Abstract
PI3K is a downstream target of multiple cell-surface receptors, which acts as a crucial modulator of both cell polarization and survival. PI3K/AKT signaling pathway is commonly involved in cancer, atherosclerosis, and other diseases. However, its role in cardiovascular diseases, especially in atherosclerosis, remains to be further investigated. To determine the effect of PI3K/AKT signaling pathway on cellular inflammatory response and oxidative stress, PI3K inhibitor (GDC0941) and AKT inhibitor (MK2206) were used. First, THP-1 cells were incubated with ox-LDL (100 µg/ml) to establish an in vitro atherosclerosis model. The inflammatory factors and foam cell formation were then evaluated to ascertain and compare the effects of PI3K and AKT inhibition. ApoE−/− mice fed a high-fat diet were used to assess the roles of PI3K and AKT in aortic plaque formation. Our results showed that the inhibition of PI3K or AKT could suppress the activation of NLRP3, decreased the expression levels of p-p65/p65 and reduced the production of mitochondrial reaction oxygen species (mitoROS) in THP-1 cells. Inhibition of PI3K or AKT could also reduced atherosclerosis lesion and plaque area, and decreased the levels of NLRP3 and IL-1β in ApoE−/− mice. The effect of PI3K inhibition was more significant than AKT. Therefore, PI3K inhibition can retard the progress of atherosclerosis. Besides, there may be other AKT-independent pathways that regulate the formation of atherosclerosis.
Keywords: atherosclerosis, NLRP3, PI3K/AKT
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by the activation of pro-inflammatory signaling pathway, overexpression of cytokines/chemokines and increased production of oxidative stress [1, 2]. Inflammatory macrophages are the most abundant immune cells found in the atherosclerotic plaques, which are originated from the circulating monocytes that bind to the activated endothelial cells and migrate into the intimal layer. Within the plaque, macrophages orchestrate the progression of atherosclerosis via the uptake of oxidized low-density lipoprotein (ox-LDL) particles and subsequent formation of foam cells. Oxidative stress is caused by the presence of intracellular reaction oxygen species (ROS) that impairs natural antioxidant defense system in cells [3]. NLRP3 inflammasome is a molecular platform activated upon signs of cellular ‘danger’ to trigger innate immune responses through the maturation of pro-inflammatory cytokines such as interleukin (IL)-1β [4, 5]. It consists of NOD-like receptor proteins (NLRP; the NOD, LRR and pyrin domain (PYD)-containing proteins), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC; an adaptor molecule) and pro-caspase 1 (an effector subunit) [6]. The increased production of IL-1β and IL-18 by NLRP3 inflammasome can contribute to the progression and instability of atherosclerotic plaques in atherosclerosis patients and animal models [7].
Inflammation is a direct response to the invasion of microbial pathogens in the innate immune system. Macrophages are essential components of mammalian tissues that are strategically positioned throughout the body to maintain tissue homeostasis and act as immune sentinels [8]. Thus, macrophages play an important role in different stages of atherosclerosis [9]. PI3Ks are enzymes that catalyze the phosphorylation of one or more inositol phospholipids at the 3’-position of the inositol ring [10]. PI3K signaling is a downstream target of multiple cell-surface receptors, which acts as a crucial modulator of both cell polarization and survival. Excessive activation/deactivation of PI3K signaling pathway is associated with many diseases, which highlights the importance of proper PI3K regulation [11]. Some researchers [12, 13] suggest that PI3K is involved in inflammatory response, while others [14] suggest its involvement in both inflammatory response and endothelial dysfunction. Consistent with previous research, Ong and colleagues [15] have reported that PI3K is associated with atherosclerosis in THP-1 cells. Compared to other downstream effectors, several members of the AKT sub-family of AGC serine/threonine kinases (e.g., AKT1, AKT2 and AKT3) seem to be activated more universally in response to PI3K activation [11]. Previous studies have demonstrated that AKT2-deficient macrophages reduce atherosclerosis in LDLr null mice [16, 17], AKT3 deficiency in macrophages can promote atherosclerosis [18], and AKT1 is overexpressed in the plasma samples of patients with atherosclerotic coronary artery disease [19]. Considering that AKT subunits have differential effects on atherosclerosis, the roles of PI3K and AKT in regulating inflammation may be not the same. At present, the association between PI3K/AKT signaling pathway and cancer development has been deeply studied [20]. However, its role in cardiovascular diseases, especially in atherosclerosis, remains to be further investigated.
In this study, an in vitro model of foam cell formation was prepared by exposing ox-LDL to human myeloid leukemia mononuclear cells (THP-1), in order to determine the effects of PI3K and AKT inhibition on cellular inflammation and oxidative stress. Apolipoprotein E (ApoE)-knockout mice fed a high-fat diet were used as an in vivo animal model to explore the roles of PI3K and AKT in aortic plaque formation. Therefore, this study aimed to evaluate the feasibility of PI3K and AKT inhibition as therapeutic or preventive targets for inflammation-related atherosclerosis.
Materials and Methods
Cell culture and reagents
Human acute monocytic leukemia cell line (THP-1) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in RPMI 1640 (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 100 U/ml Penicillin-Streptomycin (Gibco) at 37°C under a humidified 5% CO2 atmosphere. GDC-0941 and MK2206 were purchased from MedChemexpress and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Darmstadt, Germany). Oil red O was purchased from Solarbio (Beijing, China). ox-LDL was purchased from Peking Union-Biololgy (Beijing, China).
Western blot
Total protein was extracted from THP-1 cells using protein lysis solution. After protein quantification, electrophoresis and transmembrane, the protein samples were incubated with primary antibodies against vinculin (1:10,000 dilution; ab129002; Abcam, Cambridge, UK), AKT (1:1,000 dilution; 9271; CST, Danvers, MA, USA), phospho-AKT (1:1,000 dilution; 2211; CST), IL-1β (1:1,000 dilution; 12242; CST), IL-18 (1:1,000 dilution; ab243091; Abcam), nucleotide‐binding oligomerization domain (NOD)‐like receptor protein 3 (NLRP3; 1:1,000 dilution; AG-20B-0014-C100; AdipoGen, San Diego, CA, USA), caspase‐1 (1:10,000 dilution; ab108362; Abcam), NF‐κB p65 (1:1,000 dilution; AB32360; Abcam), or phospho‐NF‐κB p65 (1:1,000 dilution; 3033; CST) overnight at 4°C. Secondary antibodies conjugated with horseradish peroxidase (1:5,000 dilution; SA00001-1; SA00001-2; Proteintech, Rosemont, PA, USA) were incubated at room temperature for 1 h. The protein bands were visualized using an enhanced chemiluminescence kit (ECL; Advansta, Menlo Park, CA, USA). Data acquisition and analysis were performed on a gel image analyzing system equipped with Image Lab Software (Bio‐Rad, Hercules, CA, USA).
Mitochondrial reactive oxygen species (ROS)
After completing the desired drug treatment, THP-1 cells were stained with a 5 µM MitoSOXTM Red mitochondrial superoxide indicator (M36008; Invitrogen, Carlsbad, CA, USA) at 37°C in the dark for 15 min. The levels of reaction oxygen species (ROS) were analyzed by flow cytometry (Beckman Coulter MoFlo Astrios EQ, Brea, CA, USA). Image processing was carried out using an Olympus FluoView software.
RT-PCR
Total RNA was extracted using the Trizol reagent (Invitrogen). Nano-Drop ND-2000 spectrophotometer (NanoDrop Products, Wilmington, DE, USA) was used to assess the concentrations and ratios of RNA samples. The RNA samples were then reversely transcribed using SuperScript III Reverse Transcriptase (Life Technologies, Carlsbad, CA, USA). The corresponding cDNA was used as a template for real-time PCR assay. The reaction was performed using LightCycler 480 SYBR Green I master kit (Roche, Basel, Switzerland) and primer pairs as follows: GAPDH sense, 5’-ATGACATCAAGAGGTGGTG-3’ and antisense, 5’-CATACCAGGAAATGAGCTTG-3’; NLRP3 sense, 5’-GTGTTTCGAAATCCCACTGTG-3’ and antisense, 5’-TCTGCTTCTCACGTACTTTCTG-3’; IL-1β sense, 5’-CCTCCATTGATCATCTGTCTCTG3’ and antisense, 5’-GCTTGGATGTTTTAGAGGTTTCAG-3’; IL-18 sense, 5’-GCCTAGAGGTATGGCTGTAA-3’, antisense, 5’-GCGTCACTACACTCAGCTAA-3’.
Animal experiment
ApoE knockout male mice (6‐week old) with C57BL/6 background were obtained from HFK Bioscience (Beijing, China) and maintained on a high-fat diet (34% fat/1.25% cholesterol, Trophic Animal Feed High-Tech Co., Ltd., Nantong, China). All experimental procedures were approved by the Institutional Animal Care Committee of Dalian Medical University (Dalian, China). The mice (n=24) were divided into three groups randomly (n=8 per group): high-fat diet (HFD), GDC0941 + high-fat diet (GDC0941 + HFD) and MK2206 + high-fat diet (MK2206 + HFD). The mice in GDC0941 + HFD and MK2206 + HFD groups were administered daily by oral gavage. After 12 weeks on a high-fat diet, the mice were euthanized using 1% sodium pentobarbital (50 mg/kg; Sigma-Aldrich) and tissue samples were collected for further analyses. Histological images of aortic sinus lesions were obtained by hematoxylin and eosin (HE) and oil red O staining. Total cholesterol (TC), triglycerides (TG), low‐density lipid cholesterol (LDL‐C), and high‐density lipid cholesterol (HDL‐C) in serum samples were detected using a Hitachi 7600 automated biochemistry analyzer. NLRP3 and IL-1β were determined by immunohistochemistry and photographed using an inverted microscope (Olympus).
ELISA
Medium supernatant and serum samples were collected for the measurement of inflammatory factors. Detection of IL-18 and IL-1βwas performed using the corresponding ELISA Development Kits (Peprotech, Rehovot, Israel) in accordance with the manufacturer’s instructions. The color development was monitored using an ELISA plate reader (iMark, Bio-Rad) at 405 nm with wavelength correction set at 650 nm.
Histological observation and immunohistochemistry (IHC) via HE staining
After sacrificing the mice, the heart specimens were collected, fixed with 4% paraformaldehyde for 24 h and embedded in paraffin. The heart tissues were cut into 4-um sections for HE staining and IHC analysis. Antigen retrieval was performed after dewaxing the paraffin sections. After that, the sections were sealed with 5% goat serum and incubated overnight with NLRP3 (1:200 dilution; AG-20B-0014-C100; AdipoGen) and IL-1β (1:100 dilution; 12242; CST) at 4°C. After washing three times with PBS, the sections were incubated with secondary antibodies at 37°C for 1 h. Finally, the sections were stained with DAB (Solarbio), and the nuclei were stained with hematoxylin solution. The stained sections were then observed under a light microscope at 200× magnification. The atherosclerotic plaque areas of each mouse were measured by Image-ProPlus software.
Statistics
Data are expressed as mean ± SD. Statistical significance was assessed with analysis of variance (ANOVA), followed by Tukey’s honest significant difference (HSD) test. A P-value of <0.05 was considered statistically significant. All statistical tests were performed using the GraphPad Prism sofware version 8.0.2.
Results
1. Inhibition of PI3K or AKT in THP-1 macrophage suppresses the activation of NF-κB signaling pathway induced by ox-LDL, and the inhibitory effect of PI3K is more effective
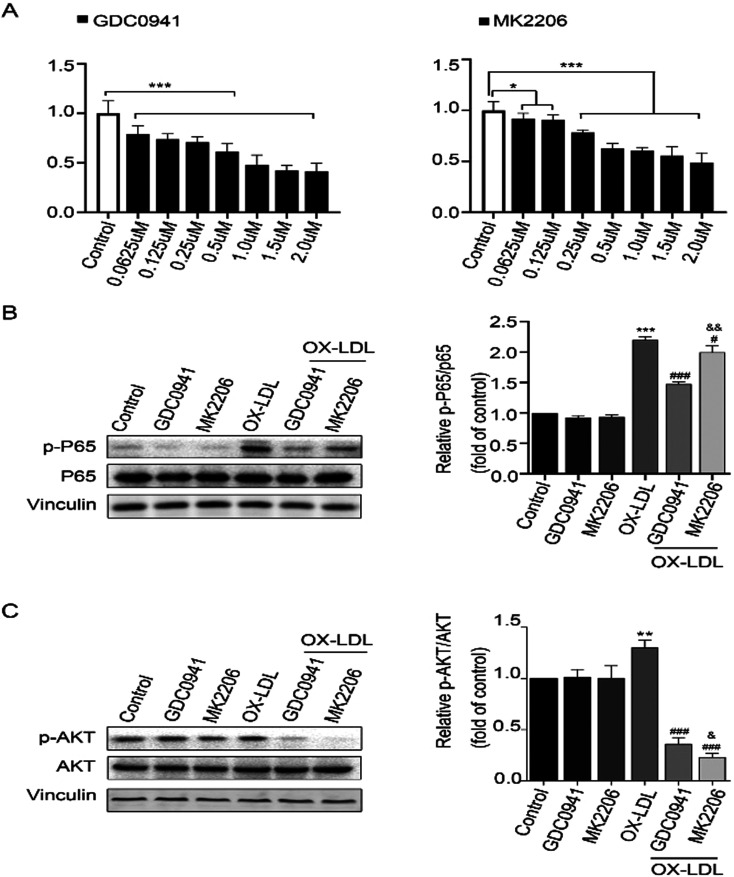
To determine the appropriate inhibitor concentrations, the proliferation rates of THP-1 cells treated with GDC0941 (a PI3K inhibitor) and MK2206 (an AKT inhibitor) were evaluated through CCK-8 assay. Based on the obtained results, an inhibitory concentration of 0.25 uM was selected (Fig. 1A). After 24 h of treatment with GDC0941 and MK2206, THP-1 cells were incubated with ox-LDL (100 ug/ml) for another 24 h. To determine the effects of PI3K and AKT on NF-κB signaling pathway, the expression levels of p-P65, P65, p-AKT and AKT were detected by Western blot analysis. It was found that ox-LDL could increase the expression levels of p-P65 and p-AKT, suggesting that inflammation is activated in ox-LDL-induced THP-1 cells. Interestingly, the expression levels of p-P65 were significantly decreased in GDC0941 + ox-LDL treatment group compared to MK2206 + ox-LDL treatment group (Figs. 1B and C). These results show that PI3K inhibition has much stronger inhibitory effect on the activation of NF-κB than AKT inhibition, indicating that PI3K can regulate NF-κB activation through part-dependent AKT signaling pathway.
Fig. 1.
(A) THP-1 cells were treated with different concentrations (0.0625, 0.125, 0.25, 0.5, 1.0, 1.5 and 2.0 uM) of GDC0941 and MK2206 for 24 h. CCK-8 assay was used to assess cell viability and cytotoxicity. *P<0.05, **P<0.01, ***P<0.001 vs. control group. (B) Protein levels of p-P65 and P65 in the cells exposed to different treatments, as determined by Western blot analysis, n=3. (C) Protein levels of p-AKT and AKT in the cells exposed to different treatments, as determined by Western blot analysis, n=3. *P<0.05, **P<0.01, ***P<0.001 vs. control group. #P<0.05, ###P<0.001 vs. ox-LDL exposure group. &P<0.05, &&P<0.01 vs. GDC0941+ox-LDL exposure group.
2. Inhibition of PI3K or AKT in THP-1 macrophages suppresses NLRP3 inflammasome activation and reduces mitochondrial ROS (mitoROS) production, and the inhibitory effects of PI3K are more effective
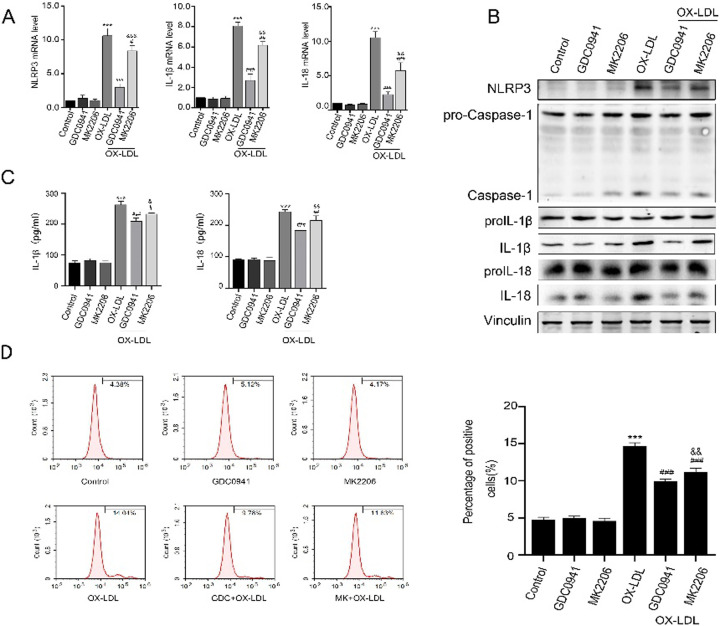
To investigate the effects of PI3K and AKT inhibition on THP-1 cell inflammatory response, the protein levels of NLRP3, Caspase-1, IL-1β and IL-18 in each group were detected by Western blot analysis, while the mRNA levels of NLRP3, IL-18 and IL-1β were detected by RT-PCR. The results showed that ox-LDL increased the expression levels of NLRP3, Caspase-1, IL-1β and IL-18, suggesting that inflammation is activated in ox-LDL group. To our expectation, the expression levels of these proteins were significantly lower in GDC0941 + ox-LDL and MK2206 + ox-LDL groups than in ox-LDL group. Particularly, the expression levels of these proteins were markedly decreased in THP-1 cells treated with GDC0941 + ox-LDL compared to those treated with MK2206 + ox-LDL (Figs. 2A and B). Moreover, the expression levels of inflammatory factors (such as IL-1β and IL-18) were significantly reduced in GDC0941 + HFD treatment group compared to those in other groups (Fig. 2C). It has been previously reported that mitoROS can induce inflammation by directly binding and activating NLRP3 inflammasome [21]. In this study, mitoROS was used as an index to reflect the cellular levels of ROS in each group, and its levels were analyzed by flow cytometry. Our results demonstrated that the content of mitoROS in ox-LDL-exposed THP-1 cells was significantly higher than that in control group. On the contrary, the levels of mitoROS in GDC0941 + ox-LDL and MK2206 + ox-LDL groups were significantly decreased compared to ox-LDL group. Furthermore, the levels of mitoROS were significantly lower in GDC0941 + ox-LDL treatment group than those in MK2206 + ox-LDL treatment group (Fig. 2D). These results indicate that PI3K inhibition has stronger inhibitory effect on the activation of NLRP3 inflammasome than AKT inhibition, suggesting that PI3K can regulate NLRP3 inflammasome activation through part-dependent AKT signaling pathway.
Fig. 2.
Effects of PI3K and AKT inhibition on NLRP3 inflammasome in ox-LDL-induced THP-1 cells. ox-LDL (100 mg/ml) was added to the medium and incubated for 24 h. (A) The mRNA levels of NLRP3, IL-1β and IL-18 were determined by PCR. (B) The protein levels of NLRP3, caspase-1, IL-18 and IL-1β were evaluated by Western blot analysis, n=3. (C) The serum levels of IL-1β and IL-18 were determined with ELISA assay. (D) The levels of mitoROS were analyzed by flow cytometry. ***P<0.001 vs. control group. #P<0.05, ##P<0.01, ###P<0.001 vs. ox-LDL exposure group. &&P<0.01, &&&P<0.001 vs. GDC0941+ox-LDL exposure group.
3. Effects of PI3K and AKT inhibition on atherosclerotic lesion and plaque area in ApoE−/− mouse model
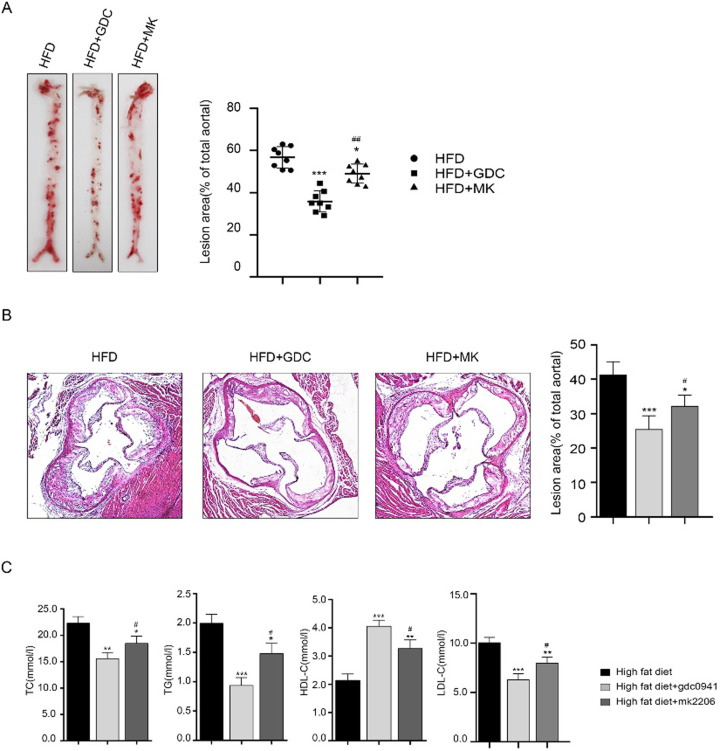
To determine the roles of PI3K and AKT inhibition in atherosclerosis, ApoE−/− mice were fed a high-fat diet (n=24) and then treated with and without GDC0941 or MK2206. After 12 weeks of treatment, the mice were anesthetized and executed, and their blood and aorta were subsequently collected. Through oil red staining, the atherosclerotic lesions in mice were significantly more severe in HFD group than in other groups. The aortic plaque area of mice treated with GDC0941 + HFD was remarkably smaller compared to HFD and MK2206 + HFD treatment groups (Figs. 3A and B). The serum levels of TC, TG and LDL-C in GDC0941 + HFD group were lower than those in the other two groups, while the concentrations of HDL-C were markedly increased (Fig. 3C). These results indicate that PI3K inhibition has much stronger inhibitory effect on atherosclerosis than AKT inhibition.
Fig. 3.
Effects of PI3K and AKT inhibition on APOE−/− mice fed a high-fat diet (n=24). (A and B) Histological images of aortic vessels and lesions in aortic sinuses were obtained by hematoxylin and eosin (HE) and oil red O staining. (C) The serum levels of total cholesterol (TC), triglycerides (TG), low-density lipid cholesterol (LDL-C) and high-density lipid cholesterol (HDL-C) were detected. *P<0.05, **P<0.01, ***P<0.001 vs. high-fat diet group. #P<0.05 vs. high-fat diet + GDC0941 treatment group.
4. Effects of PI3K and AKT inhibition on the expression levels of NLRP3 and IL-1β in ApoE−/− mouse model
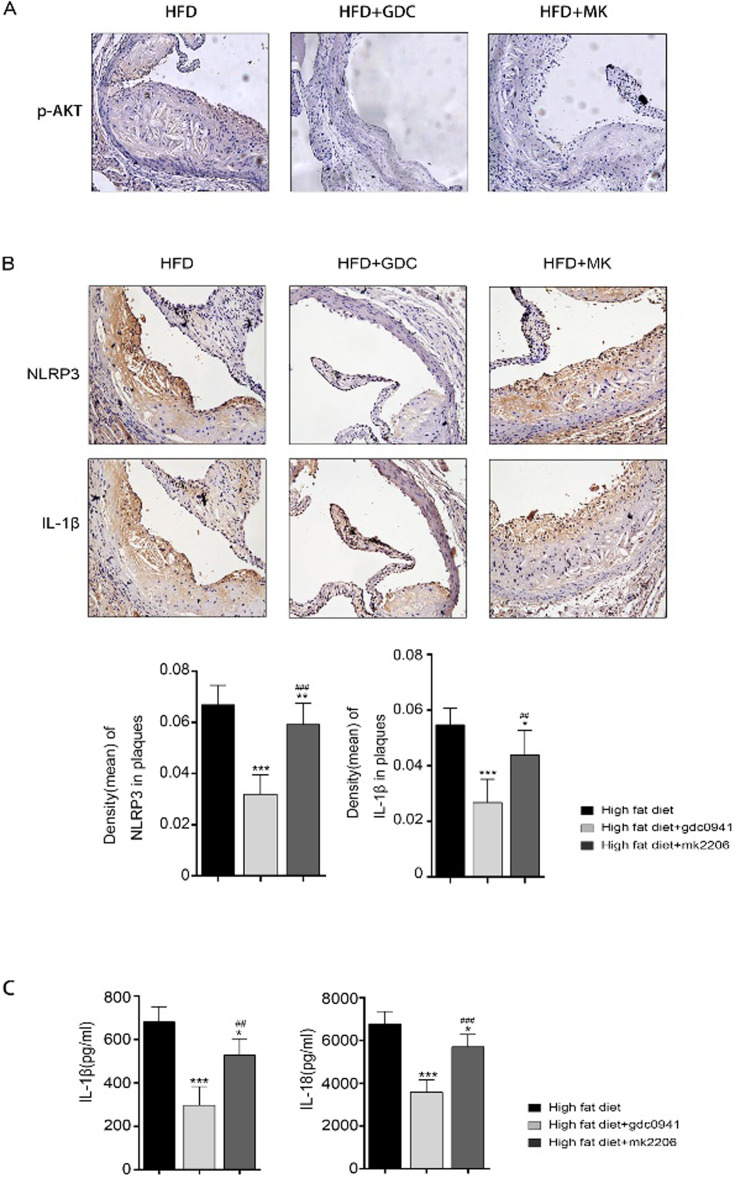
To explore the roles of PI3K and AKT in atherosclerosis, immunohistochemical staining was carried out. We observed that the expression of p-Akt in plaque was very weak in GDC0941+HFD treatment group and MK2206+HFD treatment group, suggesting that GDC0941 and MK2206 could inhibit the activation of PI3K and Akt (Fig. 4A). Besides, the expression levels of NLRP3 and IL-1βin atherosclerotic lesions were significantly decreased in GDC0941 + HFD treatment group compared to the other groups (Fig. 4B). Similarly, the expression levels of inflammatory factors (such as IL-1β and IL-18) were significantly reduced in GDC0941 + HFD treatment group compared to the other groups (Fig. 4C). These findings are consistent with those of in vitro experiments, which demonstrate that PI3K inhibition has much stronger inhibitory effect on atherosclerosis than AKT inhibition.
Fig. 4.
Effects of PI3K and AKT inhibition on APOE−/− mice fed a high-fat diet (n=24). (A) The level of p-AKT was assessed by immunohistochemical staining, and then photographed using an inverted microscope. (B) The levels of NLRP3 and IL-1β were assessed by immunohistochemical staining, and then photographed using an inverted microscope. (C) The serum levels of IL-1β and IL-18 were determined with ELISA assay. *P<0.05, **P<0.01, ***P<0.001 vs. high-fat diet group. ##P<0.01, ###P<0.001 vs. high-fat diet + GDC0941 treatment group.
Discussion
Our findings revealed that ox-LDL could trigger the inflammation of THP-1 cells by increasing the expression levels of NF-κB and NLRP3, indicating that ox-LDL successfully activates inflammatory response in macrophages. In addition, PI3K and AKT were inhibited in order to explore their roles in the development of atherosclerosis. The results were unexpected. In particular, there was a significant association between PI3K, AKT and atherosclerosis. NLRP3 inflammasomes are activated by various danger signals (such as cholesterol crystals, calcium phosphate crystals, and oxidized low-density lipoprotein) in macrophages to trigger inflammatory responses during atherosclerotic lesion [22]. Previous studies have shown that, in NLRP3−/−, caspase-1−/− deficiency mice, inflammation was significantly inhibited and the disease was significantly alleviated [23, 24]. The NLRP3 inflammasome contains NLRP3 associated with an apoptosis associated speck-like protein containing a caspase recruitment domain (ASC), which recruits caspase-1 and induces its activation. Two-step model in which both priming and activating signals are required to produce a functional inflammasome. The initial inflammasome priming step upstream of activation affects NLRP3 at the transcriptional level and also acts to initiate the posttranslational modification of inflammasome components that allow for oligomerization. The second step in the activation of NLRP3 inflammasome is provided by one of a diverse group of agonists that triggers the specific activation of NLRP3, assembly of the inflammasome complex, and finally culminates in the activation of caspase-1. The activated caspase-1 cleaves the precursors of IL-1β and IL-18 to produce mature cytokines [25, 26]. In our study, we used ox-LDL to induce inflammatory responses in THP-1 cells. The results demonstrated that PI3K inhibition could block the overexpression of NLRP3 in cell model. Moreover, the expression levels of IL-1 and IL-18, the markers of NLRP3 activation, were also inhibited. Our research suggests that PI3K signaling pathway not only affects the priming of NLRP3, but also regulates its activation. Therefore, we believe that intervention of PI3K signaling pathway can influence the activation of NLRP3 inflammasome in atherosclerosis, which is of great significance in the treatment of atherosclerosis. The expression levels of NF-κB and NLRP3 were decreased significantly in GDC0941 + ox-LDL group, but these changes were not so obvious in MK2206 + ox-LDL group. Oxidative stress is considered an imbalance in favor of the increased production of ROS and/or reduced antioxidant defense [27]. Previous studies have also shown that ROS can trigger the activation of NLRP3 inflammasome in human macrophages and animal models [28, 29]. In the present study, ROS production was increased when macrophages were stimulated with ox-LDL, but decreased after treatment with GDC0941 and MK2206. However, the effects of these two inhibitors were apparently different, in which ROS levels were decreased more obviously in GDC0941 + ox-LDL group than in MK2206 + ox-LDL group.
The findings of animal experiments are consistent with those of cell experiments. The formation of atherosclerotic lesion in ApoE−/−mice treated with GDC0941 + HFD was significantly reduced compared to the mice fed a high-fat diet alone. However, the inhibitory effect of MK2206 + HFD group on atherosclerotic plaque formation did not reach a statistically significant level. Besides, the expression levels of IL-18 and IL-1β were remarkably decreased in high-fat diet-induced atherosclerotic mice after treatment with GDC0941 or MK2206, especially in GDC0941 + HFD group. In addition, the inhibitory effects of PI3K or Akt on the regulation of blood lipids are indeed different, but the underlying mechanisms are still being explored. In previous studies, insulin acts through the insulin receptor, which undergoes auto-phosphorylation and subsequently phosphorylates IRS-1, followed by activating PI3K/Akt pathway, thus increasing the accumulation of intracellular lipids in 3T3-L1 cells [30]. Pi and colleagues [31] found that the inhibition of PI3K/Akt pathway could reduce lipid deposition, increase lipid efflux and reduce total cholesterol level in smooth muscle cells. Another study found that, MK-2206 induced the proteolytic processing of SREBP-2, upregulated LDLR expression and stimulated LDL uptake in human hepatoma cells [32]. On the basis of the above research, we hope to further explore the mechanism of PI3K regulating lipid metabolism.
PI3K signaling pathway plays central roles in regulating cellular physiology, coordinating organismal growth via insulin signaling, and mediating critical cellular processes such as glucose homeostasis, protein synthesis, cell proliferation, and survival [33]. This signaling pathway is becoming a key topic of research due to its roles in the occurrence and development of various diseases. Over the past decades, this pathway has been widely studied in different types of cancers. A number of studies have shown that PI3K/AKT signaling pathway can contribute to atherosclerosis by regulating macrophage polarization, apoptosis and survival [34]. The central role of AKT in PI3K activation makes it one of the most prominent downstream effectors [35]. In this study, the regulatory effects of PI3K and AKT on atherosclerosis were primarily discussed. Combining the in vivo and in vitro results, it was found that the inhibition of AKT and PI3K could suppress inflammatory responses and atherosclerotic lesion development, but the effect of AKT was weaker than that of PI3K. This indicates that PI3K signaling pathway may reduce the levels of inflammation and oxidative stress by regulating NLRP3 expression, which in turn leads to the prevention of atherosclerosis, via part-dependent AKT signaling pathway (including SGK and RAC). However, the specific mechanism needs to be further clarified.
In conclusion, our study indicates that PI3K and AKT have different inhibitory effects on inflammation-related atherosclerosis, and there may be similarity and specificity in the regulation of cellular function through different downstream effectors. Nevertheless, a more detailed understanding of the molecular mechanisms underlying their activation and regulation of cellular functions will be of great benefit for better prevention and treatment of atherosclerosis and other cardiovascular diseases.
Funding
This study was supported by National Natural Science Foundation of China (NSFC; Grant No. 91739119, No. 81670406, No. 82000415).
Supplementary
Acknowledgments
Not applicable.
References
- 1.Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond). 2018; 132: 1243–1252. doi: 10.1042/CS20180306 [DOI] [PubMed] [Google Scholar]
- 2.Foks AC, Bot I. Preface: Pathology and Pharmacology of Atherosclerosis. Eur J Pharmacol. 2017; 816: 1–2. doi: 10.1016/j.ejphar.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013; 13: 709–721. doi: 10.1038/nri3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019; 20: 3328. doi: 10.3390/ijms20133328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder K, Tschopp J. The inflammasomes. Cell. 2010; 140: 821–832. doi: 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 6.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016; 16: 407–420. doi: 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- 7.Peng K, Liu L, Wei D, Lv Y, Wang G, Xiong W, et al. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med. 2015; 35: 1179–1188. doi: 10.3892/ijmm.2015.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018; 19: 526–537. doi: 10.1038/s41590-018-0113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia SJ, Gao KQ, Zhao M. Epigenetic regulation in monocyte/macrophage: A key player during atherosclerosis. Cardiovasc Ther. 2017; 35: e12262. doi: 10.1111/1755-5922.12262 [DOI] [PubMed] [Google Scholar]
- 10.Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochim Biophys Acta. 2015; 1851: 882–897. doi: 10.1016/j.bbalip.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 11.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell. 2017; 170: 605–635. doi: 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oka A, Mishima Y, Liu B, Herzog JW, Steinbach EC, Kobayashi T, et al. Phosphoinositide 3-Kinase P110δ-Signaling Is Critical for Microbiota-Activated IL-10 Production by B Cells that Regulate Intestinal Inflammation. Cells. 2019; 8: 1121. doi: 10.3390/cells8101121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Fang L, Wu H, Mei X, He F, Ding P, et al. TLR2 Regulates Allergic Airway Inflammation and Autophagy Through PI3K/Akt Signaling Pathway. Inflammation. 2017; 40: 1382–1392. doi: 10.1007/s10753-017-0581-x [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Qin L, Liu X, Meng X. CTRP3 Alleviates Ox-LDL-Induced Inflammatory Response and Endothelial Dysfunction in Mouse Aortic Endothelial Cells by Activating the PI3K/Akt/eNOS Pathway. Inflammation. 2019; 42: 1350–1359. doi: 10.1007/s10753-019-00996-1 [DOI] [PubMed] [Google Scholar]
- 15.Ong MH, Wong HK, Tengku-Muhammad TS, Choo QC, Chew CH. Pro-atherogenic proteoglycanase ADAMTS-1 is down-regulated by lauric acid through PI3K and JNK signaling pathways in THP-1 derived macrophages. Mol Biol Rep. 2019; 46: 2631–2641. doi: 10.1007/s11033-019-04661-6 [DOI] [PubMed] [Google Scholar]
- 16.Babaev VR, Hebron KE, Wiese CB, Toth CL, Ding L, Zhang Y, et al. Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice. J Lipid Res. 2014; 55: 2296–2308. doi: 10.1194/jlr.M050633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babaev VR, Ding L, Zhang Y, May JM, Ramsey SA, Vickers KC, et al. Loss of 2 Akt (Protein Kinase B) Isoforms in Hematopoietic Cells Diminished Monocyte and Macrophage Survival and Reduces Atherosclerosis in Ldl Receptor-Null Mice. Arterioscler Thromb Vasc Biol. 2019; 39: 156–169. doi: 10.1161/ATVBAHA.118.312206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Biswas S, Morton RE, Smith JD, Hay N, Byzova TV, et al. Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell Metab. 2012; 15: 861–872. doi: 10.1016/j.cmet.2012.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Zheng SY, Yang CQ, Ma BM, Jiang D. MiR-155-5p inhibits the proliferation and migration of VSMCs and HUVECs in atherosclerosis by targeting AKT1. Eur Rev Med Pharmacol Sci. 2019; 23: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 20.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol. 2019; 59: 125–132. doi: 10.1016/j.semcancer.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 21.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011; 469: 221–225. doi: 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 22.Karasawa T, Takahashi M. Role of NLRP3 Inflammasomes in Atherosclerosis. J Atheroscler Thromb. 2017; 24: 443–451. doi: 10.5551/jat.RV17001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, et al. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2012; 425: 162–168. doi: 10.1016/j.bbrc.2012.07.058 [DOI] [PubMed] [Google Scholar]
- 24.Allen IC, Jania CM, Wilson JE, Tekeppe EM, Hua X, Brickey WJ, et al. Analysis of NLRP3 in the development of allergic airway disease in mice. J Immunol. 2012; 188: 2884–2893. doi: 10.4049/jimmunol.1102488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010; 464: 1357–1361. doi: 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014; 1319: 82–95. doi: 10.1111/nyas.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep. 2017; 19: 42. doi: 10.1007/s11883-017-0678-6 [DOI] [PubMed] [Google Scholar]
- 28.Dai J, Zhang X, Li L, Chen H, Chai Y. Autophagy Inhibition Contributes to ROS-Producing NLRP3-Dependent Inflammasome Activation and Cytokine Secretion in High Glucose-Induced Macrophages. Cell Physiol Biochem. 2017; 43: 247–256. doi: 10.1159/000480367 [DOI] [PubMed] [Google Scholar]
- 29.Minutoli L, Puzzolo D, Rinaldi M, Irrera N, Marini H, Arcoraci V, et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid Med Cell Longev. 2016; 2016: 2183026. doi: 10.1155/2016/2183026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto C, Koike A, Tanaka R, Fujimori K. A Limonoid, 7-Deacetoxy-7-Oxogedunin (CG-1) from Andiroba (Carapa guianensis, Meliaceae) Lowers the Accumulation of Intracellular Lipids in Adipocytes via Suppression of IRS-1/Akt-Mediated Glucose Uptake and a Decrease in GLUT4 Expression. Molecules. 2019; 24: 1668. doi: 10.3390/molecules24091668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pi S, Mao L, Chen J, Shi H, Liu Y, Guo X, et al. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy. 2021; 17: 980–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjune K, Sundvold H, Leren TP, Naderi S. MK-2206, an allosteric inhibitor of AKT, stimulates LDLR expression and LDL uptake: A potential hypocholesterolemic agent. Atherosclerosis. 2018; 276: 28–38. doi: 10.1016/j.atherosclerosis.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 33.Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017; 45: 62–71. doi: 10.1016/j.ceb.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linton MF, Moslehi JJ, Babaev VR. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int J Mol Sci. 2019; 20: 2703. doi: 10.3390/ijms20112703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017; 169: 381–405. doi: 10.1016/j.cell.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.