Abstract
Ras-induced cell transformation is mediated through distinct downstream signaling pathways, including Raf, Ral-GEFs-, and phosphatidylinositol 3-kinase (PI 3-kinase)-dependent pathways. In some cell types, strong activation of the Ras–Raf–MEK–extracellular signal-regulated kinase (ERK) cascade leads to cell cycle arrest rather than cell division. We previously reported that constitutive activation of this pathway induces sustained proliferation of primary cultures of postmitotic chicken neuroretina (NR) cells. We used this model system to investigate the respective contributions of Ras downstream signaling pathways in Ras-induced cell proliferation. Three RasV12 mutants (S35, G37, and C40) which differ by their ability to bind to Ras effectors (Raf, Ral-GEFs, and the p110 subunit of PI 3-kinase, respectively) were able to induce sustained NR cell proliferation, although none of these mutants was reported to transform NIH 3T3 cells. Furthermore, they all repressed the promoter of QR1, a neuroretina growth arrest-specific gene. Overexpression of B-Raf or activated versions of Ras effectors Rlf-CAAX and p110-CAAX also induced NR cell division. The mitogenic effect of the RasC40–PI 3-kinase pathway appears to involve Rac and RhoA GTPases but not the antiapoptotic Akt (protein kinase B) signaling. Division induced by RasG37-Rlf appears to be independent of Ral GTPase activation and presumably requires an unidentified mechanism. Activation of either Ras downstream pathway resulted in ERK activation, and coexpression of a dominant negative MEK mutant or mKsr-1 kinase domain strongly inhibited proliferation induced by the three Ras mutants or by their effectors. Similar effects were observed with dominant negative mutants of Rac and Rho. Thus, both the Raf-MEK-ERK and Rac-Rho pathways are absolutely required for Ras-induced NR cell division. Activation of these two pathways by the three distinct Ras downstream effectors possibly relies on an autocrine or paracrine loop, implicating endogenous Ras, since the mitogenic effect of each Ras effector mutant was inhibited by RasN17.
The Ras and Raf oncogene products are potent agents in neoplastic transformation. Activation of the downstream mitogen-activated protein kinase (MAPK)–extracellular signal-regulated kinase (ERK) cascade by these oncoproteins results in the up-regulation of immediate-early genes through the ability of activated ERK to phosphorylate and modulate the activity of transcription factors, thereby increasing AP1 activity and cyclin D1 accumulation (1, 10, 41, 45, 62). While the ras and raf oncogene products were initially isolated from tumors, their role in cell transformation has been mostly studied in established fibroblastic cell lines that are actively dividing upon stimulation by growth factors present in serum-containing media. In such cells, the MAPK-ERK pathway is required for cell proliferation (50). However, strong activation of the Ras-MAPK pathway was recently found to arrest the cell cycle in NIH 3T3 cells (55, 61, 72) and to cause senescence of normal human fibroblasts (74), leading to the notion that the effects of this pathway on key regulators of the cell cycle, including cyclin-dependent kinase inhibitors, depend both on the host cell and the level of ERK activation.
In addition to Raf proteins, other direct Ras effectors have been shown to contribute to the transformation of mammalian cells. These include the catalytic subunit of phosphatidylinositol 3-kinase (PI 3-kinase) (58) and the family of exchange factors for Ral (30, 40, 63, 68). Recently, these effectors were shown to contribute to G1 cell cycle progression by cooperating in the induction of E2F activity and cyclin D1 transcription in NIH 3T3 cells (23). Ras mutants that differ by their ability to bind to and activate these different effectors have been used to evaluate the contribution of each distinct signaling pathway to the transformation of established fibroblast cell lines (38, 59, 66, 67). These mutants contain a second point mutation in the Ras effector loop, in addition to the V12 activating mutation. Thus, RasS35 mutant binds only to Raf proteins, RasG37 binds only to Ral-GEFs, and RasC40 binds only to the p110 catalytic subunit of PI 3-kinase. Interestingly, each of these mutants poorly transforms NIH 3T3 cells by itself, but they can cooperate to induce a fully transformed phenotype in these cells (38, 59, 66, 73). While these studies investigated the contribution of the different Ras downstream effectors to the transformation of spontaneously dividing cells, their capacity to induce the proliferation of primary cultures of postmitotic cells has not yet been investigated.
We have shown that primary cultures of differentiating chicken embryonic neuroretina (NR) cells represent a sensitive biological system for the detection of mitogenic signals. Despite the presence of serum growth factors, these cells can be maintained in a nondividing state for several weeks under culture conditions that normally promote the division of primary or established fibroblasts (7, 52). NR cells are induced to proliferate upon constitutive expression of activated oncogenes, such as v-src (7). However, continuous NR cell division not only depends on oncogene expression but also requires the presence of serum and is therefore sustained by two types of signals (24, 54). Using this model system, we provided the first evidence that an activated raf oncogene was able to induce cell cycle reentry of postmitotic cells (3). Similarly, we reported that constitutively activated Ras and MEK, the respective activator and effector of Raf proteins, also promoted NR cell division (12). In addition, we showed that NR cell division, following infection with retroviruses that do not carry an oncogene, was correlated with the transcriptional activation of mRNAs encoding truncated forms of Raf proteins (17, 18, 42). This led to the identification of B-raf, a novel member of the raf family, as a retrovirally transduced oncogene in NR cells (42). More recently, we reported that overexpression of full-length B-Raf proteins was sufficient to induce NR cell division in the absence of an activating mutation (51). Taken together, our previous studies established that constitutive activation of the Raf-MEK-ERK cascade results in cell cycle reentry and sustained division of these postmitotic neuroepithelial cells.
NR cell division results in the down-regulation of QR1, a retina-specific gene exclusively expressed during late stages of NR development (25, 26). Transcription of this gene is strictly correlated with growth arrest both in vivo and in cultured cells expressing a v-Src mutant conditionally defective in its mitogenic capacity (25, 54). This allowed us to identify a quiescence-responsive element in the QR1 promoter (53, 54) that could serve as sensor for the early detection of mitogenic signals in the NR.
In the present study, we investigated the contribution of the different Ras downstream signaling pathways to NR cell division induced by oncogenic Ras. We show that each of the Ras effector mutants displayed strong mitogenic capacity and repressed the activity of the QR1 promoter. We also show that constitutive activation not only of the Raf-MEK pathway but also of the Ras effectors PI 3-kinase and Rlf, an exchange factor for Ral, results in sustained NR cell proliferation. The mitogenic effect of RasC40–PI 3-kinase appears to be mediated through a Rac-Rho pathway but not to involve Akt (protein kinase B [PKB]). Division induced by RasG37-Rlf appears to be independent of Ral GTPase activation and presumably requires an unidentified mechanism. However, our results also indicate that none of the three Ras downstream pathways is sufficient to induce NR cell division and that this process requires, at least, the cooperation of the Raf-MEK-ERK and Rac-Rho pathways, since cell proliferation was inhibited by dominant negative mutants of both pathways. We finally show that NR cell proliferation resulting from any of the Ras effectors depends on a feedback mechanism, possibly an autocrine or paracrine loop, involving endogenous Ras, since the mitogenic property of all Ras effector mutants was inhibited by the RasN17 dominant negative mutant.
MATERIALS AND METHODS
Plasmid constructions.
To compare the mitogenic properties of Ras mutants and Ras downstream effectors B-Raf, MEK-1, Rlf, PI 3-kinase (p110), and Rac1, all derivative mutants of these molecules were expressed from the cytomegalovirus promoter. pcDNA3-derived constructs encoding the RasV12 single mutant (V12) or Ras double mutants containing a second mutation (S35, G37, or C40) in addition to the V12 mutation (59) were a kind gift from Julian Downward. The pcDNA3/Rlf-CAAX construct was a kind gift from Rob Wolthuis (69). The pcDNA3/RlfΔCAT-CAAX construct expressing a hemagglutinin type 1 (HA1)-tagged Rlf-CAAX mutant with a deletion in the scr-1 region of the Rlf catalytic domain (69) was obtained by subcloning the HindIII-XbaI fragment from pMT2SM/RlfΔCAT-CAAX (kindly provided by Rob Wolthuis) into pcDNA3. The pcDNA3/RalA-L72 and pcDNA3/myc-RacV12 constructs were obtained by subcloning the EcoRI fragments of pMT3/RalA-L72 and pcEXV-3/myc-Rac1V12 (12), respectively, into the EcoRI site of pcDNA3. The pcDNA3-derived constructs encoding HA1-tagged wild-type and myristylated Akt (2) were a kind gift from Alfonso Bellacosa.
In cotransfection experiments, dominant negative mutants were expressed from the Rous sarcoma virus (RSV) long terminal repeat promoter present in the pRcRSV vector (Invitrogen), which allows G418 selection, while mitogenic mutants were expressed from pcDNA3(NeoR−), a modified pcDNA3 vector, in which the open reading frame of the neomycin resistance gene was disrupted by NarI digestion followed by filling in with the Klenow fragment. The Ras effector mutants (S35, G37, and C40) were transferred from pcDNA3 to pcDNA3(NeoR−) by a BamHI-XbaI digestion. RasV12 was transferred from pcDNA3 to pcDNA3(NeoR−) by a BamHI-XhoI digestion. B-Raf was transferred from pcDNA3 to pcDNA3(NeoR−) by a BamHI-XhoI digestion of pcDNA3/HA1-B1-Raf (51). HA-Rlf-CAAX was transferred from pcDNA3 to pcDNA3(NeoR−) by a HindIII-XbaI digestion. The p110α subunit of PI 3-kinase fused to the Ras CAAX sequence (64) and the K227E mutant of p110 (59) in pcDNA3 were kindly provided by Julian Downward. p110-CAAX was transferred from pcDNA3 to pcDNA3(NeoR−) by a SpeI-ApaI digestion. A constitutively activated HA1-tagged MEK mutant (MEKDD) in which serines 218 and 222 were replaced by aspartic acid residues (5) was subcloned from pECE/MEKDD (a kind gift from Jacques Pouyssegur) into pcDNA3(NeoR−) as a HindIII-XbaI fragment.
pRcRSV-derived constructs expressing dominant negative mutants were obtained as follows. The HA1-tagged MEKS222A mutant (49) was subcloned from pECE/HA1-MEKS222A (a kind gift from Gilles Pages) into pRcRSV as a HindIII-XbaI fragment to generate pRcRSV/HA1-MEKS222A. The pRcRSV/KSRΔNaeI construct expressing the kinase domain (CA5) of mKsr-1 was derived from pRcRSV/mKsr-1 (12) by an internal NaeI deletion that removed most of the N terminus of mKsr-1, including the CA2, CA3, and CA4 domains. The KpnI-XbaI fragment of pcDNA3/RasN17 (kindly provided by Jean de Gunzburg) was cloned into pRcRSV digested by KpnI (partial) and XbaI to generate pRcRSV/RasN17. The pRcRSV/RacN17 and pRcRSV/RhoN19 myc-tagged constructs were obtained as follows. The EcoRI fragments from pcEXV-3/RacN17 and pcEXV-3/RhoN19 (kindly provided by Julian Downward and Roser Busca, respectively) were subcloned into the EcoRI site of pcDNA3, and then HindIII-XbaI fragments from the resulting constructs were subcloned into pRcRSV. The pRcRSV/HA1-C3 construct was obtained by subcloning the XbaI fragment from pEF/C3 (29) in place of the NheI-XbaI fragment of pRcRSV/HA1-MEKS222A. The SalI-EcoRI fragment of pMT2/AH-Akt containing the myc-tagged pleckstrin homology (PH) domain of Akt (PKB) (39) was cloned into the pECE vector; the HindIII-XbaI fragment of the resulting pECE/AH-Akt construct was then subcloned into pRcRSV to generate pRcRSV/AH-Akt. The pEF/C3 and pMT2/AH-Akt constructs were kind gifts from Julian Downward. All the cloning procedures were verified by sequencing.
Transfection of chicken NR cells.
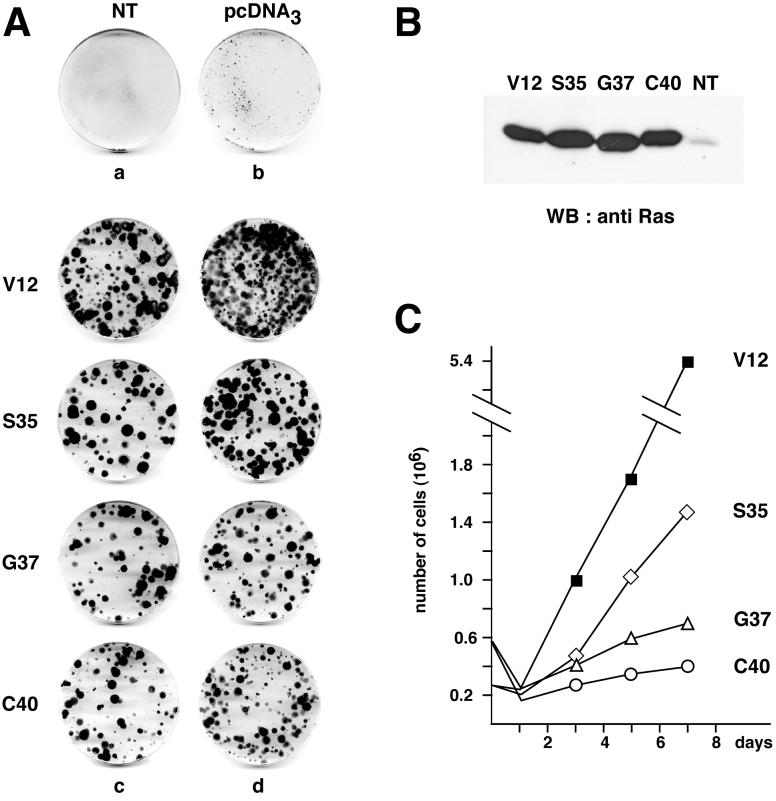
NR cell cultures were prepared from 8-day-old Brown Leghorn chicken embryos as previously described (51) and seeded in 100-mm-diameter dishes. Cultures were maintained and passaged in Eagle basal medium supplemented with 10% fetal calf serum. Cells were transfected by the calcium phosphate method as previously described (51), and G418 selection (600 μg/ml) was applied 5 days later for 15 days. The cultures were then rinsed with phosphate-buffered saline, and foci of proliferating cells were stained with 1.0% crystal violet (in 20% ethanol). For growth curve analysis, NR cell cultures, obtained after passaging of proliferating foci, were seeded at 2.5 × 105 cells (for RasV12 and RasS35 cells) or 5 × 106 cells (for RasG37 and RasC40 cells) per 60-mm dish, and the number of cells was counted every 2 days.
In transient-transfection experiments, protein analysis was performed 48 h after transfection without G418 selection.
Transient transfections and CAT assays.
Promoter activity was assayed in quail neuroretina (QNR) cells infected by tsNY68, a temperature-sensitive mutant of RSV (36) which enabled us to control the proliferation state of infected QNR cells and consequently the expression of the quiescence-specific QR1 gene (25). Induction of endogenous QR1 transcription upon a shift to 41°C was assayed in these cells prior to transfection, as previously described (54). CAT5/QR1 is a chloramphenicol acetyltransferase (CAT) reporter construct containing the −1265 to +55 (with respect to the transcriptional start site) fragment of the QR1 promoter in the CAT5 vector (53). tsNY68-infected QNR cells were seeded at 1.5 × 106 cells per 100-mm dish in Eagle basal medium supplemented with 10% fetal calf serum and maintained at 37°C. At 48 h later, the cells were transfected, by standard calcium phosphate coprecipitation, with 10 μg of reporter construct (CAT5 or CAT5/QR1), 0.5, 1, 3, or 6 μg of pcDNA3-derived constructs expressing the different Ras effector mutants or the empty pcDNA3, and pBluescript as a carrier DNA (up to a total of 20 μg). At 5 h later, the precipitates were washed with phosphate-buffered saline and the plates were incubated in Eagle basal medium supplemented with 10% fetal calf serum at 37°C for 2 h and then shifted to 41°C for 48 h. Cell extracts were produced and CAT assays were performed as previously described (53). The percent chloramphenicol conversion was calculated by scanning the thin-layer chromatographic plates with a PhosphorImager instrument (Molecular Dynamics) and ImageQuant software.
Ral and Rac pull-down experiments.
In vivo GTP loading of Ral and Rac GTPases was analyzed by pull-down experiments using the Ral binding domain of RLIP76 (35) as a glutathione S-transferase (GST) fusion protein (GST-RalBD) and the p21 binding domain of PAK-1 (amino acids 67 to 150) as a GST fusion protein (GST-PAK-PBD), respectively (4, 70). The Ral activation assay was performed as previously described (70): the pGEX-4T/GST-RalBD construct (70), kindly provided by Jacques Camonis, was used to transform Escherichia coli, and the fusion protein was prepared as previously described (70). A 1-mg portion of protein extracts prepared in the Ral buffer (70) was incubated for 1 h at 4°C with the GST-RalBD protein precoupled to glutathione beads. The beads were then washed four times in the Ral buffer, and samples were analyzed by Western blotting as described below, using an anti-Ral-A monoclonal antibody (Transduction Laboratories) at a 1/5,000 dilution. Rac activation was assayed by using the Rac activation assay kit from Upstate Biotechnology. NR cell extracts were prepared as recommended by the manufacturer, using the lysis/wash buffer provided. A 1-mg portion of protein extracts was incubated for 1 h at 4°C with the GST-PAK-PBD coupled to agarose beads. The beads were then washed three times in the lysis/wash buffer, and samples were analyzed by Western blotting as described below, using an anti-Rac monoclonal antibody (provided by the manufacturer) at a 1/1,000 dilution.
Akt kinase assays.
Akt kinase activity in transient or stable transfections was assayed by using the Akt kinase assay kit from New England Biolabs Inc. as described below. NR cell extracts were prepared as recommended by the manufacturer using the cell lysis buffer provided. Ectopically expressed HA-Akt was immunoprecipitated with the 12CA5 monoclonal antibody directed against the HA1 epitope (Boehringer Mannheim) and Pansorbin (Calbiochem). Endogenous Akt was immunoprecipitated with an immobilized Akt monoclonal antibody provided by the manufacturer (New England Biolabs). The immunoprecipitates were then washed and resuspended for 30 min at 30°C in a kinase assay buffer containing 1 μg of GSK-3 fusion protein (paramyosin fused to GSK-3α/β crosstide corresponding to residues surrounding Ser21/9) and 200 μM ATP. Phosphorylation of GSK-3 was then analyzed by Western blotting as described below, using an anti-phospho-GSK-3 polyclonal antibody (provided by the manufacturer, New England Biolabs) at a 1/1,000 dilution.
Western blot analysis.
Protein expression was assayed by Western blot analysis from total-cell lysates as follows. Foci of proliferating NR cells or NR cultures transiently transfected were lysed in 1% (wt/vol) Triton X-100–50 mM Tris-HCl (pH 7.5)–100 mM NaCl–50 mM NaF–5 mM EDTA–100 U of aprotinin per ml–10 mM NaPPi mM 4-(2-aminoethyl)benzene sulfonyl fluoride HCl–1 mM sodium orthovanadate. Insoluble material was removed by centrifugation at 21,000 × g for 20 min at 4°C, and cell lysates were normalized for protein concentration. Samples (100 to 200 μg) of protein extracts were separated on sodium dodecyl sulfate (SDS)–8 to 12% polyacrylamide gels and transferred to Immobilon-P membranes. The membranes were then probed with appropriate antibodies, and proteins were visualized by the enhanced chemiluminescence system from Amersham Pharmacia Biotech as previously described (51). The total amount of ERK was detected using a polyclonal antibody (Santa Cruz Biotechnology) at a 1/2,000 dilution. Activated ERK was detected with a polyclonal antibody directed against the phosphorylated forms of ERK (New England Biolabs Inc.) at a 1/1,000 dilution. Ras mutant expression was detected with an anti-Ras monoclonal antibody (Santa Cruz Biotechnology) at a 1/500 dilution. The KSRΔNaeI mutant was detected with the previously described anti-mKsr-1 antibody (12) at a 1/2,000 dilution. Myc-tagged proteins were detected with a monoclonal antibody directed against the Myc epitope (Invitrogen) at a 1/5,000 dilution. HA-tagged proteins were detected with the 12CA5 monoclonal antibody directed against the HA1 epitope (Boehringer Mannheim) at a 1/1,000 dilution. Peroxidase-conjugated anti-mouse and anti-rabbit antibodies were used as secondary antibodies at 1/10,000 and 1/20,000 dilutions, respectively.
RESULTS
Distinct Ras downstream signaling pathways induce sustained NR cell proliferation.
To investigate the mitogenic signaling pathways downstream of Ras, we tested the capacity of Ras double mutants, which differ in their ability to bind to distinct effectors, to induce NR cell proliferation. NR cells dissected from 8-day-old chicken embryos were transfected with DNA constructs expressing each of the Ras effector mutants, and cultures were examined for the presence of foci of proliferating cells 2 weeks after G418 selection. NR cells transfected with control plasmid pcDNA3, containing only the selection marker, remained isolated and could not give rise to foci (Fig. 1A). In contrast, NR cultures transfected with an expression vector for RasV12 contained numerous and large foci of dividing cells. In agreement with our previous results on the mitogenic capacity of activated Raf kinases and MEK (3, 12, 42), the RasS35 mutant, which binds only to Raf proteins, also displayed a strong mitogenic effect in NR cells (Fig. 1A). Interestingly, both G37 and C40 mutants, which bind to Ral-GEFs and the catalytic subunit of PI 3-kinase (p110), respectively, were also able to induce the formation of foci of proliferating cells. Thus, each of the Ras effector mutants was able to induce the division of postmitotic NR cells, although they were all shown to be poorly transforming when tested alone in NIH 3T3 cells. In addition, cultures induced to proliferate by each mutant were able to sustain several passages. However, comparison of the growth properties of NR cells expressing the various Ras mutants showed differences depending on the mutant. Clearly, the growth rate of RasS35-transfected cells was significantly higher than that of cells expressing RasG37 and RasC40 (Fig. 1C). We also found that, although each mutant induced NR cell division, none of them was able to induce the morphological transformation observed in NR cells expressing RasV12 (data not shown).
FIG. 1.
The three Ras effector mutants induce sustained NR cell proliferation. (A) Primary cultures of chicken embryonic NR cells were transfected with 5 μg (c) or 10 μg (d) of pcDNA3-derived constructs encoding the RasV12 single mutant (V12) or Ras double mutants containing a second mutation (S35, G37, or C40) in addition to the V12 mutation, as indicated. Controls were nontransfected NR cell cultures (NT) (a) and NR cell cultures transfected with 10 μg of the empty vector pcDNA3 (b). After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet. The data presented are representative of eight independent experiments. (B) Western blot analysis of Ras protein expression in NR cell cultures induced to proliferate by the different Ras mutants. Equal amounts of protein extracts from cultures obtained as described for panel A were resolved by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to Immobilon-P membranes, and probed with an anti-Ras monoclonal antibody. Nontransfected NR cells maintained in culture in the absence of G418 were used as a control (NT). (C) Growth curves of NR cell cultures induced to proliferate by the different Ras mutants. Proliferating G418-resistant cells obtained as in panel A were pooled, seeded at a density of 2.5 × 105 cells (for V12 and S35 Ras mutants) or 5 × 105 cells (for G37 and C40 Ras mutants) in 60-mm dishes, and counted at the indicated intervals.
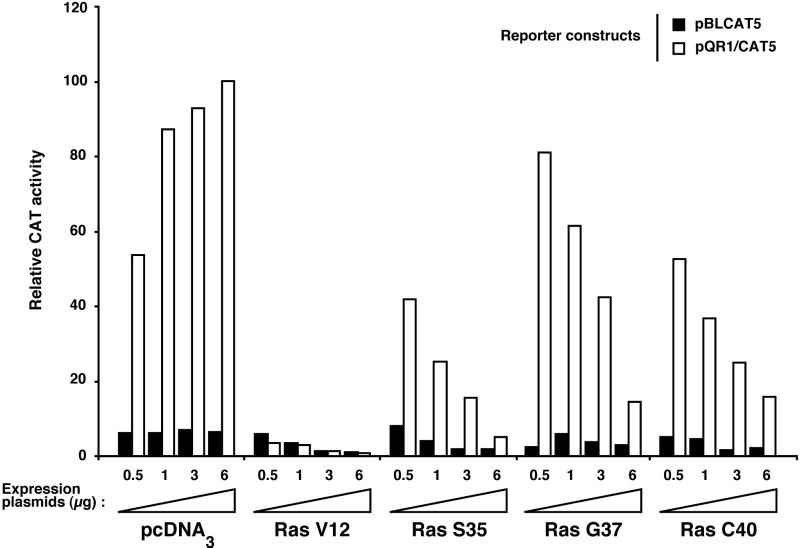
Proliferation of NR cells induced by v-Src leads to the down-regulation of an NR-specific gene, QR1, whose expression is correlated with growth arrest (25). Accordingly, the transcriptional activity of a reporter construct containing the QR1 regulatory sequences is repressed in proliferating NR cells transformed by a temperature-sensitive (ts) v-Src protein and is restored when these cells are growth arrested at the nonpermissive temperature (53, 54). We used this system to study the effects of Ras effector mutants on QR1 transcription in a short-term assay. Therefore, proliferating NR cells infected with tsNY68 RSV were cotransfected with the constructs described in Fig. 1 and with a QR1 reporter plasmid. Cell division was then arrested at the nonpermissive temperature (41°C), and promoter activity was assayed 48 h later. As expected, expression of the reporter was strongly induced in cells cotransfected with the empty vector, pcDNA3 (Fig. 2). Consistent with its potent mitogenic effect, we found that transfection of RasV12 resulted in the complete repression of the QR1 promoter. Similarly, the three Ras effector mutants were able to repress the activity of this promoter in a dose-dependent manner. Interestingly, we observed that none of the double mutants was as efficient as RasV12 and that RasS35 displayed the strongest repressing activity. Therefore, there was a tight correlation between the mitogenic properties of the different Ras mutants in NR cells and their ability to repress the promoter of the growth arrest-specific QR1 gene.
FIG. 2.
The mitogenic capacity of Ras effector mutants correlates with their ability to repress the quiescence-inducible promoter of QR1. tsNY68-infected QNR cells were cotransfected with the QR1 reporter construct (CAT5/QR1) or the CAT5-negative control and increasing amounts of either pcDNA3 or pcDNA3-derived constructs expressing Ras mutants described for Fig. 1. CAT activity was determined after a 48-h incubation at the nonpermissive temperature (41°C), as described in Materials and Methods. Relative activities are given with respect to the effect of 6 μg of pcDNA3 on CAT5/QR1 (100%). The results of a representative experiment are shown; similar results were reproducibly obtained in three independent cotransfections.
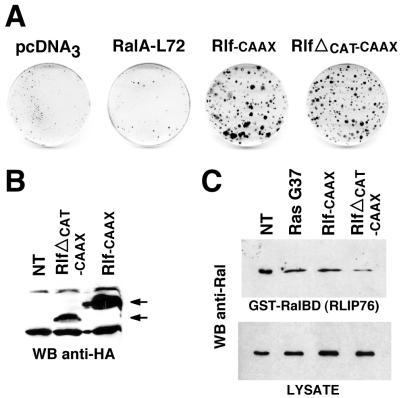
Since these mutants were expressed at high levels in NR cells (Fig. 1B), we considered the possibility that G37 and C40 could induce cell division because of their residual ability to bind Raf. To rule out this possibility, we investigated the mitogenic capacity of Ras downstream effectors. Overexpression of full-length B-Raf or of its constitutively activated direct downstream effector MEK (MEKDD) resulted in the formation of a large number of foci of proliferating NR cells (Fig. 3A), in agreement with our previous reports (12, 51). The family of exchange factors for Ral GTPase has several members, of which Rlf displays the highest affinity for Ras-GTP (71). A membrane-targeted form of Rlf, fused to the Ras CAAX sequence (69), was also able to elicit the formation of foci (Fig. 3A). Finally, a similar constitutive activation of the catalytic subunit of PI 3-kinase (p110-CAAX) (64) also resulted in NR cell division (Fig. 3A). Similar results were obtained with the K227E mutant of p110, another activated version of PI 3- kinase (data not shown). We conclude that the three Ras mutants, as well as their respective effectors, are able to induce the division of postmitotic NR cells.
FIG. 3.
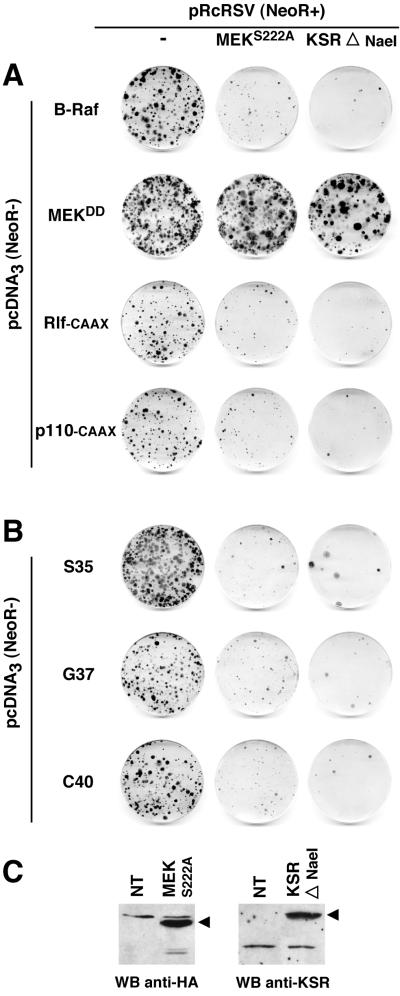
The MEK-ERK pathway is required for NR cell proliferation induced by Ras effectors and Ras double mutants. (A) NR cells were cotransfected with 10 μg of pcDNA3(NeoR−)-derived constructs expressing activated MEK-1 (MEKDD) or the different Ras direct effectors: B-Raf and activated versions of Rlf (Rlf-CAAX) and PI 3-kinase (p110-CAAX), and 10 μg of pRcRSV vector or pRcRSV-derived constructs expressing an HA1-tagged dominant negative mutant of MEK-1 (MEKS222A) or the kinase domain of mKsr-1 (KSRΔNaeI). The G418 resistance is provided by the pRcRSV-derived constructs (NeoR+), whereas the pcDNA3-derived constructs alone do not allow G418 selection (NeoR−). After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet. (B) Same as in panel A except that the Ras double mutants (S35, G37, and C40) were used instead of the Ras effectors. Data presented in panels A and B are representative of three independent experiments. (C) Expression of MEK and KSR mutants in NR cells. NR cells were transfected with pRcRSV/HA1-MEKS222A and pRcRSV/KSRΔNaeI constructs, and protein expression was analyzed 48 h later by Western blotting (WB), as described in Materials and Methods.
The MEK-ERK pathway is required for Ras-induced NR cell proliferation.
Previous studies using the Ras effector mutants demonstrated that fibroblast transformation by oncogenic Ras requires the cooperation of at least two downstream signaling pathways to induce a fully transformed phenotype (38, 59, 66, 73). We considered the possibility that such cooperative effects could also exist downstream of Ras for NR cell proliferation. Since the MAPK-ERK cascade was reported to be required for the proliferation of fibroblast cell lines (50), we first examined the contribution of this pathway to NR cell division induced by the three Ras effector pathways. Therefore, we tested the ability of a dominant negative mutant of MEK to interfere with the mitogenic effect of the Ras double mutants and with their direct effectors in cotransfection experiments (Fig. 3A and B). As expected, a mutant of MEK-1 in which serine 222, a residue phosphorylated by Raf, was replaced by an alanine (49) markedly inhibited the formation of foci induced by RasS35 (Fig. 3B) and B-Raf (Fig. 3A). Surprisingly, proliferation induced by RasG37/Rlf-CAAX and by RasC40/p110-CAAX was also strongly inhibited by MEKS222A. As expected, MEKS222A did not inhibit the proliferation induced by constitutively activated MEK (MEKDD).
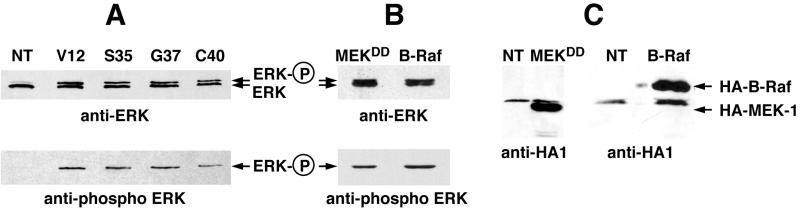
We confirmed these results by using a mutant of kinase suppressor of Ras (KSR). We previously reported that the kinase domain (CA5) of murine KSR forms a stable complex with MEK, thereby inhibiting Ras- and Raf-induced NR cell proliferation (12). Coexpression of the kinase domain of mKsr-1 markedly inhibited the proliferation induced by the three Ras mutants, as well as by their downstream effectors, whereas it had only a weak inhibitory effect on proliferation induced by MEKDD (Fig. 3A). Accordingly, we found that ERK-2, the only ERK isotype detected in avian NR cells, was phosphorylated in cultures induced to proliferate by all Ras effector mutants, as well as by B-Raf and MEKDD, but not in normal NR cells (Fig. 4). The p110-CAAX and Rlf-CAAX constitutive mutants were also found to induce ERK phosphorylation in proliferating NR cells (data not shown). Taken together, our results indicate that the three distinct Ras signaling pathways lead to ERK activation and that the MEK-ERK cascade is absolutely required for Ras-induced NR cell division.
FIG. 4.
ERK activation in NR cell cultures induced to proliferate by the Ras double mutants. (A) Western blot analysis of ERK phosphorylation in NR cell cultures induced to proliferate by the different Ras mutants. Equal amounts of protein extracts from cultures obtained as described in Fig. 1A were resolved by SDS-PAGE, transferred to Immobilon-P membranes, and probed with an anti-ERK polyclonal antibody. The membranes were then stripped and reprobed with an anti-phospho-ERK polyclonal antibody. Nontransfected NR cells maintained in culture in the absence of G418 were used as a control (NT). (B) Same as in panel A for the pcDNA3(NeoR−)-derived constructs encoding HA1-tagged MEKDD and B-Raf used in the experiments shown in Fig. 3. (C) Expression of these constructs in NR cells was controlled by Western blotting using an anti-HA1 antibody.
The three Ras effector mutants require endogenous Ras activation for NR cell proliferation.
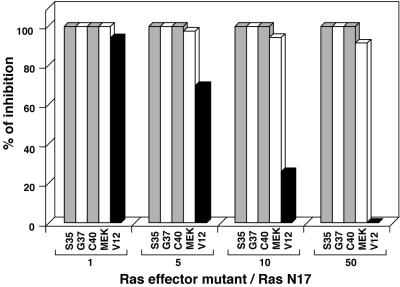
Activation of ERK by the three Ras mutants could be due to a direct effect of these mutants or their effectors on the ERK pathway, although such a cross talk between the different pathways has not thus far been reported. Alternatively, it could result from a feedback mechanism, possibly an autocrine-paracrine loop, that would activate endogenous Ras. To test whether activation of endogenous Ras was required, we cotransfected the various Ras mutants with RasN17, a dominant negative mutant of Ras (Fig. 5). In these experiments, neomycin resistance was provided by the construct expressing the Ras dominant negative allele. We found that under these conditions, large amounts of RasN17 DNA inhibited the mitogenic property of all Ras mutants, including that of RasV12. However, at reduced levels of expression, RasN17 still fully inhibited the proliferation induced by the three Ras effector mutants whereas that induced by RasV12 remained unaffected. Moreover, NR cell proliferation induced by MEKDD was also strongly inhibited by RasN17 (Fig. 5). These findings suggest that none of the three distinct pathways can independently induce NR cell proliferation. Thus, while activation of the Raf-ERK pathway is indispensable for cell division, it also requires the cooperation of an additional Ras downstream pathway.
FIG. 5.
Endogenous Ras activation is required for NR cell proliferation induced by Ras effector mutants and MEK. NR cells were cotransfected with pcDNA3(NeoR−)-derived constructs expressing the different Ras mutants (V12, S35, G37, or C40) or MEKDD and with either the pRcRSV vector or the pRcRSV/RasN17 construct expressing a dominant negative mutant of Ras. The different ratios of pcDNA3- to pRcRSV-derived plasmid DNAs used are indicated (Ras effector mutant/RasN17). As in the experiment shown in Fig. 3, G418 resistance is provided by the pRcRSV-derived constructs. After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet and counted. Results are presented as the percent inhibition obtained from the number of foci in cultures cotransfected with the pRcRSV empty vector compared to that obtained in cultures transfected with pRcRSV/RasN17 for each Ras mutant or MEKDD. Data presented are representative of seven independent experiments.
The RasG37–Ral-GEF pathway induces NR cell proliferation in a Ral-independent manner.
To further characterize the mitogenic pathways downstream of Ras in NR cells, we investigated the mechanism(s) by which RasG37 and its effector Rlf induced NR cell proliferation and we specifically investigated the possible involvement of Ral GTPase, the only known effector for Ral-GEFs. We found that RalL72, a RalA mutant constitutively bound to GTP, failed to induce the formation of proliferating foci (Fig. 6A). In addition, RlfΔCAT-CAAX, an Rlf mutant with a deletion in a conserved domain required for its exchange factor activity on Ral (69), retained a significant mitogenic effect in NR cells (Fig. 6A). These observations suggested that the mitogenic signals downstream of RasG37 and Rlf in NR cells were mediated by a Ral-independent mechanism. To confirm this possibility, we examined the levels of GTP loading of endogenous Ral in NR cells induced to proliferate by RasG37, Rlf-CAAX, and RlfΔCAT-CAAX by using pull-down experiments with the Ral binding domain of RLIP76, an effector of Ral (35, 70). Surprisingly, we detected a high level of GTP-bound Ral in cultured quiescent NR cells (Fig. 6C). In addition, RasG37 and Rlf-CAAX did not increase Ral activation in proliferating foci, confirming that NR cell division was not correlated with Ral activation. Finally, the RlfΔCAT-CAAX mutant, which retained a mitogenic effect in NR cells, apparently displayed a slight dominant negative effect on endogenous Ral activation (Fig. 6C). Taken together, these results strongly suggest that induction of NR cell proliferation by the RasG37–Ral-GEF pathway is not mediated by Ral.
FIG. 6.
NR cell proliferation induced by the G37-Rlf pathway is mediated by a Ral-independent mechanism. (A) Cultures of NR cells were transfected with 20 μg of pcDNA3 or pcDNA3-derived constructs encoding an activated RalA mutant (RalA-L72), an activated Rlf mutant (Rlf-CAAX), or an Rlf-CAAX mutant with a deletion in the scr-1 region of the catalytic domain responsible for the exchange factor activity on Ral (RlfΔCAT-CAAX), as indicated. After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet. Data presented are representative of five independent experiments. (B) Expression of HA1-tagged Rlf mutants in NR cells was controlled by Western blotting (WB) using an anti-HA1 antibody as indicated. (C) Endogenous Ral GTP loading in nontransfected NR cells and in NR cells induced to proliferate by the G37-Rlf pathway. Equal amounts of cell extracts from nontransfected NR cell cultures or from G418-resistant foci of NR cells induced to proliferate upon transfection with RasG37, Rlf-CAAX, and RlfΔCAT-CAAX were incubated with a GST-RalBD fusion protein containing the Ral binding domain of RLIP76, precoupled to glutathione beads, to recover GTP-bound Ral. The beads were washed four times, and collected Ral was identified by Western blotting analysis with a monoclonal anti-Ral antibody (top panel). The level of total Ral in whole lysates (50 μg of protein extracts) is also shown (bottom panel).
The Rac-Rho pathway is required for Ras-induced NR cell proliferation.
We next investigated the mechanisms by which RasC40 and activated PI 3-kinase induce NR cell proliferation. Two major effectors were shown to act downstream of PI 3-kinase in Ras-induced cell transformation: Akt (PKB), containing a PH domain (6, 19, 20), and Rac1, a member of the Rho subfamily of small GTPases (28, 37, 56, 59).
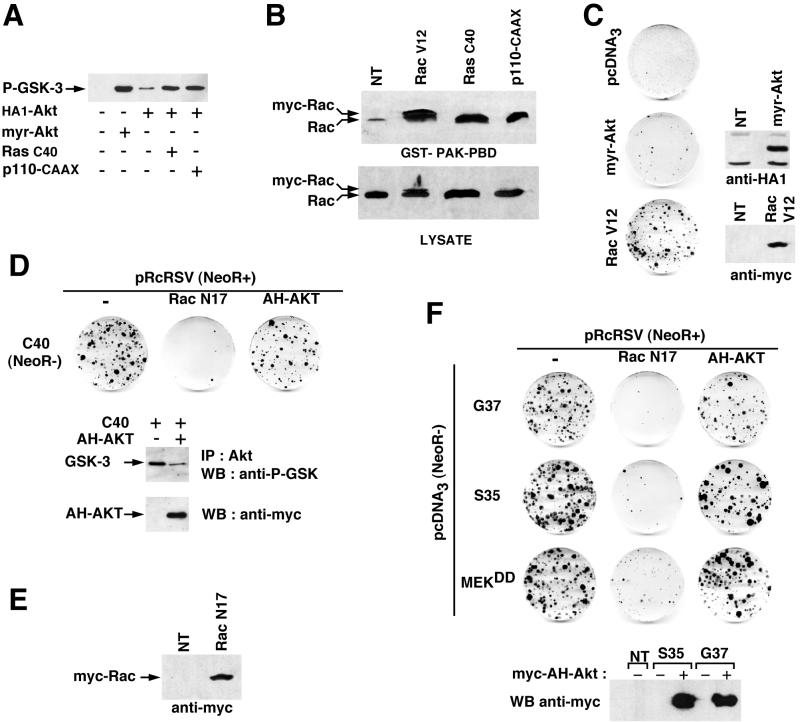
We first examined whether RasC40 and activated PI 3-kinase were able to activate these two downstream effectors in chicken NR cells. Activation of Akt was assayed in transient-transfection experiments in which a HA1-tagged Akt was coexpressed with RasC40 or p110-CAAX. Following immunoprecipitation with an anti-HA1 antibody, the kinase activity of Akt was measured in an in vitro kinase assay using a GSK-3α/β peptide as a substrate. As shown in Fig. 7A, both RasC40 and p110-CAAX were able to activate the kinase activity of Akt. Activation of endogenous Rac was assayed in G418-resistant NR cells induced to proliferate by RasC40 and p110-CAAX by using pull-down experiments with the p21-binding domain of PAK-1, a downstream effector of Rac (4). A strong Rac activation was detected in NR cells expressing RasC40 and p110-CAAX compared to that in quiescent NR cells maintained in culture in the absence of G418 (Fig. 7B). We next investigated the capacity of activated mutants of Akt and Rac to induce NR cell proliferation. Cells transfected with myristylated Akt, a constitutively activated version of Akt (PKB) (2) which displayed strong kinase activity in an in vitro kinase assay (Fig. 7A), did not give rise to proliferating foci (Fig. 7C). Similar results were obtained with v-Akt (2), another constitutively activated mutant of Akt (data not shown). Moreover, AH-Akt, a dominant negative mutant of Akt which had its kinase domain deleted but retained the AH/PH domain (39), had little if any effect on NR cell proliferation induced by RasC40 in cotransfection assays, although it was able to inhibit endogenous Akt activity in these cells (Fig. 7D). We obtained similar results using a kinase-inactive mutant of full-length Akt as a dominant negative allele (data not shown). In contrast, we found that RacV12, a constitutively activated mutant of Rac1, displayed mitogenic capacity in NR cells (Fig. 7C), consistent with its high level of GTP loading in these cells (Fig. 7B). Although weak in comparison with that of constitutive activation of the MEK-ERK pathway, this effect was comparable to that observed for p110-CAAX or p110K227E. Furthermore, cultures of NR cells induced to proliferate by RacV12 sustained several passages (data not shown). In addition, the RacN17 dominant negative mutant of Rac1 strongly inhibited NR cell proliferation induced by RasC40 (Fig. 7D). In agreement with the finding that each individual Ras downstream signaling pathway could not act independently to induce NR cell division, we found that RacN17 also inhibited the mitogenic effects of RasS35, RasG37, and MEKDD (Fig. 7F). These observations suggest that Rac but not Akt activation is required for Ras-induced NR cell proliferation, in addition to the MEK-ERK pathway.
FIG. 7.
Ras-induced NR cell proliferation requires Rac but not Akt activation. (A) Activation of Akt by the C40–PI 3-kinase pathway in NR cells. NR cell cultures were cotransfected with HA1-tagged wild-type Akt and either RasC40 or constitutively activated PI 3-kinase (p110-CAAX), as indicated. NR cultures that were not transfected, transfected with HA1-tagged myristylated Akt (myr-Akt), or cotransfected with HA1-Akt and the empty pcDNA3 vector were used as controls. Akt proteins were immunoprecipitated with a monoclonal anti-HA1 antibody, and immune complexes were incubated with a GSK-3α/β peptide as a substrate. Phosphorylation of GSK-3 (P-GSK-3) was then analyzed by Western blotting using an anti-phospho-GSK-3 polyclonal antibody. (B) Rac GTP loading in NR cells induced to proliferate by the C40–PI 3-kinase pathway. Equal amounts of cell extracts from G418-resistant foci of NR cells induced to proliferate upon transfection with RasC40, p110-CAAX, and Myc-tagged RacV12 were incubated with the p21-binding domain of PAK-1 as a GST-PAK-PBD fusion protein coupled to agarose beads, to recover GTP-bound Rac. The beads were washed three times, and collected Rac was identified by Western blot analysis with an anti-Rac monoclonal antibody (top panel). The level of total Rac in the whole lysates (50 μg of protein extracts) is also shown (bottom panel). Nontransfected NR cells maintained in culture in the absence of G418 were used as a control (NT). (C) Cultures of NR cells were transfected with 20 μg of pcDNA3, pcDNA3/myr-Akt (HA1-tagged myristylated Akt), or pcDNA3/myc-RacV12 (encoding an activated mutant of Rac1), as indicated. After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet. Expression of these constructs in NR cells was analyzed by Western blotting using anti-HA1 and anti-Myc monoclonal antibodies in transient-transfection experiments. Data presented are representative of five independent experiments for RacV12 and two independent experiments for myr-Akt. (D) NR cells were cotransfected with 10 μg of pcDNA3(NeoR−)/RasC40 and 10 μg of pRcRSV vector or pRcRSV-derived constructs expressing a Myc-tagged dominant negative mutant of Rac1 (RacN17) or the Myc-tagged PH domain of Akt (PKB) (AH-AKT). The G418 resistance is provided by the pRcRSV-derived constructs (NeoR+), whereas the RasC40 construct alone do not allow G418 selection (NeoR−). After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet. Data presented are representative of four independent experiments. Inhibition of endogenous Akt kinase activity by the Akt dominant negative mutant was analyzed in G418-resistant foci of NR cells induced to proliferate upon cotransfection with both pcDNA3/RasC40 and pRcRSV/AH-Akt compared with those obtained in the absence of AH-Akt (empty pRcRSV vector), using the same experimental procedure as described for panel A, except that endogenous Akt was immunoprecipitated with an anti-Akt monoclonal antibody (IP). (E) Expression of the Myc-tagged dominant negative mutant of Rac1 (RacN17) in NR cells was analyzed by Western blotting using an anti-Myc monoclonal antibody in a transient-transfection experiment. (F) NR cells were cotransfected with 10 μg of pcDNA3(NeoR−)-derived constructs expressing the Ras double mutants (S35 and G37) or activated MEK-1 (MEKDD) and 10 μg of pRcRSV vector, pRcRSV/RacN17, or pRcRSV/AH-AKT, as described for panel D. After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet. Data presented are representative of four independent experiments. Expression of the Myc-AH-Akt protein was analyzed by Western blot analysis. Equal amounts of protein extracts from NR cell cultures were resolved on SDS-PAGE, transferred to Immobilon-P membranes, and probed with an anti-Myc monoclonal antibody. Nontransfected NR cells maintained in culture in the absence of G418 were used as a control (NT).
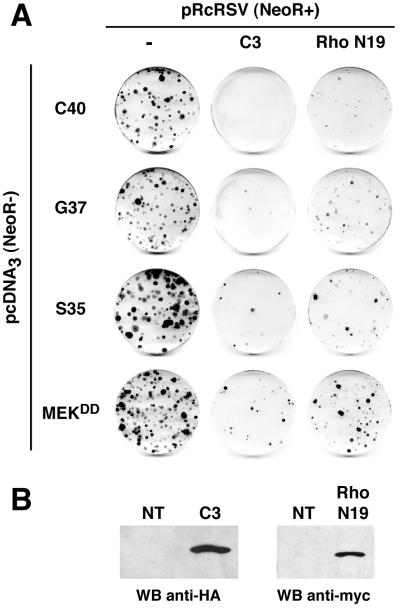
The contribution of Rac to Ras-induced cell transformation is not restricted to cytoskeleton rearrangements and could be mediated by distinct downstream signaling pathways. For example, Rac was reported to activate the JNK signaling cascade (11, 34, 46). However, we found that NR cell proliferation induced by the three Ras effector mutants was not inhibited by dominant negative versions of components of this cascade, such as JNKAPF or the JNK binding domain of JNK-interacting protein (references 13 and 14 and data not shown). The RhoA small GTPase, another downstream effector of Rac1, was also reported to play a key role in the Ras-induced transformation of murine fibroblasts (37, 38, 48, 57). To test whether the Rho pathway was required for Ras-induced NR cell proliferation, we examined the effects of C3 transferase expression in cotransfection experiments with the Ras effector mutants. Clostridium botulinum C3 transferase specifically inactivates RhoA by ADP-ribosylation at Asn41 (8, 29). Coexpression of the cDNA encoding C3 transferase with any of the three Ras effector mutants or MEKDD in NR cell cultures sharply inhibited the formation of proliferating foci (Fig. 8). Although the specificity of C3 transferase on RhoA activity, upon intracellular expression, was reported previously (29), we could not exclude a more general effect on other GTPases of the Rho family, such as Rac1 and CDC42. Therefore, we also tested the effect of RhoN19, a dominant negative mutant of RhoA (57). This mutant significantly inhibited cell proliferation induced by the three Ras effector mutants and by MEKDD, although its inhibitory effect was less marked than that of C3 transferase (Fig. 8).
FIG. 8.
Inhibition of the Rho pathway prevents Ras-induced NR cell proliferation. NR cells were cotransfected with 10 μg of pcDNA3(NeoR−)-derived constructs expressing the different Ras double mutants (S35, G37, and C40) or activated MEK-1 (MEKDD) and 10 μg of pRcRSV vector or pRcRSV-derived constructs expressing a HA1-tagged C. botulinum C3 transferase (C3) or a Myc-tagged dominant negative mutant of RhoA (RhoN19), as indicated. The G418 resistance is provided by the pRcRSV-derived constructs (NeoR+), whereas the pcDNA3-derived constructs do not allow G418 selection (NeoR−). After selection for G418-resistant cells, foci of proliferating NR cells were stained with crystal violet. Data presented are representative of three independent experiments. (B) Expression of C3 transferase and RhoN19 proteins in NR cells was analyzed by Western blotting using anti-HA1 and anti-Myc monoclonal antibodies in transient-transfection experiments. NT, control.
DISCUSSION
Loss of growth control is a major characteristic of cell transformation. In vitro studies on the mechanisms of transformation have been conducted mostly with established fibroblasts, that is, in cells able to divide spontaneously under standard culture conditions. In these cells, transformation results in the acquisition of growth capacity under restrictive conditions that do not allow the division of normal cells, such as the capacity to divide in low serum concentrations or in semisolid medium. Cultures of differentiating NR cells represent a model system characterized by the irreversible loss of growth capacity under normal culture conditions that allow the division of primary or established fibroblasts. In the present study, we have investigated the ability of the distinct Ras downstream signaling pathways to induce the proliferation of primary cultures of chicken embryonic NR cells. We had previously reported that constitutive activation of the Raf-MEK-ERK pathway was a potent inducer of NR cell division. We now demonstrate that constitutive activation of other Ras downstream signaling pathways, including Ral exchange factor Rlf and PI 3-kinase, also induces the proliferation of postmitotic neural cells. We also found that these pathways cannot act independently but that they require the activation of endogenous Ras signaling and cooperation of Raf-ERK and Rac-Rho signaling pathways in the induction of NR cell proliferation.
Induction of NR cell division by distinct Ras downstream signaling pathways.
The capacity of Ras downstream signaling pathways to induce NR cell proliferation was investigated by using different Ras effector mutants. While the properties of these mutants were previously shown to differ significantly depending on the host cell line, they were found, in general, to transform fibroblasts poorly when tested alone. However, pairwise cotransfections restored efficient cell transformation (33, 38, 59, 66, 73). These previous studies confirmed that Ras-induced transformation results from the combination of multiple cellular processes including cytoskeleton rearrangement, loss of cell adhesion, and deregulation of cell growth and survival. Paradoxically, the specific effects of Ras mutants on cell division per se were poorly documented. We show here that each Ras effector mutant displays a mitogenic effect in NR cells, by using three criteria: the induction of foci of proliferating cells under conditions where control cells do not divide, the capacity of proliferating cultures to sustain multiple passages, and the ability of Ras mutants to repress the promoter activity of QR1, a gene specifically expressed in growth-arrested NR cells. Furthermore, we showed that not only the Ras mutants but also their respective direct effectors were able to induce NR cell proliferation.
This study provides the first demonstration that the RasG37-Rlf pathway can induce cell proliferation in primary cultures of quiescent and differentiating cells. The contribution of Ral exchange factors in Ras signaling is not fully understood (71), but part of their effect is mediated by the activation of Ral GTPase (63, 69). For example, the constitutive Rlf-CAAX mutant used in this study was previously shown to stimulate the promoter activity of c-fos in a Ral-dependent manner and to confer upon NIH 3T3 cells the ability to proliferate under low-serum conditions (69). We found that the ability of the RasG37-Rlf pathway to induce NR cell proliferation does not appear to be mediated through Ral activation. Thus, RalL72, an activated mutant of Ral, did not induce NR cell division and, furthermore, a Rlf-CAAX mutant lacking exchange factor activity retained substantial mitogenic effects in NR cells. In addition, we observed that the level of Ral GTP loading in nondividing NR cells is relatively high and is not further increased in cells induced to divide by RasG37 or Rlf-CAAX. While the mechanisms which sustain an elevated level of GTP-bound Ral in quiescent NR cells are unknown, there is obviously no correlation between Ral activity and NR cell division. It remains possible that the Ral pathway is required to establish or maintain differentiation programs in these neural cells. These findings strongly suggest the requirement of a presently undefined Ral-independent mitogenic pathway downstream of Ral-GEFs. The existence of this Ral-independent pathway has already been postulated (67, 71), and it awaits further investigation.
This study also demonstrates that the activated PI 3-kinase catalytic subunit, as well as RasC40, can induce cell proliferation in primary cultures of differentiating neural cells. This strengthens the notion that activated forms of the catalytic subunit of PI 3-kinase, which arose as a retroviral oncogene in a chicken tumor (9), behave as a dominant oncogene, not only in immortalized cell lines but also in quiescent normal cells. The role of PI 3-kinase in Ras-induced cell transformation was reported to involve distinct downstream signaling pathways including Rac- and Akt-dependent pathways (6, 19, 20, 28, 37, 56, 59). We found that dominant negative mutants of Akt do not inhibit NR cell proliferation induced by any of the Ras effector mutants, suggesting that the Akt (PKB) pathway is not required for Ras-induced NR cell division. In addition, constitutively activated forms of Akt were not able to induce NR cell proliferation. Akt (PKB) signaling was commonly found to be involved in cell protection against apoptosis (15, 39). Our observations confirm that this pathway does not play a major role in cell cycle reentry induced by Ras. In contrast, we found that an activated form of Rac, another downstream target of PI 3-kinase, induces NR cell division and that, furthermore, a dominant negative mutant of Rac has a strong inhibitory effect. Microinjection of activated Rac into Swiss 3T3 fibroblasts was previously shown to stimulate cell cycle progression through G1 and subsequent DNA synthesis (47). Our data obtained with NR cells further show that Rac is also able to induce the proliferation of primary quiescent cells. Interestingly, dominant negative mutants of the JNK pathway did not inhibit NR cell proliferation induced by Ras effector mutants (data not shown), although this pathway was reported to be activated by Rac (11, 46) as well as by RasC40 and RasG37 mutants (38). However, the requirement of the JNK pathway in cell proliferation was not directly addressed in these studies, and it was furthermore reported that JNK activation is dispensable for Rac-induced NIH 3T3 cell transformation (32, 65). Another candidate downstream of Rac in NR cell proliferation could be Rho, a GTPase that does not activate the JNK pathway (47). We found that inhibition of RhoA activity by a dominant negative mutant or by the C3 transferase inhibited proliferation induced by RasC40 and PI 3-kinase. Activation of this small GTPase was shown to be required for Ras-induced morphological transformation of fibroblasts (37, 57). More recently, activation of Rho was reported to suppress p21Waf/Cip induction by the Ras-ERK pathway, thereby allowing Ras-induced DNA synthesis in murine fibroblasts (48).
Although the three Ras mutants and their direct effectors possess mitogenic capacity in NR cells, our results clearly show that constitutive activation of the Raf-MEK-ERK pathway is most efficient in inducing sustained NR cell proliferation. These findings apparently contradict other studies showing that strong activation of the MAPK pathway results in cell cycle arrest of normal cells by up-regulating the expression of CDK inhibitors (55, 61, 72, 74). Since the formation of proliferating foci was observed 20 days after transfection, we could not exclude the existence of feedback mechanisms that would down-regulate the MAPK pathway to a level compatible with cell division. However, we do not favor this hypothesis for the following reasons. First, we previously reported that infection of NR cultures with retroviruses expressing activated forms of Raf or MEK resulted in a rapid and massive induction of cell division (3, 12). Second, we were able to correlate the mitogenic property of the three Ras effector mutants with their capacity to repress the activity of the QR1 promoter in transient-transfection assays. Finally, we detected a constitutive phosphorylation of about 50% of the ERK molecules in these proliferating NR cell cultures. Thus, our data indicate that NR cell division induced by the MAPK pathway requires the cooperation of additional signals, as discussed below, rather than the MAPK pathway's down-regulation.
Cooperation of distinct signaling pathways downstream of Ras is required for NR cell proliferation.
Our study also demonstrates that none of the three Ras downstream pathways can independently induce NR cell proliferation. Indeed, we found that dominant negative mutants of both the MEK-ERK and Rac-Rho pathways strongly inhibited NR cell division induced by each of the Ras effector mutants, demonstrating that cooperation between these two pathways is required. It is plausible that RhoA contributes to Ras-induced NR cell division by down-regulating p21Waf/Cip, a mechanism similar to that described in murine fibroblasts (48).
Our observations suggest that the three Ras mutants, as well as their effectors, are able to activate the MEK-ERK and Rac-Rho pathways by a mechanism which remains to be clarified. It is possible that serum, which is required for proliferation of NR cell cultures induced by activated oncogenes, is responsible for these effects. However, we observed that serum treatment of NR cell cultures following overnight starvation results in ERK activation but not in cell division (unpublished results). Thus, in this cell system, unlike in fibroblasts, serum alone is not able to activate both the ERK and Rac-Rho pathways at the threshold or for the duration required for cell proliferation. Our data rather suggest that NR cell proliferation induced by each Ras effector mutant requires the recruitment of the additional signaling pathway through activation of endogenous Ras by a feedback mechanism. This is supported by the observation that none of the Ras effector mutants can induce NR cell division in the presence of RasN17 whereas proliferation induced by RasV12 is not inhibited under the same conditions. Therefore, cooperation between the distinct Ras downstream signaling pathways through the activation of endogenous Ras appears to be required. Accordingly, NR cell proliferation induced by constitutively activated MEK was also inhibited by Ras N17, as well as by dominant negative mutants of Rac and Rho. One possible mechanism by which endogenous Ras is activated could be the establishment of an autocrine loop. It could explain how both RasG37 and RasC40 mutants are able to induce ERK activation in NR cells. This represents a major difference between NR cells and murine fibroblasts, in which neither RasG37 nor RasC40 was found to activate ERK (33, 38, 67). Ral-GEFs such as Ral-GDS and Rlf were also found to be unable to activate ERK (67, 69). However, PI 3-kinase, in contrast to RasC40, was reported to activate the ERK pathway by acting either downstream or upstream of Ras, depending on the cell type and the level of PI 3-kinase activity (16, 31, 64). PI 3-kinase was recently implicated in an insulin-like growth factor (IGF) autocrine loop required for sustained ERK activation during myogenic differentiation (60). Conversely, induction of cyclin D1 and DNA synthesis by an inducible activated MEK in growth-arrested NIH 3T3 cells was recently found to require the indirect activation of PI 3-kinase through the autocrine production of growth factors (62).
An increasing number of studies points to the existence of autocrine-paracrine mechanisms induced by Ras downstream signaling and required for cell morphological transformation. For example, transformation of rat intestinal epithelial cells by Ras was found to depend on the stimulation of the epidermal growth factor (EGF) receptor through an autocrine loop (22). Similarly, autocrine activation of the EGF receptor is required for ERK activation in C3H10T1/2 fibroblasts transformed by a minimal expression of oncogenic Ha-Ras (27). Finally, heparin-binding EGF (HB-EGF) gene transcription is rapidly activated in NIH 3T3 cells transformed by oncogenic Ras and Raf (43, 44), as well as in chicken embryo fibroblasts transformed by several nuclear oncogenes of the bZip family, including Jun, Fos, and Maf (21). However, we did not observe an up-regulation of HB-EGF transcription in NR cells induced to proliferate by Ras mutants, in comparison with normal NR cultures, by Northern blot analysis (data not shown). In addition, infection of NR cultures with an avian retrovirus containing the chicken HB-EGF did not lead to NR cell proliferation (data not shown), whereas the same virus was shown to cause morphological transformation of chicken embryo fibroblasts (21). Further experiments are required to establish the existence of an autocrine-paracrine mechanism responsible for endogenous Ras activation in NR cells and to identify the putative factor(s) involved.
In conclusion, our observations demonstrate that the distinct Ras downstream signaling pathways, including Raf-ERK and PI 3-kinase, not only contribute to cell morphological transformation in fibroblasts but also cooperate and cannot act independently to induce proliferation in primary cultures of postmitotic and differentiating neural cells.
ACKNOWLEDGMENTS
We thank Julian Downward, Rob Wolthuis, Hans Bos, Jacques Pouyssegur, Roser Busca, Jean de Gunzburg, Jacques Camonis, Marc Symons, and Alfonso Bellacosa for providing reagents used in this study. We also thank Julian Downward, Rob Wolthuis, and Hans Bos for helpful discussions and Céline Alleaume for help in pull-down experiments.
This work was funded by the Centre National de la Recherche Scientifique, by the Institut Curie, and by grants from the Ligue Nationale Contre le Cancer (Comité des Yvelines) and the Association pour la Recherche sur le Cancer (grant 5276). C.P. and S.P. were supported by fellowships from the Ministère de l'Education Nationale de la Recherche et de la Technologie.
REFERENCES
- 1.Aktas H, Cai H, Cooper G M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt P K. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Béchade C, Calothy G, Pessac B, Martin P, Coll J, Denhez F, Saule S, Ghysdael J, Stehelin D. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature (London) 1985;316:559–562. doi: 10.1038/316559a0. [DOI] [PubMed] [Google Scholar]
- 4.Benard V, Bohl B P, Bokoch G M. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Pages G, Pouyssegur J. Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 1994;9:3379–3387. [PubMed] [Google Scholar]
- 6.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature (London) 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 7.Calothy G, Poirier F, Dambrine G, Mignatti P, Combes P, Pessac B. Expression of viral oncogenes in differentiating chick embryo neuroretinal cells infected with avian tumor viruses. Cold Spring Harbor Symp Quant Biol. 1980;44:983–990. doi: 10.1101/sqb.1980.044.01.106. [DOI] [PubMed] [Google Scholar]
- 8.Chardin P, Boquet P, Madaule P, Popoff M R, Rubin E J, Gill D M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 10.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 12.Denouel-Galy A, Douville E M, Warne P H, Papin C, Laugier D, Calothy G, Downward J, Eychène A. Murine Ksr interacts with MEK and inhibits Ras-induced transformation. Curr Biol. 1998;8:46–55. doi: 10.1016/s0960-9822(98)70019-3. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 14.Dickens M, Rogers J S, Cavanagh J, Raitano A, Xia Z, Halpern J R, Greenberg M E, Sawyers C L, Davis R J. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 15.Downward J. Ras signalling and apoptosis. Curr Opin Genet Dev. 1998;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 16.Duckworth B C, Cantley L C. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. Dependence on signal strength. J Biol Chem. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- 17.Eychène A, Béchade C, Marx M, Laugier D, Dezélée P, Calothy G. Molecular and biological properties of c-mil transducing retroviruses generated during passage of Rous-associated virus type 1 in chicken neuroretina cells. J Virol. 1990;64:231–238. doi: 10.1128/jvi.64.1.231-238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felder M-P, Laugier D, Eychène A, Calothy G, Marx M. Occurrence of alternatively spliced leader-Δ-onc-poly(A) transcripts in chicken neuroretina cells infected with Rous-associated virus type 1: implication in transduction of the c-mil/c-raf and c-Rmil/B-raf oncogenes. J Virol. 1993;67:6853–6856. doi: 10.1128/jvi.67.11.6853-6856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 20.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 21.Fu S L, Bottoli I, Goller M, Vogt P K. Heparin-binding epidermal growth factor-like growth factor, a v-Jun target gene, induces oncogenic transformation. Proc Natl Acad Sci USA. 1999;96:5716–5721. doi: 10.1073/pnas.96.10.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangarosa L M, Sizemore N, Graves-Deal R, Oldham S M, Der C J, Coffey R J. A raf-independent epidermal growth factor receptor autocrine loop is necessary for Ras transformation of rat intestinal epithelial cells. J Biol Chem. 1997;272:18926–18931. doi: 10.1074/jbc.272.30.18926. [DOI] [PubMed] [Google Scholar]
- 23.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 24.Gillet G, Michel D, Crisanti P, Guerin M, Herault Y, Pessac B, Calothy G, Brun G, Volovitch M. Serum factors and v-src control two complementary mitogenic pathways in quail neuroretinal cells in culture. Oncogene. 1993;8:565–574. [PubMed] [Google Scholar]
- 25.Guermah M, Crisanti P, Laugier D, Dezelee P, Bidou L, Pessac B, Calothy G. Transcription of a quail gene expressed in embryonic retinal cells is shut off sharply at hatching. Proc Natl Acad Sci USA. 1991;88:4503–4507. doi: 10.1073/pnas.88.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guermah M, Gillet G, Michel D, Laugier D, Brun G, Calothy G. Down regulation by p60v-src of genes specifically expressed and developmentally regulated in postmitotic quail neuroretina cells. Mol Cell Biol. 1990;10:3584–3590. doi: 10.1128/mcb.10.7.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton M, Wolfman A. Oncogenic Ha-Ras-dependent mitogen-activated protein kinase activity requires signaling through the epidermal growth factor receptor. J Biol Chem. 1998;273:28155–28162. doi: 10.1074/jbc.273.43.28155. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins P T, Eguinoa A, Qiu R G, Stokoe D, Cooke F T, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 29.Hill C S, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 30.Hofer F, Fields S, Schneider C, Martin G S. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci USA. 1994;91:11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Q, Klippel A, Muslin A J, Fantl W J, Williams L T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 32.Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996a;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 33.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996b;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 34.Joneson T, Bar-Sagi D. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol Cell Biol. 1999;19:5892–5901. doi: 10.1128/mcb.19.9.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis J H. Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. J Biol Chem. 1995;270:22473–22477. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 36.Kawai S, Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a Rous sarcoma virus mutant. Virology. 1971;46:470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- 37.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Van Aelst L, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikuchi A, Demo S D, Ye Z H, Chen Y W, Williams L T. ralGDS family members interact with the effector loop of ras p21. Mol Cell Biol. 1994;14:7483–7491. doi: 10.1128/mcb.14.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavoie J N, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 42.Marx M, Eychène A, Laugier D, Béchade C, Crisanti P, Dezélée P, Pessac B, Calothy G. A novel oncogene related to c-mil is transduced in chicken neuroretina cells induced to proliferate by infection with an avian lymphomatosis virus. EMBO J. 1988;7:3369–3373. doi: 10.1002/j.1460-2075.1988.tb03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy S A, Chen D, Yang B S, Garcia Ramirez J J, Cherwinski H, Chen X R, Klagsbrun M, Hauser C A, Ostrowski M C, McMahon M. Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol Cell Biol. 1997;17:2401–2412. doi: 10.1128/mcb.17.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mechta F, Lallemand D, Pfarr C M, Yaniv M. Transformation by ras modifies AP1 composition and activity. Oncogene. 1997;14:837–847. doi: 10.1038/sj.onc.1200900. [DOI] [PubMed] [Google Scholar]
- 46.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 47.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 48.Olson M F, Paterson H F, Marshall C J. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature (London) 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 49.Pages G, Brunet A, L'Allemain G, Pouyssegur J. Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1) EMBO J. 1994;13:3003–3010. doi: 10.1002/j.1460-2075.1994.tb06599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pages G, Lenormand P, L'Allemain G, Chambard J C, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papin C, Denouel-Galy A, Laugier D, Calothy G, Eychène A. Modulation of kinase activity and oncogenic properties by alternative splicing reveals a novel regulatory mechanism for B-Raf. J Biol Chem. 1998;273:24939–24947. doi: 10.1074/jbc.273.38.24939. [DOI] [PubMed] [Google Scholar]
- 52.Pessac B, Calothy G. Transformation of chick embryo neuroretinal cells by Rous sarcoma virus in vitro: induction of cell proliferation. Science. 1974;185:709–710. doi: 10.1126/science.185.4152.709. [DOI] [PubMed] [Google Scholar]
- 53.Pierani A, Pouponnot C, Calothy G. Transcriptional downregulation of the retina-specific QR1 gene by pp60v-src and identification of a novel v-src-responsive unit. Mol Cell Biol. 1993;13:3401–3414. doi: 10.1128/mcb.13.6.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pouponnot C, Nishizawa M, Calothy G, Pierani A. Transcriptional stimulation of the retina-specific QR1 gene upon growth arrest involves a Maf-related protein. Mol Cell Biol. 1995;15:5563–5575. doi: 10.1128/mcb.15.10.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pumiglia K M, Decker S J. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 1997;94:448–452. doi: 10.1073/pnas.94.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu R G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature (London) 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 57.Qiu R G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature (London) 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 60.Sarbassov D D, Peterson C A. Insulin receptor substrate-1 and phosphatidylinositol 3-kinase regulate extracellular signal-regulated kinase-dependent and -independent signaling pathways during myogenic differentiation. Mol Endocrinol. 1998;12:1870–1878. doi: 10.1210/mend.12.12.0205. [DOI] [PubMed] [Google Scholar]
- 61.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treinies I, Paterson H F, Hooper S, Wilson R, Marshall C J. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal to stimulate DNA synthesis. Mol Cell Biol. 1999;19:321–329. doi: 10.1128/mcb.19.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 64.Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of Ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westwick J K, Lambert Q T, Clark G J, Symons M, Van Aelst L, Pestell R G, Der C J. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 67.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 68.Wolthuis R M, Bauer B, van't Veer L J, de Vries-Smits A M, Cool R H, Spaargaren M, Wittinghofer A, Burgering B M, Bos J L. RalGDS-like factor (Rlf) is a novel Ras and Rap 1A-associating protein. Oncogene. 1996;13:353–362. [PubMed] [Google Scholar]
- 69.Wolthuis R M, de Ruiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolthuis R M, Franke B, van Triest M, Bauer B, Cool R H, Camonis J, Akkerman J W, Bos J L. Activation of the small GTPase Ral in platelets. Mol Cell Biol. 1998;18:2486–2491. doi: 10.1128/mcb.18.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolthuis R M, Bos J L. Ras caught in another affair: the exchange factors for Ral. Curr Opin Genet Dev. 1999;9:112–117. doi: 10.1016/s0959-437x(99)80016-1. [DOI] [PubMed] [Google Scholar]
- 72.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J J, Kang J S, Krauss R S. Ras signals to the cell cycle machinery via multiple pathways to induce anchorage-independent growth. Mol Cell Biol. 1998;18:2586–2595. doi: 10.1128/mcb.18.5.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu J, Woods D, McMahon M, Bishop J M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]