Abstract
Brain cancers carry bleak prognoses, with therapeutic advances helping only a minority of patients over the past decade. The brain tumour microenvironment (TME) is highly immunosuppressive and differs from that of other malignancies as a result of the glial, neural and immune cell populations that constitute it. Until recently, the study of the brain TME was limited by the lack of methods to de-convolute this complex system at the single-cell level. However, novel technical approaches have begun to reveal the immunosuppressive and tumour-promoting properties of distinct glial and myeloid cell populations in the TME, identifying new therapeutic opportunities. Here, we discuss the immune modulatory functions of microglia, monocyte-derived macrophages and astrocytes in brain metastases and glioma, highlighting their disease-associated heterogeneity and drawing from the insights gained by studying these malignancies and other neurological disorders. Lastly, we consider potential approaches for the therapeutic modulation of the brain TME.
Malignant brain tumours make up a diverse group that can be coarsely divided into primary tumours, originating in the brain, and brain metastases (BrMs). Prognosis for most patients is poor, with a median survival of less than 2 years for patients with glioblastoma (GBM) or most types of BrMs. In the past decade, immune-checkpoint blockade therapies modulating T cell responses have improved survival in patients with melanoma and lung cancer, even when metastasized to the brain1-3. Valuable insights have been gained from the study of T cell biology in brain tumours4-6, but less is known about other cell populations contributing to immune suppression in the brain tumour microenvironment (TME)7-12. The modulation of these other cell populations in the brain TME could improve the efficacy of immunotherapy against brain malignancies.
The brain TME is highly heterogeneous both in its evolution from early to late disease and its architecture, with differences detected across tumour type, between individuals with the same diagnosis, non-neoplastic cell types and cell states, and among individual tumour cell clones10,13-15. Fibroblasts, pericytes, endothelial cells, glial cells and leukocytes interact with one another and with tumour cells to perpetuate brain tumour growth13. Dendritic cells13,16,17, neutrophils10,18, natural killer (NK) cells7,19,20 (BOX 1), macrophages10,11,21-24 and astrocytes12,25 have been implicated in the modulation of the TME to impact T cell responses in brain cancer. Tumour-associated macrophages (TAMs), comprised of monocyte-derived macrophages (MDMs) and microglia, can represent up to 50% of the cells in the brain TME10,26-29. While TAMs appear to be more abundant in primary brain tumours than BrMs7,10, various TAM-targeting approaches in animal models lead to primary and metastatic brain tumour regression11,23,30. Brain tumour-associated microglia and MDMs have distinct origins31 and incompletely overlapping functions23. Their differential investigation has been hampered by technical difficulties such as the lack of differentiating markers32, the perturbation of their phenotype and function by isolation methods33-35, and the permeabilization of the brain vasculature with irradiation, which enables monocytes to engraft into the brains of healthy adult mice21,33,36. In addition, astrocytes are the most abundant glial cells in the central nervous system (CNS), and while they have emerged alongside microglia as key orchestrators of neuroinflammatory responses37, their heterogeneity has been largely understudied in brain malignancies.
Box 1 ∣. Neutrophils, dendritic cells and natural killer cells in the brain tumour microenvironment.
In addition to brain tumour-associated macrophages and astrocytes, other cells participate in the inflammatory response in the brain tumour microenvironment (TME) (reviewed in REFS38,226) (FIG. 1a).
Neutrophils
Neutrophils are the most numerous granulocytes and are early responders to injury and infection. Tumour-associated neutrophils are observed in isocitrate dehydrogenase wild-type (IDHwt) and IDH mutant (IDHmut) glioma10,227, and brain metastases (BrMs)10 and are particularly numerous in BrMs10. BrM-associated neutrophils display phenotypic changes, including expression of the adenosine receptor and the receptor tyrosine kinase MET10, associated with neutrophil immune suppressive activity in other contexts228,229. Neutrophils were recently found to amplify necrosis and promote tumour progression in a xenograft model of glioblastoma (GBM)230. Little is known about brain tumour-associated neutrophil subsets.
Dendritic cells
Dendritic cells (DCs) are myeloid cells capable of internalizing, processing and presenting antigens to tumour-reactive T cells in lymph nodes and numerous tissues231, including the brain232. At least five DC subsets have been identified in human GBM: conventional DC1 (cDC1), cDC2, migratory DCs, pre-DCs and plasmacytoid DCs (pDCs)7,16. Moreover, cDC1s, cDC2s and pDCs were also identified in BrMs7. Mechanisms such as pDC-mediated expansion of regulatory T cells233 and T cell exhaustion via the immune-checkpoint protein PDL1 (REF.234) have yet to be studied in brain cancer, but numerous cell-contact and paracrine mechanisms could participate in brain malignancies231.
Natural killer cells
Natural killer (NK) cells are innate lymphocytes capable of cytokine secretion and direct cytolytic activity against tumour cells when engaged by the proper combination of activating ligands235. Single-cell proteomic analysis classified brain tumour-associated NK cells into immature, highly cytotoxic and intraepithelial group 1 innate lymphoid-like cells7. NK cells from GBM appear predominantly immature, while NK cells from low-grade glioma and BrMs appear cytotoxic7. Early pre-clinical evidence demonstrates the potential for NK cell-mediated anti-tumour activity in the brain236. Therefore, the functional investigation of individual disease-associated NK cell subsets will be valuable.
Recent technological advances have enabled in-depth multi-omic studies of MDMs, microglia and astrocytes at single-cell resolution (BOX 2), revealing multiple cell subsets and activation states in development, health, and neurodegenerative and neuroinflammatory diseases. In this Review, we explore the diversity of microglia, MDMs and astrocytes in the TME of glioma and BrMs. A better understanding of cell heterogeneity within the TME could provide novel insights into the pathogenesis of brain malignancies and guide the development of efficacious immunotherapeutic approaches.
Box 2 ∣. Models to study brain tumour-associated astrocytes and macrophages.
Brain tumour researchers are daunted by a history of repeated clinical trial failures, particularly in malignant glioma, which call into question numerous aspects of drug development, including the pre-clinical models used to identify and evaluate therapeutic targets. The lack of in vitro models recapitulating the complexity of the brain environment has made in vivo models a critical component for the investigation of brain malignancies. These include orthotopically implanted, patient-derived or syngeneic tumours, genetically engineered mouse models237, and numerous models of metastasis (the majority of which are in vivo selected and implanted in various locations: the brain, other organs or intra-arterial14). As observed in several cancer models, the complex genetic landscape of human glioma is not fully recapitulated in genetically engineered mouse models237, and major differences in the evolution of xenografted brain tumours at the genomic level exist237,238. Indeed, a recent comparison of the immune infiltrate in syngeneic orthotopic glioma models and human tumours concluded that the spontaneous 005 model, followed by the carcinogen-induced models GL261 and then CT2A, recapitulated some but not all aspects of the glioblastoma tumour microenvironment (TME)223. Humanized mouse models may more faithfully recapitulate tumour immunology239 as recently demonstrated for T cell suppression in glioblastoma6, but the humanized components are limited to haematopoietic cell compartments, and their widespread adoption has been limited by technical limitations and costs.
Once a pre-clinical mod el is chosen, numerous tools exist to characterize tumour-associated macrophages, astrocytes and other TME components240. Rare cell populations can be investigated using multi-omic and multidimensional profiling of single cells. Genomic241,242, epigenomic243, transcriptomic6,27,29,50,51, proteomic7,22,23 and metabolomic244 profiles of individual cells can be obtained and compared with available datasets, while retaining information about the spatial organization of the TME. Single-cell transcriptomics are now well established for the identification of cell types, cell subsets and cell activation states in the TME245,246. Going further, the prediction of molecular interactions and the computational inference of regulatory networks and cell state trajectories247 based on epigenetic and gene expression data may enable the unbiased detection of biological pathways of interest and candidate therapeutic targets248. Finally, novel approaches for in situ transcriptomics249-252 allow the investigation of tumour architecture and spatial heterogeneity. Hence, efforts to integrate high-dimensional datasets with spatial genomic, proteomic and metabolomic approaches are needed to maximize our understanding of the brain TME103,253. As compartment-specific, high-resolution molecular data become available, researchers may welcome the use of databases, such as Single Cell Portal at the Broad Institute50, RecuR55, GBMseq27, Glioma Microglia RNA Expression87, Brain TIME10, Adult Astrocyte RNA-Seq Explorer150, Myeloid Single-cell RNA-Seq59, DropViz254, Brain Immune Atlas16 and UCSC Cell Browser43, that facilitate independent validation across multiple datasets.
Brain tumour-associated macrophages
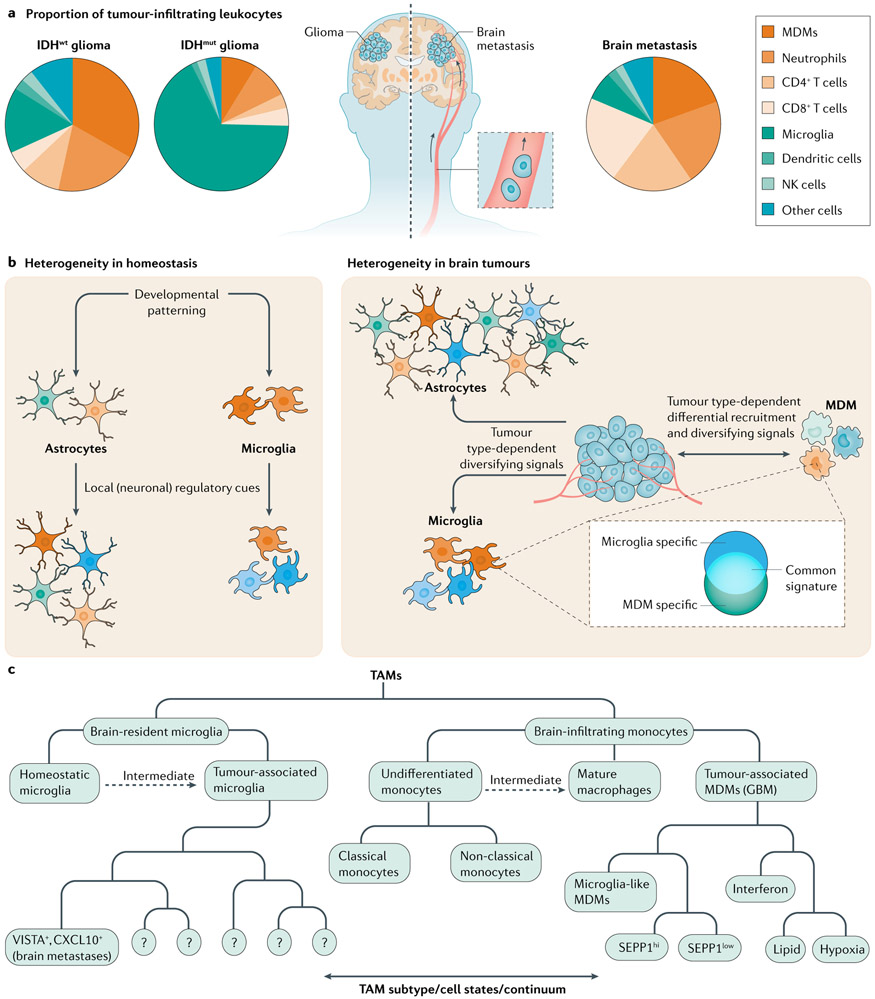
The prevalence and plasticity of TAMs makes them attractive therapeutic targets for the modulation of tumour-specific immunity in the brain38-40. However, the proper investigation of TAM heterogeneity and its implications for brain tumour pathology should be based on the differentiation of microglia and MDMs — cell subsets with different origins and functions that have been usually commingled in TME studies (BOX 3).
Box 3 ∣. Ontogeny and functions of brain TAMs.
Tumour-associated macrophages (TAMs), consisting of brain-infiltrating monocyte-derived macrophages (MDMs) and microglia are frequently grouped together in brain tumour microenvironment (TME) studies42,94,188,205,207 despite their distinct origins and development31. However, microglia derive from embryonic yolk sac erythro-myeloid progenitors that seed the central nervous system during development and self-renew in the adult brain21,31,255-257. By contrast, monocytes originate from the postnatal bone marrow myeloid lineage and renew from haematopoietic stem cells258. While monocytes are reported to acquire a more immunosuppressive phenotype than microglia21,43, both cell types exert immune suppressive or stimulatory functions depending on the context8,44,259,260.
Despite similarities between monocyte-derived macrophages (MDMs) and microglia, their distinct ontogenies can lead to different roles in the brain TME23. MDMs traffic to the TME, where they shift their transcriptional programmes in response to the local microenvironment261,262. If microglia are depleted, monocytes re-populate the microglial niche and acquire a microglia-like gene expression signature262. Nevertheless, core differences exist in ontogeny-specific transcription factors and the spatial distribution of brain tumour-associated microglia and brain tumour-associated MDMs21,43. In addition, functional differences exist between tumour-associated microglia and MDMs. For example, minimizing MDM infiltration via deletion of the gene encoding CC-chemokine receptor 2 (Ccr2) in mice was not sufficient to prevent brain metastasis (BrM) formation, whereas depletion of microglia ameliorated T cell suppression in the BrM microenvironment23. Multimodal single-cell analyses will likely enable further studies clarifying the individual functions of glioma-associated MDMs and glioma-associated microglia.
While recent studies employing single-cell analysis7,8,16,22 and fate-mapping tools in mice21 distinguish tumour-associated microglia from MDMs, earlier methods, including irradiation and labelling of individual canonical markers (including CD45, CD11b, AIF1, CD68 and CX3C-chemokine receptor 1 (CX3CR1)), are now considered less reliable21. It was recently reported that the novel antigen CD49d is upregulated in glioblastoma-associated MDMs and downregulated in glioblastoma-associated microglia in mice and humans21 but usually numerous antigens or gene signatures ought to be analysed to confirm the identity of brain TAMs100.
Novel methods (BOXES 2,3) have uncovered the broad diversity of TAM activation states in the TME, which extend beyond the dichotomous model of pro-inflammatory or anti-inflammatory phenotypes10,41-44 (TABLE 1). In glioma and BrMs, TAMs outnumber other leukocyte populations such as T cells, NK cells and neutrophils. TAM composition in gliomas is influenced by mutations in the genes encoding isocitrate dehydrogenase (IDH1 or IDH2; hereafter referred to as IDH), which lead to the production of the oncometabolite 2-hydroxyglutarate and are highly prevalent in grade 2–3 gliomas7,10,45,46 (FIG. 1a). Specifically, microglia comprise the majority of CD45+ cells in human IDH mutant (IDHmut) gliomas, which contain smaller numbers of MDMs and few lymphocytes or neutrophils45. However, human IDH wild-type (IDHwt) gliomas harbour fewer microglia than IDHmut gliomas but greater numbers of microglia than BrMs7,10,24 (FIG. 1a). Human IDHwt gliomas and BrMs contain a large number of MDMs and a moderate number of neutrophils7,10. In addition, BrMs contain larger numbers of lymphocytes in the TME than do IDHwt gliomas10. Furthermore, immunostaining studies established that glioma-associated microglia show an even distribution throughout the brain parenchyma10 and do not populate the perivascular space like MDMs. Mouse models of lung cancer-to-BrMs suggest that microglia make up 80% or more of the TAM compartment in BrMs47 but their prevalence in human BrMs is much lower10. Of note, in both BrMs7,10 and GBM from patients27, microglia are more abundant at the tumour margin compared to the tumour core. These findings illustrate how tumour origin and genomic and metabolic aberrancies shape the composition of the TAM compartment (FIG. 1b,c).
Table 1 ∣.
Microglia-derived and monocyte-derived TAM subsets identified by single-cell technologies
| Technique | Reported subsets | Disease or Model | Functional or prognostic evaluation |
|---|---|---|---|
| Monocyte-derived TAMs (MDMs) | |||
| scRNA-seq and CITE-seq16 | Transitory/differentiating; phagocytic/lipid metabolic; hypoxic/glycolytic; SEPP1low/microglia-like; interferon-induced | ND and recurrent GBM; GL261 (GBM model) | No |
| SEPP1high/anti-inflammatory | Recurrent GBM, GL261 (GBM model) | ||
| CyTOF7 | MDM2 | IDHwt glioma, melanoma BrM, carcinoma BrM | Positive correlation with OS in WHO grade II and III glioma |
| MDM3 | IDHwt and IDHmut glioma, melanoma BrM, carcinoma BrM | ||
| MDM4 | IDHwt glioma | No | |
| scRNA-seq95 | Microglia-like; glioma-associated; actively differentiating; interferon-induced | U87 (GBM model) | No |
| scRNA-seq and CyTOF8 | CD73hi ICI-resistant, hypoxic, immunosuppressive | GBM | Negative correlation with survival (TCGA GBM); Cd73 KO in GL261 mice improved survival in combination with ICI |
| CyTOF223 | Macrophages-monocytes; microglia-like; SIGLECF+ macrophages | GL261 (GBM model) | No |
| scRNA-seq47 | MDM; radiation-induced MDM | H2030 (lung cancer BrM model) | No |
| CyTOF and CITE-seq23 | Ly6Chigh BrM-enriched; Ly6Clow microglia-like; Ly6Chigh MDM | E0771 (breast cancer BrM model) | No |
| Microglia-derived TAMs | |||
| scRNA-seq16 | Phagocytic/lipid metabolic; IFNγ-induced | ND and recurrent GBM, GL261 GBM model | No |
| Hypoxic | Recurrent GBM and GL261 GBM model | ||
| scRNA-seq95 | Homeostatic; glioma-associated; cycling/proliferating; undefined; glioma-associated; MHC II high | U87 (GBM model) | No |
| scRNA-seq and CyTOF22 | Glioma-associated; ageing-associated and glioma-associated; homeostatic | IDHwt GBM | No |
| CyTOF223 | Two undefined | GL261 (GBM model) | No |
| scRNA-seq47 | TAM microglia; radiation-induced TAM microglia | H2030 (lung cancer BrM model) | No |
| CyTOF and CITE-seq23 | Homeostatic; primed; TNF+ inflammatory state; undefined inflammatory state; migratory; migratory and inflammatory; S100+ LGALS1+, LGALS3+ | E0771 (breast cancer BrM model) | No |
| VISTA+ PDL1+ BrM-promoting CNS myeloid cells | Targetable with anti-VISTA and anti-PDL1 | ||
| scRNA-seq100 | Homeostatic; transcriptionally active; MHC I and MHC II high; proliferative | GL261 (GBM model) | No |
Studies where TAM compartments were not further subclustered beyond all tumour-associated MDMs or microglia are not shown, although their single-cell data may be a useful resource7,43,100. Further populations of myeloid cells that are not shown include immature/less differentiated monocytes, intermediate macrophages, mature macrophages and border-associated macrophages23,100,223. BrM, brain metastasis; CITE-seq, cellular indexing of transcriptomes and epitopes by sequencing; CyTOF, cytometry by time of flight; GBM, glioblastoma; ICI, immune-checkpoint inhibitor; IDHmut, isocitrate dehydrogenase mutant; IDHwt, isocitrate dehydrogenase wild type; KO, knockout; MDMs, monocyte-derived macrophages; MHC, major histocompatibility complex; ND, newly diagnosed; OS, overall survival; scRNA-seq, single-cell RNA sequencing; TAMs, tumour-associated macrophages; TCGA, The Cancer Genome Atlas.
Fig. 1 ∣. Microglia, MDM and astrocyte diversity in brain tumours.
a ∣ Pie charts represent the composition of leukocytes in isocitrate dehydrogenase wild-type (IDHwt) and IDH mutant (IDHmut) glioma and brain metastases as determined by flow cytometry10 and cytometry by time of flight (CyTOF)7. Leukocyte composition varies by tumour type7,10. The pie charts represent general trends from multiple tumours. b ∣ Non-malignant cell types in the tumour microenvironment (TME) have the potential for diverse activation states during both homeostasis (left panel) and cancer (right panel). Environmental cues and differential recruitment to the TME are the major drivers of heterogeneity in tumour-associated macrophages (TAMs; comprised of monocyte-derived macrophages (MDMs) and microglia). The plasticity of TME-infiltrating MDMs results in their acquisition of microglia-like transcriptional programmes. Astrocytes are highly heterogeneous, which is amplified upon activation. c ∣ Organizational structure for TAM nomenclature. Notable subsets are listed from recent reports16,23. Intermediate states represent subsets that exist on a continuum between undifferentiated monocytes and macrophages or homeostatic microglia and tumour-associated microglia. GBM, glioblastoma; NK, natural killer.
The intratumoural heterogeneity of GBM plays a role in its resistance to therapy48,49, while also shaping the inflammatory TAM infiltrate27,50. For instance, four transcriptional GBM cell states (neural progenitor-like, oligodendrocyte progenitor-like, astrocyte-like and mesenchymal-like) are detected in different tumour areas50,51 and other epigenetic states have also been described52-54. A transcriptional mesenchymal-like signature is correlated with an enrichment in TAMs exhibiting anti-inflammatory transcriptional signatures and worse clinical outcomes55. Moreover, non-mesenchymal-like GBMs that transition to mesenchymal-like GBMs harbour increased immunosuppressive TAMs at recurrence. Thus, while the regulation and plasticity of different GBM cell states is still unclear, tumour cell diversity has strong (and likely reciprocal) effects on the immune microenvironment. In this context, future studies should leverage the power of new in situ transcriptomic techniques to determine how the transcriptional and epigenetic heterogeneity of GBM cells controls TAM subsets in different tumour regions.
Microglia
Physiological functions and heterogeneity.
Microglia arise from yolk sac haematopoietic progenitors31, which self-renew in situ via mechanisms dependent on colony-stimulating factor 1 receptor (CSF1R) signalling31,56,57. Microglia mature prior to the differentiation of other glia or neurons33 and thus they are crucial for normal CNS development and homeostasis as they prune synapses through a mechanism that involves complement proteins58. While studies in healthy adult mice did not detect extensive spatial heterogeneity59,60, human microglia display region-specific transcriptional programmes, for example, associated with grey and white matter22,61,62 (FIG. 1b). In addition, microglial transcriptional programmes diversify as humans age22,63,64. Indeed, the comparison of microglia from ten species identified greater microglial diversity in humans than in all other species analysed, including macaque, marmoset, sheep, hamster and mice65. This higher microglial diversity was attributed to evolutionary biological complexity rather than to microbial exposure for two reasons: (1) all individual human samples shared the transcriptional signatures of most microglial subtypes and (2) microglial diversity was the same between wild mice and mice housed in specific pathogen-free facilities65. However, the roles of most of these microglial subsets in health and disease are still unknown.
Neurological disorders are associated with changes in microglial activation states related to immune cell recruitment, cell debris removal and cytokine production but typically not antigen presentation33. Several microglial subsets have been identified based on their transcriptional signatures detected in neurological diseases such as Alzheimer disease and multiple sclerosis66-70. In the context of neurologic disease, at least two microglial subsets have been identified in mice: (1) microglia associated with neurodegeneration driven by TREM2 receptor–apolipoprotein E (APOE) signalling and depicting the increased expression of genes believed to perpetuate disease (Apoe, Axl, Clec7a, Csf1 and Ccl2)70; and (2) homeostatic microglia, featuring upregulation of the phagocytosis and lipid metabolism pathways thought to ameliorate disease through clearance of pathogenic cell debris67. The roles of these microglial subsets in the pathogenesis of neurological diseases are still under investigation as are the location-specific cues responsible for inducing disease-associated transcriptional and epigenetic changes34,60,71.
Evidence for microglia–brain tumour interactions.
In brain malignancies, tumour-associated microglia display substantial transcriptional heterogeneity, potentially associated with specific functions and therapeutic targets10 (FIG. 1b,c). For example, it was recently reported that microglial neuropilin 1 (NRP1), which acts as a receptor for placental growth factor, semaphorin 3A, vascular endothelial growth factor A (VEGFA) and tuftsin, could be a therapeutic target in GBM72-76 (FIG. 2). NRP1 amplifies transforming growth factor-β (TGFβ) signalling75, driving the expression of anti-inflammatory genes that limit glioma-specific immunity77,78. The administration of the selective NRP1 inhibitor EG00229 modulated microglial gene expression, increased glioma-specific CD8+ T cell immunity and extended survival in a mouse model of GBM79. Moreover, elevated NRP1 expression is associated with poor prognosis in patients with GBM, suggesting that the administration of NRP1 inhibitors in combination with antibodies targeting the immune-checkpoint protein PD1 may successfully activate GBM-specific T cells80.
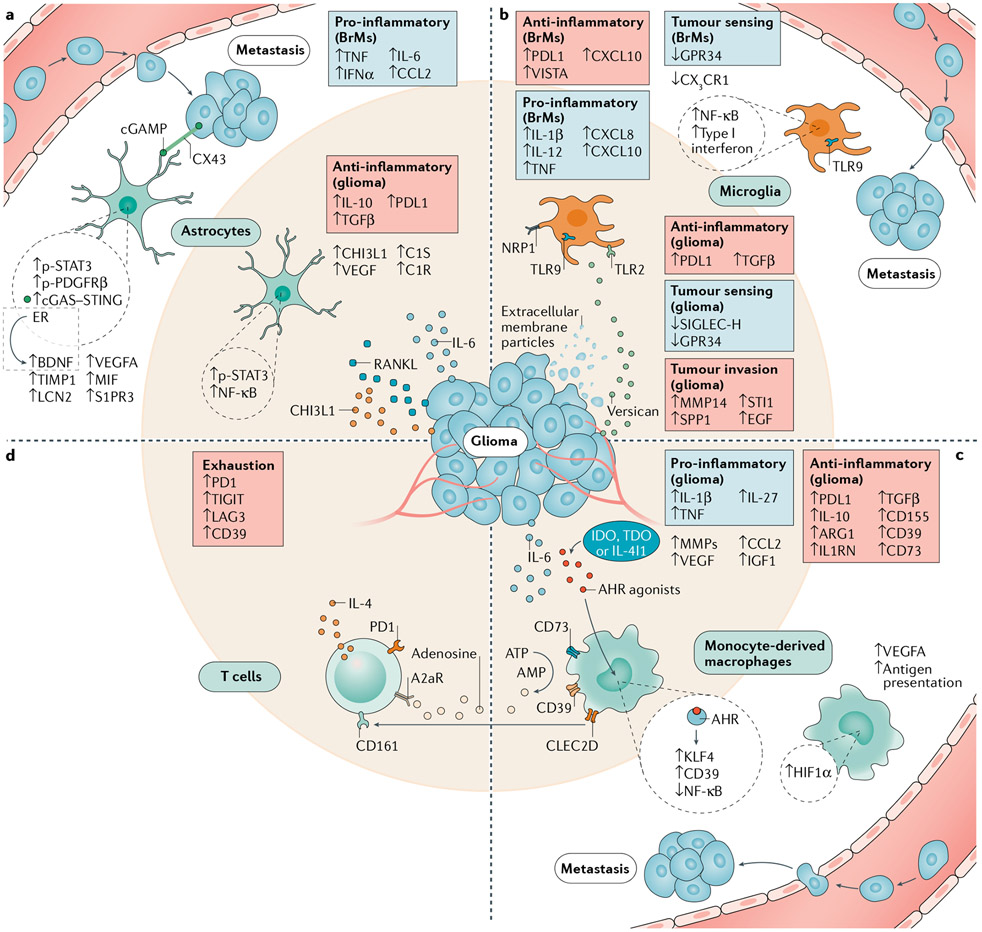
Fig. 2 ∣. Mechanisms of cell communication involved in tumour establishment, invasion and immune escape.
Multiple signalling pathways are activated in brain tumour microenvironment astrocytes and tumour-associated macrophages, but their contribution to immunosuppression as well as the dominance of a specific pathway within specific subpopulations (not shown) are unknown. Astrocytes (part a) upregulate genes encoding immunosuppressive (IL-10, transforming growth factor-β (TGFβ) and PDL1) and pro-oncogenic (CHI3L1) factors in the glioma tumour microenvironment, while nuclear factor-κB (NF-κB) signalling is concomitantly induced by tumour-derived RANKL. STAT3 is a central regulator of astrocyte reactivity programmes and characterizes a brain metastasis-promoting astrocyte subset. Other subsets promote tumour growth through paracrine factors such as brain-derived neurotrophic factor (BDNF) or TNF through cell contact-dependent (CX43) or cell contact-independent mechanisms. Microglia (part b) and monocyte-derived macrophages (MDMs) (part c) can have both pro-inflammatory and anti-inflammatory functions. In microglia (part b), tumour sensing (SIGLEC-H and GPR34) and homeostatic (CX3C-chemokine receptor 1 (CX3CR1)) programmes are disrupted, while TLR2 and NRP1 signalling respectively increase tumour invasion and immunosuppression. In monocyte-derived macrophages (part c), an anti-inflammatory phenotype supported by aryl hydrocarbon receptor (AHR) activation suppresses T cell responses (part d) via the catabolism of ATP. T cells supply tumour-associated macrophages with a CSF1R-independent survival signal by inducing IGF1 via IL-4. Anti-tumour T cell responses are suppressed by cytokines such as IL-10 and TGFβ, checkpoint proteins such as PDL1 and VISTA, and adenosine signalling. Overall T cell exhaustion is evidenced by increased immune-checkpoint expression and the inhibitory receptors A2aR and CD161 are broadly present in glioblastoma-infiltrating T cells. ARGl, arginase 1; cGAMP, cyclic GMP–AMP; EGF, epidermal growth factor; ER, oestrogen receptor; HIF1α, hypoxia-inducible factor 1α; IGF1, insulin-like growth factor 1; MIF, macrophage inhibitory factor; MMPs, matrix metalloproteinases; NRP1, neuropilin 1; p-PDGFRβ, phosphorylated platelet-derived growth factor receptor-β; p-STAT3, phosphorylated signal transducer and activator of transcription 3; TLR, Toll-like receptor; VEGFA, vascular endothelial growth factor A.
Several glioma-derived factors have been shown to induce pro-tumorigenic functions in brain tumour-associated microglia. Toll-like receptor 2 (TLR2) is upregulated in glioma-associated microglia and has been shown to support tumour progression and invasion81. Glioma cells express the endogenous TLR2 ligand versican, which upregulates the microglial expression of matrix metalloproteinase 14 (MMP14), linked to increased tumour invasiveness and growth82 (FIG. 2). Therefore, the therapeutic inhibition of TLR2 using O-Vanillin and other antagonists has been proposed as an approach to reduce microglia-dependent glioma invasiveness83. Conversely, systemic TLR9 stimulation via type B84 and C85 CpG oligodeoxynucleotides inhibited glioma growth84,86 and lung cancer and melanoma BrM formation85 in mice. While the precise mechanisms involved were not determined, TLR9 stimulation upregulated the phagocytic machinery in microglia, and contact between microglia and tumour cells was required for tumour cell death85. Further work in organotypic glioma cultures detected enhanced microglial phagocytosis following TLR3 and TLR9 co-activation86. Thus, innate sensors represent candidate targets to augment tumour-associated microglial phagocytic and cytotoxic programmes.
Tumour-derived extracellular membrane particles are also important microglial modulators in the TME. It was recently reported that the microglial uptake of fluorescently labelled membrane material from mouse glioma cells in vivo triggers functional modifications, including the upregulation of the immune-checkpoint protein PDL1 and multiple MMP-encoding genes, concomitant with the downregulation of pathways involved in tumour sensing such as SIGLEC-H and the G protein-coupled receptor GPR34 (REFS87,88) (FIG. 2). Indeed, the analysis of human glioma samples detected the downregulation of two-thirds of the known microglial sensome87, the set of receptors that sense the local microenvironment89. This downregulation of sensome-encoding genes is more pronounced at the tumour core27,87 and in microglia that have taken up GBM extracellular membrane particles87. Further investigations of the cargo of tumour-derived extracellular membrane particles are needed to identify additional molecules (for example, proteins, microRNAs and metabolites) involved in the modulation of microglial responses in the TME and potentially at distant sites.
Recent work reported preliminary comparisons of microglia in glioma and BrMs7,10. As mentioned previously, microglia comprise a smaller proportion of the TAM compartment in BrMs than in glioma10 (FIG. 1a). In addition, three findings support a more activated microglial phenotype in IDHwt glioma and BrM than in IDHmut glioma: (1) microglia from IDHwt gliomas and BrMs express increased levels of CD14 and the scavenger receptor CD64; (2) microglia from IDHwt gliomas and BrMs show an amoeboid morphology, associated with activation; and (3) microglia from IDHmut gliomas display a ramified (branched) morphology associated with homeostatic microglia7. Further observations were made through bulk RNA sequencing of microglia and MDMs in human BrMs and gliomas. First, BrM-associated microglia express gene sets associated with type I interferon signalling and nuclear factor-κB (NF-κB) signalling, whereas glioma-associated microglia lack expression of these gene sets. Second, BrM-associated microglia distinctly showed increased expression of CXC-chemokine ligand 8 (CXCL8, also known as IL-8), a known neutrophil attractant and, accordingly, neutrophils more robustly infiltrated BrMs than gliomas10. Taken together, these findings suggest complex tumour-specific functional interactions between microglia and tumour cells in the TME.
Microglial heterogeneity in brain tumours.
Single-cell studies have made considerable progress in revealing TAM transcriptional diversity, paving the way for the analysis of the functional roles of TAM subpopulations in BrMs and other CNS malignancies. An analysis of microglial heterogeneity in human IDHwt GBM and matched controls combined single-cell RNA sequencing (scRNA-seq) with mass cytometry, identifying nine microglial clusters in published scRNA-seq datasets and surgical controls22 (TABLE 1). Gene ontology analyses detected gene expression signatures suggestive of diverging functions in different subsets: antigen processing and presentation, production of pro-inflammatory cytokines, responses to type I interferons, and chemotaxis22. GBMs contained all microglial clusters identified in control tissue (albeit with different frequencies) and at least two additional unique transcriptional clusters; one such tumour-associated microglial cluster was also detected in an independent IDHwt GBM dataset27. GBM-specific microglia clusters had downregulated microglial core gene transcripts and upregulated metabolic, inflammatory and interferon-related transcripts22. Interestingly, mass cytometry studies detected preserved moderate-level expression of the protein products of microglial core genes, suggesting that post-transcriptional regulatory mechanisms play an important role in the control of microglial responses in the TME22. Collectively, these findings identify novel microglial subsets associated with GBM22; the study of their functions and regulatory mechanisms may guide new approaches for their therapeutic modulation.
In a mouse model of non-small-cell lung cancer BrM, the diversity of BrM-associated microglia was lower than that of MDMs, yet scRNA-seq identified four clusters that had upregulated pro-inflammatory genes, including Il1b (encoding IL-1β), Ifnb1 (encoding IFNβ1), Il12 (encoding IL-12) and Tnf (encoding TNF), along with downregulated homeostatic genes47 (TABLE 1). In addition, radiotherapy amplified inflammatory responses and stress-associated programmes in microglia47. While distinct gene signatures were detected during the establishment of metastasis and response to radiation, this study did not investigate the roles of these transcriptional programmes in functional experiments47.
Another study combined cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) and cytometry by time of flight (CyTOF) to define seven clusters of BrM-associated microglia in mice and humans23. Intriguingly, CNS-resident myeloid cells promote BrM growth23. By contrast, depletion of CC-chemokine receptor 2 (CCR2)+ tumour-associated MDMs did not reduce tumour growth as demonstrated in mouse models of glioma32. A subset of CNS-resident myeloid cells, which consists primarily of microglia, secrete CXCL10 and are enriched for the T cell-suppressive molecules VISTA and PDL1. Blockade of CXCL10 or VISTA in combination with anti-PDL1 treatment led to the control of BrM in mice23 (TABLE 1; FIGS 1c,2). This study demonstrates the importance of tumour-associated microglia in brain malignancies and the complex role of CXCL10, which promotes T cell recruitment90 but also immune-suppression through the microglial expression of VISTA and PDL1 (REF.23). Moreover, this study described a key difference between CCR2+ macrophages in BrM and glioma, where CCR2+ macrophage depletion impairs tumour growth9. Taken together, these studies exemplify how single-cell technologies may identify novel therapeutic targets in unique TAM subsets across tumour types.
Monocyte-derived macrophages
Recruitment and education.
Monocytes do not infiltrate the healthy brain parenchyma but, in the context of inflammatory conditions such as cancer, monocytes are recruited to the CNS through mechanisms mediated by the CCR2 (REF.16) ligands CCL2 (REF.91) and CXCL12 (REF.92). This process is facilitated by the breakdown of the blood–brain barrier93. In malignant gliomas, MDMs represent over a third of the absolute tumour mass and their abundance correlates with glioma grade94. Hence, it is not surprising that some of the earliest TAM-targeting drugs tested clinically in brain cancer were chemokine receptor blockers such as maraviroc and plerixafor (TABLE 2). More recently, high IL-33 expression was associated with increased tumour-associated MDM infiltration, possibly as a result of the control of microglial chemokine production95. The roles of these pathways in the control of microglia and MDMs in the brain TME should be further investigated.
Table 2 ∣.
Clinical trials targeting MDMs, microglia and astrocytes in brain cancer
| Targeted pathways |
Therapeutic agent and preclinical ref.a |
Trial IDb and/ or ref. |
Date initiated |
Phase | Tumour type | Result | Designed to target TAMs or astrocytes? |
|---|---|---|---|---|---|---|---|
| Microglia, MDM | |||||||
| MIF–CXCR4 and CXCL12–CXCR4 | Plerixafor + bevacizumab | NCT01339039 | 2011 | 1 | HGG (R) | Terminated, low accrual | Yes |
| Chemoradiation followed by plerixafor | NCT01977677 c | 2013 | 1 | GBM (ND) | Completed, safe | ||
| RT followed by plerixafor + TMZ | NCT03746080 (REF.224)c | 2018 | 2 | GBM (ND) | Recruiting | ||
| PDL1–PD1 and PDL2–PD1 | Several8,178,180,d | NCT03047473 | 2017 | 2 | GBM (ND) | Accrual met, ongoing | No |
| NCT02968940 | 2016 | 2 | IDHmt glioma (R) | Completed, results not published | |||
| NCT03174197 | 2017 | 1/2 | GBM (ND) | Active, not recruiting | |||
| NCT02550249 (REFS179,225) | 2015 | 2 | GBM (ND, R) | Completed, possible benefit | |||
| NCT02617589 (REF.178) | 2015 | 3 | GBM (ND) | Completed, did not meet primary endpoint (OS) | |||
| CCL5–CCR5 | Leronlimab | NCT04504942 | 2020 | 2 | Advanced cancer (R; stable BrM) | Recruiting | Yes |
| CD39–CD73–AMP–adenosine | Oleclumab8 (with durvalumab + paclitaxel + carboplatin) | NCT03616886 | 2018 | 1/2 | Triple-negative breast cancer, advanced (R, stable BrM) | Recruiting | No |
| CSF1–CSFR1 | Pexidartinib (also known as PLX3397) | NCT01349036 (REF.204)c | 2011 | 1 | GBM (R) | Terminated by sponsor | Yes |
| Pexidartinib + RT/TMZ | NCT01790503 c | 2013 | 1b/2 | GBM (ND) | Completed, safe but no evidence of efficacy | ||
| AXL kinase | Bemcentinib188 (pre- or post-surgery) | NCT03965494 | 2019 | 1 | GBM (R) | Recruiting | Yes |
| SIRPα–CD47 | BI 765063 +/− anti-PD1 | NCT03990233 | 2019 | 2 | Advanced solid tumours (stable BrM allowed) | Recruiting | Yes |
| MDMs | |||||||
| IDO1 | Epacadostat197 (with RT + bevacizumab +/− anti-PD1) | NCT03532295 | 2018 | 2 | GBM (R) | Recruiting | No |
| Linrodostat (with anti-PD1 + RT +/− TMZ) | NCT04047706 | 2019 | 1 | GBM (ND) | Recruiting | ||
| Indoximod + TMZ | NCT02052648 | 2014 | 1/2 | HGG (R) | Completed, results not published | ||
| Indoximod + RT + chemotherapy | NCT02502708 c | 2015 | 1 | Paediatric HGG (R) | Completed, safe | ||
| Indoximod + RT + chemotherapy | NCT04049669 c | 2019 | 2 | Paediatric HGG (R) | Recruiting | ||
| PF-06840003 | NCT02764151 (REF.198) | 2016 | 1 | Glioma (R) | Completed, safe but sponsor not pursuing | ||
| Brain tumour-associated astrocytes in contact with BrM | |||||||
| Connexin 43 | Meclofenamate175 | NCT02429570 | 2015 | 1 | BrM (R) | Accrual met, ongoing | Yes |
| STAT3-high tumour-associated astrocytes | |||||||
| STAT3 | WP1066 | NCT01904123 | 2013 | 1 | GBM (R) or melanoma BrM (R) | Recruiting | No |
| NCT04334863 | 2020 | 1 | Paediatric medulloblastoma or HGG | Recruiting | No | ||
| Silibinin12 | NA | 2016 | 1 | Lung cancer BrM (R) | Completed, 75% overall response rate | No | |
BrM, brain metastasis; GBM, glioblastoma; HGG, high-grade glioma; IDHmut, isocitrate dehydrogenase mutant; IDO1, indoleamine 2,3-dioxygenase 1; MDMs, monocyte-derived macrophages; NA, not available; ND, newly diagnosed; R, recurrent; RT, radiation; TAMs, tumour-associated macrophages; TMZ, temozolomide; +/− indicates treatment with or without the drug that follows this abbreviation.
Drug class can be determined by conventional nomenclature: drugs ending in ‘-mab’ are monoclonal antibodies; those ending in ‘-ib’ are small-molecule inhibitors. Other drugs not following this convention are small-molecule inhibitors, with the exception of BI765063, which is a monoclonal antibody. Preclinical references are given where available.
NCT Trial IDs can be accessed at https://clinicaltrials.gov/.
Clinical trials that are linked (phase I and II of the same agent).
In the brain TME, monocytes assume activation states that result from an adaptation to both the CNS and the TME (FIG. 1b,c; TABLE 1). Among them, RNA expression signatures containing genes encoding anti-inflammatory molecules, such as arginase 1 (ARG1), CD39, TGFβ, IL-10, IL-1 receptor antagonist (IL1RN), CD155, macrophage scavenger receptor 1 (MSR1) and PDL1 (REFS10,21,27,43,96,97), are of particular interest for therapeutic immunomodulation (FIG. 2). TME-infiltrating monocytes also have increased expression of major histocompatibility complex (MHC) class II molecules, proteins involved in the modification of the extracellular matrix and proangiogenic factors such as VEGFA. Taken together, these findings suggest that brain tumours control not only the recruitment but also the phenotype of MDMs in the TME.
MDM heterogeneity.
Numerous studies analysed MDMs in bulk following the isolation of defined cell populations using CD45, CD11b or lineage tracing in mouse models in combination with canonical markers to distinguish MDM from microglia10,21,24,41,42,44,96,98. Unbiased single-cell technologies have recently identified additional MDM subsets (TABLE 1). Notably, the acquisition of a microglia-like signature in both the glioma and BrM TME suggests an adaptation of MDM to the brain microenvironment regardless of tumour presence (TABLE 1; BOX 3), similar to previous findings35,99.
Most recently, single-cell transcriptomic and proteomic approaches were used to comprehensively profile MDM subsets in human GBM and the mouse model GL261 (REF.16). In addition to undifferentiated as well as transitory monocytes, the authors identified six monocyte-derived TAM clusters with distinct signatures, including phagocytosis/lipid metabolism, hypoxia/glycolysis, microglia-like (selenoprotein P (SEPP1)low), anti-inflammatory (SEPP1high) and IFNγ-induced MDMs16 (TABLE 1). A specific MDM subset featuring hypoxia-related signalling was exclusively present in recurrent GBM, suggesting that this signalling pathway may enable therapeutic resistance16. BrM-associated MDMs could also be classified into different subsets and had upregulated antigen presentation pathways as well as responses to a hypoxic environment characterized by increased hypoxia-inducible factor 1α (HIF1α) and VEGFA47. Heterogeneity was also detected along the macrophage differentiation axis, and sex-specific transcriptional differences in the expression of MHC class II genes and the gene encoding macrophage inhibitory factor (MIF)100 exist. Overall, the detection of diverse MDM subpopulations and tumour type-specific MDM education hint at specific roles performed by different MDM subsets in the TME, which require further functional investigation.
MDSCs and their overlap with MDMs.
Myeloid-derived suppressor cells (MDSCs), derived from monocytic or granulocytic cells, are detected in the TME of many cancers, including GBM101. MDSCs were previously distinguished from MDMs and microglia based on the expression of CD14, CD45 and CD11b, though highly specific markers are still lacking41,101. The ability of MDSCs to suppress GBM-specific CD8+ T cell responses via ARG1, IL-10, TGFβ and other molecules101,102 has raised interest in this TAM population. Indeed, a granulocyte–macrophage colony-stimulating factor (GM-CSF)-induced subset of glioma-associated IL-4Rα+ MDSCs suppressed T cells via ARG1 in mice30. Thus, the investigation of brain tumour-associated MDSCs at single-cell resolution is likely to reveal heterogeneous cell states and shed light on therapeutically relevant mechanisms driving specific MDSC subsets, as recently reported in fibrosarcoma103.
Mechanisms of MDM-mediated immune suppression.
MDMs participate in multiple immunosuppressive mechanisms that limit tumour-specific T cell responses (FIG. 2). The aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor expressed by TAMs, T cells and GBM cells, is activated by numerous ligands, including GBM-derived kynurenine and other oncometabolites, through IDO1, TDO2 or IL-4I1 (REFS104,105). AHR activation in monocytes boosts their recruitment to the brain and upregulates immunosuppressive gene expression in MDMs via the induction of the transcription factor KLF4, the suppressor of cytokine signalling 2 (SOCS2)-dependent downregulation of NF-κB signalling44 and the expression of the ectoenzyme CD39, which cooperates with CD73 to catalyse the production of the T cell-suppressive metabolite adenosine106. In addition, AHR activation is involved in the induction of forkhead box P3 (FOXP3)-negative type 1 regulatory T cells107,108, which limit effector T cell responses and are enriched in peripheral blood mononuclear cell and brain samples from patients with GBM109. These findings suggest that AHR signalling in TAMs and potentially in T cells contributes to the suppression of brain tumour-specific immunity.
Further mechanisms of MDM-mediated suppression of antitumour immunity have been proposed and demonstrated to varying degrees in brain tumour-associated MDMs, other TAMs or MDSCs. These mechanisms include the expression of PDL1 (REFS7,97,100,110-113) and PDL2 (REF.97), soluble factors such as IL-10 (REFS41,111), VEGFA114 and TGFβ97,100,114, catabolic enzymes such as ARG1 (REFS30,41), and indoleamine 2,3-dioxygenase 1 (IDO1)8, chemokines9,44 and IL-33 (REF.95) (FIG. 2). While multiple mechanisms are common to both human and mouse cells, increased ARG1 expression was detected in mouse but not human glioma-associated MDMs16. Most of these mechanisms have not been definitively investigated in loss-of-function studies but inferred based on gene expression analyses and functional studies in tumours outside the CNS115-117.
Roles of astrocytes in brain tumours
Astrocytes are the most abundant glial cells in the CNS, with remarkable functional diversity in health and disease. During homeostasis, astrocytes regulate synaptogenesis and neurotransmission, provide essential metabolic support to neurons, and modulate blood flow as well as the blood–brain barrier118. These homeostatic functions are disrupted in disease119,120. In addition, astrocytes secrete multiple immunomodulatory molecules such as cytokines, chemokines and metabolites with important effects in inflammation and neurodegeneration121-128.
Astrocytes communicate with microglia, monocytes and T cells among other cell types, playing important roles in infection, neurodegeneration and autoimmunity37,122,129-135. In addition, astrocytes have been reported to both inhibit136 and promote137-141 brain tumour growth in mice in addition to human in vitro systems but less is known about astrocyte subsets and their potential roles in the TME and brain tumour-directed immunity.
Potential astrocyte subpopulations
Astrocytes exhibit substantial heterogeneity, which is further amplified by region-specific transcriptional signatures in health and disease142-146. Specifically, trophic signals induce regional transcriptional programmes during development that remain stable into adulthood147-149. In addition, astrocytes acquire specialized functions as a result of neural circuit-specific modulation150-153 (FIG. 2). Indeed, often, the maintenance of a non-activated state in astrocytes depends on signals produced by local neurons154,155. The role of these region-specific astrocyte subsets on tumour development and their regulation by tumour cells is poorly characterized.
Trauma, infection, stroke, neurodegeneration, inflammatory disease and malignancy alter astrocyte phenotype and function. Bulk transcriptional analyses identified Janus kinase (JAK)–signal transducer and activator of transcription 3 (STAT3) signalling as a master activator of astrocytic neurotoxic and anti-inflammatory or reparative responses156,157. Moreover, NF-κB activation is reported to potentiate the neurotoxic functions of STAT3-positive astrocytes in some neurological diseases such as multiple sclerosis158. Conversely, the activation of p38 MAPK159, the deubiquitinating enzyme A20 (also known as TNFAIP3)160, otubain 1 (REF.161), SOCS1 (REF.162), SOCS2 (REFS122,163) and SOCS3 (REFS162,164) decreases astrocyte neurotoxic and pro-inflammatory activities122,159-166 (FIG. 2). Collectively, these findings identify signalling pathways involved in the control of astrocyte responses, although the specific astrocyte subsets involved are still mostly unknown.
Single-cell analyses (BOX 2) are beginning to identify functionally relevant astrocyte subsets in neurological diseases127,128,167, including brain cancers25,168. Indeed, the heterogeneity of astrocyte functional responses suggest that astrocyte subsets specialized in promoting CNS repair and limiting inflammation promote tumour growth and the generation of an immunosuppressive TME in GBM and BrMs. While tumour-associated astrocyte subsets have not been defined using single-cell analyses, changes in peritumoural astrocytes have been described with traditional immunohistochemistry12,168-170 and flow cytometry142. In pioneering studies, reporter mouse strains and flow cytometry antibody screens were used to define five astrocyte subsets conserved across different brain regions, with region-specific variations in their relative abundance142. Interestingly, the transcriptional programme of malignant glioma cells was closely related to that of a synapse-promoting astrocyte subset142, setting the stage for the study of astrocyte heterogeneity and function in CNS malignancies.
Astrocyte subsets in brain tumours
Several studies have recently analysed the roles of astrocyte subsets in CNS malignancies and their potential as therapeutic targets12,25,168-170. As with macrophages, some reports describe subsets without identification of a clear functional role. For example, one study identified a subset of astrocytes that expresses phosphorylated platelet-derived growth factor receptor-β (p-PDGFRβ) and is located at the borders of brain micrometastases of HER2-amplified breast cancer170. The authors reasoned that, as p-PDGFRβ participates in the recovery from stroke171 and penetrating brain injury172, p-PDGFRβ may also participate in the early stages of breast cancer BrM. While the exact role of this astrocyte subset remains unknown, it could be depleted by the inhibition of p-PDGFRβ with the tyrosine kinase inhibitors pazopanib170 or crenolanib173.
A second study focused on the role of astrocyte subsets in regulating blood–tumour barrier permeability. First, the authors performed RNA microarray gene expression analysis on metastases in mice displaying varying degrees of blood–tumour barrier breakdown168. This study detected the upregulation of sphingosine 1 phosphate receptor 3 (S1PR3) expression in astrocytes in mice bearing breast cancer BrMs and in patients with BrMs. Interestingly, S1PR3 inhibition restored blood–tumour barrier integrity in mice. Conversely, S1PR3 activation in cultured human astrocytes induced IL-6 and CCL2 production, and S1PR3 expression in human BrMs was predictive of blood–tumour barrier permeability as determined by contrast enhancement168. Therefore, an S1PR3-induced astrocyte state may be responsible for the disruption of vascular integrity, affect recruitment of leukocytes from the circulation and play a role in the establishment of BrMs.
Additionally, a recent study investigated the role of oestrogen receptor (ER)-expressing BrM-associated astrocytes to provide paracrine signals promoting tumour cell invasion169. Prior studies reported an increased risk of BrM in premenopausal women with HER2+ breast cancer or triple-negative breast cancer (TNBC) (in which cancer cells lack ER, HER2 and the progesterone receptor) compared with postmenopausal women with either cancer type174. Initial studies confirmed the expression of ERα and ERβ in astrocytes, which produced brain-derived neurotrophic factor (BDNF), a ligand for epidermal growth factor receptor (EGFR) and tropomyosin-related kinase-β (TRKβ). EGFR and TRKβ signalling induced the migration and invasion of TNBC BrM cells, which could be inhibited by depletion of oestrogen or inhibition of TRKβ and BDNF with small molecules169. These studies suggest that BrM-associated astrocytes could be manipulated with hormonal therapies.
In a fourth study, BrM cells induced a subset of tumour-associated astrocytes to produce pro-inflammatory cytokines through cell contact and gap junctions formed by connexin 43 (CX43)175. In particular, the translocation of cyclic GMP–AMP (cGAMP) into the astrocyte cytosol via CX43 channels activates stimulator of interferon genes (STING), inducing the production of IFNα and TNF, which promoted metastatic growth175. Astrocyte–tumour cell communication through CX43 also plays a role in GBM, although further molecular mechanisms mediated by CX43 are still unknown176.
Two recent studies12,25 investigated the role of brain tumour-associated astrocytes in shaping the neuroinflammatory milieu. In the first, bulk astrocytes isolated from GBM and epilepsy tissue samples were analysed by bulk RNA sequencing25. Gene set enrichment analysis detected increased IFNγ and JAK–STAT signalling in GBM-associated astrocytes; the expression of pro-inflammatory, neurotoxic molecules such as CD37, PDL1, chitinase 3-like protein 1 (CHI3L1), and complement components C1S and C1R was increased in purified astrocytes from tumour specimens25. The gene expression profiles of GBM-associated astrocytes differed from those of progenitor, mature, neurotoxic or neuroprotective astrocytes. Microglia were found to be important regulators of astrocyte responses in GBM as described in other neurological disorders37,124,158. Indeed, astrocytes isolated from tumours secreted anti-inflammatory cytokines such as IL-10 and TGFβ but only in the presence of microglia or macrophages25. Interestingly, the JAK inhibitor ruxolitinib boosted astrocyte secretion of pro-inflammatory cytokines such as IFNγ and TNF, suggesting that the modulation of astrocyte responses can abrogate immunosuppressive mechanisms operating in the TME25.
A second study described an anti-inflammatory astrocyte subset driven by STAT3 in the BrM TME in patient samples and mouse models of BrM12. STAT3+ astrocytes from the margins of the human and mouse BrM TME secreted MIF, modulated TAM responses and suppressed tumour-specific T cells. These studies raise several important points for consideration for further studies. First, STAT3 is a therapeutic target in a subset of BrM-associated and potentially GBM-associated astrocytes. As STAT3 is linked to both neurodegeneration-associated and BrM-associated astrocytes, single-cell studies are needed to characterize STAT3+ astrocytes associated with brain malignancies to investigate further defining and function-driving gene programmes for targeting. In addition, the location of STAT3+ astrocytes at the margins of BrMs calls for further studies of the spatial heterogeneity and cell–cell interactions in the TME. Although numerous differences exist regarding the TME of primary brain tumours and BrMs (FIG. 1a), the activation of JAK–STAT signalling in astrocytes associated with malignancies of different ontogenies highlights the potential to identify common therapeutic targets.
Taken together, these studies highlight the need for a better characterization of astrocyte subsets in the TME to identify novel mechanisms of disease pathogenesis and candidate targets for therapeutic intervention.
Targeting TAMs and astrocytes
The therapeutic targeting of TAMs and astrocytes for the treatment of glioma and BrM has been attempted in many clinical trials. Thus far, these trials have shown modest responses, and multi-target approaches will likely be required to shift the neuroinflammatory environment of the TME (TABLE 2).
The PD1–PDL1 axis
Antibodies blocking T cell inhibitory checkpoints induce remarkable clinical responses in numerous malignancies. In particular, antibodies blocking PDL1 and PD1 are efficacious in patients with metastatic cancer (TABLE 2), but their efficacy for the treatment of brain malignancies varies widely1-3,177. Anti-PD1 antibodies induce the regression of melanoma and lung cancer BrMs with similar degrees of success to those achieved with metastases to other organs, but patients with more rapidly growing tumours respond less often1-3. Renal cell carcinoma BrMs show positive responses in only 12% of patients, whereas 25% of patients with renal cell carcinoma metastases respond elsewhere177. As with systemic metastases, BrMs respond more often when additional T cell checkpoints, such as CTLA4, are targeted in addition to the PD1–PDL1 axis1,2. In the context of GBM, standard checkpoint blocking antibody treatments induce positive responses in roughly 8% of patients178. The timing of PD1 blockade may be relevant as a recent clinical trial in recurrent GBM reported a significant extension of median survival when anti-PD1 treatment was initiated prior to tumour resection179 (TABLE 2). Potential mechanisms involved in resistance to checkpoint blockade therapy in the brain include bioavailability, intratumoural heterogeneity and a relative paucity of tumour neoantigens.
PDL1 is expressed in GBM and BrM tumour cells, but numerous researchers have also reported its expression on microglia and MDMs87,110,180. PDL1 expression in tumour cells and TAMs is heterogeneous181 and may be induced in TAM subsets by local paracrine signals such as tumour-derived prostaglandin E2 (REF.182), CXCL10 (REF.23) or IL-6 (REF.183). In a mouse model of hypermutated GBM, myeloid PDL1 expression negatively correlated with the clinical response to anti-PD1 antibody treatment, suggesting that mechanisms other than canonical PD1-mediated T cell suppression may be at play97. Indeed, PDL1 also interacts with CD80 (also known as B7-1), which is expressed on CD4+ and CD8+ T cells184. Furthermore, PDL1-expressing MDMs increased CD4+ FoxP3+ regulatory T cell numbers in vitro97, further supporting a role for PDL1 as an important mechanism of MDM-mediated immunosuppression.
PDL1 expression in TAMs from non-brain metastases predicts response to antibodies against PDL1 or PD1 in TNBC185 and non-small-cell lung cancer186 but correlative data on PDL1 expression in BrM TAMs are currently lacking. The heterogeneity of PDL1+ and PDL1− TAM subsets in BrM and GBM also remains unknown. Nevertheless, ongoing investigations are focused on targeting the PD1 axis in combination with microglial signalling pathways such as the receptor tyrosine kinases AXL, TYRO3 and MER187-189. The AXL inhibitor bemcentinib (also known as BGB324) is now being tested in a phase I trial for recurrent GBM as a monotherapy190 (TABLE 2), but dual inhibition of PD1 and AXL has been shown to extend survival in preclinical models of melanoma BrMs and GBM187,188.
CD39 and CD73 ectoenzymes
CD39 cooperates with CD73 to produce the immunosuppressive metabolite adenosine106. Mass cytometry in combination with scRNA-seq was recently used to analyse various patient tumours after anti-CTLA4 and/or anti-PD1 treatment, resulting in the identification of a CD73high population of TAMs8 depicting a transcriptional signature closer to that of MDMs than microglia. Indeed, the combination of CD73 targeting with CTLA4 and/or PD1 blockade improved survival in GBM mouse models8.
Pharmacological inhibitors and blocking antibodies targeting CD73, CD39 and adenosine receptors have been developed to interfere with the immunosuppressive mechanisms driven by CD39 and CD73 (REF.106). However, extensive blockade of this axis did not show efficacy in mouse models of GBM, dampening enthusiasm for clinical trials targeting CD73, CD39 or adenosine in GBM191. Thus far, the only active clinical trial in this area is focused on the effects of CD73-specific antibodies in combination with anti-PDL1 therapy in patients with metastatic TNBC (TABLE 2). However, unless clinically stable, BrMs are an exclusion criterion. Two studies have documented the association between soluble CD73 and immunotherapy-refractory disease, which is sometimes associated with systemic metastases192. However, there are no reports of the specific upregulation of CD73 and CD39 in BrMs, with one study reporting CD73 expression only in endothelial cells and not in melanoma BrM-associated leukocytes193. While there may be a rationale for targeting the CD39–CD73 axis for systemic anti-cancer immunity, and not all types of BrMs may possess the same biology, currently the evidence for targeting this axis for brain tumours remains weak.
IDO1
GBM cells194 and associated TAMs10 express IDO1, the rate-limiting enzyme metabolizing tryptophan into kynurenine8. IDO1 limits T cell responses through multiple mechanisms44-195, including the expansion of regulatory T cells196 and the activation of AHR in TAMs and other cell types44 (FIG. 2). IDO1 expression by non-malignant cells was sufficient to drive tumour progression in mouse models of GBM, supporting therapeutic interventions based on IDO1 inhibition in combination with radiation, cytotoxic chemotherapy and immune-checkpoint blockade197. While initial clinical trials with IDO1 inhibitor monotherapy in GBM did not show efficacy198 (TABLE 2), two ongoing phase II trials are investigating the combination of IDO1 inhibitors with anti-PD1 antibodies, radiation or other systemic therapies199,200 (TABLE 2). Moreover, a recent report described IL-4I1 as the main tryptophan-catabolizing enzyme that leads to AHR activation in glioma and numerous other malignancies105. Thus, the future of IDO1 inhibition in GBM is uncertain as tryptophan-catabolizing enzymes other than IDO1 may have compensatory effects and IDO1 inhibitors may not adequately suppress AHR agonist production in GBM. An alternative approach may be the blockade of AHR, which mediates the immunosuppressive activities of the products of IDO1, tryptophan 2,3-dioxygenase (TDO) and IL-4I144,201,202. Moreover, while tryptophan metabolism has been targeted in GBM and IDO1 inhibition has been tested in metastatic cancer in general, the effect of IDO1 inhibition on BrMs has not been evaluated.
The CSF1–CSF1R axis
The disruption of colony-stimulating factor 1 (CSF1) signalling via CSF1R can lead to the depletion of certain subsets of macrophages16,56,57. Consequently, CSF1R inhibition has been explored for the therapeutic modulation of the GBM TME (TABLE 2). CSF1R inhibition reduced tumour growth in a mouse model of the proneural subtype of GBM11,203. Interestingly, instead of the expected depletion of GBM TAMs, it was their phenotypic reprogramming that diminished pro-tumour functions and increased sensitivity to chemotherapy11,203. A more recent study reported that CSF1R inhibition interferes with the maturation of tumour-associated MDMs in a mouse model of GBM that resembles the mesenchymal cellular state16. Unfortunately, a previous clinical trial failed to show efficacy of the CSF1R inhibitor pexidartinib to improve survival in GBM50,204 (TABLE 2), even when pharmacodynamic studies confirmed adequate drug concentrations in tumours and microglia depletion204. While the investigators hypothesized that the relative proportion of GBM subtypes could have led to treatment resistance, no biomarker or correlative studies were performed to demonstrate this mechanism of resistance204.
Resistance to CSF1R inhibition may develop as a result of the local production of other macrophage or microglial survival factors98. Indeed, resistance to CSF1R inhibition in a mouse model of proneural GBM developed through the production of insulin-like growth factor 1 (IGF1) by CD45low CD11b+ TAMs98. Despite strong evidence showing resistance to CSF1R blockade as a monotherapy, CSF1R inhibition could provide greater efficacy in combination with other treatments such as radiotherapy24. For example, resistance to radiation is characterized by the progressive influx of MDMs, which is prevented by CSF1R inhibition16,24. In experimental melanoma BrM models, the depletion of brain TAMs with pexidartinib prevented metastasis formation, which was attributed to a reduction in TAM-derived MMP3. However, MMP inhibitors led to a modest decrease in metastases, suggesting that TAMs contribute to metastatic colonization only partially through MMPs205. Thus, future studies should investigate the combination of CSF1R blockade with additional therapies or its use in specific patient subsets.
The SIRPα-CD47 axis
Phagocytic programmes in TAMs bridge innate and adaptive anti-tumour immunity via the clearance of malignant cells and the presentation of tumour-associated antigens. The phagocytic state of TAMs is controlled by numerous pro-phagocytic signals (such as calreticulin, SLAMF7, opsonizing antibodies and phosphatidyl serine) and anti-phagocytic signals (such as IgG4 antibodies, MHC class I, PDL1 and CD47)206. CD47, which is frequently upregulated in primary brain cancer cells207, binds the receptor signal-regulatory protein-α (SIRPα), inhibiting the phosphorylation of myosin II required for phagocytosis208. CD47 expression is widespread in primary brain cancers207,209 with IDHmut gliomas expressing lower levels of CD47 (REF.210). Many types of glioma, including those in children and GBM, upregulate CD47, which triggers signalling through SIRPα in TAMs209. Several studies in mice have demonstrated efficacy of anti-CD47 antibodies against primary brain tumours207,209,211,212, which in particular generated enthusiasm for the potential of this treatment in four paediatric tumour types207. Anti-CD47 antibodies enhance phagocytosis in MDMs207,211 and microglia86,209 and can synergize with anti-PD1 antibodies211, temozolomide211, and TLR3 and TLR9 activation86 in mouse GBM models. The majority of studies identify microglia as the main targets of anti-CD47 antibodies in primary brain cancer86,209. Although anti-CD47 antibodies are being tested in advanced solid tumours, including in patients with stable BrMs213 (TABLE 2), the targeting of the CD47–SIRPα axis has not been investigated in primary brain tumour clinical trials.
STAT3
STAT3+ astrocytes can promote BrMs via immune suppression, which is reversed by STAT3 inhibitors in mouse models12. The natural polyphenolic flavonoid STAT3 inhibitor derived from milk thistle seeds, silibinin, induced the regression of BrMs in the majority of patients in a phase I clinical trial12 (TABLE 2). Silibinin administration was safe and it appeared to selectively target BrMs, leaving metastases outside the brain unaffected12. This selective inhibition of BrMs argues that the beneficial effects of STAT3 inhibition result from the modulation of the brain TME and not the inhibition of STAT3 signalling in tumour cells12. STAT3 activation may also characterize a subset of astrocytes that cooperate with microglia to maintain an immune suppressive TME in GBM25. STAT3 inhibitors have been effective in mouse models of melanoma BrM, although they are thought to act directly on tumour cells and cells in the TME other than astrocytes214. A phase I clinical trial using the STAT3 inhibitor WP1066 as a monotherapy in recurrent GBM or melanoma BrM is currently recruiting patients215 (TABLE 2).
CX43
Gap junction formation via CX43 is inhibited by the anti-inflammatory drug meclofenamate, which is used for the treatment of migraine175. A phase I clinical trial evaluating the use of meclofenamate in patients with BrMs is progressing216. Enrolment has been completed, but the results have not been published (TABLE 2). However, it should be noted that, although meclofenamate was selected for BrM treatment because of its effects on gap junctions, its anti-tumour effects may also involve the inhibition of cyclo-oxygenase activity217 or tumour cell–tumour cell connections218.
Conclusion
The brain TME includes heterogeneous populations of tumour cells, astrocytes, TAMs and other immune cells with sometimes opposing roles in tumour initiation, progression and response to therapy219,220. Thus, we believe it is most appropriate to study the TME of brain malignancies at the level of single cells and not as bulk tissue. Technological advances now enable the interrogation of thousands of individual brain tumour29,50 and TME cells7,16,22,23 (BOX 2). Several TAM subsets have been recently associated to brain malignancies7,16,22 (TABLE 1) but our knowledge on tumour-associated astrocytes is still limited. Determining which subpopulations of TAMs or tumour-associated astrocytes are functionally important for brain malignancies remains challenging as these studies require the mechanistic investigation of candidate cell subsets defined by dozens of gene, protein or metabolic changes. In addition, the crosstalk between astrocytes, microglia, MDMs and other cells in the brain TME plays a central role in the control of tumour-specific immunity. Ideally, TAMs and tumour-associated astrocyte subsets should be studied in parallel with tumour cell heterogeneity to identify candidate interactions of therapeutic interest as was recently done in GBM-associated macrophages221. We recently combined rabies virus-based tracing of cell interactions with scRNA-seq to develop a novel approach (named RABID-seq) to study CNS cell interactions, including the mechanisms mediating them and their effects on interacting cells in vivo222. RABID-seq offers a unique opportunity to study TAM and astrocyte subsets and their interactions with brain tumour cells in the TME. TAM and tumour-associated astrocyte heterogeneity and interactions must be studied separately in BrMs, GBM and other brain malignancies because the tumour type has a major influence on the TME7,10. The study of TAM–astrocyte–tumour cell interactions will have important implications for our understanding of the pathogenesis of brain malignancies and the development of novel therapeutic approaches.
Acknowledgements
This work was supported by grants NS102807, ES02530, ES029136, AI126880 and AI149699 from the NIH; RG4111A1 and JF2161-A-5 from the NMSS, PA-1604-08459 from the International Progressive MS Alliance and the Jennifer Oppenheimer Cancer Research Initiative to F.J.Q. B.M.A. was supported by grant K12CA090354 from NIH. C.F.A. was supported by a scholarship from the German Academic Exchange Service (DAAD). M.A.W. was supported by NIH (1K99NS114111, F32NS101790), a training grant from the NIH and Dana-Farber Cancer Institute (T32CA207201), a traveling neuroscience fellowship from the Program in Interdisciplinary Neuroscience at BWH, and the Women’s Brain Initiative at BWH. EAC acknowledges support from R01NS110942, P01CA163205, P01CA069246, P01CA236749 and DoD WB1XW1H2010017. DAR acknowledges support from the Jennifer Oppenheimer Cancer Research Initiative, the Ben and Catherine Ivy Foundation, and PO1CA236749.
Glossary
- Brain tumour microenvironment
(TME). The dynamic, heterogeneous mixture of extracellular matrix and malignant and non-malignant cells, including neurons, glia, leukocytes and endothelial cells, that make up the gross brain tumour mass. Half or more of the cells within the brain tumour microenvironment, in particular in glioma, are non-neoplastic.
- Cell types
The stable identities of cells, primarily defined by intrinsic gene expression programmes, which contain permanent traits, including the potential to express certain genes and exert certain functions in response to external signals.
- Cell states
Within cell types, states are defined by the current gene expression, metabolic or functional conditions, which are a product of external signals from infection, inflammation, ageing or other disease. The duration of each cell state may be a function of ongoing signalling.
- Microglia
CNS-resident phagocytic cells primarily derived from the yolk sac with multiple roles in the development of disease, including synapse, surveillance and cell debris engulfment, cytokine production, and cross-talk with astrocytes and oligodendrocytes.
- Multi-omic studies
Approaches that include the quantification and characterization of data from various molecular domains such as the genome, transcriptome, proteome, metabolome and epigenome.
- Isocitrate dehydrogenase
(IDH1 or IDH2). Enzymes in the tricarboxylic acid cycle that catalyse the decarboxylation of isocitrate, producing alpha-ketoglutarate and carbon dioxide. Some mutations in IDH lead to the production of 2-hydroxyglutarate, a metabolite whose accumulation leads to genomic instability of glial cells through DNA methylation.
- Mesenchymal-like
One of the four transcriptional meta-signatures of GBM defined by bulk RNA sequencing featuring loss of neurofibromin 1 (NF1) expression and increased expression of mesenchymal-related, hypoxia-response, stress and glycolytic genes. It was associated with increased tumour-associated macrophage infiltration and a more immunosuppressive tumour-associated macrophage phenotype, suggesting its relevance for tumour–tumour microenvironment cross-talk.
- Extracellular membrane particles
Subcellular particles shed from malignant or non-malignant cells that are composed of a lipid bilayer with variable size and cargo, including proteins, metabolites and nucleic acids. Communication with neighbouring and sometimes distant cells is possible through their contents.
- Mass cytometry
A cytometric technique that quantifies protein expression at the single-cell level by combining the use of rare earth metal isotope-conjugated antibodies and cytometry by time of flight (CyTOF).
- Cellular indexing of transcriptomes and epitopes by sequencing
(CITE-seq). Combining DNA barcode-conjugated antibody labelling with single-cell RNA sequencing to enable immunophenotyping and unbiased transcriptome analysis.
- Blood–brain barrier
A variety of features specific to brain vasculature that limit the influx of chemicals or immune cells in addition to the active efflux of chemical molecules, thereby limiting neurotoxicity.
- Myeloid-derived suppressor cells
(MDSCs). A heterogeneous group of immature myeloid cells that contain monocyte-like and granulocyte-like subsets, which inhibit anti-tumour T cell responses through ARG1, IL-10 and TGFβ.
- Ectoenzyme
An extracellular enzyme that can be membrane bound or secreted.
- Blood–tumour barrier
Heterogeneous impairment of the blood–brain barrier and the interface between the blood and tumour due to tissue destruction by tumour growth and neoangiogenesis, resulting in altered but not necessarily efficient penetration of drug molecules and immune molecules.
- Ontogenies
The origins and development of specific cell types.
- Proneural subtype of GBM
One of four subtypes defined by bulk RNA sequencing of GBM through the Cancer Genome Atlas (TCGA), characterized by elevated expression of PDGFRA and CDK4. Originally, it was thought that each tumour represents a distinct subtype, but single-cell RNAseq of GBM revealed that all four subtypes can be detected within each tumour with varying proportions. More recently, the proneural subtype has been hypothesized to coincide with the oligodendrocyte-like and neural progenitor-like cellular states identified by single-cell RNA sequencing.
Footnotes
RELATED LINKS
Adult Astrocyte RNA-Seq Explorer: http://astrocyternaseq.org
Brain Immune Atlas: http://www.brainimmuneatlas.org
Brain TIME: https://joycelab.shinyapps.io/braintime
DropViz: http://dropviz.org
GBMseq: http://GBMseq.org
Glioma Microglia RNA Expression: http://www.glioma-microglia.com
Myeloid Single-cell RNA-Seq: https://myeloidsc.appspot.com
RecuR: http://recur.bioinfo.cnio.es/
Single Cell Portal at the Broad Institute: https://singlecell.broadinstitute.org/single_cell/study/SCP393/single-cell-rna-seq-of-adult-and-pediatric-glioblastoma
UCSC Cell Browser: http://gbm.cells.ucsc.edu
Competing interests
The authors declare no competing interests.
References
- 1.Tawbi HA et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med 379, 722–730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long GV et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 19, 672–681 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SB et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 17, 976–983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fecci PE et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 66, 3294–3302 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Kwok D & Okada H T-cell based therapies for overcoming neuroanatomical and immunosuppressive challenges within the glioma microenvironment. J. Neurooncol 147, 281–295 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathewson ND et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell 184, 1281–1298.e26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friebel E et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 181, 1626–1642.e20 (2020). This paper, published simultaneously with Klemm et al.10, demonstrated that the brain tumour immune microenvironment differs among human IDHwt glioma, IDHmut glioma and BrMs.
- 8. Goswami S et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat. Med 26, 39–46 (2020). This publication highlights the potential of targeting purinergic signalling as an immunotherapeutic approach for GBM.
- 9.Fujita M et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 71, 2664–2674 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klemm F et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181, 1643–1660.e17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pyonteck SM et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med 19, 1264–1272 (2013). This paper was the first major publication demonstrating that modulation of the CSF1R axis in TAMs in a mouse model of glioma can impact tumour growth and survival.
- 12. Priego N et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med 24, 1024–1035 (2018). This paper demonstrated in mouse models that modulation of a subpopulation of BrM-associated astrocytes through STAT3 inhibition can alter the immune microenvironment and extend survival in mice, and that an early-phase clinical trial demonstrated STAT3 inhibition can slow the growth of BrMs in patients.
- 13.Quail DF & Joyce JA The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valiente M et al. Brain metastasis cell lines panel: a public resource of organotropic cell lines. Cancer Res. 80, 4314–4323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masmudi-Martin M et al. Brain metastasis models: What should we aim to achieve better treatments? Adv. Drug Deliv. Rev 169, 79–99 (2021). [DOI] [PubMed] [Google Scholar]
- 16. Pombo Antunes AR et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat. Neurosci 24, 595–610 (2021). This publication demonstrated, with scRNA-seq and CITE-seq, subsets of glioma-associated microglia, MDMs and dendritic cells in mouse models and patients.
- 17.Yan J et al. FGL2 promotes tumor progression in the CNS by suppressing CD103+ dendritic cell differentiation. Nat. Commun 10, 448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L et al. Blocking immunosuppressive neutrophils deters pY696-EZH2-driven brain metastases. Sci. Transl. Med 12, eaaz5387 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castriconi R et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J. Immunol 182, 3530–3539 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Breckenridge CA et al. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat. Med 18, 1827–1834 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowman RL et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 17, 2445–2459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sankowski R et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat. Neurosci 22, 2098–2110 (2019). This publication described microglial subsets in patients with glioma for the first time using scRNA-seq and CyTOF.
- 23.Guldner IH et al. CNS-native myeloid cells drive immune suppression in the brain metastatic niche through Cxcl10. Cell 183, 1234–1248.e25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akkari L et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med 12, eaaw7843 (2020). [DOI] [PubMed] [Google Scholar]
- 25. Heiland DH et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun 10, 2541 (2019). This publication describes a subset of glioma-associated astrocytes characterized by the upregulation of STAT3 in surgical specimen-derived cultures, with some evidence that induction of immune-suppressive cytokines requires interactions with microglia.
- 26.Phillips JP, Eremin O & Anderson JR Lymphoreticular cells in human brain tumours and in normal brain. Br. J. Cancer 45, 61–69 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darmanis S et al. Single-cell RNA-Seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 21, 1399–1410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gieryng A et al. Immune microenvironment of experimental rat C6 gliomas resembles human glioblastomas. Sci. Rep 7, 17556 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venteicher AS et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 355, eaai8478 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohanbash G et al. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-alpha. Cancer Res. 73, 6413–6423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginhoux F et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 77, 2266–2278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prinz M, Jung S & Priller J Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Ayata P et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci 21, 1049–1060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosselin D et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mildner A et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci 10, 1544–1553 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Linnerbauer M, Wheeler MA & Quintana FJ Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broekman ML et al. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol 14, 482–495 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hambardzumyan D, Gutmann DH & Kettenmann H The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci 19, 20–27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pires-Afonso Y, Niclou SP & Michelucci A Revealing and harnessing tumour-associated microglia/macrophage heterogeneity in glioblastoma. Int. J. Mol. Sci 21, 689 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabrusiewicz K et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 1, e85841 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szulzewsky F et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One 10, e0116644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller S et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 18, 234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takenaka MC et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci 22, 729–740 (2019). This study demonstrated in detail a specific immune-suppressive mechanism in mouse glioma-associated MDMs: activation of AHR by tumour-derived kynurenine, resulting in the upregulation of CD39 and production of T cell-suppressive adenosine.
- 45.Poon CC et al. Differential microglia and macrophage profiles in human IDH-mutant and -wild type glioblastoma. Oncotarget 10, 3129–3143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amankulor NM et al. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 31, 774–786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz M et al. Cellular and molecular changes of brain metastases-associated myeloid cells during disease progression and therapeutic response. iScience 23, 101178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osuka S & Van Meir EG Overcoming therapeutic resistance in glioblastoma: the way forward. J. Clin. Invest 127, 415–426 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Rourke DM et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med 9, eaaa0984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neftel C et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849.e21 (2019). This publication demonstrated with scRNA-seq that human GBM cells exist in four plastic cellular states, which are analogous to the GBM subtypes previously described with bulk RNA sequencing, and that the mesenchymal state is associated with TAMs.
- 51.Patel AP et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capper D et al. DNA methylation-based classification of central nervous system tumours. Nature 555, 469–474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orozco JIJ et al. Epigenetic profiling for the molecular classification of metastatic brain tumors. Nat. Commun 9, 4627 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenger A et al. Intratumor DNA methylation heterogeneity in glioblastoma: implications for DNA methylation-based classification. Neuro-Oncology 21, 616–627 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elmore MR et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Y et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci 21, 530–540 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Wilton DK, Dissing-Olesen L & Stevens B Neuron-glia signaling in synapse elimination. Annu. Rev Neurosci 42, 107–127 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Li Q et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammond TR et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masuda T et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Böttcher C et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci 22, 78–90 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Marschallinger J et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci 23, 194–208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Safaiyan S et al. White matter aging drives microglial diversity. Neuron 109, 1100–1117.e10 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Geirsdottir L et al. Cross-species single-cell analysis reveals divergence of the primate microglia program. Cell 179, 1609–1622.e16 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Jordão MJC et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Keren-Shaul H et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Zhou Y et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med 26, 131–142 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schirmer L et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krasemann S et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gosselin D et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyauchi JT et al. Deletion of neuropilin 1 from microglia or bone marrow–derived macrophages slows glioma progression. Cancer Res. 78, 685–694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glinka Y & Prud’homme GJ Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol 84, 302–310 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gelfand MV et al. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. eLife 3, e03720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nissen JC, Selwood DL & Tsirka SE Tuftsin signals through its receptor neuropilin-1 via the transforming growth factor beta pathway. J. Neurochem 127, 394–402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majed HH et al. A novel role for Sema3A in neuroprotection from injury mediated by activated microglia. J. Neurosci 26, 1730–1738 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Friese MA et al. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 64, 7596–7603 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Uhl M et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 64, 7954–7961 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Miyauchi JT et al. Ablation of Neuropilin 1 from glioma-associated microglia and macrophages slows tumor progression. Oncotarget 7, 9801–9814 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leclerc M et al. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat. Commun 10, 3345 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vinnakota K et al. Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neuro-Oncology 15, 1457–1468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu F et al. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages Toll-like receptor 2 signaling. Neuro-Oncology 17, 200–210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Triller P, Bachorz J, Synowitz M, Kettenmann H & Markovic D O-Vanillin attenuates the TLR2 mediated tumor-promoting phenotype of microglia. Int. J. Mol. Sci 21, 2959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Andaloussi A, Sonabend AM, Han Y & Lesniak MS Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia 54, 526–535 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Benbenishty A et al. Prophylactic TLR9 stimulation reduces brain metastasis through microglia activation. PLoS Biol. 17, e2006859 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang Y et al. Synergistic toll-like receptor 3/9 signaling affects properties and impairs glioma-promoting activity of microglia. J. Neurosci 40, 6428–6443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maas SLN et al. Glioblastoma hijacks microglial gene expression to support tumor growth. J. Neuroinflammation 17, 120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kopatz J et al. Siglec-h on activated microglia for recognition and engulfment of glioma cells: Siglec-h on activated microglia. Glia 61, 1122–1133 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Hickman SE et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci 16, 1896–1905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu X et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-alpha and IFN-gamma dependent manners. Cancer Immunol. Immunother 59, 1401–1409 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu X, Fujita M, Snyder LA & Okada H Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J. Neurooncol 104, 83–92 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mercurio L et al. Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. J. Exp. Clin. Cancer Res 35, 55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watkins S et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun 5, 4196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sørensen MD, Dahlrot RH, Boldt HB, Hansen S & Kristensen BW Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathol. Appl. Neurobiol 44, 185–206 (2018). [DOI] [PubMed] [Google Scholar]
- 95.De Boeck A et al. Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat. Commun 11, 4997 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abels ER et al. GlioM&M: Web-based tool for studying circulating and infiltrating monocytes and macrophages in glioma. Sci. Rep 10, 9898 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aslan K et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat. Commun 11, 931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quail DF et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lavin Y et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ochocka N et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat. Commun 12, 1151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raychaudhuri B et al. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J. Neurooncol 122, 293–301 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Gabrusiewicz K, Colwell NA & Heimberger AB in Translational Immunotherapy of Brain Tumors 63–82 (Elsevier, 2017). [Google Scholar]
- 103.Katzenelenbogen Y et al. Coupled scRNA-seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell 182, 872–885.e19 (2020). [DOI] [PubMed] [Google Scholar]
- 104. Opitz CA et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011). This publication, together with Takenaka et al.106 and Sadik et al.105 reported important roles for AHR in GBM, guiding new lines of research and potential therapeutic approaches.
- 105.Sadik A et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 182, 1252–1270.e34 (2020). [DOI] [PubMed] [Google Scholar]
- 106.Takenaka MC, Robson S & Quintana FJ Regulation of the T cell response by CD39. Trends Immunol. 37, 427–439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Apetoh L et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol 11, 854–861 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mascanfroni ID et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med 21, 638–646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Z, Liu X, Guo R & Wang P CD4+Foxp3− type 1 regulatory T cells in glioblastoma multiforme suppress T cell responses through multiple pathways and are regulated by tumor-associated macrophages. Int. J. Biochem. Cell Biol 81, 1–9 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Ene CI et al. Anti-PD-L1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro-Oncology 22, 639–651 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bloch O et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res 19, 3165–3175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berghoff AS et al. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J. Neurooncol 130, 19–29 (2016). [DOI] [PubMed] [Google Scholar]
- 113.Dusoswa SA et al. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc. Natl Acad. Sci. USA 117, 3693–3703 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Q et al. Immunogenomic analysis reveals LGALS1 contributes to the immune heterogeneity and immunosuppression in glioma. Int. J. Cancer 145, 517–530 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Ruffell B et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodriguez PC et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004). [DOI] [PubMed] [Google Scholar]
- 117.Movahedi K et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 70, 5728–5739 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Verkhratsky A & Nedergaard M Physiology of astroglia. Physiol. Rev 98, 239–389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sofroniew MV & Vinters HV Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao P-S et al. Glutamate/glutamine metabolism coupling between astrocytes and glioma cells: Neuroprotection and inhibition of glioma growth. Biochem. Biophys. Res. Commun 450, 295–299 (2014). [DOI] [PubMed] [Google Scholar]
- 121.Mayo L et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med 20, 1147–1156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rothhammer V et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 22, 586–597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rothhammer V et al. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc. Natl Acad. Sci. USA 114, 2012–2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rothhammer V et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chao C-C et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell 179, 1483–1498.e22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wheeler MA et al. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wheeler MA et al. MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanmarco LM et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 590, 473–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gibson EM et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176, 43–55.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bardehle S et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat. Neurosci 16, 580–586 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Saijo K et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kano S-I et al. Glutathione S-transferases promote proinflammatory astrocyte-microglia communication during brain inflammation. Sci. Signal 12, eaar2124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frik J et al. Cross-talk between monocyte invasion and astrocyte proliferation regulates scarring in brain injury. EMBO Reports 19, e45294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ito M et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Mayo L et al. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain 139, 1939–1957 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Valiente M et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mega A et al. Astrocytes enhance glioblastoma growth. Glia 68, 316–327 (2020). [DOI] [PubMed] [Google Scholar]
- 138.Okolie O et al. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neurooncology 18, 1622–1633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim J-K et al. Tumoral RANKL activates astrocytes that promote glioma cell invasion through cytokine signaling. Cancer Lett. 353, 194–200 (2014). [DOI] [PubMed] [Google Scholar]
- 140.Loeffler S, Fayard B, Weis J & Weissenberger J Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocyte sin vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int. J. Cancer 115, 202–213 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Wurm J et al. Astrogliosis releases pro-oncogenic chitinase 3-Like 1 causing MAPK signaling in glioblastoma. Cancers 11, 1437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. John Lin C-C et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci 20, 396–405 (2017). This paper described subsets of astrocytes for the first time, using fluorescence-activated cell sorting, and compared transcriptomes of astrocytes to those of mouse and human glioma cells.
- 143.Batiuk MY et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun 11, 1220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morel L et al. Molecular and functional properties of regional astrocytes in the adult brain. J. Neurosci 37, 8706–8717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bayraktar OA et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci 23, 500–509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeisel A et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Rusnakova V et al. Heterogeneity of astrocytes: from development to injury – single cell gene expression. PLoS One 8, e69734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsai HH et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358–362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Molofsky AV et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509, 189–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chai H et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hasel P et al. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat. Commun 8, 15132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lanjakornsiripan D et al. Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat. Commun 9, 1623 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin R, Bajo-Graneras R, Moratalla R, Perea G & Araque A Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Farmer WT & Murai K Resolving astrocyte heterogeneity in the CNS. Front. Cell. Neurosci 11, 300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Farmer WT et al. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351, 849–854 (2016). [DOI] [PubMed] [Google Scholar]
- 156.Herrmann JE et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci 28, 7231–7243 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ben Haim L et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci 35, 2817–2829 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roy Choudhury G et al. Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke. Brain Res. 1551, 45–58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X et al. Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-κB- and STAT1-dependent chemokine production in astrocytes. Acta Neuropathol. 126, 711–724 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Wang X et al. OTUB1 inhibits CNS autoimmunity by preventing IFN-γ-induced hyperactivation of astrocytes. EMBO J. 38, e100947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin H, Niyongere SA, Lee SJ, Baker BJ & Benveniste EN Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J. Immunol 181, 3167–3176 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yeste A et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci. Signal 9, ra61–ra61 (2016). [DOI] [PubMed] [Google Scholar]
- 164.Okada S et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med 12, 829–834 (2006). [DOI] [PubMed] [Google Scholar]
- 165.Norris CM et al. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J. Neurosci 25, 4649–4658 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fernandez AM et al. Regulation of the phosphatase calcineurin by insulin-like growth factor I unveils a key role of astrocytes in Alzheimer’s pathology. Mol. Psychiatry 17, 705–718 (2012). [DOI] [PubMed] [Google Scholar]
- 167.Habib N et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci 23, 701–706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gril B et al. Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases. Nat. Commun 9, 2705 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Contreras-Zarate MJ et al. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene 38, 4685–4699 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gril B et al. Pazopanib inhibits the activation of PDGFRbeta-expressing astrocytes in the brain metastatic microenvironment of breast cancer cells. Am. J. Pathol 182, 2368–2379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krupinski J et al. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke 28, 564–573 (1997). [DOI] [PubMed] [Google Scholar]
- 172.Norazit A et al. Vascular endothelial growth factor and platelet derived growth factor modulates the glial response to a cortical stab injury. Neuroscience 192, 652–660 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Thies KA et al. Stromal platelet-derived growth factor receptor-beta signaling promotes breast cancer metastasis in the brain. Cancer Res. 81, 606–618 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hung MH et al. Effect of age and biological subtype on the risk and timing of brain metastasis in breast cancer patients. PLoS One 9, e89389 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chen Q et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016). This publication provided novel and transformative insights on the role of astrocytes in BrMs.
- 176.Sin WC et al. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 35, 1504–1516 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Flippot R et al. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: results of the GETUG-AFU 26 NIVOREN multicenter phase II study. J. Clin. Oncol 37, 2008–2016 (2019). [DOI] [PubMed] [Google Scholar]
- 178.Reardon DA et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 6, 1003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Cloughesy TF et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med 25, 477–486 (2019). Unlike numerous preceding studies, this clinical report demonstrated that anti-PD1 therapy given in a neoadjuvant fashion (prior to re-operation) could lead to a survival benefit in recurrent GBM. This study is currently being validated with larger trials.
- 180.Qian J et al. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflammation 15, 290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McLaughlin J et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2, 46–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Prima V, Kaliberova LN, Kaliberov S, Curiel DT & Kusmartsev S COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl Acad. Sci. USA 114, 1117–1122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lamano JB et al. Glioblastoma-derived IL6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes tumor growth. Clin. Cancer Res 25, 3643–3657 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Butte MJ, Keir ME, Phamduy TB, Sharpe AH & Freeman GJ Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ahmed FS et al. PD-L1 protein expression on both tumor cells and macrophages are associated with response to neoadjuvant durvalumab with chemotherapy in triple-negative breast cancer. Clin. Cancer Res 26, 5456–5461 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu Y et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin. Cancer Res 26, 970–977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Holtzhausen A et al. TAM family receptor kinase inhibition reverses MDSC-mediated suppression and augments anti-PD-1 therapy in melanoma. Cancer Immunol. Res 7, 1672–1686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sadahiro H et al. Activation of the receptor tyrosine kinase AXL regulates the immune microenvironment in glioblastoma. Cancer Res. 78, 3002–3013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu J et al. MerTK as a therapeutic target in glioblastoma. Neuro-Oncology 20, 92–102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.US National Library of Medicine. Clinicaltrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03965494 (2019). [DOI] [PubMed]
- 191. Ott M et al. Profiling of patients with glioma reveals the dominant immunosuppressive axis is refractory to immune function restoration. JCI Insight 5, e134386 (2020). This publication, in contrast to other studies, showed that modulating the adenosine axis was ineffective in a mouse model of GBM, potentially due to T cell exhaustion.
- 192.Turiello R et al. Serum CD73 is a prognostic factor in patients with metastatic melanoma and is associated with response to anti-PD-1 therapy. J. Immunother. Cancer 8, e001689 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mayer A et al. Role of hypoxia and the adenosine system in immune evasion and prognosis of patients with brain metastases of melanoma: a multiplex whole slide immunofluorescence study. Cancers 12, 3753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhai L et al. Infiltrating T cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clin. Cancer Res 23, 6650–6660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brenk M et al. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+Foxp3+T regulatory cells. J. Immunol 183, 145–154 (2009). [DOI] [PubMed] [Google Scholar]
- 196.Wainwright DA et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res 18, 6110–6121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ladomersky E et al. IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin. Cancer Res 24, 2559–2573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reardon DA et al. A phase 1 study of PF-06840003, an oral indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor in patients with recurrent malignant glioma. Invest. New Drugs 38, 1784–1795 (2020). [DOI] [PubMed] [Google Scholar]
- 199.US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT03532295 (2018). [DOI] [PubMed]
- 200.US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT04047706 (2019). [DOI] [PubMed]
- 201.Gabriely G & Quintana FJ Role of AHR in the control of GBM-associated myeloid cells. Semin. Cancer Biol 64, 13–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rothhammer V & Quintana FJ The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev Immunol 19, 184–197 (2019). [DOI] [PubMed] [Google Scholar]
- 203.Yan D et al. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene 36, 6049–6058 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Butowski N et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-Oncology 18, 557–564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Qiao S, Qian Y, Xu G, Luo Q & Zhang Z Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J. Neuroinflammation 16, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lecoultre M, Dutoit V & Walker PR Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J. Immunother. Cancer 8, e001408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gholamin S et al. Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med 9, eaaf2968 (2017). [DOI] [PubMed] [Google Scholar]
- 208.Tsai RK & Discher DE Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol 180, 989–1003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hutter G et al. Microglia are effector cells of CD47-SIRPalpha antiphagocytic axis disruption against glioblastoma. Proc. Natl Acad. Sci. USA 116, 997–1006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gowda P, Patrick S, Singh A, Sheikh T & Sen E Mutant isocitrate dehydrogenase 1 disrupts PKM2-beta-catenin-BRG1 transcriptional network-driven CD47 expression. Mol. Cell Biol 38, e00001–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.von Roemeling CA et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun 11, 1508 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang M et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS One 11, e0153550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT03990233 (2019). [DOI] [PubMed]
- 214.Kong LY et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol. Immunother 58, 1023–1032 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT01904123 (2013). [DOI] [PubMed]
- 216.US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT02429570 (2015). [DOI] [PubMed]
- 217.Duffy CP et al. Enhancement of chemotherapeutic drug toxicity to human tumour cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs). Eur. J. Cancer 34, 1250–1259 (1998). [DOI] [PubMed] [Google Scholar]
- 218.Osswald M et al. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98 (2015). [DOI] [PubMed] [Google Scholar]
- 219.Maire CL et al. Glioma escape signature and clonal development under immune pressure. J. Clin. Invest 130, 5257–5271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Paik PK et al. Next-generation sequencing of stage IV squamous cell lung cancers reveals an association of PI3K aberrations and evidence of clonal heterogeneity in patients with brain metastases. Cancer Discov. 5, 610–621 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hara T et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell 39, 779–792.e11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Clark IC et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Khalsa JK et al. Immune phenotyping of diverse syngeneic murine brain tumors identifies immunologically distinct types. Nat. Commun 11, 3912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thomas RP et al. Macrophage exclusion after radiation therapy (MERT): a first in human phase I/II trial using a CXCR4 inhibitor in glioblastoma. Clin. Cancer Res 25, 6948–6957 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schalper KA et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med 25, 470–476 (2019). [DOI] [PubMed] [Google Scholar]
- 226.Pasqualini C et al. Modeling the interaction between the microenvironment and tumor cells in brain tumors. Neuron 108, 1025–1044 (2020). [DOI] [PubMed] [Google Scholar]
- 227.Fossati G et al. Neutrophil infiltration into human gliomas. Acta Neuropathol. 98, 349–354 (1999). [DOI] [PubMed] [Google Scholar]
- 228.Barletta KE, Ley K & Mehrad B Regulation of neutrophil function by adenosine. Arterioscler. Thromb. Vasc. Biol 32, 856–864 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Glodde N et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 47, 789–802.e9 (2017). [DOI] [PubMed] [Google Scholar]
- 230.Yee PP et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun 11, 5424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wculek SK et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol 20, 7–24 (2020). [DOI] [PubMed] [Google Scholar]
- 232.Bailey SL, Schreiner B, McMahon EJ & Miller SD CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ TH-17 cells in relapsing EAE. Nat. Immunol 8, 172–180 (2007). [DOI] [PubMed] [Google Scholar]
- 233.Faget J et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 72, 6130–6141 (2012). [DOI] [PubMed] [Google Scholar]
- 234.Oh SA et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 1, 681–691 (2020). [DOI] [PubMed] [Google Scholar]
- 235.Huntington ND, Cursons J & Rautela J The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 20, 437–454 (2020). [DOI] [PubMed] [Google Scholar]
- 236.Fares J, Fares MY & Fares Y Natural killer cells in the brain tumor microenvironment: Defining a new era in neuro-oncology. Surg. Neurol. Int 10, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miyai M et al. Current trends in mouse models of glioblastoma. J. Neurooncol 135, 423–432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ben-David U et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet 49, 1567–1575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Morton JJ, Bird G, Refaeli Y & Jimeno A Humanized mouse xenograft models: narrowing the tumor–microenvironment gap. Cancer Res. 76, 6153–6158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N & Werb Z Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol 20, 1349–1360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Eyler CE et al. Single-cell lineage analysis reveals genetic and epigenetic interplay in glioblastoma drug resistance. Genome Biol. 21, 174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li Y et al. Murine models of IDH-wild-type glioblastoma exhibit spatial segregation of tumor initiation and manifestation during evolution. Nat. Commun 11, 3669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guilhamon P et al. Single-cell chromatin accessibility profiling of glioblastoma identifies an invasive cancer stem cell population associated with lower survival. eLife 10, e64090 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gularyan SK et al. Investigation of inter- and intratumoral heterogeneity of glioblastoma using TOF-SIMS. Mol. Cell Proteom 19, 960–970 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Svensson V, Vento-Tormo R & Teichmann SA Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc 13, 599–604 (2018). [DOI] [PubMed] [Google Scholar]
- 246.Zhang J et al. Single-cell transcriptome-based multilayer network biomarker for predicting prognosis and therapeutic response of gliomas. Brief. Bioinforma 21, 1080–1097 (2020). [DOI] [PubMed] [Google Scholar]
- 247.Saelens W, Cannoodt R, Todorov H & Saeys Y A comparison of single-cell trajectory inference methods. Nat. Biotechnol 37, 547–554 (2019). [DOI] [PubMed] [Google Scholar]
- 248.Tanay A & Regev A Scaling single-cell genomics from phenomenology to mechanism. Nature 541, 331–338 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rodriques SG et al. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang X et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, eaat5691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Keren L et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174, 1373–1387.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Eng C-HL et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH+. Nature 568, 235–239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Stuart T et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Saunders A et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kennedy DW & Abkowitz JL Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood 90, 986–993 (1997). [PubMed] [Google Scholar]
- 256.Hoeffel G et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gomez Perdiguero E et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Akashi K, Traver D, Miyamoto T & Weissman IL A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000). [DOI] [PubMed] [Google Scholar]
- 259.Wendeln A-C et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hussain SF et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 67, 9630–9636 (2007). [DOI] [PubMed] [Google Scholar]
- 261.Grassivaro F et al. Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J. Neurosci 40, 784–795 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Shemer A et al. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat. Commun 9, 5206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]