Abstract
Electrochemical CO2 reduction (CO2RR) in a product-orientated and energy-efficient manner relies on rational catalyst design guided by mechanistic understandings. In this study, the effect of conducting support on the CO2RR behaviors of semi-conductive metal-organic framework (MOF) — Cu3(HITP)2 are carefully investigated. Compared to the stand-alone MOF, adding Ketjen Black greatly promotes C2H4 production with a stabilized Faradaic efficiency between 60-70% in a wide potential range and prolonged period. Multicrystalline Cu nano-crystallites in the reconstructed MOF are induced and stabilized by the conducting support via current shock and charge delocalization, which is analogous to the mechanism of dendrite prevention through conductive scaffolds in metal ion batteries. Density functional theory calculations elucidate that the contained multi-facets and rich grain boundaries promote C–C coupling while suppressing HER. This study underlines the key role of substrate-catalyst interaction, and the regulation of Cu crystalline states via conditioning the charge transport, in steering the CO2RR pathway.
Subject terms: Electrocatalysis, Electrocatalysis, Electrocatalysis
The substrate-catalyst interaction plays a critical role in steering the pathway of electrochemical CO2 reduction. Here the authors show the crystalline states of copper in reconstructed metal-organic frameworks were induced and stabilized by Ketjen Black conducting support, promoting C2H4 production.
Introduction
The escalating climate change originating from the greenhouse effect due to excessive CO2 emission urges the mankind to realize carbon neutrality as early as possible1,2. Among the multiple strategic route maps, electrocatalytic reduction of carbon dioxide (CO2RR) driven by renewable energy is especially tempting, as it simultaneously addresses both the environmental and resource crises3,4. Copper in various forms have been demonstrated highly potent in catalyzing CO2 conversion into a diversity of hydrocarbons and oxygenates, owing to its modest binding to many of the key CO2RR intermediates5,6. This, however, also leads to the lack of product selectivity in Cu-catalyzed CO2RR as a result of the scaling relation among those intermediate binding7,8. To take advantage of the product diversity while maximizing the selectivity of individual target, many structural mutations of Cu have been explored, including morphology and facet control9,10, creation of grain boundaries11,12, under-coordinated sites13,14, and mixed valence states15,16, as well as surface functionalization and segregation17,18. Nonetheless, energy efficiency and product selectivity, especially on those value-added multiple-carbon products, remain as the major hurdles for ultimately deploying the technology in a techno-economic fashion.
Previous studies showed that the substrates for catalyst loading, in most cases the carbon support, play a critical role in steering the CO2RR pathway and stabilizing the catalytic process19,20. For instance, Strasser and colleagues21 showed that the dispersion of Cu2O nanocubes on a carbon support sharply shifted the selectivity pattern toward C1, whereas its unsupported counterpart achieved a sustained C2+ Faradaic efficiency (FE). In situ formation of small Cu seeds with larger interparticle distance were attributed to the witnessed C1 selectivity on the supported Cu2O. Differently, Hwang and colleagues showed that Cu2O nanoparticles immobilized on a carbon support via cysteamine doubled the FE of C2H4 in comparison to the unsupported catalyst and ascribed this selectivity enhancement to the electrochemical fragmentation of Cu2O nanoparticles into compactly stacked smaller particles that are more liable to oxidation22. In another study, by pre-reducing the Cu nodes in a conductive metal organic framework (MOF) (CuHHTP) into Cu2O quantum dots, Cao and co-workers achieved a high selectivity of CH4 formation, which was attributed to ample hydrogen bonding from the substrate in stabilizing key intermediates23. Thus, it is vitally important to assess the effect of carbon support on CO2RR and scrutinize the substrate–catalyst interaction, as well as the charge transport behavior, for a better mechanistic understanding.
MOFs have been perceived as a unique category of CO2RR catalyst since they offer a tunable platform to systematically alter the metal site coordination24, regulate CO2 and electrolyte counterions in the Helmholtz layer25, and control over the intermediate binding26. However, the stability of MOFs during the electrolytic process has been always a limiting issue, so that their electrochemical reconstruction (which is almost unavoidable, especially under high current conditions) to derive more robust catalyst ensembles is gaining increasing attentions27,28. Consequently, when the controllable electrochemical reconstruction of MOFs is the subject of study, their chemical stability in the electrolyte is a premise29. For that, in the current study we set to investigate the CO2RR behaviors of a semi-conductive MOF—Cu3(HITP)2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene) with and without adding the conducting support of Ketjen Black (KB). Cu3(HITP)2 was synthesized in an alkaline environment and thus, unlike many carboxylic MOFs, was born chemically resilient to the common electrolytes of CO2RR. This should endow a unique opportunity to allow us to focus on their electrochemical reconstruction and catalyst–support interaction during the electrochemical process (Fig. 1a). We found that adding KB enabled greatly promoting the C2H4 production with stabilized FE between 60 and 70% in a wide potential range and prolonged testing period, whereas the stand-alone MOF yielded more mixed reduction products. Operando X-ray absorption spectroscopy (XAS), serial post-reaction X-ray diffraction (XRD), and transmission electron microscope (TEM) analyses in conjunction with density functional theory (DFT) calculations were further carried out to correlate the product selectivity with structural characteristics of the reduced Cu moieties, furnishing fresh insights into the substrate–catalyst interaction and promoted C–C coupling on multicrystalline Cu nano-crystallites.
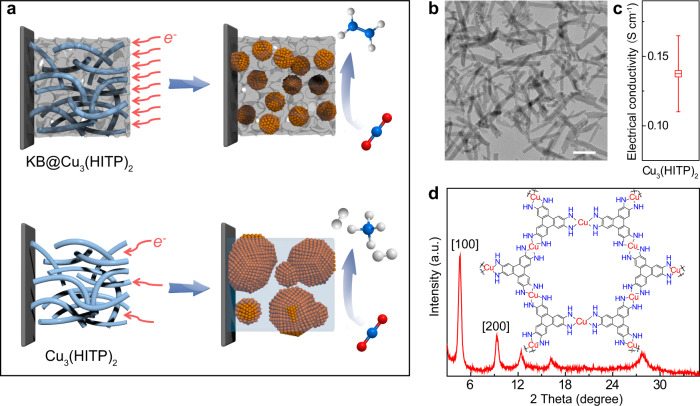
Fig. 1. Schematic representation and structure characterization.
a Schematic diagrams showing the modulation of CO2RR product selectivity by Cu3(HITP)2-derived Cu0 entities in the presence (top) or absence (bottom) of carbon support. b TEM image, c electrical conductivity measured by the four-probe method, and d XRD pattern of the as-synthesized Cu3(HITP)2. The scale bar in b is 200 nm. Error bars represent the standard deviation of three independent measurements.
Results
Structural characterization of Cu3(HITP)2
Cu3(HITP)2 with high crystallinity was synthesized following the method developed by Dincă and co-workers recently30. TEM taken on Cu3(HITP)2 revealed rod-like crystals of 30–40 nm in diameter (Fig. 1b), while energy-dispersive X-ray elemental mapping by high-angle annular dark-field scanning TEM confirmed the homogeneous elemental distribution of Cu, N, and C (Supplementary Fig. 1). Owing to the in-plane electron delocalization and through-space charge transport31, Cu3(HITP)2 demonstrated a semi-conductivity of 0.14 S cm−1 (Fig. 1c), as measured at room temperature by the four-point probe method and in line with the previous reported values31. The XRD pattern in Fig. 1d exhibiting prominent diffraction peaks between 3° and 30° well matched that previously reported for Cu3(HITP)230–32, affirming the successful synthesis of the MOF structure with high crystallinity. X-ray photoelectron spectroscopy of Cu 2p3/2 exhibited a mixture of Cu2+ (933.5 eV) and Cu+ (931.8 eV) states in Cu3(HITP)2 (Supplementary Fig. 2)31, which was further corroborated by the X-ray absorption near edge structure (XANES, Supplementary Fig. 3). While the main peak at 398.5 eV in the N 1s spectrum was attributed to the Cu-N motif (Supplementary Fig. 4)31, the shoulder at 396.7 eV corresponded to the decrease in the N valence caused by the uncoordinated amines at the edge or defect sites of Cu3(HITP)233.
CO2RR of Cu3(HITP)2 with or without KB
Considering the alkaline stability and intrinsic conductivity of Cu3(HITP)2, electrochemical CO2RR performance was evaluated without or with adding KB as the conducting agent in CO2-saturated KHCO3 electrolyte. Correspondingly, the catalysts were denoted as Cu3(HITP)2 and KB@Cu3(HITP)2, respectively. First of all, linear sweep voltammetries (LSVs, with a rate of 50 mV s−1) taken on KB@Cu3(HITP)2 showed notably higher current densities than those of Cu3(HITP)2 in both N2- and CO2-purged electrolytes (Supplementary Fig. 5), indicating the addition of KB helped to promote the Faradaic processes including both hydrogen evolution reaction (HER) and CO2RR. In addition, both KB@Cu3(HITP)2 and Cu3(HITP)2 gave higher current densities under CO2RR conditions, affirming that the Cu-containing MOF was effective in catalyzing electrochemical CO2 reduction. When normalized to the electrochemically active surface area estimated by measuring the double-layer capacitance (Cdl), the LSV current densities of KB@Cu3(HITP)2 were still higher than those of Cu3(HITP)2 (Supplementary Fig. 6), suggesting the intrinsic higher catalytic activity of the former.
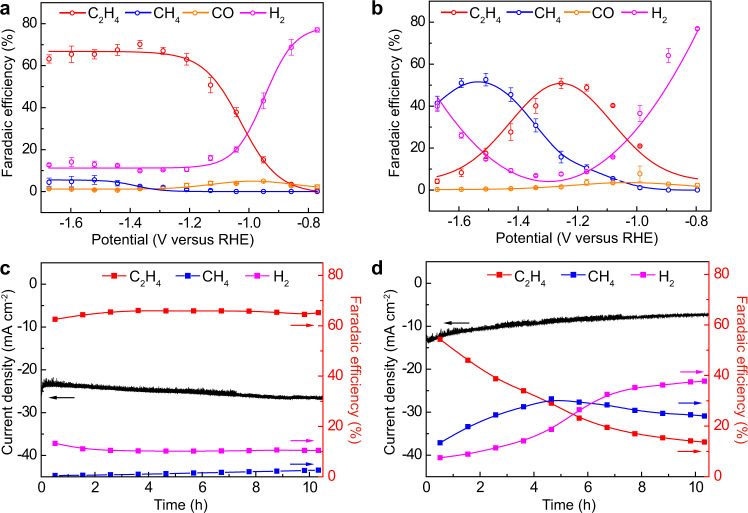
On analyzing the reduction products, it was impressive to see KB@Cu3(HITP)2 exhibiting a higher and more stable C2H4 selectivity and yield (Fig. 2a, b and Supplementary Fig. 7). For KB@Cu3(HITP)2, the production of C2H4 started at −0.85 V (reversible hydrogen electrode (RHE), hereafter all the potential mentioned are referenced to this format) and reached a maximum FE of 70% at −1.37 V with concomitantly decreased H2 yield (Fig. 2a). Of note, the FEs of C2H4 maintained over 63% within the wide potential range from −1.20 to −1.67 V, whereas those of CH4 and CO were trivial during the entire CO2RR test. In comparison, the FEs of C2H4 for Cu3(HITP)2 displayed a maximum of 51% at −1.25 V and thereafter decreased continuously (Fig. 2b). Meanwhile, a notable CH4 production was observed attaining its maximum FE of 53% at −1.51 V, accompanied by a continuous increase in H2 yield (Fig. 2b). The different CO2RR behaviors between KB@Cu3(HITP)2 and Cu3(HITP)2, especially on the varied C2H4 and CH4 production, implied a different structural mutation of the Cu3(HITP)2 MOF during CO2RR, which will be closely inspected later.
Fig. 2. Electrochemical CO2RR performances.
FEs of C2H4, CH4, CO, and H2 on a KB@Cu3(HITP)2 and b Cu3(HITP)2. The 10-h chronoamperometric test at −1.25 V for c KB@Cu3(HITP)2 and d Cu3(HITP)2 showing the evolution of total current density and FEs of C2H4, CH4, and H2. Error bars represent the standard deviation of three independent measurements.
In addition to the C2H4 FE of >60% in a wide potential range, KB@Cu3(HITP)2 also demonstrated a high partial current density reaching 37.4 mA cm−2 at −1.67 V (Supplementary Fig. 7), which is seldom seen in H cells (Supplementary Table 1). Moreover, at the applied potential of −1.25 V, KB@Cu3(HITP)2 showed a good CO2RR stability, with the total current density increased slightly from 23.3 to 26.3 mA cm−2 and C2H4 FE constantly above 64% for a duration of 10 h (Fig. 2c). By contrast, in the same testing period the C2H4 FE of Cu3(HITP)2 decreased continuously from 52 to 13%, concomitant with a drop of total current density from 13.3 to 7.2 mA cm−2 (Fig. 2d). As for CH4 production, it was intriguing to see both its FE and partial current density on Cu3(HITP)2 were higher than those on KB@Cu3(HITP)2, suggesting that the former was less competent in triggering C–C coupling. The liquid reduction products, analyzed by nuclear magnetic resonance (NMR), were also collected for both KB@Cu3(HITP)2 and Cu3(HITP)2, revealing a mixture of formate, ethanol, and propanol with a total FE <30% at all potentials (Supplementary Fig. 8). Thus, our focus of this study will be mainly placed on comparing the main gaseous products.
Operando XAS on the Cu states
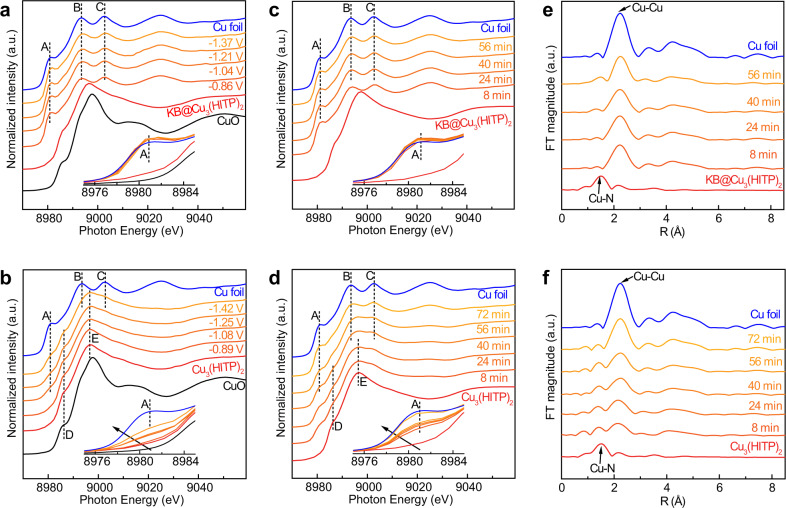
To track the change of Cu states for KB@Cu3(HITP)2 and Cu3(HITP)2 during CO2RR and elucidate the origin of their catalytic difference, both potential- and time-dependent operando XAS were carried out by looking into the Cu K-edges. For KB@Cu3(HITP)2 (Fig. 3a), upon the application of a negative potential at −0.86 V, the positive Cuδ+ (1 < δ < 2) state of the MOF turned into Cu0, showing consistent pre-edge and white-line features (marked A, B, C in the XANES spectra of Fig. 3a) to those of Cu foil. In stark contrast, Cu3(HITP)2 showed a more gradual change of Cu states as the applied potential decreased from −0.86 to −1.37 V (Fig. 3b). While the pre-edge and white-line peaks common to Cu2+ in CuO and Cu3(HITP)2 (marked as D and E in Fig. 3b) were still retained at −1.42 V, those of Cu0 also started to evolve at the applied potential of −1.08 V, especially for the absorption edge at 8981 eV (feature A, due to the 1s → 4pz transition)34. Clearly, these observations indicated that the Cuδ+ state in KB@Cu3(HITP)2 was more readily reduced than that in Cu3(HITP)2, likely due to the high current density from KB addition.
Fig. 3. Operando XAS.
Operando Cu K-edge XAS of a KB@Cu3(HITP)2 and b Cu3(HITP)2 at different potentials during CO2RR. Operando Cu K-edge XAS of c KB@Cu3(HITP)2 and d Cu3(HITP)2 as a function of reaction time at −1.25 V. The corresponding time-dependent FT-EXAFS for e KB@Cu3(HITP)2 and f Cu3(HITP)2.
Similarly, the time-dependent XANES spectra of KB@Cu3(HITP)2 collected at a constant potential of −1.25 V showed the Cuδ+ state in KB@Cu3(HITP)2 was reduced to Cu0 within only 8 min (Fig. 3c), whereas that in Cu3(HITP)2 took 72 min to match the XANES spectrum of Cu foil (Fig. 3d). Furthermore, the serial Fourier-transform extended XAFS (FT-EXAFS) spectra of KB@Cu3(HITP)2 showed a rapid evolution of the metallic Cu-Cu bond at 2.2 Å and the disappearance of the MOF-related Cu-N peak at 1.5 Å within 8 min (Fig. 3e), corroborating that the Cuδ+ nodes in the MOF were quickly and fully reduced. In the next hour, the intensity of the Cu-Cu coordination peak did not change much, indicating that the as-formed Cu0 moieties remained mostly stable. On the other hand, the sequential FT-EXAFS spectra of Cu3(HITP)2 displayed a gradual decay of the Cu-N coordination but intensification of the Cu-Cu peak within the whole testing period of 72 min (Fig. 3f), attesting to slower change of the MOF structure with gradually reduced Cu states. Taken together from the above electrochemical and XAS studies, it becomes evident that a high current shock endowed by adding the more conductive KB enables to quickly reduce the Cu nodes in the semi-conductive MOF of Cu3(HITP)2.
Ex situ time-lapse XRD and TEM
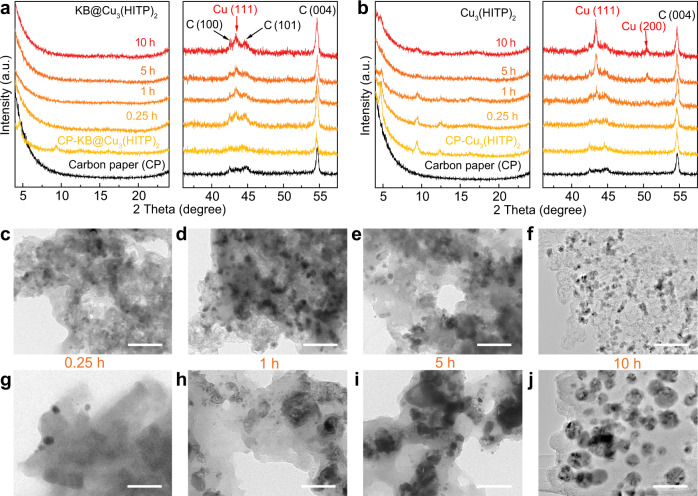
Ex situ time-lapse XRD and TEM were used to track the structural changes of KB@Cu3(HITP)2 and Cu3(HITP)2 under a fixed CO2RR potential of −1.25 V. As shown in the left panel of Fig. 4a, for KB@Cu3(HITP)2 the characteristic XRD peaks of the MOF disappeared completely after just 0.25 h of testing. In the meantime, a Cu(111) peak at 43.3° can be distinguished sandwiched between the C(100) and C(101) diffractions, and its intensity changed only marginally in the next 10 h (Fig. 4a, right panel). This observation is in good agreement with the previous XAS results, corroborating the quick reduction of Cuδ+ centers in the reticular network of the MOF to form metallic Cu species. By contrast, for the stand-alone Cu3(HITP)2, diffraction peaks specific to the MOF were still distinguishable after even a reaction period of 10 h (Fig. 4b, left panel), indicating that not all the catalyst was decomposed. Different from that of KB@Cu3(HITP)2, the intensity of the Cu(111) peak observed on Cu3(HITP)2 grew continuously along the reaction time, together with the gradual emergence of a Cu(200) peak at 50.4° (Fig. 4b, right panel). Again, the different trend of XRD evolution observed above on KB@Cu3(HITP)2 and Cu3(HITP)2 suggested that in the former the metallic Cu species were quickly formed and remained relatively stable, whereas in the latter Cuδ+ were reduced more gradually but aggregated into larger Cu particles of higher crystallinity (according to the Debye–Scherrer rule, Supplementary Fig. 9 and Table 2)35, which will be further examined by the following TEM studies.
Fig. 4. Ex situ time-lapse XRD and TEM.
Sequential XRD patterns of a KB@Cu3(HITP)2 and b Cu3(HITP)2 at −1.25 V. TEM images taken on c–f KB@Cu3(HITP)2 and g–j Cu3(HITP)2 after a chronoamperometric testing period of 0.25, 1, 5, and 10 h at −1.25 V, respectively. The scale bars in c–j are 100 nm.
Figure 4c–f, g–j displayed the TEM images taken on KB@Cu3(HITP)2 and Cu3(HITP)2 after a chronoamperometric testing period of 0.25, 1, 5, and 10 h at −1.25 V, respectively. The nano-rod morphology of the as-prepared Cu3(HITP)2 was absent in all post-electrolytic KB@Cu3(HITP)2 and Cu3(HITP)2 samples, indicating the corruption of the MOF topologic structure. Particularly, for KB@Cu3(HITP)2, Cu nanoparticles of 10 ± 2 nm were visible after 0.25 h, and beyond that the particle size remained mostly unchanged (Fig. 4c–f). As summarized in Supplementary Fig. 10a, the insignificant change of Cu particle size observed here coincided well with the stable C2H4 production seen in Fig. 2c. In contrast, for Cu3(HITP)2, the average size of Cu particles reduced out from the MOF increased from 11 ± 3 nm at 0.25 h to 39 ± 8 nm after 10 h (Fig. 4g–j), consistent with the above XRD observations (Fig. 4b and Supplementary Table 2). Correspondingly, the FE of C2H4 was reduced from the initial 52 to 13%, while that of H2 increased from 7.5 to 37% (Fig. 2d). This suggests that the growth of Cu particle size correlates to the decrease in C2H4 production and increase in H2 yield (Supplementary Fig. 10b). Similar evolution of Cu particles was also observed in the serial potential-dependent TEM images (Supplementary Fig. 11), showing the severe aggregation of Cu particles in Cu3(HITP)2 and mostly unchanged Cu particle size in KB@Cu3(HITP)2 while ramping down the potential from −1.21 to −1.75 V. Here, once again, the large Cu particle size in Cu3(HITP)2 correlated to the increase in H2 FE and decrease in C2H4 FE (Supplementary Fig. 12).
Collectively from the above observations, two facts about the CO2RR processes on KB@Cu3(HITP)2 and Cu3(HITP)2 become clear. First, the addition of KB caused the quick reduction of Cuδ+ nodes from the MOF into small Cu0 crystallites and helped stabilize them thereafter. Without the conducting support, the metal nodes were reduced more slowly but easily aggregated. We attribute this phenomenon mainly to the high current density and homogenized charge distribution on the electrode endowed by the conducting support. This is indeed analogous to the process of dendrite formation that is often observed on the anodes of metal ion batteries, where improved charge delocalization on the less-conductive solid–electrolyte interface (SEI) would greatly suppress dendrite growth36,37. Likewise, in the current case of Cu3(HITP)2 the gradually reduced Cu species tend to form aggregates due to inhomogeneous surface polarization and inadequate nuclei seeding, whereas for KB@Cu3(HITP)2 the conducting substrate can afford better charge transport to effectively reduce the metal nodes and dissipate localized charges for mitigating Cu agglomeration. Second, smaller crystallites of Cu reduced out from the MOF were more beneficial for C2H4 production22,28, whereas larger Cu particles tend to induce severe hydrogen evolution reaction. In between, a transition phase might be favorable for yielding CH4.
Control studies with naked Cu nanoparticles
In order to see whether similar FE trends could also be observed for naked Cu nanocrystals when dispersed over a carbon support or whether it is related to the utilization of MOF precursors, we mixed commercial Cu nanoparticles (10–30 nm) with KB (denoted as KB@CuNPs) and tested their CO2RR activity in H-cell. With the same mass loading, KB@CuNPs exhibited lower total current densities than those of KB@Cu3(HITP)2 at all applied potentials (Supplementary Fig. 13), indicative of inferior catalytic activity. The FEs of C2H4 for KB@CuNPs reached a maximum of 48% at −1.56 V (Supplementary Fig. 14a), which is lower than that of KB@Cu3(HITP)2 (~65%) at the same potential. At lower potentials, KB@CuNPs showed higher H2 selectivity with FEs 3–4 times higher than those of KB@Cu3(HITP)2. In addition, the C2H4 selectivity of KB@CuNPs was unstable on the time scale, with the FE dropped from 47 to 11% within 8 h at the applied potential of −1.56 V (Supplementary Fig. 14b). Meanwhile, the FE of H2 increased from 39 to 74%. Such differences in activity and selectivity between KB@Cu3(HITP)2 and KB@CuNPs underline the value of using MOF precursors to derive active Cu moieties toward enhanced CO2RR performance. Based on this comparative study, we surmise that the crystalline structure of the MOF-derived Cu crystallites and their interaction with residual ligands both contribute to the observed performance enhancement. As part of the evidence, TEM images showed that the Cu nanoparticles in KB@CuNPs agglomerated into larger nanoparticles after 8 h of electrolysis (Supplementary Fig. 15), coinciding with the decrease in C2H4 and increase in H2 selectivity. By contrast, the Cu crystallites in KB@Cu3(HITP)2 did not agglomerate over time, which implies the possible tethering effect of the residual ligands in addition to the effect of KB-promoted charge delocalization. The agglomeration of Cu nanoparticles on carbon paper after prolonged CO2RR was also previously observed by Yang and colleagues38 Above all, our control study with naked Cu nanoparticles underscores the unique advantage of MOF precursors to derive highly active Cu motifs upon reconstruction.
Mechanistic comprehension of the C2H4 selectivity on Cu nano-crystallites
To help further comprehend the observed structure–performance correlations from the aspect of atomic corrugations, we sought spherical aberration-corrected TEM (Cs-TEM) to dissect the lattice structure of the Cu nano-crystallites and nanoparticles derived from KB@Cu3(HITP)2 and Cu3(HITP)2, respectively, as well as DFT calculations to rationalize the associated C–C coupling process. Despite the much smaller particle size, Cu crystallites derived from KB@Cu3(HITP)2 after 10-h CO2RR test exhibited rich facets and grain boundaries, as well as lattice defects, in TEM images. For instance, as shown in Fig. 5a and insets39, various lattice planes of (111), (100), (220), etc. converged and were adjacent to each other, forming a highly complex lattice structure that are characteristic of pre-matured crystallites40,41. The small Cu crystallites were then stacked upon one and another, dispersed in the amorphous carbon matrix containing evenly distributed N elements that were inherited from the MOF ligands (Supplementary Figs. 16 and 17). On the other hand, the lattice structure of Cu particles reduced from Cu3(HITP)2 was relatively dull, containing less grain boundaries and defects. As shown in Fig. 5b and Supplementary Fig. 18, lattice fringes of the (111) plane with a d-spacing of 0.21 nm composed the main theme of the images, manifesting a more matured crystallinity. We attribute the observed difference in lattice diversity mainly to the relatively kinetics-driven Cu nucleation in KB@Cu3(HITP)2 but more of the thermodynamics-driven Cu growth in Cu3(HITP)2. That is to say, the as-formed Cu crystallites in KB@Cu3(HITP)2 were “frozen” in the earlier nucleation stage of crystal growth, where the packing of Cu atoms was more chaotic, leading to diverse lattice features40. In contrast, the reduced Cu nuclei in Cu3(HITP)2, with less population, tend to grow into lager conglomerates exposing less lattice features owing to Ostwald ripening41.
Fig. 5. CS-TEM images and DFT simulation.
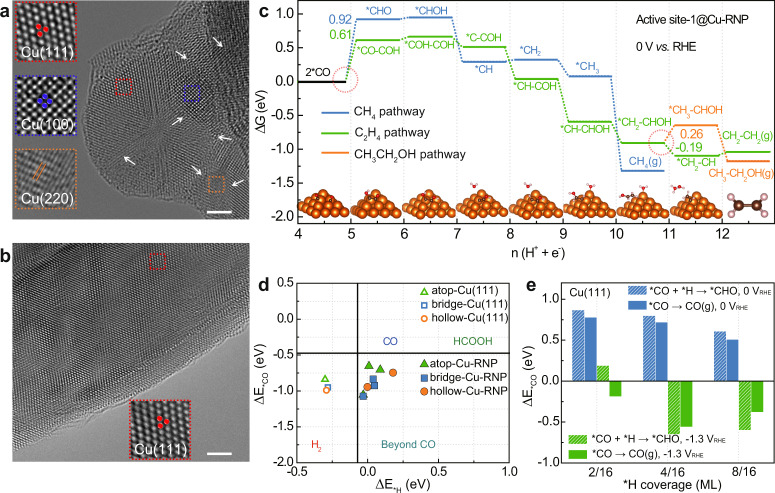
TEM images of a KB@Cu3(HITP)2 and b Cu3(HITP)2 after CO2RR conducted at −1.25 V for 10 h in CO2-saturated 0.1 M KHCO3 electrolyte. c Reaction pathways starting with 2 *CO identified on active site-1 of [101] Cu rectangular nanopyramid (Cu-RNP) surface to model the post-electrolytic KB@Cu3(HITP)2 at 0 V. Insets are the atomic structures of intermediates along the C2H4 pathway. The key bifurcating points are highlighted with red circles and the unit of energy is eV. d Relation between ΔE*CO and ΔE*H at various sites on the Cu-RNP and Cu (111) surfaces. Vertical and horizontal black lines represent the equilibrium potential for *H ↔ ½ H2 and *CO ↔ CO, respectively and e the energetics of reactions *CO + *H → *CHO and *CO → CO (g) on the modeled post-electrolytic Cu3(HITP)2 surface of Cu (111) under various *H coverages and bias potentials. The scale bars in a, b are 2 nm.
Learning the highly diverse lattice features of Cu nano-crystallites from post-electrolytic KB@Cu3(HITP)2 and the relatively dull lattice structure of Cu particles reduced from Cu3(HITP)2, we now stand on a good position to construct atomic models for simulating the CO2RR process. The difference in lattice configuration may result in non-identical electrochemical selectivity during CO2RR as suggested by previous research42. Therefore, in the following DFT study we focus on the effect of lattice configuration on the selectivity for C2 (e.g., ethylene) versus C1 (e.g., methane) production.
The post-electrolytic KB@Cu3(HITP)2 sample was modeled by [101] Cu rectangular nanopyramids (Cu-RNPs)43 as shown in Supplementary Fig. 19. The single Cu-RNP model is based on a rectangular 5 × 5 × 1 Cu (101) surface; the four triangular sidewalls of the nanopyramid are (100) and (111) facets at intervals. This model is in good agreement with Cs-TEM observation that (100) and (111) facets are converged and adjacent to each other (Fig. 5a). Moreover, the Cu-RNPs are in dense array with 5.1 Å between adjacent nanopyramids, which represents the small Cu crystallites stacked and dispersed on the substrate (Supplementary Fig. 16). Meanwhile, the post-electrolytic Cu3(HITP)2 surface with a uniform lattice structure was represented by a 4 × 4 × 4 Cu(111) supercell (Supplementary Fig. 20).
Three active sites were identified on Cu-RNP that evenly distribute on the symmetrical sidewalls (Supplementary Fig. 21). We then carried out DFT calculations to acquire the full reaction pathway as proposed by Cheng et al.44 toward main products observed in the experiment, including CH4 and C2H4. The reaction pathways are summarized in Fig. 5c and Supplementary Figs. 22 and 23, with free energy for each reaction step given in Supplementary Tables 3 and 4. From these reaction pathways, further reduction of 2*CO on Cu-RNP proceeds in two major pathways, namely, (1) C1 pathway (blue)—a conventional pathway toward CH4 formation and (2) C2 pathway which bifurcates from *CH2–CHOH to C2H4 (green) and C2H5OH (orange). The direct hydrogenation after *CO adsorption (2*CO + H+ + e– → *CHO + *CO) has the highest endothermic energy in C1 pathway, serving as the potential-determining step (PDS). PDS for the C2 pathway is *CO dimerization and subsequent proton-coupled electron transfer (PCET) to *COH–CO (2*CO + H+ + e– → *COH–CO). We also found that the limiting potential for C2 pathway on all active sites of Cu-RNP are significantly lower than those of C1 pathway (−0.61 to −0.68 V vs −0.84 to −0.92 V), which indicates that CO2RR can proceed preferentially toward C2 with an inhibited CH4 selectivity on Cu-RNP surface. In addition, for Cu-RNP C2 pathway, the proceeding of 11th PCET to *CH3–CHOH is endergonic with an uphill ΔG = 0.26 eV, in contrast to the exergonic protonation to *CH2–CH (ΔG = −0.19 eV), suggesting a low C2H5OH selectivity on the surface. All these are consistent with the experimental observation that on KB@Cu3(HITP)2 the FE for C2H4 production is significantly higher than those of CH4 and C2H5OH (Supplementary Fig. 8a).
The promoted C–C coupling and consequent high C2H4 selectivity on KB@Cu3(HITP)2 can be attributed to the enhanced *CO adsorption and geometric effect induced by rich grain boundaries and multi-facets on the reduced Cu nano-crystallites. As shown in Supplementary Fig. 24, the *CO binding on the boundary sites of Cu-RNP is significantly enhanced compared with those on basal sites and Cu(111). A strong *CO binding is highly sought after; for example, experimental efforts attribute the high C–C coupling activity to the strong *CO binding and the resulting high *CO coverage45,46. More importantly, on Cu-RNP along the grain boundaries, the strong *CO adsorption site and the adjacent weak adsorption site coexist within an under-coordinated surface square area (Supplementary Fig. 21), which meets the extended square principles criteria toward a promoted C–C coupling43. Consequently, the formation of *COH–CO on Cu-RNP is highly favored over that of *CHO and the C2 pathway is thermodynamically more selective than C1 pathway.
In addition to the promoted C–C coupling, the rich grain boundaries and multi-facets of Cu nano-crystallites on post-electrolytic KB@Cu3(HITP)2 can further contribute to higher C2 selectivity by inhibiting competitive HER. We adopted the selectivity determining method proposed by Rossmeisl and colleagues6 to indicate product selectivity on the constructed models by *H and *CO binding energy. The results are summarized in Fig. 5e, which shows that all active sites from the Cu(111) surface (Supplementary Fig. 25a) fall in the HER dominant zone. In contrast, all active sites from the Cu-RNP surface (Supplementary Fig. 25b) fall in the CO2RR dominant zone. The calculated selectivity on the two models is in good agreement with the experimental observation of stable C2H4 production with suppressed H2 yield on KB@Cu3(HITP)2.
It is worth noting that on Cu3(HITP)2 the selectivity of C2H4 starts to decrease from −1.3 V with a continuous increase in H2 and CH4 (Fig. 2b). We attribute the decline of C2H4 to the *H site-blocking effects under negative potentials. It was reported that dominant *H can partially displace *CO under very negative potential, and the resultant low *CO coverage impedes the *CO dimerization, hence C2H4 production44. We also observed that CH4 production attained the maximum FE of 53% after −1.0 V vs RHE with negligent amount of CO (Fig. 2b). The main reason lies in that Cu3(HITP)2 exposes mainly Cu(111) facets (Fig. 5b), which are more selective toward CH4 than CO under negative potentials according to previous experimental research47,48. To reveal the origin of CH4 selectivity over CO on Cu(111) surfaces, we simulated the key reaction step toward CH4 formation: *CO + *H → *CHO, and competing *CO desorption step: *CO → CO(g) on Cu(111) (Supplementary Fig. 26). During the simulation, different electrode potentials (0 and −1.3 V vs RHE) were considered by applying the constant electrode potential (CEP) method49. At the same time, different surface *H coverages were considered (i.e., 2/16 ML, 4/16 ML and 8/16 ML), because previous experimental studies suggest that the coverage will increase to over 0.3 ML at high overpotential50,51. We found that, on Cu(111) at 0 V vs RHE, both the *CHO formation and *CO desorption activities improve from low *H coverage (2/16 ML) through medium *H coverage (4/16 ML) to high *H coverage (8/16 ML), as shown in Fig. 5e. With bias potential of −1.3 V vs RHE, both reaction steps become exergonic, while the former reaction is more thermodynamically favorable with more negative energy changes (∼−0.64 vs −0.38 eV). The difference in energy change means that the reaction pathway proceeds toward CH4 production at very negative potential via the *CHO formation, instead of CO release through *CO desorption. This conclusion is consistent with previous experimental research47,48, as summarized in Supplementary Table 5.
Universality of the observations by extending to flow cell and Cu3(HHTP)2
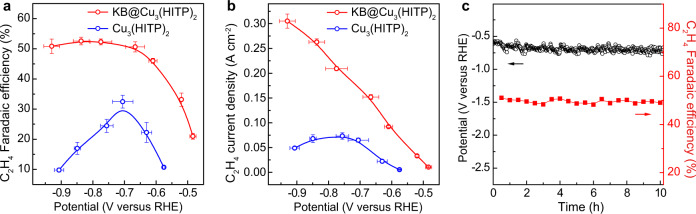
As the CO2RR kinetics are restricted by mass transportation in the H cell due to limited solubility of CO2 in aqueous electrolytes52, flow-cell tests were further carried out in 1 M KOH to achieve high current densities (>100 mA cm−2, Supplementary Fig. 27). For a better quantification of FEs, the chronopotentiometric mode was employed. Despite the different electrolyte from that utilized in H cell, KB@Cu3(HITP)2 still showed notably higher current densities than those of Cu3(HITP)2 (Supplementary Fig. 28). In addition, the onset potential of C2H4 production for KB@Cu3(HITP)2 was −0.48 V, which is earlier than that for Cu3(HITP)2 (−0.58 V). For KB@Cu3(HITP)2, the FE of C2H4 reached 51% at −0.67 V and stayed above it till −0.93 V (Fig. 6a), whereas that for Cu3(HITP)2 displayed a maximum of 32% at −0.71 V but thereafter gradually decreased to below 5% at −1.02 V (Fig. 6a and Supplementary Fig. 29). As for CH4 production, the FE for KB@Cu3(HITP)2 was trivial and remained <9% during the entire CO2RR test, while that for Cu3(HITP)2 continued to increase with decreasing potentials until a maximum of 53% at −0.85 V (Supplementary Fig. 29). Besides, the presence of KB inhibited HER, especially at more negative potentials. Consistently, KB@Cu3(HITP)2 exhibited higher C2H4 partial current densities than Cu3(HITP)2 did at all applied potentials and reached 305 mA cm−2 at −0.93 V (Fig. 6b). KB@Cu3(HITP)2 also showed good electrocatalytic stability in the flow cell, which stably operated over 10 h at a current density of 300 mA cm−2 (Fig. 6c). In general, the flow-cell tests on KB@Cu3(HITP)2 and Cu3(HITP)2, despite involving much higher current densities, showed similar trends of product selectivity to those in the H cell, corroborating the positive role of KB in promoting and stabilizing the C2H4 selectivity.
Fig. 6. Electrocatalytic CO2RR performance in the flow cell.
a FEs and b partial current densities of C2H4 at different potentials tested in the flow cell with 1 M KOH electrolyte for KB@Cu3(HITP)2 and Cu3(HITP)2. c Stability test of KB@Cu3(HITP)2 during 10 h of electrolysis under the current density of 300 mA cm−2. Error bars represent the standard deviation of three independent measurements.
To testify the universality of the above phenomenon with respect to the KB effect on promoting and stabilizing ethylene production for semi-conductive Cu MOFs that are stable in alkaline conditions, we synthesized another similar MOF of Cu3(HHTP)2 (HHTP = 2,3,6,7,10,11-hexahydroxytriphenylene) by replacing the amine groups in HITP with hydroxyls (Supplementary Figs. 30 and 31). As expected, similar CO2RR behaviors to that of Cu3(HITP)2 were also observed for Cu3(HHTP)2 (Supplementary Fig. 32). On KB@Cu3(HHTP)2, the FE of C2H4 was plateaued after −1.29 V (Supplementary Fig. 32a), coinciding with the stabilized Cu particle size at varying potentials from −1.22 to −1.65 V (Supplementary Fig. 33a–d). As for the stand-alone Cu3(HHTP)2, both the FEs of C2H4 and CH4 increased first and then decreased, concomitant with the “V”-shape voltage dependence of H2 FEs (Supplementary Fig. 32b), which was also observed previously for Cu3(HITP)2 (Fig. 2b). Correspondingly, the serial TEM images taken on the post-electrolytic Cu3(HHTP)2 samples at different potentials showed the prominent growth of Cu particle size with increasing bias (Supplementary Fig. 33e–h). Thus, for both Cu3(HITP)2 and Cu3(HHTP)2, the addition of KB enables steering the CO2RR pathway toward ethylene production by preserving the reduced Cu in their nano-crystallite state, which, as aforementioned, is induced and stabilized via high current shock and surface charge delocalization.
Discussion
CO2RR behaviors of the semi-conductive MOF—Cu3(HITP)2—were studied with and without conducting support. It was found adding KB greatly promoted the C2H4 production with a stabilized FE between 60 and 70% in a wide potential range and prolonged test, whereas the stand-alone MOF produced more mixed reduction products during the reaction course. Operando XAS in conjunction with ex situ time-lapse XRD and TEM analyses clearly revealed that in the presence of KB tiny Cu crystallites were quickly reduced from the MOF and stabilized thereafter. By contrast, on Cu3(HITP)2 in the absence of KB, Cu nanoparticles were gradually reduced out and aggregated into larger sizes, which can be attributed to the poor surface charge delocalization, analogous to the process of dendrite growth at the SEI of metal ion batteries. By scrutinizing the detailed lattice structure using CS-TEM, a structural model comprising multi-facets and grain boundaries was established via DFT to successfully rationalize the promoted C–C coupling on the Cu nano-crystallites derived from KB@Cu3(HITP)2. As a result, our study furnishes fresh insights into the steering of CO2RR pathway by regulating and stabilizing Cu crystalline states via conditioning the charge transport on electrodes, which can be possibly extended to other metal–organic complexes.
Methods
Materials
Copper(II) sulfate pentahydrate (CuSO4·5H2O) was purchased from Sinopharm Chemical Reagent Co., Ltd. Triphenylene-2,3,6,7,10,11-hexaamine 6HCl (HATP·6HCl ≥98%) was provided by Shanghai Kaiyulin Co., Ltd. Triphenylene-2,3,6,7,10,11-hexaol (HHTP, ≥97%) was provided by Bide Pharmatech Ltd. Sodium acetate trihydrate (C2H3O2Na·3H2O, ≥99.5%) and cupric acetate anhydrous (Cu(C2H3O2)2) were obtained from Aladdin. Potassium bicarbonate (KHCO3, ≥99.5%) were purchased from J&K Scientific Co., Ltd. N,N-dimethylmethanamide (DMF, ≥99.5%) and N,N-dimethylacetamide (DMA, ≥99.5%) were purchased from Greagent. Ethanol (C2H6O, ≥99.7%) was provided by Shanghai Lingfeng Chemical Reagent Co., LTD. The CO2 gas (99.995%) was supplied by Suzhou Jinhong Gas Co. Ltd. All materials were used as received without further purification. The Cu NPs were purchased from Macklin. Deionized (DI) water was purified with a Sartorius arium mini ultrapure water system.
Synthesis of Cu3(HITP)2
In all, 7 mg (0.028 mmol) of CuSO4·5H2O was suspended in 3 mL of DMA and sonicated for 10 min. Then a solution of 10 mg (0.0186 mmol) of HATP·6HCl in 3 mL of water was added and sonicated for 10 min. A total of 4 mL of C2H3O2Na aqueous solution (2 M) was then added at room temperature, and the mixture was heated in a 50 mL open glass vial with stirring for 2 h at 65 °C. The resulting black powder was filtered, washed with large amount of water and methanol, and dried under vacuum.
Synthesis of Cu3(HHTP)2
In all, 28.3 mg (0.156 mmol) of Cu(C2H3O2)2 and 14 mg (0.044 mmol) of HHTP was suspended in 3 mL of DI water and sonicated for 5 min. A total of 0.3 ml DMF was then added dropwise to this mixture followed by 5 min of sonication. The capped vial was heated at 80 °C for 6 h. The resulting blue powder was centrifuged, washed with large amount of water and ethanol, and dried under vacuum.
Characterizations
The crystalline phases of the products were analyzed by powder XRD measurements with a Bruker D8 Advance X-ray diffractometer using Cu-Ka radiation. One milligram of powder catalyst was loaded on 1 × 1 cm2 of carbon paper for ex situ time-lapse XRD testing. The surface composition and valence states were analyzed with XPS, using an Escalab 250Xi X-ray photoelectron spectrometer (Thermo Fisher) with Al Ka (1486.6 eV) X-rays as the excitation source, and the binding energy of the C 1s peak at 284.8 eV was taken as an internal reference. The pass energy was 30 eV and the photoemission angle was 45°. The energy linearity detection was calibrated with Au 4f (83.96 eV), Ag 3d5 (368.21), and Cu 2p (932.62 eV). The morphologies were examined by SEM conducted on a Hitachi SU8010 scanning electron microanalyzer with an accelerating voltage of 10 kV. The microstructures of the products were further characterized by TEM measured at 200 kV with a FEI TECNAI G20 field-emission TEM. Cs-TEM (FEI Titan Themis Cubed G2 300) was used to inspect atomic orientation of samples.
Electrochemical measurements
The CO2 electro-reduction reactions were performed in a gas-tight, two-compartment H-cell controlled with an electrochemical workstation (CHI760E). Thereinto, the two compartments of H-cell are separated by an ion exchange membrane (Nafion perfluorinated membrane), and each compartment was filled with 40 mL of 0.1 M KHCO3. A carbon rod was used as the counter electrode, and a calibrated Ag/AgCl electrode was used as the reference electrode. To prepare the catalyst ink for H-cell studies, 4 mg of the sample powder and 1 mg KB were mixed with 50 µL Nafion solution (5%) in 1 mL ethanol by ultrasonic dispersion for 15 min. Next, 10 µL of the as-prepared ink was drop-coated onto the glassy carbon electrode with a surface area of 0.197 cm−2. The working electrode was then dried at room temperature for subsequent testing. Before electrochemical measurements, a continuous CO2 was purged into the cathodic compartment for at least 30 min. During the measurements, the CO2 was bubbled into the electrolyte with a constant flow rate of 20 cm3 min−1 controlled by a digital mass flow controller. The activation of the working electrode was carried out by repeatedly running cyclic voltammetry within the potential range from −0.5 to −1.8 V with a rate of 10 mV s−1 for 20 min.
The flow cell comprises three chambers: gas, catholyte (gas diffusion electrode, YLS-30T), and anolyte (nickel foam, 0.8 mm thickness). The Ag/AgCl reference electrode was plugged in the center of catholyte chamber via a top drill hole. Catholyte and anolyte were separated by anion exchange membrane (Fumasep FAB-PK-130). Gas and catholyte were separated by the gas diffusion electrode. Using an electric airbrush, the catalyst dispersed in ethanol was spray-coated on the side of the gas diffusion electrode facing the electrolyte (0.8 mg cm−2), with the opposite side facing the gas chamber. Silicone gaskets with 2 × 0.5 cm2 window were placed to assure adequate sealing of each chamber. The high-purity CO2 was supplied to the gas chamber with a constant flow rate of 30 cm3 min−1 monitored by a digital mass flow controller (Horiba). The flow-cell measurements were conducted in 1 M KOH with a constant flow rate of 20 ml min−1 through a dual-channel peristaltic pump. The pH of 1 M KOH decreased slightly from 13.7 to 13.3 after 10 h of reaction (Supplementary Fig. 34). The cell resistances were measured via electrochemical impedance spectroscopy under open circuit, and 85% ohmic resistance correction was applied in all H-cell and flow-cell measurements.
The gas products were quantitatively analyzed using gas chromatography equipped with both flame ionization and thermal conductivity detectors (Agilent 7890B). The liquid products were collected after at least 5 h of electrolysis and quantitatively analyzed using 1H NMR spectroscopy with H2O suppression. In all, 400 µL electrolyte mixed with 50 µL dimethyl sulfoxide (20 mM) and 100 µL D2O was used as the internal standard. All the potentials were converted to RHE, according to the equation E (vs RHE) = E (vs Ag/AgCl) + 0.059 × pH + 0.198.
Operando XAS measurements
The operando XAS spectra at the Cu K-edge were recorded at the BL11B beamline of Shanghai Synchrotron Radiation Facility. The electron storage ring operated at 3.5 GeV. The beam current of the storage ring was 220 mA in a top-up mode. The incident photons were monochromatized by a Si(111) double-crystal monochromator, with an energy resolution ΔE/E ~ 1.4 × 10−4. The rejection of higher harmonics was achieved by a pair of Rh-coated mirrors at 4 mrad. The spot size at the sample was ~200 μm × 250 μm (H × V). The energy calibration was performed using a Cu foil. The operando XAS spectra were recorded in the fluorescence mode using a Lytle detector. The electrolysis was performed in CO2-saturated 0.1 M KHCO3 solution in a two-compartment H-shape cell. The sample was at 45° with respect to the incident beam direction. The electrodes were prepared as described in the “Electrochemical measurement” section. CO2 was bubbled into the cell with a constant flow rate during the operando experiments. Kapton tape was used to seal the cell. The operando XAS spectra were measured at a potential range from 0 to −1.42 V (keeping a constant potential when recording the spectrum). The running time for each spectrum was 8 min. XAS data were analyzed using the Athena software. All the XAS spectra were normalized to unity in Athena. The FT-EXAFS spectra were calculated between 3.0 and 11.0 Å−1 after weighting by k3.
Computational methods
All DFT calculations were performed with the Vienna Ab Initio Simulation Package (VASP) code53. The Perdew–Burke–Ernzerhof was employed for electron exchange–correlation54. Projector Augmented Wave potentials were used to describe the ionic cores55. The atomic relaxations were carried out with the quasi-Newton minimization scheme until the maximum force on any atom was <0.03 eV Å−1. The geometry optimizations were performed with a plane-wave cutoff of 400 eV. Irreducible 2 × 3 × 1 or 2 × 2 × 1 Monkhorst Pack k-point grid was used56, with the center shifted to the gamma point. The Fermi level was smeared with the Methfessel–Paxton approach with a smearing of 0.1 eV. Dipole corrections were included in all the calculations to minimize the inaccuracies in the total energy due to the simulated slab interactions. The dipole moment was calculated parallel to the z-direction.
The implicit solvent effect was considered by using VASPsol57. The solvent dielectric constant was set to be 78.4, the width of dielectric cavity was 0.6 Å, the cutoff charge density was set to be 0.0025, and the effective cavity surface tension was 0.000525.
The lattice constant of Cu was optimized to be 3.64 Å in its fcc crystal structure. The Cu [101] RNPs were built based on a rectangular 5 × 5 × 1 Cu (101) surface; the four triangular sidewalls of the nanopyramid are (100) and (111) facets at intervals. The base layers were fixed while nanopyramids and adsorbates were permitted to fully relax in all configurations. The Cu (111) surfaces was constructed with 4 × 4 × 4 supercells, respectively, both with two bottom layers fixed. Two topmost layers and adsorbates were free to move in all directions. The vertical separation between periodically repeated images was set to be at least 15 Å in all cases, to ensure no interaction between images.
The free energies of adsorbed species, calculated based on the computational hydrogen electrode model55, are defined as:
| 1 |
where ZPE is the zero-point energy correction and T∆S is the entropy correction, which were computed with standard methods58.
The formation free energy of *COH–CO (ΔG*COH–CO) is calculated as
| 2 |
where Gtotal is the free energy of a surface with adsorbed *COH–CO, G2*CO is the free energy of a surface with two adsorbed CO molecules, and GH2 is the energy of a hydrogen molecule, which is −6.83 eV.
The formation free energy of *CHO (ΔG*CHO) is calculated as
| 3 |
where Gtotal is the free energy of a surface with adsorbed *CHO and G*CO is the free energy of a surface with one adsorbed CO molecule. The free energy of relevant gas phases are listed in Supplementary Table 6.
Limiting potential (Ulimiting) is used to describe the lowest potential requirement to eliminate the free energy difference of the PDS, calculated as:
| 4 |
We applied the CEP model proposed by Head-Gordon and colleagues49 to consider the effect of applied potential on reactions. We simulated each surface with different electron numbers, i.e., −2|e|, 0|e|, and +2|e|. We then carried out potential calculation to obtain work function of each surface and corresponding electrode potentials. The electrode potential (U) of the slab relative to the normal hydrogen electrode (NHE) can be estimated from the work function, φ, relative to that of the NHE (φNHE).
| 5 |
where φNHE = 4.5 V as the work function of the reference system59. The linear relationship of electrode potentials and electron numbers on different models are presented in Supplementary Fig. 26. From Supplementary Fig. 26, different numbers of electrons were assigned to corresponding surfaces to simulate the desired electrode potential as shown in Supplementary Table 7.
Supplementary information
Acknowledgements
This work is supported by National Key R&D Program of China (Grant No. 2020YFB1505703), National Natural Science Foundation of China (Nos. 22072101, 22075193, U1932211), Natural Science Foundation of Jiangsu Province (No. BK20211306), Six Talent Peaks Project in Jiangsu Province (No. TD-XCL-006), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. This research is also supported by the Australian Research Council through these programs: FT190100636 and DP190103472. DFT computations for this research were undertaken with the assistance of supercomputing resources provided by the Phoenix HPC service at the University of Adelaide and the National Computational Infrastructure (NCI), which is supported by the Australian Government. The authors also thank for the support from the Honorary Professor Program of Jiangsu Province, National Key R&D Program of China (2020YFA0406103), and Collaborative Innovation Center of Suzhou Nano Science & Technology and Users with Excellence Program of Hefei Science Center CAS (2019HSC-UE002).
Author contributions
Y.P. conceived the idea and led the project. H.S. designed and performed the majority of the experiments and obtained most of the results, including material synthesis, characterization, and electrochemical tests. L.C. and Y.J. carried out the DFT calculations. K.F., Y.C. and J.Z. carried out XAS characterizations. Y.L. and M.H.R. took the Cs-TEM images and analyzed the data. Z.D., X.Y. and B.Y. helped to polish the paper. Z.D., L.X. and X.Z. devoted to the electrochemical data analysis. H.S., L.C., Z.D., Y. J. and Y.P. wrote the paper. All the authors have discussed the results.
Data availability
The data that support the findings of this study are available from the authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Christoph Baeumer and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hao Sun, Ling Chen.
Contributor Information
Jun Zhong, Email: jzhong@suda.edu.cn.
Yan Jiao, Email: yan.jiao@adelaide.edu.au.
Yang Peng, Email: ypeng@suda.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-27169-9.
References
- 1.Obama B. The irreversible momentum of clean energy. Science. 2017;355:126–129. doi: 10.1126/science.aam6284. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong RC, et al. The frontiers of energy. Nat. Energy. 2016;1:15020. [Google Scholar]
- 3.De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science364, eaav3506 (2019). [DOI] [PubMed]
- 4.Bushuyev OS, et al. What should we make with CO2 and how can we make it? Joule. 2018;2:825–832. [Google Scholar]
- 5.Nitopi S, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019;119:7610–7672. doi: 10.1021/acs.chemrev.8b00705. [DOI] [PubMed] [Google Scholar]
- 6.Bagger A, Ju W, Varela AS, Strasser P, Rossmeisl J. Electrochemical CO2 reduction: a classification problem. Chemphyschem. 2017;18:3266–3273. doi: 10.1002/cphc.201700736. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Ramirez J, Lopez N. Strategies to break linear scaling relationships. Nat. Catal. 2019;2:971–976. [Google Scholar]
- 8.Ross MB, et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2019;2:648–658. [Google Scholar]
- 9.Choi C, et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 2020;3:804–812. [Google Scholar]
- 10.Kim MJ, et al. Ethylenediamine promotes Cu nanowire growth by inhibiting oxidation of Cu(111) J. Am. Chem. Soc. 2017;139:277–284. doi: 10.1021/jacs.6b10653. [DOI] [PubMed] [Google Scholar]
- 11.Cheng T, Xiao H, Goddard WA. Nature of the active sites for CO reduction on copper nanoparticles; suggestions for optimizing performance. J. Am. Chem. Soc. 2017;139:11642–11645. doi: 10.1021/jacs.7b03300. [DOI] [PubMed] [Google Scholar]
- 12.Zhong D, et al. Coupling of Cu(100) and (110) facets promotes carbon dioxide conversion to hydrocarbons and alcohols. Angew. Chem. Int. Ed. 2021;60:4879–4885. doi: 10.1002/anie.202015159. [DOI] [PubMed] [Google Scholar]
- 13.Xiong L, et al. Breaking the linear scaling relationship by compositional and structural crafting of ternary Cu-Au/Ag nanoframes for electrocatalytic ethylene production. Angew. Chem. Int. Ed. 2021;60:2508–2518. doi: 10.1002/anie.202012631. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy. 2020;5:623–632. [Google Scholar]
- 15.De Luna P, et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018;1:103–110. [Google Scholar]
- 16.Favaro M, et al. Subsurface oxide plays a critical role in CO2 activation by Cu(111) surfaces to form chemisorbed CO2, the first step in reduction of CO2. Proc. Natl Acad. Sci. USA. 2017;114:6706–6711. doi: 10.1073/pnas.1701405114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy. 2020;5:478–486. [Google Scholar]
- 18.Nam DH, et al. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020;19:266–276. doi: 10.1038/s41563-020-0610-2. [DOI] [PubMed] [Google Scholar]
- 19.Gu H, et al. Graphdiyne/graphene heterostructure: a universal 2D scaffold anchoring monodispersed transition-metal phthalocyanines for selective and durable CO2 electroreduction. J. Am. Chem. Soc. 2021;143:8679–8688. doi: 10.1021/jacs.1c02326. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Jiang Z, Lu X, Liang Y, Wang H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature. 2019;575:639–642. doi: 10.1038/s41586-019-1760-8. [DOI] [PubMed] [Google Scholar]
- 21.Moller T, et al. Electrocatalytic CO2 reduction on CuOx nanocubes: tracking the evolution of chemical state, geometric structure, and catalytic selectivity using operando spectroscopy. Angew. Chem. Int. Ed. 2020;59:17974–17983. doi: 10.1002/anie.202007136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung H, et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C-C coupling from CO2 reduction reaction. J. Am. Chem. Soc. 2019;141:4624–4633. doi: 10.1021/jacs.8b11237. [DOI] [PubMed] [Google Scholar]
- 23.Yi JD, et al. Highly selective CO2 electroreduction to CH4 by in situ generated Cu2O single-type sites on a conductive MOF: stabilizing key intermediates with hydrogen bonding. Angew. Chem. Int. Ed. 2020;59:23641–23648. doi: 10.1002/anie.202010601. [DOI] [PubMed] [Google Scholar]
- 24.Wang YR, et al. Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat. Commun. 2018;9:4466. doi: 10.1038/s41467-018-06938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay S, et al. Assembly of a metal-organic framework (MOF) membrane on a solid electrocatalyst: introducing molecular-level control over heterogeneous CO2 reduction. Angew. Chem. Int. Ed. 2021;60:13423–13429. doi: 10.1002/anie.202102320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam DH, et al. Intermediate binding control using metal-organic frameworks enhances electrochemical CO2 reduction. J. Am. Chem. Soc. 2020;142:21513–21521. doi: 10.1021/jacs.0c10774. [DOI] [PubMed] [Google Scholar]
- 27.Yao D, et al. The controllable reconstruction of Bi-MOFs for electrochemical CO2 reduction through electrolyte and potential mediation. Angew. Chem. Int. Ed. 2021;60:18178–18184. doi: 10.1002/anie.202104747. [DOI] [PubMed] [Google Scholar]
- 28.Nam DH, et al. Metal-organic frameworks mediate Cu coordination for selective CO2 electroreduction. J. Am. Chem. Soc. 2018;140:11378–11386. doi: 10.1021/jacs.8b06407. [DOI] [PubMed] [Google Scholar]
- 29.Zheng W, Lee LYS. Metal–organic frameworks for electrocatalysis: catalyst or precatalyst? ACS Energy Lett. 2021;6:2838–2843. [Google Scholar]
- 30.Chen T, et al. Continuous electrical conductivity variation in M3(hexaiminotriphenylene)2 (M = Co, Ni, Cu) MOF alloys. J. Am. Chem. Soc. 2020;142:12367–12373. doi: 10.1021/jacs.0c04458. [DOI] [PubMed] [Google Scholar]
- 31.Campbell MG, Sheberla D, Liu SF, Swager TM, Dinca M. Cu3(hexaiminotriphenylene)2: an electrically conductive 2D metal-organic framework for chemiresistive sensing. Angew. Chem. Int. Ed. 2015;54:4349–4352. doi: 10.1002/anie.201411854. [DOI] [PubMed] [Google Scholar]
- 32.Campbell MG, Liu SF, Swager TM, Dinca M. Chemiresistive sensor arrays from conductive 2D metal-organic frameworks. J. Am. Chem. Soc. 2015;137:13780–13783. doi: 10.1021/jacs.5b09600. [DOI] [PubMed] [Google Scholar]
- 33.Lian Y, et al. Unpaired 3d electrons on atomically dispersed cobalt centres in coordination polymers regulate both oxygen reduction reaction (ORR) activity and selectivity for use in zinc-air batteries. Angew. Chem. Int. Ed. 2020;59:286–294. doi: 10.1002/anie.201910879. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, et al. Atypical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction. J. Am. Chem. Soc. 2020;142:11417–11427. doi: 10.1021/jacs.0c01562. [DOI] [PubMed] [Google Scholar]
- 35.Lian Y, et al. Carved nanoframes of cobalt-iron bimetal phosphide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2019;10:464–474. doi: 10.1039/c8sc03877e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin D, Liu Y, Cui Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017;12:194–206. doi: 10.1038/nnano.2017.16. [DOI] [PubMed] [Google Scholar]
- 37.Lin D, et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016;11:626–632. doi: 10.1038/nnano.2016.32. [DOI] [PubMed] [Google Scholar]
- 38.Kim D, Kley CS, Li Y, Yang P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2-C3 products. Proc. Natl Acad. Sci. USA. 2017;114:10560–10565. doi: 10.1073/pnas.1711493114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagger A, Ju W, Varela AS, Strasser P, Rossmeisl J. Electrochemical CO2 reduction: classifying Cu facets. ACS Catal. 2019;9:7894–7899. [Google Scholar]
- 40.Desgranges C, Delhommelle J. Molecular insight into the pathway to crystallization of aluminum. J. Am. Chem. Soc. 2007;129:7012–7013. doi: 10.1021/ja072260n. [DOI] [PubMed] [Google Scholar]
- 41.Leyssale JM, Delhommelle J, Millot C. Reorganization and growth of metastable alpha-N2 critical nuclei into stable beta-N2 crystals. J. Am. Chem. Soc. 2004;126:12286–17027. doi: 10.1021/ja046700b. [DOI] [PubMed] [Google Scholar]
- 42.Luo W, Nie X, Janik MJ, Asthagiri A. Facet dependence of CO2 reduction paths on Cu electrodes. ACS Catal. 2015;6:219–229. [Google Scholar]
- 43.Chen L, Tang C, Jiao Y, Qiao SZ. Anomalous C-C coupling on under-coordinated Cu (111): a case study of Cu nanopyramids for CO2 reduction reaction by molecular modelling. ChemSusChem. 2021;14:671–678. doi: 10.1002/cssc.202002036. [DOI] [PubMed] [Google Scholar]
- 44.Cheng T, Xiao H, Goddard WA., 3rd Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl Acad. Sci. USA. 2017;114:1795–1800. doi: 10.1073/pnas.1612106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang K, et al. Effects of surface roughness on the electrochemical reduction of CO2 over Cu. ACS Energy Lett. 2020;5:1206–1214. [Google Scholar]
- 46.Verdaguer-Casadevall A, et al. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 2015;137:9808–9811. doi: 10.1021/jacs.5b06227. [DOI] [PubMed] [Google Scholar]
- 47.De Gregorio GL, et al. Facet-dependent selectivity of Cu catalysts in electrochemical CO2 reduction at commercially viable current densities. ACS Catal. 2020;10:4854–4862. doi: 10.1021/acscatal.0c00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hori Y, Takahashi I, Koga O, Hoshi N. Selective formation of C2 compounds from electrochemical reduction of CO2 at a series of copper single crystal electrodes. J. Phys. Chem. B. 2002;106:15–17. [Google Scholar]
- 49.Garza AJ, Bell AT, Head-Gordon M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products. ACS Catal. 2018;8:1490–1499. [Google Scholar]
- 50.Elhamid MHA, Ateya BG, Weil KG, Pickering HW. Calculation of the hydrogen surface coverage and rate constants of the hydrogen evolution reaction from polarization data. J. Electrochem. Soc. 2000;147:2148–2150. [Google Scholar]
- 51.Jerkiewicz G. Electrochemical hydrogen adsorption and absorption. Part 1: Under-potential deposition of hydrogen. Electrocatalysis. 2010;1:179–199. [Google Scholar]
- 52.Liu K, Smith WA, Burdyny T. Introductory guide to assembling and operating gas diffusion electrodes for electrochemical CO2 reduction. ACS Energy Lett. 2019;4:639–643. doi: 10.1021/acsenergylett.9b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kresse G, Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 54.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997;78:1396–1396. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 55.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758–1775. [Google Scholar]
- 56.Monkhorst HJ, Pack JD. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976;13:5188–5192. [Google Scholar]
- 57.Mathew K, Sundararaman R, Letchworth-Weaver K, Arias TA, Hennig RG. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014;140:084106. doi: 10.1063/1.4865107. [DOI] [PubMed] [Google Scholar]
- 58.Norskov JK, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B. 2004;108:17886–17892. [Google Scholar]
- 59.Skulason E, et al. Density functional theory calculations for the hydrogen evolution reaction in an electrochemical double layer on the Pt(111) electrode. Phys. Chem. Chem. Phys. 2007;9:3241–3250. doi: 10.1039/b700099e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the authors upon request.