Abstract
As part of an infection management protocol, antimicrobial dressings offer an appropriate, cost‐effective choice for the management of localised bioburden in chronic wounds. The choice of antimicrobial can impact significantly not only on the treatment outcomes and cost but also on the safety and well‐being of the patient. This retrospective study investigates these outcomes comparing health care records of 2572 patients with open chronic wounds, who were treated either with an Integrated Care Wound Bundle (ICB) including nanocrystalline silver (NCS) dressings (n = 330) or without NCS dressings and not on a ICB (n = 2242) in the community from March 2016 to March 2018. Wounds treated in the NCS dressing treatment bundle had a mean healing time of 10.46 weeks, vs 25.49 weeks for the non‐ICB treated wounds. In addition, the average interval time between dressing changes was in favour of the NCS dressing treatment bundle (3.98 vs 1.87 days), contributing to a substantial reduction in mean treatment labour costs ($1251 vs $6488). The use of a NCS dressing demonstrated improved efficacy and cost effectiveness of labour required for chronic wound management; highlighting the importance of choosing an effective antimicrobial dressing as part of an infection management protocol.
Keywords: chronic wounds, community care, cost effective, integrated care bundle, nanocrystalline silver dressings
1. INTRODUCTION
Chronic wounds are those that do not progress through the normal physiological healing trajectory within an expected timeframe. This lengthened healing process, combined with the care that they require, is a major challenge faced by health care organisations around the world. Chronic wounds are frequently accompanied by comorbidities, such as diabetes, vascular disease and obesity, that can inhibit healing and add to the complexity of treatment. 1 Although the true global economic impact of chronic wounds is unknown, it is estimated that in the United States alone, approximately 6.5 to 8.2 million people are affected by chronic wounds. 2 , 3 Given the aging population, it is becoming increasingly likely that the prevalence and incidence of chronic wounds, in combination with comorbidities that are likely to affect healing, will continue to increase; as such the cost of wound care will inevitably rise.
Patients presenting with chronic wounds provide a prime environment for the colonisation of bacteria and are often a high‐risk group for acquiring and harbouring antibiotic‐resistant bacteria. 4 This colonisation of bacteria in chronic wounds, such as venous leg ulcers, pressure injuries and diabetic foot ulcers, often predispose the already compromised patient to life threatening or limb threatening infections. 5 The global threat of antibiotic resistance has led to health care professionals and organisations seeking other means to manage bacteria in the chronic wound to help prevent further medical issues. Silver, for example, has been used in the treatment of burns, ulcerations and infected wounds for hundreds of years, dating as far back as the 17th century. Since the 1960s, other forms of silver such as silver sulphadiazine (SSD), have been incorporated into wound dressings and used to manage bacteria in wounds and further advances in wound care dressings have seen the use of nanocrystalline silver structures integrated into wound dressings. 6 , 7 , 8 Silver is an antimicrobial with broad‐spectrum activity against bacteria (including bacteria resistant to antibiotics), fungi and viruses. Antiseptics such as silver act on multiple targets in a bacterial cell therefore are less likely to cause development of resistance if used appropriately, providing a suitable adjunct to antibiotics for the management of wound infection. 8 , 9 In addition to antimicrobial properties that have been well demonstrated in vitro in the literature, silver dressings are proven to reduce the bacterial load present in an infected wound, in turn reducing the inflammatory response and further aiding the healing process. 10
NCS dressings (ACTICOAT™ Flex 7, Smith+Nephew Ltd., Hull, UK) are highly conformable, single layer polyester dressings coated with nanocrystalline silver technology which provide the sustained release of silver over a 7 day period. 11 The aim of this study was to compare the rate of wound healing, the cost of wound care delivery and the safety for patients receiving the NCS dressing and Integrated Care Wound Bundle (ICB) compared with those not on a bundle and not using a NCS dressing.
2. METHODS
2.1. Institutional review board
Ethics approval for the study was requested and received from the Institutional Review Board (IRB) of D'Youville University prior to conducting the study.
2.2. Participants
This non‐experimental, retrospective study included a total of 2572 patients who were treated for open chronic wounds from admission to healing, either on an ICB containing NCS dressing (n = 330) or not on an ICB and not using a NCS dressing (n = 2242). ICBs drive evidenced‐based practice for wound management as well as triggers to drive the choice of an antimicrobial dressing, gathering standardised clinical assessments and interventions to ensure that their application is consistent for all patients. Those not being treated on an ICB were treated with standard non‐advanced wound care treatments e.g. gauze dressings. All subjects in this study were patients within the service area of the two Community Care Access Centres (CCAC) operating in Toronto, Canada who were receiving care from community nurses for a wound (pressure injuries, diabetic foot ulcer, venous leg ulcers and surgical ulcers (open incision) between March 31, 2016 and March 31, 2018. Baseline data was also collected in December 2015 from patients who received wound care prior to programme implementation. Patients were not contacted during the study and data extracted and analysed does not contain patient identifiers.
The demographic variables included in this study were: age, gender and comorbidities such as diabetes mellitus, cardiac conditions and renal conditions. The Charlson Comorbidity Index was used to apply a systematic and comparable measure of comorbidities.
Excluded from the patient population were those patients who were under 18 years of age, taking immunosuppressant drugs or receiving palliative care, and those patients who had an active infection, positive HIV status or scheduled chemotherapy. Patients with an established non‐healable wound were excluded from the study.
2.3. Wound assessment details
Comparative data on overall healing times and nursing visits were collected following programme implementation. In addition, a standardised wound status continuum score using the Bates‐Jensen Wound Assessment Tool (BWAT) was used to measure healing rates and establish acuity of wounds. The BWAT is recognised as a valid and reliable tool used to assess and monitor the healing of all types of wound. The BWAT consists of 13 assessment parameters, measured on a scale of 1 to 5. Two additional parameters are measured by a simple check system. The wound location is assessed, recorded and marked on a body diagram. The shape of the wound is described by its overall pattern, such as round or oval and linear or elongated. Once the numbers are recorded and the scale is complete, a total is calculated using all 13 parameters and then placed on a linear chart. The total ranges from 1 (Tissue Health) to 13 (Wound Regeneration) to 65 (Wound Degeneration). The higher the total score, the more severe the wound status.
2.4. Data collection and analysis
Nurses submitted electronic reports initially on admission, on an interim basis at three‐week intervals, when any variances from expected outcomes were observed, and at discharge. Patient records analysed for this study were extracted from a database managed by Nursing Practise Solutions (NPS) Inc. Data were systematically extracted from electronic reports that are submitted and uploaded into the internal computer system. A retrospective review of secondary data, electronic health records, wound progression continuum scores, and age of wound on wound closure was performed. All data were quantified per treatment group and stratified according to wound type. Data were managed and analysed using Microsoft Excel 2010. The margin of error for the mean comorbidity index and mean healing time by wound type to a confidence interval of 95% for statistical testing were determined. All variables were described using descriptive statistics.
3. RESULTS
3.1. Study population characteristics
Data on patient age and comorbidities are presented in Tables 1 and 2. Mean comorbidity scores are based on the Charlson Comorbidity Index. These data were included as indicators of key patient characteristics that may influence wound healing.
TABLE 1.
Characteristics of patient data: age and comorbidities for wounds using an ICB containing NCS dressings (n = 330) vs a non‐ICB treatment approach (n = 2242) across all chronic wounds and per wound type
| Factor | NCS dressing and ICB treatment | Non‐ICB treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Mean age (years) | Mean comorbidity index | Mean BWAT Score | No. of patients | Mean age (years) | Mean comorbidity index | Mean BWAT Score | |
| All wound patients | 330 | 56.35 | 2.55 | 31.4 + 12.5 | 2242 | 56.67 | 2.40 | 33.2 + 9.2 |
| Diabetic foot ulcer | 1 | 80.00 | 6.00 | 33.6 | 179 | 59.44 | 3.82 | 32.4 + 9.7 |
| Venous leg ulcer | 196 | 57.10 | 2.80 | 32.3 + 9.2 | 708 | 60.40 | 2.58 | 36.9 + 8.3 |
| Pressure injuries | 21 | 57.00 | 2.33 | 33.9 + 9.1 | 309 | 61.51 | 2.72 | 34.0 + 8.9 |
| Surgical wound | 92 | 54.92 | 2.10 | 30.1 + 8.3 | 1019 | 52.17 | 1.92 | 35.2 + 9.1 |
| Burn | 20 | 53.75 | 2.20 | 33.5 + 9.3 | 27 | 54.93 | 2.48 | 40.2 + 1.7 |
Note: Patients treated with an ICB including a NCS dressing were statistically older than patients not on a using a NCS dressing and not on an ICB; (P < .001).
Abbreviations: ICB, Integrated Care Wound Bundle; NCS, nanocrystalline silver.
TABLE 2.
Mean comorbidity index using an ICB containing NCS dressings (n = 330) vs a non‐ICB treatment approach (n = 2242) across all chronic wounds and per wound type
| Factor | NCS dressing and ICB Treatment | Non‐ICB treatment | ||||
|---|---|---|---|---|---|---|
| No. of patients | SD | Margin of error (±) | No. of patients | SD | Margin of error (±) | |
| All wound patients | 330 | 1.733 | 0.187 | 2242 | 1.803 | 0.075 |
| Diabetic foot ulcer | 1 | – | – | 179 | 2.160 | 0.316 |
| Venous leg ulcer | 196 | 1.597 | 0.224 | 708 | 1.442 | 0.106 |
| Pressure injuries | 21 | 1.354 | 0.579 | 309 | 1.810 | 0.202 |
| Surgical wound | 92 | 1.828 | 0.374 | 1019 | 1.785 | 0.110 |
| Burn | 20 | 2.331 | 1.022 | 27 | 1.847 | 0.697 |
Note: Margin of error for 95% confidence interval. For 95% CI, Z = 1.960.
Abbreviations: ICB, Integrated Care Wound Bundle; NCS, nanocrystalline silver; SD, standard deviation.
The mean patient age and comorbidity score showed some variation for all patients on a NCS dressing and ICB compared with those patients not on a care bundle and without a NCS dressing. Because of the uneven sample size, patients treated with an ICB including a NCS dressing were statistically older (mean age of 56.35 years) than patients not on a using a NCS dressing and not on an ICB (56.67 years; P < .001). The mean comorbidity index is higher for those on NCS dressing and ICB (mean 2.55, 95% CI: 2.36‐2.74) than those not treated with NCS dressing and not on a bundle (mean 2.40, 95% CI: 2.33‐2.48). Patients on an NCS dressing and ICB also had a slightly higher (P < .001) comorbidity score (median 3, interquartile range (2, 4) compared with patients not on a bundle (median 2, interquartile range [0, 3]).
3.2. Wound healing
A healed wound was defined as a wound that has healed to complete closure, demonstrated by complete reepithelialisation. Wound healing is indicated by the mean length of time (weeks) taken for the subjects to achieve wound closure from admission and for those patients included in the post‐implementation phase of the study, by mean score acuity using the BWAT. Patients treated with NCS dressing and ICB demonstrated a reduced mean BWAT score (31.4) compared with those not being treated with a NCS dressing (33.2).
Any increase in acuity (indicated by an increasing BWAT score) or failure of the wound to heal according to expected wound healing pathways generated a variance and, in most cases, referral to a Nurse Practitioner or another appropriate member of the multi‐disciplinary team (physician, wound nurse, physiotherapist).
The mean healing time for all wound types was reduced by more than half for patients on an ICB containing NCS dressings (mean 10.46 weeks, 95% CI: 9.86‐11.06) compared with those in the comparative treatment group (Mean 25.49 weeks, 95% CI: 24.72‐26.26). Wound healing results are presented in Figure 1 and Table 3.
FIGURE 1.
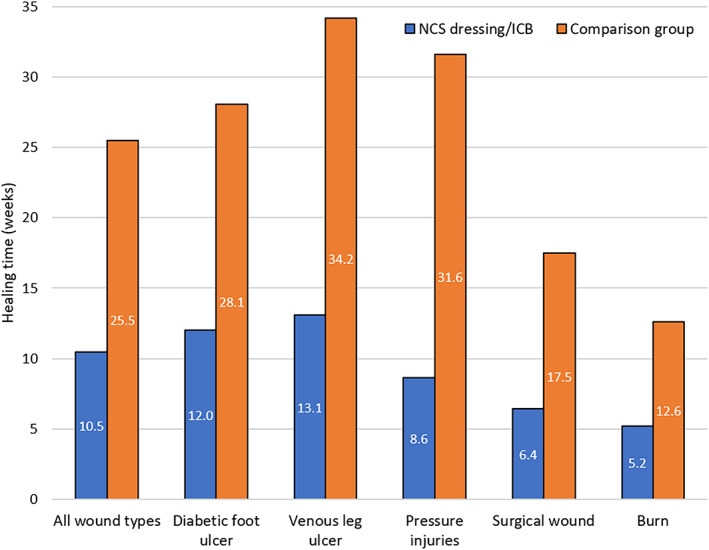
Mean wound healing time using an Integrated Care Wound Bundle (ICB) containing nanocrystalline silver (NCS) dressings (n = 330) vs a non‐ICB treatment approach (n = 2242) across all chronic wounds and per wound type
TABLE 3.
Mean healing time (weeks) using an ICB containing NCS dressings (n = 330) vs a non‐ICB treatment approach (n = 2242) across all chronic wounds and per wound type
| Factor | NCS dressing and ICB Treatment | Non‐ICB treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean healing time (weeks) | No. of patients | SD | Margin of error (±) | Mean healing time (weeks) | No. of patients | SD | Margin of error (±) | |
| All wound patients | 10.46 | 330 | 5.524 | 0.596 | 25.49 | 2242 | 18.593 | 0.770 |
| Diabetic foot ulcer | 12.00 | 1 | ‐ | ‐ | 28.08 | 179 | 15.785 | 2.312 |
| Venous leg ulcer | 13.08 | 196 | 4.382 | 0.613 | 34.18 | 708 | 19.568 | 1.441 |
| Pressure injuries | 8.62 | 21 | 6.305 | 2.697 | 31.63 | 309 | 19.562 | 2.181 |
| Surgical wound | 6.43 | 92 | 4.269 | 0.872 | 17.47 | 1019 | 14.131 | 0.868 |
| Burn | 5.20 | 20 | 4.456 | 1.953 | 12.59 | 27 | 9.394 | 3.543 |
Note: Margin of error for 95% confidence interval. For 95% CI, Z = 1.960.
Abbreviations: ICB, Integrated Care Wound Bundle; NCS, nanocrystalline silver; SD, standard deviation.
3.3. Dressing changes/nursing visits
The number of dressing changes required during the wound healing process is a key determinant of the total cost of wound treatment. Each dressing change required a visit to a home or community care setting by a Registered Nurse (RN) or Registered Practical Nurse (RPN). Nursing time required for travel and clinical care represented the single largest cost in wound care at a mean of C$68 per nursing visit.
The average number of days between dressing changes per treatment group is shown in Figure 2. Overall, the difference in the average interval between dressing changes was significantly in favour of the NCS and treatment bundle (3.98 days) compared with those treated without NCS and not on an ICB (1.87 days; P < .001). Coupled with the increased healing times, this represents a significant reduction in the frequency of dressing changes and therefore nursing time, indicating considerable potential to reduce wound care costs. ACTICOAT Flex 7 provides a sustained release of silver over a 7‐day period, thereby allowing longer wear time and reduced dressing change frequency. It is possible that the time between dressing changes could be extended even further beyond the mean time of 3.98 days as stated in this study.
FIGURE 2.
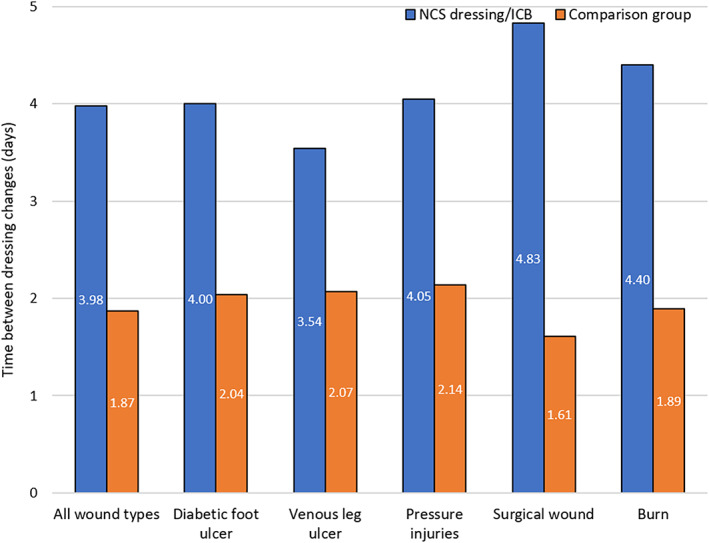
Average number of days between dressing changes using an Integrated Care Wound Bundle (ICB) containing nanocrystalline silver (NCS) dressings vs a non‐ICB treatment approach across all chronic wounds and per wound type. *P < .001
3.4. Total cost of wound healing
The difference in the mean labour cost of wound management for all wound types (Figure 3) using NCS dressings as part of an ICB (C$1251) was shown to be significantly less (P = .001) than the cost of wound management not using a NCS and not on an ICB (C$6488).
FIGURE 3.
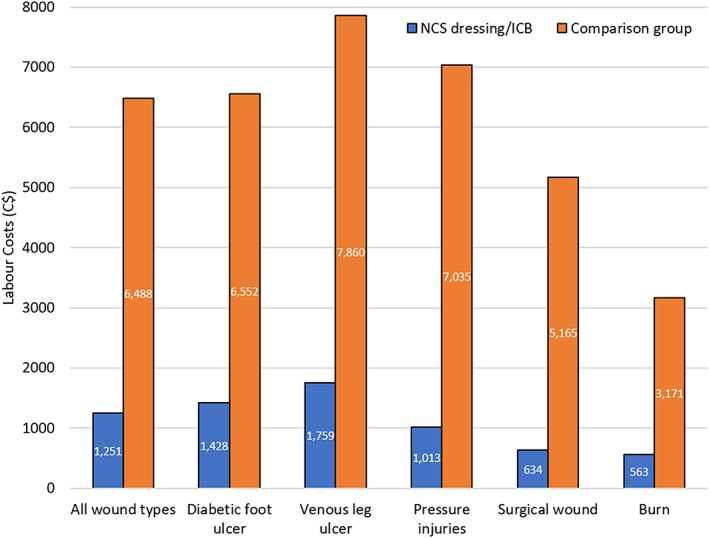
Mean labour cost (Canadian dollars) to healing using an Integrated Care Wound Bundle (ICB) containing nanocrystalline silver (NCS) dressings vs a non‐ICB treatment approach across all chronic wounds and per wound type. *P < .001
3.5. Variance reports
Table 4 summarises the variance reports that were submitted for the patients in each treatment group, identifying product adverse effects, wound infections and hospital admissions per wound type. Only one account of product adverse reactions was reported for a patient with a venous leg ulcer (VLU) undergoing treatment with NCS dressings and an ICB. The patient reported that the dressing caused a burning sensation and no other effects; once INTRASITE™ Gel (Smith+Nephew Ltd., Hull, UK) was applied under the treatment product the burning subsided and the patient had no further issues.
TABLE 4.
Impact of treatment group on product adverse reactions, systemic infection and hospital admissions per wound type
| Factor | NCS dressing and ICB treatment | Non‐ICB treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Product adverse reaction | Systemic infection | Hospital admissions | No. of patients | Product adverse reaction | Systemic infection | Hospital admissions | |
| All wound patients | 330 | 1 | 3 | 0 | 2242 | 12 | 70 | 12 |
| Diabetic foot ulcer | 1 | 0 | 1 | 0 | 179 | 0 | 36 | 8 |
| Venous leg ulcer | 196 | 1 | 1 | 0 | 708 | 6 | 2 | 0 |
| Pressure injuries | 21 | 0 | 0 | 0 | 309 | 1 | 1 | 1 |
| Surgical wound | 92 | 0 | 1 | 0 | 1019 | 3 | 31 | 1 |
| Burn | 20 | 0 | 0 | 0 | 27 | 3 | 0 | 1 |
Abbreviations: ICB, Integrated Care Wound Bundle; NCS, nanocrystalline silver.
The incidence of systemic infection was reduced for patients treated with ICB containing NCS dressings (n = 3, 0.9%) compared with those not using NCS dressings (n = 70, 3.1%).
It should also be noted that no patients required admission to hospital during their treatment with NCS dressings.
4. DISCUSSION
Wounds of a chronic nature are classed as hard‐to heal wounds, and incur considerable time and expense to review and manage the wound. Furthermore, an infected chronic wound can take up to three times the cost to heal compared with a none‐infected wound. 12 As such, the choice of wound dressing can have a marked impact on not only the healing of the wound and the psychosocial effect on the patient, but the financial burden on the health care system. The use of alternative antimicrobial treatments wherever possible is becoming an essential part of infection management protocols. Topical antimicrobials exist in many forms, including silver, iodine, antibiotics and can be used in the prevention and management of infections. By reducing local bioburden, these antimicrobial dressings can aid wound healing progress, reduce the risk of complications such as infection and ultimately can have a positive impact on the cost of treatment as a whole.
Appropriate early use of silver antimicrobial dressings for local infection control and management can help to prevent biofilm formation and reserve antibiotics for spreading or systemic infections. 13 , 14 , 15 Clinically this early intervention, with an effective silver dressing is recommended to resolve local infection rapidly 15 , 16 , 17 and is advocated in infection management pathways. 18 , 19 In addition, studies incorporating NCS antimicrobial barrier dressings into infection management protocols have demonstrated reduced antibiotic use and length of hospital stay with subsequent impact on costs and moreover a reduction in antibiotic resistant organisms. 20 , 21 , 22 , 23
Choosing to use antimicrobial dressings for local infection management can help to reduce the impact that the inappropriate and over‐use of antibiotics may have on the future use of antibiotics, whilst also potentially reducing the cost of health care. 24
The financial pressure on wound management is heavily influenced by wound infection control and management, in particular the cost of dressings. Whilst the cost of many advanced antimicrobial wound dressings varies and is often more than the cost of standard dressings, it is important to also factor in the total cost of wound management; including physician labour costs, hospital admissions and additional wound care treatment.
A previous study has shown that within 2 weeks of treatment for infected, chronic wounds, clinical signs of infection was resolved in 60% of cases treated with NCS antimicrobial barrier dressings compared with 4% to 8% of cases treated with two other silver dressings. This in turn reduced the total number of dressings used and the reduced level of infection was thought to encourage faster healing, with at least twice as many wounds healed in the NCS dressing group by week eight compared with the other two silver dressings. 16 Further analysis of this data went on to highlight the reduction in treatment costs as an overall result. 25 Additional studies with NCS dressings have shown positive healing outcomes within the first 2 to 4 weeks of treatment also. 17 A ‘Two Week Challenge’ treatment recommendation suggests that antimicrobial dressings should be used for an initial two‐week period and then the wound management should be re‐evaluated. 15 This allows physicians to assess whether there are signs of improvement of the wound with the silver dressings and to consider whether the treatment is still suitable or if an alternative should be used. This method can help physicians to ensure that patients are receiving the most appropriate care for the wound, minimising the inappropriate use of antimicrobials whilst also having a positive impact on the financial cost of wound care.
In agreement with these previous studies, the data presented in this study demonstrates a large reduction in the mean labour cost to healing for patients on the NCS and ICB treatment ($1251) compared with the cost for those not using a NCS dressing ($6488) across all wound types. Whilst there is already a clear cost benefit in using a NCS dressing for the treatment of chronic wounds in this retrospective study, there is potential for a further reduction in treatment cost provided by the longer dressing wear time afforded by the sustained availability and antimicrobial activity of the NCS dressing, leading to a reduction in dressing changes and fewer nursing or hospital visits. Nursing providers are paid for each visit and whilst they are encouraged to allow time between visits, this practice is not always adopted. It is possible that the time between dressing changes could be extended even further beyond the mean time of 3.98 days as stated in this study.
The reduced mean labour cost to healing would have also been largely influenced by the considerably faster healing times for patients on the NCS dressing and ICB (10.46 weeks) as opposed to those not using a NCS dressing and not on an ICB (25.49 weeks). These factors, combined with the reduced incidence of product adverse reactions, systemic infection and hospital admissions for those treated with a NCS dressing, would have all contributed to the reduced labour cost. It cannot be underestimated the costs and significance of utilising a wound dressing to manage localised wound bioburden vs, managing systemic infections both from a patient outcome perspective and a clinical perspective to health care organisations. Wound infections range from minor, localised infections that heal without consequence to more severe and disfiguring as well as even life threatening. Having a silver dressing such as the NCS dressing used in this study, as a foundation for health care professionals to better and more predictably control bioburden in chronic wounds is tremendously beneficial to not just patients but health care organisations. Systemic wound infections often lead to exorbitant hospitalizations and lengthy/costly wound healing times and add to the clinical burden of excessive use of antibiotics that has now become a public health threat. This can have a huge impact on wound care management and intervention, particularly as a recent report has shown that treatment for antibiotic‐resistant infections and exceeds $2billion annually, with an additional cost of $1383 for each individual patient. 26
Furthermore, this study has demonstrated that only 3 out of 330 (0.9%) patients undergoing treatment with NCS dressing and ICB developed a systemic infection, compared with 70 (3%) patients on the comparative treatment bundle, suggesting that NCS dressings may potentially reduce the progression of infection in patients through prevention and management of local infection. This aligns with previous reports where, following introduction of an early intervention treatment strategy in which NCS dressings were used, the progression of wound‐associated MRSA bacteraemia was considerably reduced, with eventually no patients presenting with wound associated MRSA. 27
Considering recent concern for the overuse of antibiotics, NCS dressings can provide a tool in managing a variety of chronic wounds by providing rapid bioburden management and facilitating closure, thereby allowing for a reduction in antibiotic use. The CDC and WHO organisations have published reports and proposed strategies to combat further antimicrobial resistance. 28 Every antibiotic class has developed resistance which has lead health care organisations around the world to use antimicrobial stewardship programmes to preserve the current antibiotic armamentarium. 29 The avoidance of systemic wound infections as well as healing times demonstrate quality indicators for health care organisations that then further benefits the whole clinical environment.
As demonstrated in this study, silver dressings can be used on a variety of wounds types, including diabetic foot ulcers, venous leg ulcers, surgical wounds, burns and pressure injuries for safe and effective infection management. Depending on the severity and age of the wound, healing rate and complications differ greatly, often affecting the number of hospital admissions, dressing changes and consequently the cost of treatment. The mean healing time, as well as the number of dressing changes was reduced for all individual wound types recorded in this study, showing that by using NCS dressings and ICBs, mean labour costs were substantially reduced; in some cases this was by approximately C$6000. This provides a promising outlook for the future of NCS dressings as part of wound management protocols, particularly as a recent review of economic costs of wounds demonstrated that in the United States, mean Medicare spending in 2014 for individual primary diagnoses of different wound types ranging up to $20 000 in some cases; with arterial ulcers and pressure ulcers having the highest treatment costs. Depending on the severity of the wound, for instance grade III and IV pressure injuries, the cost of treatment can vary greatly particularly as these are at greater risk of infection. 2 , 30
5. STRENGTH AND LIMITATIONS OF RETROSPECTIVE METHODS OF DATA COLLECTION
One limitation of a retrospective cohort study design is the potential for erroneous or missing information. It may also have been beneficial to have sufficient sample numbers in all wound classifications to analyse any differences in wound healing trends. Whilst the sample size was uneven between the two treatment groups, this has been taken into account when performing statistical analysis. In addition, due to the high risk patient group that received the ICB including NCS dressing, the bundle was continued until healing; future studies in lower risk groups may look at earlier cessation of the antimicrobial intervention to provide further efficiencies in practice and antimicrobial stewardship benefits. Nevertheless, this study does show real‐life results looking at both healing time as well as health care associated costs. The data presented does suggest that NCS dressings can help to reduce financial pressures that health care systems may be subject to in the cost of controlling infection in wounds.
6. CONCLUSION
This study was a non‐experimental, retrospective evaluation of the effectiveness of a silver nanocrystalline barrier dressing, in the treatment of open chronic wounds in the community. This evaluation has provided a rare opportunity to review the utilisation of a silver dressing on patients that have healed.
The results of the evaluation have demonstrated that the ICB model including NCS dressings can have a rapid, significant and predictable impact on wound care by reducing chronic wound healing times (an indicator of the quality and clinical effectiveness of care) and frequency of wound dressing changes (an indicator of the cost of care delivery). This data, combined with the reduced incidence of systemic infections and hospital admissions demonstrates that the NCS dressing has proven to not only be effective and efficient, but safe for clinicians to use in many types of open chronic wounds.
When deciding on antimicrobial dressing choice, this study provides a useful guide for health care professionals; the clinical, economic and patient impact of antimicrobial dressing choice highlights a role for NCS dressings as part of an infection management protocol in the management of hard‐to‐heal, chronic wounds.
ACKNOWLEDGEMENTS
Hilary Watkins and Dr. Emma J. Woodmansey are employees of Smith+Nephew and assisted with the data interpretation and manuscript preparation. Dr. Theresa Hurd was responsible for the design of the study, extracting and interpreting the data and manuscript preparation. The dressings used in this study were purchased through the hospital, with no financial assistance from Smith and Nephew. Dr. T. Hurd received a fee paid by Smith+Nephew for providing data.
Hurd T, Woodmansey EJ, Watkins HMA. A retrospective review of the use of a nanocrystalline silver dressing in the management of open chronic wounds in the community. Int Wound J. 2021;18:753–762. 10.1111/iwj.13576
Funding information Smith and Nephew
DATA AVAILABILITY STATEMENT
Author elects to not share data.
REFERENCES
- 1. Anderson K, Hamm RL. Factors that impair wound healing. J Am Coll Clin Wound Spec. 2014;4(4):84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nussbaum SR, Carter M, Fife C, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. J Int Soc Pharmacoeconom Outcome Res. 2018;21:27‐32. [DOI] [PubMed] [Google Scholar]
- 3. Järbrink K et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown MS, Ashley B, Koh A. Wearable technology for chronic wound monitoring: current dressings, advancements, and future prospects. Front Bioeng Biotechnol. 2018;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gould L, Abadir P, Brem H. Chronic wound repair and healing in adults: current status and future research. Wound Repair Regen. 2015;23:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Politano AD, Campbell KT, Rosenberger LH, Sawyer RG. Use of silver in the prevention and treatment of infections: silver review. Surg Infect (Larchmt). 2013;14(1):8‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woodmansey EJ, Roberts CD. Appropriate use of dressings containing nanocrystalline silver to support antimicrobial stewardship in wounds. Int Wound J. 2018;15(6):1025‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin Y, Hsu W, Chung W, Ko T, Lin J. Silver‐based wound dressings reduce bacterial burden and promote wound healing. Int Wound J. 2016;13:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daubney L. Silver release testing of ACTICOAT Flex 7 dressings. Smith&Nephew Data on File # DS/08/062/R2. 2008.
- 12. Guest JF, Fuller GW, Vowden P. Diabetic foot ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J. 2018;15:43‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Driffield K, Woodmansey E, Floyd H. The use of silver‐containing dressings to prevent biofilm formation by single and mixed bacterial flora. Poster Presentation in SAWC. 2007.
- 14. Schultz G, Bjarnsholt T, James GA, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744‐757. [DOI] [PubMed] [Google Scholar]
- 15. Ayello EA, Carville K, Fletcher J, et al. International consensus. Appropriate use of silver dressings in wounds. An expert working group consensus. Int Wound J. 2012;1‐24. [Google Scholar]
- 16. Gago M, Garcia F, Gaztelu V, Verdu J, Lopez P, Nolasco AA. Comparison of three silver‐containing dressings in the treatment of infected, chronic wounds. Wounds. 2008;20(10):273‐278. [PubMed] [Google Scholar]
- 17. Miller CN, Newall N, Kapp SE, et al. A randomized‐controlled trial comparing cadexomer iodine and nanocrystalline silver on the healing of leg ulcers. Wound Repair Regen. 2010;18(4):359‐367. [DOI] [PubMed] [Google Scholar]
- 18. Dowsett C, Bellingeri A, Carville K, Garten A, Woo K. A route to more effective infection management: the infection management pathway. Wounds Int. 2020;11(3):50‐57. [Google Scholar]
- 19. Woo K. Implementing the new infection management pathway to optimise outcomes: real‐world case series. Wounds Int. 2020;11(4):50‐57. [Google Scholar]
- 20. Fong J, Wood F, Fowler B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: comparative patient care audits. Burns. 2005;31:562‐567. [DOI] [PubMed] [Google Scholar]
- 21. Tonkin C, Wood F. Nanocrystalline silver reduces the need for antibiotic therapy in burn wounds. Prim Intent. 2005;13(4):163‐168. [Google Scholar]
- 22. Strand O, San Migue L, Rowan S, Sahlqvist A. Retrospective comparison of two years in a paediatric burns unit, with and without acticoat as a standard dressing. Ann Burns Fire Disasters. 2010;23(4):182‐185. [PMC free article] [PubMed] [Google Scholar]
- 23. Glik J, Łabuś W, Kitala D, et al. A 2000 patient retrospective assessment of a new strategy for burn wound management in view of infection prevention and treatment. Int Wound J. 2017;15(3):344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharmacy Therapeut. 2015;40(4):277‐283. [PMC free article] [PubMed] [Google Scholar]
- 25. Searle R., Bielby A. Dressing strategies for the management of infected wounds in community wound care: impacts and implications. Poster Presentation in Wounds UK. 2010.
- 26. Thorpe KE, Joski P, Johnston KJ. Antibiotic‐resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Aff. 2018;37:662‐669. [DOI] [PubMed] [Google Scholar]
- 27. Newton H. Reducing MRSA bacteraemias associated with wounds. Wounds UK. 2010;6(1):56‐65. [Google Scholar]
- 28. Centers for Disease Control and Prevention (CDC) . A Public Health Action Plan to Combat Antimicrobial Resistance. 2011.
- 29. Centers for Disease Control and Prevention (CDC) . Antibiotic Resistance Threats in the United States. 2013.
- 30. Bauer KW, Rock K, Nazzal MM, Jones OC, Qu W. Pressure ulcers in the United States' inpatient population from 2008 to 2012: results of a retrospective Nationwide study. Ostomy Wound Manag. 2016;62(11):30‐38. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Author elects to not share data.


