Abstract
Background:
Several Canadian provinces have introduced reimbursement policies mandating substitution of innovator biologics with lower-cost biosimilars. We estimated the number of patients affected and cost implications if such policy changes were to be implemented in Ontario, Canada.
Methods:
We conducted a cross-sectional time series analysis of Ontarians dispensed publicly funded biologics indicated for inflammatory diseases (rheumatic conditions, inflammatory bowel disease: infliximab, etanercept, adalimumab) between January 2018 and December 2019, and forecasted trends to Dec. 31, 2020. The primary source of data was pharmacy claims data for all biologics reimbursed by the public drug program. We modelled the number of patients affected and government expenditures (in nominal Canadian dollars) of several biosimilar policy options, including mandatory nonmedical biosimilar substitution, substitution in new users, introduction of a biosimilar for adalimumab, and price negotiations. In a secondary analysis, we included insulin glargine.
Results:
In 2018, 14 089 individuals were prescribed a publicly funded biologic for inflammatory diseases. A mandatory nonmedical biosimilar substitution would potentially have affected 7209 patients and saved $238.6 million from 2018 to 2020. A new-user substitution would have affected 757 patients and saved $34.2 million. If an adalimumab biosimilar were to become available, 12 928 patients would be affected by a mandatory nonmedical substitution and the 3-year savings would increase to $645.9 million (all biosimilars priced at 25% of innovator biologics). Finally, an expanded nonmedical substitution policy including insulin glargine would affect 115 895 patients and save $288.7 million (not including adalimumab).
Interpretation:
Policies designed to curb rising costs of biologics can have substantially different effects on patients and government expenditures. Such analyses warrant careful consideration of the balance between cost savings and effects on patients.
Biologic drugs have improved outcomes for individuals across a range of chronic medical conditions, including diabetes and rheumatic and gastrointestinal diseases. 1,2 However, unlike conventional small-molecule pharmaceuticals, biologics are derived from living organisms, are structurally more complex, and have substantially higher costs.1 In 2018, biologics represented only 1.5% of Canadian public drug plan claims, but accounted for 27.3% of public drug costs.3 In addition to their generally higher list prices, utilization of biologics has grown substantially in the past decade. In Ontario, the total number of people taking biologics increased by 462% between 2010 and 2019, and total annual spending on these products was anticipated to reach $1.4 billion by 2021.4 Although biologics are improving outcomes for patients, their increasing use and high costs threaten the financial sustainability of public drug programs.
The recent expiration of patents for some biologic drugs has created opportunities for the approval of new, lower-cost “biosimilars” — biologic medicines that are highly similar to an existing innovator biologic drug, with no clinically meaningful differences in efficacy, safety or immunogenicity.1,2 In 2010, Health Canada released a regulatory framework outlining the approval process for biosimilars.5 By building on the foundation of research and development already established by innovator biologics, biosimilars offer an opportunity for substantial cost savings for public and private drug plans.6,7 The first biosimilar was marketed more than a decade ago, but uptake of biosimilars has been modest in Canada relative to other Organisation for Economic Co-operation and Development countries.8,9 Consequently, Canadian public and private drug plans have begun to implement policies aimed at expanding the use of biosimilars.
In 2019, the Canadian provincial governments of British Columbia (BC) and Alberta announced policies mandating nonmedical substitution with biosimilars among people with rheumatic conditions and inflammatory bowel disease (IBD).7,10 It is estimated that these polices will save the BC and Alberta governments nearly $100 million each over the first 3 years of implementation.7,11 Despite these anticipated cost savings, concerns have been raised regarding potential destabilization of well-managed disease when medications are switched.12,13
The objective of this study was to estimate the number of patients potentially affected by different biosimilar policy options and the cost implications of these policies in Ontario.
Methods
Design, setting and study population
We conducted a cross-sectional time series analysis of all Ontarians dispensed a publicly funded prescription for infliximab, etanercept or adalimumab, to manage rheumatic conditions (i.e., rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, and severe plaque psoriasis) or IBD (i.e., ulcerative colitis, Crohn disease) between Jan. 1, 2018, and Dec. 31, 2019. We analyzed data by month and projected forward to forecast utilization up to Dec. 31, 2020.
Three people with lived experience of using biologics participated on the study team. Their engagement included meetings throughout the project, input on the study design, interpretation of results and manuscript content.
Data sources
We used the IQVIA Drug Information File to identify relevant drug identification numbers for biologics and to categorize biologics into innovators and biosimilars, and the Ontario Drug Benefit (ODB) Program database to capture prescriptions for biologics reimbursed by the public drug program. In Ontario, individuals are eligible for ODB if they are older than 65 years, reside in a long-term care home, receive income or disability support, or have drug costs that are high relative to their income. Prescription cost data in the ODB Program database include the total amount paid by the government, and copayments and deductibles paid by the patient.
We excluded individuals who newly received public coverage for biologics during the extended Ontario Health Insurance Program (OHIP+) drug program (which temporarily covered all children and youth) on Jan. 1, 2018, but subsequently appeared to lose coverage after changes to the program on Apr. 1, 2019, so as to ensure projected costs reflected the current OHIP+ program. We used the OHIP Registered Persons Database to determine the age and sex of individuals included in the study.
The data sets we used have been shown to be of high quality (Appendix 1, eTable 1, available at www.cmajopen.ca/content/9/4/E1055/suppl/DC1),14 and were linked using unique encoded identifiers and analyzed at ICES.
Policy definitions and cost adjustments
We calculated total monthly costs for study biologics (infliximab, etanercept and adalimumab) in nominal Canadian dollars. This was calculated as the sum of the total paid by the Ontario Ministry of Health (i.e., the sum of the drug ingredient cost, compounding fee [if applicable], pharmacy markup and dispensing fee) and the copayments and deductibles paid by the patient. We adjusted these costs according to 2 potential reimbursement policy options, and 3 pricing considerations.
Reimbursement policy options
We considered 2 policy options that are aligned with those introduced elsewhere and that were found to be feasible and applicable within the Ontario public drug program.7,10,15 Specifically, these were a mandatory nonmedical substitution, whereby any patient receiving an innovator biologic has therapy substituted with the relevant biosimilar; and an enforced biosimilar requirement among new users of biologics only.
We modelled the mandatory nonmedical substitution by identifying all innovator biologic prescriptions dispensed each month and multiplying the medication ingredient costs by an adjustment factor (calculated as the median price paid by the Ontario Ministry of Health for biosimilar prescriptions reimbursed over the study period as a proportion of the cost reimbursed for the innovator biologic) to reduce the cost to that of the relevant biosimilar (Table 1). We then calculated the new pharmacy markup (6% for claims above $1000 and 8% for claims below $1000, aligning with current markup policies) and added it to the adjusted costs, along with dispensing fees.
Table 1:
Adjustment factors for biologic prices
| Biologic | Primary analysis: adjustment factor, % | Policy consideration #1: include insulin glargine, % | Policy consideration #2: include biosimilar for adalimumab, % | Policy consideration #3: negotiated price reductions below threshold, % |
|---|---|---|---|---|
| Etanercept | 62.0 | 62.8 | 62.8 | 75, 50 |
| Infliximab | 53.2 | 53.2 | 53.2 | 75, 50 |
| Adalimumab | NA | NA | 60.0 | 75, 50 |
| Insulin glargine | NA | 75.0 | NA | NA |
Note: NA = not applicable.
In contrast, when modelling the biosimilar requirement among new users only, we applied these adjusted costs only to people newly starting an innovator biologic in the month of interest, or to people who had previously started an innovator biologic during our study period. This will accumulate cost implications over time as we assumed that new users from earlier months continued using the biosimilar in future months.
Pricing and policy expansion considerations
We combined the 2 policy options above with 3 policy considerations. First, we modelled the impact of the introduction of a biosimilar for adalimumab (which did not have a marketed biosimilar in Canada during the study period). In this analysis, we adjusted the price of the innovator to align with the price of the newly approved adalimumab biosimilar (60%; Table 1). Second, we modelled the implications of price negotiations across all biologics for IBD and rheumatic conditions, setting biosimilar cost thresholds at 25% and 50% of the innovator price. Finally, we modelled the impact of adding insulin glargine, a long-acting insulin, to the list of currently available innovator biologics with an assumed biosimilar cost of 75% of the innovator cost. These policy considerations were informed by previous policies introduced in BC (which include insulin and adalimumab) and Alberta (which includes adalimumab),7,10 and through discussions with managers of public drug plans across Canada to establish estimates of cost thresholds.
Statistical analysis
We summarized patient- and prescription-level characteristics for all biologics indicated for rheumatic conditions or IBD dispensed in calendar year 2018 overall and stratified by biologic type. In the time series analysis, we modelled and forecasted monthly costs of biologics based on current trends (calendar years 2018/19), under each of the policy options and considerations up to Dec. 31, 2020, using a Holt–Winters exponential smoothing model with the additive method, selected to provide the optimal model fit.16,17 To estimate the 3-year cost implications of each policy option, we summed the adjusted actual and forecasted costs from January 2018 to December 2020 in each model. We estimated the number of individuals affected by each policy option according to the real-world prescribing patterns in 2018. In 2 sensitivity analyses, we expanded our cohort definition to include Ontarians dispensed insulin glargine over the same study period, to align with similar policies introduced in BC, and replicated our primary analysis considering only costs to the public payer.
Analyses were conducted at ICES using SAS Enterprise Guide, version 7.1 (SAS Institute, Inc. Cary, NC) and used a type 1 error rate of 0.05 to determine statistical significance.
Ethics approval
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Results
In 2018, 14 089 individuals received a publicly funded biologic indicated for rheumatic conditions or IBD (Table 2). Adalimumab was prescribed most frequently (n = 5782, 41.0%), followed by infliximab (n = 4558, 32.4%) and etanercept (n = 3872, 27.5%). Overall, 54.3% (n = 7656) of users of biologics were women and 61.8% (n = 8703) were younger than 65 years, although these patterns differed by drug. For example, 63.4% (n = 2454) of users of etanercept were women, and 58.3% (n = 2258) were older than 65 years.
Table 2:
Characteristics of biologics use among people with rheumatic or gastrointestinal conditions, 2018
| Characteristic | No. (%)* of people prescribed a biologic | |||
|---|---|---|---|---|
| Any biologic n = 14 089 |
Etanercept n = 3872 |
Adalimumab n = 5782 |
Infliximab n = 4558 |
|
| Sex | ||||
| Male | 6433 (45.7) | 1418 (36.6) | 2664 (46.1) | 2405 (52.8) |
| Female | 7656 (54.3) | 2454 (63.4) | 3118 (53.9) | 2153 (47.2) |
| Age, yr | ||||
| < 18 | 441 (3.1) | 51 (1.3) | 179 (3.1) | 224 (4.9) |
| 18–44 | 4310 (30.6) | 456 (11.8) | 1838 (31.8) | 2049 (45.0) |
| 45–64 | 3952 (28.1) | 1107 (28.6) | 1682 (29.1) | 1195 (26.2) |
| ≥ 65 | 5386 (38.2) | 2258 (58.3) | 2083 (36.0) | 1090 (23.9) |
| Patients treated with any innovator biologics | 12 928 (91.8) | 3256 (84.1) | 5782 (100) | 3954 (86.7) |
| New users | ||||
| Biologics | 3219 | 773 | 1708 | 767 |
| Innovator biologics | 2924 (90.8) | 305 (39.5) | 1708 (100) | 459 (59.8) |
| Prescriptions dispensed | ||||
| Biologics | 98 070 | 27 920 | 41 582 | 28 568 |
| Innovator biologics | 91 261 (93.1) | 24 270 (86.9) | 41 582 (100) | 25 409 (88.9) |
| Total cost, $ | ||||
| Biologics | 280 782 091 | 62 083 387 | 94 391 665 | 124 307 040 |
| Innovator biologics | 268 348 355 | 57 336 774 | 94 391 665 | 116 619 916 |
| Average no. biologic prescriptions/person | 7.0 | 7.2 | 7.2 | 6.3 |
| Average cost of biologics per person, $ | 19 929 | 16 034 | 16 325 | 27 272 |
Unless otherwise specified.
Among biologics with a biosimilar available in Ontario, 84.1% (n = 3256) of users of etanercept and 86.7% (n = 3954) of users of infliximab were treated with an innovator. However, when we considered new use, 39.5% (n = 305) of people starting etanercept and 59.8% (n = 459) of those starting infliximab began on an innovator.
Overall, the cost of biologics in 2018 was $280 782 091, and the average cost of biologics per person was $19 929, ranging from $16 034 per person treated with etanercept to $27 272 per person treated with infliximab.
Trends in monthly costs
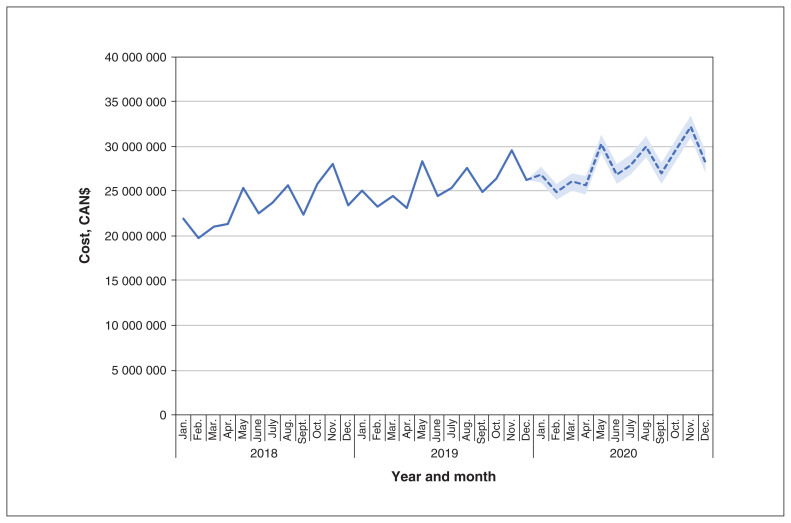
The monthly costs of biologics for rheumatic conditions and IBD increased over our study period, rising from $21 883 713 in January 2018 to $26 331 208 in December 2019 (Figure 1). Monthly costs were forecasted to reach $28 246 752 (95% confidence interval [CI] $26 984 908 to $29 508 595) by December 2020 if current trends continued. In the sensitivity analysis that considered only public payer costs, monthly costs and patterns were similar over time (rise from $21 682 705 to $25 678 079 from January 2018 to December 2019). Assuming current reimbursement policies for biologics indicated for IBD and rheumatic conditions remained the same in Ontario, we anticipated that these medications would cost a total of $925 266 759 from 2018 to 2020.
Figure 1:
Forecasted trends in monthly biologics costs over time if current trends continue. Actual data are presented with a solid line from January 2018 to December 2019, with projected estimates presented with a dashed line for calendar year 2020. The shaded area indicates the 95% confidence intervals for these estimates.
Policy impact
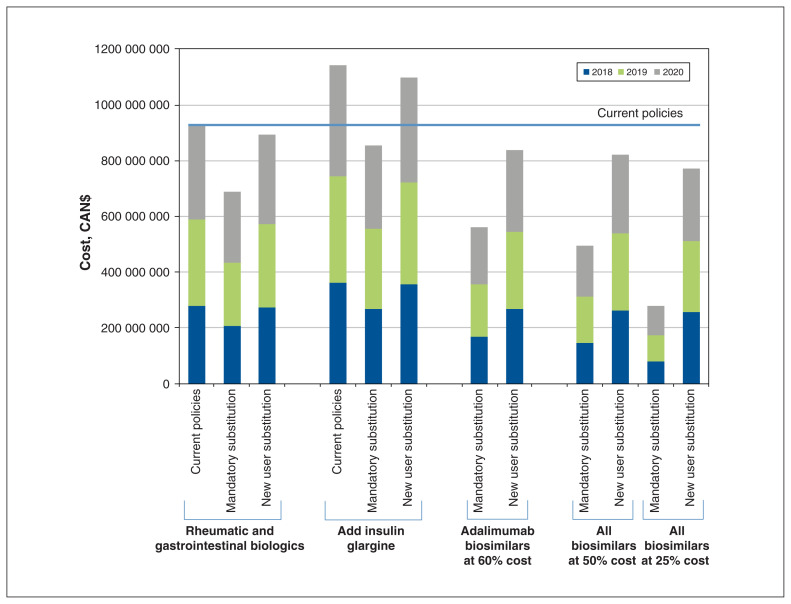
The impact of policies on the number of patients affected and the resulting cost savings varied considerably depending on the policy selected (Table 3 and Figure 2). The fewest patients were affected if only new users of etanercept and infliximab were required to use a biosimilar (n = 757 in 2018). This policy also led to the smallest percentage reduction in costs between 2018 and 2020 (3.7% reduction; $34 236 463 in savings over 3 years). We estimated that a policy mandating nonmedical substitution for all users of etanercept and infliximab innovators would affect 7209 patients upon implementation, and save $238 589 858 over 3 years (25.8% cost reduction). In policies including insulin glargine, the number of patients affected would be considerably higher, reaching 115 895 in 2018 for mandatory nonmedical substitution, and 23 680 for a new user substitution. The percentage price reductions are similar for these policies as for those focusing on etanercept and infliximab; however, the absolute cost savings over 3 years are higher ($288 733 259 and $45 341 592 for mandatory nonmedical substitution and new user substitution, respectively).
Table 3:
Cost implications of different policy scenarios, 2018–2020*
| Cost implications | No. patients affected (2018)† | Savings 2018, $ | Savings 2019, $ | Savings 2020, $ | Total 3-year savings, $ | % Reduction costs |
|---|---|---|---|---|---|---|
| Etanercept and infliximab only | ||||||
| Everyone switches to currently available biosimilar | 7209 | 75 711 829 | 79 753 211 | 83 124 819 | 238 589 858 | −25.8 |
| Only new users required to use currently available biosimilar | 757 | 6 386 595 | 11 810 257 | 16 039 611 | 34 236 463 | −3.7 |
| Including adalimumab biosimilar† | ||||||
| Everyone switches to biosimilar (adalimumab @ 60% innovator cost) | 12 928 | 112 774 144 | 122 160 470 | 130 684 224 | 365 618 838 | −39.5 |
| Only new users are required to use biosimilar (adalimumab @ 60% innovator cost) | 2443 | 14 456 007 | 28 967 204 | 42 164 844 | 85 588 055 | −9.3 |
| New cost thresholds for all biologics (etanercept, infliximab and adalimumab)† | ||||||
| Everyone switches to biosimilar (all biologics @ 50% innovator cost) | 12 928 | 133 002 041 | 143 605 795 | 153 276 509 | 429 884 345 | −46.5 |
| Only new users are required to use biosimilar (all biologics @ 50% innovator cost) | 2443 | 17 273 891 | 34 758 620 | 50 939 083 | 102 971 594 | −11.1 |
| Everyone switches to biosimilar (all biologics @ 25% innovator cost) | 12 928 | 199 861 495 | 215 784 478 | 230 233 627 | 645 879 599 | −69.8 |
| Only new users are required to use biosimilar (all biologics @ 25% innovator cost) | 2443 | 25 965 104 | 52 250 289 | 76 614 092 | 154 829 485 | −16.7 |
| Etanercept, infliximab and insulin glargine† | ||||||
| Everyone switches to currently available biosimilar | 115 895 | 94 857 347 | 96 465 505 | 97 410 407 | 288 733 259 | −25.3 |
| Only new users required to use currently available biosimilar | 23 680 | 8 541 779 | 15 386 458 | 21 413 355 | 45 341 592 | −4.0 |
Represents approximate numbers of people affected based on prevalence of new use of innovators or use of only innovators over the year.
Secondary analysis.
Figure 2:
Expected 3-year costs of biologics after modelling different policy scenarios, 2018–2020.
The impact of policies on costs varied depending on the availability of an adalimumab biosimilar and the degree of price negotiations, with the policy leading to the largest 3-year cost savings being a mandatory nonmedical substitution of 12 928 users of etanercept, infliximab and adalimumab innovators where prices are negotiated to 25% of the innovator cost (69.8% reduction; $645 879 599 over 3 years; Table 3).
Interpretation
In this population-based study, we found that policies designed to increase uptake of biosimilars differed substantially in their impact on patients and government costs. In 2018, infliximab, etanercept and adalimumab cost the Ontario public drug program $280.8 million, 95.6% of which was attributed to innovator biologics. Depending on the policy implemented and negotiated biosimilar prices, we estimated the potential 3-year (2018–2020) cost savings of biosimilar reimbursement policies to range between $34.2 million (3.7% savings; enforced new user substitution for etanercept and infliximab only) and $645.9 million (69.8% savings; mandatory nonmedical substitution for etanercept, infliximab and adalimumab, each priced at 25% of innovator biologics). Similarly, the number of patients affected by the policies ranged from 757 to 115 895 annually, depending on the policy selected.
Overall, the considerable cost savings and number of patients affected by the biosimilar policy changes examined in this study are within the range of estimates found in other jurisdictions, both nationally and internationally. In Canada, BC and Alberta estimated their biosimilar policies would affect between 40 and 60 patients per 10 000 population and save about $1500 and $3000 per patient, respectively.7,10,11 Our analysis found that a nonmedical substitution policy for etanercept, infliximab and insulin glargine (which is most similar to BC and Alberta’s policies) would affect about 80 patients per 10 000 population and save nearly $900 per patient in Ontario.
Although there have been many international studies examining the effect of biosimilars on the budget of public drug programs, primarily in Europe, many of these analyses are not directly comparable with this study owing to the variability of policies, product availability, populations studied and research methodology.18 A recent systematic review compiled 15 international studies and found that nonmedical substitution of biosimilars for etanercept, infliximab or adalimumab resulted in a wide range of cost savings (about €7 to €13 739 per patient per year).19 The variation between provincial estimates in Canada and international comparisons is likely a result of differences in the medications included in the biosimilar policies,7 the prevalence of associated diseases,20–22 and drug coverage before policy implementation.23 However, this international research suggests that policies requiring non-medical switches or automatic substitutions with biosimilars generally lead to rapid shifts in dispensing patterns and large cost reductions for public payers, but potentially increased costs related to health services utilization.15,19,24
Although cost considerations can be an important driver of policy change, the way in which biologics are dispensed introduces an additional layer of complexity for optimal reimbursement policy. For example, although biosimilars have been shown to be effective and safe,25 some clinicians are concerned that substituting treatment for patients already stable on one therapy could cause anxiety among those who are experiencing benefit from their current medication and could destabilize their condition. This could both affect patient outcomes and incur costs to the health care system. This concern appears to be greater for patients with IBD, owing to uncertainty about destabilization of their condition and the more limited number of biologic options.13,26
A unique aspect of biologic provision is that some patient care and medication administration costs (e.g., infusion clinics, laboratory tests, patient support nurses) are funded by biologic drug manufacturers. In addition, drug manufacturers often assist patients with their copayments. Therefore, any policies introducing mandatory changes in therapy need to allow for scaling-up of these services for the corresponding biosimilars. This includes anticipating funding to provide clinical support to patients when undergoing a change in therapy, and identifying potential implications for the patient copayments and financial support often provided by innovator biologics manufacturers.
Given the limited real-world evidence regarding the safety of mandatory nonmedical biosimilar substitution, particularly for patients with IBD, jurisdictions introducing these policies should monitor patient outcomes, including clinical consequences and costs, out-of-pocket expenses and quality of life.
Limitations
Although we used real-world data on publicly funded biologics to estimate the potential impacts of different biosimilar policies in Ontario, several limitations to this study merit discussion. In the absence of an available biosimilar for adalimumab, it would be possible that biologics prescribing could be channelled toward this product if a mandatory nonmedical substitution policy was introduced. Although we are unable to estimate the cost implications of such a change in clinical practice in our models, data after a similar policy change in BC suggest this did not occur.27 Furthermore, in February 2021, adalimumab biosimilars became available on the Canadian market and were added to the Ontario public drug formulary in March 2021 at 60% of the price of the innovator. Therefore, all available innovator biologics now have a biosimilar available, thus reducing the potential for channelling.
The Ontario Public Drug Programs already has a policy requiring biosimilars among new users of infliximab or etanercept; however, when patients are started on medications in hospital or they receive their first dose at low cost from the manufacturer, these policies are circumvented. Therefore, although new-user policies are potentially more acceptable to patients, they may have limited effectiveness for public payers. As our model indicates, considerable additional savings could be achieved if the intended new-user biosimilar policy was fully enforceable, although it is not known whether this can be achieved when other factors remain outside government control.
Our study is limited to estimating the cost implications of biosimilar policy changes applied to the public drug program in Ontario, and therefore does not provide estimates of cost implications if similar policies were introduced by private drug insurers who typically provide coverage to younger (i.e., < 65 yr) populations. However, younger patients with high drug costs are increasingly accessing Ontario’s catastrophic drug program (Trillium), which means that drug policy decisions made by public drug programs will affect them.28
We were unable to incorporate negotiated price reductions (rebates) already implemented in Ontario as these are confidential; the cost savings reported here therefore used the list price of the medications. Hence, we determined 2 potential thresholds for price reductions (25% and 50% of innovator cost) through consultation with policy-makers across Canada. Although achieving price reductions as low as 25% of the innovator cost may be unlikely, this provides a wide array of cost implications that can inform future price negotiations by public drug programs in Canada.
Conclusion
In this large population-based study, we found that policies designed to address the rising costs of biologics differ substantially in their impact on patients and cost savings. Given the complexity of the supply chain for these medications, including the role of manufacturers in drug provision, careful consideration of the balance between cost savings and patient access is warranted. Plans for enacting specific initiatives should consider forming partnerships with key stakeholder groups to ensure that patient and provider perspectives are incorporated.
Supplementary Material
Acknowledgement
The authors thank IQVIA Solutions Canada Inc. for use of its Drug Information File.
Footnotes
Competing interests: Muhammad Mamdani reports receiving honoraria from Neurocrine Biosciences, and reports being a one-time advisory board member for Roche. Laurie Proulx reports membershp of the executive of the Canadian Arthritis Patient Alliance. Tara Gomes reports receiving a grant from the Ontario Ministry of Health. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, and the acquisition and interpretation of data. Daniel McCormack, Mina Tadrous and Tara Gomes contributed to the analysis of data. Tara Gomes drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by a grant from the Ontario Ministry of Health. This study was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care.
Data sharing: The data set from this study is held securely in coded form at ICES. While data-sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/9/4/E1055/suppl/DC1
Disclaimer: The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario Ministry of Health is intended or should be inferred.
References
- 1.Biosimilar biologic drugs in Canada: fact sheet. Ottawa: Health Canada; 2019. [Google Scholar]
- 2.ICMRA statement about confidence in biosimilar products (for healthcare professionals) San Diego (CA): International Coalition of Medicines Regulatory Authorities (ICMRA); 2019. [Google Scholar]
- 3.Biologics in Canada: part 1: market trends. Ottawa: Patented Medicine Prices Review Board; 2020. [Google Scholar]
- 4.Tadrous M, McCormack D, Martins D, et al. Current and prospective utilization of innovator biologics and biosimilars in Ontario. Toronto: Ontario Drug Policy Research Network; 2020. [Google Scholar]
- 5.Guidance document: information and submission requirements for biosimilar biologic drugs. Ottawa: Health Canada; 2016. [Google Scholar]
- 6.Kabir ER, Moreino SS, Sharif Siam MK. The breakthrough of biosimilars: a twist in the narrative of biological therapy. Biomolecules. 2019;9:410. doi: 10.3390/biom9090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biosimilars initiative for patients. Vancouver: Government of British Columbia; 2019. [accessed 2020 May 14]. Available: https://www2.gov.bc.ca/gov/content/health/health-drug-coverage/pharmacare-for-bc-residents/what-we-cover/drug-coverage/biosimilars-initiative-patients. [Google Scholar]
- 8.Biosimilars in Canada: current environment and future opportunity. Ottawa: Patented Medicine Prices Review Board; 2019. [Google Scholar]
- 9.Biologics in Canada: part 2: biosimilar savings. Ottawa: Patented Medicine Prices Review Board; 2020. [Google Scholar]
- 10.Biosimilar drugs. Alberta: Government of Alberta; 2020. [accessed 2020 May 14]. Available: https://www.alberta.ca/biosimilar-drugs.aspx. [Google Scholar]
- 11.Biosimilar initiative. Edmonton: Alberta Health; 2020. [Google Scholar]
- 12.Kaplan GG, Ma C, Seow CH, et al. The argument against a biosimilar switch policy for infliximab in patients with inflammatory bowel disease living in Alberta. J Can Assoc Gastroenterol. 2020;3:234–42. doi: 10.1093/jcag/gwz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crain J, Mawani M, Lee K, et al. Patient and health care provider input: non-medical biosimilar switch policy for patients with inflammatory bowel disease. Toronto: Crohn’s and Colitis Canada; 2019. [Google Scholar]
- 14.Levy AR, O’Brien BJ, Sellors C, et al. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10:67–71. [PubMed] [Google Scholar]
- 15.Jensen TB, Bartels D, Saedder EA, et al. The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol. 2020;76:35–40. doi: 10.1007/s00228-019-02765-3. [DOI] [PubMed] [Google Scholar]
- 16.Kalekar PS. Time series forecasting using Holt-Winters exponential smoothing. Mumbai: Kanwal Rekhi School of Information Technology; 2004. pp. 1–13. [Google Scholar]
- 17.Fuertes EI, Henry B, Marra F, et al. Trends in antibiotic utilization in Vancouver associated with a community education program on antibiotic use. Can J Public Health. 2010;101:304–8. doi: 10.1007/BF03405291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moorkens E, Vulto A, Huys I, et al. Policies for biosimilar uptake in Europe: an overview. PLoS One. 2017;12:e0190147. doi: 10.1371/journal.pone.0190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Yang M, Garg V, et al. Economic impact of non-medical switching from originator biologics to biosimilars: a systematic literature review. Adv Ther. 2019;36:1851–77. doi: 10.1007/s12325-019-00998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broten L, Aviña-Zubieta JA, Lacaille D, et al. Systemic autoimmune rheumatic disease prevalence in canada: updated analyses across 7 provinces. J Rheumatol. 2014;41:673–9. doi: 10.3899/jrheum.130667. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan GG, Bernstein CN, Coward S, et al. The impact of inflammatory bowel disease in Canada 2018: Epidemiology. J Can Assoc Gastroenterol. 2019;2(Suppl 1):S1–S5. doi: 10.1093/jcag/gwy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health Fact Sheets: Diabetes, 2017. Ottawa: Statistics Canada; 2018. [Google Scholar]
- 23.Campbell DJ, Manns BJ, Soril LJ, et al. Comparison of Canadian public medication insurance plans and the impact on out-of-pocket costs. CMAJ Open. 2017;5:E808. doi: 10.9778/cmajo.20170065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen TB, Kim SC, Jimenez-Solem E, et al. Shift from adalimumab originator to biosimilars in Denmark. JAMA Intern Med. 2020;180:902–3. doi: 10.1001/jamainternmed.2020.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbier L, Ebbers HC, Declerck P, et al. The efficacy, safety, and immunogenicity of switching between reference biopharmaceuticals and biosimilars: a systematic review. Clin Pharm Therapeut. 2020;108:734–55. doi: 10.1002/cpt.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canadian Rheumatology Association (CRA) position statement on biosimilars. Mississauga (ON): Canadian Rheumatology Association; 2019. [Google Scholar]
- 27.Crosby M, Tadrous M, Gomes T. Potential cost implications of mandatory non-medical switching policies for biologics for rheumatic conditions and inflammatory bowel disease in Canada. Clin Pharmacol Ther. 2021;109:739–45. doi: 10.1002/cpt.2042. [DOI] [PubMed] [Google Scholar]
- 28.Tadrous M, Greaves S, Martins D, et al. Catastrophic drug coverage: utilization insights from the Ontario Trillium Drug Program. CMAJ Open. 2018;6:E132–8. doi: 10.9778/cmajo.20170132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.