Supplemental Digital Content is available in the text.
Background.
Uncontrolled donation after circulatory death (DCD) donors are an extraordinary resource to increase the number of lungs available for transplantation. However, the risk of the warm ischemia resulting from cardiac arrest to irreversibly damage the organs is considerable. Moreover, graft preservation issues and organizational problems often worsen the dangerous effects of warm ischemia. Ex vivo lung perfusion (EVLP) enables us to evaluate and recondition lungs whose functionality is doubtful, as well as to overcome the difficulties related to time and logistics.
Methods.
We report the case of uncontrolled DCD lungs successfully treated with an exceptionally prolonged EVLP. Because the donor’s blood count and liver biopsy showed signs of possible leukemia, EVLP was protracted up to 17 h while waiting for immunohistochemical analyses to rule out this diagnosis; eventually, the results came back negative, and the lungs were judged suitable for transplantation.
Results.
The recipient was a 32-y-old male individual with cystic fibrosis, colonized by Pandoraea pnomenusa. Bilateral transplantation required central extracorporeal membrane oxygenation. The patient was extubated after 36 h and was discharged 21 d after the operation. Despite early recolonization by Pandoraea pnomenusa and airway complications requiring pneumatic dilatation, he is alive and has a satisfactory respiratory function 15 mo after transplantation.
Conclusions.
Uncontrolled DCD represents a challenge due to both logistical issues and the complexity of graft evaluation before procurement. EVLP with cellular perfusate could be a valuable tool to overcome these limits. Nonetheless, caution should be exercised when interpreting the effects of this technique on airway healing.
INTRODUCTION
Lung procurement from donation after circulatory death (DCD) donors is currently under development for increasing the organ pool.1 In this scenario, the uncontrolled (u-) setting is the most promising for its potential. Nevertheless, the challenges to overcome organizational problems and those related to graft preservation and evaluation are undeniable.2-4 In 2014, our Lung-DCD project started, including in situ preservation with normothermic open-lung approach and ex situ assessment with ex vivo lung perfusion (EVLP).5 We present a case that required an extraordinary long period of EVLP to overcome the unpredictability of the uDCD setting.
MATERIALS AND METHODS
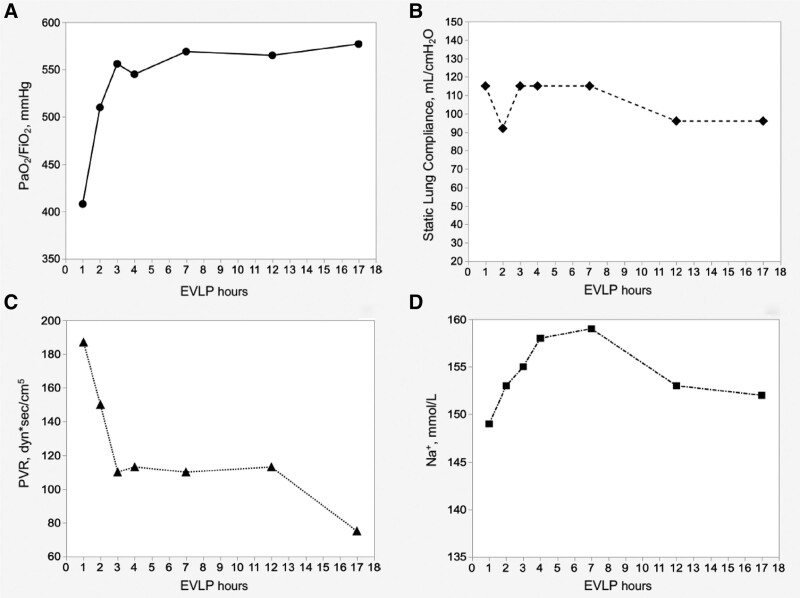
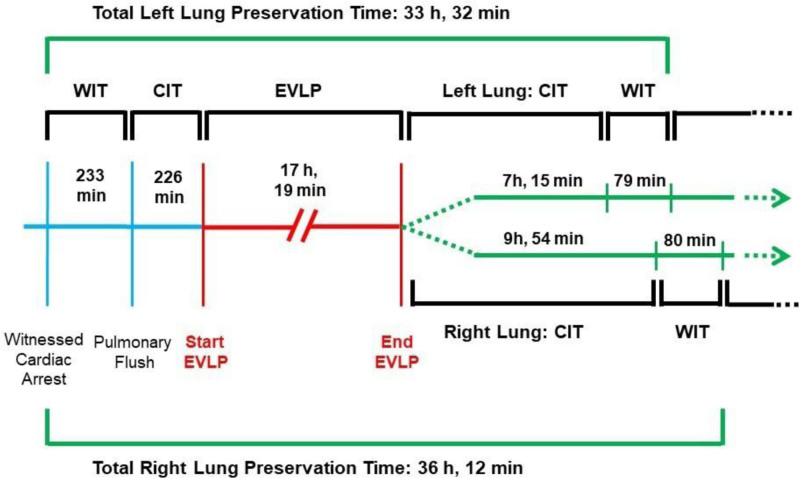
This study was approved by the ethics committee of Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico of Milan (Ref. no. 181; January 01, 2017). A 57-y-old nonsmoking male individual had a witnessed cardiac arrest at home and immediately received basic life support by bystanders. Brought to the San Gerardo Hospital (Monza), after 78 min of cardiopulmonary resuscitation (3 shocks, 12 adrenaline boluses, 69 min of manual chest compressions, and 9 min of mechanical chest compressions), the patient was declared dead. The only known disease in his medical history was systemic hypertension. The local team considered the subject as a possible lung donor and started the in situ protocol preservation.5 Briefly, after the clinical diagnosis of death, a recruitment maneuver was performed and continuous positive end-expiratory pressure (10 cmH2O, 100% FiO2) was applied until death confirmation (20 min of flat electrocardiogram). After the consent from the relatives, heparin was given (10 000 units endovenous), a new recruitment maneuver performed, and the in situ preservation with protective ventilation (tidal volume [TV] 6 mL/kg ideal body weight, positive end-expiratory pressure 8 cmH2O, respiratory rate, respiratory rate [RR] of 4 bpm, FiO2 100%) completed until the arrival of the retrieval team from the Milan Lung Transplant Center. The chest radiograph showed right upper lobe opacity (Figure S1, SDC, http://links.lww.com/TP/C111); bronchoscopy revealed abundant frothy secretions. At inspection, the lungs were judged suitable for EVLP. The main pulmonary artery was cannulated, and 500 µg of prostaglandins E1 was administered. Anterograde cold flush with low-molecular-weight dextran solution with 15 mg of recombinant tissue plasminogen activator was started together with topical cooling. In situ retrograde flushing was completed, and the lung block was stored on ice after usual retrieval. In the meantime, the first results available showed a B blood group and an inversion of the CD4+ to CD8+ lymphocyte ratio; upon suspicion of a previously unknown lymphoproliferative disease, with the features of T-large granular lymphocyte leukemia (T-LGL), the retrieval team obtained lymph node, spleen, hepatic, and renal biopsies. Grafts were transported to our center for the usual evaluation.6,7 In short, our EVLP system consists of a custom-made circuit. The lungs are accommodated in a dedicated organ chamber from which the perfusate is drained by gravity in a venous reservoir and then drawn by a centrifugal pump and pushed into a deoxygenator connected to a heat exchanger. The perfusate then reaches a pulmonary cannula fastened to the pulmonary artery, crosses the lung vasculature, and is drained from the left atrium left open to return to the organ chamber. All the circuit elements are connected by a 0.375-inch heparin-coated polyvinyl tubing system. EVLP lasts 4 h, including a perfusate flow of 40% of donor cardiac output with target pulmonary artery pressure of <15 mm Hg, an open atrium, and a cellular perfusate. The ventilatory setting provides for a TV of 7 mL/kg (donor ideal body weight) with a RR of 7 bpm (FiO2 21%). The pulmonary functional assessment is made hourly at FiO2 of 100%. The first lung evaluation after 1 h showed a good lung function in terms of oxygenation, lung mechanics, and hemodynamic: PaO2/FiO2 407 mm Hg, static lung compliance 115 mL/cmH2O, and pulmonary vascular resistance (PVR) 187 dyn*s/cm5. Simultaneously, however, the detection of a mononuclear cell infiltrate in the portal spaces but, most notably, in the sinusoids of the donor’s liver (Figure 1) supported the suspect of a T-LGL, which could be excluded only upon immunohistochemical analysis, the results of which would have only been available on the next morning. At this point, we were faced with a difficult choice: wait several hours for the definitive histological diagnosis, suspend the EVLP and prolong the cold ischemic time (CIT), or discard the grafts. The partial results supporting the suspicion of a T-LGL came when the EVLP was already underway. At that point, stopping the EVLP would have meant a very prolonged cold storage, the first CIT of 3 h 46 min plus the second CIT of around 13-h waiting for anatomopathological definitive results. Even though experimental8 and clinical9 studies reported successful transplantation after prolonged preservation time, in this case, a total CIT of almost 25 h for the left lung and 28 h for the right lung would have been added to the 223 min of warm ischemic time of a uDCD. We believed that it would have been too great a risk. Moreover, in this peculiar scenario of prolonged CIT in a uDCD, for extreme caution, we would have reassessed the lungs through a second EVLP before transplant. The option of a double EVLP and a triple CIT appeared to be even less predictable and unexplored than the one we adopted. We decided to continue the EVLP beyond our standard 4-h protocol. The circuit was primed with 2000 mL of STEEN solution plus 2 units of packed red blood cells (PRBC). Assessment of respiratory, hemodynamic, and metabolic parameters was performed throughout the entire EVLP duration (Figure 2). Blood gas analyses were collected hourly for the first 4 h and then every 2–3 h. Before any evaluation, recruitment maneuvres were performed. PaO2/FiO2 ratio improved steadily from 407 to 577 mm Hg; the static lung compliance maintained good stable values (106 ± 11 mL/cmH2O) during all 17 h. PVR remained constantly below 200 dyn*s/cm5. Careful perfusate changes were made by monitoring sodium, glucose, and lactate levels. Regular replacements of 250 mL of perfusate every hour for the first 4 h and subsequently every 3 h were performed, while 1 unit of PRBC was added around every 4 h for a total of 4500 mL of STEEN solution and 1800 mL of PRBC (ie, 6 units) throughout the whole EVLP perfusion. The hematocrit of the perfusate was maintained within 10% and 15% for the entire EVLP but at the 17th hour, when we recorded 17% of hematocrit. Thamesol 3.6% was given in repeated boluses for a total of 120 mL, and continuous infusion of free water was started from the seventh hour of the EVLP (500 mL total). Supplements of antibiotics and steroids were made for a total of 2 g of imipenem and 750 mg of methylprednisolone. At 8:30 am, the pathologist reported a global picture consistent with a reactive lymphocyte expansion. The final lung function evaluation showed a PaO2/FiO2 of 577 mm Hg, static lung compliance of 96 mL/cmH2O, and 75 dyn*s/cm5 of PVR. Sodium, glucose, and lactate levels were 152 mmol/L, 162 mg/dL, and 13 mmol/L, respectively. There was no sign of edema at macroscopic visual and palpatory assessment, and the x-rays showed the resolution of the right upper lobe infiltrate (Figure S2, SDC, http://links.lww.com/TP/C111). The bronchoscopy showed no secretions, bleeding, or plasmorrhea. After a collective discussion, the lungs were judged suitable for transplantation. EVLP duration was 17 h and 19 min. The recipient was a 32-y-old patient with cystic fibrosis, identical blood group, and on the waiting list for 13 mo; his last lung allocation score was 37.12. The patient was on 24/24-h oxygen supply and nocturnal noninvasive ventilatory support, recovering from recent pulmonary exacerbation. His lower airways were colonized by Pandoraea pnomenusa. Our immunosuppressive regimen consists of triple-drug therapy (calcineurin inhibitor, antiproliferative agent, corticosteroids) without induction therapy. The bilateral transplantation required a central extracorporeal membrane oxygenation support before the first pneumonectomy, which was ceased after the reperfusion of the second graft. Airway anastomoses were covered with vital recipient tissue. Seven units of PRBC and 8 units of fresh frozen plasma were administered. At the end of surgery, the patient was ventilated with a TV of 7 mL/kg with a RR of 15 bpm. He had a PaO2/FiO2 of 212 mm Hg, a PaCO2 of 44 mm Hg, and static lung compliance of 39 mL/cmH2O. Stable hemodynamic was recorded with minimum vasoconstrictor support (noradrenaline 0.06 µg/kg/min). Figure 3 depicts the timeline of the process.
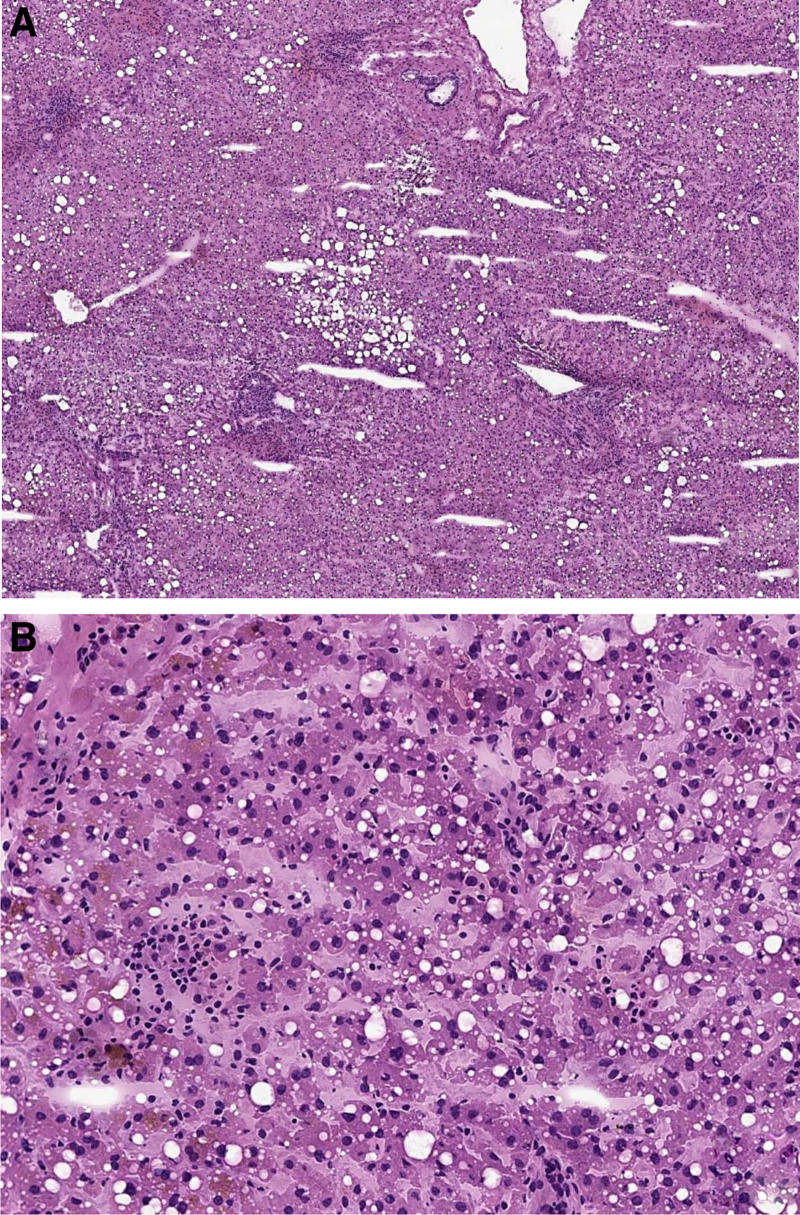
FIGURE 1.
Frozen section of the donor liver showing, at low magnification (A; hematoxylin-eosin, ×4), a preserved lobular architecture with mild macrovescicular steatosis, absence of fibrosis and a moderate small lymphocyte infiltrate in the portal spaces, which is also noted in the sinusoids (B; hematoxylin-eosin, ×20), together with sparse granulocytes.
FIGURE 2.
Trends during 17 h of EVLP. A, PaO2/FiO2. B, Static lung compliance. C, Pulmonary vascular resistances. D, Na+ concentration. EVLP, ex vivo lung perfusion.
FIGURE 3.
Ischemic and preservation times from donor to EVLP and during recipient management. CIT, cold ischemic time; EVLP, ex vivo lung perfusion; WIT, warm ischemic time.
RESULTS
The patient was extubated after 36 h and discharged from the intensive care unit on the third postoperative day. Primary graft dysfunction at 24, 48, and 72 h was scored to 2, 1, and 1, respectively.10 The postoperative period was uneventful, and the patient was discharged home after 21 d from transplantation with a forced expiratory volume in 1 s (FEV1) of 51%. After 3 mo, he was hospitalized for acute respiratory failure; chest radiograph revealed left basal pneumonia; bronchoalveolar lavage culture confirmed the early airway recolonization by P pnomenusa. At the same time, bronchoscopy showed expulsion of suture fragments within the bronchial lumen as well as a nonsuture–related stenosis of the bronchus intermedius (extent a) and superior left lobar bronchial stenoses distal to bronchial suture (extent b).11 He was successfully treated with an aggressive course of antibiotics (carbapenem, cotrimoxazole, ciprofloxacin), high flow nasal cannula oxygen therapy, and he underwent pneumatic dilatation. The patient was successfully weaned from oxygen supply and adapted to noninvasive ventilation. Two months after, a second pneumatic dilatation was performed. Surveillance computed tomography scans and transbronchial biopsies at 6 and 12 mo did not show any sign of lung allograft dysfunction (Figure S3, SDC, http://links.lww.com/TP/C111). The last bronchoscopy revealed satisfactory patency of the bronchial tree. The patient is currently (15 mo after surgery) eupneic on room air, with good exercise tolerance; last FEV1 is 50% of predicted (best FEV1 54%) and the distance covered at 6-min walking test is 628 m on room air. He is currently struggling to find a suitable job and practices soccer twice a week.
DISCUSSION
In the presented case of transplantation from uDCD, extending the duration of the EVLP up to 17 h proved feasible, once granted the necessary time to provide the correct answer to the clinical question. The procedure showed positive outcomes in the short-mid term. Lungs from DCD donors suffer less from the sympathetic and inflammatory cytokine storm, which is typical of donations after brain death12-14; in addition, if kept ventilated, lungs tolerate warm ischemia well.15,16 In a recently published case series, patients transplanted with lungs from uDCD donors, showed excellent long-term outcomes, comparable to those achieved with a DBD donation.17 These findings should encourage clinical utilization of lungs from DCD donors. Currently, there are roughly 350 000 cases of cardiopulmonary resuscitation per year in Europe, 40% of which result in recoveries.18 The opportunity provided by the great number of potential uDCD donors available each day is not adequately seized to date. In this context, lungs procurement from uDCD donors poses more serious logistical and safety challenges, when compared with standard procedures. We have been working on this issue for several years, managing preclinical research as well as logistical organization; the Welfare Direction of Regione Lombardia has actively endorsed our efforts. Our program includes lung procurement from different scenarios; a Full-DCD program for uDCD and controlled DCD donor combined with abdominal organs (normothermic regional perfusion) and a Lung-DCD program for uDCD donor for isolated lung.5,19 This last setting proves very promising because it does not require any extracorporeal program; thus, it can be applied virtually in all hospitals, as recently confirmed.20 Our first reported clinical case is today in good condition, showing no sign of chronic rejection (FEV1: 117%) and experiencing a self-reported high quality of life almost 5 y after transplantation.5 In the case we are now reporting, we stressed our Lung-DCD protocol by extending lung reconditioning to a previously unreported limit. EVLP was essential to increase the graft preservation time up to >36 h. In a uDCD setting, gaining time is of paramount importance to enhance the donor evaluation, optimize the logistics and organization of the transplant, and, possibly, improve the graft reconditioning. The present report is meaningful: without the opportunity to extend the lungs preservation through normothermic perfusion, the graft would have been discarded due to high oncological risk. To the best of the authors’ knowledge, human EVLP of similar duration has been reported only by the Padua group.21 It is worth mentioning how both protocols, the Padua Organ Care System and our Milan EVLP technique, used perfusate enriched with erythrocytes. Few preclinical studies on prolonged EVLP are available, and those reporting extended EVLP time beyond 12 h are even fewer. The available results after 24 h of EVLP were produced by only 2 research groups.22,23 The studies suggest that cellular normothermic EVLP, within a specific experimental design, may effectively extend lung preservation up to 24 h. However, they do not address the uDCD scenario with its own peculiarities and present experimental models with limited warm ischemic times. Finally, pulmonary functional parameters at the end of the 24 h EVLP or after subsequent transplant showed values at the limits of standard practice. Therefore, up to now, both preclinical and clinical data suggest that to prolong EVLP beyond 4–6 h is challenging. In the presented case, we were particularly concerned about the large bronchi; in fact, if the functionality of the parenchyma had been adequately evaluated during the EVLP, no information would have been available on the bronchi viability and bronchial artery circulation.24 Actually, our patient has developed bilateral airway stenosis. We can speculate on the potential role of re-colonization by P pnomenusa, a multidrug-resistant bacterium, which is emerging in the context of cystic fibrosis. Also, the findings related to the expulsion of suture fragments within the bronchial lumen are difficult to interpret. Moreover, the use of alpha agonists as noradrenaline in the perioperative time could have played a role in the development of airway complications, even though the overall duration and the average dosage used in this case were very low. Furthermore, because our surgical technique does not include bronchial circulation sparing, norepinephrine could not have theoretically reached the airway vascularization. Anyway, it is undeniable that the bronchi experienced extended low perfusion; therefore, caution with very prolonged EVLP especially with open atrium should be taken. In conclusion, we reported the first account of successful transplantation from uDCD donor employing lungs preserved with EVLP for 17 h. uDCD is frequently perceived as a challenge for increased logistical requirements and limited preprocurement assessment; in our experience, prolonged EVLP with cellular perfusate showed that those limits could possibly be overcome. Great caution is mandatory in interpreting the effect of prolonged EVLP on airway healing.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
A.P., M.N., and A.Z. participated in research design. All authors participated in article writing and were involved in performance of the research. A.P. and A.Z. participated in data analysis.
The study was approved by Ethics committee of Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico of Milan. Ref. no. 181 (January 24, 2017).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Van Raemdonck D, Keshavjee S, Levvey B, et al. ; International Society for Heart and Lung Transplantation. Donation after circulatory death in lung transplantation—five-year follow-up from ISHLT Registry. J Heart Lung Transplant. 2019;38:1235–1245. [DOI] [PubMed] [Google Scholar]
- 2.Cypel M, Levvey B, Van Raemdonck D, et al. Lung transplantation using controlled donation after circulatory death donors: trials and tribulations. J Heart Lung Transplant. 2016;35:146–147. [DOI] [PubMed] [Google Scholar]
- 3.Egan TM, Requard JJ, 3rd. Uncontrolled donation after circulatory determination of death donors (uDCDDs) as a source of lungs for transplant. Am J Transplant. 2015;15:2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venema LH, Brat A, Nijkamp DM, et al. Factors that complicated the implementation of a program of donation after unexpected circulatory death of lungs and kidneys. Lessons learned from a regional trial in the Netherlands. Transplantation. 2019;103:e256–e262. [DOI] [PubMed] [Google Scholar]
- 5.Valenza F, Citerio G, Palleschi A, et al. Successful transplantation of lungs from an uncontrolled donor after circulatory death preserved in situ by alveolar recruitment maneuvers and assessed by ex vivo lung perfusion. Am J Transplant. 2016;16:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenza F, Rosso L, Coppola S, et al. Ex vivo lung perfusion to improve donor lung function and increase the number of organs available for transplantation. Transpl Int. 2014;27:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fumagalli J, Rosso L, Gori F, et al. Early pulmonary function and mid-term outcome in lung transplantation after ex-vivo lung perfusion—a single-center, retrospective, observational, cohort study. Transpl Int. 2020;33:773–785. [DOI] [PubMed] [Google Scholar]
- 8.Hsin MKY, Iskender I, Nakajima D, et al. Extension of donor lung preservation with hypothermic storage after normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2016;35:130–136. [DOI] [PubMed] [Google Scholar]
- 9.Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med. 2017;5:119–124. [DOI] [PubMed] [Google Scholar]
- 10.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: definition and grading—a 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1097–1103. [DOI] [PubMed] [Google Scholar]
- 11.Crespo MM, McCarthy DP, Hopkins PM, et al. ISHLT consensus statement on adult and pediatric airway complications after lung transplantation: definitions, grading system, and therapeutics. J Heart Lung Transplant. 2018;37:548–563. [DOI] [PubMed] [Google Scholar]
- 12.Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009;53:425–435. [DOI] [PubMed] [Google Scholar]
- 13.Kang CH, Anraku M, Cypel M, et al. Transcriptional signatures in donor lungs from donation after cardiac death vs after brain death: a functional pathway analysis. J Heart Lung Transplant. 2011;30:289–298. [DOI] [PubMed] [Google Scholar]
- 14.Baciu C, Sage A, Zamel R, et al. Transcriptomic investigation reveals donor specific gene signatures in human lung transplants. Eur Respir J. 2020. 29:2000327. [DOI] [PubMed] [Google Scholar]
- 15.Ulicny KS, Jr, Egan TM, Lambert CJ, Jr, et al. Cadaver lung donors: effect of preharvest ventilation on graft function. Ann Thorac Surg. 1993;55:1185–1191. [DOI] [PubMed] [Google Scholar]
- 16.Greco R, Cordovilla G, Sanz E, et al. Warm ischemic time tolerance after ventilated non-heart-beating lung donation in piglets. Eur J Cardiothorac Surg. 1998;14:319–325. [DOI] [PubMed] [Google Scholar]
- 17.Suberviola B, Mons R, Ballesteros MA, et al. Excellent long-term outcome with lungs obtained from uncontrolled donation after circulatory death. Am J Transplant. 2019;19:1195–1201. [DOI] [PubMed] [Google Scholar]
- 18.Manyalich M, Nelson H, Delmonico FL. The need and opportunity for donation after circulatory death worldwide. Curr Opin Organ Transplant. 2018;23:136–141. [DOI] [PubMed] [Google Scholar]
- 19.Palleschi A, Tosi D, Rosso L, et al. Successful preservation and transplant of warm ischaemic lungs from controlled donors after circulatory death by prolonged in situ ventilation during normothermic regional perfusion of abdominal organs. Interact Cardiovasc Thorac Surg. 2019;29:699–705. [DOI] [PubMed] [Google Scholar]
- 20.Healey A, Watanabe Y, Mills C, et al. Initial lung transplantation experience with uncontrolled donation after cardiac death in North America. Am J Transplant. 2020;20:1574–1581. [DOI] [PubMed] [Google Scholar]
- 21.Schiavon M, Zampieri D, Marulli G, et al. Pushing the limits of reconditioning: extended normothermic lung perfusion in an extended criteria donor. J Thorac Dis. 2018;10:E796–E801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loor G, Howard BT, Spratt JR, et al. Prolonged EVLP using OCS lung: cellular and acellular perfusates. Transplantation. 2017;101:2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spratt JR, Mattison LM, Iaizzo PA, et al. Lung transplant after prolonged ex vivo lung perfusion: predictors of allograft function in swine. Transpl Int. 2018;31:1405–1417. [DOI] [PubMed] [Google Scholar]
- 24.Tane S, Noda K, Toyoda Y, et al. Bronchial-arterial-circulation-sparing lung preservation: a new organ protection approach for lung transplantation. Transplantation. 2020;104:490–499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.