Abstract
Introducing therapeutic ions into pulp capping materials has been considered a new approach for enhancing regeneration of dental tissues. However, no studies have been reported on its dentinogenic effects on human dental pulp cells (HDPCs). This study was designed to investigate the effects of magnesium (Mg2+) on cell attachment efficiency, proliferation, differentiation, and mineralization of HDPCs. HDPCs were cultured with 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM concentrations of supplemental Mg2+ and 0 mM (control). Cell attachment was measured at 4, 8, 12, 16, and 20 hours. Cell proliferation rate was evaluated at 3, 7, 10, 14, and 21 days. Crystal violet staining was used to determine cell attachment and proliferation rate. Alkaline phosphatase (ALP) activity was assessed using the fluorometric assay at 7, 10, and 14 days. Mineralization of cultures was measured by Alizarin red staining. Statistical analysis was done using multiway analysis of variance (multiway ANOVA) with Wilks' lambda test. Higher cell attachment was shown with 0.5 mM and 1 mM at 16 hours compared to control (P < 0.0001). Cells with 0.5 mM and 1 mM supplemental Mg2+ showed significantly higher proliferation rates than control at 7, 10, 14, and 21 days (P < 0.0001). However, cell proliferation rates decreased significantly with 4 mM and 8 mM supplemental Mg2+ at 14 and 21 days (P < 0.0001). Significantly higher levels of ALP activity and mineralization were observed in 0.5 mM, 1 mM, and 2 mM supplemental Mg2+ at 10 and 14 days (P < 0.0001). However, 8 mM supplemental Mg2+ showed lower ALP activity compared to control at 14 days (P < 0.0001), while 4 mM and 8 mM supplemental Mg2+showed less mineralization compared to control (P < 0.0001). The study indicated that the optimal (0.5–2 mM) supplemental Mg2+ concentrations significantly upregulated HDPCs by enhancing cell attachment, proliferation rate, ALP activity, and mineralization. Magnesium-containing biomaterials could be considered for a future novel dental pulp-capping additive in regenerative endodontics.
1. Introduction
More than two-thirds of the global population suffer from tooth decay, which results in cavities with various levels of lesion severity [1–3]. Although surface decay can usually be treated and arrested with a filling, a tooth with deep decay or one that has been severely injured in an accident, or the trauma of recurrent dental work may become unhealthy, inflamed, or infected. Left untreated, an infection may spread to the surrounding tissues [4]. The currently available treatment scenarios in such serious conditions are either vital pulp therapy if the pulp is still vital or root canal treatment if the pulp is irreversibly inflamed. Vital pulp therapy aims to induce dentinal bridge formation with scarred tissue to maintain pulp integrity and function. This mechanism of tissue repair is based on replacing damaged odontoblasts by newly regenerated populations of odontoblast-like cells derived from pulp stem cells in the healthy portion of the pulp [5, 6].
To date, calcium hydroxide (CH), mineral trioxide aggregate (MTA), and tricalcium silicate cement (Biodentine) are the pulp-capping biomaterials of choice mostly used in clinics [7]. Historically, calcium hydroxide (CH) has been considered the gold standard in recent decades [8]. However, CH has many disadvantages, such as high solubility and multiple tunnel defects in the induced dentin bridge [9], which allow the pulp to become infected or necrotic over time [10, 11]. The first calcium silicate cement-based material, mineral trioxide aggregate (MTA), is used in direct and indirect pulp capping in primary and permanent teeth [12, 13]. MTA has been reported superior to calcium hydroxide for pulp capping of mechanically exposed human teeth [14–16]. However, MTA exhibits many drawbacks such as difficult handling, long setting time [17], induction of tooth discoloration, and incompatibility with other dental materials when layered [18]. Therefore, a new formulation named Biodentine (Septodont, Saint Maur, France) has been launched [19, 20], with physicomechanical properties superior to those of MTA and similar to those of dentin, easier handling and shorter setting time than MTA [21], and positive effects of biodentine stimulating cell differentiation and promoting mineralization in human dental pulp cells [22, 23]. However, the main drawback is its water-based chemistry, and thus, poor bonding as the bond is mainly micromechanical to the overlying resin restoration [24]. Regardless of which materials used, however, there are notable limitations in the current biomaterials‐based strategies used for pulp capping and dentin regeneration. Some of the most significant limitations are the severe inflammatory reactions induced by the synthetic-capping materials that can cause failure. Besides, formation of the dentinal bridge can occur within the teeth with irreversible inflammation, which requires comprehensive retreatment [25, 26]. These limitations mainly derive from the fact that current biomaterials used in the clinics for healing dentin‐pulp tissues lack specific temporal and spatial control over biologic signaling required for progenitor cells' homing and differentiation to eventually fully restore structural and functional characteristics of the tissue. Moreover, the mechanistic understanding of the regenerative outcomes attained using current pulp-capping biomaterials is for the most part missing. Finally, current biomaterials are notably limited to control infection and inflammation for promoting reparative tissue formation [27].
Critical need exists to develop a novel therapy that induces wound healing and dentinogenesis similar to the natural process. The development of pulp-capping agents has been instrumental in promoting reparative dentin formation. Incorporation of Mg2+ into pulp-capping materials allowing the release of therapeutic Mg2+ions has been considered as a new approach for enhancing regeneration of dental tissues. Therapeutic ions induce cellular signaling, triggering resident pulp cells to differentiate into odontoblast-like cells, stimulation of dentin matrix secretion, and formation of tertiary dentin. Mg2+acts as an intracellular second messenger connecting cell-surface receptor induction and cytosolic effectors [28]. Mg2+ is the fourth most abundant cation in the human body and is critical for ATP-dependent phosphorylation of DNA, RNA, and enzymes. Mg2+ is present (0.5%) in the tooth enamel outer layer, and the average magnesium concentration is 1% (w/w) in dentin [29–31]. Mg2+ is involved in the biomineralization of bones and teeth and directly affects crystallization and pattern generation of the inorganic mineral phase [31–33]. Emerging evidence supports a notion that Mg2+ plays indispensable bioactive roles and not only induces bone formation and matrix mineralization but also in transduction of the intracellular signaling pathway that are involved in maintaining and regulating normal biological processes [34]. Mutation of Mg2+ transporters CNNM4 and TRPM7 resulted in mineralization defects of dentin, indicating that a disrupted Mg2+ transport was involved in the development of the dental abnormalities. Animals fed on low Mg2+ diets show deficient dentin and enamel mineralization [35].
This is the first report exploring Mg2+ application and its dentinogenic effects on normal human dental pulp cells. The mechanism behind magnesium relationship to cell behavior is intriguing largely unexplored. Thus, our objective in this unique study was to investigate the effect of different concentrations of supplemented Mg2+ on human dental pulp cells, looking specifically at cellular proliferation, differentiation, and mineralization which may contribute to a better understanding of the influence of magnesium on pulp regeneration. Its potential application in clinical practice as a promising material for clinical pulp regenerative therapy is to establish an optimal concentration that could ultimately be used to develop a new pulp-capping material.
2. Materials and Methods
2.1. Magnesium Chloride Preparation
Magnesium chloride hexahydrate (Fisher Chemical, USA) was dissolved in deionized water, and five stock solutions were prepared at concentrations of 5 mM, 10 mM, 20 mM, 40 mM, and 80 mM, respectively. Each concentration was subsequently filtered under sterile condition in the biological hood.
2.2. Human Dental Pulp Cells (HDPCs) Culture
This study was approved by Boston University IRB Committee. Human dental pulp explants were collected from young and systemically heathy patients between the age group of 15 and 25 years, requiring third molar or orthodontic premolar extractions. Patients were given the informed consent before the extraction procedure at the oral surgery clinic at Boston University. Human dental pulp cells (HDPCs) were isolated following a previously published protocol with modifications [36]. Teeth were sectioned with a #7 chandler bibevel bone chisel until the pulp tissue in the pulp chamber was exposed. The pulp pieces were removed with sterile instruments and placed immediately into a 12.5 cm2 culture flask (Thermo Fisher Scientific, USA). Culture medium consisted of 10% fetal bovine serum (FBS) (R&D Systems), 1X penicillin antibiotic (100 U/mL), 1X streptomycin (100 μg/mL) (Gibco), and amphotericin B antifungal (0.25 μg/ml) in Eagle's basal medium (BME) (Gibco). All tissues were maintained at 37°C, in a standard 5% CO2 incubator (Thermo Fisher Scientific, USA) and saturated humidity and cultured up to the second passage. Growth media was changed every 72 hours. Nearly, confluent cells were trypsinized with 0.05% trypsin (Gibco). Resuspended cells were then aspirated and collected in a sterile 15 mL disposable tube placed in the TJ-6 Beckman centrifuge at 1000 rpm for 5 minutes. After centrifugation, a pellet of cells was formed. The cells were then counted using a hemocytometer. Characterization of dentinogenic phenotype of the cells was confirmed by expression of dentinogenic markers induced by vit D3 stimulation. Human dental pulp cells were transferred to 24-well plates (Thermo Fisher Scientific, USA) and grown in the culture medium supplemented with 0 mM (control), 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM supplemental Mg2+ concentrations, respectively. For differentiation and mineralization studies, growth media were replaced with preinductive dentinogenic media at the following time intervals: 4, 7, and 11 days. Dentinogenic media consist of the following: 10% charcoal-stripped fetal bovine serum (FBS) (Life Technologies), 100 IU/ml penicillin (Gibco), 100 μg/m streptomycin (Gibco), 10−8 M menadione (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), 0.05 mg/mL L-ascorbic acid (Sigma-Aldrich), and 2 mM L-glutamine (Gibco). The next day, cells were cultured in preinductive dentinogenic media with the addition of 10 nM vitamin D3 (172 g/mol) (Sigma) for two additional days. Supernatant fluid was collected on days 7, 10, and 14. Alkaline phosphatase (ALP) activity was measured in the collected supernatants. The remaining fixed cells on the culture plates were used to perform the mineralization assay.
2.3. Determination of Human Dental Pulp Cells (HDPCs) Attachment Efficiency and Proliferation Assays
Two hundred thousand (2 × 105) normal human dental pulp cells were seeded in 24-well plates containing 1 mL media with 0 mM, 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM supplemental Mg2+ concentrations for 16 hours. Each condition was performed in six replicas. Supernatants were collected in 1.5 mL disposable tubes. Preexperiments were performed to determine the baseline of cell attachment at 16 hours. After 16 hours, the medium was discarded, and the wells were washed 3 times with phosphate-buffered saline (PBS) (Gibco). Thereafter, the cells were fixed by adding 500 μL of 10% neutral buffered formalin (Sigma) for 1 hour at room temperature. The cells were then stained by adding 500 μL of 0.2% crystal violet stain (Sigma-Aldrich) for another hour. Afterwards, all wells were washed 3 times using PBS to remove any unbound stains. The optical density of the stained cells was measured by the spectrophotometer (TECAN, Infinite 200 Pro) at wavelength 590 nm. The optical density is directly proportional to the cell numbers. Cell attachment efficiency was quantified through direct cell counts and normalized to the initial cell seeding density. Cell proliferation was monitored at the following time points: 16 hours, 3 days, 7 days, 10 days, 14 days, and 21 days. Three thousand normal human dental pulp cells were seeded in 24-well plates containing 1 mL of growth medium for the control group (0 mM) and various concentrations of supplemental Mg2+ 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM, respectively. Each condition has been repeated in six replicas. The culture plates were incubated under 37°C, 5% CO2, and growth media were changed every 3 days. At each predetermined time point, 0.2% crystal violate dye was used to stain the attached cells in the 24-well plates. Absorbance of crystal violet was measured using the spectrophotometer at 590 nm wavelength. The optical densities at each point of time were compared to the optical density at 16 hours as a baseline to determine the proliferation rates.
2.4. Measurement of Alkaline Phosphatase (ALP) Differentiation Marker Activity
An alkaline phosphatase (ALP) fluorometric assay kit (Abcam) was used to measure alkaline phosphatase activity in the cell culture supernatants. Supernatants at days 7, 10, and 14 were utilized. ALP activity was measured according to the manufacturer's instructions. 100 μL (10x diluted) culture supernatants were incubated with 20 μL of the nonfluorescent 4- methylumbelliferone phosphate disodium salt (MUP substrate) in a 96-well black plate with clear bottom (Thermo Scientific); MUP was converted into fluorescent 4-methylumbelliferone (4-MU) when dephospholated by ALP. The plate was incubated for 30 minutes at 25°C protected from light. The reaction was then terminated by 20 μL stop solution, which was added to all wells, with the exception of the blank control, and the plate was gently shaken. The emission of the fluorescent 4-MU was measured at 440 nm by excitation at 360 nm on the spectrophotometer. ALP activities were calculated by a standard curve and normalized to ALP activity on a per million cell base formula.
2.5. Detection of Human Dental Pulp Cells (HDPCs) Mineralization
Mineralization was examined through accumulation of calcium deposition using Alizarin red S staining. The attached cells in the 24-well plates at days 7, 10, and 14 were used. The crystal violet stain from the plates was removed by adding Triton-X 1% (v/v) (900 μL). The 24-well plates were placed on a shaker for 30 min at room temperature. The plates were washed cautiously four times with 1 ml deionized water to the point where colorless. Later, 1 mL of 40 mM Alizarin red S (Sigma) pH 4.3 solution was added to the plates for calcium staining. Cells were incubated at room temperature in the dark for 45 min. Then, Alizarin Red S staining was carefully aspirated, and the plates were washed four times with 1 ml distilled water until clear. Finally, absorbance was measured by the spectrophotometer at a wavelength of 405 nm. Each condition was normalized to a per million cell base formula.
2.6. Statistical Analysis
All experiments were performed in six replicates and repeated three times. Data are presented in means and standard deviations. The means and standard deviations (SD) of human dental pulp cell attachment efficiency and proliferation rates at 16 hours and 3, 7, 10, 14, and 21 days were calculated, in addition to levels of osteogenic differentiation marker (alkaline phosphatase) and mineralization at 7, 10, and 14 days. Differentiation and mineralization data were normalized on a per million cells basis at 7, 10, and 14 days. Statistical analysis was performed using software JMP Pro 13 (ver. 13.1.0). Multiway analysis of variance (multiway ANOVA) with Wilks' lambda test is used for statistical analysis between the groups. Differences at P ≤ 0.05 were considered statistically significant.
3. Results
3.1. Effect of Supplemental Mg2+ on Cell Attachment Efficiency of HDPCs at Various Concentrations
Adherence of HDPCs occurred within a few hours after cells were seeded. Cell attachments on the tissue culture plates were significantly greater in Mg2+ concentrations among certain groups (P ≤ 0.0001) after 16 hours (Figure 1). The data showed a significant increase in cell attachment for the 0.5 mM and 1 mM supplemental Mg2+concentrations compared to the negative control. Meanwhile, there was no significant difference in cell attachment between the 2 mm Mg2+ concentration compared to the negative control. However, for the 4 mM and 8 mM Mg2+concentrations, cell attachment was significantly lower than that of the negative control (P < 0.0001) (Figure 1).
Figure 1.
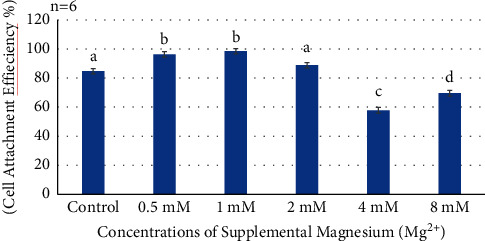
Histogram showing cell attachment efficiency at 16 hours of all supplemental Mg2+ concentrations. Note: normal human dental pulp cells were cultured for 16 hours with media containing supplemental Mg2+ concentrations 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM and 0 mM as the control group. The data are presented as means of six replicates with error bars indicating the standard deviation. Groups labeled with different letters differ statistically as compared to the control group and other study groups (P < 0.0001).
3.2. Effect of Mg2+ on Proliferation Rate of HDPCs at Various Concentrations
The proliferation rate of HDPCs at both 7 and 10 days showed a significant cell number increase in supplemental Mg2+ concentration groups 0.5 mM, 1 mM, and 2 mM compared to the negative control (P < 0.0001) (Figure 2). However, the 4 mM and 8 mM concentrations behaved similar to the control cells grown on culture media without supplements. Notably, higher supplemental Mg2+ concentrations resulted in a significant downregulation as shown in Figure 2. Wilks' lambda interaction P value for supplemental Mg2+ concentrations and their proliferation rate at 7 and 10 days showed a statistically significant value (P < 0.0001). At 14 days, the supplemental Mg2+ concentrations 0.5 mM, 1 mM, and 2 mM showed enhanced proliferation compared to the negative control (P < 0.0001). While, the 4 mM and 8 mM tested concentrations containing higher Mg2+significantly showed decreased growth (P < 0.0001). Similarly, Wilks' lambda interaction P value for these concentrations at 14 days showed a highly significant value (P < 0.0001). At 21 days, the low Mg2+ concentrations 0.5 mM, 1 mM, and 2 mM showed a significant increased proliferation rate (P < 0.001). However, the higher Mg2+ 4 mM and 8 mM concentrations significantly reduced proliferation (P < 0.0001). Wilks' lambda interaction P value for these concentrations at 21 days was highly significant (P < 0.0001).
Figure 2.
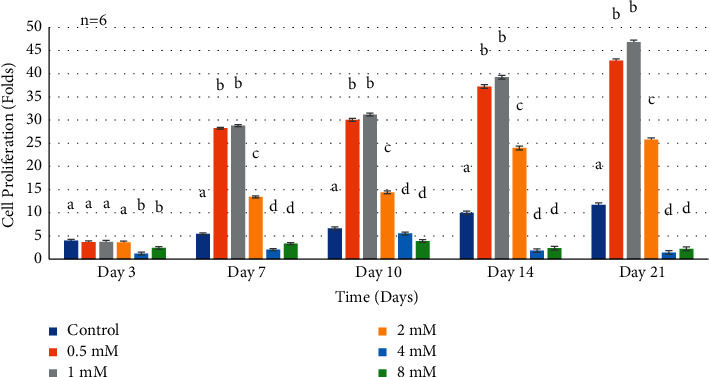
Histogram showing proliferation rates of normal human dental pulp cells in media with supplemental Mg2+ concentrations. Note: normal human dental pulp cells were cultured with media containing supplemental Mg2+concentrations 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM and 0 mM as the control group for time periods of 3, 7, 10, 14, and 21 days. Folds were calculated by dividing the cell numbers at each time interval by the seeded cell number for each condition. Cell number was calculated by using optical density measurement results dividing the constant number (per million cells' optical density). The data are presented as means of six replicates with error bars indicating the standard deviation. Groups labeled with different letters differ statistically as compared to the control group and other study groups (P < 0.0001).
3.3. Effect of Mg2+ on Proliferation Rate of HDPCs at Various Concentrations (after Addition of Preinductive-Dentinogenic Media)
At 7 days, HDPCs proliferated to a significantly higher degree in supplemental Mg2+ concentrations 0.5 mM, 1 mM, and 2 mM compared to the negative control (P < 0.0001) (Figure 3). On the other hand, the remaining two higher Mg2+ concentrations 4 and 8 mM did not proliferate the rates at day 10 but rather behaved similar to the negative control. Higher Mg2+ concentrations resulted in a significant downregulation as shown in Figure 3. Wilks' lambda interaction P value for Mg2+ concentrations and their proliferation at this time interval showed a statistically significant value (P < 0.0001). At 10 days, Mg2+ concentrations 0.5 mM, 1 mM, and 2 mM showed significantly increased proliferation rates (Figure 3) (P < 0.0001). While, 4 mM and 8 mM Mg2+concentrations revealed proliferation rates of HDPCs similar to that of the negative control (Figure 3). Wilks' lambda interaction P value at this time interval presented statistically significant values (P < 0.0001). The data at day 14 presented an enhanced proliferation (Figure 3) in the 0.5 mM, 1 mM, and 2 mM Mg2+concentrations (P < 0.0001) However, higher Mg2+concentrations 4 mM and 8 mM showed decreased proliferation rates in comparison to the negative control, with Wilks' lambda P value at this time point showing a highly statistically significant value (P < 0.0001).
Figure 3.
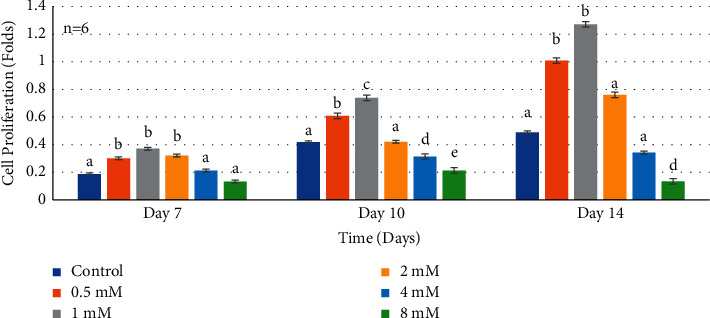
Histogram showing proliferation rates of normal human dental pulp cells in media with supplemental Mg2+ concentrations. Note: normal human dental pulp cells were cultured with media containing supplemental Mg2+concentrations 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM and 0 mM as the control group after addition of preinductive dentinogenic media for time periods of 7, 10, and 14 days. The data are presented as means of six replicates with error bars indicating the standard deviation. Groups labeled with different letters differ statistically as compared to the control group and other study groups (P < 0.0001).
3.4. Effect of Mg2+ on Alkaline Phosphatase Activity of HDPCs at Various Concentrations
Alkaline phosphatase activity observed at day 7 (Figure 4) for most groups was comparable to the negative control with a statistically significant difference. The Mg2+concentrations 0.5 mM, 1 mM, and 2 mM showed high statistically significant increase in ALP activity of HDPCs compared to the negative control (P < 0.0001). However, marked significant increase in enzymatic activity was noted with the 1 mM Mg2+concentration compared to the negative control and the other concentrations, with Wilks' lambda interaction P value for Mg2+concentrations and ALP activity at day 7 showing a statistically significant value (P < 0.0001). At day 10 (Figure 4), the 1 mM supplemental Mg2+ concentration reached a higher statistically significant value. However, cells grown in media containing 8 mM Mg2+supplement showed statistically lower ALP activity compared to the negative control. Wilks' lambda interaction P value for Mg2+concentrations and ALP activity at the 10th day timepoint showed a marked significant value (P < 0.0001). At day 14 (Figure 4), the 1 mM supplemented Mg2+concentration reached the highest value compared to the negative control. Data revealed that Mg2+concentrations in the range 0.5–2 mM have the highest effect on ALP activity. Meanwhile, cells grown in media containing higher Mg2+supplements (8 mM) showed lower ALP activity compared to the negative control. Wilks' lambda interaction P value for Mg2+concentrations and ALP activity at the 14th day timepoint showed the same statistically significant value (P < 0.0001).
Figure 4.
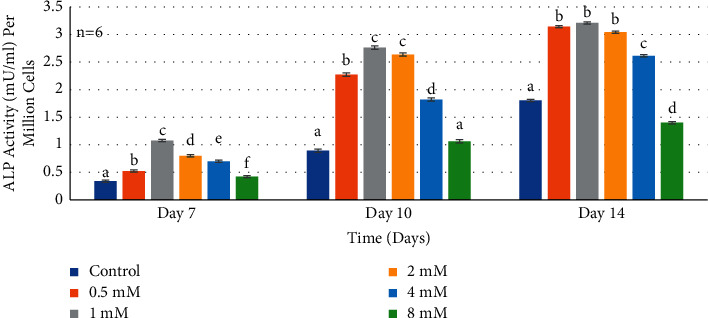
Histogram showing alkaline phosphatase activity of normal human dental pulp cells in media with supplemental Mg2+concentrations. Note: normal human dental pulp cells were cultured with media containing supplemental magnesium concentrations 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM and 0 mM as the control group for time periods of 7, 10, and 14 days. Alkaline phosphatase activity in supernatants was normalized per million cells at each time interval. The control cells were treated with growth media without supplemental Mg2+. The data are presented as means of six replicates with error bars indicating the standard deviation. Groups labeled with different letters differ statistically as compared to the control group and other study groups (P < 0.0001).
3.5. Effect of Mg2+ on Mineralization Rate of HDPCs at Various Concentrations
At day 7 (Figure 5), most Mg2+concentrations showed a statistically significant increase in the mineralization rate comparable to the negative control (P < 0.0001). Wilks' lambda interaction P value for Mg2+ concentrations and mineralization rate at day 7 presented a value of a highly statistical significance (P < 0.0001). However, at day 10, data from the Mg2+ concentrations 0.5–2 mM presented higher mineralization rates when compared to the negative control (Figure 5). As for the 1 mM Mg2+ concentration, there was a marked increase in value compared to the other Mg2+ concentrations and the negative control (P < 0.0001). Wilks' lambda interaction P value for Mg2+ concentrations and mineralization rate at day 10 pointed out a marked significant value (P < 0.0001). Similarly, the increase in mineralization rate could also be observed at day 14 (Figure 5), as Mg2+ concentration groups ranging 0.5–2 mM showed higher mineralization comparable to the negative control and the other concentrations (P < 0.0001). While, the 4 mM and 8 mM Mg2+concentrations showed a decreased mineralization rate of a statistical significance compared to the other experimental groups (P < 0.0001). Regarding, Wilks' lambda interaction P value, for Mg2+ concentrations and mineralization rate at day 14, a markedly significant value was also noted (P < 0.0001).
Figure 5.
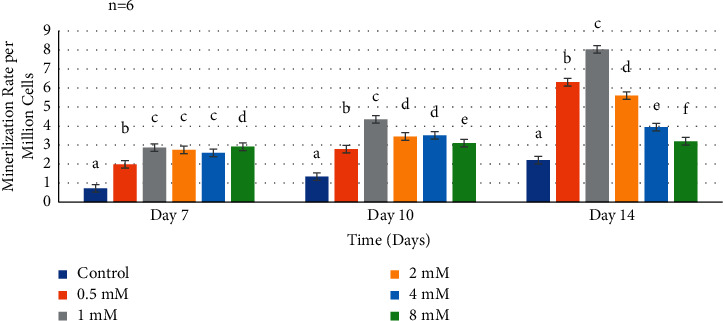
Mineralization rate of human dental pulp cells in media with supplemental Mg2+ concentrations. Normal human dental pulp cells were cultured with media containing supplemental Mg2+concentrations 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM and 0 mM as the control group for time periods of 7, 10, and 14 days. Mineralization of fixed cell samples was measured by spectroscopic analysis at 405 nm and normalized per million cells at each time interval. The control cells were treated with growth media without supplemental Mg2+. The data are presented as means of six replicates with error bars indicating the standard deviation. Groups labeled with different letters differ statistically as compared to the control group and other study groups (P < 0.0001).
4. Discussion
The vitality of the dental pulp has always been the goal for a successful long-term restorative dental treatment. In situations of deep cavitation or trauma that involves dental pulp exposure, a cascade of stem cell activation, proliferation, and differentiation into new odontoblast-like cells will culminate into reparative dentine secretion [37]. The response of the pulp to direct capping involves dentinogenesis resulting from the recruitment of odontoblasts and/or proliferation of undifferentiated mesenchymal cells [38]. By the creation of biological sealing for exposed dental pulp, the connection between dental pulp and the oral environment is eliminated, and the entry of pathogens into the dental pulp is prevented. It has been postulated that a network of interactions between extracellular matrix molecules [39, 40] and growth factors regulates odontoblast-like cell differentiation and reparative dentinogenesis in the dental pulp [41]. In spite of a wide research made in the field of pulp physiology, there is no single gold standard regimen for pulp capping materials that can achieve reliable and predictable goals preserving tooth vitality. The identification and development of appropriate biomaterials for dental pulp capping are necessary to optimize clinical approaches to dentinogenesis. Likewise, a better understanding of the interactions between the microenvironment, growth factors, and human dental pulp cells will provide design basis to fulfill this purpose. The introduction of bioactive Mg2+ ions might explore the potential to overcome challenges of dentinogenesis. Therefore, the aim of this present study was to test and determine the effects of different concentrations of supplemental Mg2+ on HDPCs in terms of cell attachment, proliferation, differentiation, and mineralization in order to establish an optimal concentration to develop a new pulp-capping material.
HDPCs are a promising cell source for dental tissue regeneration due to their ability to differentiate into odontoblast-like cells in vitro and to form dentin-pulp structures in vivo when seeded on scaffolds [42]. Most of the studies investigating the growth effects of Mg2+ were performed in cell lines, transformed cells, or highly passaged and induced pluripotent stem cells [43–48]. In this study, the explant outgrowth technique was used leading to the isolation of the HDPCs from pulp tissue on the basis of their morphological features including the fibroblast-like cells and the stellate types. For the present work, HDPCs were isolated from the pulp tissue of extracted human third molar teeth. Several studies revealed that osteogenic cells surrounding Mg-containing biomaterials showed enhanced osteogenic activities [49, 50]. It has been shown from in vitro cell line and human cell studies, as well as in vivo studies that additional Mg2+ increases bone density and bone absorption parameters [51–61]. It has been reported that the osteogenic phenotypes of osteosarcoma cell lines are significantly different from normal human osteoblast cell lines [49, 53, 59, 62–66]. Osteosarcoma cells proliferate significantly more rapidly than normal human osteoblast cells [67–69], and inversely, the differentiation rate is significantly greater in normal human osteoblasts than in any osteosarcoma cell lines tested [70–72]. Franks et al. [73] observed upregulation of cell proliferation on ATCC human osteosarcoma cell line MG-63 by increasing MgO concentration over 7 mol% in the bioglass system. In a study by Burmester et al. [74], 0.02 g/ml Mg-based extracts were used, and the results showed that the proliferation rates on the ATCC MG-63 and U20S cell lines were normal, but on ATCC human, Sa0S-2 cell line was downregulated. Moreover, Park et al. [75] found that Mg2+ and magnesium-hydroxyapatite (Mg-HA) coatings on titanium improved ALP activity in MC3T3-E1 cell line. Lü et al. [65] claimed that up to 70 mM extracellular supplemented Mg2+ originated from MgCl2 could upregulate proliferation of normal rabbit osteoblasts and reached the best result at 30 mM. Yang et al. [76] reported adult human bone marrow-derived stromal cells (hBMS) cultured in extracts of magnesium. The results indicated that 10 mm concentration of Mg+2 did not inhibit the viability and osteogenic differentiation of hBMS cells. Lu et al. [77] tested the effect of supplemented MgCl2 concentrations (0.5 mM–16 mM) on the osteogenic behaviors of normal human primary osteoblasts. They demonstrated the impact of 2 mM Mg2+on induced proliferation and differentiation of normal human osteoblasts. It is evident that Mg2+ has an influence on cell attachment, proliferation, and differentiation activity of osteoblasts, stimulating expression of growth factors and early osteogenic markers, inducing bone formation.
Incorporating Mg2+ ions into calcium phosphate cement (CPC) was reported previously. In vitro studies showed that the release of Mg2+ ions in CPC cement promoted the proliferation and differentiation of human bone marrow stem cells and enhanced the activity of osteoblast differentiation [78, 79]. One of such approaches was biomimetic nanostructured nanofibrous gelatin magnesium phosphate scaffolds (NF-gelatin/MgP) by Qu et al. [80]. This hybrid scaffold not only physically and chemically mimicked the dentin matrix but also provided sustained Mg ion release from the matrix, thus creating favorable physical and chemical cues to guide dental stem/progenitor cell growth and differentiation. In vitro and in vivo studies showed that the NF-gelatin/MgP hybrid scaffold significantly enhanced human DPSC proliferation, differentiation, and new dentin formation compared to the NF-gelatin control. The abovementioned studies emphasized that magnesium containing scaffolds are ideal tools for regenerative endodontic therapy, releasing high extracellular magnesium to enhance dentin regeneration in stem cells. However, to date, the exploration of Mg-pulp capping biomaterials for dental tissue regeneration has not been attempted. This study could be the first report on dentinogenic stimulation of HDPCs by the optimal Mg2+ concentration.
Mg2+ promotes cell adhesion via 5β1- and β1-integrin-associated signal transduction pathways, which are involved in the enhanced activation of the key signaling adaptor protein Shc (Src homology collagen), resulting in the gene expression of extracellular matrix proteins [81]. Surface chemistry modification with Mg2+ also plays an important role in focal adhesion kinase (FAK; pp125FAK) mediated signal transduction via cell-surface integrin-ECM interaction. It has been shown that FAK expression, collagen type 1, vitronectin, and fibronectin are enhanced in osteoblasts growing on Al2O3-Mg2+, suggesting that tyrosine phosphorylation of signaling proteins was enhanced by binding to Mg2+supplemented bioceramics [81]. These findings suggested that the supplemental Mg2+ ions might also deliver its effect on the attachment of HDPCs. In this study, supplemental Mg2+ was used in the following concentrations 0.5 mM, 1 mM, 2 mM, 4 mM, and 8 mM, with a control of 0 mM. The results showed that there was higher attachment when relatively optimal concentrations were used. Specifically, the highest attachment was noticed with Mg2+ in 0.5 mM and 1 mM compared to the control. However, higher concentrations (4 mM and 8 mM) of Mg2+ had no effect on attachment compared to the optimal concentrations of 0.5 mM and 1 mM. These results may be attributed to the chemical structure of Mg2+which triggers human dental pulp cell signaling pathways affecting cellular motility. In a study by Sana et al. [82], human dental pulp stem cells when placed in contact with chitosan scaffolds were not able to attach nor spread on the surface since chitosan lacks adhesion motifs. Lu et al. [77] evaluated the in vitro effect of Mg2+ (0.5–16 mM) on osteogenic phenotypic behaviors of normal human osteoblasts in terms of attachment efficiency. They concluded that no cell doubling occurred, and cell attachment efficiency was not significantly affected by any Mg2+ ion concentration when compared to the control. In contrast, the data of the present study showed the highest attachment in` HDPCs with 1 mM supplement Mg2+. In a study by Shimaya et al. [83], magnesium enhanced adherence of synovial MSCs through α3 and β1 integrins, which promoted synthesis of cartilage matrix. These results confirm with the present data demonstrating the essential role of Mg2+ in HDPCs adhesion.
Pioneering work by Rubin [84] implicated Mg2+ as a key factor of the so-called “coordinated control of cell proliferation.” Mg2+ is involved in DNA duplication and plays a role in cytoskeleton rearrangement leading to the formation of the mitotic spindle and cytokinesis [84]. At the subcellular level, Mg2+ regulates contractile proteins, modulates transmembrane transports of Ca2+, Na+, and K+, and controls metabolic regulation of the energy-dependent cytoplasmic and mitochondrial pathway [85]. Proliferating cells have more Mg2+ than nonproliferating cells and high extracellular Mg2+ [86]. Mg2+ has been shown to induce cell proliferation in mammalian cells, including neural cells, keratinocytes, endothelial cells, fibroblasts, and lymphocytes. In the present study, assessment of proliferation rate of HDPCs was upregulated as noticed during the entire experiment when the supplemented Mg2+ values ranged from 0.5 mM to 2 mM, compared to the control group. These results are in accordance with Montezano et al. [87] and Lu et al. [77] who similarly showed an upregulatory effect by 2 mM concentration of MgCl2. They reported induced cell cycle activation and increased DNA and protein synthesis in the proliferated vascular smooth muscle cells (VSMCs) and normal human osteoblasts, respectively. The concentration of Mg2+ that was focused upon in the aforementioned studies was 2 mmol/L, as this level falls within the physiological/pathophysiological range (normal serum Mg2+ 0.6–1.5 mmol/L), unlike other studies that used concentrations as high as 5–10 mmol/L, which have little physiological or pathological relevance. Whereas, higher Mg2+ concertation groups ≥4 mM showed a down regulatory effect on HDPCs proliferation rate. These results are in agreement with Lu et al. [77], Leidi et al. [88], and Kircelli et al. [89], who reported that higher Mg2+concentrations behaved similar to the control cells grown on culture media inhibiting the proliferation activity of normal human osteoblasts. Despite the fact of using normal human osteoblasts and human pulp cells, both cell types showed a similar behavior responding to the same optimal Mg2+ concentration groups and interestingly at the same time points. Higher Mg2+ concentrations in the culture medium might interfere with the ion balance in the plasma membrane, leading to cytotoxicity and therefore inhibiting cellular proliferation and differentiation activities [90].
Mg2+ can regulate its own homeostasis and, hence, its intervention in cell differentiation as an authentic cell regulator. During dentin formation, odontoblast cells synthesize and secrete noncollagenous proteins in the dentin extracellular matrix; among these, ALP plays a regulatory role in mineralization and osteo-dentin/reparative dentin and are considered specific markers for dentinogenic phenotype [91]. ALP is an early marker for dentinogenic differentiation. The activity of ALP might be a prerequisite for the differentiation of pulp cells into odontoblasts. Expression of ALP by cultured human dental pulp stem cells (hDPSCs) has been determined and used as a marker for the cells' ability to produce the mineralized matrix in vitro [92, 93]. Recent investigations have shed new light on the mechanism of ALP action in promoting mineralization [91, 94]. In addition, investigation of the key association of ALP with cell membranes and matrix vesicles and the complex interactions of lipids, proteins, and ions ultimately result in the nucleation and propagation of mineral crystals promises to reveal new insights into how cells utilize the unique properties of ALP to form mineral [91, 95]. The accelerated differentiation and biomineralization from the addition of Mg2+ ions were believed to be attributed to the activation of ALP and promotion of crystallization processes and pattern formation of the inorganic mineral phase [96]. The data of the present study revealed that at 7 days, optimal Mg 2+ concentration groups (0.5 mM, 1 mM, and 2 mM) showed an increase in the rate of ALP activity. Moreover, at days 10 and 14, the same concentration groups had a significantly higher increase in the rate of ALP activity. Notably, cells grown in media containing higher Mg2+supplements (8 mM) showed lower ALP activity compared to the control at 14 days. Comparing ALP activity at different time intervals throughout the experiment clearly demonstrated the direct stimulating effect of ALP activity with 0.5–2 mM supplemental Mg2+ concentrations on HDPCs as evidenced by increase in ALP expression among these groups. Results presented by Lu et al. [77] and Burmester et al.' [74] studies are in agreement with these experimental data in relation to the effect of MgCl2 on ALP activity of HDPCs. Contrastingly, the data presented by Yang et al. [76] and Leidi et al. [88] demonstrated that <10 mM concentration of Mg2+ did not inhibit the viability, osteogenic differentiation, and ALP activity of human bone marrow mesenchymal stem cells.
In the present study, adding optimal concentrations of supplemented Mg2+ ranging 0.5 mM–2 mM at time intervals of 7, 10, and 14 days resulted in a significantly increased effect of upregulating mineralization compared to the control group. Optimal Mg2+ concentrations could stimulate the expression of calcium deposition of HDPCs. However, higher Mg2+ concentrations 4 mM and 8 mM groups downregulated calcium deposition. In this study, it was noticed that the results of the assays of mineralization and ALP activity revealed a consistent pattern for the same concentrations at the same time points in HDPCs. These results coincide with those of Lu et al. [77], showing the same trend regarding the concentration range and the time points, but in different types of cells. Therefore, these findings confirm that both normal HDPCs and human osteoblast could exhibit similarities in biochemical responses to optimal Mg2+ supplement concentration.
Zhang et al. [97] tested the effect of extracellular Mg2+ (0.8–3.8 mM) on the deposition of calcium phosphate matrix and Ca2+ signaling on human bone marrow-derived mesenchymal stem cells (hBMSC). They reported that Mg2+ concentration over 1.3 mM suppressed mineralization of mesenchymal stem cells, which is in disagreement with our study where optimal Mg2+ concentrations (0.5–2 mM) groups had an upregulating effect on HDPCs, increasing mineralization and calcium deposition. The difference between these data could be attributed to the addition of calcium. Optimized Ca2+ is critical for ALPase activity [98], and therefore, although Mg2+ concentrations are within 0.5–1.2 mM, the physiologic range of freely available Mg2+ calcium concentrations could impact ALPase activity.
The effect of the pH on bone mineralization and repair has been previously reported [99–102]. On a cellular level, during metabolic acidosis, osteoblast functions declined, whereas during metabolic alkalosis, osteoblast functions, such as cell viability, ALP activity, and mineral deposition, increased [103–105]. The acid-base microenvironment formed by reaction of magnesium chloride with water produces magnesium cation and chloride anion (Mg2+ and Cl−) [106]. The microenvironment where the cells reside can be affected by ions arising from the material dissolution and by concomitant pH changes affecting cell behavior [103]. Studies have shown that an increment in Mg2+ concentration caused by its degradation forms a high osmotic pressure and that the alkaline environment caused by released hydroxide ions coinhibited cell proliferation [107]. However, some studies have revealed that the proliferation of rat bone marrow mesenchymal cells (rBMSCs) on Mg2+-doped TiN ((Ti, Mg) N) coatings is associated with the amount of released Mg2+. Nevertheless, none of previous studies was done to verify the effect of extracellular pH on human pulp cells. Understanding and controlling pertinent cell functions by modulating the local engineered extracellular environment, specifically pH, is certainly a critical issue in future studies, for developing suitable pulp capping materials for dental tissue regeneration.
5. Conclusions
The data of this study suggested a significant benefit imparted by Mg2+ supplements of 0.5–2 mM on HDPCs evidenced by upregulated attachment rate, proliferation, differentiation, alkaline phosphatase activity, and mineralization, leading to potential of an improved pulp-capping material. This is the first report to demonstrate the optimal Mg2+concentrations needed to significantly enhance the dentinogenic activities of normal HDPCs. Mg-containing biomaterials could be considered for a future novel dental pulp-capping material in regenerative endodontics including improving direct pulp-capping and pulpotomy procedures. Future in vitro studies would be needed to verify the biological effect of magnesium on reparative dentin formation.
Data Availability
The data used to support the findings of this study are included within the article and are available from the corresponding author upon request. The raw data used to support the findings of this study are stored at the X220 Research Lab according to Boston University policy.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Flaxman D. A., Naghavi M., Lozano R., Michaud C., Ezzati M., Memish Z. A. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet . 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NICDR. Dental caries (tooth decay) in adults (age 20 to 64) 2014. http://www.nidcr.nih.gov.ezproxy.bu.edu/DataStatistics/FindDataByTopic/DentalCaries/DentalCariesAdults20to64.htm .
- 3.Moussa D. G., Aparicio C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. Journal of Tissue Engineering and Regenerative Medicine . 2019;13(1):58–75. doi: 10.1002/term.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W., Yelick P. C. Vital pulp therapy-current progress of dental pulp regeneration and revascularization. International Journal of Dentistry . 2010;2010:9. doi: 10.1155/2010/856087.856087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang G. T. Pulp and dentin tissue engineering and regeneration: current progress. Regenerative Medicine . 2009;4(5):697–707. doi: 10.2217/rme.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheller E. L., Krebsbach P. H., Kohn D. H. Tissue engineering: state of the art in oral rehabilitation. Journal of Oral Rehabilitation . 2009;36(5):368–389. doi: 10.1111/j.1365-2842.2009.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhlaghi N., Khademi A. Outcomes of vital pulp therapy in permanent teeth with different medicaments based on review of the literature. Dental Research Journal . 2015;12:406–417. doi: 10.4103/1735-3327.166187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox C. F., Sübay R. K., Ostro E., Suzuki S., Suzuki S. H. Tunnel defects in dentin bridges: their formation following direct pulp capping. Operative Dentistry . 1996;21(1):4–11. [PubMed] [Google Scholar]
- 9.Song M. Yu B., Kim S., Hayashi M., et al. Clinical and molecular perspectives of reparative dentin formation. Dental Clinics of North America . 2017;61(1):93–110. doi: 10.1016/j.cden.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton T. J. Keys to clinical success with pulp capping: a review of the literature. Operative Dentistry . 2009;34(615):625–638. doi: 10.2341/09-132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komabayashi T., Zhu Q., Eberhart R., Imai Y. Current status of direct pulp-capping materials for permanent teeth. Dental Materials Journal . 2016;35(1):1–12. doi: 10.4012/dmj.2015-013. [DOI] [PubMed] [Google Scholar]
- 12.Parirokh M., Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part I: chemical, physical, and antibacterial properties. Journal of Endodontics . 2010;36(1):16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Torabinejad M., Watson T. F., Pitt Ford T. R. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. Journal of Endodontics . 1993;19(12):591–595. doi: 10.1016/s0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 14.Okiji T., Yoshiba K. Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. International Journal of Dentistry . 2009;2009:12. doi: 10.1155/2009/464280.464280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aeinehchi M., Eslami B., Ghanbariha M., Saffar A. S. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. International Endodontic Journal . 2003;36(3):225–235. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 16.Nair P. N., Duncan H. F., Pitt Ford T. R., Hu L. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. International Endodontic Journal . 2008;4:128–150. doi: 10.1111/j.1365-2591.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 17.Islam I., Chng H. K., Jin Yap A. U. Comparison of the physical and mechanical properties of MTA and portland cement. Journal of Endodontics . 2006;32(3):193–197. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. International Endodontic Journal . 2008;41(5):408–417. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 19.Caron G., Azérad J., Faure M.-O., Machtou P., Boucher Y. Use of a new retrograde filling material (biodentine) for endodontic surgery: two case reports. International Journal of Oral Science . 2014;6(4):250–253. doi: 10.1038/ijos.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandolfi M., Siboni F., Polimeni A., et al. In vitro screening of the apatite-forming ability, biointeractivity and physical properties of a tricalcium silicate material for endodontics and restorative dentistry. Dentistry Journal . 2013;1(4):41–60. doi: 10.3390/dj1040041. [DOI] [Google Scholar]
- 21.Attik G. N., Villat C., Hallay F., et al. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: biodentine versus MTA. International Endodontic Journal . 2014;47(12):1133–1141. doi: 10.1111/iej.12261. [DOI] [PubMed] [Google Scholar]
- 22.Ji Y. M., Jeon S. H., Park J. Y., Chung J. H., Choung Y. H., Choung P. H. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Engineering Part A . 2016;6:1823–1833. doi: 10.1089/ten.TEA.2009.0054. [DOI] [PubMed] [Google Scholar]
- 23.Narayanan K., Gajjeraman S., Ramachandran A., Hao J., George A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. Journal of Biological Chemistry . 2006;28(1):19064–19071. doi: 10.1074/jbc.m600714200. [DOI] [PubMed] [Google Scholar]
- 24.Arandi N. Z., Rabi T. TheraCal LC: from biochemical and bioactive properties to clinical applications. International Journal of Dentistry . 2018;2018:6. doi: 10.1155/2018/3484653.3484653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergenholtz G., Spångberg L. Controversies in endodontics. Critical Reviews in Oral Biology & Medicine . 2004;15(2):99–114. doi: 10.1177/154411130401500204. [DOI] [PubMed] [Google Scholar]
- 26.Farges J.-C., Alliot-Licht B., Renard E., et al. Dental pulp defence and repair mechanisms in dental caries. Mediators of Inflammation . 2015;2015:16. doi: 10.1155/2015/230251.230251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmalz G., Smith A. J. Pulp development, repair, and regeneration: challenges of the transition from traditional dentistry to biologically based therapies. Journal of Endodontia . 2014;40(4) doi: 10.1016/j.joen.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Li F.-Y., Chaigne-Delalande B., Kanellopoulou C., et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature . 2011;475(735):471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maguire M. E., Cowan J. A. Magnesium chemistry and biochemistry. Biometals . 2002;15(3):203–210. doi: 10.1023/a:1016058229972. [DOI] [PubMed] [Google Scholar]
- 30.Wolf F. I., Cittadini A. Chemistry and biochemistry of magnesium. Molecular Aspects of Medicine . 2003;24(1–3):903–914. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 31.Wiesmann H. P., Tkotz T., Joos U. J., et al. Magnesium in newly formed dentin mineral of rat incisor. Journal of Bone and Mineral Research . 1997;12(3):380–383. doi: 10.1359/jbmr.1997.12.3.380. [DOI] [PubMed] [Google Scholar]
- 32.Todorovic T., Vujanovic D. The influence of magnesium on the activity of some enzymes (AST, ALT, ALP) and lead content in some tissues. Magnesium Research . 2002;15(3–4):173–177. [PubMed] [Google Scholar]
- 33.Scibior A., Adamczyk A., Mroczka R., Niedźwiecka I., Gołębiowska D., Fornal E. Effects of vanadium (V) and magnesium (Mg) on rat bone tissue: mineral status and micromorphology. Consequences of V-Mg interactions. Metallomics . 2014;6(12):2260–2278. doi: 10.1039/c4mt00234b. [DOI] [PubMed] [Google Scholar]
- 34.Durlach J. Magnesium in Clinical Practice First English Language Edition Published in 1988 . London, UK: John Libbey & Company Ltd; 1985. [Google Scholar]
- 35.Nakano Y., Le M. H., Abduweli D., et al. A critical role of TRPM7 as an ion channel protein in mediating the mineralization of the craniofacial hard tissues. Frontiers in Physiology . 2016;7(258):1–10. doi: 10.3389/fphys.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raoof M., Yaghoobi M. M., Derakhshani A., et al. A modified efficient method for dental pulp stem cell isolation. Dental Research Journal . 2014;11(2):244–250. [PMC free article] [PubMed] [Google Scholar]
- 37.Teaford M. F., Meredith S., Ferguson M. Function and Evolution of Teeth . Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 38.Goldberg M., Smith A. J. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Critical Reviews in Oral Biology & Medicine . 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 39.Yoshiba K., Yoshiba N., Nakamura H., Iwaku M., Ozawa H. Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. Journal of Dental Research . 1996;75:1590–1597. doi: 10.1177/00220345960750081101. [DOI] [PubMed] [Google Scholar]
- 40.Tziafas D., Panagiotakopoulos N., Komnenou A. Immunolocalization of fibronectin during the early response of dog dental pulp to demineralized dentine or calcium hydroxide-containing cement. Archives of Oral Biology . 1995;40:23–31. doi: 10.1016/0003-9969(94)00148-5. [DOI] [PubMed] [Google Scholar]
- 41.Lesot H., Smith A. J., Tziafas D., Begue-Kirn C., Cassidy N., Ruch J. V. Biologically active molecules and dental tissue repair: a comparative review of reactionary and reparative dentinogenesis with the induction of odontoblast differentiation in vitro. Cells and Materials . 1994;4:199–218. [Google Scholar]
- 42.Zhang W., Walboomers X. F., van Kuppevelt T. H., Daamen W. F., Bian Z., Jansen J. A. The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterial . 2006;22(33):5658–5668. doi: 10.1016/j.biomaterials.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Inoue M., Le Geros Z. R., Inoue M., et al. Effect of magnesium, strontium or fluoride ions on in vitro activities of odontoblast-like cells (MDPC-23) Journal of Hard Tissue Biology . 2005;14(1):5–12. doi: 10.2485/jhtb.14.5. [DOI] [Google Scholar]
- 44.Rubin H. Intracellular free Mg2+ and Mg ATP2− in coordinate control of protein synthesis and cell proliferation. In: Nechifor M., editor. Magnesium in the Central Nervous System Vink . Adelaide, Australia: University of Adelaide Press; 2011. [PubMed] [Google Scholar]
- 45.Rubin H., Vidair C., Sanui H. Restoration of normal appearance, growth behavior, and calcium content to transformed 3T3 cells by magnesium deprivation. Proceedings of the National Academy of Sciences of the United States of America . 1981;78(4):2350–2354. doi: 10.1073/pnas.78.4.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y., Liu B., Bi H., et al. The degradation properties of MgO whiskers/PLLA composite in vitro. International Journal of Molecular Sciences . 2018;19(2740):5–12. doi: 10.3390/ijms19092740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belluci D., Sola A., Salvatori R., Anesi A., Chiarini L., Cannillo V. Role of magnesium oxide and strontium oxide as modifiers in silicate based bioactive glasses: effects on thermal behaviour, mechanical properties and in -vitro bioactivity. Materials Science and Engineering . 2017;72:566–575. doi: 10.1016/j.msec.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 48.Sargenti A., Castiglioni S., Olivi E., et al. Magnesium deprivation potentiates human mesenchymal stem cell transcriptional remodeling. International Journal of Molecular Sciences . 2018;9(5):1410–1420. doi: 10.3390/ijms19051410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian P., Liu X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regenerative Biomaterials . 2015;2:135–151. doi: 10.1093/rb/rbu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan G. Y., Zhang X. B., Niu J. L., et al. Research progress of new type of degradable biomedical magnesium alloys JDBM. Chinese Journal of Nonferrous Metals . 2011;10:2476–2488. [Google Scholar]
- 51.Liu Y. J., Yang Z. Y., Tan L. L., Li H., Zhang Y. Z. An animal experimental study of porous magnesium scaffold degradation and osteogenesis. Brazilian Journal of Medical and Biological Research . 2014;47:715–720. doi: 10.1590/1414-431x20144009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witte F., Fischer J., Nellesen J., et al. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials . 2006;27:1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 53.Witte F., Kaese V., Haferkamp H., et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials . 2005;26:3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 54.Zeng J., Ren L., Yuan Y., et al. Short-term effect of magnesium implantation on the osteomyelitis modeled animals induced by Staphylococcus aureus. Journal of Materials Science: Materials in Medicine . 2013;24:2405–2416. doi: 10.1007/s10856-013-4982-6. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen T. Y., Liew C. G., Liu H. An in vitro mechanism study on the proliferation and pluripotency of human embryonic stems cells in response to magnesium degradation. PLoS One . 2013;8 doi: 10.1371/journal.pone.0076547.e76547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon Y. D., Lee D. W., Hong S. O. Magnesium versus machined surfaced titanium—osteoblast and osteoclast differentiation. Journal of Advances Prosthodontics . 2014;6:157–164. doi: 10.4047/jap.2014.6.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaya R. A., Cavuşoğlu H., Tanik C., et al. The effects of magnesium particles in posterolateral spinal fusion: an experimental in vivo study in a sheep model. Journal of Neurosurgery, Spine . 2007;6 doi: 10.3171/spi.2007.6.2.141. [DOI] [PubMed] [Google Scholar]
- 58.Song G. Control of biodegradation of biocompatible magnesium alloys. Corrosion Science . 2007;49:1696–1701. doi: 10.1016/j.corsci.2007.01.001. [DOI] [Google Scholar]
- 59.Yoshizawa S., Chaya A., Verdelis K., Bilodeau E. A., Sfeir C. An in vivo model to assess magnesium alloys and their biological effect on human bone marrow stromal cells. Acta Biomaterialia . 2015;28:234–239. doi: 10.1016/j.actbio.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 60.Pichler K., Kraus T., Martinelli E., et al. Cellular reactions to biodegradable magnesium alloys on human growth plate chondrocytes and osteoblasts. International Orthopaedics . 2014;38:881–889. doi: 10.1007/s00264-013-2163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S. F., Jiang Q. H., Peel S., Wang X.-x., He F.-m. Effects of magnesium-substituted nanohydroxyapatite coating on implant osseointegration. Clinical Oral Implants Research . 2014;24:34–41. doi: 10.1111/j.1600-0501.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- 62.Abed E., Martineau C., Moreau R. Role of melastatin transient receptor potential 7 channels in the osteoblastic differentiation of murine MC3T3 cells. Calcified Tissue International . 2011;88:246–253. doi: 10.1007/s00223-010-9455-z. [DOI] [PubMed] [Google Scholar]
- 63.Cecchinato F., Xue Y., Karlsson J., et al. In vitro evaluation of human fetal osteoblast response to magnesium loaded mesoporous TiO2 coating. Journal of Biomedical Materials Research Part B: Applied Biomaterials . 2013;102:3862–3871. doi: 10.1002/jbm.a.35062. [DOI] [PubMed] [Google Scholar]
- 64.Kim H. K., Han H. S., Lee K. S., et al. Comprehensive study on the roles of released ions from biodegradable Mg-5 wt% Ca-1 wt% Zn alloy in bone regeneration. Journal of Tissue Engineering and Regenerative Medicine . 2016;11:2710–2724. doi: 10.1002/term.2166. [DOI] [PubMed] [Google Scholar]
- 65.Lü Y. M., Han P., Ji W. P., Chai Y. M. Effect of concentrations of magnesium ions on behavior of fibroblasts and osteoblasts. Chinese Journal of Orthopaedic Trauma . 2013;15:1065–1070. [Google Scholar]
- 66.Tousi N. S., Velten M. F., Bishop T. J., et al. Combinatorial effect of Si4+, Ca2+ and Mg2+ released from bioactive glasses on osteoblast osteocalcin expression and biomineralization. Materials Science and Engineering: C . 2013;33:2757–2765. doi: 10.1016/j.msec.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 67.Czekanska E. M., Stoddart M. J., Richards R. G., Hayes J. S. In search of an osteoblast cell model for in vitro research. European Cells & Materials . 2012;24:1–17. doi: 10.22203/ecm.v024a01. [DOI] [PubMed] [Google Scholar]
- 68.Wagner E. R., Luther G., Zhu G., et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma . 2011;2011:12. doi: 10.1155/2011/325238.325238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pautke C., Schieker M., Tischer T., et al. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Research . 2004;24:3743–3748. [PubMed] [Google Scholar]
- 70.Christenson R. H. Biochemical markers of bone metabolism: an overview. Clinical Biochemistry . 1997;30:573–593. doi: 10.1016/s0009-9120(97)00113-6. [DOI] [PubMed] [Google Scholar]
- 71.Robey P. G., Termine J. D. Human bone cells in vitro. Calcified Tissue International . 1985;3:453–460. doi: 10.1007/bf02557826. [DOI] [PubMed] [Google Scholar]
- 72.Ortiz J., Marquez M. G., Chou L. Survivin activity in normal human osteoblasts and osteosarcoma cells. Chinese Journal of Oral and Maxillofacial Surgery . 2010;8:241–249. [Google Scholar]
- 73.Franks K., Salih V., Knowles J. C. The effect of MgO on the solubility behavior and cell proliferation in a quaternary soluble phosphate-based glass system. Journal of Materials Science: Materials in Medicine . 2002;13:549–556. doi: 10.1023/a:1015122709576. [DOI] [PubMed] [Google Scholar]
- 74.Burmester A., Willumeit-Römer R., Feyerabend F. Behavior of bone cells in contact with magnesium implant material. Journal of Biomedical Materials Research Part B: Applied Biomaterials . 2017;105(no):165–179. doi: 10.1002/jbm.b.33542. [DOI] [PubMed] [Google Scholar]
- 75.Park K. D., Lee B. A., Piao X. H., et al. Effect of magnesium and calcium phosphate coatings on osteoblastic responses to the titanium surface. Journal of Advanced Prosthodontics . 2013;5:402–408. doi: 10.4047/jap.2013.5.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang C., Yuan G., Zhang J., Tang Z., Zhang X., Dai K. Effects of magnesium alloys extracts on adult human bone marrow-derived stromal cell viability and osteogenic differentiation. Biomedical Materials . 2010;5(4) doi: 10.1088/1748-6041/5/4/045005.045005 [DOI] [PubMed] [Google Scholar]
- 77.Lu W. C., Pringa E., Chou L. Effect of magnesium on the osteogenesis of normal human osteoblasts. Journal of Magnesium Research . 2017;30(2):42–52. doi: 10.1684/mrh.2017.0422. [DOI] [PubMed] [Google Scholar]
- 78.Sun H. L., Wu C. T., Dai K., Chang J., Tang T. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials . 2006;27:5651–5662. doi: 10.1016/j.biomaterials.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 79.Hussain A., Bessho K., Takahashi K., Tabata Y. Magnesium calcium phosphate as a novel component enhances mechanical/physical properties of gelatin scaffold and osteogenic differentiation of bone marrow mesenchymal stem cells. Tissue Engineering Part A . 2012;18:768–782. doi: 10.1089/ten.tea.2011.0310. [DOI] [PubMed] [Google Scholar]
- 80.Qu T., Jing J., Liu X. Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Engineering, Part A . 2014;20(17-18):2422–2433. doi: 10.1089/ten.TEA.2013.0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu D. D., Hoyer J. R., Smith J. W. Ca2+ suppresses cell adhesion to osteopontin by attenuating binding affinity for integrin αV3. Journal of Biological Chemistry . 1995;270:9917–9925. doi: 10.1074/jbc.270.17.9917. [DOI] [PubMed] [Google Scholar]
- 82.Sana F. A., Yurtsever M. C., Bayrak G. K., Tuncay E., Kiremitci A., Gumusderelioglu M. Spreading, proliferation and differentiation of human dental pulp stem cells on chitosan scaffolds immobilized with RGD or fibronectin. Cytotechnology . 2017;69:617–630. doi: 10.1007/s10616-017-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimaya M., Muneta T., Ichinose S., Tsuji K., Sekiya I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthritis and Cartilage . 2010;18(10):1300–1309. doi: 10.1016/j.joca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Rubin H., Terasaki M., Sanui H. Major intracellular cations and growth control: correspondence among magnesium content, protein synthesis and the onset of DNA synthesis in BALB/c3T3 cells. Proceedings of the National Academy of Sciences of the United States of America . 1979;76:3917–3921. doi: 10.1073/pnas.76.8.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pilchova I., Klacanova K., Tatarkova Z., Kaplan P., Racay P. The involvement of Mg2+ in regulation of cellular and mitochondrial functions. Oxidative Medicine and Cellular Longevity . 2017;2017:8. doi: 10.1155/2017/6797460.6797460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maguire M. E. Magnesium and cell proliferation. Annals of the New York Academy of Sciences . 1988;551:1749–6632. doi: 10.1111/j.1749-6632.1988.tb22338.x. [DOI] [PubMed] [Google Scholar]
- 87.Montezano A. C., Zimmerman D., Yusuf H., et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension . 2010;56:453–462. doi: 10.1161/hypertensionaha.110.152058. [DOI] [PubMed] [Google Scholar]
- 88.Leidi M., Dellera F., Mariotti M., Maier J. A. High magnesium inhibits human osteoblast differentiation in vitro. Magnesium Research . 2011;24:1–6. doi: 10.1684/mrh.2011.0271. [DOI] [PubMed] [Google Scholar]
- 89.Kircelli F., Peter M. E., Ok E. S., et al. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrology Dialysis Transplantation . 2012;27:514–521. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Baaij J. H. F., Hoenderop J. G. J., Bindels R. J. M. Magnesium in man. Implications for health and disease. Physiological Reviews . 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 91.Ellis E. G., Battaglia K. B. The role of alkaline phosphatase in mineralization. Current Opinion in Orthopaedics . 2007;18:444–448. [Google Scholar]
- 92.Wei X., Ling J., Wu L., Liu L., Xiao Y. Expression of mineralization markers in dental pulp cells. Journal of Endodontics . 2007;33:703–708. doi: 10.1016/j.joen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Tomlinson M. J., Dennis C., Yang X. B., Kirkham J. Tissue non-specific alkaline phosphatase production by human dental pulp stromal cells is enhanced by high density cell culture. Cell and Tissue Research . 2015;361(2):529–540. doi: 10.1007/s00441-014-2106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. Journal of Nippon Medical School . 2010;77:4–12. doi: 10.1272/jnms.77.4. [DOI] [PubMed] [Google Scholar]
- 95.Golub E. E. Role of matrix vesicles in biomineralization. Biochimica et Biophysica Acta . 2009;1790(12):1592–1598. doi: 10.1016/j.bbagen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saris N. E., Mervaala E., Karppanen H., Khawaja J. A., Lewenstam A. Magnesium: an update on physiological, clinical and analytical aspects. Clinica Chimica Acta . 2000;294(1-2):1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L., Yang C., Li J., Zhu Y., Zhang X. High extracellular magnesium inhibits mineralized matrix deposition and modulates intracellular calcium signaling in human bone marrow-derived mesenchymal stem cells. Biochemical and Biophysical Research Communications . 2014;450:1390–1395. doi: 10.1016/j.bbrc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Hoylaerts M. F., Van kerckhoven S., Kiffer-Moreira T., Sheen C., Narisawa S., Millán J. L. Functional significance of calcium binding to tissue-nonspecific alkaline phosphatase. PLoS One . 2015;10(3) doi: 10.1371/journal.pone.0119874.e0119874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swenson O., Claff C. Changes in the hydrogen ion concentration of healing fractures. Proceedings of the Society for Experimental Biology and Medicine . 1946;61:151–154. doi: 10.3181/00379727-61-15256. [DOI] [PubMed] [Google Scholar]
- 100.Arnett T. R., Dempster D. W. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology . 1986;119:119–124. doi: 10.1210/endo-119-1-119. [DOI] [PubMed] [Google Scholar]
- 101.Newman R. J., Francis M. J., Duthie R. B. Nuclear magnetic resonance studies of experimentally induced delayed fracture union. Clinical Orthopaedics and Related Research . 1987;216:253–261. doi: 10.1097/00003086-198703000-00039. [DOI] [PubMed] [Google Scholar]
- 102.Chakkalakal D. A., Mashoof A. A., Novak J., Strates B. S., McGuire H. H. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. Journal of Biomedical Materials Research . 1994;28:1439–1443. doi: 10.1002/jbm.820281209. [DOI] [PubMed] [Google Scholar]
- 103.Shen Y., Liu W., Wen C., et al. Bone regeneration: importance of local pH—strontium-doped borosilicate scaffold. Journal of Materials Chemistry . 2012;22:8662–8670. doi: 10.1039/c2jm16141a. [DOI] [Google Scholar]
- 104.Bushinsky D. A. Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. The American Journal of Physiology . 1996;271F:216–222. doi: 10.1152/ajprenal.1996.271.1.f216. [DOI] [PubMed] [Google Scholar]
- 105.Brandao-Burch A., Utting J. C., Orriss I. R., Arnett T. R. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcified Tissue International . 2005;77:167–174. doi: 10.1007/s00223-004-0285-8. [DOI] [PubMed] [Google Scholar]
- 106.Daloee T. S., Behbahani F. K. MgCl2 and its applications in organic chemistry and biochemistry: a review. Molecular Diversity . 2020;24:463–476. doi: 10.1007/s11030-019-09947-2. [DOI] [PubMed] [Google Scholar]
- 107.Luthringer B., Hereon H.-Z., Willumeit R. Effects of magnesium degradation products on mesenchymal stem cell fate and osteoblastogenesis. Gene . 2015;575:1–15. doi: 10.1016/j.gene.2015.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article and are available from the corresponding author upon request. The raw data used to support the findings of this study are stored at the X220 Research Lab according to Boston University policy.


