Abstract
Background
For a better understanding of the factors underlying the Post-Acute COVID Syndrome, we studied the relationship between symptoms and functional alterations in COVID-19 patients 10 months after hospitalization.
Methods
One-hundred-one patients hospitalized between March 1st and June 30th 2020 participated in a follow-up visit for an assessment of clinical history, comorbidities, lung function, physical capacity and symptoms, including the SGRQ for health-related quality of life, PHQ-9-D for depression, and SOMS-2 J for somatoform disorders. Data were analyzed by univariate comparisons and multiple logistic regression analyses.
Results
Median age was 60 years, 42% were female, 76% had at least one comorbidity, the median length of the hospital stay was 8 days, 19% had been on the ICU. The most prevalent symptoms included shortness of breath (49%), fatigue (49%) and cognitive impairment (39%). Signs of major depression (PHQ-9-D ≥ 10) occurred in 28%/2% (p < 0.05) of patients with/without self-reported cognitive impairment, with median total SGRQ score being 25.4/5.3 (p < 0.05). There were associations between shortness of breath and BMI, SGRQ and hemoglobin levels; between fatigue, SGRQ and PHQ-9-D; and between cognitive impairment and PHQ-9-D (p < 0.05 each) but not with lung function or physical capacity. Characteristics of the acute disease were not related to symptoms.
Conclusions
The findings demonstrate that 10 months after discharge from a hospital stay due to COVID-19, the percentages of patients with symptoms were high. Symptoms showed a consistent pattern but could not be attributed to altered lung function or physical capacity. Our results suggest a role for alternative etiologies including psychosocial factors.
Keywords: Post-Acute COVID Syndrome, Lung function, Symptoms, Depression, Somatization, HrQoL
1. Introduction
Since the beginning of the COVID-19 pandemic in 2020, in Germany almost 3.8 million inhabitants have been positively tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], and 91,803 inhabitants have been reported as having died from or in association with SARS-CoV-2 [2]. The spectrum of clinical signs and manifestations ranges from asymptomatic, mild influenza-like signs such as fever, cough and sore throat to severe pneumonias with respiratory failure, multi-organ failure and death [3], [4], [5], [6], [7], [8]. Age and comorbidities are the most important risk factors for a clinically severe course or death [9], [10], [11]. The Bavarian region of Rosenheim was among the German hotspots in the first wave, and from March to June 2020, 526 patients were hospitalized in three hospitals of the RoMed health care provider. Of these, in total 27% died, specifically 20% of patients treated on normal wards and 49% of patients of intensive care units (ICU) [9].
Many patients who survived still report complaints weeks or months after initial recovery [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. If the complaints or health-disorders persist for more than 4 weeks and cannot be explained by another disease, the set of signs and symptoms is termed „Post-Acute COVID Syndrome“ (PACS), which includes sequelae occurring after ≥ 12 weeks [23]. It can comprise impairments in cognitive function and well-being [24], [25], [26], as well as impairments of lung function [24,[27], [28], [29], [30], [31], [32]] and 6 min walk distance (6-MWD) [26,33], which only partially correlate with the severity of the acute disease [15,25,[33], [34], [35], [36], [37]]. Fatigue, shortness of breath and deteriorations of the sense of smelling and taste are the most prevalent complaints [35].
The etiology of these symptoms and their relationship to organ dysfunction are debated, including pulmonological, cardio-vascular, neurological, and psycho-social factors [38]. Early reports suggested that in patients with mild acute disease 3 months after hospital admission the subjective impairments did not correspond to impairments in functional measures [39]. This, however, might change with longer follow-up time. Recent reports already covered follow-up periods of up to 12 months [40,41], but there are no data on the relationship between symptoms and physiological impairments after the rather long follow-up time of 10 months in hospitalized patients. We addressed this question using a panel of functional assessments and questionnaires collecting data on depression, respiratory health-related quality of life (HrQoL) and potential somatization, with the specific aims, (a) to identify the prevalence of major symptoms, (b) to assess their relationship to functional and clinical characteristics.
2. Material and methods
2.1. Patient characteristics
The analysis was based on 526 patients hospitalized between March 1st and June 30th 2020 in one of three RoMed hospitals (Rosenheim, Wasserburg, Bad Aibling) with a positive PCR test for SARS-CoV-2. In the period up to 1st December 2020, finally 166/526 patients (32%) had died. Among the 360 survivors, 95 patients (26%) were excluded (61 not capable of informed consent, 23 due to positive PCR test without clinical correlate, 7 not traceable, 4 without sufficient proficiency in German or English language). Thus, 265 patients (74%) remained for follow-up.
2.2. Recruitment for follow-up
In a first step, patients were contacted by post mail and invited to participate in a follow-up visit in the Rosenheim hospital; 102 of 265 patients (38%) sent their formal informed consent, whereas 6 patients denied and 157 did not respond. One of the 102 patients did not attend the visit, thus 101 patients remained for an out-patient follow-up investigation. We additionally tried to contact the 157 non-responders by phone. This was successful in 69 patients, of whom 54 (78%) accepted a phone interview, while 15 denied any participation. In total, we thus had data from 155 of 252 patients included in the study (62%). Data on the hospital stay were taken from COVID-DB-project [9,42]. Written informed consent was obtained from all participants attending the inpatient assessment, whereas verbal informed consent was obtained from those contacted via phone. Both approaches were approved by the Ethics Committee of the University of Regensburg.
2.3. Assessments at the study visit
2.3.1. Symptoms, physical examination and medical history
In a structured manner, patients were asked for symptoms that had either newly occurred or deteriorated since discharge from the hospital in comparison to the state prior to COVID-19. The list comprised 27 symptoms selected in accordance with the current literature on PACS that were available at the time of the study [43,44] (see supplemental Table S1). The telephone interviews comprised the 9 symptoms that had turned out to be most frequent in the patients undergoing inpatient investigation. The burden from comorbidities was summarized in the Charlson Comorbidity Index (CCI) based on 20 diseases excluding the patient's age [45], as this was carried as explicit predictor. The physical examination comprised a complete physical examination according to the standards of Internal Medicine and included the assessment of blood pressure, heart rate and oxygen saturation (SpO2) by pulse oximetry at rest. Data on COVID-19 were taken from previous work [9,42]. The analysis of chest computer tomography (CT) scans was performed by an experienced radiologist following guidelines [46].
2.3.2. Functional and laboratory measures
Spirometry and bodyplethysmography (Vyaire, Höchberg, Germany) were performed by experienced personnel. We evaluated forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and their ratio FEV1/FVC to characterize airway obstruction, moreover residual volume (RV), total lung capacity (TLC) and the ratio RV/TLC to quantify lung hyperinflation or restrictive disorders. Spirometric reference values and the respective LLN (lower limit of normal, 5th percentile) were those of the Global Lung Function Initiative [47]. For bodyplethysmography, corresponding values including the ULN (upper limit of normal) for RV and RV/TLC, and the LLN for TLC were taken from the European Community for Coal and Steel (ECSC) [48].
Physical capacity was assessed by a 6 min walk test performed according to the guidelines of the American Thoracic Society (ATS) [49] by asking patients to walk as fast as possible on a course of 30 m length. The resulting 6 min walk distance (6-MWD) was expressed in relation to reference and LLN values [50].
Routine laboratory parameters were obtained from a venous blood sample, including the levels of hemoglobin (Hb), d-dimers, creatinine, gamma-glutamyltransferase (GGT) and C-reactive protein (CRP). The estimated glomerular filtration rate (eGFR) was determined from creatinine according to the CKD-EPI-formula [51]. Reference values were those used in the local medical laboratory.
2.3.3. Questionnaires
To assess and quantify symptoms of depression, the Patient Health Questionnaire for Depression (PHQ-9-D) was chosen. It comprises 9 items rated from 0 to 3 each, resulting in a maximum score of 27 [52,53]. To categorize the results regarding potential major depression, a cut-off value of ≥ 7 was chosen, as this had been shown to yield high sensitivity with still satisfactory specificity [54]. We additionally evaluated the clinically more common cut-off of ≥ 10 for major depression requiring at least a score of 2 in the first two questions.
HrQoL was determined by the St. George's Respiratory Questionnaire (SGRQ). It was chosen because it was initially assumed that impairments were mainly related to respiratory factors. Nonetheless, the questionnaire also contains general questions, for example on activity, and covers a broad range of impairments [55]. It comprises 73 items that can be summarized in a total score or three sub-scores (symptoms, activity, impact), ranging from 0 (no limitation) to 100 (maximum limitation).
Previous data suggested that PACS represents a complex situation potentially involving psycho-social factors that are known to contribute to persistent symptoms in general, even more so after severe somatic disease. This was addressed via the SOMS-2 J (Screening for Somatoform Disorders) questionnaire comprising 68 items [56]. It asks for the presence of somatic symptoms that are a burden in daily life and for which a physician could not identify an objective cause. For the present study, the SOMS-2 J general somatization index, the International Classification of Disease (ICD-10) somatization disorder (SD) index and the ICD-10 somatoform autonomous disorder (SAD) index were calculated. The SOMS-2 J general somatization index summarizes all 53 symptom items, while the SD index and the SAD index summarize the symptoms relevant for the respective diagnosis (F45) according to the ICD-10 and use additional plausibility criteria [56].
3. Data analysis
Numeric data are given as median values and quartiles, if suitable with ranges, or as numbers and percentages. Comparisons between groups of patients were performed by the Mann-Whitney U-test, correlation analyses by the Spearman rank correlation coefficient. To reveal independent predictors for key symptoms, binary logistic regression analyses were employed. We used the inclusion of predictors. These were selected according to the results of univariate analyses and pathophysiological plausibility, and we checked for predictors potentially hidden by collinearity by additional stepwise selection procedures. All analyses were performed using the software IBM SPSS (Version 26.0.0.0, Armonk, NY, US). p-values <0.05 were assumed as statistically significant, and these values are given explicitly in the tables.
4. Results
4.1. Demographic data of the clinically examined patients
Basic demographic characteristics and data on the hospital stay during COVID-19 of the 101 patients are given in Table 1A stratified according to sex. This stratification was chosen, as sex had turned out as major determinant of PACS in previous studies [57], [58], [59], [60], [61], [62]. The median age was 60 (IQR 51–66; range 28–69) years, 58% of patients were male, 35% had a history of smoking, and 76% at least one comorbidity. The median CCI was 0 (0, 7), whereby 61% of patients had a CCI of 0. A previous diagnosis of an obstructive lung disease (either asthma or chronic obstructive pulmonary disease) was present in 10 patients, and none was on long-term oxygen therapy. The median length of the hospital stay was 8 (5, 10; range 2–111) days, 20% of patients had ICU treatment, and 20% participated in an inpatient rehabilitation program after discharge. The time of follow-up was 308 (309, 346; range 226–393) days after admission to the hospital. Table 1A demonstrates significant differences between men and women regarding smoking history and the length of the hospital stay.
Table 1A.
Patients‘ characteristics grouped by sex.
| All (n = 101) | Men (n = 59) | Women (n = 42) | |
|---|---|---|---|
| Age (years) | 60.0 [50.8; 66.0] | 61.0 [52.5; 66.0] | 58.0 [49.5; 62.5] |
| Smoking history (yes) | 35 (35%) | 28 (48%) ** | 7 (17%) |
| Follow-up time (days) | 308 [309; 346] | 308 [309; 342] | 311 [309; 350] |
| Hospital stay (days) | 8.0 [5.0; 10.3] | 8.0 [6.0; 11.5] * | 7.0 [3.5; 10.5] |
| Intensive Care Unit | 20 (19.8%) | 14 (23.7%) | 6 (14.3%) |
| Intubation (yes) | 9 (9%) | 8 (14%) | 1 (1%) |
| Rehabilitation (yes) | 20 (20%) | 12 (20%) | 8 (19%) |
| Any Comorbidity (yes) | 77 (76%) | 45 (76%) | 32 (76%) |
| Charlson Score (without age) | 0 [0; 7] | 0 [0; 1] | 0 [0; 1] |
| Obstructive lung disease (yes) | 10 (10%) | 5 (9%) | 5 (12%) |
| Systemic hypertension (yes) | 41 (41%) | 28 (48%) | 13 (31%) |
| Left heart failure (yes) | 12 (12%) | 9 (9%) | 3 (7%) |
| Coronary heart disease/MI (yes) | 16 (16%) | 12 (20%) | 4 (10%) |
| Diabetes (yes) | 12 (12%) | 8 (14%) | 4 (10%) |
Baseline characteristics of the 101 follow-up participants including characteristics of their hospital stay due to COVID-19. Median values and quartiles (in brackets) are given. MI = history of myocardial infarction. Data are stratified for men and women in order to illustrate the differences. Statistical comparisons were based on the Mann-Whitney U test or Fisher's Exact test. *p < 0.05, ** p < 0.01.
4.2. Physical examination and functional status
The physical examination did not reveal pathological results except for one patient with signs of aortic valve stenosis, which was considered not to be related to COVID-19. Data are given in Table 1B including the subgroups of men and women. SpO2 showed a small but statistically significant difference between the two groups, while the statistically significant difference for diastolic blood pressure values depicted the expected dependency on sex. Data from spirometry and bodyplethysmography could be obtained in all 101 patients, whereby 21% showed abnormal findings (<LLN or >ULN, respectively) in at least one of the lung function measures FEV1, FEV1/FVC, FVC, TLC, or RV/TLC. 6-MWD was available in 99 patients, since two used wheelchairs. Laboratory values (Hb, erythrocytes, leucocytes, thrombocytes, eGFR, d-dimers, GGT) were mostly in the normal range, and the majority of clinical and functional measures showed differences between men and women as expected.
Table 1B.
Functional values grouped by sex.
| All (n = 101) | Men (n = 59) | Women (n = 42) | |
|---|---|---|---|
| Vital parameters | |||
| RRsys (mmHg) | 130.0 [110.0; 140.0] | 130.0 [120.0; 140.0] | 12.00 [110.0; 132.5] |
| RRdia (mmHg) | 80.0 [72.3; 90.0] | 80.0 [80.0; 90.0] * | 80.0 [70.0; 80.0] |
| Heart rate (1/s) | 70.0 [64.0; 75.0] | 70.0 [63.0; 74.5] | 70.0 [64.5; 76.5] |
| BMI (kg/m2) | 27.5 [25.0; 30.9] | 27.8 [24.9; 30.8] | 26.8 [24.1; 31.2] |
| SpO2 (%) | 97.0 [96.8; 98.0] | 97.0 [96.0; 97.5] | 97.0 [97.0; 99.0] * |
| Lung function/physical capacity | |||
| FEV1 (%predicted) | 91.6 [82.8; 102.6] | 91.1 [82.7; 101.1] | 92.29 [82.9; 104.1] |
| FEV1<LLN (no.) | 14 (14%) | 8 (14%) | 6 (14%) |
| FEV1/FVC (%) | 76.9 [71.8; 81.4] | 76.0 [71.5; 80.09] | 78.5 [73.1; 84.2] |
| FEV1/FVC<LLN (no.) | 5 (5%) | 4 (7%) | 1 (2%) |
| FVC (%predicted) | 94.5 [81.9; 101.6] | 94.5 [82.9; 100.3] | 93.78 [79.9; 101.8] |
| FVC<LLN (no.) | 13 (13%) | 8 (14%) | 5 (12%) |
| TLC (%predicted) | 109.5 [102.5; 121.7] | 105.2 [98.6; 121.1] | 111.6 [107.3; 122.1] * |
| TLC<LLN (no.) | 6 (6%) | 4 (7%) | 2 (5%) |
| RV/TLC (%predicted) | 44.1 [105.7; 126.5] | 43.0 [105.6; 126.0] | 47.3 [108.3; 129.3] |
| RV/TLC>ULN (no.) | 26 (26%) | 10 (17%) | 16 (38%) * |
| 6-MWD (%predicted) (n = 99) | 101.9 [88.2; 110.9] | 101.9 [90.6; 106.2] | 101.9 [88.1; 114.7] |
| 6-MWD<LLN (no.) | 5 (5%) | 5 (9%) | 0 (0%) |
| Laboratory values | |||
| Hemoglobin (g/dl) (n = 98) | 14.7 [13. 6; 15.6] | 15.3 [14.6; 16.2] *** | 13.7 [12.8; 14.1] |
| Leucocytes (x109/l) (n = 98) | 6.27 [5.38; 7.26] | 6.19 [5.27; 7.24] | 6.38 [5.49; 7.66] |
| Thrombocytes (Gpt/l) (n = 98) | 245.5 [212.3; 285.3] | 231.5 [185.5; 270.0] | 257.0 [229.0; 298.5] ** |
| gGT (U/l) (n = 98) | 30.0 [17.8; 40.5] | 35.0 [26.0; 51.0] *** | 21.5 [13.5; 29.0] |
| eGFR (ml/min) (n = 98) | 78.5 [69.0; 99.0] | 77.0 [69.0; 91.0] | 82.5 [69.0; 102.0] |
| CRP (mg/l) (n = 98) | 0.16 [0.07; 0.37] | 0.13 [0.07; 0.27] | 0.18 [0.07; 0.46] |
| D-dimers (μg/l) (n = 56) | 75.5 [0.0; 186.3] | 0.0 [0.0; 190.5] | 154.0 [0.0; 179.5] |
Results of physiological assessments in the 101 follow-up participants expressed as percent predicted, except for FEV1/FVC and SpO2. SpO2 = oxygen saturation from pulse oximetry, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, TLC = total lung capacity, RV = residual volume, 6-MWD = 6 min walk distance, LLN = lower limit of normal, ULN = upper limit of normal. Data are stratified for men and women in order to illustrate the differences. Statistical comparisons were based on the Mann-Whitney U-test or Fisher's Exact test. *p < 0.05, ** p < 0.01, *** p < 0.001.
4.3. Symptoms
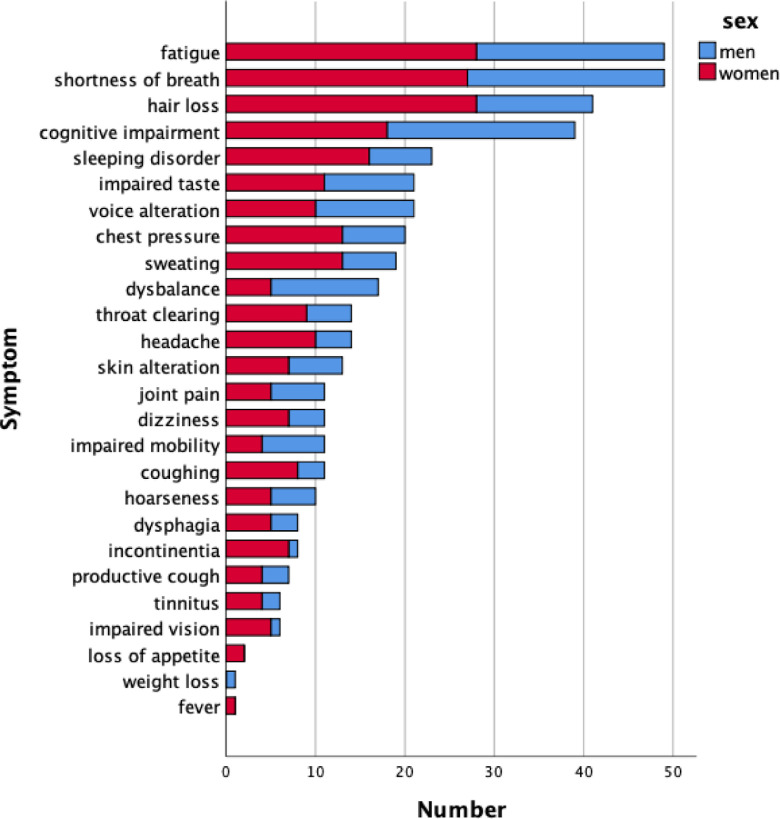
Upon follow-up, only 10% of patients reported no symptoms (see Table S1), while 51% reported 1–4 symptoms (of 26 symptoms asked) and 39% more than 4 symptoms. The most frequent symptoms were fatigue (49%), shortness of breath (49%), hair loss (41%) and cognitive impairment (39%). Data are shown in Fig. 1 stratified according to sex. Regarding the number of symptoms, women reported more symptoms than men (median 5 versus 3; p = 0.002). The results of the telephone interview can be found in the Supplement (Table S4).
Fig. 1.
Frequency of symptoms (absolute numbers) in the 101 participants of the follow-up visit. Due to the fact that the total number was n = 101, the numerical values of percentages are virtually the same. Data are given separately for males (blue) and females (red).
4.4. Mental health, health related quality of life and somatization
The respective data are given in Table 1C stratified according to sex. In the PHQ-9-D questionnaire, 20 patients (20%) had a score of ≥ 7, indicating hints for major depression, and 5 patients reported signs of a major depression (score ≥ 10). A previous diagnosis of depression was known in only 3 of the 20 patients, and in 6 of all 101 patients asked, while it was known only in 1 of the 5 patients with signs of a major depression. Women had a higher median PHQ-9-D score than men (p = 0.002) and a higher risk of suspicious values ≥ 7 (7% of men versus 38% of women; p < 0.001).
Table 1C.
Questionnaire results grouped by sex.
| All (n = 101) | Men (n = 59) | Women (n = 42) | |
|---|---|---|---|
| PHQ-9-D Score | 3.0 [1.0; 6.3] | 2.0 [0.0; 2.0] | 5.0 [3.0; 12.0] ** |
| SGRQ Activity Score | 18.4 [6.0; 43.1] | 12.17 [0.0; 29.3] | 24.32 [15.6; 56.9] * |
| SGRQ Impact Score | 3.6 [0.0; 18.7] | 0.0 [0.0; 5.6] | 10.2 [0.0; 25.1] ** |
| SGRQ Total Score | 12.2 [1.9; 25.7] | 5.7 [1.7; 15.6] | 16.7 [7.7; 34.4] * |
| SOMS-2 J general somatization index | 3.0 [0.0; 8.0] | 2.0 [0.0; 5.0] | 4.5 [0.0; 12.3] |
| SOMS-2 J SD index | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] |
| SOMS-2 J SAD index | 0.0 [0.0; 2.0] | 0.0 [0.0; 1.0] | 0.5 [0.0; 3.3] * |
Results of questionnaires in the 101 follow-up participants. Data are stratified for men and women in order to illustrate the differences. Statistical comparisons were based on the Mann-Whitney U-test. *p < 0.05, ** p < 0.01.
The total score and the sub-scores of the SGRQ questionnaire can also been found in Table 1C. There were significantly higher scores, i.e. lower quality of life, in women compared to men regarding the total score as well as all sub-scores (symptoms, activity, impact, p < 0.05 each).
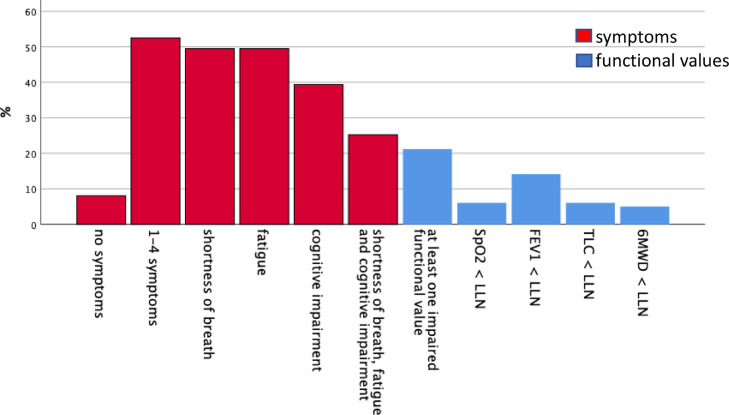
Regarding SOMS-2 J, the median (quartiles) of the general somatization index was 3 (0; 8), the SAD index was 0 (0; 2). Formally, with the SD index being 0 in all patients, no patient met the criteria for a somatization disorder according to ICD-10. A summary of symptoms, assessments of mental health and clinical and functional data is given in Fig. 2 .
Fig. 2.
Percentages of patients in the follow-up visit (n = 101) showing specific counts of symptoms (red) or abnormal values according to LLN or ULN for lung function and physical performance (blue). SpO2 = oxygen saturation from pulse oximetry, FEV1 = forced expiratory volume in 1 s, TLC = total lung capacity, 6-MWD = 6 min walk distance, LLN = lower limit of normal. For functional measures, percentages were in the range of 5% that is expected by definition in a normal population, except for FEV1, possibly because of the fact that about 10% of patients had a history of obstructive airway disease.
4.5. Stratification of characteristics according to symptoms
Major symptoms at follow-up were shortness of breath, fatigue and cognitive impairment; we omitted hair loss as it might have causes not addressed in the set of available variables. Table S2A–S2C show the same data as Table 1A–1C but stratified according to the presence of shortness of breath, fatigue and cognitive impairment. In univariate comparisons (Mann-Whitney U-test) between the groups of patients reporting one of these symptoms or not, women reported shortness of breath and fatigue more frequently than men. There were no statistically significant relationships between the three major symptoms and any functional value. Patients reporting the three selected symptoms scored significantly higher in all scores administered (PHQ-9-D, SGRQ, SOMS-2 J). The results of further comparisons are given as figures below.
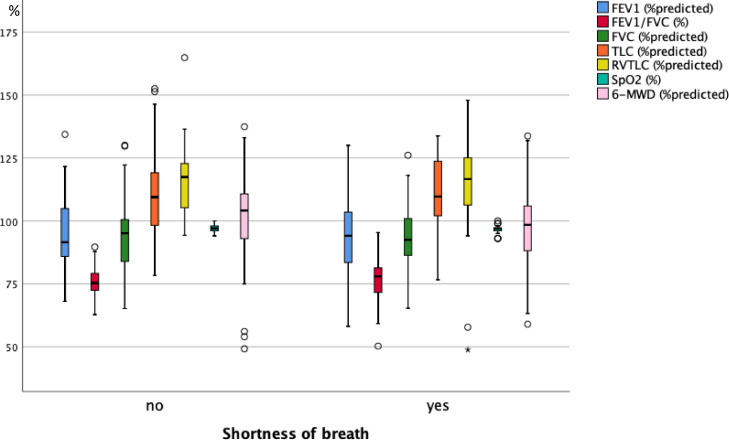
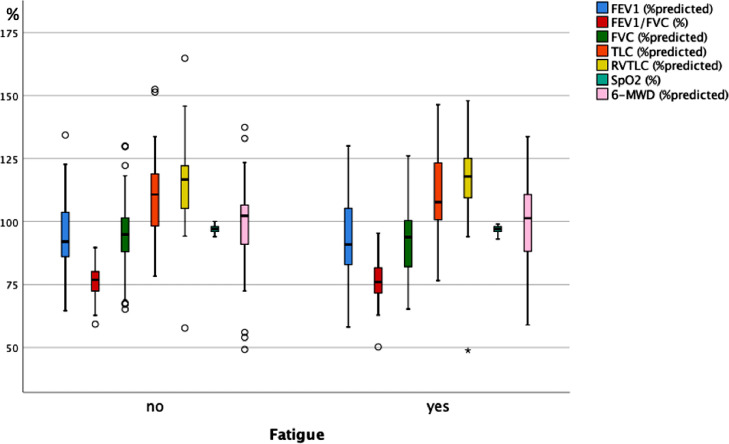
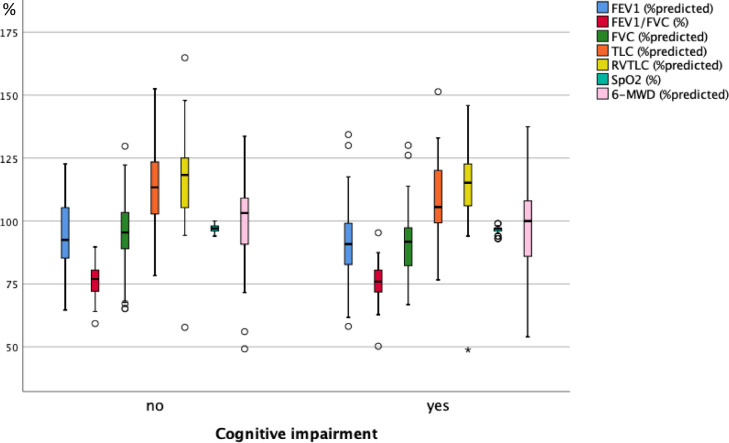
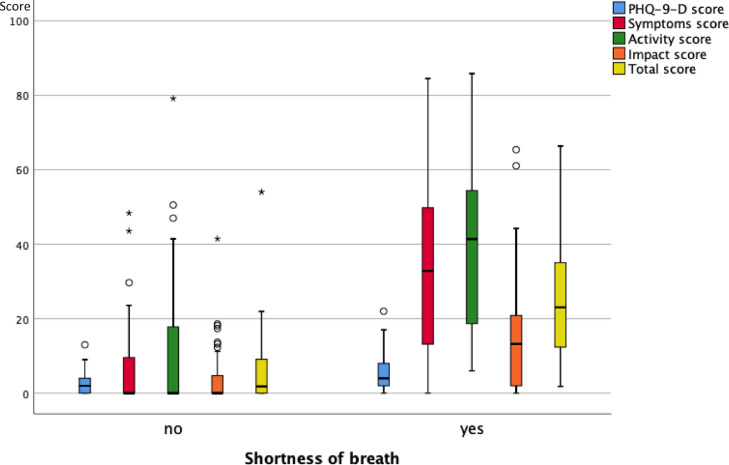
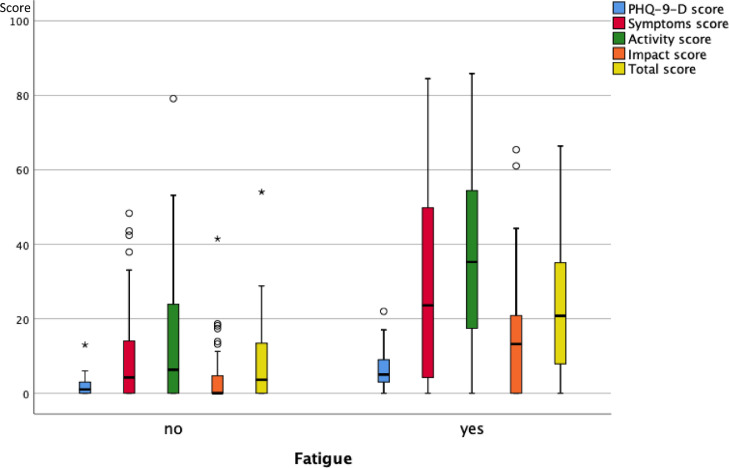
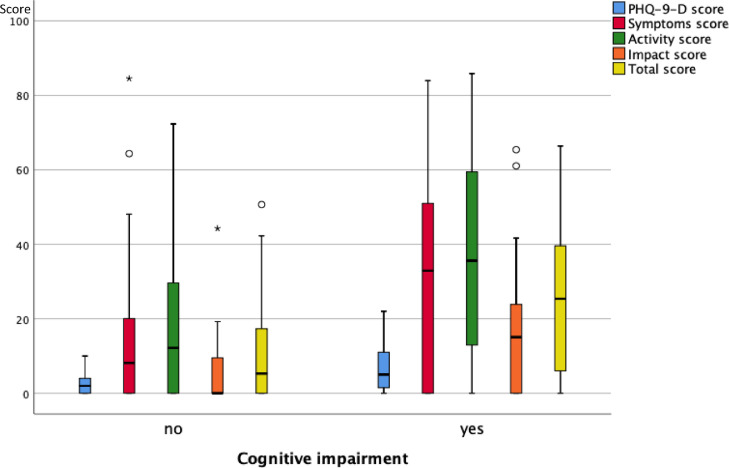
As illustrated in Fig. 3A , 3B and 3C , at follow-up none of the functional measures showed a significant difference between the groups reporting or not reporting shortness of breath, fatigue and cognitive impairment. Fig. 4A, 4B and 4C show the scores of the PHQ-9-D and SGRQ stratified in the same manner. All scores significantly differed between groups defined via shortness of breath, fatigue or cognitive impairment (p < 0.004 each).
Fig. 3A.
Box plots of functional measures in percent predicted, or percentages for SpO2 and FEV1/FVC, for the two groups of patients either reporting or not reporting shortness of breath at the follow-up visit. SpO2 = oxygen saturation from pulse oximetry, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, TLC = total lung capacity, RV = residual capacity, 6-MWD = 6 min walk distance. The boxes indicate the quartiles, the horizontal bar the median value, the whiskers the 10- and 90-percentiles, and the circles points outside of these. In none of the measures there were statistically significant differences between the two groups (Mann-Whitney U-test).
Fig. 3B.
Box plots of functional measures in percent predicted, or percentages for SpO2 and FEV1/FVC, for the two groups of patients either reporting or not reporting fatigue at the follow-up visit. SpO2 = oxygen saturation from pulse oximetry, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, TLC = total lung capacity, RV = residual capacity, 6-MWD = 6 min walk distance. The boxes indicate the quartiles, the horizontal bar the median value, the whiskers the 10- and 90-percentiles, and the circles points outside of these. In none of the measures there were statistically significant differences between the two groups (Mann-Whitney U-test).
Fig. 3C.
Box plots of functional measures in percent predicted, or percentages for SpO2 and FEV1/FVC C, for the two groups of patients either reporting or not reporting cognitive impairment at the follow-up visit. SpO2 = oxygen saturation from pulse oximetry, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, TLC = total lung capacity, RV = residual capacity, 6-MWD = 6 min walk distance. The boxes indicate the quartiles, the horizontal bar the median value, the whiskers the 10- and 90-percentiles, and the circles points outside of these. In none of the measures there were statistically significant differences between the two groups (Mann-Whitney U-test).
Fig. 4A.
Box plots of the scores of the PHQ-9-D and SGRQ (total and three sub-scores) for the two groups of patients either reporting or not reporting shortness of breath at the follow-up visit. The boxes indicate the quartiles, the horizontal bar the median value, the whiskers the 10- and 90-percentiles, and the circles points outside of these. There were statistically significant differences between the two groups (p < 0.05 each, Mann-Whitney U-test) for all of the scores.
Fig. 4B.
Box plots of the scores of the PHQ-9-D and SGRQ (total and three sub-scores) for the two groups of patients either reporting or not reporting fatigue at the follow-up visit. The boxes indicate the quartiles, the horizontal bar the median value, the whiskers the 10- and 90-percentiles, and the circles points outside of these. There were statistically significant differences between the two groups (p < 0.05 each, Mann-Whitney U-test) for all of the scores.
Fig. 4C.
Box plots of the scores of the PHQ-9-D and SGRQ (total and three sub-scores) for the two groups of patients either reporting or not reporting cognitive impairment at the follow-up visit. The boxes indicate the quartiles, the horizontal bar the median value, the whiskers the 10- and 90-percentiles, and the circles points outside of these. There were statistically significant differences between the two groups (p < 0.05 each, Mann-Whitney U-test) for all of the scores.
4.6. Associations with symptoms in multivariate analyses
As these factors were not necessarily independent of each other and differences might be due to confounding, we performed logistic regression analyses for each of the three symptoms. The predictors included those revealed as potentially relevant in the simple comparisons as well as predictors that could become relevant in multivariate analyses. As covariates we thus included age, sex, smoking status (active vs. non-active), SpO2, BMI, 6-MWD as measure of physical capacity, FEV1 (%predicted) as measure of airway obstruction, RV/TLC (%predicted) as measure of trapped air, the PHQ-9-D score, SGRQ total score, sex-adjusted hemoglobin (by multiplication of the women's Hb value with the ratio of the World Health Organization's mean reference values for men (16 g/dL) and women (14 g/dL)), and the SOMS-2 J SAD index.
Table S3 shows the Odds Ratios for the three symptoms and the predictors. Shortness of breath was related to BMI, SGRQ and Hb (p < 0.05 each), fatigue was related to PHQ-9-D and SGRQ (p < 0.05 each), and cognitive impairment was related to PHQ-9-D (p < 0.05) but not to the SOMS-2 J SAD index. There was, however, an association with the SOMS-2 J general somatization index, if this was used as predictor instead of the SAD index.
4.7. Relationship to clinical history during the hospital stay
A further factor relevant for the symptoms reported at follow-up could be the clinical history experienced during the hospital stay. For this purpose, again logistic regression analyses of the three symptoms were performed, including parameters assessed during the inpatient situation. The predictors comprised ICU treatment, length of hospital stay, invasive ventilation, CCI (without age), age, SpO2 upon admission, renal dysfunction (eGFR< 60 /min), the concentration of lactate dehydrogenase (LDH), and the administration of anti-coagulative therapy or systemic steroids. Shortness of breath was not dependent on any of these covariates (p > 0.05 each). The same was true for fatigue and cognitive impairment. Moreover, lung function values of patients with ICU treatment were not different from the values of patients treated on the normal ward (p > 0.1 for all measures evaluated).
During the hospital stay, chest CT scans had been obtained in 65 of the 101 patients. In a further logistic regression analysis, the percentage of lung affected by COVID-19 during the hospital stay according to CT imaging was added as predictor to those mentioned above, again for shortness of breath, fatigue and cognitive impairment as outcomes. None of the three symptoms showed a significant relationship to the CT parameter.
5. Discussion
The major finding of our study is that 10 months after acute COVID-19 neither the current somatic functional status of the patient nor the characteristics of the previous hospital stay were related to the presence of three major symptoms associated with PACS. To the best of our knowledge, we present the data with the longest follow-up comprising data on both functional and subjective alterations.
As major symptoms, shortness of breath, fatigue and cognitive impairment were reported by 39 to 49% of patients, and only 10% reported no symptoms at all. This result is consistent with previous findings on reduced self-reported exercise capacity (53.1%), fatigue (41.7%), sleeping problems (32.3%), concentration problems (31.3%) and dyspnea (27.1%) as major symptoms after 12 months, while 23% of patients reported no symptom [41]. However, only 30% of the patients of this previous study were hospitalised, and the median age was slightly smaller (57 vs. 60 years in our cohort).
We also found the symptom burden not to be related to characteristics of the previous hospital stay, particularly the severity of COVID-19, which goes along with data from shorter follow-up times in previous studies [36,61,[63], [64], [65]]. In some studies, however, a positive correlation between the severity of the COVID-19 disease and the symptom burden at follow-up had been found [15,26,35,58,60,66]. This heterogeneity could be due to different classifications of disease severity or not yet specified subtypes of the virus or the fact that studies employed different tools, such as personal interview, phone interview and online survey. Moreover, in our study the number of patients with invasive ventilation was too low to draw reliable conclusions.
In the present cohort, lung function as well as physical capacity assessed by 6 min walk test, showed distributions not markedly different from those in normal populations and were not related to the characteristics of the hospital stay, particularly with respect to disease severity. This is consistent with data that shows that 12 months after COVID-19 values of spirometry and bodyplethysmography were <LLN in less than 10% of patients (except for a reduction of TLC in 42%), independently from the severity of the disease during inpatient treatment (WHO guideline severity scale) [40,67]. When assessed up to 8 months after hospitalization, some studies reported a significant relationship between the current functional impairment and the previous severity of the disease [25,29,31,33,36,37,66,68], while others did not [24,26,63,69]. The situation is complicated by a potential interplay between persistent symptoms and functional alterations that could be promoted by changes in behavior, for example regarding physical capacity or anxiety and depression. This probably renders associations increasingly difficult to detect after a longer time-lag from the acute disease, suggesting to a multi-dimensional etiology.
We furthermore observed a lack of correlation between functional status and symptom burden, in line with previous findings obtained after 75 days or 3 and 6 months [39,61,70]. Other investigators, however, found that patients with a higher symptom burden had more impaired lung function after 2.5 or 3–6 months [31,71]. The lack of association in our study is illustrated in the supplemental Fig. S1A and S1B. Patients reporting either shortness of breath or fatigue were distributed over the middle part of the scatter plot, although one might have expected that, for example, patients with fatigue or shortness of breath showed a lower 6-MWD at a given FEV1.
A worsened quality of life is a fairly consistent finding for PACS [24,26,32,57,59,72,73]. In the absence of reference values for the SGRQ we could not define an overall worsened HrQoL, but data were consistent regarding their correlation with shortness of breath and fatigue, suggesting that we asked for important factors underlying the overall impairment in quality of life. Regarding fatigue and cognitive impairment, a similar tendency was observed for the PHQ-9-D as a score for depression. The PHQ-9-D is sensitive to impairments from respiratory disease in patients with COPD [74] but in our study population the percentage of patients with a history of such disease was small and lung function normal or close to normal.
Thus, it is likely that we measured the PHQ-9-D unbiased and that the scores indeed indicated depression. As the PHQ-9-D is not subject to gender bias [75], the higher prevalence of post-COVID-19 depression in women in our cohort appeared to be a valid result. The fact that HrQoL was lower and the prevalence of depression higher than in a control group never tested positive for SARS-CoV-2 [14,25], suggests that the deterioration of mental health is not simply due to the impact of the pandemic on the whole population's mental health [76], but that PACS itself bears a greater risk for psychological suffering [77].
The SOMS-2 J general index was related to cognitive impairment. This is remarkable, as by design the SOMS-2 J aims to assess symptoms that are not sufficiently explained by organic dysfunction and for which a physician could not identify an objective cause. In the current, complicated situation, one might expect that patients are inclined to attribute symptoms to somatic deteriorations associated with COVID-19, leading to a lower sensitivity of SOMS-2 J. Our findings suggest the potential relevance of psycho-social factors and somatization in addition to somatic factors.
5.1. Limitations and strengths
The present study focused on hospitalized patients and did not comprise patients without hospital admission. We had pre-COVID values (via cooperation with general practitioners) in only 17 patients, and no statistically valid, unbiased comparison was possible due to this low number. Moreover, the differences regarding laboratory parameters obtained during the hospital stay were trivial as they only indicated the end of the acute disease. Therefore, we had to perform a cross-sectional analysis. Moreover, there was no matched control group without previous COVID-19 that would have allowed to estimate the distribution of symptoms and functional measures for comparison. This could have been relevant, as depression scores, for example, might also have been affected in non-COVID patients, for example in relation to the lock-down [19,78]. On the other hand, at least for lung function measures, the percentages of potentially abnormal values were in the 5 percent range expected from the definition of the LLN or ULN. Due to organizational factors, we could not include the assessment of CO diffusing capacity of the lung, which might have been a marker of vascular alterations [26,79,80]. The results of the phone interviews (see Supplement) did not suggest a major bias in the group of subjects studied in the hospital, particularly not the selection of less symptomatic patients. A strength of the study is that we had detailed information on the COVID-19 related hospital stay and that we aimed to analyze and confirm the symptoms by using three standard questionnaires.
6. Conclusions
Our findings indicate that 10 months after discharge from a COVID-19 set off inpatient treatment, the prevalence of symptoms was high, especially that of shortness of breath, fatigue and cognitive impairment. Symptoms and scores of depression and HrQoL showed gender-dependent differences and a consistent relationship to each other, but could not be attributed to alterations in lung function or physical capacity at the time of the follow-up, or to major characteristics of the hospital stay. The associations between shortness of breath and lower hemoglobin concentration at the follow-up, and between fatigue or cognitive impairment and hints for depression, especially in women, might be helpful in the clinical assessment of post-COVID-19 patients but the results also suggest to consider alternative etiologies including psychosocial factors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Acknowledgments
We are grateful to all patients who participated in the follow-up and phone calls. We also thank the staff engaged in the coordination of the follow-up appointments (Annika Rotter, Roswitha Schmid, Christine Ehrl, Claudia Scharnagl, Veronika Ruml), the staff in the lung function laboratory (Sonja Silbernagl, Cornelia Dick), as well as Jens Deerberg-Wittram MD, CEO of RoMed, and Max v. Holleben, business administration manager Klinikum Rosenheim, for providing the technical and organizational environment needed for the study. Moreover, we thank Sevki Baş, Hedwig Grella, Ayşenur Kaya, Anja Krams, Julia Reiser, Sophie Gast, Antje Parstorfer, Sabine Leidl, Katarina Vlajic, Bardha Krivaqa and Katharina Thaler, who were involved in the updates of the database COVID-DB.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2021.10.031.
Appendix. Supplementary materials
References
- 1.Robert Koch Institut (2021), COVID-19: Fallzahlen in deutschland und weltweit, in: rki.de, 11/08/2021, https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html, accessed: 11/08/2021.
- 2.European Centre for disease prevention and control (2021), COVID-19 situation update for the EU/EEA, as of 10 August 2021, in: ecdc.europa.eu, 10/08/2021, https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea, accessed: 08/08/2021.
- 3.Richardson S., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiersinga W.J., et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 8.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budweiser S., et al. Patients' treatment limitations as predictive factor for mortality in COVID-19: results from hospitalized patients of a hotspot region for SARS-CoV-2 infections. Respir Res. 2021;22(1):168. doi: 10.1186/s12931-021-01756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorjee K., et al. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE. 2020;15(12) doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boari G.E.M., et al. Short-term consequences of SARS-CoV-2-related pneumonia: a follow up study. High Blood Press Cardiovasc Prev. 2021 doi: 10.1007/s40292-021-00454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho-Schneider C., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logue J.K., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmud R., et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS ONE. 2021;16(4) doi: 10.1371/journal.pone.0249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nehme M., et al. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med. 2021 doi: 10.7326/M21-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaes A.W., et al. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021;7(2) doi: 10.1183/23120541.00141-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carfi A., et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augustin M., et al. Post-COVID syndrome in non-hospitalized patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur, 2021;6 doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuehn B.M. Most patients hospitalized with COVID-19 have lasting symptoms. JAMA. 2021;325(11):1031. doi: 10.1001/jama.2021.2974. [DOI] [PubMed] [Google Scholar]
- 21.Nasserie T., et al. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Writing Committee for the Comebac Study Group Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multidisciplinary Collaborative Group for the Scientific Monitoring of COVID-19. Lledó, G., Sellares J., Brotons C., Sans M., Díez, J., Blanco J., Bassat Q., Sarukhan A., Campins M., Guerri R., Miró J.M., de Sanjose, S. (2021), Post-Acute COVID Syndrome (PACS): definition, impact and management, in: isglobal.org, http://hdl.handle.net/2445/178471, accessed: 11/10/2021.
- 24.Gautam N., et al. Medium-term outcome of severe to critically ill patients with SARS-CoV-2 infection. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raman B., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Borst B., et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellan M., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekbom E., et al. Impaired diffusing capacity for carbon monoxide is common in critically ill COVID-19 patients at four months post-discharge. Respir Med. 2021;182 doi: 10.1016/j.rmed.2021.106394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guler S.A., et al. Pulmonary function and radiological features four months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021 doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibila O., et al. Lung function sequelae in COVID-19 patients 3 months after hospital discharge. Arch Bronconeumol. 2021;57(Suppl 2):59–61. doi: 10.1016/j.arbres.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smet J., et al. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir Med. 2021;176 doi: 10.1016/j.rmed.2020.106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havervall S., et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325(19):2015–2016. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdallah S.J., et al. Symptoms, pulmonary function and functional capacity four months after COVID-19. Ann Am Thorac Soc. 2021 doi: 10.1513/AnnalsATS.202012-1489RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-de-Las-Penas C., et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riou M., et al. Respiratory follow-up after hospitalization for COVID-19: who and when? Eur J Clin Invest. 2021:e13603. doi: 10.1111/eci.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strumiliene E., et al. Follow-up analysis of pulmonary function, exercise capacity, radiological changes, and quality of life two months after recovery from SARS-CoV-2 pneumonia. Medicina. 2021;57(6) doi: 10.3390/medicina57060568. Kaunas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institue for Health and Care Excellence (2021), COVID-19 rapid guideline: managing the long-term effects of COVID-19, in: NICE Guidance, https://www.nice.org.uk/guidance/ng188, accessed: 11/08/2021. [PubMed]
- 39.Arnold D.T., et al. Patient outcomes after hospitalization with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan X., et al. Follow-up study of pulmonary function among COVID-19 survivors 1 year after recovery. J Infect. 2021 doi: 10.1016/j.jinf.2021.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seessle J., et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Budweiser S., et al. Comparison of the first and second waves of hospitalized patients with SARS-CoV-2. Dtsch Arztebl Int. 2021;118(18):326–327. doi: 10.3238/arztebl.m2021.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandal S., et al. Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalization for COVID-19. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen M.S., et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlson M.E., et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 46.Salehi S., et al. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol. 2020;30(9):4930–4942. doi: 10.1007/s00330-020-06863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quanjer P.H., et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quanjer P.H., et al. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6(Suppl 16):5–40. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 49.A.T.S. Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 50.Enright P.L., et al. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 51.Levey A.S., et al. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroenke K., et al. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spitzer R.L., et al. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 54.Hartung T.J., et al. The hospital anxiety and depression scale (HADS) and the 9-item patient health questionnaire (PHQ-9) as screening instruments for depression in patients with cancer. Cancer. 2017;123(21):4236–4243. doi: 10.1002/cncr.30846. [DOI] [PubMed] [Google Scholar]
- 55.Jones P.W., et al. A self-complete measure of health status for chronic airflow limitation. The St. George's respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 56.Rief W.H., et al. Huber; Bern: 1997. SOMS-das screening für somatoforme störungen. manual zum fragebogen. [Google Scholar]
- 57.Qu G., et al. Health-related quality of life of COVID-19 patients after discharge: a multicenter follow-up study. J Clin Nurs. 2021;30(11–12):1742–1750. doi: 10.1111/jocn.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peghin M., et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Todt B.C., et al. Clinical outcomes and quality of life of COVID-19 survivors: a follow-up of 3 months post hospital discharge. Respir Med. 2021;184 doi: 10.1016/j.rmed.2021.106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong Q., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-Centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aparisi A., et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med. 2021;10(12) doi: 10.3390/jcm10122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sudre C.H., et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frija-Masson J., et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56(2) doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garrigues E., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor R.R., et al. Post-COVID symptoms reported at asynchronous virtual review and stratified follow-up after COVID-19 pneumonia. Clin Med (Lond) 2021 doi: 10.7861/clinmed.2021-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang S., et al. Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep. 2021;11(1):13854. doi: 10.1038/s41598-021-93191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization (2021), Clinical management of severe acute respiratory infection when COVID-19 disease is suspected: interim guidance, in: who.int, https://www.who.int/publications/i/item/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected, accessed: 11/08/2021.
- 68.Debeaumont D., et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization-a pilot study. Phys Ther. 2021 doi: 10.1093/ptj/pzab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lerum T.V., et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J, 2021. 2021;57(4) doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Townsend L., et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fortini A., et al. COVID-19: persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection. 2021 doi: 10.1007/s15010-021-01638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gianella P., et al. Clinical, radiological and functional outcomes in patients with SARS-CoV-2 pneumonia: a prospective observational study. BMC Pulm Med. 2021;21(1):136. doi: 10.1186/s12890-021-01509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rass V., et al. Neurological outcome and quality of life 3 months after COVID-19: a prospective observational cohort study. Eur J Neurol. 2021 doi: 10.1111/ene.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Siemens S.M., et al. Effect of COPD severity and comorbidities on the result of the PHQ-9 tool for the diagnosis of depression: results from the COSYCONET cohort study. Respir Res. 2019;20(1):30. doi: 10.1186/s12931-019-0997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thibodeau M., et al. The PHQ-9 assesses depression similarly in men and women from the general population. Pers Individ Difer. 2014;56:149–153. doi: 10.1016/j.paid.2013.08.039. [DOI] [Google Scholar]
- 76.Bauerle A., et al. Increased generalized anxiety, depression and distress during the COVID-19 pandemic: a cross-sectional study in Germany. J Public Health (Oxf) 2020;42(4):672–678. doi: 10.1093/pubmed/fdaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mary-Krause M., et al. Impact of COVID-19-like symptoms on occurrence of anxiety/depression during lockdown among the French general population. PLoS ONE. 2021;16(7) doi: 10.1371/journal.pone.0255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersen A.J., et al. Symptoms of anxiety/depression during the COVID-19 pandemic and associated lockdown in the community: longitudinal data from the TEMPO cohort in France. BMC Psychiatry. 2021;21(1):381. doi: 10.1186/s12888-021-03383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Provencher S., et al. COVID-19 and the pulmonary vasculature. Pulm Circ. 2020;10(3) doi: 10.1177/2045894020933088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heckman E.J., et al. Pulmonary function tests for diagnosing lung disease. JAMA. 2015;313(22):2278–2279. doi: 10.1001/jama.2015.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.