Abstract
Two-pore-domain potassium (K2P-) channels conduct outward K+ currents that maintain the resting membrane potential and modulate action potential repolarization. Members of the K2P channel family are widely expressed among different human cell types and organs where they were shown to regulate important physiological processes. Their functional activity is controlled by a broad variety of different stimuli, like pH level, temperature, and mechanical stress but also by the presence of lipids or pharmacological agents. In patients suffering from cardiovascular diseases, alterations in K2P-channel expression and function have been observed, suggesting functional significance and a potential therapeutic role of these ion channels. For example, upregulation of atrial specific K2P3.1 (TASK-1) currents in atrial fibrillation (AF) patients was shown to contribute to atrial action potential duration shortening, a key feature of AF-associated atrial electrical remodelling. Therefore, targeting K2P3.1 (TASK-1) channels might constitute an intriguing strategy for AF treatment. Further, mechanoactive K2P2.1 (TREK-1) currents have been implicated in the development of cardiac hypertrophy, cardiac fibrosis and heart failure. Cardiovascular expression of other K2P channels has been described, functional evidence in cardiac tissue however remains sparse. In the present review, expression, function, and regulation of cardiovascular K2P channels are summarized and compared among different species. Remodelling patterns, observed in disease models are discussed and compared to findings from clinical patients to assess the therapeutic potential of K2P channels.
Keywords: K2P-channel, TASK-1, TREK-1, two-pore-domain potassium channel
1. Introduction
Two-pore-domain potassium (K2P) channels are expressed throughout the human body and contribute to background potassium conductance in many different cell types [1,2]. In the human genome 15 K2P channels have been described which differ from classical potassium channels by the fact that each subunit carries two pore-forming domains, and the channels thus assemble as dimers instead of tetramers (Figure 1). K2P channels give rise to background or “leak” potassium currents which control a multitude of physiological processes [1]. Initially, K2P currents were described as outward rectifying “leakage currents” but recent work has shown that several members of the K2P family can also be voltage activated [3].
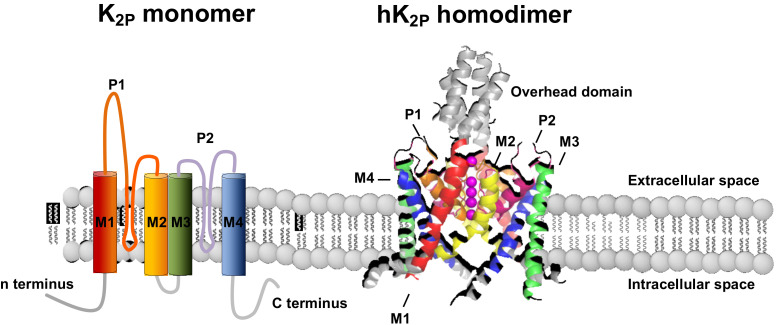
Figure 1.
Membrane topology and structure of K2P channels. K2P channel monomers (left), consisting of 4 transmembrane domains (M1–4) and 2 pore forming loops (P1–2) assemble as homo- or heterodimers. (right).
K2P currents show a high degree of similarity to the potassium plateau currents IKP, described in guinea-pig cardiomyocytes and the steady-state potassium current IK,SS, characterized in murine cardiomyocytes and the arachidonic acid-sensitive potassium current IKAA from rat ventricular cardiomyocytes [4,5,6,7]. Cardiac mRNA abundance was described for several members of the K2P family (Figure 2) In the present review, expression, function, and regulation of cardiovascular K2P channels are summarized and compared among different species. Remodelling patterns, observed in disease models are discussed and compared to findings from clinical patients to assess the therapeutic potential of K2P channels (Figure 3).
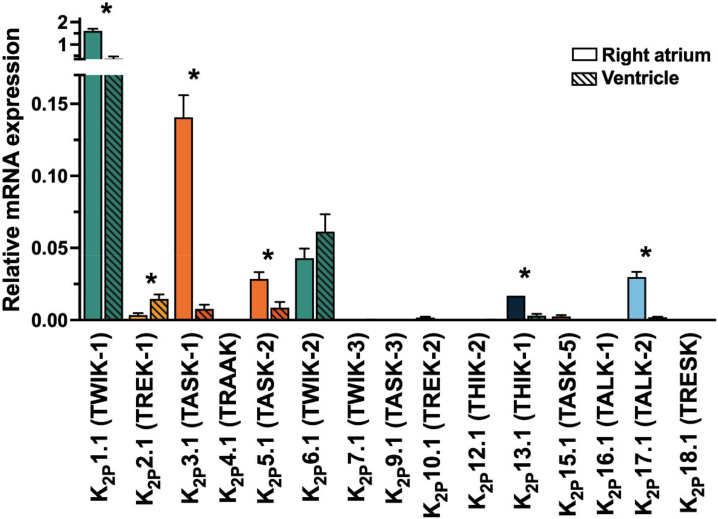
Figure 2.
Cardiac mRNA levels of K2P channels in the human heart (whole tissue). Expression of two-pore-domain potassium (K2P-) channel mRNA level in human right atrial (n = 10) and left ventricular (n = 5) tissue samples. Data are given as mean ± SEM relative to the housekeeping gene importin 8 (IPO8). * indicate p < 0.05 from Student’s t-tests. Data from Schmidt et al. 2015, Circulation [8].
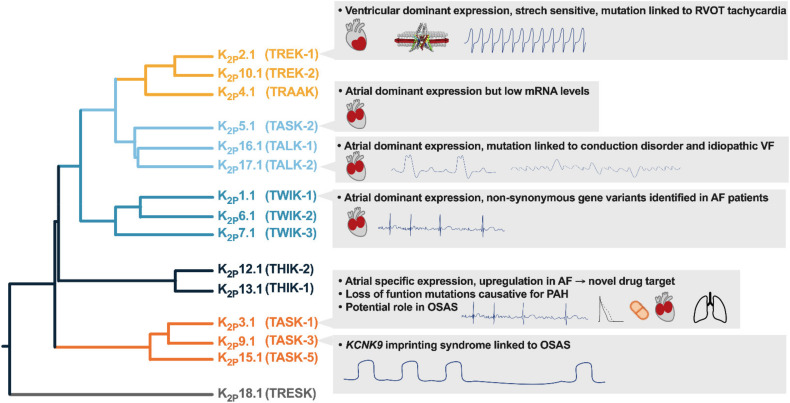
Figure 3.
Potential translational implications of cardiac K2P channel expression.AF, atrial fibrillation; OSAS, obstructive sleep apnea; PAH pulmonary arterial hypertension; RVOT, right ventricular outflow tract; VF, ventricular fibrillation.
2. Structural Assembly and Nomenclature of K2P Channels
The 15 channel subunits of the K2P family each consists of around 300 and 550 amino acids. The sequence differences between the individual subunits of the K2P channel can sometimes be as large as to other potassium channel families. K2P18.1 (TRESK) channel subunits, for example share only 19% amino acid sequence identity with the other K2P family members. But the common feature that links them is the eponymous structural motif of two pore-forming domains per subunit, which distinguishes them from all other potassium channel groups. As shown in Figure 1, the four alphahelical transmembrane domains (M1–M4) flank two pore-forming loops (P1 and P2), each containing the potassium selective filter motif (GLG, GFG, or GYG). M1 and P1 are connected by a long extracellular loop, forming an overhead cap structure. The short amino terminus and a much longer carboxy terminus, which contains a variety of regulatory phosphorylation and protein interaction motifs, are localized intracellularly. Whereas most potassium channels form tetramers with one pore-forming loop per subunit, a functional two-pore domain potassium channel is composed of two alpha subunits (Figure 1). In addition to homodimerization, certain K2P channel subunits can also assemble as heterodimers. This is mainly described within the same subfamilies (i.e., TASK-1/TASK-3, TREK-1/TRAAK, THIK-1/THIK-2), but can also occur between TWIK-1 and TREK or TASK-1, and between TASK-1/TALK-2 subunits. Physiological relevance in the perception of hypoxia has been described for TASK-1/TASK-3 heterodimers and TWIK-1/TREK-1 heterodimers have been detected in astrocytes. Apart from the TASK-1 and TALK-1 subfamilies, all K2P channel subunits possess a conserved Cys-amino acid residue of the overhead domain that is thought to play a major, although not yet conclusively elucidated, role in dimerization. The predicted membrane topology and tertiary structure have already been confirmed by X-ray structural analysis for several K2P-channels (Table 1).
Table 1.
Nomenclature of K2P-channels.
| Gene Name | IUPHAR K2P Nomenclature |
Functional Name | Other Names | Crystal Structure |
|---|---|---|---|---|
| KCNK1 | K2P1.1 | TWIK-1 (Tandem of P-domains in a weak inward-rectifying K+ channel 1) |
hOHO, DPK, KCNO1 |
|
| KCNK2 | K2P2.1 | TREK-1 (TWIK-related K+ channel 1) |
TPKC1 |
|
| KCNK3 | K2P3.1 | TASK-1 (TWIK-related acid-sensitive K+ channel 1) |
TBAK-1, OAT-1, PPH4 |
|
| KCNK4 | K2P4.1 | TRAAK (TWIK-related arachidonic acid activated K+ channel) |
FHEIG |
|
| KCNK5 | K2P5.1 | TASK-2 (TWIK-related acid-sensitive K+ channel 2) |
|
|
| KCNK6 | K2P6.1 | TWIK-2 (Tandem of P-domains in a weak inward-rectifying K+ channel 2) |
TOSS | - |
| KCNK7 | K2P7.1 | TWIK-3 (Tandem of P-domains in a weak inward-rectifying K+ channel 3) |
- | |
| The name kcnk8 was initially given to a murine K2P gene which was later identified as an ortholog of the human KCNK7 gene and therefore renamed to kcnk7 | ||||
| KCNK9 | K2P9.1 | TASK-3 (TWIK-related acid-sensitive K+ channel 3) |
KT3.2, BIBARS, TASK32 | - |
| KCNK10 | K2P10.1 | TREK-2 (TWIK-related K+ channel 2) |
PPP1R97 |
|
| KCNK11 was withdrawn due to nomenclature duplications with KCNK15 | ||||
| KCNK12 | K2P12.1 | THIK-2 (Tandem pore domain halothane inhibited K+ channel 2) |
- | |
| KCNK13 | K2P13.1 | THIK-1 (Tandem pore domain halothane inhibited K+ channel 1) |
- | |
| KCNK14 was withdrawn due to nomenclature duplications with KCNK15 | ||||
| KCNK15 | K2P15.1 | TASK-5 (TWIK-related acid-sensitive K+ channel 5) |
KT3.3, dJ781B1.1 | - |
| KCNK16 | K2P16.1 | TALK-1 (TWIK-related alkaline pH-activated K+ channel 1) |
- | |
| KCNK17 | K2P17.1 | TALK-2 (TWIK-related alkaline pH-activated K+ channel 2) |
TASK-4 | - |
| KCNK18 | K2P18.1 | TRESK (TWIK-related spinal cord K+ channel 1) |
MGR13, TRIK, TRESK2 | - |
IUPHAR, International Union of Basic and Clinical Pharmacology. Visualizations of the channel structurs were generated with PyMOL (TM) Molecular Graphics System, Version 2.3.0 (Schrodinger, LLC; New York, NY, USA) from crystall stuctures with the protein database enty numbers: 3UKM, 4TWK, 6RV2, 3UM7, 6WLV, 3UX0 and 4BW5.
Upon their discovery, the individual K2P-channels received trivial names reflecting their respective structural and regulatory properties: TWIK: “Tandem of P domains in a weak inward rectifying K+ channel”, TREK: “TWIK-related K+ channel”, TASK: “TWIK-related acid-sensitive K+ channel”, TRAAK: “TWIK-related arachidonic acid activated K+ channel”, TALK “TWIK-related alkaline pH-activated K+ channel”, THIK “tandem pore domain halothane-inhibited K+ channel”, and TRESK “TWIK-related spinal cord K+ channel”. In parallel, the channels are labeled consecutively with the designations K2P1.1 to K2P18.1 according to the Human Genome Organization name of the encoding gene (KCNK1 to KCNK18) (see Figure 2 and Table 1). Each of the 15 subfamilies members (K2P1.1 to K2P18.1) contains only one member. Unfortunately, this led to a confusing nomenclature in which channels with different functional properties such as TASK-1 and TASK-2 have similar names, while other channels are titled with acronyms of factually incorrect names (for example, TWIK-1 is not a weak inward rectifier but an open rectifier). Further, some channels carry a variety of redundant names such as in case of K2P3.1: TBAK1, TASK1 and OAT1. Several KCNKx designators were initially assigned to K2P channel transcripts that later turned out to be orthologs of other human K2P channels. Thus, KCNK8 (the murine transcript designated kcnk8 later proved to be an ortholog of human KCNK7 and was therefore renamed kcnk7), KCNK11, and KCNK14 (both orthologs of KCNK15) do not exist [9]. For better understanding, we will provide the trivial names of the channel subunits in brackets in addition to the International Union of Basic and Clinical Pharmacology IUPHAR (K2PX.1) names. Since they do not show any functional activity in heterologous expression systems, the channels KCNK7, KCNK12 and KCNK15 are referred to as silent K2P channels. It remains unclear whether these K2P channel subunits are truly nonfunctional in vivo or whether they just lack essential cofactors to achieve functionality upon heterologous expression. In fact, the functionality of the K2P16.1 channels could be restored by deletion of an n-terminal ER-retention motif [8].
3. K2P1.1 (TWIK-1)
Robust cardiac mRNA levels were consistently described for KCNK1 [10,11,12,13,14,15]. In a study from our laboratory, which examined the expression of all K2P channels in the human heart (TaqMan-qPCR; Figure 2), the highest mRNA levels were detected for KCNK1 [10]. Atrial predominant mRNA abundance was shown in patient-derived tissue samples but not in rodents (Table 2) [10,16].
Table 2.
Evidence in literature for cardiac expression of K2P channel subunits at mRNA or protein level in different species.
| K2P Channel Subunit | Species | Protein /mRNA | Observation | Citation |
|---|---|---|---|---|
|
K2P1.1
(TWIK-1) |
Zebrafish | mRNA (RT-PCR, ISH) | Ubiquitous kcnk1a and kcnk1b ortholog mRNA in embryonic heart | [11] |
| Mouse | mRNA (RT-PCR) | No cardiac mRNA abundance | [17] | |
| Mouse | mRNA (RT-qPCR, TaqMan) | Moderate cardiac mRNA abundance, V > A | [16] | |
| Rat | mRNA (RT-PCR) | Moderate cardiac mRNA abundance, A > V | [18] | |
| Rat | mRNA (RT-PCR) | Moderate cardiac mRNA abundance | [15] | |
| Rat | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance mRNA detected in sinoatrial tissue |
[19] | |
| Human | mRNA (NB) | Cardiac mRNA abundance | [20] | |
| Human | mRNA (RT-PCR) | Cardiac mRNA abundance, V > A | [21] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance, A > V Highest mRNA level among all K2P channels |
[10] | |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance, A>V | [13] | |
| Human | mRNA (RT-qPCR) | mRNA detected in human ventricular tissue mRNA detected in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance | [23] | |
| Human | mRNA (Affymetrix chip and RT-qPCR, TaqMan) | Cardiac mRNA abundance, A > Purkinje fibers > V | [12] | |
| Human | mRNA (Affymetrix chip, RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA abundance, A > Purkinje fibers > V Cardiac protein expression, A > V |
[14] | |
|
K2P2.1
(TREK-2) |
Mouse | mRNA (NB) | Cardiac mRNA abundance | [24] |
| Mouse | mRNA (RT-PCR) | Cardiac mRNA abundance, V > A | [17] | |
| Mouse | mRNA (RT-PCR) | Cardiac mRNA abundance | [25] | |
| Mouse | mRNA (RT-qPCR) and protein (WB) | Cardiac mRNA abundance, V > A Cardiac protein expression |
[26] | |
| Mouse | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA levels, V > A | [16] | |
| Mouse | Protein (IF) | Protein expression in isolated ventricular cardiomyocytes | [27] | |
| Rat | mRNA (RT-PCR) | mRNA abundance in isolated ventricular cardiomyocytes | [28] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance, A and V | [18] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance, A and V | [29] | |
| Rat | mRNA (RT-PCR) | Endocardial mRNA levels > epicardial mRNA expression | [30] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA levels, mRNA detected in cardiomyocytes |
[15] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance | [31] | |
| Rat | mRNA (RT-qPCR) | Cardiac mRNA abundance Cardiac mRNA adult heart > fetal heart |
[18] | |
| Rat | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance in sinoatrial tissue | [19] | |
| Rat | mRNA (RT-PCR)and protein (IF) | Cardiac mRNA abundance, A and V Protein expression in isolated cardiomyocytes |
[32] | |
| Rat | mRNA (RT-PCR) and protein (IF) | mRNA and protein expression in rat cardiomyocytes | [33] | |
| Rat | mRNA (RT-PCR) and protein (WB, IF) | Cardiac mRNA and protein expression, A and V | [34] | |
| Rabbit, mouse | Protein (WB) | Cardiac protein expression, SAN > A > V | [35] | |
| Pig | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA and protein expression, V = A mRNA and protein expression in sinoatrial and atrioventricular node |
[36] | |
| Pig, human | mRNA (RT-qPCR, TaqMan) | Atrial mRNA expression in human and pig | [37] | |
| Human | mRNA (RT-PCR) | Cardiac mRNA abundance | [31] | |
| Human | mRNA (RT-qPCR) | Low cardiac mRNA abundance | [38] | |
| Human | mRNA (RT-qPCR) | Low cardiac mRNA abundance | [39] | |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance, V Low mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance | [39] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance, V > A | [10] | |
| Human | mRNA (RT-qPCR, TaqMan) | Low cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA levels, V > A | [40] | |
| Human, mouse | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA and protein expression in human and mice, V > A | [41] | |
| Human | Protein (WB) | Cardiac protein expression | [42] | |
| Human | Protein (WB) | Cardiac protein expression | [43] | |
|
K2P3.1
(TASK-1) |
Chicken embryo | mRNA (ISH) and protein (IF) | Cardiac mRNA and protein expression in chicken embryos | [44] |
| Mouse, human | mRNA (NB) | Human and Mouse: Cardiac mRNA abundance | [45] | |
| Mouse | mRNA (RT-PCR) | Cardiac mRNA abundance | [17] | |
| Mouse | mRNA (RT-qPCR) | Cardiac mRNA levels, V > A | [26] | |
| Mouse | mRNA (RT-PCR) and protein (WB) | Cardiac protein expression | [25] | |
| Mouse, human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA expression | [46] | |
| Mouse | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA and protein expression, A and V | [16] | |
| Rat | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance in sinoatrial tissue | [19] | |
| Rat | mRNA (NB, RT-PCR) | Cardiac mRNA abundance, A and V | [47] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance, cardiomyocyte mRNA abundance | [15] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance | [48] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance, A > V | [18] | |
| Rat, guinea pig, human | mRNA (RT-qPCR, TaqMan) | Human: Cardiac mRNA levels, V > A Rat: Cardiac mRNA abundance, A and V Guinea pig: Cardiac mRNA levels, V > A |
[49] | |
| Dog | Protein (WB) | Atrial protein expression | [50] | |
| Pig | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA and protein expression mRNA abundance in sinoatrial and atrioventricular node |
[51] | |
| Pig | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA and protein expression | [52] | |
| Human | mRNA (RT-qPCR) | Low cardiac mRNA abundance | [38] | |
| Human | mRNA (RT-qPCR) | mRNA abundance in Purkinje fibers | [5] | |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance, A > V Cardiac mRNA adult heart > fetal heart |
[39] | |
| Human | mRNA (RT-qPCR) | Low mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR, TaqMan) | mRNA levels in isolated atrial cardiomyocytes > in isolated atrial fibroblasts | [53] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA levels, A > V | [54] | |
| Human | mRNA (Affymetrix chip and RT-qPCR, TaqMan) | Cardiac mRNA abundance, A | [55] | |
| Human | mRNA (Affymetrix chip and RT-qPCR, TaqMan) | Cardiac mRNA levels, A > V Expression in Purkinje fibers |
[14] | |
| Human | mRNA (Affymetrix chip and RT-qPCR, TaqMan) | Cardiac mRNA expression, A > V | [12] | |
| Human | mRNA (RT-qPCR) and protein (IF) | Cardiac mRNA and protein expression | [56] | |
| Human | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA levels, A > V Cardiac protein expression, A |
[40] | |
| Human | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA levels, A > V Cardiac protein expression, A |
[10] | |
| Human | mRNA (bulk RNAseq) | Cardiac mRNA levels, A > V | [57] | |
|
K2P4.1
(TRAAK) |
Mouse | mRNA (RT-PCR, NB) | No cardiac mRNA detectable | [58] |
| Mouse | mRNA (RT-qPCR) | Human: no cardiac mRNA detectable Mouse: Low cardiac mRNA abundance, A > V |
[41] | |
| Mouse | mRNA (qRT-PCR) and protein (WB) | Cardiac mRNA abundance | [26] | |
| Mouse | mRNA (RT-qPCR, TaqMan) | No cardiac mRNA levels detectable | [16] | |
| Rat | mRNA (RT-PCR) | No cardiac mRNA levels | [15] | |
| Rat | mRNA (RT-PCR) | Low cardiac mRNA levels, A and V | [18] | |
| Human | mRNA (RT-qPCR) | mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR) | Low cardiac mRNA levels | [59] | |
| Human | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA levels | [10] | |
| Human | mRNA (RT-qPCR, TaqMan) | No cardiac mRNA abundance | [23] | |
|
K2P5.1
(TASK-2) |
Mouse | mRNA (RT-PCR) | Cardiac mRNA abundance | [17] |
| Mouse | mRNA (RT-PCR) | Cardiac mRNA levels, A and V | [26] | |
| Mouse | mRNA (RT-PCR) | Low cardiac mRNA abundance | [25] | |
| Mouse | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA levels, A > V | [16] | |
| Rat | mRNA (NB) | No cardiac mRNA abundance | [60] | |
| Rat | mRNA (RT-PCR) | Low cardiac mRNA levels, A and V | [18] | |
| Rat | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance in sinoatrial tissue | [19] | |
| Human | mRNA (RT-PCR) | Cardiac mRNA abundance | [61] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR) | mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance | [56] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA levels, A > V | [10] | |
| Human | mRNA (Affymetrix chip and RT-qPCR, TaqMan) | Cardiac mRNA levels, A > V mRNA abundance in Purkinje fibers |
[14] | |
| Human | mRNA (RT-qPCR) and protein (WB) | Very low cardiac mRNA levelsDetectable protein levels | [38] | |
|
K2P6.1
(TWIK-2) |
Mouse | mRNA (RT-qPCR, TaqMan) | Moderate cardiac mRNA abundance, A and V | [16] |
| Mouse | mRNA (RT-qPCR) and protein (WB) | Low cardiac mRNA abundance, A and V Cardiac protein expression |
[26] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance Cardiac mRNA adult heart > fetal heart Highest mRNA abundance in right atrium |
[18] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance | [62] | |
| Rat | mRNA (RT-PCR) | Moderate cardiac mRNA abundance | [15] | |
| Rat | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance in sinoatrial tissue | [19] | |
| Human | mRNA (NB) | No cardiac mRNA abundance | [17] | |
| Human | mRNA (Hybridization array) | Cardiac mRNA levels, V > A | [62] | |
| Human | mRNA (RT-qPCR) | mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR, TaqMan) | Low cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA levels, V > A | [10] | |
|
K2P7.1
(TWIK-3) |
Mouse | mRNA (RT-qPCR, TaqMan) | No cardiac mRNA abundance detectable | [16] |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance | [63] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA levels, A > V | [10] | |
|
K2P9.1
(TASK-3) |
Mouse | mRNA (RT-qPCR, TaqMan) | No cardiac mRNA abundance detectable | [16] |
| Mouse | mRNA (RT-qPCR) | Low cardiac mRNA abundance | [26] | |
| Mouse | mRNA (RT-PCR) | Low cardiac mRNA abundance | [25] | |
| Rat | mRNA (RT-PCR) | Low cardiac mRNA abundance, A and V | [18] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance | [48] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance, cardiomyocyte mRNA expression | [15] | |
| Rat, guinea pig, human | mRNA (RT-qPCR, TaqMan) | Human: very low cardiac mRNA abundance Rat: no cardiac mRNA abundance Guinea pig: low cardiac mRNA abundance, V > A |
[49] | |
| Guinea pig | mRNA (RT-PCR) | No cardiac mRNA abundance | [64] | |
| Human | mRNA (RT-qPCR) | Moderate mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA levels, A > V | [10] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR) an protein (IF) | Strong cardiac mRNA and protein expression | [56] | |
|
K2P10.1
(TREK-2) |
Mouse | mRNA (RT-qPCR, TaqMan) | No cardiac mRNA abundance detectable | [16] |
| Rat | mRNA (RT-PCR) | Cardiac mRNA levels, A > V | [18] | |
| Rat | mRNA (RT-PCR) | Moderate cardiac abundance | [15] | |
| Rat | mRNA (RT-PCR, NB) | No cardiac mRNA abundance | [65] | |
| Human | mRNA (RT-qPCR) | mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR, TaqMan) | Mild cardiac mRNA abundance, A > V | [41] | |
| Human | mRNA (RT-qPCR, TaqMan) | Low cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR, TaqMan) | Low cardiac mRNA levels, A > V | [10] | |
|
K2P12.1
(THIK-2) |
Mouse | mRNA (RT-PCR) | Very low cardiac mRNA abundance | [15] |
| Mouse | mRNA (RT-qPCR, TaqMan) | No cardiac mRNA levels detectable | [16] | |
| Rat | mRNA (RT-PCR) | No cardiac mRNA abundance | [66] | |
| Human | mRNA (NB) | Cardiac mRNA abundance | [67] | |
| Human | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA abundance, A and V | [10] | |
|
K2P13.1
(THIK-1) |
Zebrafish | mRNA (RT-PCR) | Cardiac mRNA abundance | [68] |
| Mouse | mRNA (RT-PCR) | Cardiac mRNA abundance | [15] | |
| Mouse | mRNA (RT-qPCR) | Cardiac mRNA abundance | [26] | |
| Mouse | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA abundance | [16] | |
| Rat | mRNA (RT-PCR) | Cardiac mRNA abundance | [66] | |
| Human | mRNA (RT-qPCR) | mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR, TaqMan) | Low cardiac mRNA abundance | [23] | |
| Human | mRNA (RT-qPCR, TaqMan) | Low cardiac mRNA abundance, A > V | [10] | |
|
K2P15.1
(TASK-5) |
Mouse | mRNA (RT-qPCR) | Cardiac mRNA abundance | [26] |
| Mouse | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA abundance | [16] | |
| Rat | mRNA (RT-PCR) | No cardiac mRNA abundance | [48] | |
| Rat | mRNA (RT-PCR) | Moderate cardiac abundance | [15] | |
| Human | mRNA (RT-PCR) | Cardiac mRNA abundance | [69] | |
| Human | mRNA (RT-PCR, NB) | No cardiac mRNA abundance | [70] | |
| Human | mRNA (RT-qPCR, TaqMan) | Low cardiac mRNA abundance, A and V | [10] | |
|
K2P16.1
(TALK-1) |
Rat | mRNA (NB) | No cardiac mRNA abundance | [60] |
| Rat | mRNA (RT-PCR) | Moderate cardiac abundance | [15] | |
| Human | mRNA (NB) | No cardiac mRNA abundance | [67] | |
| Human | mRNA (RT-PCR, NB) | No cardiac mRNA abundance | [71] | |
| Human | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA abundance, A and V | [10] | |
|
K2P17.1
(TALK-2) |
Zebrafish | mRNA (RT-PCR) | No cardiac abundance | [72] |
| Rat | mRNA (NB) | Cardiac mRNA abundance | [60] | |
| Human | mRNA (NB) | Cardiac mRNA abundance | [67] | |
| Human | mRNA (RT-PCR) | Cardiac mRNA levels, A > V | [73] | |
| Human | mRNA (RT-qPCR) | mRNA abundance in human ventricular tissue mRNA abundance in iPS-derived cardiomyocytes |
[22] | |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance | [56] | |
| Human | mRNA (RT-qPCR) | Cardiac mRNA abundance mRNA abundance in sinoatrial and atrioventricular node Purkinje fibers > A > V |
[5] | |
| Human | mRNA (single cell RT-qPCR) and protein (IF) | mRNA and protein abundance in iPS-derived cardiomyocytes |
[74] | |
| Human | mRNA (RT-qPCR, TaqMan) | Cardiac mRNA levels, A > V | [10] | |
| Human | mRNA (RT-qPCR, TaqMan) and protein (WB) | Cardiac mRNA and protein expression | [40] | |
| Human | Protein (WB) | Cardiac protein expression, A | [75] | |
|
K2P18.1
(TRESK) |
Zebrafish | mRNA (ISH) | No cardiac abundance | [76] |
| Mouse | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA abundance | [77] | |
| Mouse | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA abundance | [16] | |
| Human | mRNA (RT-PCR) | No cardiac mRNA abundance | [61] | |
| Human | mRNA (RT-PCR) | No cardiac mRNA abundance | [78] | |
| Human | mRNA (RT-qPCR, TaqMan) | Very low cardiac mRNA abundance | [10] |
A, expression in atrial tissue; IF, immunofluorescence; iPS, induced pluripotent stem cell; ISH, in situ hybridization; LA, left atrium; NB, Northern blot; RT-PCR, reverse transcriptase PCR; RT-qPCR, reverse transcriptase quantitative PCR; RA, right atrium; TAC, transverse aortic constriction; TaqMan, reverse transcriptase quantitative PCR employing TaqMan® hydrolyse probes to increase specificity; V, expression in ventricular tissue; WB, Western blot.
The zebrafish possess two orthologues of the human KCNK1 gene, kcnk1a and kcnk1b which, most likely as the result of an ancient genome duplication, both encode functional TWIK-1 channels. Knockdown of kcnk1a or kcnk1b in zebrafish embryos resulted in a phenotype atrial dilatation and bradycardia, suggesting a role of K2P1.1 (TWIK-1) in regulation of sinus node function and structural heart development [11]. Further, downregulation of cardiac Kcnk1 mRNA levels was reported in a diabetic rat model, displaying again a phenotype of sinus bradycardia [19]. The presence of single nucleotide polymorphisms in the KCNK1 gene might be correlated with the prevalence of coronary artery disease [79]. Christensen et al. reported the identification of three non-synonymous KCNK1 gene variants (p.R171H, p.I98M, and p.G236S) in a cohort of 373 atrial fibrillation (AF) patients. Although these variants are localized in highly conserved domains, no effect on potassium current, reversal potential, or subcellular localization was detected in heterologous expression systems [11]. Pharmacological modulation of homodimeric K2P1.1 (TWIK-1) channels by quinine and quinidine was described (Table 3) [20]. In our own studies, AF and heart failure patients showed unchanged cardiac KCNK1 mRNA levels [10,40], while others reported upregulation of KCNK1 mRNA patients with atrial dilatation [11] or Brugada syndrome [80], downregulation of KCNK1 mRNA in AF [12] or mitral valve disease [81].
Table 3.
Pharmacological profile of K2P-channels.
| K2P Channel | Drug/Compound | Effect (Organism) | EC50 /IC50 (Organism) | Citation |
|---|---|---|---|---|
|
K2P1.1
(TWIK-1) |
Quinine | Inhibition (XO) | 50 µM (XO) | [20] |
| Quinidine | Inhibition (XO) | 95 µM (XO) | [20] | |
| Barium | Inhibition (XO) | 100 µM (XO) | [20] | |
| Charybdotoxin | < 10% inhibition at 3 nM (XO) | n.m. | [20] | |
| Dendrotoxin | < 10% inhibition at 100 nM (XO) | n.m. | [20] | |
| Apamin | < 10% inhibition at 300 nM (XO) | n.m. | [20] | |
| Clofilium | < 10% inhibition at 30 µM (XO) | n.m. | [20] | |
| Glibenclamid | < 10% inhibition at 30 µM (XO) | n.m. | [20] | |
| Cromakalim | No effect at 100 µM (XO) | n.m. | [20] | |
| Tedisamil | 30% inhibition at 100 µM (XO) | n.m. | [20] | |
| Dronedarone | No significant effect at 100 µM (XO) | n.m. | [82] | |
| Amiodarone | < 10% inhibition at 100 µM (XO) | n.m. | [20] | |
| Pinacidil | No effect at 100 µM (XO) | n.m. | [20] | |
| Vernakalant | No significant effect at 100 µM (XO) | n.m. | [83] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| 4-AP | < 10% inhibition at 1 mM (XO) | n.m. | [20] | |
| TEA | 30% inhibition at 10 mM (XO) | n.m. | [20] | |
|
K2P2.1
(TREK-1) |
GI-530159 | High affinity K2P2.1 activator (MC) | 890 nM (MC) | [86] |
| Copper | Activation (MC) | 3 µM (MC) | [87] | |
| Ostruthin | Activator (MC) | 5.3 µM (MC) | [88] | |
| BL-1249 | High affinity TREK-1/2 activator (XO) | 5.5 µM (XO) | [89] | |
| ML402 | High affinity TREK-1/2 activator (XO) | 13.7 µM (XO) | [90] | |
| ML335 | High affinity TREK-1/2 activator (XO) | 14.3 µM (XO) | [90] | |
| ML67-33 | High affinity TREK-1/2 activator (XO) | 36.3 µM (XO); 9.7 µM (MC) | [91] | |
| Pranlukast | 66.4% activation at 3 µM (MC) | n.m. | [92] | |
| DCPIB | ~3-fold activation at 10 µM (MC) | n.m. | [93] | |
| Morphine | ~2-fold activation at 10 µM (MC) | n.m. | [94] | |
| Flufenamic acid | ~4-fold activation at 100 µM (MC) | n.m. | [95] | |
| Niflumic acid | ~2.5-fold activation at 100 µM (MC) | n.m. | [95] | |
| Mefenamic acid | ~2-fold activation at 100 µM (MC) | n.m. | [95] | |
| Carbamazepine | 42% activation at 100 µM (MC) | n.m. | [96] | |
| Valproate | 28% activation at 100 µM (MC) | n.m. | [96] | |
| Gabapentin | 25% activation at 100 µM (MC) | n.m. | [96] | |
| Diethyl ether | ~1.75-fold activation at 600 µM (MC) | n.m. | [97] | |
| Chloroform | ~3.5-fold activation at 800 µM (MC) | n.m. | [97] | |
| Lithium | 31% activation at 1 mM (MC) | n.m. | [96] | |
| Rubidium | 27% activation at 1 mM (MC) | n.m. | [96] | |
| Halothane | ~1.4-fold activation at 1 mM (MC) | n.m. | [97] | |
| Isoflurane | ~1.5-fold activation at 2 mM (MC) | n.m. | [97] | |
| Cyclopropane | ~30% activation at 10% (MC) | n.m. | [98] | |
| Xenon | ~30% activation at 80% (MC) | n.m. | [98] | |
| Nitrous oxide | ~30% activation at 80% (MC) | n.m. | [98] | |
| Spadin | High affinity K2P2.1 inhibitor (MC) | 40 nM (MC) | [99] | |
| Amlodipin | Inhibition (MC) | 430 nM (MC) | [100] | |
| Nigludipine | Inhibition (MC) | 750 nM (MC) | [100] | |
| Pimozide | Inhibition (MC) | 1.8 µM (MC) | [101] | |
| Fluphenthixol | Inhibition (MC) | 2.0 µM (MC) | [101] | |
| Chlorpromazine | Inhibition (MC) | 2.7 µM (MC) | [96,101] | |
| Sipatrigine | 73.3% inhibition at 10 µM (MC) | 4 µM | [59] | |
| Fluphenazine | Inhibition (MC) | 4.7 µM (MC) | [101] | |
| Haloperidol | Inhibition (MC) | 5.5 µM (MC) | [101] | |
| Norfluoxetine | Inhibition (MC) | 9 µM (MC) | [102] | |
| Vernakalant | Inhibition (MC) | 13.3 µM (MC) | [84] | |
| Loxapine | Inhibition (MC) | 19.7 µM (MC) | [101] | |
| Fluoxetine | Inhibition (MC) | 19–37.9 µM (MC) | [96,102] | |
| Carvedilol | Inhibition (XO, MC) | 20.3 μM (XO); 1.6 μM (MC) | [42] | |
| A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 23.8 µM (XO) | [103] | |
| Dronedarone | Inhibition (XO, MC) | 26.7 μM (XO); 6.1 μM (MC) | [82] | |
| Propafenone | Inhibition (XO, MC) | 51.0 μM (XO); 7.9 μM (MC) | [104] | |
| Levobupivacaine | Inhibition (MC) | 126 µM (MC) | [105] | |
| Diltiazem | Inhibitor (MC) | 180 µM (MC) | [95] | |
| Lidocaine | Inhibition (MC) | 207 μM (MC) | [106] | |
| Bupivacaine | Inhibition (MC) | 370 µM (MC) | [107] | |
| Caffeine | Inhibition (MC) | 377 µM (MC) | [108] | |
| Ropivacaine | Inhibition (MC) | 402 µM (MC) | [105] | |
| Theophylline | Inhibition (MC) | 486 µM (MC) | [108] | |
| Zinc | Inhibition (MC) | 659 µM (MC) | [87] | |
| Mexiletine | Inhibition (XO, MC) | 1.3 mM (XO); 182 μM (MC); | [104] | |
| Tetramethyl-ammonium | 63% inhibition (MC) | n.m. | [24] | |
| Lamotrigine | ~10% inhibition at 10 µM (MC) | n.m. | [59] | |
| Metoprolol | ~20% inhibition at 100 µM (XO) | n.m. | [42] | |
| Propranolol | ~30% inhibition at 100 µM (XO) | n.m. | [42] | |
| Citalopram | 59% inhibition at 100 µM (MC) | n.m. | [96] | |
| Barium | 50% inhibition at 300 µM (XO) | n.m. | [24] | |
| Ranolazine | 7.35% inhibition at 300 µM (XO) | n.m. | [109] | |
| Clozapine | Inhibition (MC) | n.m. | [101] | |
| Sulpiride | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Tiapride | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Glibenclamide | No significant effect at 10 µM (XO) | n.m. | [24] | |
| Cesium | No significant effect at 100 µM (XO) | n.m. | [24] | |
| Gadolineum | No significant effect at 100 µM (XO) | n.m. | [24] | |
| TEA | No significant effect at 100 µM (XO) | n.m. | [24] | |
| Quinine | No significant effect at 100 µM (XO) | n.m. | [24] | |
| Quinidine | No significant effect at 100 µM (XO) | n.m. | [24] | |
| Tedisamil | No significant effect at 100 µM (XO) | n.m. | [24] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Flecainide | No significant effect at 100 µM (XO, MC) | n.m. | [84] | |
| Amiodarone | No significant effect (XO) | n.m. | [110] | |
| Sotalol | No significant effect (XO) | n.m. | [82] | |
| Digoxin | No significant effect (XO) | n.m. | [111] | |
| Digitoxin | No significant effect (XO) | n.m. | [111] | |
| A293 | No significant effect (XO) | n.m. | [10] | |
| Ajmaline | No significant effect (MC) | n.m. | [104] | |
| GsMTx4 | No significant effect (MC) | n.m. | [112] | |
| Magnesium | No significant effect (XO) | n.m. | [24] | |
|
K2P3.1
(TASK-1) |
Halothane | Activation (XO, MC) | 300–1000 µM (XO) | [97,113,114] |
| Sevoflurane | ~40% activation at 1 mM | n.m. | [114] | |
| Isoflurane | ~15% activation at 1 mM (XO) ~20% activation at 2 mM (MC) |
n.m. | [97,113] | |
| BAY2341237 | High affinity K2P3.1 inhibitor | 7.6 nM (XO) | [115] | |
| BAY1000493 | High affinity K2P3.1 inhibitor | 9.5 nM (XO) | [115] | |
| ML365 | High affinity K2P3.1 inhibitor | 16 nM (MC) | [116] | |
| A1899 (S20951) | High affinity K2P3.1 inhibitor | 35 nM (XO); 7 nM (MC) | [103,115] | |
| S9947 (KV1.5 blocker) |
Inhibition (XO) | 200 nM (XO) | [103,117] | |
|
A293
(AVE1231) |
High affinity K2P3.1 inhibitor | 222 nM (XO) | [10,15] | |
| PK-THPP | Inhibition (XO) | 243 nM | [118] | |
| MSD-D (KV1.5 blocker) |
Inhibition (XO) | 350 nM (XO) | [117] | |
| Amiodarone | Inhibition (XO) | 400 nM (XO) | [82,110] | |
| Doxapram | Inhibition (XO, MC) | 410 nM (XO) | [119] | |
| AVE0118 (KV1.5 blocker) |
Inhibition (XO) | 600 nM (XO) | [117] | |
| Methanandamide | Inhibition (XO) | 700 nM (MC) | [120] | |
| Digoxin | Inhibition (XO) | 900 nM (XO) | [111] | |
| ICAGEN-4 (KV1.5 blocker) |
Inhibition (XO) | 1.05 µM (XO) | [117] | |
| ML308 (High affinity K2P9.1 inhibitor) |
Inhibition (MC) | 3.2 µM (MC) | [121] | |
| Carvedilol | Inhibition (XO, MC) | 3.8 µM (XO); 0.83 µM (MC) | [42] | |
| Digitoxin | Inhibition (XO) | 7.4 µM (XO) | [111] | |
| Genistein | 81.1% inhibition at 100 µM (XO) | 12.3 µM (MC) | [85] | |
| Dronedarone | Inhibition (XO, MC) | 18.7 µM (XO); 5.2 µM (MC) | [82] | |
| Propafenone | Inhibition (XO, MC) | 18.1 μM (XO); 5.1 μM (MC); | [104] | |
| Etidocaine | Inhibition (XO) | 39 µM (XO) | [122] | |
| Ostruthin | Inhibition (MC) | 41 µM (MC) | [88] | |
| R-Ropivacaine | Inhibition (XO) | 51 µM (XO) | [122] | |
| S-Ropivacaine | Inhibition (XO) | 53 µM (XO) | [122] | |
| Bupivacaine | Inhibition (XO) | 68 µM (XO) | [123] | |
| Etomidate | Inhibition (XO) | 119 µM (XO) | [113] | |
| Zinc | Inhibition (XO) | 175 µM (XO) | [123] | |
| Ranolazine | Inhibition (XO, MC) | 198.4 µM (XO); 30.6 µM (MC) | [109] | |
| Lidocain | Inhibition (XO) | 222 µM (XO) | [122] | |
| Mexiletine | Inhibition (XO, MC) | 405 µM (XO); 97.3 μM (MC) | [104] | |
| Tetracaine | Inhibition (XO) | 668 µM | [122] | |
| Mepivacaine | Inhibition (XO) | 709 µM (XO) | [122] | |
| Agitoxin | < 15%inhibition at 1 nM (XO) | n.m. | [123] | |
| Margatoxin | < 15%inhibition at 10 nM (XO) | n.m. | [123] | |
| Dendrotoxin | < 15%inhibition at 100 nM (XO) | n.m. | [123] | |
| Charybdotoxin | < 15%inhibition at 200 nM (XO) | n.m. | [123] | |
| Anandamide | ~90% inhibition at 3 µM (MC) | n.m. | [120] | |
| CP55940 (CB1/CB2agonist) | ~50% inhibition at 10 µM (MC) | n.m. | [120] | |
| Sipatrigine | 37%inhibition at 10 µM (MC) | n.m. | [59] | |
| Glibenclamid | < 15%inhibition at 30 µM (XO) | n.m. | [123] | |
| Propranolol | ~60% inhibition at 100 µM (XO) | n.m. | [42] | |
| Cesium | 31% inhibition at 100 µM (XO) | n.m. | [45] | |
| Quinidine | < 20–71 % inhibition at 100 µM (XO) | n.m. | [45,123] | |
| Quinine | < 20 % inhibition at 100 µM (XO) | n.m. | [45] | |
| Quinacrine | < 20% inhibition at 100 µM (XO) | n.m. | [45] | |
| Barium | ~19% inhibition at 100 µM (XO) | n.m. | [45] | |
| Daidzein | 18.2% inhibition at 100 µM (XO) | n.m. | [85] | |
| Cromakalim | < 15%inhibition at 100 µM (XO) | n.m. | [123] | |
| Metoprolol | ~10% inhibition at 100 µM (XO) | n.m. | [42] | |
| Phenytoin | ~50% inhibition at 200 µM (XO) | n.m. | [123] | |
| Diethyl ether | ~45 % at 600 µM (MC) | n.m. | [97] | |
| Magnesium | ~14% inhibition at 10 mM (XO) | n.m. | [123] | |
| 4-AP | <15%inhibition at 10 mM (XO) | n.m. | [45,123] | |
| Flecainide | No significant effect at 100 µM (XO, MC) | n.m. | [84] | |
| Ouabain | No significant effect at 100 µM (XO) | n.m. | [111] | |
| Vernakalant | No significant effect at 100 µM (XO, MC) | n.m. | [84] | |
| Sotalol | No significant effect at 100 µM (XO) | n.m. | [82] | |
| Genistin | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Propofol | No significant effect at 200 µM (XO) | n.m. | [113] | |
| Chloroform | No significant effect at 800 µM (MC) | n.m. | [97] | |
| TEA | No significant effect at 1 mM (XO) | n.m. | [45] | |
|
K2P4.1
(TRAAK) |
Sipatrigine | 45%inhibition at 10 µM (MC) | 10 µM | [59] |
| ML67-33 (High affinity TREK-1/2 activator) |
Activation (XO, MC) | 27.3 µM (XO); 1.8 µM (MC) | [91] | |
| BL-1249 (High affinity TREK-1/2 activator) |
Activation (XO) | 48 µM (XO) | [89] | |
| A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | >20 µM (XO) | [103] | |
| Docosahexaenoate | ~12-fold activation at 10 µM (MC) | n.m. | [58] | |
| Eicosapentaenoate | ~8-fold activation at 10 µM (MC) | n.m. | [58] | |
| Arachidonic acid | ~5-fold activation at 10 µM (MC) | n.m. | [58] | |
| Oleate | ~1.5-fold activation at 10 µM (MC) | n.m. | [58] | |
| Linoleate | ~1.5-fold activation at 10 µM (MC) | n.m. | [58] | |
| Riluzole | 3.9-fold activation at 100 µM (MC) | n.m. | [58] | |
| Flufenamic acid | ~2-fold activation at 100 µM (MC) | n.m. | [95] | |
| Niflumic acid | ~2-fold activation at 100 µM (MC) | n.m. | [95] | |
| Mefenamic acid | ~1.6-fold activation at 100 µM (MC) | n.m. | [95] | |
| Lamotrigine | ~10% inhibition at 10 µM (MC) | n.m. | [59] | |
| Vernakalant | 17.1% inhibition at 100 µM (XO) | n.m. | [83] | |
| Barium | 56.7% inhibition at 1 mM (XO) | n.m. | [58] | |
| Charybdotoxin | No significant effect at 20 nM (XO) | n.m. | [58] | |
| Dendrotoxin | No significant effect at 100 nM (XO) | n.m. | [58] | |
| Tetrodotoxin | No significant effect at 1 µM (XO) | n.m. | [58] | |
| Tedisamil | No significant effect at 10 µM (XO) | n.m. | [58] | |
| Palmitate | No significant effect at 10 µM (MC) | n.m. | [58] | |
| Stearate | No significant effect at 10 µM (MC) | n.m. | [58] | |
| Arachidate | No significant effect at 10 µM (MC) | n.m. | [58] | |
| Fluphenazine | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Chlorpromazine | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Haloperidol | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Fluphenthixol | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Loxapine | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Pimozide | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Clozapine | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Sulpiride | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Tiapride | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Tolbutamide | No significant effect at 100 µM (XO) | n.m. | [58] | |
| Pinacidil | No significant effect at 100 µM (XO) | n.m. | [58] | |
| P1060 | No significant effect at 100 µM (XO) | n.m. | [58] | |
| Glibenclamide | No significant effect at 200 µM (XO) | n.m. | [58] | |
| Cobalt | No significant effect at 500 µM (XO) | n.m. | [58] | |
| Dronedarone | No significant effect at 100 µM (XO) | n.m. | [82] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Ranolazine | 3.32 % inhibition at 300 µM (XO) | n.m. | [109] | |
| Diethyl ether | No significant effect at 600 µM (MC) | n.m. | [97] | |
| Chloroform | No significant effect at 800 µM (MC) | n.m. | [97] | |
| Halothane | No significant effect at 1 mM (MC) | n.m. | [97] | |
| Diltiazem | No significant effect at 1 mM (MC) | n.m. | [95] | |
| TEA | No significant effect at 1 mM (XO) | n.m. | [58] | |
| 4-AP | No significant effect at 1 mM (XO) | n.m. | [58] | |
| Caesium | No significant effect at 1 mM (XO) | n.m. | [58] | |
| Isoflurane | No significant effect at 2 mM (MC) | n.m. | [97] | |
| Digoxin | No significant effect (XO) | n.m. | [111] | |
| Digitoxin | No significant effect (XO) | n.m. | [111] | |
|
K2P5.1
(TASK-2) |
A293 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 8.1 nM (XO) | [10,15] |
| A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 12 µM (XO) | [103] | |
| Quinine | Inhibition (XO) | 22.4 µM (XO) | [17] | |
| Quinidine | 65% inhibition at 100 µM (XO) | n.m. | [17] | |
| Zinc | 15.3% inhibition at 100 µM (XO) | n.m. | [17] | |
| Ranolazine | 30.02% inhibition at 300 µM (XO) | n.m. | [17] | |
| Barium | 16.9% inhibition at 1 mM (XO) | n.m. | [17] | |
| Lidocaine | 60.4% inhibition at 10 mM (XO) | n.m. | [17] | |
| Bupivacaine | 80.9% inhibition at 10 mM (XO) | n.m. | [17] | |
| Arachidonic acid | No significant effect at 10 µM (XO) | n.m. | [17] | |
| 4-AP | No significant effect at 100 µM (XO) | n.m. | [17] | |
| Dronedarone | No significant effect at 100 µM (XO) | n.m. | [82] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Vernakalant | No significant effect at 100 µM (XO) | n.m. | [83] | |
| Digoxin | No significant effect (XO) | n.m. | [111] | |
| Digitoxin | No significant effect (XO) | n.m. | [111] | |
| TEA | No significant effect at 1 mM (XO) | n.m. | [17] | |
| Cesium | No effect at 1 mM (XO) | n.m. | [17] | |
|
K2P6.1
(TWIK-2) |
Barium | Inhibition (MC) | ~100 µM (MC) | [124] |
| Quinidine | 73% inhibition at 100 µM (XO) | n.m. | [124] | |
| Quinine | 73% inhibition at 100 µM (XO) | n.m. | [124] | |
| Genistein | ~30% inhibition at 100 µM (XO) | n.m. | [85] | |
| Dronedarone | 10.7% inhibition at 100 µM (XO) | n.m. | [82] | |
| Chloroform | 32% inhibition at 300 µM (XO) | n.m. | [124] | |
| Halothane | 27% inhibition at 750 µM (XO) | n.m. | [124] | |
| Cesium | 92% inhibition of inward current at 10 mM (XO) | n.m. | [124] | |
| TEA | No significant effect at 5 mM (XO) | n.m. | [124] | |
| 4-AP | No significant effect at 3 mM (XO) | n.m. | [124] | |
| Glibenclamide | No significant effect at 10 µM (XO) | n.m. | [124] | |
| Vernakalant | No significant effect at 100 µM (XO) | n.m. | [83] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
|
K2P7.1
(TWIK-3) |
Non-functional channel | |||
|
K2P9.1
(TASK-3) |
DCPIB | ~3-fold activation at 10 µM (MC) | n.m. | [93] |
| Halothane | 65.6% activation at 1 mM (XO) | n.m. | [125] | |
| BAY2341237 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 2.3 nM (XO) | [115] | |
| BAY1000493 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 15.1 nM (XO) | [115] | |
| A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO, MC) | 318 nM (XO); 70 nM (MC) | [103] | |
| ML308 | High affinity K2P9.1 inhibitor | 413 nM (MC) | [121] | |
| A293 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 950 nM (XO) | [10,15] | |
| ML365 (High affinity K2P3.1 inhibitor) |
Inhibition (MC) | 990 nM (MC) | [116] | |
| Copper | Inhibition (MC) | 2.7 µM (MC) | [87] | |
| Zinc | Inhibition (MC) | 12.7 µM (MC) | [87] | |
| Mibefradil | Inhibition (MC) | 24.6 μM (MC) | [126] | |
| Doxapram | Inhibition (XO) | 37 µM (XO) | [119] | |
| L-703,606 oxalate | Inhibition (MC) | 45.5 μM (MC) | [126] | |
| Oligomycine A | Inhibition (MC) | 47.7 μM (MC) | [126] | |
| GW2974 | Inhibition (MC) | 50.1 µM (MC) | [126] | |
| Loratadine | Inhibition (MC) | 63.4 µM (MC) | [126] | |
| Dihydro-β-erythroidine hydrobromide | Inhibition (MC) | 73.8 µM (MC) | [126] | |
| (±)-Octoclothepin maleate | Inhibition (MC) | 73.8 µM (MC) | [126] | |
| Ruthenium red | Inhibitor (XO) | 114 µM | [127] | |
| Etomidate | Inhibition (XO) | 128 µM (XO) | [113] | |
| Mevastatin | Inhibition (MC) | 159 μM (MC) | [126] | |
| Ostruthin | Inhibition (MC) | 227 µM (MC) | [88] | |
| Barium | 11% inhibition at 100 µM (XO) | 290 µM (XO) | [64] | |
| Arachidonic acid | 4.81% inhibition at 10 µM (XO) | n.m. | [125] | |
| Genistein | ~60% inhibition at 100 µM (XO) | n.m. | [85] | |
| Bupivacaine | 50.2–56% inhibition at 100 µM (XO, MC) | n.m. | [70,125] | |
| Alphaxolone | 49.2% inhibition at 100 µM (XO) | n.m. | [125] | |
| Quinidine | 42.2% inhibition at 100 µM (XO) | n.m. | [125] | |
| Quinine | 36.9% inhibition at 100 µM (XO) | n.m. | [125] | |
| Dronedarone | 31.7% inhibition at 100 µM (XO) | n.m. | [82] | |
| Fluoxetine | 31%inhibition at 100 µM (MC) | n.m. | [102] | |
| Ketamine | 7.3% inhibition at 100 µM (XO) | n.m. | [125] | |
| Pentobarbital | 4.3% inhibition at 100 µM (XO) | n.m. | [125] | |
| Glibenclamide | 3.6% inhibition at 100 µM (XO) | n.m. | [125] | |
| Ranolazine | 28.28% inhibition at 300 µM (XO) | n.m. | [109] | |
| TEA | 6% inhibition at 1 mM (XO) | n.m. | [125] | |
| Xenon | No significant effect at 80% (MC) | n.m. | [98] | |
| Nitrous oxide | No significant effect at 80% (MC) | n.m. | [98] | |
| Cyclopropane | No significant effect at 10% (MC) | n.m. | [98] | |
| Propofol | No significant effect at 200 µM (XO) | n.m. | [113] | |
| Vernakalant | No significant effect at 100 µM (XO) | n.m. | [83] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] |
|
| Digoxin | No significant effect (XO) | n.m. | [111] | |
| Digitoxin | No significant effect (XO) | n.m. | [111] | |
| Cesium | 8–12% inhibition at 10 mM (XO) | n.m. | [64,125] | |
|
K2P10.1
(TREK-2) |
Ostruthin | Activator (MC) | 3.7 µM (MC) | [88] |
| ML335 | High affinity TREK-1/2 activator | 5.2 µM (XO) | [90] | |
| ML402 | High affinity TREK-1/2 activator | 5.9 µM (XO) | [90] | |
| Arachidonic acid | Activation (MC) | 7.3 µM (MC) | [65] | |
| BL-1249 | High affinity TREK-1/2 activator | 8.0 µM (XO) | [89] | |
| ML67-33 | High affinity TREK-1/2 activator | 30.2 µM (XO); 1.6 µM (MC) | [91] | |
| 11-deoxyprostaglandin F2α | ~5-fold activation at 2 µM (MC) | n.m. | [128] | |
| Pranlukast | 228 % activation at 3 µM (MC) | n.m. | [92] | |
| Ocosahexaenoicacid | ~5-fold activation at 20 µM (MC) | n.m. | [65] | |
| Linolenic acid | ~6-fold activation at 20 µM (MC) | n.m. | [65] | |
| Eicosapentaenoic acid | ~8-fold activation at 20 µM (MC) | n.m. | [65] | |
| Linoleic acid | ~8-fold activation at 20 µM (MC) | n.m. | [65] | |
| Flufenamic acid | ~4-fold activation at 100 µM (MC) | n.m. | [95] | |
| Niflumic acid | ~2.5-fold activation at 100 µM (MC) | n.m. | [95] | |
| Mefenamic acid | ~2-fold activation at 100 µM (MC) | n.m. | [95] | |
| Ruthenium red | Inhibition (XO) | 230 nM (XO) | [127] | |
| A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 8.4 µM (XO) | [103] | |
| Carvedilol | Inhibition (XO, MC) | 24 µM (XO); 7.6 (MC) | [43] | |
| Fluoxetine | 68% inhibition at 10 µM (MC) | 28.7 µM (MC) | [96] | |
| Diltiazem | Inhibition (MC) | 330 µM (MC) | [95] | |
| Fluphenthixol | ~80% inhibition at 10 µM (MC) | n.m. | [101] | |
| Pimozide | ~80% inhibition at 10 µM (MC) | n.m. | [101] | |
| Fluphenazine | ~70% inhibition at 10 µM (MC) | n.m. | [101] | |
| Clozapine | ~50% inhibition at 10 µM (MC) | n.m. | [101] | |
| Loxapine | ~50% inhibition at 10 µM (MC) | n.m. | [101] | |
| Haloperidol | ~50% inhibition at 10 µM (MC) | n.m. | [101] | |
| Paroxetin | 33% inhibition at 20 µM (MC) | n.m. | [96] | |
| Citalopram | 59% inhibition at 100 µM (MC) | n.m. | [96] | |
| Chlorpromazine | 57% inhibition at 100 µM (MC) | n.m. | [96,101] | |
| Vernakalant | 19.8% inhibition at 100 µM (XO) | n.m. | [83] | |
| Barium | 36% inhibition at 2 mM (MC) | n.m. | [65] | |
| Sulpiride | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Tiapride | No significant effect at 10 µM (MC) | n.m. | [101] | |
| Elaidic acid | No significant effect at 20 µM (MC) | n.m. | [65] | |
| Stearic acid | No significant effect at 100 µM (MC) | n.m. | [65] | |
| Palmitic acid | No significant effect at 100 µM (MC) | n.m. | [65] | |
| Gabapentin | No significant effect at 100 µM (MC) | n.m. | [96] | |
| Valproate | No significant effect at 100 µM (MC) | n.m. | [96] | |
| Carbamazepine | No significant effect at 100 µM (MC) | n.m. | [96] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Dronedarone | No significant effect at 100 µM (XO) | n.m. | [82] | |
| Quinidine | No significant effect at 100 µM (MC) | n.m. | [65] | |
| Bupivacaine | No significant effect at 100 µM (MC) | n.m. | [65] | |
| Gadolinium | No significant effect at 100 µM (MC) | n.m. | [65] | |
| Ranolazine | No significant effect at 300 µM (XO) | n.m. | [109] | |
| TEA | No significant effect at 1 mM (MC) | n.m. | [65] | |
| Lidocaine | No significant effect at 1 mM (MC) | n.m. | [65] | |
| Lithium | No significant effect at 1 mM (MC) | n.m. | [96] | |
| Rubidium | No significant effect at 1 mM (MC) | n.m. | [96] | |
| Digitoxin | No significant effect (XO) | n.m. | [111] | |
| Digoxin | No significant effect (XO) | n.m. | [111] | |
| K2P12.1 | Quinidine | Inhibition (XO) | 160 µM (XO) | [8] |
| Halothane | ~50% inhibition at 5 mM (XO) | n.m. | [8] | |
| Arachidonic acid | No significant effect at 5 µM (XO) | n.m. | [8] | |
|
K2P13.1
(THIK-1) |
Lysophos-phatidylcholine | ~20% activation at 10 µM (XO) | n.m. | [66] |
| Arachidonic acid | 69.6–85% activation at 5–20 µM (XO) | 980 nM (XO) | [66,68] | |
| Dronedarone | 14.9% activation at 100 µM (XO) | n.m. | [82] | |
| Quinidine | 10.9% activation at 100 µM (XO) | n.m. | [129] | |
| Amiodarone | 9.3% activation at 100 µM | n.m. | [129] | |
| Ranolazine | 4.98% activation at 300 µM (XO) | n.m. | [109] | |
| A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 2.2 µM (XO) | [103] | |
| Mexiletine | 74.6% inhibition at 1.5 mM (XO) | 356 µM (XO) | [68,129] | |
| Halothane | 56% inhibition at 5 mM (XO) | 2.8 mM (XO) | [66] | |
| Lidocaine | 59.2% inhibition at 100 µM (XO) | n.m. | [68] | |
| Carvedilol | No significant effect at 100 µM (XO) | n.m. | [129] | |
| Metoprolol | No significant effect at 100 µM (XO) | n.m. | [129] | |
| Vernakalant | No significant effect at 100 µM (XO) | n.m. | [83] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Verapamil | No significant effect at 100 µM (XO) | n.m. | [129] | |
| Propafenone | 26% inhibition at 100 µM (XO) | n.m. | [129] | |
| Genistein | ~20% inhibition at 100 µM (XO) | n.m. | [85] | |
| Propranolol | 37.6% inhibition at 200 µM (XO) | n.m. | [129] | |
| Chloroform | No significant effect at 1 mM (XO) | n.m. | [66] | |
| Barium | 88.7% inhibition at 2 mM (XO) | n.m. | [66,68] | |
| Digoxin | No significant effect (XO) | n.m. | [111] | |
| Digitoxin | No significant effect (XO) | n.m. | [111] | |
|
K2P15.1
(TASK-5) |
Non-functional channel | |||
|
K2P16.1
(THIK-1) |
Digitoxin | ~30% inhibition at 100 µM (XO) | n.m. | [111] |
| Ranolazine | 23.04% inhibition at 300 µM (XO) | n.m. | [109] | |
| Halothane | 26.8% inhibition at 800 µM (XO) | n.m. | [67] | |
| Chloroform | 21.5% inhibition at 800 µM (XO) | n.m. | [67] | |
| Barium | 51.4% inhibition at 1 mM (XO) | n.m. | [67] | |
| Quinine | 45.1% inhibition at 1 mM (XO) | n.m. | [67] | |
| Quinidine | 36.8% inhibition at 1 mM (XO) | n.m. | [67] | |
| TEA | 14.9% inhibition at 1 mM (XO) | n.m. | [67] | |
| Arachidonic acid | No significant effect at 20 µM (XO) | n.m. | [67] | |
| 4-AP | No significant effect at 100 µM (XO) | n.m. | [67] | |
| Vernakalant | No significant effect at 100 µM (XO) | n.m. | [83] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Dronedarone | No significant effect at 100 µM (XO) | n.m. | [82] | |
| Isoflurane | No significant effect at 800 µM (XO) | n.m. | [67] | |
| Cesium | No significant effect at 1 mM (XO) | n.m. | [67] | |
| Digoxin | No significant effect (XO) | n.m. | [111] | |
|
K2P17.1
(THIK-2) |
A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 8.1 µM (XO) | [103] |
| A293 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 18.1 µM (XO) | [10,15] | |
| Propafenone | 296.1% activation at 100 µM (XO, MC) | 75.4 µM (XO) | [75] | |
| Quinidine | 57.7% activation at 100 µM (XO) | n.m. | [75] | |
| Mexiletine | 20.6% activation at 100 µM (XO) | n.m. | [75] | |
| Verapamil | 20.5% inhibition at 100 µM (XO) | n.m. | [75] | |
| Amiodarone | 12.5% inhibition at 100 µM (XO) | n.m. | [75] | |
| Sotalol | 9.8% inhibition at 100 µM (XO) | n.m. | [75] | |
| Ranolazine | 8.3–34.88% inhibition at 100–300 µM (XO) | n.m. | [75,109] | |
| Barium | 81.2–82.8% inhibition at 2 mM (XO) | n.m. | [67,72,73] | |
| Cesium | No significant effect at 1–2 mM (XO) | n.m. | [67,73] | |
| Arachidonic acid | No significant effect at 100 µM (XO) | n.m. | [67,73] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Carvedilol | No significant effect at 100 µM (XO) | n.m. | [75] | |
| Amitriptyline | No significant effect at 100 µM (XO) | n.m. | [75] | |
| Ajmaline | No significant effect at 100 µM (XO) | n.m. | [75] | |
| Vernakalant | No significant effect at 100 µM (XO) | n.m. | [83] | |
| Dronedarone | No significant effect at 100 µM (XO) | n.m. | [82] | |
| Digoxin | No significant effect (XO) | n.m. | [111] | |
| Digitoxin | No significant effect (XO) | n.m. | [111] | |
| Metoprolol | 17.3% activation at 100 µM (XO) | n.m. | [75] | |
| Propranolol | 139.2% activation at 100 µM (XO) | n.m. | [75] | |
| Bupivacaine | 25.7% inhibition at 1 mM (XO) | n.m. | [73] | |
| TEA | 19.9% inhibition at 1 mM (XO) | n.m. | [67] | |
| Quinine | 17.8% inhibition at 1 mM (XO) | n.m. | [73] | |
| Lidocaine | 13.1% inhibition at 1 mM (XO) | n.m. | [73] | |
| 4-AP | No significant effect at 0.1–2 mM (XO) | n.m. | [67,73] | |
| Chloroform | 44.7% inhibition at 800 µM (XO) | n.m. | [67] | |
| Halothane | 56.4% inhibition at 800 µM (XO) | n.m. | [67] | |
| Isoflurane | 58.4% activation at 800 µM (XO) | n.m. | [67] | |
|
K2P18.1
(TRESK) |
Vernakalant | Activation (XO, MC) | 40 µM (MC) | [83] |
| Isoflurane | Activation (XO) | 162 µM (XO) | [61] | |
| Sevoflurane | Activation (XO) | 224 µM (XO) | [61] | |
| Halothane | Activation (XO) | 300 µM (XO) | [61] | |
| Desflurane | Activation (XO) | 658 µM (XO) | [61] | |
| Dronedarone | 29% activation at 100 µM (XO) | n.m. | [82] | |
| Loratadine | Inhibition (MC) | 490 nM (MC) | [126] | |
| A1899 (High affinity K2P3.1 inhibitor) |
Inhibition (XO) | 900 nM (XO) | [103] | |
| Cloxiquine | Inhibition (MC) | 3.2 µM (MC) | [130] | |
| Zinc | Inhibition (XO) | 5–10 µM for the murine but not the human ortholog | [131] | |
| Arachidonic acid | 43% inhibition at 20 µM (MC) | 6.6 µM (MC) | [73,78] | |
| Lamotrigine | Inhibition (MC) | 47 µM (MC) | [132] | |
| Bupivacaine | ~75% inhibition at 100 µM (MC) | 80.4 µM (XO) | [61,133] | |
| Tetracaine | Inhibition (XO) | 496 µM (XO) | [61] | |
| Ropivacaine | Inhibition (XO) | 610 µM (XO) | [61] | |
| Chlorprocaine | Inhibition (XO) | 832 µM (XO) | [61] | |
| Mepivacaine | Inhibition (XO) | 1300 µM (XO) | [61] | |
| Lidocaine | ~70–75% inhibition at 1 mM (MC) | 3.4 mM (XO) | [61,73,78] | |
| Mibefradil | Inhibition at 3 µM (XO) | n.m. | [131] | |
| Quinidine | 49% inhibition at 10 µM (MC) | n.m. | [133] | |
| Linoleic acid | ~35% inhibition at 20 µM (MC) | n.m. | [78] | |
| Oleatic acid | ~50% inhibition at 20 µM (MC) | n.m. | [78] | |
| Docosahexaenoic acid | ~60% inhibition at 20 µM (MC) | n.m. | [78] | |
| Propafenone | 95% inhibition at 50 µM (MC) | n.m. | [78] | |
| Glyburide | 76% inhibition at 50 µM (MC) | n.m. | [78] | |
| Quinidine | 90% inhibition at 100 µM (MC) | n.m. | [78] | |
| Quinine | 41.9–75% inhibition at 100 µM (MC) | n.m. | [61,78] | |
| Etomidate | 30.5% inhibition at 100 µM (XO) | n.m. | [61] | |
| Pentobarbital | 10.4% inhibition at 100 µM (XO) | n.m. | [61] | |
| Ketamine | 14.5% inhibition at 100 µM (XO) | n.m. | [61] | |
| Alphaxalone | 45.4% inhibition at 100 µM (XO) | n.m. | [61] | |
| Gabapentin | 4.2% inhibition at 100 µM (XO) | n.m. | [61] | |
| Barium | 38% inhibition at 3 mM (MC) | n.m. | [78,133] | |
| Ethanol | ~15% inhibition at 150 mM (MC) | n.m. | [61,133] | |
| Apamin | No significant effect at 100 nM (XO) | n.m. | [133] | |
| Ruthenium red | No significant effect at 5 µM (MC) | n.m. | [133] | |
| Glibenclamide | No significant effect at 10 µM (MC) | n.m. | [133] | |
| Stearic acid | No significant effect at 20 µM (MC) | n.m. | [78] | |
| Digoxin | No significant effect at 100 µM (XO) | n.m. | [111] | |
| Digitoxin | No significant effect at 100 µM (XO) | n.m. | [111] | |
| Flecainide | No significant effect at 100 µM (XO) | n.m. | [84] | |
| Genistein | No significant effect at 100 µM (XO) | n.m. | [85] | |
| Tolazamide | No significant effect at 100 µM (MC) | n.m. | [78] | |
| Glipizide | No significant effect at 100 µM (MC) | n.m. | [78] | |
| Paxilline | No significant effect at 100 µM (MC) | n.m. | [78] | |
| Penitrem A | No significant effect at 100 µM (MC) | n.m. | [78] | |
| Ranolazine | No significant effect at 300 µM (XO) | n.m. | [109] | |
| Cesium | No significant effect at 1 mM (MC) | n.m. | [133] | |
| 4-AP | No significant effect at 1 mM (XO) | n.m. | [73,78] | |
| TEA | No significant effect at 1 mM (XO) 30% inhibition at 2 mM (MC) |
n.m. | [61,73,78] | |
| Mercury | Inhibition (XO) | n.m. | [131] | |
| Tetrapentyl-ammonium | Inhibition (MC) | n.m. | [130] |
Potency of different drugs or compounds to activate or inhibit heterologously expressed K2P currents. Compounds that are used as experimental high-affinity inhibitors of individual K2P channels are highlighted in bold. Please note, however, that these compounds are by no means completely specific for single members of the K2P family. IC50, mean inhibitory concentration; MC, mammalian cells; n.m., not measured; XO, Xenopus laevis oocytes.
The physiological role of K2P1.1 (TWIK-1) channel subunits has not been conclusively clarified, mostly due to lack of specific inhibitors and its very low currents in heterologous expression systems [82]. If measurable, heterologously expressed K2P1.1 (TWIK-1) channel homodimers give rise to potassium currents that are sensitive to acidic pH as well as external K+ concentration [134]. Therefore, it was speculated whether K2P1.1 (TWIK-1) might contribute to cardiac IK1, IKAch, IKATP, and ITASK currents [11,12,13,14,80,135,136]. Altered ion conductivity under low extracellular potassium concentrations (for example Na+ permeability, which shifts homodimeric K2P1.1 (TWIK-1) channels from an inhibitory to an excitatory channel) could link K2P1.1 (TWIK-1) channels to the pathophysiology of hypokalemia-induced rhythm disturbances [137]. Through its ability to heterodimerize with other K2P subunits, K2P1.1 (TWIK-1) subunits could modulate the pharmacological and functional properties of atrial K2P3.1 (TASK-1) channel subunits [138,139,140].
4. K2P2.1 (TREK-1)
Mechanosensitivity is a unique feature of the TREK/TRAAK subfamily, as these K2P channels are activated by membrane stretch and osmotic swelling [141]. Temperature, lipids, extracellular or intracellular pH, anesthetics or other drugs, phosphorylation, glycosylation, G protein-coupled receptors and other protein partners represent further regulators of homodimeric K2P2.1 (TREK-1) channels [97,124,141,142,143,144]. The versatility of this channel is further enhanced by alternative translation initiation (ATI) variants that differ in spatiotemporal expression, single-channel conduction, ion selectivity and regarding their pharmacological profile [43,145,146]. Further, K2P2.1 (TREK-1) channel subunits are reported to from heterodimers with K2P1.1 (TWIK-1), K2P4.1 (TRAAK) and K2P10.1 (TREK-2) [147,148].
In the rat heart, Kcnk2 mRNA and protein expression has been described in both atrial and ventricular tissue samples (Table 2) [28,29,32,33,149]. However, in the mouse heart, most studies describe ventricular-dominant K2P2.1 (TREK-1) expression or mRNA abundance patterns [16,26,41]. Abundant K2P2.1 (TREK-1) expression was also detected in the porcine heart, with the highest expression levels in the sinoatrial and atrioventricular nodal tissue [36,37] and in human cardiac tissue samples, where again ventricular dominant K2P2.1 (TREK-1) expression could be observed [10,37,40,41]. Interestingly, a transmural gradient of ventricular K2P2.1 (TREK-1) expression levels was described with endocardial expression levels 17-fold higher than that in the epicardium, [30,149]. Strikingly, this gradient seems to parallel transmural changes in stretch-activated potassium currents, as mechanical stretch has been shown to cause increased action potential shortening in subendocardial cardiomyocytes compared to the subepicardium [150]. In a similar fashion chloroform-activated K2P2.1 (TREK-1)-like currents are significantly larger in endocardial than epicardial cells [30].
Homodimeric K2P2.1 (TREK-1) channels are inhibited by the anticonvulsant drugs valproate, gabapentin and carbamazepine [102] by the antidepressants like fluoxetine, paroxetine, citalopram or escitalopram (Table 3) [96,102], and the antipsychotics haloperidol or clozapine [101]. While some of these interactions would only be relevant at supratherapeutic plasma levels, others already have an impact in the physiological range [141]. It has therefore been speculated whether the blockade of cardiac K2P2.1 (TREK-1) channels could contribute to the proarrhythmic potential of these compounds [41,141]. Remarkably, K2P2.1 (TREK-1) knockout was shown to cause a phenotype of QT interval prolongation, linking loss of cardiac K2P2.1 (TREK-1) to QT prolongation [151]. Likewise, antiarrhythmic drugs were described to block K2P2.1 (TREK-1) channels: Vaughan Williams class I compounds lidocaine, mexiletine and propafenone, class III antiarrhythmic drugs dronedarone and vernakalant, the beta-blocker carvedilol and late sodium current inhibitor ranolazine were identified as in vitro K2P2.1 (TREK-1) inhibitors (Table 3) [43,82,84,104,106,109]. Since IC50 levels are mostly in the supratherapeutic range, it is unclear to what extent inhibition of K2P2.1 (TREK-1) contributes to the antiarrhythmic effects of these compounds.
In isolated rat ventricular cardiomyocytes the mechano-, pH-, and arachidonic acid-sensitive potassium current IKAA displays a number of further features like activation by volatile anesthetics, inhibition by cAMP analogues as well as beta-adrenergic receptor agonists, the absence of a relevant voltage dependency, a specific single-channel conductance and burst mode activity, which identify it as a K2P2.1 (TREK-1) current (Table 4) [7,28,29,32,33,149,152]. Further, resting membrane potentials of chicken embryo-derived atrial cardiomyocytes are regulated by K2P2.1 (TREK-1) [153]. Finally, cardiomyocyte-specific K2P2.1 (TREK-1) knockout mice exhibit a phenotype of stress-induced sick sinus syndrome and prolongation of QT intervals that could be reproduced in a transgenic model which employed C-terminal truncation of beta IV spectrin to disrupt its interaction with K2P2.1 (TREK-1), thereby impairing intracellular K2P2.1 (TREK-1) protein trafficking [27,151]. In a similar fashion, knockout of K2P2.1 (TREK-1) channel surface targeting by its protein partners POPDC1 or POPDC2 revealed a phenotype of exercise-induced and age-dependent sick sinus syndrome [154,155], while a double-knockout mouse displayed AV conduction disturbance [156]. Moreover, a familial autosomal recessive POPDC1 mutation has been associated with the phenotype of limb-girdle muscular dystrophy type X2 in combination with AV block [157] and POPDC2 mutations have been shown to cause AV block without a skeletal muscle phenotype [158].The fact that K2P2.1 (TREK-1) channels are activated in acidosis and by mechanical stress has given rise to speculation about a role of this channel in the development of cardiac arrhythmias for more than two decades [28]. Metabolic changes associated with myocardial ischemia lead to a decrease in pH. By activating K2P2.1 (TREK-1), this can cause a dispersion of repolarization and consecutively the development of arrhythmias. Similarly, altered wall tension due to hypertension, valvular vitiation, in the margins of myocardial scars, or AF may activate K2P2.1 (TREK-1) [141,158,159]. Recently, a heterozygous missense mutation (I267T) of K2P2.1 (TREK-1) was identified in a patient with idiopathic right ventricular outflow tract tachycardia [160]. This mutation results in an amino acid exchange from isoleucine to threonine in close proximity to the selectivity filter of the channel, leading to increased stretch sensitivity and sodium permeability.
Table 4.
Functional evidence for K2P channel expression in the cardiovascular system.
| K2P Channel Subunit | Species | Population/Model/Methodology | Observation | Citation |
|---|---|---|---|---|
|
K2P1.1
(TWIK-1) |
Zebrafish | Morpholino knockdown mRNA (RT-PCR, ISH) |
Knockdown of kcnk1a or kcnk1b in zebrafish embryos resulted in a phenotype atrial dilatation and bradycardia | [11] |
| Mouse | CREM-transgenic murine AF model mRNA (RT-qPCR, TaqMan) |
Moderate cardiac mRNA expression, V > A Ventricular mRNA downregulated in murine AF model |
[16] | |
| Rat | Goto-Kakizaki type 2 diabetic ratsmRNA (RT-qPCR, TaqMan) | Downregulation of sinoatrial mRNA levels in Goto-Kakizaki type 2 diabetic rats | [19] | |
| Human | Patient-derived tissue samples mRNA (RT-PCR) |
Identical mRNA levels in failing and healthy hearts | [21] | |
| Human | Patient-derived tissue samples | Upregulation of atrial mRNA levels in patients with atrial dilatation | [11] | |
| Human | Patient-derived tissue samples | Upregulation of atrial mRNA levels in patients with Brugada syndrome | [80] | |
| Human | Patient-derived tissue samples | Downregulation of atrial mRNA levels in AF | [12] | |
| Human | AF patients | Identification of three non-synonymous KCNK1 gene variants (p.R171H, p.I98M, and p.G236S) in a cohort of 373 atrial fibrillation (AF) patients | [11] | |
| Human | mRNA (RT-qPCR, TaqMan) | No regulation of atrial mRNA levels in AF | [10] | |
|
K2P2.1
(TREK-2) |
Mouse | CREM-transgenic murine AF model Murine TAC model mRNA (RT-qPCR, TaqMan) |
Upregulated of atrial and ventricular mRNA in a murine AF model Downregulation of atrial and ventricular mRNA in a murine TAC model |
[16] |
| Rat | Rat model of isoproterenol-induced left ventricular hypertrophy | Increased protein levels upon isoproterenol stimulation | [149] | |
| Mouse | Protein (IF) | Global K2P2.1 (TREK-1) knockout mice showed an exaggerated form of pressure overload-induced concentric ventricular hypertrophy, which could be prohibited only by fibroblast-specific deletion of K2P2.1, (TREK-1) whereas the cardiomyocyte-specific knockout of K2P2.1 (TREK-1) resulted in cardiac dysfunction under pressure-overload conditions | [161] | |
| Human | Patient-derives tissue samples mRNA (RT-qPCR, TaqMan) |
Downregulation of atrial mRNA in AF | [37] | |
| Pig | Large animal model of burst pacing-induced AF and heart failure | Downregulation of atrial mRNA and protein Attenuation of the AF phenotype by KCNK2 gene therapy |
[36,37] | |
| Rat | Goto-Kakizaki type 2 diabetic ratsmRNA (RT-qPCR, TaqMan) | Upregulation of sinuatrial mRNA levels in Goto-Kakizaki type 2 diabetic rats | [19] | |
| Human | Index patient | A heterozygous missense mutation (I267T) of K2P2.1 (TREK-1) was identified in a patient with idiopathic right ventricular outflow tract tachycardia | [160] | |
| Chicken | Isolated atrial cardiomyocytes | Resting membrane potentials of chicken embryo-derived atrial cardiomyocytes are regulated by K2P2.1 | [153] | |
| Rat | Isolated rat ventricular cardiomyocytes | In isolated rat ventricular cardiomyocytes the mechano-, pH-, and arachidonic acid-sensitive potassium current IKAA displays a number of characteristics which identify it as a K2P2.1 (TREK-1) current | [7,28,29,32,33,149,152] | |
| Mouse | Kcnk2 knockout mouse | Phenotype of QT interval prolongation and sick sinus syndrome | [35] | |
|
K2P3.1
(TASK-1) |
Rat | Isolated rat ventricular cardiomyocytes | K2P3.1 (TASK-1) currents were isolated from rat ventricular cardiomyocytes by lowering pH, activation of cardiac α1-adrenergic receptors and by administration of the inhibitor A293 | [15,162,163] |
| Mouse | Isolated cardiomyocytes | Patch-clamp measurements of K2P3.1 (TASK-1) currents (controlled by knockout mice) | [45] | |
| Pig | Isolated atrial cardiomyocytes | Patch-clamp measurements of K2P3.1 (TASK-1) currents using A293: APD prolongation via K2P3.1 (TASK-1) inhibition | [52,53,54,164] | |
| Human | Isolated atrial cardiomyocytes | Patch-clamp measurements of K2P3.1 (TASK-1) currents using A293: APD prolongation via K2P3.1 (TASK-1) inhibition ITASK-1 was identified to carry up to 28% of the background potassium current in isolated human atrial cardiomyocytes |
[10,39,40,53,56]. | |
| Human | iPSC | Prolongation of APD values by transfection of K2P3.1 (TASK-1) siRNA | [22] | |
| Zebrafish | Morpholino knockdown |
Decreased heart rate was observed after K2P3.1 (TASK-1) knockdown | [165]. | |
| Mouse | CREM-transgenic murine AF model Murine TAC model mRNA (RT-qPCR, TaqMan) and protein (WB) |
Downregulation of atrial mRNA and protein level in murine AF model Downregulation of atrial mRNA and protein level in murine TAC model |
[16] | |
| Guinea pig | Excised guinea pig hearts | Prolongation of atrial effective refractory periods upon TASK-1 inhibition at pH 7.8 | [49] | |
| Mouse | Kcnk3 knockout mouse | Phenotype of QTc prolongation (around 30%), prolongation of single cell APDs or monophasic action potentials and a broad QRS complex | [47] | |
| Rat | Kcnk3 knockout rat | Phenotype of cardiomyocyte APD prolongation as well as resting membrane depolarization | [15] | |
| Dog | Dog model of postoperative AF | Downregulation of atrial TASK-1 expression in postoperative AF | [50] | |
| Pig | Large animal model of burst pacing-induced AF | Upregulation of atrial TASK-1 expression and currents Acute cardioversion upon TASK-1 inhibition Rhythm control of AF upon TASK-1 gene therapy of pharmacological TASK-1 inhibition |
[52,141,164] | |
| Human | mRNA (RT-qPCR, TaqMan), protein (WB) | Upregulation of atrial TASK-1 expression and currents in cAF | [10,41,55,57] | |
| Human | AF patient cohort | Three genetic KCNK3 variants which reduce the expression or channel function were found in patients with familial AF | [49] | |
| Mouse | Kcnk3 knockout mouse | Compared to wild-type littermates, Kcnk3 knockout mice showed a preservation of systolic as well as diastolic function and a relative abrogation in concentric left ventricular hypertrophy upon TAC-induced pressure overload | [46] | |
| Human | Patient cohorts | KCNK3 loss-of-function mutations were found to cause idiopathic pulmonary arterial hypertension | [166] | |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) and protein (WB) | Upregulation of atrial mRNA and protein in AF Downregulation of atrial mRNA in heart failure |
[40] | |
|
K2P4.1
(TRAAK) |
Mouse | Kcnk4 knockout mice | No obvious cardiac phenotype reported | [167,168] |
| Human | Patient-derived tissue samples mRNA (RT-qPCR) | Downregulation of ventricular mRNA levels in non-ischemic heart failure | [22] | |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | No regulation of atrial mRNA levels in AF patients | [10] | |
|
K2P5.1
(TASK-2) |
Mouse | Kcnk5 knockout mice | Observation of subviable phenotype and sudden unexplained dead but association with arrhythmia or cardiomyopathy remains speculative as no detailed cardiac characterization was reported | [169] |
| Mouse | CREM-transgenic murine AF model mRNA (RT-qPCR, TaqMan) |
No regulation of atrial mRNA in murine AF model | [16] | |
| Rat | Goto-Kakizaki type 2 diabetic rats mRNA (RT-qPCR, TaqMan) | Downregulation of sinoatrial mRNA levels in Goto-Kakizaki type 2 diabetic rats | [19] | |
| Human | mRNA (RT-qPCR, TaqMan) | Trend towards downregulation of atrial mRNA levels in AF | [10] | |
|
K2P6.1
(TWIK-2) |
Physiological role under debate because of low currents upon recombinant expression | |||
| Mouse | CREM-transgenic murine AF model Murine TAC model mRNA (RT-qPCR, TaqMan) |
No regulation in murine AF model Upregulation of atrial mRNA in murine TAC model |
[16] | |
| Rat | Goto-Kakizaki type 2 diabetic rats mRNA (RT-qPCR, TaqMan) | Downregulation of sinoatrial mRNA levels in Goto-Kakizaki type 2 diabetic rats | [19] | |
| Mouse | Kcnk6 knockout mouse | Kcnk6 knockout mice are hypertensive and display elevated RV pressure level as well as enhanced vascular contractility | [170,171,172] | |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | No regulation of atrial mRNA in AF patients | [10] | |
|
K2P7.1
(TWIK-3) |
Human, Mouse | mRNA (RT-qPCR, TaqMan) | Most studies show very low cardiac mRNA levels. Functionality of the channel still under debate. | [16] al. 2015) Wang et al. 2018) |
| Human | Patient-derived tissue samples mRNA (RT-qPCR) | Upregulation of atrial mRNA levels in AF | [63] | |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | No mRNA regulation in AF | [10] | |
| Mouse | Kcnk7 knockout mouse | No cardiac phenotype of the Kcnk7 knockout mouse has been described | [173] | |
|
K2P9.1
(TASK-3) |
Human | Genetic disease | KCNK9 imprinting syndrome linked to obstructive sleep apnea | |
| Human | Patient-derived tissue samples mRNA (RT-qPCR) | Downregulation of ventricular mRNA levels in heart failure | [22] | |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | Trend towards upregulation in AF | [10] | |
| Mouse | Kcnk9 knockout mouse | Phenotype of concentric left ventricular hypertrophy with preserved ejection fraction | [46] | |
| Human | Single channel patch-clamp measurements on isolated human atrial cardiomyocytes | Evidence for heteromeric K2P9.1/ K2P3.1 but not for K2P9.1 homodimers | [56] | |
|
K2P10.1
(TREK-2) |
Human, mouse | Patient-derived tissue samples, CREM-transgenic murine AF model, Murine TAC model, mRNA (RT-qPCR, TaqMan) | No regulation of atrial mRNA levels in AF patientsNo regulation of atrial or ventricular mRNA levels in a murine AF modelNo changes in ventricular mRNA levels in a murine TAC modelUpregulation of left and right atrial mRNA in heart failure patients | [41] |
| Mouse | Kcnk10 knockout mouse | No cardiac phenotype of the Kcnk10 knockout mouse has been described | [174] | |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | No regulation of atrial mRNA levels in AF patients | [10] | |
|
K2P12.1
(THIK-2) |
Human, Rat, Mouse | mRNA (NB, RT-PCR, RT-qPCR, TaqMan) | Most studies show very low cardiac mRNA levels. Functionality of the channel still under debate. | [10,15,16,66,67] |
|
K2P13.1
(THIK-1) |
Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | Downregulation of atrial mRNA level in cAF patients | [10] |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | Trend towards downregulation of atrial mRNA level in heart failure patients | [40] | |
| Pig | Large animal model of burst-pacing induced AF and heart failure | Downregulation of atrial protein expression in combined AF and heart failure | [129] | |
|
K2P15.1
(TASK-5) |
Human, Rat, Mouse | mRNA (RT-PCR, RT-qPCR) | Most studies show rather low cardiac mRNA levels. Functionality of the channel still under debate. | [10,15,69,70] Wiedmann et al. 2018) |
| Human | Patient-derived tissue samples mRNA (RT-qPCR, TaqMan) | No regulation of atrial mRNA levels in cAF patients | [10] | |
| Mouse | CREM-transgenic murine AF model mRNA (RT-qPCR, TaqMan) | Downregulation of atrial mRNA levels in murine AF model | [16] | |
|
K2P16.1
(TALK-1) |
Human, Rat |
mRNA (NB, RT-PCR, RT-qPCR, TaqMan) | Most studies show negligible or low cardiac mRNA levels | [10,15,60,67,71] |
|
K2P17.1
(TALK-2) |
Human | Patient-derived tissue samples, iPSC mRNA (RT-qPCR) |
Downregulation of ventricular mRNA levels in non-ischemic heart failure iPSC: KCNK17 knockdown led to APD prolongation |
[22] |
| Human, Mouse | Index patient HL-1 cells (cultured cardiomyocyte cell line), mRNA (RT-qPCR) |
A patient suffering from progressive and severe cardiac conduction disorder in combination with idiopathic ventricular fibrillation was identified to carry both, a splice site mutation in the sodium channel gene SCN5A as well as a gain-of-function mutation in the KCNK17 gene HL-1 cells: KCNK17 knockdown overexpression led to APD shortening |
[5] | |
| Human | Index family Patient derived iPSC |
A common KCNK17 gain-of-function variant might be protective for LQTS by promoting APD shortening | [74] | |
| Human | Patient-derived tissue samples, mRNA (RT-qPCR, TaqMan) | Downregulation of right atrial mRNA levels in cAF | [10] | |
| Human | Patient-derived tissue samples, mRNA (RT-qPCR, TaqMan) and protein (WB) | Downregulation of left and right atrial protein and mRNA level in HF | [40] | |
|
K2P18.1
(TRESK) |
Zebrafish, Mouse, Human | mRNA (ISH, RT-PCR, RT-qPCR, TaqMan) | Most studies show negligible cardiac mRNA levels | [10,16,61,76,77,78] |
Evidence in literature for cardiac relevance of K2P channel subunits. A, expression in atrial tissue; AF, atrial fibrillation; HF, heart failure; IF, immunofluorescence; iPS, induced pluripotent stem cell; ISH, in situ hybridization; LA, left atrium; NB, Northern blot; RT-PCR, reverse transcriptase PCR; RT-qPCR, reverse transcriptase quantitative PCR; RA, right atrium; TAC, transverse aortic constriction; TaqMan, reverse transcriptase quantitative PCR employing TaqMan® hydrolyse probes to increase specificity; V, expression in ventricular tissue; WB, Western blot.
In a murine model of transverse aortic constriction (TAC)-induced pressure overload upregulation of ventricular Kcnk2 mRNA expression was described [16]. In a similar fashion, K2P2.1 (TREK-1) protein levels were increased in a rat model of isoproterenol-induced left ventricular hypertrophy [149]. Global K2P2.1 (TREK-1) knockout mice showed an exaggerated form of pressure overload-induced concentric ventricular hypertrophy, which could be prohibited only by fibroblast-specific deletion of K2P2.1, (TREK-1) whereas the cardiomyocyte-specific knockout of K2P2.1 (TREK-1) resulted in cardiac dysfunction under pressure-overload conditions [161]. In a murine atrial fibrillation (AF) model of CREM-IbΔC-X transgenic mice, downregulation of atrial K2P2.1 (TREK-1) mRNA and protein levels were observed [16,41]. It, however, remains uncertain whether this is also the case for AF patients: while one study described AF-associated downregulation of atrial K2P2.1 (TREK-1) [37] others merely describe a trend that does not reach statistical significance [10,40,41]. One possible explanation is the remote regulation of atrial K2P2.1 (TREK-1) expression by ventricular heart failure, a mechanism recently described for K2P3.1 (TASK-1) [40] and also observed for K2P2.1 (TREK-1) in another study [41]. Indeed, in contrast to the other study, the cohort of patients characterized in the former study was performed in patients who all suffered from severe heart failure. In a similar fashion, a strong trend towards downregulation of atrial Kcnk2 mRNA could be observed in a murine model of TAC-induced pressure overload [16]. Furthermore, downregulation of atrial K2P2.1 (TREK-1) protein expression was described in a porcine model of combined AF and heart failure [36] and gene therapeutic restoraton of K2P2.1 (TREK-1) expression was able to attenuate the AF phenotype [37].
For a more detailed description of the cardiac role of K2P2.1 (TREK-1), we would like to refer to the following literature [41,141,158].
5. K2P3.1 (TASK-1)
Among the entire K2P family, K2P3.1 (TASK-1) is the channel with the best characterized cardiac significance. K2P3.1 (TASK-1) channels are expressed in neuronal tissue, cardiomyocytes, vascular smooth muscle cells, the carotid body glomus, the adrenal gland, brown adipose tissue and immunocytes, where they control important physiological processes [2,115]. K2P3.1 (TASK-1) channels are regulated by a number of different stimuli, such as pH level, hypoxia, PKA, PKC, or PLC activity, and several drugs like volatile anesthetics [2].
In the murine and the rat heart, KCNK3 mRNA was detected, both in atrial as well as in ventricular tissue samples (Northern blot, RT-PCR, Taq-Man qPCR; Table 2) [15,16,18,25,26,34,44,45,47]. Humans, however, show an almost atrial-specific K2P3.1 (TASK-1) expression within the heart with 14- to 16-fold lower expression levels in ventricular tissue (RT-PCR, Taq-Man qPCR, microarray, bulk RNAseq, Western blot) [10,12,14,39,40,49,54,56,57]. In guinea pigs and domestic swine, atrial-specific K2P3.1 (TASK-1) expression was also described [49,51,52,53,54].
Several clinically relevant antiarrhythmic drugs have been identified to inhibit homodimeric K2P3.1 (TASK-1) channels at either physiological or subtherapeutic concentrations (Table 3). Among them are the class I antiarrhythmic drugs propafenone, mexiletine, lidocaine, and quinidine [104,122,123], the betablockers propranolol and carvedilol [42], class III antiarrhythmics amiodarone and dronedarone [82,110] as well as cardiac glycosides [111] and ranolazine [109]. The respiratory stimulant doxapram was further identified as a potent blocker of both K2P3.1 (TASK-1) and K2P9.1 (TASK-3) channels through which it presumably exerts the main part of its respiratory drive-increasing effect [119,175]. Furthermore, preclinical experimental antiarrhythmic drugs developed as specific inhibitors of the KV1.5 channel (A239 [AVE1231], A1899 [S20591], AVE0118, S9947, MSD-D, and ICAGEN-4) are potent K2P3.1 (TASK-1) inhibitors [117]. Although no direct structural similarities of the pore regions of both channels could be detected, these compounds were shown to be 1.4- to 70-fold more potent K2P3.1 (TASK-1) inhibitors as compared to KV1.5 [117]. In addition, bisamides represent a new class of high-affinity K2P3.1 (TASK-1) inhibitors with IC50 values in the single-digit nanomolar range, as in the case of compound ML365 (Table 3) [116].
Availability of high-affinity inhibitors enables functional detection of K2P3.1 (TASK-1) currents in isolated cardiomyocytes. K2P3.1 (TASK-1) currents were isolated from rat ventricular cardiomyocytes by lowering pH, activation of cardiac α1-adrenergic receptors and by administration of the inhibitor A293 (Table 4) [15,162,163]. Patch-clamp measurements of murine K2P3.1 (TASK-1) current could be confirmed by the use of Kcnk3 knockout mice [25] and likewise, functional detection of K2P3.1 (TASK-1) currents was achieved by patch-clamp technique in isolated porcine [52,53,54,164] and human atrial cardiomyocytes, where a significant APD prolongation could be demonstrated [10,39,40,53,56]. Under physiological conditions, ITASK-1 was identified to carry up to 28% of the background potassium current in isolated human atrial cardiomyocytes [39].
In induced pluripotent stem cell- (iPS-) derived cardiomyocytes (iPSC), APD values could be prolonged by transfection of K2P3.1 (TASK-1) siRNA [22]. In a zebrafish model, a decreased heart rate was observed after K2P3.1 (TASK-1) knockdown, which was accompanied by an increased atrial diameter [165]. In excised guinea pig hearts, APD remained unchanged upon K2P3.1 (TASK-1) inhibition with A293 or ML365. Switching the pH level from pH 7.4 to 7.8, however, resulted in significant prolongation of atrial effective refractory periods [49]. Global Kcnk3 knockout mice exhibited a phenotype of QTc prolongation (around 30%), prolongation of single cell APDs or monophasic action potentials and a broad QRS complex [25,26]. In transgenic Kcnk3 knockout rats, APD prolongation as well as resting membrane depolarization was described [163].
In a porcine large animal model of AF, atrial K2P3.1 (TASK-1) expression was found to be significantly upregulated (TaqMan qPCR, western blot, patch-clamp electrophysiology) [52,141,164]. These results could also be reproduced on atrial tissue samples from atrial fibrillation patients (TaqMan qPCR, microarray, bulk RNAseq, western blot, patch-clamp electrophysiology) [10,41,55,57]. Considering its atrial-specific expression, its effect on atrial APD, and its upregulation in patients with AF, K2P3.1 (TASK-1) channels combine several properties that make it an ideal molecular target for the treatment of AF.
Inhibition of K2P3.1 (TASK-1) in cardiomyocytes from AF patients has been shown to counteract AF-induced APD shortening [104,154]. After administration of A293 (200 nM), APDs of atrial cardiomyocytes isolated from AF patients could be prolonged around 30% to values observed in sinus rhythm controls [104,154]. After intravenous application of K2P3.1 (TASK-1) inhibitors in healthy control pigs, significant prolongation of both, atrial effective refractory periods and ADP values pointed towards class III antiarrhythmic effects of K2P3.1 (TASK-1) inhibition [53,54]. Furthermore, the inducibility of atrial arrhythmias was significantly reduced by K2P3.1 (TASK-1) inhibitors in different studies [176,177,178]. In a similar fashion, intravenous administration of K2P3.1 (TASK-1) inhibitors A293 and doxapram led to rapid, safe and successful cardioversion of artificially induced AF episodes in a porcine large animal model [53,54]. These antiarrhythmic effects could further be employed for rhythm control in a porcine model of burst pacing induced “persistent” AF, induced via implanted pacemakers using a biofeedback algorithm [53,164] and reproduced with an AAV-mediated anti-K2P3.1 (TASK-1) gene therapy approach [52]. Based on these encouraging results, the currently ongoing DOCTOS trial (doxapram conversion to sinus rhythm; EudraCT No: 2018-002979-17) was started, which investigates whether the FDA and EMA approved K2P3.1 (TASK-1) inhibitor doxapram can cardiovert AF in patients [2,179].
Interestingly, also reduction of atrial K2P3.1 (TASK-1) expression was linked to AF as in a dog model of postoperative AF, a phosphorylation dependent downregulation of K2P3.1 (TASK-1) was reported [50] and CREM-TG AF mice display atrial downregulation of K2P3.1 (TASK-1) in conjunction with massive atrial dilatation and scarring [16]. Patients who suffer from reduced left ventricular ejection fraction display reduced atrial K2P3.1 (TASK-1) expression, independently from their rhythm state [40]. Finally, three genetic variants (two kozak variants and missense variant K2P3.1 (TASK-1) V123L mutation all of which reduce the expression or channel function) were found in patients with familial AF [49].
In addition to its role in the control of heart rhythm, K2P3.1 (TASK-1) is also discussed as a regulator of cardiac energetics and metabolic function, as Kcnk3 knockout mice were protected from pressure overload-induced cardiomyopathy. Compared to wild-type littermates, Kcnk3 knockout mice showed a preservation of systolic as well as diastolic function and a relative abrogation in concentric left ventricular hypertrophy upon TAC-induced pressure overload [46].
Moreover, K2P3.1 (TASK-1) channels were described to be expressed in in human pulmonary artery smooth muscle cells, where they serve as regulators of the basal membrane potential and consecutively regulate pulmonary vascular tone [180]. Furthermore, KCNK3 loss-of-function mutations were found to cause idiopathic pulmonary arterial hypertension [166] and acute pharmacological K2P3.1 (TASK-1) inhibition in pigs led to a mild but significant increase in invasively measured pulmonary arterial pressure [164]. In the context of adrenal KCNK3 expression, a role of the K2P3.1 (TASK-1) channel in aldosterone secretion and blood pressure control is further discussed. Global Kcnk3 knockout mice display a phenotype of mild hyperaldosteronism [181] and single nucleotide polymorphisms in the KCNK3 gene were associated with plasma aldosterone levels [182]. Accordingly, elevated systolic blood pressure values were described in the Kcnk3 knockout mouse [25]. Finally, K2P3.1 (TASK-1) channels are also discussed to be involved in regulating function of immune cells and in thermogenesis in brown adipose tissue [183]. Thus, there is a need for further studies that exclude systemic side effects in the use of TASK-1 inhibitors for treatment of AF.
6. K2P4.1 (TRAAK)
Although it was suspected about 20 years ago, that the K2P4.1 (TRAAK) channel, based on northern blot analysis, might be mainly expressed in the human heart there is little evidence to date for a cardiac role of this K2P channel. Several studies reported cardiac KCNK4 mRNA expression, mostly with atrial predominant expression patterns (TaqMan qPCR; Table 2) in human as well as in murine heart tissue samples [10,22,26,41]. Compared with other cardiac ion channels, however, expression levels were relatively low [10,16,41]. A mild inhibitory effect of vernakalant and the late sodium channel blocker ranolazine has also been described for hK2P4.1 (TRAAK) homodimeric channels (Table 3) [83,109].
Kcnk4 knockout mice were reported to display smaller ischemic areas upon cerebral infarction. No obvious phenotype of heart rhythm disorder or heart failure was described, and the mice were reported as viably and healthy [167,168]. We are, however, not aware of any studies that explicitly study the cardiac phenotype of these transgenic mice (Table 4).
7. K2P5.1 (TASK-2)
Shortly after the first description of the KCNK5 gene, RT-PCR experiments had already indicated robust cardiac abundance of KCNK5 mRNA [184], while other studies (RT-PCR) considered the cardiac mRNA levels to be rather low (Table 2) [22,23,26,38]. Our own studies indicated atrial predominant KCNK5 mRNA abundance within the human and murine heart [10,16]. Further, a trend towards downregulation of atrial KCNK5 mRNA in patients, suffering from chronic AF was noted that did not reach statistical significance [10]. K2P5.1 (TASK-2) homodimers are a molecular target on volatile and amide type local anesthetics (Table 3) [185,186] and inhibited by supratherapeutic concentrations of ranolazine [109]. siRNA transfection experiments pointed towards a functional role of K2P5.1 (TASK-2) in setting the membrane potential of pulmonary artery myocytes [187]. In the diabetic rat model with sinus bradycardia, mentioned above, downregulation of cardiac Kcnk5 mRNA expression was reported (Table 4) [19]. Finally, genome-wide association studies could identify a risk locus, associated with the development of coronary artery disease and migraine within the KCNK5 gene [188].
Breeding of global Kcnk5 knockout mice resulted in a small number of female homozygous offspring, pointing towards a phenotype which might cause antenatal mortality [169]. Further, Gerstin et al. reported that one homozygote female animal was found dead in the cage at 12 days of age [169]. However, whether this was associated with cardiomyopathy or arrhythmia remains speculative.
8. K2P6.1 (TWIK-2)
Robust cardiac expressions patterns of KCNK6 mRNA, derived from RT-PCR were described [10,18,22], while others report mild to moderate cardiac expression of this channel (RT-PCR, WB; Table 2) [15,23,26]. Interestingly, mRNA levels were reported to be significantly higher in the adult as compared to the neonatal rat heart [18]. Furthermore, abundant Kcnk6 mRNA levels were found in rat saphenous arteries [189]. Upon TAC-induced pressure overload, an upregulation of murine ventricular Kcnk6 mRNA could be observed (Table 4) [16]. Kcnk6 deficient mice are hypertensive and display elevated RV pressure level as well as enhanced vascular contractility which was linked to enhanced rho kinase activity [170,171,172]. The physiological relevance of K2P6.1 (TWIK-2) is under debate because these channels conduct only low currents in the heterologous expression system [82]. It further was recently reported that K2P6.1 (TWIK-2) channel subunits give rise to functional K2P currents in endolysosomes, where they affect the size and number of lysosomes [190] so it remains unclear whether the cell membrane is indeed the actual site of action of these channels.
9. K2P7.1 (TWIK-3)
The mainly neuronally detected K2P7.1 (TWIK-3) channel is a silent K2P channel without proven potassium conductance in heterologous expression systems [191]. Only very low cardiac expression levels have been described for KCNK7 (RT-PCR, TaqMan qPCR; Table 2) [10,23]. It was, however speculated whether its mRNA expression might be upregulated in atrial tissue samples, derived from AF patients [63]. Although not explicitly cardiac characterized, a global Kcnk7 knockout mouse showed no obvious cardiac phenotype. Homozygous transgenic mice and wild-type littermates did not differ significantly in general appearance, gross anatomy, locomotion, or overt behavior (Table 4) [173].
10. K2P9.1 (TASK-3)
The cardiac relevance of K2P9.1 (TASK-3) channel subunits which are primarily known for their role in apoptosis, aldosterone secretion and tumor genesis remains controversial. Whereas most studies detected only relatively low mRNA levels in the human heart (qPCR, TaqMan qPCR; Table 2) [10,22,26,49], others showed high atrial expression, almost comparable to K2P3.1 (TASK-1) (RT qPCR, IF) [56]. In the rodent heart, low Kcnk9 (TASK-3) mRNA abundance been described [15,16,18,25,26,48].
Echocardiographic characterization of Kcnk9 knockout mice revealed a phenotype of concentric left ventricular hypertrophy with preserved ejection fraction (Table 4) [46]. In contrast to Kcnk3 knockout mice, however, these animals are not TAC resistant, and heart failure symptoms are more likely to occur at a later time point [46]. Downregulation of ventricular KCNK9 mRNA expression (TaqMan qPCR) in heart failure patients might point towards a pathophysiological role of this channel [22].
Single channel patch-clamp measurements, performed in isolated human atrial cardiomyocytes were able to detect a channel with characteristics corresponding to a heteromer of K2P3.1 (TASK-1) and K2P9.1 (TASK-3) [56]. However, besides this heteromeric and homodimeric K2P3.1 (TASK-1) channels, no current corresponding to a homodimeric K2P9.1 (TASK-3) channels could be detected. Functional studies in motoneurons or in rat carotid body glomus cells indicate that the K2P3.1 (TASK-1)/ K2P9.1 (TASK-3) heterodimer portion was about 52–75% and thus only a minority of K2P3.1 (TASK-1) channels are expressed as monomer at the cell surface [192,193]. Since the pharmacological properties of homodimeric and heterodimeric channels differ, heterodimerization has to be taken into account when targeting the K2P3.1 (TASK-1) channel in the treatment of cardiac arrhythmias.
A rare genetic disease, KCNK9 imprinting syndrome, also known as Birk-Barel Syndrome is inherited in an autosomal dominant, maternally imprinted manner and associated with congenital central hypotonia, severe feeding difficulties, delayed development, and dysmorphic manifestations [194]. While no direct cardiac manifestation has been described to date, affected individuals may develop obstructive sleep apnea syndrome, which is particularly interesting because it again links the K2P channels of the TASK subfamily to this disease entity.
Together with K2P3.1 (TASK-1), K2P9.1 (TASK-3) contributes to peripheral and central respiratory regulation [195]. Therefore, these K2P-channels are likely to constitute a molecular target of the respiratory stimulant doxapram [53]. K2P9.1 (TASK-3) homodimers are further inhibited by the class III antiarrhythmic drug dronedarone [82] and the antianginal drug ranolazine [109].
Hopefully, the recently available high-affinity K2P9.1 (TASK-3) inhibitors and activators will help to answer the question of the functional relevance of K2P9.1 (TASK-3) channels in cardiomyocytes.
11. K2P10.1 (TREK-2)
The role of K2P10.1 (TREK-2) channel subunits has so far been characterized mainly in the central nervous system (CNS), where this channel shows ubiquitous expression. However, a KCNK10 knockout mouse showed remarkably few neurobehavioral phenotypes besides discrete abnormalities in anxiety-related behavior [174]. A cardiac phenotype of this mouse has not been described yet. Pharmacological in vitro measurements revealed vernakalant and carvedilol as inhibitors of K2P10.1 (TREK-2) homodimer channels (Table 3) [43,83]. Low cardiac mRNA abundance was described by our group and others (RT-PCR, TaqMan qPCR; Table 2) [10,15,22,40]. However, the expression patterns appeared atrial-predominant both in murine and patient-derived samples [10,41]. No relevant changes of K2P10.1 (TREK-2) expression could be detected in murine disease models of TAC-induced pressure overload or CREM-TG AF (Table 4) [16]. However, in right and left atrial patient-derived tissue samples, significant mRNA upregulation was demonstrated upon systolic heart failure [41].
12. K2P12.1 (THIK-2)
K2P12.1 (THIK-2) is referred to as a silent K2P-channel. This is likely due to both, a N-terminal retention signal and a low endogenous open probability [196]. While cardiac K2P12.1 (THIK-2) mRNA levels (RT-PCR, TaqMan qPCR) were described to be rather low (Table 2) [10,15,16,67], K2P12.1 (THIK-2) expression was detected in rat saphenous arteries [189] and might therefore be of relevance in control of vascular tone.
13. K2P13.1 (THIK-1)
K2P13.1 (THIK-1) mRNA was described in the CNS, arterial smooth muscle cells, the kidney and myocardial tissue samples via RT-PCR [15,22,26,66,68]. In patient-derived myocardial tissue samples, KCNK13 mRNA abundance (TaqMan qPCR) could be demonstrated with atrial predominance (Table 2) [10]. Heterologously expressed K2P13.1 (THIK-1) channel homodimers were inhibited by the antiarrhythmic drugs lidocaine, mexiletine, propafenone and propranolol, while administration of quinidine, amiodarone, dronedarone or ranolazine resulted in a mild channel activation (Table 3) [82,109,129].
The observation of reduced KCNK13 mRNA levels in patients with chronic AF or heart failure, which could also be recapitulated in a porcine large animal model of combined AF and heart failure might point towards a physiological role of K2P13.1 (THIK-1) currents in regulating atrial electrophysiology [10,40,129]. Finally, ventricular expression levels of KCNK13 mRNA, were described as unchanged in heart failure patients (Table 4) [22].
14. K2P15.1 (TALK-5)
Data on cardiac expression of K2P15 (TASK-5) remain sparse. While some work has shown evidence of KCNK15 mRNA abundance in rodent hearts (RT-PCR), very low levels of mRNA at best have been detected in human (northern blot, RT-PCR, TaqMan qPCR; Table 2) [10,26,48,69,70] or rodent (RT-PCR, TaqMan-qPCR) [15,16,26] heart samples by other groups. Downregulation of atrial KCNK15 mRNA was reported in a murine CREM-TG model of AF (Table 4) [16]. Finally, functionality of K2P15 (TASK-5) channel subunits is still controversial, as recombinantly expressed K2P15 (TASK-5) homodimers do not give rise to potassium currents [8].
15. K2P16.1 (TALK-1)
K2P16.1 (TALK-1) is primarily expressed in pancreatic beta cells, where it is supposed to regulate insulin secretion. Recently, a gain of function mutation in KCNK16 was identified to cause maturity-onset diabetes of the young [197]. Five studies showed low to negligible abundance of KCNK16 mRNA in human or rat cardiac tissue samples (Table 2) [10,15,60,67,71]. Upon heterologous expression in Xenopus laevis oocytes, homodimeric K2P16.1 (TALK-1) channels are inhibited by ranolazine (Table 3) [109].
16. K2P17.1 (TALK-2)
K2P17.1 (TALK-2) channel subunits are expressed in the human heart (northern blot, RT-PCR, Taq-Man qPCR, western blot) [5,10,22,40,67,73,75] and in patient-derived iPSC (RT-PCR, qPCR, IF) [22,74]. Cardiac mRNA levels of KCNK17 were described as atrial predominant with highest abundance in purkinje fibers (qPCR, Taq-Man qPCR; Table 2) [5,10]. Reports of reduced KCNK17 mRNA levels in atrial fibrillation [10] and heart failure [22,40] suggest a role for K2P17.1 (TALK-2) in the pathophysiology of important cardiac pathologies. K2P17.1 (TALK-2) channel subunits were described to heterodimerize with atrial K2P3.1 (TASK-1), thereby modulating biophysical and pharmacological properties of atrial ITASK-1 [198]. In heterologous expressions systems, K2P17.1 (TALK-2) channel homodimers were reported to be activated by propafenone, quinidine, mexiletine, propranolol, vernakalant, and metoprolol [75]. Amiodarone, sotalol, verapamil, and ranolazine were further described to inhibit K2P17.1 (TALK-2) homodimers (Table 3) [75,83]. In iPSC, suppression of K2P17.1 (TALK-2) expression was shown to prolong APD (Table 4) [22] while overexpression of K2P17.1 (TALK-2) shortened APD levels in the cultured, cardiomyocyte derived HL-1 cell line [5]. Recently, a patient suffering from progressive and severe cardiac conduction disorder in combination with idiopathic ventricular fibrillation was identified to carry both, a splice site mutation in the sodium channel gene SCN5A as well as a mutation in the KCNK17 gene [5]. This K2P17.1 (TALK-2) G88R mutation, located in the first extracellular pore loop was shown to increase K2P17.1 (TALK-2) currents to about three times upon heterologous expression. Overexpression of K2P17.1 (TALK-2) G88R in spontaneously beating HL-1 cells was shown to result in a reduction of the beating frequency, hyperpolarization of the membrane potential and a strong slowing of the upstroke velocity [5].
Single nucleotide polymorphisms in the KCNK17 gene which increase K2P17.1 (TALK-2) channel subunit expression levels are associated with the occurrence of ischemic stroke in Caucasians but not in a Chinese population [137,199]. This observation links the channel once again to the pathophysiology of atrial fibrillation. KCNK17 was further proposed as a genetic modifier of long QT syndrome type 2 severity, as a common KCNK17 gain-of-function variant was shown to be LQTS protective by promoting APD shortening [74].
The cardiac characterization of the K2P17.1 (TALK-2) channel is complicated by the fact that to date no specific inhibitors are available that would allow functional studies (Table 3). Furthermore, no ortholog to the KCNK17 gene could be identified in mice and the porcine K2P17.1 (TALK-2) channel subunit does not appear to show functional activity after heterologous expression in Xenopus laevis oocytes (unpublished observation of our lab).
17. K2P18.1 (TRESK)
KCNK18 mRNA, encoding K2P18.1 (TRESK) channel subunits was detected in human spinal cord, trigeminal ganglia, and brain but not in the heart (RT-PCR and TaqMan qPCR; Table 2) [10,61,77,78]. Accordingly, K2P18.1 (TRESK) channels are supposed to play a key role in pain perception and KCNK18 was identified as a potential susceptibility gene for migraine, while a cardiac role of this channel is rather unlikely [1]. TRESK channels may nevertheless exert indirect effects on the cardiovascular system: For example, high-fat diet-induced vagal afferent dysfunction has been described to be mediated via upregulation of K2P18.1 (TRESK) [200]. Heterologously expressed K2P18.1 (TRESK) channel homodimers are inhibited by lidocaine, verapamil, quinidine and apamin (Table 3) [76,200].
18. Conclusions
Overall, K2P channels are an exciting and relevant new potassium channel class with relevance to a wide variety of disease conditions. For several members, reproducible mRNA regulation patterns in atrial fibrillation, heart failure and other cardiac disease could be described. However, the functional consequence remains difficult to assess, especially in cases where no specific channel inhibitors are available (Table 3), since surface expression and current amplitude in cardiomyocytes cannot be directly inferred from mRNA expression [11]. Further, the actual significance of the individual K2P subgroups, some of which show only weak expression patterns, merits further investigation. To date, little is also known about the differential expression of K2P channels in different cardiac cell populations and the consequence of remodelling in different cell types. In this regard, single cell next generation sequencing technology is expected to provide further evidence soon. Furthermore, computational models of cardiac electrophysiology must consider effects of K2P channels. Taken together, emerging evidence suggests that K2P channels play an important role in cardiac repolarization and in the development of various cardiac arrhythmias such as atrial fibrillation, conduction disorders, and ventricular proarrhythmia that goes far beyond the role of unspecific leak currents.
Acknowledgments
We would like to thank Manuel Kraft and Amelie Paasche for helpful comments and critical discussions.
Author Contributions
Conceptualization, F.W., N.F. and C.S.; writing—original draft preparation, F.W.; writing—review and editing, C.S. and N.F.; visualization, F.W.; supervision, N.F.; project administration, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
F.W. and C.S. have filed patent applications for pharmacological and genetic targeting of cardiac TASK-1 channels for therapy of atrial arrhythmias.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Enyedi P., Czirják G. Molecular Background of Leak K+ Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 2.Kraft M., Büscher A., Wiedmann F., L’hoste Y., Haefeli W.E., Frey N., Katus H.A., Schmidt C. Current Drug Treatment Strategies for Atrial Fibrillation and TASK-1 Inhibition as an Emerging Novel Therapy Option. Front. Pharm. 2021;12:191. doi: 10.3389/fphar.2021.638445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schewe M., Nematian-Ardestani E., Sun H., Musinszki M., Cordeiro S., Bucci G., de Groot B.L., Tucker S.J., Rapedius M., Baukrowitz T. A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K(+) Channels. Cell. 2016;164:937–949. doi: 10.1016/j.cell.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yue D.T., Marban E. A novel cardiac potassium channel that is active and conductive at depolarized potentials. Pflug. Arch. 1988;413:127–133. doi: 10.1007/BF00582522. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich C., Rinné S., Zumhagen S., Kiper A.K., Silbernagel N., Netter M.F., Stallmeyer B., Schulze-Bahr E., Decher N. Gain-of-function mutation in TASK-4 channels and severe cardiac conduction disorder. EMBO Mol. Med. 2014;6:937–951. doi: 10.15252/emmm.201303783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H., Guo W., Nerbonne J.M. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J. Gen. Physiol. 1999;113:661–678. doi: 10.1085/jgp.113.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D., Clapham D.E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989;244:1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt C., Wiedmann F., Voigt N., Zhou X.B., Heijman J., Lang S., Albert V., Kallenberger S., Ruhparwar A., Szabó G., et al. Upregulation of K(2P)3.1 K+ Current Causes Action Potential Shortening in Patients With Chronic Atrial Fibrillation. Circulation. 2015;132:82–92. doi: 10.1161/CIRCULATIONAHA.114.012657. [DOI] [PubMed] [Google Scholar]
- 9.Renigunta V., Zou X., Kling S., Schlichthörl G., Daut J. Breaking the silence: Functional expression of the two-pore-domain potassium channel THIK-2. Pflug. Arch. 2014;466:1735–1745. doi: 10.1007/s00424-013-1404-z. [DOI] [PubMed] [Google Scholar]
- 10.Christensen A.H., Chatelain F.C., Huttner I.G., Olesen M.S., Soka M., Feliciangeli S., Horvat C., Santiago C.F., Vandenberg J.I., Schmitt N., et al. The two-pore domain potassium channel, TWIK-1, has a role in the regulation of heart rate and atrial size. J. Mol. Cell Cardiol. 2016;97:24–35. doi: 10.1016/j.yjmcc.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Ellinghaus P., Scheubel R.J., Dobrev D., Ravens U., Holtz J., Huetter J., Nielsch U., Morawietz H. Comparing the global mRNA expression profile of human atrial and ventricular myocardium with high-density oligonucleotide arrays. J. Thorac. Cardiovasc. Surg. 2005;129:1383–1390. doi: 10.1016/j.jtcvs.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Ördög B., Brutyó E., Puskás L.G., Papp J.G., Varró A., Szabad J., Boldogkői Z. Gene expression profiling of human cardiac potassium and sodium channels. Int. J. Cardiol. 2006;111:386–393. doi: 10.1016/j.ijcard.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 13.Gaborit N., Le Bouter S., Szuts V., Varro A., Escande D., Nattel S., Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putzke C., Wemhöner K., Sachse F.B., Rinné S., Schlichthörl G., Li X.T., Jaé L., Eckhardt I., Wischmeyer E., Wulf H., et al. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc. Res. 2007;75:59–68. doi: 10.1016/j.cardiores.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Wiedmann F., Schulte J.S., Gomes B., Zafeiriou M.P., Ratte A., Rathjens F., Fehrmann E., Scholz B., Voigt N., Müller F.U., et al. Atrial fibrillation and heart failure-associated remodeling of two-pore-domain potassium (K(2P)) channels in murine disease models: Focus on TASK-1. Basic Res. Cardiol. 2018;113:27. doi: 10.1007/s00395-018-0687-9. [DOI] [PubMed] [Google Scholar]
- 16.Howarth F.C., Qureshi M.A., Jayaprakash P., Parekh K., Oz M., Dobrzynski H., Adrian T.E. The Pattern of mRNA Expression Is Changed in Sinoatrial Node from Goto-Kakizaki Type 2 Diabetic Rat Heart. J. Diabetes Res. 2018;2018:8454078. doi: 10.1155/2018/8454078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W., Saint D.A. Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by real-time polymerase chain reaction. Clin. Exp. Pharm. Physiol. 2004;31:174–178. doi: 10.1111/j.1440-1681.2004.03964.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Yue L., White M., Pelletier G., Nattel S. Differential Distribution of Inward Rectifier Potassium Channel Transcripts in Human Atrium Versus Ventricle. Circulation. 1998;98:2422–2428. doi: 10.1161/01.CIR.98.22.2422. [DOI] [PubMed] [Google Scholar]
- 19.McGeachie M., Ramoni R.L., Mychaleckyj J.C., Furie K.L., Dreyfuss J.M., Liu Y., Herrington D., Guo X., Lima J.A., Post W., et al. Integrative predictive model of coronary artery calcification in atherosclerosis. Circulation. 2009;120:2448–2454. doi: 10.1161/CIRCULATIONAHA.109.865501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt C., Wiedmann F., Zhou X.B., Heijman J., Voigt N., Ratte A., Lang S., Kallenberger S.M., Campana C., Weymann A., et al. Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: Implications for patient-specific antiarrhythmic drug therapy. Eur. Heart J. 2017;38:1764–1774. doi: 10.1093/eurheartj/ehw559. [DOI] [PubMed] [Google Scholar]
- 21.Chai S., Wan X., Nassal D.M., Liu H., Moravec C.S., Ramirez-Navarro A., Deschênes I. Contribution of two-pore K(+) channels to cardiac ventricular action potential revealed using human iPSC-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H1144–H1153. doi: 10.1152/ajpheart.00107.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medhurst A.D., Rennie G., Chapman C.G., Meadows H., Duckworth M.D., Kelsell R.E., Gloger I.I., Pangalos M.N. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res. Mol. Brain Res. 2001;86:101–114. doi: 10.1016/S0169-328X(00)00263-1. [DOI] [PubMed] [Google Scholar]
- 23.Fink M., Duprat F., Lesage F., Reyes R., Romey G., Heurteaux C., Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. doi: 10.1002/j.1460-2075.1996.tb01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decher N., Wemhöner K., Rinné S., Netter M.F., Zuzarte M., Aller M.I., Kaufmann S.G., Li X.T., Meuth S.G., Daut J., et al. Knock-Out of the Potassium Channel TASK-1 Leads to a Prolonged QT Interval and a Disturbed QRS Complex. Cell. Physiol. Biochem. 2011;28:77–86. doi: 10.1159/000331715. [DOI] [PubMed] [Google Scholar]
- 25.Donner B.C., Schullenberg M., Geduldig N., Hüning A., Mersmann J., Zacharowski K., Kovacevic A., Decking U., Aller M.I., Schmidt K.G. Functional role of TASK-1 in the heart: Studies in TASK-1-deficient mice show prolonged cardiac repolarization and reduced heart rate variability. Basic Res. Cardiol. 2011;106:75–87. doi: 10.1007/s00395-010-0128-x. [DOI] [PubMed] [Google Scholar]
- 26.Hund T.J., Snyder J.S., Wu X., Glynn P., Koval O.M., Onal B., Leymaster N.D., Unudurthi S.D., Curran J., Camardo C., et al. βIV-Spectrin regulates TREK-1 membrane targeting in the heart. Cardiovasc. Res. 2014;102:166–175. doi: 10.1093/cvr/cvu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aimond F., Rauzier J.-M., Bony C., Vassort G. Simultaneous Activation of p38 MAPK and p42/44 MAPK by ATP Stimulates the K+ Current ITREK in Cardiomyocytes. J. Biol. Chem. 2000;275:39110–39116. doi: 10.1074/jbc.M008192200. [DOI] [PubMed] [Google Scholar]
- 28.Tan J.H., Liu W., Saint D.A. Trek-like potassium channels in rat cardiac ventricular myocytes are activated by intracellular ATP. J. Membr. Biol. 2002;185:201–207. doi: 10.1007/s00232-001-0123-0. [DOI] [PubMed] [Google Scholar]
- 29.Tan J.H.C., Liu W., Saint D.A. Differential expression of the mechanosensitive potassium channel TREK-1 in epicardial and endocardial myocytes in rat ventricle. Exp. Physiol. 2004;89:237–242. doi: 10.1113/expphysiol.2003.027052. [DOI] [PubMed] [Google Scholar]
- 30.Rinné S., Renigunta V., Schlichthörl G., Zuzarte M., Bittner S., Meuth S.G., Decher N., Daut J., Preisig-Müller R. A splice variant of the two-pore domain potassium channel TREK-1 with only one pore domain reduces the surface expression of full-length TREK-1 channels. Pflug. Arch. 2014;466:1559–1570. doi: 10.1007/s00424-013-1384-z. [DOI] [PubMed] [Google Scholar]
- 31.Terrenoire C., Lauritzen I., Lesage F., Romey G., Lazdunski M. A TREK-1–Like Potassium Channel in Atrial Cells Inhibited by β-Adrenergic Stimulation and Activated by Volatile Anesthetics. Circ. Res. 2001;89:336–342. doi: 10.1161/hh1601.094979. [DOI] [PubMed] [Google Scholar]
- 32.Li X.T., Dyachenko V., Zuzarte M., Putzke C., Preisig-Müller R., Isenberg G., Daut J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc. Res. 2006;69:86–97. doi: 10.1016/j.cardiores.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Jones S.A., Morton M.J., Hunter M., Boyett M.R. Expression of TASK-1, a pH-sensitive twin-pore domain K(+) channel, in rat myocytes. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H181–H185. doi: 10.1152/ajpheart.00963.2001. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt C., Wiedmann F., Tristram F., Anand P., Wenzel W., Lugenbiel P., Schweizer P.A., Katus H.A., Thomas D. Cardiac expression and atrial fibrillation-associated remodeling of K2P2.1 (TREK-1) K+ channels in a porcine model. Life Sci. 2014;97:107–115. doi: 10.1016/j.lfs.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Lugenbiel P., Wenz F., Syren P., Geschwill P., Govorov K., Seyler C., Frank D., Schweizer P.A., Franke J., Weis T., et al. TREK-1 (K(2P)2.1) K(+) channels are suppressed in patients with atrial fibrillation and heart failure and provide therapeutic targets for rhythm control. Basic Res. Cardiol. 2017;112:8. doi: 10.1007/s00395-016-0597-7. [DOI] [PubMed] [Google Scholar]
- 36.Marionneau C., Aimond F., Brunet S., Niwa N., Finck B., Kelly D.P., Nerbonne J.M. PPARalpha-mediated remodeling of repolarizing voltage-gated K+ (Kv) channels in a mouse model of metabolic cardiomyopathy. J. Mol. Cell. Cardiol. 2008;44:1002–1015. doi: 10.1016/j.yjmcc.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limberg S.H., Netter M.F., Rolfes C., Rinné S., Schlichthörl G., Zuzarte M., Vassiliou T., Moosdorf R., Wulf H., Daut J., et al. TASK-1 channels may modulate action potential duration of human atrial cardiomyocytes. Cell Physiol. Biochem. 2011;28:613–624. doi: 10.1159/000335757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt C., Wiedmann F., Kallenberger S.M., Ratte A., Schulte J.S., Scholz B., Müller F.U., Voigt N., Zafeiriou M.-P., Ehrlich J.R., et al. Stretch-activated two-pore-domain (K2P) potassium channels in the heart: Focus on atrial fibrillation and heart failure. Prog. Biophys. Mol. Biol. 2017;130:233–243. doi: 10.1016/j.pbiomolbio.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Staudacher K., Staudacher I., Ficker E., Seyler C., Gierten J., Kisselbach J., Rahm A.K., Trappe K., Schweizer P.A., Becker R., et al. Carvedilol targets human K2P 3.1 (TASK1) K+ leak channels. Br. J. Pharm. 2011;163:1099–1110. doi: 10.1111/j.1476-5381.2011.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaborit N., Wichter T., Varro A., Szuts V., Lamirault G., Eckardt L., Paul M., Breithardt G., Schulze-Bahr E., Escande D., et al. Transcriptional profiling of ion channel genes in Brugada syndrome and other right ventricular arrhythmogenic diseases. Eur. Heart J. 2009;30:487–496. doi: 10.1093/eurheartj/ehn520. [DOI] [PubMed] [Google Scholar]
- 41.Kisselbach J., Seyler C., Schweizer P.A., Gerstberger R., Becker R., Katus H.A., Thomas D. Modulation of K2P 2.1 and K2P 10.1 K(+) channel sensitivity to carvedilol by alternative mRNA translation initiation. Br. J. Pharm. 2014;171:5182–5194. doi: 10.1111/bph.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham V., Zhang H., Willis S., Creazzo T.L. Expression of a two-pore domain K+ channel (TASK-1) in developing avian and mouse ventricular conduction systems. Dev. Dyn. 2006;235:143–151. doi: 10.1002/dvdy.20558. [DOI] [PubMed] [Google Scholar]
- 43.Duprat F., Lesage F., Fink M., Reyes R., Heurteaux C., Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan W., Hicks J., Makara M.A., Ilkayeva O., Abraham D.M. TASK-1 and TASK-3 channels modulate pressure overload-induced cardiac remodeling and dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H566–H580. doi: 10.1152/ajpheart.00739.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y., Bang H., Kim D. TBAK-1 and TASK-1, two-pore K(+) channel subunits: Kinetic properties and expression in rat heart. Am. J. Physiol. 1999;277:H1669–H1678. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- 46.Karschin C., Wischmeyer E., Preisig-Müller R., Rajan S., Derst C., Grzeschik K.H., Daut J., Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K(+) channel subunit, TASK-5, associated with the central auditory nervous system. Mol. Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- 47.Skarsfeldt M.A., Jepps T.A., Bomholtz S.H., Abildgaard L., Sørensen U.S., Gregers E., Svendsen J.H., Diness J.G., Grunnet M., Schmitt N., et al. pH-dependent inhibition of K₂P3.1 prolongs atrial refractoriness in whole hearts. Pflug. Arch. 2016;468:643–654. doi: 10.1007/s00424-015-1779-0. [DOI] [PubMed] [Google Scholar]
- 48.Harleton E., Besana A., Comas G.M., Danilo P., Rosen T.S., Argenziano M., Rosen M.R., Robinson R.B., Feinmark S.J. Ability to Induce Atrial Fibrillation in the Peri-operative Period Is Associated with Phosphorylation-dependent Inhibition of TWIK Protein-related Acid-sensitive Potassium Channel 1 (TASK-1)*. J. Biol. Chem. 2013;288:2829–2838. doi: 10.1074/jbc.M112.404095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt C., Wiedmann F., Langer C., Tristram F., Anand P., Wenzel W., Lugenbiel P., Schweizer P.A., Katus H.A., Thomas D. Cloning, functional characterization, and remodeling of K2P3.1 (TASK-1) potassium channels in a porcine model of atrial fibrillation and heart failure. Heart Rhythm. 2014;11:1798–1805. doi: 10.1016/j.hrthm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt C., Wiedmann F., Beyersdorf C., Zhao Z., El-Battrawy I., Lan H., Szabo G., Li X., Lang S., Korkmaz-Icöz S., et al. Genetic Ablation of TASK-1 (Tandem of P Domains in a Weak Inward Rectifying K(+) Channel-Related Acid-Sensitive K(+) Channel-1) (K(2P)3.1) K(+) Channels Suppresses Atrial Fibrillation and Prevents Electrical Remodeling. Circ. Arrhythm. Electrophysiol. 2019;12:e007465. doi: 10.1161/CIRCEP.119.007465. [DOI] [PubMed] [Google Scholar]
- 51.Wiedmann F., Beyersdorf C., Zhou X.B., Kraft M., Paasche A., Jávorszky N., Rinné S., Sutanto H., Büscher A., Foerster K.I., et al. Treatment of atrial fibrillation with doxapram: TASK-1 potassium channel inhibition as a novel pharmacological strategy. Cardiovasc. Res. 2021 doi: 10.1093/cvr/cvab177. [DOI] [PubMed] [Google Scholar]
- 52.Wiedmann F., Beyersdorf C., Zhou X., Büscher A., Kraft M., Nietfeld J., Walz T.P., Unger L.A., Loewe A., Schmack B., et al. Pharmacologic TWIK-Related Acid-Sensitive K+ Channel (TASK-1) Potassium Channel Inhibitor A293 Facilitates Acute Cardioversion of Paroxysmal Atrial Fibrillation in a Porcine Large Animal Model. J. Am. Heart Assoc. 2020;9:e015751. doi: 10.1161/JAHA.119.015751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barth A.S., Merk S., Arnoldi E., Zwermann L., Kloos P., Gebauer M., Steinmeyer K., Bleich M., Kääb S., Hinterseer M., et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: Expression of a ventricular-like genomic signature. Circ. Res. 2005;96:1022–1029. doi: 10.1161/01.RES.0000165480.82737.33. [DOI] [PubMed] [Google Scholar]
- 54.Rinné S., Kiper A.K., Schlichthörl G., Dittmann S., Netter M.F., Limberg S.H., Silbernagel N., Zuzarte M., Moosdorf R., Wulf H., et al. TASK-1 and TASK-3 may form heterodimers in human atrial cardiomyocytes. J. Mol. Cell Cardiol. 2015;81:71–80. doi: 10.1016/j.yjmcc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Darkow E., Nguyen T.T., Stolina M., Kari F.A., Schmidt C., Wiedmann F., Baczkó I., Kohl P., Rajamani S., Ravens U., et al. Small Conductance Ca(2 +)-Activated K(+) (SK) Channel mRNA Expression in Human Atrial and Ventricular Tissue: Comparison Between Donor, Atrial Fibrillation and Heart Failure Tissue. Front. Physiol. 2021;12:650964. doi: 10.3389/fphys.2021.650964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fink M., Lesage F., Duprat F., Heurteaux C., Reyes R., Fosset M., Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meadows H.J., Chapman C.G., Duckworth D.M., Kelsell R.E., Murdock P.R., Nasir S., Rennie G., Randall A.D. The neuroprotective agent sipatrigine (BW619C89) potently inhibits the human tandem pore-domain K(+) channels TREK-1 and TRAAK. Brain Res. 2001;892:94–101. doi: 10.1016/S0006-8993(00)03239-X. [DOI] [PubMed] [Google Scholar]
- 58.Kang D., Kim D. Single-channel properties and pH sensitivity of two-pore domain K+ channels of the TALK family. Biochem. Biophys. Res. Commun. 2004;315:836–844. doi: 10.1016/j.bbrc.2004.01.137. [DOI] [PubMed] [Google Scholar]
- 59.Liu C., Au J.D., Zou H.L., Cotten J.F., Yost C.S. Potent activation of the human tandem pore domain K channel TRESK with clinical concentrations of volatile anesthetics. Anesth. Analg. 2004;99:1715–1722. doi: 10.1213/01.ANE.0000136849.07384.44. [DOI] [PubMed] [Google Scholar]
- 60.Patel A.J., Maingret F., Magnone V., Fosset M., Lazdunski M., Honoré E. TWIK-2, an inactivating 2P domain K+ channel. J. Biol. Chem. 2000;275:28722–28730. doi: 10.1074/jbc.M003755200. [DOI] [PubMed] [Google Scholar]
- 61.Wang R.P., Wang S., Chen D., Yang N., Lei L.C., Wang Z.Y., Ye H.M., Ren L.H., Yang S.X. mRNA genomics change and significance of important ion channel proteins in patients with atrial fibrillation. Zhonghua Yi Xue Za Zhi. 2018;98:3171–3177. doi: 10.3760/cma.j.issn.0376-2491.2018.39.009. [DOI] [PubMed] [Google Scholar]
- 62.Rajan S., Wischmeyer E., Liu G.X., Preisig-Müller R., Daut J., Karschin A., Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J. Biol. Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- 63.Bang H., Kim Y., Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J. Biol. Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- 64.Rajan S., Wischmeyer E., Karschin C., Preisig-Müller R., Grzeschik K.H., Daut J., Karschin A., Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J. Biol. Chem. 2001;276:7302–7311. doi: 10.1074/jbc.M008985200. [DOI] [PubMed] [Google Scholar]
- 65.Girard C., Duprat F., Terrenoire C., Tinel N., Fosset M., Romey G., Lazdunski M., Lesage F. Genomic and Functional Characteristics of Novel Human Pancreatic 2P Domain K+ Channels. Biochem. Biophys. Res. Commun. 2001;282:249–256. doi: 10.1006/bbrc.2001.4562. [DOI] [PubMed] [Google Scholar]
- 66.Staudacher I., Seehausen S., Gierten J., Illg C., Schweizer P.A., Katus H.A., Thomas D. Cloning and characterization of zebrafish K(2P)13.1 (THIK-1) two-pore-domain K(+) channels. J. Mol. Cell Cardiol. 2019;126:96–104. doi: 10.1016/j.yjmcc.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Ashmole I., Goodwin P.A., Stanfield P.R. TASK-5, a novel member of the tandem pore K+ channel family. Pflügers Arch. 2001;442:828–833. doi: 10.1007/s004240100620. [DOI] [PubMed] [Google Scholar]
- 68.Kim D., Gnatenco C. TASK-5, a new member of the tandem-pore K(+) channel family. Biochem. Biophys. Res. Commun. 2001;284:923–930. doi: 10.1006/bbrc.2001.5064. [DOI] [PubMed] [Google Scholar]
- 69.Han J., Kang D., Kim D. Functional properties of four splice variants of a human pancreatic tandem-pore K+ channel, TALK-1. Am. J. Physiol. Cell Physiol. 2003;285:C529–C538. doi: 10.1152/ajpcell.00601.2002. [DOI] [PubMed] [Google Scholar]
- 70.Staudacher I., Illg C., Gierten J., Seehausen S., Schweizer P.A., Katus H.A., Thomas D. Identification and functional characterization of zebrafish K(2P)17.1 (TASK-4, TALK-2) two-pore-domain K(+) channels. Eur. J. Pharm. 2018;831:94–102. doi: 10.1016/j.ejphar.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Decher N., Maier M., Dittrich W., Gassenhuber J., Brüggemann A., Busch A.E., Steinmeyer K. Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett. 2001;492:84–89. doi: 10.1016/S0014-5793(01)02222-0. [DOI] [PubMed] [Google Scholar]
- 72.Chai S., Wan X., Ramirez-Navarro A., Tesar P.J., Kaufman E.S., Ficker E., George A.L., Jr., Deschênes I. Physiological genomics identifies genetic modifiers of long QT syndrome type 2 severity. J. Clin. Invest. 2018;128:1043–1056. doi: 10.1172/JCI94996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staudacher I., Illg C., Chai S., Deschenes I., Seehausen S., Gramlich D., Müller M.E., Wieder T., Rahm A.K., Mayer C., et al. Cardiovascular pharmacology of K(2P)17.1 (TASK-4, TALK-2) two-pore-domain K(+) channels. Naunyn Schmiedebergs Arch. Pharm. 2018;391:1119–1131. doi: 10.1007/s00210-018-1535-z. [DOI] [PubMed] [Google Scholar]
- 74.Rahm A.K., Wiedmann F., Gierten J., Schmidt C., Schweizer P.A., Becker R., Katus H.A., Thomas D. Functional characterization of zebrafish K2P18.1 (TRESK) two-pore-domain K+ channels. Naunyn Schmiedebergs Arch. Pharm. 2014;387:291–300. doi: 10.1007/s00210-013-0945-1. [DOI] [PubMed] [Google Scholar]
- 75.Keshavaprasad B., Liu C., Au J.D., Kindler C.H., Cotten J.F., Yost C.S. Species-specific differences in response to anesthetics and other modulators by the K2P channel TRESK. Anesth. Analg. 2005;101:1042–1049. doi: 10.1213/01.ane.0000168447.87557.5a. [DOI] [PubMed] [Google Scholar]
- 76.Sano Y., Inamura K., Miyake A., Mochizuki S., Kitada C., Yokoi H., Nozawa K., Okada H., Matsushime H., Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J. Biol. Chem. 2003;278:27406–27412. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- 77.Wiedmann F., Rinné S., Donner B., Decher N., Katus H.A., Schmidt C. Mechanosensitive TREK-1 two-pore-domain potassium (K(2P)) channels in the cardiovascular system. Prog. Biophys. Mol. Biol. 2021;159:126–135. doi: 10.1016/j.pbiomolbio.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Wiedmann F., Schlund D., Faustino F., Kraft M., Ratte A., Thomas D., Katus H.A., Schmidt C. N-Glycosylation of TREK-1/hK(2P)2.1 Two-Pore-Domain Potassium (K(2P)) Channels. Int. J. Mol. Sci. 2019;20:5193. doi: 10.3390/ijms20205193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15:1004–1011. doi: 10.1002/j.1460-2075.1996.tb00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaborit N., Steenman M., Lamirault G., Le Meur N., Le Bouter S., Lande G., Léger J., Charpentier F., Christ T., Dobrev D., et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt C., Wiedmann F., Schweizer P.A., Becker R., Katus H.A., Thomas D. Novel electrophysiological properties of dronedarone: Inhibition of human cardiac two-pore-domain potassium (K2P) channels. Naunyn Schmiedebergs Arch. Pharm. 2012;385:1003–1016. doi: 10.1007/s00210-012-0780-9. [DOI] [PubMed] [Google Scholar]
- 82.Lesage F., Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. Ren. Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- 83.Loucif A.J.C., Saintot P.P., Liu J., Antonio B.M., Zellmer S.G., Yoger K., Veale E.L., Wilbrey A., Omoto K., Cao L., et al. GI-530159, a novel, selective, mechanosensitive two-pore-domain potassium (K(2P) ) channel opener, reduces rat dorsal root ganglion neuron excitability. Br. J. Pharm. 2018;175:2272–2283. doi: 10.1111/bph.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goonetilleke L., Quayle J. TREK-1 K(+) channels in the cardiovascular system: Their significance and potential as a therapeutic target. Cardiovasc. Ther. 2012;30:e23–e29. doi: 10.1111/j.1755-5922.2010.00227.x. [DOI] [PubMed] [Google Scholar]
- 85.Gruss M., Mathie A., Lieb W.R., Franks N.P. The two-pore-domain K(+) channels TREK-1 and TASK-3 are differentially modulated by copper and zinc. Mol. Pharm. 2004;66:530–537. doi: 10.1124/mol.66.3. [DOI] [PubMed] [Google Scholar]
- 86.Joseph A., Thuy T.T.T., Thanh L.T., Okada M. Antidepressive and anxiolytic effects of ostruthin, a TREK-1 channel activator. PLoS ONE. 2018;13:e0201092. doi: 10.1371/journal.pone.0201092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pope L., Arrigoni C., Lou H., Bryant C., Gallardo-Godoy A., Renslo A.R., Minor D.L., Jr. Protein and Chemical Determinants of BL-1249 Action and Selectivity for K(2P) Channels. ACS Chem. Neurosci. 2018;9:3153–3165. doi: 10.1021/acschemneuro.8b00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lolicato M., Arrigoni C., Mori T., Sekioka Y., Bryant C., Clark K.A., Minor D.L., Jr. K(2P)2.1 (TREK-1)-activator complexes reveal a cryptic selectivity filter binding site. Nature. 2017;547:364–368. doi: 10.1038/nature22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bagriantsev S.N., Ang K.H., Gallardo-Godoy A., Clark K.A., Arkin M.R., Renslo A.R., Minor D.L., Jr. A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels. ACS Chem. Biol. 2013;8:1841–1851. doi: 10.1021/cb400289x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright P.D., McCoull D., Walsh Y., Large J.M., Hadrys B.W., Gaurilcikaite E., Byrom L., Veale E.L., Jerman J., Mathie A. Pranlukast is a novel small molecule activator of the two-pore domain potassium channel TREK2. Biochem. Biophys. Res. Commun. 2019;520:35–40. doi: 10.1016/j.bbrc.2019.09.093. [DOI] [PubMed] [Google Scholar]
- 91.Minieri L., Pivonkova H., Caprini M., Harantova L., Anderova M., Ferroni S. The inhibitor of volume-regulated anion channels DCPIB activates TREK potassium channels in cultured astrocytes. Br. J. Pharm. 2013;168:1240–1254. doi: 10.1111/bph.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Devilliers M., Busserolles J., Lolignier S., Deval E., Pereira V., Alloui A., Christin M., Mazet B., Delmas P., Noel J., et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat. Commun. 2013;4:2941. doi: 10.1038/ncomms3941. [DOI] [PubMed] [Google Scholar]
- 93.Takahira M., Sakurai M., Sakurada N., Sugiyama K. Fenamates and diltiazem modulate lipid-sensitive mechano-gated 2P domain K(+) channels. Pflug. Arch. 2005;451:474–478. doi: 10.1007/s00424-005-1492-5. [DOI] [PubMed] [Google Scholar]
- 94.Gruss M., Bushell T.J., Bright D.P., Lieb W.R., Mathie A., Franks N.P. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol. Pharm. 2004;65:443–452. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 95.Mazella J., Pétrault O., Lucas G., Deval E., Béraud-Dufour S., Gandin C., El-Yacoubi M., Widmann C., Guyon A., Chevet E., et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: A new concept in the antidepressant drug design. PLoS Biol. 2010;8:e1000355. doi: 10.1371/journal.pbio.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Unudurthi S.D., Wu X., Qian L., Amari F., Onal B., Li N., Makara M.A., Smith S.A., Snyder J., Fedorov V.V., et al. Two-Pore K+ Channel TREK-1 Regulates Sinoatrial Node Membrane Excitability. J. Am. Heart Assoc. 2016;5:e002865. doi: 10.1161/JAHA.115.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thomas D., Plant L.D., Wilkens C.M., McCrossan Z.A., Goldstein S.A.N. Alternative Translation Initiation in Rat Brain Yields K2P2.1 Potassium Channels Permeable to Sodium. Neuron. 2008;58:859–870. doi: 10.1016/j.neuron.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu H., Enyeart J.A., Enyeart J.J. Potent Inhibition of Native TREK-1 K+ Channels by Selected Dihydropyridine Ca2+ Channel Antagonists. J. Pharmacol. Exp. Ther. 2007;323:39–48. doi: 10.1124/jpet.107.125245. [DOI] [PubMed] [Google Scholar]
- 99.Streit A.K., Netter M.F., Kempf F., Walecki M., Rinné S., Bollepalli M.K., Preisig-Müller R., Renigunta V., Daut J., Baukrowitz T., et al. A specific two-pore domain potassium channel blocker defines the structure of the TASK-1 open pore. J. Biol. Chem. 2011;286:13977–13984. doi: 10.1074/jbc.M111.227884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin H.W., Soh J.S., Kim H.Z., Hong J., Woo D.H., Heo J.Y., Hwang E.M., Park J.Y., Lee C.J. The inhibitory effects of bupivacaine, levobupivacaine, and ropivacaine on K2P (two-pore domain potassium) channel TREK-1. J. Anesth. 2014;28:81–86. doi: 10.1007/s00540-013-1661-1. [DOI] [PubMed] [Google Scholar]
- 101.Ratte A., Wiedmann F., Kraft M., Katus H.A., Schmidt C. Antiarrhythmic Properties of Ranolazine: Inhibition of Atrial Fibrillation Associated TASK-1 Potassium Channels. Front. Pharm. 2019;10:1367. doi: 10.3389/fphar.2019.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thümmler S., Duprat F., Lazdunski M. Antipsychotics inhibit TREK but not TRAAK channels. Biochem. Biophys. Res. Commun. 2007;354:284–289. doi: 10.1016/j.bbrc.2006.12.199. [DOI] [PubMed] [Google Scholar]
- 103.Punke M.A., Licher T., Pongs O., Friederich P. Inhibition of human TREK-1 channels by bupivacaine. Anesth. Analg. 2003;96:1665–1673. doi: 10.1213/01.ANE.0000062524.90936.1F. [DOI] [PubMed] [Google Scholar]
- 104.Nayak T.K., Harinath S., Nama S., Somasundaram K., Sikdar S.K. Inhibition of Human Two-Pore Domain K+ Channel TREK1 by Local Anesthetic Lidocaine: Negative Cooperativity and Half-of-Sites Saturation Kinetics. Mol. Pharmacol. 2009;76:903–917. doi: 10.1124/mol.109.056838. [DOI] [PubMed] [Google Scholar]
- 105.Harinath S., Sikdar S.K. Inhibition of human TREK-1 channels by caffeine and theophylline. Epilepsy Res. 2005;64:127–135. doi: 10.1016/j.eplepsyres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H., Shepherd N., Creazzo T.L. Temperature-sensitive TREK currents contribute to setting the resting membrane potential in embryonic atrial myocytes. J. Physiol. 2008;586:3645–3656. doi: 10.1113/jphysiol.2008.153395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gierten J., Ficker E., Bloehs R., Schweizer P.A., Zitron E., Scholz E., Karle C., Katus H.A., Thomas D. The human cardiac K2P3.1 (TASK-1) potassium leak channel is a molecular target for the class III antiarrhythmic drug amiodarone. Naunyn Schmiedebergs Arch. Pharm. 2010;381:261–270. doi: 10.1007/s00210-009-0454-4. [DOI] [PubMed] [Google Scholar]
- 108.Schmidt C., Wiedmann F., Gaubatz A.R., Ratte A., Katus H.A., Thomas D. New Targets for Old Drugs: Cardiac Glycosides Inhibit Atrial-Specific K(2P)3.1 (TASK-1) Channels. J. Pharm. Exp. 2018;365:614–623. doi: 10.1124/jpet.118.247692. [DOI] [PubMed] [Google Scholar]
- 109.Seyler C., Li J., Schweizer P.A., Katus H.A., Thomas D. Inhibition of cardiac two-pore-domain K+ (K2P) channels by the antiarrhythmic drug vernakalant--comparison with flecainide. Eur. J. Pharm. 2014;724:51–57. doi: 10.1016/j.ejphar.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 110.Bae C., Sachs F., Gottlieb P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Putzke C., Hanley P.J., Schlichthörl G., Preisig-Müller R., Rinné S., Anetseder M., Eckenhoff R., Berkowitz C., Vassiliou T., Wulf H., et al. Differential effects of volatile and intravenous anesthetics on the activity of human TASK-1. Am. J. Physiol. Cell Physiol. 2007;293:C1319–C1326. doi: 10.1152/ajpcell.00100.2007. [DOI] [PubMed] [Google Scholar]
- 112.Sirois J.E., Lei Q., Talley E.M., Lynch C., 3rd, Bayliss D.A. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J. Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rödström K.E.J., Kiper A.K., Zhang W., Rinné S., Pike A.C.W., Goldstein M., Conrad L.J., Delbeck M., Hahn M.G., Meier H., et al. A lower X-gate in TASK channels traps inhibitors within the vestibule. Nature. 2020;582:443–447. doi: 10.1038/s41586-020-2250-8. [DOI] [PubMed] [Google Scholar]
- 114.Zou B., Flaherty D.P., Simpson D.S., Maki B.E., Miller M.R., Shi J., Wu M., McManus O.B., Golden J.E., Aubé J., et al. Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US); Bethesda, MD, USA: 2010. ML365: Development of Bis-Amides as Selective Inhibitors of the KCNK3/TASK1 Two Pore Potassium Channel. [PubMed] [Google Scholar]
- 115.Kiper A.K., Rinné S., Rolfes C., Ramírez D., Seebohm G., Netter M.F., González W., Decher N. Kv1.5 blockers preferentially inhibit TASK-1 channels: TASK-1 as a target against atrial fibrillation and obstructive sleep apnea? Pflug. Arch. 2015;467:1081–1090. doi: 10.1007/s00424-014-1665-1. [DOI] [PubMed] [Google Scholar]
- 116.Ramírez D., Bedoya M., Kiper A.K., Rinné S., Morales-Navarro S., Hernández-Rodríguez E.W., Sepúlveda F.V., Decher N., González W. Structure/Activity Analysis of TASK-3 Channel Antagonists Based on a 5,6,7,8 tetrahydropyrido[4, 3-d]pyrimidine. Int. J. Mol. Sci. 2019;20:2252. doi: 10.3390/ijms20092252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cotten J.F., Keshavaprasad B., Laster M.J., Eger E.I., 2nd, Yost C.S. The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth. Analg. 2006;102:779–785. doi: 10.1213/01.ane.0000194289.34345.63. [DOI] [PubMed] [Google Scholar]
- 118.Maingret F., Patel A.J., Lazdunski M., Honoré E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miller M.R., Zou B., Shi J., Flaherty D.P., Simpson D.S., Yao T., Maki B.E., Day V.W., Douglas J.T., Wu M., et al. Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US); Bethesda, MD, USA: 2010. Development of a Selective Chemical Inhibitor for the Two-Pore Potassium Channel, KCNK9. [PubMed] [Google Scholar]
- 120.Kindler C.H., Yost C.S., Gray A.T. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 1999;90:1092–1102. doi: 10.1097/00000542-199904000-00024. [DOI] [PubMed] [Google Scholar]
- 121.Leonoudakis D., Gray A.T., Winegar B.D., Kindler C.H., Harada M., Taylor D.M., Chavez R.A., Forsayeth J.R., Yost C.S. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J. Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meadows H.J., Randall A.D. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology. 2001;40:551–559. doi: 10.1016/S0028-3908(00)00189-1. [DOI] [PubMed] [Google Scholar]
- 123.Bruner J.K., Zou B., Zhang H., Zhang Y., Schmidt K., Li M. Identification of novel small molecule modulators of K2P18.1 two-pore potassium channel. Eur. J. Pharmacol. 2014;740:603–610. doi: 10.1016/j.ejphar.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eckert M., Egenberger B., Döring F., Wischmeyer E. TREK-1 isoforms generated by alternative translation initiation display different susceptibility to the antidepressant fluoxetine. Neuropharmacology. 2011;61:918–923. doi: 10.1016/j.neuropharm.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 125.Braun G., Lengyel M., Enyedi P., Czirják G. Differential sensitivity of TREK-1, TREK-2 and TRAAK background potassium channels to the polycationic dye ruthenium red. Br. J. Pharm. 2015;172:1728–1738. doi: 10.1111/bph.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dadi P.K., Vierra N.C., Days E., Dickerson M.T., Vinson P.N., Weaver C.D., Jacobson D.A. Selective Small Molecule Activators of TREK-2 Channels Stimulate Dorsal Root Ganglion c-Fiber Nociceptor Two-Pore-Domain Potassium Channel Currents and Limit Calcium Influx. ACS Chem. Neurosci. 2017;8:558–568. doi: 10.1021/acschemneuro.6b00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Staudacher I., Seehausen S., Illg C., Lugenbiel P., Schweizer P.A., Katus H.A., Thomas D. Cardiac K(2P)13.1 (THIK-1) two-pore-domain K(+) channels: Pharmacological regulation and remodeling in atrial fibrillation. Prog. Biophys. Mol. Biol. 2019;144:128–138. doi: 10.1016/j.pbiomolbio.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 128.Wright P.D., Weir G., Cartland J., Tickle D., Kettleborough C., Cader M.Z., Jerman J. Cloxyquin (5-chloroquinolin-8-ol) is an activator of the two-pore domain potassium channel TRESK. Biochem. Biophys. Res. Commun. 2013;441:463–468. doi: 10.1016/j.bbrc.2013.10.090. [DOI] [PubMed] [Google Scholar]
- 129.Czirják G., Enyedi P. Zinc and Mercuric Ions Distinguish TRESK from the Other Two-Pore-Domain K+ Channels. Mol. Pharmacol. 2006;69:1024–1032. doi: 10.1124/mol.105.018556. [DOI] [PubMed] [Google Scholar]
- 130.Guo Z., Cao Y.Q. Over-expression of TRESK K(+) channels reduces the excitability of trigeminal ganglion nociceptors. PLoS ONE. 2014;9:e87029. doi: 10.1371/journal.pone.0087029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang D., Mariash E., Kim D. Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J. Biol. Chem. 2004;279:28063–28070. doi: 10.1074/jbc.M402940200. [DOI] [PubMed] [Google Scholar]
- 132.Cotten J.F. TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth. Analg. 2013;116:810–816. doi: 10.1213/ANE.0b013e318284469d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lambert M., Mendes-Ferreira P., Ghigna M.R., LeRibeuz H., Adão R., Boet A., Capuano V., Rucker-Martin C., Brás-Silva C., Quarck R., et al. Kcnk3 Dysfunction Exaggerates The Development Of Pulmonary Hypertension Induced By Left Ventricular Pressure Overload. Cardiovasc. Res. 2021 doi: 10.1093/cvr/cvab016. [DOI] [PubMed] [Google Scholar]
- 134.Heidecker B., Lamirault G., Kasper E.K., Wittstein I.S., Champion H.C., Breton E., Russell S.D., Hall J., Kittleson M.M., Baughman K.L., et al. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur. Heart J. 2010;31:1188–1196. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bouter S.L., Harchi A.E., Marionneau C., Bellocq C., Chambellan A., Veen T.v., Boixel C., Gavillet B., Abriel H., Quang K.L., et al. Long-Term Amiodarone Administration Remodels Expression of Ion Channel Transcripts in the Mouse Heart. Circulation. 2004;110:3028–3035. doi: 10.1161/01.CIR.0000147187.78162.AC. [DOI] [PubMed] [Google Scholar]
- 136.Ma L., Zhang X., Chen H. TWIK-1 two-pore domain potassium channels change ion selectivity and conduct inward leak sodium currents in hypokalemia. Sci. Signal. 2011;4:ra37. doi: 10.1126/scisignal.2001726. [DOI] [PubMed] [Google Scholar]
- 137.Plant L.D., Zuniga L., Araki D., Marks J.D., Goldstein S.A.N. SUMOylation Silences Heterodimeric TASK Potassium Channels Containing K2P1 Subunits in Cerebellar Granule Neurons. Sci. Signal. 2012;5:ra84. doi: 10.1126/scisignal.2003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hwang E.M., Kim E., Yarishkin O., Woo D.H., Han K.-S., Park N., Bae Y., Woo J., Kim D., Park M. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat. Commun. 2014;5:1–15. doi: 10.1038/ncomms4227. [DOI] [PubMed] [Google Scholar]
- 139.Choi J.H., Yarishkin O., Kim E., Bae Y., Kim A., Kim S.C., Ryoo K., Cho C.H., Hwang E.M., Park J.Y. TWIK-1/TASK-3 heterodimeric channels contribute to the neurotensin-mediated excitation of hippocampal dentate gyrus granule cells. Exp. Mol. Med. 2018;50:1–13. doi: 10.1038/s12276-018-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reyes R., Duprat F., Lesage F., Fink M., Salinas M., Farman N., Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- 141.Feliciangeli S., Chatelain F.C., Bichet D., Lesage F. The family of K2P channels: Salient structural and functional properties. J. Physiol. 2015;593:2587–2603. doi: 10.1113/jphysiol.2014.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maingret F., Patel A.J., Lesage F., Lazdunski M., Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 143.Patel A.J., Honoré E., Lesage F., Fink M., Romey G., Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 144.Patel A.J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levitz J., Royal P., Comoglio Y., Wdziekonski B., Schaub S., Clemens D.M., Isacoff E.Y., Sandoz G. Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc. Natl. Acad. Sci. USA. 2016;113:4194–4199. doi: 10.1073/pnas.1522459113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blin S., Ben Soussia I., Kim E.-J., Brau F., Kang D., Lesage F., Bichet D. Mixing and matching TREK/TRAAK subunits generate heterodimeric K2P channels with unique properties. Proc. Natl. Acad. Sci. USA. 2016;113:4200–4205. doi: 10.1073/pnas.1522748113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang W., Zhang M., Li P., Yuan H., Feng N., Peng Y., Wang L., Wang X. An increased TREK-1-like potassium current in ventricular myocytes during rat cardiac hypertrophy. J. Cardiovasc. Pharm. 2013;61:302–310. doi: 10.1097/FJC.0b013e318280c5a9. [DOI] [PubMed] [Google Scholar]
- 148.Kelly D., Mackenzie L., Hunter P., Smaill B., Saint D. Gene expression of stretch-activated channels and mechanoelectric feedback in the heart. Clin. Exp. Pharmacol. Physiol. 2006;33:642–648. doi: 10.1111/j.1440-1681.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- 149.Kennard L.E., Chumbley J.R., Ranatunga K.M., Armstrong S.J., Veale E.L., Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim E.J., Lee D.K., Hong S.G., Han J., Kang D. Activation of TREK-1, but Not TREK-2, Channel by Mood Stabilizers. Int. J. Mol. Sci. 2017;18:2460. doi: 10.3390/ijms18112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmidt C., Wiedmann F., Schweizer P.A., Becker R., Katus H.A., Thomas D. Class I antiarrhythmic drugs inhibit human cardiac two-pore-domain K(+) (K2 ₂p) channels. Eur. J. Pharm. 2013;721:237–248. doi: 10.1016/j.ejphar.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 152.Froese A., Breher S.S., Waldeyer C., Schindler R.F.R., Nikolaev V.O., Rinné S., Wischmeyer E., Schlueter J., Becher J., Simrick S., et al. Popeye domain containing proteins are essential for stress-mediated modulation of cardiac pacemaking in mice. J. Clin. Investig. 2012;122:1119–1130. doi: 10.1172/JCI59410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kirchmaier B.C., Poon K.L., Schwerte T., Huisken J., Winkler C., Jungblut B., Stainier D.Y., Brand T. The Popeye domain containing 2 (popdc2) gene in zebrafish is required for heart and skeletal muscle development. Dev. Biol. 2012;363:438–450. doi: 10.1016/j.ydbio.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Simrick S., Schindler R.F., Poon K.-L., Brand T. Popeye domain-containing proteins and stress-mediated modulation of cardiac pacemaking. Trends Cardiovasc. Med. 2013;23:257–263. doi: 10.1016/j.tcm.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schindler R.F., Scotton C., Zhang J., Passarelli C., Ortiz-Bonnin B., Simrick S., Schwerte T., Poon K.L., Fang M., Rinné S., et al. POPDC1(S201F) causes muscular dystrophy and arrhythmia by affecting protein trafficking. J. Clin. Invest. 2016;126:239–253. doi: 10.1172/JCI79562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Decher N., Kiper A.K., Rinné S. Stretch-activated potassium currents in the heart: Focus on TREK-1 and arrhythmias. Prog. Biophys. Mol. Biol. 2017;130:223–232. doi: 10.1016/j.pbiomolbio.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 157.Peyronnet R., Nerbonne J.M., Kohl P. Cardiac Mechano-Gated Ion Channels and Arrhythmias. Circ. Res. 2016;118:311–329. doi: 10.1161/CIRCRESAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Decher N., Ortiz-Bonnin B., Friedrich C., Schewe M., Kiper A.K., Rinné S., Seemann G., Peyronnet R., Zumhagen S., Bustos D., et al. Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol. Med. 2017;9:403–414. doi: 10.15252/emmm.201606690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abraham D.M., Lee T.E., Watson L.J., Mao L., Chandok G., Wang H.-G., Frangakis S., Pitt G.S., Shah S.H., Wolf M.J., et al. The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J. Clin. Investig. 2018;128:4843–4855. doi: 10.1172/JCI95945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seyler C., Schweizer P.A., Zitron E., Katus H.A., Thomas D. Vernakalant activates human cardiac K(2P)17.1 background K(+) channels. Biochem. Biophys. Res. Commun. 2014;451:415–420. doi: 10.1016/j.bbrc.2014.07.133. [DOI] [PubMed] [Google Scholar]
- 161.Gierten J., Ficker E., Bloehs R., Schlömer K., Kathöfer S., Scholz E., Zitron E., Kiesecker C., Bauer A., Becker R., et al. Regulation of two-pore-domain (K2P) potassium leak channels by the tyrosine kinase inhibitor genistein. Br. J. Pharm. 2008;154:1680–1690. doi: 10.1038/bjp.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wiedmann F., Beyersdorf C., Zhou X.-B., Kraft M., Foerster K.I., El-Battrawy I., Lang S., Borggrefe M., Haefeli W.E., Frey N. The Experimental TASK-1 Potassium Channel Inhibitor A293 Can Be Employed for Rhythm Control of Persistent Atrial Fibrillation in a Translational Large Animal Model. Front. Physiol. 2021;11:1869. doi: 10.3389/fphys.2020.629421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liang B., Soka M., Christensen A.H., Olesen M.S., Larsen A.P., Knop F.K., Wang F., Nielsen J.B., Andersen M.N., Humphreys D., et al. Genetic variation in the two-pore domain potassium channel, TASK-1, may contribute to an atrial substrate for arrhythmogenesis. J. Mol. Cell Cardiol. 2014;67:69–76. doi: 10.1016/j.yjmcc.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 164.Wirth K.J., Brendel J., Steinmeyer K., Linz D.K., Rütten H., Gögelein H. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J. Cardiovasc. Pharm. 2007;49:197–206. doi: 10.1097/FJC.0b013e318032002f. [DOI] [PubMed] [Google Scholar]
- 165.Knobloch K., Brendel J., Peukert S., Rosenstein B., Busch A.E., Wirth K.J. Electrophysiological and antiarrhythmic effects of the novel I(Kur) channel blockers, S9947 and S20951, on left vs. right pig atrium in vivo in comparison with the I(Kr) blockers dofetilide, azimilide, d,l-sotalol and ibutilide. Naunyn Schmiedebergs Arch. Pharm. 2002;366:482–487. doi: 10.1007/s00210-002-0599-x. [DOI] [PubMed] [Google Scholar]
- 166.Manichaikul A., Rich S.S., Allison M.A., Guagliardo N.A., Bayliss D.A., Carey R.M., Barrett P.Q. KCNK3 Variants Are Associated With Hyperaldosteronism and Hypertension. Hypertension. 2016;68:356–364. doi: 10.1161/HYPERTENSIONAHA.116.07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gerstin K.M., Gong D.H., Abdallah M., Winegar B.D., Eger E.I.I., Gray A.T. Mutation of KCNK5 or Kir3.2 Potassium Channels in Mice Does Not Change Minimum Alveolar Anesthetic Concentration. Anesth. Analg. 2003;96:1345–1349. doi: 10.1213/01.ANE.0000056921.15974.EC. [DOI] [PubMed] [Google Scholar]
- 168.Lloyd E.E., Crossland R.F., Phillips S.C., Marrelli S.P., Reddy A.K., Taffet G.E., Hartley C.J., Bryan R.M., Jr. Disruption of K(2P)6.1 produces vascular dysfunction and hypertension in mice. Hypertension. 2011;58:672–678. doi: 10.1161/HYPERTENSIONAHA.111.175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lloyd E.E., Pandit L.M., Crossland R.F., Marrelli S.P., Bryan R.M., Jr. Endothelium-dependent relaxations in the aorta from K(2p)6.1 knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R60–R67. doi: 10.1152/ajpregu.00126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pandit L.M., Lloyd E.E., Reynolds J.O., Lawrence W.S., Reynolds C., Wehrens X.H., Bryan R.M. TWIK-2 channel deficiency leads to pulmonary hypertension through a rho-kinase-mediated process. Hypertension. 2014;64:1260–1265. doi: 10.1161/HYPERTENSIONAHA.114.03406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yost C.S., Oh I., Eger E.I., 2nd, Sonner J.M. Knockout of the gene encoding the K(2P) channel KCNK7 does not alter volatile anesthetic sensitivity. Behav. Brain Res. 2008;193:192–196. doi: 10.1016/j.bbr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mirkovic K., Palmersheim J., Lesage F., Wickman K. Behavioral characterization of mice lacking Trek channels. Front. Behav. Neurosci. 2012;6:60. doi: 10.3389/fnbeh.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Niemeyer M.I., Cid L.P., Barros L.F., Sepúlveda F.V. Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J. Biol. Chem. 2001;276:43166–43174. doi: 10.1074/jbc.M107192200. [DOI] [PubMed] [Google Scholar]
- 174.Gray A.T., Zhao B.B., Kindler C.H., Winegar B.D., Mazurek M.J., Xu J., Chavez R.A., Forsayeth J.R., Yost C.S. Volatile anesthetics activate the human tandem pore domain baseline K+ channel KCNK5. Anesthesiology. 2000;92:1722–1730. doi: 10.1097/00000542-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 175.Lambert M., Capuano V., Boet A., Tesson L., Bertero T., Nakhleh M.K., Remy S., Anegon I., Pechoux C., Hautefort A., et al. Characterization of Kcnk3-Mutated Rat, a Novel Model of Pulmonary Hypertension. Circ. Res. 2019;125:678–695. doi: 10.1161/CIRCRESAHA.119.314793. [DOI] [PubMed] [Google Scholar]
- 176.Knobloch K., Brendel J., Rosenstein B., Bleich M., Busch A.E., Wirth K.J. Atrial-selective antiarrhythmic actions of novel Ikur vs. Ikr, Iks, and IKAch class Ic drugs and beta blockers in pigs. Med. Sci. Monit. 2004;10:Br221–Br228. [PubMed] [Google Scholar]
- 177.Peyronnet R., Ravens U. Atria-selective antiarrhythmic drugs in need of alliance partners. Pharm. Res. 2019;145:104262. doi: 10.1016/j.phrs.2019.104262. [DOI] [PubMed] [Google Scholar]
- 178.Olschewski A., Li Y., Tang B., Hanze J.R., Eul B., Bohle R.M., Wilhelm J., Morty R.E., Brau M.E., Weir E.K. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ. Res. 2006;98:1072–1080. doi: 10.1161/01.RES.0000219677.12988.e9. [DOI] [PubMed] [Google Scholar]
- 179.Ma L., Roman-Campos D., Austin E.D., Eyries M., Sampson K.S., Soubrier F., Germain M., Trégouët D.A., Borczuk A., Rosenzweig E.B., et al. A novel channelopathy in pulmonary arterial hypertension. N. Engl. J. Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Davies L.A., Hu C., Guagliardo N.A., Sen N., Chen X., Talley E.M., Carey R.M., Bayliss D.A., Barrett P.Q. TASK channel deletion in mice causes primary hyperaldosteronism. Proc. Natl. Acad. Sci. USA. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.West J.D., Austin E.D., Rizzi E.M., Yan L., Tanjore H., Crabtree A.L., Moore C.S., Muthian G., Carrier E.J., Jacobson D.A., et al. KCNK3 Mutation Causes Altered Immune Function in Pulmonary Arterial Hypertension Patients and Mouse Models. Int. J. Mol. Sci. 2021;22:5014. doi: 10.3390/ijms22095014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Heurteaux C., Guy N., Laigle C., Blondeau N., Duprat F., Mazzuca M., Lang-Lazdunski L., Widmann C., Zanzouri M., Romey G., et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Laigle C., Confort-Gouny S., Le Fur Y., Cozzone P.J., Viola A. Deletion of TRAAK potassium channel affects brain metabolism and protects against ischemia. PLoS ONE. 2012;7:e53266. doi: 10.1371/journal.pone.0053266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kindler C.H., Paul M., Zou H., Liu C., Winegar B.D., Gray A.T., Yost C.S. Amide local anesthetics potently inhibit the human tandem pore domain background K+ channel TASK-2 (KCNK5) J. Pharm. Exp. 2003;306:84–92. doi: 10.1124/jpet.103.049809. [DOI] [PubMed] [Google Scholar]
- 185.Gönczi M., Szentandrássy N., Johnson I.T., Heagerty A.M., Weston A.H. Investigation of the role of TASK-2 channels in rat pulmonary arteries; pharmacological and functional studies following RNA interference procedures. Br. J. Pharmacol. 2006;147:496–505. doi: 10.1038/sj.bjp.0706649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Winsvold B.S., Bettella F., Witoelar A., Anttila V., Gormley P., Kurth T., Terwindt G.M., Freilinger T.M., Frei O., Shadrin A., et al. Shared genetic risk between migraine and coronary artery disease: A genome-wide analysis of common variants. PLoS ONE. 2017;12:e0185663. doi: 10.1371/journal.pone.0185663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shvetsova A.A., Gaynullina D.K., Schmidt N., Bugert P., Lukoshkova E.V., Tarasova O.S., Schubert R. TASK-1 channel blockade by AVE1231 increases vasocontractile responses and BP in 1- to 2-week-old but not adult rats. Br. J. Pharm. 2020;177:5148–5162. doi: 10.1111/bph.15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bobak N., Feliciangeli S., Chen C.C., Ben Soussia I., Bittner S., Pagnotta S., Ruck T., Biel M., Wahl-Schott C., Grimm C., et al. Recombinant tandem of pore-domains in a Weakly Inward rectifying K(+) channel 2 (TWIK2) forms active lysosomal channels. Sci. Rep. 2017;7:649. doi: 10.1038/s41598-017-00640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Salinas M., Reyes R., Lesage F., Fosset M., Heurteaux C., Romey G., Lazdunski M. Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J. Biol. Chem. 1999;274:11751–11760. doi: 10.1074/jbc.274.17.11751. [DOI] [PubMed] [Google Scholar]
- 190.Berg A.P., Talley E.M., Manger J.P., Bayliss D.A. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J. Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim D., Cavanaugh E.J., Kim I., Carroll J.L. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J. Physiol. 2009;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zadeh N., Graham J.M., Jr. KCNK9 Imprinting Syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Mirzaa G., Amemiya A., editors. GeneReviews(®) University of Washington; Seattle, WA, USA: 2017. [PubMed] [Google Scholar]
- 193.Yost C.S. A new look at the respiratory stimulant doxapram. CNS Drug Rev. 2006;12:236–249. doi: 10.1111/j.1527-3458.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chatelain F.C., Bichet D., Feliciangeli S., Larroque M.M., Braud V.M., Douguet D., Lesage F. Silencing of the tandem pore domain halothane-inhibited K+ channel 2 (THIK2) relies on combined intracellular retention and low intrinsic activity at the plasma membrane. J. Biol. Chem. 2013;288:35081–35092. doi: 10.1074/jbc.M113.503318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Graff S.M., Johnson S.R., Leo P.J., Dadi P.K., Dickerson M.T., Nakhe A.Y., McInerney-Leo A.M., Marshall M., Zaborska K.E., Schaub C.M., et al. A KCNK16 mutation causing TALK-1 gain of function is associated with maturity-onset diabetes of the young. JCI Insight. 2021;6:e138057. doi: 10.1172/jci.insight.138057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Suzuki Y., Tsutsumi K., Miyamoto T., Yamamura H., Imaizumi Y. Heterodimerization of two pore domain K+ channel TASK1 and TALK2 in living heterologous expression systems. PLoS ONE. 2017;12:e0186252. doi: 10.1371/journal.pone.0186252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Domingues-Montanari S., Fernández-Cadenas I., Del Río-Espinola A., Mendioroz M., Fernandez-Morales J., Corbeto N., Delgado P., Ribó M., Rubiera M., Obach V., et al. KCNK17 genetic variants in ischemic stroke. Atherosclerosis. 2010;208:203–209. doi: 10.1016/j.atherosclerosis.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 198.Grabauskas G., Wu X., Zhou S., Li J., Gao J., Owyang C. High-fat diet-induced vagal afferent dysfunction via upregulation of 2-pore domain potassium TRESK channel. JCI Insight. 2019;4:e130402. doi: 10.1172/jci.insight.130402. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 199.Park H., Kim E.J., Ryu J.H., Lee D.K., Hong S.G., Han J., Han J., Kang D. Verapamil Inhibits TRESK (K(2P)18.1) Current in Trigeminal Ganglion Neurons Independently of the Blockade of Ca(2+) Influx. Int. J. Mol. Sci. 2018;19:1961. doi: 10.3390/ijms19071961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Enyedi P., Czirják G. Properties, regulation, pharmacology, and functions of the K2P channel, TRESK. Pflügers Arch. Eur. J. Physiol. 2015;467:945–958. doi: 10.1007/s00424-014-1634-8. [DOI] [PubMed] [Google Scholar]