Abstract
Background
Etrolizumab (rhuMAb beta7) is an anti‐integrin that selectively targets the β7 subunits of the α4β7 and αEβ7 integrins, which are involved in the pathogenesis of ulcerative colitis.
Objectives
The objectives of this review were to assess the efficacy and safety of etrolizumab for induction of remission in ulcerative colitis.
Search methods
We searched PubMed, MEDLINE, EMBASE, and the Cochrane Library (CENTRAL) from inception to 12 March 2015. References and conference abstracts were searched to identify additional studies.
Selection criteria
Randomized controlled trials (RCTs) trials in which etrolizumab was compared to placebo or another active comparator in patients with active ulcerative colitis were included.
Data collection and analysis
Two authors independently screened studies for inclusion, assessed methodological quality and extracted data. We assessed methodological quality using the Cochrane risk of bias tool. The primary outcome was failure to induce clinical remission (as defined by the primary studies). Secondary outcomes included failure to induce clinical improvement (as defined by the primary studies), failure to induce endoscopic remission (as defined by the primary studies), adverse events, serious adverse events, withdrawal due to adverse events, and health‐related quality of life (as defined by the primary studies). We assessed the overall quality of the evidence using the GRADE criteria. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for each dichotomous outcome.
Main results
Two RCTs including 172 patients with moderate to severe UC who failed conventional therapy met the inclusion criteria. Both studies were rated as low risk of bias. We did not pool efficacy data from the two included studies due to differences in dose and route of administration. The small phase I study found no statistically significant differences between etrolizumab and placebo in the proportion of patients who failed to enter remission (RR 1.04, 95% CI 1.04 to 1.69; participants = 23) or respond at week 10 (RR 1.67, 95% CI 0.26 to 10.82; participants = 23). The phase II study reported on failure to enter clinical remission at weeks 6 and 10. In the etrolizumab group 91% (71/78) of patients failed to enter remission at week 6 compared to 95% (39/41) of placebo patients (RR 0.96, 95% CI 0.87 to 1.06). Subgroup analysis revealed no statistically significant differences by dose. At week 10, there was a statistically significant difference in clinical remission rates favouring etrolizumab over placebo. Of the patients who received etrolizumab, 85% (66/78) failed to enter remission at week 10 compared to 100% (41/41) patients in the placebo group (RR 0.86, 95% CI 0.77 to 0.95). A subgroup analysis by dose found a statistically significant difference in clinical remission rates favoring 100 mg etrolizumab over placebo (RR 0.81 CI 95% 0.68 to 0.96), but not 300 mg etrolizumab over placebo (RR 0.91, 95% CI 0.80 to 1.03). No significant heterogeneity was detected for this comparison (P = 0.28, I2 = 13.5%). GRADE analyses indicated that the overall quality of evidence for the clinical remission outcomes was moderate due to sparse data. Both of the included studies reported on safety. The outcome adverse events was initially pooled, however this analysis was removed due to high heterogeneity (I2 = 88%). The phase I study found no statistically significant difference between etrolizumab and placebo in the proportion of patients who had at least one adverse event. Ninety‐five per cent (36/38) of etrolizumab patients had at least one adverse event compared to 100% (10/10) of placebo patients (RR 0.98, 95% CI 0.84 to 1.14). Common adverse events reported in the phase I study included exacerbation of UC, headache, fatigue, abdominal pain, dizziness, nasopharyngitis, nausea, arthralgia and urinary tract infection. There was a statistically significant difference between etrolizumab and placebo in the proportion of patients who had at least one adverse event. Fifty‐six per cent (44/78) of etrolizumab patients had at least one adverse event compared to 79% of placebo patients (RR 0.71, 95% CI 0.55 to 0.91). A GRADE analysis indicates that the overall quality of the evidence for this outcome was moderate due to sparse data. Common adverse events reported in the phase II study included worsening UC, nasopharyngitis, nervous system disorders, headache and arthralgia . A pooled analysis of two studies indicates that there was no statistically significant difference in the proportion of patients who had a serious adverse event. Twelve per cent (14/116) of etrolizumab patients had a serious adverse event compared to 12% of placebo patients (6/49) (RR 0.92, 95% CI 0.36 to 2.34). A GRADE analysis indicated that the overall quality of the evidence for this outcome was low due to very sparse data (20 events). Common serious adverse events included worsening of UC, impaired wound healing and bacterial peritonitis.
Authors' conclusions
Moderate quality evidence suggests that etrolizumab may be an effective induction therapy for some patients with moderate to severe ulcerative colitis who have failed conventional therapy. Due to small numbers of patients in dose subgroups the optimal dosage of etrolizumab is unclear. Due to sparse data we are uncertain regarding the risk of adverse events and serious adverse events. Further studies are needed to determine the efficacy and safety of etrolizumab in this patient population. There are five ongoing phase III etrolizumab trials and two ongoing open‐label extension studies that will provide important new information on the efficacy, safety and optimal dose of this drug for the treatment of UC.
Plain language summary
Etrolizumab for the treatment of active ulcerative colitis
What is ulcerative colitis? Ulcerative colitis is a long‐term (chronic) inflammatory bowel disease. Symptoms include pain (abdominal cramping), a frequent need to defecate (fecal urgency) and bloody diarrhoea. When people with ulcerative colitis are experiencing symptoms the disease is said to be "active" and when symptoms stop this is called "remission".
What is etrolizumab? Etrolizumab is a biologic medication. This medication is either injected under the skin with a syringe or infused into a vein (intravenous). Biologics suppress the immune system and lessen the inflammation associated with ulcerative colitis.
What did the researchers investigate? The researchers investigated whether etrolizumab can stop symptoms of ulcerative colitis in people with active disease, and whether this medication causes harm (side effects). The researchers searched the medical literature up to March 12, 2015.
What did the researchers find? The researchers identified two studies that included a total of 172 participants with moderate to severe ulcerative colitis who have failed treatment with immunosuppressives (e.g. steroids) or another biologic drug. Both studies compared etrolizumab to placebo (a fake medicine). Both studies were of high quality. The smaller study (48 participants) found no difference in remission rates between etrolizumab and placebo at week 10. The larger study (124 participants) found no difference between etrolizumab and placebo in the proportion of participants who achieved remission at week 6. However, there was a statistically meaningful difference in remission rates at week 10 favoring etrolizumab over placebo. In the larger study (124 participants) placebo participants were significantly more likely to have at least one side effect compared to those who took etrolizumab. Common side effects in this study included worsening ulcerative colitis, nasopharyngitis (common cold), nervous system disorders, headache and arthralgia (joint pain). In the other study (48 participants) there was no difference in the side effect rates between the placebo and etrolizumab groups. Common side effects in this study included worsening of ulcerative colitis, headache, fatigue (tiredness), abdominal pain, dizziness, nasopharyngitis (common cold), nausea, arthralgia (joint pain) and urinary tract infection. There was no meaningful difference between etrolizumab and placebo in the proportion of patients who experienced serious side effects. Serious side effects included worsening of ulcerative colitis and infection.
Etrolizumab may be better than placebo for producing remission in people with moderate to severe ulcerative colitis who have failed other treatments. Different doses of etrolizumab were investigated but it is unclear what dose is most effective. More studies are required to determine the effectiveness and safety of etrolizumab in patients with moderate to severe ulcerative colitis. Currently there are seven ongoing studies investigating etrolizumab treatment for ulcerative colitis. These studies will provide important new information on the effectiveness, safety and ideal dose of etrolizumab for the treatment of people with moderate to severe ulcerative colitis.
Summary of findings
Summary of findings for the main comparison. Etrolizumab versus placebo for induction of remission in ulcerative colitis.
| Etrolizumab versus placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis Settings: Intervention: Etrolizumab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Etrolizumab versus placebo | |||||
| Failure to enter clinical remission at week 10 | 1000 per 10001 | 860 per 1000 (770 to 950) | RR 0.86 (0.77 to 0.95) | 119 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| Failure to enter clinical remission at week 10 ‐ 100 mg | 1000 per 10001 | 810 per 1000 (680 to 960) | RR 0.81 (0.68 to 0.96) | 59 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| Failure to respond at week 10 | 707 per 10001 | 679 per 1000 (530 to 870) | RR 0.96 (0.75 to 1.23) | 119 (1 study) | ⊕⊕⊕⊝ moderate4 | |
| Failure to enter endoscopic remission at week 6 | 976 per 10001 | 956 per 1000 (878 to 1000) | RR 0.98 (0.9 to 1.06) | 119 (1 study) | ⊕⊕⊕⊝ moderate5 | |
| Failure to enter endoscopic remission at week 10 | 1000 per 10001 | 920 per 1000 (850 to 1000) | RR 0.92 (0.85 to 1) | 119 (1 study) | ⊕⊕⊕⊝ moderate6 | |
| Adverse events | 721 per 10001 | 541 per 1000 (411 to 714) | RR 0.75 (0.57 to 0.99) | 124 (1 study) | ⊕⊕⊕⊝ moderate7 | |
| Serious adverse events | 122 per 10001 | 113 per 1000 (44 to 287) | RR 0.92 (0.36 to 2.34) | 165 (2 studies) | ⊕⊕⊝⊝ low8 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials.
2 Downgraded one level due to sparse data (107 events).
3 Downgraded one level due to sparse data (51 events).
4 Downgraded one level due to sparse data (82 events).
5 Downgraded one level due to sparse data (114 events).
6 Downgraded one level due to sparse data (112 events).
7Downgraded one level due to sparse data (75 events).
8 Downgraded two levels due to very sparse data (20 events).
Background
Description of the condition
Ulcerative colitis (UC) is a chronic inflammatory disease of unknown etiology characterized by bloody diarrhoea, abdominal pain, and fecal urgency. Worldwide incidence rates of UC range between 1.25 to 20.3 new cases per 100,000 persons per year, with approximately 10 to 12 new cases per 100,000 persons per year in North America and Europe (Danese 2011; Fedorak 2010; Molodecky 2012). In the United States it is estimated that the direct and indirect costs associated with the disease range between 8.1 billion USD and 14.9 billion USD per annum (Cohen 2010). Evidence suggests that UC is caused by an inappropriate immune response that is triggered by a combination of environmental, genetic and immunological factors (Bouma 2003).
UC is treated with broad‐spectrum anti‐inflammatory drugs including corticosteroids, 5‐aminosalicylic acid (5‐ASA) products, immunosuppressive therapies (e.g. azathioprine, 6‐mercaptopurine and methotrexate) and biologics such as tumor necrosis factor alpha (TNF‐α) antagonists (e.g. infliximab (Remicade®), adalimumab (Humira®), certolizumab pegol (Cimzia®) and golimumab (Simponi®)) and alpha4beta7 (α4β7) inhibitors (e.g. vedolizumab) (Bickston 2014; Feagan 2012a; Feagan 2012b; Ford 2011a; Ford 2011b; Kornbluth 2010; Lawson 2006; Timmer 2012). These medications are effective to varying degrees, however patients often do not respond, become corticosteroid dependent, fail therapy or experience significant drug‐related adverse events (Faubion 2001; Gisbert 2015).
Aminosalicylates are effective for mild to moderate disease (Feagan 2012a; Feagan 2012b; Ford 2011a), while corticosteroids are often required for those who fail to respond to 5‐ASAs (Ford 2011b; Turner 2007). Corticosteroids are highly effective for induction of remission, but are not useful for maintenance of remission and carry significant adverse effects, including osteoporosis, glucose intolerance, and increased risk of infection (Bjarnason 1997; Dignass 2010; Lichtenstein 2006). Immunosuppressives, including 6‐mercaptopurine and azathioprine, play a limited role in maintenance of remission in UC (Podolsky 2002; Timmer 2012). Furthermore, these drugs may increase the risk of lymphoma and non‐melanoma skin cancer in people with inflammatory bowel disease (IBD) (Ariyaratnam 2014; Smith 2010). TNF‐α antagonists are useful for both induction and maintenance of remission in UC (Reinisch 2011; Sandborn 2012; Sandborn 2014), however their use has been associated with a number of serious adverse events involving both hypersensitivity and opportunistic infection (Ford 2013). Patients who fail therapy, develop toxic megacolon or have severe attacks of ulcerative colitis require colectomy, which frequently results in post‐operative complications including infection, pouchitis, fistula formation, and bowel obstruction (Loftus 2008). New pharmaceutical agents, particularly those specific to the intestinal tract, may be more effective than conventional therapies and reduce the need for surgery.
Description of the intervention
Anti‐adhesion molecules such as natalizumab and vedolizumab represent a novel biologic option for the treatment of UC. Natalizumab was shown to be effective in patients with Crohn's disease, however its use has been associated with immunosuppression of the central nervous system and the development of progressive multifocal leukoencephalopathy (PML) (Van Assche 2005). Conversely, vedolizumab has proven to be effective for induction and maintenance of remission in UC and well‐tolerated by patients (Bickston 2014; Feagan 2013). While natalizumab regulates leukocyte trafficking by blocking both the alpha4beta1 (α4β1) and alpha4beta7 (α4β7) integrins, vedolizumab selectively targets the latter, thereby exclusively inhibiting T‐cell homing to the gut. Etrolizumab is a novel anti‐integrin that selectively targets the β7 subunits of the α4β7 and αEβ7 integrins that regulate trafficking and retention of T‐cell subset lymphocytes in the intestinal mucosa.
How the intervention might work
Etrolizumab binds with high affinity to α4β7 (Holzmann 1989; Hu 1992), and αEβ7 (Cepek 1993). By this mechanism, it blocks the homing and retention of leukocyte subpopulations in the intestinal mucosa, which occur via binding with the cell adhesion molecules (MAdCAM‐1) and E‐cadherin, respectively. Since these cell adhesion molecules are found mostly in the intestinal mucosa, etrolizumab is felt to be gut specific. In a mouse model, etrolizumab selectively blocks lymphocyte homing to the gastrointestinal tract, with no apparent effect on lymphocyte trafficking to the central nervous system or non‐mucosal tissues (Stefanich 2011).
Why it is important to do this review
There is a need for targeted UC treatment options capable of achieving and sustaining remission, decreasing steroid dependence and maintaining immunocompetence while simultaneously avoiding the risk of serious adverse events. The aim of this systematic review is to summarize the currently available evidence regarding the efficacy and safety of etrolizumab for induction of clinical remission and response in patients with moderate to severe UC.
Objectives
The primary objectives were to assess the efficacy and safety of etrolizumab for induction of remission in ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) were considered for inclusion. There were no restrictions based on publication status or language of publication.
Types of participants
Adult patients (> 18 years of age) with active UC defined by a combination of clinical, radiological, endoscopic and histological criteria (at screening visit) were included.
Types of interventions
RCTs comparing etrolizumab to placebo or active comparator were considered for inclusion.
Types of outcome measures
Primary outcomes
The primary outcome measure was the proportion of patients achieving clinical remission as defined by the primary studies and expressed as a percentage of the number of patients randomized (intention‐to‐treat analysis).
Secondary outcomes
Secondary outcome measures included:
a) Clinical improvement (as defined by the primary studies);
b) Endoscopic remission (as defined by the primary studies);
c) Adverse events;
d) Serious adverse events;
e) Withdrawal due to adverse events; and
f) Health‐related quality of life (as defined by the primary studies).
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases:
a) Ovid MEDLINE (1946 ‐ current date);
b) EMBASE (1974 ‐ current date);
c) Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library, Wiley; and
d) The Cochrane IBD Group Specialized Register.
The search strategies for each database are reported in Appendix 1.
Searching other resources
We searched for ongoing studies using:
a) Clinicaltrials.gov http://clinicaltrials.gov; and
b) ICTRP http://www.who.int/ictrp/en/.
The first authors of included studies were contacted for missing data and unpublished or on‐going studies. The reference lists of all identified studies were scanned for additional applicable studies. We hand searched conference abstracts including Digestive Disease Week (DDW), United European Gastroenterology Week (UEGW) and the European Crohn's and Colitis Organisation (ECCO) from 1999 to 12 March, 2015 to identify studies published as abstracts.
Data collection and analysis
Selection of studies
Two authors (CEP and JKM) independently screened titles and abstracts to identify potentially eligible studies based on the inclusion criteria specified above. The same two authors (CEP and JKM) independently screened studies selected for full text review. Disagreements at either stage were resolved by discussion and consensus. If consensus was not reached, a third author (GR) acted as the arbitrator. The study selection process is reported in a PRISMA flow diagram (see Figure 1) .
1.

Study flow diagram.
Data extraction and management
A data extraction form was developed and used to extract data from the included studies. Two authors (CEP and JKM) independently extracted data. Disagreements were resolved by consensus. If consensus was not reached, a third author (GR) acted as the arbitrator.
Assessment of risk of bias in included studies
Two authors (CEP and JKM) independently assessed the methodological quality of the included studies using the Cochrane risk of bias tool (Higgins 2011a). Trials were rated as high, low or unclear risk of bias for each of the following criteria:
a) Random sequence generation;
b) Allocation concealment;
c) Blinding;
d) Incomplete outcome data;
e) Selective reporting; and
f) Other sources of bias.
We used the GRADE approach to assess the overall quality of evidence supporting the primary outcome and selected secondary outcomes (Guyatt 2008; Schünemann 2011). Outcome data were rated as high, moderate, low or very low quality evidence. Data from RCTs begin as high quality but can be downgraded based on the following criteria:
a) Risk of bias in the included trials;
b) Indirect evidence;
c) Inconsistency (i.e. unexplained heterogeneity);
d) Imprecision (i.e. sparse data or wide confidence interval or both); and
e) Publication bias.
The different quality ratings are interpreted as the likelihood that future research would affect the estimate of effect. An estimate of effect based on high quality evidence is unlikely to change with further research. If the overall evidence is of moderate quality further research may have an impact on our confidence in the estimate and may change the estimate. Low quality evidence is likely to have an impact on the effect estimate. Very low quality research indicates that the finding is very uncertain (Guyatt 2008; Schünemann 2011). The results of the GRADE analysis were reported in the Table 1.
Measures of treatment effect
Review Manager (Revman 5.3.5) was used to analyse the data. Analyses were performed on an intention‐to‐treat (ITT) basis whereby all drop outs were assumed to be treatment failures. We calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for dichotomous outcomes. For continuous outcomes, we planned to calculate the mean difference (MD) and corresponding 95% CI.
Unit of analysis issues
When included studies reported multiple observations for the same outcome, the outcomes were combined for fixed intervals of follow‐up (e.g., clinical remission at eight weeks). Cross‐over trials were included if data was available from the first phase of the study (i.e. before any cross‐over). Separate analyses were planned for comparisons between etrolizumab versus placebo as well as etrolizumab versus active comparator. Where studies allocated patients to more than one etrolizumab treatment arm, these groups were pooled for the primary analysis. When possible, additional subgroup analyses were performed to compare efficacy and safety among different doses of etrolizumab. Although some studies reported more than one efficacy or safety event per patient, the primary analysis considered the proportion of patients who experienced at least one event.
Dealing with missing data
We analyzed data on an ITT basis (i.e. we attempted to include all participants randomized to each group in the analyses regardless of whether they withdrew from the trial or received the allocated intervention). If there was a discrepancy between the number randomized and the number analyzed in each treatment group, we calculated the percentage lost to follow‐up in each group and considered these participants to be treatment failures.
In the case of missing data, we directly contacted the first author of the study in an attempt to obtain missing information. If we were unable to obtain missing continuous data we planned to estimate the standard deviations using other available data (e.g. standard errors) or impute the standard deviations based on the methods suggested by Higgins 2011b. We planned to perform sensitivity analyses by calculating the effect of including and excluding imputed data to determine whether this altered the effect estimates. When it was not possible to obtain or impute missing data, we planned to record this in the data extraction form and report in the 'Risk of bias' table (see Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Assessment of heterogeneity
Statistical heterogeneity was assessed using the Chi2 test and I2 statistic. Heterogeneity was considered statistically significant when P < 0.10 for Chi2. The ranges for I2 are:
a) 0% to 40%: might not be important;
b) 30% to 60%: may represent moderate heterogeneity;
c) 50% to 90%: may represent substantial heterogeneity; and
d) 75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We compared available protocols to assess potential reporting bias. When protocols were unavailable we compared outcomes listed in the methods section of published manuscripts to those reported in the results section. If there were a sufficient number of studies included in the pooled analysis (e.g. > 10), we planned to investigate potential publication bias using funnel plots (Egger 1997).
Data synthesis
Data were pooled for meta‐analysis when the interventions, patient groups, outcome measures and timing of outcome assessment were sufficiently similar (to be determined among authors by consensus). The pooled RR and corresponding 95% CI were calculated for dichotomous outcomes. A P value of < 0.05 was considered statistically significant. For continuous outcomes, we planned to calculate the pooled MD and corresponding 95% CI.
Data were not pooled for analysis when I2 > 75%. If significant heterogeneity was detected (i.e. P < 0.10) we explored possible explanations using sensitivity analysis. In the absence of statistically significant heterogeneity a fixed‐effect model was applied. Otherwise, a random‐effects model was utilized (i.e. I2 > 50%).
Subgroup analysis and investigation of heterogeneity
If possible, we performed the following subgroup analyses for the primary and secondary efficacy outcomes:
a) disease activity (e.g. mild, moderate, or severe)
b) disease location (e.g. proctitis, left‐sided, pancolitis);
c) dose of intervention;
e) previous exposure to corticosteroids; and
f) previous exposure to biologics.
Sensitivity analysis
Where possible, we conducted sensitivity analyses by replicating the meta‐analysis with new data sets that exclude lower quality studies and incorporate specific assumptions in place of missing data. We conducted sensitivity analyses where appropriate to explore potential explanations for heterogeneity.
Results
Description of studies
Results of the search
The literature search conducted on 12 March 2015 identified 97 records. After duplicates were removed, a total of 74 records were screened for inclusion. Of these, 10 studies were selected for full text review. Four studies were excluded (see Characteristics of excluded studies), leaving 6 reports of 2 studies (N = 172) that met the pre‐defined inclusion criteria (see Figure 1).
Included studies
Rutgeerts 2013 was a randomized phase I study (N = 48) consisting of one single ascending dose (SAD) stage and one multiple dose (MD) stage. This study evaluated the safety and pharmacology of etrolizumab in patients with moderate to severe UC. Efficacy outcomes included clinical response (a decrease in Mayo Clinical Score of 3 points plus a 30% reduction from baseline and a ≥ 1 point decrease in rectal bleeding or absolute bleeding score of 0 or 1) and clinical remission at week 10 (defined as the proportion of patients with a Mayo Clinical Score < 2 with no individual subscore > 1). In the SAD stage, patients received placebo (n = 5) or one of the following doses of intravenous (IV) etrolizumab: 0.3 mg/kg (n = 4), 1.0 mg/kg (n = 4), 3.0 mg/kg (n = 4), 10.0 mg/kg (n = 4), or 3.0 mg/kg subcutaneously (n = 4). In the MD stage patients received placebo (n = 5), or one of the following subcutaneous (SC) doses of etrolizumab: 0.5 mg/kg (n = 4), 1.5 mg/kg (n = 5), 3.0 mg/kg (n = 4) or 4.0 mg/kg intravenously (n = 5) every four weeks for three cycles at days 1, 29 and 57. See Characteristics of included studies for further details.
Vermeire 2014 was a double‐blind, placebo‐controlled, randomized, phase II study in which patients with moderate to severe UC who had not responded to conventional therapy were treated with SC etrolizumab at doses of 100 mg (n = 41), or 300 mg plus a loading dose (n = 40) or matched placebo (n = 43). Patients in the 100 mg etrolizumab group received injections at weeks 0, 2, 4 and 8. Patients assigned to the 300 mg dose received an initial injection of 420 mg etrolizumab at week 0, followed by 300 mg at weeks 2, 4 and 8. The primary outcome was achievement of clinical remission at week 10 (defined as the proportion of patients with a Mayo Clinical Score < 2 with no individual subscore > 1). Secondary outcomes included clinical remission at week 6, clinical response, and the achievement of an endoscopic subscore of 0 and a rectal bleeding subscore of 0 at weeks 6 and 10. See Characteristics of included studies for further details.
Excluded studies
Armuzzi 2014, Fiorino 2014, Kreutzkamp 2014 and Lin 2014 were excluded because they were not RCTs.
Ongoing studies
We identified seven ongoing studies (NCT02163759; NCT02171429; NCT02136069; NCT02165215; NCT02118584; NCT02100696; NCT01461317). Five of the ongoing studies are phase III, randomized, double‐blind, placebo‐controlled trials (NCT02163759; NCT02171429; NCT02136069; NCT02165215; NCT02100696). Two of these studies compare the efficacy and safety of etrolizumab to adalimumab in patients with moderate to severe UC who are naive to TNF‐α antagonists (NCT02163759; NCT02171429). One study compares the efficacy and safety of etrolizumab to infliximab in patients who moderate to severe UC who are naive to TNF‐α antagonists. One study compares the efficacy and safety of etrolizumab to placebo for maintenance of UC in patients who are naive to TNF‐α antagonists (NCT02165215). NCT02100696 compares the efficacy and safety of etrolizumab in UC patients who are refractory or intolerant to TNF‐α antagonists. The remaining two ongoing trials are open‐label extension studies for patients with UC who participated in etrolizumab phase III studies (NCT02118584; NCT01461317). See Characteristics of ongoing studies for further details.
Risk of bias in included studies
The risk of bias assessment is summarized in Figure 2. The two included studies were of high methodological quality. Both studies used an interactive voice response system to conduct sequence generation. Both included studies utilized a centralized randomization technique and were rated as low risk of bias for allocation concealment. Although both studies were double‐blind, Rutgeerts 2013 failed to explicitly note whether all patients, assessors and personnel were masked to treatment assignment. As a result, this study was rated as unclear risk of bias for performance and detection bias. The studies used adequate methods to deal with missing data and were rated as low risk of bias for incomplete outcome data. The studies reported results for all a priori outcomes are were both rated as low risk of bias for selective reporting. Both studies were rated as low risk of bias for other sources of bias.
Effects of interventions
See: Table 1
Etrolizumab versus placebo
The Rutgeerts 2013 study reported efficacy data for the MD stage. The MD stage involved four different dose groups and both SC and IV dosing. However, Rutgeerts 2013 only reported efficacy results for all etrolizumab dose groups combined. Thus we did not pool efficacy data from the two included studies due to differences in dose and route of administration.
Failure to enter clinical remission at week 6: Data from one randomized trial were available for this outcome (Vermeire 2014). There was no statistically significant difference in the proportion of patients who failed to enter remission at week six. Ninety‐one per cent (71/78) of patients in the etrolizumab group failed to enter clinical remission compared to 95% (39/41) of placebo patients (RR 0.96, 95% CI 0.87 to 1.06). A subgroup analysis by dose found no statistically significant difference in clinical remission rates between patients treated with 100 mg (RR=0.94, 95% CI 0.82 to 1.09) or 300 mg etrolizumab versus placebo (RR 0.97, 95% CI 0.85 to 1.11). No significant heterogeneity was detected for this comparison (P = 0.80, I2 = 0%).
Failure to enter clinical remission at week 10: Rutgeerts 2013 reported on clinical remission rates at week 10 among patients in the MD cohort. There was no statistically significant difference in the proportion of patients who failed to enter remission at week 10, Eight‐three per cent (15/18) of etrolizumab patients failed to enter remission compared to 80% (4/5) of placebo patients (RR 1.04, 95% CI 0.64 to 1.69).
Vermeire 2014 also reported on clinical remission rates at week 10. There was a statistically significant difference in clinical remission rates favouring etrolizumab over placebo. After 10 weeks of treatment 85% (66/78) of patients treated with etrolizumab failed to achieve clinical remission compared to 100% (41/41) of patients in the placebo group (RR 0.86, 95% CI 0.77 to 0.95). A GRADE analysis indicated that the overall quality of evidence for this outcome was moderate due to sparse data (107 events, see Table 1). A subgroup analysis by dose found a statistically significant difference in clinical remission rates favoring 100 mg etrolizumab over placebo (RR 0.81 CI 95% 0.68 to 0.96), but not 300 mg etrolizumab over placebo (RR 0.91, 95% CI 0.80 to 1.03). No significant heterogeneity was detected for this comparison (P = 0.28, I2 = 13.5%).
Failure to improve clinically at week 6: There was no statistically significant difference in clinical response at week 6 (Vermeire 2014). Fifty‐six per cent (44/78) of patients in the etrolizumab group failed to respond at week 6 compared to 66% (27/41) of placebo patients (RR 0.86, 95% CI 0.64 to 1.15). A subgroup analysis by dose did not demonstrate a statistically significant difference in clinical response rates between patients treated with 100 mg (RR 0.79, 95% CI 0.51 to 1.23) or 300 mg etrolizumab versus placebo (RR 0.92, 95% CI 0.62 to 1.36). No significant heterogeneity was detected for this comparison (P = 0.60, I2 = 0%).
Failure to improve clinically at week 10: Rutgeerts 2013 reported on clinical response rates at week 10 among patients in the MD cohort. There was no statistically significant difference in the proportion of patients who failed to respond at week 10. Thirty‐three per cent (6/18) of etrolizumab patients failed to have a clinical response at week 10 compared to 20% (1/5) of placebo patients (RR 1.67, 95% CI 0.26 to 10.82).
Vermeire 2014 also reported on clinical response rates at week 10. There was no statistically significant difference in clinical response at week 10. Sixty‐eight per cent (53/78) of patients in the etrolizumab group failed to respond at week 10 compared to 71% (29/41) of placebo patients (RR 0.96, 95% CI 0.75 to 1.23). A GRADE analysis indicated that the overall quality of evidence for this outcome was moderate due to sparse data (82 events, see Table 1). A subgroup analysis by dose found no statistically significant difference in clinical response rates between patients treated with 100 mg (RR 0.95, 95% CI 0.66 to 1.37) or 300 mg etrolizumab versus placebo (RR 0.97, 95% CI 0.69 to 1.36). No significant heterogeneity was detected for this comparison (P = 0.95, I2 = 0%).
Failure to enter endoscopic remission at week 6: There was no statistically significant difference in endoscopic remission rates at week 6 (Vermeire 2014). Ninety‐five per cent (74/78) of patients in the etrolizumab group failed to enter endoscopic remission at week 6 compared to 98% (40/41) of placebo patients (RR 0.98, 95% CI 0.90 to 1.06). A GRADE analysis indicated that the overall quality of evidence for this outcome was moderate due to sparse data (114 events, see Table 1). A subgroup analysis by dose found no statistically significant difference in endoscopic remission rates for patients treated with 100 mg (RR 0.93, 95% CI 0.83 to 1.05) or 300 mg etrolizumab versus placebo (RR 1.02, 95% CI 0.92 to 1.14). No significant heterogeneity was detected for this comparison (P = 0.27, I2 = 18.9%).
Failure to enter endoscopic remission at week 10: There was no statistically significant difference in endoscopic remission rates at week 10 (Vermeire 2014). Ninety‐one per cent (71/78) of patients in the etrolizumab group failed to enter endoscopic remission at week 10 compared to 100% (41/41) of placebo patients (RR 0.92, 95% CI 0.85 to 1.00). A GRADE analysis indicated that the overall quality of evidence for this outcome was moderate due to sparse data (112 events, see Table 1). A subgroup analysis by dose found no statistically significant difference in endoscopic remission rates for patients treated with 100 mg (RR 0.91, 95% CI 0.80 to 1.03) or 300 mg etrolizumab versus placebo (RR 0.93, 95% CI 0.83 to 1.05). No significant heterogeneity was detected for this comparison (P = 0.76, I2 = 0%).
Adverse Events Vermeire 2014 and Rutgeerts 2013 reported on the total number of patients who experienced at least one adverse event, the total number of patients who experienced at least one serious adverse event, and the number of patients that withdrew due to adverse events. Initially, we pooled the outcome adverse events, however we decided not to pool this outcome due to high heterogeneity (I2 = 82%). There was no statistically significant difference in the proportion of patients who experienced at least one adverse event in the Rutgeerts 2013 study. Ninety‐five per cent (36/38) of etrolizumab patients had at least one adverse event compared to 100% (10/10) of patients in the placebo group (RR 0.98, 95% CI 0.84 to 1.14). There was a statistically significant difference in the proportion of patients who experienced at least one adverse event in the Vermeire 2014 study. Fifty‐four per cent (44/81) of etrolizumab patients had at least one adverse event compared to 72% per cent (31/43) of patients in the placebo group (RR 0.75, 95% CI 0.57 to 0.99). A GRADE analysis indicated that the overall quality of evidence for this outcome was moderate due to sparse data (75 events, See Table 1). Common adverse events reported in the Rutgeerts 2013 study included exacerbation of UC, headache, fatigue, abdominal pain, dizziness, nasopharyngitis, nausea, arthralgia and urinary tract infection. Common adverse events reported in the Vermeire 2014 study included worsening UC, nasopharyngitis, nervous system disorders, headache and arthralgia.
A pooled analysis of data from Vermeire 2014 and Rutgeerts 2013 (n = 165) indicates that there was no statistically significant difference in the proportion of patients who experienced a serious adverse event. Twelve per cent (14/116) of patients receiving etrolizumab had a serious adverse event compared to 12% (6/49) of patients who received placebo (RR 0.92, 95% CI 0.36 to 2.34). Serious adverse events included exacerbation of UC, impaired wound healing and bacterial peritonitis.
A pooled analysis of data from Vermeire 2014 and Rutgeerts 2013 (n = 165) also indicates that there was no statistically significant difference in the proportion of patients who withdrew due to adverse events. Five per cent (6/116) of etrolizumab patients withdrew due to an adverse event compared to 4% (2/49) of placebo patients (RR 1.09, 95% CI 0.26 to 4.62).
Health‐related quality of life
Neither of the included studies reported on health‐related quality of life as an outcome.
Discussion
Summary of main results
Etrolizumab is a humanized monoclonal antibody that selectively binds to the β7 subunit of the α4β7 and αEβ7 integrins, which prevents these transmembrane receptors from interacting with MAdCAM‐1 and E‐cadherin ligands, respectively. This process blocks leukocyte migration and retention specifically within the intestinal mucosa. Two randomized controlled trials have studied etrolizumab for the induction of remission in UC patients with moderate to severe disease who have failed conventional therapy. Efficacy and safety data were available for both studies (Rutgeerts 2013; Vermeire 2014). We did not pool efficacy data from the two included studies due to differences in dose and route of administration.
Rutgeerts 2013 reported on clinical remission rates in the multidose cohort at 10 weeks and found no statistically significant differences in efficacy between etrolizumab and placebo. However, this phase I study was not adequately powered to detect a difference in efficacy should one exist. Vermeire 2014 reported on clinical remission rates at week 6 and 10. There was no statistically significant difference in failure to achieve clinical remission at week 6 between the etrolizumab and placebo groups. Vermeire 2014 found a statistically significant difference in remission rates at 10 weeks favouring etrolizumab over placebo. GRADE analyses indicated that the overall quality of evidence supporting this outcome was moderate due to sparse data (107 events). Subgroup analysis revealed that there was a statistically significant difference in clinical remission rates at week 10 favouring 100 mg etrolizumab over placebo, however there was no statistically significant difference between the 300 mg etrolizumab group and placebo for this outcome. This suggests that a dose of 100 mg may be effective for induction of remission in these patients. A GRADE analysis indicated that the overall quality of evidence supporting this outcome was moderate due to sparse data (51 events). More research is needed to determine the optimal dose of etrolizumab. With regard to clinical response, Vermeire 2014 reported on clinical response rates at weeks 6 and 10 and Rutgeerts 2013 reported on response rates at week 10. There was no statistically significant difference in response rates between the etrolizumab and placebo groups at week 6. Rutgeerts 2013 found no difference in clinical response rates at week 10. Likewise, Vermeire 2014 found no statistically significant difference in clinical response at week 10 between patients treated with etrolizumab versus placebo. A GRADE analysis indicated that the overall quality of evidence supporting this outcome was moderate due to sparse data (107 events).
Rutgeerts 2013 found no statistically significant difference between etrolizumab and placebo in the proportion of patients who experienced at least one adverse event. However, Vermeire 2014 found that patients in the placebo group were significantly more likely than etrolizumab patients to experience at least one adverse event. GRADE analyses indicated that the overall quality of evidence supporting this outcome was moderate due to sparse data (121 events). Common adverse events reported by Rutgeerts 2013 included exacerbation of UC, headache, fatigue, abdominal pain, dizziness, nasopharyngitis, nausea, arthralgia and urinary tract infection. Common adverse events reported by Vermeire 2014 included worsening UC, nasopharyngitis, nervous system disorders, headache and arthralgia. While etrolizumab demonstrated an acceptable safety profile in the two included studies, the number of patients investigated did not allow for the assessment of rare adverse events. Both Rutgeerts 2013 and Vermeire 2014 found no statistically significant difference in the number of withdrawals due to adverse events or the number of serious adverse events experienced by patients in the etrolizumab and placebo groups. GRADE analyses indicated that the overall quality of the evidence supporting these outcomes was low due to very sparse data (8 events and 20 events respectively). Serious adverse events reported in the studies included exacerbation of UC, impaired wound healing and bacterial peritonitis.
Overall completeness and applicability of evidence
The results of this review are applicable to patients with moderate to severe UC who have failed conventional therapy. Moderate quality evidence suggests that etrolizumab may be effective for treating moderate to severe UC in patients who have failed conventional therapy. However, both of the included studies were relatively small in size and as a result, event numbers for outcomes were low. Further research is necessary to confirm the efficacy, safety and optimal dose of etrolizumab in these patients. Etrolizumab could offer an out‐of‐class option for patients who fail TNF‐α antagonists. Currently, there are five phase III etrolizumab trials that include patients who are refractory or intolerant to TNF‐α antagonist therapy (NCT02100696; NCT02136069; NCT02163759; NCT02165215; NCT02171429), and two open‐label extension studies investigating the long‐term safety of etrolizumab (NCT01461317; NCT02118584). Although, greater evidence is required, etrolizumab could have the potential to significantly impact the treatment of UC particularly among patients who are non‐responsive to TNF‐α antagonist therapy.
Quality of the evidence
Both of the included studies were judged to be of low risk of bias. Rutgeerts 2013 was rated as unclear risk of bias for blinding and performance bias. GRADE analyses indicated that the overall quality of the evidence supporting the primary outcomes were rated as moderate quality due to sparse data (i.e. < 400 events). The secondary outcomes clinical response and endoscopic remission were also rated as moderate quality due to sparse data. Safety outcomes were rated as low quality due to sparse data and heterogeneity.
Potential biases in the review process
A comprehensive literature search was performed to reduce potential bias and identify all eligible studies. Two review authors independently assessed studies for inclusion, extracted data and rated study quality. There are several limitations to this review. The studies that investigated etrolizumab were small, and as a result they were only able to detect large effects and frequent adverse events. Also, not all groups could be pooled for analysis since one of the two included trials was a dose finding study, which decreases statistical power. The ongoing studies on etrolizumab for UC will likely provide more information on the safety and efficacy of this drug (see Characteristics of ongoing studies).
Agreements and disagreements with other studies or reviews
Etrolizumab is a relatively new therapeutic agent for ulcerative colitis. To our knowledge, there are no other systematic reviews assessing the efficacy and safety of this agent.
Authors' conclusions
Implications for practice.
Moderate quality data suggests that etrolizumab may be an effective induction therapy for some patients with moderate to severe ulcerative colitis who have failed conventional therapy. Due to small numbers of patients in dose subgroups the optimal dosage of etrolizumab is unclear. Due to sparse data we are uncertain regarding the risk of adverse events, serious adverse events or withdrawal due to adverse events.
Implications for research.
Further randomized control trials are needed to assess the efficacy and safety of etrolizumab therapy for induction of remission in ulcerative colitis. There are five ongoing phase III etrolizumab trials and two ongoing open‐label extension studies that will provide important new information on the efficacy, safety and optimal dose of this drug for the treatment of UC.
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry ofHealth and Long Term Care (HLTC3968FL‐ 2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Search Strategies for MEDLINE, EMBASE, CENTRAL and SR‐IBD/FBD databases
1. EMBASE (via OVID)
1. (colitis and ulcerat*).mp.
2. ulcerative colitis.mp. or exp ulcerative colitis/
3. (inflammatory bowel disease* or IBD).mp.
4. 1 or 2 or 3
5. etrolizumab.mp.
6. exp etrolizumab/
7. rhuMab beta7.mp.
8. PRO145223
9. (anti‐alphaE* OR (anti alphaE*) OR antialphaE* OR (alphaEbeta7)).mp.
10. RG7413
11. 5 or 6 or 7 or 8 or 9 or 10
12. 4 and 11
2. MEDLINE
1. (colitis and ulcerat*).mp.
2. ulcerative colitis.mp. or exp ulcerative colitis/
3. (inflammatory bowel disease* or IBD).mp.
4. 1 or 2 or 3
5. etrolizumab.mp.
6. exp etrolizumab/
7. rhuMab beta7.mp.
8. PRO145223
9. (anti‐alphaE* OR (anti alphaE*) OR antialphaE* OR (alphaEbeta7)).mp.
10. RG7413
11. 5 or 6 or 7 or 8 or 9 or 10
12. 4 and 11
3. Cochrane Central Register of Controlled Trials (CENTRAL)
#1 etrolizumab
#2 rhuMab beta7
#3 PRO145223
#4 RG7413
#5 (anti‐alphaE*) or (anti alphaE*) or (antialphaE*) or (alphaEbeta7)
#6 #1 or #2 or #3 or #4 or #5
4. IBD Group Specialized Register
1. (title or abstract) etrolizumab
2. (title or abstract) rhuMab beta7
3. (title or abstract) RG7413
4. (title or abstract) alphaE
5. 1 or 2 or 3 or 4
Data and analyses
Comparison 1. Etrolizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to enter clinical remission at week 6 | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 1.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 1.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| 2 Failure to enter clinical remission at week 10 (Phase 1 study) | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| 3 Failure to enter clinical remission at week 10 | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.95] |
| 3.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.68, 0.96] |
| 3.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 4 Failure to respond at week 6 | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 4.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.23] |
| 4.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] |
| 5 Failure to respond at week 10 (Phase 1 study) | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.26, 10.82] |
| 6 Failure to respond at week 10 | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| 6.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 6.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.36] |
| 7 Failure to enter endoscopic remission at week 6 | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| 7.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 7.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.14] |
| 8 Failure to enter endoscopic remission at week 10 | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 8.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 8.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 9 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Serious adverse events | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.36, 2.34] |
| 11 Withdrawal due to adverse events | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.26, 4.62] |
1.1. Analysis.
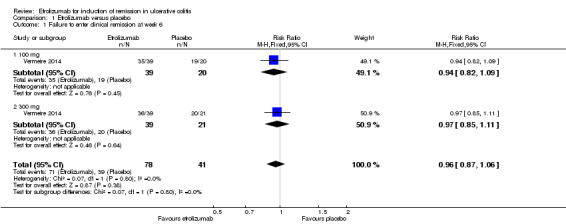
Comparison 1 Etrolizumab versus placebo, Outcome 1 Failure to enter clinical remission at week 6.
1.2. Analysis.

Comparison 1 Etrolizumab versus placebo, Outcome 2 Failure to enter clinical remission at week 10 (Phase 1 study).
1.3. Analysis.

Comparison 1 Etrolizumab versus placebo, Outcome 3 Failure to enter clinical remission at week 10.
1.4. Analysis.

Comparison 1 Etrolizumab versus placebo, Outcome 4 Failure to respond at week 6.
1.5. Analysis.
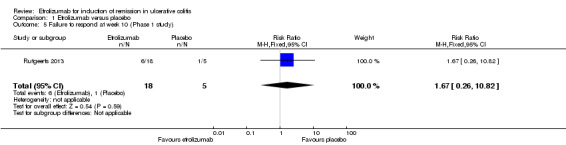
Comparison 1 Etrolizumab versus placebo, Outcome 5 Failure to respond at week 10 (Phase 1 study).
1.6. Analysis.
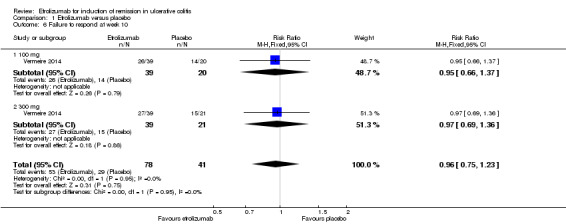
Comparison 1 Etrolizumab versus placebo, Outcome 6 Failure to respond at week 10.
1.7. Analysis.
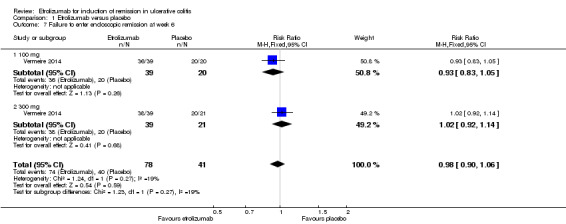
Comparison 1 Etrolizumab versus placebo, Outcome 7 Failure to enter endoscopic remission at week 6.
1.8. Analysis.
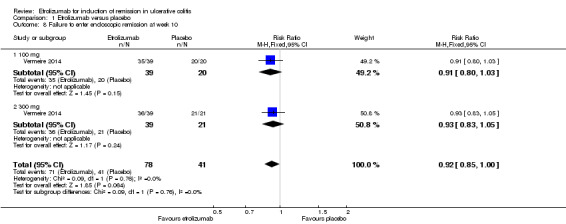
Comparison 1 Etrolizumab versus placebo, Outcome 8 Failure to enter endoscopic remission at week 10.
1.9. Analysis.

Comparison 1 Etrolizumab versus placebo, Outcome 9 Adverse events.
1.10. Analysis.
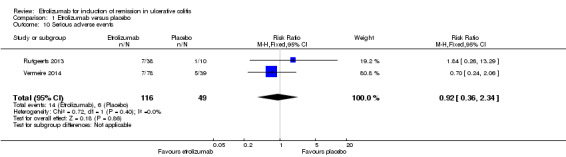
Comparison 1 Etrolizumab versus placebo, Outcome 10 Serious adverse events.
1.11. Analysis.

Comparison 1 Etrolizumab versus placebo, Outcome 11 Withdrawal due to adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Rutgeerts 2013.
| Methods | Randomised, placebo‐controlled, double‐blind within‐cohort study comparing etrolizumab to placebo (N = 48) | |
| Participants | Male and female adults (18‐70 years) with a diagnosis of UC for > 12 weeks and a Mayo Clinic Score (MCS) of > 5 points at screening | |
| Interventions | SAD stage (n = 25): 5 cohorts of patients received etrolizumab or placebo Cohort A: IV etrolizumab 0.3 mg/kg (n = 4) or placebo (n = 1) Cohort B: IV etrolizumab 1.0 mg/kg (n = 4) or placebo (n = 1) Cohort C: IV etrolizumab 3.0 mg/kg (n = 4) or placebo (n = 1) Cohort D: IV etrolizumab 10.0 mg/kg (n = 4) or placebo (n = 1) Cohort E: SC etrolizumab 3.0 mg/kg (n = 4) or placebo (n = 1) MD stage (n = 23): 5 cohorts of patients received etrolizumab or placebo Cohort F: SC etrolizumab 0.5 mg/kg (n = 4) Cohort G: SC etrolizumab 1.5 mg/kg (n = 5) Cohort H: SC etrolizumab 3.0 mg/kg (n = 4) Cohort I: IV etrolizumab 4.0 mg/kg (n = 5) placebo: (n = 5) |
|
| Outcomes | Primary outcomes: adverse events, serious adverse events, dose limiting toxicity, maximum tolerated dose
Secondary outcomes: clinical response/remission at day 29 (SAD) and days 43 and 71 (MD); pharmacokinetic serum samples (etrolizumab concentration, maximum serum concentration, area under concentration–time curve from time 0 to infinity, area under concentration–time curve during a dosing interval, total body clearance at steady state after IV doses or apparent total body clearance at steady state after SC doses, elimination half‐life, antitherapeutic antibody response); pharmacodynamics evaluations (drug occupancy on target CD4+ lymphocytes; occupancy of etrolizumab; absolute number of T lymphocyte subsets) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Conducted by an interactive voice response system based on a process designed by a biostatistician |
| Allocation concealment (selection bias) | Low risk | Conducted by an interactive voice response system based on a process designed by a biostatistician |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were similar across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Vermeire 2014.
| Methods | Randomized, double‐blind, placebo‐controlled, phase 2 study comparing SC etrolizumab to matched placebo (N = 124) | |
| Participants | Adult patients (18‐75 years) with a diagnosis of UC for > 12 weeks and MCS > 5 points at screening (> 6 points at US sites) and a centrally read MCS > 2, a rectal bleeding subscore > 1, and disease extension > 25 cm from the anal verge Patients failed to respond to prior treatment with immunosuppressants and/or TNF‐α antagonists | |
| Interventions | Etrolizumab 100 mg (n = 41): patients received 100 mg at weeks 0, 4 and 8, with placebo administered at week 2 Etrolizumab 300 mg (n = 40): patients received a 420 mg loading dose at week 0, followed by 300 mg at weeks 2, 4 and 8 Placebo (n = 43) |
|
| Outcomes | Primary outcome: clinical remission at week 10 Secondary outcomes: clinical remission at week 6; achievement of endoscopic subscore of 0 at weeks 6 and 10; achievement of rectal bleeding subscore of 0 at weeks 6 and 10; change from baseline in mucosal healing; histological active disease severity score; pharmacodymamic biomarkers in the peripheral blood and colonic tissue | |
| Notes | 124 patients were randomly assigned to placebo (n = 43), etrolizumab 100 mg (n = 41) or etrolizumab 300 mg (n = 40) 5 patients had an endoscopic subscore of 0 or 1, and were excluded from the modified intention‐to‐treat population (MITT = 119; 41 patients in the placebo group; 39 patients in the 100 mg group; 39 patients in the 300 mg group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was conducted with an interactive voice and web response system |
| Allocation concealment (selection bias) | Low risk | Randomization was conducted with an interactive voice and web response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All patients, assessing physicians, the funder and its agents and study personnel were masked to treatment assignment, except for site pharmacists who prepared drugs but did not interact with patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All patients, assessing physicians, the funder and its agents and study personnel were masked to treatment assignment, except for site pharmacists who prepared drugs but did not interact with patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were similar across groups |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armuzzi 2014 | Not RCT |
| Fiorino 2014 | Not RCT |
| Kreutzkamp 2014 | Not RCT |
| Lin 2014 | Not RCT |
Characteristics of ongoing studies [ordered by study ID]
NCT01461317.
| Trial name or title | A phase II open‐label extension study to evaluate the long‐term safety of rhuMAb beta7 in patients with moderate to severe ulcerative colitis |
| Methods | Patients will receive a repeating SC injection of etrolizumab; safety and efficacy will be assessed through 104 weeks |
| Participants | ˜ 116 patients Inclusion Criteria: Males and females between 18 to 75 years old with active ulcerative colitis Patients had failed to obtain a clinical response by week 10, or they obtained a clinical response by week 10 but they had a flare‐up between weeks 10 and 28 in a previous phase II study (ABS4986g) Patients in the Unitied States must discontinue concomitant immunosuppressive therapy before enrolment and completely taper off oral corticosteroids 24 weeks before study entry |
| Interventions | Group 1: SC injection of etrolizumab 150 mg/ml |
| Outcomes | Primary outcomes: adverse events, serious adverse events Secondary outcomes: clinically significant changes in vital signs and safety laboratory measures, discontinuation due to adverse events, incidence and nature of injection‐site reactions/hypersensitivity, incidence of infections complications, immunogenicity (incidence of anti‐therapeutic antibodies) |
| Starting date | November 2011 |
| Contact information | Genentech, Inc. |
| Notes | Study is active; enrolment is complete |
NCT02100696.
| Trial name or title | Phase III, double blind, placebo‐controlled, multicenter study of the efficacy and safety of etrolizumab during induction and maintenance in patients with moderate to severe active ulcerative colitis who are refractory to or intolerant of TNF inhibitors |
| Methods | Double‐blind, randomized, placebo‐controlled study; SC injection of placebo or etrolizumab 105 mg administered every 4 weeks |
| Participants | ˜800 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe active UC (determined by MCS score) who have experienced intolerance, loss of response or failure to respond to treatment with at least one TNF‐inhibitor in the past 5 years |
| Interventions | Group 1: Blinded (Cohort 2): etrolizumab induction (I) + maintenance (M) Group 2: Experimental: Blinded (Cohort 2): etrolizumab I + placebo M Group 3: Placebo Comparator: Blinded (Cohort 2): placebo I + M Group 4: Open‐label (Cohort 1): etrolizumab I + M Group 5: Open‐label (Cohort 1): etrolizumab I + placebo M |
| Outcomes | Primary outcomes: Clinical remission (determined by MCS) at week 14, maintenance of remission at week 66 |
| Starting date | May 2014 |
| Contact information | Reference Study ID Number: GA28950 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active; patients are being recruited |
NCT02118584.
| Trial name or title | An open label extension and safety monitoring study of moderate to severe ulcerative colitis patients previously enrolled in etrolizumab phase III studies |
| Methods | SC injection of placebo or etrolizumab 105 mg administered every 4 weeks for up to 7 years |
| Participants | ˜2600 patients Inclusion criteria: Part 1 (open‐label extension): patients are males and females over the age of 18 who were previously enrolled in a phase III study on etrolizumab who met the open‐label criteria outlined in the original study Part 2 (safety monitoring): patients are males and females over the age of 18 who previously enrolled in a phase III study on etrolizumab who were not eligible or chose not to participate in Part 1 |
| Interventions | Part 1: open‐label etrolizumab 105 mg Part 2: no intervention |
| Outcomes | Primary outcomes: long‐term efficacy as determined by partial Mayo Clinic Score (pMCS), incidence of adverse events |
| Starting date | September 2014 |
| Contact information | Reference Study ID Number: GA28951 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
NCT02136069.
| Trial name or title | Phase III, randomized, multicenter double‐blind, double dummy study to evaluate the efficacy and safety of etrolizumab compared with infliximab in patients with moderate to severe active ulcerative colitis who are naive to TNF inhibitors |
| Methods | SC injection of etrolizumab 105 mg administered every 4 weeks plus placebo IV infusions at weeks 0, 2 and 6, and then every 8 weeks, or, IV infusion of infliximab 5 mg/kg at weeks 0, 2 and 6, and then every 8 weeks plus SC placebo every 4 weeks |
| Participants | ˜720 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Patients had an inadequate response/intolerance to prior corticosteroid and/or immunosuppressant treatment |
| Interventions | Group 1 (experimental): etrolizumab + placebo Group 2 (active comparator): infliximab + placebo |
| Outcomes | Primary outcomes: proportion of patients in clinical remission (determined by MCS) Secondary outcomes: proportion of patients with clinical response (determined by MCS) at week 10, proportion of patients with sustained clinical response at weeks 10, 30 and 54 |
| Starting date | December 2014 |
| Contact information | Reference Study ID Number: GA29103 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
NCT02163759.
| Trial name or title | A phase III, randomized, double‐blind, double‐dummy, placebo‐controlled, multicenter study to evaluate the efficacy (induction of remission) and safety of etrolizumab compared with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients who are naive to TNF inhibitors (Study #1) |
| Methods | SC injection of etrolizumab 105 mg and adalimumab placebo administered at weeks 0, 2, 4, 6 and 8, or, SC injection of etrolizumab placebo and adalimumab 160 mg administered at week 0, 89 mg at week 2, and 40 mg at weeks 4, 6 and 8, or, etrolizumab placebo and adalimumab placebo administered at Weeks 0, 2, 4, 6 and 8 |
| Participants | ˜350 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Previous inadequate response to or intolerance of corticosteroids and/or immunosuppressant drugs |
| Interventions | Group 1: etrolizumab + adalimumab placebo Group 2: etrolizumab placebo + adalimumab Group 3: etrolizumab placebo + adalimumab placebo |
| Outcomes | Primary outcome: induction of remission compared with placebo (determined by MCS) Secondary outcome: induction of remission compared with adalimumab (determined by MCS) |
| Starting date | November 2014 |
| Contact information | Contact: Reference Study ID Number: GA28948 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
NCT02165215.
| Trial name or title | Phase III, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy (maintenance of remission) and safety of etrolizumab compared with placebo in patients with moderate to severe active ulcerative colitis who are naive to TNF inhibitors |
| Methods | During the open‐label phase, patients will be given SC etrolizumab 105 mg every 4 weeks During the maintenance phase, patients will be given SC etrolizumab 105 mg or placebo every 4 weeks |
| Participants | ˜350 patients Inclusion criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Previous inadequate response to or intolerance of corticosteroids and/or immunosuppressant drugs |
| Interventions | Phase 1: open‐label SC etrolizumab 105 mg Phase 2: SC etrolizumab 105 mg or placebo |
| Outcomes | Primary outcome: maintenance of clinical remission among randomized patients in clinical remission at week 10 (determined by MCS) Secondary outcomes: maintenance of clinical remission among randomized patients in clinical remission at week 10 (determined by MCS) |
| Starting date | August 2014 |
| Contact information | Contact: Reference Study ID Number: GA28949 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
NCT02171429.
| Trial name or title | Phase III, randomized, double‐blind, double‐dummy, placebo‐controlled, multicenter study to evaluate the efficacy (induction and remission) and safety of etrolizumab compared with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients who are naive to TNF inhibitors (Study #2) |
| Methods | Patients were randomized to one of three treatment groups: experimental (etrolizumab and adalimumab placebo), active comparator (etrolizumab placebo and adalimumab) or placebo comparator (etrolizumab placebo and adalimumab placebo) for 8 weeks |
| Participants | ˜350 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Previous inadequate response to or intolerance of corticosteroids and/or immunosuppressant drugs |
| Interventions | Goup 1 (experimental): SC etrolizumab 105 mg every 4 weeks, plus SC adalimumab placebo at weeks 0, 2, 4, 6 and 8 Group 2 (active comparator): SC adalimumab 160 mg administered SC at Week 0; 80 mg administered SC at Week 2; 40 mg SC at Weeks 4, 6 and 8, plus SC etrolizumab placebo every 4 weeks Group 3: SC adalimumab placebo at weeks 0, 2, 4, 6 and 8, plus SC etrolizumab placebo every 4 weeks |
| Outcomes | Primary outcome: induction of remission compared with placebo (determined by the MCS) Secondary outcome: Induction of remission compared with adalimumab (determined by MCS) |
| Starting date | November 2014 |
| Contact information | Contact: Reference Study ID Number: GA28949 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
Contributions of authors
Greg Rosenfeld contributed to planning the study and manuscript preparation.
Claire Parker contributed to planning the study, identification of relevant studies, assessment of methodological quality, data extraction, data analysis and manuscript preparation.
John MacDonald contributed to planning the study, identification of relevant studies, assessment of methodological quality, data extraction, data analysis, and manuscript preparation.
Brian Bressler contributed to planning the study and manuscript preparation.
Declarations of interest
Dr Bressler participated as a co‐investigator in Rutgeerts 2013.
Greg Rosenfeld has received fees from Abbvie, Janssen and Shire for lectures; and fees from Abbvie for development of educational presentations. All of the fees received are outside the scope of the submitted work. Dr Rosenfeld is a sub‐investigator for a Phase III clinical trial investigating etrolizumab for Crohn's disease and ulcerative colitis.
Claire Parker has no known conflicts to declare.
John MacDonald has no known conflicts to declare.
Brian Bressler has received fee(s) from Janssen, Abbvie, Celltrion, and Takeda for consultancy; has grants/grants pending from Janssen; payment for lectures from Takeda, Ferring, Actavis, Janssen, Abbvie, and Shire; stock/stock options with Qu Biologics and travel expenses from Janssen, and Abbvie. All of these activities are outside the submitted work.
New
References
References to studies included in this review
Rutgeerts 2013 {published data only}
- Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B. A phase I study of rhuMab beta7 in moderate to severe ulcerative colitis (UC). Gastroenterology 2011;140(5 Suppl 1):S‐125. [Google Scholar]
- Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, et al. A randomised phase i study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut 2013;62(8):1122‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeire 2014 {published data only}
- Keir M, Tew G, Luca W, Eastham‐Anderson D, Diehl J, Egen LG, et al. AlphaE integrin expression as a predictive biomarker for induction of clinical remission by etrolizumab: Analysis of a phase II trial in moderate‐to‐severely active ulcerative colitis. Journal of Crohn's and Colitis 2014;8:S7. [Google Scholar]
- Keir M, Tew G, Luca W, Eastham‐Anderson D, Diehl J, Egen LG, et al. Alphae integrin expression as a predictive biomarker for induction of clinical remission by etrolizumab: Analysis of a phase ii trial in moderate‐to‐severely active ulcerative colitis. Gastroenterology 2014;146(Suppl 1):S‐422. [Google Scholar]
- Vermeire S, O'Byrne S, Williams M, Mansfield JC, Feagan BG, Panes J, et al. Differentiation between etrolizumab (rhumab beta7) and placebo in the eucalyptus phase ii randomized double‐blind placebo‐controlled induction study to evaluate efficacy and safety in patients with refractory moderate‐to‐severely active ulcerative colitis. Gastroenterology 2013;144(5):S‐36. [Google Scholar]
- Vermeire S, O’Byrne S, Keir M, Williams M, Lu T, Mansfield JC, et al. Etrolizumab as induction therapy for ulcerative colitis:a randomised, controlled, phase 2 trial. Lancet 2014;384(9940):309‐18. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Armuzzi 2014 {published data only}
- Armuzzi A, Felice C. Etrolizumab in moderate‐to‐severe ulcerative colitis. Lancet 2014;384(9940):285‐6. [DOI] [PubMed] [Google Scholar]
Fiorino 2014 {published data only}
- Fiorino G, Danese S. Etrolizumab in ulcerative colitis: tightening leukocyte traffic control in the inflamed mucosa. Gastroenterology 2014;147(6):1433‐5. [DOI] [PubMed] [Google Scholar]
Kreutzkamp 2014 {published data only}
- Kreutzkamp, B. Moderate to severe treatment‐resistant ulcerative colitis: Integrin inhibitor etrolizumab leads to clinical remission [Masige bis schwere, therapierefraktare Colitis ulcerosa: Integrinhemmer Etrolizumab fuhrt in klinische Remission]. Arzneimitteltherapie 2014;32(11):309‐18. [Google Scholar]
Lin 2014 {published data only}
- Lin K K, Mahadevan, U. Etrolizumab: anti‐beta7‐a novel therapy for ulcerative colitis. Gastroenterology 2014;146(1):307‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01461317 {published data only}
- NCT01461317. A phase II open‐label extension study to evaluate the long‐term safety of rhuMAb beta7 in patients with moderate to severe ulcerative colitis. clinicaltrials.gov/ct2/show/NCT01461317 (accessed 19 November 2015).
NCT02100696 {published data only}
- NCT02100696. Phase III, double blind, placebo‐controlled, multicenter study of the efficacy and safety of etrolizumab during induction and maintenance in patients with moderate to severe active ulcerative colitis who are refractory to or intolerant of TNF inhibitors. clinicaltrials.gov/ct2/show/NCT02100696 (accessed 19 November 2015).
NCT02118584 {published data only}
- NCT02118584. An open label extension and safety monitoring study of moderate to severe ulcerative colitis patients previously enrolled in etrolizumab phase III studies. clinicaltrials.gov/ct2/show/NCT02118584 (accessed 19 November 2015).
NCT02136069 {published data only}
- NCT02136069. Phase III, randomized, multicenter double‐blind, double dummy study to evaluate the efficacy and safety of etrolizumab compared with infliximab in patients with moderate to severe active ulcerative colitis who are naive to TNF inhibitors. clinicaltrials.gov/ct2/show/NCT02136069 (accessed 19 November 2015).
NCT02163759 {published data only}
- NCT02163759. A phase III, randomized, double‐blind, double‐dummy, placebo‐controlled, multicenter study to evaluate the efficacy (induction of remission) and safety of etrolizumab compared with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients who are naive to TNF inhibitors (Study #1). clinicaltrials.gov/ct2/show/NCT02163759 (accessed 19 November 2015).
NCT02165215 {published data only}
- NCT02165215. Phase III, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy (maintenance of remission) and safety of etrolizumab compared with placebo in patients with moderate to severe active ulcerative colitis who are naive to TNF inhibitors. clinicaltrials.gov/ct2/show/NCT02165215 (accessed 19 November 2015).
NCT02171429 {published data only}
- NCT02171429. Phase III, randomized, double‐blind, double‐dummy, placebo‐controlled, multicenter study to evaluate the efficacy (induction and remission) and safety of etrolizumab compared with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients who are naive to TNF inhibitors (Study #2). clinicaltrials.gov/ct2/show/NCT02171429 (accessed 19 November 2015).
Additional references
Ariyaratnam 2014
- Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a meta‐analysis. American Journal of Gastroenterology 2014;109(2):163‐9. [DOI] [PubMed] [Google Scholar]
Bickston 2014
- Bickston SJ, Behm BW, Tsoulis DJ, Cheng J, MacDonald JK, Khanna R, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD007571.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjarnason 1997
- Bjarnason I, Macpherson A, Mackintosh C, Buxton‐Thomas M, Forgacs I, Moniz C. Reduced bone density in patients with inflammatory bowel disease. Gut 1997;40(2):228‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouma 2003
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature Reviews Immunology 2003;3(7):521‐33. [DOI] [PubMed] [Google Scholar]
Cepek 1993
- Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. Journal of Immunology 1993;150(8 Pt 1):3459‐70. [PubMed] [Google Scholar]
Cohen 2010
- Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Alimentary Pharmacology and Therapeutics 2010;31(7):693‐707. [DOI] [PubMed] [Google Scholar]
Danese 2011
- Danese S, Fiocchi C. Ulcerative colitis. New England Journal of Medicine 2011;365(18):1713‐25. [DOI] [PubMed] [Google Scholar]
Dignass 2010
- Dignass A, Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence‐based Consensus on the diagnosis and management of Crohn's disease: Current management. Journal of Crohn's and Colitis 2010;4(1):28‐62. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faubion 2001
- Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population‐based study. Gastroenterology 2001;121(2):255‐60. [DOI] [PubMed] [Google Scholar]
Feagan 2012a
- Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000543.pub3] [DOI] [PubMed] [Google Scholar]
Feagan 2012b
- Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000544.pub3] [DOI] [PubMed] [Google Scholar]
Feagan 2013
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. [DOI] [PubMed] [Google Scholar]
Fedorak 2010
- Fedorak RN, Wong K, Bridges R. Canadian Digestive Health Foundation Public Impact Series. Inflammatory bowel disease in Canada: Incidence, prevalence, and direct and indirect economic impact. Canadian Journal of Gastroenterology 2010;24(11):651‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2011a
- Ford AC, Achkar JP, Khan KJ, Kane SV, Talley NJ, Marshall JK, et al. Efficacy of 5‐aminosalicylates in ulcerative colitis: systematic review and meta‐analysis. American Journal of Gastroenterology 2011;106(4):601‐16. [DOI] [PubMed] [Google Scholar]
Ford 2011b
- Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Talley NJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta‐analysis. American Journal of Gastroenterology 2011;106(4):590‐9; quiz 600. [DOI] [PubMed] [Google Scholar]
Ford 2013
- Ford AC, Peyrin‐Biroulet L. Opportunistic infections with anti‐tumor necrosis factor‐alpha therapy in inflammatory bowel disease: meta‐analysis of randomized controlled trials. American Journal of Gastroenterology 2013;108(8):1268‐76. [DOI] [PubMed] [Google Scholar]
Gisbert 2015
- Gisbert JP, Marin AC, McNicholl AG, Chaparro M. Systematic review with meta‐analysis: the efficacy of a second anti‐TNF in patients with inflammatory bowel disease whose previous anti‐TNF treatment has failed. Alimentary Pharmacology and Therapeutics 2015;41(7):613‐23. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating high quality evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Holzmann 1989
- Holzmann B, McIntyre BW, Weissman IL. Identification of a murine Peyer's patch‐‐specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA‐4 alpha. Cell 1989;56(1):37‐46. [DOI] [PubMed] [Google Scholar]
Hu 1992
- Hu MC, Crowe DT, Weissman IL, Holzmann B. Cloning and expression of mouse integrin beta p(beta 7): a functional role in Peyer's patch‐specific lymphocyte homing. Proceedings of the National Academy of Sciences of the United States of America 1992;89(17):8254‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kornbluth 2010
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. American Journal of Gastroenterology 2010;105(3):501‐23; quiz 524. [DOI] [PubMed] [Google Scholar]
Lawson 2006
- Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005112.pub2] [DOI] [PubMed] [Google Scholar]
Lichtenstein 2006
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130(3):940‐87. [DOI] [PubMed] [Google Scholar]
Loftus 2008
- Loftus EV, Delgado DJ, Friedman HS, Sandorn WJ. Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States. American Journal of Gastroenterology 2008;103(7):1737‐45. [DOI] [PubMed] [Google Scholar]
Molodecky 2012
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46‐54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
Podolsky 2002
- Podolsky DK. Inflammatory bowel disease. New EnglandJournal of Medicine 2002;347(6):417‐29. [DOI] [PubMed] [Google Scholar]
Reinisch 2011
- Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60(6):780‐7. [PUBMED: 21209123] [DOI] [PubMed] [Google Scholar]
Sandborn 2012
- Sandborn WJ, Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology 2012;142(2):257‐65.e1‐3. [DOI] [PubMed] [Google Scholar]
Sandborn 2014
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology 2014;146(1):85‐95; quiz e14‐5. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxford AD, Visit GE, Higgins JPT, Deeks, JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook of Systematic Reviews of Intervetions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Availble from www.cochrane‐handbook.org. [Google Scholar]
Smith 2010
- Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2010;32(2):119‐30. [DOI] [PubMed] [Google Scholar]
Stefanich 2011
- Stefanich EG, Danilenko DM, Wang H, O'Byrne S, Erickson R, Gelzleichter T, et al. A humanized monoclonal antibody targeting the beta7 integrin selectively blocks intestinal homing of T lymphocytes. British Journal of Pharmacology 2011;162(8):1855‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Timmer 2012
- Timmer A, McDonald JWD, Tsoulis DJ, MacDonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000478.pub3] [DOI] [PubMed] [Google Scholar]
Turner 2007
- Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta‐regression. Clinical Gastroenterology and Hepatology 2007;5(1):103‐10. [DOI] [PubMed] [Google Scholar]
Van Assche 2005
- Assche G, Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. New England Journal of Medicine 2005;353:362‐8. [DOI] [PubMed] [Google Scholar]


