Abstract
Sickness behavior is the common denominator for a plethora of changes in normal behavioral routines and systemic metabolism during an infection. Typical symptoms include temperature, muscle weakness, and loss of appetite. Whereas we experience these changes as a pathology, in fact they are a carefully orchestrated response mediated by the immune system. Its purpose is to optimize immune cell functionality against pathogens whilst minimizing viral replication in infected cells. Sickness behavior is controlled at several levels, most notably by the central nervous system, but also by other organs that mediate systemic homeostasis, such as the liver and adipose tissue. Nevertheless, the changes mediated by these organs are ultimately initiated by immune cells, usually through local or systemic secretion of cytokines. The nature of infection determines which cytokine profile is induced by immune cells and therefore which sickness behavior ensues. In context of infection, sickness behavior is typically beneficial. However, inappropriate activation of the immune system may induce adverse aspects of sickness behavior. For example, tissue stress caused by obesity may result in chronic activation of the immune system, leading to lasting changes in systemic metabolism. Concurrently, metabolic disease prevents induction of appropriate sickness behavior following viral infection, thus impairing the normal immune response. In this article, we will revisit recent literature that elucidates both the benefits and the negative aspects of sickness behavior in context of viral infection.
Keywords: infection, sickness behavior, metabolism, appetite, cytokines, T cells, coronavirus, cytomegalovirus, anorexia, nausea, metabolic disease, diabetes
1. Introduction
Infection has a major impact on our systemic physiology. Especially when the pathogenic load is high, we display symptoms such as nausea, muscle weakness, tiredness, and temperature, which are generally referred to as sickness behavior [1]. Whereas we experience these changes as pathology, in fact, they are a carefully regulated response. Following infection, the immune system induces changes of systemic metabolism through the secretion of cytokines, which either alter metabolism of tissues directly or change normal endocrine control of systemic metabolism [2,3]. Concurrently, the immune system itself undergoes major changes in its requirement and consumption of nutrients. It has been estimated that during infection, the activated immune system may use up to 30% of all nutrients in the body [4]. Counterintuitively, despite its increased demand for nutrients, the activated immune system discourages nutrient intake whilst promoting metabolic changes that lower nutrient release in the bloodstream, thus restricting systemic nutrient availability [5]. The purpose of metabolic changes in response to infection appears to be twofold. On the one hand, the immune system restricts nutrient availability to most organs to limit pathogen replication. This is particularly true for glucose, which is a nutrient that many pathogens both of viral and bacterial origin use to promote their replication [6,7]. As a result, infection promotes a form of metabolism that is normally associated with fasting [5]. This is further enhanced by actual fasting due to reduced appetite and nausea. At the same time, the immune system aims to optimize access to nutrients by immune cells. Many activated immune cells, such as CD8 T cells and pro-inflammatory macrophages, favor glycolysis for their metabolic needs [8]. Therefore, these cells tend to highly upregulate glucose transporters on their cell surface, allowing them to take up enough glucose even when systemic concentrations are low. At the same time, many immune cells use fatty acids and ketones to fulfill their metabolic needs [9,10], allowing them to operate even in context of the fasting-type metabolism that is induced in response to infection. As such, both systemic metabolism and immunometabolism are adjusted in response to infection, and this is what we experience as sickness behavior.
The binal goals of immune-mediated changes to metabolism in response to infection can become pathological, most notably in context of metabolic syndrome (MS). MS is a condition that is typically the result of chronic obesity and is associated with increased blood pressure, high blood sugar, excess abdominal fat, hyperlipidemia, and increased blood cholesterol levels. When patients have at least three of these conditions, they are diagnosed with MS [11]. Immune-mediated changes to systemic- and immune-metabolism are negatively impacted by MS in two ways. On the one hand, because of deregulated systemic metabolism, people with MS cannot induce optimal metabolic changes to fight infection. People with MS therefore experience more infections, with longer duration and more severe symptoms [12]. For example, following infection with SARS-CoV-2 or influenza, people with diabetes mellitus type 2 (T2D) showed an increased risk of developing severe symptoms and had a higher mortality rate [13,14]. On the other hand, many immune-mediated changes of systemic metabolism are pro-diabetic. As a result, the immunological response to infection can aggravate symptoms of MS, such as defective blood glucose regulation [2,15].
In this review, we will revisit recent literature on the molecular mechanisms underlying the impact of the activated immune system on sickness behavior following infection. A particular focus will be on viral infection.
2. Immune-Mediated Sickness Behavior in Response to Infection
Upon infection, a carefully regulated cascade of events is initiated in which the immune system plays a central role. Infected tissues communicate the stress of pathogen invasion to the immune system within minutes to hours after pathogen encounter through the secretion of alarm molecules, such as type-I interferons, and by upregulating stress ligands on their cell surface [16]. These events recruit innate immune cells to the site of infection, which are further activated by recognition of pathogen-associated molecular patterns (PAMPs) on microbes or infected cells. Consequently, these cells start fighting the infection and secrete more specialized cytokines that help protect nascent cells from being infected and activate the adaptive immune system [17,18].
Sickness behavior presents in many ways, each regulated through a particular mechanism but typically initiated by cytokines. The central nervous system (CNS) plays a central role in the mediation of sickness behavior and many cytokines, most notably IFN-γ, TNF, IL-1β, and IL-6, therefore directly affect our brain. The production of these signaling molecules correlates with sickness behavior, such as fever, inability to focus, behavioral changes, and fatigue [17]. The hypothalamus is the part of the brain that is most closely associated with maintenance of systemic homeostasis. Many inflammatory signals therefore converge on this part of the CNS, which then mediates sickness behavior. Under stressful conditions, such as injury, inflammation, or infection, the hypothalamus causes an increase of the body’s temperature. Pyrogenic cytokines, including IL-1β, TNF, and IL-6, stimulate the endothelium of the brain to produce prostaglandins, most notably PGE2. These prostaglandins bind and activate the receptor EP3R on neurons, resulting in a central signal that promotes release of norepinephrine in the body [19]. This in turn leads to heat-promoting behavior, such as vasoconstriction and shivering, as well as changes in the metabolic rate of cells in the periphery, for instance, through induction of thermogenesis in brown adipose tissue [18,20,21,22]. Fever promotes the immune response against infection in several ways. Increased body temperature has been shown to promote migration and adhesion of lymphocytes to the draining lymph nodes in infection through the T-cell thermal sensory pathway [23]. NK cells show increased cytotoxic activity and increased migration to sites of inflammation under conditions of increased body temperature [20]. Finally, CD4 T cells modulate their immunological behavior under conditions of moderate fever by induction of GATA-3 and promoting the secretion of IFNγ, IL-4, and IL-13 [24].
Fatigue is a common sickness behavior following infection, which can persist for months even after the pathogen is cleared [25]. Many processes mediated by immune cell activation can lead to fatigue. Pro-inflammatory cytokines produced in infection can cause fatigue through modification of metabolic pathways through an endocrine loop, initiated in the hypothalamus and amplified by the pituitary gland. This promotes the release of corticotrophin-releasing hormone, cortisol, and adreno-corticotropic hormones and disrupts nutrient homeostasis in tissues such as liver, muscle, and adipose tissue. Intravenous injection of TNF, IL-1β, and IL-6 was therefore shown to induce fatigue in humans [26], whereas inhibition of TNF signaling prevents this symptom [18]. Other causes of fatigue include oxidative stress induction and impairment of systemic metabolism [26]. Cytokines such as type-I interferons can directly stimulate the CNS and mediate fatigue through neuropsychiatric effects [27]. In addition, cytokines such as IFNγ promote insulin resistance (IR) in many tissues, including the muscle, thus reducing its glucose uptake and anabolic activity [2]. In addition, IR is associated with a shift in serum lipid concentrations, and people with acute infection have been shown to have an increase in free fatty acids (FFA) and triglycerides in circulation [28,29]. As a result, activity of glucose- intensive organs, such as skeletal muscle, is reduced, thus contributing to fatigue.
Another frequent manifestation of sickness behavior is anorexia, i.e., the loss or lack of appetite and subsequent weight loss. During infection, our immune system requires nutrients for energy and activation, but surprisingly, we reduce our food intake when we feel sick. Systemic administration of cytokines, such as TNF and IL-18, were shown to cause anorexia by directly targeting receptors in the central nervous system, most notably in the Stria Terminalis [30,31]. As a result, instead of using external sources of energy, sickness promotes the utilization of alternative, internal nutrient sources. For example, FFA are released from adipose tissue and are used as a source of energy in the liver, which cfan lead to weight loss [18]. The pro-anorexic effects of cytokines are not restricted to the CNS. Cytokines can act directly on adipose tissue and stimulate their production of hunger-suppressing adipokines, such as leptin. In hamsters, TNF and IL-1β produced in response to LPS administration were shown to increase leptin expression in adipose tissue and induce anorexia [32]. Leptin acts on the brain, where it inhibits feeling of hunger and lipogenesis in adipose tissue. Leptin is also important for the activation and proliferation of NK cells, T cells, and dendritic cells, suggesting that the benefits of its increased concentrations in the blood reach beyond control of food intake alone [5]. Finally, the pro-inflammatory cytokine TNF can directly trigger the expression of hormone-sensitive lipase in adipocytes. This enzyme hydrolyzes triglycerides, resulting in the release of FFA and triglycerides from adipose tissue. TNF administration can therefore cause a reduction of overall adipose tissue size and a concomitant increase of these molecules in circulation [18].
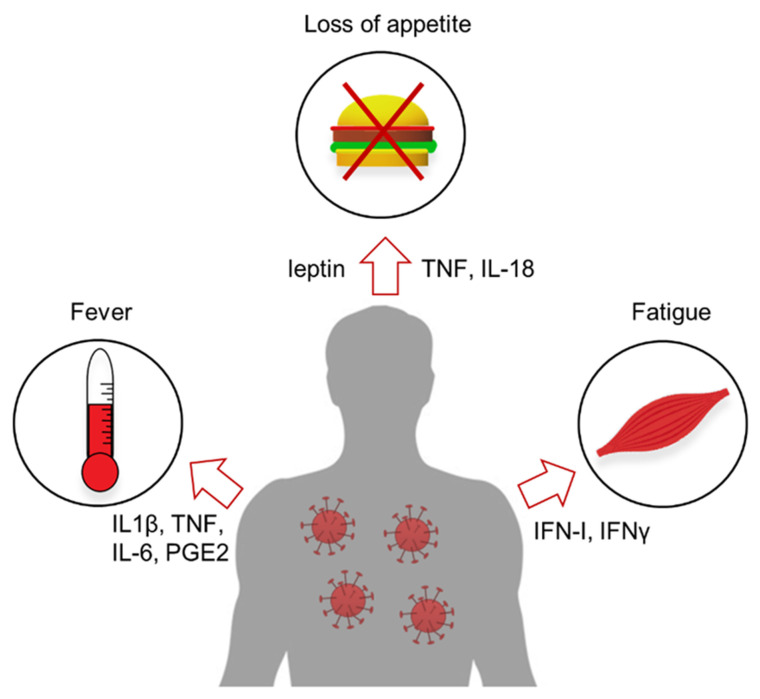
In summary, in response to infection, a complex interaction between the immune, endocrine, and CNS transiently alters the systemic nutrient availability and consumption of peripheral organs. As a result, these tissues alter their metabolic state, which leads to sickness behavior (Figure 1).
Figure 1.
Immunological mediators produced in context of viral infection induce sickness behavior by targeting various organs in our body. Prime examples are (1) the induction of fever by cytokines. TNF and IL-6, which stimulate endothelium in the brain and promote their release of prostaglandin PGE2, which directly target the thermoregulatory center in the CNS; (2) induction of anorexia by cytokines such as TNF and IL-18, which promote release of leptin and suppress appetite; and (3) induction of fatigue by Type-I interferons and IFNγ, which directly target muscle cells.
3. Molecular Mechanisms of Immune-Mediated Changes in Metabolism following Infection
Whereas the impact of individual cytokines on systemic metabolism is becoming increasingly clear, how they mediate these effects on a molecular level has long remained uncertain. However, much progress has been made in recent years, especially for adipose tissue and the liver [33]. Adipose tissue is the body’s main storage location of excess nutrients in the form of triglycerides. Much study has been devoted to the impact of cytokines on adipose tissue in context of obesity, as this organ was shown to be the source of inflammatory processes that contribute to development of metabolic disorders [34]. IFNγ is one of the most highly induced cytokines in context of viral infection and has been shown to down-regulate PPARγ in adipocytes [35]. PPARγ is a master regulator of adipose differentiation, lipogenesis, and glucose metabolism. The murine 3T3-L1 cell line is commonly used to study adipogenesis, as these cells can be differentiated into mature adipocytes in vitro. When treated with IFNγ, IFN-β, or TNF, 3T3-L1 cells showed increased insulin resistance through reduction of PPARγ levels [36,37]. In human adipocytes, IFNγ-mediated PPARγ downregulation was shown to lead to a reduction in insulin-mediated glucose uptake, reduced surface expression of the glucose transporter GLUT4, and therefore to decreased triglyceride storage [38]. Similarly, IL-1β reduces insulin-mediated glucose uptake and expression of the GLUT4 glucose transporter in adipose tissue [39]. The impact of typical Type-2 cytokines, such as IL-4, on adipose cell biology is much less clear. Whereas treatment with IL-4 reduced adipogenesis and increased lipolysis in adipose 3T3-L1 cells, in primary rat adipocytes this cytokine prevented lipolysis [40,41]. Cytokine-mediated lipolysis can become pathological if it induces a complete breakdown of adipose and muscle tissue, a state that is referred to as cachexia. Cachexia is a common phenomenon in late-stage cancer patients but can also occur in context of severe or chronic infection [42]. In a mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection, serum levels of triglycerides and FFA were strongly increased because of excessive lipid secretion and reduced lipid uptake by adipose tissue, resulting in cachexia. Chronic LCMV infection caused reduction in mRNA expression of the DGAT2 and LPL enzymes involved in triglyceride synthesis and hydrolysis of triglycerides from lipoproteins, respectively. These changes were associated with IFN-I-mediated stimulation of CD8 T cells. How they lead to a “point of no return” in lipid degradation still requires investigation [42].
The liver is one of the primary organs in the body responsible for maintenance of systemic homeostasis of nutrients, such as glucose, vitamins, and lipids. In that role, the liver is involved in a plethora of metabolic processes, including the processing and synthesis of carbohydrates, proteins, and fatty acids [43]. Not surprisingly, the liver also plays a key role in mediating systemic metabolic changes in context of infection. Cytokines such as IL-1β and IL-6, molecules for which expression is highly induced following viral infection, bind to their receptors on hepatocytes [44]. In response to these cytokines, hepatocytes upregulate expression of complement factors as well as antimicrobial proteins and peptides, such as hepcidin [45]. For example, acute influenza virus infection was shown to increase the expression of hepatic enzymes involved in metabolism of fatty acids, leading to lipid accumulation in liver cells [46]. In addition to being a metabolic organ, the liver is also an endocrine gland that impacts regulation of systemic metabolism through the secretion of hormones. Hepatocytes produce insulin-like growth factor (IGF-1), which is an anabolic hormone for which expression is strongly increased in response to infection. Moreover, in mice, IGF-1 was shown to enhance weight loss following influenza infection [47].
Typically, infection-induced changes of systemic metabolism are of transient nature, but in case of chronic infection of the liver, for example, with the hepatitis C virus (HCV), long-lasting changes and tissue damage may be induced. In chronic HCV infection, hepatocytes were shown to have impaired lipoprotein production, which could lead to dyslipidemia. Moreover, HCV infection led to increased expression of ferritin, resulting in the accumulation of iron and ultimately leading to liver toxicity [48,49,50].
Thus, both through direct and indirect actions, cytokines have a profound effect on systemic metabolism and the development of sickness behavior.
4. Nutrient Requirements of Activated Immune Cells
Following activation, immune cells undergo extensive changes in their metabolism to allow for rapid proliferation and acquisition of effector functions. Many excellent reviews on immunometabolism have been written [4,8], and we will therefore only briefly address this topic here. However, since the metabolism of immune cells is directly linked to their functionality, it is important to realize that the state of systemic metabolism therefore also impacts the efficiency of the immune system.
In absence of infection, resting immune cells, such as memory and naïve T cells, M2 macrophages, and dendritic cells, depend mostly on oxidative phosphorylation to fulfill their energetic needs, which is the most efficient way to make use of available nutrients for the generation of ATP [33,51,52]. When these cells become activated, their metabolic requirements shift, and they start using predominantly aerobic glycolysis to fulfill their energetic needs. Moreover, nutrients are shuttled into the pentose phosphate pathway to generate proteins, nucleic acids, and other building blocks that they need for their activation, proliferation, and growth [9,53]. Activated immune cells massively increase their consumption of high-energetic nutrients, such as glucose and glutamate. Following infection, therefore, up to 30% of all nutrients in the body can be directed towards the activated immune system [54,55]. As a result, in the absence glutamate, activated T cells have a strongly reduced ability to proliferate and produce cytokines [56]. Similarly, depletion of glucose inhibits production of IFN-γ, perforin, granzyme, and proliferation by activated T cells and NK cells [57].
Whereas infection causes an increase in nutrient requirements by the immune system, at the same time, the immune system reduces nutrient intake by inducing sickness behavior. This apparent paradox is resolved by a switch of systemic metabolism to a state normally associated with fasting. This form of metabolism depends on the utilization of triglyceride stores from adipose tissue and is characterized by an increased concentration of FFA and ketones in circulation [42]. At the same time, immune cells remain functioning optimally despite their increased needs of nutrients, such as glucose. One explanation is that activated immune cells strongly upregulate glucose transporters on their cell surface, allowing them to retain functionality even when glucose levels are as low as 0.5 mM [58]. Second, it appears that not all activated immune cells preferentially use glucose as a source of nutrients. B lymphocytes are essential for the generation of a high-affinity neutralizing antibody response following infection. They accomplish this after formation of a specialized structure called the germinal center, where activated B cells proliferate, differentiate, and undergo somatic hypermutation [59]. Surprisingly, B cells increase oxidative phosphorylation in the tricarboxylic acid cycle and not glycolysis early after activation [60]. Moreover, germinal center B cells predominantly use fatty acid oxidation as a primary energy source both in vitro and in vivo. Glycolysis is used minimally to fulfil metabolic needs by these cells [10]. Importantly, inhibition of fatty acid oxidation results in a strong reduction of germinal center formation in vivo [10]. Thus, the metabolism of various activated immune cells is in fact promoted by sickness behavior that mediates a switch to fasting-type metabolism.
Lipids are also important for the functionality of other immune cells. Macrophages play an important role in the early defense against pathogens. They require metabolic reprogramming to become activated, after which they produce pro-inflammatory cytokines and conduct their phagocytic activity. M1-activated macrophages were shown to increase lipid production through the mTOR signaling pathway, which increases their plasma membrane fluidity and facilitates phagocytosis of infected cells. Lipids can also increase phosphatidylcholine production, which activates the NLRP3 inflammasome and production of IL-1β and IL-18 by macrophages [61]. Cytokines themselves also play a major role in guiding the metabolic transformation of activated immune cells [62]. For example, IL-7, a cytokine important for lymphocyte development and growth, was shown to increase glucose uptake and glycolysis in T cells [63]. In contrast, IL-10, a molecule which inhibits immune cell activation and dampens the inflammatory response, impairs glycolysis and was found to inhibit maturation of activated dendritic cells [64].
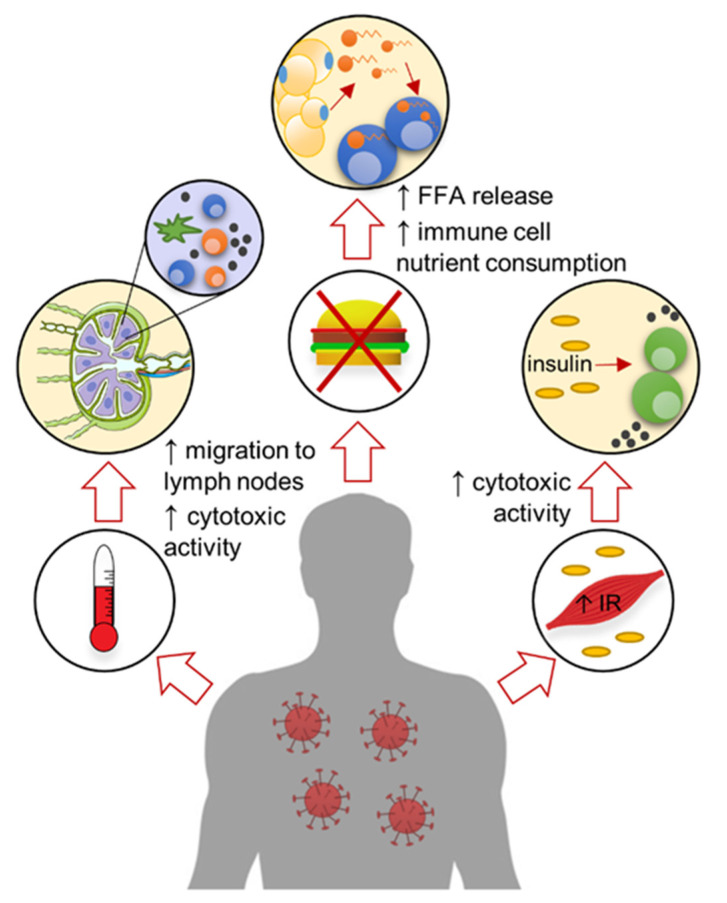
In summary, an important role of systemic metabolic changes manifested as sickness behavior is to facilitate immune cell metabolic reprogramming and activation to improve viral clearance (Figure 2).
Figure 2.
Sickness behavior is beneficial for immune cell activation. Fever can induce migration of cells to the lymph nodes and increase their cytotoxic activity. Loss of appetite induces the release of circulating free fatty acids (FFA) that are used as an energy source in immune cells. Muscle fatigue can be the consequence of insulin resistance (IR) and reduced glucose uptake, which increases systemic insulin levels and promotes the anti-viral T-cell response.
5. Pathogen-Mediated Changes of Metabolism
Whereas the immune system mediates changes in systemic homeostasis to benefit the immune response, pathogens also induce metabolic changes in their host to promote their own replication. These changes are most profound in the cells that have been directly infected, yet indirectly, they can also have a major impact on metabolite levels in entire tissues and even in circulation. At a cellular level, these changes have been characterized most extensively for viral infection. Upon entry into the cell, a virus rapidly alters the metabolism of its host to optimize production of new viral particles [65]. The nature of these changes varies greatly between pathogens [66] but typically increases the metabolic rate of the cell in order to accommodate the increased demand of anabolic processes required for viral particle assembly. A primary target of viral reprogramming is carbon metabolism. Viruses such as human cytomegalovirus (hCMV), Epstein Barr virus, influenza A, and dengue virus upregulate glucose transporters at the cell surface to boost glucose uptake and increase the rate of glycolysis in the cell [6]. Medium containing low glucose levels therefore strongly impairs replication of viruses, such as hCMV, in cell culture. Viruses also increase the efficiency of glucose utilization. hCMV, herpes simplex virus-1 (HSV-1), and zikavirus all increase oxidative phosphorylation to optimize ATP production [6,67].
Apart from glucose, viruses also promote the utilization of other molecules, such as glutamine, as an energy source. Infection with vaccinia virus, HSV-1, and adenoviruses was shown to increase glutamate uptake and enhance the activity of glutaminase and glutamate dehydrogenase [6,67,68]. Moreover, α-ketoglutarate, the product of glutaminolysis, was able to maintain viral growth and energy production under conditions of limiting glutamate availability [6,67,69]. Finally, many viruses boost lipid synthesis in the cell to promote the formation of the viral envelope. Lipids enable many viruses to infect their host by serving as receptors or co-factors for the formation of endosomes, which is usually the first step of cellular entry [66]. For hCMV and influenza A, it was shown that viral replication is reduced upon the pharmacological inhibition of acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) [70]. Thus, viruses can hijack several different metabolic pathways in order to boost cellular energy production and promote viral replication.
In addition to direct effects of the virus on the metabolism of the cells it has entered, infection may indirectly impact the metabolic state of the entire tissue in which they reside. Metabolic changes in infected cells alter the tissue-microenvironment of an organ and thereby its consumption of nutrients. This results in an altered endocrine signal coming from these organs, which causes a systemic response to restore homeostasis. For example, the liver is an important metabolic organ responsible for maintaining homeostasis of many different factors in circulation including lipids, glucose, and vitamins. Following LCMV infection of hepatocytes, the urea cycle becomes dysregulated, causing a relative decrease in ornithine in favor of arginine [71]. This shift in systemic nutrient availability has a direct negative impact on the ability of the body to fight the infection. In-vitro treatment of CD8 T cells with arginine resulted in a poor anti-viral response. Notably, arginase treatment of mice caused a reduction in TNF and IFN-γ production by CD8 T cells, which was associated with aggravated liver damage. Thus, the impact of viruses on hepatic urea metabolism appears a targeted strategy to impair the local immune response [71].
Viral infection was also shown to have a profound impact on systemic lipid levels. LCMV infection was shown to increase lipids in circulation. Trem2 is a protein that is important for lipid sensing. Animals with specific deficiency of Trem2 on myeloid cells therefore showed reduced viral titers and had less liver pathology upon LCMV infection [72]. Influenza A was shown to target the ability of the body to cope with oxygen radicals. Early after infection of mice with this virus, a reduction was observed in the levels of antioxidants, such as glutathione, in the liver [73]. Glutathione is one of the most potent antioxidants in our body and has been shown to be important for the response to viral infection [74]. Viruses, such as dengue, can target hepatocytes and cause liver damage by the induction of oxidative stress. Reactive aldehyde malondialdehyde levels and the ratio of GSSG and GSH were increased in infection, whereas superoxide dismutase was decreased, indicating increased sensitivity to oxygen radicals. Exogenous glutathione treatment impaired the reactive oxygen species production and thereby reduced viral entry into cells, thus illustrating the detrimental effect of oxygen radicals in context of infection [75].
Thus, through the induction of local changes in the cells that they infect, viruses can have a major impact on systemic metabolism, which impairs the antiviral immune response and promotes their replication.
6. Detrimental Effects of Metabolic Disease on Sickness Behavior
Patients with metabolic syndrome are well known to acquire common infections more frequently, and typically, the duration of disease is longer and with an increased risk of complications [76]. This has been best documented for patients with T2D, a disease in which people fail to maintain blood glucose levels below certain threshold values. People with T2D were shown to have more respiratory infections, urinary tract infections, and bacterial infections and showed a significant increase of infection-related death [14,77,78,79]. More recently, T2D was shown to significantly increase the risk of developing severe complications and death because of SARS-CoV-2 infection [13,80].
Various underlying causes have been proposed for the increased susceptibility of patients with metabolic syndrome to infection. These include reduced access of immune cells to infected tissues due to microvascular damage [81], disruptive immune cell skewing due to adipose tissue inflammation [3,34], and changes in endocrine hormones that can directly affect immune cell function [82,83]. However, aberrant sickness behavior appears to be a more prominent cause of immune disfunction in context of metabolic syndrome. As mentioned, a key sickness behavior is reduced nutrient intake and a shift to fasting metabolism, characterized by lower blood glucose levels [30]. Especially people with T2D have chronic hyperglycemia, meaning that they fail to lower blood glucose levels following infection. Hyperglycemia was shown to have a direct, detrimental effect on immune cell function [84,85]. Indeed, the level of glycated hemoglobin (HbA1c) positively correlates with the duration and severity of infection with a number of viral and bacterial pathogens [84]. Experimental hyperglycemia induced in mice by streptozotocin injections directly impairs macrophage activation in response to infection with Mycobacterium tuberculosis (TB). As a result, neutrophil recruitment and dendritic cell activation are reduced, causing a decrease in the cytokine production by these cells [86]. In addition, CD4 and CD8 T cells as well as NK cells showed reduced cytotoxicity and cytokine production in T2D patients infected with TB as a direct result of hyperglycemia [86].
Apart from providing an optimal metabolic environment for immune cells, a key purpose of inducing fasting metabolism in response to infection is to restrict nutrient availability to pathogens. Indeed, anorexia promotes the response to viral infection, which is counteracted by hyperglycemia [87]. At a molecular level, nutrient restriction promotes the innate stress response of cells to infection. Following a meal, muscle cells quickly take up glucose that enters the blood stream to lower glycemia, but a large fraction is returned into circulation in the form of lactate [88]. This lactate is used by many tissues as their primary source of energy [88]. In addition, lactate was shown to have an anti-inflammatory role, as it inhibits RIG-I signaling in response to viral RNA detection and impairs the subsequent type-I interferon response [89]. People with MS typically have increased levels of lactate in circulation [90], which impairs the innate response to viral infection.
Thus, metabolic syndrome negatively impacts the normal immune response to viral infection by preventing the induction of a metabolic state that is optimal for immune cell function and limits pathogen replication.
7. Detrimental Effects of Sickness Behavior on Metabolic Disease
Due to the close interaction between the immune system and tissues involved in regulating systemic metabolism, it is not surprising that infection also negatively impacts metabolic disease. Among endocrinologists, it is well known that infection causes major changes in blood values of key metabolic parameters, such as glucose, in patients with T2D. International guidelines therefore recommend screening for infection in patients that newly present with metabolic diseases, such as T2D [91]. However, the underlying molecular mechanisms only now start to become apparent. Pro-inflammatory cytokines, such as TNF and IFNγ, have long been known to induce systemic IR and patients with T2D have indeed chronic systemic presence of low levels of these cytokines [92]. Adipose tissue in obese individuals accumulates pro-inflammatory immune cells that respond to metabolic stress of hypertrophic adipocytes, resulting in the release of cytokines in the bloodstream [3,34]. However, barring severe infections, such as SARS-CoV-2 [15], most viruses do not typically induce an increase in blood glucose levels [2]. The increase of pro-inflammatory cytokines does lead to insulin resistance, most notably in skeletal muscle. This is normally compensated by increased insulin production of the pancreas [58,93,94], but it long remained unclear what the physiological benefit of this effect was to the host.
Insulin is an anabolic hormone that shares many of its downstream signaling components with those of co-stimulatory molecules, such as CD28 [95]. These two types of receptors therefore share several functional properties, such as the upregulation of glucose transporters on the cell surface upon ligand binding [95,96]. Many immune cells, including effector CD8 T cells, express the insulin receptor on their cell surface and can be stimulated by this hormone to increase their effector function (Figure 2 [61,97]). A key goal of infection-induced insulin resistance is therefore to increase systemic insulin levels in order to promote the effector CD8 T-cell response [2]. The sickness behavior associated with this phenomenon is that muscle cells take up nutrients with lower efficiency, thus contributing to muscle weakness. In metabolic disease, many people have underlying IR, which in some, has progressed to such a degree that blood glucose levels are beyond certain well-defined threshold levels, leading to the diagnosis of T2D. If people have increased blood glucose levels that do not yet reach these threshold levels, we speak of pre-diabetes, which is a high-risk state for development of T2D [98]. If metabolic changes induced by infection come on top of pre-existing IR, it may lead to progression from pre-diabetes to diabetes or worsen the current metabolic state of people with T2D [2,15]. Indeed, infection with several pathogens, such as hCMV, HCV, and SARS-CoV-2, were shown to be a risk factor for the development of T2D [15,99,100].
Apart from immune-mediated changes to systemic insulin levels, pathogen-induced alterations of insulin secretion have also been documented. Many pathogens, most notably viruses, can infect the pancreas and negatively impact insulin output by this organ. Indeed, several viruses have been associated with the destruction of pancreatic β-cells and the induction of type 1 diabetes [101]. Recently SARS-CoV-2, the virus that causes COVID-19, has been shown to infect and replicate in cells of both the exocrine and endocrine pancreas [102]. However, these observations were made on material obtained post-mortem. In addition, SARS-CoV-2 was shown to drive increased insulin output by the pancreas [15]. Whereas this pathogen therefore has a long-term detrimental effect on pancreatic β-cell numbers, its insulin output is currently unclear.
In summary, the metabolic changes induced by viral infection in order to mediate sickness behavior overlap to a certain extent with those observed in context of metabolic syndrome. Notably, it seems that some aspects of MS are in fact derailed sickness behavior reserved for the normal physiological response against viral infection. If MS and viral infection therefore co-occur in the same individual, it may lead to an aggravation of metabolic disease.
8. Conclusions
Sickness behavior in response to viral infection is not a pathology but a carefully orchestrated response mediated by the immune system. Its purpose is to optimize the immune response against the invading pathogen whilst minimizing viral replication in infected cells. The cytokine profile that is induced in response to a specific infection determines the metabolic change that is accomplished and thereby the nature of the sickness behavior. Sickness behavior can become pathological, for example when lipid degradation aggravates to cachexia in context of severe infection. In addition, inappropriate sickness behavior, such as the inflammatory response occurring in obesity, may contribute to the formation of metabolic disease. At the same time, metabolic disease prevents the development of appropriate sickness behavior following viral infection, thus impairing the immune response and increasing susceptibility to infection. In summary, immunology and metabolism are tightly interdependent in the fight against infection both at a cellular and a systemic level. Further research in this field will therefore benefit treatment of both metabolic and infectious disease.
Funding
This work was supported by a University of Rijeka Support grant (19-41-1551) and the Croatian Science Foundation (grant numbers IP-2016-06-8027 & IP-CORONA-2020-04-2045) to FMW.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Konsman J.P., Parnet P., Dantzer R. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/S0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 2.Šestan M., Marinović S., Kavazović I., Cekinović Đ., Wueest S., Turk Wensveen T., Brizić I., Jonjić S., Konrad D., Wensveen F.M., et al. Virus-Induced Interferon-γ Causes Insulin Resistance in Skeletal Muscle and Derails Glycemic Control in Obesity. Immunity. 2018;49:164–177. doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Wensveen F.M., Jelenčić V., Valentić S., Šestan M., Wensveen T.T., Theurich S., Glasner A., Mendrila D., Štimac D., Wunderich F.T., et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 4.Wensveen F.M., Valentic S., Sestan M., Turk Wensveen T., Polic B. Interactions between adipose tissue and the immune system in health and malnutrition. Semin. Immunol. 2015;27:322–333. doi: 10.1016/j.smim.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Wensveen F.M., Sestan M., Turk Wensveen T., Polic B. ‘Beauty and the beast’ in infection: How immune-endocrine interactions regulate systemic metabolism in the context of infection. Eur. J. Immunol. 2019;49:982–995. doi: 10.1002/eji.201847895. [DOI] [PubMed] [Google Scholar]
- 6.Thaker S.K., Ch’ng J., Christofk H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019;17:59. doi: 10.1186/s12915-019-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotz A., Goebel W. Glucose and glucose 6-phosphate as carbon sources in extra- and intracellular growth of enteroinvasive Escherichia coli and Salmonella enterica. Pt 4Microbiology. 2010;156:1176–1787. doi: 10.1099/mic.0.034744-0. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newsholme P., Curi R., Gordon S., Newsholme E.A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 1986;239:121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisel F.J., Mullett S.J., Elsner R.A., Menk A.V., Trivedi N., Luo W., Wikenheiser D., Hawse W.F., Chikina M., Smita S., et al. Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat. Immunol. 2020;21:331–342. doi: 10.1038/s41590-020-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 12.Turk Wensveen T., Gasparini D., Rahelic D., Wensveen F.M. Type 2 diabetes and viral infection; cause and effect of disease. Diabetes Res. Clin. Pract. 2021;172:108637. doi: 10.1016/j.diabres.2020.108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Quin R., Wang H., Shen Y., Du K., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020;36:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao Kondapally Seshasai S., Kaptoge S., Thompson A., Di Angelantonio E., Gao P., Sarwar N., Whincup P.H., Mukamal K.J., Gillum R.F., Holme I., et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montefusco L., Ben Nasr M., D’Addio F., Loretelli C., Rossi A., Pastore I., Daniele G., Abdelsalam A., Maestroni A., Dell’Acqua M., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021;3:774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wensveen F.M., Jelencic V., Polic B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018;9:441. doi: 10.3389/fimmu.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollmer-Conna U., Fazou C., Cameron B., Li H., Brennan C., Luck L., Davenport T., Wakefield D., Hickie I., Lloyd A. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol. Med. 2004;34:1289–1297. doi: 10.1017/S0033291704001953. [DOI] [PubMed] [Google Scholar]
- 18.Tizard I. Sickness behavior, its mechanisms and significance. Anim. Health Res. Rev. 2008;9:87–99. doi: 10.1017/S1466252308001448. [DOI] [PubMed] [Google Scholar]
- 19.Machado N.L.S., Bandaru S.S., Abbott S.B.G., Saper C.B. EP3R-Expressing Glutamatergic Preoptic Neurons Mediate Inflammatory Fever. J. Neurosci. 2020;40:2573–2588. doi: 10.1523/JNEUROSCI.2887-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans S.S., Repasky E.A., Fisher D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015;15:335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prajitha N., Athira S.S., Mohanan P.V. Pyrogens, a polypeptide produces fever by metabolic changes in hypothalamus: Mechanisms and detections. Immunol. Lett. 2018;204:38–46. doi: 10.1016/j.imlet.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt K.D., Chan C.W. Thermoregulation and fever in normal persons and in those with spinal cord injuries. Mayo Clin. Proc. 1992;67:469–475. doi: 10.1016/S0025-6196(12)60394-2. [DOI] [PubMed] [Google Scholar]
- 23.Lin C., Zhang Y., Zhang K., Zheng Y., Lu L., Chang H., Yang H., Yang Y., Wan Y., Wang S., et al. Fever Promotes T Lymphocyte Trafficking via a Thermal Sensory Pathway Involving Heat Shock Protein 90 and alpha4 Integrins. Immunity. 2019;50:137–151 e6. doi: 10.1016/j.immuni.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umar D., Das A., Gupta S., Chattopadhyay S., Sarkar D., Mirji G., Kalia G., Arimbasseri G.A., Durdik J.M., Rath S., et al. Febrile temperature change modulates CD4 T cell differentiation via a TRPV channel-regulated Notch-dependent pathway. Proc. Natl. Acad. Sci. USA. 2020;117:22357–22366. doi: 10.1073/pnas.1922683117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., O’Connor L., Leavy D., O’Brien K., Dowds J., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norheim K.B., Jonsson G., Omdal R. Biological mechanisms of chronic fatigue. Rheumatology. 2011;50:1009–1018. doi: 10.1093/rheumatology/keq454. [DOI] [PubMed] [Google Scholar]
- 27.Capuron L., Gumnick J.F., Musselman D.L., Lawson D.H., Reemsnyder A., Nemeroff C.B., Miller A.H. Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 28.Gallin J.I., Kaye D., O’Leary W.M. Serum lipids in infection. N. Engl. J. Med. 1969;281:1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- 29.Khovidhunkit W., Kim M.S., Memon R.A., Shigenaga J.K., Moser A.H., Feingold K.R., Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Aviello G., Cristiano C., Luckman S.M., D’Agostino G. Brain control of appetite during sickness. Br. J. Pharmacol. 2021;178:2096–2110. doi: 10.1111/bph.15189. [DOI] [PubMed] [Google Scholar]
- 31.Francesconi W., Sanchez-Alavez M., Berton F., Alboni S., Benatti C., Mori S., Nguyen W., Zorrilla E., Moroncini G., Tascedda F., et al. The Proinflammatory Cytokine Interleukin 18 Regulates Feeding by Acting on the Bed Nucleus of the Stria Terminalis. J. Neurosci. 2016;36:5170–5180. doi: 10.1523/JNEUROSCI.3919-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunfeld C., Zhao C., Fuller J., Pollack A., Moser A., Friedman J., Feingold K.R. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Investig. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alwarawrah Y., Kiernan K., MacIver N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018;9:1055. doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wensveen F.M., Valentic S., Sestan M., Turk Wensveen T., Polic B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 2015;45:2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 35.Waite K.J., Floyd Z.E., Arbour-Reily P., Stephens J.M. Interferon-gamma-induced regulation of peroxisome proliferator-activated receptor gamma and STATs in adipocytes. J. Biol. Chem. 2001;276:7062–7068. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- 36.Wada T., Hoshino M., Kimura Y., Ojima M., Nakano T., Koya D., Tsuneki H., Sasaoka T. Both type I and II IFN induce insulin resistance by inducing different isoforms of SOCS expression in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2011;300:E1112–E1123. doi: 10.1152/ajpendo.00370.2010. [DOI] [PubMed] [Google Scholar]
- 37.Xing H., Northrop J.P., Grove J.R., Kilpatrick K.E., Su J.L., Ringold G.M. TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology. 1997;138:2776–2783. doi: 10.1210/endo.138.7.5242. [DOI] [PubMed] [Google Scholar]
- 38.McGillicuddy F.C., Chiquoine E.H., Hinkle C.C., Kim R.J., Shah R., Roche H.M., Smyth E.M., Reilly M.P. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J. Biol. Chem. 2009;284:31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jager J., Gremeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J.F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szczepankiewicz D., Skrzypski M., Pruszynska-Oszmalek E., Kolodziejski P.A., Sassek M., Stefanska B., Nowak K., Szczepankiewicz A. Interleukin 4 affects lipid metabolism and the expression of pro-inflammatory factors in mature rat adipocytes. Immunobiology. 2018;223:677–683. doi: 10.1016/j.imbio.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Tsao C.H., Shiau M.Y., Chuang P.H., Chang Y.H., Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 2014;55:385–397. doi: 10.1194/jlr.M041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baazim H., Schweiger M., Moschinger M., Xu H., Scherer T., Popa A., Gallage S., Ali A., Khamina K., Kosack L., et al. CD8(+) T cells induce cachexia during chronic viral infection. Nat. Immunol. 2019;20:701–710. doi: 10.1038/s41590-019-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rui L. Energy metabolism in the liver. Compr. Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moshage H. Cytokines and the hepatic acute phase response. J. Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Z., Xu M.J., Gao B. Hepatocytes: A key cell type for innate immunity. Cell Mol. Immunol. 2016;13:301–315. doi: 10.1038/cmi.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno M., Sekiya T., Nomura N., Daito T.J., Shingai M., Kida H. Influenza virus infection affects insulin signaling, fatty acid-metabolizing enzyme expressions, and the tricarboxylic acid cycle in mice. Sci. Rep. 2020;10:10879. doi: 10.1038/s41598-020-67879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G., Zhou L., Zhang C., Shi Y., Dong D., Bai M., Wang R., Zhang C. Insulin-Like Growth Factor 1 Regulates Acute Inflammatory Lung Injury Mediated by Influenza Virus Infection. Front. Microbiol. 2019;10:2541. doi: 10.3389/fmicb.2019.02541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neves J.V., Wilson J.M., Rodrigues P.N. Transferrin and ferritin response to bacterial infection: The role of the liver and brain in fish. Dev. Comp. Immunol. 2009;33:848–857. doi: 10.1016/j.dci.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Kowdley K.V. Iron Overload in Patients with Chronic Liver Disease. Gastroenterol. Hepatol. 2016;12:695–698. [PMC free article] [PubMed] [Google Scholar]
- 50.Chaudhari R., Fouda S., Sainu A., Pappachan J.M. Metabolic complications of hepatitis C virus infection. World J. Gastroenterol. 2021;27:1267–1282. doi: 10.3748/wjg.v27.i13.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kedia-Mehta N., Finlay D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019;10:2123. doi: 10.1038/s41467-019-10015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly B., O’Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buck M.D., O’Sullivan D., Pearce E.L. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muehlenbein M.P., Hirschtick J.L., Bonner J.Z., Swartz A.M. Toward quantifying the usage costs of human immunity: Altered metabolic rates and hormone levels during acute immune activation in men. Am. J. Hum. Biol. 2010;22:546–556. doi: 10.1002/ajhb.21045. [DOI] [PubMed] [Google Scholar]
- 55.Straub R.H., Cutolo M., Buttgereit F., Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J. Intern. Med. 2010;267:543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 56.Carr E.L., Kelman A., Wu G.S., Gopaul R., Senkevitch E., Aghvanyan A., Turay A.M., Frauwirth K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cham C.M., Driessens G., O’Keefe J.P., Gajewski T.F. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobs S.R., Herman C.E., Maciver N.J., Wofford J.A., Wieman H.L., Hammen J.J., Rathmell J.C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen C.D., Okada T., Cyster J.G. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waters L.R., Ahsan F.M., Wolf D.M., Shirihai O., Teitell M.A. Initial B Cell Activation Induces Metabolic Reprogramming and Mitochondrial Remodeling. iScience. 2018;5:99–109. doi: 10.1016/j.isci.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan J., Horng T. Lipid Metabolism in Regulation of Macrophage Functions. Trends Cell Biol. 2020;30:979–989. doi: 10.1016/j.tcb.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Rathmell J.C., Vander Heiden M.G., Harris M.H., Frauwirth K.A., Thompson C.B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell. 2000;6:683–692. doi: 10.1016/S1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 63.Chehtane M., Khaled A.R. Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase II gene. Am. J. Physiol. Cell Physiol. 2010;298:C1560–C1571. doi: 10.1152/ajpcell.00506.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krawczyk C.M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R.J., Cross J.R., Jung E., Thompson C.B., Jones R.G., et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno-Altamirano M.M.B., Kolstoe S.E., Sanchez-Garcia F.J. Virus Control of Cell Metabolism for Replication and Evasion of Host Immune Responses. Front. Cell Infect. Microbiol. 2019;9:95. doi: 10.3389/fcimb.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heaton N.S., Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vastag L., Koyuncu E., Grady S.L., Shenk T.E., Rabinowitz J.D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011;7:e1002124. doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thai M., Thaker S.K., Feng J., Du Y., Hu H., Ting Wu T., Graeber T.G., Braas D., Christofk H.R. MYC-induced reprogramming of glutamine catabolism supports optimal virus replication. Nat. Commun. 2015;6:8873. doi: 10.1038/ncomms9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y., Clippinger A.J., Alwine J.C. Viral effects on metabolism: Changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011;19:360–367. doi: 10.1016/j.tim.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munger J., Bennett B.D., Parikh A., Feng X.J., McArdle J., Rabitz H.A., Shenk T., Rabinowitz J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lercher A., Bhattacharya A., Popa A.M., Caldera M., Schlapansky M.F., Baazim H., Agerer B., Gürtl B., Kosack L., Májek P., et al. Type I Interferon Signaling Disrupts the Hepatic Urea Cycle and Alters Systemic Metabolism to Suppress T Cell Function. Immunity. 2019;51:1074–1087 e9. doi: 10.1016/j.immuni.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kosack L., Gawish R., Lercher A., Vilagos B., Hladik A., Lakovits K., Bhattacharya A., Schliehe C., Mesteri I., Knapp S., et al. The lipid-sensor TREM2 aggravates disease in a model of LCMV-induced hepatitis. Sci. Rep. 2017;7:11289. doi: 10.1038/s41598-017-10637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hennet T., Peterhans E., Stocker R. Alterations in antioxidant defences in lung and liver of mice infected with influenza A virus. Pt 1J. Gen. Virol. 1992;73:39–46. doi: 10.1099/0022-1317-73-1-39. [DOI] [PubMed] [Google Scholar]
- 74.Morris D., Khurasany M., Nguyen T., Kim J., Guilford F., Mehta R., Gray D., Saviola B., Venketaraman V. Glutathione and infection. Biochim. Biophys. Acta. 2013;1830:3329–3349. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Wang J., Chen Y., Gao N., Wang Y., Tian Y., Wu J., Zhang J., Zhu J., Fan D., An J. Inhibitory effect of glutathione on oxidative liver injury induced by dengue virus serotype 2 infections in mice. PLoS ONE. 2013;8:e55407. doi: 10.1371/journal.pone.0055407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoeckle M., Kaech C., Trampuz A., Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med. Wkly. 2008;138:512–519. doi: 10.4414/smw.2008.12228. [DOI] [PubMed] [Google Scholar]
- 77.Muller L.M., Gorter K.J., Hak E., Goudzwaard W.L., Schellevis F.G., Hoepelman A.I., Rutten G.E.H.M. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 78.Gupta S., Koirala J., Khardori R., Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect. Dis. Clin. N. Am. 2007;21:617–638, vii. doi: 10.1016/j.idc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wensveen F.M., Sestan M., Turk Wensveen T., Polic B. Blood glucose regulation in context of infection. Vitam. Horm. 2021;117:253–318. doi: 10.1016/bs.vh.2021.06.009. [DOI] [PubMed] [Google Scholar]
- 82.Saisho Y. Importance of Beta Cell Function for the Treatment of Type 2 Diabetes. J. Clin. Med. 2014;3:923–943. doi: 10.3390/jcm3030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bandaru P., Shankar A. Association between plasma leptin levels and diabetes mellitus. Metab. Syndr. Relat. Disord. 2011;9:19–23. doi: 10.1089/met.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kornum J.B., Thomsen R.W., Riis A., Lervang H.H., Schonheyder H.C., Sorensen H.T. Diabetes, glycemic control, and risk of hospitalization with pneumonia: A population-based case-control study. Diabetes Care. 2008;31:1541–1545. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simonsen J.R., Harjutsalo V., Jarvinen A., Kirveskari J., Forsblom C., Groop P.H., Lehto M. Bacterial infections in patients with type 1 diabetes: A 14-year follow-up study. BMJ Open Diabetes Res. Care. 2015;3:e000067. doi: 10.1136/bmjdrc-2014-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ayelign B., Negash M., Genetu M., Wondmagegn T., Shibabaw T. Immunol.ogical Impacts of Diabetes on the Susceptibility of Mycobacterium tuberculosis. J. Immunol. Res. 2019;2019:6196532. doi: 10.1155/2019/6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang A., Huen S.C., Luan H.H., Yu S., Zhang C., Gallezot J.D., Booth C.J., Medzhitov R. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell. 2016;166:1512–1525 e12. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hui S., Ghergurovich J.M., Morscher R.J., Jang C., Teng X., Lu W., Esparza L.A., Reya T., Zhan L., Guo J.Y. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang W., Wang G., Xu Z.G., Tu H., Hu F., Dai J., Chang Y., Chen Y., Lu Y., Zeng H., et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell. 2019;178:176–189.e15. doi: 10.1016/j.cell.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y.D., Varasteh B.B., Reaven G.M. Plasma lactate concentration in obesity and type 2 diabetes. Diabete. Metab. 1993;19:348–354. [PubMed] [Google Scholar]
- 91.American Diabetes A. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S39–S41. doi: 10.2337/dc20-S004. [DOI] [PubMed] [Google Scholar]
- 92.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin. Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Fernandez-Real J.M., Lopez-Bermejo A., Vendrell J., Ferri M.J., Recasens M., Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/dc05-2068. [DOI] [PubMed] [Google Scholar]
- 94.Vafaeimanesh J., Parham M., Seyyedmajidi M., Bagherzadeh M. Helicobacter pylori infection and insulin resistance in diabetic and nondiabetic population. Sci. World J. 2014;2014:391250. doi: 10.1155/2014/391250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., Thompson C.B. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/S1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 96.Petersen M.C., Shulman G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsai S., Clemente-Casares X., Zhou A.C., Lei H., Ahn J.J., Chan Y.T., Choi O., Luck H., Woo M., Dunn S.E., et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018;28:922–934.e4. doi: 10.1016/j.cmet.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Tabak A.G., Herder C., Rathmann W., Brunner E.J., Kivimaki M. Prediabetes: A high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoo S.G., Han K.D., Lee K.H., La Y., Kwon D.E., Han S.H. Impact of Cytomegalovirus Disease on New-Onset Type 2 Diabetes Mellitus: Population-Based Matched Case-Control Cohort Study. Diabetes Metab. J. 2019;43:815–829. doi: 10.4093/dmj.2018.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salmon D., Bani-Sadr F., Loko M.A., Stitou H., Gervais A., Durant J., Rosenthal E., Quertainmont Y., Barange K., Vittecoq D., et al. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: Results from ANRS CO13 HEPAVIH. J. Hepatol. 2012;56:862–868. doi: 10.1016/j.jhep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 101.Filippi C.M., von Herrath M.G. Viral trigger for type 1 diabetes: Pros and cons. Diabetes. 2008;57:2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muller J.A., Gross R., Conzelmann C., Kruger J., Merle U., Steinhart J., Weil T., Koepke L., Bozzo C.P., Read C., et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.