Abstract
Vegetable cultivation is a promising economic activity, and vegetable consumption is important for human health due to the high nutritional content of vegetables. Vegetables are rich in vitamins, minerals, dietary fiber, and several phytochemical compounds. However, the production of vegetables is insufficient to meet the demand of the ever-increasing population. Plant-growth-promoting rhizobacteria (PGPR) facilitate the growth and production of vegetable crops by acquiring nutrients, producing phytohormones, and protecting them from various detrimental effects. In this review, we highlight well-developed and cutting-edge findings focusing on the role of a PGPR-based bioinoculant formulation in enhancing vegetable crop production. We also discuss the role of PGPR in promoting vegetable crop growth and resisting the adverse effects arising from various abiotic (drought, salinity, heat, heavy metals) and biotic (fungi, bacteria, nematodes, and insect pests) stresses.
Keywords: biofertilizer, organic farming, PGPR, vegetables, abiotic stresses, biotic stresses
1. Introduction
Vegetables are an important component of food and nutrition as they provide energy, vitamins, body-building nutrients, and minerals for human health [1]. Vegetables, fruits, and nuts now play an instrumental role in nutrition, food security, and combating the triple load of malnutrition [2]. The World Health Organization (WHO) proposed the daily consumption of 400 g of edible vegetables and fruits to fulfill the requirements of various micronutrients and prevent noncommunicable diseases [3]. In 2018, the worldwide vegetable seed market was valued at USD 9.163 billion and estimated to increase annually by 9.4% from 2019 to 2024 [4]. Commercially, potato, tomato, cabbage, lettuce, and sweet pepper are important vegetable crops in the global seed market, sharing more than 30% of the total vegetable crop production. However, a wide range of vegetables needs to be consumed to meet dietary requirements.
Potato (Solanum tuberosum L.) is a staple, nutrient-intensive, short-duration crop grown in 79% of countries [5]. Tomato (Solanum lycopersicum) is widely cultivated worldwide due to its versatility, high dietary fiber and vitamin content, and health benefits. It is a major source of lycopene and antioxidants that can potentially reduce the risk of cancer, osteoporosis, and cardiovascular disease [6]. Cabbage (Brassica oleraceae) also provides a range of nutritive and health benefits, including anticarcinogenic, antioxidant, and anti-inflamantory properties [7]. A wide variety of lettuce crops are cultivated across the world, and they are renowned for their high content of phenolic compounds that are beneficial to human health [8]. Pepper (Capsicum annuum L.) is widely cultivated in East Asia, including India [9]. It is rich in ascorbic acid, vitamins, and protein and exhibits medicinal properties. Its high ascorbic acid content and its pungent nature make it a popular herbal remedy.
In addition to the major crops, cucumbers, which belong to the Cucurbitaceae family, are important vegetables due to their economic and nutritional value. Immature cucumbers are used for pickles, and the mature fruit are used for salads. The fruit is soft, succulent, and rich in water, vitamins, and potassium (K). In addition to dietary fiber, cucumber contains copper, pantothenic acid, manganese, magnesium, and phosphorus (P) [10]. Cucumber is used in antipyretic and astringent recipes since the fruits and seeds have cooling properties [11]. Broccoli (Brassica oleracea) belongs to the Brassicaceae family and is eaten as a vegetable in many countries. It exhibits many health benefits and contains good-quality phytochemicals [12]. Broccoli inflorescences contain hydroxyl cinnamic acids, flavonoids, glucosinolates, and other beneficial compounds with antimicrobial, cardioprotective, anticancer, antioxidant, hepatoprotective, gastroprotective, and anti-inflammatory properties [13]. Several health benefits are associated with broccoli due to its high vitamin (A, B1, B2, B5, B6, C, and E) and mineral (Mg, Ca, Fe, and Zn) contents and the presence of several antioxidants [14]. Among vegetable crops grown in tropical and subtropical areas, okra (Abelmoschus esculentus L.) is a popular vegetable rich in vitamins, carbohydrates, minerals, and fats [15].
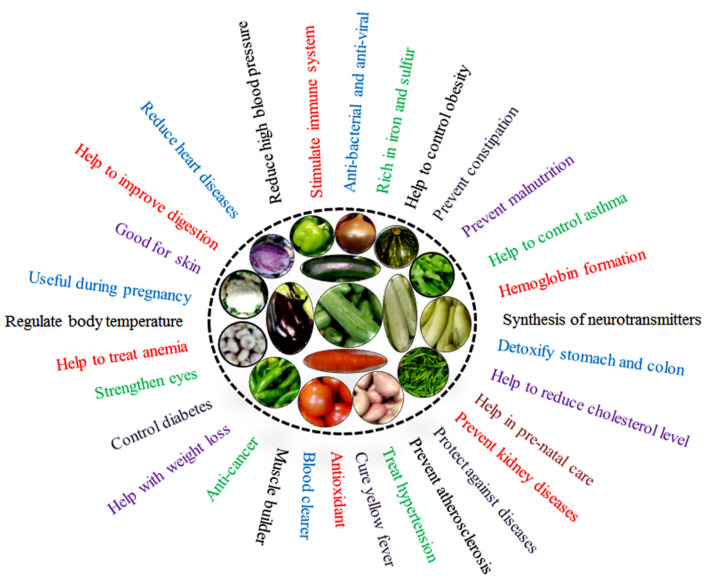
Vegetables are important for human nutrition and disease prevention as they boost the intake of calcium, dietary fiber, folate, iron, magnesium, K, and vitamin C [16]. Adequate consumption of vegetables, fruits, and whole grains reduces disease risk and all-cause mortality [17]. Green leafy vegetables have additional human health benefits [18], including a defensive effect against lung cancer [19]. Inadequate vegetable and fruit consumption can lead to chronic diseases, such as blood pressure issues, cardiovascular diseases, osteoporosis, hypercholesterolemia, various types of cancer, respiratory problems, chronic obstructive pulmonary diseases, and mental health issues [20,21,22,23,24] (Figure 1). Increased intake of cruciferous vegetables is associated with a reduced risk of bowel, thyroid, intestinal, lung, and pancreatic cancer [20]. Several varieties of Capsicum annuum, Lactuca sativa, Allium cepa, Brassica oleracea var. sabellica, and orange-fleshed Ipomoea batatas are the richest vegetable sources of phytochemicals with possible anti-obesity activity [25].
Figure 1.
Beneficial effects of vegetables on human health.
Vegetables in the Alliaceae family, including onion, garlic, leek, chive, and Welsh onion, are rich sources of thiosulfides, which are associated with a decline in several chronic diseases [26]. Tomato is the second most consumed vegetable globally after potato, with exclusive nutritional and phytochemical properties. Tomato contains key phytochemicals carotenoids: lycopene 60–64%, phytoene 10–12%, neurosporene 7–9%, and carotenes 10–15% [27]. Parsley (Petroselinum crispum) and celery (Apium graveolens) are popular vegetables and the best sources of flavonoid apigenin and vitamin E [28]. Carrot (Daucus carota) contains a unique combination of three flavonoids—quercetin, kaempferol, and luteolin [29,30,31]—that helps regulate cellular activity and reduce free radicals that cause oxidative stress.
Modern vegetable cultivation depends mainly on chemical fertilizers and pesticides. Chemical fertilizer application is one of the most endorsed systems in developing rigorous agriculture [32,33], leading to increased soil fertility and crop yields. However, the continuous use of chemical fertilizers can result in soil degradation, decreased soil organic matter content and soil quality, nutrient loss via runoff, leaching, and greenhouse gas emissions, leading to air and water pollution [34], pest resistance, and reduced food safety [35].
Organic farming can supply quality food without adversely affecting soil health or the environment. Organic fertilizer improves soil dynamics and increases the soil’s potential to retain water and nutrients in comparison to the effect of chemical fertilizers. Several studies have established that organic farming, which stringently restricts synthetic fertilizer use, is a potential substitute for minimizing the negative effect of chemical fertilizers, with the added benefit that organic farming products usually have enhanced nutritional and soil-quality properties [36,37,38,39]. However, organic farming is associated with lower crop production and higher end-product costs than conventional agriculture. Therefore, chemical fertilizers remain necessary until organic farming significantly increases food production [32,40]. Tomato (Solanum lycopersicum), a popular vegetable cultivated in more than 140 countries [41], contains several metabolites that are beneficial for health and nutrition [42]. Organically grown tomato had higher polyphenol, vitamin C, and carotenoid contents than those from conventional farming [36]. Ye et al. [43] reported that bio-organic farming, with decreased rates of chemical fertilization and enhanced soil fertility, produced higher tomato yields and quality than conventional farming. They suggested that Trichoderma spp. application as bio-organic fertilizer could be combined with chemical fertilizer application to achieve optimal yields and quality [43]. Thus, an alternative and more sustainable approach amends crops with rhizospheric microbial inoculants (bioinoculants) that promote plant growth and health.
Plant-growth-promoting rhizobacteria are free-living soil microorganisms that naturally colonize the rhizospheric zone of plant roots. These bacteria increase plant growth and control several diseases [44], and they belong to a broad taxonomic diversity, particularly Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria. Several bacteria, including Azospirillum brasilense, Azotobacter salinestris, Burkholderia phytofirmans, Bacillus megaterium, Bacillus subtilis, Paenibacillus favisporus, Paenibacillus polymyxa, Pseudomonas fluorescens, Pseudomonas stutzeri, and Rahnella aquatilis, are consistently part of the PGPR-diversified taxa [45]. These bacteria provide a plethora of plant benefits including increased root growth, nutrient uptake, and plant hormone stimulation, suppression of pathogenic activity, and restoration of soil health through the mineralization of organic pollutants [46,47]. They are not host specific, meaning that they have the advantage of being able to promote the growth of a broad range of hosts. Various rhizospheric bacteria such as Azospirillum, Azotobacter, Arthrobacter, Alcaligenes, Bacillus, Burkholderia, Enterobacter, Klebsiella, Pseudomonas, and Serratia have been linked with solanaceous vegetable crops [48]. Thus, PGPR are emerging as organic fertilizers suitable for many plant species, which could reduce chemical fertilizer application while enhancing soil quality and plant yield [49]. The PGPR species Pseudomonas putida and Bacillus amyloliquefaciens decreased the negative impact of three pesticides (carbendazim, imidacloprid, and glyphosate), maintained soil enzyme activities, and enhanced soil health and fertility [50].
Biofertilizer contains living microbes that colonize and promote plant growth by enhancing nutrient availability to the host plant [51]. The application of microbial biofertilizers to seeds or soils promotes the growth and yields of vegetable crops, such as bottlegourd [52], brinjal [53], broccoli [54], cabbage [55], carrot [56], chili [57], cucumber [58], lettuce [59], potato [60], onion [61], pumpkin [62], radish [63], and tomato [64]. The application of Bacillus strains improved growth under greenhouse/field conditions of several vegetable crops, such as broccoli, cucumber, lettuce, pepper, and tomato [65,66,67]. The positive role of PGPR on vegetable growth and production is well established [65], involving diverse mechanisms that differ according to the species of bacteria [68], such as the modulation of volatile compound production and hormone content, improvement of nutrient accessibility, and the increase of abiotic stress tolerance [69].
This review summarizes the most updated findings on the role of PGPR as biofertilizers for vegetable crop growth and production. We also discuss the impact of PGPR on vegetables under biotic and abiotic stresses and provide a mechanistic overview for ameliorating several stresses.
2. Effect of PGPR in Plant Growth Promotion
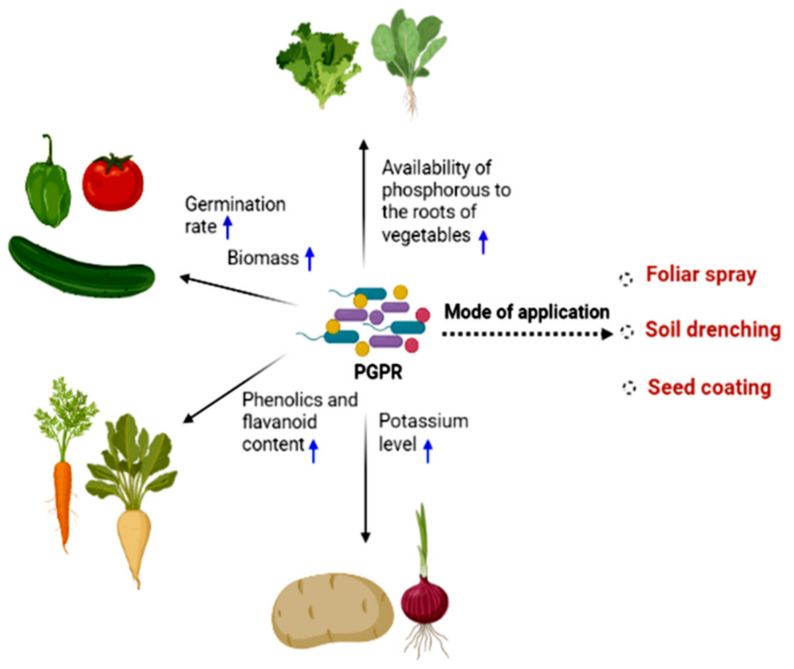
PGPR play an important role in enhancing soil quality, bioremediation, and stress control to develop eco-friendly sustainable agriculture [67]. PGPR can be used as biofertilizers and biopesticides, improving plant growth through direct mechanisms, such as nitrogen (N) fixation, phytohormone production, and phosphate solubilization (Figure 2). Figure 2 shows the application modes of PGPR bioformulations to plants. Seed coating and soil drenching are the most conventional methods of bioinoculation adopted to promote vegetable growth, whereas foliar sprays are feasible for disease protection. Phosphate-solubilizing bacteria (PSB) are PGPR that hydrolyze organic and inorganic insoluble P compounds into soluble P forms that plants readily use. Bioinoculation with PGPR can increase the germination rate and biomass content and provide essential nutrients (e.g., N, P, K) to plant roots. They also help produce hormones, such as auxin and gibberellins, siderophores, ammonia, and 1-aminocyclopropane-1-carboxylate (ACC) deaminase. Initially, it was assumed that hydrogen cyanide (HCN) production played an important role in plant growth promotion by reducing plant pathogens [70]. Later, the hypothesis changed, and it is believed that HCN production indirectly increases phosphorus accessibility by metal chelation and sequestration and indirectly induces nutrient accessibility to the rhizobacteria and host plants [71]. HCN production by PGPR is independent on genus; thus, they can be used as biofertilizers or biocontrol to increase crop production and yields [72]. The enzyme 1-aminocyclopropane1-carboxylate (ACC) deaminase cleaves the plant ethylene precursor, ACC, into ammonia and ketobutyrate [73]. Decreased ACC levels in plants by ACC deaminase-producing organisms decreased plant ethylene levels [74]; ethylene in high concentrations can lead to plant growth inhibition or even death. PGPR can also increase enzymatic activity and enhance mineral and water uptake [63]. PGPR can protect plants from biotic and abiotic stresses by using indirect mechanisms such as suppressing the growth of plant pathogens and inducing systemic resistance [75,76].
Figure 2.
Application of PGPR on vegetables and their anticipated strategies for plant growth promotion. Figure created with BioRender.com (accessed on 2 October 2021).
3. Role of PGPR in Vegetable Crop Production
Various PGPR can be used as biofertilizers in vegetable crop production. Table 1 provides a list of common PGPR used as biofertilizers on vegetable crops and their application method (seed coating, soil treatment, soil drenching, or foliar spray). Phosphorus is a major nutrient for vegetable growth; in particular, potato (Solanum tuberosum) requires high soil P for high biomass production. Limited P supply in soils reduces potato production by about 40% worldwide [77]. Potato needs higher N and P compared to other vegetables due to its tuber formation. Phosphate-solubilizing bacteria enhanced potato tuber growth and biomass production [78]. The synergy between three PSB isolates, Pantoea agglomerans strain P5, Microbacterium laevaniformans strain P7, and Pseudomonas putida, significantly impacted P solubilization and potato production [79]. Moreover, K-solubilizing bacteria can also enhance potato productivity by increasing K availability in the rhizosphere [80].
Table 1.
Plant-growth-promoting rhizobacteria (PGPR) used as biofertilizers in vegetable production.
| PGPR | Vegetable Crop | Mode of Treatment | Effect on Crops | References |
|---|---|---|---|---|
| Alcaligenes faecalis and Bacillus amyloliquefaciens | Spinacia oleracea | Soil treatment | Mitigated lead toxicity | [95] |
| B. pumilus SE34 | Solanum lycopersicum | Seed treatment | Induced systemic response during infection | [96] |
| Jeotgalicoccus huakuii NBRI 13E | S. lycopersicum, Abelmoschus esculentus, Zea mays | Seed treatment and foliar spray | Increased yield and ameliorated salt stress | [97] |
| B. pumilus strain SE34 or B. amyloliquefaciens strain IN937a or B. subtilus strain IN937 | S. lycopersicum | Seed treatment and soil drenching | Induced resistance against CMV virus | [98] |
| Rhizobium spp. | S. lycopersicum, Capsicum annuum, Daucus carota, Lactuca sativa | Seed treatment | Increased biomass | [99,100] |
| Bacillus megaterium var. phosphaticum | S. oleracea | Soil and seed treatment | Ensured efficient absorption of P, water, and other microelements to alleviate water stress and resist fungal diseases | [101,102] |
| Bacillus amyloliquefaciens | L. esculentum | Spraying on leaves | Induced systemic resistance against tomato leaf curl virus disease | [103] |
| Bacillus cereus | S. lycopersicum | Soil drenching | Biotic stress resistance against bacterial speck disease caused by Pseudomonas syringae pv | [104] |
| Paenibacillus alvei and Bacillus velezensis | Sorghum bicolor | Seed treatment | Resistance to water stress and crown rot disease caused by Fusarium pseudograminearum | [105] |
| Pseudomonas fluorescens | Arachis hypogea | Seed treatment | Produced 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase to confer resilience against salinity stress | [106] |
| PGPR Bacillus subtilis (RS2) and Bacillus spp. (RS7) | C. annuum | Seedling treatment | Increased productivity | [107] |
| Bacillus tequilensis | S. lycopersicum | Seedling and soil drenching | Produced ACC deaminase to confer resilience against salinity stress | [108] |
| Stenotrophomonas maltophilia, Achromobacter xylosoxidans, Achromobacter spp. | S. tuberosum | Potato tuber coating | Increased P solubilization, indole acetic acid, hydrogencyanide, and ammonia | [109] |
| Pseudomonas spp. PS1 | Vigna radiate | Seed treatment | Increased plant biomass, yield, and protein content | [110] |
| B. amyloliquefaciens | S. lycopersicum | Seed treatment | Resistance from bacterial wilt of tomato (Ralstonia solanacearum) | [111] |
| Bacillus cereus BC1AW and Pseudomonas putida PP3WT | S. lycopersicum | Seedling treatment | Ameliorated bacterial wilt disease | [112] |
| Pseudomonas fluorescens | Solanum tuberosum | Soil treatment | Protection from Ralstonia solanacearum pathogen. Reduced bacterial wilt incidence and improved growth | [113] |
| Trichoderma viride ES1 and Pseudomonas fluorescens Bak150 | S. tuberosum | Foliar spray | Suppressed early blight disease and increased yield | [114] |
| Trichoderma spp. | Brassica oleracea | - | [115] | |
| Trichoderma spp. | S. lycopersicum | Seed priming and soil treatment | Protection from F. oxysporum f. sp. lycopersici | [116] |
| T. harzianum+Pseudomonas spp. | S. lycopersicum | - | Protection from Sclerotium rolfsii | [117] |
| T. viride+T. harzianum+P. fluorescens+Azotobacter spp. + Azospirillum spp. + PSB | S. lycopersicum | Seed treatment and soil drenching | Disease management and protection from Pythium aphanidermatum, Ralstonia solanacearum, Fusarium oxysporum f. sp. lycopersici | [118] |
| Bacillus subtilis, Trichoderma spp. | S. lycopersicum, S. melongena | Seed treatment | Protection from Fusarium infection through secretion of extracellular cell-wall-degrading enzymes | [119,120] |
| Pseudomonas fluorescens | A. sesculentus | Seed and soil treatment | Protection from Rhizoctonia solani by the producing siderophores, HCN, and indole acetic acid | [121] |
| Lactic acid bacteria | C. annuum | Soil drenching and foliar spray | Protection from black rot by producing siderophores | [122] |
| Azospirillum brasilense, Pseudomonas fluorescens and Bacillus megaterium | Cucumis sativus | Seedling treatment and foliar spray | Improved fruit quality | [123] |
| Pseudomonas fluorescens, Pseudomonas spp., Bacillus subtilis | C. sativus | Seed treatment | Protection from damping off by producing antibiotics and metabolites and inducing systemic resistance | [124] |
| Chaetomium globosum, Burkholderia cepacia | S. tuberosum, C. annuum | Soil drenching and foliar spray | Protection from late blight disease by producing endo- and exo-glucanases; antimicrobial activity of organic acids | [125,126] |
| Trichoderma harzianum + Pseudomonas fluorescens | S. tuberosum | Seed treatment and foliar spray | Protection from early blight caused by Alternaria solani but active biomolecules not yet determined | [127] |
| Bacillus subtilis | C. sativus | Soilless potting mix drenching | Disease suppression against anthracnose disease | [128] |
| Stenotrophomonas maltophilia and Agrobacterium fabrum | Momordica charantia | Seed coating | Immobilized Cd in Cd-rich soil to improve growth | [95] |
| Bacillus velezensis isolates (Y6 and F7) | S. lycopersicum | Soil and seed treatment | Protection from fungal infections by producing antibiotic compounds | [129] |
CMV, Cucumber mosaic cucumovirus; P, Phosphorous; HCN, Hydrogen cyanide; Cd, Cadmium.
Cauliflower is an important crop due to its high dietary fiber and nutritional value and belongs to the Brassicaceae family. Cauliflower also benefits from bioinoculation with PSB and other PGPR. Kushwaha et al. [81] reported that the application of PGPR isolates enhanced cauliflower germination and growth by increasing indole acetic acid (IAA) production and P solubilization. Broccoli, known as ‘the crown jewel of nutrition’ due to its high nutritional value, is in high demand worldwide. Broccoli production in India increased after farmers became aware of its high nutritional value and improved cultivation methods. While organic farming could increase broccoli yields by improving nutrient availability to roots [54], Altuntas [82] found that the application of PGPR biofertilizers increased the yield up to 50% and 20% compared to the control and chemical fertilizers, respectively. Broccoli production relies on P absorption from the soil. Pseudomonas fluorescens, a solubilizing bacteria, increased broccoli growth when applied with a significant amount of fertilizer [83].
PGPR applied to vegetable crops can act as a biocontrol agent by protecting the plant from pathogens and pests. They achieve this directly by suppressing a broad spectrum of viral, bacterial, fungal, and nematode diseases and indirectly by altering the rhizosphere to favor beneficial microorganisms. Soilborne fungal pathogens that affect vegetable crops, such as Fusarium infection in tomato causing wilt disease, are a serious concern worldwide. Nabi et al. [84] evaluated the efficacy of the PGPR Bacillus aryabhattai to control Fusarium wilt disease in tomatoes and found higher amounts of amino acid and phytohormones in PGPR-treated plants. In addition to Fusarium, approximately 80% of tomato crop losses involve Alternaria solani, a causative agent of early blight disease [85]. The synergistic effect of green waste and wood biochar mixed with PGPR (Bacillus subtilis) inhibited the mycelial growth of A. solani by up to 55% in tomato [86]. Tariq et al. [87] evaluated the effect of PGPR on bell pepper (Capsicum annuum) yield by applying a consortium of Klebsiella, Burkholderia, Panibacillus, and Bacillus spp. in the field for up to 30 days. The results revealed steady yield increases per acre with increasing PGPR formulations. Significant phenotypic and genotypic correlations also occurred between yield per acre and yield in each treatment.
Bioinoculation of PGPR on vegetable crops can support plant growth by alleviating the impact of soil constraints (salinity, acidity, drought). Eggplant (Solanum melongena), a member of the Solanaceae family, is cultivated in tropical, subtropical, and Mediterranean countries. Increased Na+ uptake in saline soils hampers eggplant growth and yield [88]. However, eggplant seeds treated with PGPR such as Xanthobacter autotrophicus BM13, Enterobacter aerogenes BM10, and Bacillus brevis FK2 decreased Na+ uptake and increased K+ uptake, which enhanced plant growth [88]. Lettuce (Lactuca sativa L.) is sensitive to abiotic stress [89]; its shallow root system makes it sensitive to water deficit, which increases with plant growth [90]. Julia et al. [91] applied a biofertilizer of Macrocystis pyrifera algal extracts and the PGPR Azospirillum brasilense, which increased germination rate and lettuce growth in saline conditions. In another study, PGPR-inoculated lettuce had a higher phenolic and flavonoid content than uninoculated plants under greenhouse conditions [92]. Bacillus and Pseudomonas spp. increase salt tolerance in lettuce [67,89]. Okra (Abelmoschus esculentus L. Moench), a vitamin- and mineral-rich vegetable widely used by humans, is a secret weapon for diabetic people [93]. Pseudomonas spp. colonizes the rhizospheric region of okra roots and enhances plant growth [94].
4. Mechanistic Overview of PGPR-Mediated Plant Growth Promotion of Vegetable Crops under Stress Conditions
Plant–microbe PGPR interactions can be divided into two categories—symbiotic bacteria and free-living rhizobacteria, which can be further divided based on indirect or direct actions. Direct mechanisms involve biofertilization, root growth stimulation, rhizoremediation, and biotic and abiotic stress control and indirect mechanisms include disease suppression and induction of systemic resistance [130]. PGPR can be differentiated into two categories depending on their colonization: extracellular PGPR (ePGPR, which inhabit the root surface area) and intracellular PGPR (iPGPR, which colonize the intracellular space of the root cortex) [49]. Several symbiotic bacteria reside in the intercellular spaces of plant cells. Certain bacteria form mutualistic interactions with their host and enter plant cells [131]. Others have physiological interactions with plants and help in structural modifications. For example, rhizobia are well known for their mutualistic behavior, establishing symbiotic interactions with leguminous crops, forming specific root structures (nodules) to fix atmospheric N [132].
Global climate change and land degradation are increasing plant stress due to abiotic factors such as drought, salinity, cold, and heat and biotic stressors such as pathogens and herbivores [133]. PGPR can ameliorate plants from stress conditions [108,134] that affect plant growth through hormonal and nutritional imbalances and physiological and metabolic changes [135]. In addition, PGPR can initiate hydrolytic enzyme production, exopolysccharide production, heavy metal bioremediation, and induced systemic resistance (ISR) stimulation [136]. They also stimulate ISR by accelerating the physical and biochemical responses of plant cells to environmental stresses. PGPR associations with host plants enhance the biosynthesis of defense-related molecules by increasing the level of defense-responsive proteins, which provide survival support under stress conditions. Changes in biochemical and physiological parameters can account for PGPR’s ability to induce stress tolerance through osmolyte production [137], antioxidant production [138], ACC deaminase activity [76], phytohormonal content [139], and biofilm formation [140].
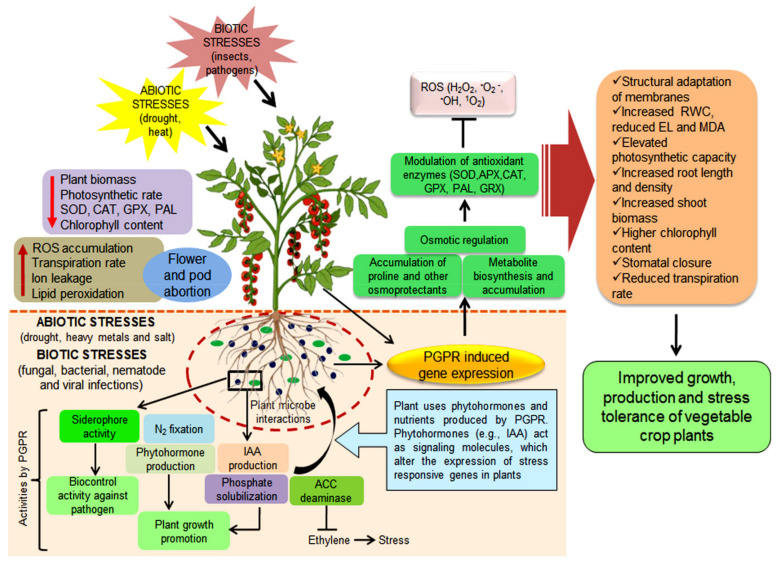
PGPR help plants to resist several abiotic stresses, including drought, salt, cold, and heavy metal toxicity (Figure 3), by colonizing the rhizosphere/endorhizosphere region and producing phytohormones, exopolysaccharides, volatile compounds, and ACC deaminase, which trigger osmolyte and antioxidant production and stress-responsive gene regulation. Salinity affects germination, plant phase transition, plant vigor, and production. Salinity-resistant PGPR induce osmotolerance in plants by improving root and shoot growth, nutrient uptake, chlorophyll content, vigor, and yield. PGPR secrete acids, phytoantibiotics, proteins, and other chemical compounds that help ameliorate toxic heavy metal stress and induce resistance in plants [135].
Figure 3.
Schematic representation of plant-growth-promoting rhizobacteria (PGPR)-mediated growth promotion and stress tolerance in vegetable crops. The model shows stress-induced reductions in plant biomass; photosynthetic rate; SOD, CAT, GPX, and PAL activities; and chlorophyll content and increases in reactive oxygen species (ROS), flower and pod abortion, transpiration rate, ion leakage, and lipid peroxidation. Plants inoculated with PGPR experience growth-promoting attributes, such as phytohormone (IAA) production and nitrogen fixation, prevent pathogen infections through biocontrol activity, and improve stress tolerance through ACC deaminase activity. PGPR also induce stress-responsive gene expression, leading to the accumulation of several osmoprotectants and defensive compounds and detoxification of ROS in cells. Modulation of antioxidants prevents cell damage and maintains homeostasis. Cellular responses, such as increased relative water content and photosynthetic capacity and reduced ion leakage and transpiration rates, and morphological changes, such as increased root and shoot biomass and reduced flower and pod abortion, occur, which improves growth, yield, and stress tolerance in vegetable crops. IAA, indole-3-acetic acid; SOD, superoxide dismutase; CAT, catalase; GPX, guaiacol peroxidase; PAL, phenylalanine ammonia-lyase. Figure created with BioRender.com (https://app.biorender.com/biorender-templates (accessed on 10 October 2021).
4.1. Role of PGPR against Biotic Stresses in Vegetable Crops
4.1.1. Role of PGPR in Fungal- and Bacterial-Induced Stress in Vegetable Crops
Pathogenic disease control can be triggered by the secretion of extracellular enzymes and other molecules that hydrolyze the microbial cell wall, compete for nutrients in the rhizosphere, and generate ISR against pathogenic infection in plants (Figure 1). For example, Bacillus xiamenensis strain PM14 has broad antifungal activity against Colletotrichum falcatum, Fusarium moniliforme, Fusarium oxysporum, Pythium splendens, Rhizoctonia solani, and Macrophomina phaseolina. PGPR produce diffusible and volatile antimicrobial compounds that exert fungicidal effects on phytopathogenic fungi by inhibiting growth or inducing the lysis of fungal mycelia [141]. In plants, PGPR can produce antibiotics (e.g., iturin, surfactins, fengycin, 2,4-diacetylphloroglucinol (DAPG), phenazine), cell-wall-degrading enzymes (protease, chitinase, and cellulase), plant-growth-promoting enzymes, hormones (indole-3-acetic acid), N-acyl-homoserine lactones, and siderophores to suppress pathogen growth [142] (Table 2).
Table 2.
Plant-growth-promoting rhizobacteria (PGPR) mediated biotic and abiotic stress tolerance in vegetable crops.
| Stress | Crops | PGPR Isolates | PGP Activity | References |
|---|---|---|---|---|
| Abiotic stress | ||||
| Salinity | Abelmoschus esculentus | Enterobacter spp. | Increased ACC deaminase activity | [148] |
| Salinity | Lycopersicum esculentum | Streptomyces spp. strain PGPA39 | Increased ACC deaminase activity, phosphate solubilization, and IAA production | [149] |
| Drought | L. esculentum | Bacillus subtilis | Cytokinin signaling | [150] |
| Drought | Capsicum annuum | Bacillus licheniformis K11 | Reduced ethylene concentration | [151] |
| Salinity and drought | Cucumis sativus | Burkholderia cepacia, Promicromonospora spp. | Increased salicylic acid and gibberellic acid | [152] |
| Salinity | Solanum melongena | Pseudomonas spp. | Produced antioxidant enzymes | [153] |
| Salinity | Pisum sativum | Bacillus spp. | Increased IAA production, phosphate solubilization, ammonia production, ACC deaminase activity, siderophore production, and antioxidant enzyme production | [154] |
| Salinity | Mentha spp. | Halomonas desiderata STR8 and Exiguobacterium oxidotolerans STR36 | Reduced harmful effects of salinity | [155,156] |
| Salinity | M. polymorpha, Medicago lupulina, Medicago truncatula, Medicago sativa | Bacillus megaterium NMp082 | Induced tolerance to salt stress | [157] |
| Heat | Solanum lycopersicum | Bacillus cereus | Extended thermotolerance in tomato seedlings | [158] |
| Biotic stress | ||||
| Damping off | L. esculentum | Streptomyces isolate DBTB 13, Trichoderma viride, T. harzianum, and P. fluorescens + Azotobacter and Azospirillum | Reduced stunting and stem collapse in infected plants | [118,159,160] |
| Bottom rot | Lactuca sativa | Bacillus amyloliquefaciens strain FZB42 | Improved the quality of lettuce by preventing wilting and rotting | [161] |
| Powdery mildew | C. sativus | Ampelomyces quisqualis Ces., B. subtilis strain GB03 | Prevented crop from tiny white superficial spots, reduced severity of angular leaf spot disease (foliar disease) | [162] |
| White rust disease, Fusarium wilts | Spinacia oleracea | B. subtilis, Pseudomonas spp., Bacillus spp., Burkholderia spp., Penicillium oxalicum, Enterobacter cloacae, Trichoderma spp. | Controlled Fusarium wilt and white rust | [78,163] |
| Colletotrichum lindemuthianum | Phaseolus vulgaris | P. fluorescens | Disease management against biotic stress | [164] |
| Damping-off | Beta vulgaris | Pseudomonas fluorescens | Disease management by producing antifungal compounds | [165] |
| Plasmodiophora brassicae | Brassicae oleraceae | Trichoderma spp. | Prevented and managed club root disease in cabbage | [115] |
| Pythium aphanidermatum, Ralstonia solanacearum, Fusarium oxysporum f. sp. lycopersici | L. esculentum | T. viride+T. harzianum+P. fluorescens+Azotobacter+Azospirillum + PSB | Disease management from several biotic stress | [118] |
| Powdery mildew, Botrytis rot | Greenhouse crops | Ampelomyces quisqualis, Pseudomonas flocculosa, Ulocladium spp. | Disease control against Botrytis rot and powdery mildew | [166] |
| Fusarium wilt, bacterial wilt | S. melongena and L. esculentum | Trichoderma spp., Bacillus subtilis, Bacillus amyloliquefaciens, Pseudomonas fluorescens | Produced antibiotics and secondary metabolites to control bacterial wilt and fusarium diseases through the secretion of enzymes that degrade extracellular wall components | [119,120,167] |
| Root rot disease | Abelmoschus esculentus | Pseudomonas fluorescens | Disease management by producing siderophores, HCN, and indole acetic acid | [121] |
| Damping off, downy mildew | Cucumis sativus | Pseudomonas spp., Bacillus subtilis, consortium of Achromobacter spp., Streptomyces spp., Bacillus licheniformis | Disease management by producing numerous antibiotics, metabolites, and induced systemic resistance | [124] |
| Bacterial spot and blight disease | C. annuum | Lactic acid bacteria, P. fluorescens | Protection by producing siderophores, numerous chemicals, and microbial fungicides | [122,168] |
| Late blight | S. tuberosum | Burkholderia cepacia; Chaetomium globosum | Protection by generating antimicrobial activity through organic acids and enzymes, such as exo- and endo-glucanases | [125,126] |
| Pythium aphanidermatum | L. esculentum Mill. | Streptomyces isolate H2 | Prevented damping off, thus acting as a biocontrol agent | [160] |
| Squash mosaic virus | C. sativus | P.fluorescens, B. polymyxa | Protection from pathogenic viruses | [169] |
| Watermelon mosaic potyvirus | C. maxima | B. subtilis, B. pumilus | Biocontrol mechanism for pathogenic viruses | [170] |
| Bacterial wilt, Fusarium wilt, leaf spot, anthracnose, Alternaria leaf blight, downy and powdery mildew | Citrullus lanatus (Thunb.) | P. polymyxa (SN-22), Sinomonas atrocyanea (NSB27) | Reduced angular leaf spot lesions and gummy stem blight lesions and inhibited bacterial fruit blotch | [156] |
| Fusarium wilt | Raphanus sativus | Pseudomonas putida strains WCS358 and RE8 | Provided biocontrol mechanism against biotic agent | [156] |
ACC, 1-aminocyclopropane-1-carboxylate; IAA, Indole acetic acid; HCN, Hydrogen cyanide.
Plant-growth-promoting rhizobacteria can be used as biocontrol agents against phytopathogens. They establish disease resistance in plants by suppressing the pathogens directly or stimulating host plant defenses and competing for nutrients with plant pathogens. Biotic and abiotic stresses confer several physiological changes in plant cells, indicated by the generation of reactive oxygen species (ROS). The accumulation of high ROS levels in plant cells is evident as oxidative damage, disrupting cellular homeostasis. Plant cells are furnished with sophisticated antioxidative mechanisms involving antioxidative defense enzymes, such as ascorbate peroxidase (APX), catalase (CAT), peroxidase (PO), superoxide dismutase (SOD), glutathione reductase, glutathione S-transferase, and guaiacol peroxidase (GPX). These defense enzymes are involved in scavenging and transforming ROS into nontoxic end-products and protecting cells from oxidative damage. In addition, plant cells induce several antioxidant molecules, such as carotenoids and phenylpropanoids, to conquer oxidative damage. Induced systemic resistance primes host plants to resist pathogen colonization through defense-related antioxidative enzymes and molecule production [136]. Other mechanisms, including the production of cell-wall-degrading enzymes, such as β-1-3-glucanase, chitinase, and β-xylosidase; volatile organic compounds; and diffusible antibiotics play key roles during biotic stresses [141].
4.1.2. PGPR against Nematode and Insect Pests
The increasing demand for agriproducts can be met by enhancing yield efficiency and minimizing losses due to plant parasites (nematodes). However, the current chemical-based strategy exerts inappropriate and adverse effects on flora and fauna. There is a need for a biocontrol agent for nematode management, such as PGPR, that can suppress nematodes directly by producing enzymes, toxins, and other metabolic products or indirectly by regulating nematode behavior and altering root diffusates. PGPR induce the production of repellents by the host plant that adversely affect host recognition and alter nematode feeding site development or sex ratios inside root tissue [131]. PGPR also enhance antioxidant activities and improve nutrient uptake by modulating plant hormone levels, increasing root proliferation. Pseudomonas aeruginosa enhances proline accumulation and modulates superoxide dismutase activity in tomato infected with Spodoptera litura, increasing root and shoot biomass [143].
4.2. Role of PGPR against Abiotic Stress in Vegetable Crops
In plants, physiological and chemical changes induced by PGPR that enhance environmental stress tolerance, including that to drought, salinity, cold, high temperature, and heavy metals, are recognized as induced systemic tolerance (IST) [144] (Table 2). These environmental stresses negatively impact endurance, biomass production, and staple food crop yields by up to 70%, affecting food security globally. Aridity stress due to drought, salinity, and high temperature is the leading abiotic stress restricting plant growth and productivity [130]. The application of PGPR against abiotic stresses has been widely studied [63,145,146,147].
4.2.1. PGPR-Mediated Drought Tolerance in Vegetable Crops
PGPR such as Achromobacter, Bacillus, Citrobacter, Mesorhizobium, Pseudomonas, and Variovorax could be used to enhance tolerance against drought stress in potato and tomato [171,172]. Tomato needs substantial irrigation water for successful growth, with drought stress significantly decreasing yields [173]. Drought affects potato growth and productivity by changing plant water relations, enhancing oxidative stress, decreasing photosynthetic capacity, inhibiting enzyme activities, and destroying membranes [174]. Drought affects the start of tuberization and decreases the rate of budding and weight of tubers [175]. Drought stress in plants is exacerbated in semi-arid areas in developing countries, leading to significant harvest losses [176]. Several PGPR, such as Pseudomonas putida, Bacillus amyloliquefaciens, Azospirillum brasilense, and Bacillus subtilis, play an important role in plants for drought tolerance [177,178,179]. For example, the application of Bacillus subtilis HAS31 reduced the impact of drought and maintained potato production (growth rate, dry matter production, leaf area, number of tubers, tuber weight, and yield) under severe water stress [180] by altering plant growth regulators and activities of superoxide dismutase (SOD), peroxidase (POD), and hydrogen peroxidase (CAT). Application of Bacillus cereus AR156 to tomato plants also maintained productivity. The mechanisms involved in drought tolerance were attributed to increased SOD, POD, and CAT synthesis and upregulation of cytosolic ascorbate peroxidase gene (cAPX) and monodehydroascorbate reductase gene (MDHAR) [181]. In another study, Bacillus licheniformis K11 reduced drought stress in pepper plants by increasing auxin and ACC deaminase production [151].
4.2.2. PGPR-Mediated Salinity Tolerance in Vegetable Crops
Most vegetable crops are affected by salinity stress [182], reducing crop growth and production through changes in morphological and physiological parameters [183]. Salinity stress affects vegetable crop growth due to osmotic or water-deficit stress, salt accumulation in shoots, nutrient imbalance, or a combination of these [184,185]. The ability of PGPR to decrease salinity stress has been evaluated for various vegetable crops [186]. PGPR enhanced salt stress tolerance in okra (Abelmoschus esculentus) through ROS-scavenging enzymes and improved water use efficiency [148]. Lettuce is one of the most consumed leafy vegetables and is a comparatively salt-sensitive crop [182,187]. Moncada et al. [67] studied the role of PGPR in enhancing the salinity stress tolerance of leaf lettuce developed in autumn and spring in a floating system by adding a PGPR-based biostimulant containing Bacillus spp. to mineral nutrient solutions (MNS) [67], which significantly alleviated salt stress and thus increased plant biomass and improved physiological and morphological parameters. In addition, Saravanakumar et al. [106] studied the effect of PGPR on groundnut in saline-affected soils. PGPR showed ACC-deaminase activity to combat salt stress by modulating antioxidant enzymatic activities. Application of PGPR confers tolerance against salinity stress in several other vegetable crops, including tomatoes, cucumbers [188], eggplant [189], tobacco, mustard, bell peppers, and radish [54].
4.2.3. PGPR-Mediated Tolerance to Heat, Metal Toxicity, and Other Stresses in Vegetable Crops
Elevated temperatures constrain vital plant functions and reduce yield in various agroclimatic zones. It is a major environmental concern globally. However, PGPR have been implicated in heat stress tolerance in several plants (see list in Table 2 and mechanistic overview in Figure 3). Bensalim et al. [190] reported that potato plants inoculated with Burkholderia phytofirmans strain PsJN had enhanced survival under high heat stress. Martin and Stutz [191] studied the role of arbuscular mycorrhizal fungi isolates that improved the growth and productivity of pepper (Capsicum annuum L.), increasing the amount of dry substance and P uptake at higher temperatures. Similarly, Mukhtar et al. [192] evaluated the efficacy of rhizobacteria Bacillus cereus for mitigating the heat stress effect in tomato and found that ACC-deaminase, exopolysaccharides, and the extracellular enzymatic attributes of PGPR modulated tomato growth traits under elevated temperature.
Heavy metals are a major environmental stress with several adverse effects on agricultural production and human health. Heavy metal accumulation in plants leads to their accumulation in the food chain and creates major health issues [193]. Plants require some metals for growth and development, but not all metals are useful. Extreme quantities of metals can act as toxicants that hamper plant growth and production [194]. The application of PGPR-based bioinoculants reduced the negative effect of metals such as copper (Cu), zinc (Zn), cadmium (Cd), nickel (Ni), and lead (Pb) in beans [195], potatoes [196], peas [197], tomato, canola, and Indian mustard [198]. Singh et al. [199] demonstrated the beneficial association of PGPR for alleviating the adverse effects of heavy metals in different crops and vegetables.
5. Conclusions and Future Perspectives
Chemical fertilizers can have detrimental effects on the soil, environment, and human health, while biofertilizers are naturally occurring products that do not negatively impact the soil ecosystem or human health. Therefore, PGPR-based biofertilizers are an indispensable and key component of sustainable agriculture to maintain long-term soil fertility and retain crop productivity. PGPR are an emerging biofertilizer alternative for chemical fertilizers to improve agricultural crop production, particularly vegetable production. PGPR promote the growth and production of vegetable crops through a variety of mechanisms, including the provision of phytohormones (e.g., IAA) and improved nutrient absorption (e.g., N, P, K). Considering the positive impact of PGPR as biofertilizer in terms of crop yield and productivity. In addition, PGPR protect plants from various abiotic and biotic stresses through osmotic adjustment, biocontrol activity, siderophore production, and ACC-deaminase production, among others. PGPR are useful soil bacteria that can stimulate biological, chemical, and physical modifications and alleviate the detrimental effects of abiotic and biotic stresses in vegetable crops. Frequent application of PGPR-mediated bioinoculants will enhance vegetable yields and production, particularly under stress conditions. Governments and private agencies should promote biofertilizer use as an environmentally friendly replacement for chemical fertilizers. In addition, farmers need to be educated on the beneficial effects of PGPR-based biofertilizers for sustainable agriculture.
Abbreviations
| AcdS | 1-Aminocyclopropane-1-carboxylate deaminase |
| CAT | Catalase |
| GPX | Guaiacol peroxidase |
| IAA | Indole-3-acetic acid |
| IST | Induced systemic tolerance |
| PAL | Phenylalanine ammonia-lyase |
| PGPR | Plant-growth-promoting rhizobacteria |
| P | Phosphorus |
| K | Potassium |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| WHO | World Health Organization |
Author Contributions
Conceptualization, M.K.; writing—original draft preparation, M.K., V.P.G., S.P. and A.G.; review and editing, M.K., V.P.G., S.P., A.G., M.K.P., A.B.B., S.J. and K.H.M.S.; supervision, K.H.M.S.; project administration, M.K. and K.H.M.S.; funding acquisition, K.H.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The publication charges were provided by the UWA Institute of Agriculture.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schreinemachers P., Simmons E.B., Wopereis M.C.S. Tapping the economic and nutritional power of vegetables. Glob. Food Secur. 2018;16:36–45. doi: 10.1016/j.gfs.2017.09.005. [DOI] [Google Scholar]
- 2.Padulosi S., Sthapit B., Lamers H., Kennedy G., Hunter D. Horticultural biodiversity to attain sustainable food and nutrition security; Proceedings of the International Symposia on Tropical and Temperate Horticulture; Cairns, QLD, Australia. 20 November 2016; Leuven, Belgium: ISHS Acta Horticulturae; 2018. pp. 21–34. [DOI] [Google Scholar]
- 3.Ebert A.W. The Role of Vegetable Genetic Resources in Nutrition Security and Vegetable Breeding. Plants. 2020;9:736. doi: 10.3390/plants9060736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Genetically Modified Seeds Market to Witness Growth Through 2020 Due to Rise in Adoption of Bio-Fuels: Reports Technavio. BusinessWire; London, UK: 2016. [Google Scholar]
- 5.FAO Crop Prospects and Food Situation . Global Information and Early Warning System on Food and Agriculture (GIEWS) Trade and Markets Division (EST) FAO; Rome, Italy: 2018. pp. 1–40. [Google Scholar]
- 6.Rasool M., Akhter A., Soja G., Haider M.S. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021;11:6092. doi: 10.1038/s41598-021-85633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drozdowska M., Leszczyńska T., Koronowicz A., Piasna-Słupecka E., Domagała D., Kusznierewicz B. Young shoots of red cabbage are a better source of selected nutrients and glucosinolates in comparison to the vegetable at full maturity. Eur. Food Res. Technol. 2020;246:2505–2515. doi: 10.1007/s00217-020-03593-x. [DOI] [Google Scholar]
- 8.Van Treuren R., Van Eekelen H.D.L.M., Wehrens R., De Vos R.C.H. Metabolite variation in the lettuce gene pool: Towards healthier crop varieties and food. Metabolomics. 2018;14:146. doi: 10.1007/s11306-018-1443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J.-A., Cho S.K., Kim J.E., Chung H.S., Hong J.-P., Hwang B., Hong C.B., Kim W.T. Isolation of cDNAs differentially expressed in response to drought stress and characterization of the Ca-LEAL1 gene encoding a new family of atypical LEA-like protein homologue in hot pepper (Capsicum annuum L. cv. Pukang) Plant Sci. 2003;165:471–481. doi: 10.1016/S0168-9452(03)00165-1. [DOI] [Google Scholar]
- 10.Vimala K., Mohan Y.M., Sivudu K.S., Varaprasad K., Ravindra S., Reddy N.N., Padma Y., Sreedhar B., MohanaRaju K. Fabrication of porous chitosan films impregnated with silver nanoparticles: A facile approach for superior antibacterial application. Colloids Surf. B Biointerfaces. 2010;76:248–258. doi: 10.1016/j.colsurfb.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Gill N.S., Sharma G., Arora R. Cucumis Trigonus Roxb: A Review. Int. J. Adv. Pharm. Sci. 2015;5:45–50. [Google Scholar]
- 12.Khan M.A.M., Ulrichs C., Mewis I. Effect of water stress and aphid herbivory on flavonoids in broccoli (Brassica oleracea var. italica Plenck) J. Appl. Bot. Food Qual. 2011;84:178–182. [Google Scholar]
- 13.Owis A.I. Broccoli; the green beauty: A review. J. Pharm. Sci. Res. 2015;7:696–703. [Google Scholar]
- 14.Talalay P., Fahey J.W. Phytochemicals from Cruciferous Plants Protect against Cancer by Modulating Carcinogen Metabolism. J. Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 15.Emuh F.N., Ofuoku A.E., Oyefia E. Effect of intercropping okra (Hibiscus esculentus) with pumpkin (Cucurbita maxima Duch Ex. Lam.) on some growth parameters and economic yield of maize (Zea mays) and maximization of land use in a Fadama soil. Res. J. Biol. Sci. 2006;1:50–54. [Google Scholar]
- 16.Storey M., Anderson P. Total fruit and vegetable consumption increases among consumers of frozen fruit and vegetables. Nutrition. 2018;46:115–121. doi: 10.1016/j.nut.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Schwingshackl L., Schwedhelm C., Hoffmann G., Lampousi A.-M., Knüppel S., Iqbal K., Bechthold A., Schlesinger S., Boeing H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017;105:1462–1473. doi: 10.3945/ajcn.117.153148. [DOI] [PubMed] [Google Scholar]
- 18.Xu C., Zeng X.-T., Liu T.-Z., Zhang C., Yang Z.-H., Li S., Chen X.-Y. Fruits and Vegetables Intake and Risk of Bladder Cancer: A PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine. 2015;94:e759. doi: 10.1097/MD.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dosil-Díaz O., Ruano-Ravina A., Gestal-Otero J.J., Barros-Dios J.M. Consumption of fruit and vegetables and risk of lung cancer: A case-control study in Galicia, Spain. Nutrition. 2008;24:407–413. doi: 10.1016/j.nut.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Williamson G. Protective effects of fruits and vegetables in the diet. Nutr. Food Sci. 1996;96:6–10. doi: 10.1108/00346659610105806. [DOI] [Google Scholar]
- 21.Adebawo O., Salau B., Ezima E., Oyefuga O., Ajani E., Idowu G., Famodu A., Osilesi O. Fruits and vegetables moderate lipid cardiovascular risk factor in hypertensive patients. Lipids Health Dis. 2006;5:14. doi: 10.1186/1476-511X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celik F., Topcu F. Nutritional risk factors for the development of chronic obstructive pulmonary disease (COPD) in male smokers. Clin. Nutr. 2006;25:955–961. doi: 10.1016/j.clnu.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Payne M.E., Steck S.E., George R.R., Steffens D.C. Fruit, Vegetable, and Antioxidant Intakes Are Lower in Older Adults with Depression. J. Acad. Nutr. Diet. 2012;112:2022–2027. doi: 10.1016/j.jand.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMartin S.E., Jacka F.N., Colman I. The association between fruit and vegetable consumption and mental health disorders: Evidence from five waves of a national survey of Canadians. Prev. Med. 2013;56:225–230. doi: 10.1016/j.ypmed.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Williams D.J., Edwards I., Hamernig I., Jian L., James A., Johnson S., Tapsell L.C. Vegetables containing phytochemicals with potential anti-obesity properties: A review. Food Res. Int. 2013;52:323–333. doi: 10.1016/j.foodres.2013.03.015. [DOI] [Google Scholar]
- 26.Kubec R., Svobodová M., Velíšek J. Distribution of S-Alk(en)ylcysteine Sulfoxides in Some Allium Species. Identification of a New Flavor Precursor: S-Ethylcysteine Sulfoxide (Ethiin) J. Agric. Food Chem. 2000;48:428–433. doi: 10.1021/jf990938f. [DOI] [PubMed] [Google Scholar]
- 27.Clinton S.K. Lycopene: Chemistry, Biology, and Implications for Human Health and Disease. Nutr. Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S.E., Young J.F., Daneshvar B., Lauridsen S.T., Knuthsen P., Sandström B., Dragsted L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br. J. Nutr. 1999;81:447–455. doi: 10.1017/S000711459900080X. [DOI] [PubMed] [Google Scholar]
- 29.Ching L.S., Mohamed S. Alpha-Tocopherol Content in 62 Edible Tropical Plants. J. Agric. Food Chem. 2001;49:3101–3105. doi: 10.1021/jf000891u. [DOI] [PubMed] [Google Scholar]
- 30.Lila M.A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004;5:306–313. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horbowicz M., Kosson R., Grzesiuk A., Dębski H. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human Nutrition. J. Fruit Ornam. Plant Res. 2008;68:5–22. doi: 10.2478/v10032-008-0001-8. [DOI] [Google Scholar]
- 32.Adesemoye A.O., Kloepper J.W. Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009;85:1–12. doi: 10.1007/s00253-009-2196-0. [DOI] [PubMed] [Google Scholar]
- 33.Da Costa P.B., Beneduzi A., De Souza R., Schoenfeld R., Vargas L., Passaglia L.M.P. The effects of different fertilization conditions on bacterial plant growth promoting traits: Guidelines for directed bacterial prospection and testing. Plant Soil. 2012;368:267–280. doi: 10.1007/s11104-012-1513-z. [DOI] [Google Scholar]
- 34.Pahalvi H.N., Rafiya L., Rashid S., Nisar B., Kamili A.N. Chemical Fertilizers and Their Impact on Soil Health. Microbiota Biofertil. 2021;2:1–20. doi: 10.1007/978-3-030-61010-4_1. [DOI] [Google Scholar]
- 35.Ye H., Cheng J., Yu K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2018;121:633–642. doi: 10.1016/j.ijbiomac.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 36.Caris-Veyrat C., Amiot M.-J., Tyssandier V., Grasselly D., Buret M., Mikolajczak M., Guilland J.-C., Bouteloup-Demange C., Borel P. Influence of Organic versus Conventional Agricultural Practice on the Antioxidant Microconstituent Content of Tomatoes and Derived Purees; Consequences on Antioxidant Plasma Status in Humans. J. Agric. Food Chem. 2004;52:6503–6509. doi: 10.1021/jf0346861. [DOI] [PubMed] [Google Scholar]
- 37.Luthria D., Singh A.P., Wilson T., Vorsa N., Banuelos G.S., Vinyard B.T. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: Plant-to-plant variation. Food Chem. 2010;121:406–411. doi: 10.1016/j.foodchem.2009.12.055. [DOI] [Google Scholar]
- 38.Vallverdu-Queralt A., Medina-Remón A., Casals-Ribes I., Lamuela-Raventos R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012;130:222–227. doi: 10.1016/j.foodchem.2011.07.017. [DOI] [Google Scholar]
- 39.Oliveira A.B., Moura C.F.H., Gomes-Filho E., Marco C.A., Urban L., Miranda M.R.A. The Impact of Organic Farming on Quality of Tomatoes Is Associated to Increased Oxidative Stress during Fruit Development. PLoS ONE. 2013;8:e56354. doi: 10.1371/journal.pone.0056354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruano-Rosa D., Mercado-Blanco J. Organic Amendments and Soil Suppressiveness in Plant Disease Management. Springer; Cham, Switzerland: 2015. Combining Biocontrol Agents and Organics Amendments to Manage Soil-Borne Phytopathogens; pp. 457–478. [DOI] [Google Scholar]
- 41.Beckles D. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012;63:129–140. doi: 10.1016/j.postharvbio.2011.05.016. [DOI] [Google Scholar]
- 42.Ezura H. Tomato is a Next-generation Model Plant for Research and Development. J. Jpn. Soc. Hortic. Sci. 2009;78:1–2. doi: 10.2503/jjshs1.78.1. [DOI] [Google Scholar]
- 43.Ye L., Zhao X., Bao E., Li J., Zou Z., Cao K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020;10:177. doi: 10.1038/s41598-019-56954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weller D.M. Biological Control of Soilborne Plant Pathogens in the Rhizosphere with Bacteria. Annu. Rev. Phytopathol. 1988;26:379–407. doi: 10.1146/annurev.py.26.090188.002115. [DOI] [Google Scholar]
- 45.Ferchichi N., Toukabri W., Boularess M., Smaoui A., Mhamdi R., Trabelsi D. Isolation, identification and plant growth promotion ability of endophytic bacteria associated with lupine root nodule grown in Tunisian soil. Arch. Microbiol. 2019;201:1333–1349. doi: 10.1007/s00203-019-01702-3. [DOI] [PubMed] [Google Scholar]
- 46.Artyszak A., Gozdowski D. The Effect of Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR) on the Soil Properties, Root Yield, and Technological Quality of Sugar Beet. Agronomy. 2020;10:1262. doi: 10.3390/agronomy10091262. [DOI] [Google Scholar]
- 47.Kour D., Rana K.L., Yadav N., Yadav A.N., Kumar A., Meena V.S., Singh B., Chauhan V.S., Dhaliwal H.S., Saxena A.K. Plant Growth Promoting Rhizobacteria for Agricultural Sustainability. Springer; Singapore: 2019. Rhizospheric Microbiomes: Biodiversity, Mechanisms of Plant Growth Promotion, and Biotechnological Applications for Sustainable Agriculture; pp. 19–65. [DOI] [Google Scholar]
- 48.Gupta S., Kaushal R., Gupta S. Plant Growth Promoting Rhizobacteria: Bioresouce for Enhanced Productivity of Solanaceous Vegetable Crops. Acta Sci. Agric. 2017;1:10–15. [Google Scholar]
- 49.Sudewi S., Ala A., Patandjengi B., BDR M.F., Rahim A. Scereening of Plant Growth Promotion Rhizobacteria (PGPR) to increase local aromatic rice plant growth. Int. J. Pharm. Res. 2020;13 doi: 10.31838/ijpr/2021.13.01.151. [DOI] [Google Scholar]
- 50.Kumar M., Yusuf M.A., Chauhan P.S., Nigam M. Pseudomonas putida and Bacillus amyloliquefaciens alleviates the adverse effect of pesticides and poise soil enzymes activities in chickpea (Cicer arietinum L.) rhizosphere. Trop. Plant Res. 2017;4:405–418. doi: 10.22271/tpr.2017.v4.i3.054. [DOI] [Google Scholar]
- 51.Vessey J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 52.Patle B.J., Wagh A.P., Umbarkar P.S., Bondre S. V Integrated nutrient management studies in bottle gourd. J. Pharmacogn. Phytochem. 2018;7:1383–1385. [Google Scholar]
- 53.Seymen M., Türkmen Ö., Dursun A., Paksoy M., Dönmez M.F. Effects of Bacteria Inoculation on Yield, Yield Components and Mineral Composition in Eggplant (Solanum melongena L.); Proceedings of the ICOEST Conference; Urgüp, Turkey. 18–21 June 2013; pp. 403–413. [Google Scholar]
- 54.Yildirim E., Karlidag H., Turan M., Dursun A., Goktepe F. Promoition of Broccoli by Plant Growth Promoting Rhizobacteria. Hort. Sci. 2011;46:932–936. [Google Scholar]
- 55.Gajbhiye V.T., Gupta S., Gupta R.K. Persistence of Imidacloprid in/on Cabbage and Cauliflower. Bull. Environ. Contam. Toxicol. 2004;72:283–288. doi: 10.1007/s00128-003-9103-7. [DOI] [PubMed] [Google Scholar]
- 56.Physiology C., Mog B., Ad D. Ph.D. Thesis. UAS Dharwad; Dharwad, India: 2007. Effect of Organics and Biofertilizers on Productivity Potential in Carrot (Daucus carota L.) [Google Scholar]
- 57.Silva L.R., Azevedo J., Pereira M.J., Valentão P., Andrade P.B. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem. Toxicol. 2012;53:240–248. doi: 10.1016/j.fct.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 58.Gül A., Özaktan H., Kıdoğlu F., Tüzel Y. Rhizobacteria promoted yield of cucumber plants grown in perlite under Fusarium wilt stress. Sci. Hortic. 2013;153:22–25. doi: 10.1016/j.scienta.2013.01.004. [DOI] [Google Scholar]
- 59.Chamangasht S., Ardakani M.R., Khavazi K. Improving Lettuce (Lactuca sativa L.) Growth and Yield by the Application of Biofertilizers. Ann. Biol. Res. 2012;3:1876–1879. [Google Scholar]
- 60.Kumar M., Baishya L.K., Ghosh D.C., Ghosh M., Gupta V.K., Verma M.R. Effects of organic manures, chemical fertilizers and biofertilizers on growth and productivity of rainfed potato in the eastern himalayas. J. Plant Nutr. 2013;36:1065–1082. doi: 10.1080/01904167.2013.770021. [DOI] [Google Scholar]
- 61.Lee J. Effect of application methods of organic fertilizer on growth, soil chemical properties and microbial densities in organic bulb onion production. Sci. Hortic. 2010;124:299–305. doi: 10.1016/j.scienta.2010.01.004. [DOI] [Google Scholar]
- 62.Habibi A., Heidari G.R., Sohrabi Y., Mohamadi K. Effect of biofertilizers and chemical fertilizers on yield and yield components of pumpkin (Cucurbita pepo L. Convar. pepo Var. styriaca) Iran. J. Med. Aromat. Plants. 2013;28:604–615. [Google Scholar]
- 63.Kumar S., Kumar S., Maji S., Pandey V.K. Effect of inorganic fertilizers and bio-fertilizers on growth, yield and quality of radish (Raphanus sativus L.) Int. J. Plant Sci. 2016;11:71–74. doi: 10.15740/HAS/IJPS/11.1/71-74. [DOI] [Google Scholar]
- 64.Bernabeu P.R., Pistorio M., Tejerizo G.T., Santos P.E.D.L., Galar M.L., Boiardi J.L., Luna M.F. Colonization and plant growth-promotion of tomato by Burkholderia tropica. Sci. Hortic. 2015;191:113–120. doi: 10.1016/j.scienta.2015.05.014. [DOI] [Google Scholar]
- 65.Ruzzi M., Aroca R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015;196:124–134. doi: 10.1016/j.scienta.2015.08.042. [DOI] [Google Scholar]
- 66.Vetrano F., Miceli C., Angileri V., Frangipane B., Moncada A., Miceli A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy. 2020;10:1477. doi: 10.3390/agronomy10101477. [DOI] [Google Scholar]
- 67.Moncada A., Vetrano F., Esposito A., Miceli A. Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant. Agronomy. 2020;10:1504. doi: 10.3390/agronomy10101504. [DOI] [Google Scholar]
- 68.Dey R., Pal K.K., Bhatt D.M., Chauhan S.M. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 2004;159:371–394. doi: 10.1016/j.micres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Choudhary D.K., Sharma K.P., Gaur R.K. Biotechnological perspectives of microbes in agro-ecosystems. Biotechnol. Lett. 2011;33:1905–1910. doi: 10.1007/s10529-011-0662-0. [DOI] [PubMed] [Google Scholar]
- 70.Voisard C., Keel C., Haas D., Dèfago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989;8:351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rijavec T., Lapanje A. Hydrogen Cyanide in the Rhizosphere: Not Suppressing Plant Pathogens, but Rather Regulating Availability of Phosphate. Front. Microbiol. 2016;7:1785. doi: 10.3389/fmicb.2016.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agbodjato N.A., Noumavo P.A., Baba-Moussa F., Salami H.A., Sina H., Sèzan A., Bankolé H., Adjanohoun A., Baba-Moussa L. Characterization of Potential Plant Growth Promoting Rhizobacteria Isolated from Maize (Zea mays L.) in Central and Northern Benin (West Africa) Appl. Environ. Soil Sci. 2015;2015:1–9. doi: 10.1155/2015/901656. [DOI] [Google Scholar]
- 73.Honma M., Shimomura T. Metabolism of 1-Aminocyclopropane-1-carboxylic Acid. Agric. Biol. Chem. 1978;42:1825–1831. doi: 10.1080/00021369.1978.10863261. [DOI] [Google Scholar]
- 74.Glick B.R., Cheng Z., Czarny J., Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007;119:329–339. doi: 10.1007/s10658-007-9162-4. [DOI] [Google Scholar]
- 75.Singh R.P., Jha P.N. The PGPR Stenotrophomonas maltophilia SBP-9 Augments Resistance against Biotic and Abiotic Stress in Wheat Plants. Front. Microbiol. 2017;8:1945. doi: 10.3389/fmicb.2017.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J., Kloepper J.W., Ryu C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Igual J.M., Valverde A., Cervantes E., Velázquez E. Phosphate-solubilizing bacteria as inoculants for agriculture: Use of updated molecular techniques in their study. Agronomie. 2001;21:561–568. doi: 10.1051/agro:2001145. [DOI] [Google Scholar]
- 78.Shahid M., Zaidi A., Khan M.S., Rizvi A., Saif S., Ahmed B. Recent Advances in Management Strategies of Vegetable Diseases. Microbial Strategies for Vegetable Production. Springer; Cham, Switzerland: 2017. pp. 197–226. [DOI] [Google Scholar]
- 79.Malboobi M.A., Owlia P., Behbahani M., Sarokhani E., Moradi S., Yakhchali B., Deljou A., Heravi K.M. Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World J. Microbiol. Biotechnol. 2009;25:1471–1477. doi: 10.1007/s11274-009-0037-z. [DOI] [Google Scholar]
- 80.Ali A.M., Awad M.Y.M., Hegab S.A., El Gawad A.M.A., Eissa M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2020;44:411–420. doi: 10.1080/01904167.2020.1822399. [DOI] [Google Scholar]
- 81.Kushwaha A., Baily S.B., Maxton A., Ram G.D. Isolation and characterization of PGPR associated with cauliflower roots and its effect on plant growth. Bioscan. 2013;8:95–99. [Google Scholar]
- 82.Altuntaş A. Comparative study on the effects of different conventional, organic and bio-fertilizers on broccoli yield and quality. Appl. Ecol. Environ. Res. 2018;16:1595–1608. doi: 10.15666/aeer/1602_15951608. [DOI] [Google Scholar]
- 83.Tanwar A., Aggarwal A., Parkash V. Effect of bioinoculants and superphosphate fertilizer on the growth and yield of broccoli (Brassica oleracea L. var. italica Plenck) N. Zealand J. Crop. Hortic. Sci. 2014;42:288–302. doi: 10.1080/01140671.2014.924537. [DOI] [Google Scholar]
- 84.Nabi R.B.S., Shahzad R., Tayade R., Shahid M., Hussain A., Ali M.W., Yun B.-W. Evaluation potential of PGPR to protect tomato against Fusarium wilt and promote plant growth. PeerJ. 2021;9:e11194. doi: 10.7717/peerj.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adhikari P., Oh Y., Panthee D.R. Current Status of Early Blight Resistance in Tomato: An Update. Int. J. Mol. Sci. 2017;18:2019. doi: 10.3390/ijms18102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rasool M., Akhter A., Haider M.S. Molecular and biochemical insight into biochar and Bacillus subtilis induced defense in tomatoes against Alternaria solani. Sci. Hortic. 2021;285:110203. doi: 10.1016/j.scienta.2021.110203. [DOI] [Google Scholar]
- 87.Tariq M., Ali Q., Khan A., Khan G.A., Rashid B. “Advancements in Life Sciences” Yield potential study of Capsicum annuum L. under the application of PGPR. Adv. Life Sci. 2014;1:202–207. [Google Scholar]
- 88.El-Azeem S.A.M.A., Elwan M.W.M., Sung J.-K., Ok Y.S. Alleviation of Salt Stress in Eggplant (Solanum melongena L.) by Plant-Growth-Promoting Rhizobacteria. Commun. Soil Sci. Plant Anal. 2012;43:1303–1315. doi: 10.1080/00103624.2012.666305. [DOI] [Google Scholar]
- 89.Azarmi-Atajan F., Sayyari-Zohan M.H. Alleviation of salt stress in lettuce (Lactuca sativa L.) by plant growth-promoting rhizobacteria. J. Hortic. Postharvest. Res. 2020;3:67–78. doi: 10.22077/JHPR.2020.3013.1114. [DOI] [Google Scholar]
- 90.Ünlükara A., Cemek B., Karaman S., Ersahin S. Response of lettuce (Lactuca sativa var. crispa) to salinity of irrigation water. N. Zealand J. Crop. Hortic. Sci. 2008;36:265–273. doi: 10.1080/01140670809510243. [DOI] [Google Scholar]
- 91.Julia I., Oscar M., Analía L., Guilherme J.Z., Virginia L. Biofertilization with Macrocystis pyrifera algae extracts combined with PGPR-enhanced growth in Lactuca sativa seedlings. Environ. Boil. Fishes. 2020;32:4361–4371. doi: 10.1007/s10811-020-02202-4. [DOI] [Google Scholar]
- 92.Ayuso-Calles M., García-Estévez I., Jiménez-Gómez A., Flores-Félix J.D., Escribano-Bailón M.T., Rivas R. Rhizobium laguerreae Improves Productivity and Phenolic Compound Content of Lettuce (Lactuca sativa L.) under Saline Stress Conditions. Foods. 2020;9:1166. doi: 10.3390/foods9091166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramachandran S., Naveen K., Panneerselvam K., Sabitha V. Investigation of in vivo antioxidant property of Abelmoschus esculentus (L) moench. fruit seed and peel powders in streptozotocin-induced diabetic rats. J. Ayurveda Integr. Med. 2012;3:188–193. doi: 10.4103/0975-9476.104432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adesemoye A.O., Ugoji E.O. Evaluating Pseudomonas aeruginosaas plant growth-promoting rhizobacteria in West Africa. Arch. Phytopathol. Plant Prot. 2009;42:188–200. doi: 10.1080/03235400601014791. [DOI] [Google Scholar]
- 95.Zafar-Ul-Hye M., Tahzeeb-Ul-Hassan M., Abid M., Fahad S., Brtnicky M., Dokulilova T., Datta R., Danish S. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020;10:12159. doi: 10.1038/s41598-020-69183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan Z., Reddy M.S., Ryu C.-M., McInroy J.A., Wilson M., Kloepper J.W. Induced Systemic Protection Against Tomato Late Blight Elicited by Plant Growth-Promoting Rhizobacteria. Phytopathology. 2002;92:1329–1333. doi: 10.1094/PHYTO.2002.92.12.1329. [DOI] [PubMed] [Google Scholar]
- 97.Misra S., Dixit V.K., Mishra S.K., Chauhan P.S. Demonstrating the potential of abiotic stress-tolerant Jeotgalicoccus huakuii NBRI 13E for plant growth promotion and salt stress amelioration. Ann. Microbiol. 2019;69:419–434. doi: 10.1007/s13213-018-1428-x. [DOI] [Google Scholar]
- 98.Zehnder G.W., Yao C., Murphy J.F., Sikora E.D.R., Kloepper J.W. Induction of resistance in tomato against cucumber mosaic cucumovirus by plant growth-promoting rhizobacteria. Entomophaga. 2000;45:127–137. doi: 10.1023/a:1009923702103. [DOI] [Google Scholar]
- 99.García-Pinilla S., Villalobos-Espinosa J.C., Cornejo-Mazón M., Gutiérrez-López G.F. Advances in Processing Technologies Bio-Based Nanosystem in Food. CRC Press; Boca Raton, FL, USA: 2019. Nanotechnology in food processing; pp. 259–276. [DOI] [Google Scholar]
- 100.Felix J.D.F., Menendez E., Rivera L.P., Marcos-García M., Martínez-Hidalgo P., Mateos P., Martínez-Molina E., Velázquez M.D.L.E., García-Fraile P., Rivas R. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativaand Daucus carota crops. J. Plant Nutr. Soil Sci. 2013;176:876–882. doi: 10.1002/jpln.201300116. [DOI] [Google Scholar]
- 101.Khalid M., Hassani D., Bilal M., Asad F., Huang D. Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot. Stud. 2017;58:1–9. doi: 10.1186/s40529-017-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mishra B.K., Lal G., Sharma Y.K., Kant K., Saxena S.N., Dubey P.N. Effect of microbial inoculants on cumin (Cuminum cyminum Linn.) growth and yield. Int. J. Seed Spices. 2019;53:53–56. [Google Scholar]
- 103.Guo Q., Li Y., Lou Y., Shi M., Jiang Y., Zhou J., Sun Y., Xue Q., Lai H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere microecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019;137:154–166. doi: 10.1016/j.apsoil.2019.01.015. [DOI] [Google Scholar]
- 104.Niu D.-D., Wang C.-J., Guo Y.-H., Jiang C.-H., Zhang W.-Z., Wang Y.-P., Guo J.-H. The plant growth-promoting rhizobacteriumBacillus cereusAR156 induces resistance in tomato with induction and priming of defence response. Biocontrol Sci. Technol. 2012;22:991–1004. doi: 10.1080/09583157.2012.706595. [DOI] [Google Scholar]
- 105.Carlson R., Tugizimana F., Steenkamp P.A., Dubery I.A., Hassen A.I., Labuschagne N. Rhizobacteria-induced systemic resilience in Sorghum bicolor (L.) moench against Fusarium pseudograminearum crown rot under drought stress conditions. Biol. Control. 2020;151:104395. doi: 10.1016/j.biocontrol.2020.104395. [DOI] [Google Scholar]
- 106.Saravanakumar D., Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbiol. 2006;102:1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 107.Gupta S., Kaushal R., Spehia R.S., Pathania S.S., Sharma V. Productivity of capsicum influenced by conjoint application of isolated indigenous PGPR and chemical fertilizers. J. Plant Nutr. 2017;40:921–927. doi: 10.1080/01904167.2015.1093139. [DOI] [Google Scholar]
- 108.Bhattacharya A., Giri V.P., Singh S.P., Pandey S., Chauhan P., Soni S.K., Srivastava S., Singh P.C., Mishra A. Intervention of bio-protective endophyte Bacillus tequilensis enhance physiological strength of tomato during Fusarium wilt infection. Biol. Control. 2019;139:104074. doi: 10.1016/j.biocontrol.2019.104074. [DOI] [Google Scholar]
- 109.Dawwam G.E., Elbeltagy A., Emara H.M., Abbas I.H., Hassan M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci. 2013;58:195–201. doi: 10.1016/j.aoas.2013.07.007. [DOI] [Google Scholar]
- 110.Ahemad M., Khan M.S. Productivity of greengram in tebuconazole-stressed soil, by using a tolerant and plant growth-promoting Bradyrhizobium sp. MRM6 strain. Acta Physiol. Plant. 2011;34:245–254. doi: 10.1007/s11738-011-0823-8. [DOI] [Google Scholar]
- 111.Tan S., Jiang Y., Song S., Huang J., Ling N., Xu Y., Shen Q. Two Bacillus amyloliquefaciens strains isolated using the competitive tomato root enrichment method and their effects on suppressing Ralstonia solanacearum and promoting tomato plant growth. Crop. Prot. 2013;43:134–140. doi: 10.1016/j.cropro.2012.08.003. [DOI] [Google Scholar]
- 112.Kurabachew H., Wydra K. Characterization of plant growth promoting rhizobacteria and their potential as bioprotectant against tomato bacterial wilt caused by Ralstonia solanacearum. Biol. Control. 2013;67:75–83. doi: 10.1016/j.biocontrol.2013.07.004. [DOI] [Google Scholar]
- 113.Kuarabachew H., Assefa F., Hiskias Y. Evaluation of ethiopian isolates of Pseudomonas fluorescens as biocontrol agent against potato bacterial wilt caused by Ralstonia (Pseudomonas) solanacearum. Acta Agric. Slov. 2007;2:125–135. [Google Scholar]
- 114.Zegeye E.D., Santhanam A., Gorfu D., Kassa B. Biocontrol activity of Trichoderma viride and Pseudomonas fluorescens against Phytophthora infestans under greenhouse conditions. J. Agric. Technol. 2011;7:1589–1602. [Google Scholar]
- 115.Cuevas V.C., Kebasen S.B. Ecological approach in the control of club root disease of cabbage; Proceedings of the 7th Annual Scientific Meeting and Symposium, Mycological Society of the Philippines; Laguna, Philippines. 8 April 2005. [Google Scholar]
- 116.Bhagat S., Bambawale O.M., Tripathi A.K., Ahmad I., Srivastava R.C. Biological management of fusarial wilt of tomato by Trichoderma spp. in Andamans. Indian J. Hortic. 2013;70:397–403. [Google Scholar]
- 117.Singh S.P., Singh H.B., Singh D.K. Biocontrol potential of mixture of trichoderma isolates on damping-off and collar rot of tomato. Bioscan. 2014;9:1301–1304. [Google Scholar]
- 118.Thakur N., Tripathi A. Biological Management of Damping-Off, Buckeye Rot and Fusarial Wilt of Tomato (cv. Solan Lalima) under Mid-Hill Conditions of Himachal Pradesh. Agric. Sci. 2015;6:535–544. doi: 10.4236/as.2015.65053. [DOI] [Google Scholar]
- 119.Loganathan M., Garg R., Venkataravanappa V., Saha S., Rai A.B. Plant growth promoting rhizobacteria (PGPR) induces resistance against Fusarium wilt and improves lycopene content and texture in tomato. Afr. J. Microbiol. Res. 2014;8:1105–1111. doi: 10.5897/ajmr2013.5653. [DOI] [Google Scholar]
- 120.Abdel-Monaim M.F., Abdel-Gaid M.A., Zayan S.A., Nassef D.M.T. Enhancement of Growth Parameters and Yield Components in Eggplant using Antagonism of Trichoderma spp. Against Fusarium Wilt Disease. Int. J. Phytopathol. 2014;3:33–40. doi: 10.33687/phytopath.003.01.0510. [DOI] [Google Scholar]
- 121.Adhikari A., Dutta S., Nandi S., Bhattacharya I., De Roy M., Sarkar G., Mandal T. Antagonistic potentiality of native rhizobacterial isolates against root rot disease of okra, incited by Rhizoctonia solani. Afr. J. Agric. Res. 2013;8:405–412. doi: 10.5897/ajar13.003. [DOI] [Google Scholar]
- 122.Shrestha A., Kim B.S., Park D.H. Biological control of bacterial spot disease and plant growth-promoting effects of lactic acid bacteria on pepper. Biocontrol Sci. Technol. 2014;24:763–779. doi: 10.1080/09583157.2014.894495. [DOI] [Google Scholar]
- 123.Salim H.A., Kadhum A.A., Ali A.F., Saleh U.N., Jassim N.H., Hamad A.R., Attia J.A., Darwish J.J., Hassan A.F. Response of cucumber plants to PGPR bacteria (Azospirillum brasilense, Pseudomonas fluorescens and Bacillus megaterium) and bread yeast (Saccharomyces cerevisiae) Syst. Rev. Pharm. 2021;12:969–975. doi: 10.31838/srp.2021.1.135. [DOI] [Google Scholar]
- 124.Khabbaz S.E., Abbasi P.A. Isolation, characterization, and formulation of antagonistic bacteria for the management of seedlings damping-off and root rot disease of cucumber. Can. J. Microbiol. 2014;60:25–33. doi: 10.1139/cjm-2013-0675. [DOI] [PubMed] [Google Scholar]
- 125.Shanthiyaa V., Saravanakumar D., Rajendran L., Karthikeyan G., Prabakar K., Raguchander T. Use of Chaetomium globosum for biocontrol of potato late blight disease. Crop. Prot. 2013;52:33–38. doi: 10.1016/j.cropro.2013.05.006. [DOI] [Google Scholar]
- 126.Sopheareth M., Chan S., Naing K.W., Lee Y.S., Hyun H.N., Kim Y.C., Kim K.Y. Biocontrol of Late Blight (Phytophthora capsici) Disease and Growth Promotion of Pepper by Burkholderia cepacia MPC-7. Plant Pathol. J. 2013;29:67–76. doi: 10.5423/PPJ.OA.07.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mane M.M., Lal A., Zghair Q.N., Simon S. Efficacy of certain bio agents and fungicides against early blight of potato (Solanum tuberosum L.) Int. J. Plant Prot. 2014;7:433–436. doi: 10.15740/HAS/IJPP/7.2/433-436. [DOI] [Google Scholar]
- 128.Park K., Park J.-W., Lee S.-W., Balaraju K. Disease suppression and growth promotion in cucumbers induced by integrating PGPR agent Bacillus subtilis strain B4 and chemical elicitor ASM. Crop. Prot. 2013;54:199–205. doi: 10.1016/j.cropro.2013.08.017. [DOI] [Google Scholar]
- 129.Cao Y., Pi H., Chandrangsu P., Li Y., Wang Y., Zhou H., Xiong H., Helmann J.D., Cai Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-22782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vejan P., Abdullah R., Khadiran T., Ismail S., Boyce A.N. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules. 2016;21:573. doi: 10.3390/molecules21050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mhatre P.H., Karthik C., Kadirvelu K., Divya K.L., Venkatasalam E.P., Srinivasan S., Ramkumar G., Saranya C., Shanmuganathan R. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocatal. Agric. Biotechnol. 2018;17:119–128. doi: 10.1016/j.bcab.2018.11.009. [DOI] [Google Scholar]
- 132.Etesami H., Adl S.M. Can interaction between silicon and non–rhizobial bacteria help in improving nodulation and nitrogen fixation in salinity–stressed legumes? A review. Rhizosphere. 2020;15:100229. doi: 10.1016/j.rhisph.2020.100229. [DOI] [Google Scholar]
- 133.Porter S.S., Bantay R., Friel C.A., Garoutte A., Gdanetz K., Ibarreta K., Moore B.M., Shetty P., Siler E., Friesen M.L. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 2019;34:2075–2086. doi: 10.1111/1365-2435.13499. [DOI] [Google Scholar]
- 134.Kalozoumis P., Savvas D., Aliferis K., Ntatsi G., Marakis G., Simou E., Tampakaki A., Karapanos I. Impact of Plant Growth-Promoting Rhizobacteria Inoculation and Grafting on Tolerance of Tomato to Combined Water and Nutrient Stress Assessed via Metabolomics Analysis. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.670236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Subiramani S., Ramalingam S., Muthu T., Nile S.H., Venkidasamy B. Phyto-Microbiome Stress Regulation. Springer; Singapore: 2020. Development of Abiotic Stress Tolerance in Crops by Plant Growth-Promoting Rhizobacteria (PGPR) pp. 125–145. [DOI] [Google Scholar]
- 136.Bhattacharyya C., Banerjee S., Acharya U., Mitra A., Mallick I., Haldar A., Haldar S., Ghosh A., Ghosh A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020;10:1–19. doi: 10.1038/s41598-020-72439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paul D., Lade H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014;34:737–752. doi: 10.1007/s13593-014-0233-6. [DOI] [Google Scholar]
- 138.Jyothsna P., Murthy S.D.S. A review on effect of senescence in plants and the role of phytohormones in delaying senescence. Int. J. Plant Anim. Environ. Sci. 2016;6:152–162. [Google Scholar]
- 139.Kaushal M., Wani S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2015;66:35–42. doi: 10.1007/s13213-015-1112-3. [DOI] [Google Scholar]
- 140.Slettengren M., Mohanty S., Kamolvit W., van der Linden J., Brauner A. Making medical devices safer: Impact of plastic and silicone oil on microbial biofilm formation. J. Hosp. Infect. 2020;106:155–162. doi: 10.1016/j.jhin.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 141.Xia Y., Farooq A., Javed M.T., Kamran M.A., Mukhtar T., Ali J., Tabassum T., Rehman S.U., Munis M.F.H., Sultan T., et al. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol. Biochem. 2020;151:640–649. doi: 10.1016/j.plaphy.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 142.Ali S., Hameed S., Shahid M., Iqbal M., Lazarovits G., Imran A. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res. 2019;232:126389. doi: 10.1016/j.micres.2019.126389. [DOI] [PubMed] [Google Scholar]
- 143.Kousar B., Bano A., Khan N. PGPR Modulation of Secondary Metabolites in Tomato Infested with Spodoptera litura. Agronomy. 2020;10:778. doi: 10.3390/agronomy10060778. [DOI] [Google Scholar]
- 144.Choudhary D.K., Varma A. Microbial-Mediated Induced Systemic Resistance in Plants. Springer; Singapore: 2016. Microbial-mediated induced systemic resistance in plants; pp. 147–162. [DOI] [Google Scholar]
- 145.Calvo-Polanco M., Romera B.S., Aroca R., Asins M.J., Declerck S., Dodd I.C., Martínez-Andújar C., Albacete A., Ruiz-Lozano J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016;131:47–57. doi: 10.1016/j.envexpbot.2016.06.015. [DOI] [Google Scholar]
- 146.Lastochkina O., Pusenkova L., Yuldashev R., Babaev M., Garipova S., Blagova D., Khairullin R., Aliniaeifard S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol. Biochem. 2017;121:80–88. doi: 10.1016/j.plaphy.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 147.Sarkar A., Ghosh P.K., Pramanik K., Mitra S., Soren T., Pandey S., Mondal M.H., Maiti T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018;169:20–32. doi: 10.1016/j.resmic.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 148.Habib S.H., Kausar H., Saud H.M. Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. BioMed Res. Int. 2016;2016:1–10. doi: 10.1155/2016/6284547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Palaniyandi S.A., Damodharan K., Yang S.H., Suh J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014;117:766–773. doi: 10.1111/jam.12563. [DOI] [PubMed] [Google Scholar]
- 150.Arkhipova T.N., Prinsen E., Veselov S.U., Martinenko E.V., Melentiev A.I., Kudoyarova G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil. 2007;292:305–315. doi: 10.1007/s11104-007-9233-5. [DOI] [Google Scholar]
- 151.Lim J.-H., Kim S.-D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus licheniformis K11 in Pepper. Plant Pathol. J. 2013;29:201–208. doi: 10.5423/PPJ.SI.02.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang S.-M., Radhakrishnan R., Khan A.L., Kim M.-J., Park J.-M., Kim B.-R., Shin D.-H., Lee I.-J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Fu Q., Liu C., Ding N., Lin Y., Guo B. Ameliorative effects of inoculation with the plant growth-promoting rhizobacterium Pseudomonas sp. DW1 on growth of eggplant (Solanum melongena L.) seedlings under salt stress. Agric. Water Manag. 2010;97:1994–2000. doi: 10.1016/j.agwat.2010.02.003. [DOI] [Google Scholar]
- 154.Gupta A., Rai S., Bano A., Khanam A., Sharma S., Pathak N. Comparative Evaluation of Different Salt-Tolerant Plant Growth-Promoting Bacterial Isolates in Mitigating the Induced Adverse Effect of Salinity in Pisum sativum. Biointerface Res. Appl. Chem. 2021;11:13141–13154. doi: 10.33263/briac115.1314113154. [DOI] [Google Scholar]
- 155.Bharti N., Barnawal D., Awasthi A., Yadav A., Kalra A. Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Mentha arvensis. Acta Physiol. Plant. 2013;36:45–60. doi: 10.1007/s11738-013-1385-8. [DOI] [Google Scholar]
- 156.Kaymak H.C. Field Crops: Sustainable Management by PGPR. Springer; Cham, Switzerland: 2019. Potential of PGPR in Improvement of Environmental-Friendly Vegetable Production. [DOI] [Google Scholar]
- 157.Chinnaswamy A., de la Peña T.C., Stoll A., de la Peña Rojo D., Bravo J., Rincón A., Lucas M.M., Pueyo J.J. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation byEnsifer medicaeand alleviates salt stress in alfalfa plants. Ann. Appl. Biol. 2018;172:295–308. doi: 10.1111/aab.12420. [DOI] [Google Scholar]
- 158.Khan M.A., Asaf S., Khan A.L., Jan R., Kang S.-M., Kim K.-M., Lee I.-J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE. 2020;15:e0232228. doi: 10.1371/journal.pone.0232228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dhanasekar D., Sivamani P., Panneersel A., Thajuddin N., Rajakumar G., Selvamani S. Biological Control of Tomato Seedling Damping off with Streptomyces sp. Plant Pathol. J. 2005;4:91–95. doi: 10.3923/ppj.2005.91.95. [DOI] [Google Scholar]
- 160.Hassanisaadi M., Bonjar G.S., Hosseinipour A., Abdolshahi R., Barka E.A., Saadoun I. Biological Control of Pythium aphanidermatum, the Causal Agent of Tomato Root Rot by two Streptomyces Root Symbionts. Agronomy. 2021;11:846. doi: 10.3390/agronomy11050846. [DOI] [Google Scholar]
- 161.Chowdhury S.P., Dietel K., Rändler M., Schmid M., Junge H., Borriss R., Hartmann A., Grosch R. Effects of Bacillus amyloliquefaciens FZB42 on Lettuce Growth and Health under Pathogen Pressure and Its Impact on the Rhizosphere Bacterial Community. PLoS ONE. 2013;8:e68818. doi: 10.1371/journal.pone.0068818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.William Q. Least-Toxic Controls of Plant Diseases. Nat. Dis. Control. 2000;11:225. [Google Scholar]
- 163.Rose S., Parker M., Punja Z. Efficacy of Biological and Chemical Treatments for Control of Fusarium Root and Stem Rot on Greenhouse Cucumber. Plant Dis. 2003;87:1462–1470. doi: 10.1094/PDIS.2003.87.12.1462. [DOI] [PubMed] [Google Scholar]
- 164.Ravi S., Sabitha D., Valluvaparidasan V., Jeyalakshmi C., Doraiswamy S. Effect of biocontrol agents on seed-borne Colletotrichum in French bean. Plant Dis. Res. 1999;14:146–151. [Google Scholar]
- 165.Ramesh Kumar N., Thirumalai Arasu V., Gunasekaran P. Genotyping of antifungal compounds producing plant growth-promoting rhizobacteria, Pseudomonas fluorescens. Curr. Sci. 2002;82:1463–1466. [Google Scholar]
- 166.Paulitz T.C., Bélanger R.R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001;39:103–133. doi: 10.1146/annurev.phyto.39.1.103. [DOI] [PubMed] [Google Scholar]
- 167.Singh D., Yadav D.K., Chaudhary G., Rana V.S., Sharma R.K. Potential of Bacillus amyloliquefaciens for Biocontrol of Bacterial Wilt of Tomato Incited by Ralstonia solanacearum. J. Plant Pathol. Microbiol. 2016;7:1–6. doi: 10.4172/2157-7471.1000327. [DOI] [Google Scholar]
- 168.Hausbeck M.K., Lamour K.H. Phytophthora capsici on Vegetable Crops: Research Progress and Management Challenges. Plant Dis. 2004;88:1292–1303. doi: 10.1094/PDIS.2004.88.12.1292. [DOI] [PubMed] [Google Scholar]
- 169.Firmansyah D., Hidayat S.H. Chitosan and Plant Growth Promoting Rhizobacteria Application to Control Squash mosaic virus on Cucumber Plants. Asian J. Plant Pathol. 2017;11:148–155. doi: 10.3923/ajppaj.2017.148.155. [DOI] [Google Scholar]
- 170.Elbeshehy E.K.F., Youssef S.A., Elazzazy A.M. Resistance induction in pumpkin Cucurbita maxima L. against Watermelon mosaic potyvirus by plant growth-promoting rhizobacteria. Biocontrol Sci. Technol. 2015;25:525–542. doi: 10.1080/09583157.2014.994198. [DOI] [Google Scholar]
- 171.Belimov A.A., Dodd I.C., Safronova V.I., Shaposhnikov A.I., Azarova T.S., Makarova N.M., Davies W.J., Tikhonovich I. Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum) Ann. Appl. Biol. 2015;167:11–25. doi: 10.1111/aab.12203. [DOI] [Google Scholar]
- 172.Ullah U., Ashraf M., Shahzad S.M., Siddiqui A.R., Piracha M.A., Suleman M. Growth behavior of tomato (Solanum lycopersicum L.) under drought stress in the presence of silicon and plant growth promoting rhizobacteria. Soil Environ. 2016;35:65–75. [Google Scholar]
- 173.Kuşçu H., Turhan A., Demir A.O. The response of processing tomato to deficit irrigation at various phenological stages in a sub-humid environment. Agric. Water Manag. 2014;133:92–103. doi: 10.1016/j.agwat.2013.11.008. [DOI] [Google Scholar]
- 174.Lulsdorf M.M., Yuan H.Y., Slater S.M.H., Vandenberg A., Han X., Zaharia L.I., Abrams S.R. Endogenous hormone profiles during early seed development of C. arietinum and C. anatolicum. Plant Growth Regul. 2013;71:191–198. doi: 10.1007/s10725-013-9819-2. [DOI] [Google Scholar]
- 175.Lidon Z.Z., Cebola F. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emirates J. Food Agric. 2012;24:57–72. doi: 10.9755/ejfa.v24i1.10599. [DOI] [Google Scholar]
- 176.Mahajan S., Tuteja N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 177.Kumar M., Mishra S., Dixit V., Agarwal L., Chauhan P.S., Nautiyal C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.) Plant Signal. Behav. 2016;11:e1071004. doi: 10.1080/15592324.2015.1071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu F., Xing S., Ma H., Du Z., Ma B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013;97:9155–9164. doi: 10.1007/s00253-013-5193-2. [DOI] [PubMed] [Google Scholar]
- 179.Creus C.M., Graziano M., Casanovas E.M., Pereyra M.A., Simontacchi M., Puntarulo S., Barassi C.A., LaMattina L. Nitric Oxide is Involved in the Azospirillum brasilense-induced Lateral Root Formation in Tomato. Planta. 2005;221:297–303. doi: 10.1007/s00425-005-1523-7. [DOI] [PubMed] [Google Scholar]
- 180.Batool T., Ali S., Seleiman M.F., Naveed N.H., Ali A., Ahmed K., Abid M., Rizwan M., Shahid M.R., Alotaibi M., et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020;10:1–19. doi: 10.1038/s41598-020-73489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang C., Guo Y., Wang C., Liu H., Niu D., Wang Y., Guo J. Enhancement of tomato (Lycopersicon esculentum) tolerance to drought stress by plant-growth-promoting rhizobacterium (PGPR) Bacillus cereus AR156. J. Agric. Biotechnol. 2012;20:1097–1105. [Google Scholar]
- 182.Shannon M.C., Grieve C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998;78:5–38. doi: 10.1016/S0304-4238(98)00189-7. [DOI] [Google Scholar]
- 183.Shahbaz M., Ashraf M., Al-Qurainy F., Harris P.J.C. Salt Tolerance in Selected Vegetable Crops. Crit. Rev. Plant Sci. 2012;31:303–320. doi: 10.1080/07352689.2012.656496. [DOI] [Google Scholar]
- 184.Munns R., Tester M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 185.Lauchli A., Epstein E. Plant Responses to Saline and Sodic Conditions and Agricultural Salinity Assessment and Management. ASCE; New York, NY, USA: 1990. pp. 113–137. [Google Scholar]
- 186.Ekinci M., Turan M., Yildirim E., Güneş A., Kotan R., Dursun A. Effect of plant growth promoting rhizobacteria on growth, nutrient, organic acid, amino acid and hormone content of cauliflower (Brassica oleracea L. var. botrytis) transplants. Acta Sci. Pol. Hortorum Cultus. 2014;13:71–85. [Google Scholar]
- 187.Xu C., Mou B. Evaluation of Lettuce Genotypes for Salinity Tolerance. HortScience. 2015;50:1441–1446. doi: 10.21273/HORTSCI.50.10.1441. [DOI] [Google Scholar]
- 188.Kidoglu F., Gül A., Özaktan H., Tüzel Y. Effect of rhizobacteria on plant growth of different vegetables. Acta Hortic. 2008;801:1471–1478. doi: 10.17660/ActaHortic.2008.801.181. [DOI] [Google Scholar]
- 189.Bochow H., El-Sayed S.F., Junge H., Stavropoulou A., Schmiedeknecht G. Use of Bacillus subtilis as biocontrol agent. IV. Salt-stress tolerance induction by Bacillus subtilis FZB24 seed treatment in tropical vegetable field crops, and its mode of action. Z. Pflanzenkrankh. Pflanzenschutz. 2001;108:21–30. [Google Scholar]
- 190.Bensalim S., Nowak J., Asiedu S.K. A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am. J. Potato Res. 1998;75:145–152. doi: 10.1007/BF02895849. [DOI] [Google Scholar]
- 191.Martin C.A., Stutz J.C. Interactive effects of temperature and arbuscular mycorrhizal fungi on growth, P uptake and root respiration of Capsicum annuum L. Mycorrhiza. 2003;14:241–244. doi: 10.1007/s00572-003-0261-6. [DOI] [PubMed] [Google Scholar]
- 192.Mukhtar T., Rehman S.U., Smith D., Sultan T., Seleiman M.F., Alsadon A.A., Ali S., Chaudhary H.J., Solieman T.H.I., Saad M.A., et al. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability. 2020;12:2159. doi: 10.3390/su12062159. [DOI] [Google Scholar]
- 193.Rubio M.I., Escrig I., Martínez-Cortina C., López-Benet F.J., Sanz A. Cadmium and nickel accumulation in rice plants. Effects on mineral nutrition and possible interactions of abscisic and gibberellic acids. Plant Growth Regul. 1994;14:151–157. doi: 10.1007/BF00025217. [DOI] [Google Scholar]
- 194.Schuurmann G., Markert B. Ecotoxicology. John Wiley and Sons; Heidelberg, Germany: 1998. Effects of heavy metals in plants at the cellular and organismic level; pp. 587–620. [Google Scholar]
- 195.Fatnassi I.C., Chiboub M., Saadani O., Jebara M., Jebara S.H. Impact of dual inoculation with Rhizobium and PGPR on growth and antioxidant status of Vicia faba L. under copper stress. Comptes Rendus Biol. 2015;338:241–254. doi: 10.1016/j.crvi.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 196.Gururani M.A., Upadhyaya C.P., Baskar V., Venkatesh J., Nookaraju A., Park S.W. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum tuberosum Through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J. Plant Growth Regul. 2012;32:245–258. doi: 10.1007/s00344-012-9292-6. [DOI] [Google Scholar]
- 197.Safronova V.I., Stepanok V.V., Engqvist G.L., Alekseyev Y.V., Belimov A.A. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol. Fertil. Soils. 2005;42:267–272. doi: 10.1007/s00374-005-0024-y. [DOI] [Google Scholar]
- 198.I Burd G., Dixon D.G., Glick B.R. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- 199.Singh A.D., Sharma P., Kohli S.K., Kumar P., Singh R., Arora P., Sharma P., Kaur R., Sharma A., Bhardwaj R. Cellular and Molecular Phytotoxicity of Heavy Metals. Springer; Cham, Switzerland: 2020. Role of Plant Growth Regulators (PGRs) in Mitigation of Heavy Metal Phytotoxicity in Plants; pp. 263–304. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study are available in the article.