Abstract
Purpose
To make up for the deficiency of the distribution characteristics of mercury (Hg) pollution in soil and rice in a specific area, the relationship between more than ten soil indices and Hg in soil-rice system was analysed, and the main factors affecting mercury accumulation in rice were screened out. So as to provide reliable theoretical and scientific basis for the regulation and safe utilization of Hg-contaminated soil.
Methods
The Hg-polluted area of Siqian Dam, with a paddy field area of 1.34 million square meters, was selected as the research unit. Soil and corresponding rice samples were collected and analysed. Then, common Kriging interpolation was used to explore the spatial distribution differences of mercury content between soil and rice, Pearson correlation analysis and stepwise linear regression were used to analyse the relationship between mercury content and 14 soil indices.
Results
In the study area, the total mercury(THg) content in soil and rice was as high as 30.60 mg/kg and 160.19 µg/kg, respectively, and the methyl mercury(MeHg) content was as high as 14.56 µg/kg and 40.32 µg/kg, respectively, indicating that mercury pollution in soil and rice was serious. The horizontal spatial distribution of soil THg and MeHg was different. Flood with its sediment and topography were the main reasons for the uneven distribution of Hg content in the region. The spatial distribution of Hg was different between rice and soil. There was no significant correlation between rice and soil THg, but there was a significant correlation between rice and soil MeHg content. Among the 14 soil indices, available potassium was a vital index affecting the accumulation of Hg in rice, followed by pH, Zn, Mn and Fe.
Conclusions
The results showed that in weakly acidic and fertile soil, the appropriate reduction of soil pH, OM and available Se and Cr contents could inhibit soil Hg methylation, the reduction of potassium fertilizer application could further reduce rice Hg accumulation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40201-021-00711-z.
Keywords: Horizontal spatial distribution, Paddy field, Rice, Accumulation, THg, MeHg
Introduction
Agencies such as the United Nations Environment Programme, the World Health Organization (WHO), the European Union (EU) and the United States Environmental Protection Agency (USEPA) have made mercury (Hg) a priority for controlling contaminants [1, 2]. Hg is a global pollutant [3], due to it has highly bio-toxicity, persistence, bio-accumulation and widespread contamination [4, 5]. The bioavailability, migration, toxic and ecological effects of mercury strongly rely on its chemical speciation [6]. There are different forms in the environment, such as metal, inorganic and organic mercury compounds. Methylmercury (MeHg) is the most toxic mercury compound with high neurotoxicity, carcinogenicity, cardiovascular toxicity, reproductive toxicity, immune system effects and renal toxicity [7, 8]. Inorganic mercury (IHg), undergoing biotic and abiotic processes, can be transformed to MeHg [6, 9, 10]. Recently, studies indicated that rice consumption, which grown in Hg-contaminated paddy soil, was the major MeHg route to inhabitants [7, 11]. Rice is a vital food crop worldwide, and intake of polluted rice could pose a severe potential health threat to human beings [12], especially pregnant women and infants [13].
The distribution of Hg resources is relatively concentrated, and large mercury deposits are mostly distributed in southwest China. The Guizhou Province was a major Hg production region in China [14]. Tongren City was the most active mercury industry in history. It was considered the third-largest mining area in the world and the largest Hg production area in China [14, 15]. Operation of Tongren mine ceased in 2001 due to the Hg resource was exhausted and environmental concerns [14]. Discharge of Hg-containing wastewater and leaching of smelting slag into nearby rivers resulted in elevated concentrations of Hg in the water with the average total mercury (THg) concentration of 1.22 mg/L [16]. Irrigation of Hg-containing wastewater caused severe Hg contamination of surrounding farmland.
In the past two decades, many scholars have investigated mercury pollution in the soil, rivers and crops around the Wanshan mercury mine. The results showed that the contents of THg and MeHg in the soil around the Wanshan mercury mining and smelting area are in the range of 0.01 ~ 4400 mg/kg and 0.1 ~ 67 µg/kg [15, 17]. There was a significant negative correlation between soil total mercury content (THgsoil) and the distance from pollution sources [17–19]. According to the investigation, the THg content of riparian soil near the slag of the Wanshan mercury mining area was as high as 790 mg/kg, and its range was still 16 ~ 89 mg/kg of 10 ~ 30 km along the river bank [15]. The highest contents of THg in vegetables and fruits were 1075 µg/kg and 160 µg/kg [20], respectively. The contents of total mercury (THgrice) and methylmercury (MeHgrice) in rice reached 1120 µg/kg and 174 µg/kg [21], respectively. Therefore, there is a relatively great risk of mercury pollution in soil, vegetables, fruits and rice around the Wanshan mercury mine.
Most of the above studies were based on the investigation and analysis of 8 ~ 30 soil and crop samples collected around the Wanshan mining area to evaluate the level of mercury pollution in soil and crop. Few field studies have systematically studied the horizontal spatial distribution of Hg in soil and rice in a specific environmental unit and the factors affecting the transport and transformation of mercury in soil-rice system.
Due to the seasonable irrigation during the periods of rice-growing, paddy fields are usually recognized as an intermittent wetland ecosystem [22]. Thus, the accumulation, migration and transport of THg and MeHg in soil-rice system are more complex. Previous studies revealed that IHg methylation process was a crucial link of mercury form transformation in paddy soil, which directly affected the content of soil MeHg, and ultimately affected the enrichment degree of MeHg in rice [23, 24].
Many scholars have studied the influencing factors of soil mercury methylation through field investigation and pot experiment. The statistical analysis revealed that the influencing factors of soil MeHg content mainly include soil pH, organic matter, soil temperature, redox potential, sulfur (S) and iron (Fe) content, species and abundance of methylated microorganisms, atmospheric mercury deposition and so on [25–27]. However, some studies have shown that nitrogen may improve the uptake of MeHg from paddy soil and translocation in rice plants [28, 29]. Nutrient elements such as nitrogen(N), phosphorus(P), potassium(K) and metals such as manganese (Mn) and selenium (Se) have antagonistic/synergistic effects with Hg [30–32], respectively. Therefore, the analysis of pH, OM and other indicators on the transport process of Hg in soil-rice system also has some limitations. In this study, 108 soil samples and 71 pairs of soil-rice samples were collected from Siqian Dam with a paddy field area of 1.34 million square meters. Based on previous studies, the following two issues are further discussed: (1) To evaluate the horizontal spatial distribution and influencing factors of Hg in soil and rice in a specific region by using the common Kriging interpolation method, and (2) Pearson correlation analysis and Stepwise linear regression were used to analyse the association of Hg in the soil-rice system, as well as the correlation between accumulated THg and MeHg in rice and 14 soil indices, including pH, OM, nutrient elements of N, P, K, sulfur and available Fe, Mn, Cu, Cd, Se, Zn, Pb and Cr in soil, and further screening out the key influencing factors.
Materials and methods
Study sites
Siqian Dam is situated in the northeast of Wanshan Hg mining area, south of Wawu River in Wawu Township. The area is a subtropical humid climate, rich in precipitation, mild temperature, annual average temperature of 13.5 ~ 17.6 ℃, and annual average rainfall of 1200 ~ 1400 mm. The soil is mainly tidal soil, paddy soil and yellow soil, more than 99 % of cultivated land belongs to the paddy field. Siqian Dam uses the dry-wet alternate planting method, planting rice in summer and autumn and planting rape in winter and spring.
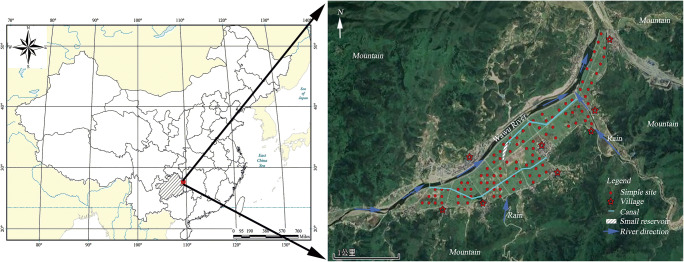
The south side of the Siqian Dam is a high mountain. The Wawu River is the major source of irrigation and the key source of Hg pollution in the region due to the influence of the upstream Wanshan Hg mining. There are two main canals in this area, the source of which is the northwest corner and the small reservoir in the middle of the area, respectively. At the junction of the lower and middle paddy fields, there is a wider canal, a tributary of the Wawu River. The geographical location of the samples is shown in Fig. 1.
Fig. 1.
Study area and sampling locations
Sample collection and measurement
Sample collection and preparation. In September 2019, rice and soil samples were collected. At each sampling site, more than five sub-samples of the edible parts of rice plant were collected and combined for further treatment and analysis. The soil sub-sample was taken for each one sub-sample of rice and then mixed thoroughly as a bulk sample at each sampling site. The collected rice and soil samples were stored in sealed polyethylene plastic bags and brought back to the laboratory as soon as possible. The specific sampling points are shown in Fig. 1. The samples were taken along a rectangular grid in 100 m intervals, with the corresponding latitude and longitude coordinates recorded using a portable GPS.
For soils, all visible roots, macro fauna, and stones were removed from soils prior to processing. Then soil samples were freeze-dried using a lyophilizer (XO−10 N), homogenized, and subsequently passed through a 100-mesh nylon sieve. Rice samples were washed with distilled water three times and freeze-dried using a lyophilizer operated at−80 ℃. Freeze-dried plant samples were husked by a rice huller (JLG-II), then, rice were crushed into powder by an electronic grinder (200T). After the samples were processed, they were put in cold storage for testing.
Analytical methods
For THg analysis, 0.2 g of rice powder was prepared and digested with a freshly prepared mixture of HNO3 : H2O2 (v/v 5:3) present in oven for 3.5 h at 125 ℃. 0.1 g samples were digested in water bath (95 ℃) using a fresh mixture of HCL:HNO3:H2O (v/v 3:1:4) for 2 h. The Hg concentration in the digested solutions was determined by atomic fluorescence spectrophotometer using a Hg analyzer (Beijing Baode Instrument-AFS−2000).
For MeHg analysis, 0.2 g rice samples were digested using a KOH-methanol/solvent extraction technique. Firstly, the samples were digested with KOH-CH3OH and heated in a water bath. Then, the digests were acidified with concentrated HCL. For soil samples, 0.2 g of soil sample was prepared using CuSO4 solution and HNO3 extraction. For both, MeHg in samples was extracted by methylene chloride as well as back-extracted from the solvent phase into aqueous phase ethylation. The ethyl analogue of MeHg-methylethyl mercury (CH3CH3CH2Hg), was decoupled from solution by purging with N2 onto a Tenax trap. The trapped CH3CH3CH2Hg was then thermally desorbed, separated from other Hg species by an isothermal gas chromatography (GC) column, disintegrated to Hg0 in a pyrolytic decomposition column (800 ℃) and analysed using CVAFS.
Soil pH and OM were measured by methods reported previously [33]. The determination of available nitrogen (N), available phosphorus (P) and available potassium (K) in soil was carried out according to the method of the Soil Agrochemical Analysis Course. Soil available iron (Fe), available manganese (Mn), available copper (Cu), available zinc (Zn), available selenium (Se), available cadmium (Cd), available lead (Pb) and available chromium (Cr) were extracted by DTPA solution and analysed using ICP-MS (Thermofisher X2, Germany).
Quality control
Both the THg and MeHg determination in samples were conducted using sample duplicates, reagent and method blanks, matrix spikes, and certified reference materials (CRMs). GSS−5 (Soil, GBW07405, institute of geophysical and geochemical exploration, GAGS Co., Ltd., China) and GSB−26 (Apium graveolens, GBW10048, institute of geophysical and geochemical exploration, GAGS Co., Ltd., China) were used as CRMs during the THsoil and THgrice analysis, respectively. Recoveries for THg analysis ranged from 85 to 110 %, and the relative standard deviations of the duplicates were in the range of 1.6 ~ 7.8 % for the soil and 2.2 ~ 19 % for the rice.
The CRMs was ERM-CC580 (estuarine sediment) for MeHgsoil analysis. Recover for MeHgsoil analysis were between 87 and 115 %, and the relative standard deviations in duplicates were lower than 9 %. The response rates of MeHg in the matrix spikes ranged from 90 to 120 %. The recovery percentage of blank tag recovery in the process of rice ranged from 85 to 120 %.
Data analysis and statistical analysis
The normplot (QQ graph) was checked the normality of data. The logarithmic transformation was applied to transform skewed data to normality. The Pearson’s correlation analysis and Stepwise analysis among the parameters were conducted by Microsoft Excel 2007 and SPSS 13.0. Correlations were considered statistically significant when p < 0.05. Figures were drawn with Origin 2017 software and Arc GIS 10.3 software. Omap was used for the export of sampling location and distance data.
The bioconcentration factor (BCF) represents the ability of plants to absorb and accumulate heavy metals in soil. The bioconcentration factors of Hg in rice were calculated using the following equation:
| 1 |
Where, Crice represents rice THg or MeHg concentration, µg/kg; Csoil represents soil THg or MeHg concentration, µg/kg. The bioconcentration factors for THg and MeHg were expressed in BCFTHg and BCFMeHg, respectively.
Results
THg and MeHg concentrations in paddy field
As can be seen from Table 1, THgsoil contents ranged from 1.09 to 30.60 mg/kg with an average of 8.80 mg/kg, which significantly exceeded the maximum THg value (1.0 mg/kg, pH > 7.5) restricted for Chinese agricultural soils (State Environmental Protection Administration of China, 2018) [34]. The MeHgsoil concentrations ranged from 0.218 to 14.56 µg/kg with an average of 1.83 µg/kg; the maximum ratio of MeHgsoil to THgsoil was 1.75 %, far lower than the THg content in soil. The concentration of available Hg in soil ranged from 0.034 to 1.72 µg/kg, with an average of 0.340 µg/kg, accounting for about 1/10,000 of THgsoil. The results showed that IHg with low mobility and low bioavailability was the main form of soil mercury.
Table. 1.
Statistical summary of Hg concentrations in soil and rice, and other soil indices
| Index | Mean ± S.D. | Min. | Max. | Median | |
|---|---|---|---|---|---|
| Soil Hg concentration | THgsoil(mg/kg) | 8.80 ± 5.68 | 1.09 | 30.60 | 7.58 |
| MeHgsoil (µg/kg) | 1.83 ± 1.97 | 0.218 | 14.56 | 1.16 | |
| Available Hg (µg/kg) | 0.340 ± 0.294 | 0.034 | 1.72 | 0.302 | |
| Soil indices | pH (unitless) | 6.52 ± 0.791 | 5.27 | 7.78 | 6.55 |
| OM (g/kg) | 26.36 ± 8.44 | 12.18 | 51.53 | 24.61 | |
| N (mg/kg) | 300.02 ± 127.37 | 82.60 | 655.20 | 285.60 | |
| P (mg/kg) | 12.52 ± 5.66 | 4.76 | 41.66 | 11.51 | |
| K (mg/kg) | 210.75 ± 66.39 | 135.35 | 401.14 | 228.06 | |
| S (mg/kg) | 43.33 ± 26.69 | 5.32 | 113.31 | 35.74 | |
| Fe (mg/kg) | 99.38 ± 50.22 | 17.19 | 222.78 | 91.38 | |
| Mn (mg/kg) | 47.48 ± 19.71 | 8.03 | 115.13 | 48.38 | |
| Cu (mg/kg) | 3.86 ± 1.63 | 1.20 | 8.48 | 3.41 | |
| Zn (mg/kg) | 1.61 ± 0.60 | 0.764 | 4.072 | 1.43 | |
| Se (mg/kg) | 0.056 ± 0.028 | 0.021 | 0.251 | 0.051 | |
| Cd (mg/kg) | 0.167 ± 0.065 | 0.046 | 0.399 | 0.151 | |
| Pb (mg/kg) | 4.29 ± 1.12 | 1.95 | 7.72 | 4.16 | |
| Cr (mg/kg) | 0.074 ± 0.004 | 0.064 | 0.091 | 0.074 | |
| Rice Hg concentration | THgrice grain (µg/kg) | 71.07 ± 32.77 | 10.70 | 160.19 | 73.81 |
| MeHgrice grain(µg/kg) | 11.91 ± 9.55 | 0.747 | 40.32 | 8.23 | |
| Bioconcentration factor(BCF) | BCFTHg | 0.128 ± 0.010 | 0.0005 | 0.050 | 0.010 |
| BCFMeHg | 11.01 ± 12.21 | 0.351 | 50.32 | 6.214 | |
Notes: N—Alkali-hydrolyzed nitrogen, P—Available phosphorus, K—Available potassium, S—Available S, Fe—Available Fe, Mn—Available Mn, Cu—Available Cu, Zn—Available Zn, Se—Available Se, Cd—Available Cd, Pb—Available Pb, Cr—Available Cr
Horizontal spatial distribution of Hg contents in paddy soil and their influencing factors
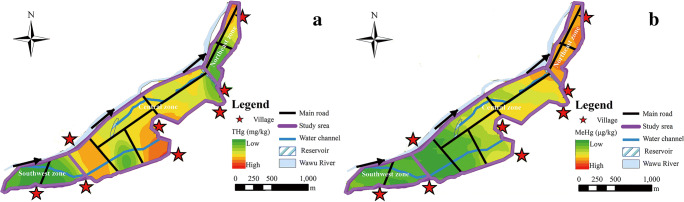
The distribution characteristics of THgsoil and MeHgsoil levels in the region were different. Based on the concentrations of THgsoil and MeHgsoil, logarithmic transformation was carried out to make them normal distribution, then the distributions of both in the region were predicted by Kriging interpolation method. From Fig. 2(a), it can be seen that the THgsoil distribution of soil was low in the paddy fields of upper and lower reaches and high in the middle reaches. In the downstream and upstream regions, the THgsoil concentration increased gradually along the direction of canal flow. The THgsoil concentration in the middle not show obvious regular distribution. According to Fig. 2(b), the distribution of MeHgsoil was different from that of THgsoil, and it increased gradually from southwest to northeast along the flow direction of the Wawu River.
Fig. 2.
The distributions of THgsoil (a) and MeHgsoil (b) in paddy soil
The flood caused by frequent rainfall in summer and the canal water were important factors affecting soil Hg methylation and MeHg accumulation. In addition, the distribution of MeHgsoil was similar to that of soil S, especially in the northeast region, which indicated that S also played an essential factor influencing the distribution of MeHg in paddy field.
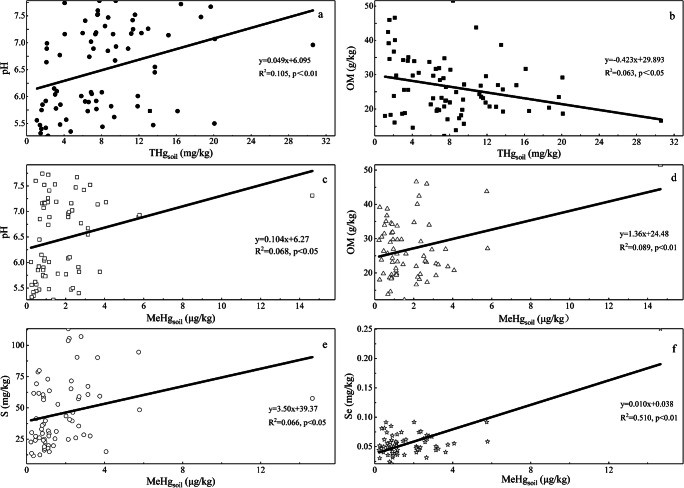
Pearson’s correlation analysis showed that soil pH (P < 0.01, r = 0.343) and OM (P < 0.05, r=−0.276) were important factors affecting Hg migration/transformation, and were significantly correlated with THgsoil (Fig. 3). In addition, THgsoil was significantly correlated with soil available Pb (P < 0.01, r=−0.366), Cu (P < 0.01, r=−0.342), Fe (P < 0.01, r=−0.327) and Zn (P < 0.01, r=−0.310) (Fig. S1). MeHgsoil and available Se (P < 0.01, r = 0.719), OM (P < 0.01, r = 0.321), available Cr (P < 0.01, r = 0.317), pH (P < 0.05, r = 0.269) and available S (P < 0.05, r = 0.266) showed a positive correlation (Fig. 3).
Fig. 3.
The correlations of THgsoil and MeHgsoil with soil pH (a,b), OM (c,d), S (e) and Se (f)
Accumulation of THg and MeHg in rice
The concentrations of THgrice and MeHgrice in rice were shown in Table 1. The THgrice concentrations ranged from 10.70 µg/kg to 160.19 µg/kg with an average of 71.07 µg/kg, and over 90 % of samples had THgrice higher than the maximum Hg levels (20 µg/kg) restricted for food in China (Ministry of Health Standardization Administration of China, 2017) [35]. The MeHgrice concentrations were between 0.75 µg/kg and 40.32 µg/kg, with an average of 11.91 µg/kg. About 20 % of rice samples had MeHgrice higher than 20 µg/kg. The ratio of MeHgrice to THgrice ranged from 2.51 to 90.26 %, with a median of 13.62 %. It can be seen that IHg is the main form of mercury in rice.
Horizontal spatial distribution of Hg concentrations and their influencing factors in rice
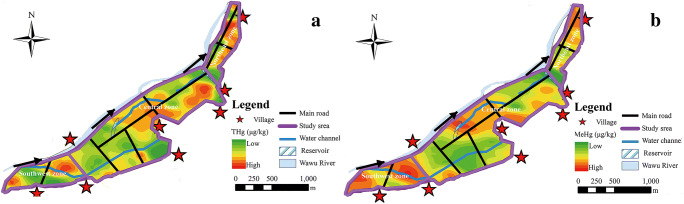
A method for predicting the spatial distribution of THgrice and MeHgrice levels in rice was the same as that in soil. The results are shown in Fig. 4. As can be seen from Fig. 4, the THgrice and MeHgrice showed uneven distribution in the study area. In the upstream and mid-stream paddy fields, the THgrice and MeHgrice decreased gradually along the direction of canal flow. In contrast, the downstream rice field was farther away from the tributary of Wawu River, the higher the Hg content of rice was.
Fig. 4.
The distributions of THgrice (a) and MeHgrice (b) in rice
The ability of rice to enrich Hg was also a crucial factor affecting the distribution of THgrice and MeHgrice. According to formula (1), the BCFTHg and BCFMeHg in rice were calculated. The BCFTHg was between 0.0005 and 0.050, the mean value was 0.0128, and the median value was 0.010. The BCFMeHg ranged from 0.351 to 50.32 with a mean of 11.01 and a median of 6.214. In contrast, rice had a strong ability to accumulate MeHg.
The results of Pearson’s correlation analysis showed that there was no significant correlation between rice Hg and soil Hg. Therefore, We used THgrice (Y1) and MeHgrice (Y2) as dependent variables, with soil pH (X1), OM (X2), available S (X3), N (X4), P (X5), K (X6), Fe (X7), Mn (X8), Cu(X9), Zn (X10), Se(X11), Cd (X12), Pb (X13), Cr (X14), available Hg (X15), THgsoil (X16) and MeHgsoil (X17) as independent variables for stepwise regression analysis (Table 2). The result found that accumulation of THg in rice was mainly affected by K, followed by pH and Zn. With the increase of soil K content or pH, the accumulation of THgrice increased; THgrice decreased with the increase of Zn content. K was the main factor affecting the accumulation of MeHgrice, followed by Mn, MeHgsoil and Fe. Except Fe, the K, Mn and MeHgsoil were positively correlated with MeHgrice, respectively.
Table. 2.
Regression equation, variance analysis and regression coefficient of Hg contents and soil indexes
| Regression equation | R | Adj-R2 | F | P1 | Independent variable | B | Std. Error | Beta | P2 | Collinearity statistics | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tolerance | VIF | ||||||||||
| Y1=-16.84X10+0.201X6+13.69X1-33.79 | 0.314 | 0.265 | 6.42 | 0.001 | Constant | -33.79 | 48.52 | 0.490 | |||
| X10 | -16.84 | 8.57 | -0.261 | 0.056 | 0.923 | 1.084 | |||||
| X6 | 0.201 | 0.069 | 0.284 | 0.006 | 0.931 | 1.074 | |||||
| X1 | 13.69 | 5.66 | 0.332 | 0.020 | 0.868 | 1.152 | |||||
| Y2=0.096X6+0.161X8+1.26X17-0.054X7-13.21 | 0.777 | 0.564 | 15.58 | 0.000 | Constant | -13.21 | 4.42 | 0.005 | |||
| X6 | 0.096 | 0.016 | 0.602 | 0.000 | 0.941 | 1.063 | |||||
| X8 | 0.161 | 0.047 | 0.342 | 0.001 | 0.979 | 1.021 | |||||
| Y2 | 1.26 | 0..430 | 0.295 | 0.005 | 0.963 | 1.039 | |||||
| X7 | -0.054 | 0.020 | -0.269 | 0.010 | 0.976 | 1.025 | |||||
Notes: P1 value of F-test; P2 value of T-test; R—Pearson correlation coefficient; Adj-R2—Adjust determination factor; F—The value of F-test; B—Unstandardized coefficient; Beta—Standardized coefficient; VIF—Variance inflation factor. Y1—THgrice, Y2—MeHgrice. X1—pH, X6—K, X7—Fe, X8—Mn, X10—Zn, X17—MeHgsoil
Discussion
Sources of soil Hg and factors influencing their transformation/ migration
Over 90 % of all samples THgsoil contents exceeded the action value of 2.0 mg/kg set by the German Federal Soil Protection Ordinance (BBodSchV 1999) [36], as well as 87.26 % beyond the limit, recommended (2.5 mg/kg) by the Company of Technology of Environmental Sanitation (CETESB) [37] for agricultural soil. Most samples of MeHgsoil concentrations were higher than the background levels in the Wanshan Hg mine (0.1 ~ 0.3 µg/kg) [15]. These results showed that there were serious ecological risks in paddy fields in this study area. Wang et al. [38] studied mercury pollution in the soil of Wanshan Greenhouse and found that the average content of available mercury in the soil was 1.0 µg/kg. The maximum content of available mercury in this study was higher than this value. This result indicated that the soil mercury pollution in this area was serious.
A previous study found that the THg concentrations in surface water (such as Meizi River, etc.) around the Wanshan Hg mining area ranged from 0.019 to 12 µg/L [39], and the Hg content in water sediments was as high as 30.10 mg/kg [17]. Through sampled and analysed irrigation water in the region from May to August 2019, we found that the water in Wawu River contained Hg, and the Hg contents ranged from 0.008 to 0.374 µg/L with an average of 0.072 µg/L (Table S1). Therefore, the leachate of waste water, waste rock and waste residue from the activities of the Wanshan Hg mine in the upper reaches entered the river and caused the increase of Hg concentration in the river.
Three profiles (Fig. S2) were also collected for the Siqian Dam in December 2018. The characteristics of the profiles showed that the soil was a relatively typical latent paddy soil formed by alluvial river deposits, indicating that the flood and its carrying sediment played an important role in the formation of soil in the region. In addition, summer rain flowed from the valley into lower paddy fields. Rain water flowed mainly from the tributaries of the Wawu River (which is at the junction of the middle and lower paddy fields) and from the middle reaches near the lower paddy fields (Fig. 1). The THg content of rain water flowed into the paddy fields nearby was low, but it had little effect on MeHgsoil (Fig. 2). Therefore, Hg-containing floods with carrying sediments and prolonged use of Hg-containing irrigation water were the main sources of Hg in the Siqian Dam. Flood and its carrying sediments were the main reasons for the different distribution of THgsoil and MeHgsoil concentrations in horizontal space.
Qiu et al. [40] collected 17 soil samples from paddy fields, dry lands and vegetable fields on both sides of the river in the Wanshan mining area. Their results found that most paddy soil distributed along the river in the mining area was affected by upstream polluted water sources. Frequent floods in summer would migrate and carry a large amount of Hg-rich particles from slag to the lower reaches of the river, resulting in secondary pollution. Dai et al. [16] collected riverbank soil, shrub soil and woodland soil within 10 km along the river in the Wanshan Wukeng mercury smelting area. They found that the Hg-pollution river was the direct source of mercury pollution in the surrounding soil. These two results also clearly supported the conclusion of this study. Qiu et al. [40] also showed that the soil MeHg content always increased with the increase of the Hg content, which was different from this study. It may be that the sampling areas of Qiu et al. [40] were paddy fields, dry lands, vegetable fields soil and natural soil within about 3 km of the mercury mining area, which is different from the geographical location and land use type of our sampling. Hence, the correlation between THgsoil and MeHgsoil was different.
The contents of THgsoil and MeHgsoil in the upstream paddy field were relatively low, and the concentrations of THgrice and MeHgrice were high. Along the direction of the channel in the paddy field, the THgsoil, MeHgsoil and MeHgrice showed a gradually increasing trend. It may be due to the upstream paddy field used the terrace planting mode, the relatively high topography, the longer land tillage time, and the better protection of the residents. It was less affected by the flood. Moreover, rain water and irrigation water scoured the higher terrain area Hg into the low terrain rice field, caused high Hg levels in low-lying areas. Sediments carried by flood outbreaks were deposited, which was also responsible for higher levels of Hg in low-lying areas. The high concentrations of Hg, especially MeHg, in rice may also be one reason for the low concentration of Hg in rice fields.
The concentrations of THgsoil and THgrice in the middle paddy field changed greatly, and there was no obvious regular characteristic. Moreover, the horizontal spatial distributions between them were inconsistent. The concentrations of MeHgsoil and MeHgrice increased and decreased trend along the flow direction of the canal, respectively. In the middle rice field, the terrain was relatively flat, but there were fluctuation changes so that the Hg retention in different plots were different after being eroded by the flood. The low-lying areas were affected by the combined action of water flow and sediment, which lead to higher Hg retention. High elevation areas were mainly influenced by water flow, and Hg retention was relatively small. Previous study has shown that the THg contents in the river beach soil in the Wanshan Hg mining area were higher than that in the forest land covered by vegetation [16]. After the dam was built, the Hg contents of the flat tidal area might be higher than the grassland, which lead to the uneven distribution of Hg in the area after they changed to cultivated land.
High levels of MeHg in the lower Wawu River soil might be the main cause of high MeHgrice levels in the region. The change trends of Hg in soil and rice in the downstream region were similar. Because of the tributaries of Wawu River at the junction of downstream and middle rice fields, the floods scoured in this area were severe. As the flow velocity slowed down, the sediments were deposited far away from the canal, and the floods inundation time was longer due to the lower terrain downstream, which lead to the hypermethylation of Hg in rice fields.
Factors influencing soil mercury transformation/ migration
The soil pH values ranged from 5.27 to 7.78 with an average of 6.52; about 64 % of samples were less than 7, suggesting that most of the rice paddy soil is slightly acidic. The OM concentrations (12.18 to 51.53 g/kg with an average of 26.36 g/kg), the N, P and K concentrations ranged from 82.60 mg/kg to 655.20 mg/kg, 4.76 mg/kg to 41.66 mg/kg and 135.35 mg/kg to 401.14 mg/kg. Most samples were generally higher than the other soils in China according to the national survey standard (Office of national soil survey,1979) [41]. These results indicated that soil fertility in this area is sufficient.
Soil pH, OM, N, P, K, S, Fe, Mn and other indices also impacted the transfer and transformation of mercury. Among them, soil pH was an important factor affecting the distribution of THsoil content in paddy field, and it also had a significant effect on MeHgsoil in paddy field. It is difficult for heavy metals to migrate when the soil pH exceeds 7. A few samples were less than 7, so the migration ability of Hg in paddy field was strong. The pH values of the study area were between 4.5 and 9.0, the best value of biological activity [25, 40], which was conducive to promoting methylation of Hg. Low pH is generally considered to be conducive to stimulating methylation of Hg [42]. However, the content of MeHg in paddy fields in this study increased with the increase of pH. The possible reason was that the high microbial activity under high pH conditions (pH < 7.0) improved the methylation process of Hg [43, 44]. Gilmour and Henry [42] studies on anaerobic sediments and the effects of Zhang et al. [45] on acid soil methylation by different irrigation measures have shown that low pH conditions can reduce mercury methylation by inhibiting bacterial activity, and our conclusion was consistent with that.
Secondly, OM also played an essential role in distributing THgsoil and MeHgsoil contents in paddy soil, especially MeHgsoil. Some studies have shown that the correlation between soil methyl mercury and OM is weak [46, 47]. However, some studies have found that the THgsoil is positively correlated with OM, and there is also a significant correlation between MeHgsoil and OM [48]. Pei et al. [49] study on related mercury and organic carbon in typical farmland soil aggregates showed a significant positive correlation between related mercury in soil aggregates and organic carbon, so organic carbon was involved in the participation and enrichment of soil mercury. The above research results were different from this study. The possible reason is that the soil methylation process is affected by multiple factors, and the role of OM varies on mercury in different soil environments. In this study, the content of THgsoil decreased with the increase of OM, which might be due to the coordination or complexation of Hg with carboxyl, hydroxyl and other functional groups in dissolved OM [26]. This process increased the migration capacity of Hg by increasing the solubility of Hg in soil solution [50].
However, the increase of OM content might increase the content of MeHgsoil in paddy soil. The possible reason was that abundant OM provided sufficient carbon source [51] and increased the activity of Hg methylated microorganisms [52], so that promoted the methylation process of Hg. In this study, there was a significant correlation among OM, N and S (Fig. S3). This is similar to the conclusion that Yin et al. sampled and analyzed the paddy soil within 500 m around the abandoned Hg mining sites and active Hg smelting site. This indicated that OM contained abundant N- and S-containing groups, forming stable Hg-N-OM or Hg-S-OM complex with Hg [53]. With the increase of OM, the contents of MeHgsoil decreased. At the same time, the inhibiting effect of S, N groups in OM on Hg methylation might be lower than the MeHg yield of methylated microorganisms. Hence, the content of MeHgsoil increased with the increase of OM content. However, the mechanism of action between the inhibition of Hg methylation and the stimulation of microbial methylation by OM is not very clear, which requires further investigation.
In addition, the slag from the upstream Wanshan Hg mine contained fine-grained Hg-inert sulfide and sulphates, which were easily dissolved in water and enter the surface runoff in the form of suspended particles [54]. Fine S-bearing Hg deposits were deposited in paddy fields of the middle and lower reaches after the rainstorm washed them out of the river banks. The high S content deposited stimulated the activity of methylation bacteria and the availability of Hg, which were conducive to promoting the methylation process of Hg in the paddy field. It has been shown that the formation of a complex between mercury and polysulfide compounds facilitates the dissolution of HgS. It converts stable mercury into bioavailable forms of mercury (e.g. HgS22−), thereby promoting methylation of mercury [27, 55–57]. Therefore, this may be the reason for the similarity of the spatial distribution of S and MeHg to some extent.
Except for available Mn, other available metals in soil were significantly correlated with THgsoil and MeHgsoil. Zhao et al. [58] analysis of pollution levels of various heavy metals in typical ecologically fragile areas found no correlation between mercury in soil and other elements such as zinc, cadmium and lead, which was inconsistent with this study. In the study, the lower the contents of available Fe, Cu, Zn and Pb, the higher contents of THg in soil. It might be that the existence of low contents of Fe, Cu, Zn and Pb reduced the migration of Hg in soil. The higher the contents of Se and Cr in soil, the higher the contents of MeHg in paddy field. Se can form a stable compound Hg-Se with Hg [59], which can reduce the mobility and availability of Hg and inhibit the absorption/transport of MeHg in rice. In this study, the high Se concentration after rice planting indicated that the content of Hg-Se complex formed in the soil was low, and the methylation degree of Hg was great, thus leading to the increase of the content of MeHg in the soil with the increase of Se. As for the relationship between Cr and MeHgsoil, there were relatively few studies, and the interaction between the two remains to be studied.
Characteristics and influencing factors of Hg enrichment in rice
The contents of THgrice and MeHgrice in the study area were significantly higher than those in the non-mercury polluted areas of Guizhou, such as Leigongshan [47], Huaxi [33], Guiyang [60], and also higher than the results of Li et al. [61] survey of rice around Wanshan mercury mine in Guizhou in 2013 (THgrice: ranging from 13 to 52 µg/kg with an average value of 26 µg/kg; MeHgrice: ranging from 3.5 to 2.3 µg/kg with an average value of 9.4 µg/kg). IHg in rice is less harmful to the human body, and the intake of MeHg will cause great damage to human organs and tissues. However, the tolerable intake for MeHg recommended by the USEPA (1997) [62] reference dose (RfD) for MeHg is 0.1 µg/kg body weight per day. According to health risk studies, the maximum daily intake of MeHg in humans was 0.202 µg/kg body weight based on average adult body weight (60 kg) and daily intake of rice (300 g) [63], which exceeded the USEPA (1997) [62] reference limit for MeHg. Therefore, there was a health risk from MeHg contamination in rice.
The Siqian Dam used alternate rapese-rice planting to reduce Hg pollution in agricultural products. Samples of rape and rice were taken in May 2019 and September 2019, respectively. The two sampling sites were the same, and the middle interval was the whole period of rice growth. The results showed that the THg content in rapeseed ranged from 10.03 µg/kg to 100.05 µg/kg, with an average of 37.27 µg/kg. The Hg content in rice was higher than that in rapeseed. Qiu et al. [21] studied the crops in the Wanshan mining area and found that the content of THg in rice was as high as 1120 µg/kg, and the concentration of MeHg was as high as 174 µg/kg. Moreover, rice had the highest levels of MeHg relative to other crops in the region, such as corn. Seasonal irrigation in rice fields provided favourable conditions for Hg uptake/transport in rice plant, resulting in a strong Hg accumulation capacity in rice.
Soil K was the main factor affecting the THgrice and MeHgrice contents in rice. Studies have found that K can alleviate the toxic effect of heavy metals on plants [64, 65]. A low concentration of K had no obvious effect on the accumulation of cadmium in the plant, while the high concentration of K inhibited the accumulation of cadmium in the plant [64, 66]. At present, there are few studies on the effect of K on plant Hg enrichment. In this study, high K content promoted the accumulation of THg and MeHg in rice. The possible reasons were that the presence of K did not effectively hinder the absorption and enrichment of Hg in rice. Studies have shown that the cosplast and ectoplasmic pathway of Casparian bands and suberin piece formed in rice roots can reduce the absorption and transport of macromolecular Hg complexes [67, 68]. When the rice seedlings were treated with KCl solution instead of selenite, it was found that there was no Casparian band and uberin piece formation in the rice seedlings, indicating that K had no significant inhibition effect on Hg uptake by rice [68]. Furthermore, K is a nutrient element. The uptake and transport of K by rice roots might promote the uptake of Hg, which lead to the accumulation of Hg in rice. The effect of K on Hg uptake/transport in rice needs further experimental investigation.
Soil pH was the second factor affecting the THg in rice. As mentioned above, high pH increased the abundance and activity of methylating microorganisms, leading to increased MeHg content, thus to increased rice ability to accumulate THg. Therefore, with the increase of pH, the contents of THg and MeHg in rice increased. Some researchers failed to find any relationship between MeHg concentration and pH values (ranged: 4.47 ~ 7.39) in rice paddy soil across the Wanshan Hg mining area [23, 24, 53]. Huang et al. [69] collected alkaline soil and corresponding rice samples for research and found that pH was negatively correlated with the concentration of MeHgrice. Tang et al. [28] research showed that the concentration of MeHgsoil was controlled by numerous indices, in which pH accounted for 52 %. The contribution of soil pH to the accumulation of MeHg in rice was different due to different study regions, leading to different conclusions in this study.
Finally, Zn also effected on the THgrice. The presence of Zn hindered the uptake and transport of Hg in rice, which may lead to the decrease of Hg accumulation in rice with the increase of Zn in soil. Previous studies have found that OsHMA3, a member of heavy metal-transporting ATPase family, is localized at the tonoplast and shows activity for Cd and Zn [70–72]. It may indicate that the rice roots have the same absorption/transport pathway for Cd and Zn. Gupta and Chatterjee [73] found that exposure of rice roots to a Hg (II) solution induced the opening of Ca2 + channels in root membranes. Therefore, it can be inferred that Hg and Zn may have similar absorption/ transport pathways in rice plant [29]. The increase of Zn content in soil indicated that the uptake of Zn by rice roots was small, and the transport of Hg from soil to rice was reduced to a certain extent.
Except for K, Mn and Fe could affect the accumulation of MeHg in rice. Studies have shown that the oxygen secretion process of rice roots can produce oxidation zones in the root surface microenvironment. Reducing substances such as Fe2 + and Mn2 + were oxidized and deposited on the surface of rice roots, forming Fe and Mn oxide plaques [74]. Fe and Mn oxide plaques can reduce free Hg ions in soil pore water through adsorption [75], which reduces the bioavailability of Hg and acts as a barrier for Hg to enter rice tissues, thus reducing the accumulation of Hg in rice. The research results of Cai et al. [76] showed that there was a significant positive correlation between the dissolved amount of Fe and the content of MeHg in the soil solution, there was no obvious correlation between the dissolved amount of Mn and the content of MeHg, which was inconsistent with our conclusions. It has also been shown that the addition of ferric salt reduces the generation of MeHg in the sediment, which may be due to the presence of polysulfide compounds that affect the formation of soluble Hg-S complexes such as HgS0 and Hg(HS)20 in the system [75–77].
In this study, with the increase of Mn content, the concentration of MeHgrice increased. With the increase of Fe content, the concentration of MeHgrice showed a decreasing trend. The possible reason for this result was the increase of Mn content, indicating that the root surface formed less Mn oxide plaque so that a large amount of MeHg was absorbed by the rice plant and then accumulated in rice. The increase of Fe content resulted in the formation of thin iron plaque on the root surface, which promoted the absorption of MeHg in rice, but also accelerated the formation of Fe-S compounds between Fe and S. Fe-S compounds inhibited methylation of Hg by forming charged Hg(II)-sulfide compounds that reduced the bioavailability of Hg [78]. The latter played a major role in reducing the content of MeHgrice with high Fe content. In addition, MeHgsoil also affected the accumulation of MeHg in rice. The higher content of MeHgsoil, the higher the content of MeHgrice, which indicated that the accumulation of MeHg in rice was controlled by soil methylation rate, and soil MeHg was the main source of MeHg in rice. This observation is consistent with previous results that rice paddies were hotspots for Hg methylation, and rice mainly received MeHg from soils.
Conclusions
Based on the sampling investigation and analysis of paddy soil in Siqian Dam, the following conclusions are drawn:
The maximum concentrations of THg and MeHg in paddy soil were 30.60 mg/kg and 14.56 µg/kg, respectively. The concentrations of THg and MeHg in rice were 160.29 µg/kg and 40.32 µg/kg, respectively. Hg pollution in this area may have a greater ecological risk.
The horizontal space distributions of THg and MeHg were different in paddy field. The Hg-laden flood with carrying sediment and long-time use of irrigation water containing Hg were the main sources of Hg in Siqian Dam. Hg-containing floods and their sediment, as well as topography, were the main reasons for the different horizontal spatial distributions of THg and MeHg concentrations in paddy fields. In addition, soil pH, OM, S, available Fe, Mn, Se and other indices also influenced the distribution of soil Hg. The THgsoil was greatly affected by pH followed by soil OM. The THgsoil content increased with the increase of pH, but decreased with the increase of OM content. MeHgsoil was mainly affected by OM, followed by S and pH. MeHgsoil increased with the increase of the three indices. In addition, the relationships of soil available Fe, Cu, Zn, Pb and THgsoil, Se, Cr and MeHgsoil were closely related. The higher the content of available elements in the former, the lower the content of THgsoil, while the content of MeHgsoil increased with the increase of Se and Cr content.
Stepwise regression analysis showed no significant correlation between THgsoil and THgrice, but there was a certain correlation between MeHgsoil and MeHgrice. The content of MeHgrice would increase with the increase of MeHgsoil content. K was the main factor affecting Hg accumulation in rice. High K content was conducive to promoting Hg absorption and enrichment in rice. Secondly, THgrice had a significant positive correlation with pH and a clear negative correlation with Zn. Mn and Fe were the factors that affected the accumulation of MeHg in rice. The higher the content of Mn in soil, the more beneficial the accumulation of Hg in rice, while the higher content of Fe inhibited the accumulation of Hg in rice.
Therefore, soil Hg methylation could be inhibited in weakly acidic and fertile paddy fields by appropriately reducing soil pH, OM and available S contents, as well as available Se and Cr contents. The accumulation of Hg in rice could be further reduced by reducing potassium fertilizer application. It can provide reliable theoretical and scientific basis for the regulation and safe utilization of Hg-contaminated soil.
Supplementary Information
(PDF 951 KB)
Acknowledgements
This research work was supported jointly by the National Soil Pollution Prevention and Control Pilot Area Project (No.WHC18128-1), China National Natural Fund Committee - Guizhou Karst Science Research Center Project (U1612442-3). The authors acknowledge the technical support of the Key Laboratory of Karst in Guizhou University.
Author contributions
Y. Teng and L. Zhao designed the experiments; Z. Fu, C. Liu and J. Du did filed sampling; J. Du analyzed the data; F. Liu and J. Du wrote paper; all authors read and approved the final version.
Data availability
Raw data are available upon request.
Code availability
The authors used Arc GIS 10.3 software to perform analysis and to generate maps and Origin 2017 software to generate linear regression analysis diagrams.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng S, Wang Q, Yuan Y, Sun W. Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chem. 2020;316:126213. [DOI] [PubMed]
- 2.Qin C. Effect of low-molecular-weight organic acids on the activation and methylation of mercury in the soils of the water-level-fluctuating zone of the Three Gorges Reservoir. Chongqing: Southwest University; 2016.
- 3.Pang J, Han J, Fan X, Li C, Dong X, Liang L, et al. Mercury speciation, bioavailability and risk assessment on soil–rice systems from a watershed impacted by abandoned Hg mine-waste tailings. Acta Geochimica. 2019;38:391–403. [Google Scholar]
- 4.Liu Y. A primary study on mercury in soil of different agricultural ecosystem. Wuhan: Huazhong Agricultural University; 2007.
- 5.Wang X. Distribution characteristics of methylmercury in paddy soil under different farming systems. Chongqing: Southwest University; 2016.
- 6.Ullrich SM, Tanton TW, Abdrashitova SA. Mercury in the aquatic environment: a review of factors affecting methylation. CRC Crit Rev Environ Control. 2001;31:241–93.
- 7.Zhang H, Feng X, Larssen T, Qiu G, Vogt RD. Inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ Health Persp. 2010;118:1183–8. doi: 10.1289/ehp.1001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng X, Chen J, Fu X, Hu Y, Li P, Qiu G, et al. Progresses on environmental geochemistry of mercury. Bull Mineral Petrol Geochem. 2013;32:503–30. [Google Scholar]
- 9.Boening DW. Ecological effects, transport, and fate of mercury: a general review. Chemosphere. 2000;40:1335–51. doi: 10.1016/s0045-6535(99)00283-0. [DOI] [PubMed] [Google Scholar]
- 10.King JK, Harmon SM, Fu TT, Gladden JB. Mercury removal, methylmercury formation, and sulfate-reducing bacteria profiles in wetland mesocosms. Chemosphere. 2002;46:859–70. doi: 10.1016/s0045-6535(01)00135-7. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Li P, Qiu G, Wang S, Li G, Shang L, et al. Human exposure to methylmercury through rice intake in mercury mining areas, guizhou province, China. Environ Sci Technol. 2008;42:326–32. doi: 10.1021/es071948x. [DOI] [PubMed] [Google Scholar]
- 12.Meng M, Li B, Shao J, Wang T, He B, Shi J, et al. Accumulation of total mercury and methylmercury in rice plants collected from different mining areas in China. Environ Pollut. 2014;184:179–86. doi: 10.1016/j.envpol.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoudi N, Jonidi JA, Moradi Y, Esrafili A. The mercury level in hair and breast milk of lactating mothers in Iran: a systematic review and meta-analysis. J Environ Health Sci Eng. 2020;18:355–66. doi: 10.1007/s40201-020-00460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan J, Wang C, Wang Z, Yang S, Li P. Mercury concentration and speciation in mine wastes in Tongren mercury mining area, southwest China and environmental effects. Appl Geochem. 2019;106:112–9. [Google Scholar]
- 15.Qiu G, Feng X, Wang S, Shang L. Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou Province, Southwestern China. Appl Geochem. 2005;20:627–38. [Google Scholar]
- 16.Dai Z, Feng X, Li P, Qiu G, Shang L. Natural soil mercury pollution in Wanshan mercury mining area, Guizhou Province. J Ecol. 2011;30:902–6. [Google Scholar]
- 17.Horvat M, Nolde N, Fajon V, Jereb V, Logar M, Lojen S, et al. Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Total Environ. 2003;304:231–56. doi: 10.1016/S0048-9697(02)00572-7. [DOI] [PubMed] [Google Scholar]
- 18.Dai Z, Feng X, Zhang C, Wang J, Jiang T, Xiao H, et al. Assessing anthropogenic sources of mercury in soil in Wanshan Hg mining area, Guizhou, China. Environ Sci Pollut Res Int. 2013;20:7560–9. doi: 10.1007/s11356-013-1616-y. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Mao K, Zhang W, Cui Z, Lu B, Huang G, et al. Distribution and pollution characteristics of mercury in paddy soil of Wanshan mercury mining area, Guizhou Province. Bull Mineral Petrol Geochem 2021;40:148–54.
- 20.Xia J, Wang J, Zhang L, Anderson CWN, Wang X, Zhang H, et al. Screening of native low mercury accumulation crops in a mercury - polluted mining region: Agricultural planning to manage mercury risk in farming communities. J Clean Prod. 2020;262.:121324.
- 21.Qiu G, Feng X, Li P, Wang S, Li G, Shang L, et al. Methylmercury accumulation in rice (Oryza sativa L.) grown at abandoned mercury mines in Guizhou, China. J Agric Food Chem. 2008;56:2465–8. doi: 10.1021/jf073391a. [DOI] [PubMed] [Google Scholar]
- 22.Yu RQ, Flanders JR, Mack EE, Turner R, Mirza MB, Barkay T. Contribution of coexisting sulfate and iron reducing bacteria to methylmercury production in freshwater river sediments. Environ Sci Technol. 2012;46:2684–91. doi: 10.1021/es2033718. [DOI] [PubMed] [Google Scholar]
- 23.Bigham GN, Murray KJ, Masue-Slowey Y, Henry EA. Biogeochemical controls on methylmercury in soils and sediments: implications for site management. Integr Environ Asses. 2017;13:249–63. doi: 10.1002/ieam.1822. [DOI] [PubMed] [Google Scholar]
- 24.Meng Q, Qian X, Chen M, Zhao L, Feng X, Meng B. Biogeochemical cycle of mercury in rice paddy ecosystem: A critical review. Chin J Ecol. 2018;37:1556–73. [Google Scholar]
- 25.Zhao L, Meng B, Feng X. Mercury methylation in rice paddy and accumulation in rice plant: A review. Ecotoxicol Environ Saf. 2020;195:110462. doi: 10.1016/j.ecoenv.2020.110462. [DOI] [PubMed] [Google Scholar]
- 26.Tang Z, Fan F, Wang X, Shi X, Deng S, Wang D. Mercury in rice (Oryza sativa L.) and rice-paddy soils under long-term fertilizer and organic amendment. Ecotoxicol Environ Saf. 2018;150:116–22. doi: 10.1016/j.ecoenv.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Tang Z, Fan F, Deng S, Wang D. Mercury in rice paddy fields and how does some agricultural activities affect the translocation and transformation of mercury - A critical review. Ecotox Environ Safe. 2020;202:110950. doi: 10.1016/j.ecoenv.2020.110950. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Wei Z, Zeng Q, Zhong H. Amendment of sulfate with Se into soils further reduces methylmercury accumulation in rice. J Soil Sediment. 2016;16:2720–7. [Google Scholar]
- 29.Zhang Y. Effects of biochar on soil mercury inactivation, migration and transformation. Harbin: Northeast Agricultural University; 2015.
- 30.Hu Y. Study on remediation of mercury contaminated soil by biochar prepared from malt root. Hangzhou: Zhejiang University; 2018.
- 31.Meng B, Feng X, Qiu G, Liang P, Li P, Chen C, et al. The process of methylmercury accumulation in rice (Oryza sativa L.) Environ Sci Technol. 2011;45:2711–7. doi: 10.1021/es103384v. [DOI] [PubMed] [Google Scholar]
- 32.State Environmental Protection Administration of China. Soil environmental quality-risk control standard for soil contamination of agricultural land GB 15618 – 2018, Beijing. 2018.
- 33.Ministry of Health Standardization Administration of China. Food safety national standard-limit of contaminants in food, GB2762-2017, Beijing. 2017.
- 34.BBodSchV. Directive of the execution of the federal protection act (Federal soil protection and hazardous waste directive–BBodSchV) (in German). Bundesgesetzblatt I. 1999:1554–1582.
- 35.CETESB/Companhia de Tecnologia de Saneamento Ambiental.Manual de gerenciamento de areas contaminadas. http//www.cetesb.sp.gov.br/Solo. Accessed Jan 2007.
- 36.Wang J, Feng X, Anderson CWN, Qiu G, Ping L, Bao Z. Ammonium thiosulphate enhanced phytoextraction from mercury contaminated soil – Results from a greenhouse study. J Hazard Mater. 2011;186:119–27. doi: 10.1016/j.jhazmat.2010.10.097. [DOI] [PubMed] [Google Scholar]
- 37.Qiu G, Feng X, Wang S, Fu X, Shang L. Mercury distribution and speciation in water and fish from abandoned Hg mines in Wanshan, Guizhou province, China. Sci Total Environ. 2009;407:5162–8. doi: 10.1016/j.scitotenv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Qiu G, Feng X, Wang S, Shang L. Total mercury and methylmercury in soils collected from Guizhou Hg-mined areas. Environment Science (China). 2006:3550–5.
- 39.Office of national soil survey. Soil census data of China. China Agriculture Press. 1979.
- 40.Gilmour CC, Henry EA. Mercury methylation in aquatic systems affected by acid deposition. Environ Pollut (Barking, Essex: 1987). 1991;71:131–69. [DOI] [PubMed]
- 41.Xu Y, Wu J. Effect of pH value on performance of sulfate-reducing bacterial granular sludge. Ind Water Wastewater. 2010;41:32–5. [Google Scholar]
- 42.Gao R, Luo W, Hu H, Zhu J, Jin Y. Research progress on microbial methylation of mercury in paddy soil. Ningxia Agric For Sci Technol. 2020;61:46–9. [Google Scholar]
- 43.Zhang J, Zhu J, Li L, Zhang H. Research progress on effects of different agronomic measures on mercury methylation in paddy soil. South China Agriculture. 2021;15:30–4.
- 44.Durao JW, Palmieri HE, Trindade MC, de Aquino BO, Filho CA, Fleming PM, et al. Speciation, distribution, and transport of mercury in contaminated soils from Descoberto, Minas Gerais, Brazil. J Environ Monit. 2009;11:1056–63. doi: 10.1039/b813997k. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Feng R, Larssen T, Shang R, Ping LI. Bioaccumulation of Methylmercury versus Inorganic Mercury in Rice (Oryza sativa L.) Grain. Environ Sci Technol. 2010;44:4499–504. doi: 10.1021/es903565t. [DOI] [PubMed] [Google Scholar]
- 46.Li P, Feng X, Qiu G, Shang L, Wang S. Mercury pollution in Wuchuan mercury mining area, Guizhou, Southwestern China: the impacts from large scale and artisanal mercury mining. Environ Int. 2012;42:59–66. doi: 10.1016/j.envint.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Pei P, Sun T, Xu Y, Sun Y. Soil aggregate-associated mercury, Herausgeber and organic carbon distribution and microbial community characteristics under typical farmland-use types. Chemosphere. 2021;275:129987. doi: 10.1016/j.chemosphere.2021.129987. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Sun T, Driscoll CT, Yin Y, Zhang X. Mechanism of accumulation of methylmercury in rice (Oryza sativa L.) in a mercury mining area. Environ Sci Technol. 2018;52:9749–57. doi: 10.1021/acs.est.8b01783. [DOI] [PubMed] [Google Scholar]
- 49.Lambertsson L, Nilsson M. Organic material: the primary control on mercury methylation and ambient methyl mercury concentrations in estuarine sediments. Environ Sci Technol. 2006;40:1822–9. doi: 10.1021/es051785h. [DOI] [PubMed] [Google Scholar]
- 50.Pallud C, Cappellen PV. Kinetics of microbial sulfate reduction in estuarine sediments. Geochim Cosmochim Acta. 2006;70:1148–62. [Google Scholar]
- 51.Yin D, He T, Yin R, Zeng L. Effects of soil properties on production and bioaccumulation of methylmercury in rice paddies at a mercury mining area, China. J Environ Sci-China. 2018;68:194–205. doi: 10.1016/j.jes.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 52.Yang H, Li P, Qiu G, Feng X. Mercury pollution in mercury mining areas throughout the world: an overview. Earth Environ. 2009;37:80–5. [Google Scholar]
- 53.Liu J, Jiang T, Huang R, Wang D, Zhang J, Qian S, et al. A simulation study of inorganic sulfur cycling in the water level fluctuation zone of the Three Gorges Reservoir, China and the implications for mercury methylation. Chemosphere. 2017;166:31–40. doi: 10.1016/j.chemosphere.2016.09.079. [DOI] [PubMed] [Google Scholar]
- 54.Liem-Nguyen V, Jonsson S, Skyllberg U, Nilsson MB, Andersson A, Lundberg E, et al. Effects of nutrient loading and mercury chemical speciation on the formation and degradation of methylmercury in estuarine sediment. Environ Sci Technol. 2016;50:6983–90. doi: 10.1021/acs.est.6b01567. [DOI] [PubMed] [Google Scholar]
- 55.Lei P, Tang C, Wang Y, Wu M, Kwong R, Jiang T, et al. Understanding the effects of sulfur input on mercury methylation in rice paddy soils. Sci Total Environ. 2021;778:146325. doi: 10.1016/j.scitotenv.2021.146325. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, Gao L, Zha F, Chen X, Pan X. Research on heavy metal level and co-occurrence network in typical ecological fragile area. J Environ Health Sci Engineer. 2021;19(1):531–40. [DOI] [PMC free article] [PubMed]
- 57.Zhang H, Feng X, Zhu J, Sapkota A, Meng B, Yao H, et al. Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativaL.). Environ Sci Technol. 2012;46,18:10040–46. [DOI] [PubMed]
- 58.Ping L, Feng X, Qiu G, Shang L, Wang S. Mercury exposure in the population from Wuchuan mercury mining area, Guizhou, China. Sci Total Environ. 2008;395:72–9. doi: 10.1016/j.scitotenv.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Shi JB, Wang X, Meng M, Huang L, Qi XL, et al. Variations and constancy of mercury and methylmercury accumulation in rice grown at contaminated paddy field sites in three Provinces of China. Environ Pollut. 2013;181:91–7. doi: 10.1016/j.envpol.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 60.USEPA . Mercury study report to the congress, EPA 452/R-97-0003. Washington, DC: USEPA; 1997. [Google Scholar]
- 61.Xu J, Wu S, Liang P, Zhang J, Wu S. Heavy metal pollution in rice of Gaohong Town with a health risk assessment. J Zhejiang A F Univ. 2017;34:983–90. [Google Scholar]
- 62.Imran H, Shamsi L, Jiang K, et al. Alleviation of cadmium toxicity in soybean by potassium supplementation. J Plant Nutr. 2010;33:1926–38. [Google Scholar]
- 63.Zörb C, Senbayram M, Peiter E. Potassium in agriculture – Status and perspectives. J Plant Physiol. 2014;171(9):656–69. [DOI] [PubMed]
- 64.Liu CH, Chao YY, Kao CH. Effect of potassium deficiency on antioxidant status and cadmium toxicity in rice seedlings. Bot Stud. 2013;54:2. doi: 10.1186/1999-3110-54-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ping L, Du B, Chan HM, Feng X. Human inorganic mercury exposure, renal effects and possible pathways in Wanshan mercury mining area, China. Environ Res. 2015;140:198–204. doi: 10.1016/j.envres.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 66.Wang YD, Wang X, Wong YS. Generation of selenium-enriched rice with enhanced grain yield, selenium content and bioavailability through fertilisation with selenite. Food Chem. 2013;141:2385–93. doi: 10.1016/j.foodchem.2013.05.095. [DOI] [PubMed] [Google Scholar]
- 67.Huang L, Li B, Tam FY, Wang X, Ye Z. Effects of environment and genotype on mercury and methylmercury accumulation in rice (Oryza sativa L.). Plant and Soil. 2018;427(1):269–80.
- 68.Cai H, Huang S, Che J, Yamaji N, Ma JF. The tonoplast-localized transporter OsHMA3 plays an important role in maintaining Zn homeostasis in rice. J Exp Bot. 2019;70:2717–25. doi: 10.1093/jxb/erz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, et al. Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci U S A. 2010;107:16500–5. doi: 10.1073/pnas.1005396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jian F, Ren F, Ji F. Transport of cadmium from soil to grain in cereal crops: A review. Pedosphere. 2021;31:3–10. [Google Scholar]
- 71.Gupta DK, Chatterjee S. Heavy metal remediation: transport and accumulation in plants. Hauppauge: Nova Science Publishers Incorporated; 2014. [Google Scholar]
- 72.Crowder AA, Coltman DW. Formation of manganese oxide plaque on rice roots in solution culture under varying pH and manganese (Mn2+) concentration conditions. J Plant Nutr. 1993;16:589–99. [Google Scholar]
- 73.Trivedi P, Axe L. Modeling Cd and Zn sorption to hydrous metal oxides. Environ Sci Technol. 2000;34:2215–23. doi: 10.1021/es001644+. [DOI] [PubMed] [Google Scholar]
- 74.Cai Z. Organic sulfur affects the production of methyl mercury in paddy soil. Journal of Agro-Environment Science (China). 2021:1–8.
- 75.Mehrotra AS, Sedlak DL. Decrease in net mercury methylation rates following iron amendment to anoxic wetland sediment slurries. Environ Sci Technol. 2005;39:2564–70. doi: 10.1021/es049096d. [DOI] [PubMed] [Google Scholar]
- 76.Mehrotra AS, Horne AJ, Sedlak DL. Reduction of net mercury methylation by iron in Desulfobulbus propionicus (1pr3) cultures: implications for engineered wetlands. Environ Sci Technol. 2003;37:3018–23. doi: 10.1021/es0262838. [DOI] [PubMed] [Google Scholar]
- 77.Zhong S, Qiu G, Xin F. Coupling of iron and sulfur on the migration and transformation of methylmercury in soilrice system: A review. J Ecol. 2017;36:2351–7. [Google Scholar]
- 78.Liu J, Valsaraj KT, Delaune RD. Inhibition of mercury methylation by iron sulfides in an anoxic sediment. Environ Eng Sci. 2009;26:833–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 951 KB)
Data Availability Statement
Raw data are available upon request.
The authors used Arc GIS 10.3 software to perform analysis and to generate maps and Origin 2017 software to generate linear regression analysis diagrams.