Abstract
Osteoarthritis (OA) is a common joint condition and one of the greatest causes of disability worldwide. The role of vitamin D in the origin and development of the disease is not clear, although it could have important implications for diagnosis and treatment. For this proposal, a cross-sectional study with a non-probabilistic sample was performed. In total, 48 with early osteoarthritis (EOA) and 48 matched controls were selected, and serum 25(OH)D and parathyroid hormone (PTH) levels were analyzed. In addition, physical and psychological variables were measured to establish their relationship with vitamin D levels. Patients with EOA showed lower levels (22.3 ± 7.3 ng/mL) in comparison to matched controls (29.31 ± 9.2 ng/mL). A statistically significant higher number (Chi-squared = 8.525; p = 0.004) of patients with EOA had deficiency levels (<20 ng/mL) compared to the control group. Patients with lower vitamin D levels showed higher levels of pain intensity, disability, and anxiety, as well as poorer values for sit-to-stand, walking speed, and social participation. Correlation analysis showed a relationship between serum 25(OH)D, PTH and pain intensity, and social participation. These results highlight the relevance of vitamin D in the early diagnosis and prevention of EOA.
Keywords: osteoarthritis, early osteoarthritis, vitamin D
1. Introduction
Osteoarthritis (OA) is defined as a common joint condition that typically causes chronic pain and is one of the greatest causes of disability worldwide, being considered a major public health challenge [1]. Knee OA has been associated with degenerative changes due to the progressive loss of articular cartilage, subchondral bone changes, synovial inflammation, and meniscus degeneration [2,3]. It is of prime importance to find predictors for knee OA progression to improve treatment options and to prevent this disabling disease [4,5].
Unfortunately, although some theories regarding the pathogenesis of OA do exist, the exact mechanism is not well charted [6]. The most frequent nutritional deficiency worldwide is vitamin D deficiency (defined as serum level of 25-hydroxyvitamin D [25-(OH) D] < 20 nmol/L) [7]. Specifically, more than 40% of the adult population over the age of 50 has this deficiency, being lower in women [8]. It is well known that vitamin D has an important influence on the state of many articular structures, such as cartilage and subchondral bone, as well as muscle tissues, all of which play a part in the progression of knee OA [9]. Similarly, parathyroid hormone levels (PTH) could play an important role in the loss and formation of bone and these levels have been related to subchondral bone remodeling, and therefore, to the radiological and clinical progression of knee OA [10].
In relation to this, previous studies suggest that vitamin D could stimulate proteoglycan synthesis in mature chondrocytes through a metabolic transformation via vitamin D receptors [11,12]. Moreover, vitamin D deficiency could impede bone remodeling causing OA through complex pathophysiological processes, and other studies suggested that low serum vitamin D could be associated with an increased risk of knee OA progression [13]. Conversely, vitamin D supplementation could be able to prevent the progression of OA by increasing bone remodeling and also by reducing the abnormal pathophysiological process [14]. Additionally, it was suggested that supplementation may help improve disease progression in patients with vitamin D deficiency but does not have any additional benefits on those with optimal levels of vitamin D [15]. However, recent studies showed that low levels of vitamin D are not associated with the risks of developing knee OA, except perhaps with the progression of knee OA [12,16]. In addition, vitamin D supplementation may not help to control pain or to reduce structural disease progression in this condition [16,17].
It has been suggested that the structural findings in OA are a late phenomenon [18,19]. For this reason, non-surgical treatments for OA are often ineffective, which may be due in part to its use late in the course of the disease, when structural deterioration is usually advanced [18]. Therefore, there is a great scientific and social interest in ‘early osteoarthritis’ (EOA), as it is believed that identifying patients affected by EOA to initiate early interventions and therapeutic approaches could prevent progression and severe structural changes in the joint associated with later stages of OA [20].
Most of the studies that have reported a link between vitamin D and OA have been conducted in patients with advanced stages of the disease; however, little is understood regarding the effect that vitamin D and PTH could have on the initiation and progression of EOA. Early identification of these factors could be critical for early diagnosis, which would facilitate early treatment to prevent the dramatic development of knee OA. Therefore, the aim of this cross-sectional study was to study the association of serum concentrations of vitamin D with knee EOA. The secondary objective was to determine the association of serum concentrations of PTH with knee EOA and to determine the relationship between vitamin D deficiency, PTH level, and pain intensity, disability, psychological, and functional variables in patients with knee EOA.
2. Materials and Methods
2.1. Study Design
A cross-sectional study with a non-probabilistic sample was designed. The international recommendations for Strengthening the Reporting of Observational Studies in Epidemiology were followed [20]. The research procedures were performed according to the ethical standards of the Declaration of Helsinki and approved by an Ethics Committee (CEIm La Fe 2017/0147). All participants received an explanation and signed a written informed consent.
2.2. Participants
Subjects were recruited at Hospital La Fe, Valencia, Spain, within the H2020 project OACTIVE. The design of the data collection protocol started in November 2017 and lasted until July 2018.
The subjects recruited were evaluated by a group of three experienced physical medicine and rehabilitation clinicians. The inclusion criteria for EOA patients were based on Luyten’s proposal for EOA classification, due to the criteria used found a specificity of 76.5% for detection of clinical progression, being valid criteria for research use [21]. Criteria were as follows: (a) Patient-based questionnaires: knee Injury and osteoarthritis outcome score: 2 out of the 4 KOOS subscales (pain, symptoms, function, or knee-related quality of life) need to score “positive” (≤85%); (b) patients should present joint line tenderness or crepitus in the clinical examination; (c) X-rays: Kellgren and Lawrence (KL) grade 0–1 standing, weight-bearing (at least 2 projections: PA fixed flexion and skyline for patellofemoral OA) [22]. To match with EOA patients, controls healthy subjects with similar descriptive and sociodemographic characteristics were selected. The inclusion criteria were (a) patient age greater than or equal to 40 years; (b) KL grade 0–1.
Exclusion criteria were: (a) Any cognitive disability that hindered viewing of the audio-visual material; (b) illiteracy; (c) comprehension or communication difficulties, (d) insufficient Spanish language comprehension to follow measurement instructions; (e) presence of any rheumatic, autoimmune or infectious pathology.
2.3. Outcome Measures
2.3.1. Vitamin D and Parathyroid Hormone Levels
For the collection of blood, separation of plasma, and long storage of the samples, we followed the rules proposed by the Standard Operating Procedures Internal Working Group (SOPIWG)/Early Detection Research Network (EDRN) for specimen collection. Four aliquots were sent on dry ice by courier to the Biobanco La Fe and kept at −50 °C until measurements of serum intact PTH and 25OHD were taken using commercial ELISA kits (IDS Co., Bolden, UK), with intraassay coefficients of variation ranging from 5% to 15%.
Serum 25(OH)D and PTH concentrations were determined by radioimmunoassay. Vitamin D levels were classified as follows: vitamin D deficiency, serum 25(OH)D concentration < 20 ng/mL; vitamin D insufficiency, serum 25(OH)D concentration 20–29 ng/mL; and vitamin D sufficiency, serum 25(OH)D concentration ≥ 30 ng/mL [23,24].
2.3.2. Pain and Disability Variables
Pain Intensity
To measure pain intensity, the Visual Analogue Scale (VAS) was used. The scale is a 100-mm line with two endpoints representing the extreme states “no pain” (0) and “the maximal pain imaginable” (10). The VAS scale has been shown to have good retest reliability (r = 0.94, p < 0.001) [25,26].
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
The WOMAC questionnaire is self-administered and is used to assess OA disability. It is composed of 24 items divided into 3 aspects: functional pain (5 items), stiffness (2 items), and activities of daily life difficulties (17 items). Higher values mean poorer WOMAC subscales scores of pain and physical function. The Spanish version of the WOMAC questionnaire has an internal consistency of 0.82 for pain and 0.93 for physical function subscales [27].
2.3.3. Functional Variables
Five-Time Sit to Stand
To assess functional capacity, the sit-to-stand test was employed. The test was performed as follows: with the patient seated with their back against the back of the chair, the clinician counted each stand aloud so that the patient remained oriented. The clinician stopped the test when the patient achieved the standing position on the 5th repetition [28]. The amount of time needed to realize the test was extracted in seconds.
Walking Speed
The subject walked without assistance 10 m (32.8 feet) and the time was measured for the intermediate 6 m (19.7 feet). Assistive devices could be used but had to be kept consistent and documented from test to test. It was performed at the fastest speed possible. There were three trials collected and the average of the three trials was calculated for the measurement [29,30].
2.3.4. Psychological Variables
Anxiety and Depression Symptoms
To assess anxiety and depression symptoms, the Hospital Anxiety and Depression Scale (HADS) was used. HADS includes 14 items in a 4-point Likert-type scale. For the full scale, the internal consistency is 0.90. For the depression subscale, the internal consistency is 0.84, and 0.85 for the anxiety subscale [31].
Social Participation
To determine the frequency and diversity of social involvement, the Maastricht Social Participation Profile was used [32]. The scale includes 9 items: ‘How often in the past four weeks have you (taken part in/been to): (1) a club, interest group or activity group, church or other similar activity; (2) a cultural or educational event such as the cinema, theatre, museum, talk or course; (3) eaten out; (4) out to a pub, cafe or tearoom; (5) a public event; (6) an organized games afternoon or evening; (7) a day trip organized by a club or society; (8) committee work for a club, society or another group; (9) any organized voluntary work?’ The response categories range from zero (‘not at all’) to three (‘more than twice a week’). The psychometric properties were appropriate (Cronbach’s αbaseline = 0.64; αfollow-up = 0.64), and higher scores equated to higher social participation [32].
2.4. Procedures
The procedure and an informed consent form were explained and given to all the participants. The included subjects filled out the sociodemographic questionnaire and self-reported measures of disability, pain, and psychological variables. Finally, the serum 25(OH)D concentrations and functional tests were assessed. This procedure was identical for both groups.
2.5. Statistical Analysis
The sociodemographic data of the participants were analyzed. The data were summarized using frequency counts, descriptive statistics, and summary tables. Statistics Package for Social Sciences (SPSS 24, IBM Inc., Armonk, NY, USA) was used for data analysis. The categorical variables are shown as frequencies and percentages. The quantitative results are represented by descriptive statistics (confidence interval, mean, and standard deviation). Student’s t-test was used for the group comparisons. Cohen’s d effect sizes were calculated for multiple comparisons of the outcome variables. According to Cohen’s method, the magnitude of the effect was classified as small (0.20–0.49), medium (0.50–0.79), or large (0.80).
The relationships between vitamin D and PTH measures with psychological and physical measurements were analyzed using Pearson’s correlation coefficients. A Pearson’s correlation coefficient greater than 0.60 indicated a strong correlation, a coefficient between 0.30 and 0.60 indicated a moderate correlation, and a coefficient below 0.30 indicated a low or very low correlation [33].
3. Results
A total of 96 participants were included in the study, with a mean age of 51.81 ± 5.59 (39 men and 58 women). In total, 48 of the participants met the criteria to be classified as EOA and 48 participants as matched controls. No abandonment of any study participant was recorded nor were adverse effects reported during the assessments. There were no statistically significant differences between the groups in terms of descriptive and demographic variables (Table 1).
Table 1.
Descriptive and demographic variables.
| Measures | EOA (n = 48) |
Controls (n = 48) |
p Value |
|---|---|---|---|
| Age (years) | 52.36 ± 5.02 | 50.72 ± 6.87 | 0.46 |
| BMI (kg/m2) | 26.98 ± 4.36 | 27.50 ± 3.89 | 0.43 |
| Gender | 0.17 | ||
| Women | 39 (81.3) | 43 (89.6) | |
| Men | 9 (18.7) | 5 (10.4) | |
| Economic status | 0.86 | ||
| Easy | 8 (16.7) | 3 (6.3) | |
| Fairly easy | 35 (72.9) | 38 (79.2) | |
| With some difficulties | 3 (6.2) | 7 (14.5) | |
| With great difficulties | 2 (4.2) | 0 (0) | |
| Alcohol | 0.65 | ||
| Never | 10 (22.2) | 8 (16.7) | |
| Seldom | 20 (41.7) | 18 (37.5) | |
| 1–2 times/month | 4 (8.3) | 8 (16.7) | |
| 1–2 times/week | 7 (14.3) | 12 (25) | |
| 1 time day | 5 (10.3) | 2 (4.1) | |
| More than 1 a day | 2 (4.1) | 0 (0) | |
| Smoking | 0.27 | ||
| Yes | 3 (6.25) | 6 (12.5) | |
| No | 10 (22.2) | 20 (41.7) | |
| Ex | 35 (72.9) | 22 (45.8) |
Data are expressed as mean ± standard deviation or n (%). BMI: Body Mass Index; EOA: Early Osteoarthritis.
3.1. Vitamin D and PTH Levels
Regarding serum 25(OH)D concentrations, patients with EOA showed lower levels (22.3 ± 7.3 ng/mL) in comparison to matched controls (29.31 ± 9.2 ng/mL), showing statistically significant differences between groups.
Student’s t-test showed statistically significant differences (p = 0.03), with a medium effect size (MD: −7.01; d = 0.72). However, no statistically significant differences were found for PTH levels between both groups (MD: −1.42, p > 0.05) (Table 2).
Table 2.
Vitamin D and PTH levels.
| Measures | EOA | Controls | Mean Difference (95% CI) |
Effect Size (d) |
|---|---|---|---|---|
| Vitamin D (Serum 25(OH)D concentration) |
22.30 ± 7.3 | 29.31 ± 2.59 | −7.01 ** (−10.39 to −1.23) | 0.88 |
| PTH concentration | 40.78 ± 15.53 | 39.36 ± 14.76 | −1.42 (3.15 to −7.69) | - |
Data are expressed as mean ± standard deviation. EOA: Early Osteoarthritis; PTH: Parathyroid Hormone Levels. ** p < 0.01
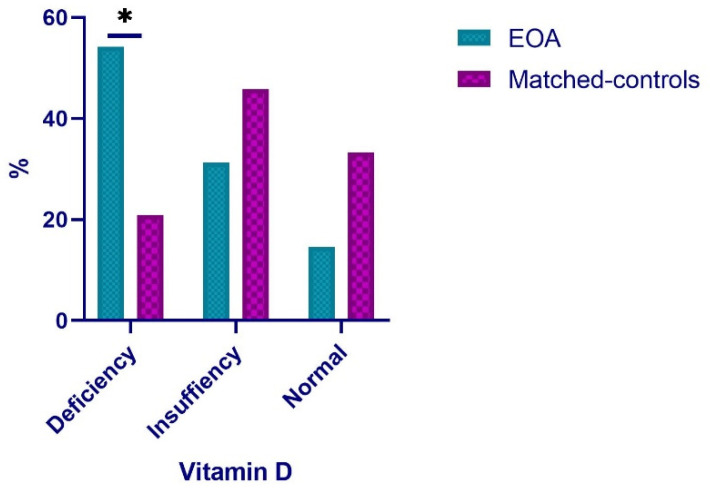
Participants in both groups were stratified according to baseline levels based on serum 25(OH)D concentration. A statistically significant higher number (Chi-squared = 8.525; p = 0.004) of patients with EOA had deficiency levels compared to the control group. For patients with EOA, 54.2% had values below 20 ng/mL (deficiency), 31.3% had values below 30 ng/mL (insufficiency), and only 14.5% had normal values (<30 mg/mL). Regarding the matched controls, the highest proportion (45.8%) presented values of insufficiency (<30 ng/mL) and normality (33.3%). Only 20.9% presented deficiency in this value (<20 ng/mL) (Figure 1).
Figure 1.
Distribution of EOA patients and matched controls according to vitamin D levels. * p < 0.05; EOA: Early Osteoarthritis.
3.2. Physical and Psychological Status
The 48 patients with EOA were divided according to their vitamin D levels. In total, 26 participants presented deficiency in their levels (<20 ng/mL) and 22 presented higher values of 20 ng/mL.
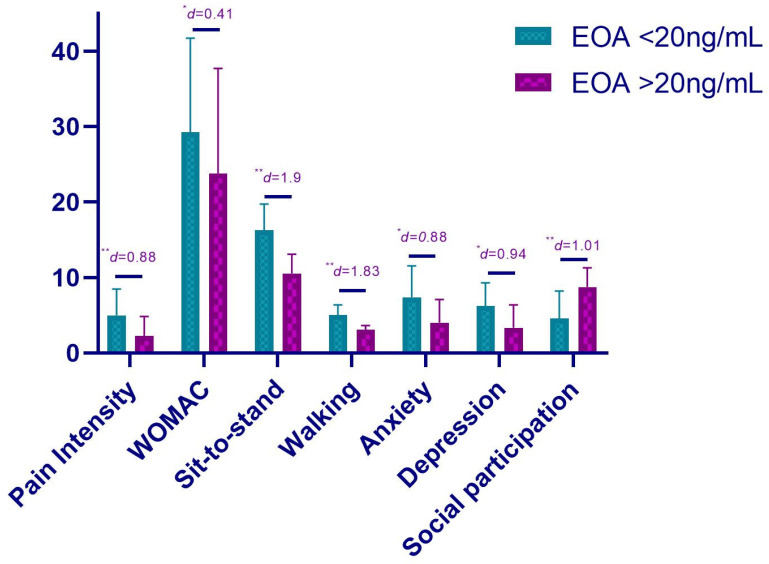
About pain intensity levels, the t-test showed statistically significant differences, showing higher levels of pain intensity in patients with lower vitamin D levels (MD = 2.73; p < 0.01; d = 0.88). Similarly, statistically significant differences were found in the WOMAC values with a higher score in the deficient group (MD = 5.51; p = 0.032; d = 0.41) (Table 3).
Table 3.
Physical and psychological status in patients with EOA according to vitamin D levels.
| Measures | EOA Deficiency (<20 ng/mL) |
EOA Sufficiency (>20 ng/mL) |
Mean Difference (95% CI) |
Effect Size (d) |
|---|---|---|---|---|
| Pain intensity | 4.95 ± 3.52 | 2.22 ± 2.59 | 2.73 ** (−3.39 to −0.76) | 0.88 |
| WOMAC Total | 29.27 ± 12.45 | 23.76 ± 13.93 | 5.51 * (−14.65 to −6.64) | 0.41 |
| Sit-to-stand (s) | 16.29 ± 3.44 | 10.53 ± 2.54 | 5.76 ** (−6.34 to −0.81) | 1.90 |
| Walking speed (km/h) | 4.99 ± 1.38 | 3.07 ± 0.54 | 1.92 ** (−1.34 to −0.11) | 1.83 |
| Anxiety | 7.36 ± 4.17 | 4.01 ± 3.05 | 4.35 * (−3.86 to −2.15) | 0.88 |
| Depression | 6.21 ± 3.06 | 3.31 ± 3.06 | 2.9 * (−2.96 to −0.15) | 0.94 |
| Social participation | 4.57 ± 3.61 | 8.73 ± 2.55 | −4.16 ** (−2.82 to −1.14) | 1.01 |
Data are expressed as mean ± standard deviation. ** p < 0.01; * p < 0.05.
Regarding functional tests, the t-test showed statistically significant differences for sit-to-stand and walking speed, showing poorer values for patients with vitamin D deficiency (MD = 5.76; p < 0.01; d = 1.90 and MD = 1.92; p < 0.01; d = 1.83, respectively) (Table 3).
Finally, regarding psychological variables, patients with vitamin D deficiency showed statistically significant differences for anxiety and depression in comparison to patients with higher vitamin D levels (MD = 3.35; p =0.028; d = 0.88 and MD = 2.9; p = 0.038; d = 0.94, respectively). In relation to social participation, statistically significant differences were also found, with lower levels for patients with vitamin D deficiency (MD = −4.16; p < 0.01; d = 1.01) (Table 3).
The physical and psychological status according to vitamin D levels is summarized in Figure 2.
Figure 2.
Physical and psychological status according to vitamin D levels. ** p < 0.01; * p < 0.05.
3.3. Correlation Analysis
The correlation analysis was performed in patients with EOA, showing strong correlations between vitamin D levels and pain intensity (r = −0.651; p < 0.01) and between vitamin D levels and anxiety (r = −0.623; p < 0.01). In addition, moderate correlations were found between vitamin D levels and disability (r = −0.443; p < 0.05), depression (r = −0.351; p < 0.05), and social participation (r = 0.355; p < 0.05). Regarding PTH levels, the strongest correlation was found with pain intensity (r = −0.686; p < 0.01). Additionally, a moderate correlation between PTH and social participation was found (r = 0.331; p < 0.05) (Table 4).
Table 4.
Correlation analysis.
| Vitamin D (Serum 25(OH)D Concentration) | PTH | |
|---|---|---|
| Pain intensity | −0.651 ** | −0.686 ** |
| WOMAC Total | −0.443 * | −0.234 |
| Anxiety | −0.623 ** | −0.153 |
| Depression | −0.351 * | −0.041 |
| Social participation | 0.355 * | 0.331 * |
** p < 0.01; * p < 0.05. PTH: Parathyroid Hormone Levels.
4. Discussion
The primary aim of this cross-sectional study was to compare the level of vitamin D of patients with EOA with matched controls. The secondary objectives were to compare the level of PTH of patients with EOA with matched controls and to determine the relationship between vitamin D deficiency, PTH level, and pain intensity, disability, psychological, and functional variables in patients with knee EOA. Results showed that EOA patients showed statistically significant lower levels of vitamin D than matched controls, but not PTH levels. It was also found that vitamin D deficiency had a statistically significant relationship with a higher level of pain intensity, disability, anxiety, and depressive symptoms, and lower social participation and physical performance. A lower PTH level is associated with higher pain intensity and lower social participation.
Patients with EOA had vitamin D insufficiency with a mean of 22.3 ng/mL, with a majority of patients having deficiency (54.2%). Similar to these results, Wu et al. found in their meta-analysis that patients with chronic pain, including OA, had a lower average vitamin D concentration than controls [21]. They found that patients with OA had a 20% difference in concentration [21]. Patients with a deficiency of vitamin D showed a statistically significant negative strong relationship between vitamin D concentration and pain intensity. The relationship between pain intensity and vitamin D is controversial [22,23,24]. For example, Cakar et al. found no relationship between vitamin D concentration and pain intensity. However, they included patients with KL grade 2 or more [22]. Contrary to them, our study included patients with EOA, so, a deficiency in vitamin D might have greater implication in the early stage of knee OA.
Vitamin D has been shown to influence the nociceptive pathway. Tague et al. found that vitamin D deficiency leads to hyperinnervation by nociceptors and hypersensitivity of mice skeletal muscles [34]. Lower levels of vitamin D are associated with higher mechanical sensitivity in patients with chronic pain [26]. Roesel hypothesized that a vitamin-D deficiency state might alter the functioning of descending inhibitory control pathways [35]. It also seems to have anti-inflammatory properties [36]. Amer and Quayyum found an inverse relationship between vitamin D and C-reactive protein in participants with a low level of vitamin D [37]. Similarly, Sun et al., 2021 showed that PTH attenuates OA pain by inhibiting subchondral sensory innervation, suggesting a critical role of PTH in OA pain [38]. Nevertheless, it is necessary to highlight that pain perception is a multifactorial experience involving the biological body state, cognitive and motivational-affective state, and the context of the individual [31]. Future studies should therefore interpret our results on vitamin D deficiency and PTH only as possible factors involved in the complexity of chronic pain.
In those patients with deficiency, we also found a statistically significant negative moderate relationship between the level of vitamin D and the level of disability. Vitamin D is essential for muscle health, as it plays a role in the maintenance of an adequate calcium plasma concentration and the differentiation of myoblast cells [35,36]. A deficiency might have altered physical performance during physical tests. Actually, Javadian et al. found a positive relationship between vitamin D concentration and quadriceps femoris muscle strength in patients with OA [23]. Future studies should evaluate the specific influence of vitamin D deficiency on the skeletal muscle properties of patients with EOA.
Finally, we also found a statistically significant positive moderate relationship between vitamin D concentration, PTH level, and social participation and a statistically significant negative strong to moderate relationship between vitamin D concentration and the presence of anxiety and depressive symptoms in that subgroup of patients. Sun exposure is the major source of vitamin D. Holick et al. warn that without sufficient sun exposure, it is not possible to produce the right amount of vitamin D [39]. Patients with higher anxiety levels, depressive symptoms, and level of pain intensity may therefore diminish their social participation and interaction, and consequently their sun exposure. Actually, Knippenberg et al. reported that there is a relationship between higher depressive symptoms and lower reports of sun exposure [40]. Future studies should assess the influence of psychological and social variables on vitamin D levels in patients with OA.
Our results on the relationship with social participation bring another factor into play, the level of physical activity [41]. It has been shown that most patients with early OA do not follow the recommendations from the Centers for Disease Control and Prevention and the American College of Sports Medicine for physical activity [42]. Higher increases of the self-reported or objective level of physical activity are associated with a higher increase of vitamin D, and participation in physical activity may increase the opportunity for sun exposure [34,43] Similarly, the practice of physical training has been shown to increase the concentration of PTH [35,36,37,38]. Future studies should focus on the interaction between the vitamin D concentration, PTH level, and the level of physical activity in patients with EOA.
Limitations
Due to the nature of the study, it is not possible to assess any cause-and-effect relationship nor the temporal relationship between the deficiency of vitamin D, PTH level, and the different variables. It is not possible to determine the direction of the relationship (e.g., if the deficiency of vitamin D is the result of the presence of OA or a responsible for its incidence). We included only Caucasian participants from the Spanish population. Because skin color hugely influences the production of vitamin D, it is not possible to extrapolate results to the worldwide population [39].
5. Conclusions
Patients with knee EOA show a lower level of vitamin D than matched controls. The presence of vitamin D deficiency among patients with knee EOA is significantly related to a higher level of pain intensity, disability, anxiety and depressive symptoms, lower social participation, and physical performance. Lower PTH level is associated with higher pain intensity and lower social participation. These results highlight the relevance of vitamin D in the early diagnosis and prevention of EOA.
Author Contributions
Conceptualization, A.A.-C., E.V.-H., and L.H.-M.; methodology, A.A.-C., F.C.-M. and L.S.-M.; formal analysis, L.S.-M., C.V.-R., F.C.-M. and J.C. (Jose Casaña); investigation, L.S.-M., C.V.-R., L.H.-M., J.C. (Joaquín Calatayud), and F.C.-M.; resources, A.A.-C., L.H.-M., M.B.-D. and E.V.-H.; data curation, L.S.-M., J.C. (Jose Casaña), J.C. (Joaquín Calatayud), C.V.-R., M.B.-D. and F.C.-M.; writing—original draft preparation, A.A.-C., L.S.-M., C.V.-R. and L.H.-M.; writing—review and editing, A.A.-C., F.C.-M.; J.C. (Jose Casaña), E.V.-H.; J.C. (Joaquín Calatayud), M.B.-D. and L.S.-M.; visualization, A.A.-C., L.H.-M., C.V.-R., M.B.-D.; L.S.-M. and J.C. (Joaquín Calatayud); supervision, E.V.-H., J.C., M.B.-D. and J.C. (José Casaña); project administration, E.V.-H., A.A.-C. and L.H.-M.; funding acquisition, E.V.-H., M.B.-D. and L.H.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 777159.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Ethics Committee (CEIm La Fe 2017/0147).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., et al. Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990-2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mora J.C., Przkora R., Cruz-Almeida Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain Res. 2018;11:2189. doi: 10.2147/JPR.S154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salaffi F., Carotti M., Stancati A., Grassi W. Health-Related Quality of Life in Older Adults with Symptomatic Hip and Knee Osteoarthritis: A Comparison with Matched Healthy Controls. Aging Clin. Exp. Res. 2005;17:255–263. doi: 10.1007/BF03324607. [DOI] [PubMed] [Google Scholar]
- 4.Chiba D., Nakamura T., Fu F.H. Early Osteoarthritis—Definition, Pathogenesis, Diagnosis, Management and Prevention: Management. Ann. Jt. 2019;4:5. doi: 10.21037/aoj.2019.01.03. [DOI] [Google Scholar]
- 5.Neogi T., Zhang Y. Osteoarthritis Prevention. Curr. Opin. Rheumatol. 2011;23:185–191. doi: 10.1097/BOR.0b013e32834307eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favero M., Ramonda R., Goldring M.B., Goldring S.R., Punzi L. Early Knee Osteoarthritis. RMD Open. 2015;1:e000062. doi: 10.1136/rmdopen-2015-000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick M.F., Chen T. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 8.Cashman K.D., Dowling K.G., Škrabáková Z., Gonzalez-Gross M., Valtueña J., De Henauw S., Moreno L., Damsgaard C.T., Michaelsen K.F., Mølgaard C., et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016;103:1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAlindon T., LaValley M., Schneider E., Nuite M., Lee J.Y., Price L.L., Lo G., Dawson-Hughes B. Effect of Vitamin D Supplementation on Progression of Knee Pain and Cartilage Volume Loss in Patients With Symptomatic Osteoarthritis: A Randomized Controlled Trial. JAMA. 2013;309:155–162. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souberbielle J.-C., Brazier F., Piketty M.-L., Cormier C., Minisola S., Cavalier E. How the Reference Values for Serum Parathyroid Hormone Concentration Are (or Should Be) Established? J. Endocrinol. Investig. 2016;40:241–256. doi: 10.1007/s40618-016-0553-2. [DOI] [PubMed] [Google Scholar]
- 11.Anderson P.H., Turner A.G., Morris H.A. Vitamin D Actions to Regulate Calcium and Skeletal Homeostasis. Clin. Biochem. 2012;45:880–886. doi: 10.1016/j.clinbiochem.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Mabey T., Honsawek S. Role of Vitamin D in Osteoarthritis: Molecular, Cellular, and Clinical Perspectives. Int. J. Endocrinol. 2015;2015:383918. doi: 10.1155/2015/383918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park C.Y. Vitamin D in the Prevention and Treatment of Osteoarthritis: From Clinical Interventions to Cellular Evidence. Nutrients. 2019;11:243. doi: 10.3390/nu11020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick M.F. High Prevalence of Vitamin D Inadequacy and Implications for Health. Mayo Clin. Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 15.Antony B., Ding C. Vitamin D and Osteoarthritis: Disparity between Observational Studies and Clinical Trials. Int. J. Rheum. Dis. 2017;20:671–674. doi: 10.1111/1756-185X.13133. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y., Liu D., Feng D., Zhao J. Association between Vitamin D and Knee Osteoarthritis: A PRISMA-Compliant Meta-Analysis. Z. Fur Orthop. Und Unf. 2021;159:281–287. doi: 10.1055/a-1098-8815. [DOI] [PubMed] [Google Scholar]
- 17.Hussain S., Singh A., Akhtar M., Najmi A.K. Vitamin D Supplementation for the Management of Knee Osteoarthritis: A Systematic Review of Randomized Controlled Trials. Rheumatol. Int. 2017;37:1489–1498. doi: 10.1007/s00296-017-3719-0. [DOI] [PubMed] [Google Scholar]
- 18.Felson D.T., Hodgson R. Identifying and Treating Preclinical and Early Osteoarthritis. Rheum. Dis. Clin. N. Am. 2014;40:699–710. doi: 10.1016/j.rdc.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neogi T., Bowes M.A., Niu J., De Souza K.M., Vincent G.R., Goggins J., Zhang Y., Felson D.T. Magnetic Resonance Imaging-Based Three-Dimensional Bone Shape of the Knee Predicts Onset of Knee Osteoarthritis: Data from the Osteoarthritis Initiative. Arthritis Rheum. 2013;65:2048–2058. doi: 10.1002/art.37987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madry H., Kon E., Condello V., Peretti G.M., Steinwachs M., Seil R., Berruto M., Engebretsen L., Filardo G., Angele P. Early Osteoarthritis of the Knee. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1753–1762. doi: 10.1007/s00167-016-4068-3. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z., Malihi Z., Stewart A.W., Lawes C.M.M., Scragg R. The Association between Vitamin D Concentration and Pain: A Systematic Review and Meta-Analysis. Public Health Nutr. 2018;21:2022–2037. doi: 10.1017/S1368980018000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cakar M., Ayanoglu S., Cabuk H., Seyran M., Dedeoglu S.S., Gurbuz H. Association between Vitamin D Concentrations and Knee Pain in Patients with Osteoarthritis. PeerJ. 2018;6:e4670. doi: 10.7717/peerj.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javadian Y., Adabi M., Heidari B., Babaei M., Firouzjahi A., Ghahhari B.Y., Hajian-Tilaki K. Quadriceps Muscle Strength Correlates With Serum Vitamin D and Knee Pain in Knee Osteoarthritis. Clin. J. Pain. 2017;33:67–70. doi: 10.1097/AJP.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 24.Muraki S., Dennison E., Jameson K., Boucher B.J., Akune T., Yoshimura N., Judge A., Arden N.K., Javaid K., Cooper C. Association of Vitamin D Status with Knee Pain and Radiographic Knee Osteoarthritis. Osteoarthr. Cartil. 2011;19:1301–1306. doi: 10.1016/j.joca.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Tague S.E., Clarke G.L., Winter M.K., McCarson K.E., Wright D.E., Smith P.G. Vitamin D Deficiency Promotes Skeletal Muscle Hypersensitivity and Sensory Hyperinnervation. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:13728–13738. doi: 10.1523/JNEUROSCI.3637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Känel R., Müller-Hartmannsgruber V., Kokinogenis G., Egloff N. Vitamin D and Central Hypersensitivity in Patients with Chronic Pain. Pain Med. 2014;15:1609–1618. doi: 10.1111/pme.12454. [DOI] [PubMed] [Google Scholar]
- 27.Roesel T.R. Does the Central Nervous System Play a Role in Vitamin D Deficiency-Related Chronic Pain? Pain. 2009;143:159–160. doi: 10.1016/j.pain.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Cantorna M.T., Humpal-Winter J., DeLuca H.F. In Vivo Upregulation of Interleukin-4 Is One Mechanism Underlying the Immunoregulatory Effects of 1,25-Dihydroxyvitamin D(3) Arch. Biochem. Biophys. 2000;377:135–138. doi: 10.1006/abbi.2000.1765. [DOI] [PubMed] [Google Scholar]
- 29.Amer M., Qayyum R. Relation between Serum 25-Hydroxyvitamin D and C-Reactive Protein in Asymptomatic Adults (from the Continuous National Health and Nutrition Examination Survey 2001 to 2006) Am. J. Cardiol. 2012;109:226–230. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 30.Sun Q., Zhen G., Li T.P., Guo Q., Li Y., Su W., Xue P., Wang X., Wan M., Guan Y., et al. Parathyroid Hormone Attenuates Osteoarthritis Pain by Remodeling Subchondral Bone in Mice. eLife. 2021;10:e66532. doi: 10.7554/eLife.66532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A., et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson R.U., Thomas G.A., Arnold A.J. Identification of 1,25-Dihydroxyvitamin D3 Receptors and Activities in Muscle. J. Biol. Chem. 1985;260:8882–8891. doi: 10.1016/S0021-9258(17)39433-4. [DOI] [PubMed] [Google Scholar]
- 33.Wassner S.J., Li J.B., Sperduto A., Norman M.E. Vitamin D Deficiency, Hypocalcemia, and Increased Skeletal Muscle Degradation in Rats. J. Clin. Investig. 1983;72:102–112. doi: 10.1172/JCI110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scragg R., Camargo C.A.J. Frequency of Leisure-Time Physical Activity and Serum 25-Hydroxyvitamin D Levels in the US Population: Results from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2008;168:577–591. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouassida A., Latiri I., Bouassida S., Zalleg D., Zaouali M., Feki Y., Gharbi N., Zbidi A., Tabka Z. Parathyroid Hormone and Physical Exercise: A Brief Review. J. Sports Sci. Med. 2006;5:367–374. [PMC free article] [PubMed] [Google Scholar]
- 36.Brahm H., Piehl-Aulin K., Ljunghall S. Bone Metabolism during Exercise and Recovery: The Influence of Plasma Volume and Physical Fitness. Calcif. Tissue Int. 1997;61:192–198. doi: 10.1007/s002239900322. [DOI] [PubMed] [Google Scholar]
- 37.Thorsen K., Kristoffersson A., Hultdin J., Lorentzon R. Effects of Moderate Endurance Exercise on Calcium, Parathyroid Hormone, and Markers of Bone Metabolism in Young Women. Calcif. Tissue Int. 1997;60:16–20. doi: 10.1007/s002239900179. [DOI] [PubMed] [Google Scholar]
- 38.Maïmoun L., Manetta J., Couret I., Dupuy A.M., Mariano-Goulart D., Micallef J.P., Peruchon E., Rossi M. The Intensity Level of Physical Exercise and the Bone Metabolism Response. Int. J. Sports Med. 2006;27:105–111. doi: 10.1055/s-2005-837621. [DOI] [PubMed] [Google Scholar]
- 39.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 40.Knippenberg S., Damoiseaux J., Bol Y., Hupperts R., Taylor B.V., Ponsonby A.-L., Dwyer T., Simpson S., van der Mei I.A.F. Higher Levels of Reported Sun Exposure, and Not Vitamin D Status, Are Associated with Less Depressive Symptoms and Fatigue in Multiple Sclerosis. Acta Neurol. Scand. 2014;129:123–131. doi: 10.1111/ane.12155. [DOI] [PubMed] [Google Scholar]
- 41.Lindström M., Hanson B.S., Östergren P.-O. Socioeconomic Differences in Leisure-Time Physical Activity: The Role of Social Participation and Social Capital in Shaping Health Related Behaviour. Soc. Sci. Med. 2001;52:441–451. doi: 10.1016/S0277-9536(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 42.Farr J.N., Going S.B., Lohman T.G., Rankin L., Kasle S., Cornett M., Cussler E. Physical Activity Levels in Patients with Early Knee Osteoarthritis Measured by Accelerometry. Arthritis Care Res. 2008;59:1229–1236. doi: 10.1002/art.24007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanner M., Richard A., Martin B., Linseisen J., Rohrmann S. Associations between Objective and Self-Reported Physical Activity and Vitamin D Serum Levels in the US Population. Cancer Causes Control. 2015;26:881–891. doi: 10.1007/s10552-015-0563-y. [DOI] [PubMed] [Google Scholar]