Abstract
Cardiovascular diseases are the leading causes of the death around the world. An elevation of the low-density lipoprotein cholesterol (LDL-C) level is one of the most important risk factors for cardiovascular diseases. To achieve optimal plasma LDL-C levels, clinal therapies were investigated which targeted different metabolism pathways. However, some therapies also caused various adverse effects. Thus, there is a need for new treatment options and/or combination therapies to inhibit the LDL-C level. Dietary polyphenols have received much attention in the prevention of cardiovascular diseases due to their potential LDL-C lowering effects. However, the effectiveness and potential mechanisms of polyphenols in lowering LDL-C is not comprehensively summarized. This review focused on dietary polyphenols that could reduce LDL-C and their mechanisms of action. This review also discussed the limitations and suggestions regarding previous studies.
Keywords: cardiovascular diseases, LDL-C, metabolism pathways, dietary polyphenols
1. Introduction
Cardiovascular diseases (CVDs) have now become the leading cause of morbidity and mortality worldwide [1]. Some studies indicate that atherosclerosis is the principal pathogenic mechanism of CVDs [2,3]. An elevation of the low-density lipoprotein (LDL) cholesterol level in plasma is one of the most important risk factors for atherosclerosis [4]. To achieve optimal plasma LDL-C levels, huge amounts of therapies were investigated and implemented which targeted different metabolism pathways. For example, statins, the first-line drugs for lowering LDL-C levels, could inhibit the expression and activity of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) and upregulate the expression and activity of the LDL receptor (LDLR) [5]. In clinical practice, however, about 50% of statins-treated patients do not achieve a desirable LDL-C level [6]. Thus, there is a need for new treatment options and/or combination therapies to suppress the plasma LDL-C level.
Studies have found that the intake of fruits and processed foods could directly reduce the LDL-C level, both of which were associated with their bioactive components like polyphenols [7,8,9]. Polyphenols refer to a large group of compounds containing aromatic ring(s) with one or more hydroxyl functional groups [10]. Based on their chemical structure, dietary polyphenols are mainly classified into phenolic acids, flavonoids, lignans, stilbenes, and phenolic polymers [11]. Flavonoids can be further divided into six subgroups: flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols [12]. In recent years, dietary polyphenols have received much attention in diseases prevention, such as type 2 diabetes, osteoarthritis, obesity, hyperlipidemia, hyperuricemia, etc. [13,14,15,16,17]. Our laboratory studies indicated that polyphenols could decrease plasma LDL-C levels in animal trials [18,19,20,21]. However, the effectiveness and potential mechanisms of polyphenols in lowering LDL-C is not comprehensively summarized. Therefore, in this review, we will discuss the effects and modes of action of dietary polyphenols on lowering LDL-C.
2. Metabolism Pathways for Lowering LDL-C
To achieve optimal plasma LDL-C levels, different metabolism pathways need to be considered. Currently, the metabolism pathways include the production and elimination of plasma LDL-C, the absorption of intestinal cholesterol, the production of very low-density lipoprotein (VLDL), the transfer of cholesterol esters through cholesterol esters transfer protein (CETP), the endocytosis of LDL via LDLR, and the formation and enterohepatic circulation of bile acids. Further details are mentioned below.
2.1. Intestinal Cholesterol Absorption
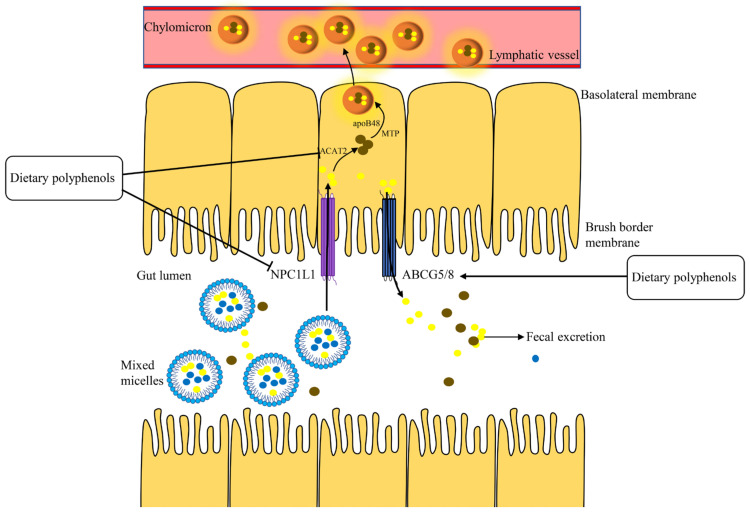
There are two recognized sources of cholesterol in the body: biosynthesis and the intestinal absorption of dietary cholesterol [22,23]. Inhibiting cholesterol absorption had been shown to reduce the levels of cholesterol in the liver, thereby promoting the synthesis of LDLR (Section 2.4, below) and a subsequent reduction of plasma LDL-C levels [24]. Cholesterol absorption is a complex process which is regulated by a set of proteins: NPC1-like transporter 1 (NPC1L1), acyl-coenzyme A: cholesterol O-acyltransferase 2 (ACAT2), microsomal triglyceride transfer protein (MTP), and apolipoprotein B-48 (apoB48) (Figure 1) [25,26,27]. By contrast, some cholesterols are secreted back through adenosine triphosphate-binding cassette transporters G5 and G8 (ABCG5/8) [27]. Subsequently, the cholesterol is passed out from intestine [28].
Figure 1.
Intestinal cholesterol absorption. ACAT2, acyl-coenzyme A: cholesterol O-acyltransferase 2; MTP, microsomal triglyceride transfer protein; NPC1L1, NPC1-like transporter 1; ABCG5/8, adenosine triphosphate-binding cassette transporters G5 and G8.
2.2. VLDL Assembly and Secretion
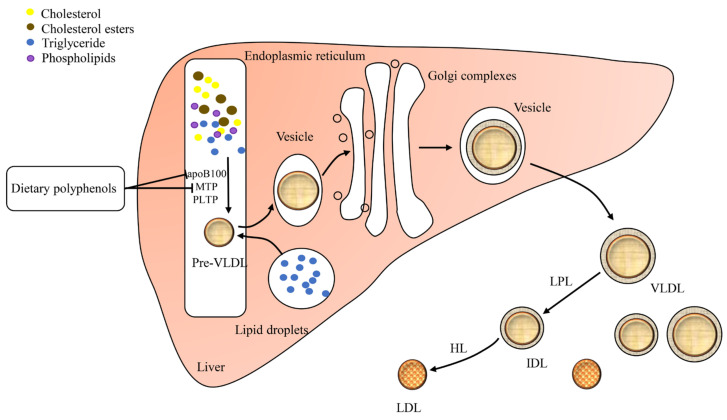
VLDL mainly delivers cholesterol and triglyceride from the liver to the bloodstream [29]. After processing in the bloodstream, VLDL is further translated into intermediate density lipoprotein (IDL) and then LDL particles by lipoprotein lipase (LPL) and hepatic lipase (HL) [30,31]. Thus, impairing liver VLDL production may reduce the rate of LDL production and the LDL-C level [32,33]. The assembly of VLDL involves a stepwise lipidation of apolipoprotein B100 (apoB100) in the liver (Figure 2) [34]. The first step of VLDL assembly is lipidation of apoB100 by MTP to generate pre-VLDL in the rough endoplasmic reticulum [35]. Moreover, the phospholipid transfer protein (PLTP) is also implicated in VLDL assembly and secretion through the blockade of apoB100 destruction [36,37]. The second step is the fusion of pre-VLDL with triglyceride-rich lipid droplets in the lumen of the smooth endoplasmic reticulum [38]. The nascent VLDL further moves from the endoplasmic reticulum to the Golgi and then transports from the Golgi to the plasma membrane [39].
Figure 2.
VLDL assembly and secretion. PLTP, phospholipid transfer protein; VLDL, very low-density lipoprotein; LPL, lipoprotein lipase; IDL, intermediate density lipoprotein; HL, hepatic lipase; LDL, low-density lipoprotein.
2.3. CETP in LDL Metabolism
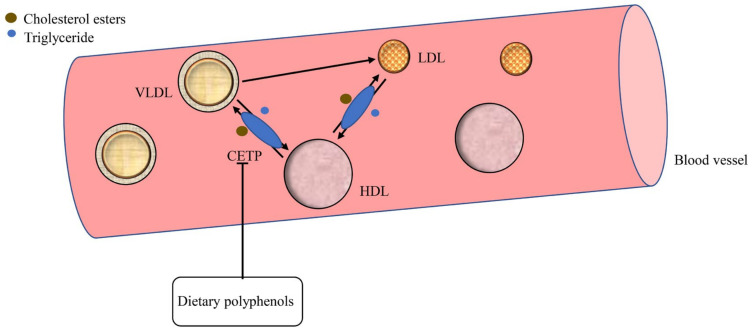
Cholesterol esters transfer protein (CETP), a 476-residue hydrophobic glycoprotein, is synthesized mainly in the liver that engages the bidirectional transfer of cholesterol esters and triglycerides between lipoproteins [40]. The CETP promotes the transfer of cholesterol esters from high-density lipoprotein (HDL) to VLDL and LDL to exchange triglycerides (Figure 3). This produces triglycerides-enriched HDL and cholesterol-enriched LDL and VLDL are produced [41]. Inhibition of CETP activity can increase the concentration of HDL-C and lowers the concentration of LDL-C and VLDL-C in the plasma [42].
Figure 3.
CETP-mediated LDL metabolism. CETP, cholesterol esters transfer protein; HDL, high-density lipoprotein.
2.4. LDLR Mediates LDL Endocytosis
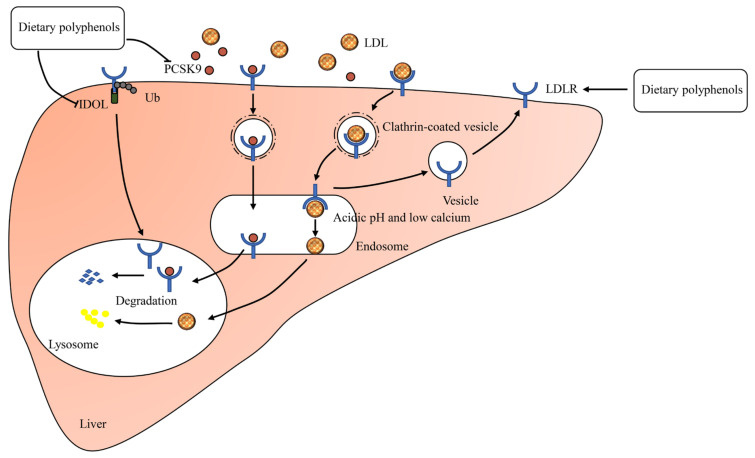
LDLR is a multidomain transmembrane protein, which is responsible for removing 70% of LDL-C from plasma [43]. Consequently, increased hepatic LDLR expression results in improved clearance of plasma LDL-C. The LDLR adhering to the liver surface binds LDL in the blood and LDL-LDLR complexes are internalized (Figure 4) [44]. Following internalization, the complexes are trafficked to sorting early endosomes where LDL release occurs [45]. Meanwhile, LDLR is either recycled back to the cell surface or transported to the lysosomes for degradation [46]. Degradation of LDLR is respectively regulated by two proteins: IDOL (inducible degrader of the LDLR) and PCSK9 (proprotein convertase subtilisin/kexin type 9) (Figure 4) [47,48].
Figure 4.
The regulatory mechanisms of LDL endocytosis. PCSK9, proprotein convertase subtilisin/kexin type 9; IDOL, inducible degrader of the LDLR; Ub, ubiquitin.
2.5. Bile Acid Metabolism
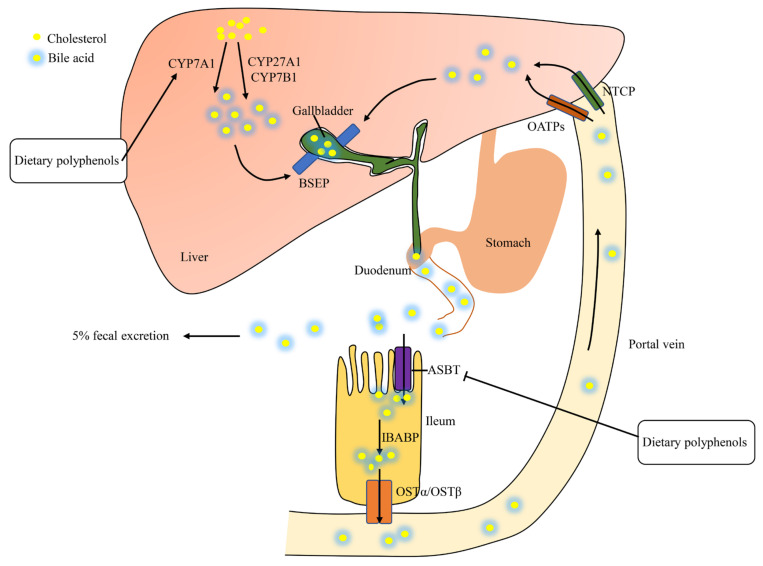
Bile acids, synthesized from cholesterol in hepatocytes, play a pivotal role in the absorption of dietary fats via the formation of micelles [49]. Hepatic cholesterol is transformed to bile acids via a variety of enzymes such as cholesterol 7 α-hydroxylase (CYP7A1), sterol 27-hydroxylase (CYP27A1), oxysterol 7 α-hydroxylase (CYP7B1), and so on (Figure 5) [50]. The newly synthesized bile acids are secreted to the canalicular space via the bile salt export pump (BSEP) to form bile [51]. Newly formed bile is stored in the gallbladder which is secreted into the duodenum in response to cholecystokinin [49]. A total of 95% of intestinal bile acids are reabsorbed via the apical sodium-dependent bile acid transporter (ASBT), the ileal bile acid binding protein (IBABP), and subsequently released into the portal vein through the organic solute transporters alpha and beta (OSTα/OSTβ) [52,53]. Hepatic uptake of bile acids is mediated by the sodium-dependent taurocholate co-transporting peptide (NTCP) and organic anion transporting polypeptides (OATPs) [52,54]. This recycling process is called enterohepatic circulation, which includes the liver, the biliary system, and the intestine [55]. Unabsorbed bile acids (about 5%) are excreted in feces, which stimulates the de novo synthesis of bile acids from cholesterol in liver [56]. Consequently, decreased hepatic cholesterol causes more LDL-C absorption from plasma to the liver [57,58].
Figure 5.
Enterohepatic circulation of bile acids. CYP7A1. cholesterol 7 α-hydroxylase; CYP27A1, sterol 27-hydroxylase; CYP7B1, oxysterol 7 α-hydroxylase; BSEP, bile salt export pump; OATPs, organic anion transporting polypeptides; NTCP, sodium-dependent taurocholate co-transporting peptide; ASBT, apical sodium-dependent bile acid transporter; IBABP, ileal bile acid binding protein; OSTα/OSTβ, organic solute transporters alpha and beta.
3. Current Strategies for LDL-C Lowering
Up to now, numerous therapies have been developed to lower the LDL-C level. For example, statins are the first-line therapy in lowering LDL-C and can reduce the LDL-C level by about 60% [59]. Other strategies for LDL-C lowering include ezetimibe, bile acid sequestrants, anti-PCSK9 monoclonal antibodies (mAbs), bempedoic acid, squalene synthase inhibitors, mipomersen, lomitapide, ASBT inhibitors, and CETP inhibitors [59,60]. These strategies are mainly categorized into increasing the LDLR level, disturbing enterohepatic circulation, interfering VLDL assembly, and the CETP inhibitor [24,60,61,62,63,64,65,66,67]. Although these therapies are now reasonably effective, they have some shortcomings and adverse effects. For instance, anti-PCSK9 mAbs, mipomersen, and lomitapide are expensive, while statins and ezetimibe are relatively inexpensive as a series of generic drugs [59,68]. The long-term use of statins can also lead to muscle symptoms [69]. The details of current therapies are presented in Table 1.
Table 1.
Current strategies for lowering the LDL-C level.
| Drug | Mechanism of Action | Adverse Effects | References |
|---|---|---|---|
| Ezetimibe | Inhibition of intestine cholesterol absorption | - | [24] |
| Mipomersen | ApoB synthesis inhibition | Injection site reactions; Influenza-like symptoms; Fatty liver | [61] |
| Lomitapide | Reduced secretion of apoB-containing lipoproteins by MTP inhibition | Gastrointestinal tract diarrhea, nausea, vomiting, and dyspepsia; Fatty liver | [70,71] |
| Statins | Reduced hepatic cholesterol synthesis and increased LDLR expression by blocking hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) | Statin-associated muscle symptoms (myalgia, myopathy, and myositis with elevated creatinine kinase rhabdomyolysis) | [62,69] |
| Anti-PCSK9 monoclonal antibodies (mAbs) | Blockade of circulating PCSK9 interaction with LDLR | Injection site reactions | [63] |
| Bempedoic acid | Reduced hepatic cholesterol synthesis and increased LDLR expression by blocking ATP-citrate lyase (ACLY) | Elevated uric acid levels; Tendon rupture; Increased serum creatinine | [59] |
| Bile acid sequestrants | Reduced bile acid reabsorption and increased fecal elimination | Gastrointestinal side effects: constipation, bloating, abdominal discomfort, and aggravation of hemorrhoids | [64,72] |
| CETP inhibitors | Inhibition of CETP | Increase of plasma aldosterone and blood pressure | [60] |
| Squalene synthase inhibitors | Reduced hepatic cholesterol synthesis and increased LDLR expression by blocking squalene synthase | Acidosis; Elevation of alanine aminotransferase and total bilirubin | [66,73] |
| ASBT inhibitors | Reduced bile acid reabsorption | Abdominal pain and diarrhea | [67] |
4. Dietary Polyphenols: Potential LDL-C Lowering Agents
As previously mentioned, some therapies lowering the LDL-C level are effective but associated with adverse effects. Hence, alternative strategies are needed to reduce the high level of LDL-C effectively without any adverse effect. In recent years, dietary polyphenols have received much attention in diseases prevention due to their potential therapeutic effects. Dietary polyphenols are a class of phytochemicals containing phenol rings, mainly from fruits, vegetables, legumes, cereals, nuts, plant-derived beverages, and chocolate [13,74]. From the last few decades, numerous studies have suggested that dietary polyphenols have exhibited the potential of reducing plasma LDL-C levels [75,76,77]. In a recent animal study, polyphenols-rich Perilla frutescens leaf extracts (250 mg/kg body weight (bw)) were orally administrated to high-fat-diet-fed rats for 4 weeks. The results revealed that Perilla frutescens significantly reduced the plasma LDL-C level by 53% (p < 0.05) [78]. In an animal model study conducted by Li and colleagues revealed that the decrease of the plasma LDL-C content by 45% in the onion-fed group was associated with the inhibition of HMGR and the upregulation of LDLR expression, which was a plausible mechanism for its LDL-C lowering effect. Researchers concluded that this effect was due to the presence of quercetin and isoquercitrin in onion [79]. Polyphenols-rich grape extract capsules (300 mg/day) were orally given to obese individuals for a period of 12 weeks. The results predicted that grape extracts decreased the plasma LDL-C level from 131 ± 4.98 to 110.28 ± 5.8 mg/dl [80]. Luteolin, a bioactive compound present in chrysanthemum, was evaluated for its LDL-C lowering effect on hyperlipidemia rats. Luteolin (50 mg/kg bw) was orally given to male Sprague Dawley rats for 6 weeks. The results divulged that luteolin reduced the level of plasma LDL-C by 34% and increased the liver CYP7A1 activity in rats [81]. In this section, we will review the effect of diets rich in polyphenols on lowering LDL-C and focus on the underlying molecular mechanism of LDL-C lowering by dietary polyphenols based on in vitro or in vivo models.
4.1. Diets Rich in Polyphenols
Polyphenol supplementation in numerous studies contained mainly extracts (mulberry water extracts, aloe vera extracts, green tea extract, grape extract, etc.), juices (raspberry juice, apple juice, and chokeberry juice), wines, and an increased intake of polyphenol-rich foods (cacao, blueberry, and bilberry). In Table 2 and Table 3, we will focus on research published from 2011 to 2021 about the effect of polyphenol consumption on lowering LDL-C in animal and clinical studies, respectively.
Table 2.
Dietary sources, polyphenols, and cholesterol-lowering activities in animal models.
| Dietary Sources | Polyphenols Components | Treatment | Effect | p-Value (vs. Model) | References |
|---|---|---|---|---|---|
| White tea (Camellia sinensis) | - | 0.5% aqueous extracts of white tea to diabetes rats for 4 weeks | Decreases in plasma LDL-C | p < 0.05 | [82] |
| Green tea (Camellia sinensis) | Epigallocatechin gallate, epicatechin, epicatechin gallate, epigallocatechin, gallocatechin gallate, and catechin | 0.2% green tea extract was given to atherogenic-diet-fed to SD rats for 4 weeks |
Decreases in plasma LDL-C by 40% | p < 0.05 | [83] |
| Fuzhuan brick tea (Camellia sinensis) | Catechin, epicatechin, epigallocatechin, gallocatechin gallate, epigallocatechin gallate, epicatechin, gallate, rutin, gallic acid, and chlorogenic acid | 75 mg/kg, 300 mg Fuzhuan brick tea water extract/kg bw were given to obese rats for 40 days | Reduction in plasma LDL-C by 38% | p < 0.05 | [84] |
| Kombucha (Camellia sinensis) | - | Kombucha (5 mL/kg bw) was given to hypercholesterolemic-diet-fed rats per day for 16 weeks | Decreases in plasma LDL-C by 36% | p < 0.05 | [85] |
| Youcha (Camellia sinensis) | - | 1500 mg/kg·bw Youcha were respectively given to hyperlipidemia rats for 32 days | Decreases in plasma LDL-C by 24% | p > 0.05 | [86] |
| Sanglan tea (Camellia sinensis) | 15 kinds of flavonoids such as quercetin and kaempferol | Sanglan tea (200 mg/kg bw, respectively) was given to obese mice for 28 weeks | Reduction in plasma LDL-C | p < 0.05 | [87] |
| Oil tea (Camellia sinensis) | - | 4 g/kg bw oil tea was given to type 2 diabetic mice | Lowers plasma LDL-C | p < 0.05 | [88] |
| Bowl tea (Camellia sinensis) | Gallic acid, epigallocatechin, catechin, L-epicatechin, epigallocatechin gallate, gallocatechin gallate, and epicatechin gallate | Bowl tea (50 mg/kg bw) was given to high-fat-diet-fed mice for 12 weeks | Decreases in plasma LDL-C by 24% | p < 0.05 | [89] |
| Persimmon (Diospyros kaki) wine and Grape (Vitis vinifera) wine | Persimmon wine: epicatechin, epigallocatechin-gallate; Grape wine: catechin, epicatechin, epicatechin-3-O-gallate, and epigallocatechin-gallate |
Wine (7.4 ml/kg bw) to atherogenic-diet-fed hamsters for 12 weeks | Decreases in plasma LDL-C levels by 38% | p < 0.05 | [75] |
| Yellow wine (Oryza sativa L.) | - | Yellow wine polyphenolic compounds (30 mg/kg bw) were given to LDL receptor knockout mice per day for 14 weeks | Decreases in plasma LDL-C by 41% | p < 0.05 | [90] |
| Mulberry (Morus alba L.) | Gallic acid, protocatechuic acid, 3-caffeoylquinic acid, chlorogenic acid, 4-caffeoyl quinic acid, caffeic acid, rutin, and quercetin-3-O-glucoside | 0.5%, 1%, and 2% mulberry water extracts were given to high-fat-diet-fed hamsters for 12 weeks | Lowers plasma LDL-C | p < 0.05 | [91] |
| Raspberry (Rubus idaeus) juice | Anthocyanins, ellagitannins, and ellagic acid-like compounds | Equivalent to a consumption of 275 mL/day by a 70 kg human to hypercholesterolemic golden Syrian hamsters for 12 weeks | Decreases in plasma LDL-C by 41% | p < 0.05 | [92] |
| Bilberry (Vaccinuim myrtillus) | Anthocyanins, quercetin-3-O-glucoside, and chlorogenic acid | 5 g bilberry powder orally given to Zucker diabetic fatty rats for 8 weeks | Ameliorates LDL-C level by 60% | p < 0.05 | [8] |
| Berry (Lonicera caerulea L. var. edulis) | Cyanidin-3-glucoside, catechin, and chlorogenic acid | Lonicera caerulea berry extract (300 mg/kg bw) was given to high-fat-diet-fed rats for 12 weeks | Decreases in plasma LDL-C by 48% | p < 0.05 | [93] |
| White bayberry (Morella rubra Sieb. et Zucc.) | Epigallocatechin gallate, epigallocatechin, myricetin-3-O-α-l-rhamnoside, quercetin-3-O-rhamnoside, and kaempferol-3-O-rhamnoside | 200 mg/kg bw white bayberry fruit extracts were given to diabetic KK-Ay mice for 5 weeks |
Ameliorates plasma LDL-C by 58% | p < 0.05 | [94] |
| Kiwifruit (Actinidia deliciosa) | - | 5% of lyophilized kiwifruits were given to atherogenic-diet-fed rats for 33 days | Decreases in plasma LDL-C by 41% | p < 0.05 | [95] |
| Tamarindus indica | - | 500 mg Tamarindus indica fruit pulp extract/kg bw to high-cholesterol-diet-fed hamsters for 10 weeks | Lowers plasma LDL-C by 60% | p < 0.05 | [96] |
| Apple (Malus ssp.) | - | Apple polyphenols (100 mg/kg bw) were given to apolipoprotein-E deficient (ApoE-/-) mice for 12 weeks | Decreases in plasma LDL-C | p < 0.05 | [97] |
| Apple (Malus) juice | Cloudy apple juice: emodin, kaempferol, cyanidin cation, stevioside, and butylated hydroxytoluene |
Cloudy apple juice (15 ml/kg bw) was given to diabetic rats for 21 days | Decreases in plasma LDL-C by 74% | p < 0.05 | [98] |
| Hawthorn (Crataegus oxyacantha) | Chlorogenic acid, epicatechin, rutin, and hyperoside | 400 mg/kg bw extracts from hawthorn fruit peel and flesh were respectively given to high-fructose-diet-fed mice for 8 weeks | Reduction in plasma LDL-C by 39% | p < 0.05 | [99] |
| Ajwa date (Phoenix dactylifera L.) | - | Ajwa date extract (25 mg/kg bw) was given to hypercholestrolemic rats for 28 days | Ameliorates plasma LDL-C | p < 0.05 | [100] |
| Hibiscus sabdariffa | 18 phenolic compounds |
100 mg/kg bw Hibiscus sabdariffa polyphenolic extract to diabetic rats for 7 weeks | Reduction in plasma LDL/HDL ratio | p < 0.05 | [101] |
| Hibiscus sabdariffa | Protocatechuic acid, catechin, gallocatechins, caffeic acid, and gallocatechin gallates | 0.5% Hibiscus sabdariffa extract were given to obese hamsters for 10 weeks | Ameliorates plasma LDL-C | p < 0.05 | [102] |
| Propolis | Green propolis: artepellin c, pinocembrin, and kampferol; Red propolis: 3-Hydroxy-8,9-dimethoxypterocarpan, medicarpin, and daidzein; Brown propolis: pinocembrin, caffeic acid phenyl ester, quercetin, and galangin |
Green, red, or brown propolis extract (250 mg/kg bw) to cholesterol-enriched-diet-fed LDLR knockout mice for 4 weeks | Reduces plasma non-HDL-C treated with the red propolis by 17% | p < 0.05 | [103] |
| Aloe vera | - | 1.25 g Aloe vera extracts/kg bw was given to type 2 diabetic rats for 28 days | Lowers plasma LDL-C | p > 0.05 | [104] |
| Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) | Gallic acid and catechin | 40 mg total phenolics/kg bw to high-cholesterol-diet-fed rats for 28 days | Decreases in plasma LDL-C by 30% | p < 0.05 | [105] |
| Bergamot (Citrus bergamia Risso et Poiteau) | - | 50 mg/kg bw bergamot polyphenolic formulation was given to high-fat-diet-fed mice for 16 weeks | Reduction in plasma LDL-C by 40% | p < 0.05 | [106] |
| Perilla. frutescens (L.) Britt. | - | Leaf extracts (250 mg/kg bw) were given to high-fat-diet-fed rats for 4 weeks | Lowers plasma LDL-C by 53% | p < 0.05 | [78] |
| Onion (Allium cepa L.) | Quercetin and isoquercitrin | Hyperlipidemic rats treated with onion extract at 4.5g/kg bw for 4 weeks | Ameliorates plasma LDL-C by 45% | p < 0.05 | [79] |
| Rhodomyrtus tomentosa fruit juice | - | 2 g/kg bw frozen fruit juice was given to high-fat-diet-fed rats for 75 days | Reduces plasma LDL-C | p < 0.05 | [107] |
| Astragalus radix | - | 25 mg Astragalus radix total flavones/kg bw was given to diabetic mice for 8 weeks | Lowers plasma LDL-C | p < 0.05 | [108] |
| Turmeric (Curcuma longa) | - | 2.0% turmeric powder was given to high-fat-diet-fed mice for 8 weeks | Lowers plasma LDL-C | p < 0.05 | [109] |
| Schinus terebinthifolius Raddi | Gallic acid, catechin, naringenin, and kaempferol | 50 mg Schinus terebinthifolius Raddi extract/kg bw to high-cholesterol-diet-fed rats for 9 weeks | Ameliorates plasma LDL-C by 41% | p < 0.05 | [110] |
Table 3.
Dietary sources, polyphenols, and cholesterol-lowering activities in clinical studies.
| Dietary Sources | Polyphenols Components | Treatment | Effect | p-Value | References |
|---|---|---|---|---|---|
| Green tea (Camellia sinesis) | Epigallocatechin gallate, epicatechin, epigallocatechin, epicatechin gallate, and gallocatechin gallate | Green tea capsules were given to healthy postmenopausal women for 2 months | Reduction in plasma LDL-C by 8% from baseline |
p < 0.05 (vs. placebo group) |
[76] |
| Green tea (Camellia sinesis) | Epigallocatechin gallate, epicatechin gallate, epigallocatechin, epicatechin, and gallocatechin gallate | 1500 mg green tea extract was given to women with central obesity for 12 weeks | Decreases in plasma LDL-C by 10% from baseline |
p < 0.05 (vs. baseline) |
[111] |
| Green tea (Camellia sinesis) | Epigallocatechin 3-gallate, epigallocatechin, catechin, epicatechin, gallocatechin gallate, and epicatechin-3-gallate | 1500 mg STA-2 (a pharmaceutical preparation of green tea polyphenols) daily was given to patients with chronic stable angina for 6 weeks |
Decreases in plasma LDL-C |
p < 0.05 (vs. placebo group) |
[112] |
| Green tea (Camellia sinesis) | Catechins and epigallocatechin-3-gallate | 400 mg of decaffeinated green tea extract daily was given to patients with type 2 diabetes mellitu for 12 weeks | Decreases in plasma LDL-C by 9% from baseline |
p > 0.05 (vs. placebo group) |
[113] |
| Goishi tea (Camellia sinesis) | - | 195 ml Goishi tea drink was given to hypercholesterolaemia individuals for 12 weeks | No changes in plasma LDL-C |
p > 0.05 (vs. placebo group) |
[114] |
| Red wine (Vitis vinifera L.) | Resveratrol | 2 capsules daily nonalcoholic red wine extract were given to nondiabetic humans for 8 weeks | Decreases in plasma LDL-C by 4% from baseline |
p < 0.05 (vs. baseline) |
[115] |
| Chokeberry (Aronia melanocarpa L.) juice | Cyanidin 3-galactoside, cyanidin 3-arabinoside, and cyanidin 3-xyloside | 200 mL chokeberry juice given to hypertensive subjects per day for 4 weeks | Reduction in plasma LDL-C by 7% from baseline |
p > 0.05 (vs. baseline) |
[9] |
| Aronia berry (Aronia melanocarpa) | Anthocyanins, hydroxycinnamic acids, and proanthocyanidins | 500 mg aronia extract per day was given to healthy adults for 12 weeks | Reduction in plasma LDL-C by 11% from baseline |
p < 0.05 (vs. placebo group) |
[116] |
| Blueberry (Vaccinium virgatum.) | - | 22 g freeze-dried blueberries daily were given to men with type 2 diabetes for 8 weeks | Decreases in plasma LDL-C by 25% from baseline |
p < 0.05 (vs. placebo group) |
[117] |
| Grape (Vitis vinifera L.) | Myricetin, quercetin, catechin, epicatechin, epigallocatechin, catechin gallate, and ellagic acid | 700 mg grape extract was given to healthy volunteers for 56 days | Reduces plasma LDL-C by 15% from baseline |
p < 0.05 (vs. placebo group) |
[118] |
| Grape (Vitis vinifera L.) | - | 500 g Condori red grapes daily were given to hypercholesterolemic humans for 8 weeks | Reduces plasma LDL-C with grapes treatment by 15% |
p < 0.01 (vs. baseline) |
[119] |
| Grapefruit (Vitis vinifera L.), bitter orange (Citrus aurantium Linné), and olive (Olea europaea) | Naringin, narirutin, rhoifolin, poncirin, apigenin, neohesperidin, neodiosmin, luteolin, and oleuropein | 1000 mg grapefruit, bitter orange, and olive leaf extracts were given to healthy subjects for 8 weeks | Reduces plasma LDL-C by 9% from baseline |
p < 0.05 (vs. placebo group) |
[120] |
| Grape (Vitis vinifera L.) | - | Overweight individuals were assigned to receive grape seed extract (300 mg/day) for 12 weeks | Reduces plasma LDL-C by 16% from baseline |
p < 0.05 (vs. placebo group) |
[80] |
| Olive (Olea europaea) | - | 250 mg/day olive extract was given to postmenopausal women for 12 months | Decreases in plasma LDL-C by 21% from baseline |
p < 0.05 (vs. placebo group) |
[121] |
| Olive (Olea europaea) | - | 400 g yogurt with 50 mg of encapsulated olive polyphenols was given to volunteers for 2 weeks | Ameliorates plasma LDL-C by 6% from baseline |
p < 0.05 (vs. baseline) |
[122] |
| Pomegranate (Punica granatum L.) | Ellagic acid | 1000 mg pomegranate extract was given to obese individuals for 30 days | Reduction in plasma LDL-C by 10% from baseline |
p < 0.01 (vs. baseline) |
[123] |
| Pomegranate (Punica granatum L.) juice | - | 200 mL/day pomegranate juice was given to type 2 diabetes patients for 6 weeks | Reduces plasma LDL-C by 10% from baseline |
p < 0.05 (vs. baseline) |
[124] |
| Cherry (Prunus avium) juice | - | 480 mL cherry juice drink daily to older adults for 12 weeks | Decreases in plasma LDL-C by 3% from baseline |
p < 0.05 (vs. placebo group) |
[125] |
| Tart cherry (Prunus cerasus) | Cyanidin sophoroside, cyanidin glucosylrutinoside, cyanidin-glucoside, cyanidin xylosylrutinoside, cyanidin rutinoside, and peonidin rutinoside | 240 mL of tart cherry juice twice daily was given to adults with metabolic syndrome for 12 weeks | Reduction in plasma LDL-C by 15% from baseline |
p > 0.05 (vs. baseline) |
[77] |
| Ecklonia cava | Dieckol, 8,8′-bieckol, 6,6′-bieckol, and phlorofurofucoeckol A | 72 mg Ecklonia cava polyphenols per day to overweight Korean individuals for 12 weeks |
Ameliorates plasma LDL-C by 10% from baseline |
p < 0.05 (vs. placebo group) |
[126] |
p < 0.05 considered to be statistically significant.
4.2. Flavonoids
4.2.1. Flavonols
Quercetin is a common plant flavonol which is widely present in vegetables, fruits, tea, and red wine [127]. In plants, partial quercetin has existed in the form quercetin-3-glucoside [128]. Quercetin exhibits favorable anti-atherosclerotic, anti-inflammatory, and antioxidant activities [129,130,131]. Interestingly, quercetin and its glycoside have attracted attention to the hypolipidemic effect [132,133,134]. In animal studies, treatment with quercetin and quercetin-3-glucoside significantly decreased the plasma LDL-C level in mice fed with a high-fat diet [133,134]. The quercetin group was administrated with quercetin in an aqueous solution through oral gavage with the dose of 12.5 mg/kg bw. Compared with the model group, the LDL-C level in quercetin group was reduced by 49% (p < 0.01). A total of 0.05% quercetin-3-glucoside supplementation significantly reduced the LDL-C level in comparison with the high-fat-fed control group. In a human study involving 24 subjects with mild hypercholesterolemia, the LDL-C level was greatly reduced by 9.2% after consuming 500 mL of quercetin-rich onion juice for 10 weeks (p < 0.01) [135]. The mechanisms by which quercetin and its glycoside inhibited the LDL-C level may involve several aspects. Firstly, quercetin reduced cholesterol absorption. The expression of NPC1L1, a critical protein in cholesterol absorption, was decreased in Caco-2 cells treated with 100 μM quercetin [136]. Secondly, both quercetin and its glycoside regulate LDLR expression. Moon demonstrated that 75 μM of quercetin upregulated the LDLR expression in HepG2 cells via the enhanced processing of the sterol regulatory element-binding protein 2 (SREBP2) following the sequential activation of c-Jun N-terminal kinases (JNK) and the extracellular signal-related kinase (ERK) signaling pathways [137]. In the Huh7 cells (human hepatocytes), treatment with 5 μM quercetin-3-glucoside accelerated the LDL uptake by increasing LDLR expression and attenuating PCSK9 secretion [138]. Similarly, Mbikay’s study showed that 0.05% quercetin-3-glucoside reduced PCSK9 secretion and increased the hepatic LDLR expression in mice fed with a high-cholesterol diet [134]. Thirdly, quercetin facilitates bile acid biosynthesis and excretion. In a Zhang’s study, it was suggested that a dietary supplementation of 0.4% quercetin increased the excretion of fecal bile acid and the level of CYP7A1, a critical enzyme promoting cholesterol-to-bile acid conversion in bile acid biosynthesis [139].
4.2.2. Flavan-3-ols
Epigallocatechin-3-gallate (EGCG), which is an ester that forms through the reaction of epigallocatechin and gallic acid, is the major catechin in tea [140]. Substantial evidence suggests that EGCG elicit a wide range of properties, including antioxidant and anti-lipid deposition and anti-inflammation [141,142,143]. Extensive studies have shown that the consumption of EGCG exhibited an LDL-C reducing effect [144,145,146,147]. A systematic review found that EGCG could significantly decrease the plasma LDL-C level at doses between 107 and 856 mg/d [148,149,150]. Several mechanisms by which EGCG lowers the content of LDL-C involve cholesterol absorption, VLDL assembly, LDLR, and bile acid metabolism. Huang and colleagues [151] found that 0.32% EGCG reduced LDL-C by 28% in mice fed with a high-fat diet by increasing the fecal excretion of bile acids and cholesterol and decreasing cholesterol and bile acid reabsorption. Raederstorff and colleagues [152] found that EGCG affected intestinal cholesterol absorption by interfering with the micellar solubilization of cholesterol in a dose-dependent manner. Researchers [145] have pointed out that the enhanced biliary secretion of cholesterol was associated with the increased expression of ABCG5/ABCG8 and the decreased expression of ACAT2 by EGCG at a dose of 50 mg/kg bw. Moreover, evidence indicated that EGCG could suppress the LDL-C content by inducing an upregulation of LDLR [153]. A total of 25 μM EGCG could upregulate the level of LDLR through the ERK signaling pathways and downregulate the expression of PCSK9 in the HepG2 cell model [153]. In an animal study [147], rats fed with high-fat diet supplementation with 50 mg/kg bw of EGCG could weaken the concentration of circulation PCSK9 and nuclear factor-1α (HNF-1α) and the expression of PCSK9, as well as raise forkhead box class O (FoxO)3a and LDLR levels. Circulation PCSK9 could accelerate the degradation of LDLR, resulting in an elevated level of LDL-C in plasma [154]. HNF1α and FoXO3a are two transcription factors that regulate the expression of PCSK9 [147]. Thereby, EGCG acts on HNF1α and FoXO3a to reduce PCSK9 expression [147]. In a study by Kuhn, it was found that 1 μM EGCG directly inhibited ubiquitin/proteasome-mediated degradation of the active SREBP2, resulting in an elevated expression of LDLR [155].
4.2.3. Anthocyanins and Proanthocyanidins
Anthocyanins are plentiful flavonoid pigments that occur in fruits, vegetables, and other plant-derived foods with a dark color, including blueberries, bilberries, blackcurrants, cranberries, cherries, and black rice [156]. Anthocyanins have been proven to possess antioxidant, anti-inflammatory, anti-atherosclerotic, and antiobesity properties [157,158,159,160]. Several epidemiologic studies have reported a marked decrease in LDL-C by the anthocyanin intervention [161,162,163,164,165]. According to previous research, daily supplementation of 90 mg, 320 mg, and 350 mg of purified anthocyanins or extracts could reduce the LDL-C level in hyperlipidemic patients by 8%, 13%, and 26.3%, respectively. No significant difference was observed in the plasma LDL-C level between patients with nonalcoholic fatty liver disease receiving anthocyanin or the placebo [166]. However, meta-analyses have found a statistically significant reduction of LDL-C in those individuals consuming anthocyanins [167,168]. Several mechanisms have been proposed to explain the effect of anthocyanins on lowering LDL-C. One possibility is that anthocyanins inhibit the activity of plasma CETP [162]. In a double-blind, randomized, and placebo-controlled trial, Qin and colleagues reported that the intake of 160 mg of anthocyanins twice daily significantly decreased the LDL-C concentration and the activity of CETP [162]. A second possibility is that anthocyanins could reduce the absorption of cholesterol [169]. In a hamster model of diet-induced dyslipidemia, anthocyanins at doses of 0.5% and 1% enhanced the fecal excretion of cholesterol accompanied with the downregulation on the gene expression of intestinal NPC1L1, ACAT-2, MTP, and ABCG8 [169]. Studies have consistently found that anthocyanins inhibited the formation of cholesterol micelles and increased the excretion of fecal sterols to lower the plasma LDL-C level [170,171,172]. The third possibility is that anthocyanin mediate bile acid metabolism [173]. In a study by Wang, it was found that dietary supplementation of 0.06% cyanidin-3-o-β-glucoside promoted fecal bile acid excretion and upregulated hepatic CYP7A1 expression compared with the control group [173].
Proanthocyanidins are known as condensed tannins, mainly present in fruits (berries, grapes, and apples), cereals, beans, and beverages (wine and tea) [174]. Proanthocyanidins were reported to consist of catechin, epicatechin, gallocatechin, and epigallocatechin-3-gallate, which divided into oligomers and polymers proanthocyanidins in terms of degree of polymerization [175]. Proanthocyanidins are believed to possess antiobesity, antioxidant, anti-inflammatory, anticancer, and hypolipidemic properties [176,177]. Previous studies showed that supplementation of proanthocyanidins could reduce both plasma TC and LDL-C levels [176,178]. The proanthocyanidins group was administrated with purified proanthocyanidins from lotus (Nelumbo nucifera, Gaertn) seed pot in an aqueous solution through an oral gavage with a dose of 25 mg/kg bw. Compared with the model group, the LDL-C level in the proanthocyanidins group was reduced by 48% (p < 0.05). The underlying mechanisms for lowering the plasma LDL-C level by proanthocyanidins are as follows. Firstly, proanthocyanidins were involved in the regulation of bile acid metabolism. For example, dietary supplementation of 1% grape seed proanthocyanidin increased the excretion of fecal bile acids and the upregulation of CYP7A1 in both the transcriptional and translational levels [179]. Similarly, supplementation with a high-cholesterol diet containing 1% cacao procyanidins could also enhance the fecal bile acid excretion in rats [180]. Heidker pointed out that grape seed procyanidin could not only enhance the expression of CYP7A1 but also decrease the absorption of intestinal bile acid by downregulating ASBT expression [181]. Secondly, the reduction of the plasma LDL-C level by proanthocyanidins (250 mg/kg bw) was associated with promoting the excretion of fecal cholesterol. The consumption of proanthocyanidins from cacao could inhibit the intestinal absorption of cholesterol through decreasing micellar cholesterol solubility and enhancing cholesterol excretion [180]. Thirdly, daily administration of 25 mg/kg bw of grape seed proanthocyanidins in rats had been shown to decrease the LDL-C level by 57%. The underlying mechanism involved disturbing VLDL assembling by repressing the expression of MTP and apoB [182].
4.2.4. Curcumin
Curcumin, a hydrophobic polyphenol extracted from turmeric, has been widely used as a dietary spice by many cultures [183]. Curcumin was reported to exhibit broad spectral biological and pharmacologic activities including antioxidant, anti-inflammatory, antidiabetic, hypoglycemic, and antimicrobial capacities [184]. Moreover, curcumin was also shown to possess antihyperlipidemic activity [185,186]. Animal studies indicated that curcumin consumption could inhibit the LDL-C level in rats and hamsters fed with a high-fat diet [186,187,188,189]. After supplementation with 0.05% curcumin in male hamsters for 12 weeks, the LDL-C level was decreased by 34% compared to model group. Moreover, curcumin supplementation at a dose of 80 mg/kg bw could reduce the LDL-C level by 53% in obese rats [190]. Several human studies showed that curcumin could significantly decrease the plasma level of LDL-C in healthy middle-aged people and in patients with polycystic ovary syndrome, nonalcoholic fatty liver disease, mild chronic obstructive pulmonary disease, or type 2 diabetes [185,191,192,193,194]. Daily 180 mg curcumin supplementation in subjects with mild COPD was effective in diminishing the LDL-C level by approximately 11%. The LDL-C level in patients with nonalcoholic fatty liver disease was decreased by 23% after 1000 mg of curcumin intervention. Supplementation with curcumin decreased intestinal cholesterol absorption and further plasma LDL-C levels by inhibiting the intestinal expression of NPC1L1 [186,195]. Curcumin could also elevate the expression of hepatic LDLR via the sterol regulatory element (SRE) pathway [196,197]. However, Tai proved that a 20 μM curcumin treatment enhanced LDL uptake in HepG2 cell by approximately 23%. The mechanism was that curcumin enhanced the density and activity of LDLR through the inhibition of PCSK9 [198]. Tai’s results were supported by another study that curcumin could promote LDLR expression by inhibiting the expression of PCSK9 [199]. Moreover, curcumin was found to decrease PCSK9 expression via reducing the nuclear abundance of HNF-1α, an important PCSK9 regulator [198]. Moreover, curcumin regulated the metabolism of LDL by improving the C-to-U RNA edition of apoB, which is major component of LDL [200]. A total of 0.1% curcumin consumption could also stimulate the conversion of hepatic cholesterol to bile acids via the upregulation of CYP7A1, and by further promoting the removal of LDL (about 56%) [188].
4.2.5. Isoflavone
Genistein, a principal bioactive soy isoflavone, has received great attention due to antioxidative, anti-inflammatory and lipid-lowering effects [201,202]. There exists a considerable amount of evidence supporting the role of genistein in the prevention of cardiovascular disease, osteoporosis, diabetes, and hyperlipemia [203,204,205]. Growing experimental data has showed that genistein consumption made a reduction in LDL-C levels [206,207,208,209]. Genistein supplementation at a dose of 2 g/kg bw significantly reduced the LDL-C level by 40% in high-fat-diet-fed hamsters. In a randomized, double-blind, and placebo-controlled trial, 54 mg of genistein daily for one year were found to decrease the LDL-C level (mean from 108.8 to 78.7 mg/dL) in Caucasian postmenopausal subjects with metabolic syndrome [210]. Similarly, a meta-analysis found that genistein consumption could significantly decrease the LDL-C level in postmenopausal women with metabolic syndrome [211]. Several mechanisms have been proposed to explain the effect of genistein on decreasing the LDL-C level. Genistein could upregulate the expression of hepatic LDLR, thereby inducing the clearance of LDL-C [207]. This result was also verified in vitro studies [212,213]. Moreover, Kartawijaya supported that 40 μM genistein treatment could activate the JNK signaling pathway and SREBP2 processing, which was followed by the upregulation of LDLR [213]. Genistein could also interfere with VLDL assembly. Borradaile found that 50 μM genistein could decrease the secretion of apoB through multiple mechanisms, including inhibiting the expression of HMGR, the activity of ACAT, and the expression and activity of MTP [214].
4.3. Stilbenes
Resveratrol is a polyphenolic compound produced by grapes, peanuts, vegetables and other plant species [215]. Polydatin, as the glycoside of resveratrol, is the main bioactive constituent of Polygonum cuspidatum Sieb. et Zucc [216]. Studies have demonstrated that resveratrol and its glycoside exhibit a lipid-lowering effect [217,218]. Daily supplementation with 0.02% resveratrol in apoE-deficient mice had been shown to decrease the LDL-C content by 15%. The level of LDL-C was evidently decreased after 3 weeks of treatment with polydatin (25, 50, and 100 mg/kg bw) by 27%, 30%, and 33%, respectively [219]. The underlying mechanisms by which resveratrol and its glycoside mitigate the LDL-C level have been investigated in cell cultures and in animals. Principally, resveratrol and its glycoside increased the expression and activity of hepatic LDLR [220]. Evidence indicated that a high-fat diet plus resveratrol (50 and 100 mg/kg bw) could downregulate the expression of LDLR in rats and further reduce the level of LDL-C [220]. Resveratrol regulated the expression of LDLR via two ways. On the one hand, a 50 μM resveratrol treatment enhanced LDLR transcription via the proteolytic activation of SREBPs and exhibited a 59% increase in the LDL uptake [221]. On the other hand, both 10 μM and 20 μM resveratrol downregulated PCSK9 expression to maintain LDLR levels on the surface of cells [222]. Similarly, 20 μM polydatin upregulated the protein expression level of LDLR and inhibited PCSK9 protein expression, as well as the combination between PCSK9 and LDLR [223]. To a lesser extent, resveratrol also involved bile acid metabolism. Chaothe and Swaan found that resveratrol promoted bile acid transporter ASBT degradation via the ubiquitin–proteasome pathway, which might be associated with an increase in fecal bile acid excretion [224,225]. Shao and colleagues found resveratrol and its liver metabolite resveratrol glucuronide at a dose of 25 μM caused a significant increase in hepatic CYP7A1 and BSEP, indicating the increase in the synthesis and efflux of bile acids [56].
4.4. Effect of Other Polyphenols on Lowering LDL-C and Mode of Action
In addition to the above mentioned, polyphenols which exerted LDL-C lowering effects also included protocatechuic acid, vanillic acid, puerarin, kaempferol, and so on. Herein, we focus on the mechanisms by which other dietary polyphenols alleviated the LDL-C level. In vivo study reported that vanillic acid possessed LDL-C lowering potential by inhibiting the HMGR activity [226]. Vanillic acid was orally given (50 mg/kg bw) to hypertensive rats for 4 weeks. The results revealed that vanillic acid reduced the plasma LDL-C level by 64% and inhibited HMGR activity (Table 4). Moreover, an animal study conducted by Ma et al. revealed that the decrease of the plasma LDL-C concentration in the puerarin intervention group was associated with the induction of CYP7A1 [227]. In another study, naringin (25 mg/kg bw) could reduce the plasma LDL-C level in obese mice via inhibiting the expression of SREBP2 and PCSK9 and inducing the expression of LDLR [228]. Kubota and colleagues elucidated the underlying mechanism of lowering LDL-C by ellagic acid [229]. In the presence of 25 μM ellagic acid, the expression of LDLR was significantly increased while extracellular apoB protein and MTP mRNA levels were decreased (p < 0.05) (Table 5). Another in vitro study conducted by Ochaiai et al. reported that the uptake of fluorescent-labeled LDL in HepG2 cells was increased after treatment with 100 μM kaempferol for 24 h [230]. The results were attributed to the promotion of LDLR expression and activity in the presence of kaempferol. Moreover, luteolin could affect the cholesterol absorption by regulating the expression of NPC1L1 in Caco-2 cells [136]. Table 4 and Table 5 respectively summarizes the results and details of the listed references based on in vitro or in vivo models.
Table 4.
Effect of other polyphenols on LDL-C and their mechanism based on in vivo models.
| Subclasses | Polyphenol | Study Type | Results | Mechanism | References |
|---|---|---|---|---|---|
| Phenolic acid | Protocatechuic acid | 0.05% (w/w) given to cholesterol-fed rats for 4 weeks | Lowers levels of non-HDL-C from 0.88 ± 014 mmol/L to 0.74 ± 0.04 mmol/L (p < 0.05) | Increases the expression of hepatic LDLR | [231] |
| Phenolic acid | Vanillic acid | Vanillic acid (50 mg/kg bw) to hypertensive rats for 4 weeks | Decreases in plasma LDL-C by 64% (p < 0.05) | Inhibits HMGR activity | [226] |
| Isoflavone | Puerarin glycosides | Puerarin glycosides (0.1%) was given to mice for 3 weeks | Reduction in plasma TC levels (p < 0.05) | Increases the expression of LDLR; Downregulates the transcription and translation of HMGR | [232] |
| Isoflavone | Puerarin | Orally (200 mg/kg bw and 400 mg/kg bw) puerarin administered to hyperlipidaemia mice for 8 weeks | Decreases in plasma LDL-C level (p < 0.05) | Regulates the expression of phosphorylated JNK, phosphorylated c-Jun protein, and CYP7A1 | [227] |
| Flavones | Apigenin | Apigenin was orally administrated to high-fat-fed mice | Decreases the level of LDL-C in plasma by 19%, 16%, and 55%, respectively (p < 0.05) | Promotes the absorption of hepatic LDL-C and increases the transformation of hepatic cholesterol into bile acid by regulating LDLR and CYP7A1 expression | [233] |
| Flavones | Apigenin | Apigenin (60 ppm and 300 ppm) given orally to hamsters with hypercholesterolaemia | Reduces the nonHDL-C level by 40% and 41% (p < 0.05) | Reduces the uptake of dietary cholesterol by inhibiting NPC1L1; Stimulates hepatic LDLR expression | [234] |
| Flavones | Luteolin | Mice were administered daily 50 mg/kg bw of luteolin in addition to ethanol exposure | Reduces LDL-C level in plasma by 52% (p < 0.05) | Inhibits cholesterol biosynthesis by regulating SREBP2 and HMGR | [235] |
| Flavones | Luteolin | Luteolin (1.5%) was given to high-fat-fed mice for 57 days | Reduces LDL-C levels by 33% (p < 0.05) | Suppresses HNF4α targeted genes, such as MTP, apoB | [236] |
| Flavones | Luteolin | Luteolin (50 mg/kg bw) given orally to hyperlipidemia rats for 6 weeks | Reduces LDL-C levels by 34% (p < 0.05) | Increases CYP7A1 activities in liver | [81] |
| Flavones | Luteolin-7-glucoside | Luteolin-7-glucoside (2 mg/kg bw) orally to rats | Decreases levels of LDL-C in plasma by 40% (p < 0.05) | Decreases cholesterol synthesis via decreasing HMGR expression | [237] |
| Flavanones | Naringin | Naringin (25 mg/kg bw) given orally to obese mice for 8 weeks | Decreases the level of LDL-C in plasma (p < 0.05) | Downregulates the expression of SREBP2 and PCSK9, and upregulates the expression of LDLR through AMPK activation | [228] |
| Ligans | Leoligin | 0.14 mg leoligin was given to CETP transgenic mice for 7 days | Reduces LDL-C level in plasma | Activates CETP activity | [238] |
| Ligans | Sesamin | Hamsters were fed two experimental diets containing 0.02% sesamin or 0.5% sesamin for 6 weeks | Lowers plasma non-HDL-C level by 25% and 32% (p < 0.05) | Reduces cholesterol absorption by inhibiting intestinal NPC1L1, ACAT2, MTP, and ABCG5/8; Stimulates LDLR and CYP7A1 expression | [239] |
Table 5.
Effect of other polyphenols on LDL-C and their mechanism based on in vitro models.
| Subclasses | Polyphenol | Study Type | Results | Mechanism | References |
|---|---|---|---|---|---|
| Phenolic acid | Ellagic acid | 25 μM ellagic acid to HepG2 cells | Regulates cholesterol metabolism | Upregulates of LDLR, downregulates MTP mRNA and extracellular apoB levels | [229] |
| Flavonols | Kaempferol | The HepG2 cells were incubated for 24 h with kaempferol (100 μM) | Increases fluorescent-labeled LDL uptake (p < 0.05) | Stimulates the expression of LDLR through activating LDLR transcription factor Sp1 | [230] |
| Flavones | Luteolin | Caco-2 cells were incubated with 100 μM luteolin | Inhibits cholesterol uptake in Caco-2 cells (p < 0.05) | Inhibits intestinal cholesterol absorption mediated by NPC1L1 | [136] |
| Flavones | Rutin | Caco-2 cells were treated with 115.7 μM rutin; Rutin (17.85 μM) was used to measure HMGR activity inhibition | Lowers the amount of cholesterol in the intracellular compartment; Reduces HMGR activity by 50% (p < 0.05) | Inhibits the uptake of dietary cholesterol and the activity of HMGR | [240] |
| Flavanones | Hesperetin | HepG2 cells were exposed to hesperetin (100 μM) | Induces the activity of LDLR promoter (p < 0.05) | Induces the transcription of LDLR through SREBPs | [241] |
| Flavanones | Glucosyl hesperidin | HepG2 cells were treated with 0.8 mM and 1.2 mM glucosyl hesperidin | Reduces cellular cholesteryl ester content (p < 0.05) | Suppresses the secretion of apoB | [242] |
5. Limitations and Suggestions
From the above studies, dietary polyphenols have exhibited the potential effect on lowering the level of LDL-C. However, there are many limitations, which are as follows:
In some studies, the crude extracts from food origin were applied to study the LDL-C-reducing activities without determining the active ingredients;
In most previous studies, researchers only focused on a partial mechanism about a particular polyphenol without conducting a comprehensive study involved in polyphenol’s potential regulatory pathways;
Bioactivity investigations using cell lines have made an extensive use of polyphenols at concentrations in the low-μM-to-mM range. However, after ingestion the dietary polyphenols appear in the circulatory system as phase II metabolites, and their presence in plasma rarely exceeds nM concentrations [12]. There is lack of data which explores the effect of polyphenols metabolites on lowering LDL-C;
Researchers paid more attention to in vitro and animal studies but less attention to clinal studies.
Thus, further studies are required to separate and identify the active components in the presence of crude extracts. Moreover, all the potential regulatory pathways about inhibiting the LDL-C level should be adequately considered during research. Furthermore, a comparative study of the effect of polyphenols and their metabolites on lowering LDL-C is needed. Finally, we should focus on clinal studies to provide a necessary dietary reference for people.
6. Conclusions
LDL-C-causing CVDs were repeatedly demonstrated in different experimental studies. LDL-C lowering therapies have become a preferred target for CVDs resulting from liner relationship between LDL-C and CVDs risk. LDL-C lowering therapies were mainly involved in hepatic and intestinal target organs. In hepatocytes, the molecular targets contained HMGR, LDLR, PCSK9, IDOL, MTP, apoB, CYP7A1, CETP, SREBPs, squalene synthase, and ACLY, while ABCG5/8, NPC1L1, MTP, ACAT, and ASBT were believed to be associated with the absorption and excretion of cholesterol and bile acid in the gut. From the above studies, dietary polyphenols have exhibited a potential effect on lowering the level of LDL-C via various molecular targets. However, further deep studies are required to elucidate how polyphenols interact with the targets.
Acknowledgments
We are grateful to the study participants.
Author Contributions
Conceptualization, B.J. and F.Z.; writing—original draft preparation, P.S.; writing—review and editing, L.Z. (Liang Zhao) and N.Z.; visualization, J.Z.; supervision, L.Z. (Liebing Zhang) and W.W.; project administration, F.Z.; funding acquisition, L.Z. (Liang Zhao) All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Postdoctoral Science Foundation, grant number 2019TQ0011 to L.Z. (Liang Zhao).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Şahin B., İlgün G. Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries. Health Soc. Care Community. 2020 doi: 10.1111/hsc.13156. [DOI] [PubMed] [Google Scholar]
- 2.McLaren J.E., Michael D.R., Ashlin T.G., Ramji D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Hung J., Miscianinov V., Sluimer J.C., Newby D.E., Baker A.H. Targeting non-coding RNA in vascular biology and disease. Front. Physiol. 2018;9:1655. doi: 10.3389/fphys.2018.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ference B.A., Mahajan N. The role of early LDL lowering to prevent the onset of atherosclerotic disease. Curr. Atheroscler. Rep. 2013;15:312. doi: 10.1007/s11883-013-0312-1. [DOI] [PubMed] [Google Scholar]
- 5.Pirillo A., Catapano A.L., Norata G.D. Recent insights into low-density lipoprotein metabolism and therapy. Curr. Opin. Clin. Nutr. Metab. Care. 2021;24:120–126. doi: 10.1097/MCO.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 6.Gitt A.K., Drexel H., Feely J., Ferrières J., Gonzalez-Juanatey J.R., Thomsen K.K., Leiter L.A., Lundman P., da Silva P.M., Pedersen T. Persistent lipid abnormalities in statin-treated patients and predictors of LDL-cholesterol goal achievement in clinical practice in Europe and Canada. Eur. J. Prev. Cardiol. 2012;19:221–230. doi: 10.1177/1741826711400545. [DOI] [PubMed] [Google Scholar]
- 7.Ravn-Haren G., Dragsted L.O., Buch-Andersen T., Jensen E.N., Jensen R.I., Németh-Balogh M., Paulovicsová B., Bergström A., Wilcks A., Licht T.R. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013;52:1875–1889. doi: 10.1007/s00394-012-0489-z. [DOI] [PubMed] [Google Scholar]
- 8.Brader L., Overgaard A., Christensen L.P., Jeppesen P.B., Hermansen K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud. 2013;10:270. doi: 10.1900/RDS.2013.10.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kardum N., Milovanović B., Šavikin K., Zdunić G., Mutavdžin S., Gligorijević T., Spasić S. Beneficial effects of polyphenol-rich chokeberry juice consumption on blood pressure level and lipid status in hypertensive subjects. J. Med. Food. 2015;18:1231–1238. doi: 10.1089/jmf.2014.0171. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y., Zheng J., Li Y., Xu D.P., Li S., Chen Y.M., Li H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients. 2016;8:515. doi: 10.3390/nu8080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers K.F., Day P.E., Aboufarrag H.T., Kroon P.A. Polyphenol effects on cholesterol metabolism via bile acid biosynthesis, CYP7A1: A review. Nutrients. 2019;11:2588. doi: 10.3390/nu11112588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Rio D., Rodriguez-Mateos A., Spencer J.P., Tognolini M., Borges G., Crozier A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao J., Hogger P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015;22:23–38. doi: 10.2174/0929867321666140706130807. [DOI] [PubMed] [Google Scholar]
- 14.Shen C.-L., Smith B.J., Lo D.-F., Chyu M.-C., Dunn D.M., Chen C.-H., Kwun I.-S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012;23:1367–1377. doi: 10.1016/j.jnutbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R., Bapat P., Kwun I., Shen C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G., Wang H., Zhang X., Yang S.-T. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit. Rev. Food Sci. Nutr. 2014;54:1180–1201. doi: 10.1080/10408398.2011.629354. [DOI] [PubMed] [Google Scholar]
- 17.Mehmood A., Zhao L., Wang C., Nadeem M., Raza A., Ali N., Shah A.A. Management of hyperuricemia through dietary polyphenols as a natural medicament: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019;59:1433–1455. doi: 10.1080/10408398.2017.1412939. [DOI] [PubMed] [Google Scholar]
- 18.Wang O., Liu J., Cheng Q., Guo X., Wang Y., Zhao L., Zhou F., Ji B. Effects of Ferulic Acid and gamma-Oryzanol on High-Fat and High-Fructose Diet-Induced Metabolic Syndrome in Rats. PLoS ONE. 2015;10:e0118135. doi: 10.1371/journal.pone.0118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J., Zhao L., Cheng Q., Ji B., Yang M., Sanidad K.Z., Wang C., Zhou F. Structurally Different Flavonoid Subclasses Attenuate High-Fat and High-Fructose Diet Induced Metabolic Syndrome in Rats. J. Agric. Food Chem. 2018;66:12412–12420. doi: 10.1021/acs.jafc.8b03574. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L., Wang Y., Liu J., Wang K., Guo X., Ji B., Wu W., Zhou F. Protective Effects of Genistein and Puerarin against Chronic Alcohol-Induced Liver Injury in Mice via Antioxidant, Antiinflammatory, and Anti-apoptotic Mechanisms (vol 64, pg 7291, 2016) J. Agric. Food Chem. 2016;64:8463. doi: 10.1021/acs.jafc.6b04807. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L., Zhang N., Yang D., Yang M., Guo X., He J., Wu W., Ji B., Cheng Q., Zhou F. Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model. Nutrients. 2018;10:1754. doi: 10.3390/nu10111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atici E.B., Karlığa B. Identification, synthesis and characterization of process related desfluoro impurity of ezetimibe and HPLC method validations. J. Pharm. Anal. 2015;5:356–370. doi: 10.1016/j.jpha.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemes K., Åberg F., Gylling H., Isoniemi H. Cholesterol metabolism in cholestatic liver disease and liver transplantation: From molecular mechanisms to clinical implications. World J. Hepatol. 2016;8:924. doi: 10.4254/wjh.v8.i22.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruckert E., Giral P., Tellier P. Perspectives in cholesterol-lowering therapy: The role of ezetimibe, a new selective inhibitor of intestinal cholesterol absorption. Circulation. 2003;107:3124–3128. doi: 10.1161/01.CIR.0000072345.98581.24. [DOI] [PubMed] [Google Scholar]
- 25.Wei J., Fu Z.-Y., Li P.-S., Miao H.-H., Li B.-L., Ma Y.-T., Song B.-L. The clathrin adaptor proteins ARH, Dab2, and numb play distinct roles in Niemann-Pick C1-Like 1 versus low density lipoprotein receptor-mediated cholesterol uptake. J. Biol. Chem. 2014;289:33689–33700. doi: 10.1074/jbc.M114.593764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen T.M., Sawyer J.K., Kelley K.L., Davis M.A., Rudel L.L. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: Evidence from thoracic lymph duct cannulation. J. Lipid Res. 2012;53:95–104. doi: 10.1194/jlr.M018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D.Q.-H. Regulation of intestinal cholesterol absorption. Annu. Rev. Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 28.Visser M.E., Jakulj L., Kastelein J.J., Stroes E.S. LDL-C-lowering therapy: Current and future therapeutic targets. Curr. Cardiol. Rep. 2008;10:512. doi: 10.1007/s11886-008-0080-7. [DOI] [PubMed] [Google Scholar]
- 29.Mensenkamp A.R., Van Luyn M.J., Havinga R., Teusink B., Waterman I.J., Mann C.J., Elzinga B.M., Verkade H.J., Zammit V.A., Havekes L.M. The transport of triglycerides through the secretory pathway of hepatocytes is impaired in apolipoprotein E deficient mice. J. Hepatol. 2004;40:599–606. doi: 10.1016/j.jhep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari S., Siddiqi S.A. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feingold K.R., Grunfeld C. Introduction to Lipids and Lipoproteins. MDText.com, Inc.; South Dartmouth, MA, USA: 2021. [Google Scholar]
- 32.Dietschy J.M., Turley S.D., Spady D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993;34:1637–1659. doi: 10.1016/S0022-2275(20)35728-X. [DOI] [PubMed] [Google Scholar]
- 33.Cedó L., Farràs M., Lee-Rueckert M. Molecular insights into the mechanisms underlying the cholesterol-lowering effects of phytosterols. Curr. Med. Chem. 2019;26:6704–6723. doi: 10.2174/0929867326666190822154701. [DOI] [PubMed] [Google Scholar]
- 34.Olofsson S.O., Boren J. Apolipoprotein B: A clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 2005;258:395–410. doi: 10.1111/j.1365-2796.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 35.Boren J., Wettesten M., Sjöberg A., Thorlin T., Bondjers G., Wiklund O., Olofsson S. The assembly and secretion of apoB 100 containing lipoproteins in Hep G2 cells. Evidence for different sites for protein synthesis and lipoprotein assembly. J. Biol. Chem. 1990;265:10556–10564. doi: 10.1016/S0021-9258(18)86983-6. [DOI] [PubMed] [Google Scholar]
- 36.Lagrost L. Plasma phospholipid transfer protein: A multifaceted protein with a key role in the assembly and secretion of apolipoprotein B–containing lipoproteins by the liver. Hepatology. 2012;56:415–418. doi: 10.1002/hep.25725. [DOI] [PubMed] [Google Scholar]
- 37.Manchekar M., Liu Y., Sun Z., Richardson P.E., Dashti N. Phospholipid transfer protein plays a major role in the initiation of apolipoprotein B-containing lipoprotein assembly in mouse primary hepatocytes. J. Biol. Chem. 2015;290:8196–8205. doi: 10.1074/jbc.M114.602748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye J., Li J.Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. Cideb, an ER-and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–190. doi: 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Hossain T., Riad A., Siddiqi S., Parthasarathy S., Siddiqi S.A. Mature VLDL triggers the biogenesis of a distinct vesicle from the trans-Golgi network for its export to the plasma membrane. Biochem. J. 2014;459:47–58. doi: 10.1042/BJ20131215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masson W., Lobo M., Siniawski D., Huerín M., Molinero G., Valero R., Nogueira J.P. Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Diabetes Metab. 2018;44:508–513. doi: 10.1016/j.diabet.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Barter P.J., Brewer H.B., Jr., Chapman M.J., Hennekens C.H., Rader D.J., Tall A.R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003;23:160–167. doi: 10.1161/01.ATV.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 42.Shrestha S., Wu B.J., Guiney L., Barter P.J., Rye K.-A. Cholesteryl ester transfer protein and its inhibitors. J. Lipid Res. 2018;59:772–783. doi: 10.1194/jlr.R082735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown M.S., Goldstein J.L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 44.Esser V., Limbird L., Brown M.S., Goldstein J.L., Russell D.W. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J. Biol. Chem. 1988;263:13282–13290. doi: 10.1016/S0021-9258(18)37702-0. [DOI] [PubMed] [Google Scholar]
- 45.Maxfield F.R., McGraw T.E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein J.L., Brown M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scotti E., Calamai M., Goulbourne C.N., Zhang L., Hong C., Lin R.R., Choi J., Pilch P.F., Fong L.G., Zou P. IDOL stimulates clathrin-independent endocytosis and multivesicular body-mediated lysosomal degradation of the low-density lipoprotein receptor. Mol. Cell. Biochem. 2013;33:1503–1514. doi: 10.1128/MCB.01716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poirier S., Mayer G., Poupon V., McPherson P.S., Desjardins R., Ly K., Asselin M.-C., Day R., Duclos F.J., Witmer M. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: Evidence for an intracellular route. J. Biol. Chem. 2009;284:28856–28864. doi: 10.1074/jbc.M109.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donkers J.M., Abbing R.L.R., Van de Graaf S.F. Developments in bile salt based therapies: A critical overview. Biochem. Pharmacol. 2019;161:1–13. doi: 10.1016/j.bcp.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Russell D.W. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 2009;50:S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi H., Takada T., Suzuki H., Onuki R., Hofmann A.F., Sugiyama Y. Transport by vesicles of glycine-and taurine-conjugated bile salts and taurolithocholate 3-sulfate: A comparison of human BSEP with rat Bsep. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2005;1738:54–62. doi: 10.1016/j.bbalip.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Dawson P.A., Lan T., Rao A. Bile acid transporters. J. Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawson P.A., Hubbert M.L., Rao A. Getting the mOST from OST: Role of organic solute transporter, OSTα-OSTβ, in bile acid and steroid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2010;1801:994–1004. doi: 10.1016/j.bbalip.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slijepcevic D., Roscam Abbing R.L., Katafuchi T., Blank A., Donkers J.M., van Hoppe S., de Waart D.R., Tolenaars D., van der Meer J.H., Wildenberg M. Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology. 2017;66:1631–1643. doi: 10.1002/hep.29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.JG Marin J., IR Macias R., Briz O., M Banales J., J Monte M. Bile acids in physiology, pathology and pharmacology. Curr. Drug Metab. 2016;17:4–29. doi: 10.2174/1389200216666151103115454. [DOI] [PubMed] [Google Scholar]
- 56.Shao D., Wang Y., Huang Q., Shi J., Yang H., Pan Z., Jin M., Zhao H., Xu X. Cholesterol-Lowering Effects and Mechanisms in View of Bile Acid Pathway of Resveratrol and Resveratrol Glucuronides. J. Food Sci. 2016;81:H2841–H2848. doi: 10.1111/1750-3841.13528. [DOI] [PubMed] [Google Scholar]
- 57.Huff M.W., Telford D.E., Edwards J.Y., Burnett J.R., Barrett P.H.R., Rapp S.R., Napawan N., Keller B.T. Inhibition of the apical sodium-dependent bile acid transporter reduces LDL cholesterol and apoB by enhanced plasma clearance of LDL apoB. Arterioscler. Thromb. Vasc. Biol. 2002;22:1884–1891. doi: 10.1161/01.ATV.0000035390.87288.26. [DOI] [PubMed] [Google Scholar]
- 58.Al-Dury S., Marschall H.-U. Ileal bile acid transporter inhibition for the treatment of chronic constipation, cholestatic pruritus, and NASH. Front. Pharmacol. 2018;9:931. doi: 10.3389/fphar.2018.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feingold K.R.J.E. Cholesterol Lowering Drugs. MDText.com, Inc.; South Dartmouth, MA, USA: 2021. [Google Scholar]
- 60.Clerc R.G., Stauffer A., Weibel F., Hainaut E., Perez A., Hoflack J.-C., Bénardeau A., Pflieger P., Garriz J.M., Funder J.W. Mechanisms underlying off-target effects of the cholesteryl ester transfer protein inhibitor torcetrapib involve L-type calcium channels. J. Hypertens. 2010;28:1676–1686. doi: 10.1097/HJH.0b013e32833b1f8e. [DOI] [PubMed] [Google Scholar]
- 61.Rader D.J., Kastelein J.J. Lomitapide and mipomersen: Two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129:1022–1032. doi: 10.1161/CIRCULATIONAHA.113.001292. [DOI] [PubMed] [Google Scholar]
- 62.Adhyaru B.B., Jacobson T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018;15:757–769. doi: 10.1038/s41569-018-0098-5. [DOI] [PubMed] [Google Scholar]
- 63.Noto D., Cefalù A.B., Averna M.R. Beyond statins: New lipid lowering strategies to reduce cardiovascular risk. Curr. Atheroscler. Rep. 2014;16:414. doi: 10.1007/s11883-014-0414-4. [DOI] [PubMed] [Google Scholar]
- 64.Insull W., Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: A scientific review. South. Med. J. 2006;99:257–274. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- 65.Feng X., Zhang L., Xu S., Shen A.-Z. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog. Lipid Res. 2020;77:101006. doi: 10.1016/j.plipres.2019.101006. [DOI] [PubMed] [Google Scholar]
- 66.Vaidya S., Bostedor R., Kurtz M.M., Bergstrom J.D., Bansal V.S. Massive production of farnesol-derived dicarboxylic acids in mice treated with the squalene synthase inhibitor zaragozic acid A. Arch. Biochem. Biophys. 1998;355:84–92. doi: 10.1006/abbi.1998.0704. [DOI] [PubMed] [Google Scholar]
- 67.Al-Dury S., Wahlström A., Wahlin S., Langedijk J., Elferink R.O., Ståhlman M., Marschall H.-U. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci. Rep. 2018;8:6658. doi: 10.1038/s41598-018-25214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu M., Xu K., Guo Y., Yu J., Wu Y., Lin L. Lipoprotein (a) and atherosclerotic cardiovascular disease: Current understanding and future perspectives. Cardiovasc. Drugs Ther. 2019;33:739–748. doi: 10.1007/s10557-019-06906-9. [DOI] [PubMed] [Google Scholar]
- 69.Ward N.C., Watts G.F., Eckel R.H. Statin toxicity: Mechanistic insights and clinical implications. Circ. Res. 2019;124:328–350. doi: 10.1161/CIRCRESAHA.118.312782. [DOI] [PubMed] [Google Scholar]
- 70.Neef D., Berthold H.K., Gouni-Berthold I. Lomitapide for use in patients with homozygous familial hypercholesterolemia: A narrative review. Expert Rev. Clin. Pharmacol. 2016;9:655–663. doi: 10.1586/17512433.2016.1162095. [DOI] [PubMed] [Google Scholar]
- 71.Gouni-Berthold I., Berthold H.K. Mipomersen and lomitapide: Two new drugs for the treatment of homozygous familial hypercholesterolemia. Atheroscler. Suppl. 2015;18:28–34. doi: 10.1016/j.atherosclerosissup.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Aldridge M.A., Ito M.K. Colesevelam hydrochloride: A novel bile acid-binding resin. Ann. Pharmacother. 2001;35:898–907. doi: 10.1345/aph.10263. [DOI] [PubMed] [Google Scholar]
- 73.Stein E.A., Bays H., O’Brien D., Pedicano J., Piper E., Spezzi A. Lapaquistat acetate: Development of a squalene synthase inhibitor for the treatment of hypercholesterolemia. Circulation. 2011;123:1974–1985. doi: 10.1161/CIRCULATIONAHA.110.975284. [DOI] [PubMed] [Google Scholar]
- 74.Kim Y., Keogh J.B., Clifton P.M. Polyphenols and glycemic control. Nutrients. 2016;8:17. doi: 10.3390/nu8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suh J.-H., Virsolvy A., Goux A., Cassan C., Richard S., Cristol J.-P., Teissedre P.-L., Rouanet J.-M. Polyphenols prevent lipid abnormalities and arterial dysfunction in hamsters on a high-fat diet: A comparative study of red grape and white persimmon wines. Food Funct. 2011;2:555–561. doi: 10.1039/c1fo10066a. [DOI] [PubMed] [Google Scholar]
- 76.Wu A.H., Spicer D., Stanczyk F.Z., Tseng C.-C., Yang C.S., Pike M.C. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev. Res. 2012;5:393–402. doi: 10.1158/1940-6207.CAPR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson S.A., Navaei N., Pourafshar S., Jaime S.J., Akhavan N.S., Alvarez-Alvarado S., Proano G.V., Litwin N.S., Clark E.A., Foley E.M., et al. Effects of Montmorency Tart Cherry Juice Consumption on Cardiometabolic Biomarkers in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Trial. J. Med. Food. 2020;23:1238–1247. doi: 10.1089/jmf.2019.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z., Tu Z., Xie X., Cui H., Kong K.W., Zhang L. Perilla frutescens Leaf Extract and Fractions: Polyphenol Composition, Antioxidant, Enzymes (alpha-Glucosidase, Acetylcholinesterase, and Tyrosinase) Inhibitory, Anticancer, and Antidiabetic Activities. Foods. 2021;10:315. doi: 10.3390/foods10020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li W., Yang C., Mei X., Huang R., Zhang S., Tang Y., Dong Q., Zhou C. Effect of the polyphenol-rich extract from Allium cepa on hyperlipidemic sprague-dawley rats. J. Food Biochem. 2021;45:e13565. doi: 10.1111/jfbc.13565. [DOI] [PubMed] [Google Scholar]
- 80.Yousefi R., Parandoosh M., Khorsandi H., Hosseinzadeh N., Tonekaboni M.M., Saidpour A., Babaei H., Ghorbani A. Grape seed extract supplementation along with a restricted-calorie diet improves cardiovascular risk factors in obese or overweight adult individuals: A randomized, placebo-controlled trial. Phytother. Res. 2021;35:987–995. doi: 10.1002/ptr.6859. [DOI] [PubMed] [Google Scholar]
- 81.Sun J., Wang Z., Chen L., Sun G. Hypolipidemic Effects and Preliminary Mechanism of Chrysanthemum Flavonoids, Its Main Components Luteolin and Luteoloside in Hyperlipidemia Rats. Antioxidants. 2021;10:1309. doi: 10.3390/antiox10081309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Islam M.S. Effects of the aqueous extract of white tea (Camellia sinensis) in a streptozotocin-induced diabetes model of rats. Phytomedicine. 2011;19:25–31. doi: 10.1016/j.phymed.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 83.Bornhoeft J., Castaneda D., Nemoseck T., Wang P., Henning S.M., Hong M.Y. The protective effects of green tea polyphenols: Lipid profile, inflammation, and antioxidant capacity in rats fed an atherogenic diet and dextran sodium sulfate. J. Med. Food. 2012;15:726–732. doi: 10.1089/jmf.2011.0258. [DOI] [PubMed] [Google Scholar]
- 84.Li Q., Liu Z., Huang J., Luo G., Liang Q., Wang D., Ye X., Wu C., Wang L., Hu J. Anti-obesity and hypolipidemic effects of Fuzhuan brick tea water extract in high-fat diet-induced obese rats. J. Sci. Food Agric. 2013;93:1310–1316. doi: 10.1002/jsfa.5887. [DOI] [PubMed] [Google Scholar]
- 85.Bellassoued K., Ghrab F., Makni-Ayadi F., Pelt J.V., Elfeki A., Ammar E. Protective effect of kombucha on rats fed a hypercholesterolemic diet is mediated by its antioxidant activity. Pharm. Biol. 2015;53:1699–1709. doi: 10.3109/13880209.2014.1001408. [DOI] [PubMed] [Google Scholar]
- 86.Zhu Z., Lin Z., Jiang H., Jiang Y., Zhao M., Liu X. Hypolipidemic effect of Youcha in hyperlipidemia rats induced by high-fat diet. Food Funct. 2017;8:1680–1687. doi: 10.1039/C7FO00089H. [DOI] [PubMed] [Google Scholar]
- 87.Guruvaiah P., Guo H., Li D., Xie Z. Preventive effect of flavonol derivatives abundant sanglan tea on long-term high-fat-diet-induced obesity complications in c57bl/6 mice. Nutrients. 2018;10:1276. doi: 10.3390/nu10091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin R., He X., Chen H., He Q., Yao Z., Li Y., Yang H., Simpson S., Jr. Oil tea improves glucose and lipid levels and alters gut microbiota in type 2 diabetic mice. Nutr. Res. 2018;57:67–77. doi: 10.1016/j.nutres.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 89.Liu B., Zhang J., Sun P., Yi R., Han X., Zhao X. Raw Bowl Tea (Tuocha) Polyphenol Prevention of Nonalcoholic Fatty Liver Disease by Regulating Intestinal Function in Mice. Biomolecules. 2019;9:435. doi: 10.3390/biom9090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhai X., Chi J., Tang W., Ji Z., Zhao F., Jiang C., Lv H., Guo H. Yellow wine polyphenolic compounds inhibit matrix metalloproteinase-2,-9 expression and improve atherosclerotic plaque in LDL-receptor–knockout mice. J. Pharmacol. Sci. 2014;125:132–141. doi: 10.1254/jphs.13263FP. [DOI] [PubMed] [Google Scholar]
- 91.Peng C.-H., Liu L.-K., Chuang C.-M., Chyau C.-C., Huang C.-N., Wang C.-J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011;59:2663–2671. doi: 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]
- 92.Suh J.-H., Romain C., González-Barrio R., Cristol J.-P., Teissèdre P.-L., Crozier A., Rouanet J.-M. Raspberry juice consumption, oxidative stress and reduction of atherosclerosis risk factors in hypercholesterolemic golden Syrian hamsters. Food Funct. 2011;2:400–405. doi: 10.1039/c1fo10047e. [DOI] [PubMed] [Google Scholar]
- 93.Liu S., You L., Zhao Y., Chang X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res. Int. 2018;107:73–83. doi: 10.1016/j.foodres.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y., Zhang X., Zhan L., Xu C., Sun L., Jiang H., Sun C., Li X. LC-Q-TOF-MS Characterization of Polyphenols from White Bayberry Fruit and Its Antidiabetic Effect in KK-A(y) Mice. ACS Omega. 2020;5:17839–17849. doi: 10.1021/acsomega.0c02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leontowicz M., Jesion I., Leontowicz H., Park Y.-S., Namiesnik J., Rombolà A.D., Weisz M., Gorinstein S. Health-promoting effects of ethylene-treated kiwifruit ‘Hayward’ from conventional and organic crops in rats fed an atherogenic diet. J. Agric. Food Chem. 2013;61:3661–3668. doi: 10.1021/jf400165k. [DOI] [PubMed] [Google Scholar]
- 96.Lim C.Y., Junit S.M., Abdulla M.A., Aziz A.A. In vivo biochemical and gene expression analyses of the antioxidant activities and hypocholesterolaemic properties of Tamarindus indica fruit pulp extract. PLoS ONE. 2013;8:e70058. doi: 10.1371/journal.pone.0070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Z.-R., Li J.-Y., Dong X.-W., Tan Z.-J., Wu W.-Z., Xie Q.-M., Yang Y.-M. Apple polyphenols decrease atherosclerosis and hepatic steatosis in ApoE−/− mice through the ROS/MAPK/NF-κB pathway. Nutrients. 2015;7:7085–7105. doi: 10.3390/nu7085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fathy S.M., Drees E.A. Protective effects of Egyptian cloudy apple juice and apple peel extract on lipid peroxidation, antioxidant enzymes and inflammatory status in diabetic rat pancreas. BMC Complement. Med. Ther. 2015;16:8. doi: 10.1186/s12906-015-0957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han X., Li W., Huang D., Yang X. Polyphenols from hawthorn peels and fleshes differently mitigate dyslipidemia, inflammation and oxidative stress in association with modulation of liver injury in high fructose diet-fed mice. Chem. Biol. Interact. 2016;257:132–140. doi: 10.1016/j.cbi.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Alqarni M.M.M., Osman M.A., Al-Tamimi D.S., Gassem M.A., Al-Khalifa A.S., Al-Juhaimi F., Ahmed I.A.M. Antioxidant and antihyperlipidemic effects of Ajwa date (Phoenix dactylifera L.) extracts in rats fed a cholesterol-rich diet. J. Food Biochem. 2019;43:e12933. doi: 10.1111/jfbc.12933. [DOI] [PubMed] [Google Scholar]
- 101.Peng C.-H., Chyau C.-C., Chan K.-C., Chan T.-H., Wang C.-J., Huang C.-N. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J. Agric. Food Chem. 2011;59:9901–9909. doi: 10.1021/jf2022379. [DOI] [PubMed] [Google Scholar]
- 102.Kao E.-S., Yang M.-Y., Hung C.-H., Huang C.-N., Wang C.-J. Polyphenolic extract from Hibiscus sabdariffa reduces body fat by inhibiting hepatic lipogenesis and preadipocyte adipogenesis. Food Funct. 2016;7:171–182. doi: 10.1039/C5FO00714C. [DOI] [PubMed] [Google Scholar]
- 103.Daleprane J.B., Freitas V.d.S., Pacheco A., Rudnicki M., Faine L.A., Dörr F.A., Ikegaki M., Salazar L.A., Ong T.P., Abdalla D.S.P. Anti-atherogenic and anti-angiogenic activities of polyphenols from propolis. J. Nutr. Biochem. 2012;23:557–566. doi: 10.1016/j.jnutbio.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 104.Moniruzzaman M., Rokeya B., Ahmed S., Bhowmik A., Khalil M., Gan S.H. In vitro antioxidant effects of Aloe barbadensis Miller extracts and the potential role of these extracts as antidiabetic and antilipidemic agents on streptozotocin-induced type 2 diabetic model rats. Molecules. 2012;17:12851–12867. doi: 10.3390/molecules171112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L., Sun J., Yi Q., Wang X., Ju X. Protective effect of polyphenols extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on hypercholesterolemia-induced oxidative stress in rats. Molecules. 2012;17:8886–8897. doi: 10.3390/molecules17088886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Musolino V., Gliozzi M., Scarano F., Bosco F., Scicchitano M., Nucera S., Carresi C., Ruga S., Zito M.C., Maiuolo J., et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020;10:2565. doi: 10.1038/s41598-020-59485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sinaga E., Suprihatin, Yenisbar, Iswahyudi M., Setyowati S., Prasasty V.D. Effect of supplementation of Rhodomyrtus tomentosa fruit juice in preventing hypercholesterolemia and atherosclerosis development in rats fed with fat cholesterol diet. Biomed. Pharmacother. 2021;142:111996. doi: 10.1016/j.biopha.2021.111996. [DOI] [PubMed] [Google Scholar]
- 108.Wang Z., Li X.-L., Hong K.-F., Zhao T.-T., Dong R.-X., Wang W.-M., Li Y.-T., Zhang G.-L., Lin J., Gui D.-K., et al. Total flavonoids of Astragalus Ameliorated Bile Acid Metabolism Dysfunction in Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2021;2021:6675567. doi: 10.1155/2021/6675567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang M., Wang R., Li L., Yan Y., Jia S., Jiang H., Du Z. Quantitative proteomics of plasma and liver reveals the mechanism of turmeric in preventing hyperlipidemia in mice. Food Funct. 2021;12:10484–10499. doi: 10.1039/D1FO01849C. [DOI] [PubMed] [Google Scholar]
- 110.Feriani A., Tir M., Arafah M., Gomez-Caravaca A.M., Contreras M.d.M., Nahdi S., Taamalli A., Allagui M.S., Alwasel S., Segura-Carretero A., et al. Schinus terebinthifolius fruits intake ameliorates metabolic disorders, inflammation, oxidative stress, and related vascular dysfunction, in atherogenic diet-induced obese rats. Insight of their chemical characterization using HPLC-ESI-QTOF-MS/MS. J. Ethnopharmacol. 2021;269:113701. doi: 10.1016/j.jep.2020.113701. [DOI] [PubMed] [Google Scholar]
- 111.Chen I.-J., Liu C.-Y., Chiu J.-P., Hsu C.-H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016;35:592–599. doi: 10.1016/j.clnu.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 112.Lee T.-M., Charng M.-J., Tseng C.-D., Lai L.-P. A Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of STA-2 (Green Tea Polyphenols) in Patients with Chronic Stable Angina. Acta Cardiol. Sin. 2016;32:439–449. doi: 10.6515/acs20150708d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quezada-Fernandez P., Trujillo-Quiros J., Pascoe-Gonzalez S., Trujillo-Rangel W.A., Cardona-Mueller D., Ramos-Becerra C.G., Barocio-Pantoja M., Rodriguez-de la Cerda M., Nerida Sanchez-Rodriguez E., Cardona-Munoz E.G., et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2019;70:977–985. doi: 10.1080/09637486.2019.1589430. [DOI] [PubMed] [Google Scholar]
- 114.Ishida N., Iizuka M., Kataoka K., Okazaki M., Shiraishi K., Yagi Y., Jobu K., Yokota J., Oishi M., Moriyama H. Improvement of blood lipid profiles by goishi tea polyphenols in a randomised, double-blind, placebo-controlled clinical study. Int. J. Food Sci. Nutr. 2018;69:598–607. doi: 10.1080/09637486.2017.1386629. [DOI] [PubMed] [Google Scholar]
- 115.Kitada M., Ogura Y., Monno I., Koya D. Supplementation with Red Wine Extract Increases Insulin Sensitivity and Peripheral Blood Mononuclear Sirt1 Expression in Nondiabetic Humans. Nutrients. 2020;12:3108. doi: 10.3390/nu12103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie L., Vance T., Kim B., Lee S.G., Caceres C., Wang Y., Hubert P.A., Lee J.-Y., Chun O.K., Bolling B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017;37:67–77. doi: 10.1016/j.nutres.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 117.Stote K.S., Wilson M.M., Hallenbeck D., Thomas K., Rourke J.M., Sweeney M.I., Gottschall-Pass K.T., Gosmanov A.R. Effect of Blueberry Consumption on Cardiometabolic Health Parameters in Men with Type 2 Diabetes: An 8-Week, Double-Blind, Randomized, Placebo-Controlled Trial. Curr. Dev. Nutr. 2020;4:nzaa030. doi: 10.1093/cdn/nzaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yubero N., Sanz-Buenhombre M., Guadarrama A., Villanueva S., Carrión J.M., Larrarte E., Moro C. LDL cholesterol-lowering effects of grape extract used as a dietary supplement on healthy volunteers. Int. J. Food Sci. Nutr. 2013;64:400–406. doi: 10.3109/09637486.2012.753040. [DOI] [PubMed] [Google Scholar]
- 119.Rahbar A.R., Mahmoudabadi M.M.S., Islam M.S. Comparative effects of red and white grapes on oxidative markers and lipidemic parameters in adult hypercholesterolemic humans. Food Funct. 2015;6:1992–1998. doi: 10.1039/C5FO00100E. [DOI] [PubMed] [Google Scholar]
- 120.Sanchez Macarro M., Martinez Rodriguez J.P., Bernal Morell E., Perez-Pinero S., Victoria-Montesinos D., Maria Garcia-Munoz A., Canovas Garcia F., Castillo Sanchez J., Javier Lopez-Roman F. Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients. 2020;12:1475. doi: 10.3390/nu12051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Filip R., Possemiers S., Heyerick A., Pinheiro I., Raszewski G., Davicco M.-J., Coxam V. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J. Nutr. Health Aging. 2015;19:77–86. doi: 10.1007/s12603-014-0480-x. [DOI] [PubMed] [Google Scholar]
- 122.Georgakouli K., Mpesios A., Kouretas D., Petrotos K., Mitsagga C., Giavasis I., Jamurtas A.Z. The effects of an olive fruit polyphenol-enriched yogurt on body composition, blood redox status, physiological and metabolic parameters and yogurt microflora. Nutrients. 2016;8:344. doi: 10.3390/nu8060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hosseini B., Saedisomeolia A., Wood L.G., Yaseri M., Tavasoli S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016;22:44–50. doi: 10.1016/j.ctcp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Sohrab G., Roshan H., Ebrahimof S., Nikpayam O., Sotoudeh G., Siasi F. Effects of pomegranate juice consumption on blood pressure and lipid profile in patients with type 2 diabetes: A single-blind randomized clinical trial. Clin. Nutr. ESPEN. 2019;29:30–35. doi: 10.1016/j.clnesp.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 125.Chai S.C., Davis K., Wright R.S., Kuczmarski M.F., Zhang Z. Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: A randomized controlled trial. Food Funct. 2018;9:3185–3194. doi: 10.1039/C8FO00468D. [DOI] [PubMed] [Google Scholar]
- 126.Shin H.C., Kim S.H., Park Y., Lee B.H., Hwang H.J. Effects of 12-week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean individuals: A double-blind randomized clinical trial. Phytother. Res. 2012;26:363–368. doi: 10.1002/ptr.3559. [DOI] [PubMed] [Google Scholar]
- 127.Boots A.W., Haenen G.R.M.M., Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 128.Valentova K., Vrba J., Bancirova M., Ulrichova J., Kren V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014;68:267–282. doi: 10.1016/j.fct.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 129.Salvamani S., Gunasekaran B., Shaharuddin N.A., Ahmad S.A., Shukor M.Y. Antiartherosclerotic Effects of Plant Flavonoids. Biomed. Res. Int. 2014;2014:48058. doi: 10.1155/2014/480258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nickel T., Hanssen H., Sisic Z., Pfeiler S., Summo C., Schmauss D., Hoster E., Weis M. Immunoregulatory effects of the flavonol quercetin in vitro and in vivo. Eur. J. Nutr. 2011;50:163–172. doi: 10.1007/s00394-010-0125-8. [DOI] [PubMed] [Google Scholar]
- 131.Jeong S.-M., Kang M.-J., Choi H.-N., Kim J.-H., Kim J.-I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012;6:201–207. doi: 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ying H.-Z., Liu Y.-H., Yu B., Wang Z.-Y., Zang J.-N., Yu C.-H. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 2013;52:53–60. doi: 10.1016/j.fct.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 133.Li S.-S., Cao H., Shen D.-Z., Chen C., Xing S.-L., Dou F.-F., Jia Q.-L. Effect of Quercetin on Atherosclerosis Based on Expressions of ABCA1, LXR-alpha and PCSK9 in ApoE(-/-) Mice. Chin. J. Integr. Med. 2020;26:114–121. doi: 10.1007/s11655-019-2942-9. [DOI] [PubMed] [Google Scholar]
- 134.Mbikay M., Mayne J., Sirois F., Fedoryak O., Raymond A., Noad J., Chretien M. Mice Fed a High-Cholesterol Diet Supplemented with Quercetin-3-Glucoside Show Attenuated Hyperlipidemia and Hyperinsulinemia Associated with Differential Regulation of PCSK9 and LDLR in their Liver and Pancreas. Mol. Nutr. Food Res. 2018;62:e1700729. doi: 10.1002/mnfr.201700729. [DOI] [PubMed] [Google Scholar]
- 135.Lu T.-M., Chiu H.-F., Shen Y.-C., Chung C.-C., Venkatakrishnan K., Wang C.-K. Hypocholesterolemic Efficacy of Quercetin Rich Onion Juice in Healthy Mild Hypercholesterolemic Adults: A Pilot Study. Plant Foods Hum. Nutr. 2015;70:395–400. doi: 10.1007/s11130-015-0507-4. [DOI] [PubMed] [Google Scholar]
- 136.Nekohashi M., Ogawa M., Ogihara T., Nakazawa K., Kato H., Misaka T., Abe K., Kobayashi S. Luteolin and Quercetin Affect the Cholesterol Absorption Mediated by Epithelial Cholesterol Transporter Niemann-Pick C1-Like 1 in Caco-2 Cells and Rats. PLoS ONE. 2014;9:e97901. doi: 10.1371/journal.pone.0097901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moon J., Lee S.-M., Do H.J., Cho Y., Chung J.H., Shin M.-J. Quercetin Up-regulates LDL Receptor Expression in HepG2 Cells. Phytother. Res. 2012;26:1688–1694. doi: 10.1002/ptr.4646. [DOI] [PubMed] [Google Scholar]
- 138.Mbikay M., Sirois F., Simoes S., Mayne J., Chretien M. Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture. FEBS Open Bio. 2014;4:755–762. doi: 10.1016/j.fob.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang M., Xie Z., Gao W., Pu L., Wei J., Guo C. Quercetin regulates hepatic cholesterol metabolism by promoting cholesterol-to-bile acid conversion and cholesterol efflux in rats. Nutr. Res. 2016;36:271–279. doi: 10.1016/j.nutres.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 140.Eng Q.Y., Thanikachalam P.V., Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018;210:296–310. doi: 10.1016/j.jep.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 141.Moon H.-S., Chung C.-S., Lee H.-G., Kim T.-G., Choi Y.-J., Cho C.-S. Inhibitory effect of (-)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity. 2007;15:2571–2582. doi: 10.1038/oby.2007.309. [DOI] [PubMed] [Google Scholar]
- 142.Guo Q.N., Zhao B.L., Li M.F., Shen S.R., Xin W.J. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 1996;1304:210–222. doi: 10.1016/S0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 143.Khalatbary A.R., Ahmadvand H. Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran. Biomed. J. 2011;15:31–37. [PMC free article] [PubMed] [Google Scholar]
- 144.Ren Z., Yang Z., Lu Y., Zhang R., Yang H. Anti-glycolipid disorder effect of epigallocatechin-3-gallate on high-fat diet and STZ-induced T2DM in mice. Mol. Med. Rep. 2020;21:2475–2483. doi: 10.3892/mmr.2020.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hirsova P., Kolouchova G., Dolezelova E., Cermanova J., Hyspler R., Kadova Z., Micuda S. Epigallocatechin gallate enhances biliary cholesterol secretion in healthy rats and lowers plasma and liver cholesterol in ethinylestradiol-treated rats. Eur. J. Pharmacol. 2012;691:38–45. doi: 10.1016/j.ejphar.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 146.Li Y., Wu S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol. Cell. Biochem. 2018;448:175–185. doi: 10.1007/s11010-018-3324-x. [DOI] [PubMed] [Google Scholar]
- 147.Cui C.-J., Jin J.-L., Guo L.-N., Sun J., Wu N.-Q., Guo Y.-L., Liu G., Dong Q., Li J.-J. Beneficial impact of epigallocatechingallate on LDL-C through PCSK9/LDLR pathway by blocking HNF1α and activating FoxO3a. J. Transl. Med. 2020;18:195. doi: 10.1186/s12967-020-02362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mielgo-Ayuso J., Barrenechea L., Alcorta P., Larrarte E., Margareto J., Labayen I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014;111:1263–1271. doi: 10.1017/S0007114513003784. [DOI] [PubMed] [Google Scholar]
- 149.Brown A.L., Lane J., Coverly J., Stocks J., Jackson S., Stephen A., Bluck L., Coward A., Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Momose Y., Maeda-Yamamoto M., Nabetani H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int. J. Food Sci. Nutr. 2016;67:606–613. doi: 10.1080/09637486.2016.1196655. [DOI] [PubMed] [Google Scholar]
- 151.Huang J., Feng S., Liu A., Dai Z., Wang H., Reuhl K., Lu W., Yang C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018;62:1700696. doi: 10.1002/mnfr.201700696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Raederstorff D.G., Schlachter M.F., Elste V., Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003;14:326–332. doi: 10.1016/S0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 153.Kitamura K., Okada Y., Okada K., Kawaguchi Y., Nagaoka S. Epigallocatechin gallate induces an up-regulation of LDL receptor accompanied by a reduction of PCSK9 via the annexin A2-independent pathway in HepG2 cells. Mol. Nutr. Food Res. 2017;61:1600836. doi: 10.1002/mnfr.201600836. [DOI] [PubMed] [Google Scholar]
- 154.Poirier S., Mayer G., Benjannet S., Bergeron E., Marcinkiewicz J., Nassoury N., Mayer H., Nimpf J., Prat A., Seidah N.G. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 2008;283:2363–2372. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]
- 155.Kuhn D.J., Burns A.C., Kazi A., Dou Q.P. Direct inhibition of the ubiquitin-proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2004;1682:1–10. doi: 10.1016/j.bbalip.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 156.He J., Giusti M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y., Zhao L., Wang D., Huo Y., Ji B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: Antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J. Sci. Food Agric. 2016;96:2494–2503. doi: 10.1002/jsfa.7370. [DOI] [PubMed] [Google Scholar]
- 158.Hassellund S.S., Flaa A., Kjeldsen S.E., Seljeflot I., Karlsen A., Erlund I., Rostrup M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: A double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2013;27:100–106. doi: 10.1038/jhh.2012.4. [DOI] [PubMed] [Google Scholar]
- 159.Aboonabi A., Meyer R.R., Gaiz A., Singh I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020;76:82–93. doi: 10.1016/j.nutres.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 160.Xia M., Hou M., Zhu H., Ma J., Tang Z., Wang Q., Li Y., Chi D., Yu X., Zhao T., et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: The role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J. Biol. Chem. 2005;280:36792–36801. doi: 10.1074/jbc.M505047200. [DOI] [PubMed] [Google Scholar]
- 161.Kianbakht S., Abasi B., Dabaghian F.H. Improved Lipid Profile in Hyperlipidemic Patients Taking Vaccinium arctostaphylos Fruit Hydroalcoholic Extract: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytother. Res. 2014;28:432–436. doi: 10.1002/ptr.5011. [DOI] [PubMed] [Google Scholar]
- 162.Qin Y., Xia M., Ma J., Hao Y., Liu J., Mou H., Cao L., Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009;90:485–492. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- 163.Soltani R., Hakimi M., Asgary S., Ghanadian S.M., Keshvari M., Sarrafzadegan N. Evaluation of the Effects of Vaccinium arctostaphylos L. Fruit Extract on Serum Lipids and hs-CRP Levels and Oxidative Stress in Adult Patients with Hyperlipidemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Evid. Based Complement. Altern. Med. 2014;2014:217451. doi: 10.1155/2014/217451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu Y., Ling W., Guo H., Song F., Ye Q., Zou T., Li D., Zhang Y., Li G., Xiao Y., et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013;23:843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 165.Bakuradze T., Tausend A., Galan J., Groh I.A.M., Berry D., Tur J.A., Marko D., Richling E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019;53:1045–1055. doi: 10.1080/10715762.2019.1618851. [DOI] [PubMed] [Google Scholar]
- 166.Zhang P.-W., Chen F.-X., Li D., Ling W.-H., Guo H.-H. A CONSORT-Compliant, Randomized, Double-Blind, Placebo-Controlled Pilot Trial of Purified Anthocyanin in Patients with Nonalcoholic Fatty Liver Disease. Medicine. 2015;94:e758. doi: 10.1097/MD.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang L., Ling W., Du Z., Chen Y., Li D., Deng S., Liu Z., Yang L. Effects of Anthocyanins on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2017;8:684–693. doi: 10.3945/an.116.014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu C., Sun J., Lu Y., Bo Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0162089. doi: 10.1371/journal.pone.0162089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liang Y., Chen J., Zuo Y., Ma K.Y., Jiang Y., Huang Y., Chen Z.-Y. Blueberry anthocyanins at doses of 0.5 and 1 % lowered plasma cholesterol by increasing fecal excretion of acidic and neutral sterols in hamsters fed a cholesterol-enriched diet. Eur. J. Nutr. 2013;52:869–875. doi: 10.1007/s00394-012-0393-6. [DOI] [PubMed] [Google Scholar]
- 170.Wang L., Zhu H., Zhao Y., Jiao R., Lei L., Chen J., Wang X., Zhang Z., Huang Y., Wang T., et al. Cranberry anthocyanin as an herbal medicine lowers plasma cholesterol by increasing excretion of fecal sterols. Phytomedicine. 2018;38:98–106. doi: 10.1016/j.phymed.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 171.Thilavech T., Adisakwattana S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement. Med. Ther. 2019;19:242. doi: 10.1186/s12906-019-2664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yao S.-L., Xu Y., Zhang Y.-Y., Lu Y.-H. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. 2013;4:1602–1608. doi: 10.1039/c3fo60196j. [DOI] [PubMed] [Google Scholar]
- 173.Wang D., Xia M., Gao S., Li D., Zhang Y., Jin T., Ling W. Cyanidin-3-O-β-glucoside upregulates hepatic cholesterol 7α-hydroxylase expression and reduces hypercholesterolemia in mice. Mol. Nutr. Food Res. 2012;56:610–621. doi: 10.1002/mnfr.201100659. [DOI] [PubMed] [Google Scholar]
- 174.Aron P.M., Kennedy J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008;52:79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 175.De la Iglesia R., Milagro F.I., Campión J., Boqué N., Martínez J.A. Healthy properties of proanthocyanidins. BioFactors. 2010;36:159–168. doi: 10.1002/biof.79. [DOI] [PubMed] [Google Scholar]
- 176.Cao J., Yu X., Deng Z., Pan Y., Zhang B., Tsao R., Li H. Chemical Compositions, Antiobesity, and Antioxidant Effects of Proanthocyanidins from Lotus Seed Epicarp and Lotus Seed Pot. J. Agric. Food Chem. 2018;66:13492–13502. doi: 10.1021/acs.jafc.8b05137. [DOI] [PubMed] [Google Scholar]
- 177.Zhou Q., Han X., Li R., Zhao W., Bai B., Yan C., Dong X. Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomedicine Int. J. Phytother. Phytopharm. 2018;51:171–180. doi: 10.1016/j.phymed.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 178.Wang Z., Su B., Fan S., Fei H., Zhao W. Protective effect of oligomeric proanthocyanidins against alcohol-induced liver steatosis and injury in mice. Biochem. Biophys. Res. Commun. 2015;458:757–762. doi: 10.1016/j.bbrc.2015.01.153. [DOI] [PubMed] [Google Scholar]
- 179.Jiao R., Zhang Z., Yu H., Huang Y., Chen Z.-Y. Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and up-regulation of CYP7A1. J. Nutr. Biochem. 2010;21:1134–1139. doi: 10.1016/j.jnutbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 180.Yasuda A., Natsume M., Sasaki K., Baba S., Nakamura Y., Kanegae M., Nagaoka S. Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet. BioFactors. 2008;33:211–223. doi: 10.1002/biof.5520330307. [DOI] [PubMed] [Google Scholar]
- 181.Heidker R.M., Caiozzi G.C., Ricketts M.-L. Dietary procyanidins selectively modulate intestinal farnesoid X receptor-regulated gene expression to alter enterohepatic bile acid recirculation: Elucidation of a novel mechanism to reduce triglyceridemia. Mol. Nutr. Food Res. 2016;60:727–736. doi: 10.1002/mnfr.201500795. [DOI] [PubMed] [Google Scholar]
- 182.Quesada H., del Bas J.M., Pajuelo D., Diaz S., Fernandez-Larrea J., Pinent M., Arola L., Salvado M.J., Blade C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. 2009;33:1007–1012. doi: 10.1038/ijo.2009.136. [DOI] [PubMed] [Google Scholar]
- 183.Prasad S., Gupta S.C., Tyagi A.K., Aggarwal B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014;32:1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 184.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Panahi Y., Kianpour P., Mohtashami R., Jafari R., Simental-Mendía L.E., Sahebkar A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016;68:223–229. doi: 10.1097/FJC.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 186.Feng D., Zou J., Zhang S., Li X., Lu M. Hypocholesterolemic Activity of Curcumin Is Mediated by Down-regulating the Expression of Niemann-Pick C1-like 1 in Hamsters. J. Agric. Food Chem. 2017;65:276–280. doi: 10.1021/acs.jafc.6b04102. [DOI] [PubMed] [Google Scholar]
- 187.Zou J., Zhang S., Li P., Zheng X., Feng D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018;56:32–40. doi: 10.1016/j.nutres.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 188.Kim M., Kim Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010;4:191–195. doi: 10.4162/nrp.2010.4.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Momtazi-Borojeni A.A., Zabihi N.A., Bagheri R.K., Majeed M., Jamialahmadi T., Sahebkar A. Intravenous Curcumin Mitigates Atherosclerosis Progression in Cholesterol-Fed Rabbits. In: Barreto G.E., Sahebkar A., editors. Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health. Volume 1308. Springer; Berlin/Heidelberg, Germany: 2021. pp. 45–54. [DOI] [PubMed] [Google Scholar]
- 190.Labban R.S.M., Alfawaz H.A., Almnaizel A.T., Al-Muammar M.N., Bhat R.S., El-Ansary A. Garcinia mangostana extract and curcumin ameliorate oxidative stress, dyslipidemia, and hyperglycemia in high fat diet-induced obese Wistar albino rats. Sci. Rep. 2021;11:7278. doi: 10.1038/s41598-021-86545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jamilian M., Foroozanfard F., Kavossian E., Aghadavod E., Shafabakhsh R., Hoseini A., Asemi Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN. 2020;36:128–133. doi: 10.1016/j.clnesp.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 192.Jalali M., Mahmoodi M., Mosallanezhad Z., Jalali R., Imanieh M.H., Moosavian S.P. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020;48:102283. doi: 10.1016/j.ctim.2019.102283. [DOI] [PubMed] [Google Scholar]
- 193.Funamoto M., Sunagawa Y., Katanasaka Y., Miyazaki Y., Imaizumi A., Kakeya H., Yamakage H., Satoh-Asahara N., Komiyama M., Wada H., et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:2029–2034. doi: 10.2147/COPD.S104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Adibian M., Hodaei H., Nikpayam O., Sohrab G., Hekmatdoost A., Hedayati M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019;33:1374–1383. doi: 10.1002/ptr.6328. [DOI] [PubMed] [Google Scholar]
- 195.Feng D., Ohlsson L., Duan R.-D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis. 2010;9:40. doi: 10.1186/1476-511X-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fan C., Wo X., Dou X., Xu L., Qian Y., Luo Y., Yan J. Regulation of LDL receptor expression by the effect of curcumin on sterol regulatory element pathway. Pharmacol. Rep. 2006;58:577–581. [PubMed] [Google Scholar]
- 197.Dou X., Fan C., Wo L., Yan J., Qian Y., Wo X. Curcumin Up-Regulates LDL Receptor Expression via the Sterol Regulatory Element Pathway in HepG2 Cells. Planta Med. 2008;74:1374–1379. doi: 10.1055/s-2008-1081316. [DOI] [PubMed] [Google Scholar]
- 198.Tai M.-H., Chen P.-K., Chen P.-Y., Wu M.-J., Ho C.-T., Yen J.-H. Curcumin enhances cell-surface LDLR level and promotes LDL uptake through downregulation of PCSK9 gene expression in HepG2 cells. Mol. Nutr. Food Res. 2014;58:2133–2145. doi: 10.1002/mnfr.201400366. [DOI] [PubMed] [Google Scholar]
- 199.Cai Y., Lu D., Zou Y., Zhou C., Liu H., Tu C., Li F., Liu L., Zhang S. Curcumin Protects Against Intestinal Origin Endotoxemia in Rat Liver Cirrhosis by Targeting PCSK9. J. Food Sci. 2017;82:772–780. doi: 10.1111/1750-3841.13647. [DOI] [PubMed] [Google Scholar]
- 200.Tian N., Li X., Luo Y., Han Z., Li Z., Fan C. Curcumin regulates the metabolism of low density lipoproteins by improving the C-to-U RNA editing efficiency of apolipoprotein B in primary rat hepatocytes. Mol. Med. Rep. 2014;9:132–136. doi: 10.3892/mmr.2013.1754. [DOI] [PubMed] [Google Scholar]
- 201.Hussain H., Green I.R. A patent review of the therapeutic potential of isoflavones (2012–2016) Expert Opin. Ther. Pat. 2017;27:1135–1146. doi: 10.1080/13543776.2017.1339791. [DOI] [PubMed] [Google Scholar]
- 202.Mazumder M.A.R., Hongsprabhas P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed. Pharmacother. 2016;82:379–392. doi: 10.1016/j.biopha.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 203.Usui T. Pharmaceutical prospects of phytoestrogens. Endocr. J. 2006;53:7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- 204.Behloul N., Wu G. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013;698:31–38. doi: 10.1016/j.ejphar.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 205.Yousefinejad A., Siassi F., Javanbakht M.H., Mohammadi H., Ghaedi E., Zarei M., Djalali E., Djalali M. Effect of Genistein and L-carnitine and Their Combination on Lipid Profile and Inflammatory Cytokines in Experimental Nephrotic Syndrome. Rep. Biochem. Mol. Biol. 2018;7:1–8. [PMC free article] [PubMed] [Google Scholar]
- 206.Incir S., Bolayirli I.M., Inan O., Aydin M.S., Bilgin I.A., Sayan I., Esrefoglu M., Seven A. The effects of genistein supplementation on fructose induced insulin resistance, oxidative stress and inflammation. Life Sci. 2016;158:57–62. doi: 10.1016/j.lfs.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 207.Tang C., Zhang K., Zhao Q., Zhang J. Effects of Dietary Genistein on Plasma and Liver Lipids, Hepatic Gene Expression, and Plasma Metabolic Profiles of Hamsters with Diet-Induced Hyperlipidemia. J. Agric. Food Chem. 2015;63:7929–7936. doi: 10.1021/acs.jafc.5b01590. [DOI] [PubMed] [Google Scholar]
- 208.Yin Y., Liu H., Zheng Z., Lu R., Jiang Z. Genistein can ameliorate hepatic inflammatory reaction in nonalcoholic steatohepatitis rats. Biomed. Pharmacother. 2019;111:1290–1296. doi: 10.1016/j.biopha.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 209.Amerizadeh A., Asgary S., Vaseghi G., Farajzadegan Z. Effect of Genistein Intake on Some Cardiovascular Risk Factors: An Updated Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2021;1:100902. doi: 10.1016/j.cpcardiol.2021.100902. [DOI] [PubMed] [Google Scholar]
- 210.Squadrito F., Marini H., Bitto A., Altavilla D., Polito F., Adamo E.B., D’Anna R., Arcoraci V., Burnett B.P., Minutoli L., et al. Genistein in the Metabolic Syndrome: Results of a Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2013;98:3366–3374. doi: 10.1210/jc.2013-1180. [DOI] [PubMed] [Google Scholar]
- 211.Li J., Liu Y., Wang T., Zhao L., Feng W. Does genistein lower plasma lipids and homocysteine levels in postmenopausal women? A meta-analysis. Climacteric. 2016;19:440–447. doi: 10.1080/13697137.2016.1194388. [DOI] [PubMed] [Google Scholar]
- 212.Lu R., Zheng Z., Yin Y., Jiang Z. Effect of Genistein on Cholesterol Metabolism-Related Genes in HepG2 Cell. J. Food Sci. 2019;84:2330–2336. doi: 10.1111/1750-3841.14725. [DOI] [PubMed] [Google Scholar]
- 213.Kartawijaya M., Han H.W., Kim Y., Lee S.-M. Genistein upregulates LDLR levels via JNK-mediated activation of SREBP-2. Food Nutr. Res. 2016;60:31120. doi: 10.3402/fnr.v60.31120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Borradaile N.M., de Dreu L.E., Wilcox L.J., Edwards J.Y., Huff M.W. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem. J. 2002;366:531–539. doi: 10.1042/bj20020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Giovinazzo G., Ingrosso I., Paradiso A., De Gara L., Santino A. Resveratrol Biosynthesis: Plant Metabolic Engineering for Nutritional Improvement of Food. Plant Foods Hum. Nutr. 2012;67:191–199. doi: 10.1007/s11130-012-0299-8. [DOI] [PubMed] [Google Scholar]
- 216.Ravagnan G., De Filippis A., Carteni M., De Maria S., Cozza V., Petrazzuolo M., Tufano M.A., Donnarumma G. Polydatin, A Natural Precursor of Resveratrol, Induces beta-Defensin Production and Reduces Inflammatory Response. Inflammation. 2013;36:26–34. doi: 10.1007/s10753-012-9516-8. [DOI] [PubMed] [Google Scholar]
- 217.Jeon S.-M., Lee S.-A., Choi M.-S. Antiobesity and Vasoprotective Effects of Resveratrol in ApoE-Deficient Mice. J. Med. Food. 2014;17:310–316. doi: 10.1089/jmf.2013.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Du J., Sun L.-N., Xing W.-W., Huang B.-K., Jia M., Wu J.-Z., Zhang H., Qin L.-P. Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine. 2009;16:652–658. doi: 10.1016/j.phymed.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 219.Xing W.-W., Wu J.-Z., Jia M., Du J., Zhang H., Qin L.-P. Effects of polydatin from Polygonum cuspidatum on lipid profile in hyperlipidemic rabbits. Biomed. Pharmacother. 2009;63:457–462. doi: 10.1016/j.biopha.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 220.Xin P., Han H., Gao D., Cui W., Yang X., Ying C., Sun X., Hao L. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats. Food Chem. Toxicol. 2013;52:12–18. doi: 10.1016/j.fct.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 221.Yashiro T., Nanmoku M., Shimizu M., Inoue J., Sato R. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis. 2012;220:369–374. doi: 10.1016/j.atherosclerosis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 222.Jing Y., Hu T., Lin C., Xiong Q., Liu F., Yuan J., Zhao X., Wang R. Resveratrol downregulates PCSK9 expression and attenuates steatosis through estrogen receptor alpha-mediated pathway in L02 cells. Eur. J. Pharmacol. 2019;855:216–226. doi: 10.1016/j.ejphar.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 223.Wang Y., Ye J., Li J., Chen C., Huang J., Liu P., Huang H. Polydatin ameliorates lipid and glucose metabolism in type 2 diabetes mellitus by downregulating proprotein convertase subtilisin/kexin type 9 (PCSK9) Cardiovasc. Diabetol. 2016;15:19. doi: 10.1186/s12933-015-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chothe P.P., Swaan P.W. Resveratrol promotes degradation of the human bile acid transporter ASBT (SLC10A2) Biochem. J. 2014;459:301–312. doi: 10.1042/BJ20131428. [DOI] [PubMed] [Google Scholar]
- 225.Miura D., Miura Y., Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393–1400. doi: 10.1016/S0024-3205(03)00469-7. [DOI] [PubMed] [Google Scholar]
- 226.Kumar S., Prahalathan P., Saravanakumar M., Raja B. Vanillic acid prevents the deregulation of lipid metabolism, endothelin 1 and up regulation of endothelial nitric oxide synthase in nitric oxide deficient hypertensive rats. Eur. J. Pharmacol. 2014;743:117–125. doi: 10.1016/j.ejphar.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 227.Ma J.-Q., Ding J., Zhao H., Liu C.-M. Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin. Pharmacol. Toxicol. 2014;115:389–395. doi: 10.1111/bcpt.12245. [DOI] [PubMed] [Google Scholar]
- 228.Sui G.-G., Xiao H.-B., Lu X.-Y., Sun Z.-L. Naringin Activates AMPK Resulting in Altered Expression of SREBPs, PCSK9, and LDLR To Reduce Body Weight in Obese C57BL/6J Mice. J. Agric. Food Chem. 2018;66:8983–8990. doi: 10.1021/acs.jafc.8b02696. [DOI] [PubMed] [Google Scholar]
- 229.Kubota S., Tanaka Y., Nagaoka S. Ellagic acid affects mRNA expression levels of genes that regulate cholesterol metabolism in HepG2 cells. Biosci. Biotechnol. Biochem. 2019;83:952–959. doi: 10.1080/09168451.2019.1576498. [DOI] [PubMed] [Google Scholar]
- 230.Ochiai A., Miyata S., Iwase M., Shimizu M., Inoue J., Sato R. Kaempferol stimulates gene expression of low-density lipoprotein receptor through activation of Sp1 in cultured hepatocytes. Sci. Rep. 2016;6:24940. doi: 10.1038/srep24940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tamura A., Fukushima M., Shimada K.-i., Han K.-H., Sekikawa M., Watanabe S., Nakano M., Matsumoto M., Chiji H. Cholesterol metabolism in rat is affected by protocatechuic acid. J. Nutr. Sci. Vitaminol. 2004;50:13–18. [PubMed] [Google Scholar]
- 232.Chung M.J., Sung N.-J., Park C.-S., Kweon D.-K., Mantovani A., Moon T.-W., Lee S.-J., Park K.H. Antioxidative and hypocholesterolemic activities of water-soluble puerarin glycosides in HepG2 cells and in C57BL/6J mice. Eur. J. Pharmacol. 2008;578:159–170. doi: 10.1016/j.ejphar.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 233.Zhang K., Song W., Li D., Jin X. Apigenin in the regulation of cholesterol metabolism and protection of blood vessels. Exp. Ther. Med. 2017;13:1719–1724. doi: 10.3892/etm.2017.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wong T.Y., Tan Y.Q., Lin S.-m., Leung L.K. Co-administrating apigenin in a high-cholesterol diet prevents hypercholesterolaemia in golden hamsters. J. Pharm. Pharmacol. 2018;70:1253–1261. doi: 10.1111/jphp.12953. [DOI] [PubMed] [Google Scholar]
- 235.Liu G., Zhang Y., Liu C., Xu D., Zhang R., Cheng Y., Pan Y., Huang C., Chen Y. Luteolin Alleviates Alcoholic Liver Disease Induced by Chronic and Binge Ethanol Feeding in Mice. J. Nutr. 2014;144:1009–1015. doi: 10.3945/jn.114.193128. [DOI] [PubMed] [Google Scholar]
- 236.Li J., Inoue J., Choi J.-M., Nakamura S., Yan Z., Fushinobu S., Kamada H., Kato H., Hashidume T., Shimizu M., et al. Identification of the Flavonoid Luteolin as a Repressor of the Transcription Factor Hepatocyte Nuclear Factor 4 alpha. J. Biol. Chem. 2015;290:24021–24035. doi: 10.1074/jbc.M115.645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sa C., Oliveira A.R., Machado C., Azevedo M., Pereira-Wilson C. Effects on Liver Lipid Metabolism of the Naturally Occurring Dietary Flavone Luteolin-7-glucoside. Evid. Based Complement. Altern. Med. 2015;2015:647832. doi: 10.1155/2015/647832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Duwensee K., Schwaiger S., Tancevski I., Eller K., van Eck M., Markt P., Linder T., Stanzl U., Ritsch A., Patsch J.R., et al. Leoligin, the major lignan from Edelweiss, activates cholesteryl ester transfer protein. Atherosclerosis. 2011;219:109–115. doi: 10.1016/j.atherosclerosis.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Liang Y.T., Chen J., Jiao R., Peng C., Zuo Y., Lei L., Liu Y., Wang X., Ma K.Y., Huang Y., et al. Cholesterol-Lowering Activity of Sesamin Is Associated with Down-Regulation on Genes of Sterol Transporters Involved in Cholesterol Absorption. J. Agric. Food Chem. 2015;63:2963–2969. doi: 10.1021/jf5063606. [DOI] [PubMed] [Google Scholar]
- 240.Fale P.L., Ferreira C., Maruzzella F., Florencio M.H., Frazao F.N., Serralheiro M.L.M. Evaluation of cholesterol absorption and biosynthesis by decoctions of Annona cherimola leaves. J. Ethnopharmacol. 2013;150:718–723. doi: 10.1016/j.jep.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 241.Morin B., Nichols L.A., Zalasky K.M., Davis J.W., Manthey J.A., Holland L.J. The citrus flavonoids hesperetin and nobiletin differentially regulate low density lipoprotein receptor gene transcription in HepG2 liver cells. J. Nutr. 2008;138:1274–1281. doi: 10.1093/jn/138.7.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Miwa Y., Mitsuzumi H., Yamada M., Arai N., Tanabe F., Okada K., Kubota M., Chaen H., Sunayama T., Kibata M. Suppression of apolipoprotein B secretion from HepG2 cells by glucosyl hesperidin. J. Nutr. Sci. Vitaminol. 2006;52:223–231. doi: 10.3177/jnsv.52.223. [DOI] [PubMed] [Google Scholar]