Abstract
Irreversible G1 arrest in senescent human fibroblasts is mediated by two inhibitors of cyclin-dependent kinases (Cdks), p21Cip1/SDI1/WAF1 and p16Ink4A. To determine the physiological and molecular events that specifically require p21, we studied senescence in human diploid fibroblasts expressing the human papillomavirus type 16 E6 oncogene, which confers low p21 levels via enhanced p53 degradation. We show that in late-passage E6 cells, high Cdk activity drives the cell cycle, but population expansion is slowed down by crisis-like events, probably owing to defective cell cycle checkpoints. At the end of lifespan, terminal-passage E6 cells exhibited several aspects of the senescent phenotype and accumulated unphosphorylated pRb and p16. However, both replication and cyclin-Cdk2 kinase activity were still not blocked, demonstrating that phenotypic and replicative senescence are uncoupled in the absence of normal p21 levels. At this stage, E6 cells also failed to upregulate p27 and inactivate cyclin-Cdk complexes in response to serum deprivation. Eventually, irreversible G1 arrest occurred coincident with inactivation of cyclin E-Cdk2 owing to association with p21. Similarly, when p21−/− mouse embryo fibroblasts reached the end of their lifespan, they had the appearance of senescent cells yet, in contrast to their wild-type counterparts, they were deficient in downregulating bromodeoxyuridine incorporation, cyclin E- and cyclin A-Cdk2 activity, and inhibiting pRb hyperphosphorylation. These data support the model that the critical event ensuring G1 arrest in senescence is p21-dependent Cdk inactivation, while other aspects of senescent phenotype appear to occur independently of p21.
Human diploid fibroblasts (HDFs) have a finite proliferative lifespan, at the end of which they cease irreversibly to divide and they undergo a series of phenotypic changes that distinguish senescence from quiescence (26). These phenotypic changes include altered morphology, increased cell volume, expression of a neutral senescence-associated β-galactosidase activity (SA–β-Gal), and increased production of extracellular matrix degradative enzymes such as collagenase and stromelysin (26, 40, 61). It is now generally accepted that two inhibitors of cyclin-dependent kinases (Cdks), p16Ink4a (p16) and p21Cip1/Waf1/Sdi1 (p21), whose amounts increase with age, have an essential role in inactivating Cdks in senescent fibroblasts (1, 24, 42, 44, 60). Cdk inactivation, in turn, allows the accumulation of unphosphorylated retinoblastoma protein (pRb) (59), a growth suppressor whose function is modulated by Cdks. Unphosphorylated pRb exerts negative regulation of cell cycle progression by forming complexes with members of the E2F transcription factor family (23, 28).
In spite of their undisputed role in mediating senescence, the precise contribution of each Cdk inhibitor (CKI) is not fully established. The CKI p21 binds to and inactivates most cyclin-Cdk complexes, whereas p16 blocks cyclin D-Cdk activation by binding specifically to Cdk4 and Cdk6, thus preventing their association with cyclin D (57). Although several investigators proposed that both inhibitors play a role in causing the senescent G1 arrest (1, 24), our recent results raised the possibility that inactivation of Cdk-cyclin complexes and subsequent G1 arrest in senescent fibroblasts is initially accomplished by p21 alone and occurs prior to p16 accumulation (60). Therefore, we proposed that p16 is upregulated as part of a program terminal initiated at the end of lifespan and that it is involved in maintenance of the senescent arrest (60). The predominant role of p21 in senescence is also supported by results showing that specific inactivation of p21 in HDFs bypasses senescence in spite of p16 accumulation (7).
The antiproliferative signals provoking the elevation of p21 levels in senescent cells are thought to be generated by telomere shortening, but the precise mechanism for this is not known. Factors that compromise p53 activity, such as simian virus 40 large T antigen or human papillomavirus type 16 (HPV-16) E6 oncogene, interfere with the accumulation of p21, suggesting that the age-dependent p21 increase is p53 dependent (5, 49, 53, 55). In this way, cellular senescence is similar to radiation-induced cell cycle arrest, which is also mediated by p53-dependent accumulation of p21 (18).
HDFs expressing HPV-16 E6 oncoprotein have an extended lifespan (55), but the behavior of these cells at the end of the lifespan is not understood. For example, Filatov et al. (21) reported that replicative senescence was inactivated in HDFs expressing HPV-16 E6 and, consequently, these cells had an extended lifespan that ended in crisis without expression of the senescent phenotype, as measured by SA–β-Gal activity. In contrast, Bond et al. (4) found that HDFs expressing HPV-16 E6 had an extended lifespan that ended with arrest in a senescence-like state, characterized by the senescent morphology and expression of SA–β-Gal activity. Thus, conflicting outcomes were obtained in these two studies.
The mechanism for the senescent cell cycle arrest in E6 cells is also not well defined. Bond et al. (4) suggested that a senescence-associated increase in p21 does not occur in E6 cells because senescent E6 cells have less p21 than wild-type controls and that the senescence-like cell cycle arrest of E6 cells is probably mediated by the increase in p16 that occurs at the end of lifespan. Although those experiments indicated clearly that senescent E6 cells have less p21 than wild-type controls, they could not show directly whether there is an age-associated increase in p21 in E6 cells because the HDFs were grown to near senescence before they were transfected with HPV-16 E6. Moreover, the effect of p16 on the formation of cyclin D1–Cdk4 and -Cdk6 complexes was not assessed, thereby leaving unanswered the question of whether elevated p16 is sufficient to mediate the senescence-like cell cycle arrest of E6 cells.
Finally, our previous results indicated that elevated p16, increased cell volume, and expression of SA–β-Gal activity occur primarily after the senescent cell cycle arrest is initiated in wild-type HDFs, suggesting that these senescence-associated changes might be dependent on prior elevation of p21 and initiation of the senescent cell cycle arrest (60). Likewise, Millis et al. (40) showed that there is a dramatic increase in the expression of collagenase at the very end of the lifespan in HDFs, suggesting that fibroblast functions are also altered in response to the senescent cell cycle arrest. These results raised the question of whether senescence-associated changes can take place in E6 cells independently of a high p21-mediated senescent cell cycle arrest. Since our work was submitted, Wei and Sedivy (65) reported that p21−/− HDFs express SA–β-Gal activity while remaining replicative, thereby supporting the hypothesis that the senescence program is not attenuated by downregulation of p21.
We have studied the cell cycle machinery and the phenotype of E6 cells throughout their lifespan to answer the questions raised above. We have also studied wild-type and p21−/− mouse embryo fibroblasts (MEFs) to test our hypotheses further. Our results show that a single population of E6 cells can exhibit both crisis and senescence and demonstrate how the cell cycle machinery is affected in each of these stages, with several surprising results. First, the small amount of p21 in E6 cells plays a crucial role in mediating their senescent cell cycle arrest. Second, in terminal passage E6 cultures that are not yet senescent the cyclin E-Cdk2 and cyclin A-Cdk2 kinase activities are high and the cells are replicative in spite of a lack of pRb hyperphosphorylation. Third, terminal-passage E6 cells express the senescence-associated phenotype even though the population is still replicative. Fourth, in contrast to wild-type cells and in spite of high p16 amounts, late-passage and senescence-like p21−/− MEFs are not able to block DNA synthesis.
MATERIALS AND METHODS
Cell culture and markers of the senescence phenotype.
IMR90 HDFs from the fetal lung were cultured as described earlier (60). DNA synthesis (percent labeled nuclei) was determined by autoradiography of cells labeled with [3H]thymidine for 24 h. The relative cell volume was calculated from the volume of the pellet formed by 4 × 106 freshly trypsinized cells centrifuged at 100 × g for 5 min. The results were consistent with the cell volume calculated on the basis of cell diameter in a hemacytometer. SA–β-Gal activity was determined by the method of Dimri et al. (16). Steady-state amounts of p21, collagenase (MMP-1), and β-actin mRNAs were determined by Northern blotting with cDNA probes, as previously described (17, 40).
IMR90-E6 cells, prepared by retroviral transfer of the HPV-16 E6 oncogene in the PLXSN vector, were provided generously by Jerry Shay (The University of Texas SW Medical Center, Dallas) (55). These cells were passaged according to the same protocol used for wild-type HDFs. MEFs from p21−/− mice (8) were provided generously by Tyler Jacks (Massachusetts Institute of Technology, Cambridge).
Immunofluorescence microscopy.
The conditions for the cell fixation, double-immunolabeling, and mounting were as described previously (19). Immunofluorescence and phase-contrast photomicrographs were image captured, and a composite generated using Adobe Macintosh Photoshop or Microsoft Powerpoint software. For the time-lapse microscopy experiments (Hamamatsu software), cells were kept in a temperature- and CO2-controlled chamber.
Immunoprecipitations, immunoblot analysis, and antibodies.
Preparation of whole-cell lysates from frozen cell pellets, conditions for immunoprecipitation, histone H1 kinase assays, and immunoblotting have been described previously (17). For p21 or p27 depletion, total cell extracts (usually 100 to 150 μg) were incubated with saturating amounts of p21- and p27-specific antibodies, whereas mock samples were incubated with protein A-Sepharose beads only. The resulting supernatants were analyzed by immunoblotting and/or used for further immunoprecipitation with cyclin-specific antibodies as described previously (60). Proteins were visualized by enhanced chemiluminescence (ECL; Amersham) and quantitated by densitometry using Adobe Photoshop software. In some cases the same immunoprecipitates were used both for the determination of histone H1 kinase activity and for the immunoblot detection of various components. This was accomplished by a controlled partial transfer of 12% gels following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The resulting membrane was then used for immunoblot analysis using the indicated antibodies, whereas the gel was stained with Coomassie blue and dried, and the histone H1 bands were excised and counted by the Cerenkov method as described previously (17).
The round mitotic-cell-like cells from late passage E6 cultures (population-doubling numbers [PDs] of 77 and 78) were harvested by vigorous pipetting and pelleted by low-speed centrifugation. The equivalent of 20 100-mm dishes was needed for approximately 200 μg of total cell extracts.
Most of the primary antibodies used in this study were the same as described previously (19, 60). Anti-cyclin D2 antibody was from Gilles Ponzio (INSERM, Nice, France) (48), anti-Skp2 antibody was from Thierry Lorca (CRBM, Montpellier, France), and anti-HPV16-E6 antibody was from Martin Mueller (Heidelberg, Germany). In addition, commercially available antibodies used were anti-p27 (sc-528), anti-p53 (DO-1, sc-126), anti-HPV16-E6 (C-19, sc-1583), and anti-p107 (C-18, SC-318) from Santa-Cruz Biotechnology and anti-phospho-pRb (Ser780) from MBL, Watertown, Mass. Secondary antibodies were anti-mouse and anti-rabbit immunoglobulin G-horseradish peroxidase conjugates (Promega).
RESULTS
Replication-associated events do not decrease in late-passage HDFs expressing HPV-16 E6 oncogene.
To understand whether senescence is possible in the absence of high amounts of p21 and, if so, what the alternative mechanisms are whereby the cell accomplishes the cell cycle arrest, we studied the in vitro aging of HDFs (IMR-90) expressing the HPV-16 E6 oncogene (55). Since senescence is a dynamic process, which includes both a block of replication events and numerous morphological and biochemical changes conferring the senescence phenotype, we first sought to define different stages during the cellular aging of E6 cells compared with wild-type HDFs. We measured several parameters, including [3H]thymidine incorporation, serum response, SA–β-Gal staining, cell volume, and population density that could help in determining both the onset of senescence, defined as an irreversible cell cycle arrest in G1 (<5% labeled nuclei) and the appearance of the senescence phenotype. In wild-type cells, the end of lifespan (EOL) stage, i.e., the stage when the population undergoes no further expansion, coincides with cell cycle arrest, but a complete expression of the senescence phenotype, characterized by strong SA–β-Gal staining (16), a fivefold increase in cell volume, and maximal p16 elevation, is achieved more slowly with kinetics that suggest that these changes occur after the senescent cell cycle arrest is achieved (60). We termed this stage “late senescence” (Fig. 1).
FIG. 1.
In vitro aging of wild-type and E6 HDFs. (A) Growth curves (PD versus time) for wild-type HDF (WT) and E6 cells were aligned at the EOL, defined as the time when no further PDs were achieved. The percentages of quiescent or senescent cells able to synthesize DNA after serum stimulation were measured by autoradiography of cells labeled with [3H]thymidine for 24 h. EOL coincided with senescent cell cycle arrest (SEN) in wild-type HDFs but not in E6 cells. Terminal-passage (T.p.) E6 cells at EOL have distinct characteristics that are described in the text. EOL, no further population doublings; SEN, senescence; −, irreversible G1 arrest; L.S., late senescent stage, characterized by elevated p16 and positive SA–β-Gal staining. (B) Relative cell size and SA–β-Gal staining (16) of the same populations as in panel A. (C) Northern blot analysis showing mRNA levels of p21, collagenase, and β-actin in asynchronous early-passage (EP) and terminal-passage (TP) wild-type and E6 HDFs. The amount of p21 mRNA normalized to β-actin mRNA increased six- to sevenfold in terminal-passage cells versus early-passage cells for both wild-type and E6 cells, even though E6 cells have much lower amounts of p21 mRNA overall.
Even though most growth properties of early-passage E6 cells did not differ significantly from their wild-type counterparts, late-passage (PD > 60) E6 cells exhibited several characteristics that suggested a greatly perturbed senescence process (Fig. 1). First, late-passage E6 cells became more replicative in response to serum stimulation, in contrast to the decreased replicative response of comparably passaged wild-type cells. Second, E6 cells continued to replicate even in terminal-passage cultures, i.e., the point at which no more population doublings could be achieved. Third, terminal-passage E6 cells acquired several phenotypic characteristics of senescent wild-type cells, such as increased cell volume, SA–β-Gal accumulation and increased expression of collagenase (Fig. 1). These results demonstrated that cell cycle arrest and the senescence-related phenotype are not necessarily coupled. Eventually, and at a somewhat variable PD (PDs of 79 to 87), E6 cells ceased to replicate, a finding which was in agreement with the results published earlier by several groups (4, 10).
Our observations raised two questions. First, how can sustained replication take place at the same time as decreasing population expansion? Second, what are the mechanisms whereby senescent E6 cells finally achieve cell cycle arrest?
Abortive mitoses increase in aging E6 cells.
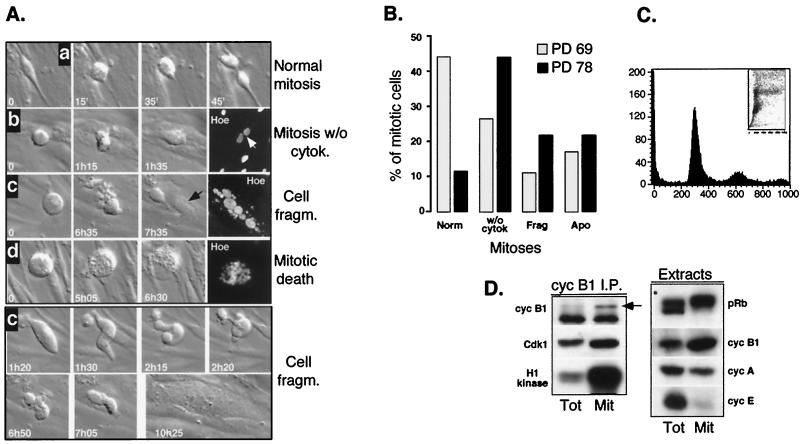
Late-passage E6 cells exhibited both DNA replication (Fig. 1A) and an accumulation of large, round, mitotic-cell-like cells, whose numbers increased significantly toward the EOL, in parallel with a decrease of the population expansion. The fate of these cells was determined by using time-lapse microscopy, a technique that enables continuous viewing of individual cells. By comparing two different late passage E6 populations (PDs of 69 and 78), we found that the number of mitotic cells undergoing successful cytokinesis decreased dramatically as the cells approached their terminal passage. Instead, an increasing number of cells underwent: (i) mitosis without cytokinesis, resulting in binucleate cells; (ii) transient fragmentation followed by resealing, giving rise to cells with fragmented nuclei; and (iii) cell death, presumably by apoptosis (Fig. 2A and B). This mitotic-cell-like state often lasted for several hours, during which the cell exhibited rapid mobility and changing of shape (Fig. 2A, lower panel). Microscopic analysis of Hoechst- or orcein-stained cells indicated that most mitotic cells were in a metaphase-like stage (data not shown). In addition, the later-passage E6 population became increasingly aneuploid, presumably owing to increasing endoreplication events in the absence of the G2/M checkpoint (Fig. 2C) (21, 67). In contrast to late-passage E6 cells, in late-passage wild-type HDF populations virtually all mitotic cells completed cytokinesis, and very few binucleate cells could be observed (data not shown).
FIG. 2.
Increasing abortive mitoses in old E6 populations. (A) In the upper panel are shown the fates of individual mitotic cells from two different old E6 populations (PD 69 and PD 78) monitored at 5-min intervals by time-lapse microscopy. Representative micrographs of mitoses giving rise to normal (a), binucleate (b), multinucleate (c), and apoptotic (d) cells are shown. All micrographs were taken at the same magnification. Hoechst 33258-stained micrographs (corresponding to these situations) were taken from formaldehyde-fixed cells. In the lower panel, a more-detailed sequence of the cell presented in the c panel showing rapid changing of shape and position is presented. Cell movements were obvious even on a “real-time” scale. (B) Quantitative analysis of time-lapse microscopy-acquired data showing different outcomes of mitotic events observed in old E6 populations. Norm, normal; w/o cytok, without cytokinesis; Frag, fragmented; Apo, apoptotic. Note that aberrant mitoses and cell death were also frequently observed in the early-old E6 cells (PD 69). (C) Fluorescence-activated cell sorter (FACS) analysis of old (PD 78) E6 cells. The cells were serum deprived for 48 h to show the absence of cell cycle arrest. (D) Western blot and histone H1 kinase analysis of cyclin B1 immunoprecipitates (I.P.) isolated from lysates prepared from whole-cell population (Tot) and round mitotic (Mit) E6 cells (PD 77). Floating cells corresponded to the mitotic population examined in panels A and B. Total cell extracts were also analyzed for pRb, cyclin A, cyclin E, and cyclin B1 content, as indicated.
To analyze further the actual cell cycle stage of the round cells, their extracts were assayed for cyclin B1-associated kinase activity and for the presence of several cell cycle regulators. Indeed, when compared to the total cell population (Tot), the round cells (mitotic-Mit) contained extremely high cyclin B1-associated kinase activity, and all Cdk1 (cyclin B1-associated and total) was in its fully dephosphorylated/active state (Fig. 2D). Importantly, the presence of hyperphosphorylated pRb suggested that the events leading to aberrant mitoses occurred during a stage of the lifespan when the Cdks were fully active. Thus, these results provide an explanation for the slowdown and cessation of population doublings in late-passage E6 populations in the absence of cell cycle arrest.
“Uncoupling” between pRb phosphorylation, G1 arrest, and cyclin-Cdk2 activity in terminal-passage E6 cells.
As mentioned earlier, the accumulation of underphosphorylated pRb in wild-type senescent cells occurs concomitantly with p21-mediated inactivation of cyclin E-Cdk2 kinase and with increased association of p21 with cyclin D1-Cdk complexes, such that all cyclin D1-Cdk6 and almost all cyclin D1-Cdk4 complexes are p21 bound (17, 59, 60). Therefore, one would not expect a comparable inhibition of p21-deficient E6 cells at the EOL. The presence of hyperphosphorylated pRb in late-passage E6 cells at the EOL stage (Fig. 2D) was in agreement with this expectation and with their lack of cell cycle arrest as measured by continued DNA synthesis and mitosis (Fig. 1A and 2A). However, after EOL, the terminal-passage E6 cells started to accumulate underphosphorylated pRb (Fig. 3A), suggesting that cyclin-Cdk complexes were being inactivated. To test this hypothesis directly, we examined the cyclin E- and cyclin A-associated kinase activities in immunoprecipitates isolated from cell extracts prepared from wild-type and E6 cells at the indicated stages. In addition, total cyclin and Cdk contents were assessed by Western blot analysis. The results presented in Fig. 3B revealed several important findings.
FIG. 3.
Cyclin-Cdk2 inactivation is uncoupled from pRb dephosphorylation in senescing E6 cells. (A) Western blot analysis of pRb, p107, and P-S780 in total cell lysates prepared from early-passage (EP), late-passage (LPa), terminal-passage (TPa), and two senescent (Sa and Sb) populations of E6-expressing HDFs that were serum starved (lanes 0) or serum stimulated for 16 h (lanes 16). P-S780 denotes an antibody directed against phosphoserine 780 of pRb, which is specifically phosphorylated by cyclin D1 (30). (B) Histone H1 kinase activity and Western blot analysis of cyclin E and cyclin A immunocomplexes from wild-type HDFs and E6 cells. Cyclin E and cyclin A immunoprecipitates, isolated from the designated cell extracts, were assayed for their kinase activities using histone H1 as a substrate and then subjected to SDS-PAGE. Proteins were partially transferred to polyvinylidene difluoride (PVDF) membrane and analyzed by Western blot for their cyclin and Cdk contents. The remaining gel was subjected to autoradiography. Cell lysates were prepared from the following wild-type (W.T.) or E6 cell populations: wild type, early-passage (PD 27.5), senescent (PD 76), and late senescent (PD 78); E6, early-passage (PD 45), late passage (LPa; PD 74.5), terminal passage (TPa; PD 83), and three different senescent populations designated Sa (PD 87), Sb (PD 80), and Sc (PD 79). (C and D) Western blot analysis of designated cell extracts using anti-p27 and anti-Skp2 antibodies. As a positive control for Skp2, HDFs expressing HPV E7 oncogene and HeLa cells were used.
Terminal-passage E6 cells contained very high cyclin E- and cyclin A-associated kinase activities in both serum-starved and serum-stimulated cells and were actively engaged in DNA synthesis under both conditions (24 and 28% [3H]thymidine-labeled nuclei, respectively). Moreover, the levels of p107, the pRb-related pocket protein whose protein amounts and phosphorylation by Cdks greatly increase during G1/S-phase progression (3, 66), were highly upregulated even in the absence of serum (Fig. 3A). These results were very surprising for three reasons. First, they indicate that there is a dramatic loss of serum regulation in terminal-passage E6 cells. Second, high cyclin E- and cyclin A-associated kinase activities are at odds with the strongly diminished hyperphosphorylation of pRb in these cells, implying that pRb phosphorylation is blocked before the inactivation of cyclin-Cdk2 complexes. Third, since the cells were able to initiate replication events and synthesize DNA in the presence of underphosphorylated pRb, these results suggest that at the end of lifespan, E6 cells experience an uncoupling between the pRb pathway (but not the p107 pathway) and the replication machinery. A similar effect is seen to a lesser degree in late-passage E6 cells when pRb hyperphosphorylation is decreased even though cyclin E- and A-Cdk2 activity is high. However, when E6 cells eventually became senescent, both cyclin E- and cyclin A-associated kinases had low activity, although their inactivation was not as efficient as in wild-type cells. Moreover, p107 protein amounts in these cells are comparable to those of quiescent young cells (Fig. 3A). Thus, terminal-passage E6 cells revealed the unexpected result that DNA synthesis and high cyclin-Cdk2 activity are uncoupled from pRb hyperphosphorylation, whereas senescent E6 cells revealed that their cell cycle arrest occurred in conjunction with reduced cyclin-Cdk2 activity in spite of their low amounts of p21 and p27.
The lack of cyclin E- and A-Cdk2 inactivation in serum-deprived terminal-passage E6 cells was unexpected because p27Kip1, rather than p21, is usually responsible for Cdk inactivation in serum-starved cells (57). However, in contrast to early- and late-passage E6 cells, terminal-passage E6 cells fail to upregulate p27 when serum-deprived (Fig. 3C). Recently, p45Skp2 was shown to be the substrate recognition subunit of the ubiquitin-protein ligase that targets p27 for degradation (13, 62). Moreover, when overexpressed, p45Skp2 prevented exit from the cell cycle upon serum withdrawal (69). Because this mimicked the behavior of terminal-passage E6 cells, we investigated whether p45Skp2 overexpression might be responsible for the lack of p27 in these cells. Our data indicate that there is no significant difference in the amount of p45Skp2 in early-, late-, and terminal-passage E6 cells, suggesting that a simple increase in p45Skp2 is not the mechanism involved (Fig. 3D). Note that there were high Skp2 levels in late-passage E6 cells, as well as in tumor-derived HeLa cells and in HDF-expressing HPV-E7 oncoprotein. Interestingly, these experiments revealed that both wild-type and E6 cells have greatly reduced amounts of both p45Skp2 and p27 when they are senescent. These results suggest that, at senescence, factors other than p45Skp2 promoted degradation are responsible for the low amount of p27 in both serum-deprived and serum-stimulated cells. On the other hand, decreased p45Skp2 in senescent cells might play a role in the accumulation of p21 and cyclin D1 because Yu et al. found that inhibition of human p19Skp1–p45Skp2–CUL-1 complexes led to an increase of p21 and cyclin D1, while having no effect on amount of p27 (68).
Cell cycle arrest in senescent E6 cells is mediated by p21-dependent cyclin E inactivation.
The results showing inactivation of cyclin E-Cdk2 and irreversible cell cycle arrest in senescent E6 cells raised several questions regarding the mechanism underlying these events. Since the presence of hyperphosphorylated Cdk2 in cyclin E complexes excluded inactivation of Cdk-activating kinase as a mechanism for decreased cyclin E-Cdk2 activity (Fig. 3B), we predicted that their inactivation could be mediated by CKIs. However, as shown in Fig. 4A, although late-passage and senescent E6 cells contained somewhat increased p21 levels, these were still very low compared to wild-type cells and were not compensated for by p27 upregulation.
FIG. 4.
Cyclin E-Cdk2 inactivation in senescing E6 fibroblasts is p21 dependent. (A) Western blot analysis of total cell extracts prepared from designated cell populations described in the legend for Fig. 3, except for late-passage E6 cells (LPb; PD 79). (B) Western blot analysis of p21 immunoprecipitates isolated from the extracts prepared from senescent wild-type (WT), serum-starved (0 h) and serum-stimulated (16 h) early-passage (EP; PD 45), and stimulated (16 h) senescent (S) E6 cells. (C) p21 immunodepletion experiments. Cell extracts from serum starved (0 h) and serum stimulated (16 h) early-passage (EP; PD 45), and stimulated (16 h) senescent (S; PD 79 + PD 80) E6 cells were depleted for p21 by using specific antibodies. Mock-treated extracts are designated “−”. Following depletion, supernatants were tested for immunodepletion efficiency (not shown) and the presence of cyclin E. Cyclin E complexes (cyc E I.P.) were precipitated from all supernatants, and the Cdk2 content was determined by Western blotting using anti-PSTAIRE antibody. In addition, the same immunoprecipitates were tested for histone H1 kinase activity (H1 kinase).
To determine whether the low amount of p21 in senescent E6 cells could be responsible for inactivation of cyclin E-Cdk2, we examined the effect of complete removal of p21-containing complexes by immunoprecipitation (Fig. 4C). In early-passage E6 cells, as expected, there was no effect of p21 depletion on either the amount of cyclin E-Cdk2 or its activity. In contrast, in serum-stimulated senescent E6 cells, where the cells have only one-third as much cyclin E-associated kinase activity as in early passage cells, p21 depletion removed the vast majority of the cyclin E-associated Cdk2. As seen previously in wild-type HDFs, only p21-free cyclin E-Cdk2 complexes have activity (60). Taken together, these data indicate that the majority of the cyclin E-Cdk2 complexes in senescent E6 cells are inhibited owing to their association with p21. The related CKI p27 may also play a modest role because a smaller fraction of cyclin E-associated Cdk2 is removed by p27 depletion (data not shown). Thus, our results strongly suggest that increasing association with p21 is predominantly responsible for inactivation of cyclin E-Cdk2 kinase and cell cycle arrest in senescent E6 cells.
In contrast to the continued abundance of cyclin E-Cdk2 complexes in senescent E6 cells, there is a dramatic decrease in the amount of cyclin A and therefore of cyclin A-Cdk2 complexes in these cells (Fig. 3B). Moreover, p21 and p27 immunodepletion did not significantly affect the amount of cyclin A-Cdk complexes in serum-stimulated senescent E6 cells (data not shown). These results imply that in senescent E6 cells, as in senescent wild-type HDFs, cyclin A-associated kinase activity is reduced primarily through decreased complex formation.
Accumulation of underphosphorylated pRb correlates with increasing p21-cyclin D1-Cdk association.
Even though Cdk-dependent pRb phosphorylation takes place throughout the cell cycle, it is widely accepted that pRb is initially phosphorylated at mid-G1 by cyclin D-Cdk4 and cyclin D-Cdk6 complexes (cyclin D-Cdk4/Cdk6 complexes) (25, 48). Consequently, the accumulation of underphosphorylated pRb in terminal-passage E6 cells containing high cyclin E- and cyclin A-associated kinase activities might occur because prior phosphorylation of pRb by cyclin D-Cdk complexes is reduced. Indeed, previous studies had suggested that a lack of cyclin D-Cdk complex formation owing to accumulation of p16 might be the mechanism for the senescent cell cycle arrest in p21-deficient E6 cells (4). To determine whether cyclin D1-dependent pRb phosphorylation occurred in terminal-passage and senescent E6 cells, we analyzed the phosphorylation of pRb on Ser780, a site known to be phosphorylated specifically by cyclin D-Cdk4/Cdk6 complexes (30). Our data indicate that phosphorylation at pRb-Ser780 is greatly reduced in terminal-passage E6 cells and is negligible in senescent E6 cells (Fig. 5A; cf. Fig. 3A). In all of our experiments, phosphorylation of pRb-Ser780 and hyperphosphorylation of pRb changed coordinately, a result consistent with the idea that cyclin D-Cdk4/Cdk6 complex phosphorylation of Ser780 may be necessary for subsequent hyperphosphorylation of pRb.
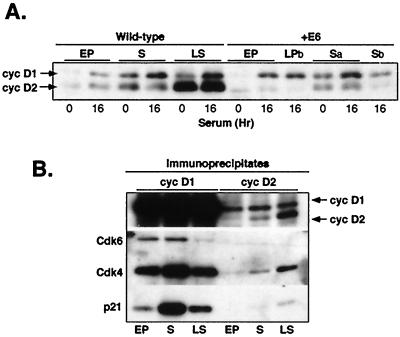
FIG. 5.
Accumulation of underphosphorylated Rb correlates with increasing p21-cyclin D1-Cdk association in late-passage and senescent wild-type and E6 cells. (A) Western blot analysis of extracts prepared from designated cell populations (see legend for Fig. 3) using a pRb-specific antibody (see above) and the polyclonal antibody directed against phosphoserine 780 of pRb which is specifically phosphorylated by cyclin D1 (S-780 [30]). Arrows show a band corresponding to pRb phosphorylated on S-780 and a 100-kDa band (?), recognized by the same antibody, that accumulates in late senescent HDFs. (B and C) Western blot analysis of p16 (above) and cyclin D1 (below) immunoprecipitates isolated from the indicated cell extracts (see legend to Fig. 3). The resulting immunoprecipitates were electrophoresed by SDS–12% PAGE, transferred onto PVDF membranes, and analyzed for the indicated proteins using specific antibodies. The black dot indicates the Cdk6-specific band. pRb pattern corresponding to LPa cells is shown in Fig. 3A. (D) CKI immunodepletion experiment. The indicated cell extracts were immunodepleted for p21 and p27, and the resulting immunoprecipitates [CKI I.P. (pellet)] were analyzed for the presence of Cdk6 and Cdk4, respectively. After immunodepletion, cyclin D1 immunoprecipitates were isolated from supernatants (Sup), and their content was assessed by Western blotting. A “−” denotes mock-treated extracts. Arrows indicate weak Cdk6-specific bands. EP, early-passage E6 cells; TP, terminal-passage E6 cells; S, senescent E6 cells.
Phosphorylation of pRb-Ser780 was strongly diminished in terminal passage E6 cells and completely lacking in senescent E6 cells (Fig. 5A), suggesting that either the total amount or the kinase activity of the cyclin D1-Cdk complexes was reduced in these cells (1, 4, 24). To address this question, we analyzed both p16 and cyclin D1 immunoprecipitates isolated from extracts prepared at different life stages of wild-type and E6 cells. Our results show that p16 and p16-Cdk4/Cdk6 complexes increased in terminal-passage and senescent E6 cells, albeit to a lesser extent than in the wild-type cells (Fig. 5B). To test the effect of this increase on the formation of cyclin D1-Cdk4/Cdk6 complexes, we analyzed cyclin D1 immunoprecipitates from different lifetime stages of E6 cells (Fig. 5C and D). Early-passage E6 cells have fewer cyclin D1-Cdk4/Cdk6 complexes than the wild-type cells, which is consistent with the role of p21 in stabilizing cyclin D1-Cdk4/Cdk6 complexes. Overall, late-passage, terminal-passage, and senescent E6 cells have reduced amounts of cyclin D1-Cdk4/Cdk6 complexes, but the reduction is <50% for cyclin D1-Cdk4. Therefore, p16 appears to make a contribution to the senescent cell cycle arrest in senescent E6 cells but is not sufficient to account for the lack of cyclin D1-mediated phosphorylation of pRb.
Since we have shown previously that the accumulation of underphosphorylated pRb in senescent wild-type cells occurred when virtually all cyclin D1-Cdk complexes became associated with p21 (60), we examined the cyclin D1 immunoprecipitates from E6 cells for the presence of p21 and p27. These results showed that p21, though virtually absent in early-passage cells, was readily detected in cyclin D1 immunoprecipitates from terminal-passage and senescent cells (Fig. 5C). By contrast, the presence of p27 in cyclin D1-Cdk immunoprecipitates was strongly diminished in terminal-passage and senescent E6 cells. Thus, the increasing association between cyclin D1-Cdk complexes and p21 correlated well with the accumulation of underphosphorylated pRb and pRb-Ser780 shown in Fig. 3A and 5A.
Finally, to assess the relative contribution of p21 versus p27 in associating with cyclin D1-Cdk4/Cdk6 complexes, we analyzed cyclin D1 immunoprecipitates after immunodepletion of p21 or p27 (Fig. 5D). These experiments showed that in early-passage cells both Cdk4 and Cdk6 were readily detected in p27 immunoprecipitates but that in terminal-passage and senescent cells these Cdks were predominantly associated with p21 (Fig. 5D, pellet). Consistent with this, p21 depletion removed progressively more cyclin D1-Cdk4/Cdk6 complexes from terminal-passage and senescent E6 cells than from early-passage cells, whereas the proportion of the complexes associated with p27 decreased or remained the same. Thus, the diminished phosphorylation of pRb and pRb-Ser780 in terminal-passage and senescent E6 cells occurs in conjunction with increased binding of p21 to cyclin D1-Cdk4/Cdk6 complexes, together with continued binding of p27, in spite of the fact that both of these CKIs are present at low levels in these populations.
In summary, we suggest that the lack of pRb phosphorylation in terminal-passage and senescent E6 cells is a consequence of both downregulation of cyclin D1-Cdk4/Cdk6 complex formation by p16 and increasing p21-mediated cyclin D1-Cdk4/Cdk6 complex inactivation. Because cyclin E-Cdk2 and cyclin A-Cdk2 activities are not concomitantly downregulated in terminal-passage cells, these cells exhibited the surprising uncoupling of pRb phosphorylation and cell cycle progression described above. In other words, pRb hyperphosphorylation depends on cyclin D1-dependent hypophosphorylation (20, 25), which does not occur in terminal-passage E6 cells, whereas cyclin E-Cdk2 and cyclin A-Cdk2 kinase activities persist in those cells and are sufficient to allow them to progress to S phase.
Evidence for p53-dependent p21 accumulation.
Although modest compared to wild-type cells, the age-dependent increase of p21 in E6 cells was reproducible and was also observed by others (4). Western blot analysis confirmed that in most cases p21 upregulation coincided with a small but detectable p53 increase (Fig. 6A and results not shown). These results suggested that p53-dependent accumulation of p21 might be essential for the onset of events leading to cell cycle arrest. To test this hypothesis, we determined p53 levels in a late-passage E6 population (LPc; PD 77) exhibiting aberrant mitoses (Fig. 2). We found that even though the population as a whole contained modestly elevated p53 levels (and p21 [data not shown]), no p53 was detectable in the cells undergoing aberrant mitotic events (Fig. 6B), a result consistent with the notion that some p53 is needed to avoid this outcome.
FIG. 6.
Increase of p21 in senescent E6 correlates with rise of p53. (A) Western blot analysis of cell lysates prepared from early-passage (EP) and senescent (S) wild-type HDFs and from early-passage, terminal-passage (TPb) and senescent (Sa and Sc) E6 cells. Where indicated, the cells were serum starved (lanes 0) or serum stimulated for 16 h (lanes 16). A prolonged ECL exposure was used to detect p53, whose level varies among different terminal passage and senescent E6 populations. In the presented Sc extract, the p53 band was too faint to be seen in the photograph but it is clearly detectable on the original fluorograph, as well as in other Western blots. (B) Absence of p53 in aberrant mitotic cells shown in Fig. 2. Western blot analysis of cell lysates prepared from senescent wild-type (PD 76), early-passage (PD 42), and late-passage (LPc; PD 77) cells. M, round mitotic cells; T, total cell population. (C) Age-related chromosome condensation in E6 cells is p21 and p53 dependent. Indirect immunofluorescence of exponentially growing populations of early-passage and senescent E6 cells. The cells were fixed in paraformaldehyde and then simultaneously stained with rabbit polyclonal anti-p21 and anti-BrdU (Br; early passage) or monoclonal anti-p53 antibodies (senescent; Sen). The nuclei were counterstained with Hoechst 33258 (Hoe). Arrows indicate a p53-expressing cell.
These observations raised several questions regarding the origin of p53-containing E6 cells. Although p53 amounts were still low in senescent E6 populations, it is possible that a population of the cells with the lowest level of E6 expression became enriched by positive selection. Unfortunately, we could not test this possibility directly because we could not detect E6 protein with several available anti-E6 antibodies. However, we did examine the expression of p53 and p21 in individual E6 cells by indirect immunofluorescence. These data indicated that a senescent E6 population contained a very small population of cells (<5%) that was labeled strongly both by anti-p21 and anti-p53 antibody (Fig. 6C). These cells were also unique in having a “speckled” distribution of DNA, as revealed by Hoechst staining. This speckled distribution of DNA is characteristic of wild-type senescent HDFs (data not shown) and may reflect a senescence-associated condensation of chromatin. Thus, these data suggest that at most a tiny minority of senescent E6 cells may have had unusually low E6 expression. In addition to the lack of speckled nuclei, senescent E6 populations have other features that further distinguish them from senescent wild-type cells (see below).
Absence of senescence-dependent increase of cyclin D2-Cdk in E6 cells.
While analyzing pRb phosphorylation in E6 cells, we observed that senescent E6 cells failed to accumulate a presumably pRb-related phosphoprotein (ca. 100 kDa), which was recognized by anti-pRbS780 antibody and which increased dramatically in late senescent wild-type cells (Fig. 5A). Since this antibody was raised against a cyclin D-specific phospho-pRb peptide, we speculated that this protein might be a potential substrate of D-type cyclin-Cdk complexes. Given our results suggesting that cyclin D1-Cdk complexes in wild-type late senescent cells are inactive, we examined protein levels of cyclin D2, a D-type cyclin recently found to increase in senescent cells (39, 56). Interestingly, we found that cyclin D2 accumulated very strongly in late senescent wild-type cells, whereas it increased to a much smaller extent in senescent E6 cells (Fig. 7A). In addition, Western blot analysis of cyclin D immunoprecipitates showed a specific increase of cyclin D2-Cdk4 complexes in late senescent HDFs (Fig. 7B). Even though these results do not show that the 100-kDa putative pRb-related protein is indeed a cyclin D2-Cdk4 substrate, they suggest that this is a possibility. They also demonstrate that E6 cells do not attain the wild-type senescent stage characterized by elevated cyclin D2 levels. Thus, cyclin D2 may specifically play a role during the late stages of senescence.
FIG. 7.
Cyclin D2/Cdk4 complexes accumulate in senescent wild-type but not E6 cells. (A) Western blot analysis of cell lysates prepared from indicated cell extracts which are described in the legend for Fig. 3. Note that anti-cyclin D2 antibody (47) cross-reacts with cyclin D1. (B) Western blot analysis of cyclin D1 and cyclin D2 immunoprecipitates isolated from early-passage (EP), senescent (S), and late senescent (LS) wild-type HDFs. Cyclin D1 and cyclin D2 were sequentially immunoprecipitated from the same extracts. Cyclin D1-specific band visible in cyclin D2 IP is either a “leftover” from cyclin D1 immunoprecipitation or it results from a cross-reaction.
Terminal-passage p21−/− MEFs are deficient in G1 arrest.
To test further the idea that p21 is required for inactivation of Cdks, inhibition of pRb phosphorylation, and prevention of DNA synthesis in senescent cells, we analyzed cell cycle regulation in fibroblasts derived from wild-type and p21 nullizygous mouse embryos (p21−/− MEFs [8]). The starting cultures of both wild-type and p21−/− MEFs (p5 and p6, respectively) consisted of small, vigorously proliferating cells of rather uniform size. With increasing passage, the wild-type cultures gradually became enriched for large, often binucleated cells, and strongly reduced bromodeoxyuridine (BrdU) incorporation at passage 9 (Fig. 8C and D), with no outgrowth of an immortal line. In contrast to the senescent cell cycle arrest of wild-type MEFs at passage 9, p21−/− MEFs continued to proliferate and underwent a dramatic crisis after passage 10 (which precluded further biochemical analysis owing to low cell numbers). At this stage, the p21−/− culture contained many large cells, but most of them were smaller than those observed in wild-type cultures, and they lacked the increase in SA–β-Gal activity that occurred in the wild-type cultures (data not shown). A small population of p21−/− MEFs survived the crisis at p10-11, but even though they resembled senescent cells, they continued to incorporate BrdU at a high level (Fig. 8C and D), until the culture eventually died at passage 14. Thus, our data indicate that p21−/− MEFs do not achieve the cell cycle arrest characteristic of senescence even though they acquire a senescent morphology.
FIG. 8.
Sustained pRb hyperphosphorylation, cyclin A- and cyclin E-associated kinase activity, and BrdU incorporation in senescent p21−/− MEFs. (A) Western blot analysis of cell lysates prepared from asynchronously growing wild-type (WT) and p21−/− MEF at indicated passages. Passages 9 and 10.5 were terminal for WT and p21−/− MEFs, respectively. (B) Histone H1 kinase activity of cyclin A and cyclin E immunoprecipitates isolated from the designated cell extracts. (C and D) BrdU incorporation. Prior to fixation, assynchronously growing wild-type and p21−/− MEFs at the indicated passages were incubated for 45 min in the presence of BrdU. At least 200 cells were scored for BrdU staining for each passage. Passages 11.5, 12, 13, and 14 present the 21−/− MEFs that were “rescued” after a substantial cell death that occurred after passage 10. (D) Immunofluorescence microscopy showing BrdU incorporation and DNA staining (Hoechst) of early- and late-passage wild-type (p6 and p9) and 21−/− (p6 and p13) MEFs. Note the large 21−/− MEFs still incorporating BrdU at passage 13.
Role of CKI: p21 versus p16.
In terminal-passage wild-type MEFs, pRb is underphosphorylated, cyclin E-associated kinase activity is diminished even though cyclin E-Cdk2 complexes are still abundant, and cyclin A-Cdk2 kinase activity is diminished in conjunction with a reduction in the amount of cyclin A (Fig. 8A and 9A). Although these changes parallel the behavior of senescent HDFs, they are not the result of a comparable age-related increase in p21. Rather, Western blot analysis revealed no significant difference in p21 between early-passage and late-passage wild-type MEFs (Fig. 9A), a result in agreement with the results of Pantoja and Serrano (45). Nevertheless, indirect immunofluorescence analysis using a p21-specific antibody showed that most of the senescent wild-type MEFs contained nuclear p21 (not shown), a finding consistent with their decreased BrdU incorporation (Fig. 8C and D).
FIG. 9.
In late-passage MEFs p16 increase do not block cyclin D1-Cdk4 association. (A) Western blot analysis of p16, p21, and p27 in cell extracts prepared from wild-type and p21−/− MEFs at the indicated passages. The 10* and 10.5 values indicate different passages that preceded crisis. (B) Western blot analysis of p16 immunoprecipitates isolated from cell extracts prepared from wild-type and p21−/− MEFs at the indicated passages. (C) Western blot analysis of cyclin D1 and cyclin D2 immunoprecipitates isolated from cell extracts prepared from wild-type and p21−/− MEFs at the indicated passages. In the cyclin D2 immunoprecipitates isolated from p21−/− MEFs, a higher exposure (H.E.) was necessary to visualize the Cdk4.
In contrast to p21, the amount of p27 is decreased and the amount of p16 is increased in late-passage wild-type MEFs (Fig. 9A and B). However, the increase in p16 had only a small effect, i.e., there was a modest increase in Cdk4 and no increase in Cdk6 associated with this inhibitor in terminal-passage MEFs (Fig. 9B). Consequently, the total amount of cyclin D1-Cdk4 complexes remained high (Fig. 9C), although a moderate decrease of cyclin D2-Cdk4 complexes occurred (Fig. 9C; in MEFs we could not detect cyclin D1-Cdk6 complexes). In p21−/− MEFs, p16 amounts increased only moderately between passages 6 and 10 (Fig. 9A and B), and these cells did not become senescent in spite of having higher levels of p16 and lower levels of cyclin D1- and cyclin D2-Cdk4 complexes than comparably aged wild-type MEFs. These observations suggest that in wild-type MEFs, as in HDFs, p16 does not play a significant role in mediating the initial senescent cell cycle arrest; and, furthermore, that extensively reduced cyclin D-Cdk complex formation alone is not sufficient to cause a senescent cell cycle arrest in cells lacking p21.
In order to elucidate the mechanism whereby cyclin-Cdk complexes are inactivated in senescent MEFs, we performed CKI immunodepletion experiments using anti-p21 and anti-p27 antibodies (as in Fig. 4 and 5). After CKI removal, we compared cyclin A, cyclin E, and cyclin D1 immunoprecipitates from early- and late-passage wild-type and p21−/− MEFs. The results presented in Fig. 10A show that most of the cyclin A-Cdk2 and cyclin E-Cdk2 complexes in senescent wild-type MEFs were removed when p21 was depleted, whereas the removal of p27 did not significantly affect these complexes. These results imply that association with p21 is primarily responsible for the inhibition of the cyclin E- and cyclin A-Cdk2 kinase activity in senescent MEFs. Likewise, even though the majority of cyclin D1-Cdk4 is already associated with p21 in early-passage MEFs, this association increases further in senescent MEFs (Fig. 10A, cyclin D immunoprecipitation). In contrast, p27, rather than p21, is associated with about half of the cyclin E-Cdk2 and cyclin A-Cdk2 in early-passage p21−/− MEFs. However, the amount of p27-free cyclin E-Cdk2 and cyclin A-Cdk2 is the same in early- and late-passage p21−/− MEFs, a result consistent with the undiminished activity of these complexes. Taken together, these results further support the hypothesis that p21 is necessary for the senescent cell cycle arrest, regardless of whether the net amount of p21 is increased in the senescent cells.
FIG. 10.
Cyclin-Cdk complexes increasingly associate with p21 in late-passage wild-type MEFs. (A) p21 versus p27 immunodepletion. Cell extracts from early (p5, W.T.; p6, p21−/−)- and late (p9, W.T.; p10, p21−/−)-passage MEFs were immunodepleted for indicated CKI by repeated incubation with appropriate antibody (p21 and p27). Control extracts (−) were incubated with protein A beads alone. After immunodepletion, aliquots of the resulting supernatants were assessed for the presence of indicated CKI (Sup), whereas the remaining extracts were used for cyclin A, E, and D1 immunoprecipitation (cyclin I.P.). The resulting cyclin immunoprecipitates were separated on 12% SDS-PAGE gels and analyzed for Cdk content. All Western blot signals were revealed by ECL. Note that owing to low amounts of cyclin D1-Cdk complexes in p21−/− MEFs, we had to show higher-exposure (H.E.) ECL fluorographs. (B) p21 and p27 immunodepletion: evidence for CKI-free cyclin D-Cdk4 complexes. The immunodepletion experiment was carried out as described above except that cell extracts from replicating wild-type (p7) and p21−/− (p8) MEFs were immunodepleted simultaneously for both p21 and p27. In the latter cells, only p27-specific antibodies were used. In the upper panel, a Western blot analysis of the resulting supernatants (Sup) shows that incubation with p21- and p27-specific antibodies removed virtually all detectable p21 and p27. In the lower panel, a Western blot analysis of cyclin D1 and cyclin D2 immunoprecipitates isolated from the extracts incubated with protein A beads alone (−) or with anti-CKI mix (+) is shown. A higher exposure (H.E.) of ECL fluorographs was necessary to visualize the cyclin D2-associated Cdk4.
Western blot analysis of cyclin D1 immunoprecipitates following the immunodepletion in Fig. 10A suggested that CKI-free cyclin D-Cdk4 complexes may exist. To further substantiate this possibility, we carried out a simultaneous immunodepletion of both p21 and p27 (CKI) in early-passage wild-type and p21−/− MEFs and analyzed the cyclin D1 and cyclin D2 immunoprecipitates in the resulting supernatants. Note that, as far as our results reveal, immunodepletion was complete (Fig. 10B, upper panel). Nevertheless, cyclin D1- and cyclin D2-Cdk4 complexes could be readily detected in CKI-depleted extracts prepared from either wild-type or p21−/− MEFs (Fig. 10B, lower panel). These results are in agreement with those shown in Fig. 5D for cyclin D1 immunoprecipitates in E6 cells and are consistent with the existence of CKI-free cyclin D-Cdk complexes.
DISCUSSION
The aim of the present study was to determine which physiological and biochemical events occurring during cellular aging specifically require the presence of p21. To this end, we chose to study HDFs expressing the HPV16 E6 oncogene (55) and MEFs derived from p21−/− mice (8). In addition, we sought to delineate the respective roles of p21 and p16 in conferring the senescence-mediated G1 arrest. Even though increasing evidence suggests that both Cdk inhibitors play important roles in replicative senescence (7, 52), a recent study has suggested that p16 could replace p21 in Cdk inactivation in E6 cells (4). However, the results presented here provide strong evidence that p21 is still required for inactivation of cyclin E-Cdk2 complex and probably of cyclin D1-Cdk4/Cdk6 complexes at the onset of senescence in E6 cells with low amounts of p21. In addition, our results pinpoint the cell cycle events occurring at the transition between an initial crisis-like phase and the eventual senescence of E6 populations.
Effects of E6 on early-passage cells.
Early- to mid-passage E6 cells exhibit a normally regulated cell cycle and are phenotypically the same as wild-type cells in their morphology, cell volume, and low amounts of SA–β-Gal activity and collagenase. However, their content of p53, p21, and cyclin D1-Cdk4/Cdk6 complexes is reduced relative to wild-type cells. The reduction in p21 occurs at both the mRNA and protein level, which is consistent with the role of p53 as a positive transactivator of p21. The reduction in cyclin D1 and its kinase partners is consistent with the hypothesis that p21 stabilizes cyclin D1-Cdk4/Cdk6 complexes in vivo and thereby also protects each of the components from degradation (32). Thus, even though expression of HPV-16 E6 can affect the activity or stability of other proteins beyond p53, e.g., paxillin, CBP/p300, Bak, and a putative GAP protein (22, 63, 64, 71), the effects on the cell cycle machinery appear to derive from the decreased amounts of p53 and p21 in these cells.
Cell cycle deregulation begins in late-passage E6 cells.
In late-passage populations, the behavior of E6 cells begins to deviate from that of their wild-type counterparts. There is an accumulation of abortive mitotic cells due to the lack of p53-mediated G1/S and G2/M checkpoints that gradually slows down population expansion. Although late-passage E6 cells contain more p21 than early-passage E6 cells, it is still quite low in comparison to wild-type cells, thereby allowing cell cycle progression in spite of an increasing occurrence of chromosomal aberrations (21). In addition, serum-stimulated DNA synthesis is increased in these cells even though pRb hyperphosphorylation is partially decreased. Although cell cycle regulation is still relatively normal at this stage, it is not as stringent as in wild-type cells or early-passage E6 cells, e.g., cyclin E- and cyclin A-associated kinase activity are downregulated in serum-deprived quiescent E6 cells and p27 is upregulated, but not as extensively as in earlier passages. All of these trends become magnified as the cells reach their terminal passage, where there is no net increase in cell number.
Lack of pRb hyperphosphorylation despite high cyclin E- and cyclin A-associated kinase activity.
When E6 cells reach their terminal passage, a steady cell number is maintained by a balance between cell death, abortive mitoses, and successful divisions. Replicative E6 cells in these populations exhibited several characteristics that indicate a dramatically perturbed cell cycle and altered response to serum. First, both G1 cyclin expression (D1, E, and A) and cyclin E- and cyclin A-Cdk2 kinase activity became serum independent, presumably because the cells failed to upregulate p27 after serum deprivation. Most importantly, in spite of high cyclin E- and cyclin A-Cdk2 activity, terminal-passage E6 cells accumulate underphosphorylated pRb. We suggest that this is due to increasing inactivation of cyclin D-Cdk complexes, because the lack of pRb hyperphosphorylation coincided with (i) a lack of phosphorylation at a cyclin D1-Cdk4/Cdk6-specific site (pRb-Ser780), (ii) an accumulation of p21-associated cyclin D1-Cdk4/Cdk6 complexes, and (iii) reduced cyclin D1-Cdk4/Cdk6 levels. Alternatively, pRb could be dephosphorylated in these cells as a result of increasing phosphatase activity, but this event usually occurs at the M/G1 transition (50). Several recent studies suggest that cyclin D-mediated phosphorylation of pRb is a prerequisite for its phosphorylation by cyclin E-Cdk2, owing to a conformational change induced in pRb by cyclin D-Cdk4/Cdk6 kinases (20, 25, 36). This model strongly supports the interpretation that pRb hyperphosphorylation fails to take place in terminal-passage E6 cells because they lack cyclin D-associated kinase activity, even though they still have abundant cyclin E- and cyclin A-associated kinase activity.
Uncoupling of DNA synthesis from pRb hyperphosphorylation.
The uncoupling of DNA synthesis from pRb hyperphosphorylation in terminal-passage E6 cells is quite striking and implies that the accumulation of underphosphorylated pRb is not sufficient to block DNA synthesis. An interesting precedent for this was reported by Lukas et al. (35), who showed that ectopic expression of constitutively active, nonphosphorylatable pRb (pRbΔCdk) in human osteosarcoma cells (U2OS) conferred only a transient G1 arrest. The pRbΔCdk-expressing cells entered S phase but failed to divide and consequently became increasingly aneuploid, as occurs in E6 cells. Moreover, ectopic expression of cyclin E, but not cyclin D1, could override the G1 arrest imposed by either pRbΔCdk or overexpression of p16, independent of pRb hyperphosphorylation and E2F activation (34). These data suggested that cyclin E controls an S-phase-promoting event that is independent of phosphorylation of pRb. This hypothesis is consistent with recent results showing that overexpression of cyclin E provokes chromosome instability (59) and with earlier experiments indicating that cyclin E and cyclin D control different rate-limiting steps in G1 and that cyclin E is essential for the G1/S transition in Rb-negative as well as Rb-positive cells, whereas cyclin D is not (34, 43, 48). Thus, our results with terminal-passage E6 cells provide further support for the hypothesis that entry into S phase can be controlled by high amounts of endogenous cyclin E-associated kinase activity in the absence of pRb phosphorylation.
Essential role of p21 in the cell cycle arrest in senescent E6 cells.
The eventual senescent cell cycle arrest in E6 cells coincides with p21-dependent inactivation of cyclin E-Cdk2 complexes and decreased cyclin A expression. This occurs in spite of the fact that senescent E6 cells contain less p21 than early-passage wild-type cells. Our data indicate that when E6 cells become senescent, the majority of their cyclin D1-Cdk4/Cdk6 and cyclin E-Cdk2 complexes are associated with p21, as is also the case for wild-type senescent cells. We suggest that this occurs with a smaller amount of p21 in E6 cells because cyclin D1-Cdk complexes, which normally sequester the vast majority of p21 (60), are present in low amounts in these cells regardless of their passage number. In addition, the increase in p16 that is initiated at end of lifespan contributes to a further reduction of cyclin D1-Cdk4/Cdk6 complexes and hence renders more p21 available to bind cyclin E-Cdk2. Similarly, even though p27 is at its lowest level in senescent E6 cells, it may also contribute to inactivation of the G1 cyclin-Cdk complexes through its association with a minority of the cyclin D1-Cdk4/Cdk6 complexes and cyclin E-Cdk2 complexes.
In summary, our studies of the cell cycle machinery in terminal-passage and senescent E6 cells suggest that p21 plays an essential role in the mechanism for the senescent cell cycle arrest despite the fact that it is always significantly inferior to the amount of p21 found in early passage wild-type HDFs. This conclusion argues against the simpler hypothesis that elevated p16 is responsible for the senescent arrest in E6 cells but is consistent with the idea that p16 plays an important role by reducing the number of targets for p21 (4). Although ectopic expression of active Raf-1 or p16 alone can induce a senescence-like state in early-passage HDFs (37, 70), this does not argue that p16 alone is sufficient because the recipient cells were not p21−/− but rather E6-expressing HDFs and therefore had low levels of p21 to contribute to the arrest process. In agreement with this interpretation, ectopic expression of p16 could not confer G1 arrest in p21-null colorectal carcinoma cells (41). Likewise, the finding that ectopic expression of Raf can induce a senescence-like arrest in early-passage E6 cells does not necessarily mean that p21 is not required for senescence because it is highly likely that some p21 was present in the E6 cells used in those experiments. Similarly, the fact that Li-Fraumeni fibroblasts become senescent with significantly decreased amounts of p21 mRNA and protein does not mean that p21 is unnecessary for the senescence of HDFs (38). On the other hand, HDFs completely lacking p21 (p21−/−) do not become senescent, implying that p21 is essential for that arrest (7). By carrying out a detailed analysis of the cell cycle machinery in p21-deficient E6 cells, we have shown that a surprisingly small amount of p21 still plays a key role in mediating the senescent cell cycle arrest.
Uncoupling of the senescence phenotype from the senescent cell cycle arrest.
Many aspects of the cellular aging process seem to be unaffected by the expression of E6 and the attendant decreases in p53 and p21. Consequently, the elevation of p16 and the expression of the senescence-associated phenotype (altered morphology, increased cell volume, SA–β-Gal activity, and collagenase) occur at the end of lifespan in both wild-type HDF and E6 cells even though only the former cells are senescent at that point. Likewise, elevated p16 and SA–β-Gal activity are present in late-passage p21−/− HDFs even though these cells do not become senescent (7, 65). Taken together, these results suggest that expression of p16 and the senescence-associated phenotype are regulated by the mitotic clock (e.g., telomere shortening) independently of the senescent cell cycle arrest.
An increased amount of p21 in later-passage E6 cells is also consistent with the notion that the basic cellular aging processes are unchanged in E6 cells. For example, if the aging process causes an increase in the amount of p53 (31) or in the activity of p53 (2), this proportional change should occur equally well in E6 cells, albeit starting from a much smaller amount of p53. Similarly, if p21 increases owing to increased stabilization of p21 mRNA (11), then the smaller amount of p21 mRNA in E6 cells should be stabilized to the same extent. Thus, we suggest that the modest increase in p21 in late-passage, terminal-passage, and senescent E6 cells is consistent with the notion that the mechanism for the age-related increase in p21 is intact in these cells.
p21 is also essential for the senescent cell cycle arrest in MEFs.
Our studies of wild-type and p21−/− MEFs at the EOL point provide additional compelling evidence that p21 plays an essential role in Cdk2 inactivation and inhibition of pRb phosphorylation. Although wild-type MEFs become senescent without experiencing an increase in p21 relative to total protein, p21 is responsible for the inhibition of the abundant cyclin E-Cdk2 complexes and remaining cyclin A-Cdk2 complexes in senescent MEFs, just as it is in senescent HDFs (with high p21) and senescent E6 cells (with low p21). On the other hand, p21−/− MEFs, like p21−/− HDFs, are unable to achieve a senescent cell cycle arrest at the EOL point. Rather, cyclin E- and cyclin A-associated kinase activities remain high in late-passage p21−/− MEFs, as expected if p21 is essential for the inhibition of these complexes. In addition, pRb phosphorylation continues unabated in late passage p21−/− MEFs, whereas phosphorylation of pRb is reduced in terminal-passage E6 cells. These results suggest that a small amount of p21 is needed to block pRb hyperphosphorylation in cells with high cyclin E and cyclin A kinase activity, presumably through an inhibitory effect on cyclin D-associated kinase activity. Nevertheless, as described previously (45), late-passage p21−/− MEFs have a senescent morphology (but not SA–β-Gal) when their population expansion ceases (or reaches a plateau in the case of cultures that eventually immortalize) owing to crisis-like events. Overall, our data imply that, even though p21 may be dispensable for some aspects of senescence, its presence is necessary for inactivation of cyclin-Cdk complexes and that this function is not replaced by the endogenous activity of p27 and/or p16.
Role of p16 in the senescence of MEFs.
Until recently, the role of p16 in the senescence of MEFs was ambiguous for several reasons. First, although p16 increases as MEFs are passaged in culture, much of the increase occurs in early-passage cells well before senescence (45, 72; this study). This is in strong contrast to the situation observed in HDFs, where accumulation of p16 occurs after senescent cell cycle arrest (60). Second, earlier studies showing that senescence is abrogated in MEFs when the INK4A gene is altered were complicated by the fact that both p16 and p19Arf were inactivated (33, 51). Third, inactivation of p19Arf alone was sufficient to allow escape from senescence, such that it could account for above results (29). However, Carnero et al. have now shown that inactivation of p16 alone is also sufficient to extend the lifespan of MEFs (12). In addition, reexpression of p16 in immortalized populations of p16-deficient MEFs caused a loss of proliferative capacity as long as functional pRb was present. Thus, these data strongly imply that p16 plays a role in cellular senescence in MEFs. How can these results be reconciled with our data showing that there is only a small to moderate decrease in cyclin D-Cdk4 complexes in newly senescent MEFs compared to MEFs in mid-lifespan? One possibility is that p16 functions primarily in the maintenance of the senescent cell cycle arrest but not in its initiation. This hypothesis is consistent with our previous studies of early and late senescent HDFs, where p16 had little or no effect on the amount of cyclin D1-Cdk4/Cdk6 complexes in early senescent cells but caused a much greater reduction in those complexes in late senescent cells (60; this study). Alternatively, because p16 becomes upregulated in early-passage and still-proliferating MEFs, it may contribute to senescence in these cells by reducing the amount of cyclin D-Cdk complexes in mid-lifespan, thus sensitizing the cells to the inhibitory effects of p21 at the EOL. The absence of a strong increase in p16 in senescent MEFs per se may partially explain why MEFs arrest less efficiently and immortalize more readily than senescent HDFs.
As mentioned above, abrogation of p19Arf also promotes the immortalization of MEFs, and its reexpression in the immortalized cells causes the cessation of proliferation (12, 29). Likewise, p14Arf has been shown to be elevated in senescent HDFs, and its overexpression in young HDFs causes a senescence-like cell cycle arrest (15). In MEFs, p19Arf appears to affect both the p53 and pRb pathways through its inhibitory effect on MDM2 (12). Because active MDM2 promotes p53 degradation and inhibits pRb activity, these data suggest that elevated levels of p14Arf or p19Arf may contribute to senescence through increased p53-mediated transactivation of p21 and decreased MDM2-mediated inhibition of pRb activity.
Effects of p21 on cyclin D1-Cdk4/Cdk6 complexes.
The regulation of cyclin D-associated kinase activity is quite complex, and many paradoxes remain to be solved. For example, the currently accepted model emphasizes that in most cases association with p21 or p27 serves to activate Cdk4 and Cdk6 kinase activity by stabilizing the formation of cyclin D-Cdk4/Cdk6 complexes (14, 57). Inhibition of cyclin D-Cdk4/Cdk6 complexes could occur by “titration,” in which association with one molecule of p21 would promote activity, whereas binding of an additional molecule would be inhibitory (32). However, in our studies of wild-type and E6 HDFs, there is an intriguing correlation between the accumulation of underphosphorylated pRb (Fig. 3A and 5A) and the association of an increased fraction of the cyclin D1-Cdk4/Cdk6 complexes with p21 (Fig. 5C and D), suggesting that association with p21 per se may mediate inactivation of these complexes in vivo.
A further paradox is that neither pRb phosphorylation nor cell cycle progression seems to be affected in p21−/− p27−/− double null MEFs that fail to assemble detectable amounts of cyclin D1-Cdk4/Cdk6 complexes and lack cyclin D1-associated kinase activity in an in vitro assay (14). These results imply that if p21- and p27-cyclin D1-Cdk4/Cdk6 complexes are in vivo pRb kinases, they may be replaced in some way in the doubly null MEFs. One possibility suggested by Cheng et al., is that elevated cyclin E-Cdk2 or cyclin A-Cdk2 activity (increased owing to the lack of inhibition by p21 or p27) might compensate for cyclin D-Cdk activity. However, our data indicate that in terminal-passage E6 cells, pRb is neither hyperphosphorylated nor phosphorylated at a cyclin D1-specific site (Ser780), even though these cells contain fully active cyclin E- and cyclin A-Cdk2 complexes. Thus, these data imply that neither cyclin E-Cdk2 nor cyclin A-Cdk2 activity can replace cyclin D1-Cdk activity in phosphorylating pRb in these cells. This conclusion is consistent with recent results showing that cyclin D1-mediated pRb phosphorylation is necessary for subsequent phosphorylation by cyclin E-Cdk2 (25, 36). On the other hand, most of these results could be reconciled if one assumes that CKI-free cyclin D-Cdk complexes do exist in vivo, albeit in very low levels, as is the case with CKI-free cyclin E- and A-Cdk2 complexes (6, 19, 27, 46, 60). Indeed, our immunodepletion experiments carried out in both HDFs (Fig. 5) and MEFs (Fig. 10) demonstrate that this indeed may be the case.
In conclusion, we hypothesize that the paucity of p21 strongly impedes the senescent cell cycle arrest in E6 cells and that, consequently, the most severely p53- and p21-deficient cells in the population are eliminated in the course of aging by events that resemble crisis (9, 21, 54). Even though some aspects of phenotypic senescence are expressed in the absence of p21, we propose that the senescent cell cycle arrest occurs only in cells that are able to inactivate cyclin E-Cdk2 through the action of p21.
ACKNOWLEDGMENTS
We thank Marcel Dorée (CRBM, Montpellier, France), Daniel Fisher (IGH, Montpellier, France), Annick Péléraux (Sanofi-Synthelabo, Montpellier, France), and anonymous reviewers for invaluable comments and critical suggestions. We are also grateful to Jerry Shay (Dallas, Tex.) for HDF-expressing HPV-16 E6, Tyler Jacks (Cambridge, Mass.) for p21−/− MEFs, Gilles Ponzio (INSERM, Nice, France) for anti-cyclin D2 antibody, Thierry Lorca (CRBM) for anti-Skp2 antibody, Martin Mueller (Heidelberg, Germany) for anti-E6 antibody, and Pierre Travo (CRBM) for his enthusiastic help with the time-lapse microscopy. V.D. also acknowledges the skillful technical help of Delphine Claudet.
This work was supported by a grant from l'Association pour la Recherche sur le Cancer (ARC-9737; V.D.) and by Public Health Service grant AG00947 from the National Institute on Aging (G.H.S.).
REFERENCES
- 1.Alcorta D A, Xiong Y, Phelps D, Hannon G, Beach D, Barrett J C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atadja P, Wong H, Garkavtsev I, Veillette C, Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beijersbergen R L, Carlee L, Kerkhoven R M, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- 4.Bond J A, Haughton M F, Rowson J M, Smith P J, Gire V, Wynford-Thomas D, Wyllie F S. Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis. Mol Cell Biol. 1999;19:3103–3114. doi: 10.1128/mcb.19.4.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bond J A, Wyllie F S, Wynford T D. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene. 1994;9:1885–1889. [PubMed] [Google Scholar]
- 6.Bresnahan W A, Boldogh I, Ma T, Albrecht T, Thompson E A. Cyclin E/Cdk2 activity is controlled by different mechanisms in the G0 and G1 phases of the cell cycle. Cell Growth Differ. 1996;7:1283–1290. [PubMed] [Google Scholar]
- 7.Brown J P, Wei W, Sedivy J M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 8.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 9.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 10.Burkhart B A, Alcorta D A, Chiao C, Isaacs J S, Barrett J C. Two posttranscriptional pathways that regulate p21(Cip1/Waf1/Sdi1) are identified by HPV16-E6 interaction and correlate with life span and cellular senescence. Exp Cell Res. 1999;247:168–175. doi: 10.1006/excr.1998.4345. [DOI] [PubMed] [Google Scholar]
- 11.Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 12.Carnero A, Hudson J D, Price C M, Beach D H. p16INK4A and p19ARF act in overlapping pathways in cellular immortalization. Nat Cell Biol. 2000;2:148–155. doi: 10.1038/35004020. [DOI] [PubMed] [Google Scholar]
- 13.Carrano A C, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 14.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimri G P, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol. 2000;20:273–285. doi: 10.1128/mcb.20.1.273-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulić V, Drullinger L F, Lees E, Reed S I, Stein G H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci USA. 1993;90:11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulić V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 19.Dulić V, Stein G H, Far D F, Reed S I. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezhevsky S A, Nagahara H, Vocero A A, Gius D R, Wei M C, Dowdy S F. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc Natl Acad Sci USA. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filatov L, Golubovskaya V, Hurt J C, Byrd L L, Phillips J M, Kaufmann W K. Chromosomal instability is correlated with telomere erosion and inactivation of G2 checkpoint function in human fibroblasts expressing human papillomavirus type 16 E6 oncoprotein. Oncogene. 1998;16:1825–1838. doi: 10.1038/sj.onc.1201711. [DOI] [PubMed] [Google Scholar]
- 22.Gao Q, Srinivasan S, Boyer S N, Wazer D E, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19:733–744. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grana X, Garriga J, Mayol X. Role of the retinoblastoma protein family, pRb, p107, and p130 in the negative control of cell growth. Oncogene. 1998;17:3365–3383. doi: 10.1038/sj.onc.1202575. [DOI] [PubMed] [Google Scholar]
- 24.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbour J W, Luo R X, Dei Santi A, Postigo A A, Dean D C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 26.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 27.Hengst L, Gopfert U, Lashuel H A, Reed S I. Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1) Genes Dev. 1998;12:3882–3888. doi: 10.1101/gad.12.24.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaelin W G., Jr Functions of the retinoblastoma protein. Bioessays. 1999;21:950–958. doi: 10.1002/(SICI)1521-1878(199911)21:11<950::AID-BIES7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 31.Kulju K S, Lehman J M. Increased p53 protein associated with aging in human diploid fibroblasts. Exp Cell Res. 1995;217:336–345. doi: 10.1006/excr.1995.1095. [DOI] [PubMed] [Google Scholar]
- 32.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd A C. p53: only ARF the story. Nat Cell Biol. 2000;2:48–50. doi: 10.1038/35004078. [DOI] [PubMed] [Google Scholar]
- 34.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 35.Lukas J, Sorensen C S, Lukas C, Santoni-Rugiu E, Bartek J. p16INK4a, but not constitutively active pRb, can impose a sustained G1 arrest: molecular mechanisms and implications for oncogenesis. Oncogene. 1999;18:3930–3935. doi: 10.1038/sj.onc.1202777. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McConnell B B, Starborg M, Brookes S, Peters G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol. 1998;8:351–354. doi: 10.1016/s0960-9822(98)70137-x. [DOI] [PubMed] [Google Scholar]
- 38.Medcalf A S, Klein-Szanto A J, Cristofalo V J. Expression of p21 is not required for senescence of human fibroblasts. Cancer Res. 1996;56:4582–4585. [PubMed] [Google Scholar]
- 39.Meyyappan M, Wong H, Hull C, Riabowol K T. Increased expression of cyclin D2 during multiple states of growth arrest in primary and established cells. Mol Cell Biol. 1998;18:3163–3172. doi: 10.1128/mcb.18.6.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millis A J, Hoyle M, McCue H M, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- 41.Mitra J, Dai C Y, Somasundaram K, El-Deiry W S, Satyamoorthy K, Herlyn M, Enders G H. Induction of p21(WAF1/CIP1) and inhibition of Cdk2 mediated by the tumor suppressor p16(INK4a) Mol Cell Biol. 1999;19:3916–3928. doi: 10.1128/mcb.19.5.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noda A, Ning Y, Venable S F, Pereira S O, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 43.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmero I, McConnell B, Parry D, Brookes S, Hara E, Bates S, Jat P, Peters G. Accumulation of p16INK4a in mouse fibroblasts as a function of replicative senescence and not of retinoblastoma gene status. Oncogene. 1997;15:495–503. doi: 10.1038/sj.onc.1201212. [DOI] [PubMed] [Google Scholar]
- 45.Pantoja C, Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene. 1999;18:4974–4982. doi: 10.1038/sj.onc.1202880. [DOI] [PubMed] [Google Scholar]
- 46.Planas-Silva M D, Weinberg R A. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponzio G, Loubat A, Rochet N, Turchi L, Rezzonico R, Farahi Far D, Dulić V, Rossi B. Early G1 growth arrest of hybridoma B cells by DMSO involves cyclin D2 inhibition and p21[CIP1] induction. Oncogene. 1998;17:1159–1166. doi: 10.1038/sj.onc.1202040. [DOI] [PubMed] [Google Scholar]
- 48.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogan E M, Bryan T M, Hukku B, Maclean K, Chang A C, Moy E L, Englezou A, Warneford S G, Dalla-Pozza L, Reddel R R. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol Cell Biol. 1995;15:4745–4753. doi: 10.1128/mcb.15.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin E, Tamrakar S, Ludlow J W. Protein phosphatase type 1, the product of the retinoblastoma susceptibility gene, and cell cycle control. Front Biosci. 1998;3:D1209–D1219. doi: 10.2741/a357. [DOI] [PubMed] [Google Scholar]
- 51.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho R A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 52.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 53.Shay J W, Pereira S O, Wright W E. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 54.Shay J W, Van der Haegen B, Ying Y, Wright W E. The frequency of immortalization of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp Cell Res. 1993;209:45–52. doi: 10.1006/excr.1993.1283. [DOI] [PubMed] [Google Scholar]
- 55.Shay J W, Wright W E, Brasiskyte D, van der Haegen B. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993;8:1407–1413. [PubMed] [Google Scholar]
- 56.Shelton D N, Chang E, Whittier P S, Choi D, Funk W D. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 57.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 58.Spruck C H, Won K A, Reed S I. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 59.Stein G H, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249:666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- 60.Stein G H, Drullinger L F, Soulard A, Dulić V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein G H, Dulić V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 62.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 63.Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol. 1999;80:1513–1517. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 64.Tong X, Salgia R, Li J L, Griffin J D, Howley P M. The bovine papillomavirus E6 protein binds to the LD motif repeats of paxillin and blocks its interaction with vinculin and the focal adhesion kinase. J Biol Chem. 1997;272:33373–33376. doi: 10.1074/jbc.272.52.33373. [DOI] [PubMed] [Google Scholar]
- 65.Wei W, Sedivy J M. Differentiation between senescence (M1) and crisis (M2) in human fibroblast cultures. Exp Cell Res. 1999;253:519–522. doi: 10.1006/excr.1999.4665. [DOI] [PubMed] [Google Scholar]
- 66.Xiao Z X, Ginsberg D, Ewen M, Livingston D M. Regulation of the retinoblastoma protein-related protein p107 by G1 cyclin-associated kinases. Proc Natl Acad Sci USA. 1996;93:4633–4637. doi: 10.1073/pnas.93.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong Y, Kuppuswamy D, Li Y, Livanos E M, Hixon M, White A, Beach D, Tlsty T D. Alteration of cell cycle kinase complexes in human papillomavirus E6- and E7-expressing fibroblasts precedes neoplastic transformation. J Virol. 1996;70:999–1008. doi: 10.1128/jvi.70.2.999-1008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Z K, Gervais J L, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 70.Zhu J, Woods D, McMahon M, Bishop J M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmermann H, Degenkolbe R, Bernard H U, O'Connor M J. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zindy F, Quelle D E, Roussel M F, Sherr C J. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]