Abstract
Background
Medication errors are preventable events that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional or patient. Medication errors in hospitalised adults may cause harm, additional costs, and even death.
Objectives
To determine the effectiveness of interventions to reduce medication errors in adults in hospital settings.
Search methods
We searched CENTRAL, MEDLINE, Embase, five other databases and two trials registers on 16 January 2020.
Selection criteria
We included randomised controlled trials (RCTs) and interrupted time series (ITS) studies investigating interventions aimed at reducing medication errors in hospitalised adults, compared with usual care or other interventions. Outcome measures included adverse drug events (ADEs), potential ADEs, preventable ADEs, medication errors, mortality, morbidity, length of stay, quality of life and identified/solved discrepancies. We included any hospital setting, such as inpatient care units, outpatient care settings, and accident and emergency departments.
Data collection and analysis
We followed the standard methodological procedures expected by Cochrane and the Effective Practice and Organisation of Care (EPOC) Group. Where necessary, we extracted and reanalysed ITS study data using piecewise linear regression, corrected for autocorrelation and seasonality, where possible.
Main results
We included 65 studies: 51 RCTs and 14 ITS studies, involving 110,875 participants. About half of trials gave rise to 'some concerns' for risk of bias during the randomisation process and one‐third lacked blinding of outcome assessment. Most ITS studies presented low risk of bias. Most studies came from high‐income countries or high‐resource settings. Medication reconciliation –the process of comparing a patient's medication orders to the medications that the patient has been taking– was the most common type of intervention studied. Electronic prescribing systems, barcoding for correct administering of medications, organisational changes, feedback on medication errors, education of professionals and improved medication dispensing systems were other interventions studied.
Medication reconciliation
Low‐certainty evidence suggests that medication reconciliation (MR) versus no‐MR may reduce medication errors (odds ratio [OR] 0.55, 95% confidence interval (CI) 0.17 to 1.74; 3 studies; n=379). Compared to no‐MR, MR probably reduces ADEs (OR 0.38, 95%CI 0.18 to 0.80; 3 studies, n=1336 ; moderate‐certainty evidence), but has little to no effect on length of stay (mean difference (MD) ‐0.30 days, 95%CI ‐1.93 to 1.33 days; 3 studies, n=527) and quality of life (MD ‐1.51, 95%CI ‐10.04 to 7.02; 1 study, n=131).
Low‐certainty evidence suggests that, compared to MR by other professionals, MR by pharmacists may reduce medication errors (OR 0.21, 95%CI 0.09 to 0.48; 8 studies, n=2648) and may increase ADEs (OR 1.34, 95%CI 0.73 to 2.44; 3 studies, n=2873). Compared to MR by other professionals, MR by pharmacists may have little to no effect on length of stay (MD ‐0.25, 95%CI ‐1.05 to 0.56; 6 studies, 3983). Moderate‐certainty evidence shows that this intervention probably has little to no effect on mortality during hospitalisation (risk ratio (RR) 0.99, 95%CI 0.57 to 1.7; 2 studies, n=1000), and on readmissions at one month (RR 0.93, 95%CI 0.76 to 1.14; 2 studies, n=997); and low‐certainty evidence suggests that the intervention may have little to no effect on quality of life (MD 0.00, 95%CI ‐14.09 to 14.09; 1 study, n=724).
Low‐certainty evidence suggests that database‐assisted MR conducted by pharmacists, versus unassisted MR conducted by pharmacists, may reduce potential ADEs (OR 0.26, 95%CI 0.10 to 0.64; 2 studies, n=3326), and may have no effect on length of stay (MD 1.00, 95%CI ‐0.17 to 2.17; 1 study, n=311).
Low‐certainty evidence suggests that MR performed by trained pharmacist technicians, versus pharmacists, may have little to no difference on length of stay (MD ‐0.30, 95%CI ‐2.12 to 1.52; 1 study, n=183). However, the CI is compatible with important beneficial and detrimental effects.
Low‐certainty evidence suggests that MR before admission may increase the identification of discrepancies compared with MR after admission (MD 1.27, 95%CI 0.46 to 2.08; 1 study, n=307). However, the CI is compatible with important beneficial and detrimental effects.
Moderate‐certainty evidence shows that multimodal interventions probably increase discrepancy resolutions compared to usual care (RR 2.14, 95%CI 1.81 to 2.53; 1 study, n=487).
Computerised physician order entry (CPOE)/clinical decision support systems (CDSS)
Moderate‐certainty evidence shows that CPOE/CDSS probably reduce medication errors compared to paper‐based systems (OR 0.74, 95%CI 0.31 to 1.79; 2 studies, n=88).
Moderate‐certainty evidence shows that, compared with standard CPOE/CDSS, improved CPOE/CDSS probably reduce medication errors (OR 0.85, 95%CI 0.74 to 0.97; 2 studies, n=630).
Low‐certainty evidence suggests that prioritised alerts provided by CPOE/CDSS may prevent ADEs compared to non‐prioritised (inconsequential) alerts (MD 1.98, 95%CI 1.65 to 2.31; 1 study; participant numbers unavailable).
Barcode identification of participants/medications
Low‐certainty evidence suggests that barcoding may reduce medication errors (OR 0.69, 95%CI 0.59 to 0.79; 2 studies, n=50,545).
Reduced working hours
Low‐certainty evidence suggests that reduced working hours may reduce serious medication errors (RR 0.83, 95%CI 0.63 to 1.09; 1 study, n=634). However, the CI is compatible with important beneficial and detrimental effects.
Feedback on prescribing errors
Low‐certainty evidence suggests that feedback on prescribing errors may reduce medication errors (OR 0.47, 95%CI 0.33 to 0.67; 4 studies, n=384).
Dispensing system
Low‐certainty evidence suggests that dispensing systems in surgical wards may reduce medication errors (OR 0.61, 95%CI 0.47 to 0.79; 2 studies, n=1775).
Authors' conclusions
Low‐ to moderate‐certainty evidence suggests that, compared to usual care, medication reconciliation, CPOE/CDSS, barcoding, feedback and dispensing systems in surgical wards may reduce medication errors and ADEs. However, the results are imprecise for some outcomes related to medication reconciliation and CPOE/CDSS. The evidence for other interventions is very uncertain. Powered and methodologically sound studies are needed to address the identified evidence gaps. Innovative, synergistic strategies –including those that involve patients– should also be evaluated.
Keywords: Adult, Humans, Hospitalization, Hospitals, Medication Errors, Medication Errors/prevention & control, Medication Reconciliation, Pharmacists
Plain language summary
Interventions for reducing medication errors in adults in hospital settings
Background to the question
An adverse drug event (ADE) is an injury resulting from a medical intervention related to a drug. ADEs are sometimes associated with medication errors. ADEs and medication errors may cause important harm, costs and even death.
Interventions for reducing medication errors include medication reconciliation, which is the process of comparing a patient's medication orders to the medications that the patient has been taking. Medication reconciliation can be performed jointly with other interventions, such as electronic prescribing systems, barcoding for a correct administering of medications, organisational changes, feedback on medication errors, education of professionals and improved medication dispensing systems.
Review question
What is the effectiveness of interventions to reduce medication errors for adults in hospital settings?
We included inpatient care settings (secondary or tertiary units, intensive care units, operating theatres), outpatient care settings, and accident and emergency departments.
Study characteristics
We searched databases of scientific studies. We included 65 studies, 51 of which were randomised trials, involving 23,182 adults in hospital settings. The remaining 14 studies were large interrupted time‐series that concern long‐term period before and after a point of intervention to assess the intervention's effects, involving more than 87,000 participants.
Certainty of the evidence
We assessed the included evidence to establish how certain we are that the effects are true and would not be altered with the addition of more evidence. In general, we judged the certainty of the evidence to be low to moderate, but it was very low for some outcomes.
Key results
Medication reconciliation compared with no medication reconciliation probably reduce ADEs and may reduce medication errors. It may have little to no effect on length of stay or quality of life. However, the effect of medication reconciliation on these latter outcomes is imprecise; it is not clear if the effects are beneficial or detrimental (low‐ to moderate‐certainty evidence).
Compared to medication reconciliation by other professionals, medication reconciliation performed by pharmacists may increase ADEs (but this result is imprecise); may reduce medication errors; and may have little to no effect on length of stay, mortality during hospitalisation, and readmissions. However, these effects are imprecise (low‐certainty evidence).
Compared to no assistance, database‐assisted medication reconciliation conducted by pharmacists may reduce potential ADEs and may have no effect on length of stay, but the last effect is imprecise (low‐certainty evidence).
Medication reconciliation performed by trained pharmacist technicians instead of pharmacists, may have no effect on length of stay, but this effect is imprecise (low‐certainty evidence).
Medication reconciliation before admission, versus after admission, may increase identified discrepancies; however, the effect is imprecise (low‐certainty evidence).
Compared to usual care, some interventions have different effects:
Multimodal interventions probably increase discrepancy resolutions (moderate‐certainty evidence).
Electronic prescribing systems probably reduce medication errors and ADEs. Prioritised alerts may additionally prevent ADEs (low‐ to moderate‐certainty evidence).
Barcode identification of participants or medications may reduce medication errors (low‐certainty evidence).
Reduced working hours and feedback on medication errors may reduce serious medication errors; however, the effect is imprecise (low‐certainty evidence).
Authors' conclusions
Compared to usual care, medication reconciliation, electronic prescribing systems, barcoding and feedback to professionals may reduce ADEs or medication errors, or both. Nonetheless, the best modalities to deliver these interventions, and the effect of other interventions, are less clear.
How up to date is this review?
The review authors searched for studies that had been published up to January 2020.
Summary of findings
Summary of findings 1. Medication reconciliation versus no medication reconciliation.
| Medication reconciliation versus no medication reconciliation for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: medication reconciliation (MR) Comparison: no medication reconciliation | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 17 to 27 months) |
OR 0.55 (0.17 to 1.74) | Not estimable | 379 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | Grouped outcomes Analysis 1.1 |
| ADEs (Follow‐up 7 to 16 months) |
OR 0.38 (0.18 to 0.80) | Not estimable | 1336 (3 RCTs) |
⊕⊕⊕⊝ Moderatec | Grouped outcomes Analysis 1.2 |
| Mortality during hospitalisation (Follow‐up 9 months) |
RR 3.85 (0.44 to 33.89) |
27 more per 1000 (from 5 fewer to 316 more) |
212 (1 RCT) |
⊕⊝⊝⊝ Very lowd,e | Baseline risk 1.0% Analysis 1.3 |
| Length of stay (days) (Follow‐up 9 to 13 months) |
Not estimable |
MD ‐0.30 (‐1.93 to 1.33) |
527 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | Analysis 1.4 |
| Quality of life (VAS 0‐10; EQ‐5D‐3L) (Follow‐up 10 months) |
Not estimable |
MD ‐1.51 (‐10.04 to 7.02) |
131 (1 RCT) |
⊕⊕⊝⊝ Lowa,b | (high score better) Analysis 1.5 |
| Discrepancy resolution (Follow‐up 10 months) |
RR 7.48 (5.62 to 9.95) |
860 more per 1000 (from 613 more to 1000 more) |
564 (1 RCT) |
⊕⊕⊕⊝ Moderateb | Analysis 1.6 |
| ADEs: adverse drug events; CI: confidence interval; EQ‐5D‐3L: EuroQol 5‐dimension survey; OR: odds ratio; RR: risk ratio; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data for readmissions. aDowngraded one level due to imprecision. bDowngraded one level due to risk of bias. cDowngraded one level due to inconsistency among the studies. dDowngraded two levels due to a high level of inconsistency. eDowngraded two levels due to very serious risk of bias.
1.1. Analysis.

Comparison 1: Medication reconciliation versus no medication reconciliation, Outcome 1: Medication errors
1.2. Analysis.

Comparison 1: Medication reconciliation versus no medication reconciliation, Outcome 2: ADEs
1.3. Analysis.

Comparison 1: Medication reconciliation versus no medication reconciliation, Outcome 3: Mortality during hospitalisation
1.4. Analysis.

Comparison 1: Medication reconciliation versus no medication reconciliation, Outcome 4: Length of Stay (days)
1.5. Analysis.

Comparison 1: Medication reconciliation versus no medication reconciliation, Outcome 5: QoL (VAS 0‐10 ‐ EQ‐5D‐3L ‐ high score better)
1.6. Analysis.

Comparison 1: Medication reconciliation versus no medication reconciliation, Outcome 6: Discrepancy resolutions (per discrepancies at discharge)
Summary of findings 2. Medication reconciliation: pharmacist versus other professionals.
| Medication reconciliation: pharmacist versus other professionals for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: medication reconciliation by pharmacist Comparison: medication reconciliation by other professionals | |||||
| Outcomes | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 1 to 5 months) |
OR 0.21 (0.09 to 0.48) | Not estimable | 2648 (8 RCTs) |
⊕⊕⊝⊝ Lowa | Grouped outcomes Analysis 2.1 |
| ADEs (Follow‐up 18 months to 5 years) |
OR 1.34 (0.73 to 2.44) | Not estimable | 2873 (3 RCTs) |
⊕⊕⊝⊝ Lowb,c | Grouped outcomes Analysis 2.2 |
| Mortality during hospitalisation (Follow‐up 13 to 21 months) |
RR 0.99 (0.57 to 1.73) |
0 fewer per 1000 (from 20 fewer to 34 more) |
1000 (2 RCTs) |
⊕⊕⊕⊝ Moderatec | Baseline risk 4.6% Analysis 2.3 Mortality at six months RR 0.54 (95% CI 0.22 to 1.32) |
| Readmission at 1 month (Follow‐up 13 to 21 months) |
RR 0.93 (0.76 to 1.14) |
20 fewer per 1000 (from 67 fewer to 39 more) |
997 (2 RCTs) |
⊕⊕⊕⊝ Moderatec | Baseline risk 28% Analysis 2.4 |
| Length of stay (days) (Follow‐up 18 to 21 months) |
Not estimable |
MD ‐0.25 (‐1.05 to 0.56) |
3983 (6 RCTs) |
⊕⊕⊝⊝ Lowb,c | General wards inpatients (MD ‐0.25, 95% CI ‐1.09 to 0.59) Inpatients coming from ICU (MD ‐0.30, 95% CI ‐6.71 to 6.11) Test for subgroup differences: I² = 0% Analysis 2.5 |
| Quality of life (VAS 0‐10; EQ‐5D‐3L) (Follow‐up 18 months) |
Not estimable |
MD 0.00 (‐14.09 to 14.09) |
724 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | (High score better) Analysis 2.6 |
| Discrepancy resolution (Follow‐up 6 to 13 months) |
OR 4.80 (1.81 to 12.76) | Not estimable | 1449 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | Grouped outcomes Analysis 2.7 |
| ADEs: adverse drug events; CI: Confidence interval; EQ‐5D‐3L: EuroQol 5‐dimension survey; ICU: intensive care unit; MD: mean difference; OR: odds ratio; RR: risk ratio; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to a high level of inconsistency among the studies. bDowngraded one level due to serious risk of bias. cDowngraded one level due to imprecision.
2.1. Analysis.

Comparison 2: Medication reconciliation: pharmacist versus other professionals, Outcome 1: Medication errors
2.2. Analysis.

Comparison 2: Medication reconciliation: pharmacist versus other professionals, Outcome 2: ADEs
2.3. Analysis.

Comparison 2: Medication reconciliation: pharmacist versus other professionals, Outcome 3: Mortality during hospitalisation
2.4. Analysis.

Comparison 2: Medication reconciliation: pharmacist versus other professionals, Outcome 4: Readmisson at 1 month
2.5. Analysis.

Comparison 2: Medication reconciliation: pharmacist versus other professionals, Outcome 5: Length of stay (days)
2.6. Analysis.

Comparison 2: Medication reconciliation: pharmacist versus other professionals, Outcome 6: QoL (VAS 0‐10 ‐ EQ‐5D‐3L, high score is better)
2.7. Analysis.

Comparison 2: Medication reconciliation: pharmacist versus other professionals, Outcome 7: Discrepancy resolution
Summary of findings 3. Medication reconciliation by pharmacist: database‐assisted MR versus unassisted MR.
| Medication reconciliation by pharmacist: database‐assisted versus unassisted MR for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: database‐assisted medication reconciliation performed by pharmacists Comparison: unassisted nedication reconciliation performed by pharmacists | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Potential ADEs ( ≥ 1 per patient) (Follow‐up 3 to 20 months) |
OR 0.26 (0.10 to 0.64) |
77 more per 1000 (from 7 fewer to 163 more) |
3326 (2 RCTs) |
⊕⊕⊝⊝ Lowa,b | Baseline risk 39.8% Analysis 3.1 |
| Length of stay (days) (Follow‐up 31 months) |
Not estimable |
MD 1.00 (‐0.17 to 2.17) |
311 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | Analysis 3.2 |
| Discrepancy resolution (Follow‐up 3 to 31 months) |
OR 1.37 (0.97 to 1.93) |
1 fewer per 1000 (from 2 fewer to 1 fewer) |
791 (2 RCTs) |
⊕⊕⊝⊝ Lowa,c | Analysis 3.3 |
| ADEs: adverse drug events; CI: confidence interval; OR: odds ratio; MD: Mean difference | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for medication errors, mortality, readmissions, and quality of life (QoL). aDowngraded one level due to inconsistency among studies. bDowngraded one level due to risk of bias. cDowngraded one level due to imprecision.
3.1. Analysis.

Comparison 3: Medication reconciliation by pharmacist: database‐assisted versus not‐assisted, Outcome 1: Potential ADEs (≥1 per patient)
3.2. Analysis.

Comparison 3: Medication reconciliation by pharmacist: database‐assisted versus not‐assisted, Outcome 2: Lenght of stay (days)
3.3. Analysis.

Comparison 3: Medication reconciliation by pharmacist: database‐assisted versus not‐assisted, Outcome 3: Discrepancy resolution (higher number is better)
Summary of findings 4. Medication reconciliation by trained pharmacist technician versus pharmacist.
| Medication reconciliation by trained pharmacist technician versus pharmacist for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: medication reconciliation by trained pharmacist technician Comparison: medication reconciliation by pharmacist | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 7 months) |
OR 0.65 (0.25 to 1.70) | Not estimable | 306ƚ (2 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | Grouped outcomes. Analysis 4.1 ƚThe number of participants in 1 of the studies is unknown because the study analysed prescriptions. |
| Length of stay (days) (Follow‐up not available) |
Not estimable |
MD ‐0.30 (‐2.12 to 1.52) |
183 (1 RCT) |
⊕⊕⊝⊝ Lowa,c | Analysis 4.2 |
| CI: confidence interval; MD: mean difference; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for adverse drug events, mortality, readmissions, quality of life (QoL), and discrepancy resolution. aDowngraded one level due to risk of bias. bDowngraded one level due to inconsistency among studies. cDowngraded one level due to imprecision.
4.1. Analysis.

Comparison 4: Medication reconciliation by trained pharmacist technicians versus by pharmacists, Outcome 1: Medication errors
4.2. Analysis.

Comparison 4: Medication reconciliation by trained pharmacist technicians versus by pharmacists, Outcome 2: Length of stay (days)
Summary of findings 5. Medication reconciliation: before versus at admission.
| Medication reconciliation: before versus at admission for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals (emergency department) Intervention: medication reconciliation before admission Comparison: medication reconciliation after admission | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Identified discrepancies per patient (Follow‐up 1 month) |
Not estimable |
MD 1.27 (0.46, 2.08) |
307 (1 RCT) |
⊕⊕⊝⊝ Lowa,b | Analysis 5.1 |
| CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for medication errors, adverse drug events, mortality, readmissions, length of stay, quality of life (QoL), and discrepancy resolution. aDowngraded one level due to risk of bias. bDowngraded one level due to imprecision.
5.1. Analysis.

Comparison 5: Medication reconciliation: before versus at admission, Outcome 1: Identified discrepancies per patient (higher number is better)
Summary of findings 6. Medication reconciliation: 1 or 2 versus 4 charts open simultaneously.
| Medication reconciliation: 1 or 2 versus 4 charts open simultaneously for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: 1 or 2 charts open simultaneously Comparison: 4 charts open simultaneously | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (ITS study) (Follow‐up 70 months) |
Not estimable |
MD ‐0.19 (‐0.58, 0.20) |
11,504 (1 ITS study) |
⊕⊝⊝⊝ Very lowa | Analysis 6.1 |
| CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for adverse drug events, mortality, readmissions, length of stay, quality of life (QoL), and discrepancy resolution. aDowngraded one level due to imprecision.
6.1. Analysis.

Comparison 6: Medication reconciliation: 1 or 2 versus 4 charts open simultaneously, Outcome 1: Prescribing error (per order session)
Summary of findings 7. Medication reconciliation: multimodal intervention versus usual care.
| Medication reconciliation: multimodal intervention vs usual care for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: medication reconciliation: multimodal intervention Comparison: medication reconciliation: usual care | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95%CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication error (Follow‐up 24 months) |
RR 0.92 (0.87, 0.97) |
Not estimable | 1648 (1 ITS study) |
⊕⊝⊝⊝ Very lowa | Unintended discrepancies ( ≥ 1 per patient) Analysis 7.1 |
| Potential ADEs (Follow‐up 24 months) |
RR 0.97 (0.86, 1.09) |
Not estimable | 1648 (1 ITS study) |
⊕⊝⊝⊝ Very lowa,b | Analysis 7.2 |
| Discrepancy resolution (Follow‐up 6 months) |
RR 2.14 (1.81 to 2.53) |
417 more per 1000 (from 297 more to 560 more) |
487 (1 RCT) |
⊕⊕⊕⊝ Moderatea | Analysis 7.3 |
| ADEs: adverse drug events; CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for mortality, readmissions, length of stay, and quality of life. aDowngraded one level due to risk of bias. Moderate‐certainty evidence coming from one RCT shows that, compared with usual care, a multimodal intervention probably increases discrepancy resolutions (RR 2.14, 95% CI 1.81 to 2.53; 487 participants; Analysis 7.3). bDowngraded one level due to imprecision.
7.1. Analysis.

Comparison 7: Medication reconciliation: multimodal intervention versus usual care, Outcome 1: Unintended discrepancies (≥1 per patient)
7.2. Analysis.

Comparison 7: Medication reconciliation: multimodal intervention versus usual care, Outcome 2: Potential ADEs (≥ 1 per patient)
7.3. Analysis.

Comparison 7: Medication reconciliation: multimodal intervention versus usual care, Outcome 3: Discrepancies resolutions (≥1 per patient, higher number is better)
Summary of findings 8. CPOE/CDSS compared to control/paper‐based.
| CPOE/CDSS compared to control/paper‐based systems for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: computerised physician order entry (CPOE)/clinical decision support systems (CDSS) Comparison: control/paper‐based system | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 4 months) |
OR 0.74 (0.31 to 1.79) | Not estimable | 88 (2 RCTs) |
⊕⊕⊕⊝ Moderatea | Grouped outcomes. In fact, this is one RCT but with results separated for first‐year doctors and other doctors. Analysis 8.1 |
| ADEs (Follow‐up 1 to 12 months) |
OR 0.24 (0.04 to 1.50) | Not estimable | 827 (2 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | Grouped outcomes. The ITS study Ongering 2019 favours the paper‐based arm on serious Preventable ADEs per prescriptions (MD 0.12, 95% CI ‐0.03 to 0.27; n = 2711 patients). Analysis 8.2 |
| Mortality during hospitalisation (Follow‐up 12 months) |
RR 1.04 (0.54 to 2.01) |
2 more per 1000 (from 21 fewer to 46 more) |
737 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | Analysis 8.3 |
| Length of stay (days) (Follow‐up 12 months) |
Not estimable |
MD ‐1.00 (‐2.05 to 0.05) |
737 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | Analysis 8.4 |
| ADEs: adverse drug events; CI: confidence interval; OR: odds ratio; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for mortality, readmissions, quality of life, and discrepancy resolution. aDowngraded one level due to imprecision. bDowngraded two levels due to very serious risk of bias. cDowngraded two levels due to high level of inconsistency among studies.
8.1. Analysis.

Comparison 8: CPOE/CDSS versus control/paper‐based system, Outcome 1: Medication error
8.2. Analysis.

Comparison 8: CPOE/CDSS versus control/paper‐based system, Outcome 2: ADEs
8.3. Analysis.

Comparison 8: CPOE/CDSS versus control/paper‐based system, Outcome 3: Mortality
8.4. Analysis.

Comparison 8: CPOE/CDSS versus control/paper‐based system, Outcome 4: Length of stay (days)
Summary of findings 9. Improved CPOE/CDSS versus standard CPOE/CDSS.
| CPOE/CDSS: improved compared to standard CPOE/CDSS for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: improved CPOE/CDSS Comparison: standard CPOE/CDSS | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 3 to 47 months) |
OR 0.85 (0.74 to 0.97) | Not estimable | 630 (2 RCTs) |
⊕⊕⊕⊝ Moderatea |
Analysis 9.1.1 2 ITS studies (OR 0.77, 95% CI 0.37 to 1.62; participants = 2382 + ƚGreen 2015 sample not reported) |
|
OR 0.77 0.37 to 1.62 |
Not estimable | 2382ƚ (2 ITS studies) |
Analysis 9.1.2 ƚGreen 2015 sample not reported |
||
| ADEs (Follow‐up 1 to 3 months) |
OR 0.82 (0.71 to 0.94) |
Not estimable | 2382ƚ (2 ITS studies) |
⊕⊕⊕⊝ Moderatea |
Analysis 9.2 ƚGreen 2015 sample was not reported |
| ADEs: adverse drug events; CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for mortality, readmissions, length of stay, quality of life, and discrepancy resolution. aThe certainty of evidence was driven by the RCTs, and downgraded one level due to risk of bias.
9.1. Analysis.

Comparison 9: CPOE/CDSS: improved versus standard CPOE/CDSS, Outcome 1: Medication errors
9.2. Analysis.

Comparison 9: CPOE/CDSS: improved versus standard CPOE/CDSS, Outcome 2: ADEs
Summary of findings 10. CPOE/CDSS: prioritised versus non‐prioritised alerts.
| CPOE/CDSS: prioritised versus no prioritised alerts for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: CPOE/CDSS: prioritised alerts Comparison: CPOE/CDSS: non‐prioritised alerts | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Resolved potential ADEs (per prescriptions) (Follow‐up 21 months) |
Not estimable |
MD 1.98 (1.65 to 2.31) |
Not available (1 ITS study) |
⊕⊕⊝⊝ Lowa | The unit of analysis was prescriptions Analysis 10.1 |
| ADEs: adverse drug events; CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for medication errors, mortality, readmissions, length of stay, quality of life, and discrepancy resolution. aThe certainty of evidence was low because it was drawn from non‐randomised studies, but it was not downgraded due to risk of bias.
10.1. Analysis.

Comparison 10: CPOE/CDSS: prioritised versus no prioritised alerts, Outcome 1: Resolved potential ADEs (per prescriptions, higher is better)
Summary of findings 11. Barcoding versus no barcoding.
| Barcoding versus no barcoding for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: barcoding Comparison: no barcoding | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 22 to 79 months) |
OR 0.69 (0.59 to 0.79) | Not estimable | 50,545ƚ (2 ITS studies) |
⊕⊕⊝⊝ Lowa | Grouped outcomes Analysis 11.1 ƚThe number of participants is unknown for 1 study because it used prescriptions as the unit of analysis. |
| CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
# There were no data available for adverse drug events, mortality, readmissions, length of stay, quality of life, and discrepancy resolution. aThe certainty of evidence was low because it was drawn from non‐randomised studies, but it was not downgraded due to risk of bias.
11.1. Analysis.

Comparison 11: Barcoding versus no barcoding, Outcome 1: Medication errors
Summary of findings 12. Organisational changes: reduced versus unreduced working hours.
| Organisational changes: reduced versus unreduced working hours for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals (intensive care unit) Intervention: reduced working hours Comparison: unreduced working hours | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up not available) |
RR 0.83 (0.63 to 1.09) |
17 fewer per 1000 (from 37 fewer to 9 more) |
634 (1 RCT) |
⊕⊕⊝⊝ Lowa,b | Serious medication errors per patient‐days Analysis 12.1 |
| CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for adverse drug events, mortality, readmissions, length of stay, quality of life and discrepancy resolution. aDowngraded one level due to risk of bias. bDowngraded one level due to imprecision.
12.1. Analysis.

Comparison 12: Organisational changes: reduced versus unreduced work hours, Outcome 1: Serious medication errors per patient‐days
Summary of findings 13. Feedback on prescribing errors versus no feedback.
| Feedback on prescribing errors versus no feedback for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: feedback on prescribing errors Comparison: no feedback | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 1 to 4 months) |
OR 0.47 (0.33 to 0.67) | Not estimable | 384ƚ (4 RCTs) |
⊕⊕⊝⊝ Lowa | Grouped outcomes Analysis 13.1 ƚOnly 1 out of 4 RCTs reported participants. |
| CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for adverse drug events, mortality, readmissions, length of stay, quality of life and discrepancy resolution. aDowngraded two levels because of very serious inconsistency amongst the studies.
13.1. Analysis.

Comparison 13: Feedback on prescribing errors versus no feedback, Outcome 1: Medication errors
Summary of findings 14. Feedback on prescribing errors versus education on prescribing errors.
| Feedback on prescribing errors versus education for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: feedback on prescribing errors Comparison: education | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 1 to 4 months) |
OR 0.59 (0.20 to 1.76) | Not estimable | Not available (2 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | Grouped outcomes. The unit of analysis was prescriptions. Analysis 14.1 |
| CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for adverse drug events, mortality, readmissions, length of stay, quality of life and discrepancy resolution. aDowngraded one level due to risk of bias. bDowngraded two levels due to the high level of inconsistency amongst studies. cDowngraded one level due to imprecision.
14.1. Analysis.

Comparison 14: Feedback on prescribing errors versus education, Outcome 1: Medication errors
Summary of findings 15. Education versus no education on prescribing or administration.
| Education versus no education on prescribing or administration for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults Setting: hospitals Intervention: education on prescribing or administration Comparison: no education on prescribing or administration | |||||
| Outcomes# | Relative effect (95%CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (Follow‐up 1 to 4 months) |
OR 1.21 (0.93 to 1.58) |
Not estimable | 30ƚ (4 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | Grouped outcomes Analysis 15.1 ƚOnly 1 out of 4 RCTs reported participants. Education on prescriptions (physicians) OR 1.11 (95% CI 0.88 to 1.39) Education on administration (nurses) OR 1.64 (95% CI 0.88 to 3.08) |
| CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for adverse drug events, mortality, readmissions, length of stay, quality of life and discrepancy resolution. aDowngraded one level due to risk of bias. bDowngraded two levels due to very serious inconsistency amongst the studies. cDowngraded one level due to imprecision.
15.1. Analysis.

Comparison 15: Education versus no education on prescribing, Outcome 1: Medication errors
Summary of findings 16. Dispensing system versus control.
| Dispensing system versus control for reducing medication errors in adults in hospital settings | |||||
| Patient or population: adults | |||||
| Outcomes# | Relative effect (95% CI) | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Medication errors (surgical wards) (Follow‐up 1 month) |
OR 0.61 (0.47 to 0.79) | Not estimable | 1775ƚ (2 RCTs) |
⊕⊕⊝⊝ Lowa | Grouped outcomes Analysis 16.1 ƚ1 out of 2 RCTs did not report participants. |
| Medication errors (operating rooms) (Follow‐up 5 to 12 months) |
OR 0.92 (0.75 to 1.13) | Not estimable | 2310 (2 RCTs) |
⊕⊝⊝⊝ Very lowb,c,d | Grouped outcomes Analysis 16.2 |
| CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||
#There were no data available for adverse drug events, mortality, readmissions, length of stay, quality of life and discrepancy resolution. aDowngraded two levels due to very serious risk of bias. bDowngraded one level due to risk of bias. cDowngraded one level due to inconsistency amongst the studies. dDowngraded one level due to imprecision.
16.1. Analysis.

Comparison 16: Dispensing system versus no dispensing system, Outcome 1: Medication errors
16.2. Analysis.

Comparison 16: Dispensing system versus no dispensing system, Outcome 2: Medication errors (per prescriptions)
Background
Description of the condition
The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) defines a medication error as “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient or consumer” (NCC‐MERP 2021; see also Lisby 2012). Medication errors can be associated with adverse drug events (ADEs), defined as unwanted occurrences after exposure to a drug that are not necessarily caused by the drug. ADEs include adverse drug reactions as well as 'preventable ADEs' and 'ameliorable ADEs', which are ADEs due to medication error (Figure 1). More specifically, an ameliorable ADE is an injury whose severity or duration could have been substantially reduced if different actions had been taken. A preventable ADE is an injury that is the result of an error at any stage in the medication use (Morimoto 2004). An adverse drug reaction is defined as any response to a drug which is noxious and unintended that occurs at doses normally used for prophylaxis, diagnosis or therapy of the disease (European Council 2005; Falconer 2019). Potential ADEs are defined as medication errors with high likelihood to cause harm (Bates 1995).
1.
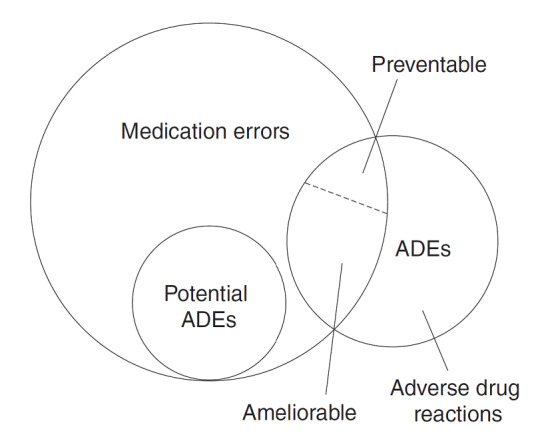
Medication error framework (from Morimoto 2004 (Licence: 4295121359710) that modified Bates 1995, with permission)
The severity of ADEs has been classified as follows (ISMP 2011).
Category 1: circumstances or processes that have the potential to cause an adverse drug event.
Category 2: an event occurred, but the patient was not harmed.
Category 3: an event occurred that resulted in the need for increased patient assessments but no change in vital signs and no patient harm.
Category 4: an event occurred that resulted in the need for treatment or intervention, or both, and caused temporary patient harm.
Category 5: an event occurred that resulted in initial or prolonged hospitalisation, affected patient participation in an investigational drug study, and/or caused temporary patient harm.
Category 6: an event occurred that resulted in permanent patient harm or a near‐death event, such as anaphylaxis.
Category 7: an event occurred that resulted in patient death.
Such events may be related to professional practice; healthcare products, procedures and systems, including prescribing; order communications; product labelling, packaging and nomenclature; compounding; dispensing; distribution; administration; and education and monitoring (Nebeker 2004). However, most of the literature on medication errors suggests that prescribing errors are the most prevalent cause (Kohn 2000; World Alliance for Patient Safety 2008).
The burden of medication errors and adverse drug events in hospitals is especially important. Medication errors and adverse drug events are associated with substantial death and injury (Kohn 2000). More than 500,000 people are injured or die each year in hospitals from adverse drug events (ADEs), which may cost up to USD 5.6 million annually per hospital (Classen 2005).
One systematic review found that the prevalence of prescribing errors ranged widely, from 2% to 94% (Assiri 2018).
The Canadian Adverse Events Study showed an adverse event rate of 7.5 per 100 hospital admissions, of which 37% were judged to be preventable (Baker 2004). Another multicenter study in the USA found that medication errors occurred in 5.3 of each 100 medication orders written, half of which were caused by missing medication dosages, 15% involved dose errors, and 13% involved route or frequency errors (Bates 1995). Five of the 25 adverse drug events (20%) identified during the study period were directly associated with medication errors, all of them judged as preventable. A systematic review of studies on adverse events in hospitalised people showed that 1 in 10 is affected by an adverse event, with a median percentage of 43% being preventable and a rate of lethal events of 7.4 per 100 adverse events (de Vries 2008). Other studies suggest that medication errors and adverse drug events are associated with 140,000 deaths annually, and occur in 1 in 16 hospitalised people (Classen 1997; World Alliance for Patient Safety 2008). Classen and colleagues estimated that the additional costs of hospitalisation for each person with an adverse drug event were USD 2000 (Classen 1997). Two recent systematic reviews found considerable variability between studies in terms of financial cost, patients, settings and errors included (Vilela 2018; Walsh 2017).
Description of the intervention
Attention has been paid to this patient safety issue, and the literature identifying the causes, frequency and consequences of ADEs and medication errors, as well as the effects of interventions to prevent them, has grown (World Alliance for Patient Safety 2008).
In this review, we adopt the Cochrane Effective Practice and Organisation of Care (EPOC) group's taxonomy of health systems interventions to conceptualise and organise interventions used to try to reduce medication errors in hospitals (EPOC 2015). The taxonomy identifies four main domains of interventions: delivery arrangements, financial arrangements, governance arrangements, and implementation strategies, defined as follows.
Delivery arrangement interventions involve changes in how, when and where health care is organised and delivered, and who delivers health care.
Financial arrangement interventions involve changes in: how funds are collected; how services are purchased; insurance schemes; and the use of targeted financial incentives or disincentives.
Governance arrangement interventions involve rules or processes that affect the way in which powers are exercised, particularly with regard to authority, accountability, openness, participation, and coherence.
Implementation strategy interventions are those designed to bring about changes in healthcare organisations, the behaviour of healthcare professionals or the use of health services by healthcare recipients (EPOC 2015).
Reviews of medication safety intervention evidence have identified more than 20 distinct practices, healthcare professionals and technologies that have the potential to improve medication safety (de Vries 2008; Hodgkinson 2006; Ioannidis 2001; Shojania 2001). The following is a non‐exhaustive list of examples.
Medication reconciliation: the process of comparing a patient's medication orders in hospital to the medications that the patient has been taking. Medication reconciliation can be performed by individual healthcare professionals (such as pharmacists or pharmacist technicians) or teams, or both, trained to prevent or manage medication errors.
Database‐assisted medication reconciliation by using prescription databases to assist professionals in obtaining medication histories upon hospital admission.
Electronic prescribing systems, including computerised physician ordering entry (CPOE) and clinical decision support systems (CDSS). In general, these refer to the process of a medical professional entering and sending medication orders and treatment instructions electronically via a computer application instead of on paper charts. They are computer‐based programs that analyse data within electronic health records to provide prompts and reminders to assist healthcare providers in implementing treatments at the point of care.
Electronic prescription: the computer‐based electronic generation, transmission and filling of a medical prescription.
Automated dispensing systems, including devices that dispense medications and fill prescriptions. These systems also communicate with the pharmacy and its information management system to track medications removed and support inventory replenishment.
Bedside terminal systems: bedside computers to provide access to hospital resources.
Computer‐generated medication administration records (MARs): these synchronise data throughout an organisation; for example, they can interface with the pharmacy system, the computerised prescriber order entry system, and any admission‐discharge‐transfer system.
Computer alert systems.
Barcodes for identification of patients or medications.
Education and training.
Pharmacists.
Dedicated nurses.
Double‐checking.
Medication administration review and safety.
Utilisation of standardised checklists (protocols) by triage nurses.
Syringes marked with doses to reduce mistakes in identifying the right medication or doses.
Self‐medication programmes to reduce errors by healthcare workers.
Illumination in the workplace to reduce mistakes in identifying the right medication or doses.
Reduced working hours by eliminating extended work shifts and reducing the number of hours worked per week.
Education interventions to improve medication prescription or administration.
Multidisciplinary approaches.
How the intervention might work
The interventions applied at different hospital care levels, including delivery, financial and governance arrangements as well as implementation strategies, are expected to improve patient safety in terms of medication errors. The interventions are mainly directed to human resources, use different technologies and structural or organisational changes, or a combination of some or all of these. Interventions directed to improve human resources performance may include medication reconciliation, training, education, and feedback on medication errors, or having dedicated health professionals. Technological interventions may reduce human medication errors through electronic prescribing systems, such as CPOE and CDSS, electronic medication administration records (e‐MARs), automated dispensing or barcodes for identification of patients or medications. Structural or organisational changes may include reduced working hours or decentralised pharmacy systems.
Why it is important to do this review
Medication errors are a leading, avoidable, source of harm to hospital patients. Some authors have called for the implementation of evidence‐based practices to find solutions to this patient safety problem (Brennan 2005). Several systematic reviews, published prior to our protocol, partially addressed this topic (de Vries 2008; Hodgkinson 2006; Ioannidis 2001; Shojania 2001; Wong 2010). But none of these reviews, nor more recent ones (Ahtiainen 2020; Eng 2018; Khalil 2020; Korb‐Savoldelli 2018; Redmond 2018; Roumeliotis 2019; Shitu 2019), have comprehensively covered the wide range of interventions used to reduce medication errors at different points of care, precluding comparisons of the clinical utility of separate interventions and strong recommendations.
Our systematic review provides an exhaustive and up‐to‐date analysis of the available evidence for interventions devoted to preventing medication errors in hospital settings.
Objectives
To determine the effectiveness of interventions to reduce medication errors in adults in hospital settings.
Methods
Criteria for considering studies for this review
Types of studies
We included study designs that met the explicit criteria used by the Cochrane Effective Practice and Organisation of Care (EPOC) group: randomised controlled trials (RCTs), quasi‐randomised controlled trials (quasi‐RCTs), interrupted time series (ITS) studies with at least three data points before and three after the intervention, and controlled before‐and‐after (CBA) studies, with more than one intervention or control site, that could be analysed as ITS studies.
A quasi‐randomised trial is one in which participants are allocated to different arms of the trial using a method of allocation that is not truly random (e.g. sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number).
Types of participants
Setting
We included studies conducted in a hospital. We further classified studies by these setting categories: (i) inpatient care (secondary or tertiary units, intensive care units, operating theatres); (ii) outpatient care; and (iii) accident and emergency departments.
Healthcare professionals
We considered a study for inclusion if it involved healthcare professionals responsible for prescribing, dispensing or administering medications, in charge of care of adult (> 18 years old) hospitalised patients. When studies also included participants under 18 years old, we extracted data only for the adult population.
We excluded studies based in geriatric, institutional settings caring for the elderly, psychiatric institutions and in settings that provide care to children. The last of these is the focus of another Cochrane Review (Maaskant 2015).
Types of interventions
We included studies of interventions applied in hospital care to improve patient safety in terms of medication errors, compared to no intervention, other intervention, or usual care. Studies might have described one intervention, or a package of interventions, which we refer to as 'multifaceted'. The types of interventions we anticipated finding are listed in the Description of the intervention. We categorised these interventions ‐ applied at the hospital care level ‐ according to the EPOC taxonomy of four domains of interventions aimed at achieving practice change (EPOC 2015).
Delivery arrangements
Health service delivery arrangements include changes in who receives care and when, who provides care, the working conditions of those who provide care, co‐ordination of care amongst different providers, where care is provided, the use of health information and communication technology to deliver care, and quality and safety systems. Listed below are some of these delivery arrangement subcategories, together with examples of interventions to reduce medication errors in hospital settings.
Who receives care and when? Example: medication reconciliation before versus at admission.
Who provides care? Example: medication reconciliation performed by pharmacist versus by other professionals.
Who provides care, or co‐ordination of care? Example: medication reconciliation by a multidisciplinary team or trained pharmacist or pharmacist technicians versus standard pharmacist.
Health information and communication technology. Examples: electronic prescribing systems such as CPOE and CDSS; barcoding; dispensing systems, database‐assisted medication reconciliation; one or two versus four charts open simultaneously for medication reconciliation.
Working conditions of healthcare workers. Example: reduced versus unreduced working hours.
Co‐ordination of care / integration. Example: integrated multimodal intervention.
Financial arrangements
Health financing arrangements comprise the collection of funds, insurance schemes, the purchasing of services, and the use of targeted financial incentives or disincentives.
Governance arrangements
The term 'governance' can be defined in several, sometimes overlapping, ways. Its use differs within the social sciences, especially between economics and political science. We have defined governance here as rules or processes that affect the way in which powers are exercised, particularly with regard to authority, accountability, openness, participation, effectiveness and coherence. Governance arrangements subcategories could include:
Interagency collaboration. Example: collaboration and partnerships, for example, using big data;
Policies that regulate programme monitoring and evaluation;
Processes for accrediting healthcare providers in patient safety;
Policies that regulate the sale and dispensing of drugs or other healthcare products;
Policies that regulate training and licensing requirements for health professionals or what they can do.
Implementation strategies
Implementation strategies include interventions designed to bring about changes in healthcare organisations or the behaviour of healthcare professionals or recipients. Implementation strategy subcategories could include:
Interventions targeted at healthcare worker practice. Examples: feedback on prescribing errors; education.
Types of problems targeted at healthcare worker practice. Example: medication reconciliation.
Nevertheless, medication reconciliation is an intervention that crosscuts the EPOC taxonomy categories, including also delivery arrangements.
The comparison groups in the studies could have been another intervention, no intervention or usual care.
Types of outcome measures
Our initial approach was to extract each outcome with the exact name given by the authors of the included studies. However, these studies assessed the impacts of interventions to reduce medication errors in a wide range of ways (70 different outcomes). In order to organise and prioritise these outcomes for analysis in each comparison, we sought the input of a group of expert pharmacists, and we arrived at a consensus (Appendix 1 describes the outcomes as reported by authors of the included studies and grouped outcomes for this systematic review). We also analysed separately the ungrouped outcomes in natural units, or in the way that the authors of primary studies originally reported the outcomes, and we have reported them as additional figures. We did not pre‐specify time points for the outcomes; instead, we reported every available result.
Primary outcomes
Medication errors (grouped outcomes)
Proportion of patients with a medication error (i.e. administration, discrepancy, dispensing or duplication errors)
Incidence of medication errors
Adverse drug events (grouped outcomes)
Proportion of patients with serious adverse drug events, defined as categories 6 and 7 (see Description of the condition): that is, adverse drug events that are permanently disabling, require or prolong hospitalisation or are lethal or potentially life‐threatening (ISMP 2011)
Proportion of ADEs and preventable ADEs, defined as undesired reaction to medication that may have been prevented by appropriate drug selection or management (Hodgkinson 2006)
Secondary outcomes
Adverse drug events (grouped outcomes)
Total number of adverse drug events
Incidence of serious ADEs
Non‐grouped outcomes
Mortality
Morbidity
Hospitalisations
Length of stay
Resource use
Quality of life
Identified discrepancies and discrepancy resolutions
We used in this review a taxonomy for medication error proposed by Bates 1995 and modified by Morimoto 2004 (see Figure 1).
Medication errors (MEs) include any errors that occur during any of the processes involved in medicines management (e.g. prescribing, transcribing, dispensing, administration, documentation and monitoring).
Potential adverse drug events (ADEs) are defined as medication errors with a high likelihood to cause harm. Medication errors cause around 30% of ADEs.
Adverse drug events (ADEs) are defined as injuries resulting from medical interventions related to a drug.
A discrepancy is defined as an inconsistency between two medication lists of a patient, regarding the presence, absence, dosage, route, frequency or formulation of a medication during a transition of care between home and hospital or between different hospital settings. Unintended medication discrepancy is a type of medication error not detected by medication reconciliation. Thus, discrepancy resolution and identified discrepancies ‐ as a proxy of the former outcome ‐ are beneficial outcomes oriented to resolve medication errors.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) for primary studies included in related systematic reviews.
We searched the following databases on 16 January 2020.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 1), in the Cochrane Library.
MEDLINE, Ovid (including Epub ahead of print, in‐process and other non‐indexed citations, 1946 onwards).
Embase, Ovid (1974 onwards).
CINAHL (Cumulative Index to Nursing and Allied Health Literature), EBSCOHost (1980 onwards).
Conference Proceedings Citation Index ‐ Science, Web of Science, Clarivate Analytics (1990 onwards).
COS Conference Papers Index, ProQuest (1995 onwards).
Dissertations and Theses, Global, ProQuest (1861 onwards).
The EPOC Cochrane Information Specialist (CIS), in consultation with the authors, developed the search strategies. Broad initial searches were subsequently revised in an iterative process, following peer review by a second information specialist, to produce a more specific set of search terms. Search strategies are comprised of keywords and controlled vocabulary terms. We applied no language or time limits. For translations of publications, we contacted native‐speaker collaborators. We searched all databases from database start date to the date of search (16 January 2020). All strategies used are provided in Appendix 2.
Searching other resources
We also:
Reviewed reference lists of relevant systematic reviews or other publications;
Contacted authors of relevant studies or reviews to clarify reported published information or to seek unpublished results/data;
Contacted researchers with expertise relevant to the review topic or EPOC interventions; and
Conducted cited reference searches in Science Citation Index, Web of Science.
Trials Registries
We searched these trials registers on 16 January 2020:
ClinicalTrials.gov (www.clinicaltrials.gov/); and
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
Working in pairs, the review authors independently screened all titles and abstracts retrieved from the search strategy, using software for systematic reviews (Covidence), to assess which studies met the inclusion criteria. We obtained copies of all references considered potentially relevant. We resolved any disagreement between the pairs of review authors through discussion. If consensus could not be reached, we involved an EPOC Group editor to resolve the disagreement.
Data extraction and management
Pairs of review authors independently undertook data extraction using a modified and piloted version of the EPOC Group data collection checklist. We resolved any disagreement between the review author pairs through discussion.
A statistician extracted data from the included interrupted time series (ITS) studies using WebPlotDigitizer (accessed in March and April 2020). He estimated pre‐interruption level and slope, post‐interruption change in level, post‐interruption slope using piecewise linear regression, adjusted for autocorrelated disturbances and seasonality, using the ITSA add‐on command (Linden 2016), for Stata (StataCorp 2015). We adjusted for autocorrelated disturbances by setting the maximum lag option to a value determined by visual inspection of autocorrelation and partial correlation plots, and by using Cumby‐Huizinga general tests for autocorrelation with a significance threshold of 0.05 (Cumby 1992). We adjusted for seasonality by modelling the effect of each quarter as a fixed effect if at least three observations were available for each quarter. We modelled ITS data on the natural logarithmic scale to constrain the error distribution to positive values, to stabilise variance, and to facilitate meta‐analysis (see Measures of treatment effect). None of the included ITS studies included controls in which no intervention (or a substantively different intervention) was used in the post‐interruption period, so we could not adjust for other possible explanations for the observed changes.
Assessment of risk of bias in included studies
Pairs of review authors independently assessed the risk of bias of the included studies.
For RCTs, we used the Cochrane risk of bias tool (Higgins 2011), paying special attention to the following domains: sequence generation, allocation concealment, blinding and incomplete outcome data. For the other eligible designs, we assessed their quality using pre‐established criteria used by the EPOC group (EPOC 2017). We resolved any discrepancies in quality ratings through discussion and the involvement of an arbitrator where necessary. For all study designs, we added a conflict of interest domain ('unclear risk' of bias for studies sponsored by industry and 'high risk' of bias only when there was evidence of causal bias).
Measures of treatment effect
Reporting
We tabulated data in natural units for each study. We reported pre‐intervention and post‐intervention means or proportions where baseline results were available for both study and control groups from RCTs, quasi‐RCTs and CBAs. We calculated the absolute change from baseline with 95% confidence limits. For ITS studies, we reported the main outcomes in natural units with two indicators of the effects of the intervention being documented: the change in the level of outcome immediately after the intervention and the change in the slope of the regression lines.
Analytical approach
Primary analyses
We based the primary analyses on consideration of dichotomous process measures (for example, proportion of participants experiencing an adverse reaction). When studies reported more than one measure for each endpoint, we extracted the primary measure (as defined by the authors of the study) or the median measure identified. We presented the results for all comparisons using a standard method of presentation where possible. For comparisons of RCTs or quasi‐RCTs and ITS studies, we reported (separately for each study design):
median effect size across included studies;
interquartile ranges of effect sizes across included studies;
range of effect sizes across included studies.
Secondary analyses
Secondary analyses explored the consistency of primary analyses with other types of endpoints. We calculated standardised mean differences (SMD) for continuous measures by dividing the difference in mean scores between the intervention and comparison group in each study by an estimate of the (pooled) standard deviation. In order to gain comparability between combined SMDs, we also transformed MD of single studies to SMDs.
Confounding variables considered for ITS analysis included patient level variables (sex, age, and ethnicity), provider role (attending physician, resident, medical student, nurse, pharmacist or other), type of setting (inpatient care settings such as secondary or tertiary units, intensive care units, operating theatres, outpatient care settings and accident and emergency departments) or practice context (i.e. order placed during a day or a night shift).
Methods for reanalysis
We reanalysed RCTs and quasi‐RCTs with potential unit of analysis errors, where possible, by recalculating results using the appropriate unit of analysis; otherwise, we contacted the authors of such studies for clarification. For the ITS studies, we exponentiated change in level and slope (which were estimated on the logarithmic scale, see Data extraction and management) to obtain estimates of ratios of post‐ to pre‐interruption levels and slopes. These estimates describe the nature of any change in reporting. In principle, however, genuine changes in level and slope can lead to no overall change (i.e. a change in slope can effectively cancel a change in level). We therefore measure change as the ratio of expected events by extrapolating the pre‐interruption curve into the post‐interruption period and treating it as a counterfactual. Because this ratio is a function of time, we estimated it at one and two years post‐intervention. We excluded a study if it would be necessary to extrapolate beyond the end of follow‐up for that study.
Where appropriate, we used Cochrane's standard statistical methods for pooling of data from randomised and quasi‐randomised controlled trials. For categorical and continuous data, we calculated the risk ratio (RR) or odds ratio (OR) and mean difference (MD), respectively, with 95% confidence intervals (CIs). We used a random‐effects model to take into account the heterogeneity of the various studies.
We reported data in individual tables comparing effect sizes of interventions for grouped outcomes according to the EPOC group taxonomy (delivery, financial and governance arrangements, and implementation standards) (EPOC 2015). We examined data from ITS studies and cluster‐randomised trials with unit of analysis errors according to the EPOC Group guidelines and used absolute risk differences.
We created summary of findings tables for the main comparisons in the review to interpret the results and draw conclusions about the effects (benefits, potential harms and costs) of different interventions, including the size of effects and quality of the evidence for outcomes for which there is evidence.
Unit of analysis issues
We reanalysed the study if data were available (i.e. using intracluster correlation). If not, we reported the unit of analysis error for each study and classified it as high risk of bias in the 'other bias' domain. For cluster‐randomised trials, we appraised whether an appropriate analysis had been done that adjusted for clustering in results. If the analysis did not appear to have adjusted for clustering appropriately, we considered whether the effect estimate was likely to be affected by such issues and, as appropriate, noted this as a potential source of bias relating to the outcome in question.
We described the unit of analysis of each study and only combined them using the generic inverse‐variance method with specific standard errors.
Dealing with missing data
If information was missing or unclear, we contacted the study investigators for additional information or clarification. To reduce the risk of overly positive answers, we used open‐ended questions.
Assessment of heterogeneity
We obtained an initial visual overview of heterogeneity through scrutinising the forest plots and looking at the overlap between CIs around the estimate for each included study. To quantify the inconsistency across studies, and thus the impact of heterogeneity on the meta‐analysis, we used the I2 statistic to detect heterogeneity (Higgins 2003). In the latter case, we defined an I2 of higher than 60% as revealing substantial heterogeneity. We also interpreted the significance of the I2 test in light of: (i) the magnitude and direction of effects; and (ii) the strength of evidence for heterogeneity (for example, a CI for the I2, or the P value for the Chi2 test).
We assessed observable heterogeneity amongst the study questions and methods, to determine whether a meta‐analysis was appropriate. We also looked at the study participants, settings, interventions, and reported outcomes. We paid particular attention to the homogeneity of the methodology (such as variances in blinding and concealment of allocation) within and across included studies.
If we found evidence of statistical heterogeneity, we examined it in a subgroup analysis and a sensitivity analysis, as outlined in the respective sections below (Subgroup analysis and investigation of heterogeneity; Sensitivity analysis).
Assessment of reporting biases
To reduce possible publication bias, we employed strategies to search for and include relevant unpublished studies. These strategies included searching the grey literature and prospective trial registration databases to overcome time‐lag bias.
To investigate the likelihood of overt publication bias, we planned to draw a funnel plot, plotting trial effects against inverse standard errors of the effects for any outcome with more than eight studies. In the event, this step was unnecessary.
Data synthesis
For each comparison, we reported summary statistics for each of the included studies (RCTs or quasi‐RCTs and ITS studies). We used forest plots to display the data graphically.
For dichotomous data, we used the Mantel‐Haenszel method, and for continuous data, we used the inverse‐variance method.
We pooled the results from individual studies in a meta‐analysis using the random‐effects model by DerSimonian and Laird (DerSimonian 1986). We chose this method because we could not assume a single, underlying (fixed) treatment effect. When the impact of the intervention was assessed in individual studies on more than one outcome measure, we selected the outcome that best reflected the targeted intervention for pooling data.
We used generic inverse‐variance when we only had results expressed as adjusted relative effects or to combine different types of outcomes, following the expert advice of the pharmacists we consulted about outcome groupings. We gave priority to risk ratios (RRs; for easier interpretation), but if data did not allow this approach, we reported odds ratios (ORs).
When a study compared more than one arm, we only analysed the intervention arm that fitted most closely with the comparison and with the studies included under it. For example, we excluded from the analysis any multimodal interventions besides the intervention under study.
We analysed ITS studies separately to RCTs.
We analysed ITS study data using the guidelines of the EPOC group (EPOC 1998), and reported outcomes in natural units. We reported pre‐intervention and post‐intervention means or proportions for both study and control groups, and calculated the unadjusted and adjusted (for any baseline imbalance) absolute change from baseline with 95% CIs. We used either a regression analysis with time trends before and after the intervention, which adjust for autocorrelation and any periodic changes, or an autoregressive, integrated, moving average (ARIMA) model to isolate the effect of the intervention from existing time trends.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses, where possible, to check if the intervention effect varied with different populations, interventions, or settings.
Type of setting (general wards, emergency department, intensive care units).
Type of provider (less trained, more trained, etc.).
Type of outcome (all errors, prescribing errors, etc.).
Type of outcome measure (per patients, per admissions, per prescriptions, etc.).
Time points of outcomes (during hospitalisation, post‐hospitalisation).
When we were not able to perform a meta‐analysis, we summarised the results for these subgroups within the text of the review.
Sensitivity analysis
We performed sensitivity analysis based on the method of meta‐analysis. That is, we compared the results from the random‐effects and fixed‐effect models if there was unexplained heterogeneity between studies, to assess the robustness of the results.
We also planned to analyse only studies at low risk of bias for both random sequence generation and allocation concealment. However, we were unable to conduct this analysis due to an insufficient number of such studies for each comparison.
Summary of findings and assessment of the certainty of the evidence
We imported data from Review Manager 5.4 (Review Manager 2020) to GRADE profiler (GRADEpro GDT), and created summary of findings tables for the following 16 comparisons.
Comparison 1: medication reconciliation (MR) versus no MR.
Comparison 2: MR performed by pharmacist versus other professionals.
Comparison 3: MR by pharmacist: database‐assisted versus unassisted.
Comparison 4: MR by pharmacist: trained pharmacist technician versus pharmacist.
Comparison 5: MR: before versus at admission.
Comparison 6: MR: one to two versus four charts open simultaneously.
Comparison 7: MR: multimodal intervention versus usual care.
Comparison 8: computerised physician order entry (CPOE)/clinical decision support systems (CDSS) versus control/paper‐based system.
Comparison 9: CPOE/CDSS: improved versus standard CPOE/CDSS.
Comparison 10: CPOE/CDSS: prioritised versus non‐prioritised alerts.
Comparison 11: barcoding versus no barcoding.
Comparison 12: organisational changes: reduced versus unreduced working hours.
Comparison 13: feedback on prescribing errors versus no feedback.
Comparison 14: feedback on prescribing errors versus education.
Comparison 15: education versus no education on prescribing or administration.
Comparison 16: dispensing system versus control.
In the summary of findings tables, for each comparison, we have presented seven of eight primary and secondary outcomes, listed below. We prioritised these in consultation with a group of expert pharmacists.
Medication errors (MEs; primary outcome)
Adverse drug events (ADEs) / preventable ADEs (primary outcome)
Mortality (secondary outcome)
Readmission (secondary outcome)
Length of stay (LoS; secondary outcome)
Quality of life (QoL; secondary outcome)
Discrepancy resolution (secondary outcome)
Identified discrepancies per patient (secondary outcome). This outcome was presented only if the previous seven outcomes were not reported.
We reported separately the evidence from RCTs or ITS studies which evaluated the same outcome.
Pairs of review authors independently graded the certainty of the evidence for each outcome using the GRADE approach (Guyatt 2011; Hultcrantz 2017; Schünemann 2013); we resolved discrepancies by reaching consensus. For assessments of the overall certainty of the evidence for outcomes that included pooled data from RCTs, we initially graded the evidence as high certainty, downgrading the rating (by one level from high to moderate certainty, by two levels to low certainty, or three levels to very low‐certainty evidence) depending on the extent of accomplishment across the following criteria: study limitations (risk of bias); indirectness of evidence; inconsistency; imprecision of effect estimates; or publication bias. For certainty ratings for outcomes that included pooled data from ITS studies, we initially graded the evidence as low certainty, upgrading the rating to moderate or high certainty if the pooled estimates revealed a large magnitude of effect, negligible concerns about confounders, or a strong dose‐response gradient. We used these assessments, along with the evidence (or lack thereof) for absolute benefit or harm of the interventions, and the sum of available data on all primary and secondary outcomes from each study included for each comparison, to draw conclusions about the effectiveness of the interventions.
Results
Description of studies
See Characteristics of included studies for more information.
Results of the search
We screened a total of 21,545 titles and abstracts, and from these, identified 1066 full‐text publications for further screening. Of these full‐text publications, we excluded 985 reports. The majority of these involved an ineligible study design (n = 619), an ineligible intervention (n = 125) or other disqualifier (n = 99) (e.g. non‐separated adult and paediatric population or insufficient information to decide). Ultimately, we included 65 studies, four secondary references and 12 ongoing studies (see Figure 2).
2.

Study flow diagram
Included studies
We included 65 studies and 4 secondary references (see Characteristics of included studies).
Of the included studies, 51 were RCTs: Aag 2014; Adelman 2013; Adelman 2019; Al‐Hashar 2018; Barker 1984; Becerra‐Camargo 2015; Beckett 2012; Bell 2016; Bolas 2004; Boockvar 2017; Cadman 2017; Chiu 2018; Colpaert 2006; De Winter 2011; Ding 2012; Farris 2014; Fernandes 2011; George 2011; Gordon 2017; Graabaek 2019; Greengold 2003; Gursanscky 2018; Hale 2013; Heselmans 2015; Hickman 2018; Juanes 2018; Khalil 2016; Kwan 2007; Landrigan 2004; Leung 2017; Lind 2017; Marotti 2011; McCoy 2012; Merry 2011; Nielsen 2017; O'Sullivan 2016; Pevnick 2018; Piqueras Romero 2015; Quach 2015; Redwood 2013; Schmader 2004; Schneider 2006; Schnipper 2009; Scullin 2007; SUREPILL 2015; Tamblyn 2018; Tompson 2012; Tong 2016; Vega 2016; Wang 2017; Willoch 2012.
The 14 remaining included studies included seven ITS studies (Agrawal 2009; Bhakta 2019; Burkoski 2019; Kannampallil 2018; Ongering 2019; Schnipper 2018; Van Doormaal 2009), one controlled ITS study (Thompson 2018), and six CBA studies, reanalysed as ITS studies (Bowdle 2018; Furuya 2013; Green 2015; Higgins 2010; Narang 2013; Seibert 2014).
Population
The total number of included participants was 110,875. The RCTs included a total of 23,182 participants (some studies used prescriptions or providers as the unit of analysis, and are not included in this total: Adelman 2013; Adelman 2019; Ding 2012; Gordon 2017; Greengold 2003; Gursanscky 2018; Hickman 2018; Leung 2017). ITS studies included a total of 87,692 participants (some studies used medication alerts or prescriptions as the unit of analysis, and are not included in this total: Bhakta 2019; Burkoski 2019; Green 2015; Higgins 2010; Narang 2013; Seibert 2014; Thompson 2018).
Fifty‐five studies included inpatient adults (from18 years old up to no age limit), five studies included only elderly inpatients (age more than 65 years old) (Beckett 2012; Chiu 2018; Piqueras Romero 2015; Quach 2015; Schmader 2004), and five studies included both inpatient and outpatients adults (Adelman 2013; Adelman 2019; Agrawal 2009; Farris 2014; Vega 2016).
Setting
The most frequent setting was medical and surgical wards (17 studies). The remaining studies' settings were: medical wards (13 studies), surgical wards (9), emergency departments (7), intensive care units (ICUs; 3), operating rooms (2) and other settings (14).
Most of the RCTs (47/51) were conducted in high‐income countries: 16 in the USA (Adelman 2013; Adelman 2019; Barker 1984; Beckett 2012; Bell 2016; Boockvar 2017; Farris 2014; Greengold 2003; Landrigan 2004; McCoy 2012; Pevnick 2018; Quach 2015; Schmader 2004; Schneider 2006; Schnipper 2009; Tompson 2012); eight in Australia (George 2011; Gursanscky 2018; Hale 2013; Hickman 2018; Khalil 2016; Leung 2017; Marotti 2011; Tong 2016); five in the United Kingdom (Bolas 2004; Cadman 2017; Gordon 2017; Redwood 2013; Scullin 2007); three in Belgium (Colpaert 2006; De Winter 2011; Heselmans 2015); three in Canada (Fernandes 2011; Kwan 2007; Tamblyn 2018); three in Denmark (Graabaek 2019; Lind 2017; Nielsen 2017); three in Spain (Juanes 2018; Piqueras Romero 2015; Vega 2016); two in Norway (Aag 2014; Willoch 2012); and one each in Ireland (O'Sullivan 2016), the Netherlands (SUREPILL 2015), New Zealand (Merry 2011) and Oman (Al‐Hashar 2018). The four remaining RCTs were conducted in middle‐income countries: three in China (Chiu 2018; Ding 2012; Wang 2017), and one in Colombia (Becerra‐Camargo 2015).
All the 14 ITS studies were conducted in high‐income countries: 10 in the USA (Agrawal 2009; Bhakta 2019; Bowdle 2018; Green 2015; Higgins 2010; Kannampallil 2018; Narang 2013; Schnipper 2018; Seibert 2014; Thompson 2018); two in the Netherlands (Ongering 2019; Van Doormaal 2009); one in Canada (Burkoski 2019); and one in Japan (Furuya 2013).
Interventions and comparisons
We categorised all the interventions in the included studies into two of the four EPOC taxonomy categories (EPOC 2015), as described in the Description of the intervention and in the Types of interventions sections; namely, delivery arrangements and implementation strategies (see Appendix 3 for categorisations). Thus, we did not categorise any interventions in the included studies as falling under the two remaining categories of financial arrangements or governance arrangements.
In order to further categorise the interventions, we classified each study by EPOC group taxonomy and the comparison number (See Appendix 4).
In Appendix 5 and Appendix 6, we describe the study designs, populations, settings and countries, and the study level contribution by comparison.
Ongoing Studies
We identified 12 ongoing studies, which we describe in the Characteristics of ongoing studies tables.
Excluded studies
In the Characteristics of excluded studies tables, we present the details of the 12 excluded studies (Farley 2014; Franklin 2019; Gillespie 2009; Heng 2013; Kripalani 2012; Kucukarslan 2003; Makowsky 2009; Pellegrin 2017; Shah 2013; Singh 2012; Stowasser 2002; Whittington 2004).
Risk of bias in included studies
We assessed the risk of bias for RCTs and ITS studies separately. We provide a summary of the results of our assessment below and graphically in Figure 3 and Figure 4 (for RCTs) and Figure 5 and Figure 6 (for ITS studies). Further details can be found in the risk of bias tables for each study (see the Characteristics of included studies tables).
3.
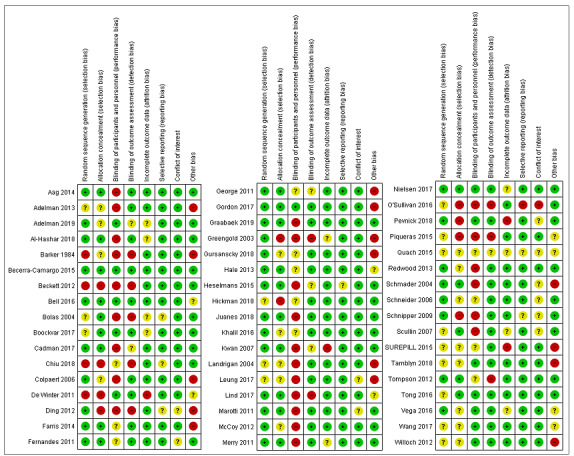
Risk of bias summary for RCTs: review authors' judgements about each risk of bias item for each included study
4.
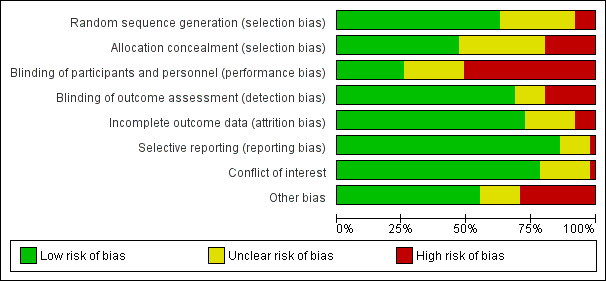
Risk of bias graph for RCTs: review authors' judgements about each risk of bias item presented as percentages across all included studies
5.
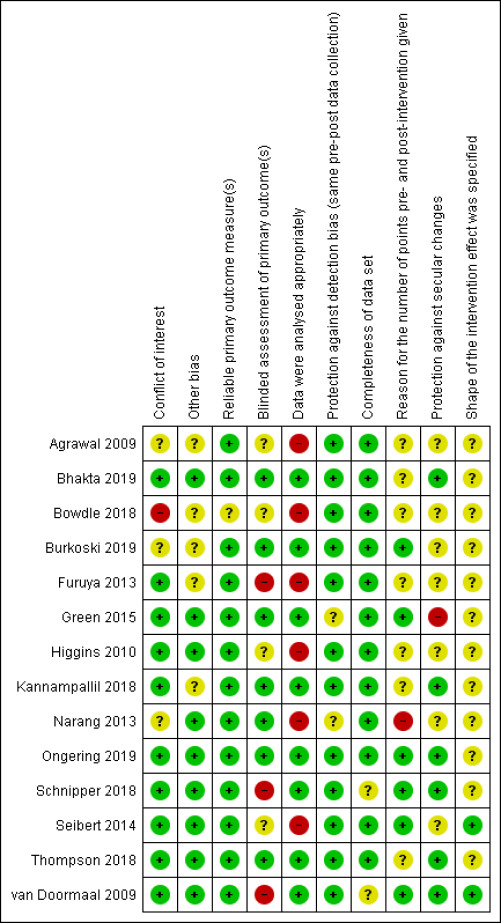
Risk of bias summary for CBA and ITS studies: review authors' judgements about each risk of bias item for each included study
6.
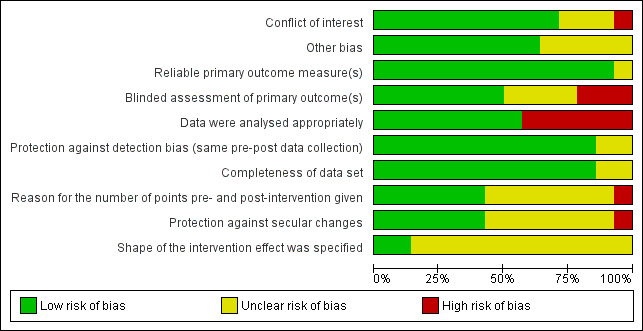
Risk of bias graph for CBA and ITS studies: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
We rated 32 studies as low risk of bias for random sequence generation (Aag 2014; Adelman 2019; Al‐Hashar 2018; Becerra‐Camargo 2015; Bell 2016; Cadman 2017; Colpaert 2006; Ding 2012; Farris 2014; Fernandes 2011; George 2011; Gordon 2017; Graabaek 2019; Greengold 2003; Gursanscky 2018; Hale 2013; Heselmans 2015; Juanes 2018; Khalil 2016; Kwan 2007; Lind 2017; Marotti 2011; McCoy 2012; Merry 2011; Nielsen 2017; Pevnick 2018; Redwood 2013; Schmader 2004; Schneider 2006; Schnipper 2009; Tompson 2012; Vega 2016), and 14 as unclear risk of bias (Adelman 2013; Bolas 2004; Boockvar 2017; Hickman 2018; Landrigan 2004; Leung 2017; O'Sullivan 2016; Quach 2015; Scullin 2007; SUREPILL 2015; Tamblyn 2018; Tong 2016; Wang 2017; Willoch 2012). We assessed five studies as high risk of bias in this domain because they randomised based on record numbers or days of the week (Barker 1984; Beckett 2012; Chiu 2018; De Winter 2011; Piqueras Romero 2015).
We rated 24 studies as low risk of bias for allocation concealment (Aag 2014; Al‐Hashar 2018; Becerra‐Camargo 2015; Bell 2016; Bolas 2004; Boockvar 2017; Cadman 2017; Farris 2014; Fernandes 2011; George 2011; Gordon 2017; Graabaek 2019; Hale 2013; Heselmans 2015; Juanes 2018; Kwan 2007; Lind 2017; Marotti 2011; Merry 2011; Nielsen 2017; Schmader 2004; Scullin 2007; Tompson 2012; Tong 2016), and 17 as unclear risk of bias (Adelman 2013; Adelman 2019; Barker 1984; Colpaert 2006; Gursanscky 2018; Khalil 2016; Landrigan 2004; Leung 2017; McCoy 2012; Quach 2015; Redwood 2013; Schneider 2006; SUREPILL 2015; Tamblyn 2018; Vega 2016; Wang 2017; Willoch 2012). We judged 10 studies as high risk of bias in this domain because they used open randomisation or predictable allocation procedures (Beckett 2012; Chiu 2018; De Winter 2011; Ding 2012; Greengold 2003; Hickman 2018; O'Sullivan 2016; Pevnick 2018; Piqueras Romero 2015; Schnipper 2009).
Blinding
We rated 13 studies as low risk of bias for blinding of participants and personnel (Adelman 2019; Becerra‐Camargo 2015; Bell 2016; Boockvar 2017; De Winter 2011; Gordon 2017; Nielsen 2017; Pevnick 2018; Tamblyn 2018; Tong 2016; Vega 2016; Wang 2017; Willoch 2012), and 12 as unclear risk of bias (Chiu 2018; Farris 2014; Fernandes 2011; George 2011; Gursanscky 2018; Hale 2013; Hickman 2018; Khalil 2016; Quach 2015; Schneider 2006; SUREPILL 2015; Tompson 2012). We judged 26 studies as high risk of bias in this domain because they were non‐blinded studies (Aag 2014; Adelman 2013; Al‐Hashar 2018; Barker 1984; Beckett 2012; Bolas 2004; Cadman 2017; Colpaert 2006; Ding 2012; Graabaek 2019; Greengold 2003; Heselmans 2015; Juanes 2018; Kwan 2007; Landrigan 2004; Leung 2017; Lind 2017; Marotti 2011; McCoy 2012; Merry 2011; O'Sullivan 2016; Piqueras Romero 2015; Redwood 2013; Schmader 2004; Schnipper 2009; Scullin 2007).
We rated 35 studies as low risk of bias for blinding of outcome assessment (Aag 2014; Adelman 2013; Al‐Hashar 2018; Becerra‐Camargo 2015; Bell 2016; Boockvar 2017; Colpaert 2006; De Winter 2011; Farris 2014; Fernandes 2011; Gordon 2017; Graabaek 2019; Gursanscky 2018; Hale 2013; Hickman 2018; Juanes 2018; Khalil 2016; Landrigan 2004; Leung 2017; Marotti 2011; McCoy 2012; Merry 2011; Nielsen 2017; Pevnick 2018; Redwood 2013; Schmader 2004; Schneider 2006; Schnipper 2009; Scullin 2007; SUREPILL 2015; Tamblyn 2018; Tong 2016; Vega 2016; Wang 2017; Willoch 2012), and six as unclear risk of bias (Adelman 2019; Cadman 2017; George 2011; Heselmans 2015; Kwan 2007; Quach 2015). We rated 10 studies as high risk of bias in this domain because outcome assessors were not blinded (Barker 1984; Beckett 2012; Bolas 2004; Chiu 2018; Ding 2012; Greengold 2003; Lind 2017; O'Sullivan 2016; Piqueras Romero 2015; Tompson 2012).
Incomplete outcome data
We rated 37 studies as low risk of bias for incomplete outcome data (Aag 2014; Adelman 2013; Barker 1984; Becerra‐Camargo 2015; Beckett 2012; Bell 2016; Cadman 2017; Chiu 2018; Colpaert 2006; Ding 2012; Farris 2014; Fernandes 2011; George 2011; Gordon 2017; Graabaek 2019; Gursanscky 2018; Hale 2013; Heselmans 2015; Hickman 2018; Juanes 2018; Khalil 2016; Landrigan 2004; Leung 2017; Lind 2017; Marotti 2011; McCoy 2012; O'Sullivan 2016; Piqueras Romero 2015; Redwood 2013; Schmader 2004; Schneider 2006; Schnipper 2009; Tamblyn 2018; Tompson 2012; Tong 2016; Wang 2017; Willoch 2012), and 10 as unclear risk of bias (Adelman 2019; Al‐Hashar 2018; Bolas 2004; Boockvar 2017; Greengold 2003; Merry 2011; Nielsen 2017; Quach 2015; Scullin 2007; Vega 2016). We assessed four studies as high risk of bias because they had a high proportion of missing outcomes or imbalances in numbers or reasons for missing data across intervention groups (De Winter 2011; Kwan 2007; Pevnick 2018; SUREPILL 2015).
Selective reporting
We rated 43 studies as low risk of bias for selective reporting (Aag 2014; Adelman 2013; Adelman 2019; Al‐Hashar 2018; Barker 1984; Becerra‐Camargo 2015; Beckett 2012; Bell 2016; Boockvar 2017; Cadman 2017; Colpaert 2006; De Winter 2011; Farris 2014; Fernandes 2011; George 2011; Gordon 2017; Graabaek 2019; Greengold 2003; Gursanscky 2018; Hale 2013; Hickman 2018; Juanes 2018; Khalil 2016; Kwan 2007; Landrigan 2004; Leung 2017; Lind 2017; Marotti 2011; McCoy 2012; Merry 2011; Nielsen 2017; Pevnick 2018; Redwood 2013; Schmader 2004; Schneider 2006; Scullin 2007; SUREPILL 2015; Tamblyn 2018; Tompson 2012; Tong 2016; Vega 2016; Wang 2017; Willoch 2012), and seven as unclear risk of bias (Bolas 2004; Chiu 2018; Ding 2012; Heselmans 2015; Piqueras Romero 2015; Quach 2015; Schnipper 2009). We rated one study as high risk of bias in this domain (O'Sullivan 2016).
Other potential sources of bias
We rated 40 studies as low risk of bias for conflict of interest (Aag 2014; Adelman 2013; Adelman 2019; Al‐Hashar 2018; Barker 1984; Becerra‐Camargo 2015; Beckett 2012; Bell 2016; Bolas 2004; Boockvar 2017; Cadman 2017; Chiu 2018; Colpaert 2006; De Winter 2011; Farris 2014; George 2011; Gordon 2017; Graabaek 2019; Greengold 2003; Gursanscky 2018; Hale 2013; Heselmans 2015; Hickman 2018; Juanes 2018; Khalil 2016; Kwan 2007; Landrigan 2004; Lind 2017; McCoy 2012; Merry 2011; Nielsen 2017; Piqueras Romero 2015; Redwood 2013; SUREPILL 2015; Tamblyn 2018; Tompson 2012; Tong 2016; Vega 2016; Wang 2017; Willoch 2012), and 10 as unclear risk of bias (Ding 2012; Fernandes 2011; Leung 2017; Marotti 2011; Pevnick 2018; Quach 2015; Schmader 2004; Schneider 2006; Schnipper 2009; Scullin 2007). We assessed one study as high risk of bias because secondary outcomes presented in the clinical trial registration were not reported in the manuscript (O'Sullivan 2016).
We rated 28 studies as low risk of bias for other bias (Aag 2014; Adelman 2019; Al‐Hashar 2018; Becerra‐Camargo 2015; Beckett 2012; Bolas 2004; Boockvar 2017; Cadman 2017; Chiu 2018; Fernandes 2011; Graabaek 2019; Heselmans 2015; Hickman 2018; Juanes 2018; Khalil 2016; Kwan 2007; Marotti 2011; McCoy 2012; Merry 2011; Nielsen 2017; O'Sullivan 2016; Pevnick 2018; Redwood 2013; Schneider 2006; Schnipper 2009; Scullin 2007; Tompson 2012; Tong 2016), 8 studies as unclear risk of bias (Bell 2016; De Winter 2011; Hale 2013; Lind 2017; Piqueras Romero 2015; Quach 2015; Vega 2016; Wang 2017), and 15 as high risk of bias (Adelman 2013; Barker 1984; Colpaert 2006; Ding 2012; Farris 2014; George 2011; Gordon 2017; Greengold 2003; Gursanscky 2018; Landrigan 2004; Leung 2017; Schmader 2004; SUREPILL 2015; Tamblyn 2018; Willoch 2012).
The main causes for high risk of other bias were: the analysis method did not account for the cluster design (10 studies: Barker 1984; Colpaert 2006; Ding 2012; Gordon 2017; Greengold 2003; Gursanscky 2018; Landrigan 2004; Leung 2017; SUREPILL 2015; Tamblyn 2018); the specific effect of the intervention could not be isolated (Farris 2014); recruitment was performed on certain days of the week (George 2011); retrospective methods were used to identify adverse drug reactions (Schmader 2004); contamination bias (Adelman 2013); and temporal difference between arms in the identification of outcomes (Willoch 2012).
Interrupted time series studies
Reliable primary outcome measure(s)
We rated 13 studies as low risk of bias for reliable primary outcome measure (Agrawal 2009; Bhakta 2019; Burkoski 2019; Furuya 2013; Green 2015; Higgins 2010; Kannampallil 2018; Narang 2013; Ongering 2019; Schnipper 2018; Seibert 2014; Thompson 2018; Van Doormaal 2009), and one as unclear risk of bias (Bowdle 2018).
Blinded assessment of primary outcome(s)
We rated seven studies as low risk of bias for blinded assessment of primary outcomes (Bhakta 2019; Burkoski 2019; Green 2015; Kannampallil 2018; Narang 2013; Ongering 2019; Thompson 2018), four as unclear risk of bias (Agrawal 2009; Bowdle 2018; Higgins 2010; Seibert 2014), and three as high risk of bias because they used non‐blinded assessment or were open trials (Furuya 2013; Schnipper 2018; Van Doormaal 2009).
Data were analysed appropriately
We rated nine studies as low risk of bias for appropriate data analysis (Bhakta 2019; Burkoski 2019; Green 2015; Kannampallil 2018; Ongering 2019; Schnipper 2018; Seibert 2014; Thompson 2018; Van Doormaal 2009), and five as high risk of bias because they did not use ARIMA models or time series regression models to analyse the data (Agrawal 2009; Bowdle 2018; Furuya 2013; Higgins 2010; Narang 2013).
Protection against detection bias (same pre‐post data collection)
We rated 12 studies as low risk of bias for protection against detection bias (Agrawal 2009; Bhakta 2019; Bowdle 2018; Burkoski 2019; Furuya 2013; Higgins 2010; Kannampallil 2018; Ongering 2019; Schnipper 2018; Seibert 2014; Thompson 2018; Van Doormaal 2009), and two as unclear risk of bias (Green 2015; Narang 2013).
Completeness of data set
We rated 12 studies as low risk of bias for completeness of data set (Agrawal 2009; Bhakta 2019; Bowdle 2018; Burkoski 2019; Furuya 2013; Green 2015; Higgins 2010; Kannampallil 2018; Narang 2013; Ongering 2019; Seibert 2014; Thompson 2018), and two as unclear risk of bias (Schnipper 2018; Van Doormaal 2009).
Reason for the number of points pre‐ and post‐intervention given
We rated six studies as low risk of bias for giving reasons for the number of points pre‐ and post‐intervention (Burkoski 2019; Green 2015; Ongering 2019; Schnipper 2018; Seibert 2014; Van Doormaal 2009), and seven as unclear risk of bias (Agrawal 2009; Bhakta 2019; Bowdle 2018; Furuya 2013; Higgins 2010; Kannampallil 2018; Thompson 2018). We rated one study as high risk of bias in this domain because it did not present a rationale for the numbers of data points (Narang 2013).
Protection against secular changes
We rated six studies as low risk of bias for protection against secular changes (Bhakta 2019; Kannampallil 2018; Ongering 2019; Schnipper 2018; Thompson 2018; Van Doormaal 2009), and seven as unclear risk of bias Agrawal 2009; Bowdle 2018; Burkoski 2019; Furuya 2013; Higgins 2010; Narang 2013; Seibert 2014). We assessed one study as high risk of bias because it used a before‐and‐after design, and the results could potentially be confounded by an unknown simultaneous intervention that was not measured in the analyses (Green 2015).
Shape of the intervention effect was specified
We rated two studies as low risk of bias for specifying the shape of the intervention effect (Seibert 2014; Van Doormaal 2009), and 12 as unclear risk of bias (Agrawal 2009; Bhakta 2019; Bowdle 2018; Burkoski 2019; Furuya 2013; Green 2015; Higgins 2010; Kannampallil 2018; Narang 2013; Ongering 2019; Schnipper 2018; Thompson 2018).
Conflict of interest
We rated 10 studies as low risk of bias for conflict of interest (Bhakta 2019; Furuya 2013; Green 2015; Higgins 2010; Kannampallil 2018; Ongering 2019; Schnipper 2018; Seibert 2014; Thompson 2018; Van Doormaal 2009), and three as unclear risk of bias (Agrawal 2009; Burkoski 2019; Narang 2013). We assessed one study as high risk of bias for conflict of interest because one of the study authors was the director and a shareholder of the company that supported the study and another author was a consultant for the same company (Bowdle 2018).
Other bias
We rated nine studies as low risk of bias for other bias (Bhakta 2019; Green 2015; Higgins 2010; Narang 2013; Ongering 2019; Schnipper 2018; Seibert 2014; Thompson 2018; Van Doormaal 2009), and five as unclear risk of bias (Agrawal 2009; Bowdle 2018; Burkoski 2019; Furuya 2013; Kannampallil 2018).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16
In the summary of findings tables 1 to 16, we describe the effects of interventions for reducing medication errors in adults in hospital settings for the identified comparisons. Appendix 5 and Appendix 6 detail the evidence map of identified comparisons by study. The effect of each comparison is detailed below.
The included studies assessed medication errors and adverse events in different ways. Therefore, we grouped studies if they used the same outcome measures, as described in the Methods section and in Appendix 2. These grouped outcomes are the outcomes presented in each summary of findings table, and in the related comments, we indicate "grouped outcomes". We do not include the non‐grouped outcomes in the summary of findings tables, nor provide narrative descriptions, but for transparency, we moved from the Data and analyses section to Figures 7 to 25, and we referenced them at the end of each comparison.
1. Medication reconciliation (MR) compared with no MR (Delivery arrangement, Implementation strategies)
This comparison, described in Table 1, includes 9 RCTs and 2243 participants (Al‐Hashar 2018; Bolas 2004; Cadman 2017; Chiu 2018; Juanes 2018; Nielsen 2017; Piqueras Romero 2015; Vega 2016; Willoch 2012)
Compared with no MR, MR may reduce medication errors (OR 0.55, 95% CI 0.17 to 1.74; I² = 28%; 3 studies, 379 participants; low‐certainty evidence; Analysis 1.1), probably reduces adverse drug events (ADEs) (OR 0.38, 95% CI 0.18 to 0.80; I² = 69%; 3 studies, 1336 participants; moderate‐certainty evidence; Analysis 1.2), and probably increases discrepancy resolutions (RR 7.48, 95% CI 5.62 to 9.95; 1 study, 564 participants; Analysis 1.6). Low‐certainty evidence suggests that MR may have little to no effect on length of stay (MD ‐0.30 days, 95% CI ‐1.93 to 1.33 days; I2 = 0%; 3 studies, 527 participants; Analysis 1.4), and on quality of life (MD ‐1.51, 95% CI ‐10.04 to 7.02; I2 = 0%; 1 study, 131 participants; Analysis 1.5). However, the confidence intervals for these outcomes are compatible with important beneficial and detrimental effects. The effect of medication reconciliation on mortality during hospitalisation was very uncertain (RR 3.85, 95% CI 0.44 to 33.89; I2 = 0%; 1 study, 212 participants; Analysis 1.3).
We grouped medication errors and ADEs (as described in the Methods, and in the Appendix 1). The specific outcomes contained in these grouped outcomes, and other secondary outcomes, are presented in Figure 7 (Analysis 1.7 to 1.12) and Figure 8 (Analysis 1.2 to 1.16).
7.

Comparison 1. Medication reconciliation (MR) versus no MR ‐ Ungrouped outcomes 1.7 to 1.11
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
8.

Comparison 1. MR versus no MR ‐ Ungrouped outcomes 1.12 to 1.16
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
2. MR: pharmacist compared with other professionals (Delivery arrangements)
This comparison, described in Table 2, includes 19 RCTs and 9854 participants (Aag 2014; Becerra‐Camargo 2015; Beckett 2012; Bell 2016; De Winter 2011; Farris 2014; George 2011; Graabaek 2019; Hale 2013; Heselmans 2015; Khalil 2016; Kwan 2007; Lind 2017; Marotti 2011; Pevnick 2018; Schmader 2004; Scullin 2007; SUREPILL 2015; Tong 2016).
Graabaek 2019 compared three arms. We excluded from the analysis the arm that combined MR with patient counselling and a medication report at discharge.
Low‐certainty evidence suggests that medication reconciliation performed by pharmacists, instead of other professionals, may reduce medication errors (OR 0.21, 95% CI 0.09 to 0.48; I² = 92%; 8 studies, 2648 participants; Analysis 2.1), and may increase ADEs (OR 1.34, 95% CI 0.73 to 2.44; I² = 12%; 3 studies, 2873 participants; Analysis 2.2). However, the confidence interval for the latter is compatible with important beneficial and detrimental effects. MR performed by pharmacists may increase discrepancy resolutions (OR 4.80, 95% CI 1.81 to 12.76; I² = 93%; 3 studies, 1449 participants; Analysis 2.7), and may have little to no effect on length of stay (MD ‐0.25, 95% CI ‐1.05 to 0.56; I2 = 63%; 6 studies, 3983 participants; Analysis 2.5), both for inpatients on general wards (MD ‐0.25, 95% CI ‐1.09 to 0.59) and inpatients coming from an ICU (MD ‐0.30, 95% CI ‐6.71 to 6.11) (test for subgroup differences: I² = 0%).
Moderate‐certainty evidence shows that medication reconciliation performed by pharmacists, instead of other professionals, probably has little to no effect on: mortality during hospitalisation (RR 0.99, 95% CI 0.57 to 1.73; I2 = 0%; 2 studies, 1000 participants; Analysis 2.3); lower mortality at six months (RR 0.54, 95% CI 0.22 to 1.32; I2 = 0%; 1 study, 400 participants; Analysis 2.25); and readmissions at one month (RR 0.93, 95% CI 0.76 to 1.14; I2 = 0%; 2 studies, 997 participants; Analysis 2.4). Low‐certainty evidence suggests that, compared with other professionals, medication reconciliation by pharmacists may have little to no effect on quality of life (MD 0.00, 95% CI ‐14.09 to 14.09; 1 study, 724 participants; Analysis 2.6). However, the confidence intervals of these outcomes are compatible with important beneficial and detrimental effects.
We grouped medication errors and ADEs (as described in the Methods, and in the Appendix 1). The specific outcomes contained in these grouped outcomes and secondary outcomes are presented in Figure 9 (Analysis 2.8 and 2.9); Figure 10 (Analysis 2.10 to 2.13); Figure 11 (Analysis 2.14 to 2.18); Figure 12 (Analysis 2.19 to 2.22); and Figure 13 (Analysis 2.23 to 2.25).
9.
Comparison 2. Medication reconciliation: pharmacist compared to other professionals ‐ Ungrouped outcomes 2.8 to 2.9
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
10.

Comparison 2. Medication reconciliation: pharmacist compared to other professionals ‐ Ungrouped outcomes 2.10 to 2.13
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
11.

Comparison 2. Medication reconciliation: pharmacist compared to other professionals ‐ Ungrouped outcomes 2.14 to 2.18
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
12.

Comparison 2. Medication reconciliation: pharmacist compared to other professionals ‐ Ungrouped outcomes 2.19 to 2.22
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
13.

Comparison 2. Medication reconciliation: pharmacist compared to other professionals ‐ Ungrouped outcomes 2.23 to 2.25
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
Beckett 2012 randomised 81 geriatric patients to receive MR according to current hospital practice or to pharmacist‐led MR at admission, but could not be meta‐analysed because the study did not provide CIs. Pharmacist‐led MR was superior to standard hospital practice (71% versus 48% appropriate medication profiles at 48 hours post‐admission, respectively; P = 0.033; 1.1 solved discrepancies per patient versus 0.8, respectively; P = 0.097).
3. MR by pharmacist: database‐assisted MR compared to unassisted MR (Delivery arrangements)
This comparison, described in Table 3, includes three RCTs and 3713 participants (Boockvar 2017; Fernandes 2011; Tamblyn 2018).
Low‐certainty evidence suggests that database‐assisted MR, compared to unassisted MR, may reduce potential ADEs per patient (OR 0.26, 95% CI 0.10 to 0.64; I2 = 49%; 2 studies, 3326 participants; Analysis 3.1), and may increase discrepancy resolutions (OR 1.37, 95% CI 0.97 to 1.93; I² = 41%; 2 studies, 797 participants; Analysis 3.3). However, the confidence interval for the latter is compatible with important beneficial and no effects. Database‐assisted MR may have no effect on length of stay, but the confidence interval is compatible with no effect and with important increase (MD 1.00, 95% CI ‐0.17 to 2.17; 1 study, 311 participants; low‐certainty evidence; Analysis 3.2).
We grouped discrepancy resolution outcomes (as described in the Methods, and in the Appendix 1). The specific outcomes contained in this grouped outcome and other secondary outcomes are presented in Figure 14 (Analysis 3.4 to 3.6).
14.

Comparison 3. Medication reconciliation by pharmacist: database‐assisted medication reconciliation compared to unassisted medication reconciliation ‐ Ungrouped outcomes 3.4 to 3.6
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
4. MR by pharmacist: trained pharmacist technician versus pharmacist (Delivery arrangements)
This comparison, described in Table 4, includes two RCTs: Pevnick 2018 (306 participants) and Hickman 2018 (unknown number of participants because it only reported prescriptions).
The effect of team/highly‐trained pharmacist MR versus standard pharmacist MR is very uncertain regarding medication errors (OR 0.65, 95% CI 0.25 to 1.70; 2 studies; 306 participants plus Hickman 2018 sample; very low‐certainty evidence; Analysis 4.1). Low‐certainty evidence suggests there may be little to no difference on length of stay (MD ‐0.30, 95% CI ‐2.12 to 1.52; 1 study, 183 participants; Analysis 4.2). However, the confidence intervals of these outcomes are compatible with important beneficial and detrimental effects.
We grouped medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in this grouped outcome and other secondary outcomes are presented in Figure 15 (Analysis 4.3 to 4.5).
15.

Comparison 4. Medication reconciliation by trained pharmacist technician compared to pharmacist ‐ Ungrouped outcomes 4.3 to 4.5
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
5. MR: before versus at admission (Delivery arrangements)
This comparison, described in Table 5, includes one RCT and 307 participants (Quach 2015).
Low‐certainty evidence suggests that MR before admission may increase the identification of discrepancies (MD 1.27, 95% CI 0.46 to 2.08; 1 study, 307 participants; Analysis 5.1). However, the confidence interval is compatible with important beneficial and detrimental effects.
6. MR: one to two versus four medical charts open simultaneously (Delivery arrangements)
This comparison, described in Table 6, includes one RCT involving 3356 clinicians and 543,490 participants (Adelman 2019), and one ITS study involving 11,504 participants (Kannampallil 2018).
Even though medication orders were the most frequent component of orders analysed (45%), we excluded Adelman 2019 from the meta‐analysis because it also included orders for laboratory tests and imaging.
The certainty of evidence provided by one ITS study was very low (MD ‐0.19, 95% CI ‐0.58 to 0.20; 1 study, 11,504 participants; Analysis 6.1).
7. MR: multimodal intervention versus usual care (Delivery arrangements, Implementation strategies)
This comparison, described in Table 7, includes one RCT involving 539 participants (Tompson 2012), and one ITS study involving 1648 participants (Schnipper 2018).
The certainty of evidence provided by one ITS study was very low for both medication errors (RR 0.92, 95% CI 0.87 to 0.97; 1 study, 1648 participants; Analysis 7.1) and potential ADEs (RR 0.97, 95% CI 0.86 to 1.09; 1 study, 539 participants; Analysis 7.2).
Moderate‐certainty evidence from one RCT shows that, compared with usual care, a multimodal intervention probably increases discrepancy resolutions (RR 2.14, 95% CI 1.81 to 2.53; 1 study, 487 participants; Analysis 7.3).
8. Computerised physician order entry (CPOE)/clinical decision support systems (CDSS) compared to control/paper‐based systems (Delivery arrangements)
This comparison, described in Table 8, includes three RCTs involving 915 participants (Colpaert 2006; O'Sullivan 2016; Redwood 2013), and 3 ITS studies involving 3906 participants (Burkoski 2019; Ongering 2019; Van Doormaal 2009).
Moderate‐certainty evidence from two RCTs shows that, compared with control/paper‐based, CPOE/CDSS probably reduce medication errors (OR 0.74, 95% CI 0.31 to 1.79; 2 studies, 88 participants; Analysis 8.1). The effect of the intervention on: ADEs (OR 0.24, 95% CI 0.04 to 1.50; 2 studies, 827 participants; Analysis 8.2); mortality during hospitalisation (RR 1.04, 95% CI 0.54 to 2.01; 1 study, 737 participants; Analysis 8.3); and length of stay (MD ‐1.00, 95% CI ‐2.05 to 0.05; 1 study, 737 participants; Analysis 8.4) was very uncertain. The effect on medication errors assessed by the ITS study, Ongering 2019, was RD 0.12 (95% CI ‐0.03 to 0.27; Analysis 8.5).
We grouped ADEs and medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in these grouped outcomes and other secondary outcomes are presented in Figure 16 (Analysis 8.6 to 8.8) and Figure 17 (Analysis 8.9 to 8.14).
16.

Comparison 8. CPOE/CDSS compared to control/paper‐based systems ‐ Ungrouped outcomes 8.5 to 8.8
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
17.

Comparison 8. CPOE/CDSS compared to control/paper‐based systems ‐ Ungrouped outcomes 8.9 to 8.14
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
9. Improved CPOE/CDSS compared to standard CPOE/CDSS (Delivery arrangements)
This comparison, described in Table 9, includes three RCTs involving 952 participants (Colpaert 2006; McCoy 2012; Schnipper 2009), one RCT involving 4264 providers and an unknown number of participants (Adelman 2013), two ITS studies involving 20,551 participants (Agrawal 2009; Van Doormaal 2009), and two CBA studies reanalysed as ITS studies, involving 2382 participants in Furuya 2013 and an unknown number of participants in Green 2015 because it measured prescriptions.
Moderate‐certainty evidence from two RCTs shows that, compared with standard CPOE/CDSS, improved CPOE/CDSS probably reduce medication errors (OR 0.84, 95% CI 0.73 to 0.97; 2 studies, 630 participants; Analysis 9.1.1), and could reduce medications errors (OR 0.77, 95% CI 0.37 to 1.62; participants = 2382 and Green 2015 sample; ITSs = 2 ; Analysis 9.1.2, very low certainty evidence). Test for subgroup differences: Chi² = 0.05, degrees of freedom (df) = 1 (P = 0.82), I² = 0%.
Improved CPOE/CDSS probably reduce ADEs (OR 0.82, 95% CI 0.71 to 0.94; 2 studies, 2382 participants plus Green 2015 sample; moderate certainty evidence; Analysis 9.2).
We grouped ADEs and medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in these grouped outcomes and other secondary outcomes are presented in Figure 18 (Analysis 9.3 to 9.8) and Figure 19 (Analysis 9.9 to 9.14).
18.

Comparison 9. CPOE/CDSS: improved compared to standard CPOE/CDSS ‐ Ungrouped outcomes 9.3 to 9.8
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
19.
Comparison 9. CPOE/CDSS: improved compared to standard CPOE/CDSS ‐ Ungrouped outcomes 9.9 to 9.14
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
10. CPOE/CDSS: prioritised versus non‐prioritised alerts (Delivery arrangements)
This comparison, described in Table 10, includes one ITS study that did not report participant numbers (Bhakta 2019).
Low‐certainty evidence suggests that, compared with non‐prioritised alerts, prioritised alerts provided by CPOE/CDSS may prevent ADEs (MD 1.98, 95% CI 1.65 to 2.31; 1 study; Analysis 10.1).
11. Barcoding versus no barcoding (Delivery arrangements)
This comparison, described in Table 11, includes two ITS studies (Burkoski 2019; Thompson 2018), and four CBA studies reanalysed as ITS studies (Bowdle 2018; Higgins 2010; Narang 2013; Seibert 2014). Bowdle 2018 was the only study in this comparison that reported participant numbers (50,545 participants). The other studies reported only prescriptions.
Low‐certainty evidence suggests that, compared with no‐barcoding, barcoding may reduce medication errors (OR 0.69, 95% CI 0.59 to 0.79; 2 studies, 50,545 participants in 1 study; Analysis 11.1).
We grouped medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in this grouped outcome and other secondary outcomes are presented in Figure 20 (Analysis 11.2 to 11.6); and ADEs in Figure 21 (Analysis 11.7).
20.

Comparison 11. Barcoding compared to no barcoding ‐ Ungrouped outcomes 11.2 to 11.16
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
21.

Comparison 11. Barcoding compared to no barcoding ‐ Ungrouped outcomes 11.7
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
12. Organisational changes: reduced versus unreduced working hours (Delivery arrangements)
This comparison, described in Table 12, includes one RCT involving 634 participants (Landrigan 2004).
Low‐certainty evidence suggests that, compared with unreduced working hours, reduced working hours may reduce serious medication errors (RR 0.83, 95% CI 0.63 to 1.09; 1 study, 634 participants, 2203 patient‐days; Analysis 12.1). However, the confidence interval for this result is compatible with important beneficial and detrimental effects.
13. Feedback on prescribing errors versus no feedback (Implementation strategies)
This comparison, described in Table 13, includes four RCTs (Gordon 2017; Gursanscky 2018; Hale 2013; Leung 2017). Only Hale 2013 reported randomising 384 participants; the other studies did not report participant numbers.
Low‐certainty evidence suggests that, compared with not providing feedback, feedback on prescribing errors may reduce medication errors (OR 0.47, 95% CI 0.33 to 0.67; 4 studies, 384 participants plus the other 3 RCTs samples; Analysis 13.1).
We grouped medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in this grouped outcome and other secondary outcomes are presented in Figure 22 (Analysis 13.2 to 13.4).
22.

Comparison 13. Feedback on prescribing errors compared to no feedback ‐ Ungrouped outcomes 13.3 to 13.4
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
14. Feedback on prescribing errors versus education (Implementation strategies)
This comparison, described in Table 14, includes two RCTs (Gursanscky 2018; Leung 2017). These studies reported prescriptions, not participants.
Compared with education, the effect of feedback on prescribing errors on medication errors is very uncertain (OR 0.59, 95% CI 0.20 to 1.76; 2 studies; Analysis 14.1).
We grouped medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in this grouped outcome and other secondary outcomes are presented in Figure 23 (Analysis 14.2 to 14.3).
23.

Comparison 14. Feedback on prescribing errors compared to education ‐ Ungrouped outcomes 14.2 to 14.3
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
15. Education compared to no education on prescribing (Implementation strategies)
This comparison, described in Table 15, includes four RCTs (Greengold 2003; Gursanscky 2018; Leung 2017; Schneider 2006). Only Schneider 2006 reported randomising participants (N = 30).
The effect of education on prescribing compared with no education is very uncertain (OR 1.21, 95% CI 0.93 to 1.58; 5 studies, 30 participants (available from only 1 study); Analysis 15.1; very low certainty evidence). The subgroup analysis by type of professional and type of education content is described below:
Education on prescriptions (physicians) OR 1.11 (95% CI 0.88 to 1.39; 2 studies; Analysis 15.1.1; very low certainty evidence).
Education on administration (nurses) OR 1.64 (95% CI 0.88 to 3.08; 3 studies; Analysis 15.1.2; very low certainty evidence).
Test for subgroup differences: Chi² = 0.73, df = 1 (P = 0.25), I² = 24.8%.
We grouped medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in this grouped outcome and other secondary outcomes are presented in Figure 24 (Analysis 15.2 to 15.6).
24.

Comparison 15. Education compared to no education on prescribing or administration ‐ Ungrouped outcomes 15.3 to 15.6
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
16. Dispensing systems compared with control (Delivery arrangements)
This comparison, described in Table 16, includes four RCTs (Barker 1984; Ding 2012; Merry 2011; Wang 2017), involving a total of 4085 participants. Ding 2012 reported only prescriptions.
Low‐certainty evidence suggests that, compared with no intervention, dispensing systems in the setting of surgical wards may reduce medication errors (OR 0.61, 95% CI 0.47 to 0.79; I2 = 0%; 2 studies, 1775 participants; Analysis 16.1.1).
The effect of dispensing systems on medication errors in operating rooms is very uncertain (OR 0.92, 95% CI 0.75 to 1.13; participants = 2310; studies = 2; I2 = 45%) (Analysis 16.1.2).
The test for subgroup differences was: Chi² = 6.00, df = 1 (P = 0.01), I² = 83.3%.
We grouped medication errors (as described in the Methods, and in the Appendix 1). The specific outcomes contained in this grouped outcome and other secondary outcomes are presented in Figure 25 (Analysis 16.3 to 16.4).
25.

Comparison 16. Dispensing system compared to control ‐ Ungrouped outcomes 16.3 to 16.4
(A) Random sequence generation (selection bias), (B) Allocation concealment (selection bias), (C) Blinding of participants and personnel (performance bias), (D) Blinding of outcome assessment (detection bias),(E) Incomplete outcome data (attrition bias), (F) Selective reporting (reporting bias), (G) Conflict of interest, (H) Other bias
Discussion
Summary of main results
The review includes 65 studies (51 RCTs and 14 ITS studies). Below, we summarise the main findings and remaining uncertainties by comparison. In order to facilitate the interpretation of findings, it is important to consider that ADEs include adverse drug reactions as well as preventable and ameliorable ADEs, which are ADEs due to medication error (Figure 1). Potential ADEs are defined as medication errors with a high likelihood to cause harm (Bates 1995). More important than the variability in the definitions of medication errors and ADEs within the studies, are the differences in the methods used to identify them and the subtypes of medication errors analysed by the researchers. Unfortunately, it was not possible to summarise the intervention effects by the severity of ADEs because this information was not provided in the original studies. We were aware of the limitations posed by the heterogeneity of populations, settings, interventions and outcome measures identified in our review. We used a framework validated by expert pharmacists to group outcomes, and when possible, we performed subgroup analysis to deal with the identified heterogeneity. For some specific outcomes, we did not pool results. When interpreting our findings, we suggest that the confidence interval limits should be given at least as much consideration as the effect estimates themselves.
We reanalysed RCTs and quasi‐RCTs with potential unit of analysis errors (i.e. cluster or prescriptions), where possible, by recalculating results using the appropriate unit of analysis.
Moderate‐certainty evidence showed that, compared with no medication reconciliation (MR), MR probably reduces ADEs (OR 0.38, 95% CI 0.18 to 0.80) and increases discrepancy resolutions (RR 7.48, 95% CI 5.62 to 9.95). Low‐certainty evidence suggests that MR may reduce medication errors (OR 0.55, 95% CI 0.17 to 1.74), and may have little to no effect on length of stay (MD ‐0.30 days, 95% CI ‐1.93 to 1.33 days) and on quality of life (MD ‐1.51, 95% CI ‐10.04 to 7.02), although the confidence intervals are compatible with important beneficial and detrimental effects. The effect of MR on mortality during hospitalisation was very uncertain. Single studies suggested that MR may reduce hospitalisations and serious ADEs with uncertain effects on discrepancy errors per prescriptions and resolved Preventable ADEs per prescriptions.
Low‐certainty evidence suggests that MR performed by pharmacists, instead of other professionals, may reduce medication errors (OR 0.21, 95% CI 0.09 to 0.48), and may increase ADEs (OR 1.34, 95% CI 0.73 to 2.44); however, the last confidence interval is compatible with important beneficial and detrimental effects. MR performed by pharmacists may increase discrepancy resolutions (OR 4.80, 95% CI 1.81 to 12.76) and may have little to no effect on length of stay (MD ‐0.25, 95% CI ‐1.05 to 0.56). Although the point estimate for ADEs suggest a worse effect with pharmacists than other professionals, this is an imprecise estimation indicating that caution should be used. This counterintuitive finding could be explained by the submaximal certainty of evidence, differences in the vulnerability of the populations studied to ADEs, methodologies employed for error detection, or because the interventions were aimed at reducing medications errors and a reduction in ADEs is not necessarily a fixed consequence. Moderate‐certainty evidence shows that MR performed by pharmacists probably has little to no effect on mortality during hospitalisation (RR 0.99, 95% CI 0.57 to 1.7), and on readmissions at one month (RR 0.93, 95% CI 0.76 to 1.14). Low‐certainty evidence suggests that MR by pharmacist may have little to no effect on quality of life (MD 0.00, 95% CI ‐14.09 to 14.09). However, the confidence intervals of the outcomes for this comparison are compatible with important beneficial and detrimental effects.
Low‐certainty evidence suggests that database‐assisted MR performed by pharmacists, instead of unassisted MR, may reduce potential ADEs per patient (OR 0.26, 95% CI 0.10 to 0.64) and may increase discrepancy resolutions (OR 1.37, 95% CI 0.97 to 1.93). However, the confidence interval of the last outcome is compatible with important beneficial and no effects. Database‐assisted MR may have no effect on length of stay (MD 1.00, 95% CI ‐0.17 to 2.17), but this confidence interval is compatible with no effect and with important increase.
The effect of medication reconciliation by trained pharmacist technicians versus pharmacist is very uncertain. Low‐certainty evidence suggests that there may be little to no difference on length of stay (MD ‐0.30, 95% CI ‐2.12 to 1.52). However, the confidence interval of this outcome is compatible with important beneficial and detrimental effects.
Low‐certainty evidence suggests that MR before admission, versus at admission, may increase the identification of discrepancies (MD 1.27, 95% CI 0.46 to 2.08); however, the confidence interval is compatible with important beneficial and detrimental effects.
Moderate‐certainty evidence from one RCT shows that, compared with MR allowing four charts open simultaneously, MR allowing only one or two charts open simultaneously probably has little to no effect on medication errors (MD 0.19, 95% CI ‐0.58 to 0.20).
Moderate‐certainty evidence shows that compared with usual care, a multimodal intervention probably increases discrepancy resolutions (RR 2.14, 95% CI 1.81 to 2.53). The evidence for effect on potential ADEs and medication errors is very uncertain.
Moderate‐certainty evidence from two RCTs shows that compared with control/paper‐based systems, CPOE/CDSS probably reduce medication errors (OR 0.74, 95% CI 0.31 to 1.79). The effect on ADEs, mortality during hospitalisation and on length of stay was very uncertain.
Moderate‐certainty evidence from two RCTs shows that compared with standard CPOE/CDSS, improved CPOE/CDSS probably reduce medication errors (OR 0.85, 0.74 to 0.97) and ADEs (OR 0.82, 0.71 to 0.94).
Low‐certainty evidence suggests that compared with non‐prioritised alerts, prioritised alerts provided by CPOE/CDSS may prevent ADEs (MD 1.98, 95% CI 1.65 to 2.31).
Low‐certainty evidence suggests that compared with no barcoding, barcoding may reduce medication errors (OR 0.69, 95% CI 0.59 to 0.79).
Low‐certainty evidence suggests that compared with unreduced working hours, reduced working hours may reduce serious medication errors (RR 0.83, 95% CI 0.63 to 1.09); however, the confidence interval is compatible with important beneficial and detrimental effects.
Low‐certainty evidence suggests that compared with not providing feedback, feedback on prescribing errors may reduce medication errors (OR 0.47, 95% CI 0.33 to 0.67). Compared with education, the effect of feedback on prescribing errors on medication errors is very uncertain.
The effect of education on prescribing compared to no education, and the effect of dispensing systems compared to control, on medication errors are very uncertain.
Overall completeness and applicability of evidence
The growing burden of medication errors reinforces the rationale of our review. Several recent systematic reviews reported the prevalence or incidence of medication errors. The prevalence of prescribing errors found in a systematic review that included 46 studies ranged widely, from 2% to 94% (Assiri 2018). This wide range may be at least partially due to the inconsistency in the definitions of medication errors used in the studies, differences in populations studied, methodologies employed for error detection and different outcome measures. Inappropriate prescribing was the most common type of error reported. The incidence of Preventable ADEs was estimated as 15/1000 person‐years and the prevalence as 0.4%. Alanazi 2016 included eight studies and found 0.24 to 89.6 errors per 100 orders in high‐risk medicines.
In order to avoid underestimation of medication errors' epidemiology, counting with a proper reporting system is critical. Even the most sophisticated health information and communication technologies could be insufficient (Korb‐Savoldelli 2018). One systematic review suggests that organisational and cultural barriers, including fear, accountability and characteristics of professionals, are additional barriers to reporting medication errors (Vrbnjak 2016). Other authors have suggested ways to improve reporting. A systematic review by Young and colleagues found that natural language processing (NLP) can generate meaningful information about medication errors and ADEs and could be a promising complementary method to deal with underreporting (Young 2019). A systematic review of patients' perspectives found that patients were able to identify medication errors, ADEs and their contributing factors in health care (Villar 2020).
Our exhaustive search strategy identified a very large number of references, and we are confident that we have not missed important pieces of evidence. The search results have allowed us to report many comparisons and outcomes involving interventions aimed at reducing medication errors in adults in hospital settings.
The relevance of the evidence identified in this review is very applicable to the research question with respect to participants and interventions, and partially applicable with respect to our prespecified primary outcomes, since the included studies did not always report on all of these outcomes.
Considering that medication errors are more frequent in elderly patients with multiple comorbidities, and we do not have separate evidence for this group, the applicability for this population is limited.
Our systematic review included the most reliable study designs. The included RCTs provided a considerable body of evidence about medication errors and ADEs. The included ITS studies, assessing CPOE/CDSS and barcoding, provided low‐certainty evidence about these interventions.
In order to combine the great diversity of outcomes assessing broader medication errors and ADEs concepts, we developed, and validated with highly trained pharmacists, a reasonable outcome grouping approach that allowed us to improve precision and to explore heterogeneity when it was identified. However, the low number of included studies by comparison limited the number of subgroup and sensitivity analyses.
Most studies were conducted in high‐income countries in reference hospitals. Therefore, the external validity of our review is good for these settings and limited for lower‐resource settings. The setting is very relevant for applicability issues, because medication errors can generate high costs, and that they represent an important source of medical waste and hospital inefficiency. None of the included studies presented economic analyses. One systematic review that included 16 studies (many of poor quality), found a mean cost per error per study ranging from 2.58 to 111,727 euros, highlighting a considerable variability between studies in terms of financial cost, patients, settings and errors included (Walsh 2017). Another systematic review also found huge variability in the estimation of avoidable cost per medication error (Vilela 2018).
Quality of the evidence
This review included 65 studies, 51 of which were RCTs and 14 were ITS studies.
Below, we describe the key risk of bias of the studies (see also Figure 3 and Figure 4 for RCTs; Figure 5 and Figure 6 for CBA and ITS studies; and Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16.)
We rated no outcome as high‐certainty evidence. In general, for ADEs and medication errors, the certainty of evidence was low to moderate, and for the other outcomes, it was of very low certainty.
RCTs (n = 51)
The number of studies at low risk of bias varied across the eight domains, as follows: 32 studies for random sequence generation; 24 studies for allocation concealment; 13 studies for blinding of participants and personnel; 35 studies for blinding of outcome assessment; 37 studies for incomplete outcome data; 43 studies for selective reporting; 40 studies for potential bias related to conflict of interests; and 28 studies appeared to be free of other sources of bias.
ITS studies (n = 14)
The number of studies at low risk of bias varied across the ten domains: 13 studies for reliable primary outcome measure; 7 studies for blinded assessment of primary outcomes; 9 studies for having analysed data appropriately; 12 studies for protection against detection bias; 12 studies for completeness of data set; 6 studies for the number of points given pre‐ and post‐intervention; 6 studies for protection against secular changes; 2 studies at low risk of bias for specifying the shape of the intervention effect; 10 studies for conflict of interest; and 9 studies for other bias.
Potential biases in the review process
We followed Cochrane guidelines to prevent bias in the review process. We conducted a comprehensive search without restriction on date or language, and we undertook independent screening of eligible studies. Although we are confident we were able to obtain most of the relevant data, our review may have omitted important unpublished data not reported from several hospitals worldwide.
Another potential source of bias is that we were unable to obtain additional data from many authors of included studies to clarify certain aspects of methodology that would have enabled a more thorough assessment of the risk of bias.
In order to capture most of the body of evidence, we accepted any medication error‐related outcome meeting our criteria. To handle this limitation, we received input from a group of trained pharmacists on how to group the outcomes. Additionally, we reported both grouped and non‐grouped outcomes, and have provided in Appendix 1 a list linking both types of outcomes to improve transparency.
We published the protocol in 2012. Because there have been many methodological advances since then, we have had to include some unplanned analyses. Additionally, we have run the literature searches several times, with small differences in the search strategies required by database updates.
Finally, several authors left the review and new ones were recruited, and we cannot discount minor inconsistencies in the process arising from these transitions.
Agreements and disagreements with other studies or reviews
Several systematic reviews that included fewer studies and patients than our review are nonetheless largely consistent with our findings. Below, we describe the systematic reviews or overviews assessing Interventions for reducing medication errors in adults in hospital settings published in the last five years.
Khalil 2020, an umbrella review that included 23 systematic reviews, found four effective interventions in reducing medication errors: education, medication reconciliation (MR), specialist pharmacists' roles and physical or design modifications. The certainty of their conclusions was limited due to high heterogeneity.
A Cochrane Review of medication review in hospitalised patients to reduce morbidity and mortality found no evidence that this intervention reduces mortality or hospital readmissions, but may reduce emergency department contacts (Christensen 2016).
Shitu 2019, a systematic review that included 20 studies, found that most interventions seem effective at reducing the occurrence of medication errors, with CPOE being the most effective one, followed by clinical pharmacist, computerisation, automatic dispensing cabinets, and barcoding. Manias 2020, another systematic review, evaluated the effectiveness of 12 different interventions in reducing prescribing, dispensing and administration medication errors in acute medical and surgical settings. It included 34 articles (9 RCTs), and showed that prescribing errors were reduced by pharmacist‐led MR, computerised MR, pharmacist partnership, prescriber education, MR by trained mentors and CPOE as single interventions. Cheema 2018, another systematic review about pharmacist‐led MR that included 18 RCTs, concluded that pharmacist‐led interventions were effective in reducing medication discrepancies but not ADEs or healthcare utilisation.
Administration errors were reduced by CPOE and the use of an automated drug distribution system as single interventions. Combined interventions were also found to be effective in reducing prescribing or administration medication errors (Manias 2020). Berdot 2016, a systematic review, evaluated interventions to reduce only nurses' medication administration errors in inpatient settings, and found that interventions may decrease administration errors, but the confidence interval is compatible with beneficial and detrimental effects.
Anderson 2019, an overview, summarised the evidence from systematic reviews examining MR and included 11 reviews, five of which included meta‐analysis. The reviews largely focused on transitions into and out of hospital settings, but five focused exclusively on pharmacist‐led interventions. Three reviews found very low‐quality evidence that interventions reduced medication discrepancies but neither of the two reviews that examined clinically significant medication discrepancies found any intervention effect. One out of the five reviews that examined healthcare utilisation outcomes, found low‐ to very low‐quality evidence of intervention effect. Four reviews considered clinical outcomes, but none found any intervention effect.
Wang 2018, a systematic review, evaluated the available electronic MR tools and their effect on unintended discrepancies that occur in hospital institutions. A total of 13 studies (three RCTs and 10 non‐RCTs) were identified. A total of 12 electronic tools were reported and were mostly integrated into the hospitals' information systems. Most were shown to reduce the incidence of medication with unintended discrepancies and improve medication safety.
Redmond 2018, a Cochrane Review, assessed the effect of MR on medication discrepancies, patient‐related outcomes and healthcare utilisation during care transitions, and included 25 RCTs involving 6995 participants. The authors concluded that the effect of MR, in particular pharmacist‐led MR, on medication discrepancies, ADEs, Preventable ADEs and healthcare utilisation, is uncertain due to very low certainty of evidence.
Choi 2019, a systematic review, found that pharmacy‐led MR significantly decreased the number of discrepancies, but only one study investigated ADEs in patients from emergency departments.
Eng 2018, a systematic review that assessed the effects of pharmacist prescribing on patient outcomes in the hospital setting, found three studies suggesting that pharmacist prescribers made 20 to 25 times fewer prescribing errors, and 3 to 116 times fewer omissions than doctors. A systematic review (Gillani 2020) included seven studies was also consistent with this finding.
Jia 2016, an overview that included 20 systematic reviews, found that CDSS reduces medication errors by improving process of care, but with inconsistent effects on patient outcomes.
Devin 2020, a systematic review that included 20 non‐randomised studies focused on adults, found that prescribing health information technology reduced the median OR of prescribing errors.
Mekonnen 2016, a systematic review that included 10 studies, showed a reduction of unintentional discrepancies and omission errors with electronic MR.
Roumeliotis 2019, another systematic review focused on electronic prescribing strategies on medication errors and patient harm in hospitalised patients, included 11 RCTs (all of which reported on patient outcomes for specific conditions and none on medication errors) and one ITS, which was also included in our review. Roumeliotis and colleagues found very low‐certainty evidence of a reduction on ADEs and Preventable ADEs and a small effect on length of stay and mortality.
A systematic review that included 19 CBA studies and one RCT also included by us (Prgomet 2017), found that the transition from paper‐based ordering to commercial CPOE systems in intensive care units was associated with an important reduction in medication prescribing error rates and in ICU mortality rates and no important effect on length of stay and hospital mortality.
A systematic review of CPOE/CDSS that included only one RCT and no ITS study (Vélez‐Díaz‐Pallarés 2018), could not pool data on medication errors and ADEs, mainly due to heterogeneity in outcome definitions and study methodologies, but found an overall reduction in prescribing errors.
The correct classification of prescriptions is also a key process to prevent medication errors. Sloss 2020, a systematic review that included eight observational studies, found that the frequency of alert generation varied across studies during barcode‐assisted medication administration, and not all alerts were clinically meaningful. Larmené‐Beld 2018, another systematic literature review on strategies to avoid look‐alike errors of labels, included 11 studies that evaluated Tall Man lettering (capitalising parts of the drug name, two colour‐coding). Six of these studies showed that this intervention reduced medication errors due to better readability of medication labels.
Although simulation‐based learning to prevent medication errors was outside our scope, Sarfati 2019, a systematic review, found it to be a good method to train staff in events that happen only exceptionally, as well as in standard daily activities. Another systematic review found positive effects of educational interventions, but it could not define the best strategy (Harkanen 2016).
Ahtiainen 2020, a systematic review of automated and semi‐automated drug distribution systems in hospitals, found ‐ consistently with our findings ‐ that these systems reduced medication errors and none was found to be better than another.
Finally, Maaskant 2015, another Cochrane Review that asks the same question as our review but in hospitalised children, included seven studies. They found that some interventions may decrease medication errors, but the results were not consistent. They found that any study resulted in a significant reduction in patient harm.
Authors' conclusions
Implications for practice.
Low‐ to moderate‐certainty evidence suggests that compared to usual care, medication reconciliation, computerised physician order entry (CPOE)/clinical decision support systems (CDSS), barcoding, feedback and dispensing systems in surgical wards may reduce adverse drug events (ADEs), medication errors, or both. It is less clear which are the best ways to conduct the medication reconciliation or the levels of functionalities that CPOE/CDSS should provide. The certainty of evidence for other interventions is very uncertain.
This systematic review found evidence that it is possible to reduce medication errors by an adequate medication reconciliation process conducted by teams composed of different professionals, including nurses, pharmacists, pharmacist technicians and physicians. These interventions are potentially affordable in low‐resource settings. In higher‐resources settings, there is evidence that many technological aids, such as CPOE/CDSS, barcoding, alert systems and dispensing systems, could obtain some additional benefits in reducing medication errors.
Implications for research.
Our systematic review highlighted remarkable evidence gaps for most studied interventions, particularly in low‐resource settings, in low income countries, or in both. Powered and methodologically sound experimental and quasi‐experimental studies are needed before deciding which strategy should be scaled‐up. To find the most impactful interventions to reduce medication errors, further studies should compare them in different settings and populations. It is very important to include the most critical outcomes for patients and health systems in future studies. Researchers should use validated frameworks for medication errors to standardise outcome measures. Additionally, it is important to study the effects of interventions in the high‐risk group of poly‐medicated elderly people.
It is also critical to improve medication error reporting and registers, and to assess the effectiveness, safety, and cost‐effectiveness of interventions to reduce medication errors in different settings to extend the external validity. Continuous development of health information technology could probably greatly improve patient safety, but other innovative solutions ‐ such as multiple synergistic strategies, including patient involvement wherever possible ‐ also deserve to be evaluated.
History
Protocol first published: Issue 7, 2012
Acknowledgements
To Daniel Comande and Paul Miler for their contribution to the search strategy; to Pierre Durieux, Simon Arnold Lewin, Sasha Shepperd, Jen Hilgart and Christopher Cooper for methodological support; to Cathal Cadogan, Carmel Hughes and Farouk Chughlay for their expert pharmacist guidance regarding the strategy for outcome grouping; to our contact editor Mary Ann O'Brien; to our copy editor Faith Armitage; and to the researchers who contributed during the systematic review process, including Analia S. Lopez, Florencia Koch and Lana M. Chagas.
Appendices
Appendix 1. Outcome grouping
| Outcomes reported by authors of included studies | Outcome group | Type of medication error |
| Administration errors (per monthly administered doses) | Medication errors | Administration errors |
| Administration (per prescription) | Medication errors | Administration errors |
| Administration errors (per weekly anaesthetised patients) | Medication errors | Administration errors |
| Administration errors (per opportunities for error, by nurse) | Medication errors | Administration errors |
| Medication errors (per administered doses) | Medication errors | Administration errors |
| Medication errors (per administration) | Medication errors | Administration errors |
| Medication errors (per monthly administered doses) | Medication errors | Administration errors |
| All errors | Medication errors | All type of medication errors |
| Medication errors (per prescriptions) | Medication errors | All type of medication errors |
| Medication errors (per weekly prescriptions) | Medication errors | All type of medication errors |
| Medication errors per patient at discharge | Medication errors | All type of medication errors |
| Pharmacist only perform MR (all errors) | Medication errors | All type of medication errors |
| Serious medication errors per patient‐days by interns | Medication errors | All type of medication errors |
| Dispensing errors (per monthly administered doses) | Medication errors | Dispensing error |
| Dispensing errors (per prescriptions) | Medication errors | Dispensing error |
| Potential ADEs (≥ 1 per patient) | Medication errors | Potential ADEs |
| Potential ADEs (≥ 1 per patient) | Medication errors | Potential ADEs |
| Potential ADEs (per patient) | Medication errors | Potential ADEs |
| Potential ADEs (per prescriptions) | Medication errors | Potential ADEs |
| Potential ADEs + ADEs (≥ 1 per patient) | Medication errors | Potential ADEs |
| Serious PADEs (≥ 1 per patient) | Medication errors | Potential ADEs |
| Serious PADEs (per prescriptions) | Medication errors | Potential ADEs |
| Discrepancies errors (≥ 1 per patient) | Medication errors | Prescribing errors |
| Discrepancy errors | Medication errors | Prescribing errors |
| Discrepancy errors (per prescriptions) | Medication errors | Prescribing errors |
| Discrepancy errors per patient | Medication errors | Prescribing errors |
| Duplication errors (per prescription) | Medication errors | Prescribing errors |
| ID‐reentry function | Medication errors | Prescribing errors |
| ID‐verify alert | Medication errors | Prescribing errors |
| Medications omitted (per prescriptions) | Medication errors | Prescribing errors |
| Only omissions | Medication errors | Prescribing errors |
| Prescribing errors (per doctor) | Medication errors | Prescribing errors |
| Pharmacist history and supplementary prescribing | Medication errors | Prescribing errors |
| Pharmacist only perform MR (dosing errors) | Medication errors | Prescribing errors |
| Pharmacist only perform MR (frequency dosing errors) | Medication errors | Prescribing errors |
| Pharmacists perform MR + prescribing (dosing errors) | Medication errors | Prescribing errors |
| Pharmacists perform MR + prescribing (frequency dosing errors) | Medication errors | Prescribing errors |
| Prescribing error (per order session) | Medication errors | Prescribing errors |
| Prescribing errors (per prescriptions) | Medication errors | Prescribing errors |
| Serious discrepancy errors per patient | Medication errors | Prescribing errors |
| Serious prescribing errors (per prescriptions) | Medication errors | Prescribing errors |
| Unintended discrepancies (≥ 1 per patient) | Medication errors | Prescribing errors |
| ADEs | Adverse drug events (ADEs) | |
| ADEs (≥ 1 per patient) | Adverse drug events (ADEs) | |
| ADEs (per monthly administered doses) | Adverse drug events (ADEs) | |
| ADEs (per prescriptions) | Adverse drug events (ADEs) | |
| ADEs due to discrepancies per patient | Adverse drug events (ADEs) | |
| ADEs due to medication error | Adverse drug events (ADEs) | |
| ADEs per admissions | Adverse drug events (ADEs) | |
| Medication error + ADEs (per monthly administered medication doses) | Adverse drug events (ADEs) | |
| Preventable ADEs (≥ 1 per patient) | Adverse drug events (ADEs) | |
| Preventable ADEs (per monthly patients admitted) | Adverse drug events (ADEs) | |
| Preventable ADEs per admissions | Adverse drug events (ADEs) | |
| Serious ADEs | Adverse drug events (ADEs) | |
| Serious ADEs per admissions | Adverse drug events (ADEs) | |
| Mortality | Mortality | |
| Mortality at 6 months | Mortality | |
| Mortality during hospitalisation | Mortality | |
| Hospitalisations due to ADEs | Hospitalisations | |
| Readmisson at 1 month | Hospitalisations | |
| Length of stay (days) | LoS | |
| Length of stay (days) | LoS | |
| Quality of life (visual analogue scale (VAS) 0‐10; EQ‐5D‐3L) | QoL | |
| Discrepancies resolutions (≥ 1 per patient) | Discrepancies resolutions | |
| Discrepancy resolution (≥ 1 per patient) | Discrepancies resolutions | |
| Discrepancy resolutions (per discrepancies at discharge) | Discrepancies resolutions | |
| Discrepancy resolutions (per discrepancies) | Discrepancies resolutions | |
| Resolved Potential ADEs (per prescriptions) | Resolution of MEs | |
| Identified discrepancies (≥ 1 per patient) | Identified discrepancies | |
| Identified discrepancies per patient | Identified discrepancies |
Appendix 2. Search strategies
Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® 1946‐)
Search date: 16 January 2020
| No. | Search terms | Results |
| 1 | medication errors/ | 13014 |
| 2 | inappropriate prescribing/ | 2991 |
| 3 | medication reconciliation/ | 1066 |
| 4 | ((drug? or prescri* or medicat*) adj3 alert*).ti,ab,kf. | 1119 |
| 5 | ((drug? or medication? or medicine? or dose or dosage? or dosing or prescri* or order?) adj2 wrong*).ti,ab,kf. | 722 |
| 6 | (medication adj1 (review? or reconcil* or counsel* or error? or safety)).ti,ab,kf. | 9945 |
| 7 | ((inappropriate* or appropriate*) adj1 prescri*).ti,ab,kf. | 3282 |
| 8 | (prescri* adj4 (safe* or error*)).ti,ab,kf. | 4055 |
| 9 | decision support systems, clinical/ or medical order entry systems/ | 9292 |
| 10 | prescri*.ti,ab,kf,hw. | 222837 |
| 11 | 9 and 10 | 1433 |
| 12 | or/1‐8,11 | 28110 |
| 13 | (unit or units).ti,ab,kf,hw. | 662106 |
| 14 | hospital*.ti,ab,kf,hw,in. | 4988144 |
| 15 | or/13‐14 | 5415306 |
| 16 | 12 and 15 | 14195 |
| 17 | randomized controlled trial.pt. | 498732 |
| 18 | controlled clinical trial.pt. | 93522 |
| 19 | multicenter study.pt. | 264836 |
| 20 | pragmatic clinical trial.pt. | 1277 |
| 21 | (randomis* or randomiz* or randomly).ti,ab,kf. | 872592 |
| 22 | groups.ab. | 1998032 |
| 23 | (trial or multicenter or multi center or multicentre or multi centre).ti. | 250811 |
| 24 | (intervention? or effect? or impact? or controlled or control group? or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab,kf. | 9334407 |
| 25 | non‐randomized controlled trials as topic/ | 610 |
| 26 | interrupted time series analysis/ | 750 |
| 27 | or/17‐26 | 10423000 |
| 28 | exp animals/ | 22903001 |
| 29 | humans/ | 18238668 |
| 30 | 28 not (28 and 29) | 4664333 |
| 31 | review.pt. | 2600548 |
| 32 | meta analysis.pt. | 109833 |
| 33 | news.pt. | 198904 |
| 34 | comment.pt. | 824770 |
| 35 | editorial.pt. | 515077 |
| 36 | cochrane database of systematic reviews.jn. | 14808 |
| 37 | comment on.cm. | 824716 |
| 38 | (systematic review or literature review).ti. | 148362 |
| 39 | or/30‐38 | 8493610 |
| 40 | 27 not 39 | 7351460 |
| 41 | 16 and 40 | 6830 |
Embase (OVID, 1974‐)
Search date: 16 January 2020
| No. | Search terms | Results |
| 1 | *medication error/ | 8365 |
| 2 | *inappropriate prescribing/ | 1423 |
| 3 | *medication therapy management/ | 4166 |
| 4 | ((drug? or prescri* or medicat*) adj3 alert*).ti,ab,kw. | 1889 |
| 5 | ((drug? or medication? or medicine? or dose or dosage? or dosing or prescri* or order?) adj2 wrong*).ti,ab,kw. | 1502 |
| 6 | (medication adj1 (review? or reconcil* or counsel* or error? or safety)).ti,ab,kw. | 18748 |
| 7 | ((inappropriate* or appropriate*) adj1 prescri*).ti,ab,kw. | 5693 |
| 8 | (prescri* adj4 (safe* or error*)).ti,ab,kw. | 7279 |
| 9 | *clinical decision support system/ | 1316 |
| 10 | *physician order entry system/ | 98 |
| 11 | prescri*.ti,ab,kw,hw. | 418777 |
| 12 | or/9‐10 | 1401 |
| 13 | 11 and 12 | 246 |
| 14 | or/1‐8,13 | 38313 |
| 15 | (unit or units).ti,ab,kw,hw. | 913799 |
| 16 | hospital*.ti,ab,kw,hw,in. | 8046625 |
| 17 | or/15‐16 | 8545861 |
| 18 | 14 and 17 | 22561 |
| 19 | randomized controlled trial/ | 586757 |
| 20 | controlled clinical trial/ | 463266 |
| 21 | quasi experimental study/ | 6357 |
| 22 | pretest posttest control group design/ | 441 |
| 23 | time series analysis/ | 24966 |
| 24 | experimental design/ | 17983 |
| 25 | multicenter study/ | 240605 |
| 26 | (randomis* or randomiz* or randomly).ti,ab. | 1223708 |
| 27 | groups.ab. | 2778000 |
| 28 | (trial or multicentre or multicenter or multi centre or multi center).ti. | 351927 |
| 29 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 11970676 |
| 30 | or/19‐29 | 13353223 |
| 31 | (systematic review or literature review).ti. | 178369 |
| 32 | "cochrane database of systematic reviews".jn. | 13931 |
| 33 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 26825418 |
| 34 | human/ or normal human/ or human cell/ | 20517865 |
| 35 | 33 not (33 and 34) | 6370493 |
| 36 | 31 or 32 or 35 | 6561150 |
| 37 | 30 not 36 | 10292608 |
| 38 | 18 and 37 | 13764 |
The Cochrane Library
Search date: 16 January 2020
| No. | Search terms | Results |
| #1 | [mh “medication errors”] | 407 |
| #2 | [mh “inappropriate prescribing”] | 130 |
| #3 | [mh “medication reconciliation”] | 76 |
| #4 | ((drug? or prescri* or medicat*) near/3 alert*):ti,ab,kw | 247 |
| #5 | ((drug? or medication? or medicine? or dose or dosage? or dosing or prescri* or order?) near/2 wrong*):ti,ab,kw | 55 |
| #6 | (medication near/1 (review? or reconcil* or counsel* or error? or safety)):ti,ab,kw | 1565 |
| #7 | ((inappropriate* or appropriate*) near/1 prescri*):ti,ab,kw | 518 |
| #8 | (prescri* near/4 (safe* or error*)):ti,ab,kw | 574 |
| #9 | [mh “decision support systems, clinical”] or [mh “medical order entry systems”] | 393 |
| #10 | prescri* | 35146 |
| #11 | #9 and #10 | 87 |
| #12 | {or #1‐#8, #11} | 2695 |
| #13 | (unit or units) | 107814 |
| #14 | hospital* | 335426 |
| #15 | #13 or #14 | 396828 |
| #16 | #12 and #15 | 1478 |
CINAHL (EBSCO)
Search date: 16 January 2020
| No. | Search terms | Results |
| S1 | (MH "Medication Errors+") | 15,070 |
| S2 | (MH "Medication Reconciliation") | 1,518 |
| S3 | TI ((drug? or prescri* or medicat*) N3 alert*) OR AB ((drug? or prescri* or medicat*) N3 alert*) | 888 |
| S4 | TI ((drug? or medication? or medicine? or dose or dosage? or dosing or prescri* or order?) N2 wrong*) OR AB ((drug? or medication? or medicine? or dose or dosage? or dosing or prescri* or order?) N2 wrong*) | 446 |
| S5 | TI ((inappropriate* or appropriate*) N1 prescri*) OR AB ((inappropriate* or appropriate*) N1 prescri*) | 2,092 |
| S6 | (MH "Decision Support Systems, Clinical") | 4,682 |
| S7 | (MH "Electronic Order Entry") | 2,978 |
| S8 | S6 OR S7 | 7,207 |
| S9 | TX prescri* | 115,470 |
| S10 | S8 AND S9 | 1,299 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S10 | 18,919 |
| S12 | TX (unit or units) | 348,687 |
| S13 | TX hospital* | 1,473,988 |
| S14 | S12 OR S13 | 1,629,464 |
| S15 | S11 AND S14 | 8,059 |
| S16 | PT randomized controlled trial | 86,198 |
| S17 | PT clinical trial | 85,810 |
| S18 | PT research | 1,949,263 |
| S19 | (MH "Randomized Controlled Trials") | 89,376 |
| S20 | (MH "Clinical Trials") | 151,432 |
| S21 | (MH "Intervention Trials") | 7,249 |
| S22 | (MH "Nonrandomized Trials") | 455 |
| S23 | (MH "Experimental Studies") | 23,867 |
| S24 | (MH "Pretest‐Posttest Design+") | 40,092 |
| S25 | (MH "Quasi‐Experimental Studies+") | 14,063 |
| S26 | (MH "Multicenter Studies") | 158,080 |
| S27 | (MH "Health Services Research") | 13,903 |
| S28 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 273,171 |
| S29 | TI (trial or effect* or impact* or intervention* or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 1,830,248 |
| S30 | S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 | 2,883,722 |
| S31 | S15 AND S30 | 5,097 |
Conference Proceedings Citation Index‐ Science (CPCI‐S)
Search date: 16 January 2020
| No. | Search terms | Results |
| #1 | TS=((drug? OR prescri* OR medicat*) NEAR/3 alert*) | 12 |
| #2 | TS= ((drug? OR medication? OR medicine? OR dose OR dosage? OR dosing OR prescri* OR order?) NEAR/2 wrong*) | 11 |
| #3 | TS= (medication NEAR/1 (review? OR reconcil* OR counsel* OR error? OR safety)) | 203 |
| #4 | TS= ((inappropriate* OR appropriate*) NEAR/1 prescri*) | 51 |
| #5 | TS= (prescri* NEAR/4 (safe* OR error*)) | 186 |
| #6 | TS=((decision support systems OR order entry systems) AND (prescri*)) | 98 |
| #7 | #6 OR #5 OR #4 OR #3 OR #2 OR #1 | 530 |
| #8 | TS=(unit OR units OR hospital*) | 66,515 |
| #9 | #8 AND #7 | 97 |
| #10 | TS=(randomis* OR randomiz* OR randomly OR groups OR trial OR multicenter OR multi center OR multicentre OR multi centre OR intervention? OR effect? OR impact? OR controlled OR control group? OR (pre NEAR/5 post) OR ((pretest OR pre test) and (posttest OR post test)) OR quasiexperiment* OR quasi experiment* OR pseudo experiment* OR pseudoexperiment* OR evaluat* OR time series OR time point? OR repeated measur*) | 566,221 |
| #11 | #10 AND #9 | 44 |
ProQuest Dissertations & Theses Global COS Conference Papers Index
Search date: 16 January 2020
| Search terms | Results |
| TI,AB,SU(((drug? OR prescri* OR medicat*) NEAR/3 alert*) OR ((drug? OR medication? OR medicine? OR dose OR dosage? OR dosing OR prescri* OR order?) NEAR/2 wrong*) OR (medication NEAR/1 (review? OR reconcil* OR counsel* OR error? OR safety)) OR ((inappropriate* OR appropriate*) NEAR/1 prescri*) OR (prescri* NEAR/4 (safe* OR error*)) OR ((decision support systems OR order entry systems) AND (prescri*))) AND TI,AB,SU(unit OR units OR hospital*) AND TI,AB,SU(randomis* OR randomiz* OR randomly OR groups OR trial OR multicenter OR "multi center" OR multicentre OR "multi centre" OR intervention? OR effect? OR impact? OR controlled OR "control group?" OR pretest OR "pre test" OR posttest OR "post test" OR quasiexperiment* OR "quasi experiment*" OR "pseudo experiment*" OR "pseudoexperiment*" OR evaluat* OR "time series" OR "time point?" OR "repeated measur*") | 93 |
ClinicalTrials.gov
Search date: 16 January 2020
| Field | Search terms |
| Other terms | medication error AND hospital |
| Study type | interventional studies |
| Age | adult, older adult |
WHO International Clinical Trials Registry Platform (ICTRP)
Search date: 16 January 2020
| Search terms |
| medication error* AND hospital* |
| prescri* error* AND hospital* |
Appendix 3. Interventions included, by EPOC group taxonomy
| EPOC group taxonomy categories | Intervention included (comparison #) |
| Delivery arrangements | |
| Who receives care and when | MR: before versus at admission (#5) |
| Who provides care | MR: pharmacist versus other professionals (#2) |
| Who provides care/Co‐ordination of care | MR by pharmacist: team/highly trained pharmacist versus standard pharmacist (#4) |
| Health Information and communication technology | CPOE/CDSS (#8, #9, #10); barcoding (#11); dispensing systems (#16); database‐assisted medication reconciliation conducted by pharmacists (#3); one to two charts versus four charts open simultaneously for MR (#6) |
| Working conditions of health workers | Organisational changes: reduced versus unreduced working hours (#12) |
| Coordination of care / Integration | Multimodal intervention (#7) |
| Implementation strategies | |
| Interventions targeted at healthcare worker practice | Feedback on prescribing errors; education (#13, #14, #15) |
| Types of problems targeted at healthcare worker practice | Medication reconciliation (#1) |
| Multifaceted interventions | Multimodal intervention (#7) |
| Financial arrangements | |
| ‐ | No included interventions |
| Governance arrangements | |
| ‐ | No included interventions |
Appendix 4. EPOC taxonomy and comparison number, by study
| Study ID | EPOC taxonomy | Comparison # |
| Greengold 2003 | Implementation strategy | 15 |
| Schneider 2006 | Implementation strategy | 15 |
| Aag 2014 | Delivery arrangement | 2 |
| Adelman 2019 | Delivery arrangement | 6 |
| Al‐Hashar 2018 | Implementation strategy | 1 |
| Becerra‐Camargo 2015 | Delivery arrangement | 2 |
| Beckett 2012 | Delivery arrangement | 2 |
| Bell 2016 | Delivery arrangement | 2 |
| Cadman 2017 | Implementation strategy | 1 |
| Chiu 2018 | Implementation strategy | 1 |
| De winter 2011 | Delivery arrangement | 2 |
| George 2011 | Delivery arrangement | 2 |
| Graabaek 2019 | Delivery arrangement | 2 |
| Heselmans 2015 | Delivery arrangement | 2 |
| Hickman 2018 | Delivery arrangement | 4 |
| Khalil 2016 | Delivery arrangement | 2 |
| Scullin 2007 | Delivery arrangement | 2 |
| Lind 2017 | Delivery arrangement | 2 |
| Piqueras 2015 | Implementation strategy | 1 |
| Pevnick 2018 | Delivery arrangement | 2, 4 |
| Schmader 2004 | Delivery arrangement | 2 |
| Nielsen 2017 | Implementation strategy | 1 |
| Bolas 2004 | Implementation strategy | 1 |
| Juanes 2018 | Implementation strategy | 1 |
| Leung 2017 | Implementation strategy | 13, 14, 15 |
| Gursanscky 2018 | Implementation strategy | 13, 14, 16 |
| Farris 2014 | Delivery arrangement | 2 |
| Kwan 2007 | Delivery arrangement | 2 |
| Hale 2013 | Delivery arrangement + Implementation strategy | 2, 13 |
| Marotti 2011 | Delivery arrangement | 2 |
| Quach 2015 | Delivery arrangement | 5 |
| Tong 2016 | Delivery arrangement | 2 |
| Willoch 2012 | Implementation strategy | 1 |
| SUPERPILL 2015 | Delivery arrangement | 2 |
| Vega 2016 | Implementation strategy | 1 |
| Tompson 2012 | Delivery arrangement | 7 |
| Merry 2011 | Delivery arrangement | 16 |
| McCoy 2012 | Delivery arrangement | 9 |
| Landrigan 2004 | Delivery arrangement | 12 |
| Wang 2017 | Delivery arrangement | 16 |
| Boockvar 2017 | Delivery arrangement | 3 |
| Fernandes 2011 | Delivery arrangement | 3 |
| Tamblyn 2018 | Delivery arrangement | 3 |
| OSullivan 2015 | Delivery arrangement | 8 |
| Schnipper 2009 | Delivery arrangement | 9 |
| Ding 2012 | Delivery arrangement | 16 |
| Barker 1984 | Delivery arrangement | 16 |
| Colpaert 2006 | Delivery arrangement | 8 |
| Redwood 2013 | Delivery arrangement | 8 |
| Adelman 2013 | Delivery arrangement | 9 |
| Gordon 2017 | Implementation strategy | 13 |
| Seibert 2014 | Delivery arrangement | 11 |
| Schnipper 2018 | Implementation strategy | 7 |
| Agrawal 2009 | Delivery arrangement | 9 |
| Narang 2013 | Delivery arrangement | 11 |
| Bhakta 2019 | Delivery arrangement | 10 |
| Green 2015 | Delivery arrangement | 9 |
| Kannampallil 2018 | Delivery arrangement | 6 |
| Ongering 2019 | Delivery arrangement | 8 |
| Furuya 2013 | Delivery arrangement | 9 |
| van Doormaal 2009 | Delivery arrangement | 8 |
| Bowdle 2018 | Delivery arrangement | 11 |
| Higgins 2010 | Delivery arrangement | 11 |
| Burkoski 2019 | Delivery arrangement | 8, 11 |
| Thompson 2018 | Implementation strategy | 11 |
Comparison #
| ||
Appendix 5. Identified evidence mapping: Comparisons 1 to 8
| Comparison level ↓____________________ Comparison # → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Study designs | RCT | RCT | RCT | RCT | RCT | RCT ‐ ITS | RCT ‐ ITS | RCT ‐ ITS | ||
| Target population | Adults. Older adults | Adults. Older adults | Adults | Adults | Old adults with high alert medications | Adults | RCT Adults, Adults with 2 two chronic conditions ITS Adults | Adults | ||
| Setting | Wards, ED | Wards, ED, Surgery units, pre‐admission clinic | General hospital, Surgery units, Anesthesia units | Hospital/ED | Emergency department (ED) | RCT Hospital ‐ ITS ED | General hospital | RCT General hospital ‐ ITS General hospital, ICU | ||
| Countries | China, Denmark, Norway, Oman, Spain(3), UK (2) | Australia (5), Belgium (2), Canada, Colombia, Denmark (2), Netherlands, Norway, United Kingdom, USA (5) | Canada (2), USA (1) | Netherlands, USA | USA | RCT USA ‐ ITS USA | RCT New Zealand, USA ‐ ITS USA | RCT Belgium, Ireland, UK ‐ ITS Canada, Japan Netherlands (2) | ||
| Study level | Comparisons description | |||||||||
| Study ID | Study design | Unit of analysis | MR vs No MR | MR: pharmacist vs other professionals | MR: database assisted vs not‐assisted. | MR by pharmacist: team/highly trained pharmacist vs standard pharmacist | MR: before vs after admission | MR: 1‐2 vs 4 charts open | MR: Multimodal intervention vs Usual care | CPOE/CDSS vs control/paper based |
| Aag 2014 | RCT‐ individual | Patients | x | |||||||
| Adelman 2013 | RCT‐ individual | Patients | ||||||||
| Adelman 2019 | RCT‐ individual | Order session | x | |||||||
| Agrawal 2009 | ITS | Unintended discrepancy per admission | ||||||||
| Al‐Hashar 2018 | RCT‐ individual | Patients | x | |||||||
| Barker 1984 | RCT‐ individual | Prescriptions | ||||||||
| Becerra‐Camargo 2015 | RCT‐ individual | Patients | x | |||||||
| Beckett 2012 | RCT‐ individual | Patients | x | |||||||
| Bell 2015 | RCT‐ individual | Patients | x | |||||||
| Bhakta 2019 | ITS | Weekly prescription | ||||||||
| Bolas 2004 | RCT‐ individual | Patients | x | |||||||
| Boockvar 2017 | RCT‐ cluster | Patients | x | |||||||
| Bowdle 2018 | ITS | Patients receiving anaesthesia | ||||||||
| Burkoski 2019 | ITS | Monthly medication doses administered | x | |||||||
| Cadman 2017 | RCT‐ individual | Patients/Unintended discrepancies | x | |||||||
| Chiu 2018 | Quasi‐RCT | Patients | x | |||||||
| Colpaert 2006 | RCT‐ individual | Prescription | x | |||||||
| De winter 2011 | Quasi‐RCT individual | Patients | x | |||||||
| Ding 2012 | RCT‐ cluster | Prescriptions | ||||||||
| Farris 2014 | RCT‐ individual | Patients | x | |||||||
| Fernandes 2011 | RCT‐ individual | Patients | x | |||||||
| Furuya 2013 | ITS | Patient‐days | x | |||||||
| George 2011 | RCT‐ individual | Patients | x | |||||||
| Gordon 2017 | RCT‐ cluster | Prescriptions | ||||||||
| Graabaek 2019 | RCT‐ individual | Patients | x | |||||||
| Green 2015 | ITS | Prescriptions | ||||||||
| Greengold 2003 | RCT‐ individual | Administered doses | ||||||||
| Gursanscky 2018 | RCT‐ cluster | Prescriptions | ||||||||
| Hale 2013 | RCT‐ individual | Prescriptions | x | |||||||
| Heselmans 2015 | RCT‐ individual | Patients/Prescriptions | x | |||||||
| Hickman 2018 | RCT‐ individual | Prescriptions | x | |||||||
| Higgins 2010 (Heelon) | ITS | Monthly administered doses | ||||||||
| Juanes 2018 | RCT‐ individual | Patients | x | |||||||
| Kannampallil 2018 | ITS | Order session | x | |||||||
| Khalil 2016 | RCT‐ individual | Patients | x | |||||||
| Kwan 2007 | RCT‐ individual | Patients | x | |||||||
| Landrigan 2004 | RCT‐ cluster | Patients | ||||||||
| Leung 2017 | RCT‐ individual | Prescriptions | ||||||||
| Lind 2017 | RCT‐ individual | Patients | x | |||||||
| Marotti 2011 | RCT‐ individual | Patients | x | |||||||
| McCoy 2012 | RCT‐ individual | Patients/Prescriptions | ||||||||
| Merry 2011 | Quasi‐RCT | Patients | ||||||||
| Narang 2013 | ITS | Probably monthly administered doses (it is unclear we cannot discard that were patients) | ||||||||
| Nielsen 2017 | RCT‐ cluster | Patients | x | |||||||
| Ongering 2019 | ITS | Prescription | x | |||||||
| OSullivan 2016 | RCT‐ cluster | Patients | x | |||||||
| Pevnick 2018 | RCT‐ individual | Patients | x | x | ||||||
| Piqueras 2015 | RCT‐ individual | Prescriptions | x | |||||||
| Quach 2015 | RCT‐ individual | Patients | x | |||||||
| Redwood 2013 | RCT‐ individual | Doctors | x | |||||||
| Schmader 2004 | RCT‐ individual | Patients | x | |||||||
| Schneider 2006 | RCT‐ individual | Opportunities for error by nurse | ||||||||
| Schnipper 2009 | RCT‐ cluster | Patients | ||||||||
| Schnipper 2018 | CITS | Patients | x | |||||||
| Scullin 2007 | RCT‐ individual | Patients | x | |||||||
| Seibert 2014 | ITS | Monthly administered doses | ||||||||
| SUREPILL 2015 | RCT‐ cluster | Patients | x | |||||||
| Tamblyn 2018 | RCT‐ cluster | Patients | x | |||||||
| Thompson 2018 | ITS | Monthly administered doses | ||||||||
| Tompson 2012 | RCT‐ individual | Patients | x | |||||||
| Tong 2016 | RCT‐ cluster | Patients | x | |||||||
| van Doormaal 2009 | ITS | Prescriptions (MO) Patients | x | |||||||
| Vega 2016 | RCT‐ individual | Patients | x | |||||||
| Wang 2017 | RCT‐ individual | Prescriptions | ||||||||
| Willoch 2012 | RCT‐ individual | Patients | x | |||||||
Appendix 6. Identified evidence mapping: Comparisons 9 to 16
| Comparison level ↓_______________Comparison # → | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Study designs | RCT ‐ ITS | ITS | ITS | RCT | RCT | RCT | RCT | RCT | ||
| Target population | Adults | Adults | Adults | Adults | Adults | Adults | Adults | Adults | ||
| Setting | RCT Hospital ‐ ITS Hospital/ED | Hospital | Hospital, Anesthesia units | ICU | Hospital | Hospital | Hospital | Hospital, Surgery units | ||
| Countries | RCT USA (3) ‐ ITS USA (2) | USA | Canada, USA (5) | USA | Australia (2), UK | Australia (2) | Australia (2), USA (2) | China (2), USA | ||
| Study level | Comparisons description | |||||||||
| Study ID | Study design | Unit of analysis | CPOE/CDSS: Improved vs standard CPOE/CDSS | CPOE/CDSS: prioritised vs no prioritised alerts | Barcoding vs no barcoding S1 No CPOE/CDSS S2: with CPOE/CDSS + CDSS | Organisational changes: reduced vs not reduced work hours | Feedback on prescribing errors vs no feedback | Feedback vs prescribing education | Education vs no education | Dispensing system vs control |
| Aag 2014 | RCT‐ individual | Patients | ||||||||
| Adelman 2013 | RCT‐ individual | Patients | x | |||||||
| Adelman 2019 | RCT‐ individual | Order session | ||||||||
| Agrawal 2009 | ITS | Unintended discrepancy per admission | x | |||||||
| Al‐Hashar 2018 | RCT‐ individual | Patients | ||||||||
| Barker 1984 | RCT‐ individual | Prescriptions | x | |||||||
| Becerra‐Camargo 2015 | RCT‐ individual | Patients | ||||||||
| Beckett 2012 | RCT‐ individual | Patients | ||||||||
| Bell 2015 | RCT‐ individual | Patients | ||||||||
| Bhakta 2019 | ITS | Weekly prescription | x | |||||||
| Bolas 2004 | RCT‐ individual | Patients | ||||||||
| Boockvar 2017 | RCT‐ cluster | Patients | ||||||||
| Bowdle 2018 | ITS | Weekly anaesthestized patients | x | |||||||
| Burkoski 2019 | ITS | Monthly medication doses administered | x | |||||||
| Cadman 2017 | RCT‐ individual | Patients/Unintended discrepancies | ||||||||
| Chiu 2018 | Quasi‐RCT | Patients | ||||||||
| Colpaert 2006 | RCT‐ individual | Prescription | ||||||||
| De winter 2011 | Quasi‐RCT individual | Patients | ||||||||
| Ding 2012 | RCT‐ cluster | Prescriptions | x | |||||||
| Farris 2014 | RCT‐ individual | Patients | ||||||||
| Fernandes 2011 | RCT‐ individual | Patients | ||||||||
| Furuya 2013 | ITS | Patient‐days | ||||||||
| George 2011 | RCT‐ individual | Patients | ||||||||
| Gordon 2017 | RCT‐ cluster | Prescriptions | x | |||||||
| Graabaek 2019 | RCT‐ individual | Patients | ||||||||
| Green 2015 | ITS | Prescriptions | x | |||||||
| Greengold 2003 | RCT‐ individual | Administered doses | x | |||||||
| Gursanscky 2018 | RCT‐ cluster | Prescriptions | x | x | x | |||||
| Hale 2013 | RCT‐ individual | Prescriptions | ||||||||
| Heselmans 2015 | RCT‐ individual | Patients/Prescriptions | ||||||||
| Hickman 2018 | RCT‐ individual | Prescriptions | ||||||||
| Higgins 2010 (Heelon) | ITS | Monthly administered doses | x | |||||||
| Juanes 2018 | RCT‐ individual | Patients | ||||||||
| Kannampallil 2018 | ITS | Order session | ||||||||
| Khalil 2016 | RCT‐ individual | Patients | ||||||||
| Kwan 2007 | RCT‐ individual | Patients | ||||||||
| Landrigan 2004 | RCT‐ cluster | Patients | x | |||||||
| Leung 2017 | RCT‐ individual | Prescriptions | x | x | x | |||||
| Lind 2017 | RCT‐ individual | Patients | ||||||||
| Marotti 2011 | RCT‐ individual | Patients | ||||||||
| McCoy 2012 | RCT‐ individual | Patients/Prescriptions | x | |||||||
| Merry 2011 | Quasi‐RCT | Patients | x | |||||||
| Narang 2013 | ITS | Probably monthly administered doses (it is unclear we cannot discard that were patients) | x | |||||||
| Nielsen 2017 | RCT‐ cluster | Patients | ||||||||
| Ongering 2019 | ITS | Prescription | ||||||||
| OSullivan 2016 | RCT‐ cluster | Patients | ||||||||
| Pevnick 2018 | RCT‐ individual | Patients | ||||||||
| Piqueras 2015 | RCT‐ individual | Prescriptions | ||||||||
| Quach 2015 | RCT‐ individual | Patients | ||||||||
| Redwood 2013 | RCT‐ individual | Doctors | ||||||||
| Schmader 2004 | RCT‐ individual | Patients | ||||||||
| Schneider 2006 | RCT‐ individual | Opportunities for error by nurse | x | |||||||
| Schnipper 2009 | RCT‐ cluster | Patients | x | |||||||
| Schnipper 2018 | CITS | Patients | ||||||||
| Scullin 2007 | RCT‐ individual | Patients | ||||||||
| Seibert 2014 | ITS | Monthly administered doses | x | |||||||
| SUREPILL 2015 | RCT‐ cluster | Patients | ||||||||
| Tamblyn 2018 | RCT‐ cluster | Patients | ||||||||
| Thompson 2018 | ITS | Monthly administered doses | x | |||||||
| Tompson 2012 | RCT‐ individual | Patients | ||||||||
| Tong 2016 | RCT‐ cluster | Patients | ||||||||
| van Doormaal 2009 | ITS | Prescriptions (MO) Patients | ||||||||
| Vega 2016 | RCT‐ individual | Patients | ||||||||
| Wang 2017 | RCT‐ individual | Prescriptions | x | |||||||
| Willoch 2012 | RCT‐ individual | Patients | ||||||||
Data and analyses
Comparison 1. Medication reconciliation versus no medication reconciliation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Medication errors | 3 | Odds Ratio (IV, Random, 95% CI) | 0.55 [0.17, 1.74] | |
| 1.2 ADEs | 3 | Odds Ratio (IV, Random, 95% CI) | 0.38 [0.18, 0.80] | |
| 1.3 Mortality during hospitalisation | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 3.85 [0.44, 33.89] |
| 1.4 Length of Stay (days) | 3 | 527 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.93, 1.33] |
| 1.5 QoL (VAS 0‐10 ‐ EQ‐5D‐3L ‐ high score better) | 1 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐10.04, 7.02] |
| 1.6 Discrepancy resolutions (per discrepancies at discharge) | 1 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 7.48 [5.62, 9.95] |
Comparison 2. Medication reconciliation: pharmacist versus other professionals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Medication errors | 8 | Odds Ratio (IV, Random, 95% CI) | 0.21 [0.09, 0.48] | |
| 2.2 ADEs | 3 | Odds Ratio (IV, Random, 95% CI) | 1.34 [0.73, 2.44] | |
| 2.3 Mortality during hospitalisation | 2 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.57, 1.73] |
| 2.4 Readmisson at 1 month | 2 | 997 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 2.5 Length of stay (days) | 6 | 3983 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.05, 0.56] |
| 2.5.1 General ward inpatients | 5 | 3383 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.09, 0.59] |
| 2.5.2 Inpatients coming from ICU | 1 | 600 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐6.71, 6.11] |
| 2.6 QoL (VAS 0‐10 ‐ EQ‐5D‐3L, high score is better) | 1 | 724 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐14.09, 14.09] |
| 2.7 Discrepancy resolution | 3 | Odds Ratio (IV, Random, 95% CI) | 4.80 [1.81, 12.76] |
Comparison 3. Medication reconciliation by pharmacist: database‐assisted versus not‐assisted.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Potential ADEs (≥1 per patient) | 2 | 3326 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.10, 0.64] |
| 3.2 Lenght of stay (days) | 1 | 311 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.17, 2.17] |
| 3.3 Discrepancy resolution (higher number is better) | 2 | Odds Ratio (IV, Random, 95% CI) | 1.37 [0.97, 1.93] |
Comparison 4. Medication reconciliation by trained pharmacist technicians versus by pharmacists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Medication errors | 2 | Odds Ratio (IV, Random, 95% CI) | 0.65 [0.25, 1.70] | |
| 4.2 Length of stay (days) | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.12, 1.52] |
Comparison 5. Medication reconciliation: before versus at admission.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Identified discrepancies per patient (higher number is better) | 1 | 307 | Mean Difference (IV, Random, 95% CI) | 1.27 [0.46, 2.08] |
Comparison 6. Medication reconciliation: 1 or 2 versus 4 charts open simultaneously.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Prescribing error (per order session) | 1 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.58, 0.20] |
Comparison 7. Medication reconciliation: multimodal intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Unintended discrepancies (≥1 per patient) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.87, 0.97] | |
| 7.2 Potential ADEs (≥ 1 per patient) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.86, 1.09] | |
| 7.3 Discrepancies resolutions (≥1 per patient, higher number is better) | 1 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.81, 2.53] |
Comparison 8. CPOE/CDSS versus control/paper‐based system.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Medication error | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.74 [0.31, 1.79] | |
| 8.2 ADEs | 2 | Odds Ratio (IV, Random, 95% CI) | 0.24 [0.04, 1.50] | |
| 8.3 Mortality | 1 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 2.01] |
| 8.4 Length of stay (days) | 1 | 737 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.05, 0.05] |
Comparison 9. CPOE/CDSS: improved versus standard CPOE/CDSS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Medication errors | 4 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9.1.1 RCTs | 2 | Odds Ratio (IV, Random, 95% CI) | 0.84 [0.73, 0.97] | |
| 9.1.2 ITSs | 2 | Odds Ratio (IV, Random, 95% CI) | 0.77 [0.37, 1.62] | |
| 9.2 ADEs | 2 | Odds Ratio (IV, Random, 95% CI) | 0.82 [0.71, 0.94] |
Comparison 10. CPOE/CDSS: prioritised versus no prioritised alerts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Resolved potential ADEs (per prescriptions, higher is better) | 1 | Mean Difference (IV, Random, 95% CI) | 1.98 [1.65, 2.31] |
Comparison 11. Barcoding versus no barcoding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Medication errors | 2 | Odds Ratio (IV, Random, 95% CI) | 0.69 [0.59, 0.79] |
Comparison 12. Organisational changes: reduced versus unreduced work hours.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Serious medication errors per patient‐days | 1 | 2203 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.09] |
Comparison 13. Feedback on prescribing errors versus no feedback.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Medication errors | 4 | Odds Ratio (IV, Random, 95% CI) | 0.47 [0.33, 0.67] |
Comparison 14. Feedback on prescribing errors versus education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Medication errors | 2 | Odds Ratio (IV, Random, 95% CI) | 0.59 [0.20, 1.76] |
Comparison 15. Education versus no education on prescribing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Medication errors | 4 | Odds Ratio (IV, Random, 95% CI) | 1.21 [0.93, 1.58] | |
| 15.1.1 Education on prescriptions (physicians) | 2 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.88, 1.39] | |
| 15.1.2 Education on administration (nurses) | 2 | Odds Ratio (IV, Random, 95% CI) | 1.64 [0.88, 3.08] |
Comparison 16. Dispensing system versus no dispensing system.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Medication errors | 4 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 16.1.1 Surgical wards | 2 | Odds Ratio (IV, Random, 95% CI) | 0.61 [0.47, 0.79] | |
| 16.1.2 Operating rooms | 2 | Odds Ratio (IV, Random, 95% CI) | 0.92 [0.75, 1.13] | |
| 16.2 Medication errors (per prescriptions) | 1 | Mean Difference (IV, Random, 95% CI) | ‐8.66 [‐12.77, ‐4.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aag 2014.
| Study characteristics | ||
| Methods |
RCT. Non‐blinded, two‐armed, randomised controlled trial conducted by the Department of Cardiology at the University Hospital of North Norway. An expert team comprising a ward resident in cardiology and two clinical pharmacists retrospectively rated the clinical relevance of the identified medications discrepancies (MDs) using the classification system for clinical relevance described by Scullin and colleagues (Scullin 2007), where 1 = no relevance to patient care, 2 = relevant but does not lead to an improvement in patient care, 3 = relevant and results in an improvement in the standard of care, 4 = very relevant and prevents major organ failure or adverse reaction of similar importance and 5 = potentially lifesaving [2]. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | People aged 18 years or more admitted to the ward during a five‐week period. IP adults (Department of Cardiology) (N = 206) Oncological patients (tertiary care centre) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: reconciliation: medication reconciliation (MR) performed by pharmacists Control: reconciliation: medication reconciliation performed by nurses |
|
| Outcomes | Mean errors (medications discrepancies) per patient Mean time spent during MR, minutes. |
|
| Notes | No funding information No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to PG or NG in a 1:1 relationship, block randomized in block sizes six to ten, and stratified on gender only" "An online randomization procedure was applied to randomize eligible patients in two groups: PG (clinical pharmacist performing MR) and NG (nurse performing MR)" (randomization service from the Norwegian University of Science and Technology. https://www.ntnu.no/dmf/akf/randomisering. Accessed 25 March 2014) |
| Allocation concealment (selection bias) | Low risk | An online randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded, two‐armed, randomised controlled trial. "The expert team was blinded to the patients' group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An expert team retrospectively rated the clinical relevance of the identified medications discrepancies (MDs) using the classification system for clinical relevance described by Scullin et al. "The expert team was blinded to the patients' group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is unlikely that missing data (PG 1% and NG 6%) had a great impact on outcomes. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | None detected |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Adelman 2013.
| Study characteristics | ||
| Methods |
RCT. "Understanding and preventing wrong‐patient electronic orders: a randomized controlled trial." After establishing the effectiveness of the measurement tool in phase 1, they performed a three‐armed randomised controlled concurrent trial to investigate the effectiveness of both interventions in preventing wrong‐patient electronic orders compared with controls. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | The research protocol was designed as a 2‐phase study within Montefiore Medical Center, an academic medical center in the Bronx, New York, consisting of three general hospitals and one children's hospital, 1500 inpatient beds, using a Centricity CPOE system (N not available) IP/OP adults (medical wards, ED, office) |
|
| Interventions | Intervention Technology Verification of order communication, Computerized Physician Order Entry (CPOE), Intervention: they developed to prevent wrong‐patient electronic orders: an ‘ID‐verify alert’ and an ‘ID‐reentry function’. The ID‐verify alert is triggered on opening the order entry screen, and displays the patient’s name, gender and age. Using a single click response, a provider must acknowledge they are ordering on the correct patient before they can proceed. The ID‐reentry function blocks access to the order entry screen until the provider actively re‐enters the patient’s initials, gender and age. Intervention 1: CPOE + ID‐verify alert. Passive intervention: when a user is about to place orders on a patient, a pop‐up alert will show the user the name, age, sex, room number and MR# of the patient who is currently activated. Intervention 2: CPOE + ID‐reentry function. Active intervention: the user will be required to enter the initials, age and sex of the activated patient prior to placing any orders. Control: CPOE with no intervention |
|
| Outcomes |
The unit of analysis was ordering session Prescribing errors per patient The primary endpoints of phase 1 included the proportion of retract‐and‐reorder events that were true positive wrong‐patient electronic orders based on the provider interviews, and the overall frequency of retract‐and‐reorder events. The primary endpoint of phase 2 was the proportion of ordering sessions that contained retract‐and‐reorder events as a marker for wrong‐patient electronic orders. |
|
| Notes | NCT01262053 Funding: this work was supported by institutional funds from Montefiore Medical Center, and in part by the CTSA grant UL1RR025750, KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH roadmap for medical research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk". |
| Allocation concealment (selection bias) | Unclear risk | Although it was not possible to blind the participants to their study group assignment, all data extraction, management, and analyses were carried out with study personnel unaware of study group assignment. All providers, including attending physicians, residents, physician assistants, registered nurses, nurse practitioners and pharmacists who placed orders on inpatients from 16 December 2010 to 17 June 2011 were randomly assigned always to receive either the ID‐verify alert, the ID‐reentry function, or no intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind the participants to their study group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All data extraction, management, and analyses were carried out with study personnel unaware of study group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. The automated retract‐and‐reorder tool provided the reliable data needed to power a large‐scale randomised controlled trial testing multiple interventions. |
| Selective reporting (reporting bias) | Low risk | All expected results are included. |
| Conflict of interest | Low risk | The authors declare that they have no conflicts of interest. |
| Other bias | High risk | Providers in the control group may have been educated to the importance of reverifying patient identification before placing orders by observing their colleagues in the intervention groups, potentially causing a contamination bias. |
Adelman 2019.
| Study characteristics | ||
| Methods |
RCT. Non‐blinded, two‐armed, randomised controlled trial. This randomised clinical trial was conducted from October 2015 to April 2017 at a large academic medical centre in New York to assess the risk of wrong‐patient electronic order errors in an EHR system configured to display only 1 vs a maximum of 4 patient records at once. Trial sites included 4 hospitals with a total of 1536 beds, 5 emergency departments (EDs), and 144 outpatient facilities. Unit of allocation: clinicians Unit of analysis: order session |
|
| Participants | This randomised trial included 3356 clinicians and 4,486,631 order sessions (N not available) IP/OP adults (medical wards, ED, office) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention1: EHR configuration limiting to 1 patient record open at a time Intervention2: EHR configuration allowing up to 4 patient records open concurrently |
|
| Outcomes | The primary outcome was order sessions that included 1 or more wrong‐patient orders identified by the Wrong‐Patient Retract‐and‐Reorder measure (an electronic query that identifies orders placed for a patient, retracted, and then reordered shortly thereafter by the same clinician for a different patient). | |
| Notes | NCT02876588 Funding/Support: this project was supported by grant R01HS023704 from the Agency for Healthcare Research and Quality. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All clinicians with the authority to place electronic orders were randomly assigned in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | The assignment method is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The expert team was blinded to patients' group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no blinding but it was not likely to affect the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unlikely that the cause of the missing outcome data is related to the true outcome. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | Not detected |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Agrawal 2009.
| Study characteristics | ||
| Methods |
ITS: interrupted time series study An electronic MedRecon system was designed and implemented in an acute inpatient care facility. Two analyses were performed: (1) one based on a 2‐week pilot evaluation of the system based on 120 MedRecon events, and (2) a more comprehensive 17‐month evaluation of the system, based on 19,356 MedRecon events. Unit of analysis: unintended discrepancy per admission |
|
| Participants | Kings County Hospital Center (KCHC), a member of the New York City Health and Hospitals Corporation, is a 630‐bed acute tertiary care academic facility providing inpatient, outpatient and emergency services. KCHC currently supports approximately 25,000 admissions, 750,000 outpatient visits, and 100,000 emergency room visits per year. The staff includes 640 attending physicians, 700 nurses, and 28 pharmacists, and
approximately 893 house officers rotate through various services (N = 19,356). IP adults (acute inpatient care facility) |
|
| Interventions | Intervention Technology, medication reconciliation, computerized Physician Order Entry (CPOE). Intervention: electronic health record (EHR), including CPOE + improved MR (MedRecon processes) Control: electronic health record (EHR), including CPOE The inpatient system incorporates MedRecon processes for all three stages: admission, transfer, and discharge. The admission MedRecon process involves three steps: 1) Comprehensive home medication history complementing the MedRecon application; 2) a physician documents the “intended action” for each medication in the MedRecon application by selecting one of these options: “continue,” “discontinue,” “substitute,” or “unable to verify”. This reconciliation documentation is then automatically routed to an electronic work queue for pharmacy; 3) a pharmacist performs reconciliation. If a discrepancy is found, the pharmacist categorises it and communicates with the provider to resolve any discrepancies found. |
|
| Outcomes | Prescribing errors per admission Total no. errors (including discrepancies) Discrepancy resolution |
|
| Notes | No funding information No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Unclear risk | No funding information |
| Other bias | Unclear risk | No information |
| Reliable primary outcome measure(s) | Low risk | Objective outcome |
| Blinded assessment of primary outcome(s) | Unclear risk | No description |
| Data were analysed appropriately | High risk | Chi2 analysis was used for comparisons of proportions. Patient, clinician, and environment‐of‐care characteristics were also analysed using logistic regression. These characteristics were entered into a logistic regression model, and adjusted odds ratios with 95% confidence intervals were calculated. All P values were 2‐sided, and a significance level of 0.05 was used. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Only one source |
| Completeness of data set | Low risk | More than 80% |
| Reason for the number of points pre‐ and post‐intervention given | Unclear risk | No description |
| Protection against secular changes | Unclear risk | No description |
| Shape of the intervention effect was specified | Unclear risk | No description |
Al‐Hashar 2018.
| Study characteristics | ||
| Methods |
RCT. Non‐blinded randomised controlled study with intention‐to‐treat analysis comparing standard care, which includes some degree of pharmacist involvement, to an approach featuring a more intensive pharmacist contribution.
The study was undertaken at Sultan Qaboos University Hospital, a tertiary care academic hospital in Oman with a bed capacity of 500. Patient recruitment took place from end of January 2014 to end of January 2015. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients were eligible for inclusion if they were ≥ 18 years old, admitted to medical wards, on at least one medication prior to admission, admitted for at least 24 h, had not been included in this study during a previous admission, and they or their caregiver spoke Arabic or English and could be interviewed for medication history (N = 622). Patients were excluded if they were: admitted under surgical specialties but then admitted to medical wards because of lack of beds in their respective wards; discharged on no chronic medication (a medication taken continuously for at least a month, i.e. the follow‐up period) and not otherwise on any chronic medication (whether in the current discharge prescription list or not); transferred/discharged to other specialties/hospitals; pregnant; or if they had length of stay (LOS) of more than 60 days or left against medical advice (LAMA). IP adults (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: medication reconciliation + identification of unintentional discrepancies + medical history Control: simple to moderate medication review The intervention consisted of several components: (1) Interviewing patients on admission to obtain medication history and identify counselling needs. (2) Identifying and resolving medication discrepancies (i.e. unexplained differences between medication orders and medication history). Discrepancies were judged to be unintentional after discussion with the prescriber, and efforts were made to reconcile those discrepancies. (3) Reviewing discharge medications: as with admission, medication discrepancies were identified and an attempt to reconcile them was made. (4) Dispensing and bringing discharge medications to the bedside and providing bedside counselling by a pharmacist while addressing any adherence concerns that were identified on admission. (5) Issuing a medication list with take‐home educational material if needed. Patients were informed that they would receive a phone call after 1 month to discuss their experience with their medications. Standard care included ward‐based pharmacist coverage in the form of a general medication review; that is, a simple to moderate medication review during admission and dispensing discharge medications at pharmacy window with basic instructions. All steps in each arm were carried out by the same pharmacist for all patients. |
|
| Outcomes | Percentage of preventable adverse drug events as primary outcome and healthcare resource utilisation as secondary outcome at 30 days post discharge (rates of readmission, emergency department (ED) visits, unplanned visits to hospitals or health centres, and the three healthcare resources combined). All outcome measures were identified at 30 days following discharge. | |
| Notes | NCT02805270 Funding: the work was funded by a doctoral grant provided by Sultan Qaboos University’s College of Medicine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer tables (#10) of 64 labels each were generated (using Stata statistical software), randomising patients into intervention (1) and standard care (0) groups. Labels were covered and opened only after obtaining patients' written consent and contact details. |
| Allocation concealment (selection bias) | Low risk | Individuals were then randomly assigned to either of two groups, the intervention or the standard care, using the sealed envelope method. Labels were covered and opened only after obtaining patients' written consent and contact details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients were contacted by a research assistant 30 days (+7 days) after discharge to enquire about their experience with the medications. The research assistant was masked to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data balanced in numbers across intervention (7%) and control (5%) groups, with similar reasons for missing data across groups, but it is unclear if the proportion of missing data had a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | Not detected |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Barker 1984.
| Study characteristics | ||
| Methods |
RCT ‐ individual. A crossover study design with random assignment of subjects and treatments was used. Unit of allocation: nurses Unit of analysis: prescriptions |
|
| Participants | The study was conducted in a 32‐bed general surgery unit of an 848‐bed acute‐care, not‐for‐profit general hospital in a large metropolitan area in the USA. It is a decentralised unit dose dispensing system with a single pharmacy satellite on each floor serving three different nursing units of comparable size. Two separate medication carts are provided for each unit (N = 1775). IP adults (surgical wards) |
|
| Interventions | Intervention Technology Prescribing and order communication systems. Intervention: Automated dispensing system that included the following components: a bedside dispenser with removable tray, a magnetic program card, and the pharmacy computer system. The bedside dispenser is a locked medication cabinet kept at the bedside of each patient. Control: no automated dispensing. The current medication system served as the control system. It is a decentralized unit dose dispensing system with a single pharmacy satellite on each floor serving three different nursing units of comparable size. Two separate medication carts provided for each unit. These are filled daily and also adjusted whenever changes (e.g. new orders) occur. Flow charts illustrating use of the medication dispensing system and the current (control) system are available from the authors. |
|
| Outcomes | Medical error % of total opportunities for error The dependent variable was the medication error rate. A medication error was defined as "a dose of medication that deviates from the physician's medication order on the patient's chart," and an error was viewed as an instance of failure of the medication system (as measured by its outcome). |
|
| Notes | No funding information No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All nurses were randomly assigned to work with either the experimental or control system beds during the first seven days and then were switched to the other system for the remaining seven days of the study period. |
| Allocation concealment (selection bias) | Unclear risk | "All nurses were randomly assigned to work with either the experimental or control system beds." But no information on the generation of the randomisation sequence is given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the participants (nurses) and study personnel was not possible given the intervention, and the outcome could be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | All data were collected by a single observer, who was a pharmacist trained and experienced in the observation technique. He accompanied each nurse during preparation of medications and witnessed the actual administration of each dose to each patient. He then reviewed the charts of the study patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not detected |
| Selective reporting (reporting bias) | Low risk | Prespecified (primary and secondary) outcomes that are of interest in the review have been reported. |
| Conflict of interest | Low risk | Not detected |
| Other bias | High risk | The investigators randomised the intervention to either the left or right side of a hallway, and randomised each nurse to work on the right or left side (i.e. intervention or control) for the first 7 days, and nurses switched to the other side of the hallway for the following 7 days (i.e. crossover design with each nurse serving as their own control). Patients were not randomised to beds, and it is hard to believe that the actual process of assigning beds mimicked random assignment (e.g. patients were segregated by sex). The outcome was measured as error rate, defined as the number of errors per opportunity. Crossover studies should be analysed with respect to treatment sequence (i.e. control‐treatment and treatment‐control), the design and analysis should consider clustering effects, there should be consideration given to wash‐in and wash‐out effects, and the specific analysis performed (t‐test) could definitely be improved upon. The result is likely to be at high risk of bias, and is likely to misstate precision. |
Becerra‐Camargo 2015.
| Study characteristics | ||
| Methods |
RCT. Multicentre, double‐blind, randomised, controlled parallel‐group study The study was conducted from 26 October to 30 November 30 2012 at 3 large teaching hospitals in Bogotá, Colombia. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | 270 patients who had been admitted to an ED were enrolled; each had a standardised, comprehensive MH interview, focusing on a patient’s current home medication regimen prior to being seen by a doctor (N = 270). IP adults (ED) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: the intervention consisted of a pharmacist acquiring patients’ medication histories in an ED prior to their being seen by a doctor. It focused on a patient’s current home medication regimen which was documented on an admission medication order form which was available for use by a doctor when consulting a patient in an ED. The admitting doctors verified the data with patients and indicated which home medications were to be reordered, suspended or discontinued. Control: standard of care. Control group patients received standard care; this included doctors documenting medication histories in admission notes and nurses reviewing medication orders for appropriateness. The admission medication order form was given to the doctors at a later stage for them to amend prescriptions made on admission. Pharmacists would not have been routinely involved in documenting patients’ medication histories on admission to the institutions involved in the present study; this function is primarily the admitting resident doctor or a medical student’s responsibility. |
|
| Outcomes | The intervention dealt with comparing the percentage of patients in the intervention and control groups having at least 1 potential adverse drug event (Potential ADE). A secondary outcome was recording the number of Potential ADEs per patient using Poisson regression analysis. | |
| Notes | Trial registration: 28/10/2012, ISRCTN63455839. MF provided mentorship for our research team and acquired funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to an intervention or standard care arm using computer‐generated random numbers (Microsoft Excel). |
| Allocation concealment (selection bias) | Low risk | The study population’s baseline demographic and clinical characteristics were similar. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Doctors who received patients were also randomly allocated; each randomisation manager made a daily allocation which depended on the number of doctors and residents per shift. A nurse (epidemiologist) at each site who was not involved in caring for the trial patients and independent of the site investigator was responsible for trial allocation and record‐keeping (i.e. the randomisation manager). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A patient’s current home medications were compared to medications prescribed 24 h after having been admitted to an ED to see whether a patient’s home medications had also been prescribed by a doctor in an ED. This was done by an independent team consisting of a pharmacist and a doctor blinded to intervention status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% incomplete data for intervention and 7% for control group |
| Selective reporting (reporting bias) | Low risk | Data were objective: primary outcome: having at least 1 Potential ADE |
| Conflict of interest | Low risk | The authors declare that they have no competing interests. |
| Other bias | Low risk | No other biases detected |
Beckett 2012.
| Study characteristics | ||
| Methods |
RCT. Non‐blinded, quasi‐randomised, controlled trial 1 general medicine floor or 1 general surgery floor during the study period (1 December 2009 through 31 March 2010). USA Staff: pharmacists Unit of allocation: patients Unit of analysis: patients |
|
| Participants | 81 geriatric patients > 70 years of age Elderly IP (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: pharmacist‐led medication reconciliation Control: medication reconciliation per current hospital practice, followed by additional quality assurance performed by a pharmacist at 48 hours after admission, to determine whether the original medication list was reconciled correctly and to allow for comparison to the intervention group. |
|
| Outcomes | The primary endpoint was medication profile appropriateness by pharmacist review at 48 hours post‐admission. Secondary endpoints involved determining the impact and feasibility of this program. | |
| Notes | The authors received no financial support for the research, authorship, and/or publication of this article. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All participants were randomly assigned to either control or pharmacist‐led medication reconciliation based on the last digit of their medical record number (i.e. control, odds; intervention, evens). |
| Allocation concealment (selection bias) | High risk | Not described, but based on the reported random sequence generation, it was likely not performed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was a non‐blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified |
| Conflict of interest | Low risk | The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bell 2016.
| Study characteristics | ||
| Methods |
RCT. The PILL‐CVD study was a randomised controlled trial conducted at two academic medical centers—Vanderbilt University Hospital (VUH) in Nashville and Brigham and Women’s Hospital (BWH) in Boston. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Adults hospitalised with a diagnosis of acute coronary syndrome (ACS) and acute decompensated heart failure (ADHF) (N = 851). IP adults with cardiovascular conditions (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: a tailored, pharmacist‐delivered intervention including medication reconciliation, inpatient counselling, low‐literacy adherence aids, and individualised telephone follow‐up after discharge. Control: usual care. At each hospital, the nurses, pharmacists, and physicians involved in the patients’ care performed medication reconciliation and counselling. Post‐discharge follow‐up calls were not routinely performed. |
|
| Outcomes | The aim of this study was to determine the effect of a tailored, pharmacist‐delivered, health literacy intervention on unplanned health care utilisation, including hospital readmission or emergency room (ER) visit, following discharge. The primary outcome was time to first unplanned health care event, defined as hospital readmission or an ER visit within 30 days of discharge. | |
| Notes | This study was funded by grants R01 HL989755 (SK), K23 HL077597 (SK), and K08 HL072806 (JS) 2K24 HL077506 (VV) from the National Heart, Lung, and Blood Institute. Dr. Bell is supported by K12HD043483‐11 from NIH/NICHD and by the Eisenstein Women’s Heart Fund. TRIAL NUMBER: NCT00632021 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to receive usual care or intervention in a 1‐to‐1 ratio. The randomisation sequence was computer‐generated in permuted blocks of 2 to 6 patients and was stratified by patient diagnosis and study site. Assignment was managed by a computer program that maintained concealment of treatment allocation and by one unblinded research coordinator at each site who did not play a role in outcome assessment. |
| Allocation concealment (selection bias) | Low risk | Assignment was managed by a computer program that maintained concealment of treatment allocation. To avoid biased enrollment, the order in which patients were approached to participate was randomised each day. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators, outcome assessors, and biostatisticians were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators, outcome assessors, and biostatisticians were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 189 individuals (97 intervention, 92 usual‐care) who reached the primary composite outcome of time to unplanned health care utilisation during the 30 days following discharge. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Low risk | Authors declare no potential conflicts of interest. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
Bhakta 2019.
| Study characteristics | ||
| Methods |
ITS study. This quasi‐experimental study evaluated the impact of a risk‐based systematic intervention designed to streamline medication‐related alerts and warnings. The University of Houston and Houston Methodist Hospital institutional review boards designated this study as exempt from their review as it did not involve human subjects. The study was performed at an academic, quaternary care institution in Texas between June 2016 and January 2018. The institution implemented a new EHR system with CPOE and CDS features in May 2016 and in January 2017 (intervention began on week 31), the medication‐related clinical decision support (MRCDS) committee made their first major interventions to suppress drug–drug interactions and duplicate therapy alerts within order sets built in the EHR. The study period included 29 weeks pre‐intervention and 52 weeks post‐intervention. Unit of analysis: weekly prescription |
|
| Participants | Inpatients from the Houston Methodist Hospital (N not available) IP adults (quaternary care centre) |
|
| Interventions | Intervention Technology, Prescribing and order communication systems (CPOE + CDSS) Control: the institution implemented a new EHR system with CPOE and CDSS features in May 2016 with commercial knowledge‐base support. During the order‐entry and verification processes, providers and pharmacists received unfiltered drug–drug interaction, drug allergy, dose, drug–inactive ingredient allergy, duplicate therapy, duplicate medication order, pregnancy, lactation, drug–disease interaction, i.v. incompatibility, and total parenteral nutrition alerts. Intervention: in January 2017, the drug–drug interactions and duplicate therapy alerts were suppressed within order sets built in the EHR system with CPOE and CDSS |
|
| Outcomes | The primary endpoint was weekly overall, modification, and acknowledgement rates of medication alerts after drug–drug interaction reclassification. Secondary endpoints included subanalysis of types of medication alerts (drug–drug interaction and duplicate therapy alerts) and alert use by providers (pharmacist and prescribers). Data was analysed using interrupted time‐series regression analysis. | |
| Notes | No financial support stated. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | The authors have declared no potential conflicts of interest. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Reliable primary outcome measure(s) | Low risk | The institution implemented a new EHR system with CPOE and CDS features in May 2016 with commercial knowledge‐base support. Alert modifications were defined as alert actions that directly led to the discontinuation of an offending medication order as a result of the medication alert. |
| Blinded assessment of primary outcome(s) | Low risk | The institution implemented a new EHR system with CPOE and CDS features in May 2016 with commercial knowledge‐base support. |
| Data were analysed appropriately | Low risk | Interrupted time‐series regression analysis was used to assess both primary and secondary endpoints over the study period. Autocorrelation was assessed using the Durbin‐Watson statistic, and positive autocorrelation was evaluated through autoregressive modelling. All statistical analyses were performed using the statistical software package STATA, version 15 (StataCorp, College Station, TX). A P value of < 0.05 was considered significant for all endpoints evaluated. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention. |
| Completeness of data set | Low risk | Data were obtained by the system. |
| Reason for the number of points pre‐ and post‐intervention given | Unclear risk | Not described; there is no rationale presented for the number of data points. |
| Protection against secular changes | Low risk | Changes in other outcomes, such as a decrease in the number of alerts while modified alerts increased, in some ways reduce the possibility of secular changes affecting the estimation. The strength of the multidisciplinary committee that included dedicated IT support allowed the committee to overcome these hurdles and react to unanticipated findings when they arose. |
| Shape of the intervention effect was specified | Unclear risk | Not described |
Bolas 2004.
| Study characteristics | ||
| Methods |
RCT ‐ individual. Randomised controlled clinical trial. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | People were considered suitable for inclusion if they were aged 55 years or over, receiving more than 3 drugs, and had been admitted to the medical unit of a district general hospital in Northern Ireland (N = 162). IP adults (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation, clinical pharmacy services. Intervention: patients received an enhanced service involving the community liaison pharmacist. Interventions made by this pharmacist include an intensive clinical pharmacy service to the study patients including management of Pharmacist On Demand Services (PODs) and patient counselling to explain changes to therapy and at discharge. The inpatient interventions were: a full medication history was taken by comparing the GP referral letter, the initial inpatient prescription, the GP surgery record, the community pharmacy PMR, the patient's own drugs brought into hospital and the patient or carer as sources of information; unintentional discrepancies were recorded; daily contact with the patient to explain changes made to their treatment as they happened and preparation of the discharge letter Control: patients received the standard clinical pharmacy service, which at the time of study, did not include discharge counselling. |
|
| Outcomes | Average no. of medication changes during hospital stay. | |
| Notes | Financial support from the DHSSPS Primary Care Development Fund (Northern Ireland). No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Allocation concealment (selection bias) | Low risk | Patients were randomised into study or control group by allocation of a computer‐generated random number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of 'Low risk' or 'High risk'. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of 'Low risk' or 'High risk'. |
| Conflict of interest | Low risk | Financial support from the DHSSPS Primary Care Development Fund (N. Ireland). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Boockvar 2017.
| Study characteristics | ||
| Methods |
RCT‐Cluster. Cluster‐randomised controlled trial aimed to determine the effect of real‐time health information exchanges (HIEs) on medication reconciliation in hospitalised patients at a US Department of Veterans Affairs (VA) hospital that is an early adopter of HIE. Patients admitted to 1 of the 4 inpatient units at the James J Peters VA Medical Center (JJP VA), Bronx, NY, USA. between 25 January 2012 and 25 August 2014, were screened for study enrolment. For primary outcome, we used generalised linear models (SAS Inc., Cary, NC, USA) and generated robust variance estimates to account for within‐provider correlations, since some providers had more than 1 patient. Similar models were estimated for secondary outcome measures (e.g. MAI). Multivariable logistic regression was used for the outcome of ADE (yes/no). Main models were intention‐to‐treat models. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients were eligible if they used non‐VA health care services in the last 2 years, as indicated by an identity match in the Bronx Regional Health Information Organization (RHIO) system, a regional HIE. Identity matching in the HIE was based on name and birth date. Patients were excluded if they were admitted to an intensive care unit, were transferred to a study unit from a non‐study unit, or did not remain in the hospital at least 24 hours (N = 387). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Technology, medication reconciliation. Intervention: patients admitted to an urban hospital received structured medication reconciliation by a pharmacist with access to regional health information exchanges (HIEs) that combine multiple medication sources. Control: usual care without access to HIEs. For patients assigned to usual care, the intervention pharmacist performed the structured medication reconciliation protocol but without access to the Bronx RHIO HIE. |
|
| Outcomes | The primary endpoint was discrepancies between pre‐admission and inpatient medication regimens, and secondary endpoints included adverse drug events (ADEs) and proportions of rectified discrepancies. | |
| Notes | NCT01239121 Financial support for the study was provided by the US Department of Veterans Affairs Health Services Research and Development Service (grant no. IIR‐10‐146). This work was supported with resources and the use of facilities at the James J Peters VA Medical Center, Bronx, NY, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process not described. Patients were assigned to intervention or control according to the unit to which they were admitted. At study start, 2 units were randomly assigned to intervention and 2 to control. Subsequently, units crossed over between intervention and control every 3 months, such that 2 of the 4 units were always intervention units and 2 were control units. |
| Allocation concealment (selection bias) | Low risk | Admitted patients were recruited on business days by a research assistant who was blinded to study hypotheses and group assignment. Patients admitted on non‐business days were recruited the next business day. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To preserve blinding of the house staff and the outcomes assessors, the intervention pharmacist did not indicate in his medication reconciliation note whether he had accessed the Bronx RHIO HIE. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors were 5 research pharmacists who were separate from the intervention pharmacist and were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The publication include all the expected results, reported in NCT01239121 |
| Conflict of interest | Low risk | All authors have no competing interests to declare. The study sponsor and the Bronx RHIO had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bowdle 2018.
| Study characteristics | ||
| Methods |
ITS study. Facilitated self‐reporting of errors was carried out in 2002‐2003. Subsequently, a medication safety bundle, including ‘smart’ infusion pumps, was implemented. During 2014, facilitated self‐reporting commenced again. A barcode‐based medication safety system was then implemented and the facilitated self‐reporting was continued through 2015. Unit of analysis: weekly anaesthetised patients |
|
| Participants | Anaesthesia service from the University of Washington Medical Center. Anaesthesia care was provided using the anaesthesia care team model, including attending anaesthesiologists, nurse anaesthetists, residents, and fellows (N = 50,545). IP adults (surgical wards) |
|
| Interventions | Intervention Technology V+A: Verification (V) +Administration (A) A1 Barcoding Control: after implementation of a computerised Anaesthesia Information Management System (AIMS) and a computerised decision support system (CDSS) software tool (Smart Anaesthesia Manager; SAM), we reinstituted the medication error survey in February 2014 as a computerised reporting form that must be completed in order to close the anaesthesia record (a so‐called ‘hard stop’). The computerised form looks different from the preceding paper form but seeks to collect essentially the same information. Intervention: In November 2014, after 10 months of computerised medication error data collection, a previously described barcode‐based medication safety system was implemented, and data collection was continued for another 13 months, through December 2015. (At the time of medication preparation, the Codonics vial barcode scanner reads the barcode on a medication vial, speaks the name of the medication, displays the name of the medication on a splash screen, and prints a syringe label that is compliant with international and local standards for syringe labels. |
|
| Outcomes | "We utilised facilitated self‐reporting of anaesthesia medication administration errors to compare the rates of errors before and after implementation of a medication safety bundle including ‘smart’ infusion pumps with built‐in medication libraries, and a barcode‐based medication safety system....Medication administration errors were classified using the original system devised by Webster and colleagues [...] with several modifications. The results are expressed as the rate of cases with an error reported per 100 cases (%) (i.e. number of cases with a reported error divided by the total numbers of cases x 100). An intercepted error (near misses) was defined as any incident with the potential to become an error." | |
| Notes | No financial support stated. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | High risk | AFM is a director and shareholder in Safer Sleep LLC (that uses barcode technology) and is a consultant to Fisher and Paykel Healthcare. CSW is a shareholder in Safer Sleep LLC. The other authors have no interests to declare. |
| Other bias | Unclear risk | No information |
| Reliable primary outcome measure(s) | Unclear risk | Medication error survey as computerised reporting form |
| Blinded assessment of primary outcome(s) | Unclear risk | Not described |
| Data were analysed appropriately | High risk | A two‐sample test of proportion was used to compare the incidence of error or intercepted error before and after implementation of smart infusion pumps (2002‐2003 vs 2014) and a barcode‐based medication safety system (2014 vs 2014‐2015). To account for multiple comparisons, the HolmeBonferroni method was applied to the six primary analyses which compared errors, intercepted errors and the sum of errors and intercepted errors in 2002‐2003 vs 2014 and 2014 vs 2014‐2015. Secondary outcomes were also evaluated using a two‐sample test of proportion. All statistical comparisons were performed using STATA version 11.0 (StataCorp LP, College Station, TX, USA). A control (Shewhart) chart showing the biweekly incidence of error during the three phases of the study served as a secondary form of statistical analysis. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention. |
| Completeness of data set | Low risk | During the period from February 2014 through November 2014, 14,572 computerised medication error survey forms were completed; the response rate was 100% because the anaesthetic record cannot be closed without completion of the medication error form. |
| Reason for the number of points pre‐ and post‐intervention given | Unclear risk | Not well described. |
| Protection against secular changes | Unclear risk | "We cannot exclude the possibility that there was a decline in reporting of medication errors over time, although we have no particular reason to suspect that this occurred." |
| Shape of the intervention effect was specified | Unclear risk | Not described |
Burkoski 2019.
| Study characteristics | ||
| Methods |
ITS study. The aim of this study was to evaluate the effects of barcode medication administration (BCMA) and the closed‐loop medication system (CLMS) interventions on medication errors and adverse drug event (ADE) rates. An autoregressive integrated moving average model for interrupted time series design was used to evaluate the impact of the BCMA and CLMS interventions on the monthly reported medication error and ADE rates at the HRRH Network and HRH sites between September 2013 and August 2018. Descriptive statistics were generated to evaluate the types of error and their gravity. Unit of analysis: monthly medication doses adminstered |
|
| Participants | Inpatients and staff (physicians, pharmacists, nurses) of the hospital (N not available) IP adults (community care hospitals) |
|
| Interventions | Intervention Technology Highly automated systems: Verification +Administration. Bar‐coding + electronic medication management system. Intervention: training in the use of barcode medication administration (BCMA) technology was provided to all nurses and other healthcare professionals (as required) at the HRRH Network sites prior to implementation. The closed‐loop medication system (CLMS) technology provides an end‐to‐end, safe and efficient electronic medication management system across the full cycle of the medication ordering to administration processes. CLMS was then rolled out over four months between May and August 2014. Training in CLMS technology was provided to all nurses and involved hospital staff prior to the relocation of the HRRH Network sites to the HRH site in October 2015. 05/01/2014 (started BMCA) 10/01/2015 (started CLMS) Control 1: no barcoding, no electronic medication management system Control 2: no electronic medication management system |
|
| Outcomes | A retrospective audit of self‐reported incidence of patient‐related medication errors and ADEs submitted through the hospital’s EMR into an electronic database was conducted over a five‐year period between September 2013 and August 2018. The system is used to report any medication errors and ADEs that caused or had the potential to cause patient harm whether they were preventable or non‐preventable. The main outcome measure was the monthly reported medication error and ADE rate, which was calculated by dividing the total number of reported medication errors and ADEs per month by the number of medication doses administered that month. The monthly number of doses administered was obtained from electronic pharmacy records. Information regarding incident classification (e.g. wrong dose, known medication allergy, etc.) and severity of harm (e.g. no harm, moderate harm) were also extracted from the reporting database. | |
| Notes | No financial support stated. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Unclear risk | No financial support stated. |
| Other bias | Unclear risk | No information |
| Reliable primary outcome measure(s) | Low risk | A retrospective audit of self‐reported incidence of patient‐related medication errors and ADEs submitted through the hospital’s EMR into an electronic database was conducted over a 5‐year period between September 2013 and August 2018. The system is used to report any medication errors and ADEs that caused or had the potential to cause patient harm whether they were preventable or non‐preventable. |
| Blinded assessment of primary outcome(s) | Low risk | A retrospective audit of self‐reported incidence of patient‐related medication errors and ADEs submitted through the hospital’s EMR into an electronic database was conducted over a 5‐year period between September 2013 and August 2018. The system is used to report any medication errors and ADEs that caused or had the potential to cause patient harm whether they were preventable or non‐preventable. |
| Data were analysed appropriately | Low risk | To evaluate the effects of the BCMA and CLMS interventions on the reported medication error and ADE rate, interrupted time series (ITS) analysis was performed using the autoregressive integrated moving average (ARIMA) model |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention. |
| Completeness of data set | Low risk | Data were obtained by the system. |
| Reason for the number of points pre‐ and post‐intervention given | Low risk | No reason presented, but there were a total of 56 monthly intervals, providing 8 pre‐intervention, 13 post‐BCMA intervention and 35 post‐CLMS intervention data points. ITS analysis was used to estimate the changes in level and trend following each intervention. Ljung‐Box Q fit statistic and visual inspection of autocorrelation (ACF) and partial autocorrelation (PACF) plots were used to assess for autocorrelation, seasonality and stationarity. Ljung‐Box Q fit statistic and visual inspection of the ACF and PACF plots did not indicate the presence of autocorrelation. Examination of the series ACF plot for cyclical or periodic fluctuations at four, six and 12 lags indicated that seasonality was absent. Lastly, the ACF patterns show a clear exponential decay indicative of stationarity. Therefore, adjustments to and transformation of the data were not necessary. |
| Protection against secular changes | Unclear risk | Not described and it is a long period, but there were a total of 56 monthly intervals, providing 8 pre‐intervention, 13 post‐BCMA intervention and 35 post‐CLMS intervention data points. ITS analysis was used to estimate the changes in level and trend following each intervention. Ljung‐Box Q fit statistic and visual inspection of autocorrelation (ACF) and partial autocorrelation (PACF) plots were used to assess for autocorrelation, seasonality and stationarity. Ljung‐Box Q fit statistic and visual inspection of the ACF and PACF plots did not indicate the presence of autocorrelation. Examination of the series ACF plot for cyclical or periodic fluctuations at four, six and 12 lags indicated that seasonality was absent. Lastly, the ACF patterns show a clear exponential decay indicative of stationarity. Therefore, adjustments to and transformation of the data were not necessary. |
| Shape of the intervention effect was specified | Unclear risk | Not specified |
Cadman 2017.
| Study characteristics | ||
| Methods |
RCT. Randomised controlled pilot study undertaken at Cambridge University Hospitals NHS Foundation Trust (CUHFT) on five adult medical wards from a range of medical specialities where patients did not routinely receive medication reconciliation (MR) from a pharmacist within 24 hours of admission. One similar ward was identified as a ‘backup’, in the eventuality that one of the study wards was closed for any reason (e.g. norovirus outbreak) during the recruitment period. Recruitment took place between July 2012 and April 2013 (9 months and 2 weeks), resulting in a recruitment rate of 5.2 patients per 7 days. Unit of allocation: patients Unit of analysis: patients/unintended discrepancies |
|
| Participants | Patients were recruited based on the following inclusion and exclusion criteria: adult ( ≥ 18 years of age); admitted with at least one prescribed medicine to one of the five medical wards; patient had not already received MR from the pharmacy team as part of routine pharmaceutical input at the time of recruitment; identified from hospital computer system as having been admitted straight from the ED to one of the five participating wards within the previous 24 hours. (N = 198). IP adults (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: a standard operating procedure (SOP) based on hospital guidelines was used to deliver MR by a trained MR pharmacist (MRP) within 24 hours of admission (including weekends) and at the point of transfer of care out of hospital, or as soon as possible following patient discharge from hospital to the next care provider. The five MRPs, all clinical pharmacists employed within the hospital, covered for each other's holidays, sick leave and absences wherever possible. MRPs recorded all unintentional discrepancies (UDs), defined as differences between patient records with no identifiable rationale, they identified between the information they collated and the inpatient medication chart on admission and again any differences between the inpatient chart and discharge letter. MRPs followed up on all identified UDs to ensure that they were addressed prior to discharge. To enhance intervention fidelity, all MRPs were observed by the principal investigator on at least three occasions to confirm adherence to the SOP. All MRPs had provided MR to more than 30 patients in the year previous to delivering the intervention for the trial. Control: patients in the control arm received usual care which may or may not consist of MR and where it was provided it may not have occurred within 24 hours and could either be delivered by a pharmacist or pharmacy technician. The MRPs within the intervention arm did not deliver MR to control patients and the SOP used for study intervention purposes was not automatically followed within the control arm. For the purposes of the study, all MR details regarding interventions undertaken within the control arm were recorded and costed. |
|
| Outcomes | Although undertaken as a pilot study with study aims to identify the most suitable outcome measure, length of stay (LOS) was nominally selected as the primary outcome measure for this pilot trial. Secondary outcome measures were unplanned (emergency) readmission at 3 months, quality of life (EQ‐5D‐3L) and unintentional discrepancies (UDs). | |
| Notes | Trial registration number: ISRCTN23949491. This article presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0110‐20116). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using the Norwich Clinical Trials Unit automated service with patients stratified by ward. When wards were later closed for infection control reasons, participants on the ‘backup’ ward were randomised and stratified as if they had entered the closed ward. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using the Norwich Clinical Trials Unit automated service with patients stratified by ward. When wards were later closed for infection control reasons, participants on the ‘backup’ ward were randomised and stratified as if they had entered the closed ward. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was a non‐blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | When identifying UDs, we have assumed that the MRP‐generated list in the intervention arm and the research assistant (RA)‐generated list in the control arm were accurate. Both are unrealistic assumptions. The unblinded identification of MRs and inability to confirm intentional or unintentional nature of errors in many instances also means that the data on UDs must be treated with further caution. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome across intervention (1%) and control (0%) groups had no relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | The publication include all the expected results, reported in ISRCTN2394949. |
| Conflict of interest | Low risk | The author(s) declared no competing interests. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Chiu 2018.
| Study characteristics | ||
| Methods |
Quasi‐RCT Quasi‐randomised controlled study conducted in the geriatric unit of a regional hospital in Hong Kong. All patients admitted to the unit during December 2013 to September 2014 were included. The allocation was done according to the day of admission. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | People aged 65 years or above who were transferred from an acute hospital after initial stabilisation of medical and/or geriatric problems. Patients were excluded if they refused to participate, were terminally ill with a life expectancy of less than 3 months, or if they had already received pharmacist intervention in another hospital prior to this admission (N = 212). Elderly IP (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: the intervention was conducted by a pharmacist who was present in the unit from Monday to Saturday. The pharmacist provided pharmaceutical care from admission to discharge. Interventions performed by the pharmacist consisted of the following: (1) medication reconciliation on admission to identify unintended discrepancies; (2) medication review to check for medication appropriateness on admission and also at discharge; (3) pharmacist counselling on admission and also at discharge was provided to improve patients’ drug knowledge to ensure proper use of drugs and compliance after discharge. Control: the control group received routine clinical services. |
|
| Outcomes | The primary outcome measure was the appropriateness of prescription as measured by the MAI. Secondary outcomes included the acceptance rate by physicians, number of subjects with unintended discrepancies, patient satisfaction with the programme (for those home‐living only), and unplanned hospitalisations 1 and 3 months after discharge. | |
| Notes | No financial support stated. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Eligible subjects were assigned to an intervention or control group according to the admission day of the week. Those who were admitted on Monday through Thursday were assigned to the intervention group, and those admitted on Friday through Sunday to the control group. |
| Allocation concealment (selection bias) | High risk | Eligible subjects were assigned to an intervention or control group according to the admission day of the week. Those who were admitted on Monday through Thursday were assigned to the intervention group, and those admitted on Friday through Sunday to the control group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The pharmacist who carried out the review and data extraction was not blinded to the study hypothesis and the group status of the subjects. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The pharmacist who carried out the review and data extraction was not blinded to the study hypothesis and the group status of the subjects. Records of the control group were retrospectively reviewed by the pharmacist after patient discharge to check for medication appropriateness on admission and also at discharge. This could potentially lead to information bias, although this might be partially offset by the fact that the majority of the information or data on the outcome measures were taken with reference to a well‐established and validated tool. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The low loss of data at discharge does not seem to be influential. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | All authors have disclosed no conflicts of interest. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Colpaert 2006.
| Study characteristics | ||
| Methods |
RCT ‐ individual. A prospective, controlled trial was conducted in two paper‐based units (PBUs; total of 14 beds (8 + 6)) versus one computerised unit (CU; 8 beds), 10 months after implementation of the intensive care information system (ICIS) in the latter unit. The objective of this study was to evaluate and compare the incidence and severity of medication prescribing errors (MPEs) between this CPOE unit and paper‐based units. Unit of allocation: patients Unit of analysis: prescriptions |
|
| Participants | 22‐bed ICU of a tertiary university hospital, Centricity Critical Care Clinisoft (N = 90) IP adults (ICU) |
|
| Interventions | Intervention Technology Prescribing and order communication systems + Intensive care information system (ICIS). Intervention: an intensive care information system (ICIS); that is, a computerised system specifically designed for the ICU that combines CPOE and a moderate level of CDSS. Control: paper‐based unit. |
|
| Outcomes | Prescribing errors Serious prescribing errors (potential to cause, or actually causing patient harm) The primary outcome measure was the difference in incidence and severity of medication prescribing errors (MPEs) in the CU versus the PBU. Secondary endpoints were univariate correlations between patient characteristics (APACHE II, renal failure, number of drug prescriptions (at screening day) and the number of MPEs. |
|
| Notes | No financial support stated. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to either of these units by an independent nurse. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent panel, consisting of one clinical pharmacist, not involved in the registration part of the study, and two intensive care specialists, evaluated independently the severity of MPEs at least one month after screening. The panel was blinded for specific patient Grabar characteristics, as well as for patient group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up was reported. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Low risk | Financial support not described |
| Other bias | High risk | Patients were randomly assigned to units, where there were two units for one arm and one unit for the other arm — i.e. patients were randomly assigned to study arms. Medical staff moved between the units (arms) on a one‐week basis. The outcome was measured as errors per prescription. There are possible clustering effects (e.g. of prescription within patient, and patient within unit). These possible effects are not accounted for by the analysis used. |
De Winter 2011.
| Study characteristics | ||
| Methods | Quasi‐randomised trial This prospective study enrolled adults presenting to a tertiary care emergency department. In the control group, medication histories were conducted by physicians of general internal medicine in conformity with standard care. In the intervention group, the physicians were obliged to use, besides the standard care, the ‘limited questions list’ for medication history acquisition. The clinical pharmacist re‐obtained medication histories of the patients in both groups using a standardised approach. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | The study was conducted at the ED of a 1900‐bed, tertiary care teaching hospital. The ED admits around 150 patients per day, totaling up to 55,000 patient visits per year. Approximately 30% of patients are hospitalised. About 10,000 of the 55,000 patients are treated in the ED by the division of general internal medicine (GIM) and 45% of these cases are admitted to the hospital. Adult patients ( > 16 years old) who are brought in for medical problems (non‐trauma patients) and who are not referred to a specific department (N = 260). IP/OP adults (ED) |
|
| Interventions |
Intervention: a clinical pharmacist and a pharmacy technician are attached to the ED from 8.30 a.m. up to 17 p.m. during the week. One of the pharmacy services is medication reconciliation. A structured form, containing a checklist, a table and a standardised list of questions, is used to guide the pharmacy staff to ensure a standardised approach. Control: medication histories were conducted by physicians of general internal medicine in conformity with standard care. |
|
| Outcomes | The primary endpoint was to evaluate if drug omission rate decreased when a simple list of limited questions was used during anamnesis. The secondary objective was to demonstrate the clinical impact of the tool by describing the difference in omitted drug classes in both study arms. | |
| Notes | Funded by the Health Department of the Belgian government as part of a national project on implementation of clinical pharmacy in hospitals. No trial number. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Consecutive patients were included in the standard care group or in the intervention group if they were admitted to the ED by a physician of GIM, between 16 p.m. and 11 a.m. and hospitalised. A computer‐generated admission roster, which is daily reviewed by the admitting team, was used to identify the patients. |
| Allocation concealment (selection bias) | High risk | Using an open random allocation schedule. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The pharmacist was not blinded to the results of the ‘limited questions list’, as obtained by the physician. The physicians were not explicitly informed about the objective of this study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 151 patients were excluded in the intervention group as the ‘limited questions list’ was not solicited in these patients. Reasons may have included diagnostic and treatment priorities that prohibited gathering a detailed medication history and additionally, the patient may not have been capable of providing an accurate history upon admission. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Low risk | All authors have declared no conflict of interest. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
Ding 2012.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. Cluster‐randomised control‐experimental design in a general surgery patient ward in a tertiary hospital in Beijing. Unit of allocation: nurses Unit of analysis: prescriptions |
|
| Participants | Medication nurses and pharmacists in the chosen patient wards (N not available) IP adults (surgical wards) |
|
| Interventions | Intervention Technology Dispensing systems (for "processing" of the order). Automated dispensing Intervention: the Unit Dose Dispensing System, which was installed in the experimental group. The Unit Dose Dispensing System was installed only on TPN (total parenteral nutrition) doses. The data analysis was limited to TPN doses. Control: hand‐written patient charts were the primary method of prescription. |
|
| Outcomes | Total no. errors (including discrepancies) The ultimate outcome measure of the medication use system from the patient’s perspective is the rate of errors which reach the patient at the point of administration. |
|
| Notes | No financial support stated. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The clusters of doses for two units with 29 beds in one unit and 24 beds in the other unit on the general surgery ward were randomly assigned to the control group or experimental group by flipping a coin. |
| Allocation concealment (selection bias) | High risk | The clusters of doses for two units with 29 beds in one unit and 24 beds in the other unit on the general surgery ward were randomly assigned to the control group or experimental group by flipping a coin. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participating nurses were informed that their normal medication preparation and administration processes would be observed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The direct observation method was used to detect and measure medication errors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The Principal Investigator excluded 7 doses (pre‐test) and 11 doses (post‐test) from the TOEs in the control group because they did not meet a priori operational definitions. A final total of 517 ordered doses plus 4 unordered doses were analysed for the statistical analysis in the control group, 41.7% of the total prescribed TPN doses. The Principal Investigator excluded 14 doses (post‐test) from the TOEs because they did not meet a priori operational definitions. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was not available. |
| Conflict of interest | Unclear risk | The authors did not address this issue. |
| Other bias | High risk | This is a cluster‐RCT with two clusters, where each cluster is randomly assigned. The authors argued that there cannot be any cluster effects because nurses worked across the two clusters. However, this argument does not consider any other factors that might have cluster‐level effects (e.g. unobsevable variables that might differ between the clusters), so it is unconvincing. It is also not immediately clear whether within‐patient clustering is possible. |
Farris 2014.
| Study characteristics | ||
| Methods |
RCT. Randomised, controlled trial of 945 participants assigned to enhanced, minimal and usual care groups conducted 2007 to 2012. To test if continuity of pharmacy care, including increased communication between inpatient and outpatient settings, will improve the appropriateness of medication therapy and reduce the number of serious adverse drug events, hospitalisations and unscheduled office visits in vulnerable patients with cardiovascular disease, pulmonary disease or diabetes. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Participants were recruited from general medicine, family medicine, cardiology or orthopedics. The inclusion criteria were: English or Spanish speaker; 18 years or older; admitted with diagnosis of hypertension, hyperlipidemia, heart failure, coronary artery disease, myocardial infarction, stroke, transient ischaemic attack, asthma, chronic obstructive pulmonary disease or receiving oral anticoagulation. These conditions were focused on in this study because of previous work completed among patients with cardiovascular conditions where pharmacists had impacted their clinical outcomes. Individuals were excluded if they were admitted to psychiatry, surgery or hematology/oncology service, could not use a telephone, had life expectancy < 6 months, had dementia or cognitive impairment or had a severe psychiatric diagnosis (N = 945). IP adults with cardiovascular conditions (medical wards) |
|
| Interventions | Participants in the enhanced intervention group received medication reconciliation, pharmacist visits every 2 to 3 days for patient education during inpatient stay, discharge counselling and discharge medication list, plus a telephone call at 3 to 5 days post‐discharge and primary care physician and community pharmacist received a discharge care plan focused on medication changes and recommendations. The care plan was faxed to the primary care physician and community pharmacist within 24 hours of discharge but usually within 6 hours. The care plan included the discharge medication list, plans for dosage adjustments and monitoring, recommendations for preventing adverse drug events, with patient specific concerns such as adherence or cost issues highlighted. Minimal intervention group patients were seen by a clinical pharmacist in the hospital but did not receive follow‐up after hospital discharge. Enhanced intervention patients received care from a clinical pharmacist during hospitalisation and follow‐up by phone after hospitalisation. Control arm patients were not seen by the clinical pharmacist. |
|
| Outcomes | Primary outcomes at 30 and 90 days after hospital discharge:
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were typically enrolled into the study within 1 day after admission and randomised to study group using the statistician‐generated blinded randomisation scheme with sequentially numbered envelopes. |
| Allocation concealment (selection bias) | Low risk | Participants were typically enrolled into the study within 1 day after admission and randomised to study group using the statistician‐generated blinded randomisation scheme with sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded research staff collect the data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Conflict of interest | Low risk | The author(s) declared no competiting interests. |
| Other bias | High risk | This study has limitations. At baseline, forgetting medications was not well randomised. Yet, it is unlikely that this single aspect of medication management would change the impact of the intervention on medication appropriateness or adverse events to a great degree across the three study groups. The intervention fidelity was good but not without some issues. We cannot separate the effect of any specific component of the intervention, such as patient counselling, on the outcomes of the study. |
Fernandes 2011.
| Study characteristics | ||
| Methods |
RCT ‐ individual. Prospective, dual‐centre RCT with blinded independent observer assessments conducted to determine whether clinician access to medication‐related information from the Drug Profile Viewer (DPV) System in a surgical pre‐admission clinic, as part of a structured best possible medication history (BPMH) and multidisciplinary medication reconciliation process, would reduce the number of patients with at least one unintentional BPMH medication. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Surgical pre‐admission clinics of two tertiary care teaching hospitals. The targeted clinics already employed a pro‐active, sustained inter‐professional medication reconciliation model in which a structured best possible medication history (BPMH) is taken prior to writing admissions orders. Participants: all consecutive elective patients, at least 65 years old, who had a surgical pre‐admission clinic visit prior to undergoing surgical procedures. Patients were excluded if they were scheduled for discharge on the same day of surgery, from out of province (information not contained in DPV), or had remote telehealth pre‐admission assessments. The surgical pre‐admission best possible medication history (BPMH) was conducted by a pre‐admission clinic (PAC) staff pharmacist who completed a standardised medication reconciliation training program, had access to a standardised World Health Organization (WHO) endorsed BPMH interview guide and participated in central Ontario DPV clinician training (N = 410). IP adults (surgical wards) |
|
| Interventions | Intervention Technology medication reconciliation. A pharmacist conducted BPMH as described above but also had access to a printed copy of the medication information contained in the DPV database which was actively used as part of the BPMH assessment. |
|
| Outcomes | Discrepancy resolution The primary endpoint, number of patients with at least one unintentional BPMH discrepancy at the time of pre‐admission clinic assessment, was assessed by an independent pharmacist study coordinator who did not participate in the informed consent process for the patient and was blinded to treatment assignment. The primary outcome was systematically determined by comparing the printed clinician BPMH medical chart note with the DPV printout to initially identify medication incongruencies along with any other clinical information in the chart. An “unintentional BPMH medication discrepancy” was defined as any medication entry that required correction (prior to surgery) after the incongruency clarification occurred, to reflect the most accurate representation of the patient’s medication‐taking practice. Secondary endpoints were the discrepancy characteristics, time required to complete the BPMH, unique discrepancies prevented by the DPV and clinical significance assessment for potential adverse drug events (Potential ADEs). |
|
| Notes | Funded by: Canada Health Infoway (Co‐funder) Ontario Ministry of Health and Long‐Term Care (Co‐funder) No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomised treatment assignments were centrally prepared by an independent clinician using a random number computer generator and sealed in sequentially numbered, identical, opaque envelopes according to the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | No blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The independent observer assessing the primary outcome was blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Unclear risk | Sponsored by Baxter Corporation |
| Other bias | Low risk | The study seems to be free of other bias. |
Furuya 2013.
| Study characteristics | ||
| Methods |
ITS study Unit of analysis: patient‐days |
|
| Participants | The study was conducted in a teaching hospital with 804 beds. In this hospital, 913, 969 and 996 doctors and 956, 996 and 1011 nurses worked. There were 783, 799 and 800 inpatients being treated per day, and the average length of hospital stay was 13, 12 and 12 days in 2008, 2009 and 2010, respectively (N = 2382). IP adults (teaching hospital) |
|
| Interventions | Intervention Technology: Electronic Medication Administration Records (e‐MARs) and profiles. The commercial e‐prescribing system (MegaOak Assist Rakuraku Kanngoshisan; NEC, Tokyo) was implemented in inpatient wards in November 2009, and includes barcode scanning technology for patient identification. Barcode wristbands are now given to blood transfusion and chemotherapy patients, while either visual or verbal identication is used to identify patients for other types of treatment. |
|
| Outcomes | Total no. errors (including discrepancies) Number of error reports monthly before and after the e‐prescribing system was implemented Monthly error rates were calculated from the number of both medical and medication errors divided by the number of patient‐days. |
|
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | No authors have any conflicts of interest to declare. |
| Other bias | Unclear risk | No information |
| Reliable primary outcome measure(s) | Low risk | Error reports were gathered and investigated in the patient safety division of the hospital. After validation of these reports, the number of errors related to patient safety was reported to the committee every month. |
| Blinded assessment of primary outcome(s) | High risk | The outcomes were not assessed blindly. |
| Data were analysed appropriately | High risk | The U control chart was used to evaluate the performance of the e‐prescribing system. The U control chart is used for ratio data, and the upper control limit (UCL) was calculated by adding three times the standard deviation (SD) to the overall process mean. The lower control limit (LCL) was calculated by subtracting three times the SD from the overall process mean. Wilcoxon rank sum test was used to compare the mean error rates between pre‐ and post‐intervention. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Error reports were gathered and investigated in the patient safety division of the hospital. After validation of these reports, the number of errors related to patient safety was reported to the committee every month. The monthly error rate was then calculated based on the number of errors divided by the number of patient days. Data collected from April 2008 to March 2012 were used for analysis. |
| Completeness of data set | Low risk | The hospital used a standard method of collection for every medication error reported. |
| Reason for the number of points pre‐ and post‐intervention given | Unclear risk | There is no rationale for the number of points stated. |
| Protection against secular changes | Unclear risk | It is not specified if the intervention was independent of other changes in time. |
| Shape of the intervention effect was specified | Unclear risk | Not described |
George 2011.
| Study characteristics | ||
| Methods |
RCT. A prospective, randomised, controlled design was used to assign patients to either the intervention or control groups. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients were eligible if they attended a pre‐admission clinic (PAC) at a large metropolitan teaching hospital in Melbourne, Australia prior to orthopaedic, colorectal and vascular surgery. Patients from these surgery types were selected as they would benefit from a surgical PAC pharmacist’s input, due to their age, length of inpatient stay, potential for comorbidities and complex medication regimens. Patients were eligible if they were either aged 60 years or over, with or without comorbidities or current medication use, or under 60 years of age, with at least one pre‐existing comorbidity and taking regular prescribed medication. 401 participants (intervention: 192; control: 209). Participants were eligible if they attended the surgical PAC at a large metropolitan teaching hospital in Melbourne prior to orthopaedic, colorectal and vascular surgery.
Inclusion criteria: aged > 60 years, with or without comorbidities or current medication use, or < 60 years of age, with at least 1 pre‐existing comorbidity and taking regular prescribed medication. Exclusion criteria: people for non‐elective, day and other surgical procedures and people unable to give written informed consent.
Transition of care: pre‐admission clinic to admission
Age (median): intervention: 68 (interquartile range (IQR) 61‐75) years; control: 67 (IQR 60‐76) years; Female (%): intervention: 54%; control: 51%; Ethnicity: not reported but non‐English speaking: intervention: 17%; control: 10% (N = 401). IP adults (surgical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. The study hospital has a well established surgical pre‐admission clinic (PAC), where patients are assessed, approximately 2 weeks prior to surgery, by nurses, surgeons, anaesthetists and two pharmacists. Two pharmacists on rotation 3 days each week: 2 and 8 years of clinical pharmacy experience, although no previous experience in PAC. Intervention: standard PAC care plus assessment by a PAC pharmacist Control: received standard PAC care only Both groups received standard inpatient care on admission, including clinical pharmacy services from the rostered clinical pharmacist. Important to note that standard care involved a ward pharmacist involved in building the pre‐admission medication list. |
|
| Outcomes | Interventions: pharmacist interventions were any actions that resulted in a change in medication management or therapy Intervention severity assessment: visual analogue scale (0 = no potential adverse effect to 10 = potential for causing death or lasting impairment) MR at admission and discharge: process of checking that the medicines the participant was taking prior to hospital admission correlated with medicines prescribed during the admission and on discharge, and any discrepancies were intentional. Further communication with the author clarified exactly what this outcome reported: "It means the percentage [of participants] that had accurate medications as an outcome assessment... inaccurate meaning at least one unintended medication discrepancy". |
|
| Notes | No financial support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation numbers" |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation numbers and group assignments were presealed in sequentially numbered, opaque envelopes held by the pharmacy technician. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Did not specify if outcomes were assessed blindly. The PAC, pharmacy and ward staff were aware that a study was underway, but were not privy to the study protocol or patient allocation. Both groups also received standard inpatient care, and were followed from PAC to discharge, and data collected on pharmacist interventions, medication reconciliation and medication history documentation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The PAC, pharmacy and ward staff were aware that a study was underway, but were not privy to the study protocol or patient allocation. Both groups also received standard inpatient care, and were followed from PAC to discharge, and data collected on pharmacist interventions, medication reconciliation and medication history documentation. "Interventions were classified by the researchers" but the article does not mention whether they were blind to the group allocation or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 6% lost to follow‐up out of eligible patients for the analysis in a balanced way between groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes (confirmed in a personal communication). |
| Conflict of interest | Low risk | The author(s) declared no competing interests. |
| Other bias | High risk | Participants were only recruited on certain days: "Eligible patients attending clinic days when the PAC pharmacist was in attendance were invited to participate". |
Gordon 2017.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. Cluster‐randomised controlled trial was conducted in a UK teaching hospital (Blackpool Victoria Hospital), including all medical prescribers in four randomised inpatient ward areas. Four inpatient wards were purposefully selected for inclusion in the study: a children’s ward, an orthopaedic ward, an endocrine ward and a cardiology ward. These were selected as they represent a range of clinical specialisms, as well as all having almost mutually exclusive clinical teams, with the aim of preventing contamination. All medical staff who prescribe in each of these clinical areas were contacted by email, at departmental meetings and through on‐ward recruitment over an 8‐week period during March–April 2016. The number of clusters was fixed at four ward areas, two in each group, which all use paper‐based prescribing. Unit of allocation: wards Unit of analysis: prescriptions |
|
| Participants | All medical prescribers in four randomised inpatient ward areas of a UK teaching hospital. Consent was obtained from 55 prescribing doctors out of a possible 123 in those areas (44.7%). No one withdrew consent during the study (N not available). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Structural/organizational, Organizational changes. Intervention Technology. The commercial e‐prescribing system was implemented in inpatient wards in November 2009, and includes barcode scanning technology for patient identification. Barcode wristbands are now given to blood transfusion and chemotherapy patients, while either visual or verbal identication is used to identify patients for other types of treatment. After an assessment of prescribing on each ward, a ward‐specific feedback document was prepared, giving general and anonymous feedback, and forwarded to all consenting participants in the intervention areas. Intervention wards: prospective ongoing prescribing error feedback Control wards: no feedback. No e‐prescribing system |
|
| Outcomes | The primary outcome was total prescribing order error rates (calculated as the number of medication orders with any error as a percentage of the total medication orders audited); secondary outcome measures included clinical order error rates, technical order error rates and cost per error prevented. Prescriptions were eligible for assessment, if they were active on the day of data collection: "once only" drugs, regular medication orders, "when required" drugs and continuous infusions. Errors were not recorded if they had been corrected by the prescriber immediately, but were recorded if they had been corrected by other staff. |
|
| Notes | No registration was obtained. Funding: Blackpool Victoria Hospital (host organisation) provided a pump priming grant of £6900 to support this work internally. The department had no involvement in the carrying out or writeup of the study, but did peer review the protocol before funding and as part of internal and ethics approval. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completed used a computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using sealed opaque envelopes, with assignment to the next sealed envelope as per the random number list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Involvement in the trial would not impact on the routine screening, quality assurance and intervention processes conducted by the pharmacists. Participants were not aware of which group their area would be randomised to on enrolment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ward pharmacists in each of the study groups began collecting data using a bespoke electronic pro‐forma, with several changes made to the interface and content based on feedback. A senior pharmacist acting as principal investigator performed reliability checks during this period to confirm the appropriate and consistent recording of data. The error data was aligned with the previously published EQUIP trial, in which this hospital participated for data collection. All interventions on the ward by the pharmacist were maintained as normal during this process. There is no mention regarding blinded assessment of outcomes but the process is very transparent and supervised. The outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No one withdrew consent during the study. There were no missing data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | Authors have completed the International Committee of Medical Journal Editors (ICMJE) disclosure form. With the exception of the declared funding, there has been no other financial support for this work, no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years, no other relationships or activities that could appear to have influenced the submitted work. |
| Other bias | High risk | The outcome was measured for each prescription, prescriptions seem to be clustered within audits, which were clustered within wards; wards were randomised. The analysis does not account for the possible cluster effects of ward or audit. It may be possible to use the published P value under the assumption that there is no effect of audit. |
Graabaek 2019.
| Study characteristics | ||
| Methods |
RCT. Unblinded randomised controlled study. Patients were included from the medical acute admission unit at Hospital South West Jutland, Denmark. From April 2013 to December 2014, the pharmacist was present on the ward 267 days. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Acutely admitted medical patients (not surgical) aged 65 years or above, able to speak and understand Danish, and holding a Danish personal registration number. Patients were excluded if they were extremely ill, terminal, had not been seen by either a nurse or physician yet, or were not accessible (N = 600). IP adults (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. Two intervention groups: pharmacist‐led medication review and patient interview upon admission (intervention 'ED') or pharmacist‐led medication review and patient interview upon admission, medication review during inpatient stay, and medication report and patient counselling at discharge (intervention 'STAY'). Control group named 'Control' (usual care) |
|
| Outcomes | The primary outcome was number of patients with a medication‐related re‐admission within 30 days from discharge. The assessment of whether a re‐admission was medication‐related or not followed a strict procedure based on WHO‐UCM internationally agreed criteria for causality and Hallas' criteria for contribution. Secondary outcomes included mortality (overall, during index admission, within 30 days after discharge or 31 to 180 days after discharge), patients with re‐admissions (acute and planned, both including medication‐related re‐admissions) within 30 days after discharge, and number of visits to the emergency department, the hospital, or a general practitioner within 180 days after discharge. These data were collected from the nationwide registers from the Danish Health Authorities: the Civil Registration System, the National Health Insurance Service Registry, and the National Patient Registry. |
|
| Notes | The study protocol was approved by the Danish Data Protection Agency and the Regional Scientific Ethics Committees for Southern Denmark (registration number S‐20110161). This work was supported by Hospital South West Jutland, University of Southern Denmark, Region of Southern Denmark, Sygehusapotekernes og Amgros' forsknings‐ og udviklingspulje, Actavis Legat, Karola Jørgensens Forskningsfond, and Edith & Vagn Hedegaard Jensens Fond. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomised using a 1:1:1 allocation ratio to one of three groups in blocks of 15 (each block contained five patients from each group) using the opaque closed envelope technique. The randomisation process was performed at Odense University Hospital. The patients were included consecutively. Details about the generation sequence are not specified but it is very likely that this second hospital in charge of randomisation used an appropiate method. |
| Allocation concealment (selection bias) | Low risk | The patients were randomised using a 1:1:1 allocation ratio to one of three groups in blocks of 15 (each block contained five patients from each group) using the opaque closed envelope technique. The pharmacist opened the envelope at the bedside after patient consent was obtained, and the patient was informed immediately about allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The group allocation was not blinded to the patient, the pharmacist, or other healthcare professionals present at the ward. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two researchers, with expertise in clinical pharmacology and geriatrics, individually conducted the analysis of the primary outcome. Information about group allocation was blinded to these researchers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low percent of patients lost. ITT analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Green 2015.
| Study characteristics | ||
| Methods |
ITS study. Monthly measurements of wrong‐patient order rate were obtained before and after the implementation of the computerised provider order entry (CPOE)–based patient verification process. Five emergency department (EDs) were included: 2 adult EDs, 2 pediatric EDs, and 1 combined ED. The EDs serve a socioeconomically, racially, and ethnically diverse population in New York City and have a combined annual visit volume of 250,000 patients. The EDs support pediatrics and emergency medicine residency and pediatric emergency medicine fellowship programs. Unit of analysis: prescriptions |
|
| Participants | Adult and paediatric ED patients (N not available) IP/OP adults (ED) | |
| Interventions |
Intervention: Technology Prescribing and order communication systems. Computerised physician order entry (CPOE). As part of a quality improvement initiative, a custom patient verification module was integrated into the computerised provider order entry system with the intent of helping practitioners intercept wrong‐patient selection errors before order entry. Three patient identifiers were prominently displayed: full name, birth date, and medical record number. Additional information that could facilitate patient identification was also included, such as ED length of stay, chief complaint, bed location, and recent medication orders. |
|
| Outcomes | The primary outcome was intercepted wrong‐patient orders (expressed as a rate per 1000 orders), which was calculated with the retract‐and‐reorder method. The electronic health record system was fully implemented by January 2011 and all order entry was performed electronically in the study sites. A record of each order entry was obtained from electronic health record system logs. Additionally, the actions taken by providers within the patient verification module were also electronically recorded. We used the data from the electronic health record logs to perform our analysis. | |
| Notes | This study was supported in part by National Library of Medicine grants 5 T15 LM007079 and LM006910. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | No conflict of interest |
| Other bias | Low risk | No other biases detected |
| Reliable primary outcome measure(s) | Low risk | The primary outcome was intercepted wrong‐patient orders (expressed as a rate per 1000 orders), which was calculated with the retract‐and‐reorder method described by Adelman et al. This method identifies orders placed for a patient but then rapidly discontinued by the same practitioner (i.e. the retract event); it then checks to determine whether an identical order was subsequently entered by the same provider for a different patient (i.e. the reorder event) within a short period after the retract event. Adelman et al. evaluated the accuracy of the retract‐and‐reorder method by interviewing the provider after a retract‐and‐reorder event occurred. The authors defined the method positive predictive value as the percentage of retract‐and‐reorder events that were reported because of a wrong‐patient order by the interviewed providers and estimated a positive predictive value of 76.2% (95% confidence interval (CI) 70.6% to 81.9%). |
| Blinded assessment of primary outcome(s) | Low risk | Not blinded but objective method. Medication orders are placed via computerised provider order entry (CPOE) |
| Data were analysed appropriately | Low risk | Primary data analysis: the authors assessed the potential effect of different confounding variables, using a logistic regression model. Confounding variables included in the model consisted of patient level variables (sex, age, and race), provider role (attending physician, resident, medical student, or other), and whether the order was placed during a day or a night shift. Furthermore, they compared the effect of intervention across the 5 sites included in this study. In a secondary analysis, they used the rate of wrong‐patient orders in the 5 facilities' 2019 inpatient settings to standardise the rate of such orders in the ED data. Standardisation was accomplished by dividing the rate of wrong‐patient orders in the ED setting for each study period by dividing the rate of such orders in the inpatient setting within the same period. This was done to eliminate the potential effect of secular trends, assuming that the influence of these trends was proportionally the same in inpatient and ED settings. The adjusted rate was then compared across study periods with the X2 test. They used change‐point analysis to study the longitudinal trends of wrong‐patient orders to identify whether the effect of intervention was sustained over time. |
| Protection against detection bias (same pre‐post data collection) | Unclear risk | "Wrong‐patient orders that remain unnoticed or are intercepted by a different clinician are not identified with this method, which may lead to an underestimation of the wrong‐patient order rate." "The retract‐and‐reorder method can identify only wrong‐patient orders that were identified and corrected by the same provider" Comment: same method applied pre & post. Potential detection bias could have had similar effect in pre & post measurement. Adjustment by provider role was performed (to account for better practices in more experienced doctors). No description of doctors provided in article |
| Completeness of data set | Low risk | "A record of each order entry was obtained from electronic health record system logs. Additionally, the actions taken by providers within the patient verification module were also electronically recorded. We used the data from the electronic health record logs to perform our analysis." |
| Reason for the number of points pre‐ and post‐intervention given | Low risk | The study sample included all orders written at these sites from January 2011 through April 2013. The pre‐intervention phase included orders written from January to April 2011. They used 2 different periods for the post‐intervention phase of the study: to assess short‐term effect of intervention, they used orders written in the 4 months after the intervention (June 2011 to September 2011); they excluded May from this analysis because the module was being gradually rolled out then. To evaluate the long‐term effect of the intervention, they used orders written between January 2013 and April 2013. |
| Protection against secular changes | High risk | "Additionally, our study used a before‐after design, and the results can be potentially confounded by an unknown simultaneous intervention that was not measured in the analyses; the use of a parallel control group can reduce the effect of unknown confounders, but because our control group was not matched with the study group (i.e. inpatient versus ED), we are reporting the result of our controlled analysis only as a secondary outcome and encourage the readers to interpret it with caution." |
| Shape of the intervention effect was specified | Unclear risk | Not stated in the article |
Greengold 2003.
| Study characteristics | ||
| Methods |
RCT‐ individual Unit of allocation: nurses Unit of analysis: administered doses |
|
| Participants | Study participants were registered nurses who had at least 1 year of acute care nursing experience and a minimum of 6 months of full‐time employment at the hospital (N not available). IP adults (teaching hospital) |
|
| Interventions | Intervention Human resources. Administration. Drug administration models (primary vs. functional, Registered nurses vs.unlicensed, etc) The drug administration error rate could be decreased by having "dedicated medication nurses", who had received a brief review course on pharmacology and safe medication use, focus exclusively on administering drugs during their nursing shifts without increasing the existing complement of nursing staff. |
|
| Outcomes | Total error rate | |
| Notes | No trial number Supported by National Patient Safety Fundation, Chicago and by the two hospitals participating in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Nurses were randomly assigned using a random number generator. |
| Allocation concealment (selection bias) | High risk | Random allocation was broken for the general nurses group. "It was occasionally necessary to recruit nurses to serve in the general nurse role when the randomized backup general nurses were unavailable. This occurred 12% of the time for total days worked. These nurses were not randomized." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | In addition, although the specific hypotheses of the research were not shared with the study participants, the study was not masked, and it is believed that most of the nurses knew or inferred the purpose of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not masked, and the observers were aware of study design, so they might have interjected their own biases in documenting the errors made. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The analysis plan was not described. |
| Selective reporting (reporting bias) | Low risk | Prespecified (primary and secondary) outcomes that are of interest in the review have been reported. |
| Conflict of interest | Low risk | Funded by a national foundation |
| Other bias | High risk | Nurses were randomised to one of the two arms. Nurses worked within a total of 8 units across 2 hospitals (it is not clear if nurses work in more than one unit). So, the clustering structure might be nurse within unit within hospital. Error rates were computed for each nursing unit‐week, but then appear to be pooled. There is no detailed description of the statistical analysis. It seems as if the authors do not account for the factors they have identified as potentially important. |
Gursanscky 2018.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. Cluster‐randomised trial in 2014 involving 16 prescribers in four general medical units of a large tertiary referral centre in Melbourne, Australia. One unit was randomised to regular prescribing feedback and targeted education; another unit was randomised to the intervention whereby junior doctors completed the NPS National Inpatient Medication Chart Training e‐learning course and the two remaining units were randomised to no intervention. Statistical analysis was by Chi2 comparison of each unit’s error rate pre‐intervention to post‐intervention. Unit of allocation: units Unit of analysis: prescriptions |
|
| Participants | All junior doctors working in the general medical units at the time of the study participated, consisting of 12 interns and 4 registrars. Each unit had 1 registrar and 3 interns. All units were made aware of the study before it began and doctors were informed that they were expected to participate as part of an ongoing quality assurance process (N not available). IP adults (medical wards) |
|
| Interventions |
Intervention 1: one unit was randomised to prescribing feedback and targeted education by a clinical pharmacist Intervention 2: another unit was randomised to an e‐learning intervention on safe prescribing Control: two units were randomised to no intervention. |
|
| Outcomes | Prescription writing errors, error rate A prescription writing error was deemed to have occurred if patient or prescriber details were incomplete, or if a medication order was illegible, incomplete or incorrect. Data were collected via daily audit of paper medication charts. Using a systematic process, each part of the medication chart was evaluated for errors identified by the pharmacist, conventionally identified at the study hospital by chart annotations in purple ink. |
|
| Notes | No registration reported. Financial support not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The four general medical units were randomised to two intervention groups (one unit each) and control groups (two units) using a random number generator and a predefined sequence of allocation. |
| Allocation concealment (selection bias) | Unclear risk | All units were made aware of the study before it began and doctors were informed that they were expected to participate as part of an ongoing quality assurance process. Clinical pharmacists remained blinded to intervention unit allocation and a rotating ward roster meant that each pharmacist reviewed charts from all four units. All senior medical staff remained blinded to intervention unit allocation and junior medical staff were asked not to discuss the interventions. The investigator responsible for data collection and the intervention pharmacist were unable to be blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All units were made aware of the study before it began and doctors were informed that they were expected to participate as part of an ongoing quality assurance process. All senior medical staff remained blinded to intervention unit allocation and junior medical staff were asked not to discuss the interventions. The investigator responsible for data collection and the intervention pharmacist were unable to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Clinical pharmacists remained blinded to intervention unit allocation and a rotating ward roster meant that each pharmacist reviewed charts from all four units. Clinical pharmacists had reviewed charts and identified prescription writing errors each day, which occurred as part of their usual ward duties." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
| Other bias | High risk | Units were randomised and the analysis does not model this because comparisons are made within study arms. While there is a control arm, it is not used in the analysis for that purpose. They concluded that the interventions have an effect, but the analysis and its results do not support this (note that similar changes are inferred in the control arm). |
Hale 2013.
| Study characteristics | ||
| Methods |
RCT ‐ individual. Single centre, randomised, controlled, two‐arm trial Unit of allocation: patients Unit of analysis: prescriptions |
|
| Participants | Elective surgery PAC in a Brisbane‐based tertiary hospital. Participants: 400 adults scheduled for elective surgery were randomised to intervention or control (N = 384). IP adults (surgical wards) |
|
| Interventions | Prescribing and order communication systems. Clinical pharmacy services Intervention: a pharmacist generated the inpatient medication chart to reflect the patient’s regular medication, made a plan for medication perioperatively and prescribed venous thromboembolism (VTE) prophylaxis. Control: the medication chart was generated by the Resident Medical Officers (RMOs). |
|
| Outcomes | Omissions Prescribing errors Primary outcome was frequency of omissions and prescribing errors when compared against the medication history. The clinical significance of omissions was also analysed. Secondary outcome was appropriateness of VTE prophylaxis prescribing |
|
| Notes | Trial Registration: Registered with ANZCTR—ACTR Number ACTRN12609000426280 Funding: this research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After consent, patients were randomised using a computer generated randomisation list, in blocks of 10 (Microsoft Excel)" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes (not prepared by the recruiting researcher) contained a zero or one as per the computer list; the next envelope was opened after consent to determine whether a patient entered the control or intervention arm, respectively". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors made an effort to keep the participants blinded in both arms. The pharmacist and resident in charge were not blinded, and that could have affected the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Analysis of scanned copies of medication charts, for the primary outcomes of omissions and errors, was conducted in tandem by two assessors, one a member of the research team and the other an external assessor, both trained in the use of validated audit tools and blinded to randomisation. An expert panel, comprising a surgeon, a clinical pharmacologist, an anaesthetist, a RMO, a pharmacist and a nurse, was convened to assess the clinical significance of omissions in a randomly selected 5% sample of the total cohort of patients from both arms (N = 10 control, N = 9 intervention). Panel members were blinded to randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 400 patients randomised and 384 were analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes described in ACTRN1260900042628 were reported |
| Conflict of interest | Low risk | The authors stated that they did not have a conflict of interest. |
| Other bias | Unclear risk | While the paper makes clear that orders cluster within patient, and the authors seem to account for this in their analysis, it is not clear to us whether opportunities for omission also cluster within patient. For example, if there is exactly one opportunity per patient, then the analysis would not need to model clustering for this outcome. |
Heselmans 2015.
| Study characteristics | ||
| Methods |
RCT Randomised controlled multicentre trial conducted at the Hospital Network of Antwerp, Belgium, between December 2010 and January 2012. During the study period, six pharmacists (one pharmacist and a backup pharmacist at each general hospital) were assigned to the project to review the medication list of all patients transferred from ICU to wards. Unit of allocation: patients Unit of analysis: patients/prescriptions |
|
| Participants | 1. Hospitalised patients above 15 years of age. Participants had a mean age of 65.4 years and 37.8% were women. 2. Patients should have stayed a minimum of three days in intensive care and then undergo a transfer to a ward with surgical, medical or geriatric beds (N = 600). IP adults (ICU and medical ward) |
|
| Interventions | Intervention Human resources, medication reconciliation Participants were assigned either to usual care or usual care plus intervention. Intervention: clinical pharmacist performed a medical review and used a Case Report Form (CRF). Recommendations for drug therapy changes were immediately communicated to the ward physician. Control: there was no intervention. |
|
| Outcomes | The primary outcome was expressed as the number of implemented recommendations for drug therapy changes. Differences between groups were calculated using mixed effects binary logistic regression. Secondary outcomes were the number of implemented recommendations of drug therapy changes for each type of DRP and each type of intensive care (surgery/internal medicine), length of stay in the hospital, hospital discharge mortality and ICU re‐admission rates. | |
| Notes | The clinical trial was registered in the International Standard Randomized Controlled Trial Number register (ISRCTN40005781 Ref: CCT‐NAPN‐20967). The project was funded by the National Institute of Disability and Health care Insurance (RIZIV, NIDHI). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned either to usual care or usual care plus intervention in a 1:1 ratio based on the last digit of their computer‐generated admission number." |
| Allocation concealment (selection bias) | Low risk | Patients with even numbers were assigned to the intervention group, and patients with odd admission numbers were assigned to the observation group during the first 6 months. The assignment procedure was reversed at 6 months. Although it was not explicity masked, it is unlikely that there was influence on the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physicians and pharmacists were aware of the allocated arm, but patients were not informed about the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data analysts were kept blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We analysed outcomes on an intention‐to‐treat basis. Intervention and control groups were compared on baseline variables to evaluate the randomization". There were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | The publication included all the expected results, reported in ISRCTN40005781, but also reported non‐prespecified outcomes such as length of stay in the hospital, hospital discharge mortality and ICU re‐admission rates. |
| Conflict of interest | Low risk | The author(s) declared no competing interests. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Hickman 2018.
| Study characteristics | ||
| Methods |
RCT. Randomised controlled trial, conducted in the inpatient dispensary of a major tertiary‐referral hospital in Melbourne, Australia, between February and August 2014. On a daily basis, the dispensary is staffed by four pharmacists (including one ’in charge’) and three to four technicians. Unit of allocation: patients Unit of analysis: prescriptions |
|
| Participants | All pharmacists (N = 12) and UK‐trained Accuracy Checking Pharmacy Technicians (ACPTs) (N = 3) working in the inpatient dispensary at the time of the study were invited and chose to participate. The ACPTs had all previously completed UK technician training programs (UK National Vocational Qualification/Business and Technology Education Council Extended Diploma) and had been practicing for between 2 and 6 years in the UK prior to commencing practice in Australia. Medication orders for inpatient use were included. Medications are distributed to wards twice daily, and generally ordered for the next delivery period. Medications that were required by the ward immediately, such as emergency supply or medications for a deteriorating patient, were excluded, as were discharge prescriptions, compounded products and controlled drugs (N not available). IP adults (tertiary care center) |
|
| Interventions | Intervention Human resources, medication reconciliation Inpatient medication orders were received by the dispensary from the wards, typed and assembled by technicians as per standard operating procedures, and then queued for checking in order of completion. Intervention 1: pharmacists, highly trained (usual training) Intervention 2: UK‐trained Accuracy Checking Pharmacy Technicians (ACPTs), highly trained. |
|
| Outcomes | Errors identified by the reviewing pharmacist were documented and severity was assessed by an independent Medication Safety pharmacist. | |
| Notes | No registration reported Financial support not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random sequence generation process not described. Allocation was according to a simple randomisation allocation strategy, where the next available pharmacist or ACPT received the next order ready to be checked from the study coordinator." |
| Allocation concealment (selection bias) | High risk | "Random sequence generation process not described. Allocation was according to a simple randomisation allocation strategy, where the next available pharmacist or ACPT received the next order ready to be checked from the study coordinator." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All errors identified by the research pharmacist were evaluated by a Medication Safety Pharmacist, also blinded to study allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | The author(s) declared no competing interests. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Higgins 2010.
| Study characteristics | ||
| Methods |
ITS study. Retrospective analysis of data from an existing safety reporting system with anonymous and non‐punitive self‐reporting. Units of analysis: monthly administered doses |
|
| Participants | This study was conducted at Baystate Medical Center, a 655‐bed general, acute care tertiary care teaching hospital (N not available). IP adults (tertiary care center) |
|
| Interventions | Intervention Technology, Barcoding Intervention: barcode scanning and positive patient identification (PPID) in a large teaching hospital already using computerised provider order entry (CPOE) Control: only computerised provider order entry (CPOE) |
|
| Outcomes | Near‐miss errors Reached‐patients errors Total errors (near‐miss + reached‐patients) Medication safety events were categorised as “near‐miss” (unsafe conditions or caught before reaching the patient) or reaching the patient, with requisite additional monitoring or treatment. Baseline and post‐PPID implementation data on events per 1 million drug dministrations. An existing on‐line safety reporting system (UHC Patient Safety Net) was used to capture baseline and post‐implementation data on incidence and severity of medication events. |
|
| Notes |
Higgins 2010 No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | None of the authors report any conflicts of interest. |
| Other bias | Low risk | No other biases detected. |
| Reliable primary outcome measure(s) | Low risk | Medication errors reaching patients averaged and near misses per million. "Data analyzed for this study were collected routinely for clinical care and quality improvement,and beyond introduction of bar‐code scanning, clinical practice was not affected in any way by the study collection were the same before and after the intervention" |
| Blinded assessment of primary outcome(s) | Unclear risk | Not disclosed in the article |
| Data were analysed appropriately | High risk | Baseline and post‐implementation data were compared by Chi2 with P < 0.05 considered significant. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data: "Data analyzed for this study were collected routinely for clinical care and quality improvement, and beyond introduction of bar‐code scanning, clinical practice was not affected in any way by the study collection were the same before and after the intervention" "An existing on‐line safety reporting system (UHC Patient Safety Net) [5] was used to capture baseline and post‐implementation data on incidence and severity of medication events. [...] Safety Reporting System events are filed on‐line by hospital personnel (physicians, nurses, allied health professionals) and reviewed daily by a pharmacy medication safety specialist. Any adverse drug events thus identified would be reviewed and when appropriate" |
| Completeness of data set | Low risk | Data set covers the total number of participants |
| Reason for the number of points pre‐ and post‐intervention given | Unclear risk | The rationale for the number of points was not stated. |
| Protection against secular changes | Unclear risk | Not described |
| Shape of the intervention effect was specified | Unclear risk | Partially described in the background section |
Juanes 2018.
| Study characteristics | ||
| Methods |
RCT. A randomised controlled trial to assess the clinical impact on drug‐related negative outcomes of a pharmaceutical care programme focusing on the resolution of potential drug‐related problems, initiated in the emergency department for patients with heart failure (HF) and/or chronic obstructive pulmonary disease (COPD). Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients who met the following criteria were eligible for inclusion: 65 years or older, length of stay in ED longer than 12 hours, decompensation of HF and/or COPD and polypharmacy (four or more drugs). Hospital de la Santa Creu i Sant Pau, Barcelona, Spain (N = 118). IP adults (ED) |
|
| Interventions | Clinical pharmacy services, medication reconciliation Intervention: pharmaceutical care programme focusing on resolving potential drug‐related problems initiated at the emergency department (intervention group (IG)). The pharmaceutical care programme comprised the following steps: 1. Obtaining and recording the medication chart. As part of this process, the pharmacist confirmed, by interviewing the patient or caregiver, the medication taken at home as listed in the electronic health records. 2. Medication reconciliation in each of the care transitions. Medication reconciliation is defined by the Institute for Healthcare Improvement as ‘the process of creating the most accurate list possible of all medications a patient is taking—including drug name, dosage, frequency and route—and comparing that list against the physician’s admission, transfer and/or discharge orders, with the goal of providing correct medications to the patient at all transition points within the hospital’. 3. Medicine review and validation of physician prescriptions during the stay at the ED and during hospitalisation. This consisted of reviewing the following aspects of the patient’s medication: (a) the indication for each medication in relation to the patient’s condition; and (b) the appropriateness of each medication, dose, schedule, duration of the treatment for the patient’s age and/or clinical status (renal function or liver function). In addition, therapeutic drug monitoring was performed for drugs with a narrow therapeutic range. 4. Patient follow‐up. This consisted of evaluation of the effectiveness and safety of the treatment according to standard clinical practice and patients’ objective data from clinical records. 5. Provision of additional written information at discharge, with clear indications for drug therapy regimen using software tools provided by the Catalan Drug Information Centre (CedimCat). Control: standard care excluding medication reconciliation (medication review and prescriptions' validation, analogous to step 3 in the intervention group). |
|
| Outcomes | Drug‐related negative outcomes, 180‐day mortality, mean stay, revisits | |
| Notes | Trial registration number NCT02368548 No financial support stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the hospital’s pharmacology department using SPSS software V.18 (SPSS, Chicago, Illinois, USA) to create a dedicated application to randomise patients to one of the two study groups (distribution 1:1). The application used a seed obtained by rolling two dice to select the row and column from a random‐number table; therefore, while replicable but unpredictable, the series was perfectly balanced between groups in 10‐case blocks. |
| Allocation concealment (selection bias) | Low risk | Participants or investigators enrolling participants could possibly foresee assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither patients nor healthcare professionals were blinded to the treatment group, in accordance with the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Conflict of interest | Low risk | None declared |
| Other bias | Low risk | None detected |
Kannampallil 2018.
| Study characteristics | ||
| Methods |
ITS study. They used a quasi‐experimental ITS design to characterise the temporal course of changes in the number of RAR (rate of retract‐and‐reorder) events in relation to changes in the maximum number of allowable open charts. The ED made 2 changes during the considered period: from 4 to 2 charts in November 2012, and from 2 to 4 charts in September 2014. Unit of analysis: order session |
|
| Participants | Adult ED patients (range of means 34 to 37 years old) receiving health care at the ED at the University of Illinois Hospital (UIH). UIH ED is part of a 495‐bed tertiary urban hospital associated with an academic medical centre (N = 11,504). IP/OP adults (ED) |
|
| Interventions | Intervention Technology, Prescribing and order communication systems, Computerized Physician Order Entry (CPOE). Medication orders are placed via computerised provider order entry using Cerner FirstNet or Cerner PowerChart The ED made 2 changes during the considered period: Intervention 1: 4 charts Intervention 1: from 4 to 2 charts in November 2012 Intervention 2: from 2 to 4 charts in September 2014 |
|
| Outcomes | The primary outcome variable was the rate of retract‐and‐reorder (RAR) events. RAR is a surrogate measure for wrong‐patient orders, developed by Adelman and colleagues, and is endorsed by the National Quality Forum. A RAR event is triggered when a medication order is cancelled by an ordering clinician within 10 minutes of an order and then reordered by the same clinician for a different patient within the next 10 minutes. Based on a single‐institution study, a RAR event was found to have a 76% positive predictive value (PPV) for identifying intercepted wrong‐patient orders. The RAR measure has been used to study intercepted wrong‐patient errors in a variety of settings. | |
| Notes | This project was supported in part by grants from the Agency for Healthcare Research and Quality (AHRQ) (Nos. R01HS024945, R21HS023704, and R01HS024945‐01). The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | BLL provides software and consulting services designed to prevent wrong‐drug medication errors. His companies had no access to the data or involvement in the study. |
| Other bias | Unclear risk | No other biases detected |
| Reliable primary outcome measure(s) | Low risk | Obtained from an automated system |
| Blinded assessment of primary outcome(s) | Low risk | Not blinded but objective method. Medication orders are placed via computerised provider order entry (CPOE) |
| Data were analysed appropriately | Low risk | We used a segmented quasi‐Poisson regression (accounting for overdispersion) at monthly intervals, measuring the changes in intercept and slope after each transition: from 4 charts to 2 charts, then from 2 charts to 4 charts. A change in the intercept corresponds to the magnitude of the difference between the periods immediately before and after the intervention. A change in slope corresponds to a change in trend between periods. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention. |
| Completeness of data set | Low risk | Data were obtained by the system. |
| Reason for the number of points pre‐ and post‐intervention given | Unclear risk | Not described |
| Protection against secular changes | Low risk | Segmented regression analysis helps in determining how an intervention has affected an outcome of interest “immediately and over time; instantly or with delay; transiently or long‐term.” This approach can account for secular trends over time, such as increased number of orders. |
| Shape of the intervention effect was specified | Unclear risk | Not described |
Khalil 2016.
| Study characteristics | ||
| Methods |
RCT. The aim of the study was to develop, implement and evaluate the role of pharmacist‐led medication reconciliation and charting service for patients admitted to an acute assessment and admission unit via the emergency department in an electronic medication management environment at a metropolitan Australian hospital. Following the credentialing of an experienced clinical pharmacist to perform collaborative medication charting, a prospective parallel study of medication errors was undertaken. Patients were randomly allocated to an intervention (n = 56) or a usual care (control) (n = 54) arm. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Although the mean age of patients in the intervention group was younger (65.1 vs. 74.8 years, P < 0.005), there were no significant differences in the mean number of medications per patient (10.66 vs. 10.26, P = 0.71) or mean length of stay (5.87 vs. 6.08 days, P = 0.81) (N = 110). IP adults (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation Intervention: medication orders charted by pharmacist Control: medication orders charted by medical staff in the usual care An independent clinical pharmacist reviewed all the medication orders at 24 h after admission and errors recorded. The severity of errors was rated by a ‘blinded’ consultant physician and an independent senior pharmacist according to a standardised matrix. |
|
| Outcomes | Medication errors. The aim of the study was to develop, implement and evaluate the role of pharmacist‐led medication reconciliation and charting service for patients admitted to an acute assessment and admission unit via the emergency department. | |
| Notes | The study was funded by a grant from the Victorian Department of Health and Human Services for the Advanced Practice Allied Health Workforce Program. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated using a random number generator to the intervention. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Potential limitations to this study include that blinding of the reviewing pharmacist was not possible as patients interviewed by the project pharmacist were readily identifiable during data collection on the electronic prescribing clinical system. No blinding, but the review authors judge that the outcome is not very likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The severity of all errors was then rated by a ‘blinded’ consultant physician and an independent senior pharmacist according to a standardised matrix and recorded for analysis. "Secondary endpoints included the types of errors based on an inhouse classification system and their severity which were rated by a blinded independent physician and a senior pharmacist using the risk assessment tool from the Society of Hospital Pharmacists of Australia standards of practice of clinical pharmacy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Low risk | None declared. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kwan 2007.
| Study characteristics | ||
| Methods |
RCT. Randomised controlled trial. The primary objective of the 'Surgical Pharmacist in PreAdmission Clinic Evaluation' (SPPACE) study was to evaluate whether structured pharmacist medication history interviews with assessments in the surgical pre‐admission clinic and the use of a postoperative medication order form reduces the number of patients with at least 1 post‐operative medication discrepancy related to home medications. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | The study was conducted at a tertiary care university‐affiliated teaching hospital in Toronto, Ontario. Between 19 April 2005, and 3 June 2005, all consecutive patients who had a surgical pre‐admission clinic visit before undergoing surgical procedures from the urology, plastic surgery, general surgery, thoracic surgery, gynecology, oncology, and ear, nose, and throat services were eligible for inclusion. Patients were excluded if they were scheduled for discharge on the same day as their surgery. Eligible patients were centrally randomised by an independent ward clerk to the intervention or standard care arm using a random number computer generator in blocks of 24 (the daily maximum number of patients seen at the clinic). The treatment assignments were sealed in sequentially numbered, identical, opaque envelopes according to the allocation sequence. For practical reasons, the patients and clinicians were not blinded to treatment assignment. (N = 464). IP adults (surgical wards) |
|
| Interventions | Intervention Human resources, Clinical pharmacy services Intervention: structured pharmacist medication history interview with assessment and generation of a post‐operative medication order form Control: standard care arm (nurse‐conducted medication histories and surgeon‐generated medication orders). Standard care consisted of nurses conducting medication histories with patients at the surgical pre‐admission clinic or occasionally over the telephone. Medication history information was entered in the hospital electronic health record and printed. Surgeons could refer to this printout to generate their post‐operative medication orders. The patient’s community pharmacy or family physician was contacted for additional medication clarifications if needed. It was not standard practice to routinely follow‐up after surgery to clarify medication changes since the clinic assessment. |
|
| Outcomes | Discrepancy resolution Medications discrepancy related to home medications The primary endpoint was the number of patients with at least 1 post‐operative medication discrepancy related to home medications |
|
| Notes | Financial disclosure: none reported No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment assignments were sealed in sequentially numbered, identical, opaque envelopes according to the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Eligible patients were centrally randomised by an independent ward clerk to the intervention or standard care arm using a random number computer generator in blocks of 24 (the daily maximum number of patients seen at the clinic). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For practical reasons, the patients and clinicians were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although every effort was made to conceal the treatment arms during the clinical assessment, the assignment of the patient was unblinded if the independent assessors thought they needed to look into the medication discrepancy in more detail. Although blinding was not carried out, a systematic approach was used to identify medication discrepancies in a reproducible format through the comparison of admission orders with the home medication regimens. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per‐protocol analysis was performed among the remaining 416 patients. 10% of patients were not included in the analisys in both arms. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Low risk | The authors have not disclosed any potential conflicts of interest. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Landrigan 2004.
| Study characteristics | ||
| Methods |
RCT‐ individual. A prospective, randomised study comparing the rates of serious medical errors made by interns while they were working according to a traditional schedule with extended (24 hours or more) work shifts every other shift (an “every third night” call schedule) and while they were working according to an intervention schedule that eliminated extended work shifts and reduced the number of hours worked per week. Unit of allocation: doctors Unit of analysis: patients |
|
| Participants | Medical intensive care unit (MICU) and coronary care unit (CCU) of Brigham and Women's Hospital, a large academic hospital in Boston (N = 634). IP adults (ICU) |
|
| Interventions | Intervention: Structural changes/Organizational changes Intervention:limited work time. During the intervention schedule, interns’ work hours and overnight work schedules were changed. Interns’ traditional extended work shifts were divided in two: a “day‐call” intern worked the first half of a traditional call (from 7 a.m. to 10 p.m.); a “night‐call” intern worked the second half (from 9 p.m. to 1 p.m. the following day). To effect this schedule, four interns shared patient care responsibilities during the rotation. The maximum scheduled hours of work were 60 to 63 per week, with consecutive hours of work limited to approximately 16 hours. The intervention did not alter the schedules or staffing of second‐ or third‐year residents or other clinical personnel. Control: nomal work time.The traditional MICU house‐staff team consisted of three interns and three third‐year residents, whereas the CCU team consisted of three interns and two second‐year residents. Each intern and resident on these teams worked overnight in the hospital every third night. A resident from another hospital service assumed patient care responsibilities in the CCU on nights when neither of the daytime CCU residents was working. Under this rotation, interns’ scheduled workweeks averaged 77 to 81 hours, depending on the clinic assignment, with up to 34 continuous hours of scheduled work when clinic occurred after they were on call. |
|
| Outcomes | Medication error per 1000 patient‐days rate (number of errors/1000 patient‐days) Medical error: any error in the delivery of medical care, whether harmful or trivial |
|
| Notes | Supported by a grant (RO1 HS12032) from the Agency for Healthcare Research and Quality (AHRQ); by a grant (RO1 OH07567) from the National Institute for Occupational Safety and Health, by the Department of Medicine, Brigham and Women’s Hospital; by the Division of Sleep Medicine, Harvard Medical School; by the Brigham and Women’s Hospital; and by a General Clinical Research Center grant (M01RR02635) from the National Center for Research Resources. Dr. Landrigan is the recipient of an AHRQ career development award (K08 HS13333); Dr. Cronin is the recipient of an AHRQ National Research Service Award (F32 HS14130) and a National Heart, Lung, and Blood Institute fellowship in the program of training in Sleep, Circadian, and Respiratory Neurobiology at Brigham and Women’s Hospital (T32 HL079010) No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the interventions, participants may not have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Each suspected error or adverse event identified was independently rated by two physician investigators who were unaware of the identity of those involved or whether the incident occurred during the traditional or intervention schedule. Blinded reviewers categorized each incident as an adverse event, nonintercepted serious error, intercepted serious error, or error with little potential for harm." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Low risk | None detected |
| Other bias | High risk | Interns were randomised to work the traditional schedule in the CCU and the intervention schedule in the MICU, or vice versa. The outcome is error (for example, a medication error). Each opportunity for error is not independent, as they cluster within doctor (i.e. each doctor may be more or less likely to make errors). The analyses do not appear to account for this, so it seems that a unit of analysis error was made. |
Leung 2017.
| Study characteristics | ||
| Methods |
RCT. The study utilised a randomised controlled design, with three study groups: Feedback, Training and Control. All doctors, regardless of level or specialty, who had prescribed more than 80 medications in a 4‐month period, were randomised to one of the three study groups and invited to take part in the study. Unit of allocation: doctors Unit of analysis: prescriptions |
|
| Participants | This study was conducted at a 320‐bed teaching hospital in Sydney, Australia. Fifty doctors were randomised (N not available). IP adults (medical and surgical wards) |
|
| Interventions | Education + Error feedback Control: doctors in the control group did not receive any intervention over the course of the study. Intervention 1: Doctors in the Feedback group were sent an email containing an individualised feedback report. This report contained information on the number of duplication alerts triggered by the doctor in the 4‐month period, as well as information (written guide and screenshots) on how to use the ePS shortcut functions to avoid duplication alerts being triggered. In the report, doctors were also provided a contact email for any queries on ePS use or to provide feedback. Information on whether or not participants accessed the feedback document was not able to be collected. Intervention2: doctors in the Training group participated in a 5‐minute face‐to‐face refresher training session. |
|
| Outcomes | The primary outcome measure for the study was the proportion of medication orders which triggered at least one duplication alert (i.e. orders with a duplication alert/total medication orders prescribed). The secondary outcome measure was the average number of duplication alerts per order (i.e. number of duplication alerts triggered/number of medication orders prescribed). A sample of prescription data was extracted from the ePS four months prior to (2/2/2015–2/6/2015) and four months following (5/10/2015–5/2/16) the implementation of interventions. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Fifty doctors were randomised to one of three groups: Control, Feedback or Training. The randomization method was not explained. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the interventions, participants may not have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although not mentioned, data collection was done with electronic records for an objective outcome in a prespecified period of time, so low chances of affecting the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. Data collection was done with electronic records. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Conflict of interest | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | High risk | The unit of randomisation is doctor, but the unit of analysis is order, so a unit of analysis error has been made. |
Lind 2017.
| Study characteristics | ||
| Methods |
RCT ‐ cluster The study was designed as a prospective, cluster‐randomised study. Weekdays were randomised to control or intervention. Clinical Pharmacists (CP) intervention consisted of obtaining medication history and performing medication reconciliation and review. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients were included on weekdays from 09.00 to 16.15 in the acute assessment unit (AAU) at Randers Regional Hospital,Denmark, from 22 October 2013 until 1 May 2014. Eligible for inclusion were medical or surgical patients aged ≥ 18 years, taking ≥ 4 drugs daily (including over‐the‐counter (OTC) drugs, herbals and supplements).The clusters consisted of patients arriving at the AAU at Randers Regional Hospital, Denmark, from 22 October 2013 until 1 May 2014 on weekdays from 09:00 to 16:15. 232 and 216 patients, respectively, were included in control and intervention (N = 448). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation Intervention:clinical pharmacists (CPs) obtained medication history and performed medication reconciliation and review. CPs updated the electronic medication module (EMM) more thoroughly than physicians, especially entering new prescriptions, substitutions and changing instructions for use. Half of the written proposals were accepted. Control: besides examination, the physician was responsible for obtaining a medication history, reconciling and assessing overall medication treatment, and entering approved prescriptions into the electronic medication module (EMM). |
|
| Outcomes | The primary outcome was changes in the Electronic Medication Module (EMM) and changes proposed by CPs. Discrepancy resolutions, length of stay in the AAU. Secondary outcomes were other time‐related measures—for example, physicians’ self‐reported time spent on medication topics |
|
| Notes | ClinicalTrials.gov Identifier: NCT02223676 Funding: Research Center for Emergency Medicine at Aarhus University Hospital, Denmark, The Hospital Pharmacy of Aarhus and Randers Regional Hospital, Denmark |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to either control or intervention using www.randomization.com and block sizes from 8 to 18 to avoid possible prediction of the distribution. |
| Allocation concealment (selection bias) | Low risk | For each cluster, the allocations were written down and placed in a sealed opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Each morning, the AAU staff were informed whether the day was allocated to control or intervention." No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | All changes in the EMM made by physicians and CPs as well as the proposed changes were collected from the EMR and EMM by the first author (KBL) a few days after the intervention. The classification of PCNE codes was performed by the first author (KBL) and a trained CP (CAS). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. Data collected from the EMR and EMM. |
| Selective reporting (reporting bias) | Low risk | The outcomes that are of interest in the review have been reported in the prespecified way. (NCT02223676) |
| Conflict of interest | Low risk | None declared |
| Other bias | Unclear risk | The generalisability of the study is somewhat limited due to the single‐centre focus, the recruitment of patients during office hours only and the alternative interpretation of the PCNE classification. |
Marotti 2011.
| Study characteristics | ||
| Methods |
RCT. This study was a randomised, three‐arm, prospective, parallel group trial. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | All adult elective surgery patients admitted to the John Hunter Hospital on the day of surgery were candidates for inclusion in the study. John Hunter Hospital is a 750‐bed regional tertiary referral hospital in Newcastle, New South Wales, Australia (N = 357). IP adults (surgical wards) |
|
| Interventions | Prescribing and order communication systems, Clinical pharmacy services This randomised controlled three‐arm parallel‐group trial examined the impact of pharmacist medication history taking and pharmacist supplementary prescribing on unintentional omissions of postoperative medications in a large perioperative service. Intervention 1: pharmacist medication history only Intervention 2: pharmacist taking both the history and prescribing medications on their medication chart at surgery. Control: ‘usual care’ involved no clinical pharmacist consultation prior to surgery. These patients had their medications charted immediately prior to surgery or post‐operatively by the medical officer in the normal time frame. |
|
| Outcomes | Prescribing errors Medications charted at incorrect dose Primary aim was to determine whether the number of missed doses of regular medication were significantly different between the three allocated interventions: 1) usual care (control), 2) pre‐operative pharmacist medication history only, and 3) pre‐operative pharmacist medication history and supplementary prescribing on the day of surgery. |
|
| Notes | Australian New Zealand Clinical Trials Registry ACTRN1260900868280 No financial support stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation. Permuted blocks |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | A limitation of this study was that patients, pharmacists and clinicians could not be blinded to intervention group, introducing the opportunity for bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. Outcome measures were collected after discharge by an independent technician through retrospective chart review and patient administration system records. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available, but the important outcomes were described. |
| Conflict of interest | Unclear risk | The authors did not disclose if they had any conflicts of interest. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
McCoy 2012.
| Study characteristics | ||
| Methods |
RCT. A prospective, randomised, controlled study, comparing the effect of enhanced clinical pharmacist surveillance of patients in the intervention group with existing clinical decision support (CDS), and standard pharmacy services on the occurrence, preventability, and severity of ADEs. Unit of allocation: patients Unit of analysis: patients/prescriptions |
|
| Participants | 278 participants were randomised to the control group, and 262 were randomised to the intervention group. The patients were admitted to an academic tertiary care hospital between 1 June 2010 and 31 August 2010 with an acute 0.5 mg/dL change in serum creatinine over 48 hours and a nephrotoxic or renally cleared medication order. (N = 540). IP adults (tertiary care center) |
|
| Interventions | Intervention mixed, Prescribing and order communication systems, Computerized Physician Order Entry (CPOE) + Enhanced clinical pharmacist sevice Intervention: enhanced clinical pharmacist surveillance with existing clinical decision support (CDS) alerts Control: standard pharmacy services also with existing CDS alerts |
|
| Outcomes | Primary outcome was the rate of acute kidney injury‐related ADEs and Potential ADEs, and severity of ADEs. | |
| Notes | The authors were funded in part by National Library of Medicine grants T15 LM007450 and R01 LM009965. Some data collection was supported by NCRR/NIH grant UL1 RR024975. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were automatically assigned to a study group using a pseudo‐random number function within the surveillance tool at the time that he or she first met eligibility criteria and remained in the assigned group until discharge. |
| Allocation concealment (selection bias) | Unclear risk | While the article states that allocation happened automatically, it did not describe anything else about the procedure. Doubts remains to whether this was a centralised allocation or not. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Formal recommendations to doctors treating intervention patients was given so masking was not possible in those cases. Risk of cross‐over also present because neither patients nor doctors were the ones randomised. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors were blinded to patient intervention status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Because everything was registered by the surveillance tool, it seems unlikely to have missing information. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Low risk | The authors declare that they have no conflicts of interest in the research. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Merry 2011.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. Prospective randomised open label clinical trial Unit of allocation: patients Unit of analysis: patients |
|
| Participants | 89 consenting anaesthetists from 5 operating theatres in a major tertiary referral hospital, managing 1075 cases in which there were 10,764 drug administrations (N = 1244). IP adults (operating room) |
|
| Interventions | Intervention mixed (dispensing + Barcoding + Verification + Organizational change) Intervention: use of the new system (which included customised drug trays and purpose‐designed drugtrolley drawers to promote a well‐organised anaesthetic workspace and aseptic technique; pre‐filled syringes for commonly used anaesthetic drugs; large legible colour‐coded drug labels; a barcode reader linked to a computer, speakers, and touch screen to provide automatic auditory and visual verification of selected drugs immediately before each administration; automatic compilation of an anaesthetic record; an on‐screen and audible warning if an antibiotic has not been administered within 15 minutes of the start of anaesthesia; and certain procedural rules—notably, scanning the label before each drug administration) versus conventional practice in drug administration with a manually compiled anaesthetic record. Control: the conventional management option included the following elements. • A standard drug tray to hold the syringes and ampoules. • A standard fully‐stocked drug trolley. • All drugs drawn up by the anaesthetist. • Small standardised colour‐coded drug labels, to be applied by the anaesthetists. • Standard anaesthetic record chart to be filled in by hand, with usual access to data routinely logged by the anaesthetic monitor if desired. |
|
| Outcomes | Total error rate Total nº errors Primary: composite of errors in the recording and administration of intravenous drugs detected by direct observation and by detailed reconciliation of the contents of used drug vials against recorded administrations; and lapses in responding to an intermittent visual stimulus (vigilance latency task). Secondary: outcomes in patients; analyses of anaesthetists’ tasks and assessments of workload; evaluation of the legibility of anaesthetic records; evaluation of compliance with the procedural rules of the new system; and questionnaire‐based ratings of the respective systems by participants. |
|
| Notes | Funding: this project was supported by grant 07/269R from the Health Research Council of New Zealand and a supplementary grant from the Green Lane Research and Educational Fund. These funding organisations were not involved in the study design; in collection, analysis and interpretation of data; in writing the report; or in the decision to submit the article for publication. Australian New Zealand Clinical Trials Registry No 12608000068369 https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000068369 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study's statistician (CF) performed randomisation by week, with treatment allocation codes in blocks of four, and with stratification for study theatre, with a computer‐generated random sequence (Microsoft Excel, Redmond, WA).Theatres were set up for provision of anaesthesia with either the new system or conventional methods, according to the randomisation schedule at the start of each week and remained so for that week. |
| Allocation concealment (selection bias) | Low risk | The study's statistician (CF) performed randomisation by week, with treatment allocation codes in blocks of four and with stratification for study theatre, with a computer‐generated random sequence (Microsoft Excel, Redmond, WA). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the intervention, masking was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although blinding was not possible due to the nature of intervention, researchers made all efforts to ensure objective data collection. Direct observation is an adequate method for data collection. For the assessment of dose discrepancies, a panel of four anaesthetists blinded to the treatment arm in which the discrepancies occurred evaluated dose discrepancies. However, it is also stated that "anaesthetists were less likely to consent to taking part in the study when anaesthetising complex cases, when there was a preference for using the new system", which could have introduced bias. Two investigators (RH and PR) with no relevant conflict of interest were explicitly asked to oversee the study processes; among other things, they made several visits to the study theatres to personally inspect the processes of observation and data collection. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Because of the occasional unavailability of our observers, we were unable to collect data on all cases." |
| Selective reporting (reporting bias) | Low risk | The study protocol and registration details were updated twice before the finalisation of data entry and subsequent analysis of results, to add the terms and for clarification, and because it became apparent that components of the combined primary outcome variable, as initially defined, had different denominators and could not be added to each other. |
| Conflict of interest | Low risk | No competing interest. |
| Other bias | Low risk | This risk was explicitly addressed by the inclusion of senior co‐investigators with no conflicts of interest and by asking independent overseas collaborators to visit and review the study processes. |
Narang 2013.
| Study characteristics | ||
| Methods |
ITS study. This was a before‐and‐after, nonexperimental comparison study that started with a presumed cause and then went forward to evaluate a presumed effect. Unit of analysis: probably monthly administered doses (it is unclear; we cannot discount that unit of analysis was patients) |
|
| Participants | The study was conducted in a 183‐bed for‐profit hospital located in the city of Long Beach, California, USA. The maximum nurse‐to‐patient ratios for the medical‐surgical unit are 1:5. The hospital has 12‐hour shifts (N not available). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Technology (CPOE + eMARs + Barcoding) Intervention: BCMA, barcode‐assisted medication administration and CPOE technology. Barcode on the patient's armband and on the medication were scanned by the nurse using laptops placed on a rolling cart with a barcode scanning device attached to it. Control: usual care without BCMA and CPOE |
|
| Outcomes | Medication error rates Medical error % of total opportunities for error % total reported medication errors Adverse drugs event Dispensing error Administration error The objective of the study was to determine the effect of the BCMA‐CPOE system on medication administration accuracy and medication administration error in an acute care hospital with a highly computerised setting. eMAR was used in conjunction with BCMA‐CPOE in this study. eMAR is updated by the pharmacy continuously with orders received from the individual floors using the scanning system, where 1 indicates routine and 7 would imply stat. The orders scanned to the pharmacy were obtained by nurses as telephone orders, and CPOE physicians had the ability to put in their own medication orders. Once updated by the pharmacy, the eMAR automatically updates the BCMA system when new orders are sent. |
|
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Unclear risk | No statement |
| Other bias | Low risk | No other biases detected |
| Reliable primary outcome measure(s) | Low risk | The outcome was obtained from an automated system (Medication administration reports). |
| Blinded assessment of primary outcome(s) | Low risk | Not blinded but objective method. Medication orders are placed via computerised provider order entry (CPOE) |
| Data were analysed appropriately | High risk | No ARIMA analysis |
| Protection against detection bias (same pre‐post data collection) | Unclear risk | Not described |
| Completeness of data set | Low risk | Data were obtained from an automated system (Medication administration reports). |
| Reason for the number of points pre‐ and post‐intervention given | High risk | Not described; no rationale presented for the numbers of data points |
| Protection against secular changes | Unclear risk | Not described |
| Shape of the intervention effect was specified | Unclear risk | Not described |
Nielsen 2017.
| Study characteristics | ||
| Methods |
RCT. Over 16 months, 593 adult patients taking ≥ 4 medications daily were included from three Danish acute medicine wards. Patients were randomised to either the clinical pharmacist (CP) intervention or the usual care (prospective control). Unit of allocation: patients Unit of analysis: patients |
|
| Participants | The setting was the acute medicine wards of three non‐university hospitals in Region Zealand, one in five regions of Denmark (N = 542 analysed). IP adults (medical wards) |
|
| Interventions | The purpose of the study was to investigate the clinical effect of a clinical pharmacist (CP) intervention upon admission to hospital. Intervention: clinical pharmacist (CP) intervention upon admission to hospital on inpatient harm and to assess a potential educational bias. 1. Review and use of patient's own drugs by clinical pharmacist. 2. Clinical pharmacist taking secondary medication history. 3. Medication review by clinical pharmacist. 4. Entry of proposed prescriptions in the electronic medication system by pharmacist, ready for approval by doctor. The intervention took place on the day the patient was admitted, and the duration of the intervention was approximately 1.5 hours. Control: standard care with no pharmacist involvement (prospective control). |
|
| Outcomes | Primary outcome measure: number of patients with in‐hospital adverse drug events, detected by Adverse Drug Event Trigger Tool. Secondary outcome measures: 1. Length of hospital stay 2. Number of readmissions during the first year after admission |
|
| Notes | ISRCTN08043800 The study was supported by grants from Hospital Pharmacies and Amgros’ Research and Development Foundation, The Health Foundation (Helsefonden) and Region Zealand Health Scientific Research Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In the prospective periods, the patients were stratified by centre and randomised to the intervention or the prospective control using computer‐generated block randomisation with a block size of six. |
| Allocation concealment (selection bias) | Low risk | Allocation was revealed to the CP by telephone whenever the CP had enrolled a patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither the CPs nor the healthcare personnel or patients were blinded to the patient allocation. The patients were informed of their allocation on request, although few actually asked." Since standard care did not include pharmacists, perfomance bias is unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the assessment of the primary outcome, a trigger panel and two outcome panels, all blinded to the allocation of patients, were formed. The trigger panel consisted of two nurses, with 7 and 15 years of clinical experience, both trained in Global Trigger Tool (GTT) as a whole and in the selected medication triggers in particular. The nurses independently reviewed the medical records of all included patients and recorded all triggers. The patients’ records were reviewed in the same order determined by a pre‐made, randomised list. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intervention arm lost 40 participants (20%), proactive control arm lost 49 participants (25%). However, all patients were excluded for the same reason, and baseline characteristics seem balanced except for 1 aspect. |
| Selective reporting (reporting bias) | Low risk | All outcomes except direct cost for the hospital were reported. |
| Conflict of interest | Low risk | None of the authors are affiliated or involved in any organization or entity with a direct or indirect financial interest in the manuscript. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
O'Sullivan 2016.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. Cluster‐randomised controlled trial comparing a clinical decision support software (CDSS)‐supported structured pharmacist review of medication (SPRM) intervention with standard pharmaceutical care in older patients hospitalised with an acute unselected illness. To allow for autocorrelation within the randomisation scheme, which was clustered by clinical specialty service, we quantified the significance of the intervention’s effect on the occurrence of ADRs using generalised estimating equations. Unit of allocation: admitting consultants and their teams Unit of analysis: patients |
|
| Participants | 810‐bed teaching hospital in the Munster region of southern Ireland. All patients aged 65 years admitted under the care of the medical or surgical services through the emergency department were eligible for inclusion. Patients excluded if they were (1) aged < 65 years; (2) admitted to psychiatric services; (3) admitted directly to the intensive care unit; (4) admitted to specialist geriatric medicine or clinical oncology services or had attended these services in the previous 12 months; (5) terminally ill; (6) expected to have a length of stay < 48 h; (7) previously recruited into the study; or (viii) admitted electively (N = 737). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Technology (CPOE + CDSS) Intervention: various interventions have been designed to minimise inappropriate prescribing and curtail hospital‐acquired ADRs in older individuals, e.g. Comprehensive Geriatric Assessment, computerised clinical decision support software (CDSS), prescriber education initiatives and structured pharmacist review of medication (SPRM). Control: control patients received usual care, i.e. routine medical and pharmacist review, depending on their presenting clinical problem(s). The hospital pharmacists performed pharmaceutical reviews within 24–72 h of admission for the majority of trial patients throughout the study period. |
|
| Outcomes | Adverse drug events Median length of stay (days) Hospital mortality The primary outcome was adverse drug reactions (ADRs) |
|
| Notes | Funding: a funding body (Health Research Board of Ireland: HRA_HSR/2010/14) grant funded this work. ClinicalTrials.gov identifier NCT01467128 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on this item. "We cluster‐randomised the admitting consultants and their teams into two groups prior to study initiation, i.e. intervention or control consultants. The research pharmacist was responsible for screening, enrolment and randomisation of patients to the trial. Due to the nature of the intervention, it was not possible to blind participating attending doctors. At admission, we allocated patients to one of two groups (...) based on the particular consultant with primary responsibility for the patient’s care during the index hospital admission." |
| Allocation concealment (selection bias) | High risk | "Once the composition of the clusters was finalized, one group (cluster) of specialist consultants was allocated the intervention arm of the study while the other group (cluster) of specialist consultants was allocated the control arm." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients were unmasked. "Due to the nature of the intervention, it was not possible to blind participating attending doctors." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. "For each putative ADR, the primary researcher recorded details of the suspect medication(s), i.e. dose, formulation and duration, as well as a description of the putative ADR and any actions taken to resolve it. A physician trained in geriatric medicine and experienced in geriatric pharmacology/ therapeutics reviewed and verified all putative ADRs identified by the primary researcher." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Secondary outcomes in clinical trial registration included drug ingredient cost at hospital discharge, Medication Appropriateness Index score, and composite health resource utilisation including hospital admissions and primary care consultations were not reported. |
| Conflict of interest | High risk | David Sullivan and Marie Connor were funded by a Health Research Board Ireland grant to conduct this research using the STOPP/START criteria. Denis Mahony and Stephen Byrne were members of the development and validation team that created the STOPP/START criteria and are named on a patent of computer software which used these criteria. Paul Gallagher was a member of the development and validation team that created the STOPP/START criteria. Shane Cullinan, Richard Sullivan, James Gallagher and Joseph Eustace have no conflicts of interest relevant to the content of this study. |
| Other bias | Low risk | The study appears to be free of other sources of bias. A cluster design was used and an appropriate method (generalized estimating equations ‐ GEEs), was used to account for this (i.e. no unit of analysis error). |
Ongering 2019.
| Study characteristics | ||
| Methods |
ITS study. Interrupted time series analysis was used to evaluate the effect of a CDSS (clinical decision support system). Unit of analysis: prescription |
|
| Participants | This study was conducted at the ICU department of the Amsterdam UMC (location AMC) in the Netherlands (N = 2,711). IP adults (ICU) |
|
| Interventions | On 12 April 2012, the medication interaction module (MiM) was implemented, and medication monitoring systems (MBS) that, as an add‐on module, is compatible with Metavision. The medication‐interactions (MIAs) reports in the MiM were based on the information from the G‐Standaard. The ICU doctors were able to accept the reports (cancel interacting order) or transfer (still prescribe interacting order). The doctor could optionally insert the reasons of their decisions. Each report was provided with information about the type of interaction, advice for handling and severity level. Intervention: medication interaction module (MiM) + CDSS Control: medication interaction module (MiM) but no CDSS |
|
| Outcomes | To evaluate the effect of a CDSS on the incidence of serious potential drug‐drug interactions (pDDIs) in the ICU of an academic hospital. The primary outcome measure was the number of D, E and F potential MIAs per 100 drug administrations. The secondary outcome measures were: • proportion overwritten D, E, and F potential MIA reports; • proportion of monitoring actions in response to (vitamin K antagonist, QTc and nephrotoxic) pMIA reports; • number and type of motivation texts for the overwritten pMIA reports. A potential MIA has been defined as administering a combination of two potentially interacting medicines which could lead to an actual interaction. | |
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | No conflict of interest reported |
| Other bias | Low risk | Not detected |
| Reliable primary outcome measure(s) | Low risk | Obtained from an automated system |
| Blinded assessment of primary outcome(s) | Low risk | Not blinded but objective method. Medication orders were placed via computerised provider order entry (CPOE) |
| Data were analysed appropriately | Low risk | To assess the effect of MiM on the number of D, E and F level potential drug‐drug interactions (pDDIs) per 100 drug administrations, we performed an interrupted time series (ITS) analysis. We tested the difference in trend statistical significance with a generalised linear model with negative binomial link function. The independent variables time (continuous), intervention (0/1) and period after the intervention (0.14 to 27) were included in the ITS model for the analysis. In addition, the following demographic variables were included in the model to correct for differences in patient composition on IC (case mix): age, acute physiology and chronic health evaluation 4 (APACHE IV) score, duration of admission, number of unique medicines (based on Anatomical Therapeutic Chemical code) and number of unique medication administrations (based on Generic Product Code). |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention |
| Completeness of data set | Low risk | Data were obtained by the system |
| Reason for the number of points pre‐ and post‐intervention given | Low risk | To demonstrate a 20% reduction in D, E and F pMIAs per 100 drug administrations, assuming an incidence of 0.11 D, E and F pMIAs per 100 drug administrations (period for the implementation of MiM), 928 patients were needed. These had to be evenly distributed over the period before and after the implementation of MiM (α = 0.05, β = 0.8, based on a negative binomial distribution). |
| Protection against secular changes | Low risk | Patients in the period after MiM implementation had a significantly lower APACHE IV score based on data from the first 24 hours of IC recording (P = 0.006). The other patient characteristics did not differ. |
| Shape of the intervention effect was specified | Unclear risk | Not described |
Pevnick 2018.
| Study characteristics | ||
| Methods |
RCT. This was a three‐arm randomised controlled trial of 306 inpatients. In one intervention arm, pharmacists, and in the second intervention arm, pharmacy technicians, obtained initial admission medication history (AMHs) prior to admission. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Eligible participants were medically complex patients admitted to CSMC through the ED (N=306). IP adults (medical and surgical wards) |
|
| Interventions | Medication reconciliation, Clinical pharmacy services Patients were randomly allocated to usual care or to one of two intervention arms in which either a pharmacist or a pharmacist‐supervised pharmacy technician (PSPT) had primary responsibility for obtaining the AMH. Intervention 1: pharmacist Intervention 2: PSPT (pharmacist‐supervised pharmacy technician) Control: usual care |
|
| Outcomes | The primary outcome was severity‐weighted mean admission medication history (AMH) error score | |
| Notes | Trial registration number NCT02026453 Funding: Joshua Pevnick was supported by the National Institute On Aging and the National Center for Advancing Translational Science of the National Institutes of Health under awards K23AG049181 and UCLA CTSI KL2TR000122 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After enrolling patients meeting criteria, investigators used RANDI2 randomisation software to randomise each patient." |
| Allocation concealment (selection bias) | High risk | "Each block of six consecutively enrolled patients was allocated in a 2:2:2 distribution across the three study arms" "(...) not all aspects of randomisation were masked from study personnel. Because block size was not masked, selection bias could have occurred." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but the outcome is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "In obtaining reference standard AMHs, expert pharmacists identified AMH errors in the initial AMHs and classified each error according to a previously developed taxonomy as significant, serious or life threatening." "A second pharmacist reviewed classifications, and a physician adjudicated disagreements." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The primary outcome was not measurable for 9/103 (8.7%) participants receiving pharmacist AMH. 14/102 (13.7%) participants receiving PSPT AMH, and 6/102 (5.9%) patients receiving usual care, with a total of 28/306 (9.2%) patients lacking a reference standard AMH. Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified in the clinical trial registry. |
| Conflict of interest | Unclear risk | JP currently receives funding from the American Society for Health‐System Pharmacists Research and Education Foundation to design a toolkit for pharmacists to use in post‐discharge medication management. |
| Other bias | Low risk | No other sources of bias were detected. |
Piqueras Romero 2015.
| Study characteristics | ||
| Methods |
RCT. Randomised clinical trial of 17 months (February 2013 to June 2014) in the SSU of a hospital emergency department Unit of allocation: patient Unit of analysis: prescription |
|
| Participants | Patients were aged 65 years or older at high risk of medication‐related problems (MRPs). A total of 130 patients were analysed in the control group (n = 65) or the intervention group (n = 65) and 10 participants were excluded. (N = 140). Elderly IP (ED) |
|
| Interventions | Intervention Human resources, medication reconciliation Intervention: the reconciliation process (intervention) was carried out by a specialised pharmacist Control: no reconciliation |
|
| Outcomes | The main outcome was the number of MRPs in each group. The MRPs are elements of the process (that happen before the result) that, for the drug user, pose a greater risk of negative drug results. | |
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patient selection was made by the study pharmacist immediately in the morning on working days, following the consecutive numerical order of the beds located in the UCE. In this order, patients who met the selection criteria and who consented to participate in the study were randomly assigned to the control group or intervention group according to the balanced block method. |
| Allocation concealment (selection bias) | High risk | Participants or investigator enrolling participants could possibly foresee assignments |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The research pharmacist was responsible for screening, enrolment and randomisation of patients to the trial, providing the intervention and recording of patient data. Due to the nature of the intervention, it was not possible to blind participating attending doctors, patients or outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The research pharmacist was responsible for screening, enrolment and randomisation of patients to the trial, providing the intervention and recording of patient data. Due to the nature of the intervention, it was not possible to blind participating attending doctors, patients or outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Conflict of interest | Low risk | None declared |
| Other bias | Unclear risk | The unit of randomisation was patient, and outcomes were measured on prescriptions. If each patient could have more than one prescription, then there was clustering of prescription within patient. We used the ORs from a logistic mixed‐effects regression model, with random effects used to account for within‐patient grouping. This is an appropriate method for analysing discrepancy resolutions, but there was no adjustment for discrepancy errors and Potential ADEs per prescriptions. |
Quach 2015.
| Study characteristics | ||
| Methods |
RCT. RCT ‐ individual to determine the impact of an early medication reconciliation (MR) in patients evaluated in the emergency department (ED) and identify barriers to reconciling medication in the ED. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients ≥ 65 years old, taking a high alert medication (i.e. anticoagulants, opioids, insulin), or if the patient’s physician deemed it necessary, from the University of California Davis Medical Center (UCDMC) in Sacramento (N = 307). Elderly IP (ED) |
|
| Interventions | Intervention Human resources, medication reconciliation Patients agreeing to receive MR were randomly assigned to receive either: Intervention: MR completed prior to admission Control: MR standard of care |
|
| Outcomes | Discrepancies in prescriptions | |
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All unintentional discrepancies were regarded as errors and were then given to a panel of experts for severity ranking (1 = severe error, 4 = non‐significant error). No information provided on the main outcome assessment (unintentional discrepancy) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Conflict of interest | Unclear risk | No description |
| Other bias | Unclear risk | No information on baseline characteristics of included patients was provided. "A total of 307 patients were enrolled in the study (treatment = 134 and control = 173". The authors mentioned "barriers to MR included: constant movement of patients on the floor, frequent room changes, patients unable to give history due to acuity, inability to reach family or caregiver, and patients discharge before MR can be completed". |
Redwood 2013.
| Study characteristics | ||
| Methods |
RCT. A mixed‐methods approach was employed which included a parallel group randomised controlled trial, and individual and focus group interviews. A power calculation which took into account within‐doctor correlation found the detectable difference in the rate of ignoring password warnings to be < 10% for both grades of doctor (at 80% power with 5% alpha). During the power calculation, non‐trivial levels of correlation were detected in the doctors’ responses to laboratory alerts and alarms. In order to account for this, the analyses were performed using generalised estimating equations with an exchangeable correlation structure. This controlled for the potential non‐independence of repeated measures on the same junior doctor. Binary logistic models were used, with the dependent variable being whether a warning was generated at the relevant level for the prescribing data, and whether a message was ignored for laboratory alert and alarm data. No factors in the generalised estimating equations were found to be significant for the prescribing outcomes. Unit of allocation: doctors Unit of analysis: doctors |
|
| Participants | The study was carried out in a large UK National Health Service (NHS) Foundation Trust teaching hospital (N = 88). IP adults (medical and surgical wards) | |
| Interventions | Intervention Technology Prescribing and order communication systems, Computerized/Clinical Decision Support Systems (CDSS) They used the PICS (Prescribing, Information and Communication System) database to develop the Junior Doctors’ Dashboard (JDD), based on the two highest warning levels for prescribers – disallow and password warnings – which indicate that there is potential for patient harm. Intervention: CDSS Control: No CDSS |
|
| Outcomes | Difference in responses to prescribing warnings (password or disallow level warnings) and laboratory alerting (message ignored and signed off) between the months before and the months during the intervention, analysed as the difference in performance between the intervention and the control groups. Disallow warning Password warning Laboratory alert |
|
| Notes | ISRCTN: ISRCTN72253051 Funded by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care for Birmingham and Black Country |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician randomly assigned the doctors in the trial to the intervention and control groups using random number function in Microsoft Excel and stratification by doctor grade. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "It was not possible to conduct a blinded randomisation due to the nature of the intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinded but warnings generated by Clinical Decision Support Systems and electronic laboratory reporting system are objective automated systems. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44/44 participants in the control group and 42/44 participants in the intervention group were analysed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Conflict of interest | Low risk | Not declared |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Schmader 2004.
| Study characteristics | ||
| Methods | The study employed a randomised 2x2 factorial controlled design. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients were eligible if they were ≥ 65 years old, hospitalised on a medical or surgical ward, had an expected length of stay ≥ 3 days, and met criteria for frailty (N = 834). Elderly IP (medical and surgical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation Intervention: pharmacists performed regular assessments and recommendations regarding medications Control: usual inpatient care that was the customary medical or surgical treatment by attending physicians |
|
| Outcomes | The primary outcomes were related to adverse drug reactions, which were assumed when the relation between an event (i.e. symptoms, signs, laboratory values) and a drug was determined to be causally related to a drug. Secondary outcomes were polypharmacy, inappropriate prescribing, and underuse, which were measured at baseline, hospital discharge, and closeout or date of death, dropout, or institutionalisation | |
| Notes | Financial support was provided by grant AG‐15432 and the Veterans Affairs Cooperative Study Program 006. Additional support was provided by grant AG‐14158 from the National Institute on Aging, Washington, D.C.; grant AI‐51324 from the National Institute of Allergy and Infectious Diseases, Washington, D.C.; the VFW Endowed Chair in Pharmacotherapy for the Elderly, College of Pharmacy, University of Minnesota; and the Veterans Affairs Cooperative HSR&D Service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The coordinating centre used a computer‐generated random allocation sequence to assign patients, stratified by age and functional status, to one of four groups. |
| Allocation concealment (selection bias) | Low risk | The centre notified site research assistants of patients’ inpatient assignment by telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study charts were mailed to the Durham VA. A trained research assistant, blinded to group assignment, conducted closeout telephone interviews 12 months after randomisation and screened for potential drug‐related adverse effects using standardised methods. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it seems that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Unclear risk | Not specified |
| Other bias | High risk | Had a potential source of bias related to the specific study design used. Retrospective methods were used to identify adverse drug reactions, which could have led to underestimation |
Schneider 2006.
| Study characteristics | ||
| Methods |
RCT ‐ individual. This randomised, controlled, non‐blinded study was conducted at three community hospitals. Study participants included 30 registered nurses who had at least one year of nursing experience in acute care and who worked on medical or medical–surgical units. Nurses were randomised to an intervention group that completed an interactive CD‐ROM program on safe medication practices or to a control group. Unit of allocation: nurses Unit of analysis: opportunities for error by nurse |
|
| Participants | Three hospitals of Ohio State University and 30 registered nurses who had worked in medical or medical–surgical units (at least one year of nursing experience in acute care and who worked on medical or medical–surgical units) (N = 30). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Human resources Administration, Training Nurses were randomised to an intervention group that completed an interactive CD‐ROM program on safe medication practices Intervention: interactive CD‐ROM program on safe medication practices Control: no programme |
|
| Outcomes | Total no. errors (including discrepancies) Aggregate‐level error rates were determined using the total number of opportunities for error as the total number of doses administered plus the number of omitted doses during pre‐intervention and post‐intervention periods for each group. |
|
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Nurses were randomly assigned (using a random‐number generator) to either a study or control group |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two blinded observers participated in this study and followed study and control groups during different days and weeks. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All measures were reported. |
| Conflict of interest | Unclear risk | Not specified |
| Other bias | Low risk | The study seems free of other bias. |
Schnipper 2009.
| Study characteristics | ||
| Methods |
RCT ‐ cluster Cluster‐randomised controlled trial A controlled trial, randomised by medical team, on general medical inpatient units at 2 academic hospitals from May to June 2006. Enrolled patients were admitted to 14 medical teams, for whom a medication history could be obtained before discharge. The intervention was a computerised medication reconciliation tool and process redesign involving physicians, nurses, and pharmacists. The main outcome was unintentional discrepancies between pre‐admission medications and admission or discharge medications that had potential for harm (Potential ADEs). Generalised estimating equations, using a robust covariance matrix, were applied to adjust for clustering of results by the admitting physician. Model fit for the propensity score model of the primary outcome was assessed based on aggregates of residuals using the ASSESS statement in SAS statistical software (SAS Institute Inc, Cary, North Carolina), with a P value computed based on 10,000 simulated paths (P = 0.60, suggesting good model fit). Analyses were intention to treat. P = 0.05 (2 sided) was considered significant. Unit of allocation: medical teams and floors Unit of analysis: patients |
|
| Participants | 2 large academic hospitals in Boston, Massachusetts. Participants: eligible patients were admitted to one of several general medicine teams and floors of each hospital, according to a rotating call cycle. Professionals: each team (6 at hospital 1 and 8 at hospital 2) consisted of 1 attending physician, 1 junior or senior resident, 2 to 4 interns, and 1 or 2 medical students. Patients were enrolled if study pharmacists (generally 1 pharmacist per weekday per hospital) had time to obtain a medication history prior to discharge. Patients admitted to 1 of 7 randomly chosen medical teams and floors were assigned to the intervention, while patients admitted to the other teams and on different floors received usual care. (N = 322). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Technology V+C P2 + V3 Intervention:IT applicationdesigned to facilitate medication reconciliation, integrated into the newly developed computerised provider order entry (CPOE) systems at the 2 hospitals, and process redesign involving physicians, nurses, and pharmacists. Control: CPOE without IT application |
|
| Outcomes | Potential adverse drug events (Potential ADEs) errors per patient. Readmission or emergency department visit within 30 days Number of unintentional medication discrepancies with potential for causing harm (Potential ADEs) per patient. Defined as “incidents with potential for injury related to a drug.” |
|
| Notes | NCT00296426 Funded in part by an investigator‐initiated grant from the Harvard Risk Management Foundation, including compensation for Elisabeth Burdick, MS, Amy Bloom, MPH, and Emily Barsky, BA, as well as internal funding from Brigham and Women’s Hospital (BWH), Massachusetts General Hospital, and Partners HealthCare. Dr Schnipper was supported by a mentored clinical scientist award from the National Heart, Lung, and Blood Institute (K08 HL072806). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by study hospital and assigned by the principal investigator (JLS) using random number generation in Microsoft Excel (Microsoft Corp, Redmond, Washington) |
| Allocation concealment (selection bias) | High risk | Patients admitted to 1 of 7 randomly chosen medical teams and floors were assigned to the intervention, while patients admitted to the other teams and on different floors received usual care. Thus, patients in the 2 arms were cared for by different physicians and nurses. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | A gold standard preadmission medication history was taken of all study patients by 1 of 2 study pharmacists at each hospital, following a strict protocol but not blinded to intervention status. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Recorded discrepancies were shown by the study pharmacist to rotating adjudication teams of 2 physicians (from a pool of 6) blinded to intervention status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. Figure 2. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess whether an important risk of bias exists. Outcomes not reported. |
| Conflict of interest | Unclear risk | Insufficient information to permit judgement of 'Low risk'. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Schnipper 2018.
| Study characteristics | ||
| Methods |
CITS. A pragmatic quality improvement (QI) study with concurrent controls, using time series methodology. Unit of analysis: patients |
|
| Participants | Across the five participating sites, patients were enrolled from September 2011 to July 2014, including 613 patients during the pre‐implementation period and 1035 patients during the post‐implementation period, of whom 791 were on intervention units and 244 on control units (N = 1648). IP adults (medical and surgical wards) |
|
| Interventions | A multifaceted medicationreconciliation quality improvement intervention at five US hospitals. Intervention: local implementation of medication reconciliation best practices, utilising an evidence‐based toolkit with 11 intervention components:
Control: pre‐intervention usual care regarding medication reconciliation as currently practiced at each participating site. |
|
| Outcomes | The primary outcome was number of potentially harmful unintentional medication discrepancies per patient; secondary outcome was total discrepancies regardless of potential for harm. | |
| Notes | ClinicalTrials.gov NCT01337063 Funding: this study was supported by the Agency for Healthcare Research and Quality (grant number: R18 HS019598). JLS has received funding from (1) Mallinckrodt Pharmaceuticals for an investigator‐initiated study of opioid‐related adverse drug events in postsurgical patients; (2) Horizon Blue Cross Blue Shield for an honorarium and travel expenses for workshop on medication reconciliation; (3) Island Peer Review Organization for an honorarium and travel expenses for workshop on medication reconciliation; and (4) Portola Pharmaceuticals for investigator‐initiated study of inpatients who decline subcutaneous medications for venous thromboembolism prophylaxis. ASM was funded by a VA HSR&D Career Development Award (12‐168). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | JLS has received funding from Mallinckrodt Pharmaceuticals for an investigator‐initiated study of opioid‐related adverse drug events in postsurgical patients. AM was funded by a VA HSR&D Career Development Award (12‐168). SK has served as a consultant to Verustat. |
| Other bias | Low risk | Not detected. |
| Reliable primary outcome measure(s) | Low risk | The primary outcome was determined by a study pharmacist who took a "gold standard" medication history on 5 patients per week, then compared that history to the medical team's medication history, to admission orders, and to discharge orders. Any unintentional medication discrepancies in orders were recorded. A physician adjudicator then made a final determination regarding whether an error occurred, the type of error, the potential for patient harm, and the potential severity. To ensure consistency in outcome assessment across pharmacists, the research team: (1) provided baseline training; (2) led monthly phone meetings to discuss a patient case and its medication discrepancies; (3) provided an updated 'frequently asked questions' document for managing new situations; and (4) conducted site visits by the research team’s pharmacist (SL) to observe data collection processes and provide feedback. Inter‐rater reliability of discrepancies exceeded 80% across sites |
| Blinded assessment of primary outcome(s) | High risk | Open label |
| Data were analysed appropriately | Low risk | The study used a time series regression model. The outcome was assessed as both a change from site‐specific baseline temporal trends (i.e. change in slope) and sudden improvement with implementation of the intervention as a whole (i.e. change in y‐intercept). To adjust for concurrent controls, we also entered into the model any baseline differences in discrepancy rates and in temporal trends between intervention and control units, as well as sudden improvement in control units at the time when interventions started on other units (i.e. to adjust for the effect of contamination). Additionally, we adjusted for patient demographic, socioeconomic and clinical variables, then manually eliminated non‐significant collinear variables. We used general estimating equations to cluster by site. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention. |
| Completeness of data set | Unclear risk | We used multiple imputation for missing administrative data (which varied by site and characteristic: approximately 26% for marital status; 17%–19% for age, sex, prior admissions, insurance, length of stay and discharge destination; less than 2% for all other demographic variables). Due to restrictions on sharing patient‐level billing data from sites, Elixhauser score and diagnosis‐related group weight were missing in 60% and 54% of patients, respectively, but we received aggregated data by site for these variables to improve our imputation calculations. |
| Reason for the number of points pre‐ and post‐intervention given | Low risk | For a stable estimate of temporal trends, each site’s data collection goal was 22 patients per month, beginning 6 months before implementation through a minimum of 21 months after implementation. |
| Protection against secular changes | Low risk | Our modelling approach allowed us to reduce confounding by comparing each unit to itself over time, adjusting for temporal trends and adjusting for patient case mix. |
| Shape of the intervention effect was specified | Unclear risk | Not described |
Scullin 2007.
| Study characteristics | ||
| Methods |
RCT ‐ individual. Patients meeting the eligibility criteria were randomly assigned to the integrated medicines management (IMM) group or normal care group. Three general hospital sites of the United Hospitals Trust: Antrim Area Hospital (426 beds), Mid‐Ulster Hospital (194 beds) and Whiteabbey Hospital (176 beds) from Ireland. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Patients deemed at risk of drug‐related problems according to a list of drugs (391 (192 male; 199 female) normal care; 371 (167 male; 204 female) IMM) were involved in this service development project over a period of 1.5 years. Patients were eligible for the receipt of the new IMM service if they met any one of the following criteria on admission: were taking at least four regular medications, were taking any high‐risk drugs, were taking antidepressants and were 65 years old or older, and/or had a previous hospital admission within the last 6 months. Scheduled admissions and patients admitted from private nursing homes were excluded. The average age (± SD) of the population who received normal care was 69.9 ± 14.8, compared with an average age of 70.3 ± 13.8 for the IMM population. (N = 762). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation. Intervention: integrated medicines management (IMM) service group. The IMM service involved comprehensive pharmaceutical care provided by a pharmacy team throughout each of three stages: patient admission, inpatient monitoring and counselling, and patient discharge. The IMM team consisted of five pairs of clinical pharmacists and pharmacy technicians. Each pharmacist/technician pair were assigned to wards within the three general hospital sites of the United Hospitals Trust. Each IMM patient received, as time permitted, pharmaceutical care provided by a project pharmacist throughout each of the three IMM stages: admission (medical reconciliation), inpatient monitoring and counselling, and discharge (prescription). Inpatient monitoring and counselling included an intensive clinical pharmacy service throughout their hospital stay. Drug treatment was reviewed daily, taking into account therapeutic goals, relevant clinical chemistry and haematology results, and, where appropriate, therapeutic drug monitoring and counselling focused on drugs which had been commenced or discontinued, and high‐risk drugs. Project technicians implemented an enhanced management of stock on the wards. Control: standard of care |
|
| Outcomes | The primary outcome measure was the difference in the length of hospital stay between the IMM patients and normal care patients. As a secondary outcome measure, over a 12‐month follow‐up period, readmission data for the two groups were collected from the hospital computer system, and included assessment of the time to a further hospital admission as well as the number of readmissions. Further outcomes included an assessment of health care practitioner satisfaction with the new model of care (using custom‐designed satisfaction questionnaires). | |
| Notes | Funding for this project was obtained from the Department of Health, Social Services and Public Safety (Northern Ireland) under its Executive Programme Fund scheme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Patients meeting the eligibility criteria were randomly assigned to the IMM group or normal care group, using block randomisation coupled with a closed envelope technique. |
| Allocation concealment (selection bias) | Low risk | Patients meeting the eligibility criteria were randomly assigned to the IMM group or normal care group, using block randomisation coupled with a closed envelope technique. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessment but is unlikely to be influenced by lack of blinding, since length of stay was not decided by the IMM team |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Conflict of interest | Unclear risk | Not specified |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Seibert 2014.
| Study characteristics | ||
| Methods |
ITS study. A pretest–post‐test nonequivalent comparison group was used to investigate the effect of barcode‐assisted medication administration (BCMA) with electronic medication administration record (eMAR) on the medication administration accuracy rates at two community‐based hospitals. Unit of analysis: monthly administered doses |
|
| Participants | Setting. St. Joseph’s/Candler Health System comprises two tertiary care, community hospitals totaling 644 beds, with an annual patient volume of 22,807. The hospital staff includes 455 community‐based, private practice physicians, 1245 nurses, and 53 pharmacists (N not available). IP adults (community care hospitals) |
|
| Interventions | Intervention Technology, Administration, Barcoding Intervention: the barcode‐assisted medication administration (BCMA) with electronic medication administration record (eMAR) technology on the occurrence of medication administration errors was evaluated. Control: pre‐intervention no BCMA‐eMAR |
|
| Outcomes | Administration error rate Total error rate / 100 administrations Effect of barcode technology with electronic medication administration record on medication accuracy rates Medication administration accuracy rates were observed and recorded before (phase 1) and approximately 6 and 12 months after (phases 2 and 3, respectively) the implementation of BCMA‐eMAR |
|
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | The authors have declared no potential conflicts of interest. |
| Other bias | Low risk | Not detected |
| Reliable primary outcome measure(s) | Low risk | The outcome was obtained from an automated system. |
| Blinded assessment of primary outcome(s) | Unclear risk | Not described |
| Data were analysed appropriately | High risk | Chi2 analysis with Yates correction was used to compare phases 1 and 3 to determine whether the BCMA‐eMAR system was associated with accurate medication administration in each patient care unit. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention. |
| Completeness of data set | Low risk | Data were obtained from an automated system. |
| Reason for the number of points pre‐ and post‐intervention given | Low risk | Observations of medication administration errors were made before (phase 1) and approximately 6 and 12 months after (phases 2 and 3, respectively) implementation of the BCMA‐eMAR system. Post‐implementation data were collected via direct observation after the study unit staff were fully trained, the system was operational for at least 6 months, and study unit nurses achieved an electronic scanning rate of at least 80%. Post‐implementation data were not collected at the same time for all study units. Study units were re‐evaluated approximately 12 months after BCMA‐eMAR implementation. |
| Protection against secular changes | Unclear risk | Not reported |
| Shape of the intervention effect was specified | Low risk | After BCMA‐eMAR was implemented, the number of doses administered showed little change (Figure 1). The number of averted events far exceeded both voluntarily reported and directly observed medication errors. |
SUREPILL 2015.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. In the study interval, randomisation at ward level allocated one ward in each participating centre as the intervention ward (receiving ward‐based pharmacy care), whereas the other ward(s) served as a control (receiving standard care similar to that in the baseline interval). At least two surgical wards from three different types of hospital participated. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Consecutive patients admitted for elective surgery with expected hospital stay longer than 48 h were included. At least two surgical wards from three different types of hospital participated: an academic hospital (Academic Medical Centre, Amsterdam, the Netherlands), a tertiary teaching hospital (Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands) and a community teaching hospital (Diakonessenhuis, Utrecht, the Netherlands) (N = 1094) IP adults (surgical wards) |
|
| Interventions | Intervention Human resources Verification of order communication, Decentralized (satellite) pharmacy systems. Intervention: on admission, the pharmacy practitioner performed medication reconciliation including medication verification of the actual medication use, in consultation with the patient, using a standard questionnaire. During admission, the hospital pharmacist reviewed medication charts and (electronic) patient medical records, and optimised drug therapy when needed. The goal was to perform daily medication reviews of all included patients during the week. At discharge, the pharmacy paractioner performed MR and provided counselling. Control: standard pharmaceuticalcare from a pharmacy team in their traditional role of taking responsibility for the appropriate, safe and cost‐effective use of medication from a central pharmacy. This did not include patient contact or direct access to patients’ medical records; nor did it include regular face‐to‐face contact with ward doctors or nurses. Ward doctors, without consultation with a pharmacist, checked actual medication use on admission and at discharge. These activities were continued at the control wards. |
|
| Outcomes | Preventable ADEs per 100 admissions Length of stay (days) Rehospitalisations Complications (serious) Primary outcome: mean number of preventable ADEs per 100 admissions by hospital department during the baseline and study intervals Secondary outcomes: duration of hospital days; complications per 100 patients; severity of complications; readmissions; health‐related quality of life |
|
| Notes | Funding from ZonMw, the Dutch Organization for Health Research and Development
(project number 170882706). ZonMw approved the SUREPILL study protocol 10.1186/1472-6963-11-55. Netherlands Trial Register (NTR): NTR2258 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two expert panels, each with a consultant surgeon and a clinical pharmacologist, determined causality, preventability and severity of the ADE. The expert panels were blinded to allocation of intervention or control group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Description of 265 patients out of 547 |
| Selective reporting (reporting bias) | Low risk | The protocol describe all reported outcomes. |
| Conflict of interest | Low risk | The authors declared no conflict of interest. |
| Other bias | High risk | The unit of randomisation was ward (i.e. multiple patients), and Poisson regression was used to model the numbers of ADEs for each patient. The analysis did not account for the clustering of patients within wards, so a unit of analysis error was made. |
Tamblyn 2018.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. Cluster‐randomised trial. Pragmatic randomised trial with medical and surgical unit pairs randomised to intervention and control between 2014‐2016. Setting: academic health center including 5 tertiary hospitals for adults and children in Montreal, Quebec, Canada. Unit of allocation: medical units Unit of analysis: patients |
|
| Participants | Among the patients admitted to the intervention and control units, 41.6% were female, and the mean age was 69.6 years (Table 3). Intervention unit patients were slightly older, and there was a higher proportion of male patients, mainly attributable to a higher proportion of male patients being admitted to the cardiac surgery unit. While 14.5% of patients had no prescription medication prior to admission, 15.8% in the control units and 13.0% in the intervention units had 16 prescribed medications (N = 2916). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation Intervention: the automated MedRec application retrieved community‐based medications from the provincial insurance agency and aligned it with in‐hospital medications from the hospital drug information system. The discharge prescription was generated using a one‐click action bar, where the community and hospital drugs to be continued, stopped, modified or started were determined. Control: the units used a fillable PDF form to complete medication reconciliation. |
|
| Outcomes | PADEs included errors in omission of community medications not continued and therapy duplications of 2 or more medications from the same therapeutic class. Potential ADEs were measured at discharge. | |
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Data were extracted from the hospital pharmacy system (GE Centricity) by using the built‐in re‐ port generator. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The outcomes that are of interest in the review have been reported in the prespecified way; |
| Conflict of interest | Low risk | None declared |
| Other bias | High risk | The analysis method does not account for the cluster design. |
Thompson 2018.
| Study characteristics | ||
| Methods |
ITS study. Time series graphs of medication‐related adverse events. Unit of analysis: monthly administered doses |
|
| Participants | The study included all inpatient nursing units at a large academic medical center with recognition as a Magnet organisation (N not available). IP adults (medical and surgical wards) |
|
| Interventions | Intervention Technology, Administration, Barcoding Intervention: barcode medication administration (BCMA) technology Control: no BCMA |
|
| Outcomes | The number of events over time per 100,000 medications administered and the number of days between events for events with harm (category E or higher) or major harm (category F or higher) | |
| Notes | No financial support stated No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | The authors report no competing interests. |
| Other bias | Low risk | Not detected |
| Reliable primary outcome measure(s) | Low risk | Data on these adverse events were collected from the Midasþ system, which includes all relevant characteristics pertaining to the event reported to the system by nursing staff (e.g. event date, harm type, harm level, and nursing unit). |
| Blinded assessment of primary outcome(s) | Low risk | Not blinded but objective method. Medication orders are placed via computerised provider order entry (CPOE) |
| Data were analysed appropriately | Low risk | To assess the effect on medication‐related adverse events, an interrupted time series analysis (ITSA) was performed incorporating a step‐wedge design for the barcoding implementation. The ITSA model was fit using Markov chain Monte‐Carlo via an interface to the JAGS software through the R statistical programming language. The Markov chain Monte‐Carlo is well known to be a reliable and robust approach to fitting complex mixed‐effects models. |
| Protection against detection bias (same pre‐post data collection) | Low risk | Sources and methods of data collection were the same before and after the intervention. To provide consistent measures over time, data on reported medication events were obtained to ensure that the analysis was not influenced by a change in event‐reporting behavior, changes were assessed in all reported medication events and total events (adverse events, potential events that involved the patient, and near misses) in addition to all harmful medication events. |
| Completeness of data set | Low risk | Data were obtained by the system. Data set covers total episodes of care in the study |
| Reason for the number of points pre‐ and post‐intervention given | Unclear risk | Not described |
| Protection against secular changes | Low risk | The intervention occurred independently of other changes over time. Reported errors for medication events decreased over 17% while reporting of nonmedication events increased by 20% after the barcoding system was fully implemented. |
| Shape of the intervention effect was specified | Unclear risk | Not described |
Tompson 2012.
| Study characteristics | ||
| Methods |
RCT ‐ individual. The study was a multicentered, single‐blinded, randomised controlled trial involving “high‐risk” medical patients. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | Eligible patients (2 or more chronic conditions, 3 or more chronic medications and aged over 50 years) were randomised to the intervention or control group. Within 24 hours of admission, the patient’s nominated community pharmacy was contacted, a 6‐month dispensing history obtained, patient was interviewed and a current medication list compiled (N = 539). IP adults (medical and surgical wards) |
|
| Interventions | Intervention mixed (Clinical pharmacy services, medication reconciliation) Intervention: multifaceted intervention to reduce the ADEs associated with transitional care between the community and hospital settings. The interventions included (i) provision of a secure electronic pathway for medication profiles between community and hospital pharmacies, (ii) supply of a comprehensive medication information sheet to the patient/carer, GP and community pharmacist at time of discharge, (iii) upload of the discharge medication informationto a secure website for later viewing and printing by the patient/carer, GP or community pharmacist, and (iv) a model whereby suitable patients were automatically referred for a home medicines review (HMR), to be undertaken shortly after discharge. Control: MR without multifaceted intervention |
|
| Outcomes | Discrepancy resolution Identify and resolve discrepancies in admission medication histories, utilising community pharmacy dispensing data, in newly hospitalised patients, and investigate the relationship between unresolved discrepancies and length of hospital stay. |
|
| Notes | Funding for this project was provided by the Commonwealth Department of Health and Aging through the Community Pharmacy Agreement Grants Program, managed by the Pharmacy Guild of Australia. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolled patients were randomised centrally using computer‐generated randomisation tables to an intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Enrolled patients were randomised centrally using computer‐generated randomisation tables. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was unblinded, but considering the description of each arm it is unclear if perfomance bias exists. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There are some limitations to this randomised controlled trial. The trial was non‐blinded to group allocation and outcome assessment. Allocation concealment would have been difficult to conduct in this type of project. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the publications include all the expected results, including those that were prespecified. |
| Conflict of interest | Low risk | Probably none. Funding for this project was provided by the Commonwealth Department of Health and Aging through the Community Pharmacy Agreement Grants Program, managed by the Pharmacy Guild of Australia. |
| Other bias | Low risk | The study appears to be free from other sources of bias. |
Tong 2016.
| Study characteristics | ||
| Methods |
RCT ‐ cluster. Unblinded cluster‐randomised controlled trial comparing partnered pharmacist charting to standard medical charting among patients admitted to general medical units (GMUs) and emergency short‐stay units with complex medication regimens or polypharmacy. The four GMU subunits were randomised to receiving partnered pharmacist charting among eligible patients in one cluster of two sub‐units, with standard medical charting continuing in the other cluster of the remaining two sub‐units. Unit of allocation: clinical units Unit of analysis: patients |
|
| Participants | Patients admitted to general medical units and emergency short‐stay units with complex medication regimens or polypharmacy. The study was conducted at the Alfred Hospital, Melbourne, Australia (N = 881). IP adults (medical wards) |
|
| Interventions | Intervention Human resources, medication reconciliation Intervention: test the effectiveness of partnered pharmacist charting Control: standard medical charting in preventing inpatient medication errors without pharmacist |
|
| Outcomes | The primary outcome variable was a patient’s medication chart with a medication error detected within 24 h of the patient’s admission, identified by an independent pharmacist assessor. Errors identified were classified as omitted drug, incorrect dose/frequency, incorrect/unnecessary drug or incorrect route of prescription | |
| Notes | Funding provided by the Department of Health and Human Services, Victoria. No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. The four GMU sub‐units were randomised to receiving partnered pharmacist charting among eligible patients in one cluster of two sub‐units, with standard medical charting continuing in the other cluster of the remaining two sub‐units. |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment because central allocation was used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All errors identified were reviewed and assigned a risk rating by a blinded independent expert panel comprising a general physician, an emergency physician and a senior clinical pharmacist. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary outcome variable was a medication error identified by an independent assessor within 24 h of admission, who was not part of the patient's admission process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. The intercluster correlation coefficient was very small (0.0007; 95% CI 0.0 to 0.0009), approaching zero and an adjustment for the design effect was not performed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Conflict of interest | Low risk | None |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Van Doormaal 2009.
| Study characteristics | ||
| Methods |
ITS study. Data collection took place during a 5‐month pre‐implementation period (during which the handwritten medication order system continued to be used) and during a 5‐month post‐implementation period (when the CPOE/CDSS system continued to be used). The post‐implementation data collection period started 8 weeks after finishing the implementation process in order to make sure that initial problems were solved. Because CPOE/CDSS was not simultaneously implemented in all study wards, the starting date for the post‐implementation period was different for each ward. Unit of analysis: prescriptions (Medication Orders) patients |
|
| Participants | Two medical wards of the 1300‐bed University Medical Center Groningen (a general internal medicine and a gastroenterology/rheumatology ward) and in two medical wards (a geriatric and a general internal medicine ward) of the 600‐bed teaching hospital “TweeSteden” in Tilburg and Waalwijk, the Netherlands (N = 1195). IP adults (medical wards) |
|
| Interventions | Intervention Technology Prescribing and order communication systems (CPOE + CDSS) Intervention: the hospitals used the CPOE/CDSS system only for ordering medication. In the system, medication can be selected from menus in which medication from the local ward stock or from the pharmacy drug database is shown. Physicians are obliged to complete fields with key prescription characteristics (such as frequency and administration route). Moreover, standardised prescriptions and medication protocols (a set of prescriptions belonging to one protocol) can be programmed. In this system, transcription of medication orders by both the nurses and the pharmacy staff was no longer necessary. The CDSS system used was basic: safety alerts were rather straightforward and were only generated in case of drug–drug interactions, overdosing, and allergies. Control: pre‐intervention paper‐based |
|
| Outcomes | Total error rate / 100 administrations Preventable ADE per 100 admissions The primary outcome measurements comprised the percentage of medication orders with one or more medication errors (MEs) and the percentage of patients with one or more preventable adverse drug events. |
|
| Notes | Funded by an unconditional grant from the Netherlands Organization for Health Research and Development (ZonMw). file Number 94504109 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Conflict of interest | Low risk | No conflict of interest declared |
| Other bias | Low risk | Not detected |
| Reliable primary outcome measure(s) | Low risk | Data were extracted from the hospital information system, medical charts, and the medication order and administration charts, and, during the post‐intervention period, from the CPOE/CDSS system. |
| Blinded assessment of primary outcome(s) | High risk | After collecting the data, the two research pharmacists, in parallel, individually reviewed the medication orders and identified medication errors according to the classification scheme for medication errors developed by the Netherlands Association of Hospital Pharmacists. They were not blinded as to whether they assessed data before or after the introduction of CPOE/CDSS. The two research pharmacists were thoroughly trained in the classification scheme before the data collection. |
| Data were analysed appropriately | Low risk | Segmented linear regression analysis |
| Protection against detection bias (same pre‐post data collection) | Low risk | The patient data were collected prospectively by two research pharmacists. |
| Completeness of data set | Unclear risk | The dataset seems to be complete (only 4 exclusions because consent was not privided) |
| Reason for the number of points pre‐ and post‐intervention given | Low risk | The reason is provided |
| Protection against secular changes | Low risk | The intervention occurred independently of other changes over time. |
| Shape of the intervention effect was specified | Low risk | Author explained the shape of intervention effect |
Vega 2016.
| Study characteristics | ||
| Methods |
RCT ‐ individual. A randomised, controlled, open‐label clinical trial was designed. To identify the proportion of patients with at least 1 reconciliation error that reached the patient (RERP). Medication reconciliation (intervention group) was compared with standard practice (control group) in patients starting new chemotherapy and who were receiving at least 1 home medication before the start of chemotherapy. A prespecified analysis of factors capable of influencing the occurrence of reconcilation error (RE) in oncological patients was also carried out. Unit of allocation: patients Unit of analysis: patients |
|
| Participants | This study was carried out in Puerta del Mar University Hospital (Cádiz, Spain), a tertiary care center with 620 beds. Patients over 18 years of age who started or changed chemotherapy in an outpatient setting for some oncological disorder and who were also receiving at least 1 additional outpatient medication on a chronic basis (prescription or over‐the‐counter medication) were included (N = 172). | |
| Interventions | Intervention Human resources. Medication reconciliation. Additional components: Verification of order communication and Clinical pharmacy services Intervention: the patients in the intervention group entered a pharmacist‐led medication reconciliation program that was specifically developed for cancer patients during the first cycle of chemotherapy. Control: standard practice for the control and intervention groups included validation of chemotherapy and supportive care medications in the treatment protocol: indication, dose, route and administration sequence, dose adjustments based on toxicity, and stability of intravenous preparations. Standard practice did not include medication reconciliation. |
|
| Outcomes | Total no. errors Reached‐patients errors A 'reconcilation error' was defined as any discrepancy reported to the physician in charge of patient care that resulted in a change in treatment in accordance with the clinical recommendation provided by the pharmacist. |
|
| Notes | No financial support stated No trial register number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by random number assignment. |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. Since the intervention was a professional act, blind patient assignment was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The pharmacist compiled information about medications from the Unique Digital Health Story. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appropriate description of data |
| Selective reporting (reporting bias) | Low risk | The study protocol approved by the Clinical Research Ethics Committee of Puerta del Mar University Hospital and Distrito Bahía de Cádiz‐La Janda (Spain) is not available. However, it seems that all important outcomes were reported. |
| Conflict of interest | Low risk | Not detected |
| Other bias | Unclear risk | There were differing diagnoses between the intervention and control groups, as well as a different gender distribution, with more women than men in the control group (61% vs. 51%). No information was available on whether these differences affected the incidence of REs. The number of patient losses was higher in the control group than in the intervention group. |
Wang 2017.
| Study characteristics | ||
| Methods |
RCT. This was a prospectively randomised open label clinical trial, in 10 designated operating suites in the First Affiliated Hospital of Zhengzhou University, in China. 1066 cases originating from 10,812 medication administrations in anaesthesia were randomised. 78 registered anaesthesiologists managed the medication. The patients received medication administrations in anaesthesia with either an automated or a conventional manual cart. American Society of Anesthesiologists (ASA) score, sex, duration of anaesthesia and surgical specialty, errors in administration of medications (incorrect medication given (substitution), medication not given (omission) and drug recordings errors), compliance and satisfaction were recorded. Unit of allocation: patient Unit of analysis: prescription |
|
| Participants | Data were collected from 10 designated operating suites in the First Affiliated Hospital of Zhengzhou University from May to October 2015. Participants were 1066 patients (533 with the new automated anaesthesia carts and 533 with conventional manual carts) (N = 1066). IP adults (operating room) |
|
| Interventions | Intervention Technology Administration (A)P5 Electronic Medication Administration Records (e‐MARs) and profiles Intervention: automated anaesthesia carts. All participating anaesthesiologists in the intervention group had received formal training on using the automated anaesthesia carts. Medication record is automatically compiled by computer for a real‐time read‐out and a hardcopy of the complete record could be printed out at the end of anaesthesia. Control: conventional manual carts |
|
| Outcomes | Total numbers of medication administrations errors | |
| Notes | This study was supported by the Youth Foundation of the First Affiliated Hospital of Zhengzhou University for Medical Scientific and Technological Project of Henan Province (Grant No. 201403079). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Surgical suites were set up for provision of anaesthesia with either the automated anaesthesia carts or conventional manual carts according to the randomisation schedule at the beginning of each week. Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but the outcome is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At the end of each case, the drugs used were identified by the same means as stated above and the remaining contents of the drug drawers against the pre‐operative inventory were reconciliated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Conflict of interest | Low risk | The study appears to be free of conflicts of interest. |
| Other bias | Unclear risk | Even though the analysis did not model the clustering of opportunity for error within patient, it is plausible that within‐patient ICC would be very close to zero. Further, if very few patients received anaesthesia more than once, each anaesthesia is essentially independent. |
Willoch 2012.
| Study characteristics | ||
| Methods |
RCT ‐ individual. A prospective, randomised controlled trial was designed. Block randomisation. Intervention group (IG) or usual care group (control, CG). Unit of allocation: patients Unit of analysis: patients |
|
| Participants | The rehabilitation ward of a general hospital in Oslo, Norway (N = 77). IP adults (medical and surgical wards) | |
| Interventions | Intervention Human resources, Prescribing and order communication systems, Clinical pharmacy services Intervention reconciliation Patients were randomised into an intervention group (IG) or a usual care group (CG). Intervention: the IG patients were followed prospectively by a pharmacist, who reviewed the patients’ drug therapies using information from their medical records and patient interviews. The pharmacist identified drug‐related problems (DRPs) and suggested solutions during multidisciplinary team meetings. The IG patients received targeted drug counselling from the pharmacist before discharge. The drug therapy in the CG, for the period from study randomisation to discharge, was assessed retrospectively by the pharmacist, who identified DRPs and recorded how they were acted upon. Control: the CG patients were given usual care, insofar as a pharmacist was not part of their treatment teams. No pharmacist counselling was given at discharge. |
|
| Outcomes | Drug‐related problems Number of DRPs DRP / patients Types and frequencies of DRPs in the IG and CG were compared at hospital admission, at discharge, and 3 months after discharge |
|
| Notes | Funded by the Norwegian Directorate of Health No trial number |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After enrolment in the study, the participants were randomized to either the IG or the CG. Block randomisation was applied, with blocks of 20 patients. Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | After enrolment in the study, the participants were randomised to either the IG or the CG. Block randomisation was applied, with blocks of 20 patients. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacists who visited the patients at home were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The pharmacists who visited the patients at home were blinded to whether the patients belonged to the IG or CG. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 77 patients were included, 40 in the IG and 37 in the CG; three patients, all belonging to a total of 40 in the CG group, were lost to follow‐up immediately after the randomisation and data on these were not included in the result analyses. |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) study results that are of interest for review were described; it is clear that publications include all expected results. |
| Conflict of interest | Low risk | No conflicts of interest detected |
| Other bias | High risk | One limitation of the study was the temporal difference between the two groups in the identification of the DRPs present in hospital: the IG was followed prospectively and the DRPs of the CG were assessed retrospectively after discharge. This could have led to fewer observed DRPs in the CG, because less information was available to the pharmacist who identified the DRPs retrospectively. |
AAU: acute assessment unit; BCMA: barcode‐assisted medication administration; CDS(S): clinical decision support (system); CPOE: computerised physician ordering entry; CU: computerised unit; DRP: drug‐related problem; ED: emergency department; EHR: electronic health record; eMAR: electronic medication administration report/record; EMR: electronic medication record; ePS: electronic prescribing system; ICIS: intensive care information system; ICU: intensive care unit; IP: inpatient; ITT: intention‐to‐treat; LOS: length of stay; MAI: Medication Appropriateness Index; MD: medications discrepancy(ies); MH: medical/medications history; MR: medication reconciliation; OP: outpatient; PAC: pre‐admission clinic; Potential ADE: potential adverse drug event; Preventable ADE: preventable adverse drug event; PBU: paper‐based unit; PCNE: Pharmaceutical Care Network Europe; PMR: Pharmacy Management Records; RAR: retract and reorder; TOEs: Total Opportunity for Error; UD: unintentional discrepancies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Farley 2014 | Transition care, intervention at discharge |
| Franklin 2019 | Non‐randomised stepped wedge study |
| Gillespie 2009 | Transition care, main intervention at discharge focused on reducing one‐year mortality and morbidity |
| Heng 2013 | Intervention delivered in endocrine outpatient clinics, not directed at hospitalised patients |
| Kripalani 2012 | Transition care, intervention at discharge |
| Kucukarslan 2003 | Non‐randomised study |
| Makowsky 2009 | The intervention included transition care at discharge and the readmission rate cannot be separated from the intervention directed at reducing inpatient medication errors |
| Pellegrin 2017 | Transition care after discharge to a community consulting pharmacist |
| Shah 2013 | Transition care, intervention at discharge |
| Singh 2012 | Ambulatory setting |
| Stowasser 2002 | Transition care, intervention at discharge. Intervention aimed at reducing medication errors during outpatient setting |
| Whittington 2004 | Time series without basal data |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618000067279.
| Study name | Evaluation of the implementation of electronic prescribing on prescribing errors using interrupted time‐series analysis at two hospitals in Queensland |
| Methods | Interrupted time‐series analysis for the primary outcome (and some secondary outcomes). "We will estimate the level and trend of the primary and secondary outcomes pre‐ and post‐implementation of the electronic prescribing system, using a linear regression model. We will compare differences in means with T‐tests (or non‐parametric alternative, where necessary) and differences in proportions with Chi‐square test. We will perform pre‐specified subgroup analysis across the two different hospitals." |
| Participants | The electronic system will be implemented in three medical wards at Caboolture Hospital, Queensland, Australia (a 265‐bed secondary referral centre) and one geriatrics ward at Royal Brisbane and Women's Hospital (a 926‐bed tertiary referral centre in Brisbane, Queensland, Australia). The electronic prescribing system will be in addition to standard care (see comparator). |
| Interventions |
Intervention The implementation of an electronic prescribing system (MedChart version 9.1), which will, at the time of implementation, contain basic decision support (link to electronic formulary, pregnancy category X warnings), in addition to usual practice. Prescription of infusions, insulin, patient‐controlled analgesia and intravenous heparin will continue to be performed on paper charts. Comparator / control treatment (active) Usual care will consist of prescribing of medications on a standard medication chart (National Inpatient Medication Chart), which contains sections for regular and as‐required medications, and a specific section for variable dose medications, warfarin and venous thromboembolism prophylaxis. There are separate charts for intravenous fluids, patient‐controlled analgesia, intravenous heparin and insulin (subcutaneous and intravenous), with the latter forms having in‐built decision support. In addition, clinicians have access to a range of online and hard copy decision support, including MIMS, Therapeutic Guidelines, Australian Medicines Handbook, Injectables Handbook, plus numerous locally developed guidelines and protocols (e.g. for warfarin and other oral anticoagulants, fluid management). Clinical pharmacists, where possible, perform daily reviews of medication charts. |
| Outcomes | The methods used to collect the data will include review of the medical notes and medication chart, review of reported clinical incidents, and use of hospital coding data which identifies an adverse effect of a medication which has occurred for a patient. A panel of pharmacists and doctors will review all of the identified incidents and potential incidents to determine the severity. The appropriateness of the medications prescribed will also be reviewed using a common tool. |
| Starting date | 24 January 2018 |
| Contact information | Principal investigator Dr Peter Donovan Address Department of Clinical Pharmacology Royal Brisbane and Women's Hospital Butterfield Street, Herston, Queensland 4029, Australia Phone+61 7 3646 8111 Email: peter.donovan@health.qld.gov.au |
| Notes | ACTRN12618000067279 |
ACTRN12619001757101.
| Study name | A stepped‐wedge trial of efficacy and scalability of a virtual clinical pharmacy service (VCPS) in rural and remote New South Wales health facilities |
| Methods | Stepped‐wedge randomised trial. The virtual pharmacy intervention will be delivered using a stepped‐wedge cluster randomised trial design, where the intervention is sequentially implemented in the eight facilities. The ‘steps’ are the order in which each site cross‐over from the control condition (pre‐VCPS) to the intervention condition (VCPS). The sequence of the steps is also randomised, allowing for control of potential confounding temporal trends. This cross‐over will occur across 8 steps (one site per step), each one month apart (with a two month ‘in‐transition’ period). The VCPS will be fully implemented after 11 months with all 8 hospitals receiving the VCPS. Process and outcome measures such as medication reconciliation, hospital readmissions, length of stay and falls data will be collected for baseline data from time period 1 and intervention data from time period 4. |
| Participants | 8 hospitals in Western and Far West New South Wales Local Health Districts, Australia |
| Interventions |
Intervention Virtual Clinical Pharmacy Service (VCPS), that is being delivered to 8 hospitals via a video link. The aim of the virtual pharmacy is to improve medication management, reduce medication harm, help patients manage their medications, and support staff with patients. Comparator / control treatment (active) The hospital sites are their own controls. The virtual pharmacy intervention will be delivered using a stepped‐wedge cluster‐randomised trial design, where the intervention is sequentially implemented in the eight facilities. The ‘steps’ are the order in which each site cross‐over from the control condition (pre‐VCPS) to the intervention condition (VCPS). |
| Outcomes | Primary outcomes The proportion of separations (“discharged home by the hospital”) where the medical reconciliation occurred on admission and discharge. Medical reconciliation is assessed via routine reports on entries in the electronic medical record. Secondary outcomes 28‐day readmission to hospital. The outcome is assessed via routine reports on patient records comparing those who saw a pharmacist with those who did not. Number of falls in hospital. The outcome is assessed via routine reports on patient records comparing those who saw a pharmacist with those who did not. Falls are reported in an on‐line incident management system (IMS) that is connected to the eMR. Detection of medication‐related errors. Medication‐related errors are identified through the medication reconciliation process and recorded on the patient's eMR. |
| Starting date | 03 February 2020 |
| Contact information | Dr Shannon Nott Address Western New South Wales Local Health District, 29 Hawthorn St, Dubbo NSW 2830Country Australia Phone+61 2 68098600 Email shannon.nott@health.nsw.gov.au |
| Notes | ACTRN12619001757101 |
Bakker 2019.
| Study name | The effect of ICU‐tailored drug‐drug interaction alerts on medication prescribing and monitoring: protocol for a cluster randomized stepped‐wedge trial |
| Methods | Stepped‐wedge randomised trial. To define the clinically relevant potential drug‐drug interactions (pDDIs), the authors will follow a rigorous two‐step Delphi procedure in which a national expert panel will assess which pDDIs are perceived clinically relevant for the Dutch ICU setting. Of the 12 ICUs, 9 agreed to participate and will be enrolled in the trial. Our primary outcome measure is the incidence of clinically relevant pDDIs per 1000 medication administrations. |
| Participants | A total of 12 Dutch ICUs using Metavision as a patient data management system in which the clinical decision support system (CDSS) will operate, were invited to participate in the trial. Patients admitted to one of the participating ICUs under the age of 18 will be excluded. |
| Interventions | A clinical decision support system will be implemented that produces alerts to warn for DDIs that are clinically relevant for the ICU setting. Participating ICUs will receive a training for use of the clinical decision support system. |
| Outcomes | Primary outcome Change in the incidence of clinically relevant potential drug‐drug interactions per 1000 medication administrations Secondary outcomes ‐ The number of (clinically relevant) potential drug‐drug interactions per patient ‐ The proportion of patients admitted to the ICU with at least one (clinically relevant) potential drug‐drug interaction ‐ ICU length of stay ‐ The override rate of (clinically relevant) potential drug‐drug interaction alerts ‐ The number of ADEs related to drug‐drug interactions per 1000 medication administrations ‐ The proportion of appropriately‐handled clinically relevant potential drug‐drug interactions |
| Starting date | 01 November 2018 |
| Contact information | Correspondence: t.bakker@amc.nl Department of Medical Informatics, Amsterdam UMC (location AMC), Amsterdam, the Netherlands |
| Notes | Nederlands Trial register Identifier: NL6762 |
Granados 2020.
| Study name | Effect and associated factors of a clinical pharmacy model in the incidence of medication errors (EACPharModel) in the Hospital Pablo Tobón Uribe: study protocol for a stepped wedge randomized controlled trial |
| Methods | A prospective, stepped‐wedge, cluster‐randomised, controlled trial with a duration of 14 months will be performed to compare the effect of a clinical pharmacy practice model (CPPM) along with the usual care process of patients in the Pablo Tobón Uribe Hospital (Medellin, Colombia). The study is designed as a cluster‐randomised controlled trial, involving five hospital wards (clusters) and 720 patients. Medical wards are allocated to interventions using a stepped‐wedge design. Clusters are initially assigned to the control group. After a 2‐month observation period, hospital clusters were randomly allocated to the intervention group. Study outcomes will be assessed at baseline and at 2, 4, 6, 8, 10, and 12 months after randomisation. Statistical analyses will be performed using a mixed model, with the treatment group and time as fixed effects and the clustering structure as a random effect. Statistical analysis will be performed using Pearson Chi2 tests and Student’s t‐tests, and a P value. |
| Participants | 720 patients admitted to five hospital wards (clusters) of the Pablo Tobón Uribe Hospital (Medellin, Colombia) A pharmacist will evaluate whether each patient meets all the inclusion criteria. The inclusion criteria will be the following: patients at least 18 years old; hospitalised patients in the Pablo Tobón Uribe Hospital; patients receive at least five drugs in their pharmacological therapy The exclusion criterion is a ward stay of less than 24 h. |
| Interventions |
Intervention: clinical pharmacy practice model (CPPM) Comparator / control treatment (active): the hospital sites are their own controls. |
| Outcomes | The primary outcome will be to assess the effect of a CPPM on the incidence of medication errors associated with the medication use process. Drug‐related problems and factors that contribute to the occurrence of MEs will be assessed as secondary outcomes. |
| Starting date | 01 February 2018 |
| Contact information | Correspondence: elkyn.granados@udea.edu.co +573185864419 Grupo Promoción y Prevención Farmacéutica, Facultad de Ciencias Farmacéuticas y Alimentarias, Universidad de Antioquia, Calle 70 No 52‐21, Medellín, Colombia Pedro Amariles pedro.amariles@udea.edu.co |
| Notes | NCT03338725 |
IRCT20181213041949N1.
| Study name | Investigation of the effectiveness of a training course for management of common diseases on knowledge and medication error of nurses of Akbar Children's Hospital, Islamic Republic of Iran |
| Methods | Unblinded parallel randomised controlled trial. Placebo: not used Simple randomisation is done through table of random numbers. The researcher at first determines the direction of reading the numbers (for example from top, bottom, left or right). Then the researcher will assign the numbers to the groups (for example, odd ones to intervention group A and even ones to intervention group B). |
| Participants | Inclusion criteria: all nurses with work experience of less than 5 years working in Akbar Hospital, Iran. Exclusion criteria: nurses who are reluctant to participate No age limit, both genders |
| Interventions |
Intervention group: six nurses are randomly selected from each department and based on the table of random numbers are assigned to two groups of 3 people, one receiving training and one without passing a course. Each nurse receives 3 months of technical training by the responsible staff regarding a common disease (determined by the staff using available records and statistics in the related department) in the related field. Each nurse's theoretical and practical training will consist of two 3‐hour training sessions each month (total of 18 hours each). In order to assess the knowledge of nurses in the field of related diseases, before and after training, a questionnaire will be provided from the topics discussed and the average score of nurses before and after training and also in comparison with the control group will be reported. Control group: includes nurses who are randomly selected for the control group without passing a training course. |
| Outcomes | Evaluation of nurses' knowledge level, before and after the intervention. Method of measurement: self‐reported questionnaire about the materials taught Rate of nursing errors. Timepoint: before and after the intervention. Method of measurement: standard questionnaire of nursing medication error validated by Baghaei et al. |
| Starting date | 21 January 2019 |
| Contact information | Name: Ali Khakshour Address: Akbar Children's Hospital, In front of Shahid kave 14, Shahid Kaveh sq 9139963185 Mashhad Iran (Islamic Republic of) Telephone:+98 51 3870 9202 Email:khakshoura@mums.ac.ir Affiliation: Mashhad University of Medical Sciences |
| Notes | IRCT20181213041949N1 |
ISRCTN01624723.
| Study name | Medication error and adverse event detection and resolution by a pharmacist in the Emergency Department at Southampton General Hospital. Sub‐study on patient views about medication |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: patients admitted through the Emergency Department consuming three or more medications Exclusion criteria: not provided at time of registration No age limit, both genders |
| Interventions | Patients who are consuming more than three medications and who are being admitted will be randomised into two groups. Intervention: intensive medication review. Control: the current system of doctors recording medication histories. |
| Outcomes | 1. Detection of medications errors of prescribing, administration or supply 2. Patient side‐effects, or interactions related to admission or adverse events related to medication 3. Early investigation and resolution of these events 4. Documentation of medication errors |
| Starting date | 01 January 2004 |
| Contact information | Mark Tomlin Cardiac Intensive Care Unit Southampton General Hospital Tremona Road SO16 6YD, Southampton, United Kingdom |
| Notes | ISRCTN01624723 |
Lavan 2019.
| Study name | The effect of SENATOR (Software ENgine for the Assessment and optimisation of drug and non‐drug Therapy in Older peRsons) on incident adverse drug reactions (ADRs) in an older hospital cohort ‐ trial protocol |
| Methods | Multinational, pragmatic, parallel arm Prospective Randomised Open‐label, Blinded Endpoint (PROBE) controlled trial. Randomisation is stratified by site and medical versus surgical admission, and uses random block sizes. For outcome data, details were extracted from patients' case records to determine if trigger list adverse clinical events had occurred following randomisation. These trigger list events represented the great majority of adverse drug reactions (ADRs) and were independently adjudicated by a blinded endpoint committee comprised of the co‐PI's, such that no co‐PI adjudicated potential ADRs at his own site. |
| Participants | The trial includes six large university‐affiliated hospitals from across Europe (Ireland, Scotland, Iceland, Spain, Italy and Belgium). Patient inclusion criteria • ≥ 65 years • Admitted with an acute illness under the care of a specialist other than a geriatrician OR clinical pharmacologist OR palliative care physician OR oncologist OR haematologist • Consented into the study ≤ 60 h from time of arrival to the hospital • Anticipated in‐hospital stay of > 48 h, in the opinion of the treating physician • ≥ 3 active (requiring current medication) chronic medical disorders |
| Interventions | All patients randomised to either arm receive standard routine pharmaceutical clinical care as it exists in each site. Intervention: additionally, in the intervention arm, an individualised SENATOR‐generated medication advice report based on the participant's clinical and medication data is placed in their medical record and a senior medical staff member is requested to review it and adopt any of its recommendations that they judge appropriate. Control: standard pharmaceutical care as per local practice. |
| Outcomes | Primary outcome is the proportion of patients experiencing at least one adjudicated probable or certain, non‐trivial ADR, during the index hospitalisation, assessed at 14 days post‐randomisation or at index hospital discharge if it occurs earlier. Potential ADRs are identified retrospectively by the site researchers who complete a Potential Endpoint Form (one per type of event) that is adjudicated by a blinded, expert committee. All occurrences of 12 prespecified events, which represent the majority of ADRs, are reported to the committee along with other suspected ADRs. Participants are followed up 12 (+/‐ 4) weeks post‐index hospital discharge to assess medication quality and healthcare utilisation. |
| Starting date | 09 July 2014 |
| Contact information | Joseph A. Eustace j.eustace@ucc.ie Health Research Board Clinical Research Facility‐Cork, University College Cork, Cork University Hospital, Wilton, Cork, IrelandT12 DC4A. Full list of author information is available at the end of the article. |
| Notes | NCT02097654 |
Leguelinel‐Blache 2018.
| Study name | Impact of collaborative pharmaceutical care on in‐patients’ medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study) |
| Methods | This is a multicentric stepped‐wedge cluster‐randomised study involving six care units from six French University Hospitals (each unit corresponding to a cluster) over seven consecutive 14‐day periods. Each hospital unit will start with a control period and switch to an experimental period after a randomised number of 14‐day periods. For each 14‐day period, 15 patients will be recruited in each care unit to obtain a total of 630 patients enrolled in all centres. During the control period, there will be no clinical pharmacist in the care unit, whereas during the experimental period, a clinical pharmacist will perform medication reconciliation and review with the healthcare team. |
| Participants | Patients aged at least 65 years hospitalised in one of the participating care units and having given their consent to be called for a 30‐day and 90‐day follow‐up can be enrolled. Finally, a total of 630 patients will be enrolled in the study. Patients with a hospital stay of more than 21 days will be excluded. |
| Interventions |
Intervention: the pharmacist performs collaborative pharmaceutical care in the ward: reconciliation of drug treatments and revision of drug prescriptions indicated on the admission drug prescription. All the pharmaceutical interventions, i.e. the medication errors detected and the pharmaceutical suggestions of order modification, will be collected and characterized in a standardized form according to the French Society of Clinical Pharmacy. The pharmaceutical interventions are discussed during a collaborative interview. Control: during the control period, there will be no clinical pharmacist in the care unit, whereas during the experimental period a clinical pharmacist will perform medication reconciliation and review with the healthcare team. |
| Outcomes | Primary outcomes Number of patients with at least one preventable medication error [Time frame: Day 1 (medical prescription at hospital admission)] Number of patients with at least one preventable medication error [Time frame: Phase 2 (maximum 105 days)] Number of patients with at least one preventable medication error not accepted by the prescribing doctor during the interventional phase Secondary outcomes Preventable medication error rate [Time frame: Day 1 (medical prescription at hospital admission)] Potential clinical impact: preventable medication error rate detected in the medical prescription at admission (MPA) according to the level of criticality 1, 2 or 3. This error rate is defined by the ratio of the number of avoidable errors to the number of unrevised lines in the MPA. Number of patients at high risk for adverse drug events [Time frame: Day 1 (medical prescription at hospital admission)] Potential clinical impact: number of patients at high risk for adverse drug events (Trivalle score calculated on the medical prescription at hospital admission) Readmission rate for in‐patient hospitalisation [Time frame: 30 days after hospital discharge (expected maximum of 21 days of hospitalisation)] Clinical impact observed: readmission rate for in‐patient hospitalisation Readmission rate for in‐patient hospitalisation [Time frame: 90 days after hospital discharge (expected maximum of 21 days of hospitalisation)] Clinical impact observed: readmission rate for in‐patient hospitalisation Mortality rate [Time frame: 30 days after hospital discharge (expected maximum of 21 days of hospitalisation)] Mortality rate [Time frame: 90 days after hospital discharge (expected maximum of 21 days of hospitalisation)] Length of hospital stay [Time frame: hospital discharge (expected maximum of 21 days of hospitalisation)] Acceptance rate of pharmaceutical interventions during collaborative interview. [Time frame: Day 1, hospital admission] Avoided costs related to the occurrence of medication errors (criticality 3) [Time frame: 90 days after hospital discharge (expected maximum of 21 days of hospitalisation)] Satisfaction questionnaire (for health care professionals) on the implementation of collaborative pharmaceutical care [Time frame: end of study (expected at 195 days)] |
| Starting date | 4 November 2015 (Final data collection date for primary outcome measure submitted: 27 February 2020) |
| Contact information | Géraldine Leguelinel‐Blache geraldine.leguelinel@chu‐nimes.fr Department of Pharmacy, Nîmes University Hospital, Nîmes, France UPRES EA 2415, Laboratory of Biostatistics, Epidemiology, Clinical Research and Health Economics, Clinical Research University Institute, Montpellier University, Montpellier, France |
| Notes | NCT02598115 |
NCT02999412.
| Study name | Medication reviews bridging healthcare: a cluster‐randomised crossover trial |
| Methods | Multicentre, three‐treatment, replicated, cluster‐randomised, crossover trial. Setting: 8 wards with a multidisciplinary team within 4 hospitals in 3 Swedish counties. |
| Participants | Patients aged 65 years or older, admitted to one of the study wards. Exclusion criteria: palliative stage; residing in other than the hospital's county; medication review within the last 30days; one‐day admission. Estimated enrolment: 2310 participants |
| Interventions |
Intervention 1: comprehensive medication review during hospital stay Intervention 2: same as 1 with the addition of active follow‐up into primary care Control: usual care |
| Outcomes | Primary outcome measure: incidence of unplanned hospital visits during a 12‐month follow‐up period. Secondary outcomes (n = 26) about healthcare utilisation Extraction and collection from the counties' medical record system into a GCP compliant electronic data capture system. Intention‐to‐treat‐analyses using hierarchical models. |
| Starting date | 06 February 2017 |
| Contact information | Thomas G.H. Kempen, thomas.kempen@medsci.uu.se Pharmacy Department, Uppsala University Hospital, Ing.13 2 tr, 751 85 Uppsala, Sweden |
| Notes | NCT02999412 |
NCT03062852.
| Study name | Preventing drug errors related to caregiver interruptions (PERMIS) |
| Methods | The study is a randomised controlled trial in 30 care units of four hospitals in France. Each unit will be randomised in either the control group or the experimental group using the medication safety vest. Nurses of the unit will be selected at random to determine who will be observed during the administration rounds.The observation method will be used to evaluate the error rates in the 2 groups. The number of interruptions and error rates will be evaluated. |
| Participants | Inclusion criteria: ‐ Voluntary nurses of the 30 care units who have drugs to deliver during medication administration rounds will be included. Exclusion criteria:
|
| Interventions |
Intervention: medication safety vest. During administration rounds, nurses will wear the medication safety vest. Control: during administration rounds, nurses will be dressed as usual without a safety vest. |
| Outcomes | The primary outcome is the medication errors rate measured by the observation technique two weeks after implementation of the medication safety vests and flyers. Secondary Outcomes:
|
| Starting date | 03/15/2017 |
| Contact information | Brigitte Sabatier, PharmD, PhD Assistance Publique ‐ Hôpitaux de Paris (AP‐HP), France; INSERM, UMR_S 1138, Equipe 22, Centre de Recherche des Cordeliers, F‐75006 Paris, France. Electronic address: sarah.berdot@aphp.fr. |
| Notes | NCT03062852 |
NCT03541421.
| Study name | Self‐administration of patients' own drugs during hospital stay |
| Methods | This PhD study is performed at the Department of Cardiology, Randers Regional Hospital, Denmark. The study design is "complex intervention" and the PhD study therefore consists of three studies. In study 1, the intervention is developed, investigated for feasibility and pilot‐tested in small scale. In studies 2 and 3, the intervention is evaluated within a RCT with outcomes as medication errors, medication adherence, patient satisfaction and cost‐effectiveness. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: the patients' administer own drugs during hospital stay Control: the patients receive medications from the medicine room dispensed by a nurse (standard care). No intervention. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 06 March 2017 |
| Contact information | Charlotte A. Sørensen, PhD student, Health, Aarhus University Regionshospitalet Randers Randers, Denmark, 8930 |
| Notes | NCT03541421 |
NCT03928106.
| Study name | Impact of pharmacists' directed medication reconciliation on reducing medication discrepancies in a surgery ward |
| Methods | Parallel, single‐blind randomised controlled trial in Jordan University Hospital Amman, Jordan |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: pharmacist responsible for enrolment will identify the medication discrepancies, make the recommendations to correct these discrepancies and contact the physician to resolve these discrepancies. Control: pharmacists will identify medication discrepancies. No recommendation will be written by pharmacists to solve these discrepancies. |
| Outcomes | Primary outcome: The number of accepted recommendations by the clinicians will be documented and recorded, an accepted recommendation, and implemented recommendation at 3 months |
| Starting date | 01 April 2017 |
| Contact information | Khawla Abu Hammour Jordan University Hospital Amman, Jordan, 00962 |
| Notes | NCT03928106 |
ADRs: adverse drug reactions; CDS(S): clinical decision support (system); eMR: electronic medical record; ICU: intensive care unit; pDDI: potential drug‐drug interaction; MEs: medication errors
Differences between protocol and review
We originally planned to include controlled before‐and‐after (CBA) studies. However, considering the amount of evidence from randomised controlled trials (RCTs) and interrupted time series (ITS) studies, and that CBA studies do not report different outcomes than RCTs or ITS studies, we decided to exclude CBA studies except if they could be reanalysed as ITS studies.
We planned to reanalyse ITS studies using time series regression (where possible). We estimated the best fit pre‐intervention and post‐intervention lines using linear regression and autocorrelation adjusted for using the Cochrane‐Orcutt method where appropriate (Draper 1981). At analysis stage, the EPOC group statistician, Christopher James Rose, recommended an alternative method. For the ITS studies, we exponentiated change in level and slope (which were estimated on the logarithmic scale to obtain estimates of ratios of post‐ to pre‐interruption levels and slopes. These estimates describe the nature of any change in reporting. We therefore measured change as the ratio of expected events by extrapolating the pre‐interruption curve into the post‐interruption period and treating it as a counterfactual. Because this ratio is a function of time, we estimated it at one and two years post‐intervention. We excluded a study if it would be necessary to extrapolate beyond the end of follow‐up for that study.
We included new sections on Sensitivity analysis, Subgroup analysis and investigation of heterogeneity, and 'summary of findings and assessment of the certainty of the evidence', not present in the original protocol.
We planned to report rate ratios for dichotomous outcomes. However, we presented odds ratio for most outcomes listed in the summary of findings tables because the reanalysis outputs of many studies were reported with this effect measure.
We made the following changes to the outcomes measures.
We added quality of life as an outcome measure, because it is a very important outcome for patients.
We added 'identified discrepancies' as an outcome measure, included only if no other outcomes were available.
In the protocol, we planned to evaluate costs as a composite outcome, including resource utilisation, length of stay and readmissions. In the review, we disaggregated these into separate outcomes.
Contributions of authors
All the authors contributed to the various stages of the systematic review, interpreted the findings and wrote or revised the manuscript.
Sources of support
Internal sources
-
Instituto de Efectividad Clínica y Sanitaria (IECS), Argentina
IECS protected time for its researchers involved in this review
External sources
-
None, Other
Non applicable
Declarations of interest
The authors declare that they do not have any special conflicts of interest.
New
References
References to studies included in this review
Aag 2014 {published data only}
- Aag T, Garcia BH, Viktil KK. Should nurses or clinical pharmacists perform medication reconciliation? A randomized controlled trial. European Journal of Clinical Pharmacology 2014;70:1325-32. [DOI] [PubMed] [Google Scholar]
Adelman 2013 {published data only}
- Adelman JS, Kalkut GE, Schechter CB, Weiss JM, Berger MA, Reissman SH, et al. Understanding and preventing wrong-patient electronic orders: a randomized controlled trial. Journal of the American Medical Informatics Association 2013;20(2):305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adelman 2019 {published data only}
- Adelman JS, Applebaum JR, Schechter CB, Berger MA, Reissman SH, Thota R, et al. Effect of restriction of the number of concurrently open records in an electronic health record on wrong-patient order errors: a randomized clinical trial. JAMA 2019;321(18):1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agrawal 2009 {published data only}
- Agrawal A, Wu WY. Reducing medication errors and improving systems reliability using an electronic medication reconciliation system. Joint Commission Journal on Quality and Patient Safety / Joint Commission Resources 2009;35(2):106-14. [DOI] [PubMed] [Google Scholar]
Al‐Hashar 2018 {published data only}
- Al-Hashar A, Al-Zakwani I, Eriksson T, Sarakbi A, Al-Zadjali B, Al Mubaihsi S, et al. Impact of medication reconciliation and review and counselling, on adverse drug events and healthcare resource use. International Journal of Clinical Pharmacy 2018;40(5):1154-64. [DOI] [PubMed] [Google Scholar]
Barker 1984 {published data only}
- Barker KN, Pearson RE, Hepler CD, Smith WE, Pappas CA. Effect of an automated bedside dispensing machine on medication errors. American Journal of Hospital Pharmacy 1984;41(7):1352-8. [PubMed] [Google Scholar]
Becerra‐Camargo 2015 {published data only}
- Becerra-Camargo J, Martinez-Martinez F, Garcia-Jimenez E. A multicentre, double-blind, randomised, controlled, parallel-group study of the effectiveness of a pharmacist-acquired medication history in an emergency department. BMC Health Services Research 2013;13:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Camargo J, Martinez-Martinez F, Garcia-Jimenez E. The effect on potential adverse drug events of a pharmacist-acquired medication history in an emergency department: a multicentre, double-blind, randomised, controlled, parallel-group study. BMC Health Services Research 2015;15:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beckett 2012 {published data only}
- Beckett RD, Crank CW, Wehmeyer A. Effectiveness and feasibility of pharmacist-led admission medication reconciliation for geriatric patients. Journal of Pharmacy Practice 2012;25(2):136-41. [DOI] [PubMed] [Google Scholar]
Bell 2016 {published data only}
- Bell SP, Schnipper JL, Goggins K, Bian A, Shintani A, Roumie CL, et al. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. Journal of General Internal Medicine 2016;31(5):470-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhakta 2019 {published data only}
- Bhakta SB, Colavecchia AC, Haines L, Varkey D, Garey KW. A systematic approach to optimize electronic health record medication alerts in a health system. American Journal of Health-system Pharmacy 2019;76(8):530-6. [DOI] [PubMed] [Google Scholar]
Bolas 2004 {published data only}
- Bolas H. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharmacy World & Science : PWS 2004;26(2):114-20. [DOI: 10.1023/b:phar.0000018601.11248.89] [DOI] [PubMed] [Google Scholar]
Boockvar 2017 {published data only}
- Boockvar KS, Ho W, Pruskowski J, DiPalo KE, Wong JJ, Patel J, et al. Effect of health information exchange on recognition of medication discrepancies is interrupted when data charges are introduced: results of a cluster-randomized controlled trial. Journal of the American Medical Informatics Association : JAMIA 2017;24(6):1095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowdle 2018 {published data only}
- Bowdle TA, Jelacic S, Nair B, Togashi K, Caine K, Bussey L, et al. Facilitated self-reported anaesthetic medication errors before and after implementation of a safety bundle and barcode-based safety system. British Journal of Anaesthesia 2018;121(6):1338-45. [DOI] [PubMed] [Google Scholar]
Burkoski 2019 {published data only}
- Burkoski V, Yoon J, Solomon S, Hall TN, Karas AB, Jarrett SR, et al. Closed-loop medication system: leveraging technology to elevate safety. Nursing Leadership (Toronto, Ont.) 2019;32(SP):16-28. [DOI] [PubMed] [Google Scholar]
Cadman 2017 {published data only}
- Cadman B, Wright D, Bale A, Barton G, Desborough J, Hammad EA, et al. Pharmacist provided medicines reconciliation within 24 hours of admission and on discharge: a randomised controlled pilot study. BMJ Open 2017;7(3):e013647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiu 2018 {published data only}
- Chiu KC, Lee WK, See YW, Chan HW. Outcomes of a pharmacist-led medication review programme for hospitalised elderly patients. Hong Kong Medical Journal 2018;24(2):98-106. [DOI] [PubMed] [Google Scholar]
Colpaert 2006 {published data only}
- Colpaert K, Claus B, Somers A, Vandewoude K, Robays H, Decruyenaere J. Impact of computerized physician order entry on medication prescription errors in the intensive care unit: a controlled cross-sectional trial. Critical Care (London, England) 2006;10(1):R21. [DOI: 10.1186/cc3983] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Winter 2011 {published data only}
- De Winter S, Vanbrabant P, Spriet I, Desruelles D, Indevuyst C, Knockaert D, et al. A simple tool to improve medication reconciliation at the emergency department. European Journal of Internal Medicine 2011;22(4):382-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ding 2012 {published data only}
- Ding Q. The Effect of the Unit Dose Dispensing System on Medication Preparation and Administration Errors in Intravenous (IV) Drugs in a Chinese Hospital: Inpatient [Doctoral thesis]. Auburn (AL): Auburn University Harrison School of Pharmacy, 2012. [Google Scholar]
Farris 2014 {published data only}
- Farris KB, Carter BL, Xu Y, Dawson JD, Shelsky C, Weetman DB, et al. Effect of a care transition intervention by pharmacists: an RCT. BMC Health Services Research 2014;14:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandes 2011 {published data only}
- Fernandes OA, Etchells EE, Lee AW, Siu V, Bell C, Wong G, et al. What is the impact of a centralized provincial drug profile viewer on the quality and efficiency of patient admission medication reconciliation? A randomized controlled trial. Canadian Journal of Hospital Pharmacy 2011;64(1):85. [Google Scholar]
Furuya 2013 {published data only}
- Furuya H, Morimoto T, Ogawa Y. Relationship between the use of an electronic commercial prescribing system and medical and medication errors in a teaching hospital. Tokai Journal of Experimental and Clinical Medicine 2013;38(1):33-6. [PubMed] [Google Scholar]
George 2011 {published data only}
- George LJW, Senturk-Raif R, Hodgkinson MR, Emmerton M, Larmour I. Impact of a surgical preadmission clinic pharmacist on the quality of medication management from preadmission to discharge: a randomised controlled study. Journal of Pharmacy Practice and Research 2011;41(3):212‐6. [Google Scholar]
Gordon 2017 {published data only}
- Gordon M, Jones H. Prospective ongoing prescribing error feedback to enhance safety: a randomised controlled trial. Drugs and Therapy Perspectives 2017;33(8):387-94. [Google Scholar]
Graabaek 2019 {published data only}
- Graabaek T, Hedegaard U, Christensen MB, Clemmensen MH, Knudsen T, Aagaard L. Effect of a medicines management model on medication-related readmissions in older patients admitted to a medical acute admission unit: a randomized controlled trial. Journal of Evaluation in Clinical Practice 2019;25(1):88-96. [DOI] [PubMed] [Google Scholar]
Green 2015 {published data only}
- Green RA, Hripcsak G, Salmasian H, Lazar EJ, Bostwick SB, Bakken SR, et al. Intercepting wrong-patient orders in a computerized provider order entry system. Annals of Emergency Medicine 2015;65(6):679-686.e1. [DOI: 10.1016/j.annemergmed.2014.11.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greengold 2003 {published data only}
- Greengold NL, Shane R, Schneider P, Flynn E, Elashoff J, Hoying CL, et al. The impact of dedicated medication nurses on the medication administration error rate: a randomized controlled trial. Archives of Internal Medicine 2003;163(19):2359-67. [DOI: 10.1001/archinte.163.19.2359] [DOI] [PubMed] [Google Scholar]
Gursanscky 2018 {published data only}
- Gursanscky J, Young J, Griffett K, Liew D, Smallwood D. Benefit of targeted, pharmacist-led education for junior doctors in reducing prescription writing errors – a controlled trial. Journal of Pharmacy Practice and Research 2018;48(1):26‐35. [Google Scholar]
Hale 2013 {published data only}
- Hale AR, Coombes ID, Stokes J, McDougall D, Whitfield K, Maycock E, et al. Perioperative medication management: expanding the role of the preadmission clinic pharmacist in a single centre, randomised controlled trial of collaborative prescribing. BMJ Open 2013;13(7):pii: e003027. [DOI: 10.1136/bmjopen-2013-003027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heselmans 2015 {published data only}
- Heselmans A, Van Krieken J, Cootjans S, Nagels K, Filliers D, Dillen K, et al. Medication review by a clinical pharmacist at the transfer point from ICU to ward: a randomized controlled trial. Journal of Clinical Pharmacy and Therapeutics 2015;40(5):578-83. [DOI] [PubMed] [Google Scholar]
Hickman 2018 {published data only}
- Hickman L, Poole SG, Hopkins RE, Walters D, Dooley MJ. Comparing the accuracy of medication order verification between pharmacists and a tech check tech model: a prospective randomised observational study. Research in Social & Administrative Pharmacy : RSAP 2018;14(10):931-5. [DOI] [PubMed] [Google Scholar]
Higgins 2010 {published data only}
- Higgins T, Heelon M, Siano B, Douglass L, Liebro P, Spath B, et al. Medication safety improves after implementation of positive patient identification. Applied Clinical Informatics 2010;1(3):213-20. [DOI: 10.4338/ACI-2010-02-RA-0011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Juanes 2018 {published data only}
- Juanes A, Garin N, Mangues MA, Herrera S, Puig M, Faus MJ, et al. Impact of a pharmaceutical care programme for patients with chronic disease initiated at the emergency department on drug-related negative outcomes: a randomised controlled trial. European Journal of Hospital Pharmacy 2018;25(5):274-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kannampallil 2018 {published data only}
- Kannampallil TG, Manning JD, Chestek DW, Adelman J, Salmasian H, Lambert BL, et al. Effect of number of open charts on intercepted wrong-patient medication orders in an emergency department. Journal of the American Medical Informatics Association : JAMIA 2018;25(6):739-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalil 2016 {published data only}
- Khalil V, deClifford JM, Lam S, Subramaniam A. Implementation and evaluation of a collaborative clinical pharmacist's medications reconciliation and charting service for admitted medical inpatients in a metropolitan hospital. Journal of Clinical Pharmacy and Therapeutics 2016;41(6):662-6. [DOI] [PubMed] [Google Scholar]
Kwan 2007 {published data only}
- Kwan Y, Fernandes OA, Nagge JJ, Wong GG, Huh JH, Hurn DA, et al. Pharmacist medication assessments in a surgical preadmission clinic. Archives of Internal Medicine 2007;167(10):1034-40. [DOI: 10.1001/archinte.167.10.1034] [DOI] [PubMed] [Google Scholar]
Landrigan 2004 {published data only}
- Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. New England Journal of Medicine 2004;351(18):1838-48. [DOI: 10.1056/NEJMoa041406] [DOI] [PubMed] [Google Scholar]
Leung 2017 {published data only}
- Leung S, Zheng WY, Sandhu A, Day R, Li L, Baysari M. Feedback and training to improve use of an electronic prescribing system: a randomised controlled trial. Studies in Health Technology and Informatics 2017;239:63-9. [PubMed] [Google Scholar]
Lind 2017 {published data only}
- Lind KB, Soerensen CA, Salamon SA, Jensen TM, Kirkegaard H, Lisby M. Impact of clinical pharmacist intervention on length of stay in an acute admission unit: a cluster randomised study. European Journal of Hospital Pharmacy 2016;23(3):171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind KB, Soerensen CA, Salamon SA, Kirkegaard H, Lisby M. Consequence of delegating medication-related tasks from physician to clinical pharmacist in an acute admission unit: an analytical study. European Journal of Hospital Pharmacy 2017;24(5):272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marotti 2011 {published data only}
- Marotti SB, Kerridge RK, Grimer MD. A randomised controlled trial of pharmacist medication histories and supplementary prescribing on medication errors in postoperative medications. Anaesthesia and Intensive Care 2011;39(6):1064-70. [DOI: 10.1177/0310057X1103900613] [DOI] [PubMed] [Google Scholar]
McCoy 2012 {published data only}
- McCoy AB, Cox ZL, Neal EB, Waitman LR, Peterson NB, Bhave G, et al. Real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: a randomized, controlled trial. Applied Clinical Informatics 2012;3(2):221‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merry 2011 {published data only}
- Merry AF, Webster CS, Hannam J, Mitchell SJ, Henderson R, Reid P, et al. Multimodal system designed to reduce errors in recording and administration of drugs in anaesthesia: prospective randomised clinical evaluation. BMJ (Online) 2011;343:d5543. [DOI: 10.1136/bmj.d5543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Narang 2013 {published data only}
- Narang S. Use of technology to reduce the occurrence of medication errors in a U.S. hospital: a project report. ProQuest Dissertations and Theses 2013:45. [ISBN 978-1-303-76609-1] [Publication Number: 1527334]
Nielsen 2017 {published data only}
- Nielsen TR, Honore PH, Rasmussen M, Andersen SE. Clinical effects of a pharmacist intervention in acute wards - a randomized controlled trial. Basic & Clinical Pharmacology & Toxicology 2017;121(4):325-33. [DOI] [PubMed] [Google Scholar]
O'Sullivan 2016 {published data only}
- O'Sullivan D, Mahony D, Connor MN, Gallagher P, Gallagher J, Cullinan S, et al. Prevention of adverse drug reactions in hospitalised older patients using a software-supported structured pharmacist intervention: a cluster randomised controlled trial. Drugs & Aging 2016;33(1):63-73. [DOI: 10.1007/s40266-015-0329-y.] [DOI] [PubMed] [Google Scholar]
Ongering 2019 {published data only}
- Ongering MS, Bakker T, Dongelmans DA, De Keizer NF, Abu-Hanna A, Klopotowska JE. Effect of a clinical decision support system on reducing drug-drug interactions in the intensive care unit. Pharmaceutisch Weekblad 2019;154(39):17-22. [Google Scholar]
Pevnick 2018 {published data only}
- Pevnick JM, Nguyen C, Jackevicius CA, Palmer KA, Shane R, Cook-Wiens G, et al. Improving admission medication reconciliation with pharmacists or pharmacy technicians in the emergency department: a randomised controlled trial. BMJ Quality & Safety 2018;27(7):512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piqueras Romero 2015 {published data only}
- Piqueras Romero C, Calderon Hernanz B, Segura Fragoso A, Juarez Gonzalez R, Berrocal Javato MA, Calleja Hernandez MA. Efficacy of a reconciliation intervention by a specialized pharmacist to resolve medication-related problems of elderly patients admitted to an emergency department short-stay unit: a randomized clinical trial. Emergencias 2015;27(6):364-70. [PubMed] [Google Scholar]
Quach 2015 {published data only}
- Quach J, Hua S, Traylor B, Burton J. Reduce medication errors by doing early medication reconciliation in the emergency department. Pharmacotherapy 2015;35:E197. [Google Scholar]
Redwood 2013 {published data only}
- Redwood S, Ngwenya NB, Hodson J, Ferner RE, Coleman JJ. Effects of a computerized feedback intervention on safety performance by junior doctors: results from a randomized mixed method study. BMC Medical Informatics & Decision Making 2013;13:63. [DOI: 10.1186/1472-6947-13-63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmader 2004 {published data only}
- Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. American Journal of Medicine 2004;116(6):394-401. [DOI] [PubMed] [Google Scholar]
Schneider 2006 {published data only}
- Schneider PJ, Pedersen CA, Montanya KR, Curran CR, Harpe SE, Bohenek W, et al. Improving the safety of medication administration using an interactive CD-ROM program. American Journal of Health-System Pharmacy 2006;63(1):59-64. [DOI: 10.2146/ajhp040609] [DOI] [PubMed] [Google Scholar]
Schnipper 2009 {published data only}
- Schnipper JL, Hamann C, Ndumele CD, Liang CL, Carty MG, Karson AS, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Archives of Internal Medicine 2009;169(8):771-80. [DOI] [PubMed] [Google Scholar]
Schnipper 2018 {published data only}
- Mixon AS, Kripalani S, Stein J, Wetterneck TB, Kaboli P, Mueller S, et al. An on-treatment analysis of the MARQUIS study: interventions to improve inpatient medication reconciliation. Journal of Hospital Medicine 2019;14(10):614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper JL, Mixon A, Stein J, Wetterneck TB, Kaboli PJ, Mueller S, et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: final results of the MARQUIS study. BMJ Quality & Safety 2018;27(12):954-64. [DOI] [PubMed] [Google Scholar]
Scullin 2007 {published data only}
- Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. Journal of Evaluation in Clinical Practice 2007;13(5):781-8. [DOI] [PubMed] [Google Scholar]
Seibert 2014 {published data only}
- Seibert HH, Maddox RR, Flynn EA, Williams CK. Effect of barcode technology with electronic medication administration record on medication accuracy rates. American Journal of Health-System Pharmacy 2014;71(3):209-18. [DOI: 10.2146/ajhp130332] [DOI] [PubMed] [Google Scholar]
SUREPILL 2015 {published data only}
- Surgery and Pharmacy in Liaison (SUREPILL) Study Group. Effect of a ward-based pharmacy team on preventable adverse drug events in surgical patients (SUREPILL study). British Journal of Surgery 2015;102(10):1204-12. [DOI: 10.1002/bjs.9876] [DOI] [PubMed] [Google Scholar]
Tamblyn 2018 {published data only}
- Tamblyn R, Huang AR, Motulsky A, Meguerditchian AN, Winslade NE, Buckeridge DL, et al. The Right-Rx medication reconciliation trial: impact on potential adverse drug events. Pharmacoepidemiology and Drug Safety 2018;27:366-7. [Google Scholar]
- Tamblyn R, Winslade N, Lee TC, Motulsky A, Meguerditchian A, Bustillo M, et al. Improving patient safety and efficiency of medication reconciliation through the development and adoption of a computer-assisted tool with automated electronic integration of population-based community drug data: the RightRx project. Journal of the American Medical Informatics Association : JAMIA 2018;25(5):482-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2018 {published data only}
- Thompson KM, Swanson KM, Cox DL, Kirchner RB, Russell JJ, Wermers RA, et al. Implementation of bar-code medication administration to reduce patient harm. Mayo Clinic Proceedings: Innovations, Quality & Outcomes 2018;2(4):342-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tompson 2012 {published data only}
- Tompson AJ, Peterson GM, Jackson SL, Hughes JD, Raymond K. Utilizing community pharmacy dispensing records to disclose errors in hospital admission drug charts. International Journal of Clinical Pharmacology & Therapeutics 2012;50(9):639-46. [DOI: 10.5414/CP201720] [DOI] [PubMed] [Google Scholar]
Tong 2016 {published data only}
- Tong EY, Roman C, Mitra B, Yip G, Gibbs H, Newnham H, et al. Partnered pharmacist charting on admission in the General Medical and Emergency Short-stay Unit - a cluster-randomised controlled trial in patients with complex medication regimens. Journal of Clinical Pharmacy and Therapeutics 2016;41(4):414-8. [DOI] [PubMed] [Google Scholar]
Van Doormaal 2009 {published data only}
- Van Doormaal JE, Van den Bemt PM, Zaal RJ, Egberts AC, Lenderink BW, Kosterink JG, et al. The influence that electronic prescribing has on medication errors and preventable adverse drug events: an interrupted time-series study. Journal of the American Medical Informatics Association 2009;16(6):816-25. [DOI: 10.1197/jamia.M3099 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vega 2016 {published data only}
- Vega TG, Sierra-Sanchez JF, Martinez-Bautista MJ, Garcia-Martin F, Suarez-Carrascosa F, Baena-Canada JM. Medication reconciliation in oncological patients: a randomized clinical trial. Journal of Managed Care & Specialty Pharmacy 2016;22(6):734-40. [DOI: 10.18553/jmcp.2016.15248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang Y, Du Y, Zhao Y, Ren Y, Zhang W. Automated anesthesia carts reduce drug recording errors in medication administrations: a single center study in the largest tertiary referral hospital in China. Journal of Clinical Anesthesia 2017;40:11-5. [DOI] [PubMed] [Google Scholar]
Willoch 2012 {published data only}
- Willoch K, Blix HS, Pedersen-Bjergaard AM, Eek AK, Reikvam A. Handling drug-related problems in rehabilitation patients: a randomized study. International Journal of Clinical Pharmacy 2012;34:382-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Farley 2014 {published data only}
- Farley TM, Shelsky C, Powell S, Farris KB, Carter BL. Effect of clinical pharmacist intervention on medication discrepancies following hospital discharge. International Journal of Clinical Pharmacy 2014;36(2):430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franklin 2019 {published data only}
- Franklin BD, Puaar S. What is the impact of introducing inpatient electronic prescribing on prescribing errors? A naturalistic stepped wedge study in an English teaching hospital. Health Informatics Journal 2019;26(4):3152-62. [DOI: 10.1177/1460458219833112] [DOI] [PubMed] [Google Scholar]
Gillespie 2009 {published data only}
- Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Archives of Internal Medicine 2009;169(9):894-900. [DOI] [PubMed] [Google Scholar]
Heng 2013 {published data only}
- Heng ST, Lee JC. Medication reconciliation in outpatient hospital clinics. Annals of the Academy of Medicine, Singapore 2013;42(9 Suppl 1):S7. [Google Scholar]
Kripalani 2012 {published data only}
- Kripalani S, Roumie CL, Dalal AK, Cawthon C, Businger A, Eden SK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Annals of Internal Medicine 2012;157(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kucukarslan 2003 {published data only}
- Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Archives of Internal Medicine 2003;163(17):2014-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Makowsky 2009 {published data only}
- Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676]. Medical Care 2009;47(6):642-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pellegrin 2017 {published data only}
- Pellegrin KL, Krenk L, Oakes SJ, Ciarleglio A, Lynn J, McInnis T, et al. Reductions in medication-related hospitalizations in older adults with medication management by hospital and community pharmacists: a quasi-experimental study. Journal of the American Geriatrics Society 2017;65(1):212-9. [DOI] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah M, Norwood CA, Farias S, Ibrahim S, Chong PH, Fogelfeld L. Diabetes transitional care from inpatient to outpatient setting: pharmacist discharge counseling. Journal of Pharmacy Practice 2013;26(2):120-4. [DOI] [PubMed] [Google Scholar]
Singh 2012 {published data only}
- Singh R, Anderson D, McLean-Plunkett E, Brooks R, Wisniewski A, Satchidanand N, et al. IT-enabled systems engineering approach to monitoring and reducing ADEs. American Journal of Managed Care 2012;18(3):169-75. [PMID: ] [PubMed] [Google Scholar]
Stowasser 2002 {published data only}
- Stowasser DA, Collins DM, Stowasser M. A randomised controlled trial of medication liaison services - patient outcomes. Journal of Pharmacy Practice and Research 2002;32(2):133-40. [Google Scholar]
Whittington 2004 {published data only}
- Whittington J, Cohen H. OSF healthcare's journey in patient safety. Quality Management in Health Care 2004;13(1):53-9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12618000067279 {published data only}
- ACTRN12618000067279. Evaluation of the implementation of electronic prescribing on prescribing errors using interrupted time-series analysis at two hospitals in Queensland. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374237 (first received 29 December 2017).
ACTRN12619001757101 {published data only}
- ACTRN12619001757101. Realising the benefits of clinical pharmacy in the bush: the efficacy and scalability of a virtual clinical pharmacy service (VCPS) in rural and remote New South Wales health facilities. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378878 (first received 5 December 2019).
Bakker 2019 {published data only}
- Bakker T, Klopotowska JE, Eslami S, Lange DW, Van Marum R, Van der Sijs H, et al. The effect of ICU-tailored drug-drug interaction alerts on medication prescribing and monitoring: protocol for a cluster randomized stepped-wedge trial. BMC Medical Informatics and Decision Making 2019;19(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Granados 2020 {published data only}
- Granados J, Salazar-Ospina A, Botero-Aguirre JP, Valencia-Quintero AF, Ortiz N, Amariles P. Effect and associated factors of a clinical pharmacy model in the incidence of medication errors (EACPharModel) in the Hospital Pablo Tobon Uribe: study protocol for a stepped wedge randomized controlled trial (NCT03338725). Trials 2020;21(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20181213041949N1 {published data only}
- IRCT20181213041949N1. Assessment of the effectiveness of a training course on knowledge and medication error of nurses. trialsearch.who.int/?TrialID=IRCT20181213041949N1 (first received 21 January 2019).
ISRCTN01624723 {published data only}
- ISRCTN01624723. Medication error and adverse event detection and resolution by a pharmacist in the Emergency Department at Southampton General Hospital. Sub-study on patient views about medication. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN01624723 (first received 28 September 2007).
Lavan 2019 {published data only}
- Lavan AH, O'Mahony D, Gallagher P, Fordham R, Flanagan E, Dahly D, et al. The effect of SENATOR (Software ENgine for the Assessment and optimisation of drug and non-drug Therapy in Older peRsons) on incident adverse drug reactions (ADRs) in an older hospital cohort - Trial Protocol. BMC Geriatrics 2019;19(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leguelinel‐Blache 2018 {published data only}
- Leguelinel-Blache G, Castelli C, Roux-Marson C, Bouvet S, Andrieu S, Cestac P, et al. Impact of collaborative pharmaceutical care on in-patients' medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study). Trials 2018;19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02999412 {published data only}
- NCT02999412. Medication reviews bridging healthcare: a cluster-randomised crossover trial. clinicaltrials.gov/ct2/show/NCT02999412 (first received 21 December 2016).
NCT03062852 {published data only}
- NCT03062852. Preventing drug errors related to caregiver interruptions (PERMIS). clinicaltrials.gov/ct2/show/NCT03062852 (first received 23 February 2017).
NCT03541421 {published data only}
- NCT03541421. Self-administration of patients' own drugs during hospital stay. clinicaltrials.gov/ct2/show/NCT03541421 (first received 30 May 2018).
NCT03928106 {published data only}
- NCT03928106. Impact of pharmacists' directed medication reconciliation on reducing medication discrepancies in a surgery ward. clinicaltrials.gov/ct2/show/NCT03928106 (first received 25 April 2019).
Additional references
Ahtiainen 2020
- Ahtiainen HK, Kallio MM, Airaksinen M, Holmström AR. Safety, time and cost evaluation of automated and semi-automated drug distribution systems in hospitals: a systematic review. European Journal of Hospital Pharmacy: Science and Practice 2020;27(5):253-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alanazi 2016
- Alanazi MA, Tully MP, Lewis PJ. A systematic review of the prevalence and incidence of prescribing errors with high-risk medicines in hospitals. Journal of Clinical Pharmacy and Therapeutics 2016;41(3):239-45. [DOI] [PubMed] [Google Scholar]
Anderson 2019
- Anderson LJ, Schnipper JL, Nuckols TK, Shane R, Le MM, Robbins K, et al. Effect of medication reconciliation interventions on outcomes: A systematic overview of systematic reviews. American Journal of Health-system Pharmacy 2019;76(24):2028-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Assiri 2018
- Assiri GA, Shebl NA, Mahmoud MA, Aloudah N, Grant E, Aljadhey H, et al. What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ Open 2018;8(5):e019101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2004
- Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. Canadian Medical Association Journal 2004;170(11):1678-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bates 1995
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. Journal of General Internal Medicine 1995;10(4):199-205. [PMID: ] [DOI] [PubMed] [Google Scholar]
Berdot 2016
- Berdot S, Roudot M, Schramm C, Katsahian S, Durieux P, Sabatier B. Interventions to reduce nurses' medication administration errors in inpatient settings: a systematic review and meta-analysis. International Journal of Nursing Studies 2016;53:342-50. [DOI] [PubMed] [Google Scholar]
Brennan 2005
- Brennan TA, Gawande A, Thomas E, Studdert D. Accidental deaths, saved lives, and improved quality. New England Journal of Medicine 2005;353(13):1405-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cheema 2018
- Cheema E, Alhomoud FK, Kinsara ASA, Alsiddik J, Barnawi MH, Al-Muwallad MA, et al. The impact of pharmacists-led medicines reconciliation on healthcare outcomes in secondary care: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2018;13(3):e0193510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2019
- Choi YJ, Kim H. Effect of pharmacy-led medication reconciliation in emergency departments: a systematic review and meta-analysis. Journal of Clinical Pharmacy and Therapeutics 2019;44(6):932-45. [DOI] [PubMed] [Google Scholar]
Christensen 2016
- Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD008986. [DOI: 10.1002/14651858.CD008986.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Classen 1997
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997;277(4):301-6. [PMID: ] [PubMed] [Google Scholar]
Classen 2005
- Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. 1991. Quality & Safety in Health Care 2005;14(3):221-5; discussion 225-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed prior to 4 November 2021. Melbourne, Australia: Veritas Health Innovation, 2021. Available at covidence.org.
Cumby 1992
- Cumby RE, Huizinga J. Testing the autocorrelation structure of disturbances in ordinary least squares and instrumental variables regressions. Econometrica 1992;60(1):185-95. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Devin 2020
- Devin J, Cleary BJ, Cullinan S. The impact of health information technology on prescribing errors in hospitals: a systematic review and behaviour change technique analysis. Systematic Reviews 2020;9(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vries 2008
- Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Quality & Safety in Health Care 2008;17(3):216-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Draper 1981
- Draper N, Smith H. Applied Regression Analysis. New York (NY): Wiley, 1981. [Google Scholar]
Eng 2018
- Poh EW, McArthur A, Stephenson M, Roughead EE. Effects of pharmacist prescribing on patient outcomes in the hospital setting: a systematic review. JBI Database of Systematic Reviews & Implementation Reports 2018;16(9):1823-73. [DOI] [PubMed] [Google Scholar]
EPOC 1998
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. EPOC methods paper: interrupted time series (ITS) designs for EPOC reviews; April 1998. Available at epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/inttime.pdf.
EPOC 2015
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. EPOC Taxonomy; 2015. epoc.cochrane.org/epoc-taxonomy (accessed prior to 4 November 2021).
EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. Suggested risk of bias criteria for EPOC reviews; August 2017. Available at www.epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.pdf 2017.
European Council 2005
- Council of Europe Committee of Experts on Management of Safety and Quality in Health Care, Expert Group on Safe Medicine Practices. Glossary of terms related to patient and medication safety – approved terms; October 2005. Available at www.who.int/patientsafety/highlights/COE_patient_and_medication_safety_gl.pdf.
Falconer 2019
- Falconer N, Barras M, Martin J, Cottrell N. Defining and classifying terminology for medication harm: a call for consensus. European Journal of Clinical Pharmacology 2019;75(2):137-45. [DOI] [PubMed] [Google Scholar]
Gillani 2020
GRADEpro GDT [Computer program]
- GRADEpro Guideline Development Tool (GDT). Version accessed prior to 4 November 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brożek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Harkanen 2016
- Harkanen M, Voutilainen A, Turunen E, Vehvilainen-Julkunen K. Systematic review and meta-analysis of educational interventions designed to improve medication administration skills and safety of registered nurses. Nurse Education Today 2016;41:36-43. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodgkinson 2006
- Hodgkinson B, Koch S, Nay R, Nichols K. Strategies to reduce medication errors with reference to older adults. International Journal of Evidence-based Healthcare 2006;4(1):2-41. [DOI] [PubMed] [Google Scholar]
Hultcrantz 2017
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. Journal of Clinical Epidemiology 2017;87:4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2001
- Ioannidis J P, Lau J. Evidence on interventions to reduce medical errors: an overview and recommendations for future research. J Gen Intern Med 2001;16(5):325-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISMP 2011
- Institute for Safe Medicine Practices. Adverse Drug Events and Potential Adverse Drug Events. Horsham (PA): Institute for Safe Medication Practices, 2011. [Google Scholar]
Jia 2016
- Jia P, Zhang L, Chen J, Zhao P, Zhang M. The effects of clinical decision support systems on medication safety: an overview. PloS One 2016;11(12):e0167683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalil 2020
- Khalil H, Kynoch K, Hines S. Interventions to ensure medication safety in acute care: an umbrella review. International Journal of Evidence-based Healthcare 2020;18(2):188-211. [DOI] [PubMed] [Google Scholar]
Kohn 2000
- Institute of Medicine Committee on Quality of Health Care in America. To Err is Human: Building a Safer Health System. Washington (DC): National Academies Press (US) Copyright 2000 by the National Academy of Sciences. All rights reserved., 2000. [PMID: 25077248] [Google Scholar]
Korb‐Savoldelli 2018
- Korb-Savoldelli V, Boussadi A, Durieux P, Sabatier B. Prevalence of computerized physician order entry systems-related medication prescription errors: a systematic review. International Journal of Medical Informatics 2018;111:112-22. [DOI] [PubMed] [Google Scholar]
Larmené‐Beld 2018
- Larmené-Beld KHM, Alting EK, Taxis K. A systematic literature review on strategies to avoid look-alike errors of labels. European Journal of Clinical Pharmacology 2018;74(8):985-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linden 2016
- Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata Journal 2016;15(2):480–500. [Google Scholar]
Lisby 2012
- Lisby M, Nielsen LP, Brock B, Mainz J. How should medication errors be defined? Development and test of a definition. Scandinavian Journal of Public Health 2012;40(2):203-10. [DOI] [PubMed] [Google Scholar]
Maaskant 2015
- Maaskant JM, Vermeulen H, Apampa B, Fernando B, Ghaleb MA, Neubert A, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD006208. [DOI: 10.1002/14651858.CD006208.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manias 2020
- Manias E, Kusljic S, Wu A. Interventions to reduce medication errors in adult medical and surgical settings: a systematic review. Therapeutic Advances in Drug Safety 2020;11:[29 p.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mekonnen 2016
- Mekonnen AB, Abebe TB, McLachlan AJ, Brien JA. Impact of electronic medication reconciliation interventions on medication discrepancies at hospital transitions: a systematic review and meta-analysis. BMC Medical Informatics and Decision Making 2016;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morimoto 2004
- Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Quality & Safety in Health Care 2004;13(4):306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCC‐MERP 2021
- National Coordinating Council for Medication Error Reporting and Prevention. About Medication Errors: What is a Medication Error.. www.nccmerp.org/about-medication-errors (accessed prior to 4 November 2021).
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795-801. [PMID: ] [DOI] [PubMed] [Google Scholar]
Prgomet 2017
- Prgomet M, Ling L, Niazkhani Z, Georgiou A, Westbrook JI. Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. Journal of the American Medical Informatics Association 2017;24(2):413-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redmond 2018
- Redmond P, Grimes TC, McDonnell R, Boland F, Hughes C, Fahey T. Impact of medication reconciliation for improving transitions of care. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD010791. [DOI: 10.1002/14651858.CD010791.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roumeliotis 2019
- Roumeliotis N, Sniderman J, Adams-Webber T, Addo N, Anand V, Rochon P, et al. Effect of electronic prescribing strategies on medication error and harm in hospital: a systematic review and meta-analysis. Journal of General Internal Medicine 2019;34(10):2210-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarfati 2019
- Sarfati L, Ranchon F, Vantard N, Schwiertz V, Larbre V, Parat S, et al. Human-simulation-based learning to prevent medication error: a systematic review. Journal of Evaluation in Clinical Practice 2019;25(1):11-20. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Shitu 2019
- Shitu Z, Aung MM, Kamauzaman TH, Bhagat V, Rahman AF. Medication error in hospitals and effective intervention strategies: a systematic review. Research Journal of Pharmacy and Technology 2019;12(10):4669-77. [Google Scholar]
Shojania 2001
- Shojania KG, Duncan BW, McDonald KM, Wachter RM (editors). Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment No. 43 edition. Vol. AHRQ Publication No. 01-E058. San Francisco (CA): Agency for Healthcare Research and Quality, July 2001. [PMC free article] [PubMed] [Google Scholar]
Sloss 2020
- Sloss EA, Jones TL. Alert types and frequencies during bar code-assisted medication administration: a systematic review. Journal of Nursing Care Quality 2020;35(3):265-9. [DOI] [PubMed] [Google Scholar]
StataCorp 2015 [Computer program]
- Stata. Version 14. College Station, Tx, USA: StataCorp, 2015. Available at www.stata.com.
Vélez‐Díaz‐Pallarés 2018
- Vélez-Díaz-Pallarés M, Pérez-Menéndez-Conde C, Bermejo-Vicedo T. Systematic review of computerized prescriber order entry and clinical decision support. American Journal of Health-system Pharmacy 2018;75(23):1909-21. [DOI] [PubMed] [Google Scholar]
Vilela 2018
- Vilela R, Pompeo D, Jericó M, Werneck A. Cost of the medication error and adverse drug events in the medication therapy chain: review literature as a topic. Jornal Brasileiro de Economia da Saúde 2018;10(2):179-89. [Google Scholar]
Villar 2020
- Villar V, Duarte S, Martins M. Patient safety in hospital care: a review of the patient's perspective. Cadernos de Saúde Publica 2020;36(12):e00223019. [DOI] [PubMed] [Google Scholar]
Vrbnjak 2016
- Vrbnjak D, Denieffe S, O'Gorman C, Pajnkihar M. Barriers to reporting medication errors and near misses among nurses: a systematic review. International Journal of Nursing Studies 2016;63:162-78. [DOI] [PubMed] [Google Scholar]
Walsh 2017
- Walsh EK, Hansen CR, Sahm LJ, Kearney PM, Doherty E, Bradley CP. Economic impact of medication error: a systematic review. Pharmacoepidemiology and Drug Safety 2017;26(5):481-97. [DOI] [PubMed] [Google Scholar]
Wang 2018
- Wang H, Meng L, Song J, Yang J, Li J, Qiu F. Electronic medication reconciliation in hospitals: a systematic review and meta-analysis. European Journal of Hospital Pharmacy 2018;25(5):245-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2010
- Wong K, Yu SK, Holbrook A. A systematic review of medication safety outcomes related to drug interaction software. Journal of Population Therapeutics and Clinical Pharmacology [Journal de la Therapeutique des Populations et de la Pharamcologie Clinique] 2010;17(2):e243-55. [PubMed] [Google Scholar]
World Alliance for Patient Safety 2008
- World Alliance for Patient Safety. Summary of the Evidence on Patient Safety: Implications for Research. Geneva, Switzerland: World Health Organization, 2008. [Google Scholar]
Young 2019
- Young IJ, Luz S, Lone N. A systematic review of natural language processing for classification tasks in the field of incident reporting and adverse event analysis. International Journal of Medical Informatics 2019;132:103971. [DOI] [PubMed] [Google Scholar]




