Abstract
Background
Progesterone is essential for the maintenance of a healthy pregnancy. Any defect in the secretion of human chorionic gonadotropin or progesterone is associated with a significantly increased risk of first-trimester abortion. Progesterone is frequently prescribed to patients presenting with per vaginal (PV) bleeding in early pregnancy and a history of recurrent pregnancy loss.
Methods
Pregnant women up to 12 weeks of gestation with a history of more than two early pregnancy losses and presenting with vaginal bleeding were included in this study. All subjects were randomized to receive either vaginal progesterone 600 mg/day or oral dydrogesterone 30 mg/day. A detailed history—including menstrual history, previous pregnancies, previous miscarriages, and other risk factors–was obtained. The mean time required for the cessation of PV bleeding and continuation of pregnancy up to 24 weeks and till term was compared.
Results
A total of 200 patients were randomized to vaginal progesterone 600 mg/day (n = 100) or oral dydrogesterone 30 mg/day (n = 100). While 74 patients had two miscarriages in the progesterone group, 68 patients had two miscarriages in the dydrogesterone group. The time required for complete cessation of bleeding was significantly lesser among patients who received oral dydrogesterone compared to those who received intravaginal progesterone (53.90 ± 9.09 vs. 94.60 ± 7.29 h, p < 0.0001). Numerically higher number of patients receiving oral dydrogesterone had a successful continuation of pregnancy up to 24 weeks of gestation, as well as till full term compared to progesterone group (70 vs. 75).
Conclusion
Oral dydrogesterone is preferred over vaginal progesterone in patients presenting with vaginal bleeding during early pregnancy and a history of recurrent early pregnancy loss.
Keywords: Recurrent pregnancy loss, Progesterone, Vaginal bleeding, Outcome
Introduction
Progesterone plays a key role in the maintenance of a healthy pregnancy. During early pregnancy, human chorionic gonadotropin (hCG) is secreted by the syncytiotrophoblast, which, in turn, is responsible for the production and secretion of progesterone from the corpus luteum. The corpus luteum is prevented from regression by hCG till the time syncytiotrophoblast starts directly secreting progesterone [1]. Any defect in the secretion of hCG or progesterone is associated with a significantly increased risk of first-trimester abortion, and many studies have found that progesterone plays a vital role in the sustenance of pregnancy till the fetus becomes viable. Studies suggest that progesterone levels are considerably lower in women with early first-trimester abortions compared to those in women with healthy pregnancies reaching the stage of fetal viability without any complications [2].
Threatened abortion is usually manifested by vaginal bleeding in the first trimester. Abortions have a considerable impact on the psychology of pregnant women, and in many cases, on caregivers as well, particularly in the developing world. The management of abortion remains challenging. Threatened abortion, as defined by the World Health Organization (WHO), is “pregnancy-related bloody vaginal discharge or frank bleeding during the first half of pregnancy without cervical dilatation [3].” Although abdominal pain may occur, its presence is not a prerequisite for the diagnosis of threatened abortion. It needs to be emphasized that around one-fourth of pregnant women will experience some degree of vaginal bleeding in the first trimester of pregnancy, and around half of these patients will progress to abortion [4]. The usual causes of first-trimester abortion include chromosomal anomalies, maternal thyroid disorders, maternal infections, and chronic systemic illnesses. A few of these etiologies, such as undiagnosed maternal thyroid disorders and chromosomal and genetic abnormalities, may result in recurrent pregnancy loss, which remains an important cause of morbidity in women of childbearing age [5].
Management of women with vaginal bleeding with a history of one or more miscarriages is a common challenge facing obstetricians and is a common reason for seeking obstetric consultation [6]. Approximately 50% of patients presenting with vaginal bleeding in the first trimester will eventually have an abortion. Since progesterone is known to play a vital role in the maintenance of pregnancy, it is commonly prescribed for the prevention of miscarriage in women presenting with any degree of vaginal bleeding during early pregnancy [7]. Various studies have reported that progesterone reduces the risk of eventual miscarriage in women presenting with vaginal bleeding in early pregnancy. Progesterone can be administered by various routes, such as oral, intramuscular, rectal, or vaginal [8]. Various studies have reported mixed results concerning the benefits of various routes. Although oral progesterone is easily administered and has better compliance, it is associated with reduced bioavailability due to its first-pass metabolism in the liver [9].
Vaginal progesterone does not have the disadvantage of reduced bioavailability, but may be associated with irritation, vaginal discharge and bleeding [10]. We, therefore, undertook this comparative study to compare the efficacy of micronized vaginal progesterone versus oral dydrogesterone in pregnant women presenting with a history of two abortions and per vaginal (PV) bleeding.
Materials and Methods
This was a comparative study in which 200 pregnant women up to 12 weeks of gestation were included based on the following predefined inclusion criteria: (1) a history of more than two miscarriages and presenting with PV bleeding; (2) age 28–35 years; (3) fetal pole and heart activity confirmed by ultrasound and closed OS. Pregnant women with systemic illnesses (such as diabetes, hypertension, and chronic obstructive airway disease), absence of fetal pole or heart activity on ultrasound, presence of autoimmune disorders (such as antiphospholipid antibody syndrome, lupus erythematosus) and Müllerian abnormalities (diagnosed based on ultrasound) were excluded from the study. The study was conducted in the Department of Obstetrics and Gynecology of a tertiary care infertility center situated in an urban area. The study publication was approved by Institutional Ethics Committee, Govt Medical College, Aurangabad. Informed consent was obtained from the participants. Demographic data such as age, education, religion, occupation, obstetrical history, gravida, and economic status were obtained from all participants. A detailed history was obtained—particularly menstrual history, the outcome of previous pregnancies, the number of previous miscarriages, and presence of risk factors that might contribute to threatened abortion. Specific importance was given to the history of recurrent miscarriage and mental health issues in other family members, delayed developmental milestones, or any diagnosed chromosomal disorders. The presence of systemic diseases such as hypertension, diabetes, or chronic obstructive airway disease was ruled out by a thorough clinical examination and appropriate laboratory tests.
A per speculum examination was done in all cases to determine the status of the os. During per speculum examination, evidence of infection, nature of bleeding, and presence of any discharge per vaginum were also examined and noted.
Complete blood count, renal function tests, liver function tests, blood grouping and Rhesus (Rh) factor typing, urine routine microscopy and culture sensitivity, thyroid function tests, human immunodeficiency virus (HIV), hepatitis B surface antigen (HBsAg), Venereal Disease Research Laboratory (VDRL) test, fasting as well as postprandial blood sugar levels were performed for all patients. An obstetric ultrasound examination was performed to determine the location of the placenta, the status of the pregnancy, and the viability of the fetus, as well as to rule out the presence of Müllerian abnormalities, which are a cause of recurrent abortion.
The study population was randomly divided into two groups of equal size: group A consisting of 100 patients receiving vaginal progesterone 600 mg once a day and group B consisting of 100 patients receiving oral dydrogesterone (tablet) 30 mg once a day. The outcome was assessed based on the time required for the stoppage of bleeding and the continuation of pregnancy until 24 weeks.
Statistical analysis was done using SSPS 21.0 software. A p-value < 0.05 was considered statistically significant.
Results
A total of 200 patients were included in this study; 100 patients were randomly assigned to receive vaginal progesterone 600 mg suppository once a day and remaining 100 patients were given oral dydrogesterone tablets 30 mg once a day.
The baseline and demographic characteristics of the enrolled patients are presented in Table 1. The mean age in groups A and B was found to be comparable, at 31.12 ± 4.12 and 29.74 ± 3.68 years, respectively, with no statistically significant difference noted between the groups (p > 0.05). The mean body mass index (BMI) was also comparable between the groups (22.42 ± 3.98 kg/m2 in group A and 21.94 ± 3.88 kg/m2 in group B), with no statistically significant difference noted between the groups (p > 0.05).
Table 1.
Demographic analysis of patients
| Group A | Group B | p-value | 95% CI* | |
|---|---|---|---|---|
| Age (years), Mean ± SD | 31.12 ± 4.12 | 29.74 ± 3.68 | 0.0804 | − 2.9303–0.1703 |
| BMI (kg/m2), Mean ± SD | 22.42 ± 3.98 | 21.94 ± 3.88 | 0.3889 | − 1.5761–0.6161 |
| Progesterone levels (ng/mL), Mean ± SD | 22.24 ± 8.64 | 19.98 ± 7.12 | 0.1566 | − 5.4020–0.8820 |
BMI Body mass index; SD Standard deviation
*CI Confidence interval
In group A, 74 patients had two miscarriages and 26 patients had ≥ 3 miscarriages; in group B, 68 patients had two miscarriages and 32 patients had ≥ 3 miscarriages (Fig. 1).
Fig. 1.
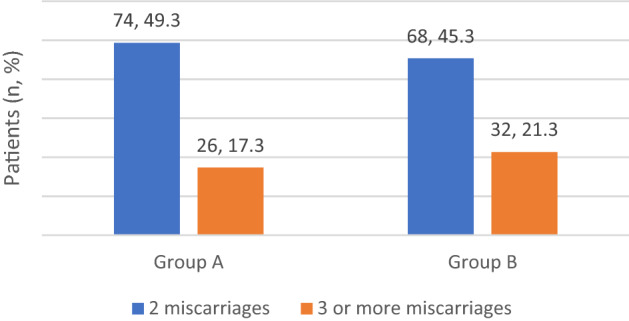
History of previous miscarriages in studied cases
Serum progesterone levels were estimated at the first consultation. Analysis of serum progesterone levels was performed at a fixed time in the afternoon (2:00–4:00 PM), as the serum progesterone level varied during different periods of the day. The mean serum progesterone level was 22.24 ± 8.64 ng/mL in group A and 19.98 ± 7.12 ng/mL in group B (Table 1). The difference in mean serum progesterone was found to be comparable, with no statistically significant difference noted between the groups (p > 0.05).
The time required for complete stoppage of bleeding was significantly lower after receiving the first dose of oral dydrogesterone compared to intravaginal progesterone (53.90 ± 9.09 vs. 94.60 ± 7.29 h; p < 0.0001).
Continuation of pregnancy (fetal viability confirmed with ultrasound) until 24 weeks of gestation was observed in 70 patients in group A (52 patients with a history of two miscarriages and 18 patients with ≥ 3 miscarriages) and 75 patients in group B (48 patients with a history of two miscarriages and 27 patients with a history of ≥ 3 miscarriages); however, the difference between the two groups was not statistically significant (p = 0.5267) (Table 2).
Table 2.
Continuation of pregnancy in studied cases
| Group A | Group B | p-value | ||
|---|---|---|---|---|
| Continuation of pregnancy up to 24 weeks of gestation | History of two miscarriages | 52 | 48 | 0.5267 |
| History of ≥ 3 miscarriages | 18 | 27 | ||
| Total | 70 | 75 | ||
| Continuation of pregnancy up to term (37 weeks of gestation) | History of two miscarriages | 45 | 48 | 0.2270 |
| History of ≥ 3 miscarriages | 18 | 24 | ||
| Total | 63 | 72 |
Continuation of pregnancy till term, i.e., 37 weeks of gestation (fetal viability confirmed with ultrasound), was noted in 63 patients in group A (45 patients with a history of two miscarriages and 18 patients with ≥ 3 miscarriages) and 72 patients in group B (48 patients with a history of two miscarriages and 24 patients with ≥ 3 miscarriages); however, there was no statistical significance between the groups (p = 0.5267).
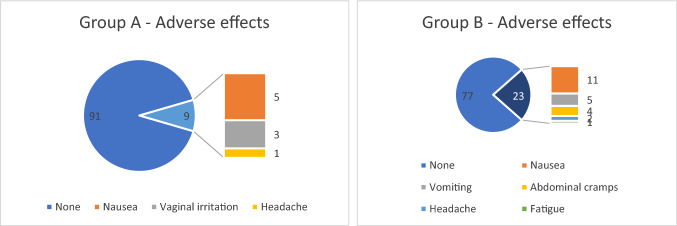
Nausea (11%), vomiting (5%), and abdominal cramps (4%) were the most common adverse events, while headache (2%) and fatigue (1%) were the least common adverse events observed in women receiving oral dydrogesterone. In progesterone group, the observed side effects included nausea (5%), vaginal irritation (3%), and headache (1%) (Fig. 2).
Fig. 2.
Adverse effects of oral dydrogesterone and vaginal progesterone
Discussion
Progesterone is important for the maintenance of pregnancy, and several studies have reported its role in preventing spontaneous miscarriage. Progesterone also induces secretory changes in uterine endothelial cells and makes them receptive to implantation of the fertilized embryo. Hence, it is also frequently prescribed to patients with a history of recurrent abortions. Progesterone can be administered by the oral, vaginal, or intramuscular routes. The oral route is the easiest to administer with better patient compliance, but it may be associated with side effects such as nausea, vomiting, and headache. Intravaginal administration of progesterone is associated with a reduced likelihood of these side effects [11].
Since early pregnancy loss is a common occurrence, affecting at least 15%–20% of pregnancies, its management is a common challenge faced by obstetricians [12]. The WHO defines recurrent pregnancy loss as three or more consecutive episodes of spontaneous pregnancy loss with the same biological father. Recurrent pregnancy loss is a difficult problem faced by couples and has a considerable psychological impact [13]. Various studies have compared the effectiveness of progesterone in patients with a history of recurrent early pregnancy loss presenting with vaginal bleeding during early pregnancy [14].
Yassaee et al. conducted a single-blind clinical trial with 60 pregnant women with threatened abortion. The study included women with vaginal bleeding up to 20 weeks of their pregnancy and divided them into control and case groups. The case group was administered 400 mg of vaginal progesterone suppository (Cyclogest®) each day until the bleeding stopped; the group was followed until the completion of pregnancy. Treatment success was considered as continuation of pregnancy beyond 20 weeks. The authors found that the number of abortions in the case group was lower than that in the control group (20% vs. 33.3%, respectively). The study results indicate that progesterone might reduce the incidence of spontaneous abortion in patients presenting with vaginal bleeding in early pregnancy [15]. Similarly, Wahabi et al. [16] and Ahmed et al. [17] have also reported the beneficial effects of progesterone in the prevention of early pregnancy loss.
Lee et al. conducted a meta-analysis to evaluate the efficacy of progesterone therapy for the prevention of miscarriage in pregnant women experiencing threatened abortion. The study included nine randomized trials with 913 pregnant women (including 322 treated with oral dydrogesterone, 213 treated with vaginal progesterone, and 378 control subjects). The study reported a significantly lower incidence of miscarriage in the total progesterone group than in the control group (13.0% vs. 21.7%; odds ratio [OR] 0.53; 95% confidence interval [CI] 0.36–0.78; p = 0.001). Additionally, both the oral dydrogesterone and vaginal progesterone groups were associated with a lower incidence of miscarriage compared to the control group (11.7% vs. 22.6%, OR 0.43, 95% CI 0.26–0.71; p = 0.001 and 15.4% vs. 20.3%, OR 0.72, 95% CI 0.39–1.34; p = 0.3, respectively). However, there was no difference in the incidence of miscarriage between the oral dydrogesterone and vaginal progesterone groups [18]. Based on the results of this study, it was evident that progesterone therapy, especially oral dydrogesterone, was effective in preventing miscarriage in pregnant women experiencing threatened abortion. Similar results have been reported by Czyzyk et al. [19] and Mesen et al. [20]. These results are similar to the results of our study, in which we found oral dydrogesterone supplementation to be more effective than vaginal progesterone for the prevention of miscarriage in women presenting with vaginal bleeding during early pregnancy.
Conclusion
Oral dydrogesterone was found to be more effective than vaginal progesterone for the management of patients presenting with vaginal bleeding during early pregnancy. Dydrogesterone therapy was associated with significantly lesser time for complete stoppage of bleeding. It was also associated with higher viable pregnancy rates at 24 weeks, as well as at term, compared to intravaginal progesterone—although the difference was not found to be statistically significant.
Acknowledgements
We would like to thank BioQuest Solutions for Editorial support
Dr. Ashish Kale
He was an associate professor at Dr. D. Y. Patil Medical College, Hospital & Research Centre, Vice President at POGS, Executive Vice President at POGS, Treasurer at MSR, Executive Managing Committee member at ISAR and IAGE. Dr. Ashish is a trained laparoscopic surgeon from Germany. He obtained training in IVF from Cleveland University, USA, and National University Hospital, Singapore. At present, he is working as an IVF consultant and is the Founder Director of Ashakiran Group of Hospitals and Asha IVF Centre. He is also an examiner at the College of Physicians & Surgeons of Mumbai for postgraduates in obstetrics and gynecology. He has to his credit the first state-of-the-art IVF laboratory in the state of Arunachal Pradesh.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Ashish Ramchandra Kale is a Consultant, Ashakiran hospitals and Asha IVF Centre, # 555, Narsinha Chintamani Kelkar Road, Narayan Peth, Pune, Maharashtra 411030 India. Ashwini Ashish Kale is a Consultant, Ashakiran hospitals and Asha IVF Centre, # 555, Narsinha Chintamani Kelkar Road, Narayan Peth, Pune, Maharashtra 411030 India. Kanan Yelikar is a Dean in Ashwini Hospital, Govt Medical College & Hospital Aurangabad (MS), India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ashish Ramchandra Kale, Email: drashishkale1978@yahoo.com.
Ashwini Ashish Kale, Email: ashwini13@rediffmail.com.
Kanan Yelikar, Email: drkananyelinkar2016@gmail.com.
References
- 1.Paul S, Das C, Jailkhani BL, Talwar GP. Progesterone synthesis by human placental syncytiotrophoblast in vitro–preferred precursor and effect of human chorionic gonadotropin. J Steroid Biochem. 1981;14:311–313. doi: 10.1016/0022-4731(81)90142-4. [DOI] [PubMed] [Google Scholar]
- 2.Ku CW, Allen JC, Jr, Lek SM, Chia ML, Tan NS, Tan TC. Serum progesterone distribution in normal pregnancies compared to pregnancies complicated by threatened miscarriage from 5 to 13 weeks gestation: a prospective cohort study. BMC Pregnancy Childbirth. 2018;18:360. doi: 10.1186/s12884-018-2002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sotiriadis A, Papatheodorou S, Makrydimas G. Threatened miscarriage: evaluation and management. BMJ. 2004;329:152–155. doi: 10.1136/bmj.329.7458.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sapra KJ, Joseph KS, Galea S, Bates LM, Louis GM, Ananth CV. Signs and symptoms of early pregnancy loss. Reprod Sci. 2017;24:502–513. doi: 10.1177/1933719116654994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2:76–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Szabó I, Szilágyi A. Management of threatened abortion. Early Pregnancy. 1996;2:233–240. [PubMed] [Google Scholar]
- 7.Dante G, Vaccaro V, Facchinetti F. Use of progestagens during early pregnancy. Facts Views Vis Obgyn. 2013;5:66–71. [PMC free article] [PubMed] [Google Scholar]
- 8.Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update. 2000;6:139–148. doi: 10.1093/humupd/6.2.139. [DOI] [PubMed] [Google Scholar]
- 9.Warren MP, Shantha S. Uses of progesterone in clinical practice. Int J Fertil Womens Med. 1999;44:96–103. [PubMed] [Google Scholar]
- 10.Cicinelli E. Intravaginal oestrogen and progestin administration: advantages and disadvantages. Best Pract Res Clin Obstet Gynaecol. 2008;22:391–405. doi: 10.1016/j.bpobgyn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Warren MP. Vaginal progesterone and the vaginal first-pass effect. Climacteric. 2018;21:355–357. doi: 10.1080/13697137.2018.1450856. [DOI] [PubMed] [Google Scholar]
- 12.Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154. doi: 10.1186/1741-7015-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farren J, Mitchell-Jones N, Verbakel JY, Timmerman D, Jalmbrant M, Bourne T. The psychological impact of early pregnancy loss. Hum Reprod Update. 2018;24:731749. doi: 10.1093/humupd/dmy025. [DOI] [PubMed] [Google Scholar]
- 14.El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Women’s Health. 2017;9:331–345. doi: 10.2147/IJWH.S100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yassaee F, Shekarriz-Foumani R, Afsari S, Fallahian M. The effect of progesterone suppositories on threatened abortion: a randomized clinical trial. J Reprod Infertil. 2014;15:147–151. [PMC free article] [PubMed] [Google Scholar]
- 16.Wahabi HA, Fayed AA, Esmaeil SA, Al Zeidan RA. Progestogen for treating threatened miscarriage. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005943.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed SR, Mel-K E-S, Al-Sheeha MA, Aitallah AS, Jabin Khan F, Ahmed SR. Pregnancy outcome in women with threatened miscarriage: a year study. Mater Sociomed. 2012;24:26–28. doi: 10.5455/msm.2012.24.26-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Park TC, Kim JH, Norwitz E, Lee B. The influence of oral dydrogesterone and vaginal progesterone on threatened abortion: a systematic review and meta-analysis. Biomed Res Int. 2017;2017:3616875. doi: 10.1155/2017/3616875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czyzyk A, Podfigurna A, Genazzani AR, Meczekalski B. The role of progesterone therapy in early pregnancy: from physiological role to therapeutic utility. Gynecol Endocrinol. 2017;33:421–424. doi: 10.1080/09513590.2017.1291615. [DOI] [PubMed] [Google Scholar]
- 20.Mesen TB, Young SL. Progesterone and the luteal phase: a requisite to reproduction. Obstet Gynecol Clin North Am. 2015;42:135–151. doi: 10.1016/j.ogc.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]