Abstract
Our purpose was to perform a meta‐analysis to evaluate the effect of Low‐level laser therapy (LLLT) on diabetic foot ulcers (DFUs). The PubMed, Cochrane, Embase, Web of Science, Chinese BioMedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), VIP and Wanfang databases were searched systematically up to August 27, 2020. Studies that met the inclusion criteria were included in the analysis. A total of 13 randomised controlled trials (RCTs) and 413 patients were analysed. Compared with the control group, LLLT significantly increased the complete healing rate (risk ratio [RR] = 2.10, 95% confidence interval [CI] 1.56‐2.83, P < .00001), reduced the ulcer area (standardised mean difference [SMD] = 3.52, 95% CI 1.65‐5.38, P = .0002), and shortened the mean healing time (SMD = −1.40, 95% CI −1.90 to −0.91, P < .00001) of patients with DFUs. The quality of the evidence was very low according to the GRADE system. LLLT is a promising and effective adjuvant treatment to accelerate the healing of DFUs. Further evidence from larger samples and higher quality RCTs is needed to prove the effect of LLLT and to determine the most appropriate parameters for the healing of DFUs.
Keywords: diabetic foot ulcer, low‐level laser therapy, meta‐analysis, randomised control trials
Abbreviations
- ABI
ankle‐brachial index
- CBM
Chinese BioMedical Literature Database
- CI
confidence interval
- CNKI
China National Knowledge Infrastructure
- df
degrees of freedom
- DFU
diabetic foot ulcer
- GRADE
the Grading of Recommendations, Assessment, Development and Evaluations
- IL‐1α
interleukin‐1 alpha
- IL‐8
interleukin‐8
- LLLT
low‐level laser therapy
- PDGF
platelet‐derived growth factor
- PRISMA
the preferred reporting items for systematic reviews and meta‐analyses
- RCT
randomised controlled trials
- RR
risk ratio
- SMD
standardised mean difference
- TGF‐β
transforming growth factor‐β
- TNF‐α
tumour necrosis factor‐alpha
- VAS
Visual Analog Scale
1. INTRODUCTION
DFU is one of the major complications of diabetes, which is a destructive factor in diabetes progression. It has been estimated that the lifetime risk of patients with diabetes developing a foot ulcer may be as high as 25%. 1 Chronic nonhealing ulcers are an advanced indication of infection and amputation, 2 bringing a great economic burden to patients, 3 and dramatically impacting the quality of life of patients. 4 Patients with DFUs are nearly 2.5 times more likely to die than patients with diabetes without DFUs. 5 The five‐year mortality rate in patients with DFUs has previously been shown to be more than 40%. 5 , 6
Despite the high prevalence and great burden of DFUs, the current treatment strategies for DFUs are not very satisfactory. It is estimated that 7% to 20% of patients with DFUs will subsequently need an amputation after standard care treatment. 7 , 8 Even with a multidisciplinary approach, including glycemic control, daily local care, foot offloading, antibiotic therapy, and surgical revascularization, chronic DFUs require a long time to heal completely. 9 Ulcer healing requires good integration of the complex biological and molecular events of cell migration, cell proliferation, and extracellular matrix deposition. 10 , 11 In this context, the tissue repair process has been the focus of many studies looking for therapeutic treatments that can increase the speed of ulcer healing. 12 Some adjuvant therapies have emerged, 13 such as epidermal growth factor, 14 hyperbaric oxygen therapy, 15 negative‐pressure wound therapy, 16 and LLLT. 12 Among them, LLLT has high potential as a noninvasive and nonpharmacological therapy for the treatment of DFUs.
LLLT often includes wavelengths between 500 and 1100 nm and involves the delivery of 1–4 J/cm2 to treatment sites with lasers having output powers between 10 and 90 mW. 17 LLLT is known to directly provide biological stimulation with light energy to body cells, thereby promoting cell function and tissue repair. 18 The absorbed laser energy stimulates the molecules and atoms of cells without a significant tissue temperature increase. 19 , 20 In 1968, Mester unexpectedly found that the laser could promote hair growth in mice 21 ; then, he continued the clinical study of LLLT on skin ulcers and found that LLLT had potential benefits for ulcer healing. 22 , 23 In recent years, cell studies have indicated that LLLT may moderate the adverse effects of hyperglycemia on vascular endothelial cells and lead to a reduction in TNF‐α concentration and enhancement of fibroblast proliferation. 24 , 25 Animal studies have indicated that LLLT can accelerate cutaneous wound healing, 26 even if a single laser treatment is performed. 27 Some clinical trials indicated that LLLT can accelerate the tissue repair process of DFUs. 12 , 28
Considering the acceptability, availability, and negligible adverse effects, the effect of LLLT on DFUs has caught the attention of researchers. The sample size of previous independent studies was limited and not sufficiently representative. Furthermore, through the search strategy we used, we found three systematic reviews related to LLLT treatment of DFUs. 29 , 30 , 31 However, these reviews performed a meta‐analysis of a limited number of studies or did not apply meta‐analysis methods. These studies also did not evaluate the quality of the evidence. Therefore, more comprehensive studies are needed to determine the efficacy of LLLT on DFUs. In this paper, we conducted a meta‐analysis of randomised controlled trials to estimate the treatment efficacy of LLLT for DFU based on currently available RCTs.
2. MATERIALS AND METHODS
The present study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 32 This study was registered at https://www.crd.york.ac.uk/prospero/ (registration number: CRD42020220496).
2.1. Inclusion criteria
Eligible studies had to meet the following criteria.
RCTs that explored the effect of LLLT on DFUs.
LLLT was compared with traditional treatment or placebo.
The study provided available results about DFU healing.
2.2. Exclusion criteria
Studies were excluded if they met either one of the following criteria.
Studies reporting the same sample; in this case, the most recent and most complete paper was chosen.
Studies that were not RCTs (i.e., review articles, editorials, case reports, or case series); studies that were not human studies (i.e., vitro or animal).
2.3. Literature search
A systematic review was conducted on August 27, 2020 by searching eight databases, namely, PubMed, Cochrane, Embase, Web of Science, CBM, CNKI, VIP and Wanfang. The search terms are as follows: “diabetic ulcer”, “diabetic foot”, “diabetic foot ulcer”, “foot ulcer” and “low‐level light therapy”, “low‐level laser therapy”, “LLLT”, “phototherapy”, and “laser”. There were no language restrictions. References from these relevant studies were also reviewed to identify additional studies.
2.4. Data extraction
Data relating to the effects of LLLT on DFUs were extracted using a predetermined form and checked by the second author (HJ and CJQ). Discrepancies in the extracted data were settled by discussion among three authors (HJ, CJQ, and XSY). The authors extracted the following information from each included study: the first author's name, year, country, study design, demographic information, sample size, duration of diabetes, inclusion criteria, characteristics of the ulcers, LLLT parameters, treatment time, outcomes of treatment (i.e., complete healing rate, ulcer area reduction percentage and mean healing time), and adverse events.
2.5. Assessment of risk of bias and strength of evidence
Risk of bias in each of the included studies was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions. 33 Each study was assessed for the following six aspects: randomization generation, allocation concealment, blindness of participants and personnel, blindness of outcome assessment, incomplete outcome data, and selective reporting. The results of the risk of bias assessment were pooled into the Review Manager statistical software package (version 5.3), and a “risk of bias summary” figure was generated. Further, the quality of the evidence was judged to be high, moderate, low or very low according to the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system, based on the risk of bias, inconsistency, indirectness, imprecision, and publication bias (GRADEpro/GDT, https://gdt.gradepro.org/). 34 The assessment was conducted independently by two authors (HJ and CJQ). Different opinions were settled by discussion among three authors (HJ, CJQ, and XSY).
2.6. Statistical analysis
RRs and 95% CIs were calculated for dichotomous data. Because of the difference in the parameters of the laser in the included studies, SMD and 95% CI were calculated for continuous data. The SMD and RR were tested by Z statistic, and a two‐tailed P < .05 was considered statistically significant. Heterogeneity among the included studies was quantified by calculating the Q and I 2 statistics. For the Q statistic, P < .10 was considered to indicate statistical heterogeneity. For I 2 statistics, 0% to 24% = no heterogeneity; 25% to 49% = moderate heterogeneity; 50% to 74% = large heterogeneity; and 75% to 100% = extreme heterogeneity. If a χ 2 statistic had a P < .10 35 or an I 2 statistic >50%, 36 the heterogeneity between studies was considered statistically significant. In this case, we used the random‐effects model. Otherwise, we used the fixed‐effects model. Because of the heterogeneity of the percentage reduction in ulcer area, we conducted subgroup analyses to determine the reason for the heterogeneity, which were based on the control groups' intervention, sample size, Wagner grade and treatment time. Sensitivity analysis was performed based on the leave‐one‐out approach. Funnel plots and Egger's test were applied to assess any potential publication bias. 37 We also used trim‐and‐fill analysis to assess sensitivity and the impact of heterogeneity. The statistical analyses were performed in the Review Manager statistical software package (version 5.3) and STATA statistical software package (version 15.1).
3. RESULTS
3.1. Search results
A preliminary search of the databases identified 1461 records. After 436 duplicates were removed, we manually searched nine articles through other sources. The titles and abstracts were screened for a total of 1034 records; 889 studies were subsequently excluded. A total of 145 studies were submitted to full‐text screening. Finally, 13 studies that met our criteria were identified. The literature selection process is shown in Figure 1. PubMed search process is shown in Table S1.
FIGURE 1.
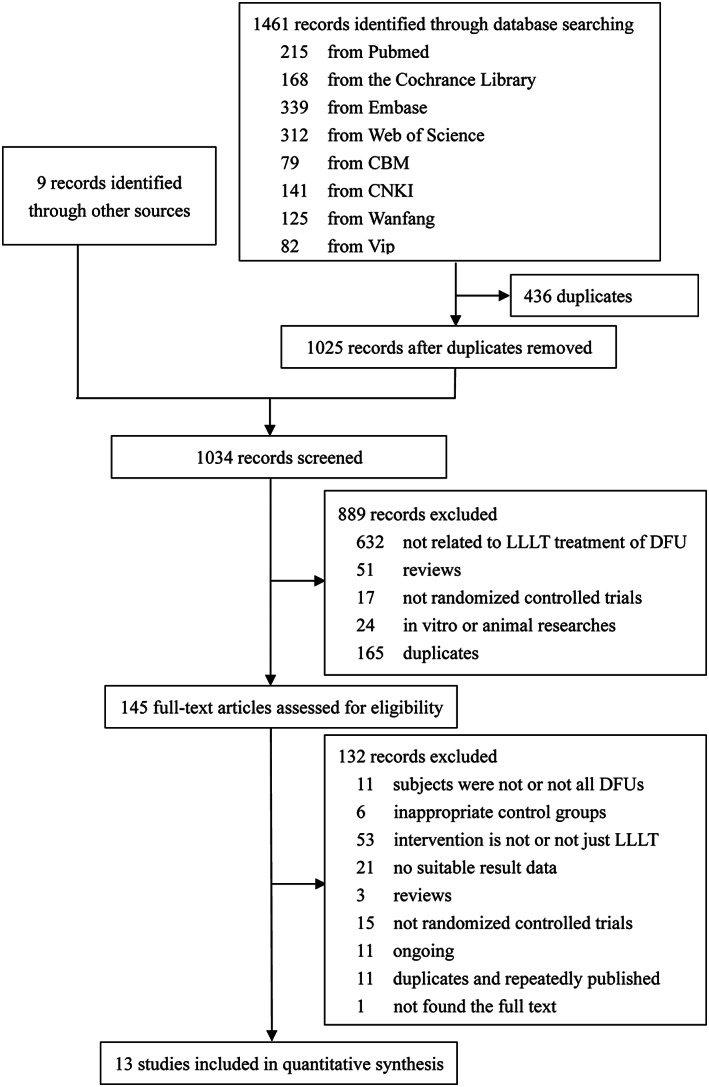
Flow diagram for the selection of studies for systematic review
3.2. Study characteristics
The year of publication range from 2002 to 2018. In these 13 RCTs, there were 227 patients in the intervention groups and 186 patients in the control groups. Most studies recruited a higher proportion of males than females, except for one study 38 that had a slightly higher proportion of females, and we did not obtain sex information in the three studies. 12 , 39 , 40 Among the 13 studies, the interventions in the control groups of nine studies 12 , 28 , 38 , 41 , 42 , 43 , 44 , 45 , 46 were traditional treatments, and the remaining four studies 11 , 39 , 40 , 47 used placebos. The placebo in the two studies 11 , 39 was sham irradiation, and the placebo in the other two studies was nontherapeutic 11 , 47 and lower‐energy 40 light. There were five studies on type 2 diabetes. 11 , 28 , 40 , 43 , 45 The duration of ulcers ranged from 1 week to 95 months, and the severity of DFUs ranged from Wagner I to Wagner III. The follow‐up time varied from 15 days to 20 weeks. The wavelength of LLLT in the included studies ranged from 400 to 904 nm. There were three groups in one study 38 ; in addition to the control group, there was also the high voltage group and the LLLT group. The characteristics of the 13 included studies are shown in Tables 1 and 2.
TABLE 1.
Characteristics of the included studies (a)
| First author (year) | Country | Groups | Number of participants | Sex (M/F) | Age (year) | Duration of diabetes (year) | Characteristics of ulcers | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Initial size (cm2) | Final size (cm2) | Duration | Wagner grade | |||||||
| Chi LX (2002) | China | LLLT | 24 | 13/11 | 56.89 ± 1.08 | NA | 24 | NA | NA | NA | II‐III |
| TT | 16 | 9/7 | 53.54 ± 11.20 | 16 | |||||||
| Chen HJ (2003) | China | LLLT | 36 | 30/6 | 40‐72 | 2‐10 | >36 | NA | NA | 10 days‐6 months | I‐III |
| TT | 13 | 9/4 | 40‐72 | 2‐10 | >13 | 10 days‐6 months | |||||
| Cui ZH (2009) | China | LLLT | 23 | 14/9 | 68.50 ± 5.53 | 5‐20 | 23 | NA | NA | NA | I‐II |
| TT | 23 | 13/10 | 68.12 ± 5.06 | 4‐21 | 23 | ||||||
| Ouyang ZS (2010) | China | LLLT | 20 | 12/8 | 59.6 ± 6.5 | NA | 20 | NA | NA | 1 week‐3 months | I‐III |
| TT | 20 | 9/11 | 56.8 ± 6.4 | 20 | |||||||
| Zhang LJ (2012) | China | LLLT | 12 | 15/9 | 45‐65 | 10‐20 | 12 | 2 × 2‐6 × 6 | NA | 1 week‐2 months | I‐III |
| TT | 12 | 12 | |||||||||
| Minatel DG (2009) | Brazil | LLLT | 7 | NA | 66.3 ± 14.73 | 11.4 ± 11.39 | 13 | 11.8 ± 20.46 | NA | 95.5 ± 33.0 months | NA |
| PLA | 7 | 63.4 ± 11.26 | 11.30 ± 7.40 | 10 | 3.8 ± 4.13 | 28.1 ± 23.6 months | |||||
| Kaviani A (2011) | Iran | LLLT | 13 | 8/3 | 60.2 ± 9.0 | 19.5 ± 6.2 | 13 | 10.7 ± 25.7 | NA | 11.4 ± 8.5 months | I‐II |
| PLA | 10 | 4/3 | 59.4 ± 3.7 | 19.0 ± 4.1 | 10 | 7.8 ± 11.0 | 8.8 ± 3.6 months | ||||
| Landau Z (2011) | Israel | LLLT | 10 | 5/5 | 62.6 | NA | 19 | 1.08 | 0.12 | >8 weeks | I‐II |
| PLA | 6 | 4/2 | 63.4 | NA | 6 | 0.45 | 0.21 | ||||
| Ortíz MCS (2014) | Colombia | LLLT | 9 | 42.9%/57.1% | 59.3 ± 11.8 | 11.2 ± 10.1 | 14 | 62.9* | NA | 16.2 ± 34.6 months | I‐II |
| TT | 9 | 13 | 41.6* | ||||||||
| Kajagar BM (2012) | India | LLLT | 34 | 22/12 | 54.35 ± 6.84 | 5* | 34 | 26.1 ± 6.8 | 15.65 | 5 weeks* | I |
| TT | 34 | 21/13 | 50.94 ± 8.11 | 10* | 34 | 27.5 ± 6.0 | 24.25 | 4 weeks* | |||
| Hoseini SM (2016) | Iran | LLLT | 15 | NA | NA | NA | NA | NA | NA | NA | II |
| PLA | 12 | ||||||||||
| Mathur RK (2017) | India | LLLT | 15 | 9/6 | 54 | ~5.2 | 15 | ~14.84 | ~9.3 | 56 days | I |
| TT | 15 | 11/4 | 49 | ~5.0 | 15 | ~13.52 | ~11.46 | 51 days | |||
| Santos JAF (2018) | Brazil | LLLT | 9 | NA | 53.11 ± 8.85 | NA | 9 | 1.83 ± 1.08 | 0.32 ± 0.26 | 6.00 ± 7.23 months | II‐III |
| TT | 9 | 48.33 ± 2.09 | 9 | 2.97 ± 1.66 | 1.63 ± 1.57 | 13.00 ± 13.58 months | |||||
Abbreviations: NA, not available; LLLT, low‐level laser therapy; TT, traditional treatment; PLA, placebo; *, median; PCBG, postprandial capillary blood glucose.
TABLE 2.
Characteristics of the included studies (b)
| First author (year) | Intervention group | |
|---|---|---|
| LLLT parameters | Treatment time | |
| Chi LX (2002) | <25 mW | Daily for 10 days as a course of treatment; several courses of treatment in total |
| Chen HJ (2003) | 830 nm, 250‐350 mW | Daily for 15 days as a course of treatment; 1‐3 courses of treatment in total |
| Cui ZH (2009) | 632.8 nm, 0‐30 mW | Daily for 7‐10 days as a course of treatment, with an interval of 5‐7 days for a second course of treatment; 2 months in total |
| Ouyang ZS (2010) | 650 nm, 500 mW | Daily for 10 days as a course of treatment, with an interval of 3 days for a second course of treatment; 4 courses of treatment in total |
| Zhang LJ (2012) | 10‐20 mW | Daily for 10 days as a course of treatment, 20 days in total |
| Minatel DG (2009) | LLLT: a probe with 36 diodes, 4 red (660 nm) and 32 infrared (890 nm), 500 mW, 100 mW/cm2, 3 J/cm2 PLA: diodes of 890 nm and 3 of 660 nm were disabled and added one resistor, <1 mW/cm2 | Twice per week, 90 days in total |
| Kaviani A (2011) | LLLT: 685 nm; 50 mW/cm2, 10 J/cm2 PLA: sham irradiation under strictly controlled double‐blinded condition | Six times a week, for at least two successive weeks and then every other day up to complete healing, 20 weeks in total |
| Landau Z (2011) | LLLT: 400‐800 nm, 180 mW/cm2 PLA: non‐therapeutic light, 10 mW/cm2 | Twice a day, 12 weeks in total |
| Ortíz MCS (2014) | 685 nm, 30 mW, 2 J/cm2 on the edges of the ulcer, 1.5 J/cm2 in the wound bed | Three times a week, 16 weeks in total |
| Kajagar BM (2012) | 60 mW/cm2, 2‐4 J/cm2 | Daily for 15 days |
| Hoseini SM (2016) | LLLT: 904 nm, 90 mW, 2 J/cm2 PLA: laser probe was set similar to the laser group, but the power was off | Three times a week for 12 sessions, 4 weeks in total |
| Mathur RK (2017) | 660 ± 20 nm, 50 mW/cm2, 3 J/cm2 | Daily for 15 days |
| Santos JAF (2018) | 660 nm; 30 mW; 6 J/cm2 | Once per 48 hours, totaliy16 sessions in 4 weeks |
| First author (year) | Adverse events | Ulcer area reduction percentage | Complete healing rate | Mean healing time | Other outcomes (laser vs control) | |||
|---|---|---|---|---|---|---|---|---|
| Laser | Control | Laser | Control | Laser | Control | |||
| Chi LX (2002) | NA | ‐ | ‐ | 18/24 | 7/16 | 34.42 ± 8.20 days | 46.26 ± 10.43 days | Efficiency: 23/24 vs 14/16 |
| Chen HJ (2003) | NA | ‐ | ‐ | 21/36 | 3/13 | ‐ | ‐ | Improvement rate:14/36 vs 5/13; Inefficiency:1/36 vs 5/13 |
| Cui ZH (2009) | NA | ‐ | ‐ | 12/23 | 7/23 | ‐ | ‐ | Obvious efficiency:7/23 vs 8/23; Improvement rate:3/23 vs 4/23; Inefficiency:1/23 vs 4/23 |
| OuyangZS (2010) | None | ‐ | ‐ | 14/20 | 3/20 | 38.08 ± 3.57 days | 50.20 ± 10.25 days | Efficiency: 4/20 vs 8/20; Inefficiency:2/20 vs 9/20 |
| Zhang LJ (2012) | None | ‐ | ‐ | 6/12 | 4/12 | ‐ | ‐ | Efficiency: 5/12 vs 3/12; Inefficiency:1/12 vs 5/12 |
| Minatel DG (2009) | None | ‐ | ‐ | 7/13 | 1/10 | ‐ | ‐ | Ulcer granulation rate: 87.0 ± 4.96% vs 30.8 ± 11.24%; Pain relief within 1 week in LLLT group |
| Kaviani A (2011) | None | 73.7 ± 10.2 | 47.3 ± 15.4 | 8/13 | 3/9 | 11 weeks, 95% CI, 7.3‐14.7 | 14 weeks, 95% CI, 8.76‐19.2 | ‐ |
| Landau Z (2011) | None | 89 | 54 | 9/10 | 2/6 | 7.14* weeks | 11.5* weeks | ‐ |
| Ortíz MCS (2014) | None | ‐ | ‐ | 7/9 | 6/9 | ‐ | ‐ | Abnormal protective sensation: P > .05; Health status (EQ VAS): P > .05 |
| Kajagar BM (2012) | NA | 40.24 ± 6.30 | 11.87 ± 4.28 | ‐ | ‐ | ‐ | ‐ | Reduction in ulcer area (mm2): 1043.20 ± 266.62 vs 322.44 ± 85.84 |
| Hoseini SM (2016) | NA | 72.08 ± 7.22 | 12.69 ± 9.05 | ‐ | ‐ | ‐ | ‐ | The skin temperature and ABI values did not show any significant difference |
| Mathur RK (2017) | None | 37.3 ± 9 | 15 ± 5 | ‐ | ‐ | ‐ | ‐ | Average final ulcer area: 9.3 vs 11.46; the wound that received conventional treatment showed more pus and lesser granulation |
| Santos JAF (2018) | NA | 76.45 ± 18.30 | 51.29 ± 31.61 | ‐ | ‐ | ‐ | ‐ | PUSH scales: 2.88 ± 1.45 vs 7.00 ± 2.59; VAS scales: 0.77 ± 1.71 vs 2.33 ± 2.29 |
Abbreviations: NA, not available; *, median; ABI, ankle brachial index; PUSH, pressure ulcer scale for healing; VAS, visual analog scale.
3.3. Risk of bias assessment
Among the 13 included studies, five 42 , 43 , 44 , 45 , 46 RCTs did not report the method of generating random sequences, nine 11 , 12 , 28 , 40 , 42 , 43 , 44 , 45 , 46 studies offered no information on allocation concealment, and four 11 , 39 , 40 , 47 studies were double‐blind. One study 39 was unable to judge whether patients were lost to follow‐up because of incomplete information, and this study was recorded as having an unclear attrition bias. Another study's 46 baseline data were not reported in detail, and this study was recorded as having an unclear bias. The results of the risk of bias assessment are shown in Figure 2.
FIGURE 2.
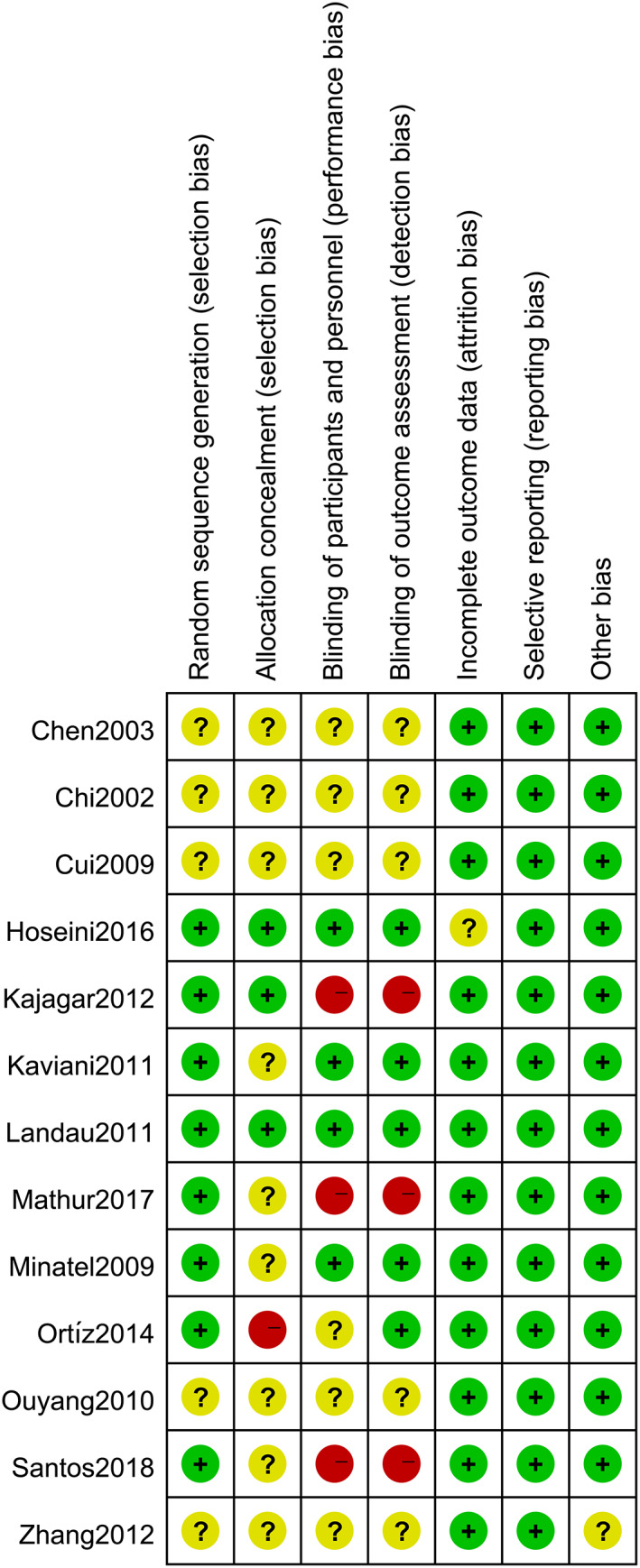
Risk of bias summary. Green: low risk of bias; Red: high risk of bias; Yellow: unclear risk of bias
3.4. Meta‐analysis results
3.4.1. Complete healing rate
Nine studies provided data on the complete healing rate of patients with DFUs. No heterogeneity was found after pooling the data from these nine studies (χ 2 = 8.52, degrees of freedom [df] = 8, P = .38, I 2 = 6%); thus, the fixed‐effects model was used for analysis. Compared with the control group, LLLT significantly increased the complete healing rate of patients with DFUs (RR = 2.10, 95% CI 1.56‐2.83, P < .00001). The forest plot of the complete healing rate is shown in Figure 3. The stability of the results was tested by sensitivity analysis. We deleted each study one by one; this analysis showed that the results of our meta‐analysis were not significantly unstable. The sensitivity analysis results are shown in Table S2.
FIGURE 3.
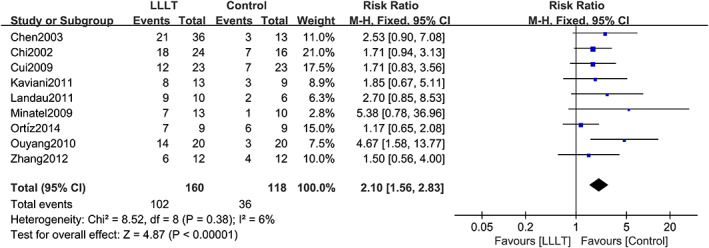
Forest plot of LLLT vs control for complete healing rate. Compared with the control group, LLLT significantly increased the complete healing rate in patients with DFUs (P < .00001). M‐H: mantel‐haenszel; Fixed: fixed‐effects model
3.4.2. Ulcer area reduction percentage
Five studies provided data on the ulcer area reduction percentage in patients with DFUs. Heterogeneity between the included studies was high (χ 2 = 52.13, df = 4, P < .00001, I 2 = 92%), so a random‐effects model was used for analysis. Compared with the control group, LLLT significantly reduced the ulcer area of patients with DFUs (SMD = 3.52, 95% CI 1.65‐5.38, P = .0002). The forest plot of the ulcer area reduction percentage is shown in Figure 4. The analysis of each subgroup did not significantly reduce heterogeneity. The placebo subgroup showed an opposite result that the P‐value changed from <.05 to >.05. The results of the subgroup analysis are shown in Table S3; forest plots of subgroup analyses are shown in Figures S1 to S4. Then, a sensitivity analysis was performed to evaluate the impact of a single study on the results. The analysis showed that no study significantly changed the advantages of LLLT or significantly reduced the heterogeneity. The sensitivity analysis results are shown in Table S4.
FIGURE 4.
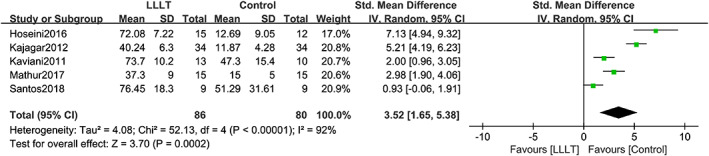
Forest plot of LLLT vs control for ulcer area reduction percentage. Compared with the control group, LLLT significantly reduced the ulcer area in patients with DFUs (P = .0002). The heterogeneity between the included studies was relatively high (I 2 = 92%). IV: inverse variance method; Random: random‐effects model
3.4.3. Mean healing time
Two studies reported the mean healing time of patients with DFUs. No heterogeneity was found after pooling the data (χ 2 = 0.30, df = 1, P = .58, I 2 = 0%); therefore, the fixed‐effects model was used for analysis. Compared with the control group, LLLT significantly decreased the mean healing time of patients with DFUs (SMD = −1.40, 95% CI −1.90 to −0.91, P < .00001). The forest plot of the mean healing time is shown in Figure 5.
FIGURE 5.
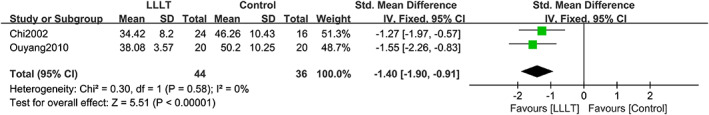
Forest plot of LLLT vs control for mean healing time. Compared with the control group, LLLT significantly shortened the mean healing time in patients with DFUs (P < .00001). IV: inverse variance method; Fixed: fixed‐effects model
3.5. Strength of evidence and publication bias assessment
According to the GRADE system, quality of the evidence was considered “very low” because of the imperfect study design, small sample size, significant heterogeneity, and potential publication bias. The results are summarised in Table 3. For the complete healing rate, the funnel plot was mildly asymmetric (Figure 6), and the Egger's test showed that there may be publication bias (P = .012). Regarding the ulcer area reduction percentage, the funnel plot was also mildly asymmetric (Figure S5), whereas Egger's test showed no significant publication bias (P = .316). Then, we performed a trim‐and‐fill analysis to evaluate the impact of publication bias on these results. The results showed that the combined effect size after trim‐and‐fill did not change significantly, indicating that publication bias had little effect on the results and that the meta‐analysis results had good authenticity. However, publication bias is inevitable because of the small number of included studies. Funnel plots after trim‐and‐fill are shown in Figures S6 and S7.
TABLE 3.
GRADE assessment for the effect of LLLT on the healing of diabetic foot ulcer
| Outcome | Number of studies | Study design | Certainty assessment | Effect | Certainty | ||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||||
| Complete healing rate | Nine | Randomised trials | Serious a | Not serious b | Not serious c | Serious d | Publication bias strongly suspected d | RR (95% CI): 2.10 (1.56, 2.83) |
⊕◯◯◯ Very Low |
| Ulcer area reduction percentage | Five | Randomised trials | Serious a | Serious e | Not serious c | Serious d | Publication bias strongly suspected d | SMD (95% CI): 3.52 (1.65, 5.38) |
⊕◯◯◯ Very Low |
| Mean healing time | Two | Randomised trials | Serious a | Not serious b | Not serious c | Serious d | Publication bias strongly suspected d | SMD (95% CI): −1.40 (−1.90, −0.91) |
⊕◯◯◯ Very Low |
Abbreviations: CI, confidence interval; RR, risk ratio; SMD, standardised mean difference.
The randomization method, allocation concealment and blinding method of some included studies were not clear, and some studies did not carry out allocation concealment and blinding method.
The 95% CI of each study overlapped, and the heterogeneity test showed no significant heterogeneity.
All included studies were related to research questions and no indirect comparisons were made.
The sample size is small.
The heterogeneity test showed that P < .00001, I 2 = 92%, and the subgroup analysis was conducted according to the control groups' intervention, sample size, Wagner grade and treatment time, but the results showed that the heterogeneity did not decrease.
FIGURE 6.
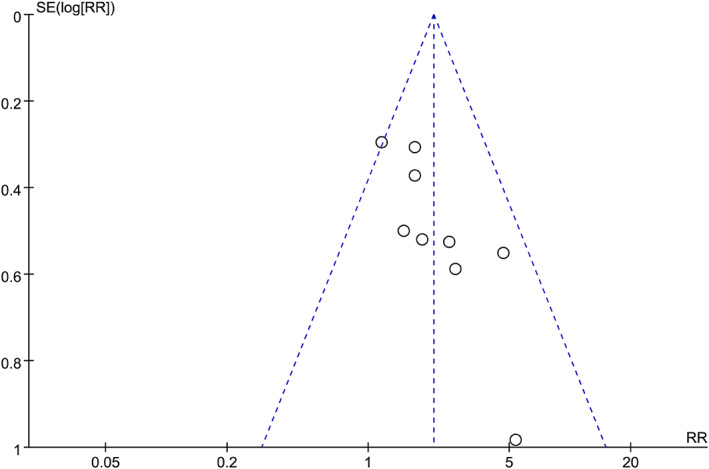
Funnel plot analysis of the complete healing rate
4. DISCUSSION
This meta‐analysis demonstrated that LLLT significantly improved the complete healing rate, reduced the areas of the ulcers, and shortened the mean healing time in patients with DFUs compared with the control group. Egger's test and trim‐and‐fill analysis showed that the potential risk of publication bias was low, and sensitivity analysis suggested the reliability of the results. To the best of our knowledge, this is currently the largest sample size, the only research that has evaluated the quality of evidence and has a rigorous evaluation process.
The main considerations of the mechanism of LLLT in the treatment of DFU as following. Collagen I is a major protein in the extracellular matrix that constitutes most of the connective tissue during wound healing. 48 , 49 However, diabetes can cause fibroblast proliferation disorders and impaired collagen synthesis. 50 An in vitro study found that LLLT could increase cell viability, cell migration, proliferation, and collagen synthesis. 51 LLLT induces macrophages to release factors that stimulate fibroblast proliferation. 52 In addition, LLLT can promote the production of interleukin‐1 alpha (IL‐1α) and interleukin‐8 (IL‐8), which can stimulate the migration of keratinocytes. 53 LLLT also increases the expression of platelet‐derived growth factor (PDGF) and transforming growth factor‐β (TGF‐β). 54 PDGF stimulates mitogenicity and chemotaxis of fibroblasts and smooth muscle cells and chemotaxis of neutrophils and macrophages, 55 playing a role in wound healing. Wound healing is a complex process, and TGF‐β has been shown to regulate these different steps by acting on multiple cell types and to promote the wound healing process in the body. 56 Moreover, LLLT leads to a reduction in tumour necrosis factor‐alpha (TNF‐α) concentration. 24 TNF‐α reduces cell migration and proliferation while encouraging apoptosis, 57 so its reduction helps wound healing. LLLT also increases the ulcer granulation rate of patients with DFUs. 40 In general, LLLT promotes ulcer healing by increasing the synthesis of collagen and extracellular matrix, recruiting‐related cytokines and growth factors, and promoting the migration, proliferation and differentiation of different cell types. 30 , 58
The therapeutic effect of LLLT depends on parameters such as power density, wavelength, fluence, irradiation time, and treatment duration. The recommended LLLT parameters in a previous review were as follows: wavelength of 660 or 890 nm, power density of 50 mW/cm2, fluence of 2 J/cm2, irradiation time of 30 seconds, and a distance of 1 cm away from the wound. 31 In our study, the wavelength range of LLLT was 400–904 nm, and the parameters of power density and fluence were roughly in line with the recommended parameters. In our included studies, the treatment time ranged from 15 days to 20 weeks. In the subgroup analysis of the ulcer area reduction percentage according to treatment time, it was found that the combined effect size in the 4‐week subgroup was higher than that in the 15‐day subgroup. This may mean that under certain conditions, the longer the treatment time, the better the ulcer healing.
It is worth noting that in the subgroup analysis of the ulcer area reduction percentage based on the control groups' intervention, the placebo subgroup showed the opposite result. However, there were only two studies 11 , 39 in this subgroup, and the P‐value (P = .08) was only slightly higher than .05. Based on this, we currently consider this result to be unclear, and we look forward to continued verification in high‐quality research in the future. Minatel et al 40 used a lower dose of placebo light as a control group. The ulcers in this group worsened during the initial 30 days; however, they turned slowly but steadily positive after the first 45 days of treatment. This indicates that a small amount of light has a cumulative effect, changing the trend of ulcer deterioration. However, in the two studies included in the placebo subgroup analysis, the control group received sham irradiation instead of a lower dose of light.
Among the included studies, two studies reported results related to pain. Minatel et al 40 showed that patients began to report pain relief as early as 1 week of LLLT. Santos et al 12 used the Visual Analog Scale (VAS) to assess pain intensity and found that there was no significant difference between the LLLT group and the control group in improving pain. A study that was not included in our analysis also used the VAS and found that after LLLT, the patient's pain improved. 59 Another study used the Brief Pain Inventory Questionnaire and the VAS, and found that pain was significantly improved after LLLT. 60 In other aspects of pain, studies showed that LLLT reduced the pain of eating, drinking, and toothbrushing for patients with recurrent aphthous stomatitis, 61 and LLLT could also benefit pain in patients with osteoarthritis. 62 However, another study showed that LLLT did not improve pain in postpartum women with a right mediolateral episiotomy after normal birth. 63 Therefore, the effect of LLLT in improving pain may be related to the nature and source of the pain. Current studies show that the effect of LLLT in improving pain in patients with DFUs is not clear, and more research is needed. In addition, Hoseini et al 39 reported that the ankle‐brachial index (ABI) values and skin temperature did not show any significant difference after LLLT compared with baseline, consistent with the results published by Carvalho et al. 60 However, another study indicated that LLLT accelerated collateral circulation and enhanced microcirculation. 64 Therefore, LLLT may promote angiogenesis and improve the microcirculation of patients with DFUs. Future studies should consider microcirculation as one of the potential indicators for evaluating the efficacy of LLLT, such as ABI, to clarify the effect of LLLT on the microcirculation of patients with DFUs. To the best of our knowledge, none of the included studies reported adverse effects of LLLT, indicating that LLLT was relatively safe.
Our findings are consistent with the results of previous systematic reviews 29 , 30 , 31 that LLLT is a very promising therapy for the treatment of DFUs, but the quality of the evidence is very low. Tchanque‐Fossuo et al 31 included four studies in their systematic review without meta‐analysis. Li et al 30 included seven studies in their systematic review and performed a meta‐analysis on the ulcer area reduction percentage and the complete healing rate. Santos et al 29 included 13 studies in their systematic review and performed a meta‐analysis on the ulcer area reduction percentage. In these systematic reviews, the sample sizes was small, and the quality of evidence was not rated, which weakened the impact of the results. In addition, Beckmann et al 18 reviewed the clinical studies of LLLT in the treatment of DFUs, but this study did not follow the reporting norms for systematic reviews, so the conclusions needed to be treated with caution.
Our study has several limitations. First, the pooled effect of ulcer area reduction percentage had significant heterogeneity (I 2 = 92%). Subgroup analyses were performed to explore the reasons for the heterogeneity, but it did not improve according to the control groups' intervention, sample size, treatment time or Wagner grade. The sensitivity analysis also did not find the source of heterogeneity by eliminating the included studies one by one. We did not perform subgroup analyses based on the parameters of LLLT because some of the included studies did not report complete LLLT parameters. Second, the LLLT parameters, treatment time, and baseline characteristics of ulcers in the included studies were not uniform, and these factors may affect the healing of ulcers. Finally, the small number of included studies and the small sample size would make possibility of publication bias inevitable.
5. CONCLUSION
The healing of DFUs is the focus of continuous exploration by researchers. This meta‐analysis demonstrates that LLLT is a promising and effective treatment for DFUs. Further evidence from larger samples and higher quality RCTs is needed to prove the effect of LLLT and to determine the most appropriate parameters for the healing of DFUs.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interests.
Supporting information
Figure S1: Forest plot of subgroup by control groups' intervention. IV: inverse variance method; Random: random‐effects model.
Figure S2: Forest plot of subgroup by sample size. IV: inverse variance method; Random: random‐effects model.
Figure S3: Forest plot of subgroup by treatment time. IV: inverse variance method; Random: random‐effects model.
Figure S4: Forest plot of subgroup by Wagner grade. IV: inverse variance method; Random: random‐effects model.
Figure S5: Funnel plot of ulcer area reduction percentage.
Figure S6 Funnel plots after trim‐and‐fill (complete healing rate).
Figure S7: Funnel plots after trim‐and‐fill (ulcer area reduction percentage).
Table S1: PubMed's detailed search process and search results.
Table S2: Sensitivity analysis results (complete healing rate). RR, risk ratio.
Table S3: Subgroup analysis of ulcer area reduction percentage.
Table S4: Sensitivity analysis (ulcer area reduction percentage). RR, risk ratio.
ACKNOWLEDGEMENTS
Thanks to the teachers of the library of Chongqing Medical University for guiding us to collect literatures. Thanks to Dr. Pangbo Wang for the language check. This work was supported by Nursing Science Research Fund of Chongqing Medical University (2019hlxk07).
Huang J, Chen J, Xiong S, Huang J, Liu Z. The effect of low‐level laser therapy on diabetic foot ulcers: A meta‐analysis of randomised controlled trials. Int Wound J. 2021;18:763–776. 10.1111/iwj.13577
Jing Huang and Jiangqiong Chen contributed equally to the study.
Funding information This work was supported by Nursing Science Research Fund of Chongqing Medical University, Grant/Award Number: 2019hlxk07
Contributor Information
Jiangqiong Chen, Email: jiangqiongchen@sina.com.
Zhiping Liu, Email: nfmlzp@163.com.
DATA AVAILABILITY STATEMENT
Data can be obtained from the original articles included in this study and the corresponding author of this study.
REFERENCES
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217‐228. [DOI] [PubMed] [Google Scholar]
- 2. Pickwell K, Siersma V, Kars M, et al. Predictors of lower‐extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38(5):852‐857. [DOI] [PubMed] [Google Scholar]
- 3. Cavanagh P, Attinger C, Abbas Z, Bal A, Rojas N, Xu ZR. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;28(Suppl 1):107‐111. [DOI] [PubMed] [Google Scholar]
- 4. Wukich DK, Raspovic KM. Assessing health‐related quality of life in patients with diabetic foot disease: why is it important and how can we improve? The 2017 Roger E Pecoraro Award Lecture. Diabetes Care. 2018;41(3):391‐397. [DOI] [PubMed] [Google Scholar]
- 5. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493‐1498. [DOI] [PubMed] [Google Scholar]
- 6. Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: from ulceration to death, a systematic review. Int Wound J. 2016;13(5):892‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5):S1‐S66. [DOI] [PubMed] [Google Scholar]
- 8. Zheng YJ, Ji SZ, Wu HB, Tian S, Wang XT. Acceleration of diabetic wound healing by a cryopreserved living dermal substitute created by micronized amnion seeded with fibroblasts. Am J Transl Res. 2015;7(12):2683‐2693. [PMC free article] [PubMed] [Google Scholar]
- 9. Lim JZM, Ng NSL, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110(3):104‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736‐1743. [DOI] [PubMed] [Google Scholar]
- 11. Kaviani A, Djavid GE, Ataie‐Fashtami L, et al. A randomized clinical trial on the effect of low‐level laser therapy on chronic diabetic foot wound healing: a preliminary report. Photomed Laser Surg. 2011;29(2):109‐114. [DOI] [PubMed] [Google Scholar]
- 12. de Alencar Fonseca Santos J, Campelo MBD, de Oliveira RA, Nicolau RA, Rezende VEA, Arisawa E. Effects of low‐power light therapy on the tissue repair process of chronic wounds in diabetic feet. Photomed Laser Surg. 2018;36(6):298‐304. [DOI] [PubMed] [Google Scholar]
- 13. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sridharan K, Sivaramakrishnan G. Growth factors for diabetic foot ulcers: mixed treatment comparison analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(3):434‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao D, Luo S, Xu W, Hu J, Lin S, Wang N. Efficacy and safety of hyperbaric oxygen therapy used in patients with diabetic foot: a meta‐analysis of randomized clinical trials. Clin Ther. 2017;39(10):2088‐2094.e2082. [DOI] [PubMed] [Google Scholar]
- 16. Borys S, Hohendorff J, Frankfurter C, Kiec‐Wilk B, Malecki MT. Negative pressure wound therapy use in diabetic foot syndrome‐from mechanisms of action to clinical practice. Eur J Clin Invest. 2019;49(4):e13067. [DOI] [PubMed] [Google Scholar]
- 17. Hawkins D, Houreld N, Abrahamse H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann N Y Acad Sci. 2005;1056:486‐493. [DOI] [PubMed] [Google Scholar]
- 18. Beckmann KH, Meyer‐Hamme G, Schröder S. Low level laser therapy for the treatment of diabetic foot ulcers: a critical survey. Evid Based Complement Alternat Med. 2014;2014:626127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karu T. Photobiology of low‐power laser effects. Heulfh Physfrs. 1989;56(5):691‐704. [DOI] [PubMed] [Google Scholar]
- 20. Taradaj J, Franek A, Blaszczak E, et al. Physical therapy in the treatment of venous leg ulcers: biophysical mechanisms. Wounds. 2012;24(5):138‐145. [PubMed] [Google Scholar]
- 21. Mester E, Szende B, Gärtner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl). 1968;9(5):621‐626. [PubMed] [Google Scholar]
- 22. Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971;122:532‐535. [DOI] [PubMed] [Google Scholar]
- 23. Mester E, Nagylucskay S, Döklen A, Tisza S. Laser stimulation of wound healing. Acta Chir Acad Sci Hung. 1976;17(1):49‐55. [PubMed] [Google Scholar]
- 24. Góralczyk K, Szymańska J, Szot K, Fisz J, Rość D. Low‐level laser irradiation effect on endothelial cells under conditions of hyperglycemia. Lasers Med Sci. 2016;31(5):825‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mirzae M, Bayat M, Mosafa N, et al. Effect of low‐level laser therapy on skin fibroblasts of streptozotocin‐diabetic rats. Photomed Laser Surg. 2007;25(6):519‐525. [DOI] [PubMed] [Google Scholar]
- 26. Dancakova L, Vasilenko T, Kovac I, et al. Low‐level laser therapy with 810 nm wavelength improves skin wound healing in rats with Streptozotocin‐induced diabetes. Photomed Laser Surg. 2014;32(4):198‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rezende SB, Ribeiro MS, Nunez SC, Garcia VG, Maldonado EP. Effects of a single near‐infrared laser treatment on cutaneous wound healing: biometrical and histological study in rats. J Photochem Photobiol B. 2007;87(3):145‐153. [DOI] [PubMed] [Google Scholar]
- 28. Mathur RK, Sahu K, Saraf S, Patheja P, Khan F, Gupta PK. Low‐level laser therapy as an adjunct to conventional therapy in the treatment of diabetic foot ulcers. Lasers Med Sci. 2017;32(2):275‐282. [DOI] [PubMed] [Google Scholar]
- 29. Santos CMD, Rocha RBD, Hazime FA, Cardoso VS. A systematic review and meta‐analysis of the effects of low‐level laser therapy in the treatment of diabetic foot ulcers. Int J Low Extrem Wounds. 2020;1534734620914439. https://10.1177/1534734620914439. [DOI] [PubMed] [Google Scholar]
- 30. Li S, Wang C, Wang B, et al. Efficacy of low‐level light therapy for treatment of diabetic foot ulcer: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Res Clin Pract. 2018;143:215‐224. [DOI] [PubMed] [Google Scholar]
- 31. Tchanque‐Fossuo CN, Ho D, Dahle SE, et al. A systematic review of low‐level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen. 2016;24(2):418‐426. [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guyatt GH, Oxman AD, Vist GE, et al. GRADE an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820‐826. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 37. Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ Open Diabetes Res Care. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ortíz MCS, Villabona EH, Lemos DMC, Castellanos R. Effects of low level laser therapy and high voltage stimulation on diabetic wound healing. Rev Univ Ind Santander Salud. 2014;46(3):107‐117. [Google Scholar]
- 39. Hoseini Sanati M, Torkaman G, Hedayati M. M IAEffect of Ga‐as laser on decrease of wound surface area and ABI value in diabetic foot ulcers. J Zanjan Univ Med Sci Health Serv. 2016;25(103):20‐21. [Google Scholar]
- 40. Minatel DG, Frade MA, França SC, Enwemeka CS. Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers Surg Med. 2009;41(6):433‐441. [DOI] [PubMed] [Google Scholar]
- 41. Kajagar BM, Godhi AS, Pandit A, Khatri S. Efficacy of low level laser therapy on wound healing in patients with chronic diabetic foot ulcers‐a randomised control trial. Indian J Surg. 2012;74(5):359‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen HJ, Wang GF, Gao M. Application of semiconductor laser to accelerate the healing of diabetic foot ulcers. Chin J Phys Med Rehab. 2003;25(2):104. [Google Scholar]
- 43. Chi LX, Huang JQ, Li JH, Teng YP, Xue JS, Zhang Q. The efficiency of laser on the treatment of diabetic foot ulcer. Hainan Med J. 2002;13(8):20‐21. [Google Scholar]
- 44. Cui ZH, Tang FF. Low‐intensity he‐ne laser irradiation assisted treatment of diabetic foot comprehensive nursing experience. IMHGN. 2009;15(13):126‐128. [Google Scholar]
- 45. Ouyang ZS, Li WZ. Diabetic foot ulcer treated with 650 nm semiconductor laser after surgical treatment:a clinical study. Clin Focus. 2010;25(16):1388‐1389. [Google Scholar]
- 46. Zhang LJ. Observation on the effect of he‐ne laser irradiation combined with surgical debridement in the treatment of 24 cases of diabetic foot ulcer. Chin Commun Doctors. 2012;14(11):92. [Google Scholar]
- 47. Landau Z, Migdal M, Lipovsky A, Lubart R. Visible light‐induced healing of diabetic or venous foot ulcers: a placebo‐controlled double‐blind study. Photomed Laser Surg. 2011;29(6):399‐404. [DOI] [PubMed] [Google Scholar]
- 48. Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S‐34S. [DOI] [PubMed] [Google Scholar]
- 49. Ricard‐Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris). 2005;53(7):430‐442. [DOI] [PubMed] [Google Scholar]
- 50. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ayuk SM, Houreld NN, Abrahamse H. Collagen production in diabetic wounded fibroblasts in response to low‐intensity laser irradiation at 660 nm. Diabetes Technol Ther. 2012;14(12):1110‐1117. [DOI] [PubMed] [Google Scholar]
- 52. Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C. Macrophage responsiveness to light therapy. Lasers Surg Med. 1989;9(5):497‐505. [DOI] [PubMed] [Google Scholar]
- 53. Yu HS, Chang KL, Yu CL, Chen JW, Chen GS. Low‐energy helium‐neon laser irradiation stimulates interleukin‐1 alpha and interleukin‐8 release from cultured human keratinocytes. J Invest Dermatol. 1996;107(4):593‐596. [DOI] [PubMed] [Google Scholar]
- 54. Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, Mir M. Effects of low‐level he‐ne laser irradiation on the gene expression of IL‐1beta, TNF‐alpha, IFN‐gamma, TGF‐beta, bFGF, and PDGF in rat's gingiva. Lasers Med Sci. 2008;23(3):331‐335. [DOI] [PubMed] [Google Scholar]
- 55. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet‐derived growth factor. Physiol Rev. 1999;79(4):1283‐1316. [DOI] [PubMed] [Google Scholar]
- 56. Morikawa M, Derynck R, Miyazono K. TGF‐beta and the TGF‐beta family: context‐dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8(5):a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor alpha to improve wound healing in diabetic mice. Bioeng Transl Med. 2019;4(1):75‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Freitas LF, Hamblin MR. Proposed mechanisms of Photobiomodulation or low‐level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):7000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Feitosa MC, Carvalho AF, Feitosa VC, Coelho IM, Oliveira RA, Arisawa E. Effects of the low‐level laser therapy (LLLT) in the process of healing diabetic foot ulcers. Acta Cir Bras. 2015;30(12):852‐857. [DOI] [PubMed] [Google Scholar]
- 60. Carvalho AF, Feitosa MC, Coelho NP, et al. Low‐level laser therapy and Calendula officinalis in repairing diabetic foot ulcers. Rev Esc Enferm USP. 2016;50(4):628‐634. [DOI] [PubMed] [Google Scholar]
- 61. Albrektson M, Hedstrom L, Bergh H. Recurrent aphthous stomatitis and pain management with low‐level laser therapy: a randomized controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):590‐594. [DOI] [PubMed] [Google Scholar]
- 62. Dima R, Francio VT, Towery C, Davani S. Review of literature on low‐level laser therapy benefits for nonpharmacological pain control in chronic pain and osteoarthritis. Altern Ther Health Med. 2018;24(5):8‐10. [PubMed] [Google Scholar]
- 63. Alvarenga MB, de Oliveira SM, Francisco AA, da Silva FM, Sousa M, Nobre MR. Effect of low‐level laser therapy on pain and perineal healing after episiotomy: a triple‐blind randomized controlled trial. Lasers Surg Med. 2017;49(2):181‐188. [DOI] [PubMed] [Google Scholar]
- 64. Ihsan FRM. Low‐level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg. 2005;23(3):289‐294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Forest plot of subgroup by control groups' intervention. IV: inverse variance method; Random: random‐effects model.
Figure S2: Forest plot of subgroup by sample size. IV: inverse variance method; Random: random‐effects model.
Figure S3: Forest plot of subgroup by treatment time. IV: inverse variance method; Random: random‐effects model.
Figure S4: Forest plot of subgroup by Wagner grade. IV: inverse variance method; Random: random‐effects model.
Figure S5: Funnel plot of ulcer area reduction percentage.
Figure S6 Funnel plots after trim‐and‐fill (complete healing rate).
Figure S7: Funnel plots after trim‐and‐fill (ulcer area reduction percentage).
Table S1: PubMed's detailed search process and search results.
Table S2: Sensitivity analysis results (complete healing rate). RR, risk ratio.
Table S3: Subgroup analysis of ulcer area reduction percentage.
Table S4: Sensitivity analysis (ulcer area reduction percentage). RR, risk ratio.
Data Availability Statement
Data can be obtained from the original articles included in this study and the corresponding author of this study.


