Abstract
The histone H10-encoding gene is expressed in vertebrates in differentiating cells during the arrest of proliferation. In the H10 promoter, a specific regulatory element, which we named the H4 box, exhibits features which implicate a role in mediating H10 gene expression in response to both differentiation and cell cycle control signals. For instance, within the linker histone gene family, the H4 box is found only in the promoters of differentiation-associated subtypes, suggesting that it is specifically involved in differentiation-dependent expression of these genes. In addition, an element nearly identical to the H4 box is conserved in the promoters of histone H4-encoding genes and is known to be involved in their cell cycle-dependent expression. The transcription factors interacting with the H10 H4 box were therefore expected to link differentiation-dependent expression of H10 to the cell cycle control machinery. The aim of this work was to identify such transcription factors and to obtain information concerning the regulatory pathway involved. Interestingly, our cloning strategy led to the isolation of a retinoblastoma protein (RB) partner known as HBP1. HBP1, a high-mobility group box transcription factor, interacted specifically with the H10 H4 box and moreover was expressed in a differentiation-dependent manner. We also showed that the HBP1-encoding gene is able to produce different forms of HBP1. Finally, we demonstrated that both HBP1 and RB were involved in the activation of H10 gene expression. We therefore propose that HBP1 mediates a link between the cell cycle control machinery and cell differentiation signals. Through modulating the expression of specific chromatin-associated proteins such as histone H10, HBP1 plays a vital role in chromatin remodeling events during the arrest of cell proliferation in differentiating cells.
During embryonic development and cell differentiation, specific transitions in gene expression are associated with chromatin remodeling (39, 58). One important aspect of these remodeling events is the synthesis of specific core and linker histones (11, 30, 25, 33, 40, 51). For instance, in many organisms embryonic- and adult-type histone H1s characterize the chromatin of proliferating and differentiated cells, respectively (23). In vertebrates, the histone H10 gene encodes a linker histone variant which is expressed in terminally differentiated cells concomitant with the arrest of cell proliferation (23, 61). The specific role of this linker histone is not clearly established, but the timing and pattern of its expression during early embryogenesis strongly suggest a role for the protein in the organization of chromatin in arrested and differentiated cells (61). It is therefore of great interest to discover the regulatory cascade that induces the expression of this gene in differentiated cells. We believe that molecules involved in this cascade interact with different regulatory pathways, leading to a general control of chromatin remodeling during cell differentiation. Indeed, through the control of a specific group of genes encoding chromatin-associated proteins (such as H10), these molecules may regulate chromatin structure and function. Transcription factors interacting with the H10 promoter are presumably part of this regulatory cascade and are believed to link cell cycle control machinery to chromatin remodeling. The discovery of these transcription factors would therefore be useful for gaining an understanding of the interaction between these two important biological processes.
The first step in this work was a detailed study of all the cis-acting regulatory elements involved in the control of H10 gene expression and to define those that were sensitive to differentiation signals. Previously, we and others defined major cis-acting regulatory elements involved in the expression of the histone H10 gene (4, 7, 21). Besides the TATA box, essentially three major cis-acting regulatory elements contribute to maximal H10 promoter activity (21, 22). Two of these elements, the upstream conserved element (UCE) and the so-called H1 box, are located 435 and 100 bp, respectively, upstream of the initiation site, (4, 21). Both elements reside at the same relative position in all vertebrate replication-dependent H1 genes (15). The third element, located almost immediately upstream of the TATA box, is intriguing because of its similarity to H4 site II, a highly conserved promoter element located at the same position of almost all vertebrate histone H4-encoding genes (23). H4 site II is involved in the cell cycle-dependent control of the H4 promoter (24, 43). These two elements share extensive sequence homology and reside at corresponding positions in their respective promoters (23). For this reason, we named this third element the H4 box (22). Among the linker histone genes, the H4 box is a unique feature of the differentiation-dependent H10 and H5 genes (23, 41). Almost all of the replication-dependent histone H1-encoding genes have a CAAT box at this position (41). The unusual characteristics of the H10 H4 box make this a potential response element to both cell cycle control machinery and differentiation signals. We used a yeast one-hybrid screen strategy to isolate H10 H4-box-interacting factors. Interestingly, this approach allowed us to identify the high-mobility-group (HMG) box protein HBP1 as an H4-box-binding transcription factor. HBP1 is a partner of the retinoblastoma protein (RB), and we showed that both RB and HBP1 control H10 gene expression. These findings confirmed the function of RB in the control of cell differentiation and, most importantly, established a link between cell cycle control machinery and chromatin remodeling during differentiation.
MATERIALS AND METHODS
Cell culture conditions.
Murine erythroleukemia (MEL) cells from clone G9, a subclone of F4NW0, were maintained in minimum essential medium (MEM; Gibco) containing 10% (vol/vol) fetal calf serum. Murine B16 melanoma cells were grown in Dulbecco's DMEM supplemented with 5% fetal calf serum and 2 mM l-glutamine. The Clone 6 (Cl6) cell line, a rat embryonic fibroblast line transformed by ras (34), was maintained in RPMI 1640 (Boehringer) supplemented with 5% fetal calf serum and 4 mM l-glutamine, grown normally at 37°C, or shifted to 32°C to induce cell growth arrest. SAOS-2 cells, an RB-deficient osteosarcoma cell line which is stably transfected with a tetracycline-inhibitable RB expression vector, were cultured in DMEM–10% fetal calf serum supplemented with tetracycline (1 μg/ml), puromycin (1 μg/ml), and G418 (400 μg/ml).
Northern blot analysis. (i) Cells in culture.
Total RNA was purified from MEL, B16, and SAOS-2 cells using Tri-reagent (Sigma) according to the manufacturer's recommendations.
(ii) Rat partial hepatectomy and RNA preparation.
Male Wistar rats were hepatectomized, and RNA was purified from control liver or from liver at different times after the surgery exactly as described by Khochbin et al. (20).
(iii) Adult mouse and human tissues.
Mouse and human multiple-tissue Northern blots were obtained from Clontech and analyzed using different probes as indicated.
One-hybrid screen.
Saccharomyces cerevisiae HIS3-lacZ double-reporter strains were created with the aid of the MATCHMAKER one-hybrid system (Clontech), using procedures essentially as described in the supplied protocol (PT1031-1). Oligonucleotides containing three tandem copies of the human H10 H4 box were cloned upstream of the reporter genes, which were then stably integrated into the genome of yeast strain YM4271. The sequence of this oligonucleotide is AATTCCTGTCCTCACCGCGGTCCGCTGTCCTCACCGCGGTCCGCTGTCCTCACCGCGGTCCGCCC. The H4 box strain was used to screen a MATCHMAKER GAL4 activation domain (AD)-cDNA fusion library from adult human brain (HL4004AB; Clontech). Approximately 4.0 × 106 recombinants were screened. On selective media lacking histidine and containing 30 mM-aminotriazole, (3-AT), we selected two fast-growing His+ clones which also tested positive in the β-galactosidase assay. Plasmid DNA from the positive clones was amplified in Escherichia coli and retransformed into the H4 box reporter strain, as well as control reporter strains containing either three tandem copies of the p53-binding site or a minimal strain which lacked a defined DNA-binding site.
Plasmids and transfection.
Mouse and Xenopus H10 gene promoters (fragments from −610 to +210 and −860 to +30, respectively), were cloned into a chloramphenicol acetyltransferase (CAT) reporter plasmid (pCAT-Basic; Promega). Site-directed mutagenesis was performed by overlapping PCR (18). Human and rat HBP1 cDNAs were cloned into pcDNA3.1 expression vector (Invitrogen) and used in transfection assays. The HMG-box-containing region of HBP1 (amino acids 396 to 513) was cloned into pGEX-5X-3 (Pharmacia). The full-length HBP1 cDNA was cloned into the same vector. The recombinant vectors were introduced into E. coli strain BL21, and the fusion proteins were purified using glutathione-Sepharose 4B beads (Pharmacia) according to the supplier's instructions. The HBP1 HMG-less construct (HBP1dHMG) was obtained by PCR amplification of the region encoding amino acids 1 to 427 and cloning of this fragment into an expression vector. The HBP1d expression vector produces a protein containing the putative AD of HBP1 (26) fused to the DNA-binding domain and was constructed as follows. The region encoding amino acids 37 to 120 of HBP1 was PCR amplified and fused to the HMG-box-containing domain (amino acids 394 to 513). The Mist1 reporter plasmid has been described elsewhere (27). For transfections, Lipofectin reagent (Gibco-BRL) was used; CAT assays were performed according to the protocol published by Nordeen et al. (37).
Footprinting and gel shift assays.
DNase I footprinting was performed as follows. 32P (50,000 cpm)-labeled restriction fragments corresponding to the Xenopus H10 sequence (−120 to +30) from either wild-type, TCA-mutated, or GT-mutated promoters were incubated with increasing amounts of purified glutathione S-transferase (GST)-HBP1 DNA-binding domain in DBB buffer (10 mM Tris HCl [pH 7.8], 15 mM HEPES [pH 7.8], 50 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol bovine serum albumin [100 μg/ml], 5% glycerol for 30 min on ice. After this incubation period, DNase I was added, and incubation was carried out for an additional 5 min on ice. The digestion was stopped by the addition of EDTA and phenol extraction. The products of DNase digestion were then analyzed on a 6% sequencing gel.
For photofootprinting, an oligonucleotide covering the region of Xenopus H10 H4 box (CAGCCGCTAGTCCTCAACTCGGTCCGACCCCA) was end labeled, annealed, gel purified, and incubated with GST-HBP1 fusion protein as above. After the incubation period, the samples were UV irradiated in siliconized 0.65-ml Eppendorf tubes with a single pulse from the fourth harmonic (266 nm) of a Surelite II (Continuum) Nd-YAG laser (maximum energy, 60 mJ; pulse duration, 5 ns). The diameter of the laser beam was adjusted to fit that of the sample surface by means of a set of circular diaphragms. The pulse energy of radiation was measured with a calibrated pyroelectrical detector (Ophir Optronics Ltd.) using an 8% deviation beam splitter. The irradiation dose (pulse energy divided by beam surface) did not exceed 1 kJ/m2 (this dose has been previously determined as required for a 35-bp DNA single-hit experiment (48). After irradiation, the samples were treated with 1 M piperidine for 30 min at 90°C. The piperidine was removed by five successive evaporations in a Speed-Vac. Finally, the samples were dissolved in 3 μl of formamide loading buffer and analyzed on a 15% sequencing gel.
Gel shift assays were performed as follows. 32P-labeled oligonucleotides (32 bp) representing the wild-type Xenopus H10 H4 box sequence (see above) or the same sequence containing a TCA mutation were incubated with bacterially expressed GST-tagged full-length HBP1 alone or with increasing amounts of bacterially expressed His-tagged RB in DBB buffer (20 μl) containing 0.5 μg of poly(dI-C) for 30 min on ice. The mixture was loaded onto a 4% polyacrylamide gel containing 5% glycerol and 1× electrophoresis buffer (10 mM HEPES, 10 mM Tris HCl [pH 8], 1 mM EDTA), and electrophoresis was carried out at 4°C.
[3H]thymidine incorporation.
After different times of induction, [3H]thymidine was introduced to the culture medium (10 μCi/25-mm-diameter dish) for a period of 15 min. Cells were collected, washed in phosphate-buffered saline, and lysed in a lysis buffer containing 7.6 M guanidine hydrochloride in 0.1 M potassium acetate (pH 5). Trichloroacetic acid was added to a final concentration of 10%; after 30 min at 0°C, insoluble material was washed three times with 5% trichloroacetic acid (0°C) and twice with ethanol.
Nucleotide sequence accession number.
The novel nucleotide sequence reported here (HBP1a) has been deposited with GenBank under accession number AF182038.
RESULTS
The H4 box is a proximal cis-acting element that specifies differentiation-dependent linker histone-encoding genes.
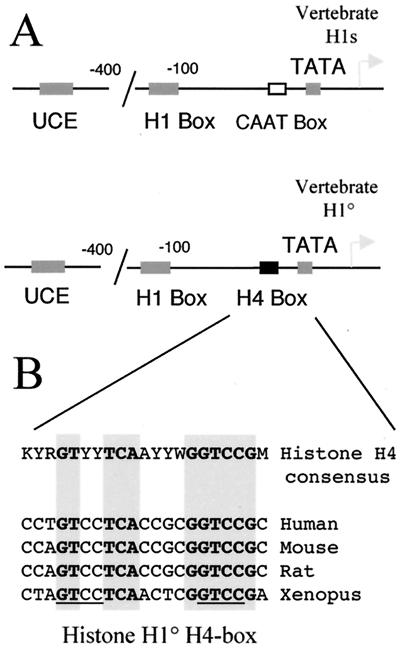
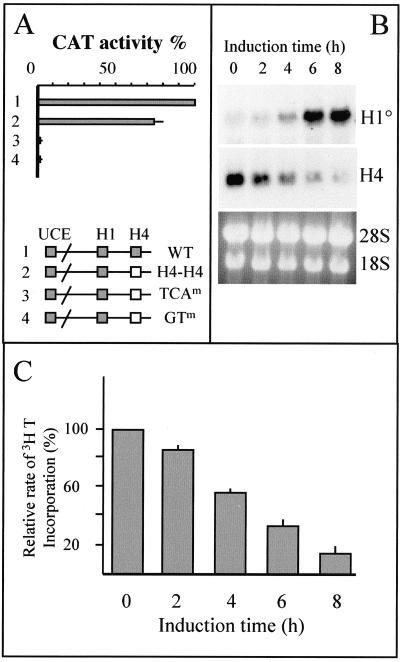
Among the three functionally defined cis-regulatory elements involved in H10 gene expression, two, the UCE and H1 box, are also present in vertebrate replication-dependent histone H1-encoding genes (Fig. 1A). The H4 box thus defines a class of H1 genes that are expressed in differentiated- and growth-arrested cells, because it is found only in the proximal promoter region of the differentiation-specific H1 genes, H10 and H5 (23, 41). Indeed, all vertebrate replication-dependent H1 genes possess a CAAT box at this position (Fig. 1A). In fact, the H4 box shows high sequence similarity with H4 site II, one of the cis-acting regulatory elements of histone H4-encoding genes (38). van Wijnen et al. established a consensus sequence for H4 site II after the alignment of vertebrate histone H4 promoter regions (53). Figure 1B shows that the H10 H4 box is almost identical to H4 site II, which is an essential and highly conserved promoter element involved in the cell cycle-dependent activity of the histone H4 gene promoter (24, 43). All highly conserved nucleotide motifs in the consensus H4 site II sequence are absolutely conserved in the proximal promoter region of all known vertebrate H10 genes (Fig. 1B). These observations strongly suggest that at least in proliferating cells, H4 site II and the H10 H4 box should be functionally equivalent. To confirm this hypothesis, we converted the H4 box from the Xenopus H10 promoter to human H4 site II (derived from the H4 gene FO108 promoter) and cloned the promoter upstream of a CAT reporter gene. Transient transfection assays showed that in proliferating cells, the human H4 site II was fully functional in the Xenopus H10 gene promoter and could maintain efficient transcription of this gene (Fig. 2A, H4H4 construct). To show the specificity of this element, we converted the two highly conserved TCA and GT motifs (Fig. 1B) to unrelated GTC and AA, respectively. These mutations almost completely abolished the activity of the H10 promoter (Fig. 2A, TCAm and GTm constructs). These experiments showed that in exponentially growing cells, histone H4 site II binding factors can participate to maintain the transcriptional activity of both H10 and H4 genes. However, in differentiating cells, endogenous histone H4 and histone H10 genes show different patterns of expression. Histone H10 gene expression is induced during the early stages of cell differentiation and expression is maintained in fully arrested, differentiated cells, while histone H4 expression decreases rapidly. Figure 2B illustrates this situation. The induced differentiation of murine melanoma cells (line B16) is accompanied by a rapid exit of cells from the cell cycle (45). Incorporation of [3H]thymidine into DNA was measured in order to visualize this phenomenon. Different times after the induction, [3H]thymidine was added to the culture medium for 15 min; then cells were lysed and the rate of thymidine incorporation into trichloroacetic acid-insoluble material was measured (Fig. 2C). Two hours after the induced differentiation, the rate of DNA synthesis started to decrease, reflecting an exit from the cell cycle. This phenomenon became more and more pronounced as the time of induction proceeded (Fig. 2C, 4, 6, and 8 h). This event is associated with an induction of H10 gene activity and a concomitant dramatic decrease of histone H4 gene expression. As the H4 box is the only element that specifies differentiation-specific H1 variants, one can suggest that during cell differentiation, specific H4-box-interacting factors are activated, leading to induction of the H10 promoter.
FIG. 1.
The H4 box cis-acting regulatory element is a distinctive feature of differentiation-dependent histone H1 genes. (A) The schematic representation of the vertebrate histone H1-encoding gene proximal promoter region illustrates that the nature of regulatory elements present is relatively conserved. However, upstream of the TATA box, a CAAT box is found in almost all vertebrate replication-dependent H1 genes, while an H4 box is found at this position in all known histone H10 genes. (B) The histone H10 H4 box is essentially identical to the histone H4 site II regulatory element. The sequence of the H4 box region of all known H10 gene promoters was compared with that of the H4 site II consensus established by van Wijnen et al. (53). Y, C or T; R, A or G; K, G or T; W, A or T; M, A or C. The shadowed sequences are absolutely conserved between almost all H4 genes and all H10 genes. The underlined sequence highlights the repetition of the GTCC motif, discussed in the text.
FIG. 2.
(A) Histone H4 site II and H10 H4 box are functionally equivalent in proliferating cells. The H4 box from the Xenopus histone H10 gene promoter was replaced by the H4 site II sequence from a human histone H4 promoter (construct 2, H4H4). Conserved TCA and GT motifs in the H10 H4 box were replaced by GTC and AA, respectively (TCAm and GTm constructs). Construct 1 is the wild-type Xenopus histone H10 promoter. One microgram of each plasmid was used to transfect Cl6 cells; 48 h after transfection, CAT activity was measured. Plasmid uptake was controlled by Southern blot analysis of DNA extracted from transfected cells and hybridization with a 32P-labeled plasmid probe. The measured CAT activity was then normalized with respect to the plasmid uptake. Data are presented as percentage of the activity detected from the wild-type promoter, and bars indicate standard deviations of two independent experiments. (B) Different transcriptional activities of histone H4 and H10 genes during cell cycle exit in differentiating cells. B16 cells were induced to differentiate with 5 mM butyrate, and cells were taken at indicated times. A Northern blot containing these RNAs was probed successively with 32P-labeled H10 and H4 probes. The ethidium bromide-stained gel before the transfer of RNAs onto the membrane is also shown. (C) Early cell cycle exit after the induced differentiation of B16 cells. B16 cells were pulse-labeled with [3H]thymidine at the indicated times after the induced differentiation and analyzed; 100% represents [3H]thymidine incorporation in noninduced cells (means of three independent counts); bars indicate standard deviations.
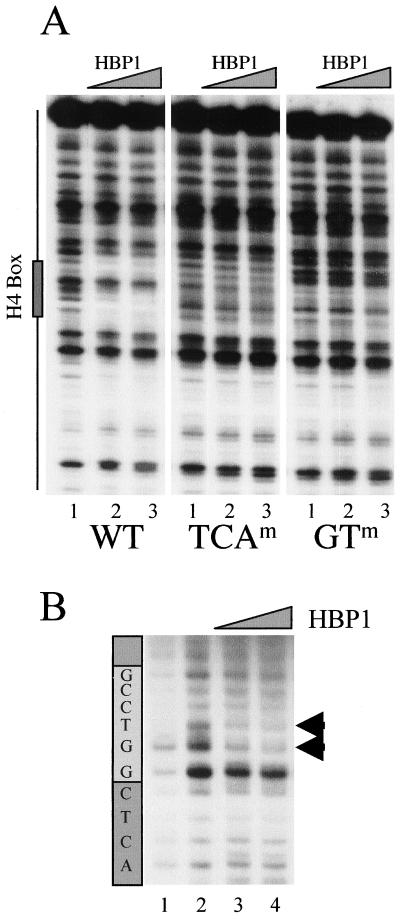
FIG. 4.
DNA-binding analysis of HBP1 on the H10 promoter. (A) A 150-bp DraIII-XbaI fragment was excised from the wild-type Xenopus H10 gene promoter, or promoters containing mutations in TCA and GT subelements (WT, TCAm, and GTm, respectively; see the legend to Fig. 2), end labeled, and incubated with 100 and 500 ng of GST-HBP1 fusion protein (lane 2 and 3 for each panel). Lane 1 shows the pattern of DNase I digestion in the absence of the protein. The position of the H4 box is indicated on the left. (B) UV laser footprinting of H4 box HBP1 complex. An end-labeled H4-box-containing oligonucleotide (32 bp) was complexed with 100 and 500 ng of purified GST-HBP1 fusion protein and irradiated with a single 266-nm laser pulse. The irradiated samples were then treated with hot piperidine, and the cleaved products were analyzed on a sequencing gel. The patterns of the cleavage of nonirradiated (lane 1) and irradiated (lane 2) naked DNA are also shown. The light gray box indicates the position of the highly conserved GGTCC motif. Arrowheads show a modified photoreactivity of the GT nucleotides in the presence of HBP1.
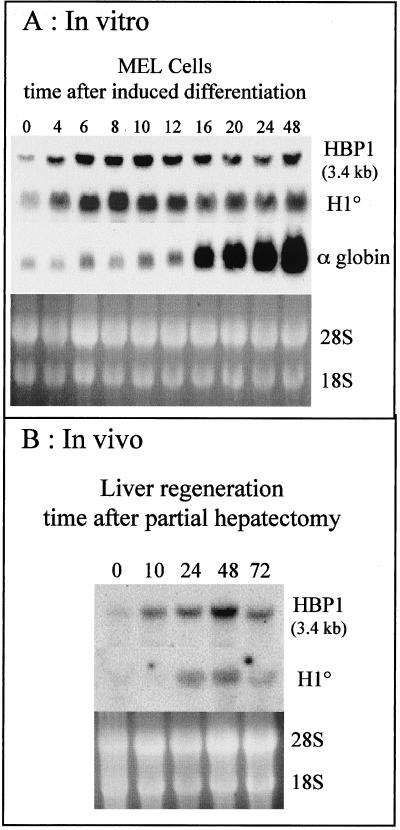
FIG. 6.
Differentiation-dependent expression of HBP1. (A) MEL cells were induced to differentiate with 4 mM hexamethylene bisacetamide. At the indicated times after induction, RNA was prepared and a Northern blot was obtained. The blot was probed successively with HBP1, H10, and α-globin probes. (B) Rats were partially hepatectomized, and regeneration was allowed to proceed for the indicated times; then RNA was purified and analyzed as above. The ethidium bromide-stained gels before the transfer of RNAs onto the membrane are also shown.
FIG. 8.
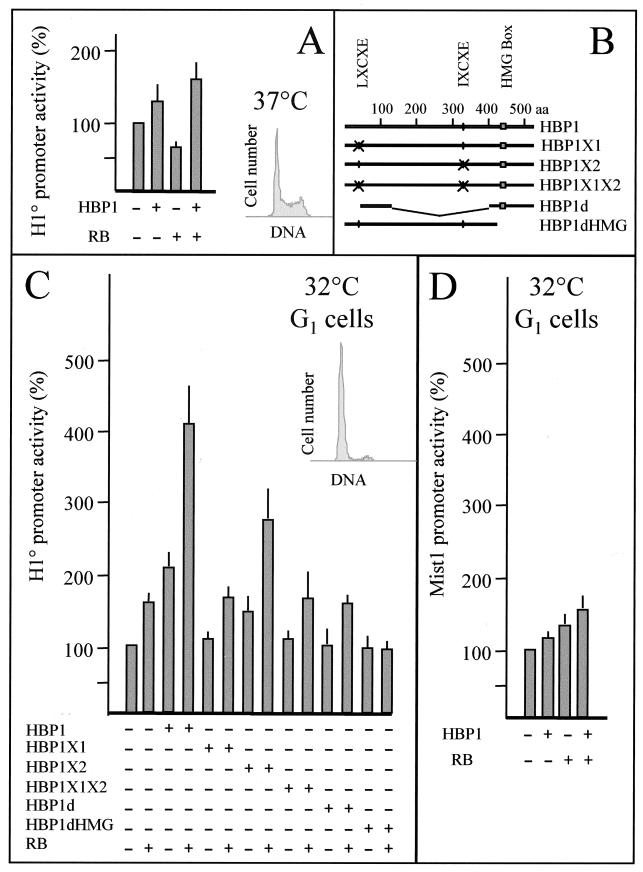
HBP1 and RB cooperate to activate the H10 gene promoter in growth-arrested cells. (A) Proliferative Cl6 cells (cultured at 37°C) were transfected with 1 μg of mouse H10 promoter-CAT reporter construct in the absence (−) or presence (+) of 500 ng of the indicated expression vectors. (B) Schematic representation of various HBP1 mutants expressed in cells. aa, amino acids. (C) Growth-arrested Cl6 cells cultured at 32°C were transfected with the indicated HBP1 expression vectors together with a H10 promoter-CAT construct. In the HBP1X1 construct, the putative RB-binding site (LXCXE; black circle) is replaced by RPCRP encoded by an SfiI restriction site (crossed circle). In the HBP1X2 mutant, the second putative RB-binding site (IXCXE; black circle) is replaced by the SfiI sequence (crossed circle). HBP1X1X2 harbors both mutations. The HBP1d expression vector expresses a protein containing amino acids 37 to 120 of HBP1 fused to the HMG-box-containing domain (amino acids 394 to 513), lacking the two putative RB-binding sites. The HBP1dHMG construct expresses a truncated HBP1 protein (amino acids 1 to 427) lacking the HMG box domain. (D) Growth-arrested Cl6 cells were transfected with HBP1, RB, or both expression vectors with a reporter plasmid containing the Mist1 gene promoter. A 500-ng aliquot of each expression vector was used for each transfection, and in every transfection the total amount of expression vector was kept constant to 500 ng using a CMV-directed green fluorescent protein expression vector. Data summarize results of three to six independent experiments and are normalized and represented as in Fig. 2. The histograms (cell number/DNA) show the state of cell proliferation at the moment of transfection.
Identification of H4-box-interacting factors.
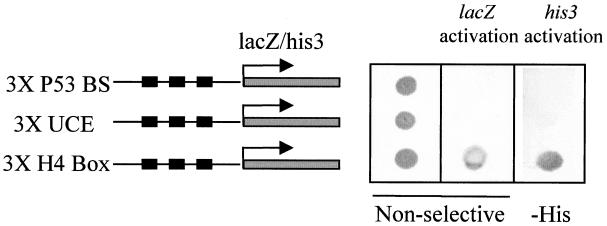
A yeast one-hybrid screening strategy was adopted to clone factors interacting with the histone H10 H4 box. We generated a yeast reporter strain in which three tandem copies of the H4 box from the human H10 promoter were inserted upstream of His3 and lacZ genes. A human brain cDNA library cloned in the yeast expression vector pGAD10, which expresses the cDNA insert as a fusion protein containing the GAL4 AD, was transformed into the reporter strain. Adult brain was selected as the source of cDNA to minimize the cloning of histone H4-specific transcription factors, since this tissue is constituted of mostly differentiated and arrested cells which express a high level of H10 (13, 42). Two cDNA clones able to confer a high rate of growth on selective media (lacking histidine) and also capable of activating β-galactosidase production were isolated. These two clones had the same sequence and encoded an HMG-box-containing protein known as HBP1 (29). We also used two established yeast strains harboring either three copies of the UCE from the human H10 promoter or three copies of a p53-binding site, upstream of the HIS3 and lacZ genes. One of the GAL4 AD-HBP1-expressing plasmids isolated after the library screening was introduced into these lines to check the specificity of the interaction of HBP1 with the H4 box element. Figure 3 shows that while the three strains (H4 box, UCE, and p53-binding sites) grow well on nonselective media (left panel), only the H4-box-containing strain can efficiently activate lacZ gene expression (middle panel). On selective media lacking histidine and containing 30 mM 3-AT, only the H4 box strain was able to grow (right panel). This experiment demonstrated that in vivo, HBP1 is able to specifically recognize the H4 box. Interestingly, two groups using the yeast two-hybrid system have independently identified HBP1 as an RB-interacting protein and also as a potential transcription factor (26, 50). Indeed, two distinct RB-binding sites are present in HBP1 (50).
FIG. 3.
HBP1 interacts specifically with the H10 H4 box in vivo. A GAL4 AD-HBP1 expression vector, isolated from the one-hybrid screening, was introduced into three different yeast strains harboring three copies of either the H4 box, UCE, or a p53-binding site (P53 BS) upstream of each of two reporter genes (lacZ and HIS3). After transfection and selection of HBP1-expressing clones, one clone of each strain was spread on nonselective medium (left panel) or histidine-deficient medium containing 30 mM 3-AT (-His panel). Yeast grown on nonselective medium were transferred to a membrane and assayed for β-galactosidase activity (middle panel).
HBP1 interacts specifically with the H10 H4 box element.
To confirm the observed interaction of HBP1 with the H10 H4-box in vivo, we studied in vitro HBP1-H4 box interaction. Figure 4A represents a standard DNase I footprinting experiment with a fragment covering the H4 box region of the wild-type Xenopus H10 gene or the same fragment isolated from the mutated H10 promoters, TCA and GT (Fig. 1). As shown in Fig. 1 and 2, these motifs are highly conserved in all H4 boxes and are necessary to maintain H10 promoter activity. An HBP1 DNA-binding domain–GST fusion protein was expressed in bacteria, purified, and used for this study. The HBP1–DNA-binding domain fusion protected several nucleotides within the wild-type H4 box sequence (Fig. 4A, left panel). Interestingly, when the corresponding fragments isolated from the TCA- and GT-mutated H4 boxes were used, HBP1 could not protect these regions against DNase I digestion (Fig. 4A, compare WT, TCA, and GT constructs). To obtain information on specific regions within the H4 box affected by the interaction with HBP1, we used the powerful UV laser footprinting strategy (2). The HBP1 DNA-binding domain–GST fusion protein was incubated with a 32P-labeled oligonucleotide (32 bp) corresponding to the H4 box sequence. Irradiation of the complex by the UV laser followed by a hot piperidine treatment revealed bases presenting a modified photoreactivity due to the presence of the protein. This methodology allowed us to visualize, with high precision, bases within the H4 box that were the most affected by HBP1 interaction. The photoreactivity of the GT nucleotides located in the highly conserved GGTCC motif was the most severely affected by the presence of HBP1 (Fig. 4B, arrows). Although the photofootprinting did not show significant modification of the photoreactivity of TCA and GT motifs (not shown), the DNase I footprinting using the promoter fragments mutated at these sites showed that they were also important for HBP1 interaction. It is interesting that the GTCC motif is present twice in the H10 H4 box, once in the highly conserved GGTCC element and once just upstream of the TCA motif and including the highly conserved GT dinucleotide (Fig. 1B, underlined).
Different forms of HBP1 are expressed in various adult tissues.
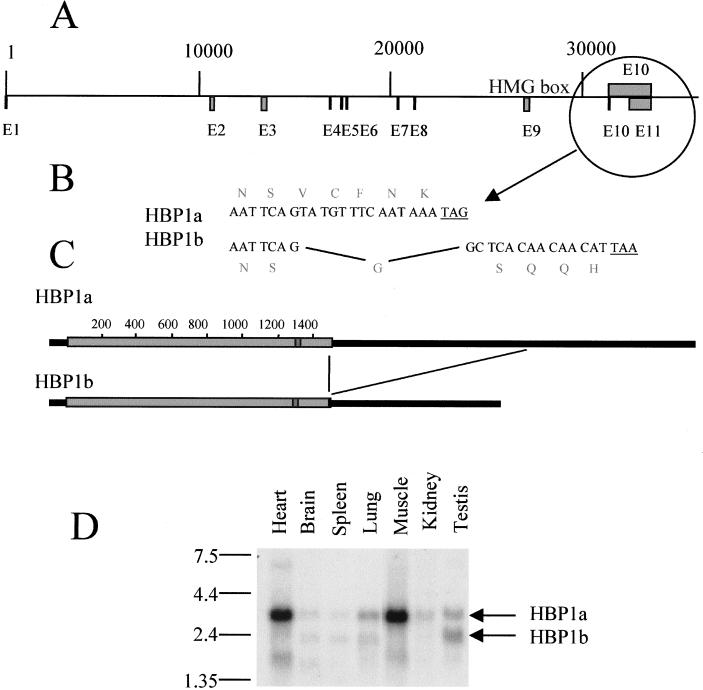
The HBP1 clone that we isolated after one-hybrid screening possesses five different amino acids at its C-terminal extremity compared to the reported human HBP1 (accession no. AF019214). This observation suggested that different HBP1-related mRNAs were expressed. To elucidate this issue and to establish the pattern of different HBP1-related mRNAs in adult tissues, Northern blots containing mRNA samples from various mouse and human adult tissues were probed with an HBP1 probe. In mouse cells, at least three different HBP1-related mRNAs (3.4, 2.4, and 1.7 kb), expressed at different levels, were observed (Fig. 5D). In human cells, although the same HBP1-related mRNAs were observed, the 3.4-kb species constituted the most abundant form (not shown). In our search of the sequence databanks, we noticed the presence of the human HBP1-encoding gene whose sequence is now available thanks to the chromosome 7 mapping project (GenBank accession no. AC004492). We used this sequence to establish the intron/exon organization of the gene (Fig. 5A) and to identify the nature of different HBP1-related mRNAs. Sequence comparison of the two available human HBP1 cDNAs (ours and the previously reported one, AF019214) showed that the two cDNAs were the result of an alternative splicing. Intron 10 (1 kb long), spliced in the AF019214 sequence (named here HBP1b), was found unspliced in our cDNA (HBP1a). This event results in the alteration of the five most C-terminal amino acids (Fig. 5B) as well as in a longer 3′ untranslated region in our cDNA (Fig. 5C). This alternative splicing explained the two upper mRNA bands observed in the Northern blot (3.4 and 2.4 kb). We do not know the nature of the smaller HBP1-related mRNA. However, a search of the EST (expressed sequence tag) databank revealed the existence of an EST of 600 bp (GenBank accession no. AI186052) showing sequence identity with both the N- and C-terminal parts of the HBP1-encoding sequence. A detailed analysis of this EST (not shown) revealed that a splicing event removed exons 2 to 7, leading to an mRNA that potentially encodes a protein containing essentially the HMG box domain.
FIG. 5.
Structure of the human HBP1-encoding gene. (A) The sequence of a human bacterial artificial chromosome clone, RG363E19 (accession no. AC004492), from chromosome 7q31.1 was compared with the sequence of the cDNA cloned in this work as well as that of human HBP1 available in the GenBank database (accession no. AF019214). The positions of exons and introns were determined, and a map was obtained. (B and C) An alternative splicing event produces two types of HBP1-encoding mRNA. Intron 10 remained unspliced in the cDNA that we cloned in this work and is removed in the HBP1 sequence available in the database (AF0192114). This alternative splicing alters the sequence of the five most C-terminal amino acids (B) and includes (HBP1a) or omits (HBP1b) 1 kb of 3′ untranslated region to the HBP1-encoding mRNAs (C). (D) Three HBP1-related mRNAs are expressed in different mouse adult tissues. A Northern blot containing 2 μg of poly(A)+ RNAs from indicated mouse tissues was probed a 32P-labeled HBP1 probe. The positions of HBP1a and HBP1b at 3.4 and 2.4 kb, respectively, are indicated.
The differentiation-dependent expression of HBP1 correlates with the induction of H10 gene expression.
We have identified HBP1 as a transcription factor that is potentially involved in the control of H10 gene expression during cell differentiation. Moreover, different HBP1-related mRNAs have been observed. Previously, several groups demonstrated the accumulation of HBP1 mRNA during the induced differentiation of two cell lines (29, 50). Here we tried to correlate the expression of HBP1 mRNAs with that of H10 in systems where the pattern of H10 gene expression was precisely established during cell differentiation. Previously, we showed that the induction of MEL cell differentiation by hexamethylene bisacetamide treatment efficiently induced the expression of H10 (44). We used the same system to examine the timing of HBP1 expression. Figure 6A shows that the expression of HBP1 correlated very well with that of H10 mRNA, and the only form of messenger detected was that corresponding to HBP1a (3.4 kb). Moreover, this event takes place before the commitment of cells to differentiate, as judged by the timing of globin gene expression (Fig. 6A). To exclude any artifactual expression of these genes in vitro (due to the treatment with chemicals), we tested an inducible in vivo system: rat liver regeneration after partial hepatectomy. This procedure induces liver cell proliferation to regenerate the functional differentiated tissue (1), a process accompanied by an induction of H10 mRNA (20). Interestingly, in this case the induction of HBP1 expression preceded that of H10 gene expression, and the expression of both genes decreased 72 h after the hepatectomy (Fig. 6B). Here again the only form detected corresponded to HBP1a. These data identify HBP1 as a candidate regulator of H10 gene expression during cell differentiation.
Involvement of RB in the control of H10 gene expression in vivo.
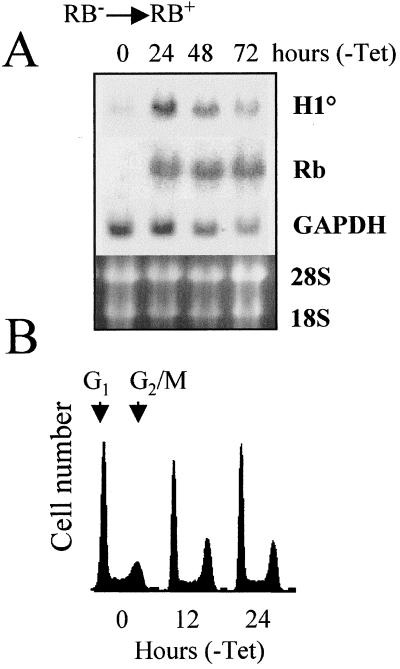
The fact that the expression of H10 is activated during induced differentiation, concomitant with the exit of cells from the cell cycle, suggests strongly that there should exist a link between the cell cycle control machinery and the expression of H10. Since RB is a partner of HBP1 (26, 50), one can suggest that it provides the link between arrest of cell proliferation and the expression of H10. To confirm this hypothesis, we used the RB-deficient osteosarcoma cell line SAOS-2, which is stably transfected with a tetracycline-inhibitable RB expression vector. The removal of tetracycline leads to the induction of the RB transgene. SAOS-2 cells were cultured for various periods in the absence of tetracycline, and expression of the endogenous H10 gene was monitored. The Northern blot presented in Fig. 7 shows that 24 h after the removal of tetracycline, RB expression is efficiently induced. Interestingly, the expression of endogenous H10 is sensitive to this event and a significant accumulation of H10 mRNA can be observed concomitant with that of RB. At 48 and 72 h after withdrawal of tetracycline, decreases in both H10 and GAPDH mRNA levels were observed, probably due to the toxicity of RB overexpression. As expected, the induction of RB is accompanied with a modification of cell cycle parameters. Interestingly, the first visible event is an accumulation of cells in the G2/M phase of the cell cycle (Fig. 7B, 12 and 24 h), followed by an arrest of cells in both G1 and G2/M phases after 24 h of RB induction (not shown). These results show that RB, a well-known regulator of the cell cycle, is able to communicate with the H10 promoter and therefore provides a link between cell cycle regulation and H10 gene expression.
FIG. 7.
RB modulates the expression of the endogenous H10 gene. The RB-deficient SAOS-2 cell line, harboring an inducible RB-encoding transgene, was cultured in the absence of tetracycline to induce the expression of RB. (A) At the indicated times, RNA was prepared from these cells, and the Northern blot obtained was probed successively with 32P-labeled H10, RB, and GAPDH probes. The ethidium bromide-stained gel before the transfer of RNAs onto the membrane is shown. (B) The induced accumulation of RB is associated with the modification of cell cycle parameters. SAOS-2 cells were fixed at the indicated times after the removal of tetracycline, stained with propidium iodide, and analyzed using a flow cytofluorimeter. Histograms represent the number of cells as a function of DNA content (DNA fluorescence).
Involvement of RB and HBP1 in the control of H10 gene expression.
To show directly the involvement of HBP1 and RB in the control of H10 gene expression, we introduced a mouse H10 promoter-CAT reporter construct into cells expressing HBP1 and RB proteins. The expression of HBP1 alone or together with RB only slightly stimulated the activity of the H10 promoter using various exponentially growing cell lines in transient transfection assays (Fig. 8A and data not shown). Because of the abundance of histone H4 transcriptional regulatory factors in proliferative cells (19, 52) and a possible interference of these factors with HBP1 and thereby H10 expression, we decided to transfect arrested cells where H4 site II binding activity is greatly reduced (49). A very convenient cell line for this purpose is Cl6, a ras- and thermosensitive p53 mutant-transformed line. At 37°C, p53 is in a mutated conformation which is responsible for the appearance of a transformed phenotype. At 32°C, p53 exhibits the property of the wild-type protein and triggers an arrest of cell proliferation (34). After the temperature shift from 37 to 32°C and arrest of cell proliferation, cells were transfected with the H10 promoter-CAT reporter gene. The cotransfection of an HBP1 expression vector led to a 2-fold activation of H10 activity compared with transfection of the reporter construct alone, while cotransfection of an RB expression vector stimulated promoter activity 1.5-fold. Interestingly, when HBP1 and RB were introduced simultaneously in cells, the transactivation observed was approximately fourfold. This activation is dependent on RB-HBP1 interaction. Replacement of the first RB-binding site on HBP1 (LXCXE) by an unrelated sequence (Fig. 8B, HBP1X1 construct) completely abolished HBP1-RB cooperation in H10 activation (Fig. 8C). The destruction of the second RB-binding site, IXCXE, did not strongly affect the RB-HBP1 cooperativity in activating the H10 gene promoter (Fig. 8C, HBP1X2 construct).
We also prepared a construct expressing a fusion protein containing the 93-amino-acid putative AD of HBP1 (26) fused to the HMG box domain (HBP1d construct). The expressed protein lacked both RB-binding sites. Expression of this protein did not activate the H10 promoter; moreover, it could not cooperate with RB (Fig. 8C, HBP1d construct). The same situation was observed when a truncated form of HBP1, lacking the HMG box domain, was expressed (Fig. 8C, HBP1dHMG construct).
To show the specific involvement of HBP1 and RB in H10 gene expression, we used another cellular promoter controlling expression of Mist1 gene, which does not contain any H4-box-like element (27). Mist1, a gene expressed in various cell types, encodes a DNA-binding protein of the basic-helix-loop-helix family capable of dimerization with other members of this family and specifically inhibits MyoD transcriptional activity (28). Figure 8D shows that upon the expression of RB and HBP1 in arrested Cl6 cells, while H10 promoter activity is significantly stimulated, that of Mist1 is only slightly affected. In these experiments we also used a reporter gene under the control of cytomegalovirus (CMV) promoter and found that HBP1 alone or together with RB inhibited the CMV promoter activity by about 20 to 30% (not shown). These results showed the specific involvement of both HBP1 and RB in the control of H10 gene expression.
RB enhances the binding of HBP1 to H10 H4 box.
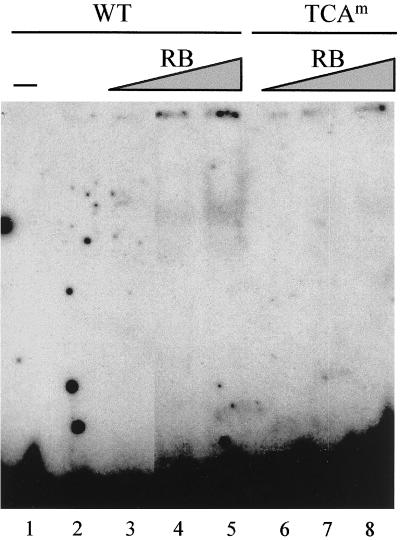
To investigate whether RB might in some way facilitate or stabilize binding of HBP1 to H4 box site, we considered the capacity of RB to influence the binding of HBP1 to its target sequence. A gel shift assay was carried out using a 32-mer oligonucleotide containing the wild-type Xenopus H10 H4 box. In the presence of suboptimum binding levels of GST-HBP1 (Fig. 9, lane 2), the addition of an increasing amount of RB led to an enhancement of the HBP1-dependent DNA-binding activity (Fig. 9, lanes 4 to 5). When the same experiment was performed with an oligonucleotide containing a TCA mutation (Fig. 9, lanes 6 to 8), the effect of RB in HBP1 DNA-binding activity was greatly reduced. These experiments demonstrated that the DNA-binding activity of HBP1, like that of c-Jun and C/EBP members (9, 10, 35, 36), can be enhanced upon its interaction with RB, and the results supported the observed cooperativity between RB and HBP1 in the activation of H10 gene expression.
FIG. 9.
Modulation of HBP1 DNA-binding activity by RB. Gel shift assays were performed with a constant amount (200 ng) of bacterially expressed GST-HBP1 in the absence (lanes 2) or in the presence of increased amounts of His-RB (100 ng, lanes 3 and 6; 200 ng, lanes 4 and 7; 300 ng, lanes 5 and 8). WT indicates the wild-type H10 H4 box sequence, and TCAm indicates the same sequence bearing a mutation at the TCA subelement level.
DISCUSSION
Detailed analysis of factors interacting with histone H4 site II, known to be involved in the S-phase-dependent activation of histone H4 genes (24, 43), showed the obvious link between the cell cycle control machinery and histone H4 gene expression. Indeed, the H4 site II element interacts with at least three distinct complexes, named HiNF-D, HiNF-M, and HiNF-P (53). Extensive research on the nature of these complexes has led to the identification of several interacting subunits. Interferon regulatory factor 2 is the DNA-binding subunit of the HiNF-M complex (56), while HiNF-D, a complex that is associated with the S-phase activation of H4 genes (52), harbors the transcription factor CDP/cut (55) in addition to Cdc2, cyclin A, and RB, three major cell cycle regulatory factors (54). The DNA-binding subunit of the HiNF-P complex (H4TF2 [12]) has not been yet identified. Here we have shown that in proliferating cells, the H10 H4 box can be replaced by H4 site II from the H4 gene FO108, demonstrating that these two elements are functionally equivalent. However, during embryonic development and cell differentiation, H10 and H4 genes present opposite patterns of expression. H4 genes are sensitive to signals inducing the S phase of the cell cycle, while the H10 gene is activated when growth arrest signals become functional. This observation allows one to propose a hypothesis for the presence of the H4 box in the promoter of the H10 gene. Indeed, this element could be considered as a site of connection with the cell cycle control machinery, informing the H10 gene about the state of cell proliferation. In the same way as H4 site II links H4 gene expression to S-phase-inducing signals, the H10 H4 box could render the H10 gene responsive to growth arrest signals. All of these considerations prompted us to search for novel H4-box-binding proteins from fully differentiated and growth-arrested cells. For this reason we used a one-hybrid screen of a human adult brain cDNA library. The H4-box-interacting factor cloned was HBP1, an HMG box transcription factor (29). This factor was able to interact with the H4 box in vivo and in vitro. Previously, a detailed analysis of the N-myc promoter showed that the TCCTTCAATGGGGA motif is the HBP1-binding site on this promoter (50). Interestingly, there exists some sequence homology between this site and the Xenopus H10 H4 box, TCCTCAACTCGGTC. The TCC and TCAA submotifs are found in both sites. The TCA trinucleotide motif, conserved between these two sites, appears therefore to play an important role in HBP1 recognition. Indeed, our data showed that the destruction of this motif abolished HBP1 binding. Moreover, very recently a high-affinity HBP1-binding site, TTCATTCATTCA, containing a triple copy of the TCA motif, has been reported (60). All of these observations point to the crucial role of the TCA trinucleotide motif in HBP1 binding. However, the laser photofootprinting approach showed that the binding of HBP1 more specifically affects the GT dinucleotide in the GGTCC element, which is the other highly conserved submotif in all histone H4-encoding genes (53) and in all differentiation-specific H1s (41). It is important to note that the photoreactivity of bases in DNA is very sensitive to local conformational changes in DNA, such as those induced by protein-DNA interactions (2). Considering the DNA-bending activity of HMG-box-containing proteins (8, 59), it is very probable that the modification of the photoreactivity observed at the GT dinucleotide is due to the bending of DNA at this level.
The expression of HBP1 was found to be positively controlled by cell differentiation, suggesting its involvement in the expression of differentiation-dependent genes. Most interestingly, HBP1 is a partner of RB (26, 50) and therefore establishes a link between transcriptional regulation and control of the cell cycle (46, 50). Moreover, the expression of HBP1 leads to an arrest of cell proliferation (50). These observations prompted Tevosian et al. (50) to propose that HBP1 functions in the initiation and the maintenance of irreversible cell cycle arrest during cell differentiation. HBP1 is therefore an excellent candidate as a regulator of the H10 gene, since H10 expression is activated after induced differentiation (61), precisely during the early cell cycle modifications suggested to be controlled by HBP1 (50). We were also able to show that the expression of RB in an RB-deficient cell line leads to induced expression of the endogenous H10 gene. This observation has been confirmed in transient transfection assays. RB, more generally known as a repressor of transcription, has been shown to be the activator of several differentiation-dependent genes. For instance, in adipocytes RB binds to C/EBPs in differentiating cells and enhances the binding of these factors to the target element, leading to the activation of a C/EBP-responsive reporter (10). The interaction of RB with NF-IL6, also a member of C/EBP family, was found to enhance its transactivation capacity and to be involved in monocyte/macrophage differentiation (9). In muscle cells, RB binds MyoD and mediates differentiation (16). Moreover, the interaction of RB with c-Jun and the enhancement of its transactivator capacity implicates RB as a transcriptional activator in the early G1 phase of the cell cycle (35, 36) and more specifically during keratinocyte differentiation (35). This transcriptional stimulatory effect of RB might be, at least in part, due to the enhancement of DNA-binding activity of target transcription factors upon their interaction with RB. Indeed, it has been shown that the interaction of RB with c-Jun stimulates its DNA-binding activity (35, 36). Similar results have been obtained with members of the C/EBP transcription factor family, in the presence of RB (9, 10). Interestingly, here we could show that RB can also stimulate the DNA-binding activity of HBP1. It appears therefore that the stimulation of transcription by RB generally involves an increase in the DNA-binding activity of partner transcription factors.
In this work, we showed that HBP1 is a ubiquitous transcription factor expressed in various tissues. Histone H10 is also expressed in almost all arrested and fully differentiated cells. Our findings therefore provide evidence for a more general involvement of RB in cell differentiation rather than in the control of specific programs. Data presented here also indicate a role for RB in chromatin remodeling during cell differentiation through the control of histone H10 gene activity. A role for RB in modulation of chromatin structure has already been postulated (6). This activity of RB could either be indirect or direct. A permanent hyperphosphorylation of histone H1, associated with relaxed chromatin structure, has been found in RB-deficient fibroblasts, indicating an involvement of RB in the regulation of those kinases responsible for the phosphorylation of chromatin proteins (17). Besides this indirect role, RB may have a direct role in chromatin remodeling through its interaction with a human SWI2/SNF2 homologue, hBrm (47), and through the recruiting of histone deacetylase, HDAC1 (5, 31, 32). The interaction of RB with topoisomerase IIα, shown recently, is also a possible means by which RB could modulate chromatin structure (3).
Finally, this work constitutes the first attempt to identify a regulatory pathway involved in the control of the differentiation-dependent expression of H10. However, results reported here do not exclude a role for other factors, interacting with key regulatory elements in H10 promoter, in the differentiation-dependent expression of this gene. It is important to consider the fact that the basal H10 promoter activity is dependent on the concerted action of at least three cis-acting regulatory elements, UCE, H1 box, and H4 box (21, 22). Therefore, besides the H4 box and transcription factors interacting with this element, other yet unknown factors are expected to participate in the organization of the active H10 promoter during cell differentiation. Some of these factors may be also involved in the regulation of expression of replication-dependent H1s. Indeed, one of these elements, also present in replication-dependent H1 promoter (15) and known as the UCE (21) or site IV (14), has been shown to play an important role in the activation of H10 gene expression in differentiating Friend cells (14). However, the identification of the H10 H4 box as a unique cis-acting regulatory element, linked to both differentiation and cell cycle control signals, allowed us to search for a key regulator responding to both of these processes. Interestingly, the transcription factor identified, HBP1, shows exactly the characteristics of the expected regulator; it is expressed in differentiated cells, and interacts with the cell cycle regulator RB. Moreover, the presence of an HMG box in this factor strongly suggests that HBP1 may be involved in an architectural regulation, organizing the active H10 promoter and facilitating the recruitment of other factors such as those discussed above.
These findings place also RB at the center of the putative chromatin remodeling command system that we were looking for. Recently, we have identified a new family of HDACs related to yeast HDA1 deacetylase (57). We showed that the expression of these genes is strictly coordinated with that of histone H10. These observations suggest that the regulatory mechanism presented here may control the expression of other differentiation-dependent chromatin modifiers and therefore identify RB as an essential regulator of chromatin structure during cell differentiation.
ACKNOWLEDGMENTS
We are grateful to Jean-Jacques Lawrence for encouraging this work and to Marie-Paule Brocard and Sandrine Curtet for technical assistance. We are deeply indebted to Annick Harel-Belan and Didier Trouche for RB expression vectors and other materials and helpful discussions and to E. Harlow for the Saos-2 cell line.
A.V. was a recipient of a fellowship from the Ligue Nationale Contre le Cancer, comité de la Haute Savoie. This work was supported by Association pour la Recherche sur le Cancer (ARC) and Groupement des Entreprises Françaises et Monegasques dans la Lutte Contre le Cancer (GEFLUC).
REFERENCES
- 1.Alison M R. Regulation of hepatic growth. Physiol Rev. 1986;66:499–541. doi: 10.1152/physrev.1986.66.3.499. [DOI] [PubMed] [Google Scholar]
- 2.Angelov D, Khochbin S, Dimitrov S. UV laser footprinting and protein-DNA crosslinking. In: Becker P B, editor. Chromatin protocols. Totowa, N.J: Humana Press; 1999. pp. 481–495. [DOI] [PubMed] [Google Scholar]
- 3.Bhat U G, Raychaudhuri P, Beck W T. Functional interaction between human topoisomerase IIalpha and retinoblastoma protein. Proc Natl Acad Sci USA. 1999;96:7859–7864. doi: 10.1073/pnas.96.14.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouterfa H L, Triebe S M, Doenecke D R. Differential regulation of the human H10 histone gene transcription in human tumor cell line. Eur J Biochem. 1993;217:353–360. doi: 10.1111/j.1432-1033.1993.tb18253.x. [DOI] [PubMed] [Google Scholar]
- 5.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 6.Brehm A, Kouzarides T. Retinoblastoma protein meets chromatin. Trends Biochem Sci. 1999;24:142–145. doi: 10.1016/s0968-0004(99)01368-7. [DOI] [PubMed] [Google Scholar]
- 7.Breuer B, Steuer B, Alonso A. Basal level of transcription of H10 gene is mediated by 80 bp promoter fragment. Nucleic Acids Res. 1993;21:927–934. doi: 10.1093/nar/21.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustin M. Regulation of DNA-dependent activities by functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen P L, Riley D J, Chen-Kiang S, Lee W H. Retinoblastoma protein directly interacts with and activates the transcription factor NF-IL6. Proc Natl Acad Sci USA. 1996;93:465–469. doi: 10.1073/pnas.93.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P L, Riley D J, Chen Y, Lee W H. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson M J, Wells J R, Gibson F, Saint R, Tremethick D J. Regions of variant histone His2AvD required for Drosophila development. Nature. 1999;399:694–697. doi: 10.1038/21436. [DOI] [PubMed] [Google Scholar]
- 12.Dailey L, Roberts S B, Heintz N. Purification of the human histone H4 gene-specific transcription factors H4TF-1 and H4TF-2. Genes Dev. 1988;2:1700–1712. doi: 10.1101/gad.2.12b.1700. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez V, Pina B, Suau P. Histone H1 subtype synthesis in neurons and neuroblasts. Development. 1992;115:181–185. doi: 10.1242/dev.115.1.181. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y, Liu D, Skoultchi A I. An upstream control region required for inducible transcription of the mouse H10 histone gene during terminal differentiation. Mol Cell Biol. 1995;15:1889–1900. doi: 10.1128/mcb.15.4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncliffe K N, Rondahl M E, Wells J R. A H1 histone gene-specific AC-box-related element influences transcription from a major chicken H1 promoter. Gene. 1995;163:227–232. doi: 10.1016/0378-1119(95)00370-l. [DOI] [PubMed] [Google Scholar]
- 16.Gu W, Schneider J W, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 17.Herrera R E, Chen F, Weinberg R A. Increased histone H1 phosphorylation and relaxed chromatin structure in Rb-deficient fibroblasts. Proc Natl Acad Sci USA. 1996;93:11510–11515. doi: 10.1073/pnas.93.21.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using PCR. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 19.Holthuis J, Owen T A, van Wijnen A J, Wright K L, Ramsey-Ewing A, Kennedy M B, Carter R, Cosenza S C, Soprano K J, Lian J B, Stein J L, Stein G S. Tumor cells exhibit deregulation of the cell cycle histone gene promoter factor HiNF-D. Science. 1990;247:1454–4547. doi: 10.1126/science.247.4949.1454. [DOI] [PubMed] [Google Scholar]
- 20.Khochbin S, Gorka C, Lawrence J J. Multiple control level governing H10 mRNA and protein accumulation. FEBS Lett. 1991;238:65–67. doi: 10.1016/0014-5793(91)80554-g. [DOI] [PubMed] [Google Scholar]
- 21.Khochbin S, Wolffe A P. Developmental regulation and butyrate inducible transcription of the Xenopus histone H10 promoter. Gene. 1993;128:173–180. doi: 10.1016/0378-1119(93)90560-p. [DOI] [PubMed] [Google Scholar]
- 22.Khochbin S, Lawrence J J. Molecular basis of the activation of basal histone H10 gene expression. Nucleic Acids Res. 1994;22:2887–2893. doi: 10.1093/nar/22.15.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khochbin S, Wolffe A P. Developmentally regulated expression of linker histone variants in vertebrates. Eur J Biochem. 1994;225:501–510. doi: 10.1111/j.1432-1033.1994.00501.x. [DOI] [PubMed] [Google Scholar]
- 24.Kroeger P, Stewart T, Schaap T, van Wijnen J, Hirshman S, Helms G, Stein G, Stein J. Proximal and distal regulatory elements that influence in vivo expression of a cell cycle-dependent human H4 histone gene. Proc Natl Acad Sci USA. 1987;84:3982–3986. doi: 10.1073/pnas.84.12.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai Z C, Maxson R, Childs G. Both basal and ontogenic promoter elements affect the timing and level of expression of a sea urchin H1 gene during early embryogenesis. Genes Dev. 1988;2:173–183. doi: 10.1101/gad.2.2.173. [DOI] [PubMed] [Google Scholar]
- 26.Lavender P, Vandel L, Bannister A J, Kouzarides T. The HMG-box transcription factor HBP1 is targeted by the pocket proteins and E1A. Oncogene. 1997;14:2721–2728. doi: 10.1038/sj.onc.1201243. [DOI] [PubMed] [Google Scholar]
- 27.Lemercier C, Brown A, Mamani M, Ripoche J, Reiffers J. The rat Mist1 gene: structure and promoter characterization. Gene. 2000;242:209–218. doi: 10.1016/s0378-1119(99)00523-5. [DOI] [PubMed] [Google Scholar]
- 28.Lemercier C, To R Q, Carrasco R A, Konieczny S F. The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of MyoD. EMBO J. 1998;17:1412–1422. doi: 10.1093/emboj/17.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesage F, Hugnot J P, Amri E Z, Grimaldi P, Barhanin J, Lazdunski M. Expression cloning in K+ transport defective yeast and distribution of HBP1, a new putative HMG transcriptional regulator. Nucleic Acids Res. 1994;22:3685–3688. doi: 10.1093/nar/22.18.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieber T, Weisser K, Childs G. Analysis of histone gene expression in adult tissues of the sea urchins Strongylocentrotus purpuratus and Lytechinus pictus: tissue-specific expression of sperm histone genes. Mol Cell Biol. 1986;7:2602–2612. doi: 10.1128/mcb.6.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo R X, Postigo A A, Dean D C. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 32.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain J P, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 33.Mandl B, Brandt W F, Superti-Furga G, Graninger P G, Birnstiel M L, Busslinger M. The five cleavage-stage (CS) histones of the sea urchin are encoded by a maternally expressed family of replacement histone genes: functional equivalence of the CS H1 and frog H1M (B4) proteins. Mol Cell Biol. 1997;3:1189–1200. doi: 10.1128/mcb.17.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalovitz D, Halevy O, Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990;62:671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- 35.Nead M A, Baglia L A, Antinore M J, Ludlow J W, McCance D J. Rb binds c-Jun and activates transcription. EMBO J. 1988;17:2342–2352. doi: 10.1093/emboj/17.8.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishitani J, Nishinaka T, Cheng C H, Rong W, Yokoyama K K, Chiu R. Recruitment of the retinoblastoma protein to c-Jun enhances transcription activity mediated through the AP-1 binding site. J Biol Chem. 1999;274:5454–5461. doi: 10.1074/jbc.274.9.5454. [DOI] [PubMed] [Google Scholar]
- 37.Nordeen S, Green III P P, Fowlkes D M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987;6:173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- 38.Pauli U, Chrysogelos S, Stein G, Stein J, Nick H. Protein-DNA interactions in vivo upstream of a cell cycle-regulated human H4 histone gene. Science. 1987;236:1308–1311. doi: 10.1126/science.3035717. [DOI] [PubMed] [Google Scholar]
- 39.Patterton D, Wolffe A P. Developmental role for chromatin and chromosomal structure. Dev Biol. 1996;173:2–13. doi: 10.1006/dbio.1996.0002. [DOI] [PubMed] [Google Scholar]
- 40.Pehrson J R, Costanzi C, Dharia C. Developmental and tissue expression patterns of histone macroH2A1 subtypes. J Cell Biochem. 1997;65:107–13. doi: 10.1002/(sici)1097-4644(199704)65:1<107::aid-jcb11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 41.Peretti M, Khochbin S. The evolution of the differentiation-specific histone H1 gene basal promoter. J Mol Evol. 1997;44:128–134. doi: 10.1007/pl00006129. [DOI] [PubMed] [Google Scholar]
- 42.Ponte I, Martinez P, Ramirez A, Jorcano J L, Monzo M, Suau P. Transcriptional activation of histone H10 during neuronal terminal differentiation. Dev Brain Res. 1994;80:35–44. doi: 10.1016/0165-3806(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 43.Ramsey-Ewing A, van Wijnen A J, Stein G S, Stein J L. Delineation of a human histone H4 cell cycle element in vivo: the master switch for H4 gene transcription. Proc Natl Acad Sci USA. 1994;91:4475–4479. doi: 10.1073/pnas.91.10.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rousseau D, Khochbin S, Gorka C, Lawrence J J. Regulation of H10 accumulation during induced differentiation of murine erythroleukemia cells. J Mol Biol. 1991;194:174–179. doi: 10.1016/0022-2836(91)90613-b. [DOI] [PubMed] [Google Scholar]
- 45.Rousseau D, Khochbin S, Gorka C, Lawrence J J. Induction of H10 gene expression in B16 murine melanoma. Eur J Biochem. 1992;208:775–779. doi: 10.1111/j.1432-1033.1992.tb17247.x. [DOI] [PubMed] [Google Scholar]
- 46.Shih H, Tevosian S G, Yee A. Regulation of differentiation by HBP1, a target of the retinoblastoma protein. Mol Cell Biol. 1998;18:4732–4743. doi: 10.1128/mcb.18.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh P, Coe J, Hong W. A role for retinoblastoma protein in potentiating transcriptional activation by the glucocorticoid receptor. Nature. 1995;374:562–565. doi: 10.1038/374562a0. [DOI] [PubMed] [Google Scholar]
- 48.Spassky A, Angelov D. Influence of the local helical conformation on the guanine modifications generated from one-electron DNA oxidation. Biochemistry. 1997;36:6571–6576. doi: 10.1021/bi962761d. [DOI] [PubMed] [Google Scholar]
- 49.Stein G, Lian J, Stein J, Briggs R, Shalhoub V, Wright K, Pauli U, van Wijnen A. Altered binding of human histone gene transcription factors during the shutdown of proliferation and onset of differentiation in HL-60 cells. Proc Natl Acad Sci USA. 1989;86:865–869. doi: 10.1073/pnas.86.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tevosian S G, Shih H H, Mendelson K G, Sheppard K A, Paulson K E, Yee A S. HBP1: a HMG-box transcriptional repressor that is targeted by the retinoblastoma family. Genes Dev. 1997;11:383–396. doi: 10.1101/gad.11.3.383. [DOI] [PubMed] [Google Scholar]
- 51.Trieschmann L, Schulze E, Schulze B, Grossbach U. The histone H1 genes of the dipteran insect, Chironomus thummi, fall under two divergent classes and encode proteins with distinct intranuclear distribution and potentially different functions. Eur J Biochem. 1997;250:184–196. doi: 10.1111/j.1432-1033.1997.00184.x. [DOI] [PubMed] [Google Scholar]
- 52.van Wijnen A J, Wright K L, Lian J B, Stein J L, Stein G S. Human H4 histone gene transcription requires the proliferation-specific nuclear factor HiNF-D. Auxiliary roles for HiNF-C (Sp1-like) and HiNF-A (high mobility group-like) J Biol Chem. 1989;264:15034–15042. [PubMed] [Google Scholar]
- 53.van Wijnen A J, Van den Ent F M I, Lian J B, Stein J L, Stein G S. Overlapping and CpG methylation-sensitive protein-DNA interaction at the histone H4 transcriptional cell cycle domain: distinctions between two human H4 gene promoters. Mol Cell Biol. 1992;12:3273–3287. doi: 10.1128/mcb.12.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Wijnen A J, Aziz F, Grana X, De Luca A, Desai R K, Jaarsveld K, Last T J, Soprano K, Giordano A, Lian J B, Stein J L, Stein G S. Transcription of histone H4, H3, and H1 cell cycle genes: promoter factor HiNF-D contains CDC2, cyclin A, and an RB-related protein. Proc Natl Acad Sci USA. 1994;91:12882–12886. doi: 10.1073/pnas.91.26.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Wijnen A J, van Gurp M F, de Ridder M C, Tufarelli C, Last T J, Birnbaum M, Vaughan P S, Giordano A, Krek W, Neufeld E J, Stein J L, Stein G S. CDP/cut is the DNA-binding subunit of histone gene transcription factor HiNF-D: a mechanism for gene regulation at the G1/S phase cell cycle transition point independent of transcription factor E2F. Proc Natl Acad Sci USA. 1996;93:11516–11521. doi: 10.1073/pnas.93.21.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaughan P S, Aziz F, van Wijnen A J, Wu S, Harada H, Taniguchi T, Soprano K J, Stein J L, Stein G S. Activation of a cell-cycle-regulated histone gene by the oncogenic transcription factor IRF-2. Nature. 1995;377:362–365. doi: 10.1038/377362a0. [DOI] [PubMed] [Google Scholar]
- 57.Verdel A, Khochbin S. Identification of a new family of higher eukaryotic histone deacetylases. J Biol Chem. 1999;274:2440–2445. doi: 10.1074/jbc.274.4.2440. [DOI] [PubMed] [Google Scholar]
- 58.Wolffe A P. Chromatin and gene regulation at the onset of embryonic development. Reprod Nutr Dev. 1996;36:581–606. [PubMed] [Google Scholar]
- 59.Wolffe A P. Architectural regulation and Hmg1. Nat Genet. 1999;22:215–217. doi: 10.1038/10267. [DOI] [PubMed] [Google Scholar]
- 60.Zhuma T, Tyrrell R, Sekkali B, Skavdis G, Saveliev A, Tolaini M, Roderick K, Norton T, Smerdon S, Sedgwick S, Festenstein R, Kioussis D. Human HMG box transcription factor HBP1: a role in hCD2 LCR function. EMBO J. 1999;18:6306–6406. doi: 10.1093/emboj/18.22.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zlatanova J, Doenecke D. Histone H10: a major player in cell differentiation? FASEB J. 1994;15:1260–1268. doi: 10.1096/fasebj.8.15.8001738. [DOI] [PubMed] [Google Scholar]