Abstract
Several species of avian schistosomes are known to cause dermatitis in humans worldwide. In Europe, this applies above all to species of the genus Trichobilharzia. For Austria, a lot of data are available on cercarial dermatitis and on the occurrence of Trichobilharzia, yet species identification of trematodes in most cases is doubtful due to the challenging morphological determination of cercariae. During a survey of trematodes in freshwater snails, we were able to detect a species in the snail Physella acuta (Draparnaud, 1805) hitherto unknown for Austria, Trichobilharzia physellae; this is also the first time this species has been reported in Europe. Species identification was performed by integrative taxonomy combining morphological investigations with molecular genetic analyses. The results show a very close relationship between the parasite found in Austria and North American specimens (similarity found in CO1 ≥99.57%). Therefore, a recent introduction of T. physellae into Europe can be assumed.
Keywords: trematodes, Schistosomatidae, DNA barcoding, cercariae, swimmer’s itch, Trichobilharzia physellae, Europe
1. Introduction
Digenean trematodes are parasitic worms, and some of them have considerable medical and economic relevance [1]. Among them are members of the family Schistosomatidae that includes mammal parasites, some of which cause severe human diseases particularly in tropical and subtropical regions [2], and birds schistosomes, etiological agents of avian cercarial dermatitis worldwide [3]. Avian schistosomes use birds as final hosts. Eggs contain and release miracidia which infect freshwater snails, the first intermediate hosts. In the snails, further larval stages—sporocysts and cercariae—propagate and develop, respectively. Swimming invasive larvae, the cercariae, finally leave the snail host and, if they find a suitable bird host, they enter it by penetrating the skin. Adults settle in blood vessels of visceral organs or mucus tissue of the bird host species, where they can complete their life cycle and reproduce sexually. Yet, cercariae may also penetrate the skin of unsuitable “aberrant” hosts, such as humans. In such a case, the life cycle cannot be completed, but the immune response of the host to the invading worms may cause an exanthema called “cercarial dermatitis” or “swimmer’s itch”. Generally, the dermatitis is harmless but awkward due to heavy pruritus which may last several days. This causes inconvenience and may—due to scratching—lead to secondary bacterial infections [4,5]. However, in some cases cercarial infection can also lead to more serious symptoms such as anaphylaxis or disorders of the respiratory system [6]. There have also been reports of other symptoms such as nausea, diarrhea, swollen glands, insomnia, and fever as well as reports of finding schistosomula in different organs of mammals which are summarized in Marszewska et al. and Horák et al. [3,7]. Worldwide, several genera are known to cause these symptoms [3,8]. Overviews of cercarial dermatitis outbreaks and avian schistosome occurrences in Europe have been provided by several papers [4,9,10]. The causative agents of cercarial dermatitis in Europe are mostly species of the genus Trichobilharzia. This genus comprises, depending on differences in genus assignments, 30–40 known species worldwide [11,12,13,14]. Six species are known in Europe, namely Trichobilharzia szidati Neuhaus, 1952; Trichobilharzia regenti Horák, Kolářová, and Dvořák, 1998; Trichobilharzia franki Müller and Kimmig, 1994; Trichobilharzia salmanticensis Simon-Vicente and Simon-Martin, 1999; Trichobilharzia anseri Jouet, Kolářová, Patrelle, Ferté, and Skírnisson, 2015; and Trichobilharzia mergi Kolářová, Skírnisson, Ferté, and Jouet, 2013 [3,15,16]. Besides Trichobilharzia, five other genera of avian schistosomes are reported to infect aquatic birds in Europe: Allobilharzia, Bilharziella, Dendritobilharzia, Gigantobilharzia, and Ornithobilharzia [10].
In Austria, to date, T. szidati from Lymnaea stagnalis (Linnaeus, 1758), T. franki from Radix auricularia (Linnaeus, 1758), and Bilharziella polonica (Kowalewski, 1895) from Planorbarius corneus (Linnaeus, 1758) have been recorded as potential causative agents of human cercarial dermatitis. More findings of (presumed) Trichobilharzia cercariae were reported from Aplexa hypnorum (Linnaeus, 1758), Gyraulus parvus (Say, 1817), Lymnaea stagnalis, Stagnicola sp., Radix auricularia, and Ampullaceana balthica (Linnaeus, 1758) (syn., e.g., Radix ovata (Draparnaud, 1805), Radix balthica (Linnaeus, 1758)) [17,18], yet without species assignment. The very first records from the 1970s were tentatively assigned to T. szidati due to the swimming behavior of the cercariae and the occurrence in L. stagnalis [19,20]. Later, investigations of Trichobilharzia from Lower Austria evidenced the species identity as T. szidati by rearing adults via life cycle performance in the laboratory [21]. Recently, T. franki was reported for the first time for Austria by Reier et al. [22]. Species identification was performed by employing morphological and molecular genetic methods (DNA barcoding). Similarly, Gaub et al. [23] verified the occurrence of T. szidati from eastern Austria using DNA sequence data. In view of the scarce data and considering the availability of a diversity of host species, it can be assumed that still other (avian) schistosomes may occur in Austria [10,24].
The invasive snail Physella acuta (Draparnaud, 1805) is native to North America but was long thought to be a European species since it was first described in 1805 from France [25]. However, it may be one of the earliest cases of a successful biological invasion that started in Europe [26] and resulted in the species now having a global distribution with the exception of Antarctica [27]. Physella acuta is known to be capable of a very high reproduction rate (as short as 4 weeks) and even to alter the number of generations per year [27,28]. This effectively enables P. acuta to displace native gastropods in a very short time [28]. In recent years, P. acuta has been investigated in Upper and Lower Austria at several localities (but in moderate numbers) at Danube backwaters and tributaries as well as in Upper Austrian lakes, but without any trematode evidence until now.
In the framework of a survey of trematodes in freshwater snails in eastern Austria, avian schistosomes were collected from infected freshwater snails [29]. During that study, schistosome cercariae were found also in physid snails. We report here (1) the first finding of Trichobilharzia physellae in Austria/Europe in the intermediate host snail P. acuta in the field, (2) provide first DNA barcode sequences of this species (for Europe) and (3) compare it with earlier published sequences. We also (4) assessed its phylogenetic position among other Trichobilharzia species as well as the intraspecific genetic diversity found in the mitochondrial marker sequence. Furthermore (5) we provide some general morphometric data and photomicrographs of the cercariae of this European isolate of T. physellae.
2. Results
2.1. Morphology
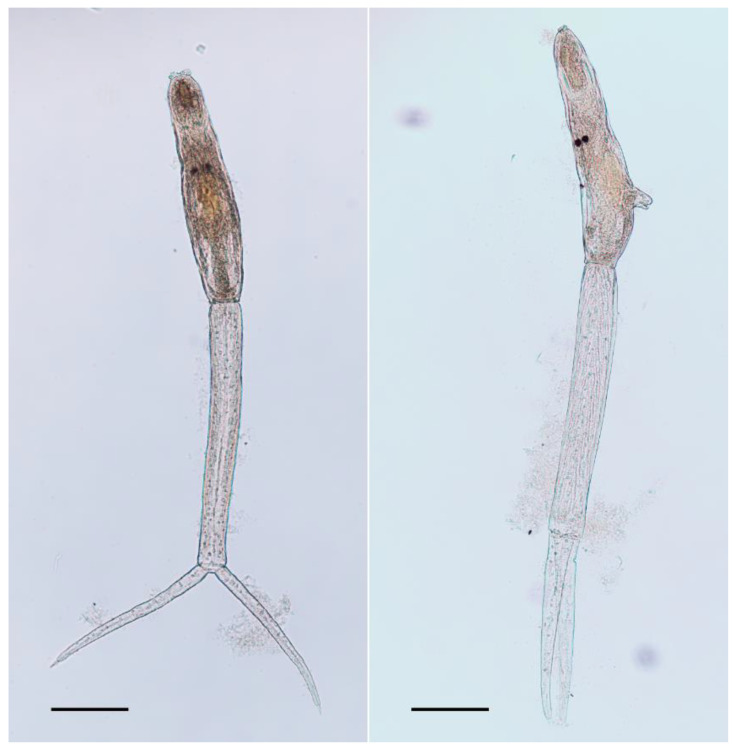
The measurements of 25 T. physellae specimens (Figure 1) found on 16 September 2020 (NHMW, Collection Evertebrata Varia, inventory number 5858) in Upper Austria can be seen in Table 1 in comparison with previously published measurements. The measured specimens were similar in every measurement and there were no outliers in the data (Supplementary Spreadsheet S1). In particular, the diameter of ventral sucker, the width of tail stem, and the width of tail furca were very consistent. In comparison with previously published data, there is a good consensus with the measurements reported by Talbot [30] except for length of body, length of tail stem, and length of tail furca. The values reported by Tanaka [31] and Pence and Rhodes [32] were more deviant in most measurements, especially in diameter of sucker, distance from ventral sucker to posterior of body, and all width measurements.
Figure 1.
Photomicrographs of two of the measured Trichobilharzia physellae cercariae elucidated by glycerol and stained by borax carmine. Left = ventral, right = lateral. Scale = 100 µm.
Table 1.
Morphological measurements of Trichobilharzia physellae specimens of this study (n = 25) in comparison with previously published measurements. Our measurements and those by Pence and Rhodes [32] give mean values in µm with standard error; measurements by Talbot [30] are mean values in µm with probable error; measurements by Tanaka [31] are only mean values in µm. Abbreviations: MI = Michigan, OI = Oki Islands, TX = Texas, UA = Upper Austria, Formalin = hot formalin solution (10%), AFA = alcohol formalin acetic acid mixture, EtOH = ethanol.
| Reference | Talbot (1936) | Tanaka (1960) | Pence and Rhodes (1982) | This Study |
|---|---|---|---|---|
| Host | Physella parkeri, P. magnalacustris | Radix japonica | Physa anatina | Physella acuta |
| Locality | MI, USA | OI, JPN | TX, USA | UA, AUT |
| Fixation | Formalin | Formalin | AFA | 96% EtOH |
| Length of body | 265 ± 8.4 | 281 | 244 ± 15 | 306.5 ± 3.1 |
| Width of body | 60 ± 4.5 | 51 | 65 ± 4 | 60.5 ± 1.4 |
| Diameter of ventral sucker | 29 ± 2.4 | 27 | 18 ± 3 | 29.2 ± 0.7 |
| Distance from ventral sucker to the posterior end of the body | 80 ± 5.2 | 95 | 68 ± 6 | 83.6 ± 1.2 |
| Length of tail stem | 374 ± 10.6 | 361 | 301 ± 7 | 343.8 ± 3.1 |
| Width of tail stem | 40 ± 3.6 | 35 | 36 ± 4 | 43.5 ± 0.8 |
| Length of tail furca | 196 ± 7.8 | 221 | 157 ± 4 | 225.9 ± 1.8 |
| Width of tail furca | 32 ± 0.9 | 39 | 18 ± 1 | 26.3 ± 0.8 |
2.2. Molecular Genetic Results
Sequencing of the two cercariae specimens from the lake Pleschinger See from 16 September 2020 (NHMW, Collection Evertebrata Varia, inventory number 5858) resulted in long mitochondrial cytochrome c oxidase subunit 1 gene (CO1) fragments of 1130 bp (Pha1-21-001) and 1143 bp (Pha1-21-002), respectively. In addition, CO1 DNA barcode sequences (Folmer region) of a length of 591 bp were obtained from independent PCR reactions from both specimens. The sequences from both specimens gave a clear result, both gave hits with 99.88% similarity with T. physellae, using NCBI (National Center for Biotechnology Information) BLAST (Basic Local Alignment Search Tool). From the two cercariae specimens (Pha2-21-001 and Pha2-21-002) from 11 November, 2020 (NHMW, Collection Evertebrata Varia, inventory number 5859) only short sequences could be obtained due to bad DNA quality. One was obtained with the primers Tricho_tRNA_fw and CO1560R_modif (length of sequence 576 bp) and one with ZDOE-COI-fw and Tricho_rev_20 (460 bp). We were not able to amplify the section in between those two sections. Additionally, the sequences were not compliant with the quality criteria for DNA barcodes. Yet, the NCBI BLAST with the sequences gave a clear result for both specimens (T. physellae with 99.57% and 99.78%, respectively). Sequencing of both host snails (NHMW, Collection Evertebrata Varia, inventory numbers 21338 and 21339) resulted in CO1 DNA barcode sequences of a length of 655 bp (BOLD (Barcode of Life Data System) ID: NHBP005-21 and NHBP006-21; NCBI GenBank accession numbers: OL434666 and OL434667). The sequences from both host snail specimens gave a clear result for P. acuta (99.24% and 99.39%, respectively). The comparison of the two host snail sequences to the data published by Moore et al. [33] also showed a clear assignment to the P. acuta lineage.
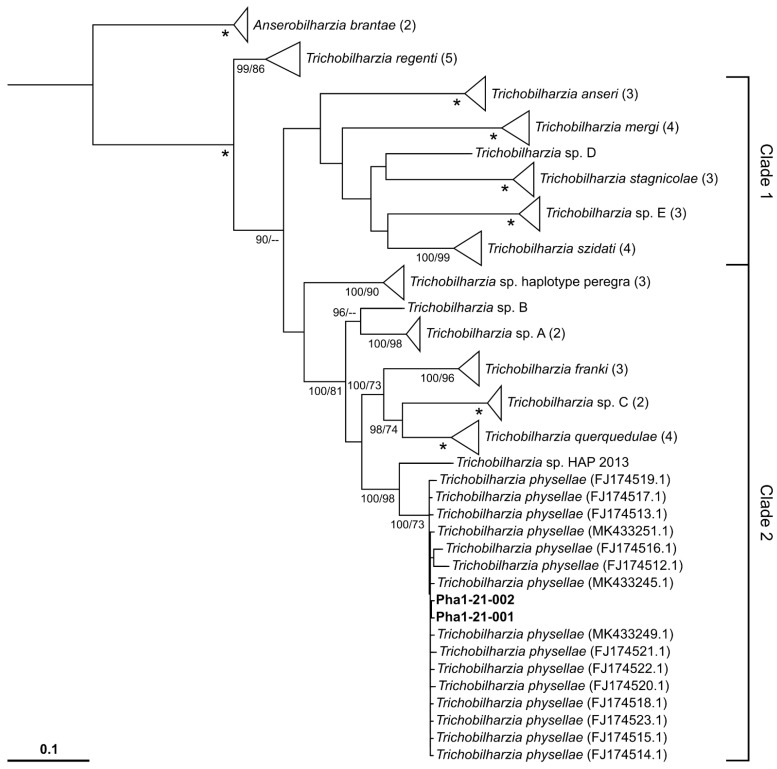
The maximum likelihood (ML) and Bayesian Inference (BI) trees based on the Trichobilharzia data both have the same overall topology, differing only in support for some branches (Figure 2). Trichobilharzia regenti was the sister group to all other lineages. The tree displays a separation of the remaining Trichobilharzia species into two main clades. Clade 1 comprises the highly supported species T. anseri, T. mergi, T. stagnicolae, T. szidati and two not yet described Trichobilharzia species (Trichobilharzia sp. D, E; [34]). The relationships within this clade did not receive considerable support values and have to be considered as unresolved. Clade 2 comprised T. franki, T. querquedulae, T. physellae plus five not yet described species (Trichobilharzia sp. haplotype peregra; Trichobilharzia sp. A, B, and C; Trichobilharzia sp. HAP 2013; [15,34,35]). Nodes within clade 2 are generally much better supported. Our specimens of T. physellae originating from Austria cluster within the other sequences of this species (Figure 2).
Figure 2.
Maximum likelihood (ML) tree of Trichobilharzia species based on mitochondrial cytochrome c oxidase subunit 1 gene (CO1) sequences. The Bayesian Inference (BI) tree had the same topology. Bootstrap values ≥70 % (right, in %) and posterior probabilities ≥90 (left) are presented at the nodes. A star indicates full support (100/100). All species except T. physellae are presented as collapsed nodes (if more than one sequence is present). Sequences obtained in this study are in bold. The size of the collapsed nodes reflects the number of sequences (2–5), which is also given in parentheses behind the species names.
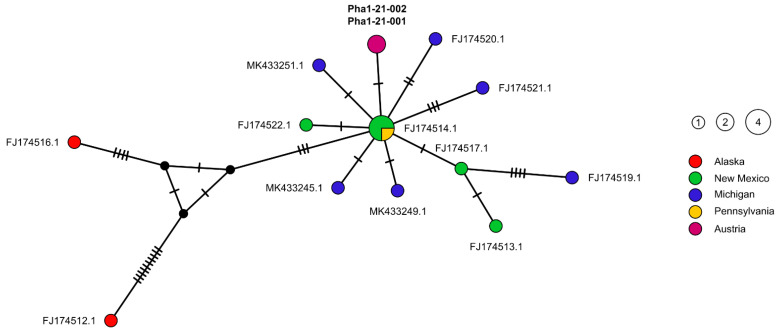
The median-joining (MJ) haplotype network (Figure 3) illustrates the high diversity of haplotypes within T. physellae with 13 different haplotypes present in 17 specimens. The haplotype diversity was accordingly high with 0.95, whereas the nucleotide diversity was very low (Pi = 0.006). The two specimens from Austria correspond to the same haplotype and are separated by one substitution from the most common haplotype shared by three specimens collected in New Mexico and one in Pennsylvania (FJ174514.1, FJ174515.1, FJ174518.1, and FJ174523.1). The specimens collected in New Mexico and Michigan both have a large haplotype diversity with different sets of substitutional steps. The haplotypes of the specimens from Alaska (FJ174512.1 and FJ174516.1) differ considerably from the rest of the presented haplotypes (Figure 3) as well as from each other. An examination of the sequences showed that the two sequences from Alaska each have a different short region with a high aggregation of autapomorphic substitutions, while in the remaining parts the sequences are rather similar to the other T. physellae sequences. FJ174512.1 has seven autapomorphic substitutions between position 708 and 782 of the alignment and FJ174516.1 has five autapomorphic substitutions between position 546 and 568 of the alignment.
Figure 3.
Median-joining network (MJ) based on CO1 sequences of T. physellae. NCBI GenBank accession numbers/specimen Lab-IDs representing different haplotypes are given near the dots. The central shared haplotype consists of FJ174514.1, FJ174515.1, FJ174518.1, and FJ174523.1. Haplotypes from the USA were coded at the level of the U.S. states. All haplotypes are marked in different colors and are constituted of one to four sequences (representative circle sizes in the legend). Sequences obtained in the present study are in bold. Orthogonal lines on the connecting lines between the haplotypes indicate the number of mutation steps; black dots indicate missing haplotypes.
3. Discussion
This study represents the first report of Trichobilharzia physellae in Europe. Species assignment is based on a combination of morphology, DNA sequence comparison, and determination of the host snail. So far, the species was recorded unambiguously only in North America by means of morphology [30,32] and DNA sequence data [34,36,37]. There are also reports of T. physellae from Japan with studies on the general morphology of adult flukes and cercariae as well as on the fine structure of cercariae [31,38,39]. Another study from India also reported T. physellae, but in this study no data were provided to support this assumption [40]. A study from Brazil [35] found a genetically similar Trichobilharzia lineage in Stenophysa marmorata (Guilding, 1828) (mentioned in that publication under its synonym Physa marmorata Guilding, 1828). This lineage (Figure 2, sequence HAP 2013) might be, according to the data of that paper and our data, a sister species of T. physellae.
Our morphological measurements are highly congruent with the original species description by Talbot [30] in most measurements taken (despite different fixation), which confirms our morphological determination. In contrast, the specimens of T. physellae measured by Pence and Rhodes [32] differ in most points. However, higher deviation and variability, especially of the length of body, tail stem, and furca has often been observed and, therefore, body dimensions are not considered as reliable tools for species determination of cercariae [11,41]. This is due to the contractibility of cercariae, the temperature of the environment, and the snail size during the development of cercariae, as well the influence of varying fixatives [41,42]. Based on our data and the data from Talbot [30], the diameter of sucker, distance from ventral sucker to the posterior end of the body, and all width measurements seem to be more stable and informative. The information on host species neither helps to explain the considerable differences in measurements, nor allows straightforward interpretations concerning species assignment of the cercariae. Talbot [30] collected T. physellae (morphologically the most similar to ours) from Physella parkeri (Currier in DeCamp, 1881) and Physella magnalacustris (Walker, 1901), which later were considered as synonyms of Physella ancillaria (Say, 1825) [43]. On the other hand, Pence and Rhodes [32] collected the cercariae (morphologically quite distinct from ours) from Physa anatina I. Lea, 1864 (syn. Haitia mexicana (Philippi in Küster, 1841)), which later was synonymized with Physella acuta [33,43]. The cercariae collected by Tanaka [31] differ strongly in measurements and were released from Radix japonica (Jay, 1857) which belongs to the family Lymnaeidae while the other above-mentioned host snails belong to Physidae. The same is true for cercariae which have been reported as hatching from R. auricularia (Linnaeus, 1758) in India by Dutt [40]. Based on the data provided, species identification as T. physellae by both Tanaka [31] and Dutt [40] is highly doubtful and has been questioned by other authors as well [41]. Similarly, the comparison with measurements of other Trichobilharzia species from Austria and other European countries did not allow clear delimitation of the species as can be seen in the Supplementary Spreadsheet S1 [15,22,42]. More detailed characters, which could optimize the differentiation, would require living cercariae and special staining [44], which was not performed in this study, since it was based on ethanol fixed material. In summary, it appears that determination solely on morphological measurements or the host snail species is not sufficient for T. physellae.
The sequence data of our specimens give a much clearer result when compared to data available in GenBank and we could exemplify that CO1 is a good marker for identifying species. Concerning the topology of our tree (Figure 2) we may state here that it matches that of published CO1 trees including Trichobilharzia species [15,22,34,35,45]. However, in comparison with published trees based on other genetic markers, the deeper nodes of Clade 1 and the position of T. regenti differ, seemingly depending on the genetic markers used and the chosen outgroup [12,14,34]. Yet, for a solid phylogenetic reconstruction of the relationships within the genus Trichobilharzia (which was not the aim of the study) CO1 sequences are certainly not sufficient.
The MJ network presented here (Figure 3) gives an interesting picture concerning the distribution of T. physellae. Our specimens from Austria are only separated by one substitution from the most common haplotype in North America, whereas many other haplotypes were found in North America. Especially the haplotypes from Alaska are very different. However, despite the larger geographic separation of these two specimens, these sequences appear doubtful since it appears strange for a coding gene to have a tight aggregation of autapomorphic substitutions within a short section. Thus, it would need more data from specimens from Alaska to confirm these conspicuous mutations. The high overall haplotype diversity and the very low overall nucleotide diversity of the T. physellae sequences are similar to those of other Trichobilharzia species found in the literature [22,46,47].
Since the CO1 haplotype of the Austrian specimens is very closely related to the most common North American haplotype, a recent introduction of the species to Europe may be assumed. The most plausible vector seems to be the host snail P. acuta. Originally native to North America it was accidentally introduced to reach a nowadays worldwide distribution. The introduction of P. acuta in Europe started over 200 years ago and the population today is considered to be the result of multiple invasions into Europe from where the snail subsequently spread eastwards [27]. The phases of invasion and distribution through Europe are summarized by Vinarski [26] and indicate that P. acuta was present in Austria at least by 1925. Findings in southern Germany and in the Czech Republic suggest an introduction into northern Austria even earlier in the twentieth century [26] which would imply that a suitable host for T. physellae has already been established in Austria and Europe for some time. A plausible transmission route of aquatic snails and therefore probably also for T. physellae is nowadays the international aquarium trade and trade of water plants [48]. A good example is another invasive snail species, Melanoides tuberculata (O. F. Müller, 1774), which was originally described from India and has since reached a pantropical distribution mainly due to the trade of aquarium plants and its ability to essentially reproduce clonally [49]. Additionally, M. tuberculata is reported to be the host of 37 trematode species in 26 countries [50]. Yet, it should be considered that snails may be transported mainly as eggs or small/young snails which have a much lower prevalence for cercarial infection [27,51]. Therefore, the introduction of infected snails might be a rare event.
Several migrating Anatidae may also be considered as possible vectors for Trichobilharzia from North America to Europe. For example, Anatidae (mostly the genus Anas) have been reported for the transcontinental dispersal of T. querquedulae [45]. All hosts of adult T. physellae recorded so far in North America belong to Anatidae [32,34,37]. For example, the lesser scaup Aythya affinis (Eyton, 1838) and the ring-necked duck Aythya collaris (Donovan, 1809) are both common in North America and vagrants in Europe. The sightings accepted by local avifauna committees in countries around Austria and in Austria of wild birds are scarce but regular (most recent sightings of A. affinis: Germany [52]; Switzerland [53]; of A. collaris: Austria [54]; Slovakia [55]; Hungary [56]; Italy [57]; Germany [58]; Switzerland [59]; Czech Republic [60]). Within Europe, the goosander (Mergus merganser Linnaeus, 1758) could be a possible avian vector since it is also a known host in North America [34]. The goosander has known populations in the Swiss Alps, Bavaria (Germany) and Austria; the male birds from these populations seem to migrate regularly to northern Europe [61]. The Austrian population of the goosander is distributed along rivers in Upper Austria and one of the highest densities is in the area of the city Linz [62] where our specimens of T. physellae were collected. Whether our record of T. physellae is part of a single population of this species or the species has a wider distribution in Austria or even in Europe (and simply was not detected so far) remains to be investigated.
4. Materials and Methods
4.1. Sampling
While performing a study dedicated to eDNA detection of cercarial dermatitis agents in Upper Austrian water bodies, different species of potential host snails were collected at locations where cercarial dermatitis cases were reported. Among them were 36 P. acuta (Draparnaud, 1805) specimens collected at the lake Pleschinger See in Linz (Upper Austria) on 16 September, 2020 (6 specimens) and on 11 November, 2020 (30 specimens). This quarry lake has a surface area of 0.13 km², a maximum depth of 8 m, and an altitude of 248 m above sea level (Coordinates: 48°19′9″ N, 14°19′57″ E). The lake temperatures were estimated at approximately 22 °C in September and 8 °C in November. In both cases, the snails were transported within lake water to the lab and subsequently isolated in individual jars with lake water, which were placed near a window (but not in direct sunlight) at room temperature. Cercarial infection of the snail (detected by release of cercariae) was found after one day in one snail collected on 16 September 2020 and after three days in one snail collected on the 11 November 2020. This results in a prevalence of 5.6%. The cercariae released from two P. acuta specimens were fixed in molecular grade ethanol (96%) immediately after discovery. The corresponding infected snails were also fixed in molecular grade ethanol (96%) after death (18 September 2020 and 16 November 2020). Uninfected snails were released at the collecting site.
4.2. Morphological Examination
A part of the fixed cercariae obtained from several infected snails was placed in a 50:50 ethanol (80%)/glycerol mixture, which was complemented with 1 mL borax-carmine solution (according to Grenacher from Sigma Aldrich) per 100 mL. The ethanol was than evaporated for 48 hours in a thermo-incubator (at 40 °C) pre-mounting. After evaporation of the ethanol, the cercariae were mounted in glycerol on micro slides and covered with cover glasses. This procedure results in increased lucency from glycerol and slight staining from Borax-Carmine for better visibility of the anatomy. The mounted cercariae were morphologically examined through a Nikon Eclipse Ni-U microscope (Nikon Instruments Inc., New York, NY, USA). Subsequently, photomicrographs were made using the mounted Nikon DSRi2 microscope camera unit. Based on the photomicrographs, measurements of the body length and width, diameter of ventral sucker, distance from ventral sucker to the posterior end of the body, stem length and width, furca length, and width [30,32] were taken in the corresponding application NIS-Elements BR v.5.02.00. For some specimens, there were missing data caused by non-visibility of some characters due to the position of the specimens on the microscopic slide. Finally, the mean and the standard errors were calculated, and the measurement data were tested for outliers. The final photomicrographs were edited using Gimp 2.10.24 (https://www.gimp.org, accessed on 28 May 2021). The corresponding host snails were morphologically examined, identified based on their shell anatomy, and photographed using a stereo microscope (Wild-Leica Heerbrugg M420 Makroskop, Leica, Wetzlar, Germany). The mounted slides, fixated cercariae and host snails are deposited in the collection of the Natural History Museum Vienna (NHMW, Collection Evertebrata Varia, inventory numbers 5858 and 5859 for the microslides/cercariae and inventory numbers 21338 and 21339 for the host snails, respectively).
4.3. Analysis of the Mitochondrial cytochrome c oxidase subunit 1 Gene
For the molecular genetic analysis, DNA of single cercaria of every infected snail was extracted using the QIAmp DNA Micro Kit (Qiagen, Hilden, Germany). Altogether, four cercariae were analyzed genetically. Cercariae were isolated with stainless insect needles under a stereo microscope (Wild-Leica Heerbrugg M420 Makroskop, Leica, Wetzlar, Germany), dried for approximately 10 seconds on the needle to remove the ethanol, and subsequently transferred into the lysis buffer. The extraction was performed according to the manufacturers’ protocol. In the final step, the DNA was eluted with 25 µL AE buffer. Additionally, small tissue samples of the foot of both host snails were taken and extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Lysis of the tissue samples was conducted overnight. The extraction was performed according to the manufacturers’ protocol. In the final step, the DNA was eluted with 40 µL AE buffer.
Fragments of the mitochondrial cytochrome c oxidase subunit 1 gene (CO1) were amplified. For the cercariae, the first primer pair (Cox1_schist_5k/Cox1_schist_3k) was used to gain a 1244 bp amplicon of the CO1 gene. Additional primers optimized for Trichobilharzia were designed to amplify four overlapping fragments in specimens with poor DNA quality and/or for sequencing: Tricho_tRNA_fw/Tricho_tRNA_rv_2, 401-482 bp amplicon length (depending on an indel between tRNA-Ser and CO1); Cox1_schist_5_trich/CO1560R_modif, 612 bp amplicon length (corresponding to the so-called “Folmer region”, covering 88,6% of this sequence); Tricho_Fw2/Tricho_Rv2_2, 491 bp amplicon length; ZDOE-COI-fw/Tricho_rev_20, 486 bp amplicon length). For the host snails, the primer pair LCO1490_ABOL_Moll_1/HCO2198_ABOL_Moll¬_1 was used to gain a 704 bp amplicon of the CO1 gene. Primer sequences are given in Table 2.
Table 2.
List of used primers and their sequences.
| Name | Sequence 5′–3′ | Reference |
|---|---|---|
| Schisto-COI-5-Fw | TCTTTRGATCATAAGCG | [63] |
| Schisto-COI-3-Rv | TAATGCATMGGAAAAAAACA | [63] |
| Tricho_tRNA_fw | GGTTGTCGCTGCTAACGA | This study |
| Tricho_tRNA_rv_2 | CCATATAAAACATTGAAGGAACC | This study |
| Cox1_schist_5_trich | GTTRGTTTCTTTGGATCATAAGCG | This study |
| CO1560R_modif | GCAGTACCAAATTTTCGATC | This study |
| Tricho_Fw2 | GGTTCTGTAAAATTTATAACTAC | This study |
| Tricho_Rv2_2 | CCTAACATATACAACCAAG | This study |
| ZDOE-COI-fw | TAGTTTGTGCTATGGGTTCTATAGT | This study |
| Tricho_rev_20 | GCATTCCTAAATAATGCATAGG | This study |
| LCO1490_ABOL_Moll_1 | TCAACAAAYCATAARGAYATTGG | [64] |
| HCO2198_ABOL_Moll_1 | TAAACTTCTGGRTGACCAAAAAAYCA | [64] |
PCR amplification was performed in 25 µL reaction volume including 18.4 µL distilled water, 2.5 µL of 10× TopTaq PCR buffer, 1.5 µL of 25 mM MgCl¬2 (only for the cercariae), 0.5 µL of 50 µM primers (0.25 µL each), 0.5 µL dNTP (10 mM each), 0.5 units of TopTaq DNA polymerase (Qiagen, Hilden, Germany), and 1.5 µL template DNA. The conditions used in the PCR reactions were 94 °C for 180 s, 35–40× of (94 °C for 30 s, Tann for 30 s, 72 °C for 60–90 s), 72 °C for 420 s; elongation time and annealing temperature (Tann) can be found in Table 3.
Table 3.
Conditions of PCR reactions of the different primer pairs. Tann = annealing temperature.
| Primer Combination | Amplicon Length | Tann/Elongation Time |
|---|---|---|
| Cox1_schist_5k/Cox1_schist_3k | 1244 bp | 50 °C/90 s |
| Tricho_tRNA_fw/Tricho_tRNA_rv_2 | 401–482 bp | 54 °C/60 s |
| Cox1_schist_5_trich/CO1560R_modif | 612 bp | 52 °C/60 s |
| Tricho_Fw2/Tricho_Rv2_2 | 491 bp | 49 °C/60 s |
| ZDOE-COI-fw/Tricho_rev_20 | 486 bp | 53 °C/60 s |
| LCO1490_ABOL_Moll_1/HCO2198_ABOL_Moll _1 | 704 bp | 50 °C/60 s |
The amplified DNA products were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) according to the manufacturers´ protocol. Purified PCR products were sequenced in both directions using the PCR primers by Microsynth Austria (Vienna, Austria).
Chromatograms of the sequencing results were checked using FinchTV 1.4.0 (Geospiza Inc.) and sequences were edited using GeneDoc 2.7.0 [65]. The final sequences were blasted using NCBI BLAST search. Sequences obtained in the present study were deposited in the NCBI GenBank database and in the BOLD database (see Table A1). Additionally, 159 CO1 sequences of Trichobilharzia species (representing all species and clades currently available) were downloaded from GenBank and added to the preliminary alignment. Anserobilharzia brantae (Farr and Blankemeyer, 1956) was used as outgroup. The alignment was built using MEGA-X 10.0.5 [66] and MAFFT 7.481 with the L-INS-i algorithm [67]. Due to different lengths of published sequences, the alignment was reduced to a length of 782 sites with all shorter sequences excluded. Furthermore, a selection of 1–5 sequences was chosen from the various species (except T. physellae), depending on the availability in the NCBI database, in an attempt to cover the genetic variability of each species. For that purpose, the genetic variability was checked by calculating a neighbor-joining test tree in MEGA-X and a ML test tree with 1000 ultrafast bootstrap approximations [68] in the software IQ-Tree (for details see below). The final alignment consisted of 58 sequences including the two T. physellae sequences sampled from 16 September 2020 (Appendix A Table A1). The sequences from the specimens from 11 November 2020 were not included in the tree and network calculations as only short sections of the CO1 gene could be obtained from them. The sequencing results of the host snails have been additionally checked against the data published by Moore et al. [33] to assess which genetic lineage our snail specimens belong to.
The best fitting evolutionary models for the phylogenetic tree searches were selected for every codon position by using ModelFinder [69] with the BIC criterion implemented in the IQ-Tree software (1. Pos.: TN+F+I; 2. Pos.: TN+F+I, 3. Pos.: TIM2+F+I+G4). For the ML analyses, the software IQ-Tree 2.1.1. [70] was used with edge-linked partition models [71]. Branch support was assessed by calculating 1000 standard bootstrap iterations. Bayesian Inference (BI) was conducted using MrBayes 3.2.6 [72] with 2 × 4 Markov Chain Monte Carlo iterations of 1 × 107 generations and sampling every 200th generation. After inspecting the log-likelihood values, a 10% burn-in was chosen. The final trees were visualized by using iTOL v5 [73].
The sequences of the species T. physellae (782 bp alignment) were also used to produce a median-joining (MJ) haplotype network [74] using PopART 1.7 (http://www.popart.otago.ac.nz, accessed on 6 July 2021) to compare the specimens found in Austria with already published sequences from the USA (accession numbers FJ174512-FJ174523.1, MK433245.1, MK433249.1, MK433251.1, see Table A1). In addition, the haplotype diversity (Hd) and the nucleotide diversity (Pi) were calculated using DnaSP 6.12.03 [75]. The final tree and the network were graphically edited using Inkscape 1.0.2 (https://inkscape.org, accessed on 17 May 2021) and exported using Gimp 2.10.24.
Acknowledgments
We are grateful to the Upper Austrian Provincial Government and the “Freunde des Naturhistorischen Museums Wien” for financial support. Wolfgang Heinisch (Department of Water Management, Upper Austria Provincial Government) is thanked for initiating and promoting the Upper Austrian cercaria project. We are grateful to ABOL (Austrian Barcode of Life) initiative and especially Oliver Macek (Natural History Museum, Vienna) for technical help concerning the BOLD database as well as Michael Duda (Natural History Museum, Vienna) for help concerning information on the snails. We thank Marcia Sittenthaler and Alexandra Wanka for technical assistance in the lab. Ivan Maggini (University of Veterinary Medicine, Vienna) contributed valuable information and suggestions in conversations.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10111473/s1, Spreadsheet S1: Morphological measurements and test for outliers.
Appendix A
Table A1.
List of sequences that have been used in the present study. § refers to corrected data (Host, Life Cycle Stage, Locality) based on personal communication with Sara Brant concerning differences between data in the NCBI GenBank Database and the cited paper [34,76]. Accession numbers (Acc.No) are in general GenBank accession numbers, those marked with an asterisk are the DNA barcodes generated in the present study (Lab-ID, BOLD as well as GenBank accession numbers are provided).
| Genus/ Species |
Acc.No/ Lab-ID |
Host | Life Cycle Stage | Locality | Reference |
|---|---|---|---|---|---|
| Anserobilharzia | |||||
| Anserobilharzia brantae | |||||
| MK433247.1 | Gyraulus sp. | cercariae | USA, Michigan | [37] | |
| KC570954.1 | Anser anser | egg | France, Der-Chantecoq Lake | [14] | |
| Trichobilharzia | |||||
| Trichobilharzia anseri | |||||
| KP901380.1 | Radix balthica | cercariae | Iceland, Family park, Reykjavik | [77] | |
| KP901382.1 | Anser anser | egg | France, Der-Chantecoq Lake | [77] | |
| KP901381.1 | Radix balthica | cercariae | Iceland, Family park, Reykjavik | [77] | |
| Trichobilharzia franki | |||||
| § FJ174530.1 | Radix sp. | cercariae | Czech Republic | [34] | |
| HM131200.1 | Radix auricularia | cercariae | France, Beauvais | [15] | |
| HM131197.1 | Radix auricularia | cercariae | France, Der-Chantecoq Lake | [15] | |
| Trichobilharzia mergi | |||||
| JQ681535.1 | Radix ampla | cercariae | Belarus, Naroch Lake | [78] | |
| JQ681536.1 | Radix ampla | cercariae | Belarus, Naroch Lake | [78] | |
| JX456172.1 | Mergus serrator | adult | Iceland, Botsvatn Lake | [79] | |
| JX456171.1 | Mergus serrator | adult | Iceland, Botsvatn Lake | [79] | |
| Trichobilharzia physellae | |||||
| FJ174512.1 | Aythya affinis | adult | USA, Alaska | [34] | |
| FJ174513.1 | Physa gyrina | cercaria | USA, New Mexico | [34] | |
| FJ174514.1 | Bucephala albeola | miracidia | USA, New Mexico | [34] | |
| FJ174515.1 | Aythya affinis | adult | USA, Pennsylvania | [34] | |
| FJ174516.1 | Clangula hyemalis | adult | USA, Alaska | [34] | |
| FJ174517.1 | Aythya collaris | adult | USA, New Mexico | [34] | |
| FJ174518.1 | Aythya affinis | adult | USA, New Mexico | [34] | |
| FJ174519.1 | Mergus merganser | miracidia | USA, Michigan | [34] | |
| § FJ174520.1 | Mergus merganser/Physa parkeri | miracidia/cercariae | USA, Michigan | [34] | |
| FJ174521.1 | Mergus merganser | miracidia | USA, Michigan | [34] | |
| FJ174522.1 | Aythya affinis | adult | USA, New Mexico | [34] | |
| FJ174523.1 | Physa gyrina | cercaria | USA, New Mexico | [34] | |
| MK433245.1 | Physa sp. | cercariae | USA, Michigan | [37] | |
| MK433249.1 | Common Merganser | miracidia | USA, Michigan | [37] | |
| MK433251.1 | Mallard | miracidia | USA, Michigan | [37] | |
|
* Pha1-21-001/NHBP001-21/
OL434665 |
Physella acuta | cercaria | Austria, Upper Austria | Present Study | |
|
* Pha1-21-002/NHBP002-21/
OL434663 |
Physella acuta | cercaria | Austria, Upper Austria | Present Study | |
|
* Pha2-21-001/NHBP003-21/
OL434664 |
Physella acuta | cercaria | Austria, Upper Austria | Present Study | |
|
* Pha2-21-002/NHBP004-21/
OL434662 |
Physella acuta | cercaria | Austria, Upper Austria | Present Study | |
| Trichobilharzia querquedulae | |||||
| KU057181.1 | Anas rhynchotis | adult | New Zealand, South Island | [45] | |
| FJ174498.1 | Anas discors | adult | USA, Louisiana | [34] | |
| KU057183.1 | Anas rhynchotis | adult | New Zealand, South Island | [45] | |
| FJ174497.1 | Anas clypeata | adult | USA, Louisiana | [34] | |
| Trichobilharzia regenti | |||||
| MN337555.1 | Anas platyrhynchos | egg | Iran, Azbaran | unpublished | |
| MN337560.1 | Radix auricularia | cercariae | Iran, Azbaran | unpublished | |
| NC_009680.1 | Radix peregra | cercariae | Laboratory Snail | [80] | |
| MN337557.1 | Anas platyrhynchos domesticus | egg | Iran, Sari | unpublished | |
| HM439501.1 | Mergus merganser | adult | France, Annecy Lake | [81] | |
| Trichobilharzia sp. A | |||||
| FJ174527.1 | Anas americana | adult | USA, Alaska | [34] | |
| FJ174524.1 | Anas americana | adult | USA, New Mexico | [34] | |
| Trichobilharzia sp. B | |||||
| FJ174528.1 | Anas americana | adult | USA, Alaska | [34] | |
| Trichobilharzia sp. C | |||||
| KJ855996.1 | Aix sponsa | adult | USA | [35] | |
| FJ174529.1 | Lophodytes cucullatus | adult | USA | [34] | |
| Trichobilharzia sp. D | |||||
| FJ174485.1 | Stagnicola sp. | cercariae | Canada | [34] | |
| Trichobilharzia sp. E | |||||
| FJ174483.1 | Stagnicola sp. | cercariae | Canada | [34] | |
| FJ174487.1 | Anas acuta | adult | Canada | [34] | |
| FJ174486.1 | Stagnicola sp. | cercariae | Canada | [34] | |
| Trichobilharzia sp. HAP_2013 | |||||
| KJ855995.1 | Physa marmorata | cercariae | Brazil, Espírito Santo | [35] | |
| Trichobilharzia sp. haplotype peregra | |||||
| HM131205.1 | Radix peregra | cercariae | France, Annecy Lake | [15] | |
| HM131204.1 | Radix peregra | cercariae | France, Annecy Lake | [15] | |
| HM131203.1 | Radix peregra | cercariae | France, Annecy Lake | [15] | |
| Trichobilharzia stagnicolae | |||||
| FJ174488.1 | Stagnicola sp. | cercariae | USA, Montana | [34] | |
| KT831352.1 | Stagnicola elodes | cercariae | Canada, Alberta | [36] | |
| FJ174492.1 | Stagnicola sp. | cercariae | USA, New Mexico | [34] | |
| Trichobilharzia szidati | |||||
| NC_036411.1 | Lymnaea stagnalis | cercariae | Belarus, Naroch Lake | [82] | |
| MT708493.1 | Lymnaea stagnalis | cercariae | Belarus, Naroch Lake | [83] | |
| MG570047.1 | - | - | China | unpublished | |
| JF838200.1 | Lymnaea stagnalis | cercariae | Russia, Moscow, Olympiyskaya derevnya ponds | [47] | |
Author Contributions
Conceptualization, N.H., C.H., H.S. and E.H.; field work, N.H, H.B., C.H., S.R., H.S., N.U.S. and E.H.; morphological investigations, N.H., H.B., C.H. and H.S.; laboratory procedures, N.H., S.R. and J.S.; data analysis, N.H., C.H., S.R., H.S. and E.H.; writing—original draft preparation, N.H. and H.S.; writing—review and editing, N.H, H.B., C.H., S.R., H.S., J.S., N.U.S. and E.H.; project administration, E.H. and N.U.S.; funding acquisition, E.H., C.H. and N.U.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Upper Austrian Provincial Government (Reference number: WW-583711-2019/KR) and the “Freunde des Naturhistorischen Museums Wien”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data (sequences generated in the course of the present study as well as previously published once) is available online in the NCBI Database. Additional, detailed information on the specimens sequenced in the present study is available in the BOLD Database. Appendix A Table A1 provides accession numbers for both GenBank and BOLD entries. Raw morphological measurements mentioned in the present article are provided in Supplementary Spreadsheet 1. Photomicrographs taken for the morphological measurements and used sequence alignments can be provided by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kearn G.C. Parasitism and the Platyhelminths. Chapman & Hall; London, UK: 1998. [Google Scholar]
- 2.Jamieson B.G.M. Schistosoma: Biology, Pathology, and Control. CRC Press; Boca Raton, FL, USA: 2017. [Google Scholar]
- 3.Horák P., Mikeš L., Lichtenbergová L., Skála V., Soldánová M., Brant S.V. Avian schistosomes and outbreaks of cercarial dermatitis. Clin. Microbiol. Rev. 2015;28:165–190. doi: 10.1128/CMR.00043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horák P., Kolářová L. Snails, waterfowl and cercarial dermatitis. Freshw. Biol. 2010;56:779–790. doi: 10.1111/j.1365-2427.2010.02545.x. [DOI] [Google Scholar]
- 5.Auer H., Aspöck H. Helminths and helminthoses in Central Europe: General overview and diseases caused by trematodes (flukes) Wien. Med. Wochenschr. 2014;164:405–413. doi: 10.1007/s10354-014-0316-7. [DOI] [PubMed] [Google Scholar]
- 6.Bayssade-Dufour C., Martins C., Vuong P.N. Histopathologie pulmonaire d’un modèle mammifère et dermatite cercarienne humaine. Méd. Mal. Infect. 2001;31:713–722. doi: 10.1016/S0399-077X(01)00297-9. [DOI] [Google Scholar]
- 7.Marszewska A., Cichy A., Heese T., Żbikowska E. The real threat of swimmers’ itch in anthropogenic recreational water body of the Polish Lowland. Parasitol. Res. 2016;115:3049–3056. doi: 10.1007/s00436-016-5060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macháček T., Turjanicová L., Bulantová J., Hrdý J., Horák P., Mikeš L. Cercarial dermatitis: A systematic follow-up study of human cases with implications for diagnostics. Parasitol. Res. 2018;117:3881–3895. doi: 10.1007/s00436-018-6095-0. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenbergová L., Horák P. Pathogenicity of Trichobilharzia spp. for Vertebrates. J. Parasitol. Res. 2012;2012:1–9. doi: 10.1155/2012/761968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soldánová M., Selbach C., Kalbe M., Kostadinova A., Sures B. Swimmer’s itch: Etiology, impact, and risk factors in Europe. Trends Parasitol. 2013;29:65–74. doi: 10.1016/j.pt.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Horák P., Kolářová L., Adema C. Biology of the schistosome genus Trichobilharzia. Adv. Parasitol. 2002;52:155–233. doi: 10.1016/s0065-308x(02)52012-1. [DOI] [PubMed] [Google Scholar]
- 12.Brant S.V. The occurrence of the avian schistosome Allobilharzia visceralis Kolarova, Rudolfova, Hampl et Skirnisson, 2006 (Schistosomatidae) in the tundra swan, Cygnus columbianus (Anatidae), from North America. Folia Parasitol. 2007;54:99–104. doi: 10.14411/fp.2007.013. [DOI] [PubMed] [Google Scholar]
- 13.Brant S.V., Loker E.S. Discovery-based studies of schistosome diversity stimulate new hypotheses about parasite biology. Trends Parasitol. 2013;29:449–459. doi: 10.1016/j.pt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brant S.V., Jouet D., Ferté H., Loker E.S. Anserobilharzia gen. n. (Digenea, Schistosomatidae) and redescription of A. brantae (Farr & Blankemeyer, 1956) comb. n. (syn. Trichobilharzia brantae), a parasite of geese (Anseriformes) Zootaxa. 2013;3670:193–206. doi: 10.11646/zootaxa.3670.2.5. [DOI] [PubMed] [Google Scholar]
- 15.Jouet D., Skírnisson K., Kolářová L., Ferté H. Molecular diversity of Trichobilharzia franki in two intermediate hosts (Radix auricularia and Radix peregra): A complex of species. Infect. Genet. Evol. 2010;10:1218–1227. doi: 10.1016/j.meegid.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen A., Olsen A., Buchmann K., Kania P.W., Nejsum P., Vennervald B. Molecular diversity of avian schistosomes in Danish freshwater snails. Parasitol. Res. 2015;115:1027–1037. doi: 10.1007/s00436-015-4830-3. [DOI] [PubMed] [Google Scholar]
- 17.Sattmann H., Hörweg C., Konecny R. Zerkariendermatitis in Österreich–Rückblick und Perspektiven. Denisia. 2004;13:457–461. [Google Scholar]
- 18.Hörweg C., Sattmann H., Auer H. Cercarial dermatitis in Austria: Questionnaires as useful tools to estimate risk factors? Wien. Klin. Wochenschr. 2006;118:77–80. doi: 10.1007/s00508-006-0674-2. [DOI] [PubMed] [Google Scholar]
- 19.Graefe G. Experimenteller Nachweis einer von Cercarien verursachten Dermatitis am Neusiedler See. Sitz. Akad. Wiss. Math. Klasse. Abt. 1. 1971;179:73–79. [Google Scholar]
- 20.Graefe G., Aspöck H., Picher O. Auftreten von Bade-Dermatitis in Österreich und Möglichkeiten ihrer Bekämpfung. Zentralbl. Bakteriol. Orig. A. 1973;225:398–405. [PubMed] [Google Scholar]
- 21.Dvořák J., Sattmann H., Horák P., Konecny R. Bird schistosomes from freshwater snails in Austria, with some notes on current problems (Digenea, Schistosomatidae) Mitt. Österr. Ges. Tropenmed. Parasitol. 1999;21:69–76. [Google Scholar]
- 22.Reier S., Haring E., Billinger F., Blatterer H., Duda M., Gorofsky C., Grasser H.-P., Heinisch W., Hörweg C., Kruckenhauser L., et al. First confirmed record of Trichobilharzia franki Müller & Kimmig, 1994, from Radix auricularia (Linnaeus, 1758) for Austria. Parasitol. Res. 2020;119:4135–4141. doi: 10.1007/s00436-020-06938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaub L., Sattmann H., Hörweg C., Walochnik J. Molecular validation of species determination of larval trematodes from freshwater snail hosts in Austria, with special emphasis on the genus Trichobilharzia Skrjabin & Zakharow, 1920. Arianta. 2020;8:13–19. [Google Scholar]
- 24.Auer H., Aspöck H. “Vogelbilharzien” als Erreger einer Hautkrankheit: Die Zerkarien-Dermatitis. Denisia. 2002;6:321–331. [Google Scholar]
- 25.Lydeard C., Campbell D., Golz M. Physa acuta Draparnaud, 1805 should be treated as a native of North America, not Europe. Malacologia. 2016;59:347–350. doi: 10.4002/040.059.0213. [DOI] [Google Scholar]
- 26.Vinarski M.V. The history of an invasion: Phases of the explosive spread of the physid snail Physella acuta through Europe, Transcaucasia and Central Asia. Biol. Invasions. 2017;19:1299–1314. doi: 10.1007/s10530-016-1339-3. [DOI] [Google Scholar]
- 27.Ebbs E.T., Loker E.S., Brant S.V. Phylogeography and genetics of the globally invasive snail Physa acuta Draparnaud 1805, and its potential to serve as an intermediate host to larval digenetic trematodes. BMC Evol. Biol. 2018;18:103. doi: 10.1186/s12862-018-1208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appleton C.C. Alien and invasive fresh water Gastropoda in South Africa. Afr. J. Aquat. Sci. 2003;28:69–81. doi: 10.2989/16085914.2003.9626602. [DOI] [Google Scholar]
- 29.Helmer N., Haring E., Hörweg C., Sattmann H., Szucsich N. Sensitivität des eDNA-Nachweises von Zerkariendermatitis- Erregern in oberösterreichischen Gewässern; Wien. 2021. [(accessed on 1 September 2021)]. Available online: https://www.land-oberoesterreich.gv.at/Mediendateien/Formulare/DokumenteUWDAbt_WW/ProjektberichtZerkarieneDNA2020-v2.pdf.
- 30.Talbot S.B. Studies on schistosome dermatitis: II. Morphological and life history studies on three dermatitis-producing schis-tosome cercariae, C. elvae Miller, 1923, C. stagnicolae n. sp., and C. physellae n. sp. Am. J. Epidemiol. 1936;23:372–384. doi: 10.1093/oxfordjournals.aje.a118224. [DOI] [Google Scholar]
- 31.Tanaka M. Studies on Trichobilharzia physellae in Oki Islands. 2. Four kinds of schistosome cercariae parasitic in Lymnaea japonica in Oki Island. Jpn. J. Parasitol. 1960;9:604–609. [Google Scholar]
- 32.Pence D.B., Rhodes M.J. Trichobilharzia physellae (Digenea: Schistosomatidae) from endemic waterfowl on the high plains of texas. J. Wildl. Dis. 1982;18:69–74. doi: 10.7589/0090-3558-18.1.69. [DOI] [PubMed] [Google Scholar]
- 33.Moore A.C., Burch J.B., Duda T.F. Recognition of a highly restricted freshwater snail lineage (Physidae: Physella) in southeastern Oregon: Convergent evolution, historical context, and conservation considerations. Conserv. Genet. 2014;16:113–123. doi: 10.1007/s10592-014-0645-5. [DOI] [Google Scholar]
- 34.Brant S.V., Loker E.S. Molecular systematics of the avian schistosome genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. J. Parasitol. 2009;95:941–963. doi: 10.1645/GE-1870.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto H.A., Brant S.V., De Melo A.L. Physa marmorata (Mollusca: Physidae) as a natural intermediate host of Trichobilharzia (Trematoda: Schistosomatidae), a potential causative agent of avian cercarial dermatitis in Brazil. Acta Trop. 2014;138:38–43. doi: 10.1016/j.actatropica.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Gordy M.A., Kish L., Tarrabain M., Hanington P.C. A comprehensive survey of larval digenean trematodes and their snail hosts in central Alberta, Canada. Parasitol. Res. 2016;115:3867–3880. doi: 10.1007/s00436-016-5152-9. [DOI] [PubMed] [Google Scholar]
- 37.Rudko S.P., Turnbull A., Reimink R.L., Froelich K., Hanington P.C. Species-specific qPCR assays allow for high-resolution population assessment of four species avian schistosome that cause swimmer’s itch in recreational lakes. Int. J. Parasitol. Parasit. Wildl. 2019;9:122–129. doi: 10.1016/j.ijppaw.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misaki S. Studies on the fine structure of cercariae of Trichobilharzia physellae (Talbot 1936) and Trichobilharzia ocellata (La-Valette 1855) Jpn. J. Parasitol. 1960;9:744–759. [Google Scholar]
- 39.Tanaka M. Studies on Trichobilharzia physellae in Oki Island. 1. Trichobilharzia physellae found in wild ducks in Oki Island. Jpn. J. Parasitol. 1960;9:596–603. [Google Scholar]
- 40.Dutt S.C. The Occurrence of Trichobilharzia physellae in India. Sci. Cult. 1965;31:320. [Google Scholar]
- 41.Blair D., Islam K. The life-cycle and morphology of Trichobilharzia australis n. sp. (Digenea: Schistosomatidae) from the nasal blood vessels of the black duck (Anas superciliosa) in Australia, with a review of the genus Trichobilharzia. Syst. Parasitol. 1983;5:89–117. doi: 10.1007/BF00049237. [DOI] [Google Scholar]
- 42.Podhorský M., Huůzová Z., Mikeš L., Horák P. Cercarial dimensions and surface structures as a tool for species determination of Trichobilharzia spp. Acta Parasitol. 2009;54:28–36. doi: 10.2478/s11686-009-0011-9. [DOI] [Google Scholar]
- 43.Wethington A.R., Lydeard C. A molecular phylogeny of Physidae (Gastropoda: Basommatophora) based on mitochondrial DNA sequences. J. Molluscan Stud. 2007;73:241–257. doi: 10.1093/mollus/eym021. [DOI] [Google Scholar]
- 44.Lawton S.P., Lim R.M., Dukes J.P., Cook R.T., Walker A.J., Kirk R.S. Identification of a major causative agent of human cercarial dermatitis, Trichobilharzia franki (Müller and Kimmig 1994), in southern England and its evolutionary relationships with other European populations. Parasit. Vectors. 2014;7:277. doi: 10.1186/1756-3305-7-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebbs E.T., Loker E.S., Davis N.E., Flores V., Veleizan A., Brant S.V. Schistosomes with wings: How host phylogeny and ecology shape the global distribution of Trichobilharzia querquedulae (Schistosomatidae) Int. J. Parasitol. 2016;46:669–677. doi: 10.1016/j.ijpara.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Lopatkin A.A., Chrisanfova G.G., Voronin M.V., Zazornova O.P., Beer S.A., Semyenova S.K. Polymorphism of the cox1 gene in cercariae isolates of bird schistosomes (Trematoda: Schistosomatidae) from ponds of Moscow and Moscow region. Russ. J. Genet. 2010;46:873–880. doi: 10.1134/S1022795410070136. [DOI] [PubMed] [Google Scholar]
- 47.Korsunenko A., Chrisanfova G., Lopatkin A., Beer S.A., Voronin M., Ryskov A., Semyenova S.K. Genetic differentiation of cercariae infrapopulations of the avian schistosome Trichobilharzia szidati based on RAPD markers and mitochondrial cox1 gene. Parasitol. Res. 2012;110:833–841. doi: 10.1007/s00436-011-2562-6. [DOI] [PubMed] [Google Scholar]
- 48.Duggan I.C. The freshwater aquarium trade as a vector for incidental invertebrate fauna. Biol. Invasions. 2010;12:3757–3770. doi: 10.1007/s10530-010-9768-x. [DOI] [Google Scholar]
- 49.Facon B., Pointier J.-P., Glaubrecht M., Poux C., Jarne P., David P. A molecular phylogeography approach to biological invasions of the New World by parthenogenetic Thiarid snails. Mol. Ecol. 2003;12:3027–3039. doi: 10.1046/j.1365-294X.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- 50.Pinto H., de Melo A. A checklist of trematodes (Platyhelminthes) transmitted by Melanoides tuberculata (Mollusca: Thiaridae) Zootaxa. 2011;2799:15–28. doi: 10.11646/zootaxa.2799.1.2. [DOI] [Google Scholar]
- 51.Graham A.L. Effects of snail size and age on the prevalence and intensity of avian schistosome infection: Relating laboratory to field studies. J. Parasitol. 2003;89:458–463. doi: 10.1645/0022-3395(2003)089[0458:EOSSAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.DAK Seltene Vögel in Deutschland 2013. 2014. [(accessed on 11 August 2021)]. pp. 2–39. Available online: https://www.dda-web.de/downloads/publications/statusreports/svid_2013_seltenheitenbericht.pdf.
- 53.Maumary L., Martinez N. Seltene Vogelarten und ungewöhnliche Vogelbeobachtungen in der Schweiz im Jahre 2018. Der Ornithol. Beobachter. 2019;116:241–274. [Google Scholar]
- 54.Ranner A., Khil L. Records of rare and remarkable bird species in Austria 2007–2009. Sixth report of the Avifaunistic Commission of BirdLife Austria. Egretta. 2011;52:13–32. [Google Scholar]
- 55.Šrank V. The 6th Report of the Slovak Rarities Committee. Tichodroma. 2006;18:145–147. [Google Scholar]
- 56.MME Nomenclator Committee MME Nomenclator Committee Annual Report 2014 on the Occurrence of Rare Bird Species in Hungary. 2014. [(accessed on 11 August 2021)]. pp. 1–15. Available online: http://www.birding.hu/doc/NB2014.pdf.
- 57.Fracasso G., Janni O., Fulco E., Liuzzi C. Commissione Ornitologica Italiana (COI)—Report 27. Avocetta. 2018;42:45–54. [Google Scholar]
- 58.DAK Seltene Vögel in Deutschland 2017. 2019. [(accessed on 11 August 2021)]. pp. 2–34. Available online: https://www.dda-web.de/downloads/publications/statusreports/svid_2017_seltenheitenbericht.pdf.
- 59.Marques D.A., Jaquier S. Seltene Vogelarten und ungewöhnliche Vogelbeobachtungen in der Schweiz im Jahre 2019. Ornithol. Beob. 2020;117:312–337. [Google Scholar]
- 60.Vavřík M., Šírek J. FK Čso Rare birds in the Czech Republic in 2019. Sylvia. 2020;56:93–114. [Google Scholar]
- 61.Keller V. The Goosander Mergus merganser population breeding in the Alps and its connections to the rest of Europe. Wildfowl. 2009;special issue 2:60–73. [Google Scholar]
- 62.Weißmair W. The goosander (Mergus merganser) in Upper-Austria–Breeding population in 2016/2017 and wintering population 1996–2016. Vogelkdl. Nachr. aus Oberösterr. Nat. Aktuell. 2019;27:3–35. [Google Scholar]
- 63.Lockyer A.E., Olson P.D., Østergaard P., Rollinson D., Johnston D.A., Attwood S.W., Southgate V.R., Horak P., Snyder S.D., LE T.H., et al. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology. 2003;126:203–224. doi: 10.1017/S0031182002002792. [DOI] [PubMed] [Google Scholar]
- 64.Duda M., Schindelar J., Macek O., Eschner A., Kruckenhauser L. First record of Trochulus clandestinus (Hartmann, 1821) in Austria (Gastropoda: Eupulmonata: Hygromiidae) Malacol. Bohemoslov. 2016;16:37–43. [Google Scholar]
- 65.Nicholas K.B., Nicholas H.B.J. GeneDoc: A tool for editing and annotating multiple sequence alignments. Embnet. News. 1997;4:1–4. [Google Scholar]
- 66.Kumar S., Stecher G., Li M., Knyaz C., Tamura K., Battistuzzi F.U. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoang D.T., Chernomor O., Von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalyaanamoorthy S., Minh B.Q., Wong T., Von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., von Haeseler A., Lanfear R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chernomor O., Von Haeseler A., Minh B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016;65:997–1008. doi: 10.1093/sysbio/syw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Hoehna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Letunic I., Bork P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bandelt H.J., Forster P., Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 75.Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J.C., Guirao-Rico S., Librado P., Ramos-Onsins S., Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 76.Brant S.V. ((University of New Mexico, Albuquerque, NM, USA)). Personal communication. 2021.
- 77.Jouet D., Kolářová L., Patrelle C., Ferté H., Skírnisson K. Trichobilharzia anseri n. sp. (Schistosomatidae: Digenea), a new visceral species of avian schistosomes isolated from greylag goose (Anser anser L.) in Iceland and France. Infect. Genet. Evol. 2015;34:298–306. doi: 10.1016/j.meegid.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 78.Chrisanfova G.G., Lopatkin A.A., Mishchenkov V.A., Kheidorova E.E., Dorozhenkova T.E., Zhukova T.V., Ryskov A., Semyenova S.K. Genetic variability of bird schistosomes (class Trematoda, family Schistosomatidae) of Naroch Lake: Identification of a new species in the Trichobilharzia ocellata group. Dokl. Biochem. Biophys. 2009;428:268–272. doi: 10.1134/S1607672909050123. [DOI] [PubMed] [Google Scholar]
- 79.Kolářová L., Skírnisson K., Ferté H., Jouet D. Trichobilharzia mergi sp. nov. (Trematoda: Digenea: Schistosomatidae), a visceral schistosome of Mergus serrator (L.) (Aves: Anatidae) Parasitol. Int. 2013;62:300–308. doi: 10.1016/j.parint.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Webster B.L., Rudolfová J., Horák P., Littlewood D.T.J. The complete mitochondrial genome of the bird schistosome Trichobilharzia regenti (Platyhelminthes: Digenea), causative agent of cercarial dermatitis. J. Parasitol. 2007;93:553–561. doi: 10.1645/GE-1072R.1. [DOI] [PubMed] [Google Scholar]
- 81.Jouet D., Skírnisson K., Kolářová L., Ferté H. Final hosts and variability of Trichobilharzia regenti under natural conditions. Parasitol. Res. 2010;107:923–930. doi: 10.1007/s00436-010-1953-4. [DOI] [PubMed] [Google Scholar]
- 82.Semyenova S., Chrisanfova G., Mozharovskaya L., Guliaev A., Ryskov A. The complete mitochondrial genome of the causative agent of the human cercarial dermatitis, the visceral bird schistosome species Trichobilharzia szidati (platyhelminthes: Trematoda: Schistosomatidae) Mitochondrial DNA Part B. 2017;2:469–470. doi: 10.1080/23802359.2017.1347833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chrisanfova G., Mozharovskaya L., Zhukova T., Nefedova D., Semyenova S. Non-coding Regions of mitochondrial DNA and the cox1 gene reveal genetic variability among local Belarusian populations of the causative agent of cercarial dermatitis, bird schistosome Trichobilharzia szidati (Digenea: Schistosomatidae) Acta Parasitol. 2021:1–11. doi: 10.1007/s11686-021-00371-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data (sequences generated in the course of the present study as well as previously published once) is available online in the NCBI Database. Additional, detailed information on the specimens sequenced in the present study is available in the BOLD Database. Appendix A Table A1 provides accession numbers for both GenBank and BOLD entries. Raw morphological measurements mentioned in the present article are provided in Supplementary Spreadsheet 1. Photomicrographs taken for the morphological measurements and used sequence alignments can be provided by the corresponding author upon request.