Abstract
Background
Delirium is an acute neuropsychological disorder that is common in hospitalised patients. It can be distressing to patients and carers and it is associated with serious adverse outcomes. Treatment options for established delirium are limited and so prevention of delirium is desirable. Non‐pharmacological interventions are thought to be important in delirium prevention.
Objectives
To assess the effectiveness of non‐pharmacological interventions designed to prevent delirium in hospitalised patients outside intensive care units (ICU).
Search methods
We searched ALOIS, the specialised register of the Cochrane Dementia and Cognitive Improvement Group, with additional searches conducted in MEDLINE, Embase, PsycINFO, CINAHL, LILACS, Web of Science Core Collection, ClinicalTrials.gov and the World Health Organization Portal/ICTRP to 16 September 2020. There were no language or date restrictions applied to the electronic searches, and no methodological filters were used to restrict the search.
Selection criteria
We included randomised controlled trials (RCTs) of single and multicomponent non‐pharmacological interventions for preventing delirium in hospitalised adults cared for outside intensive care or high dependency settings. We only included non‐pharmacological interventions which were designed and implemented to prevent delirium.
Data collection and analysis
Two review authors independently examined titles and abstracts identified by the search for eligibility and extracted data from full‐text articles. Any disagreements on eligibility and inclusion were resolved by consensus. We used standard Cochrane methodological procedures. The primary outcomes were: incidence of delirium; inpatient and later mortality; and new diagnosis of dementia. We included secondary and adverse outcomes as pre‐specified in the review protocol. We used risk ratios (RRs) as measures of treatment effect for dichotomous outcomes and between‐group mean differences for continuous outcomes. The certainty of the evidence was assessed using GRADE. A complementary exploratory analysis was undertaker using a Bayesian component network meta‐analysis fixed‐effect model to evaluate the comparative effectiveness of the individual components of multicomponent interventions and describe which components were most strongly associated with reducing the incidence of delirium.
Main results
We included 22 RCTs that recruited a total of 5718 adult participants. Fourteen trials compared a multicomponent delirium prevention intervention with usual care. Two trials compared liberal and restrictive blood transfusion thresholds. The remaining six trials each investigated a different non‐pharmacological intervention. Incidence of delirium was reported in all studies.
Using the Cochrane risk of bias tool, we identified risks of bias in all included trials. All were at high risk of performance bias as participants and personnel were not blinded to the interventions. Nine trials were at high risk of detection bias due to lack of blinding of outcome assessors and three more were at unclear risk in this domain.
Pooled data showed that multi‐component non‐pharmacological interventions probably reduce the incidence of delirium compared to usual care (10.5% incidence in the intervention group, compared to 18.4% in the control group, risk ratio (RR) 0.57, 95% confidence interval (CI) 0.46 to 0.71, I2 = 39%; 14 studies; 3693 participants; moderate‐certainty evidence, downgraded due to risk of bias).
There may be little or no effect of multicomponent interventions on inpatient mortality compared to usual care (5.2% in the intervention group, compared to 4.5% in the control group, RR 1.17, 95% CI 0.79 to 1.74, I2 = 15%; 10 studies; 2640 participants; low‐certainty evidence downgraded due to inconsistency and imprecision).
No studies of multicomponent interventions reported data on new diagnoses of dementia.
Multicomponent interventions may result in a small reduction of around a day in the duration of a delirium episode (mean difference (MD) ‐0.93, 95% CI ‐2.01 to 0.14 days, I2 = 65%; 351 participants; low‐certainty evidence downgraded due to risk of bias and imprecision). The evidence is very uncertain about the effect of multicomponent interventions on delirium severity (standardised mean difference (SMD) ‐0.49, 95% CI ‐1.13 to 0.14, I2=64%; 147 participants; very low‐certainty evidence downgraded due to risk of bias and serious imprecision). Multicomponent interventions may result in a reduction in hospital length of stay compared to usual care (MD ‐1.30 days, 95% CI ‐2.56 to ‐0.04 days, I2=91%; 3351 participants; low‐certainty evidence downgraded due to risk of bias and inconsistency), but little to no difference in new care home admission at the time of hospital discharge (RR 0.77, 95% CI 0.55 to 1.07; 536 participants; low‐certainty evidence downgraded due to risk of bias and imprecision). Reporting of other adverse outcomes was limited.
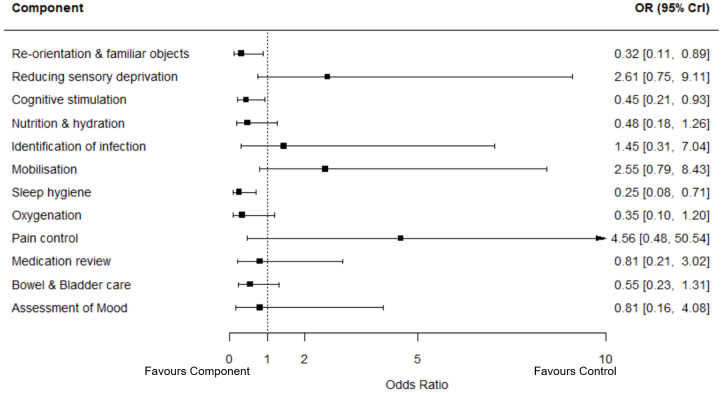
Our exploratory component network meta‐analysis found that re‐orientation (including use of familiar objects), cognitive stimulation and sleep hygiene were associated with reduced risk of incident delirium. Attention to nutrition and hydration, oxygenation, medication review, assessment of mood and bowel and bladder care were probably associated with a reduction in incident delirium but estimates included the possibility of no benefit or harm. Reducing sensory deprivation, identification of infection, mobilisation and pain control all had summary estimates that suggested potential increases in delirium incidence, but the uncertainty in the estimates was substantial.
Evidence from two trials suggests that use of a liberal transfusion threshold over a restrictive transfusion threshold probably results in little to no difference in incident delirium (RR 0.92, 95% CI 0.62 to 1.36; I2 = 9%; 294 participants; moderate‐certainty evidence downgraded due to risk of bias).
Six other interventions were examined, but evidence for each was limited to single studies and we identified no evidence of delirium prevention.
Authors' conclusions
There is moderate‐certainty evidence regarding the benefit of multicomponent non‐pharmacological interventions for the prevention of delirium in hospitalised adults, estimated to reduce incidence by 43% compared to usual care. We found no evidence of an effect on mortality. There is emerging evidence that these interventions may reduce hospital length of stay, with a trend towards reduced delirium duration, although the effect on delirium severity remains uncertain. Further research should focus on implementation and detailed analysis of the components of the interventions to support more effective, tailored practice recommendations.
Plain language summary
Non‐drug approaches for preventing delirium in adults receiving care in hospital outside of intensive care and high dependency units
Review question
We reviewed the evidence for non‐pharmacological (non‐medication‐based) approaches to prevent delirium in adults in hospital, not including those treated in intensive care units (ICU, specialised wards for the care of critically ill patients).
Background
Delirium is an important illness which is common among adults, especially older adults who are in hospital. It is sometimes referred to as an 'acute confusional state'. Typically, a person with delirium has sudden onset of confusion, which fluctuates, and often includes impaired concentration, memory and thinking skills; reduced awareness of surroundings; drowsiness or agitation and restlessness; and hallucinations, which are usually visual (seeing things which are not really there). It can be distressing for the individual with delirium and their family. It is also associated with increased risks of complications, such as dying in hospital, having a longer hospital stay, and requiring more care after discharge. Increasingly, there is evidence that delirium is associated with an increased risk of permanent worsening of memory and thinking skills, including development or worsening of dementia.
Non‐pharmacological approaches are approaches which do not use medications, but which focus on other aspects of care. They are already recognised as important in reducing the risk of delirium, particularly multicomponent interventions which target several of the common risk factors for delirium. It is not known which components of these complex interventions are most important in preventing delirium and this was something we wanted to find out.
Study characteristics
We searched up to 16 September 2020 for reports of studies in which people in hospital were randomly allocated to a non‐pharmacological intervention intended to prevent delirium or to usual hospital care. We found 22 studies with 5718 participants. Fourteen of the studies were of multicomponent approaches; two studies looked at different cut‐offs for giving a blood transfusion after an orthopaedic operation; the remaining six studies all considered different approaches.
Key findings
Multicomponent approaches probably reduce occurrence of delirium by 43% compared to usual hospital care. This means that two in five cases of delirium in adults in hospital wards (other than ICU) can be prevented by multicomponent, non‐pharmacological approaches. These interventions may also reduce the length of time people stay in hospital and, if delirium does occur, they may reduce the duration of the delirium episode by about a day. However, these approaches may have little or no effect on the risk of dying in hospital. The studies did not investigate the effect of multicomponent interventions on the development or worsening of dementia. There was little information about whether the interventions had any harmful effects.
Using a new statistical technique, we found that the following components within each intervention were most important for preventing delirium: (a) trying to keep people well‐oriented to their surroundings and making their surroundings more familiar, (b) providing stimulation to memory and thinking skills, and (c) trying to improve sleep (through sleep hygiene measures). We could not be so certain about the effect of other components, largely because not enough evidence was available. More research is needed comparing the specific components included in multicomponent interventions to help determine the most effective and efficient ways to prevent delirium.
The evidence for other, single‐component, non‐pharmacological interventions was very limited.
Certainty of the evidence
There were some limitations in the studies which may affect the results. In many included studies the people in the study and sometimes researchers were aware of who was and was not receiving the intervention.
There was very little information about people living with dementia, who are at greater risk of experiencing delirium.
External funding
Funding to support researchers to undertake this review was received from the National Institute for Health Research (Incentive Award 130725) and Medical Research Scotland (Vacation Scholarship).
Summary of findings
Summary of findings 1. Non‐pharmacological multicomponent interventions for preventing delirium in hospitalised non‐ICU patients.
| Multicomponent delirium prevention intervention compared with usual care for hospitalised adults | |||||
|
Patients: adults (aged 18 years and over) in hospital for any reason Settings: receiving care in general hospital settings (excluding those in intensive care or high dependency units; also known as level 3 and level 2 critical care settings) Intervention: multicomponent interventions designed to prevent delirium Comparison: usual hospital care | |||||
|
Outcomes No of participants (studies) |
Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
Certainty of the evidence (GRADE) |
Comments | |
|
Assumed risk Risk with usual care |
Corresponding risk Risk with multicomponent intervention |
||||
|
Incidence of delirium during hospital admission validated diagnostic instruments1 3693 participants (14 studies) |
184 per 10002 |
105 per 1000 (85 to 216) |
RR 0.57 (0.46 to 0.71) |
⨁⨁⨁◯ MODERATE3 | |
|
Inpatient mortality 2640 participants (10 studies) |
45 per 10002 |
52 per 1000 (37 to 73) |
RR 1.17 (0.79 to 1.74) |
⊕⊕◯◯ LOW4 | |
|
New diagnosis of dementia (at any time point after randomisation) Not measured |
No relevant studies | No relevant studies | No relevant studies | No relevant studies | |
|
Duration of delirium (days) (any time during hospital admission) 351 participants (6 studies) |
The mean duration of delirium in the control groups ranged from 2.1 to 10.2 days | The mean duration of delirium in the intervention groups was 0.93 days shorter (2.01 days shorter to 0.14 days longer) | ⊕⊕◯◯ LOW5 |
||
|
Delirium severity (any time during hospital admission) validated diagnostic instruments6 147 participants (5 studies) |
The standardised mean severity of delirium in the intervention groups was 0.49 standard deviations lower (1.13 lower to 0.14 higher)10 | ⊕◯◯◯ VERY LOW7 |
A standardised mean severity of 0.49 standard deviations represents a moderate effect. The 95% confidence interval encompasses a very large effect and little or no effect, indicating serious imprecision. | ||
|
Length of hospital admission (days) 3351 participants (10 studies) |
The mean length of hospital admission in the control groups ranged from 5 to 38 days | The mean length of admission in the intervention groups was 1.30 days shorter (2.56 days shorter to 0.04 days shorter) | ⨁⨁◯◯ LOW8 | ||
|
Discharge from hospital to new long‐term care placement 536 participants (1 study) |
247 per 10002 |
190 per 1000 (136 to 264) |
RR 0.77 (0.55 to 1.07) |
⊕⊕◯◯ LOW9 | |
* The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95%CI). CI: Confidence interval; RR: Risk ratio;
GRADE Working Group grades of evidence HHigh certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
- Delirium was diagnosed using the CAM, DRS‐R‐98, DSM‐IV, DSM‐V criteria
- The assumed risk is the risk in the control group
- Downgraded one level for study limitations (high risk of performance bias due to the lack of blinding of participants and personnel in all studies (due to the nature of the intervention) and outcome assessors unblinded in 6 studies)
- Downgraded one level for inconsistency and one level for imprecision (pooled estimate includes both no effect, appreciable benefit and appreciable harm)
- Downgraded one level for study limitations (high risk of performance bias due to lack of blinding of participants and personnel) and one level for imprecision (Minimal important difference (MID) of 1 day assumed. 95% confidence limits around the pooled estimate of mean difference includes both ’no difference’, and the MID)
- Delirium severity was assessed using CAM, CAM‐S, DRS‐R‐98
- Downgraded one level for study limitations (high risk of performance bias due to lack of blinding of participants and personnel and outcome assessors unblinded in 3 studies) and two levels for serious imprecision (based on small, pooled sample size of 147 participants)
- Downgraded one level for study limitations (high risk of performance bias due to lack of blinding of participants and personnel; outcome assessors unblinded in 4 studies) and one level for inconsistency (significant statistical heterogeneity, with I2 = 91%)
- Downgraded one level for study limitations (high risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (based on results from a single study)
Background
Description of the condition
Delirium is a disturbance of consciousness and cognition, which usually has a rapid onset and a fluctuating course. The core features of delirium are defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, and include "disturbance in attention, awareness and cognition, which develops over a short period of time and tends to fluctuate in severity during the course of a day. It represents an acute change from baseline and is not better explained by a pre‐existing, established or evolving neurocognitive disorder or a severely reduced level of arousal such as coma. There should be evidence from history, physical examination or laboratory findings that the disturbance is a direct physiological consequence of another medical condition, substance intoxication or withdrawal, or exposure to a toxin, or is due to multiple etiologies" (American Psychiatric Association 2013). The International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10) definition of delirium is similar, but also includes disturbance of the sleep‐wake cycle and does not specify that there is a definitive underlying aetiology (World Health Organization 2016).
Delirium is highly prevalent across all inpatient hospital settings, with an estimated occurrence of 23% (Gibb 2020). The highest prevalence rates were found in patients who had experienced cardiac surgery, neurosurgery, trauma, radiotherapy and neurology (36% to 41%) (Schubert 2018). However, delirium was also common in geriatric medicine, internal medicine, general surgery, reconstructive plastic surgery and cranio‐maxillo‐facial surgery (22% to 29%) (Schubert 2018). A point prevalence study conducted in Ireland found that 20% of adult hospital inpatients had delirium on a single day and that age was associated with higher prevalence (5% in those under 50 years of age, versus 35% in those aged over 80) (Ryan 2013). Pooled prevalence of delirium from meta‐analysis of 25 studies was 15% with cumulative incidence of new delirium of 9% over two weeks (Gibb 2020). This ranges from 11% to 14% in general medicine, 20% to 29% in geriatric medicine, 10% to 27% in stroke units, 47% in palliative care settings and 12% to 51% in orthopaedic units (Inouye 2014).
Delirium is associated with a range of serious adverse health outcomes. Factors associated with poorer outcomes after an episode of delirium include: longer duration and severity; hypoactive delirium subtype; and the presence of comorbid dementia and depression (Jackson 2016a). A meta‐analysis of observational study data from older adults found those with delirium were at an increased risk of death (hazard ratio (HR) 1.95, 95% confidence interval (CI) 1.51 to 2.52), after adjusting for age, sex, comorbidity illness or illness severity and baseline dementia (Witlox 2010). Evidence indicates increased hospital length of stay is both a risk factor for developing delirium and an outcome associated with experiencing delirium (Ahmed 2014; Aitken 2017; Pendlebury 2015).
Delirium can have irreversible effects on an individual's function. A UK cohort of hospital admissions with mental health problems found only 25% of those experiencing delirium had a clinically important recovery in their activities of daily living six months after the episode (Whittamore 2013). Delirium is also associated with an increased risk in overall dependency (odds ratio (OR) 2.56, 95% CI 1.37 to 4.76) (Pendlebury 2015). This can lead to an increased risk of requiring formal institutional care (Witlox 2010), particularly for those with delirium superimposed on an existing dementia (Burton 2018).
Undiagnosed cognitive impairment and dementia are common in older adults presenting with delirium (Jackson 2016b). In adults with Alzheimer's disease, an episode of delirium was found to accelerate cognitive decline, compared to those who did not experience delirium (Fong 2009). Combined neuropathological and clinical cohort study data have confirmed that delirium both accelerates existing cognitive decline and is a risk factor for developing dementia (Davis 2012). Delirium symptoms experienced in early older age (60 to 69 years) are associated with poorer cognitive function after adjustment for other dementia risk factors (Tsui 2018).
An important consideration in evaluating the impact of an episode of delirium is both the duration of the episode and the severity, and validated measures are available to quantitatively assess both parameters (Vasunilashorn 2016). Persistent delirium (lasting beyond hospital discharge) is common, estimated to affect "25.6% (95% CI 7.9% to 43.3%)" of older hospitalised patients at three months follow‐up (Cole 2009). Dementia, malignancy, multi morbidity, increased delirium severity, hypoactive subtype and hypoxic illness have been independently associated with persistent delirium (Cole 2015; Dasgupta 2010).
Significantly, delirium is distressing, particularly to family members who witness episodes (Finucane 2017), and also may have lasting effects on the individual patient if they recall their in‐hospital experiences (Grover 2015; Partridge 2013). It can also cause distress to staff caring for these patients (Agar 2012; Partridge 2013; Waterfield 2018).
Delirium has considerable economic impact on healthcare systems and society (Leslie 2011). Estimates suggest the costs for those with delirium are two and a half times greater per day than for those without delirium (Leslie 2008). The cost‐effectiveness of multicomponent delirium‐prevention interventions has been demonstrated using data from a non‐randomised study (Akunne 2012), however there is a lack of data on cost‐effectiveness from randomised trials (Siddiqi 2016).
Description of the intervention
This review assesses the effectiveness of non‐pharmacological interventions for preventing delirium in hospitalised patients outside the intensive care unit (ICU) and high dependency unit (HDU) setting. Non‐pharmacological interventions can be broadly divided into single component interventions, which often target a specific risk factor, and multicomponent interventions, which target multiple risk factors for delirium. Multicomponent interventions are often based around care delivered according to specific protocols, and target risk factors such as sleep deprivation, immobility, dehydration and sensory impairment (Inouye 1999a). The National Institute for Health and Care Excellence (NICE) recommend assessing for the presence of delirium risk factors in adults aged 65 years and older; those with cognitive impairment; those with a hip fracture; and those with severe illness at the time of hospital presentation (NICE 2010). Thereafter, it recommends a multicomponent intervention tailored to needs and care setting, delivered by a multidisciplinary team (NICE 2010).
How the intervention might work
A number of risk factors for delirium have been identified (Ahmed 2014; Pendlebury 2015; Wilson 2020). While some of these are non‐modifiable factors such as age and comorbidity, there are others which are potentially modifiable, including dehydration, sensory impairment and urinary catheterisation (Ahmed 2014). Predictors of incident delirium during a hospital admission include dementia, dependence in activities of daily living, and increased illness severity (O'Regan 2018). Delirium has been described as the interaction between an individual's baseline vulnerability (based on predisposing factors such as age and cognitive function) and precipitating factors or insults occurring during the hospital admission (Inouye 1996). These precipitating factors can be further divided into those which are related to the presenting illness an individual is experiencing and those occurring after admission, which include environmental factors, pain management interventions, and sleep deprivation (Wilson 2020). Furthermore, it has been suggested that a combination of risk factors for delirium may interact to increase vulnerability and that susceptibility can be scored at the time of admission (Pendlebury 2017). It is thought that non‐pharmacological interventions can be used to address these risk factors, targeting those vulnerable to developing delirium, as an effective prevention strategy.
Why it is important to do this review
Delirium is common across all inpatient settings and, in view of the serious complications, costs and consequences arising, it is a priority for healthcare practitioners and providers. Establishing the degree to which delirium can be prevented, and identifying evidence‐based strategies for prevention, will help inform evidence‐based care pathways.
Multicomponent interventions have been shown in randomised controlled trials to reduce the incidence of delirium (Martinez 2015; Siddiqi 2016). However, the reductions seen in delirium incidence have not been associated with statistically significant reductions in length of stay or in longer‐term sequelae, including mortality or the need for admission to long‐term care (Hshieh 2015; Martinez 2015). There is uncertainty about the precision and certainty of these findings and the extent to which frailty influences outcomes (Teale 2015). Delirium and frailty (defined as '"a diminished ability to compensate for stressors") are conditions associated with poor outcomes in older people and they have been postulated to be different manifestations of "shared vulnerability to stress" (Quinlan 2011). This relationship is complex and poorly understood; recent evidence suggests that mortality risk in delirium is greatest in those with lower levels of frailty (Dani 2018), although the role of illness severity in mediating this association is not known. It would, however, be helpful to identify if those with frailty are differentially affected by delirium‐prevention interventions.
Some of the risk factors for delirium — including malnutrition, dehydration, restraint use, and iatrogenic events (condition caused by medical or surgical interventions) — can be seen as measures of the quality of hospital care. The occurrence of delirium has been linked to the quality of care delivered to inpatients, which can highlight areas for improvement (Inouye 1999b). Clinical adverse events which have been associated with delirium, such as falls and pressure ulcers, are also priorities for reduction within inpatient settings. The associations between delirium and dementia mean that interventions to prevent delirium are of interest to the wider public health agenda of dementia prevention (Fong 2015).
Over the past decade there has been a rapid increase in the number of randomised trials of delirium‐prevention interventions. In 2007, a Cochrane Review identified six trials evaluating six interventions to prevent delirium, only one of which was a non‐pharmacological intervention (Siddiqi 2007). The 2016 update identified 39 trials of 22 interventions, including seven trials of multicomponent interventions and two other non‐pharmacological interventions (Siddiqi 2016). There was heterogeneity among the multicomponent interventions studied, with the number of components ranging from two to 13. In this review, we focused on non‐pharmacological interventions only to allow a more detailed synthesis of the current evidence in this area. We also add a component network meta‐analysis to try to develop understanding of which components are necessary and most effective. This should allow more robust recommendations for practice and future research to be made.
Objectives
To assess the effectiveness of non‐pharmacological interventions designed to prevent delirium in hospitalised non‐intensive care unit (ICU) patients.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐RCTs.
Types of participants
We included studies of adult participants (aged 18 years and over) who were admitted to general hospital settings. This included acute and rehabilitation hospitals and sub‐acute care provided in hospital. We excluded studies conducted in community settings, such as long‐term care or nursing homes; these are considered in a separate Cochrane Review (Woodhouse 2019). If settings were mixed, we only included the study if data could be extracted specifically for the hospitalised patients.
We excluded studies conducted in intensive care unit (ICU) and high dependency unit (HDU) settings, due to the different populations and interventions likely to be found in such environments. ICU settings, also known as Level 3 settings, are those where patients require either respiratory support alone, or support of a minimum of two organs (Intensive Care Society 2009). HDU settings, also known as Level 2 settings, are those where patients either receive single‐organ support or are stepping down from Level 3 care; need preoperative optimisation using invasive monitoring; or need extended postoperative care (Intensive Care Society 2009). The evidence for delirium prevention in ICU settings is evaluated in a separate Cochrane Review (Herling 2018).
We excluded studies of delirium associated with psychoactive substance misuse or withdrawal, as these presentations are clinically distinct.
We considered studies of delirium prevention in patients receiving only in‐hospital specialist palliative care and evaluated them using a sensitivity analysis within this review. Delirium prevalence in specialist palliative care settings can be very high (approximately 42% of admissions to specialist palliative care units) and the goals of care may be different in this context (Bush 2017).
Types of interventions
We only included non‐pharmacological interventions which were designed and implemented to prevent delirium. We did not include studies targeting those with "geriatric syndromes", rather than delirium specifically.
Eligible interventions were multicomponent interventions or single‐component interventions targeting a specific risk factor for delirium (e.g. sleep disturbance, dehydration, disorientation). Interventions could be implemented at the level of the ward or department providing care, or at the individual level.
We excluded studies of pharmacological interventions to prevent delirium. Specifically, this included tablets, infusions, injectable medications, inhaled medications, or anaesthetic gases, given to all participants in active treatment arms with the intention of preventing delirium. Studies that included correction of abnormal physiology using a pharmacological intervention as part of a multicomponent intervention, e.g. administration of oxygen in presence of low oxygen saturations, were eligible for inclusion.
Comparators could be usual care or an active control intervention.
Types of outcome measures
We included all studies which fulfilled our other eligibility criteria and which measured any of the primary or secondary outcomes. We prespecified clinically important secondary outcomes and adverse outcomes which are relevant to patients, families and healthcare providers.
Primary outcomes
Incidence of delirium during hospital admission, using a validated diagnostic method. (Studies using only a positive screening test in the absence of a formal diagnosis were excluded.)
Mortality as an inpatient, between one and three months, six and 12 months, and beyond 12 months from randomisation.
New diagnosis of dementia, made between one and three months, six and 12 months, and beyond 12 months from randomisation.
Secondary outcomes
Duration of delirium episode, measured in days.
Severity of delirium, measured using validated instruments including the Memorial Delirium Assessment Scale (MDAS) (Breitbart 1997), Delirium Rating Scale (DRS) (Trzepacz 1988), and Delirium Rating Scale Revised 1998 (DRS‐R‐98) (Trzepacz 2001).
Length of hospital admission, measured in days.
Use of new psychotropic medication during hospital admission.
Activities of daily living, measured using a validated instrument including the Barthel Index (Mahoney 1965) and Katz Index (Katz 1963), between one and three months, six and 12 months, and beyond 12 months from randomisation.
Quality of life, measured using a validated patient reported measure, between one and three months, six and 12 months, and beyond 12 months from randomisation.
Carer's quality of life, using a validated carer‐reported measure, between one and three months, six and 12 months, and beyond 12 months from randomisation.
Withdrawal from protocol by participants.
Adverse outcomes
Readmission to hospital within 30 days of discharge.
Progression of existing dementia, measured using a validated instrument, between one and three months, six and 12 months, and beyond 12 months from randomisation.
New care‐home admission at discharge and between one and three months, six and 12 months, and beyond 12 months from randomisation.
Falls.
Pressure ulcers.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register, up to the 16th September 2020. ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia (prevention and treatment), mild cognitive impairment and cognitive improvement. The studies are identified from: 1. monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS; 2. monthly searches of the trial registers: the WHO International Clinical Trials Registry Platform (which covers ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others) and ClinicalTrials.gov; 3. quarterly search of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL); 4. six‐monthly searches of a number of grey literature sources from ISI Web of Science Core Collection. Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group. We performed additional searches in many of the sources listed above, to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used are described in Appendix 1. The most recent search was carried out on the 16 September 2020.
Searching other resources
We examined reference lists from identified articles and relevant systematic reviews to identify any additional potential trials to review for eligibility. We searched the ClinicalTrials.gov database, to identify any relevant ongoing trials. We compared the trials that meet our review inclusion criteria with the trials register to identify any trials where results have been unpublished. We contacted the lead author of any unpublished trials, to ask if they are prepared to share their results (we examined these against the published protocols to ensure they have been consistently analysed).
Data collection and analysis
Selection of studies
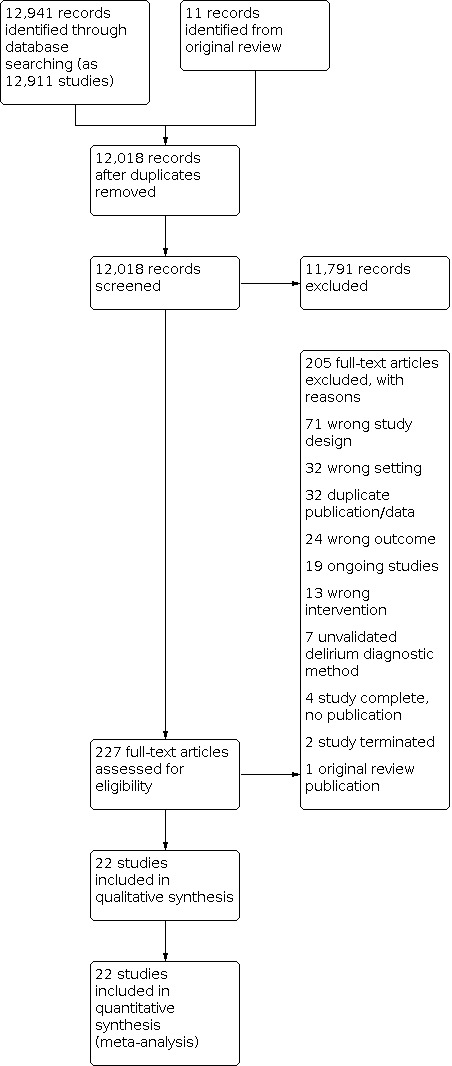
We directly imported the results of the literature searches into Covidence software (Covidence 2017). This automatically removed direct duplicate records. Thereafter, two review authors, with experience in conducting systematic reviews, independently screened the titles and abstracts of all identified articles and removed irrelevant results. We resolved any disagreements by discussion, involving a third review author if necessary. Two review authors then independently examined the full‐text articles of potentially relevant articles against the review eligibility criteria. We resolved any disagreements by consensus with a third review author. If we were unable to determine eligibility based on the available information, for example if only an abstract was identified, we contacted the study authors for clarification and additional data as necessary. We listed all articles excluded after full‐text assessment in the Characteristics of included studies table, with reasons for exclusion. We present a PRISMA diagram to summarise the study selection process (Figure 1).
1.
Data extraction and management
We created a data extraction tool, adapted from the version used in the previous version of this review (Siddiqi 2016). Two review authors extracted data using this tool, discussing any disagreements and involving a third author if necessary.
To allow use of more of the reported data for syntheses, where medians and Interquartile ranges (IQR) or ranges were presented rather than means and standard deviations, we converted values as follows. We assumed the median value was equivalent to the mean. We estimated the standard deviation as ’IQR/1.35’ or ’range/4’ (small studies, n < 70) or ’range/6’ (larger studies, n > 70).
For delirium incidence and severity, where results were presented for multiple time points and no summary data were available, we used the highest recorded number or peak values for the intervention and control arm. This was because we were interested in interventions that reduced the overall burden of delirium. For example, if delirium severity was ascertained on days one, three, and five of the hospital stay, then we included only the highest of the three scores (most severe) in our analysis of delirium severity. For severity and duration of delirium, data were included only from patients with delirium.
We used RevMan Web to produce tables documenting the characteristics of included, excluded and ongoing trials (RevMan Web 2021). We created a summary of findings table using GRADE Pro Software (GRADEpro 2014).
Component network meta‐analysis
The previous version of this review (Siddiqi 2016), included a descriptive table of multicomponent intervention components, with 20 components described from the seven included studies (Abizanda 2011; Bonaventura 2007; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012). These 20 'components' were: individualised care; checklists/protocols; structured education/training of staff or carers; reorientation; attention to sensory deprivation; familiar objects; cognitive stimulation; nutrition/hydration; identification of infection; mobilisation; sleep hygiene; multidisciplinary care; comprehensive geriatric assessment; oxygenation; electrolytes; pain control; medication review; mood (assessment for depression/anxiety); bowel/bladder care and postoperative complications.
To undertake our exploratory component network meta‐analysis we extracted text from each of the included studies about their specific multicomponent intervention and the components included in each study ( Appendix 2). We mapped the 14 included studies to the originally described 20 components and then reviewed these. Due to the small number of identified studies relative to the number of potential components, components had to be grouped to enable analysis to be undertaken. This was done by clinical review authors and experts in network meta‐analysis to ensure clinical and methodological integrity in the approach.
We recognised that some of these components described how the intervention was delivered (e.g. individualised care (often described as tailoring), or use of checklists/protocols) as distinct from components of the intervention itself. Thus we considered tailored/individualised care, use of protocol/checklist, staff education and multidisciplinary care as modes of delivery, rather than components. We also considered comprehensive geriatric assessment for inclusion in the network as it was reported in three trials (Hempenius 2013; Lundstrom 2007; Partridge 2017), but clinically this entity encapsulated several of the components already specified (Welsh 2014) and thus we classified it as a mode of delivery, rather than an individual component. Similarly, family involvement, reported in three studies, covered both the delivery of the intervention (Hosie 2020; Martinez 2012; Wang 2020) and a component of the intervention itself (Hosie 2020) so we did not include it as a component in the analysis. Inclusion of family involvement resulted in the model failing to achieve convergence.
Management of postoperative complications was identified in a single study (Marcantonio 2001) and we did not consider it to be analogous to other components, so excluded it. Fluid and electrolyte balance was also only considered in a single study (Marcantonio 2001). Considering the clinical context, we combined this with the nutrition/hydration component and included it in the analysis in this format. Finally, use of familiar objects was reported in two studies (Bonaventura 2007; Martinez 2012). These were only delivered in studies which included a reorientation component and we considered it clinically appropriate to combine these components as reorientation (including use of familiar objects).
Assessment of risk of bias in included studies
Two review authors independently performed a risk of bias assessment. We evaluated each study using the criteria described in the original Cochrane tool for assessing risk of bias (Higgins 2011). We assessed trials for the domains of: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting and other bias.
Cluster‐RCTs are subject to additional biases: recruitment bias (recruitment of individual study participants after randomisation of clusters), chance between‐cluster baseline imbalances due to a small number of clusters, loss of clusters (e.g. withdrawal of a study site), not accounting for clustering during the analysis (incorrect unit of analysis issues), or bias introduced through combining data from cluster‐randomised and individually‐randomised trials in meta analyses (risk of underestimation of treatment effects). We considered those which extended beyond the traditional risk of bias domains under the heading of 'other bias'.
We judged each study as being at either high, low or unclear risk of bias in each domain. We resolved any disagreements by discussion between the two review authors, involving a third author if necessary. We produced summary tables and figures of the risk of bias assessment, with justification, in RevMan Web (RevMan Web 2021). When using GRADE methods to assess the certainty of the evidence from pooled analyses, we downgraded certainty for risk of bias, or study limitations, where the majority or all of included studies had limitations likely to be relevant to the outcome of interest (e.g. we downgraded for high risk of performance and detection bias for outcomes such as delirium incidence; whereas ascertainment of mortality would probably not be affected by lack of blinding but could be subject to important selection or attrition bias).
Measures of treatment effect
For continuous outcomes, we calculated between‐group (intervention versus control) mean differences (MDs) with 95% confidence intervals (CIs). If studies used different instruments to measure the same continuous outcome, we calculated the standardised mean difference (SMD). SMD was interpreted in accordance with the Cochrane Handbook. For dichotomous outcomes, we calculated risk ratios (RRs) with 95% CIs.
Unit of analysis issues
Where cluster‐randomised studies had analysed data using statistical methods that account for clustering, we extracted the adjusted effect measures (RRs) and their 95% CIs. If an included study reported only unadjusted analyses, then we approximated corrected analyses by extracting data on the number of clusters, mean size of each cluster, primary outcome data and estimates of intra‐cluster correlation coefficient (ICC). If approximately corrected analyses were not possible, then we extracted the primary data and calculated RRs with 95% CIs.
Dealing with missing data
We contacted study authors to try to obtain data not reported in the publication. We reported missing data for each included study, including reporting the number of participants included in the final analysis as a proportion of all participants in the study. We performed available‐case analysis, including data on those participants whose outcomes were known. We reported incomplete outcome assessment in the risk of bias table for each study, including an assessment of the potential impact of missing data on the results.
Assessment of heterogeneity
We described clinical heterogeneity. If we considered the data to be appropriate for quantitative synthesis, we calculated statistical heterogeneity and described it using the I2 statistic (Higgins 2002). Interpretation of the I2 statistic was in accordance with guidance in the Cochrane Handbook (Deeks 2019). Assessment of heterogeneity was based on visual analysis of the forest plot, directions of effect at individual study level and the I2 statistic, with I2 of 75% to 100% indicating considerable heterogeneity (Guyatt 2011).
Assessment of reporting biases
We compared the studies included in our review with clinical trial registries, to identify trials with unpublished results. We compared the published studies included in the review against their protocols to check adherence to planned methods.
We used funnel plots to assess for possible publication bias for our two primary outcomes with pooled data (incidence of delirium and in‐hospital mortality) for multicomponent interventions and used these to inform our GRADE assessments.
Data synthesis
Where it was appropriate, we performed meta‐analysis of extracted data using RevMan Web (RevMan Web 2021). We used a random‐effects model. We calculated pooled RRs with 95% CIs for dichotomous outcomes (intervention versus control), and pooled MDs with 95% CIs for continuous outcomes. If studies used different instruments to measure the same continuous outcome, we calculated the SMD. We synthesised outcomes from appropriately adjusted cluster‐RCTs. We performed data synthesis only where it was considered that the identified studies were clinically homogenous, such that pooling of data was appropriate and valid comparisons could be made. If the clinical heterogeneity was significant, we reported a narrative evidence synthesis.
Component network meta‐analysis
We used a Bayesian component network meta‐analysis fixed‐effect model to evaluate the comparative effectiveness of the individual components of interventions and to draw conclusions about which components were most strongly associated with reducing the incidence of delirium. A fixed‐effect model was used because there were not enough data to estimate between‐study heterogeneity. Models were constructed as described by Welton 2009, using code from Freeman 2018 adapted to include a binomial likelihood with logit link for binary outcomes. We fitted an additive effects model which assumes the effects of components add together directly when combined. If data allowed we planned to fit a model relaxing the assumption of additivity through the inclusion of pairwise interactions between components which would allow combinations of components to have synergistic or antagonistic effects. However, due to the small number of trials relative to the number of component parameters in the model we were unable to fit this model.
Bayesian analyses were run using WinBUGS version 1.4.3 and R version 4.0.1 through the R2WinBUGS package (Sturtz 2005). Models were run with a burn in of at least 20,000 iterations and a sample of 30,000 iterations. Convergence was assessed through history and density plots. We used vague prior distributions for trial‐specific baselines (e.g. the log‐odds of the outcome in the control group) and component effects. Results are reported as odds ratios (ORs) with 95% credibility intervals with component effects reported relative to treatment‐as‐usual.
Subgroup analysis and investigation of heterogeneity
From our main meta‐analysis results we performed subgroup analyses for participants in trials conducted in medical versus surgical inpatient settings; and for those with and without a diagnosis of dementia (measured using a validated diagnostic instrument). We were unable to undertake the planned analysis of those who were considered to have frailty versus those who were not (measured using a validated instrument) due to lack of available data in the included studies.
Sensitivity analysis
Sensitivity analysis to remove studies in which participants were receiving palliative care only versus those receiving other medical or surgical treatment was undertaken as planned.
We did not undertake our planned sensitivity analysis around risk of methodological bias, as all studies were considered at high risk of bias in at least one domain.
In the protocol, we planned sensitivity analyses to address two possible scenarios: uncertainty about the optimal way to define components, and an intervention component being delivered to only a fraction of a trial arm. However, we did not encounter either of these scenarios.
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro Guideline Development Tool software (GRADEpro 2014) to determine the overall certainty of the evidence and to generate a summary of findings table for the outcomes: incidence of delirium, inpatient mortality, new diagnosis of dementia (at any time point after randomisation), duration of delirium, peak delirium severity, length of hospital admission, and discharge to new long‐term care placement. We created a summary of findings table only for the multicomponent intervention analysis as the other interventions had too few included studies to draw conclusions.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
The search results are summarised in a PRISMA diagram (Figure 1). Of the 227 full‐text articles retrieved, 26 were considered eligible for inclusion; 182 were excluded (see Excluded studies); and 19 are ongoing (see Characteristics of ongoing studies). Of the 26 studies considered eligible for inclusion, four had no published results available including searching for publications based on author names and study titles from within trial registry entries. We were unsuccessful in attempts to contact the named study contacts identified in the Trial Registry entries by email. These four studies have been listed as 'Studies awaiting classification' and are described in Characteristics of studies awaiting classification. Twenty‐two studies are included in the review.
Included studies
The 22 studies included a total study population of 5718 randomised participants. The trials assessed multicomponent and seven different single‐component non‐pharmacological interventions.
Study design
All 22 studies were randomised controlled trials (RCTs). Four of them were cluster‐randomised in design (Chen 2017; Hosie 2020; Wang 2020; Young 2020).
Eighteen studies evaluated a delirium prevention intervention against usual care (Abizanda 2011; Avendano‐Cespedes 2016; Bonaventura 2007; Boustani 2012; Cetinkaya 2019; Chen 2017; Dong 2020; Hempenius 2013; Hosie 2020; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Martinez‐Velilla 2019; Nadler 2017; Partridge 2017; Wang 2020; Young 2020). Two studies compared use of different thresholds for physiological correction (Fan 2014; Gruber‐Baldini 2013). One study compared a delirium prevention intervention to a placebo (Gao 2018). One study compared two different interventions (Watne 2014).
Sample size
The sample size of included studies ranged from 50 to 713 randomised participants. Five studies randomised fewer than 100 participants (Avendano‐Cespedes 2016; Bonaventura 2007; Cetinkaya 2019; Gao 2018; Hosie 2020).
Setting
Thirteen studies were conducted in patients under the care of surgical teams for elective or emergency surgical or procedural interventions and care. Orthopaedic settings were the commonest, in eight of the included studies (Cetinkaya 2019; Fan 2014; Gao 2018; Gruber‐Baldini 2013; Lundstrom 2007; Marcantonio 2001; Nadler 2017; Watne 2014). In one study, participants were undergoing elective surgery for known cancer (Hempenius 2013). Four studies were conducted in other surgical settings (Chen 2017; Dong 2020; Partridge 2017; Wang 2020). Seven studies were conducted in a general medical or specialist geriatric medical hospital environment (Abizanda 2011; Avendano‐Cespedes 2016; Bonaventura 2007; Boustani 2012; Jeffs 2013; Martinez 2012; Martinez‐Velilla 2019). One study was conducted in inpatient palliative care settings for individuals with a diagnosis of cancer (Hosie 2020). One study was conducted in both specialist wards for older adults and orthopaedic trauma wards (Young 2020).
Participants
Age
In 12 studies the mean age of included participants was between 70 to 79 years in one or both arms. Seven studies had a mean age in both allocation arms of more than 80 years (Abizanda 2011; Avendano‐Cespedes 2016; Gruber‐Baldini 2013; Lundstrom 2007; Martinez‐Velilla 2019; Watne 2014; Young 2020). Two studies had a mean age of less than 70 years in both allocation arms (Cetinkaya 2019; Nadler 2017). One study did not report data on the mean age of included participants (Bonaventura 2007).
Co‐morbidities
Eight studies used the Charlson Index (Charlson 1994) to compare co‐morbidities between intervention and control groups (Avendano‐Cespedes 2016; Boustani 2012; Chen 2017; Jeffs 2013; Marcantonio 2001; Martinez 2012; Wang 2020; Young 2020). One study (Boustani 2012) reported higher Charlson Index scores in the usual care group. One study used the Cumulative Illness Rating Scale (Martinez‐Velilla 2019), and another the American Society of Anesthesiologists (ASA) score (Gao 2018) to quantify the co‐morbidity of participants. Four studies reported a count of conditions experienced by participants (Abizanda 2011; Bonaventura 2007; Cetinkaya 2019; Hempenius 2013). Three studies considered specific co‐morbidities and described the distribution of these among recruited participants at baseline (Fan 2014; Gruber‐Baldini 2013; Lundstrom 2007). Lundstrom 2007 reported a higher rate of depression among those allocated to the control arm of their study. Four studies did not report co‐morbidities at baseline (Dong 2020; Hosie 2020; Nadler 2017; Watne 2014).
Dementia
Three studies excluded all participants with dementia (Bonaventura 2007; Dong 2020; Gao 2018), and three excluded those assessed as having severe dementia (Avendano‐Cespedes 2016; Martinez‐Velilla 2019; Wang 2020). Six studies reported an imbalance in the proportion of those with dementia between their intervention and control arms, with higher rates in the control arms in Gruber‐Baldini 2013; Lundstrom 2007; Marcantonio 2001; Nadler 2017; Partridge 2017 and higher rates in the intervention arm in Young 2020. Three studies did not report specifically on dementia (Boustani 2012; Cetinkaya 2019; Hosie 2020).
Frailty
Only one study included a baseline assessment of the frailty of recruited participants (Wang 2020). This used the Chinese adaptation of the FRAIL scale score (Dong 2018) ‐ with a higher proportion of the intervention group classed as healthy and a higher proportion of the control group considered as frail (Wang 2020).
Interventions
Multicomponent interventions versus usual care
Fourteen studies evaluate multicomponent interventions for delirium prevention, compared to usual hospital care.
We identified characteristics associated with the delivery of the intervention, with the use of tailored interventions mentioned in nine trials (Abizanda 2011; Avendano‐Cespedes 2016; Dong 2020; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Partridge 2017; Wang 2020); protocols/checklists used in 10 trials (Chen 2017; Dong 2020; Hempenius 2013; Hosie 2020; Jeffs 2013; Lundstrom 2007; Martinez 2012; Partridge 2017; Wang 2020; Young 2020). Ten trials had a specific education component as part of the intervention (Abizanda 2011; Avendano‐Cespedes 2016; Bonaventura 2007; Chen 2017; Hempenius 2013; Hosie 2020; Lundstrom 2007; Martinez 2012; Wang 2020; Young 2020) and five specified multidisciplinary involvement (Hosie 2020; Lundstrom 2007; Martinez 2012; Partridge 2017; Wang 2020) with three trials specifying family involvement as a key characteristic (Hosie 2020; Martinez 2012; Wang 2020). Many of the delirium risk factors targeted with multi‐component interventions relate to good fundamental care, supporting staff within the care team to deliver these aspects consistently.
We identified 12 distinct components of the interventions that could be entered in the network meta‐analysis: re‐orientation (including use of familiar objects); reducing sensory deprivation (for example hearing aids, spectacles); cognitive stimulation; nutrition and hydration (including electrolyte balance); identification of infection; mobilisation; sleep hygiene; oxygenation; pain control; medication review; bladder and bowel care and assessment of mood. Individual studies included between two and 10 components with a mean and median of six components included in each study. The distribution of these components across the included studies is summarised in Table 2 and Appendix 2 summarises how components were selected.
1. Distribution of components across included studies.
Liberal versus restrictive blood transfusion thresholds
Intraoperative blood transfusion has been implicated as a risk factor for postoperative delirium (Carson 2011). Subset analysis from a multicentre RCT identified that anaemia was associated with delirium and blood transfusion was associated with reduced risk of delirium (van der Zanden 2016). Gruber‐Baldini 2013 and Fan 2014 tested the use of liberal versus restrictive blood transfusion thresholds on risk of incident delirium. Fan 2014 classified liberal transfusion strategy as transfusing to maintain haemoglobin ≥10g/dL and restrictive strategy as only transfusing when haemoglobin < 8 g/dL or when symptoms of anaemia developed. Gruber‐Baldini 2013 gave their liberal transfusion group one unit of packed red blood cells and as much as needed to maintain haemoglobin ≥10 g/dL; their restrictive group was treated in the same way as described by Fan 2014.
Care in geriatric medicine unit versus in orthopaedic unit following hip fracture
Individuals admitted following a fracture are typically placed under the care of an orthopaedic surgeon, pending operative intervention. However, the complex nature of the predominantly older adult population who experience a hip fracture has led to the emergence of orthogeriatric medicine services, where input is also received from physicians specialist in the care of older adults. Comprehensive geriatric assessment (CGA) is an evidence‐based “multidimensional interdisciplinary diagnostic process used to determine the medical, psychological and functional capabilities of a frail older person to develop a coordinated and integrated plan for treatment and long‐term follow‐up” associated with improved outcomes, particularly when delivered in a dedicated ward (Ellis 2017). Watne 2014 designed their trial around their local service reconfiguration where older adults were admitted to their specialist geriatric medicine unit and received CGA comparing this to the care received in the orthopaedic unit.
Exercise therapy versus usual care
Observational data support a link between physical activity and incidence of delirium in hospitalised adults (Yang 2008), with those unable to undertake such activity at increased risk (Marcantonio 1998). Ability to undertake physical activity while in hospital is likely to be complex, with associations with illness severity important to consider. Emerging evidence from intensive care unit settings supports mobilisation strategies to reduce delirium (Banerjee 2011). Martinez‐Velilla 2019 undertook a multicomponent exercise intervention targeted towards hospitalised older adults and prevention of delirium was one of their secondary end‐points of interest.
Computerised clinical decision support system versus usual care
Computerised clinical decision support software (CCDS) has been reported as an effective tool in prompting healthcare practitioners to comply with established protocols and preventive measures (Dexter 2001). One study in our review (Boustani 2012), investigated the use of CCDS in medical inpatients with alerts to identify cognitive impairment or individuals who would benefit from specialist assessment and prompts around urinary catheters, physical restraints and anticholinergic medications.
Listening to music versus usual care
Cetinkaya 2019 evaluated listening to classical Turkish music as a postoperative intervention to reduce delirium. Music has been proposed as a potential intervention for delirium research in intensive care unit settings, with limited empirical data (Guerra 2019) and for postoperative orthopaedic surgery (Sibanda 2019).
Transcutaneous electrical acupoint stimulation versus placebo
Complementary medicine approaches, including techniques such as acupoint stimulation, have been postulated as helpful in the management of agitation and delirium, although evidence of their effectiveness has been lacking (Levy 2017). Gao 2018 examined the use of transcutaneous electrical acupoint stimulation among older adults with evidence of silent lacunar infarction on imaging as a modality to prevent postoperative delirium.
Continuous positive airway pressure versus usual care (CPAP)
Nadler 2017 evaluated the use of continuous positive airway pressure (CPAP) for those identified as at risk of obstructive sleep apnoea (OSA) as a potential intervention to prevent postoperative delirium. CPAP is an evidence‐based treatment for OSA, known to reduce sleepiness symptoms and improve quality of life (Giles 2006). An association between postoperative delirium and OSA has been identified in elective surgical patients (Flink 2012).
Outcomes
Primary outcomes
Incidence of delirium was measured using a range of validated diagnostic methods. The commonest approach was use of the Confusion Assessment Method (CAM) (Inouye 1990), used in 15 of the included studies (Abizanda 2011; Avendano‐Cespedes 2016; Boustani 2012; Chen 2017; Dong 2020; Gruber‐Baldini 2013; Jeffs 2013; Marcantonio 2001; Martinez 2012; Martinez‐Velilla 2019; Nadler 2017; Partridge 2017; Wang 2020; Watne 2014; Young 2020). The CAM‐ICU (Ely 2001) was used in two studies (Fan 2014; Gao 2018). Diagnostic and Statistical Manual (DSM‐IV) criteria were used in Lundstrom 2007. Hempenius 2013 used theDelirium. Observation Screening) Scale (DOSS) which, if positive, resulted in an assessment using DSM‐IV criteria and the Delirium Rating Scale Revised 1998 (DRS‐R‐98). Bonaventura 2007 used the CAM and DRS‐R‐98 (Trzepacz 2001). Cetinkaya 2019 used the NEECHAM confusion scale (Nelson 1996), assessed on postoperative days one, two and three, comparing scores between intervention and control groups. They categorise the score as 0 to 19 indicating moderate to severe confusion, 20 to 24 moderate or early confusion, 25 to 26 as high risk for confusion and 27 to 30 as normal function (Cetinkaya 2019). Hosie 2020 used the Nursing Delirium Screening Scale (NuDESC) (Gaudreau 2005) and the DSM‐V criteria and DRS‐R‐98.
Only 13 studies reported data on mortality, either in‐hospital or at follow‐up of one and three, six and 12 months (Abizanda 2011; Avendano‐Cespedes 2016; Boustani 2012; Chen 2017; Dong 2020; Hempenius 2013; Hosie 2020; Lundstrom 2007; Martinez‐Velilla 2019; Partridge 2017; Wang 2020; Watne 2014; Young 2020). No study evaluated mortality beyond 12 months from randomisation.
One study evaluated new diagnosis of dementia at 12 months (Watne 2014).
Secondary outcomes
Seven studies reported on the duration of delirium (in days) experienced by participants (Avendano‐Cespedes 2016; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Watne 2014; Young 2020).
Eight studies reported on severity of delirium episodes (Avendano‐Cespedes 2016; Dong 2020; Hempenius 2013; Hosie 2020; Jeffs 2013; Wang 2020; Watne 2014; Young 2020) using the CAM, CAM‐S, DRS‐R‐98 and Memorial Delirium Assessment Scale (MDAS). Only one study (Hempenius 2013) reported the peak severity of delirium, with others reporting mean or median over the duration of the study. Avendano‐Cespedes 2016 reported mean severity data at multiple time points, but the denominator for analysis was not clear and thus these figures were not included in the quantitative synthesis. Wang 2020 reported severe delirium (defined as MDAS ≥18) as a dichotomous outcome only.
Length of hospital admission was reported by 16 studies (Abizanda 2011; Boustani 2012; Chen 2017; Dong 2020; Fan 2014; Gruber‐Baldini 2013; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Martinez‐Velilla 2019; Partridge 2017; Wang 2020; Watne 2014; Young 2020). Partridge 2017 reported only the mean length of stay, without standard deviation, so this could not be included in quantitative synthesis. Avendano‐Cespedes 2016 reported data on length of stay for their whole sample and then for those who did or did not experience delirium, rather than those in the intervention and control groups, again these data could not be pooled.
None of the included studies evaluated use of new psychotropic medications during admission.
Activities of daily living were reported in six studies (Abizanda 2011; Dong 2020; Martinez‐Velilla 2019 Wang 2020; Watne 2014; Young 2020).
Quality of life was reported by only two studies (Hempenius 2013; Martinez‐Velilla 2019).
None of the included studies evaluated carer's quality of life.
Seven studies included data on individuals withdrawal from protocol (Chen 2017; Fan 2014; Hosie 2020; Marcantonio 2001; Partridge 2017; Wang 2020; Young 2020).
Adverse outcomes
Only two studies examined hospital readmission (Hempenius 2013; Partridge 2017).
None of the included studies evaluated progression of existing dementia.
Two studies reported on new care home admission, one at the time of hospital discharge (Young 2020) and one at follow‐up of four and 12 months (Watne 2014).
Nine of the included studies looked at the incidence of in‐hospital falls (Boustani 2012; Hempenius 2013; Hosie 2020; Lundstrom 2007; Martinez 2012; Martinez‐Velilla 2019; Partridge 2017; Watne 2014; Young 2020), and four evaluated the incidence of in‐hospital pressure ulcers (Boustani 2012; Hempenius 2013; Lundstrom 2007; Watne 2014).
Exclusion of prevalent delirium at baseline
Failure to exclude delirium at enrolment to the study was a common problem. Only seven studies clearly excluded or accounted for prevalent cases of delirium at baseline (Abizanda 2011; Fan 2014; Gao 2018; Jeffs 2013; Martinez 2012; Wang 2020; Young 2020). Avendano‐Cespedes 2016 reported multiple measures of delirium, including exclusion of delirium present on the first day of admission, but there was uncertainty around the denominators for each group, making it difficult to use in pooled comparisons.
Funding sources and declarations of interest
The majority of included studies (18 of 22) were funded through academic or governmental research institutions or grant funding schemes. In three studies, the source of funding was not reported (Boustani 2012; Cetinkaya 2019; Martinez 2012), and one study received no specific funding, but was loaned equipment from a health technology company (Nadler 2017).
Four studies reported potential interest to declare related to their study (Boustani 2012; Gruber‐Baldini 2013; Hosie 2020; Wang 2020). Three studies did not provide a declaration of interest statement in their publication (Bonaventura 2007; Lundstrom 2007; Marcantonio 2001).
Excluded studies
We excluded 182 records. Thirty‐two duplicate records (either duplicate publications or publications reporting the same underlying data) were excluded. Studies were excluded for the following reasons: n=71 wrong study design; n=32 wrong setting; n=24 wrong outcome (not delirium prevention); n=13 wrong intervention (not non‐pharmacological); n=7 unvalidated delirium diagnostic method; n=2 study terminated and n=1 previous version of review (Figure 1). Excluded studies for which a full text was available are listed in Characteristics of excluded studies. Details of 19 studies identified as ongoing are given in Characteristics of ongoing studies.
Risk of bias in included studies
Risk of bias assessments are presented for each study in the Characteristics of included studies table and are summarised in the text below and graphically in Figure 2. We assessed no study to be at low risk of bias across all domains.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered 12 of the included studies to be at low risk of selection bias with appropriate random methods for sequence generation and allocation concealment reported (Abizanda 2011; Avendano‐Cespedes 2016; Boustani 2012; Cetinkaya 2019; Chen 2017; Gruber‐Baldini 2013; Hempenius 2013; Hosie 2020; Marcantonio 2001; Martinez 2012; Partridge 2017; Watne 2014; Young 2020).
Bonaventura 2007 was at high risk of selection bias due to use of day of admission in allocation to intervention or control. Wang 2020 was at unclear risk of selection bias due to the method of allocating individuals to groups and concealment of the allocation. Two studies were at unclear risk in terms of their random sequence generation (Jeffs 2013; Lundstrom 2007). Six studies were at unclear risk in their allocation concealment (Cetinkaya 2019; Dong 2020; Fan 2014; Gao 2018; Martinez‐Velilla 2019; Nadler 2017).
Blinding
All studies were at high risk of performance bias as none were able to blind participants and study personnel.
Ten studies were at low risk of detection bias due to blinding of outcome assessors (Abizanda 2011; Avendano‐Cespedes 2016; Chen 2017; Gao 2018; Hempenius 2013; Jeffs 2013; Marcantonio 2001; Martinez‐Velilla 2019; Nadler 2017; Wang 2020). Three studies were at unclear risk of detection bias (Bonaventura 2007; Boustani 2012; Cetinkaya 2019). The remaining nine studies were at high risk of detection bias (Dong 2020; Fan 2014; Gruber‐Baldini 2013; Hosie 2020; Lundstrom 2007; Martinez 2012; Partridge 2017; Watne 2014; Young 2020).
Incomplete outcome data
Seventeen studies were at low risk of attrition bias (Abizanda 2011; Avendano‐Cespedes 2016; Bonaventura 2007; Boustani 2012; Cetinkaya 2019; Chen 2017; Fan 2014; Gao 2018; Gruber‐Baldini 2013; Hosie 2020; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Wang 2020; Watne 2014; Young 2020). Two studies were at unclear risk of attrition bias (Dong 2020; Hempenius 2013) and the remaining three studies were considered to be at high risk of attrition bias (Martinez‐Velilla 2019; Nadler 2017; Partridge 2017).
Selective reporting
Eleven studies were at low risk of reporting bias having published protocols and reporting as per their protocol (Abizanda 2011; Avendano‐Cespedes 2016; Gruber‐Baldini 2013; Hempenius 2013; Hosie 2020; Jeffs 2013; Martinez‐Velilla 2019; Nadler 2017; Partridge 2017; Watne 2014; Young 2020). Eight studies were at unclear risk of reporting bias as a result of an absence of a published protocol (Bonaventura 2007; Boustani 2012; Cetinkaya 2019; Fan 2014; Gao 2018; Lundstrom 2007; Marcantonio 2001; Martinez 2012). Three studies were at high risk of reporting bias due to inconsistency in reporting between protocol and paper or between methods and results (Chen 2017; Dong 2020; Wang 2020).
Other potential sources of bias
The other bias domain was used to assess the four cluster‐randomised trials (Chen 2017; Hosie 2020; Wang 2020; Young 2020). These were assessed for recruitment bias, baseline imbalance, loss of clusters and incorrect analysis, with full details provided in the study‐level risk of bias tables. Three of the cluster‐randomised trials were considered at low risk of bias (Chen 2017; Wang 2020; Young 2020), and one study (Hosie 2020) was considered at high risk, as no specific analytical consideration was made to account for the cluster design. To investigate the fifth parameter of assessing risk of bias in cluster‐randomised trials, comparability with individually randomised trials, a sensitivity analysis was undertaken of the primary outcome (incidence of delirium), removing the cluster‐randomised trials. Removing the cluster‐randomised trials results in a change to the effect estimate (risk ratio (RR) 0.65 compared to 0.57) and associated uncertainty (95%CI 0.55 to 0.77, compared to 0.46 to 0.71 for all studies), but the direction and nature of the effect was the same. This analysis does not suggest an important bias from inclusion of the cluster‐randomised trials in the summary estimate.
Visual inspection of funnel plots for incidence of delirium and inpatient mortality for multicomponent interventions did not suggest publication bias.
The four studies in which trials are completed but the results are not publicly available are summarised in Studies awaiting classification. They include four interventions (family intervention (n = 79 participants), care bundle (n = 80 participants), preventative care protocol (n = 80 participants) and passive cycling (n = 230 participants) with a total planned sample size of 469 participants. From the information available in the trial registry entries it is difficult to categorise these studies and estimate how they would influence the published results.
Effects of interventions
See: Table 1
1. Multicomponent interventions versus usual care
Fourteen trials investigated the effectiveness of multicomponent interventions for the prevention of delirium (Abizanda 2011; Avendano‐Cespedes 2016; Bonaventura 2007; Chen 2017; Dong 2020; Hempenius 2013; Hosie 2020; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Partridge 2017; Wang 2020; Young 2020). A summary of findings table for the seven key outcomes is presented in Table 1.
a. Primary outcomes
Pooled analysis showed that multi‐component non‐pharmacological interventions probably reduce the incidence of delirium compared to usual care (risk ratio (RR) 0.57, 95% confidence interval (CI) 0.46 to 0.71, I2=39%; 3693 participants; downgraded to moderate certainty due to risk of bias) (Analysis 1.1, Figure 3).
1.1. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 1: Incident Delirium
3.

Forest plot: Multi‐component delirium prevention intervention (MCI) versus usual care for incident delirium
Ten studies reported data on inpatient mortality; there may be little or no effect of multicomponent interventions on inpatient mortality compared to usual care (RR 1.17, 95% CI 0.79 to 1.74, I2=15%; 2640 participants; low‐certainty evidence downgraded due to inconsistency and imprecision) (Analysis 1.2; Figure 4).
1.2. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 2: Inpatient mortality
4.

Forest plot: Multi‐component delirium prevention intervention (MCI) versus usual care for inpatient mortality
Three studies reported mortality data between one and three months. Multicomponent interventions likely result in little to no difference in mortality at one to three months compared to usual care (RR 1.26, 95% CI 0.92 to 1.75, I2=0%; 1200 participants; moderate‐certainty evidence downgraded due to imprecision) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 3: Mortality at 1 to 3 months
Only one study (Lundstrom 2007) reported mortality data between six and 12 months. There may be little or no effect of multicomponent interventions on mortality at 12 months compared to usual care (RR 0.85, 95% CI 0.46 to 1.56; 199 participants; low‐certainty evidence downgraded due to imprecision and risk of bias within the study) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 4: Mortality at 12 months
None of the included studies reported mortality data beyond 12 months from randomisation.
None of the included studies of multicomponent interventions reported data on new diagnosis of dementia at any point following randomisation.
b. Secondary outcomes
Six studies reported data on the duration of delirium episodes. Multicomponent interventions may result in a small reduction of around a day in the duration of a delirium episode (mean difference (MD) ‐0.93, 95% CI ‐2.01 to 0.14 days, I2 = 65%; 351 participants; low‐certainty evidence downgraded due to risk of bias and imprecision) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 5: Duration of delirium episode
Five studies compared delirium severity between intervention and usual care groups. The evidence is very uncertain about the effect of multicomponent interventions on delirium severity (standardised mean difference (SMD) ‐0.49, 95% CI ‐1.13 to 0.14, I2 = 64%; 147 participants; very low‐certainty evidence downgraded due to risk of bias and serious imprecision) (Analysis 1.6). A standardised mean severity of 0.49 standard deviations represents a moderate effect. The 95% confidence interval encompasses a very large effect and little or no effect, indicating serious imprecision.
1.6. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 6: Peak severity of delirium
Pooled analysis of 10 studies showed multicomponent interventions may result in a reduction in hospital length of stay compared to usual care (MD ‐1.30 days, 95% CI ‐2.56 to ‐0.04 days, I2=91%; 3351 participants; low‐certainty evidence downgraded due to risk of bias and inconsistency) (Analysis 1.7; Figure 5).
1.7. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 7: Length of hospital stay
5.

Forest plot: Multi‐component delirium prevention intervention (MCI) versus usual care for length of hospital stay
None of the included studies evaluated use of new psychotropic medication during hospital admission.
Activities of daily living, measured using a validated instrument were evaluated in four trials (Abizanda 2011; Dong 2020 Wang 2020; Young 2020). However, these were reported using different measures as post‐intervention values and change scores or as a dichotomous variable determining improvement/not (Abizanda 2011) and it was not considered appropriate to pool these data.
Quality of life, measured using a validated patient reported measure was reported in two trials (Hempenius 2013; Martinez‐Velilla 2019). However, these data were not considered suitable to pool as Hempenius 2013 dichotomised results based on presence/absence of change and Martinez‐Velilla 2019 derived results from a linear mixed‐effects model.
None of the included studies evaluated carer's quality of life.
Withdrawal from protocol by participants was reported in six studies. The evidence suggests that multicomponent interventions result in little to no difference in withdrawal from protocol compared to usual care (RR 1.03, 95% CI 0.60 to 1.75, I2=0%; 1751 participants; low‐certainty evidence downgraded due to risk of bias and imprecision) (Analysis 1.8).
1.8. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 8: Withdrawal from protocol
c. Adverse outcomes
Only two studies reported on readmission to hospital (Hempenius 2013; Partridge 2017). The evidence is very uncertain about the effect of multicomponent interventions on hospital readmission (RR 1.35, 95% CI 0.89 to 2.07, I2=0%; 401 participants; very low‐certainty evidence downgraded due to risk of bias and serious imprecision) (Analysis 1.9).
1.9. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 9: Readmission to hospital
None of the included studies evaluated progression of existing dementia.
New care home admission at the time of hospital discharge was only reported in a single study (Young 2020). The evidence suggests that multicomponent interventions result in little to no difference in new care home admission at the time of hospital discharge compared to usual care (RR 0.77, 95% CI 0.55 to 1.07; 536 participants; low‐certainty evidence downgraded due to risk of bias and imprecision) ( Analysis 1.10).
1.10. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 10: New care home admission on discharge
Rates of falls were reported in six studies, the evidence suggests multicomponent interventions result in a reduced rate of falls compared to usual care (RR 0.56, 95% CI 0.35 to 0.90, I2=8%; 1680 participants; low‐certainty evidence downgraded due to risk of bias and imprecision) (Analysis 1.11).
1.11. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 11: Falls
Rates of pressure ulcers were only reported in two studies (Hempenius 2013; Lundstrom 2007). The evidence suggests multicomponent interventions result in a reduced risk of pressure ulcer formation compared to usual care (RR 0.48, 95% CI 0.26 to 0.89, I2 = 0%; 457 participants; low‐certainty evidence downgraded, due to risk of bias and imprecision) (Analysis 1.12).
1.12. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 12: Pressure ulcers
Subgroup analysis by setting
Pre‐planned subgroup analysis was conducted to evaluate the effectiveness of delirium prevention interventions based on clinical setting. The 14 trials were divided into the six conducted in medical settings (Abizanda 2011; Avendano‐Cespedes 2016; Bonaventura 2007; Hosie 2020; Jeffs 2013; Martinez 2012), seven conducted in surgical settings including orthopaedics (Chen 2017; Dong 2020; Hempenius 2013; Lundstrom 2007; Marcantonio 2001; Partridge 2017; Wang 2020) and one conducted in both medical and surgical settings (Young 2020). There were similar effect sizes in medical (RR 0.61, 95% CI 0.45 to 0.83; I2= 0%; 1460 participants) and surgical including orthopaedic (RR 0.49, 95% CI 0.34 to 0.72; I2 = 65%; 1520 participants) settings in favour of multicomponent interventions in reducing incidence delirium (moderate certainty evidence downgraded due to risk of bias. (Analysis 1.1)
Subgroup analysis by dementia diagnosis
Only one trial (Marcantonio 2001) reported incident delirium in patients with pre‐existing dementia. Delirium incidence estimates appeared different between individuals with dementia (RR 0.90, 95% CI 0.59 to 1.36; 50 participants; low‐certainty evidence, downgraded due to risk of bias and imprecision) and those without dementia (RR 0.50, 95% CI 0.22 to 1.13; 76 participants; low‐certainty evidence, downgraded due to risk of bias and imprecision). However, the results are too imprecise to allow a conclusion to be drawn.
Subgroup analysis by frailty status
It was not possible to evaluate the impact of frailty as no studies reported numerical delirium data stratified by frailty status.
Sensitivity analysis removing specialist palliative care
Pre‐planned sensitivity analysis to remove the trial conducted in specialist palliative care (Hosie 2020) did not make any significant difference to the observed estimate of the effectiveness of multicomponent delirium prevention interventions (RR 0.56, 95% CI 0.45 to 0.71; 3648 participants; moderate‐certainty evidence downgraded due to risk of bias).
Component network meta‐analysis
We created a component network using data from the 14 trials (n = 3693 participants) of multicomponent interventions included in the main review.
The forest plot of component effects is given in Figure 6. Based on our data, re‐orientation (odds ratio (OR) 0.32, 95% Credible Intervals (CrI) 0.11 to 0.89), cognitive stimulation (OR 0.45, 95% CrI 0.21 to 0.93) and sleep hygiene (OR 0.25, 95% CrI 0.08 to 0.71) were associated with reduced risk of incident delirium. Attention to nutrition and hydration (OR 0.48, 95% CrI 0.18 to 1.26), oxygenation (OR 0.35, 95% CrI 0.10 to 1.20), and bowel and bladder care (OR 0.55, 95% CrI 0.23 to 1.31) suggested probable reduction in incident delirium, but estimates included the possibility of no effect or harm. For most other components the 95% credible intervals were too wide to comment on potential effects. This is likely a result of lack of data, both a low number of trials and low heterogeneity in the composition of components included in the trials. Medication review and assessment of mood both had imprecise summary estimates, favouring the interventions, although harms could not be excluded. On the other hand, reducing sensory deprivation, identification of infection, mobilisation, pain control and assessment of mood all had summary estimates that suggested potential increase in delirium, but the uncertainty in these estimates was very substantial, including potential benefits as well as harms. We compared estimates based on the network against data from the original trials and found that most of the component network meta‐analysis estimates were similar to the individual trial estimates, providing some evidence of validity to the component network analysis.
6.
Forest plot summarising component network meta‐analysis results
2. Liberal versus restrictive blood transfusion thresholds
Two trials (Fan 2014; Gruber‐Baldini 2013) evaluated blood transfusion thresholds. Fan 2014 included 192 participants undergoing elective hip replacement and Gruber‐Baldini 2013 included 139 participants undergoing surgical repair of hip fracture. Both compared liberal versus restrictive blood transfusion thresholds. There was significant overlap in the volume of blood received by participants in both studies and in other products administered.
a. Primary outcomes
The evidence suggests that use of a liberal transfusion threshold over a restrictive transfusion threshold results in little to no difference in incident delirium (RR 0.92, 95% CI 0.62 to 1.36; I2 = 9%; 294 participants; moderate‐certainty evidence downgraded due to risk of bias) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Liberal versus restrictive blood transfusion thresholds, Outcome 1: Incident delirium
Neither study reported on mortality or new diagnosis of dementia.
b. Secondary outcomes
The evidence suggests that liberal transfusion thresholds does not affect the severity of delirium (MD ‐ 0.10, 95% CI ‐2.99 to 2.79; 38 participants; low‐certainty evidence downgraded due to imprecision and risk of bias) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Liberal versus restrictive blood transfusion thresholds, Outcome 2: Delirium severity
The evidence suggests that liberal transfusion thresholds do not affect the length of hospital stay (MD 0.28, 95% CI ‐0.49 to 1.04 days; I2 = 0%; 324 participants; low‐certainty evidence downgraded due to imprecision and risk of bias) (Analysis 2.3).
2.3. Analysis.

Comparison 2: Liberal versus restrictive blood transfusion thresholds, Outcome 3: Length of hospital stay
The evidence is very uncertain about the effect of liberal transfusion thresholds on the risk of withdrawal from the study (RR 2.00, 95% CI 0.38 to 10.66; 192 participants; very low‐certainty evidence downgraded due to serious imprecision and risk of bias) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Liberal versus restrictive blood transfusion thresholds, Outcome 4: Withdrawal
The studies did not report on duration of delirium, use of new psychotropic medications, activities of daily living, quality of life or carer's quality of life.
c. Adverse outcomes
Neither study reported on hospital readmission, progression of existing dementia, new care home admission at discharge, falls or pressure ulcers.
3. Geriatric unit care versus orthopaedic unit care
One trial of 329 older adults following hip fracture compared care in a specialist geriatric unit to care in their orthopaedic unit (Watne 2014).
a. Primary outcomes
The evidence suggests that care in the geriatric unit does not affect the incidence of delirium compared to care in the orthopaedic unit (RR 0.98, 95% CI 0.79 to 1.22; 329 participants; low‐certainty evidence downgraded due to risk of bias and imprecision) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 1: Incident delirium
Care in the geriatric unit likely results in little to no difference in the rate of in‐hospital mortality (RR 0.56, 95% CI 0.21 to 1.47; 329 participants; moderate‐certainty evidence downgraded due to imprecision) compared to the orthopaedic unit. (Analysis 3.2).
3.2. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 2: Inpatient mortality
Care in the geriatric unit appeared to increase the rate of incident dementia at 12 months (RR 2.26, 95% CI 0.60 to 8.49; 193 participants). However, the evidence was deemed to be of low certainty and was downgraded two levels due to serious imprecision. (Analysis 3.3).
3.3. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 3: Incident dementia at 12 months
b. Secondary outcomes
The evidence suggests that care in the geriatric unit results in little to no difference in the duration of delirium (MD ‐1.00 days, 95% CI ‐2.03 to 0.03 days; 166 participants) (Analysis 3.4), or severity of delirium episodes (MD 1.50 points, 95% CI ‐0.97 to 3.97 points; 166 participants) (Analysis 3.5) compared to the orthopaedic unit, low‐certainty evidence for both outcomes, downgraded due to risk of bias and imprecision.
3.4. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 4: Duration of delirium
3.5. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 5: Severity of delirium
Care in the geriatric unit probably increases length of hospital admission by a mean of three days (RR 3.00, 95% CI 1.94 to 4.06 days; 329 participants; moderate‐certainty evidence downgraded due to risk of bias) compared to the orthopaedic unit (Analysis 3.6).
3.6. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 6: Length of hospital stay
The study did not report on use of new psychotropic medications, quality of life, carer's quality of life or withdrawal from protocol by participants.
c. Adverse outcomes
The evidence suggests that care in the geriatric unit does not affect the rate of falls (RR 1.30, 95% CI 0.61 to 2.77; 329 participants; low‐certainty evidence downgraded due to risk of bias and imprecision) (Analysis 3.7), or pressure ulcer formation (RR 0.38, 95% CI 0.10 to 1.41; 329 participants; low‐certainty evidence downgraded due to risk of bias and imprecision) (Analysis 3.8).
3.7. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 7: Falls
3.8. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 8: Pressure ulcers
Care in the geriatric unit probably does not affect the risk of new care home admission at 12 months (RR 0.86, 95% CI 0.47 to 1.59; 193 participants; moderate‐certainty evidence downgraded due to imprecision) (Analysis 3.9).
3.9. Analysis.

Comparison 3: Geriatric unit care versus orthopaedic unit care, Outcome 9: New care home admission at 12 months
The study did not report on hospital readmission or progression of existing dementia.
4. Exercise therapy versus usual care
One trial Martinez‐Velilla 2019 evaluated the effect of an exercise intervention on 370 older adults hospitalised in an acute elderly care unit.
a. Primary outcomes
The evidence suggests that an exercise intervention does not affect the incidence of delirium compared to usual care (RR 1.80, 95% CI 0.99 to 3.27; 370 participants; lo‐ certainty evidence downgraded due to risk of bias and imprecision) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Exercise therapy versus usual care, Outcome 1: Incident Delirium
Exercise intervention likely results in little to no difference on mortality at one to three months (RR 1.22, 95% CI 0.68 to 2.20; 370 participants; moderate‐certainty evidence downgraded due to imprecision). (Analysis 4.2)
4.2. Analysis.

Comparison 4: Exercise therapy versus usual care, Outcome 2: Mortality at 1 to 3 months
The study did not report on new diagnosis of dementia.
b. Secondary outcomes
Exercise intervention results in little to no difference on length of hospital admission compared to usual care (MD 0.00 days, 95%CI ‐0.60 to 0.60; 370 participants; high‐certainty evidence). (Analysis 4.3)
4.3. Analysis.

Comparison 4: Exercise therapy versus usual care, Outcome 3: Length of hospital stay
Activities of daily living data were reported, as a change in Barthel Index score from two weeks prior to hospital admission to hospital discharge. These data were derived from linear mixed‐effects modelling and reported as time coefficient and 95% CI, thus they could not be entered into RevMan Web.
The study did not report on duration or severity of delirium, use of new psychotropic medications, quality of life, carer's quality of life or withdrawal from protocol by participants.
c. Adverse outcomes
The evidence suggests that an exercise intervention does not affect the likelihood of new care home admission at hospital discharge (RR 2.00, 95% CI 0.37 to 10.79; 370 participants; low‐certainty evidence downgraded due to serious imprecision). (Analysis 4.4)
4.4. Analysis.

Comparison 4: Exercise therapy versus usual care, Outcome 4: New care home admission on discharge
The evidence suggests that an exercise intervention does not affect the rate of falls experienced by participants (RR 8.57, 95% CI 0.47 to 157.75; 285 participants; low‐certainty evidence downgraded due to serious imprecision). (Analysis 4.5)
4.5. Analysis.

Comparison 4: Exercise therapy versus usual care, Outcome 5: Falls
The study did not report on hospital readmission, progression of existing dementia or pressure ulcers.
5. Computerised clinical decision support system versus usual care
One trial Boustani 2012 assessed the use of a computerised clinical decision support system (CCDSS) on the management of 427 older adults with cognitive impairment, compared to usual care.
a. Primary outcomes
Use of CCDSS probably results in little to no difference in delirium incidence (RR 1.08, 95% CI 0.82 to 1.43; 424 participants; moderate‐certainty evidence downgraded due to risk of bias) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Computerised clinical decision support system (CCDS) versus usual care, Outcome 1: Incident delirium
The evidence suggests that use of CCDSS does not affect the rate of mortality within one to three months (30 days of discharge) (RR 1.04, 95% CI 0.49 to 2.23; 424 participants; low‐certainty evidence downgraded due to serious imprecision) (Analysis 5.2).
5.2. Analysis.

Comparison 5: Computerised clinical decision support system (CCDS) versus usual care, Outcome 2: Mortality at 1 to 3 months
The study did not report on new diagnosis of dementia.
b. Secondary outcomes
The evidence suggests that CCDSS does not affect the length of admission (MD 0.90 days, 95% CI ‐0.35 to 2.15 days; 424 participants; low‐certainty evidence, downgraded due to serious imprecision (Analysis 5.3).
5.3. Analysis.

Comparison 5: Computerised clinical decision support system (CCDS) versus usual care, Outcome 3: Length of hospital stay
The study did not report on duration of delirium, severity of delirium, use of new psychotropic medications, activities of daily living, quality of life, carer's quality of life or withdrawal from protocol by participants.
c. Adverse outcomes
Use of CCDSS probably does not affect rates of falls (RR 0.93, 95% CI 0.39 to 2.19; 424 participants; moderate‐certainty evidence downgraded due to imprecision) or pressure ulcers (RR 1.09, 95% CI 0.64 to 1.84; 424 participants; moderate‐certainty evidence downgraded due to imprecision) (Analysis 5.4; Analysis 5.5).
5.4. Analysis.

Comparison 5: Computerised clinical decision support system (CCDS) versus usual care, Outcome 4: Falls
5.5. Analysis.

Comparison 5: Computerised clinical decision support system (CCDS) versus usual care, Outcome 5: Pressure sores
The study did not report on hospital readmission, progression of existing dementia or new care home admission at discharge.
6. Listening to music versus usual care
One trial Cetinkaya 2019 included 60 individuals undergoing hip or knee surgery and evaluated listening to classical Turkish music in the postoperative period, compared to usual care.
a. Primary outcomes
Using the postoperative day one (peak in severity) NEECHAM scores, the evidence is very uncertain about the effect of music listening on the incidence of delirium (MD 1.47, 95% CI 0.16 to 2.78; 60 participants; very low‐certainty evidence downgraded due to risk of bias, imprecision and indirectness due to type of music) (Analysis 6.1).
6.1. Analysis.

Comparison 6: Listening to music verus usual care, Outcome 1: Incident delirium
The study did not report on mortality or new diagnosis of dementia.
b. Secondary outcomes
The study did not report on duration of delirium, severity of delirium, length of hospital admission, use of new psychotropic medications, activities of daily living, quality of life, carer's quality of life or withdrawal from protocol by participants.
c. Adverse outcomes
The study did not report on hospital readmission, progression of existing dementia, new care home admission at discharge, falls or pressure ulcers.
7. Transcutaneous electrical acupoint stimulation versus placebo
One trial Gao 2018 evaluated the use of transcutaneous electrical acupoint stimulation during surgery in a sample of 64 adults who had experienced silent lacunar infarction and were undergoing spinal surgery, compared to placebo.
a. Primary outcomes
The evidence is very uncertain about the effect of transcutaneous electrical acupoint stimulation on incident delirium (RR 0.25, 95% CI 0.06 to 1.09; 64 participants; very low‐certainty evidence downgraded due to risk of bias, indirectness and imprecision) (Analysis 7.1).
7.1. Analysis.

Comparison 7: Transcutaneous electrical acupoint stimulation versus placebo, Outcome 1: Incident delirium
The study did not report on mortality or new diagnosis of dementia.
b. Secondary outcomes
The study did not report on duration of delirium, severity of delirium, length of hospital admission, use of new psychotropic medications, activities of daily living, quality of life, carer's quality of life or withdrawal from protocol by participants.
c. Adverse outcomes
The study did not report on hospital readmission, progression of existing dementia, new care home admission at discharge, falls or pressure ulcers.
8. Continuous positive airway pressure versus usual care
One trial Nadler 2017 evaluated the use of peri‐operative continuous positive airway pressure (CPAP) for 135 adults considered at risk of obstructive sleep apnoea, undergoing elective hip or knee arthroplasty, compared to usual care. CPAP was given during sleep before surgery and on postoperative days zero, one1 and tw0.
a. Primary outcomes
The evidence is very uncertain about the effect of peri‐operative CPAP on incident delirium (RR 1.29, 95% CI 0.59 to 2.82; 114 participants; very low‐certainty evidence downgraded due to risk of bias and serious imprecision) (Analysis 8.1).
8.1. Analysis.

Comparison 8: Continuous positive airway pressure (CPAP) verus usual care, Outcome 1: Incident delirium
The study did not report on mortality or new diagnosis of dementia.
b. Secondary outcomes
The study did not report on duration of delirium, severity of delirium, length of hospital admission, use of new psychotropic medications, activities of daily living, quality of life, carer's quality of life or withdrawal from protocol by participants.
c. Adverse outcomes
The study did not report on hospital readmission, progression of existing dementia, new care home admission at discharge, falls or pressure ulcers.
Discussion
Summary of main results
We identified 22 randomised trials of eight non‐pharmacological interventions for the prevention of delirium in hospitalised adults, not in intensive care unit (ICU) or high dependency unit (HDU) settings. Most of these evaluated multicomponent interventions, with two trials evaluating the use of blood transfusion thresholds and the others evaluating interventions in a single study.
We found moderate‐certainty evidence from 14 randomised controlled trials that multicomponent interventions probably reduce delirium incidence in hospitalised adults by 40% compared with usual care. This evidence holds across different settings and populations within the hospital, broadly categorised as medical versus surgical (including orthopaedics). We found low‐certainty evidence that these interventions may result in a reduction in hospital length of stay. Delirium duration may be reduced by around a day, although evidence was of low certainty. The evidence is very uncertain around the effect on delirium severity. There may be little or no effect of multicomponent interventions on inpatient mortality. The need for care home placement at discharge was only evaluated in a single multicomponent intervention study.
Our component network meta‐analysis identified 12 distinct components of the interventions; studies included between two and 10 components with a mean of six components in each study. Re‐orientation, cognitive stimulation and sleep hygiene were associated with reduced risk of incident delirium. Attention to nutrition and hydration, oxygenation, medication review, assessment of mood and bowel and bladder care suggested probable reduction in incident delirium, but estimates included the possibility of no benefit or harm. For most other components the 95% credible intervals were too wide to comment on potential effect.
We found no evidence for any single component intervention affecting delirium incidence.
Overall completeness and applicability of evidence
The majority of the evidence in this review is about the use of multicomponent delirium prevention interventions (14 of the 22 included trials). This reflects a significant increase in research evidence in this area, with the previous iteration of this review published five years ago identifying only seven trials (Siddiqi 2016). The other eight interventions identified in this review were investigated in only one or two small studies each, precluding meaningful synthesis of these results.
Multicomponent interventions have previously been shown to be effective in reducing the incidence of delirium, however, this is the first review that has attempted to define the components which should be considered for inclusion. This question is relevant for clinical practice in terms of operationalising the implementation of delirium prevention interventions as part of hospital care. This exploratory analysis sought to describe if there are some components which are necessary and if any are harmful or non‐contributory to the effectiveness of prevention. Our analysis was novel, but limited by the total number of included studies and the range of components included. Aspects of intervention delivery were not considered as components in the model, but these are clearly worthy of further systematic exploration. Interventions in future trials could include re‐orientation, cognitive stimulation and sleep hygiene. However, it would be helpful to have future trials directly comparing different combinations of components, rather than one combination compared with usual care, as having trials directly comparing interventions would increase the benefit of the component network meta‐analysis approach over pairwise meta‐analyses.
Implementation of evidence‐based delirium prevention interventions in healthcare settings globally is recognised to be complex, but critical to improve outcomes for individuals (Wilson 2020). One of the included studies (Young 2020) had a parallel implementation study using Normalization Process Theory to articulate how the intervention was implemented and delivered within clinical hospital settings, identifying key contextual factors for successful implementation (Godfrey 2019). Implementation science approaches such as this are likely to be required to understand how to apply evidence from randomised trials in clinical care settings, ensuring the fundamental aspects of care are delivered consistently for all.
Only one study specifically reported on the impact of their intervention on adults living with dementia, an important subgroup to study in delirium prevention. Four studies actively excluded those with dementia and a further three excluded those with severe dementia.The effectiveness of delirium interventions might be expected to differ given the higher prevalence of delirium and poorer outcomes in dementia. Only one study reported progression of existing dementia and no studies evaluated new diagnosis of dementia. This is an important limitation in light of the growing epidemiological evidence associating delirium with the development of dementia (Richardson 2020), and recurrent delirium with worsening cognitive decline (Richardson 2021). Only one study included a measure of the frailty of recruited participants and results were not presented stratified by frailty. This may be an important variable to consider within the population targeted by delirium prevention interventions. There were limited data on quality of life of patients and no data on quality of life of carers/families. Data on new care home admission were limited with one study reporting need for care at time of hospital discharge and another at later follow‐up. These outcomes are important for individuals, their families and healthcare services and would benefit from further research.
A core outcomes set for studies evaluating interventions to prevent delirium among adults requiring an acute care hospital admission has recently been published, incorporating the perspectives of multiple stakeholders (Rose 2021). This includes cognition, emotional distress and health‐related quality of life. It is hoped that this will inform future data collection in delirium prevention research, with researchers focusing on measuring outcomes which matter and in a less heterogeneous way to support evidence synthesis.
We note there are 19 ongoing studies whose findings may be eligible for inclusion in future updates of this review.
Future trials and reviews should consider the health economic implications of multicomponent interventions.
Quality of the evidence
We undertook risk of bias assessment for each included trial and used GRADEpro software (GRADEpro 2014) to inform the generation of evidence certainty statements. None of the included trials were considered to be at low risk of bias across all domains. All of the included interventions were conducted without blinding of participants and personnel and fewer than half of the studies attempted to reduce detection bias through use of blinded outcome assessors. Multi‐component delirium prevention interventions are complex and thus arguably a double‐blind design is not realistic. However, independent outcome assessment may be feasible.
Evidence is typically of moderate or low certainty, downgraded as a result of the risk of bias in the included studies, imprecision or inconsistency of results. Delirium incidence was the only outcome used in all of the included studies and reporting on other delirium variables, such as duration and severity were more limited. Delirium incidence was measured at any time point during hospital admission. It is therefore possible that where interventions were effective in reducing length of hospital stay, delirium may not be detected. Hospital readmission with delirium would be a way to identify if this was occurring, however this outcome was not commonly reported in the included studies.
Heterogeneity in the measurement of outcomes limited the pooling of results.
We note that there were four studies identified which cannot be classified for inclusion or exclusion (Characteristics of studies awaiting classification). These studies appear to have been completed based on information in trial registry entries. However, results remain unpublished and no further information was identified from correspondence using contact details recorded in the trial registries.
Failure to exclude prevalent delirium at enrolment was a common limitation in the majority of studies (16/22). This has the potential to reduce precision in the results as interventions cannot prevent cases of delirium already present in recruited participants. However, ruling out prevalent delirium in busy, clinical settings is difficult and it is perhaps more representative of real‐world delirium care that those with and without delirium are included at the baseline of intervention studies. This likely increases external validity of the evidence.
Potential biases in the review process
This review was conducted in accordance with Cochrane procedures and there were only a small number of amendments to the review process, which are outlined in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
Our findings are consistent with the previous version of this review which included all interventions to prevent delirium, pharmacological and non‐pharmacological (Siddiqi 2016). That version included seven randomised controlled trials ( RCTs) of multicomponent interventions; the additional seven trials now included provide consistent and stronger results than the earlier estimate of a reduction of a third in the rate of incident delirium (Siddiqi 2016).
These data from RCTs are consistent with the evidence seen in non‐randomised studies, most notably studies of the Hospital Elder Life Program (HELP) (Inouye 1999). The programme targets six delirium risk factors (cognitive impairment, sleep deprivation, immobility, dehydration, vision or hearing impairment) and provides targeted interventions to address these factors involving specialist nurses and clinicians and volunteers (Inouye 2000a). The HELP approach has been studied extensively, with 44 articles informing a review of effectiveness and implementation (Hshieh 2018). The data from these clinical studies are consistent with reduced costs and reduced rate of falls as well as reduced delirium incidence (Hshieh 2018).
Heim 2017 reported on the experience of undertaking a planned, stepped‐wedge design, randomised trial of the HELP intervention within Dutch hospitals. Difficulty accessing electronic records to ascertain outcomes and missing data from these care records were identified as particular challenges which contributed to the termination of their study. The authors note the challenges of trying to evaluate a complex intervention using a pragmatic study design, highlighting selection bias due to recruitment procedures as another contributing factor (Heim 2017). This is an important contribution to the delirium prevention research landscape in terms of generating evidence in clinical practice.
One study included in the previous version of the review was excluded as it reported a mixed pharmacological and non‐pharmacological intervention (Jia 2014). The 'fast‐track surgery' intervention described shared components with those studies which described themselves as multicomponent delirium prevention studies, including focus on bowels/bladder care, nutrition and hydration, and early mobilisation (Jia 2014). However, the intervention also included a different mode of anaesthesia and type of analgesia regimen from the control group, rendering it ineligible for inclusion in this non‐pharmacological review. This multicomponent, mixed pharmacological and non‐pharmacological intervention was also reported to be associated with reductions in the incidence of delirium and the length of hospital stay.
Other systematic reviews have found similar results to those reported here. Most recently these include León‐Salas 2020 pooling data in older adults (aged ≥ 65 years) across medical, surgical and ICU populations and identifying 10 randomised trials, and Ludolph 2020 who included studies of adults in all hospital settings, and similarly found no evidence of an effect of interventions intended to prevent delirium on either duration of delirium or mortality. Martinez 2015 identified that multi‐component interventions were effective in reducing incident delirium and accidental falls among hospitalised older adults (aged > 60 years), compared to usual care.
Evidence for delirium prevention in other settings does not allow such consistent conclusions. The Cochrane review of delirium prevention in long‐term care settings identified only three trials for inclusion, each considering different non‐pharmacological interventions, with considerable uncertainty about the results (Woodhouse 2019). In ICU settings, most studies have been conducted on the effects of pharmacological interventions. A Cochrane Review in the ICU setting, included four non‐pharmacological intervention studies, but there was heterogeneity in the interventions and outcomes and no clear conclusions could be drawn from the available evidence (Herling 2018).
Authors' conclusions
Implications for practice.
Non‐pharmacological delirium prevention interventions are probably effective across all (non‐intensive care unit (ICU)) hospital settings in reducing the incidence of delirium by around 43%. These interventions may include reorientation and use of familiar objects, cognitive stimulation and sleep hygiene, with consideration for support for nutrition, hydration and electrolyte balance, oxygenation and bowel and bladder care.
Implications for research.
Given the strength of evidence to support non‐pharmacological multicomponent interventions, there is a need for research to understand how these can be implemented in practice. The randomised‐trial evidence to support single‐component interventions for delirium prevention in non‐ICU settings remains limited and this may reflect the nature of the condition, necessitating complex intervention approaches. Future evidence synthesis may benefit from focusing on multicomponent approaches alone, taking time and attention on the specific included components in greater detail, both their content and delivery. There is a need to evaluate cost‐effectiveness to make the case for investment to implement interventions.
There is a lack of evidence involving people with delirium superimposed on dementia, who are often excluded from trials and for whom specific subgroup reporting would be beneficial. These individuals must be included in delirium prevention research.
Routine assessment of frailty status and reporting stratified by this variable would also be helpful in future research studies to test hypotheses about the role of frailty in delirium.
Outcome assessment focused on variables which matter to individuals, particularly around cognitive outcomes including progression of dementia and the development of dementia would be informative as part of the wider dementia prevention agenda.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2021 | New citation required but conclusions have not changed | A data entry error has been corrected, and the results and discussion text amended accordingly. The Acknowledgements text has been updated. |
| 4 November 2021 | Amended | A data entry error impacting Adverse Outcome Falls (Analyses 1.11) has been corrected, with concomitant updates to results and discussion text. The Acknowledgements text updated. |
History
Protocol first published: Issue 4, 2019 Review first published: Issue 7, 2021
Acknowledgements
The authors would like to thank those who responded to enquiries for additional information (Dr Lars Bergmann, Dr Monidipa Dasgupta, Dr John Green, Dr Annmarie Hosie, Professor Finbarr Martin, Dr Judith Partridge, Dr Eduardo Tobar, Dr Liesbeth van Heel) and for translation support (Dr Jeff Kim).
The authors would like to thank Professor Kenneth Boockvar, Dr Lisa Burry and Catherine Hofstetter for their constructive peer and consumer review comments.
The authors would like to thank Steven He and colleagues for their comment, identifying two data entry errors in the reporting of the adverse outcome Falls (https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013307.pub2/detailed‐comment/en?messageId=326085441). We have corrected these errors and updated the review text and results accordingly.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| ALOIS (Cochrane Dementia and Cognitive Improvement Group Specialised Regsiter, searched via the Cochrane Register of Studies) [Date of most recent search: 16 September 2020] |
Deliri* OR DEL [studies that are about delirium trestment or prevention are coded DEL in ALOIS] |
June 2019: 240 Jan 2020: 106 Sep 2020: 67 |
| CENTRAL (the Cochrane Library) http://crso.cochrane.org/SearchSimple.php [Date of most recent search: 16 September 2020] |
#1 MESH DESCRIPTOR Delirium EXPLODE ALL TREES #2 deliri*:TI,AB,KY #3 ("acute confusion*" ):TI,AB,KY #4 ("acute confusion*" ):TI,AB,KY #5 ("acute organic psychosyndrome" ):TI,AB,KY #6 ("acute brain syndrome" ):TI,AB,KY #7 ("metabolic encephalopathy" ):TI,AB,KY #8 ("acute psycho‐organic syndrome" ):TI,AB,KY #9 ("clouded state" ):TI,AB,KY #10 ("clouding of consciousness" ):TI,AB,KY #11 ("exogenous psychosis" ):TI,AB,KY #12 ("toxic psychosis" ):TI,AB,KY #13 ("toxic confusion" ):TI,AB,KY #14 obnubilat*:TI,AB,KY #15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 #16 MESH DESCRIPTOR Hospitals EXPLODE ALL TREES #17 MESH DESCRIPTOR Patient Care EXPLODE ALL TREES #18 MESH DESCRIPTOR Inpatients EXPLODE ALL TREES #19 Hospital*:TI,AB,KY #20 "In‐patient":TI,AB,KY #21 Ward*:TI,AB,KY #22 Inpatient*:TI,AB,KY #23 #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 #24 #15 AND #23 |
June 2019: 1642 Jan 2020: 531 Sep 2020:157 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 16 September 2020] |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. Delirium, Dementia, Amnestic, Cognitive Disorders/su [Surgery] 14. obnubilat*.ti,ab. 15. or/1‐14 16. Hospitals/ 17. Inpatients/ 18. Patient Care/ 19. Hospital*.ti,ab. 20. "In‐patient".ti,ab. 21. Ward*.ti,ab. 22. Inpatient*.ti,ab. 23. 16 or 17 or 18 or 19 or 20 or 21 or 22 24. randomized controlled trial.pt. 25. controlled clinical trial.pt. 26. randomi?ed.ab. 27. placebo.ab. 28. drug therapy.fs. 29. randomly.ab. 30. trial.ab. 31. groups.ab. 32. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33. (animals not (humans and animals)).sh. 34. 32 not 33 35. 15 and 23 and 34 |
June 2019: 1873 Jan 2020: 216 Sep 2020: 136 |
| EMBASE (Ovid SP) 1974 to 15 January 2020 [Date of most recent search: 16 September 2020] |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. Delirium, Dementia, Amnestic, Cognitive Disorders/su [Surgery] 14. obnubilat*.ti,ab. 15. or/1‐14 16. Hospitals/ 17. Inpatients/ 18. Patient Care/ 19. Hospital*.ti,ab. 20. "In‐patient".ti,ab. 21. Ward*.ti,ab. 22. Inpatient*.ti,ab. 23. 16 or 17 or 18 or 19 or 20 or 21 or 22 24. randomized controlled trial.pt. 25. controlled clinical trial.pt. 26. randomi?ed.ab. 27. placebo.ab. 28. drug therapy.fs. 29. randomly.ab. 30. trial.ab. 31. groups.ab. 32. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33. (animals not (humans and animals)).sh. 34. 32 not 33 35. 15 and 23 and 34 |
June 2019: 6907 Jan 2020: 1117 Sep 2020:748 |
| PsycINFO (Ovid SP) [Date of most recent search: 16 September 2020] |
1 Delirium/ 2 deliri*.mp. 3 "acute confusion*".ti,ab. 4 "acute organic psychosyndrome".ti,ab. 5 "acute brain syndrome".ti,ab. 6 "metabolic encephalopathy".ti,ab. 7 "acute psycho‐organic syndrome".ti,ab. 8 "clouded state".ti,ab. 9 "clouding of consciousness".ti,ab. 10 "exogenous psychosis".ti,ab. 11 "toxic psychosis".ti,ab. 12 "toxic confusion".ti,ab. 13 obnubilat*.ti,ab. 14 or/1‐13 15 exp HOSPITALS/ 16 exp Hospitalized Patients/ 17 Hospital*.ti,ab. 18 "In‐patient".ti,ab. 19 Ward*.ti,ab. 20 Inpatient*.ti,ab. 21 or/15‐20 22 exp Clinical Trials/ 23 randomly.ab. 24 randomi?ed.ti,ab. 25 placebo.ti,ab. 26 groups.ab. 27 "double‐blind*".ti,ab. 28 "single‐blind*".ti,ab. 29 RCT.ti,ab. 30 or/22‐29 31 14 and 21 and 30 32 from 31 keep 1‐288 |
June 2019: 332 Jan 2020: 21 Sep 2020:365 |
| CINAHL (EBSCOhost) [Date of most recent search: 16 September 2020] |
1 deliri* 2 "acute psycho‐organic syndrome" or "clouded state" or "clouding of consciousness" or "exogenous psychosis" or "toxic psychosis" or "toxic confusion" 3 "acute brain confusion" or "acute brain failure" or "acute organic psychosyndrome" or "acute brain syndrome" or "metabolic encephalopathy" 4 "Delirium"/ 5 (S1 OR S2 OR S3 OR S4) 6 (MH "Hospitals+") 7 (MH "Inpatients") 8 TX Hospital* 9 TX "In‐patient" 10 Ward* 11 Inpatient* 12 (MH "Patient Care") 13 (S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12) 14 MH "Clinical Trials" 15 TX trial 16 TX "single‐blind*" 17 TX "double‐blind*" 18 TX "treatment as usual" 19 TX randomly 20 S14 OR S15 OR S16 OR S17 OR S18 OR S19 21 S5 AND S13 AND S20 |
June 2019: 1,498 Jan 2020: 461 Sep 2020: 144 |
| ISI Web of Science – core collection [Date of most recent search: 16 September 2020] |
TOPIC:(deliri* OR "acute confusion*" OR "acute organic psychosyndrome" OR "acute brain syndrome" OR "metabolic encephalopathy" OR "acute psycho‐organic syndrome" OR "clouded state" OR "clouding of consciousness" OR "exogenous psychosis" OR "toxic psychosis" OR "toxic confusion" OR obnubilat*)ANDTOPIC:(hospital* OR Inpatient* OR In‐patient* OR ward OR "In patient*")AND TOPIC:(randomly OR randomised OR randomized OR "random allocat*" OR RCT OR CCT OR "double blind*" OR "single blind*" OR "double blind*" OR "single blind*" OR trial) | June 2019: 1612 Jan 2020: 186 Sep 2020: 321 |
| LILACS (BIREME) [Date of most recent search: 16 September 2020] |
deliri$ OR delirio OR loucura [Words]and hospital$ OR inpatient$ [Words] | June 2019: 417 Jan 2020: 13 Sep 2020: 9 |
| ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 16 September 2020] |
HOSPITAL OR INPATIENT | delirium OR toxic psychosis OR toxic confusion | June 2019: 540 Jan 2020: 97 Sep 2020: 68 |
| ICTRP [Date of most recent search: 15 January 2020. Database not available 16 September 2020] |
HOSPITAL OR INPATIENT | delirium OR toxic psychosis OR toxic confusion | June 2019: 89 Jan 2020: 18 |
| TOTAL before de‐duplication | June 2019: 15,150 Jan 2020: 2766 Sep 2020: 2015 |
|
| TOTAL after de‐duplication | June 2019: 10,810 Jan 2020: 2246 Sep 2020:1682 |
|
Appendix 2. Table reporting original study‐level description of components forming their multicomponent interventions mapped across to included components in the analysis
| Study ID | Description of components within study | Components not included or combined with others | Intervention delivery | Included components |
| Abizanda 2011 | Occupational therapy intervention consisted of a daily session with patient and relative/caregiver Monday‐Friday for the duration of admission. Activities were carried out according to needs and day of admission. Therapeutic plan included: cognitive stimulation; instruction on preventing complications including immobility, confusion, falls, urinary incontinence, pressure sores; retraining in ADL; assessment of technical aids for home |
Education Tailored | Bowel/bladder care Cognitive stimulation Mobilisation |
|
| Avendano‐Cespedes 2016 | The intervention was carried out exclusively by the intervention nurses and was composed of two main parts, being the first one a risk factor analysis, and the second one the intervention on the risk factors detected. Risk factors: orientation, sensorial deficit, sleep, mobilisation, hydration, nutrition, drugs, oxygenation, elimination, and pain |
Education Tailored |
Bowel/bladder care Medication review Mobilisation Nutrition & hydration Oxygenation Pain control Re‐orientation & familiar objects Reducing sensory deprivation Sleep hygiene |
|
| Bonaventura 2007 | Intervention to Prevent Delirium (IPD), a series of structured and standardised welfare actions based on existing guidelines, including support in the following areas: cognitive re‐orientation, sensory and environmental, mobilisation, hydration, and ’socio‐emotional’ | Familiar objects | Education | Mobilisation Nutrition & hydration Re‐orientation & familiar objects Reducing sensory deprivation Sleep hygiene |
| Chen 2017 | Modified Hospital Elder Life Program comprising of three standardised protocols: orienting communication (i.e., orientation and engaged conversation), oral and nutritional assistance (i.e., brushing teeth, oral‐facial exercise, and postoperative dietary education), and early mobilization. | Education Protocol/checklist |
Mobilisation Nutrition & hydration Re‐orientation & familiar objects |
|
| Dong 2020 | All patients received 1. Directional communication plan 2. Cognitive therapy activity plan 3. Early activity plan The following schemes are implemented as needed based on the evaluation results 4. Pain improvement program 5. Sleep improvement program 6. Assisted feeding plan 7. Rehydration program 8. Constipation improvement plan 9. Hearing/vision improvement program 10. Hypoxic improvement program 11. Aspiration pneumonia prevention program 12. Urine‐related infection prevention program 13. Delirium improvement program 14. Dementia improvement program 15. Multiple medication management plan |
Protocol/checklist Tailored |
Bowel/bladder care Medication review Mobilisation Nutrition & hydration Oxygenation Pain control Reducing sensory deprivation Sleep hygiene |
|
| Hempenius 2013 | Multi‐component intervention focused on best supportive care and the prevention of delirium. Preoperative geriatric team assessment with daily monitoring during hospital stay, supported by the use of standardised checklists. This checklist consisted of nine items: orientation, mobility, anxiety, senses, pain, sleep, intake, defecation and infection. |
Comprehensive Geriatric Assessment Education Protocol/checklist Tailored |
Assessment of mood Bowel/bladder care Identification of infection Medication review Mobilisation Nutrition & hydration Pain control Re‐orientation & familiar objects Reducing sensory deprivation Sleep hygiene |
|
| Hosie 2020 | The intervention had six domains 1. Preserve natural sleep 2. Maintain optimal sensory perception 3. Optimise hydration 4. Stimulate communication, orientation and cognition 5. Optimise mobility 6. Family partnership For each domain there are 4‐12 strategies provided and their implementation is described We asked team members to enlist family and volunteers and tailor the intervention to patients’ needs and wishes. |
Education Family involvement Multidisciplinary Protocol/checklist |
Mobilisation Nutrition & hydration Re‐orientation & familiar objects Reducing sensory deprivation Sleep hygiene |
|
| Jeffs 2013 | Participants received a graded physical activity and orientation programme twice daily, which was delivered in addition to usual care. A certified Allied Health Assistant, trained in administering exercise programmes, delivered the intervention after initial assessment of the participant by a physiotherapist. Commensurate with ability, participants were prescribed one of four exercise programmes: bed, seated, standing or rails. All programmes were customised to the participant’s ability and were reviewed daily. Exercise programmes were modified to ensure suitable progression for those participants who made significant gains. The orientation programme comprised formal and informal elements. The formal element of the programme comprised a series of seven questions aimed at assessing and improving orientation (day, month, year, date, ward, bed number and name of primary nurse). The participant was asked the questions in sequence and prompted with the correct answer if they were not able to give a correct response. The informal element of the programme related to engaging in the exercise programme and in the social interaction with the Allied Health Assistant and/or Physiotherapist. |
Protocol/checklist | Mobilisation Re‐orientation & familiar objects |
|
| Lundstrom 2007 | The staff worked as a team, applying comprehensive geriatric assessment, management and rehabilitation. Main content of the intervention ‐ Prevention and treatment of complications, bowel and bladder function, sleep, decubitus ulcers, pain, saturation, body temperature, Blood pressure, nutrition, rehabilitation, Secondary prevention of falls and fractures and osteoporosis prophylaxis. Other – staff education, teamwork, individual care planning, delirium |
Comprehensive Geriatric Assessment Education Multidisciplinary Protocol/checklist Tailored |
Bowel/bladder care Identification of infection Mobilisation Nutrition & hydration Oxygenation Pain control Sleep hygiene |
|
| Marcantonio 2001 | Proactive geriatrics consultation. The consultation included 10 modules – adequate CNS oxygen delivery, fluid electrolyte balance, treatment of severe pain, elimination of unnecessary medications, regulation of bowel/bladder function, adequate nutritional intake, early mobilisation and rehabilitation, prevention, early detection and treatment of major postoperative complications, appropriate environmental stimuli, treatment of agitated delirium. |
Electrolytes Postoperative complications |
Tailored | Bowel/bladder care Cognitive stimulation Identification of infection Medication review Mobilisation Nutrition & hydration (including electrolyte balance) Oxygenation Pain control Re‐orientation & familiar objects Reducing sensory deprivation |
| Martinez 2012 | Multicomponent management protocol. The intervention was delivered by family members and consisted of 6 elements: 1. Education 2. Provision of a clock 3. Avoidance of sensory deprivation 4. Presence of familiar objects in the room 5. Reorientation of patient provided by family members 6. Extended visitation time. |
Familiar objects | Education Family involvement Multidisciplinary Protocol/checklist |
Cognitive stimulation Re‐orientation & familiar objects Reducing sensory deprivation |
| Partridge 2017 | Comprehensive geriatric assessment delivered by a multidisciplinary team (geriatrician, clinical nurse specialist, social worker, occupational therapist) according to individual patient. Patients were assessed and optimised according to peer‐reviewed protocols based on current evidence, national and hospital guidelines, and expert opinion. The domains that were included cognition, general health status, function independence, social support, medication use, nutrition and mood. |
Comprehensive Geriatric Assessment Multidisciplinary Tailored |
Assessment of mood Cognitive stimulation Medication review Mobilisation Nutrition & hydration |
|
| Wang 2020 | t‐Hospital Elder Life Program (HELP) (tailored, family‐involved HELP), which involved family members instead of volunteers and applied a tailored approach to assigning HELP protocols. The t‐HELP intervention consisted of 3 universal protocols and 8 targeted protocols. The universal protocols, including orientation, therapeutic activities, and early mobilisation protocol (universal protocols), were given to all t‐HELP participants. The targeted protocols were tailored for each patient on the basis of delirium related risk factors, which were assessed daily and comprised of pain management, sleep enhancement, nutrition assistance/aspiration prevention, fluid repletion/constipation, vision/hearing enhancement, hypoxia Improvement, catheter associated UTI (CAUTI) prevention and multiple medications management. |
Education Family involvement Multidisciplinary Protocol/checklist Tailored |
Bowel/bladder care Identification of infection Medication review Mobilisation Nutrition & hydration Oxygenation Pain control Re‐orientation & familiar objects Sleep hygiene |
|
| Young 2020 | The Prevention of Delirium (POD) programme is a manualised, multicomponent intervention and systematic implementation process designed to secure ward practice changes consistent with a reduction in delirium. POD comprises actions centred on ten risk factors associated with the development of delirium among those who are vulnerable on of the basis of predisposing risk (NICE 2010). These interventions directly affect the patient experience of care and include optimising hydration and nutrition, reducing environmental triggers (excessive noise, multiple moves), increasing orientation to time and place, improving communicative practices (personally meaningful interaction and cognitive stimulation), supporting and/or encouraging mobility and better management of pain and infection. |
Education Protocol/checklist |
Cognitive stimulation Identification of infection Mobilisation Nutrition & hydration Pain control Re‐orientation & familiar objects Reducing sensory deprivation Sleep hygiene |
Additional detail on each of the multicomponent interventions is provided at study level within Characteristics of included studies.
ADL: activities of daily living; CNS: central nervous system.
Data and analyses
Comparison 1. Multi‐component delirium prevention intervention (MCI) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incident Delirium | 14 | 3693 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.46, 0.71] |
| 1.1.1 Medical patients | 6 | 1460 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.83] |
| 1.1.2 Surgical patients | 7 | 1520 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.34, 0.72] |
| 1.1.3 Mixed medical and surgical | 1 | 713 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.30] |
| 1.2 Inpatient mortality | 10 | 2640 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.79, 1.74] |
| 1.3 Mortality at 1 to 3 months | 3 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.92, 1.75] |
| 1.4 Mortality at 12 months | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 1.5 Duration of delirium episode | 6 | 351 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐2.01, 0.14] |
| 1.6 Peak severity of delirium | 5 | 147 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.13, 0.14] |
| 1.7 Length of hospital stay | 10 | 3351 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.56, ‐0.04] |
| 1.8 Withdrawal from protocol | 6 | 1751 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.60, 1.75] |
| 1.9 Readmission to hospital | 2 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.89, 2.07] |
| 1.10 New care home admission on discharge | 1 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.07] |
| 1.11 Falls | 6 | 1680 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.35, 0.90] |
| 1.12 Pressure ulcers | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 1.13 Incidence of delirium in patients with dementia | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.41, 1.32] |
| 1.13.1 Individuals with dementia | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 1.13.2 Individuals without dementia | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.13] |
1.13. Analysis.

Comparison 1: Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 13: Incidence of delirium in patients with dementia
Comparison 2. Liberal versus restrictive blood transfusion thresholds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incident delirium | 2 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.62, 1.36] |
| 2.2 Delirium severity | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.99, 2.79] |
| 2.3 Length of hospital stay | 2 | 324 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.49, 1.04] |
| 2.4 Withdrawal | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.38, 10.66] |
Comparison 3. Geriatric unit care versus orthopaedic unit care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incident delirium | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 3.2 Inpatient mortality | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.21, 1.47] |
| 3.3 Incident dementia at 12 months | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.60, 8.49] |
| 3.4 Duration of delirium | 1 | 166 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.03, 0.03] |
| 3.5 Severity of delirium | 1 | 166 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐0.97, 3.97] |
| 3.6 Length of hospital stay | 1 | 329 | Mean Difference (IV, Random, 95% CI) | 3.00 [1.94, 4.06] |
| 3.7 Falls | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.61, 2.77] |
| 3.8 Pressure ulcers | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.10, 1.41] |
| 3.9 New care home admission at 12 months | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
Comparison 4. Exercise therapy versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Incident Delirium | 1 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.99, 3.27] |
| 4.2 Mortality at 1 to 3 months | 1 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.68, 2.20] |
| 4.3 Length of hospital stay | 1 | 370 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.60, 0.60] |
| 4.4 New care home admission on discharge | 1 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.37, 10.79] |
| 4.5 Falls | 1 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 8.57 [0.47, 157.75] |
Comparison 5. Computerised clinical decision support system (CCDS) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Incident delirium | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.43] |
| 5.2 Mortality at 1 to 3 months | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.49, 2.23] |
| 5.3 Length of hospital stay | 1 | 424 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.35, 2.15] |
| 5.4 Falls | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.19] |
| 5.5 Pressure sores | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.84] |
Comparison 6. Listening to music verus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Incident delirium | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.47 [0.16, 2.78] |
Comparison 7. Transcutaneous electrical acupoint stimulation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Incident delirium | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.09] |
Comparison 8. Continuous positive airway pressure (CPAP) verus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Incident delirium | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.59, 2.82] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abizanda 2011.
| Study characteristics | ||
| Methods | Design: RCTof a short‐term occupational therapy intervention in an acute geriatric unit Date of study: November 2002 to June 2003 Power calculation: yes Inclusion criteria: all patients aged 65 and over consecutively admitted to the acute geriatric unit with an acute medical illness or exacerbation of existing chronic condition Exclusion criteria: none reported |
|
| Participants | Number in study: 400 Country: Spain Setting: one acute geriatric unit Age: mean age 83.7 years (SD 6.1) in intervention group, 83.3 years (SD 6.5) in control group Sex: 43.4% male in intervention group, 43.1% male in control group Co‐morbidity: number of previous chronic conditions 3.8 in intervention group, 3.5 in control group Dementia: 35.3% in intervention group, 31.4% in control group Frailty: not reported |
|
| Interventions | Intervention: occupational therapy intervention schedule consisted of a daily 45‐minute session with patient and relative/caregiver Monday‐Friday for the duration of admission. Activities were carried out according to needs and day of admission. Therapeutic plan included: cognitive stimulation; instruction on preventing complications including immobility, confusion, falls, urinary incontinence, pressure sores; retraining in ADL; assessment of technical aids for home. Control: all participants received medical treatment, nursing care, physical therapy and social assistance. |
|
| Outcomes | Outcomes reported: ‐ Incident delirium, measured daily using CAM ‐ In‐hospital mortality ‐ Length of admission ‐ Activities of daily living (ADL), measured using Barthel index ‐ Adverse events Outcomes not reported: none Frequency of outcomes assessment: daily during hospitalisation |
|
| Notes | Funding source: Institute of Health Sciences, Junta de Comunidades de Castilla‐La Mancha. Declarations of interest: quote: "All authors declare that there is not any personal, financial or potential conflict of interest, and therefore have nothing to declare." Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation system |
| Allocation concealment (selection bias) | Low risk | Assignment to randomised group by a geriatrician who did not participate in the clinical management of participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The geriatricians caring for the patients and providing their routine care were blinded to allocated group. Participants were not blinded due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor and the individual performing data analysis were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number with missing data are balanced between groups and there do not appear to be any systematic differences between the groups |
| Selective reporting (reporting bias) | Low risk | No changes were made to trial outcomes after the trial was initiated |
| Other bias | Low risk | No evidence of other bias |
Avendano‐Cespedes 2016.
| Study characteristics | ||
| Methods | Design: RCT Date of study: Oct 13 to Feb 14 Power calculation: pilot sample calculated to detect 10% reduction in delirium from 20% in control group with upper 1 sided 80% confidence limit (power not specified) – sample size of 50 selected Inclusion criteria: >=65, Hospitalised on acute geriatric unit of participating hospital (single‐site) between Oct 13 and Feb 14, Valid informed consent (patient or their legal representative) Exclusion criteria: quote: "Agonic situation" (presume palliative care), Non‐Spanish speaking Severe cognitive decline (Reisberg's Global Deterioration Scale = 7), patient sharing a room with a previously included participant (to avoid contamination bias) |
|
| Participants | Sample size: 50 Country: Spain Setting: acute geriatric unit in one tertiary University hospital Age: Mean age 85.8 (SD = 6.2) in intervention, mean age 87.0 (SD 4.9) in control Overall 86.5 (5.5) Sex: Males, 10 (47.6%) in intervention, males 16 (32%) in control Overall males 26 (52%) Co‐morbidity: mean Charlson comorbidity index score 2.1 (1.7) in intervention group, 2.2 (1.3) in control group. Data reported on physiological parameters including blood pressure, temperature and oxygen saturation– no major imbalances between groups. Dementia: patients with ‘severe’ cognitive impairment were excluded – Reisberg's Global Deterioration scale = 7 (end‐stage dementia). Other stages of dementia are included. Frailty: not reported |
|
| Interventions | Intervention:the intervention was carried out exclusively by the “intervention nurses”, and was composed of two main parts, being the first one a risk factor analysis, and the second one the a daily multicomponent non‐pharmacologic intervention (orientation, sensorial deficit, sleep, mobilisation, hydration, nutrition, drug chart review, elimination, oxygenation, pain), on the risk factors detected. The intervention nurses identified the principal caregiver in the first 24 hours from admission, and provided an informative booklet about strategies and recommendations to prevent delirium incidence, including ambient strategies, orientation abilities, and identification of alert signs. Participants received the initial intervention in the first 24 hours from admission, and thereafter daily until hospital discharge. Control: Usual medical and nursing care throughout the hospitalisation process. No booklet |
|
| Outcomes | Outcomes reported: ‐ Incident delirium using CAM ‐ Prevalent delirium, at any point during hospitalisation, using CAM ‐ New diagnosis of dementia using Pfeiffer Short Portable Mental Status Questionnaire and Reisberg Global Deterioration Scale ‐ Duration of delirium episode (days) ‐ Peak severity of delirium using validated instruments ‐ Length of hospital admission (days) ‐ Use of new psychotropic medication during admission ‐ Withdrawal from protocol by participants Outcomes not reported: none Frequency of outcomes assessment: daily assessment for delirium whilst in hospital |
|
| Notes | Funding source: Funded by RD12/0043RETICEF, Instituto de Salud Carlos III, Ministerio de Economíay Competitividad Declarations of interest: none declared Multiple measures of delirium reported. Those with delirium on first day are highlighted in various analyses presented, but difficult to ascertain denominator and use the data presented in narrative and tables as some inconsistency in reporting. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐based using computer‐generated random numbers with a proportion of 1:1 between control group and intervention group. |
| Allocation concealment (selection bias) | Low risk | After randomisation before participant allocation, opaque envelopes were used to store the data with sequential study numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of investigator and participants was not possible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was conducted blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Reporting in accordance with pre‐registered trial protocol |
| Other bias | Low risk | No evidence of other bias |
Bonaventura 2007.
| Study characteristics | ||
| Methods | Design: RCT of a multi‐component intervention, the Intervention to Prevent Delirium (IPD) in older patients admitted to medical and geriatric wards Date of study: 2005 to 2006 Power calculation: no Inclusion criteria: age > or = to 65 years admitted to medical and geriatric wards in one hospital Exclusion criteria: MMSE score < or =25, at least 1 relative not present, transfer out of ward, pre‐existing dementia, blindness, deafness, aphasia or unable to understand Italian |
|
| Participants | Number in study: 60 Country: Italy Setting: medical and geriatric wards Age: not given Sex M:F: Intervention 12/18, Control 12/18 Co‐morbidity: comparable P = 0.77 Dementia: excluded Frailty: not reported |
|
| Interventions | Intervention: Intervention to Prevent Delirium (IPD), a series of structured and standardised welfare actions based on existing guidelines, including support in the following areas: cognitive re‐orientation, sensory and environmental, mobilisation, hydration, and 'socio‐emotional' Control: usual care, not described further |
|
| Outcomes | Outcomes reported: ‐ Incident delirium measured using CAM & DRS‐R‐98 ‐ Functional performance using Barthel Index Outcomes not reported: none Frequency of outcomes assessment: days 1, 2, 4 and 7 of admission |
|
| Notes | Funding source: not reported Declarations of interest: not reported Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated using day of admission |
| Allocation concealment (selection bias) | High risk | Odd and even days of admission used so concealment unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded, not possible given nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Boustani 2012.
| Study characteristics | ||
| Methods | Design: RCT of a clinical decision support system to improve the care of hospitalised older adults with cognitive impairment Date of study: July 2006 to March 2008 Power calculation: no Inclusion criteria: at least 65 years of age, hospitalised on a medical ward, English‐speaking, and cognitive impairment at the time of hospital admission. Exclusion criteria: pPatients were excluded if they had previously been enrolled in the study, were aphasic, or unresponsive at the time of screening |
|
| Participants | Number in study: 427 Country: USA Setting: medical wards of Wishard Memorial University Hospital Age: Mean age 76.8 years (SD 7.9 years) in intervention group, 77.6 years (SD 8.3 years) in control group Sex: 39.7% male in intervention group, 28.9% male in control group Co‐morbidity: mean Charlson comorbidity index 1.8 (SD 1.8) in intervention group, 2.4 (SD 2.1) in control group Dementia: not reported Frailty: not reported |
|
| Interventions | Intervention: electronically‐delivered clinical decision support system (CDSS) (1) Each time a physician enters an order for a patient randomised to the intervention arm, the physician received non‐interruptive alerts of the presence of CI, catheter, physical restraints, anticholinergic drugs, or the need for ACE services; (2) If the physician orders a urinary catheter, s/he will receive interruptive alerts to recommending discontinuing the catheter; (3) If the physician orders physical restraints, s/he will receive interruptive alerts recommending substituting physical restraints with the use of a professional sitter or low dose trazodone; (4) If the physician orders any of the 18 inappropriate anticholinergics, s/he will receive interruptive alerts recommending stopping the drug, suggesting an alternative, or recommending dose modification. (5) The physician was required to make a decision to accept, reject, or modify any of the interruptive alerts. Control: patients randomised into usual care did not receive CDSS |
|
| Outcomes | Outcomes reported: ‐ Incident delirium, measured using CAM ‐ Mortality ‐ Length of hospital stay ‐ Falls ‐ Pressure ulcers Outcomes not reported: None Frequency of outcomes assessment: every weekday during hospital admission |
|
| Notes | Funding source: NIA Paul B. Beeson K23 Career Development Award Declarations of interest: quote: "Dr Boustani has work supported by grants from the NIA and AHRQ. He is also a member of the Pfizer speakers' bureau. Dr Buckley has provided expert testimony for local law firms. Mr Perkins owns stock in several pharmaceutical firms" Delirium assessed but not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated process was employed for sequence generation in a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Central process following computer generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind personnel treating the patients in the CDSS group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of research assistants conducting outcome assessments not known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 427 enrolled into trial, outcome data available for 424 with no account given for missing participants or to which group they were assigned. However, small as proportion of total sample |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Cetinkaya 2019.
| Study characteristics | ||
| Methods | Design: RCT Date of study: February and June 2018 Power calculation: no Inclusion criteria: the inclusion criteria for the study were 65 years of age or older, no complications during the 3 days of the postoperative period, and willingness to participate in the study. Exclusion criteria: the exclusion criteria were mental retardation that hinders communication, dementia (defined as a Mini‐Mental State Examination [MMSE] score of <23), age < 65 years, hearing problem, development of postoperative complications and unable to speak Turkish. |
|
| Participants | Sample size: 60 Country: Turkey Setting: orthopaedics clinic in an educational research hospital Age: Mean age 69.86 (± 7.59) Sex: male, 10 (33.3%) in Intervention group, male, 5 (16.7%) in control group Overall: male (15, 25%) Co‐morbidity: there were no differences between groups for the number of chronic diseases, previous surgery or regular use if medication. Dementia: not reported Frailty: not reported |
|
| Interventions | Intervention: patients were exposed to music for 3 postoperative days after hip or knee surgery. The patients listening to music were supplied with an Mp3 player in their room, in bed. A separate headset was used for each patient. The patients listened to Acemasiran‐type classical Turkish music. Acemasiran‐type music affects the human brain and provides a sense of creativity of people. Each patient in the intervention group listened to the music for 20‐minute sessions three times a day for 3 postoperative days. Control: routine nursing care – no other description provided. |
|
| Outcomes | Outcomes reported: ‐Incident delirium using The Neecham Confusion Scale Outcomes from study not reported: none Frequency of outcomes assessment: three days postoperatively |
|
| Notes | Funding source: not reported Declarations of interest: none reported by authors Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated into two groups by drawing lots using closed envelopes with numbers from 0 to 9. However, authors state – quote: "Those who selected single numbers were allocated to the control group, and those with double numbers formed the intervention group". Assumed to reflect typographical error in the paper. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how the allocation to groups was concealed based on method used to generate random allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients receiving the intervention were supplied with an MP3 player in their room, blinding not possible. States nurses were blinded, although their role in study is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Those in the intervention group visited by the researcher. States statistician was blinded to assignment, but role of researcher and statistician not defined in terms of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported – no losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available for review |
| Other bias | Low risk | No evidence of other bias |
Chen 2017.
| Study characteristics | ||
| Methods | Design: cluster‐RCT Date of study: August 1 2009 to October 31 Power calculation: not calculated before data collection Inclusion criteria: scheduled for elective abdominal surgery and expected LOS longer than 6 days (≥ 65 years of age) Exclusion criteria: not reported |
|
| Participants | Sample size: 557 Country: Taiwan Setting: two 36‐bed gastrointestinal wards of a 2000‐bed urban medical centre in Taipei Age: mean age 74.3 years (SD= 5.8) in intervention group, Mean age 74.8 (SD=6.0) in control group Sex: male, 111 (56.4%) in intervention group, male, 103 (57.2%) in control group Co‐morbidity: mean Charlson comorbidity index ‐ IG (1.6, 1.9), CG (1.5, 1.7) Dementia: cognitive, MMSE score range, 0‐30; 30 indicates no impairment, (mean, SD): IG (27.0, 3.8), CG (26.8, 3.1) P‐value 0.61 Frailty: not reported |
|
| Interventions | Intervention: the intervention was implemented by a mHELP nurse which is registered nurse who had 2 years of medical surgical experience and who was trained on site for 1 month before the intervention start. The intervention consisted of 3 protocols administered daily: orienting communication, oral and nutritional assistance, and early mobilisation. Intervention group participants received all 3 mHELP protocols with a median start time of postoperative day 1 (IQR 1‐3), in addition to usual care, as soon as they arrived in the inpatient ward and until hospital discharge. Control: usual care consisted of standard hospital care provided by surgeons, residents, nurses, and physical therapists (as needed) in the general surgery wards. All participants were encouraged to ambulate and did so as tolerated. The mHELP nurses did not provide services to participants assigned to the control group. However, the same attending physicians provided care to participants in the mHELP and control groups. |
|
| Outcomes | Outcomes reported: ‐ Incident delirium using CAM ‐ Inpatient mortality ‐ Length of hospital admission (days) ‐ Withdrawals (not explicitly included as an outcome but reported) Outcomes from study not reported: ‐ New diagnosis of dementia using change in MMSE at baseline, discharge, 4 and 6 weeks ‐ ADL using Barthel Index Frequency of outcomes assessment: daily from Monday to Saturday |
|
| Notes | Funding source: This study was supported in part by grants 98‐2314‐B‐002‐113‐MY3 from the Ministry of Science and Technology and NHRIEX‐9820PC from the National Health Research Institute in Taiwan (Dr C.C.‐H. Chen). Dr Inouye’s time was covered in part by grants R24AG054259, P01AG031720, K07AG041835, and R01AG044518 from the National Institute on Aging. Dr Inouye holds the Milton and Shirley F. Levy Family Chair. Declarations of interest: none reported Delirium not excluded at enrolment Intervention only delivered once participants discharged from ITU so varied from first postoperative day to after three days postoperatively |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated based on computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Cluster‐randomised to groups with an allocation ratio of 1:1. Cluster randomisation used to reduce risk of cross‐contamination due to shared occupancy rooms. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, participants and personnel were unblinded. Intervention delivery was separate from outcome assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were masked to group assignment and room assignments were re‐randomised every 20 patients to minimise potential unmasking of the randomisation scheme |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted‐for and low levels of attrition from analysis of outcomes, clearly described with reasons documented. |
| Selective reporting (reporting bias) | High risk | NCT protocol specifies other primary outcomes (frailty and bowel dysmotility) not reported here. |
| Other bias | Low risk | No evidence of recruitment bias or evidence of baseline imbalance associated with cluster methods. No clusters lost. Authors report that the intracluster correlation coefficient (ICC) was calculated for each outcome and were not significantly different from 0 and some were even less than 0, suggesting that the true ICCs are small and adjustment for cluster effect is not indicated. We thus analysed treatment effects using standard statistical methods not accounting for within‐cluster correlation. |
Dong 2020.
| Study characteristics | ||
| Methods | Design: RCT Date of study: December 2016 to December 2019 Power calculation: not calculated Inclusion criteria: meets the Severe Acute Pancreatitis diagnostic criteria in the 2013 Chinese Guidelines for the Diagnosis and Treatment of Acute Pancreatitis, aged 70 years or older, expected hospital stay >2 weeks and provision of written informed consent. Exclusion criteria: the exclusion criteria were history of severe acute pancreatitis, coma, complicated with mental disorders or disorders, dementia, low immune function (such as neutrophil deficiency) and end‐stage disease. |
|
| Participants | Sample size: 106 Country: China Setting: Affiliated Hospital of Jiangnan University, Age: mean age in intervention group 75.87 [+/‐ 4.32], mean age in control group 76.23 [+/‐ 4.58] Sex: male, 32 (64%) in intervention group, male 34 (65%), in control group Co‐morbidity: not reported Dementia: excluded Frailty: not reported |
|
| Interventions | Intervention: all patients received 1. Directional communication plan 2. Cognitive therapy activity plan 3. Early activity plan The following schemes are implemented as needed based on the evaluation results 4. Pain improvement program 5. Sleep improvement program 6. Assisted feeding plan 7. Rehydration program 8. Constipation improvement plan 9. Hearing/vision improvement program 10. Hypoxic improvement program 11. Aspiration pneumonia prevention program 12. Urine‐related infection prevention program 13. Delirium improvement program14. Dementia improvement program 15. Multiple medication management plan Control: Routine nursing programmes and procedures. |
|
| Outcomes | Outcomes reported: ‐ Incident delirium using CAM ‐ Inpatient mortality ‐ Length of hospital admission (days) ‐ ADL using Barthel Index (pre and post 2‐week intervention period only) Outcomes from study not reported: None Frequency of outcomes assessment: pre and post 2 week intervention period |
|
| Notes | Funding source:this work was supported by the Translational Medicine Specialty of Wuxi Municipal Health Committee (ZM006). Declarations of interest: No conflicts of interest to declare Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of random number table to determine if subjects for test group or control |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three participants in intervention group died, no data presented on them, even at baseline |
| Selective reporting (reporting bias) | High risk | No protocol available. Length of stay was not pre‐specified in methods. Incomplete reporting for cognition and self‐care as categories used are not referenced or specified as data derived. |
| Other bias | Low risk | No evidence of other bias |
Fan 2014.
| Study characteristics | ||
| Methods | Design: RCT Date of study: October 2011 to May 2013 Power calculation: no Inclusion criteria: patients older than 65 years undergoing elective unilateral total hip replacement surgery with spinal anaesthetic Exclusion criteria: ASA physical status 3 IV; preoperative delirium; unwilling to comply with the procedures; inability to understand the language (Mandarin Chinese); hearing loss, or a failure in spinal anaesthesia |
|
| Participants | Sample size:192 Country: China Setting: hospital inpatient – elective orthopaedics Age: mean age 73 (+/‐ 7) in the intervention group, 75 (+/‐ 6) in the control group Sex: male, 30, (31.9%) in the Intervention group, male, 32, (35.9%) in the control group Co‐morbidity: no baseline between‐group differences in CVD, IHD, CHF, hypertension, pulmonary disease, renal insufficiency, PVD, diabetes mellitus, liver disease. Dementia: not mentioned explicitly but cognitive assessment undertaken using MMSE Frailty: not reported |
|
| Interventions | Intervention: patients older than 65 years undergoing elective unilateral total hip replacement surgery with spinal anaesthetic. Control: ASA physical status 3 IV; preoperative delirium; unwilling to comply with the procedures; inability to understand the language (Mandarin Chinese); hearing loss, or a failure in spinal anaesthesia |
|
| Outcomes | Outcomes reported ‐ Incident delirium using the Confusion Assessment Method for the intensive care unit (CAM‐ICU) ‐ Length of stay ‐ Withdrawal Outcomes from study not reported: none Frequency of outcomes assessment: Delirium was assessed by the same attending anaesthesiologist between 8 a.m. and 9 a.m. preoperatively, and 1, 2, 3 days after surgery. |
|
| Notes | Funding source: this work was supported by the grants from the National Natural Science Foundation of China (No. 81300946) and the Natural Science Foundation of Jiangsu Province (BK2012778). Declarations of interest: no conflict of interest stated by authors Delirium excluded at enrolment using the (CAM‐ ICU) criteria (Chinese version) Imbalance between groups om other substances transfused – restrictive group received more Ringer’s lactate and hydroxyethyl starch |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to restrictive or liberal group using a random number table |
| Allocation concealment (selection bias) | Unclear risk | The method used to was sealed envelope technique, however, there is insufficient detail as to whether these were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessments described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear accounting for all participants at follow‐up. Loss of 2 from restrictive and 4 from liberal transfusion groups at follow‐up due to declined consent for transfusion. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess as no published protocol |
| Other bias | Low risk | No evidence of other bias |
Gao 2018.
| Study characteristics | ||
| Methods | Design: RCT Date of study: July 2017 Power calculation: not reported Inclusion criteria: aged 65+, with silent lacunar infarct, who underwent spinal surgery in July 2017 (at Third Hospital of Hebei Medical University) Exclusion criteria: MMSE score of less than 24 or dementia, due to various aetiologies, preoperative delirium, history of neurological or mental illness, current use of tranquillisers or antidepressants, history of an endocrine or metabolic disorder, recent use of glucocorticoids or other hormones, suffering from infections or chronic inflammatory conditions, intake of anti‐inflammatory drugs, unwillingness to complete the experimental procedures, inability to communicate in the preoperative period (language barrier or severe hearing or visual impairment), and alcohol or drug dependence. |
|
| Participants | Sample size: 64 Country: China Setting: hospital ‐post surgery at Third Hospital of Hebei Medical University (China) Age: mean age 71 (SD = 5) in the intervention group, mean age 73 (SD = 4) in the control group Sex: male, 15, 47% in the intervention group, male, 18, 56% in the control group Co‐morbidity: no significant differences for the American Society of Anaesthesiologists (physical status) and for BMI Dementia: excluded Frailty: not reported |
|
| Interventions | Intervention: transcutaneous electrical acupoint stimulation: TEAS (disperse‐dense waves; frequency, 2/100 Hz) on acupoints Hegu and Neiguan of both sides starting from 30 minutes before induction of anaesthesia until the end of surgery, and the intensity was the maximum current that could be tolerated. Control: in the control group, electrodes were placed on the same acupoints before anaesthesia induction, but no current was given. |
|
| Outcomes | Outcomes reported ‐Incident delirium using CAM‐ICU/RASS Outcomes from study not reported: none Frequency of outcomes assessment: assessed on day of surgery and twice daily on the 3 days following surgery |
|
| Notes | Funding source: National Natural Science Foundation of China (81771134), Natural Science Foundation of Hebei Province (H2018206305), and Hebei Provincial Government Funded Clinical Talents Cultivation and Basic Research Projects (361005). Declarations of interest: authors report no conflicts of interest. Delirium excluded at enrolment Statistically significant imbalance in administration of Propofol and Remifentanil with those in the control group receiving more of both. May be related to observed rates of delirium. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Divided into two groups using a random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided around how allocations were concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants unclear as intervention delivered while under anaesthesia and electrodes places for both groups. Blinding of personnel not described, but intervention would need to be actively administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Delirium assessment conducted by researchers blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess as no published protocol |
| Other bias | Low risk | No evidence of other bias |
Gruber‐Baldini 2013.
| Study characteristics | ||
| Methods | Design: RCT of liberal blood transfusion thresholds compared to restrictive transfusion practice for hip fracture patients Date of study: April 2008‐February 2009 Power calculation: yes Frequency of outcomes assessment: multiple times within 5 days after randomisation or up to hospital discharge (if hospital stay was shorter) Inclusion criteria: aged 50 and older; undergoing surgical repair of hip fracture; Hb < 10 g/dL within 3 days after surgery; clinical evidence of cardiovascular disease or cardiovascular disease risk factors Exclusion criteria: non‐English speaking; unable to walk unaided before fracture; declined blood transfusions; multiple traumas; pathological hip fracture; clinical acute myocardial infarction within 30 days pre‐randomisation; previous participants in the trial; symptoms associated with anaemia; actively bleeding at time of potential randomisation |
|
| Participants | Number in study: 139 Country: USA and Canada Setting: 13 hospitals Age: mean age 82.4 (SD 7.4) in intervention group compared to 80.6 (SD 10.4) in control group Sex: 81.8% of intervention group were female compared to 47% of control group Co‐morbidity: numbers and percentages of common co‐morbidities reported in paper (stroke/TIA, chronic lung disease, cancer, diabetes, atrial fibrillation, Parkinson's disease, hearing problems, visual problems and alcohol abuse or withdrawal) Dementia: 27.3% of intervention group had dementia compared to 36.1% of the control group Frailty: not reported |
|
| Interventions | Intervention (aka liberal treatment): one unit of packed red blood cells and as much blood as needed to maintain a Hb concentration >10 g/dL Control (aka restrictive treatment): only transfused if symptoms of anaemia developed or at the study physicians discretion or if Hb < 8 g/dL |
|
| Outcomes | Outcomes reported: ‐ Incident delirium, using CAM ‐ Delirium severity, using MDAS ‐ Length of admission ‐ Physical morbidity (post‐randomisation adverse events) ‐ Psychoactive medication use Outcomes from study not reported: none Frequency of outcomes assessment: multiple times within 5 days after randomisation or up to hospital discharge. |
|
| Notes | Funding source: Research grant from National Heart Lung and Blood Institute Declarations of interest: quote: "Dr Magaziner received support from Amgen, Eli Lilly, Glaxo SmithKline, Merck, Novartis and Sanofi Aventis to conduct research through his institution, provide academic consultation, or serve on an advisory board. Dr Roffey reports working as a consultant for Palladian Health. Dr Cardson reports receiving grant support to his institution from Amgen. Dr Marcantionio is a recipient of a Mid‐Career Investigator Award in Patient‐Oriented Research from the National Institute on Aging" Delirium assessed at baseline but not excluded >1/3 of the restrictive group received transfusion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated central telephone randomisation system |
| Allocation concealment (selection bias) | Low risk | No evidence to suggest allocations revealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research staff unblinded to treatment status except at one site |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 139 randomised, outcome assessment data available for 138 |
| Selective reporting (reporting bias) | Low risk | Data reported for all participants included in the study |
| Other bias | Low risk | No evidence of other bias |
Hempenius 2013.
| Study characteristics | ||
| Methods | Design: multi‐centre, RCT Date of study: June 2007‐June 2010 Power calculation: yes but study underpowered Inclusion criteria: over 65 yrs; due to undergo elective surgery for a solid tumour, deemed to be frail (using Groningen Frailty Indicator >3) Exclusion criteria: unable to complete protocol; unable to complete follow‐up; unable to complete questionnaire | |
| Participants | Sample size: 297
Country: the Netherlands
Setting: 3 hospitals (1 university medical centre, 1 teaching hospital and 1 community hospital)
Age: Mean age 77.45 (SD 6.72) in intervention group; 77.63 (SD 7.69) in usual care group
Sex: 62.2% of intervention group were female compared with 65.8%of usual care group
Co‐morbidity: stratified into < or equal to 2 co‐morbidities (39.6% of intervention group 40.4% of usual care group) or >2 co‐morbidities (60.4% in intervention group 59.6% of usual care group)
Dementia: MMSE performed at baseline; mean score 26.6 in intervention group versus 26. 33 in usual care group (P = 0.49) Frailty: not reported |
|
| Interventions | Intervention: multi‐component intervention focused on best supportive care and the prevention of delirium. Preoperative geriatric team assessment with daily monitoring during hospital stay, supported by the use of standardised checklists Control: only had access to geriatric care if treating physician requested referral | |
| Outcomes | Outcomes reported: ‐ Incident delirium, using DOSS ‐ if > 3 then had specialist assessment using DSM‐IV. ‐ Delirium severity, using DRS‐R‐98 ‐ Length of admission ‐ Mortality ‐ Return to independent living ‐ Postoperative complications ‐ Quality of life using Short‐Form‐36 ‐ Falls ‐ ADL using validated instrument using the Care Dependency Scale (CDS) ‐ Withdrawals Outcomes not reported: none Frequency of outcomes assessment: days 1‐10 postoperatively, 3 times per day |
|
| Notes | Funding source: Netherlands Organisation for Health Research and Development Declarations of interest: quote: “The authors declared that no competing interests exist” Delirium not excluded at enrolment No record of how many in usual care group received geriatrician input |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive voice response telephone system for randomisation provided by university |
| Allocation concealment (selection bias) | Low risk | Central allocation system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and research nurses unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Delirium assessment blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 297 participants randomised, outcome assessments available for 260 (n = 127 in intervention group and n = 133 in control group) ‐ no information provided, described as ’lost to follow‐up’ |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per original protocol |
| Other bias | Low risk | No evidence of other bias |
Hosie 2020.
| Study characteristics | ||
| Methods | Design: cluster‐RCT Date of study: 2017 Power calculation:dDetails of formal calculation for future phase III cluster study are detailed in the published protocol; Inclusion criteria: patients eligible for enrolment were adults (i.e. 18 years of age or older) with advanced (stage 4) cancer. Exclusion criteria: none specified. |
|
| Participants | Sample size: 65 Country: Australia Setting: four specialist palliative care inpatient units within hospitals in metropolitan Australia. Age: mean age of 76.0 (SD 11.2) in intervention group, 70.5 (15.5) in control group and 68.1 (12.5) in waitlisted group Sex: 41% of intervention group were male, compared to 65% of the control group and 66% in waitlisted group. Co‐morbidity: no specific measure of co‐morbidity or health conditions listed by group. Provided breakdown of Australian‐modified Karnofsky Performance Scale which suggests there are differences between the group, however, numbers are very small Dementia: not reported Frailty: not reported |
|
| Interventions | Intervention: the intervention had six domains (eating and drinking, sleep, exercise, reorientation, vision and hearing, and family partnership), containing 36 strategies overall (4–12 per domain). Team members were asked to enlist family and volunteers and tailor the intervention to patients’ needs and wishes. A two‐month site engagement and training period, guided by customised information manuals , preceded control and intervention conditions. Sites formed working groups of interested team members to plan implementation in line with their resources and systems. University‐based researchers attended working group meetings to ensure intervention fidelity, trial integrity, and timely progress. Sites shared meeting records whenever researchers could not attend in person. Training was provided through four discrete 30– to 40‐minute sessions using Biggs’ educational model, delivered multiple times for broadest reach. Control: control sites received information about delirium prevention strategies when they transitioned to the intervention phase, along with a summary of learnings from intervention sites about optimising trial processes. A key message was that the checklist was not the intervention per se, but essential to measuring the primary outcome of adherence. |
|
| Outcomes | Outcomes reported: ‐ Incident delirium using Nurses Delirium Screening Scale (Nu‐DESC) and DSM‐V with DRS‐R‐98. ‐ Inpatient mortality ‐ Severity of delirium (mean, using DRS‐R‐98) ‐ Falls Outcomes from study not reported: none Frequency of outcomes assessment: each eight‐hour shift |
|
| Notes | Funding source: the trial was funded by an Australian National Breast Cancer Foundation (NBCF) 2017 Pilot Study Grant (Grant code PS‐17‐030). Declarations of interest: Drs. A.H., J.P., L.L., S.K., S.L.C., A.G., and M.A. and Ms. L.B., B.F., L.E., J.H., R.A., T.A., M.G. and J.W. report a grant from the National Breast Cancer Foundation during the conduct of the study. Dr. A.H. also reports personal fees from Medtronic, outside the submitted work. Dr. G.A.C. reports grants from Bionomics Pty Ltd., outside the submitted work. Dr. E.W.E. reports personal fees from Masimo, grants from VA/NIH, personal fees from Pfizer/Orion, and grants from Koheler, outside the submitted work. All remaining authors have no disclosures to report. Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation method used |
| Allocation concealment (selection bias) | Low risk | Allocation performed by trial statistician at University using above randomisation method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not possible due to study design and nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment also unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for – high proportion in waitlisted site (7/27) did not receive intervention or have data collected but reasons provided, and majority relate to nature of the unit of care (palliative/end of life), rather than the intervention |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in accordance with published protocol (BMJ Open citation) |
| Other bias | High risk | No evidence of recruitment bias or baseline imbalance associated with cluster design. No loss of clusters. No account made for cluster design in the analytical methods reported. |
Jeffs 2013.
| Study characteristics | ||
| Methods | Design: RCT Date of study: May 2005‐December 2007 Power calculation: yes ‐ incorporating incident delirium and absolute risk reduction of 6% Inclusion criteria: aged 65 years or older; admitted to a medical unit in the study area; in hospital < 48 hours Exclusion criteria: severe dysphasia rendering communication impossible; death expected within 24 hours; isolation for infection control; documented contraindication to mobilisation; admission to the Stroke Unit or to critical care; planned admission of < 48 hours; major psychiatric diagnosis; previous inclusion in the study; delirium documented in the admission notes; transfer from another hospital. |
|
| Participants | Number in study: 649 Country: Australia Setting: acute medical wards, secondary referral centre Age: mean age of 79.6 (SD 7.5) in intervention group, 79.1 (7.9) in control group Sex: 45% of intervention group were male, compared to 50% of control group Co‐morbidity: Charlson index of 2 (1‐3) in both groups at baseline Dementia: MMSE recorded at baseline in both groups: 25 (20 to 28) in intervention group versus 26 (19‐28) in control group Frailty: not reported |
|
| Interventions | Intervention: participants randomised to the intervention arm received a graded physical activity and orientation programme twice daily, which was delivered in addition to usual care. A certified Allied Health Assistant, trained in administering exercise programmes, delivered the intervention after initial assessment of the participant by a physiotherapist. The programme started on the same day as the participant was randomised. Commensurate with ability, participants were prescribed one of four exercise programmes: bed, seated, standing or rails. All programmes were customised to the participant’s ability and were reviewed daily. Exercise programmes were modified to ensure suitable progression for those participants who made significant gains. The orientation programme comprised formal and informal elements. The formal element of the programme comprised a series of seven questions aimed at assessing and improving orientation (day, month, year, date, ward, bed number and name of primary nurse). The participant was asked the questions in sequence and prompted with the correct answer if they were not able to give a correct response. The informal element of the programme related to engaging in the exercise programme and in the social interaction with the Allied Health Assistant and/or Physiotherapist. Control: Usual care included 24‐hour nursing care, daily medical assessment and allied health referral by medical, nursing or other staff. Allied health input was provided on referral only, but daily ward meetings were held to review patient progress and facilitate referrals. Patients with significant functional, cognitive or social issues could be referred to the Aged Care medical consultation service that performed a daily round and could offer advice regarding the recognition, investigation and management of geriatric syndromes including delirium. |
|
| Outcomes | Outcomes reported: ‐ Incidence of delirium, using CAM ‐ Duration of delirium ‐ Severity of delirium, using CAM ‐ Length of stay ‐ Return to previous residence Outcomes not reported: none Frequency of outcomes assessment: every 48 hours |
|
| Notes | Funding source: HCF Health and Medical Research Foundation Declarations of interest: quote: "No competing interests" Very low rates of delirium in both arms. Authors suggest may be due to 48 hourly assessments or not selecting those at high risk. Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not clear, just states ’randomisation was achieved using sealed opaque envelopes’ |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes for allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not informed of allocation, but unable to fully blind due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n = 17 in intervention and n = 18 in control did not receive the intervention, but were assessed on an intention‐to‐treat analysis basis |
| Selective reporting (reporting bias) | Low risk | Trial protocol retrospectively registered with Australian New Zealand Clinical Trials Registry ACTRN 012605000044628; outcomes reported in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
Lundstrom 2007.
| Study characteristics | ||
| Methods | Design: RCT of multi‐component delirium prevention intervention for older hip fracture patients Date of study: May 2000 to December 2002 Power calculation: yes Inclusion criteria: patients aged 70 years and older consecutively admitted to the orthopaedic department in Umea hospital, Sweden. Exclusion criteria: age under 70, severe rheumatoid arthritis, severe hip osteoarthritis, severe renal failure, pathological fracture and patients who were bedridden before the fracture | |
| Participants | Sample size: 199
Country: Sweden
Setting: orthopaedic hip fracture patients
Age: Mean age 82 years
Sex: 74% female
Co‐morbidity: no baseline between group differences in cardiovascular disease, respiratory disease, hypertension or diabetes. More patients in control group with depression (46% versus 32%, P = 0.03)
Dementia: 27.5 % in intervention group, 37.1% in control group Frailty: not reported |
|
| Interventions | Intervention: multi‐disciplinary team providing comprehensive geriatric assessment, management and rehabilitation on a geriatric ward. Intervention comprising: staff education; teamwork; individual care planning; delirium prevention detection and treatment; prevention and treatment of complications; bowel/bladder function; sleep; decubitus ulcer prevention/treatment; pain management; oxygenation; body temperature measurement; nutrition; rehabilitation; secondary prevention of falls/fractures and osteoporosis prophylaxis Control: usual care on orthopaedic ward. |
|
| Outcomes | Outcomes reported: ‐ Incident delirium, diagnosed retrospectively using DSM‐IV based on nursing notes (for the duration of the inpatient stay) and organic brain scale (measured once between the 3rd and 5th postoperative day) ‐ Duration of delirium, diagnosed retrospectively using DSM‐IV based on nursing notes and OBS ‐ Length of admission ‐ Cognitive status, measured using MMSE ‐ Falls ‐ New pressure ulcers ‐ Psychological morbidity (Depression) ‐ Mortality ‐ inpatient and at 12 months Outcomes not reported: None Frequency of outcomes assessment: all patients tested once between day 3 and day 5 postoperatively using organic brain scale, MMSE and geriatric depression scale. Delirium diagnosed retrospectively after the study had finished by specialist in geriatric medicine blind to allocation group on the basis of the nursing assessments by applying the DSM IV criteria |
|
| Notes | Funding source: Swedish Research Council & Vardal Foundation Declarations of interest: not reported Prevalent delirium not excluded at enrolment (21.8% intervention group, 30.9% control group) but, patients with prevalent delirium appear to have been included in outcome data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on how randomisation sequence generated |
| Allocation concealment (selection bias) | Low risk | Sealed‐opaque envelopes to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All staff aware of allocation group, patients potentially aware due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff recording outcome measurements not blind to study arm. Blinded specialist made diagnosis of delirium retrospectively based on staff measurements and medical/ nursing records |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
Marcantonio 2001.
| Study characteristics | ||
| Methods | Design: RCT of proactive geriatric consultation in patients with hip fracture Date of study: study dates not reported Power calculation: yes. Study adequately powered for bivariate analyses but not for the multivariate or stratified analyses. Inclusion criteria: all patients aged 65 years and older, admitted for primary surgical repair of hip fracture, who were at intermediate or high risk of delirium (presence of 1 or more delirium risk factors) Exclusion criteria: metastatic cancer or comorbid illness reducing life expectancy to less than 6 months; Unable to obtain consent (or proxy assent) within 24 hours of surgery, or 48 hours of admission |
|
| Participants | Number in study: 126 Country: USA Setting: one academic centre orthopaedic department Age mean (SD): Intervention 78 (8), Control 80 (8); P = 0.39 Sex M:F: Intervention 21%, Control 22%; P = 0.9 Co‐morbidity: Charlson Index > 4 Intervention 39%, Control 33%; P = 0.49 Dementia: Intervention 37%, Control 51%; P = 0.13. However, dementia assessment only reported for 90% of participants Frailty: not reported |
|
| Interventions | Intervention: Proactive consultation by Consultant Geriatrician, with daily visits starting preoperatively or within 24 hours postoperatively for duration of admission. Protocol based targeted recommendations over and above what was already being done by team, limited to 5 at initial visit and 3 at follow‐up visits. Controls: usual care, consisting of management by orthopaedic team and consultation by internal medicine or geriatrics on reactive rather than proactive basis. | |
| Outcomes | Outcomes reported: ‐ Delirium incidence‐ total cumulative during admission, using CAM (performed daily throughout inpatient stay) ‐ Delirium duration ‐ Length of admission ‐ Return to independent living ‐ Withdrawals from protocol Outcomes not reported: none Frequency of outcomes assessment: daily interviews from enrolment to discharge to complete MMSE, DSI, CAM, MDAS |
|
| Notes | Funding source: older Americans Independence Center; Charles Farnworth Trust;
Declarations of interest: not reported Delirium examined but not reported at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used to generate sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared with allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nature of intervention precluded blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent researchers conducted delirium assessments and timed not to coincide with Geriatrician consultation. States blinding successfully maintained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
Martinez 2012.
| Study characteristics | ||
| Methods | Design: RCT of a multi‐component delirium prevention intervention provided by family members Date of study: September 2009‐June 2010 Power calculation: yes Inclusion criteria: all patients at risk for delirium (> 70 years, cognitive impairment (MMSE < 24 prior to admission) alcoholism or metabolic imbalance at admission) Exclusion criteria: delirium at admission, no family support, admitted to ward other than general medicine, those in a room with more than two beds |
|
| Participants | Number in study: 287 Country: Chile Setting: internal medicine ward of acute hospital Age: mean age 78.1 years (SD 6.3) in intervention group; 78.3 years (6.1) in control group Sex: 42% female in intervention group; 33% female in control group Co‐morbidity: median Charlson comorbidity index (CCI) 2 (interquartile range, IQR, 1‐4) in intervention group, median CCI 2 (IQR 1‐3) in control group Dementia: 9% in intervention group, 8% in control group Frailty: not reported |
|
| Interventions | Intervention: multi‐component non‐pharmacological intervention provided by family members, including education regarding confusional syndromes; provision of a clock and calendar; avoidance of sensory deprivation (glasses, denture and hearing aids available as needed); presence of familiar objects in the room; re‐orientation of patient provided by family members; extended visiting times (5 hours daily). Control: usual care from the attending physician |
|
| Outcomes | Outcomes reported: ‐ Incident delirium, measured using CAM performed daily, throughout admission ‐ Duration of delirium ‐ Length of admission ‐ Falls Outcomes not reported: none Frequency of outcomes assessment: Daily during hospital stay |
|
| Notes | Funding source: not reported Declarations of interest: quote: "No conflicts of interest declared" Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by a statistician who was not involved in data collection |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unblinded due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed, 5% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
Martinez‐Velilla 2019.
| Study characteristics | ||
| Methods | Design: RCT Date of study: Feb 1 2015 to Aug 30 2017 Power calculation: not for delirium outcome Inclusion criteria: Age >=75, Barthel index >=60, mobile with/without assistance, able to communicate Exclusion criteria: LOS< 6 days, severe cognitive decline, terminal illness, uncontrolled arrhythmias, acute PE, recent MI, recent major surgery, extremity bone fracture in the last 3 months |
|
| Participants | Sample size: 370 Country: Spain Setting: Acute elderly care unit Age: Mean age 87.6 years (SD 4.6) in intervention group; 87.1 years (SD 5.2) in control group Sex: male, 76 (41.1%) in the intervention group, male, 85 (45.9%) in the control group Co‐morbidity: cumulative Illness Rating Scale similar between groups = Intervention group, 13(5), Control group 12(5). No baseline between‐group differences in demographic variables including BMI and number of diseases (includes hypertension, congestive heart failure, osteoarthritis, COPD). Dementia: mean score 22 (SD = 5) in the intervention group, Mean score 23 (SD = 45) in the control group Frailty: not reported |
|
| Interventions | Intervention: intervention session supervised by an experienced fitness specialist was conducted twice daily (morning and evening) of 20 minutes’ duration during 5 to 7 consecutive days (including weekends). A session was considered completed when 90% or more of the Programmed exercises were successfully performed. Exercises were adapted from the multicomponent physical exercise program Vivifrail to prevent weakness and falls. The morning sessions included individualized supervised progressive resistance, balance, and walking training exercises. The evening session consisted of functional unsupervised exercises using light loads Control: usual care is offered to the patient by the geriatricians of the geriatrics department and consists of standard physiotherapy focused on walking exercises for restoring the functionality conditioned by potentially reversible abnormalities. A formal exercise prescription was not provided at study entry and patients were instructed to continue with the current activity practices through the duration of the study. |
|
| Outcomes | Outcomes reported ‐Incident delirium using CAM ‐Mortality at 3 months ‐Length of hospital admission (days) ‐ ADL using Barthel index (2 weeks prior to admission to hospital discharge) ‐ Quality of life using EQ5D (baseline to discharge) ‐ New care home admission at discharge (reported as home/institution) ‐ Falls Outcomes from study not reported: none Frequency of outcomes assessment: not reported. |
|
| Notes | Funding source: Gobierno de Navarra project Resolucion grant., Ministerio de Economia, Industria y Cometitividad, ISCIII and Fondos FEDER. Declarations of interest: none reported Delirium measured but not excluded at baseline (control group 12 %, intervention group 17%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation based on use of online calculator (www.randomizer.org) on a 1:1 ratio without restrictions |
| Allocation concealment (selection bias) | Unclear risk | No description provided as to how allocations concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were explicitly informed and reminded not to discuss assignment with the assessment staff. Staff providing intervention were unblinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment staff were blinded to main study design and group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in discontinuation of intervention with 47% of those dropouts in intervention group due to ‘clinical worsening’ versus 21% in control – unclear if this due to intervention. |
| Selective reporting (reporting bias) | Low risk | Study reported in accordance with trial protocol |
| Other bias | Low risk | No evidence of other bias |
Nadler 2017.
| Study characteristics | ||
| Methods | Design: RCT Date of study: 2014 (unclear ‐ not explicitly reported) Power calculation: yes (study underpowered, baseline rate of delirium lower than expected) Inclusion criteria: aged 50 years or older, at risk of obstructive sleep apnoea as defined by a STOP‐Bang score ≥ 3, scheduled for elective knee or hip arthroplasty, able to speak English, understand consent forms and give informed consent. Exclusion criteria: patients with severe tracheal or lung disease (e.g. bullous lung disease, pneumothorax, recent tracheal anastomosis) or contra‐indications to nasal‐mask CPAP (e.g. facial fractures/lacerations/burns, recent ENT surgery, basilar skull fracture, tracheostomy); Patients with previously diagnosed obstructive sleep apnoea |
|
| Participants | Sample size: 135 Country: USA Setting: perioperative hospitalised patients undergoing joint arthroplasty population could be shortened to elective orthopaedic Age: mean age 65.1 (SD = 8.4) in intervention group, mean age 66.3 (SD = 9.4) in control group Sex: male, 22 (32.4%) in intervention group, male 24 (35.8%) in control group Co‐morbidity: imbalance in rates of depression and visual or hearing impairment in intervention group compared to control. Dementia: dementia or significant cognitive impairment, 0 in intervention group, 2 (3%) in control group Frailty: not reported |
|
| Interventions | Intervention: CPAP before surgery and days 0 1 and 2 postoperative (variable amount of time between enrolment and surgery) Control: usual care |
|
| Outcomes | Outcomes reported: ‐Incident delirium using DRS‐R‐98 diagnostic assessment tool Outcomes from study not reported: ‐Incident delirium using CAM Frequency of outcomes assessment: once post op day 2 |
|
| Notes | Funding source: Equipment loaned by Philips Respironics, Amsterdam. No other funding source Supported by the Department of Psychiatry Duke University Medical Centre, Durham, NC, USA Declarations of interest: none stated. Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed by consulting a computer‐permuted sequence that guaranteed an equal number of patients in each arm within blocks of 20 |
| Allocation concealment (selection bias) | Unclear risk | No information provided around how allocations were concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators performing assessments were blinded to study group assignment and CPA devices were recovered from the group before assessment took place. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Assessments not performed in 10% of intervention group and 13% of control group and unclear why. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per NCT protocol |
| Other bias | Low risk | No evidence of other bias |
Partridge 2017.
| Study characteristics | ||
| Methods | Design: RCT Date of study: Nov 2012 to Feb 2014 Power calculation: yes, Assuming 80% power and a two‐sided significance level of 5%, a total sample size of 198 patients was required (99 per group). Attrition rates were expected to be negligible from previous observational work that showed no dropouts10; the target sample size was inflated (by 5%) to 208. Inclusion criteria: patients aged at least 65 years scheduled for elective endovascular/open aortic aneurysm repair or lower‐limb arterial bypass surgery. Exclusion criteria: admitted directly to the ward from the surgical clinic or emergency department for emergency or very urgent surgery, which precluded the opportunity for outpatient preoperative assessment and optimisation. |
|
| Participants | Sample size: 209 Country: UK Setting: teaching hospital with a tertiary referral practice for vascular arterial surgery Age: mean age 75.5 (SD = 6.6) in intervention, Mean age 75.5 (SD = 6.3) Sex: males, 80 (76.9%) in intervention, males, 79 (75.2%) in control Co‐morbidity: there were some differences between the randomised groups in relation to CVD (C: 21 of 100 (21⋅0) versus I: 10 (9⋅6), falls C: 10(9.5) versus I: 26 of 100 (26.0) Dementia: 2 (1.9%) in intervention, 5 (4.8) in control Frailty: not reported |
|
| Interventions | Intervention: comprehensive geriatric assessment and optimisation in an outpatient clinic setting. Patients were assessed and optimised according to peer‐reviewed protocols based on current evidence, national and hospital guidelines, and expert opinion. Control: the control group received standard preoperative care. Within the participating centre, this consisted of a nurse led preoperative assessment clinic where a protocolised appraisal of anaesthetic and medical issues was conducted. |
|
| Outcomes | Outcomes reported: ‐Incident delirium using CAM ‐Inpatient mortality (not explicit included as outcome but data reported in study flow chart) ‐Length of hospital admission ‐Withdrawal ‐Readmission to hospital within 30 days of discharge (unplanned 30‐day readmission) ‐Falls Outcomes from study not reported: None Frequency of outcomes assessment: recorded routinely by hospital staff |
|
| Notes | Funding source: Research Into Ageing–Age UK–British Geriatrics Society grant (reference 366) and the Guy’s and St Thomas’ Charity (EFT120610). Declarations of interest:No conflicts of interest Delirium is not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based randomisation using a 1:1 allocation and stratified according to sex and site of surgical procedure |
| Allocation concealment (selection bias) | Low risk | Performed independently by the King’s Clinical Trials Unit |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded due to nature of the intervention. Those providing their postoperative care were unaware of the patient’s involvement in study, however they had access to individualised care plans generated as part of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were recorded by an unblinded research nurse using data collected by the routine clinical teams. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Primary outcome data missing for significant proportion of those randomised. Figures in CONSORT diagram do not account for all individuals from allocation to analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per ISRCTN protocol, retrospectively registered. |
| Other bias | Low risk | No evidence of other bias |
Wang 2020.
| Study characteristics | ||
| Methods | Design: cluster‐RCT Date of study: 24/08/2015‐28/02/2016 Power calculation: yes Inclusion criteria: patients aged 70 years or older and scheduled for an elective surgical procedure with an anticipated LOS longer than 2 days were eligible for inclusion. Exclusion criteria: included (1) delirium at baseline as assessed with the Confusion Assessment Method (CAM); (2) a terminal condition with life expectancy of less than 6 months (e,g, metastatic cancer, pancreatic cancer, or receiving end‐of‐life care); (3) inability to perform cognitive tests be‐ cause of severe dementia, legal blindness, or severe deafness; (4) a documented history of schizophrenia or psychosis; and (5) a documented history of alcohol abuse or withdrawal within the past 6 months and/or reporting consumption of more than 5 drinks per day for men (4 for women). |
|
| Participants | Sample size: 281 Country: China Setting: Hospital Age: overall, mean: 74.7 (5.2) years. Mean age 74.20 (SD = 5.33) in intervention group, mean age 75.28 (SD = 4.73) in control group Sex: overall, male: 171 (60.9%). Male, 96 (63.2%) in intervention group, male 75.28 (58.1%) in control group Co‐morbidity: no clinically significant differences between groups using the Charlson comorbidity index score Score 0: I: 38(25.0), S: 27 (20.9), Score 1‐2: I:56 (36.8), 52(40.3), Score >2: I:58(38.2), C:50 (38.8) Dementia: exclusion included inability to perform cognitive tests because of severe dementia but the number of patients with any level of dementia not defined. Frailty: healthy 59.2% of intervention versus 52.7% of control, prefrail32.9% of intervention versus 34.1% of control and frail 7.9% of intervention versus 13.2% of control, assessed using FRAIL scale |
|
| Interventions | Intervention: the t‐HELP intervention consisted of 3 universal protocols and 8 targeted protocols. The universal protocols, including orientation, therapeutic activities, and early mobilisation protocol, were given to all t‐HELP participants. The targeted protocols) were tailored for each patient on the basis of delirium‐ related risk factors, which were assessed daily. Control: usual care |
|
| Outcomes | Outcomes reported: ‐ Incident delirium using CAM ‐ Inpatient mortality ‐ Peak severity of delirium (incidence of severe delirium) ‐ Length of hospital admission ‐ ADL ‐ Withdrawal ‐ Falls Outcomes not reported: ADL at 30 days. Frequency of outcomes assessment: daily up to 7 days, discharge and 30 days |
|
| Notes | Funding source: this study was funded by grant 2018YFC1312300 from the National Key Research and Development Program of the Ministry of Science and Technology of China; grant H1403014 from the Milstein Medical Asian American Partnership Foundation; grant 81800092 from the National Natural Science Foundation of China; grant Z2018B03 from the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University; grant 2018SZ0252 from the Sichuan Science and Technology Program; and grant 2019‐109, 2017‐111 from the Health Research of Cadres in Sichuan province. Dr Inouye was supported in part by grants P01AG031720, K07AG041835, R24AG054259, and R01AG044518 from the National Institutes of Health and by the Milton and Shirley F. Levy Family Chair. Declarations of interest: Dr Inouye was the creator of the Hospital Elder Life Program (HELP) but receives no income or royalties from the program. The American Geriatrics Society holds the exclusive license to HELP. no other disclosures were reported. Delirium excluded at enrolment using the Confusion Assessment Method (CAM) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Two‐step randomisation process ‐ standard computerised randomisation for the nursing units. Random assignment of participants to units co‐ordinated by member of staff not involved in study. Unclear how participant sequence generated. |
| Allocation concealment (selection bias) | Unclear risk | Opening sealed envelopes containing the random assignments’. Unclear if these envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and intervention personnel were not blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor and statistical analysts were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted‐for via CONSORT diagram. Those discontinuing intervention and lost to 30‐day follow‐up are balanced between groups. |
| Selective reporting (reporting bias) | High risk | Adverse events are not reported by group. ADL only reported at discharge |
| Other bias | Low risk | No evidence of recruitment bias or baseline imbalance associated with cluster design. No loss of clusters reported. Statistical analysis planned and performed to take account of the cluster design including use of multilevel binomial regression model in which two nested models were fitted. |
Watne 2014.
| Study characteristics | ||
| Methods | Design: RCT comparing care in an acute geriatric ward or standard orthopaedic ward following hip fracture Date of study: September 2009 ‐ January 2012 Power calculation: yes but powered for primary outcome of cognitive function not delirium Inclusion criteria: all acute admissions to Oslo University Hospital with a hip fracture Exclusion criteria: hip fracture due to high energy trauma (defined as a fall from higher than one metre) or if they were moribund on admission |
|
| Participants | Number in study: 332 randomised; 329 included in analyses Country: Norway Setting: university hospital Age: mean age 84 years (range: 55 to 99) for intervention group and 85 years (range: 46 to 101) Sex: male 42 (26%) for intervention group; 38 (23%) for controls Co‐morbidity: not reported Dementia: 49% in both intervention and control groups diagnosis by expert evaluation Frailty: not reported |
|
| Interventions | Intervention: acute geriatric ward – 20 bed ward mainly admitting patients suffering from acute medical disorder superimposed upon frailty, co‐morbidities and polypharmacy. Comprehensive Geriatric Assessment was the basis for treatment planning. Assessment by geriatrician, nurse, physiotherapist and occupational therapists was expected during their first day on the ward and this team had daily meetings to plan discharge. Checklists and clinical routines based on published literature and previous experience. These included medication reviews, optimal pain control, correction of physiological disturbances preoperatively and postoperatively (hypoxaemia, anaemia, electrolyte disturbances, acid–base disturbances, dehydration, hypotension, blood sugar etc), early and intensive mobilisation, optimising pre‐ and postoperative nutrition and early discharge planning. Outpatient orthopaedic clinic at 4 months. Control: usual care in orthopaedic ward setting. Staffing levels were similar but there was no multidisciplinary meetings and no geriatric assessments. Early mobilisation was emphasised and patients were seen by a physiotherapist soon after surgery. Outpatient orthopaedic clinic at 4 months. |
|
| Outcomes | Outcomes reported:
‐ Incident delirium using CAM ‐ In‐hospital mortality ‐ Incident dementia at 12 months ‐ Delirium duration (days) ‐ Delirium severity using MDAS ‐ Length of stay ‐ ADL function using Barthel Index at four months ‐ New care home residence at four and 12 months ‐ Falls ‐ Pressure ulcers ‐ Postoperative complications Outcomes not reported: None Frequency of outcomes assessment: daily using CAM preoperatively and until the fifth postoperative day or for patients with delirium until discharge |
|
| Notes | Funding source: Research Council of Norway through the program ‘Improving mental health of older people through multidisciplinary efforts’ (Grant No: 187980/H10) plus Oslo University Hospital, The Sophies Minde Foundation, The Norweigan Association for Public Health and Civitan’s Research Foundation Declaration of interest: the authors declare ‘they have no competing interests’ Delirium not excluded at enrolment There are concerns about the fidelity of the intervention received as, when a bed was not available in the unit, care was provided in a corridor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (blocks of variable and unknown size) carried‐ out by statistician not involved in clinical service |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed‐opaque numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Delirium assessments were performed by study nurse/geriatrician aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three moribund patients erroneously randomised were excluded from the analysis (2 from intervention and 1 from control arm) |
| Selective reporting (reporting bias) | Low risk | Study reported in accordance with published protocol |
| Other bias | Low risk | No evidence of other bias |
Young 2020.
| Study characteristics | ||
| Methods | Design: cluster‐randomised controlled feasibility trial Date of study: August 2014‐Feb 2015 Power calculation: no Inclusion criteria: patients were eligible for trial recruitment if they were aged over 65 years and admitted to the study wards during the study period. Exclusion criteria: patients were excluded if delirium was present on admission to the ward, discharge was planned within 48 hours of admission, delirium assessment had not been performed by an RA within 24 hours of admission (older people’s care patients) or preoperatively (orthopaedic trauma patients), consent had not been obtained with 48 hours of admission to the ward, end of life care was being provided or the patient was under the care of another ward. |
|
| Participants | Sample size: 713 Country: UK Setting: hospital wards ‐ orthopaedic trauma and older people care wards. Imbalance in clinical setting between intervention and control group – 62% in intervention group in Older People’s wards and 38% in Orthopaedic Trauma compared to 49% and 51% of those in the control group. Age: 82.5 (7.9) in intervention group, 83.0 (7.8) in intervention group Sex: males, 112 (23.7) in intervention group, males, 114 (31.8) in control group Co‐morbidity: no clinically significant difference between groups for overall comorbidities. Comorbidities 236 (68.8) in intervention group 244 (65.9) in control group; Mean (SD) Charlson comorbidity index score 1.7 (2.0) in intervention group, 1.7 (1.9) in control group Dementia: cognitive impairment and/or dementia 83 (24.2) in intervention group, 67 (18.1) in control group Frailty: not reported |
|
| Interventions | Intervention: prevention of delirium programme – manualised, multicomponent intervention and systematic implementation process designed to secure ward practice changes, potentially enhanced by the involvement of hospital volunteers. Comprises of actions directly affected to optimise nutrition & hydration, reduce environmental threats, increase orientation to time and place, improve communicative practices, supporting/encouraging mobility and better management of pain and infection. Implementation is supported through raising awareness and training of staff. Control: Usual care |
|
| Outcomes | Outcomes reported: ‐ Incident delirium using CAM ‐ Inpatient mortality (within 10 days) ‐ Mortality (overall) ‐ Peak severity of delirium ( mean severity of delirium episode and mean severity of delirium at 30 days) ‐ Length of stay ‐ ADL function using NEADL at 3 months ‐ Withdrawals (not explicitly included as an outcome but reported) ‐ New care home admission at discharge ‐ Falls Outcomes from study not reported: ‐ Quality of Life using EuroQoL EQ‐5D Frequency of outcomes assessment: daily up to 10 days from admission, discharge, 30 days and 3 months |
|
| Notes | Funding source: National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant RP‐PG‐0108‐10037). Declarations of interest: no conflicts to declare Delirium excluded at enrolment using CAM |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratified by ward type in a two‐stage process, randomised 1:1 between site‐level allocation and ward level allocation. Those selected for site‐level allocation further randomised 1:1 for their wards to receive intervention or control. Those selected for ward‐level allocation randomised 1:1 for intervention or control. |
| Allocation concealment (selection bias) | Low risk | Performed centrally by the statistician at the Clinical Trials Unit. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were unblinded due to nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment conducted by research assistants not involved in intervention development or delivery, but unblinded to treatment allocation. Post‐discharge outcomes were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up at day 10 is comparable between groups. Longer term (30 day and 3 months) follow‐up affected by missing questionnaire data and deaths. |
| Selective reporting (reporting bias) | Low risk | Study reported as per published protocol (Trials) |
| Other bias | Low risk | No evidence of recruitment bias. Baseline imbalance in study populations (orthopaedic versus older adult wards) between intervention and control noted by reviewers and authors of the study. Not thought to relate to underlying bias associated with randomisation. No loss of clusters reported. Statistical analysis performed to take account of cluster design including calculation of the intracluster correlation coefficient using the incidence of new‐onset delirium expressed as a proportion of the recruited study population |
ADL: activities of daily living;BMI: body mass index; CAM: Confusion Assessment Method;CAM‐ICU: Confusion Assessment Method for Intensive Care Unit; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure;CVD: cardiovascular disease; DOSS: Delirium. Observation Screening) Scale; DRS‐R‐98: Delirium Rating Scale Revised 98; DSI: Delirium Symptom Interview;DSM: Diagnostic and Statistical Manual; ENT: ear nose and throat;Hb: haemoglobin; IQR: interquartile range;IV: intravascular; MDAS: Memorial Delirium Assessment Scale; mHELP: modified Hospital Elder Life Program; MMSE: Mini Mental State Examination; Nu_DESC: Nurses Delirium Screening Scale; PVD: peripheral vascular disease; SD: standard deviation; RCT: randomised controlled trial; TIA: transient Ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2012 | Wrong setting |
| Asplund 2000 | Wrong outcome |
| Astaneh 2007 | Wrong study design |
| Avidan 2018 | Wrong outcome |
| Baldwin 2004 | Wrong outcome |
| Bjorkelund 2010 | Wrong study design |
| Blandfort 2017 | Unvalidated delirium diagnostic method |
| Boltz 2014 | Wrong study design |
| Bruera 2013 | Wrong setting |
| Cavalcante 2014 | Wrong outcome |
| Cole 1998 | Wrong study design |
| Cole 1999 | Wrong study design |
| Dalal 2012 | Wrong setting |
| Davies 2015 | Wrong setting |
| Davies 2018 | Wrong setting |
| Deschodt 2012 | Wrong study design |
| Dharmarajan 2017 | Wrong study design |
| Epling 1999 | Wrong study design |
| Ettema 2014 | Wrong study design |
| Fish‐Trotter 2018 | Wrong intervention |
| Freter 2017 | Wrong intervention |
| Gorski 2017 | Wrong study design |
| Greaves 2020 | Wrong setting |
| Groshaus 2012 | Wrong study design |
| Gustafson 1991 | Wrong study design |
| Hammond 2017 | Wrong outcome |
| Hea‐Jeong 2014 | Wrong study design |
| Heim 2017 | Unvalidated delirium diagnostic method |
| Holly 2019 | Wrong study design |
| Holroyd‐Leduc 2010 | Wrong study design |
| Hoolahan 2011 | Wrong study design |
| Hudetz 2015 | Wrong setting |
| Illioska 2014 | Wrong setting |
| Inouye 1999 | Wrong study design |
| Inouye 2000b | Wrong study design |
| Jia 2014 | Wrong intervention (included pharmacological measures) |
| Ko 2019 | Wrong study design |
| Lei 2017 | Wrong setting |
| Li 2017 | Wrong outcome |
| Lisann 2016 | Wrong setting |
| Llera 2005 | Wrong study design |
| Lundstrom 2005 | Wrong outcome |
| McCaffrey 2004 | Unvalidated delirium diagnostic method |
| Moppett 2017 | Unvalidated delirium diagnostic method |
| Mudge 2008 | Wrong study design |
| Mudge 2017 | Wrong outcome |
| Nikelski 2019 | Unvalidated delirium diagnostic method |
| O'Gara 2020 | Wrong setting |
| Pitkala 2004 | Wrong outcome |
| Rice 2017 | Wrong setting |
| Saltvedt 2012 | Wrong outcome |
| Sandberg 2001 | Wrong outcome |
| Shirvani 2020 | Wrong setting |
| Stromberg 1999 | Wrong outcome |
| Vlisides 2019 | Wrong setting |
| Wang 2018 | Wrong setting |
| Xin 2017 | Wrong intervention |
| Yoo 2013 | Wrong study design |
| Zamvar 2002 | Wrong outcome |
| Zhao 2018 | Wrong outcome |
Characteristics of studies awaiting classification [ordered by study ID]
NCT01998997 2013.
| Methods | Randomised controlled trial |
| Participants | 79 hospitalised older (≥70 years) medical inpatients |
| Interventions | Group 1: a family educational, non‐pharmacologic intervention will be administered to educate family members on how to prevent delirium. Family members will be encouraged to actively participate in this non‐pharmacologic intervention Group 2: the placebo group will be given a brochure on good health habits |
| Outcomes | Primary outcome: acceptance rate of intervention over 14 weeks Secondary outcome (s): date of incident delirium (14 weeks) Other outcomes: difficulties in performing the intervention (14 weeks) |
| Notes | ClinicalTrials.gov Identifier: NCT01998997 Status: completed (last update 24/12/2015) |
NCT03470662 2018.
| Methods | Randomised controlled study |
| Participants | 80 patients (≥65 years) with hip fractures treated surgically |
| Interventions | Group 1: care bundle Group 2: standard care |
| Outcomes | Primary outcome: incidence of delirium within 3 weeks Secondary outcome(s): VAS, perioperative complications and adverse events |
| Notes | ClinicalTrials.gov Identifier: NCT03470662 Status: completed |
NCT04188795 2019.
| Methods | Randomised controlled study |
| Participants | 80 patients with hip fracture |
| Interventions | Group 1: nursing care in accordance with the delirium preventive care protocol developed with the support of literature Group 2: routine nursing care |
| Outcomes | Primary outcome: Richards‐Campbell Sleep Questionnaire on admission, 1st day and 3rd day postoperatively, Barthel Index (BI) on admission, 1st day and 3rd day postoperatively, VAS on admission, 1st day and 3rd day postoperatively, Mini Nutritional Assesment‐ Short Form on admission Other outcome(s): Confusion Assessment Method‐ Intensive Care Unit (CAM‐ICU) on admission, 1st day and 3rd day postoperatively |
| Notes | ClinicalTrials.gov Identifier: NCT04188795 Status: completed |
UMIN000027181 2017.
| Methods | Randomised controlled study |
| Participants | 230 patients, (≥ 50 years) undergoing planned head and neck surgery |
| Interventions | Group 1: passive cycling exercise by bedside ergometer for 20 minutes Group 2: early mobilisation |
| Outcomes | Primary outcomes: incidence of delirium as determined by the DSM‐5 Secondary outcomes: change in cognitive function, depression incidence rate, number of hospital days, mortality rate, presence or absence of other adverse events |
| Notes | UMIN‐CTR Clinical Trial Identifer: UMIN000027181 Status: completed (last modified 28/10/2017) |
DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition;VAS: visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
Boltz 2018.
| Study name | Reducing disability via a family centered intervention for acutely ill persons with Alzheimer's disease and related dementias: protocol of a cluster‐randomized controlled trial (Fam‐FFC study) |
| Methods | Cluster randomised trial |
| Participants | 438 patients (≥65 years) with a diagnosis of very mild to moderate stage dementia |
| Interventions | Group 1: Family centred Function Focused Care nurse (Fam‐FFC nurse) which involves the staff nurse, along with the patient and family care givers, in the care planning, delivery, and evaluation process of Fam‐FFC interventions. Group 2: control condition (Fam‐ FFC Ed‐only) consists of education of the nursing staff with no other intervention |
| Outcomes | Primary outcome(s): physical function (activities of daily living, functional performance/ chair rise, physical activity), delirium (occurrence and severity), mood and behavior. All at 6 months post‐discharge. Secondary outcome(s): preparedness for caregiving, caregiver strain, caregiver burden, desire to institutionalise scale. All at 6 months post‐discharge. Other outcome measures: healthcare cost 12 months after enrolment, post‐acute health care utilisation at 6 months after discharge. |
| Starting date | November 2017 |
| Contact information | Marie Boltz: mpb40@psu.edu The Pennsylvania State University, College of Nursing, 306 Nursing Sciences Building, University Park, PA 16802, USA |
| Notes | ClinicalTrials.gov, ID: NCT03046121 Status: recruiting Boltz et al. Trials (2018) 19:496 https://doi.org/10.1186/s13063‐018‐2875‐1 |
ChiCTR1900027115 2019.
| Study name | Effects of acupuncture on postoperative delirium in elderly patients after laparoscopic surgery |
| Methods | Randomised controlled trial |
| Participants | 240 (≥ 65 years) undergoing laparoscopic surgery |
| Interventions | Group 1: acupuncture Group 2: sham acupuncture Group 3: control group |
| Outcomes | Primary outcome: incidence of postoperative delirium Secondary outcome(s): VAS |
| Starting date | 1/11/2019 |
| Contact information | Shen Qihong, shenqihong1989@163.com The First Hospital of Jiaxing |
| Notes | ClinicalTrials.gov Identifier: ChiCTR1900027115 Status: recruiting |
DRKS00013158 2017.
| Study name | Influence of perinterventional acupuncture on the incidence of postoperative delirium after elective, endoprosthetic replacement of the hip joint |
| Methods | Randomised controlled trial |
| Participants | 135 patients (≥ 65 years) scheduled for total endoprosthetic hip |
| Interventions | Group 1: acupuncture Group 2: control (no treatment) |
| Outcomes | Primary outcome: incidence of postoperative delirium after total hip joint replacement at 24 hours and 48 hours postoperatively using the CAM for intensive care units. Secondary outcome: activity of acetylcholinesterase (ACHE) and butyrylcholinesterase (BCHE) in the blood, Determination TNFa, NSE, S‐100 ß protein, Epigenetic markers such as DNA methylation, acetylation, and histone modifications, hospital length of stay. All measured 4 times (during premedication, directly postoperatively, first and second postoperatively) |
| Starting date | 01/01/2018 |
| Contact information | Lars Bergmann, lars.bergmann@kk‐bochum.de. Universitätsklinikum Knappschaftskrankenhaus Bochum, Klinik für Anästhesiologie, Intensivmedizin und Schmerztherapie |
| Notes | German Clinical Trials Register, Main ID: DRKS00013158 Status: recruiting planned |
DRKS00016352 2019.
| Study name | Evaluation of incidence of delirium in an acute care hospital by engaging an innovative and multidisciplinary approach |
| Methods | Randomised controlled study |
| Participants | 190 patients (≥ 65 years) who have been hospitalised for 3 days or longer in trauma surgery. |
| Interventions | Group 1: innovative standardised management of delirium Group 2: standard care |
| Outcomes | Primary outcome: incidence of delirium using the CAM (Confusion Assessment Method) on the first three postoperative days/ on the first three days of hospitalisation Secondary outcome(s): cognitive outcome 12 months after discharge by using the MoCA (Montreal Cognitive Assessment) and the i‐ADL (instrumental ‐ Activity of Daily Living Scale) |
| Starting date | 14/01/2016 |
| Contact information | Ms. Katharina Iltingreuke, katharina.ilting‐reuke@ukmuenster.de Universitätsklinikum Münster |
| Notes | German Clinical Trials Register ID: DRKS00016352 Status: Complete |
Humeidan 2015.
| Study name | Perioperative cognitivepProtection ‐ Cognitive Exercise and Cognitive Reserve (The Neurobics Trial) |
| Methods | Randomised clinical trial |
| Participants | 322 patients (≥60 years) who underwent non‐cardiac/non‐neurological surgery with expected hospital stay of at least 72 hours. |
| Interventions | Group 1: cognitive exercises consisting of a series of computer games focusing on five categories: memory, speed, attention, flexibility and problem‐solving Group 2: standard care |
| Outcomes | Primary outcome(s): reduction in the incidence of PostOperative Delirium (%) [ Time Frame: Postoperative period (Day 0 through Day 7 or discharge, whichever comes first)] as detected by the Confusion Assessment Method (CAM) / Memorial Delirium Assessment Scale (MDAS) Secondary outcome(s): Mini‐Mental State Examination, Self‐Administered Gerocognitive Examination, Geriatric Depression Scale, Charlson Comorbidity Index, Short Form 36 Health Survey, Confusion Assessment Method, Memorial Delirium Assessment Scale, Postoperative Quality of Recovery Scale |
| Starting date | July 2015 |
| Contact information | Michelle L Humeidan, michelle.humeidan@osumc.edu Department of Anesthesiology, The Ohio State University Wexner Medical Center, Columbus, Ohio |
| Notes | ClinicalTrials.gov Identifier: NCT02230605 Status: completed |
IRCT20180910040995N1 2019.
| Study name | Effect of HELP model on prevention of delirium |
| Methods | Randomised clinical trial |
| Participants | 110 patients (≥70 years) admitted to the internal wards with at least one risk factor for delirium at admission |
| Interventions | Group 1: interventions such as therapeutic activity, early mobilisation, daily orientation, sleep enhancement, feeding assistance/fluid repletion, and helping to resolve visual and auditory disorders. Group 2: standard care |
| Outcomes | Primary outcome: incidence of delirium using CAM on everyday until discharge Secondary outcome(s): activities of daily living (Barthel Index) on admission and on discharge, level of frailty (clinical frailty index) on admission and on discharge, number of falls, use of anti‐psychotic drugs, number of readmissions after discharge until 3 months after discharge |
| Starting date | 07/10/2018 |
| Contact information | Afsaneh Kogaie Bidgoli, kojaiibidgoli@yahoo.com University of social welfare and rehabilitation sciences |
| Notes | IRCT registration number: IRCT20180910040995N1 Status: recruitment complete |
JPRN 2017.
| Study name | A multi‐center, cluster randomized controlled study comparing usual care and a multidisciplinary intervention such as the DELirium Team Approach program to manage delirium among hospitalized cancer patients |
| Methods | Cluster‐randomised controlled trial |
| Participants | 9600 Hospitalised cancer patients (≥50 years) |
| Interventions | Group 1: implementation of the DELTA program with six components: (1) education of healthcare providers, (2) screening of delirium, (3) planning for delirium care, (4) prevention of occurrence and worsening of delirium, (5) scheduled assessment of delirium symptoms or risk factors, and (6) management and treatment of delirium. Group 2: standard care |
| Outcomes | Primary outcome (s): the incidence in events in medical safety such as falls, self‐removal of drip infusion or drain tube, restraint Secondary outcome (s): falls, self‐removal of drip infusion or drain tube, restraint, Barthel Index, level of nursing care needs, antipsychotic drug use, opioid use, duration of hospital stay, cost of medical care, hospital readmission within 1 month after discharge, mortality within 1 year after discharge |
| Starting date | 11/12/2017 |
| Contact information | Asao Ogawa, asogawa@east.ncc.go.jp National Cancer Center, Division of Psycho‐Oncology, Exploratory Oncology Research&Clinical Trial Center |
| Notes | Japan Primary Registries Network‐UMIN000030062 Status: no longer recruiting |
NCT03060174 2017.
| Study name | Study of prevention of postoperative delirium to reduce Incidence of postoperative cognitive dysfunction |
| Methods | Randomised controlled trial |
| Participants | 638 patients (≥ 60 years) to undergo cardiac surgery (on‐pump/off‐pump, standard/minimal invasive) |
| Interventions | Group 1: monitoring and non‐medical prophylaxis of delirium which incorporates reorientation (watches, calendar, family photos, use of hearing aids, glasses and dentures, cognitive stimulation (newspaper, magazines, radio, television), early mobilisation, early enteral nutrition, early removal of drains or catheters, normalizing sleep‐awake‐rhythm. Group 2: standard care |
| Outcomes | Primary outcome: postoperative cognitive deficit (POCD) measured by neuropsychological test battery, analysis (change from baseline in cognitive function at day 7, 3 months and 1 year after operation) Secondary outcome: incidence and severity of postoperative delirium (from day of operation until the 7th postoperative day) measured 3 times per day via CAM‐ICU, number of patients with cardiac complications (day of operation until 7th postoperative day), length of hospital stay (from day of admission until day of discharge, up to 24 weeks), mortality (1 years), health related quality of life ( months, 1 year after operation) using short form health survey, number of patients with respiratory complications (day of operation until 7th postoperative day), number of patients with renal complications (day of operation until 7th postoperative day) daily documentation of renal complications (creatinine, haemo(dia)filtration or haemodialysis), number of patients with complications in the immuno system (day of operation until 7th postoperative day), daily documentation of parameters mirroring the immune answer (C‐reactive protein, leukocytes, procalcitonin) |
| Starting date | May 2014 |
| Contact information | Prof. Alwin E. Goetz Department of Anaesthesiology and Intensive Care Medicine, University Hospital Hamburg Eppendorf |
| Notes | ClinicalTrials.gov Identifier: NCT03060174 Status: active, not recruiting Estimated completion date: May 2019 |
NCT03158909 2017.
| Study name | Trial of a non‐pharmacological Intervention to prevent delirium among elderly in‐patients |
| Methods | Randomised controlled study |
| Participants | 284 patients (≥ 60 years) for inpatient stay at a Brazilian Hospital |
| Interventions | Group 1: patients in this group will receive eyemask and earplugs, for use during the night, and orientations about space and time, every night Group 2: this group will receive orientations about space and time only, every night. |
| Outcomes | Primary outcome: incidence cases of delirium up to 15 days from the inclusion in the study using the Confusion Assessment Method (Short‐CAM) Secondary outcome(s): sleep quality up to 15 days, safety of the intervention up to 15 days, acceptance, comfort and adherence to the intervention up to 15 days, use of psychotropic drugs up to 15 days, time of hospital stay up to 6 months, evaluation of the sleep‐wake cycle up to 15 days, urinary 6‐sulfatoxymelatonin levels 48 hours after admission |
| Starting date | 15/01/2020 |
| Contact information | Artur Schuh, schuh.afs@gmail.com Hospital de Clínicas de Porto Alegre |
| Notes | ClinicalTrials.gov Identifier: NCT03158909 Status: recruiting (last update, 14/01/2020) |
NCT03541408 2018.
| Study name | Preventative delirium protocol in elderly patients |
| Methods | Randomised controlled trial |
| Participants | Patients (≥65 years) of age undergoing elective surgery. |
| Interventions | Group 1: preventative delirium protocol Group 2: standard of care without preventative delirium protocol |
| Outcomes | Primary outcome: presence or absence of delirium (CAM_ICU) (Within one postoperative day) using the validated CAM‐ICU measure Secondary outcome:pPostoperative nausea and vomiting within one day postoperatively, numerical rating scale of pain intensity within one postoperative day. |
| Starting date | May 2016 |
| Contact information | Robert McCarthy, Robert_J_McCarthy@40rush.edu Rush University Medical Center, Chicago, Illinois, United States, 60612 |
| Notes | ClinicalTrials.gov Identifier: NCT03541408 Status: recruiting Estimated study completion: 01/12/2020 |
NCT03573843 2018.
| Study name | Software‐guided cognitive stimulation to prevent delirium (Prevedel) |
| Methods | Pilot randomised controlled trial |
| Participants | 60 older patients (≥ 65 years) and admitted to medicine room or intermediate care unit > 48 hours |
| Interventions | Group 1: receive standard prevention measures plus the use of software installed on a mobile device designed to support the prevention of delirium (Prevention software) Group 2: receive the standard prevention measures plus the use of a mobile device without installed delirium prevention software (placebo). |
| Outcomes | Primary outcome: difference in delirium incidence between both groups at day 5 using the with CAM twice a day Secondary outcome(s): length of stay at 5 days, severity of delirium at 5 days using the CAM‐S, time of use of electronic device at 5 days, functionality at discharge at 5 days and at discharge using the Barthel index |
| Starting date | 15/09/2018 |
| Contact information | Eduardo A Tobar, etobar@40hcuch.cl University of Chile |
| Notes | ClinicalTrials.gov Identifier: NCT03573843 Status: recruitment completed |
NCT03704090 2018.
| Study name | Non‐pharmacological Prevention of Postoperative Delirium by Occupational Therapy Teams (PREPODOT) |
| Methods | Randomised controlled trial |
| Participants | 160 patients (≥75 years) and older admitted to hospital for highly complex elective surgery |
| Interventions | Group 1: occupational therapy intervention twice a day plus standard non‐pharmacological prevention intervention during 5 days after surgery Group 2: standard non‐pharmacological intervention during 5 days after surgery |
| Outcomes | Primary outcome: delirium at 5 days using the CAM, subsyndromal delirium at 5 days using the CAM Secondary outcome(s): length of hospital stay at 30 days, mortality, severity of delirium at 5 days using the CAM‐S, duration of delirium at 5 days. |
| Starting date | 1/10/2018 |
| Contact information | Antonello Penna, apenna@40uchile.cl University of Chile |
| Notes | ClinicalTrials.gov Identifier: NCT03704090 Status: recruiting Estimated completion date: 30/12/2020 |
NCT03832192 2019.
| Study name | Care.Coach Avatars for improvement of outcomes in hospitalized elders,iIncluding mitigation of falls and delirium: a multi‐site clinical study |
| Methods | Randomised controlled trial |
| Participants | 2400 patients (≥18 years) at risk of fall/delirium |
| Interventions | Group 1: care.coach human‐in‐the‐loop avatar system with software‐directed protocols based on the Hospital Elder Life Program (HELP) Group 2: standard care |
| Outcomes | Primary outcome: incidence delirium until day 4 using the CAM, average number of falls until day 4 Secondary outcome(s): delirium resolution until day 4, change in delirium severity until day 4 using the memorial delirium assessment scale (MDAS), patient sitter utilisation until day 4, change in cognitive function until day 4 using the short portable mental status questionnaire, falls with injury until day 4 |
| Starting date | 08/01/2019 |
| Contact information | Victor Wang, victor@40care.coach Jamaica Hospital Medical Center |
| Notes | ClinicalTrials.gov Identifier: NCT03832192 Status: recruiting Estimated completion date: 07/01/2021 |
NCT03894709 2019.
| Study name | A care model for elderly hip‐fractured persons with cognitive impairment and their family caregivers |
| Methods | Randomised controlled trial |
| Participants | 304 patients (≥60 years) admitted with one‐side hip fracture and requiring surgery. |
| Interventions | Group 1:family‐centred approach to interdisciplinary care and a family caregiving‐training component to enhance family caregivers' competence in providing postoperative care and handling behavioural problems of adults with cognitive impairment Group 2: patients receive health teaching for exercise while still in bed. The usual care does not involve interdisciplinary care protocols, continuity of care, or specific care for hip‐fractured patients with cognitive impairment. |
| Outcomes | Primary outcome: change from baseline in range of motion to 1 year, change from baseline muscle strength to 1 year, change from baseline flexibility to 1 year, change from baseline physical function to 1 year using the activities of daily livings change from baseline cognitive function to 1 year using the Chinese version Cohen‐mansfield agitation inventory, change from baseline caregiver competence to 1 year, change from baseline delirium to 1 year Secondary outcome(s): change from one month service utilisation to 1 year, change from baseline health‐related quality of life using the SF‐36 Taian version, change from baseline cost of care to 1 year |
| Starting date | 1/1/2015 |
| Contact information | Yea‐Ing Lotus Shyu, yeaing@mail.cgu.edu.tw Chang Gung Memorial Hospital |
| Notes | ClinicalTrials.gov Identifier: NCT03894709 Status: Active, not recruiting Estimated completion date: 31/10/2019 |
NCT03980782 2019.
| Study name | The effect of music therapy on delirium |
| Methods | Randomised controlled trial |
| Participants | 44 acutely ill patients (≥ 65 years) admitted to the progressive care unit |
| Interventions | Group 1: each participant will receive a 30‐minute individual music intervention twice daily Group 2: standard care |
| Outcomes | Primary outcome: Incidence of delirium at 2‐3 months using the confusion assessment method Secondary outcome(s): Severity of delirium at 2‐3 months using the CAM‐S, |
| Starting date | 5/6/2019 |
| Contact information | Mary Kovaleski Geisinger Clinic |
| Notes | ClinicalTrials.gov Identifier: NCT03980782 Status: completed |
NTR7036 2018.
| Study name | Effect of Music on the clinical outcome after Hip fracture OPeratIoNs (MCHOPIN): a multicenter randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants | 508 patients (≥ 65 years) with a proximal femur fracture undergoing surgical treatment |
| Interventions | Group 1: Perioperative recorded music Group 2: Standard care |
| Outcomes | Primary outcome: delirium (DOS scale and clinical diagnosis by geriatrician) Secondary outcome(s): pain (NRS), Anxiety (STAI‐6), medication use, postoperative complications, neurohormonal stress response (serum cortisol), hospital length of stay, 30‐day mortality, nursing home length of stay, 90‐day readmission, 90‐day functional ability to perform daily living activities (Katz‐ADL6), cost analysis (direct medical costs) |
| Starting date | 1/7/2018 |
| Contact information | V.X. Fu, v.fu@erasmusmc.nl Department of Surgery, Erasmus MC University Medical Center |
| Notes | Netherlands Trial Register Identifier: NTR7036 Status: pPending Estimated completion date: not reported |
Piotrowicz 2018.
| Study name | The “Wholesome Contact” non‐pharmacological, volunteer‐delivered multidisciplinary programme to prevent hospital delirium in elderly patients: study protocol for a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | 416 patients (≥70 years) and have been hospitalised for medical reasons. |
| Interventions | Group 1: structured, non‐pharmacological care delivered by students of medicine, psychology and nursing, together with standard medical treatment Group 2: standard medical treatment |
| Outcomes | Primary outcome: incidence of delirium using the CAM Secondary outcome(s): occurrence of in‐hospital adverse health outcomes, such as falls and in‐hospital deaths, In‐hospital changes (i.e. the difference noted between the day of baseline assessment and the day of discharge) in cognition (difference in the Mini‐ Mental State Examination (MMSE) score), mood and anxiety (difference in the Hospital Anxiety and Depression Scale score (HADS)) and functional status (difference in the Activities of Daily Living Scale (ADL) and the Instrumental Activities of Daily Living Scale score (IADL)), |
| Starting date | May 2018 |
| Contact information | Karolina Piotrowicz, karolina.piotrowicz@uj.edu.pl Department of Internal Medicine and Gerontology, Faculty of Medicine, Jagiellonian University Medical College |
| Notes | Polish Science Database Identifer: 317484 Estimated completion date: not reported Piotrowicz et al. Trials (2018) 19:439 https://doi.org/10.1186/s13063‐018‐2781‐6 |
Sanchez 2019.
| Study name | Patient safety, cost‐effectiveness, and quality of life: reduction of delirium risk and postoperative cognitive dysfunction after elective procedures in older adults‐study protocol for a stepped‐wedge cluster randomized trial (PAWEL Study) |
| Methods | Stepped‐wedge cluster randomised trial |
| Participants | 1500 patients (≥70 years) undergoing elective operative procedures (cardiac, thoracic, vascular, proximal big joints and spine, genitourinary, gastrointestinal, and general elective surgery procedures) |
| Interventions | Group 1: cross‐sectorial all‐encompassing multimodal delirium prevention and management approach Group 2: standard care |
| Outcomes | Primary outcome: delirium prevalence using the delirium screening (I‐Confusion Assessment Method‐based scoring system for delirium severity (I‐CAM)/CAM‐S)) [over 7 days after surgery and after 2 and 6 months, the Nursing Delirium Screening Scale (NuDESC) [(days 2 and 6 after surgery), a chart review at discharge applying the DSM‐V delirium criteria as a reference standard and the clinical evaluation. Secondary outcome(s): delirium duration as described in the primary outcome assessment; prevalence of POCD 2 and 6 months after surgery; and persistence of POCD after 12 months. The prevalence of POCD will be measured by the following neuropsychological test battery: the Montreal Cognitive Assessment (MoCA), the digit span backwards, the Trail Making Test A and B (TMT A and B), and cognitive performance measured with the continuous non standardised test values of these scales. |
| Starting date | November 2017 |
| Contact information | Michael Rapp, michael.rapp@uni‐potsdam.de Department of Social and Preventive Medicine, University of Potsdam |
| Notes | German Clinical Trials Register Identifer: DRKS00013311 Status: not reported. Estimated completion date: December 2020 |
Wong 2018.
| Study name | The prevention of delirium in elderly with obstructive sleep apnea (PODESA) study: protocol for a multi‐centre prospective randomized, controlled trial |
| Methods | Randomised controlled trial |
| Participants | 304 patients (≥60 years) scheduled for elective hip or knee replacement surgery at least 4 working days after the preadmission clinic visit |
| Interventions | Group 1: auto‐titrating Continuous Positive Airway Pressure (CPAP) treatment will be given on postoperative days 1, 2, and 3. Group 2: standard care |
| Outcomes | Primary outcome: incidence of postoperative delirium over 2 months Secondary outcome(s): length of hospital stay, time to ambulate (1 week to 2 months) perioperative complications at 10‐14 days |
| Starting date | 24/03/2016 |
| Contact information | Jean Wong, University Health Network, Toronto |
| Notes | ClinicalTrials.gov Identifier: NCT02954224 Status: Active, not recruiting Estimated completion date: 30/08/2020 |
CAM: Confusion Assessment Method; SF‐36: Short Form survey; STAI‐6: State‐Trait Anxiety Inventory;TNFa: tumour necrosis factor alpha; VAS: visual analogue scale.
Differences between protocol and review
The review protocol (Burton 2019) specified the primary outcome was 'Incidence of delirium, using a validated diagnostic method'. Following a peer review comment, we added text to clarify that this outcome is measured during hospital admission.
The review protocol specified 'peak severity of delirium' as a defined secondary outcome. It was not possible to evaluate this outcome due to the heterogeneity of methods to report delirium severity. Thus, the review includes delirium severity and comments on how this has been measured.
The review protocol referenced using the Risk of Bias‐2 (RoB‐2) (Higgins 2018) and listed the domains for RoB‐2 utilisation. The review has used the original risk of bias tool (Higgins 2011) ‐ the references and domains have been updated to reflect this change.
The review protocol referenced use of Review Manager 5 (Review Manager 2014), however the review has been drafted and submitted using RevMan Web (RevMan Web 2021).
The review protocol described subgroup analysis to remove studies in which individuals were receiving palliative care only versus those receiving other medical or surgical treatment. This should have been described as a sensitivity analysis and is reported here correctly as a sensitivity analysis.
An additional sensitivity analysis was undertaken removing studies which used a cluster‐randomised design from the analysis of delirium incidence; this was not planned in the protocol, but done to address reviewer comments.
Contributions of authors
JKB ‐ title and abstract screening; full‐text retrieval and review; data extraction; risk of bias assessment; data entry; network meta‐analysis and interpretation of findings; drafting of review and co‐ordination of comments
LC ‐ data extraction; risk of bias assessment; data entry; network meta‐analysis and interpretation of findings
SQY ‐ title and abstract screening; full‐text retrieval and review; data extraction
NS ‐ title and abstract screening; full‐text review; data extraction
EAT ‐ title and abstract screening; full‐text review
RW ‐ full text review; data extraction
AJB ‐ title and abstract screening; data extraction
AMS ‐ full‐text review; data extraction
AB ‐ data extraction
SCF ‐ network meta‐analysis and interpretation of findings
AJS ‐ network meta‐analysis and interpretation of findings
TJQ ‐ network meta‐analysis and interpretation of findings; support in responding to editorial and peer‐reviewer comments
LC, NS, AJB, AB, SCF, AJS, TJQ provided comments and critical review of the manuscript
All review authors approved the manuscript
Sources of support
Internal sources
-
Academic Section of Geriatric Medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow, UK
Support for author JKB
-
Department of Health Sciences, University of York and Hull York Medical School, UK
Support for author NS
-
Academic Unit of Elderly Care and Rehabilitation, University of Leeds, Bradford, UK
Support for author EAT
-
Department of Geriatric Medicine, University of Edinburgh, UK
Support for author AJB
-
Department of Health Sciences, University of Leicester, UK
Support for authors SCF and AJS
External sources
-
Medical Research Scotland, UK
Shun Qi Yong received a Vacation Scholarship from Medical Research Scotland. The funder played no role in the conduct of the review.
-
National Institute for Health Research Incentive Award (130725), UK
The Cochrane Dementia and Cognitive Improvement Group received an NIHR Cochrane Incentive Award to support this review. This review presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
-
NHS Education for Scotland (NES) Scottish Clinical Research Excellence Development Scheme (SCREDS), UK
Jennifer K Burton is funded by an NES SCREDS Clinical Lectureship. The funder played no role in the conduct of the review.
Declarations of interest
JKB has no known conflicts of interest
LC has no known conflicts of interest
SQY has no known conflicts of interest
NS is an author on a study included in the review (Young 2020), she played no part in the study selection, data extraction or quality assessment of the work
EAT is an author on a study included in the review (Young 2020), she played no part in the study selection, data extraction or quality assessment of the work
RW has no known conflicts of interest
AJB has no known conflicts of interest
AMS has no known conflicts of interest
AB has no known conflicts of interest
SCF has no known conflicts of interest
AJS has no known conflicts of interest
TJQ has no known conflicts of interest
Edited (no change to conclusions)
References
References to studies included in this review
Abizanda 2011 {published data only}
- Abizanda P, León M, Domínguez-Martín L, Lozano-Berrio V, Romero L, Luengo C, et al. Effects of a short-term occupational therapy intervention in an acute geriatric unit. Maturitas May 2011 Epub;69(3):273-8. [DOI] [PubMed] [Google Scholar]
Avendano‐Cespedes 2016 {published data only}
- Avendano-Cespedes A, Garcia-Cantos N, Gonzalez-Teruel M, Martinez-Garcia M, Villarreal-Bocanegra E, Oliver-Carbonell JL, et al. Pilot study of a preventive multicomponent nurse intervention to reduce the incidence and severity of delirium in hospitalized older adults: MID-Nurse-P. Maturitas 2016;86:86-94. [DOI] [PubMed] [Google Scholar]
- Pilot study of a Multicomponent nurse intervention to reduce delirium in hospitalizedoOlder adults. https://clinicaltrials.gov/show/nct02558777 2015.
Bonaventura 2007 {published data only (unpublished sought but not used)}
- Bonaventura M, Zanotti, R. Effectiveness of "IPD" treatment for delirium prevention in hospitalized elderly. A controlled randomized clinical trial [Italian]. Professioni Infermieristiche 2007;60(4):230-6. [PubMed] [Google Scholar]
Boustani 2012 {published data only}
- Boustani MA, Campbell NL, Khan BA, Abernathy G, Zawahiri M, Campbell T, et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. Journal of General Internal Medicine 2012;27(5):561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cetinkaya 2019 {published data only}
- Cetinkaya, F. Effect of listening to music on postoperative cognitive function in older adults after hip or knee surgery: a randomized controlled trial. Journal of Perianesthesia Nursing 2019;34(5):919-28. [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen CC, Li HC, Liang JT, Lai IR, Purnomo JDT, Yang YT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surgery 2017;152(9):827-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dong 2020 {published data only}
- Dong Z, Song J, Ge M, Lin C, Zhang J, Chen J, et al. Effectiveness of a multidisciplinary comprehensive intervention model based on the Hospital Elderly Life Program to prevent delirium in patients with severe acute pancreatitis. Annals of Palliative Medicine 2020;9(4):2221-8. [DOI] [PubMed] [Google Scholar]
Fan 2014 {published data only}
- Fan YX, Liu FF, Jia M, Yang JJ, Shen JC, Zhu GM, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Archives of Gerontology and Geriatrics 2014;59(1):181-5. [DOI] [PubMed] [Google Scholar]
Gao 2018 {published data only}
- Gao F, Zhang Q, Li YA, Tai Y, Xin X, Wang XL, et al. Transcutaneous electrical acupoint stimulation for prevention of postoperative delirium in geriatric patients with silent lacunar infarction: a preliminary study. Clinical Interventions in Aging 2018;13:2127-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruber‐Baldini 2013 {published data only}
- Gruber-Baldini AL, Marcantonio E, Orwig D, Magaziner J, Terrin M, Barr E, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. Journal of the American Geriatrics Society 2013;61:1286-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hempenius 2013 {published data only}
- A randomised controlled trial of geriatric liaison intervention in frail surgical oncology patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN46161863 2006.
- Hempenius L, Slaets JP, Asselt D, Bock GH, Wiggers T, Leeuwen BL. Outcomes of a geriatric liaison intervention to prevent the development of postoperative delirium in frail elderly cancer patients: report on a multicentre, randomized, controlled trial. PLOS One 2013;8(6):e64834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempenius L, Slaets JP, Asselt D, Bock TH, Wiggers T, Leeuwen BL. Long term outcomes of a geriatric liaison intervention in frail elderly cancer patients. PLOS One 2016;11(2):e0143364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosie 2020 {published data only}
- A pilot study to determine the feasibility and acceptability of a non-pharmacological intervention to prevent delirium for people with advanced cancer in hospital. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373168.
- Hosie A, Philips J, Lam L, Kochovska S, Noble B, Brassil M, et al. A multicomponent nonpharmacological intervention to prevent delirium for hospitalized people with advanced cancer: a phase ii cluster randomized waitlist controlled trial (The PRESERVE pilot study). Journal of Palliative Medicine 2020;23(10):1314-22. [DOI] [PubMed] [Google Scholar]
- Hosie A, Phillips J, Lam L, Kochovska S, Brasil M, Noble B, et al. A phase II cluster randomised controlled trial of a multi-component non-pharmacological intervention to prevent delirium for hospitalised people with advanced cancer: study protocol. In: Palliative Medicine. Supplement 1 edition. Vol. 32. 2018:136. [DOI] [PMC free article] [PubMed]
- Hosie A, Phillips J, Lam L, Kochovska S, Brassil M, Noble B, et al. A phase II cluster randomised controlled trial of a multi component non-pharmacological intervention to prevent delirium for in-patients with advanced cancer (The PRESERVE pilot study). In: Asia-Pacific Journal of Clinical Oncology. Vol. 14. 2018:166.
- Hosie A, Phillips J, Lam L, Kochovska S, Noble B, Brassil M, et al. Multicomponent non-pharmacological intervention to prevent delirium for hospitalised people with advanced cancer: study protocol for a phase II cluster randomised controlled trial. BMJ Open 2019;9(1):e026177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffs 2013 {published data only}
- Jeffs KJ, Berlowitz DJ, Grant S, Lawlor V, Graco M, Morton NA, et al. An enhanced exercise and cognitive programme does not appear to reduce incident delirium in hospitalised patients: a randomised controlled trial. BMJ Open 2013;3:e002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs KJ, Berlowitz DJ, Savige JA, Lim WK. Does an enhanced exercise and cognitive program reduce incident delirium in older hospital patients: results of a randomised controlled trial. Internal Medicine Journal 2008;38(Suppl 5):A121. [Google Scholar]
Lundstrom 2007 {published data only}
- Gustafson Y. Outcomes of hip fractures: Rehabilitation programmes: Comprehensive Geriatric Assessment and Rehabilitation-a prerequisite for successful treatment of people who have suffered a hip-fracture. European Geriatric Medicine 2012;3:S19.. [Google Scholar]
- Lundstrom M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Ageing Clinical and Experimental Research 2007;19(3):178-86. [DOI] [PubMed] [Google Scholar]
- Stenvall M, Berggren M, Lundstrom M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia--subgroup analyses of a randomized controlled trial. Archives of Gerontology and Geriatrics 2012;54(3):e284-9. [DOI] [PubMed] [Google Scholar]
Marcantonio 2001 {published data only}
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. Journal of the American Geriatrics Society 2001;49(5):516-22. [DOI] [PubMed] [Google Scholar]
Martinez 2012 {published data only}
- Martinez F. Prophylactic environmental management of delirium. https://clinicaltrials.gov/ct2/show/NCT01356810 2011.
- Martinez FT, Tobar C, Beddings CI, Vallejo G. Preventing delirium in an acute hospital using a non-pharmacological intervention. Age Ageing 2012;41(5):629-34. [DOI] [PubMed] [Google Scholar]
Martinez‐Velilla 2019 {published data only}
- Martinez-Velilla N, Casas-Herrero A, Zambon-Ferraresi F, Saez fe Asteasu M, Lucia A, Galbete A, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA Internal Medicine 2019;179(1):28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadler 2017 {published data only}
- Nadler JW, Evans JL, Fang E, Preud'Homme XA, Daughtry RL, Chapman JB, et al. A randomised trial of peri-operative positive airway pressure for postoperative delirium in patients at risk for obstructive sleep apnoea after regional anaesthesia with sedation or general anaesthesia for joint arthroplasty. Anaesthesia 2017;72(6):729-36. [DOI] [PubMed] [Google Scholar]
Partridge 2017 {published and unpublished data}
- Partridge JS, Harari D, Martin FC, Peacock JL, Bell R, Mohammed A, et al. Randomized clinical trial of comprehensive geriatric assessment and optimization in vascular surgery. British Journal of Surgery 2017;104(6):679-87. [DOI] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- ChiCTR-POR-15006944. A perioperative multidisciplinary intervention to prevent postoperative delirium in elderly patients: a cluster randomized controlled study. Chinese Clinical Trial Registry 2015.
- Wang Y-Y, Yue J-R, Xie D-M, Carter P, Li Q-L, Gartaganis SL, et al. Effect of the tailored, family-involved hospital elder life program on postoperative delirium and function in older adults: a randomized clinical trial. JAMA Internal Medicine 2020;180(1):17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watne 2014 {published data only}
- Watne LO, Torbergsen AC, Conroy S, Engedal K, Frihagen F, Hjorthaug GA, et al. The effect of a pre- and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial (Oslo Orthogeriatric Trial). BMC Medicine 2014;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyller TB, Watne LO, Torbergsen A, Engedal K, Frihagen F, Juliebø V, et al. The effect of a pre- and post-operative orthogeriatric service on cognitive function in patients with hip fracture. The protocol of the Oslo Orthogeriatrics Trial. BMC Geriatrics 2012;12(36):doi:10.1186/1471-2318-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2020 {published data only}
- Godfrey M, Green J, Smith J, Cheater F, Inouye SK, Hurst K et al. Process of implementing and delivering the Prevention of Delirium system of care: a mixed method preliminary study. BMC Geriatrics 2019;20(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Teale E. A cluster, randomised feasibility study of the prevention of delirium (POD) programme for elderly patients admitted to hospital. In: Age and Ageing. Supplement 1 edition. Vol. 46. 2016:i35.
- Prevention of Delirium (POD) for older people in hospital - a feasibility study. http://isrctn.com/ISRCTN01187372 2014.
- Smith J, Green J, Siddiqi N, Inouye SK, Collinson M, Farrin A, Young J. Investigation of ward fidelity to a multicomponent delirium prevention intervention during a multicentre, pragmatic, cluster randomised, controlled feasibility trial. Age and Ageing 2020;49(4):648-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Cheater F, Collinson M, Fletcher M, Forster A, Godfrey M, et al. Prevention of delirium (POD) for older people in hospital: study protocol for a randomised controlled feasibility trial. Trials 2015;16:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Green J, Farrin A, Collinson M, Hartley S, Smith J, et al. A multicentre, pragmatic, cluster randomised, controlled feasibility trial of the POD system of care. Age and Ageing 2020;49(4):640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 2012 {published data only}
- Alvarez E, Garrido M, González F, Guzmán E, DonosoT, Gallegos S, et al. Early and intensive occupational therapy in the prevention of delirium in older adults admitted to critical patient units. randomized clinical trial: preliminary results. Chilean Journal of Occupational Therapy 2012;12(1):44-59. [Google Scholar]
Asplund 2000 {published data only}
- Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. Journal of the American Geriatrics Society 2000;48(11):1381-8. [DOI] [PubMed] [Google Scholar]
Astaneh 2007 {published data only}
- Astaneh A, Khajehmougahi N, Pakseresht S. The multicomponent intervention to prevent postoperative delirium after open-heart surgery. Pakistan Journal of Medical Sciences 2007;23(2):188-92. [Google Scholar]
Avidan 2018 {published data only}
- Avidan MS, Gregory S, Murray-Torres TM, Fritz BA, Ben Abdallah A, Helsten DL, et al. Study protocol for the Anesthesiology Control Tower-Feedback Alerts to Supplement Treatments (ACTFAST-3) trial: a pilot randomized controlled trial in intraoperative telemedicine. F1000Research 2018;7:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldwin 2004 {published data only}
- Baldwin R, Pratt H, Goring H, Marriott A, Roberts C. Does a nurse-led mental health liaison service for older people reduce psychiatric morbidity in acute general medical wards? A randomised controlled trial. Age and Ageing 2004;33(5):472-8. [DOI] [PubMed] [Google Scholar]
- Baldwin R. Evaluation of a nurse-led psychogeriatric liaison service. The Research Findings Register 2003.
Bjorkelund 2010 {published data only}
- Bjorkelund KB, Hommel A, Thorngren KG, Gustafson L, Larsson S, Lundberg D. Reducing delirium in elderly patients with hip fracture: a multi-factorial intervention study. Acta Anaesthesiologica Scandinavica 2010;54(6):678-88. [DOI] [PubMed] [Google Scholar]
Blandfort 2017 {published data only}
- Blandfort S, Damsgaard EM, Gregersen M. Blood transfusion strategy and risk of postoperative delirium in nursing homes residents with hip fracture. In: European Geriatric Medicine. 2015:S77. [DOI] [PubMed]
- Blandfort S, Gregersen M, Borris LC, Damsgaard EM. Blood transfusion strategy and risk of postoperative delirium in nursing homes residents with hip fracture. A post hoc analysis based on the TRIFE randomized controlled trial. Aging Clinical and Experimental Research 2017;29(3):459-66. [DOI] [PubMed] [Google Scholar]
Boltz 2014 {published data only}
- Boltz M, Resnick B, Chippendale T, Galvin J. Testing a family-centered intervention to promote functional and cognitive recovery in hospitalized older adults. Journal of the American Geriatrics Society 2014;62:2398-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruera 2013 {published data only}
- Bruera E, Hui D, Dalal S, Torres-Vigil I, Trumble J, Roosth J, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. Journal of Clinical Oncology 2013;31(1):111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavalcante 2014 {published data only}
- Cavalcante ES, Magario R, Conforti CA, Junior GC, Arena R, Carvalho ACC, et al. Impact of intensive physiotherapy on cognitive function after coronary artery bypass graft surgery. Arquivos brasileiros de Cardiologia 2014;103(5):391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 1998 {published data only}
- Cole MG, Primeau FJ, Elie LM. Delirium: prevention, treatment, and outcome studies. Journal of Geriatric Psychiatry and Neurology 1998;11(3):126-37. [DOI] [PubMed] [Google Scholar]
Cole 1999 {published data only}
- Cole MG. Delirium: effectiveness of systematic interventions. Dementia and Geriatric Cognitive Disorders 1999;10(5):406-11. [DOI] [PubMed] [Google Scholar]
Dalal 2012 {published data only}
- Dalal S, Hui D, Isabel T-V, Palmer JL, Allo J, Susan F-H, et al. Parenteral hydration (PH) in advanced cancer patients: A multi-center, double-blind, placebo-controlled randomized trial. In: Journal of Clinical Oncology. Vol. 30. 2012. [DOI] [PMC free article] [PubMed]
Davies 2015 {published data only}
- Davies A, Waghorn M, Boyle J, Gallagher A, Johnsen S. Alternative forms of hydration in patients with cancer in the last days of life: study protocol for a randomised controlled trial. Trials 2015;16(1):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2018 {published data only}
- Davies AN, Waghorn M, Webber K, Johnsen S, Mendis J, Boyle J. A cluster randomised feasibility trial of clinically assisted hydration in cancer patients in the last days of life. Palliative Medicine 2018;32(4):733-43. [DOI] [PubMed] [Google Scholar]
Deschodt 2012 {published data only}
- Deschodt M, Braes T, Flamaing J, Detroyer E, Broos P, Haentjens P, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. Journal of the American Geriatrics Society 2012;60(4):733-9. [DOI] [PubMed] [Google Scholar]
Dharmarajan 2017 {published data only}
- Dharmarajan K, Swami S, Gou RY, Jones RN, Inouye SK. Pathway from delirium to death: potential in-hospital mediators of excess mortality. Journal of the American Geriatrics Society 2017;65(5):1026-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epling 1999 {published data only}
- Epling J, Taylor H. Preventing delirium in hospitalized older patients. Journal of Family Practice 1999;48(6):417-8. [PubMed] [Google Scholar]
Ettema 2014 {published data only}
- Ettema RG, Hoogendoorn ME, Kalkman CJ, Schuurmans MJ. Development of a nursing intervention to prepare frail older patients for cardiac surgery (the PREDOCS programme), following phase one of the guidelines of the Medical Research Council. European Journal of Cardiovascular Nursing 2014;13(6):494-505. [DOI] [PubMed] [Google Scholar]
Fish‐Trotter 2018 {published data only}
- Fish-Trotter H, Collins SP, Danagoulian S, Hunter B, Li X, Levy PD, et al. Design and rationale of a randomized trial: using short stay units instead of routine admission to improve patient centered health outcomes for acute heart failure patients (SSU-AHF). Contemporary Clinical Trials 2018;72:137-45. [DOI] [PubMed] [Google Scholar]
Freter 2017 {published data only}
- Freter S, Koller K, Dunbar M, MacKnight C, Rockwood K. Translating delirium prevention strategies for elderly adults with hip fracture into routine clinical care: a pragmatic clinical trial. Journal of the American Geriatrics Society 2017;65(3):567-73. [DOI] [PubMed] [Google Scholar]
Gorski 2017 {published data only}
- Gorski S, Piotrowicz K, Rewiuk K, Halicka M, Kalwak W, Rybak P, et al. Nonpharmacological interventions targeted at delirium risk factors, delivered by trained volunteers (medical and psychology students), reduced need for antipsychotic medications and the length of hospital stay in aged patients admitted to an acute internal medicine ward: pilot study. Biomedical Research International 2017;2017:1297164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greaves 2020 {published data only}
- Greaves D, Psaltis PJ, Lampit A, Davis DH, Smith AE, Bourke A, et al. Computerised cognitive training to improve cognition including delirium following coronary artery bypass grafting surgery: protocol for a blinded randomised controlled trial. BMJ Open 2020;10(2):e034551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groshaus 2012 {published data only}
- Groshaus H, Boscan A, Khandwala F, Holroyd-Leduc J. Use of clinical decision support to improve the quality of care provided to older hospitalized patients. Applied Clinical Informatics 2012;3(1):94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustafson 1991 {published data only}
- Gustafson Y, Brannstrom B, Berggren D, Ragnarsson JI, Sigaard J, Bucht G, et al. A geriatric-anesthesiologic program to reduce acute confusional states in elderly patients treated for femoral neck fractures. Journal of the American Geriatrics Society 1991;39(7):655-62. [DOI] [PubMed] [Google Scholar]
Hammond 2017 {published data only}
- Hammond SP, Cross JL, Shepstone L, Backhouse T, Henderson C, Poland F, et al. PERFECTED enhanced recovery (PERFECT-ER) care versus standard acute care for patients admitted to acute settings with hip fracture identified as experiencing confusion: study protocol for a feasibility cluster randomized controlled trial. Trials 2017;18(1):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hea‐Jeong 2014 {published data only}
- Hea-Jeong Hwang, Yeonghee Shin. Effects of nursing intervention program on reducing acute confusion in hospitalized older adults. Korean Journal of Adult Nursing 2014;26(1):89-97. [Google Scholar]
Heim 2017 {published data only}
- Heim N, Stel HF, Ettema RG, Mast RC, Inouye SK, Schuurmans MJ. HELP! Problems in executing a pragmatic, randomized, stepped wedge trial on the Hospital Elder Life Program to prevent delirium in older patients. Trials 2017;18(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchoux C, Duclos A, Krolak-Salmon P, Rippert P. Methodology for assessing the impact of a multidisciplinary prevention program to prevent postoperative delirium in the elderly. In: European Geriatric Medicine. 2010:S63-4.
- Mouchoux C, Rippert P, Duclos A, Fassier T, Bonnefoy M, Comte B, et al. Impact of a multifaceted program to prevent postoperative delirium in the elderly: the CONFUCIUS stepped wedge protocol. https://clinicaltrials.gov/show/nct01316965. [DOI] [PMC free article] [PubMed]
- NTR3842. The Hospital Elder Life Program (HELP) een interventie ter voorkoming van acute verwardheid (delier) bij ouderen tijdens een ziekenhuisopname. https://trialregister.nl/trial/3672 2013.
- Strijbos MJ, Steunenberg B, Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatrics 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holly 2019 {published data only}
- Holly C. Primary prevention to maintain cognition and prevent acute delirium following orthopaedic surgery. Orthopaedic Nursing 2019;38(4):244-52. [DOI] [PubMed] [Google Scholar]
Holroyd‐Leduc 2010 {published data only}
- Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? Canadian Medical Association Journal 2010;182(5):465-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoolahan 2011 {published data only}
- Hoolahan A. OVoiD delirium and improved outcomes in acute care. Introducing a model of care. Australian Journal of Advanced Nursing 2011;29(2):30-5. [Google Scholar]
Hudetz 2015 {published data only}
- Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Pagel PS. Remote ischemic preconditioning prevents deterioration of short-term postoperative cognitive function after cardiac surgery using cardiopulmonary bypass: results of a pilot investigation. Journal of Cardiothoracic and Vascular Anesthesia 2015;29(2):382-8. [DOI] [PubMed] [Google Scholar]
Illioska 2014 {published data only}
- Illioska P, Brendel L, Kruger N, Navratil D, Kiessling AH, Moritz A, et al. Neurologic outcome following axillary artery versus classic aortic cannulation in high risk patients: preliminary results from a prospective randomized study. Thoracic and Cardiovascular Surgeon 2014;62:S01. [Google Scholar]
Inouye 1999 {published data only}
- Inouye SK, Bogardus ST Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. New England Journal of Medicine 1999;340(9):669-76. [DOI] [PubMed] [Google Scholar]
Inouye 2000b {published data only}
- Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Annals of Medicine 2000;32(4):257-63. [DOI] [PubMed] [Google Scholar]
Jia 2014 {published data only}
- Jia Y, Jin G, Guo S, Gu B, Jin Z, Gao X, et al. Fast-track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Archives of Surgery 2014;399:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2019 {published data only}
- Ko F. Multi component exercise program can reverse hospitalization-associated functional decline in elderly patients. Journal of Clinical Outcomes Management 2019;26(2):57-9. [Google Scholar]
Lei 2017 {published data only}
- Lei L, Katznelson R, Fedorko L, Carroll J, Poonawala H, Machina M, et al. Cerebral oximetry and postoperative delirium after cardiac surgery: a randomised, controlled trial. Anaesthesia 2017;72(12):1456-66. [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li Q-P, Lin L, Yang L, Jiang P-F, Yang Y. Effect of nursing intervention based on Roy adaptation model on postoperative gastrointestinal function recovery and incidence of postoperative delirium in patients with colorectal cancer. Wolrd Chinese Journal of Digestology 2017;25(7):632-7. [Google Scholar]
Lisann 2016 {published data only}
- Lisann L, Pagnini F, Langer E, Deiner S. Remind: reducing delirium and improving patient satisfaction with a perioperative mindfulness intervention. In: Journal of Alternative and Complementary Medicine. Vol. 22. 2016:A94-5.
Llera 2005 {published data only}
- Llera FG. Delirium in hospitalized elderly patients. Medicina Clinica 2005;124(14):538-40. [DOI] [PubMed] [Google Scholar]
Lundstrom 2005 {published data only}
- Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. Journal of the American Geriatrics Society 2005;53(4):622-8. [DOI] [PubMed] [Google Scholar]
McCaffrey 2004 {published data only}
- McCaffrey R, Locsin R. The effect of music listening on acute confusion and delirium in elders undergoing elective hip and knee surgery. Journal of Clinical Nursing 2004;13(6B):91-6. [DOI] [PubMed] [Google Scholar]
Moppett 2017 {published data only}
- Moppett IK, White S, Griffiths R, Buggy D. Tight intra-operative blood pressure control versus standard care for patients undergoing hip fracture repair - Hip Fracture Intervention Study for Prevention of Hypotension (HIP-HOP) trial: study protocol for a randomised controlled trial. Trials 2017;18(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mudge 2008 {published data only}
- Mudge AM, Giebel AJ, Cutler AJ. Exercising body and mind: an integrated approach to functional independence in hospitalized older people. Journal of the American Geriatrics Society 2008;56(4):630-5. [DOI] [PubMed] [Google Scholar]
Mudge 2017 {published data only}
- Mudge AM, Banks MD, Barnett AG, Blackberry I, Graves N, Green T, et al. CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatrics 2017;17(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge AM, McRae P, Barnett A, Inouye S. Reducing hospital associated complications in older people: results from the cherish cluster randomised controlled study. In: Journal of the American Geriatrics Society. Vol. 67 (Supplement 1). 2019:S142. [DOI] [PMC free article] [PubMed]
Nikelski 2019 {published data only}
- Nikelski A, Keller A, Schumacher-Schonert F, Dehl T, Laufer J, Sauerbrey U, et al. Supporting elderly people with cognitive impairment during and after hospital stays with intersectoral care management: study protocol for a randomized controlled trial. Trials 2019;20:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Gara 2020 {published data only}
- O'Gara B, Marcantonio ER, Pascual-Leone A, Shaefi S, Mueller A, Banner-Goodspeed V, et al. Prevention of early postoperative decline (peapod): protocol for a randomized, controlled feasibility trial. Trials 2018;19(1):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara BP, Mueller A, Gasangwa DV, Patxot M, Shaefi S, Khabbaz K, et al. Prevention of early postoperative decline: a randomized, controlled feasibility trial of perioperative cognitive training. Anesthesia and Analgesia 2020;130(3):586-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitkala 2004 {published data only}
- Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS, Helsinki University Hospital. Geriatric consultation in delirium - a randomized, controlled trial. Journal of Nurition, Health & Aging 2004;8(4):268. [Google Scholar]
Rice 2017 {published data only}
- Rice KL, Bennett MJ, Berger L, Jennings B, Eckhardt L, Fabre-LaCoste N, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. Journal of Cardiovascular Nursing 2017;32(1):E1-E10. [DOI] [PubMed] [Google Scholar]
Saltvedt 2012 {published data only}
- Saltvedt I, Prestmo A, Einarsen E, Johnsen LG, Helbostad JL, Sletvold O. Development and delivery of patient treatment in the Trondheim Hip Fracture Trial. A new geriatric in-hospital pathway for elderly patients with hip fracture. BMC Research Notes 2012;5:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandberg 2001 {published data only}
- Sandberg O, Franklin KA, Bucht G, Eriksson S, Gustafson Y. Nasal continuous positive airway pressure in stroke patients with sleep apnoea: a randomized treatment study. The European Respiratory Journal 2001;18(4):630-4. [DOI] [PubMed] [Google Scholar]
Shirvani 2020 {published data only}
- Shirvani F, Naji SA, Davari E, Sedighi M. Early mobilization reduces delirium after coronary artery bypass graft surgery. Asian Cardiovascular & Thoracic Annals 2020;28(9):566-71. [DOI] [PubMed] [Google Scholar]
Stromberg 1999 {published data only}
- Stromberg L, Ohlen G, Nordin C, Lindgren U, Svensson O. Postoperative mental impairment in hip fracture patients. A randomized study of reorientation measures in 223 patients. Acta Orthopaedica Scandinavica 1999;70(3):250-5. [DOI] [PubMed] [Google Scholar]
Vlisides 2019 {published data only}
- Vlisides PE, Das AR, Thompson AM, Kunkler B, Zierau M, Cantley MJ, et al. Home-based cognitive prehabilitation in older surgical patients: a feasibility study. Journal of Neurosurgical Anesthesiology 2019;31(2):212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018 {published data only}
- Wang Y, Duan X, Zhuang Y. Research progress on prevention measures of delirium in patients after coronary artery bypass grafting surgery. Chinese Nursing Research 2018;32(13):2006-8. [Google Scholar]
Xin 2017 {published data only}
- Xin X, Xin F, Chen X, Zhang Q, Li Y, Huo S, et al. Hypertonic saline for prevention of delirium in geriatric patients who underwent hip surgery. Journal of Neuroinflammation 2017;14(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoo 2013 {published data only}
- Yoo JW, Nakagawa S, Kim S. Delirium and transition to a nursing home of hospitalized older adults: a controlled trial of assessing the interdisciplinary team-based "geriatric" care and care coordination by non-geriatrics specialist physicians. Geriatrics and Gerontology International 2013;13(2):342-50. [DOI] [PubMed] [Google Scholar]
Zamvar 2002 {published data only}
- Zamvar V, Williams D, Hall J, Payne N, Cann C, Young K, et al. Assessment of neurocognitive impairment after off-pump and on-pump techniques for coronary artery bypass graft surgery: prospective randomised controlled trial. BMJ 2002;325(7375):1268-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2018 {published data only}
- Zhao FY, Zhang ZY, Zhao YX, Yan HX, Hong YF, Xia XJ, et al. The effect of electroacupuncture preconditioning on cognitive impairments following knee replacement among elderly: a randomized controlled trial. World Journal of Acupuncture-Moxibustion 2018;28(4):231-6. [Google Scholar]
References to studies awaiting assessment
NCT01998997 2013 {published data only}
- NCT01998997. A Family intervention for delirium prevention. Trial Registry 2013.
NCT03470662 2018 {published data only}
- NCT03470662. Prevention of delirium among elderly patients with hip fractures. https://clinicaltrials.gov/show/nct03470662 2018.
NCT04188795 2019 {published data only}
- NCT04188795 2019. Evaluation of the effectiveness of Delirium Preventive Care protocol. https://clinicaltrials.gov/ct2/show/NCT04188795 2019.
UMIN000027181 2017 {published data only}
- UMIN000027181. Impact of perioperative passive cycling exercise on postoperative delirium, cognitive function: randomized controlled trial. Trial Registry 2017.
References to ongoing studies
Boltz 2018 {published data only}
- Boltz M, Kuzmik A, Resnick B, Trotta R, Mogle J, Belue R, et al. Reducing disability via a family centered intervention for acutely ill persons with Alzheimer's disease and related dementias: protocol of a cluster-randomized controlled trial (Fam-FFC study) 11 Medical and Health Sciences 1117 Public Health and Health Services. Trials 2018;19:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR1900027115 2019 {published data only}
- Effects of acupuncture on postoperative delirium in elderly patients after laparoscopic surgery. Ongoing study. 1/11/2019. Contact author for more information.
DRKS00013158 2017 {published data only}
- DRKS00013158. Influence of perinterventional acupuncture on the incidence of postoperative delirium after elective, endoprosthetic replacement of the hip joint. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00013158 2017.
DRKS00016352 2019 {published data only}
- DRKS00016352. Evaluation of incidence of delirium in an acute care hospital by engaging an innovative and multidisciplinary approach. http://www.drks.de/DRKS00016352 2019.
Humeidan 2015 {published data only}
- Humeidan ML, Otey A, Zuleta-Alarcon A, Mavarez-Martinez A, Stoicea N, Bergese S. Perioperative cognitive protection-cognitive Exercise and cognitive reserve (The Neurobics Trial): a single-blind randomized trial. Clinical Therapeutics 2015;37(12):2641-50. [DOI] [PubMed] [Google Scholar]
- NCT02230605. Perioperative Cognitive Protection - Cognitive Exercise and Cognitive Reserve (The Neurobics Trial). https://clinicaltrials.gov/show/nct02230605 2014. [DOI] [PubMed]
IRCT20180910040995N1 2019 {published data only}
- IRCT20180910040995N1. Effect of HELP model on prevention of delirium. http://en.irct.ir/trial/33830.
JPRN 2017 {published data only}
- JPRN 2017. A multi-center, cluster randomized controlled study comparing usual care and a multidisciplinary intervention such as the DELirium Team Approach program to manage delirium among hospitalized cancer patients. http://www.whoint/trialsearch/Trial2aspx?TrialID=JPRN-UMIN000030062 2017.
NCT03060174 2017 {published data only}
- NCT03060174. Study of prevention of postoperative delirium to reduce incidence of postoperative cognitive dysfunction. https://clinicaltrials.gov/show/nct03060174 2017.
NCT03158909 2017 {published data only}
- NCT03158909. Trial of a non-pharmacological intervention to prevent delirium among elderly in-patients. https://clinicaltrials.gov/show/NCT03158909 2017.
NCT03541408 2018 {published data only}
- NCT03541408. Preventative delirium protocol in elderly patients. https://clinicaltrials.gov/ct2/show/nct03541408 2018.
NCT03573843 2018 {published data only}
- NCT03573843. Software-guided cognitive stimulation to prevent delirium. https://clinicaltrialsgov/show/NCT03573843 2018.
NCT03704090 2018 {published data only}
- NCT03704090. Non-pharmacological Prevention of Postoperative Delirium by Occupational Therapy Teams (PREPODOT). https://clinicaltrials.gov/ct2/show/NCT03704090 2018. [DOI] [PMC free article] [PubMed]
NCT03832192 2019 {published data only}
- NCT03832192. Care.Coach Avatars for improvement of outcomes in hospitalized elders, including mitigation of falls and delirium: a multi-site clinical study (AvatarHELP). https://clinicaltrials.gov/ct2/show/NCT03832192 2019.
NCT03894709 2019 {published data only}
- NCT03894709. A care model for elderly hip-fractured persons with cognitive impairment and their family caregivers. https://clinicaltrials.gov/ct2/show/nct03894709 2019.
NCT03980782 2019 {published data only}
- NCT03980782 2019. The effect of music therapy on delirium. https://clinicaltrials.gov/ct2/show/NCT03980782 2019.
NTR7036 2018 {published data only}
- NTR7036. Effect van muziek op de klinische uitkomst na heupfractuur operaties (MCHOPIN): een multicenter gerandomiseerde studie. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR7036 2018.
Piotrowicz 2018 {published data only}
- Piotrowicz K, Rewiuk K, Gorski S, Kalwak W, Wizner B, Pac A, et al. The "Wholesome Contact" non-pharmacological, volunteer-delivered multidisciplinary programme to prevent hospital delirium in elderly patients: study protocol for a randomised controlled trial. Trials 2018;19(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez 2019 {published data only}
- DRKS00013311. Patient safety, cost-effectiveness and quality of life: reduction of delirium risk and post-operative cognitive dysfunction (POCD) after elective procedures in the elderly. German Clinical Trials Register 2017.
- Sanchez A, Thomas C, Deeken F, Wagner S, Kloppel S, Kentischer F, et al. Patient safety, cost-effectiveness, and quality of life: reduction of delirium risk and postoperative cognitive dysfunction after elective procedures in older adults-study protocol for a stepped-wedge cluster randomized trial (PAWEL Study). Trials 2019;20(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2018 {published data only}
- NCT02954224. Prevention of delirium in elderly with obstructive sleep apnea (PODESA). https://clinicaltrials.gov/show/nct02954224.
- Wong J, Lam D, Choi S, Singh M, Siddiqui N, Sockalingam S, et al. The prevention of delirium in elderly with obstructive sleep apnea (PODESA) study: protocol for a multi-centre prospective randomized, controlled trial. BMC Anesthesiology 2018;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agar 2012
- Agar M, Draper B, Phillips PA, Phillips J, Collier A, Harlum J, et al. Making decisions about delirium: a qualitative comparison of decision making between nurses working in palliative care, aged care, aged care psychiatry, and oncology. Palliative Medicine 2012;26(7):887-96. [DOI: 10.1177/0269216311419884] [DOI] [PubMed] [Google Scholar]
Ahmed 2014
- Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age and Ageing 2014;43(3):326-33. [DOI: 10.1093/ageing/afu022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aitken 2017
- Aitken SJ, Blyth FM, Naganathan V. Incidence, prognostic factors and impact of postoperative delirium after major vascular surgery: a meta-analysis and systematic review. Vascular Medicine 2017;22(5):387-97. [DOI: 10.1177/1358863X17721639] [DOI] [PubMed] [Google Scholar]
Akunne 2012
- Akunne A, Murthy L, Young J. Cost-effectiveness of multi-component interventions to prevent delirium in older people admitted to medical wards. Age and Ageing 2012;41(3):285-91. [DOI: 10.1093/ageing/afr147] [DOI] [PubMed] [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA, 2015. [Google Scholar]
Banerjee 2011
- Banerjee A, Girard TD, Pandharipande P. The complex interplay between delirium, sedation, and early mobility during critical illness: applications in the trauma unit. Current Opinion in Anaesthesiology 2011;24(2):195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breitbart 1997
- Breitbart W, Rosenfield B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. Journal of Pain & Symptom Management 1997;13(3):128-37. [DOI] [PubMed] [Google Scholar]
Burton 2018
- Burton JK, Guthrie B, Hapca SM, Cvoro V, Donnan PT, Reynish EL. Living at home after emergency hospital admission: prospective study in older adults with and without cognitive spectrum disorder. BMC Medicine 2018;16:231. [DOI: 10.1186/s12916-018-1199-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bush 2017
- Bush SH, Tierney S, Lawlor PG. Clinical assessment and management of delirium in the palliative care setting. Drugs 2017;77(15):1623-43. [DOI: 10.1007/s40265-017-0804-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2011
- Carson JL, Terrin ML, Noveck H, Sanders DW, ChaitmanBR, Rhoads GG, et al. FOCUS Investigators. Liberal orrestrictive transfusion in high-risk patients after hip surgery. New England Journal of Medicine 2011;365(26):2453-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charlson 1994
- Charlsons M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology 1994;47(11):1245-51. [DOI] [PubMed] [Google Scholar]
Cole 2009
- Cole MG, Ciampi A, Belzile E, Zhong L. Persistent delirium in older hospital patients: a systematic review of frequency and prognosis. Age and Ageing 2009;38:19-26. [DOI: 10.1093/ageing/afn253] [DOI] [PubMed] [Google Scholar]
Cole 2015
- Cole MG, Bailey R, Bonnycastle M, McCusker J, Fung S, Ciampi A, et al. Partial and no recovery from delirium in older hospitalized adults: frequency and baseline risk factors. Journal of the American Geriatrics Society 2015;63(11):2340-8. [DOI: 10.1111/jgs.13791] [DOI] [PubMed] [Google Scholar]
Covidence 2017 [Computer program]
- Veritas Health Innovation Covidence systematic review software. Available at www.covidence.org. Melbourne, Australia: Veritas Health Innovation, 2017.
Dani 2018
- Dani M, Owen LH, Jackson TA, Rockwood K, Sampson EL, Davis D. Delirium, frailty, and mortality: interactions in a prospective study of hospitalized older people. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences 2018;73(3):415-8. [DOI: 10.1093/gerona/glx214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dasgupta 2010
- Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. International Psychogeriatrics 2010;22(3):373-94. [DOI: ] [DOI] [PubMed] [Google Scholar]
Davis 2012
- Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain 2012;135:2809-16. [DOI: 10.1093/brain/aws190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Chapter 10: Analysing data and undertaking meta-analyses. Draft version (29 January 2019). In: Deeks JJ, Higgins JP, Altman DG, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane, 2019. [Google Scholar]
Dexter 2001
- Dexter PR, Perkins S, Overhage JM, Maharry K, KohlerRB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. New England Journal of Medicine 2001;345:965-70. [DOI] [PubMed] [Google Scholar]
Dong 2018
- Dong L, Qiao X, Tian X, Liu N, Jin Y, Si H, Wang C. Cross-cultural adaptation and Validation of the FRAIL Scale in Chinese community-dwelling older adults. Journal of the American Medical Directors Association 2018;19(1):12-7. [DOI] [PubMed] [Google Scholar]
Ellis 2017
- Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD006211. [DOI: 10.1002/14651858.CD006211.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 2001
- Ely EW, Margolin R, Francis J, May L, Truman B, DittusR, et al. Evaluation of delirium in critically ill patients:validation of the Confusion Assessment method for theIntensive Care Unit (CAM-ICU). Critical Care Medicine 2001;27(7):1370-9. [DOI] [PubMed] [Google Scholar]
Finucane 2017
- Finucane AM, Lugton J, Kennedy C, Spiller JA. The experiences of caregivers of patients with delirium, and their role in its management in palliative care settings: an integrative literature review. Psycho-oncology 2017;26(3):291-300. [DOI: 10.1002/pon.4140] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flink 2012
- Flink BJ, Rivelli SK, Cox EA, White WD, Falcone G, Vail TP, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012;116(4):788-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fong 2009
- Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009;72:1570-5. [DOI: 10.1212/WNL.0b013e3181a4129a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fong 2015
- Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurology 2015;14(8):823-32. [DOI: 10.1016/S1474-4422(15)00101-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 2018
- Freeman SC, Scott NW, Powell R, Johnston M, Sutton AJ, Cooper NJ. Component network meta-analysis identifies the most effective components of psychological preparation for adults undergoing surgery under general anesthesia. Journal of Clinical Epidemiology 2018;98:105-16. [DOI] [PubMed] [Google Scholar]
Gaudreau 2005
- Gaudreau J-D, Gagnon P, Harel F, Tremblay A, Roy M-A. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. Journal of Pain and Symptom Management 2005;29(4):368-75. [DOI] [PubMed] [Google Scholar]
Gibb 2020
- Gibb K, Seeley A, Quinn T, Siddiqi N, Shenkin S, Rockwood K, Davis D. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age and Ageing 2020;49(3):352-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giles 2006
- Giles TL, Lasserson TJ, Smith B, White J, Wright JJ, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD001106. [DOI: 10.1002/14651858.CD001106.pub2] [DOI] [PubMed] [Google Scholar]
Godfrey 2019
- Godfrey M, Green J, Smith J, Cheater F, Inouye SK, Hurst K, et al. Process of implementing and delivering the Prevention of Delirium system of care: a mixed method preliminary study. BMC Geriatrics 2019;20(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University GRADEpro. McMaster University. McMaster University, 2014.
Grover 2015
- Grover S, Ghosh A, Ghormode D. Experience in delirium: is it distressing? Journal of Neuropsychiatry and Clinical Neurosciences 2015;27(2):139-46. [DOI: 10.1176/appi.neuropsych.13110329] [DOI] [PubMed] [Google Scholar]
Guerra 2019
- Guerra GG, Almeida L, Zorzela L, King-Jones S, Joffe AR, Hartling L, et al. Efficacy of music on sedation, analgesia and delirium in critically ill patients. A systematic review of randomized controlled trials. Journal of Critical Care 2019;53:75-80. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-1302. [DOI] [PubMed] [Google Scholar]
Herling 2018
- Herling SF, Greve IE, Vasilevskis EE, Egerod I, Bekker Mortensen C, Møller AM, et al. Interventions for preventing intensive care unit delirium in adults. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD009783. [DOI: 10.1002/14651858.CD009783.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2018
- Higgins JP, Savovic J, Page MJ, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. Draft version (16 September 2018) for inclusion in: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane 2018.
Hshieh 2015
- Hsheieh TT, Yue J, Oh E, Puelle M, Dowal S, Travison T, Inouye SK. Effectiveness of multicomponent nonpharmacological delirium interventions. Journal of the American Medical Association: Internal Medicine 2015;175(4):512-20. [DOI: 10.1001/jamainternmed.2014.7779] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hshieh 2018
- Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: Systematic Review and Meta-analysis of Effectiveness. American Journal of Geriatric Psychiatry 2018;26(10):1015-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inouye 1990
- Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessmentmethod. A new method for detection of delirium. Annals of Internal Medicine 1990;113(12):941-8. [DOI] [PubMed] [Google Scholar]
Inouye 1996
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7. [PubMed] [Google Scholar]
Inouye 1999a
- Inouye SK, Bogardus ST, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. New England Journal of Medicine 1999;340(9):669-76. [DOI] [PubMed] [Google Scholar]
Inouye 1999b
- Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care Is failing older persons and a window to improve quality of hospital care. American Journal of Medicine 1999;106:565-73. [DOI] [PubMed] [Google Scholar]
Inouye 2000a
- Inouye SK, Bogardus ST jR, Baker DI, Leo-Summers L, Cooney LM jR. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Journal of the American Geriatrics Society 2000;48(12):1697-706. [DOI] [PubMed] [Google Scholar]
Inouye 2014
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-22. [DOI: 10.1016/S0140-6736(13)60688-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Intensive Care Society 2009
- Intensive Care Society. Levels of Critical Care for Adult Patients. https://www.ics.ac.uk/ICS/guidelines-and-standards.aspx 2009.
Jackson 2016a
- Jackson TA, Wilson D, Richardson S, Lord JM. Predicting outcome in older hospitalised patients with delirium: a systematic literature review. International Journal of Geriatric Psychiatry 2016;31(4):392-9. [DOI: 10.1002/gps.4344] [DOI] [PubMed] [Google Scholar]
Jackson 2016b
- Jackson TA, MacLullich AM, Gladman JR, Lord JM, Sheehan B. Undiagnosed long-term cognitive impairment in acutely hospitalised older medical patients with delirium: a prospective cohort study. Age and Ageing 2016;45(4):493-9. [DOI: 10.1093/ageing/afw064] [DOI] [PubMed] [Google Scholar]
Katz 1963
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914-9. [DOI] [PubMed] [Google Scholar]
León‐Salas 2020
- León-Salas B, Trujillo-Martín MM, Martínez Del Castillo LP, García-García J, Pérez-Ros P, et al. Multicomponent interventions for the prevention of delirium in hospitalized older people: a meta-analysis. Journal of the American Geriatrics Society 2020;68(12):2947-54. [DOI] [PubMed] [Google Scholar]
Leslie 2008
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Archives of Internal Medicine 2008;168(1):27-32. [DOI: 10.1001/archinternmed.2007.4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leslie 2011
- Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. Journal of the American Geriatrics Society 2011;59(Suppl 2):S241-243. [DOI: 10.1111/j.1532-5415.2011.03671.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2017
- Levy I, Attias S, Ben-Arye E, Bloch B, Schiff E. Complementary medicine for treatment of agitation and delirium in older persons: a systematic review and narrative synthesis. International Journal of Geriatric Psychiatry 2017;32(5):492-508. [DOI] [PubMed] [Google Scholar]
Ludolph 2020
- Ludolph P, Stoffers-Winterling J, Kunzler AM, Rosch R, Geschke K, Vahl CF, et al. Non- pharmacologic multicomponent interventions preventing delirium in hospitalized people. Journal of the American Geriatrics Society 2020;68(8):1864-71. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel D. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56-61. [PubMed] [Google Scholar]
Marcantonio 1998
- Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. American Journal of Medicine 1998;105:380-4. [DOI] [PubMed] [Google Scholar]
Martinez 2015
- Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological, multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age and Ageing 2015;44(2):196-204. [DOI: 10.1093/ageing/afu173] [DOI] [PubMed] [Google Scholar]
Nelson 1996
- Neelon VJ, Champagne MT, Carlson JR, Funk SG. The NEECHAM Confusion Scale: construction, validation, and clinical testing. Nursing Research 1996;45(6):324-30. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence. Delirium: diagnosis, prevention and management. Clinical guideline [CG103] 2010;https://www.nice.org.uk/guidance/cg103:(accessed 29 July 2018).
O'Regan 2018
- O'Regan NA, Fitzgerald J, Adamis D, Molloy DW, Meagher D, Timmons S. Predictors of delirium development in older medical inpatients: readily identifiable factors at admission. Journal of Alzheimer's Disease 2018;64(3):775-85. [DOI: 10.3233/JAD-180178.] [DOI] [PubMed] [Google Scholar]
Partridge 2013
- Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? International Journal of Geriatric Psychiatry 2013;28(8):804-12. [DOI: 10.1002/gps.3900] [DOI] [PubMed] [Google Scholar]
Pendlebury 2015
- Pendlebury ST, Lovett NG, Smith SC, Dutta N, Bendon C, Lloyd-Lavery A, et al. Observational, longitudinal study of delirium in consecutive unselected acute medical admissions: age-specific rates and associated factors, mortality and re-admission. BMJ Open 2015;5:e007808. [DOI: 10.1136/bmjopen-2015-007808] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pendlebury 2017
- Pendlebury ST, Lovett NG, Smith SC, Wharton R, Rothwell PM. Delirium risk stratification in consecutive unselected admissions to acute medicine: validation of a susceptibility score based on factors identified externally in pooled data for use at entry to the acute care pathway. Age and Ageing 2017;46(2):226-31. [DOI: 10.1093/ageing/afw198] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinlan 2011
- Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. Journal of the American Geriatrics Society 2011;59(Suppl 2):S262-268. [DOI: 10.1111/j.1532-5415.2011.03674.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3.. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2021 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 2.5.0. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Richardson 2020
- Richardson S, Davis DH, Stephan BC, Robinson L, Brayne C, Barnes LE, et al. Recurrent delirium over 12 months predicts dementia: results of the Delirium and Cognitive Impact in Dementia (DECIDE) study. Age and Ageing 2020;TBC:TBC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2021
- Richardson S, Lawson R, Davis DHJ, Stephan BCM, Robinson L, Matthews FE, et al. Hospitalisation without delirium is not associated with cognitive decline in a population-based sample of older people—results from a nested, longitudinal cohort study. Age and Ageing 2021;TBC:TBC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2021
- Rose L, Burry LD, Agar M, Blackwood B, Campbell NL, Clarke M, et al. A core outcome set for studies evaluatinginterventions to prevent and/or treatdelirium for adults requiring an acute carehospital admission: an international keystakeholder informed consensus study. BMC Medicine 2021;19:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2013
- Ryan DJ, O'Regan NA, Caoimh RÓ, Clare J, O'Connor M, Leonard M, et al. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open 2013;3:e001772. [DOI: 10.1136/bmjopen-2012-001772] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schubert 2018
- Schubert M, Schürch R, Boettger S, Garcia Nuñez D, Schwarz U, Bettex D, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - a cohort study. BMC Health Services Research 2018;18:550. [DOI: 10.1186/s12913-018-3345-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sibanda 2019
- Sibanda A, Carnes D, Visentin D, Cleary M. A systematic review of the use of music interventions to improve outcomes for patients undergoing hip or knee surgery. Journal of Advanced Nursing 2019;75(3):502-16. [DOI] [PubMed] [Google Scholar]
Sturtz 2005
- Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. Journal of Statistical Software 2005;12(3):1-16. [Google Scholar]
Teale 2015
- Teale E, Young J. Multicomponent delirium prevention: not as effective as NICE suggest? Age and Ageing 2015;44(6):915-7. [DOI: 10.1093/ageing/afv120] [DOI] [PubMed] [Google Scholar]
Trzepacz 1988
- Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Research 1988;23(1):89-97. [DOI] [PubMed] [Google Scholar]
Trzepacz 2001
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. Journal of Neuropsychiatry and Clinical Neurosciences 2001;13(2):229-42. [DOI: 10.1176/jnp.13.2.229] [DOI] [PubMed] [Google Scholar]
Tsui 2018
- Tsui A, Kuh D, Richards M, Davis D. Delirium symptoms are associated with decline in cognitive function between ages 53 and 69 years: findings from a British birth cohort study. Alzheimers & Dementia 2018;14(5):617-22. [DOI: 10.1016/j.jalz.2017.08.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Zanden 2016
- Zanden V, Beishuizen SJ, Scholtens RM, Jonghe A, Rooij SE, Munster BC. The Effects of Blood Transfusion on Delirium Incidence. Journal of the American Medical Directors Association 2016;17(8):748-53. [DOI] [PubMed] [Google Scholar]
Vasunilashorn 2016
- Vasunilashorn SM, Marcantonio ER, Gou Y, Pisani MA, Travison TG, Schmitt EM, et al. Quantifying the severity of a delirium episode throughout hospitalization: the combined importance of intensity and duration. Journal of General and Internal Medicine 2016;31(10):1164-71. [DOI: 10.1007/s11606-016-3671-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterfield 2018
- Waterfield K, Weiand D, Dewhurst F, Kiltie R, Pickard J, Karandikar U, et al. A qualitative study of nursing staff experiences of delirium in the hospice setting. International Journal of Palliative Nursing 2018;24(11):524-34. [DOI: 10.12968/ijpn.2018.24.11.524] [DOI] [PubMed] [Google Scholar]
Welsh 2014
- Welsh TJ, Gordon AL, Gladman JR. Comprehensive geriatric assessment--a guide for the non-specialist. International Journal of Clinical Practice 2014;68(3):290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welton 2009
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. American Journal of Epidemiology 2009;169(9):1158-65. [DOI: 10.1093/aje/kwp014] [DOI] [PubMed] [Google Scholar]
Whittamore 2013
- Whittamore KH, Goldberg SE, Gladman JRF, Bradshaw LE, Jones RG, Harwood RH. The diagnosis, prevalence and outcomes of delirium in a cohort of older people with mental health problems on general hospital wards. International Journal of Geriatric Psychiatry 2013;29:32-40. [DOI: 10.1002/gps.3961] [DOI] [PubMed] [Google Scholar]
Wilson 2020
- Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, Maclullich AM, et al. Delirium. Nature Reviews Disease Primers 2020;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
WinBUGS [Computer program]
- WinBUGS Package 1.4.3. MRC Biostatistics Unit. MRC Biostatistics Unit, 2019.
Witlox 2010
- Witlox J, Eurelings LS, Jonghe JF, Kalisvaart KJ, Eikelenboom P, Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia. JAMA 2010;304(4):443-51. [DOI: 10.1001/jama.2010.1013] [DOI] [PubMed] [Google Scholar]
Woodhouse 2019
- Woodhouse R, Burton JK, Rana N, Pang YL, Lister JE, Siddiqi N. Interventions for preventing delirium in older people in institutional long-term care. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD009537. [DOI: 10.1002/14651858.CD009537] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Health Organization 2016
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en 2016.
Yang 2008
- Yang FM, Inouye SK, Fearing MA, Kiely DK, Marcantonio ER, Jones RN. Participation in activity and risk for incident delirium. Journal of the American Geriatrics Society 2008;56(8):1479-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Burton 2019
- Burton JK, Siddiqi N, Teale EA, Barugh A, Sutton AJ. Non‐pharmacological interventions for preventing delirium in hospitalised non‐ICU patients. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013307. [DOI: 10.1002/14651858.CD013307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddiqi 2007
- Siddiqi N, Holt R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD005563. [DOI: 10.1002/14651858.CD005563.pub2] [DOI] [PubMed] [Google Scholar]
Siddiqi 2016
- Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, Simpkins SA. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD005563. [DOI: 10.1002/14651858.CD005563.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]