Summary
Some Bacillus-infecting bacteriophages use a peptide-based communication system, termed arbitrium, to coordinate the lysis-lysogeny decision. In this system, the phage produces AimP peptide during the lytic cycle. Once internalized by the host cell, AimP binds to the transcription factor AimR, reducing aimX expression and promoting lysogeny. Although these systems are present in a variety of mobile genetic elements, their role in the phage life cycle has only been characterized in phage phi3T during phage infection. Here, using the B. subtilis SPβ prophage, we show that the arbitrium system is also required for normal prophage induction. Deletion of the aimP gene increased phage reproduction, although the aimR deletion significantly reduced the number of phage particles produced after prophage induction. Moreover, our results indicated that AimR is involved in a complex network of regulation and brought forward two new players in the SPβ lysis-lysogeny decision system, YopN and the phage repressor YopR. Importantly, these proteins are encoded in an operon, the function of which is conserved across all SPβ-like phages encoding the arbitrium system. Finally, we obtained mutant phages in the arbitrium system, which behaved almost identically to the wild-type (WT) phage, indicating that the arbitrium system is not essential in the laboratory but is likely beneficial for phage fitness in nature. In support of this, by possessing a functional arbitrium system, the SPβ phage can optimize production of infective particles while also preserving the number of cells that survive after prophage induction, a strategy that increases phage persistence in nature.
Keywords: bacteriophage, SPβ phages, phi3T, AimR, AimP, SOS response, lysis, lysogeny, repressor, lysis/lysogeny
Graphical abstract
Highlights
-
•
The arbitrium system controls prophage induction in B. subtilis
-
•
An operon downstream of the arbitrium system is involved in controlling lysogeny
-
•
The operon is functionally conserved in SPβ-like phages encoding arbitrium systems
-
•
YopR acts as the phage repressor in SPβ
Bacillus subtilis phages from the SPβ family use the arbitrium system to communicate during infection of the host. Brady et al. show that this system is also required for induction of the resident prophage after activation of the host SOS response and identify a key operon involved in the control of the lytic/lysogenic cycle.
Introduction
Deciphering the basis of communication is essential for understanding the communities where organisms live and their ecological behaviors. The ability to communicate is not restricted to highly evolved animals; bacteria and unicellular eukaryotes also possess sophisticated mechanisms of communication. However, it has recently been described that viruses also have communication mechanisms that allow them to make collective decisions. Quorum-sensing communication mechanisms in bacteriophages (phages)—such as the arbitrium system—to make lysis-lysogeny decisions represent a breakthrough confirming viruses as sophisticated social agents in the microbial world.1,2 In addition, other social behaviors, such as cooperation, where different viruses co-infect a host,3,4 or altruism to defeat the CRISPR-Cas-mediated immune defense of bacteria5,6 confirm that viruses have different communication skills that may have a crucial role in establishing sophisticated social microbial networks.
The novel arbitrium system was described in the Bacillus subtillis SPβ group of phages using phi3T as a model. In this elegant system, phages communicate during the infection cycle using a six-amino-acid (aa) peptide (AimP) as a signal.2 Depending on the concentration of peptide present, phages undergo either a lytic or lysogenic cycle. The arbitrium system is composed of three genes: aimP, which encodes the arbitrium peptide; aimR, encoding a transcriptional factor that binds to AimP; and aimX, which produces a small non-coding RNA that exerts a negative regulatory effect on lysogeny, inducing lysis by a mechanism that has not been deciphered yet.2 AimP is produced as a 43-aa pro-peptide that is released from the bacterial cell into the surrounding medium. The pro-peptide is then processed into the mature 6-aa AimP before it is imported into neighboring bacteria via the oligopeptide permease (OPP) transporter channel. Once internalized, the mature AimP binds to the AimR receptor and controls its DNA regulatory activity.2 AimR is a transcriptional factor and, in its apo peptide-free form, promotes aimX expression. During the initial stages of infection, when the number of active phages is low, the arbitrium peptide is absent and AimR activates aimX expression, promoting the lytic cycle of the phage (Figure S1). After intense phage replication, AimP will accumulate in the medium, increasing the intracellular concentration of the mature AimP peptide until it reaches the threshold level required to bind to its cognate AimR receptor. When this occurs, AimR cannot activate aimX expression. This promotes the lysogenic cycle and the integration of the prophage into the bacterial chromosome, thus preventing eradication of the entire bacterial population by the phage (Figure S1).2 This simple and elegant communication system allows infecting phages to “decide” between lytic and lysogenic life cycles.
Although the ecological impact of the arbitrium system in phage infection has clearly been established, whether this system has a role in prophage induction or not remains to be determined. Here, we solve this mystery, providing evidence that the arbitrium system has an important role in prophage induction and cellular survival.
Results
Analysis of aimR and aimP mutants
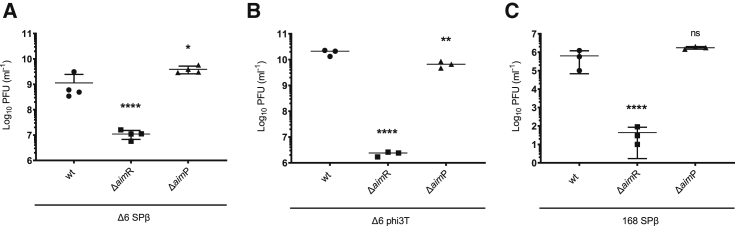
SPβ is one of the prophages present in the B. subtillis 168 strain and was selected as the phage model used to study the impact of the arbitrium system in prophage induction. Several studies have analyzed the transcriptomic landscape of this strain in response to different stimuli, including induction of the SOS response by treating the lysogenic cells with mitomycin C (MC).7,8 When we analyzed these transcriptomic data in relation to the SPβ prophage, one result raised our curiosity: the expression of the aimP gene is relatively high compared to other genes in the uninduced SPβ lysogenic strain,7,8 suggesting that aimP (and therefore the arbitrium system) might have a role during lysogeny. To test this hypothesis, we initially made use of the B. subtillis 168 Δ6 strain, in which all the mobile genetic elements (MGEs) present in the original B. subtillis 168 strain have been deleted.9 The strain was lysogenized with SPβ and subsequently used to generate derivative ΔaimP or ΔaimR mutants. We next tested whether aimR or aimP impacted SPβ prophage induction. Lysogenic strains carrying either the wild type (WT), the ΔaimR, or the ΔaimP SPβ prophages were induced with MC, and after leaving them overnight to complete the lysis, the phages present in the lysates were quantified. The titer of the SPβ ΔaimP was slightly higher than that observed for the WT SPβ (Figure 1A). Alongside an increased titer, the culture carrying the SPβ ΔaimP prophage was significantly more lysed than that carrying the WT prophage after prophage induction (Figure S2A). Importantly, our results also demonstrate that AimR is required for SPβ induction. Thus, after induction, the titer of the SPβ ΔaimR phage was significantly reduced compared to the SPβ WT (Figure 1A). Importantly, both the aimP and aimR mutations could be complemented (Figure S2), confirming the validity of these results.
Figure 1.
Effect of aimR and aimP mutations on phage titer
(A) 168 Δ6 strains lysogenic for phage SPβ WT, ΔaimR, and ΔaimP were MC induced (0.5 μg/mL), and the number of resulting phages were quantified by titering using 168 Δ6 as the recipient strain. The results are represented as the plaque-forming units (PFUs) mL−1. The means and SDs are presented (n = 4). An ordinary one-way ANOVA of transformed data was performed to compare mean differences between SPβ WT, ΔaimR, and ΔaimP titers. Adjusted p values were as follows: SPβ ΔaimR ∗∗∗∗p ≤ 0.0001; SPβ ΔaimP ∗p = 0.0115.
(B) 168 Δ6 strains lysogenic for phages phi3T WT, ΔaimR, and ΔaimP were MC induced (0.5 μg/mL), and the number of resulting phages were quantified by titering using 168 Δ6 as the recipient strain. The results are represented as PFUs/mL−1. The means and SDs are presented (n = 3). An ordinary one-way ANOVA of transformed data was performed to compare mean differences between SPβ WT, ΔaimR, and ΔaimP titers. Adjusted p values were as follows: SPβ ΔaimR ∗∗∗∗p ≤ 0.0001; SPβ ΔaimP ∗∗p = 0.0058.
(C) Strain 168 lysogenic for phages SPβ WT, ΔaimR, and ΔaimP were MC induced (0.5 μg/mL), and the number of resulting phages was quantified by titering using 168 Δ6 as the recipient strain. The results are represented as PFUs/mL−1. The means and SDs are presented (n = 3). An ordinary one-way ANOVA of transformed data was performed to compare mean differences between SPβ WT, ΔaimR, and ΔaimP titers. Adjusted p values were as follows: SPβ ΔaimR ∗∗∗∗p ≤ 0.0001; SPβ ΔaimP ns, not significant.
In support of the role of the arbitrium system during SPβ infection, the plaque morphology of the phages analyzed were different. Although the SPβ aimP mutant produced the sharpest plaques, the ones produced by the aimR mutants were more diffuse, confirming that the absence of AimP or AimR promotes lysis or lysogeny, respectively (Figure S2D). In fact, when the different lysates were used to analyze lysogenization, the SPβ ΔaimR mutant generated more lysogenic cells than the WT after infection of the recipient cells (Figure 4B).
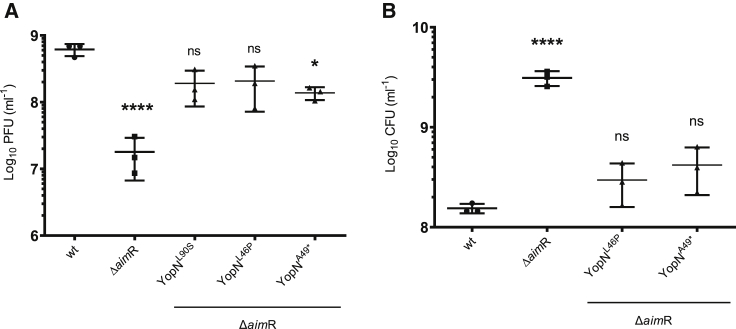
Figure 4.
Titer and lysogenization of SPβ WT, ΔaimR, ΔaimP, and evolved phages
Strains lysogenic for phages SPβ WT, ΔaimR, and evolved aimR phages were MC induced (0.5 μg/mL).
(A) The number of resulting phages were quantified using 168 Δ6 as the recipient strain. The results are represented as PFUs mL−1. The means and SDs are presented (n = 3). An ordinary one-way ANOVA of transformed data was performed to compare mean differences between titers. Adjusted p values were as follows: SPβ ΔaimR ∗∗∗∗p ≤ 0.0001; YopNL90S and YopNL46P ns; YopNA49∗∗p = 0.0324.
(B) The number of resulting lysogens were quantified using 168 Δ6 as the recipient strain. The results are represented as colony-forming units (CFUs) mL−1 normalized by PFUs per milliliter and represented as the log CFU of an average phage titer (1 × 109 PFUs). The means and SDs are presented (n = 3). An ordinary one-way ANOVA of transformed data was performed to compare mean differences in lysogenization. Adjusted p values were as follows: SPβ ΔaimR ∗∗∗∗p ≤ 0.0001; YopNL46P and YopNA49∗ ns.
Importantly, and because the aimR mutation increases lysogenization, it could be possible that the aimR mutant did not generate less infective particles than the WT phage, but these could not be properly quantified because most of the aimR mutant phages could integrate after infection. To analyze this possibility, the SPβ WT and ΔaimR lysates obtained were used to infect either the B. subtillis 168 Δ6 strain or its derivative expressing aimRSPβ. As shown in Figure S3A, although the plaques were sharper in the strain expressing aimR (Figure S3C), no differences in the number of plaques formed were observed when the different lysates were plated in either the WT or in the AimR-expressing strain, confirming that aimR is required for SPβ prophage induction (Figure 1).
Next, we analyzed whether overexpression of AimR would per se induce the resident SPβ prophage. To do that, we overexpressed the aimRSPβ gene in the strain lysogenic for SPβ, and after 12 h, we quantified the number of phages present in the lysate. We did not observe significant differences between the number of phages obtained from the strain carrying the empty vector versus the one that overexpressed aimR (Figure S3D), suggesting that AimR is required once the SOS response has been activated.
Because the arbitrium system played an important role in SPβ, we extended our studies by analyzing the impact of the aimR and aimP mutations in the phi3T prophage. Note that, although SPβ and phi3T belong to the same family of SPβ phages,10,11 they encode arbitrium systems that are different in sequence. Our results demonstrated that, in this prophage, the role of AimR seemed to be more relevant, and the titer obtained after induction of the phi3T ΔaimR prophage was reduced 10,000 times compared to that seen in the WT phi3T (Figure 1B). Interestingly, and contrary to what is seen with SPβ, the phi3T ΔaimP showed a slightly reduced titer after induction, compared to the WT (Figure 1B). Why the aimP mutations have different consequences in both phages is an intriguing question that is currently under investigation. Complementation of the aimR or aimP mutations restored the phage titers, confirming that the observed phenotypes were consequence of the mutations (Figure S2).
Finally, we analyzed the impact of the arbitrium system in prophage induction using a more natural scenario. To do that, we used B. subtillis 168 strain, which, in addition to the SPβ prophage, contains 4 other prophages and the integrative conjugative element ICEBs1.12 We obtained SPβ aimR and aimP derivative mutants of this strain, and after MC induction of the WT and mutant strains, the SPβ titers were quantified using B. subtillis 168 Δ6 as recipient. Note that, in the B. subtillis 168 strain, none of the other phages present except SPβ can produce plaques. In support with the fact that ICEBs1 and PBSX (one of the defective prophages present in this strain) interfere with SPβ reproduction,13,14 the titer of SPβ was significantly reduced (more than 3 logs) after induction of prophage from the B. subtillis 168 strain, compared with the induction of the SPβ prophage from B. subtillis 168 Δ6 strain (Figure 1). Although the aimP mutant did not show any difference in titer, the aimR deletion had a more pronounced effect in the B. subtillis 168 background compared to what is seen in the B. subtillis 168 Δ6 strain, with a reduction in the phage titer higher than 10,000 times (Figure 1C). This result suggests that the arbitrium system may be even more important in strains carrying multiple mobile genetic elements, where these elements compete for resources in terms of induction and transmissibility.
Impact of AimR on phage replication
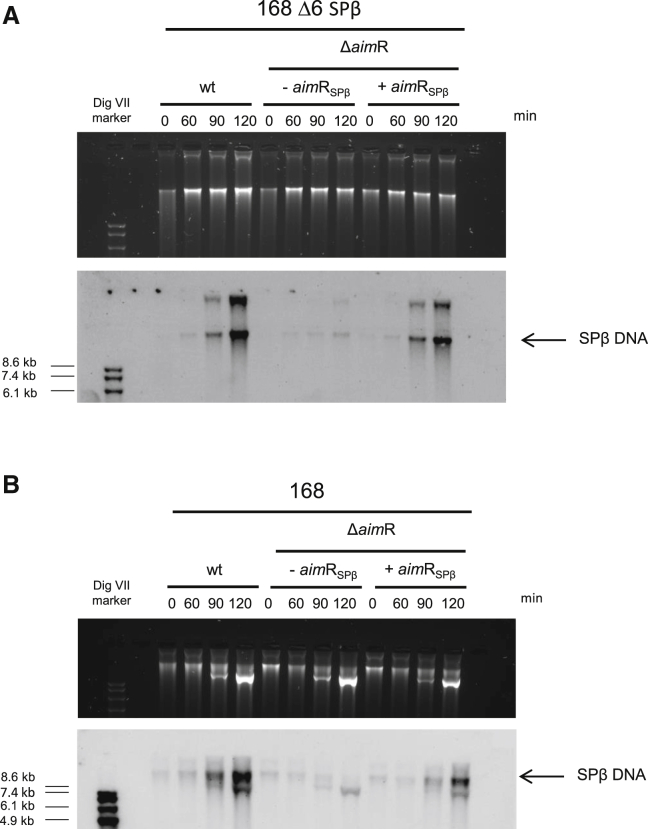
Although the SPβ master repressor has not yet been identified, the existing results suggested a cascade in prophage activation, starting with the elimination of the SPβ repressor after activation of the cellular SOS response. Once this occurs, the role of AimR in prophage induction turns essential. To acquire a better understanding of what was occurring with the aimR mutant, we took samples at different time points of the WT, ΔaimR mutant, and complemented SPβ prophages, present in either B. subtillis 168 or its derivative Δ6 strain, after MC (SOS) induction of the lysogenic cells and analyzed phage replication. As shown in Figure 2, replication of the SPβ aimR mutant was delayed and significantly reduced.
Figure 2.
Phage replication of SPβ WT, ΔaimR, and ΔaimR complemented
(A) Strains Δ6 lysogenic for phages SPβ WT, ΔaimR, and ΔaimR complemented with aimRSPβ were MC induced (0.5 μg/mL), and 1 mL of each culture at different time points after induction was collected. Samples were loaded in a 0.7% agarose gel, Southern blotted, and probed for phage DNA.
(B) Strains 168 lysogenic for phages SPβ WT, ΔaimR, and ΔaimR complemented with aimRSPβ were MC induced (0.5 μg/mL), and 5 mL of each culture at different time points after induction was collected. Samples were loaded in a 0.7% agarose gel, Southern blotted, and probed for phage SPβ DNA.
Evolved phage mutants provide insights into AimR function
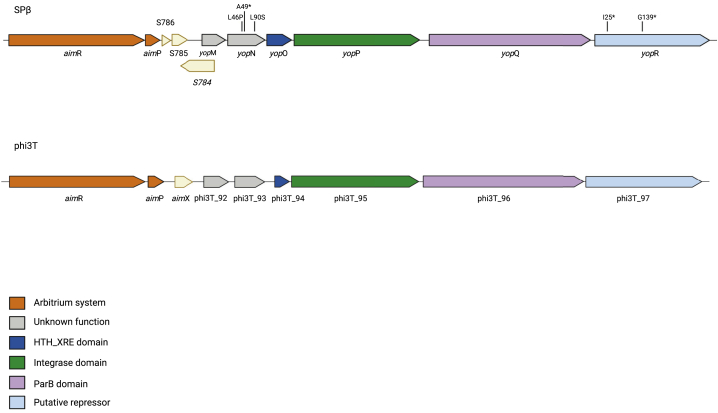
The previous results suggested that AimR functions either by controlling expression of the genes involved in SPβ replication or by promoting the removal of the phage-encoded master repressor. To gain more of an insight into AimR function, we evolved the SPβ aimR mutant in the B. subtillis 168 Δ6 background until it produced plaques identical in morphology to those of the WT SPβ phage (see scheme in Figure S4). As previously mentioned, the plaques produced by the SPβ aimR mutant have a diffuse (cloudy) morphology (Figure S2D). Different evolved phages, from independent experiments, were obtained and sequenced (Table S1). In three evolved phages, the mutations affected yopN, a gene localized in an operon next to the aimP gene in the SPβ genome (Figure 3) and encoded a protein with no known function. The mutations identified in the independently evolved phages were different. However, because one of the mutations generated a nucleotide deletion that created an early stop codon in yopN, we assumed that, in all cases, the reversion of the aimR mutant phenotype was consequence of a loss of function in the YopN protein.
Figure 3.
Schematic representation of the SPβ and phi3T arbitrium and operon genetic layout
Diagram shows the genetic organization of the arbitrium genes, aimR and aimP, followed by the operon directly downstream. Colors denote putative functions according to BLAST results; orange, arbitrium genes; light yellow, sRNAs; gray, unknown function; navy blue, HTH_XRE domain; green, integrase domain; purple, ParB domain; light blue, putative repressor. The mutations obtained during the evolution experiments are marked. Shown was created with BioRender.com. See also Figures S1 and S7 and Tables S1 and S2.
To analyze the behavior of these evolved phages, we lysogenized the B. subtilis 168 Δ6 strain with the different evolved phages. Following MC induction of the lysogenic cells, the number of phage particles present in the lysates were quantified. Because the WT and two of the evolved phages have a kanamycin marker inserted in their genome, the number of lysogens obtained after induction of the B. subtilis 168 Δ6 derivative strain was also quantified. Note that one of the evolved phages originates from a strain carrying SPβ without a kanamycin marker. In support of the idea that these evolved phages had bypassed the defect generated by the absence of AimR, MC induction of the lysogenic strains carrying these evolved prophages generated phage titers that were significantly higher than that observed for the aimR mutant prophage and similar to those observed for the WT SPβ phage (Figure 4A). Interestingly, these evolved phages maintained the ability to lysogenize as observed in the SPβ WT (Figure 4B). Taken together, these results indicated that the evolved phages, which are defective in the arbitrium system, behaved as the WT phage in the lab conditions, suggesting that this system is dispensable in these conditions, but not in nature.
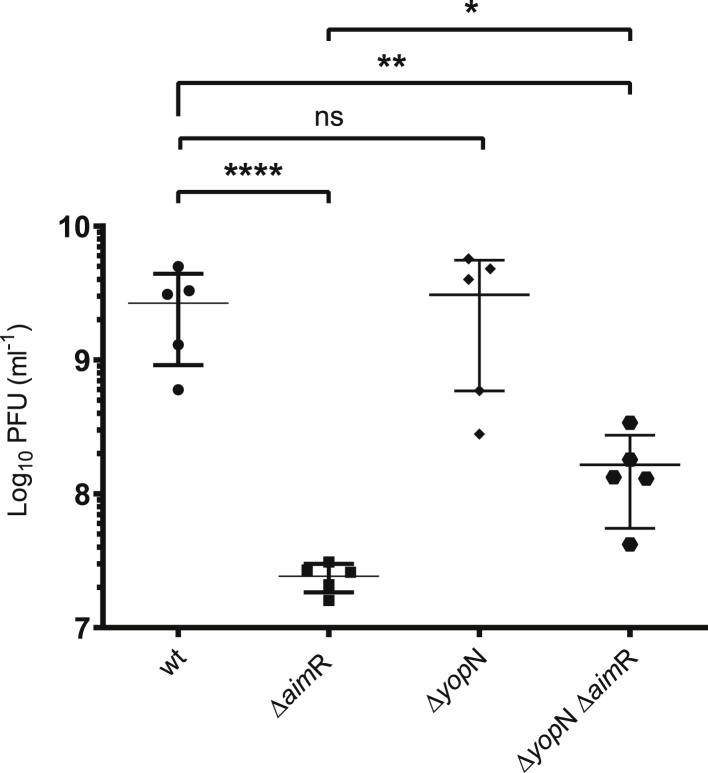
Next, because YopN has no assigned function, and its role in the phage cycle remains undetermined, we generated a yopN deletion mutant in the SPβ prophage and tested its impact on the phage cycle. Deletion of yopN did not modify the titer of the mutant after MC induction, compared to that of the WT phage (Figure 5). However, the plaques obtained with this mutant were significantly sharper than those obtained with the WT SPβ (Figure S5), with a morphology similar to that generated by the aimP mutant (Figure S2D).
Figure 5.
Titer of SPβ WT, ΔaimR, ΔyopN, and double mutant ΔaimR-yopN
Strains lysogenic for phages SPβ WT, ΔaimR, ΔyopN, and ΔaimR-yopN were MC induced (0.5 μg/mL). The number of resulting phages were quantified using 168 Δ6 as the recipient strain. The results are represented as PFUs mL−1. The means and SDs are presented (n = 5). An ordinary one-way ANOVA of transformed data was performed to compare mean differences between titers. Adjusted p values were as follows: SPβ WT versus SPβ ΔaimR ∗∗∗∗p ≤ 0.0001; SPβ ΔyopN ns; SPβ ΔyopN ΔaimR ∗∗p = 0.0010. SPβ ΔaimR versus SPβ ΔyopN ΔaimR ∗p = 0.0351. See also Figures S2, S3, and S5.
Next, and to confirm the results obtained with the evolved phages, we generated a SPβ prophage carrying the double aimR/yopN deletion and tested its titer after SOS induction of this mutant. As shown in Figure 5, in accordance with the results obtained with the evolved phages, the titer of the double mutant was slightly (but significantly) higher than that observed for the single SPβ ΔaimR mutant (Figure 5). The difference observed between the evolved phages and the double SPβ aimR/yopN are likely to be the consequence of the elimination of the yopN coding sequence in the latter, which may have an impact in the stability of the transcript of the operon containing yopN. Importantly, and as observed with the single yopN mutant, the plaques produced by the double SPβ aimR/yopN mutant were sharper than those produced either by the WT or the ΔaimR SPβ phages (Figure S5). Taken together, these results involve YopN in the process controlling lysis/lysogeny in the SPβ phage.
In addition to the yopN mutants, two of the evolved phages characterized in this study presented mutations in yopR (Table S1), a gene that is also contained in the same putative operon as yopN (Figure 3). The plaques produced by these mutants were even sharper than those produced by the yopN mutant, suggesting that these phages had activated their lytic pathway. Confirming this idea, we were not able to obtain lysogens of these evolved phages. Although an initial BLAST analysis showed that YopR has an integrase domain, we propose here that this protein is not required for prophage integration but is the SPβ master repressor. Previous studies have characterized the SPβ protein SprA, which is the recombinase involved in the integration and excision of the SPβ prophage.15
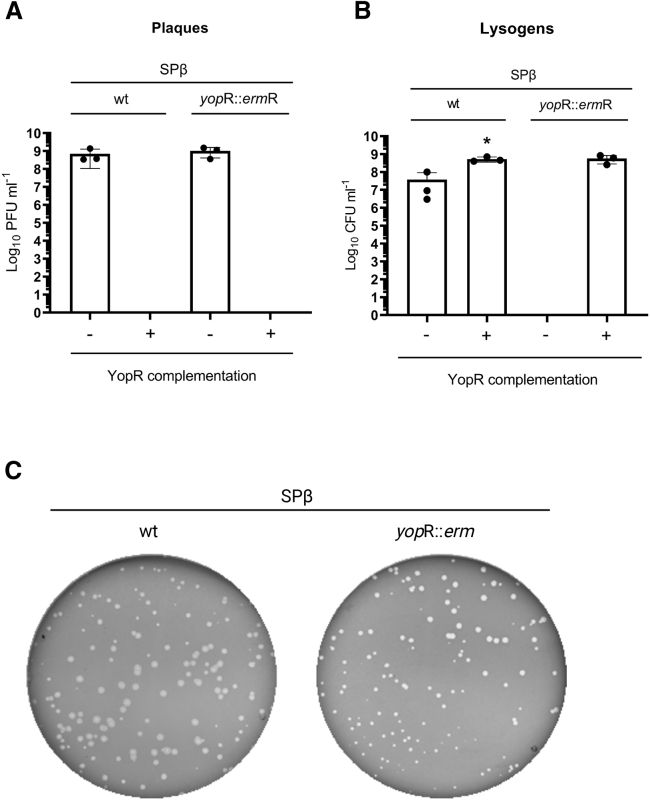
To test the function of YopR, we initially tried to make a yopR mutant by inserting an erythromycin marker. The fact that we got a few erythromycin-resistant colonies was unexpected for us, as deletion of the putative phage master repressor would kill the lysogenic cells as a consequence of the induction of the resident prophage. Even more surprising was the fact that the putative yopR mutant did not show a reduction in the titer after induction with MC, but it showed plaques with two very distinctive phenotypes, suggesting a mixed population of phages (Figure S5). Concurrently, we tested the original yopR::erm mutant from the BKE genome-scale deletion library (BKE20790),16 and we obtained the same mixed population producing two different plaque phenotypes. Although some plaques looked as the WT SPβ prophage, others had the same phenotype previously observed for the evolved SPβ yopR mutants (sharper). Because these results suggested the presence of two different phages, we hypothesized that, to maintain integrated the SPβ yopR::erm prophage, another copy of SPβ would have integrated elsewhere in the bacterial chromosome, complementing the yopR mutation. In support of this, PCR analyses confirmed that all the plaques with the cloudy-diffuse morphology carried the WT SPβ phage, although the plaques with the sharp morphology were produced by the SPβ yopR mutant. This result suggests that YopR is acting as the repressor of the system, and its deletion abolishes the capacity of the phage to remain integrated as a prophage.
Alternatively, we tried to generate a yopR mutant by introducing a second copy of yopR elsewhere in the chromosome of the lysogenic strain. Using this strain, we were able to delete yopR from the SPβ genome. This mutant phage remained inactive and integrated in the lysogenic strain, because of the complementation with the ectopic copy of yopR. However, when this strain was MC induced, the analysis of the lysate showed that these phages produced sharp and clear plaques, as observed with the double aimR-yopR mutant, and it was unable to lysogenize. Finally, to clearly confirm the role of YopR as the SPβ repressor, we tried to infect the B. subtilis 168 Δ6 strain expressing yopR with the SPβ phage. As expected for the function of a master repressor, YopR expression in the recipient cells completely blocked plaque formation but increased the number of lysogens generated after infection of the WT or the yopR mutant (Figure 6). In summary, these results indicate that AimR is required to remove YopR repression. The molecular details of this interaction and control are currently under study.
Figure 6.
Titer and lysogenization of SPβ WT and yopR mutant
Strains lysogenic for phages SPβ WT and SPβ amyE::Pspank-YopR yopR::ermR were MC induced (0.5 μg/mL).
(A) The number of resulting phages were quantified using 168 Δ6 or 168 Δ6 amyE::Pspank-YopR as the recipient strain. The results are represented as PFUs mL−1. The means and SDs are presented (n = 3).
(B) The number of resulting lysogens were quantified using 168 Δ6 or 168 Δ6 amyE::Pspank-YopR as the recipient strain. The results are represented as CFUs mL−1. The means and SDs are presented (n = 3). An ordinary one-way ANOVA of transformed data was performed to compare mean differences between SPβ lysogen titers obtained using 168 Δ6 or 168 Δ6 amyE::Pspank-YopR recipient strains (adjusted p = 0.0171).
(C) The lysates were titered using 168 Δ6 as the recipient strain. The resulting plaque morphologies were photographed. Shown was created with BioRender.com.
See also Figure S5.
The structure of the yopN-yopR operon is conserved in phages that use the arbitrium system
Because yopN and yopR seem to belong to the same operon, we scrutinized the rest of the genes forming this transcriptional unit. Four additional genes were annotated in the SPβ genome: yopM; yopO; yopP; and yopQ (Figure 3). As occurred with YopN, YopM is a hypothetical protein with no assigned function, YopO seems to be a transcriptional regulator of the Xre family, YopP has a XerC superfamily integrase domain, and YopQ has a ParB_N_Srx superfamily domain (Table S2). Importantly, this operon was uniquely found in B. subtilis phages belonging to the SPβ-like family that encode the arbitrium system.
When we examined the genetic layout of the SPβ-like phages carrying the arbitrium system,2,10 we discovered that, in the majority of the cases, phages carrying this operon also encoded an AimR almost identical to that expressed by SPβ (Table S2). In the exceptions where this did not occur (Table S2), these phages encoded a chimeric AimR, carrying an N-terminal region, responsible of the recognition of the AimR boxes in the phage genome,17 identical to that present in the SPβ AimR. However, the rest of the protein, involved in AimP recognition, was different, suggesting that these phages encode a different AimP (Figure S6). In support of these ideas, we were able to identify the SPβ AimR boxes in all the analyzed phage genomes carrying the yopN-yopR operon, although the putative AimP peptide produced by those phages expressing the chimeric AimR was different to that produced by SPβ (GMPRGA versus GIVRGA; mature peptide sequence).
We next scrutinized the region localized 3′ of aimP in the phi3T genome to see what genes were located there. As with SPβ, phi3T also carries in this region an operon composed of 6 genes (phi3T_92 to phi3T_97; Figure 3). Importantly, although different in sequence, the proteins encoded by this operon have identical predicted functions than those encoded by SPβ (Table S2; Figure 3). In light of these results, we decided to analyze in more detail whether this region was conserved in phages carrying different arbitrium systems. In our analysis, we were able to identify at least 9 families of SPβ-like phages carrying different arbitrium systems with differentiated AimR and AimP genes. After analyzing representatives of each family where the complete sequence of the phage is available, we determined that the genes downstream form part of an operon with genes with conserved functions in the same position as seen for SPβ and phi3T (Table S2; Figure S7). These analyses suggest that this genetic organization and gene composition are important for arbitrium function and prophage induction.
The arbitrium system increases survival of the lysogenic cells after induction
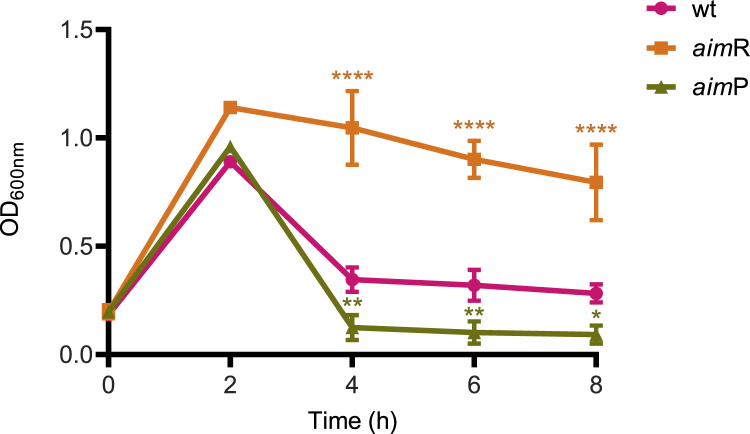
The fact that the SPβ aimP mutant, compared to the WT phage, showed higher levels of phage titer after induction raised an interesting question: why is aimP highly expressed in the SPβ lysogen? Because aimP expression reduces prophage induction, one would expect that this gene would not be expressed during lysogeny. Because AimP expression during infection protects cells from phage killing by promoting lysogenization,2 we hypothesized that aimP expression could also increase cell survival after prophage induction by limiting prophage activation. To test this, we measured cell growth after MC induction of the lysogenic cells carrying either the WT, the ΔaimR, or the ΔaimP SPβ prophages. As shown in Figure 7, the growth of the different lysogenic strains was inversely proportional to the ability of the different prophage to be SOS induced: thus, the number of lysogenic cells carrying the ΔaimR prophage were almost not affected by the induction of the mutant prophage, although the induction of the ΔaimP prophage significantly reduced the number of the lysogenic cells (Figure 7). In summary, the arbitrium system provides an interesting equilibrium between prophage induction and cellular survival by providing an almost optimal prophage induction by preserving better the population of lysogenic cells. In other words, our results indicate that the arbitrium system provides a “bet-hedging” strategy that retains some active lysogens during stress conditions.
Figure 7.
Growth curves of SPβ WT, ΔaimR, and ΔaimP after MC induction
Strains lysogenic for phages SPβ WT, ΔaimR, and ΔaimP were MC induced (0.5 μg/mL). Optical density 600 nm (OD600nm) was monitored over time, and cells were collected at time points 0, 2, 4, 6, and 8 h. The means and SDs are presented (n = 3). A two-way ANOVA was performed to compare mean differences in OD600nm values. Adjusted p values were as follows: time 4 h SPβ ΔaimR ∗∗∗∗p ≤ 0.0001, SPβ ΔaimP ∗∗p = 0.0077; time 6 h SPβ ΔaimR ∗∗∗∗p ≤ 0.0001, SPβ ΔaimP ∗∗p = 0.0085; time 8 h SPβ ΔaimR ∗∗∗∗p ≤ 0.0001, SPβ ΔaimP ∗p = 0.0226.
Discussion
The results presented here demonstrate that the arbitrium system is not only required for phage infection but is also essential for prophage induction. Interestingly, we were able to obtain laboratory-evolved phages that bypassed the necessity to encode an arbitrium system. These results imply that the arbitrium system is not the primary mechanism controlling lysogeny and lysis. However, and mirroring the results obtained during infection, our results demonstrate that the arbitrium system provides an essential ecological role in vivo after prophage induction by providing a mechanism that promotes efficient phage reproduction linked to an increased survival of the bacterial population. Because lysogenic cells also contain the intact prophage, by protecting the cells from the lysis that would occur after the activation of the lytic cycle, the arbitrium system provides phages with two alternative and complementary strategies to persist in nature, either as infective particle or as a prophage. One can hypothesize that, when the cellular damage is intense, provoking cell death, an increased production of infective particles could be a better strategy for the phage. However, it is likely that, in many scenarios and after induction of the SOS response, the non-lysogenic cells would be able to repair the damage. However, in this scenario, the presence of a very active and induced prophage would be detrimental for the population by promoting their lysis in circumstances where the cellular damage would be able to prevent the death of the cells. An example of this scenario occurs in the interaction between Streptococcus pneumoniae and Staphylococcus aureus, where the hydrogen peroxide produced by S. pneumoniae kills lysogenic S. aureus cells, after activation of the resident prophage, but not the non-lysogenic ones.18 In this scenario, the presence of an arbitrium system would minimize the damage created by the activation of the resident prophage.
We have yet to decipher how the arbitrium system works mechanistically, but we have been able to identify two additional players in this intriguing system. One is YopN. Although this protein does not seem to have a role in the induction of the WT prophage (at least in the laboratory conditions), this mutant behaves as the aimP mutant during infection. The evidence that YopN is an important player of the arbitrium system came from the results from the evolved phages, which indicated that the yopN mutation compensates the ΔaimR defect. The second player identified corresponds to YopR, which works as the master repressor of the phage. In this moment, we cannot anticipate how AimR, YopN, and YopR interact. Because yopN and yopR are part of an operon containing additional genes (Figure 3), our hypothesis is that some of these genes would be also involved in prophage induction. Therefore, our current working hypothesis is AimR controls the expression of additional gene/s encoding protein/s that, by modulating YopN function, remove YopR activity and induce the lytic cycle of the phage after prophage induction (Figure S1B). In support of this idea, we have previously shown that, in addition to aimX promoter, AimR plasticity allows the recognition of additional phage operators. Remarkably, one of the operators proved to be specifically recognized by AimR maps between yopS and yopR genes,17 suggesting a direct role in the control of the expression of these genes.
Another interesting feature of the phages carrying the arbitrium system is that they required the activation of the SOS response for induction. Classically, phages have sensed the cellular SOS response by encoding repressors that mimic LexA structure. These repressors, exemplified by the λ cI or the P22 c2, are recognized by the activated RecA∗ protein that appears as a consequence of the cellular damage, activating the autocleavage and the eliminations of these repressors.19 Surprisingly, the analysis of SPβ and phi3T genomes failed to reveal open reading frames (ORFs) with the cI architecture, which has precluded the assignment of a putative repressor for these phages. Moreover, it seems a general feature for SPβ-like phages because we have not been able to detect cI-like repressors in other members of this family, indicating that these phages must encode for a different type of repressor. Interestingly, the results of the present work points to YopR as the putative repressor for the SPβ phage family. YopR sequence analysis by PFAM or SMART servers does not find any match with annotated domains, even with low confidence or those annotated as unknown function (DUF). Therefore, it seems that YopR lacks the characteristic motifs and fold of cI repressor. However, our in silico analyses with different structural prediction software (Phyre2, Robetta, and RaptorX) confirm this fact, proposing for this protein an architecture with structural homology to the tyrosine recombinase superfamily that includes different families of integrases, transposases, and recombinases.20 The confidence scores of these models are higher at the N-terminal portion, which corresponds to the integrase core-binding domain, than the C-terminal portion corresponding to the catalytic domain. The low confidence of the C-terminal domain hampers to locate the putative catalytic elements, including the conserved Tyr residue that covalently binds to DNA and is usually placed at the most C-terminal portion of the enzyme. Therefore, from the models, it is not possible to discern whether YopR could act as a functional integrase. In any case, the function of YopR as an active integrase seems not to be required because SPβ encodes SprA, whose genetic and enzymatic characterization has confirmed as the SPβ prophage integrase/excisionase,15 suggesting an alternative function for YopR. Conversely, the high confidence of the models for N-terminal, core-binding domain supports the DNA-binding capacity of YopR. This domain presents a four-helix bundle fold that includes a prototypical helix-turn-helix DNA-binding motif that mediates the interaction at the integration sites. It has been shown that different integrases have repressor capacity by binding their own promoters.21,22 For the integrase of P4, this capacity has been restricted to the integrase N-terminal portion,21 supporting our proposed repressor activity for YopR. Importantly, in this new system, how the SOS response promotes the elimination of this repressor remains to be determined.
Our observations also open an interesting possibility involving the arbitrium system in phage interference. We have demonstrated that AimP expression may have an impact by protecting the lysogenic cells of a massive prophage induction. Another possibility is that this expression may protect the lysogenic cells from an attack of a different phage encoding an AimR protein, which activity would be blocked by the AimP peptide expressed from the lysogen. In this scenario, the presence of AimP would promote lysogenization of the infecting phage, preserving both the lysogenic cells and the resident prophage. Although it is assumed that there is not crosstalk among different arbitrium systems, with the reduced number of studies that have analyzed we cannot discard completely that this process exists in nature.
The existence of communication systems in phages and other MGEs represents a paradigm shift requiring investigation. Here, we have provided insights into the molecular basis of this novel concept, providing knowledge that we anticipate will be relevant not just for understanding this specific system but also for many biological and evolutionary processes, including the emergence of virulent and multi-resistant bacterial clones. Of note is the fact that not only phages but also plasmids and other MGEs encode arbitrium systems.10
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Lysogeny broth (LB), Miller | Sigma – Aldrich | Cat. # L3522-1KG |
| Lysogeny broth (LB), Lennox | Sigma – Aldrich | Cat. # L3022-1KG |
| Agar | Formedium | Cat. # AGA02 |
| Spectinomycin dihydrochloride pentahydrate | Sigma – Aldrich | Cat. # S4014-5G |
| Erythromycin | Sigma – Aldrich | Cat. # E6376-25G |
| Kanamycin Sulfate | Sigma – Aldrich | Cat. # 60615-5G |
| Ampicillin Sodium Salt | Sigma – Aldrich | Cat. # A9518-25G |
| Isopropyl-β-D-thio-galactopyranoside (IPTG) | Melford | Cat. # 156000-5.0 |
| Ammonium sulfate | Sigma – Aldrich | Cat. # A4915-500G |
| K2HPO4 | Fisher scientific | Cat. # 10509263 |
| KH2PO4 | Fisher scientific | Cat. # 10573181 |
| Tri-sodium citrate dihydrate | Fisher scientific | Cat. # 10396430 |
| D-(+)-Glucose | Sigma – Aldrich | Cat. # G7021-1KG |
| Yeast extract | Fisher scientific | Cat. # 11407541 |
| Casein hydrolysate | Sigma – Aldrich | Cat. # 22090-100G |
| Magnesium sulfate heptahydrate | VWR | Cat. # 25165.26 |
| L-tryptophan | Sigma – Aldrich | Cat. # T8941-25G |
| L-methionine | Sigma – Aldrich | Cat. # M9625-25G |
| CaCl2 | VWR | Cat. # 190464K |
| Manganese II chloride dihydrate | Sigma – Aldrich | Cat. # 1059340100 |
| Mitomycin C | Sigma – Aldrich | Cat. # M0503-5X2MG |
| NaCl | VWR | Cat. # 27810.295 |
| Tris Base | Fisher scientific | Cat. # 10376743 |
| Gen Elute Bacterial genomic DNA Kit | Sigma – Aldrich | Cat. # NA2120-1KT |
| Nylon membrane | Sigma – Aldrich | Cat. # 11417240001 |
| Digoxigenin-11-dUTP, alkali-stable | Sigma – Aldrich | Cat. # 11093088910 |
| Anti-Digoxigenin-AP | Sigma – Aldrich | Cat. # 11093274910; RRID:AB_2734716 |
| UltraPure Agarose | Thermo Fisher | Cat. # 16500-500 |
| Lysozyme | Sigma – Aldrich | Cat. # 10837059001 |
| Proteinase K | Sigma – Aldrich | Cat. # P2308-500MG |
| Experimental models: Organisms/strains | ||
| Bacillus subtilis subsp. subtilis str. 168 (1A700) | Burkholder and Giles23 | Bacillus Genetic Stock Centre (http://bgsc.org) |
| Bacillus subtilis subsp. Subtilis str. 168 (1A700) derivatives (listed in Table S3) | N/A | N/A |
| Bacillus subtilis subsp. Subtilis str. 168 IL26 | Dean et al.24 | Bacillus Genetic Stock Centre (http://bgsc.org) |
| Bacillus subtilis subsp. Subtilis str. 168 IL26 derivatives (listed in Table S3) | N/A | N/A |
| Bacillus subtilis subsp. Subtilis str. 168 Δ6 (1A1299) | Westers et al.9 | Bacillus Genetic Stock Centre (http://bgsc.org) |
| Bacillus subtilis subsp. subtilis str. 168 Δ6 (1A1299) derivatives (listed in Table S3) | N/A | N/A |
| Oligonucleotides | ||
| See Tables S5 and S6 for list of oligonucleotides used in this study | N/A | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, José R Penadés (j.penades@imperial.ac.uk).
Materials availability
All bacterial strains and plasmids generated during this work are freely available from José R. Penadés (j.penades@imperial.ac.uk). The study did not generate new reagents.
Experimental model and subject details
All bacterial strains used in this study belong to Bacillus subtilis or Escherichia coli species. B. subtilis strains were routinely grown at 37°C on LB (Miller) agar plates or in LB (Miller) broth liquid medium shaking at 200 rpm. E. coli DH5α was grown at 37°C on LB (Lennox) agar plates or in LB (Lennox) broth shaking at 180 rpm. When required, antibiotics were utilized at the following concentrations: erythromycin (1 μg ml-1), kanamycin (10 μg ml-1), ampicillin (100 μg ml-1) or spectinomycin (100 μg ml-1).
Strain construction
The SPβ phage (accession number NC_001884) has been recently established in our lab as our model to study arbitrium communication. Bacterial strains used in this study are listed in Table S3. B. subtilis strains 168, Δ6, 1L26 (phi3T, accession number KY030782) and the BKE Genome-Scale deletion library mutants were obtained from the Bacillus Genetic Stock Centre (BGSC).
To generate the deletion mutants in phage SPβ, the corresponding mutant strain from the BKE collection was used as a template for a PCR using primers amplifying the desired gene plus 1 Kb of flanking region. In the case of phi3T, we generated overlapping PCRs containing the erythromycin marker (including the lox sites) and 1 Kb of flanking region for the desired gene. These PCRs were transformed into the Δ6 SPβ or phi3T strain and selected for erythromycin. Once the insertion of the erythromycin cassette was confirmed by PCR and sequencing, the antibiotic resistance cassette was removed as previously described16. Briefly, plasmid pDR244 was transformed into strains harboring the loxP-flanked antibiotic resistance cassette with selection for spectinomycin resistance at 30°C to allow for cre/lox-mediated loop-out of the cassette. Transformant colonies were then streaked onto LB plates and incubated overnight at 42°C for removal of the temperature-sensitive plasmid. Resulting strains were screened for plasmid curing (loss of spectinomycin resistance) and the antibiotic resistance cassette (loss of erythromycin resistance). Strains were streaked to single colonies and confirmation of the clean mutant was performed using PCR. Similarly, we introduced into the SPβ and phi3T genomes a kanamycin cassette replacing the yokI gene that was not essential for the phage, by amplifying the marker without including the lox sites from one of the BKK Genome-Scale deletion library mutants (BGSC).
Method details
Plasmids and cloning
Plasmids generated in this study are listed in Table S4. The AimRSPβ and AimRphi3T and the yopR genes were cloned into the amyE integration vector pDR110 under the control of the IPTG inducible promoter Pspank25. Cloning was performed after PCR amplification of the appropriate template DNA using primers listed in Table S5. Competent cell preparation and transformation was performed as described26. Briefly, B. subtilis cells were grown in GM1 minimum medium to early stationary phase to induce natural competence and 1 μg of plasmid was added and incubated at 37°C for 1 h with shaking at 210 rpm. The culture was centrifuged at 6000 g for 1 min, 800 μL of the supernatant removed, and the pellet re-suspended in 400 μL and plated out onto the relevant antibiotic plates. Plates were incubated at 37°C for 24 h.
Bacteriophage induction assay
For induction, an overnight culture was diluted 1:100 in LB media supplemented with 0.1 mM MnCl2 and 5 mM MgCl2 and then grown at 37°C with 210 rpm shacking until reaching absorbance 0.2 at 600 nm. This step was repeated twice to ensure the cells were in exponential growth. After the second growth Mitomycin C (MC) at 0.5 μg ml-1 was added to the culture. Where experiments were performed to test the complementation of the mutants, 1 mM of IPTG was added at the same time as MC induction. The induced cultures were incubated at 30 °C with 80 rpm shaking for 4 h and then left overnight at room temperature. Following lysis, samples were filtered using 0.2 μm filters and lysates were stored at 4°C until use.
Bacteriophage titering assay
The number of phage particles contained in the phage lysate of interest were quantified by a titering assay. An overnight culture of the relevant recipient strain (normally B. subtilis Δ6 or with the corresponding integration vector) was diluted 1/100 in LB supplemented with 0.1 mM MnCl2 and 5 mM MgCl2 and then grown at 37°C with 210 rpm shacking until reaching absorbance 0.2 at 600 nm. If needed 0.1 mM IPTG was added. Then, 100 μL of recipient bacteria was infected with 100 μL of serial dilutions of phage lysate in phage buffer (PhB; 1 mM NaCl, 0.05 M Tris pH 7.8, 0.1 mM MnCl2, 5 mM MgCl2) at room temperature for 10 min and 3 mL of phage top agar (LB media supplemented with 0.1 mM MnCl2 and 5 mM MgCl2 and 0.7% agar) at 55°C was added to the culture-phage mix and immediately poured over phage base agar plates (LB media supplemented with 0.1 mM MnCl2 and 5 mM MgCl2 and 1.5% agar). Plaques were counted after overnight growth at 37°C temperature and photographed. To obtain evolved SPβ ΔaimR phages, plaque lawns from SPβ ΔaimR titrations using Δ6 as a recipient strain were collected and added to 4 mL of PhB followed by centrifugation and filtration to acquire new SPβ ΔaimR phage lysates. The resulting lysates were used to infect fresh cultures of recipient bacteria and the process was repeated until plaques showing wt morphology were observed as a majority in the phage population (Figure S4). Individual plaques were isolated and evolved phages were subsequently verified as ΔaimR mutants by PCR, titered and sent for whole-genome sequencing (MicrobesNG, University of Birmingham).
Lysogenisation assays
The number of lysogens were quantified by growing a recipient strain to OD600nm = 0.2. Lysates of interest that contain the kanamycin marker were serially diluted in PhB and 100 μL was added to 1 mL of the recipient bacteria in 12 mL tubes. The mixture was incubated at 37°C for 30 min to allow the phage to infect bacteria. The bacteria-phage mixture was then transferred to 1.5 mL Eppendorf tubes and centrifuged at 6,600 rpm for 1 min. The supernatant was removed, and the bacterial pellet was resuspended in 400 μL of fresh LB broth before plating onto selective antibiotic LB agar plates. Plates were incubated overnight at 37°C. The number of colony forming units (CFU) was calculated.
Southern blotting
Samples were taken at 0, 60, 90 and 120 min after adding MC (M0503, Sigma-Aldrich). For 168 Δ6 lysogenic strains, 1 mL of sample was pelleted and frozen at −20°C until all samples were obtained. For 168 lysogenic strains 5 mL was pelleted. The samples were processed for total bacterial DNA extraction using Gen Elute Bacterial genomic DNA Kit (NA2120, Sigma-Aldrich). Afterward, 5 μL of sample was mixed with 5 μL of 2X loading dye and run on a 0.7% agarose gel at 25V overnight. DNA was transferred to a nylon membrane (0.45 mm hybond-N pore diameter, Amersham Life Science) and exposed using a DIG-labeled probe (Digoxigenin-11-dUTP alkali-labile, Roche) and anti-DIG antibody (Anti-Digoxigenin-AP Fab fragments, Roche) as per the suppliers’ protocol, before washing and visualization with Chemdoc imager. The primers used to obtain the labeled probes are shown in Table S5.
Bioinformatic analyses
Alignment of the AimRSPβ and AimRKATMIRA1933 sequences was performed using the PRALINE27 server (Figure S6).
Quantification and statistical analysis
Statistical analysis
Statistical analysis was performed as indicated in the figure legends. Briefly, phage and lysogenisation titers were log10-transformed and analyzed by a One-Way ANOVA followed by an appropriate multiple comparisons test (Dunnett’s or Tukey’s). For analysis of AimR overexpression data, titers were log10-transformed and analyzed by a Two-Way ANOVA followed by Bonferroni’s multiple comparisons test. All analysis was done using GraphPad Prism 9 software. The p values represented in each figure are shown in the figure legends.
Acknowledgments
This work was supported by grants MR/M003876/1, MR/V000772/1, and MR/S00940X/1 from the Medical Research Council (UK); BB/N002873/1, BB/V002376/1, and BB/S003835/1 from the Biotechnology and Biological Sciences Research Council (BBSRC, UK); ERC-ADG-2014 proposal no. 670932 Dut-signal (from EU); and Wellcome Trust 201531/Z/16/Z to J.R.P. and grants PID2019-108541GB-I00 from Spanish government (Ministerio de Ciencia e Innovación) and PROMETEO/2020/012 from Valencian government to A.M. A.F.-R. is the recipient of fellowship FPU19/00433 from Ministerio de Ciencia, Innovación y Universidades (Spanish government). Some figures in this manuscript have been created with BioRender.com.
Author contributions
A.M. and J.R.P. conceived the study; A.B., N.Q.-P., F.G.d.S., S.Z.-C., A.F.-R., and J.V.-C. conducted the experiments; and A.B., N.Q.-P., F.G.d.S., S.Z.-C., A.F.-R., J.V.-C., W.J.J.M., A.M., and J.R.P. analyzed the data. A.B., N.Q.-P., and J.R.P. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: September 24, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2021.08.072.
Contributor Information
Alberto Marina, Email: amarina@ibv.csic.es.
José R. Penadés, Email: j.penades@imperial.ac.uk.
Supplemental information
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
References
- 1.Silpe J.E., Bassler B.L. A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell. 2019;176:268–280.e13. doi: 10.1016/j.cell.2018.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erez Z., Steinberger-Levy I., Shamir M., Doron S., Stokar-Avihail A., Peleg Y., Melamed S., Leavitt A., Savidor A., Albeck S., et al. Communication between viruses guides lysis-lysogeny decisions. Nature. 2017;541:488–493. doi: 10.1038/nature21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey L., Wald A., Celum C.L., Quinn T.C. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Xue K.S., Hooper K.A., Ollodart A.R., Dingens A.S., Bloom J.D. Cooperation between distinct viral variants promotes growth of H3N2 influenza in cell culture. eLife. 2016;5:e13974. doi: 10.7554/eLife.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borges A.L., Zhang J.Y., Rollins M.F., Osuna B.A., Wiedenheft B., Bondy-Denomy J. Bacteriophage cooperation suppresses CRISPR-Cas3 and Cas9 immunity. Cell. 2018;174:917–925.e10. doi: 10.1016/j.cell.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landsberger M., Gandon S., Meaden S., Rollie C., Chevallereau A., Chabas H., Buckling A., Westra E.R., van Houte S. Anti-CRISPR phages cooperate to overcome CRISPR-Cas immunity. Cell. 2018;174:908–916.e12. doi: 10.1016/j.cell.2018.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolas P., Mäder U., Dervyn E., Rochat T., Leduc A., Pigeonneau N., Bidnenko E., Marchadier E., Hoebeke M., Aymerich S., et al. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science. 2012;335:1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- 8.Buescher J.M., Liebermeister W., Jules M., Uhr M., Muntel J., Botella E., Hessling B., Kleijn R.J., Le Chat L., Lecointe F., et al. Global network reorganization during dynamic adaptations of Bacillus subtilis metabolism. Science. 2012;335:1099–1103. doi: 10.1126/science.1206871. [DOI] [PubMed] [Google Scholar]
- 9.Westers H., Dorenbos R., van Dijl J.M., Kabel J., Flanagan T., Devine K.M., Jude F., Seror S.J., Beekman A.C., Darmon E., et al. Genome engineering reveals large dispensable regions in Bacillus subtilis. Mol. Biol. Evol. 2003;20:2076–2090. doi: 10.1093/molbev/msg219. [DOI] [PubMed] [Google Scholar]
- 10.Stokar-Avihail A., Tal N., Erez Z., Lopatina A., Sorek R. Widespread utilization of peptide communication in phages infecting soil and pathogenic bacteria. Cell Host Microbe. 2019;25:746–755.e5. doi: 10.1016/j.chom.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dragoš A., Priyadarshini B., Hasan Z., Strube M.L., Kempen P.J., Maróti G., Kaspar C., Bose B., Burton B.M., Bischofs I.B., Kovács Á.T. Pervasive prophage recombination occurs during evolution of spore-forming Bacilli. ISME J. 2021;15:1344–1358. doi: 10.1038/s41396-020-00854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohm K., Hertel R. The life cycle of SPβ and related phages. Arch. Virol. 2021;166:2119–2130. doi: 10.1007/s00705-021-05116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson C.M., Harden M.M., Grossman A.D. An integrative and conjugative element encodes an abortive infection system to protect host cells from predation by a bacteriophage. Biorxiv. 2020 2020.12.13.422588. [Google Scholar]
- 14.Martin M., Dragoš A., Hölscher T., Maróti G., Bálint B., Westermann M., Kovács Á.T. De novo evolved interference competition promotes the spread of biofilm defectors. Nat. Commun. 2017;8:15127. doi: 10.1038/ncomms15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe K., Takamatsu T., Sato T. Mechanism of bacterial gene rearrangement: SprA-catalyzed precise DNA recombination and its directionality control by SprB ensure the gene rearrangement and stable expression of spsM during sporulation in Bacillus subtilis. Nucleic Acids Res. 2017;45:6669–6683. doi: 10.1093/nar/gkx466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo B.-M., Kritikos G., Farelli J.D., Todor H., Tong K., Kimsey H., Wapinski I., Galardini M., Cabal A., Peters J.M., et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst. 2017;4:291–305.e7. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego Del Sol F., Penadés J.R., Marina A. Deciphering the molecular mechanism underpinning phage arbitrium communication systems. Mol. Cell. 2019;74:59–72.e3. doi: 10.1016/j.molcel.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selva L., Viana D., Regev-Yochay G., Trzcinski K., Corpa J.M., Lasa I., Novick R.P., Penadés J.R. Killing niche competitors by remote-control bacteriophage induction. Proc. Natl. Acad. Sci. USA. 2009;106:1234–1238. doi: 10.1073/pnas.0809600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little J.W. Autodigestion of lexA and phage lambda repressors. Proc. Natl. Acad. Sci. USA. 1984;81:1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaram M., Ma C.-H., Kachroo A.H., Rowley P.A., Guga P., Fan H.-F., Voziyanov Y. An overview of tyrosine site-specific recombination: from an Flp perspective. Microbiol. Spectr. 2015;3 doi: 10.1128/microbiolspec.MDNA3-0021-2014. 10.1128/microbiolspec.MDNA3-0021-2014. [DOI] [PubMed] [Google Scholar]
- 21.Piazzolla D., Calì S., Spoldi E., Forti F., Sala C., Magnoni F., Dehò G., Ghisotti D. Expression of phage P4 integrase is regulated negatively by both Int and Vis. J. Gen. Virol. 2006;87:2423–2431. doi: 10.1099/vir.0.81875-0. [DOI] [PubMed] [Google Scholar]
- 22.Chittò M., Berger M., Berger P., Klotz L., Dröge P., Dobrindt U. IHF stabilizes pathogenicity island I of uropathogenic Escherichia coli strain 536 by attenuating integrase I promoter activity. Sci. Rep. 2020;10:9397. doi: 10.1038/s41598-020-66215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkholder P.R., Giles N.H., Jr. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 1947;34:345–348. [PubMed] [Google Scholar]
- 24.Dean D.H., Orrego J.C., Hutchison K.W., Halvorson H.O. New temperate bacteriophage for Bacillus subtilis, rho 11. J. Virol. 1976;20:509–519. doi: 10.1128/jvi.20.2.509-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carniol K., Ben-Yehuda S., King N., Losick R. Genetic dissection of the sporulation protein SpoIIE and its role in asymmetric division in Bacillus subtilis. J. Bacteriol. 2005;187:3511–3520. doi: 10.1128/JB.187.10.3511-3520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano E., Carrasco B. Measurement of the length of the integrated donor DNA during Bacillus subtilis natural chromosomal transformation. Bio Protoc. 2019;9:e3338. doi: 10.21769/BioProtoc.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simossis V.A., Heringa J. PRALINE: a multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res. 2005;33:W289–W294. doi: 10.1093/nar/gki390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.