Abstract
The extracellular calcium-sensing receptor (CaSR) controls vital bone cell functions such as cell growth, differentiation and apoptosis. The binding of the native agonist (Ca2+) to CaSR activates the receptor, which undergoes structural changes that trigger a cascade of events along the cellular signaling pathways. Strontium (in the form of soluble salts) has been found to also be a CaSR agonist. The activation of the receptor by Sr2+ is considered to be the major mechanism through which strontium exerts its anti-osteoporosis effect, mostly in postmenopausal women. Strontium-activated CaSR initiates a series of signal transduction events resulting in both osteoclast apoptosis and osteoblast differentiation, thus strengthening the bone tissue. The intimate mechanism of Sr2+ activation of CaSR is still enigmatic. Herewith, by employing a combination of density functional theory (DFT) calculations and polarizable continuum model (PCM) computations, we have found that the Ca2+ binding sites 1, 3, and 4 in the activated CaSR, although possessing a different number and type of protein ligands, overall structure and charge state, are all selective for Ca2+ over Sr2+. The three binding sites, regardless of their structural differences, exhibit almost equal metal selectivity if they are flexible and have no geometrical constraints on the incoming Sr2+. In contrast to Ca2+ and Sr2+, Mg2+ constructs, when allowed to fully relax during the optimization process, adopt their stringent six-coordinated octahedral structure at the expense of detaching a one-backbone carbonyl ligand and shifting it to the second coordination layer of the metal. The binding of Mg2+ and Sr2+ to a rigid/inflexible calcium-designed binding pocket requires an additional energy penalty for the binding ion; however, the price for doing so (to be paid by Sr2+) is much less than that of Mg2+. The results obtained delineate the key factors controlling the competition between metal cations for the receptor and shed light on some aspects of strontium’s therapeutic effects.
Keywords: calcium-sensing receptor (CaSR), Ca2+/Sr2+ selectivity, Mg2+, DFT/PCM calculations, CaSR agonists, osteoporosis
1. Introduction
The extracellular calcium-sensing receptor (CaSR), a member of the G protein-coupled receptor superfamily, plays a key role in regulating Ca2+ concentration in extracellular fluids, which (in resting conditions) are usually within the 1.1–1.3 mM range [1]. It is expressed in a variety of tissues, such as parathyroid glands, kidneys and bone cells, and has been assigned to perform an active role in maintaining skeletal homeostasis [2,3]. CaSR senses alterations in Ca2+ concentration in blood and tightly controls Ca2+ levels by modulating the synthesis of parathyroid hormone in the parathyroid glands and regulating the reabsorption of Ca2+ in the kidneys and bones [4]. In different bone cells (osteoblasts, osteoclast precursors and mature osteoclasts), CaSR governs vital cellular functions such as cell growth, differentiation and apoptosis [2]. The binding of the native agonist, Ca2+, to CaSR activates the receptor, which undergoes structural changes, thus, triggering a cascade of events along the phospholipase C and cAMP-dependent signal transduction pathways [1].
The CaSR is a homodimer with a molecular mass of 240-310 kDa [5]. A recent X-ray study of the extracellular domain of the human CaSR revealed four metal-binding sites in each monomer, one of which (Site 2) is loaded with Ca2+ in both the inactive and active states of the receptor, whereas, the other three sites (Site 1, Site 3 and Site 4) are populated by the cognate Ca2+ in the activated state only [6]. The presence of the metal-loaded Site 2 in both inactive and active states of the CaSR implies that it is an integral part of the receptor’s structure. The experiments have suggested that Site 2 provides a crucial framework for the recognition of the receptor co-activator, L-Trp [6]. Crystallographic data have also shown that the bound Ca2+ ions have different peak heights in the anomalous difference maps, which suggests different Ca2+-occupancies (and respectively, affinities) of the metal-binding sites. The Ca2+ ions at Sites 1 and 2 have strong peaks, suggesting that these are high-occupancy sites. The Ca2+ ions at Sites 3 and 4 have weaker anomalous peaks, which implies lower occupancy/metal affinity of these sites. The Ca2+ ion at Site 4 has the weakest peak compared with the other sites, which is consistent with the site being loaded only at elevated concentrations of the activator [6].
Site 1 is located in a loop region and comprises backbone carbonyl groups of Ile81, Ser84, Leu87 and Leu88 lining the binding pocket. The metal cation in Site 2 is directly coordinated by the side chain of Thr100 and indirectly (via a water molecule) by the side chain of Asn102. The calcium ion in Site 3 is bound in an outer-shell mode (via water molecules) to Ser302 and Ser303, whereas the side chain of Asp234 and backbone carbonyl groups of Glu231 and Gly557 surround the metal cation in Site 4. Note that these binding pockets are somewhat atypical (less crowded) for the calcium ion, which usually binds to constellations of 5 or 6 protein ligands [7,8]. The role of bound Ca2+ ions in activating CaSR has been found to be mostly structural: they stabilize the active state by enhancing homodimer interactions between membrane-proximal domains [6].
The CaSR can be activated by other divalent/trivalent metal cations and organic polycations as well, though with lesser effectiveness compared to that of the cognate activator [1]. The activation of the receptor by Sr2+ is of particular interest since it is considered as the major mechanism through which strontium (in the form of soluble salts) exerts its anti-osteoporosis effect, mostly in postmenopausal women [1,9,10]. Strontium-activated CaSR initiates a series of signal relay processes promoting both osteoclast apoptosis and osteoblast differentiation, thus strengthening the bone material. In support of this mechanism, it has been experimentally demonstrated that Sr2+, unlike other divalent metal cations, such as Mg2+, is a full agonist of CaSR with efficacy close to that of Ca2+ [1,10]. The rationale behind this lies in the fact that the properties of Ca2+ and Sr2+ ions are rather close. They are spherical, doubly charged, alkali-earth metal cations with similar physico-chemical properties and marked affinity toward “hard” (less polarizable) oxygen-containing ligands. Their ionic radii are also similar: 1.0/1.06 Å for Ca2+ and 1.18/1.21 Å for Sr2+ in hexacoordinated/heptacoordinated complexes, respectively [11]. The respective hydration-free energies are not very different either: −359.7 kcal/mol for Ca2+ and −329.8 kcal/mol for Sr2+ [12]. In the human body, the two metals behave similarly, exhibiting distinct bone-seeking properties [13].
The intimate mechanism of Sr2+ activation of CaSR is, however, not fully understood. Several outstanding questions remain to be answered. To what extent are Ca2+/Sr2+-selective metal binding sites of the activated CaSR? How effectively could the “alien” Sr2+ compete with the native Ca2+ for binding sites of the receptor? What are the key determinants of the metal affinity/selectivity of CaSR in the activated state? Why is Sr2+ a full CaSR agonist while other divalent cations, like Mg2+, are much less effective in activating the host protein?
Herein, we try to answer these questions by studying the Ca2+/Sr2+ competition in the metal-binding sites of the activated CaSR, employing density functional theory (DFT) calculations combined with polarizable continuum model (PCM) computations. The first/second-shell ligands and metal cations are treated explicitly using quantum chemical methods to account for electronic effects such as the polarization of the participating entities and charge transfer from the ligands to the metal cation, whereas the rest of the protein is represented by a continuum dielectric constant ranging from 4 (buried binding sites) to 29 (binding pockets with high solvent exposure). Such an approach allows treating the strong electrostatic interactions between the two competing metal ions and protein ligands in computing the Ca2+ → Sr2+ exchange free energies and assessing the metal’s affinity toward the coordinating ligands as accurately as possible. The findings of this work, which are consistent with available experimental data, help delineate the key factors making Sr2+ a potent agonist for CaSR.
2. Materials and Methods
2.1. Models Used
The Ca2+/Sr2+/Mg2+-loaded binding sites of CaSR were modeled, and their thermodynamic characteristics were evaluated. The models were built based on the X-ray structure of the Ca2+-bound active-state of human CaSR (PDB entry 5K5S; resolution 2.60 Å) [6]. The metal cation and its first/second shell ligands (see Introduction) were incised from the protein structure and further modified by capping the amino acid side chains at the Cα atom with a methyl group. Thus, the side chains of Asp−, Ser, Thr and Asn were represented by CH3CH2COO−, CH3CH2OH, CH3CH(OH)CH3 and CH3CH2CONH2, respectively, while the metal-coordinated backbone peptide group was modeled by N-methylacetamide (CH3CONHCH3). Since the Ca2+ coordination number in proteins is typically seven [14], the calcium coordination sphere in each binding site was accordingly complemented with the respective number of water molecules. The initial Ca2+-bound constructs created were thus subjected to geometry optimization (see below). Consequently, Ca2+ in the optimized structures was replaced by Sr2+ and Mg2+ and the resulting strontium/magnesium-containing constructs were fully optimized. The optimized structures of Ca2+, Sr2+ and Mg2+ complexes are shown in Figure 1 and Figure 2.
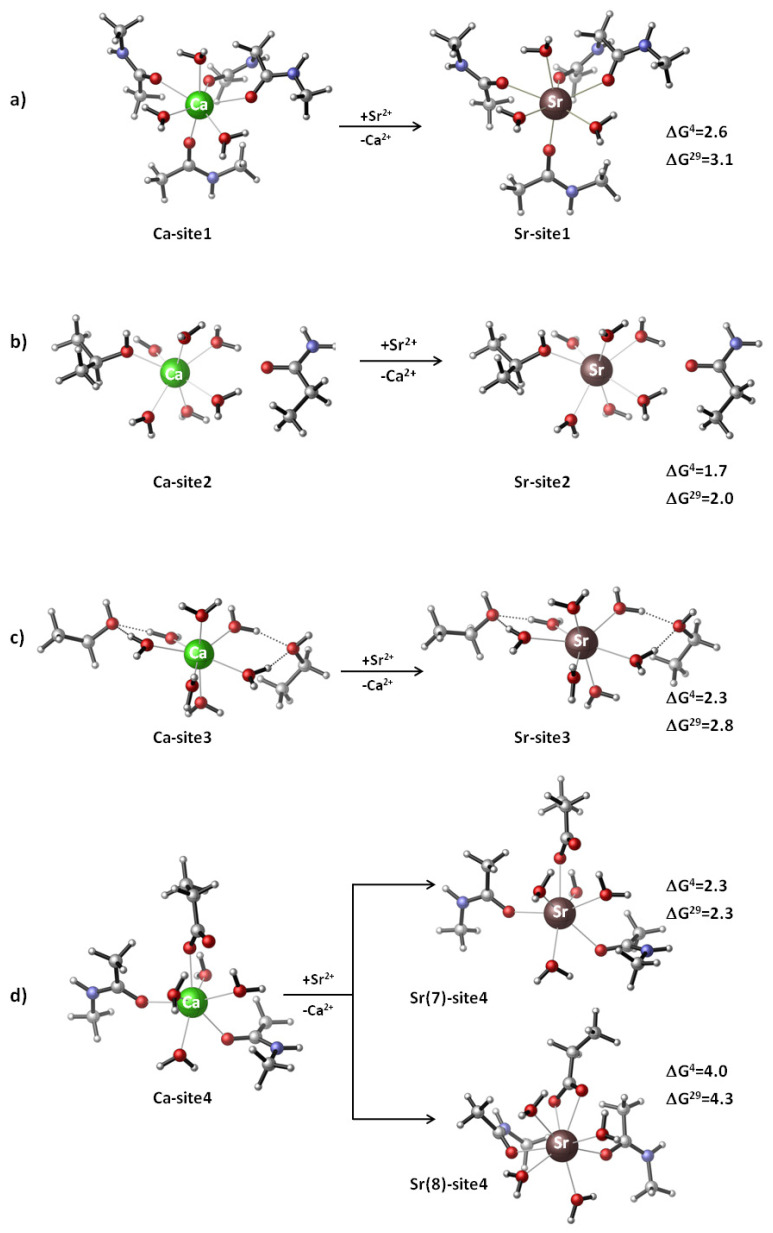
Figure 1.
Fully optimized Ca2+ and Sr2+-loaded metal-binding sites of CaSR: (a) Site 1, (b) Site 2, (c) Site 3 and (d) Site 4 (drawn by CYLview visualization software [23]) along with the Gibbs free energies (in kcal/mol) of the Ca2+ → Sr2+ substitution.
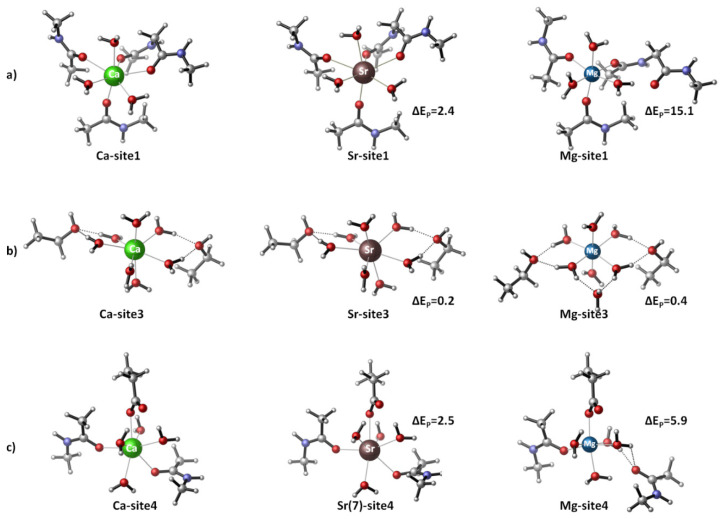
Figure 2.
Fully optimized Ca2+, Mg2+ and Sr2+-loaded metal binding sites of the active-state CaSR: (a) Site 1, (b) Site 3 and (c) Site 4 (drawn by CYLview visualization software [23]) and energy penalties (in kcal/mol) for Sr2+ and Mg2+ structures (∆Ep = E(partial optimization) − E(full optimization); see text).
The competition between Sr2+ and Ca2+ can be expressed in terms of the Gibbs free energy of displacement of the native agonist, Ca2+, bound to CaSR, by its rival, Sr2+:
| [Sr2+-aq] + [Ca2+-CaSR] ↔ [Sr2+-CaSR] + [Ca2+-aq] | (1) |
In Equation (1), [Ca2+/Sr2+-CaSR] and [Ca2+/Sr2+-aq] represent the metal cation bound to the receptor ligands inside the binding pockets and unbound outside the binding cavity (in the bulk solvent), respectively. A positive free energy value for Equation (1) implies a Ca2+-selective site, whereas a negative value suggests a Sr2+-selective one.
2.2. DFT/PCM Calculations
The M06-2X method [15] in conjunction with Pople’s triple zeta 6-311++G(d,p) basis set for C, H, N, O, Ca and Mg atoms, and SDD basis set/effective core potential for Sr was employed in the calculations. This combination of theoretical method/basis sets has been thoroughly calibrated and validated in our previous studies with respect to available experimental data and proven to be reliable as it properly reproduced the geometry of a series of representative metal structures [16] as well as the Gibbs free energies of metal substitution in acetate, imidazole and glycine complexes [17].
Each metal-loaded binding site was optimized in the gas phase by employing the Gaussian 09 package of programs [18]. Electronic energies, Eel, were evaluated for each optimized complex. Subsequent frequency calculations were performed at the same M06-2X/6-311++G(d,p)//SDD level of theory to prove a local minimum on the potential energy surface—no imaginary frequency was found for any of the structures studied. The frequencies were scaled by an empirical factor of 0.983 [15] and employed to calculate the thermal energies, Eth, including zero-point energy, and entropies, S. The differences Eel, Eth and S between the products and reactants in Equation (1) were used to evaluate the metal exchange Gibbs free energy in the gas phase, ∆G1, at T = 298.15 K according to:
| ∆G1 = ∆Eel1 + ∆Eth1 − T∆S1 | (2) |
The basis set superposition error for the type of reactions modeled by Equation (1) is negligible [19] and, thus, was not considered in the present evaluations.
Metal-binding sites in metalloproteins are located in cavities/crevices of the protein structure whose dielectric properties differ from those in the bulk water [20] and behave similarly to the low-polarity solvents [21]. Thus, condensed-phase calculations were conducted in solvents mimicking the dielectric properties of buried and solvent-accessible binding sites, diethyl ether (ε = 4) and propanonitrile (ε = 29), respectively. Solvation effects were accounted for by employing the solvation model based on the density (SMD) scheme [22] as implemented in the Gaussian 09 program. In doing so, the optimized structure of each metal complex in the gas phase was subjected to single-point calculations in the respective solvent at M06-2X/6-311++G(d,p)//SDD level of theory. The differences between the gas-phase and SMD energies were used to compute the solvation free energy, ΔGsolvε, of each metal construct. The incoming Sr2+ and outgoing Ca2+ metal species were considered to be in a bulk aqueous environment (ε = 78) outside the binding pocket. Accordingly, their experimentally determined hydration free energies of −329.8 kcal/mol and −359.7 kcal/mol, respectively [12], were used in the computations. The cation exchange free energy in a protein cavity characterized by an effective dielectric constant ε was evaluated as:
| ∆Gε = ∆G1 + ∆Gsolvε ([Sr2+-CaSR]) − ∆Gsolvε ([Ca2+-CaSR]) − ∆Gsolv78 ([Sr2+-aq]) + ∆Gsolv78 ([Ca2+-aq]) | (3) |
3. Results
3.1. Competition between Ca2+ and Sr2+ for CaSR Binding Sites
Fully optimized (without any constraints) Ca2+- and Sr2+-loaded metal-binding sites of the CaSR along with the Gibbs free energies of the Ca2+ → Sr2+ substitution are presented in Figure 1. Generally, strontium, upon metal exchange, preserves the overall shape and metal coordination number seven of the “mother” calcium site (Figure 1a–d (upper part)) but, as expected, expands the size of the metal construct by elongating the bond distances with protein ligands: the average Ca2+-O(ligand) bond distance is 2.358 Å, whereas the respective Sr2+-O(ligand) bond distance is 2.515 Å. In all cases, Sr2+ cannot outcompete Ca2+ in the binding to metal centers, and this is evidenced by positive free energies of the metal exchange ranging in the narrow limits between 1.7 and 3.1 kcal/mol (Figure 1a–d (upper part)).
The results obtained imply that, although the free energies of metal exchange are not very large, these metal-binding sites are selective for Ca2+ over Sr2+ regardless of the overall net charge of the binding pockets (0 for Sites 1, 2 and 3, and −1 for Site 4) and their solvent exposure (very similar ∆G4 and ∆G29 for buried and solventaccessible metal centers, respectively). Note that Site 2, which is occupied by Ca2+ in both the inactive and active states of the receptor (see Introduction), behaves very similarly to Sites 1, 3 and 4. It is not severely disrupted by the incoming Sr2+ (Figure 1b) and is selective for Ca2+/Sr2+ with free energies of metal exchange ranging between 1.7 (∆G4) and 2.0 kcal/mol (∆G29).
Strontium, being bulkier than calcium, may adopt greater coordination numbers in its complexes. To assess the role of the increased coordination number of Sr2+ on the Ca2+/Sr2+ competition, we modeled a Site 4 complex where the Asp- residue changed its binding mode from monodentate (Figure 1d (upper part)) to bidentate (Figure 1d (lower part)); thus, increasing the strontium coordination number from seven to eight. The resultant free energies of metal exchange for the eight-coordinated strontium (∆G4/∆G29 = 4.0/4.5 kcal/mol; Figure 1d (lower part)) are more positive (meaning decreased strontium competitiveness) than those for the seven-coordinated strontium (∆G4/∆G29 = 2.3/2.3 kcal/mol; Figure 1d (upper part)), suggesting that a significant departing from the inherited seven-coordinated structure of the metal center is unfavorable for strontium binding to CaSR. Note that increasing the metal’s coordination number (which results in increased steric repulsion between its ligands) reflects on the metal-ligand bond distances, which become longer. Indeed, the average metal-ligand bond distance in the 7-coordinated Sr2+ complex in Figure 1d is 2.542 Å, whereas that of its 8-coordinated counterpart (with bidentate aspartate) is 2.579 Å. Moreover, the hydrogen bonds between the second oxygen atom of Asp- and the two neighboring water molecules weaken in the 8-coordinated structure as (Asp)O is now engaged with a coordinative bond with the metal: the two hydrogen bonds (Asp)-O…H2O elongate from 1.756/1.737 Å in the 7-coordinated (monodentate) structure to 2.175/1.948 Å in the 8-coordinated counterpart. All these factors decrease the overall stability of the latter as compared to the former.
3.2. Magnesium Binding to CaSR
Magnesium, unlike the full agonist strontium, has been found to be only a partial agonist of CaSR [1,10]. Why is that? Why is strontium more effective than magnesium in activating the receptor? To shed light on these issues, we modeled and fully optimized active-state CaSR binding sites loaded with Mg2+. The resultant structures are shown in Figure 2, where they are compared with those optimized for the native Ca2+ and “alien” Sr2+. The inspection of the structures clearly shows that the Mg2+ constructs, when allowed to fully relax during the optimization process, differ quite significantly from the optimized Ca2+ and Sr2+ counterparts. Mg2+ adopts its stringent six-coordinated octahedral structure at the expense of detaching one backbone carbonyl ligand (Site 1 and Site 4; Figure 2a,c) or a water molecule (Site 3; Figure 2b), which have been shifted to the metal second coordination layer. At the same time, Sr2+, as already mentioned (see above), preserves the overall structures of the binding sites, which appear to be close to those of the “mother” Ca2+ constructs.
The above results were obtained by assuming flexible calcium-binding sites that allow the incoming competitor to rearrange the protein ligands lining the binding pocket in accordance with its physico-chemical preferences. In many cases, however, calcium-binding sites are known to be quite rigid, forcing the attacking metal cation to adapt to their native arrangement [7,8]. The rigidity of the binding site is often considered to be one of the key factors governing the metal selectivity in calcium-binding sites [7,8]. To study the effect of the metal-binding site rigidity on the metal affinity in CaSR, we performed partial optimizations of Sr2+- and Mg2+-loaded Sites 1, 3 and 4, where the protein ligands were frozen at their original positions in the respective Ca2+-coordinated structures and only the metal cations and water molecules were allowed to relax. The electronic energies evaluated (designated as energy penalty for each structure, ∆Ep) were compared with those obtained from the respective full optimizations (summarized in Figure 1). The results demonstrate that the binding of both metals to a rigid/inflexible calcium-designed binding pocket requires an extra energy penalty for the attacker (positive numbers in Figure 2). However, the price to be paid by Sr2+ (a couple of kcal/mol) is much less than that of Mg2+ for doing so (15.1 kcal/mol for Site 1 and 5.9 kcal/mol for Site 4). Thus, it appears that Sr2+ has an advantage over Mg2+ in populating relatively inflexible CaSR binding sites.
4. Discussion
4.1. Ca2+/Sr2+ Selectivity of CaSR Binding Sites
The Ca2+/Sr2+ selectivity of the active-state CaSR binding sites was assessed by DFT calculations combined with PCM computations. The results reveal that these sites, although possessing varying metal affinities, have a different number and type of protein ligands, different overall structure and charge state, and are all selective for Ca2+ over Sr2+ (Figure 1). Thus, strontium (at therapeutic concentrations in the blood of ~0.1 mM [1]) may not be able to displace the native calcium (with plasma levels of 1.1–1.3 mM [1]) from the respective metal centers. However, the exchange free energy of ~2-3 kcal/mol between Sr2+ and Ca2+ (Figure 1) is not very high and could be surmounted (in favor of strontium) in the case of Sr2+ concentration increases. The literature suggests that the concentration of strontium ions present locally within the bone may significantly exceed the levels present in the blood [1,10]. Furthermore, cooperativity has been found between the CaSR agonists (Sr2+ and Ca2+) in the activation of the receptor that allows the secondary activator (Sr2+) to activate the receptor at relatively low concentrations of the primary agonist (Ca2+) [9,10]. Strontium, being a calcium mimetic species, can populate (though with lower efficiency than calcium) empty metal-binding sites in inactive CaSRs and trigger the respective cellular response.
The three binding sites of the activated receptor, regardless of their structural differences, exhibit almost equal metal selectivity if they are flexible and do not impose any geometrical constraints on the incoming Sr2+ (very similar ∆G4/29 in Figure 1a,c,d (upper part)). Rigidifying the binding pockets, however, would decrease Sr2+ competitiveness of Site 1 and Site 4 (making them less attractive for Sr2+) to a greater extent than that of Site 3, as the energy penalty to be paid by the attacking Sr2+ for binding to the former (2.4/2.5 kcal/mol) would be higher relative to that of the latter (0.2 kcal/mol; Figure 2).
Data analysis suggests that factors, such as the number and type of protein ligands, charge state of the binding pocket and its solvent exposure do not seem to play a significant role in governing the competition between Ca2+ and Sr2+ in CaSR. Rather, these are the intrinsic physico-chemical properties of the two competing metal species (Ca2+ is a better Lewis acid than Sr2+) and the rigidity of binding sites that orchestrates the process. Similar conclusions have been drawn on the competition between Ca2+ and Sr2+ in a representative of the EF-hand family calcium protein, parvalbumin [24].
4.2. Sr2+ as a CaSR Agonist
Strontium has been found to be a full CaSR agonist, though with slightly lower efficacy than the native Ca2+ [1,10]. Indeed, Sr2+, as a Ca2+-mimetic species, reproduces very closely the structure and overall shape of the calcium-binding sites of CaSR, which results in strontium-loaded constructs that energetically are only a few kcal/mol away from their calcium-loaded counterparts. Note that any deviation from the native calcium structure, for example, increasing the Sr2+ coordination number from seven to eight (Figure 1d (lower part)) or the significant rearrangement of the binding site buildup, as in the case of Mg2+ (Figure 2), is severely penalized. For Mg2+, this compromises its ability to act as an effective CaSR activator, which, as some experiments show, renders magnesium as a partial agonist of the receptor [1,10]. Thus, we may hypothesize that Sr2+ is superior to Mg2+ in binding/activating CaSR as it (i) better mimics the calcium-binding sites in the structure, shape and, to some extent, energetics and (ii) incurs a lower energy penalty upon fitting into calcium-designed binding pockets (see above).
4.3. Sr2+ Mechanism of Therapeutic Action as Compared to Other Metallodrugs
Other abiogenic metal species (in cationic forms), such as Li+ and Ga3+, are used in medicinal practice to fight various medical conditions such as psychiatric disorders [25], neoplastic formations and bacterial infections [26,27,28]. The competition between the non-native Li+ and cognate Na+/Mg2+/Ca2+, as well as in the case of abiogenic Ga3+ and native Fe3+, in the key biological systems involved in the respective pathogeneses, have been found to constitute the core of these metals’ therapeutic actions [29,30,31]. In both cases, the “alien” cations, Li+ and Ga3+, substitute for the native metal species (Na+/Mg2+/Ca2+ and Fe3+, respectively) in the respective metalloenzymes/receptors and, as the resulting metal constructs are either structurally altered (lithium’s effect) or redox silent (gallium therapy), they suppress the activity of overexpressed disease-related proteins. The mechanism of the Sr2+ anti-osteoporosis action appears to be different from those mentioned above: it does not fully substitute for the native Ca2+ in CaSR but rather binds and activates the receptor as it closely mimics the basic physico-chemical and structural features of the native agonist. Strontium activation of CaSR is aided by a synergistic calcium binding to the receptor [9,10] and increased Sr2+ concentration achieved locally within the bone microenvironment [1,10].
Author Contributions
Conceptualization, T.D., E.A.P. and S.E.P.; methodology, T.D. and S.E.P.; calculations, D.C. and S.I., visualization, D.C.; writing, T.D., E.A.P. and S.E.P.; project administration, S.E.P. and T.D.; funding acquisition, T.D. and S.E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Foundation of Basic Research (S.E.P. project # 19-54-18014) and the Bilateral Bulgaria-Russia Science Cooperation Program KP-06-Russia/30–2019.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Optimized structures of the metal complexes can be obtained from DC or TD upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brown E.M. Is the calcium receptor a molecular target for the actions of strontium on bone? Osteoporos Int. 2003;14:25–34. doi: 10.1007/s00198-002-1343-6. [DOI] [PubMed] [Google Scholar]
- 2.Marx D., Yazdi A.R., Papini M., Towler M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020;12:100273–100288. doi: 10.1016/j.bonr.2020.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goltzman D., Hendy G.N. The calcium-sensing receptor in bone -mechanistic and therapeutic insights. Nat. Rev. Endocrinol. 2015;11:298–307. doi: 10.1038/nrendo.2015.30. [DOI] [PubMed] [Google Scholar]
- 4.Brown E.M. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:333–343. doi: 10.1016/j.beem.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Ward D.T., Brown E.M., Harris H.W. Disulfide Bonds in the Extracellular Calcium-Polyvalent Cation-sensing Receptor Correlate with Dimer Formation and Its Response to Divalent Cations in Vitro. J. Biol. Chem. 1998;273:14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- 6.Geng Y., Mosyak L., Kurinov I., Zuo H., Sturchler E., Cheng T.C., Subramanyam P., Brown A.P., Brennan S.C., Man H.-C., et al. Structural mechanism of ligand activation in human calcium-sensing receptor. eLife. 2016;5:e13662. doi: 10.7554/eLife.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudev T., Lim C. Calcium Ion Selectivity in Biological Systems. In: Uversky V.N., Kretsinger R.H., Permyakov E.A., editors. Encyclopedia of Metalloproteins. Springer Science; New York, NY, USA: 2013. pp. 478–484. [Google Scholar]
- 8.Dudev T., Lim C. Principles Governing Mg, Ca and Zn Selectivity in Proteins. Chem. Rev. 2003;103:773–787. doi: 10.1021/cr020467n. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay N., Quinn S.J., Kifor O., Ye C., Brown E.M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007;74:438–447. doi: 10.1016/j.bcp.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Coulombe J., Faure H., Robin B., Ruat M. In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2004;323:1184–1190. doi: 10.1016/j.bbrc.2004.08.209. [DOI] [PubMed] [Google Scholar]
- 11.Shannon R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976;A32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 12.Marcus Y. Thermodynamics of solvation of ions. 5. Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991;87:2995–2999. doi: 10.1039/FT9918702995. [DOI] [Google Scholar]
- 13.Nielsen S.P. The biological role of strontium. Bone. 2004;35:583–588. doi: 10.1016/j.bone.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Jernigan R., Raghunathan G., Bahar I. Characterization of interactions and metal ion binding sites in proteins. Curr. Opin. Struct. Biol. 1994;4:256–263. doi: 10.1016/S0959-440X(94)90317-4. [DOI] [Google Scholar]
- 15.Zhao Y., Truhlar D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215–241. [Google Scholar]
- 16.Dudev T., Cheshmedzhieva D., Doudeva L. Competition between abiogenic Al3+ and native Mg2+, Fe2+ and Zn2+ ions in protein binding sites: Implications for aluminum toxicity. J. Mol. Model. 2018;24:55. doi: 10.1007/s00894-018-3592-0. [DOI] [PubMed] [Google Scholar]
- 17.Dudev T., Nikolova V. Determinants of Fe2+ over M2+ (M=Mg, Mn, Zn) Selectivity in Non-Heme Iron Proteins. Inorg. Chem. 2016;55:12644–12650. doi: 10.1021/acs.inorgchem.6b01822. [DOI] [PubMed] [Google Scholar]
- 18.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09, Revision A.02. Gaussian, Inc.; Wallingford, CT, USA: 2009. [Google Scholar]
- 19.Dudev T., Lim C. Determinants of K+ vs Na+ Selectivity in potassium channels. J. Am. Chem. Soc. 2009;131:8092–8101. doi: 10.1021/ja900168k. [DOI] [PubMed] [Google Scholar]
- 20.Li L., Li C., Zhang Z., Alexov E. On the Dielectric Constant of Proteins: Smooth Dielectric Function for Macromolecular Modeling and Its Implementation in DelPhi. J. Chem. Theory Comput. 2013;9:c2126–c2136. doi: 10.1021/ct400065j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertz E.L., Krishtalik L.I. Low dielectric response in enzyme active site. Proc. Natl. Acad. Sci. USA. 2000;97:2081–2086. doi: 10.1073/pnas.050316997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marenich A.V., Cramer C.J., Truhlar D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B. 2009;113:6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- 23.CYLview, 1.0b; Claude Y. Legault. Université de Sherbrooke. 2009. [(accessed on 7 October 2021)]. Available online: http://www.cylview.org.
- 24.Vologzhannikova A.A., Shevelyova M.P., Kazakov A.S., Sokolov A.S., Borisova N.I., Permyakov E.A., Kircheva N., Nikolova V., Dudev T., Permyakov S.E. Strontium Binding to α-Parvalbumin, a Canonical Calcium-Binding Protein of the “EF-Hand” Family. Biomolecules. 2021;11:1158. doi: 10.3390/biom11081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould T.D., Quiroz J.A., Singh J., Zarate C.A., Manji H.K. Emerging experimental therapeutics for bipolar disorder: Insights from the molecular and cellular actions of current mood stabilizers. Mol. Psychiatry. 2004;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- 26.Chitambar C.R. Gallium Complexes as Anticancer Drugs. Met. Ions Life Sci. 2018;18:281–301. doi: 10.1515/9783110470734-016. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein L.R. Gallium, Therapeutic Effects. In: Uversky V.N., Kretsinger R.H., Permyakov E.A., editors. Encyclopedia of Metalloproteins. Springer Science; New York, NY, USA: 2013. pp. 823–835. [Google Scholar]
- 28.Auger C., Lemire J., Appanna V., Appanna V.D. Encyclopedia of Metalloproteins. Springer Science; New York, NY, USA: 2013. Gallium in Bacteria, Metabolic and Medical Implications; pp. 800–807. [Google Scholar]
- 29.Dudev T., Mazmanian K., Weng W.-H., Grauffel C., Lim C. Free and bound lithium in brain signaling. Acc. Chem. Res. 2019;52:2960–2970. doi: 10.1021/acs.accounts.9b00389. [DOI] [PubMed] [Google Scholar]
- 30.Kircheva N., Dudev T. Competition between abiogenic and biogenic metal cations in biological systems: Mechanisms of gallium‘s anticancer and antibacterial effect. J. Inorg. Biochem. 2021;214:111309. doi: 10.1016/j.jinorgbio.2020.111309. [DOI] [PubMed] [Google Scholar]
- 31.Grauffel C., Weng W.-H., Dudev T., Lim C. Trinuclear Calcium Site in the C2 Domain of PKCα/γ Is Prone toLithium Attack. ACS Omega. 2021;6:20657–20666. doi: 10.1021/acsomega.1c02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Optimized structures of the metal complexes can be obtained from DC or TD upon request.