Abstract
According to a variety of experiments, Rose damascene may lead to memory enhancement and acetylcholine esterase inhibition. However, Rose damascene cannot pass through the blood-brain barrier due to its hydrophilic contents. Solid lipid nanoparticles (SLNs) are suitable carriers for brain drug delivery. Herein, SLNs were made by micro-emulsion method. Then, lactoferrin was covalently attached to the surface of the nanoparticles by amide bond interaction for targeted delivery. The nanoparticle properties and the amount of attached lactoferrin were calculated. The effect of the selected compounds on scopolamine-induced animals was also measured by Y-maze, passive avoidance test, elevated plus maze, and forced swim test. The results revealed that the size and zeta potential of nanoparticles were 52 nm and − 13 mV before conjugation, and 161 nm and − 16 mV after conjugation, respectively. The percentage of entrapment efficiency and drug loading before conjugation was 98 ,93.6 and, after conjugation, was 11.2, 15.9, respectively. According to Y-maze and passive avoidance test results, Rose damascene can enhance short-term memory and may also reduce anxiety and depression in scopolamine-induced animals.
Keywords: Dementia, Solid lipid nanoparticles (SLN), Rose damascene Mill L., Behavioral tests, Biochemical assessments
Introduction
The prevalence of dementia has been on the rise and Alzheimer’s disease (AD) is the most prevalent form of dementia. According to the World Health Organization’s (WHO) reports, 13 % of 65-year-old people are at the risk of dementia and the number of patients with dementia will reach 34 million by 2025. The most common symptoms of dementia are memory loss, loss of focus ability, and visual perception disability [1].
Scopolamine is believed to have several toxic effects on the nervous system. There are several pathways influenced by scopolamine, e.g. the decrease in the N-methyl scopolamine binding site may increase H2O2 as an inducer of oxidation damage. Oxidative stress could affect the nervous system due to its toxicity and may cause cognitive dysfunction [2].
Different acetylcholine esterase and NMAD inhibitor drugs such as rivastigmine, galantamine, donepezil, and memantine have been used for AD. However, these medications do not seem to change the final outcome and also have various side effects [3]. Therefore, research has been conducted for investigating the best natural resources such as piperine, quercetin, frankincense, and Rose damascene extract for treating dementia [4–8]. Rose damascene is a holy plant belonging to the Rosacea family, which is cultivated in Europe and Middle Eastern countries such as Iran (city of Kashan), Turkey, and India. These plants have been mostly used for decorative and perfumery purposes. The colorful flower has been exploited for producing essential oil and aqueous extract. In the traditional medicine, Rose damascene has been used for enhancing memory and treating fever, gastrointestinal complaints, and anxiety. It has been suggested that its chloroform extract can outgrow the neurons as an infant’s neurons. Also, it may modulate the expression of BDNF, NGF, CREB, and EGR-1 transcription factors, significantly reduce lipid peroxidation, and protect neurons from oxidative damage [6]. Perfusing Rose damascene extract to the brain may be limited due to liver metabolism and overconsuming rose damascene may have some gastric dysfunctions [6].
The advantage of nanotechnology for drug delivery, especially brain delivery, is evident. Dendrimers, carbon nanotubes, poly cyanoacrylates, chitosan, and PLGA are the nano-carriers for brain delivery, but they have some disadvantages such as lack of biocompatibility and leakage.
The solid lipid nanoparticle (SLN) is a new-generation method for brain delivery. SLNs combine the advantages of polymers, emulsions, and liposomal nanoparticles and lack some of their disadvantages. Their merits include a high payload of both hydrophilic and hydrophobic drugs, excellent reproducibility in drug loadings, safe transport through bio-systems, and an industrially scalable preparation method. The targeted delivery of SLNs may reduce extrapyramidal side effects resulting from non-specific delivery [9].
Different receptors such as transferrin (TF), insulin, epidermal growth factor, and insulin-like growth factor receptors have so far been used for BBB drug delivery [10]. Lactoferrin is 81 kDa glycoprotein which belongs to the transferrin family. This protein consists of 690 amino acids folded in two globular lobes with one iron-binding side. It has been localized in a special part of the brain such as glial cells, neurons, and microvasculature. The uptake of lactoferrin is significantly greater than that of transferrin. The lactoferrin receptor (LfR) has been shown to exist on the BBB of different species [11]. Lactoferrin has anti-inflammatory effects on the brain and the lactoferrin receptors have been increased in dementia diseases such as AD and Parkinson’s disease [12].
As no similar study has been conducted before, the present study aims to target Rose damascene via the lactoferrin receptor in the brain and evaluate its efficiency against dementia, stress, anxiety, and enzymatic stress using behavioral and enzyme activity tests.
Materials and methods
Materials
D-alpha tocopherol succinate, lactoferrin, trehalose, N-hydroxysuccihnimide (NHS), 1-ethyl-3-[3-dimethylaminopropyl] carboamid hydrochloride (EDC), and cellulose dialysis tubing (Molecular weight cut-off: 12 kDa) were purchased from Sigma Aldrich Company. DMSO (dimethylsulfoxide), DMF (dimethyl formamide), and sodium citrate were purchased from Merck company.
Methods
Preparing Rose damascene extract
Rose damascene was purchased from Alzahra Factory, Kashan, Iran. The ethanolic extract was obtained by percolation with ethanol 70 %.
Preparing Rose damascene-loaded SLN
Rose damascene-loaded SLN was prepared using the micro-emulsion method [13]. Briefly, Rose damascene extract (0.15 g), D-alpha tocopherol succinate (0.3 g), Tween 80 (0.642 g), and soy lecithin (0.03 g) were mixed as the lipid phase. The aqueous phase was prepared by dissolving sodium citrate in water (pH: 5.5). Both phases were heated to 72 °C on magnetic stirring for 30 min. Then, the water phase was added to the lipid phase and kept on constant striating for 30 min. The emulsion was added to cold water and homogenized for 50 min at 2000 rpm. The above-mentioned emulsion was subjected to probe sonication for 5 min at 50 W. The solution was lyophilized by adding 15 % trehalose solution to its pellets as a cryo protectant [14].
Lactoferrin-SLN conjugation
Lactoferrin (Lf) was conjugated to the SLN surface by carboamide coupling and was covalently coupled by its amine group to free carboxylic acids that were present on the SLN surface. For this purpose, 10 mg of SLN was dissolved in 600 µl of DMSO, and EDC and NHS with the 3:2 molar ratio were dissolved in DMSO and DMF with the 70:30 ratio. These two solutions were poured together and kept on gentle stirring for 1 h at the room temperature. Next, 4.5 mg of Lf (dissolved in 0.1 M PBS and 0.5 M NaCl) was added dropwise, mixed well, and kept on stirring for 5 h. Excessive unbound Lf and EDC were removed by centrifugation using a high-speed tabletop centrifuge at 13,000 rpm for 30 min. The SLN pellets were then lyophilized and Lf- SLN was characterized using various techniques [15].
Qualitative and quantitative determination of conjugation
The number of Lf conjugated to SLN was measured by Bradford assay. The unbound Lf was separated from bounded Lf via centrifugation at 12,000 rpm for 30 min. The supernatant was mixed well with the Bradford reagent in a 96-well plate and kept for 20 min at the room temperature. The amount of Lf was evaluated at the wavelength of 595nm by using a UV-VIS spectrophotometer. This amount was compared to the blank which contained the same amount of Bradford reagent. The efficiency was expressed as the percentage of bounded Lf [16].
The FTIR (Fourier-transform infrared) spectra of Rose damascene were obtained by means of an FTIR spectrophotometer. The sample was prepared by accumulating 10 scans with the resolution of 4 cm-1 over the range of 600 to 3600 cm-1 [17].
Characterizing solid lipid nanoparticles
Particle size and zeta potential were measured by photon correlation spectroscopy using a Zeta sizer, Nano ZS (Malvern Instruments, Malvern, UK). The morphology of different formulations was measured by FE-SEM. Entrapment efficiency was measured by an Amicon filter. The SLN dispersion in water was poured on the Amicon filter (10 KD) and centrifuged by a refrigerated table-top centrifuge for 40 min at 5000 rpm and 14 °C. Then, the amount of unentrapped Rose damascene was measured by UV-VIS and compared to the blank [13]. The percentage of drug loading capacity was determined using the following Eq. (1):
| 1 |
In vitro drug release studies
In vitro release of Rose damascene from SLN was measured by dialysis bag diffusion techniques in PBS (pH = 7.4). The SLN drug suspension was centrifuged at 12,000 rpm for 30 min and the pellets were dispersed by vortexing in deionized water twice. The solution was placed on a pre-swollen dialysis bag (12 kDa cut-off) and immersed in 20 ml of the release medium. This was placed in the 37 °C shaker incubators at 100 rpm. Finally, the specific amount of PBS was withdrawn at 15 min, 30 min, 1, 2, 4, 8, 24, and 48 h intervals and the same amount of PBS was replaced. The Rose damascene concentration was measured by UV-VIS at 270 nm.
In vivo studies
Thirty-six adult male Wister rats (200 ± 50) were used in this study. The animals were kept at the room temperature and 12 h light/dark with food and water access. Animals were randomly divided into six groups (eight in each) and treated for two weeks as mentioned below:
Group 1: Saline.
Group 2: 1.5 mg/kg scopolamine (ip).
Group 3: Scopolamine + SLN without drug (E-SLN).
Group 4: Scopolamine + SLN with Rose damascene extract (R-SLN).
Group 5: Lf- SLN every other day (Lf-SLN).
Finally, the behavioral tests including Y-maze, passive avoidance test, elevated plus maze (EPM), forced swim maze, and biochemical assays were performed 30 min after scopolamine injection (i.p.) after two weeks of training. The ethical use of the animal models was approved by Ethics Committee, Shahid Beheshti University of Medical Sciences (National Institutes of Health Publication NO.1399.066).
Y-maze
Short-term memory was assessed by the spontaneous alternation behavior, which reflects short-term memory on the Y-maze task. The Y-maze used here consisted of three black plexuses (35 cm long, 25 cm high, and 10 cm wide), which formed a 120° angle. Thirty minutes after inhaling the compounds, the rats were placed at the end of one arm and allowed to move freely through the arms for 8 min. The arm entry was counted when the hind paws of the rats were entirely within the arm. Spontaneous alternation behavior was calculated. The maze was cleaned with a 70 % ethanol solution and dried with a cloth before each animal entered [18].
Passive avoidance test
According to Aydin et al. [18], the passive avoidance test was performed to describe long-term memory assessments. The passive avoidance test has three steps on two consecutive days; two of them were conducted within 30-min intervals and the last one was done after 24 h. The cage was divided into two equal-sized compartments (which were dark and light). Rats preferred to enter the dark compartment. The compound for each group was injected 30 min before each trial. On the first day of training, the rats were put in the light compartment and, after 30 s, the guillotine door was opened and the rats were allowed to enter the dark compartment. Then, the rats were removed from the cage. In the training step, the rats were placed in the light compartment. When the hind paws of the rats entered the dark compartment, the door was closed immediately and, then, an electric foot shock (0.2 mA, 2 s) occurred via the grid floor. After 20 s, the animal was placed in the cage. After 24 h in the testing step, the animal was placed in the light compartment and the latency of the rat’s entry into the dark chamber was calculated within 300 s.
Elevated plus maze
Behavior in the EPM test is utilized to assess anxiety. The EPM consists of four arms, 49 cm long and 10 cm wide, elevated 50 cm above the ground. The two arms are enclosed by 30-cm-high walls and the other two arms are exposed. Thirty minutes after inhaling the compound by each group, each rat was placed in the exposed arms of the maze facing the center of the maze. Behavior was observed for 6 min, and the time spent and number of entry into the open and enclosed arms were measured [19]. The duration of staying on the open arm indicates the anxiety level of the rats. In addition, the total number of open- and enclosed-arm entries (number of crossings) indicates the exploratory activity of the animals [20]. An entry was defined as an animal placing all four paws into an arm and no time was recorded when the animal was in the central area. The maze floor was cleaned with a cotton cloth and a 70 % ethanol solution between the subjects’ entries.
Forced swim test
The forced swim test (FST) is the most widely used model for assessing depressive-like response [21] which was basically assessed using the same method described by Campos et al. [22], but with modifications. Rats were individually placed into cylindrical recipients (diameter of 30 cm, height of 59 cm) containing 25 cm of water at 26 ± 1 °C. The animals were left to swim for 300 s before being removed and, then, dried and returned to their cages. During the test session, the following behavioral responses were recorded: (1) Immobility (time spent floating with minimal movements to keep the head above the water) which represents depressive-like behavior, and (2) Swimming (time spent with active swimming movements).
Biochemical assessments
Removing the hippocampus and cortex
The animals on each group were anesthetized using CO2 and rapidly decapitated after the behavioral study. The hippocampus and cortex were removed and conserved in the refrigerator at -80 °C for biochemical assessments. Before the biochemical tests, the brains were weighed and homogenized in PBS with the 1:3 ratio.
Measuring acetylcholine esterase activity
Ellman’s modified method was used for acetylcholine esterase measurement [23]. In this experiment, acetyl thiocholine iodide (ATCI) was used as a substrate of the reaction and 5, 5-dithiobis (2-nitrobenzioc) acid (DTNB) was used for measuring the AChE activity. In this procedure, 150 µl of 0.1 M sodium phosphate buffer (pH 8.0), the test compound solution, and the enzyme solution were mixed and incubated for 15 min at 25 °C. DTNB (0.1 M) was then added and the reaction was initiated by adding the substrate (8 µl of ATCI, 0.075 M solution). The hydrolysis of the ATCI can be measured by forming the colored product 5- thio-2-nitrobenzoate anion formed by the reaction of DTNB and thiocholine, released by the hydrolysis of the enzyme. The formation of the colored product was measured at the wavelength of 410 nm after 10 min.
Analysis of glutathione (GSH)
GSH was measured to determine oxidative stress. For this procedure, Ellman’s method was followed [23]. Briefly, the test compound was mixed in 96-well plates and, then, 100 µl of DTNB was added to each compound. The formation of the colored product was measured at 405nm wavelength.
Statistical analysis
Statistical analysis was performed by applying one-way analysis of variance (ANOVA) and the data were expressed as mean ± SEM ((GraphPad Prism, V 5.02, San Diego, CA, USA). Values < 0.05 were deemed statistically significant.
Results and discussion
Characterizing solid lipid nanoparticles
The particle size of R-SLN and Lf-SLN was 50.2 and 161.3 nm, respectively (Table 1). The smaller particle size than other experiments may be due to the high percentage of Tween 80 which decreases the interaction of nanoparticles with their environment. As expected, the size of Lf-SLN was increased due to its binding to the surface of the nanoparticles. The zeta potential of R-SLN and LF-SLN was measured and equaled − 13 and − 15 mV, respectively. The Zeta potential was decreased; therefore, the interaction and aggregation of the nanoparticles were reduced. As a result, ligand binding to the nanoparticle may stabilize them and increase the hydrodynamic radius. The entrapment and loading efficiency of SLN was 98 and 15 %, respectively, but was decreased to 93 and 11 % in Lf-SLN, respectively. The reduction in the latter case was due to drug release during the binding protocol (Table 1) [11].
Table 1.
Size, zeta potential, entrapment efficiency, and loading efficiency of R-SLN and Lf-SLN
| Size (nm) | Zeta potential | EE | LE | |
|---|---|---|---|---|
| R-SLN | 50.2 | -13 | %98 | %15 |
| Lf-SLN | 161.3 | -15 | %93 | %11 |
FE-SEM image
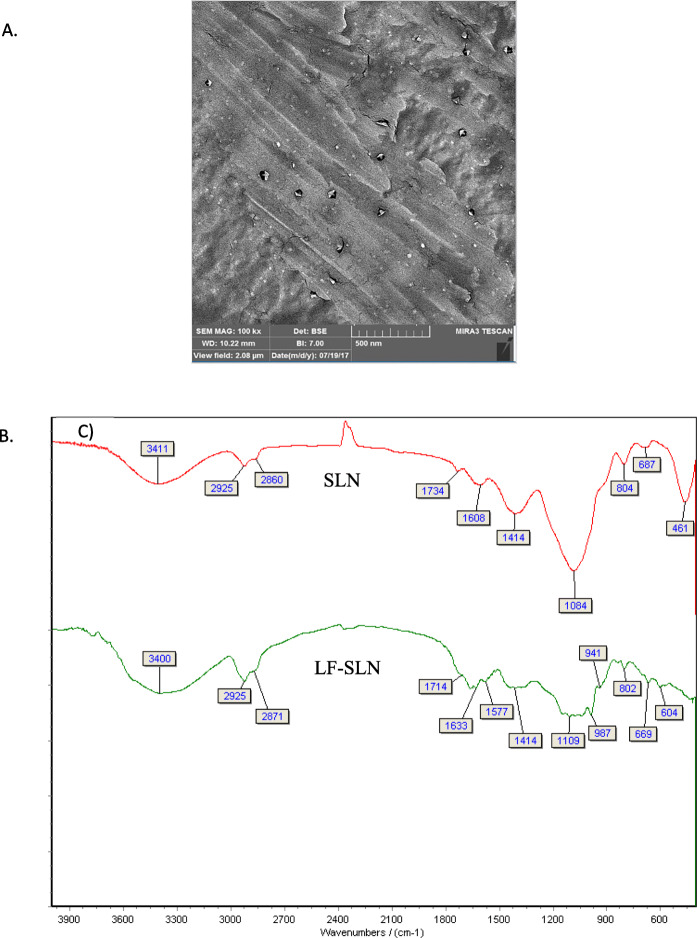
The FE-SEM image confirmed the spherical shape of the entrapped SLN (Fig. 1).
Fig. 1.
A) FE-SEM from the nanoparticle after freeze-drying. B) Amide bond formation between the NH2 group of Lf and the COOH group of tocopherol succinate proved by LF-SLN spectra peaks at 1633cm-1 (C¼O, amide stretching), 1577 cm-1 (NH bend)]
Qualitative and quantitative determination of lactoferrin conjugation
Lf conjugation was confirmed using the FTIR method (Fig. 1.2). This technique confirmed the surface interaction between the amine group of Lf and the carboxyl group of tocopherol succinate (Fig. 1.2). The peak at 1653cm-1 confirmed the amid group, clearly confirming the conjugation of Lf and TS. The absence of a strong peak at 1734cm-1 strongly suggested conjugation. Finally, the peak at 1577cm-1 further confirmed this coupling [11].
The amount of conjugation was quantified using the Bradford method. In one study, 30 % conjugation was considered to be satisfactory for brain targeting. However, in this study, the amount of conjugation was significantly increased to ~ 73.2 % [16].
Drug release profile
The in vitro drug release profile was represented as the cumulative drug release percentage with respect to time. As depicted in Fig. 2, R-SLN and Lf-SLN exhibited 31.56 and 21.9 % of drug release within 48 h, respectively. However, the drug release profile of Lf-SLN was considerably slower than that of R-SLN due to the increased hydrodynamic radius and the distance between the core and the environment [24], Also, there was less surface area and drug release in proportion to the surface area [25]. The high drug release in the first 2 h was due to the electrostatic interaction of Rose damascene with the nanoparticle surface.
Fig. 2.
In vitro release profile of the drug and nanoparticles in PBS (pH 7.4). Data suggesting the sustained release of Rose damascene Mill from SLN and Lf-SLN formulations compared to R-SLN
Behavioral tests
Y-maze
The Y-maze has been validated for short-term assessment in rodents. In this test, the spontaneous alteration is measured relying on the tendency of rats to explore the iconic place. Based on Fig. 3.B, the significant (p < 0.0001) reduction of spontaneous alteration in the scopolamine group compared to the saline group confirmed short-term memory reduction. Moreover, the non-loaded SLN (E-SLN) had no significant effect on scopolamine-treated rats, so the tocopherol succinate did not enhance the spatial memory. Moreover, Rose damascene significantly improved (p < 0.05) the short-term memory in comparison to saline. A low dose of Rose damascene may be needed for memory enhancement. The effect of R-SLN and Lf-SLN on memory was significant (p < 0.0001) compared to the scopolamine group. Furthermore, both of the above-mentioned groups affected the acquisition of the short-term memory of scopolamine-treated rats. The lack of significant differences between R-SLN and Lf-SLN may be due to the loss of Rose damascene during Lf-SLN preparation; however, it is yet to be confirmed and more research is required. Developing short-term memory in different groups did not affect their locomotor activity (Fig. 3.A). This was confirmed by the non-significant difference in locomotor activity in different groups.
Fig. 3.
Effects of Rose damascene extract in the Y-maze test on (A) Number of entries, and (B) spontaneous alternation result. Values are mean ± SEM (six animals per group). For one-way ANOVA P++++ < 0.0001 in comparison to saline and P*< 0.05, P***<0.001 in comparison to scopolamine
Passive avoidance test
The passive avoidance test is a precious test for long-term memory evaluation. In this test, the time through the latency of rats to the dark compartment is measured as a criterion for long-term memory. The time through latency in scopolamine-treated rats was significantly shorter (p < 0.05) than that of the control group (Fig. 4). The non-loaded SLN (E-SLN) had no significant influence on the long-term memory. The effect of Rose damascene alone (Rose) on the long-term memory was insignificant compared to the scopolamine group, which was due to the insufficient dosage required to affect long-term memory. R-SLN and Lf-SLN significantly increased the time through latency (p < 0.05), so the impact of these two was accepted. As mentioned above, the non-significant difference between R-SLN and Lf-SLN may be because of the leakage of Rose damascene in the preparation steps. Moreover, further research is needed to estimate the body distribution of Rose damascene in different organs and different parts of the brain.
Fig. 4.

Effects of Rose damascene in the passive avoidance test on time through latency. Values are mean ± SEM (six animals per group). For one-way ANOVA P + < 0.05 compared to saline and P*<0.05 compared to scopolamine
Elevated plus maze
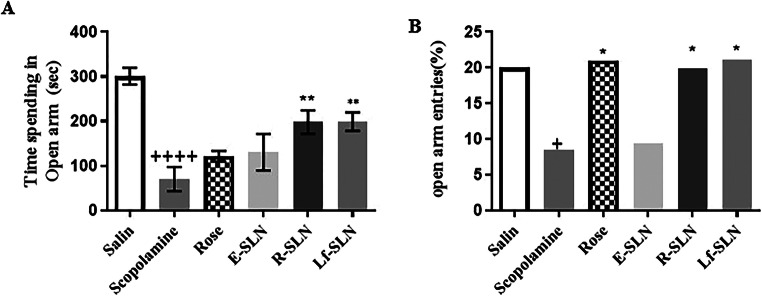
The EPM is recognized as a powerful model for predicting the anxiolytic or anxiogenic-like effect of different compounds on rodents. In this test, the more time the rat spends on the open arm, the greater the anxiety would be. The scopolamine-alone treated group had a significantly decreased percentage of the time spent on the open arm and number of entries (p < 0.0001); this indicates the anxiogenic-like effect of scopolamine on rats (Fig. 5). Rose damascene had anxiolytic effects on scopolamine-treated rats and a low dose of Rose damascene affected anxiety (p < 0.05). Tocopherol succinate (E-SLN) alone had no significant effect compared to scopolamine. The R-SLN and Lf-SLN had significant anxiolytic impacts on the rats.
Fig. 5.
Effects of Rose damascene in the EPM on (A) Time spent in the open arm and (B) Number of open arm entries. Values are mean ± SEM. For one-way ANOVA P + < 0.05 and P++++<0.0001 compared to saline and P*<0.05, P**<0.01 compared to scopolamine
FST
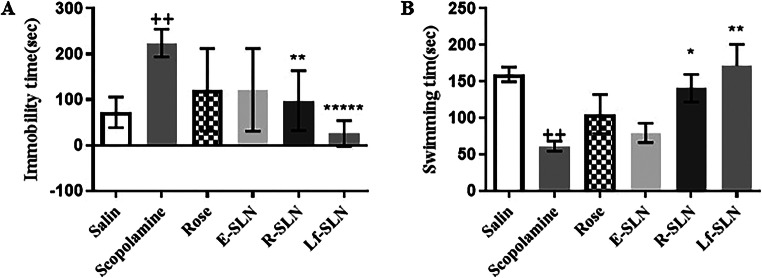
As mentioned above, the FST is a valuable test for depressive and anti-depressive-like effects of different compounds in rats. In Fig. 6, the influence of various compounds on depressive-like behavior is reported. Experimental depressive-like behavior was confirmed in the scopolamine group which showed a significant increase (p < 0.01) in immobility time during the FST compared to the saline group. E-SLN and Rose damascene did not affect the anti-depressive-like behavior. However, in both R-SLN and Lf-SLN, the immobility time was significantly decreased (p < 0.001 and p < 0.05, respectively) compared to the scopolamine group, proving the effect of untargeted and targeted SLN-loaded Rose damascene on the depressive-like behavior. The swimming time was also significantly increased in comparison to the scopolamine group. In addition, the effect of R-SLN on the depressive-like behavior may be due to polysorbate 80 coating which enters the brain by endocytosis from apolipoprotein E receptors on BBB [26].
Fig. 6.
Effects of Rose damascene in the FST in (A) Immobility test and (B) Swimming time. Values are mean ± SEM. For one-way ANOVA P++<0.01 compared to saline and P*<0.05, P**<0.01, P***<0.001 compared to scopolamine
Biochemical assessment
ACHE assessment
The dementia development in the scopolamine groups was also confirmed by the significant increase in acetylcholine esterase activity per gram of tissue per min compared to the control group (P < 0.01). R-SLN demonstrated a significant decrease in acetylcholine esterase in both the hippocampus and cortex (P < 0.01 and P < 0.05, respectively). Animals receiving Lf-SLN showed a significant decrease in AchE activity in both the hippocampus and cortex (P < 0.01). Also, the Rose group displayed a significant reduction in AchE activity in the hippocampus tissue compared to the scopolamine group (P < 0.05). The effect of Rose damascene was significantly higher in the hippocampus compared to the cortex, suggesting that the hippocampus is more sensitive than the cortex (Fig. 7A and C) [27].
Fig. 7.
Effects of Rose damascene on Acetylcholine Esterase Activity/min/g and GSH µg/g of tissue on A & B) Hippocampus and C & D) Cerebral cortex. Values are mean ± SEM. For one-way ANOVA P++<0.01 compared to saline and P*<0.05, P*<0.05, P**<0.01, P***<0.001 compared to scopolamine
GSH concentration assessments
Administering scopolamine significantly decreased the total thiol concentration (P < 0.01). R-SLN significantly increased the GSH level in both the hippocampus and cortex (P < 0.01 and P < 0.05, respectively). The GSH level was significantly increased in the animals receiving Lf-SLN in both the hippocampus and cortex (P < 0.001 and P < 0.01, respectively) (Fig. 7B and D). Indeed, glutathione peroxidase reduced toxic radicals using GSH as a substrate, subsequent to the oxidation of GSH to GSSG. This supported the hypothesis regarding the activity of the oxidase enzyme. GSH reduction because of scopolamine administration was enhanced by Rose damascene. As a result, R-SLN and Lf-SLN may reduce oxidative damage, while there is no significant difference in the other groups compared to the scopolamine group [28].
Conclusions
The effect of Rose damascene Mill L on memory enhancement, anxiolytic effects, and anti-depressive-like behaviors was examined by biochemical assays on scopolamine-treated rats based on specific behavioral and biochemical tests (Y-maze, radial arm maze, EPM, and FST).
SLNs are suitable nanoparticles for brain delivery due to their small size, targeting effects, and hydrophobic nature [24]. Indeed, this nanoparticle does not have many side effects due to its natural lipid compartment. The high loading capacity makes this nanoparticle suitable for targeting [9]. In this experiment, the anxiolytic, anti-acetylcholine esterase, and antioxidant effects of Rose damascene Mill L were confirmed. Tocopherol succinate did not affect behavioral and biochemical impairment, which may be due to the low dose of tocopherol succinate. In addition, there was no significant difference between R-SLN and Lf-SLN, which may be due to the targeting properties of polysorbate 80 that adsorbs ApoE and leads to the transcytosis of nanoparticles to the BBB.
Acknowledgements
The authors would like to acknowledge the financial support of Science and Technology Park, University of Tehran, under grant number 5436201.
Declarations
Conflicts of interest
The authors of this article declare that they have no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 2.Rakhshandah H, Boskabady MH, Mossavi Z, Gholami M, Saberi Z. The differences in the relaxant effects of different fractions of Rosa damascena on guinea pig tracheal smooth muscle. Iran J Basic Med Sci. 2010;13(3):126–32. [Google Scholar]
- 3.Di Stefano† A, Laserra S, Sozio P. Drug delivery strategies for Alzheimer’s disease treatment; 2011. Expert Opin Drug Deliv 5:581–603. [DOI] [PubMed]
- 4.Yusuf M, Khan M, Khan RA, Ahmed B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J Drug Target. 2012 doi: 10.3109/1061186X.2012.747529. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63(3):342–51. doi: 10.1111/j.2042-7158.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 6.Esfandiary E, Karimipour M, Mardani M, Alaei H, Ghannadian M, Kazemi M, Mohammadnejad D, Hosseini N, Esmaeili A. Novel effects of Rosa damascena extract on memory and neurogenesis in a rat model of Alzheimer’s disease. J Neurosci Res. 2014;92(4):517–30. doi: 10.1002/jnr.23319. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadpour T, Hosseini M, Naderi A, Karami R, Sadeghnia HR, Soukhtanloo M, Vafaee F. Protection against brain tissues oxidative damage as a possible mechanism for the beneficial effects of Rosa damascena hydroalcoholic extract on scopolamine induced memory impairment in rats. Nutr Neurosci. 2015;18(7):329–36. doi: 10.1179/1476830514Y.0000000137. [DOI] [PubMed] [Google Scholar]
- 8.Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M. Frankincense (Rǔ Xiāng; Boswellia Species): From the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J Tradit Complement Med. 2013;3(4):221. doi: 10.4103/2225-4110.119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MuÈller RH, MaÈder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 10.Huang R, Ke W, Liu Y, Jiang C, Pei Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials. 2008;29(2):238–46. doi: 10.1016/j.biomaterials.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Singh I, Swami R, Pooja D, Jeengar MK, Khan W, Sistla R. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. J Drug Target. 2016;24(3):212–23. doi: 10.3109/1061186X.2015.1068320. [DOI] [PubMed] [Google Scholar]
- 12.Ward P, Paz E, Conneely O. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62(22):2540–8. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malekpour-Galogahi F, Hatamian-Zarmi A, Ganji F, Ebrahimi-Hosseinzadeh B, Nojoki F, Sahraeian R, Mokhtari-Hosseini ZB. Preparation and optimization of rivastigmine-loaded tocopherol succinate-based solid lipid nanoparticles. J Liposome Res. 2017:1–10. 10.1080/08982104.2017.1349143. [DOI] [PubMed]
- 14.Schwarz C, Mehnert W. Freeze-drying of drug-free and drug-loaded solid lipid nanoparticles (SLN) Int J Pharm. 1997;157(2):171–9. doi: 10.1016/S0378-5173(97)00222-6. [DOI] [PubMed] [Google Scholar]
- 15.Kamalinia G, Khodagholi F, Shaerzadeh F, Tavssolian F, Chaharband F, Atyabi F, Sharifzadeh M, Amini M, Dinarvand R. Cationic albumin-conjugated chelating agent as a novel brain drug delivery system in neurodegeneration. Chem Biol Drug Des. 2015;86(5):1203–14. doi: 10.1111/cbdd.12586. [DOI] [PubMed] [Google Scholar]
- 16.Manoocheheri S, Darvishi B, Kamalinia G, Amini M, Fallah M, Ostad SN, Atyabi F, Dinarvand R. Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel. Daru. 2013;21(7):1. doi: 10.1186/2008-2231-21-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Aydin E, Hritcu L, Dogan G, Hayta S, Bagci E. The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol Neurobiol. 2016;53(9):6557–67. doi: 10.1007/s12035-016-9693-9. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y, Sogabe S, Hattori Y, Tanaka J. Anxiolytic and hypnotic effects in mice of roasted coffee bean volatile compounds. Neurosci Lett. 2012;531(2):166–9. doi: 10.1016/j.neulet.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols. 2007;2(2):322. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23(5):238–45. doi: 10.1016/S0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 22.Campos MM, Fernandes ES, Ferreira J, Santos AR, Calixto JB. Antidepressant-like effects of Trichilia catigua (Catuaba) extract: evidence for dopaminergic-mediated mechanisms. Psychopharmacology. 2005;182(1):45–53. doi: 10.1007/s00213-005-0052-1. [DOI] [PubMed] [Google Scholar]
- 23.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 24.Venishetty VK, Samala R, Komuravelli R, Kuncha M, Sistla R, Diwan PV. β-Hydroxybutyric acid grafted solid lipid nanoparticles: A novel strategy to improve drug delivery to brain. Nanomed Nanotechnol Biol Med. 2013;9(3):388–97. doi: 10.1016/j.nano.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas A, Ithakissios D. PLGA–mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Controlled Release. 2002;79(1):123–35. doi: 10.1016/S0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Xie C, Wang H, Hu Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials. 2004;25(15):3065–71. doi: 10.1016/j.biomaterials.2003.09.087. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed T, Gilani AH. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol Biochem Behav. 2009;91(4):554–9. doi: 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat Protoc. 2013;8(9):1660. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]