Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a fatal multifactorial neurodegenerative disease characterized by the selective death of motor neurons. Cytosolic phospholipase A2 alpha (cPLA2α) upregulation and activation in the spinal cord of ALS patients has been reported. We have previously shown that cPLA2α upregulation in the spinal cord of mutant SOD1 transgenic mice (SOD1G93A) was detected long before the development of the disease, and inhibition of cPLA2α upregulation delayed the disease’s onset. The aim of the present study was to determine the mechanism for cPLA2α upregulation.
Methods
Immunofluorescence analysis and western blot analysis of misfolded SOD1, cPLA2α and inflammatory markers were performed in the spinal cord sections of SOD1G93A transgenic mice and in primary motor neurons. Over expression of mutant SOD1 was performed by induction or transfection in primary motor neurons and in differentiated NSC34 motor neuron like cells.
Results
Misfolded SOD1 was detected in the spinal cord of 3 weeks old mutant SOD1G93A mice before cPLA2α upregulation. Elevated expression of both misfolded SOD1 and cPLA2α was specifically detected in the motor neurons at 6 weeks with a high correlation between them. Elevated TNFα levels were detected in the spinal cord lysates of 6 weeks old mutant SOD1G93A mice. Elevated TNFα was specifically detected in the motor neurons and its expression was highly correlated with cPLA2α expression at 6 weeks. Induction of mutant SOD1 in primary motor neurons induced cPLA2α and TNFα upregulation. Over expression of mutant SOD1 in NSC34 cells caused cPLA2α upregulation which was prevented by antibodies against TNFα. The addition of TNFα to NSC34 cells caused cPLA2α upregulation in a dose dependent manner.
Conclusions
Motor neurons expressing elevated cPLA2α and TNFα are in an inflammatory state as early as at 6 weeks old mutant SOD1G93A mice long before the development of the disease. Accumulated misfolded SOD1 in the motor neurons induced cPLA2α upregulation via induction of TNFα.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-021-02326-5.
Keywords: Cytosolic phospholipase A2α, ALS, Mutant SOD1G93A, Motor neurons, TNFα, Misfolded SOD1
Background
Amyotrophic lateral sclerosis (ALS) is a severe degenerative disorder, mainly affecting the motor neurons. Most of the cases (about 90%) are sporadic. Familial cases have been linked to mutations in several genes, including chromosome 9 open reading frame 72 (C9ORF72) repeat expansions, Cu/Zn superoxide dismutase (SOD1), TAR DNA binding protein (TDP-43) and others [1]. Mutant SOD1 is the best characterized form of familial ALS, accounting for 20% of familial cases [2]. It is generally believed that sporadic and familial ALS may share pathological mechanisms. The pathophysiology of the multifactorial-multisystemic ALS disease includes various mechanisms. Although ALS is not primarily considered an inflammatory or immune-mediated disease, inflammation appears to play a role in the pathogenesis of the disease in both ALS patients and animal models, inflammatory responses have been observed [3–5]. Microglia [6] and astrocytes [7] are activated during the progression of the disease, and evidence suggests that they contribute to neuronal death.
Previous findings suggested that cytosolic phospholipase A2α (cPLA2α) is regarded as an essential source of inflammation. cPLA2α specifically hydrolyzes phospholipids containing arachidonic acid at the sn-2 position [8, 9] and is the rate-limiting step in the generating eicosanoids and a platelet activating factor. These lipid mediators play critical roles in the initiation and modulation of inflammation and oxidative stress. cPLA2α is ubiquitous in all cells and is essential for their physiological regulation. However, elevated cPLA2α expression and activity were detected in the inflammatory sites in a vast array of inflammatory diseases, including neurodegenerative diseases [10–12]. Increased expression and activity of cPLA2α has been detected in neurons, astrocytes and microglia in the spinal cord, brainstem and cortex of sporadic ALS patients [13] and in the spinal cord of mutant SOD1G93A transgenic mice [14], suggesting that cPLA2α may have an important role in the pathogenesis of the disease in all ALS patients. Our previous study [15] demonstrated that cPLA2α is upregulated in the spinal cord of 6 weeks old SOD1G93A mice long before the appearance of the disease symptoms, neuronal death or gliosis, and remained elevated during the whole life span of the mice. Prevention of cPLA2α upregulation shortly before the onset of the disease symptoms, significantly delayed the loss of motor neuronal function, suggesting that cPLA2α upregulation in the spinal cord plays a role in the disease pathology. The mechanism that induces cPLA2α elevation in the spinal cord of as early as 6 weeks old mice, is not yet clear. SOD1 insoluble protein complexes (IPCs) were detected in motor neurons of 30 days old SOD1G93A mice [16], before the manifestation of ALS pathology, and several months before the appearance of inclusion bodies. The present study aims to determine whether accumulated misfolded SOD1 triggers cPLA2α upregulation in motor neurons in the spinal cord of 6 weeks old ALS mice.
Methods
Animals
B6.Cg-Tg(SOD1G93A)1Gur/J hemizygous transgenic male mice were obtained from Jackson Laboratory (Bar Harbor, ME, U.S.A). The hemizygous transgenic male mice were also obtained by mating hemizygous transgenic males with C57BL/6J females (Jackson Laboratory). Each litter would generate hemizygous SOD1G93A transgenic mice and littermate wild type controls as done before [15]. Transgenic male offspring were genotyped by PCR assay of DNA obtained from tail tissue (according to Jackson Laboratory). The study included male mice to avoid the estrogen effect. The study was approved by Ben-Gurion University Institutional Animal Care and Use Committee (IL-40-07-2016) and was conducted according to the Israeli Animal Welfare Act following the Guide for Care and Use of Laboratory Animal (National Research Council, 1996).
Motor function measurement by Rotarod A Rotarod test was used to evaluate the motor performance of the mice using an accelerating paradigm of 0.12 rpm/s as described before [15]. After a learning period of several days, mice were able to stay on the Rotarod (Rotamex-5, Columbus instruments, Columbus, OH, USA) for up to 150 s. Each mouse was given 3 trials and the best performance was used as a measure for motor function ability. Mice were tested twice a week from age of the 7 weeks-old until they could no longer perform the task.
Spinal cord tissue preparation Mice were deeply anesthetized and transcardially perfused with 20 ml of PBS [17].
For immunoblot analysis or immunoprecipitation Spinal cords were harvested in Lysis buffer containing 20 mM Tris pH7.5, 150 mM NaCl, 0.5% Sodium deoxycholate, 0.1% SDS, 0.1% Triton, 1 mM Phenylmethylsulfonyl fluoride and 1% protease inhibitors (Roche, Mannheim, Germany). The suspensions were sonicated 3 times for 20 s with Microsom Heatsystem Sonicator and centrifugated at 13,000×g for 20 min at 4 °C. Immunoprecipitation of cPLA2α or misfolded SOD1 was performed as described earlier [18, 19]. Spinal cords (100 µg) were solubilized in IP buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, plus 1 × protease inhibitors) and incubated overnight with B8H10 antibodies (MédiMabs) or cPLA2α antibodies previously cross-linked to magnetic beads (Invitrogen, Waltham, Massachusetts, USA) with dimethyl pimelimidate (Pierce) according to the manufacturer’s instructions. The beads were magnetically isolated and washed three times with IP buffer. Samples were eluted by boiling in a 2 × SDS sample buffer. Lysate protein or resolved proteins were separated on 7% or 15% SDS-PAGE electrophoresis and transferred to nitrocellulose or PVDF membranes. Membranes were incubated in Tris-buffered saline (10 mM Tris, 135 mM NaCl, pH 7.4), with 0.1% Tween 20 (TBS-T) containing 5% non-fat milk for 1.5 h at 25 °C. The blots were then incubated with primary antibodies: 1:1000 rabbit anti-cPLA2 (Cell Signaling Danvers, MA USA), 1:250 mouse B8H10 anti-misfolded human SOD1 (Medimabs, Quebec, Canada), 1:1000 rabbit anti-calreticulin (Thermo Scientiific, IL, USA) as primary antibodies for overnight at 4 °C. After washing with TBS-T, they were incubated with secondary antibody: peroxidase conjugated goat anti-rabbit or anti mouse (Amersham Biosciences, Buckinghamshire, United Kingdom) for 1 h at 25 °C and developed using the enhanced chemiluminescence (ECL) detection system (PerkinElmer, Waltham, MA, USA). Proteins were quantified using video densitometry analysis (ImageJ version 4.0 Fuji).
For immunostaining—The spinal cords were fixed [15] in paraformaldehyde 4%/PBS solution overnight at 4 °C. The spinal cords were then transferred to PBS containing 30% sucrose for 24 h and then embedded in a 1:2 mixture of 30% sucrose in PBS:Tissue-Tek OCT (VWR, Radnor, PA), frozen in liquid nitrogen and stored at − 80 °C. Sections were made by cryostat (Leica Biosystems, Vienna, Austria) at 12 µm thickness, washed in PBS/tween 0.05%, incubated in PBS/Glycine 0.1% for 5 min and incubated in blocking solution (3% normal donkey serum and 2% BSA) at room temperature for 1 h. Then, these sections were incubated with primary antibodies diluted in blocking solution overnight at 4 °C. The primary antibodies used in the study were: 1:100 rabbit anti-cPLA2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-pcPLA2α (Cell Signaling Danvers, MA USA), 1:100 mouse anti-misfolded human SOD1 (Medimabs, Quebec, Canada), 1:100 mouse anti-TNFα (Novus Biological USA, CO, USA), 1:100 goat anti-choline Acetyl-transferase (ChAT) (Millipore, CA, USA), 1:1000 rabbit anti-Iba-1 (Wako Pure Chemical Industries, Osaka, Japan), 1:500 rabbit anti-GFAP (Dako Glostrup Denmark), 1:100 mouse anti-GFAP (Millipore Darmstadt, Germany). Sections were washed with PBS/tween 0.05%, and incubated with 1:200 Cy3 or 1:100 anti-mouse Alexa488 or anti-rabbit Dylight conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, USA) for 1 h at room temperature. The staining of samples from the different treatments was performed in parallel. For each treatment, a negative control was prepared by omitting the primary antibody. Sections were mounted with anti-fading mounting medium (Electron Microscopy Sciences (EMS), Hatfield, PA, USA) and photographed in a blinded fashion using a fluorescent microscope (Olympus, BX60, Hamburg, Germany) or with confocal microscopy (Olympus, FluoView 1000, Tokyo, Japan). Using a confocal microscope, Z-sections were taken at 0.5 μm intervals and the results present Z-stack images. Fluorescence intensity was determined for cPLA2α using CellProfiler program. The % of fluorescence intensity of cell area was determined for the different cell types using CellProfiler program. LSM880 inverted laser-scanning confocal microscope (Zena, Germany) equipped with an Airyscan high-resolution detection unit and under identical acquisition conditions was also used. A Plain-Apochromat 63x/1.4 Oil DIC M27 objective was used, and parameters were set to avoid pixel intensity saturation and to ensure Nyquist sampling in the XY plane. Excitation of lasers for DAPI, Alexa 488 and Cy3 were 405 nm, 488 nm and 561 nm, respectively.
Cell cultures
A Motor neurons were isolated as described before with few modifications. Briefly, spinal cords were dissociated from C57BL/6J mouse embryos at day 13.5 (E13.5) with 2 mg/ml papain for 25 min at 37 °C, and triturated with 0.5% bovine serum albumin (BSA) and 0.01 mg/ml DNase I in Leibovitz’s L-15 medium (Gibco). Cells were then triturated with Leibovitz’s L-15 media and the single-cells suspension was separated through Optiprep gradient (Sigma) and seeded at a density of 50,000 cells/ 24 well on coverslips pre-covered polyornithine (Sigma) and laminin (Sigma). The motor neurons were cultured with Neurobasal media (Gibco) supplemented with B27 (Gibco), 2% horse serum (Sigma), 1 ng/ml CNTF (R&D systems), and 1 ng/ml GDNF (R&D systems) and maintained at 37 °C in a 5% CO2 humidified incubator. At 6 DIV, glial cells were inhibited using 5-Fluoro-2′-deoxyuridine (Sigma) and uridine (Sigma). For expression of human SOD1 in motor neurons, the cells were infected at 7 DIV using AAV1/2-SOD1WT or AAV1/2-SOD1G93A as previously described [20].
B Motor neuron like NSC34 cell line—were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin solution at 37 °C with 5% CO2 and were sub-cultured every 2–3 days [21]. For differentiation, the proliferation medium (DMEM, 10% FCS, 1% P/S) was exchanged 24 h after seeding with a differentiation medium containing 1:1 DMEM/F-12 (Ham), 1% FCS, 1% modified Eagle’s medium nonessential amino acids (NEAA), 1% P/S and 1 μM all- trans retinoic acid. SOD1WT and SOD1G93A were constructed and purified as described before [22]. Transfection was performed by using TurboFect (Thermo) according to the manufacturer’s protocol. TNF-α-neutralizing antibody (Cell Signaling Technology, Danvers, MA, USA) was used to study its effect.
Cell lysates—were prepared using lysis buffer containing: 2% Triton X-100, 50 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 10 μm MgCl2, 10 μg/ml leupeptin, 1 mM phenyl-methylsulphonylfluoride, 10 μg/ml aprotonin, 1 mM benzamidine, 20 mM para-nitrophenyl phosphate, 5 mM sodium orthovanadate, 10 mM sodium fluoride, and 50 mM β-glycerophosphate). Cell lysates were analyzed by SDS-PAGE on 15–10% gels. The amount of protein in each sample was quantified with the Pierce BCA Proteins Assay using BSA standards. The resolved proteins were transferred to PVDF membrane and blocked in 5% BSA in TBS-T (10 mM Tris, 135 mM NaCl, pH 7.4, 0.1% Tween 20). The detection of immunoreactive bands was carried out as described above but for cPLA2α, using 1:500 rabbit anti cPLA2α (GeneTex Inc, Alton Pkwy Irvine, CA, USA).
TNFα levels—were measured by a TNFα high sensitivity ELISA, eBioscience, Vienna, Austria.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Statistical significance was determined by either one- or two-way analysis of variance (ANOVA) followed by a posteriori Bonferroni’s test for multiple comparisons provided by GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Pearson coefficient correlation (r) was used to study the relationships between the variables.
Results
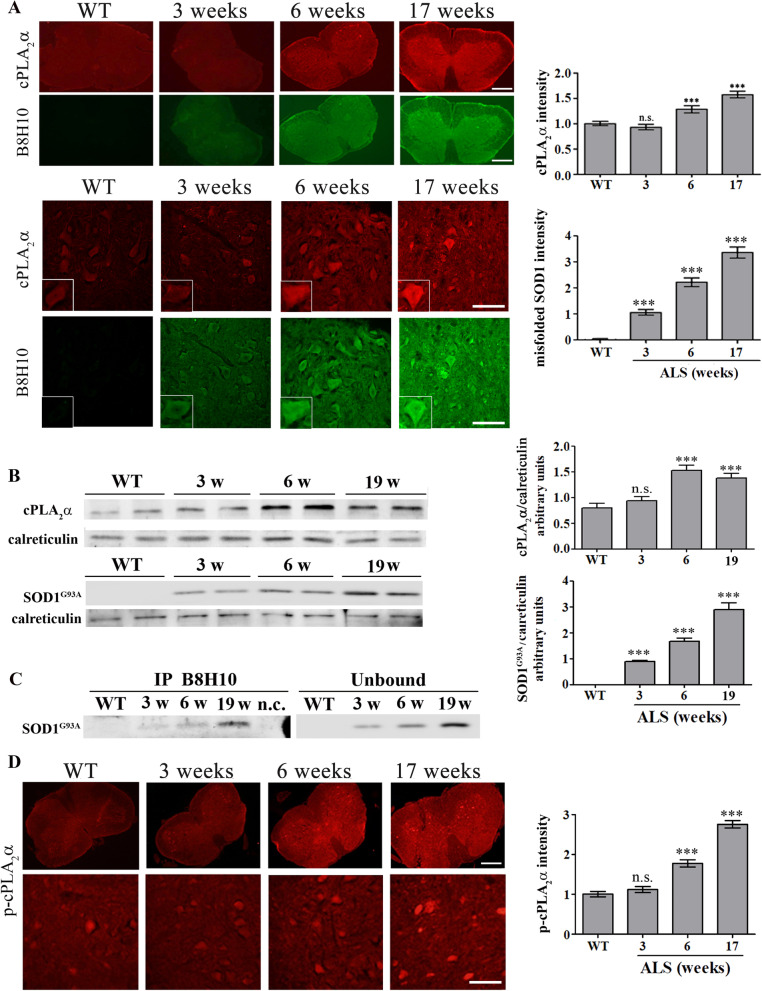
In our previous study we reported that cPLA2α is elevated in the spinal cord of 6 weeks old mutant SOD1G93A mice but not at 3 weeks. To study whether cPLA2α is affected by the accumulation of misfolded SOD1 in the cells, cPLA2α and misfolded SOD1 proteins expression and accumulation were analyzed in the spinal cord of SOD1G93A mice. Immunofluorescence staining and quantitation showed a significant (p < 0.001) elevation of cPLA2α protein expression in the spinal cord sections (Fig. 1A) of 6 weeks old SOD1G93A mice, as shown in our previous study [15]. Immunofluorescence staining and quantitation of misfolded SOD1 showed that it was significantly (p < 0.001) detected in the spinal cord at 3 weeks old SOD1G93A mice, before the elevation of cPLA2α. The expression of cPLA2α and mutant SOD1G93A was also determined by western blot analysis and showed that mutant SOD1G93A was detected at 3 weeks preceding the elevation of cPLA2α (Fig. 1B). Moreover, misfolded SOD1 determined by immunoprecipitation with anti B8H10 was detected at 3 weeks in the spinal cord of SOD1G93A mice and gradually increased at a later age (Fig. 1C). Activation of cPLA2α analyzed by immunostaining of phosphor- cPLA2α was detected at the spinal cord section of 6 weeks old SOD1G93A mice but not at 3 weeks (Fig. 1D).
Fig. 1.
Misfolded SOD1 accumulation precedes cPLA2α upregulation and activation. A A representative double immunofluorescence staining of cPLA2α (red) and misfolded SOD1 (B8H10, green) proteins in the lumbar spinal cord sections of WT and mutant SOD1G93A mice during the development of the disease (3, 6 and 17 weeks). Scale bars = 500 μm (upper panel), 100 μm (lower panel) and inset 20 μm. The means ± SEM fluorescence intensity for both magnifications are presented in the bar graph as arbitrary units. Four mice for each time point and five fields for each mouse were analyzed. ***p < 0.001 compared to control mice (WT). n.s. non-significant. B A representative immunoblot analysis of cPLA2α, mutant SOD1G93A and their corresponding calreticulin protein expression in the spinal cord lysates of WT and mutant SOD1G93A mice during the development of the disease. cPLA2α protein expression was determined by dividing the intensity of each cPLA2α or mutant SOD1G93A (SOD1G93A) by the intensity of the corresponding calreticulin band after quantitation by densitometry and expressed in the bar graph as arbitrary units. The bar graphs are the means ± SE of 4 mice in each group. ***p < 0.001 significance—compared to control mice (WT). n.s. non-significant. C. Misfolded SOD1 was immunoprecipitated using anti-B8H10 antibodies from spinal cord lysates of WT and mutant SOD1G93A mice during the development of the disease. A representative immunoblot analysis of the misfolded SOD1 (IP B8H10, left) and 10% of the unbound (Unbound, right) fractions are shown. nc- negative control, immunoprecipitation without anti-B8H10 antibodies. D A representative immunofluorescence staining of phosphor-cPLA2α (p-cPLA2α) in the lumbar spinal cord sections of WT and mutant SOD1G93A mice during the development of the disease (3, 6 and 17 weeks). Scale bars = 500 μm (upper panel) and 100 μm (lower panel). The means ± SEM fluorescence intensity for both magnifications are presented in the bar graph in arbitrary units. Four mice for each time point and five fields for each mouse were analyzed. ***p < 0.001 significance compared to WT mice. n.s. non-significant
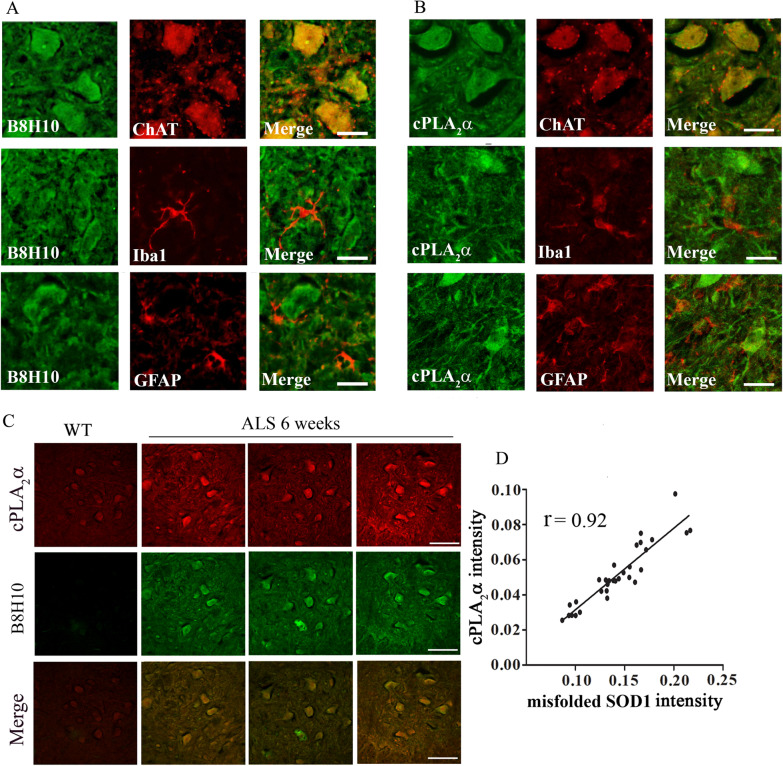
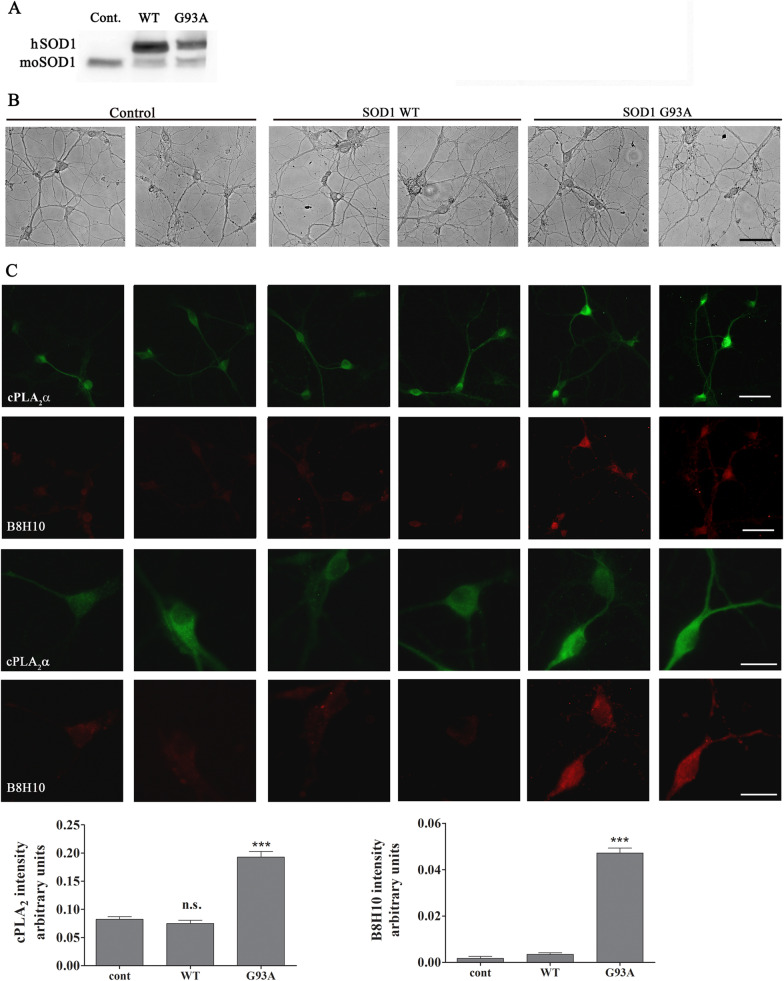
The elevated accumulation of misfolded SOD1 and the elevated cPLA2α protein expression in the spinal cord sections of 6 weeks old mutant SOD1G93A mice were detected specifically in motor neurons as determined by co-immunofluorescence staining of misfolded SOD1 and the marker of motor neurons ChAT (Fig. 2A). Using specific antibodies against misfolded SOD1 showed that elevated misfolded SOD1 was already detected at 3 weeks (Additional file 1: Fig. S1), and co-staining with ChAT showed that it is expressed in the motor neurons (Additional file 2: Fig. S2). Co-immunofluorescence staining of misfolded SOD1 and Iba1 or GFAP, the markers of microglia or astrocytes, respectively, showed that misfolded SOD1 is not accumulated in these cells at 6 weeks (Fig. 2A). As shown by co-immunofluorescence staining, elevated cPLA2α expression was also detected specifically in the motor neurons and not in microglia or astrocytes (Fig. 2B). Co-immunostaining of cPLA2α and misfolded SOD1 showed co-localization and overlapping between cPLA2α and misfolded SOD1 in the motor neurons in the spinal cord of 6 weeks old mutant SOD1G93A mice (Fig. 2C). cPLA2α upregulation showed some variation that highly correlated with the accumulation of misfolded SOD1 with a correlation coefficient of 0.92 between both proteins, suggesting that the level of misfolded SOD1 in the motor neurons defines the level of cPLA2α protein expression (Fig. 2D). To determine whether the accumulation of misfolded SOD1 in the motor neurons is responsible for cPLA2α upregulation, human SOD1WT or mutant SOD1G93A were expressed in primary motor neurons isolated from the spinal cord from C57BL/6J mouse embryos as shown in the western blot analysis (Fig. 3A). The expression of human SOD1 did not affect the morphology of the motor neurons and their number (Fig. 3B). Double staining with anti -B8H10 and anti-cPLA2α showed elevated cPLA2α protein expression in motor neurons expressing mutant SOD1G93A and accumulating misfolded SOD1 (Fig. 3C). In motor neurons that did not accumulate misfolded SOD1 (as shown in Fig. 3A), cPLA2α expression did not change and was similar to that detected in control motor neurons (without induction). These results clearly indicate that misfolded SOD1 induced cPLA2α upregulation.
Fig. 2.
cPLA2α and misfolded SOD1 accumulates specifically in motor neurons of young pre-symptomatic SOD1G93A mice. A Double staining (red) of motor neurons (ChAT), microglia (Iba-1) or astrocytes (GFAP) and misfolded SOD1 (B8H10, green) in the spinal cord sections of 6 weeks old pre-symptomatic SOD1G93A mice. Scale bars = 20 μm. Shown a representative out of five mice. B Double staining (red) of motor neurons (ChAT), microglia (Iba-1) or astrocytes (GFAP) and cPLA2α (green) in the spinal cord sections of 6 weeks old pre-symptomatic SOD1G93A mice. Scale bars = 20 μm. Shown a representative out of five mice. C Double immunofluorescence staining of cPLA2α (red) with misfolded SOD1 (B8H10, green) proteins in the spinal cord sections of control mice (WT) and 6 weeks old pre-symptomatic mutant SOD1G93A mice. Scale bars = 100 μm. D The Pearson coefficient correlation (r) between cPLA2α and misfolded SOD1 in the motor neurons of the intensity of cPLA2α and misfolded SOD1 expressed in arbitrary units of immunostaining as presented in the representative results in C. Four filed in each of the 8 different mice analyzed
Fig. 3.
Accumulation of misfolded SOD1 in primary motor neurons induced cPLA2α upregulation. A Immunoblot analysis of human SOD1WT or mutant SOD1G93A (hSOD1), expressed in primary motor neurons by infection of AAV1/2 as described in materials and methods. Immunoblot analysis of mouse SOD1 (mSOD1) is shown as a control. B Light microscope pictures of primary motor neurons. Control—without infection, motor neurons expressing human wild type SOD1 (SOD1 WT) or mutant SOD1 (SOD1 G93A). Scale bars = 50 μm. C Double immunofluorescence staining of cPLA2α (green) with misfolded SOD1 (B8H10, red) in motor neurons expressing SOD1WT, mutant SOD1G93A and control cells. Two upper panels scale bars = 50 μm and two lower panels scale bars = 20 μm. 3 different independent experiments were analyzed and showed similar results. The means ± SEM fluorescence intensity for cPLA2α and misfolded SOD1 is presented in the bar graphs as arbitrary units. Five fields in each of the 3 different treatments of motor neurons in each experiment was analyzed. Significance compared to control ***p < 0.001, n.s. non-significant
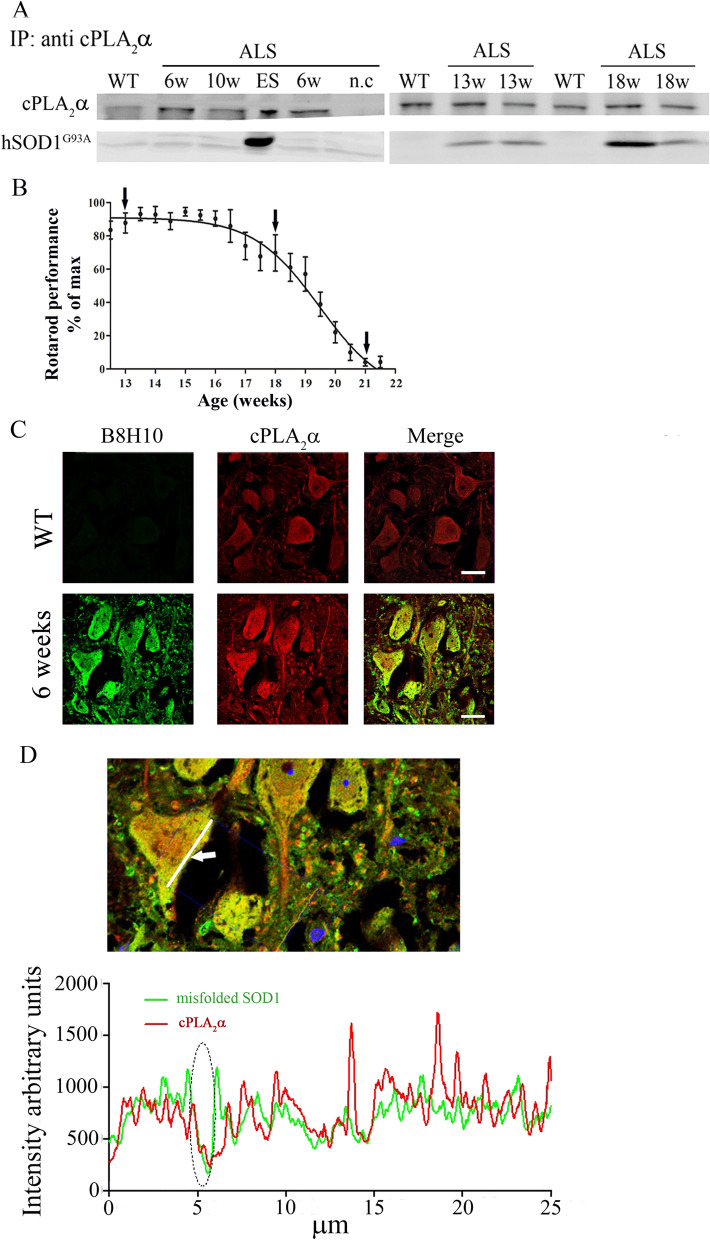
To study whether the elevated cPLA2α protein expression is triggered or stabilized by an interaction between misfolded SOD1 and cPLA2α, the binding between both proteins was determined by co-immunoprecipitation experiments. As shown in Fig. 4A, immunoprecipitation of cPLA2α in the spinal cord lysates at disease onset (13 weeks), symptomatic stage (18 weeks) or end stage (Fig. 4B) resulted in a significant co-immunoprecipitation of mutant SOD1G93A (Fig. 4A), suggesting a binding between them. In contrast, there was no co-immunoprecipitation of mutant SOD1G93A and cPLA2α in the spinal cord lysates of 6 weeks old mutant SOD1G93A mice, suggesting that there is no binding between the proteins in that early stage. We then used an Airyscan detector, a sub-diffraction high-resolution laser-scanning confocal microscope to examine the binding between both proteins. The Airyscan detector used in the current study provides improved lateral resolution (~ 150 nm) and signal to noise ratio, as compared with conventional confocal microscopes. Under these conditions, accurate and straight forward analysis of the interaction between misfolded SOD1 and cPLA2α in the motor neurons in the spinal cord section was allowed. Airyscan high-resolution detection showed that there is only partial overlapping indicating partial binding between both proteins at 6 weeks (Fig. 4C, D).
Fig. 4.
Partial binding between cPLA2α and mutant SOD1G93A in motor neurons of 6 weeks old mutant SOD1G93A mice. A Immunoprecipitation with antibody against cPLA2α and Western Blot analysis for cPLA2α and mutant SOD1 in the spinal cord lysate of control (WT) and mutant SOD1G93A mice during the development of the disease shown by a representative immunoblot. Negative control (n.c). -without antibodies against cPLA2α. B Motor performance on accelerating Rotarod test (n = 10 mice). The arrows show the immunoprecipitation analysis along the development of the disease. C Airyscan detector high resolution confocal microscopy images of double immunofluorescence staining of cPLA2α (red) with misfolded SOD1 (B8H10, green) proteins in the spinal cord sections of 6 weeks old pre-symptomatic SOD1G93A mouse. Scale bars = 20 μm. D The coupling of both proteins (cPLA2α and misfolded SOD1) in the line shown by the arrow was analyzed and presented as intensity along the line. The ellipse shows an example of binding between both proteins
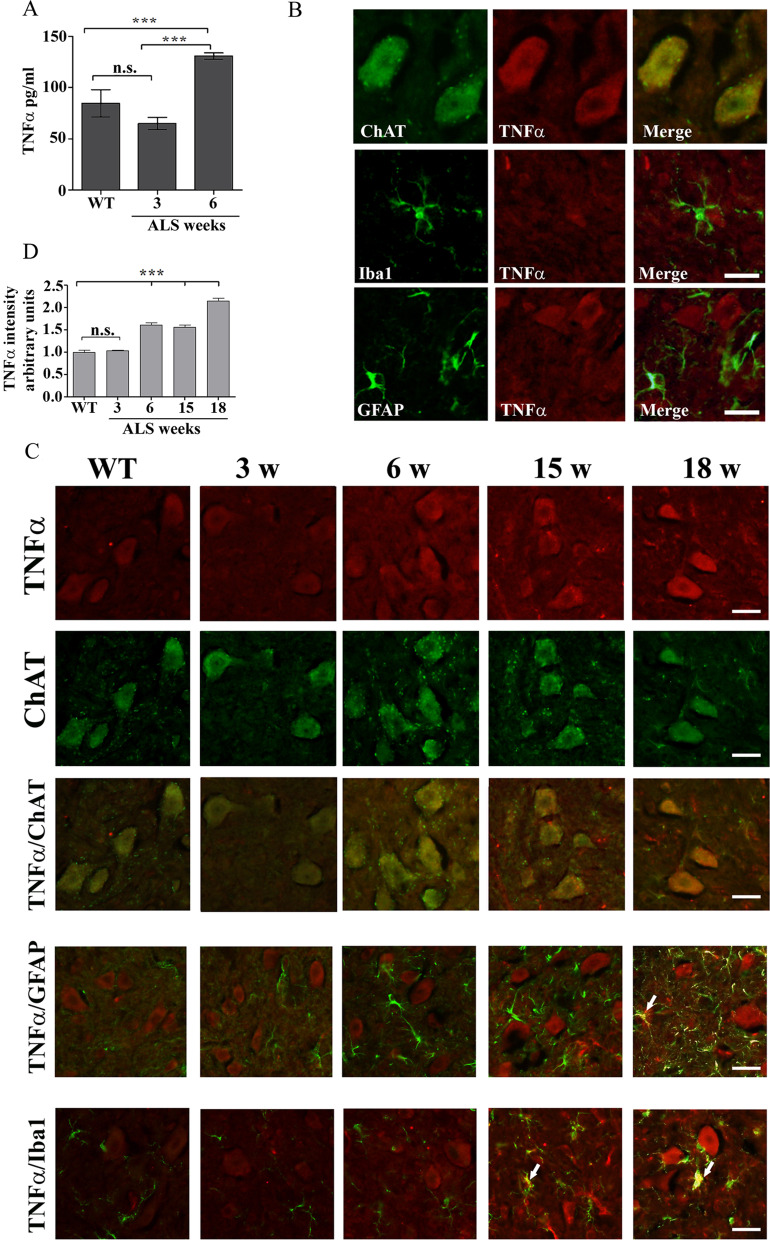
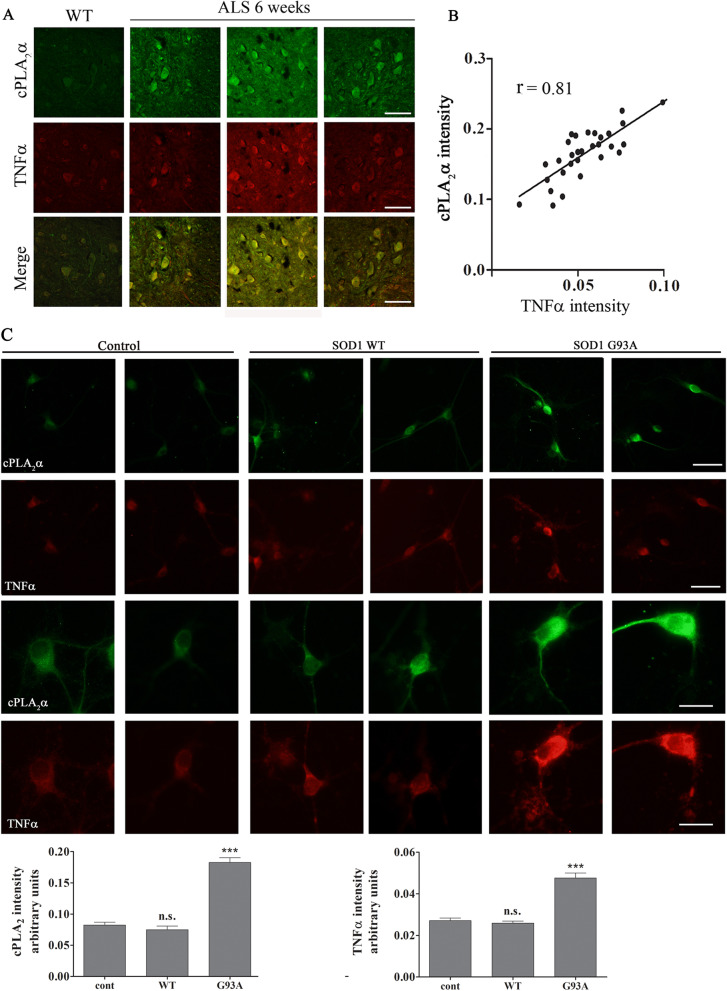
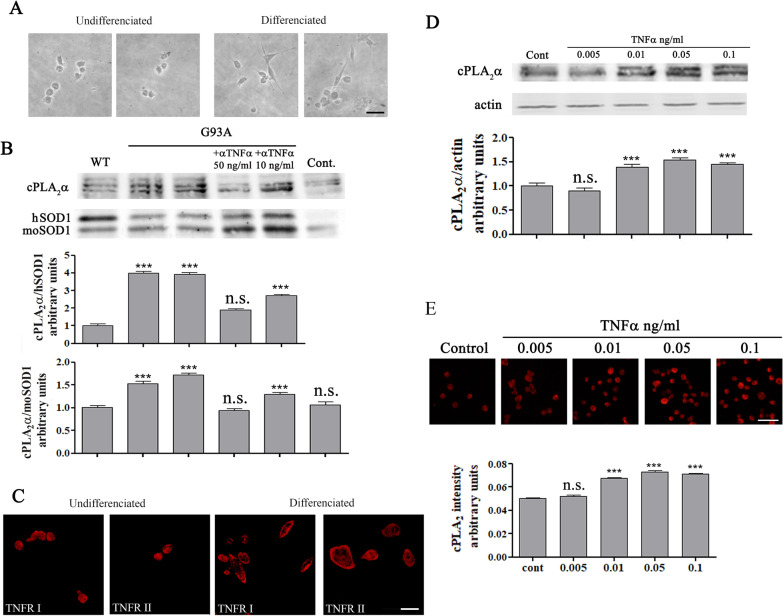
Proinflammatory cytokines such as TNFα [23, 24] are shown to induce elevation of cPLA2α protein expression, thus, we examined whether increased levels of TNFα could be detected in the spinal cord of 6 weeks old mutant SOD1G93A mice. As shown in Fig. 5A, there is a significant (p < 0.001) elevation of TNFα in the spinal cord lysates of 6 weeks old mutant SOD1G93A mice in comparison with spinal cord lysates of WT or 3 weeks old SOD1G93A mice (130.0 ± 3.0 pg/ml compared with 85.5 ± 13.3 and 73.6 ± 6.7 pg/ml, respectively). To determine which type of cell produces TNFα, co-immunofluorescence staining of TNFα using anti-TNFα antibodies that show specific staining (Additional file 3: Fig. S3) and the different cell markers was performed in the spinal cord sections of 6 weeks old SOD1G93A mice. Co-immunofluorescence staining of TNFα and ChAT clearly showed that TNFα was detected in the motor neurons in the spinal cords of 6 weeks old mutant SOD1G93A mice (Fig. 5B). Immunofluorescence staining of the motor neurons in the spinal cord of 6 weeks old SOD1G93A mice showed elevation of both TNFα receptors expression in comparison to WT mice (Additional file 4: Fig. S4). Co- immunofluorescence staining of TNFα and either Iba1 or GFAP showed that TNFα was not detected in the microglia or astrocytes at 6 weeks (Fig. 5B). A time course of co-immunofluorescence staining of TNFα and the different cell markers in the spinal cords of SOD1G93A mice clearly shows elevated TNFα levels in the motor neurons at 6 weeks which was gradually increased at a later stage (Fig. 5C). TNFα was not detected in glia cells at 6 weeks, but was detected in microglia at 15 weeks and in astrocytes at 18 weeks (Fig. 5C). In accordance with these results, immunofluorescence staining of Iba1 or GFAP, to determine glia activation, showed no significant activation in the spinal cord section of mutant SOD1G93A mice at these early stages (3 and 6 weeks), although misfolded SOD1 was already accumulated in the spinal cord, but did show a significant activation in the spinal cord sections of 17 weeks old SOD1G93A mice (Additional file 5: Fig. S5) as reported in our previous study [15]. Co-immunofluorescence staining and densitometry analysis of cPLA2α and TNFα in the spinal cord sections of 6 weeks old SOD1G93A mice showed as above that there is a variation of elevated cPLA2α protein expression in the different mice (Fig. 6A), which was highly correlated with TNFα (coefficient correlation, r = 0.81) in the motor neurons (Fig. 6B), suggesting that TNFα produced in the motor neurons is responsible for cPLA2α upregulation. We next studied the effect of misfolded SOD1 accumulation on TNFα expression using motor neurons that were viral induced with human SOD1WT or mutant SOD1G93A (described in Fig. 3). As shown in Fig. 6C, double staining of cPLA2α and TNFα showed that motor neurons with accumulated mutant SOD1G93A (Fig. 3C) expressed elevated levels of both cPLA2α and TNFα. Over expression of SOD1WT did not affect cPLA2α and TNFα compared with the control cells. The comparison between motor neurons expressing SOD1WT and those expressing the mutant SOD1G93A clearly shows that of the accumulation of misfolded SOD1 induces the elevation of cPLA2α and TNFα (Additional file 6: Fig. S6). To determine whether elevated TNFα is responsible for cPLA2α upregulation, human SOD1WT or SOD1G93A were expressed in the motor neurons like NSC34 cells. NSC34 is an hybrid cell line produced by the fusion of motor neurons from the spinal cords of mouse embryos with mouse neuroblastoma cells N18TG2 that exhibit properties of motor neurons after differentiation [21] as presented by shape change (Fig. 7A). As clearly shown in the western blot analysis (Fig. 7B), accumulation of mutant SOD1 caused significant elevation of cPLA2α, while accumulation of SOD1WT did not affect on cPLA2α expression in NSC34 cells compared with the control (untransfected cells). The presence of neutralizing TNFα antibodies (50 ng/ml) prevented cPLA2α upregulation, indicating that TNFα is responsible for cPLA2α upregulation by an autocrine mechanism. In accord with these results, although very low levels of TNFα were detected in the supernatant of the cells, they were significantly higher in supernatant of cells transfected with human SOD1G93A compared with cells transfected with SOD1WT or control cells (Additional file 7: Fig. S7). The differentiated neuron motors NSC34 cells express both TNF receptors as shown by immunofluoresnce staining (Fig. 7C). The addition of TNFα to differentiated NSC34 cells caused cPLA2α upregulation in a dose dependent manner, as shown by western blot analysis (Fig. 7D) and immunofluorescence staining cPLA2α (Fig. 7E).
Fig. 5.
Increased TNFα is restricted to the motor neurons of pre-symptomatic 6 weeks old mutant SOD1G93A mice. A The levels of TNFα in the spinal cord lysate of WT and of 3 and 6 weeks old pre-symptomatic mutant SOD1G93A mice detected by ELISA. Significance—***p < 0.001, n.s. = non-significant. The bar graph is the mean ± SE of 8 mice in each group. B Representative double immunofluorescence staining cell markers (green) of motor neurons (ChAT), microglia (Iba-1) or astrocytes (GFAP) and TNFα (red) in the spinal cord sections of 6 weeks old pre-symptomatic SOD1G93A mice. Scale bars = 20 μm. 3 other mice in each group were analyzed and showed similar results. C A representative time course of double immunofluorescence staining of TNFα (red) and cell markers (green) of motor neurons (ChAT), microglia (Iba-1) or astrocytes (GFAP) proteins in the lumbar spinal cord sections of WT and mutant SOD1G93A mice during the course of the disease (3, 6, 15 and 18 weeks). Scale bars = 20 μm. 3 other mice in each group were analyzed and showed similar results. D. The means ± SEM fluorescence intensity for TNFα is presented in the bar graph as arbitrary units. Four mice for each time point and five fields for each mouse were analyzed. Significance compared to control ***p < 0.001, n.s. non-significant
Fig. 6.
Elevated cPLA2α protein expression in the motor neurons is highly correlated with TNFα. A Double immunofluorescence staining of cPLA2α (green) and TNFα (red) proteins in the lumbar spinal cord sections of WT and 6 weeks old mutant SOD1G93A mice. Scale bars = 100 μm. B The Pearson coefficient correlation (r) between cPLA2α and TNFα in the spinal motor neurons of mutant SOD1G93A mice was analyzed. Florescence intensity is expressed in arbitrary units of immunostaining as presented in the representative results in A. Four fields in each of the 8 different mice analyzed. C Elevated cPLA2α and TNFα in primary motor neurons expressing mutant SOD1G93A. Double immunofluorescence staining of cPLA2α (green) and TNFα (red) in primary motor neurons expressing human SOD1WT, mutant SOD1G93A and control cells described in Fig. 3. Two upper panels, scale bars = 50 μm and two lower panels, scale bar s = 20 μm. 3 different independent experiments were analyzed and showed similar results. The means ± SEM fluorescence intensity for cPLA2α and TNFα is presented in the bar graphs as arbitrary units. Five fields in each of the 3 different treatments of motor neurons in each experiment was analyzed. Significance compared to control ***p < 0.001, n.s. non-significant
Fig. 7.
Secreted TNFα is responsible for cPLA2α upregulation. A Representative light microscope pictures of undifferentiated and differentiated motor like NSC34 cells, scale bars = 50 μm. B cPLA2α upregulation induced by mutant SOD1G93A expressing cells is prevented in the presence of anti-TNFα antibodies. NSC-34 cells were transfected with human SOD1WT or mutant SOD1G93A plasmids for 72 h before lysates preparations. TNFα neutralizing antibodies were added at 48 h. A representative immunoblot analysis of cPLA2α in NSC34 cells transfected with human SOD1WT (WT) or mutant SOD1G93A (G93A) in the absence or presence of anti-TNFα. Control cells without transfection (Cont.). cPLA2α protein expression was determined by dividing the intensity of each cPLA2α with the intensity of the corresponding human SOD1 or mouse SOD1 after quantitation by densitometry and expressed in the bar graph as arbitrary units. The bar graphs are the means ± SE of three experiments. Significance compared to control ***p < 0.001, n.s. non-significant. C Representative confocal pictures of immunofluorescence staining of TNFRI and TNFRII in undifferentiated and differentiated motor like NSC34 cells, scale bars = 20 μm. Two other experiments showed similar results. D A representative immunoblot analysis of a dose dependent effect of TNFα (0.005–0.1 ng/ml) for 24 h on cPLA2α expression in differentiated motor neuron-like NSC34 cells. cPLA2α protein expression was determined by dividing the intensity of each cPLA2α with the intensity of the corresponding actin after quantitation by densitometry and expressed in the bar graph as arbitrary units. The bar graphs are the means ± SE of 3 experiments. Significance compared to control ***p < 0.001, n.s. non-significant. E A representative immunofluorescence staining of a dose dependent effect of TNFα (0.05–0.1 ng/ml) for 24 h on cPLA2α expression in differentiated motor neuron-like NSC34 cells. Scale bars = 50 μm. The bar graph is the mean ± SE of 3 different experiments. Significance compared to control ***p < 0.001, n.s. non-significant
Discussion
The present study clearly demonstrates that misfolded SOD1 is significantly detected in the spinal cords of 3 weeks old mutant SOD1G93A mice preceding the elevated expression of cPLA2α and its activation at 6 weeks. The elevation of cPLA2α in the spinal cords at 6 weeks, was detected specifically in motor neurons and not in microglia or astrocytes. While, at the symptomatic stage, elevated cPLA2α was also detected in the glia cells in accordance with our and others previous studies [14, 15]. Similar to our results, activation and elevation of cPLA2α protein expression were mainly detected in motor neurons in other pathological conditions such as after spinal cord injury and in spinal inflammatory hyperalgesia [25–29]. cPLA2α is a major inflammatory enzyme-producing arachidonic acid, a substrate for the formation of eicosanoids and a platelet-activating factor which are well-known mediators of inflammation and tissue damage implicated in pathological states of several acute and chronic neurological disorders [26, 30–32]. The detection of elevated and activated cPLA2α in the motor neurons at 6 weeks old mutant SOD1G93A mice and long before any neuronal damage or sign of the disease is evident, indicates an inflammatory state of the motor neurons at this very early stage. Misfolded SOD1 accumulation in the spinal cord of 3 weeks old SOD1G93A mice was detected in motor neurons but not in astrocytes or microglia. The significant accumulation of misfolded SOD1 in the motor neurons before the appearance of elevated cPLA2α expression and the significant correlation (r = 0.92) between both proteins at 6 weeks shown in the present study raised the possibility that the accumulation of misfolded SOD1 in the cells dictates the expression of cPLA2α. Indeed, the accumulation of misfolded SOD1 (determined by B8H10 staining) in primary motor neurons isolated from the mouse spinal cord or expression of mutant SOD1 in NSC34 motor neuron-like cells caused a significant elevation of cPLA2α protein expression. In contrast, expression of human SOD1WT did not affect cPLA2α expression, indicating that intracellular misfolded SOD1 induced cPLA2α upregulation. Although the role of glial cells in neuronal damage and disease progression is well established [33], we show here that the elevation of cPLA2α in the motor neurons at 6 weeks is independent of glia cells and occurs long before any neuronal damage. In accordance with our results, the effect of accumulated mutant SOD1 on cPLA2α in NSC34 cells was reported recently [34]. They showed that expression of SOD1G93A in motor neuron cell line NSC34 for long time induced cell death mediated by cPLA2α. The elevated levels of cPLA2α in familial and sporadic ALS [13] together with the observations that inclusions containing misfolded SOD1 are regularly present in motor neurons of ALS patients, both with and without SOD1 mutations [35], support the notion that misfolded SOD1 accumulated in the motor neurons contributes to the elevated cPLA2α expression.
Induction or stabilization of proteins by other proteins within the cells via an interaction mechanism such as induction of P53 by elevated amyloid beta [36] and stabilization of EGFR or Kit C by HIP1 binding have been reported [37, 38]. In addition, binding and co-precipitation of oligomeric or misfolded SOD1 with other proteins including voltage dependent anion channel (VDAC1) [39, 40], macrophage migration inhibitory factor (MIF) [22, 41], heat shock protein [42], glutathione peroxidase 1 [43] or Bcl-2 [44] have been documented. Taken together, these observations and the results demonstrating high overlapping of misfolded SOD1 and cPLA2α in the motor neurons in spinal cord sections by confocal microscopy could suggest that cPLA2α upregulation is induced by its interaction with misfolded SOD1 at 6 weeks old mutant SOD1G93A mice. Co-immunoprecipitation of both proteins was detected only at the symptomatic stage but not at 6 weeks, indicating no direct interaction at this stage. Using the Airyscan detector, a sub-diffraction high-resolution laser-scanning confocal microscope [45, 46], we showed that only partial binding between both proteins in motor neurons at 6 weeks, explains the absence of co-immunoprecipitation at this stage, and questioning the possibility that the interaction between misfolded SOD1 and cPLA2α induced the elevation of cPLA2α expression.
cPLA2α was shown to be induced by different proinflammatory mediators and insults through specific receptors or scavenger receptors [23, 47–50]. Ours and other studies reported that TNFα induced cPLA2α upregulation in various systems [23, 51, 52] and in motor neuron-like NSC34 cells, as demonstrated in the present study. In the neural environment, constitutive physiological levels of TNFα regulate synaptic plasticity, modulates dendritic maturation, pruning, and synaptic connectivity to respond to alterations in sensory stimuli to maintain homeostatic plasticity [53, 54]. Overexpression of TNFα has been associated with neuronal excitotoxicity, synapse loss, and propagation of the inflammatory state [55]. TNFα elicits its wide range of biological responses by activating two distinct receptors, TNF-R1 and TNF-R2 [56–58]. Antigenic TNFα and its soluble receptors measured by ELISA were significantly higher in ALS patients than in healthy controls [59]. To our knowledge, the present study is the first to show a significant elevation of TNFα protein in the spinal cord of mutant SOD1G93A mice as early as at 6 weeks, that was about 150% of the levels of TNFα in the spinal cord of WT mice and of 3 weeks old mutant SOD1G93A mice. Our results are supported by other studies reporting that TNFα was detected in the spinal cord of late pre-symptomatic stage ALS mice. TNFα was the sole cytokine whose mRNA could be observed in the spinal cord of young pre-symptomatic SOD1G93A mice [60]. A microarray survey of 1081 gene products expressed in spinal cords of SOD1G93A mice reported that TNFα was the only inflammatory cytokine found to be differentially expressed [4]. Upregulation of TNFα and its proapoptotic receptors mRNA were detected at late pre-symptomatic stages and preceded transcriptional upregulation of other pro-inflammatory gene products and temporally correlates with the progression of the disease in SOD1G93A mice [61, 62]. TNFα was reported to be elevated in the spinal cord of SOD1G93A transgenic mice in the early life span, at 80 days [63]. We also show here for the first time that elevated TNFα is expressed specifically in spinal motor neurons of 6 weeks old SOD1G93A mice but not in microglia or astrocytes, although glia cells are reported to be the major cell type to secrete TNFα [64]. In agreement with our results, immunohistochemical analysis showed little TNFα immunoreactivity in motor neurons from 60 days old SOD1G93A transgenic mice with a healthy appearance [65] and FasL as early as day 40 [65]. Since FasL is upregulated by TNFα [66], it is possible that due to the methodology sensitivity TNFα was not detected in the spinal cord of 40 days old SOD1G93A mice but at 60 days in their study [65]. We show here a high correlation (r = 0.81) between cPLA2α and TNFα expressed in the spinal motor neurons of 6 weeks old SOD1G93A mice, suggesting that misfolded SOD1 induced the elevation of cPLA2α via production of TNFα. Indeed, as we show in the present study, that expression of mutant SOD1 but not SOD1WT in motor neurons induced both cPLA2α and TNFα upregulation. Moreover, the presence of neutralizing TNFα antibodies prevented the elevation of cPLA2α expression NSC34 like motor neurons, indicating that TNFα is responsible for cPLA2α upregulation, acting via its autocrine effect. Likewise, addition of TNFα (10–100 pg/ml) to differentiated NSC34 cells for 24 h caused cPLA2α upregulation in a dose dependent manner similar to the concentration detected in the spinal cord of 6 weeks of SOD1G93A mice. In agreement with our report, addition of soluble TNFα (acting through a reverse signaling) for 6 days affected motor neurons, inducing a marked motor neuron loss in SOD1-G93A monocultures [33]. Since elevated TNFα receptors were detected in motor neurons in the spinal cord of 6 weeks old SOD1G93A mice, in accordance with others that reported elevated TNFR in the pre-symptomatic stage [67], the elevated TNFα in the spinal cord at this time point probably acts through its receptors to induce cPLA2α upregulation.
Our results, suggesting that motor neurons have a crucial role in inflammatory state (demonstrating elevated both cPLA2α and TNFα) during the early stage of the disease, are in accordance with the specific activation of motor neurons but not glia cells in the pre-symptomatic stage (at 8 weeks) of the disease in mutant SOD1G93A mice evident by the increased p38MAPK [67, 68], activation of ASK1, MKK3,4,6, overexpression of both TNFα receptors (TNFR1 and TNFR2) [67] and TNFα accumulation in transgenic motor neurons [33]. In addition, motor neurons were reported as a primary determinant of disease onset and early disease progression by selective mutant gene inactivation within the cells [69]. Moreover, it was shown [70] that neuronal expression of mutant SOD1 was sufficient to cause motor neuron degeneration and paralysis in transgenic mice with cytosolic dendritic ubiquitinated SOD1 aggregates as the dominant pathological feature. Crossing neuron-specific mutant SOD1 mice with ubiquitously wild-type SOD1-expressing mice led to dramatic wild-type SOD1 aggregation in oligodendroglia after the onset of neuronal degeneration suggesting that mutant SOD1 in neurons triggers neuronal degeneration, which in turn may facilitate aggregates formation in surrounding glial cells. In contrast, cell-specific deletion of mutant SOD1 in genetically altered mice has implicated microglia and astrocytes as contributors to the late disease progression but not the onset of disease [71–73].
Conclusions
We show here that elevated protein expression of both cPLA2α and TNFα were detected specifically in motor neurons and not in glial cells in the spinal cord of 6 weeks old SOD1G93A mice, indicating the inflammatory state of the motor neurons long before the development of signs of the disease. Misfolded SOD1 is accumulated in the spinal cord motor neurons of 3 weeks old SOD1G93A mice, preceding cPLA2α and TNFα upregulation. The results of the present study show: a. The high correlation between cPLA2α and misfolded SOD1 levels and between cPLA2α and TNFα levels in the motor neurons at 6 weeks, b. cPLA2α and TNFα upregulation by expressing mutant SOD1 in primary motor neurons and in NSC34 motor neurons like cells, c. the prevention of cPLA2α upregulation in the presence of TNFα neutralizing antibodies and d. the induction of cPLA2α upregulation by addition of TNFα and the presence of TNFR receptors. Based on these results we can conclude that accumulated misfolded SOD1, in the motor neurons in the spinal cord of 6 weeks old SOD1G93A mice, induced cPLA2α upregulation mediated by TNFα via its autocrine effect.
Supplementary Information
Additional file 1: Figure S1. Specificity of immunofluorescence analysis of misfolded SOD1. Representative results of immunofluorescence analysis of misfolded SOD1, using B8H10 antibodies, versus negative control (n.c.) and wild type (WT) in mice spinal cords. Scale bar = 100 μm.
Additional file 2: Figure S2. Misfolded SOD1 could be detected at 3 weeks in motor neurons. A representative double immunofluorescence of motor neurons marker (ChAT) and misfolded SOD1 in the spinal cord of 3 weeks old SOD1G93A mice. Scale bar = 100 μm
Additional file 3: Figure S3. Specificity of immunofluorescence analysis of TNFα. Representative results of immunofluorescence staining of TNFα versus negative control (n.c.) in the spinal cord of WT mice and 6 weeks old SOD1G93A mice. Scale bar = 100 μm. There is a low staining of TNFα in the spinal cord of WT mice in accordance with the low level of TNFα in their lysates.
Additional file 4: Figure S4. TNFα receptors are elevated in motor neurons in the spinal cord of 6 weeks old mutant SOD1G93A mice. Representative immunofluorescence staining of TNFRI and TNFRII in the spinal cord of WT mice and mutant SOD1G93A mice. Scale bars = 50 μm. The elevated receptors are detected in motor neurons as determined by the cell shape.
Additional file 5: Figure S5. The accumulation of misfolded SOD1 precedes glia activation. Representative immunofluorescence staining of Iba1, GFAP (red) or misfolded SOD1 (B8H10, green) proteins in the lumbar spinal cord sections of WT and mutant SOD1G93A mice during the course of the disease (3, 6 and 17 weeks). Scale bars = 100 μm. The mean ± SEM fluorescence intensity expressed by arbitrary units is presented in the bar graph (n = 4 mice for each time point, five fields were analyzed for each mouse). ***p < 0.001—compared to control mice (WT). n.s. = non significant.
Additional file 6: Figure S6. Detection of cPLA2α and TNFα in motor neurons expressing hSOD1WT or mutant hSOD1G93A. The Pearson coefficient correlation between cPLA2α and misfolded SOD1 (r = 0.87) and between cPLA2α and TNFα (r = 0.96) in motor neurons expressing mutant SOD1G93A. Representative results of florescence intensity of immunostaining presented in Figs. 3C and 6C is expressed in arbitrary units.
Additional file 7: Figure S7. Elevated TNFα levels in supernatant of NSC34 cells expressing SOD1G93A. The levels of TNFα in the supernatants of NSC34 cells transfected with human SOD1WT or mutant SOD1G93A plasmids (as described in Fig. 6B) detected by ELISA. The bar graph is the mean ± SE of three experiments. Significance compared to control ***p < 0.001, n.s. non-significant.
Acknowledgements
We thank Miss Dorothy Polayes, Florida, USA, for her a generous donation to support research on Degenerative Diseases at Ben-Gurion University of the Negev. We thank Ms. Shir Barel for helping with the purification of the plasmids used for transfection.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- cPLA2α
Cytosolic phospholipase A2 alpha
- SOD1G93A
SOD1 transgenic mice
- TNFα
Tumor necrosis factor alpha
- hSOD1G93A
Recombinant human mutant SOD1G93A
- WT
Wild type
Authors’ contributions
YME searched the literature, designed and carried out experiments, researched the data, prepared Figures. YS carried out experiments, researched the data, prepared Figures. LA carried out experiments, researched the data. NH and AI participated in the design of the study and guided some methodologies. RL designed the study, directed the study and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by a generous donation from Miss Dorothy Polayes, Florida, USA, during 2016–2019.
Availability of data and materials
There is no data, software, databases, and application/tool available apart from the reported in the present study. All data generated or analyzed during this study are included in this published article are provided in the manuscript and its Additional files.
Declarations
Ethics approval and consent to participate
The study was approved by Ben-Gurion University Institutional Animal Care and Use Committee (IL-40-07-2016) and was conducted according to the Israeli Animal Welfare Act following the guidelines of the Guide for Care and Use of Laboratory Animal (National Research Council, 1996).
Consent for publication
Not applicative.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yafa Fetfet Malada Edelstein, Email: yafamalada@gmail.com.
Yulia Solomonov, Email: yuliasol@bgu.ac.il.
Nurit Hadad, Email: nurith@bgu.ac.il.
Leenor Alfahel, Email: leenor@post.bgu.ac.il.
Adrian Israelson, Email: adriani@bgu.ac.il.
Rachel Levy, Email: ral@bgu.ac.il.
References
- 1.Ince P, Highley J, Kirby J, Wharton S, Takahashi H, Strong M, Shaw P. Molecular pathology and genetic advances in amyotrophic lateral sclerosis: an emerging molecular pathway and the significance of glial pathology. Acta Neuropathol. 2011;122:657–671. doi: 10.1007/s00401-011-0913-0. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P. Genetics of sporadic ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:S37–41. doi: 10.1080/14660820152415726. [DOI] [PubMed] [Google Scholar]
- 3.Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- 4.Yoshihara T, Ishigaki S, Yamamoto M, Liang Y, Niwa J, Takeuchi H, Doyu M, Sobue G. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 2002;80:158–167. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiu I, Phatnani H, Kuligowski M, Tapia J, Carrasco M, Zhang M, Maniatis T, Carroll M. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci U S A. 2009;106:20960–20965. doi: 10.1073/pnas.0911405106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beers D, Henkel J, Xiao Q, Zhao W, Wang J, Yen A, Siklos L, McKercher S, Appel S. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai M, Re D, Nagata T, Chalazonitis A, Jessell T, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer RM, Roberts EF, Manetta J, Putnam JE. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J Biol Chem. 1991;266:5268–5272. [PubMed] [Google Scholar]
- 9.Clark JD, Milona N, Knopf JL. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci USA. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 11.Clemens JA, et al. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–535. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J, Little S, Kramer R, Clemens J. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27:110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Shibata N, Kakita A, Takahashi H, Ihara Y, Nobukuni K, Fujimura H, Sakoda S, Kobayashi M. Increased expression and activation of cytosolic phospholipase A2 in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. Acta Neuropathol. 2010;119:345–354. doi: 10.1007/s00401-009-0636-7. [DOI] [PubMed] [Google Scholar]
- 14.Kiaei M, Kipiani K, Petri S, Choi D, Chen J, Calingasan N, Beal M. Integrative role of cPLA with COX-2 and the effect of non-steriodal anti-inflammatory drugs in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2005;93:403–411. doi: 10.1111/j.1471-4159.2005.03024.x. [DOI] [PubMed] [Google Scholar]
- 15.Solomonov Y, Hadad N, Levy R. Reduction of cytosolic phospholipase A2alpha upregulation delays the onset of symptoms in SOD1G93A mouse model of amyotrophic lateral sclerosis. J Neuroinflammation. 2016;13:134. doi: 10.1186/s12974-016-0602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97:12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron R, Babcock AA, Nemirovsky A, Finsen B, Monsonego A. Accelerated microglial pathology is associated with Abeta plaques in mouse models of Alzheimer’s disease. Aging Cell. 2014;13:584–595. doi: 10.1111/acel.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shmelzer Z, Haddad N, Admon E, Pessach I, Leto TL, Eitan-Hazan Z, Hershfinkel M, Levy R. Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. J Cell Biol. 2003;162:683–692. doi: 10.1083/jcb.200211056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyton-Jaimes MF, Benaim C, Abu-Hamad S, Kahn J, Guetta A, Bucala R, Israelson A. Endogenous macrophage migration inhibitory factor reduces the accumulation and toxicity of misfolded SOD1 in a mouse model of ALS. Proc Natl Acad Sci USA. 2016;113:10198–10203. doi: 10.1073/pnas.1604600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royo NC, Vandenberghe LH, Ma JY, Hauspurg A, Yu L, Maronski M, Johnston J, Dichter MA, Wilson JM, Watson DJ. Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res. 2008;1190:15–22. doi: 10.1016/j.brainres.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier O, Bohm J, Dahm M, Bruck S, Beyer C, Johann S. Differentiated NSC-34 motoneuron-like cells as experimental model for cholinergic neurodegeneration. Neurochem Int. 2013;62:1029–1038. doi: 10.1016/j.neuint.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Shvil N, Banerjee V, Zoltsman G, Shani T, Kahn J, Abu-Hamad S, Papo N, Engel S, Bernhagen J, Israelson A. MIF inhibits the formation and toxicity of misfolded SOD1 amyloid aggregates: implications for familial ALS. Cell Death Dis. 2018;9:107. doi: 10.1038/s41419-017-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadad N, Tuval L, Elgazar-Carmom V, Levy R, Levy R. Endothelial ICAM-1 protein induction is regulated by cytosolic phospholipase A2alpha via both NF-kappaB and CREB transcription factors. J Immunol. 2011;186:1816–1827. doi: 10.4049/jimmunol.1000193. [DOI] [PubMed] [Google Scholar]
- 24.Yang CM, Lee IT, Chi PL, Cheng SE, Hsiao LD, Hsu CK. TNF-alpha induces cytosolic phospholipase A2 expression via Jak2/PDGFR-dependent Elk-1/p300 activation in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2014;306:L543–551. doi: 10.1152/ajplung.00320.2013. [DOI] [PubMed] [Google Scholar]
- 25.Liu NK, Deng LX, Zhang YP, Lu QB, Wang XF, Hu JG, Oakes E, Bonventre JV, Shields CB, Xu XM. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann Neurol. 2014;75:644–658. doi: 10.1002/ana.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu NK, Xu XM. Phospholipase A2 and its molecular mechanism after spinal cord injury. Mol Neurobiol. 2010;41:197–205. doi: 10.1007/s12035-010-8101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu NK, Zhang YP, Han S, Pei J, Xu LY, Lu PH, Shields CB, Xu XM. Annexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injury. J Neuropathol Exp Neurol. 2007;66:932–943. doi: 10.1097/nen.0b013e3181567d59. [DOI] [PubMed] [Google Scholar]
- 28.Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, Shields CB, Xu XM. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- 29.Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA. Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol. 2005;144:940–952. doi: 10.1038/sj.bjp.0706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonventre JV. Roles of phospholipases A2 in brain cell and tissue injury associated with ischemia and excitotoxicity. J Lipid Mediat Cell Signal. 1997;16:199–208. doi: 10.1016/s0929-7855(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 31.Farooqui AA, Yang HC, Rosenberger TA, Horrocks LA. Phospholipase A2 and its role in brain tissue. J Neurochem. 1997;69:889–901. doi: 10.1046/j.1471-4159.1997.69030889.x. [DOI] [PubMed] [Google Scholar]
- 32.Titsworth WL, Liu NK, Xu XM. Role of secretory phospholipase a(2) in CNS inflammation: implications in traumatic spinal cord injury. CNS Neurol Disord Drug Targets. 2008;7:254–269. doi: 10.2174/187152708784936671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tortarolo M, Vallarola A, Lidonnici D, Battaglia E, Gensano F, Spaltro G, Fiordaliso F, Corbelli A, Garetto S, Martini E, et al. Lack of TNF-alpha receptor type 2 protects motor neurons in a cellular model of amyotrophic lateral sclerosis and in mutant SOD1 mice but does not affect disease progression. J Neurochem. 2015;135:109–124. doi: 10.1111/jnc.13154. [DOI] [PubMed] [Google Scholar]
- 34.Kazuki Ohuchi K, Tsuruma K, Masamitsu Shimazawa M, Junji Nakamura J, Hideaki HH. The novel cPLA2 inhibitor AK106-001616 has a protective effect on SOD1G93A-induced cell death in NSC34 murine motor neuron-like cell. Pharmacol Pharmacy. 2016;7:193–199. [Google Scholar]
- 35.Forsberg K, Jonsson PA, Andersen PM, Bergemalm D, Graffmo KS, Hultdin M, Jacobsson J, Rosquist R, Marklund SL, Brannstrom T. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS ONE. 2010;5:e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, et al. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- 37.Bradley SV, Holland EC, Liu GY, Thomas D, Hyun TS, Ross TS. Huntingtin interacting protein 1 is a novel brain tumor marker that associates with epidermal growth factor receptor. Cancer Res. 2007;67:3609–3615. doi: 10.1158/0008-5472.CAN-06-4803. [DOI] [PubMed] [Google Scholar]
- 38.Ames HM, Bichakjian CK, Liu GY, Oravecz-Wilson KI, Fullen DR, Verhaegen ME, Johnson TM, Dlugosz AA, Ross TS. Huntingtin-interacting protein 1: a Merkel cell carcinoma marker that interacts with c-Kit. J Invest Dermatol. 2011;131:2113–2120. doi: 10.1038/jid.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Israelson A, Arbel N, Da Cruz S, Ilieva H, Yamanaka K, Shoshan-Barmatz V, Cleveland DW. Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron. 2010;67:575–587. doi: 10.1016/j.neuron.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shteinfer-Kuzmine A, Argueti S, Gupta R, Shvil N, Abu-Hamad S, Gropper Y, Hoeber J, Magri A, Messina A, Kozlova EN, et al. A VDAC1-derived N-terminal peptide inhibits mutant SOD1-VDAC1 interactions and toxicity in the SOD1 model of ALS. Front Cell Neurosci. 2019;13:346. doi: 10.3389/fncel.2019.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Israelson A, Ditsworth D, Sun S, Song S, Liang J, Hruska-Plochan M, McAlonis-Downes M, Abu-Hamad S, Zoltsman G, Shani T, et al. Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1. Neuron. 2015;86:218–232. doi: 10.1016/j.neuron.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maatkamp A, Vlug A, Haasdijk E, Troost D, French PJ, Jaarsma D. Decrease of Hsp25 protein expression precedes degeneration of motoneurons in ALS-SOD1 mice. Eur J Neurosci. 2004;20:14–28. doi: 10.1111/j.1460-9568.2004.03430.x. [DOI] [PubMed] [Google Scholar]
- 43.Kato S, Saeki Y, Aoki M, Nagai M, Ishigaki A, Itoyama Y, Kato M, Asayama K, Awaya A, Hirano A, Ohama E. Histological evidence of redox system breakdown caused by superoxide dismutase 1 (SOD1) aggregation is common to SOD1-mutated motor neurons in humans and animal models. Acta Neuropathol. 2004;107:149–158. doi: 10.1007/s00401-003-0791-1. [DOI] [PubMed] [Google Scholar]
- 44.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Kolossov VL, Sivaguru M, Huff J, Luby K, Kanakaraju K, Gaskins HR. Airyscan super-resolution microscopy of mitochondrial morphology and dynamics in living tumor cells. Microsc Res Tech. 2018;81:115–128. doi: 10.1002/jemt.22968. [DOI] [PubMed] [Google Scholar]
- 46.Sieben C, Douglass KM, Guichard P, Manley S. Super-resolution microscopy to decipher multi-molecular assemblies. Curr Opin Struct Biol. 2018;49:169–176. doi: 10.1016/j.sbi.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Hazan-Halevy I, Seger R, Levy R. The requirement of both extracellular regulated kinase and p38 mitogen-activated protein kinase for stimulation of cytosolic phospholipase A(2) activity by either FcgammaRIIA or FcgammaRIIIB in human neutrophils. A possible role for Pyk2 but not for the Grb2-Sos-Shc complex. J Biol Chem. 2000;275:12416–12423. doi: 10.1074/jbc.275.17.12416. [DOI] [PubMed] [Google Scholar]
- 48.Szaingurten-Solodkin I, Hadad N, Levy R. Regulatory role of cytosolic phospholipase A2alpha in NADPH oxidase activity and in inducible nitric oxide synthase induction by aggregated Abeta1-42 in microglia. Glia. 2009;57:1727–1740. doi: 10.1002/glia.20886. [DOI] [PubMed] [Google Scholar]
- 49.Sagy-Bross C, Hadad N, Levy R. Cytosolic phospholipase Alpha upregulation mediates apoptotic neuronal death induced by aggregated amyloid-beta peptide. Neurochem Int. 2013;63:541–550. doi: 10.1016/j.neuint.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Sagy-Bross C, Kasianov K, Solomonov Y, Braiman A, Friedman A, Hadad N, Levy R. The role of cytosolic phospholipase A alpha in amyloid precursor protein induction by amyloid beta: implication for neurodegeneration. J Neurochem. 2014;132:559–571. doi: 10.1111/jnc.13012. [DOI] [PubMed] [Google Scholar]
- 51.Lee CW, Lin CC, Lee IT, Lee HC, Yang CM. Activation and induction of cytosolic phospholipase A2 by TNF-alpha mediated through Nox2, MAPKs, NF-kappaB, and p300 in human tracheal smooth muscle cells. J Cell Physiol. 2011;226:2103–2114. doi: 10.1002/jcp.22537. [DOI] [PubMed] [Google Scholar]
- 52.Malada-Edelstein YF, Hadad N, Levy R. Regulatory role of cytosolic phospholipase A2 alpha in the induction of CD40 in microglia. J Neuroinflammation. 2017;14:33–59. doi: 10.1186/s12974-017-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yee AX, Hsu YT, Chen L. A metaplasticity view of the interaction between homeostatic and Hebbian plasticity. Philos Trans R Soc Lond B Biol Sci 2017; 372. [DOI] [PMC free article] [PubMed]
- 55.Jung YJ, Tweedie D, Scerba MT, Greig NH. Neuroinflammation as a factor of neurodegenerative disease: thalidomide analogs as treatments. Front Cell Dev Biol. 2019;7:313. doi: 10.3389/fcell.2019.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 57.Tewari M, Dixit VM. Recent advances in tumor necrosis factor and CD40 signaling. Curr Opin Genet Dev. 1996;6:39–44. doi: 10.1016/s0959-437x(96)90008-8. [DOI] [PubMed] [Google Scholar]
- 58.Venters HD, Dantzer R, Kelley KW. A new concept in neurodegeneration: TNFalpha is a silencer of survival signals. Trends Neurosci. 2000;23:175–180. doi: 10.1016/s0166-2236(99)01533-7. [DOI] [PubMed] [Google Scholar]
- 59.Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, Francolini G, Mora G, Camana C, Mazzini L, Bachetti T. Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Lett. 2000;287:211–214. doi: 10.1016/s0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- 60.Elliott JL. Cytokine upregulation in a murine model of familial amyotrophic lateral sclerosis. Brain Res Mol Brain Res. 2001;95:172–178. doi: 10.1016/s0169-328x(01)00242-x. [DOI] [PubMed] [Google Scholar]
- 61.Hensley K, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, Hamdheydari L, Mhatre M, Mou S, Pye QN, et al. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 62.Hensley K, Floyd RA, Gordon B, Mou S, Pye QN, Stewart C, West M, Williamson K. Temporal patterns of cytokine and apoptosis-related gene expression in spinal cords of the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. J Neurochem. 2002;82:365–374. doi: 10.1046/j.1471-4159.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 63.Jeyachandran A, Mertens B, McKissick EA, Mitchell CS. Type I vs. type II cytokine levels as a function of SOD1 G93A mouse amyotrophic lateral sclerosis disease progression. Front Cell Neurosci. 2015;9:462–471. doi: 10.3389/fncel.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welser-Alves JV, Milner R. Microglia are the major source of TNF-alpha and TGF-beta1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem Int. 2013;63:47–53. doi: 10.1016/j.neuint.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiaei M, Petri S, Kipiani K, Gardian G, Choi DK, Chen J, Calingasan NY, Schafer P, Muller GW, Stewart C, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2006;26:2467–2473. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinkoski MJ, Droin NM, Green DR. Tumor necrosis factor alpha up-regulates non-lymphoid Fas-ligand following superantigen-induced peripheral lymphocyte activation. J Biol Chem. 2002;277:42380–42385. doi: 10.1074/jbc.M208167200. [DOI] [PubMed] [Google Scholar]
- 67.Veglianese P, Lo Coco D, Bao Cutrona M, Magnoni R, Pennacchini D, Pozzi B, Gowing G, Julien JP, Tortarolo M, Bendotti C. Activation of the p38MAPK cascade is associated with upregulation of TNF alpha receptors in the spinal motor neurons of mouse models of familial ALS. Mol Cell Neurosci. 2006;31:218–231. doi: 10.1016/j.mcn.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Tortarolo M, Veglianese P, Calvaresi N, Botturi A, Rossi C, Giorgini A, Migheli A, Bendotti C. Persistent activation of p38 mitogen-activated protein kinase in a mouse model of familial amyotrophic lateral sclerosis correlates with disease progression. Mol Cell Neurosci. 2003;23:180–192. doi: 10.1016/s1044-7431(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 69.Boillee S, Yamanaka K, Lobsiger C, Copeland N, Jenkins N, Kassiotis G, Kollias G, Cleveland D. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 70.Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 72.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 73.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Specificity of immunofluorescence analysis of misfolded SOD1. Representative results of immunofluorescence analysis of misfolded SOD1, using B8H10 antibodies, versus negative control (n.c.) and wild type (WT) in mice spinal cords. Scale bar = 100 μm.
Additional file 2: Figure S2. Misfolded SOD1 could be detected at 3 weeks in motor neurons. A representative double immunofluorescence of motor neurons marker (ChAT) and misfolded SOD1 in the spinal cord of 3 weeks old SOD1G93A mice. Scale bar = 100 μm
Additional file 3: Figure S3. Specificity of immunofluorescence analysis of TNFα. Representative results of immunofluorescence staining of TNFα versus negative control (n.c.) in the spinal cord of WT mice and 6 weeks old SOD1G93A mice. Scale bar = 100 μm. There is a low staining of TNFα in the spinal cord of WT mice in accordance with the low level of TNFα in their lysates.
Additional file 4: Figure S4. TNFα receptors are elevated in motor neurons in the spinal cord of 6 weeks old mutant SOD1G93A mice. Representative immunofluorescence staining of TNFRI and TNFRII in the spinal cord of WT mice and mutant SOD1G93A mice. Scale bars = 50 μm. The elevated receptors are detected in motor neurons as determined by the cell shape.
Additional file 5: Figure S5. The accumulation of misfolded SOD1 precedes glia activation. Representative immunofluorescence staining of Iba1, GFAP (red) or misfolded SOD1 (B8H10, green) proteins in the lumbar spinal cord sections of WT and mutant SOD1G93A mice during the course of the disease (3, 6 and 17 weeks). Scale bars = 100 μm. The mean ± SEM fluorescence intensity expressed by arbitrary units is presented in the bar graph (n = 4 mice for each time point, five fields were analyzed for each mouse). ***p < 0.001—compared to control mice (WT). n.s. = non significant.
Additional file 6: Figure S6. Detection of cPLA2α and TNFα in motor neurons expressing hSOD1WT or mutant hSOD1G93A. The Pearson coefficient correlation between cPLA2α and misfolded SOD1 (r = 0.87) and between cPLA2α and TNFα (r = 0.96) in motor neurons expressing mutant SOD1G93A. Representative results of florescence intensity of immunostaining presented in Figs. 3C and 6C is expressed in arbitrary units.
Additional file 7: Figure S7. Elevated TNFα levels in supernatant of NSC34 cells expressing SOD1G93A. The levels of TNFα in the supernatants of NSC34 cells transfected with human SOD1WT or mutant SOD1G93A plasmids (as described in Fig. 6B) detected by ELISA. The bar graph is the mean ± SE of three experiments. Significance compared to control ***p < 0.001, n.s. non-significant.
Data Availability Statement
There is no data, software, databases, and application/tool available apart from the reported in the present study. All data generated or analyzed during this study are included in this published article are provided in the manuscript and its Additional files.