Abstract
Cordyceps spp. mushrooms have a long tradition of use as a natural raw material in Asian ethnomedicine because of their adaptogenic, tonic effects and their ability to reduce fatigue and stimulate the immune system in humans. This review aims to present the chemical composition and medicinal properties of Cordyceps militaris fruiting bodies and mycelium, as well as mycelium from in vitro cultures. The analytical results of the composition of C. militaris grown in culture media show the bioactive components such as cordycepin, polysaccharides, γ-aminobutyric acid (GABA), ergothioneine and others described in the review. To summarize, based on the presence of several bioactive compounds that contribute to biological activity, C. militaris mushrooms definitely deserve to be considered as functional foods and also have great potential for medicinal use. Recent scientific reports indicate the potential of cordycepin in antiviral activity, particularly against COVID-19.
Keywords: Cordyceps militaris, bioactive compounds, biological activities, cordycepin, ergothioneine, polysaccharides
1. Introduction
Cordyceps spp. mushrooms, classified into the Ascomycota group, have a long tradition of use as a natural agent in Asian ethnomedicine because of their adaptogenic and tonic effects and their ability to reduce fatigue and stimulate the immune system in humans. Among the numerous, approximately 500, species of Cordyceps, most scientific research has been conducted on Cordyceps sinensis, which is included in the Chinese Pharmacopoeia (2015), and its adenosine concentration is considered as the main quality indicator. Considering several complications (explained further in this review) regarding C. sinensis, the species C. militaris has attracted more attention from scientists and the industry over recent years [1,2].
C. sinensis is an authorized ingredient in food and dietary supplements in the European Union (EU), and it is included in European Commission (EC) and European Food Safety Authority (EFSA) documents such as the Novel Foods Catalogue, Compendium of Botanicals. Health claims for C. sinensis have been proposed in the past, such as that C. sinensis possesses antioxidant properties (400–800 mg/day), stimulates the immune system and increases performance and endurance during exercise (3 g dried powder or equivalent extracts); however, these claims have not been approved by EFSA and EC, because the cause-and-effect relationship between the consumption of C. sinensis and the corresponding health benefit has not yet been established. Meanwhile, C. militaris is not included in the abovementioned documents in the EU, meaning it is considered an unauthorized ingredient of foods or dietary supplements [3].
The inhabitants of China, Tibet, Nepal and India have consumed Cordyceps spp. for centuries in order to adapt their bodies to difficult high mountain conditions such as low ambient temperature, high atmospheric pressure and reduced oxygen content in the environment. Traditional Chinese medicine recommends the use of Cordyceps spp. for treating several human disorders such as cardiovascular and respiratory diseases, disorders of the liver and kidney, cancers, diabetes, infectious and parasitic diseases and sexual dysfunctions [1,2].
A unique aspect of Cordyceps spp. is its existence as an entomopathogenic fungus in the natural environment. These species are endemic to the Himalayas at locations 2000 m above sea level. C. sinensis is a parasitic fungus that attacks the larvae of moths Thitarodes spp. and Lepidoptera spp. These larvae reside approximately 15 cm underground, where they become “infected” with C. sinensis, which gradually uses the entire interior part of the larvae and then produces fruiting bodies on their shells so that these are visible above the soil surface. The indigenous people collect C. sinensis with the larva in the season from May to June, dry it, pulverize it and then prepare an extract with hot water. After that, the preparation can be consumed as a hot water beverage [2].
Because of the limited resources of C. sinensis, endemic occurrence, unique growth pattern and biology of its life cycle in the ecosystem, the long-term process of symbiosis between the fungus and the larva (from autumn to spring) is time-consuming, and the harvesting process of this species from its natural site results in a very high price. Obtaining this species from the natural environment has become insufficient to meet the growing demand for raw materials for the production of dietary supplements, nutraceuticals and functional foods, especially in Asia, the United States and Europe [2,4,5].
Since historical times, C. sinensis has been used as an ingredient of food and medicine and is therefore more recognizable by consumers. The aforementioned difficulties in obtaining C. sinensis from culture media, the growing demand for this species and its high prices due to limited natural resources have led to the search for alternative sources. The solution to this issue is a related species, namely C. militaris (L.) Fr., which can be grown and obtained in vitro. Importantly, it was observed that under in vitro culture conditions, C. sinensis mycelium cannot synthesize cordycepin (or can synthesize only in a negligible amount) and has a lower content of amino acids and D-mannitol than those in fruiting bodies of this species obtained from natural sites [6].
C. militaris is an alternative to C. sinensis because the qualitative and quantitative composition of bioactive substances from in vitro-cultivated C. militaris does not differ from the content of these substances in C. sinensis fruiting bodies. Therefore, the aim of this work was to review the composition of C. militaris. The analysis of the composition of C. militaris from the culture media showed that the concentration of cordycepin and polysaccharides is higher than that in C. sinensis from the natural environment [7,8]. Both species show a similar profile of 17 amino acids, with the predominance of L-arginine and L-proline in C. militaris from the culture media [2,5].
2. Materials and Methods
Keywords used for data collection were: Cordyceps militaris, bioactive compounds, biological activities and cordycepin. The following databases were examined: US National Library of Medicine (PubMed), Medical Literature Analysis and Retrieval System Online (MEDLINE), EMBASE, Scopus and Google Scholar. The search terms were applied as keywords in the titles, abstracts and body of the journal articles in academic databases, including Cochrane Library, Frontiers, Jagiellonian University Repository, PLoS ONE, MDPI, Wiley Online Library, SAGE Journals, ScienceDirect, Springer Link and Taylor and Francis. The selection criteria for studies to be considered in the review include that the study is written in English (unless, for relevance to the review, it was available in a different language), the study is published in a peer-reviewed journal and the study is published between 1991 and 2021 (unless its historical value and, therefore, older scientific works were also used).
3. Bioactive Compounds from C. militaris
Based on the results of the scientific research, it is possible to obtain information on bioactive compounds from the group of nucleosides and polysaccharides contained in C. militaris. Nucleosides—cordycepin and adenosine—have been confirmed to be present in C. militaris, and the content of these ingredients in C. militaris is higher than those in C. sinensis. The analytical results showed the presence of the following biologically active substances in C. militaris: γ-aminobutyric acid (GABA) and ergothioneine; glycolipids (cerebrosides), glycoproteins (lectins), D-mannitol (referred as cordycepic acid), xanthophylls including carotenoids (lutein and zeaxanthin), sterols (ergosterol), statins (lovastatin), phenolic compounds (including phenolic acids and flavonoids), vitamins and biominerals/bioelements (magnesium, potassium, selenium, and sulfur) [7,8,9].
Previous studies have shown that the concentration and distribution of bioactive compounds is not uniform in the fruiting bodies. The outer parts of C. militaris fruiting bodies have the highest concentration of nucleosides, polysaccharides, carotenoids and selenium organic compounds. The comparison of the content of bioactive ingredients in the mycelium and fruiting bodies of C. militaris is presented in Table 1. The optimal drying temperature for C. militaris is 60 °C. A higher temperature causes a loss of the content of cordycepin and phenolic compounds [10].
Table 1.
Content of bioactive compounds and nutrients present in the mycelium and fruiting bodies of C. militaris.
| Content | |||
|---|---|---|---|
| Bioactive Compound | Mycelium | Fruiting Bodies | References |
| Cordycepin | 1.82 mg/g | 1.10 mg/g | [9] |
| Cordycepin | 1.74 mg/g | 5.28 mg/g (Water extrct) |
[7] |
| Cordycepin | 8.37 mg/g (Ethanol extract) |
[7] | |
| D-mannitol | 5.2 mg/kg | 4.7 mg/kg | [9] |
| Ergothioneine | 130.6 mg/kg | 782.3 mg/kg | [9] |
| Ergothioneine | 123.4–785.1 mg/kg | 409.8 μg/g | [7,8] |
| GABA | 68.6–180.1 mg/kg | 756.30 μg/g | [7,8] |
| Lovastatin | 37.7–57.3 mg/kg | 2.76 μg/g | [7,8] |
| Vitamins | |||
| Vit. A | 100 mg/kg | 96 mg/kg | [9] |
| Vit. E (tocopherols) | 1.3 mg/kg | 3.6 mg/kg | [9] |
| Vit. B2 (riboflavin) | 0.32 mg/kg | 0.16 mg/kg | [9] |
| Vit. B3 (niacin) | 15.2 mg/kg | 4.9 mg/kg | [9] |
| Vit. C | Not detected | <2 mg/kg | [9] |
| Bioelements | |||
| Magnesium | 3414 mg/kg | 4227 mg/kg | [9] |
| Sulfur | 2558 mg/kg | 5088 mg/kg | [9] |
| Potassium | 12,183 mg/kg | 15,938 mg/kg | [9] |
| Selenium | <0.5 mg/kg | 0.4 mg/kg | [9] |
| Iron | 9 mg/kg | 31 mg/kg | [9] |
| Calcium | 11 mg/kg | 797 mg/kg | [9] |
| Zinc | 10 mg/kg | Not detected | [9] |
| Nutrients | |||
| Protein | 39.5% | 59.8% | [9] |
| Protein | Not analyzed | 29.7% | [7] |
| Fat | 2.2% | 8.8% | [9] |
| Fat | Not analyzed | 2.9% | [7] |
| Carbohydrate | 39.6% | 29.1% | [9] |
| Carbohydrate | Not analyzed | 54.3% | [7] |
3.1. Nucleosides
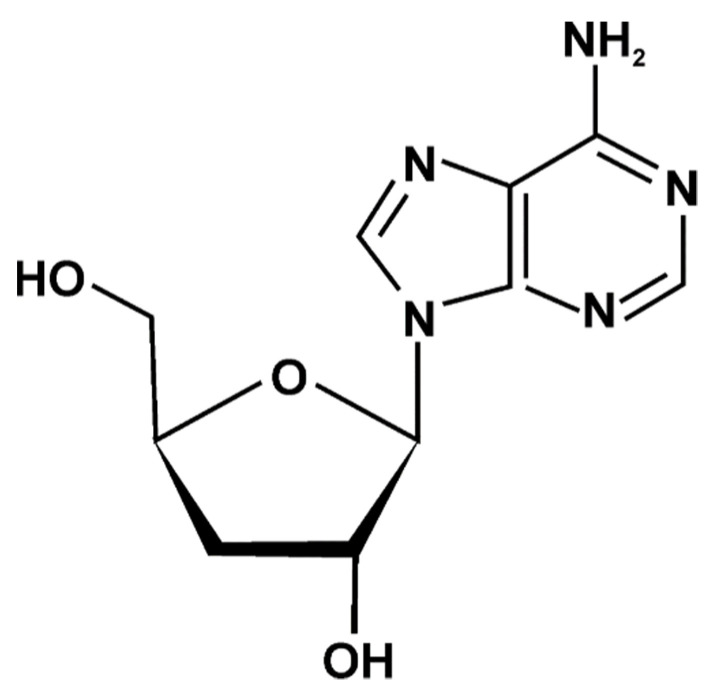
Cordycepin (3ʹ-deoxyadenosine) is a water-insoluble organic compound and a structural analog of the nucleoside adenosine (Figure 1).
Figure 1.
Chemical structure of cordycepin.
Cordycepin was isolated from C. militaris in 1950. On the basis of in vitro and in vivo studies performed to date, this compound has been confirmed to exhibit the following activities: immunostimulating, anti-inflammatory, antiviral, antitumor, ergogenic, hypolipidemic, hypoglycemic and regulation of steroidogenesis and spermatogenesis. Few studies have demonstrated the antioxidant activity of cordycepin. The antioxidant activity has been explained in scientific studies based on the mechanisms of biological activity of polysaccharide fractions contained in fruiting bodies of C. militaris. Experiments conducted on rodents demonstrated that the immunostimulatory activity of cordycepin results from its ability to induce cellular and humoral immune response. The studies revealed an increase in the concentration of interleukins IL-4, IL-10 and IL-12, and Th1 and Th2 cytokines, a decrease in the concentration of IL-2 and transforming growth factor-β (TGF-β) and an increase in the level of T lymphocytes (CD4 and CD8). One of the mechanisms of action of cordycepin is supplementing “produced” energy in the form of adenosine-5′-triphosphate (ATP) molecules. Cordycepin can also increase the concentration of nitric oxide (NO) [11,12].
3.2. Carbohydrates
D-Mannitol is one of the most important products of C. militaris metabolism and is classified as a polyhydric alcohol (polyol). D-Mannitol from C. militaris is commonly referred to as cordycepic acid. It is used by C. militaris as a carbohydrate reserve and as a transporter of other compounds in the osmoregulation and control of metabolic pathways. D-mannitol shows osmotic activity, thus it can be used in clinical practice as a diuretic and anti-edematous drug [7,13].
In addition to D-mannitol, C. militaris contains saccharides with more complex structures—polysaccharides. Depending on the site of polysaccharide biosynthesis in the hyphae of C. militaris, these may occur as secondary intracellular (IPS) or extracellular (EPS) metabolites. Polysaccharides isolated from the mycelium of C. militaris have a different chemical structure. The chemical structure is determined by the type of monosaccharides that form the polysaccharides, their linear sequence, spatial configuration, location of glycosidic bonds and degree of branching (cross-linking) of the chain [14].
Significant differences have been observed in the chemical structure as well as the qualitative and quantitative composition of monosaccharides obtained from the fraction of polysaccharides present in C. militaris growing in the natural environment, and cultivated under laboratory conditions. Mannose, glucose and galactose are the most important monosaccharides which form saccharide polymers. In subsequent scientific studies on the polysaccharides of C. militaris, the following other monosaccharides were detected: arabinose, rhamnose and xylose [14].
In the CPSN Fr II polysaccharide obtained from the culture broth of C. militaris, the percentage content of monosaccharides is as follows: mannose (65.12%), galactose (28.72%) and glucose (6.12%) [15]. The WCBP50 polysaccharide structurally contains α-D-glucose, α-D-mannose and α-D-arabinose units linked by an α-glycosidic bond [16]. CMN1 is a 37.8 kDa polysaccharide isolated from C. militaris mycelium. CMN1 is composed of the subunits of D-galactose, D-mannose, L-arabinose and L-rhamnose linked by a glycosidic bond (1 → 2) and (1 → 3). The branching of the side substituents is in the (1 → 4) and (1 → 6) positions [17].
The basis of the structure of LCMPs-II is (1 → 4)-α-D-glucose with side substituents (1 → 3) of rhamnose, xylose and glucose [18]. In subsequent studies, the SeCSP-I polysaccharide fortified with selenium was obtained, and the structure of this polysaccharide was shown as (1 → 4) galactose, (1 → 3) and (1 → 6) mannose and (1 → 4) glucose [19]. Subsequent studies showed that the CMP-W1 polysaccharide consists of 55.4% D-mannose, 19.5% D-glucose and 25.1% D-galactose. The types of glycosidic bonds identified in the chain are (1 → 3), (1 → 4) and (1 → 6) glycosidic bonds [20].
The CMP-1 polysaccharide has a low molecular weight of 4.4 kDa and consists of the following combined units: (1 → 4) α-D-glucose, (1 → 6) β-D-glucose and (1 → 4) β-D-glucose, and the side branches contain (1 → 3) α-L-rhamnose [21]. The new structure CMPB90-1 isolated from C. militaris was found to be composed of α-D-glucose linked by (1 → 6) and (1 → 3) side branches containing (1 → 4) β-D-mannose [22].
To summarize, the differences in the obtained results for the chemical structure of polysaccharides are due to the source of C. militaris, the cultivation methods and conditions and the extraction method. The molecular weight, spatial conformation, type of glycosidic bonds and the degree of branching (cross-linking) of polysaccharides influence the biological activity of C. militaris polysaccharides [14].
In vitro and/or in vivo experiments showed that the polysaccharides contained in C. militaris exhibit immunostimulatory, antitumor, anti-inflammatory, antioxidant, hypoglycemic, hypolipidemic, and hepatoprotective activities. These studies proved the immunostimulatory activity of the polysaccharide fractions isolated from C. militaris, and the tested polysaccharides were shown to induce an immune response in vitro after stimulating the activity of macrophages to produce NO, IL-1β, interferon (IFN -γ), tumor necrosis factor (TNF-α), T and B lymphocytes and natural killer (NK) cells as well as increased phagocytosis by macrophages [14].
The experiments confirmed that the polysaccharides exerted antitumor activity through growth inhibition and induction of apoptosis of tumor cells. Inhibition of carcinogenesis involved inhibition of cyclin-dependent kinase and arrest of the tumor cell cycle in the G0/G1 and G2/M phases [14].
3.3. Amino Acids
The total content of amino acids in fruiting bodies of C. militaris is 57.39 mg/g dry weight. In addition to protein amino acids, the fruiting bodies contain non-protein amino acids, such as GABA and ergothioneine [9].
GABA is a non-protein amino acid biosynthesized in the human body from glutamic acid (glutamate). GABA is an inhibitory neurotransmitter in the central nervous system and affects various parts of the nervous system, including the cerebellum, hippocampus, hypothalamus, striatum and spinal cord. GABA interferes with the activity of GABAergic receptors subtype A, B and C. It regulates sleep, memory and learning processes and emotional processes such as anxiety and stress. GABA also shows myorelaxant and anticonvulsant activities [23].
Previous scientific research has shown discrepancies in the results on the pharmacokinetic and pharmacodynamic parameters of GABA after oral administration in humans. Some studies indicate the low bioavailability of GABA after oral administration. Other complications related to GABA include difficulty in penetrating the blood–brain barrier and short biological half-life [24].
Some scientific publications have confirmed the bioavailability and effectiveness of oral GABA supplementation in humans [25,26].
In studies on mycelium and fruiting bodies of C. militaris, the concentration of GABA was determined, respectively, at 68.6–180.1 mg/kg and 756.30 μg/g dry weight [7,8].
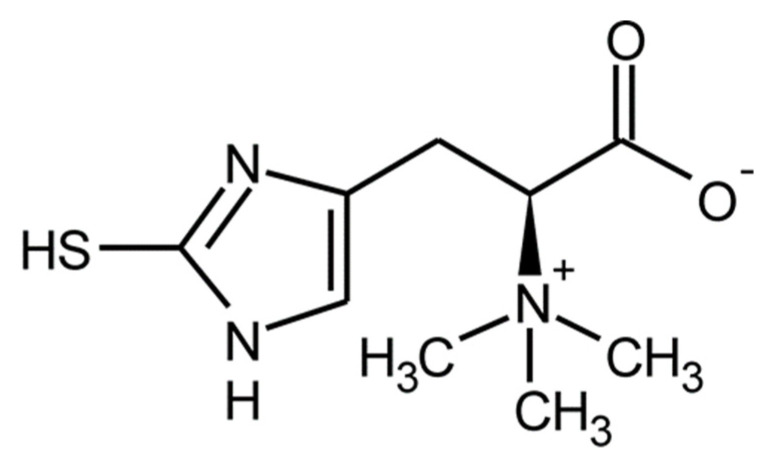
Ergothioneine (2-thiol-L-histidine-betaine) is a water-soluble sulfur analog of the amino acid L-histidine with an attached fragment of the betaine molecule (Figure 2).
Figure 2.
Chemical structure of ergothioneine.
Ergothioneine is a non-protein amino acid produced by certain bacteria, plants, and fungi, but it is not produced by mammals and therefore must be provided through the diet [27].
A valuable source of ergothioneine are various species of fungi, in which the concentration of this substance ranges from 0.2 mg/g to 2.6 mg/g dry weight. The content of ergothioneine in Agaricus bisporus is approximately 0.55 mg/g dry weight. The concentration of ergothioneine has been confirmed in the following species: Lentinula edodes (shiitake), 1.98 mg/g dry weight; Pleurotus osteratus (oyster), 2.59 mg/g dry weight, and Grifola frondosa (maitake), 1.13 mg/g dry weight [27,28,29].
The concentration of ergothioneine in the fruiting bodies of C. militaris was determined to be 782.3 mg/kg dry weight, whereas in mycelium it achieves range 130.6 mg/kg dry weight [9]. In another study, it was estimated to be 409.8 μg/g dry weight in the fruiting bodies of C. militaris [8].
The human body is unable to biosynthesize ergothioneine. A specific transporter carnitine/organic cation transporter 1 (OCTN1) for this substance has been identified, and a high concentration of ergothioneine has been confirmed in some tissues and cells, including erythrocytes, spleen, liver, and eyes. The antioxidant, cytoprotective, and radioprotective activities of ergothioneine have been demonstrated in in vitro and in vivo experiments on animal models [30].
In healthy volunteers aged 21–35 years, supplementation with ergothioneine (at the dose of 5 mg/day and 25 mg/day) was found to correlate with a slight reduction in the level of oxidative stress markers. Presumably, the antioxidant activity of ergothioneine may be more important in situations predisposing to the generation of oxygen free radicals, e.g., diseases with inflammatory processes or physical exercise [31].
The concentration of ergothioneine in the human body decreases with age [32]. Ergothioneine concentration significantly decreases in the elderly people after 60 years of age, which may correlate with the development of neurodegenerative diseases [33].
3.4. Carotenoids
The presence of carotenoids, including xanthophyll derivatives, has been confirmed in fruiting bodies of various species of fungi, including C. militaris. Carotenoids are responsible for an intense yellow-orange color of C. militaris fruiting bodies. The main xanthophylls present in the fruiting bodies of C. militaris are β-carotene, lycopene, lutein and zeaxanthin [34,35]. Lutein and zeaxanthin are also found in the macula of the human eye, which is a cluster of cones responsible for color (daytime) vision [36]. In addition to research on the protective effect of lutein and zeaxanthin on eye structures, previous scientific reports have indicated that supplementation with carotenoid pigments improves cognitive functions, reduces the level of cortisol and stress symptoms in young and adult people, and induces antioxidant effects [36,37,38].
It has been proved that a diet rich in carotenoids, such as the consumption of tomato juice (high concentration of lycopene) and carrot juice (high concentration of β-carotene), correlates with a positive effect on immune functions in healthy males who previously showed a low level of carotenoids in their diet [39]. There are discrepancies in the effects of β-carotene on human health. Some reviews indicate a benefit of β-carotene supplementation in the area of cancer diseases [40], whereas in some scientific reviews contradictory information can be found in that supplementation of β-carotene does not have a significant effect in the prevention of cancer, and conversely, in some types of cancer, such as lung or stomach cancer, it may increase the risk of cancer. It should be noted that the increased risk of lung or stomach cancer was found in the case of supplementation with high doses of β-carotene, in the range of 20–30 mg per day [41].
It was confirmed that lycopene supplementation has a positive effect on the functions of the vascular endothelium in patients with cardiovascular disease (CVD). However, no significant impact on endothelium function in healthy volunteers [42]. The correlation between the supply of lycopene and the prevention of the metabolic syndrome was confirmed [43]. In vitro and in vivo studies have shown that lycopene inhibits the progression and induces apoptosis of prostate cancer cells. Lycopene is an auxiliary agent supporting basic chemotherapy and hormone therapy in patients with prostate cancer [44].
The concentration of β-carotene and lycopene in the extract of fruiting bodies of C. militaris was determined. The content of β-carotene and lycopene was 0.328 mg/g and 0.277 mg/g, respectively [45]. Choi et al. [46] noted that in the aqueous extract of C. militaris, the concentration of β-carotene was determined at 24.51 μg/g, whereas lycopene was 3.42 μg/g.
For comparison, the concentration of β-carotene in A. bisporus (Turkey) is 0.04 mg/g, whereas in Pleurotus ostreatus (India) it is 0.03 mg/g [35]. Content of β-carotene has been demonstrated in the species Tricholoma acerbum at 75.48 μg/g, whereas lycopene is at 39.65 μg/g [47].
In fruiting bodies of C. militaris, the presence a novel class of carotenoids was confirmed such as cordyxanthins. Four cordyxanthins have been identified in fruiting bodies of C. militaris, which are marked numerically as cordyxanthin I–IV. Concentration of cordyxanthin I, cordyxanthin II, cordyxanthin III and cordyxanthin IV was, respectively, 0.289 mg/g, 0.235 mg/g, 0.401 mg/g, 0.175 mg/g. Unlike lutein, zeaxanthin, β-carotene and lycopene, cordyxanthins show better solubility in water, which results from differences in the chemical structure—they contain less lipophilic methyl groups and more hydroxyl substituents. Among the marked cordyxanthins, cortixanthin II deserves attention because unlike most carotenoids, it does not present in two, six-membered carbon rings, but one six-membered and the second five-membered carbon structure [34].
3.5. Statins
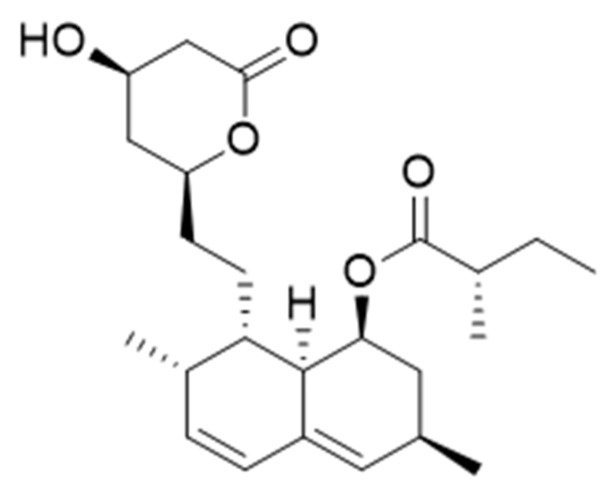
The fruiting bodies of C. militaris are a valuable source of lovastatin that belongs to a group of compounds called statins, which are commonly used as cholesterol-lowering drugs [7,8]. Lovastatin is a naturally occurring compound that selectively blocks the synthesis of endogenous cholesterol. It was first isolated from Aspergillus terreus in 1978 and introduced as a medicinal compound by Merck in 1987. Lovastatin contains a six-part lactone ring with a hydroxyl group and partially hydrogenated naphthalene with a hydroxyl substituent esterified with a 2-methylbutyric acid residue (Figure 3). Lovastatin is a precursor converted from a lactone form to a hydroxy acid during enzymatic reactions, which competitively blocks 3-hydroxymethylglutaryl-coenzyme A (HMG-CoA). Consequently, the conversion of HMG-CoA to mevalonate, which is a key step in cholesterol synthesis, is blocked. Drugs from the statin group also show pleiotropic effects, which include protection of the vascular endothelium. Lovastatin is an approved drug at the dose of 20 mg and is indicated in the primary treatment of hypercholesterolemia in patients and in reducing the development of coronary atherosclerosis in patients with coronary artery disease [48,49].
Figure 3.
Chemical structure of lovastatin.
The concentration of lovastatin in mycelium of C. militaris is defined in the range from 37.7 mg/kg to 57.3 mg/kg dry weight. In this respect, C. militaris has a lower content of lovastatin than that determined in C. sinensis at 1365.3 mg/kg [8]. For comparison, in fruiting bodies of C. militaris the concentration of lovastatin is 2.76 μg/g. However, one of the highest contents of lovastatin was confirmed in fruiting bodies of Hericium erinaceus 14.38 μg/g and Ganoderma lucidum 11.54 μg/g [7].
3.6. Phenolic Compounds
An important group of phenolic compounds present in mushrooms are phenolic acids and flavonoids. Their potential is associated with a strong antioxidant effect and the ability to protect important structures such as proteins, enzymes, lipids or nucleic acids against oxidative damage. The strongest antioxidant properties are exhibited by phenolic acids such as vanillic and caffeic acids. p-Hydroxybenzoic, gallic and protocatechuic acids found in edible mushrooms not only show antioxidant activity but also exert antibacterial, antifungal, antiviral and anti-inflammatory effects as demonstrated by in vitro and in vivo studies [50].
The total content of phenolic acids and organic acids in A. bisporus is 23.43 µg/g, with the concentration of gallic acid reaching only 0.83 µg/g. The total amount of phenolic acids and organic acids in P. ostreatus was determined to be 10.41 µg/g. The concentration of gallic acid and protocatechuic acid was determined at 1.38 µg/g and 2.07 µg/g, respectively [51]. The concentration of phenolic acids in fruiting bodies of C. militaris was also analyzed. The concentration of p-hydroxybenzoic acid is 0.02 mg/100 g dry weight and cinnamic acid 0.11 mg/100g dry weight were determined [52].
The concentration of flavonoids was determined in the extract of fruiting bodies of C. militaris in the amount of 1.56 mg/g [45]. The concentration of flavonoids was calculated as rutin equivalent (RE). The content of flavonoids in fruiting bodies and mycelium was determined, respectively, as 5.54 mg RE/g and 2.26 mg RE/g [53]. In the aqueous extract of C. militaris, the concentration of flavonoids was 275.52 mg/g, whereas polyphenols were 19.79 mg/g [46]. The concentration of flavonoids is higher in the extract than in fresh fruiting bodies of C. militaris. The total content of flavonoids was 6.6 mg RE/100 g and 4.5 mg RE/100 g respectively in the extract and fresh fruiting bodies of C. militaris [54].
3.7. Other Bioactive Compounds in C. militaris Fruiting Bodies
Lectins, detected in fruiting bodies of C. militaris on the basis of their chemical structure, are protein molecules with attached saccharide fragments (glycoproteins). Lectins have been demonstrated to possess mitogenic activity. These bind to sugar residues on the surface of cells to initiate the process of cell clumping—the agglutination of cells [55,56]. Another group of compounds found in this species are beauveriolides, characterized by a complex chemical structure—cyclodepsipeptides. Beauveriolides exhibited antiatherosclerotic activity and the ability to reduce β-amyloid concentration [57]. Militarinones are a less known group of chemical compounds classified as alkaloids. Structurally, these are pyridine derivatives or tetramic acids (pyrrolidine-2,4-dione) and have been shown to possess antimicrobial and cytotoxic activities [57].
Pentostatin isolated from the fruiting bodies of the described species is an analog of the purine base hypoxanthine. It exhibits inhibitory activity for the enzyme adenine deaminase. It also shows antitumor and immunosuppressive activity. Pentostatin has been registered as a drug from the group of chemotherapeutic agents, indicated in the treatment of cancer in oncology and hematology hospital departments [57,58]. Cordymin is a compound found in fruiting bodies of C. militaris and is classified as a peptide with antimicrobial activity [59].
Fruiting bodies of C. militaris contain water-soluble vitamins (vitamin B2, vitamin B3, and vitamin C) and fat-soluble vitamins (vitamin A and vitamin E) [9]. Vitamin B2 (riboflavin) and vitamin B3 (niacin) reduce the feeling of tiredness and fatigue and are responsible for normal energy-yielding metabolism. Vitamin C protects cells from oxidative stress and contributes to maintain the normal function of the immune system, support collagen formation and increase the absorption of iron from the gastrointestinal tract. Vitamin A plays a role in the process of cell specialization and maintenance of normal vision. Vitamins E protects cells against oxidative stress [60].
Bioelements such as magnesium, potassium, selenium and sulfur have been detected in fruiting bodies of C. militaris. The high concentration of these bioelements in C. militaris makes it an alternative source of these minerals for human diet. The content of minerals present in C. militaris is summarized in Table 1. The concentration of biominerals such as boron, manganese, copper and iron is relatively negligible and ranges from 5 to 31 mg/kg dry weight [7].
In fruiting bodies of C. militaris, selenium occurs in an organic form and is bound to amino acids or proteins. Selenium is chelated with the amino acid L-methionine (selenomethionine) or L-cysteine (selenocysteine). It combines with proteins to form methylselenocysteine. The addition of selenium (sodium selenate) to C. militaris medium increases the concentration of active compounds in fruiting bodies—nucleosides, polysaccharides, amino acids, and organic selenium [61,62]. Enrichment of the C. militaris substrate with organic or inorganic selenium (sodium selenate(IV); sodium selenate(VI)) increases the concentration of cordycepin and adenosine in fruiting bodies [63]. Selenium-fortified polysaccharide was obtained from the mycelium of C. militaris (SeCSP -I). An in vitro experiment demonstrated the antioxidant activity of SeCSP-I [19].
4. Biological Activity
Scientific studies have shown significantly more diverse biological activities of C. militaris than those of C. sinensis. C. militaris has been shown to exert the following activities: ergogenic, immunostimulating, antitumor, antioxidant, anti-inflammatory, antiviral, neuroprotective and hypolipemic [11,14].
4.1. Ergogenic and Anti-Fatigue Activity
The activity of reducing the feeling of fatigue correlates with the strengthening and ergogenic effect, i.e., the improvement of physical performance. Cordycepin is the main bioactive compound with ergogenic potential in C. militaris. The ergogenic activity of cordycepin is related to its physiological function as indirect precursor of ATP and NO [11,12].
Cordycepin, similar to creatine, is indirect precursor of ATP. The ergogenic activity of creatine is supported by numerous pieces of scientific evidence. Creatine increases physical performance in successive bursts of short-term, high intensity exercise [64]. In a group of 11 men, who were physically active recreationally, immediate consumption of ATP at a dose of 400 mg/day contributed to the improvement of physical performance and oxygen and energy consumption in lower body exercises [65].
In Vivo Research
In experiments conducted on rodents, the anti-fatigue activity of C. militaris was demonstrated. In the swimming test, there was a prolongation of time to fatigue in mice. The reduction in fatigue caused by C. militaris in experimental animals was due to an increase in the concentration of ATP and antioxidant enzymes, a decrease in the concentration of lactate and ROS, and activation of the 5ʹ-AMP-activated kinase (AMPK) and AKT/mTOR pathways [66,67,68]. Mice fed with the extract of C. militaris with cordycepin showed an improvement in exercise performance in a grip strength test [69].
Hirsch et al. [70] demonstrated an improvement in exercise performance in recreationally active subjects following the administration of 4 g/day of a mushroom mixture (containing C. militaris) for 3 weeks. The effect of mushroom mixture with C. militaris on exercise capacity is associated with an improvement in maximum oxygen consumption (VO2max), an increase in time to exhaustion, and a reduction in lactic acid concentration.
Hirsch et al. [71] verified the effect of using a mushroom mixture (containing C. militaris) on physical performance during high-intensity exercise after 1 week and 3 weeks of supplementation. Twenty-eight recreationally active people in aged 18–27 years were subjected to this experiment. VO2max, time to exhaustion and ventilation threshold (VT) were measured during an exercise test on a cycling ergometer. The intake of the mushroom mixture with C. militaris improved the tolerance of effort in the performed exercises. In another study, Dudgeon et al. [72] demonstrated that the consumption of a mushroom mixture with C. militaris in a group of healthy subjects aged 19–34 years contributed to an improvement in physical endurance, an increase in time to exhaustion, an increase in VO2max and a decrease in blood lactate concentration. In the aforementioned scientific works, C. militaris was a component of a mixture of adaptogenic mushrooms (trade name PeakO2®), which also contained Ganoderma lucidum, Pleutorus eryngii, Lentinula edodes, Hericium erinaceus and Trametes versicolor [73].
4.2. Immunostimulating Activity
Extracts from fresh C. militaris fruiting bodies exhibit greater immunostimulatory activity (in vitro and in animal model) than those from dried fruiting bodies. There was no significant difference in the content of cordycepin and adenosine between fresh and dried raw material, but a significant discrepancy was noted in the concentration of polysaccharides, polyphenols and flavonoids, which were greater in fresh C. militaris [74].
It has been proven that, depending on the solvent used and thus the active substances contained in the C. militaris extract, there are two pathways to stimulate the immune system function. Water or ethanol extract (50%) of C. militaris containing polysaccharides stimulates type 1 immune response, whereas ethanol extract (70–80%) containing cordycepin stimulates type 2 immune response [75].
4.2.1. In Vitro Research
C. militaris extract containing cordycepin exhibits immunostimulatory effects on mouse macrophages. The mechanism of the immunostimulatory activity of C. militaris and cordycepin is based on the activation of macrophages to produce NO and proinflammatory cytokines IL-1β, IL-6, TNF-α and prostaglandin-2 (PGE2), as well as an increase in the activity of induced nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). The stimulation of macrophages to produce proinflammatory mediators was due to the activation of the nuclear transcription factor (NF-κB) by C. militaris/cordycepin [11,12].
Another experiment found that the CMP-W1 polysaccharide could stimulate the proliferation of lymphocytes [20]. The novel low-molecular-weight polysaccharide (CMPB90-1) isolated from C. militaris was shown to have immunostimulatory activity. In in vitro tests, the production of lymphocytes and NK cells and stimulation of phagocytosis by macrophages were demonstrated in mouse spleen cells treated with C. militaris polysaccharide [22].
It was shown that the CPMN Fr III polysaccharide (β-1,4-branched-β-1,6-galactoglucomannans) obtained from the cultured mycelia of C. militaris induced the production of NO, IL-1β and TNF-α by macrophage cells [76]. Furthermore, it was demonstrated that the polysaccharides found in fruiting bodies of C. militaris induced an immune response in murine macrophages. The experiment showed the intensification of the activity of NO, and TNF-α [77]. A novel polysaccharide (signed as PLCM) isolated from culture broth of C. militaris can induce immune response via MAPK and NF-κB signaling pathways [78].
4.2.2. In Vivo Research
Tests on rodents showed an increase in phagocytosis and an increase in the production of lymphocytes in spleen cells. The immunostimulatory activity was explained by the action of polysaccharides present in the tested mushroom material [14]. Immunostimulatory activity, through macrophage activation, was confirmed for the polysaccharide fraction from fruiting bodies of C. militaris [77].
Jeong et al. [79] demonstrated the immunomodulatory activity of cordycepin and C. militaris, which was associated with the inhibition of tumor growth in experimental mice. The study showed an increase in the production of IL-4, a decrease in the concentration of IL-2 and TGF-β and an increase in the level of T lymphocytes (CD4 and CD8).
In studies on healthy adult males supplemented with 1.5 g/day of C. militaris (in capsules) for 4 weeks, the immunostimulatory activity was found to be enhanced due to an increase in the levels of IL-2, IL-12, NK, TNF-α and IFN-γ [80].
Another experiment analyzed the effect of a 12-week supplementation of C. militaris on the course of upper respiratory tract infection and the immune response in a group of 100 patients aged 20–70 years. The study showed that the use of C. militaris did not have a significant effect on the frequency and symptoms of colds. However, an increase in NK cell activity and an increase in IgA concentration were observed, which indicated the immunostimulatory effect of C. militaris [81].
4.3. Antitumor Activity
4.3.1. In Vitro Research
In a study by Yoo et al. 2004 [82], the antitumor activity of C. militaris extract was confirmed. The cytotoxic activity of C. militaris aqueous extract against human breast cancer cells have been demonstrated. The promotion of apoptosis in tumor cells resulted from the activation of caspase-3 [83].
In scientific work by Park et al. [84], the authors demonstrated that C. militaris aqueous extract inhibits proliferation and induces apoptosis in human lung cancer cells. The antitumor activity of C. militaris was associated with an increase in the enzymatic activity of caspase-3, caspase-8 and caspase-9, and inhibition of the telomerase enzyme. The study reported an increase in the concentration of the Fas protein, which is associated with the “death receptor” of cancer cells.
Cordycepin and ergosterol isolated from C. militaris have shown antiproliferative activity against human colon cancer cells. Proliferative activity was associated with anti-inflammatory activity [85].
The in vitro cytotoxic activity of cordycepin extracted from C. militaris was tested. The results revealed that cordycepin inhibits the proliferation and migration of human bladder cancer cells. The cytotoxic activity of cordycepin has been linked with its effect on various signaling pathways, namely metalloproteinase-9 (MMP-9), TNF-α, NF-κB and protein activator-1 (AP-1) [86].
The apoptotic activity of C. militaris against glioblastoma tumor cells was also confirmed, and the C. militaris extract was shown to restrict the angiogenesis of human malignant melanoma cells [87,88]. In another study, the CMPS-II polysaccharide in the extract was shown to inhibit the growth of lung cancer cells [89].
A more recent study showed that cordycepin suppressed the growth of human liver cancer cells. The mechanism of antitumor activity of cordycepin has been linked with a reduction in the expression of the chemokine CxCR4, which promotes invasiveness and migration of liver cancer cells [90].
C. militaris induces apoptosis in ovarian cancer cells. The mechanism of antitumor activity was shown to involve the activation of TNF-α, TNFR1, NF-κB, caspase-3 and caspase-9, and reduction in the levels of Bcl-2 and BclxL [91].
C. militaris has been shown to induce apoptosis of cancer cells in non-small cell lung cancer. The basis of apoptotic activity its associated with inhibition of tectonic-3 protein (TCTN3) expression. Reduction in TCTN3 expression correlated with the suppression of different signaling pathways such as: Smoothened (SMO), Patched1 (PTCH1) and glioma-associated (GLI1) pathways. The aforementioned processes correlate with decreased Bcl-2, BclxL, and increased Bak, cleaved caspase-3 and caspase-9 levels [92].
4.3.2. In Vivo Research
Park et al. [84] reported that the aqueous extract of C. militaris reduced the mass and volume of the tumor and also extended the survival time (viability) of mice with lung cancer. Similar results were obtained by Jeong et al. [79], wherein C. militaris additionally enriched with cordycepin inhibited tumor growth and increased the viability of tumor-bearing mice. The study showed the immunotropic effect of cordycepin, which correlated with its antitumor activity.
4.4. Antioxidant Activity
The antioxidant activity was confirmed mainly for polysaccharides present in C. militaris. Few studies have shown the antioxidant properties of cordycepin [93]. The antioxidant activity of C. militaris may also be influenced by other chemical constituents present in fruiting bodies, e.g., ergothioneine, phenolic compounds, carotenoids and selenium [7,8,9].
4.4.1. In Vitro Research
The antioxidant potential of C. militaris in terms of inhibiting lipid peroxidation exceeds that of C. sinensis. The antioxidant activity was related to the content of polysaccharides and phenolic compounds in C. militaris fruiting bodies [94]. Previous studies confirmed the ability of the polysaccharide P70-1 and CBP-1 obtained from C. militaris to eliminate hydroxyl radicals [95,96]. The antioxidant activity and the ability to chelate iron ions (Fe2+) were also proven for the polysaccharide designated as CM-hs-CPS2 [97].
In many in vitro experiments, the antioxidant activity of the polysaccharide fractions was observed for the components WCBP50, CMP, CMP-1 and SeCSP-I [16,19,21,98]. The addition of selenium to the medium of C. militaris was found to increase the antioxidant activity of the polysaccharide fractions [63].
4.4.2. In Vivo Research
Experiments on rodents fed C. militaris containing polysaccharides showed an increase in the activity of enzymes with antioxidant potential, namely superoxide dismutase (SOD), catalase and GPX, and/or a reduction in the level of malondialdehyde (MDA) [14,99]. Antioxidant activity was also observed in other experiments on rodents and correlated with the neuroprotective or hepatoprotective activities, described in subsequent sections of this review.
4.5. Anti-Inflammatory Activity
4.5.1. In Vitro Research
In a model of lipopolysaccharide (LPS)-induced inflammation in macrophages, cordycepin was shown to reduce the expression of TNF-α, COX-2, iNOS and NF-κB [100]. In subsequent in vitro tests, the anti-inflammatory activity of C. militaris was confirmed to result from the inhibition of production of proinflammatory mediators, namely NO, TNF-α and IL-6, which were induced by LPS in murine macrophages. The group of bioactive compounds of C. militaris was not defined in the study [101].
It was proven through in vitro experiment that cordycepin and ergosterol from C. militaris inhibit the release of inflammatory mediators: NO, TNF-α and IL-12, which correlates with antiproliferative activity in colon tumor cells [85].
In vitro experiments using mouse microglia demonstrated that cordycepin (from C. militaris) inhibits the activity of COX-2 and iNOS enzymes and lowers the levels of inflammatory mediators: NO, TNF-α, PGE2 and IL-1β. Cordycepin was also shown to inhibit the activity of NF-κB and to inhibit the phosphorylation of mitogen-activated protein kinases (MAPKs). These results confirmed the neuroprotective effect of cordycepin and C. militaris extracts and have opened up new opportunities for further use of C. militaris in research and therapy of neurodegenerative diseases [102].
4.5.2. In Vivo Research
In an experimental mice model of colitis induction, the administration of C. militaris was shown to contribute to the inhibition of the activity of iNOS and TNF-α and the reduction in mast cell degranulation. The study, however, did not identify a specific group of bioactive compounds responsible for anti-inflammatory activity [103].
In scientific work Won and Park [104] was confirmed in vivo to ability of cordycepin contained in mycelium C. militaris to inhibit the activity of iNOS and reduce the concentration of NO in inflammatory reaction. The study also demonstrated analgesic activity.
In a study conducted on mice, Smiderle et al. [105], concluded that a polysaccharide with a linear structure of β-(1 → 3)-D-glucan obtained from C. militaris exhibited anti-inflammatory and antinociceptive activity. The mechanism of the biological activity of this polysaccharide resulted from its inhibition of COX-2, TNF-α and IL-1β activity. The anti-inflammatory and antinociceptive activity of the polysaccharide was similar to that of the reference compounds from the group of non-steroidal anti-inflammatory drugs (acetylsalicylic acid, indomethacin and diclofenac).
Cerebrosides, a new group of compounds, were indicated as a component with anti-inflammatory activity. The mechanism of suppression of the inflammatory process was associated with the inhibition of expression of COX-2, iNOS, NF-κB, IL-1β and IL-6 [106].
Cai et al. and Gai et al. [107,108] confirmed the possibility of using C. militaris for treating chronic bronchitis in humans.
4.6. Hypoglycemic Activity
In experiments on rats, C. militaris extract was found to influence the use of glucose by tissues and reduce insulin resistance. In another study on rats, the hypoglycemic activity of C. militaris extract containing polysaccharides was demonstrated [14]. Furthermore, the ability of the LCMPs-II polysaccharide to inhibit the α-glucosidase enzyme was demonstrated by Zhu et al. [18]. In contrast, the cerebroside fraction inhibited PTP1B activity [109]. Exopolysaccharide (EPS III) isolated from culture broth of C. militaris demonstrated hypoglycemic activity in streptozotocin-induced diabetic mice. EPS III showed antagonist activity against the enzyme on α-glucosidase with the dose–effect relationship [110].
In an animal model on diabetes, 21 days of treatment with cordycepin (from C. militaris) in alloxan-induced diabetic mice contributed to the improvement of many symptoms of metabolic syndrome, including regulation of glucose tolerance and glucose absorption, and decreased blood glucose concentration [111]. Current scientific studies also indicate the potential of cordycepin in the treatment of diabetes by the regulation of expression liver proteins such as Nfat3, Flcn and Psma3. These proteins are correlated with generation of energy (ATP), AMPK signaling pathway and ubiquitin proteasome system (UPS) [112].
4.7. Antimicrobial Activity
Cordymin is a peptide isolated from C. militaris. It was shown to have antifungal and antiviral activity in in vitro tests. Cordymin inhibited the growth of various fungal species, including Bipolaris maydis and Candida albicans. It also inhibited reverse transcriptase of human immunodeficiency virus (HIV). Several scientific studies have confirmed the anti-inflammatory and antinociceptive activity of cordymin [59].
In vitro studies have demonstrated the antiviral activity of cordycepin and its derivatives. The antiviral activity was confirmed for the influenza virus, Epstein–Barr virus (EBV), herpes simplex virus (HSV) and HIV. The mechanism of antiviral activity of cordycepin is related to the inhibition of reverse transcriptase and RNA polymerase of the virus [113,114,115].
In the United States in 2020, the Food and Drug Administration (FDA) repurposed potential and investigational drug or molecule candidates and included cordycepin as a chemical structure with antiviral activity. Precursor research in India on cordycepin created new possibilities for the use of this molecule in the treatment of COVID-19. Cordycepin exhibited strong chemical interactions with SARS-CoV-2, with receptor-binding domain (RBD) at the spike protein (S) and the main protease (Mpro) of the virus in in silico study. Anti-SARS-CoV-2 mechanisms of the action of cordycepin correlated with inhibited viral replication [116]. Another in silico study using pharmacological and molecular modeling, simulation and estimation indicated that cordycepin is the potential inhibitor for RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 [117].
4.8. Effect of C. militaris on the Endocrine System
C. militaris has been shown to stimulate hormonal activity in rodents. There has been an increase in testosterone levels in plasma [118,119].
Cordycepin has been shown to stimulate steroidogenesis in rodents and increase testosterone and progesterone levels [120].
Sohn et al. [121] showed that the administration of cordycepin (from C. militaris) in a group of experimental rats improved testicular function, stimulated spermatogenesis and increased sperm motility. The study also showed that cordycepin administration increased calcium concentration and decreased urea and creatinine levels in the blood of rats. Similar results in terms of improvement in sperm quality were reported in rodent studies by Kopalli et al. [122].
Improvement of quality parameters of semen in infertile boars to which C. militiaris mycelium was given has been demonstrated. The improvement was connected with the increase in the amount of sperm and its motility [123].
In vitro and in vivo experiments on rodents revealed that the C. militaris extract containing cordycepin reduced the proliferation of prostate cells and regulated the concentration of androgens [124].
Research concerning the effect of Cordyceps spp. or cordycepin on the endocrine system of human objects are limited. The promising effects of Cordyceps spp. or cordycepin on steroidogenesis in rodents have not yet been confirmed in humans. In a group of young male adults, it has been proven that 8-week supplementation of 2.4 g per day of C. sinensis (containing 5.92 µmol/g of adenosine, 1.23 µmol/g of cordycepin and 8.81 µmol/g of ergosterol) does not significantly affect testosterone levels in volunteers. So far, no studies have been conducted to analyze the effect of C. militaris on testosterone concentration in men. One can only speculate in the area of potential results due to the fact that C. miliatris presents a higher concentration of cordycepin than C. sinensis [125].
4.9. Effect of C. militaris on the Respiratory System
In vitro experiments provided evidence that cordycepin from C. militaris affects the transport of sodium, potassium and chloride ions in the epithelial cells of the respiratory tract [126].
Early publications found that extracts from fruiting bodies of C. militaris are less effective than the reference compounds—prednisolone and montelukast—in reducing the inflammatory process in a mouse model of asthma. C. militaris extracts showed negligible effects on the reduction in IgE concentration, eosinophilia and inhibition of leukotriene synthesis [127]. However, later scientific works demonstrated in an asthma model in mice that cordycepin alleviates airway hyperresponsiveness, reduces inflammation and decreases IgE and eosinophil levels. A decrease in the expression of IL-4, IL-5, IL-13 and NF-κB was observed [12].
Cordycepin was also shown to reduce airway remodeling in an asthma model in rats. A decrease in the levels of IgE, eosinophils and neutrophils and a decrease in the expression of TNF-α, TGF-β1, IL-5 and IL-13 were also observed [128].
The polysaccharide, designated as CPS, reduced IgE levels in a mouse model of asthma, inhibited cell proliferation and infiltration and alleviated inflammation and airway hyperresponsiveness. CPS also inhibited the secretion of IL-4, IL-5, IL-13 and IFN-γ and reduced the expression of TGF-β1 [129].
In human studies by Cai et al. and Gai et al. [107,108] some efficacy of C. militaris was demonstrated in the treatment of chronic bronchitis. Jung et al. [81] found that although C. militaris did not have a significant effect on the course of upper respiratory tract infection in a group of volunteers, an immunostimulatory effect was observed.
4.10. Effect of C. militaris on the Locomotor System
Scientific research indicated the potential benefits of C. militaris in the treatment of osteoporosis. An in vitro experiment showed that C. militaris inhibited osteoclast differentiation and reduced the expression of genes encoding this process [130]. It has been proved that cordycepin exhibits anti-inflammatory activity in human osteoarthritis chondrocytes. Cordycepin inhibited the production of PGE2 and NO, and decreased the expression of NF-κB, induced by IL-1β [131].
In an inflammatory-induced osteoporosis model in experimental rats, cordycepin showed anti-inflammatory activity and limited bone loss [132].
Cordycepin and C. militaris inhibited osteoclast differentiation in in vitro tests. The mechanism of their biological activity has been linked to the inhibition of the NF-κB ligand receptor activator (RANKL). In addition, the mRNA expression of genes related to osteoclastogenesis (TRAP, Cathepsin K, MMP-9 and NFATc1) was also inhibited by CME and cordycepin. In addition, cordycepin significantly inhibited RANKL-induced p38 and NF-κB phosphorylation. Moreover, in a mouse model of LPS-induced osteoporosis, cordycepin and C. militaris limited bone loss [133]. In an experimental model of osteoarthritis in rats, cordycepin as a polyadenylation inhibitor was shown to reduce pain and inflammation in the synovium [134].
4.11. Effect of C. militaris on the Nervous System
The neuroprotective effect of cordycepin was confirmed in an in vitro study on mouse microglia cells. The protective effect of cordycepin on neurons was dependent on its anti-inflammatory activity [102] or antioxidant activity [135].
Lee et al. [136] proved that C. militaris antagonized the scopolamine-induced memory deficit effect in experimental rats. The neuroprotective effect of C. militaris was also confirmed in a rodent model of dementia and ischemic brain damage [137]. Yuan et al. [138] showed that a polypeptide isolated from C. militaris improves memory in mice. Decreased activity of acetylcholinesterase (AChE) and enhanced GABA neurotransmission were also detected. Field work also showed antioxidant and neuroprotective activity, increased SOD activity and reduced MDA concentration. In a model of Alzheimer’s disease induced by the β-amyloid protein (Aβ1-42) in experimental mice, C. militaris improved procognitive functions in the following tests: water maze, new route and object recognition tests [135]. In rodents treated with doxorubicin chemotherapy, C. militaris extract was shown to reduce chemotherapy-induced oxidative stress. Postmortem of the experimental rodents showed that the C. militaris extract increased the concentration of ATP in the brain and inhibited the activity of AChE [139].
One study verified the effect of supplementation of a mushroom beverage (containing C. militaris with cordycepin, polysaccharides and mannitol) on the regulation of emotions in humans. The potential effect on alleviating the symptoms of depression has not been confirmed so far; that is, the results of the research have not been published yet [140].
4.12. Effect of C. militaris on the Cardiovascular System
Cordycepin is a candidate for development of potential antiatherosclerotic agents, because it improves vascular responses in smooth muscle cells [141].
In experiments with rodents, cordycepin from C. militaris has been shown to lower triglycerides, total cholesterol, low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) level, in an animal model of hyperlipidemia. Cordycepin showed the features of an AMPK activator and an inhibitor of lipoprotein and hepatic lipase [142,143].
Cordycepin inhibited adipogenesis and lipid deposition in adipocytes in in vitro experiments. The mechanism of the biological activity of cordycepin was found to be associated with the suppression of the C/EBPβ, PPARγ and mTORC1 pathways and activation of AMPK [144]. The hypolipidemic activity of cordycepin and its ability to activate AMPK, the γ1 region, were also confirmed in in vitro tests [145].
An in vitro experiment and an in vivo study in rodents also confirmed the hypolipidemic activity of the polysaccharide fractions isolated from C. militaris [146,147]. Similarly, Huang et al. [148] demonstrated the hypolipidemic activity of intracellular (IPCM) and extracellular polysaccharides (EPCM) from C. militaris in mice fed a high-fat diet.
An in vitro experiment confirmed the antiaggregation activity of cordycepin isolated from C. militaris. Inhibition of aggregation of human platelets resulted from an increase in the activity of cyclic guanosine-3ʹ,5ʹ-monophosphate (cGMP) and cyclic adenosine-3ʹ,5ʹ-monophosphate (cAMP), a decrease in the intracellular concentration of calcium ions and inhibition of the synthesis of thromboxane A2 (TXA2) by cordycepin [149]. Cordycepin exhibited cardioprotective activity in an isolated ischemic rat heart [150].
In an in vivo experiment conducted in mice, the sodium nitrite-induced toxicity test and the ischemia test showed that the fraction of CMN1 polysaccharides isolated from fruiting bodies of C. militaris prevented hypoxia [17].
C. militaris extract enriched with cordycepin inhibited collagen-induced platelet aggregation in in vitro tests. The antiaggregation activity resulted from the inhibition of fibrinogen attachment to glycoprotein IIb/IIIa, as well as the stimulation of VASP phosphorylation, inhibition of PI3K/A-kinase phosphorylation and increase in cAMP concentration [151].
Further in vitro and in vivo experiments demonstrated the neuroprotective effect of C. militaris (WIB801C) extract with a quantified cordycepin concentration. Cordycepin reduced the ischemic area, reduced brain swelling and restricted damage to the blood–brain barrier in experimental rats. The neuroprotective effect was related to the anti-inflammatory activity of cordycepin [152]. More recent studies indicate greater antiplatelet activity than anticoagulant activity of C. militaris [153].
4.13. Other Biological Activity of C. militaris
In in vitro experiments, C. sinensis showed a greater ability than C. militaris to affect the gut microbiota [154].
In rodent experiments, C. militaris polysaccharides minimized damage to hepatocytes. Hepatoprotective activity of C. militaris extract correlated with its antioxidant and hypolipemic activity [147]. In a study conducted in patients with impaired liver function, supplementation with 1.5 g/day C. militaris (cordycepin) for 8 weeks protected the liver against lipid accumulation [155].
The nephroprotective activity of C. militaris was demonstrated by inhibiting the hypertrophy of glomerular cells induced by LDL in in vitro [156]. The limitation of proliferation on glomerular mesangium cells was confirmed for C. militaris extracts [157]. The nephroprotective effect of C. militaris or cordycepin was confirmed in a diabetic animal model in mice [111,158].
5. Safety Assessment and Toxicology
In vivo experiments showed that high-dose administration of C. militaris (4.9 × 108 spores/mouse) for 7 days in rodents did not induce toxic effects. No significant differences were observed between the control group and the experimental group fed with C. militaris in terms of body weight, blood hematological parameters, and food and water consumption [159].
In experimental rats, no toxic effect of C. militaris was observed at the dose of 3000 mg/kg/day in the sub-acute oral toxicity test. No genotoxic and teratogenic effects were found. In the sub-chronic toxicity test (90-day) in experimental rats fed with C. militaris, no changes were found in clinical, histopathological and hematological parameters. The no-observed-adverse-effect level (NOAEL) was determined for C. militaris mycelium at 4000 mg/kg/day in experimental rats [160]. The maximum tolerance dose of cordycepin has been confirmed at 3600 mg/kg/day in mice without any adverse effects [111].
In human studies, C. militaris supplementation did not correlate with the occurrence of serious adverse events. Few of the reported mild adverse events were associated with gastrointestinal disturbances (Table 2).
Table 2.
Details of C. militaris research on human participants.
| Research Group | Quantity of Raw Material C. militaris |
Supplementation Time | Biological Activity | Bioactive Compound | Adverse Events | References |
|---|---|---|---|---|---|---|
| 10 healthy individuals of both sexes aged 19–24 years | 4 g/day 1 | 3 weeks | Ergogenic | Undefined | Undefined | [70] |
| 28 healthy individuals of both sexes aged 18–27 years | 4 g/day 1 | 1–3 weeks | Ergogenic | Undefined | Undefined | [71] |
| 43 healthy individuals of both sexes aged 19–34 years | 1–12 g/day 1 | 1–4 weeks | Ergogenic | Undefined | Gastrointestinal | [72] |
| 79 healthy males aged 19–64 years | 1.5 g/day | 4 weeks | Immunostimulation | Cordycepin | No serious adverse events | [80] |
| 100 healthy volunteers aged 20–70 years | 1.5 g/day | 12 weeks | Immunostimulation | Cordycepin | No serious adverse events | [81] |
| 510 patients with chronic bronchitis | Undefined 2 | Undefined | Anti-inflammatory | Undefined | Undefined | [107] |
| 425 patients with chronic bronchitis | 3 g/day 2 | 8 weeks | Anti-inflammatory | Undefined | Undefined | [108] |
| 57 patients with mild hepatic impairment aged 31–61 years | 1.5 g/day | 8 weeks | Hepatoprotective | Cordycepin | No serious adverse events | [155] |
1 Adaptogenic mushroom mixture (trade name: PeakO2®) 2 Access to publications only as abstract.
Beauveriolides and militarinones are among the compounds present in C. militaris with cytotoxic potential. Pentostatin and cordycepin have been shown to exert gastrotoxic effects. Pentostatin is also toxic to the lungs and nervous system [57].
Bai and Sheu [161] isolated an 18-kDa nonglycosylated protein (CMP) that induces mitochondrial-dependent apoptosis in murine cells. CMP is unstable and degrades in a high temperature or alkaline environment. Heat treatment of C. militaris is therefore necessary to deactivate CMP and further process the C. militaris raw material for food, dietary supplements and medicines.
Heavy metals such as cadmium, lead, mercury and arsenic pose toxicological hazards to human health. Although these metals may be accumulated in the fruiting bodies of various species of fungi, these do not occur in mushrooms used for cultivation. A quantitative analysis of heavy metals in fruiting bodies of wild-growing C. militaris showed that lead concentration did not exceed 0.2 mg/kg dry weight, whereas mercury concentration did not exceed 0.5 mg/kg dry weight. Relatively high levels of arsenic and cadmium were noted, amounting to 0.4 mg/kg and 0.2 mg/kg dry weight, respectively [7,162].
6. Conclusions
Strengthening endurance of the human body includes stimulation of immune functions and reduction in fatigue. To summarize, the presence of several bioactive compounds in edible fungi such as C. militaris makes them worthy to be considered as a functional food. The inclusion of C. militaris extract in the diet can have several health benefits; moreover, the consumption of many mushroom species that can be grown on household waste will popularize the mushroom sector in the sustainable economy of many regions of the world. At the same time, a large number of bioactive compounds hampers clinical trials and creates problems for designing drugs based on mushroom extracts. A single substance, however, may have a less therapeutic effect and may not show synergistic effects with other compounds present in mushroom fruiting bodies. Among all bioactive substances mentioned in the studies, the greatest therapeutic potential is associated with cordycepin. Recent scientific reports indicate the potential of cordycepin as antiviral agent against SARS-CoV-2 in COVID-19.
Author Contributions
Conceptualization, B.M. and K.J.J.; data curation, K.J.J., J.L. and B.M.; writing—original draft preparation, K.J.J.; writing—review and editing, K.J.J. and B.M.; project administration B.M.; B.M., J.L. and K.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Das S.K., Masuda M., Sakurai A., Sakakibara M. Medicinal Uses of the Mushroom Cordyceps militaris: Current State and Prospects. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Holliday J. Medicinal Plants and Fungi: Recent Advances in Research and Development. Springer; Singapore: 2017. Cordyceps: A Highly Coveted Medicinal Mushroom; pp. 59–91. [Google Scholar]
- 3.European Commission. [(accessed on 15 August 2021)]. Available online: https://ec.europa.eu/search/
- 4.Reis F.S., Martins A., Vasconcelos M.H., Morales P., Ferreira I.C.F.R. Functional Foods Based on Extracts or Compounds Derived from Mushrooms. Trends Food Sci. Technol. 2017;66:48–62. doi: 10.1016/j.tifs.2017.05.010. [DOI] [Google Scholar]
- 5.Cheong P.C.H., Tan C.S., Fung S.Y. Biology of Macrofungi. Fungal Biology. Springer; Cham, switzerland: 2018. Medicinal Mushrooms: Cultivation and Pharmaceutical Impact; pp. 287–304. [Google Scholar]
- 6.Wang J., Kan L., Nie S., Chen H., Cui S.W., Phillips A.O., Phillips G.O., Li Y., Xie M. A Comparison of Chemical Composition, Bioactive Components and Antioxidant Activity of Natural and Cultured Cordyceps sinensis. LWT. 2015;63:2–7. doi: 10.1016/j.lwt.2015.03.109. [DOI] [Google Scholar]
- 7.Cohen N., Cohen J., Asatiani M.D., Varshney V.K., Yu H.-T., Yang Y.-C., Li Y.-H., Mau J.-L., Wasser S.P. Chemical Composition and Nutritional and Medicinal Value of Fruit Bodies and Submerged Cultured Mycelia of Culinary-Medicinal Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms. 2014;16:273–291. doi: 10.1615/IntJMedMushr.v16.i3.80. [DOI] [PubMed] [Google Scholar]
- 8.Chen S.Y., Ho K.J., Hsieh Y.J., Wang L.T., Mau J.L. Contents of Lovastatin, γ-Aminobutyric Acid and Ergothioneine in Mushroom Fruiting Bodies and Mycelia. LWT. 2012;47:274–278. doi: 10.1016/j.lwt.2012.01.019. [DOI] [Google Scholar]
- 9.Chan J.S.L., Barseghyan G.S., Asatiani M.D., Wasser S.P. Chemical Composition and Medicinal Value of Fruiting Bodies and Submerged Cultured Mycelia of Caterpillar Medicinal Fungus Cordyceps militaris CBS-132098 (Ascomycetes) Int. J. Med. Mushrooms. 2015;17:649–659. doi: 10.1615/IntJMedMushrooms.v17.i7.50. [DOI] [PubMed] [Google Scholar]
- 10.Wu X.F., Zhang M., Li Z. Influence of Infrared Drying on the Drying Kinetics, Bioactive Compounds and Flavor of Cordyceps militaris. LWT. 2019;111:790–798. doi: 10.1016/j.lwt.2019.05.108. [DOI] [Google Scholar]
- 11.Tuli H.S., Sandhu S.S., Sharma A.K. Pharmacological and Therapeutic Potential of Cordyceps with Special Reference to Cordycepin. 3 Biotech. 2014;4:1–12. doi: 10.1007/s13205-013-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin P., Li X., Yang H., Wang Z.-Y., Lu D. Therapeutic Potential and Biological Applications of Cordycepin and Metabolic Mechanisms in Cordycepin-Producing Fungi. Molecules. 2019;24:2231. doi: 10.3390/molecules24122231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shawkat H., Westwood M.-M., Mortimer A. Mannitol: A Review of Its Clinical Uses. BJA Edu. 2012;12:82–85. doi: 10.1093/bjaceaccp/mkr063. [DOI] [Google Scholar]
- 14.Zhang J., Wen C., Duan Y., Zhang H., Ma H. Advance in Cordyceps militaris (Linn) Link Polysaccharides: Isolation, Structure, and Bioactivities: A Review. Int. J. Biol. Macromol. 2019;132:906–914. doi: 10.1016/j.ijbiomac.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.S., Kwon J.S., Won D.P., Lee K.E., Shin W.C., Hong E.K. Study on Macrophage Activation and Structural Characteristics of Purified Polysaccharide from the Liquid Culture Broth of Cordyceps militaris. Carbohydr. Polym. 2010;82:982–988. doi: 10.1016/j.carbpol.2010.06.025. [DOI] [Google Scholar]
- 16.Chen X., Wu G., Huang Z. Structural Analysis and Antioxidant Activities of Polysaccharides from Cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013;58:18–22. doi: 10.1016/j.ijbiomac.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y., Hu S., Liu C., Meng Q., Song J., Lu J., ChenG Y., Gao C., Liu Y., Wang D., et al. Purification of Polysaccharides from Cordyceps militaris and Their Anti-Hypoxic Effect. Mol. Med. Rep. 2015;11:1312–1317. doi: 10.3892/mmr.2014.2786. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Z.Y., Guo M.Z., Liu F., Luo Y., Chen L., Meng M., Wang X.T., Zhang Y.M. Preparation and Inhibition on α-D-Glucosidase of Low Molecular Weight Polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2016;93:27–33. doi: 10.1016/j.ijbiomac.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Z.Y., Liu F., Gao H., Sun H., Meng M., Zhang Y.M. Synthesis, Characterization and Antioxidant Activity of Selenium Polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2016;93:1090–1099. doi: 10.1016/j.ijbiomac.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 20.Luo X., Duan Y., Yang W., Zhang H., Li C., Zhang J. Structural Elucidation and Immunostimulatory Activity of Polysaccharide Isolated by Subcritical Water Extraction from Cordyceps militaris. Carbohydr. Polym. 2017;157:794–802. doi: 10.1016/j.carbpol.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 21.Jing Y., Cui X., Chen Z., Huang L., Song L., Liu T., Lv W., Yu R. Elucidation and Biological Activities of a New Polysaccharide from Cultured Cordyceps militaris. Carbohydr. Polym. 2014;102:288–296. doi: 10.1016/j.carbpol.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 22.Bi S., Jing Y., Zhou Q., Hu X., Zhu J., Guo Z., Song L., Yu R. Structural Elucidation and Immunostimulatory Activity of a New Polysaccharide from Cordyceps militaris. Food Funct. 2018;9:279–293. doi: 10.1039/C7FO01147D. [DOI] [PubMed] [Google Scholar]
- 23.Boonstra E., de Kleijn R., Colzato L.S., Alkemade A., Forstmann B.U., Nieuwenhuis S. Neurotransmitters as Food Supplements: The Effects of GABA on Brain and Behavior. Front. Psychol. 2015;6:1520. doi: 10.3389/fpsyg.2015.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hepsomali P., Groeger J.A., Nishihira J., Scholey A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020;14:923. doi: 10.3389/fnins.2020.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Zhang Z., Liu X., Wang Y., Mao F., Mao J., Lu X., Jiang D., Wan Y., Lv J.-Y., et al. Study of GABA in Healthy Volunteers: Pharmacokinetics and Pharmacodynamics. Front. Pharmacol. 2015;6:260. doi: 10.3389/fphar.2015.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamatsu A., Yamashita Y., Pandharipande T., Maru I., Kim M. Effect of Oral γ-Aminobutyric Acid (GABA) Administration on Sleep and Its Absorption in Humans. Food Sci. Biotechnol. 2016;25:547–551. doi: 10.1007/s10068-016-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muszyńska B., Kała K., Rojowski J., Grzywacz A., Opoka W. Composition and Biological Properties of Agaricus bisporus Fruiting Bodies- a Review. Polish J. Food Nutr. Sci. 2017;67:173–181. doi: 10.1515/pjfns-2016-0032. [DOI] [Google Scholar]
- 28.Dubost N.J., Ou B., Beelman R.B. Quantification of Polyphenols and Ergothioneine in Cultivated Mushrooms and Correlation to Total Antioxidant Capacity. Food Chem. 2007;105:727–735. doi: 10.1016/j.foodchem.2007.01.030. [DOI] [Google Scholar]
- 29.Weigand-Heller A.J., Kris-Etherton P.M., Beelman R.B. The Bioavailability of Ergothioneine from Mushrooms (Agaricus bisporus) and the Acute Effects on Antioxidant Capacity and Biomarkers of Inflammation. Prev. Med. 2012;54:S75–S78. doi: 10.1016/j.ypmed.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Borodina I., Kenny L.C., McCarthy C.M., Paramasivan K., Pretorius E., Roberts T.J., van der Hoek S.A., Kell D.B. The Biology of Ergothioneine, an Antioxidant Nutraceutical. Nutr. Res. Rev. 2020;33:190–217. doi: 10.1017/S0954422419000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheah I.K., Tang R.M.Y., Yew T.S.Z., Lim K.H.C., Halliwell B. Administration of Pure Ergothioneine to Healthy Human Subjects: Uptake, Metabolism, and Effects on Biomarkers of Oxidative Damage and Inflammation. Antioxid. Redox Signal. 2017;26:193–206. doi: 10.1089/ars.2016.6778. [DOI] [PubMed] [Google Scholar]
- 32.Sotgia S., Zinellu A., Mangoni A.A., Pintus G., Attia J., Carru C., McEvoy M. Clinical and Biochemical Correlates of Serum L-Ergothioneine Concentrations in Community-Dwelling Middle-Aged and Older Adults. PLoS ONE. 2014;9:e84918. doi: 10.1371/journal.pone.0084918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheah I.K., Feng L., Tang R.M.Y., Lim K.H.C., Halliwell B. Ergothioneine Levels in an Elderly Population Decrease with Age and Incidence of Cognitive Decline; a Risk Factor for Neurodegeneration? Biochem. Biophys. Res. Commun. 2016;478:162–167. doi: 10.1016/j.bbrc.2016.07.074. [DOI] [PubMed] [Google Scholar]
- 34.Dong J.Z., Wang S.H., Ai X.R., Yao L., Sun Z.W., Lei C., Wang Y., Wang Q. Composition and Characterization of Cordyxanthins from Cordyceps militaris Fruit Bodies. J. Funct. Foods. 2013;5:1450–1455. doi: 10.1016/j.jff.2013.06.002. [DOI] [Google Scholar]
- 35.Muszyńska B., Mastej M., Sułkowska-Ziaja K. Biological Function of Carotenoids and Their Occurrence in the Fruiting Bodies of Mushrooms. MIR. 2016;107:113–122. [Google Scholar]
- 36.Bovier E.R., Hammond B.R. A Randomized Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Visual Processing Speed in Young Healthy Subjects. Arch. Biochem. Biophys. 2015;572:54–57. doi: 10.1016/j.abb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Renzi-Hammond L., Bovier E., Fletcher L., Miller L., Mewborn C., Lindbergh C., Baxter J., Hammond B. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients. 2017;9:1246. doi: 10.3390/nu9111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stringham N.T., Holmes P.V., Stringham J.M. Supplementation with Macular Carotenoids Reduces Psychological Stress, Serum Cortisol, and Sub-Optimal Symptoms of Physical and Emotional Health in Young Adults. Nutr. Neurosci. 2018;21:286–296. doi: 10.1080/1028415X.2017.1286445. [DOI] [PubMed] [Google Scholar]
- 39.Watzl B., Bub A., Briviba K., Rechkemmer G. Supplementation of a Low-Carotenoid Diet with Tomato or Carrot Juice Modulates Immune Functions in Healthy Men. Ann. Nutr. Metab. 2003;47:255–261. doi: 10.1159/000072397. [DOI] [PubMed] [Google Scholar]
- 40.Gul K., Tak A., Singh A.K., Singh P., Yousuf B., Wani A.A. Chemistry, Encapsulation, and Health Benefits of β-Carotene-A Review. Cogent Food Agric. 2015;1:1018696. doi: 10.1080/23311932.2015.1018696. [DOI] [Google Scholar]
- 41.Druesne-Pecollo N., Latino-Martel P., Norat T., Barrandon E., Bertrais S., Galan P., Hercberg S. Beta-carotene Supplementation and Cancer Risk: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Int. J. Cancer Res. 2010;127:172–184. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 42.Gajendragadkar P.R., Hubsch A., Mäki-Petäjä K.M., Serg M., Wilkinson I.B., Cheriyan J. Effects of Oral Lycopene Supplementation on Vascular Function in Patients with Cardiovascular Disease and Healthy Volunteers: A Randomised Controlled Trial. PLoS ONE. 2014;9:e99070. doi: 10.1371/journal.pone.0099070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senkus K.E., Tan L., Crowe-White K.M. Lycopene and Metabolic Syndrome: A Systematic Review of the Literature. Adv. Nutr. 2019;10:19–29. doi: 10.1093/advances/nmy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirahmadi M., Azimi-Hashemi S., Saburi E., Kamali H., Pishbin M., Hadizadeh F. Potential Inhibitory Effect of Lycopene on Prostate Cancer. Biomed. Pharmacother. 2020;129:110459. doi: 10.1016/j.biopha.2020.110459. [DOI] [PubMed] [Google Scholar]
- 45.Joshi M., Sagar A., Kanwar S.S., Singh S. Anticancer, Antibacterial and Antioxidant Activities of Cordyceps militaris. Indian J. Exp. Biol. 2019;57:15–20. [Google Scholar]
- 46.Choi E.-J., Park B., Lee J., Kim J. Anti-Atopic Dermatitis Properties of Cordyceps militaris on TNFα/IFNγ-Stimulated HaCaT Cells and Experimentally Induced Atopic Dermatitis in Mice. Phys. Act. Nutr. 2020;24:7. doi: 10.20463/pan.2020.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barros L., Venturini B.A., Baptista P., Estevinho L.M., Ferreira I.C.F.R. Chemical Composition and Biological Properties of Portuguese Wild Mushrooms: A Comprehensive Study. J. Agric. Food Chem. 2008;56:3856–3862. doi: 10.1021/jf8003114. [DOI] [PubMed] [Google Scholar]
- 48.Oesterle A., Laufs U., Liao J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tobert J.A. Efficacy and Long-Term Adverse Effect Pattern of Lovastatin. Am. J. Cardiol. 1988;62:J28–J34. doi: 10.1016/0002-9149(88)90004-5. [DOI] [PubMed] [Google Scholar]
- 50.Muszyńska B., Sułkowska-Ziaja K., Ekiert H. Phenolic Acids in Selected Edible Basidiomycota Species: Armillaria mellea, Boletus badius, Boletus edulis, Cantharellus cibarius, Lactarius deliciosus and Pleurotus ostreatus. Acta Sci. Pol. Hortorum Cultus. 2013;12:107–116. [Google Scholar]
- 51.Çayan F., Deveci E., Tel-Çayan G., Duru M.E. Identification and Quantification of Phenolic Acid Compounds of Twenty-Six Mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020;14:1690–1698. doi: 10.1007/s11694-020-00417-0. [DOI] [Google Scholar]
- 52.Reis F.S., Barros L., Calhelha R.C., Ćirić A., van Griensven L.J.L.D., Soković M., Ferreira I.C.F.R. The Methanolic Extract of Cordyceps militaris (L.) Link Fruiting Body Shows Antioxidant, Antibacterial, Antifungal and Antihuman Tumor Cell Lines Properties. Food Chem. Toxicol. 2013;62:91–98. doi: 10.1016/j.fct.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 53.Huang S.J., Lin C.P., Mau J.L., Li Y.S., Tsai S.Y. Effect of UV-B irradiation on physiologically active substance content and antioxidant properties of the medicinal caterpillar fungus Cordyceps militaris (Ascomycetes) Int J. Med. Mushrooms. 2015;17:241–253. doi: 10.1615/IntJMedMushrooms.v17.i3.40. [DOI] [PubMed] [Google Scholar]
- 54.Awang M.A., Daud N.N.N.N.M., Ismail N.I.M., Cheng P.G., Ismail M.F., Ramaiya S.D. Antioxidant and Cytotoxicity Activity of Cordyceps Militaris Extracts against Human Colorectal Cancer Cell Line. J App Pharm Sci. 2021;11:105–109. [Google Scholar]
- 55.Singh R.S., Bhari R., Kaur H.P. Mushroom Lectins: Current Status and Future Perspectives. Crit. Rev. Biotechnol. 2010;30:99–126. doi: 10.3109/07388550903365048. [DOI] [PubMed] [Google Scholar]
- 56.Hassan M., Rouf R., Tiralongo E., May T., Tiralongo J. Mushroom Lectins: Specificity, Structure and Bioactivity Relevant to Human Disease. Int. J. Mol. Sci. 2015;16:7802–7838. doi: 10.3390/ijms16047802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen B., Sun Y., Luo F., Wang C. Bioactive Metabolites and Potential Mycotoxins Produced by Cordyceps Fungi: A Review of Safety. Toxins. 2020;12:410. doi: 10.3390/toxins12060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsimberidou A., Giles F., Duvic M., Fayad L., Kurzrock MD R. Phase II Study of Pentostatin in Advanced T-cell Lymphoid Malignancies: Update of an MD Anderson Cancer Center Series. Cancer. 2004;100:342–349. doi: 10.1002/cncr.11899. [DOI] [PubMed] [Google Scholar]
- 59.Wong J.H., Ng T.B., Wang H., Sze S.C.W., Zhang K.Y., Li Q., Lu X. Cordymin, an Antifungal Peptide from the Medicinal Fungus Cordyceps militaris. Phytomed. 2011;18:387–392. doi: 10.1016/j.phymed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Commission Regulation (EU) No 432/2012 Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. OJEU. 2012;136:1–40. [Google Scholar]
- 61.Dong J.Z., Lei C., Ai X.R., Wang Y. Selenium Enrichment on Cordyceps militaris Link and Analysis on Its Main Active Components. Appl. Biochem. 2012;166 doi: 10.1007/s12010-011-9506-6. [DOI] [PubMed] [Google Scholar]
- 62.Dong J.Z., Ding J., Yu P.Z., Lei C., Zheng X.J., Wang Y. Composition and Distribution of the Main Active Components in Selenium-Enriched Fruit Bodies of Cordyceps militaris Link. Food Chem. 2013;137:164–167. doi: 10.1016/j.foodchem.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Hu T., Liang Y., Zhao G., Wu W., Li H., Guo Y. Selenium Biofortification and Antioxidant Activity in Cordyceps militaris Supplied with Selenate, Selenite, or Selenomethionine. Biol. Trace Elem. Res. 2019;187:553–561. doi: 10.1007/s12011-018-1386-y. [DOI] [PubMed] [Google Scholar]
- 64.Maughan R.J., Burke L.M., Dvorak J., Larson-Meyer D.E., Peeling P., Phillips S.M., Rawson E.S., Walsh N.P., Garthe I., Geyer H. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018;28:104–125. doi: 10.1123/ijsnem.2018-0020. [DOI] [PubMed] [Google Scholar]
- 65.Freitas M.C., Cholewa J.M., Gerosa-Neto J., Gonçalves D.C., Caperuto E.C., Lira F.S., Rossi F.E. A Single Dose of Oral ATP Supplementation Improves Performance and Physiological Response During Lower Body Resistance Exercise in Recreational Resistance-Trained Males. J. Strength Cond. Res. 2019;33:3345–3352. doi: 10.1519/JSC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 66.Xu Y.-F. Effect of Polysaccharide from Cordyceps militaris (Ascomycetes) on Physical Fatigue Induced by Forced Swimming. Int. J. Med. Mushrooms. 2016;18:1083–1092. doi: 10.1615/IntJMedMushrooms.v18.i12.30. [DOI] [PubMed] [Google Scholar]
- 67.Zhong L., Zhao L., Yang F., Yang W., Sun Y., Hu Q. Evaluation of Anti-Fatigue Property of the Extruded Product of Cereal Grains Mixed with Cordyceps militaris on Mice. J. Int. Soc. Sports Nutr. 2017;14:15. doi: 10.1186/s12970-017-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song J., Wang Y., Teng M., Cai G., Xu H., Guo H., Liu Y., Wang D., Teng L. Studies on the Antifatigue Activities of Cordyceps militaris Fruit Body Extract in Mouse Model. ECAM. 2015;2015:174616. doi: 10.1155/2015/174616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi E., Oh J., Sung G.-H. Beneficial Effect of Cordyceps militaris on Exercise Performance via Promoting Cellular Energy Production. Mycobiology. 2020;48:512–517. doi: 10.1080/12298093.2020.1831135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirsch K.R., Mock M.G., Roelofs E.J., Trexler E.T., Smith-Ryan A.E. Chronic Supplementation of a Mushroom Blend on Oxygen Kinetics, Peak Power, and Time to Exhaustion. J. Int. Soc. Sports Nutr. 2015;12:1. doi: 10.1186/1550-2783-12-S1-P45. [DOI] [Google Scholar]
- 71.Hirsch K.R., Smith-Ryan A.E., Roelofs E.J., Trexler E.T., Mock M.G. Cordyceps militaris Improves Tolerance to High-Intensity Exercise after Acute and Chronic Supplementation. J. Diet. Suppl. 2017;14:42–53. doi: 10.1080/19390211.2016.1203386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudgeon W.D., Thomas D.D., Dauch W., Scheett T.P., Webster M.J. The Effects of High and Low-Dose Cordyceps militaris-Containing Mushroom Blend Supplementation After Seven and Twenty-Eight Days. Am. J. Sports Sci. 2018;6:1–7. doi: 10.11648/j.ajss.20180601.11. [DOI] [Google Scholar]
- 73.Compound Solutions, PeakO2®. [(accessed on 15 August 2021)]. Available online: https://compoundsolutions.com/ingredients/peako2/
- 74.Zhu S., Pan J., Zhao B., Liang J., Ze-Yu W., Yang J. Comparisons on Enhancing the Immunity of Fresh and Dry Cordyceps militaris in Vivo and in Vitro. J. Ethnopharmacol. 2013;149:713–719. doi: 10.1016/j.jep.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 75.Lee C.-T., Huang K.-S., Shaw J.-F., Chen J.-R., Kuo W.-S., Shen G., Grumezescu A.M., Holban A.M., Wang Y.-T., Wang J.-S. Trends in the Immunomodulatory Effects of Cordyceps militaris: Total Extracts, Polysaccharides and Cordycepin. Front. Pharmacol. 2020;11:1824. doi: 10.3389/fphar.2020.575704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J.S., Kwon J.S., Yun J.S., Pahk J.W., Shin W.C., Lee S.Y., Hong E.K. Structural Characterization of Immunostimulating Polysaccharide from Cultured Mycelia of Cordyceps militaris. Carbohydr. Polym. 2010;80:1011–1017. doi: 10.1016/j.carbpol.2010.01.017. [DOI] [Google Scholar]
- 77.Lee J.S., Hong E.K. Immunostimulating Activity of the Polysaccharides Isolated from Cordyceps militaris. Int. Immunopharmacol. 2011;11:1226–1233. doi: 10.1016/j.intimp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Lee J.S., Kwon D.S., Lee K.R., Park J.M., Ha S.-J., Hong E.K. Mechanism of Macrophage Activation Induced by Polysaccharide from Cordyceps militaris Culture Broth. Carbohydr. Polym. 2015;120:29–37. doi: 10.1016/j.carbpol.2014.11.059. [DOI] [PubMed] [Google Scholar]
- 79.Jeong M.-H., Lee C.-M., Lee S.-W., Seo S.-Y., Seo M.-J., Kang B.-W., Jeong Y.-K., Choi Y.-J., Yang K.-M., Jo W.-S. Cordycepin-Enriched Cordyceps militaris Induces Immunomodulation and Tumor Growth Delay in Mouse-Derived Breast Cancer. Oncol. Rep. 2013;30:1996–2002. doi: 10.3892/or.2013.2660. [DOI] [PubMed] [Google Scholar]
- 80.Kang H.J., Baik H.W., Kim S.J., Lee S.G., Ahn H.Y., Park J.S., Park S.J., Jang E.J., Park S.W., Choi J.Y. Cordyceps militaris Enhances Cell-Mediated Immunity in Healthy Korean Men. J. Med. Food. 2015;18:1164–1172. doi: 10.1089/jmf.2014.3350. [DOI] [PubMed] [Google Scholar]
- 81.Jung S.J., Hwang J.H., Oh M.R., Chae S.W. Effects of Cordyceps militaris Supplementation on the Immune Response and Upper Respiratory Infection in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. J. Nutr. Health. 2019;52:258–267. doi: 10.4163/jnh.2019.52.3.258. [DOI] [Google Scholar]
- 82.Yoo H.-S., Shin J., Cho J.-H., Son C.-G., Lee Y.-W., Park S., Cho C.-K. Effects of Cordyceps militaris Extract on Angiogenesis and Tumor Growth. Acta Pharmacol. Sin. 2004;25:657–665. [PubMed] [Google Scholar]
- 83.Jin C.-Y., Kim G.-Y., Choi Y.-H. Induction of Apoptosis by Aqueous Extract of Cordyceps militaris through Activation of Caspases and Inactivation of Akt in Human Breast Cancer MDA-MB-231 Cells. J. Microbiol. Biotechnol. 2008;18:1997–2003. [PubMed] [Google Scholar]
- 84.Park S.E., Yoo H.S., Jin C.-Y., Hong S.H., Lee Y.-W., Kim B.W., Lee S.H., Kim W.-J., Cho C.K., Choi Y.H. Induction of Apoptosis and Inhibition of Telomerase Activity in Human Lung Carcinoma Cells by the Water Extract of Cordyceps militaris. Food Chem. Toxicol. 2009;47:1667–1675. doi: 10.1016/j.fct.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 85.Rao Y.K., Fang S.-H., Wu W.-S., Tzeng Y.-M. Constituents Isolated from Cordyceps militaris Suppress Enhanced Inflammatory Mediator’s Production and Human Cancer Cell Proliferation. J. Ethnopharmacol. 2010;131:363–367. doi: 10.1016/j.jep.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 86.Lee E., Kim W., Moon S. Cordycepin Suppresses TNF-alpha-induced Invasion, Migration and Matrix Metalloproteinase-9 Expression in Human Bladder Cancer Cells. Phytother. Res. 2010;24:1755–1761. doi: 10.1002/ptr.3132. [DOI] [PubMed] [Google Scholar]
- 87.Yang C.H., Kao Y.H., Huang K.S., Wang C.Y., Lin L.W. Cordyceps militaris and Mycelial Fermentation Induced Apoptosis and Autophagy of Human Glioblastoma Cells. Cell. Death Discov. 2012;3:e431. doi: 10.1038/cddis.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruma I., Putranto E.W., Kondo E., Watanabe R., Saito K., Inoue Y., Yamamoto K.-I., Nakata S., Kaihata M., Murata H. Extract of Cordyceps militaris Inhibits Angiogenesis and Suppresses Tumor Growth of Human Malignant Melanoma Cells. Int. J. Oncol. 2014;45:209–218. doi: 10.3892/ijo.2014.2397. [DOI] [PubMed] [Google Scholar]
- 89.Liu X.-C., Zhu Z.-Y., Liu Y.-L., Sun H.-Q. Comparisons of the Anti-Tumor Activity of Polysaccharides from Fermented Mycelia and Cultivated Fruiting Bodies of Cordyceps militaris in Vitro. Int. J. Biol. Macromol. 2019;130:307–314. doi: 10.1016/j.ijbiomac.2019.02.155. [DOI] [PubMed] [Google Scholar]
- 90.Guo Z., Chen W., Dai G., Huang Y. Cordycepin Suppresses the Migration and Invasion of Human Liver Cancer Cells by Downregulating the Expression of CXCR4. Int. J. Mol. 2020;45:141–150. doi: 10.3892/ijmm.2019.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jo E., Jang H.-J., Yang K.E., Jang M.S., Huh Y.H., Yoo H.-S., Park J.S., Jang I.-S., Park S.J. Cordyceps militaris Induces Apoptosis in Ovarian Cancer Cells through TNF-α/TNFR1-Mediated Inhibition of NF-ΚB Phosphorylation. BMC Complement. Med. Ther. 2020;20:1–12. doi: 10.1186/s12906-019-2780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jo E., Jang H.-J., Shen L., Yang K.E., Jang M.S., Huh Y.H., Yoo H.-S., Park J., Jang I.S., Park S.J. Cordyceps militaris Exerts Anticancer Effect on Non–Small Cell Lung Cancer by Inhibiting Hedgehog Signaling via Suppression of TCTN3. Integr. Cancer Ther. 2020;19:1–14. doi: 10.1177/1534735420923756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He Y.T., Zhang X.L., Xie Y.M., Xu Y.X., Li J.R. Extraction and Antioxidant Property in Vitro of Cordycepin in Artificially Cultivated Cordyceps militaris. Adv. Mater. Res. 2013;750–752:1593–1596. doi: 10.4028/www.scientific.net/AMR.750-752.1593. [DOI] [Google Scholar]
- 94.Yu H.M., Wang B.-S., Huang S.C., Duh P.-D. Comparison of Protective Effects between Cultured Cordyceps militaris and Natural Cordyceps sinensis against Oxidative Damage. J. Agric. Food Chem. 2006;54:3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- 95.Yu R., Yang W., Song L., Yan C., Zhang Z., Zhao Y. Structural Characterization and Antioxidant Activity of a Polysaccharide from the Fruiting Bodies of Cultured Cordyceps militaris. Carbohydr. Polym. 2007;70:430–436. doi: 10.1016/j.carbpol.2007.05.005. [DOI] [Google Scholar]
- 96.Yu R., Yin Y., Yang W., Ma W., Yang L., Chen X., Zhang Z., Ye B., Song L. Structural Elucidation and Biological Activity of a Novel Polysaccharide by Alkaline Extraction from Cultured Cordyceps militaris. Carbohydr. Polym. 2009;75:166–171. doi: 10.1016/j.carbpol.2008.07.023. [DOI] [Google Scholar]
- 97.Fengyao W., Hui Y., Xiaoning M., Junqing J., Guozheng Z., Xijie G., Zhongzheng G. Structural Characterization and Antioxidant Activity of Purified Polysaccharide from Cultured Cordyceps militaris. Afr. J. Microbiol. Res. 2011;5:2743–2751. doi: 10.5897/AJMR11.548. [DOI] [Google Scholar]
- 98.Chen R., Jin C., Li H., Liu Z., Lu J., Li S., Yang S. Ultrahigh Pressure Extraction of Polysaccharides from Cordyceps militaris and Evaluation of Antioxidant Activity. Sep. Purif. Technol. 2014;134:90–99. doi: 10.1016/j.seppur.2014.07.017. [DOI] [Google Scholar]
- 99.Liu J., Feng C., Li X., Chang M., Meng J., Xu L. Immunomodulatory and Antioxidative Activity of Cordyceps militaris Polysaccharides in Mice. Int. J. Biol. Macromol. 2016;86:594–598. doi: 10.1016/j.ijbiomac.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 100.Kim H.G., Shrestha B., Lim S.Y., Yoon D.H., Chang W.C., Shin D.-J., Han S.K., Park S.M., Park J.H., Park H. il Cordycepin Inhibits Lipopolysaccharide-Induced Inflammation by the Suppression of NF-ΚB through Akt and P38 Inhibition in RAW 264.7 Macrophage Cells. Eur. J. Pharmacol. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 101.Jo W.S., Choi Y.J., Mm H.J., Lee J.Y., Nam B.H., Lee J.D., Lee S.W., Seo S.Y., Jeong M.H. The Anti-Inflammatory Effects of Water Extract from Cordyceps militaris in Murine Macrophage. Mycobiology. 2010;38:46–51. doi: 10.4489/MYCO.2010.38.1.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jeong J.-W., Jin C.-Y., Kim G.-Y., Lee J.-D., Park C., Kim G.-D., Kim W.-J., Jung W.-K., Seo S.K., Choi I.-W. Anti-Inflammatory Effects of Cordycepin via Suppression of Inflammatory Mediators in BV2 Microglial Cells. Int. Immunopharmacol. 2010;10:1580–1586. doi: 10.1016/j.intimp.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Han E.S., Oh J.Y., Park H.-J. Cordyceps militaris Extract Suppresses Dextran Sodium Sulfate-Induced Acute Colitis in Mice and Production of Inflammatory Mediators from Macrophages and Mast Cells. J. Ethnopharmacol. 2011;134:703–710. doi: 10.1016/j.jep.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 104.Won S.-Y., Park E.-H. Anti-Inflammatory and Related Pharmacological Activities of Cultured Mycelia and Fruiting Bodies of Cordyceps militaris. J. Ethnopharmacol. 2005;96:555–561. doi: 10.1016/j.jep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 105.Smiderle F.R., Baggio C.H., Borato D.G., Santana-Filho A.P., Sassaki G.L., Iacomini M., van Griensven L.J.L.D. Anti-Inflammatory Properties of the Medicinal Mushroom Cordyceps militaris Might Be Related to Its Linear (1→ 3)-β-D-Glucan. PLoS ONE. 2014;9:e110266. doi: 10.1371/journal.pone.0110266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiu C.-P., Liu S.-C., Tang C.-H., Chan Y., El-Shazly M., Lee C.-L., Du Y.-C., Wu T.-Y., Chang F.-R., Wu Y.-C. Anti-Inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016;64:1540–1548. doi: 10.1021/acs.jafc.5b05931. [DOI] [PubMed] [Google Scholar]
- 107.Cai H., Wang Y., Pang J. Efficacy of Cordyceps militaris Capsules in Treatment of Chronic Bronchitis. Chin. J. New Drugs. 2004;13:171–174. [Google Scholar]
- 108.Gai G., Jin S., Wang B., Li Y., Li C. The Efficacy of Cordyceps militaris Capsules in Treatment of Chronic Bronchitis in Comparison with Jinshuibao Capsules. Chin. J. New Drugs. 2004;13:169–170. [Google Scholar]
- 109.Sun J., Xu J., Wang S., Hou Z., Lu X., An L., Du P. A New Cerebroside from Cordyceps militaris with Anti-PTP1B Activity. Fitoterapia. 2019;138:104342. doi: 10.1016/j.fitote.2019.104342. [DOI] [PubMed] [Google Scholar]
- 110.Sun H., Yu X., Li T., Zhu Z. Structure and Hypoglycemic Activity of a Novel Exopolysaccharide of Cordyceps militaris. Int. J. Biol. Macromol. 2021;166:496–508. doi: 10.1016/j.ijbiomac.2020.10.207. [DOI] [PubMed] [Google Scholar]
- 111.Ma L., Zhang S., Du M. Cordycepin from Cordyceps militaris Prevents Hyperglycemia in Alloxan-Induced Diabetic Mice. Nutr. Res. 2015;35:431–439. doi: 10.1016/j.nutres.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 112.Parunyakul K., Srisuksai K., Charoenlappanit S., Phaonakrop N., Roytrakul S., Fungfuang W. Metabolic Impacts of Cordycepin on Hepatic Proteomic Expression in Streptozotocin-Induced Type 1 Diabetic Mice. PLoS ONE. 2021;16:e0256140. doi: 10.1371/journal.pone.0256140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Montefiori D.C., Sobol R.W., Li S.W., Reichenbach N.L., Suhadolnik R.J., Charubala R., Pfleiderer W., Modliszewski A., Robinson W.E., Mitchell W.M. Phosphorothioate and Cordycepin Analogues of 2′, 5′-Oligoadenylate: Inhibition of Human Immunodeficiency Virus Type 1 Reverse Transcriptase and Infection in Vitro. Proc. Natl. Acad. Sci. USA. 1989;86:7191–7194. doi: 10.1073/pnas.86.18.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mueller W.E.G., Weiler B.E., Charubala R., Pfleiderer W., Leserman L., Sobol R.W., Suhadolnik R.J., Schroeder H.C. Cordycepin Analogs of 2′, 5′-Oligoadenylate Inhibit Human Immunodeficiency Virus Infection via Inhibition of Reverse Transcriptase. Biochemistry. 1991;30:2027–2033. doi: 10.1021/bi00222a004. [DOI] [PubMed] [Google Scholar]
- 115.Ryu E., Son M., Lee M., Lee K., Cho J.Y., Cho S., Lee S.K., Lee Y.M., Cho H., Sung G.-H. Cordycepin Is a Novel Chemical Suppressor of Epstein-Barr Virus Replication. Oncoscience. 2014;1:866. doi: 10.18632/oncoscience.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Verma A.K., Aggarwal R. Repurposing Potential of FDA Approved and Investigational Drugs for COVID-19 Targeting SARS-CoV-2 Spike and Main Protease and Validation by Machine Learning Algorithm. Chem. Biol. Drug Des. 2020;97:836–853. doi: 10.1111/cbdd.13812. [DOI] [PubMed] [Google Scholar]
- 117.Bibi S., Hasan M.M., Wang Y.-B., Papadakos S.P., Yu H. Cordycepin as a Promising Inhibitor of SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp) Curr. Med. Chem. 2021;28:1–11. doi: 10.2174/0929867328666210820114025. [DOI] [PubMed] [Google Scholar]
- 118.Liu J., Yang S., Yang X., Chen Z., Li J. Anticarcinogenic Effect and Hormonal Effect of Cordyceps militaris Link. Chin. Med. J. 1997;22:111–113. [PubMed] [Google Scholar]
- 119.Hong I.-P., Choi Y.-S., Woo S.-O., Han S.-M., Kim H.-K., Lee M.-R., Nam S.-H., Ha N.-G. Stimulatory Effect of Cordyceps militaris on Testosterone Production in Male Mouse. Kor. J. Mycol. 2011;39:148–150. doi: 10.4489/KJM.2010.39.2.148. [DOI] [Google Scholar]
- 120.Leu S.-F., Poon S.L., Pao H.-Y., Huang B.-M. The in Vivo and in Vitro Stimulatory Effects of Cordycepin on Mouse Leydig Cell Steroidogenesis. Biosci. Biotechnol. 2011;75:723–731. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 121.Sohn S.-H., Lee S.-C., Hwang S.-Y., Kim S.-W., Kim I.-W., Michael B.Y., Kim S.-K. Effect of Long-Term Administration of Cordycepin from Cordyceps militaris on Testicular Function in Middle-Aged Rats. Planta Med. 2012;78:1620–1625. doi: 10.1055/s-0032-1315212. [DOI] [PubMed] [Google Scholar]
- 122.Kopalli S.R., Cha K.-M., Lee S.-H., Hwang S.-Y., Lee Y.-J., Koppula S., Kim S.-K. Cordycepin, an Active Constituent of Nutrient Powerhouse and Potential Medicinal Mushroom Cordyceps militaris Linn., Ameliorates Age-Related Testicular Dysfunction in Rats. Nutrients. 2019;11:906. doi: 10.3390/nu11040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin W.-H., Tsai M.-T., Chen Y.-S., Hou R.C.-W., Hung H.-F., Li C.-H., Wang H.-K., Lai M.-N., Jeng K.-C.G. Improvement of Sperm Production in Subfertile Boars by Cordyceps militaris Supplement. Am. J. Chinese Med. 2007;35:631–641. doi: 10.1142/S0192415X07005120. [DOI] [PubMed] [Google Scholar]
- 124.Kusama K., Miyagawa M., Ota K., Kuwabara N., Saeki K., Ohnishi Y., Kumaki Y., Aizawa T., Nakasone T., Okamatsu S. Cordyceps militaris Fruit Body Extract Decreases Testosterone Catabolism and Testosterone-Stimulated Prostate Hypertrophy. Nutrients. 2021;13:50. doi: 10.3390/nu13010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsu C.C., Lin Y.A., Su B., Li J.H., Huang H.Y., Hsu M.C. No Effect of Cordyceps sinensis Supplementation on Testosterone Level and Muscle Strength in Healthy Young Adults for Resistance Training. Biol. Sport. 2011;28:107. doi: 10.5604/942739. [DOI] [Google Scholar]
- 126.Yue G.G.-L., Bik-San Lau C., Fung K.-P., Leung P.-C., Ko W.-H. Effects of Cordyceps sinensis, Cordyceps militaris and Their Isolated Compounds on Ion Transport in Calu-3 Human Airway Epithelial Cells. J. Ethnopharmacol. 2008;117:92–101. doi: 10.1016/j.jep.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 127.Hsu C.-H., Sun H.-L., Sheu J.-N., Ku M.-S., Hu C.-M., Chan Y., Lue K.-H. Effects of the Immunomodulatory Agent Cordyceps militaris on Airway Inflammation in a Mouse Asthma Model. Pediatr. Neonatol. 2008;49:171–178. doi: 10.1016/S1875-9572(09)60004-8. [DOI] [PubMed] [Google Scholar]
- 128.Fei X., Zhang X., Zhang G., Bao W., Zhang Y., Zhang M., Zhou X. Cordycepin Inhibits Airway Remodeling in a Rat Model of Chronic Asthma. Biomed. Pharmacother. 2017;88:335–341. doi: 10.1016/j.biopha.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 129.Zheng Y., Li L., Cai T. Cordyceps Polysaccharide Ameliorates Airway Inflammation in an Ovalbumin-Induced Mouse Model of Asthma via TGF-Β1/Smad Signaling Pathway. Respir. Physiol. Neurobiol. 2020;276:103412. doi: 10.1016/j.resp.2020.103412. [DOI] [PubMed] [Google Scholar]
- 130.Choi K.-H., Yoo J.-E., Hwang G.-S., Yoo D.-Y. Effects of Cordyceps militaris (CM) on Osteoclastogenesis and Gene Expression. Obstet. Gynecol. Sci. 2012;25:16–26. [Google Scholar]
- 131.Ying X., Peng L., Chen H., Shen Y., Yu K., Cheng S. Cordycepin Prevented IL-β-Induced Expression of Inflammatory Mediators in Human Osteoarthritis Chondrocytes. Int. Ort. 2014;38:1519–1526. doi: 10.1007/s00264-013-2219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang D., Wang Z., Qi W., Lei W., Zhao G. Cordycepin (3′-Deoxyadenosine) down-Regulates the Proinflammatory Cytokines in Inflammation-Induced Osteoporosis Model. Inflammation. 2014;37:1044–1049. doi: 10.1007/s10753-014-9827-z. [DOI] [PubMed] [Google Scholar]
- 133.Kim J., Lee H., Kang K.S., Chun K.-H., Hwang G.S. Cordyceps militaris Mushroom and Cordycepin Inhibit RANKL-Induced Osteoclast Differentiation. J. Med. Food. 2015;18:446–452. doi: 10.1089/jmf.2014.3215. [DOI] [PubMed] [Google Scholar]
- 134.Ashraf S., Radhi M., Gowler P., Burston J.J., Gandhi R.D., Thorn G.J., Piccinini A.M., Walsh D.A., Chapman V., de Moor C.H. The Polyadenylation Inhibitor Cordycepin Reduces Pain, Inflammation and Joint Pathology in Rodent Models of Osteoarthritis. Sci. Rep. 2019;9:4696. doi: 10.1038/s41598-019-41140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He M.T., Lee A.Y., Kim J.H., Park C.H., Shin Y.S., Cho E.J. Protective Role of Cordyceps militaris in Aβ 1–42-Induced Alzheimer’s Disease in Vivo. Food Sci. Biotechnol. 2019;28:865–872. doi: 10.1007/s10068-018-0521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee B., Park J., Park J., Shin H.-J., Kwon S., Yeom M., Sur B., Kim S., Kim M., Lee H. Cordyceps militaris Improves Neurite Outgrowth in Neuro2a Cells and Reverses Memory Impairment in Rats. Food Sci. Biotechnol. 2011;20:1599–1608. doi: 10.1007/s10068-011-0221-4. [DOI] [Google Scholar]
- 137.Kim Y.O., Kim H.J., Abu-Taweel G.M., Oh J., Sung G.-H. Neuroprotective and Therapeutic Effect of Cordyceps militaris on Ischemia-Induced Neuronal Death and Cognitive Impairments. Saudi J. Biol. Sci. 2019;26:1352–1357. doi: 10.1016/j.sjbs.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yuan G., An L., Sun Y., Xu G., Du P. Improvement of Learning and Memory Induced by Cordyceps Polypeptide Treatment and the Underlying Mechanism. ECAM. 2018;2018:9419264. doi: 10.1155/2018/9419264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Veena R.K., Carmel E.J., Ramya H., Ajith T.A., Wasser S.P., Janardhanan K.K. Caterpillar Medicinal Mushroom, Cordyceps militaris (Ascomycetes), Mycelia Attenuates Doxorubicin-Induced Oxidative Stress and Upregulates Krebs Cycle Dehydrogenases Activity and ATP Level in Rat Brain. Int. J. Med. Mushrooms. 2020;22:593–604. doi: 10.1615/IntJMedMushrooms.2020035093. [DOI] [PubMed] [Google Scholar]
- 140.U.S. National Library of Medicine Clinical Trials. [(accessed on 15 August 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04002219/
- 141.Won K.-J., Lee S.-C., Lee C.-K., Lee H.M., Lee S.H., Fang Z., Choi O.B., Jin M., Kim J., Park T. Cordycepin Attenuates Neointimal Formation by Inhibiting Reactive Oxygen Species–Mediated Responses in Vascular Smooth Muscle Cells in Rats. J. Pharmacol. Sci. 2009;109:403–412. doi: 10.1254/jphs.08308FP. [DOI] [PubMed] [Google Scholar]
- 142.Gao J., Lian Z.-Q., Zhu P., Zhu H.-B. Lipid-Lowering Effect of Cordycepin (3′-Deoxyadenosine) from Cordyceps militaris on Hyperlipidemic Hamsters and Rats. Acta Pharm. Sin. 2011;46:669–676. [PubMed] [Google Scholar]
- 143.Guo P., Kai Q., Gao J., Lian Z., Wu C., Wu C., Zhu H. Cordycepin Prevents Hyperlipidemia in Hamsters Fed a High-Fat Diet via Activation of AMP-Activated Protein Kinase. J. Pharmacol. Sci. 2010;113:395–403. doi: 10.1254/jphs.10041FP. [DOI] [PubMed] [Google Scholar]
- 144.Takahashi S., Tamai M., Nakajima S., Kato H., Johno H., Nakamura T., Kitamura M. Blockade of Adipocyte Differentiation by Cordycepin. Br. J. Pharmacol. 2012;167:561–575. doi: 10.1111/j.1476-5381.2012.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu C., Guo Y., Su Y., Zhang X., Luan H., Zhang X., Zhu H., He H., Wang X., Sun G. Cordycepin Activates AMP-activated Protein Kinase (AMPK) via Interaction with the Γ1 Subunit. J. Cell. Mol. Med. 2014;18:293–304. doi: 10.1111/jcmm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu S., Wang J., Li F., Hou P., Yin J., Yang Z., Yang X., Li T., Xia B., Zhou G. Structural Characterisation and Cholesterol Efflux Improving Capacity of the Novel Polysaccharides from Cordyceps militaris. Int. J. Biol. Macromol. 2019;131:264–272. doi: 10.1016/j.ijbiomac.2019.03.078. [DOI] [PubMed] [Google Scholar]
- 147.Wang L., Xu N., Zhang J., Zhao H., Lin L., Jia S., Jia L. Antihyperlipidemic and Hepatoprotective Activities of Residue Polysaccharide from Cordyceps militaris SU-12. Carbohydr. Polym. 2015;131:355–362. doi: 10.1016/j.carbpol.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 148.Huang Z., Zhang M., Zhang S., Wang Y., Jiang X. Structural Characterization of Polysaccharides from Cordyceps militaris and Their Hypolipidemic Effects in High Fat Diet Fed Mice. RSC Adv. 2018;8:41012–41022. doi: 10.1039/C8RA09068H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cho H.-J., Cho J.Y., Rhee M.H., Park H.-J. Cordycepin (3′-Deoxyadenosine) Inhibits Human Platelet Aggregation in a Cyclic AMP-and Cyclic GMP-Dependent Manner. Eur. J. Pharmacol. 2007;558:43–51. doi: 10.1016/j.ejphar.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 150.Park E.-S., Kang D.-H., Yang M.-K., Kang J.C., Jang Y.C., Park J.S., Kim S.-K., Shin H.-S. Cordycepin, 3′-Deoxyadenosine, Prevents Rat Hearts from Ischemia/Reperfusion Injury via Activation of Akt/GSK-3β/P70S6K Signaling Pathway and HO-1 Expression. Cardiovasc. Toxicol. 2014;14:1–9. doi: 10.1007/s12012-013-9232-0. [DOI] [PubMed] [Google Scholar]
- 151.Lee D.-H., Kim H.-H., Lim D.H., Kim J.-L., Park H.-J. Effect of Cordycepin-Enriched WIB801C from Cordyceps militaris Suppressing Fibrinogen Binding to Glycoprotein IIb/IIIa. Biomol. Ther. 2015;23:60. doi: 10.4062/biomolther.2014.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hwang S., Cho G.-S., Ryu S., Kim H.J., Song H.Y., Yune T.Y., Ju C., Kim W.-K. Post-Ischemic Treatment of WIB801C, Standardized Cordyceps Extract, Reduces Cerebral Ischemic Injury via Inhibition of Inflammatory Cell Migration. J. Ethnopharmacol. 2016;186:169–180. doi: 10.1016/j.jep.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 153.Choi E., Oh J., Sung G.-H. Antithrombotic and Antiplatelet Effects of Cordyceps militaris. Mycobiology. 2020;48:228–232. doi: 10.1080/12298093.2020.1763115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ji Y., Su A., Ma G., Tao T., Fang D., Zhao L., Hu Q. Comparison of Bioactive Constituents and Effects on Gut Microbiota by in Vitro Fermentation between Ophicordyceps sinensis and Cordyceps militaris. J. Funct. Foods. 2020;68:103901. doi: 10.1016/j.jff.2020.103901. [DOI] [Google Scholar]
- 155.Heo J.Y., Baik H.W., Kim H.J., Lee J.M., Kim H.W., Choi Y.S., Won J.H., Hyun Mi Kim W.I.P., Kim C.Y. The Efficacy and Safety of Cordyceps militaris in Korean Adults Who Have Mild Liver Dysfunction. J. Clin. Nutr. 2015;7:81–86. doi: 10.15747/jcn.2015.7.3.81. [DOI] [Google Scholar]
- 156.Zhao-Long W., Xiao-Xia W., Wei-Ying C. Inhibitory Effect of Cordyceps sinensis and Cordyceps militaris on Human Glomerular Mesangial Cell Proliferation Induced by Native LDL. Cell Biochem. Funct. 2000;18:93–97. doi: 10.1002/(SICI)1099-0844(200006)18:2<93::AID-CBF854>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 157.Sun T., Dong W., Jiang G., Yang J., Liu J., Zhao L., Ma P. Cordyceps militaris Improves Chronic Kidney Disease by Affecting TLR4/NF-ΚB Redox Signaling Pathway. Oxid. Med. Cell. Longev. 2019;2019:7850863. doi: 10.1155/2019/7850863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yu S.-H., Dubey N.K., Li W.-S., Liu M.-C., Chiang H.-S., Leu S.-J., Shieh Y.-H., Tsai F.-C., Deng W.-P. Cordyceps militaris Treatment Preserves Renal Function in Type 2 Diabetic Nephropathy Mice. PLoS ONE. 2016;11:e0166342. doi: 10.1371/journal.pone.0166342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hartmann G.C., Wasti S.S., Hendrickson D.L. Murine Safety of Two Species of Entomogenous Fungi, Cordyceps militaris (FRIES) LINK and Paecilomyces Fumoso-Roseus (WIZE) BROWN and SMITH. Appl. Entomol. Zool. 1979;14:217–220. doi: 10.1303/aez.14.217. [DOI] [Google Scholar]
- 160.Jhou B.-Y., Fang W.-C., Chen Y.-L., Chen C.-C. A 90-Day Subchronic Toxicity Study of Submerged Mycelial Culture of Cordyceps militaris in Rats. Toxicol. Res. 2018;7:977–986. doi: 10.1039/C8TX00075A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bai K.-C., Sheu F. A Novel Protein from Edible Fungi Cordyceps militaris That Induces Apoptosis. J. Food Drug Anal. 2018;26:21–30. doi: 10.1016/j.jfda.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gençcelep H., Uzun Y., Tunçtürk Y., Demirel K. Determination of Mineral Contents of Wild-Grown Edible Mushrooms. Food Chem. 2009;113:1033–1036. doi: 10.1016/j.foodchem.2008.08.058. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.