Abstract
Latent sensitization (LS) of pain engages pronociceptive signaling pathways in the dorsal horn that include NMDA receptor (NMDAR)→adenylyl cyclase-1 (AC1)→protein kinase A (PKA), and exchange proteins directly activated by cyclic AMP (Epacs). To determine whether these pathways operate similarly between males and females or are under the inhibitory control of spinal κ opioid receptors (KOR), we allowed hyperalgesia to resolve after plantar incision and then blocked KOR with intrathecal administration of LY2456302, which reinstated hyperalgesia and facilitated touch-evoked immunoreactivity of phosphorylated extracellular signal-regulated kinase (pERK) in neurons (NeuN) but not astrocytes (GFAPs) nor microglia (Iba1). LY2456302 reinstated hyperalgesia even when administered 13 months later, indicating that chronic postoperative pain vulnerability persists for over a year in a latent state of remission. In both sexes, intrathecal MK-801 (an NMDAR competitive antagonist) prevented LY2456302-evoked reinstatement of hyperalgesia as did AC1 gene deletion or the AC1 inhibitor NB001. NB001 also prevented stimulus-evoked pERK. In both sexes, the Epac inhibitor ESI-09 prevented reinstatement, whereas the Epac activator 8-CPT reinstated hyperalgesia. By contrast, the PKA inhibitor H89 prevented reinstatement only in male mice, whereas the PKA activator 6-bnz-cAMP itself evoked reinstatement at all doses tested (3–30 nmol, i.t.). In neither sex did incision change gene expression of KOR, GluN1, PKA, or Epac1 in dorsal horn. We conclude that sustained KOR signaling inhibits spinal PKA-dependent mechanisms that drive postoperative LS in a sex-dependent manner. Our findings support the development of AC1, PKA, and Epac inhibitors toward a new pharmacotherapy for chronic postoperative pain.
SIGNIFICANCE STATEMENT Because of neural mechanisms that are not well understood, men and women respond differently to treatments for chronic pain. We report that surgical incision recruits a pronociceptive latent pain sensitization that persisted for over a year and was kept in check by the sustained analgesic activity of κ opioid receptors. NMDAR→AC1→cAMP→Epac signaling pathways in the dorsal horn of the spinal cord maintain latent sensitization in both males and females; however, only males recruit a PKA-dependent mechanism. This work presents a novel male-specific mechanism for the promotion of chronic postoperative pain.
Keywords: adenylyl cyclase 1, exchange protein activated by cAMP, kappa opioid receptor, latent sensitization, N-methyl-D-aspartate receptor, protein kinase A
Introduction
Chronic postoperative pain develops in millions of patients who undergo surgical procedures (Kehlet et al., 2006), yet there are no safe and effective treatment options. Although opioids remain the mainstay analgesics for acute postoperative pain, prolonged therapy leads to serious adverse effects including dependence, addiction liability, and paradoxical pain. A better understanding of the cellular mechanisms that drive chronic postoperative pain states, and the engagement of opposing endogenous analgesic systems, will lead to the development of novel pharmacotherapies. To this end, we used a murine model of chronic postoperative pain that involves plantar incision of the hindpaw, followed by a waiting period of several weeks. Although hyperalgesia has resolved, a silent, long-lasting latent sensitization of nociceptive neurons in the dorsal horn remains (Solway et al., 2011; Taylor and Corder, 2014). Unlike models of hyperalgesic priming (Kandasamy and Price, 2015; Araldi et al., 2017), latent sensitization (LS) is necessarily tonically opposed, or masked, by compensatory activity of pain-inhibitory G-protein coupled receptors (GPCRs). These GPCRs include µ-opioid receptor constitutive activity (MORCA; Corder et al., 2013), the neuropeptide Y (NPY) Y1 receptor (Fu et al., 2019), and, most recently, the κ opioid receptor (KOR; Custodio-Patsey et al., 2020). LS has been demonstrated in humans (Pereira et al., 2015) and may represent the transition from acute to chronic pain as a latent predisposition to relapse, which might explain the episodic nature and vulnerability to stressors that accompany chronic pain conditions in humans (Reichling and Levine, 2009; Taylor and Corder, 2014, for a comprehensive review of the implications and significance of latent sensitization).
We previously reported that inflammation triggers a pronociceptive NMDA receptor (NMDAR)→adenylyl cyclase-1 (AC1) signaling mechanism of LS that is masked by MORCA or Y1R in male mice (Corder et al., 2013; Fu et al., 2019). Whether this regulatory system operates in female mice and is also under the control of KOR is unknown. Therefore, the first part of the current study was designed to test the hypothesis that surgical incision establishes a long-lasting activation of KOR signaling that masks LS in part through tonic inhibition of NMDA receptor→AC1 signaling in both male and female mice.
AC1-mediated production of cyclic AMP (cAMP) activates multiple downstream receptor targets, including the canonical protein kinase A (PKA) and the relatively recently described family known as exchange protein directly activated by AMP (Epac; Cheng et al., 2008). Epac isoforms 1 and 2 act as guanine nucleotide exchange factors for small GTPases (de Rooij et al., 1998; Bos, 2003), that can activate downstream PKC-dependent pathways (Griffin, 2005; Hucho et al., 2005). Both PKA and Epac contribute to persistent hyperalgesia after inflammation or tissue injury (Wang et al., 2013; Gu et al., 2016; Matsuda et al., 2017; Singhmar et al., 2016, 2018), and we previously reported that inflammation triggers pronociceptive PKA and Epac mechanisms of LS that are masked by Y1R in male mice (Fu et al., 2019). Therefore, the second part of the current study was designed to test the hypothesis that surgical incision establishes a long-lasting activation of KOR signaling that masks LS in part through tonic inhibition of the pronociceptive downstream cAMP targets: PKA and/or Epac.
To address these questions, we performed incisions in male and/or female mice, waited for behavioral signs of hyperalgesia to resolve (21 d), targeted spinal KOR, NMDA, AC1, and cAMP receptors with the intrathecal delivery of pharmacological antagonists (and in the case of AC1, we also used AC1−/− mice), and then evaluated behavioral signs of hyperalgesia and nociceptive sensitization of dorsal horn neurons. The latter was assessed by the immunohistochemical expression of the phosphorylated extracellular-signal-regulated kinase (pERK), a marker of neuronal activation in the dorsal horn. Furthermore, we used quantitative real-time polymerase chain reaction (qPCR) to determine whether peak hyperalgesia and its resolution at day 21 was correlated with the gene expression of KOR, GluN1, AC1, PKA, or Epac1.
Materials and Methods
Animals
Male and female mice, 8–12 weeks of age, were obtained from Charles Rivers Laboratories (C57Bl/6J) or bred in-house (AC1−/− deletion mutant × wild-type littermate AC1+/+ controls, originally provided as a gift from Dan Storm, University of Washington). In accordance with the consensus report of the Sex, Gender, and Pain Special Interest Group of the International Association for the Study of Pain, we used females without considering their stage in the estrous cycle (Greenspan et al., 2007). Animals were housed in a temperature-controlled room on a 14/10 h light/dark cycle and were provided food and water ad libitum. Mice were acclimated to the colony housing room for at least 4 d and then acclimated to handling for 3 min per day on each of 4 d before the initiation of the experiments. All animal-use protocols were approved by the Institutional Animal Care and Use Committee at the University of Kentucky (AC1, PKA, and Epac experiments) and University of Pittsburgh (NMDAR and qPCR experiments). The studies in Figures 4, 6–8, and 10–12 were conducted at the University of Kentucky by L.C.-P., whereas Figures 1–3, 5, 9, and 13 were conducted at the University of Pittsburgh by P.B. and P.P.
Figure 4.
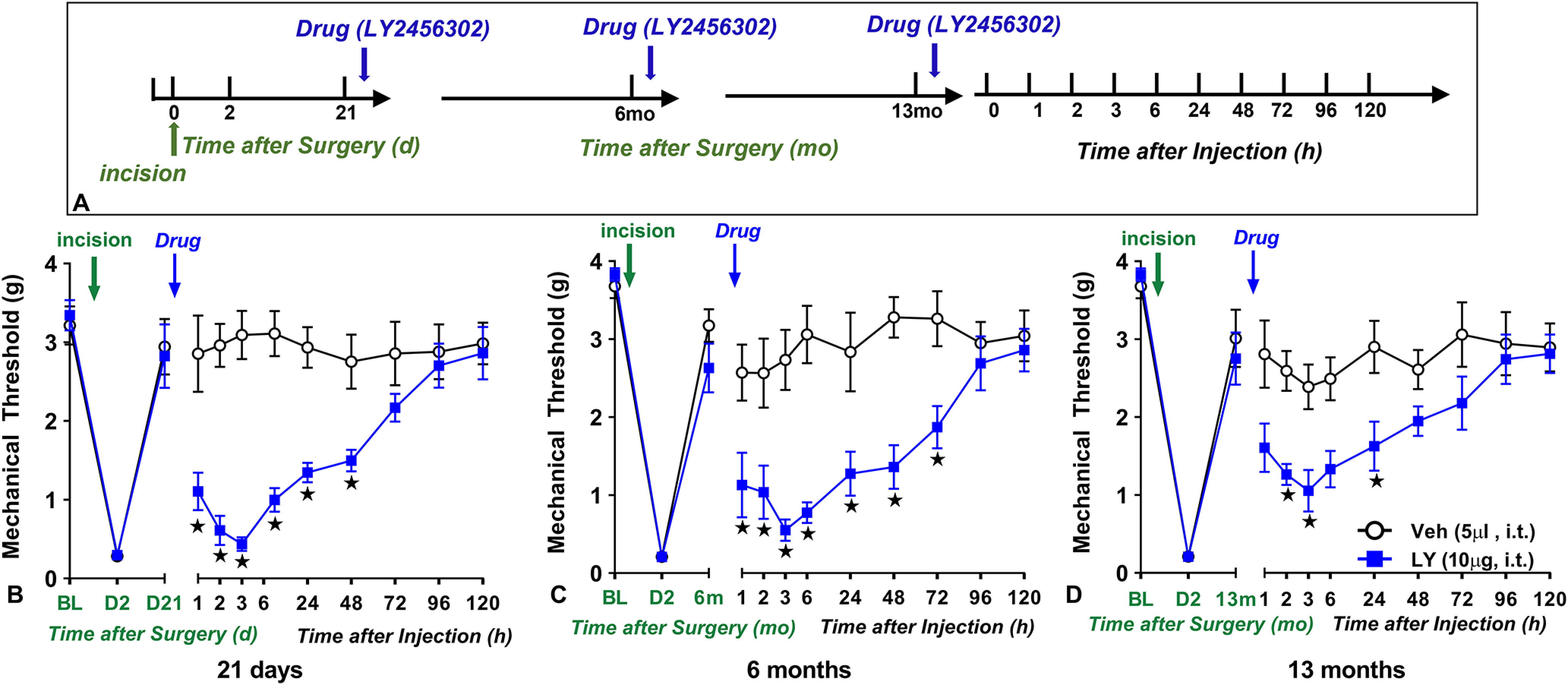
Latent sensitization persists 13 months after surgical incision. A, Timeline of the experiment. B–D, Left, Mechanical thresholds at baseline (BL), 2 d, 21 d (B), 6 months (C), and 13 months (D) after plantar incision in male C57Bl/6 mice. Right, Intrathecal administration of selective KOR antagonist LY2456302 (10 µg) reinstated mechanical hypersensitivity when given 21 d (B), 6 months (C), or 13 months (D) after incision; n = 6–8. BL indicates baseline behavior before incision. *p < 0.05 vehicle versus drug. Data represented as mean ± SEM. Veh, Vehicle; LY, LY2456302.
Figure 6.
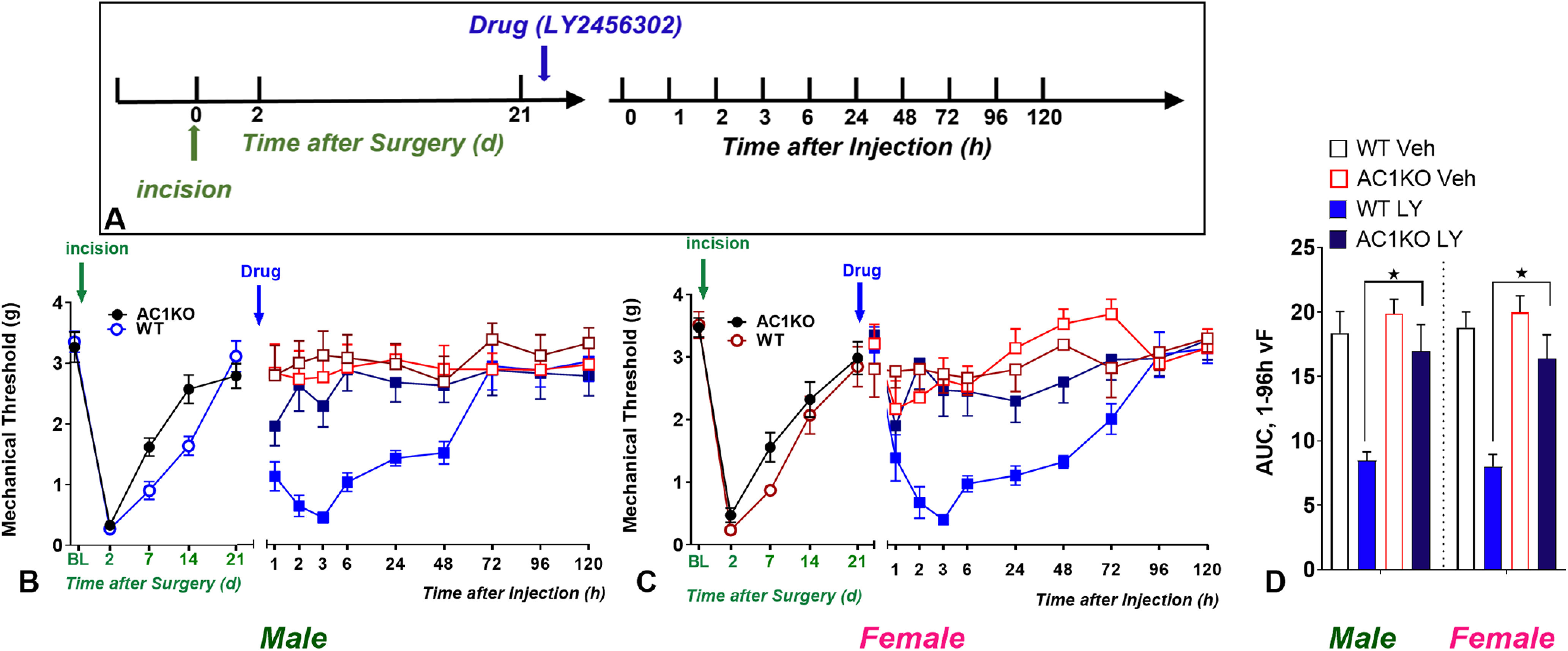
AC-1 gene deletion precludes KOR antagonist-induced postoperative hyperalgesia. A, Timeline of the experiment. B–C, Line graphs describing mechanical thresholds at baseline (BL), 2 d, and 21 d after plantar incision in (B) male and (C) female C57Bl/6 mice. Gene deletion of AC1 accelerates the recovery of mechanical thresholds after surgery in (B) male but not in (C) female C57Bl/6 mice. D, Histograms showing that AC1 gene deletion prevented LY2456302 (10 µg)-induced reinstatement of hyperalgesia in male and female mice. n = 6–8 per group/sex. *p < 0.05. BL indicates baseline behavior before incision. Data represented as mean ± SEM. Veh, Vehicle; LY, LY2456302.
Figure 8.
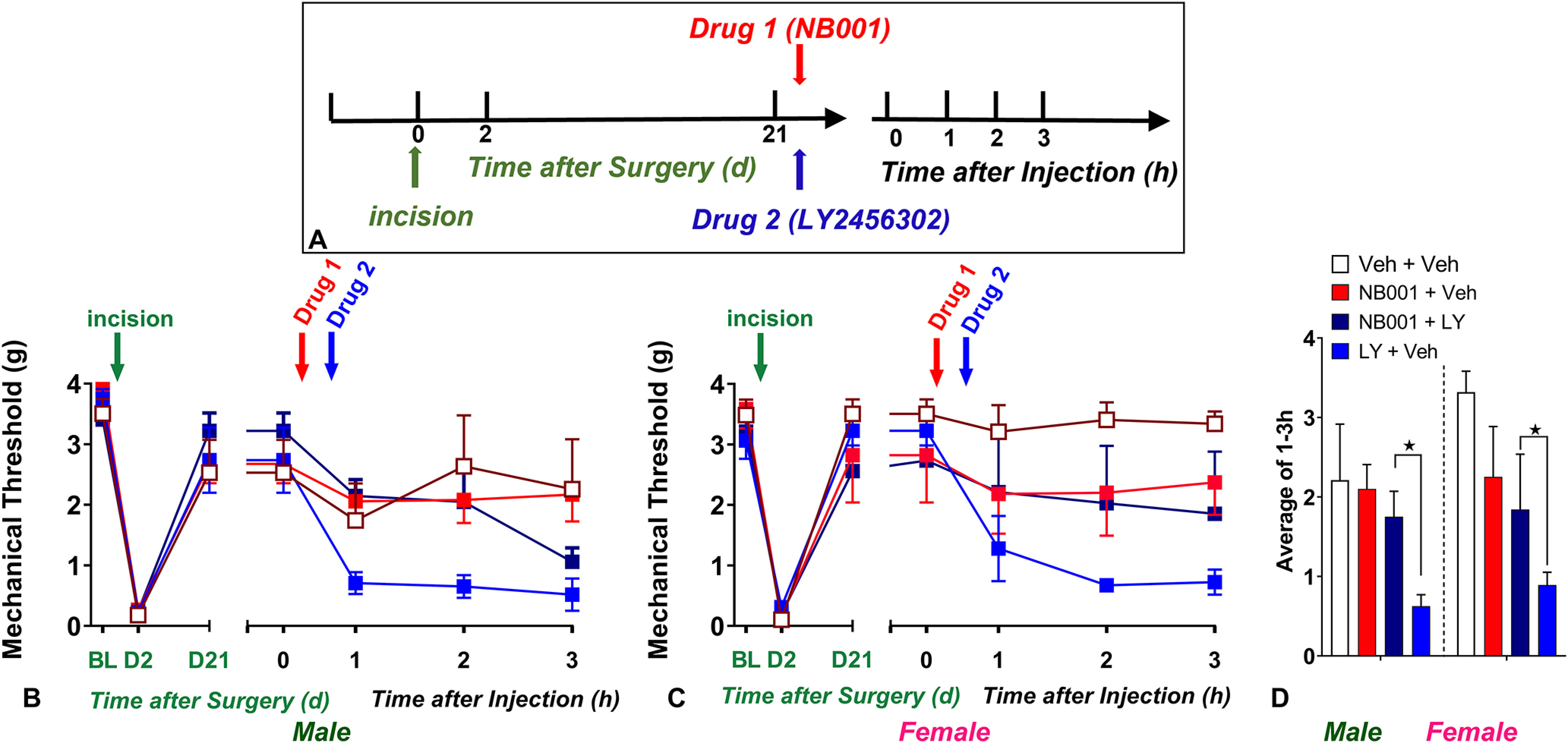
Pharmacological inhibition of AC1 prevented LY2456302-induced reinstatement of postoperative hyperalgesia. A, Timeline of the experiment. B–C, Line graphs describing mechanical thresholds at baseline (BL), 2 d, and 21 d after plantar incision in (B) male and (C) female C57Bl/6 mice. D Histograms showing that preadministration of the AC1 inhibitor NB001 (1.5 µg, i.t.) prevented LY2456302 (10 µg)-induced reinstatement of hyperalgesia in male and female mice; n = 3–5 per group. *p < 0.05. BL indicates baseline behavior before drug administration. Data represented as mean ± SEM. Veh, Vehicle; LY, LY2456302.
Figure 10.
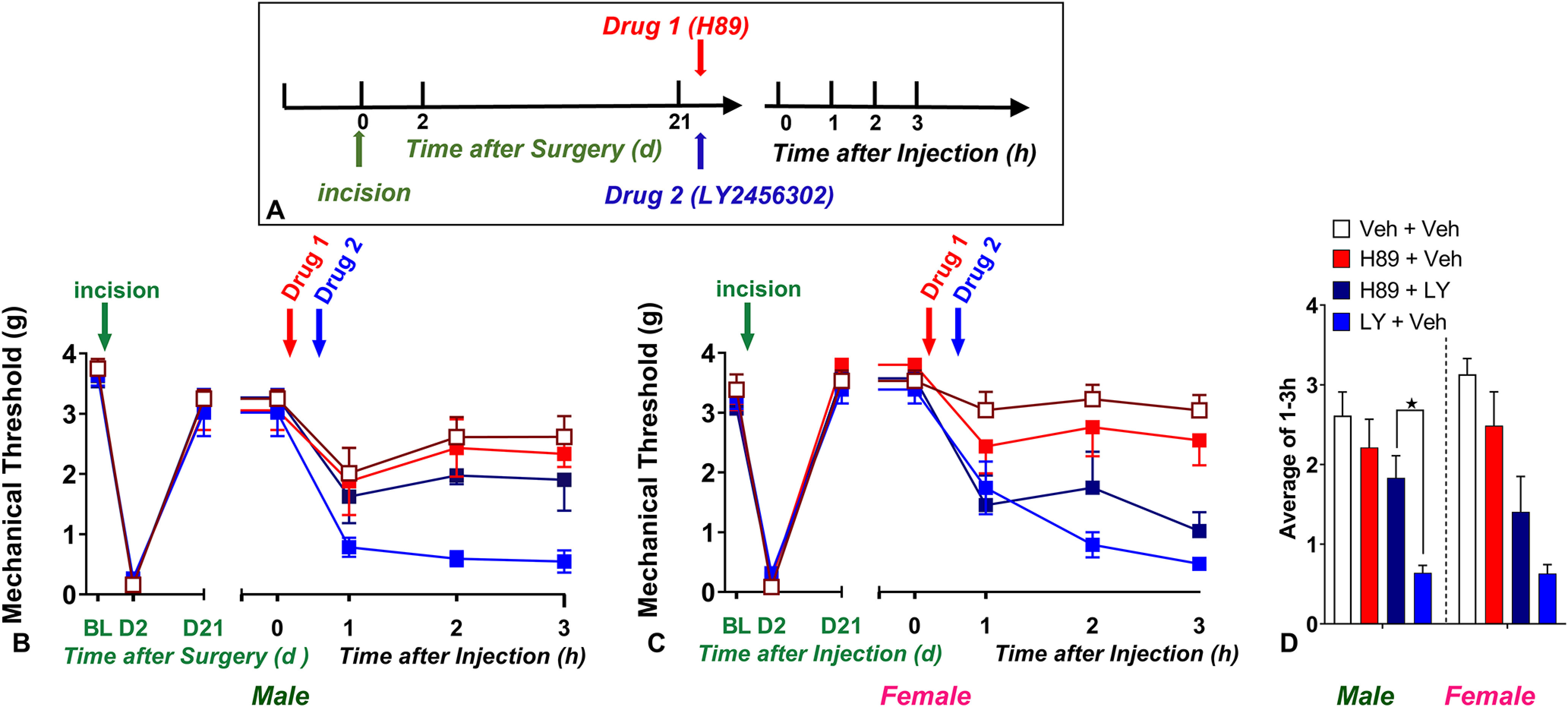
PKA inhibition prevents the reinstatement of KOR-antagonist-induced postoperative hyperalgesia in male but not female mice. A, Timeline of the experiment. B–C, Line graphs describing mechanical thresholds at baseline (BL), 2 d, and 21 d after plantar incision in (B) male and (C) female C57Bl/6 mice. D, Histograms showing that preadministration of the selective PKA inhibitor H89 prevented LY2456302 (10 µg)-induced reinstatement of hyperalgesia in male and female mice; n = 6–12. ★p < 0.05. BL indicates baseline behavior before drug administration. Data represented as mean ± SEM. Veh, Vehicle; LY, LY2456302.
Figure 12.
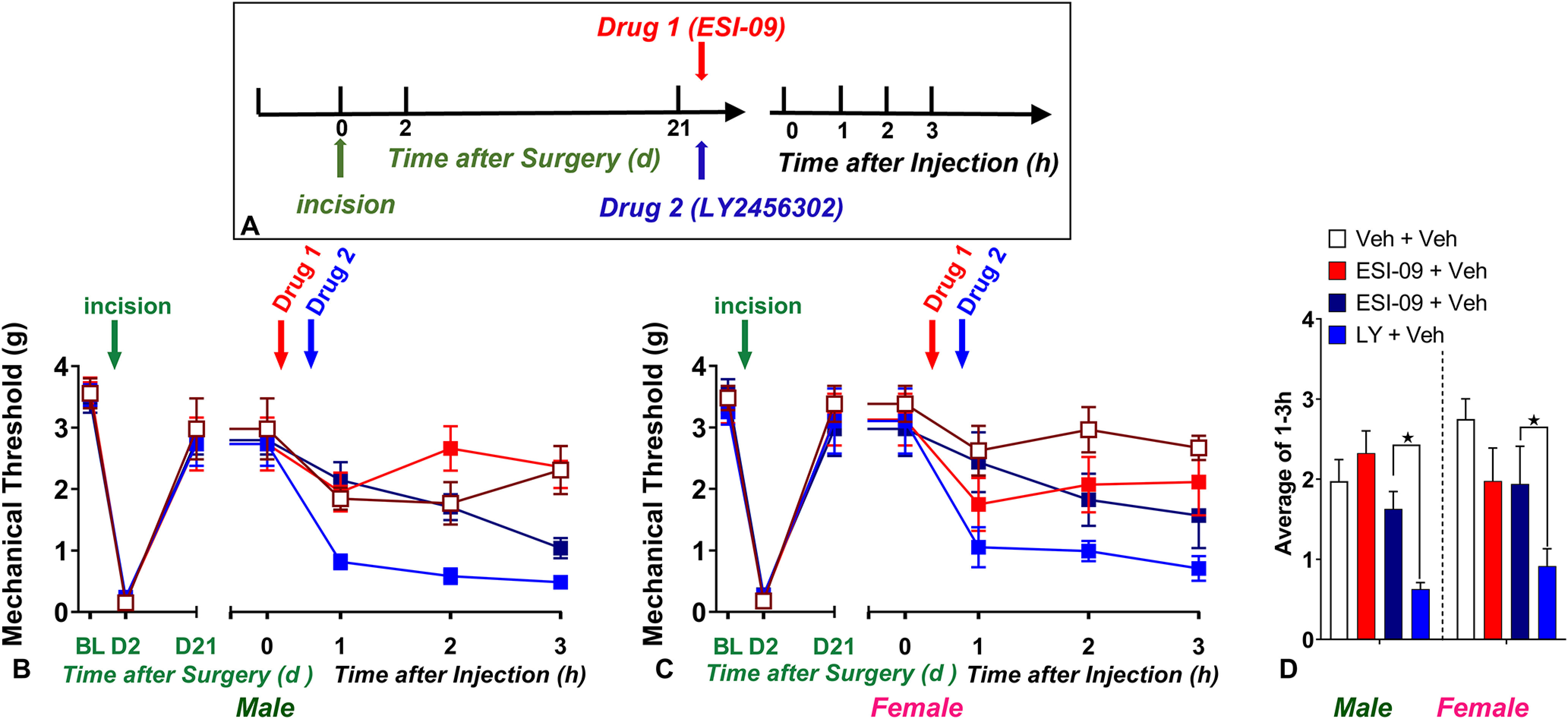
Pharmacological inhibition of Epac1-2 prevents the reinstatement of KOR-antagonist-induced postoperative hyperalgesia. A, Timeline of the experiment. B–C, Line graphs describing mechanical thresholds at baseline (BL), 2 d, and 21 d after plantar incision in (B) male and (C) female C57Bl/6 mice. D, Histograms showing that preadministration of the nonselective Epac inhibitor ESI-09 (10 µg, i.t.) prevented LY2456302 (10 µg)-induced reinstatement of hyperalgesia in male and female mice; n = 6 per group/sex. ★p < 0.05. BL indicates baseline behavior before incision. Data represented as mean ± SEM. Veh, Vehicle; LY, LY2456302.
Figure 1.
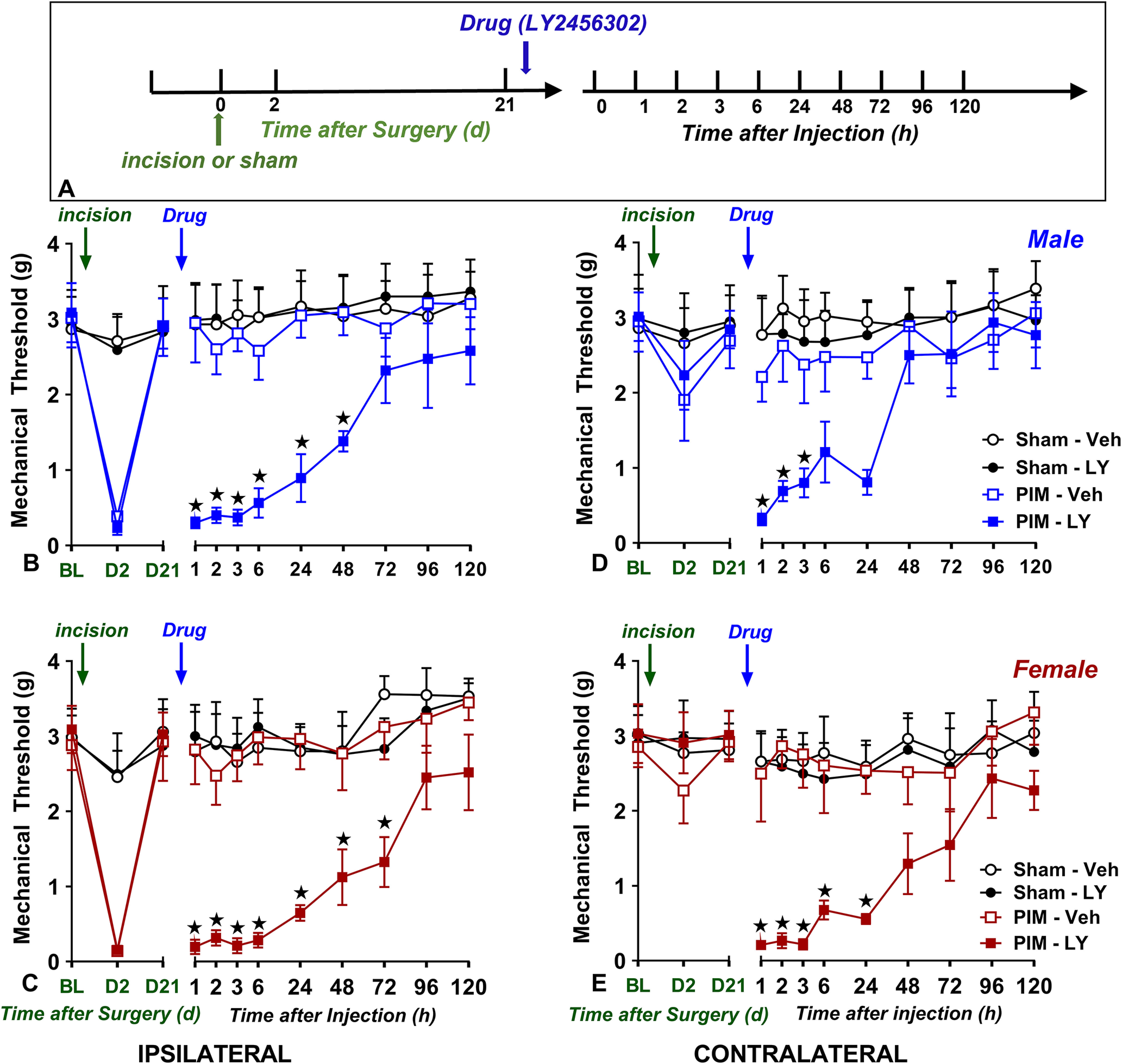
LY2456302 bilaterally reinstates mechanical hypersensitivity in male and female mice after plantar incision but not sham surgery. A, Timeline of the experiment. B–E, Mechanical thresholds at the plantar hindpaw on the side ipsilateral (B, C) and contralateral (D, E) to incision in male (B, D) and female (C, E) mice when tested at baseline (BL) just before surgery or 2 d (D2) or 21 d (D21) after surgery. On day 21, intrathecal administration of the KOR antagonist LY2456302 (10 µg) but not vehicle reinstated mechanical hypersensitivity at ipsilateral and contralateral sides of both sexes that received incision but not sham surgery; n = 4–6 per group. *p < 0.05 vehicle versus drug after ANOVA. Data represented as mean ± SEM. Veh, Vehicle; LY, LY2456302.
Figure 3.
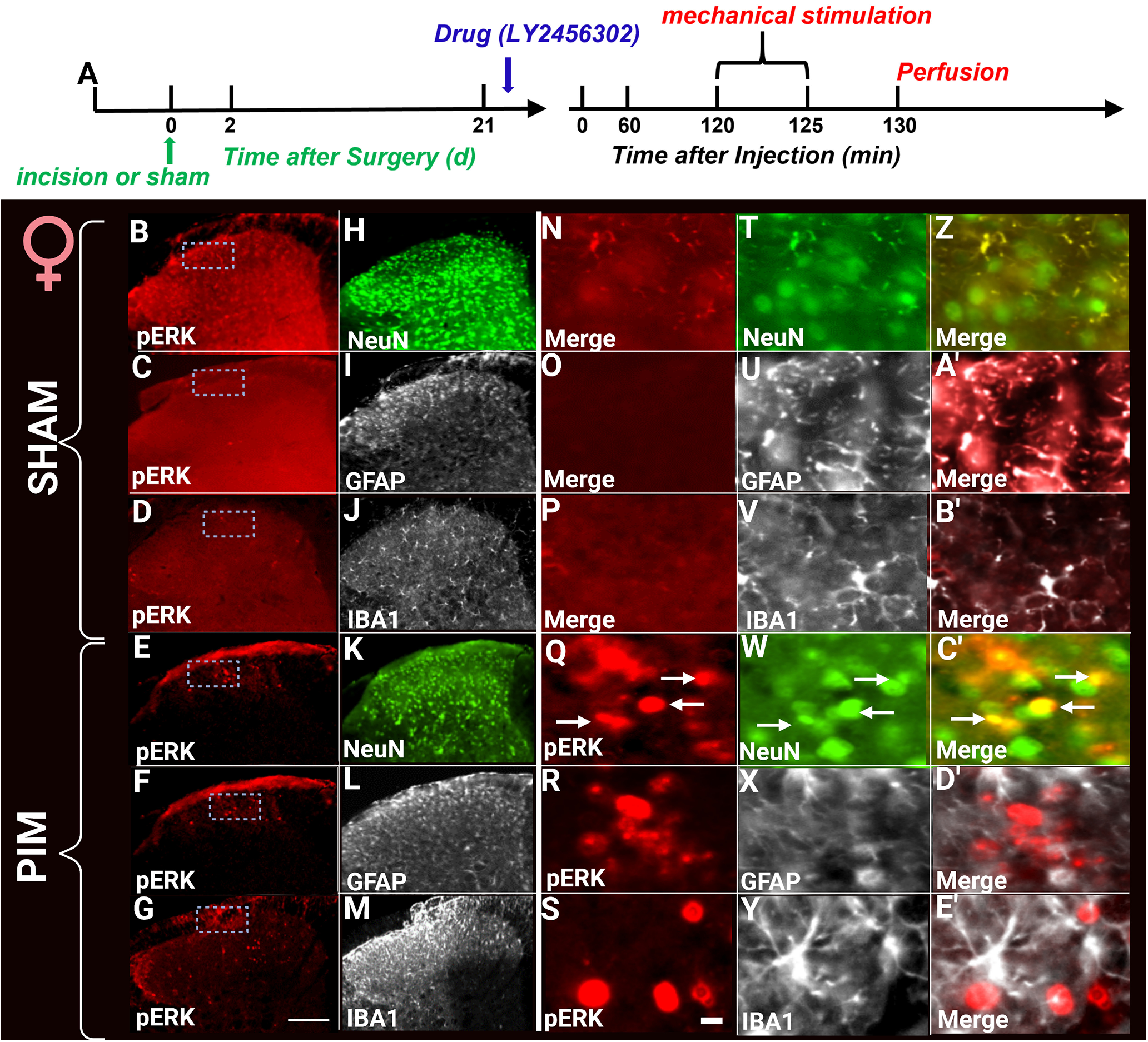
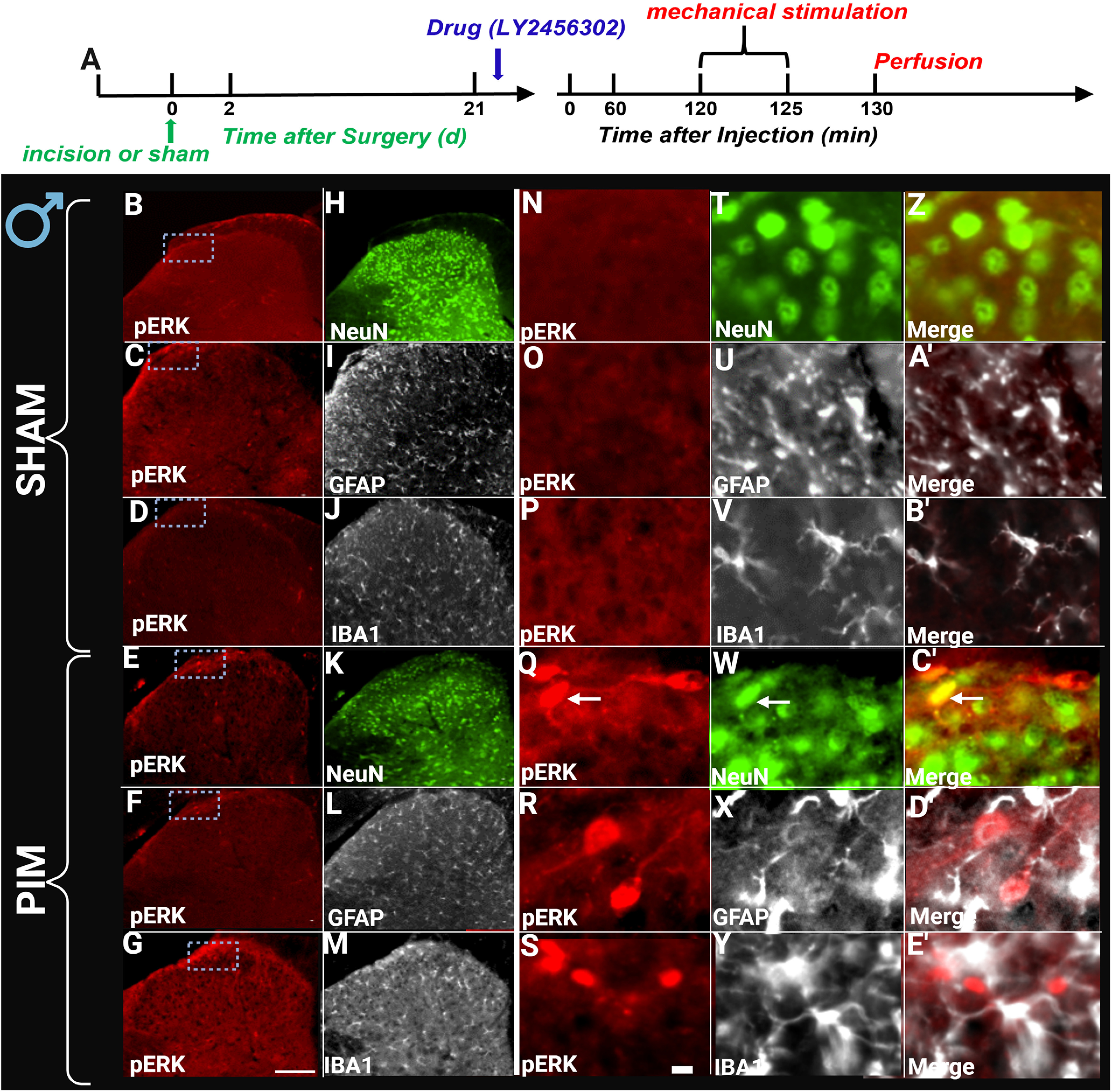
LY2456302 elicited spinal pERK expression in female mice after plantar incision but not sham surgery. A, Timeline of the experiment. LY2456302 (10 μg) or vehicle was intrathecally administered in incision and sham animals of female mice. Two hours after drug or vehicle administration, ipsilateral light-touch paw stimulation was performed for 5 min. Representative images of the ipsilateral sides of dorsal horn treated with either LY2456302 (B–E') isolated from female mice with either sham (B–B') or incision (E–E') surgery. Sections were colabeled with either pERK (B–G and N–S), NeuN (H, K, T, W, Z, C'), GFAP (I, L, U, X, A', D'), or IBA1 (J, M, V, Y, B', E'). Arrows indicate pERK colabeling. Touch stimulation did not evoke pERK expression in sham surgery controls and did not evoke pERK expression in cells that stained for GFAP or IBA1. These are representative examples from sections taken from 2–3 mice per group. Low-magnification images (left 2 columns) were taken with a 20× objective, whereas high-magnification images (right 3 columns) were taken with a 40× objective. Scale bars: B–M, 100 μm; N–E', 10 μm.
Figure 5.
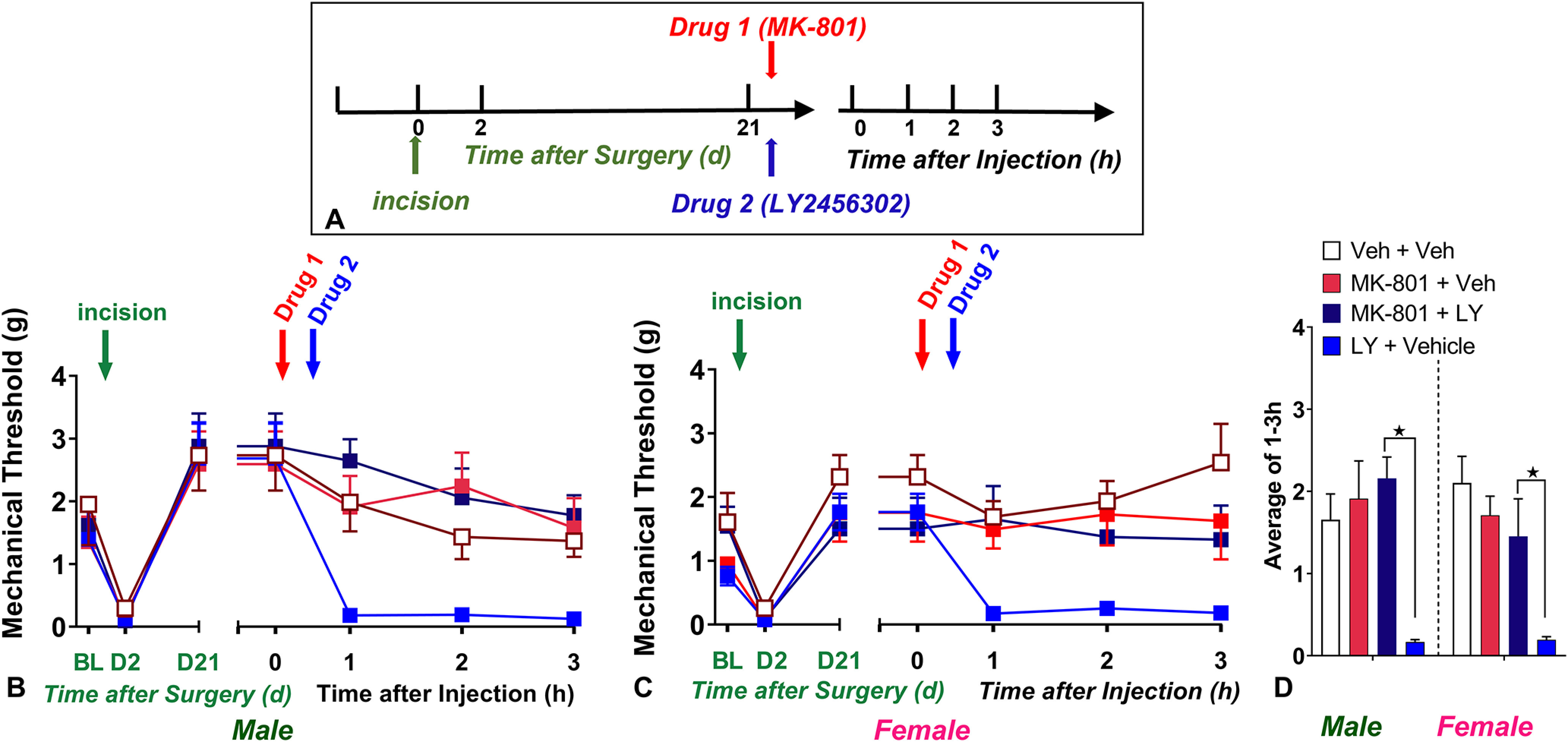
Pharmacological inhibition of NMDA receptor signaling prevented LY2456302-induced reinstatement of postoperative hyperalgesia. A, Timeline of the experiment. B–C, Line graphs describing mechanical thresholds at baseline (BL), 2 d, and 21 d after incision in (B) male and (C) female C57Bl/6 mice; n = 4–6 per group/sex. D, Histograms showing that preadministration of the NMDA receptor inhibitor MK-801 (1 µg/5 µL, i.t.) prevented LY2456302 (10 µg)-induced reinstatement of hyperalgesia in male and female mice; *p < 0.05. BL indicates baseline behavior before incision. Data represented as mean ± SEM. Veh, Vehicle; LY, LY2456302.
Figure 9.
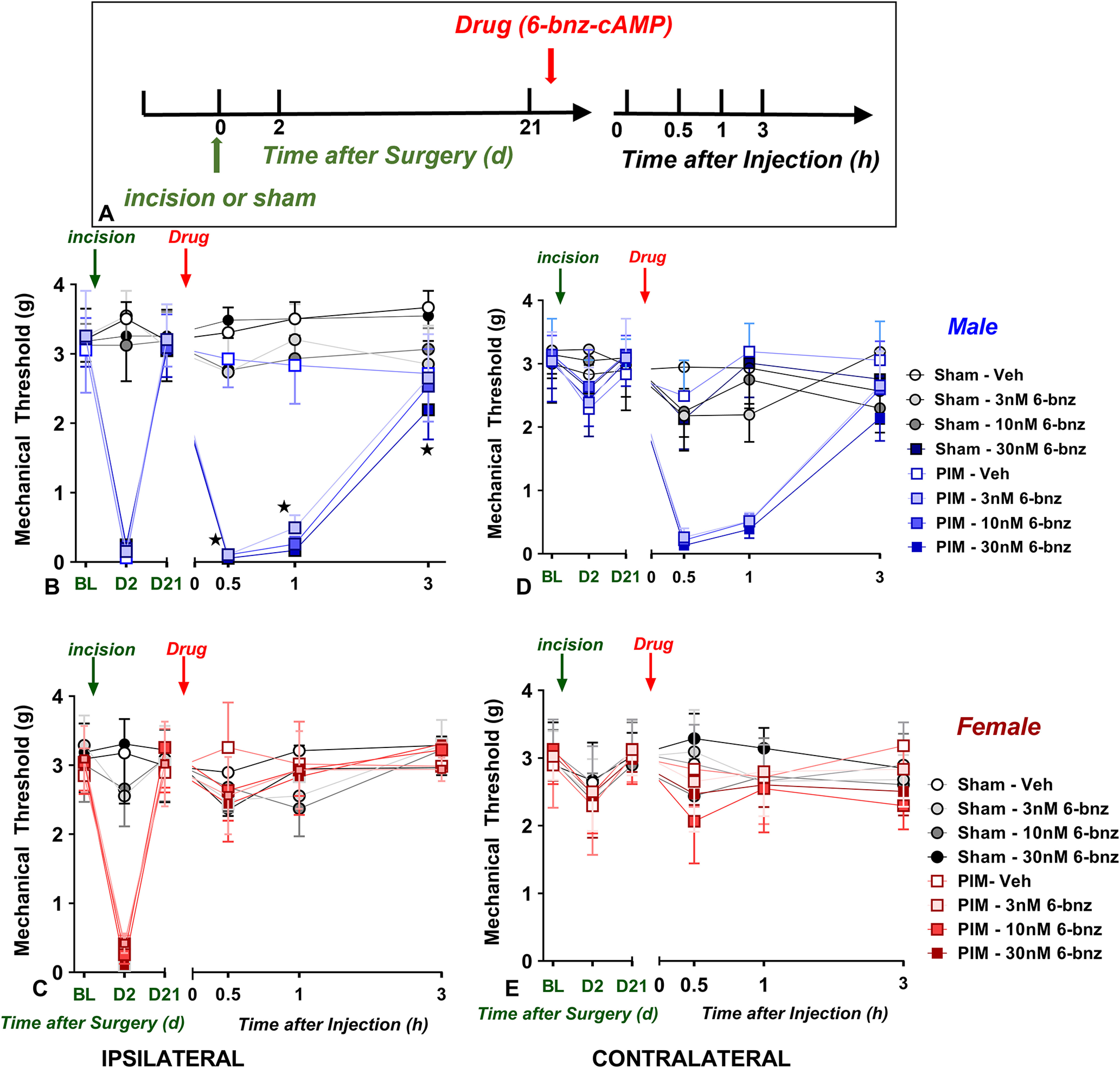
PKA activation reinstates behavioral signs of postsurgical pain in male but not female mice. A, Timeline of the experiment. B–E, Line graphs describing mechanical thresholds at baseline (BL), 2 d, and 21 d after incision or sham surgery when tested at either ipsilateral (B–C) or contralateral (D–E) sides of male (B, D) and female (C, E) C57BL/6J mice. On day 21 after incision, intrathecal administration of the PKA activator 6-Bnz-cAMP (3, 10, 30 nmol) reinstated mechanical hypersensitivity at the ipsilateral (B) and contralateral (D) sides of male (B, D) but not female mice (C, E); 6-Bnz-cAMP did not reinstate mechanical hypersensitivity in mice that received sham surgery; n = 4 males and 4 females per group; *p < 0.05. BL indicates baseline behavior before incision. Data represented as mean ± SEM. Veh, Vehicle.
Figure 13.
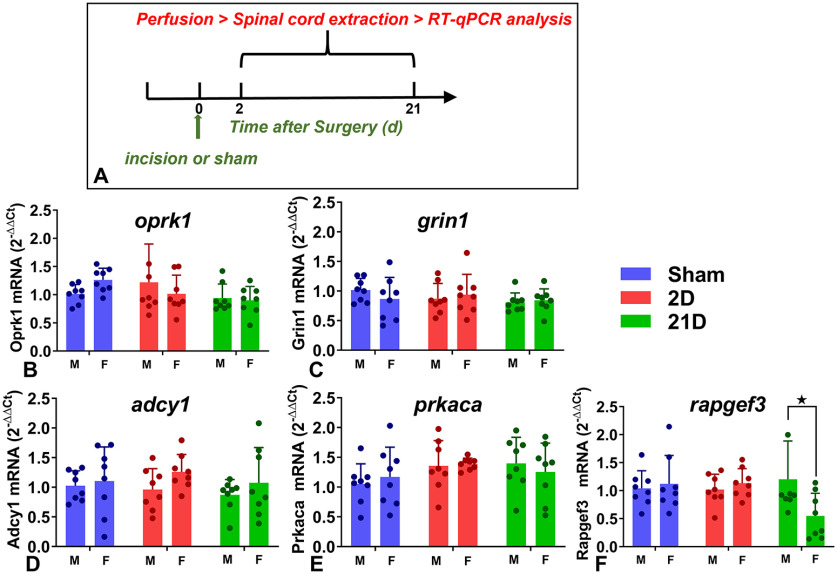
Plantar incision does not change KOR, GluN1, AC1, PKA, or Epac1 gene expression in dorsal horn. A, Timeline of the experiment. B–F, Histograms showing the mRNA quantification relative to GAPDH of (B) KOR (oprk1), (C) GluN1 (grin1), (D) AC1 (adcy1), (E) PKA (prkaca), and (F) Epac1 (rapgef3) in male and female mice after sham surgery or 2 d (2D) or 21 d (21D) after incision. Dots represent data points from individual animals, and bars and error bars indicate mean ± SEM; n = 8 mice per group. Except for a sex difference in rapgef3 expression at 21 d (★p > 0.05, Bonferroni post hoc tests following 2-way ANOVA) no differences were observed between groups (p < 0.05). Data represented as mean ± SEM.
Plantar incision model of postoperative pain
Postoperative hyperalgesia was induced by longitudinal incision of the plantaris muscle as previously described (Pogatzki and Raja, 2003; Jang et al., 2011). Under isoflurane anesthesia (5% induction and 1.5–2% maintenance via a nose cone) and antisepsis of the left hindpaw with Chlorascrub then alcohol, a no. 11 scalpel blade was used to make a 5 mm incision through the skin and fascia, beginning 2 mm from the proximal edge of the heel and extending toward the digits. The underlying muscle was raised with a curved forceps, extended 4 mm and then incised longitudinally, leaving the origin and insertion of the muscle intact. The overlying skin was closed with synthetic 5–0 sutures (PDS* II, Ethicon), followed by application of antibiotic ointment. Surgery was typically completed within 5-10 min. Sutures were removed on postoperative day 10. Sham controls received anesthesia for 2 min but no surgical incision.
Intrathecal drug delivery
As previously described (Fairbanks, 2003), nonanesthetized mice were lightly restrained in a towel, and a 30 ga × 1/2 inch needle (Becton Dickinson) attached to a 25 µl Hamilton microsyringe was inserted into the subarachnoid space between the L4/L5 vertebrae. The needle was angled ∼30–45 degrees to the horizontal plane and slightly advanced until a reflexive tail flick was confirmed, and then 5 µl of drug or vehicle was slowly injected. The needle was held in place for 30 s to ensure the solution fully entered the spinal canal, was withdrawn, and then the mouse was returned to its testing chamber.
Drug administration
We administered drugs at least 21 d after incision. LY2456302 (Rorick-Kehn et al., 2014a,b) was received either as a gift (National Institute on Drug Abuse Drug Supply Program) and dissolved in ddH20 and sonicated at 30°C for 30 min until complete homogenization of the drug was achieved; or it was received from MedChem Express and dissolved in 1:1:8 (EtOH/chremophor/saline), and injected at a dose of 10 µg. NB001 (1.5 µg; Tocris Bioscience), 6-Bnz-cAMP (10 nmol, Biolog), H89 (10 nmol; Tocris Bioscience), and 8-(4-Chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic Monophosphate sodium salt (8-CPT; 3 nmol; Enzo Life Sciences) were dissolved in saline. Other drugs were dissolved in deionized (DI) water/EtOH/chremophor/saline, including (+)−MK-801 maleate (1 µg/5 µl; Tocris Bioscience) and ESI-09 (10 µg; gift from Jia Zhuo, University of Texas Medical Branch; Almahariq et al., 2013; Chen et al., 2013). The dose of LY2456302 was informed by our previously published dose–response studies (Custodio-Patsey et al., 2020). The doses of NB001 and MK-801 were informed by Corder et al. (2013). Doses of 6-bnz-cAMP, H89, 8-CPT, and ESI-09 were informed by Fu et al. (2019). Control animals for the behavioral pharmacology experiments received 5 µl, i.t., of vehicle [sterile saline or DI water or 1:1:8 EtOH:chremophor (castor oil and ethylene oxide at a 1:35 ratio)]. All compounds were injected after mechanical paw withdrawal thresholds had returned to baseline levels at least 21 d postincision. While keeping the experimenter blind, another experimenter assigned animals to groups in a balanced fashion to avoid significant differences in baseline thresholds.
Mechanical thresholds
Animals were acclimated to a temperature- and light-controlled room within individual Plexiglas boxes placed on the top of a stainless steel mesh platform for 30–60 min before behavioral testing. Mechanical thresholds were assessed using an incremental series of eight von Frey filaments (Stoelting) of logarithmic stiffness (∼0.008–6 g). The 50% withdrawal threshold was determined using an up-down method (Chaplan et al., 1994). Each filament was applied perpendicular to the surface of the skin just lateral to the incision site with enough force to cause a slight bending of the filament. A positive response was defined by a rapid withdrawal of the paw within 5 s. Using the up-down method (Chaplan et al., 1994), gram force was logarithmically converted to 50% mechanical threshold. Thresholds were measured from baseline through 21 d after the plantar incision model (PIM) to confirm the development and resolution of mechanical allodynia and then again after intrathecal injection. Baseline mechanical thresholds recorded at the University of Kentucky (3–4 × g) were higher than those recorded at the University of Pittsburgh (2–3 × g). We speculate that this could be because of differences in the testing environment, which include lighting, temperature, humidity, bedding, cage enrichment, and/or personnel.
pERK
At 21 d after surgery, mice received either vehicle or LY2456302 (10 µg). Two hours after injection, mice were lightly anesthetized with isoflurane (5% induction and then 1.5%) maintenance, and the ventral surface of the ipsilateral hindpaw was mechanically stimulated with a gentle 2 s stroke with a cotton swab from heel to toe. This was repeated every 5 s for 5 min. After an additional 5 min pause, mice were deeply anesthetized with isoflurane and transcardially perfused with cold 0.01 m PBS (Fischer Scientific)/heparin buffer (10,000 USP units/L), followed by 10% phosphate formalin buffer. Lumbar spinal cords were harvested and postfixed in the same fixative overnight at 4°C and then cryoprotected with 30% sucrose until equilibrated (1–3 d).
Immunohistochemistry
Transverse sections (35 µm) from L3–L5 segments were obtained with a sliding microtome (SM2000 R, Leica). Sections were washed in 0.01 m PBS, blocked in 3% normal goat serum (GeminiBio) containing 0.3% Triton X-100 (Sigma-Aldrich) in 0.01 m PBS for 1 h and then incubated with rabbit primary antibody to phosphorylated-ERK1/2 (1:800; catalog #4370S, Cell Signaling; Custodio-Patsey et al., 2020), goat anti-AIF1/Iba1 (Isoforms 1 and 3, 1:500; catalog #SAB2500041, Sigma-Aldrich; Custodio-Patsey et al., 2020), and chicken anti-GFAP (1:1000; catalog #ab4674, Abcam; Custodio-Patsey et al., 2020) at 4°C for 24 h on a shaker. For neuronal costaining, sections were incubated with Alexa Fluor 488 mouse NeuN (1:500; catalog #MAB377X, EMD Milipore; Custodio-Patsey et al., 2020) at 4°C for 24 h on a shaker. The following day, sections were washed in 0.01 m PBS and then incubated with the following secondary conjugated antibodies: donkey anti-Goat Alexa Fluor Plus 647 (catalog #A32849), donkey anti-rabbit Alexa Fluor 568 (catalog #10042), goat anti-chicken Alexa Fluor Plus 680 (catalog #A32934), and goat anti-rabbit Alexa Fluor 568 (1:1000, catalog #A-11011, Invitrogen; Custodio-Patsey et al., 2020) for 1 h at room temperature. The sections were washed in 0.01 m phosphate buffer, mounted, and then coverslipped with ProLong Glass Antifade mounting medium (catalog #P36984, Thermo Fisher Scientific).
Five high-quality sections per animal from spinal cord segments L4, separated by at least 240 µm, were randomly selected for pERK analyses. We focused our quantification of p-ERK within lamina I–II, where most nociceptive peripheral afferents terminate within the dorsal horn (Corder et al., 2013). The number of pERK-positive cells in laminae I–II as well as III–V were counted by a blinded examiner. All images were captured with a Nikon TE-2000 microscope equipped with Nikon Instruments Software (NIS) Elements Advanced Research 4.13.05 (Nikon), which was used to perform the analyses off-line.
qPCR
Following decapitation of unanesthetized animals, the spinal cord was extruded by pressure ejection with a blunt 20–22-gauge needle attached to a 10 ml syringe filled with cold saline. The lumbar enlargement (L3–L5) was removed rapidly, quickly sectioned in the horizontal and vertical planes, and then the dorsal horn quadrant ipsilateral to incision was submerged in RNAlater stabilization solution (Thermo Fisher Scientific) and stored at −20°C. RNA was purified as previously described (Üçeyler et al., 2011; França et al., 2012). First, quadrants were placed in RLT lysis buffer with β-ME, disrupted and homogenized thoroughly with a rotor–stator homogenizer, and the lysate was centrifuged for 3 min. The supernatant was carefully removed and transferred to a new microcentrifuge tube. One volume of 70% ethanol was added to the lysate, transferred to an RNeasy spin column placed in a 2 ml collection tube, and centrifuged for 15 s at ≥10,000 rpm. Buffer RW1 was added followed by RPE to wash the spin column membrane and then centrifuged. Finally, 30 µl RNase-free water was added directly to the spin column membrane and centrifuged for 1 min at ≥10,000 rpm to elute the RNA. The extracted RNA was assessed for yield and purity with a NanoDrop 2000/2000c Spectrophotometer (Thermo Fisher Scientific). All samples were of sufficient yield and purity (260 nm/280 nm ratio >2.0). To produce a purified cDNA template, extracted RNA samples were reverse transcribed with a SuperScript IV VILO Master Mix with ezDNase Enzyme Kit (catalog #1176050, Invitrogen) using the following thermal cycling steps: (1) anneal primers at 25°C for 10 min, (2) reverse transcribe RNA at 50°C for 10 min, and (3) inactivate enzyme at 85°C for 5 min.
Gene expression of KOR (Mm00440561_m1), GluN1 (Mm00433790_m1), AC1 (Mm01187829_m1), PKA (Mm00660092_m1), Epac 1 (Mm00522941_m1), or the internal reference gene glyceraldehyde 3-phosphate dehydrogenase GAPDH (Mm99999915_g1) was evaluated with Applied Biosystems QuantStudio 3 Real-Time PCR system using TaqMan gene expression probes (Thermo Fisher Scientific). Real-time PCRs included one cycle of predegeneration at 95°C for 30 s, 40 cycles of PCR reaction at 95°C for 5 s, and dissociation at 60°C for 34 s. The relative gene expression was calculated based on the 2ΔΔ Ct method (Üçeyler et al., 2007; Rao et al., 2013). The abundance of each transcript was normalized to the internal control housekeeping gene, GAPDH, which rarely changes in response to system perturbation (Vandesompele et al., 2002), and so, as expected, spinal cord levels were similar between sham and PIM mice.
Statistical analysis
Data were graphed and analyzed using Prism software (version 8.0, GraphPad). In most experiments, we conducted a complex experimental design that balanced four factors: Sex, Drug 1 (MK-801, NB001, H89, ESI-09, or vehicle), Drug 2 [either LY2456302 (10 µg, i.t.) or vehicle), and Time as a repeated measure. To analyze these results, we conducted two statistical analyses. First, to evaluate any Sex × Drug 1 interaction, two-way ANOVA (Sex × Drug 1) with Time collapsed as either the average of postinjection hours 1–3 or Time integrated across additional time points using the trapezoidal method for calculation of area under the curve (AUC). Second, to determine the effects of Drug 1 and Drug 2 for each sex separately at each time point, we conducted a three-way ANOVA (Drug 1 × Drug 2 × Time). If a main effect was found, then subsequent analyses for each sex included two-way Drug 1 × Time ANOVA with Time as a repeated measure. When main effects were significant, ANOVAs were followed by Bonferroni post hoc analyses at each time point. Statistical significance was set at p < 0.05. For qPCR data, two-way ANOVA (Sex × Drug) was used, and if a main effect was found then this was followed by Bonferroni post hoc analysis. All data are presented as mean ± SEM.
Results
Latent postoperative pain sensitization is bilateral, robust, and very long-lasting
Custodio-Patsey et al. (2020) reported that intrathecal LY2456302 rinstated mechanical hypersensitivity when injected 3 weeks after plantar incision, indicating the KOR activity inhibits latent postoperative pain sensitization. However, one could argue that KOR activity inhibited transient nociception as well, thus confounding interpretation of the plantar incision studies. To test this alternative hypothesis, we repeated the Custodio-Patsey et al. (2020) study with the addition of sham controls in an experimental design that included four independent variables: Surgery (sham vs incision), Drug, Sex, and Side. We evaluated behavior for 120 h because of the long functional half-life of LY2456302 (Rorick-Kehn et al., 2014a,b). As illustrated in Figure 1, three-way ANOVA revealed that 10 µg LY2456302 did not induce reinstatement of hyperalgesia in a sex-specific manner on the ipsilateral side (Sex × Drug: F(1,40) = 0.03,828; p = 0.8459; Sex × Surgery: F(1,40) = 0.4318; p = 0.5149). This is reminiscent of our previous report that 0.3 µg but not 10 µg LY2456302 induced a greater degree of hyperalgesia in female compared with male mice on the ipsilateral side (Custodio-Patsey et al., 2020). Two-way ANOVA (Drug × Surgery) revealed that LY2456302 reinstated mechanical hyperalgesia in mice with incision but not sham surgery, and this was true in both males (F(3,20) = 20.40; p < 0.0001; Fig. 1B) and females (F(3,20) = 29.65; p < 0.0001, Fig. 1C). Two-way ANOVA (Drug × Side) revealed that LY2456302 reinstated hypersensitivity in both ipsilateral and contralateral sides of males (Drug × Side: F(3,20) = 30.02; p < 0.0001, Fig, 1B,D) and females (Drug × Side: F(3,20) = 35.63; p < 0.0001, Fig. 1C,E) with incision but not sham surgery. Subsequent one-way ANOVA confirmed that LY2456302 but not vehicle reinstated mechanical hyperalgesia in both males (Drug: F(1,10) = 62.09; p < 0.0001, Fig. 1B) and females (Drug: F(1,10) = 49.74; p < 0.0001, Fig. 1C) with incision.
We previously reported that naltrexone can increase touch-evoked pERK immunoreactivity, a marker of increased cellular responses to non-noxious stimuli during pathologic conditions or itch (Ji et al., 1999, 2009; Jiang et al., 2015) when tested during the remission phase of Complete Freund's Adjuvant (CFA)-induced inflammatory pain (Fu et al., 2019). Here, we asked a similar question using LY2456302 in the PIM. As illustrated in Figures 2 and 3, intrathecal LY2456302 increased touch-evoked pERK expression in both male and female mice when tested 21 d after incision but not sham surgery. Costaining with NeuN, GFAP, and Iba1, pERK expression was restricted to neurons but not astrocytes nor microglia, respectively, consistent with previous studies (Corder et al., 2013; Custodio-Patsey et al., 2020).
Figure 2.
LY2456302 elicited spinal pERK expression in male mice after plantar incision but not sham surgery. A, Timeline of the experiment. LY2456302 (10 μg) or vehicle was intrathecally administered in incision and sham animals of male mice. Two hours after drug or vehicle administration, ipsilateral light-touch paw stimulation was performed for 5 min. Representative images of the ipsilateral sides of dorsal horn treated with either LY2456302 (B–E') isolated from male mice with either sham (B, B') or incision (E, E') surgery. Sections were colabeled with either pERK (B–G and N–S), NeuN (H, K, T, W, Z, C'), GFAP (I, L, U, X, A', D'), or IBA1 (J, M, V, Y, B', E'). Arrows indicate pERK colabeling. Touch stimulation did not evoke pERK expression in sham surgery controls and did not evoke pERK expression in cells that stained for GFAP or IBA1. These are representative examples from sections taken from 2–3 mice per group. Low-magnification images (left 2 columns) were taken with a 20× objective, whereas high-magnification images (right 3 columns) were taken with a 40× objective. Scale bars: B–M, 100 μm; N–E', 10 μm.
We previously reported that latent sensitization in male mice can be unmasked with naltrexone as early as 3 weeks and for up to 5 months after the induction of peripheral inflammation with intraplantar CFA (Corder et al., 2013). To determine whether this could be replicated with a KOR-selective antagonist in the plantar incision model at even later time points, we asked whether a single intrathecal injection of LY2456302 could reinstate mechanical hypersensitivity in one group of male mice tested 21 d, 6 months, and then again 13 months after plantar incision. As illustrated in Figure 4, two-way ANOVA revealed main effects of Drug × Time, Drug, and Time (p < 0.05). LY2456302 but not vehicle reinstated mechanical hyperalgesia when administered either 21 d, 6 months, or 13 months after surgery (main effect of Drug: F(1,10) = 31.4, p = 0.0002 (21 d); F(1,13) = 23.53, p = 0.0003 (6 months); and F(1,13) = 7.88, p = 0.0148 (13 months). Our findings indicate that LS and opposing KOR signaling persists for more than a year after surgery.
Inhibition of NMDA receptor signaling prevents LY2456302-induced reinstatement of postoperative hyperalgesia
NMDAR and its downstream signaling pathways contribute to the spinal sensitization of nociceptive circuits after tissue injury (Woolf, 2007; Zhuo, 2012), including in male mouse models of latent sensitization (Campillo et al., 2011; Corder et al., 2013; Fu et al., 2019). Here, we used the noncompetitive NMDA receptor antagonist MK-801 to test the hypothesis that NMDA receptors contribute to KOR-masked LS in females as well. To address this question, we conducted a complex experimental design that balanced four factors: Sex, Drug 1 [either MK-801 (1 µg/5 µL, i.t.) or vehicle], Drug 2 [either LY2456302 (10 µg, i.t.) or vehicle], and Time as a repeated measure. To analyze the results of Figure 5, we conducted two statistical analyses. We analyzed any effect of just MK-801 (MK-801 + LY2456302 vs vehicle + LY2456302) by two-way ANOVA (Sex × Drug 1) with Time collapsed as the average of time points 1, 2, and 3 h (Fig. 5D). The Sex × Drug 1 analysis revealed a main effect of Drug (F(1,10) = 39.7, p < 0.0001) but not Sex (F(1,10) = 1.6, p = 0.2345]. Figure 5, B and C, illustrates that MK-801 blocked LY2456302-induced reinstatement in both male and female cohorts.
To determine the effects of Drug Injection 1 (MK-801) and Drug Injection 2 (LY2456302) for each sex separately at each time point, we conducted a three-way ANOVA (Drug 1 × Drug 2 × Time) on the data as plotted in Figures 5, B and C. This revealed a main effect of the MK-801 × LY2456302 interaction (F(1,40) = 15.42, p = 0.0003). Subsequent Drug 1 × Time two-way ANOVA of the MK-801 + LY2456302 and vehicle + LY2456302 group indicated that MK-801 prevented LY2456302-induced mechanical hypersensitivity in both male (F(1,10) = 58.68, p < 0.0001, Drug 1; Fig. 5B) and female F(1,9) = 6.11, p = 0.0355, Drug 1; Fig. 5C) mice.
Inhibition of AC1 prevents LY2456302-induced reinstatement of chronic postoperative hyperalgesia and spinal neuron activation
Neither genetic nor pharmacological interruption of AC1 changes transient nociception in uninjured animals (Wei et al., 2002) but prevents CFA-induced hyperalgesia (Wei et al., 2002; Wang et al., 2011) and pain-related sensitization (Xu et al., 2008; Yamanaka et al., 2017) including the latent sensitization that is masked by MORCA (Corder et al., 2013). For the following reasons we hypothesized that AC1 also mediates the LS that is masked by KOR. First, KOR is an inhibitory GPCR and as such inhibits the activity of the ACs (Childers et al., 1992). AC1 is a Ca2+-activated protein that acts as a key modulator for the induction of pathologic pain in the CNS (Wu et al., 1995; Liauw et al., 2005; Wei et al., 2006; Zhuo, 2012). It couples with the intracellular Ca2+ derived from NMDAR activation to downstream activation of an array of downstream proteins and kinases (Zhuo, 2012). Similar to MOR, persistent activation of KOR by an exogenous drug can also lead to an AC overshoot (Avidor-Reiss et al., 1997; Nakagawa et al., 1999). Therefore, to test the hypothesis that KOR inhibits AC1 to maintain latent sensitization in a state of remission, we evaluated LY2456302-induced reinstatement of hyperalgesia in AC1 knockout mice and after intrathecal administration of the AC1 inhibitor NB001.
AC1 deletion mutant mice: behavior
As illustrated in Figure 6, AC1 knockout mice and their wild-type controls exhibited similar baseline mechanical thresholds, regardless of sex. Figure 6D illustrates the data with Time collapsed across the 1–96 h time points. A two-way Sex × Genotype ANOVA revealed a main effect of Genotype (F(3,30) = 29.5, p < 0.0001) but not Sex (p > 0.05) on LY2456302-induced reinstatement of mechanical hypersensitivity. A three-way Genotype × Drug 2 × Time ANOVA revealed a main effect of the Genotype × Drug interaction (F(1,40) = 12.54, p = 0.0010). A two-way Genotype × Time ANOVA indicated significant mechanical hypersensitivity in male AC1KO (F(1,16) = 4.774, p = 0.0441, Genotype; Fig. 6B) compared with wild-type mice, whereas the effect in female AC1KO did not reach significance (F(1,15) = 3.240, p = 0.0920, Genotype; Fig. 6C). Subsequent analysis revealed that LY2456302 reinstated hyperalgesia in wild-type mice of either sex as previously described (Custodio-Patsey et al., 2020), and this was absent in both AC1 knock-out males (F(1,12) = 9.64, p = 0.0091, Drug; Fig. 6B) and females (F(1,13) = 20.08, p = 0.0006, Drug; Fig. 6C).
AC1 deletion mutant mice: pERK expression
To test the hypothesis that AC1 contributes to latent sensitization of dorsal horn neurons, we evaluated the effect of AC1 deletion on a measure of neuronal activation, pERK. We administered LY2456302 intrathecally, and then 2 h later, at the peak of hyperalgesia, the ventral surface of the ipsilateral hindpaw was mechanically stimulated for 5 min to evoke pERK. As illustrated in Figure 7, we found that the number of stimulus-evoked pERK+ profiles in laminae I–II of the ipsilateral dorsal horn was significantly reduced in both sexes of AC1KO compared with WT mice (F(1,6) = 15.41, p = 0.0077, Genotype; Fig. 7J).
Figure 7.
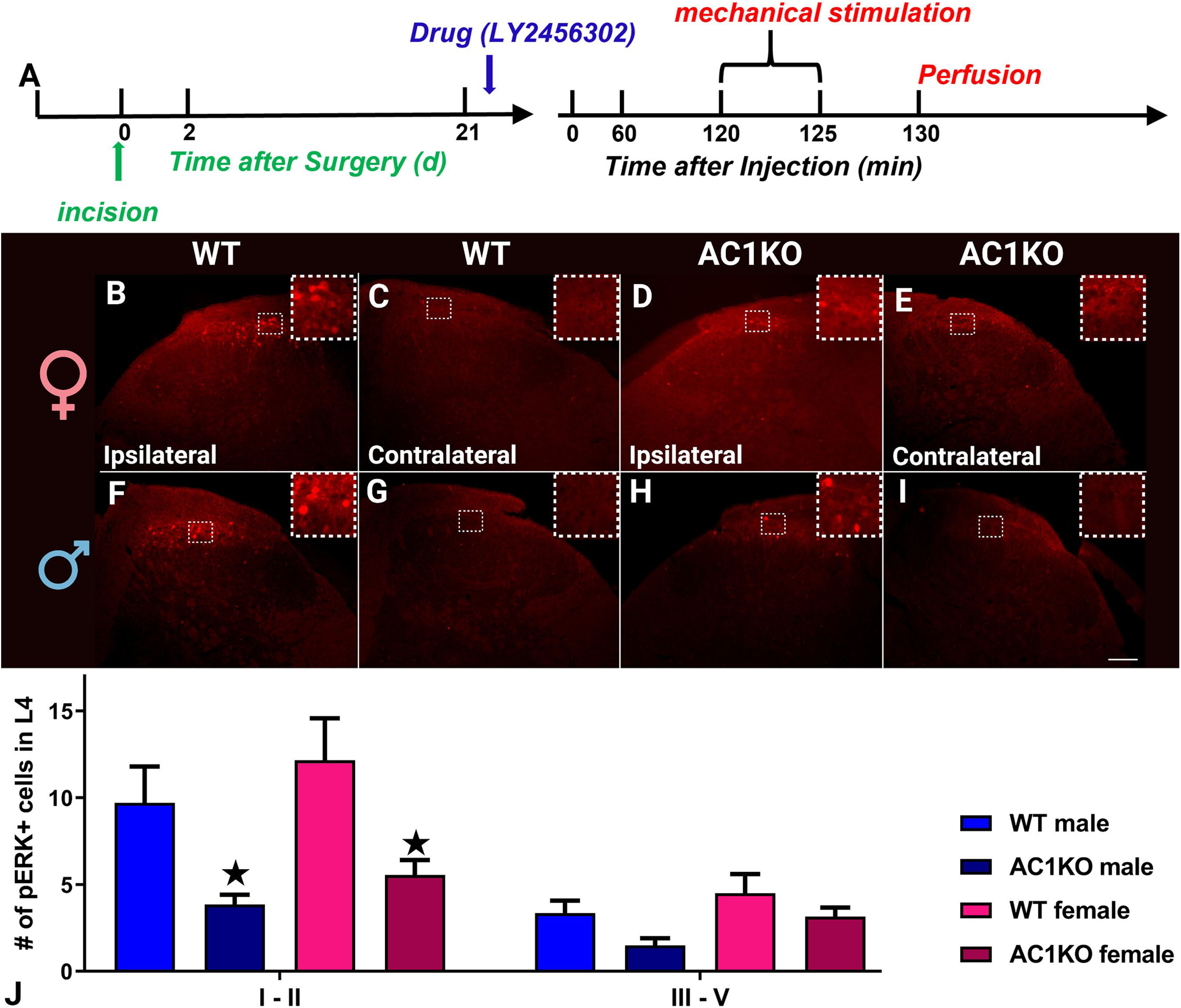
AC1 gene deletion prevented LY2456302-induced pERK expression in dorsal horn. A, Timeline of the experiment. B–I, Representative images of pERK immunoreactivity, 21 d after incision in female (B–E) and male (F–I) mice, in the dorsal horn ipsilateral (B, D, F, H) or contralateral (C, E, G, I) to surgery in WT (B, C, F, G) or AC1 knock-out mice (D, E, H, I) after LY2456302 (10 µg, i.t.). J, Histograms illustrating the number of pERK+ profiles in lamina I–II or III–IV on the side ipsilateral to incision. Images were taken with a 10× objective; n = 4 per group. Insets are magnified images of the boxed region to better show pERK immunoreactivity. *p < 0.05 AC1KO compared with WT mice. Scale bar (lower right corner), 100 μm. Data represent mean ± SEM. Ipsi, Ipsilateral.
Pharmacological inhibition of AC1
As illustrated in Figure 8, pharmacological inhibition of AC1 prevented LY2456302-induced reinstatement of postoperative hyperalgesia, regardless of sex. We used an experimental design with four factors: Sex, Drug 1 [either NB001(1.5 µg, i.t.) or vehicle], Drug 2 [either LY2456302 (10 µg, i.t.) or vehicle], and Time as a repeated measure. A two-way Sex × Drug 1 ANOVA revealed a main effect of Drug (F(1,6) = 10.31, p = 0.0183). Figure 8D illustrates that NB001 blocked LY2456302-induced reinstatement of mechanical hypersensitivity in both male and female cohorts.
A three-way Drug 1 × Drug 2 × Time ANOVA revealed a NB001 × LY interaction (F(1,16) = 4.159, p = 0.05). Subsequent two-way Drug 1 × Time ANOVA of the NB001 + LY and vehicle + LY groups indicated that NB001 prevented LY2456302-induced mechanical hypersensitivity in male (F(1,6) = 10.28, p = 0.0184, Drug 1; Fig. 8B) but not female (F(1,6) = 1.962, p = 0.2202, Drug 1; Fig. 8C) mice.
PKA activation reinstates postoperative hyperalgesia in male but not female mice
Adenylyl cyclase synthesizes cAMP, an intracellular second messenger that stimulates PKA. The canonical cAMP–PKA pathway is critically involved in the development (Taiwo et al., 1989; Malmberg et al., 1997; Yajima et al., 2003) and maintenance of hyperalgesia after inflammation (Sluka, 1997; Aley and Levine, 1999; Aley et al., 2000; Tumati et al., 2011) or prolonged opioid exposure (Tumati et al., 2011; Araldi et al., 2015; W. Chen et al., 2018). We reported that intrathecal administration of the selective PKA activator 6-bnz-cAMP reinstated mechanical hyperalgesia in male mice and that the PKA inhibitor H89 prevented Y1-antagonist-induced reinstatement of inflammatory hyperalgesia in the CFA model (Fu et al., 2019). To determine whether PKA persistent activity also contributes to postoperative latent sensitization masked by KOR, we administered the selective PKA activator 6-bnz-cAMP (6-bnz), 21 d following surgical incision with an 8-group experimental design in 64 mice that balanced four independent variables: Sham versus Incision, 6-bnz-cAMP versus vehicle, male versus female, and ipsilateral versus contralateral paw (Fig. 9). Three-way ANOVA (Drug × Sex × Surgery) revealed a main effect of the Drug × Sex interaction when tested 21 d at the side ipsilateral to incision (F(1,24) = 7.543; p = 0.0112) but not sham surgery (F(1,24) = 0.1875; p = 0.6689). As illustrated by studies in male mice, as depicted in Figure 9B, subsequent two-way ANOVA revealed that 6-bnz-cAMP reinstated mechanical hyperalgesia when tested 21 d at the side ipsilateral to incision but not sham surgery (Drug × Surgery: F(7,24) = 14.19; p < 0.0001). This is consistent with our previous report (Fu et al., 2019). By contrast, Figure 9C illustrates that 6-bnz-cAMP did not reinstate hyperalgesia in females after incision or sham surgery (Drug × Surgery: F(7,24) = 0.26; p = 0.96). A subsequent one-way ANOVA confirmed that 6-bnz-cAMP but not vehicle reinstated mechanical hyperalgesia in males (Drug: F(3,12) = 11.53; p = 0.0008; Fig. 9B) but not females (Drug: F(3,12) = 0.24; p = 0.86; Fig. 9C). Similar results were observed at the contralateral side (Fig. 9D,E). These data indicate that PKA signaling is sensitized during the remission phase of LS in male but not female mice.
Inhibition of PKA prevents reinstatement of chronic postoperative hyperalgesia in male but not female mice
Next, we tested the hypothesis that PKA is necessary for the LS that is masked by KOR. As illustrated in Figure 10, two-way Sex × Drug ANOVA (with Time collapsed as the average of time points 1, 2, and 3 h) revealed a main effect of Drug (H89; F(1,10) = 9.507, p = 0.0116; Fig. 10D). Three-way Drug 1 × Drug 2 × Time ANOVA revealed a main effect of the Drug 1 × Drug 2 interaction (F(1,40) = 12.51, p = 0.0010, H89 × LY2456302). Subsequent Drug 1 × Time two-way ANOVA of the H89-801 + LY2456302 and vehicle + LY2456302 groups revealed that H89 prevented LY2456302-induced mechanical hypersensitivity in male mice (F(1,10) = 16.69, p = 0.0022, Drug 1; Fig. 10B). These data are consistent with our previous report using H89 in the CFA model of latent sensitization (Fu et al., 2019). By contrast, Figure 10C illustrates that H89 did not change LY2456302-induced mechanical hypersensitivity in female mice (F(1,10) = 0.6929, p = 0.4246, Drug 1; Fig. 10C). The absence of 6-Bnz-induced pain reinstatement in female mice confirms the sex difference in PKA signaling of LS masked by KOR in the spinal cord.
Epac activation reinstates chronic postoperative hyperalgesia in male and female mice
Cyclic AMP stimulates not only PKA-dependent but also Epac-dependent intracellular signaling. We have reported that intrathecal administration of the selective Epac activator 8-CPT (Rehmann et al., 2003) reinstated mechanical hyperalgesia (Fu et al., 2019), but this study was restricted to male mice in the CFA model of LS. Therefore, we tested 8-CPT in male and female mice 21 d following surgical incision. As illustrated in Figure 11, two-way Sex × Drug ANOVA (with Time collapsed as the average of time points 0.5 and 1 h) revealed a main effect of Drug (8-CPT; F(2,20) = 12.76, p = 0.0003, Fig. 11D) on 8-CPT induced-reinstatement of mechanical hypersensitivity.
Figure 11.
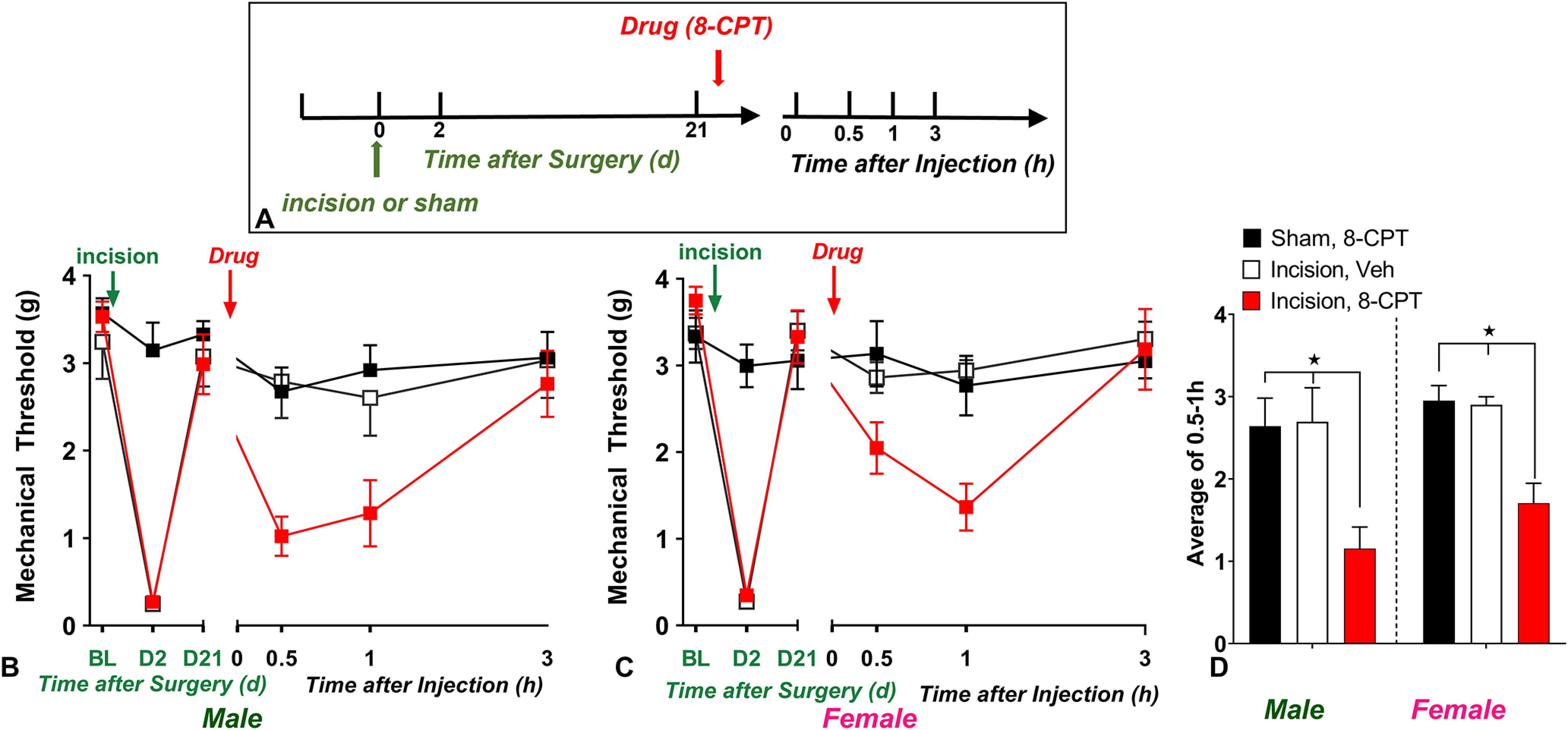
Epac drives postoperative latent sensitization in male and female mice. A, Timeline of the experiment. B, C, Line graphs describing mechanical thresholds at baseline (BL), 2 d, and 21 d after incision in (B) male and (C) female C57Bl/6 mice; n = 6 per group/sex. D, Histograms showing that preadministration of the Epac activator 8-CPT (3 nmol, i.t.) induced mechanical hypersensitivity in male and female mice. ★p < 0.05. BL indicates baseline behavior before drug administration. Data represented as mean ± SEM. Veh, Vehicle.
To determine the effect of 8-CPT for each sex separately at each time point, we conducted a two-way ANOVA with Drug as the main factor and Time as the repeated measure, finding that 8-CPT reinstates mechanical hypersensitivity in 21 d PIM male mice (F(2,18) = 6.076, p = 0.0096, Drug; Fig. 11B), and this finding extends to 21 d PIM female mice (F(2,15) = 5.22, p = 0.0190, Drug; Fig. 11C). These data indicate that Epac signaling is sensitized during the remission phase of LS in both males and females.
Inhibition of Epac prevents LY2456302-induced reinstatement of behavioral signs of postoperative hyperalgesia
Next, we tested the hypothesis that Epac is necessary for the LS that is masked by KOR. As illustrated in Figure 12, two-way Sex × Drug ANOVA (with Time collapsed as the average of time points 1, 2, and 3 h) revealed a main effect of Drug (ESI-09; F(1,10) = 11.03, p = 0.0077; Fig. 12D).
Three-way Drug 1 × Drug 2 × Time ANOVA revealed a main effect of the Drug 1 × Drug 2 interaction (F(1,40) = 13.5, p = 0.0007, ESI-09 × LY2456302]. A subsequent Drug 1 × Time two-way ANOVA revealed that ESI-09 prevented LY2456302-induced mechanical hypersensitivity in male mice (F(1,10) = 19.06, p = 0.0014, Drug 1; Fig. 12B), consistent with our previous report in the CFA model of latent sensitization (Fu et al., 2019). In female mice, ESI-09 numerically reduced LY2456302-induced mechanical hypersensitivity (Fig. 12C), although the effect did not reach statistical significance (F(1,10) = 3.924, p = 0.0758, Drug 1).
Plantar incision differently alters the dorsal horn gene expression of Epac1 in male and female mice
Dorsal horn gene expression of KOR, GluN1, AC1, PKA, and/or Epac1 has been studied in numerous rodent pain models, including CFA inflammation (Obara et al., 2009; Yang et al., 2009), carrageenan inflammation (Lawson et al., 2010), sciatic nerve transection (Eijkelkamp et al., 2013), and plantar incision (Matsuda et al., 2017), but few if any have compared males versus females or early versus late time points. To address these gaps and determine whether incision alters the expression of these genes during initial hyperalgesia or during LS remission, we conducted qPCR at postsurgical days 2 or 21, respectively. As illustrated in Figure 13, incision did not alter the gene expression of KOR, GluN1, AC1, or PKA when measured in the ipsilateral dorsal horn at either 2 d or 21 d after surgery (all main effects p > 0.05, two-way ANOVA). Incision did not change Epac1 expression at day 2 (p > 0.05) but did lead to a Sex × Surgery interaction at day 21 (F(1,14) = 4.77, p = 0.0465). As illustrated in Figure 13F, Epac1 expression was lower in females compared with males at 21 d after incision (p = 0.0269).
Discussion
Surgery engages indices of latent postoperative pain that are tonically inhibited by KOR
We report that intrathecal administration of the KOR-selective inhibitor LY2456302 reinstated hyperalgesia and neuronal pERK expression in both sexes when administered 3 weeks after incision but not sham surgery. This is consistent with reinstatement of hyperalgesia after (1) intrathecal LY2456302 or nor-BNI (Custodio-Patsey et al., 2020), (2) subcutaneous nor-BNI (Campillo et al., 2011), and (3) subcutaneous KOR inhibitor JDTic 6 weeks after CFA (Walwyn et al., 2016). LY2456302 also produced a remarkably robust hypersensitivity at the contralateral hindpaw as observed following naltrexone (Corder et al., 2013; Cooper et al., 2021) and in CFA rats after nor-BNI (Walwyn et al., 2016) These studies provide clear and convincing evidence that KOR activity maintains bilateral LS within a state of remission regardless of sex, mouse strain, or model of inflammatory pain.
Plantar incision can engage ligand-dependent, Gi-protein coupled, neuropeptide Y receptor mechanisms, indicating that NPY release can tonically inhibit LS (Solway et al., 2011). However, germline deletion of dynorphin did not change NTX-induced reinstatement in a CFA model of LS (Walwyn et al., 2016). Furthermore, spinal dynorphin levels did not change during antagonist-induced reinstatement of hyperalgesia in mice with incision (Campillo et al., 2011). These results argue against tonic dynorphin release onto KORs as a mechanism that maintains LS in remission. An intriguing alternative hypothesis is that incision engages a ligand-independent form of signaling termed “constitutive activity” (Costa and Herz, 1989; Kenakin, 2001, 2004; Seifert and Wenzel-Seifert, 2002). Our laboratory reported that CFA inflammation establishes µ opioid receptor constitutive activity (MORCA; Corder et al., 2013). An important unresolved question is whether incision similarly establishes constitutive activity at KOR. Rigorous testing of this hypothesis awaits further development of neutral antagonists and inverse agonists at KOR (Walwyn et al., 2016). Neurophysiological studies in molecularly defined KOR-expressing neurons would be of particular interest; fluorescent in situ hybridization studies indicate that they are heterogeneous, with ∼50% excitatory and 50% inhibitory (T. D. Sheahan, personal communication).
Kappa-opioid receptors maintain LS in remission for at least 1 year after tissue injury
Nor-BNI or naltrexone can reinstate hyperalgesia when administered ∼5 months after plantar incision or intraplantar CFA, respectively (Campillo et al., 2011; Corder et al., 2013). The present study shows that LY2456302 reinstated hyperalgesia in male mice when administered not just 6 months but even 13 months after incision. This extends the known duration of LS and its opposing inhibition to more than a year, making LS among the longest-lasting rodent models of chronic pain, rivaling the time course of the tibia fracture model of complex regional pain syndrome (Tajerian et al., 2015; Wang et al., 2016; Guo et al., 2018) and the spared nerve injury model of neuropathic pain (Decosterd and Woolf, 2000; Intondi et al., 2010).
NMDA receptors contribute to the latent postoperative pain that is masked by KOR
In male mice, pharmacological inhibition of spinal NMDA receptors with intrathecal MK-801 prevented reinstatement of KOR-antagonist-induced mechanical hypersensitivity on plantar incision (Campillo et al., 2011) and also prevented reinstatement of mechanical hypersensitivity in the CFA model of LS after either naltrexone (Corder et al., 2013) or the NPY Y1 antagonist BIBO3304 (Fu et al., 2019). The current results demonstrate that MK-801 prevented LY2456302-induced reinstatement of mechanical hypersensitivity in female mice as well.
Incision did not change GluN1 gene expression in either sex during the peak of hyperalgesia (2 d postsurgery) or during remission (21 d), consistent with reports of no change in dorsal horn GluN1 gene expression when measured 1 d into CFA inflammation (Yang et al., 2009). On the other hand, Du et al. (2003) reported an upregulation of spinal GluN1 mRNA following CFA inflammation. Thus, NMDA receptors contribute to LS in both sexes, but this is unrelated to spinal GluN1 gene expression.
AC1 contributes to the latent postoperative pain that is masked by KOR
We found that either genetic or pharmacological disruption of AC1 prevented LY2456302-induced reinstatement of mechanical hypersensitivity and spinal pERK expression in both sexes with plantar incision but not sham surgery. These results agree with previous studies suggesting that AC1 modulates inflammatory and persistent pain states (Wei et al., 2002; Wang et al., 2011; Corder et al., 2013), including latent inflammatory pain (Corder et al., 2013) and hyperalgesic priming (Parada et al., 2005; Araldi et al., 2015). Because incision did not change AC1 gene expression of either sex during the peak of hyperalgesia (2 d postsurgery) or during remission (21 d postsurgery), the AC1 contribution to LS is unrelated to its gene expression.
Surgical incision produces a long-lasting sensitization of PKA signaling that contributes to latent postoperative pain in a sex-dependent manner
The current 6-bnz-cAMP studies extend our previous findings in male mice that used a single dose of 6-bnz-cAMP (10 nmol, i.t.) in the CFA model of LS without sham surgery controls (Fu et al., 2019). Here we studied both male and female mice using multiple doses of 6-bnz-cAMP (3–30 nmol) in the incision model of LS along with sham surgery controls. In males, 6-Bnz-cAMP reinstated mechanical hypersensitivity in incision but not sham surgical controls when tested 21 d after incision during the remission phase of LS, indicating a sensitization of PKA signaling. Also, 6-Bnz-cAMP reinstated mechanical hypersensitivity at both sides of the body, indicating that PKA contributes to the bilateral nature of LS, as we have previously described (Corder et al., 2013). Doses as low as 3 nmol produced robust hyperalgesia (further studies with even lower doses are needed to determine ED50). Remarkably, 6-bnz-cAMP completely failed to reinstate mechanical hypersensitivity in females, even at a 10-fold higher dose (30 nmol).
H89 reversed LY2456302-induced reinstatement of hypersensitivity in male but not female mice, indicating a sex difference in long-lasting sensitization of spinal PKA signaling. This is consistent with reports that the development of hyperalgesic priming and consolidation of chronic musculoskeletal pain is independent of PKA signaling in female mice (male mice were not studied; W. H. Chen et al., 2018). On the other hand, in a type II hyperalgesic priming model involving repeated intradermal injection of DAMGO, administration of the PKA activator 8-bromo cAMP prolonged hyperalgesia, and H89 reduced priming in both male and female rats (Araldi et al., 2015). Possible reasons for this discrepancy include species differences or mechanistic differences between latent sensitization and hyperalgesic priming.
Because incision did not change PKA gene expression in the ipsilateral dorsal horn of either sex during the peak of hyperalgesia or remission, we conclude that incision sensitizes spinal PKA signaling and a PKA-dependent LS that is masked by KOR activity, but this is unrelated to gene expression.
Surgical incision produces a long-lasting sensitization of Epac signaling that contributes to latent postoperative pain in both sexes
As previously described in the male CFA model of LS (Fu et al., 2019), we report that Epac activation with 8-CPT reinstates mechanical hypersensitivity in the incision model of LS in both males and females. These results indicate that incision sensitizes spinal Epac signaling in both sexes.
Intrathecal ESI-09 prevented the ability of LY2456302 to reinstate postoperative hyperalgesia, just as it attenuated BIBO3304-evoked reinstatement of hyperalgesia in the CFA model of LS (Fu et al., 2019), and it prevented prostaglandin E2-induced hyperalgesia when tested 14 d after incision-induced hyperalgesic priming (Matsuda et al., 2017). We conclude that Epac is required for the maintenance of LS under multiple conditions.
Incision decreased dorsal horn Epac1 gene expression in female compared with male mice when measured 21 d but not 2 d after incision. By contrast, studies in male Sprague Dawley rats indicate either no change or an increase in spinal Epac 1 after tissue injury (Vasko et al., 2014; Cao et al., 2016; Matsuda et al., 2017). A species difference might account for this discrepancy.
Plantar incision does not change bulk gene expression of KOR in the dorsal horn
We found that incision did not change ipsilateral KOR gene expression in dorsal horn in either sex or at either time point postsurgery, consistent with reports in CFA-induced hyperalgesia (Massaly et al., 2019) and the formalin test (Yoon et al., 2011) in male rats and in nerve injury-induced allodynia in male rats (Obara et al., 2009) or mice (Pol et al., 2006).
Summary
We conclude that long-lasting KOR signaling provides endogenous analgesia via inhibition of AC1 signaling in a preclinical LS model of chronic postoperative pain and that the mechanism of latent postoperative pain is different between male and female mice. As illustrated in Figure 14, NMDAR→AC1→cAMP→Epac + PKA mechanisms drive LS in males, whereas just an NMDAR→AC1→cAMP→Epac signaling mechanism drives LS in females. Both Epac and PKA pathways are kept in remission by sustained KOR activation. We propose that AC1 and Epac represent promising therapeutic targets for chronic postoperative pain in men, whereas just Epac represents a target in women. Our results underscore the importance of biological sex in the identification of molecular targets for chronic pain management.
Figure 14.
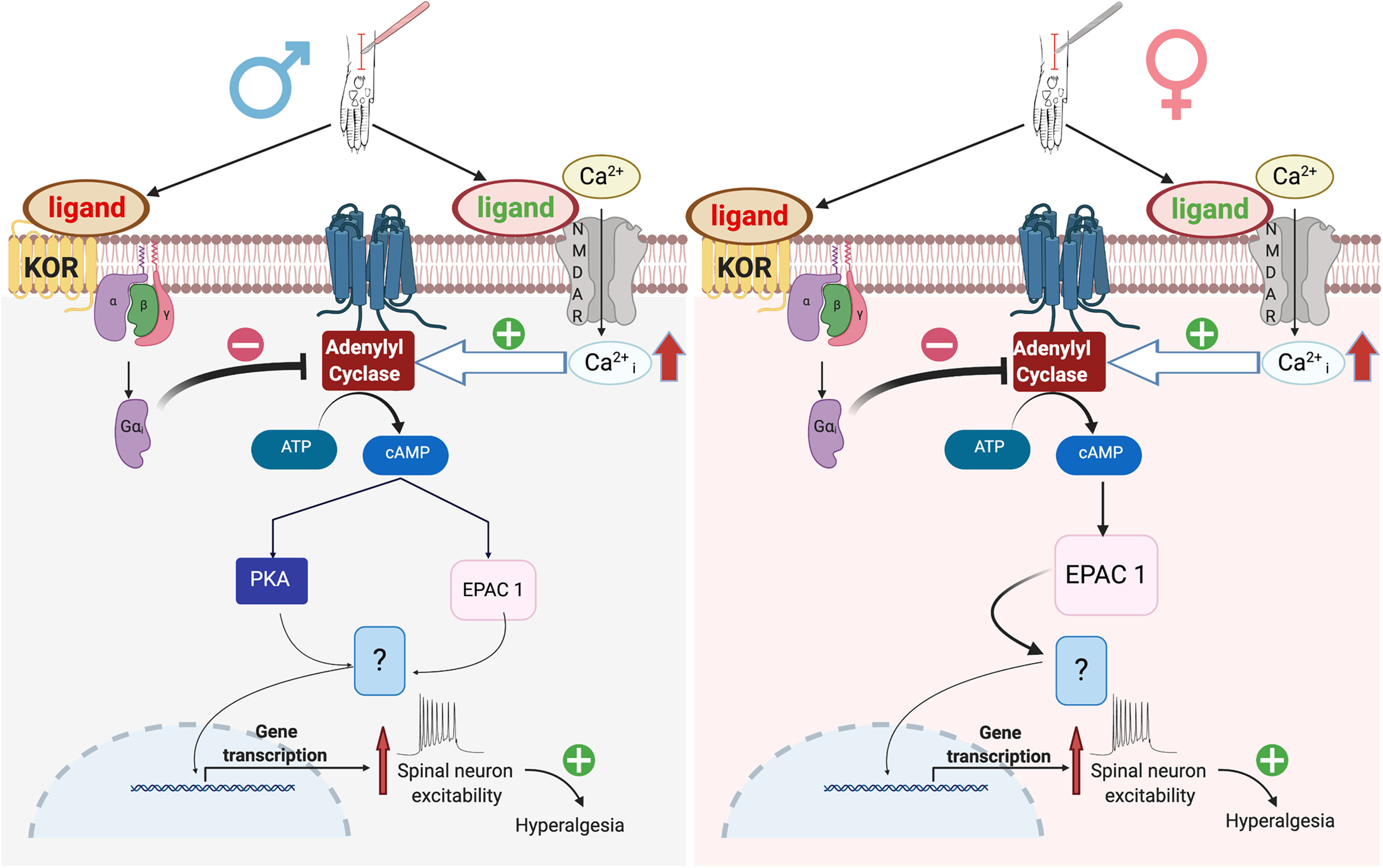
A conceptual model of latent incision-induced chronic pronociceptive signaling that is kept in remission by tonic endogenous KOR activity in the dorsal horn. KOR is an inhibitory GPCR and as such inhibits the activity of the AC. AC1 is a Ca2+-activated protein, which converts the ATP to cAMP and acts as a key modulator for the induction of pathologic pain. It couples the intracellular Ca2+ derived from NMDAR activation to downstream activation of an array of downstream proteins and kinases. AC1–cAMP signaling activates multiple downstream signal molecules including the canonical PKA as well as the Epac protein family. In male mice (left), incision induces both NMDAR→AC1→PKA and NMDAR→AC1→Epac signaling pathways, leading to changes in gene transcription through undetermined transcription factors (?) and ultimately long-term changes in spinal neuron hyperexcitability, hyperalgesia, and affective pain. By contrast, in female mice (right), KOR inhibition operates through just the NMDAR→AC1→Epac signaling pathway.
Footnotes
This work was supported by National Institutes of Health Grants R01DA37621, R01NS45954, and R01NS62306 to B.K.T. and T32DA016176 to L.C.-P. We thank Renee Donahue and Diogo dos Santos for technical assistance; University of Kentucky faculty members Dr. Linda Dwoskin, director of the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant (T32), who supported L.C.-P. after B.K.T. moved to the University of Pittsburgh; Dr. Daniel Storm (Washington University, Seattle) for providing the AC1 knock-out mice; and Dr. Jia Zhuo (University of Texas Medical Branch, Galveston, Texas) for providing the ESI-09.
The authors declare no competing financial interests.
References
- Aley KO, Levine JD (1999) Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci 19:2181–2186. 10.1523/JNEUROSCI.19-06-02181.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD (2000) Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci 20:4680–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X (2013) A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 83:122–128. 10.1124/mol.112.080689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2015) Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci 35:12502–12517. 10.1523/JNEUROSCI.1673-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2017) Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain 158:1204–1216. 10.1097/j.pain.0000000000000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Nevo I, Saya D, Bayewitch M, Vogel Z (1997) Opiate-induced adenylyl cyclase superactivation is isozyme-specific. J Biol Chem 272:5040–5047. 10.1074/jbc.272.8.5040 [DOI] [PubMed] [Google Scholar]
- Bos JL (2003) Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4:733–738. 10.1038/nrm1197 [DOI] [PubMed] [Google Scholar]
- Campillo A, Cabañero D, Romero A, García-Nogales P, Puig MM (2011) Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol 657:89–96. 10.1016/j.ejphar.2011.01.059 [DOI] [PubMed] [Google Scholar]
- Cao S, Bian Z, Zhu X, Shen SR (2016) Effect of Epac1 on pERK and VEGF activation in postoperative persistent pain in rats. J Mol Neurosci 59:554–564. 10.1007/s12031-016-0776-x [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- Chen H, Ding C, Wild C, Liu H, Wang T, White MA, Cheng X, Zhou J (2013) Efficient synthesis of ESI-09, a novel non-cyclic nucleotide EPAC antagonist. Tetrahedron Lett 54:1546–1549. 10.1016/j.tetlet.2013.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ennes HS, McRoberts JA, Marvizón JC (2018) Mechanisms of mu-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord. Neuropharmacology 128:255–268. 10.1016/j.neuropharm.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Chang YT, Chen YC, Cheng SJ, Chen CC (2018) Spinal PKC/ERK signal pathway mediates hyperalgesia priming. Pain 159:907–918. 10.1097/j.pain.0000000000001162 [DOI] [PubMed] [Google Scholar]
- Cheng X, Ji Z, Tsalkova T, Mei F (2008) Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 40:651–662. 10.1111/j.1745-7270.2008.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Fleming L, Konkoy C, Marckel D, Pacheco M, Sexton T, Ward S (1992) Opioid and cannabinoid receptor inhibition of adenylyl cyclase in brain. Ann N Y Acad Sci 654:33–51. 10.1111/j.1749-6632.1992.tb25954.x [DOI] [PubMed] [Google Scholar]
- Cooper AH, Hedden NS, Corder G, Lamerand SR, Donahue RR, Morales-Medina JC, Selan L, Prasoon P, Taylor BK (2021) Endogenous µ-opioid receptor activity in the lateral and capsular subdivisions of the right central nucleus of the amygdala prevents chronic postoperative pain. J Neurosci Res 10.1002/jnr.24846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK (2013) Constitutive µ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341:1394–1399. 10.1126/science.1239403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Herz A (1989) Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A 86:7321–7325. 10.1073/pnas.86.19.7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodio-Patsey L, Donahue RR, Fu W, Lambert J, Smith BN, Taylor BK (2020) Sex differences in kappa opioid receptor inhibition of latent postoperative pain sensitization in dorsal horn. Neuropharmacology 163:107726. 10.1016/j.neuropharm.2019.107726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477. 10.1038/24884 [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158. 10.1016/S0304-3959(00)00276-1 [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM (2003) N-methyl-d-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience 118:547–562. 10.1016/s0306-4522(03)00009-5 [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN (2013) A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun 4:1682. 10.1038/ncomms2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA (2003) Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev 55:1007–1041. 10.1016/s0169-409x(03)00101-7 [DOI] [PubMed] [Google Scholar]
- França A, Freitas AI, Henriques AF, Cerca N (2012) Optimizing a qPCR gene expression quantification assay for S. epidermidis biofilms: a comparison between commercial kits and a customized protocol. PLoS One 7:e37480. 10.1371/journal.pone.0037480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Nelson TS, Santos DF, Doolen S, Gutierrez JJP, Ye N, Zhou J, Taylor BK (2019) An NPY Y1 receptor antagonist unmasks latent sensitization and reveals the contribution of protein kinase A and Epac to chronic inflammatory pain. Pain 160:1754–1765. 10.1097/j.pain.0000000000001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ (2007) Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132 Suppl 1:S26–S45. 10.1016/j.pain.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin RS (2005) An Epac-dependent pain pathway. J Neurosci 25:8113–8114. 10.1523/JNEUROSCI.3044-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li G, Chen Y, Huang LY (2016) Epac-protein kinase C alpha signaling in purinergic P2X3R-mediated hyperalgesia after inflammation. Pain 157:1541–1550. 10.1097/j.pain.0000000000000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T-Z, Wei T, Huang T-T, Kingery WS, Clark JD (2018) Oxidative stress contributes to fracture/cast-induced inflammation and pain in a rat model of complex regional pain syndrome. J Pain 19:1147–1156. 10.1016/j.jpain.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD (2005) Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci 25:6119–6126. 10.1523/JNEUROSCI.0285-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intondi AB, Zadina JE, Zhang X, Taylor BK (2010) Topography and time course of changes in spinal neuropeptide Y immunoreactivity after spared nerve injury. Neuroscience 165:914–922. 10.1016/j.neuroscience.2009.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Liang D, Kido K, Sun Y, Clark DJ, Brennan TJ (2011) Increased local concentration of complement C5a contributes to incisional pain in mice. J Neuroinflammation 8:80. 10.1186/1742-2094-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R-R, Baba H, Brenner GJ, Woolf CJ (1999) Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 2:1114–1119. 10.1038/16040 [DOI] [PubMed] [Google Scholar]
- Ji R-R, Gereau IR, Malcangio M, Strichartz GR (2009) MAP kinase and pain. Brain Res Rev 60:135–148. 10.1016/j.brainresrev.2008.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G-Y, Dai M-H, Huang K, Chai G-D, Chen J-Y, Chen L, Lang B, Wang Q-X, St Clair D, McCaig C, Ding Y-Q, Zhang L (2015) Neurochemical characterization of pERK-expressing spinal neurons in histamine-induced itch. Sci Rep 5:12787–12710. 10.1038/srep12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Price TJ (2015) The pharmacology of nociceptor priming. Handb Exp Pharmacol 227:15–37. 10.1007/978-3-662-46450-2_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. Lancet 367:1618–1625. 10.1016/S0140-6736(06)68700-X [DOI] [PubMed] [Google Scholar]
- Kenakin T (2001) Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J 15:598–611. 10.1096/fj.00-0438rev [DOI] [PubMed] [Google Scholar]
- Kenakin T (2004) Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25:186–192. 10.1016/j.tips.2004.02.012 [DOI] [PubMed] [Google Scholar]
- Lawson KP, Nag S, Thompson AD, Mokha SS (2010) Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. PAIN 151:806–815. 10.1016/j.pain.2010.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liauw J, Wu LJ, Zhuo M (2005) Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J Neurophysiol 94:878–882. 10.1152/jn.01205.2004 [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI (1997) Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci 17:7462–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RW, McCall JG, Al-Hasani R, Bruchas MR, Morón JA (2019) Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102:564–573.e6. 10.1016/j.neuron.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Oh-Hashi K, Yokota I, Sawa T, Amaya F (2017) Acquired exchange protein directly activated by cyclic adenosine monophosphate activity induced by p38 mitogen-activated protein kinase in primary afferent neurons contributes to sustaining postincisional nociception. Anesthesiology 126:150–162. 10.1097/ALN.0000000000001401 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ozawa T, Watanabe T, Minami M, Satoh M (1999) Sensitization of the adenylyl cyclase system in cloned kappa-opioid receptor-transfected cells following sustained agonist treatment: a chimeric study using G protein alpha(i)2/alpha(q) subunits. Jpn J Pharmacol 81:353–361. 10.1016/S0021-5198(19)30746-2 [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R (2009) Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 141:283–291. 10.1016/j.pain.2008.12.006 [DOI] [PubMed] [Google Scholar]
- Parada CA, Reichling DB, Levine JD (2005) Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain 113:185–190. 10.1016/j.pain.2004.10.021 [DOI] [PubMed] [Google Scholar]
- Pereira MP, Donahue RR, Dahl JB, Werner M, Taylor BK, Werner MU (2015) Endogenous opioid-masked latent pain sensitization: studies from mouse to human. PLoS One 10:e0134441. e0134441. 10.1371/journal.pone.0134441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki EM, Raja SN (2003) A mouse model of incisional pain. Anesthesiology 99:1023–1027. 10.1097/00000542-200310000-00041 [DOI] [PubMed] [Google Scholar]
- Pol O, Murtra P, Caracuel L, Valverde O, Puig MM, Maldonado R (2006) Expression of opioid receptors and c-fos in CB1 knockout mice exposed to neuropathic pain. Neuropharmacology 50:123–132. 10.1016/j.neuropharm.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2ˆ(−delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 3:71–85. [PMC free article] [PubMed] [Google Scholar]
- Rehmann H, Schwede F, Døskeland SO, Wittinghofer A, Bos JL (2003) Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem 278:38548–38556. 10.1074/jbc.M306292200 [DOI] [PubMed] [Google Scholar]
- Reichling D, Levine J (2009) Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 32:611–618. 10.1016/j.tins.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL (2014a) LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 77:131–144. 10.1016/j.neuropharm.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witcher JW, Lowe SL, Gonzales CR, Weller MA, Bell RL, Hart JC, Need AB, McKinzie JH, Statnick MA (2014b) Determining pharmacological selectivity of the kappa opioid receptor antagonist LY2456302 using pupillometry as a translational biomarker in rat and human. Int J Neuropsychopharmacol 18: 10.1093/ijnp/pyu036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366:381–416. 10.1007/s00210-002-0588-0 [DOI] [PubMed] [Google Scholar]
- Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr., Murga C, Heijnen CJ, Kavelaars A (2016) Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci U S A 113:3036–3041. 10.1073/pnas.1516036113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhmar P, Huo X, Li Y, Dougherty PM, Mei F, Cheng X, Heijnen CJ, Kavelaars A (2018) Orally active Epac inhibitor reverses mechanical allodynia and loss of intraepidermal nerve fibers in a mouse model of chemotherapy-induced peripheral neuropathy. Pain 159:884–893. 10.1097/j.pain.0000000000001160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA (1997) Activation of the cAMP transduction cascade contributes to the mechanical hyperalgesia and allodynia induced by intradermal injection of capsaicin. Br J Pharmacol 122:1165–1173. 10.1038/sj.bjp.0701486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK (2011) Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A 108:7224–7229. 10.1073/pnas.1017719108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD (1989) Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience 32:577–580. 10.1016/0306-4522(89)90280-7 [DOI] [PubMed] [Google Scholar]
- Tajerian M, Sahbaie P, Sun Y, Leu D, Yang HY, Li W, Huang TT, Kingery W, David Clark J (2015) Sex differences in a murine model of complex regional pain syndrome. Neurobiol Learn Mem 123:100–109. 10.1016/j.nlm.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Corder G (2014) Endogenous analgesia, dependence, and latent pain sensitization. Curr Top Behav Neurosci 20:283–325. 10.1007/7854_2014_351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumati S, Roeske WR, Largent-Milnes TM, Vanderah TW, Varga EV (2011) Intrathecal PKA-selective siRNA treatment blocks sustained morphine-mediated pain sensitization and antinociceptive tolerance in rats. J Neurosci Methods 199:62–68. 10.1016/j.jneumeth.2011.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üçeyler N, Tscharke A, Sommer C (2007) Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun 21:553–560. 10.1016/j.bbi.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Üçeyler N, Topuzoğlu T, Schiesser P, Hahnenkamp S, Sommer C (2011) IL-4 deficiency is associated with mechanical hypersensitivity in mice. PloS One 6:e28205–e28205. 10.1371/journal.pone.0028205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko MR, Habashy Malty R, Guo C, Duarte DB, Zhang Y, Nicol GD (2014) Nerve growth factor mediates a switch in intracellular signaling for PGE2-induced sensitization of sensory neurons from protein kinase A to Epac. PLoS One 9:e104529. 10.1371/journal.pone.0104529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn WM, Chen W, Kim H, Minasyan A, Ennes HS, McRoberts JA, Marvizon JC (2016) Sustained suppression of hyperalgesia during latent sensitization by µ-, δ-, and κ-opioid receptors and α2A adrenergic receptors: role of constitutive activity. J Neurosci 36:204–221. 10.1523/JNEUROSCI.1751-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, Kaang BK, Zhuo M (2011) Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med 3:65ra3–ra65r63. [DOI] [PubMed] [Google Scholar]
- Wang H, Heijnen CJ, van Velthoven CTJ, Willemen HLDM, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A (2013) Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest 123:5023–5034. 10.1172/JCI66241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Guo T-Z, Hou S, Wei T, Li W-W, Shi X, Clark JD, Kingery WS (2016) Bisphosphonates inhibit pain, bone loss, and inflammation in a rat tibia fracture model of complex regional pain syndrome. Anesth Analg 123:1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ, Zhuo M (2002) Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 36:713–726. 10.1016/s0896-6273(02)01019-x [DOI] [PubMed] [Google Scholar]
- Wei F, Vadakkan KI, Toyoda H, Wu LJ, Zhao MG, Xu H, Shum FW, Jia YH, Zhuo M (2006) Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci 26:851–861. 10.1523/JNEUROSCI.3292-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2007) Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 106:864–867. 10.1097/01.anes.0000264769.87038.55 [DOI] [PubMed] [Google Scholar]
- Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR (1995) Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci U S A 92:220–224. 10.1073/pnas.92.1.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M (2008) Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 28:7445–7453. 10.1523/JNEUROSCI.1812-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Shimamura M, Narita M, Kubota C, Suzuki T (2003) Differential involvement of spinal protein kinase C and protein kinase A in neuropathic and inflammatory pain in mice. Brain Res 992:288–293. 10.1016/j.brainres.2003.08.042 [DOI] [PubMed] [Google Scholar]
- Yamanaka M, Matsuura T, Pan H, Zhuo M (2017) Calcium-stimulated adenylyl cyclase subtype 1 (AC1) contributes to LTP in the insular cortex of adult mice. Heliyon 3:e00338. 10.1016/j.heliyon.2017.e00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang HB, Xie QJ, Liu XH, Hu XD (2009) Peripheral inflammation increased the synaptic expression of NMDA receptors in spinal dorsal horn. Pain 144:162–169. 10.1016/j.pain.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Yoon MH, Kim KS, Lee HG, Kim CM, Kim WM, Choi JI, Kim YO (2011) Synergistic interaction between intrathecal ginsenosides and morphine on formalin-induced nociception in rats. J Pain 12:774–781. 10.1016/j.jpain.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Zhuo M (2012) Targeting neuronal adenylyl cyclase for the treatment of chronic pain. Drug Discov Today 17:573–582. 10.1016/j.drudis.2012.01.009 [DOI] [PubMed] [Google Scholar]