Abstract
Background:
In the subclinical hypothyroidism, T4 or T3 levels are normal and thyroid-stimulating hormone (TSH) is slightly high. Selenium deficiency can lead to thyroid dysfunction. The present study aims to investigate the effect of selenium supplementation on the thyroid hormone and anti-thyroid peroxidase antibody (anti-TPO AB) levels.
Materials and Methods:
In this double-blinded, randomized, placebo-controlled clinical trial, 42 patients with subclinical hypothyroidism were randomly assigned to receive 200 μg selenium or placebo for 8 weeks. In the both groups, the serum TSH and anti-TPO antibody levels were measured and assessed before and after the intervention.
Results:
After the interventions, the mean serum TSH reduction in the intervention and placebo groups was −10.98 ± 33.31 and −3.20 ± 38.36, respectively, which were not statistically significant. However, the mean serum anti-TPO Ab concentration increased in the intervention and placebo groups (109.81 ± 51.49% vs. 173.17 ± 96.26%), between which the difference was not statistically significant (P >0.05) despite a slight increase in the mean anti-TPO level in the intervention group.
Conclusion:
The results of the current study indicated that selenium supplementation has no significant effect on serum anti-TPO Ab and TSH levels in the patients with subclinical hypothyroidism. Studies with larger sample size and with different doses of selenium are needed to reach more precise results.
Keywords: Hashimoto Thyroiditides, hypothyroidism, selenium
Introduction
Hashimoto's thyroiditis (HT) is the most common thyroid disorder which affects 1%–2 % of the population.[1] It is a gender-specific disorder, which affects 5%–10% of the female population in the childbearing age.[2] It is characterized by the presence of anti-thyroid peroxidase autoantibodies that are closely associated with overt thyroid dysfunction and correlated with progressive thyroidal damage and lymphocytic inflammation.[3,4] In subclinical hypothyroidism, T4 or T3 levels are normal and TSH is slightly elevated. Some patients, but not all, will have overt hypothyroidism.[5]
Along with several genetic and environmental factors, selenium deficiency has been implicated in its pathogenesis. Selenium is the essential micronutrient for the synthesis, activation, and metabolism of thyroid hormones. The highest amount of selenium per gram of tissue is accumulated in the thyroid gland. However, selenium plays a major role in antioxidant defense in the structure of selenoproteins.[3]
Some recent studies have found that selenium deficiency is associated with the increased risk of thyroid disease, especially in the conversion of T4 into T3.[6,7,8,9]
According to the available published data, no consistent health effects of selenium supplementation in autoimmune disorders can be deduced. In the patients with HT as well as in pregnant women with elevated anti-thyroid peroxidase antibody (anti-TPO Ab), selenium supplementation leads to a decrease in the anti-thyroid antibody concentrations.[5,10] In the pregnant women, who have been treated with selenium supplementation, lower percentage of postpartum and hypothyroid thyroiditis has been observed. However, adding selenium to the diet in the patients with HT has resulted in significantly decreased clinical symptoms in comparison with the control group.[5,10,11] In fact, epidemiological studies have shown that adequate selenium intake is necessary for thyroid function.Diet enrichment with selenium in the patients with subclinical hypothyroidism has also reduced the level of antibodies and increased levels of thyroid hormones and, subsequently, improved the quality of life.[11,12]
In contrast to the studies published in 2005 by Moncayo et al., only a few cases of patients with autoimmune hypothyroidism exhibited marked recovery of thyroid function after selenium treatment.[13] However, selenium supplementation had no positive effect on thyroid echogenicity or TPO Ab levels in the patients with normal selenium concentration.[14]
Furthermore, in another report, 200 μg/day selenium supplementation in the women with autoimmune hypothyroidism did not induce significant immunological changes, neither in terms of cytokine production patterns of peripheral T lymphocytes, nor anti-TPO Ab levels.[15]
According to the previous works, it seems that administering selenium supplements in individuals with subclinical hypothyroidism may improve their health status and does not lead to clinical hypothyroidism. Due to insufficient trial evaluation of clinically relevant outcomes, the present study aims to evaluate the effect of selenium-enriched yeast supplementation on the serum of anti-TPO Ab and TSH levels among subclinical hypothyroid patients.
Materials and Methods
Study design
This double-blind randomized controlled clinical trial was carried out on 42 patients with subclinical hypothyroidism. The study protocol was approved Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1398.339) and was registered on the website of Iranian Registry of Clinical Trials (IRCT20190212042686N2), available in https://www.irct.ir/.
Study population
Patients were recruited from the outpatient ward of Endocrine Clinic, Emam Reza Hospital, Tabriz City, from July 2019 to October 2019, on receiving their written consent forms.
Study inclusion criteria were patients with subclinical hypothyroidism, aged 18–60-year-old. Diagnoses were made by elevated basal TSH levels in two consecutive tests as well as examination by expert endocrinologist.[16] The exclusion criteria included consuming trace element and antioxidant supplements in the previous 6 months, pregnancy and lactation, having acute and chronic renal disease, heart problems (ischemic heart diseases, congenital adrenal hyperplasia, and coronary heart diseases), renal failure, proteinuria, liver disease, and proteinuria.
Randomization
A computer-generated random sequence was kept in a secure remote location and administered by an independent third party who was not involved in the clinical conduct of the study until all the data were collected and verified. Patients and those involved in enrolling the participants, administering the interventions, and assessing the outcomes were blind to group assignments.
Sample size
The sample size was determined based on the results of a pilot study conducted on the scale of 5 samples per group. In this study, TSH changes were considered as the primary outcome. Based on the results of the pilot study, the amount of TSH changes before and after intervention in the intervention and placebo groups were 1.28 ± 0.25 and 1.09 ± 0.36, respectively. The sample size was calculated to be 20 for each group using standard formula for randomized controlled trial based on the first type error (α) of 0.05 and 80% power anticipating an approximate drop-out rate of 10% during the study; thus, 21 participants were recruited in each group.
Trial procedure
The patients were randomly assigned to the supplemented group (n = 21), receiving 200 μg selenium capsule (each capsule contained Saccharomyces cerevisiae yeast, which is permitted for human consumption) once a day, or a control group (n = 21) receiving one placebo capsule for 8 weeks. Each selenium capsule contained 200 μg of selenium selenite and 1800 μg Saccharomyces cerevisiae yeast, which is permitted for human consumption. The selenium supplement was produced in Nutrition Research Center, Tabriz University of medical sciences, examined for heavy metal and other mineral contaminations and filled in capsules in the laboratory of Faculty of Pharmacy. The participants were asked to keep their usual dietary intake, physical activity, and medication during the study unchanged. Patients were monitored weakly for any side-effects of the supplementation.
Outcome measures
At the onset and end of the intervention, 5 mL of venous blood sample was collected. The serum samples of the patients were kept at −80°C until the biochemical analysis. The serum anti-TPO Ab and TSH, T3, and T4 concentrations were measured by Liaison with DiaSorin kits by electrochemiluminescence method. The TSH and anti-TPO Ab levels of above 4.4 and 34 mg/dl were considered as the abnormal values, respectively.
Statistical analysis
Descriptive parameters were obtained for all the study variables in each study group. The normality of the variables was tested by Kolmogorov–Smirnov test. ANCOVA test was used to compare the findings before and after the intervention in each group. The McNemar and Chi-square tests were also employed to compare the qualitative findings between the two groups. A P < 0.05 was considered statistically significant. Data were presented as mean ± standard deviation. Statistical analysis was performed using SPSS software (version 17; SPSS, Inc., Chicago, IL, USA).
Results
Baseline data outcomes
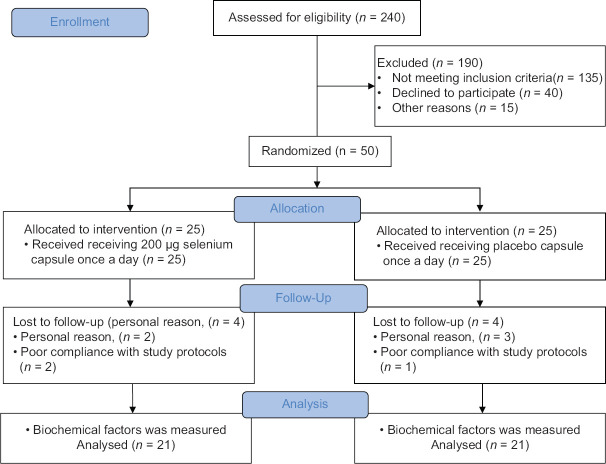
In total, eight patients did not complete the study for reasons as follows: three patients due to poor compliance with study protocols and five patients due to personal reasons [Figure 1]. Baseline characteristics of the patients are presented in Table 1. At the beginning of the study, the two groups were similar based on the mean age and biochemical parameters levels. Furthermore, the two groups were similar based on the mean age, gender, and biochemical parameters.
Figure 1.
CONSORT follow diagram
Table 1.
Baseline characteristics of participants
| Variables | Intervention (n=21) | Placebo (n=21) | P |
|---|---|---|---|
| Age (year) | 44.04±9.80 | 40.28±11.44 | 0.26 |
| Gender, n (%) | |||
| Men | 1 (4.8) | 4 (19) | 0.15 |
| Women | 20 (95.2) | 17 (81) | |
| TSH (mg/dl) | 6.85±2.97 | 5.63±1.83 | 0.11 |
| Anti-TPO Ab (mg/dl) | 421.79±399.45 | 452.44±366.68 | 0.15 |
TPO-Ab: Thyroid peroxidase antibody, TSH: Thyroid-stimulating hormone
Laboratory findings
The mean TSH and anti-TPO Ab levels of the studied groups at the baseline and after intervention are presented in Table 2. There was no difference in the mean serum TSH and anti-TPO Ab concentration between the two groups before and after the intervention.
Table 2.
Mean (standard deviation) level of thyroid-stimulating hormone and anti-thyroid peroxidase antibody of the participants throughout the study
| Variables | Intervention (n=21) | Placebo (n=21) | P |
|---|---|---|---|
| TSH pretest | 85.97±6.2 | 63.83±5.1 | 0.11 |
| TSH posttest | 39.95±6.39 | 01.78±5.1 | 0.8 |
| Anti-TPO Ab pretest | 79345±421.399 | 44.68±452.366 | 0.15 |
| Anti-TPO Ab posttest | 19.21±516.390 | 60.87±524.380 | 0.94 |
TPO-Ab: Thyroid peroxidase antibody, TSH: Thyroid-stimulating hormone
The within-group comparison showed that the mean TSH level decreased (P = 0.38) and the mean anti-TPO Ab increased after the intervention (P = 0.14) insignificantly in the intervention group. However, the elevation in the mean serum TSH (P = 0.24) and anti-TPO Ab (P = 0.37) levels were not statistically significant in the placebo group.
The mean difference of TSH level in the intervention group was −10.98 ± 33.31 and, in the placebo group, it was −3.20 ± 38.36%, which was not statistically significant despite more reduction in the intervention group (P = 0.48).
The mean difference of anti-TPO Ab level in the intervention and placebo groups was 109.81 ± 51.49% and 173.17 ± 96.26%, respectively. In this case as well, the mean changes were not statistically significant (P = 0.56) despite a small increase in the mean level of anti-TPO in the intervention group [Table 3].
Table 3.
Frequency of high level of thyroid-stimulating hormone and anti-thyroid peroxidase antibody of the participants throughout the study
| Variables | Intervention (n=21), n (%) | Placebo (n=21), n (%) | Placebo (n=21), n (%) |
|---|---|---|---|
| TSH pretest | 18 (87.5) | 16 (76.2) | 16 (76.2) |
| TSH posttest | 14 (66.7) | 14 (66.7) | 14 (66.7) |
| Anti-TPO Ab posttest | 20 (95.2) | 17 (81) | 0.15 |
TPO-Ab: Thyroid peroxidase antibody, TSH: Thyroid-stimulating hormone
The frequency of high level of TSH and anti-TPO Ab concentrations is shown in Table 3. The insignificant reduction in abnormal TSH levels was observed in both the groups (P > 0.05). The rate of cases with abnormal anti-TPO levels did not differ before and after the intervention in both groups.
Discussion
The main finding of this study was that TSH and anti TPOAb concentrations were not statistically different at the baseline level and after 2 months of SE or placebo supplementation, respectively.
Recent research has shown that selenium not only helps restore and balance the thyroid, but also is effective in balancing the immune system. Selenium regulates the immune system and selenium deficiency in the body causes the immune system to dysfunction. Mild selenium deficiency worsens autoimmune disease, but severe deficiency leads to destroying the thyroid cells and increasing the macrophages levels.[17]
Our result seems to be, somehow, similar to what observed in a previous study showing that selenium supplementation (166 mg sodium selenite/day) in a group of 38 Italian participants with HT had no significantly in serum concentrations of TSH.[18]
On the contrary, another study conducted by Nordio and Basciani showed that TSH levels were significantly reduced in the patients receiving selenium and Myo-inositol (MI) supplements.[17] Mahmoodianfard et al.[19] and Pirola et al.[2] have also reported that if selenium is used in combination with levothyroxine, TSH levels would be significantly reduced.
We can speculate that the discrepancy of our results compared to that of previous studies could be probably due to different time of SE supplementation associated with lower selenium dietary intake, different dose of SE supplement, and short duration of our trial. Knowledge about background SE level was limited among our participants.
Future trials of selenium supplementation in HT should pay close attention to the selenium status of the study populations. It seems that selenium supplementation should be long-term and with high doses of selenium to reach favorable results on TSH levels.
In addition, in the present study, it was found that the level of anti-TPO Ab in the patients after taking selenium did not differ significantly from those taking placebo.
In addition to the present study, Kyrgios et al. reported that if high-dose selenium was added, although anti-TPO and anti-Tg levels were reduced, the change did not differ significantly from that in the control group.[20]
Kachouie et al. reported similar results and found no significant difference between pre- and post-intervention results in mean serum anti-TPO Ab levels.[21]
However, our results were not similar to those of Nordio and Basciani and Yu et al., who have shown different results.[17,22] Nordio et al. reported that the anti-TPO Abs and anti-thyroglobulin levels in the patients treated with selenium and MI decreased significantly after 6 months of treatment.[17]
Furthermore, Yu et al. showed that if selenium was added to the treatment of chronic lymphocytic thyroiditis in the patients receiving selenium and levothyroxine, the serum concentrations of TPO antibody, Tg antibody, interleukin (IL-2), and IL-10 were decreased.[22]
On the other hand, Santos et al. obtained confusing results and concluded that, in several studies, selenium consumption had no effect on anti-TPO Abs, whereas in others, selenium supplementation led to a decrease in anti-TPO Abs and anti-Tg depletion.[23]
The reason for this inconsistency could be that the initial values of TPOAb were considerably higher in the previous works which reported significant reduction in the TPOAb levels after selenium supplementation. For example, in Gärtner et al.,[24], Duntas et al.[25] and Turker et al.,[26] the anti-TPO Abs levels have been 904 ± 205, 1875 ± 1039, and 804 ± 484 IU/mL, respectively in comparison with our patients (421.79 ± 399.45 IU/mL). The result of this evidence suggested that higher anti-TPO Abs titers at the baseline may result in the maximum response to the supplementation.[3]
The limitations of the present study were its short duration and small sample size of the participants. However, the strength was in using organic selenium in formulating supplement, which had better absorption and preferable formulation.[27,28]
Furthermore, most of the patients in the selenium supplemented group reported improvement in the mood and quality of their life. Previous trial results give some promise of beneficial effects of SE supplementation on health-related quality of life.[29] The future trials should be designed to evaluate this outcome using validated quality-of-life measurement to reach more concise results.
Conclusion
The results of the present study showed that selenium supplementation had no significant effect on serum anti-TPO Ab and TSH levels in the patients with subclinical hypothyroidism.
Considering the inconsistent results in several studies and lack of evidence, especially systematic review studies and meta-analyses in relation to the effect of selenium supplement on the serum levels of anti-TPO Abs and TSH, further studies with more sample size and different doses of selenium are necessary to achieve more precise results.
Financial support and sponsorship
We kindly acknowledge Endocrine Research Center of Tabriz University of Medical Sciences for their financial support.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors are deeply indebted to all the patients who participated in this study. This work was financially supported by Endocrine Research Center, Tabriz University of Medical Sciences. The results of this paper are extracted from the dissertation of (Dr. Leila Mahmoudi), the resident of internal medicine, which was registered at Tabriz University of Medical Sciences. The authors like to thank Dr. Morteza Ghojazadeh for contributing in data analysis.
References
- 1.Ott J, Promberger R, Kober F, Neuhold N, Tea M, Huber JC, et al. Hashimoto's thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: A prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011;21:161–7. doi: 10.1089/thy.2010.0191. [DOI] [PubMed] [Google Scholar]
- 2.Pirola I, Gandossi E, Agosti B, Delbarba A, Cappelli C. Selenium supplementation could restore euthyroidism in subclinical hypothyroid patients with autoimmune thyroiditis. Endokrynol Pol. 2016;67:567–71. doi: 10.5603/EP.2016.0064. [DOI] [PubMed] [Google Scholar]
- 3.Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: A systematic review and a meta-analysis. Thyroid. 2010;20:1163–73. doi: 10.1089/thy.2009.0351. [DOI] [PubMed] [Google Scholar]
- 4.Liontiris MI, Mazokopakis EE. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients.Points that need more investigation. Hell J Nucl Med. 2017;20:51–6. doi: 10.1967/s002449910507. [DOI] [PubMed] [Google Scholar]
- 5.Tan L, Sang ZN, Shen J, Wu YT, Yao ZX, Zhang JX, et al. Selenium supplementation alleviates autoimmune thyroiditis by regulating expression of TH1/TH2 cytokines. Biomed Environ Sci. 2013;26:920–5. doi: 10.3967/bes2013.022. [DOI] [PubMed] [Google Scholar]
- 6.Contempré B, de Escobar GM, Denef JF, Dumont JE, Many MC. Thiocyanate induces cell necrosis and fibrosis in selenium-and iodine-deficient rat thyroids: A potential experimental model for myxedematous endemic cretinism in central Africa. Endocrinology. 2004;145:994–1002. doi: 10.1210/en.2003-0886. [DOI] [PubMed] [Google Scholar]
- 7.Stuss M, Michalska-Kasiczak M, Sewerynek E. The role of selenium in thyroid gland pathophysiology. Endokrynol Pol. 2017;68:440–65. doi: 10.5603/EP.2017.0051. [DOI] [PubMed] [Google Scholar]
- 8.Ventura M, Melo M, Carrilho F. Selenium and thyroid disease: From pathophysiology to treatment. Int J Endocrinol. 2017;2017:1–9. doi: 10.1155/2017/1297658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegedüs L, Bonnema SJ, Winther KH. Selenium in the treatment of thyroid diseases: An element in search of the relevant indications? Eur Thyroid J. 2016;5:149–51. doi: 10.1159/000448002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidder GW, Montgomery CW. Oxygenation of frog gastric mucosa in vitro. Am J Physiol. 1975;229:1510–3. doi: 10.1152/ajplegacy.1975.229.6.1510. [DOI] [PubMed] [Google Scholar]
- 11.Köhrle J. Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes. 2015;22:394–401. doi: 10.1097/MED.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 12.Andrade GR, Gorgulho B, Lotufo PA, Bensenor IM, Marchioni DM. Dietary selenium intake and subclinical hypothyroidism: A cross-sectional analysis of the ELSA-Brasil study. Nutrients. 2018;10:E693. doi: 10.3390/nu10060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moncayo R, Moncayo H, Kapelari K. Nutritional treatment of incipient thyroid autoimmune disease.Influence of selenium supplementation on thyroid function and morphology in children and young adults. Clin Nutr. 2005;24:530–1. doi: 10.1016/j.clnu.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Pilli T, Cantara S, Schomburg L, Cenci V, Cardinale S, Heid EC, et al. IFNγ-inducible chemokines decrease upon selenomethionine supplementation in women with euthyroid autoimmune thyroiditis: Comparison between two doses of selenomethionine (80 or 160 μg) versus placebo. Eur Thyroid J. 2015;4:226–33. doi: 10.1159/000439589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karanikas G, Schuetz M, Kontur S, Duan H, Kommata S, Schoen R, et al. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid. 2008;18:7–12. doi: 10.1089/thy.2007.0127. [DOI] [PubMed] [Google Scholar]
- 16.Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun Rev. 2014;13:391–7. doi: 10.1016/j.autrev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Nordio M, Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto's patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. 2017;21:51–9. [PubMed] [Google Scholar]
- 18.Esposito D, Rotondi M, Accardo G, Vallone G, Conzo G, Docimo G, et al. Influence of short-term selenium supplementation on the natural course of Hashimoto's thyroiditis: Clinical results of a blinded placebo-controlled randomized prospective trial. J Endocrinol Invest. 2017;40:83–9. doi: 10.1007/s40618-016-0535-4. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoodianfard S, Vafa M, Golgiri F, Khoshniat M, Gohari M, Solati Z, et al. Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: A randomized double-blind controlled trial. J Am Coll Nutr. 2015;34:391–9. doi: 10.1080/07315724.2014.926161. [DOI] [PubMed] [Google Scholar]
- 20.Kyrgios I, Giza S, Kotanidou EP, Kleisarchaki A, Tsinopoulou VR, Papadopoulou A, et al. l-selenomethionine supplementation in children and adolescents with autoimmune thyroiditis: A randomized double-blind placebo-controlled clinical trial. J Clin Pharm Ther. 2019;44:102–8. doi: 10.1111/jcpt.12765. [DOI] [PubMed] [Google Scholar]
- 21.Kachouei A, Rezvanian H, Amini M, Aminorroaya A, Moradi E. The effect of levothyroxine and selenium versus levothyroxine alone on reducing the level of anti-thyroid peroxidase antibody in autoimmune hypothyroid patients. Adv Biomed Res. 2018;7:1. doi: 10.4103/2277-9175.223735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu L, Zhou L, Xu E, Bi Y, Hu X, Pei X, et al. Levothyroxine monotherapy versus levothyroxine and selenium combination therapy in chronic lymphocytic thyroiditis. J Endocrinol Invest. 2017;40:1243–50. doi: 10.1007/s40618-017-0693-z. [DOI] [PubMed] [Google Scholar]
- 23.Santos LR, Neves C, Melo M, Soares P. Selenium and selenoproteins in immune mediated thyroid disorders. Diagnostics. 2018;8:E70. doi: 10.3390/diagnostics8040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gärtner R, Gasnier BC, Dietrich JW, Krebs B, Angstwurm MW. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J Clin Endocrinol Metab. 2002;87:1687–91. doi: 10.1210/jcem.87.4.8421. [DOI] [PubMed] [Google Scholar]
- 25.Duntas L, Mantzou E, Koutras D. Effects of a six months treatment with selenometheionine in patients with autoimmune thyroiditis. Eur J Endocrinol. 2003;148:389–93. doi: 10.1530/eje.0.1480389. [DOI] [PubMed] [Google Scholar]
- 26.Turker O, Kumanlioglu K, Karapolat I, Dogan I. Selenium treatment in autoimmune thyroiditis: 9-month follow-up with variable doses. J Endocrinol. 2006;190:151–6. doi: 10.1677/joe.1.06661. [DOI] [PubMed] [Google Scholar]
- 27.Wojciechowska-Durczynska K, Lewinski A. Search for relevant indications for selenium supplementation in thyroid diseases. Neuro Endocrinol Lett. 2017;38:237–41. [PubMed] [Google Scholar]
- 28.Winther KH, Papini E, Attanasio R, Negro R, Hegedüs L. A 2018 European Thyroid Association survey on the use of selenium supplementation in Hashimoto's thyroiditis. Eur Thyroid J. 2020;9:99–105. doi: 10.1159/000504781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winther KH, Wichman JE, Bonnema SJ, Hegedüs L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376–85. doi: 10.1007/s12020-016-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]