Abstract
The differentiation and maturation of skeletal muscle cells into functional fibers is coordinated largely by inductive signals which act through discrete intracellular signal transduction pathways. Recently, the calcium-activated phosphatase calcineurin (PP2B) and the family of transcription factors known as NFAT have been implicated in the regulation of myocyte hypertrophy and fiber type specificity. Here we present an analysis of the intracellular mechanisms which underlie myocyte differentiation and fiber type specificity due to an insulinlike growth factor 1 (IGF-1)–calcineurin–NFAT signal transduction pathway. We demonstrate that calcineurin enzymatic activity is transiently increased during the initiation of myogenic differentiation in cultured C2C12 cells and that this increase is associated with NFATc3 nuclear translocation. Adenovirus-mediated gene transfer of an activated calcineurin protein (AdCnA) potentiates C2C12 and Sol8 myocyte differentiation, while adenovirus-mediated gene transfer of noncompetitive calcineurin-inhibitory peptides (cain or ΔAKAP79) attenuates differentiation. AdCnA infection was also sufficient to rescue myocyte differentiation in an IGF-depleted myoblast cell line. Using 10T1/2 cells, we demonstrate that MyoD-directed myogenesis is dramatically enhanced by either calcineurin or NFATc3 cotransfection, while a calcineurin inhibitory peptide (cain) blocks differentiation. Enhanced myogenic differentiation directed by calcineurin, but not NFATc3, preferentially specifies slow myosin heavy-chain expression, while enhanced differentiation through mitogen-activated protein kinase kinase 6 (MKK6) promotes fast myosin heavy-chain expression. These data indicate that a signaling pathway involving IGF-calcineurin-NFATc3 enhances myogenic differentiation whereas calcineurin acts through other factors to promote the slow fiber type program.
Skeletal muscle cell differentiation is coordinated by endocrine, paracrine, and autocrine inductive factors that activate discrete intracellular signal transduction pathways, resulting in the modulation of transcription factor activity and the reprogramming of gene expression. During embryonic development, the MyoD family of basic helix-loop-helix transcription factors directly regulate myocyte cell specification and differentiation (reviewed in reference 33). The myogenic basic helix-loop-helix proteins operate in concert with other transcriptional regulators such as MEF2, serum response factor, and CBP/p300 to promote myocyte differentiation (17, 34, 44, 49, 60). In turn, these transcriptional regulators are themselves regulated by intracellular signaling pathways and phosphorylation cascades.
In general, growth factors such as fibroblast growth factor and transforming growth factor β antagonize myocyte differentiation through signaling pathways involving ras, mitogen-activated protein kinase, and protein kinase C (14, 28, 41). Proliferation-inducing transduction pathways enhance AP-1 activity, increase Id expression, and directly attenuate the activity of the myogenic basic helix-loop-helix transcription factors through cell cycle-dependent mechanisms (20, 33, 48). In contrast, inductive factors such as insulin-like growth factor 1 (IGF-1) promote myocyte differentiation or hypertrophy (4, 38, 39, 43, 47, 55), partly through a transduction pathway involving phosphatidylinositol 3-kinase (24, 25, 38).
Superimposed on the myocyte differentiation program are molecular pathways which regulate fiber type specificity. During development, maturing myofibers first express embryonic myosin, followed by neonatal myosin, followed again by various isoforms of fast myosin and then slow myosin (reviewed in reference 51). Less is known about the intracellular regulatory pathways that control fiber type specificity, although evidence has accumulated implicating a calcium-dependent pathway (10, 16). Calcium levels in resting fast fibers are reported to be 50 nM, while prolonged or chronic stimulation of fast fibers, associated with increased intracellular calcium levels, induces slow-fiber transformation (3, 8, 45, 50, 56, 58).
Recent data have implicated calcineurin, a calcium-calmodulin-regulated serine/threonine phosphatase, in the control of IGF-1-dependent myocyte hypertrophy and fiber type specificity (10, 16, 38, 46). Calcineurin participates in the transduction of extracellular signals to the nucleus by targeting members of the NFAT family of transcription factors (reviewed in references 13 and 42). Calcineurin-directed dephosphorylation of NFAT factors unmasks their nuclear localization signal, resulting in nuclear translocation and gene activation. Five NFAT genes have thus far been identified, NFATc1 (NFATc or NFAT2), NFATc2 (NFATp or NFAT1), NFATc3 (NFAT4 or NFATx), NFATc4 (NFAT3), and NFAT5 (29, 42). Calcineurin-mediated signaling pathways contribute to T-cell activation, to the establishment of cardiac hypertrophy, and to the regulation of neuronal activity (13).
Treatment of cultured human myoblasts with the calcineurin inhibitor cyclosporine A attenuates myogenic differentiation in culture and cyclosporine administration inhibits regeneration in response to acute injury in the mouse (1). Cyclosporine also prevents skeletal muscle hypertrophy in response to muscle overloading in vivo (16). More recently, myoblast cell lines expressing the local form of IGF-1 were shown to utilize a calcineurin-dependent pathway to promote hypertrophy in culture (39, 46). Lastly, cyclosporine induced fast-fiber-type switching in rodent skeletal muscle (10, 16), and transgenic mice expressing an activated calcineurin cDNA in skeletal muscle had increased numbers of slow fibers (39a).
In the present study, we used four different model systems to examine the mechanisms whereby calcineurin and NFAT regulate myocyte differentiation and/or fiber type specificity. We demonstrate that calcineurin directly potentiates myocyte differentiation through a slow-fiber-type program in C2C12 myoblasts, Sol8 myoblasts, and MyoD-converted fibroblasts. Inhibition of calcineurin with a noncompetitive peptide inhibitor (cain) delays the differentiation of C2C12 and Sol8 cells and prevents MyoD-directed differentiation of 10T1/2 fibroblasts. Expression of activated calcineurin in C2C12 myoblasts overexpressing an inhibitory IGF binding protein overrides blocked differentiation. We demonstrate that NFATc3 is a specific target of calcineurin and translocates to the nucleus at the onset of myoblast differentiation, enhancing myogenesis.
MATERIALS AND METHODS
Cells and transfections.
C2C12, Sol8, and 10T1/2 cells were maintained in growth medium (GM) consisting of Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL, Gaithersburg, Md.) supplemented with 15% fetal bovine serum (FBS). C2BP5 myocytes were described previously and were grown in DMEM supplemented with 10% heat-inactivated fetal calf serum, 10% heat-inactivated newborn calf serum, and 300 μg of G418 per ml (22). C2BP-5 cells were subsequently placed in DMEM supplemented with 2% horse serum for differentiation inducing experiments. Transient transfections of 10T1/2 fibroblasts were done in 60-mm tissue culture dishes containing a total of 2 μg of DNA per transfection, using Fugene 6 transfection reagent as specified by the manufacturer (Roche Diagnostics Corporation, Indianapolis, Ind.). At 24 h after transfection, the cells were washed and transferred to differentiation medium (DM) consisting of DMEM supplemented with 4% horse serum. The cells were grown for up to an additional 6 days in DM before being subjected to immunocytochemistry or Western blot analysis.
Western blotting.
Extracts were prepared in cell lysis buffer (20 mM sodium phosphate [pH 7.0], 150 mM NaCl, 2 mM MgCl2, 10 mM NaF, 0.1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM dithiothreitol, 1% NP-40, 10% glycerol, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of pepstatin per ml, 10 μg of tolylsulfonyl phenylalanyl chloromethyl ketone [TPCK] per ml, 10 μg of Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK] per ml), and proteins were resolved on a sodium dodecyl sulfate–10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and immunodetected using an enhanced chemifluorescence (ECF) kit as specified by the manufacturer (Amersham). The following antibodies were used: MF20 monoclonal antibody (MAb) (Developmental Studies Hybridoma Bank, University of Iowa) against sarcomeric myosin heavy chain, the NOQ7.5.4D MAb (Sigma, St. Louis, Mo.) against slow skeletal myosin heavy chain (MyHC), a MAb against fast MyHC (Novocastra Laboratories), an antibody reactive to both calcineurin Aα and Aβ (Transduction Labs), and calcineurin Aα and Aβ isoform-specific antibodies (Santa Cruz). Western blot reactivity was quantified on a Storm 860 PhosphorImager (Molecular Dynamics) using the Imagequant software. All acrylamide gels were loaded in the linear range of Western blot signal reactivity for each protein assayed.
Immunocytochemistry.
Cells were fixed in 3.7% formaldehyde–phosphate-buffered saline and blocked in phosphate-buffered saline containing 2% bovine serum albumin, 2% horse serum, and 0.1% NP-40. All antibody incubations were done in the blocking solution. Cells were treated with antibodies for 2 h at room temperature or overnight at 4°C at a dilution of 1:100 for primary antibodies and 1:400 for secondary antibodies. Secondary antibodies used were Alexa 488 or Alexa 594 (Molecular Probes). When indicated, the cells were incubated for 1 h at 37°C with 0.1 or 5 μM thapsigargin directly before fixation.
Calcineurin phosphatase assay.
Extracts were prepared in phosphatase lysis buffer (50 mM Tris-Cl [pH 7.5], 0.1 mM NaCl, 5 mM dithiothreitol, 1 mM EDTA [pH 8.0], 1 mM phenylmethylsulfonyl fluoride, 5 μg of pepstatin per ml, 5 μg of leupeptin per ml, 5 μg of aprotinin per ml). Calcineurin enzymatic activity was measured in phosphatase buffer (20 mM Tris-Cl [pH 7.5], 50 mM NaCl, 6 mM MgCl2, 0.5 mM CaCl2, 1 mM dithiothreitol, 50 μg of bovine serum albumin per ml). Phosphatase activity was determined as the dephosphorylation rate of a synthetic [32P]ATP-labeled phosphopeptide substrate (R-II peptide; Peninsula Labs) in the presence of 0.5 mM CaCl2, 1.0 μM calmodulin, and 1.0 μM okadaic acid. Control assays were performed in parallel and blocked with 500 μM calcineurin autoinhibitory peptide (Calbiochem), and total activity was determined as the difference between the reaction and the control. Assays were performed in duplicate at each time point in three separate experiments.
Adenovirus constructs and infections.
The adenovirus E1 to E3 genes in pACCMVpLpA (18) were replaced by sequences encoding either a constitutively active form of mouse calcineurin Aα (AdCnA) (amino acids 1 to 398), β-galactosidase (Adβgal) (53), or the inhibitory domain (amino acids 1989 to 2182) of the calcineurin-interacting protein cain (27, 52). Infectious virus was produced by cotransfection of HEK293 cells with recombinant pACCMV-pLpA and pJM17 vectors as described previously (18). All initial recombinants were plaque purified, expanded, and subjected to titer determination by duplicate plaque assays in monolayers of HEK293 cells by using the agarose gel overlay method (32). Recombinants were tested for appropriate expression in Cos-7 and 10T1/2 cells by immunofluorescence and Western blotting. Adenovirus infection of C2C12 and Sol8 cells (80 to 90% confluence) was performed for 2 h at 37°C at a multiplicity of infection of 50 to 100 PFU in 1.6 ml (6-cm culture dishes) of DMEM supplemented with 2% FBS in a humidified, 5% CO2 incubator. The infection medium was replaced by DM, and cells were cultured for the indicated time. Under these conditions, approximately 95 to 99% of the cells were positive for protein expression by immunocytochemistry or stained β-galactosidase positive after 24 h.
For adenovirus gene transfer in vivo, 1.0 × 109 PFU of AdCnA or Adβgal was injected in a volume of 50 μl into the gastrocnemius of 4-day-old neonatal rat pups. One or two weeks later, the animals were sacrificed, the injected tissue was collected, cryopreserved, and cryosectioned (7 μm), and immunocytochemistry was performed.
Plasmids.
The following plasmids were used: the pEMSVMyoD expression vector for wild-type MyoD under the control of the EMSV long terminal repeat, PECE-flagCnA, which contains the activated form of mouse calcineurin Aα corresponding to amino acids 1 to 398, which was generated by PCR with EcoRI linkers; and PECE-flag cain, which contains a 582-bp fragment corresponding to amino acids 1989 to 2182 of the mouse calcineurin-inhibitory protein identified in the rat as cain (27). This fragment was generated by PCR from a mouse expressed sequence tag (accession number 406188). The MKK6 expression vector encodes a mutated, constitutively active protein which was described previously (36). The NFATc4 expression vector was described previously (21). NFATc1 expression vectors were described previously and were kindly provided by Rhonda Bassel-Duby (10, 40). NFATc3 was a kind gift of Naoko Arai (30).
Statistical analysis.
Data are expressed as means and standard errors of the mean (SEM). Differences between groups were evaluated for statistical significance using Student's t test for unpaired data or by analysis of variance followed by Bonferroni's post-test when appropriate. P < 0.05 was considered to be statistically significant.
RESULTS
Calcineurin is transiently activated early in C2C12 myotube formation.
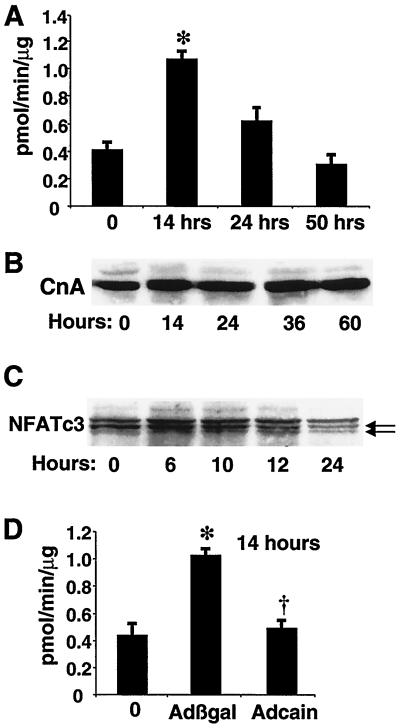
To investigate the role of calcineurin in myogenic differentiation, we measured calcineurin phosphatase activity in extracts from C2C12 cells prepared 14, 24, or 50 h after transfer into DM (Fig. 1A). Interestingly, calcineurin activity was increased nearly threefold after 14 h in DM compared to myoblasts in GM (P < 0.05). Calcineurin activity decreased at 24 h and returned to basal levels at 50 h. We also directly measured the calcineurin protein content by Western blot analysis with antiserum that detects both CnAα and CnAβ gene products (Fig. 1B). No differences in calcineurin A protein levels were identified between C2C12 myoblasts and differentiating myotubes at 14, 24, 36, or 60 h. Members of the NFAT transcription factor family are important targets of calcineurin dephosphorylation in multiple cell types. Western blot analysis of NFATc3 migration from C2C12 cells harvested at 6, 10, 12, or 24 h in DM revealed the gradual appearance of a faster-migrating band between 10 and 24 h (Fig. 1C). The appearance of a faster-migrating form of NFATc3 is indicative of dephosphorylation, suggesting an increase in calcineurin activity in vivo. Collectively, these results demonstrate a specific activation of calcineurin coincident with initiation of myogenic differentiation in C2C12 cells.
FIG. 1.
Calcineurin phosphatase activity peaks at an early stage of myocyte differentiation. (A) Calcineurin phosphatase activity assays were performed in C2C12 extracts prepared at the indicated times after transfer into DM as described in Materials and Methods. After 14 h in DM, calcineurin activity was increased nearly threefold. The data represent the means of three independent experiments, each done in duplicate, and SEM. ∗, P < 0.05. (B) Representative Western blot of total calcineurin A protein from myoblasts in GM (0 h) or at the indicated times in DM. Identical results were obtained in three independent experiments. (C) C2C12 cells were harvested at 0, 6, 10, 12, and 24 h after switching to DM, and whole-cell protein extracts were generated for NFATc3 Western blotting. The data demonstrate the appearance of a faster-migrating band (lower arrow), suggestive of enhanced calcineurin activity in vivo as differentiation progresses. (D) Infection of C2C12 cells with a calcineurin-inhibitory adenovirus, Adcain, blocked the increase in calcineurin activity at 14 h, while Adβgal infection had no effect. ∗, P < 0.05 versus the zero-time point; †, P < 0.05 versus Adβgal infection.
Calcineurin promotes differentiation of C2C12 and Sol8 myoblasts.
Previous studies have shown that cyclosporine attenuates myocyte differentiation and regeneration in vivo (1, 16). However, cyclosporine has a number of calcineurin-independent effects which might influence myoblast differentiation (5, 31), and a number of mechanistic questions remain to be answered. To begin to address these issues, we used adenovirus-mediated gene transfer of an activated form of calcineurin or a calcineurin-specific inhibitory peptide in C2C12 and Sol8 myoblasts. Approximately 95 to 99% adenovirus infection rates were routinely obtained, so that uniform effects could be examined in culture.
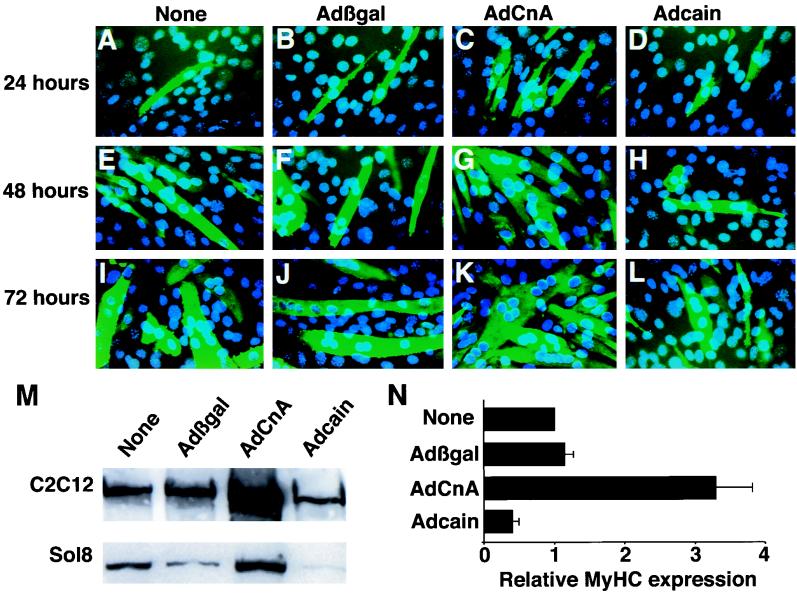
C2C12 cells were infected with AdCnA or a control adenovirus (Adβgal) for 24, 48, and 72 h, and immunocytochemistry was performed with anti-sarcomeric myosin heavy-chain antibody to evaluate differentiation. The data demonstrate enhanced myotube formation in AdCnA-infected myocytes (Fig. 2C, G, and K) compared with Adβgal infection (Fig. 2B, F, and J) at each time point. At 48 and 72 h, AdCnA-infected myocytes also demonstrated a more mature phenotype with an increased size and number of nuclei compared to Adβgal-infected myocytes. Adβgal infection alone did not affect differentiation compared with uninfected controls (Fig. 2A, E, and I), nor did infection with an adenovirus expressing another phosphatase, MKP-1 (data not shown). A similar visual enhancement of differentiation was also seen in Sol8 myoblasts infected with AdCnA (data not shown).
FIG. 2.
Calcineurin enhances myogenic differentiation in myoblast cell lines. (A, E, and I) C2C12 myocytes were placed in DM for 24, 48, or 72 h without adenovirus infection. (B, F, and J) Adβgal infection did not influence the degree of myotube formation, the size of myotubes, or their degree of multinucleation. (C, G, and K) AdCnA-infected C2C12 myocytes displayed enhanced differentiation characterized by increased numbers of myosin-expressing cells and increased multinucleated cells. (D, H, and L) Inhibition of calcineurin activity by Adcain infection attenuated myocyte differentiation. Total myosin (MF-20 antibody) is shown in green, and nuclei are shown in blue. (M) Western blotting for total MyHC protein was assessed 72 h after adenovirus infection of either C2C12 or Sol8 myocytes. (N) Western blot quantitation of MyHC protein expression in C2C12 cells was averaged from five independent experiments, and the mean values and SEM are shown.
Recently, a calcineurin-interacting protein was identified by yeast two-hybrid screening that acts as a noncompetitive inhibitory factor (cain or Cabin-1) (27, 52). We generated an adenovirus that expresses the calcineurin-inhibitory domain of cain and determined that it significantly reduces calcineurin enzymatic activity in cultured cardiomyocytes in response to hypertrophic agonists (54). Adcain infection of C2C12 cells also inhibited the increase in calcineurin enzymatic activity at 14 h of differentiation, while Adβgal infection had no effect (Fig. 1D). Associated with inhibited calcineurin activity, Adcain also decreased myogenic differentiation, as shown by a reduced number of myotubes, decreased myotube fusion, and less multinucleation at 24, 48, and 72 h postinfection (Fig. 2D, H, and L). We further determined that an adenovirus expressing a different calcineurin-inhibitory peptide, from the AKAP79 protein, also blunted C2C12 cell differentiation (data not shown). These results demonstrate that calcineurin is a regulator of myocyte differentiation and confirm the specificity of cyclosporine in attenuating myocyte differentiation.
To quantify the effects of Adcain and AdCnA infection on myocyte differentiation, we performed Western blotting of total MyHC levels in extracts of C2C12 or Sol8 cells. Consistent with immunocytochemistry data, we observed that AdCnA infection induced a quantitative increase in the total MyHC protein level at 72 h compared with the level induced in uninfected or Adβgal-infected C2C12 cells (Fig. 2M). A similar increase in the MyHC protein level was also observed in AdCnA-infected Sol8 myocytes (Fig. 2M). Conversely, inhibition of calcineurin activity with Adcain reduced MyHC protein expression (Fig. 2M). These data were quantified in five individual experiments which demonstrated a greater than threefold increase in the total MyHC protein level at 72 h in AdCnA-infected C2C12 cells, while Adcain-infected C2C12 cells had twofold-less MyHC protein (P < 0.05) (Fig. 2N).
Calcineurin induces NFATc3 nuclear translocation in myoblasts.
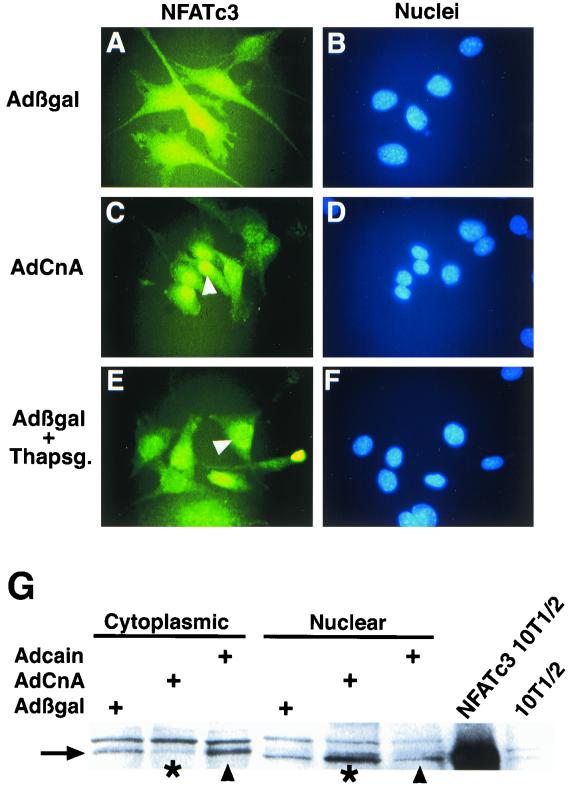
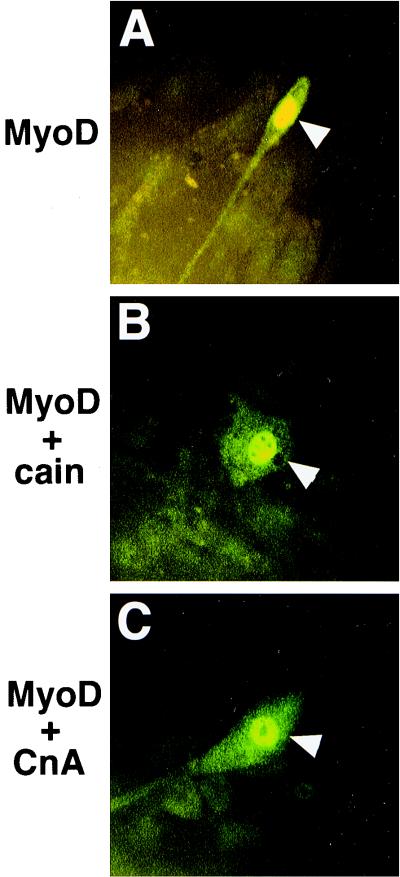
Members of the NFAT family of transcriptional activators are targets of calcineurin in multiple cell types (reviewed in reference 13). Using adenovirus-mediated gene transfer, we determined that AdCnA induced NFATc3 nuclear translocation in C2C12 myoblasts while NFATc2 and NFATc1 remained cytoplasmic (Fig. 3C and data not shown). AdCnA-induced NFATc3 nuclear translocation was similar to nuclear translocation induced by thapsigargin (Fig. 3E). Infection with control Adβgal did not induce NFATc3 nuclear translocation (Fig. 3A). These data indicate that only NFATc3 is regulated by calcineurin in pre-differentiated C2C12 myoblasts, implicating NFATc3 as an early regulator of differentiated gene expression. We also examined the effects of AdCnA infection on NFAT nuclear translocation in differentiated C2C12 myotubes. While only NFATc3 was regulated by calcineurin in myoblasts, we observed that NFATc1 was regulated by calcineurin in differentiated C2C12 myotubes (data not shown).
FIG. 3.
Calcineurin induces nuclear translocation of NFATc3 in C2C12 myoblasts. C2C12 myoblasts were immunostained 13 h after adenovirus infection with an antibody against NFATc3, and nuclei were counterstained with bisbenzimide (blue). (A and B) In Adβgal-infected cells, NFATc3 was localized mostly to the cytoplasm. (C to F) Infection with AdCnA induced nuclear translocation of NFATc3 (C and D), similar to calcium mobilization by thapsigargin treatment (E and F). (G) Protein fractionation followed by Western blotting revealed a loss of NFATc3 (arrow) from the cytoplasm and a redistribution to the nucleus in AdCnA-infected C2C12 cells (asterisks). In contrast, Adcain infection was associated with a mild increase in cytoplasmic NFATc3 and less in the nucleus (arrowheads). NFATc3-transfected 10T1/2 cell extract is shown as a mobility control.
To extend these observations, cytoplasmic or nuclear protein fractions were generated from adenovirus-infected C2C12 cells for Western blot analysis. The data demonstrate that AdCnA infection resulted in a loss of NFATc3 from the cytoplasm and an increase in its level in the nucleus (Fig. 3G). In contrast, Adcain infection resulted in a relative increase in the level of NFATc3 in the cytoplasm and a decrease in its level in the nucleus (Fig. 3G). We also noted that nuclear NFATc3 protein had a slightly faster migration than the cytoplasmic fraction, consistent with its dephosphorylation (Fig. 3G). To properly identify NFATc3 on a Western blot, protein extracts were generated from NFATc3-transfected 10T1/2 cells, which revealed a size of 130 kDa (Fig. 3G).
Calcineurin is sufficient to induce myogenic differentiation in the absence of IGFs.
To further characterize the involvement of calcineurin in IGF-induced myogenesis, we employed C2BP-5 cells which stably express IGFBP-5 (22). IGFBP-5 is a highly conserved IGF binding protein that is expressed during muscle differentiation in vitro and in vivo (19, 23). Our previous studies indicate that forced expression of IGFBP-5 inhibits muscle cell differentiation, which can be rescued by exogenous IGF-1 treatment (22) (Fig. 4A to D). We first characterized calcineurin activity in C2BP-5 cells, which demonstrated activity of 0.46 ± 0.09 pmol/min/μg in unstimulated cells compared with a value of 1.03 ± 0.01 pmol/min/μg in cells treated with IGF-1 for 24 h.
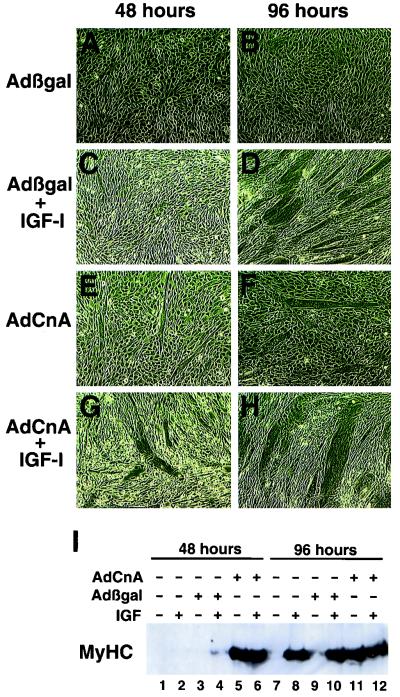
FIG. 4.
Calcineurin is sufficient to induce differentiation in an IGF-inhibited cell line. (A to D) C2BP-5 cells failed to differentiate in DM after 48 or 96 h in the absence of exogenous IGF-1 (A and B); however, supplementation with IGF-1 rescued differentiation by 96 h but not by 48 h (C and D). (E and F) AdCnA infection of C2BP-5 myoblasts maintained in DM demonstrated noticeable differentiation by 48 and 96 h. (G and H) AdCnA infection in the presence of IGF-1 promoted even greater differentiation and increased the myotube size by 96 h. (I) Western blotting for total MyHC protein demonstrated that Adβgal-infected C2BP-5 cells completely lacked MyHC protein at 48 or 96 h in the absence of IGF-1 (lanes 3 and 9). However, AdCnA-infected cells displayed abundant MyHC protein expression at 48 and 96 h in the absence of IGF-1 (lanes 5 and 11).
Remarkably, AdCnA, but not Adβgal, infection of C2BP-5 cells was sufficient to rescue differentiation in the absence of IGF signaling (Fig. 4E and F). Furthermore, AdCnA potentiated the IGF-1-induced hypertrophy of C2BP-5 cells (Fig. 4G and H). Myocyte differentiation was quantified by Western blotting for MyHC protein levels. The data demonstrate that in the absence of IGF-1 stimulation (in DM), no MyHC protein was expressed (Fig. 4I, lanes 3 and 9). However, AdCnA infection was sufficient to induce myosin expression in the absence of IGF-1 at either 48 or 96 h in DM (lanes 5 and 11). These data indicate that calcineurin is sufficient to induce myocyte differentiation in the absence of IGF signaling, implicating calcineurin as a sufficient regulator of IGF-induced differentiation.
Despite the ability of calcineurin to promote differentiation in the absence of IGF-1 signaling in C2BP-5 cells, inhibition of calcineurin with cyclosporine or Adcain did not block IGF-1-induced differentiation (data not shown). These data indicate that while calcineurin is sufficient to act in the absence of IGF-1 signaling, other IGF-1-stimulated signaling pathways (e.g., phosphatidylinositol 3-kinase) are sufficient to promote myogenic differentiation in the absence of calcineurin. Indeed, Adcain infection of C2C12 cells only mildly attenuated differentiation in response to growth factor withdrawal (Fig. 2).
Calcineurin cooperates with MyoD to induce myogenic conversion of 10T1/2 fibroblasts.
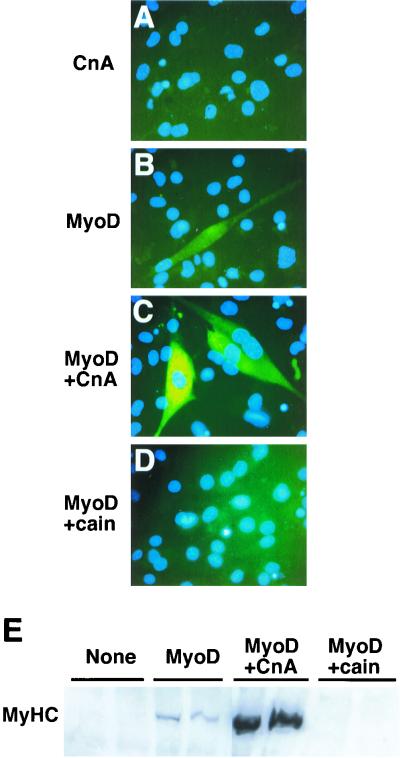
C2C12 and Sol8 are satellite cell lines derived from adult mouse limb muscle and 4-week-old mouse soleus muscle, respectively (7, 37). Since satellite cell lines have an inherent predisposition to differentiate into myotubes, we sought another model system that was unbiased as to its cell fate. Therefore, we used MyoD-induced conversion of 10T1/2 fibroblasts to confirm the role of calcineurin and NFATc3 in myogenesis. 10T1/2 fibroblasts were transfected with an expression vector encoding the myogenic transcription factor MyoD or cotransfected with MyoD and a constitutively active form of calcineurin. Immunocytochemistry for total MyHC demonstrated that MyoD expression in 10T1/2 cells induced myogenic conversion (Fig. 5B) while calcineurin cotransfection significantly enhanced differentiation such that each myotube was larger and more likely to be multinucleated (Fig. 5C).
FIG. 5.
Calcineurin enhances the differentiation of 10T1/2 cells transfected with MyoD. (A) 10T1/2 fibroblasts were transiently transfected with an expression vector encoding a constitutively active calcineurin Aα protein and subsequently immunostained for total MyHC protein (MF20 antibody). No MyHC was detected after 6 days in DM. (B) In contrast, transient transfection of an MyoD-encoding expression vector induced the conversion of fibroblasts into MyHC-expressing myotubes (green stain). (C) Cotransfection of MyoD and calcineurin resulted in a dramatic enhancement in the MyHC immunoreactivity and size of each converted cell. (D) However, inhibition of calcineurin activity with a cain (194-amino-acid fragment) expression vector blocked MyoD-directed differentiation. (E) Western blot quantitation revealed a dramatic increase in the amount of total MyHC protein between MyoD and calcineurin, while cain blocked MyHC expression. Identical results were obtained in four independent experiments.
To examine the effects of calcineurin cotransfection in more detail, myogenin immunocytochemistry was performed. Myogenin immunocytochemistry demonstrated that calcineurin cotransfection did not increase the total number of specified myocytes (myogenin-positive cells) but that by enhancing differentiation, more MyHC-expressing cells were visible (Fig. 6 and data not shown). We also observed that calcineurin transfection alone (Fig. 5A) or calcineurin cotransfection with NFATc1, NFATc3, or NFATc4 failed to induce myogenesis without MyoD (data not shown).
FIG. 6.
Myogenin immunocytochemistry reveals that calcineurin does not alter myocyte commitment. (A) 10T1/2 cells transiently transfected with MyoD become immunoreactive for the muscle-specific marker myogenin (arrowhead, green staining). (B) Even though cain cotransfection blocks MyoD-directed MyHC expression, these cells still express myogenin, suggesting no loss of transfected cells or in their respecification to the myogenic lineage. (C) Cotransfection of MyoD and calcineurin (CnA) also does not alter the number of myogenin-positive cells. Transfected cells were shifted to DM for 6 days before being immunostained for myogenin.
To examine the necessity of calcineurin activity in MyoD-induced 10T1/2 cell myogenic conversion, an expression vector encoding the noncompetitive calcineurin-inhibitory domain of cain was utilized. Cotransfection of MyoD with cain blocked MyHC protein expression and myotube formation (Fig. 5D). However, MyoD-cain cotransfection did not inhibit the number of myogenin-positive cells, suggesting that calcineurin was not regulating myocyte specification but instead was regulating the subsequent differentiation (Fig. 6B).
To quantify myogenic conversion, we performed Western blotting for total MyHC protein. In the presence of calcineurin, MyoD-induced myogenesis of 10T1/2 cells was typically characterized by a 20-fold increase in the MyHC protein level (Fig. 5E). In contrast, no MyHC expression was detected in extracts from 10T1/2 cells cotransfected with MyoD and cain (Fig. 5E). These results were confirmed in four separate experiments. Taken together, these findings indicate that calcineurin signaling is necessary for MyoD-induced myogenic differentiation of uncommitted fibroblasts.
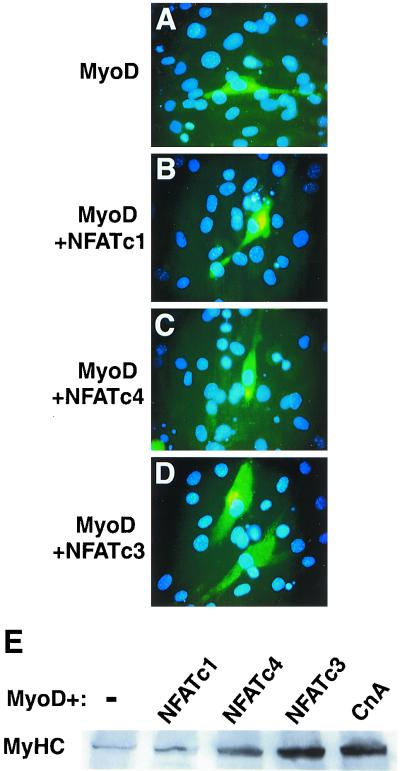
NFATc3 enhances the myogenic activity of MyoD.
To further examine the mechanism whereby calcineurin-signaling pathways might cooperate with MyoD, we assayed the ability of different NFAT factors to enhance differentiation (Fig. 7). Using cotransfection assays, we observed that NFATc3, but not NFATc4 or NFATc1, enhanced the differentiation of MyoD-specified 10T1/2 cells (Fig. 7A to D). We also determined that even a constitutively nuclear form of NFATc4 or NFATc1 (10, 35) was incapable of significantly enhancing myogenesis to the extent observed with wild-type NFATc3 (data not shown). Western blotting for total MyHC was performed to quantify enhanced myogenesis afforded by NFATc3 cotransfection with MyoD (Fig. 7E). The data demonstrated a reproducible 10- to 20-fold increase in MyHC protein expression with NFATc3 cotransfection, similar to calcineurin cotransfection. These data were confirmed in three separate experiments.
FIG. 7.
NFATc3 collaborates with MyoD to induce myogenic conversion in transiently transfected 10T1/2 cells. (A) 10T1/2 cells were grown in DM for 6 days after transfection with MyoD and stained for total MyHC protein (MF20 antibody). (B and C) Cotransfection of MyoD with either a constitutively nuclear or wild-type (not shown) NFATc1- or NFATc4-encoding vector did not significantly enhance 10T1/2 cell differentiation or MyHC immunostaining. (D) In contrast, cotransfection of a expression vector encoding full-length NFATc3 produced a noticeable enhancement of myogenesis (extent of differentiation). (E) Western blot analysis for total MyHC protein levels demonstrated that only NFATc3 enhanced the effects of MyoD. These results were similar in three independent experiments. CnA, calcineurin.
Calcineurin promotes slow-fiber-specific MyHC expression in vitro and in vivo.
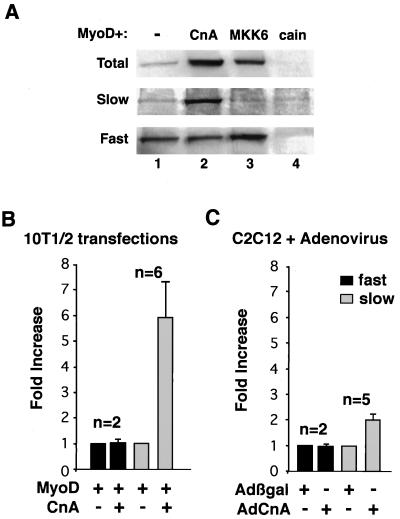
It was previously reported that cyclosporine induced fast-fiber-type switching in the muscles of rodents over 2 to 6 weeks of treatment (10, 16). These data imply that calcineurin normally plays a role in promoting slow-fiber-type specificity. To directly test this notion, we assayed the distribution of slow- or fast-fiber-specific MyHC isoforms in 10T1/2 fibroblasts transfected with MyoD and calcineurin (Fig. 8A). Western blotting demonstrated a pronounced increase in slow MyHC protein content in MyoD-calcineurin-cotransfected 10T1/2 cells compared to that in MyoD-transfected cells alone (Fig. 8A). In contrast, fast MyHC protein levels were not increased by calcineurin cotransfection (Fig. 8A, lanes 1 and 2). Quantitation of multiple experiments revealed an approximately sixfold increase in slow MyHC protein expression by calcineurin cotransfection in 10T1/2 cells (n = 6) with no change in fast MyHC protein levels (Fig. 8B). Similarly, AdCnA infection of C2C12 cells promoted a twofold increase in slow MyHC protein expression (n = 5) without causing a change in fast MyHC protein expression (Fig. 8C).
FIG. 8.
Calcineurin promotes the expression of slow fiber MyHC isoform in C2C12 and 10T1/2 cells. (A) Extracts from transiently transfected 10T1/2 cells were probed with antibodies against total, slow, or fast MyHC antibody. Calcineurin (CnA) cotransfection induced slow myosin but not fast myosin. In contrast, mitogen-activated protein kinase kinase 6 (MKK6) cotransfection induced fast myosin but not slow myosin. These data indicate that increased slow MyHC protein expression is specific to calcineurin and is not the result of a general enhancement of differentiation. (B and C) Quantitation of these effects from multiple independent experiments demonstrates augmented slow MyHC protein levels in both 10T1/2 cells and C2C12 cells infected with AdCnA.
Since calcineurin potentiates myogenic differentiation, it is feasible that any enhancement of differentiation in C2C12, Sol8, or MyoD-converted fibroblasts might result in preferential slow MyHC protein expression. To assess specificity, we sought to enhance MyoD-directed differentiation of 10T1/2 cells in a calcineurin-independent manner. Previous studies have shown that p38 activation also enhances myogenic differentiation (61). As a control, we cotransfected MyoD with an expression vector encoding constitutively active MKK6 (which activates p38), which dramatically increased total MyHC protein expression (Fig. 8A, lane 3). However, enhanced myogenesis directed by MKK6 did not promote slow MyHC protein expression but instead promoted fast MyHC expression (Fig. 8A, lane 3). These data indicate that calcineurin activation promotes slow-fiber-type specificity in vitro while p38 activation enhances a fast-fiber-type program in the presence of transfected MyoD (see Discussion).
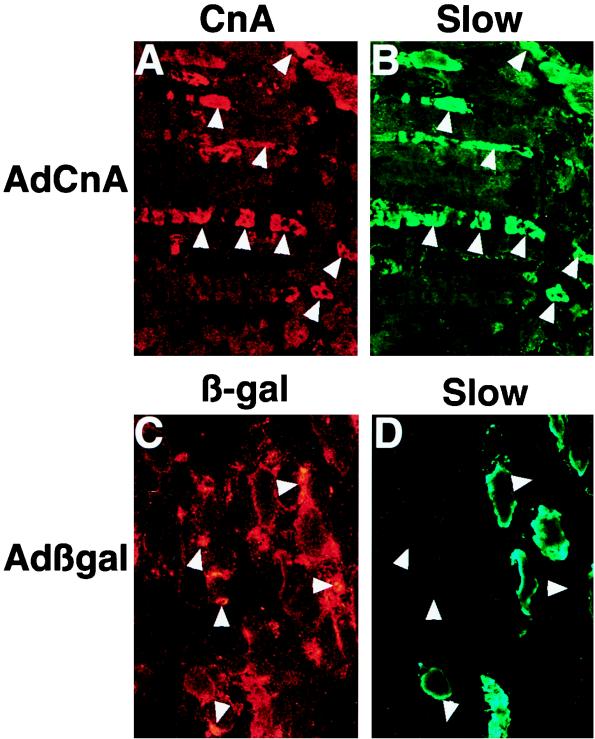
To extend these in vitro observations, AdCnA was injected into the gastrocnemius muscle of 4-day-old neonatal rat pups for subsequent immunohistochemical analysis of slow MyHC protein expression in vivo. Two weeks after AdCnA injection (1.0 × 109 PFU), we observed induction of slow MyHC protein in predominantly fast MyHC-expressing areas of the gastrocnemius muscle that was coincident with expression of the activated calcineurin protein (Fig. 9A and B). In contrast, Adβgal infection (nucleus localized) did not correlate with slow MyHC protein expression (Fig. 9C and D). These data indicate that calcineurin activation is sufficient to induce slow MyHC in vivo.
FIG. 9.
Adenovirus-mediated gene transfer of activated calcineurin in the rat gastrocnemius induces slow MyHC expression in vivo. (A) Immunostaining with calcineurin (CnA)-specific antibody (which readily detects the activated form of calcineurin) on histological sections from an injected rat gastrocnemius demonstrates a large region of expression (red). (B) Coimmunostaining with slow MyHC antibody (green) demonstrates largely coincident staining (see arrowheads). (C) As a control, Adβgal infection was performed followed by immunostaining with a β-galactosidase (β-gal) antibody (nuclear staining in red). (D) Slow MyHC protein (green) was not coincident with Adβgal infection.
DISCUSSION
In this study we demonstrate that calcineurin signaling contributes to the initial events of myogenic differentiation through an NFATc3-dependent mechanism. Calcineurin augmented the differentiation of C2C12 myoblasts, Sol8 myoblasts, 10T1/2 fibroblasts transfected with MyoD, and modified C2C12 myoblasts expressing an inhibitor of IGF signaling. The transcription factor NFATc3 is implicated as the critical downstream mediator of the effects of calcineurin in four ways. First, NFATc3 is the only NFAT factor which translocates to the nucleus in response to calcineurin in C2C12 myoblasts and 10T1/2 fibroblasts at the onset of differentiation. Second, NFATc3 cotransfection with MyoD increases MyHC expression in 10T1/2 fibroblasts. Third, inhibition of NFATc3 nuclear translocation in 10T1/2 fibroblasts using a calcineurin inhibitory peptide (cain) blocks differentiation. Fourth, initiation of differentiation in C2C12 cells is coincident with the appearance of a faster-migrating isoform of NFATc3, suggesting specific dephosphorylation by calcineurin. In contrast, NFATc3 did not promote slow-MyHC expression, indicating that calcineurin specifies a slow-fiber-type program through other factors.
Endogenous calcineurin is activated at the onset of myocyte differentiation.
Removal of growth factors from the culture medium results in the initiation of myogenic differentiation of most primary and established myoblast cell lines. This event is associated with altered intracellular signaling such that some pathways are inhibited while others are activated. Growth factor withdrawal is also associated with the production of locally acting IGF proteins which are thought to promote myocyte differentiation (43, 55). We observed a transient, 2.7-fold increase in calcineurin phosphatase activity at the onset of myoblast differentiation in the absence of any change in calcineurin protein content (catalytic subunit, molecular mass of 61 kDa) (Fig. 1). This increase in calcineurin enzymatic activity gradually decreased as differentiation progressed in C2C12 myocytes and was completely inhibited by Adcain infection.
Two recent studies have also shown increased calcineurin activity or protein content during myocyte differentiation (39, 46). However, our data differed slightly from those in both reports. Semsarian et al. demonstrated that IGF-1-induced C2C12 differentiation resulted in a sustained increase in calcineurin enzymatic activity throughout differentiation, without a corresponding change in calcineurin catalytic protein levels (46). In contrast, using a stable IGF-1-expressing L6E9 cell line, Musaró et al. found a sustained increase in calcineurin protein levels during differentiation (39). While augmentation of calcineurin was identified in both studies, the exact details are different, possibly due to differences in cell lines and/or culture conditions.
The underlying molecular mechanisms whereby endogenous calcineurin is activated by growth factor withdrawal or by IGFs is unknown. Calcineurin activation is typically calcium and calmodulin dependent, although long-chain fatty acids and arachidonic acid have recently been shown to potently activate calcineurin, independent of calcium/calmodulin (26). Local or subcellular alterations in calcium concentrations might also be sufficient to activate calcineurin. Indeed, insulin stimulation of myocytes was shown to produce a 70% increase in the subsarcolemma calcium concentration through L-type channels without affecting basal calcium levels (9). In addition, IGF-1-transfected C2C12 myotubes demonstrated higher intracellular calcium concentrations than did control myotubes (46). Alternatively, calcineurin might be regulated by the loss of an inhibitory factor or signal at the onset of differentiation.
Calcineurin and IGF-1 signaling.
The molecular alterations which initiate myocyte differentiation in culture are largely complete within 24 h of growth factor withdrawal or IGF-1 stimulation. During this critical period, calcineurin is activated and NFATc3 is translocated to the nucleus. Musaró et al. reported that a delay of cyclosporine administration until 48 h after the initiation of differentiation was ineffective in blocking differentiation, suggesting that calcineurin activity is required only at the onset of differentiation (39). Similarly, our data demonstrated that Adcain or AdAKAP (calcineurin-inhibitory domain of AKAP79) only mildly inhibited (twofold) C2C12 or Sol8 cell differentiation and did not affect the differentiation of C2BP-5 cells treated with exogenous IGF-1. Collectively, these data suggest that while calcineurin is sufficient to promote differentiation in the absence of IGF-1 signaling, inhibition of calcineurin cannot override the effects of other signaling pathways downstream of IGF-1. Despite this conclusion, we also noted that cain blocked the differentiation of 10T1/2 fibroblasts when cotransfected with MyoD. These data suggest that conversion of 10T1/2 cells by MyoD is more sensitive to calcineurin inhibition. Alternatively, the inability of cain to completely abolish differentiation in C2C12 and Sol8 cells, compared with converted 10T1/2 cells, may be due to the presence of an intrinsic program and numerous myogenic promoting factors in C2C12 and Sol8 myoblasts.
NFATc3 enhances myocyte differentiation.
Although three NFAT family members are present in the cytoplasm of undifferentiated human myoblasts, only NFATc3 translocates to the nucleus during the onset of differentiation whereas NFATc1 and NFATc2 translocate to the nucleus of more mature myotubes (1). We observed an identical regulatory hierarchy among the NFAT family members in differentiating C2C12 myocytes (Fig. 2 and data not shown). This raises the possibility that NFATc3 activates genes essential for the initiation of myogenesis while NFATc1 and NFATc2 function in the maintenance of the myogenic state or are involved in secondary myofiber formation. We also observed that only NFATc3 enhanced MyoD-induced conversion of 10T1/2 fibroblasts and that blocking NFATc3 nuclear translocation with cain prevented 10T1/2 myogenic differentiation. It is unclear why NFATc3 is dramatically more efficient in promoting myocyte differentiation than are the other NFAT factors tested. However, NFATc3 may interact with different cofactors or bind slightly different sequence elements in gene promoters to provide specificity. It will be interesting to monitor the kinetics of muscle development or myofiber regeneration in NFATc3-targeted mice. Indeed, NFATc3 knockout mice appear to have a muscle defect related to differentiation (U. Delling and J. D. Molkentin, unpublished data).
Slow- versus fast-fiber-type programs.
Two previous studies have demonstrated that cyclosporine administration augments fast MyHC protein expression in skeletal muscle (10, 16), while a third study reported no effect of cyclosporine on fiber specificity (6). The data presented in this report support the notion that calcineurin regulates fiber specificity. In two of three previous studies, calcineurin inhibition was associated with decreased slow-fiber-type specificity, suggesting that basal calcineurin activity normally promotes the slow-fiber-type program. In this study, we directly demonstrated that enhanced calcineurin activity promotes slow MyHC protein expression in 10T1/2 cells and C2C12 myocytes and in skeletal muscle in vivo.
Conversion of 10T1/2 cells with MyoD represents an ideal experimental system to examine fiber type gene expression because 10T1/2 cells have no predetermined specificity for fast or slow programs. However, MyoD enhances the expression of fast type IIB expression in C2C12 and HepG2 cells and is associated with fast-fiber-type switching in unloaded soleus muscle (57). We also observed that MKK6 was capable of enhancing the MyoD-induced conversion of 10T1/2 fibroblasts according to a fast program (Fig. 8). Despite a propensity for MyoD to promote fast MyHC protein expression, calcineurin cotransfection preferentially induced endogenous slow MyHC protein expression. This mechanism is consistent with the data of Chin et al., who demonstrated in transient-transfection experiments that calcineurin potentiated the expression of the slow TnI and myoglobin promoters but not of fast associated promoters such as muscle creatine kinase (10).
The intrinsic specificity of C2C12 or Sol8 myocytes for fast or slow fiber types has not been previously examined. Both cell lines were derived from satellite cells in the mouse (7, 37). Recent investigation has suggested that satellite cells remain unspecified toward a fast- or slow-lineage commitment, regardless of their origins (12). The commitment to a slow or fast fiber type is thought to be plastic, such that changes in motor nerve activity transform the specificity (reviewed in reference 59). As proposed previously (10), calcineurin represents an ideal signaling factor to integrate changes in gene expression in response to cumulative changes in muscle activity. Specifically, slow fibers are associated with chronic workloads and tonic motor nerve activity, which result in higher basal calcium concentrations (11). In contrast, fast fibers are thought to have substantially lower levels of calcium at rest and generally have lower tonic motor nerve activity (56). Since calcineurin is activated primarily by sustained elevations in intracellular calcium levels (15), slow fibers may preferentially utilize calcineurin to integrate calcium concentration with slow-fiber-type gene expression.
In vivo, we observed that adenovirus-mediated gene transfer of activated calcineurin promotes slow-MyHC expression after 2 weeks. AdCnA was injected into the gastrocnemius, which is a predominantly fast muscle in the rat. We observed that more than 90% of the muscle cells that expressed the activated calcineurin protein were also positive for slow MyHC, as assessed by double-antibody labeling (Fig. 9). In contrast, Adβgal injection did not induce slow MyHC protein expression. Recently, transgenic mice expressing an activated calcineurin cDNA under control of the muscle creatine kinase promoter were reported (39a). These mice were characterized by increased numbers of slow fibers in specific regions of the body. Our study confirms these results and extends the paradigm such that only 2 weeks of activated calcineurin expression is sufficient to cause fiber switching.
While calcineurin is activated downstream of IGF-1 in the induction of myocyte differentiation, it is not certain how IGF-1 stimulation relates to fiber type specificity. IGF-1 was reported to induce a phenotype in skeletal muscle that is consistent with the fast-fiber program (47); however, another study reported that IGF-1 had no inherent ability to specify slow or fast fibers (2). It is likely that calcineurin is but one component of a multicomponent signaling pathway which integrates IGF-1 signaling in myocytes. It is also likely that IGF-1 operates in concert with other growth factors and intrinsic mechanisms to promote myocyte differentiation and fiber type specificity in vivo.
Consistent with the notion that multiple signaling pathways mediate myocyte differentiation and fast or slow fiber types, we determined that NFATc3 functioned only in the enhancement of myogenic differentiation and not in fiber type specificity. These data indicate that calcineurin acts through NFATc3 to regulate myocyte differentiation but that calcineurin acts independent of NFATc3 in promoting slow-fiber specificity. This conclusion does not rule out a role for NFATc1 or NFATc2 as downstream effectors of the slow-fiber-type program. Alternatively, calcineurin may function through other intracellular mediators and signaling pathways to promote the slow-fiber-type program.
ACKNOWLEDGMENTS
We thank Bruce C. Trapnell for assistance with adenovirus.
This work was supported by National Institutes of Health (NIH) grants HL-69562, HL-62927 (J.D.M.) and DK-42748 (P.R.). The work was also supported by a Scholar award from the Pew Foundation and by local American Heart Affiliate grant-in-aid to J.D.M. U.D. and L.J.D.W. were each supported by a local American Heart Affiliate postdoctoral grant.
REFERENCES
- 1.Abbott K L, Friday B B, Thaloor D, Murphy T J, Pavlath G K. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle. Mol Biol Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barjot C, Navarro M, Cotten M L, Garandel V, Bernardi H, Bacou F, Barenton B. Rabbit slow and fast skeletal muscle-derived satellite myoblast phenotypes do not involve constitutive differences in the components of the insulin-like growth factor system. J Cell Physiol. 1996;169:227–234. doi: 10.1002/(SICI)1097-4652(199611)169:2<227::AID-JCP1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Barton-Davis E R, LaFramboise W A, Kushmerick M J. Activity-dependent induction of slow myosin gene expression in isolated fast-twitch mouse muscle. Am J Physiol. 1996;271:C1409–C1414. doi: 10.1152/ajpcell.1996.271.4.C1409. [DOI] [PubMed] [Google Scholar]
- 4.Barton-Davis E R, Shoturma D I, Musaro A, Rosenthal N, Sweeney H L. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors. 1998;8:273–281. doi: 10.1002/biof.5520080315. [DOI] [PubMed] [Google Scholar]
- 6.Biring M S, Fournier M, Ross D J, Lewis M I. Cellular adaptations of skeletal muscles to cyclosporine. J Appl Physiol. 1998;84:1967–1975. doi: 10.1152/jappl.1998.84.6.1967. [DOI] [PubMed] [Google Scholar]
- 7.Blau H M, Chiu C-P, Webster C. Cytoplasmic activation of human nuclear genes in stable heterokaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- 8.Brunetti O, Barazzoni A M, Dell Torre G, Clavenzani P, Pettorossi V E, Bortolami R. Partial transformation from fast to slow muscle fibers induced by deafferentation of capsaicin-sensitive muscle afferents. Muscle Nerve. 1997;20:1404–1413. doi: 10.1002/(sici)1097-4598(199711)20:11<1404::aid-mus8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Bruton J D, Katz A, Westerblad H. Insulin increases near-membrane but not global Ca2+ in isolated skeletal muscle. Proc Natl Acad Sci USA. 1999;96:3281–3286. doi: 10.1073/pnas.96.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin E R, Olson E N, Richardson J A, Yang Q, Humphries C, Shelton J M, Wu H, Zhu W, Bassel-Duby R, Williams R S. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin E R, Allen D G. Changes in intracellular free Ca2+ concentration during constant 10 Hz stimulation of mouse single skeletal muscle fibers. Physiologist. 1996;39:A75. [Google Scholar]
- 12.Cooper R N, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne G S. In vivo satellite cell activation via myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci. 1999;112:2895–2901. doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree G R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NFAT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 14.De Angelis L, Borghi S, Melchionna R, Berghella L, Baccarani-Contri M, Parise F, Farrari S, Cossu G. Inhibition of myogenesis by transforming growth factor beta is density-dependent and related to the translocation of transcription factor MEF2 to the cytoplasm. Proc Natl Acad Sci USA. 1998;95:12358–12363. doi: 10.1073/pnas.95.21.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 16.Dunn S E, Burns J L, Michel R N. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- 17.Eckner R, Yao T P, Oldread E, Livingston D M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Foix A M, Coats W S, Baque S, Alam T, Gerard R D, Newgard C B. Adenovirus-mediated transfer of muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J Biol Chem. 1992;267:25129–25134. [PubMed] [Google Scholar]
- 19.Green B N, Jones S B, Streck R D, Wood T L, Rotwein P, Pintar J E. Distinct expression patterns of insulin-like growth factor binding proteins 2 and 5 during fetal and post-natal development. Endocrinology. 1994;133:954–962. doi: 10.1210/endo.134.2.7507840. [DOI] [PubMed] [Google Scholar]
- 20.Guo K, Wang J, Andrés V, Smith R C, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoey T, Sun Y-L, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 22.James P L, Stewart C E H, Rotwein P. Insulin-like growth factor binding protein-5 modulates muscle differentiation through an insulin-like growth factor-dependent mechanism. J Cell Biol. 1996;133:683–694. doi: 10.1083/jcb.133.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James P L, Busby W C, Clemmons D, Rotwein P. A highly conserved insulin-like growth factor binding protein (IGFBP-5) that is expressed during myoblast differentiation. J Biol Chem. 1993;268:22305–22312. [PubMed] [Google Scholar]
- 24.Jiang B H, Zheng J Z, Vogt P K. An essential role of phosphatidylinositol 3-kinase in myogenic differentiation. Proc Natl Acad Sci USA. 1998;95:14179–14183. doi: 10.1073/pnas.95.24.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaliman P, Canicio J, Shepherd P R, Beeton C A, Testar X, Palacin M, Zorzano A. Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol Endocrinol. 1998;12:66–77. doi: 10.1210/mend.12.1.0047. [DOI] [PubMed] [Google Scholar]
- 26.Kessen U, Schaloske R, Aichem A, Mutzel R. Ca2+/calmodulin-independent activation of calcineurin from Dictyostelium by unsaturated long chain fatty acids. J Biol Chem. 1999;274:37821–37826. doi: 10.1074/jbc.274.53.37821. [DOI] [PubMed] [Google Scholar]
- 27.Lai M M, Burnett P E, Wolosker H, Blackshaw S, Snyder S H. Cain, a novel physiologic protein inhibitor of calcineurin. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Zhou J, James G, Heller-Harrison R, Czech M P, Olson E N. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992;71:1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- 29.Lopéz-Rodríguez C, Aramburu J, Rakeman A S, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci USA. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masadu E S, Naito Y, Tokumitsu H, Campbell D, Saito F, Hannum C, Arai K, Arai N. NFATx, a novel member of the nuclear factor of activated T-cells family that is expressed predominantly in the thymus. Mol Cell Biol. 1995;15:2697–2706. doi: 10.1128/mcb.15.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer S, Kohler N G, Joly A. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-kappaB activation. FEBS Lett. 1997;413:354–358. doi: 10.1016/s0014-5793(97)00930-7. [DOI] [PubMed] [Google Scholar]
- 32.Mittereder N, March K L, Trapnell B C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molkentin J D, Olson E N. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- 34.Molkentin J D, Black B L, Martin J F, Olson E N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 35.Molkentin J D, Lu J R, Antos C L, Markham B E, Richardson J, Robbins J, Grant S R, Olson E N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Matusumoto M, Nishida E. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-alpha, and H2O2. J Biol Chem. 1996;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- 37.Mulle C, Benoit P, Pinset C, Roa M, Changeux J-P. Calcitonin gene-related peptide enhances the rate of desensitization of the nicotinic acetylcholine receptor in cultured mouse muscle cells. Proc Natl Acad Sci USA. 1988;85:5728–5732. doi: 10.1073/pnas.85.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musaró A, Rosenthal N. Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I. Mol Cell Biol. 1999;19:3115–3124. doi: 10.1128/mcb.19.4.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musaró A, McCullagh K J A, Naya F J, Olson E N, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 39a.Naya F J, Mercer B, Shelton J, Richardson J A, Williams R S, Olson E N. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- 40.Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 41.Ramocki M B, White M A, Konieczny S F, Taparowsky E J. A role for RalGDS and a novel Ras effector in the Ras-mediated inhibition of skeletal myogenesis. J Biol Chem. 1998;273:17696–17701. doi: 10.1074/jbc.273.28.17696. [DOI] [PubMed] [Google Scholar]
- 42.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 43.Rosen K M, Wentworth B M, Rosenthal N, Villa-Komaroff L. Specific, temporally regulated expression of the insulin-like growth factor II gene during muscle cell differentiation. Endocrinology. 1993;133:474–481. doi: 10.1210/endo.133.2.8393762. [DOI] [PubMed] [Google Scholar]
- 44.Sartorelli V, Haung J, Hamamori Y, Kedes L. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol Cell Biol. 1997;17:1010–1026. doi: 10.1128/mcb.17.2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuler M, Pette D. Fiber transformation and replacement in low-frequency stimulated rabbit fast-twitch muscles. Cell Tissue Res. 1996;285:297–303. doi: 10.1007/s004410050647. [DOI] [PubMed] [Google Scholar]
- 46.Semsarian C, Wu M-J, Ju Y-K, Marciniec T, Yeoh T, Allen D G, Harvey R P, Graham R M. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signaling pathway. Nature. 1999;400:576–580. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- 47.Semsarian C, Sutrave P, Richmond D R, Graham R M. Insulin-like growth factor (IGF-I) induces myotube hypertrophy associated with an increase in anaerobic glycolysis in a clonal skeletal-muscle cell model. Biochem J. 1999;339:443–451. [PMC free article] [PubMed] [Google Scholar]
- 48.Skapek S X, Rhee J, Spicer D B, Lassar A B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 49.Soulez M, Rouviere C G, Chafey P, Hentzen D, Vandromme M, Lautredou N, Lamb N, Kahn A, Tuil D. Growth and differentiation of C2 myogenic cells are dependent on serum response factor. Mol Cell Biol. 1996;16:6065–6074. doi: 10.1128/mcb.16.11.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sreter F A, Lopez J R, Alamo L, Mabuchi K, Gergely J. Changes in intracellular ionized calcium concentration associated with muscle fiber type transformation. Am J Physiol. 1987;253:C296–C300. doi: 10.1152/ajpcell.1987.253.2.C296. [DOI] [PubMed] [Google Scholar]
- 51.Stockdale F E. Mechanisms of formation of muscle fiber types. Cell Struct Funct. 1997;22:37–43. doi: 10.1247/csf.22.37. [DOI] [PubMed] [Google Scholar]
- 52.Sun L, Youn H D, Loh C, Stolow M, He W, Liu J O. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 53.Sussman M A, Baque S, Uhm C S, Daniels M P, Price R L, Simpson D, Terracio L, Kedes L. Altered expression of tropomodulin in cardiomyocytes disrupts the sarcomeric structure of myofibrils. Circ Res. 1998;82:94–105. doi: 10.1161/01.res.82.1.94. [DOI] [PubMed] [Google Scholar]
- 54.Taigen T, De Windt L J, Lim H W, Molkentin J D. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tollefsen S E, Lajara R, McClusker R H, Clemmons D R, Rotwein P. Insulin-like growth factors (IGF) in muscle development. Expression of IGF-I, IGF-I receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem. 1989;264:13810–13817. [PubMed] [Google Scholar]
- 56.Westerbald H, Allen D G. Changes in myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wheeler M T, Snyder E C, Patterson M N, Swoap S J. An E-box within the MHC IIB gene is bound by MyoD and is required for gene expression in fast muscle. Am J Physiol. 1999;276:C1067–C1078. doi: 10.1152/ajpcell.1999.276.5.C1069. [DOI] [PubMed] [Google Scholar]
- 58.Williams R S, Salmons S, Newsholme E A, Kaufman R E, Mellor J. Regulation of nuclear and mitochondrial gene expression by contractile activity in skeletal muscle. J Biol Chem. 1986;261:376–380. [PubMed] [Google Scholar]
- 59.Williams R S, Neufer P D. Regulation of gene expression in skeletal muscle by contractile activity. In: Rowell L B, Shepard J T, editors. Handbook of physiology: integration of motor, circulatory, respiratory and metabolic control during exercise. Bethesda, Md: American Physiological Society; 1996. pp. 1124–1150. [Google Scholar]
- 60.Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem. 1996;271:9009–9013. doi: 10.1074/jbc.271.15.9009. [DOI] [PubMed] [Google Scholar]
- 61.Zester A, Gredinger E, Bengal E. P38 mitogen-activated kinase pathways promotes skeletal muscle differentiation. J Biol Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]