using a Sepharose CL-4B spun column (Pharmacia) according to manufacturer's specifications. The chromatin was then treated for 1 min on ice with NaCl (final concentration, 200 mM) and was Sepharose CL-4B spun column purified again. Yield after purification was determined by comparing radioactivity before and after passage through a spun column. Mixtures (40 μl) containing 0.04 μg of chromatin were incubated at room temperature for 10 min with indicated amounts of GR or GR buffer in EX-N buffer, 0.1 mg of BSA per ml, 0.5 mM 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), and 0.05 μg of poly dI · dC and run for 4 h in 4°C on a 1.0% agarose gel that had been equilibrated for 2 h in 0.5× Tris-borate-EDTA plus 0.5 mM CHAPS.
DNase I-hypersensitive region detection.
DNase I-hypersensitive regions were detected by using two methods. In the first, MMTV LTR chromatin was reconstituted with the pGEM3zf(−)-LTRCAT plasmid DNA by using Drosophila late embryo extract as described above. Following assembly, the chromatin template was treated with 0.05% Sarkosyl at room temperature for 5 min. The chromatin template was then partially purified two times by using Sepharose CL-4B spun column as recommended by manufacturer (Pharmacia). The purified chromatin template (100 ng in 25 μl) was incubated for 20 min at room temperature with and without 0.6 μM GR, 4.5 μg of HeLa nuclear extract, and 1.5 mM ATP, as described in the figure legends. Following incubation of the chromatin with purified receptor, transcription factor (NF-1), and HeLa nuclear extract proteins, the chromatin was digested with DNase I for 2 min at room temperature and treated with proteinase K. Purified DNA was digested with NcoI, separated on a 1.5% agarose gel, and analyzed by Southern blot hybridization with random prime-labeled 481-bp EcoRI-SacI fragment from the pGEM3zf(−)-LTRCAT plasmid.
The DNase I hypersensitivity site was also mapped by using the bead-immobilized template. The purified bead-immobilized chromatin template (100 ng in 40 μl) was incubated for 10 min at room temperature with or without 0.6 μM GR, 4.5 μg of HeLa nuclear extract, and 1.5 mM ATP. Following incubation of the chromatin with purified receptor, transcription factor (NF-1), and HeLa nuclear extract proteins, the chromatin was digested with DNase I for 2 min at room temperature and processed in the same manner as described in the restriction enzyme accessibility assay. Location of the DNase I-hypersensitive site was determined by using 32P-labeled 100-bp ladders (RTS Ready-Label ladder; Gibco BRL).
Low-resolution nucleosome mapping.
A reaction mixture containing 50 μl of reconstitute in 0.3 mM CaCl2 was digested with 1 U of micrococcal nuclease per ml for 0.5 or 1 min at room temperature. Reactions were stopped with 6.3 μl of stop buffer and were deproteinated as in the restriction accessibility assay. The products remaining to the beads were 32P end-labeled by using 10 U of T4 polynucleotide kinase (New England Biolabs) and 2 μl of [γ-32P]ATP (6,000 Ci/mmol) then washed extensively with 50 μl of TE plus 0.1% NP-40 to remove unreacted nucleotides. Labeled DNA was digested from the beads with EcoRI and run on a 1.5% agarose gel which was dried then exposed to a Molecular Dynamics PhosphorImager screen. Nucleosome positions were obtained by comparing mobility of cleavage sites within the linker regions to 1-kb (with 22 fragments ranging from 75 to 12 kb) and 100-bp ladder size markers (Gibco BRL). The NcoI-SphI fragment used in reconstitution was digested with either SacI-EcoRI or AlwNI-EcoRI to compare the nucleosome positions to the SacI (−108 from transcription start site) and AlwNI (−293 from the transcription start site). The SacI-EcoRI digest liberated fragments of 1,092, 481 (−108 from start site)-, and 301-bp fragments. The AlwNI-EcoRI digest liberated 891-, 665 (−293 from start site), and 301 bp.
Transient transfection assays with GRE mutants.
Transient transfections of 34i mouse mammary and NIH 3T3 fibroblast cells were conducted as described previously by using calcium phosphate (28). The plasmid pCH110 (Pharmacia Biotech, Inc.) containing simian virus 40–β-galactosidase was cotransfected with the LTR-luciferase constructions to provide a normalization control. The enzymatic assays for β-galactosidase (fluorometric) and luciferase (chemiluminescence) were conducted as described (28).
RESULTS
MMTV-reconstituted chromatin.
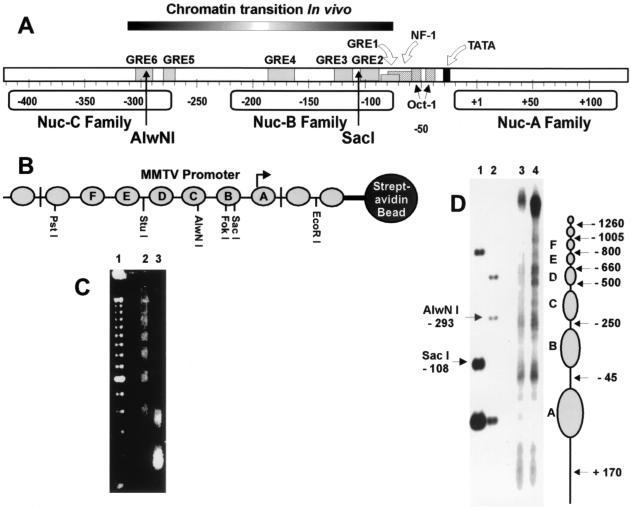
It was recently reported that the MMTV region of GR-dependent chromatin remodeling extends beyond the Nuc-B region in vivo and is asymmetrically positioned with respect to the positioned nucleosome families (Fig. 1A) (15). This finding prompted us to analyze, in detail, the location of GR binding and the relationship between GR binding and chromatin remodeling in vitro. The DNA used for reconstitution consisted of either a 2.1- or 1.8-kb fragment with the MMTV LTR centrally located to minimize possible end effects, or steric hindrance caused by the streptavidin beads (Fig. 1B). Drosophila embryo extracts (3) provide an effective method for reconstituting physiologically spaced nucleosomal arrays in vitro (Fig. 1C). MMTV chromatin has been previously reconstituted by using this system (10). In addition, chromatin reconstituted in Drosophila embryo extracts with DNA immobilized on streptavidin beads has been used previously to study regulation of the Hsp70 gene (42, 50). Immobilization of the chromatin allows for effective elimination of proteins present in the Drosophila extracts, leaving a template that is largely comprised of histones at a histone-to-DNA ratio of approximately 1:1, as judged by Coomassie blue staining (42; data not shown). Moreover, treatment with Sarkosyl has been effective in the removal of nucleosome remodeling and assembly activities from the chromatin template (9, 48, 49). Low-resolution mapping (Fig. 1D) revealed positioned nucleosomes similar to that obtained in vivo (14, 38). This places both the SacI and AlwNI sites within Nuc-B and -C families, respectively, and located 3′ from their respective nucleosome centers. MMTV chromatin was also prepared with DNA templates containing mutations in each of the GREs (Table 1) to determine the association of GR binding and chromatin remodeling, including the putative GREs (5 and 6) located upstream of the Nuc-B region.
FIG. 1.
MMTV LTR chromatin structure. (A) Location of chromatin transition determined in vivo (15) and GREs in relation to positions of the Nuc-A, -B, and -C families. (B) Reconstituted nucleosomal array containing an MMTV LTR fragment (2.1 kb), including nucleosome families A to F, immobilized on streptavidin beads. (C) Micrococcal nuclease digestion of reconstituted chromatin at 0 (lane 2), 1 (lane 3), and 5 (lane 4) min; lane 1 contains a 100-bp periodic repeat ladder as marker. (D) Low-resolution mapping of nucleosome positions on reconstituted MMTV chromatin using micrococcal nuclease digestion at 1 min (lane 3) and 0.5 min (lane 4) as described in Materials and Methods. To the right of the gel are the approximate nucleosome positions. The numbers refer to the micrococcal cleavage sites in the linker regions relative to the transcription start site. The fragment used in reconstitution was digested with either SacI-EcoRI (lane 1) or AlwNI-EcoRI (lane 2) to illustrate the SacI (−108 relative to start site) and AlwNI (−293 relative to start site) cleavage sites in relation to the nucleosome centers (see Materials and Methods).
TABLE 1.
GRE mutationsa
| Nucleosome family | GRE | Sequence of:
|
|
|---|---|---|---|
| Wild type | Mutantb | ||
| Nuc-B | 2 | −102 GAGTGTTCTAT −92 | −102 GAGTGTGACAT −92 |
| 3 | −123 AAAGGTTCTGATCTGAG −107 | −123 AAAGGTTCTGACTCGAG −107 | |
| 4 | −188 TTGGTTACAAACTGTTCTTAAAAC −165 | −188 TTGGTTGTTAACGTCTCTTAAAAC −165 | |
| Nuc-C | 5 | −283 TAATAGAACATTATTCTGC −269 | −283 TAATGAATTCTTATTCTGC −269 |
| 6 | −305 AAGGAGGGGACAGTGG −289 | −305 AAGGAGATCTCAGTGG −289 | |
GRE site-specific mutations are designated as follows: proximal halfsites 2 and 3 (GRE2/3m), the distal site 4 (GRE4m), and putative upstream GREs (GRE5/6m).
Mutant sequences are designated by boldface.
Site-specific binding of GR to reconstituted MMTV chromatin.
Electrophoretic mobility shifts were performed with agarose gels to detect GR binding to the complete three million-dalton MMTV chromatin fragment. We found that the presence of CHAPS detergent in the sample, combined with preequilibration of the gels in CHAPS buffer, stabilized the GR-chromatin interactions. A mobility shift of wild-type MMTV chromatin in the presence of GR was observed for 1.0% agarose gels (Fig. 2, lanes 2 to 4). As the concentration of GR in the mixture was increased, a majority of the chromatin was shifted into a population of slower-migrating species. The mobility shift with the GRE5 and -6 sites mutated (GRE5/6) was nearly indistinguishable from wild-type chromatin (Fig. 2, lanes 5 to 7). Mutations in the distal GRE4, and two of the proximal GREs, GRE2 and -3 (GRE2/3/4m), disable all the major binding sites on the Nuc-B family. Surprisingly, chromatin reconstituted with this mutant also bound GR, resulting in a retarded complex, although this shift was not as large as with wild-type or GRE5/6m chromatin (Fig. 2, lanes 8 to 10). Finally, GRE2/3/4/5/6m chromatin was unable to bind GR in this concentration range (Fig. 2, lanes 11 to 13). GR also interacted specifically with 200-bp mononucleosomes and naked DNA formed from the Nuc-B region (−40 to −250) and the upstream Nuc-C region (−250 to −440), resulting in mobility-shifted species in 5% polyacrylamide gels (data not shown). However, compared to the Nuc-B region, 10-fold higher GR concentrations were required to cause a mobility shift with Nuc-C region sequences.
FIG. 2.
GR binding to wild-type or GRE mutant MMTV chromatin fragments containing 9 to 10 nucleosomes analyzed by electrophoretic mobility shift in 1% agarose gels. Mobility shift of chromatin on 1% agarose gels. GRE mutants are as described in Table 1. GR concentrations for each chromatin fragment were 0 (lanes 2, 5, 8, and 11), 0.3 (lanes 3, 6, 9, and 12), and 0.5 nM (lanes 4, 7, 10, and 13). Chromatin concentration was 1 ng/μl. Lane 1 contains the DNA fragment alone as marker.
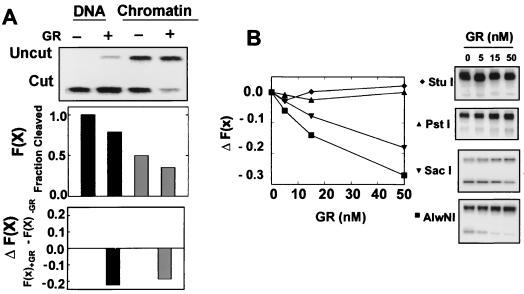
Restriction enzyme access has been extensively used to monitor the MMTV chromatin transition in the presence of dexamethasone (14, 15, 18–20, 34, 45). Accessibility is monitored by the fractional cleavage [F(x)], which is the amount of material cleaved by the enzyme divided by the total amount of material. Reconstituting MMTV DNA into chromatin results in a 50% loss of SacI access to the template (Fig. 3A, top graph). The change in fractional cleavage, ΔF(x), was found to be highly effective in the detailed mapping of the region of the chromatin transition in vivo (15). We also found this relationship to be useful for these studies. The effect of GR on MMTV naked DNA or chromatin was determined by monitoring the ΔF(x) in the presence or absence of GR (Fig. 3A). Addition of GR resulted in a loss of SacI (located between GRE2 and GRE3) access revealed by a drop in cleavage of DNA from 100 to 80% and digestion of chromatin from 50 to 30%. This corresponds to a ΔF(x) of −0.22 and −0.18 for DNA and chromatin, respectively, with GR addition (Fig. 3A, bottom graph). Both SacI (located between GRE2 and -3) and AlwNI (located in GRE6) cleavage decreased with increasing concentration of GR from 5 to 50 nM (Fig. 3B). FokI (located near GRE4) cleavage was similarly affected (data not shown). Enzyme cleavage sites located upstream of the GREs, such as StuI and PstI, were unaffected by the presence of GR. Moreover, mutation of GREs at or near a specific enzyme site resulted in a recovery of accessibility. For example, accessibility of SacI to GRE2/3m and GRE2/3/4/5/6m chromatin was unaffected by increasing concentrations of GR (Fig. 4A). However, GR still had an effect on the accessibility of SacI in the GRE4m chromatin. Similarly, mutation of GRE5 and -6 restored accessibility of chromatin to AlwNI, whereas GRE2/3m and GRE4m were still affected by GR (Fig. 4B). These data demonstrate that static binding of GR to HREs in reconstituted MMTV LTR chromatin prevents a restriction enzyme from gaining access to a recognition site located at the same position, almost certainly by steric hindrance. Together with the mobility shift results, these experiments demonstrate a significant contribution of the upstream GRE5 and -6 to association of GR with MMTV chromatin.
FIG. 3.
Restriction enzyme accessibility in the presence of GR. (A) GR induces a reduction in SacI cleavage [a reduction in fractional cleavage, F(x)] in both naked DNA and reconstituted chromatin. The change in fractional cleavage in the presence of GR (50 nM), ΔF(x), was determined by subtracting the F(x) in the presence of GR from the F(x) in the absence of GR. (B) GR induces a loss in fractional cleavage, F(x), for SacI (1 U) and AlwNI (10 U), but not StuI (10 U) and PstI (2 U). GR concentrations from left to right on all gels were 0, 5, 15, and 50 nM. Chromatin concentration was 1 ng/μl.
FIG. 4.
GR-dependent SacI (C) and AlwNI (D) ΔF(x) of chromatin with mutant GREs. See Table 1 for description of GRE mutants. GR concentrations from left to right on all gels were 0, 5, 15, and 50 nM. Chromatin concentration was 1 ng/μl.
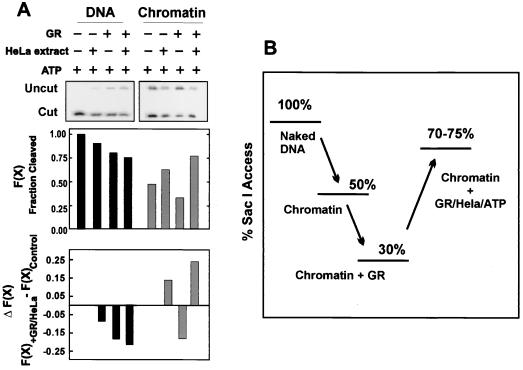
A GR-specific chromatin transition occurs with HeLa nuclear extracts and ATP.
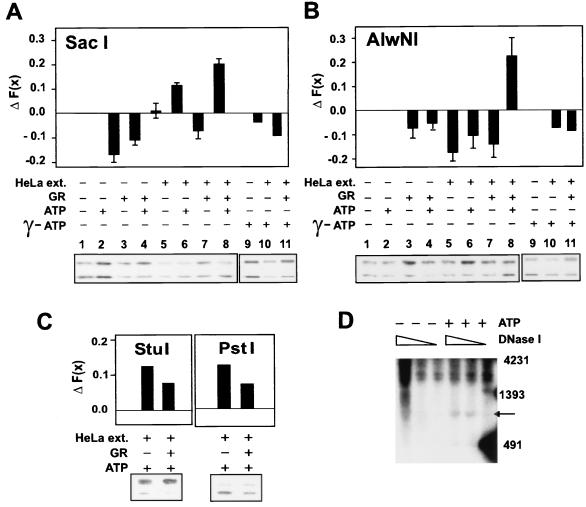
As shown in Fig. 3, addition of GR alone resulted in a decrease in accessibility [−ΔF(x)] of SacI to its site in both DNA and chromatin. This was true even in the presence of ATP (Fig. 5A). Since GR alone did not cause an increase in SacI or AlwNI cleavage, a nuclear extract from HeLa cells was added to the reaction to provide potential factors required for chromatin remodeling. The presence of either HeLa extract alone or GR plus HeLa extract blocked SacI access to its site on a naked DNA template, resulting in ΔF(x)'s of −0.1 and −0.2, respectively. However, an increase in SacI access to its site on a chromatin template was observed in the presence of HeLa plus ATP [+0.15 ΔF(x)]. This effect was potentiated by GR [ΔF(x) of 0.25]. As mentioned in the last section, reconstituting nucleosomes onto DNA resulted in a structure that is 50% less accessible to SacI (Fig. 5B). The presence of GR on the template resulted in another 20% loss of access. A similar level of access was also observed with GR and HeLa extract, indicating that GR was still on the template (see Fig. 6A, lane 7) (discussed in next section). However, the chromatin in the presence of GR, HeLa nuclear extract, and ATP was accessible midway between that of naked DNA and fully reconstituted chromatin (75% access). A similar change in fractional cleavage was obtained when purified SWI-SNF is recruited by GAL4-VP16 to 5S nucleosomal arrays containing the GAL4 element (52). Interestingly, this is also the same ΔF(x) achieved in vivo with dexamethasone treatment (15).
FIG. 5.
GR-specific SacI hypersensitivity on MMTV chromatin but not naked DNA requires HeLa nuclear extract and ATP (A). The change in fractional cleavage, ΔF(x), was determined by subtracting the fractional cleavage [F(x)] in the absence of GR and ATP or GR, HeLa extract, and ATP from the control (ATP alone). Reaction mixtures contained 1 ng of chromatin per μl, 0 or 10 nM GR, 0 or 0.075 mg of HeLa nuclear extract per ml, and 0 or 1 mM ATP. A comparison of SacI accessibilities of MMTV naked DNA, reconstituted chromatin, chromatin in the presence of GR, and chromatin in the presence of GR, HeLa extract, and ATP (B).
FIG. 6.
A GR-specific chromatin transition occurs at SacI (Nuc-B) and AlwNI (Nuc-C), not PstI nor StuI sites, and requires ATP hydrolysis. The ΔF(x) is presented for restriction enzyme access at SacI (A) and AlwNI (B). Reaction mixtures contained 1 ng of chromatin per μl, 0 or 10 nM GR, 0 or 0.075 mg of HeLa nuclear extract per ml, and 0 or 1 mM ATP. Effect of replacing ATP (A and B, lanes 1 to 8) with [γ-S]-ATP (A and B, lanes 9 to 11). Error bars represent the standard error of five independent determinations. GR-specific chromatin remodeling does not occur at StuI and PstI (C) sites. DNase I hypersensitivity induced by HeLa nuclear extract, GR, and ATP maps to the same region as determined in vivo (D). Triangles indicate the increased amount of DNase I. Chromatin reactions (100 ng/25 μl) containing GR (0.6 μM), HeLa extract (0.075 mg/ml), NF-1, and 0 (−) or 1 (+) mM ATP were digested with 0, 0.1, and 0.5 U of DNase I, and purified DNA was digested with NcoI, separated in 1.5% agarose gel, and analyzed by Southern blot hybridization as described in Materials and Methods. DNA size markers were prepared by end-labeling of SacI- and EcoRI-digested fragments of pGEM3zf(−)-LTRCAT plasmid; the 4,231-, 1,393-, and 481-bp fragments are indicated. The arrow indicates the DNase I-hypersensitive site, which is located precisely at the Nuc-B and -C region of MMTV LTR promoter.
Chromatin remodeling extends beyond the Nuc-B region.
The region of in vitro chromatin remodeling was further characterized by restriction enzyme accessibility. Addition of HeLa nuclear extract (1 to 3 μg) alone to reconstituted chromatin did not affect accessibility of SacI (Fig. 6A, compare lanes 1 and 5). However, addition of both HeLa extract and ATP resulted in a ΔF(x) of about 0.1 (compare lanes 5 and 6). The decrease in SacI access in the presence of GR and HeLa extract in the absence of ATP compared to HeLa alone indicated the continued presence of GR on the template (compare lanes 5 and 7). However, the presence of GR, HeLa extract, and ATP resulted in an increase in accessibility, shown by a ΔF(x) of 0.2 (compare lanes 2 and 8). Interestingly, this is the same ΔF(x) achieved in vivo with dexamethasone treatment (15). In contrast to the results for SacI, the presence of HeLa nuclear extract caused a decrease in AlwNI accessibility (Fig. 6B, lane 5 compared to lane 1) that was not restored by ATP (lane 6 compared to lane 2). A slight decrease in AlwNI accessibility was also observed when HeLa nuclear extract was added to naked DNA (data not shown). This result was not attributable to an effect of HeLa extract on the enzyme itself, since AlwNI accessibility was unaffected by HeLa extract on a reconstituted chromatin fragment containing an AlwNI site from another region of the plasmid (data not shown). Although HeLa extract alone caused a decrease in AlwNI accessibility, the presence of GR, HeLa nuclear extract, and ATP resulted in a large increase [ΔF(x) of 0.2; lane 8 compared to lane 7], demonstrating that chromatin remodeling extends beyond the Nuc-B region. The SacI and AlwNI hypersensitivity required ATP hydrolysis since no increase in accessibility was observed when ATP was replaced with [γ-S]-ATP (Fig. 6A and B, compare lanes 10 and 11 to lane 9). Enzymes acting further upstream did not display a GR-dependent hypersensitivity (Fig. 6C). Chromatin was more accessible to both StuI and PstI in the presence of HeLa and ATP, suggesting that this non-GR-dependent chromatin remodeling is not restricted to SacI and AlwNI (Fig. 6C). However, a further increase in access with GR was not observed, demonstrating that the GR-specific nuclease hypersensitivity is localized to the Nuc-B and Nuc-C regions.
A GR-specific and ATP-dependent DNase I hypersensitivity was also observed. Hormone activation of the MMTV LTR in vivo results in DNase I hypersensitivity in the Nuc-B region (38, 39, 53). The presence of GR, HeLa nuclear extract, and ATP produced a DNase I-hypersensitive region centering at −170 from the transcription start site (Fig. 6D). Thus, DNase I hypersensitivity generated in vitro with GR in the presence of ATP and the HeLa nuclear extract maps to the same region (−162 from the start site) characterized in vivo (38).
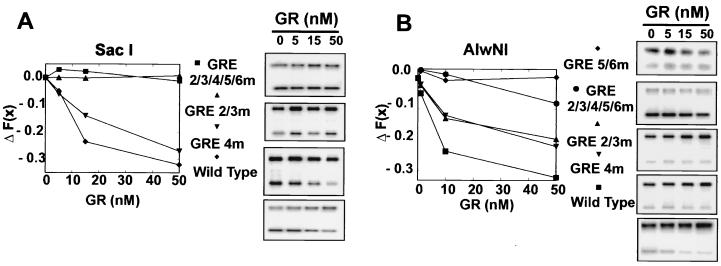
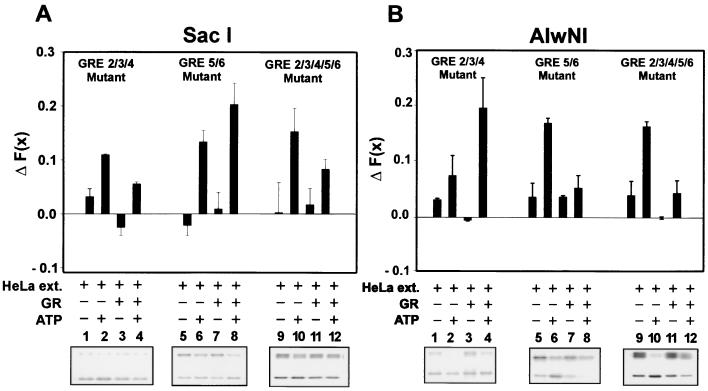
GR-dependent chromatin remodeling of a specific region requires GREs in that region.
Since mutation of the proximal GREs near the SacI site resulted in loss of GR binding in that region (Fig. 4), these mutants were analyzed to investigate the relationship between GREs and local chromatin remodeling. The GR-dependent SacI hypersensitivity observed in the presence of HeLa and ATP was abolished when chromatin contained the GRE2, -3, and -4 (Fig. 7A, GRE 2/3/4 mutant, compare lanes 2 and 4) mutants alone, or in concert with GRE5 and -6 (Fig. 7, GRE 2/3/4/5/6 mutants, compare lanes 10 and 12). However, hypersensitivity at the SacI site is still present in the GRE5/6m chromatin (compare 6 and 8). Similarly, GR-dependent AlwNI hypersensitivity was reduced by mutation of GRE5 and -6 (Fig. 7B), while GRE2/3/4m chromatin still retained the GR-dependent AlwNI hypersensitivity. It is important to note that in all cases where a GRE has been removed, the HeLa- and ATP-induced enzyme hypersensitivity in that region was reduced upon addition of GR. This effect was also observed at the StuI and PstI sites (Fig. 6C). Thus, the presence of an intact set of GR binding sites is required for GR-dependent enzyme access both in the Nuc-B region (SacI) and in the upstream region (AlwNI).
FIG. 7.
GR-dependent chromatin remodeling requires the presence of GREs. ΔF(x) cleavage values are shown for SacI (A) and AlwNI (B) for chromatin assembled with templates mutant at each of the GRE sites. Reaction conditions were as indicated in the legend of Fig. 6. See Table 1 for description of GRE mutants. Error bars represent the standard error of three independent determinations.
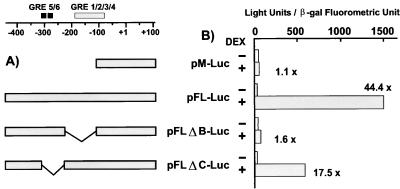
Mutation of upstream GREs results in substantial loss in hormone induction of transcription in mouse mammary 34i and NIH 3T3 cells.
To determine the potential function of upstream GRE5 and -6 in hormone stimulation of transcription, MMTV-luc plasmids containing wild-type LTR sequences, proximal and distal GRE deletion mutants (GRE 2/3/4), or upstream GRE deletion mutants (GRE 5/6) were transfected into 34i and NIH 3T3 cells, and the response to hormone induction was determined (Fig. 8). The parental vector used for the constructions, pM-Luc, contains GRE1 and -2 but is not hormone inducible (31). In addition to the parental vector, the constructs tested were pFL-Luc (containing the full-length wild-type LTR), pFLΔB-Luc (lacking GRE sites 3 and 4 in the HRE [−110 to −229 deletion]), and pFLΔC-Luc (lacking putative GRE sites 5 and 6 in the HRE, from −230 to −309). As expected, the parental vector was not hormone inducible (pM-Luc), but reconstruction of the LTR results in an approximate 44-fold induction (pFL-Luc). Deletion of the GREs in Nuc-B, GRE3 and -4 (construct pFLΔB-Luc), resulted in a complete loss of hormone inducibility. In addition, deleting a region containing GRE5 and -6 (pFLΔC-Luc) reduced the level of inducibility by 50%. The data are consistent with GR binding and hypersensitivity described above. It is important to note that although hormone induction of transcription is observed with these transiently transfected plasmids, they do not adopt the regularly spaced nucleosomal arrays observed in cells containing stably replicating MMTV chromatin (2). Nevertheless, these results clearly show that the upstream GREs are not only important for GR binding and the chromatin transition in vitro, but that this region is critical for full transcriptional activation by GR in vivo.
FIG. 8.
Transient transfection assay for functional elements upstream of the B-region GREs. (A) Sequence organization of LTR-luciferase constructions in the Nuc-B and -C region. Shown are the B-region GREs (gray bar) and two binding sites for GR in the 3′ region of the Nuc-C family (black bars) (33). MMTV sequences present in the pM-Luc, pFL-Luc, pFLΔB-Luc, and pFLΔC-Luc constructions are schematized. (B) Activity for each of the constructs is presented with or without the addition of dexamethasone. Luciferase expression from the LTR constructions is shown normalized to the expression of a cotransfected simian virus 40–β-galactosidase plasmid.
DISCUSSION
Binding of GR to reconstituted nucleosomal arrays.
A purified system has allowed us to demonstrate specific GR binding to a chromatin fragment containing the complete MMTV promoter. A complete analysis of each of the six independent GR binding sites indicates that the putative upstream GRE5 and -6 play a significant role in binding of GR to chromatin. In addition, steric hindrance caused by GR binding to GREs resulted in a reduction in accessibility of local restriction enzymes to their respective cleavage sites. This phenomenon is not observed in vivo and provides preliminary evidence for a previously undetected intermediate state in GR-induced chromatin remodeling (see below). In agreement with the electrophoretic mobility shift analysis, results from the enzyme access assay demonstrate that GR binds to GRE5 and -6. Further studies should establish if cooperativity exists between the Nuc-B GREs and those located upstream, either in terms of chromatin GR binding or chromatin remodeling.
It has been shown that the nucleosomal structure is altered through interactions with GR (35, 36). These alterations are likely due to DNA conformational changes (12, 13, 26) and changes in histone-DNA contacts upon GR binding. The chromatin fragment utilized in these experiments contains approximately 3 × 106 Da in nucleoprotein mass. It is difficult to explain the large-scale mobility shifts observed with such a massive structure purely in terms of a molecular weight change. Thus, the mobility shifts of MMTV chromatin in agarose gels caused by GR may indicate that a significant chromatin conformational change occurs upon GR binding. The system described here now provides the opportunity to study in detail the potential role of higher-order, internucleosomal, interactions in chromatin transitions induced by the GR.
GR-dependent remodeling of MMTV chromatin in vitro is not restricted to the Nuc-B region.
In findings remarkably similar to those recently observed in vivo (15), we show that the GR-dependent chromatin remodeling extends beyond the Nuc-B region, into the Nuc-C family. Regulation of the MMTV promoter has been shown to be dependent on a variety of sequences upstream of the distal GRE (6, 7, 17, 23, 24, 29). MMTV constructs containing deletion mutants in the region that includes the putative GRE5 and -6 exhibited a significant loss in hormone-dependent activation in NIH 3T3 cells (17). However, this deletion encompassed a larger region than the mutation in Fig. 8. In addition, a negative regulatory element has been described in the vicinity of the putative GRE5. A complex containing the AT sequence binding protein, SATB1, was found to interact with this sequence, resulting in repression of basal transcription (24).
The finding that the hormone-induced nuclease hypersensitive region extended beyond Nuc-B in vivo suggested two models (15). The extension of remodeling beyond a single nucleosome could potentially result from an alteration in chromatin higher-order structure, that is, the disruption of internucleosomal structures, as opposed to single-nucleosome events. Alternatively, the extended hypersensitive region could reflect the binding of factors both upstream and within the Nuc-B region. The results shown in Fig. 5 and 6 show that the upstream GR binding sites are indeed involved in the extended transition. That is, nuclease hypersensitivity of a particular region requires the presence of GREs in that region. GR-dependent AlwNI hypersensitivity requires GRE5 and -6, and SacI hypersensitivity requires GRE2, -3, and -4. In addition, mutation of GRE2, -3, and -4 does not appear to greatly affect AlwNI hypersensitivity, nor does mutation of GRE5 and -6 significantly diminish SacI hypersensitivity. These findings clearly support a model in which the extent of the remodeled region is dependent on the location of the cis-acting regulatory elements, as opposed to a unique underlying chromatin structure. It should now be possible to critically address the potential participation of internucleosomal, or multinucleosome, structures in the chromatin transition state.
Possible mechanisms for GR-dependent remodeling of MMTV chromatin.
There is substantial in vivo and in vitro evidence for targeted chromatin reorganization by transcriptional activator recruitment of remodeling complexes to the template. Our results demonstrate that GR alone does not cause an increase in nuclease accessibility. The synergistic increase in both SacI and AlwNI cleavage observed with GR-HeLa nuclear extract-ATP, compared to the combination of GR and HeLa extract, or HeLa extract and ATP, is likely due to recruitment of remodeling factors by GR to MMTV chromatin. As described above, recruitment to a specific region requires the presence of GREs. This is demonstrated by our finding that there is decreased nuclease access in the presence of GR-HeLa-ATP compared to HeLa-ATP when there are no GREs present (compare StuI and PstI [Fig. 6C], SacI for GRE2/3/4m and GRE2/3/4/5/6m [Fig. 7A], and AlwNI for GRE5/6m and GRE2/3/4/5/6m [Fig. 7B]). A likely explanation for these results is that GR in solution and chromatin compete for binding with the chromatin remodeling machinery in the absence of a GRE.
Which of the many described chromatin-remodeling complexes are involved with GR or steroid receptors? It was recently reported that a Drosophila ISWI-containing complex is capable of facilitating a progesterone receptor-dependent change in DNA topology of minichromosomes and transcriptional activation in vitro (10). Similarly, chromatin remodeling and transcriptional activation by either progesterone receptor or RAR-RXR heterodimers may have been facilitated by ISWI-containing chromatin-remodeling complexes present in the Drosophila chromatin assembly extracts (11, 25). In mammalian cells, a significant amount of evidence suggests that the complexes containing BRG1 and/or human Brm (hBrm) (components of mammalian SWI-SNF) are involved in interacting with steroid receptors (either directly or indirectly) and potentiating transcription (16, 30). In yeast, another SWI-SNF gene, SWP73 (homolog of human BAF60), potentiated glucocorticoid activation of transcription (5). Also, the presence of rat GR enhanced the ability of purified rat liver SWI-SNF complex to remodel GRE-containing nucleosomes and to facilitate binding of NF-1 in vitro (32). There is increasing evidence that BRG1 and hBrm may be present in a large variety of different complexes that are specific to particular promoters, cell types, or are present in response to specific signal transduction events. The purified in vitro system described here recapitulates precisely the in vivo remodeling event and should now allow a detailed comparison between known remodeling complexes for their effect on GR-specific chromatin remodeling in conjunction with transcriptional activation and a determination of potential specificity with respect to the contribution of each activity.
Transient binding by the GR.
An unexpected finding in the present study is that binding of GR to the Nuc-B–Nuc-C domain blocks access of restriction nucleases to sequences that are coincident with GR recognition sites in the absence of ATP. Action of the remodeling complex with ATP induces an open chromatin configuration with increased access of nuclease probes to the DNA template. The loss of the restriction enzyme blockage is also consistent with ejection of GR from the template. A link between loss of receptor from the template in the presence of extract plus ATP and a chromatin transition is strengthened by the observation that GR, HeLa extract, and ATP block SacI access to a naked DNA template (Fig. 5A). This is compatible with a “hit-and-run” mechanism for receptor action (40) and transcription factors in general (43). These conclusions are also consistent with recent findings from living cell experiments which reveal that the receptor is not statically bound to the LTR in the continued presence of ligand, but rather exchanges at a high rate between the chromatin target and the nucleoplasmic compartment (27). It is expected that the extension of these complimentary approaches, both the in vitro remodeling system, and the real-time living cell approach, should elaborate considerable detail concerning the unexpected dynamics of steroid receptor action.
ACKNOWLEDGMENTS
T.M.F. and B.W.R. contributed equally to this work.
We gratefully acknowledge the assistance of Carl Wu, Raphael Sandaltzopoulos, and Ju-Gyeong Kang, who generously provided the Drosophila embryos for preparation of the assembly extracts used throughout this work. We also thank Ronald Wolford for technical assistance in preparation of GRE5/6m DNAs.
REFERENCES
- 1.Archer T K, Cordingley M G, Wolford R G, Hager G L. Transcription factor access is mediated by accurately positioned nucleosomes on the mouse mammary tumor virus promoter. Mol Cell Biol. 1991;11:688–698. doi: 10.1128/mcb.11.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer T K, Lefebvre P, Wolford R G, Hager G L. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science. 1992;255:1573–1576. doi: 10.1126/science.1347958. [DOI] [PubMed] [Google Scholar]
- 3.Becker P B, Wu C. Cell-free system for assembly of transcriptionally repressed chromatin from Drosophila embryos. Mol Cell Biol. 1992;12:2241–2249. doi: 10.1128/mcb.12.5.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomquist P, Li Q, Wrange O. The affinity of nuclear factor 1 for its DNA site is drastically reduced by nucleosome organization irrespective of its rotational or translational position. J Biol Chem. 1996;271:153–159. doi: 10.1074/jbc.271.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Cairns B R, Levinson R S, Yamamoto K R, Kornberg R D. Essential role of Swp73p in the function of yeast Swi/Snf complex. Genes Dev. 1996;10:2131–2144. doi: 10.1101/gad.10.17.2131. [DOI] [PubMed] [Google Scholar]
- 6.Cato A C, Skroch P, Weinmann J, Butkeraitis P, Ponta H. DNA sequences outside the receptor-binding sites differently modulate the responsiveness of the mouse mammary tumor virus to various steroid hormones. EMBO J. 1988;7:1403–1410. doi: 10.1002/j.1460-2075.1988.tb02957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavin C, Buetti E. Tissue-specific and ubiquitous factors binding next to the glucocorticoid receptor modulate transcription from the mouse mammary tumor virus promoter. J Virol. 1995;69:3759–3770. doi: 10.1128/jvi.69.6.3759-3770.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordingley M G, Riegel A T, Hager G L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987;48:261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- 9.Di Croce L, Koop R, Beato M. Rapid purification of intact minichromosomes over a glycerol cushion. Nucleic Acids Res. 1999;27:11. doi: 10.1093/nar/27.16.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Croce L, Koop R, Venditti P, Westphal H M, Nightingale K P, Corona D F, Becker P B, Beato M. Two-step synergism between the progesterone receptor and the DNA-binding domain of nuclear factor 1 on MMTV minichromosomes. Mol Cell. 1999;4:45–54. doi: 10.1016/s1097-2765(00)80186-0. [DOI] [PubMed] [Google Scholar]
- 11.Dilworth F J, Fromental-Ramain C, Remboutsika E, Benecke A, Chambon P. Ligand-dependent activation of transcription in vitro by retinoic acid receptor alpha/retinoid X receptor alpha heterodimers that mimics transactivation by retinoids in vivo. Proc Natl Acad Sci USA. 1999;96:1995–2000. doi: 10.1073/pnas.96.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson M A, Hard T, Nilsson L. Molecular dynamics simulations of the glucocorticoid receptor DNA-binding domain in complex with DNA and free in solution. Biophys J. 1995;68:402–426. doi: 10.1016/S0006-3495(95)80203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson M A, Nilsson L. Structure, thermodynamics and cooperativity of the glucocorticoid receptor DNA-binding domain in complex with different response elements. Molecular dynamics simulation and free energy perturbation studies. J Mol Biol. 1995;253:453–472. doi: 10.1006/jmbi.1995.0566. [DOI] [PubMed] [Google Scholar]
- 14.Fragoso G, John S, Roberts M S, Hager G L. Nucleosome positioning on the MMTV LTR results from the frequency-biased occupancy of multiple frames. Genes Dev. 1995;9:1933–1947. doi: 10.1101/gad.9.15.1933. [DOI] [PubMed] [Google Scholar]
- 15.Fragoso G, Pennie W D, John S, Hager G L. The position and length of the steroid-dependent hypersensitive region in the mouse mammary tumor virus long terminal repeat is invariant despite multiple nucleosome B frames. Mol Cell Biol. 1998;18:3633–3644. doi: 10.1128/mcb.18.6.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryer C J, Archer T K. Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature. 1998;393:88–91. doi: 10.1038/30032. [DOI] [PubMed] [Google Scholar]
- 17.Gouilleux F, Sola B, Couette B, Richard-Foy H. Cooperation between structural elements in hormone-regulated transcription from the mouse mammary tumor virus promoter. Nucleic Acids Res. 1991;19:1563–1569. doi: 10.1093/nar/19.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hager G L, Archer T K, Fragoso G, Bresnick E H, Tsukagoshi Y, John S, Smith C L. Influence of chromatin structure on the binding of transcription factors to DNA. Chromosomes and DNA. Cold Spring Harbor Symp Quant Biol. 1993;58:63–71. doi: 10.1101/sqb.1993.058.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Hager G L, Fragoso G. Analysis of nucleosome positioning in mammalian cells. In: Wassarman P M, Wolffe A P, editors. Chromatin. Orlando, Fla: Academic Press; 1999. pp. 626–638. [DOI] [PubMed] [Google Scholar]
- 20.Hager G L, Smith C L, Svaren J, Horz W. Initiation of expression: remodelling genes. In: Elgin S C R, editor. Chromatin structure and gene expression. Oxford, United Kingdom: Oxford University Press; 1994. pp. 89–103. [Google Scholar]
- 21.Hirst M A, Northrop J P, Danielsen M, Ringold G M. High level expression of wild type and variant mouse glucocorticoid receptors in Chinese hamster ovary cells. Mol Endocrinol. 1990;4:162–170. doi: 10.1210/mend-4-1-162. [DOI] [PubMed] [Google Scholar]
- 22.Lee H-L, Archer T K. Nucleosome mediated disruption of transcription factor: chromatin initiation complexes at the mouse mammary tumor virus long terminal repeat in vivo. Mol Cell Biol. 1994;14:32–41. doi: 10.1128/mcb.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Ricousse S, Gouilleux F, Fortin D, Joulin V, Richard-Foy H. Glucocorticoid and progestin receptors are differently involved in the cooperation with a structural element of the mouse mammary tumor virus promoter. Proc Natl Acad Sci USA. 1996;93:5072–5077. doi: 10.1073/pnas.93.10.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Bramblett D, Zhu Q, Lozano M, Kobayashi R, Ross S R, Dudley J P. The matrix attachment region-binding protein SATB1 participates in negative regulation of tissue-specific gene expression. Mol Cell Biol. 1997;17:5275–5287. doi: 10.1128/mcb.17.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Wong J, Tsai S Y, Tsai M J, O'Malley B W. Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptor-dependent cell-free transcription of chromatin. Proc Natl Acad Sci USA. 1999;96:9485–9490. doi: 10.1073/pnas.96.17.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luisi B F, Xu W X, Otwinowski Z, Freedman L P, Yamamoto K R, Sigler P B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991;352:497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- 27.McNally J G, Mueller W G, Walker D, Wolford R G, Hager G L. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- 28.Mellentin-Michelotti J, John S, Pennie W D, Williams T, Hager G L. The 5′ enhancer of the MMTV LTR contains a functional AP-2 element. J Biol Chem. 1994;269:31983–31990. [PubMed] [Google Scholar]
- 29.Mink S, Ponta H, Cato A C B. The long terminal repeat region of the mouse mammary tumour virus contains multiple regulatory elements. Nucleic Acids Res. 1990;18:2017–2024. doi: 10.1093/nar/18.8.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muchardt C, Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 1993;12:4279–4290. doi: 10.1002/j.1460-2075.1993.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordeen S K. Luciferase reporter gene vectors for analysis of promoters and enhancers. BioTechniques. 1988;6:454–458. [PubMed] [Google Scholar]
- 32.Ostlund Farrants A K, Blomquist P, Kwon H, Wrange O. Glucocorticoid receptor-glucocorticoid response element binding stimulates nucleosome disruption by the SWI/SNF complex. Mol Cell Biol. 1997;17:895–905. doi: 10.1128/mcb.17.2.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payvar F, DeFranco D, Firestone G L, Edgar B, Wrange O, Okret S, Gustafsson J A, Yamamoto K R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983;35:381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- 34.Pennie W D, Hager G L, Smith C L. Nucleoprotein structure influences the response of the mouse mammary tumor virus promoter to activation of the cAMP signalling pathway. Mol Cell Biol. 1995;15:2125–2134. doi: 10.1128/mcb.15.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlmann T, Eriksson P, Wrange O. Quantitative analysis of the glucocorticoid receptor-DNA interaction at the mouse mammary tumor virus glucocorticoid response element. J Biol Chem. 1990;265:17222–17229. [PubMed] [Google Scholar]
- 36.Perlmann T, Wrange O. Specific glucocorticoid receptor binding to DNA reconstituted in a nucleosome. EMBO J. 1988;7:3073–3079. doi: 10.1002/j.1460-2075.1988.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pina B, Brüggemeier U, Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990;60:719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- 38.Richard-Foy H, Hager G L. Sequence specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J. 1987;6:2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richard-Foy H, Sistare F D, Riegel A T, Simons S S, Jr, Hager G L. Mechanism of dexamethasone 21-mesylate antiglucocorticoid action. II. Receptor-antiglucocorticoid complexes are unable to interact productively with MMTV LTR chromatin in vivo. Mol Endocrinol. 1987;1:659–665. doi: 10.1210/mend-1-9-659. [DOI] [PubMed] [Google Scholar]
- 40.Rigaud G, Roux J, Pictet R, Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991;67:977–986. doi: 10.1016/0092-8674(91)90370-e. [DOI] [PubMed] [Google Scholar]
- 41.Ross S R, Hsu C-L L, Choi Y, Mok E, Dudley J P. Negative regulation in correct tissue-specific expression of mouse mammary tumor virus in transgenic mice. Mol Cell Biol. 1990;10:5822–5829. doi: 10.1128/mcb.10.11.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandaltzopoulos R, Blank T, Becker P B. Transcriptional repression by nucleosomes but not H1 in reconstituted preblastoderm Drosophila chromatin. EMBO J. 1994;13:373–379. doi: 10.1002/j.1460-2075.1994.tb06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaffner W. Gene regulation. A hit-and-run mechanism for transcriptional activation? Nature. 1988;336:427–428. doi: 10.1038/336427a0. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro D J, Sharp P A, Wahli W W, Keller M J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988;7:47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- 45.Smith C L, Hager G L. Transcriptional regulation of mammalian genes in vivo: a tale of two templates. J Biol Chem. 1997;272:27493–27496. doi: 10.1074/jbc.272.44.27493. [DOI] [PubMed] [Google Scholar]
- 46.Truss M, Bartsch J, Hache R S, Beato M. Chromatin structure modulates transcription factor binding to the mouse mammary tumor virus (MMTV) promoter. J Steroid Biochem Mol Biol. 1993;47:1–10. doi: 10.1016/0960-0760(93)90051-w. [DOI] [PubMed] [Google Scholar]
- 47.Truss M, Bartsch J, Schelbert A, Hache R J, Beato M. Hormone induces binding of receptors and transcription factors to a rearranged nucleosome on the MMTV promoter in vivo. EMBO J. 1995;14:1737–1751. doi: 10.1002/j.1460-2075.1995.tb07163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsukiyama T, Wu C. Purification and properties of an ATP-dependent nucleosome remodeling factor. Cell. 1995;83:1011–1020. doi: 10.1016/0092-8674(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 49.Varga-Weisz P D, Wilm M, Bonte E, Dumas K, Mann M, Becker P B. Chromatin-remodelling factor CHRAC contains the ATPases ISWI and topoisomerase II. Nature. 1997;388:598–602. doi: 10.1038/41587. . (Erratum, 389:1003.) [DOI] [PubMed] [Google Scholar]
- 50.Wall G, Varga-Weisz P D, Sandaltzopoulos R, Becker P B. Chromatin remodeling by GAGA factor and heat shock factor at the hypersensitive Drosophila hsp26 promoter in vitro. EMBO J. 1995;14:1727–1736. doi: 10.1002/j.1460-2075.1995.tb07162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warren B S, Kusk P, Wolford R G, Hager G L. Purification and stabilization of transcriptionally active glucocorticoid receptor. J Biol Chem. 1996;271:11434–11440. doi: 10.1074/jbc.271.19.11434. [DOI] [PubMed] [Google Scholar]
- 52.Yudkovsky N, Logie C, Hahn S, Peterson C L. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 1999;13:2369–2374. doi: 10.1101/gad.13.18.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaret K S, Yamamoto K R. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984;38:29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]